- 1Department of Pancreatobiliary Surgery, State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, China
- 2Department of Anesthesiology, State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, China
- 3Department of Nuclear Medicine, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: Irreversible electroporation (IRE) has shown promise in improving survival outcomes and activating the immune response in patients with locally advanced pancreatic cancer (LAPC). Given these immune-enhancing effects, we hypothesized that combining IRE with immune checkpoint inhibitors may further improve treatment outcomes. This study aimed to evaluate the efficacy and safety of IRE combined with anti-PD-1 immunotherapy versus IRE alone in patients with LAPC.
Methods: In this retrospective study, LAPC patients treated either with IRE plus toripalimab (240 mg administered 7 days post-IRE) or with IRE alone were included. Propensity score matching (PSM) analyses were employed for analysis. Clinical outcomes including overall survival (OS), progression-free survival (PFS), and treatment-related adverse events were analyzed and compared between the groups.
Results: A total of 108 patients from August 2015 and Match 2024 from SYSUCC cohort were identified with 76 undergoing IRE and 32 undergoing IRE and toripalimab in this study. After PSM, 96 patients consisting of 64 and 32 patients in the IRE and combination groups were enrolled. Clinical factors were all balanced between two groups. Patients receiving IRE combined with toripalimab showed significantly improved OS (35.03 months; 95% CI: 30.94-39.13 vs. 15.87 months; 95% CI: 8.99-22.74; P=0.014) and PFS (14.33months; 95% CI: 11.19-17.47 vs. 7.47 months; 95% CI: 3.86-11.08; P=0.022) compared to those receiving IRE alone. No treatment-related mortality was reported in either group and no statistically significant differences were observed in terms of complications and adverse events between two groups (all P>0.05).
Conclusions: The combination of IRE and anti-PD-1 immunotherapy was associated with improved survival outcomes and acceptable safety profiles compared to IRE alone in patients with LAPC. Further investigation through prospective trials is warranted.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive gastrointestinal malignancy characterized by rising incidence and a substantial impact on cancer-related mortality globally (1). Approximately 40% of PDAC cases present as locally advanced pancreatic cancer (LAPC), defined by the involvement of major vascular structures, resulting in unresectable yet non-metastatic disease. Despite current treatment modalities, LAPC prognosis remains poor, with a median survival around 12 months (2). Identifying an optimal therapeutic strategy for LAPC remains a significant clinical challenge. Chemotherapy has expanded treatment options, including the potential for tumor downstaging and subsequent surgical resection. Although some LAPC patients benefit from extended surgical resection following chemotherapy, the rates of successful conversion surgery vary widely (0%–43%), influenced by factors such as chemotherapy regimens, tumor heterogeneity, and surgical techniques (3). Additionally, extended surgeries are associated with relatively high postoperative complication rates, potentially diminishing survival benefits (4). Given that mortality in LAPC patients is primarily driven by local tumor progression rather than distant metastasis, local ablative therapies represent a valuable therapeutic avenue (5).
Local ablative therapies have emerged as important adjunctive treatments for LAPC. Nevertheless, conventional thermal ablative methods, such as radiofrequency ablation (RFA) and microwave ablation, are restricted due to potential thermal injury to nearby organs and vessels (6, 7). Irreversible electroporation (IRE), a non-thermal ablative technique, induces apoptosis through permeabilization of the tumor cell membranes by applying short, high-voltage electrical pulses (8). Notably, IRE’s independence from the heat sink effect makes it particularly suitable for LAPC compared to thermal methods. Furthermore, the vascular preservation associated with IRE facilitates the transport of immune cells and molecules, enhancing its immunological responsiveness relative to thermal ablation techniques.
Beyond direct tumor cell apoptosis, IRE has been demonstrated to remodel the tumor microenvironment (TME) and stimulate immune responses (9, 10). Prior research indicates that IRE reduces immune suppression and enhances T-cell activation, suggesting its potential to augment immunotherapy efficacy in PDAC (11, 12).
In recent years, immune checkpoint inhibitors (ICIs) have significantly advanced treatment outcomes in cancers such as melanoma, lung, and liver cancers (13–15). However, the therapeutic benefits of ICIs in PDAC remain limited, potentially due to low programmed cell death protein 1 (PD-1) expression, low mutational burden, limited T-cell infiltration, and increased regulatory T-cell (Treg) accumulation (16, 17). Efforts have thus focused on combination therapies aimed at modifying the immunosuppressive TME to improve ICI responsiveness.
IRE has demonstrated the ability to induce immunogenic cell death (ICD), enhancing effector CD8+ T-cell infiltration (9). Moreover, it facilitates antigen presentation by encouraging dendritic cell maturation and promoting M1 macrophage polarization (10, 18). These properties position IRE as a promising adjunct therapy capable of transforming the immunologically “cold” TME into a “hot” environment, thereby improving ICI responsiveness. Indeed, preclinical studies combining IRE and anti-PD-1 therapy have shown increased selective infiltration of CD8+ T cells and significantly prolonged survival in Kras-induced pancreatic cancer (KPC) models (19). Additionally, previous studies based on small cohorts had shown that the combination of IRE and anti-PD-1 therapy provided encouraging survival results for LAPC (20, 21). Despite these promising results, the clinical benefit of combining IRE with anti-PD-1 therapy based on relatively large cohorts with long time follow-up in LAPC remains necessary. Therefore, this study was designed to evaluate the clinical outcomes and survival of LAPC patients undergoing combined IRE and anti-PD-1 therapy, aiming to validate the potential therapeutic benefits observed in preclinical settings.
Methods
Patients
This retrospective study adhered to the ethical guidelines established by the 1964 Helsinki Declaration and received approval from the Institutional Review Board of Sun Yat-sen University Cancer Center (SYSUCC). Informed consent was obtained from all participants before initiating treatment. Eligible patients were identified through electronic medical records based on these inclusion criteria: (1) histologically confirmed pancreatic adenocarcinoma with radiologically confirmed locally advanced pancreatic cancer (LAPC), defined according to the seventh edition of the AJCC staging system, which includes arterial involvement of the celiac axis or superior mesenteric artery, or unreconstructable involvement of the superior mesenteric or portal vein without metastatic disease confirmed by abdominal and thoracic computed tomography (CT) (22); (2) Eastern Cooperative Oncology Group (ECOG) performance status score of 0–2. Patients who were lost to follow-up or had incomplete information of follow-up were excluded from this study.
Treatment procedure
The procedure for IRE followed previously reported methods (23). Two to six probes were positioned around the tumor based on its dimensions to establish an electric field, resulting in nanoscale pores in tumor cell membranes. The generator software optimized probe placement based on ultrasound data, specifying appropriate voltage and pulse duration. Standard settings included an initial voltage of 1500 V/cm with 90 pulses at pulse durations of 70–90 ms.
