- 1Department of Medicine, University of British Columbia, Vancouver, BC, Canada
- 2Department of Medicine, Western University, London, ON, Canada
- 3Department of Medicine, McMaster University, Hamilton, ON, Canada
- 4Department of Surgery, McGill University, Montreal, QC, Canada
- 5Department of Medicine, University of Toronto, Toronto, ON, Canada
- 6Department of Medicine, Dalhousie University, Halifax, NS, Canada
- 7Department of Medicine, University of Alberta, Edmonton, AB, Canada
- 8Medical Affairs, Merck Canada Inc, Kirkland, QC, Canada
Purpose: To document prophylactic practices, infection patterns, and disease burden to inform strategies for CMV management in high-risk kidney transplant recipients.
Methods: A retrospective cohort of 311 consecutive CMV D+/R- kidney recipients were enrolled from 7 Canadian programs over 4 years (2018-2021) to provide data on demographic, clinical, therapeutic and health resource use during the 1st year post-transplant.
Results: Themedian age was 58 (46, 67) years, 69% were male, and 53% were White. Diabetes was the principal cause of kidney failure (19%). 208 (69%) received a deceased donor graft; 76 (24%) had ATG induction, and 84% had maintenance therapy with tacrolimus and MMF/MPA ± prednisone. All received antiviral prophylaxis, 90% with valganciclovir, for a median of 180 days. 106 (34%) developed CMV viremia (median peak viral load 14,224 IU/ml) at a median of 218 days, of whom 46 (43%) had CMV disease and 15 (14%) had recurrent infection. Myelotoxicity occurred in 121 (39%) patients at a median of 88 days, lasting a median of 30 days. Opportunistic infections occurred in 119 patients (38%) at a median of 53 days. 141 patients (45%) were hospitalized, 50 (16%) more than once. 20 patients (6%) had biopsy-confirmed rejection, and 293 (94%) were alive with a functioning graft at 1 year.
Conclusion: Current prophylaxis strategies fail to prevent CMV infection in 34% of high-risk patients. Myelotoxicity, opportunistic infection, reduced immunosuppression, and hospitalization remain common and serious complications. More effective and less toxic personalized treatment strategies are required to minimize these risks and burdens.
Introduction
Cytomegalovirus (CMV) infection is a common complication of kidney transplantation which remains a major therapeutic challenge with both clinical and economic implications (1, 2). The risk of CMV infection is highest in immunologically naïve seronegative recipients (R-) of organs from a seropositive donor (D+), comprising approximately 10% to 20% of kidney transplants (3–5). Anti-viral prophylaxis has reduced and delayed the risk of CMV infection in these patients (5–9), which occurs in 10 - 50% (3, 10–12) of subjects throughout the first post-transplant year (1).
CMV infection may present with a broad range of disease expression from asymptomatic viremia to CMV syndrome or end-organ disease including esophagitis, enteritis, pneumonitis, encephalitis, pancreatitis, and other target organ damage (8). It may occur alone, or in a complex context of multiple disease events. For example, CMV is often diagnosed coincident with other bacterial, viral or fungal opportunistic infections in the post-transplant setting, complicating diagnosis, and management (13–15). The occurrence of CMV infection and graft rejection are also closely correlated, and models of combinatorial risk have been proposed (16). However, it remains unclear whether the immune modulating effect of the virus enhances graft rejection, or treatment of rejection increases the risk of viremia (8, 17).
Despite overall reduction in the incidence, severity, and consequences of CMV infection, the management of D+/R- patients remains challenging. CMV viremia and disease occur more commonly within this group and may result in serious complications, despite current prophylaxis strategies (1, 5, 18, 19). Prolonged prophylaxis can itself have important costs and toxicity resulting in serious clinical leukopenia (20) which may in turn lead to co-infection or secondary reduction in immune suppression resulting in breakthrough rejection.
Measurement of CMV viral load is considered the most relevant index to document viral replication, to diagnose infection, and to determine treatment effect. It has also been included as a surrogate marker in clinical trial settings (1, 7, 8, 21). Accurate monitoring of viral load and the use of anti-viral prophylaxis or pre-emptive therapy have mitigated the lethal consequences of CMV infection and diminished the early indirect consequences and costs of care (1, 8). Calculation of viral load kinetics has been proposed to guide the frequency of sample measurement and to facilitate the effective use of therapy (22, 23), but this has not yet been incorporated into clinical practice.
Despite the availability of US, European and international clinical guidelines, we lack current Canadian published data on the clinical treatment and burden of CMV infection as we optimize management strategies for preventing infection in this high-risk kidney transplant population (8, 24–26). The recommendations of the Canadian Society of Transplantation CMV Consensus Working Group were published almost two decades ago (27) and a more recent single-center Canadian pediatric study (28) does not provide evidence that is applicable to the broader landscape of transplantation. Consequently, viral testing, prophylaxis or pre-emptive therapies vary between transplant programs.
The goal of this study was to complement existing knowledge in both live donor (LD) and deceased donor (DD) D+/R- patients who were at highest risk for CMV infection and disease by providing a comprehensive understanding of current practice, to understand disease burden, provide precise and current data for economic modeling, and to highlight opportunities to improve therapy and healthcare resource utilization in this patient population. We report the dynamics and cumulative frequencies of the key outcomes of interest which will enable us to model the interplay of these events and identify groups with differential disease burden to guide therapy.
Methods
Objectives
The primary objectives of this study were to describe: (a) the incidence of CMV infections (including but not limited to: reactivations, number of recurrences as well as incidence of resistant or refractory infection or disease); (b) incidence of CMV antiviral-related myelotoxicities, including leukopenia and neutropenia, and their impact on patient’s treatment adjustments (decrease/discontinuation of immunosuppressants and CMV antiviral agents); and (c) the association of CMV management with health care resource utilization (HCRU), including the length of hospital stay (LOS) and frequency of hospital admissions.
Study design
This retrospective, consecutive subject, multicenter cohort study was designed to examine the clinical and economic burden and unmet prophylactic needs in CMV seronegative adult recipients (R-) 18 years or older of a kidney transplant from a CMV seropositive donor (D+). Recipients who participated in an interventional trial in the prior 90 days, had received another organ or stem-cell transplant, or were exposure to letermovir were excluded. The study was conducted in seven major transplant centers in Canada (Table 1) and included consecutive CMV seronegative recipients in each participating transplant program who received a kidney transplant from a CMV seropositive donor from January 1st, 2018 to December 31st, 2021, and for whom continuous follow-up data were available. A pragmatic sample of approximately 300 CMV D+/R- kidney transplant recipients was considered adequate to provide preliminary evidence of CMV treatment and burden.
The index date for each participant was the date of kidney transplant (Day 0) and data were recorded for the first year following transplantation. Primary data elements included, but were not limited to, patient demographics (age, age at time of first kidney transplant, race, proximity to transplant center); donor source (living/deceased), HLA matching; clinical therapies including induction regimen; CMV infection, syndrome, or disease; the incidence and management of CMV antiviral-related myelotoxicities; and healthcare resource use. Secondary data elements included time to CMV infection, viremia levels, viral load kinetics; event history (phlebitis, anemia, thrombocytopenia, pancytopenia, neutropenia, direct renal tubular toxicity, crystalline nephropathy, leukopenia, other); non-CMV opportunistic infections; graft outcomes; utilization patterns of CMV therapy; and incidence and management of other CMV antiviral-related toxicities.
CMV prophylaxis and treatment
The following characteristics were extracted from the patient charts to describe antiviral prophylaxis and treatment for CMV: whether anti-viral prophylaxis treatment was prescribed; the stage of use (primary [immediate post-transplant] or secondary [post-primary infection]); and the type of antiviral medication including the dose, unit; frequency and start and stop dates. The response of CMV infection to antiviral treatment was measured using the following data obtained from the patient chart: the date of the first episode of CMV infection; the duration of the episode (days); any change in treatment; (immunosuppression, antiviral prophylaxis, G-CSF, other); whether resistant or refractory CMV Infection or disease occurred (yes/no); and whether resistant infection or disease was confirmed (yes/no).
Myelotoxicity (determined as hematological values below the lower level of normal for each institution) and other adverse consequences of antiviral treatment were recorded using measures obtained from the patient chart. These included: whether CMV antiviral related myelotoxicities occurred (yes/no); the type of myelotoxicity (neutropenia, leukopenia, thrombocytopenia, pancytopenia); other antiviral toxicity recorded (nephrotoxicity, neurotoxicity, electrolyte disturbance, other); grading of the severity (using the NCI-CTCAE Grade 1 - 5) (29); whether toxicity was drug related (yes/no) and if so the drug name (ganciclovir, foscarnet sodium, immunoglobulin, valganciclovir, other); and any change in treatment (immunosuppression, antiviral prophylaxis, G-CSF, other).
Outcomes and assessments
CMV infection was measured as: (a) the incidence of the first and of each subsequent CMV episode post-transplant, including any combination of CMV infection, CMV syndrome and CMV disease; (b) the time in days to start of the first CMV reactivation (infection, syndrome or disease if noted prior to documented infection) and to each subsequent CMV infection post-transplant; and (c) the peak viral load during first and each subsequent CMV infection post-transplant.
CMV syndrome was defined as viremia accompanied by the presence of classical features of CMV infection including fever, malaise, leukopenia, thrombocytopenia, elevated hepatic transaminases and greater than or equal to 5% atypical lymphocytes (30).
CMV end-organ disease was defined as viremia, which was accompanied by gastrointestinal disease, pneumonitis, hepatitis, nephritis, myocarditis, pancreatitis, encephalitis, retinitis, pulmonary or other classical features of organ involvement (30).
Unmet needs of current prophylaxis were measured as: (a) the incidence of CMV prophylaxis, the descriptions of medications used and the duration of therapy; (b) the incidence of CMV treatment for CMV infection, the description of medications used and the duration of therapy; (c) the incidence and management of CMV antiviral related myelotoxicities and patient outcome associated with neutropenia/leukopenia and (d) the incidence and management of other CMV antiviral-related toxicities (e.g., nephrotoxicity).
Health economic burden was measured as; (a) the incidence and length of stay of all cause hospitalizations, ICU admissions, hospitalization for CMV infection, and hospitalizations associated with neutropenia/leukopenia and their consequences; (b) the number of health care visits post-transplant with a general practitioner, nephrologist and/or urologist; (c) the frequency of CMV surveillance post-transplant (e.g. PCR tests, in-person visits); (d) the number of re-admissions for CMV related morbidity; (e) CMV antiviral therapy and duration of treatment; (f) the incidence and use of Granulocyte Colony Stimulating Factor (GCSF); and (g) the proximity of the patient to the primary transplant hospital to assess feasibility of in-person follow-up visits.
Graft outcome and patient survival were assessed from the incidence and type of graft rejection, graft dysfunction and graft loss based on normal clinical, laboratory and histological parameters. Patient survival was measured using the following data from the patient chart: whether the patient was alive at end of follow up period (yes/no); if no, the date of patient death; and the primary cause of death (i.e. CMV disease, related to CMV including documented CMV infection at death, or other complications relating to the patients transplant surgery, cardiovascular event, other events, or unknown).
