- 1Department of Surgery, Chung Shan Medical University Hospital, Taichung, Taiwan
- 2School of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 3Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 4Division of Allergy, Immunology and Rheumatology, Department of Internal Medicine, Changhua Christian Hospital, Changhua, Taiwan
- 5Division of Pediatric Gastroenterology, Children’s Medical Center, Taichung Veterans General Hospital, Taichung, Taiwan
- 6Department of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan
1 Autoimmune disease pathophysiology and immune dysregulation
Hileman et al. reported that ADs arise from a complex interplay of genetic susceptibility and environmental triggers, with viral infections playing a central role (1). Viruses activate innate immunity by inducing type I interferon (IFN-α/β) production and stimulating neutrophil extracellular trap (NET) release, thereby enhancing dendritic cell maturation and antigen presentation. In genetically predisposed individuals, these events promote adaptive immune activation, leading to B- and T-cell expansion, autoantibody generation, and T-cell dysregulation.
Robinson et al. further proposed that Epstein-Barr virus (EBV) contributes to autoimmunity through multiple mechanisms: molecular mimicry of human autoantigens, reprogramming of B-cell function, and binding of EBV nuclear antigen 2 (EBNA2) to host super-enhancer regions associated with autoimmune-susceptibility genes (2). Together, these actions disrupt normal gene regulation and perpetuate chronic immune activation.
Taken together, these studies underscore the pivotal role of viral infections in both initiating and exacerbating ADs and highlight potential antiviral or immunomodulatory targets for therapeutic intervention.
2 Vitamin D deficiency in autoimmune diseases and its immunomodulatory potential
According to the recent estimation, nearly 15 million Americans live with at least one ADs. Increasing evidence suggests that vitamin D insufficiency is highly prevalent in these patients and correlates with immune dysregulation, higher disease activity, and more frequent flares (3). Vitamin D supplementation was important to prevent and treat deficiency-related conditions like rickets. However, in vitamin D-replete adults, large-scale randomized trials and Mendelian randomization studies consistently showed no significant benefit for preventing cancer, cardiovascular disease, diabetes, or fractures. High-dose supplementation may even pose risks. Thus, routine use in the general population is not supported, except to correct deficiency or in specific at-risk groups (4).
Epidemiological studies have linked low serum 25-hydroxyvitamin D [25(OH)D] levels (<20 ng/mL) to elevated risk for ADs such as psoriasis, T1D, and multiple sclerosis (MS) (5). Vitamin D contributes to immune homeostasis by promoting innate defenses, enhancing macrophage and dendritic cell function, while modulating adaptive responses through suppression of Th1- and Th17-mediated inflammation and upregulation of regulatory T cells (6).
In MS specifically, vitamin D influences lymphocyte activation, T-helper cell polarization, and cytokine production. It decreases pro-inflammatory cytokines (e.g., IFN-γ, IL-17) and increases anti-inflammatory mediators (e.g., IL-10), thereby shifting the immune milieu toward tolerance (7). Randomized trials of supplementation (e.g., 4,000 IU/day cholecalciferol) have demonstrated significant reductions in relapse rates and Magnetic Resonance Imaging (MRI) lesion burden in relapsing–remitting MS, particularly in patients with baseline 25(OH)D <30 ng/mL (8).
Taken together, these findings support a therapeutic role for vitamin D in ADs, especially MS, by rebalancing innate and adaptive immunity and modulating key cytokines such as IL-10 and IL-17. Future large-scale trials are warranted to define optimal dosing, target serum levels, and long-term safety profiles.
3 Relevance to vitamin D receptor signaling and immune regulation (T-cell modulation and cytokine suppression)
The hormonally active metabolite of vitamin D, 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], exerts its immunomodulatory functions primarily by binding the vitamin D receptor (VDR), a nuclear transcription factor expressed across diverse immune cell types (5). Upon ligand engagement, VDR heterodimerizes with retinoid X receptor and associates with vitamin D response elements in target gene promoters, thereby regulating transcription. This VDR-mediated gene regulation underlies vitamin D’s capacity to shape both innate and adaptive immune responses.
