- 1Shandong Provincial Third Hospital, Shandong University, Jinan, China
- 2Department of Otolaryngology-Head and Neck Surgery, Shandong Provincial Third Hospital, Shandong University, Jinan, China
- 3Department of Neurology, Shandong Provincial Third Hospital, Shandong University, Jinan, China
- 4Department of Gastrointestinal Surgery, Central Hospital Affiliated to Shandong First Medical University, Jinan, China
Background: Lung cancer (LC) remains the leading cause of cancer-related death worldwide. While immune checkpoint inhibitors (ICIs) have demonstrated survival benefits in advanced-stage disease, treatment responses exhibit significant heterogeneity across patients. The potential role of comorbid chronic obstructive pulmonary disease (COPD) in modulating survival outcomes from ICIs therapy remains controversial, with conflicting evidence regarding its synergistic or antagonistic effects. This meta-analysis systematically evaluates the impact of COPD on survival outcomes in lung cancer patients receiving ICIs, aiming to clarify its prognostic value and guide precision immunotherapy strategies.
Methods: We systematically searched PubMed, Cochrane Library, CNKI, and EMBASE for studies published up to March 31, 2025, to evaluate the synergistic survival impact of comorbid COPD in lung cancer patients receiving ICIs. Primary outcomes included overall survival (OS) and progression-free survival (PFS).
Results: This study pooled data from 10 studies (N = 6,909) to assess the impact of comorbid COPD on survival outcomes among LC patients receiving ICIs. The meta-analysis revealed that LC patients with comorbid COPD showed significant improvements in OS (HR = 0.90, 95% CI: 0.85–0.96, p < 0.001) and PFS (HR = 0.54, 95% CI: 0.44–0.67, p < 0.001) compared to those without COPD. Notably, while bias analysis for PFS indicated potential bias, inter-subgroup heterogeneity was negligible (I² = 0%).
Conclusions: This study demonstrated that the presence of comorbid COPD was associated with significantly improved overall survival in lung cancer patients receiving ICIs. Although a significant progression-free survival benefit was also observed, this result may be influenced by potential publication bias. Further prospective studies that incorporate comprehensive biomarker analyses are warranted to validate the observed COPD-ICIs interaction and to develop optimized, personalized treatment strategies for this patient population.
Systematic Review Registration: www.inplasy.com, identifier INPLASY202580086.
1 Introduction
Lung cancer (LC) remains the most common cause of cancer-related mortality worldwide, accounting for 18% to 22% of all cancer deaths [1, 2]. For patients with resectable stage I–III LC, radical surgery represents the cornerstone of curative-intent treatment; however, postoperative recurrence rates remain high, ranging from 30% to 55%, with stage IIIA disease carrying a 5-year recurrence risk exceeding 45%–60% (3–5). The advent of immune checkpoint inhibitors (ICIs) targeting the PD-1/PD-L1 axis has revolutionized therapeutic paradigms by reactivating cytotoxic T-cell-mediated antitumor immunity. In advanced non-small cell lung cancer (NSCLC), the combination of ICIs with chemotherapy has extended median overall survival (OS) from 10–12 months with chemotherapy alone to 17–22 months, underscoring a shift toward precision immunotherapy (6–10).
The tumor immune microenvironment (TIME), composed of malignant cells, immune infiltrates (e.g., CD8+ T cells, regulatory T cells [Tregs], tumor-associated macrophages [TAMs]), and stromal components, plays a pivotal role in modulating response to therapy through complex signaling networks such as TGF-β and IL-6 (11, 12). Chronic inflammation driven by COPD induces immunosuppressive remodeling of the TIME via activation of NF-κB and STAT3 pathways, leading to exhausted CD8+ T cells (expressing TIM-3 and LAG-3), expansion of myeloid-derived suppressor cells (MDSCs), and dysregulation of immune checkpoints including PD-L1 and CTLA-4, which may consequently attenuate ICI efficacy (13–15). Furthermore, oxidative stress and protease imbalance associated with COPD may promote genomic instability and facilitate immune evasion, thereby synergizing with lung cancer progression (16, 17). Retrospective analyses have demonstrated reduced tumor-infiltrating lymphocyte (TIL) density and heterogeneous PD-L1 expression in COPD-associated LC, which correlate with shorter recurrence-free survival (RFS) (18, 19).
