- 1Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 2Department of Urology, Taichung Veterans General Hospital, Taichung, Taiwan
- 3Department of Medicine and Nursing, Hungkuang University, Taichung, Taiwan
- 4Department of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan
- 5Department of Applied Chemistry, National Chi Nan University, Nantou, Taiwan
- 6College of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 7Jenteh Junior College of Medicine, Nursing and Management, Miaoli, Taiwan
Abbreviations: BMI, body mass index; CI, confidence interval; ESRD, end stage renal disease; HR, hazard ratio; IRB, Institutional Review Board; ICD, International Classification of Diseases; KTX, kidney transplantation; mTOR, mammalian target of rapamycin; PSM, propensity scoring matching; PTM: post-transplantation malignancy; SIR, standardized incidence ratio; SMR, standardized mortality rate.
Objective: Malignancy is a main cause of mortality and morbidity in kidney transplantation recipients. Advancements in cancer surveillance and treatment may contribute to increased incidence and improved clinical outcomes. This study aimed to investigate the trends and clinical outcomes of post-transplantation malignancies over the past two decades.
Methods: We conducted a retrospective cohort study using the TriNetX network. Common post-transplantation malignancies were identified, and outcomes were assessed using Kaplan–Meier survival analysis with propensity score matching. We compared two transplantation cohorts (2000–2010 and 2011–2021) to assess potential changes in malignancy incidence, graft survival, and patient mortality.
Results: A total of 184,267 kidney transplantation recipients were included. Compared to the general population, transplantation recipients exhibited a higher risk of malignancy [standardized incidence ratio (SIR) 1.635; 95% confidence interval (CI), 1.600–1.670] and mortality [hazard ratio (HR) 1.115; 95% CI, 1.071–1.763; P < 0.0001]. The overall incidence of post-transplantation malignancies remained stable over the past two decades. Significant reductions in graft failure (HR 0.442; 95% CI, 0.413–0.473; P < 0.0001) and all-cause mortality (HR 0.755; 95% CI, 0.713–0.801; P < 0.0001) were observed in the recent decade.
Conclusion: Kidney transplantation recipients remain at increased risk for malignancies and associated mortality. While the incidence of malignancies has not changed significantly over the past two decades, both graft failure and mortality have declined in the recent decade, potentially reflecting improvements in post-transplantation care and cancer management.
Introduction
Kidney transplantation (KTX) is a standard treatment for patients with end stage renal disease (ESRD). For patients under dialysis, KTX prolonged overall survival (OS) and improved life quality (1, 2). Malignancy, infection and cardiovascular disease are the leading causes of mortality and morbidity in KTX recipients (3–7).
KTX recipients have a cancer incidence rate approximately twofold to fourfold higher than the age- and gender- matched general population. The main mechanisms of an elevated malignancy incidence rate are likely the long-term immunosuppress agents use, carcinogenic virus infection, and donor transmission (8–17). Immunosuppressants use with an increasing risk of carcinogenic virus infection may be the cause of post-transplantation cancer occurrence, such as liver cancer caused by hepatitis B virus. Risk increased for common cancers in the general population, for example, skin, lung and kidney cancer, which may be attributable to multi-factors, including immunosuppression, carcinogenic medication, or end stage renal disease (18–20). Certain cancer types (breast and prostate cancer) have decreased incidence after kidney transplantation for unknown reasons (12, 13).
Improvement in post-kidney transplantation care results in a longer graft survival and a reduction in recipient mortality caused by infection or cardiovascular events (3–7). Meanwhile, a longer survival may contribute to an increased incidence of post-transplantation malignancy. Despite recent advancement in cancer management, it is challenging in managing transplantation recipients with cancer when preserving renal function at the same time (21–28).
In view of the increased risk of post-kidney transplantation malignancy and improved cancer treatment, we conducted here a cohort study to evaluate the variation in cancer incidence and outcomes for kidney transplantation recipients in the past two decades.
Materials and methods
Data source and study population
We conducted a retrospective cohort study on the TriNetX network, a database with more than 275 million population around the world. The TriNetX platform provided real-world and updated data on demographics, diagnosis, laboratory values, medications and procedures. Within the database, we used the US Collaborative Network, which is a sub-database of the network including 57 US healthcare organizations. Data retrieval and analysis were carried out in June, 2024.
We included patients aged ≥18 years who had received kidney transplantation during the period from January 1, 2000 to December 31, 2021. Patients with kidney transplantation were included and identified using the International Classification of Diseases, 10th edition, Clinical Modification (ICD-10-CM): ICD-10-CM Z94.0, as well as other ICD-10-CM codes to confirm the diagnosis of cancer (Supplementary Data 1). The index date was set at the date of kidney transplantation when evaluating time from transplantation to cancer diagnosis, and cancer diagnosis when calculating graft failure and overall mortality. To ensure the inclusion of all post-transplant cancer cases while minimizing discrepancies in registration timing in the database, post-transplantation malignancy (PTM) was defined as a diagnosis made at least one month following the transplantation. Recipients with cancer diagnosis before KTX were excluded, since our aim was to investigate de novo malignancy after KTX.
Statistical analyses
For baseline characteristics, mean and standard deviation were used to represent continuous variables, and number (percentage) to represent categorical variables. To evaluate the risk of specific malignancy in KTX recipients, we evaluated the standardized incidence ratios (SIR) between recipients and the general population after adjusted with age, gender (male/female) and race (Caucasian/African American/Asian). When calculating SIR, the general population was identified using ICD-10: Z00.0 (encounter for general adult medical examination) and patients with history of transplantation were excluded. Kaplan-Meier method with propensity score matching (PSM) was used to evaluate the relative risk of mortality among patients with or without PTM, and the incidence and outcomes of PTM between two different periods (2000–2010 and 2011-2021). To evaluate relative mortality, the control cohorts (the general population with cancer) were identified using corresponding ICD codes of malignancies, excluding patients with history of transplantation. For the trends over the past two decades, we compared the overall mortality and graft failure (ICD-10-CM: T86.12) between the two cohorts (2000–2010 versus 2011-2021), using 2000–2010 as a reference cohort. We performed all the statistical analyses on the platform. Statistically significance was set at P <0.05.
Ethics in research
Our study was carried out after approval from the Institutional Review Board (IRB) of Taichung Veterans General Hospital (IRB number: SE:22220A). Since all data on the database was de-identified, informed consent was waived by the ethics committee.
