- Quanzhou First Hospital Affiliated to Fujian Medical University, Quanzhou, China
Background: Immune checkpoint inhibitors (ICIs)-based neoadjuvant therapy has been regulatory approved in clinical practice since 2021. However, it is still difficult to determine which patients can benefit from it. Here, we conducted a meta-analysis to evaluate the predictive values of programmed cell death ligand 1 (PD-L1) in pan-cancer neoadjuvant immunotherapy.
Methods: We searched MEDLINE and EMBASE for randomized controlled trials (RCTs) to collect information regarding pathological complete response (pCR) and event-free survival (EFS) in patients with PD-L1-positive and PD-L1-negative tumors. Odd ratio (OR), hazard ratio (HR), and their 95% confidence intervals (CIs) were calculated.
Results: Totally, 10353 patients with 6 tumor types in 23 RCTs were included in this study. Neoadjuvant immunotherapy was associated with increased pCRs in both patients with PD-L1-positive (OR, 3.22; 95% CI, 2.25-4.61; P < 0.001) and PD-L1-negative tumors (OR, 2.07; 95% CI, 1.42-3.00; P < 0.001). However, compared with PD-L1 negative tumors, PD-L1 positive tumors benefited more from ICB-based neoadjuvant therapy (interaction effect, 0.65; 95% CI, 0.45-0.94; PInteraction = 0.01). Similarly, neoadjuvant immunotherapy resulted in favorable EFS in patients with PD-L1 positive (HR, 0.55; 95% CI, 0.46-0.66; P < 0.001) and PD-L1 negative tumors (HR, 0.70; 95% CI, 0.62-0.80; P < 0.001), the efficacy differences were also significant (interaction effect, 1.24; 95% CI, 1.03-1.50; PInteraction = 0.04).
Conclusion: Both patients with PD-L1-positive and PD-L1-negative tumors can benefit from neoadjuvant immunotherapy. However, the magnitude of efficacy is greater in patients with PD-L1-positive tumors. Accordingly, rather than serving as an independent marker for patient selection, PD-L1 expression is more effectively applied as a prognostic biomarker.
Introduction
Immune checkpoint inhibitors (ICIs) targeting programmed cell death protein 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) can significantly prolong the overall survival (OS) and have been standard therapeutic agents in a number of malignancies (1). In recent years, immune checkpoint blockade (ICB)-based neoadjuvant therapy has been gradually recognized as a potential treatment approach for various tumors. Accumulating evidence revealed that neoadjuvant immunotherapy, by promoting systemic anti-cancer immunity, was associated with eliminating potential micro-metastases, reduction of the tumor stages, improvement of the R0 resection rate, and enhancement of pathological responses (2). This notion differs greatly from the well-established paradigm of conventional neoadjuvant therapy, which is simply known as a means to decrease tumor size. Currently, neoadjuvant immunotherapy has been regulatory granted in several malignancies, including breast cancer in 2021, lung cancer in 2022, and melanoma in 2023 (2). Meanwhile, hundreds of clinical trials have been launched involving various other indications, which will undoubtedly lead to more approvals in the future.
ICB-based neoadjuvant therapy may postpone surgery if the disease advances or potentially induce life-threatening immune-related toxicities (3). However, to date, it is still difficult to determine which patients should be offered neoadjuvant immunotherapy. Considering the fundamental nature of these agents, PD-L1 expression status is usually considered biologically plausible for predicting tumor response (4). Indeed, the European Medicines Agency grants the application of nivolumab-based neoadjuvant setting exclusively for patients with PD-L1 positive lung cancer (5). However, a considerable number of exceptions are recorded in clinical practice. For example, a recent study revealed that pathologic complete responses (pCRs) were reported in over 20% patients with PD-L1 negative breast cancer treated with ICB-based neoadjuvant therapy (3). Accordingly, the predictive values of PD-L1 expression status remain undetermined. To address this issue, here, we conducted a meta-analysis to systematically assess the efficacy of neoadjuvant immunotherapy in both patients with PD-L1-positive and PD-L1-negative tumors.
Method
Search strategy and selection criteria
A systematic search of MEDLINE and EMBASE databases for published trials on neoadjuvant ICB in patients with solid tumors from inception to March 2025 was conducted without language restriction. In addition, abstracts from the American Society of Clinical Oncology conference, European Society for Medical Oncology conference, and American Association for Cancer Research conference were examined for potential updates on published trials. The keywords used for search included: cancer, clinical trial, neoadjuvant, immunotherapy, PD-1, and PD-L1.
Both inclusion and exclusion standards were pre-specified. To be eligible, studies had to meet the following criteria: (1) study design: randomized controlled trials (RCTs) irrespective of clinical phase; (2) population: over 18 years of age, had histologic confirmation resectable solid tumors; (3) intervention: at least one experimental arm of patients who were treated with neoadjuvant immunotherapy (monotherapy or combination strategy) irrespective of dosage or duration, and one control arm with treatment did not involve any ICIs; (4) outcomes: pCR and event-free survival (EFS) in both patients with PD-L1 positive and PD-L1 negative tumors. Studies were excluded if they were: (1) other studies on this topic, including review articles, retrospective studies, editorials, letters, nonclinical or pre-clinical papers, phase I and non-randomized phase II studies, comments, quality of life studies, and cost effectiveness analyses; (2) studies in the pediatric population, patients with hematological disease, or small sample size (n<50); (3) patients with active central nervous system metastases, autoimmune disease, and glucocorticoid or immunosuppressant use; (4) studies with irretrievable or insufficient information for our statistical analysis.
All investigators independently conducted the initial search, reviewed the title and abstract for relevance, and classified the potential papers as included, uncertain, and excluded. For uncertain trials, the full texts were examined for the confirmation of eligibility. When multiple publications of the same trial appeared, only the most recent and/or complete report was included in our analysis.
Data extraction and quality assessment
Relevant data were extracted independently by all investigators using a prespecified form. Extracted information were listed as follows: (1) study information, including study design, clinical phase, randomization stratified by PD-L1 expression status, PD-L1 detective method, neoadjuvant treatment regimens, and the intention-to-treat sample size; (2) baseline characteristics of the included patients, including age, cancer type, the definition of PD-L1 positive, and numbers of patients with PD-L1 positive and PD-L1 negative tumors, respectively; (3) data on treatment-related outcomes, including the number of patients who achieved pCR and information regarding EFS. Hazard ratios (HRs) for EFS and their 95% CIs stratified by PD-L1 level were extracted from each included study. Only studies that reported the outcome of interest were included in the relevant analysis.
Risk of bias was assessed by the Cochrane risk of bias tool (6). We examined every trial and scored it as high, low, or unclear risk of bias to the following criteria: random sequence generation; allocation concealment; blinding of participants and personnel to the study protocol; blinding of outcome assessment; incomplete outcome data; and selective reporting.
When disagreements occurred in terms of study selection, data extraction, and risk of bias assessment, all investigators double-checked the original data independently and discussed the potential problems together. The discrepancies were resolved when all authors came to an agreement.