Statistical analysis
All analyses were performed based on the Eligible Population (ELIG) which was a subset of all enrolled participants with complete data available for 365 days, or to the point of death or graft loss. Descriptive statistics were produced for all key study variables for the overall study population. Demographic data included baseline recipient characteristics. Therapeutic data included immunosuppressive therapy, anti-viral prophylaxis and treatment, and other principal therapies that could influence outcome or disease prevalence. Clinical and laboratory data included CMV Ig status at the time of transplant, quantitative viremia, CMV syndrome and disease, graft and participant survival and other outcome variables of interest.
Continuous variables were summarized using the number of non-missing observations, mean, standard deviation (SD), median, minimum, and maximum values and interquartile ranges. Categorical variables were summarized using the number and percentage of participants belonging to each category. Event rates (summarized as the number of participants experiencing that event) and incidence rates (summarized as the total number of events in a group divided by the time at risk), were summarized within the study population and sub- populations of interest for all the dependent variables (e.g. CMV viremia, CMV resistance, developing refractory CMV). Time to event endpoints were calculated using the Kaplan-Meier estimator, and differences between groups were evaluated using the log-rank test. Analysis was performed using SAS version 9.4.
Ethical approval
This study including all relevant documentation was approved by the Research Ethics Boards at each of the participating sites.
Results
Study population
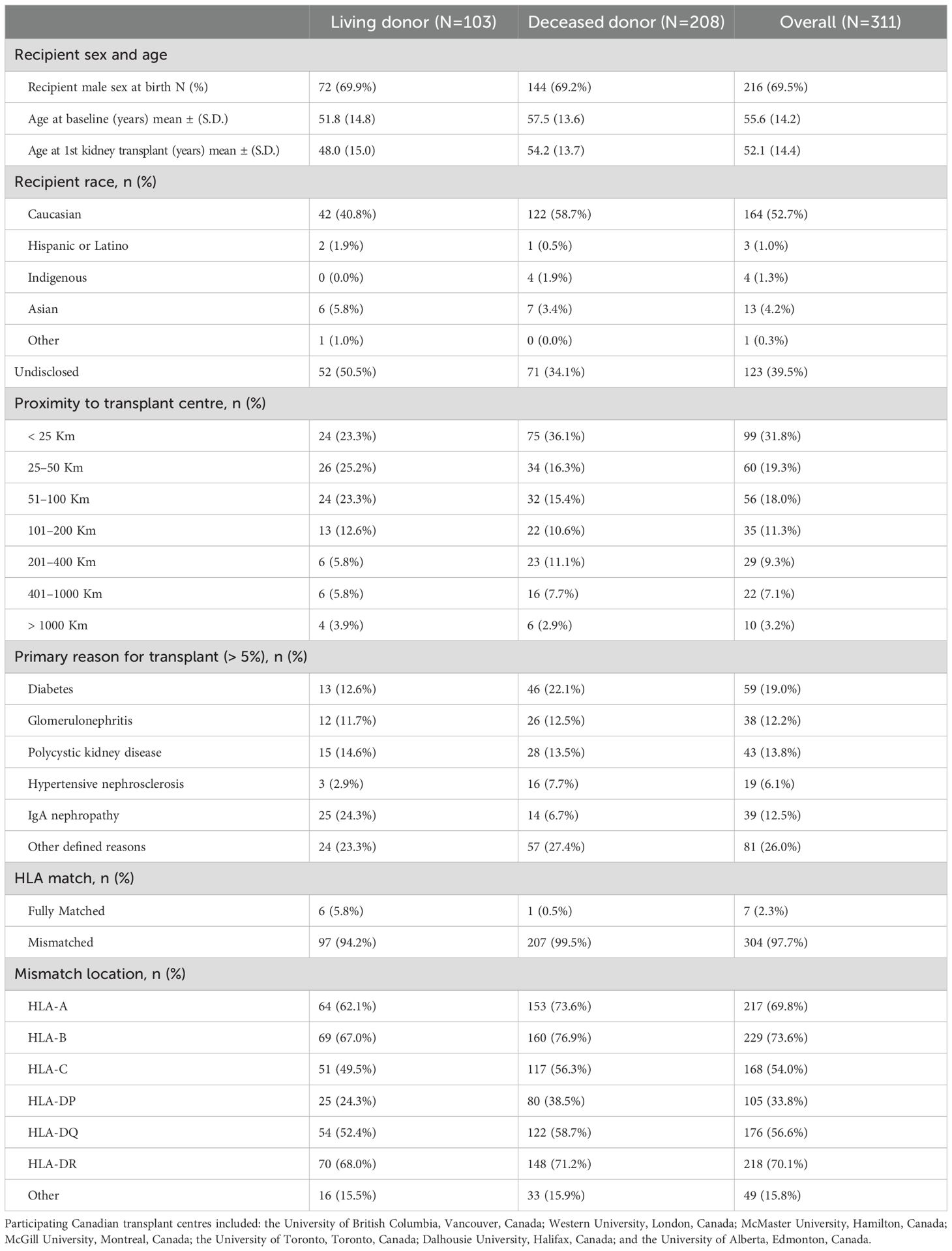
A total of 311 consecutive CMV D+/R- kidney recipients within the index period were enrolled across seven Canadian centers (Table 1). There were no screen failures or exclusions. Of these, 103 received a transplant from a living donor (LD) and 208 from a deceased donor (DD); the median age (IQR) at baseline was 58 (46-67) years and at the first kidney transplant was 55 (42, 64) years; and 69.5% were male and 52.7% were Caucasian. Most participants (68%) lived within 100 km of their transplant center. Primary reasons for kidney failure were diabetes (19.0%), polycystic kidney disease (13.8%), IgA nephropathy (12.5%), other glomerulonephritis (12.2%), or for reasons not specifically defined (26.0%). Most grafts were HLA mismatched (97.7%) with typically two mismatches at HLA-A (44.5%), HLA-B (60.1%), HLA-C (45.4%), HLA-DR (49.6%), HLA-DQ (53.5%) and HLA-DP (47.2%) for those reporting these values. No clinically meaningful differences between those receiving an LD or DD graft were observed with regards to participant baseline characteristics.
Immunosuppression and antiviral prophylaxis
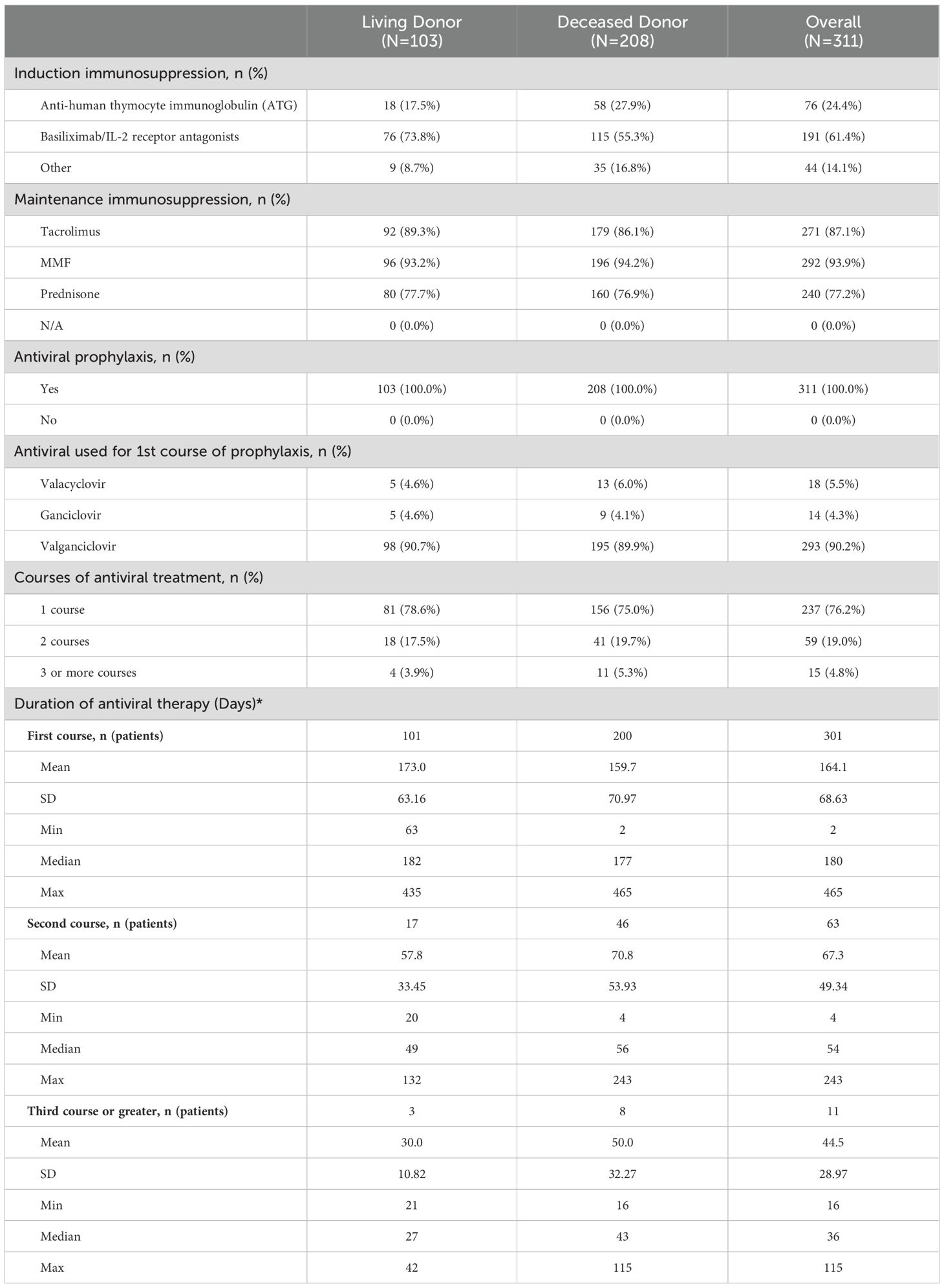
Most patients received induction with basiliximab (61.4%), ATG (24.4%) or another agent (14.1%), principally alemtuzumab, and 93.9% received mycophenolate mofetil (MMF) or mycophenolic acid (MPA) as maintenance immunosuppression, 87.1% received tacrolimus and 77.2% prednisone (Table 2). Most participants (62.4%) received all 3 maintenance agents, while 33.4% received two and 4.2% only one. All participants received antiviral CMV prophylaxis, principally with valganciclovir (293, 90.2%), started a median of 1 day post-transplant and lasting for a median of 180 days (range: 2–465 days). Most patients received only one course of antiviral therapy (76.2%), though almost one quarter received two (19.0%) or more courses (4.8%). The duration of each course is shown in Table 2. There were no clinically meaningful differences between LD and DD recipients with regards to antiviral prophylaxis. Less than 2% of participants had documented toxicities related to antiviral therapies, of which 28.6% were related to valganciclovir, 14.3% to ganciclovir and 57.1% to other agents (tacrolimus) with antivirals. Outside of myelotoxicity (discussed below), the most common toxicities were neurotoxicity, nephrotoxicity, electrolyte disturbances and other toxicities that were mild (grade 1 – 2). No participants experienced symptoms related to G-CSF administration.
