- Institute of Transplantation Medicine, The Second Affiliated Hospital of Guangxi Medical University, Guangxi Clinical Research Center for Organ Transplantation, Guangxi Key Laboratory of Organ Donation and Transplantation, Nanning, Guangxi, China
Objective: To comparatively evaluate the efficacy and safety of induction therapies in solid organ transplantation (SOT) using a Bayesian network meta-analysis (NMA).
Methods: Randomized controlled trials (RCTs) assessing induction therapies were systematically identified across major databases (up to November 20, 2024). The screening, data extraction, and risk of bias (ROB) assessment were independently conducted by two reviewers through standardized tools. Bayesian NMA synthesized outcomes, including rejection, graft/overall survival, and infection rates.
Results: Sixty-eight RCTs (9,626 patients) evaluating 12 therapies were included. Surface Under the Cumulative Ranking Area (SUCRA) probabilities identified alemtuzumab as the most effective agent for reducing rejection rates (93.9%), followed by antilymphocyte globulin (ALG, 87.0%) and belimumab (77.0%). For graft survival, OKT3 ranked highest (87.9%), with subsequent superiority for ALG (83.5%) and alemtuzumab (75.6%). Basiliximab demonstrated the highest overall survival benefit (88.0%), outperforming rabbit antithymocyte globulin (rATG, 82.1%) and inolimomab (70.3%). Belimumab showed the greatest infection risk reduction (94.4%), surpassing alemtuzumab (80.0%) and basiliximab (74.5%).
Conclusion: Alemtuzumab emerged as the optimal therapy for minimizing rejection, while OKT3 and basiliximab were superior for graft and overall survival, respectively. Belimumab exhibited the strongest potential for reducing incidence of infection. These findings highlight therapy-specific advantages for optimizing SOT outcomes.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/myprospero, identifier CRD42025634120.
1 Introduction
Solid organ transplantation (SOT) has emerged as the optimal therapy for patients with end-stage organ disease, based on both clinical and economic perspectives (1). However, a significant challenge in this field is the persistent shortage of organs (2). Mortality rates for individuals remaining on the transplant waiting list continue to be significantly elevated (3). Consequently, enhancing graft survival remains a pressing issue requiring resolution. Acute rejection following organ transplantation is the primary determinant of short-term graft survival (4). Furthermore, prolonged administration of immunosuppressive medications can progressively impair graft function, ultimately leading to graft failure (5). Additionally, chronic antibody-mediated rejection driven by donor-specific antibodies (DSA) poses a major obstacle to the long-term survival of transplanted organs (1, 6). Nonetheless, antibody induction therapy has partially mitigated this issue. Numerous prospective RCTs have confirmed that antibody induction significantly decreases acute rejection episodes (4) and markedly enhances short-term graft survival (7). This improvement can be attributed to the widespread adoption of preoperative induction protocols, which facilitate delayed initiation of postoperative immunosuppressive regimens and allow for lower doses of maintenance immunosuppressants, thus reducing the harmful impact of immunosuppressive drugs on the graft (8). The landscape of induction therapy has evolved substantially over the past several decades. Initially, many transplantation centers explored either no induction or therapies involving cyclophosphamide or anti-CD3 monoclonal antibodies (OKT3) (9); however, these approaches have gradually been phased out because of their considerable side effects. Despite the potential promise of targeting B cell activation and acknowledging the role of DSA in SOT (10), current immunosuppressive research continues to focus primarily on T cells (11, 12). Agents such as antithymocyte globulin (ATG), anti-CD52, and anti-CD25 have gained widespread acceptance. However, the efficacy and safety of these induction protocols remain unclear. Therefore, establishing a widely accepted, safe, and effective pre-transplantation induction regimen is crucial for preventing graft damage and loss due to acute rejection.
Network meta-analysis (NMA) enables comparative assessments of therapeutic interventions through indirect treatment comparisons and quantitative synthesis, thereby facilitating the optimization of therapeutic strategies. In this investigation, we employ NMA methodology to systematically evaluate the efficacy of induction therapy in preventing acute rejection episodes among SOT recipients. The findings aim to provide evidence-based recommendations to inform clinical decision-making in this patient population.
2 Data and methods
This systematic review and NMA registers on PROSPERO. The work has been reported in line with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (13) and AMSTAR (Assessing the methodological quality of systematic reviews) Guidelines (14).
2.1 Literature screening
A comprehensive search was conducted in Cochrane Library, PubMed, Embase, and Web of Science (through November 20, 2024) using MeSH and free-text keywords such as “transplantation”, “rejection”, and “randomized controlled trials”. Full search syntax is available in Supplementary Material 1.
2.2 Eligibility criteria
2.2.1 Type: randomized controlled trials (RCTs)
2.2.2 Population: SOT patients receiving induction therapy
2.2.3 Intervention
Induction therapies included basiliximab, alemtuzumab, daclizumab, horse anti-thymocyte globulin (hATG), rabbit anti-thymocyte globulin (rATG), odulimumab, inolimomab, OKT3, Lo-tact-1, rituximab, belimumab, and anti-lymphocyte globulin (ALG). The control group received standard care.
2.2.4 Outcome
Primary outcome: Rejection rate.
Secondary outcomes: (1) Graft survival rate. (2) Overall survival rate. (3) Infection rate.
2.2.5 Exclusion
(1) Non-English language publications (15). (2) Duplicate datasets. (3) Insufficient outcome reporting. (4) Unresolved data discrepancies after author contact. (5) Test or control groups receiving pharmacological agents for the treatment of rejection. (6) Cell transplantation and tissue transplantation.
2.3 Data collection
Two investigators independently screened titles/abstracts, reviewed full texts, and extracted data using standardized forms. Discrepancies were resolved via consensus or third-party adjudication. Corresponding authors were contacted for missing data. Extracted variables encompassed study metadata (authors, country, year), participant demographics, intervention protocols, and outcome metrics.
2.4 Quality assessment
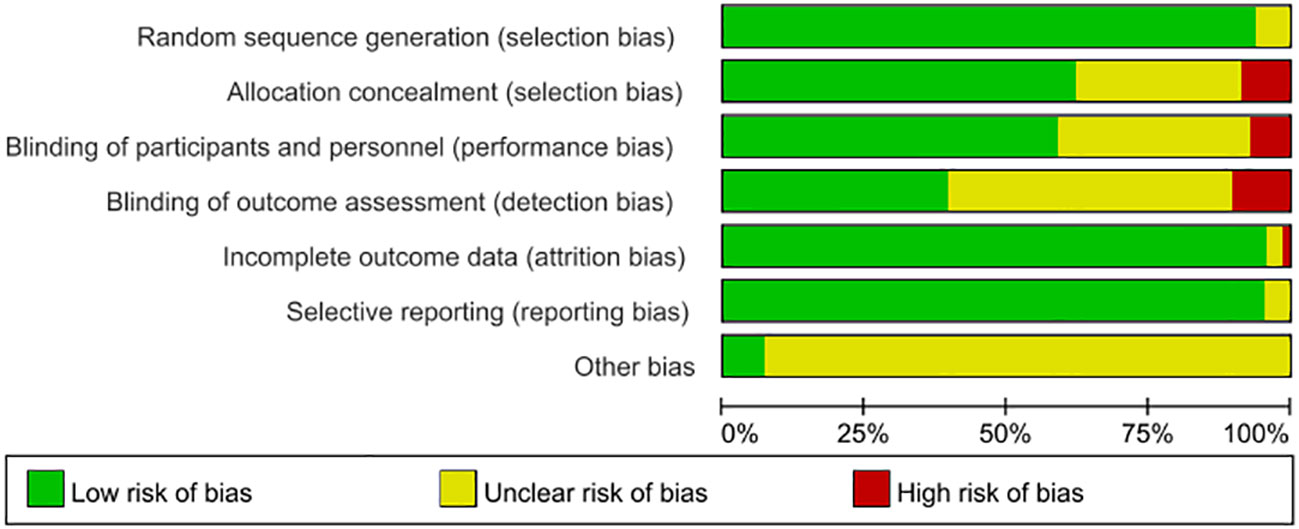
Risk of bias (ROB) assessment for included RCTs followed Cochrane Collaboration guidelines (v5.1.0), evaluating seven domains: randomization, allocation concealment, blinding, incomplete data, selective reporting, and other biases. The category “Other biases” assesses whether significant factors exist in the specific study being evaluated, considering its design and methodological details, which might systematically distort its results and are not covered by the six standard domains. Examples include bias in crossover trials, contamination, bias due to early stopping, bias due to baseline imbalances, and recruitment bias in cluster-randomized trials. Two reviewers independently categorized each domain as high, unclear risk, or low. Disagreements were resolved through discussion.
2.5 Statistical methods
Bayesian NMA was performed using R 4.4.0 (GeMTC package) with model consistency verified via Deviance Information Criterion (DIC; Supplementary Material 2). Fixed-effects models generated mean differences (95% CIs). Results were visualized via forest plots, league tables, and ranking probability diagrams. Surface Under the Cumulative Ranking Area (SUCRA) scores (0–1 scale) ranked intervention efficacy. Stata 18 generated network plots and assessed publication bias via comparison-adjusted funnel plots.
