- 1Immunohematology and Transfusion Service, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
- 2Pediatric Hematology and Oncology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
- 3Cell Factory and Pediatric Hematology/Oncology Unit, Department of Mother and Child Health, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
- 4Division of Hematology, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
1 Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) has significantly advanced the treatment of numerous hematological disorders. Advances in haploidentical transplantation have broadened access to this life-saving therapy, even for patients lacking fully matched donors (1–4). In this context, the role of donor-specific anti-HLA antibodies (DSA) in graft failure and delayed engraftment is well established, to the extent that pre-transplant screening for DSA has become standard practice in many centers (5–7). Conversely, considerably less attention has been devoted to another humoral immune factor: recipient-specific antibodies (RSA). Screening for DSA prior to transplantation—particularly those capable of complement fixation—is now standard practice and often guides interventions such as plasma exchange, administration of rituximab, and intensified immunosuppressive therapy in patients deemed at high immunological risk (8, 9). These practices underscore the clinical relevance of antibody-mediated complications in HSCT and offer a conceptual framework for evaluating the potential impact of RSA as well.
2 Clinical impact of Recipient-Specific Antibodies (RSAs)
2.1 Mechanisms of RSA-mediated damage
Recipient-specific antibodies (RSA) are antibodies present in the donor that recognize the recipient’s HLA antigens. Their development is often associated with previous allo-sensitization events, such as pregnancy in multiparous female donors, blood transfusions, or previous transplants (10). Once transferred during HSCT, RSAs can bind to recipient tissues, activate complement, and contribute to endothelial injury and inflammatory responses. Mechanistically, RSAs could act similarly to DSAs by triggering antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC), resulting in endothelial cell activation, loss of vascular integrity, and the creation of a pro-inflammatory microenvironment (11–13).
2.2 Clinical evidence and potential implications
Although the clinical relevance of RSAs is not as well established as that of DSAs, emerging evidence suggests they may play a non-negligible role in immune modulation after transplantation. Delbos et al. (14) reported an increased incidence of acute and chronic GVHD in recipients of transplants from donors harboring class II anti-HLA antibodies. Sadowska-Klasa et al. (15) hypothesized that RSAs may mediate endothelial activation via complement pathways, contributing to complications such as veno-occlusive disease (VOD) and transplant-associated thrombotic microangiopathy (TA-TMA). Post-transplant complications, such as engraftment syndrome (ES), cytokine release syndrome (CRS) in haploidentical transplantation with cyclophosphamide-based GVHD prophylaxis, cardiotoxicity, TA-TMA, and veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS), share a common pathogenic mechanism centered on endothelial injury. This injury originates from a subclinical baseline condition, which is exacerbated by pro-inflammatory and pro-thrombotic events, including cytokine release (e.g., TNF-α, IL-6), complement cascade activation, reduced nitric oxide (NO) bioavailability, and elevated levels of angiopoietin-2, von Willebrand factor (vWF), and high mobility group box 1, potentially resulting in multiorgan failure (16–18). To date, TA-TMA remains the only syndrome with a clearly demonstrated association with recipient-specific antibodies (RSA) (15), as RSA may activate complement and directly damage the endothelium. Although direct evidence linking RSA to other endothelial complications is currently lacking, their shared endothelial pathophysiology supports the hypothesis that RSA could similarly contribute to these syndromes, warranting further targeted research. Additionally, Ciurea et al. described a haploidentical transplantation case in which RSA transfer was associated with early endothelial injury and adverse outcomes (19).
This relative omission in clinical practice may stem from various factors: the perception of low RSA levels, the lack of routine testing on donor samples, or the hypothesis that their impact might be less significant compared to that of DSAs. Recent reviews (20) have mainly emphasized the need to start considering the potential clinical role of RSAs and to investigate possible management parallels with DSAs, as current evidence is still too limited to draw definitive conclusions.
2.3 Immunologic modulation and RSA pathogenicity
One possibility is that RSAs contribute to the creation of a pro-inflammatory environment in the period immediately following transplantation, amplifying tissue damage triggered by conditioning regimens or subclinical allogeneic reactivity. In particular, RSAs capable of binding complement may have greater pathogenic potential, suggesting the use of functional assays, such as the C1q binding test, to identify clinically relevant cases. RSAs could thus act more as immunological “modulators” rather than direct barriers to engraftment, influencing the threshold for the development of GVHD, endothelial dysfunction, or chronic graft failure.