CMV infections
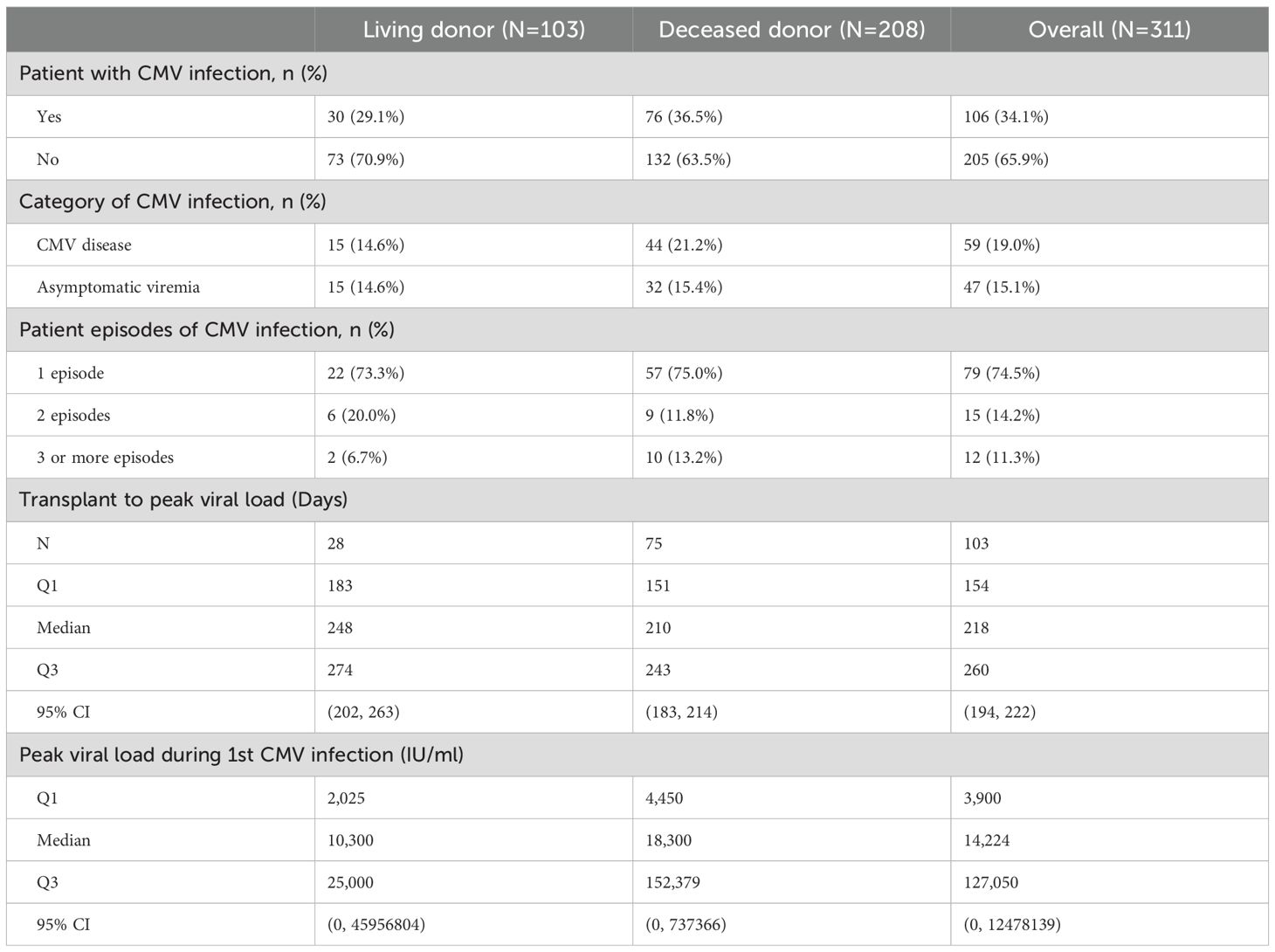
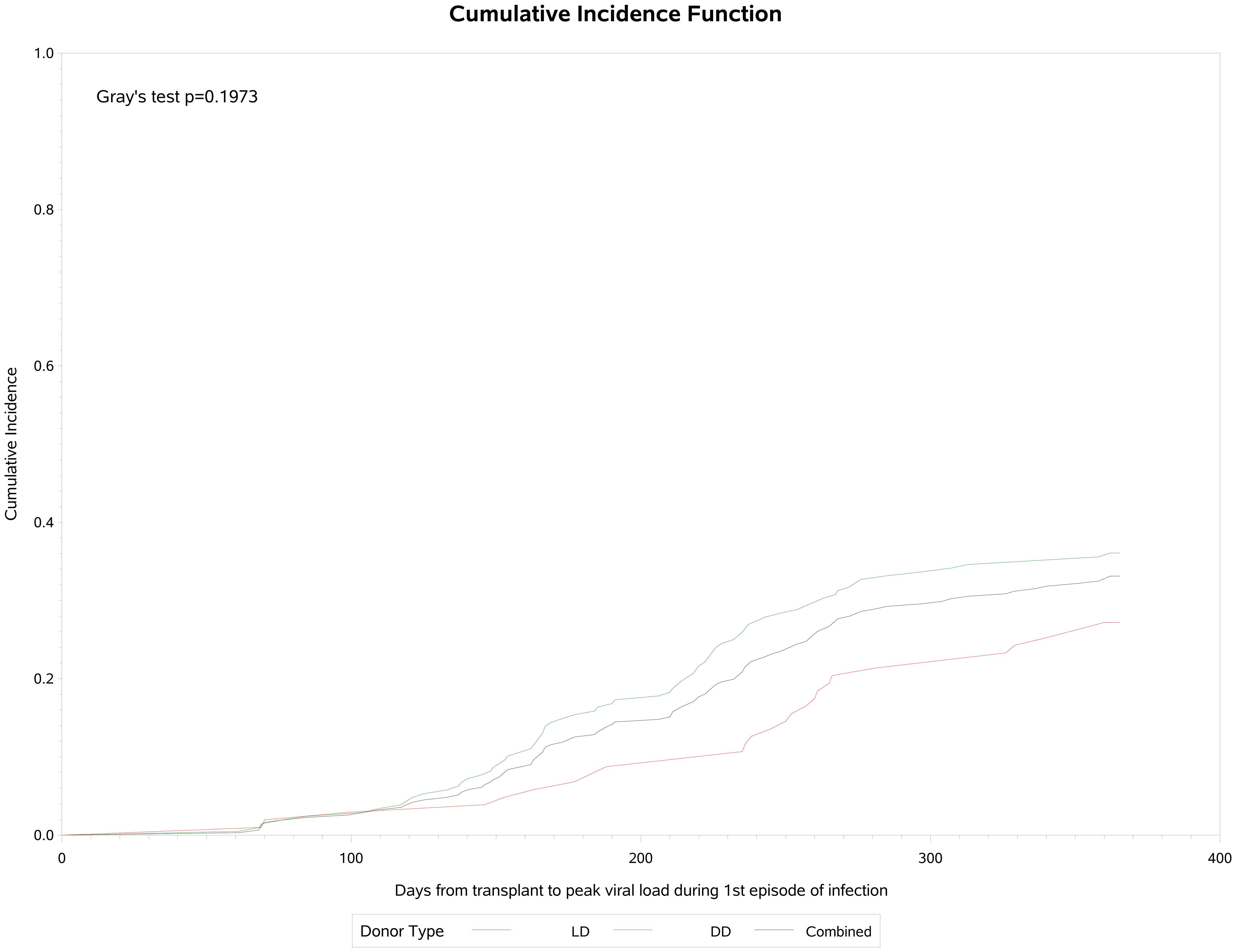
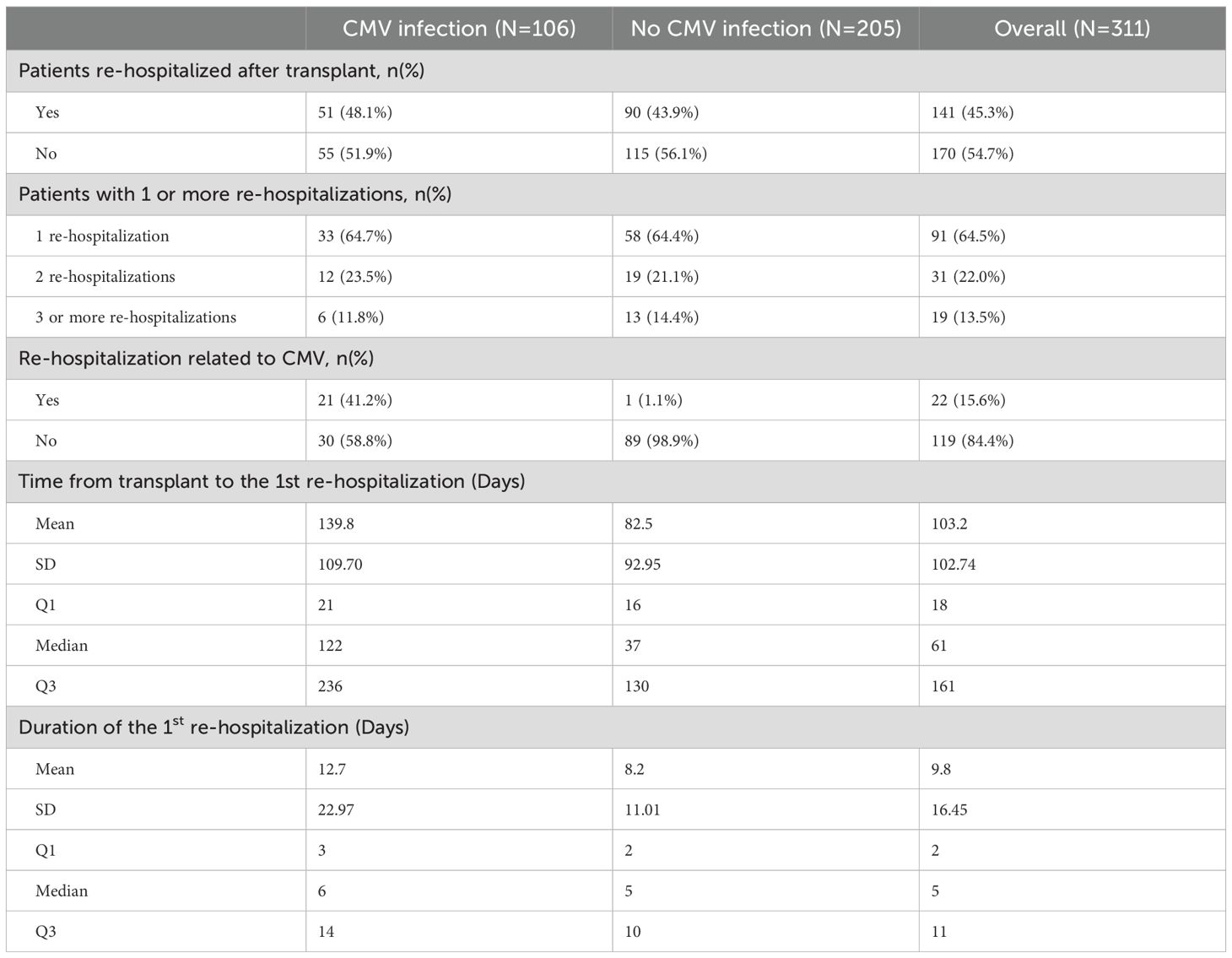
A total of 106 (34.1%) participants developed post-transplant CMV infection, of whom 15 (14.2%) (had two and 12 (11.3%) had three or more episodes of infection (Table 3). Of these 106 patients, 47 (44.3%) had asymptomatic viremia during follow-up while 59 (55.6%) had CMV disease presenting principally with fever (21.9%), malaise (33.3%), leukopenia (28%) and transaminitis (12%). CMV infection occurred approximately 90 days post-transplant and increased in frequency to a plateau at the end of the first year (Figure 1). Breakthrough CMV infection occurred during prophylaxis in approximately 23% of these patients for whom precise dates were available (n=95). CMV disease was diagnosed a median of 223 days (IQR 165, 257) post-transplant, lasted for a median of 34 days (IQR 23, 41)and resolved by 261 days (IQR 192, 306) days post-transplant. Only 7 of these patients (11.9%) had end-organ disease, manifested principally by gastrointestinal or neurological symptoms. CMV infection was slightly more common in DD than LD recipients (viremia: 36.5% vs 29.1%, symptomatic: 21.2% vs 14.6%) but there was no clinically meaningful or statistically significant difference between LD and DD recipients. Peak viral load during the first infection occurred at a median of 218 days (IQR: 154, 260) days post-transplant with a median value of 14,224 IU/ml (IQR: 3,900, 127,050 IU/ml). Peak viral loads measured in subsequent viremic episodes were later (up to 352 days) and lower (maximum 32,007 IU/ml). Eighty-one patients received a second course of antiviral treatment, including 69 (85.2%) with valganciclovir and 9 (11.1%) with ganciclovir.