Notably, the impact of COPD on ICI outcomes remains controversial. Whereas some studies report improved OS in COPD patients receiving ICIs (HR = 0.72) (14, 20), large multicenter datasets indicate a significant reduction in median OS (14.1 vs. 18.4 months, p = 0.032), highlighting substantial heterogeneity in COPD-mediated TIME modulation (21). Additionally, COPD-related imbalances in Th17/Treg cell ratios may increase the risk of immune-related adverse events such as checkpoint inhibitor pneumonitis and colitis (OR = 1.45) (22, 23). The effect of COPD on pathological response (e.g., pathological complete response [pCR] or major pathological response [MPR]) in the neoadjuvant setting with ICIs and chemotherapy also remains uncertain.
Given these conflicting observations and the potential interplay between COPD and immunotherapy outcomes, this meta-analysis aims to systematically evaluate the influence of COPD on both short-term (pathological response) and long-term (event-free survival [EFS] and OS) outcomes in LC patients undergoing neoadjuvant ICI-based therapy, while exploring pertinent molecular mechanisms underlying such effects.
2 Materials and methods
2.1 Search strategy
This systematic review and meta-analysis were conducted in strict accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (24), focusing on evaluating the survival synergy of comorbid chronic obstructive pulmonary disease (COPD) in lung cancer patients undergoing ICIs therapy. To ensure methodological rigor, two independent investigators systematically searched PubMed, Embase, CNKI, and the Cochrane Library for studies published from database inception to March 31, 2025. The search strategy employed the following key concepts and their variants:”Lung Cancer”or”Lung Neoplasms”or”Non-Small Cell Lung Cancer”or “NSCLC”or”Small Cell Lung Cancer”or “Pulmonary Disease, Chronic Obstructive”or”Chronic Obstructive Pulmonary Diseases”or”COPD”or”COAD”or”Chronic Obstructive Airway Disease”or”Chronic Airflow Obstructions”and”Immune Checkpoint Inhibitors”or”PD-1 Inhibitors”or”PD-L1 Inhibitors”or”CTLA-4 Inhibitors”or “ICI Therapy”.
2.2 Inclusion and exclusion criteria
Inclusion Criteria: (1)Lung cancer patients confirmed by histopathological diagnosis with comorbid COPD (diagnosed according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines (25) or pulmonary function tests); (2)Receiving ICIs monotherapy or combination regimens (e.g., ICIs combined with chemotherapy, radiotherapy); (3) Prospective or retrospective clinical studies investigating the impact of COPD on survival outcomes in patients treated with ICIs; (4)Direct or indirect reporting of survival endpoints, including but not limited to OS, PFS, hazard ratios (HR), and 95% confidence intervals (CI), or accessible Kaplan-Meier curve data for HR extraction.
Exclusion Criteria: (1)Studies that did not stratify lung cancer patients by COPD status or reported only systemic inflammatory markers (e.g., CRP, IL-6) without COPD-specific data; (2)Studies including patients receiving non-ICIs therapies (e.g., targeted therapy, chemotherapy/radiotherapy alone) without subgroup analysis for ICIs-treated cohorts; (3)Case reports, conference abstracts, preclinical studies (e.g., animal experiments, cell models), reviews, or commentary articles; (4)Studies lacking sufficient data to extract HR and 95% CI for survival outcomes (even after contacting authors) or duplicate publications (retaining the most recent or largest cohort).