2.4 NIMA tolerance and maternal alloimmunization: a dual immunological legacy in haploidentical transplantation
In haploidentical transplantation, the mismatched donor haplotypes are referred to as non-inherited maternal antigens (NIMA) or non-inherited paternal antigens (NIPA). Due to fetal exposure to maternal HLA antigens during pregnancy, which may induce partial immunological tolerance, grafts from NIMA-mismatched donors are generally considered less immunogenic than those from NIPA-mismatched donors. Accordingly, several studies have demonstrated that NIMA-mismatched haplo-HSCT is associated with a significantly lower incidence of acute graft-versus-host disease (aGVHD) compared to NIPA-mismatched transplants. Although this evidence supports a tolerogenic effect induced during gestation, it is important to note that a substantial proportion of pregnant women develop HLA antibodies against paternal antigens. The mother encounters inherited paternal antigens (IPA) during adulthood, when her immune system is fully mature and immunocompetent. During pregnancy, she has approximately a 50% probability of mounting both humoral and cellular immune responses against the IPA haplotype.
In this context, the development of recipient-specific antibodies (RSAs) in multiparous mothers against the child’s IPA haplotype may adversely affect transplant outcomes, potentially negating the immunological advantage often attributed to maternal donors (21–23) (Figure 1). Future studies could be useful to clarify the interplay between NIMA-induced tolerance and maternal RSA formation against paternal antigens, and how these mechanisms impact donor selection and post-transplant outcomes (Figure 1).
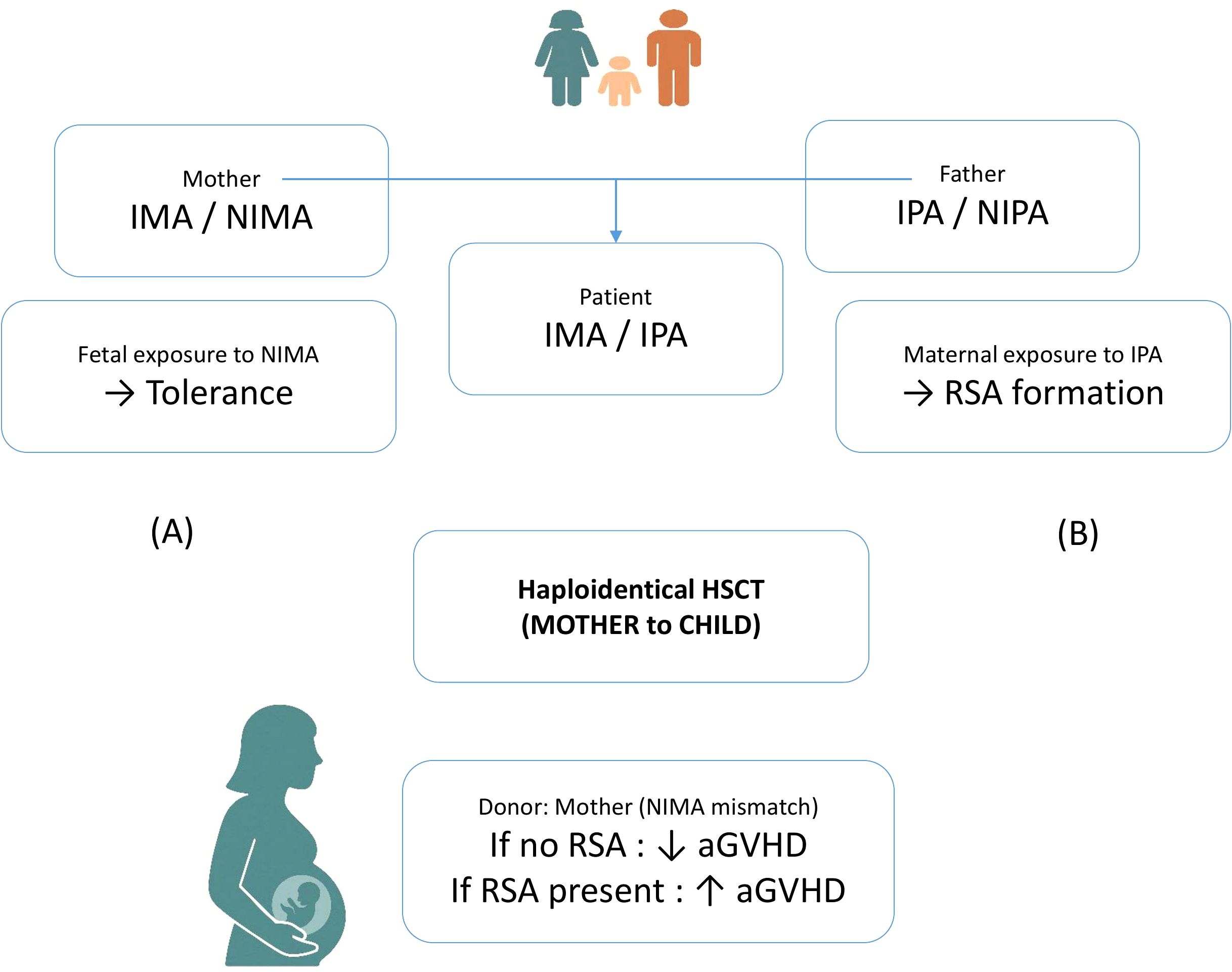
Figure 1. Schematic representation of dual immunological mechanisms occurring during pregnancy. (A) The fetus is exposed to non-inherited maternal antigens (NIMA), which may induce immune tolerance. (B) Conversely, the mother develops B e T cell immunity becoming sensitized to inherited paternal antigens (IPA) expressed by the fetus, this potentially leads to the formation of recipient-specific antibodies (RSAs).
2.5 Gender and reproductive history as risk factors
Gender-related and reproductive history–related immunologic sensitization is therefore a critical area that warrants further investigation. Should the clinical relevance of RSAs be confirmed, integrating targeted clinical strategies to mitigate the effects of prior sensitization could prove useful and might lead to modifications in current donor screening protocols and risk management approaches.
2.6 Technological advances in RSA detection
Defining clinically significant thresholds for RSAs would be crucial to standardizing diagnostic and therapeutic protocols at an international level, potentially promoting greater uniformity in the management of patients undergoing HSCT from haploidentical or partially matched donors (24).