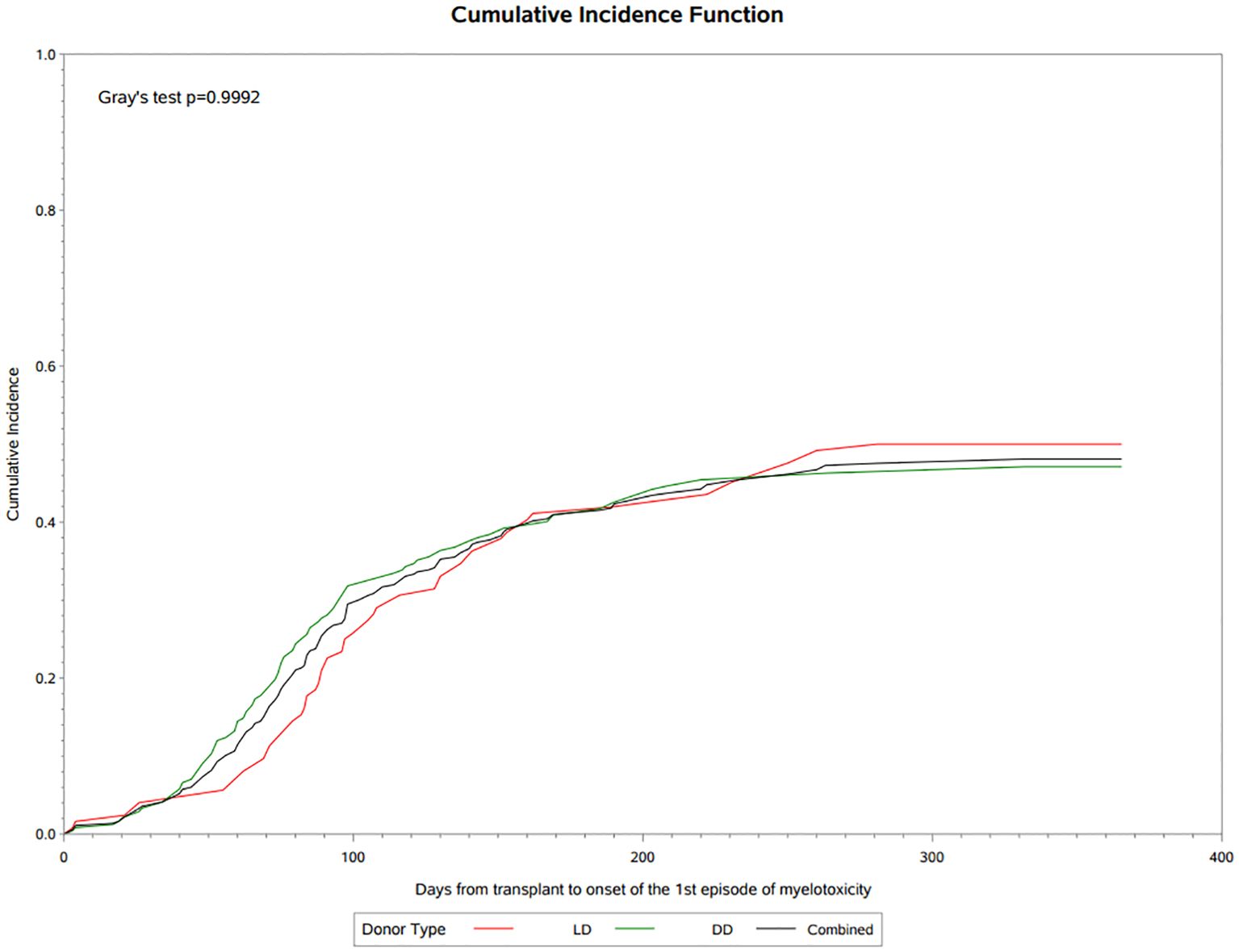
Myelotoxicities
A total of 121 (38.9%) patients experienced antiviral related myelotoxicities classified principally as leukopenia or neutropenia, with a frequency that was similar in LD (39.8%) and DD (38.5%) recipients. Cumulative incidence analysis showed that myelotoxicity was observed within 30 days post-transplant, rising rapidly during the first 3 months to 34.5% at day 100 and 46.8% by the end of the first year of follow-up (Figure 2). The median onset was 90 (IQR 65, 133) days post-transplant, median duration was 30 (IQR 14, 74) days and only 6 patients (4.9%) had an NCI-CTCAE severity grade above 3. Immunosuppressive therapy was reduced in almost 80% of the patients with myelotoxicity, being decreased on 65(53.7%) or discontinued or interrupted on 43 (35.1%) of the 134 changes recorded, and antiviral therapy was similarly decreased or discontinued in 34 of the 50 (68%) patients on treatment at the time of myelotoxicity. Other agents such as trimethoprim-sulfamethoxazole were discontinued in 9.1% of patients and G-CSF was implemented in 22 (18.2%) patients during the episode of myelotoxicity. A second episode of myelotoxicity occurred in 14 (11.5%) of these patients at a median of 136 (IQR 89, 167) days, with a duration of 39 (IQR 24, 46) days, of which none had an NCI-CTCAE severity over grade 3. Immunosuppressive therapy was decreased, interrupted or discontinued in 94.5%, antiviral therapy discontinued or interrupted in 28.6% and G-CSF was started in 14.3% of these patients. A third or greater episode of myelotoxicity occurred in 5 (4.1%) of patients a median of 174 (IQR 161, 247) days post-transplant, lasting for 70 (IQR 60,80) days, in which no events were above Grade 2 severity. Immunosuppression was decreased or discontinued/interrupted in 2 (40%) patients, and antiviral prophylaxis discontinued or interrupted in 1 (20%) patient. No patients received G-CSF.
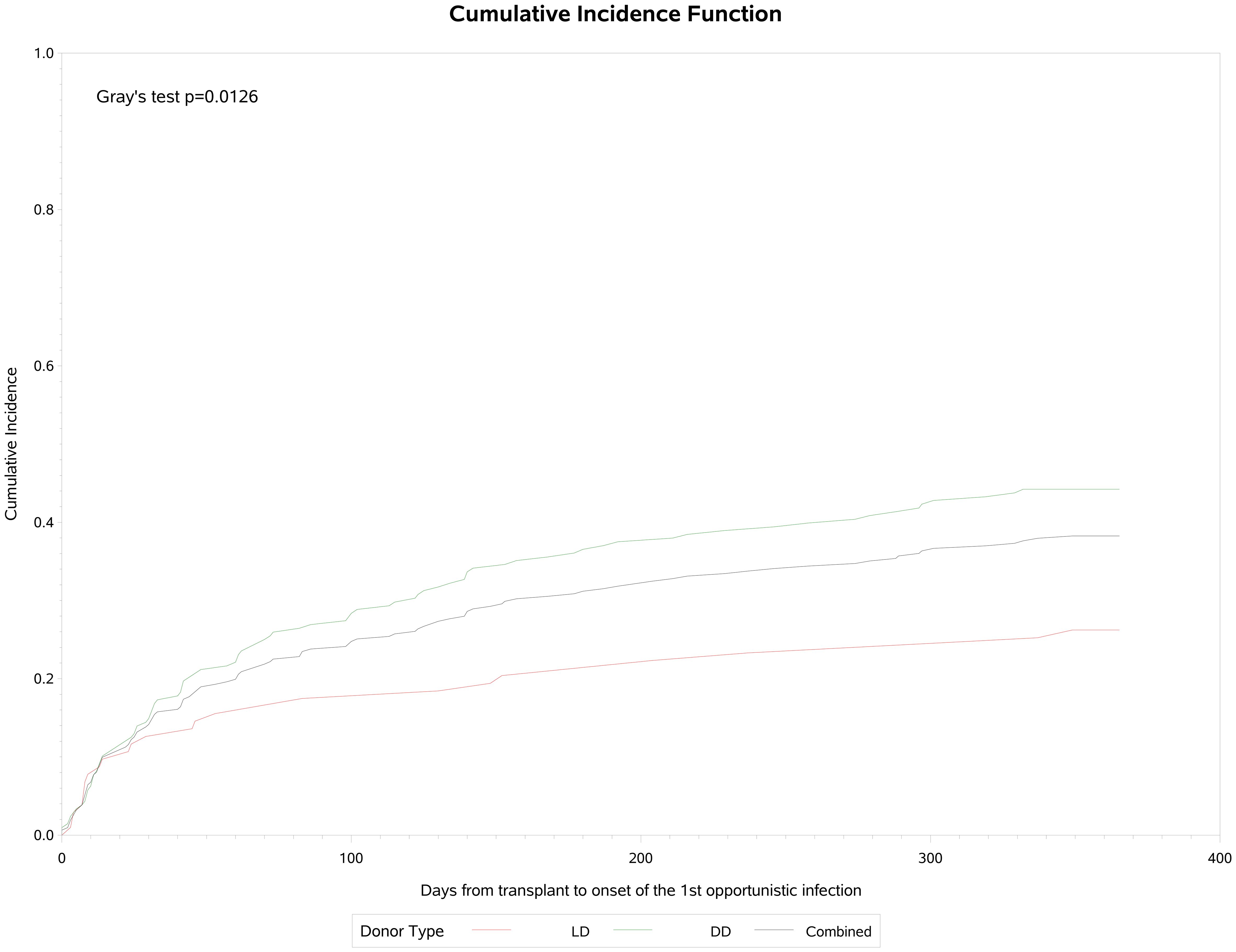
Opportunistic infections
A total of 119 (38.3%) patients experienced opportunistic infections post-transplant, which were significantly more common in DD (44.2%) than LD (26.2%) recipients (p=0.002) (Table 4). Cumulative incidence analysis (Figure 3) showed that infections were first observed within 30 days post-transplant, rising to an inflection at 60–90 days and increasing slowly thereafter to approximately 38% by the end of the first year, with a median onset of 53 (IQR: 14, 142) days post-transplant and a median duration of 28 (IQR: 15, 62). Recurrent opportunistic infections were recorded in 45 (14.5%) of these patients, of whom 15 (4.8%) had three or more episodes. Median onset for the second and third or more episodes was 137 (IQR 77, 213) days and 181 (IQR 109, 236) days respectively, with a duration of 22 (IQR 14, 49) days and 29 (IQR 18, 50) days. Of the 179 total episodes of opportunistic infection reported, 98 (54.7%) were bacterial, 56 (31.3%) were non-CMV viral infections and 30 (16.7%) were fungal in origin. Almost all (97.8%) were Grade 1–3 in severity.
