- 1Key Laboratory of Artificial Organs and Computational Medicine of Zhejiang Province, Shulan International Medical College, Zhejiang Shuren University, Hangzhou, China
- 2School of Basic Medical Sciences, Zhejiang Chinese Medical University, Hangzhou, China
- 3Zhejiang Key Laboratory of Public Health Detection and Pathogenesis Research, Department of Microbiology, Zhejiang Provincial Center for Disease Control and Prevention, Hangzhou, China
Respiratory tract infections, such as influenza, respiratory syncytial virus (RSV) infection, and COVID-19, remain a persistent threat to global public health due to their high transmissibility and disease burden. Vaccination, as a key preventive strategy, not only reduces the risk of infection but also blocks transmission by activating adaptive immunity. While traditional vaccine evaluations have primarily focused on humoral immunity, growing evidence highlights the critical role of T lymphocyte-mediated cellular immunity in clearing virus-infected cells, establishing long-term immune memory, and responding to viral mutations. This review systematically summarizes the cellular immune responses induced by vaccines against respiratory tract infections and their correlation with protective efficacy. It also outlines evaluation methodologies such as flow cytometry, providing a theoretical foundation for optimizing vaccine design and assessment, and advancing the development of effective, broad-spectrum vaccines.
1 Introduction
Upper respiratory tract viruses-such as influenza virus, SARS-CoV-2, and respiratory syncytial virus (RSV)—pose a continued threat to global public health due to their high transmissibility and potential for mutation. According to estimates by the World Health Organization, respiratory infections account for approximately 1 billion cases annually, including 3 to 5 million severe cases and 290,000 to 650,000 related deaths (1). Among them, influenza virus, adenovirus, and the novel coronavirus are the most prominent representatives (2). The 1918 Spanish flu was characterized by both high mortality and high transmissibility, resulting in the deaths of over 50 million people worldwide (3). The COVID-19 outbreak that began in 2019 rapidly evolved into a global public health crisis. As of July 7, 2024, a total of 775,754,322 confirmed COVID-19 cases have been reported to the WHO (World Health Organization), including 7,053,902 deaths worldwide. Furthermore, WHO reports that by 2025, respiratory syncytial virus (RSV) is expected to cause approximately 33 million cases of acute lower respiratory tract infections annually, resulting in over 3 million hospitalizations and 59,600 in-hospital deaths among children under the age of five.
Currently, vaccination against corresponding upper respiratory tract viruses is one of the key measures for preventing infection, reducing severe cases, and minimizing related deaths (4). Over the years, vaccines targeting various pathogens have saved hundreds of millions of lives. There is an increasing recognition of the immense potential of vaccines in controlling disease outbreaks and preventing severe cases. However, traditional vaccine evaluation systems have long focused on humoral immunity (such as neutralizing antibody titers), neglecting the crucial role of cellular immunity. Recent studies have revealed that T lymphocyte-mediated cellular immunity not only directly eliminates virus-infected host cells but also provides long-lasting protection by forming tissue-resident memory T cells (TRM) and circulating memory T cells (TCM). Furthermore, it demonstrates unique advantages in responding to viral antigenic drift or escape mutations (5, 6). For example, studies on SARS-CoV-2 variants have shown that, although the potency of neutralizing antibodies may decrease, T cells’ recognition of conserved epitopes (such as the S2 subunit and nucleocapsid protein) can still effectively reduce the risk of severe disease (7–9). This finding highlights the strategic value of cellular immunity in the design of broad-spectrum vaccines (Figure 1).
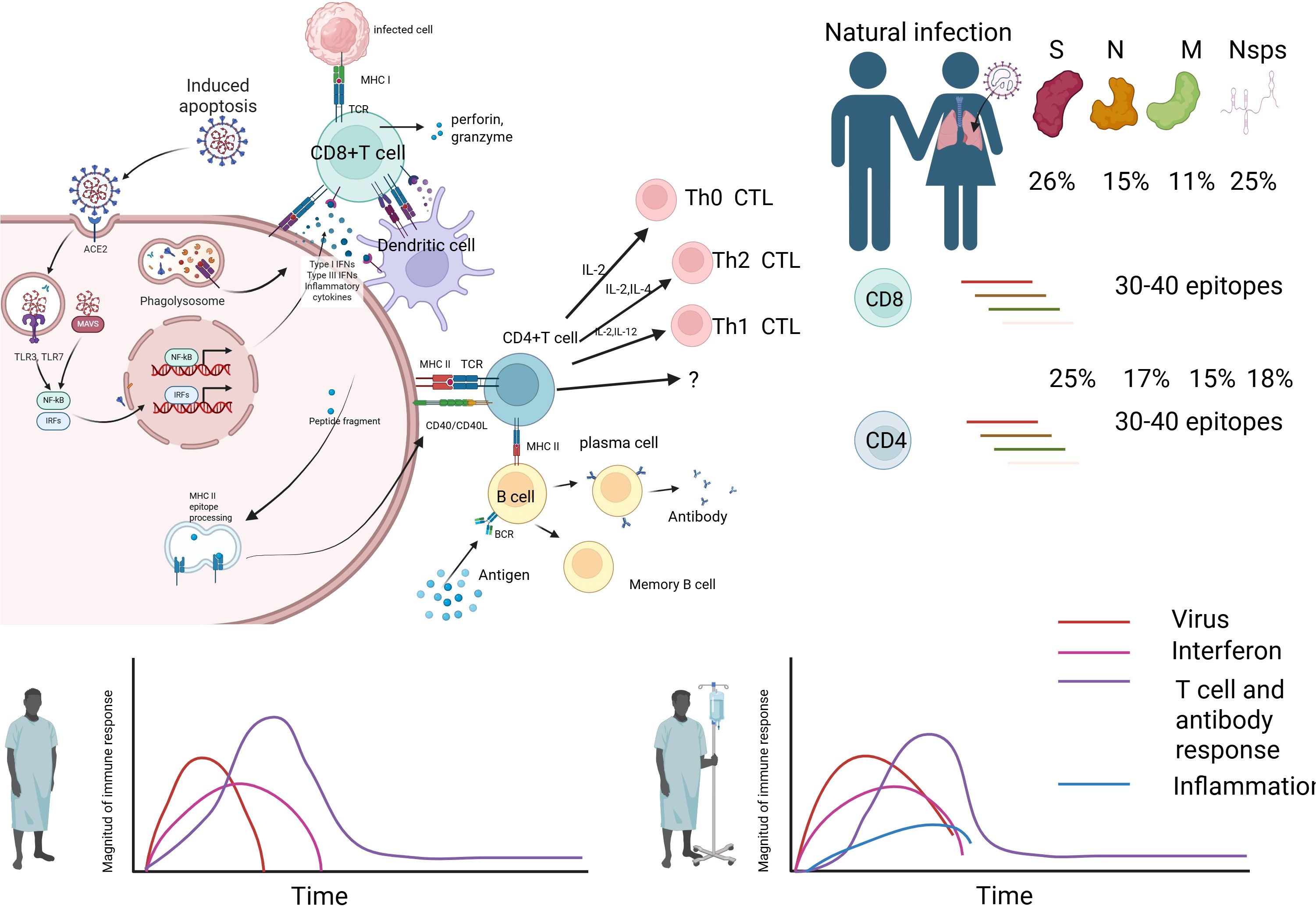
Figure 1. Immune response mechanism during natural infection. Image 1 illustrates the immune response mechanism during natural infection. The virus infects cells via the ACE2 receptor, leading to phagocytosis by macrophages (MФ), which release inflammatory mediators such as NF-κB, IRFs, and IFNs, inducing Type I IFNs and inflammatory cytokines. Infected cells present antigens via MHC class I molecules, activating CD8+ T cells (CTLs) to release perforin and granzyme, targeting infected cells for destruction. Dendritic cells (DCs) present antigens via MHC class II molecules, activating CD4+ T cells (Th0 CTL), which differentiate into Th1 and Th2 cells, regulating immunity through IL-2, IL-12, IFN-γ, and IL-2, IL-4, respectively. B cells, with help from CD4+ T cells, differentiate into plasma cells to produce antibodies and form memory B cells for future immunity. The bar chart on the right indicates the proportion of T cell and antibody responses to 30–40 epitopes on viral proteins S, N, M, and Nsps, with 26%, 15%, 11%, and 25% for S protein, and 25%, 17%, 15%, and 18% for others, respectively. The lower line graph depicts the dynamics of virus (red), interferon (pink), T cell and antibody responses (purple), and inflammation (blue) over time, reflecting the intensity and duration of the immune response.
However, there are significant differences among various vaccine platforms in inducing cellular immunity. Traditional inactivated vaccines, while highly safe, primarily activate CD4+ Th2 responses and are relatively weak in activating CD8+ cytotoxic T cells (CTLs) (10). In contrast, mRNA vaccines efficiently induce multi-epitope-specific CTLs and TRMs through endogenous antigen presentation. However, their durability and reliance on cold-chain storage remain significant challenges (11, 12). Viral vector vaccines, on the other hand, mimic the natural infection pathway, providing both mucosal and systemic immunity, but may be affected by pre-existing immunity (Figure 2).
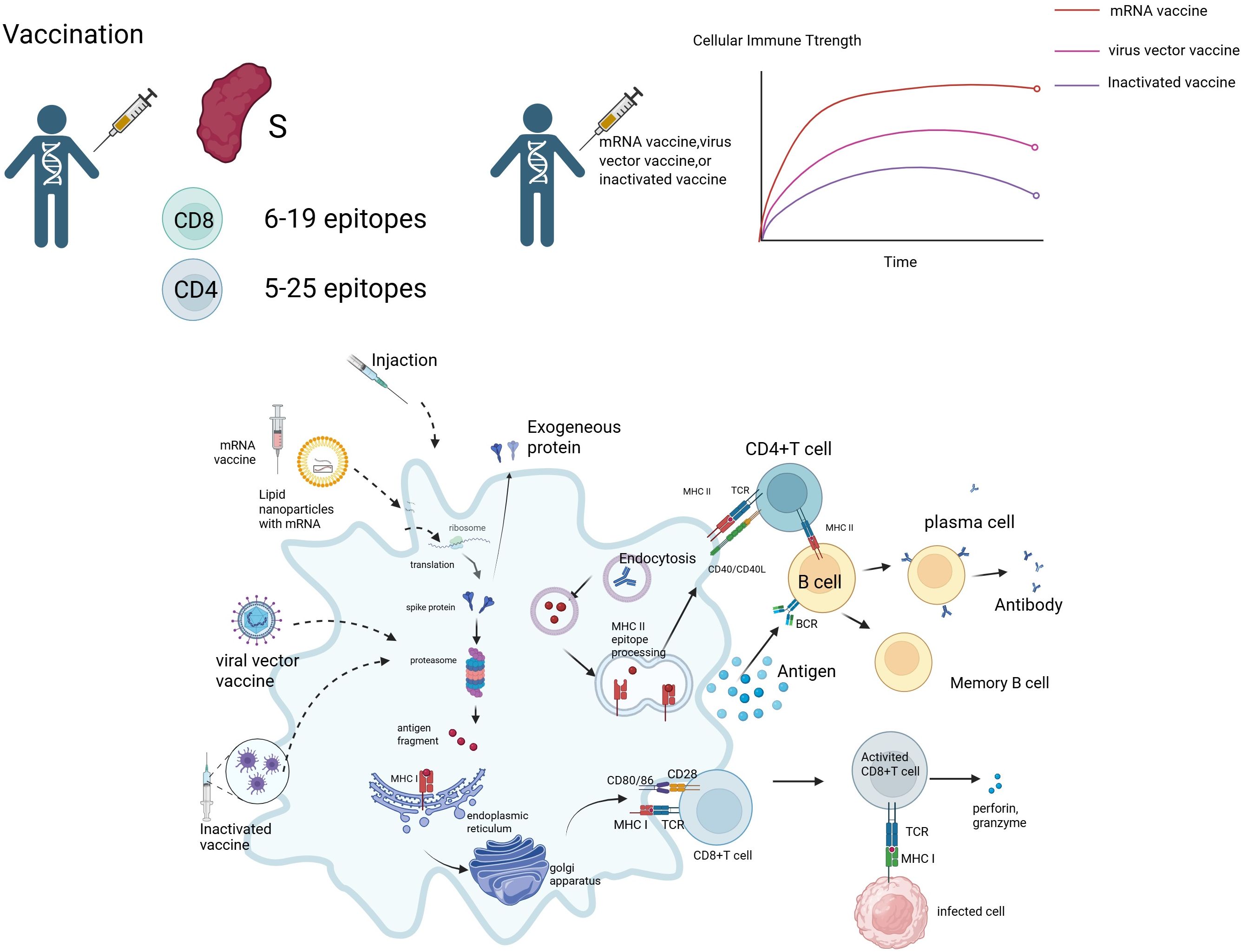
Figure 2. Vaccine-induced immune response mechanism. Image 2 depicts the immune response induced by mRNA vaccines, viral vector vaccines, and inactivated vaccines. mRNA vaccines deliver mRNA via lipid nanoparticles (LNPs), translating into S protein, while viral vector vaccines express S protein through endogenous proteins, and inactivated vaccines provide antigen fragments directly. Antigen-presenting cells (APCs) activate CD4+ T cells via MHC class II molecules, which differentiate into Th1 and Th2 cells, regulating immunity through IL-2, IL-12, and IL-2, IL-4, respectively. CD4+ T cells assist B cells in differentiating into plasma cells, producing antibodies against the S protein and forming memory B cells. MHC class I molecules activate CD8+ T cells (CTLs), which release perforin and granzyme to kill infected cells. Vaccine-induced CD8+ T cells recognize 6–19 epitopes, while CD4+ T cells recognize 5–25 epitopes. The line graph on the right shows the cellular immune strength over time for mRNA vaccines (red), viral vector vaccines (pink), and inactivated vaccines (purple), indicating that mRNA vaccines induce a stronger early response that gradually stabilizes, while other vaccines produce a lower but more sustained response.
(13, 14) These differences not only determine the protective efficacy of vaccines but also provide direction for optimization: how to coordinate mucosal local immunity and systemic immunity through adjuvant modulation, antigen targeting, and innovations in delivery systems has become a central issue in the development of next-generation vaccines.
Currently, the frequent emergence of viral variants, the low response rates in elderly individuals and immunocompromised patients, as well as the bottlenecks in mucosal immunity induction technologies, further complicate vaccine design. This paper elucidates the core mechanisms of cellular immunity in the defense against upper respiratory tract viruses, systematically compares the efficacy differences in cellular immunity induction across different vaccine platforms, and proposes optimization strategies based on the synergy between mucosal and systemic immunity, along with future directions to address clinical challenges. By integrating cutting-edge research, the goal is to provide a theoretical framework and technical insights for developing efficient, broad-spectrum vaccines against upper respiratory tract viruses, driving the paradigm shift from “population protection” to “precision immunization.”