Results
Patient characteristics
We studied a total of 184,267 patients who had received kidney transplantation in the period between 2000 and 2021. During the follow-up period, 30, 545 (16%) patients had developed de novo malignancy after kidney transplantation. When compared with those without cancer, patients who had developed cancer after kidney transplantation were older, with more male in gender, more white patients, higher body mass index (BMI), higher rates of rejection, smoking, comorbidities; they also had lower incomes, and lower serum Tacrolimus level (All P<0.0001). Table 1 shows the patients baseline demographics.
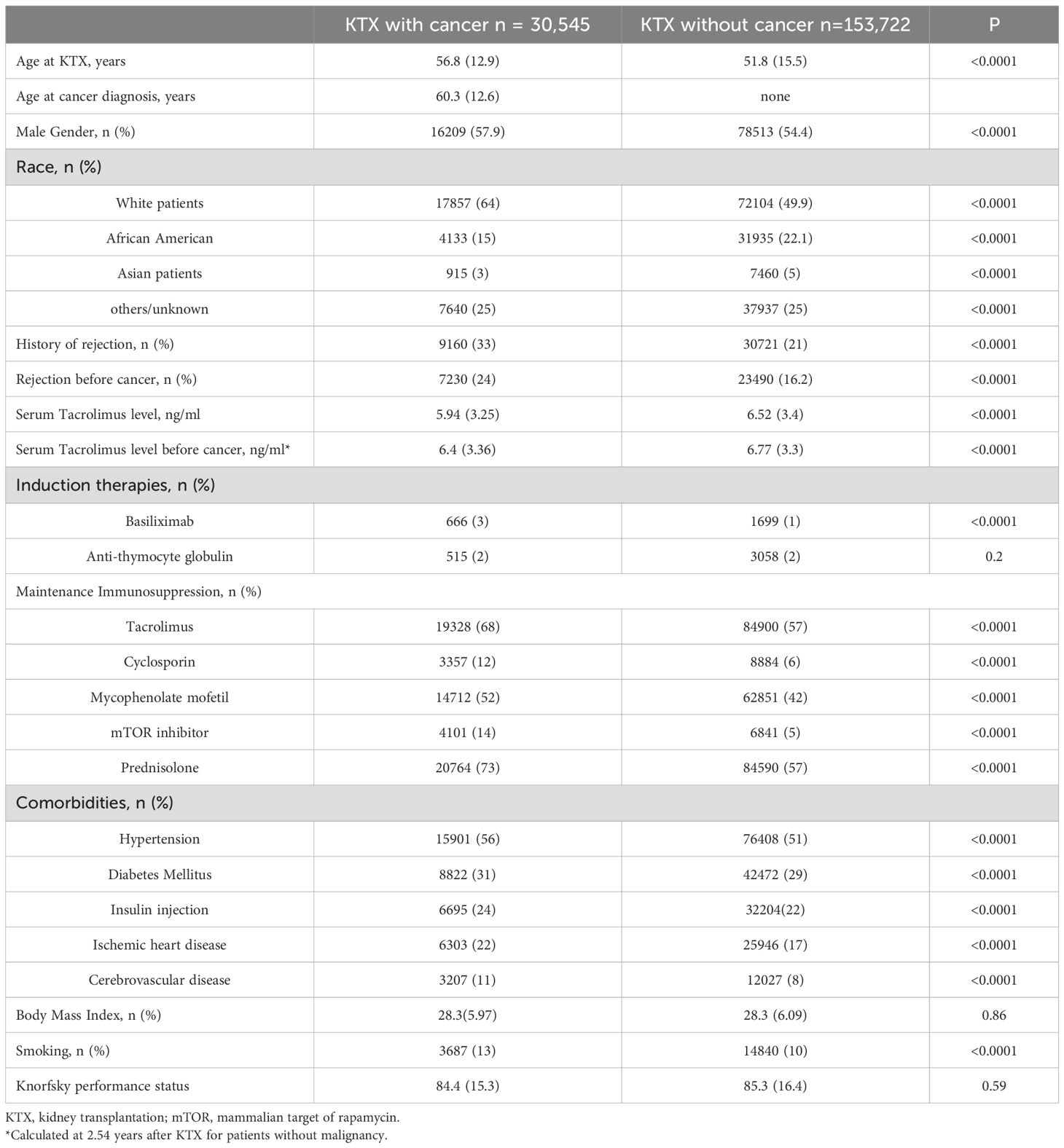
Table 1. Baseline characteristics for patients with or without post-kidney transplantation malignancy.
Incidence of various cancers
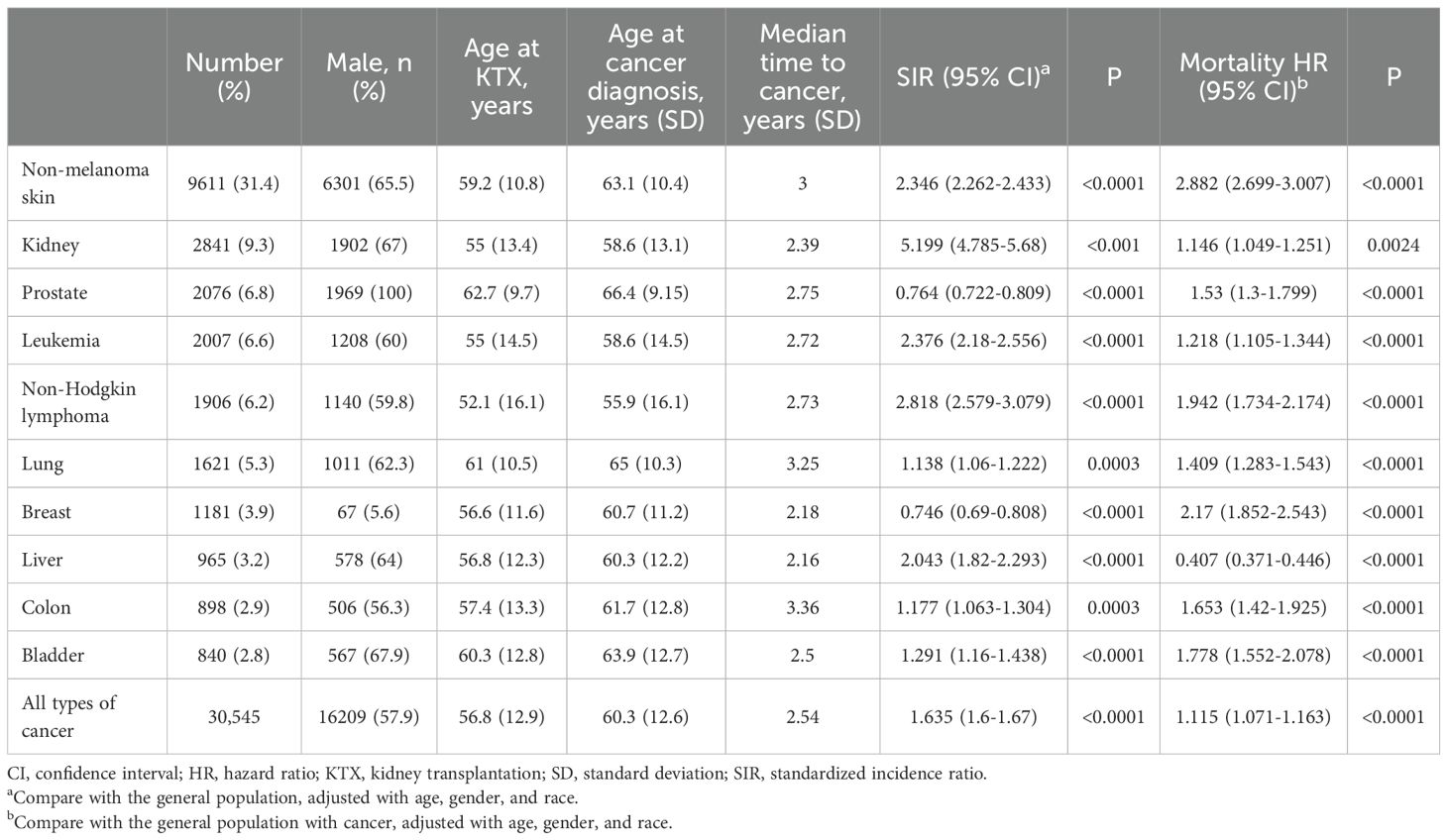
Common types of cancer that had occurred following kidney transplantation are listed on Table 2. The most common PTM was non-melanoma skin cancer (31.4%), followed by kidney cancer (9.3%), prostate cancer (6.8%), leukemia (6.6%), non-Hodgkin lymphoma (NHL) (6.2%), lung cancer (5.3%), breast cancer (3.9%), liver cancer (3.2%), colon cancer (2.9%), and bladder cancer (2.8%). Median age at cancer diagnosis was 60.3 years. Patients with NHL (52 years old for KTX and 55.9 cancer diagnosis) were younger at both KTX and the diagnosis of