- 1Department of Rheumatology, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, China
- 2Department of Dermatology, Tianjin Institute of Integrative Dermatology, Tianjin Academy of Traditional Chinese Medicine Affiliated Hospital, Tianjin, China
CAR-T therapy, an innovative immunotherapeutic approach, genetically modifies T cells to express CARs, enabling targeted destruction of specific antigen-expressing cells. Initially developed for oncology, CAR-T therapy has shown significant potential in treating autoimmune diseases. By targeting CD19+ B cells, CAR-T therapy has demonstrated rapid and sustained remission in refractory cases, with studies showing normalized laboratory parameters and reduced disease activity. At the same time, CAR-NK, CAAR-T and CAR-Treg technologies further broaden therapeutic strategies. However, some adverse effects also exist, including CRS, ICANS and so on. Despite these challenges, CAR therapy represents a promising advancement in autoimmune disease treatment, with ongoing research aimed at enhancing efficacy, durability, and safety. Continuous innovation is essential to address limitations and optimize therapeutic outcomes.
1 The application of CAR-T therapy in autoimmune diseases
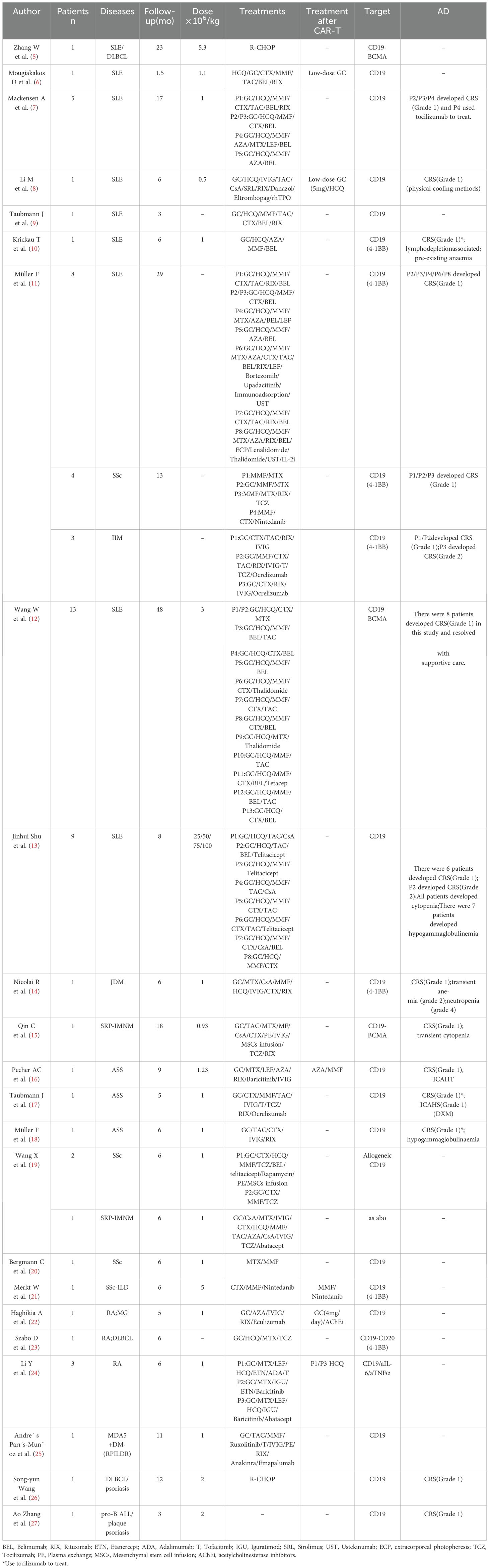
Chimeric Antigen Receptor T-cell (CAR-T) therapy represents an innovative immunotherapeutic approach wherein T lymphocytes are genetically modified to express chimeric antigen receptors (CARs), thereby enabling the precise recognition and targeted elimination of specific antigen-expressing cells (Figure 1A). Kuwana first reported the concept of chimeric receptors in 1987 (1). This therapy has demonstrated significant efficacy in oncology (2–4). With a deep understanding of the technology, it has gradually been applied to treat autoimmune diseases (Table 1). Conventional therapies for autoimmune diseases exhibit certain limitations, whereas CAR-T therapy offers a new strategy. However, the potential adverse effects associated with this treatment require consideration.