3 Results
3.1 Study selection
Initial database screening identified 17,621 English-language records. After removing 5,198 duplicates, 11,964 studies were excluded through title/abstract screening. Full-text review excluded 391 additional articles, yielding 68 eligible RCTs for final inclusion (Figure 1).
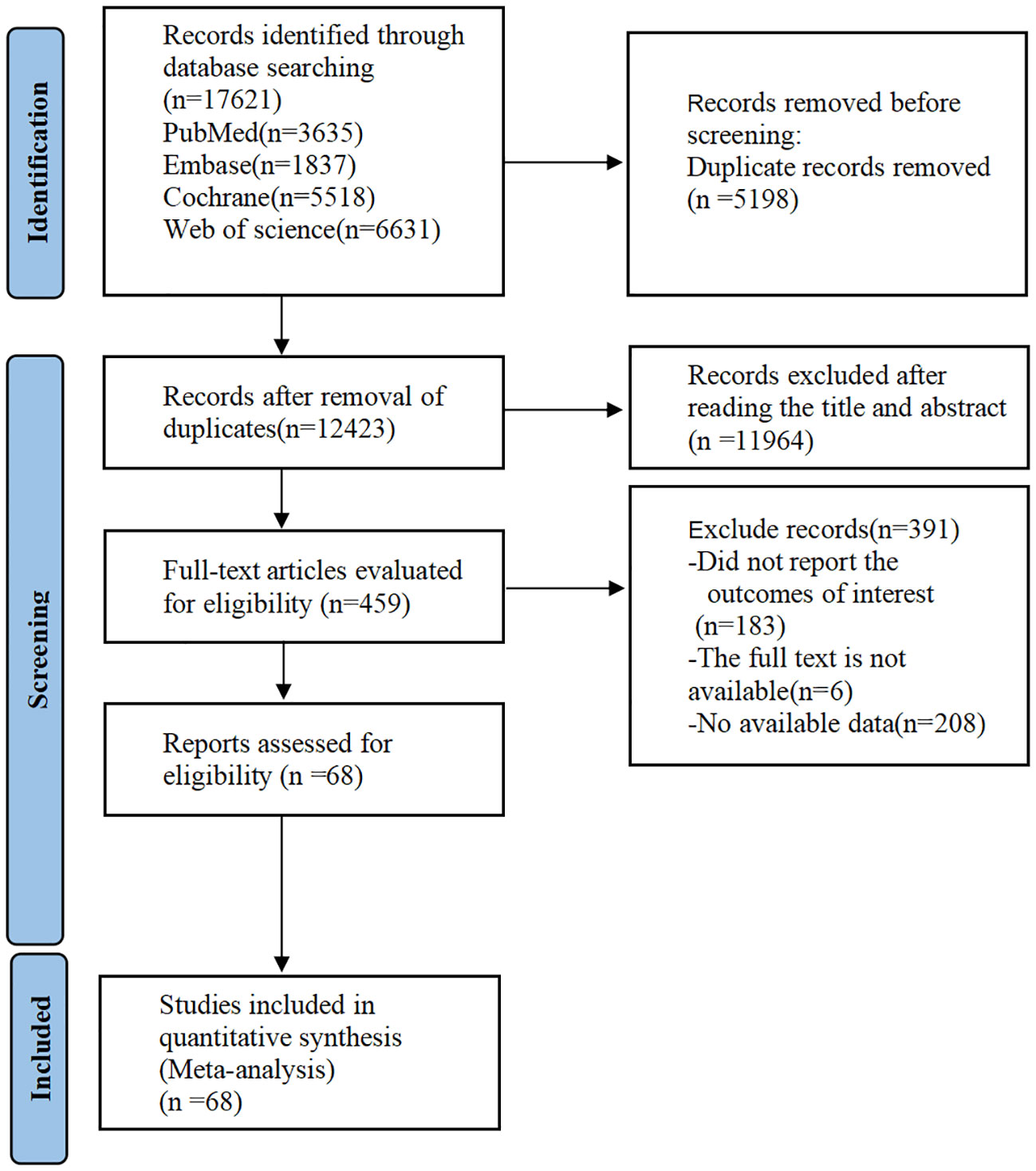
Figure 1. PRISMA flow diagram of the study process. PRISMA, Preferred Reporting Items for Meta-analysis.
3.2 Study characteristics
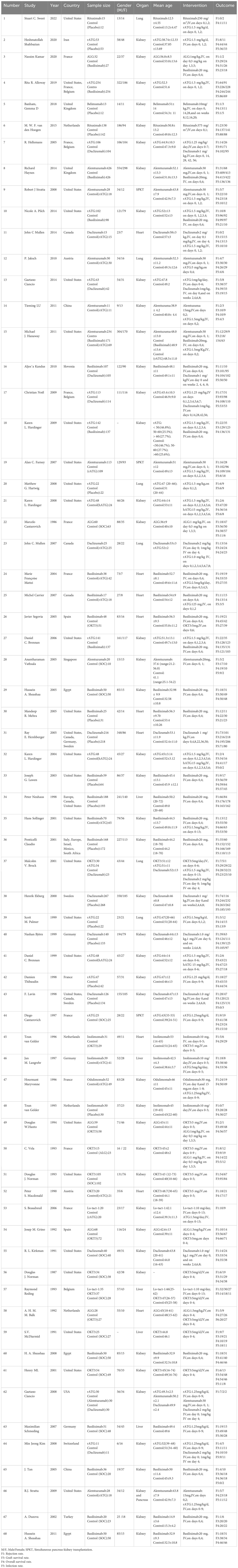
68 RCTs, comprising 9,626 patients, were included. Twelve different induction therapies were used: basiliximab, alemtuzumab, daclizumab, hATG, rATG, odulimomab, inolimomab, OKT3, Lo-tact-1, rituximab, belimumab, and ALG. The effects of each intervention were compared against the control group’s results in a pairwise fashion. The fundamental attributes of these studies are listed in Table 1.
3.3 Intervention network
Direct comparisons among therapies are visualized in Figure 2. Nodes represent interventions (size proportional to sample size), and edges denote direct comparisons (thickness indicating study count).
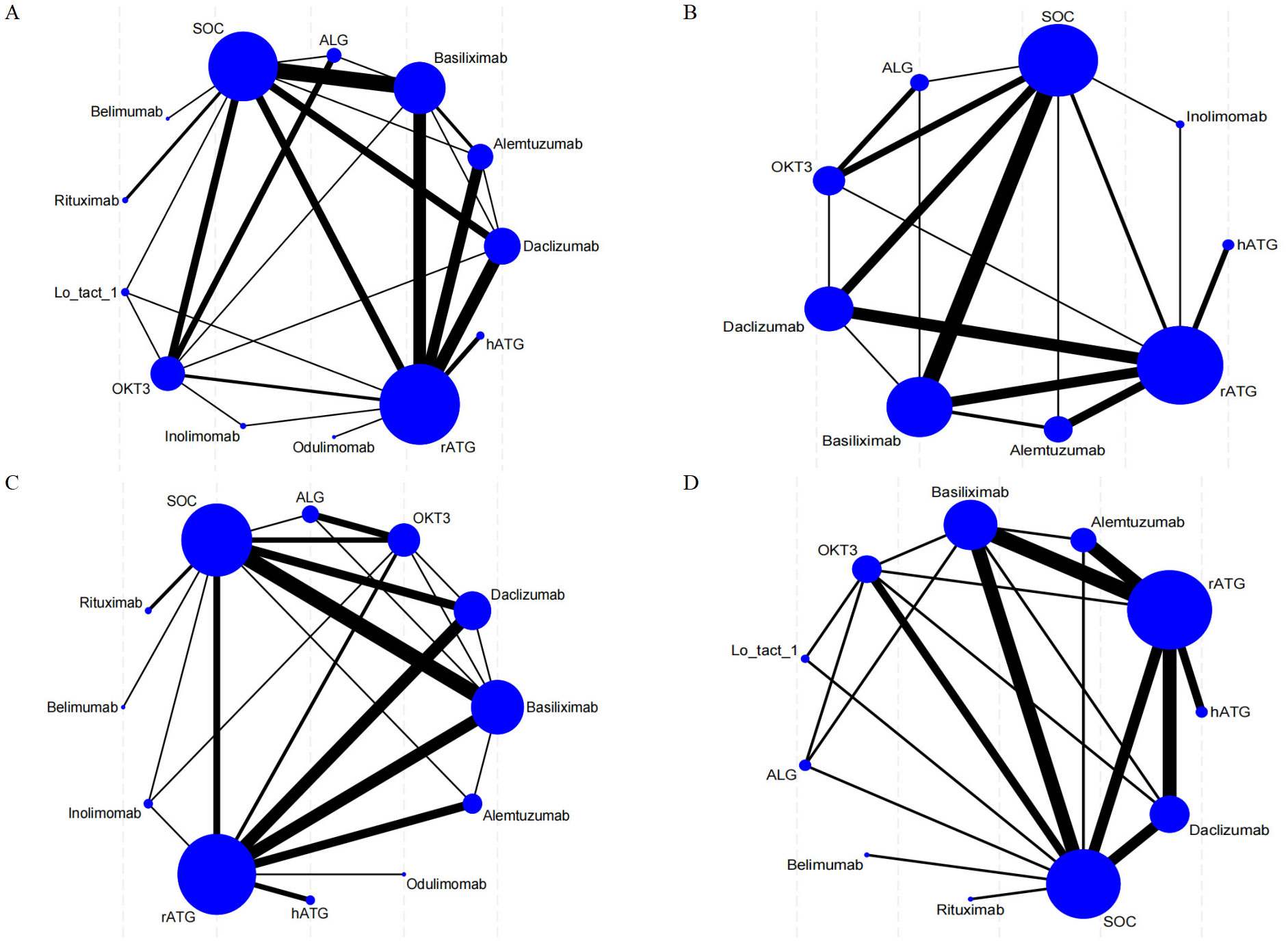
Figure 2. Network meta-analysis maps illustrate the studies assessing the efficacy and safety of induction therapy in SOT concerning (A) rejection rate, (B) graft survival rate, (C) overall survival rate, and (D) infection rate. The size of the nodes reflects the number of participants within each intervention, while the thickness of the lines connecting the interventions indicates the number of studies associated with each comparison.