2.3 Data extraction and quality assessment
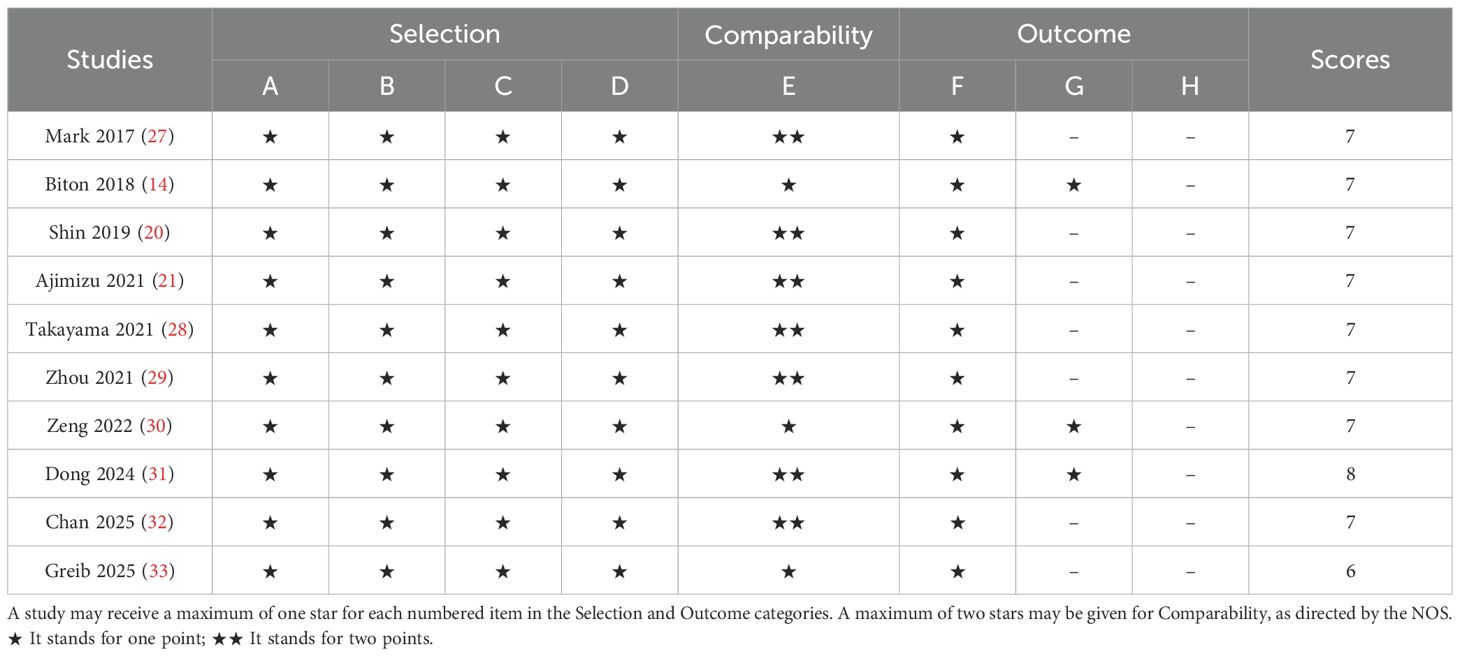
Data extraction was performed independently by two researchers, who extracted the following information from the included studies: first author, publication year, country, study design (retrospective/prospective), histological type of lung cancer (NSCLC/SCLC), diagnostic criteria for COPD (GOLD staging or pulmonary function indices), sample size (COPD group vs. non-COPD group), baseline patient characteristics (median age, gender distribution), immunotherapy regimens (PD-1/PD-L1 monotherapy or combination therapy), follow-up period (months), survival outcomes (OS, PFS), and their corresponding hazard ratios (HR) with 95% confidence intervals (CI). Study quality was assessed using the Newcastle-Ottawa Scale (NOS) for observational studies, focusing on three domains:(1)Selection of participants (0–4 points): Whether COPD (e.g., confirmed by pulmonary function tests) and lung cancer histology were clearly defined, and whether confounding factors (e.g., smoking history, COPD severity) were controlled via matching or multivariate analysis.(2)Comparability between groups (0–2 points): Balance in prognostic factors (e.g., age, cancer stage, PD-L1 expression) between COPD and non-COPD groups.(3)Outcome assessment (0–3 points): Blinding in survival endpoint adjudication and follow-up completeness (lost-to-follow-up rate <20%). The total score ranges from 0 to 9. Studies scoring above 6 points were classified as high quality (26). Any discrepancies were resolved through discussion or consultation with a third researcher.