Luminex technology has represented a significant methodological advance, enabling precise identification and quantification of RSAs thanks to its high sensitivity and specificity (25, 26). It has greatly facilitated the investigation of correlations between the presence and intensity of RSAs (measured by MFI) and post-transplant clinical outcomes. Although no validated thresholds currently exist for RSA interpretation, mean fluorescence intensity (MFI) values commonly used for DSA, typically >1,000 to indicate low-level sensitization and >5,000 for antibodies with clinical relevance, could serve as a preliminary reference. These values are supported by EBMT consensus guidelines (6). Aligning RSA interpretation with established DSA criteria may support more consistent risk assessment and guide future standardization efforts. Complement-binding functional assays, such as the C1q binding test, provide additional valuable information on the pathogenic potential of these antibodies.
2.7 Future perspectives on RSA screening and management
From a clinical perspective, the selective integration of RSA screening could represent a rational strategy. It could be especially considered for donors with a history of multiple pregnancies, and possibly for those with prior transfusion events, in whom the identification of significant RSAs might guide targeted therapeutic choices or influence donor selection.
However, the lack of large prospective studies makes it difficult to draw definitive conclusions about the clinical need for RSA screening. Prospective multicenter studies with harmonized methodologies and functional characterization of RSAs would be useful to assess whether integrating RSA screening into clinical practice is appropriate. In parallel, the development of therapeutic strategies to mitigate the effects of pathogenic RSAs—such as plasmapheresis, immunoadsorption, or complement inhibition—could offer new therapeutic options.
3 Discussion
In conclusion, recipient-specific antibodies represent a fascinating yet still underexplored aspect of transplant immunology. Preliminary evidence suggests that they may contribute to shaping the immune environment after HSCT, influencing the risk of GVHD, endothelial injury, and long-term transplant success. In contrast to donor-specific antibodies (DSAs), which are more clearly associated with graft rejection and engraftment failure, RSAs may play a distinct pathogenic role, particularly in the context of GVHD and immune modulation. Recognizing these differences could help to refine risk stratification and to outline new strategies for donor evaluation. RSAs should be considered as a potential piece of the complex mosaic of immune reactivity in HSCT. As research in this field progresses, integrating RSAs into a broader vision of transplant immunology could, in our opinion, broaden horizons for improving clinical outcomes.