3.4 ROB assessment
Among 68 studies, 60 described explicit randomization methods, while 64 reported allocation concealment. Double-blinding was implemented in 42 trials, and 29 blinded outcome assessors. Eleven studies documented participant attrition with reasons. All studies exhibited low selective reporting risk. ROB evaluations are detailed in Figure 3.
3.5 NMA outcomes
3.5.1 Rejection rate
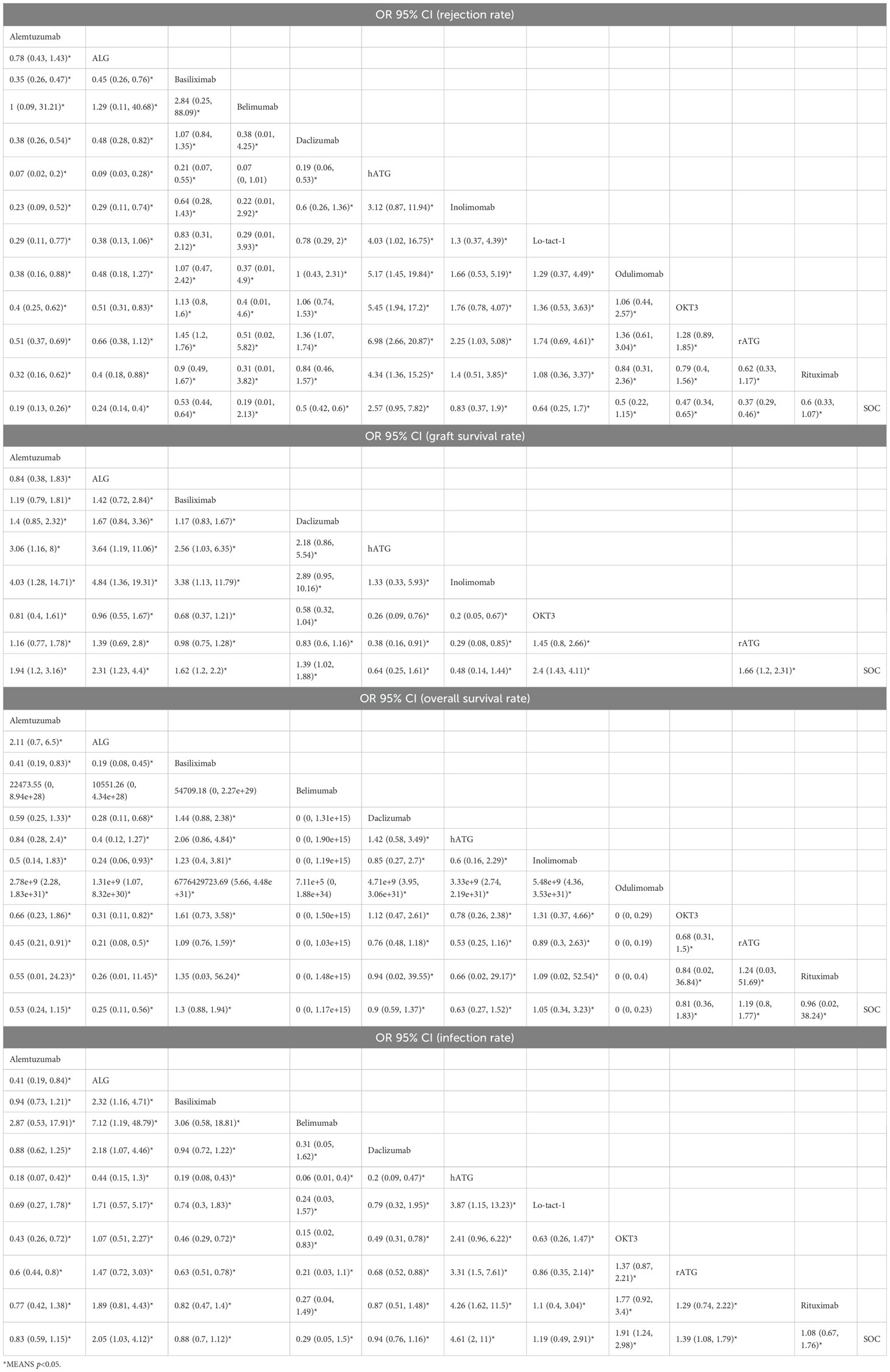
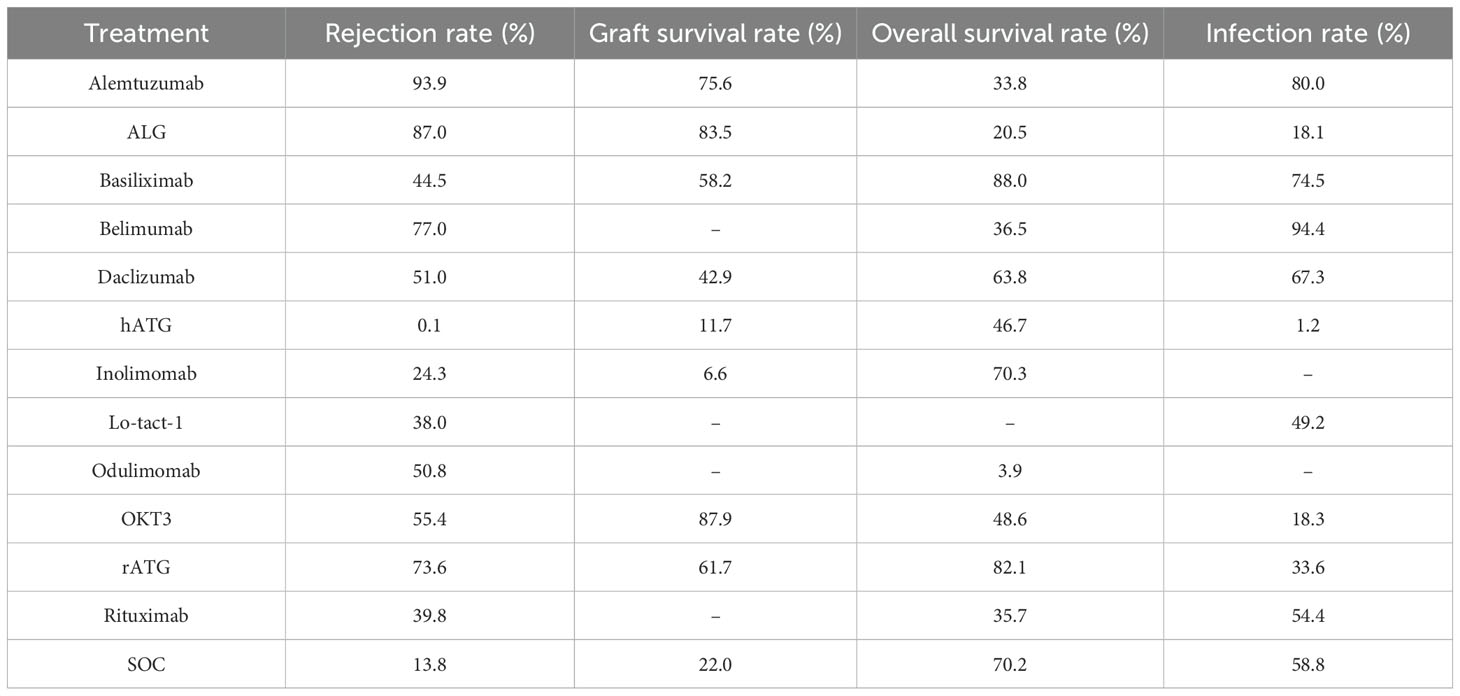
All included RCTs reported rejection rates. Network meta-analysis (NMA) revealed significant reductions in rejection rates with alemtuzumab, ALG, belimumab, rATG, OKT3, daclizumab, odulimomab, basiliximab, rituximab, Lo-tact-1, and inolimomab compared to controls, whereas hATG showed no superiority (Figure 4A, Table 2). Surface Under the Cumulative Ranking Area (SUCRA) scores ranked interventions as follows: alemtuzumab (93.9%) > ALG (87.0%) > belimumab (77.0%) > rATG (73.6%) > OKT3 (55.4%) > daclizumab (51.0%) > odulimomab (50.8%) > basiliximab (44.5%) > rituximab (39.8%) > Lo-tact-1 (38.0%) > inolimomab (24.3%) > SOC (13.8%) > hATG (0.1%) (Table 3). Alemtuzumab emerged as the most effective therapy for reducing rejection in SOT recipients undergoing induction therapy (cumulative ranking: Figure 5A).