In the innate arm, 1,25(OH)2D3–VDR signaling promotes monocyte-to-macrophage differentiation, enhances expression of antimicrobial peptides such as cathelicidin, and upregulates HLA-DR and co-stimulatory molecules on dendritic cells, facilitating more effective antigen presentation (5). Concomitantly, VDR activation modulates cytokine and chemokine profiles within the innate compartment, fostering an environment that supports pathogen clearance while limiting excessive inflammation.
Within adaptive immunity, VDR signaling exerts a potent suppressive effect on Th1- and Th17-mediated inflammation by directly downregulating transcription of IFN-γ and IL-17, respectively. At the same time, it promotes the expansion and functional stability of regulatory T cells (Tregs), enhancing IL-10 production and strengthening peripheral tolerance. (5, 9) B-cell activity is similarly restrained: VDR activation inhibits plasma cell differentiation and autoantibody secretion, thereby reducing humoral autoimmunity (10). Figure 1 revealed the relationship between vitamin D and immune modulation.
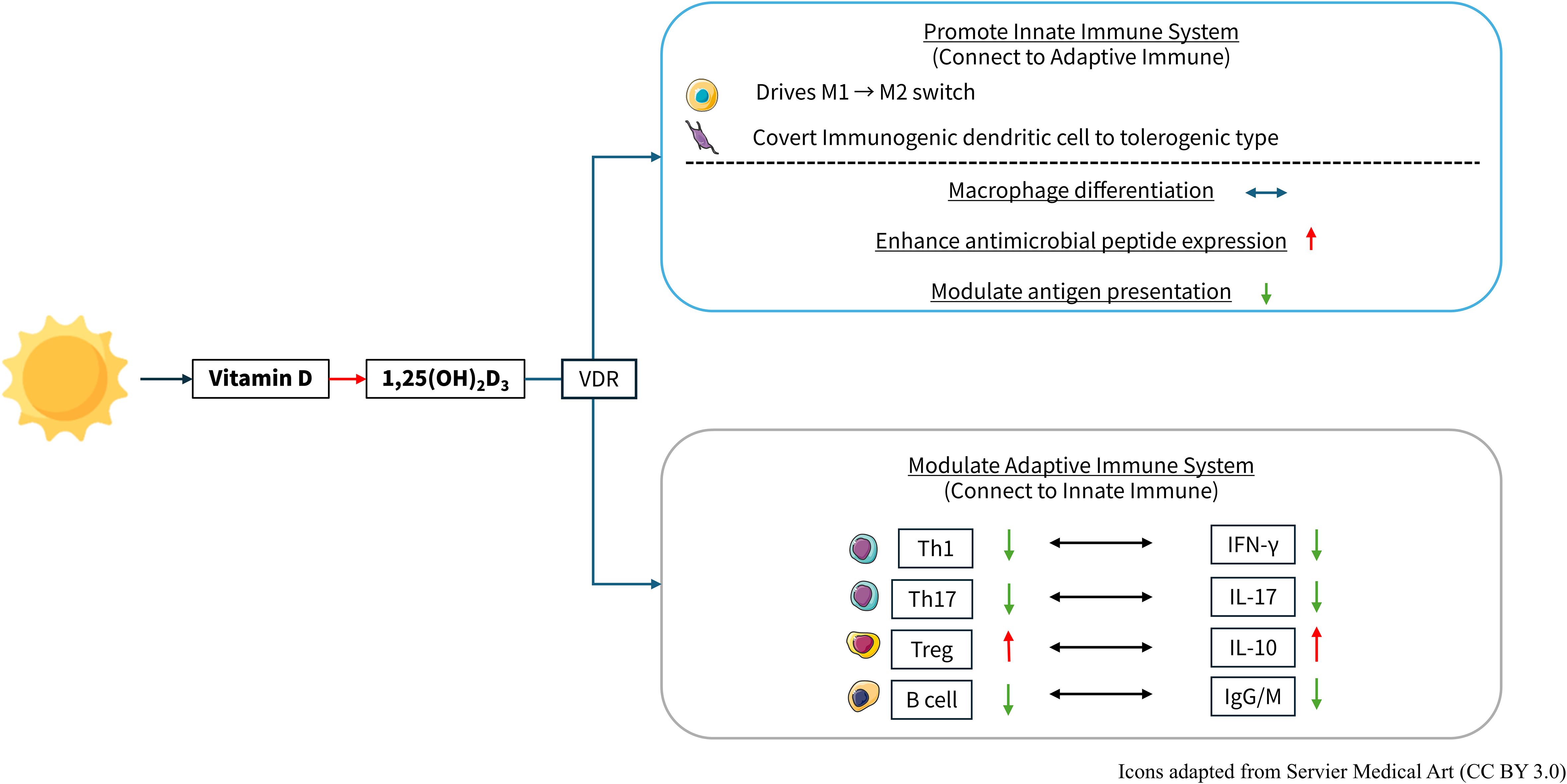
Figure 1. The active form of vitamin D, 1,25-dihydroxyvitamin D3, exerts immunomodulatory effects on both the innate and adaptive immune systems. Vitamin D and Immune Regulation. 1,25-(OH)2D3: vitamin D, 1,25-dihydroxyvitamin D3; VDR, Vitamin D receptor; Th, T helper cell; IFN-γ, interferon-γ; IL, interleukin; Treg, regulatory T cells; IgG, immunoglobulin G; IgM, immunoglobulin M. https://smart.servier.com/.