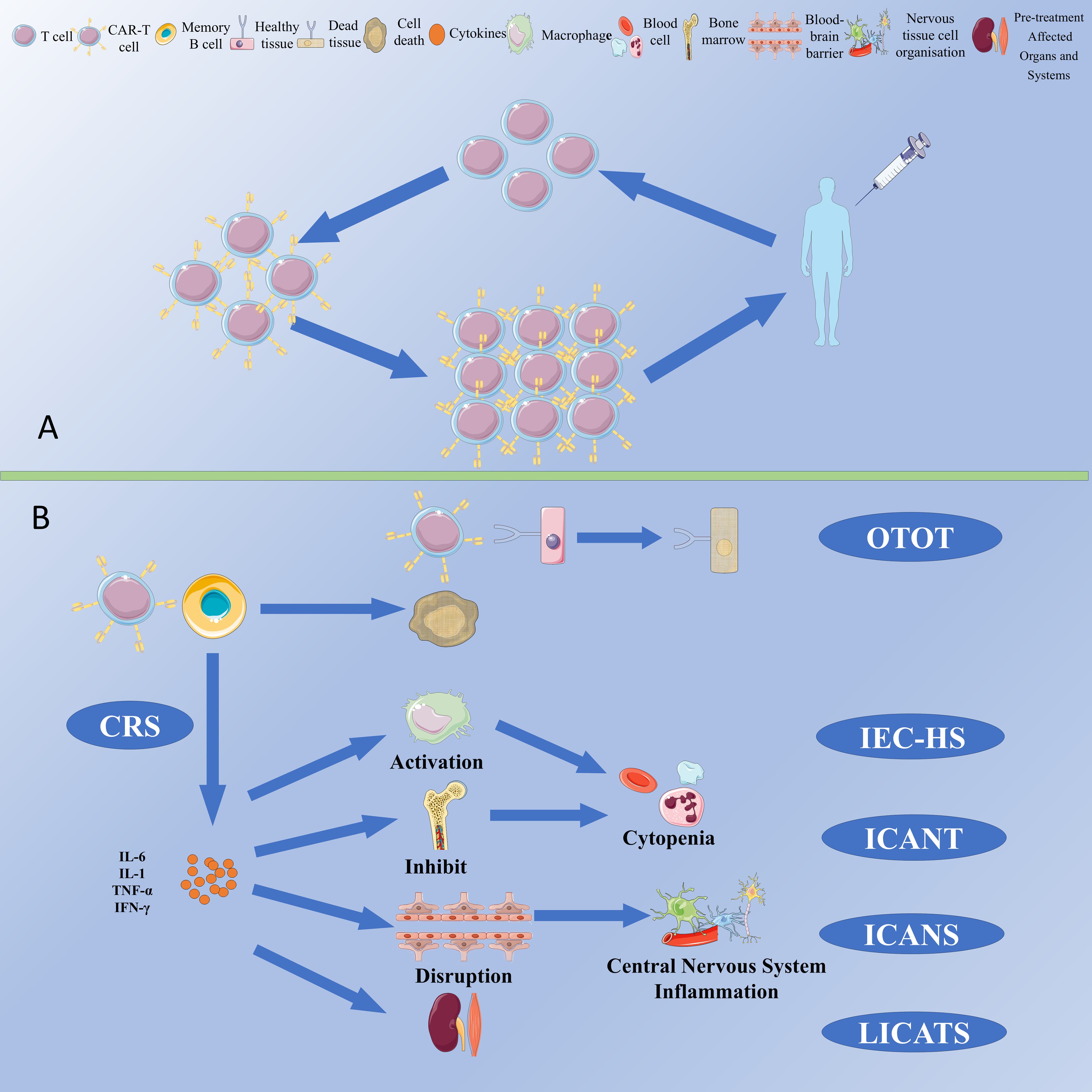
Figure 1. (A) Schematic for manufacturing and administration workflow of CAR-T Therapy. (B) CAR-T cell-associated toxicities.
1.1 Systemic lupus erythematosus
The pathogenesis of SLE is centered on the dysregulation of autoreactive B cells, which are abnormally activated and continuously produce antibodies targeting self-tissues, leading to inflammatory responses and tissue damage. Current B cell-targeted therapeutic strategies, such as rituximab and belimumab, have demonstrated some efficacy; however, some patients respond poorly to these treatments. In contrast, CAR-T therapy offers a more rapid and sustained targeting effect, providing a novel and promising option for SLE. CD19 is highly expressed at all stages of B cell differentiation and is considered a promising target for more effective and durable therapeutic responses in treating SLE (28, 29). Kansal (30) demonstrated that CD19-targeted CAR-T cells (Figure 2A) exerted therapeutic effects in a murine lupus model by depleting B cells and could prevent disease progression before the onset of illness. Subsequently, Jin (31) developed murine anti-CD19 CAR-T cells incorporating CD28 or 4-1BB co-stimulatory signals. They found that allogeneic CD19 CAR-T cell infusion prevented disease occurrence before symptom manifestation and provided therapeutic benefits in the later stages of the disease. CAR-NK therapy is another type of cell-based therapy that has also demonstrated potential for treating SLE. Specifically, anti-programmed death ligand 1 (PD-L1) CAR-NK 92 cells (Figure 2B) are capable of recognizing and eliminating follicular helper T cells with high expression of programmed death protein 1 (PD-1), thereby reducing the proliferation and differentiation of memory B cells, decreasing immunoglobulin secretion, and alleviating splenomegaly (32). Mougiakakos (6) first applied autologous anti-CD19 CAR-T therapy in patients with refractory SLE. Post-treatment, the patient’s abnormal laboratory parameters normalized, and the SLEDAI was reduced to 0. In their subsequent study, all five patients with refractory SLE achieved rapid and sustained remission (7). Zhang (5) reported a case of SLE with stage IV diffuse large B cell lymphoma (DLBCL) that achieved stable remission following anti-BCMA/CD19 CAR-T therapy (Figure 2C). Feng (33) also demonstrated effective and sustained remission in 12 patients with refractory SLE treated with anti-BCMA/CD19 CAR-T therapy. A study analyzing serum samples from six patients with refractory SLE before and after CAR-T therapy revealed decreased levels of IL-6 and TNF-α, accompanied by increased levels of IL-7 and BAFF, as well as a significant reduction in SLE-related antibodies (34). These findings all show CAR-T therapy’s potential in treating SLE.