2.4 Statistical methods
This meta-analysis employed Stata SE (version 16.0; StataCorp, Texas, USA) to statistically evaluate the survival synergy between chronic obstructive pulmonary disease (COPD)-lung cancer immunoaxis interactions and clinical outcomes in patients receiving ICIs. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated through stratification. Heterogeneity across studies was assessed using Cochran’s Q-test and I² statistics. A random-effects model was applied for pooled analysis when significant heterogeneity existed (I² > 50% or Q-test p-value < 0.10), while a fixed-effects model was used otherwise. Publication bias was preliminarily evaluated via funnel plot symmetry testing, further validated by Egger’s regression analysis and Begg’s rank correlation test (p-values < 0.05 indicated potential bias). To enhance methodological rigor, sensitivity analyses (iteratively excluding individual studies) were performed to test the stability of the pooled results, thereby confirming the robustness of the survival synergy between dynamic changes in the COPD-lung cancer immunoaxis and ICIs therapeutic efficacy.
3 Results
3.1 Study selection and characteristics
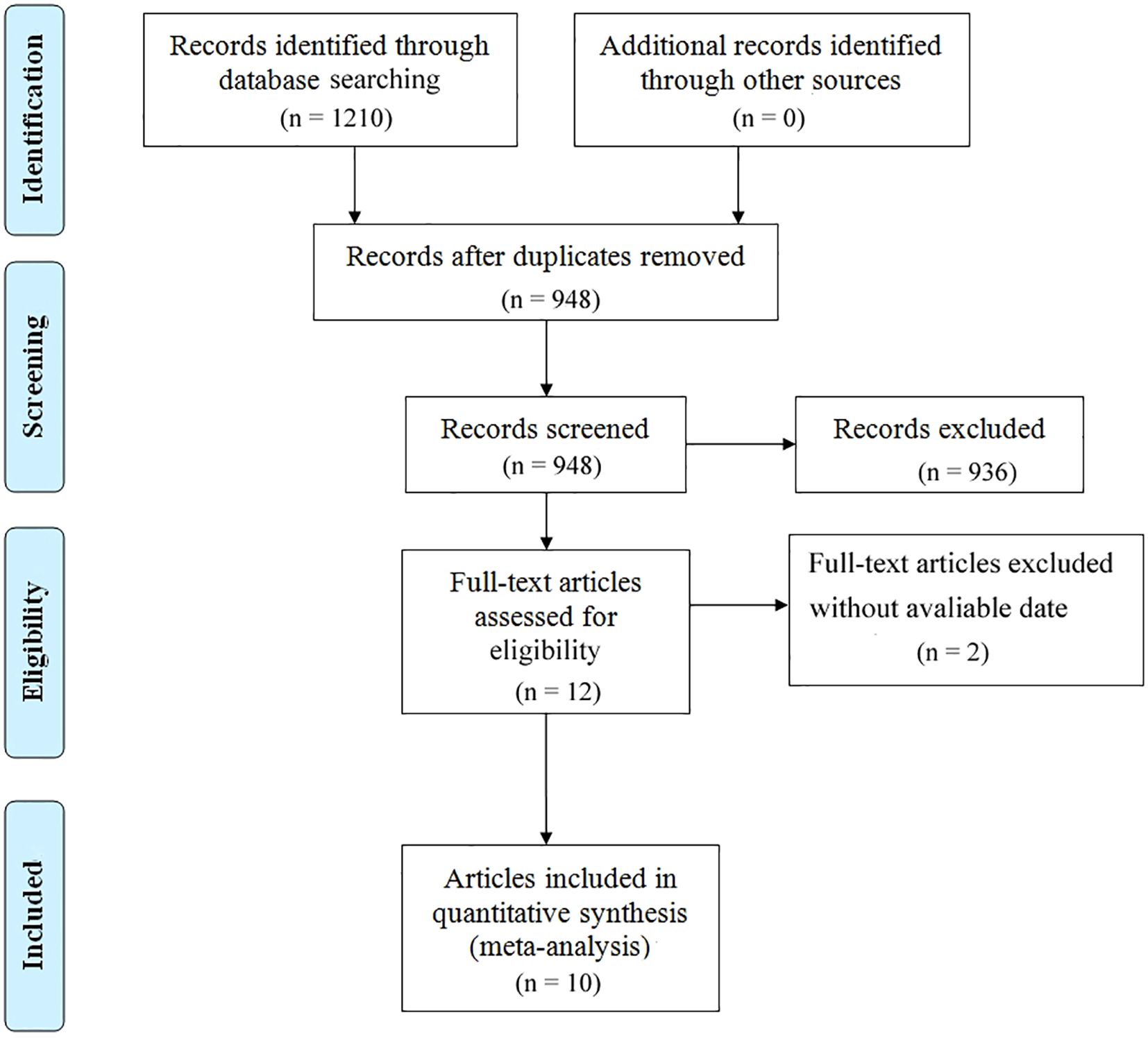
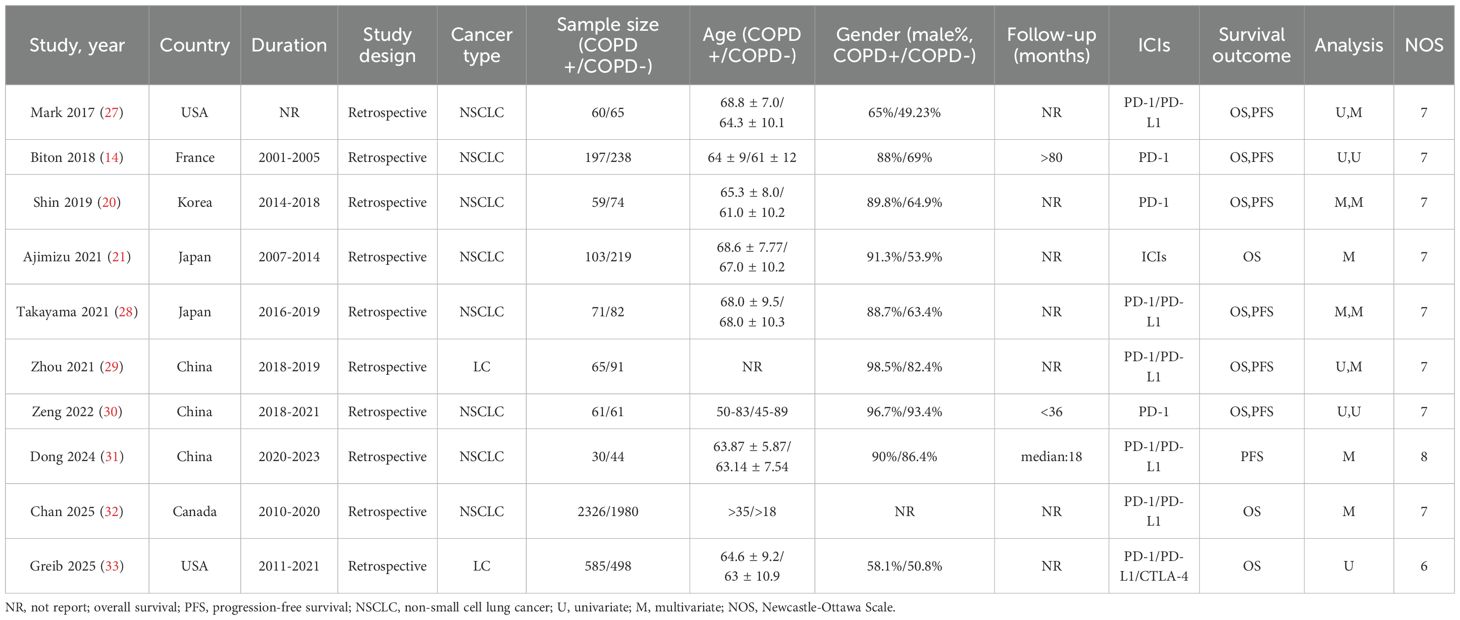
The literature selection protocol is detailed in Figure 1. An initial search across multiple academic databases yielded 1,210 records. Following the removal of 262 duplicates, 948 unique articles were retained for preliminary evaluation. Titles and abstracts of these records were systematically reviewed using predefined eligibility criteria, leading to the exclusion of 936 entries deemed irrelevant or non-compliant. Subsequently, full-text assessments were conducted on the remaining 12 articles, of which 2 were excluded due to insufficient data availability. In the final phase, 10 high-quality studies were incorporated into the meta-analysis (14, 20, 21, 27–33). A comprehensive overview of the selected studies is provided in Figure 1. The included studies, spanning