Beyond direct effects on immune cells, Sîrbe et al. (5) have reported that VDR signaling contributes to the maintenance of gut barrier integrity and the modulation of microbiota composition, mechanisms increasingly implicated in the pathogenesis of ADs. Clinical investigations echo these molecular insights. Zhao et al. (11) and Manousaki et al. (12) observed that vitamin D supplementation was associated with lower incidence rates and reduced disease severity in SLE and T1D cohorts. Taken together, these findings highlight VDR-dependent pathways as promising targets for therapeutic strategies aiming to recalibrate immune homeostasis in autoimmune disorders.
4 The efficacy and safety of high-dose vitamin D supplementation in modulating immune profiles in autoimmune disease
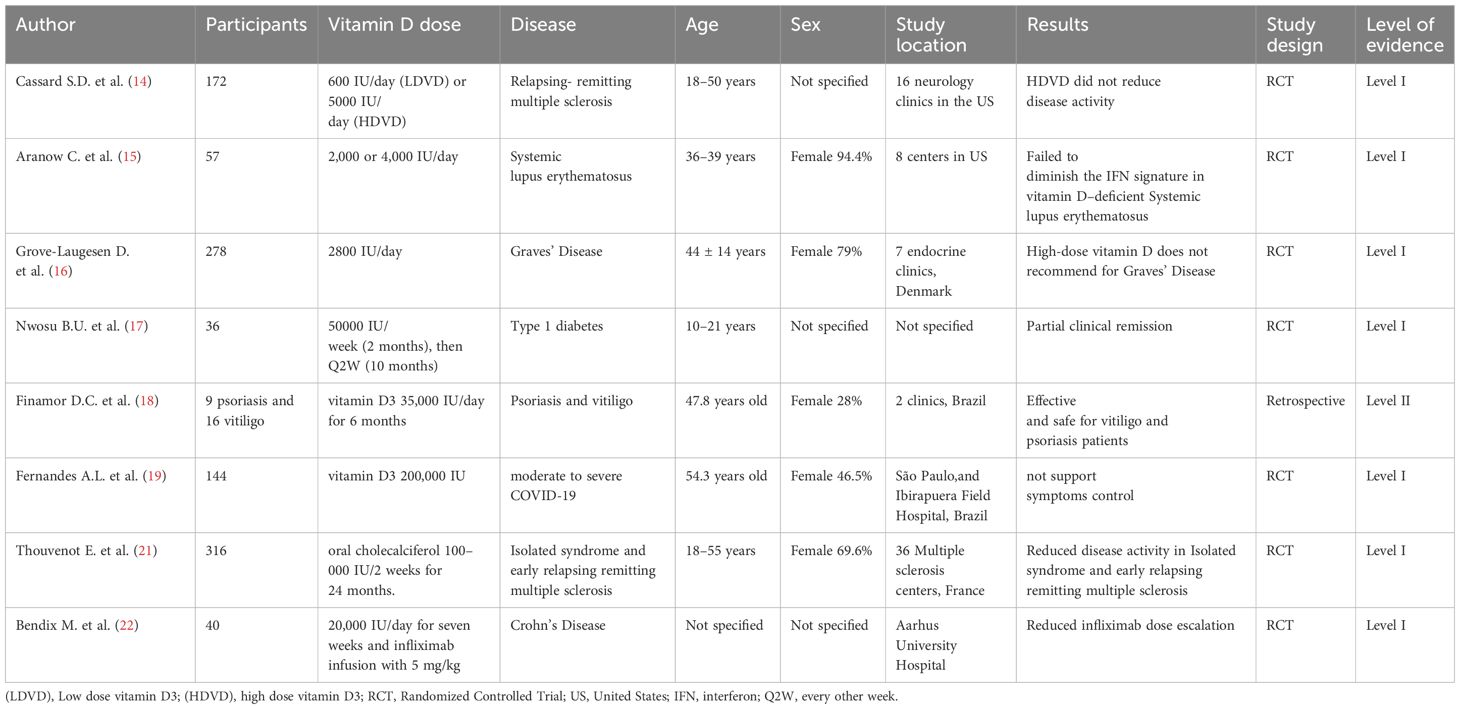
High-dose cholecalciferol is increasingly investigated to overcome VDR sensitivity loss in genetically predisposed individuals, where pathogen-mediated VDR downregulation, chronic glucocorticoid exposure, environmental toxins, low UVB exposure, and aging all contribute to impaired vitamin D signaling and elevated autoimmune risk (13). However, the efficacy of high-dose vitamin D remains debated (14–19). Table 1 summarizes clinical trials of high-dose vitamin D in autoimmune diseases.
Several studies have revealed high-dose vitamin D3 supplementation in autoimmune diseases but reported no significant clinical benefits. However, many of these studies have important methodological limitations that may affect the interpretation of their findings. For instance, the study by Cassard SD et al. (14) involved a relatively small sample size, lacked a placebo control, and was conducted only in the