- 1Department of Immunization, Shanghai Municipal Center for Disease Control and Prevention, Shanghai, China
- 2Department of Immunization, Yangpu District Center for Disease Control and Prevention, Shanghai, China
Background: To understand the immunogenicity and safety of the 23-valent pneumococcal polysaccharide vaccine in elderly individuals aged 60–70 years in Shanghai after revaccination.
Methods: A total of 330 elderly people aged 60–70 years were recruited to study the immunogenicity and safety of PPSV23 revaccination. The group with a history of PPSV23 vaccination and an interval of 5 years or more was selected as the revaccination group (n=220), and the group without any pneumococcal vaccine was selected as the first vaccination group (n=110).
Results: In terms of immunogenicity, the GMCs of all serotypes before and after immunization were 1.15-21.57 µg/ml and 1.62-33.19 µg/ml, respectively, in the revaccination group, and the GMCs of all serotypes after immunization were higher than those before immunization (P<0.0001). After immunization, the total GMI of antibodies in the revaccination group was lower than that in the first vaccination group [1.62(1.57, 1.67) vs 3.20(2.82, 3.57), P<0.0001], and the GMIs of all serotypes in the revaccination group were also lower than those in the first vaccination group [(1.40-1.92) vs (1.82-4.69), P<0.0001]. In terms of safety, no serious adverse events occurred during the study and all adverse events that occurred were mild or self-limiting. From 0–30 days after immunization, 7 patients in the revaccination group and 14 patients in the first vaccination group experienced adverse events, with incidence rates of 3.18% and 12.73%, respectively, which were lower in the revaccination group than in the first vaccination group (P=0.0008).
Conclusions: Compared with those before vaccination, the antibody levels of elderly people aged 60–70 years in Shanghai who were inoculated with PPSV23 for 5 years or more tended to increase but were lower than those in the first vaccination group. The safety of revaccination with PPSV23 was favorable.
Clinical Trial Registration: http://www.chictr.org.cn, identifier ChiCTR2100042000; http://clinicaltrials.gov, identifier NCT04701788.
Introduction
Streptococcus pneumoniae (Spn), abbreviated as pneumococcus, is a common conditionally pathogenic bacterium that can invade different parts of the body, leading to a series of diseases, such as otitis media, sinusitis, pneumonia, meningitis, bacteremia, etc., and pneumococcal disease (PD) is a serious public health problem worldwide (1, 2). According to the World Health Organization (WHO), about 75% of cases of invasive pneumococcal disease (IPD) occur in children aged <2 years, and case fatality rates from IPD in children can be high, ranging up to 20% for septicaemia and 50% for meningitis in low- and middle-income countries (3). A systematic analysis of the global burden of disease revealed that Spn was the leading cause of lower respiratory infection morbidity and mortality globally, contributing to more deaths than all other etiologies combined in 2016 (4). In China, the disease burden of PD is equally severe (5–8). About 2.5 million people suffer from PD every year, resulting in 125,000 deaths, mainly among middle-aged and elderly people over 50 years old and infants under 1 year old with relatively weak immunity (9).
Vaccination is one of the important and effective means for the prevention and control of PD. As early as 2008, the WHO classified PD as a disease requiring “very high priority” for vaccine prevention (10). The 23-valent pneumococcal polysaccharide vaccine (PPSV23) has been proven to be an effective means of preventing Spn infections, particularly for IPD (11). The application of PPSV23 in the elderly population can effectively reduce the incidence of community-acquired pneumonia and prevent diseases associated with Spn infection (12, 13). In China, PPSV23 produced by Chengdu Institute of Biological Products Limited Liability Company contains serotypes 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F And 33F. It was approved in Chinese Mainland in 2006 for susceptible people aged 2 years and above. Shanghai has fully implemented free vaccination with PPSV23 for registered elderly people aged 60 and above since 2013, and the implementation results have shown excellent vaccination safety and health economics (14, 15).
Some studies have shown that the effectiveness of PPSV23 for preventing IPD in the elderly population decreases with time. An ecological study conducted by Andrews et al. in England and Wales showed that the protective effect of PPSV23 against serotype specific IPD ranged from 48% (95% CI: 32-60%) within 2 years to 15% (95% CI: -3% -30%) after 5 years (16). Another study conducted by MANOFF et al. in the United States involving 1,008 subjects showed that the functional antibody levels of people over 65 years old decreased over time (17). The research by SHAPIROED et al. showed that the effect of PPSV23 vaccination on IPD is the best occurring at <3 years after vaccination and the worst results occurring at >5 years (18). There is still controversy over whether it is necessary to revaccinate the elderly population with PPSV23. The American Advisory Committee on Immunization Practices (ACIP) and the 2021 guidelines from the Japanese Ministry of Health, Labour and Welfare both recommend that healthy elderly individuals receive a single dose of PPSV23, while high-risk populations such as those without spleens or immunosuppressants are recommended to receive PPSV23 again (19, 20). The Chinese expert consensus on pneumococcal vaccination for PPSV23 revaccination recommends that, for those who need to be revaccinated, the vaccination should be administered according to the instructions, and the revaccination interval should be at least 5 years (2). To further validate the safety and efficacy of revaccination of domestically produced PPSV23 in elderly individuals, the population-based observational studies were conducted simultaneously in Chengdu (21) and Shanghai, China. By the end of 2023, more than 1.83 million elderly people had been vaccinated with PPSV23 free of charge in Shanghai, and approximately 3% of them had been revaccinated. In this study, we investigated the immunogenicity and safety of revaccination with PPSV23 in elderly people aged 60–70 years in Shanghai and provided a scientific basis for improving the immunization strategy for elderly people vaccinated with PPSV23.