2 Interaction between upper respiratory tract viruses and host cell immunity
2.1 Viral escape mechanisms and the defensive role of cellular
2.1.1 Immunity influenza virus
For influenza virus, CD8+ T cells initiate cytotoxic responses early in the infection by recognizing highly conserved epitopes of the viral nucleoprotein (NP) and matrix protein (M1). This leads to the direct lysis of virus-infected respiratory epithelial cells, significantly reducing viral load and the risk of severe disease (15, 16). These epitopes, due to their involvement in the assembly of the virus’s core structure, are less likely to undergo mutations, making CD8+ T cell responses have cross-strain protective potential. For example, cross-protection against different subtypes such as H1N1 and H5N1 can be mediated by these responses (17). An experiment using a single-cycle influenza vaccine (S-FLU) in female mice aged 6 to 8 weeks has confirmed that NP-specific CD8+ TRM cells deposited in the respiratory tract can recognize conserved epitopes from at least 12 different influenza strains. Their polyclonal nature ensures that mutations in a single epitope cannot completely escape immune surveillance (15, 18). At the same time, Th1 cells activate the antiviral state of alveolar macrophages by secreting cytokines such as IFN-γ. This not only directly inhibits the function of the viral polymerase but also upregulates the expression of MHC-I molecules on infected cells, enhancing the epitope presentation efficiency to CD8+ T cells (17, 19) (Table 1).
2.1.2 SARS-CoV-2
For SARS-CoV-2, the pressure to escape cellular immunity primarily comes from the CTL response targeting the S2 subunit of the spike protein (S protein), a region that plays a critical role in viral membrane fusion and, therefore, is constrained in terms of mutations (20, 21). Whole-genome T cell epitope scanning shows that approximately 60% of CD8+ T cell responses target non-structural proteins, such as ORF1ab. These regions are conserved in more than 85% of the Omicron variant (22, 23). Structural studies indicate that HLA-A*02:01-restricted epitopes of the spike protein (such as S269-277) can induce potent perforin-granzyme release. Even in the presence of key mutations like K417N and E484K, these epitopes maintain over 70% TCR recognition efficiency (24, 25). Respiratory TRM cells localize to the nasopharyngeal-associated lymphoid tissue (NALT) through CXCR3-mediated homing. Their phenotypic characteristics (CD69+CD103+) are similar to those of EBV-specific TRM cells, but their function is somewhat weaker. This may explain the phenomenon of repeated upper respiratory tract infections (26, 27). It is noteworthy that SARS-CoV-2 employs three molecular strategies to escape CTL surveillance: first, by using the NSP7 protein to mimic human T cell epitopes (for example, NSP795–102 shares 67% similarity with human PPIA), thereby achieving immune camouflage; second, by mutating the furin cleavage site of the spike protein to interfere with antigen processing; and third, by utilizing the ORF8-mediated MHC-I degradation pathway to systematically downregulate antigen presentation (25, 28, 29). Hybrid immunity (post-infection mRNA vaccination) induces the broadest TRM response. These cells exhibit unique transcriptional characteristics—high expression of TCF7 to maintain stem cell-like properties, and upregulation of KLF2 to enhance tissue residency. As a result, their survival time in bronchoalveolar lavage fluid is extended to more than 12 months after infection (30, 31). Single-cell TCR sequencing has also revealed that CD8+ T cell clones targeting SARS-CoV-2 exhibit a “layered cross-reactivity” feature: the foundational clones recognize conserved epitopes from seasonal coronaviruses, while the dominant clones specifically target unique epitopes of SARS-CoV-2. This dual recognition architecture significantly enhances the breadth of defense against variants (32, 33).
2.1.3 RSV
The core challenge in the development of respiratory syncytial virus (RSV) vaccines lies in the immune pathology induced by a Th2-type immune bias. This bias is characterized by the excessive secretion of cytokines such as IL-4, IL-5, and IL-13 following vaccination, leading to pathological features such as eosinophil infiltration, airway hyperreactivity, and excessive mucus production (34, 35). This phenomenon was particularly evident in the 1960s formalin-inactivated RSV vaccine (FI-RSV) clinical trials, which manifested as exacerbated pulmonary inflammation and defective immune memory (36, 37). This pathological reaction may stem from the damage to viral surface proteins (e.g., PreF conformation) during vaccine preparation, leading to impaired innate immune recognition (e.g., TLR signaling) and aberrant Th2 polarization (38, 39). Notably, Th2 cytokines (e.g., IL-4, IL-5) not only promote eosinophil infiltration but also synergize with the IL-17 pathway—Th17 cells further amplify the inflammatory cascade through IL-17A/F secretion. This imbalance in T-cell subsets (Th2/Th17 dominance) has been confirmed in RSV infection models to exacerbate tissue damage (40–43). However, protective immunity requires coordinated T-cell responses: CD8+ T cells can directly clear virus-infected cells, while Th1-type responses (IFN-γ-dominated) and regulatory T cells (Tregs) can suppress excessive inflammation (44–46).
2.2 Durability and cross-protection of cellular immunity
2.2.1 Memory T cell pool
In terms of cellular immunity durability and cross-protection, tissue-resident memory T cells (TRM) and central memory T cells (TCM) form a dual barrier against RSV reinfection. TRM cells (such as CD103+ CD69+ CD8+ T cells) reside in the respiratory mucosa, and their presence has been directly linked to reduced viral load in experimental RSV infections (47, 48). Their expansion depends on type I interferon signaling mediated by the MAVS and MyD88/TRIF pathways (49–51). In MAVS-deficient mice, TRM numbers can be restored to 80% of wild-type levels following IFN-α treatment (52). Single-cell RNA sequencing reveals that TCM cells initiate a transcriptional reprogramming process to differentiate into TRM cells within 2 days following intranasal LAIV booster immunization. This process involves the recruitment of chemokines dependent on CXCR3 and the upregulation of CD8+ TRM characteristic genes, such as Itgae and Cxcr6 (53). Circulating memory T cells maintain systemic immune surveillance through the TCM (CD62L+ CCR7+) and effector memory T cell (TEM) subsets. Under stimulation with RSV F protein peptides, PBMCs from uninfected individuals can generate a stable memory T cell response that lasts for more than 10 years (54). Long-lived TRM and circulating memory T cells (TCM) provide rapid recall responses upon re-exposure to the pathogen.
2.2.2 Epitope conservancy
Epitope conservancy is key to achieving cross-protection. The preF conformation epitopes of the RSV F protein (such as antigenic sites Ø and V) are conserved by more than 95% between the A and B subtypes (55). In contrast, the mutation rate of T cell epitopes (such as M187–195 of the F protein) is only one-third of that of B cell epitopes (56). This makes it an ideal target for broad-spectrum vaccines. Multi-epitope vaccine design, by integrating HLA supertype epitopes of the F protein (such as the DRB104:01-restricted CD4+ epitope F254–268 and the HLA-A02:01-restricted CD8+ epitope F85-93), can simultaneously stimulate a balanced Th1/Th2 antibody response and CTL activity in mouse models (56, 57). It is worth noting that the multi-epitope display virus-like particles (VLP) based on the Round Leaf Bat Hepatitis Core Antigen (RBHcAg) can induce cross-neutralizing antibodies against RSV A2, B18537, and the clinical isolate hRSV/C-Tan/BJ 202301. The potency of these antibodies is comparable to that of approved vaccines (57, 58).
3 Vaccine-induced cellular immune responses in upper respiratory viruses
3.1 Cellular immune characteristics of different vaccine platforms
3.1.1 mRNA vaccine
mRNA vaccines carry the information encoding specific antigens (such as the spike protein of SARS-CoV-2), which are directly translated into antigens within cells, thereby activating a strong cellular immune response (59, 60). mRNA vaccines work by injecting mRNA that encodes the pathogen’s antigen, allowing the ribosomes within the cells to translate it into antigen proteins (61, 62). The synthesized antigens are processed by the cell’s endogenous pathways, generating peptides that bind to MHC class I molecules, which primarily activate CD8+ cytotoxic T cells (CTLs). This enables CD8+ T cells to recognize and bind to the MHC I-antigen complex, leading to activation, proliferation, and differentiation, and ultimately killing the infected cells (63–65). At the same time, some of the antigens are presented by antigen-presenting cells (such as dendritic cells) via MHC class II molecules, activating helper T cells (Th cells) (66). Furthermore, mRNA vaccines can induce a strong cytokine response, including pro-inflammatory cytokines (such as IL-12 and IFN-γ), which help enhance the activation and expansion of CD8+ T cells (67, 68). Particularly, under the regulation of cytokines secreted by CD4+ T cells, the generation of memory T cells is further promoted (65, 69). After the initial immune response, a portion of the CD8+ T cells will differentiate into memory T cells. The memory T cells induced by mRNA vaccines typically exhibit stronger functionality and longer survival. These memory T cells can respond rapidly when re-exposed to the same antigen, providing quick and effective immune protection (70). Therefore, mRNA vaccines can effectively induce CD8+ T cell and Th1 cell responses, providing strong cellular immune protection, making them particularly suitable for defending against intracellular pathogens and tumor cells (71). mRNA vaccines not only effectively induce humoral immunity (antibody production), but also activate potent cytotoxic T cells (CTL) via the MHC class I pathway, thereby combating viral infections. Since mRNA vaccines do not contain live viruses or viral proteins, there is no risk of infection. As a transient molecule, mRNA degrades quickly in the body and does not alter the host’s genes, ensuring a high level of safety (15).
3.1.2 Inactivated vaccines
In contrast, traditional inactivated vaccines induce an immune response using inactivated virus particles. These vaccines inactivate the virus through physical or chemical methods, rendering it unable to cause infection (72). The vaccine contains inactivated whole viruses or viral components, and after injection, the viral antigens are directly presented, triggering an immune response. The antigens of inactivated vaccines enter cells primarily via the exogenous pathway, where they are taken up and processed, and then presented to CD4+ T cells through MHC class II molecules. This typically leads to the activation of Th2 cells. Th2 cells secrete cytokines that promote humoral immunity (such as IL-4 and IL-5), stimulating B cells to produce antibodies, particularly IgE antibodies, thereby enhancing the humoral immune response (73–75). However, the cellular immune response induced by inactivated vaccines is weaker because the antigens are mainly presented via MHC class II, which is insufficient to activate CD8+ T cells (76). Because inactivated vaccines typically induce a weaker cellular immune response, the quantity and functionality of the generated memory T cells may be insufficient. Compared to the strong immune responses induced by mRNA vaccines, inactivated vaccines may have lower durability and functionality of memory T cells (77). In addition, the persistence of antigens from inactivated vaccines in the body is relatively short, which may affect the formation and maintenance of memory T cells. The development and production cycles for traditional inactivated vaccines are longer, and the costs are higher. However, their safety profile is excellent, as the viruses in inactivated vaccines have lost their infectivity, ensuring that no infection is triggered after vaccination. Therefore, inactivated vaccines are highly safe and suitable for various populations, especially those with weakened immune systems (73, 78–80). The immune response induced by inactivated vaccines is primarily focused on humoral immunity, making them effective at inducing antibody responses, providing short-term protection against the virus. However, they are less effective at inducing cellular immunity, particularly in terms of activating CD8+ T cells (81).
3.1.3 Viral vector vaccines
Adenoviral vaccines deliver target antigen genes into host cells using non-pathogenic viral vectors, efficiently activating multilayered cellular immune responses. The core mechanism lies in the ability of the viral vector to rapidly recognize receptors on the host cell surface, such as the coxsackievirus and adenovirus receptor (CAR) (82). The vector then enters the cell via endocytosis or membrane fusion, followed by antigen protein expression in the cytoplasm (83, 84). These antigens are degraded into peptides by the proteasome via the endogenous pathway, then bind to major histocompatibility complex class I (MHC-I) molecules and are presented on the cell surface, directly activating CD8+ T cells to differentiate into cytotoxic T lymphocytes (CTLs) (85). CTLs eliminate virus-infected cells directly by releasing perforin and granzymes, while also establishing a long-lasting pool of memory T cells to respond to future infections (86, 87). In addition, antigen-presenting cells (such as dendritic cells) transfer antigens to MHC class II molecules through the classical exogenous pathway, activating CD4+ T helper cells (Th1 subtype). These cells secrete cytokines such as interferon-gamma (IFN-γ), which further enhance the cytotoxic function of CD8+ T cells and promote macrophage activation (88, 89). The direct cytotoxicity of CD8+ CTLs and the immunoregulatory function of CD4+ Th1 cells work synergistically, not only enhancing antigen delivery efficiency (90, 91), but also inducing long-lasting immune memory by mimicking the natural infection pathway, thereby conferring (92).
3.1.4 Novel nanoparticle vaccines
Novel nanoparticle vaccines efficiently activate cellular immune responses through their unique delivery systems and multifunctional designs. Nanoparticles (50–250 nm) enhance the uptake efficiency by antigen-presenting cells (APCs) through size-dependent effects and surface charge modulation, such as positive charge modifications (93, 94). Passive targeting relies on the enhanced permeability and retention (EPR) effect to accumulate in inflammatory or lymphoid tissues (94, 95). Active targeting involves surface modification with antibodies or peptide ligands (such as DC-SIGN ligands) to precisely recognize APC surface receptors (96). After the particles enter the cell, pH-sensitive materials [such as poly (β-amino esters)] disassemble in the acidic environment of the endosome, releasing the antigen into the cytoplasm and promoting cross-presentation by MHC-I molecules, which directly activates CD8+ T cells to differentiate into cytotoxic T lymphocytes (CTLs) (97, 98). If the antigen enters the lysosome, it activates CD4+ Th1 cells via MHC-II molecules, which secrete IFN-γ to enhance CTL function. Sustained-release designs (such as PLGA degradation control) can extend antigen exposure for up to 28 days, continuously stimulating the generation of memory T cells. Nanocarriers can co-deliver antigens and adjuvants (such as TLR3/7/9 agonists), enhancing synergistic effects through spatiotemporal synchronized delivery. Furthermore, nanoparticles surface-modified with mannose target APC surface C-type lectin receptors, improving drug delivery specificity, bioavailability, and therapeutic efficacy, while reducing off-target effects and systemic toxicity (99). Multifunctional nanocarriers achieve microenvironmental regulation through the co-delivery of immunomodulatory factors (100, 101). For example, PLGA nanoparticles loaded with OVA antigen and rapamycin (an mTOR inhibitor) can induce the differentiation of regulatory T cells (Tregs) and suppress Th17-mediated inflammatory responses. Th17-associated pro-inflammatory factors (IL-17, IL-1β, IL-12) are significantly reduced in the PLGA-Rapa treatment group, with some literature reporting a decrease in IL-17a of over 50% (102, 103). The ratio of anti-inflammatory factors (TGF-β1, IL-10) increases, with the secretion of TGF-β1 in the Rapa&P-50k group approximately doubling (104). In the OVA inflammation model, PLGA nanoparticles (such as IL10-AMNP) increase the proportion of Tregs and decrease the proportion of Th17 cells. The exact proportions vary depending on the model, but the trend remains consistent (105, 106). At the same time, it maintains antiviral CTL activity, achieving a balance between therapy and protection (107). This ‘immune switch’ design provides a precise intervention strategy for chronic infections or autoimmune diseases (Table 2).