Chemotherapy is the standard treatment for LAPC according to the guideline of National Comprehensive Cancer Network (NCCN) and it was adopted for all patients in this study. Patients received induction and adjuvant chemotherapy with the FOLFIRINOX (a combination of folinic acid, 5-fluorouracil, irinotecan, and oxaliplatin), gemcitabine with nab-paclitaxel (AG) or S-1 (Tegafur, Gimeracil, and Oteracil Potassium Capsules) regimen for 4 months as previously described (24, 25). On the base of the standard care (chemotherapy), the immunotherapy was recommended for part of patients according to doctors’ experience. Written informed consent of immunotherapy was obtained from these patients. In these patients, anti-PD-1 therapy (Toripalimab 240 mg) was initiated one week post-IRE and administered every three weeks thereafter. Patients who had received IRE treatment were included in the IRE group. Those who had received IRE combined with anti-PD-1 therapy were included in combination group.
Data collection
Patient data, including clinical and radiological information, were retrospectively extracted from medical records. Collected data included demographics (age, gender), tumor characteristics (size, grade, location), laboratory parameters (white blood cell count, platelet count, alanine transaminase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transpeptidase, albumin, total bilirubin, indirect bilirubin, C-reactive protein, hepatitis B surface antigen, carcinoembryonic antigen, carbohydrate antigen 19-9), and chemotherapy regimens. Primary endpoints of the study were overall survival (OS) and progression-free survival (PFS), calculated from the date of diagnosis until death from any cause, disease progression, or last follow-up. Follow-up concluded on Match 30, 2025.
Statistical analysis
Propensity score matching (PSM) analysis at a ratio of 1:2 was used to minimize selection bias and balance variables. Propensity scores for all patients were estimated by a logistic regression model using the following characteristics as covariates: age, gender, tumor size, imaging LN metastasis, response to neoadjuvant chemotherapy, adjuvant chemotherapy, CA19–9 and CA12-5. A one-to-one nearest-neighbor matching algorithm with an optimal of 0.2 without replacement was used. Categorical variables were compared using the chi-square test or Fisher’s exact test and reported as frequencies and percentages. Variables significantly correlated with OS in univariate analysis were entered into multivariate Cox regression to identify independent predictors, expressed with 95% confidence intervals (CI). OS and PFS curves were analyzed using the Kaplan-Meier method, and differences between the groups were identified using the log-rank test.
Analyses for survival curves were performed using MedCalc software version 11.4.2.0 (MedCalc, Ostend, Belgium). All statistical analyses were conducted with R software version 3.4.2 (The R Foundation for Statistical Computing, Vienna, Austria). A two-tailed P-value of <0.05 was deemed statistically significant.
