- 1Cardiovascular Research Team (UR5_3 PC2E), University of the French West Indies (Université des Antilles), Fort de France, France
- 2Department of Toxicology and Critical Care Medicine, University Hospital of Martinique (CHU Martinique), Fort-de-France, France
- 3Department of Research, University Hospital of Martinique (CHU Martinique), Fort-de-France, France
- 4Department of Emergency Medicine SAMU 972, University Hospital of Martinique (CHU Martinique), Fort-de-France, France
Snakebite envenoming remains a predominant neglected disease in tropical and subtropical regions, with high rates of morbidity and mortality worldwide. Bothrops snakebite envenoming. is characterized by severe injuries at the site of venom injection, which include tissue necrosis, hemorrhage, blistering, and edema. Haemotoxicity is typically attributed to the strong procoagulant state induced by the majority Bothrops venoms leading to coagulation factor consumption and incoagulable blood. Concomitantly with this procoagulant state, a complex host response develops in the affected tissues, accompanied by the recruitment of inflammatory and immunocompetent cells, along with the activation of resident cells, and the synthesis of a plethora of pro-inflammatory mediators and damage-associated molecular patterns from injured tissue. An increasing body of evidence suggests that this intricate response is, in fact, related to the well-documented immunothrombosis and thromboinflammation integrated features. Of note, thrombotic complications are extremely rare in Bothrops snakebite envenoming. However, in the case of Bothrops lanceolatus and B. caribbaeus, which are respectively endemic to Martinique and St. Lucia, the absence of overt consumption coagulopathy due to their weak procoagulant effects may be related to the thrombotic effects, as clotting factors are present in the bloodstream by the time the thrombogenic and inflammatory mechanisms are operating in blood vessels. Prior to the era of immunotherapy, B. lanceolatus envenoming was associated with thrombotic complications in 25% of cases and was fatal in approximately 10% of cases. This review examines the potential role of thromboinflammation as a mechanism of thrombotic accidents in B. lanceolatus snakebite envenoming.
Highlights
● Bothrops snakes are the most common venomous snakes in tropical regions.
● Venoms of Bothrops snake have a complex composition of toxins that can trigger a series of systemic effects, including haemotoxicity.
● While thrombosis is a rare feature in Bothrops envenoming, B. lanceolatus bite can elicit thrombotic complications in 25% of cases.
● Understanding the exact pathological mechanisms involved in B. lanceolatus venom-induced thrombotic events are still a challenge for the scientific community.
● Increasing evidence suggests that specific processes involved in thromboinflammation are operative in B. lanceolatus envenoming.
● Thromboinflammation may provide new options for the therapeutical approach of B. lanceolatus envenoming, such as antithrombin, activated protein C, thrombomodulin, and interleukin inhibitors.
1 Introduction
Increasing evidence suggests that specific processes involved in thromboinflammation are operative in Bothrops snakebite envenoming. The terms “immunothrombosis” and “thromboinflammation” refer to the complex interplay between thrombotic and inflammatory pathways (1, 2) Immunothrombosis has been proposed to describe an innate immune response involving intravascular thrombus formation that can lead to the recognition, containment, and destruction of pathogens (1, 3–5). Thromboinflammation is associated with specific pathways that operate through mechanisms involving platelets, leukocytes and immunocompetent cells, and the contact kinin system (3, 6–9). Thromboinflammation is increasingly recognized in several pathologies, including infection and sepsis, as well as stroke, deep vein thrombosis, and myocardial infarction (3, 6–13). Thrombo-inflammatory pathways can exacerbate inflammation and immune cell interactions, eventually leading to vascular occlusion, tissue ischemia, and ultimately irreversible organ damage (1, 3, 4, 6, 9, 13).
The procoagulant components of Bothrops venoms can cause intravascular coagulation, in most cases can induce a consumption coagulopathy, which results in defibrinogenation and incoagulability, as reflected by abnormal blood clotting tests (14). Envenomed patients present increased prothrombin time (PT) and activated partial thromboplastin time (aPTT), with low level of fibrinogen. Extensive experimental, clinical, and laboratory data underpinned the fact that Bothrops snakebite envenoming also elicits a pro-inflammatory state, along with multiple blood cell activation (15–18). However, despite procoagulant state and pro-inflammatory activation, thrombotic complications are extremely rare in Bothrops sp. snakebite envenoming, except for those associated with B. lanceolatus and B. caribbaeus snakes. B. lanceolatus and B. caribbaeus are endemic to Martinique and St. Lucia, respectively and genetically close (19–25). Better known as” trigonocephalus” or “fer-de-lance”, the Bothrops lanceolatus (Bonnaterre, 1790) is a species of snake belonging to the Crotalinae subfamily and the Vipiridae family, just like the Bothrops caribbaeus (Garman, 1887).
Prior to the era of immunotherapy, B. lanceolatus envenoming was associated with thrombotic complications in 25% of cases and was fatal in approximately 10% of cases (20–22). The proposed mechanism of these thrombotic events has been related to the combination of two processes: a weak procoagulant effect of B. lanceolatus and B. caribbaeus venoms, which does not induce coagulation factor consumption, along with the simultaneous potent activation of thrombogenic and inflammatory processes operating in blood vessels (23, 26–30).
The question of whether the thrombogenic and inflammatory processes induced by B. lanceolatus would be considered a manifestation of thromboinflammation has not been explored. This review provides an update to gain insight into the pathophysiological mechanisms involved in thrombo-inflammatory processes associated with Bothrops snakebite envenoming. We will discuss the central role of platelet activation, recruitment of peripheral leukocytes and immunocompetent cells, and activation of the contact kinin system. In addition, we will investigate whether thromboinflammation may represent a valuable mechanism of thrombotic accidents in Bothrops snakebite envenoming.
2 Overview of the toxic effects of Bothrops venoms
2.1 Local effects
The local effects of Bothrops snakebite envenoming are characterized by an intense inflammatory response, a consequence of the direct and indirect action of the venom toxins on the tissues (31). The toxins can directly activate leukocyte receptors, such as Toll-like receptors (TLRs), recognizing venom-associated molecular patterns (VAMPs), and immunological soluble molecules (32–34). In addition, venom toxin-induced tissue damage results in the release of damage-associated molecular patterns (DAMPs), which also contribute to inflammation (35). Direct activation of the complement system by the toxins represents another important mechanism in the pathogenesis of local inflammation (36). The clinical features of Bothrops envenomation are well-established, typically presenting with pain, edema, blistering, ecchymosis, local hemorrhage, and, in severe cases, compartment syndrome and necrosis (37–49).
2.2 Systemic effects
Bothrops venom also induces systemic changes, characterized mainly by coagulation disorders. These can be caused by the direct action of the toxins on coagulation factors, activating the coagulation cascade, leading to a state of blood incoagulability, and by the direct action on platelets, causing platelet death, platelet activation, or cleavage of platelet activation receptors (50). These mechanisms result in platelet consumption, and consequently thrombocytopenia. Together, these factors favor the development of hemorrhage, which can lead to death if not promptly controlled. Thrombotic microangiopathy (TMA) is another systemic manifestation, but less common. TMA appears to be triggered by thrombin generation followed by fibrin formation and deposition in the vascular bed, which are involved in microangiopathic hemolytic anemia and blood vessel wall damage in the micro-circulation. TMA hence carries a risk of organ damage and failure (51–53).
Renal dysfunction following Bothrops envenomation may result from dysregulation of the coagulation cascade, direct nephrotoxicity of venom components, systemic hemodynamic alterations such as hypotension, and, in certain cases, the development of thrombotic microangiopathy (TMA) (15, 18, 54–59). Nonetheless, clinical data indicate that the predominant mechanism of acute kidney injury (AKI) in human envenomation cases is closely associated with coagulation disturbances. AKI has been reported in patients exhibiting prolonged activated partial thromboplastin time (aPTT), hemorrhagic manifestations, elevated lactate dehydrogenase (LDH) levels, and evidence of TMA (23, 60, 61).
3 Bothrops venom induced procoagulant effects and thrombotic events
Bothrops venom has been observed to have a range of effects, including thrombotic, procoagulant, and inflammatory properties (15–18). The genus Bothrops is the most reported genus responsible for snakebites in South America. Of these, B. atrox (Linnaeus, 1758) is the species most frequently involved in cases of life-threatening envenoming in humans (17). In the French Departments of America, B. atrox is the predominant species involved in envenoming in French Guiana, whereas B. lanceolatus is the sole venomous snake present in Martinique, where it is endemic (23–25, 62). The biological active toxins responsible for these features include SVMPs, serine proteinases (SVSPs), phospholipases A2, C-type lectin-like toxins, disintegrins, and L-amino acid oxidases (15–18). Bothrops snakebite envenoming causes significant local tissue damage and systemic manifestations, including coagulopathies, bleeding, and hemorrhage related to enzymatic degradation and rupture of vessel walls (16, 18).
3.1 Procoagulant effects of Bothrops venoms
Envenomed patients typically display increased prothrombin time and activated partial thromboplastin time, along with low level of fibrinogen. Procoagulant toxins derived from Bothrops venom have been observed to activate a number of coagulation factors, including factor Factors II, V, VII, X, XIII II (26–28, 63, 64). Factors involved in the intrinsic pathway (factors VIII, IX, XI, XII) are less frequently reduced (26–28, 63, 64). Activation of the coagulation cascade ultimately results in the generation of intravascular thrombin. From a biological standpoint, one of the most notable distinctions between B. lanceolatus and B. caribbaeus, in comparison to other Bothrops species, pertains to their respective procoagulant activities. The procoagulant activities of Bothrops venoms elicit the conversion of prothrombin to thrombin, which is responsible for consumptive coagulopathy. The majority of Bothrops venoms are capable of activating thrombin without the involvement of cofactors such as calcium and phospholipids (26–28, 30).
Previous research has produced inconsistent findings on the procoagulant effects of B. lanceolatus venom (27, 29, 30, 65–69). Early studies suggested that the venom lacked procoagulant or defibrinogenating activity, as it failed to induce clot formation in citrated human plasma (65, 67, 69). However, these in vitro experiments assessed coagulation in citrated plasma without added calcium or phospholipids—key cofactors that modulate procoagulant activity. More recent thrombo-elastography studies using human plasma or whole blood have shown that B. lanceolatus venom can exhibit procoagulant effects when sufficient calcium and phospholipids are present (27, 29, 30, 66, 68). In brief, the procoagulant activity of B. lanceolatus venom appears weaker than that of other Bothrops venoms. This activity is entirely calcium-dependent, with a minor reliance on phospholipids (27). Unlike typical procoagulant Bothrops venoms—which act by directly activating thrombin (via prothrombin conversion) or indirectly by activating upstream zymogens like factor X (26)—B. lanceolatus venom displays a pseudo-coagulant effect. This results in fragile, unstable fibrin clots that degrade quickly (30), possibly explaining its limited procoagulant activity in citrated plasma.
3.2 Bothrops venom can induce thrombotic complications
Thrombotic complications induced by Bothrops venoms are less common than hemorrhagic complications in Amazonian Bothrops snakebite envenoming (26). Only a limited number of cases of ischemic strokes have been documented so far (Supplementary Table 1 summarized the reported thrombotic complications involved in Bothrops sp. envenoming).
In contrast to with the typical hemorrhagic profile, venoms of Bothrops, such as B. lanceolatus (endemic to Martinique), B. caribbaeus (endemic to St. Lucia, located approximately 40 km south of Martinique), and B. atrox can induce a thrombotic biological profile, which may result in cerebral, myocardial, and pulmonary infarctions (20–22). Prior to the advent of immunotherapy, B. lanceolatus envenoming was linked to systemic thrombotic complications in approximately 30% of cases and was fatal in approximately 10% of cases (20–22). Previous studies failed to identify a distinctive proteomic profile between B. lanceolatus and B. caribbaeus venoms compared with other Bothrops venoms (27, 29, 65). It has been, however, demonstrated that enzymatic or non-enzymatic proteins present in B. lanceolatus and B. atrox venoms may differ in their peptide sequences, which could be responsible for the different biological effects observed, namely hemorrhagic versus thrombotic profiles (29).
Laboratory test abnormalities have been documented regarding activated partial thromboplastin time (aPTT), prothrombin time (PT), prothrombin activity, International Normalized Ratio (INR), fibrinogen consumption, fibrin degradation product increase, thrombocytopenia, along with anemia and leukocytosis (21, 22, 70–79). Furthermore, fibrinogen is a determinant of blood viscosity and platelet activation, also playing a role in the inflammatory process, and is considered a marker related to ischemic stroke (80, 81). Consistent evidence has shown that high levels of fibrinogen may increase the risk of ischemic stroke (82). However, in the case of Bothrops snakebites, hypofibrinogenemia occurs as the result of the action of toxins, which are capable of directly cleaving the chains of fibrinogen releasing fibrinopeptide A and traces of fibrinopeptide B (83). In addition, fibrinogen may be also decreased or consumed as the result of coagulation cascade activation. In this regard, toxins from B. atrox, B. caribbaeus, B. lanceolatus and B. marajoensis (snakes with reports of snakebite envenoming with thrombotic complications) can activate the coagulation cascade via the intrinsic, extrinsic or common pathway. B. atrox venom activates factors II, X, XII and V, and increases the procoagulant activity of factor VIII, which, as a result, leads to the generation of intravascular thrombin and hypofibrinogenemia (84–86). In the case of B. lanceolatus venom, toxins that may alter coagulation factor activation have not been previously isolated and characterized. It is known, however, that B. lanceolatus venom can induce the formation of fibrin in plasma and in purified human fibrinogen, indicating activity similar to thrombin, as well as the degradation of fibrinogen (30). Similarly, B. caribbaeus venom can hydrolyze fibrinogen in vitro resulting in hypofibrinogenemia and increased levels of fibrin/fibrinogen degradation products in vivo, but no increase in D-dimer levels (87). In acute ischemic stroke and infarcts related to B. lanceolatus snakebite, a decrease in aPTT and an increase in PT have been observed. Thrombotic complications with (or without) hemorrhagic transformations triggered by B. atrox venom involve an increase in PT. It is noteworthy that reduced aPTT has been associated with ischemic stroke, severity and neurological worsening (88), although other studies do not corroborate this (89). Of note, the specific pathogenic mechanisms and toxins involved in thrombotic complications associated with B. lanceolatus envenoming remain unknown. Proposed mechanisms include venom-induced endothelial injury, platelet activation, involvement of von Willebrand factor, and proinflammatory activity of the venom (26, 90).