Methods
Study design and participants
This single-center, controlled clinical study was conducted by the Shanghai Municipal Center for Disease Control and Prevention in Changning, Hongkou, Jiading and Qingpu districts of Shanghai, China, from March to August 2021. A total of 330 elderly people aged 60–70 years were recruited to study the immunogenicity and safety of PPSV23 revaccination. The group with a history of PPSV23 vaccination and an interval of 5 years or more was selected as the revaccination group (n=220), and the group without any pneumococcal vaccine was selected as the first vaccination group (n=110). Subjects who are allergic to PPSV23 vaccine components, have immunodeficiency, are under treatment for malignant tumors, have received nonspecific immunoglobulin injections within 3 months prior to enrollment, have a body temperature >37.0°C, have coagulation disorders, and those the researchers believe may influence the evaluation of the trial will be excluded.
Research process and research content
After the study subjects were enrolled, their general information was collected by uniformly trained staff. With informed consent, 5ml of venous blood was collected before immunization and the serum was separated and divided into two tubes, with a minimum serum volume of 0.5ml in each tube. The tubes were stored at a temperature below -20°C. Then, one dose of PPSV23 was injected subcutaneous or intramuscularly into the lateral deltoid muscle of the upper arm according to the instruction. After an interval of 30 days, blood samples were collected again using the same method. The blood collection window period was -2 to 10 days.
For laboratory testing, an enzyme-linked immunosorbent assay (ELISA) was used to quantitatively detect 23 immunoglobulin G (IgG) antibodies against specific serotypes of Spn intercalated polysaccharide in human sera and to evaluate the geometric mean concentration (GMC), geometric mean increase (GMI) and 2-fold increase rate of the antibodies in human sera before and after inoculation with PPSV23.
For safety, the study subjects were required to stay for 30 minutes after one dose of PPSV23, and the staff issued diary cards for them to collect the occurrence of adverse events within 30 days after vaccination, in which the adverse events included localized solicited adverse events, systemic solicited adverse events, and non-solicited adverse events.
Study vaccine
The vaccine administered in this study was PPSV23 produced by the Chengdu Institute of Biological Products Limited Liability Company, lot number 2020311, specification 0.5 ml/vial.
Data sets
This study involved two types of datasets. The immunogenicity evaluation analysis used the immunogenicity Per-Protocol Set (PPS), that is, the subjects who met the inclusion/exclusion criteria, underwent full follow-up as required by the protocol, and were not excluded during the blind verification of serum results. The safety evaluation analysis used the Safety Set (SS), which included the study subjects who received the vaccine and underwent at least one safety evaluation.
Statistical analysis
The data were statistically analyzed via SAS 9.4 software. The quantitative data were presented as x ± s, and the qualitative data were presented as frequencies. The chi-square test was used to compare the mean 2-fold increase rates of antibodies between groups, and the paired t test was used to compare the GMCs and GMIs of antibodies before and after immunization in the same group. Local and systemic reactions after vaccination were statistically analyzed in all study subjects, and the incidence was compared between groups via the chi-square test. Two-sided test with test level α=0.05.
Ethics
This study was approved by the institutional review board of the Shanghai Municipal Center for Disease Control and Prevention (No. 2020-96). This study was registered in January 2021 on a foreign website (http://clinicaltrials.gov, NCT04701788) and on a domestic website (http://www.chictr.org.cn, ChiCTR2100042000).
Results
Basic situation
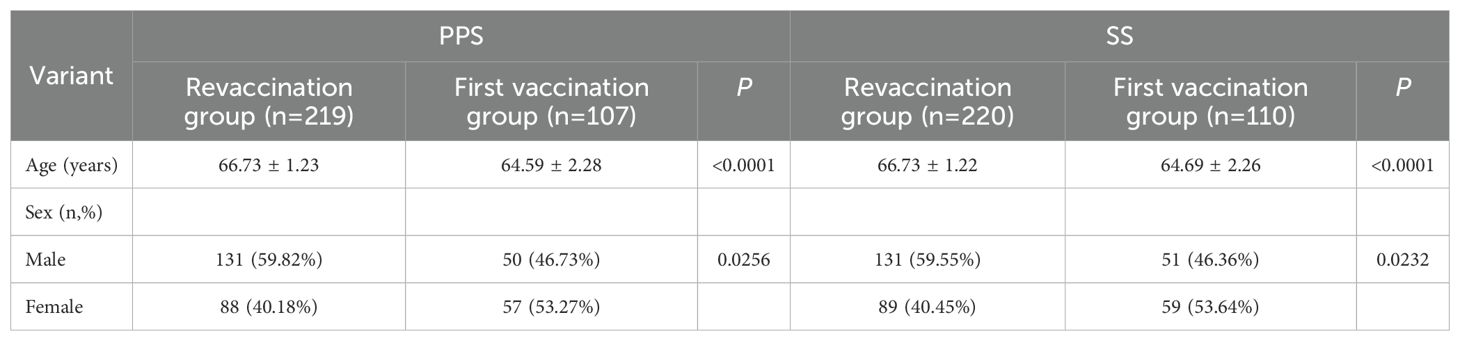
A total of 330 subjects were enrolled in this study, including 220 in the revaccination group and 110 in the first vaccination group. Four subjects were dislodged during the period, 326 subjects were included in the PPS, and 330 subjects were included in the SS. See Table 1; Figure 1.