Statistical analysis
The primary endpoints were the improvements of pCR and EFS in patients with PD-L1-positive and PD-L1-negative tumors who were treated with neoadjuvant immunotherapy compared with conventional treatment. Statistical heterogeneity between different trials and subgroups was evaluated by Cochrane’s Q statistic (7). The I2 statistic was estimated to evaluate the extent of inconsistency attributable to the heterogeneity across different trials. The assumption of homogeneity was considered invalid for I2 > 50% and P < 0.10. The heterogeneity of efficacy between patients who were PD-L1 positive and PD-L1 negative was assessed by an interaction test and expressed as P for interaction. To explore the potential sources of heterogeneity and to examine the influence of different exclusion standards on the overall efficacy of neoadjuvant immunotherapy, pre-defined subgroup analyses were also performed. In this study, the sensitivity analysis was conducted according to different masking methods, cancer type, drug target, randomization stratified by PD-L1 expression status, and clinical phase. Publication bias was evaluated through visual inspection of Begg’s funnel plots (8). The Egger linear regression test and the Begg rank correlation test were further conducted with the significance of P < 0.10 (8, 9).
All analysis was conducted by Stata version 12.0 and MedCalc 18.2.1 software. Two-sided P < 0.05 was considered statistically significant.
Results
Baseline characteristics of the included trials
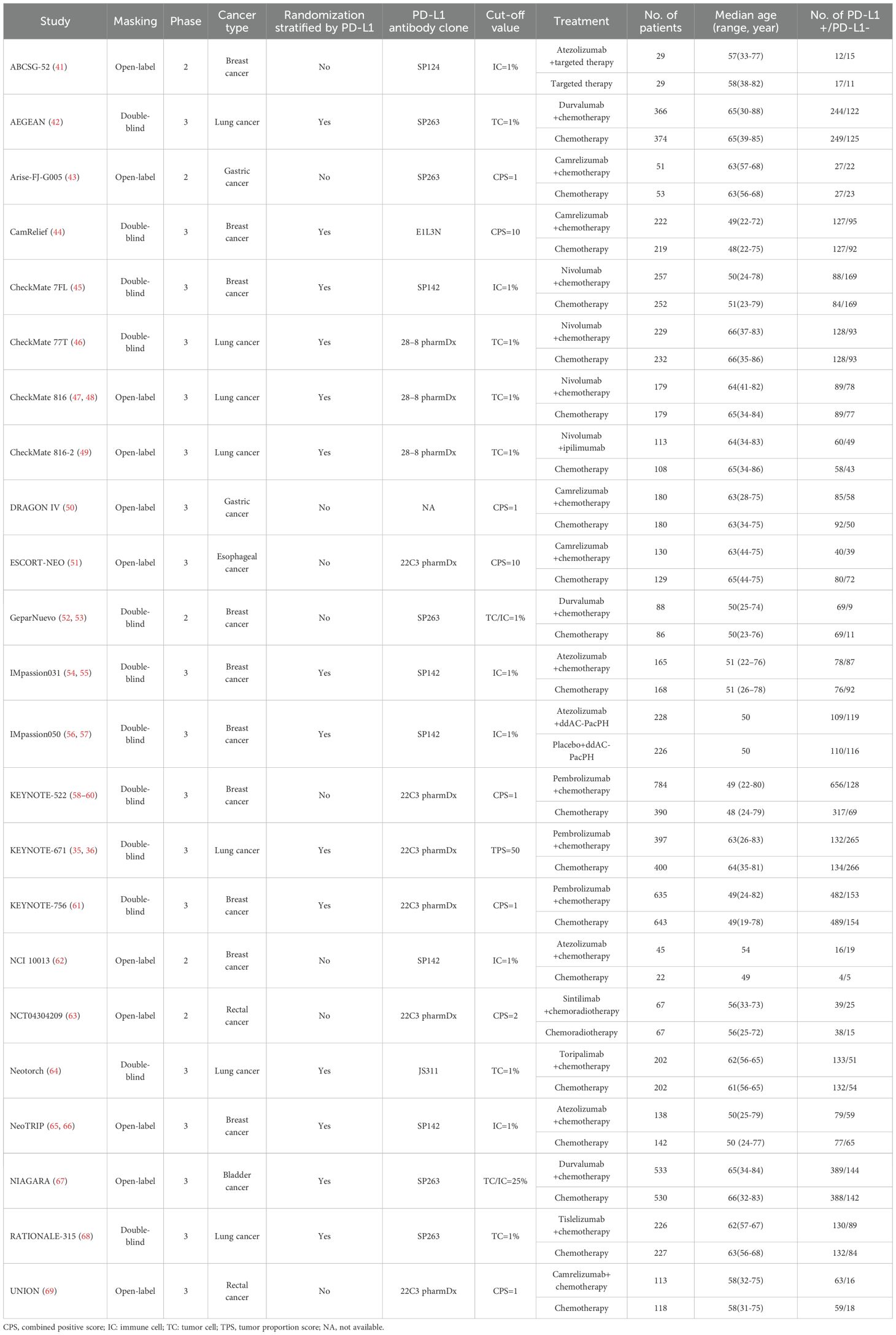
1514 relevant manuscripts were discovered from the initial search, including 879 studies from MEDLINE and 635 articles from EMBASE. Further examinations removed 1491 papers that failed to meet our inclusion criteria (Supplementary Figure S1). Totally, 10353 patients with 6 tumor types in 23 RCTs were included in this study (Table 1), with 4976 (48.1%) patients as controls, and 5377 patients (51.9%) in the experimental arms. Patients received agents targeting PD-1 in 14 trials (including camrelizumab in 5 RCTs, nivolumab in 3 trials, pembrolizumab in 3 studies, and sintilimab, toripalimab, and tislelizumab in 1 RCT each), agents targeting PD-L1 in 8 trials (including atezolizumab in 5 studies and durvalumab in 3 RCTs), and the combination of nivolumab and ipilimumab in 1 RCT. Among 23 eligible trials, 18 studies with 9816 patients (94.8%) were phase 3 RCTs, the rest 5 trials with 537 subjects (5.2%) were phase 2 studies. Breast cancer (n=4768) was investigated in 10 studies, lung cancer (n=3434) in 7 RCTs, gastric cancer (n=464) and rectal cancer (n=365) in 2 trials, and bladder cancer (n=1063) and esophageal cancer (n=259) in 1 RCT. According to the trial designs, the randomization stratified by PD-L1 expression status was conducted in 14 trials with 7792 cancer patients (75.3%). Totally, 6251 patients with PD-L1-positive tumors and 3750 subjects with PD-L1-negative tumors were identified. Among them, 3275 patients with PD-L1-positive cancer and 1904 subjects with PD-L1-negative cancer were treated with neoadjuvant ICB, while 2976 patients with PD-L1-positive diseases and 1846 subjects with PD-L1-negative diseases were in the control arms.
The method qualities of the eligible RCTs, assessed by the Cochrane risk of bias tool (6), were generally moderate to good; the major issue was lack of blinding since 11 trials were open-labelled.