Results
Patient characteristics
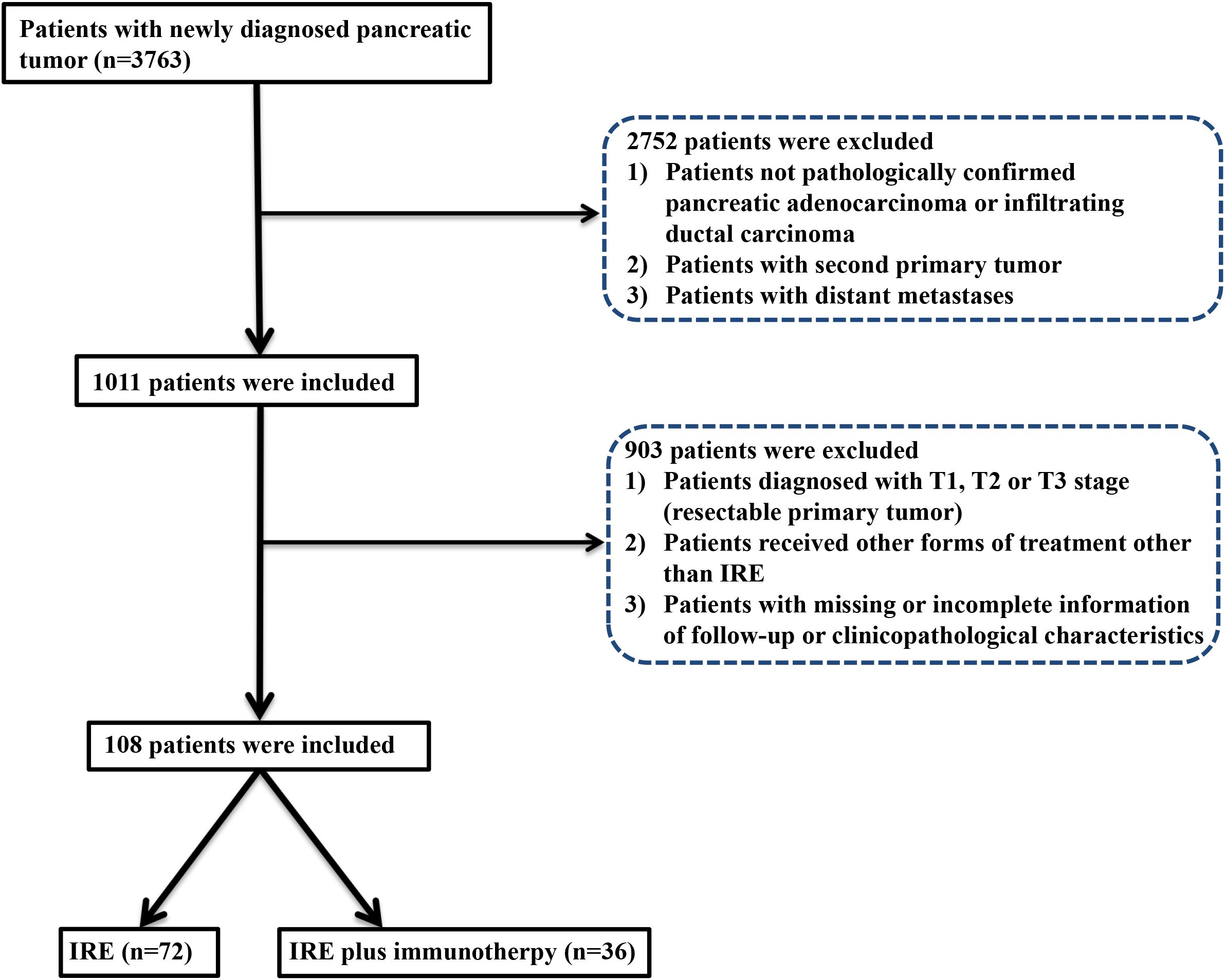
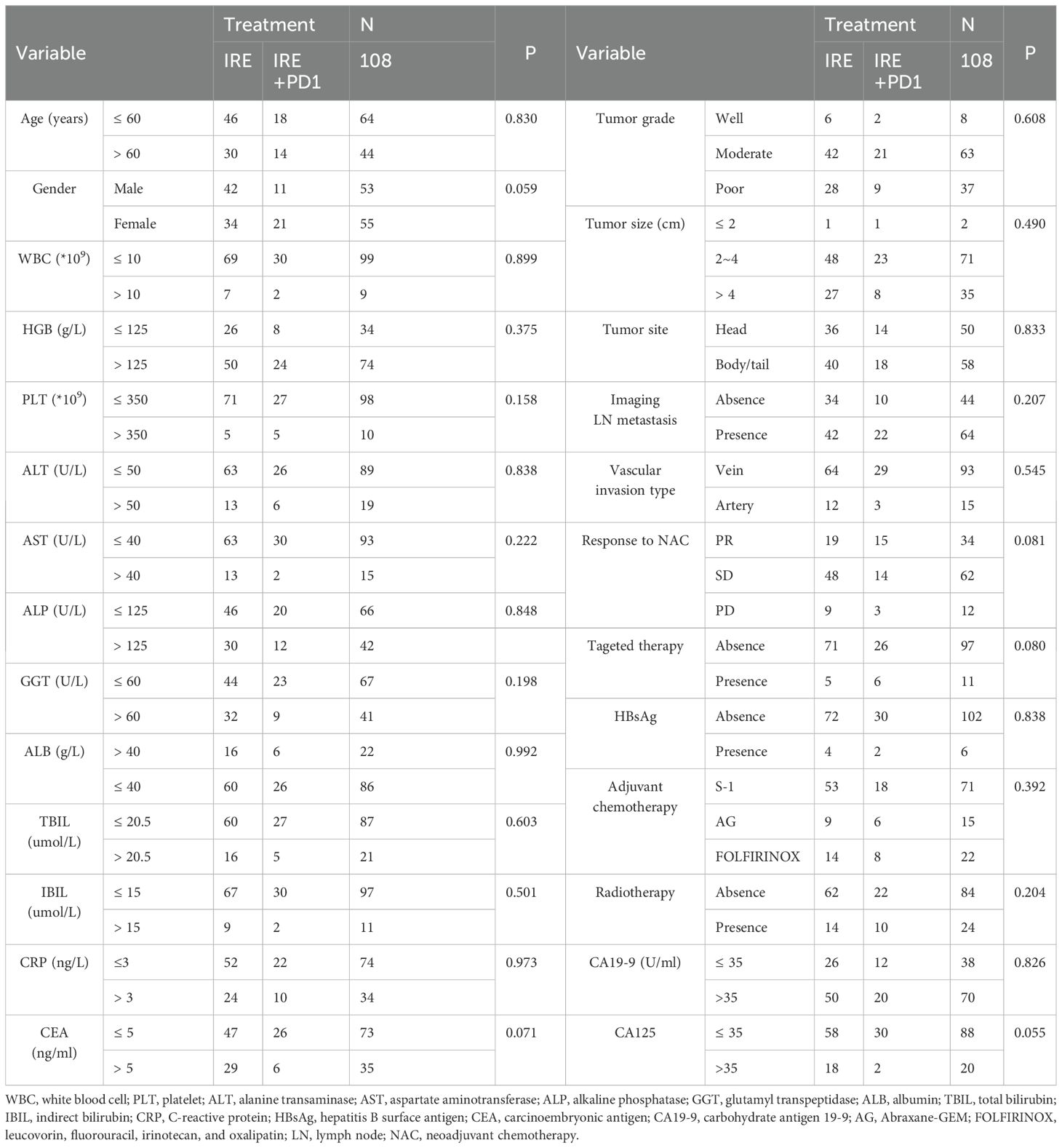
From August 2015 to Match 2023, a total of 108 eligible patients were enrolled: 32 received combined therapy with irreversible electroporation (IRE) and toripalimab (anti-PD-1 therapy), and 76 received IRE alone. A total of 21 patients with missing or incomplete information of follow-up or clinicopathological characteristics were excluded in this study (Figure 1). For patients included in this study, chemotherapy for a total of four months were adopted before IRE. Baseline clinical and pathological characteristics were balanced between groups. Nearly balanced distribution of gender was observed in the whole group. The median age was 57.5 years (range, 19–87 years), specifically 57.8 years (range, 40–76 years) for the combined treatment group and 57.3 years (range, 19–87 years) for the IRE-alone group. Tumors shared similar characteristics between two groups, including tumor size, grade, site and vascular invasion. Additionally, adjuvant therapy, such as radiotherapy, targeted therapy and chemotherapy were also similar between these two groups (Table 1). PSM was further applied to minimize the selection bias at a caliper score of 0.1 and match ratio of 2:1. After PSM, there were 32 and 64 patients in the combined treatment therapy and IRE group, respectively. No differences in the baseline characteristics after PSM across groups were observed (Supplementary Table S1).
Survival analysis
With a median follow-up of 26.5 months and the longest follow-up of 69.2 months, the median overall survival (OS) for the entire cohort was 20.93 months (95% CI: 11.38-30.49 months), and median progression-free survival (PFS) was 11.70 months (95% CI: 9.68–13.72 months). The median survival time (MST) of OS was significantly longer in the combined treatment group (35.03 months, 95% CI: 30.94–39.13 months) compared to the IRE-alone group (15.77 months, 95% CI: 10.23–21.31 months). The 1-, 2-, and 3-year OS rates were 83.7%, 67.6%, and 34.2%, respectively, in the combined group versus 63.1%, 38.1%, and 27.4% in the IRE-alone group (P=0.008, Figure 2A). The MST of PFS was also significantly improved in the combined group (14.33 months, 95% CI: 11.19-17.43 months) compared to the IRE-alone group (8.53 months, 95% CI: 4.05–13.02 months). One- and two-year PFS rates were 61.3% and 29.3% for the combined group versus 41.2% and 18.8% for the IRE-alone group (P=0.024, Figure 2B). After PSM, significantly higher OS and PFS rates were also observed in the combined treatment group, compared with IRE group [OS: MST, 35.03 months (95% CI: 11.38-30.49 months) vs. 15.87 months (95% CI: 8.99-22.74 months), P=0.014, Figure 2C; PFS: MST, 14.33 months (95% CI: 11.19-17.47 months) vs. 7.47 months (95% CI: 3.86-11.08 months), P=0.022, Figure 2D].