In addition to interspecies differences, intra-species factors such as age, gender, geographic location, diet, and captivity conditions may alter Bothrops venom composition (29, 91, 92). Accidents with juvenile Bothrops cause higher incidence of hemorrhage and coagulation disorders than snakebite with adult snakes, while the latter inflict less inflammation and more severe local tissue damage (93, 94). In the case of B. lanceolatus, thrombotic complications are more frequently observed in patients bitten by juvenile snakes (i.e., small snakes of less than 70 cm in length), while envenoming by adult snakes produce more swelling (95). In Bothrops sp., ontogenetic variation refers to changes in venom composition as the snake progresses from juvenile to adult stages, often correlating with shifts in diet and prey type (93–97). Sexual variation, conversely, denotes differences between male and female venom, potentially linked to physiological or behavioral dimorphism. Ontogenetic and/or sexual variations have been documented in several Bothrops snakes, such as B. leucurus (Wagler, 1824), B. pauloensis (Amaral, 1925), B. jararaca, B. jararacussu, B. moojeni and B. atrox (93–99). These researchers consistently demonstrate that venom from Bothrops juvenile snakes often exhibit higher coagulotoxicity with procoagulant activities, whereas venom from Bothrops adult snakes display increased hemorrhagic and proteolytic activities. Likewise, previous reports have indicated sexual variations in protein levels, like higher disintegrins in female B. atrox and higher serine protease effects in B. moojeni, which may influence venom coagulant effects (99, 100).
4 Proinflammatory effects of Bothrops venoms
4.1 The inflammatory process
Inflammation is an immune system response triggered by various factors, such as pathogens, damaged cells, and toxic compounds (101, 102). This process involves the coordinated activation of signaling pathways—primarily NF-κB, MAPK, and JAK-STAT—which regulate the release of inflammatory mediators from resident tissue cells and modulate the activity of blood-derived immune cells (103, 104). Platelets are among the first responders to endothelial injury and microbial threats. Their expression of P-selectin is crucial for forming platelet-leukocyte aggregates, facilitating leukocyte recruitment and their rolling adhesion to the vascular endothelium in the presence of activated platelets (86). Following platelet activation, circulating neutrophils and monocytes rapidly infiltrate injured tissues (105). Meanwhile, resident macrophages and dendritic cells play key roles in tissue immunosurveillance and antigen presentation. The inflammatory response is tightly controlled by mediators such as cytokines, chemokines, vasoactive amines, and eicosanoids, which act both locally and systemically. These molecules are released near the injury site by endothelial cells and resident immune cells (e.g., mast cells and macrophages) during the early inflammatory phase, preceding leukocyte infiltration (101, 102).
4.2 Inflammatory effects of specific Bothrops sp. toxins
Among biological active toxins isolated from Bothrops sp. venoms, metalloproteases, phospholipases A2 and C-type lectin-like proteins play a relevant role by activating platelet function, the coagulation cascade, and the inflammatory host response (50, 103, 104).
4.2.1 Bothrops sp. venom SVMPs
The innate immune response may be initiated by a large diversity of Bothrops SVMPs. Among them, jararhagin isolated from Bothrops jararaca (Wied, 1824), BaP1 from Bothrops asper (Garman, 1883), batroxase from B. atrox, neuwiedase from Bothrops neuwiedi (Wagler, 1824), moojenactivase from Bothrops moojeni (Hoge, 1966), HF3 from B. jararaca can activate many features of the immune response, including priming of monocyte/macrophage immune competent cells, neutrophil recruitment and activation, release of proinflammatory cytokines and chemokines, and activation of the complement cascade (C5a and C3a release) (59, 103, 104). For example, jararhagin can elicit the recruitment of inflammatory cells and induce the release of inflammatory mediators such as IL-1β, IL-6, IL-8, and IL-11 in vitro (106, 107). Likewise, batroxase can induce the release of pro-inflammatory cytokines (e.g., IL-6, IL-1β, IL-10) and increase the local release of PGE2 prostaglandins (108). BaP-1 activates the complement system (release of C5a), induces leukocyte infiltration and mast cells degranulation, as well as cytokine release (109, 110).
In addition to their direct effects on the immune competent cells, SVMPs can indirectly activate platelets by diverse mechanisms, such prothrombin activation, vWF, factor X, and II and the complement cascade (C5a and C3a) release, along with engagement of platelet glycoprotein receptors (103, 104). For example, berythractivase help to upregulate tissue factor (TF) expression in endothelial cells in vitro, which favor a systemic thrombogenic and inflammatory activities (111, 112). Likewise, moojenactivase induce factor X, and II activation and platelet tissue factor (TF) expression leading to thrombosis and inflammation (113). SVMPs, such as botrocetin and jararhagin, can engage platelet glycoprotein receptors, which also favor thrombosis and inflammation (114, 115).
4.2.2 Bothrops sp. venom phospholipases A2
Several PLA2 have been shown to induce a wide range of inflammatory effects (116). Snake venom PLA2 also play a role in inflammation, intervening in microvascular permeability, edema formation, leukocyte recruitment and cytokine release (104, 117, 118). Among snake venom PLA2, bothropstoxins from Bothrops jararacussu (Lacerda, 1884) can induce mast cell degranulation and stimulate neutrophil chemotaxis by releasing leukotriene B4 (LTB4) and platelet-activating factor (119). Snake venom PLA2 such as batrox PLA2 from B. atrox, BJ-PLA2-I from B. jararaca and piratoxin from Bothrops pirajai (Amaral, 1923) can also induce mast cell degranulation, stimulate neutrophil recruitment, and increase the production of various cytokines and chemokines (108, 119–122). Snake venom Lys49 PLA2 homologs MT-II and MT-III from B. asper activate the inflammatory process through NF-kB activation and can increase macrophage phagocytic activity (108). A purified PLA2 from the venom of B. lanceolatus was recently shown to increase the production of TNF-α, CXCL8, CCL2 and CCL5 and activate the complement system (C5a and C3a release). The venom PLA2 also triggered the generation of lipid mediators, as evidenced by the detected high levels of LTB4, PGE2 and thromboxane TXB2 prostanoid (123).
4.2.3 Bothrops sp. venom C-type lectin-like toxins
C-type lectin family comprises proteins that bind carbohydrates in a Ca2+-dependent manner and non-sugar-binding snake venom C-type lectin-related proteins (SV-CLRPs), so called snaclecs. Snaclecs from snake venom interact with several proteins or receptors having a role in thrombus formation and inflammation, which include C-type lectin-like receptor 2 (CLEC-2), coagulation and vWF factors, GPIb and GPVI receptors on platelets as well as α2β1 receptors of integrins (103, 104). Snaclecs are known to modulate platelet aggregation and their proinflammatory activities. For example, pro-inflammatory activity of B. jararaca on mouse and human platelets has been recently described (103, 104). Likewise, engagement of platelet glycoprotein receptors and prothrombin activate platelet can stimulate the pro-inflammatory host response. More specifically, snaclecs from B. jararacussu and Bothrops leucurus venoms can stimulate immune competent cells (mononuclear cells and neutrophils) to produce proinflammatory mediators (124, 125). Galatrox, a glycan-binding protein from B. atrox snake venom promotes neutrophil migration and induces the release of pro-inflammatory cytokines, such as IL-1 and IL-6 both in vitro and in vivo. Galatrox also stimulates macrophages to produce pro-inflammatory mediators through the TLR4-MyD88 signaling pathway suggesting its role in mediating the proinflammatory action of B. atrox venom (126). Likewise, BJcul from B. jararacussu venom can activate NLRP3 inflammasome through TLR4 signaling pathway and also induce the activation of NF-κB, resulting in the release of several cytokines such IL-1β and proinflammatory mediators (124–127).
4.2.4 Bothrops sp. venom serine proteases
SVSPs are monomeric glycoproteins displaying proteolytic activites that are directly involved in the coagulation machinery by inducing platelet aggregation and activation of coagulation factors. The activity of serine proteases has been mainly correlated to the thrombin-like activity of Bothrops sp. venoms, but “kininogenases” present in these venoms have also been shown to participate to inflammatory processes (103, 104). Recent findings suggest the participation of SVSPs in the local and systemic inflammation processes induced by crude Bothrops sp. venom. SVSPs from B. pirajai snake venom, BpirSP27 and BpirSP41, can promote neutrophil recruitment in the peritoneal inflammatory exudate (108). In contrast, the SVSP batroxobin from B. moojeni is a defibrinogenating agent that can inhibit human NETs induced by TNF-α (128). Beside Bothrops sp. SVSPs, KnBa from the African viper Bitis arietans can increase the production of IL-1β, TNF α, and IL-6 and also upregulated chemokines such as IL-8, RANTES and MCP-1 (129).
As stated above, the most described activity of SVSPs is thrombin-like, but theses pro-coagulant enzymes also present other activities, such as kallikrein-like, platelet aggregation, and activators of the following substrates: plasminogen, factor X, factor V, prothrombin, and protein C, which may be involved in several inflammatory processes (120). Notably, certain kallikrein-like SVSPs are known to generate vasoactive kinins from α kininogens, which are involved in the regulation of blood pressure, vascular permeability, and inflammatory processes (130). Among others, kininogenase from B. jararaca, BjussuSP-I, from B. jararacussu, leucurobin from B. leucurus, and BpSP-I from B. pauloensis venoms display kallikrein-like activity and elicit the release of kallikrein that directly liberates bradykinin and derived vasoactive proinflammatory peptides (131–133). Following B1 and B2 receptor binding, bradykinin can induce numerous pathophysiological processes, including expression of adhesion molecules, leukocyte infiltration and formation of inter-endothelial gaps and protein extravasation (134–136).
4.3 Inflammation initiated by whole Bothrops sp. venoms
The inflammatory response triggered by Bothrops venoms involves platelet activation, leukocyte recruitment (primarily polymorphonuclear and mononuclear cells at the injury site), and the participation of endothelial cells and resident immune cells, which release cytokines in response to the venom (50, 90, 103). In Bothrops envenomation, both local and systemic inflammation can occur. A hallmark feature of local tissue damage caused by Bothrops venoms is blister formation, characterized by the accumulation of protein-rich fluid due to inflammatory exudation. Analyses of wound exudates and blister fluid consistently reveal elevated levels of pro-inflammatory mediators (41, 45, 137, 138). Additionally, among the numerous DAMPs detected, the most abundant proteins in these exudates are linked to platelet degranulation, innate immune activation, complement pathways, and coagulation cascade.
4.4 The role of platelets and neutrophils in inflammation caused by Bothrops venoms
In addition to hemostasis, platelets are involved in a multitude of physiological and pathological processes, including the innate immune response induced by Bothrops venoms. It is well established that Bothrops venoms exert effects on platelets, which are known to be affected by mechanisms including binding or degradation of vWF or platelet receptors, activation of protease-activated receptors (PARs) by thrombin-like enzymes, and modulation of adenosine diphosphate (ADP) and thromboxane A2 release (26, 50, 103, 139).
Neutrophils, the primary component of the innate immune system’s initial response, have been identified as a key player in the context of inflammation induced by Bothrops venoms (140). Upon activation, neutrophils produce substantial quantities of pro-inflammatory cytokines and are capable of releasing neutrophil extracellular traps (NETs), thereby influencing the course of inflammatory processes (50, 140). The effects of Bothrops venoms on neutrophils have been extensively studied, resulting in a substantial body of knowledge accumulated over decades (140).
4.5 Activation of complement system, endothelial response and inflammatory mediator release caused by Bothrops venoms
Bothrops venoms have the capacity to activate the complement cascade, resulting in the generation of substantial quantities of anaphylatoxins, including C3a, C4a, and C5a (108, 141–145). These anaphylatoxins are regarded as the pivotal mediators between innate and adaptive immunity. Another typical characteristic of the systemic inflammatory syndrome induced by Bothrops venoms is the cytokine and chemokine storm, which reflects the emergence of multiple disorders in the regulation of the immune response. Once more, a plethora of proinflammatory activities has been observed in Bothrops sp (141–145).
B. lanceolatus venom exerts a profound impact on the complement system, revealing that the toxins activate both the alternative and classical complement pathways, unbalancing the homeostasis of this immune system (143). Activation of the alternative pathway is accompanied by a paradoxical inhibition of its lytic activity, while the classical pathway is activated by the cleavage of C1 inhibitor by proteases present in the venom. The convergence of the three complement pathways results in the formation of C5 convertase, which cleaves C5 into C5a and C5b. The C5a fragment, a potent anaphylatoxin, induces inflammation and the recruitment of inflammatory cells (146, 147). An elegant study of Delafontaine et al. indicates that metalloproteases in the venom are primarily responsible for the generation of C5a (143). The C5b portion initiates the assembly of the membrane attack complex (MAC), which forms pores in the cell membrane, leading to cell lysis. In addition to this effector function, the complement system, when activated by the venom, performs other biological functions, such as opsonization of pathogens, formation of NETs by neutrophils, and release of anaphylatoxins (C3a and C5a), which amplify the inflammatory response (148–150). On the other hand, venoms of B. atrox inhibit the activation of the complement system by the alternative pathway (144). Thus, contribution of the complement system to thromboinflammation in snakebite still remains unclear and requires further exploration.
An ex vivo model based on human whole blood demonstrated that B. lanceolatus venom elicited an inflammatory reaction comprising the production of pro-inflammatory interleukins (IL-1β, IL-6 and TNF-α), chemokine upregulation (MCP-1, RANTES and IL-8), complement activation and eicosanoid release (leukotriene, prostaglandin and thromboxane) (142). Similarly, the administration of B. lanceolatus and B. atrox venoms in rats has been observed to result in elevated levels of plasmatic proinflammatory cytokines, including interleukin-1 β (IL-1β), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and monocyte chemoattractant protein-1 (MCP-1) (28). In the latter study, plasmatic proinflammatory mediator levels were observed to be higher in rats treated with B. lanceolatus compared to those treated with B. atrox.