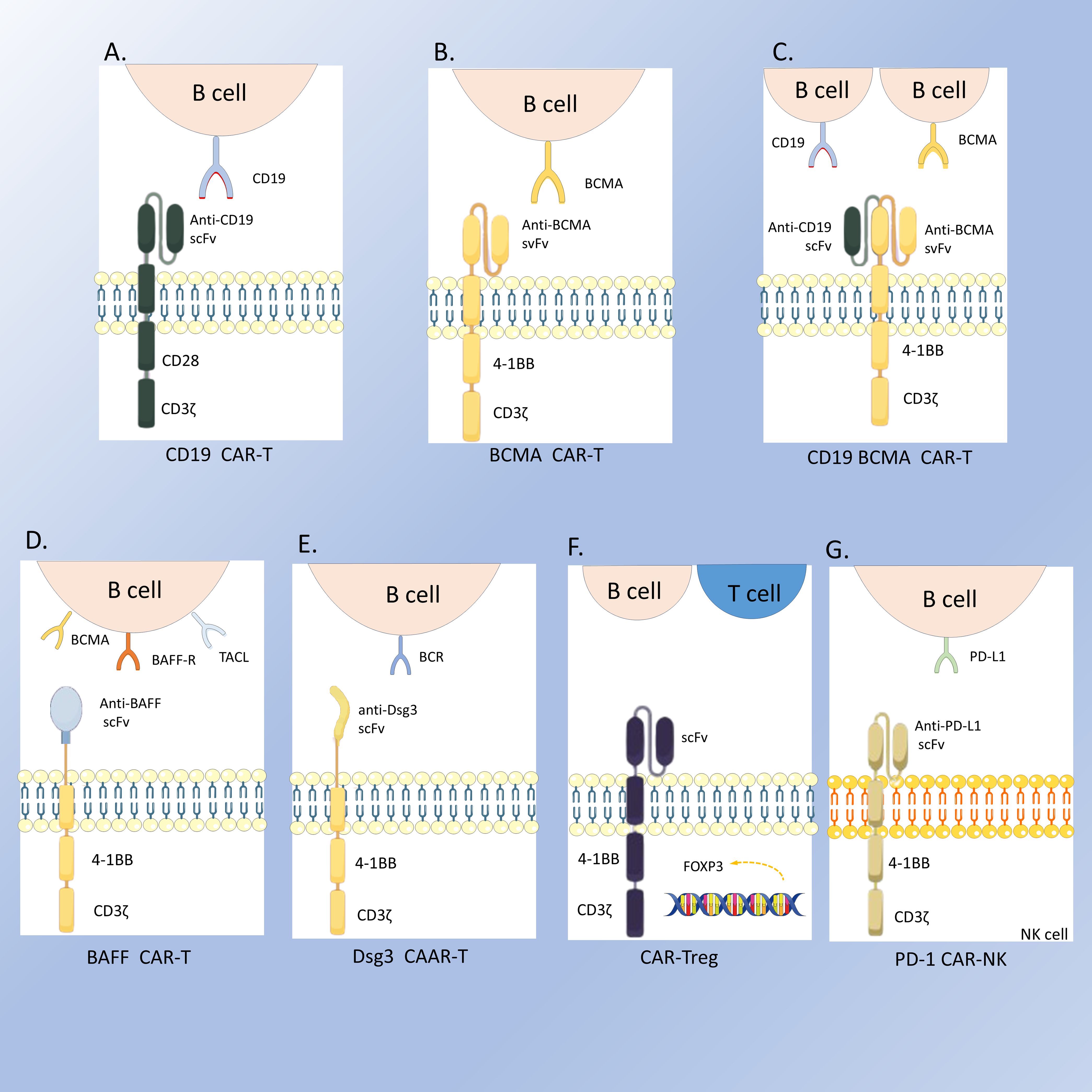
Figure 2. The structures of the CARs. (A) CD19 CAR-T; (B) BCMA CAR-T; (C) CD19-BCMA CAR-T; (D) BAFF CAR-T; (E) Dsg3 CAAR-T; (F) CAR-Treg; (G) PD-1 CAR-NK.
1.2 Idiopathic inflammatory myopathies
CAR-T therapy has also demonstrated significant efficacy in the treatment of IIM, including juvenile dermatomyositis (JDM), antisynthetase syndrome (ASS), and immune-mediated necrotizing myopathy (IMNM). There are refractory ASS cases (16, 17) that were successfully treated with CAR-T therapy. Ann-Christin Pecher proposed that the cytokine release following CAR-T therapy may promote the expansion of CD8+ Temra cells, thereby sustaining inflammation. So, she administered mycophenolate mofetil one month after CAR-T treatment to induce durable clinical remission, thereby validating the hypothesis that B cells and T cells cross-activate to cause myositis. Rebecca Nicolai (14)reported a case of refractory JDM treated with CAR-T therapy, in which MRI at the 6-month follow-up showed resolution of lesions suggestive of myositis. This was accompanied by decreased expression of interferon (IFN) and IFN-induced chemokines, such as CXCL10 and CXCL9. Additionally, overexpression of IFN-α is crucial for maintaining B cell proliferation and stimulating the expansion of short-lived plasmablasts. IFN-λ promotes the differentiation of naïve B cells into plasmablasts via the mTORC1 pathway, leading to IL-6 and IL-10 and antibody production. SRP-IMNM is a particularly refractory subgroup of IMNM. Chuan Qin (15) reported using CAR-BCMA T therapy in a patient with refractory SRP-IMNM complicated by Sjögren’s syndrome. The patient exhibited sustained clinical and radiological improvement, with seroconversion of anti-SRP antibodies to negative. Additionally, within the T cell lineage, the highly cytotoxic and inflammatory CD8+ TE-1 cells were replaced by CD8+ TE-3 cells, indicating that CAR-T therapy may help modulate immune responses and reduce inflammation. Xiaobing Wang (19) also reported a case of refractory SRP-IMNM treated with allogeneic CD19 CAR-T therapy. The thigh muscle biopsy demonstrated a marked reduction in SRP expression and decreased B cell infiltration. Moreover, CAR-T therapy has also been proven effective and safe in severe pediatric cases. Andre’s París-Munoz (25) successfully treated a 10-year-old girl with MDA5+DM-rapidly progressive interstitial lung disease (RPILD) and macrophage activation syndrome (MAS), who had not responded well to conventional treatments. After receiving CD19 CAR-T therapy, the patient did not experience any serious adverse effects. For 11 months in an immunosuppressant-free context, the patient exhibited progressive improvements in motor and lung function, as well as restoration of the B cell compartment, without any autoimmune episodes.