Immunogenicity
GMC levels
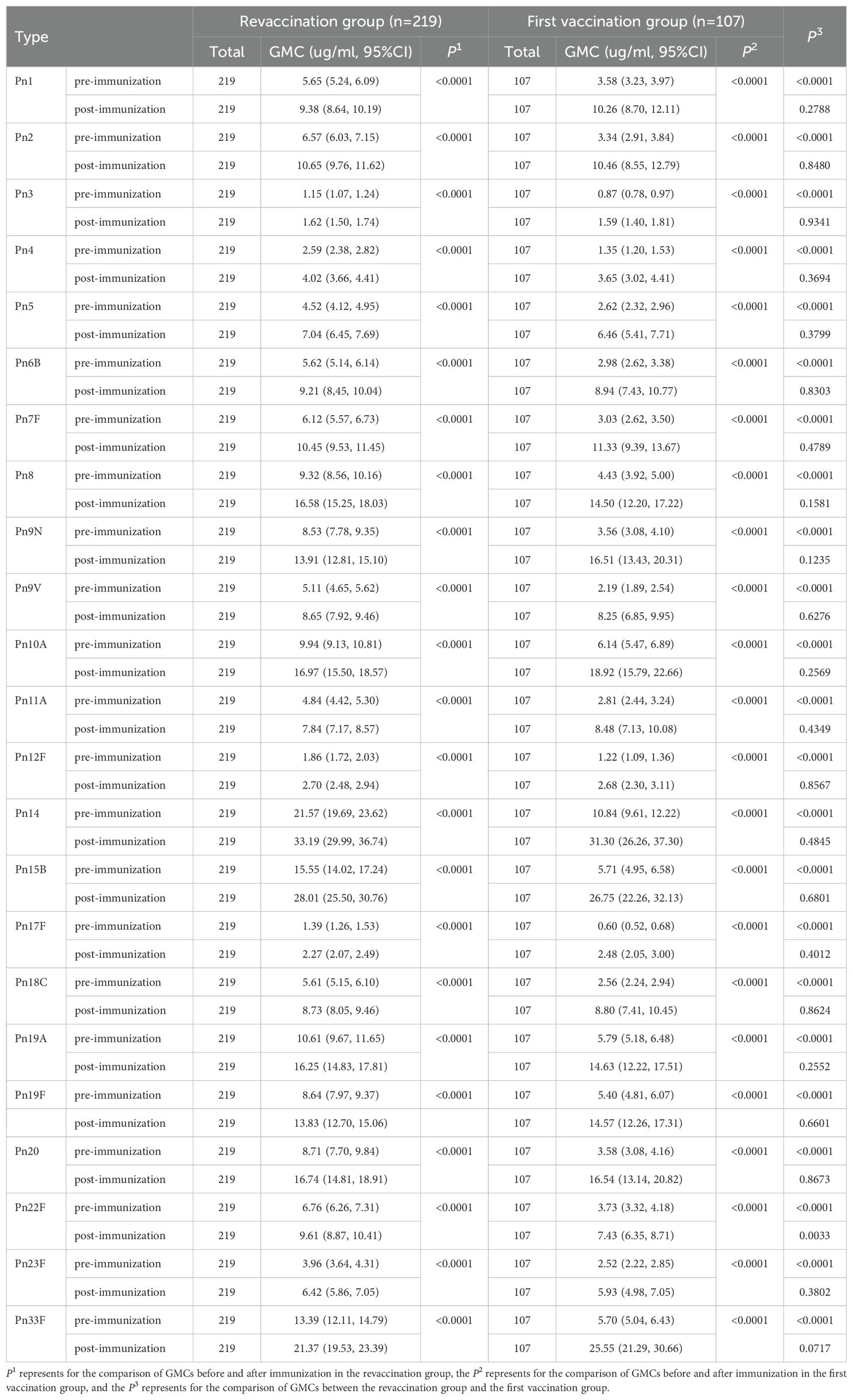
In the revaccination group, the GMCs of the 23 serotypes of Spn capsular polysaccharide IgG antibodies before and after immunization were 1.15-21.57 µg/ml and 1.62-33.19 µg/ml, respectively, and the level of the GMC of each type after immunization was higher than that before immunization, and the difference was statistically significant in all cases (P<0.0001). Similarly, in the first vaccination group, the GMCs of all serotypes were 0.60-10.84 µg/ml and 1.59-31.30 µg/ml before and after immunization, respectively, and the level of each type after immunization was significantly higher than that before immunization (P<0.0001). In addition, GMCs of all serotypes in the revaccination group before immunization were higher than those in the first vaccination group (P<0.0001), and GMC of type 22F in the revaccination group after immunization was higher than that in the first vaccination group [9.61(8.87, 10.41) vs 7.43(6.35, 8.71), P=0.0033]. See Table 2.