cancer, while patients developing lung cancer (61 for KTX and 65 for cancer diagnosis) and prostate cancer (62.7 for KTX and 66.4 years for cancer diagnosis) were older. Median time from KTX to cancer diagnosis was 2.54 years, with colon cancer having the longest time (3.3 years), and liver cancer (2.16 years) having the shortest time.
We further compared the cancer incidence between KTX patients and the general population after PSM. After PSM for age, gender and race, patients receiving KTX were associated with a higher risk for cancer development compared with the general population [SIR 1.635, 95% confidence interval (CI) 1.6-1.67]. In general, KTX patients had a higher risk for cancer occurrence for most of the cancer types. Among different sites of cancer, the highest risk of PTM was kidney cancer (SIR 5.199, 95% CI 4.785-5.68), followed by NHL (SIR 2.818, 95% CI 2.579-3.079) and leukemia (SIR 2.376, 95% CI 2.18-2.556).
Risk factors for malignancy
Through the multivariate cox regression analysis, factors revealed for PTM were as follows: older age (40–60 years HR 1.934 95% CI 1.817-2.059 P<0.0001, > 60 years HR 4.674 95% CI 4.406-4.957 P<0.0001), male gender (HR 1.193 95% CI 1.170-1.126 P<0.0001), tacrolimus (HR 1.135 95% CI 1.098-1.173 P<0.0001), cyclosporin (HR 1.284 95% CI 1.214-1.358 P<0.0001), mTOR (HR 1.306 95% CI 1.218-1.401 P<0.0001), and prednisolone (HR 1.112 95% CI 1.079-0.1.146 P<0.0001). Figure 1 presents the results of the multivariate analysis and forest plot on factors for PTM in kidney transplantation recipients.