Previous in vivo and in vitro studies have yielded consistent results regarding the deleterious effects of Bothrops venoms on endothelial cell integrity and function (26, 50, 90). Degradation of basement membrane and the subsequent disruption of endothelial cell integrity have been described (151). Detachment of endothelial cells from their surrounding basal lamina, leading to discontinuity of endothelial cell line and extravasation (111, 152, 153). While many SVMPs have no direct cytotoxic effect on capillary endothelium, jararhagin can decrease endothelial cell viability and induce cellular apoptosis, which can be reinforced by phospholipase A2 action (111).
An organ‐on‐a‐chip approach used to investigate the effects of various venoms on a perfused microfluidic blood vessel model have recently suggested that endothelial barrier function of the microvasculature can be affected by two different mechanisms, including disruption of the endothelial cell membrane and delamination of the endothelial cell monolayer from its matrix (154). SVMPs toxins, isolated from Bothrops venoms, induce the expression of adhesion molecules on the microvasculature of murine cremaster muscle. In vivo, intravenous injection of B. jararaca venom in rabbits induces endothelial injury as evidenced by increase plasma soluble thrombomodulin levels (155). Likewise, SVMPs isolated from venom can render endothelial cells highly thrombogenic, with the release of vWF and expression of tissue factor TF, ICAM-1 and E-selectin (155). SVMPs can also cleaves endothelial glycocalyx proteoglycans, which participle to the disruption of microvasculature integrity (156). In the case of B. lanceolatus venom, endothelial injury as evidenced by ICAM-1, VCAM-1, E-selectin, and TF expression, seem to be particularly low compared to B. jararaca venom, while longer times of incubation enhanced B. lanceolatus venom induced cytotoxicity (143, 157). Overall, based on these experimental findings, B. lanceolatus venom exhibits poor direct endothelial cell toxicity, while intermediate system such as the involvement of the complement system may activate endothelium in vivo.
The link between the complement system and thrombosis is complex and multifaceted. Although complement is primarily known for its immunological function, recent studies have demonstrated its involvement in inflammatory processes and blood coagulation. One point that is noteworthy is that C3 is a target of Bothrops toxins, and in stroke, elevated plasma C3 levels are markers of worse prognosis in patients with ischemic stroke. In fact, in brain tissue, C3 is produced locally and its activation contributes to ischemic injury (158). Different mechanisms by which the complement cascade can activate coagulation (immunothrombosis) have been reported. Elevated concentrations of complement C3 in the normal population are associated with an increased risk of venous thromboembolism (VTE) (159). When activated, C3 plays a crucial role in amplifying thrombus formation by activating platelets and modulating tissue factor (TF) function by inducing conformational changes in TF, increasing its procoagulant activity and facilitating the exposure of phosphatidylserine (160). C5a can promote the release of prothrombotic factors from platelets, induce the expression of endothelial tissue factor, and promote the natural production of anticoagulants. Other complement components may promote fibrinogen cleavage and increase XIIIa activity, among other mechanisms (161, 162). Therefore, complement dysregulation disorders, such as those caused by Bothrops venoms, may result in a prothrombotic state and thrombotic microangiopathy (small vessel thrombosis).
5 Immunothrombosis and thromboinflammation
5.1 Immunothrombosis
“Immune thrombosis” refers to an excessive inflammatory response that leads to thrombotic events. The concept of “immunothrombosis,” first introduced by Engelmann and Massberg (1), describes a thrombus formation process mediated by immune cells and thrombosis-related molecules. This mechanism aids in pathogen recognition, damaged cell detection, and containment of microbial dissemination in circulation (1, 3–5). Immunothrombosis thus represents an evolutionarily conserved connection between coagulation and innate immunity (163). In contrast, “thromboinflammation” denotes a concurrent inflammatory and thrombotic response occurring in microvessels following exposure to harmful stimuli, such as pathogens or DAMPs (3, 6–9).
Immunothrombosis is emerging as a distinct host defense mechanism against infection, employing specialized molecular pathways to enhance antimicrobial protection. This process enables pathogen recognition, limits microbial dissemination, and contributes to vascular immunity by integrating coagulation and immune responses within the bloodstream (1, 3–5). Microbial components, designated as pathogen-associated molecular patterns (PAMPs), are recognized by pattern recognition receptors (PRRs) on immune competent cells, such as monocytes (164). Following the recognition of the pathogen, monocytes present activated tissue factor (TF) on their surfaces, which is released in situ, thus activating the extrinsic pathway of coagulation (165, 166). Monocytes release decrypted TF in a process called pyroptosis, which provokes leakage in response to NLRP3 inflammasome and caspase pathway (IL-1β and IL-18) activation (4). Additionally, monocyte activation can result in the release of pro-inflammatory cytokines (165, 166). Proinflammatory molecules recruit neutrophils, which contribute to immunothrombosis through the release of NETs. NETs directly activate factor XII, thereby initiating the contact-dependent pathway of coagulation. NETs bind von Willebrand factor (vWF) and facilitate the recruitment and activation of platelets. NETs cleave and inactivate natural anticoagulants, including tissue factor pathway inhibitor and thrombomodulin (166–170). Additionally, NETs can externalize and bind tissue factor TF, which further promotes the activation of the extrinsic pathway of coagulation (171).
Immunothrombosis describes an overshooting inflammatory reaction that results in detrimental thrombotic activity. The major pathological outcome is thrombosis (occlusion of a blood vessel) due to platelet-involved thrombotic activity in response to initial inflammatory stimuli, such as pathogen invasion. Upon activation, platelets promote the immunothrombotic process by triggering the contact-dependent pathway of coagulation through the release of polyphosphates. In collaboration with endothelial cells, they facilitate the generation of fibrin (172, 173). Once activated, platelets release substantial quantities of pro-inflammatory cytokines, thereby contributing to the establishment of an inflammatory microenvironment. As a result of this mechanism, pathogens are trapped within the fibrin-based NETs and eliminated in this intravascular restricted compartment (3–5, 7).
In this scenario, immunothrombosis is primarily initiated by monocytes and neutrophils and is facilitated by the formation of microthrombi in microvessels (167, 168, 174–176). It is of particular importance to note the crucial role played by tissue factor, monocyte triggering via NLRP3 inflammasome activation, release of NETs, and activated platelets in the cross talk of inflammation with the coagulation processes. It is noteworthy that the term “immunothrombosis” is now employed in a more expansive manner to encompass thrombotic events driven by infection or sterile inflammation. The primary consequence of immunothrombosis is a process of microcoagulation, which does not elicit an adverse systemic response and effectively immobilizes invading pathogens or foreign “alarmin” structures (in the context of sterile inflammation) for subsequent clearance by immune competent cells (1, 3–5).
5.2 Thromboinflammation
This term denotes a process whereby inflammation and thrombosis coexist within microvessels in response to noxious stimuli, including pathogens, injured cells, and other harmful molecules. Thromboinflammation represents the manifestation of dysregulation of the two most crucial defensive and wound-healing responses of the body: inflammation and hemostasis (3, 6–9). Thromboinflammation describes the interplay of platelets and coagulation with the vascular system, resulting in the recruitment of immune cells. In this process, the initial platelet adhesion/activation pathways act in concert with key components of immune cells and the contact pathway of plasmatic coagulation (factor XII—kallikrein/kinin pathway) (5, 12, 166, 172). Intravenous thrombotic processes can, in turn, trigger aberrant complement, coagulation, platelet, and endothelial cell activation, which may ultimately result in disrupted vascular integrity. It is well-established that viral and bacterial infections, as well as ischemia–reperfusion (e.g., acute ischemic stroke, coronary heart disease), can cause microvascular thrombi and fuel inflammatory processes (3, 6–11, 13). Overall, thrombo-inflammation describes the interplay of platelets and coagulation with the immunovascular system. The major pathological outcome is resulting in the recruitment of immune cells.
6 Thromboinflammation induced by Bothrops venoms
Thromboinflammation triggered by Bothrops snakebite envenoming represents a distinct pathophysiological pathway with unique biological characteristics (3, 6–9). Supplementary Table 2 summarized the main characteristics of thromboinflammation in Bothrops sp. envenoming comparing the effects of venoms with hemorrhagic profile with those with thrombotic profile.
Previous research has suggested an interaction between inflammatory and coagulation processes in these envenomation cases (50). A recent investigation of B. atrox bite victims demonstrated that fibrinogen concentrations modulate inflammatory mediator responses (177). The researchers found that fibrinogen levels directly influence cytokine and chemokine expression patterns including CXCL-8 CXCL-9 CCL-2 and IL-6. They also documented elevated CCL-5 levels alongside decreased IFN-γ concentrations in patients with reduced plasma fibrinogen. This study provided the first evidence that thromboinflammation involving reciprocal interactions between inflammation and coagulation mechanisms occurs in Bothrops envenomation cases (177).
6.1 Role of platelets
Platelets mediate a harmful interaction between coagulation pathways and immune cells. Within the framework of thromboinflammation this dysregulated crosstalk leads to NETs formation and activation of immune-competent cells including monocytes (178). The P-selectin/PSGL-1 axis serves as a crucial mediator of cellular interactions involving endothelial cells immune cells and neutrophils. P-selectin remains stored within α-granules of resting platelets and Weibel-Palade bodies of endothelial cells. PSGL-1 functions as the principal receptor for P-selectin facilitating neutrophil recruitment and fostering a proinflammatory milieu through monocyte/macrophage activation (3 6 173). Extensive research has characterized the impact of Bothrops venoms on platelet aggregation and activation (179–181). However relatively few studies have examined adhesion molecule expression on platelet surfaces following Bothrops venom exposure. Both in vitro and in vivo experiments have demonstrated that Bothrops venoms can upregulate various adhesion molecules including L-selectin integrin αLβ2 (LFA-1) ICAM-1 PECAM-1 and CD18. Notably only a single investigation has reported P-selectin expression on platelet surfaces during the early hours following B. jararaca envenomation in rabbits (182). These findings underscore the need for greater emphasis on understanding the P-selectin/PSGL-1 pathway’s role in Bothrops envenomation pathophysiology.
6.2 Role of leukocytes
Although the interaction between platelets and monocytes/leukocytes through P-selectin-PSGL1 binding has not been specifically investigated, the increased expression of E-selectin and L-selectin induced by Bothrops venoms has been demonstrated to facilitate leukocyte rolling and adhesion to the endothelium (28, 183–185). In accordance with the aforementioned findings, an elegant study employing intravital microscopy has demonstrated that jararhagin, a multi-domain snake venom metalloproteinase isolated from B. jararaca venom, can increase the number of rolling leukocytes in post-capillary venules of mouse cremaster muscle (186). Furthermore, in vivo studies have demonstrated that Bothrops venoms can induce the migration of polymorphonuclear neutrophils to the envenoming sites and function as phagocytes and inflammatory response controllers (141, 184–186).
As part of the host defense mechanisms against snake venom reaction, neutrophils generate reactive oxygen species (ROS), produce several proinflammatory cytokines and eicosanoids, and release of NETs (140). NETs production represents a convergence point for the processes of inflammation, coagulation, and thrombosis (3–5, 12, 168, 169, 171, 176, 187, 188). NETs are regarded as a crucial element in the thrombotic process, as they intensify platelet and endothelial cell activation and facilitate fibrin formation in Bothrops envenoming (87, 88). In vitro and in vivo studies have demonstrated that Bothrops venom can induce NET formation (131, 189–191). Several toxins of snake venoms were able to induce in vitro DNA release from human neutrophils. For example, BaTX-II, a phospholipase A2 from B. atrox induced the release of double strand DNA from neutrophils collected from healthy donors (190). Similar results have been obtained with BjussuMP-II, a P–I class of SVMPs from the B. jararacussu (191).
6.3 Role of high molecular weight vWF multimers
The vascular occlusion observed in thromboinflammation depends critically on interactions between ultra-large von Willebrand factor (vWF) and NETs (2, 9, 167, 174–176). Research has shown that Bothrops snake venoms can directly influence vWF polymerization in circulating blood (189). Experimental studies in rats revealed temporary reductions in plasma ADAMTS13 concentrations following envenomation by B. jararaca and B. lanceolatus (28, 192). Interestingly these venom-induced decreases in ADAMTS13 activity did not consistently lead to elevated vWF antigen levels or a shift toward ultra large and high molecular weight vWF multimers in circulation. This apparent paradox has been explained by the proteolytic degradation of these multimers through the action of venom metalloproteinases (192–194).
The lack of detectable ultralarge vWF multimers in plasma does not exclude their potential role in thromboinflammation. These multimers may still participate in NET formation and vascular adhesion particularly when considering venom-induced endothelial activation. The combined effects of immune cell activation endothelial stimulation and impaired vWF cleavage due to reduced ADAMTS13 activity likely promote platelet-vessel wall interactions that drive microthrombus formation. Histological examinations of thrombi from both animal models and human cases consistently demonstrate colocalization of fibrin NETs and vWF within the thrombus structure (2). Supporting these findings microthrombi have been identified in pulmonary vessels of envenomed mice and in postmortem analysis of a case of B. lanceolatus envenomation (23, 29).
6.4 Thromboinflammation as a unifying mechanism of Bothrops venom-induced thrombosis
To gain a deeper understanding into underlying mechanisms of thrombotic complications in Bothrops snakebite envenoming, it is essential to consider two lines of evidence. Firstly, the absence of overt consumption coagulopathy due to the weak procoagulant effects of the venoms of these snakes may be associated with the thrombotic effects, as clotting factors are present in the bloodstream by the time the thrombogenic and inflammatory mechanisms induced by the venom are operating in blood vessels (28). Secondly, in addition to the presence of normal coagulation factors and a proinflammatory response, it is necessary to examine whether the biological response elicited by Bothrops venoms exhibits the typical signature of thrombo-inflammation.