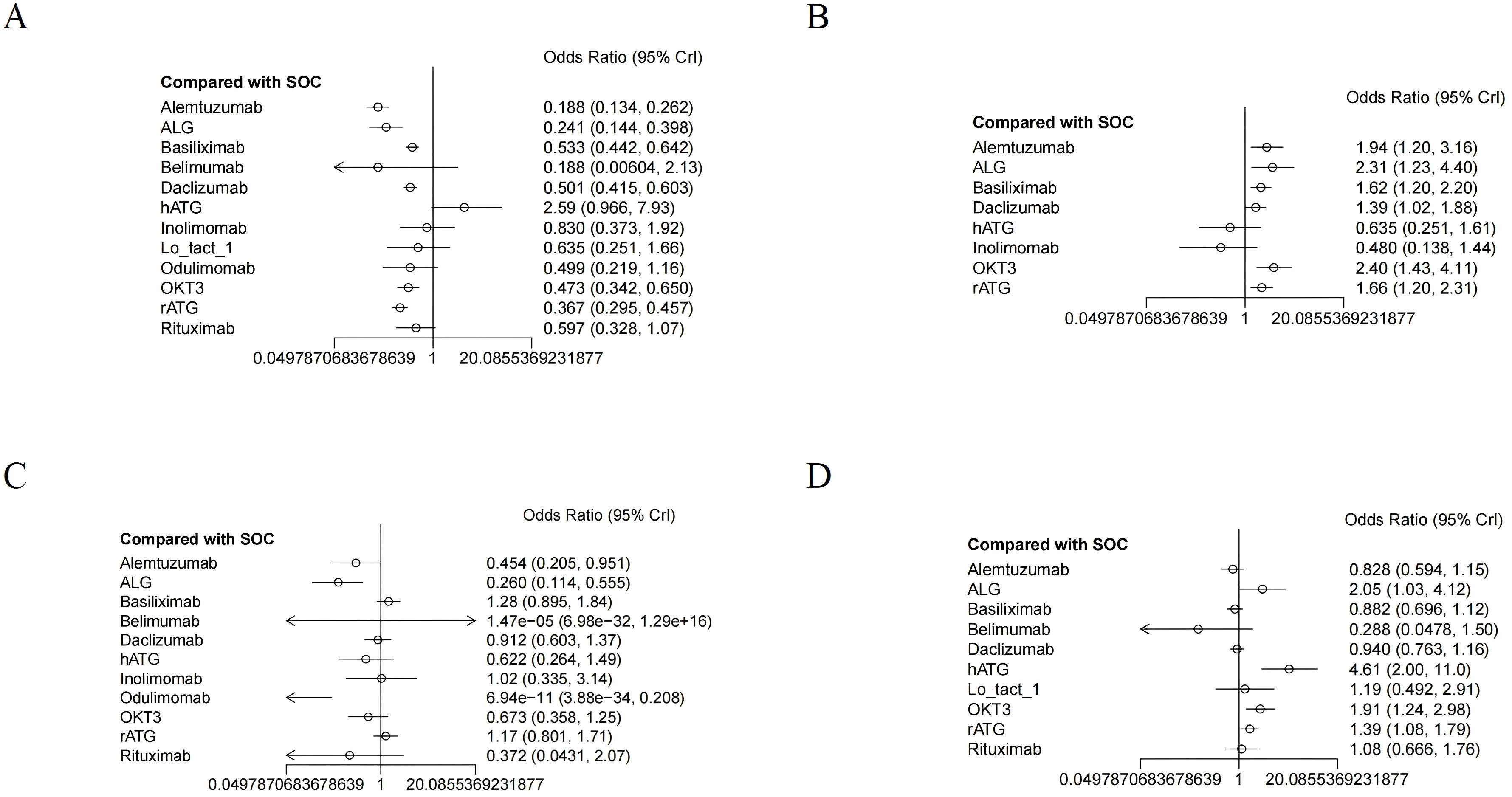
Figure 4. The forest plot illustrates the studies assessing the efficacy and safety of induction therapy in SOT concerning (A) rejection rate, (B) graft survival rate, (C) overall survival rate, and (D) infection rate.
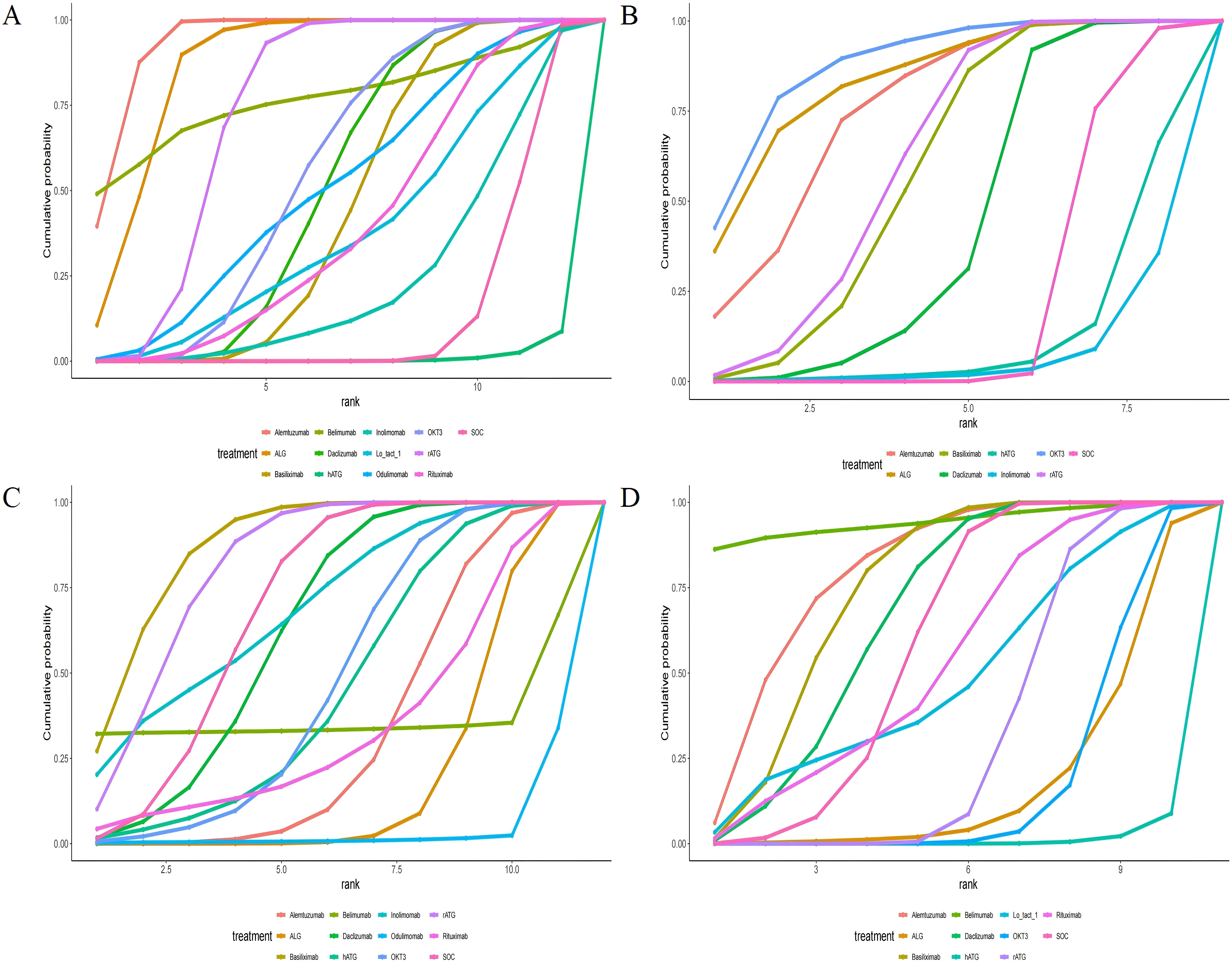
Figure 5. Area under the cumulative probability curve. (A) rejection rate, (B) graft survival rate, (C) overall survival rate, (D) infection rate.