publication years from 2017 to 2025, were conducted across diverse geographic regions, including the United States, Japan, China, France, Korea, and Canada. Cohort sizes exhibited substantial variation, ranging from 30 participants (Dong 2024) to 2,326 patients (Chan 2025), collectively representing multiple cancer subtypes, predominantly non-small cell lung cancer (NSCLC). Among these investigations, six studies evaluated survival outcomes (OS and/or PFS) in relation to PD-1/PD-L1 inhibitors, while three analyses incorporated combination therapies involving CTLA-4 inhibitors. Temporal follow-up periods ranged from a median of 18 months to over 80 months, with five studies reporting incomplete follow-up data (marked as NR). Detailed demographic characteristics, including age distributions (mean ± SD: 50–83 years) and gender disparities (male predominance: 53.9%–98.5% across cohorts), are systematically cataloged in Table 1. As shown in Table 2, the methodological quality assessed using the Newcastle-Ottawa Scale (NOS) was consistently high, with eight of the included studies scoring 7/9 and one study obtaining the maximum score of 8.
3.2 Association of COPD with OS and PFS
Heterogeneity test results indicated that neither OS (Figure 2A: I² = 29.6%, p = 0.182) nor PFS (Figure 2B: I² = 0.0%, p = 0.957) analyses reached significant heterogeneity thresholds (I² < 50%, p > 0.1), supporting the use of a fixed-effects model for data integration. The pooled results from 9 studies (OS) and 7 studies (PFS) demonstrated significant differences in survival outcomes between lung cancer patients with COPD and those without COPD following PD-1/PD-L1 inhibitor therapy. For OS analysis (Figure 2A), the hazard ratio (HR) for the COPD group was 0.90 (95% CI: 0.85–0.96, p < 0.001), indicating a statistically significant improvement in OS compared to the non-COPD group. Individual study HR distributions revealed that Shin 2019 (HR = 0.51, 95% CI: 0.28–0.90) and Greib 2025 (HR = 0.85, 95% CI: 0.75–0.97) contributed notably to the overall