1.3 Systemic sclerosis
In patients with SSc, the upregulation of B-cell activating factors and the abnormality of B-cell homeostasis make targeting CD19+ B cells a potential therapeutic strategy that could induce a more profound immune system reprogramming. Christina Bergmann (20) reported a case of a patient with SSc who received CAR-T therapy and experienced significant improvements in cardiac, joint, skin, and serological indicators. Wolfgang Merkt (21) reported a case of a patient with Scl-70 positive SSc treated with third-generation CD19 CAR-T therapy in combination with mycophenolate mofetil and nintedanib. Clinical evaluation at a five-month follow-up demonstrated a significant therapeutic response, characterized by reduced ANA titers and CRP levels, along with improved pulmonary function parameters and radiologic manifestations. Xiaobing Wang (19) reported the use of allogeneic CD19 CAR-T cells in two patients with relapsing diffuse cutaneous systemic sclerosis (dcSSc). Both patients exhibited robust responses to treatment, with skin biopsies revealing a significant reduction in B cell infiltration. These findings suggest that the depth of B cell depletion is far higher with CAR-T cell-based than antibody-based B cell depletion. Additionally, the patients exhibited potential reversal of tissue fibrosis, highlighting the therapeutic potential of this approach for treating systemic sclerosis.
1.4 Dermatology
Currently, CAR-T therapy is showing surprising effects in the treatment of psoriasis. Shaowei Qiu (27) successfully applied CD19 CAR-T therapy to treat a patient with high-risk pro-B acute lymphoblastic leukemia (pro-B ALL) complicated with severe plaque psoriasis. Notably, the patient’s psoriasis lesions completely resolved within 2 months after CAR-T infusion, and no signs of relapse were observed during the follow-up period. Huirui Wang (26) used CAR-T therapy to treat a 65-year-old male patient with a 45-year history of psoriasis and refractory DLBCL. After receiving CD19 CAR-T therapy, the patient not only achieved complete remission of refractory DLBCL but also experienced a significant improvement in his long-standing psoriasis, with only minimal residual lesions remaining on the neck. These findings suggest that CD19 CAR-T therapy may exert therapeutic effects on psoriasis by targeting B cells and modulating the immune microenvironment, offering a new research direction and potential therapeutic strategy for psoriasis treatment and highlighting the need for further exploration of the application and immunological mechanisms of CAR-T cell therapy in psoriasis.
1.5 Others
Beyond the aforementioned diseases, progress has also been made in other autoimmune conditions. For instance, a 37-year-old female patient with both Rheumatoid Arthritis (RA) and myasthenia gravis (MG) achieved complete remission of MG and a significant reduction in RA disease activity following CD19-CAR T therapy (22). In the treatment of RA, Bo Zhang (35) and colleagues combined universal anti-fluorescein isothiocyanate (FITC) CAR-T cells with FITC-T-labeled RA-T immunodominant peptides, thereby eliminating hybridoma cells from RA patients and RA patient-derived autoreactive B cell subsets. This approach offers a new direction for precise and customized treatment of RA based on the individual patient’s autoantigen profile. A study on pemphigus vulgaris reported that T cells engineered to express a chimeric autoantibody receptor (CAAR) (Figure 2D) exhibited specific cytotoxicity against cells expressing anti-Dsg3 BCRs in vitro and effectively eliminated these B cells in vivo. Cody D. Moorman (36) developed CAR-T cells and CAR-Tregs targeting X-C motif chemokine receptor 1 (XCR1) (Figure 2E) on the surface of conventional type 1 dendritic cells (DC1) to suppress experimental autoimmune encephalomyelitis (EAE). The results showed that both cell types modestly inhibited Th1-driven EAE. Dörte Lodka (37) first reported the efficacy of CD19 CAR-T cells in ANCA-associated vasculitis, demonstrating robust depletion of B cells and plasmablasts, a significant decline in MPO-ANCA levels, and protection of mice from ANCA-induced necrotizing crescentic glomerulonephritis (NCGN).
2 Adverse effects
Common cytotoxicities associated with CAR-T therapy include cytokine release syndrome (CRS), immune-cell-associated neurotoxicity syndrome (ICANS), immune effector cell-associated haematotoxicity (ICAHT), immune effector cell-associated haemophagocytic lymphohistiocytosis-like syndrome (IEC-HS), persistent B-cell aplasia, hypogammaglobulinemia (HGG), on-target off-tumor toxicity (OTOT) and local immune effector cell-associated toxicity syndrome (LICATS) (Figure 1B). Given the lower B cell burden in autoimmune diseases compared to that in neoplastic disorders, the incidence and severity of adverse effects tend to be correspondingly lower.
CRS is a systemic inflammatory syndrome mediated by the release of cytokines such as IL-6, with fever often being the primary clinical symptom (38). Most reported cases are mild and do not require medication; however, severe cases can be treated with tocilizumab, glucocorticoids, and other drugs (7). ICANS is initiated by the activation of immune cells, which release large amounts of inflammatory cytokines. This process can deplete mural cells (such as endothelial cells expressing CD19) and disrupt the blood-brain barrier, thereby activating microglia and manifesting as neurological symptoms (39). Corticosteroids are the first-line treatment for ICANS. ICAHT refers to bone marrow suppression and cytopenia after CAR-T therapy, which may be related to multiple factors such as target cell burden, hematopoietic reserve, and baseline inflammatory environment (40). After CAR-T therapy, most patients experience lymphopenia or neutropenia (grade 3 or 4), which usually recovers within 4 weeks. Thrombopoietin receptor agonists, glucocorticoids, and stem cell transplantation can improve cytopenia. OTOT is an adverse reaction caused by CAR-T cells attacking healthy tissues that express the same antigens as the pathological tissue. CAAR-T therapy, which targets and eliminates autoreactive cells secreting autoantibodies, offers a new strategy to reduce the risk of off-target effects (41). IEC-HS is a highly inflammatory syndrome resulting from macrophage activation, which has not been reported in CAR-T therapy for autoimmune diseases. Persistent CAR-T cell-induced B-cell hypoplasia and HGG are common complications of CAR-T therapy (11, 41), which may increase the risk of infection, mostly mild (42). To mitigate side effects and avoid unnecessary immune activation, the concept of on/off switches has been introduced into CAR-T therapy, enabling precise control over CAR-T cell activity and allowing them to remain quiescent during remission periods to reduce the risk of unnecessary immune activation and toxicity (43). In a recent study conducted in Germany, LICATS events were documented (44). The researchers posited that LICATS represents a form of toxicity distinct from CRS, characterized by localized and self-limiting organ dysfunction that correlates with the organs and systems affected prior to treatment. The occurrence of LICATS may be associated with the depletion of B cells in tissues by CAR T-cell therapy, thereby triggering localized inflammatory responses. Typically, LICATS can resolve spontaneously or with the administration of low-dose glucocorticoids. In clinical practice, it is crucial to differentiate LICATS from disease relapse or CRS to avert unwarranted therapeutic interventions.