3.5.2 Graft survival rate
Fifty RCTs evaluated graft survival. NMA demonstrated superior outcomes with OKT3, ALG, alemtuzumab, rATG, basiliximab, and daclizumab versus controls, while hATG and inolimomab lacked significant benefits (Figure 4B, Table 2). SUCRA rankings were: OKT3 (87.9%) > ALG (83.5%) > alemtuzumab (75.6%) > rATG (61.7%) > basiliximab (58.2%) > daclizumab (42.9%) > SOC (22.0%) > hATG (11.7%) > inolimomab (6.6%) (Table 3). OKT3 ranked highest for improving graft survival (cumulative ranking: Figure 5B).
3.5.3 Overall survival rate
Sixty-one RCTs assessed overall survival. Basiliximab, rATG, and inolimomab significantly increased survival rates compared to controls, whereas daclizumab, OKT3, hATG, belimumab, rituximab, alemtuzumab, ALG, and odulimomab showed no advantage (Figure 4C, Table 2). SUCRA scores prioritized basiliximab (88.0%) > rATG (82.1%) > inolimomab (70.3%) > SOC (70.2%) > daclizumab (63.8%) (Table 3). Basiliximab was identified as the most effective for enhancing overall survival (cumulative ranking: Figure 5C).
3.5.4 Infection rate
Forty-four RCTs evaluated infections. Belimumab, alemtuzumab, basiliximab, and daclizumab significantly reduced infection rates versus controls, while rituximab, Lo-tact-1, rATG, OKT3, ALG, and hATG exhibited higher incidences (Figure 4D, Table 2). SUCRA rankings were: belimumab (94.4%) > alemtuzumab (80.0%) > basiliximab (74.5%) > daclizumab (67.3%) > SOC (58.8%); conversely, rituximab (54.4%), Lo-tact-1 (49.2%), rATG (33.6%), OKT3 (18.3%), ALG (18.1%), and hATG (1.2%) underperformed (Table 3). Belimumab demonstrated the greatest efficacy in reducing infections (cumulative ranking: Figure 5D).
3.6 PB assessment
The observed asymmetry within the funnel plot presented in Figure 6, which constitutes a key component of our publication bias assessment, demonstrates the dispersion of numerous outlying data points positioned beyond the established funnel boundaries. This deviation is particularly pronounced within the lower contour region, a pattern consistent with Egger’s regression results (p<0.05). Such distributional irregularities substantiate the probable existence of small-study effects alongside systematic publication bias. Consequently, the preliminary nature of these findings necessitates their interpretation with substantial scientific caution due to inherent methodological limitations.
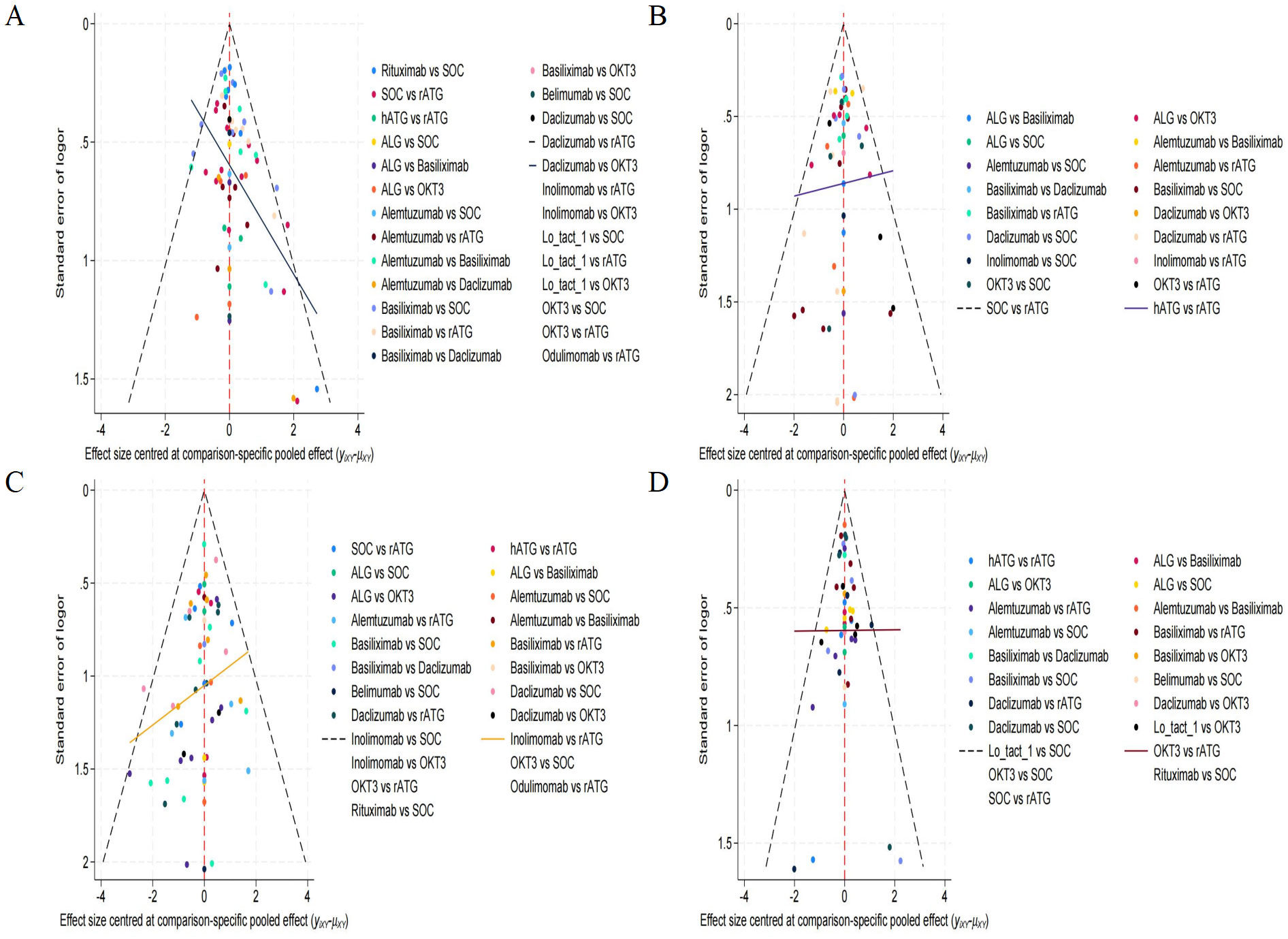
Figure 6. Funnel plot for evaluating publication bias. (A) rejection rate, (B) graft survival rate, (C) overall survival rate, (D) infection rate.
4 Discussion
4.1 Mechanisms, clinical applications, and future directions of monoclonal antibody induction therapies
Historically, polyclonal antibodies such as ATG and ALG were the mainstay of immunosuppressive therapy in pre-transplantation. Their mechanism of action involved antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity, and phagocytosis by reticuloendothelial cells in the liver, spleen, and lungs. This broad reactivity resulted in efficient elimination of T cells within the thymus and the transplanted organ. However, the xenogeneic nature of these polyclonal antibodies resulted in significant adverse effects, including cytokine release syndrome, serum sickness, anaphylaxis, and increased infection susceptibility (16). This led to their gradual displacement by monoclonal antibodies (17). At present, the advent of monoclonal antibody technology has ushered in a new era of pre-transplant induction therapy, characterized by more specific and effective agents. These monoclonal antibodies target specific lymphocyte subsets or cytokines, enabling more precise immunosuppression and reduced non-specific side effects. Commonly used examples include anti-CD3 (e.g., OKT3), anti-CD25 (e.g., basiliximab, daclizumab, Inolimomab, and Lo-tact-1), and anti-CD52 (e.g., alemtuzumab) monoclonal antibodies, which target T cells, as well as antibodies targeting adhesion molecules and chemokine receptors. Anti-CD20 (e.g., rituximab) and anti-B-lymphocyte stimulator (BLyS; e.g., belimumab) are also utilized. The mechanisms of allograft rejection and the therapeutic targets for its prevention are presented in Figure 7. Future advancements in transplant rejection prevention will focus on several key strategies: First, the development of more precisely targeted therapies utilizing monoclonal antibodies to specifically target cellular subsets or signaling pathways crucial in allograft rejection, thereby minimizing off-target effects and adverse events. Second, the implementation of combination therapies synergistically employing monoclonal antibodies with other immunosuppressants, cellular therapies, or gene therapies to enhance efficacy and overcome monotherapy limitations. Third, the use of biomarker-guided therapy, employing predictive biomarkers to personalize immunosuppressant administration, including monoclonal antibodies, based on individual patient risk profiles. Finally, the induction of immunological tolerance, which necessitates further research into tolerance mechanisms and the identification of novel therapeutic targets and strategies (18). The widespread implementation of immunoinduction therapy has significantly enhanced the short-term overall survival rates of solid organ grafts (19). However, a consensus regarding the most effective induction protocol remains elusive. Graft rejection profoundly affects both the functionality of transplanted organs and the survival of recipients (20). Therefore, enhancing graft survival is a pivotal objective in patients undergoing SOT. Despite this, numerous studies have failed to adequately differentiate between the various treatments, thereby limiting clinical applicability.