effect, accounting for 1.09% and 22.45% of the weight, respectively. Notably, the confidence intervals for Biton 2018 (HR = 0.62, 95% CI: 0.28–1.37) and Takayama 2021 (HR = 0.57, 95% CI: 0.27–1.20) crossed the null value (HR = 1), suggesting potential limitations in sample size or follow-up duration (>80 months). For PFS analysis (Figure 2B), the COPD group exhibited a pooled HR of 0.54 (95% CI: 0.44–0.87, p < 0.001), further supporting the association between COPD and superior PFS. Among these, Dong 2024 (HR = 0.32, 95% CI: 0.11–0.93) and Takayama 2021 (HR = 0.49, 95% CI: 0.28–0.84) demonstrated the most pronounced effect sizes, contributing 4.02% and 15.05% of the weight, respectively. The exclusion of the null value (HR = 1) in all confidence intervals indicated consistent results across studies. We performed subgroup analyses based on region (Asia vs. others), sample size of the COPD-positive group (≤70 vs. >70), treatment type (PD-1 inhibitor, PD-1/PD-L1 inhibitors, or ICIs broadly), and statistical method (univariate vs. multivariate analysis) to further evaluate the impact of comorbid COPD on survival outcomes among lung cancer patients treated with immune checkpoint inhibitors. As shown in Table 3, subgroup analysis of overall survival (OS) indicated that patients with COPD showed particularly improved outcomes in the following subgroups: those from non-Asian regions (HR = 0.91, p = 0.004), those in studies with larger sample sizes (COPD+ >70, HR = 0.92, p = 0.007), those receiving PD-1 inhibitor monotherapy (HR = 0.58, p = 0.004), and those analyzed using univariate methods (HR = 0.83, p = 0.001).Similarly, Table 4 shows that progression-free survival (PFS) was significantly improved among COPD patients across all subgroups, with especially pronounced benefits observed in Asian populations (HR = 0.54, p < 0.001), studies with smaller sample sizes (COPD+ ≤70, HR = 0.56, p < 0.001), patients treated with PD-1 inhibitors (HR = 0.52, p = 0.001), and those analyzed using multivariate methods (HR = 0.56, p < 0.001).