United States, limiting its generalizability. Similarly, the trial by Aranow C et al. (15) showed patients with SLE, was also restricted by a small participant pool, a short follow-up period of only 12 weeks, and a predominantly female population with varied baseline disease activity, introducing potential heterogeneity in treatment response. The study by Grove-Laugesen D et al. (16) was also limited to a single country (Denmark), and included mostly female participants, raising concerns about gender representation and external validity. In the trial by Nwosu BU. et al. (17), the limitations included a small sample size, a narrow age range, a single-country of Denmark. Lastly, the study conducted by Fernandes AL et al. (19) assessed the effects of a single high-dose intervention (200,000 IU) and faced challenges such as a limited sample size for 1 year analysis and reliance on self-reported symptoms, which may compromise the reliability of the outcome assessment.
Brustad et al. (20) conducted a systematic review and meta-analysis of 32 randomized trials (n = 8,400 children, doses 1,200–10,000 IU/day; bolus up to 600,000 IU) and found no increase in serious adverse events, including hypercalcemia or nephrolithiasis. In France, Thouvenot et al. (21) treated 316 early MS patients with 100,000 IU cholecalciferol biweekly for 24 months, observing reduced relapse rates and MRI lesion accumulation in clinically isolated syndrome and relapsing–remitting MS. Bendix et al. (22) administered 20,000 IU/day for seven weeks to 40 Crohn’s disease patients, reporting a 25% decrease in the need for infliximab dose escalation.