As shown in Table 5, there were no significant differences in the incidence, number of episodes, causal agents or severity of opportunistic infections between patients who did, or did not, experience post-transplant CMV infection (p=NS), Opportunistic infection occurred significantly later in patients with CMV infection (median: 86, IQR: 20, 180 days, p=0.037) than in those without CMV infection (median: 43, IQR: 14, 127 days) though there was no difference in duration of opportunistic infections between the two groups (median: 28 days).
Hospitalization
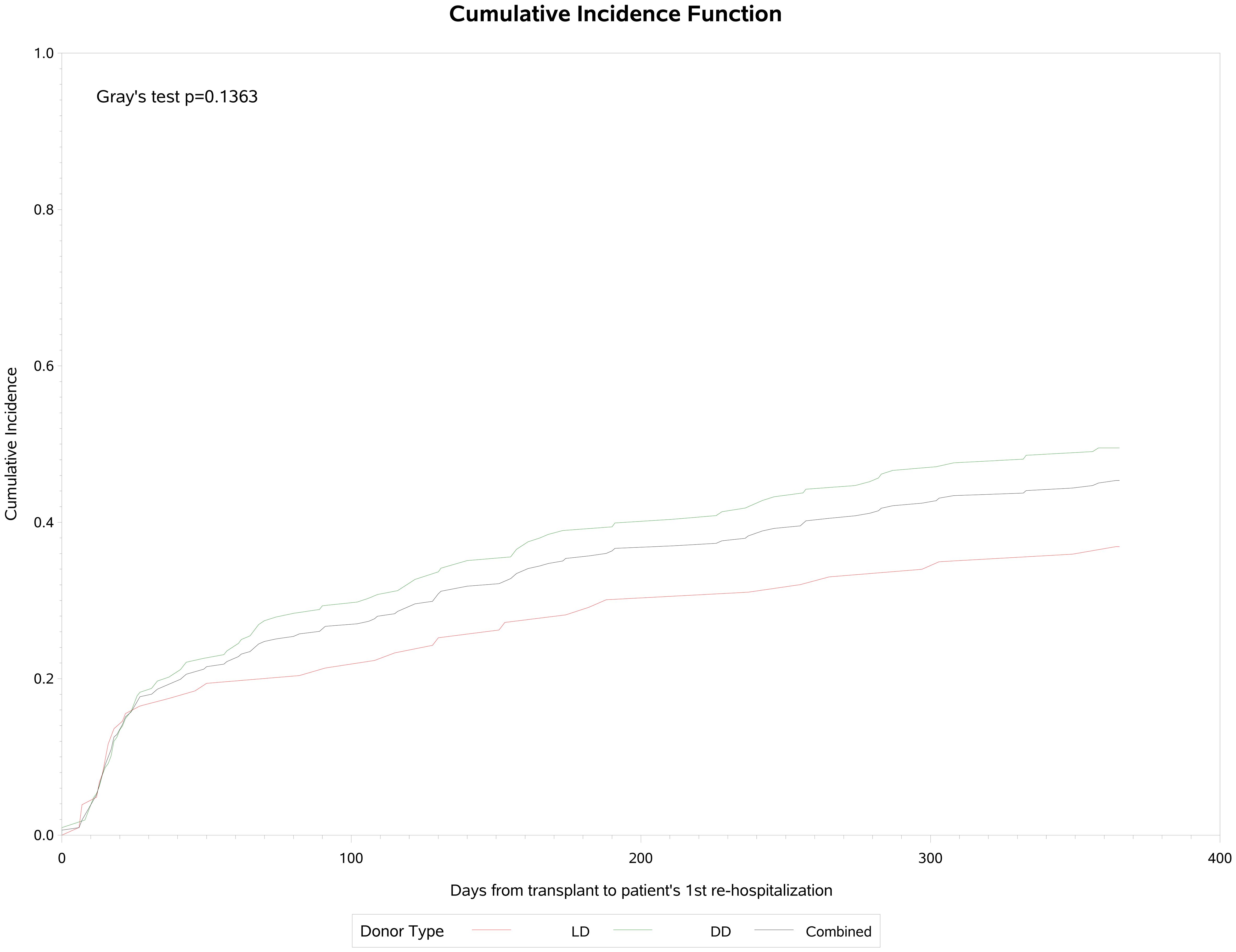
A total of 141 (45.3%) participants were re-hospitalized post-transplantation, 103 (36.9%) in the living donor and 38 (49.5%) in the deceased donor group. Of these admissions, 25 (17.7%) were related to CMV infection or leukopenia/neutropenia. Cumulative incidence analysis (Figure 4) showed that re-hospitalization commenced early, rising to 28% by day 30 and continuing to 45% by the end of the first year of observation. The median time to first re-hospitalization was 58 (IQR: 18, 158) days and the median duration was 5 (IQR: 2, 11) days. Of these patients, 9 (6.3%) required admission to ICU, for a median of 14 (IQR: 5, 14) days. Fifty (35,4%) of these 141 patients were re-hospitalized a second time, of which 6 (12%) were related to CMV infection or leukopenia/neutropenia. The median time to re-admission was 114 (IQR: 49, 205) days, and median duration was 6 (IQR: 4, 10) days. Two patients (4%) were admitted to ICU for a median of 2 days. And 31 (21.9%) patients were re-hospitalized 3 or more times of which 3 (9.6%) were for CMV infection or neutropenia. The third admission was a median of 145 (IQR: 95, 249) days post-transplant, lasting for a median of 7 (IQR: 3, 12) days and 2 were admitted to ICU for a median of 3 days.
As shown in Table 6, the proportion of patients who were re-hospitalized did not differ significantly between patients with or without CMV infection post-transplant (p=NS), But re-hospitalization occurred substantially later in patients with CMV infection (median: 122, IQR: 21, 236 days, p<0.0001) than in those without CMV infection (median: 37, IQR: 16, 130 days) but there was no difference in duration of hospital stay between the two groups (median: 5 days, p=NS).
In the year following transplantation, all participants (100%) saw a nephrologist (total 5,285 visits, mean 17.0), 82.6% a urologist (total 425 visits, mean 1.4), 57.7% another healthcare provider (total 866 visits, mean 2.8), 39.9% an ambulatory nurse (total 1,293, mean 4.1) and 20.1% a general practitioner (total 162, mean 0.5). There were no clinically meaningful differences between LD or DD recipients, with regards to health care visits.
Graft and patient survival
Biopsy-proven acute graft rejection (BPAR) occurred in 20 patients (6.4%) overall (LD 4.9% and DD 7.2%, p=NS) after a median of 107 (IQR: 36, 202) days. Three of these patients received biologic therapy with rituximab or ATG. Ten patients (3.2%) lost their graft (LD 1.0%, DD 4.3%), all for reasons other than rejection. By 1 year post-transplant, 10 patients (3.2%) had died (LD 1.0%, DD 4.3%). Death occurred at a median of 152 days (IQR: 140, 201) days post-transplant due principally to cardiovascular events (n=5) and other causes (n=3). Only 1 case was reported related to CMV infection. Overall, 293 of the 311 patients (94.2%) were alive with a functioning graft by 1 year (LD 98.1%, DD 92.3%) (Figure 5).
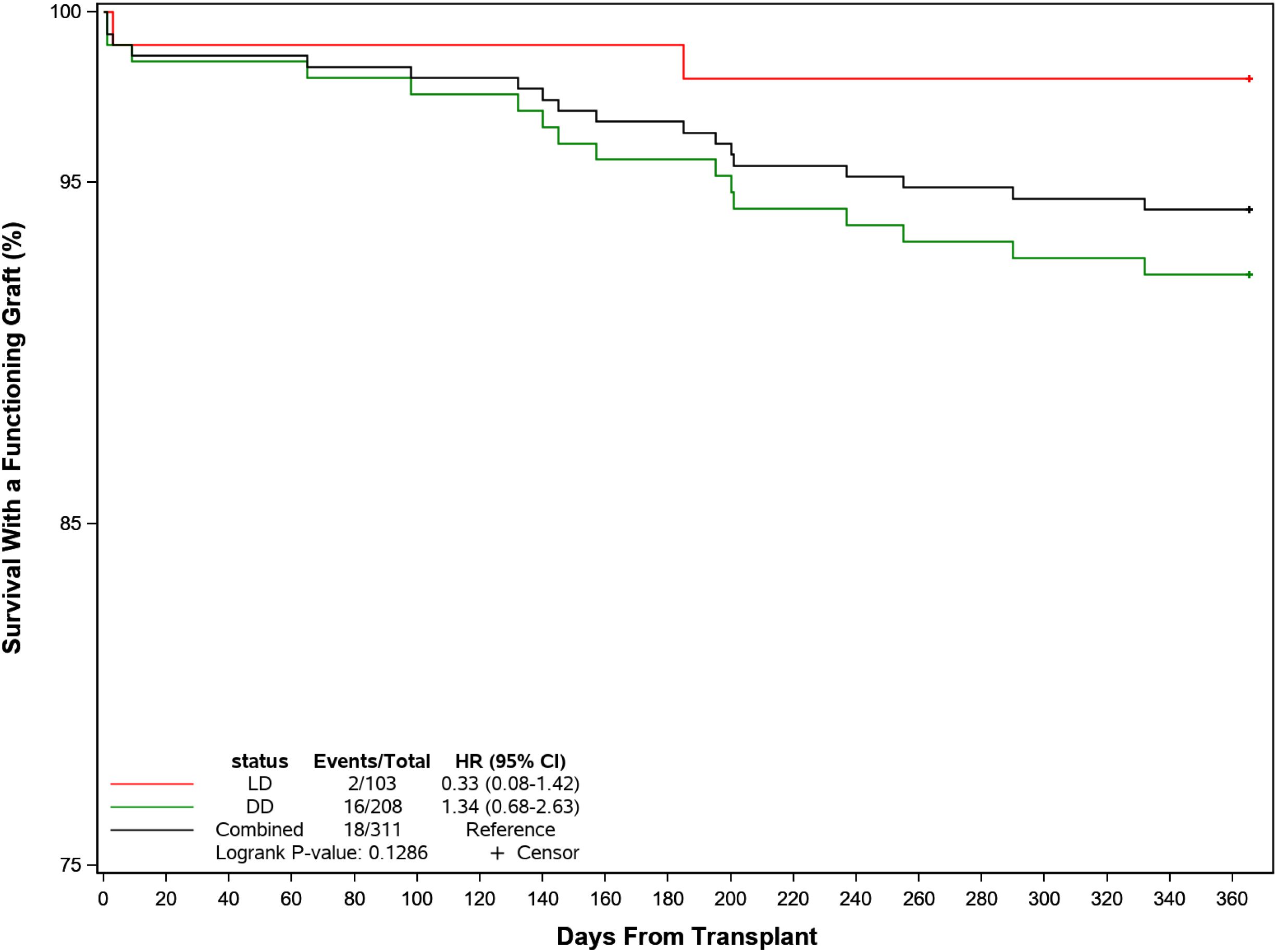
Figure 5. Probability of recipient survival with a functioning kidney graft following transplant by donor source.