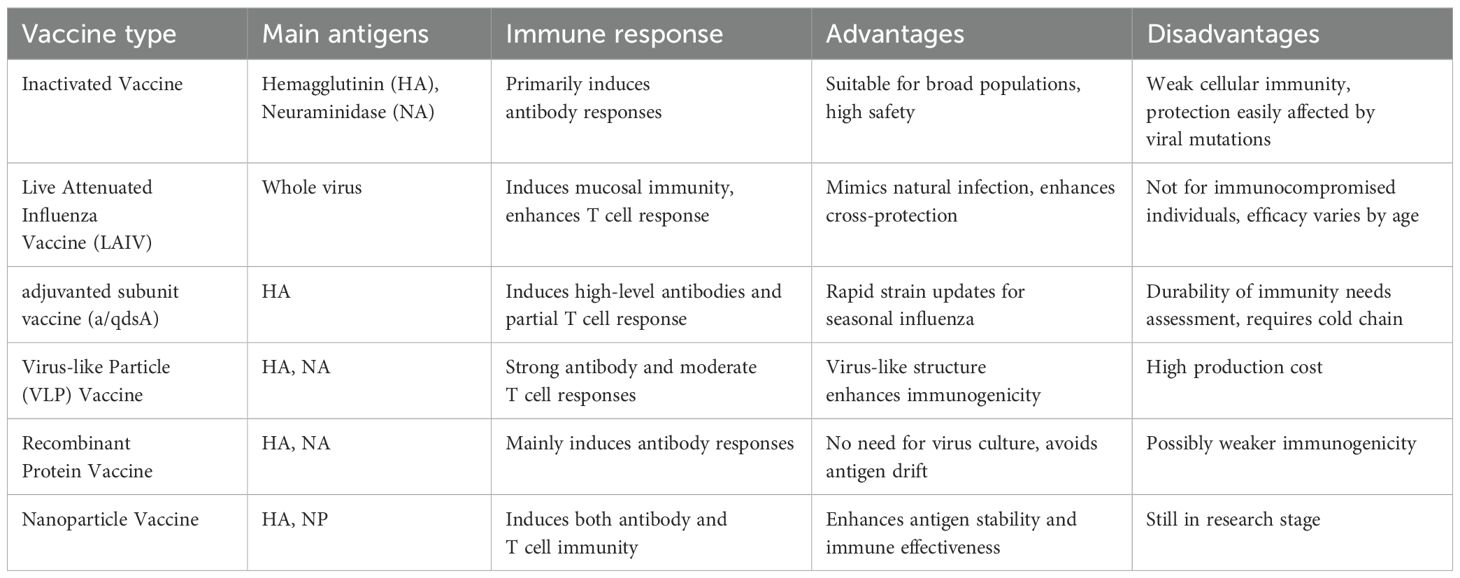
Table 2. (206).
3.2 Comparison of key indicators
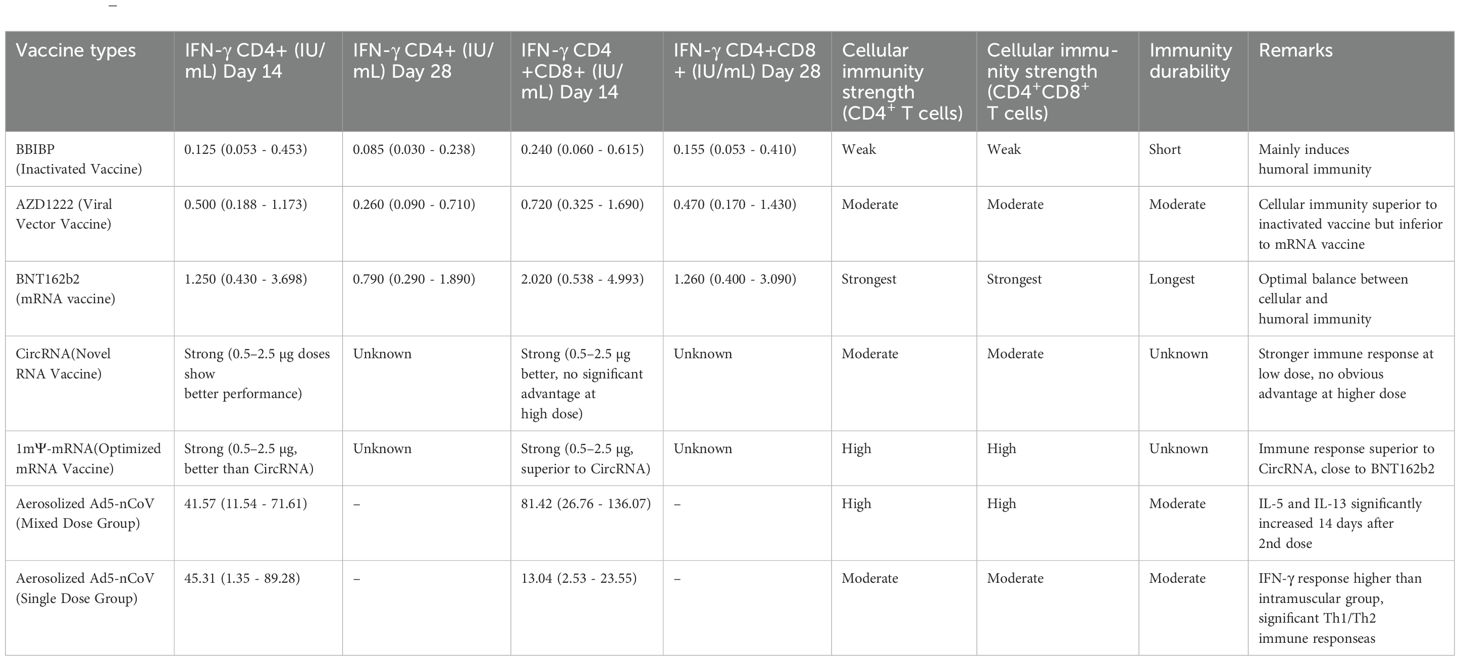
3.2.1 IFN-γ secretion levels
IFN-γ (interferon-gamma) is a key cytokine secreted by immune cells such as CD8+ T cells and NK cells, and its secretion level is an important indicator for evaluating vaccine-induced cellular immune responses. IFN-γ plays a central role in anti-infection and anti-tumor immunity by activating macrophages, enhancing antigen presentation, promoting Th1-type immune responses, and directly inhibiting viral replication or tumor growth (108–110). In vaccine immunization, high levels of IFN-γ secretion are typically associated with stronger immune protection, especially against pathogens that require cellular immunity for clearance, such as viruses or intracellular bacteria (111, 112). mThe IFN-γ secretion by CD8+ T cells induced by the RNA vaccine (BNT162b2) was significantly higher than that induced by the inactivated vaccine (BBIBP-CorV) (2.02 vs. 0.24 IU/mL), indicating that mRNA vaccines induce a stronger multifunctional CD8+ T cell response. mRNA vaccines are significantly superior to inactivated vaccines in activating Th1-type immune responses. Another study showed that CD8+ T cells induced by mRNA vaccines have a broader epitope coverage and are associated with reduced viral load in the upper respiratory tract (113, 114). This difference primarily arises from the distinct antigen designs and immune activation mechanisms of the two vaccines. mRNA vaccines (such as BNT162b2) encode a single target protein (such as the SARS-CoV-2 spike protein), efficiently activate dendritic cells, and directly present antigens through the MHC I pathway, thereby more strongly stimulating CD8+ T cell differentiation into effector cells and the secretion of IFN-γ (70, 112, 115). In contrast, although inactivated vaccines can induce CD8+ T cell responses targeting multiple viral proteins (such as S, N, and M proteins), their antigen presentation efficiency is lower and relies on cross-presentation pathways, leading to relatively weaker IFN-γ secretion by CD8+ T cells (70, 115, 116). Another comparative study showed that the IFN-γ secretion by CD8+ T cells induced by the mRNA vaccine is twice that of the inactivated vaccine (70). Moreover, the response is more focused on the S protein epitopes (115). In addition, the lipid nanoparticle (LNP) delivery system of mRNA vaccines may directly enhance T cell activation, further promoting IFN-γ production (117, 118). These differences suggest that mRNA vaccines have an advantage in stimulating Th1-type immunity and CD8+ T cell-mediated cytotoxicity.
3.2.2 Memory T cell durability
Viral vector vaccines (such as AZD1222) maintain moderate CD8+ T cell activity 28 days after vaccination, while the response from inactivated vaccines rapidly declines. The advantage of viral vector vaccines (like AZD1222) in inducing memory T cell durability compared to inactivated vaccines is primarily due to their unique antigen presentation methods and immune activation mechanisms. Viral vector vaccines mimic the natural infection process, continuously expressing the target antigen [for example, adenoviral vectors can infect fibroblasts over the long term) (119)]. This results in the phenomenon of ‘memory inflation,’ where effector memory CD8+ T cells (TEM) continue to expand and maintain high numbers in peripheral tissues (120). This persistent antigen stimulation is achieved through two mechanisms: first, viral vectors (such as adenoviruses or cytomegalovirus vectors) can persist in host cells for extended periods and express the antigen at low levels (121, 122). Second, it preferentially targets antigen-presenting cells such as dendritic cells, promoting cross-presentation and activating CD8+ T cells (123). In contrast, the antigen of inactivated vaccines has a short-lived effect (78). It cannot form persistent stimulation, leading to a rapid decline in CD8+ T cell responses. Viral vector vaccines also enhance durability by inducing tissue-resident memory T cells (TRM), which remain distributed in various tissues months after vaccination (121, 124). In contrast, inactivated vaccines primarily induce circulating memory T cells (10). In addition, viral vector vaccines can more effectively activate cytokine pathways such as IL-15, promoting the early generation of memory precursor cells (such as Tscm) (125). These stem cell-like memory T cells have self-renewal capabilities and can maintain the memory pool for a long time (126). Phenotypically, viral vector-induced CD8+ T cells exhibit high expression of cytotoxic-related genes (127). They also exhibit higher functional affinity (128). The T cell subset distribution induced by inactivated vaccines is more limited (78). It is worth noting that adenovirus vector vaccines can also drive the expansion of innate-like CD8+ T cells by inducing host IL-15 production (122). This mechanism has not been reported in inactivated vaccines. Taken together, viral vector vaccines achieve more durable memory T cell maintenance compared to inactivated vaccines through multiple mechanisms, including persistent antigen exposure, tissue-resident memory formation, cytokine signaling activation, and the induction of stem cell-like memory subsets.
4 Comparison of local and systemic immunity in upper respiratory infections
The synergistic action of cellular and humoral immunity is crucial for respiratory virus vaccines. Current research indicates that although cellular immunity plays a key role in host protection (129), relying solely on a single immune mechanism often fails to confer comprehensive protection. For instance, the PIV5-vectored SARS-CoV-2 vaccine CVXGA1, administered intranasally, can simultaneously induce durable mucosal, cellular, and humoral immune responses, demonstrating the advantage of coordinated immunity (130). Studies have confirmed that when vaccines are able to simultaneously activate CD8+ T cell responses (cellular immunity) and neutralizing antibody production (humoral immunity), they achieve optimal protection against respiratory viruses, including influenza and SARS-CoV-2 variants (131–133). This synergistic relationship is particularly important in vaccine design, as respiratory viruses often evade pre-existing antibodies to escape immune defense (134, 135), whereas cellular immunity—such as CD8+ cytotoxic T cells—can recognize conserved viral epitopes and provide broad cross-protection (136, 137). Moreover, molecular docking studies suggest that ideal vaccine constructs should be capable of simultaneously engaging pattern recognition receptors like TLR3 and TLR8 to co-activate both arms of the immune response (138). Clinical observations have shown that while humoral immunity tends to wane over time following booster vaccination, cellular immune responses are more durable (139, 140), further highlighting the necessity of inducing both immune pathways. Therefore, the development of next-generation respiratory virus vaccines should focus on optimizing antigen design [e.g., co-delivery of spike and nucleocapsid proteins (141)], delivery platforms [e.g., adenoviral vectors (142)], and adjuvants [e.g., ARNAX (143)] to synergistically enhance mucosal IgA, systemic IgG, and T cell responses (144, 145), thereby providing more effective protection against immune-evading viral variants (146).
4.1 Synergy between local and systemic immunity
Local and systemic immunity do not exist in isolation; rather, they interact and coordinate through various mechanisms to form a more comprehensive and efficient immune response. In upper respiratory tract infections, the interaction between local TRM cells and systemic immune cells (such as CD8+ T cells, Th1 cells, etc.) plays a crucial role in enhancing the overall effectiveness of the immune response. The local immune response, particularly through the rapid response of TRM cells, can provide localized immune protection during the early stages of infection, while the systemic immune response strengthens the local immune function through the T cell response across the body (147). For example, local TRM cells can recruit and activate surrounding immune cells, such as neutrophils and macrophages, by secreting cytokines (e.g., interferon-γ and tumor necrosis factor-α), which help eliminate local infection sources. The local immune response can also act on immune organs such as lymph nodes to promote the activation and proliferation of systemic T cells, thereby further enhancing the breadth and persistence of the immune response (148). Experimental studies have shown that nasal immunization can promote the activation of the systemic immune system through local immune responses, and the systemic immune response can also enhance immune defense at the local infection site through memory T cells. Karaki et al., through mouse experiments, found that nasal spray vaccines could activate TRM cells in the respiratory tract, promoting local immune responses while enhancing the systemic immune response through cytokines, particularly the activation of CD8+ T cells and Th1 cells, ultimately improving the mouse’s defense against influenza virus (48).
4.2 The combination of mucosal vaccines and systemic vaccines
The immune characteristics of upper respiratory tract viruses dictate that an effective immunization strategy needs to balance both local and systemic immunity. Traditional intramuscular vaccines mainly activate systemic immunity and struggle to effectively induce mucosal immunity. The advantage of local mucosal vaccination is that it directly stimulates immune cells in the respiratory mucosa, rapidly inducing secretory IgA production. IgA can neutralize viruses, prevent them from attaching to and entering epithelial cells, and activate tissue-resident memory T cells (TRM), which rapidly respond after infection and help limit viral spread. Systemic intramuscular vaccines primarily generate a strong IgG antibody response and can induce circulating CD8+ cytotoxic T cells to clear infected cells. When these two vaccines are used together, they can establish a local barrier defense and mobilize systemic immune resources to provide multi-layered protection. Studies have shown that this strategy provides significant advantages in various viral models. For example, experiments demonstrate that combining adenovirus vector-based nasal vaccines and intramuscular vaccines significantly enhances protection against SARS-CoV-2 in mouse and non-human primate models. The nasal vaccine effectively induces IgA and TRM cell responses in the upper respiratory tract, while the intramuscular vaccine significantly boosts systemic neutralizing antibody levels and CD8+ T cell responses (149). Similarly, Animal experiments on mice studies on influenza virus have also shown that combining nasal immunization with inactivated virus and traditional intramuscular injection can provide more comprehensive immune coverage, reduce viral load, and prevent severe disease (150). In addition, this strategy is particularly important when addressing viral variants. Mucosal immune mechanisms demonstrate strong cross-protection against viral variants. For example, researchers genetically engineered two AD vectors for a trivalent vaccine (Tri: HuAd and Tri: ChAd) and extensively compared their immunogenicity and protective effects against both the ancestral and variant strains of SARS-CoV-2. The study found that trivalent ChAd vector vaccine delivered through the respiratory mucosa is the most effective next-generation COVID-19 vaccine strategy. Their research supports its further clinical development. On the other hand, systemic antibodies may be more sensitive to variant epitopes, and nearly all first-generation gene-based COVID-19 vaccines were designed for intramuscular delivery, expressing only the S protein (151). The combined strategy can better overcome the limitations of single vaccination methods. The integration of both local and systemic vaccination approaches is one of the key directions for future vaccine design.