These data suggest that high-dose vitamin D can safely modulate immune profiles, decrease disease activity, and potentiate existing therapies in autoimmune disorders. Nonetheless, optimal dosing regimens, especially for individuals with profound deficiency or specific disease phenotypes, require further large randomized trials to balance maximal immunomodulation against potential toxicity.
5 Future research and study design
Future research should focus on well designed, randomized controlled trials that enroll patients based on their baseline 25(OH)D levels, VDR related genetic variants, and specific autoimmune phenotypes. Such trials ought to include dose finding phases to identify effective yet safe vitamin D regimens, serial immunological assessments (e.g., Th1/Th17 cytokines, Treg frequencies, autoantibody titers), and disease specific clinical endpoints (relapse rates, imaging markers, or activity indices). Close monitoring for hypercalcemia and renal effects will ensure safety, while stratified analyses will reveal which patient subgroups derive the greatest benefit from high dose supplementation.
6 Conclusion
Vitamin D is essential for immune balance, and its deficiency contributes to autoimmunity. High-dose vitamin D can rebalance Th1/Th17 versus Treg activity, lessen disease flares, and boost standard therapies without raising serious safety concerns. Tailoring supplementation to patients’ baseline levels and genetics offers a promising adjunct in managing autoimmune diseases.
Author contributions
SS: Writing – original draft, Writing – review & editing. PS: Writing – original draft, Writing – review & editing. MW: Writing – review & editing, Supervision. Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hileman CO, Malakooti SK, Patil N, Singer NG, and McComsey GA. New-onset autoimmune disease after COVID-19. Front Immunol. (2024) 15:1337406. doi: 10.3389/fimmu.2024.1337406
2. Robinson WH, Younis S, Love ZZ, et al. Epstein–Barr virus as a potentiator of autoimmune diseases. Nat Rev Rheumatol. (2024) 20:729–40. doi: 10.1038/s41584-024-01167-9
3. Abend AH, He I, Bahroos N, et al. Estimation of prevalence of autoimmune diseases in the United States using electronic health record data. J Clin Invest. (2025) 135:e178722. doi: 10.1172/JCI178722
4. Bouillon R, Manousaki D, Rosen C, et al. The health effects of vitamin D supplementation: evidence from human studies. Nat Rev Endocrinol. (2022) . 18:96–110. doi: 10.1038/s41574-021-00593-z
5. Sîrbe C, Rednic S, Grama A, et al. An update on the effects of vitamin D on the immune system and autoimmune diseases. Int J Mol Sci. (2022) 23:9784. doi: 10.3390/ijms23179784
6. Daryabor G, Gholijani N, and Rezaei Kahmini F. A review of the critical role of vitamin D axis on the immune system. Exp Mol Pathol. (2023) 132–133:104866. doi: 10.1016/j.yexmp.2023.104866
7. Artusa P and White JH. Vitamin D and its analogs in immune system regulation. Pharmacol Rev. (2025) 77:100032. doi: 10.1016/j.pharmrev.2024.100032
8. Hupperts R, Smolders J, Vieth R, Holmøy T, Marhardt K, Schluep M, et al. Randomized trial of daily high-dose vitamin D3 in patients with RRMS receiving subcutaneous interferon β-1a. Neurology. (2019) 93:e1906–16. doi: 10.1212/WNL.0000000000008445
9. Khosravi-Largani M, Pourvali-Talatappeh P, Rousta AM, et al. A review on potential roles of vitamins in incidence, progression, and improvement of multiple sclerosis. eNeurologicalSci. (2018) 10:37–44. doi: 10.1016/j.ensci.2018.01.007