Graft and patient survival were similar in patients with or without post-transplant CMV infection (Figure 6). BPAR was reported in 8 (7.5%) participants with post-transplant CMV infections compared to 12 (5.9%) without, although rejection occurred later in the former at a median of 150 days (IQR: 98, 248) days compared with a median of 64 days (IQR: 14, 199) days in those without infection. Graft loss was higher in patients without CMV infection (4.4% vs 0.9%) and 4 participants (3.8%) with CMV and 6 participants (2.9%) without CMV had died by the end of the follow-up period. Overall, 101 (95.3%) and 192 (93.7%) in each group were alive with a functioning graft by 1 year post-transplant. [Post-text Table 14.10.2].
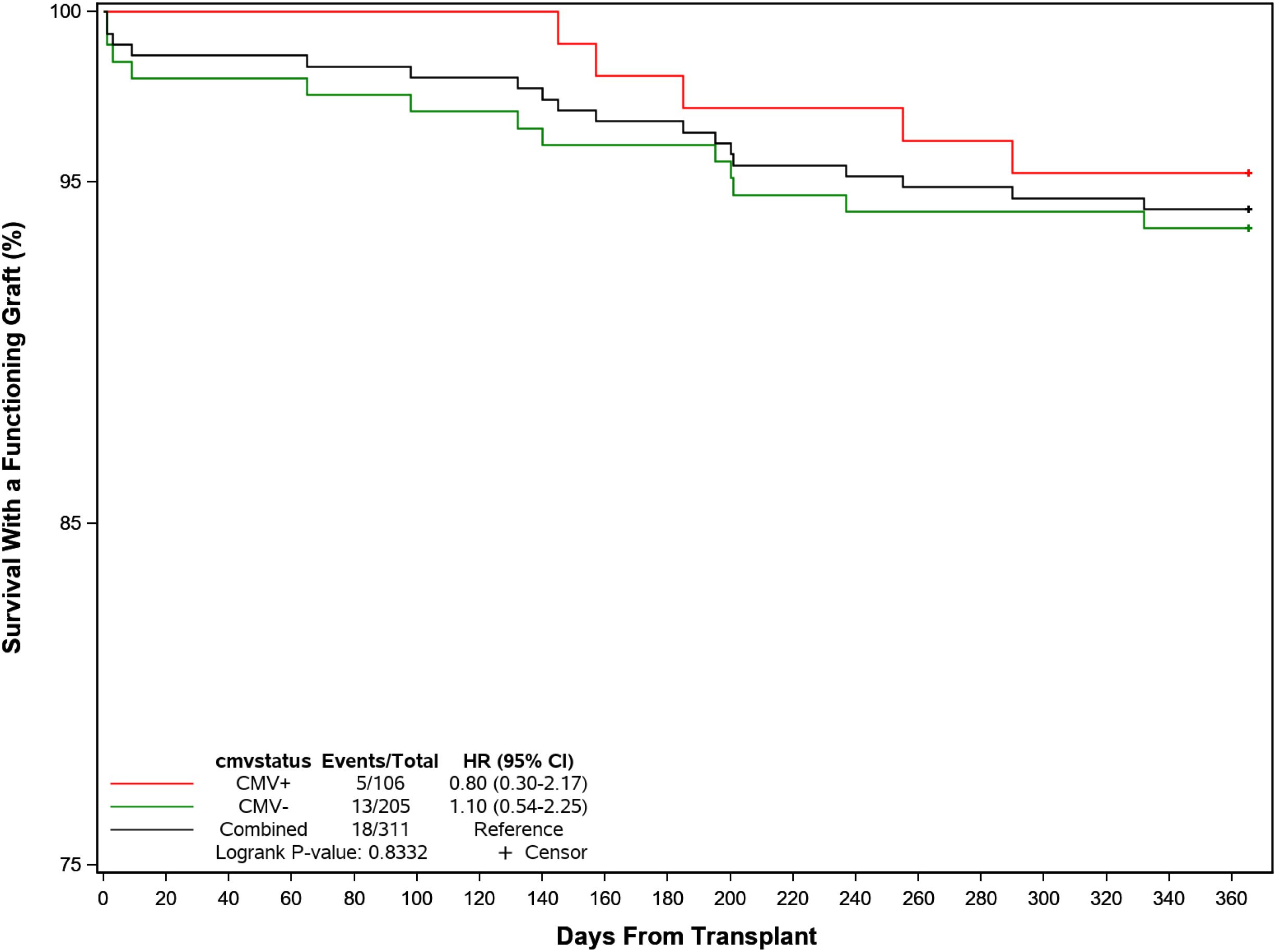
Figure 6. Probability of recipient survival with a functioning kidney graft following transplant by CMV infection.
Discussion
This study provides a detailed and comprehensive understanding of current real-world Canadian practice regarding the management, clinical outcomes and disease burden of patients at the highest risk of CMV infection as a foundation for precision medicine care. It highlights the limitations of current therapy and defines the dynamic patterns of CMV infection in relation to other cardinal events including leukopenia, rejection, infection and hospitalization, elucidating the input parameters to enable the clinical and economic modeling of strategies to optimize therapy and healthcare resource utilization in this complex aspect of transplantation medicine (17, 31–34).
CMV is a ubiquitous pathogen of enormous biological complexity and consequence (35). Primary CMV infection occurs in 40-90% of people globally (36), following which the virus establishes long-term latency by modulating innate and adaptive immunity to facilitate immune evasion (37). Reactivation may occur in the context of host immune dysfunction due to intrinsic disease or external immunosuppression, while reinfection may follow contact with another infected source despite natural immunity (37). By virtue of its frequency, biological complexity and paucity of therapy, CMV has been one of the most challenging pathogens in transplantation (38). It complicates the clinical course through direct effects (including systemic viral infection and end-organ disease) (39), and potentially via indirect consequences including opportunistic bacterial, fungal or other viral diseases, by increasing the risk of rejection and graft loss, or through thrombotic events and accelerated cardiovascular disease (6, 38, 40–42). Current anti-viral treatment has reduced the incidence and severity of CMV disease, although leukopenia and other consequences may decrease the potential benefit (43), so that routine prophylaxis is normally restricted to patients at elevated risk defined by CMV serostatus, increased age and immunocompromised state (44).
Systematic review confirms the benefit of antiviral medication in preventing CMV infection, CMV disease and all-cause death compared with placebo or no treatment (45). Universal prophylaxis and preemptive therapy are the preferred treatment strategies (46), the former offering potentially superior outcomes particularly in high-risk D+/R- subjects with fewer opportunistic infections, rejections and improved graft and patient survival, while the latter offers lower costs and drug exposure and lower rates of late CMV and of viral resistance (47, 48). The limitations of universal prophylaxis are evident in the current study where patients received treatment for a median of 180 days, consistent with current guidelines (8, 18, 25, 26, 49). CMV infection was delayed (to a median of over 200 days) but occurred in 34% of patients of whom over half had CMV disease or end-organ involvement and one quarter had recurrent episodes of infection. Because of these problems, investigators have proposed a hybrid approach in which prophylaxis is followed by long-term pre-emptive monitoring to rapidly identify and treat break-through infection (50, 51). But while appealing, this approach is not without challenge since most patients have returned home by this point, which in the current study was up to 1,000 km from the transplant clinic.
The dosing strategy and duration of CMV treatment are influenced by both costs and adverse effects, of which the most common is leukopenia occurring in 20-80% of patients (43, 52). The incidence, severity and burden depend on a combination of factors including the drug, dose, co-incident therapy, renal function and nucleotide polymorphisms which control drug metabolism and elimination (53). Almost 40% of patients in this study experienced myelotoxicities presenting as leukopenia or neutropenia (54). Myelotoxicity occurred within 30 days post-transplant, rising to almost half of all patients by the end of the first year of follow-up. While leukopenia was rarely serious by NCI-CTCAE severity grade it triggered a reduction or discontinuation of immunosuppression or antiviral therapy in up to 60% of patients increasing the potential for CMV infection, development of resistant strains (55, 56) or graft rejection (57). Systematic review confirms that such reduction in treatment is common in kidney transplantation while, as observed here, the safe and effective alternative of G-CSF is less frequently employed (54, 58). Letermovir, a new antiviral agent which inhibits the CMV viral terminase complex, offers the opportunity to change current practice (59) since antiviral efficacy is comparable to valganciclovir, but leukopenia was reduced by more than half (26% vs. 64%) and fewer patients discontinued treatment due to adverse effects (60–62). Caution may be required due to recognized drug interactions with tacrolimus and cyclosporine (61).
Prudent immune suppression, meticulous surgical intervention and improved antimicrobial therapy have reduced the incidence and impact of opportunistic infection and re-hospitalization in kidney transplantation (1, 3, 63). Most infections and hospitalizations reported here occurred in the first 100 days consistent with prior experience. Opportunistic infections occurred in almost 40% of patients, were significantly more common in DD than LD recipients, but were generally mild to moderate in severity with few patients needing ICU admission. Approximately half were bacterial, one third were viral (non-CMV) and 15% were fungal in origin. Almost half of all patients were re-hospitalized post-transplantation, 20% related to leukopenia/neutropenia or CMV infection. Despite these complications, graft and patient outcomes were excellent. Biopsy-proven acute graft rejection (BPAR) occurred in only 6% of patients overall, the majority of these within the first month long preceding CMV infection. This suggests that the broadly reported relationship between infection and BPAR may be consequential rather than causal (17), mediated through the common reduction of IST in the face of leukopenia. By the end of follow-up, 94% of patients remained alive with a functioning graft with only 1 death attributed to CMV infection.
Graft and patient survival were similar in patients with or without post-transplant CMV infection. BPAR was reported in 8 (7.5%) participants with post-transplant CMV infections compared to 12 (5.9%) without, although rejection occurred later in the former at a median of 150 days (range: 36–283 days) compared with a median of 64 days (range: 6–256 days) in those without infection. Graft loss was higher in patients without CMV infection (4.4% vs 0.9%) and 4 participants (3.8%) with CMV and 6 participants (2.9%) without CMV had died by the end of the follow-up period. Overall, 101 (95.3%) and 192 (93.7%) in each group were alive with a functioning graft by 1 year post-transplant. We do not yet have definitive explanations for these marginal differences, though are exploring whether they reflect variations in immune suppression and/or viral prophylaxis.