Author contributions
AP: Conceptualization, Writing – original draft. CTP: Conceptualization, Writing – original draft. PC: Writing – review & editing, Supervision. IS: Supervision, Writing – review & editing. RC: Writing – review & editing, Visualization. GG: Writing – review & editing. SR: Writing – review & editing. PB: Writing – review & editing, Visualization. MT: Writing – review & editing, Supervision. AT: Writing – review & editing. GL: Writing – review & editing. CZ: Writing – review & editing. AB: Writing – review & editing. ID: Writing – review & editing. NP: Writing – review & editing, Supervision. MZ: Writing – review & editing. CGP: Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The publication costs of this article were supported by institutional current research funds from the Italian Ministry of Health.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Passweg JR, Baldomero H, Bader P, Bonini C, Duarte RF, Dufour C, et al. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. (2017) 52:811–7. doi: 10.1038/bmt.2017.34
2. Fuchs EJ. Haploidentical transplantation for hematologic Malignancies: where do we stand? Hematol Am Soc Hematol Educ Program. (2012) 2012:230–6. doi: 10.1182/asheducation-2012.1.230
3. Kongtim P and Ciurea SO. Who is the best donor for haploidentical stem cell transplantation? Semin Hematol. (2019) 56:194–200. doi: 10.1053/j.seminhematol.2018.08.003
4. Chang YJ, Luznik L, Fuchs EJ, and Huang XJ. How do we choose the best donor for T-cell-replete, HLA-haploidentical transplantation? J Hematol Oncol. (2016) 9:35. doi: 10.1186/s13045-016-0265-2
5. Gladstone DE and Bettinotti MP. HLA donor-specific antibodies in allogeneic hematopoietic stem cell transplantation: challenges and opportunities. Hematol Am Soc Hematol Educ Program. (2017) 2017:645–50. doi: 10.1182/asheducation-2017.1.645
6. Ciurea SO, Cao K, Fernandez-Vina M, Kongtim P, Al Malki M, Fuchs E, et al. The european society for blood and marrow transplantation (EBMT) consensus guidelines for the detection and treatment of donor-specific anti-HLA antibodies (DSA) in haploidentical hematopoietic cell transplantation. Bone Marrow Transplant. (2018) 53:521–30. doi: 10.1038/s41409-017-0062-8
7. Gladstone DE, Zachary AA, Fuchs EJ, Luznik L, Kasamon YL, King KE, et al. Partially mismatched transplantation and human leukocyte antigen donor-specific antibodies. Biol Blood Marrow Transplant. (2013) 19:647–52. doi: 10.1016/j.bbmt.2013.01.016
8. File B, Huang Y, Peedin A, and Gergis U. The impact of HLA donor-specific antibodies on engraftment and the evolving desensitization strategies. Bone Marrow Transplant. (2022) 57:526–31. doi: 10.1038/s41409-022-01578-w
9. Zhang R, He Y, Yang D, Jiang E, Ma Q, Pang A, et al. Combination treatment of rituximab and donor platelets infusion to reduce donor-specific anti-HLA antibodies for stem cells engraftment in haploidentical transplantation. J Clin Lab Anal. (2020) 34:e23261. doi: 10.1002/jcla.23261
10. McCaughan J, Xu Q, and Tinckam K. Detecting donor-specific antibodies: the importance of sorting the wheat from the chaff. Hepatobiliary Surg Nutr. (2019) 8:37–52. doi: 10.21037/hbsn.2019.01.01
11. Ciurea SO, Thall PF, Milton DR, Barnes TH, Kongtim P, Carmazzi Y, et al. Complement-binding donor-specific anti-HLA antibodies and risk of primary graft failure in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. (2015) 21:1392–8. doi: 10.1016/j.bbmt.2015.05.001
12. Picascia A, Grimaldi V, and Napoli C. From HLA typing to anti-HLA antibody detection and beyond: The road ahead. Transplant Rev (Orlando). (2016) 30:187–94. doi: 10.1016/j.trre.2016.07.007
13. Morin-Zorman S, Loiseau P, Taupin JL, and Caillat-Zucman S. Donor-specific anti-HLA antibodies in allogeneic hematopoietic stem cell transplantation. Front Immunol. (2016) 7:307. doi: 10.3389/fimmu.2016.00307