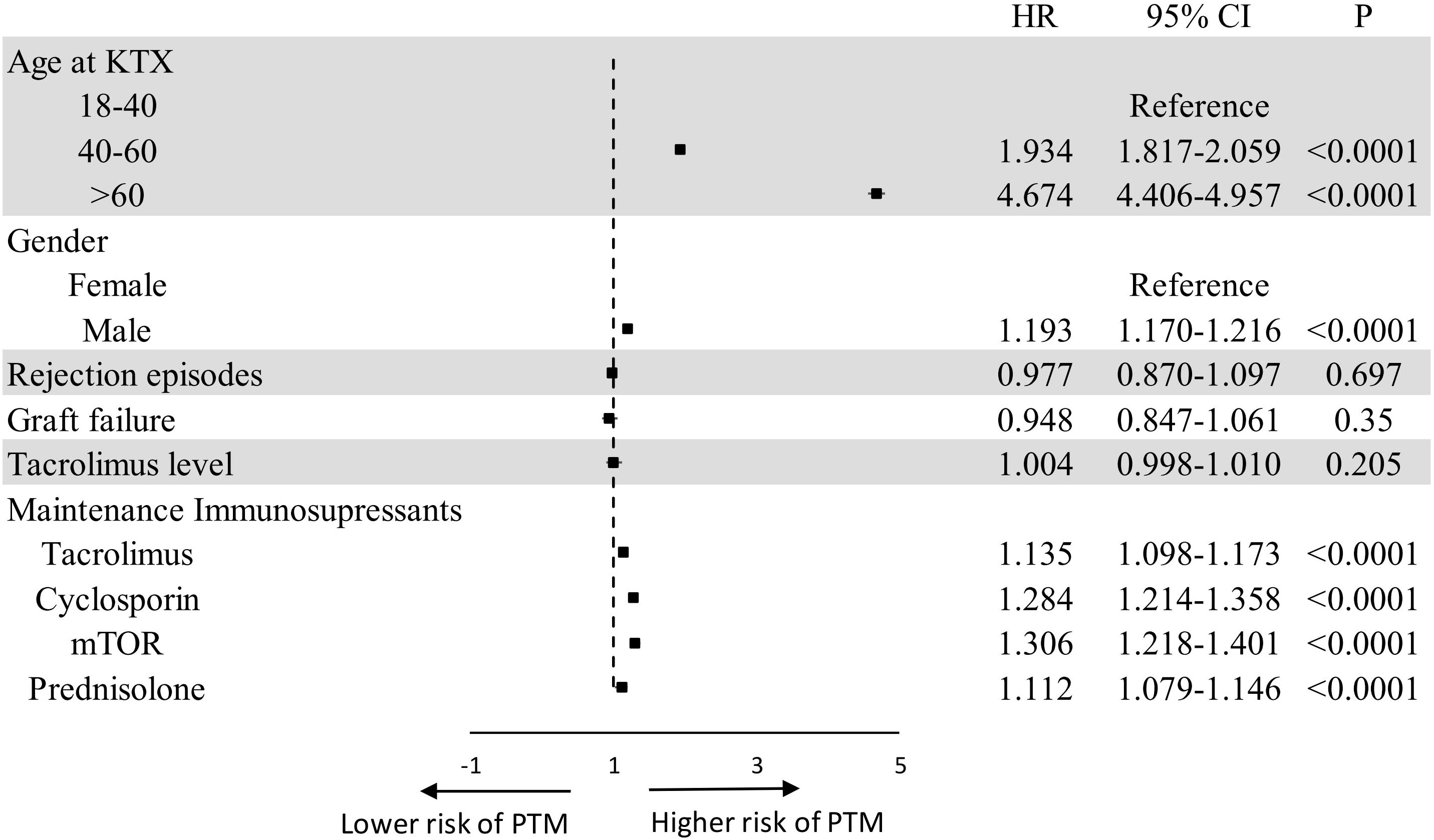
Figure 1. Forest plot of multivariate analysis on risk factors for PTM. CI, confidence interval; HR, hazard ratio; KTX, kidney transplantation; OS, overall survival; PTM, post-transplant malignancy.
Trends for post-KTX malignancy
A total of 56,922 patients who received KTX in 2000–2010 and 127,345 patients in 2011–2021 were included. During the follow-up period, 12,921 (22.7%) patients in the 2000–2010 cohort and 15,026 (11.8%) in the 2011–2021 cohort experienced PTM. After adjusted for age, gender, and race, there was no change in overall cancer incidence over the past two decades (HR 1.003 95% CI 0.956-1.053, P = 0.892). Regarding the specific site of cancer, there was a decrease in incidence for leukemia (HR 0.615 95% CI 0.518-0.729, P < 0.0001) and bladder cancer (HR 0.741 95% CI 0.576-0.954, P = 0.019). Table 3 shows the unadjusted and adjusted incidence hazard ratios of PTM between the 2000–2010 and 2011–2021 cohorts.
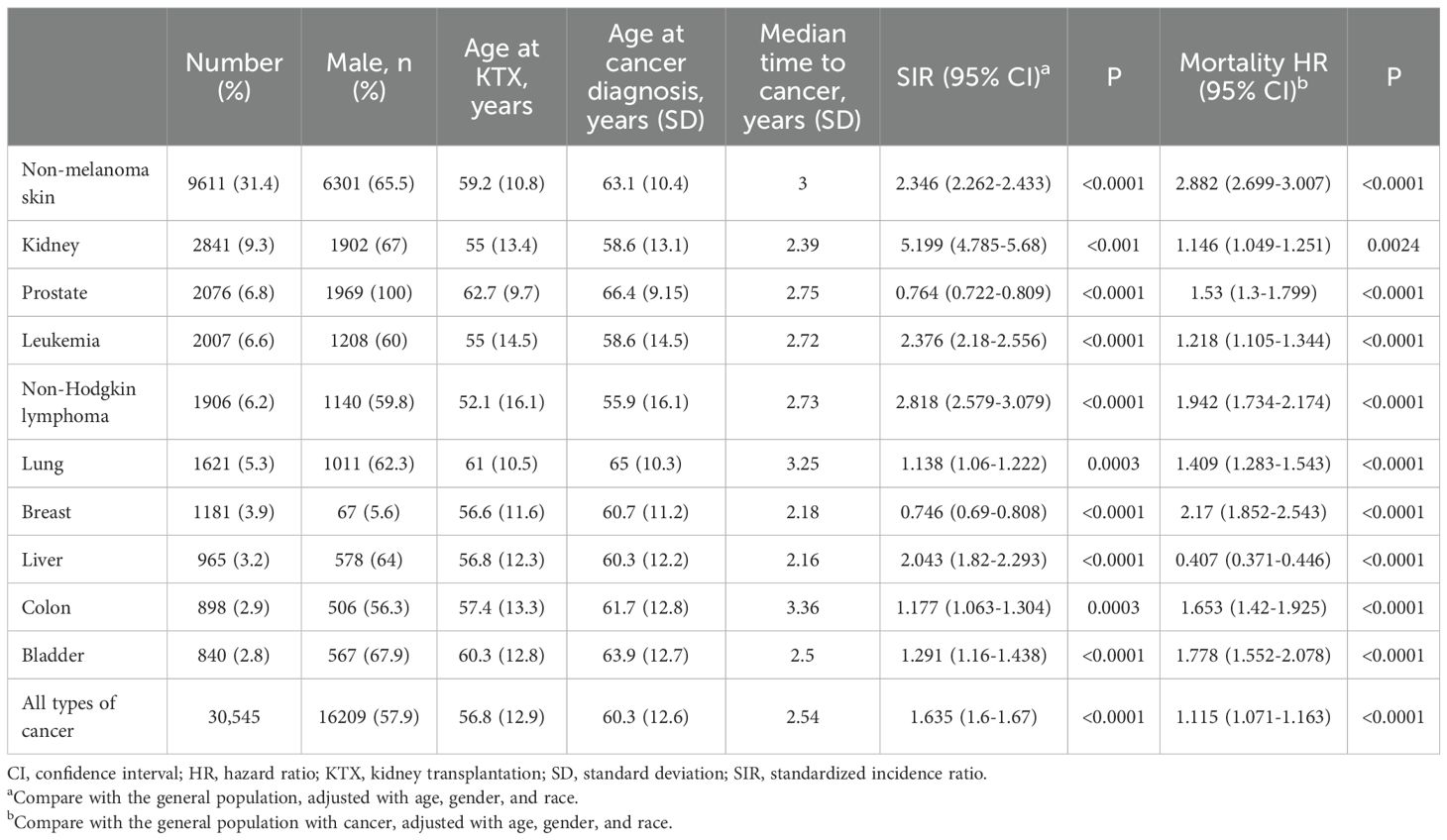
Table 3. Unadjusted and adjusted incidence hazard ratios for post-transplantation malignancy between patients receiving kidney transplantation at 2000-2010 or 2011-2021, using 2000-2010 cohort as a reference.