B. lanceolatus and B. caribbaeus venoms, that display a prothrombotic profile, can exhibit specific biological characteristics that are identical to those observed in thromboinflammation. Firstly, it can be argued that the lack of overt consumption coagulopathy due to the weak procoagulant effects of B. lanceolatus and B. caribbaeus venoms in comparison to other Bothrops venoms is a crucial factor that allows for a hypercoagulable state. Similarly, it has been demonstrated that the systemic proinflammatory response elicited by B. lanceolatus venom is more pronounced than that induced by B. atrox venom (28). Therefore, the thrombotic effects of B. lanceolatus venom may be attributable to the combined action of clotting factor activation and the concurrent operation of potent inflammatory mechanisms within the blood vessels (123, 142, 143, 157). Secondly, an increasing body of evidence indicates that B. lanceolatus and B. caribbaeus venoms exhibit distinctive characteristics of thromboinflammation, which are not observed in other Bothrops venoms. For example, distinct kallikrein-like activity and ADAMTS13/von Willebrand factor (vWF) interactions have been observed in B. lanceolatus and B. atrox venoms (30). A summary of major drivers involved in inflammation and coagulation activation eventually leading to thromboinflammatory pathways in Bothrops snakebite envenoming is displayed Figure 1.
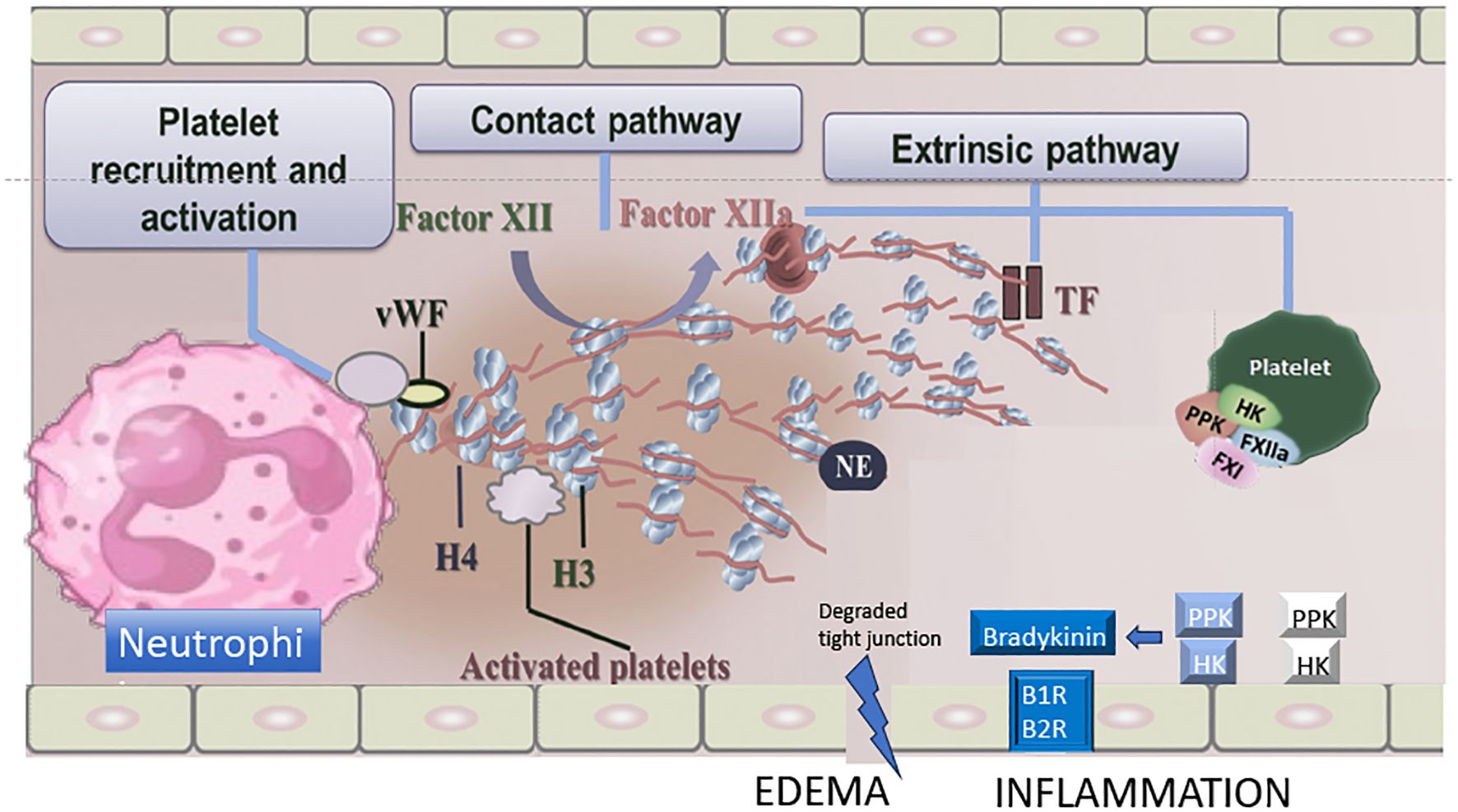
Figure 1. Major drivers involved in inflammation and coagulation activation eventually leading to thromboinflammatory pathways in Bothrops sp. envenoming. Proposed mechanisms of contact phase activation with NET formation, extrinsic coagulation, von Willebrand factor (vWF) and kallikrein/bradykinin pathway following Bothrops sp. venom exposure. H, histone; NE, neutrophil elastase; TF, tissue factor; HK, high-molecular-weight kininogen; PPK, plasma prekallikrein; B1R/B2R, bradykinin 1/2 receptors. Adapted from Teixeira C et al. Inflammation Induced by Platelet-Activating Viperid Snake Venoms: Perspectives on Thromboinflammation. Front Immunol. 2019;10:2082. doi:10.3389/fimmu.2019.02082.
Before concluding that Bothrops sp. envenoming is related to thromboinflammation, it is important to consider the dualistic effects of snake venoms on hemostasis. Snake venoms are intricate biochemical arsenals that often contain toxins with opposing effects on hemostasis, including both procoagulant and anticoagulant factors, as well as platelet-activating and platelet-inhibiting components (16, 26). The resulting disturbances in hemostasis and inflammation can arise through independent, synergistic, or antagonistic mechanisms, contributing to the diverse and sometimes paradoxical effects observed in snakebite victims. At first glance, anticoagulant toxins and thromboinflammation appear to represent opposing mechanisms—anticoagulants prevent clotting, while thromboinflammation involves thrombosis-driven inflammatory responses. Nevertheless, despite their anticoagulant effects, some venom toxins can indirectly drive thromboinflammation through the cleavage of fibrinogen that releases fibrinopeptides and other breakdown products, which may activate TLR4, elicit pro-inflammatory cytokine release and leukocyte recruitment (104). Likewise, some anticoagulant toxins (e.g., disintegrins) block platelet aggregation, but can activate platelets (179–181), which may release microparticles (procoagulant surfaces) and serotonin, along with P-selectin expression and leukocyte recruitment creating a prothrombotic microenvironment and vascular inflammation. Overall, antiplatelet toxins do not simply prevent clotting—they shift thrombosis to alternative pathways (platelet activation, NETosis, tissue factor-driven coagulation, endothelial injury).
7 Advances and perspectives for the study of thromboinflammation in snakebite envenoming
The interaction between hemostasis and activation of innate immunity is highly complex, which makes it difficult to precisely define the relative contribution of each of these two processes to the pathogenesis of different complications, possibly explaining the absence of a direct association between classical biomarkers of hemostasis activation and the risk or severity of some of these clinical manifestations. Thus, the crossover between inflammation and thrombosis is very well exemplified during snakebite envenoming, due to the presence of a wide variety of characterized proteins that can activate the innate immune system and/or hemostasis.
Evidence supports the crossover between inflammation and hemostasis: (i) studies in animal models report hemostasis disorders; despite some heterogeneity within the model and within the venom, a less effective hemostatic system is associated with an increase in hemorrhagic manifestations; (ii) it has been shown that components of the coagulation system, such as platelets, also signal through immunological pathways; (iii) there are examples of toxins and venoms whose mechanisms disrupt local hemostatic balance and induce inflammation; and (iv) studies show that leukocytes are not only found at the site of envenoming, but also in arterial and venous thrombi. However, it is noteworthy that to date, little studies about Bothrops snakebite envenoming causing thrombotic complications have been carried out.
To advance our understanding of the role of thromboinflammation in the development of thrombotic clinical complications in Bothrops snakebite envenoming, it is necessary to develop new study models and apply advanced study techniques. This limitation to date is due to the use of less robust techniques and studies that report changes in only one of the axes of thromboinflammation, resulting in incomplete reports and fragmented conclusions. It is important to mention that research on thromboinflammation involves a variety of approaches, and some are proteomics, animal models, biomarkers for thromboinflammation measure, use of image techniques and others. This will not be an easy task for the scientific community, and among the challenges we can list the difficulty associated with (i) multiple molecular interactions, considering that thromboinflammation involves a complex cascade of biochemical reactions, with the participation of several cells and molecules; (ii) heterogeneity of the disease, since the clinical manifestations of thromboinflammation vary widely between different diseases and individuals, making it difficult to create universal models, especially for snake envenoming; (iii) study of the inflammatory microenvironment, which is dynamic and heterogeneous, influencing the progression of thrombosis.
Proteomics is a useful tool for studying thromboinflammation in a more comprehensive manner, allowing us to identify changes in biochemical processes related to hemostasis and inflammation (complement system, inflammatory cells, cytokines, chemokines) and other elements to thromboinflammation (195–197). This can be done using animal models, which is a limiting factor, because other models have already been developed to study the pulmonary thrombotic effect induced by Bothrops snake venom (29), we do not yet have models for cerebral thrombotic complications. Studies characterizing biomarkers of thrombo-inflammation in patients with cerebral thrombotic complications from Bothrops snakebite envenoming will also be useful, as will the use of proteomics to study these cases (198). The use of imaging techniques, such as computed tomography and magnetic resonance imaging, can be used to visualize thrombus formation and assess the impact of thromboinflammation on different organs. The use of organoids and organ chips will allow for more realistic simulation of the in vivo microenvironment, genetically modified animal models to study the role of specific pathways and proteins in thromboinflammation, the analysis of large data sets and the application of machine learning algorithms; and finally, collaboration between different areas, such as biology, medicine, engineering, and computer science, will be essential to overcome the challenges of modeling thromboinflammation.
8 Conclusions
Bothrops snake venom, which is common in tropical regions, has a complex composition of toxins that can trigger a series of systemic effects, including coagulopathies and thrombotic events. Understanding the exact mechanisms involved in this pathogenesis is still a challenge for the scientific community, but some theories have been proposed to explain this complex interaction between the venom and the human organism. The thromboinflammation theory has emerged as one of the main hypotheses to explain the effects of Bothrops venom.
Thrombotic complications are extremely rare in Bothrops snakebite envenoming. Several factors can contribute to the development of ischemic stroke in patients bitten by Bothrops snakes, which include the procoagulant activity of venom toxins, hypovolemic shock, and endothelial dysfunction and injury. In the specific cases of B. lanceolatus and B. caribbaeus, thrombotic events are frequent. Proposed mechanism has been related to the combination of two processes: a weak procoagulant effect of B. lanceolatus and B. caribbaeus venoms, which does not induce coagulation factor consumption, along with the simultaneous activation of thrombogenic and inflammatory processes operating in blood vessels. Despite early and adequate initiation of treatment for B. lanceolatus and B. caribbaeus envenoming, the patient can develop a catastrophic stroke, resulting in significant disability. Proposal of thromboinflammation as a key pathophysiological event in these envenoming will provide options for the development of new therapeutical issues, which may target antithrombin, activated protein C, thrombomodulin, glycosaminoglycans and interleukin inhibitors.