GMI levels
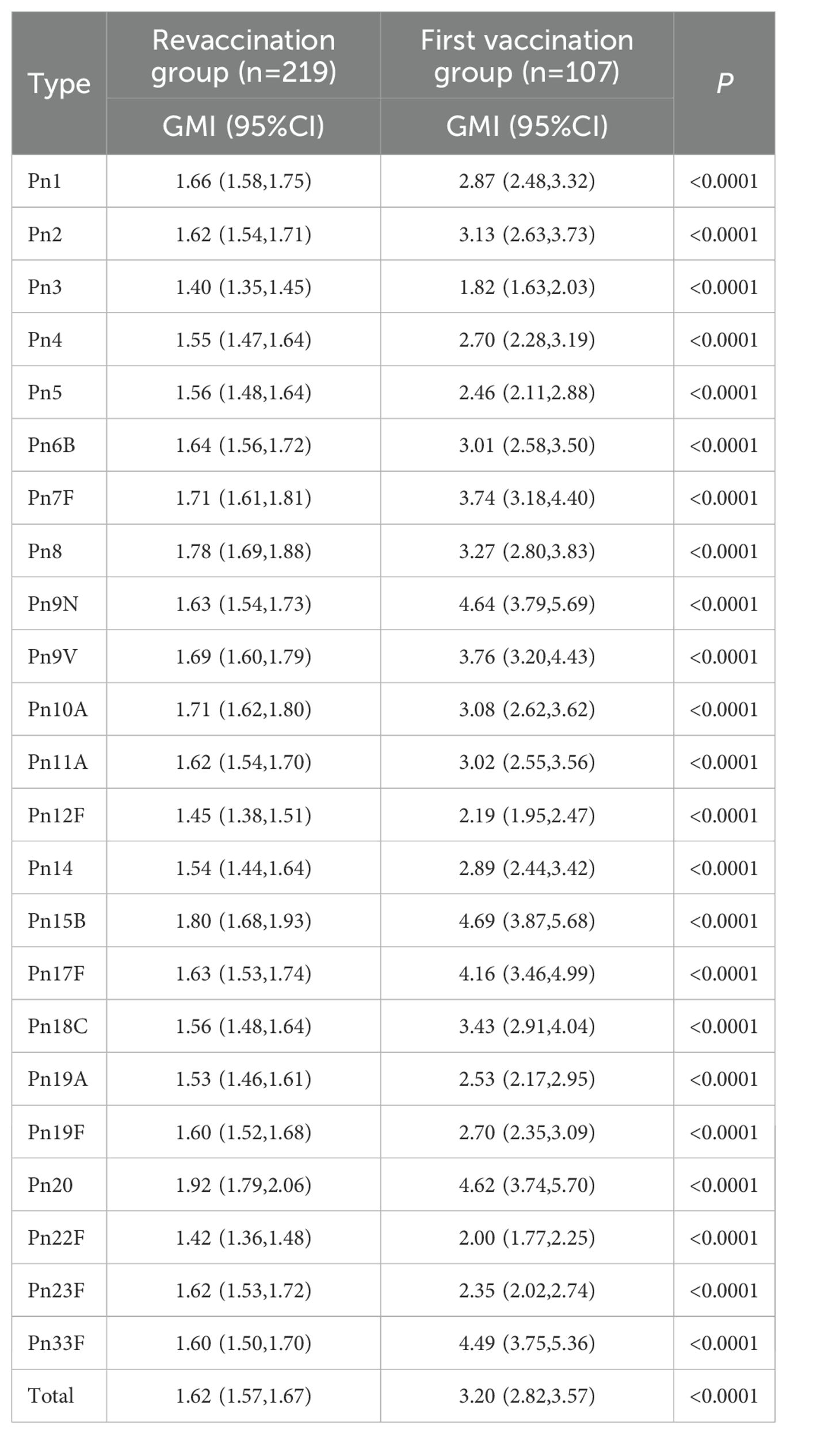
The total antibody GMI of the revaccination group and the first vaccination group was 1.62(1.57, 1.67) and 3.20(2.82, 3.57), respectively, and that of the revaccination group was lower than that of the first vaccination group (P<0.0001). The GMIs of the 23 serotypes of antibodies in the two groups were 1.40-1.92 and 1.82-4.69, respectively, and the GMI of the antibodies of each serotype in the revaccination group was lower than that in the first vaccination group (P<0.0001). See Table 3.
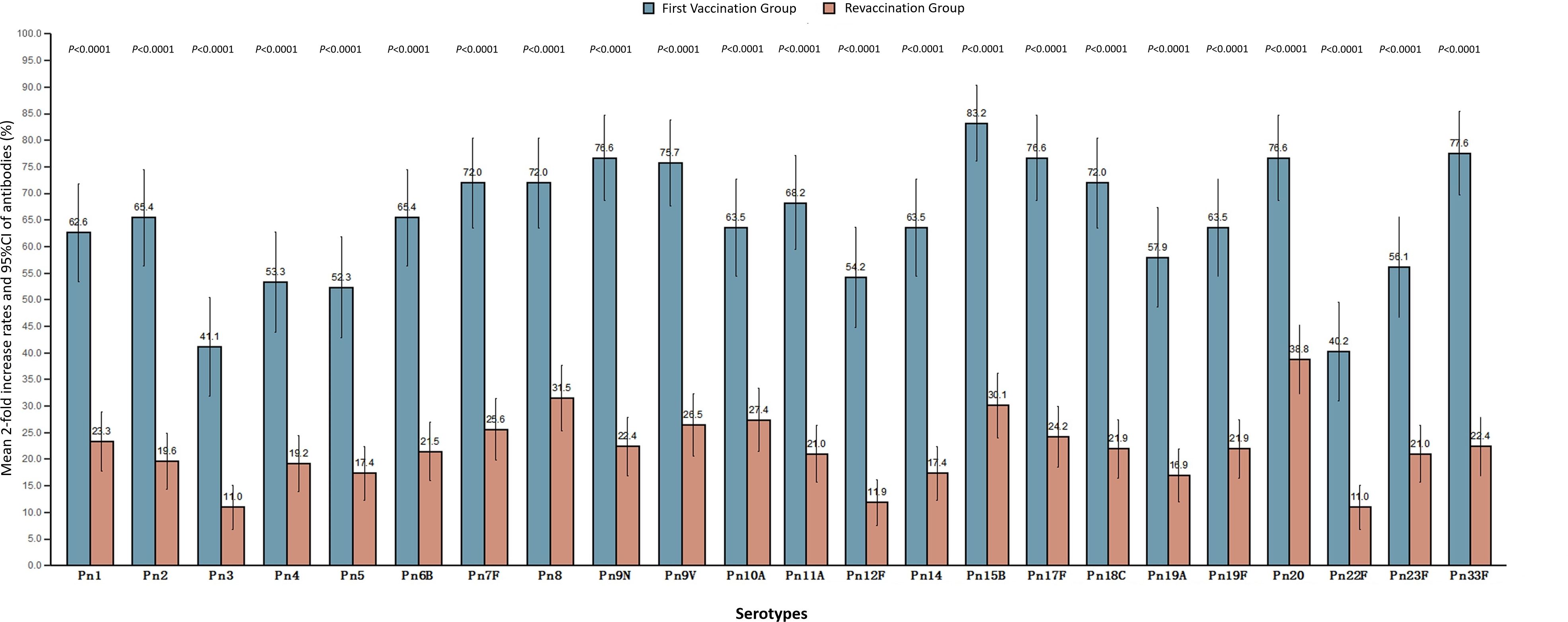
Mean 2-fold increase rates
The mean 2-fold increase rate of antibodies in the revaccination group and the first vaccination group was 21.90% (19.07%, 24.72%) and 64.77% (59.80%, 69.74%), respectively, which was lower in the revaccination group than that in the first vaccination group (P<0.0001). The average 2-fold increase rates of antibodies to 23 serotypes in the two groups were 10.96%-38.81% and 40.19%-83.18%, respectively, and the average 2-fold increase rates of antibodies to all serotypes in the revaccination group were lower than those in the first-time vaccination group, and the differences were statistically significant (P<0.0001). See Figure 2.