3 Other CAR Therapy
CAR-NK cells are NK cells that have been genetically engineered to express chimeric antigen receptors (CARs) and have shown potential in the treatment of autoimmune diseases. Research by Seth D. Reighard (32) has demonstrated that CAR-NK cells derived from the NK-92 cell line and targeting PD-L1 successfully reduced the proliferation of memory B cells as well as the secretion and differentiation of immunoglobulins in a humanized lupus-like disease mouse model. Xiaobing Wang has (45) developed an off-the-shelf allogeneic iPSC-derived CD19/BCMA CAR-NK dual-targeting cell product, which has been successfully applied to a patient with refractory dcSSc. In addition, CAR-NK cells can secrete specific cytokines such as IFN-γ and GM-CSF, which help to reduce the risk of CRS and neurotoxicity (46). Compared with traditional CAR-T cells, CAR-NK cells offer more flexibility as they can eliminate target cells not only through antigen-dependent cytotoxicity but also through antigen-independent mechanisms (47).
CAAR T therapy is an innovative immunotherapy that incorporates autoantigens into the receptors of T cells, enabling these T cells to specifically recognize and eliminate autoreactive B cells. Jie Zhou (48) constructed GPIbα CAAR T cells, which demonstrated robust persistence and specific cytotoxicity in an NSG mouse model, significantly reducing the growth of anti-GPIbα hybridoma cells without apparent organ toxicity. When applied to immune thrombocytopenia (ITP) patients, these cells could identify and eliminate autoreactive B cells from the patients’ sera. GPIbα CAAR T therapy is a precision cellular immunotherapy with the potential to induce complete and durable remission in refractory and relapsed ITP patients. CAAR T therapy was also applied to the treatment of pemphigus and muscle-specific tyrosine kinase myasthenia gravis.
CAR-Treg cells are obtained by co-culturing T cells with two vectors containing the CAR gene and the FOXP3 gene. They can not only interact with dendritic cells to convert conventional T cells into induced Treg cells, thereby expanding the effect of immune tolerance (49), but also suppress immune responses targeting antigens and regulate other related immune responses through bystander suppression mechanisms (secreting anti-inflammatory cytokines such as IL-10 and TGF-β to modulate the local immune environment) (50). In the treatment of autoimmune diseases, CAR-Treg cells have been applied. Related studies have shown that in a 2,4,6-trinitrophenol (TNP)-induced IBD mouse model, TNP-CAR-Treg cells can be observed at the inflamed colon site. This indicates that CAR-Treg cells designed against specific antigens can alleviate colitis in mice by recognizing and suppressing overactive immune responses (51). Tenspolde (52) also found that insulin CAR-Treg cells showed effective control of type 1 diabetes in both in vitro and in vivo experiments.
With the continuous expansion of CAR technology, CAR-T cells targeting the BAFF ligand have shown unique advantages. Researchers used the non-viral transposon system TcBuster (TcB) to stably integrate BAFF-CAR into T cells (Figure 2F). This method is safer and more economical than traditional viral transduction methods, and these CAR-T cells can target three receptors: BAFF-R, BCMA, and TACI simultaneously (53). Studies have shown that BAFF CAR-T cells can specifically deplete autoreactive B cells by targeting BAFF receptors, thereby reducing the production of autoantibodies. This phenomenon has been confirmed in various lupus animal models, including humanized lupus mouse models and MRL/lpr mouse models (54). As research deepens, it is hoped that more types of CAR-T cells will be applied to the treatment of autoimmune diseases in the future, providing patients with more options.
CAR-T therapy has demonstrated the potential to alleviate symptoms and improve the quality of life for patients with autoimmune diseases. However, the sustainability and stability of its therapeutic effect still pose challenges. Because CAR-T therapy cannot eliminate the patient’s pathological T cells or some CD19- plasma cells that produce antibodies, patients may experience persistent disease or recurrence after the treatment (55). It has been reported that a patient with IIM relapsed 9 months after CD19 CAR-T therapy. Reinfusion of CD19 CAR-T cells failed to expand. After the failure of the reinfusion of CD19 CAR-T cells, the patient then received BCMA CAR-T cell therapy (Figure 2G) and achieved stable drug-free remission (25). This suggests that BCMA targeting addresses autoreactive plasma cells resistant to CD19-directed therapies and switching CAR-T cell targets may be an effective strategy in the treatment of AIDs. Therefore, it is crucial to continuously innovate to address the limitations of CAR-T therapy in autoimmune diseases to enhance efficacy and durability and minimize adverse events. To further explore the potential of CAR-T therapy in autoimmune diseases, multiple registered clinical trials have been initiated worldwide (Table 2). Off-the-shelf allogeneic CAR-T therapy recently has been utilized in the treatment of autoimmune diseases with excellent therapeutic responses (19). This approach significantly reduces the treatment waiting time and demonstrates superior cost-effectiveness compared to conventional therapies. However, off-the-shelf CAR-T therapy also has disadvantages. For example, their expansion and persistence are not as good as those of autologous CAR-T cells. There is also the possibility of graft-versus-host disease(GVHD). At present, there are also studies (45) working on solving these problems, such as using iPSC-derived NK cell-based CAR therapies and knocking out certain genes. In summary, the emergence and continuous improvement of CAR-T cell therapy offers a promising and effective treatment option for patients with autoimmune diseases. It also faces many challenges and limitations that need to be solved.