14. Delbos F, Barhoumi W, Cabanne L, Beckerich F, Robin C, Redjoul R, et al. Donor immunization against human leukocyte class II antigens is a risk factor for graft-versus-host disease. Biol Blood Marrow Transplant. (2016) 22:292–9. doi: 10.1016/j.bbmt.2015.09.027
15. Sadowska-Klasa A, Dukat-Mazurek A, Zielińska H, Dębska-Zielkowska J, Piekarska A, Moszkowska G, et al. Incidence and role of recipient-specific antibodies in allogeneic hematopoietic cell transplantation from mismatched related donors. Transplant Cell Ther. (2024) 30:99.e1–99.e10. doi: 10.1016/j.jtct.2023.10.015
16. Milone G, Bellofiore C, Leotta S, Milone GA, Cupri A, Duminuco A, et al. Endothelial dysfunction after hematopoietic stem cell transplantation: a review based on physiopathology. J Clin Med. (2022) 11:623. doi: 10.3390/jcm11030623
17. Hildebrandt GC and Chao N. Endothelial cell function and endothelial-related disorders following hematopoietic cell transplantation. Br J Hematol. (2020) 190:508–19. doi: 10.1111/bjh.16621
18. Baumeister SHC, Mohan GS, Elhaddad A, and Lehmann L. Cytokine release syndrome and associated acute toxicities in pediatric patients undergoing immune effector cell therapy or hematopoietic cell transplantation. Front Oncol. (2022) 12:841117. doi: 10.3389/fonc.2022.841117
19. Ciurea SO, Cao K, and Zou J. Donor-specific anti-HLA antibodies and … recipient-specific anti-HLA antibodies? The conundrum on pregnancy in transplantation. Am J Hematol. (2020) 95:E112–4. doi: 10.1002/ajh.25762
20. Little AM. HLA antibodies in hematopoietic stem cell transplantation. HLA. (2019) 94:21–4. doi: 10.1111/tan.13741
21. van Rood JJ, Roelen DL, and Claas FHJ. The effect of noninherited maternal antigens in allogeneic transplantation. Semin Hematol. (2005) 42:104–11. doi: 10.1053/j.seminhematol.2005.01.008
22. van Rood JJ, Loberiza FR Jr, Zhang MJ, Oudshoorn M, Claas FH, Cairo MS, et al. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. (2002) 99:1572–7. doi: 10.1182/blood.V99.5.1572
23. Bracamonte-Baran W and Burlingham W. Non-inherited maternal antigens, pregnancy, and allotolerance. BioMed J. (2015) 38:39–51. doi: 10.4103/2319-4170.143498
24. Kiernan JJ, Ellison CA, and Tinckam KJ. Measuring alloantibodies: a matter of quantity and quality. Curr Opin Organ Transplant. (2019) 24:20–30. doi: 10.1097/MOT.0000000000000593
25. Ellis TM. Interpretation of HLA single antigen bead assays. Transplant Rev (Orlando). (2013) 27:108–11. doi: 10.1016/j.trre.2013.07.001
Keywords: hematopoietic stem cell transplantation, recipient-specific HLA antibodies, donor-specific anti-HLA antibodies, alloantigen-induced immune responses, complement-mediated endothelial injury, haploidentical stem cell transplantation, transplant immunology
Citation: Pasi A, Prezioso CT, Comoli P, Sbarsi I, Cacciatore R, Giorgiani G, Recupero S, Bergamaschi P, Torchio M, Taurino A, Losi G, Zerbi C, Bianchessi A, Defrancesco I, Polverelli N, Zecca M and Perotti CG (2025) Recipient-specific antibodies in HSCT: current knowledge and future perspectives. Front. Immunol. 16:1621252. doi: 10.3389/fimmu.2025.1621252
Received: 30 April 2025; Accepted: 17 June 2025;
Published: 03 July 2025.
Edited by:
Luigi Nespoli, University of Insubria, ItalyCopyright © 2025 Pasi, Prezioso, Comoli, Sbarsi, Cacciatore, Giorgiani, Recupero, Bergamaschi, Torchio, Taurino, Losi, Zerbi, Bianchessi, Defrancesco, Polverelli, Zecca and Perotti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Annamaria Pasi, YS5wYXNpQHNtYXR0ZW8ucHYuaXQ=
†These authors have contributed equally to this work
 Annamaria Pasi
Annamaria Pasi Carmen Tania Prezioso1†
Carmen Tania Prezioso1† Alessia Taurino
Alessia Taurino Irene Defrancesco
Irene Defrancesco Nicola Polverelli
Nicola Polverelli Marco Zecca
Marco Zecca