4.3 The regulatory role of vaccine adjuvants on cellular immunity
The mechanisms by which adjuvants enhance T cell responses through activation of the innate immune system vary depending on their type. Aluminum salt adjuvants (Alum), one of the earliest adjuvants used, primarily function by activating the NLRP3 inflammasome to induce the secretion of IL-1β and IL-18, thereby promoting dendritic cell (DC) maturation and enhancing antigen presentation capacity. Additionally, Alum improves antigen persistence and delivery efficiency by forming antigen depots (152). Emulsifier-based adjuvants (e.g., MF59) enhance immune responses by promoting antigen presentation and activating innate immune cells, enabling antigen dose sparing, broader response range, and fewer immunizations. Their mechanisms include improving antigen distribution and uptake, inducing inflammatory chemokine production to attract monocytes and macrophages to the injection site, thereby promoting dendritic cell (DC) recruitment and activation (153). Pathogen-associated molecular pattern (PAMP)-based adjuvants, such as CpG oligonucleotides, activate innate immunity by binding to TLR9, inducing IL-12 secretion to promote Th1-type immune responses, and enhancing dendritic cell (DC) cross-presentation, enabling exogenous antigens to activate CD8+ T cells (154). Pathogen-associated molecular pattern (PAMP)-based adjuvants, such as CpG oligonucleotides, activate innate immunity by binding to TLR9, inducing IL-12 secretion to promote Th1-type immune responses, and enhancing dendritic cell (DC) cross-presentation, enabling exogenous antigens to activate CD8+ T cells (155).
Adjuvants primarily function by activating the innate immune system and modulating the activity of antigen-presenting cells (e.g., dendritic cells). Different types of adjuvants engage specific pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), retinoic acid-inducible gene I (RIG-I), melanoma differentiation-associated protein 5 (MDA5), and laboratory of genetics and physiology 2 (LGP2), to induce distinct cytokine environments, thereby shaping T-cell differentiation. For example, in Th1 responses, TLR3 or TLR9 ligands (e.g., Poly I:C or CpG oligonucleotides) promote IL-12 and IFN-γ production, enhancing cytotoxicity and antiviral immunity (156). For Th2 responses, aluminum salt-based adjuvants (e.g., alum) tend to stimulate macrophages containing large, persistent intracellular crystalline inclusions, a characteristic feature of muscle-infiltrating macrophages described in vaccine-injected animal models and recently reported in human macrophagic myofasciitis - myofasciitis (MMF) histological responses. Experiments by Gherardi, Verdier, et al. showed that macrophages were dominant in animals injected with aluminum hydroxide vaccines (mice, cynomolgus monkeys, rabbits, etc.), and the data obtained illustrated the critical role of this cell type in the physiological response to aluminum hydroxide-containing vaccines (157, 158). For Th17 responses, TGF-β, together with IL-6 and IL-21, promotes Th17 cell development. Adjuvants capable of activating IL-6, IL-23, and TGF-β, such as β-glucan molecules, can effectively induce Th17 cell differentiation, suitable for immune responses against fungal or extracellular bacterial infections (159). Optimizing specific immune response directions requires comprehensive consideration of pathogen characteristics and the desired effector mechanisms, achieving enhanced specific immune pathways through selective adjuvant combinations.
4.4 Comprehensive enhancement of cellular immunity
Enhancing the formation and function of tissue-resident memory T cells (TRM) while increasing the quantity and activity of systemic memory T cells to achieve comprehensive immune protection is a key topic in current immunology research. In local immunity, promoting TRM formation and function requires targeted optimization of the local microenvironment and signaling molecules. TRM generation relies on specific tissue-retention signals (160), such as cytokines like TGF-β and IL-15, which drive the formation and function of resident cells in local tissues by regulating the expression of TRM signature molecules (e.g., CD69 and CD103). TGF-β can enhance TRM adhesion to epithelial cells by promoting CD103 expression, thereby strengthening their retention capacity, while IL-15 improves TRM survival by supporting metabolic adaptability and anti-apoptotic signaling (161, 162). Studies show that in specific environments like adipose tissue, TRM adapt to local nutritional conditions by upregulating genes related to fatty acid oxidation (e.g., Cpt1a and Pparg), further enhancing their persistence and effector functions. Local immune enhancement can be optimized through vaccine delivery strategies (163, 164), such as local injection of TGF-β or IL-15 agonists, to accelerate TRM generation and improve their immune response to local pathogens. Systemic immune enhancement focuses on increasing the quantity and activity of circulating memory T cells (TCM) and effector memory T cells (TEM) to provide broad systemic protection (165) (162). A key strategy for systemic immune enhancement is inducing a high-quality memory T cell pool through vaccination. Using genetically modified antigen vaccines or adjuvants (e.g., TLR agonists or IL-12) can significantly enhance T cell proliferation and effector functions. IL-12 activates T cell effector functions via the STAT4 signaling pathway and promotes their differentiation into TEM. Additionally, systemic immunity can be improved by facilitating the interconversion between circulating and resident T cells. TEM cells can enter local tissues and, upon receiving TGF-β and IL-15 signals, convert into TRM, thereby establishing long-term immune memory locally (166, 167). Local immunity promotes TRM generation and persistence by optimizing the microenvironment, while systemic immunity ensures broad defense by expanding the memory T cell pool. A combined strategy, utilizing local and systemic cytokine co-delivery or optimized vaccine platforms, can significantly enhance the functions of TRM and TCM/TEM. This ultimately achieves more comprehensive and durable protection against pathogens.
5 Clinical challenges
5.1 The impact of immune escape and variant strains on vaccine efficacy
The emergence of viral variants can lead to changes in surface antigenicity through antigenic drift or antigenic shift, thereby impacting vaccine efficacy. In terms of cellular immunity, alterations in T cell epitopes are a key factor (168). SARS-CoV-2 variants carry multiple mutations, particularly concentrated in the receptor-binding domain (RBD) and antigenic epitope regions of the spike (S) protein, which may lead to a reduced recognition efficiency of certain mutated epitopes by vaccine-induced T cells (169–171). For example, the extensive mutations in the Omicron variant not only weaken the neutralizing antibody efficacy but also affect certain CD8+ T cell epitopes, thus reducing the cytotoxic T cell killing ability. Additionally, CD4+ T cell responsiveness to variants may be diminished if the mutated epitopes cannot be effectively presented by antigen-presenting cells. Despite the strong robustness of cellular immunity, the ability to recognize conserved viral epitopes (such as the S2 region of the S protein or the nucleocapsid (N) and membrane (M) proteins) still exists across different variants, but mutations in these conserved epitopes could further threaten the stability of cellular immunity (172). For rapidly mutating viruses, such as the influenza virus and respiratory syncytial virus (RSV), similar mutations may also alter key epitopes, leading to a reduction in vaccine efficacy.
5.2 Differences in vaccine efficacy among immunocompromised populations
5.2.1 Age and immune response
There are significant differences in cellular immune responses to vaccination across different age groups. Adults generally exhibit the strongest T-cell responses, while infants and the elderly show lower reactivity due to the unique states of their immune systems. In infants, the immune system is still under development, with a higher proportion of naive T cells that are functionally immature, and limited capacity of antigen-presenting cells. This results in a significant reduction in the proliferation and effector function of CD4+ and CD8+ T cells after vaccination compared to adults. In contrast, elderly individuals experience immunosenescence, characterized by a significant reduction in the naive T cell pool. Immunosenescence weakens T cell activation and sensitivity to antigens, reduces the diversity of memory T cells, and chronic inflammation (inflammaging) further suppresses vaccine-induced cellular immune responses, thereby significantly affecting vaccine efficacy in older adults (173–175). Additionally, studies have found that elderly individuals exhibit significantly lower CD8+ T cell reactivity after receiving the pertussis vaccine compared to younger individuals, and the functionality of effector T cells is also impaired (175, 176). A study on age and immune response (with CMV infection as an immune-related phenotype) showed that the CMV positivity rate was 42.86% in young individuals with an average age of 25.3 years (aged 20–31 years at enrollment) and 55.56% in elderly individuals with an average age of 76.96 years (aged 60–96 years at enrollment). According to the IMM-AGE score, the immune aging index of a 90-year-old was approximately 3.2 times higher than that of a 66–67-year-old. The final results indicated that immune senescence begins in mid-adulthood (ages 40–60) (177). A study by Wu et al. showed that the antibody titers (GMT values) induced by the mRNA vaccine were significantly higher in younger individuals compared to the elderly. Specifically, 28 days after vaccination, the GMT for younger individuals was 302.9, while the GMT for the elderly was 173.2 (178). These studies suggest that the immune system status at different age stages significantly affects the cellular immune response induced by vaccines. Both infants and the elderly face greater immune response deficiencies, highlighting the need to specifically optimize vaccination strategies for these populations.
5.2.2 Challenges in immunosuppressed and chronic disease patients
Immunocompromised and chronic disease patients often exhibit weakened cellular immune responses following vaccination, which poses significant challenges to vaccine efficacy and clinical application. In immunocompromised patients, such as those who have undergone organ transplants, the long-term use of immunosuppressive drugs significantly reduces their T-cell-mediated immune response, leading to a weaker vaccine-induced cytotoxic T lymphocyte (CTL) response (179, 180). Studies have shown that kidney transplant recipients exhibit CD4+ and CD8+ T cell responses after receiving the mRNA COVID-19 vaccine that are only 30%-50% of those seen in healthy individuals. Chronic kidney disease patients also face similar issues, with research indicating a reduction in memory T cell function and lower protection after vaccination compared to healthy controls (181). In chronic disease patients, such as those with diabetes, vaccine-induced cellular immune responses are also significantly affected due to chronic inflammation and immune system dysfunction (182). These characteristics lead to a higher infection risk for these patient groups when facing emerging viruses like SARS-CoV-2. Even after completing vaccination, additional preventive strategies, such as booster doses and passive immunotherapy, should be considered. Yang indicated that the third or fourth dose of the SARS-CoV-2 vaccine significantly improved the immunogenicity rate in dialysis patients, and this beneficial effect was not altered by vaccine type (same or different immunogenic vaccine), dialysis method (HD or PD), or prior low response after two doses of the vaccine (183).
6 Future research directions
6.1 Emerging immuno-monitoring technologies
Emerging immuno-monitoring technologies, such as single-cell RNA sequencing (scRNA-seq) and multi-color flow cytometry, play a crucial role in studying vaccine-induced cellular immunity. Single-cell RNA sequencing (scRNA-seq) enables gene expression analysis at the single-cell level, revealing transcriptomic changes in immune cells before and after vaccination, and helping identify specific cell subpopulations and their functional states. Weng et a. us’d scRNA-seq technology to analyze peripheral blood mononuclear cells (PBMCs) from convalescent COVID-19 patients, aiming to uncover dynamic changes in immune responses and the characteristics of cell subpopulations. The study collected PBMC samples from 10 COVID-19 patients and healthy controls, isolated the cells through Ficoll-Hypaque density gradient centrifugation, and constructed single-cell RNA libraries using the 10x Genomics Chromium Single Cell 5′ system. After high-throughput sequencing, the data were processed using Cell Ranger software, barcode labeling was applied, and data integration, normalization, and dimensionality reduction were performed using the Seurat software package. The study revealed the remodeling process of the immune system in convalescent patients, including an increase in the proportion of CD4+ and CD8+ memory T cells, a tendency of B cells to differentiate into plasma cells, and specific changes in the function of monocytes and NK cells. This provided valuable insights into immune recovery following COVID-19 infection (184–187). Multi-color flow cytometry, by using a variety of fluorescent markers, can identify and characterize cell subpopulations of interest. It can rapidly analyze tens of thousands of cells per second and, through cell sorting, isolate pure, viable cell populations, enabling precise characterization and quantitative analysis of different immune cell groups (188, 189). Guo et al. used a 21-color flow cytometry panel to analyze immune cell subpopulations in human non-small cell lung cancer (NSCLC) tissues. They assessed the proportions of different cell subpopulations in the lung cancer tissues, as well as the immune phenotypes and differentiation states of major cell populations. The successfully established 21-color flow cytometry protocol is applicable for detecting PBMCs and NSCLC tissue samples, providing an effective new approach for monitoring the immune microenvironment in lung cancer (190). The combination of these technologies provides powerful tools for a deeper understanding of the mechanisms behind vaccine-induced immune responses.
6.2 Personalized vaccination strategy
Personalized vaccination strategies are an important development direction in modern medicine, aiming to design more precise and efficient vaccination plans by analyzing an individual’s immune background. An individual’s immune response to vaccines can vary significantly due to differences in genetic background, age, gender, health status, and environmental factors. In practical applications, personalized vaccination strategies not only enhance an individual’s immunity but also effectively improve overall vaccination rates. Particularly for high-risk groups, understanding and utilizing the characteristics of breakthrough infections can help public health officials create more targeted vaccination plans to better protect these vulnerable populations (191, 192). As the COVID-19 pandemic continues, the implementation of personalized vaccination strategies will be key to improving vaccine effectiveness. Thus, personalized vaccination strategies have gradually become a focus of vaccine research. The core idea is that the genomic instability of tumors leads to the production of tumor-specific neoantigens, which are absent in normal tissues and serve as ideal targets for personalized vaccines (193–195). High-throughput sequencing and bioinformatics analysis can be used to identify specific neoantigens in a patient’s tumor, providing the basis for personalized vaccine design. Vaccines can be tailored based on the patient’s tumor mutation profile, using mutation hotspots and predictive algorithms to select candidate neoantigens that effectively activate CD8+ and CD4+ T cells (196–199). Additionally, mRNA vaccines and peptide vaccines are the main technological platforms for personalized vaccines, as they are highly flexible and can quickly incorporate patient-specific neoantigens for production. To further enhance efficacy, personalized vaccines can be combined with immune checkpoint inhibitors or other immunotherapies, using synergistic effects to amplify anti-tumor immunity (200). In recent years, some literature has supported the feasibility and potential of personalized vaccination strategies. It has demonstrated the safety of two types of personalized cancer vaccines in small-scale human trials, which showed positive clinical responses in high-risk melanoma patients. These results confirm that vaccines specifically designed based on a patient’s individual cancer mutations are feasible and safe in clinical practice. They also provide valuable insights for the development of personalized cancer immunotherapy strategies (201, 202).
7 Conclusion
Different vaccines for upper respiratory viruses exhibit significant differences in inducing cellular immune responses. Live attenuated vaccines typically simulate natural infection and induce a stronger T cell immune response, particularly in the activation of effector CD8+ T cells. In contrast, inactivated vaccines and subunit vaccines mainly enhance the activity and auxiliary functions of antigen-specific CD4+ T cells to support the immune response. Emerging nucleic acid vaccines (mRNA and DNA) can also significantly induce strong and durable cellular immune responses through efficient antigen expression. The ability of different vaccines to induce mucosal immunity (such as tissue-resident memory T cells, TRM) also varies, which directly impacts the local defense against viruses. Future vaccine design and research should focus on the following key directions: optimizing mucosal immune responses, studying how to enhance vaccine-induced TRM cells and other mucosal immune effects to better prevent virus replication and transmission in the upper respiratory tract; developing multi-target, broad-spectrum antigens targeting high mutation regions of viruses to improve immune protection against variant strains; exploring the specific mechanisms by which different vaccine platforms induce cellular immunity, especially the generation and maintenance of T cell memory; designing personalized vaccines based on host immunology and genetic data to meet the immune needs of different populations; exploring the combined use of different types of vaccines (such as live attenuated vaccines and nucleic acid vaccines) or supplementation with immunomodulators to enhance the breadth and strength of immune effects. These directions are expected to drive the development of next-generation upper respiratory virus vaccines and provide scientific evidence and technical support for addressing viral mutations and potential future pandemics.