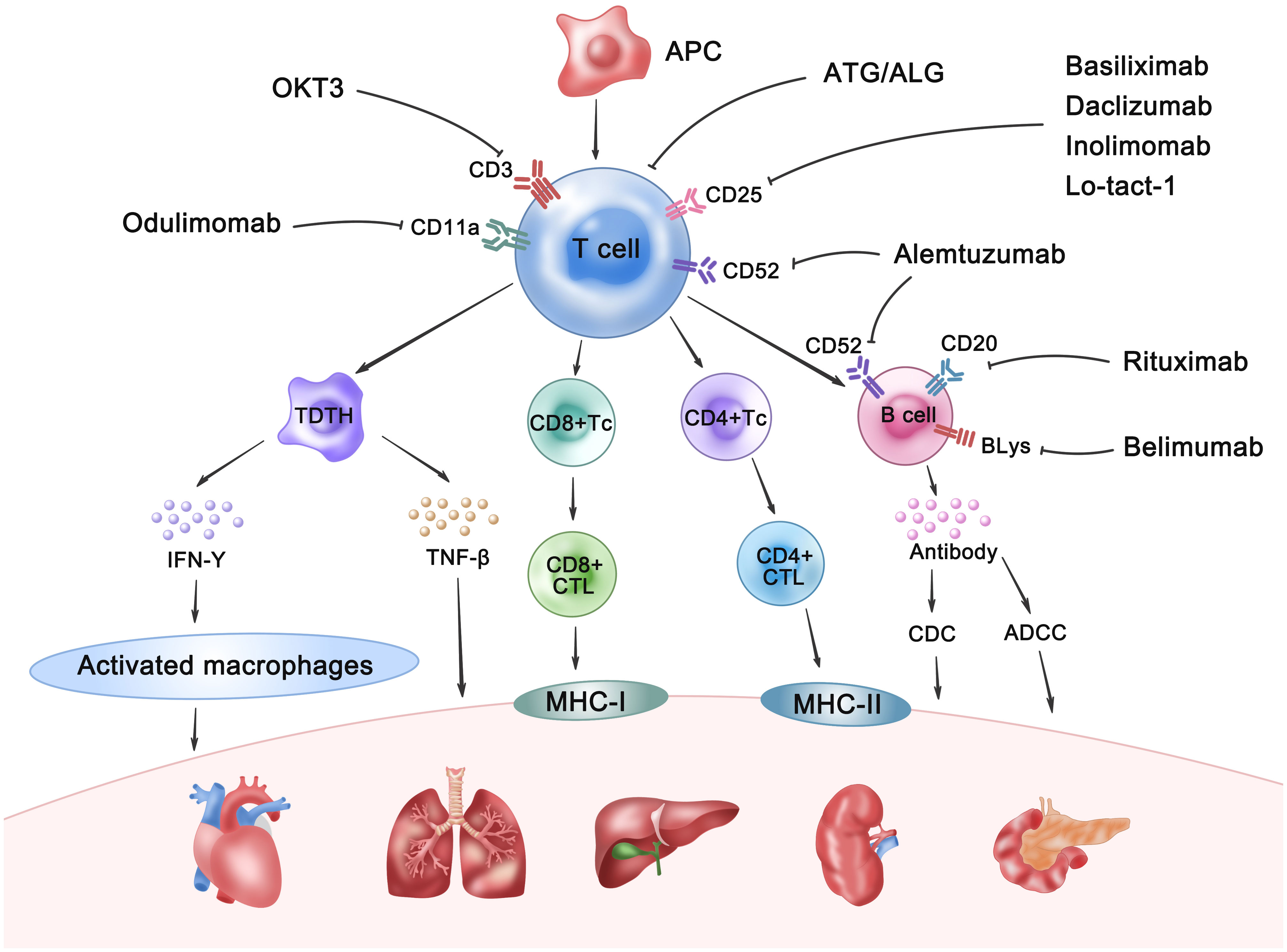
Figure 7. The mechanisms of allograft rejection and the therapeutic targets for its prevention. APC, Antigen-presenting cell; TDTH, Delayed-type hypersensitivity T cell; Tc, T cytotoxic cell; CD4+/CD8+CTL, CD4+/CD8+ cytotoxic T lymphocyte; IFN-γ, Interferon-γ; TNF-β, Tumor Necrosis Factor-β; CDC, Complement-dependent cytotoxicity; ADCC: Antibody-dependent cell-mediated cytotoxicity; BlyS, Anti-B-lymphocyte stimulator.
4.2 Efficacy-safety profiles of induction agents via NMA
This NMA comprehensively evaluates the efficacy and safety profiles of various induction therapies in SOT recipients. The findings reveal that the agents demonstrate varying performance characteristics across different critical clinical endpoints: alemtuzumab was the most effective treatment for reducing the rejection rate; basiliximab is acknowledged to be a significant treatment for improving overall survival; belimumab has been recognized for its potential to reduce infection episodes. OKT3 has been identified as the primary treatment for enhancing graft survival. These findings suggest that the selection of induction agents should be determined by weighing multiple therapeutic objectives and prioritizing them according to individualized patient factors. Although our study found that OKT3 as induction therapy significantly enhances graft survival, it has been supplanted by newer pharmacological agents due to the high incidence of adverse events, and will not be further discussed.
4.3 Mechanisms of alemtuzumab-mediated immunosuppression
Alemtuzumab targets CD52, which exerts significant immunomodulatory effects by inducing depletion and subsequent repopulation of lymphocytes, resulting in alterations in the number, proportion, and characteristics of specific lymphocyte subsets after treatment. Notably, the immunophenotypic alterations were characterized by elevated frequencies of Treg subsets and expanded populations of memory T and B lymphocytes (21). Zwan et al. (22) compared the efficacy of alemtuzumab and rATG for renal transplantation rejection. They found that alemtuzumab may serve as a viable alternative for patients with severe acute renal transplantation rejection. Furukawa et al. (23) conducted a study comparing belatacept and alemtuzumab and discovered that the alemtuzumab group experienced a significantly reduced incidence of acute rejection events. Similarly, Hanaway et al. (24) and co-authors found in a prospective RCT that compared the SOC and rATG groups, alemtuzumab markedly decreased the proportion of acute rejection among renal transplantation recipients within the first year post-surgery. Nonetheless, they noted that in high-risk groups, no significant difference was found between alemtuzumab and rATG in terms of the reduction in rejection rates. Jaksch et al. (25) conducted a prospective RCT to compare the efficacy of alemtuzumab with rATG in lung transplantation induction therapy. They found that alemtuzumab significantly reduced the occurrence of high-grade rejection events. Although alemtuzumab significantly reduces the incidence of acute rejection, this advantage fails to translate into long-term survival benefits (26). The likely reason is that its prolonged and profound immune erosion may delay immune reconstitution and lead to a significantly elevated risk of long-term opportunistic infections, which is probably a key factor underlying its failure to achieve optimal graft and patient survival outcomes (26). This underscores that rejection control is not the sole determinant of long-term success. Non-rejection-related causes of death, including infections, cardiovascular events, and malignancies, contribute disproportionately to mortality in patients treated with the potent depleting agent alemtuzumab, thereby attenuating the potential survival benefit conferred by its superior rejection control (27). This observation carries implications for clinical decision-making: alemtuzumab could be considered a preferred induction agent for highly immunogenic organ transplants (e.g., kidney, heart, lung, pancreas), particularly in recipients with a high degree of HLA mismatching and inherently low infection risk. However, clinicians must diligently manage patients’ short-term infection risks and prioritize long-term survival. In conclusion, a substantial body of research has substantiated alemtuzumab in reducing rejection, which is consistent with our findings.
4.4 Mechanisms of basiliximab-mediated immunosuppression
Interleukin-2 (IL-2) plays a critical role in the pathogenesis of acute rejection by activating donor cytotoxic T lymphocytes that specifically target receptor antigens. Consequently, blockade of IL-2 receptors has emerged as a crucial strategy in the induction therapy of organ transplantation (28). Basiliximab, a chimeric monoclonal antibody (IgG1κ) composed of murine and human components, selectively antagonizes the alpha chain of IL-2 receptor (CD25 antigen). Activated T lymphocytes demonstrate a high affinity for IL-2, enabling basiliximab to bind with remarkable specificity to the CD25 antigen, thereby significantly reducing the CD25 levels (29). This reduction influences the interactions between CD4/CD25/CD127-Foxp3/Tregs, effectively disrupting the signaling pathways that facilitate T cell proliferation (30). Importantly, the pharmacological mechanism of basiliximab is fundamentally distinct from that of polyclonal antibodies, such as ATG, because it does not induce lymphocyte depletion. This targeted mechanism, which preserves the functionality of alternative effector pathways, has a relatively minor impact on susceptibility to post-transplant infections associated with the use of anti-CD25 monoclonal antibodies as induction therapy, particularly compared with ATG (31). Furthermore, basiliximab offers substantial advantages in enhancing recipient overall survival rates, improving graft survival, and reducing rejection (32), establishing it as a preferred option in certain centers to prevent acute rejection during SOT (33). Mo et al. (34) et al. conducted a multicenter analysis involving over 900 recipients, demonstrating that basiliximab is both safe and effective for steroid-resistant acute rejection. Similarly, Kamar et al. (35) performed a RCT focusing on induction therapy. They found that both ATG and basiliximab were safe, with no significant difference in the overall recipient survival rates. In a RCT of pediatric liver transplantation, Dong et al. (36) found that basiliximab induction therapy is a safe and effective strategy that reduces acute rejection without compromising post-operative graft survival rates. Basiliximab selectively blocks T-cell activation without causing depletion. This intrinsic mechanism underlies its relatively mild anti-rejection effect and is responsible for its minimal risk of infection, which likely contributes to higher long-term patient survival rates (31). The pharmacological profile of basiliximab indicates that in low-immunogenicity organ transplants (e.g., liver transplantation, combined liver-kidney transplantation, combined heart-liver transplantation), basiliximab should be considered the induction therapy of choice for recipients with a high degree of HLA matching and/or those at potential risk of infection. Concurrently, clinicians must diligently monitor for the occurrence of early rejection episodes and promptly adjust immunosuppressive therapy if necessary.