Limitations and conclusions
This study has limitations including selection bias, information bias and confounding which are inherent to observational design. To minimize selection bias, the study was conducted over a period in which laboratory diagnosis and clinical management of CMV remained constant, from a representative cohort of regional centers performing over 60% of the transplants annually in Canada, all working within the framework of the Canadian Blood Services to ensure national coordination in laboratory and clinical practice (64, 65). While information bias may occur from many sources, stringent efforts were made to reduce these by continuous digital data screening to define data trends and identify missing, extreme and implausible values and by formal data review with the contributing investigators. Nor do we include analysis of cellular immunity, a critical defense mechanism in which a broad range of effector-memory, NK-like CD8 cells and others play an important role in controlling the expression of this virus (26). Exploring the consequences of immune suppression and leukopenia is therefore of particular importance in this aspect.
Within these limits, we may draw the following conclusions. First, CMV infection represents an important and continuing challenge to clinical care, for which current strategies are deficient. Consequently, even within the framework of highly structured and widely approved clinical guidelines, enormous variability occurs in the application of current prevention. Second, the myelotoxicity of current prophylaxis is an important and perhaps under-recognized problem, affecting as it does a high proportion of patients and resulting in reduction in dose of both antiviral agents and immune suppressants. It is feasible that this response contributes to both the persistence and recurrence of CMV infection observed in many patients and the development of acute – and perhaps chronic – rejection in these patients. Finally, the introduction of new agents such as letermovir without appreciable myelotoxicity offers the opportunity to optimize our strategies for care, potentially minimizing the burden of this disease. But the complexities of dose-related pharmacokinetic interactions with key immunosuppressive drugs, and the potential for simply delaying CMV expression require careful consideration to optimize treatment strategies. We anticipate that the granular data obtained in this study will enable detailed modeling to develop the foundation for effective precision medicine strategies.
Data availability statement
These data are confidential clinical information under the custodianship of the regional health authorities and as such are not freely available. Reasonable requests for access may be reviewed by the principal investigator and partners. Requests to access the datasets should be directed to cGF1bC5rZW93bkBzeXJlb24uY29t.
Ethics statement
Approval was provided by the Clinical Ethics Review Boards at the following universities: University of British Columbia, Western University, McMaster University, McGill University, University of Toronto, Dalhousie University and University of Alberta. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because retrospective chart review does not require formal written consent at the participating sites.
Author contributions
JG: Methodology, Validation, Data curation, Writing – review & editing, Resources, Project administration, Visualization. AH: Data curation, Writing – review & editing, Visualization, Methodology, Validation, Resources, Project administration. ZC: Methodology, Data curation, Validation, Writing – review & editing, Visualization, Resources, Project administration. JT: Validation, Project administration, Data curation, Writing – review & editing, Methodology, Resources, Visualization. SK: Writing – review & editing, Validation, Methodology, Data curation, Visualization, Resources, Project administration. AV: Resources, Data curation, Project administration, Visualization, Writing – review & editing, Methodology, Validation. CC: Data curation, Visualization, Resources, Project administration, Validation, Methodology, Writing – review & editing. PK: Methodology, Supervision, Investigation, Formal Analysis, Software, Visualization, Data curation, Writing – review & editing, Conceptualization, Resources, Writing – original draft, Project administration, Funding acquisition, Validation. SS: Methodology, Supervision, Writing – review & editing, Project administration, Conceptualization, Funding acquisition. CK: Project administration, Supervision, Conceptualization, Methodology, Writing – review & editing, Funding acquisition. CG: Writing – review & editing, Funding acquisition, Conceptualization, Methodology, Project administration, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Funding for the study was provided by Merck Canada Inc, Kirkland, QC, Canada.
Conflict of interest
PK is a Director of Syreon Corporation which conducted the study on behalf of Merck Canada Inc. and the investigators. SS, CK and CG were employees of Merck Canada Inc. at the time of the trial/study and may hold stock in the company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fimmu.2025.1712602.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fishman JA. Infection in organ transplantation. Am J Transpl. (2017) 17:856–79. doi: 10.1111/ajt.14208
2. Meesing A and Razonable RR. New developments in the management of cytomegalovirus infection after transplantation. Drugs. (2018) 78:1085–103. doi: 10.1007/s40265-018-0943-1
3. Cowan J, Bennett A, Fergusson N, McLean C, Mallick R, Cameron DW, et al. Incidence rate of post-kidney transplant infection: A retrospective cohort study examining infection rates at a large Canadian multicenter tertiary-care facility. Can J Kidney Health Dis. (2018) 5:2054358118799692. doi: 10.1177/2054358118799692
4. Leeaphorn N, Garg N, Thamcharoen N, Khankin EV, Cardarelli F, and Pavlakis M. Cytomegalovirus mismatch still negatively affects patient and graft survival in the era of routine prophylactic and preemptive therapy: A paired kidney analysis. Am J Transpl. (2019) 19:573–84. doi: 10.1111/ajt.15183
5. Jamal AJ, Husain S, Li Y, Famure O, and Kim SJ. Risk factors for late-onset cytomegalovirus infection or disease in kidney transplant recipients. Transplantation. (2014) 97:569–75. doi: 10.1097/01.tp.0000438197.38413.f2
6. Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. (2013) 96:333–60. doi: 10.1097/TP.0b013e31829df29d
7. Razonable RR, Humar A, and Practice AIDCo. Cytomegalovirus in solid organ transplantation. Am J Transpl. (2013) 13 Suppl 4:93–106. doi: 10.1111/ajt.12103
8. Razonable RR and Humar A. Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transpl. (2019) 33:e13512. doi: 10.1111/ctr.13512
9. Raval AD, Kistler KD, Tang Y, Murata Y, and Snydman DR. Epidemiology, risk factors, and outcomes associated with cytomegalovirus in adult kidney transplant recipients: A systematic literature review of real-world evidence. Transpl Infect Dis. (2021) 23:e13483. doi: 10.1111/tid.13483
10. Kumar D, Chin-Hong P, Kayler L, Wojciechowski D, Limaye AP, Osama Gaber A, et al. A prospective multicenter observational study of cell-mediated immunity as a predictor for cytomegalovirus infection in kidney transplant recipients. Am J Transpl. (2019) 19:2505–16. doi: 10.1111/ajt.15315
11. Harvala H, Stewart C, Muller K, Burns S, Marson L, MacGilchrist A, et al. High risk of cytomegalovirus infection following solid organ transplantation despite prophylactic therapy. J Med Virol. (2013) 85:893–8. doi: 10.1002/jmv.23539
12. Lumbreras C, Manuel O, Len O, ten Berge IJ, Sgarabotto D, and Hirsch HH. Cytomegalovirus infection in solid organ transplant recipients. Clin Microbiol Infect. (2014) 20 Suppl 7:19–26. doi: 10.1111/1469-0691.12594
13. Thorndyke A, Joyce C, Samra M, Cotiguala L, Trotter C, Aguirre O, et al. Risk factors for CMV and BK infections in an elderly veteran population following kidney transplantation: implications for immunosuppression induction and management. Biomedicines. (2023) 11. doi: 10.3390/biomedicines11113060
14. Rosiewicz KS, Blazquez-Navarro A, Kaliszczyk S, Bauer C, Or-Guil M, Viebahn R, et al. Interactions of TTV with BKV, CMV, EBV, and HHV-6A and their impact on post-transplant graft function in kidney transplant recipients. Front Transpl. (2024) 3:1393838. doi: 10.3389/frtra.2024.1393838
15. Jorgenson MR, Descourouez JL, Cardinale B, Lyu B, Astor BC, Garg N, et al. Risk of opportunistic infection in kidney transplant recipients with cytomegalovirus infection and associated outcomes. Transpl Infect Dis. (2019) 21:e13080. doi: 10.1111/tid.13080
16. Cippà PE, Schiesser M, Ekberg H, van Gelder T, Mueller NJ, Cao CA, et al. Risk stratification for rejection and infection after kidney transplantation. Clin J Am Soc Nephrol. (2015) 10:2213–20. doi: 10.2215/CJN.01790215
17. Dmitrienko S, Balshaw R, Machnicki G, Shapiro R, and Keown P. Probabilistic modeling of cytomegalovirus infection under consensus clinical management guidelines. Transplantation. (2009) 87:570–7. doi: 10.1097/TP.0b013e3181949e09
18. Humar A, Lebranchu Y, Vincenti F, Blumberg EA, Punch JD, Limaye AP, et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transpl. (2010) 10:1228–37. doi: 10.1111/j.1600-6143.2010.03074.x
19. Razonable RR. Oral antiviral drugs for treatment of cytomegalovirus in transplant recipients. Clin Microbiol Infect. (2023) 29:1144–9. doi: 10.1016/j.cmi.2023.03.020
20. Raval AD, Kistler K, Tang Y, Murata Y, and Snydman DR. Antiviral treatment approaches for cytomegalovirus prevention in kidney transplant recipients: A systematic review of randomized controlled trials. Transplant Rev (Orlando). (2021) 35:100587. doi: 10.1016/j.trre.2020.100587
21. Razonable RR and Hayden RT. Clinical utility of viral load in management of cytomegalovirus infection after solid organ transplantation. Clin Microbiol Rev. (2013) 26:703–27. doi: 10.1128/CMR.00015-13
22. Mueller NJ. Cytomegalovirus: why viral dynamics matter. EBioMedicine. (2015) 2:631. doi: 10.1016/j.ebiom.2015.06.004
23. Lodding IP, Sengeløv H, da Cunha-Bang C, Iversen M, Rasmussen A, Gustafsson F, et al. Clinical application of variation in replication kinetics during episodes of post-transplant cytomegalovirus infections. EBioMedicine. (2015) 2:699–705. doi: 10.1016/j.ebiom.2015.05.003
24. Navarro D, San-Juan R, Manuel O, Giménez E, Fernández-Ruiz M, Hirsch HH, et al. Cytomegalovirus infection management in solid organ transplant recipients across European centers in the time of molecular diagnostics: An ESGICH survey. Transpl Infect Dis. (2017) 19. doi: 10.1111/tid.12773
25. Ruiz-Arabi E, Torre-Cisneros J, Aguilera V, Alonso R, Berenguer M, Bestard O, et al. Management of cytomegalovirus in adult solid organ transplant patients: GESITRA-IC-SEIMC, CIBERINFEC, and SET recommendations update. Transplant Rev (Orlando). (2024) 38:100875. doi: 10.1016/j.trre.2024.100875
26. Kotton CN, Kumar D, Manuel O, Chou S, Hayden RT, Danziger-Isakov L, et al. The fourth international consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. (2025) 109(7):1066–110. doi: 10.1097/TP.0000000000005374
27. Preiksaitis JK, Brennan DC, Fishman J, and Allen U. Canadian society of transplantation consensus workshop on cytomegalovirus management in solid organ transplantation final report. Am J Transpl. (2005) 5:218–27. doi: 10.1111/j.1600-6143.2004.00692.x
28. Suresh S, Lee BE, Robinson JL, Akinwumi MS, and Preiksaitis JK. A risk-stratified approach to cytomegalovirus prevention in pediatric solid organ transplant recipients. Pediatr Transpl. (2016) 20:970–80. doi: 10.1111/petr.12786
29. National Cancer Institute, Common Terminology Criteria etc. Common terminology criteria for adverse events (CTCAE)(2010). Available online at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_4.03.xlsx (Accessed December 27, 2024).