Author contributions
KC: Writing – original draft, Visualization, Conceptualization, Writing – review & editing. JH: Conceptualization, Writing – original draft, Data curation. JXL: Visualization, Conceptualization, Writing – review & editing, Writing – original draft. GW: Writing – review & editing, Conceptualization. XT: Visualization, Conceptualization, Writing – review & editing. HW: Writing – review & editing, Data curation, Visualization. HL: Visualization, Conceptualization, Writing – review & editing. JHL: Visualization, Conceptualization, Writing – review & editing. YZ: Visualization, Writing – review & editing, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by the Provincial Industry-University Cooperation Collaborative Education Project (NO.318 (2022) of the Zhejiang Development Reform Society) and the University Level Scientific Research Project of Zhejiang Shuren University (Grant No:2024R057). The Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2022-I2M-CoV19-006) and The National Natural Science Foundation of China (U20A20410).
Acknowledgments
Authors thank all the researchers, doctors, nurses, medical technicians, front-line workers, and public health officials for their hard work during this pandemic.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhibin P, Dayan F, and Dayan W. Technical guidelines for seasonal influenza vaccination in China (2020-2021). Zhonghua Liu Xing Bing Xue Za Zhi. (2020) 41:1555–76. doi: 10.3760/cma.j.cn112338-20200904-01126
2. Clementi N, Ghosh S, De Santis M, Castelli M, Criscuolo E, Zanoni I, et al. Viral respiratory pathogens and lung injury. Clin Microbiol Rev. (2021) 34. doi: 10.1128/CMR.00103-20
3. Akin L and Gözel MG. Understanding dynamics of pandemics. Turk J Med Sci. (2020) 50:515–9. doi: 10.3906/sag-2004-133
4. Uyeki TM, Hui DS, Zambon M, Wentworth DE, and Monto AS. Influenza. Lancet. (2022) 400:693–706. doi: 10.1016/S0140-6736(22)00982-5
5. Kuse N, Zhang Y, Chikata T, Nguyen HT, Oka S, Gatanaga H, et al. Long-term memory CD8(+) T cells specific for SARS-CoV-2 in individuals who received the BNT162b2 mRNA vaccine. Nat Commun. (2022) 13:5251. doi: 10.1038/s41467-022-32989-4
6. Wu D, Efimov GA, Bogolyubova AV, Pierce BG, and Mariuzza RA. Structural insights into protection against a SARS-CoV-2 spike variant by T cell receptor diversity. J Biol Chem. (2023) 299:103035. doi: 10.1016/j.jbc.2023.103035
7. Shafqat A, Omer MH, Ahmad O, Niaz M, Abdulkader HS, Shafqat S, et al. SARS-CoV-2 epitopes inform future vaccination strategies. Front Immunol. (2022) 13:1041185. doi: 10.3389/fimmu.2022.1041185
8. Rodrigues-da-Silva RN, Conte FP, da Silva G, Carneiro-Alencar AL, Gomes PR, Kuriyama SN, et al. Identification of B-cell linear epitopes in the nucleocapsid (N) protein B-cell linear epitopes conserved among the main SARS-coV-2 variants. Viruses. (2023) 15. doi: 10.3390/v15040923
9. Tarke A, Grifoni A, and Sette A. Bioinformatic and experimental analysis of T cell immune reactivity to SARS-coV-2 and its variants. Front Bioinform. (2022) 2:876380. doi: 10.3389/fbinf.2022.876380
10. Hirai T and Yoshioka Y. Considerations of CD8(+) T cells for optimized vaccine strategies against respiratory viruses. Front Immunol. (2022) 13:918611. doi: 10.3389/fimmu.2022.918611
11. Gombolay GY, Dutt M, and Tyor W. Immune responses to SARS-CoV-2 vaccination in multiple sclerosis: a systematic review/meta-analysis. Ann Clin Transl Neurol. (2022) 9:1321–31. doi: 10.1002/acn3.51628
12. Toniolo A, Maccari G, and Camussi G. mRNA technology and mucosal immunization. Vaccines (Basel). (2024) 12. doi: 10.3390/vaccines12060670
13. Ura T, Takeuchi M, Kawagoe T, Mizuki N, Okuda K, and Shimada M. Current vaccine platforms in enhancing T-cell response. Vaccines (Basel). (2022) 10. doi: 10.3390/vaccines10081367
14. Van der Ley P and Schijns VE. Outer membrane vesicle-based intranasal vaccines. Curr Opin Immunol. (2023) 84:102376. doi: 10.1016/j.coi.2023.102376
15. Zheng MZM, Tan TK, Villalon-Letelier F, Lau H, Deng YM, Fritzlar S, et al. Single-cycle influenza virus vaccine generates lung CD8(+) Trm that cross-react against viral variants and subvert virus escape mutants. Sci Adv. (2023) 9:eadg3469. doi: 10.1126/sciadv.adg3469
16. Swarnalekha N, Schreiner D, Litzler LC, Iftikhar S, Kirchmeier D, Kunzli M, et al. T resident helper cells promote humoral responses in the lung. Sci Immunol. (2021) 6. doi: 10.1126/sciimmunol.abb6808
17. Marinaik CB, Kingstad-Bakke B, Lee W, Hatta M, Sonsalla M, Larsen A, et al. Programming multifaceted pulmonary T cell immunity by combination adjuvants. Cell Rep Med. (2020) 1:100095. doi: 10.1016/j.xcrm.2020.100095
18. Del Campo J, Bouley J, Chevandier M, Rousset C, Haller M, Indalecio A, et al. OVX836 heptameric nucleoprotein vaccine generates lung tissue-resident memory CD8+ T-cells for cross-protection against influenza. Front Immunol. (2021) 12:678483. doi: 10.3389/fimmu.2021.678483
19. Kingstad-Bakke B, Toy R, Lee W, Pradhan P, Vogel G, Marinaik CB, et al. Polymeric pathogen-Like particles-Based combination adjuvants elicit potent mucosal T cell immunity to influenza A virus. Front Immunol. (2020) 11:559382. doi: 10.3389/fimmu.2020.559382
20. Boni C, Cavazzini D, Bolchi A, Rossi M, Vecchi A, Tiezzi C, et al. Degenerate CD8 epitopes mapping to structurally constrained regions of the spike protein: A T cell-based way-out from the SARS-coV-2 variants storm. Front Immunol. (2021) 12:730051. doi: 10.3389/fimmu.2021.730051
21. Jiang S, Wu S, Zhao G, He Y, Guo X, Zhang Z, et al. Identification of a promiscuous conserved CTL epitope within the SARS-CoV-2 spike protein. Emerg Microbes Infect. (2022) 11:730–40. doi: 10.1080/22221751.2022.2043727
22. Saini SK, Hersby DS, Tamhane T, Povlsen HR, Amaya Hernandez SP, Nielsen M, et al. SARS-CoV-2 genome-wide T cell epitope mapping reveals immunodominance and substantial CD8(+) T cell activation in COVID-19 patients. Sci Immunol. (2021) 6. doi: 10.1126/sciimmunol.abf7550
23. Tarke A, Sidney J, Kidd CK, Dan JM, Ramirez SI, Yu ED, et al. Comprehensive analysis of T cell immunodominance and immunoprevalence of SARS-CoV-2 epitopes in COVID-19 cases. Cell Rep Med. (2021) 2:100204. doi: 10.1016/j.xcrm.2021.100204
24. Qiu C, Xiao C, Wang Z, Zhu G, Mao L, Chen X, et al. CD8(+) T-cell epitope variations suggest a potential antigen HLA-A2 binding deficiency for spike protein of SARS-coV-2. Front Immunol. (2021) 12:764949. doi: 10.3389/fimmu.2021.764949
25. Deng S, Xu Z, Hu J, Yang Y, Zhu F, Liu Z, et al. The molecular mechanisms of CD8(+) T cell responses to SARS-CoV-2 infection mediated by TCR-pMHC interactions. Front Immunol. (2024) 15:1468456. doi: 10.3389/fimmu.2024.1468456
26. Niessl J, Sekine T, Lange J, Konya V, Forkel M, Maric J, et al. Identification of resident memory CD8(+) T cells with functional specificity for SARS-CoV-2 in unexposed oropharyngeal lymphoid tissue. Sci Immunol. (2021) 6:eabk0894. doi: 10.1126/sciimmunol.abk0894
27. Wang Z, He Y, Wang W, Tian Y, Ge C, Jia F, et al. A novel "prime and pull" strategy mediated by the combination of two dendritic cell-targeting designs induced protective lung tissue-resident memory T cells against H1N1 influenza virus challenge. J Nanobiotechnology. (2023) 21:479. doi: 10.1186/s12951-023-02229-y
28. Miah SMS, Lelias S, Gutierrez AH, McAllister M, Boyle CM, Moise L, et al. A SARS-CoV-2 NSP7 homolog of a Treg epitope suppresses CD4+ and CD8+ T cell memory responses. Front Immunol. (2023) 14:1290688. doi: 10.3389/fimmu.2023.1290688
29. Kombe Kombe AJ, Biteghe FAN, Ndoutoume ZN, and Jin T. CD8(+) T-cell immune escape by SARS-CoV-2 variants of concern. Front Immunol. (2022) 13:962079. doi: 10.3389/fimmu.2022.962079
30. Schulien I, Kemming J, Oberhardt V, Wild K, Seidel LM, Killmer S, et al. Characterization of pre-existing and induced SARS-CoV-2-specific CD8(+) T cells. Nat Med. (2021) 27:78–85. doi: 10.1038/s41591-020-01143-2
31. Ahmed SF, Sohail MS, Quadeer AA, and McKay MR. Identification of potential SARS-coV-2 CD8(+) T cell escape mutants. Vaccines (Basel). (2022) 10. doi: 10.3390/vaccines10040542
32. Diniz MO, Mitsi E, Swadling L, Rylance J, Johnson M, Goldblatt D, et al. Airway-resident T cells from unexposed individuals cross-recognize SARS-CoV-2. Nat Immunol. (2022) 23:1324–9. doi: 10.1038/s41590-022-01292-1
33. Fahnoe U, Feng S, Underwood AP, Jacobsen K, Ameri A, Blicher TH, et al. T cell receptor usage and epitope specificity amongst CD8(+) and CD4(+) SARS-CoV-2-specific T cells. Front Immunol. (2025) 16:1510436. doi: 10.3389/fimmu.2025.1510436
34. Kosanovich JL, Eichinger KM, Lipp MA, Gidwani SV, Brahmbhatt D, Yondola MA, et al. Exacerbated lung inflammation following secondary RSV exposure is CD4+ T cell-dependent and is not mitigated in infant BALB/c mice born to PreF-vaccinated dams. Front Immunol. (2023) 14:1206026. doi: 10.3389/fimmu.2023.1206026
35. Binns E, Tuckerman J, Licciardi PV, and Wurzel D. Respiratory syncytial virus, recurrent wheeze and asthma: A narrative review of pathophysiology, prevention and future directions. J Paediatr Child Health. (2022) 58:1741–6. doi: 10.1111/jpc.v58.10
36. Wang Y, Ge F, Wang J, Li H, Zheng B, Li W, et al. Mycobacterium bovis BCG Given at Birth Followed by Inactivated Respiratory Syncytial Virus Vaccine Prevents Vaccine-Enhanced Disease by Promoting Trained Macrophages and Resident Memory T Cells. J Virol. (2023) 97:e0176422. doi: 10.1128/jvi.01764-22
37. Noor A and Krilov LR. A historical perspective on respiratory syncytial virus prevention: A journey spanning over half a century from the setback of an inactive vaccine candidate to the success of passive immunization strategy. J Pediatr Infect Dis Soc. (2024) 13:S103–s109. doi: 10.1093/jpids/piae027
38. Powell TJ, Jacobs A, Tang J, Cardenas E, Palath N, Daniels J, et al. Microparticle RSV vaccines presenting the G protein CX3C chemokine motif in the context of TLR signaling induce protective th1 immune responses and prevent pulmonary eosinophilia post-challenge. Vaccines (Basel). (2022) 10. doi: 10.3390/vaccines10122078
39. Chen F, Park HR, Ji HJ, Kwon Y, Kim MK, Song JY, et al. Gamma irradiation-inactivated respiratory syncytial virus vaccine provides protection but exacerbates pulmonary inflammation by switching from prefusion to postfusion F protein. Microbiol Spectr. (2023) 11:e0135823. doi: 10.1128/spectrum.01358-23
40. Mills KHG. IL-17 and IL-17-producing cells in protection versus pathology. Nat Rev Immunol. (2023) 23:38–54. doi: 10.1038/s41577-022-00746-9
41. Fouka E, Linden A, and Bossios A. The role of T-helper and T regulatory cells in driving neutrophilic and eosinophilic inflammation in bronchiectasis. Front Immunol. (2025) 16:1598257. doi: 10.3389/fimmu.2025.1598257
42. Zheng HT, Zhao QY, Ding Y, Ma SX, Chen WX, Qiu JL, et al. Investigation of the relationships among respiratory syncytial virus infection, T cell immune response and intestinal flora. Eur Rev Med Pharmacol Sci. (2023) 27:2671–8. doi: 10.26355/eurrev_202303_31804
43. Crawford MP, Borcherding N, and Karandikar NJ. IL-17 cytokines preferentially act on naive CD4+ T cells with the IL-17AF heterodimer inducing the greatest functional changes. PloS One. (2023) 18:e0285166. doi: 10.1371/journal.pone.0285166
44. De C, Pickles RJ, Yao W, Liao B, Boone A, Choi M, et al. Human T cells efficiently control RSV infection. JCI Insight. (2023) 8. doi: 10.1172/jci.insight.168110
45. Wang H, Li Y, Li H, Yan X, Jiang Z, Feng L, et al. T cell related osteoimmunology in fracture healing: Potential targets for augmenting bone regeneration. J Orthop Translat. (2025) 51:82–93. doi: 10.1016/j.jot.2024.12.004
46. Rixon JA, Fong KD, Morris C, Nguyen AT, Depew CE, and McSorley SJ. Elimination of Chlamydia muridarum from the female reproductive tract is IL-12p40 dependent, but independent of Th1 and Th2 cells. PloS Pathog. (2024) 20:e1011914. doi: 10.1371/journal.ppat.1011914
47. Malloy AMW, Lu Z, Kehl M, Pena DaMata J, Lau-Kilby AW, and Turfkruyer M. Increased innate immune activation induces protective RSV-specific lung-resident memory T cells in neonatal mice. Mucosal Immunol. (2023) 16:593–605. doi: 10.1016/j.mucimm.2023.05.012
48. Kawasaki T, Ikegawa M, and Kawai T. Antigen-presenting cells and lung CD8(+) resident memory T cells coordinate local immune protection and shape responses to respiratory virus infection. Int Immunol. (2025). doi: 10.1093/intimm/dxaf033
49. Alippe Y, Wang L, Coskun R, Muraro SP, Zhao FR, Elam-Noll M, et al. Fetal MAVS and type I IFN signaling pathways control ZIKV infection in the placenta and maternal decidua. J Exp Med. (2024) 221. doi: 10.1084/jem.20240694