4.5 Mechanisms of belimumab-mediated immunosuppression
Belimumab binds to and antagonizes the soluble BLyS protein, a cytokine pivotal for promoting B-cell survival and proliferation while contributing to the plasma cell niche (37). Its mechanism of action involves binding to tumor necrosis factor receptor superfamily, thereby effectively obstructing the interaction between BLyS and its receptor (38). By engaging the TNF receptor, belimumab inhibits the survival, maturation, and activation of B lymphocytes, thereby preventing their differentiation into plasma cells. Furthermore, it impedes both T lymphocyte-dependent and T lymphocyte-independent antibody responses, as well as T lymphocyte costimulation (39). A growing body of evidence suggests that regulatory B cells play a critical role in transplantation. First, the association between anti-CD20 therapy (rituximab) and T-cell mediated rejection has been documented (40). Second, renal transplantation recipients exhibiting an increased presence of migratory B cells demonstrated a decrease in rejection episodes (41). Lastly, experimental models indicate that inhibition of BLyS effectively prevents rejection and influences the survival of allografts (42). Conversely, some studies have shown that anti-BLyS therapy is ineffective in reducing DSA concentrations (43). Gemma D (44) et al. conducted a RCT comparing the efficacy and safety of belimumab to that of a placebo. The results revealed that belimumab significantly reduced the levels of immature B cells, activated memory B cells, circulating plasma cells, and DSA without increasing the risk of infection in recipients. Currently, belimumab is widely used to treat systemic lupus erythematosus (SLE). Mariette et al. (45) conducted a phase II RCT to compare the efficacy and safety of the combination of belimumab and rituximab monotherapy. Their conclusions indicated consistency in safety; however, the combination demonstrated a significantly greater effect on efficacy. Additionally, Zhang et al. (46) conducted a phase III open-label observational study, primarily assessing the safety of belimumab, with secondary endpoints focused on evaluating its efficacy. During a period of six years, belimumab was found to be both safe and effective for treating SLE. Despite the relative scarcity of research on belimumab in the context of SOT induction therapy, substantial evidence supports its safety and efficacy in SLE treatment. Future investigations should conduct prospective multicenter RCTs to validate belimumab in the context of organ transplantation induction therapy, as it may represent a promising strategy in this field.
5.6 Limitations
Although this study included 68 RCTs involving 9,626 patients, several limitations must be addressed. First, the number of RCTs examining certain intervention measures was limited and not all RCTs strictly follow a dual-phase adaptive design. Second, the sample sizes in several of these trials were relatively small, which may have introduced bias into our conclusions. Third, the pooled analysis of induction therapy for various solid organs may result in some degree of heterogeneity, necessitating subsequent validation in subgroup analyses. As emphasized in this article, the most effective approach to demonstrating the efficacy and safety of pharmacological agents is through well-designed, prospective multicenter RCTs with substantial sample sizes, which provide robust evidence necessary for clinical decision-making and regulatory approval. Therefore, these findings should be interpreted with caution.
5 Conclusion
Alemtuzumab emerged as the optimal therapy for minimizing rejection, while OKT3 and basiliximab were superior for graft and overall survival, respectively. Belimumab exhibited the strongest potential for reducing incidence of infection. These findings highlight therapy-specific advantages for optimizing SOT outcomes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
JS: Formal Analysis, Methodology, Software, Data curation, Writing – original draft, Investigation, Writing – review & editing. CH: Writing – review & editing, Methodology. QL: Writing – review & editing, Formal Analysis, Data curation, Methodology. YY: Methodology, Writing – review & editing. NW: Methodology, Supervision, Data curation, Writing – review & editing. JD: Project administration, Supervision, Methodology, Writing – review & editing. HL: Visualization, Project administration, Validation, Writing – review & editing, Resources, Supervision, Software. XS: Formal Analysis, Writing – review & editing, Resources, Funding acquisition, Project administration, Supervision, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. National Natural Science Foundation of China (82460138) and Joint Project on Regional High-Incidence Diseases Research of Guangxi Natural Science Foundation under Grant No.2024GXNSFAA010454.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1625710/full#supplementary-material
References
1. Louis K, Macedo C, Lefaucheur C, and Metes D. Adaptive immune cell responses as therapeutic targets in antibody-mediated organ rejection. Trends Mol Med. (2022) 28:237–50. doi: 10.1016/j.molmed.2022.01.002
2. Magyar CTJ, O’Kane GM, Aceituno L, Li Z, Vogel A, Bruix J, et al. Liver transplantation for hepatocellular carcinoma: an expanding cornerstone of care in the era of immunotherapy. JCO. (2024) 43:JCO2400857. doi: 10.1200/JCO.24.00857
3. Bera KD, Shah A, English MR, Harvey D, and Ploeg RJ. Optimisation of the organ donor and effects on transplanted organs: a narrative review on current practice and future directions. Anaesthesia. (2020) 75:1191–204. doi: 10.1111/anae.15037
4. Paulo Guzman J, Maklad M, Osman M, Elsherif A, and Fujiki M. Updates in induction immunosuppression regimens for intestinal transplantation. Hum Immunol. (2024) 85:110800. doi: 10.1016/j.humimm.2024.110800
5. Corr M, Walker A, Maxwell AP, and McKay GJ. Non-adherence to immunosuppressive medications in kidney transplant recipients- a systematic scoping review. Transplant Rev. (2025) 39:100900. doi: 10.1016/j.trre.2024.100900
6. Sun J, Yu Y, Huang F, Zhang Q, Zhu L, He G, et al. Network meta-analysis of pharmacological treatment for antibody-mediated rejection after organ transplantation. Front Immunol. (2024) 15:1451907. doi: 10.3389/fimmu.2024.1451907
7. Meier-Kriesche H-U, Li S, Gruessner RWG, Fung JJ, Bustami RT, Barr ML, et al. Immunosuppression: evolution in practice and trends, 1994–2004. Am J Transplant. (2006) 6:1111–31. doi: 10.1111/j.1600-6143.2006.01270.x
8. Karpe KM, Talaulikar GS, and Walters GD. Calcineurin inhibitor withdrawal or tapering for kidney transplant recipients. Cochrane Database Syst Rev. (2017) 2017):CD006750. doi: 10.1002/14651858.CD006750.pub2
9. Abu-Elmagd KM, Costa G, Bond GJ, Soltys K, Sindhi R, Wu T, et al. Five hundred intestinal and multivisceral transplantations at a single center. Ann Surg. (2009) 250:567–81. doi: 10.1097/SLA.0b013e3181b67725
10. Taner T, Stegall MD, and Heimbach JK. Antibody-mediated rejection in liver transplantation: Current controversies and future directions. Liver Transpl. (2014) 20:514–27. doi: 10.1002/lt.23826
11. Juneja T, Kazmi M, Mellace M, and Saidi RF. Utilization of treg cells in solid organ transplantation. Front Immunol. (2022) 13:746889. doi: 10.3389/fimmu.2022.746889
12. Nedredal GI, Picon RV, Chedid MF, and Foss A. Immunosuppression in liver transplantation: state of the art and future perspectives. Curr Pharm Des. (2020) 26:3389–401. doi: 10.2174/1381612826666200610183608
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
14. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017) 358:j4008. doi: 10.1136/bmj.j4008
15. Nussbaumer-Streit B, Klerings I, Dobrescu AI, Persad E, Stevens A, Garritty C, et al. Excluding non-English publications from evidence-syntheses did not change conclusions: a meta-epidemiological study. J Clin Epidemiol. (2020) 118:42–54. doi: 10.1016/j.jclinepi.2019.10.011