Efficacy of neoadjuvant immunotherapy and PD-L1 expression status
Overall, in 19 RCTs with 6600 patients, compared with conventional treatment, ICB-based neoadjuvant therapy was associated with more pCRs (34.5% vs. 20.6%; odds ratio [OR], 2.66; 95% CI, 2.04-3.46; P < 0.001; Figure 1). There were significantly increased pCRs in both patients with PD-L1-positive (40.5% vs. 22.6%; OR, 3.22; 95% CI, 2.25-4.61; P < 0.001) and patients with PD-L1-negative tumors (25.8% vs. 17.6%; OR, 2.07; 95% CI, 1.42-3.00; P < 0.001). Of note, the magnitude of efficacies was greater in patients with PD-L1 positive tumors compared with patients with PD-L1 negative tumors (interaction effect, 0.65; 95% CI, 0.45-0.94; PInteraction = 0.01). Subgroup analysis based on masking method, clinical phase, cancer type, drug target, and randomization stratified by PD-L1 status, showed similar results but to a lesser extent (Supplementary Figure S2).
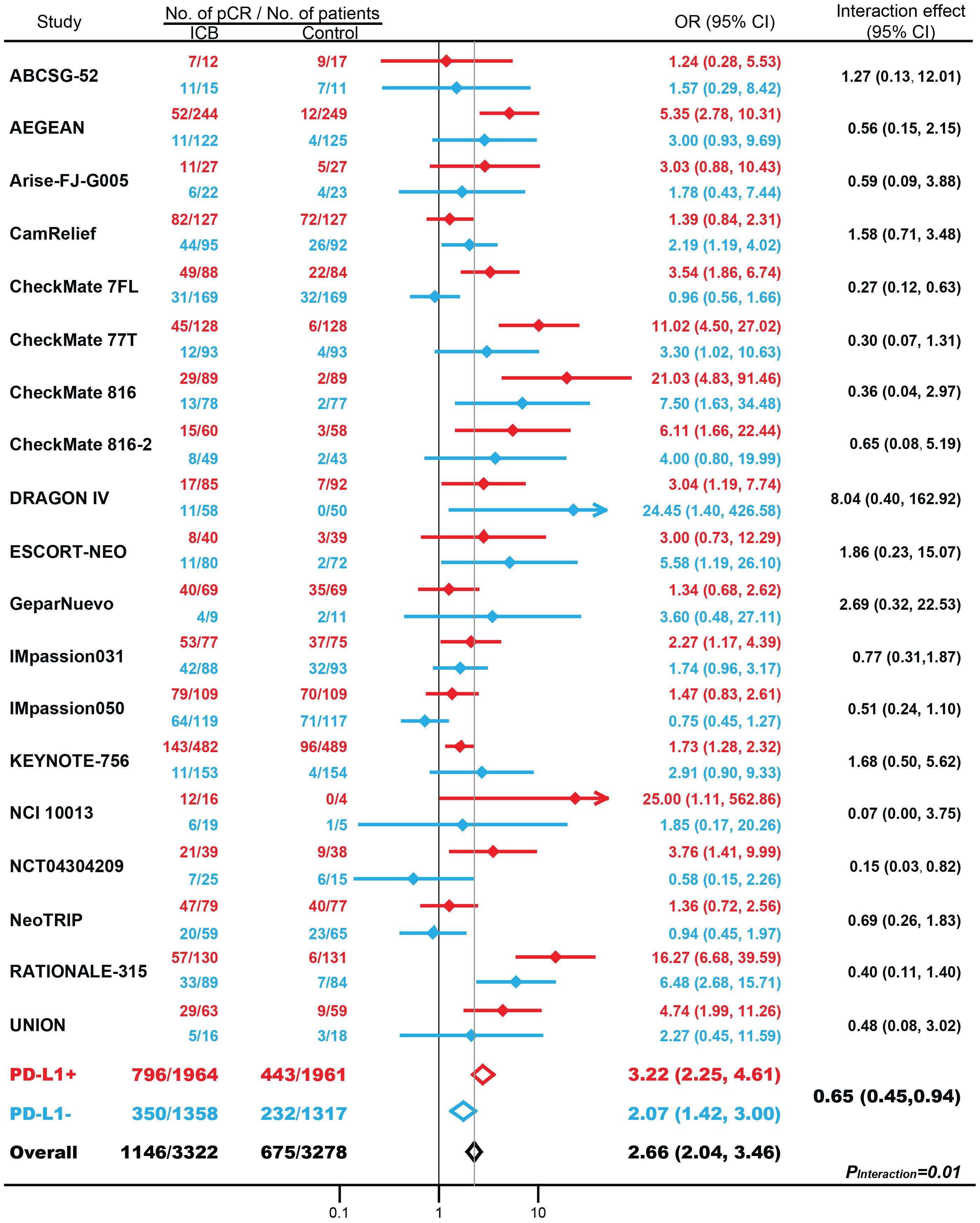
Figure 1. The association between PD-L1 expression status and pathological complete response (pCR) in patients treated with immune checkpoint blockade (ICB)-based neoadjuvant therapy. OR, odds ratio. Red indicates patients with PD-L1-positive tumors; Blue indicates patients with PD-L1-negative tumors.
Totally, 16 eligible RCT examined the association between PD-L1 expression and pCR with the thershold for PD-L1 expression was set as 1%. 1758 patients with PD-L1 positive tumors and 1158 individuals with PD-L1 negative tumors were treated with immune checkpoint inhibitors, while 1757 patients with PD-L1 positive tumors and 1138 individuals with PD-L1 negative tumors were included in the control arms. For patients with PD-L1 positive tumors, 685 patients (39.0%) responded to ICB, while 359 pCRs (20.4%) were observed in control arms. The difference was significant (OR, 2.54; 95% CI, 2.14-3.02; P < 0.001). similarly, for PD-L1 negative tumors, more pCRs were identified in patients treated with ICB (n=288, 24.9%) than in control arms (n=198, 17.4%) (OR, 1.57; 95% CI, 1.24-1.99; P = 0.002). The magnitude of efficacies was greater in patients with PD-L1 positive tumors compared with patients with PD-L1 negative tumors (PInteraction = 0.001).
In 11 studies with 6172 patients, ICB-based neoadjuvant therapy was associated with favorable EFS (hazard ratio [HR], 0.63; 95% CI, 0.56-0.71; P < 0.001; Figure 2). The efficacies of neoadjuvant immunotherapy were significantly improved in both patients with PD-L1-positive (HR, 0.55; 95% CI, 0.46-0.66; P < 0.001) and PD-L1-negative tumors (HR, 0.70; 95% CI, 0.62-0.80; P < 0.001). Patients with PD-L1-positive tumors benefit more from neoadjuvant immunotherapy compared with patients with PD-L1-negative tumors (interaction effect, 1.24; 95% CI, 1.03-1.50; PInteraction = 0.04). Further subgroup analysis based on masking method, cancer type, drug target, and randomization stratified by PD-L1 status, was shown in Supplementary Figure S3. Interestingly, partly due to fewer trials included, the superiority of EFS benefits in PD-L1-positive tumors over PD-L1-negative tumors was not as great as the pCR benefits.