50. Yang J, Li W, Zhang Z, Gong X, Chen Y, Peng X, et al. Targeting PRMT7-mediated monomethylation of MAVS enhances antiviral innate immune responses and inhibits RNA virus replication. Proc Natl Acad Sci U.S.A. (2024) 121:e2408117121. doi: 10.1073/pnas.2408117121
51. Zerillo L, Zotti T, Tutela A, Madera JR, Grasso G, Vito P, et al. The CARD14sh-BCL10-MALT1 complex regulates MAVS-mediated antiviral response in keratinocytes. Proc Natl Acad Sci U.S.A. (2025) 122:e2500711122. doi: 10.1073/pnas.2500711122
52. Varese A, Nakawesi J, Farias A, Kirsebom FCM, Paulsen M, Nuriev R, et al. Type I interferons and MAVS signaling are necessary for tissue resident memory CD8+ T cell responses to RSV infection. PloS Pathog. (2022) 18:e1010272. doi: 10.1371/journal.ppat.1010272
53. Xu H, Yue M, Zhou R, Wang P, Wong MY, Wang J, et al. A prime-boost vaccination approach induces lung resident memory CD8+ T cells derived from central memory T cells that prevent tumor lung metastasis. Cancer Res. (2024) 84:3173–88. doi: 10.1158/0008-5472.CAN-23-3257
54. Blunck BN, Angelo LS, Henke D, Avadhanula V, Cusick M, Ferlic-Stark L, et al. Adult memory T cell responses to the respiratory syncytial virus fusion protein during a single RSV season (2018-2019). Front Immunol. (2022) 13:823652. doi: 10.3389/fimmu.2022.823652
55. Langedijk AC and Bont LJ. Respiratory syncytial virus infection and novel interventions. Nat Rev Microbiol. (2023) 21:734–49. doi: 10.1038/s41579-023-00919-w
56. Dar HA, Almajhdi FN, Aziz S, and Waheed Y. Immunoinformatics-aided analysis of RSV fusion and attachment glycoproteins to design a potent multi-epitope vaccine. Vaccines (Basel). (2022) 10. doi: 10.3390/vaccines10091381
57. Shao S, Zhang XF, Hou JW, Yang SS, Han ZB, Wu HL, et al. Design of hepadnavirus core protein-based chimeric virus-like particles carrying epitopes from respiratory syncytial virus. NPJ Vaccines. (2024) 9:62. doi: 10.1038/s41541-024-00855-7
58. Bakkers MJG, Cox F, Koornneef A, Yu X, van Overveld D, Le L, et al. A foldon-free prefusion F trimer vaccine for respiratory syncytial virus to reduce off-target immune responses. Nat Microbiol. (2024) 9:3254–67. doi: 10.1038/s41564-024-01860-1
59. van den Dijssel J, Duurland MC, Konijn VA, Kummer LY, Hagen RR, Kuijper LH, et al. mRNA-1273 vaccinated inflammatory bowel disease patients receiving TNF inhibitors develop broad and robust SARS-CoV-2-specific CD8(+) T cell responses. J Autoimmun. (2024) 144:103175. doi: 10.1016/j.jaut.2024.103175
60. Bonam SR, Hazell NC, Mathew MJ, Liang Y, Zhang X, Wei Z, et al. Innate and Adaptive Immune Parameters following mRNA Vaccination in Mice. Vaccines (Basel). (2024) 12. doi: 10.3390/vaccines12050543
61. Parveen A and Elkordy AA. Brief Insights into mRNA Vaccines: Their Successful Production and Nanoformulation for Effective Response against COVID-19 and Their Potential Success for Influenza A and B. Pathogens. (2024) 13. doi: 10.3390/pathogens13060500
62. Kirtane AR and Traverso G. Improving the efficacy of cancer mRNA vaccines. Cancer J. (2025) 31. doi: 10.1097/PPO.0000000000000764
63. Ling J, Chen H, Huang M, Wang J, and Du X. An mRNA vaccine encoding proteasome-targeted antigen enhances CD8(+) T cell immunity. J Control Release. (2025) 381:113578. doi: 10.1016/j.jconrel.2025.02.074
64. Mani R, Balu KE, and Suzuki Y. Deficiencies of inducible costimulator (ICOS) during chronic infection with toxoplasma gondii upregulate the CD28-dependent cytotoxicity of CD8(+) T cells and their effector function against tissue cysts of the parasite. Cells. (2024) 13. doi: 10.3390/cells13231998
65. Kingstad-Bakke B, Cleven T, Bussan H, Yount BL Jr., Uraki R, Iwatsuki-Horimoto K, et al. Airway surveillance and lung viral control by memory T cells induced by COVID-19 mRNA vaccine. JCI Insight. (2023) 8. doi: 10.1172/jci.insight.172510
66. Abualrous ET, Stolzenberg S, Sticht J, Wieczorek M, Roske Y, Günther M, et al. MHC-II dynamics are maintained in HLA-DR allotypes to ensure catalyzed peptide exchange. Nat Chem Biol. (2023) 19:1196–204. doi: 10.1038/s41589-023-01316-3
67. Aunins EA, Phan AT, Alameh MG, Dwivedi G, Cruz-Morales E, Christian DA, et al. An Il12 mRNA-LNP adjuvant enhances mRNA vaccine-induced CD8 T cell responses. Sci Immunol. (2025) 10:eads1328. doi: 10.1126/sciimmunol.ads1328
68. Gao Y, Cai C, Wullimann D, Niessl J, Rivera-Ballesteros O, Chen P, et al. Immunodeficiency syndromes differentially impact the functional profile of SARS-CoV-2-specific T cells elicited by mRNA vaccination. Immunity. (2022) 55:1732–1746.e1735. doi: 10.1016/j.immuni.2022.07.005
69. Nogimori T, Suzuki K, Masuta Y, Washizaki A, Yagoto M, Ikeda M, et al. Functional changes in cytotoxic CD8+ T-cell cross-reactivity against the SARS-CoV-2 Omicron variant after mRNA vaccination. Front Immunol. (2022) 13:1081047. doi: 10.3389/fimmu.2022.1081047
70. Vályi-Nagy I, Matula Z, Gönczi M, Tasnády S, Bekő G, Réti M, et al. Comparison of antibody and T cell responses elicited by BBIBP-CorV (Sinopharm) and BNT162b2 (Pfizer-BioNTech) vaccines against SARS-CoV-2 in healthy adult humans. Geroscience. (2021) 43:2321–31. doi: 10.1007/s11357-021-00471-6
71. Qin S, Tang X, Chen Y, Chen K, Fan N, Xiao W, et al. mRNA-based therapeutics: powerful and versatile tools to combat diseases. Signal Transduct Target Ther. (2022) 7:166. doi: 10.1038/s41392-022-01007-w
72. Crofts KF, Page CL, Swedik SM, Holbrook BC, Meyers AK, Zhu X, et al. An analysis of linker-dependent effects on the APC activation and in vivo immunogenicity of an R848-conjugated influenza vaccine. Vaccines (Basel). (2023) 11. doi: 10.3390/vaccines11071261
73. Yin J, Zhao Y, Huang F, Yang Y, Huang Y, Zhuang Z, et al. Immune response and homeostasis mechanism following administration of BBIBP-CorV SARS-CoV-2 inactivated vaccine. Innovation (Camb). (2023) 4:100359. doi: 10.1016/j.xinn.2022.100359
74. Yi X, Wang Y, Li Q, Li X, Zhang P, Fu X, et al. Pre-existing immunity to SARS-CoV-2 associates with strong T cell responses induced by inactivated COVID-19 vaccines. J Med Virol. (2023) 95:e28642. doi: 10.1002/jmv.28642
75. Liang Z, Zhang H, Xu L, Li N, Yin Z, Sun Y, et al. Role of senescent CD4(+) T cells in breakthrough infection of the new variant strain of SARS-CoV-2 in elderly patients. J Transl Med. (2025) 23:737. doi: 10.1186/s12967-025-06756-0
76. Ling Y, Zhong J, and Luo J. Safety and effectiveness of SARS-CoV-2 vaccines: A systematic review and meta-analysis. J Med Virol. (2021) 93:6486–95. doi: 10.1002/jmv.v93.12
77. McCann N, O'Connor D, Lambe T, and Pollard AJ. Viral vector vaccines. Curr Opin Immunol. (2022) 77:102210. doi: 10.1016/j.coi.2022.102210
78. Swain SL. CD4 memory has a hierarchical structure created by requirements for infection-derived signals at an effector checkpoint. Front Immunol. (2023) 14:1306433. doi: 10.3389/fimmu.2023.1306433
79. Lee W and Suresh M. Vaccine adjuvants to engage the cross-presentation pathway. Front Immunol. (2022) 13:940047. doi: 10.3389/fimmu.2022.940047
80. Sritipsukho P, Khawcharoenporn T, Siribumrungwong B, Damronglerd P, Suwantarat N, Satdhabudha A, et al. Real-life effectiveness of COVID-19 vaccine during the Omicron variant-dominant pandemic: how many booster doses do we need? Emerg Microbes Infect. (2023) 12:2174779. doi: 10.1080/22221751.2023.2174779
81. Hu L, Sun J, Wang Y, Tan D, Cao Z, Gao L, et al. A review of inactivated COVID-19 vaccine development in China: focusing on safety and efficacy in special populations. Vaccines (Basel). (2023) 11. doi: 10.3390/vaccines11061045
82. Scarsella L, Ehrke-Schulz E, Paulussen M, Thal SC, Ehrhardt A, and Aydin M. Advances of recombinant adenoviral vectors in preclinical and clinical applications. Viruses. (2024) 16. doi: 10.3390/v16030377
83. Blanchette P and Teodoro JG. A renaissance for oncolytic adenoviruses? Viruses. (2023) 15. doi: 10.3390/v15020358
84. Rzymski P. Guillain-Barre syndrome and COVID-19 vaccines: focus on adenoviral vectors. Front Immunol. (2023) 14:1183258. doi: 10.3389/fimmu.2023.1183258
85. Mahncke C, Schmiedeke F, Simm S, Kaderali L, Broker BM, Seifert U, et al. DiscovEpi: automated whole proteome MHC-I-epitope prediction and visualization. BMC Bioinf. (2024) 25:310. doi: 10.1186/s12859-024-05931-2
86. Liu PJ, Yang TT, Fan ZX, Yuan GB, Ma L, Wang ZY, et al. Characterization of antigen-specific CD8+ memory T cell subsets in peripheral blood of patients with multiple sclerosis. Front Immunol. (2023) 14:1110672. doi: 10.3389/fimmu.2023.1110672
87. Lin G, Yan H, Sun J, Zhao J, and Zhang Y. Self-replicating RNA nanoparticle vaccine elicits protective immune responses against SARS-CoV-2. Mol Ther Nucleic Acids. (2023) 32:650–66. doi: 10.1016/j.omtn.2023.04.021
88. Calzada-Fraile D, Iborra S, Ramirez-Huesca M, Jorge I, Dotta E, Hernandez-Garcia E, et al. Immune synapse formation promotes lipid peroxidation and MHC-I upregulation in licensed dendritic cells for efficient priming of CD8(+) T cells. Nat Commun. (2023) 14:6772. doi: 10.1038/s41467-023-42480-3
89. Chatterjee F and Spranger S. MHC-dressing on dendritic cells: Boosting anti-tumor immunity via unconventional tumor antigen presentation. Semin Immunol. (2023) 66:101710. doi: 10.1016/j.smim.2023.101710
90. Macy AM, Herrmann LM, Adams AC, and Hastings KT. Major histocompatibility complex class II in the tumor microenvironment: functions of nonprofessional antigen-presenting cells. Curr Opin Immunol. (2023) 83:102330. doi: 10.1016/j.coi.2023.102330
91. D'Alise AM, Nocchi L, Garzia I, Secli L, Infante L, Troise F, et al. Adenovirus Encoded Adjuvant (AdEnA) anti-CTLA-4, a novel strategy to improve Adenovirus based vaccines against infectious diseases and cancer. Front Immunol. (2023) 14:1156714. doi: 10.3389/fimmu.2023.1156714
92. Marquez-Martinez S, Salisch N, Serroyen J, Zahn R, and Khan S. Peak transgene expression after intramuscular immunization of mice with adenovirus 26-based vector vaccines correlates with transgene-specific adaptive immune responses. PloS One. (2024) 19:e0299215. doi: 10.1371/journal.pone.0299215
93. Priyanka, Abusalah MAH, Chopra H, Sharma A, Mustafa SA, Choudhary OP, et al. Nanovaccines: A game changing approach in the fight against infectious diseases. BioMed Pharmacother. (2023) 167:115597. doi: 10.1016/j.biopha.2023.115597
94. Liao Z, Huang J, Lo PC, Lovell JF, Jin H, and Yang K. Self-adjuvanting cancer nanovaccines. J Nanobiotechnology. (2022) 20:345. doi: 10.1186/s12951-022-01545-z
95. Gurunathan S, Thangaraj P, Wang L, Cao Q, and Kim JH. Nanovaccines: An effective therapeutic approach for cancer therapy. BioMed Pharmacother. (2024) 170:115992. doi: 10.1016/j.biopha.2023.115992
96. Zaccariotto GC, Bistaffa MJ, Zapata AMM, Rodero C, Coelho F, Quitiba JVB, et al. Cancer nanovaccines: mechanisms, design principles, and clinical translation. ACS Nano. (2025). doi: 10.1021/acsnano.4c15765
97. Sowndharya CK, Mehnath S, Ponbharathi A, and Jeyaraj M. Self-adjuvanting adenoviral nanovaccine for effective T-cell-mediated immunity and long-lasting memory cell activation against tuberculosis. ACS Infect Dis. (2024) 10:3939–50. doi: 10.1021/acsinfecdis.4c00619
98. Kim CG, Lee JC, Ju DB, Kim SK, Yun CH, and Cho CS. Enhancement of immune responses elicited by nanovaccines through a cross-presentation pathway. Tissue Eng Regener Med. (2023) 20:355–70. doi: 10.1007/s13770-023-00527-y
99. Rojekar S, Gholap AD, Togre N, Bhoj P, Haeck C, Hatvate N, et al. Current status of mannose receptor-targeted drug delivery for improved anti-HIV therapy. J Control Release. (2024) 372:494–521. doi: 10.1016/j.jconrel.2024.06.002
100. Faria P, Pacheco C, Moura RP, Sarmento B, and Martins C. Multifunctional nanomedicine strategies to manage brain diseases. Drug Delivery Transl Res. (2023) 13:1322–42. doi: 10.1007/s13346-022-01256-w
101. Hao JN, Ge K, Chen G, Dai B, and Li Y. Strategies to engineer various nanocarrier-based hybrid catalysts for enhanced chemodynamic cancer therapy. Chem Soc Rev. (2023) 52:7707–36. doi: 10.1039/D3CS00356F
102. Haghnavaz N, Rezaee MA, Pordel S, Shobeiri SS, Dashti MR, Ansari B, et al. Mannose targeting of poly(lactic-co-glycolic acid): a promising approach for improving sublingual allergen-specific immunotherapy. Immunopharmacol Immunotoxicol. (2024) 46:815–28. doi: 10.1080/08923973.2024.2410291
103. Chen K, Yang Y, Wu Y, Cao W, Zhao Y, Wang S, et al. PLGA nanoparticles encapsulating TSHR-A and rapamycin enhance the induction of dendritic cell-specific immune tolerance in mice with Graves' disease. BioMed Mater. (2025) 20. doi: 10.1088/1748-605X/adbaa3