Author contributions
YHW: Conceptualization, Data curation, Methodology, Writing – original draft. LH: Investigation, Writing – original draft. YW: Investigation, Writing – review & editing. MG: Writing – original draft. YNW: Formal Analysis, Writing – original draft. JG: Writing – review & editing. HH: Writing – review & editing. CL: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (82074246, 82374272).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kuwana Y, Asakura Y, Utsunomiya N, Nakanishi M, Arata Y, Itoh S, et al. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem Biophys Res Commun. (1987) 149:960–8. doi: 10.1016/0006-291x(87)90502-x
2. June CH and Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. (2018) 379:64–73. doi: 10.1056/NEJMra1706169
3. Mikkilineni L and Kochenderfer JN. CAR T cell therapies for patients with multiple myeloma. Nat Rev Clin Oncol. (2021) 18:71–84. doi: 10.1038/s41571-020-0427-6
4. June CH, O’Connor RS, Kawalekar OU, Ghassemi S, and Milone MC. CAR T cell immunotherapy for human cancer. Science. (2018) 359:1361–5. doi: 10.1126/science.aar6711
5. Zhang W, Feng J, Cinquina A, Wang Q, Xu H, Zhang Q, et al. Treatment of systemic lupus erythematosus using BCMA-CD19 compound CAR. Stem Cell Rev Rep. (2021) 17:2120–3. doi: 10.1007/s12015-021-10251-6
6. Mougiakakos D, Krönke G, Völkl S, Kretschmann S, Aigner M, Kharboutli S, et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N Engl J Med. (2021) 385:567–9. doi: 10.1056/NEJMc2107725
7. Mackensen A, Müller F, Mougiakakos D, Böltz S, Wilhelm A, Aigner M, et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus [published correction appears in Nat Med. 2023 Nov;29(11):2956. doi: 10.1038/s41591-022-02091-9. Nat Med. (2022) 28:2124–32. doi: 10.1038/s41591-022-02017-5
8. Li M, Zhang Y, Jiang N, Ning C, Wang Q, Xu D, et al. Anti-CD19 CAR T cells in refractory immune thrombocytopenia of SLE. N Engl J Med. (2024) 391:376–8. doi: 10.1056/NEJMc2403743
9. Taubmann J, Müller F, Yalcin Mutlu M, Völkl S, Aigner M, Bozec A, et al. CD19 Chimeric Antigen Receptor T Cell Treatment: Unraveling the Role of B Cells in Systemic Lupus Erythematosus [published correction appears in Arthritis Rheumatol. 2025 Jan 31. doi: 10.1002/art.43080. Arthritis Rheumatol. (2024) 76:497–504. doi: 10.1002/art.42784
10. Krickau T, Naumann-Bartsch N, Aigner M, Kharboutli S, Kretschmann S, Spoerl S, et al. CAR T-cell therapy rescues adolescent with rapidly progressive lupus nephritis from haemodialysis. Lancet. (2024) 403:1627–30. doi: 10.1016/S0140-6736(24)00424-0
11. Müller F, Taubmann J, Bucci L, Wilhelm A, Bergmann C, Völkl S, et al. CD19 CAR T-cell therapy in autoimmune disease - A case series with follow-up. N Engl J Med. (2024) 390:687–700. doi: 10.1056/NEJMoa2308917
12. Wang W, He S, Zhang W, Zhang H, DeStefano VM, Wada M, et al. BCMA-CD19 compound CAR T cells for systemic lupus erythematosus: a phase 1 open-label clinical trial. Ann Rheum Dis. (2024) 83:1304–14. doi: 10.1136/ard-2024-225785
13. Shu J, Xie W, Mei C, Ren A, Ke S, Ma M, et al. Safety and clinical efficacy of Relmacabtagene autoleucel (relma-cel) for systemic lupus erythematosus: a phase 1 open-label clinical trial. EClinicalMedicine. (2025) 83:103229. doi: 10.1016/j.eclinm.2025.103229
14. Nicolai R, Merli P, Moran Alvarez P, Bracaglia C, Del Bufalo F, Marasco E, et al. Autologous CD19-targeting CAR T cells in a patient with refractory juvenile dermatomyositis. Arthritis Rheumatol. (2024) 76:1560–5. doi: 10.1002/art.42933
15. Qin C, Dong MH, Zhou LQ, Wang W, Cai SB, You YF, et al. Single-cell analysis of refractory anti-SRP necrotizing myopathy treated with anti-BCMA CAR-T cell therapy. Proc Natl Acad Sci U S A. (2024) 121:e2315990121. doi: 10.1073/pnas.2315990121
16. Pecher AC, Hensen L, Klein R, Schairer R, Lutz K, Atar D, et al. CD19-targeting CAR T cells for myositis and interstitial lung disease associated with antisynthetase syndrome. JAMA. (2023) 329:2154–62. doi: 10.1001/jama.2023.8753
17. Taubmann J, Knitza J, Müller F, Völkl S, Aigner M, Kleyer A, et al. Rescue therapy of antisynthetase syndrome with CD19-targeted CAR-T cells after failure of several B-cell depleting antibodies. Rheumatol (Oxford). (2024) 63:e12–4. doi: 10.1093/rheumatology/kead330
18. Müller F, Boeltz S, Knitza J, Aigner M, Völkl S, Kharboutli S, et al. CD19-targeted CAR T cells in refractory antisynthetase syndrome. Lancet. (2023) 401:815–8. doi: 10.1016/S0140-6736(23)00023-5
19. Wang X, Wu X, Tan B, Zhu L, Zhang Y, Lin L, et al. Allogeneic CD19-targeted CAR-T therapy in patients with severe myositis and systemic sclerosis. Cell. (2024) 187:4890–4904.e9. doi: 10.1016/j.cell.2024.06.027