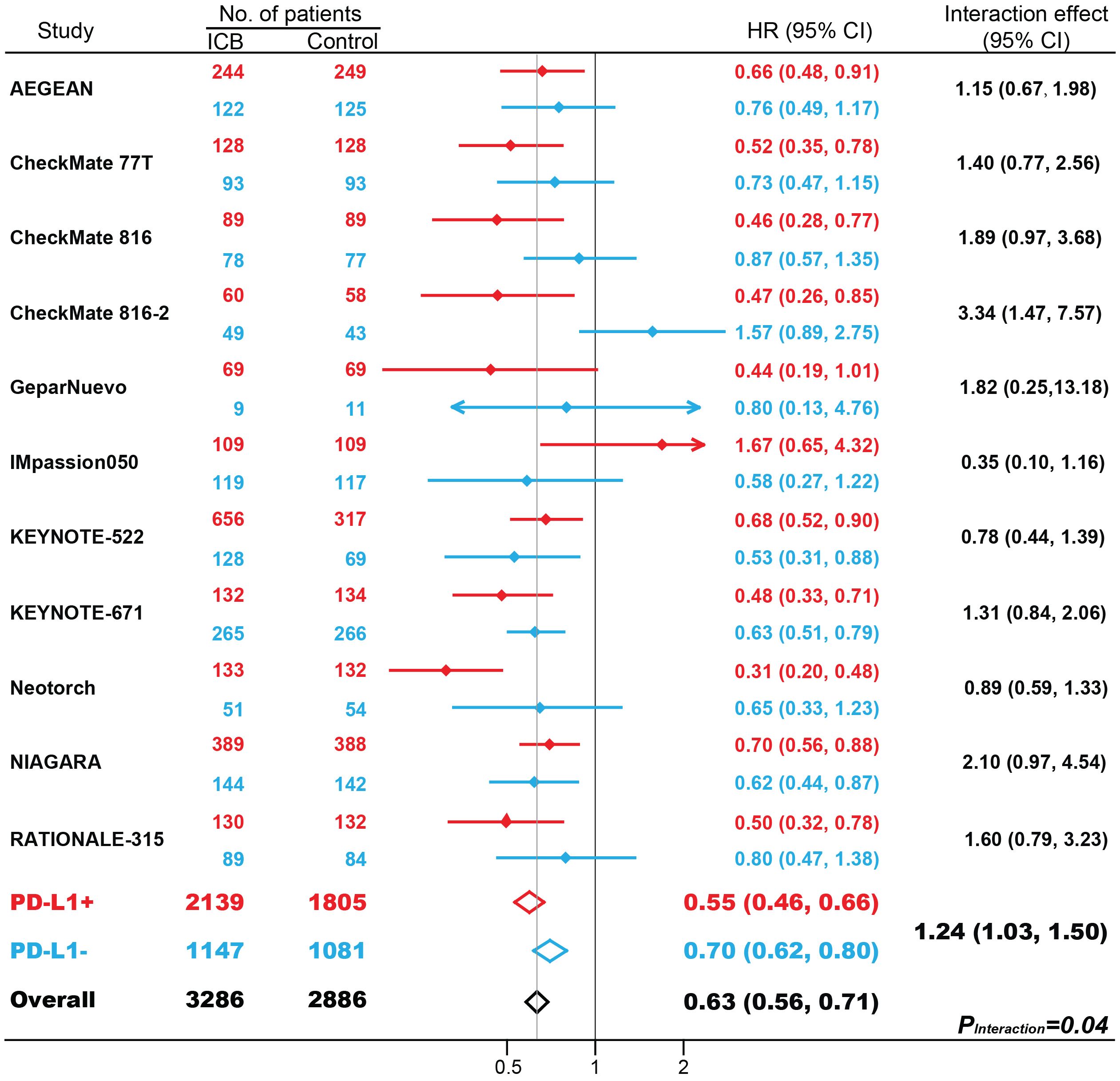
Figure 2. The impact of PD-L1 expression on event-free survival (EFS) in patients treated with immune checkpoint blockade (ICB)-based neoadjuvant therapy. HR, hazard ratio. Red indicates patients with PD-L1-positive tumors; Blue indicates patients with PD-L1-negative tumors.
No significant asymmetry was identified by visual inspection of Begg’s funnel plot (Supplementary Figure S4).
Discussion
For the first time to our knowledge, this study, based on high-quality RCTs including the largest sample size to date, reveals that both patients with PD-L1-positive and PD-L1-negative tumors can benefit from neoadjuvant immunotherapy. However, it should be noted that, compared with patients with PD-L1-negative tumors, the magnitudes of efficacy are greater in patients with PD-L1-positive tumors. Considering hundreds of neoadjuvant ICB trials are currently underway, these findings may serve as a valuable reference in the drug development process, aid in the design and interpretation of clinical trials, and provide complementary information in drafting the clinical practice guideline.
Previous investigations have validated the importance of PD-L1 expression in forecasting the effectiveness of ICIs in advanced patients, showing a positive correlation between PD-L1 levels and the benefits of immunotherapy (10). The absence of PD-L1 expression is commonly assumed to result in weak or no anti-tumor immunity induced by ICIs. In our previous study (4), we investigated 4174 patients enrolled in 8 randomized trials; 2254 had PD-L1-positive tumors, and the other 1920 individuals were PD-L1-negative. All patients had advanced or metastatic diseases, and they were diagnosed as lung cancer, melanoma, renal cell carcinoma, head and neck cancer, and urothelial carcinoma. Inhibitors targeting PD-1/PD-L1 were administered as second-line or later treatment in these subjects. Our results revealed that, compared with conventional treatment, immunotherapy was associated with favorable overall survival in patients with PD-L1-negative tumors (HR, 0.80; 95% CI, 0.71-0.90; P<0.001). Moreover, one recent study conducted in 5569 patients with lung cancer across different PD-L1 levels suggested that the improved efficacy of immunotherapy was independent of PD-L1 expression status in neoadjuvant, adjuvant, and peri-operative settings (11). Similarly, in the present study, our analysis revealed that, for subjects lacking PD-L1 expression, ICB-based neoadjuvant therapy was associated with better outcomes compared with conventional treatment. This result, based on well-defined endpoints of pCR and EFS from 3750 patients who did not express PD-L1 in high-quality RCTs, enhanced our analysis by mitigating the problem of individual trials lacking sufficient power. Technical explanations may account for why patients with negative PD-L1 expression can also gain advantages: the PD-L1 condition at treatment time may not be accurately depicted by testing archived tissues after cancer progression; the availability of tissues to examine PD-L1 expression is restricted; or there may be inconsistencies in PD-L1 expression among different tumor histologies.