104. Wu W, Liu R, Guo J, Hu Z, An C, Zhang Y, et al. Modulation of immunosuppressive effect of rapamycin via microfluidic encapsulation within PEG-PLGA nanoparticles. J Biomater Appl. (2024) 38:821–33. doi: 10.1177/08853282231223808
105. Li JD and Yin J. Interleukin-10-alveolar macrophage cell membrane-coated nanoparticles alleviate airway inflammation and regulate Th17/regulatory T cell balance in a mouse model. Front Immunol. (2023) 14:1186393. doi: 10.3389/fimmu.2023.1186393
106. Xu L and Shi M. Lipocalin 2 (LCN2) knockdown regulates treg/th17 balance to improve asthma in mice. J Asthma Allergy. (2023) 16:1323–32. doi: 10.2147/JAA.S418596
107. Zajc CU, Sylvander E, Lehner M, and Traxlmayr MW. Small molecule-regulated switches to provide functional control of CAR T cells within the patient. Expert Opin Biol Ther. (2024) 24:425–32. doi: 10.1080/14712598.2024.2371034
108. de Araujo-Souza PS, Hanschke SCH, Nardy A, Secca C, Oliveira-Vieira B, Silva KL, et al. Differential interferon-gamma production by naive and memory-like CD8 T cells. J Leukoc Biol. (2020) 108:1329–37. doi: 10.1002/JLB.2AB0420-646R
109. Stifter K, Krieger J, Ruths L, Gout J, Mulaw M, Lechel A, et al. IFN-gamma treatment protocol for MHC-I(lo)/PD-L1(+) pancreatic tumor cells selectively restores their TAP-mediated presentation competence and CD8 T-cell priming potential. J Immunother Cancer. (2020) 8. doi: 10.1136/jitc-2020-000692
110. Han S, Liu ZQ, Chung DC, Paul MS, Garcia-Batres CR, Sayad A, et al. Overproduction of IFNgamma by cbl-b-deficient CD8+ T cells provides resistance against regulatory T cells and induces potent antitumor immunity. Cancer Immunol Res. (2022) 10:437–52. doi: 10.1158/2326-6066.CIR-20-0973
111. Schuller SS, Barman S, Mendez-Giraldez R, Soni D, Daley J, Baden LR, et al. Immune profiling of age and adjuvant-specific activation of human blood mononuclear cells. vitro Commun Biol. (2024) 7:709. doi: 10.1038/s42003-024-06390-4
112. Gnazzo V, Saleh H, Castro IA, Boon ACM, Pinto AK, Brien JD, et al. DDO-adjuvanted influenza A virus nucleoprotein mRNA vaccine induces robust humoral and cellular type 1 immune responses and protects mice from challenge. mBio. (2025) 16:e0358924. doi: 10.1128/mbio.03589-24
113. Ramirez SI, Lopez PG, Faraji F, Parikh UM, Heaps A, Ritz J, et al. Early antiviral CD4+ and CD8+ T cells are associated with upper airway clearance of SARS-CoV-2. JCI Insight. (2024) 9. doi: 10.1172/jci.insight.186078
114. Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol. (2022) 23:186–93. doi: 10.1038/s41590-021-01122-w
115. Lim JME, Hang SK, Hariharaputran S, Chia A, Tan N, Lee ES, et al. A comparative characterization of SARS-CoV-2-specific T cells induced by mRNA or inactive virus COVID-19 vaccines. Cell Rep Med. (2022) 3:100793. doi: 10.1016/j.xcrm.2022.100793
116. Huang R, Ying L, Wang J, Xia J, Zhang Y, Mao H, et al. Non-spike and spike-specific memory T cell responses after the third dose of inactivated COVID-19 vaccine. Front Immunol. (2023) 14:1139620. doi: 10.3389/fimmu.2023.1139620
117. Van Hoecke L, Roose K, Ballegeer M, Zhong Z, Sanders NN, De Koker S, et al. The opposing effect of type I IFN on the T cell response by non-modified mRNA-lipoplex vaccines is determined by the route of administration. Mol Ther Nucleic Acids. (2020) 22:373–81. doi: 10.1016/j.omtn.2020.09.004
118. Yang K, Bai B, Lei J, Yu X, Qi S, Wang Y, et al. Biodegradable lipid-modified poly(Guanidine thioctic acid)s: A fortifier of lipid nanoparticles to promote the efficacy and safety of mRNA cancer vaccines. J Am Chem Soc. (2024) 146:11679–93. doi: 10.1021/jacs.3c14010
119. Cupovic J, Ring SS, Onder L, Colston JM, Lutge M, Cheng HW, et al. Adenovirus vector vaccination reprograms pulmonary fibroblastic niches to support protective inflating memory CD8(+) T cells. Nat Immunol. (2021) 22:1042–51. doi: 10.1038/s41590-021-00969-3
120. Welten SPM, Yermanos A, Baumann NS, Wagen F, Oetiker N, Sandu I, et al. Tcf1(+) cells are required to maintain the inflationary T cell pool upon MCMV infection. Nat Commun. (2020) 11:2295. doi: 10.1038/s41467-020-16219-3
121. van der Gracht ET, Schoonderwoerd MJ, van Duikeren S, Yilmaz AN, Behr FM, Colston JM, et al. Adenoviral vaccines promote protective tissue-resident memory T cell populations against cancer. J Immunother Cancer. (2020) 8. doi: 10.1136/jitc-2020-001133
122. Mendez-Lagares G, Chin N, Chang WLW, Lee J, Rosas-Umbert M, Kieu HT, et al. Cytomegalovirus mediates expansion of IL-15-responsive innate-memory cells with SIV killing function. J Clin Invest. (2021) 131. doi: 10.1172/JCI148542
123. Chiale C, Marchese AM, Furuya Y, and Robek MD. Virus-based vaccine vectors with distinct replication mechanisms differentially infect and activate dendritic cells. NPJ Vaccines. (2021) 6:138. doi: 10.1038/s41541-021-00400-w
124. Xu L, Ye L, and Huang Q. Tissue-resident memory CD8+ T cells: differentiation, phenotypic heterogeneity, biological function, disease, and therapy. MedComm (2020). (2025) 6:e70132. doi: 10.1002/mco2.70132
125. Jung S, Jung JH, Noh JY, Kim WJ, Yoon SY, Jung J, et al. The generation of stem cell-like memory cells early after BNT162b2 vaccination is associated with durability of memory CD8(+) T cell responses. Cell Rep. (2022) 40:111138. doi: 10.1016/j.celrep.2022.111138
126. Heidarian M, Griffith TS, and Badovinac VP. Sepsis-induced changes in differentiation, maintenance, and function of memory CD8 T cell subsets. Front Immunol. (2023) 14:1130009. doi: 10.3389/fimmu.2023.1130009
127. Wong YC, Liu W, Yim LY, Li X, Wang H, Yue M, et al. Sustained viremia suppression by SHIVSF162P3CN-recalled effector-memory CD8+ T cells after PD1-based vaccination. PloS Pathog. (2021) 17:e1009647. doi: 10.1371/journal.ppat.1009647
128. Holtappels R, Freitag K, Renzaho A, Becker S, Lemmermann NAW, and Reddehase MJ. Revisiting CD8 T-cell 'Memory inflation': new insights with implications for cytomegaloviruses as vaccine vectors. Vaccines (Basel). (2020) 8. doi: 10.3390/vaccines8030402
129. Yoon KW, Chu KB, Eom GD, Mao J, Kim MJ, Lee H, et al. Protective humoral immune response induced by recombinant virus-like particle vaccine expressing leishmania donovani surface antigen. ACS Infect Dis. (2023) 9:2583–92. doi: 10.1021/acsinfecdis.3c00411
130. Beavis AC, Xiao P, Gingerich MC, Briggs K, Li G, Howerth EW, et al. A parainfluenza virus 5 (PIV5)-vectored intranasal SARS-CoV-2 vaccine (CVXGA1) elicits protective and long-lasting immunity in nonhuman primates. J Virol. (2025) 99:e0199024. doi: 10.1128/jvi.01990-24
131. Doyle JD, Barbeau DJ, Cartwright HN, and McElroy AK. Immune correlates of protection following Rift Valley fever virus vaccination. NPJ Vaccines. (2022) 7:129. doi: 10.1038/s41541-022-00551-4
132. Miyakawa K, Kato H, Ohtake N, Jeremiah SS, and Ryo A. Enhancement of humoral and cellular immunity against severe acute respiratory syndrome coronavirus 2 by a third dose of BNT162b2 vaccine in Japanese healthcare workers. J Infect Dis. (2023) 227:221–5. doi: 10.1093/infdis/jiac344
133. Yu J, Thomas PV, McMahan K, Jacob-Dolan C, Liu J, He X, et al. Protection against SARS-CoV-2 Omicron BA.1 variant challenge in macaques by prime-boost vaccination with Ad26.COV2.S and SpFN. Sci Adv. (2022) 8:eade4433. doi: 10.1126/sciadv.ade4433
134. Lobby JL, Danzy S, Holmes KE, Lowen AC, and Kohlmeier JE. Both humoral and cellular immunity limit the ability of live attenuated influenza vaccines to promote T cell responses. J Immunol. (2024) 212:107–16. doi: 10.4049/jimmunol.2300343
135. Uddback I, Michalets SE, Saha A, Mattingly C, Kost KN, Williams ME, et al. Prevention of respiratory virus transmission by resident memory CD8(+) T cells. Nature. (2024) 626:392–400. doi: 10.1038/s41586-023-06937-1
136. Xiong F, Zhang C, Shang B, Zheng M, Wang Q, Ding Y, et al. An mRNA-based broad-spectrum vaccine candidate confers cross-protection against heterosubtypic influenza A viruses. Emerg Microbes Infect. (2023) 12:2256422. doi: 10.1080/22221751.2023.2256422
137. Zhang L, Yang J, Deng M, Xu C, Lai C, Deng X, et al. Blood unconjugated bilirubin and tacrolimus are negative predictors of specific cellular immunity in kidney transplant recipients after SAR-CoV-2 inactivated vaccination. Sci Rep. (2023) 13:7263. doi: 10.1038/s41598-023-29669-8
138. Ma S, Zhu F, Wen H, Rao M, Zhang P, Peng W, et al. Development of a novel multi-epitope vaccine based on capsid and envelope protein against Chikungunya virus. J Biomol Struct Dyn. (2024) 42:7024–36. doi: 10.1080/07391102.2023.2240059
139. Kobashi Y, Kawamura T, Shimazu Y, Kaneko Y, Nishikawa Y, Sugiyama A, et al. Kinetics of humoral and cellular immune responses 5 months post-COVID-19 booster dose by immune response groups at the peak immunity phase: An observational historical cohort study using the Fukushima vaccination community survey. Vaccine X. (2024) 20:100553. doi: 10.1016/j.jvacx.2024.100553
140. Breznik JA, Rahim A, Bhakta H, Clare R, Zhang A, Ang J, et al. Early humoral and cellular responses after bivalent SARS-CoV-2 mRNA-1273.214 vaccination in long-term care and retirement home residents in Ontario, Canada: An observational cohort study. J Med Virol. (2023) 95:e29170. doi: 10.1002/jmv.29170
141. Mendoza-Ramirez NJ, Garcia-Cordero J, Shrivastava G, and Cedillo-Barron L. The key to increase immunogenicity of next-generation COVID-19 vaccines lies in the inclusion of the SARS-coV-2 nucleocapsid protein. J Immunol Res. (2024) 2024:9313267. doi: 10.1155/2024/9313267
142. Afkhami S, Kang A, Jeyanathan V, Xing Z, and Jeyanathan M. Adenoviral-vectored next-generation respiratory mucosal vaccines against COVID-19. Curr Opin Virol. (2023) 61:101334. doi: 10.1016/j.coviro.2023.101334
143. Kawakita T, Sekiya T, Kameda Y, Nomura N, Ohno M, Handabile C, et al. ARNAX is an ideal adjuvant for COVID-19 vaccines to enhance antigen-specific CD4(+) and CD8(+) T-cell responses and neutralizing antibody induction. J Virol. (2025) 99:e0229024. doi: 10.1128/jvi.02290-24
144. Zhou M, Xiao H, Yang X, Cheng T, Yuan L, and Xia N. Novel vaccine strategies to induce respiratory mucosal immunity: advances and implications. MedComm (2020). (2025) 6:e70056. doi: 10.1002/mco2.70056
145. McMahan K, Wegmann F, Aid M, Sciacca M, Liu J, Hachmann NP, et al. Mucosal boosting enhances vaccine protection against SARS-CoV-2 in macaques. Nature. (2024) 626:385–91. doi: 10.1038/s41586-023-06951-3
146. Aligolighasemabadi F, Bakinowska E, Kielbowski K, Sadeghdoust M, Coombs KM, Mehrbod P, et al. Autophagy and respiratory viruses: mechanisms, viral exploitation, and therapeutic insights. Cells. (2025) 14. doi: 10.3390/cells14060418
147. Malik BT, Byrne KT, Vella JL, Zhang P, Shabaneh TB, Steinberg SM, et al. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci Immunol. (2017) 2. doi: 10.1126/sciimmunol.aam6346
148. Kok L, Masopust D, and Schumacher TN. The precursors of CD8(+) tissue resident memory T cells: from lymphoid organs to infected tissues. Nat Rev Immunol. (2022) 22:283–93. doi: 10.1038/s41577-021-00590-3
149. Afkhami S, D'Agostino MR, Zhang A, Stacey HD, Marzok A, Kang A, et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell. (2022) 185:896–915.e819. doi: 10.1016/j.cell.2022.02.005
150. Kazer SW, Match CM, Langan EM, Messou MA, LaSalle TJ, O'Leary E, et al. Primary nasal viral infection rewires the tissue-scale memory response. bioRxiv. (2024). doi: 10.1101/2023.05.11.539887
151. Hassan AO, Kafai NM, Dmitriev IP, Fox JM, Smith BK, Harvey IB, et al. A single-dose intranasal chAd vaccine protects upper and lower respiratory tracts against SARS-coV-2. Cell. (2020) 183:169–184.e113. doi: 10.1016/j.cell.2020.08.026
152. Di Q, Zhao X, Tang H, Li X, Xiao Y, Wu H, et al. USP22 suppresses the NLRP3 inflammasome by degrading NLRP3 via ATG5-dependent autophagy. Autophagy. (2023) 19:873–85. doi: 10.1080/15548627.2022.2107314
153. Lodaya RN, Kanitkar AP, Ashraf A, Bamba D, Amiji MM, and O'Hagan DT. A self-emulsified adjuvant system containing the immune potentiator alpha tocopherol induces higher neutralizing antibody responses than a squalene-only emulsion when evaluated with a recombinant cytomegalovirus (CMV) pentamer antigen in mice. Pharmaceutics. (2023) 15. doi: 10.3390/pharmaceutics15010238
154. Nigar S and Shimosato T. Cooperation of oligodeoxynucleotides and synthetic molecules as enhanced immune modulators. Front Nutr. (2019) 6:140. doi: 10.3389/fnut.2019.00140
155. Bauer DL, Bachnak L, Limbert VM, Horowitz RM, Baudier RL, D'Souza SJ, et al. The adjuvant combination of dmLT and monophosphoryl lipid A activates the canonical, nonpyroptotic NLRP3 inflammasome in dendritic cells and significantly interacts to expand antigen-specific CD4 T cells. J Immunol. (2023) 210:1519–30. doi: 10.4049/jimmunol.2200221