Regarding the variation of outcomes (graft failure and mortality) for patients with PTM over the past two decades, we found a trend toward reduction in graft failure (HR 0.442 95% CI 0.413-0.473, P < 0.000) and overall mortality (HR 0.755 95% CI 0.713-0.801, P < 0.0001 for overall mortality). Figures 2, 3 compares the graft failure and mortality rates for post-KTX malignancy between the 2000–2010 and 2011–2021 cohorts.
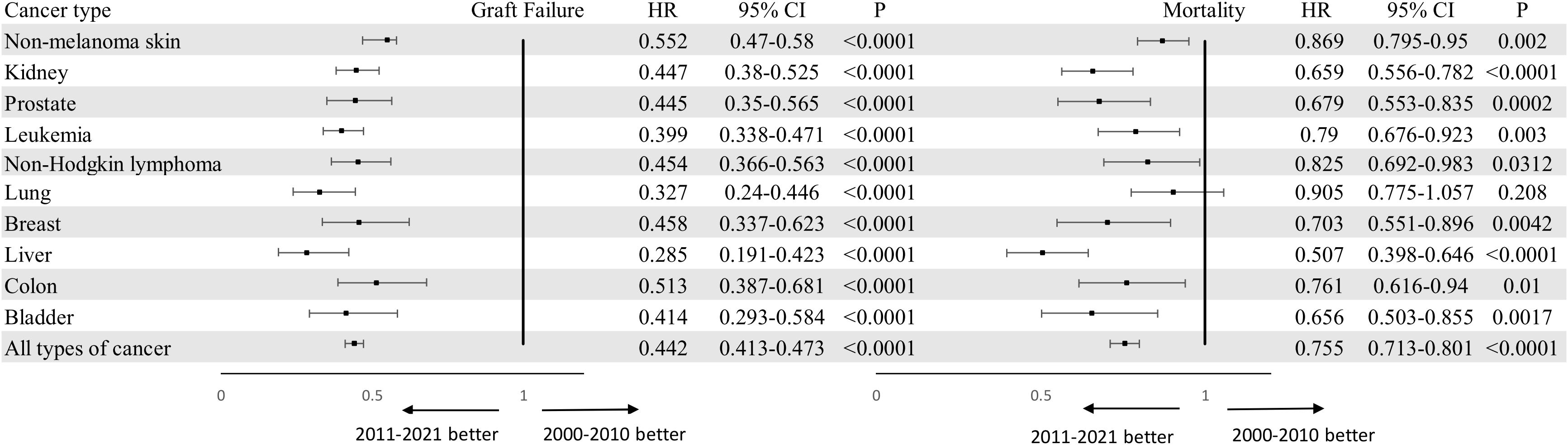
Figure 2. Forest plots of graft failure and overall mortality for kidney transplantation recipients with malignancy who underwent KTX in 2000-2010 or 2011-2021 using 2000-2010 cohort as a reference. Adjusted for age, gender and race. KTX, kidney transplantation.
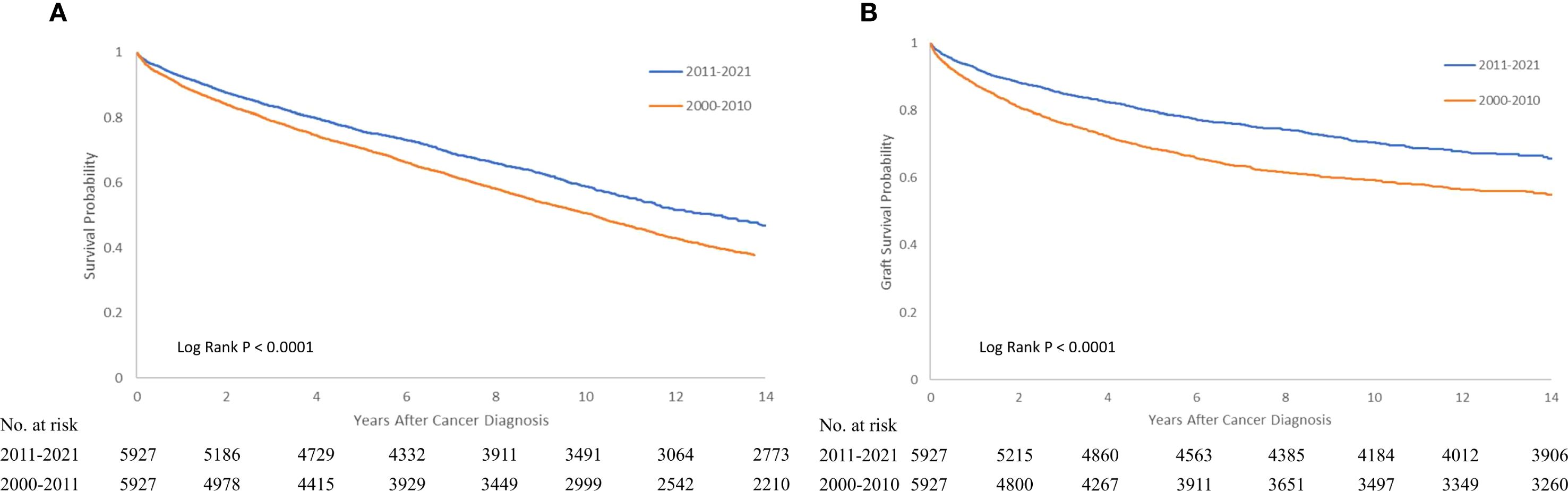
Figure 3. Kaplan-Meier survival curve of overall (A) and graft survival (B) for recipients with post-KTX malignancy who underwent KTX in 2000-2010 or 2011-2021. KTX, kidney transplantation.
Impact of malignancy on outcomes for KTX recipients
Patients with PTM were associated with a higher risk of mortality (HR 1.115 95% CI 1.071-1.763 P < 0.0001) compared with the general population with malignancy after PSM. For specific cancer: non-melanoma skin cancer (HR 2.88 95% CI 2.699-3.007) and breast cancer (HR 2.17 95% CI 1.852-2.543) were associated with the worst overall survival compared with the general population with cancers (Table 2).