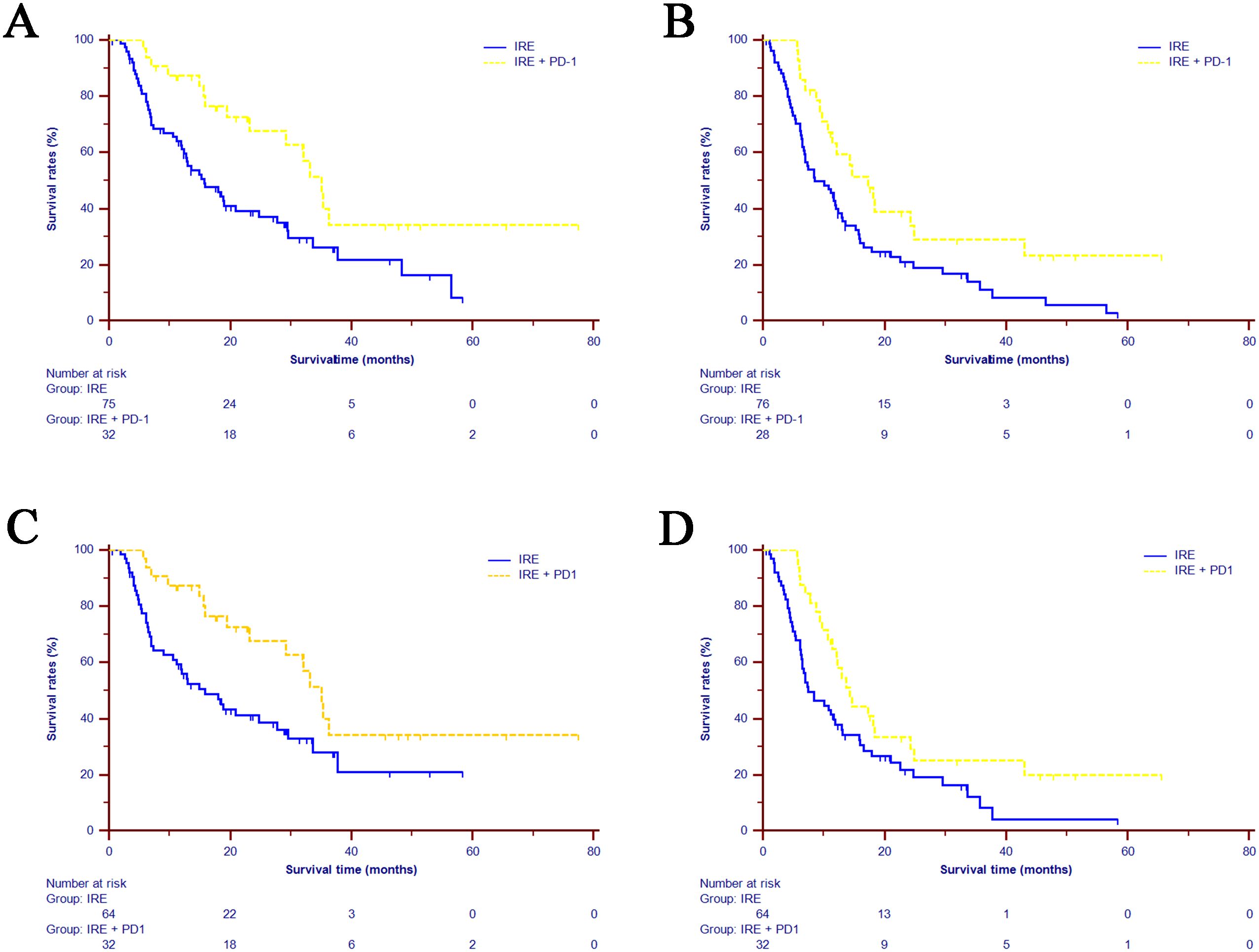
Figure 2. Kaplan-Meier estimates of survival for the LAPC patients underwent IRE and combined treatment (IRE+PD-1). (A) OS comparison in the whole cohort. (B) PFS comparison in the whole cohort. (C) OS comparison in the matched cohort. (D) PFS comparison in the matched cohort. LAPC=locally advanced pancreatic cancer; IRE=irreversible electroporation; OS=overall survival; PFS=progression-free survival.
Prognostic factors for OS and PFS
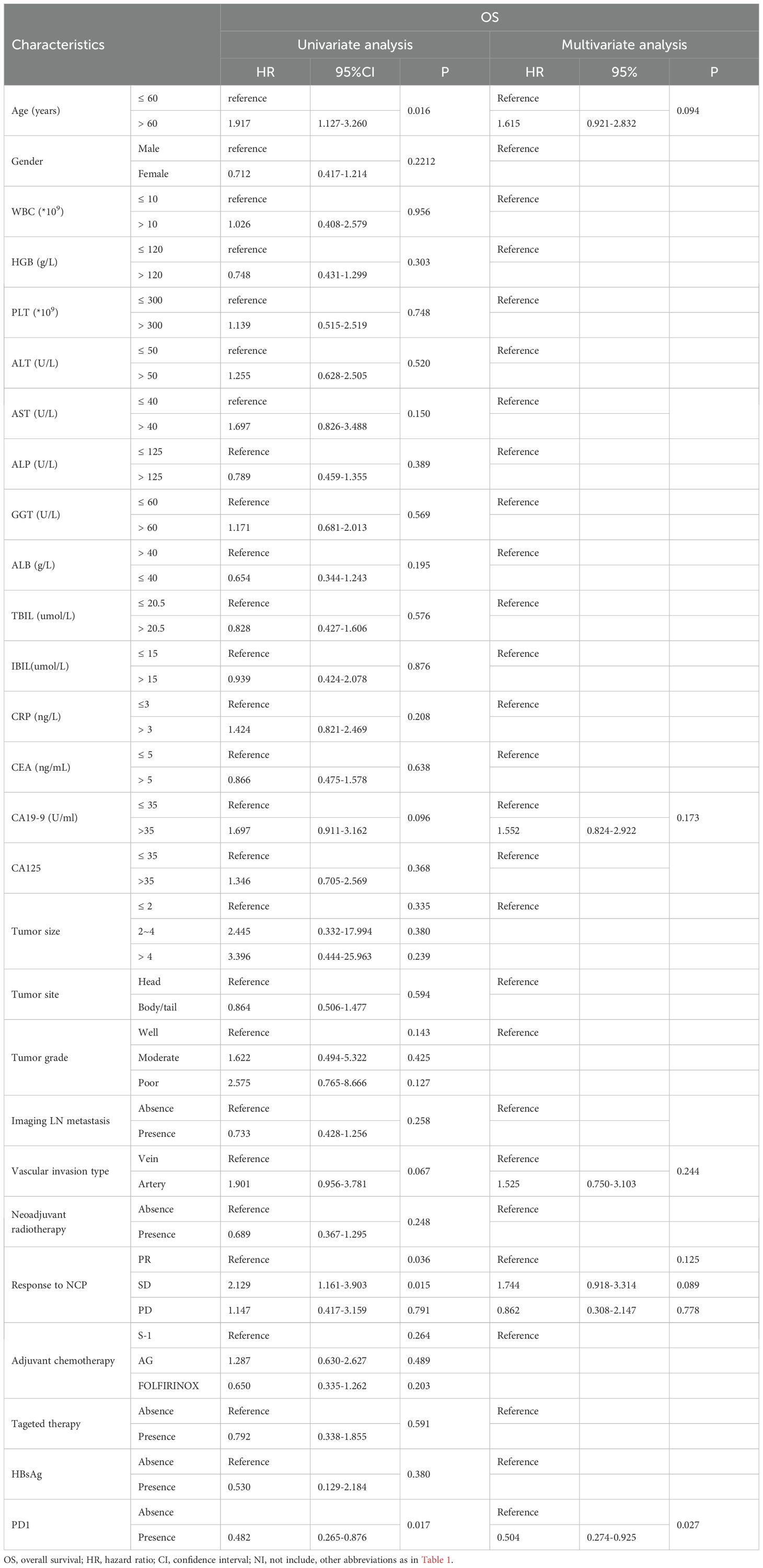
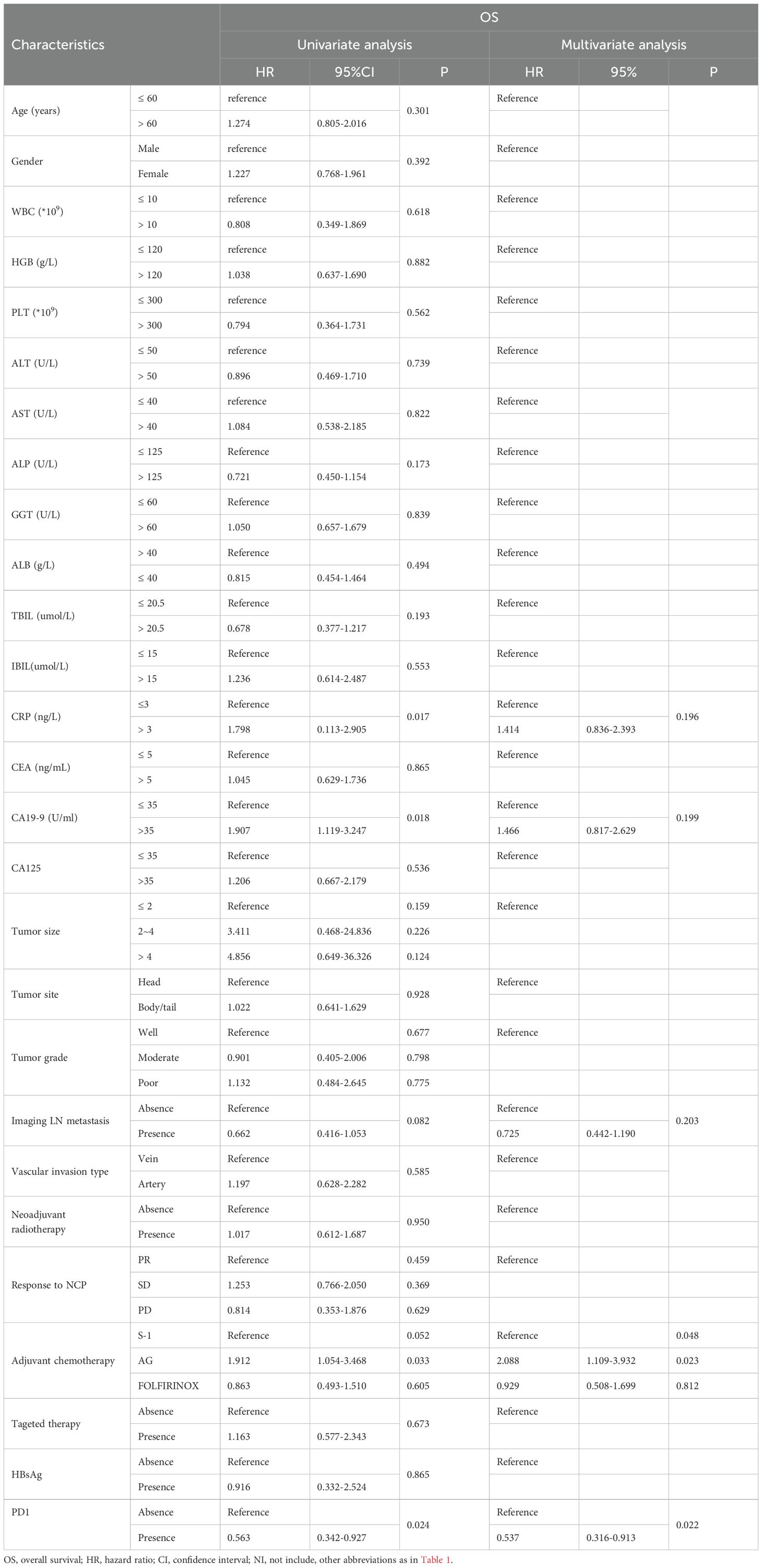
To identify risk factors for OS and PFS, all clinical and pathological factors were included and analyzed using Cox regression analysis. The results showed that anti-PD1 therapy, age, vascular invasion type, tumor response after neoadjuvant chemotherapy, AST and tumor grade were associated with OS. Further multivariate analysis revealed that age older than 60 years old (HR=1.816, 95% CI 1.056–3.124, P=0.031) predicted poorer OS compared to those with younger ages. Additionally, tumor of poor differentiation (HR=4.735, 95% CI 1.312-17.088, P=0.018) and arterial invasion (HR=2.324, 95% CI 1.166-4.633, P=0.017) were associated with worse survival, while anti-PD1 therapy (HR=0.497, 95% CI 0.269–0.917, P=0.025) was likely to prolong OS (Supplementary Table S2). After PSM, anti-PD1 therapy (HR=0.504, 95% CI 0.274–0.925, P=0.027) was significant prognostic factors for OS (Table 2). For PFS, multivariate analysis indicated that adjuvant chemotherapy regimen (AG vs. S-1, HR=2.216, 95% CI 1.190–4.129, P=0.012) and anti-PD1 therapy (HR=0.537, 95% CI 0.318–0.909, P=0.020) were significant predictive factors for PFS in patients with LAPC (Supplementary Table S3). In the matched cohorts after PSM, these two factors were also identified as prognostic factors for PFS (Table 3).