Safety
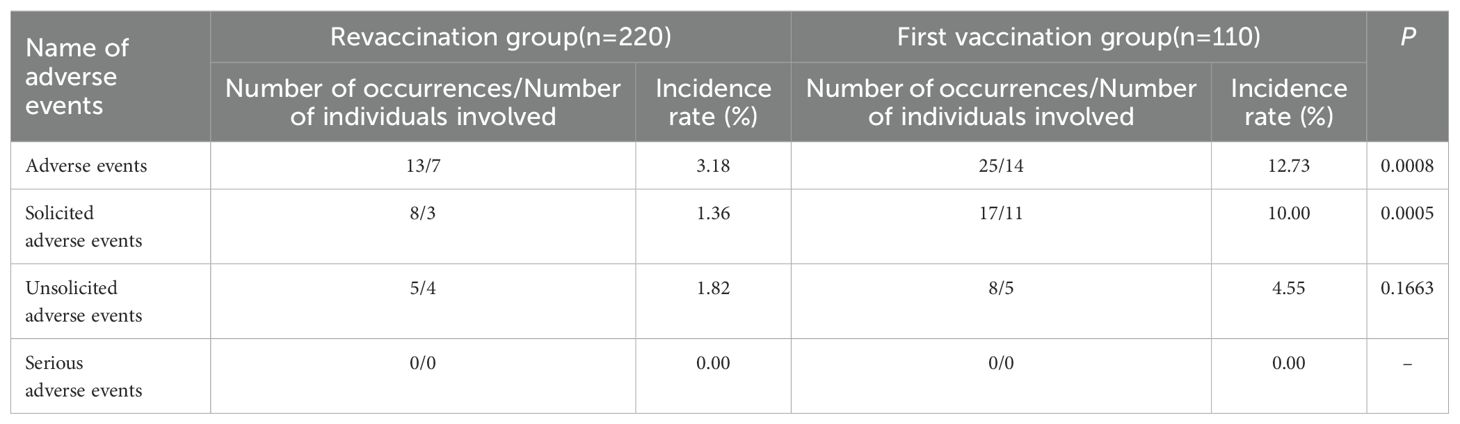
Occurrence of adverse events
From 0–30 days after PPSV23 vaccination, adverse events occurred in 7 patients in the revaccination group, with an adverse event rate of 3.18%, and 14 patients in the first vaccination group, with an adverse event rate of 12.73%. There was a statistically significant difference in incidence between two groups (P =0.0008), and the incidence in the revaccination group was lower than that in the first vaccination group. No serious adverse events occurred during the study observation period. See Table 4.
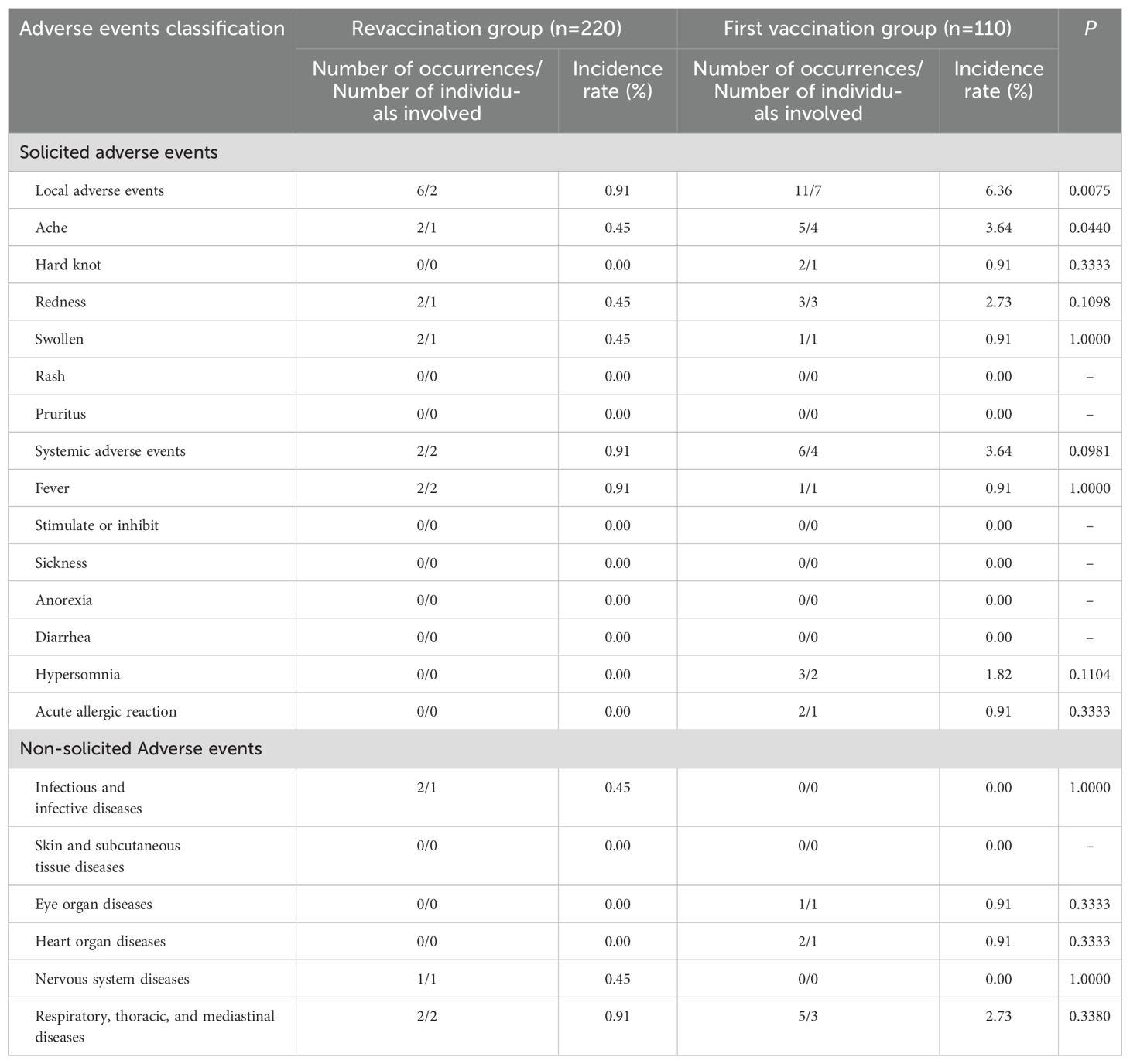
Analysis of symptoms of adverse events
From 0–30 days after PPSV23 vaccination, the number of localized symptomatic adverse events in the revaccination group and the first vaccination group was 2 (0.91%) and 7(6.36%), respectively, and the incidence rate in the revaccination group was lower than that in the first vaccination group (P=0.0075). The number of systemic solicited adverse events in the revaccination group and the first vaccination group was 2(0.91%) and 4(3.64%), respectively, and the difference in the incidence rates between two groups was not statistically significant (P=0.0981). There were no statistically significant differences in the occurrence of non-solicited adverse events between the two groups (P>0.05). See Table 5.
Discussion
The morbidity and mortality of PD and its associated complications are high in people aged 60 years and older, and pneumonia vaccination (including polysaccharide and conjugate vaccines) is one of the most cost-effective means of preventing Spn infection (22–24). A previous population–cohort study conducted in Shanghai suggested that most antibody levels of PPV23 can persist for more than 5 years (25). However, there is still controversy about the need to revaccinate the elderly population with PPSV23, and there have not been sufficient previous studies on PPSV23 revaccination in China. In this study, 330 elderly people aged 60–70 years were selected to conduct a clinical study on the immunogenicity and safety of PPSV23 revaccination in Shanghai. The results showed that the antibody GMC of elderly individuals in Shanghai increased to different degrees after 5 years of inoculation with PPSV23, and the safety of revaccination with PPSV23 was favorable. Thus, elderly people aged 60 years and older could consider revaccination with PPSV23 at least 5 years after the first vaccination, and since the PCV vaccine has been used in adults abroad with a good protective effect, we also look forward to introducing the PCV vaccine for sequential vaccination in the future.