Author contributions
CR: Writing – review & editing, Data curation, Investigation. JF: Data curation, Investigation, Writing – review & editing. FR: Data curation, Investigation, Writing – review & editing. PJ: Data curation, Investigation, Writing – review & editing. FN: Data curation, Investigation, Writing – review & editing. PG: Data curation, Investigation, Writing – review & editing. OP: Data curation, Investigation, Conceptualization, Writing – review & editing. DR: Conceptualization, Writing – review & editing. RN: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The present study benefited from grants from APIDOM GIRCI SOHO, 2019 and the French National Research Agency (ANR-25: AAPG 2025 - PRC – KariBothrops).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1625165/full#supplementary-material
References
1. Engelmann B and Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. (2013) 13:34–45. doi: 10.1038/nri3345
2. Martinod K and Wagner DD. Thrombosis: tangled up in NETs. Blood. (2014) 123:2768–76. doi: 10.1182/blood-2013-10-463646
3. Martinod K and Deppermann C. Immunothrombosis and thromboinflammation in host defense and disease. Platelets. (2021) 32:314–24. doi: 10.1080/09537104.2020.1817360
4. Marcos-Jubilar M, Lecumberri R, and Paramo JA. Immunothrombosis: molecular aspects and new therapeutic perspectives. J Clin Med. (2023) 12:1399. doi: 10.3390/jcm12041399
5. De Nardi AC, Coy-Cangucu A, Saito A, Florio MF, Marti G, Degasperi GR, et al. Immunothrombosis and its underlying biological mechanisms. Hematol Transfus Cell Ther. (2024) 46:49–57. doi: 10.1016/j.htct.2023.05.008
6. De Meyer SF, Langhauser F, Haupeltshofer S, Kleinschnitz C, and Casas AI. Thromboinflammation in brain ischemia: recent updates and future perspectives. Stroke. (2022) 53:1487–99. doi: 10.1161/STROKEAHA.122.038733
7. Stark K and Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol. (2021) 18:666–82. doi: 10.1038/s41569-021-00552-1
8. Wagner DD and Heger LA. Thromboinflammation: from atherosclerosis to COVID-19. Arterioscler Thromb Vasc Biol. (2022) 42:1103–12. doi: 10.1161/ATVBAHA.122.317162
9. Szepanowski RD, Haupeltshofer S, Vonhof SE, Frank B, Kleinschnitz C, and Casas AI. Thromboinflammatory challenges in stroke pathophysiology. Semin Immunopathol. (2023) 45:389–410. doi: 10.1007/s00281-023-00994-4
10. Cui J, Li H, Chen Z, Dong T, He X, Wei Y, et al. Thrombo-inflammation and immunological response in ischemic stroke: focusing on platelet-Tregs interaction. Front Cell Neurosci. (2022) 16:955385. doi: 10.3389/fncel.2022.955385
11. Tang X and Zheng F. A review of ischemic stroke in COVID-19: currently known pathophysiological mechanisms. Neurol Sci. (2022) 43:67–79. doi: 10.1007/s10072-021-05679-0
12. Nappi F. To gain insights into the pathophysiological mechanisms of the thrombo-inflammatory process in the atherosclerotic plaque. Int J Mol Sci. (2023) 25:47. doi: 10.3390/ijms25010047
13. Iba T, Helms J, Levi M, and Levy JH. Thromboinflammation in acute injury: infections heatstroke and trauma. J Thromb Haemost. (2024) 22:7–22. doi: 10.1016/j.jtha.2023.07.020
14. Cavalcante JS, Arruda SST, Riciopo PM, Pucca M, and Ferreira Junior RS. Diagnosis of human envenoming by terrestrial venomous animals: routine advances and perspectives. Toxicon X. (2024) 24:100211. doi: 10.1016/j.toxcx.2024.100211
15. Leon G, Herrera M, Segura A, Villalta M, Vargas M, and Gutierrez JM. Pathogenic mechanisms underlying adverse reactions induced by intravenous administration of snake antivenoms. Toxicon. (2013) 76:63–76. doi: 10.1016/j.toxicon.2013.09.010
16. Gutierrez JM, Calvete JJ, Habib AG, Harrison RA, Williams DJ, and Warrell DA. Snakebite envenoming. Nat Rev Dis Primers. (2017) 3:17063. doi: 10.1038/nrdp.2017.63
17. Monteiro WM, Contreras-Bernal JC, Bisneto PF, Sachett J, Mendonca da Silva I, Lacerda M, et al. Bothrops atrox the most important snake involved in human envenomings in the amazon: how venomics contributes to the knowledge of snake biology and clinical toxinology. Toxicon X. (2020) 6:100037. doi: 10.1016/j.toxcx.2020.100037
18. Seifert SA, Armitage JO, and Sanchez EE. Snake envenomation. N Engl J Med. (2022) 386:68–78. doi: 10.1056/NEJMra2105228
19. Thomas L, Tyburn B, Bucher B, Pecout F, Ketterle J, Rieux D, et al. Prevention of thromboses in human patients with Bothrops lanceolatus envenoming in Martinique: failure of anticoagulants and efficacy of a monospecific antivenom research group on snake bites in Martinique. Am J Trop Med Hyg. (1995) 52:419–26. doi: 10.4269/ajtmh.1995.52.419
20. Thomas L, Tyburn B, Lang J, and Ketterle J. Early infusion of a purified monospecific F(ab’)2 antivenom serum for Bothrops lanceolatus bites in Martinique. Lancet. (1996) 347:406. doi: 10.1016/S0140-6736(96)90590-5
21. Numeric P, Moravie V, Didier M, Chatot-Henry D, Cirille S, Bucher B, et al. Multiple cerebral infarctions following a snakebite by Bothrops caribbaeus. Am J Trop Med Hyg. (2002) 67:287–8. doi: 10.4269/ajtmh.2002.67.287
22. Thomas L, Chausson N, Uzan J, Kaidomar S, Vignes R, Plumelle Y, et al. Thrombotic stroke following snake bites by the “Fer-de-lance” Bothrops lanceolatus in Martinique despite antivenom treatment: A report of three recent cases. Toxicon. (2006) 48:23–8. doi: 10.1016/j.toxicon.2006.04.007
23. Malbranque S, Piercecchi-Marti MD, Thomas L, Barbey C, Courcier D, Bucher B, et al. Fatal Diffuse Thrombotic Microangiopathy after a Bite by the “Fer-de-Lance” Pit Viper (Bothrops lanceolatus) of Martinique. Am J Trop Med Hyg. (2008) 78:856–61. doi: 10.4269/ajtmh.2008.78.856
24. Resiere D, Megarbane B, Valentino R, Mehdaoui H, and Thomas L. Bothrops lanceolatus bites: guidelines for severity assessment and emergent management. Toxins. (2010) 2:163–73. doi: 10.3390/toxins2010163
25. Resiere D, Florentin J, Mehdaoui H, Kallel H, Legris-Allusson V, Gueye P, et al. Bothrops lanceolatus envenoming in Martinique: A historical perspective of the clinical effectiveness of bothrofav antivenom treatment. Toxins. (2024) 16:146. doi: 10.3390/toxins16030146
26. Larreche S, Chippaux JP, Chevillard L, Mathe S, Resiere D, Siguret V, et al. Bleeding and thrombosis: insights into pathophysiology of bothrops venom-related hemostasis disorders. Int J Mol Sci. (2021) 22:9643. doi: 10.3390/ijms22179643
27. Larreche S, Bousquet A, Chevillard L, Gahoual R, Jourdi G, Dupart AL, et al. Bothrops atrox and Bothrops lanceolatus Venoms In Vitro Investigation: Composition Procoagulant Effects Co-Factor Dependency and Correction Using Antivenoms. Toxins. (2023) 15:614. doi: 10.3390/toxins15100614
28. Larreche S, Chevillard L, Jourdi G, Mathe S, Servonnet A, Joly BS, et al. Bothrops venom-induced hemostasis disorders in the rat: between scylla and charybdis. PLoS Negl Trop Dis. (2023) 17:e0011786. doi: 10.1371/journal.pntd.0011786
29. Rucavado A, Camacho E, Escalante T, Lomonte B, Fernandez J, Solano D, et al. A murine experimental model of the pulmonary thrombotic effect induced by the venom of the snake Bothrops lanceolatus. PLoS Negl Trop Dis. (2024) 18:e0012335. doi: 10.1371/journal.pntd.0012335
30. Radouani F, Jalta P, Rapon C, Lezin C, Branford C, Florentin J, et al. The Contrasting Effects of Bothrops lanceolatus and Bothrops atrox Venom on Procoagulant Activity and Thrombus Stability under Blood Flow Conditions. Toxins. (2024) 16:400. doi: 10.3390/toxins16090400
31. Silva FS, Ibiapina HNS, Neves JCF, Coelho KF, Barbosa FBA, Lacerda MVG, et al. Severe tissue complications in patients of Bothrops snakebite at a tertiary health unit in the Brazilian amazon: clinical characteristics and associated factors. Rev Soc Bras Med Trop. (2021) 54:e03742020. doi: 10.1590/0037-8682-0374-2020
32. Ibiapina HNS, Costa AG, Sachett JAG, Silva IM, Tarragô AM, Neves JCF, et al. An immunological stairway to severe tissue complication assembly in Bothrops atrox snakebites. Front Immunol. (2019) 10:1882. doi: 10.3389/fimmu.2019.01882
33. Fontana BC, Soares AM, Zuliani JP, and Gonçalves GM. Role of toll-like receptors in local effects in a model of experimental envenoming induced by Bothrops jararacussu snake venom and by two phospholipases A2. Toxicon. (2022) 214:145–54. doi: 10.1016/j.toxicon.2022.05.043
34. Moreira V, Teixeira C, Borges da Silva H, D’Império Lima MR, and Dos-Santos MC. The role of TLR2 in the acute inflammatory response induced by Bothrops atrox snake venom. Toxicon. (2016) 118:121–8. doi: 10.1016/j.toxicon.2016.04.042
35. Rucavado A, Nicolau CA, Escalante T, Kim J, Herrera C, Gutiérrez JM, et al. Viperid envenomation wound exudate contributes to increased vascular permeability via a DAMPs/TLR-4 mediated pathway. Toxins. (2016) 8:349. doi: 10.3390/toxins8120349
36. Bittenbinder MA, van Thiel J, Cardoso FC, Casewell NR, Gutiérrez JM, Kool J, et al. Tissue damaging toxins in snake venoms: mechanisms of action pathophysiology and treatment strategies. Commun Biol. (2024) 7:358. doi: 10.1038/s42003-024-06019-6
37. Cavalcante JDS, Nogueira Júnior FA, Bezerra Jorge RJ, and Almeida C. Pain modulated by Bothrops snake venoms: mechanisms of nociceptive signaling and therapeutic perspectives. Toxicon. (2021) 201:105–14. doi: 10.1016/j.toxicon.2021.08.016
38. Kondo FV, Cabrera WHK, Ribeiro OG, De Franco M, Jensen JR, Picolo G, et al. Pain and cellular migration induced by Bothrops jararaca venom in mice selected for an acute inflammatory response: involvement of mast cells. Front Immunol. (2022) 12:779473. doi: 10.3389/fimmu.2021.779473
39. Maia-Marques R, Nascimento IMR, Lauria PSS, Silva ECPD, Silva DF, and Casais-E-Silva LL. Inflammatory mediators in the pronociceptive effects induced by Bothrops leucurus snake venom: The role of biogenic amines nitric oxide and eicosanoids. Toxicology. (2021) 448:152649. doi: 10.1016/j.tox.2020.152649
40. Zychar BC and Gonçalves LRC. Understanding local reactions induced by Bothrops jararaca venom: the role of inflammatory mediators in leukocyte-endothelium interactions. Biomedicines. (2024) 12:734. doi: 10.3390/biomedicines12040734
41. Gimenes SNC, Sachett JAG, Colombini M, Freitas-de-Sousa LA, Ibiapina HNS, Costa AG, et al. Observation of Bothrops atrox snake envenoming blister formation from five patients: pathophysiological insights. Toxins. (2021) 13:800. doi: 10.3390/toxins13110800
42. Soares Coriolano Coutinho JV, Fraga Guimarães T, and Borges Valente B. Gomes Martins de Moura Tomich L. Epidemiology of Secondary Infection after Snakebites in Center-West Brazil. PLoS Negl Trop Dis. (2023) 17:e0011167. doi: 10.1371/journal.pntd.0011167
43. Baramova EN, Shannon JD, Bjarnason JB, and Fox JW. Degradation of extracellular matrix proteins by hemorrhagic metalloproteinases. Arch Biochem Biophys. (1989) 275:63–71. doi: 10.1016/0003-9861(89)90350-0
44. Rucavado A, Escalante T, Kalogeropoulos K, Camacho E, Gutiérrez JM, and Fox JW. Analysis of Wound Exudates Reveals Differences in the Patterns of Tissue Damage and Inflammation Induced by the Venoms of Daboia russelii and Bothrops asper in Mice. Toxicon. (2020) 186:94–104. doi: 10.1016/j.toxicon.2020.07.025
45. Macêdo JKA, Joseph JK, Menon J, Escalante T, Rucavado A, Gutiérrez JM, et al. Proteomic analysis of human blister fluids following envenomation by three snake species in India: differential markers for venom mechanisms of action. Toxins. (2019) 11:246. doi: 10.3390/toxins11050246
46. Moreira V, Teixeira C, Borges da Silva H, D’Império Lima MR, and Dos-Santos MC. The crucial role of the MyD88 adaptor protein in the inflammatory response induced by Bothrops atrox venom. Toxicon. (2013) 67:37–46. doi: 10.1016/j.toxicon.2013.02.010
47. Herrera C, Voisin MB, Escalante T, Rucavado A, Nourshargh S, and Gutiérrez JM. Effects of PI and PIII snake venom haemorrhagic metalloproteinases on the microvasculature: A confocal microscopy study on the mouse cremaster muscle. PLoS One. (2016) 11:e0168643. doi: 10.1371/journal.pone.0168643
48. Baldo C, Jamora C, Yamanouye N, Zorn TM, and Moura-da-Silva AM. Mechanisms of vascular damage by hemorrhagic snake venom metalloproteinases: tissue distribution and in situ hydrolysis. PLoS Negl Trop Dis. (2010) 4:e727. doi: 10.1371/journal.pntd.0000727