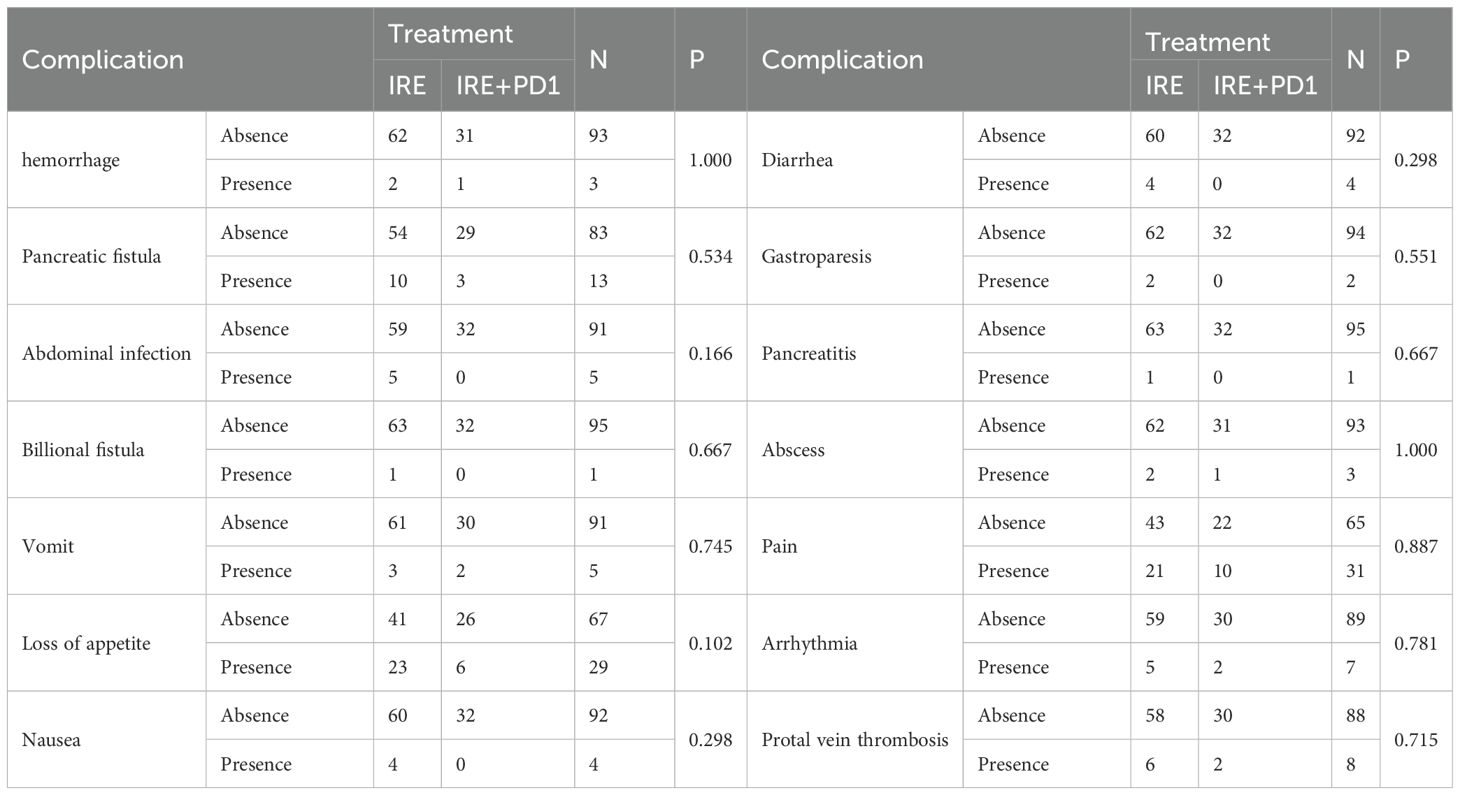
Comparisons of complications and adverse events between two groups
Complications were compared and no significant differences were identified between two groups in both of whole (Supplementary Table S4) and matched cohorts (Table 4). No treatment-related deaths occurred. In terms of surgery-related complications, similar probabilities of hemorrhage, pancreatic fistula, biliary fistula, abdominal infection, Pancreatitis, abscess, pain, cardiac arrhythmias, gastroparesis, and portal vein thrombosis were observed. Additionally, differences of incidences of immune-related adverse events, including loss of appetite, nausea, vomiting, and diarrhea were not statistically significant (all P>0.05).
Discussion
Minimally invasive local techniques such as endoscopic ultrasound-guided radiofrequency ablation (EUS-RFA) (26), high intensity focused ultrasound (HIFU) (27) and IRE have emerged as potential therapeutic options for pancreatic neoplastic lesions. Additionally, the feature of free from the heat sink effect makes IRE more appropriate in the treatment of LAPC. As a non-thermal ablation technique, irreversible IRE induces tumor cell apoptosis by irreversibly permeabilizing the cell membrane (8). Increasing clinical evidence supports the safety and efficacy of IRE in LAPC treatment (6, 25). Our previous studies further demonstrated that IRE combined with chemotherapy significantly improved patient survival compared to conventional treatments alone, including chemotherapy or conversion surgery (24, 28). These findings suggest that IRE plays a crucial role in LAPC management, and combining IRE with other modalities might further enhance therapeutic outcomes.
In recent years, breakthroughs in immunotherapies, particularly ICI and chimeric antigen receptor T-cell (CAR-T) therapies, have revolutionized cancer treatment (29, 30). However, the TME of PDAC often limits the effectiveness of immune therapies (31). Emerging studies, including our previous research, have shown that IRE not only destroys tumor cells but also modulates the local immune environment by promoting M1 macrophage polarization and increasing infiltration of tumor-specific T cells (9, 10, 19). This suggests that IRE could enhance immune responsiveness through increased antigen release and improved immune cell infiltration. Additionally, the low expression of anti-PD-1 therapy in PDAC may contribute to resistance against ICIs, but IRE-induced up-regulation of PD-1 expression on T cells might help overcome this barrier (32).
Building upon these observations, we hypothesized that IRE could enhance sensitivity to ICIs in LAPC, a concept supported by previous experimental studies demonstrating improved immunotherapy efficacy when combined with IRE (19). However, clinical validation of this combination has been lacking. To address this gap, we developed a novel treatment regimen involving IRE and chemotherapy followed by systemic administration of toripalimab, an anti-PD-1 antibody. In this study, we observed that patients receiving IRE combined with chemotherapy and toripalimab exhibited significantly improved immune profiles, tumor control, and survival compared with those undergoing IRE alone. Remarkably, median overall survival in the combination group reached near three years, highlighting its potential as an effective therapeutic strategy for LAPC.