20. Bergmann C, Müller F, Distler JHW, Györfi AH, Völkl S, Aigner M, et al. Treatment of a patient with severe systemic sclerosis (SSc) using CD19-targeted CAR T cells. Ann Rheum Dis. (2023) 82:1117–20. doi: 10.1136/ard-2023-223952
21. Merkt W, Freitag M, Claus M, Kolb P, Falcone V, Röhrich M, et al. Third-generation CD19.CAR-T cell-containing combination therapy in Scl70+ systemic sclerosis. Ann Rheum Dis. (2024) 83:543–6. doi: 10.1136/ard-2023-225174
22. Haghikia A, Hegelmaier T, Wolleschak D, Böttcher M, Pappa V, Motte J, et al. Clinical efficacy and autoantibody seroconversion with CD19-CAR T cell therapy in a patient with rheumatoid arthritis and coexisting myasthenia gravis. Ann Rheum Dis. (2024) 83:1597–8. doi: 10.1136/ard-2024-226017
23. Szabo D, Balogh A, Gopcsa L, Giba-Kiss L, Lakatos G, Paksi M, et al. Sustained drug-free remission in rheumatoid arthritis associated with diffuse large B-cell lymphoma following tandem CD20-CD19-directed non-cryopreserved CAR-T cell therapy using zamtocabtagene autoleucel. RMD Open. (2024) 10:e004727. doi: 10.1136/rmdopen-2024-004727
24. Li Y, Li S, Zhao X, Sheng J, Xue L, Schett G, et al. Fourth-generation chimeric antigen receptor T-cell therapy is tolerable and efficacious in treatment-resistant rheumatoid arthritis. Cell Res. (2025). doi: 10.1038/s41422-024-01068-2
25. París-Muñoz A, Alcobendas-Rueda RM, Verdú-Sánchez C, Udaondo C, Galán-Gómez V, González-Martínez B, et al. CD19 CAR-T cell therapy in a pediatric patient with MDA5+ dermatomyositis and rapidly progressive interstitial lung disease. Med. (2025). doi: 10.1016/j.medj.2025.100676
26. Wang SY, An WH, Wang ZS, Wang WL, Zhang B, Xu KL, et al. Incidentally cured psoriasis in a patient with refractory/relapsed diffuse large B-cell lymphoma receiving CD19 CAR-T cell therapy: a case report. Front Immunol. (2024) 15:1418768. doi: 10.3389/fimmu.2024.1418768
27. Zhang A, Zhang G, Yang H, Gong B, Li S, Wei N, et al. Treatment of pro-B acute lymphoblastic leukemia and severe plaque psoriasis with anti-CD19 CAR T cells: a case report. Front Immunol. (2025) 16:1529745. doi: 10.3389/fimmu.2025.1529745
28. Odendahl M, Jacobi A, Hansen A, Feist E, Hiepe F, Burmester GR, et al. Disturbed peripheral B lymphocyte homeostasis in systemic lupus erythematosus. J Immunol. (2000) 165:5970–9. doi: 10.4049/jimmunol.165.10.5970
29. Mei HE, Schmidt S, and Dörner T. Rationale of anti-CD19 immunotherapy: an option to target autoreactive plasma cells in autoimmunity. Arthritis Res Ther. (2012) 14 Suppl 5:S1. doi: 10.1186/ar3909
30. Kansal R, Richardson N, Neeli I, Khawaja S, Chamberlain D, Ghani M, et al. Sustained B cell depletion by CD19-targeted CAR T cells is a highly effective treatment for murine lupus. Sci Transl Med. (2019) 11:eaav1648. doi: 10.1126/scitranslmed.aav1648
31. Jin X, Xu Q, Pu C, Zhu K, Lu C, Jiang Y, et al. Therapeutic efficacy of anti-CD19 CAR-T cells in a mouse model of systemic lupus erythematosus. Cell Mol Immunol. (2021) 18:1896–903. doi: 10.1038/s41423-020-0472-1
32. Reighard SD, Cranert SA, Rangel KM, Ali A, Gyurova IE, de la Cruz-Lynch AT, et al. Therapeutic Targeting of Follicular T Cells with Chimeric Antigen Receptor-Expressing Natural Killer Cells [published correction appears in Cell Rep Med. 2020 Aug 25;1(5):100080. doi: 10.1016/j.xcrm.2020.100080. Cell Rep Med. (2020) 1:100003. doi: 10.1016/j.xcrm.2020.100003
33. Feng J, Yongxian H, Chang AH, and Huang H. CD19/BCMA CAR-T cell therapy for refractory systemic lupus erythematosus: safety and preliminary efficacy data from a phase I clinical study. Blood. (2023) 142:4835. doi: 10.1182/blood-2023-186669
34. Nunez D, Patel D, Volkov J, Wong S, Vorndran Z, Müller F, et al. Cytokine and reactivity profiles in SLE patients following anti-CD19 CART therapy. Mol Ther Methods Clin Dev. (2023) 31:101104. doi: 10.1016/j.omtm.2023.08.023
35. Zhang B, Wang Y, Yuan Y, Sun J, Liu L, Huang D, et al. In vitro elimination of autoreactive B cells from rheumatoid arthritis patients by universal chimeric antigen receptor T cells. Ann Rheum Dis. (2021) 80:176–84. doi: 10.1136/annrheumdis-2020-217844
36. Moorman CD, Yu S, Briseno CG, Phee H, Sahoo A, Ramrakhiani A, et al. CAR-T cells and CAR-Tregs targeting conventional type-1 dendritic cell suppress experimental autoimmune encephalomyelitis. Front Immunol. (2023) 14:1235222. doi: 10.3389/fimmu.2023.1235222
37. Lodka D, Zschummel M, Bunse M, Rousselle A, Sonnemann J, Kettritz R, et al. CD19-targeting CAR T cells protect from ANCA-induced acute kidney injury. Ann Rheum Dis. (2024) 83:499–507. doi: 10.1136/ard-2023-224875