49. Oliveira S, Alves EC, Santos AS, Nascimento EF, Pereira JP, Silva IM, et al. Bleeding disorders in Bothrops atrox envenomations in the Brazilian amazon: participation of hemostatic factors and the impact of tissue factor. Toxins. (2020) 12:554. doi: 10.3390/toxins12090554
50. Cavalcante JS, de Almeida DEG, Santos-Filho NA, Sartim MA, de Almeida Baldo A, Brasileiro L, et al. Crosstalk of inflammation and coagulation in Bothrops snakebite envenoming: endogenous signaling pathways and pathophysiology. Int J Mol Sci. (2023) 24:11508. doi: 10.3390/ijms241411508
51. Senise LV, Yamashita KM, and Santoro ML. Bothrops jararaca envenomation: pathogenesis of hemostatic disturbances and intravascular hemolysis. Exp Biol Med. (2015) 240:1528–36. doi: 10.1177/1535370215590818
52. Noutsos T, Currie BJ, Lek RA, and Isbister GK. Snakebite associated thrombotic microangiopathy: A systematic review of clinical features outcomes and evidence for interventions including plasmapheresis. PLoS Negl Trop Dis. (2020) 14:e0008936. doi: 10.1371/journal.pntd.0008936
53. Bentes KO, de Amorim RLO, Barbosa FBA, Ratis da Silva VCP, Valente J, Almeida-Val F, et al. Long-Term Disability after Cerebral Ischemic Stroke Following a Bothrops atrox Snakebite in the Brazilian Amazon. Toxicon. (2024) 247:107793. doi: 10.1016/j.toxicon.2024.107793
54. Barbosa PS, Havt A, Facó PE, Sousa TM, Bezerra IS, Fonteles MC, et al. Renal toxicity of Bothrops moojeni snake venom and its main myotoxins. Toxicon. (2002) 40:1427–35. doi: 10.1016/S0041-0101(02)00156-3
55. Braga MD, Martins AM, de Menezes DB, Barbosa PS, Evangelista JS, Toyama MH, et al. Purification and biological activity of the thrombin-like substance isolated from Bothrops insularis venom. Toxicon. (2007) 49:329–38. doi: 10.1016/j.toxicon.2006.10.005
56. Evangelista IL, Martins AM, Nascimento NR, Havt A, Evangelista JS, de Norões TB, et al. Renal and cardiovascular effects of Bothrops marajoensis venom and phospholipase A2. Toxicon. (2010) 55:1061–70. doi: 10.1016/j.toxicon.2009.12.004
57. Marinho AD, Morais IC, Lima DB, Jorge AR, Jorge RJ, Menezes RR, et al. Bothropoides pauloensis venom effects on isolated perfused kidney and cultured renal tubular epithelial cells. Toxicon. (2015) 108:126–33. doi: 10.1016/j.toxicon.2015.09.031
58. Júnior FAN, Jorge ARC, Marinho AD, Silveira JAM, Alves NTQ, Costa PHS, et al. Bothrops alternatus snake venom induces cytokine expression and oxidative stress on renal function. Curr Top Med Chem. (2019) 19:2058–68. doi: 10.2174/1568026619666190809100319
59. Marinho AD, Coelho Jorge AR, Nogueira Junior FA, Alison de Moraes Silveira J, Rocha DG, Negreiros Nunes Alves AP, et al. Effects of cilostazol a phosphodiesterase-3 inhibitor on kidney function and redox imbalance in acute kidney injury caused by Bothrops alternatus venom. Toxicon. (2022) 220:106922. doi: 10.1016/j.toxicon.2022.09.008
60. Malaque CMS, Duayer IF, and Santoro ML. Acute kidney injury induced by thrombotic microangiopathy in two cases of Bothrops envenomation. Clin Toxicol. (2019) 57:213–6. doi: 10.1080/15563650.2018.1510129
61. Albuquerque PLMM, Paiva JHHGL, Martins AMC, Meneses GC, da Silva GB, Buckley N, et al. Clinical assessment and pathophysiology of Bothrops venom-related acute kidney injury: A scoping review. J Venom Anim Toxins Incl Trop Dis. (2020) 26:e20190076. doi: 10.1590/1678-9199-jvatitd-2019-0076
62. Resiere D, Kallel H, Florentin J, Houcke S, Mehdaoui H, Gutierrez JM, et al. Bothrops (Fer-de-lance) snakebites in the French departments of the Americas (Martinique and Guyana): clinical and experimental studies and treatment by immunotherapy. PloS Negl Trop Dis. (2023) 17:e0011083. doi: 10.1371/journal.pntd.0011083
63. Marsh NA. Use of snake venom fractions in the coagulation laboratory. Blood Coagul Fibrinolysis. (1998) 9:395–404. doi: 10.1097/00001721-199807000-00001
64. Kamiguti AS and Cardoso JL. Haemostatic changes caused by the venoms of South American snakes. Toxicon. (1989) 27:955–63. doi: 10.1016/0041-0101(89)90146-3
65. Gutierrez JM, Sanz L, Escolano J, Fernandez J, Lomonte B, Angulo Y, et al. Snake Venomics of the Lesser Antillean Pit Vipers Bothrops caribbaeus and Bothrops lanceolatus: Correlation with Toxicological Activities and Immunoreactivity of a Heterologous Antivenom. J Proteome Res. (2008) 7:4396–408. doi: 10.1021/pr8003826
66. Alsolaiss J, Alomran N, Hawkins L, and Casewell NR. Commercial antivenoms exert broad paraspecific immunological binding and in vitro inhibition of medically important Bothrops pit viper venoms. Toxins. (2022) 15:1. doi: 10.3390/toxins15010001
67. Bogarin G, Romero M, Rojas G, Lutsch C, Casadamont M, Lang J, et al. Neutralization by a monospecific Bothrops lanceolatus antivenom of toxic activities induced by homologous and heterologous Bothrops snake venoms. Toxicon. (1999) 37:551–7. doi: 10.1016/s0041-0101(98)00193-7
68. Bourke LA, Zdenek CN, Tanaka-Azevedo AM, Silveira GPM, Sant’Anna SS, Grego KF, et al. Clinical and evolutionary implications of dynamic coagulotoxicity divergences in Bothrops (Lancehead Pit Viper) Venoms. Toxins. (2022) 14:297. doi: 10.3390/toxins14050297
69. Lobo de Araujo A, Kamiguti A, and Bon C. Coagulant and Anticoagulant Activities of Bothrops lanceolatus (Fer de Lance) Venom. Toxicon. (2001) 39:371–5. doi: 10.1016/S0041-0101(00)00139-2
70. Dellandrea H, Guidoni CM, Linck Junior A, and Girotto E. Brain death due to intracranial hemorrhage in a child following suspected Bothrops snakebite. Rev Soc Bras Med Trop. (2024) 57:e008102024. doi: 10.1590/0037-8682-0264-2024
71. Martínez-Villota VA, Mera-Martínez PF, and Portillo-Miño JD. Massive Acute Ischemic Stroke after Bothrops spp Envenomation in Southwestern Colombia: Case Report and Literature Review. Biomedica. (2022) 42:9–17. doi: 10.7705/biomedica.6114
72. Cañas CA. Brainstem Ischemic Stroke after Bothrops atrox Snakebite. Toxicon. (2016) 120:124–7. doi: 10.1016/j.toxicon.2016.08.005
73. de Oliveira Pardal PP, Pinheiro AC, Silva CT, Santos PR, and Gadelha MA. Hemorrhagic stroke in children caused by Bothrops marajoensis envenoming: A case report. J Venom Anim Toxins Incl Trop Dis. (2015) 21:53. doi: 10.1186/s40409-015-0052-5
74. Pérez-Gómez AS, Monteiro WM, João GAP, Sousa JDB, Safe IP, Damian MM, et al. Hemorrhagic stroke following viper bites and delayed antivenom administration: three case reports from the western Brazilian amazon. Rev Soc Bras Med Trop. (2019) 52:e20190115. doi: 10.1590/0037-8682-0115-2019
75. Silva de Oliveira S, Freitas-de-Sousa LA, Alves EC, de Lima Ferreira LC, da Silva IM, de Lacerda MVG, et al. Fatal stroke after Bothrops snakebite in the amazonas state Brazil: A case report. Toxicon. (2017) 138:102–6. doi: 10.1016/j.toxicon.2017.08.021
76. Sachett JAG, Mota da Silva A, Dantas AWCB, Dantas TR, Colombini M, Moura da Silva AM, et al. Cerebrovascular accidents related to snakebites in the amazon—Two case reports. Wilderness Environ Med. (2020) 31:337–43. doi: 10.1016/j.wem.2020.04.009
77. Cordero GN, Castro Ulloa G, Saborío Morales L, Angulo Morales S, Álvarez Aguilar P, and Padilla Cuadra JI. Hemorragia Cerebelosa Secundaria a Accidente Ofídico por Bothrops sp. Rev Médica Costa Rica. (2019) 627:30–3.
78. MaChado AS, Barbosa FB, Mello Gda S, and Pardal PP. Acidente Vascular Cerebral Hemorrágico Associado à Acidente Ofídico por Serpente do Gênero Bothrops: Relato de Caso. Rev Soc Bras Med Trop. (2010) 43:602–4. doi: 10.1590/S0037-86822010000500029
79. Merle H, Donnio A, Ayeboua L, Plumelle Y, Smadja D, and Thomas L. Occipital infarction revealed by quadranopsia following snakebite by Bothrops lanceolatus. Am J Trop Med Hyg. (2005) 73:583–5. doi: 10.4269/ajtmh.2005.73.583
80. Barakzie A, Jansen AJG, Ten Cate H, and de Maat MPM. Coagulation biomarkers for ischemic stroke. Res Pract Thromb Haemost. (2023) 7:100160. doi: 10.1016/j.rpth.2023.100160
81. Swarowska M, Polczak A, Pera J, Klimkowicz-Mrowiec A, Slowik A, and Dziedzic T. Hyperfibrinogenemia predicts long-term risk of death after ischemic stroke. J Thromb Thrombolysis. (2014) 38:517–21. doi: 10.1007/s11239-014-1122-1
82. Prasad MK, Marandi S, Mishra B, Guria RT, Kumar A, Birua H, et al. Association of fibrinogen with ischemic stroke: A systematic review and meta-analysis. Cureus. (2023) 15:e34335. doi: 10.7759/cureus.34335
83. Cintra AC, De Toni LG, Sartim MA, Franco JJ, Caetano RC, Murakami MT, et al. Batroxase a new metalloproteinase from B atrox snake venom with strong fibrinolytic activity. Toxicon. (2012) 60:70–82. doi: 10.1016/j.toxicon.2012.03.018
84. Hofmann H and Bon C. Blood coagulation induced by the venom of Bothrops atrox 2 identification purification and properties of two factor X activators. Biochemistry. (1987) 26:780–7. doi: 10.1021/bi00377a019
85. Rosing J, Govers-Riemslag JW, Yukelson L, and Tans G. Factor V activation and inactivation by venom proteases. Haemostasis. (2001) 31:241–6. doi: 10.1159/000048069
86. Kirby EP, Niewiarowski S, Stocker K, Kettner C, Shaw E, and Brudzynski TM. Thrombocytin a serine protease from Bothrops atrox venom 1 purification and characterization of the enzyme. Biochemistry. (1979) 18:3564–70. doi: 10.1021/bi00583a020
87. Herrera C, Rucavado A, Warrell DA, and Gutiérrez JM. Systemic effects induced by the venom of the snake Bothrops caribbaeus in a murine model. Toxicon. (2013) 63:19–31. doi: 10.1016/j.toxicon.2012.10.023
88. Lin CH, Kuo YW, Kuo CY, Huang YC, Hsu CY, Hsu HL, et al. Shortened activated partial thromboplastin time is associated with acute ischemic stroke stroke severity and neurological worsening. J Stroke Cerebrovasc Dis. (2015) 24:2270–6. doi: 10.1016/j.jstrokecerebrovasdis.2015.06.008
89. Nnachi OC, Ezenwenyi IP, Akulue JC, Nnachi OA, Nwadum FE, Onoh TJ, et al. An investigative study of prothrombin time activated partial thromboplastin time and antithrombin levels in patients with ischaemic stroke. Int Blood Res Rev. (2024) 15:22–8. doi: 10.9734/ibrr/2024/v15i3341
90. Resiere D, Mehdaoui H, and Neviere R. Inflammation and oxidative stress in snakebite envenoming: A brief descriptive review and clinical implications. Toxins. (2022) 14:802. doi: 10.3390/toxins14110802
91. Casewell NR, Jackson TNW, Laustsen AH, and Sunagar K. Causes and consequences of snake venom variation. Trends Pharmacol Sci. (2020) 41:570–81. doi: 10.1016/j.tips.2020.05.006
92. Mora-Obando D, Lomonte B, Pla D, Guerrero-Vargas JA, Ayerbe-González S, Gutiérrez JM, et al. Half a century of research on Bothrops asper venom variation: biological and biomedical implications. Toxicon. (2023) 221:106983. doi: 10.1016/j.toxicon.2022.106983
93. Hatakeyama DM, Jorge Tasima L, da Costa Galizio N, Serino-Silva C, Fabri Bittencourt Rodrigues C, Rodrigues Stuginski D, et al. From birth to adulthood: An analysis of the Brazilian lancehead (Bothrops moojeni) venom at different life stages. PLoS One. (2021) 16:e0253050. doi: 10.1371/journal.pone.0253050
94. Tasima LJ, Hatakeyama DM, Aguiar WDS, Lima EOV, Miyamoto JG, Tashima AK, et al. Analyzing the influence of age and sex in Bothrops pauloensis snake venom. Toxicon. (2022) 214:78–90. doi: 10.1016/j.toxicon.2022.05.007
95. Bucher B, Canonge D, Thomas L, Tyburn B, Robbe-Vincent A, Choumet V, et al. Clinical indicators of envenoming and serum levels of venom antigens in patients bitten by Bothrops lanceolatus in Martinique. Trans R Soc Trop Med Hyg. (1997) 91:186–90. doi: 10.1016/S0035-9203(97)90219-4
96. Antunes TC, Yamashita KM, Barbaro KC, Saiki M, and Santoro ML. Comparative analysis of newborn and adult Bothrops jararaca snake venoms. Toxicon. (2010) 56:1443–58. doi: 10.1016/j.toxicon.2010.08.011
97. Rodrigues CFB, Zdenek CN, Bourke LA, Seneci L, Chowdhury A, Freitas-de-Sousa LA, et al. Clinical implications of ontogenetic differences in the coagulotoxic activity of Bothrops jararacussu venoms. Toxicol Lett. (2021) 348:59–72. doi: 10.1016/j.toxlet.2021.05.005
98. MaChado Braga JR, de Morais-Zani K, Pereira DDS, Sant’Anna SS, da Costa Galizio N, Tanaka-Azevedo AM, et al. Sexual and ontogenetic variation of Bothrops leucurus venom. Toxicon. (2020) 184:127–35. doi: 10.1016/j.toxicon.2020.05.028
99. Guércio RA, Shevchenko A, Shevchenko A, López-Lozano JL, Paba J, Sousa MV, et al. Ontogenetic variations in the venom proteome of the Amazonian snake Bothrops atrox. Proteome Sci. (2006) 4:11. doi: 10.1186/1477-5956-4-11