The enhanced immune activity could contribute to the significantly elevated efficacy of combination therapy. In our previous studies, It was found that notable increases in circulating CD4+ helper T cells and CD8+ cytotoxic T cells, alongside decreases in immunosuppressive CD8+ regulatory T cells following combined therapy. Furthermore, significant elevations of cytokines such as IL-4, IL-6, IL-10, TNF, and IFN-γ were observed, reflecting a robust antitumor immune response. Elevated TNF and IFN-γ, primarily secreted by activated CD8+ T cells, indicate enhanced specific immune-mediated tumor killing. TNF-α also promotes M1 macrophage polarization, facilitating antigen presentation and immune activation via additional cytokines such as IL-4, IL-6, and IL-10 (21). These immunological changes likely underpin the improved survival outcomes observed with combined treatment.
Our study confirmed the synergistic benefit of adding anti-PD-1 therapy to IRE in LAPC management without significantly increasing adverse events, corroborating previous safety data (21, 33). Administering toripalimab one week after IRE treatment provided an optimal time window for immune activation and patient recovery, potentially contributing to the low incidence of adverse events.
Despite promising results, our study has several limitations. Firstly, its retrospective, non-randomized design may introduce selection bias, despite the balanced baseline characteristics and the omission of important indices, such as Quality of Life (QoL) assessments. Prospective, randomized controlled trials are necessary to validate our findings. Secondly, study cohort based on single center limits generalizability, emphasizing the need for validation in populations from multiple centers. Further randomized clinical trials with longer follow-up periods are required to confirm the enduring efficacy of this novel combination therapy.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
This study was approved by the Institutional Review Board of Sun Yat-sen University Cancer Center (approve No. G2022-170-01). Written informed consent was obtained from all individual participants included in the study.. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PX: Conceptualization, Data curation, Formal analysis, Methodology, Resources, Writing – original draft. PS: Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft. MC: Formal analysis, Funding acquisition, Investigation, Project administration, Writing – original draft. ZY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft. QZ: Conceptualization, Data curation, Project administration, Writing – original draft. SL: Resources, Supervision, Visualization, Writing – review & editing. CH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Guangdong Basic and Applied Basic Research Foundation (2023A1515010394), Guangzhou Science and Technology Program (2025A04J3552), National Natural Science Funds (No.82102166), Guangdong Basic and Applied Basic Research Foundation (2020A1515110954) and Sun Yat-sen University Grant for Medical Humanities Practice and Teaching (No. 23000-18008023). Science and Technology Bureau Dawning project of Wuhan (2023020201020536), Natural Science Foundation of Hubei Province (2023AFB454) and Research Foundation of Wuhan Central Hospital (22YJ28).
Acknowledgments
The authors thank the members of our research group for useful discussions and Dr. Jibin Li for the statistical consultation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1620988/full#supplementary-material
References
1. Siegel RL, Giaquinto AN, and Jemal A. Cancer statistics, 2024. CA: Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
2. Mukherjee S, Hurt CN, Bridgewater J, Falk S, Cummins S, Wasan H, et al. Radhakrishna G et al: Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol. (2013) 14:317–26. doi: 10.1016/S1470-2045(13)70021-4
3. Gillen S, Schuster T, Meyer Zum Büschenfelde C, Friess H, and Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PloS Med. (2010) 7:e1000267. doi: 10.1371/journal.pmed.1000267
4. Beetz O, Sarisin A, Kaltenborn A, Klempnauer J, Winkler M, and Grannas G. Multivisceral resection for adenocarcinoma of the pancreatic body and tail-a retrospective single-center analysis. World J Surg Oncol. (2020) 18:218. doi: 10.1186/s12957-020-01973-x
5. Peixoto RD, Speers C, McGahan CE, Renouf DJ, Schaeffer DF, and Kennecke HF. Prognostic factors and sites of metastasis in unresectable locally advanced pancreatic cancer. Cancer Med. (2015) 4:1171–7. doi: 10.1002/cam4.459
6. Martin RC 2nd, Kwon D, Chalikonda S, Sellers M, Kotz E, Scoggins C, et al. : Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann Surg. (2015) 262:486–94. doi: 10.1097/SLA.0000000000001441
7. Pezzilli R, Ricci C, Serra C, Casadei R, Monari F, D’Ambra M, et al. The problems of radiofrequency ablation as an approach for advanced unresectable ductal pancreatic carcinoma. Cancers. (2010) 2:1419–31. doi: 10.3390/cancers2031419
8. Al Efishat M, Wolfgang CL, and Weiss MJ. Stage III pancreatic cancer and the role of irreversible electroporation. BMJ (Clinical Res ed). (2015) 350:h521. doi: 10.1136/bmj.h521
9. He C, Huang X, Zhang Y, Lin X, and Li S. T-cell activation and immune memory enhancement induced by irreversible electroporation in pancreatic cancer. Clin Trans Med. (2020) 10:e39. doi: 10.1002/ctm2.39
10. He C, Sun S, Zhang Y, Xie F, and Li S. The role of irreversible electroporation in promoting M1 macrophage polarization via regulating the HMGB1-RAGE-MAPK axis in pancreatic cancer. Oncoimmunology. (2021) 10:1897295. doi: 10.1080/2162402X.2021.1897295
11. Imran KM, Brock RM, Beitel-White N, Powar M, Orr K, Aycock KN, et al. Tintera B et al: Irreversible electroporation promotes a pro-inflammatory tumor microenvironment and anti-tumor immunity in a mouse pancreatic cancer model. Front Immunol. (2024) 15:1352821. doi: 10.3389/fimmu.2024.1352821
12. Geboers B, Timmer F, Vos D, Scheffer H, Bakker J, Ruarus A, et al. Systemic immunomodulation by irreversible electroporation versus stereotactic ablative body radiotherapy in locally advanced pancreatic cancer: the CROSSFIRE trial. J immunotherapy Cancer. (2025) 13(3):e010222. doi: 10.1136/jitc-2024-010222