About PPSV23 revaccination, Musher et al.’s study showed that both primary vaccination and revaccination with PPSV23 induce antibody responses that persist during 5 years of observation (26). Lackner et al.’s study also showed that revaccination with PPSV23 at least 5 years after primary vaccination is associated with a significant immune response for most of the serotypes tests in frail, chronically ill older nursing facility residents and revaccination was well tolerated (27). These are similar to the results of our study. In this study, before immunization, the antibody GMC levels of all serotypes in the revaccination group were higher than those in the first vaccination group, which was similar to the research results of Musher et al (26). And it suggested that protective antibodies were still present in the organism 5 years after PPSV23 vaccination. After immunization, in the revaccination group, except for the type 22F GMC level, which was higher than that in the first vaccination group [9.61 (8.87, 10.41) vs. 7.43 (6.35, 8.71), P=0.0033], the other antibody GMC levels were not significantly different between two groups, which was similar to the results of a study conducted by Kawakami (28) et al. in people >70 years old in Japan. That is, the antibody levels in the revaccination group and the first vaccination group were comparable after immunization. The reason why the GMC level of type 22F in the revaccination group was higher than that in the first vaccination group might be that type 22F could activate B cells more effectively, while other types were inhibited due to competition. However, the antibody GMI and mean 2-fold increase rate were lower in the revaccination group than those in the first vaccination group, possibly because the baseline levels of each antibody GMC in the revaccination group were higher than those in the first vaccination group, that is, the baseline levels of the two groups were inconsistent. However, it has also been shown (29) that although revaccination with PPSV23 induced an increase in the antibody GMC, it was still lower than the level after the first vaccination, which may be related to the depletion of the memory B-cell population (30).
In terms of safety, no serious adverse events occurred in the subjects during the study observation period in either the revaccination group or the first vaccination group, and those adverse events were usually mild or self-limiting, which is similar to the findings of previous studies (31). The incidence of localized adverse events was higher in the first vaccination group than that in the revaccination group in this study (0.91% vs 6.36%, P=0.0075), especially tenderness, which may be related to the fact that the subjects in the revaccination group were older and had a poorer sense of adverse events. It is also possible that during the first vaccination, the body had no pre-stored immunity to the pneumococcal polysaccharide antigen, and the immune system would initiate a stronger inflammatory response, resulting a higher incidence of local redness, swelling, pain and other reactions. However, the results of another study (32) revealed an increase in localized reactions (≥10.2cm) within 2 days after revaccination with PPSV23 compared with the first vaccination (RR=3.3, 95%CI: 2.1-5.1).