10. Feng W, Ma XN, Wu Q, et al. Serum 25-hydroxyvitamin D levels and dermatomyositis: A 2-sample mendelian randomization study. Int J Rheum Dis. (2024) 27:e15204. doi: 10.1111/1756-185X.15204
11. Zhao SS, Mason A, Gjekmarkaj E, et al. Associations between vitamin D and autoimmune diseases: Mendelian randomization analysis. Semin Arthritis Rheum. (2023) 62:152238. doi: 10.1016/j.semarthrit.2023.152238
12. Manousaki D, Harroud A, Mitchell RE, et al. Vitamin D levels and risk of type 1 diabetes: A Mendelian randomization study. PLoS Med. (2021) 18:e1003536. doi: 10.1371/journal.pmed.1003536
13. Lemke D, Klement RJ, Schweiger F, et al. Vitamin D resistance as a possible cause of autoimmune diseases: A hypothesis confirmed by a therapeutic high-dose vitamin D protocol. Front Immunol. (2021) 12:655739. doi: 10.3389/fimmu.2021.655739
14. Cassard SD, Fitzgerald KC, Qian P, et al. High-dose vitamin D3 supplementation in relapsing-remitting multiple sclerosis: A randomized clinical trial. eClinicalMedicine. (2023) 59:101957. doi: 10.1016/j.eclinm.2023.101957
15. Aranow C, Kamen DL, Dall’Era M, et al. Randomized, double-blind, placebo-controlled trial of the effect of vitamin D3 on the interferon signature in patients with systemic lupus erythematosus. Arthritis Rheumatol. (2015) 67:1848–57. doi: 10.1002/art.39108
16. Grove-Laugesen D, Ebbehoj E, Watt T, Riis AL, Østerga° rd T, Bruun BJ, et al. Effect of vitamin D supplementation on Graves’ disease: The DAGMAR trial. Thyroid. (2023) 33:1110–8. doi: 10.1089/thy.2023.0111
17. Nwosu BU. Guidance for high-dose vitamin D supplementation for prolonging the honeymoon phase in children and adolescents with new-onset type 1 diabetes. Front Endocrinol. (2022) 13:974196. doi: 10.3389/fendo.2022.974196
18. Finamor DC, Sinigaglia-Coimbra R, Neves LCM, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. (2013) 5:222–34. doi: 10.4161/derm.23385
19. Fernandes AL, Sales LP, Santos MD, Caparbo VF, Murai IH, and Pereira RMR. Persistent or new symptoms 1 year after a single high dose of vitamin D3 in patients with moderate to severe COVID-19. Front Nutr. (2022) 9:979667. doi: 10.3389/fnut.2022.979667
20. Brustad N, Yousef S, Stokholm J, et al. Safety of high-dose vitamin D supplementation among children aged 0 to 6 years: A systematic review and meta-analysis. JAMA Netw Open. (2022) 5:e227410. doi: 10.1001/jamanetworkopen.2022.7410
21. Thouvenot E, Laplaud D, Lebrun-Frenay C, Derache N, Page EL, Maillart M, et al. High-dose Vitamin D in clinically isolated syndrome typical of multiple sclerosis: the D-LayMS randomized clinical trial. JAMA. (2025) 333(16):1413–22. doi: 10.1001/jama.2025.1604
Keywords: high-dose, vitamin D, immune, autoimmune disease, VDR
Citation: Su S-T, Shih P-C and Wu M-C (2025) High-dose Vitamin D supplementation for immune recalibration in autoimmune diseases. Front. Immunol. 16:1625769. doi: 10.3389/fimmu.2025.1625769
Received: 09 May 2025; Accepted: 25 July 2025;
Published: 12 August 2025.
Edited by:
Mourad Aribi, University of Abou Bekr Belkaïd, AlgeriaReviewed by:
Dieter Steinhilber, Goethe University Frankfurt, GermanyCopyright © 2025 Su, Shih and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Po-Cheng Shih, cm9iZXJ0cGNzaGloQGdtYWlsLmNvbQ==; Meng-Che Wu, d3VtZW5nY2hlQGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Shiuan-Tzuen Su
Shiuan-Tzuen Su Po-Cheng Shih
Po-Cheng Shih Meng-Che Wu2,5,6*†
Meng-Che Wu2,5,6*†