13. Lee CK, Yoo C, Hong JY, Park SJ, Kim JW, Tai DWM, et al. Real-World Study of Systemic Treatment after First-Line Atezolizumab plus Bevacizumab for Hepatocellular Carcinoma in Asia-Pacific Countries. Liver Cancer. (2025) 14:127–41. doi: 10.1159/000540969
14. Campbell KM, Amouzgar M, Pfeiffer SM, Howes TR, Medina E, Travers M, et al. Prior anti-CTLA-4 therapy impacts molecular characteristics associated with anti-PD-1 response in advanced melanoma. Cancer Cell. (2023) 41:791–806.e794. doi: 10.1016/j.ccell.2023.03.010
15. Zhao Y, He Y, Wang W, Cai Q, Ge F, Chen Z, et al. Efficacy and safety of immune checkpoint inhibitors for individuals with advanced EGFR-mutated non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitors: a systematic review, meta-analysis, and network meta-analysis. Lancet Oncol. (2024) 25:1347–56. doi: 10.1016/S1470-2045(24)00379-6
16. Torphy RJ, Zhu Y, and Schulick RD. Immunotherapy for pancreatic cancer: Barriers and breakthroughs. Ann gastroenterological Surg. (2018) 2:274–81. doi: 10.1002/ags3.12176
17. Henriksen A, Dyhl-Polk A, Chen I, and Nielsen D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat Rev. (2019) 78:17–30. doi: 10.1016/j.ctrv.2019.06.005
18. Yang J, Eresen A, Shangguan J, Ma Q, Yaghmai V, and Zhang Z. Irreversible electroporation ablation overcomes tumor-associated immunosuppression to improve the efficacy of DC vaccination in a mice model of pancreatic cancer. Oncoimmunology. (2021) 10:1875638. doi: 10.1080/2162402X.2021.1875638
19. Zhao J, Wen X, Tian L, Li T, Xu C, Wen X, et al. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat Commun. (2019) 10:899. doi: 10.1038/s41467-019-08782-1
20. Ma Y, Xing Y, Li H, Yuan T, Liang B, Li R, et al. Irreversible electroporation combined with chemotherapy and PD-1/PD-L1 blockade enhanced antitumor immunity for locally advanced pancreatic cancer. Front Immunol. (2023) 14:1193040. doi: 10.3389/fimmu.2023.1193040
21. He C, Sun S, Zhang Y, and Li S. Irreversible electroporation plus anti-PD-1 antibody versus irreversible electroporation alone for patients with locally advanced pancreatic cancer. J Inflammation Res. (2021) 14:4795–807. doi: 10.2147/JIR.S331023
22. Callery MP, Chang KJ, Fishman EK, Talamonti MS, William Traverso L, and Linehan DC. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. (2009) 16:1727–33. doi: 10.1245/s10434-009-0408-6
23. He C, Wang J, Mao Y, Lao X, Chen Y, Li X, et al. Chinese expert consensus on open irreversible electroporation ablation for locally advanced pancreatic cancer (version 2023). J Pancreatology. (2024) 7(4):244–50. doi: 10.1097/JP9.0000000000000176
24. He C, Sun S, Huang X, Zhang Y, Lin X, and Li S. Survival comparison of neoadjuvant chemotherapy followed by irreversible electroporation versus conversional resection for locally advanced pancreatic cancer. Front Oncol. (2020) 10:622318. doi: 10.3389/fonc.2020.622318
25. He C, Huang X, Zhang Y, Cai Z, Lin X, and Li S. Comparison of survival between irreversible electroporation followed by chemotherapy and chemotherapy alone for locally advanced pancreatic cancer. Front Oncol. (2020) 10:6. doi: 10.3389/fonc.2020.00006
26. Armellini E, Facciorusso A, and Crinò SF. Efficacy and safety of endoscopic ultrasound-guided radiofrequency ablation for pancreatic neuroendocrine tumors: A systematic review and metanalysis. Medicina (Kaunas Lithuania). (2023) 59(2):359. doi: 10.3390/medicina59020359
27. Yu L, Liu Y, Li Z, Huang Y, Tu G, Shi Q, et al. A retrospective comparative study on the treatment of non-metastatic pancreatic cancer using high-intensity focused ultrasound versus radical surgery. Int J hyperthermia: Off J Eur Soc Hyperthermic Oncology North Am Hyperthermia Group. (2024) 41:2398557. doi: 10.1080/02656736.2024.2398557
28. He C, Wang J, Zhang Y, Lin X, and Li S. Irreversible electroporation after induction chemotherapy versus chemotherapy alone for patients with locally advanced pancreatic cancer: A propensity score matching analysis. Pancreatology: Off J Int Assoc Pancreatology (IAP). (2020) 20:477–84. doi: 10.1016/j.pan.2020.02.009
29. Xu-Monette ZY, Zhou J, and Young KH. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood. (2018) 131:68–83. doi: 10.1182/blood-2017-07-740993
30. Zhang L, Lin Y, Hu L, Wang Y, Hu C, Shangguan X, et al. Transient intracellular expression of PD-L1 and VEGFR2 bispecific nanobody in cancer cells inspires long-term T cell activation and infiltration to combat tumor and inhibit cancer metastasis. Mol Cancer. (2025) 24:119. doi: 10.1186/s12943-025-02253-6
31. Thind K, Padrnos LJ, Ramanathan RK, and Borad MJ. Immunotherapy in pancreatic cancer treatment: a new frontier. Ther Adv Gastroenterol. (2017) 10:168–94. doi: 10.1177/1756283X16667909
32. Scheffer HJ, Stam AGM, Geboers B, Vroomen L, Ruarus A, de Bruijn B, et al. Irreversible electroporation of locally advanced pancreatic cancer transiently alleviates immune suppression and creates a window for antitumor T cell activation. Oncoimmunology. (2019) 8:1652532. doi: 10.1080/2162402X.2019.1652532
Keywords: locally advanced pancreatic cancer, irreversible electroporation, immunotherapy, efficacy, prognosis
Citation: Xi P, Sun P, Chen M, Yao Z, Zhu Q, Li S and He C (2025) Efficacy of irreversible electroporation combined with immunotherapy versus irreversible electroporation alone in locally advanced pancreatic cancer: a propensity score-matched retrospective study. Front. Immunol. 16:1620988. doi: 10.3389/fimmu.2025.1620988
Received: 30 April 2025; Accepted: 23 June 2025;
Published: 08 July 2025.
Edited by:
Xing-xing Fan, Macau University of Science and Technology, Macao SAR, ChinaReviewed by:
Stefano Francesco Crinò, University of Verona, ItalyBrian Davidson, University College London, United Kingdom
Copyright © 2025 Xi, Sun, Chen, Yao, Zhu, Li and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengping Li, bGlzaGVuZ3BAbWFpbC5zeXN1LmVkdS5jbg==; Chaobin He, aGVjaGJAc3lzdWNjLm9yZy5jbg==
†These authors have contributed equally to this work and share first authorship
 Pu Xi
Pu Xi Peng Sun2†
Peng Sun2† Zehui Yao
Zehui Yao Shengping Li
Shengping Li Chaobin He
Chaobin He