It is well-established that chemotherapy, radiotherapy, or even immunotherapy itself can foster the upregulation of PD-L1 expression, induce immunogenicity by improving antigen processing machinery and T-cell killing in tumor tissues (12, 13). The reason why more patients benefit from ICB-based neoadjuvant therapy may be that ICIs are combined with other agents. Indeed, certain chemotherapy drugs, like Oxaliplatin, are capable of causing immunogenic cell death (ICD) and suppressing tumor growth by enhancing T cell infiltration and activating dendritic cells within the tumor (14). Further in vivo studies demonstrated that the combination of Oxaliplatin and immune checkpoint inhibitors could improve the therapeutic outcomes (15). Interestingly, this synergistic effect of oxaliplatin-based chemotherapy and immunotherapy was not observed in the combination of cisplatin-based chemotherapy and immunotherapy in patients with gastric cancer (16). Indeed, accumulating evidence has demonstrated that various chemotherapy agents can induce immunogenic cell death (ICD) in tumors, such as Anthracyclines (doxorubicin and mitoxantrone) (17, 18), DNA-damaging agents (cyclophosphamide, platinum derivatives) (19–23), proteasome inhibitors (bortezomib) (24, 25), and paclitaxel (26). Other conventional treatments, including therapeutic oncolytic virus, targeted anti-tumor drugs, radiotherapy, external phototherapy, and photodynamic therapy, may also produce ICD (27). The immunogenic microenvironment created by drug-induced ICD can significantly enhance the efficacy of ICB. Additionally, the HMGB1 released during the ICD process may trigger the rapid endocytosis and subsequent breakdown in lysosomes, which in turn improves T cell anti-cancer activities, though inhibiting the persistent signal transduction of PD-1 (28). Apart from directly inhibiting the growth of cancer cells, chemoradiotherapy can also activate immune effectors, boosting anti-cancer immune response while the bulk tumor and tumor antigens still remain during treatment (29). Furthermore, the neoantigens resulting from neoadjuvant therapy will provoke a vigorous and enduring anti-cancer reaction, even following surgical procedures (30). Moreover, PD-L2 serves as another important ligand for PD-1, inhibiting the function of T cells and contributing to tumor immune escape, binding to PD-1 with 2–6 times greater affinity than PD-L1 (31). However, the prognostic or predictive significance of PD-L2 in cancer has yet to be determined. Therefore, future studies should prioritize promoting the whole tumor immune microenvironment, rather than solely concentrating on PD-L1 expression.
The molecular mechanisms underlying PD-L1 expression and chemotherapy are complex and still largely unclear. For example, approximately half of the patients with non-small-cell lung cancer have negative PD-L1 expression (32), but numerous studies have suggested that individuals in this subpopulation can benefit significantly from immunotherapy compared with conventional chemotherapy. This may be attributed to the unique molecular PD-L1 signaling pathways or clinical features associated with chemotherapy rather than the tumor immune microenvironment itself. Previous studies reported that Mutations in STK11 and EGFR, as well as alterations in the WNT pathway, have been strongly linked to negative PD-L1 expression, whereas mutations in TP53, KRAS, and MET have been robustly associated with high PD-L1 expression (33). Moreover, low PD-L1 expression levels are correlated with particular clinicopathological characteristics such as primary tumors, adenocarcinoma, and resected samples (34).
There are several issues regarding ICB-based neoadjuvant therapy that still need to be addressed. First, neoadjuvant immunotherapy alone and the combination of neoadjuvant and adjuvant immunotherapy are the two major treatment managements based on the eligible RCTs. Nevertheless, the selection of the most effective treatment for cancer patients is still debated. Currently, there is a deficiency of studies on whether continuing immunotherapy post-surgery prolongs survival compared to pre-operative use alone, and the best duration for post-operative immunotherapy remains unclear. It is important to identify which patients should continue immunotherapy post-surgery in future studies, and novel biomarker analysis, like circulating DNA, may be needed. Second, there is ambiguity about the requirement of chemotherapy for patients with PD-L1 expression levels of 50% or higher in the neoadjuvant settings. In KEYNOTE-671 (35, 36), the cut-off value for PD-L1 expression status was set as 50%. Immunotherapy was associated with favorable EFS in both patients with PD-L1-negative tumors (HR, 0.64; 95% CI, 0.49-0.82) and patients with PD-L1-positive tumors (HR, 0.42; 95% CI, 0.28-0.65). The findings were in agreement with our major conclusion. Unfortunately, all RCTs have been set up to make a direct comparison between ICB-based immunotherapy and conventional treatment. Of note, besides evaluating the synergistic interaction between chemotherapy and immunotherapy (37), the potential toxicities associated with chemotherapy should also be taken into account. Further clinical studies are necessary to address these concerns.
Our study also has some limitations. First, it is essential to assess whether neoadjuvant immunotherapy can convert the pCR/EFS advantage into a substantial overall survival improvement over conventional treatment, as it defines the ultimate purpose of neoadjuvant immunotherapy. However, since the information regarding overall survival in these eligible RCTs is immature, we cannot conduct such an analysis here. Second, the detection methods of PD-L1 expression status were conducted by various approaches. However, numerous studies have investigated the reproducibility of PD-L1 interpretation concordance among different tumor types. For example, the Blueprint Project phase 2 has revealed a high concordance among staining tests of Dako 22C3, Ventana SP263, and Dako 28–8 in lung cancer (38), Hodgson et al. demonstrated good analytic comparability of Ventana SP142, Ventana SP263, Dako 22C3, and CST E1L3N in urothelial carcinoma (39) and esophageal cancer (40). Hence, we believe the potential bias due to different PD-L1 antibodies was acceptable here. Third, other confounding factors, such as tumor types, drug targets, and imbalances in patients’ characteristics among PD-L1-positive and PD-L1-negative tumors, may also be the source of heterogeneity. Accordingly, we conducted pre-defined subgroup analysis based on the classifications of these features, and found no significant differences in terms of pCR and EFS among these subgroups. Fourth, the treatment regimens, patterns, cycles, and duration varied greatly in the eligible trials, which may introduce some selection bias. Moreover, our analysis was conducted at the trial level. Accordingly, individualized patient data were urgently needed to confirm our results. Fifth, although patients with PD-L1-negative tumors can benefit from immunotherapy, determining the optimal management strategy for cancer patients necessitates a multifaceted process in real-world clinical practice. The toxicity profile and financial burdens were also key factors in choosing treatment options. However, it was very difficult to address these concerns due to the limited information. Hence, the clinicians need to carefully balance efficacy, safety, and patient preferences to deliver individualized treatment.
In summary, our meta-analysis demonstrates that both patients with PD-L1-positive and PD-L1-negative tumors can benefit from neoadjuvant immunotherapy. However, compared with patients with PD-L1-negative tumors, the magnitude of efficacy is greater in patients with PD-L1-positive tumors. Hence, rather than serving as an independent marker for patient selection, PD-L1 expression status is more effectively applied as a prognostic biomarker.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
YH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. JX: Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Writing – original draft. JW: Data curation, Formal Analysis, Investigation, Methodology, Resources, Writing – original draft. JL: Formal Analysis, Methodology, Visualization, Writing – original draft. MC: Writing – original draft, Data curation, Formal Analysis, Methodology. BZ: Conceptualization, Project administration, Visualization, Writing – original draft, Writing – review & editing. ZH: Conceptualization, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1617905/full#supplementary-material
Supplementary Figure 1 | Flowchart diagram of selected clinical trials included in our study.