156. Jang HJ and Song KD. Expression patterns of innate immunity-related genes in response to polyinosinic:polycytidylic acid (poly[I:C]) stimulation in DF-1 chicken fibroblast cells. J Anim Sci Technol. (2020) 62:385–95. doi: 10.5187/jast.2020.62.3.385
157. Rimaniol AC, Gras G, Verdier F, Capel F, Grigoriev VB, Porcheray F, et al. Aluminum hydroxide adjuvant induces macrophage differentiation towards a specialized antigen-presenting cell type. Vaccine. (2004) 22:3127–35. doi: 10.1016/j.vaccine.2004.01.061
158. Gherardi RK and Authier FJ. Aluminum inclusion macrophagic myofasciitis: a recently identified condition. Immunol Allergy Clin North Am. (2003) 23:699–712. doi: 10.1016/S0889-8561(03)00095-X
159. Cardone M, Dzutsev AK, Li H, Riteau N, Gerosa F, Shenderov K, et al. Interleukin-1 and interferon-γ orchestrate β-glucan-activated human dendritic cell programming via IκB-ζ modulation. PloS One. (2014) 9:e114516. doi: 10.1371/journal.pone.0114516
160. Fung HY, Espinal AM, Teryek M, Lemenze AD, and Bergsbaken T. STAT4 increases the phenotypic and functional heterogeneity of intestinal tissue-resident memory T cells. Mucosal Immunol. (2023) 16:250–63. doi: 10.1016/j.mucimm.2023.03.002
161. Crowl JT, Heeg M, Ferry A, Milner JJ, Omilusik KD, Toma C, et al. Tissue-resident memory CD8(+) T cells possess unique transcriptional, epigenetic and functional adaptations to different tissue environments. Nat Immunol. (2022) 23:1121–31. doi: 10.1038/s41590-022-01229-8
162. Jarjour NN, Dalzell TS, Maurice NJ, Wanhainen KM, Peng C, O'Flanagan SD, et al. Collaboration between interleukin-7 and -15 enables adaptation of tissue-resident and circulating memory CD8(+) T cells to cytokine deficiency. Immunity. (2025) 58:616–631.e615. doi: 10.1016/j.immuni.2025.02.009
163. Depew CE, Rixon JA, and McSorley SJ. Optimal generation of hepatic tissue-resident memory CD4 T cells requires IL-1 and IL-2. Proc Natl Acad Sci U.S.A. (2023) 120:e2214699120. doi: 10.1073/pnas.2214699120
164. Green WD, Gomez A, Plotkin AL, Pratt BM, Merritt EF, Mullins GN, et al. Enhancer-driven gene regulatory networks reveal transcription factors governing T cell adaptation and differentiation in the tumor microenvironment. Immunity. (2025) 58:1725–1741.e1729. doi: 10.1016/j.immuni.2025.04.030
165. Powell MD, Read KA, Sreekumar BK, Jones DM, and Oestreich KJ. IL-12 signaling drives the differentiation and function of a T(H)1-derived T(FH1)-like cell population. Sci Rep. (2019) 9:13991. doi: 10.1038/s41598-019-50614-1
166. Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, et al. T-box transcription factors combine with the cytokines TGF-β and IL-15 to control tissue-resident memory T cell fate. Immunity. (2015) 43:1101–11. doi: 10.1016/j.immuni.2015.11.008
167. Tan S and Tian P. Advances in the study of tissue-resident memory T cells in lung cancer. Zhongguo Fei Ai Za Zhi. (2022) 25:862–9. doi: 10.3779/j.issn.1009-3419.2022.102.49
168. Bedi R, Bayless NL, and Glanville J. Challenges and progress in designing broad-spectrum vaccines against rapidly mutating viruses. Annu Rev BioMed Data Sci. (2023) 6:419–41. doi: 10.1146/annurev-biodatasci-020722-041304
169. Gan M, Cao J, Zhang Y, Fu H, Lin X, Ouyang Q, et al. Landscape of T cell epitopes displays hot mutations of SARS-CoV-2 variant spikes evading cellular immunity. J Med Virol. (2024) 96:e29452. doi: 10.1002/jmv.29452
170. Tye EXC, Jinks E, Haigh TA, Kaul B, Patel P, Parry HM, et al. Mutations in SARS-CoV-2 spike protein impair epitope-specific CD4(+) T cell recognition. Nat Immunol. (2022) 23:1726–34. doi: 10.1038/s41590-022-01351-7
171. Nkosi T, Chasara C, Papadopoulos AO, Nguni TL, Karim F, Moosa MS, et al. Unsuppressed HIV infection impairs T cell responses to SARS-CoV-2 infection and abrogates T cell cross-recognition. Elife. (2022) 11. doi: 10.7554/eLife.78374.sa2
172. Keeton R, Tincho MB, Ngomti A, Baguma R, Benede N, Suzuki A, et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature. (2022) 603:488–92. doi: 10.1038/s41586-022-04460-3
173. Simon AK, Hollander GA, and McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. (2015) 282:20143085. doi: 10.1098/rspb.2014.3085
174. Santoro A, Bientinesi E, and Monti D. Immunosenescence and inflammaging in the aging process: age-related diseases or longevity? Ageing Res Rev. (2021) 71:101422. doi: 10.1016/j.arr.2021.101422
175. Han WG, van Twillert I, Poelen MC, Helm K, van de Kassteele J, Verheij TJ, et al. Loss of multi-epitope specificity in memory CD4(+) T cell responses to B. pertussis with age. PloS One. (2013) 8:e83583. doi: 10.1371/journal.pone.0083583
176. Wang Y, Li R, Tong R, Chen T, Sun M, Luo L, et al. Integrating single-cell RNA and T cell/B cell receptor sequencing with mass cytometry reveals dynamic trajectories of human peripheral immune cells from birth to old age. Nat Immunol. (2025) 26:308–22. doi: 10.1038/s41590-024-02059-6
177. Alpert A, Pickman Y, Leipold M, Rosenberg-Hasson Y, Ji X, Gaujoux R, et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat Med. (2019) 25:487–95. doi: 10.1038/s41591-019-0381-y
178. Wu JD, Li JX, Liu J, Wang HM, Zhou GH, Li J, et al. Safety, immunogenicity, and efficacy of the mRNA vaccine CS-2034 as a heterologous booster versus homologous booster with BBIBP-CorV in adults aged ≥18 years: a randomised, double-blind, phase 2b trial. Lancet Infect Dis. (2023) 23:1020–30. doi: 10.1016/S1473-3099(23)00199-8
179. Rizzi M, Tonello S, Brinno C, Zecca E, Matino E, Cittone M, et al. SARS-CoV-2 infection risk is higher in vaccinated patients with inflammatory autoimmune diseases or liver transplantation treated with mycophenolate due to an impaired antiviral immune response: results of the extended follow up of the RIVALSA prospective cohort. Front Immunol. (2023) 14:1185278. doi: 10.3389/fimmu.2023.1185278
180. Ko JH, Kim CM, Bang MS, Lee DY, Kim DY, Seo JW, et al. Risk factors for impaired cellular or humoral immunity after three doses of SARS-CoV-2 vaccine in healthy and immunocompromised individuals. Vaccines (Basel). (2024) 12. doi: 10.3390/vaccines12070752
181. Benotmane I, Gautier-Vargas G, Cognard N, Olagne J, Heibel F, Braun-Parvez L, et al. Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int. (2021) 99:1487–9. doi: 10.1016/j.kint.2021.03.014
182. Berbudi A, Rahmadika N, Tjahjadi AI, and Ruslami R. Type 2 diabetes and its impact on the immune system. Curr Diabetes Rev. (2020) 16:442–9. doi: 10.2174/1573399815666191024085838
183. Yang X, Zhang H, Bao W, Fu S, and Jin H. Immunogenicity Rates after SARS-CoV-2 Three-Dose Vaccination in Patients under Dialysis: A Systematic Review and Meta-Analysis. Vaccines (Basel). (2022) 10. doi: 10.3390/vaccines10122070
184. Wen W, Su W, Tang H, Le W, Zhang X, Zheng Y, et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. (2020) 6:31. doi: 10.1038/s41421-020-0168-9
185. Chattopadhyay P, Khare K, Kumar M, Mishra P, Anand A, Maurya R, et al. Single-cell multiomics revealed the dynamics of antigen presentation, immune response and T cell activation in the COVID-19 positive and recovered individuals. Front Immunol. (2022) 13:1034159. doi: 10.3389/fimmu.2022.1034159
186. Gunasena M, Alles M, Wijewantha Y, Mulhern W, Bowman E, Gabriel J, et al. Synergy between NK cells and monocytes in potentiating cardiovascular disease risk in severe COVID-19. Arterioscler Thromb Vasc Biol. (2024) 44:e243–61. doi: 10.1161/ATVBAHA.124.321085
187. May L, Chu CF, and Zielinski CE. Single-cell RNA sequencing reveals HIF1A as a severity-sensitive immunological scar in circulating monocytes of convalescent comorbidity-free COVID-19 patients. Cells. (2024) 13. doi: 10.3390/cells13040300
188. Garcia-Aguilera G, Castillo-Robleda A, Sanz A, and Ramirez M. Validation of a spectral flow cytometry single-tube panel for the clinical diagnosis and follow-up of children and adolescents with B-cell acute lymphoblastic leukemia. Cells. (2024) 13. doi: 10.3390/cells13221891
189. Robinson JP, Ostafe R, Iyengar SN, Rajwa B, and Fischer R. Flow cytometry: the next revolution. Cells. (2023) 12. doi: 10.3390/cells12141875
190. Guo T and Xie H. Establishment of a 21-color panel for the detection of immune cell subsets in human non-small cell lung cancer tumor tissues with flow cytometry. Zhongguo Fei Ai Za Zhi. (2024) 27:56–64. doi: 10.3779/j.issn.1009-3419.2024.102.02
191. Montin D, Santilli V, Beni A, Costagliola G, Martire B, Mastrototaro MF, et al. Towards personalized vaccines. Front Immunol. (2024) 15:1436108. doi: 10.3389/fimmu.2024.1436108
192. Purcell RA, Theisen RM, Arnold KB, Chung AW, and Selva KJ. Polyfunctional antibodies: a path towards precision vaccines for vulnerable populations. Front Immunol. (2023) 14:1183727. doi: 10.3389/fimmu.2023.1183727
193. Gomes SMR, Ribeiro-Alves M, Ribeiro RSA, Brito ACS, Lisboa VDC, de Azevedo SG, et al. Evaluation of humoral and cellular immune responses in healthcare workers with varying levels of SARS-CoV-2 exposure: effects of CoronaVac vaccination followed by heterologous booster. Front Immunol. (2025) 16:1576430. doi: 10.3389/fimmu.2025.1576430
194. Patil DR, Shete AM, Yadav PD, Sapkal GN, Deshpande GR, Kaushal H, et al. Host immune responses in aged rhesus macaques against BBV152, an inactivated SARS-CoV-2 vaccine, and cross-neutralization with beta and delta variants. Front Immunol. (2023) 14:1161571. doi: 10.3389/fimmu.2023.1161571
195. Yap RXL, Lai YW, Wei C, Ng JJW, Xu D, Feng S, et al. Impact of immunomodulatory therapy on COVID-19 vaccine response in patients with autoimmune inflammatory rheumatic diseases. Vaccines (Basel). (2024) 12. doi: 10.3390/vaccines12030274
196. Aparicio B, Reparaz D, Ruiz M, Llopiz D, Silva L, Vercher E, et al. Identification of HLA class I-restricted immunogenic neoantigens in triple negative breast cancer. Front Immunol. (2022) 13:985886. doi: 10.3389/fimmu.2022.985886
197. Zhang X, Xu Z, Dai X, Zhang X, and Wang X. Research progress of neoantigen-based dendritic cell vaccines in pancreatic cancer. Front Immunol. (2023) 14:1104860. doi: 10.3389/fimmu.2023.1104860
198. Fritsch EF and Ott PA. Personalized cancer vaccines directed against tumor mutations: building evidence from mice to humans. Cancer Res. (2024) 84:953–5. doi: 10.1158/0008-5472.CAN-24-0565
199. Huff AL, Longway G, Mitchell JT, Andaloori L, Davis-Marcisak E, Chen F, et al. CD4 T cell-activating neoantigens enhance personalized cancer vaccine efficacy. JCI Insight. (2023) 8. doi: 10.1172/jci.insight.174027
200. Drew L. Turning tumours against themselves. Nature. (2024) 629:S10–s12. doi: 10.1038/d41586-024-01429-2
201. Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Löwer M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. (2017) 547:222–6. doi: 10.1038/nature23003
202. Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature. (2017) 547:217–21. doi: 10.1038/nature22991
203. Liu X, Shaw RH, Stuart ASV, Greenland M, Aley PK, Andrews NJ, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. (2021) 398:856–69. doi: 10.1016/S0140-6736(21)01694-9
204. Qu L, Yi Z, Shen Y, Lin L, Chen F, Xu Y, et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell. (2022) 185:1728–1744.e1716. doi: 10.1016/j.cell.2022.03.044
205. Zhu Y, Tang R, Li X, Chen X, Wang X, Wang Y, et al. Vaccination with adenovirus type 5 vector-based COVID-19 vaccine as the primary series in adults: A randomized, double-blind, placebo-controlled phase 1/2 clinical trial. Vaccines (Basel). (2024) 12. doi: 10.3390/vaccines12030292
Keywords: upper respiratory tract infection, vaccine, humoral immunity, cellular immunity, vaccine optimization
Citation: Chen K, Hu J, Li J, Wu G, Tie X, Wu H, Li H, Li J and Zhang Y (2025) Harnessing cellular immunity for next-generation vaccines against respiratory viruses: mechanisms, platforms, and optimization strategies. Front. Immunol. 16:1618406. doi: 10.3389/fimmu.2025.1618406
Received: 12 May 2025; Accepted: 25 July 2025;
Published: 13 August 2025.
Edited by:
John Willaim Sleasman, Duke University, United StatesReviewed by:
Mariah Hassert, The University of Iowa, United StatesBali Zhao, Wuhan Ammunition Life-tech Company Ltd., China
Copyright © 2025 Chen, Hu, Li, Wu, Tie, Wu, Li, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianhua Li, amhsaUBjZGMuemouY24=; Yanjun Zhang, eWp6aGFuZ0BjZGMuemouY24=
†These authors have contributed equally to this work and share first authorship
 Keda Chen
Keda Chen Jutao Hu1†
Jutao Hu1† Jianhua Li
Jianhua Li Yanjun Zhang
Yanjun Zhang