Discussion
In this study, we found that patients with KTX were associated with a higher risk of malignancy when compared with the general population. Recipients with post-KTX malignancy had worse overall survival compared with the general population with malignancy. There was no significant change in cancer incidence for KTX recipients over the past 20 years, but graft failure and mortality rates decreased in the recent decade.
Previous studies reported higher malignancy risks of 2 to 4-fold in kidney transplantation recipients compared with the general population (8–17). In this study, we found that such patients had an increased risk of developing malignancy following kidney transplantation (SIR:1.635) compared with the general population. The slightly lower SIR is likely related to the fact that we excluded patients with history of malignancy before transplantation. In general, most sites of cancer presented higher SIR compared with the general population. On the other hand, breast and prostate cancer did not show an elevated risk, the real reason was unclear, and this finding was consistent with the study conducted by Engels et al. (12).
A higher risk of malignancy for KTX recipients may be resulted from several factors (16). Immunosuppression may be the reason for virus induced cancers. For example, hepatitis B virus and hepatitis c virus for liver cancer, and human T cell lymphotropic virus for non-Hodgkin lymphoma. On the other hand, for non-virus mediated cancers, including common cancers in general population (lung, colon, breast, and prostate cancer) and ESRD patients (kidney and bladder cancer), closer cancer screening tests may have contributed to a higher incidence rate for those patients (18–20).
The mortality rates between KTX patients with cancer and the general population with cancer were debatable. Kiberd et al. reported that there was no difference in cancer mortality between these two populations (29). However, some studies report a higher mortality rate for patients with PTM compared with the general population (30–32). In this study, we found that the overall survival of KTX recipients was worse for those with PTM compared with the general population for most cancer types and overall malignancy (HR 1.115 95% CI 1.071-1.763 P < 0.0001). The results indicated a challenging condition in treating these KTX recipients with malignancy under immunosuppressants, which may limit the use of chemotherapy or immunotherapy.
Previous reports on the median time from kidney transplantation (KTX) to cancer diagnosis have been limited and variable, ranging from 2.6 to 4 years (32, 33). In our study, the interval from KTX to cancer diagnosis was comparatively shorter (2.54 years). This difference may be attributable to more intensive cancer screening following KTX as well as the increasing use of marginal donors and recipient. Importantly, these results highlight the need for closer surveillance for common post-transplant malignancies.
The use of mammalian target of rapamycin (mTOR) inhibitors has been associated with a reduced incidence of malignancy in several studies (28, 34, 35). However, conflicting evidence has been reported regarding the cancer risk associated with sirolimus use in recipients (36, 37). A meta-analysis by Knoll et al. indicated that the anti-cancer benefits of sirolimus were primarily observed in patients who were converted from other immunosuppressive regimens, rather than in those who received de novo sirolimus (34). Furthermore, de novo use of sirolimus was associated with an increased risk of post-transplant lymphoproliferative disorder (38). In our study, multivariate analysis revealed that the use of mTOR inhibitors was associated with an increased risk of PTM (HR 1.306–95 CI 1.218-1.401). Previous studies have shown that calcineurin inhibitors (CNIs), such as cyclosporin and tacrolimus, are associated with a heightened risk of PTM, potentially due to their immunosuppressive or carcinogenic properties (32, 39). Consistent with these findings, multivariate analysis identified tacrolimus (HR 1.135–95 CI 1.098-1.173) and cyclosporin (HR 1.284–95 CI 1.214-1.358) as risk factors for PTM when used as maintenance immunosuppressive therapies. Notably, we did not observe a significant association between elevated serum tacrolimus levels and PTM. This may be attributed to the dynamic nature of tacrolimus serum levels and the lack of standardized timing for level measurement in the TriNetX database. These findings underscore that immunosuppression remains a key factor for PTM, regardless of the specific regimen used. Therefore, enhanced cancer surveillance is warranted in kidney transplant recipients (24, 40, 41).
Blosser CD, et al. and Piselli P, et al. reported that there was no significant change in incidence of PTM over time (42, 43). Our results align with previous evidence, indicating that the overall incidence of PTM has remained relatively stable over the last twenty years. In the unadjusted analysis, increased incidences of kidney, prostate, and overall cancer were observed. These findings may be attributable to the older age of recipients and the increased intensity of cancer screening in recent years. However, after adjusting for these factors, the overall cancer incidence did not show a significant increase over the past two decades. Blosser et al. previously reported no significant improvement in graft or patient survival among kidney transplant recipients with non-Hodgkin lymphoma (non-NHL) malignancies (42). In contrast, our study demonstrated a decline in both graft failure and patient mortality among recipients diagnosed with PTM over the past two decades. Although the exact mechanisms underlying these observations remain unclear, the trends may be partially attributable to improvements in post-transplantation care, including advancements in oncologic treatments and the management of infectious and cardiovascular complications, which could positively influence graft and patient survival.
This study has several limitations inherent to its retrospective design, including the possibility of residual confounding despite adjustment for known covariates. The use of the TriNetX database, while providing a large sample size, is limited by the absence of detailed cancer-specific outcome metrics such as tumor staging, histopathology, treatment modalities, and cause-specific mortality, which restricts the depth of interpretation. Additionally, the reliance on administrative coding may introduce misclassification bias in diagnoses and outcomes. Selection bias is also a concern, as the dataset represents patients from specific healthcare organizations and may not fully generalize to the broader transplant population. Furthermore, patients in the more recent transplant cohort may have shorter follow-up durations, potentially leading to an underestimation of cancer incidence in this group. Lastly, we defined PTM as any cancer diagnosis occurring ≥1 month following kidney transplantation. It is acknowledged that this definition may inadvertently capture malignancies that were present but undiagnosed at the time of transplantation. Despite these limitations, our research was a population-based study with a large sample size, which may avoid some bias arising from its retrospective nature. Furthermore, data regarding the trends of PTM over the past decades was limited, and our research provided information on this topic.
In conclusion, KTX recipients exhibited a significantly higher risk of PTM compared with the general population. Moreover, the presence of PTM was associated with increased overall mortality. Although the incidence of PTM has remained stable over the past two decades, both graft failure and mortality risks among patients with PTM have shown a significant decline during the same period.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Taichung Veterans General Hospital (IRB number: SE:22220A). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because All data on the database was de-identified.