Supplementary Figure 2 | Subgroup analysis of the association between PD-L1 and pCR in patients treated with neoadjuvant immunotherapy. OR, odds ratio. Red indicates patients with PD-L1-positive tumors; Blue indicates patients with PD-L1-negative tumors.
Supplementary Figure 3 | Subgroup analysis of the association between PD-L1 and EFS in patients treated with neoadjuvant immunotherapy. HR, hazard ratio. Red indicates patients with PD-L1-positive tumors; Blue indicates patients with PD-L1-negative tumors.
Supplementary Figure 4 | Begg’s funnel plot for the publication bias test.
References
1. Zhao B, Zhao H, and Zhao J. Efficacy of PD-1/PD-L1 blockade monotherapy in clinical trials. Ther Adv Med Oncol. (2020) 12:1758835920937612. doi: 10.1177/1758835920937612
2. Topalian SL and Pardoll DM. Neoadjuvant anti-PD-1-based immunotherapy: evolving a new standard of care. J Immunother Cancer. (2025) 13:e010833. doi: 10.1136/jitc-2024-010833
3. Ye Y, Zhang Z, Zhao H, and Zhao B. A system review of neoadjuvant immune checkpoint blockade for breast cancer. Front Immunol. (2025) 16:1537926. doi: 10.3389/fimmu.2025.1537926
4. Shen X and Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ (Clin Res ed). (2018) 362:k3529. doi: 10.1136/bmj.k3529
5. Sorin M, Prosty C, Ghaleb L, Nie K, Katergi K, Shahzad MH, et al. Neoadjuvant chemoimmunotherapy for NSCLC: A systematic review and meta-analysis. JAMA Oncol. (2024) 10:621–33. doi: 10.1001/jamaoncol.2024.0057
6. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clin Res ed). (2011) 343:d5928. doi: 10.1136/bmj.d5928
7. Higgins JP, Thompson SG, Deeks JJ, and Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clin Res ed). (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
8. Begg CB and Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
9. Egger M, Davey Smith G, Schneider M, and Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin Res ed). (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
10. Zhang SL, Tian Y, Yu J, Zhang JH, Sun L, Huang LT, et al. Is neoadjuvant immunotherapy necessary in patients with programmed death ligand 1 expression-negative resectable non-small cell lung cancer? A systematic review and meta-analysis. Lung Cancer (Amsterdam Netherlands). (2024) 191:107799. doi: 10.1016/j.lungcan.2024.107799
11. Zhang Z, Lin Y, and Chen S. Efficacy of neoadjuvant, adjuvant, and perioperative immunotherapy in non-small cell lung cancer across different PD-L1 expression levels: a systematic review and meta-analysis. Front Immunol. (2025) 16:1569864. doi: 10.3389/fimmu.2025.1569864
12. Anagnostou V, Smith KN, Forde PM, Niknafs N, Bhattacharya R, White J, et al. Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov. (2017) 7:264–76. doi: 10.1158/2159-8290.CD-16-0828
13. Hegde PS and Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. (2020) 52:17–35. doi: 10.1016/j.immuni.2019.12.011
14. Zhang X, Wang D, Li Z, Jiao D, Jin L, Cong J, et al. Low-dose gemcitabine treatment enhances immunogenicity and natural killer cell-driven tumor immunity in lung cancer. Front Immunol. (2020) 11:331. doi: 10.3389/fimmu.2020.00331
15. Sun F, Cui L, Li T, Chen S, Song J, and Li D. Oxaliplatin induces immunogenic cells death and enhances therapeutic efficacy of checkpoint inhibitor in a model of murine lung carcinoma. J Receptor Signal Transduct Res. (2019) 39:208–14. doi: 10.1080/10799893.2019.1655050
16. Liu P, Chen J, Zhao L, Hollebecque A, Kepp O, Zitvogel L, et al. PD-1 blockade synergizes with oxaliplatin-based, but not cisplatin-based, chemotherapy of gastric cancer. Oncoimmunology. (2022) 11:2093518. doi: 10.1080/2162402X.2022.2093518
17. Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. (2007) 13:54–61. doi: 10.1038/nm1523
18. Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J, et al. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. (2011) 71:4821–33. doi: 10.1158/0008-5472.CAN-11-0950
19. Kopecka J, Salaroglio IC, Righi L, Libener R, Orecchia S, Grosso F, et al. Loss of C/EBP-β LIP drives cisplatin resistance in Malignant pleural mesothelioma. Lung Cancer (Amsterdam Netherlands). (2018) 120:34–45. doi: 10.1016/j.lungcan.2018.03.022
20. Limagne E, Thibaudin M, Nuttin L, Spill A, Derangère V, Fumet JD, et al. Trifluridine/tipiracil plus oxaliplatin improves PD-1 blockade in colorectal cancer by inducing immunogenic cell death and depleting macrophages. Cancer Immunol Res. (2019) 7:1958–69. doi: 10.1158/2326-6066.CIR-19-0228
21. Schiavoni G, Sistigu A, Valentini M, Mattei F, Sestili P, Spadaro F, et al. Cyclophosphamide synergizes with type I interferons through systemic dendritic cell reactivation and induction of immunogenic tumor apoptosis. Cancer Res. (2011) 71:768–78. doi: 10.1158/0008-5472.CAN-10-2788
22. Wang Z, Chen J, Hu J, Zhang H, Xu F, He W, et al. cGAS/STING axis mediates a topoisomerase II inhibitor-induced tumor immunogenicity. J Clin Invest. (2019) 129:4850–62. doi: 10.1172/JCI127471
23. Yamazaki T, Buqué A, Ames TD, and Galluzzi L. PT-112 induces immunogenic cell death and synergizes with immune checkpoint blockers in mouse tumor models. Oncoimmunology. (2020) 9:1721810. doi: 10.1080/2162402X.2020.1721810
24. Spisek R, Charalambous A, Mazumder A, Vesole DH, Jagannath S, and Dhodapkar MV. Bortezomib enhances dendritic cell (DC)-mediated induction of immunity to human myeloma via exposure of cell surface heat shock protein 90 on dying tumor cells: therapeutic implications. Blood. (2007) 109:4839–45. doi: 10.1182/blood-2006-10-054221
25. Gulla A, Morelli E, Samur MK, Botta C, Hideshima T, Bianchi G, et al. Bortezomib induces anti-multiple myeloma immune response mediated by cGAS/STING pathway activation. Blood Cancer Discov. (2021) 2:468–83. doi: 10.1158/2643-3230.BCD-21-0047
26. Lau TS, Chan LKY, Man GCW, Wong CH, Lee JHS, Yim SF, et al. Paclitaxel induces immunogenic cell death in ovarian cancer via TLR4/IKK2/SNARE-dependent exocytosis. Cancer Immunol Res. (2020) 8:1099–111. doi: 10.1158/2326-6066.CIR-19-0616