38. Brudno JN and Kochenderfer JN. Current understanding and management of CAR T cell-associated toxicities. Nat Rev Clin Oncol. (2024) 21:501–21. doi: 10.1038/s41571-024-00903-0
39. Velasco R, Mussetti A, Villagrán-García M, and Sureda A. CAR T-cell-associated neurotoxicity in central nervous system hematologic disease: Is it still a concern? Front Neurol. (2023) 14:1144414. doi: 10.3389/fneur.2023.1144414
40. Westin JR, Oluwole OO, Kersten MJ, Miklos DB, Perales MA, Ghobadi A, et al. Survival with axicabtagene ciloleucel in large B-cell lymphoma. N Engl J Med. (2023) 389:148–57. doi: 10.1056/NEJMoa2301665
41. Pecher AC, Hensen L, Lengerke C, and Henes J. The future of CAR T therapeutics to treat autoimmune disorders. Mol Diagn Ther. (2024) 28:593–600. doi: 10.1007/s40291-024-00730-0
42. Lee AY and Reed JH. Highlight of 2023: CAR T cells driving precision therapy for autoimmune disease. Immunol Cell Biol. (2024) 102:437–40. doi: 10.1111/imcb.12766
43. Ohno R and Nakamura A. Advancing autoimmune Rheumatic disease treatment: CAR-T Cell Therapies - Evidence, Safety, and future directions. Semin Arthritis Rheumatol. (2024) 67:152479. doi: 10.1016/j.semarthrit.2024.152479
44. Hagen M, Müller F, Wirsching A, Kharboutli S, Spoerl S, Düsing C, et al. Local immune effector cell-associated toxicity syndrome in CAR T-cell treated patients with autoimmune disease: an observational study. Lancet Rheumatol. (2025) 7:e424–33. doi: 10.1016/S2665-9913(25)00091-8
45. Wang X, Zhang Y, Jin Y, Dai L, Yue Y, Hu J, et al. An iPSC-derived CD19/BCMA CAR-NK therapy in a patient with systemic sclerosis. Cell. (2025). doi: 10.1016/j.cell.2025.05.038
46. Hassan SH, Alshahrani MY, Saleh RO, Mohammed BA, Kumar A, Almalki SG, et al. A new vision of the efficacy of both CAR-NK and CAR-T cells in treating cancers and autoimmune diseases. Med Oncol. (2024) 41:127. doi: 10.1007/s12032-024-02362-0
47. Moreno C, Haynie C, Cheever A, and Weber KS. Alternative CAR therapies: recent approaches in engineering chimeric antigen receptor immune cells to combat cancer. Biomedicines. (2022) 10:1493. doi: 10.3390/biomedicines10071493
48. Zhou J, Xu Y, Shu J, Jiang H, Huang L, Xu M, et al. GPIba CAAR T cells function like a Trojan horse to eliminate autoreactive B cells to treat immune thrombocytopenia. Haematologica. (2024) 109:2256–70. doi: 10.3324/haematol.2023.283874
49. Arjomandnejad M, Kopec AL, and Keeler AM. CAR-T regulatory (CAR-treg) cells: engineering and applications. Biomedicines. (2022) 10:287. doi: 10.3390/biomedicines10020287
50. Abraham AR, Maghsoudlou P, Copland DA, Nicholson LB, and Dick AD. CAR-Treg cell therapies and their future potential in treating ocular autoimmune conditions. Front Ophthalmol (Lausanne). (2023) 3:1184937. doi: 10.3389/fopht.2023.1184937
51. Elinav E, Waks T, and Eshhar Z. Redirection of regulatory T cells with predetermined specificity for the treatment of experimental colitis in mice. Gastroenterology. (2008) 134:2014–24. doi: 10.1053/j.gastro.2008.02.060
52. Tenspolde M, Zimmermann K, Weber LC, Hapke M, Lieber M, Dywicki J, et al. Regulatory T cells engineered with a novel insulin-specific chimeric antigen receptor as a candidate immunotherapy for type 1 diabetes. J Autoimmun. (2019) 103:102289. doi: 10.1016/j.jaut.2019.05.017
53. Wong DP, Roy NK, Zhang K, Anukanth A, Asthana A, Shirkey-Son NJ, et al. A BAFF ligand-based CAR-T cell targeting three receptors and multiple B cell cancers. Nat Commun. (2022) 13:217. doi: 10.1038/s41467-021-27853-w
54. Uppin V, Gibbons H, Troje M, Feinberg D, Webber BR, Moriarity BS, et al. CAR-T cell targeting three receptors on autoreactive B cells for systemic lupus erythematosus therapy. J Autoimmun. (2025) 151:103369. doi: 10.1016/j.jaut.2025.103369
Keywords: chimeric antigen receptor T-cell therapy, CAR (chimeric antigen receptor), SLE - systemic lupus erythematosus, autoimmune diseases, adverse (side) effects
Citation: Wu Y, Han L, Wang Y, Gu M, Wang Y, Gao J, Huang H and Li C (2025) CAR-T therapy: pioneering a new era in the treatment of autoimmune diseases. Front. Immunol. 16:1625166. doi: 10.3389/fimmu.2025.1625166
Received: 08 May 2025; Accepted: 23 July 2025;
Published: 13 August 2025.
Edited by:
Apostolos Zaravinos, European University Cyprus, CyprusReviewed by:
Rao Prabhala, Harvard Medical School, United StatesSalim H. Hassan, Al-Furat Al-Awsat Technical University, Iraq
Copyright © 2025 Wu, Han, Wang, Gu, Wang, Gao, Huang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen Li, Y2FzaW8xOTgxQDE2My5jb20=
 Yuanhao Wu
Yuanhao Wu Luyao Han
Luyao Han Yu Wang
Yu Wang Mengjiao Gu1
Mengjiao Gu1 Chen Li
Chen Li