Author contributions
G-SL: Investigation, Software, Conceptualization, Resources, Writing – original draft, Visualization, Funding acquisition, Validation, Formal analysis, Methodology, Supervision, Data curation, Project administration, Writing – review & editing. J-RL: Writing – review & editing. C-SC: Writing – review & editing. S-SW: Writing – review & editing. C-YL: Writing – review & editing. C-JY: Writing – review & editing. H-CH: Writing – review & editing. S-CH: Writing – review & editing. K-YC: Writing – review & editing. C-KY: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The data used in this study was retrieved from the TriNetX network.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1626135/full#supplementary-material
References
1. Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. (1999) 341:1725–30. doi: 10.1056/NEJM199912023412303
2. Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, and Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. (2000) 342:605–12. doi: 10.1056/NEJM200003023420901
3. Weinrauch LA, D'Elia JA, Weir MR, Bunnapradist S, Finn PV, Liu J, et al. Infection and Malignancy outweigh cardiovascular mortality in kidney transplant recipients: post hoc analysis of the FAVORIT trial. Am J Med. (2018) 131:165–72. doi: 10.1016/j.amjmed.2017.08.038
4. Awan AA, Niu J, Pan JS, Erickson KF, Mandayam S, Winkelmayer WC, et al. Trends in the causes of death among kidney transplant recipients in the United States (1996-2014). Am J Nephrol. (2018) 48:472–81. doi: 10.1159/000495081
5. Sypek MP, Dansie KB, Clayton P, Webster AC, and Mcdonald S. Comparison of cause of death between Australian and New Zealand Dialysis and Transplant Registry and the Australian National Death Index. Nephrol (Carlton). (2019) 24:322–9. doi: 10.1111/nep.13250
6. Au EH, Chapman JR, Craig JC, Lim WH, Teixeira-Pinto A, Ullah S, et al. Overall and site-specific cancer mortality in patients on dialysis and after kidney transplant. J Am Soc Nephrol. (2019) 30:471–80. doi: 10.1681/ASN.2018090906
7. Lai GS, Li JR, Wang SS, Chen CS, Yang CK, Lin CY, et al. Influence of surgical complications on outcomes in kidney transplantation patients. In Vivo. (2023) 37:2796–802. doi: 10.21873/invivo.13392
8. Adami J, Gäbel H, Lindelöf B, Ekström K, Rydh B, Glimelius B, et al. Cancer risk following organ transplantation: a nationwide cohort study in Sweden. Br J Cancer. (2003) 89:1221–7. doi: 10.1038/sj.bjc.6601219
9. Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, Law M, et al. Cancer incidence before and after kidney transplantation. JAMA. (2006) 296:2823–31. doi: 10.1001/jama.296.23.2823
10. Villeneuve PJ, Schaubel DE, Fenton SS, Shepherd FA, Jiang Y, and Mao Y. Cancer incidence among Canadian kidney transplant recipients. Am J Transplant. (2007) 7:941–8. doi: 10.1111/j.1600-6143.2007.01736.x
11. Collett D, Mumford L, Banner NR, Neuberger J, and Watson C. Comparison of the incidence of Malignancy in recipients of different types of organ: a UK Registry audit. Am J Transplant. (2010) 10:1889–96. doi: 10.1111/j.1600-6143.2010.03181.x
12. Engels EA, Pfeiffer RM, Fraumeni JF Jr, Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. (2011) 306:1891–901. doi: 10.1001/jama.2011.1592
13. Hwang JK, Moon IS, and Kim JI. Malignancy after kidney transplantation: a 40-year single-center experience in Korea. Transpl Int. (2011) 24:716–21. doi: 10.1111/j.1432-2277.2011.01270.x
14. Li WH, Chen YJ, Tseng WC, Lin MW, Chen TJ, Chu SY, et al. Malignancies after renal transplantation in Taiwan: a nationwide population-based study. Nephrol Dial Transplant. (2012) 27:833–9. doi: 10.1093/ndt/gfr277
15. Au E, Wong G, and Chapman JR. Cancer in kidney transplant recipients. Nat Rev Nephrol. (2018) 14:508–20. doi: 10.1038/s41581-018-0022-6
16. Cheung CY and Tang SCW. An update on cancer after kidney transplantation. Nephrol Dial Transplant. (2019) 34:914–20. doi: 10.1093/ndt/gfy262
17. Lu K and Chiu KY. Temporal trends of de novo urological Malignancy in renal transplant recipients without a cancer history: A longitudinal cohort study. Clin Transplant. (2023) 37:e15047. doi: 10.1111/ctr.15047
18. Massicotte-Azarniouch D, Noel JA, and Knoll GA. Epidemiology of cancer in kidney transplant recipients. Semin Nephrol. (2024) 44:151494. doi: 10.1016/j.semnephrol.2024.151494
19. Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, et al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet. (1999) 354:93–9. doi: 10.1016/s0140-6736(99)06154-1
20. Butler AM, Olshan AF, Kshirsagar AV, Edwards JK, Nielsen ME, Wheeler SB, et al. Cancer incidence among US Medicare ESRD patients receiving hemodialysis, 1996-2009. Am J Kidney Dis. (2015) 65:763–72. doi: 10.1053/j.ajkd.2014.12.013
21. Alhamad T, Venkatachalam K, Linette GP, and Brennan DC. Checkpoint inhibitors in kidney transplant recipients and the potential risk of rejection. Am J Transplant. (2016) 16:1332–3. doi: 10.1111/ajt.13711
22. Murakami N, Mulvaney P, Danesh M, Abudayyeh A, Diab A, Abdel-Wahab N, et al. Immune Checkpoint Inhibitors in Solid Organ Transplant Consortium. A multi-center study on safety and efficacy of immune checkpoint inhibitors in cancer patients with kidney transplant. Kidney Int. (2021) 100:196–205. doi: 10.1016/j.kint.2020.12.015
23. Al-Adra D, Al-Qaoud T, Fowler K, and Wong G. De novo Malignancies after kidney transplantation. Clin J Am Soc Nephrol. (2022) 17:434–43. doi: 10.2215/CJN.14570920