27. Zhai J, Gu X, Liu Y, Hu Y, Jiang Y, and Zhang Z. Chemotherapeutic and targeted drugs-induced immunogenic cell death in cancer models and antitumor therapy: An update review. Front Pharmacol. (2023) 14:1152934. doi: 10.3389/fphar.2023.1152934
28. Gao Q, Wang S, Li F, Lian J, Cheng S, Yue D, et al. High mobility group protein B1 decreases surface localization of PD-1 to augment T-cell activation. Cancer Immunol Res. (2022) 10:844–55. doi: 10.1158/2326-6066.CIR-21-0652
29. Bracci L, Schiavoni G, Sistigu A, and Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differentiation. (2014) 21:15–25. doi: 10.1038/cdd.2013.67
30. Uprety D, Mandrekar SJ, Wigle D, Roden AC, and Adjei AA. Neoadjuvant immunotherapy for NSCLC: current concepts and future approaches. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2020) 15:1281–97. doi: 10.1016/j.jtho.2020.05.020
31. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. (2001) 2:261–8. doi: 10.1038/85330
32. Dietel M, Savelov N, Salanova R, Micke P, Bigras G, Hida T, et al. Real-world prevalence of programmed death ligand 1 expression in locally advanced or metastatic non-small-cell lung cancer: The global, multicenter EXPRESS study. Lung Cancer (Amsterdam Netherlands). (2019) 134:174–9. doi: 10.1016/j.lungcan.2019.06.012
33. Bai Y, Yang W, Käsmann L, Sorich MJ, Tao H, and Hu Y. Immunotherapy for advanced non-small cell lung cancer with negative programmed death-ligand 1 expression: a literature review. Trans Lung Cancer Res. (2024) 13:398–422. doi: 10.21037/tlcr-23-144
34. Zheng Q, Huang Y, Zeng X, Chen X, Shao S, Jin Y, et al. Clinicopathological and molecular characteristics associated with PD-L1 expression in non-small cell lung cancer: a large-scale, multi-center, real-world study in China. J Cancer Res Clin Oncol. (2021) 147:1547–56. doi: 10.1007/s00432-020-03444-y
35. Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Gao S, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. New Engl J Med. (2023) 389:491–503. doi: 10.1056/NEJMoa2302983
36. Spicer JD, Garassino MC, Wakelee H, Liberman M, Kato T, Tsuboi M, et al. Neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab compared with neoadjuvant chemotherapy alone in patients with early-stage non-small-cell lung cancer (KEYNOTE-671): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London England). (2024) 404:1240–52. doi: 10.1016/S0140-6736(24)01756-2
37. Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, and Schellens JHM. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol Off J Eur Soc Med Oncol. (2019) 30:219–35. doi: 10.1093/annonc/mdy551
38. Tsao MS, Kerr KM, Kockx M, Beasley MB, Borczuk AC, Botling J, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. (2018) 13:1302–11. doi: 10.1016/j.jtho.2018.05.013
39. Hodgson A, Slodkowska E, Jungbluth A, Liu SK, Vesprini D, Enepekides D, et al. PD-L1 immunohistochemistry assay concordance in urothelial carcinoma of the bladder and hypopharyngeal squamous cell carcinoma. Am J Surg Pathol. (2018) 42:1059–66. doi: 10.1097/PAS.0000000000001084
40. Wang X, He J, Li J, Wu C, Yue M, Niu S, et al. Concordance of assessments of four PD-L1 immunohistochemical assays in esophageal squamous cell carcinoma (ESCC). J Cancer Res Clin Oncol. (2024) 150:43. doi: 10.1007/s00432-023-05595-0
41. Rinnerthaler G, Egle D, Bartsch R, Schmitt CA, Petzer A, Balic M, et al. Neoadjuvant atezolizumab in combination with dual HER2 blockade plus epirubicin in women with early HER2-positive breast cancer: the randomized phase 2 ABCSG-52/ATHENE trial. Nat Cancer. (2025) 6:41–50. doi: 10.1038/s43018-024-00890-2
42. Heymach JV, Harpole D, Mitsudomi T, Taube JM, Galffy G, Hochmair M, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. New Engl J Med. (2023) 389:1672–84. doi: 10.1056/NEJMoa2304875
43. Lin JX, Tang YH, Zheng HL, Ye K, Cai JC, Cai LS, et al. Neoadjuvant camrelizumab and apatinib combined with chemotherapy versus chemotherapy alone for locally advanced gastric cancer: a multicenter randomized phase 2 trial. Nat Commun. (2024) 15:41. doi: 10.1038/s41467-023-44309-5
44. Chen L, Li H, Zhang H, Yang H, Qian J, Li Z, et al. Camrelizumab vs placebo in combination with chemotherapy as neoadjuvant treatment in patients with early or locally advanced triple-negative breast cancer: the camRelief randomized clinical trial. Jama. (2025) 333:673–81. doi: 10.1001/jama.2024.23560
45. Loi S, Curigliano G, Salgado RF, Romero Diaz RI, Delaloge S, Rojas C, et al. LBA20 A randomized, double-blind trial of nivolumab (NIVO) vs placebo (PBO) with neoadjuvant chemotherapy (NACT) followed by adjuvant endocrine therapy (ET) &xb1; NIVO in patients (pts) with high-risk, ER+ HER2&x2212; primary breast cancer (BC). Ann Oncol. (2023) 34:S1259–S60. doi: 10.1016/j.annonc.2023.10.010
46. Cascone T, Awad MM, Spicer JD, He J, Lu S, Sepesi B, et al. Perioperative nivolumab in resectable lung cancer. New Engl J Med. (2024) 390:1756–69. doi: 10.1056/NEJMoa2311926
47. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. New Engl J Med. (2022) 386:1973–85. doi: 10.1056/NEJMoa2202170
48. Deutsch JS, Cimino-Mathews A, Thompson E, Provencio M, Forde PM, Spicer J, et al. Association between pathologic response and survival after neoadjuvant therapy in lung cancer. Nat Med. (2024) 30:218–28. doi: 10.1038/s41591-023-02660-6
49. Awad MM, Forde PM, Girard N, Spicer J, Wang C, Lu S, et al. Neoadjuvant nivolumab plus ipilimumab versus chemotherapy in resectable lung cancer. J Clin Oncol Off J Am Soc Clin Oncol. (2025) 43:1453–62. doi: 10.1200/JCO-24-02239
50. Li C, Tian Y, Zheng Y, Yuan F, Shi Z, Yang L, et al. Pathologic response of phase III study: perioperative camrelizumab plus rivoceranib and chemotherapy versus chemotherapy for locally advanced gastric cancer (DRAGON IV/CAP 05). J Clin Oncol Off J Am Soc Clin Oncol. (2025) 43:464–74. doi: 10.1200/JCO.24.00795