There are several shortcomings in this study. For instance, the sample size of this study is relatively small and stratified analyses of age, gender have not been conducted. Secondly, only ELISA for serum neutralizing antibodies was performed in this study, but functional antibody testing was not performed, nor was cellular immunity level testing. Also, the vaccination histories of herpes zoster vaccine, influenza vaccine and COVID-19 vaccine of the participants were not collected in this study. These are all the directions for subsequent in-depth research.
Conclusion
The antibody levels of 60–70 year old people in Shanghai who were inoculated with PPSV23 for 5 years or more showed an increasing trend compared with those before vaccination, but were lower than those in the first vaccination group. The safety of revaccination with PPSV23 was favorable.
Data availability statement
The datasets presented in this article are not readily available because of contractual agreements with the sponsor (China Biotechnology Co., Ltd), which stipulated that the ownership of the raw data belonged to the sponsor. Requests to access the datasets should be directed to Haiping Chen from China Biotechnology Co., Ltd, Y2hlbmhhaXBpbmdAc2lub3BoYXJtLmNvbQ==.
Ethics statement
The studies involving humans were approved by the institutional review board of the Shanghai Municipal Center for Disease Control and Prevention. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JQ: Project administration, Visualization, Software, Data curation, Methodology, Investigation, Writing – original draft. ZL: Investigation, Writing – review & editing, Data curation, Methodology, Formal analysis. FH: Validation, Visualization, Writing – review & editing. ZH: Writing – review & editing, Validation, Supervision. XL: Data curation, Project administration, Writing – review & editing, Supervision, Investigation. JL: Visualization, Validation, Writing – review & editing, Software. YL: Software, Visualization, Validation, Writing – review & editing. XG: Writing – review & editing, Project administration, Conceptualization, Validation, Supervision. XS: Resources, Conceptualization, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Key Discipline-Infectious Diseases (No. GWVI- 11.1-01) of the Three-year Action Program of Shanghai Municipality for Strengthening the Construction of the Public Health System (2023–2025), and 2025 Health Special Program for Decision-Consulting Research "Research on Optimization of Immunization Planning Strategies in Shanghai Under the High-Quality Development Background of Disease Control and Prevention" (No. 2025- LH-WJ06). XS was supported by the Talent training project for public health in China.
Acknowledgments
We thank the staff of the CDCs and community health service centers in Changning, Hongkou, Jiading, and Qingpu Districts who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP) Vol. 46. Atlanta, Georgia, USA: MMWR Recomm Rep (1997) p. 1–24.
2. Chinese Preventive Medicine Association, Vaccine and Immunology Branch of Chinese Preventive Medicine Association. Expert consensus on immunoprophylaxis of pneumococcal diseases (2020). Chin J Vaccines Immunization. (2021) 01):1–47. doi: 10.3760/cma.j.cn112338-20201111-01322
3. World Health Organization. Pneumococcal conjugate vaccines in infants and children under 5 years of age-WHO position paper. Wkly Epidemiol Rec. (2019) 94:85–104.
4. GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. (2018) 18:1191–210. doi: 10.1016/S1473-3099(18)30310-4
5. O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. (2009) 374:893–902. doi: 10.1016/S0140-6736(09)61204-6
6. Chen Y, Deng W, Wang SM, Mo QM, Jia H, Wang Q, et al. Burden of pneumonia and meningitis caused by Streptococcus pneumoniae in China among children under 5 years of age: a systematic literature review. PloS One. (2011) 6:e27333. doi: 10.1371/journal.pone.0027333
7. Xie MZ, Dong M, Du J, Zhang SS, Huang F, and Lu QB. Epidemiological features of Streptococcus pneumoniae in patients with acute respiratory tract infection in Beijing, China during 2009-2020. J Infect Public Health. (2023) 16:719–26. doi: 10.1016/j.jiph.2023.03.010
8. Jiang N, Li R, Bao J, Xie Y, Ma X, He Y, et al. Incidence and disease burden of community-acquired pneumonia in southeastern China: data from integrated medical resources. Hum Vaccines immunotherapeutics. (2021) 17:5638–45. doi: 10.1080/21645515.2021.1996151
9. Zhang Y and Shu JD. Pneumococcal pneumonia and its vaccine. Zhonghua Liu Xing Bing Xue Za Zhi. (2002) 23:78.
10. World Health Organization. Meeting of the immunization strategic advisory group of experts, November 2007-conclusions and recommendations. Wkly Epidemiol Rec. (2008) 83:1–15.
11. Moberley S, Holden J, Tatham DP, and Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. (2013) 2013:CD000422. doi: 10.1002/14651858.CD000422.pub3
12. Guo X, Qiu J, Ren J, Ma X, Huang Z, and Sun X. Evaluation of the effect of 23-valent pneumococcal polysaccharide vaccine after 5 years of vaccination in a cohort of elderly aged 60 years and above in Shanghai, 2013-2018. Chin J Prev Med. (2020) 54:923–8. doi: 10.3760/cma.j.cn112150-20200306-00262
13. Sun X, Guo X, Qiu J, Zhao G, Xu X, Wagner AL, et al. Effectiveness of 23-valent pneumococcal polysaccharide vaccine against pneumococcal diseases among the elderly aged 60 years or older: A matched test negative case-control study in shanghai, China. Front Public Health. (2021) 9:620531. doi: 10.3389/fpubh.2021.620531
14. Guo X, Qiu J, Ren J, Liu J, and Sun X. Safety evaluation of mass vaccination with 23-valent pneumococcal polysaccharide vaccine in the elderly population aged 60 years and above in Shanghai, 2013-2017. Chin J Prev Med. (2020) 54:929–33. doi: 10.3760/cma.j.cn112150-20191011-00779
15. Sun X, Tang Y, Ma X, Guo X, Huang Z, Ren J, et al. Cost-effectiveness analysis of 23-valent pneumococcal polysaccharide vaccine program for the elderly aged 60 years or older in Shanghai, China. Front Public Health. (2021) 9:647725. doi: 10.3389/fpubh.2021.647725
16. Andrews NJ, Waight PA, George RC, Slack MP, and Miller E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. (2012) 30:6802–8. doi: 10.1016/j.vaccine.2012.09.019
17. Manoff SB, Liss C, Caulfield MJ, Marchese RD, Silber J, Boslego J, et al. Revaccination with a 23-valent pneumococcal polysaccharide vaccine induces elevated and persistent functional antibody responses in adults aged 65 > or = years. J Infect Dis. (2010) 201:525–33. doi: 10.1086/651131
18. Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margolis A, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. New Engl J Med. (1991) 325:1453–60. doi: 10.1056/NEJM199111213252101.