30. Ljungman P, Chemaly RF, Khawaya F, Alain S, Avery R, Badshah C, et al. Consensus definitions of cytomegalovirus (CMV) infection and disease in transplant patients including resistant and refractory CMV for use in clinical trials: 2024 update from the transplant associated virus infections forum. Clin Infect Dis. (2024) 79:787–94. doi: 10.1093/cid/ciae321
31. Kotton CN and Kamar N. New insights on CMV management in solid organ transplant patients: prevention, treatment, and management of resistant/refractory disease. Infect Dis Ther. (2023) 12:333–42. doi: 10.1007/s40121-022-00746-1
32. Jenner AL, Aogo RA, Davis CL, Smith AM, and Craig M. Leveraging computational modeling to understand infectious diseases. Curr Pathobiol Rep. (2020) 8:149–61. doi: 10.1007/s40139-020-00213-x
33. Fojo AT, Kendall EA, Kasaie P, Shrestha S, Louis TA, and Dowdy DW. Mathematical modeling of “Chronic” Infectious diseases: unpacking the black box. Open Forum Infect Dis. (2017) 4:ofx172. doi: 10.1093/ofid/ofx172
34. Dobrer S, Sherwood KR, Hirji I, Lan J, Gill J, Matic N, et al. Viral load kinetics and the clinical consequences of cytomegalovirus in kidney transplantation. Front Immunol. (2023) 14:1302627. doi: 10.3389/fimmu.2023.1302627
35. Griffiths P and Reeves M. Pathogenesis of human cytomegalovirus in the immunocompromised host. Nat Rev Microbiol. (2021) 19:759–73. doi: 10.1038/s41579-021-00582-z
36. Zuhair M, Smit GSA, Wallis G, Jabbar F, Smith C, Devleesschauwer B, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev Med Virol. (2019) 29:e2034. doi: 10.1002/rmv.2034
37. Berry R, Watson GM, Jonjic S, Degli-Esposti MA, and Rossjohn J. Modulation of innate and adaptive immunity by cytomegaloviruses. Nat Rev Immunol. (2020) 20:113–27. doi: 10.1038/s41577-019-0225-5
38. Kotton CN, Torre-Cisneros J, Aguado JM, Alain S, Baldanti F, Baumann G, et al. Cytomegalovirus in the transplant setting: Where are we now and what happens next? A report from the International CMV Symposium 2021. Transpl Infect Dis. (2022) 24:e13977. doi: 10.1111/tid.13977
39. Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. (2017) 64:87–91. doi: 10.1093/cid/ciw668
40. Belga S, MacDonald C, Chiang D, Kabbani D, Shojai S, Abraldes JG, et al. Donor graft cytomegalovirus serostatus and the risk of arterial and venous thrombotic events in seronegative recipients after non-thoracic solid organ transplantation. Clin Infect Dis. (2021) 72:845–52. doi: 10.1093/cid/ciaa125
41. Ishikawa S, Tasaki M, Saito K, Nakagawa Y, Ikeda M, Takahashi K, et al. Long-term CMV monitoring and chronic rejection in renal transplant recipients. Front Cell Infect Microbiol. (2023) 13:1190794. doi: 10.3389/fcimb.2023.1190794
42. Rodríguez-Goncer I, Corbella L, Lora D, Redondo N, López-Medrano F, Gutiérrez E, et al. Role of cytomegalovirus infection after kidney transplantation on the subsequent risk of atherosclerotic and thrombotic events. Atheroscler Plus. (2022) 48:37–46. doi: 10.1016/j.athplu.2022.03.003
43. Belga S, Hernandez C, Kabbani D, and Cervera C. Incidence of valganciclovir-related leukopenia and neutropenia in solid organ transplant recipients at high risk of cytomegalovirus disease. Transpl Infect Dis. (2024) 26:e14227. doi: 10.1111/tid.14227
44. Kotton CN, Torre-Cisneros J, Yakoub-Agha I, and Faculty CIS. Slaying the “Troll of Transplantation”-new frontiers in cytomegalovirus management: A report from the CMV International Symposium 2023. Transpl Infect Dis. (2024) 26:e14183. doi: 10.1111/tid.14183
45. Vernooij RW, Michael M, Ladhani M, Webster AC, Strippoli GF, Craig JC, et al. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev. (2024) 5:CD003774. doi: 10.1002/14651858.CD005133.pub4
46. Grossi PA, Kamar N, Saliba F, Baldanti F, Aguado JM, Gottlieb J, et al. Cytomegalovirus management in solid organ transplant recipients: A pre-COVID-19 survey from the working group of the European society for organ transplantation. Transpl Int. (2022) 35:10332. doi: 10.3389/ti.2022.10332
47. Kotton CN. CMV: prevention, diagnosis and therapy. Am J Transpl. (2013) 13 Suppl 3:24–40; quiz. doi: 10.1111/ajt.12006
48. Dmitrienko S, Yu A, Balshaw R, Shapiro R, and Keown P. The use of consensus guidelines for management of cytomegalovirus infection in renal transplantation. Kidney Int. (2007) 72:1014–22. doi: 10.1038/sj.ki.5002464
49. Chung MC, Chen CH, Chang SS, Lee CY, Tian YC, Wu MY, et al. Prevention and management of cytomegalovirus infection and disease in kidney transplant: A consensus statement of the Transplantation Society of Taiwan. J Formos Med Assoc. (2024) 124:104–11. doi: 10.1016/j.jfma.2024.05.009
50. van der Beek MT, Berger SP, Vossen AC, van der Blij-de Brouwer CS, Press RR, de Fijter JW, et al. Preemptive versus sequential prophylactic-preemptive treatment regimens for cytomegalovirus in renal transplantation: comparison of treatment failure and antiviral resistance. Transplantation. (2010) 89:320–6. doi: 10.1097/TP.0b013e3181bc0301
51. Boillat Blanco N, Pascual M, Venetz JP, Nseir G, Meylan PR, and Manuel O. Impact of a preemptive strategy after 3 months of valganciclovir cytomegalovirus prophylaxis in kidney transplant recipients. Transplantation. (2011) 91:251–5. doi: 10.1097/TP.0b013e318200b9f0
52. Raval AD, Kistler KD, Tang Y, and Vincenti F. Burden of neutropenia and leukopenia among adult kidney transplant recipients: A systematic literature review of observational studies. Transpl Infect Dis. (2023) 25:e14000. doi: 10.1111/tid.14000
53. Oreschak K, Saba LM, Rafaels N, Ambardekar AV, Deininger KM, PageII R, et al. Variants in mycophenolate and CMV antiviral drug pharmacokinetic and pharmacodynamic genes and leukopenia in heart transplant recipients. J Heart Lung Transpl. (2021) 40:917–25. doi: 10.1016/j.healun.2021.05.020
54. Baradaran H, Hashem Zadeh A, Dashti-Khavidaki S, and Laki B. Management of drug-induced neutropenia, thrombocytopenia, and anaemia after solid organ transplantation: A comprehensive review. J Clin Pharm Ther. (2022) 47:1895–912. doi: 10.1111/jcpt.13775
55. Rolling KE, Jorgenson MR, Descourouez JL, Mandelbrot DA, Redfield RR, and Smith JA. Ganciclovir-resistant cytomegalovirus infection in abdominal solid organ transplant recipients: case series and review of the literature. Pharmacotherapy. (2017) 37:1258–71. doi: 10.1002/phar.1987
56. Stevens DR, Sawinski D, Blumberg E, Galanakis N, Bloom RD, and Trofe-Clark J. Increased risk of breakthrough infection among cytomegalovirus donor-positive/recipient-negative kidney transplant recipients receiving lower-dose valganciclovir prophylaxis. Transpl Infect Dis. (2015) 17:163–73. doi: 10.1111/tid.12349
57. Zafrani L, Truffaut L, Kreis H, Etienne D, Rafat C, Lechaton S, et al. Incidence, risk factors and clinical consequences of neutropenia following kidney transplantation: a retrospective study. Am J Transpl. (2009) 9:1816–25. doi: 10.1111/j.1600-6143.2009.02699.x
58. Sandal S, Yao H, Alam A, Arienzo D, Baran D, and Cantarovich M. Granulocyte colony-stimulating factor with or without immunosuppression reduction in neutropenic kidney transplant recipients. Clin Transpl. (2022) 36:e14766. doi: 10.1111/ctr.14766
59. Raglow Z and Kaul DR. A new antiviral option for cytomegalovirus prevention after kidney transplant. JAMA. (2023) 330:27–9. doi: 10.1001/jama.2023.9100
60. Limaye AP, Budde K, Humar A, Vincenti F, Kuypers DRJ, Carroll RP, et al. Letermovir vs valganciclovir for prophylaxis of cytomegalovirus in high-risk kidney transplant recipients: A randomized clinical trial. JAMA. (2023) 330:33–42. doi: 10.1001/jama.2023.9106
61. Linder KA, Kovacs C, Mullane KM, Wolfe C, Clark NM, La Hoz RM, et al. Letermovir treatment of cytomegalovirus infection or disease in solid organ and hematopoietic cell transplant recipients. Transpl Infect Dis. (2021) 23:e13687. doi: 10.1111/tid.13687
62. Vyas A, Raval AD, Kamat S, LaPlante K, Tang Y, and Chemaly RF. Real-world outcomes associated with letermovir use for cytomegalovirus primary prophylaxis in allogeneic hematopoietic cell transplant recipients: A systematic review and meta-analysis of observational studies. Open Forum Infect Dis. (2023) 10:ofac687. doi: 10.1093/ofid/ofac687
63. Agrawal A, Ison MG, and Danziger-Isakov L. Long-term infectious complications of kidney transplantation. Clin J Am Soc Nephrol. (2022) 17:286–95. doi: 10.2215/CJN.15971020
64. CORR. Canadian Organ Replacement Register (CORR). Ottawa, Canada: Canadian Institute for Health Information (2024). Available online at: https://www.cihi.ca/en/canadian-organ-replacement-register-corr (Accessed December 27, 2024).
65. CBS. Canadian Blood Services. Ottawa, Canada: Canadian Blood Services (2017). Available online at: https://blood.ca/en (Accessed December 27, 2024).
Keywords: cytomegalovirus, kidney transplantation, antiviral prophylaxis and treatment, immunosuppression, superinfection, leukopenia, hospitalization, graft failure
Citation: Gill J, House AA, Chagla Z, Tchervenkov J, Kim SJ, Vinson A, Cervera C, Keown PA, Sun SLW, Khoury C and Ghakis C (2025) Clinical management and burden of cytomegalovirus in D+/R-Kidney transplant recipients in Canada. Front. Immunol. 16:1618748. doi: 10.3389/fimmu.2025.1618748
Received: 26 April 2025; Accepted: 17 July 2025;
Published: 18 September 2025; Corrected: 14 October 2025.
Edited by:
Stanislaw Stepkowski, University of Toledo, United StatesReviewed by:
Elisa Ruiz-Arabi, Reina Sofia University Hospital, SpainSrijana Jonchhe, New York University, United States
Carla De Castro Sant’ Anna, Universidade da Amazônia, Brazil
Copyright © 2025 Gill, House, Chagla, Tchervenkov, Kim, Vinson, Cervera, Keown, Sun, Khoury and Ghakis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christiane Ghakis, Q2hyaXN0aWFuZS5HaGFraXNAbXNkLmNvbQ==
 John Gill1
John Gill1 Carlos Cervera
Carlos Cervera Paul A. Keown
Paul A. Keown Christiane Ghakis
Christiane Ghakis