51. Qin J, Xue L, Hao A, Guo X, Jiang T, Ni Y, et al. Neoadjuvant chemotherapy with or without camrelizumab in resectable esophageal squamous cell carcinoma: the randomized phase 3 ESCORT-NEO/NCCES01 trial. Nat Med. (2024) 30:2549–57. doi: 10.1038/s41591-024-03064-w
52. Loibl S, Schneeweiss A, Huober J, Braun M, Rey J, Blohmer JU, et al. Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann Oncol Off J Eur Soc Med Oncol. (2022) 33:1149–58. doi: 10.1016/j.annonc.2022.07.1940
53. Loibl S, Untch M, Burchardi N, Huober J, Sinn BV, Blohmer JU, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol Off J Eur Soc Med Oncol. (2019) 30:1279–88. doi: 10.1093/annonc/mdz158
54. Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet (London England). (2020) 396:1090–100. doi: 10.1016/S0140-6736(20)31953-X
55. Barrios C, Harbeck N, Zhang HA, Saji S, Jung KH, Hegg R, et al. LBA1 Final analysis of the placebo-controlled randomised phase III IMpassion031 trial evaluating neoadjuvant atezolizumab (atezo) plus chemotherapy (CT) followed by open-label adjuvant atezo in patients (pts) with early-stage triple-negative breast cancer (eTNBC). ESMO Open. (2023) 8. doi: 10.1016/j.esmoop.2023.101571
56. Huober J, Barrios CH, Niikura N, Jarząb M, Chang YC, Huggins-Puhalla SL, et al. Atezolizumab with neoadjuvant anti-human epidermal growth factor receptor 2 therapy and chemotherapy in human epidermal growth factor receptor 2-positive early breast cancer: primary results of the randomized phase III IMpassion050 trial. J Clin Oncol Off J Am Soc Clin Oncol. (2022) 40:2946–56. doi: 10.1200/JCO.21.02772
57. Huober J, Barrios CH, Niikura N, Jarzab M, Chang YC, Huggins-Puhalla SL, et al. 127P Atezolizumab (A) + pertuzumab + trastuzumab (PH) + chemotherapy (CT) in HER2-positive early breast cancer (HER2+ eBC): Final results of the phase III IMpassion050 trial. ESMO Open. (2024) 9. doi: 10.1016/j.esmoop.2024.103115
58. Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. New Engl J Med. (2022) 386:556–67. doi: 10.1056/NEJMoa2112651
59. Shah M, Osgood CL, Amatya AK, Fiero MH, Pierce WF, Nair A, et al. FDA approval summary: pembrolizumab for neoadjuvant and adjuvant treatment of patients with high-risk early-stage triple-negative breast cancer. Clin Cancer Res: Off J Am Assoc Cancer Res. (2022) 28:5249–53. doi: 10.1158/1078-0432.CCR-22-1110
60. Pusztai L, Denkert C, O’Shaughnessy J, Cortes J, Dent R, McArthur H, et al. Event-free survival by residual cancer burden with pembrolizumab in early-stage TNBC: exploratory analysis from KEYNOTE-522. Ann Oncol Off J Eur Soc Med Oncol. (2024) 35:429–36. doi: 10.1016/j.annonc.2024.02.002
61. Cardoso F, McArthur HL, Schmid P, Cortés J, Harbeck N, Telli ML, et al. LBA21 KEYNOTE-756: Phase III study of neoadjuvant pembrolizumab (pembro) or placebo (pbo) + chemotherapy (chemo), followed by adjuvant pembro or pbo + endocrine therapy (ET) for early-stage high-risk ER+/HER2&x2013; breast cancer. Ann Oncol. (2023) 34:S1260–S1. doi: 10.1016/j.annonc.2023.10.011
62. Ademuyiwa FO, Gao F, Street CR, Chen I, Northfelt DW, Wesolowski R, et al. A randomized phase 2 study of neoadjuvant carboplatin and paclitaxel with or without atezolizumab in triple negative breast cancer (TNBC) - NCI 10013. NPJ Breast Cancer. (2022) 8:134. doi: 10.1038/s41523-022-00500-3
63. Xiao WW, Chen G, Gao YH, Lin JZ, Wu XJ, Luo HL, et al. Effect of neoadjuvant chemoradiotherapy with or without PD-1 antibody sintilimab in pMMR locally advanced rectal cancer: A randomized clinical trial. Cancer Cell. (2024) 42:1570–81.e4. doi: 10.1016/j.ccell.2024.07.004
64. Lu S, Zhang W, Wu L, Wang W, Zhang P, Fang W, et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the neotorch randomized clinical trial. Jama. (2024) 331:201–11. doi: 10.1001/jama.2023.24735
65. Gianni L, Huang CS, Egle D, Bermejo B, Zamagni C, Thill M, et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Ann Oncol Off J Eur Soc Med Oncol. (2022) 33:534–43. doi: 10.1016/j.annonc.2022.02.004
66. Gianni L, Huang C, Egle D, Bermejo B, Zamagni C, Thill M, et al. LBA19 Event-free survival (EFS) analysis of neoadjuvant taxane/carboplatin with or without atezolizumab followed by an adjuvant anthracycline regimen in high-risk triple negative breast cancer (TNBC): NeoTRIP Michelangelo randomized study. Ann Oncol. (2023) 34:S1258–S9. doi: 10.1016/j.annonc.2023.10.009
67. Powles T, Catto JWF, Galsky MD, Al-Ahmadie H, Meeks JJ, Nishiyama H, et al. Perioperative durvalumab with neoadjuvant chemotherapy in operable bladder cancer. New Engl J Med. (2024) 391:1773–86. doi: 10.1056/NEJMoa2408154
68. Yue D, Wang W, Liu H, Chen Q, Chen C, Liu L, et al. Perioperative tislelizumab plus neoadjuvant chemotherapy for patients with resectable non-small-cell lung cancer (RATIONALE-315): an interim analysis of a randomised clinical trial. Lancet Respir Med. (2025) 13:119–29. doi: 10.1016/S2213-2600(24)00269-8
69. Lin ZY, Zhang P, Chi P, Xiao Y, Xu XM, Zhang AM, et al. Neoadjuvant short-course radiotherapy followed by camrelizumab and chemotherapy in locally advanced rectal cancer (UNION): early outcomes of a multicenter randomized phase III trial. Ann Oncol Off J Eur Soc Med Oncol. (2024) 35:882–91. doi: 10.1016/j.annonc.2024.06.015
Keywords: cancer, immunotherapy, PD-L1, neoadjuvant therapy, pathologic completeresponse, event-free survival
Citation: Huang Y, Xie J, Wang J, Lin J, Chen M, Zhao B and Huang Z (2025) Association between the expression status of programmed cell death ligand 1 and the efficacy of pan-cancer neoadjuvant immune checkpoint blockade. Front. Immunol. 16:1617905. doi: 10.3389/fimmu.2025.1617905
Received: 25 April 2025; Accepted: 17 September 2025;
Published: 30 September 2025.
Edited by:
Jehad Charo, Roche, SwitzerlandReviewed by:
Alessio Vagliasindi, Oncological Center of Basilicata (IRCCS), ItalyFabio Scirocchi, Bambino Gesù Children’s Hospital (IRCCS), Italy
Copyright © 2025 Huang, Xie, Wang, Lin, Chen, Zhao and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Zhao, ZG9jdG9yYmluemhhb0AxMjYuY29t; Zhiyang Huang, ZG9jdG9yaHVhbmd6aGl5YW5nQDE2My5jb20=
 Ying Huang
Ying Huang Bin Zhao
Bin Zhao