100. Ferreira-Rodrigues SC, Silva RCC, Trevisan M, Rodrigues PSM, Del-Rei THM, Sousa LF, et al. Ontogenetic and sexual differences in the venom of Bothrops moojeni: insights from a litter and its mother. Braz J Biol. (2024) 84:e279474. doi: 10.1590/1519-6984.279474
101. Cajander S, Kox M, Scicluna BP, Weigand MA, Mora RA, Flohe SB, et al. Profiling the dysregulated immune response in sepsis: overcoming challenges to achieve the goal of precision medicine. Lancet Respir Med. (2024) 12:305–22. doi: 10.1016/S2213-2600(23)00330-2
102. van der Poll T, Shankar-Hari M, and Wiersinga WJ. The immunology of sepsis. Immunity. (2021) 54:2450–64. doi: 10.1016/j.immuni.2021.10.012
103. Teixeira C, Fernandes CM, Leiguez E, and Chudzinski-Tavassi AM. Inflammation induced by platelet-activating viperid snake venoms: perspectives on thromboinflammation. Front Immunol. (2019) 10:2082. doi: 10.3389/fimmu.2019.02082
104. Rao S, Reghu N, Nair BG, and Vanuopadath M. The role of snake venom proteins in inducing inflammation post-envenomation: an overview on mechanistic insights and treatment strategies. Toxins. (2024) 16:519. doi: 10.3390/toxins16120519
105. Timmerman I, Daniel AE, Kroon J, and van Buul JD. Leukocytes crossing the endothelium: A matter of communication. Int Rev Cell Mol Biol. (2016) 322:281–329. doi: 10.1016/bs.ircmb.2015.10.005
106. Clissa PB, Laing GD, Theakston RD, Mota I, Taylor MJ, and Moura-da-Silva AM. The effect of jararhagin a metalloproteinase from Bothrops jararaca venom on pro-inflammatory cytokines released by murine peritoneal adherent cells. Toxicon. (2001) 39:1567–73. doi: 10.1016/S0041-0101(01)00131-3
107. Gallagher P, Bao Y, Serrano SM, Laing GD, Theakston RD, Gutiérrez JM, et al. Role of the snake venom toxin jararhagin in proinflammatory pathogenesis: in vitro and in vivo gene expression analysis of the effects of the toxin. Arch Biochem Biophys. (2005) 441:1–15. doi: 10.1016/j.abb.2005.06.007
108. Menaldo DL, Bernardes CP, Pereira JC, Silveira DS, Mamede CC, and Stanziola L. Effects of two serine proteases from Bothrops pirajai snake venom on the complement system and the inflammatory response. Int Immunopharmacol. (2013) 15:764–71. doi: 10.1016/j.intimp.2013.02.023
109. Farsky SH, Gonçalves LR, Gutiérrez JM, Correa AP, Rucavado A, Gasque P, et al. Bothrops asper snake venom and its metalloproteinase BaP-1 activate the complement system role in leukocyte recruitment. Mediators Inflammation. (2000) 9:213–21. doi: 10.1080/09629350020025728
110. Fernandes CM, Zamuner SR, Zuliani JP, Rucavado A, Gutiérrez JM, and Teixeira CF. Inflammatory effects of BaP1 a metalloproteinase isolated from Bothrops asper snake venom: leukocyte recruitment and release of cytokines. Toxicon. (2006) 47:549–59. doi: 10.1016/j.toxicon.2006.01.009
111. Schattner M, Fritzen M, Ventura JS, de Albuquerque Modesto JC, Pozner RG, Moura-da-Silva AM, et al. The snake venom metalloproteases berythractivase and jararhagin activate endothelial cells. Biol Chem. (2005) 386:369–74. doi: 10.1515/BC.2005.044
112. Pereira ALM, Fritzen M, Faria F, Da Motta G, and Chudzinski-Tavassi AM. Releasing or expression modulating mediator involved in hemostasis by berythractivase and jararhagin (SVMPs). Toxicon. (2006) 47:788–96. doi: 10.1016/j.toxicon.2006.02.014
113. Sartim MA, Costa TR, Laure HJ, Espíndola MS, Frantz FG, Sorgi CA, et al. Moojenactivase a novel pro-coagulant PIIId metalloprotease isolated from Bothrops moojeni snake venom activates coagulation factors II and X and induces tissue factor up-regulation in leukocytes. Arch Toxicol. (2016) 90:1261–78. doi: 10.1007/s00204-015-1533-6
114. Fujimura Y, Titani K, Usami Y, Suzuki M, Oyama R, Matsui T, et al. Isolation and chemical characterization of two structurally and functionally distinct forms of botrocetin the platelet coagglutinin isolated from the venom of Bothrops jararaca. Biochemistry. (1991) 30:1957–64. doi: 10.1021/bi00221a032
115. De Luca M, Ward CM, Ohmori K, Andrews RK, and Berndt MC. Jararhagin and Jaracetin: novel snake venom inhibitors of the integrin collagen receptor alpha 2 beta 1. Biochem Biophys Res Commun. (1995) 206:570–6. doi: 10.1006/bbrc.1995.1081
116. Khan SA and Ilies MA. The phospholipase A2 superfamily: structure isozymes catalysis physiologic and pathologic roles. Int J Mol Sci. (2023) 24:1353. doi: 10.3390/ijms24021353
117. Bickler PE. Amplification of snake venom toxicity by endogenous signaling pathways. Toxins. (2020) 12:68. doi: 10.3390/toxins12020068
118. Sampat GH, Hiremath K, Dodakallanavar J, Patil VS, Harish DR, Biradar P, et al. Unraveling snake venom phospholipase A2: an overview of its structure pharmacology and inhibitors. Pharmacol Rep. (2023) 75:1454–73. doi: 10.1007/s43440-023-00543-8
119. de Castro RC, Landucci EC, Toyama MH, Giglio JR, Marangoni S, De Nucci G, et al. Leucocyte recruitment induced by type II phospholipases A2 into the rat pleural cavity. Toxicon. (2000) 38:1773–85. doi: 10.1016/S0041-0101(00)00107-0
120. Cedro RCA, Menaldo DL, Costa TR, Zoccal KF, Sartim MA, Santos-Filho NA, et al. Cytotoxic and inflammatory potential of a phospholipase A2 from Bothrops jararaca snake venom. J Venom Anim Toxins Incl Trop Dis. (2018) 24:33. doi: 10.1186/s40409-018-0170-y
121. Landucci EC, de Castro RC, Toyama M, Giglio JR, Marangoni S, De Nucci G, et al. Inflammatory oedema induced by the lys-49 phospholipase A2 homologue piratoxin-I in the rat and rabbit effect of polyanions and p-bromophenacyl bromide. Biochem Pharmacol. (2000) 59:1289–94. doi: 10.1016/S0006-2952(00)00248-3
122. Moreira V, Lomonte B, Vinolo MAR, Curi R, Gutiérrez JM, and Teixeira C. An Asp49 Phospholipase A2 from Snake Venom Induces Cyclooxygenase-2 Expression and Prostaglandin E2 Production via Activation of NF-κB p38MAPK and PKC in Macrophages. Mediators Inflammation. (2014) 2014:105879. doi: 10.1155/2014/105879
123. Gabrili JJM, Pidde G, Magnoli FC, Marques-Porto R, Villas-Boas IM, Squaiella-Baptistão CC, et al. New Insights into Immunopathology Associated to Bothrops lanceolatus Snake Envenomation: Focus on PLA2 Toxin. Int J Mol Sci. (2023) 24:9931. doi: 10.3390/ijms24129931
124. Elifio-Esposito S, Tomazeli L, Schwartz C, Gimenez AP, Fugii GM, Fernandes LC, et al. Human neutrophil migration and activation by BJcuL a galactose binding lectin purified from Bothrops jararacussu venom. BMC Immunol. (2011) 12:10. doi: 10.1186/1471-2172-12-10
125. Pires WL, de Castro OB, Kayano AM, da Silva Setubal S, Pontes AS, Nery NM, et al. Effect of bjcuL a lectin isolated from Bothrops jararacussu on human peripheral blood mononuclear cells. Toxicol In Vitro. (2017) 41:30–41. doi: 10.1016/j.tiv.2017.02.003
126. Sartim MA, Riul TB, Del Cistia-Andrade C, Stowell SR, Arthur CM, Sorgi CA, et al. Galatrox is a C-type lectin in bothrops atrox snake venom that selectively binds lacNAc-terminated glycans and can induce acute inflammation. Glycobiology. (2014) 24:1010–21. doi: 10.1093/glycob/cwu061
127. Ikenohuchi YJ, Silva MDS, Rego CMA, Francisco AF, da Silva Setubal S, Ferreira EFAA, et al. A C-type lectin induces NLRP3 inflammasome activation via TLR4 interaction in human peripheral blood mononuclear cells. Cell Mol Life Sci. (2023) 80:188. doi: 10.1007/s00018-023-04839-z
128. Masuda H, Sato A, Shizuno T, Yokoyama K, Suzuki Y, Tokunaga M, et al. Batroxobin accelerated tissue repair via neutrophil extracellular trap regulation and defibrinogenation in a murine ischemic hind limb model. PloS One. (2019) 14:e0220898. doi: 10.1371/journal.pone.0220898
129. Megale ÂAA, Magnoli FC, Guidolin FR, Go. doi KS, Portaro FCV, Dias-da-Silva W, et al. Bitis arietans snake venom and Kn-Ba a snake venom serine protease induce the production of inflammatory mediators in THP-1 macrophages. Toxins. (2021) 13:906. doi: 10.3390/toxins13120906
130. Vaiyapuri S. Kallikrein enzymes. In: Fry B, editor. Venomous Reptiles and Their Toxins: Evolution Pathophysiology and Biodiscovery. Oxford University Press, New York NY (2015).
131. Magalhães A, Magalhães HPB, Richardson M, Gontijo S, Ferreira RN, Almeida AP, et al. Purification and properties of a coagulant thrombin-like enzyme from the venom of Bothrops leucurus. Comp Biochem Physiol A Mol Integr Physiol. (2007) 146:565–75. doi: 10.1016/j.cbpa.2005.12.033
132. Sant’Ana CD, Bernardes CP, Izidoro LFM, Mazzi MV, Soares SG, Fuly AL, et al. Molecular characterization of BjussuSP-I a new thrombin-like enzyme with procoagulant and kallikrein-like activity isolated from Bothrops jararacussu snake venom. Biochimie. (2008) 90:500–7. doi: 10.1016/j.biochi.2007.10.005
133. Costa FLS, Rodrigues RS, Izidoro LFM, Menaldo DL, Hamaguchi A, Homsi-Brandeburgo MI, et al. Biochemical and functional properties of a thrombin-like enzyme isolated from Bothrops pauloensis snake venom. Toxicon. (2009) 54:725–35. doi: 10.1016/j.toxicon.2009.05.040
134. Dagnino APA, Campos MM, and Silva RBM. Kinins and their receptors in infectious diseases. Pharmaceuticals. (2020) 13:215. doi: 10.3390/ph13090215
135. Rex DAB, Vaid N, Deepak K, Dagamajalu S, and Prasad TSK. A comprehensive review on current understanding of bradykinin in COVID-19 and inflammatory diseases. Mol Biol Rep. (2022) 49:9915–27. doi: 10.1007/s11033-022-07539-2
136. Ji M, Ran X, Zuo H, and Zhang Q. Novel insights into the kallikrein-kinin system in fulminant myocarditis: physiological basis and potential therapeutic advances. J Inflammation Res. (2024) 17:7347–60. doi: 10.2147/JIR.S488237
137. Alsolaiss J, Leeming G, Da Silva R, Alomran N, Casewell NR, Habib AG, et al. Investigating snake-venom-induced dermonecrosis and inflammation using an ex vivo human skin model. Toxins. (2024) 16:276. doi: 10.3390/toxins16060276
138. Escalante T, Rucavado A, Pinto AF, Terra RM, Gutiérrez JM, and Fox JW. Wound exudate as a proteomic window to reveal different mechanisms of tissue damage by snake venom toxins. J Proteome Res. (2009) 8:5120–31. doi: 10.1021/pr900489m
139. Matsui T, Hamako J, and Titani K. Structure and function of snake venom proteins affecting platelet plug formation. Toxins. (2010) 2:10–23. doi: 10.3390/toxins2010010
140. Zuliani JP, Soares AM, and Gutiérrez JM. Polymorphonuclear neutrophil leukocytes in snakebite envenoming. Toxicon. (2020) 187:188–97. doi: 10.1016/j.toxicon.2020.09.006
141. Leonel TB, Gabrili JJM, Squaiella-Baptistão CC, Woodruff TM, Lambris JD, and Tambourgi DV. Bothrops jararaca snake venom inflammation induced in human whole blood: role of the complement system. Front Immunol. (2022) 13:885223. doi: 10.3389/fimmu.2022.885223
142. Silva de Franca F, Gabrili JJM, Mathieu L, Burgher F, Blomet J, and Tambourgi DV. Bothrops lanceolatus snake (Fer-de-lance) venom triggers inflammatory mediators’ Storm in human blood. Arch Toxicol. (2021) 95:1129–38. doi: 10.1007/s00204-020-02959-0
143. Delafontaine M, Villas-Boas IM, Pidde G, van den Berg CW, Mathieu L, Blomet J, et al. Venom from Bothrops lanceolatus a snake species native to Martinique potently activates the complement system. J Immunol Res. (2018) 2018:3462136. doi: 10.1155/2018/3462136
144. Pidde-Queiroz G, Furtado MF, Filgueiras CF, Pessoa LA, Spadafora-Ferreira M, van den Berg CW, et al. Human complement activation and anaphylatoxins generation induced by snake venom toxins from Bothrops genus. Mol Immunol. (2010) 47:2537–44. doi: 10.1016/j.molimm.2010.07.003
145. Rodrigues FG, Petretski JH, Kanashiro MM, Lemos L, da Silva WD, and Kipnis TL. The Complement System Is Involved in Acute Inflammation but Not in the Hemorrhage Produced by a Bothrops atrox Snake Venom Low Molecular Mass Proteinase. Mol Immunol. (2004) 40:1149–56. doi: 10.1016/j.molimm.2003.11.032
146. Sarma JV and Ward PA. The complement system. Cell Tissue Res. (2011) 343:227–35. doi: 10.1007/s00441-010-1034-0
147. Bajic G, Degn SE, Thiel S, and Andersen GR. Complement activation regulation and molecular basis for complement-related diseases. EMBO J. (2015) 34:2735–57. doi: 10.15252/embj.201591881
148. Mannes M, Schmidt CQ, Nilsson B, Ekdahl KN, and Huber-Lang M. Complement as driver of systemic inflammation and organ failure in trauma burn and sepsis. Semin Immunopathol. (2021) 43:773–88. doi: 10.1007/s00281-021-00872-x