16. Crepin T, Carron C, Roubiou C, Gaugler B, Gaiffe E, Simula-Faivre D, et al. ATG-induced accelerated immune senescence: clinical implications in renal transplant recipients. Am J Transplant. (2015) 15:1028–38. doi: 10.1111/ajt.13092
17. Cordell J. Developing monoclonal antibodies for immunohistochemistry. Cells. (2022) 11:243. doi: 10.3390/cells11020243
18. Carney EF. Induction of chimerism and immune tolerance using belatacept. Nat Rev Nephrol. (2015) 11:66–6. doi: 10.1038/nrneph.2014.230
19. Pilat N, Steiner R, and Sprent J. Treg therapy for the induction of immune tolerance in transplantation—not lost in translation? IJMS. (2023) 24:1752. doi: 10.3390/ijms24021752
20. Berger M, Baliker M, Van Gelder T, Böhmig GA, Mannon RB, Kumar D, et al. Chronic active antibody-mediated rejection: opportunity to determine the role of interleukin-6 blockade. Transplantation. (2024) 108:1109–14. doi: 10.1097/TP.0000000000004822
21. van der Zwan M, Baan CC, van Gelder T, and Hesselink DA. Review of the clinical pharmacokinetics and pharmacodynamics of alemtuzumab and its use in kidney transplantation. Clin Pharmacokinet. (2018) 57:191–207. doi: 10.1007/s40262-017-0573-x
22. van der Zwan M, Clahsen-Van Groningen MC, van den Hoogen MWF, Kho MML, Roodnat JI, Mauff KAL, et al. Comparison of alemtuzumab and anti-thymocyte globulin treatment for acute kidney allograft rejection. Front Immunol. (2020) 11:1332. doi: 10.3389/fimmu.2020.01332
23. Furukawa M, Chan EG, Ryan JP, Hyzny EJ, Sacha LM, Coster JN, et al. Induction strategies in lung transplantation: alemtuzumab vs. Basiliximab a single-center experience. Front Immunol. (2022) 13:864545. doi: 10.3389/fimmu.2022.864545
24. Hanaway MJ, Woodle ES, Mulgaonkar S, Peddi VR, Kaufman DB, First MR, et al. Alemtuzumab induction in renal transplantation. N Engl J Med. (2011) 364:1909–19. doi: 10.1056/NEJMoa1009546
25. Jaksch P, Ankersmit J, Scheed A, Kocher A, Murakoezy G, Klepetko W, et al. Alemtuzumab in lung transplantation: an open-label, randomized, prospective single center study. Am J Transplant. (2014) 14:1839–45. doi: 10.1111/ajt.12824
26. Koyawala N, Silber JH, Rosenbaum PR, Wang W, Hill AS, Reiter JG, et al. Comparing outcomes between antibody induction therapies in kidney transplantation. JASN. (2017) 28:2188–200. doi: 10.1681/ASN.2016070768
27. Santos AH, Belal A, Mehta R, Ibrahim H, Leghrouz MA, and Alquadan K. Identification of non-virologic risk factors for lymphoma after the first year of kidney transplant in adults: A retrospective analysis. Cancer Epidemiol. (2025) 97:102832. doi: 10.1016/j.canep.2025.102832
28. Malard F, Huang X-J, and Sim JPY. Treatment and unmet needs in steroid-refractory acute graft-versus-host disease. Leukemia. (2020) 34:1229–40. doi: 10.1038/s41375-020-0804-2
29. Kim HD, Bae H, Yun S, Lee H, Eum SH, Yang CW, et al. Impact of induction immunosuppressants on T lymphocyte subsets after kidney transplantation: A prospective observational study with focus on anti-thymocyte globulin and basiliximab induction therapies. Int J Mol Sci. (2023) 24:1–14. doi: 10.3390/ijms241814288
30. Aly MG, Ibrahim EH, Karakizlis H, Weimer R, Opelz G, Morath C, et al. CD4+CD25+CD127-Foxp3+ and CD8+CD28- tregs in renal transplant recipients: phenotypic patterns, association with immunosuppressive drugs, and interaction with effector CD8+ T cells and CD19+IL-10+ Bregs. Front Immunol. (2021) 12:716559. doi: 10.3389/fimmu.2021.716559
31. Fernández-Ruiz M and Aguado JM. Direct T-cell inhibition and agents targeting T-cell migration and chemotaxis. Infect Dis Clin North Am. (2020) 34:191–210. doi: 10.1016/j.idc.2020.02.002
32. Kubal CA, Mangus RS, Vianna RM, Lobashevsky A, Mujtaba MA, Higgins N, et al. Impact of positive flow cytometry crossmatch on outcomes of intestinal/multivisceral transplantation. Transplantation. (2013) 95:1160–6. doi: 10.1097/TP.0b013e3182888df0
33. Elsabbagh AM, Hawksworth J, Khan KM, Kaufman SS, Yazigi NA, Kroemer A, et al. Long-term survival in visceral transplant recipients in the new era: A single-center experience. Am J Transplant. (2019) 19:2077–91. doi: 10.1111/ajt.15269
34. Mo X-D, Hong S-D, Zhao Y-L, Jiang E-L, Chen J, Xu Y, et al. Basiliximab for steroid-refractory acute graft-versus-host disease: A real-world analysis. Am J Hematol. (2022) 97:458–69. doi: 10.1002/ajh.26475
35. Kamar N, Lepage B, Couzi L, Albano L, Durrbach A, Pernin V, et al. A randomized prospective study comparing anti-T-lymphocyte Igs to basiliximab in highly sensitized kidney transplant patients. Kidney Int Rep. (2020) 5:1207–17. doi: 10.1016/j.ekir.2020.05.020
36. Dong C, Song Z, Sun C, Wang K, Zhang W, Chen J, et al. Basiliximab induction and postoperative steroid-free immunosuppression with tacrolimus in pediatric liver transplantation: A randomized clinical trial. Transplantation. (2024) 108:1769–75. doi: 10.1097/TP.0000000000004951
37. Avery DT, Kalled SL, Ellyard JI, Ambrose C, Bixler SA, Thien M, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. (2003) 112:286–97. doi: 10.1172/JCI18025
38. van Vugt LK, Schagen MR, de Weerd A, Reinders ME, de Winter BC, and Hesselink DA. Investigational drugs for the treatment of kidney transplant rejection. Expert Opin Investigational Drugs. (2022) 31:1087–100. doi: 10.1080/13543784.2022.2130751
39. Schlößer HA, Thelen M, Dieplinger G, von Bergwelt-Baildon A, Garcia-Marquez M, Reuter S, et al. Prospective analyses of circulating B cell subsets in ABO-compatible and ABO-incompatible kidney transplant recipients. Am J Transplant. (2017) 17:542–50. doi: 10.1111/ajt.14013
40. van den Hoogen MWF, Kamburova EG, Baas MC, Steenbergen EJ, Florquin S, Koenen HJPM, et al. Rituximab as induction therapy after renal transplantation: A randomized, double-blind, placebo-controlled study of efficacy and safety. Am J Transplant. (2015) 15:407–16. doi: 10.1111/ajt.13052
41. Shabir S, Girdlestone J, Briggs D, Kaul B, Smith H, Daga S, et al. Transitional B lymphocytes are associated with protection from kidney allograft rejection: A prospective study. Am J Transplant. (2015) 15:1384–91. doi: 10.1111/ajt.13122
42. Kwun J, Page E, Hong JJ, Gibby A, Yoon J, Farris AB, et al. Neutralizing BAFF/APRIL with atacicept prevents early DSA formation and AMR development in T cell depletion induced nonhuman primate AMR model. Am J Transplant. (2015) 15:815–22. doi: 10.1111/ajt.13045
43. Agarwal D, Luning Prak ET, Bharani T, Everly M, Migone T-S, Cancro M, et al. BLyS neutralization results in selective anti-HLA alloantibody depletion without successful desensitization. Transplant Immunol. (2021) 69:101465. doi: 10.1016/j.trim.2021.101465
44. Banham GD, Flint SM, Torpey N, Lyons PA, Shanahan DN, Gibson A, et al. Belimumab in kidney transplantation: an experimental medicine, randomised, placebo-controlled phase 2 trial. Lancet. (2018) 391:2619–30. doi: 10.1016/S0140-6736(18)30984-X
45. Mariette X, Barone F, Baldini C, Bootsma H, Clark KL, De Vita S, et al. A randomized, phase II study of sequential belimumab and rituximab in primary Sjögren’s syndrome. JCI Insight. (2022) 7:1–19. doi: 10.1172/jci.insight.163030
Keywords: organ transplantation, induction therapy, rejection, network meta-analysis, randomized controlled trials
Citation: Sun J, Hu C, Liang Q, Yu Y, Wen N, Dong J, Li H and Sun X (2025) Comparative efficacy and safety of induction therapy in solid organ transplantation: a systematic review and network meta-analysis. Front. Immunol. 16:1625710. doi: 10.3389/fimmu.2025.1625710
Received: 09 May 2025; Accepted: 23 June 2025;
Published: 14 July 2025.
Edited by:
Zhenhua Dai, Guangdong Provincial Academy of Chinese Medical Sciences, ChinaReviewed by:
Zhuyuan Si, Tianjin Medical University, ChinaLea Liaigre, Centre Hospitalier Universitaire de Grenoble, France
Copyright © 2025 Sun, Hu, Liang, Yu, Wen, Dong, Li and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuyong Sun, c3VueHV5b25nQGd4bXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Junjie Sun†
Junjie Sun† Xuyong Sun
Xuyong Sun