19. Centers for Disease Control and Prevention. Use of pneumococcal vaccines in adults: Updated recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2023. MMWR Morb Mortal Wkly Rep. (2023) 72:1–12. doi: 10.15585/mmwr.mm7203a1
20. Ministry of Health, Labour and Welfare, Japan. Pneumococcal vaccination guidelines (revised 2021 edition) (2021). Available online at: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/kenkou_iryou/kenkou/kekkaku-kansenshou/index.html (Accessed March 26, 2021).
21. Ma QL, Zhang M, Liu LJ, Zhou Y, Yuan W, Yang M, et al. Immunogenicity and safety of revaccination of 23-valent pneumococcal polysaccharide vaccine in people aged 60 years and above. Zhonghua Liu Xing Bing Xue Za Zhi. (2023) 44:1119–25. doi: 10.3760/cma.j.cn112338-20221130-01019
22. Briles DE, Paton JC, Mukerji R, Swiatlo E, and Crain MJ. Pneumococcal vaccines. Microbiol Spectr. (2019) 7:10. doi: 10.1128/microbiolspec.GPP3-0028-2018
23. Drijkoningen JJ and Rohde GG. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. (2014) 20 Suppl 5:45–51. doi: 10.1111/1469-0691.12461
24. Jiang Y, Yang X, Taniguchi K, Petigara T, and Abe M. A cost-effectiveness analysis of revaccination and catch-up strategies with the 23-valent pneumococcal polysaccharide vaccine (PPV23) in older adults in Japan. J Med economics. (2018) 21:687–97. doi: 10.1080/13696998.2018.1465272
25. Guo X, Li J, Qiu J, Zhang R, Ren J, Huang Z, et al. Persistence of antibody to 23-valent pneumococcal polysaccharide vaccine: a 5-year prospective follow-up cohort study. Expert Rev Vaccines. (2024) 23:237–45. doi: 10.1080/14760584.2023.2296934
26. Musher DM, Manof SB, Liss C, McFetridge RD, Marchese RD, Bushnell B, et al. Safety and antibody response, including antibody persistence for 5 years, after primary vaccination or revaccination with pneumococcal polysaccharide vaccine in middle-aged and older adults. J Infect Dis. (2010) 201:516–24. doi: 10.1086/649839
27. Lackner TE, Hamilton R G, Hill J J, Davey C, and Guay DR. Pneumococcal polysaccharide revaccination: immunoglobulin g seroconversion, persistence, and safety in frail, chronically ill older subjects. J Am Geriatrics Soc. (2003) 51:240–5. doi: 10.1046/j.1532-5415.2003.51064.x
28. Kawakami K, Kishino H, Kanazu S, Takahashi K, Iino T, Sawata M, et al. Time interval of revaccination with 23-valent pneumococcal polysaccharide vaccine more than 5 years does not affect the immunogenicity and safety in the Japanese elderly. Hum Vaccines immunotherapeutics. (2018) 14:1931–8. doi: 10.1080/21645515.2018.1456611
29. Törling J, Hedlund J, Konradsen HB, and Ortqvist A. Revaccination with the 23-valent pneumococcal polysaccharide vaccine in middle-aged and elderly persons previously treated for pneumonia. Vaccine. (2003) 22:96–103. doi: 10.1016/s0264-410x(03)00521-8
30. Clutterbuck EA, Lazarus R, Yu LM, Bowman J, Bateman EA, Diggle L, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis. (2012) 205:1408–16. doi: 10.1093/infdis/jis212
31. Remschmidt C, Harder T, Wichmann O, Bogdan C, and Falkenhorst G. Effectiveness, immunogenicity and safety of 23-valent pneumococcal polysaccharide vaccine revaccinations in the elderly: a systematic review. BMC Infect Dis. (2016) 16:711. doi: 10.1186/s12879-016-2040-y
Keywords: elderly, revaccination, 23-valent pneumococcal polysaccharide vaccine, immunogenicity, safety
Citation: Qiu J, Li Z, Huang F, Huang Z, Liang X, Li J, Liao Y, Guo X and Sun X (2025) Immunogenicity and safety study of 23-valent pneumococcal polysaccharide vaccine revaccination among elderly individuals aged 60–70 years in Shanghai, China. Front. Immunol. 16:1623611. doi: 10.3389/fimmu.2025.1623611
Received: 14 May 2025; Accepted: 01 July 2025;
Published: 17 July 2025.
Edited by:
Ritthideach Yorsaeng, Chulalongkorn University, ThailandReviewed by:
Javier Carbone, Gregorio Marañón Hospital, SpainAmornphat Kitro, Chiang Mai University, Thailand
Ravikumar K. L., Kempegowda Institute of Medical Sciences, India
Copyright © 2025 Qiu, Li, Huang, Huang, Liang, Li, Liao, Guo and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Guo, Z3VveGlhbmdAc2NkYy5zaC5jbg==
 Jing Qiu
Jing Qiu Zhi Li
Zhi Li Fang Huang
Fang Huang Zhuoying Huang1
Zhuoying Huang1 Xiang Guo
Xiang Guo