149. de Bont CM, Boelens WC, and Pruijn GJM. NETosis complement and coagulation: A triangular relationship. Cell Mol Immunol. (2019) 16:19–27. doi: 10.1038/s41423-018-0024-0
150. Markiewski MM and Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. (2007) 171:715–27. doi: 10.2353/ajpath.2007.070166
151. Moreira L, Gutiérrez JM, Borkow G, and Ovadia M. Ultrastructural Alterations in Mouse Capillary Blood Vessels after Experimental Injection of Venom from the Snake Bothrops asper (Terciopelo). Exp Mol Pathol. (1992) 57:124–33. doi: 10.1016/0014-4800(92)90004-U
152. Lomonte B, Gutiérrez JM, Borkow G, Ovadia M, Tarkowski A, and Hanson LA. Activity of hemorrhagic metalloproteinase baH-1 and myotoxin II from Bothrops asper snake venom on capillary endothelial cells in vitro. Toxicon. (1994) 32:505–10. doi: 10.1016/0041-0101(94)90302-6
153. Rucavado A, Lomonte B, Ovadia M, and Gutiérrez JM. Local tissue damage induced by BaP1 a metalloproteinase isolated from Bothrops asper (Terciopelo) snake venom. Exp Mol Pathol. (1995) 63:186–99. doi: 10.1006/exmp.1995.1042
154. Bittenbinder MA, Bonanini F, Kurek D, Vulto P, Kool J, and Vonk FJ. Using organ-on-a-chip technology to study haemorrhagic activities of snake venoms on endothelial tubules. Sci Rep. (2024) 14:11157. doi: 10.1038/s41598-024-60282-5
155. Kawano J, Anai K, Sugiki M, Yoshida E, and Maruyama M. Vascular endothelial cell injury induced by Bothrops jararaca venom, non-significance of hemorrhagic metalloproteinase. Toxicon. (2002) 40:1553–62. doi: 10.1016/S0041-0101(02)00171-X
156. Asega AF, Menezes MC, Trevisan-Silva D, Cajado-Carvalho D, Bertholim L, Oliveira AK, et al. Cleavage of proteoglycans plasma proteins and the platelet-derived growth factor receptor in the hemorrhagic process induced by snake venom metalloproteinases. Sci Rep. (2020) 10:12912. doi: 10.1038/s41598-020-69396-y
157. Delafontaine M, Villas-Boas IM, Mathieu L, Josset P, Blomet J, and Tambourgi DV. Enzymatic and pro-inflammatory activities of Bothrops lanceolatus venom: relevance for envenomation. Toxins. (2017) 9:244. doi: 10.3390/toxins9080244
158. Ma Y, Liu Y, Zhang Z, and Yang GY. Significance of complement system in ischemic stroke: A comprehensive review. Aging Dis. (2019) 10:429–62. doi: 10.14336/AD.2019.0119
159. Nørgaard I, Nielsen SF, and Nordestgaard BG. Complement C3 and high risk of venous thromboembolism: 80517 individuals from the copenhagen general population study. Clin Chem. (2016) 62:525–34. doi: 10.1373/clinchem.2015.251314
160. Ruf W. Links between complement activation and thrombosis. Blood. (2019) 134:SCI–40. doi: 10.1182/blood-2019-121113
161. Jayarangaiah A, Kariyanna PT, Chen X, Jayarangaiah A, and Kumar A. COVID-19-associated coagulopathy: an exacerbated immunothrombosis response. Clin Appl Thromb Hemost. (2020) 26:1076029620943293. doi: 10.1177/1076029620943293
162. Rawish E, Sauter M, Sauter R, Nording H, and Langer HF. Complement inflammation and thrombosis. Br J Pharmacol. (2021) 178:2892–904. doi: 10.1111/bph.15476
163. Loof TG, Schmidt O, Herwald H, and Theopold U. Coagulation systems of invertebrates and vertebrates and their roles in innate immunity: the same side of two coins? J Innate Immun. (2011) 3:34–40. doi: 10.1159/000321641
164. Jarczak D, Kluge S, and Nierhaus A. Sepsis-pathophysiology and therapeutic concepts. Front Med. (2021) 8:628302. doi: 10.3389/fmed.2021.628302
165. Musgrave KM, Scott J, Sendama W, Gardner AI, Dewar F, Lake CJ, et al. Tissue factor expression in monocyte subsets during human immunothrombosis endotoxemia and sepsis. Thromb Res. (2023) 228:10–20. doi: 10.1016/j.thromres.2023.05.018
166. Yong J and Toh CH. The convergent model of coagulation. J Thromb Haemost. (2024) 22:2140–6. doi: 10.1016/j.jtha.2024.05.014
167. Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. (2010) 107:15880–5. doi: 10.1073/pnas.1005743107
168. Zhou Y, Xu Z, and Liu Z. Impact of neutrophil extracellular traps on thrombosis formation: new findings and future perspective front cell. Infect Microbiol. (2022) 12:910908. doi: 10.3389/fcimb.2022.910908
169. Perdomo J and Leung HHL. Immune thrombosis: exploring the significance of immune complexes and NETosis. Biology. (2023) 12:1332. doi: 10.3390/biology12101332
170. Chen WA and Boskovic DS. Neutrophil extracellular DNA traps in response to infection or inflammation and the roles of platelet interactions. Int J Mol Sci. (2024) 25:3025. doi: 10.3390/ijms25053025
171. Noone D, Preston RJS, and Rehill AM. The role of myeloid cells in thromboinflammatory disease. Semin Thromb Hemost. (2024) 50:998–1011. doi: 10.1055/s-0044-1782660
172. Burkard P, Vogtle T, and Nieswandt B. Platelets in thrombo-inflammation: concepts mechanisms and therapeutic strategies for ischemic stroke. Hamostaseologie. (2020) 40:153–64. doi: 10.1055/a-1151-9519
173. Muller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, et al. Platelet polyphosphates are proinflammatory and procoagulant mediators. In Vivo Cell. (2009) 139:1143–56. doi: 10.1016/j.cell.2009.11.001
174. Ge L, Zhou X, Ji WJ, Lu RY, Zhang Y, Zhang YD, et al. Neutrophil extracellular traps in ischemia-reperfusion injury-induced myocardial no-reflow: therapeutic potential of DNase-based reperfusion strategy. Am J Physiol Heart Circ Physiol. (2015) 308:H500–9. doi: 10.1152/ajpheart.00381.2014
175. Thalin C, Demers M, Blomgren B, Wong SL, von Arbin M, von Heijne A, et al. NETosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb Res. (2016) 139:56–64. doi: 10.1016/j.thromres.2016.01.009
176. Hao X, Zeng Z, Liang L, Feng Z, Li W, Xiong B, et al. The role of neutrophil extracellular traps in early microthrombosis and brain injury after subarachnoid hemorrhage in mice. Transl Stroke Res. (2023) 14:752–65. doi: 10.1007/s12975-022-01074-9
177. Wellmann IAM, Ibiapina HNS, Sachett JAG, Sartim MA, Silva IM, Oliveira SS, et al. Correlating fibrinogen consumption and profiles of inflammatory molecules in human envenomations by Bothrops atrox in the Brazilian amazon. Front Immunol. (2020) 11:1874. doi: 10.3389/fimmu.2020.01874
178. Wienkamp AK, Erpenbeck L, and Rossaint J. Platelets in the NETworks interweaving inflammation and thrombosis. Front Immunol. (2022) 13:953129. doi: 10.3389/fimmu.2022.953129
179. de Queiroz MR, de Sousa BB, da Cunha Pereira DF, Mamede CCN, Matias MS, de Morais NCG, et al. The role of platelets in hemostasis and the effects of snake venom toxins on platelet function. Toxicon. (2017) 133:33–47. doi: 10.1016/j.toxicon.2017.04.013
180. McCleary RJ and Kini RM. Snake bites and hemostasis/thrombosis. Thromb Res. (2013) 132:642–6. doi: 10.1016/j.thromres.2013.09.031
181. Kini RM and Evans HJ. Effects of snake venom proteins on blood platelets. Toxicon. (1990) 28:1387–422. doi: 10.1016/0041-0101(90)90155-Z
182. Santoro ML and Sano-Martins IS. Platelet Dysfunction during Bothrops jararaca Snake Envenomation in Rabbits. Thromb Haemost. (2004) 92:369–83. doi: 10.1160/TH04-02-0120
183. Carneiro AS, Ribeiro OG, Cabrera WH, Vorraro F, De Franco M, Ibanez OM, et al. Bothrops jararaca venom (BjV) induces differential leukocyte accumulation in mice genetically selected for acute inflammatory reaction: the role of host genetic background on expression of adhesion molecules and release of endogenous mediators. Toxicon. (2008) 52:619–27. doi: 10.1016/j.toxicon.2008.07.012
184. Zamuner SR, Zuliani JP, Fernandes CM, Gutiérrez JM, and de Fatima Pereira Teixeira C. Inflammation induced by Bothrops asper venom: release of proinflammatory cytokines and eicosanoids and role of adhesion molecules in leukocyte infiltration. Toxicon. (2005) 46:806–13. doi: 10.1016/j.toxicon.2005.08.011
185. Farsky SH, Borelli P, Fock RA, Proto SZ, Ferreira JM, and Mello SB. Chronic blockade of nitric oxide biosynthesis in rats: effect on leukocyte endothelial interaction and on leukocyte recruitment. Inflammation Res. (2004) 53:442–52. doi: 10.1007/s00011-004-1288-7
186. Clissa PB, Lopes-Ferreira M, Della-Casa MS, Farsky SH, and Moura-da-Silva AM. Importance of jararhagin disintegrin-like and cysteine-rich domains in the early events of local inflammatory response. Toxicon. (2006) 47:591–6. doi: 10.1016/j.toxicon.2006.02.001
187. Thakur M, Junho CVC, Bernhard SM, Schindewolf M, Noels H, and Doring Y. NETs-induced thrombosis impacts on cardiovascular and chronic kidney disease. Circ Res. (2023) 132:933–49. doi: 10.1161/CIRCRESAHA.123.321750
188. Zhu Y, Chen X, and Liu X. NETosis and neutrophil extracellular traps in COVID-19: immunothrombosis and beyond. Front Immunol. (2022) 13:838011. doi: 10.3389/fimmu.2022.838011
189. Setubal SDS, Pontes AS, Nery NM, Rego CMA, Santana HM, de Lima AM, et al. Human Neutrophils Functionality under Effect of an Asp49 Phospholipase A2 Isolated from Bothrops atrox Venom. Toxicon X. (2020) 6:100032. doi: 10.1016/j.toxcx.2020.100032
190. Setubal SDS, Pontes AS, Nery NM, Bastos JS, Castro OB, Pires WL, et al. Effect of Bothrops bilineata snake venom on neutrophil function. Toxicon. (2013) 76:143–9. doi: 10.1016/j.toxicon.2013.09.019
191. Lisita K, Silva MDS, Santana HM, Ikenohuchi YJ, Paloschi MV, Rego CMA, et al. Action of bjussuMP-II a snake venom metalloproteinase isolated from Bothrops jararacussu venom on human neutrophils. Toxicon. (2023) 222:106992. doi: 10.1016/j.toxicon.2022.106992
192. Thomazini CM, Sachetto ATA, de Albuquerque CZ, de Moura Mattaraia VG, de Oliveira AK, Serrano SMT, et al. Involvement of von Willebrand Factor and Botrocetin in the Thrombocytopenia Induced by Bothrops jararaca Snake Venom. PLoS Negl Trop Dis. (2021) 15:e0009715. doi: 10.1371/journal.pntd.0009715
193. Serrano SMT, Wang D, Shannon JD, Pinto AFM, Polanowska-Grabowska RK, and Fox JW. Interaction of the Cysteine-Rich Domain of Snake Venom Metalloproteinases with the A1 Domain of von Willebrand Factor Promotes Site-Specific Proteolysis of von Willebrand Factor and Inhibition of von Willebrand Factor-Mediated Platelet Aggregation. FEBS J. (2007) 274:3611–21. doi: 10.1111/j.1742-4658.2007.05895.x
194. Thomazini CM, Soares RPS, da Rocha TRF, Sachetto ATA, and Santoro ML. Optimization of von willebrand factor multimer analysis in vertical mini-gel electrophoresis systems: A rapid procedure. Thromb Res. (2019) 175:76–83. doi: 10.1016/j.thromres.2019.01.018
195. Cavalcante JS, Borges da Silva WRG, de Oliveira LA, Brito IMC, Muller KS, Vidal IS, et al. Blood Plasma Proteome Alteration after Local Tissue Damage Induced by Bothrops erythromelas Snake Venom in Mice. J Proteomics. (2022) 269:104742. doi: 10.1016/j.jprot.2022.104742
196. Cavalcante JS, Brito IMDC, De Oliveira LA, De Barros LC, Almeida C, Rossini BC, et al. Experimental Bothrops atrox Envenomation: Blood Plasma Proteome Effects after Local Tissue Damage and Perspectives on Thromboinflammation. Toxins. (2022) 14:613. doi: 10.3390/toxins14090613
197. Cavalcante JDS, de Almeida CAS, Clasen MA, da Silva EL, de Barros LC, Marinho AD, et al. A Fingerprint of Plasma Proteome Alteration after Local Tissue Damage Induced by Bothrops leucurus Snake Venom in Mice. J Proteomics. (2022) 253:104464. doi: 10.1016/j.jprot.2021.104464
Keywords: Bothrops, envenoming, thrombosis, inflammation, NETs, kallikrein, von Willebrand factor
Citation: Rapon C, Florentin J, Radouani F, Jalta P, Negrello F, Gueye P, Pierre-Louis O, Neviere R and Resiere D (2025) Thromboinflammatory complications of Bothrops snakebite envenoming: the case of B. lanceolatus endemic to the Caribbean Island of Martinique. Front. Immunol. 16:1625165. doi: 10.3389/fimmu.2025.1625165
Received: 08 May 2025; Accepted: 11 August 2025;
Published: 10 September 2025; Corrected: 31 October 2025.
Edited by:
Christoph Reinhardt, Johannes Gutenberg University Mainz, GermanyReviewed by:
Mirta Schattner, Laboratory of Experimental Thrombosis (IMEX-ANM), ArgentinaAltair Seabra de Farias, University of the State of Amazonas, Brazil
Copyright © 2025 Rapon, Florentin, Radouani, Jalta, Negrello, Gueye, Pierre-Louis, Neviere and Resiere. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Remi Neviere, cmVtaS5uZXZpZXJlQGNodS1tYXJ0aW5pcXVlLmZy
†These authors have contributed equally to this work
 Caroline Rapon1†
Caroline Rapon1† Jonathan Florentin
Jonathan Florentin Remi Neviere
Remi Neviere