24. Schreiber B, Abdelrahim M, Abudayyeh A, and Murakami N. Emerging concepts in managing Malignancy in kidney transplant patients. Semin Nephrol. (2022) 42:63–75. doi: 10.1016/j.semnephrol.2022.01.003
25. Nada A and Jetton JG. Pediatric onco-nephrology: time to spread the word-part II: long-term kidney outcomes in survivors of childhood Malignancy and Malignancy after kidney transplant. Pediatr Nephrol. (2022) 37:1285–300. doi: 10.1007/s00467-021-05172-y
26. Alzahrani N, Al Jurdi A, and Riella LV. Immune checkpoint inhibitors in kidney transplantation. Curr Opin Organ Transplant. (2023) 28:46–54. doi: 10.1097/MOT.0000000000001036
27. Bigotte Vieira M, Arai H, Nicolau C, and Murakami N. Cancer screening and cancer treatment in kidney transplant recipients. Kidney360. (2024) 5:1569–83. doi: 10.34067/KID.0000000000000545
28. Kong D, Duan J, Chen S, Wang Z, Ren J, Lu J, et al. Transplant oncology and anti-cancer immunosuppressants. Front Immunol. (2025) 15:1520083. doi: 10.3389/fimmu.2024.1520083
29. Kiberd BA, Rose C, and Gill JS. Cancer mortality in kidney transplantation. Am J Transplant. (2009) 9:1868–75. doi: 10.1111/j.1600-6143.2009.02728.x
30. Acuna SA, Fernandes KA, Daly C, Hicks LK, Sutradhar R, Kim SJ, et al. Cancer mortality among recipients of solid-organ transplantation in ontario, Canada. JAMA Oncol. (2016) 2:463–9. doi: 10.1001/jamaoncol.2015.5137
31. Rosales BM, de la Mata N, Vajdic CM, Kelly PJ, Wyburn K, Webster AC, et al. Cancer mortality in kidney transplant recipients: An Australian and New Zealand population-based cohort study, 1980-2013. Int J Cancer. (2020) 146:2703–11. doi: 10.1002/ijc.32585
32. Jeong S, Lee HS, Kong SG, Kim DJ, Lee S, Park MJ, et al. Incidence of Malignancy and related mortality after kidney transplantation: a nationwide, population-based cohort study in Korea. Sci Rep. (2020) 10:21398. doi: 10.1038/s41598-020-78283-5
33. Teo SH, Lee KG, Lim GH, Koo SX, Ramirez ME, Chow KY, et al. Incidence, risk factors and outcomes of Malignancies after kidney transplantation in Singapore: a 12-year experience. Singapore Med J. (2019) 60:253–9. doi: 10.11622/smedj.2018122
34. Knoll GA, Kokolo MB, Mallick R, Beck A, Buenaventura CD, Ducharme R, et al. Effect of sirolimus on Malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data. BMJ. (2014) 349:g6679. doi: 10.1136/bmj.g6679
35. Re Sartò GV, Alfieri C, Cosmai L, Brigati E, Campise M, Regalia A, et al. Post-kidney transplant cancer: A real-world retrospective analysis from a single italian center. Transpl Int. (2024) 37:13220. doi: 10.3389/ti.2024.13220
36. Budde K, Lehner F, Sommerer C, Reinke P, Arns W, Eisenberger U, et al. Five-year outcomes in kidney transplant patients converted from cyclosporine to everolimus: the randomized ZEUS study. Am J Transplant. (2015) 15:119–28. doi: 10.1111/ajt.12952
37. Yanik EL, Gustafson SK, Kasiske BL, Israni AK, Snyder JJ, Hess GP, et al. Sirolimus use and cancer incidence among US kidney transplant recipients. Am J Transplant. (2015) 15:129–36. doi: 10.1111/ajt.12969
38. Nee R, Hurst FP, Dharnidharka VR, Jindal RM, Agodoa LY, and Abbott KC. Racial variation in the development of posttransplant lymphoproliferative disorders after renal transplantation. Transplantation. (2011) 92:190–5. doi: 10.1097/TP.0b013e3182200e8a
39. Massicotte-Azarniouch D, Detwiler RK, Hu Y, Falk RJ, Saha MK, Hogan SL, et al. Malignancy risk in kidney transplant recipients exposed to immunosuppression pre-transplant for the treatment of glomerulonephritis. Nephrol Dial Transplant. (2023) 38:2009–18. doi: 10.1093/ndt/gfac337
40. Munagala M and Phancao A. Malignancy: an adverse effect of immunosuppression. Handb Exp Pharmacol. (2022) 272:315–35. doi: 10.1007/164_2021_554
41. Pyrża M, Małyszko J, Głogowski T, Wieliczko M, Żebrowski P, and Małyszko J. Kidney transplant recipients have higher Malignancy prevalence than hemodialyzed patients. Transplant Proc. (2022) 54:972–5. doi: 10.1016/j.transproceed.2022.01.018
42. Blosser CD, Haber G, and Engels EA. Changes in cancer incidence and outcomes among kidney transplant recipients in the United States over a thirty-year period. Kidney Int. (2021) 99:1430–8. doi: 10.1016/j.kint.2020.10.018
Keywords: cancer, kidney transplantation, outcomes, trend, maligancy
Citation: Lai G-S, Li J-R, Chen C-S, Wang S-S, Lin C-Y, Yang C-J, Ho H-C, Hung S-C, Chiu K-Y and Yang C-K (2025) Temporal trends in malignancy incidence and outcomes among kidney transplantation recipients: a multi-center real-world evidence study using the TriNetX network (2000–2010 vs. 2010–2021). Front. Immunol. 16:1626135. doi: 10.3389/fimmu.2025.1626135
Received: 10 May 2025; Accepted: 17 September 2025;
Published: 02 October 2025.
Edited by:
Alícia Molina-Andújar, Hospital Clinic de Barcelona, SpainReviewed by:
Sheron Latcha, Memorial Sloan Kettering Cancer Center, United StatesMónica Bolufer, Hospital Germans Trias i Pujol, Spain
Copyright © 2025 Lai, Li, Chen, Wang, Lin, Yang, Ho, Hung, Chiu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng-Kuang Yang, eWFuZ2NrQGljbG91ZC5jb20=
 Gu-Shun Lai
Gu-Shun Lai Jian-Ri Li
Jian-Ri Li Chuan-Shu Chen
Chuan-Shu Chen Shian-Shiang Wang
Shian-Shiang Wang Chia-Yen Lin1,2,6
Chia-Yen Lin1,2,6 Sheng-Chun Hung
Sheng-Chun Hung Cheng-Kuang Yang
Cheng-Kuang Yang