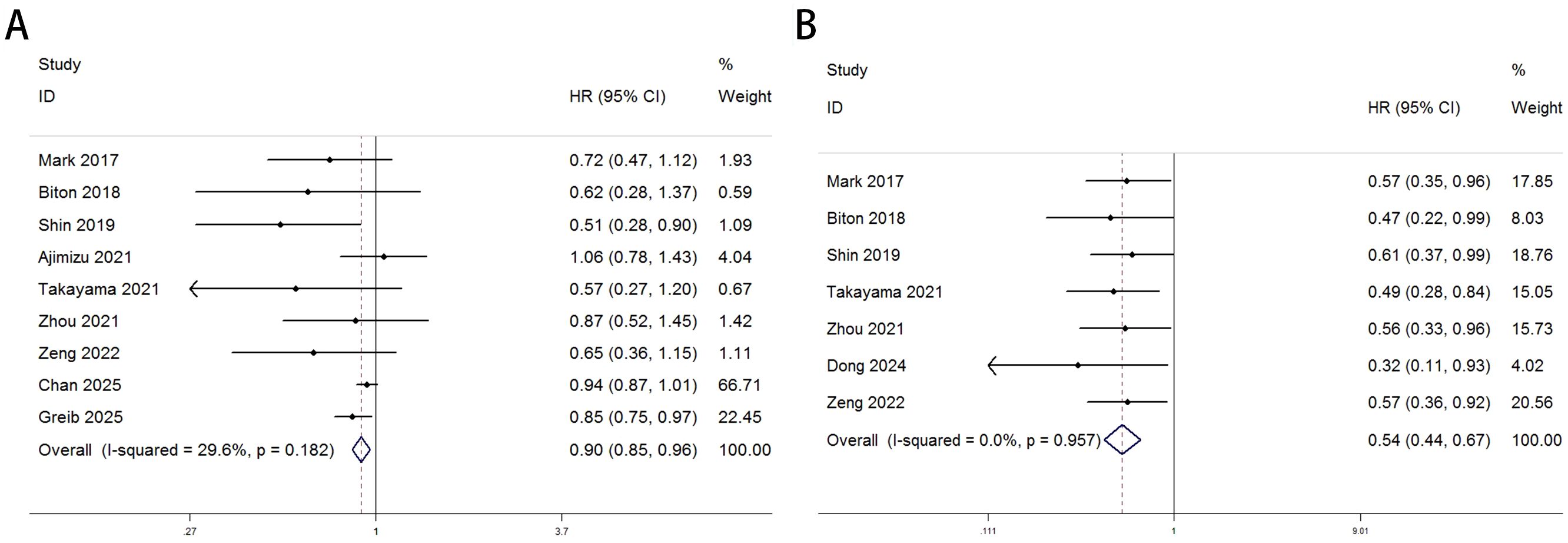
Figure 2. Forest plots delineate the association between COPD and survival outcomes in lung cancer patients treated with ICIs [pOS: (A); PFS: (B)].
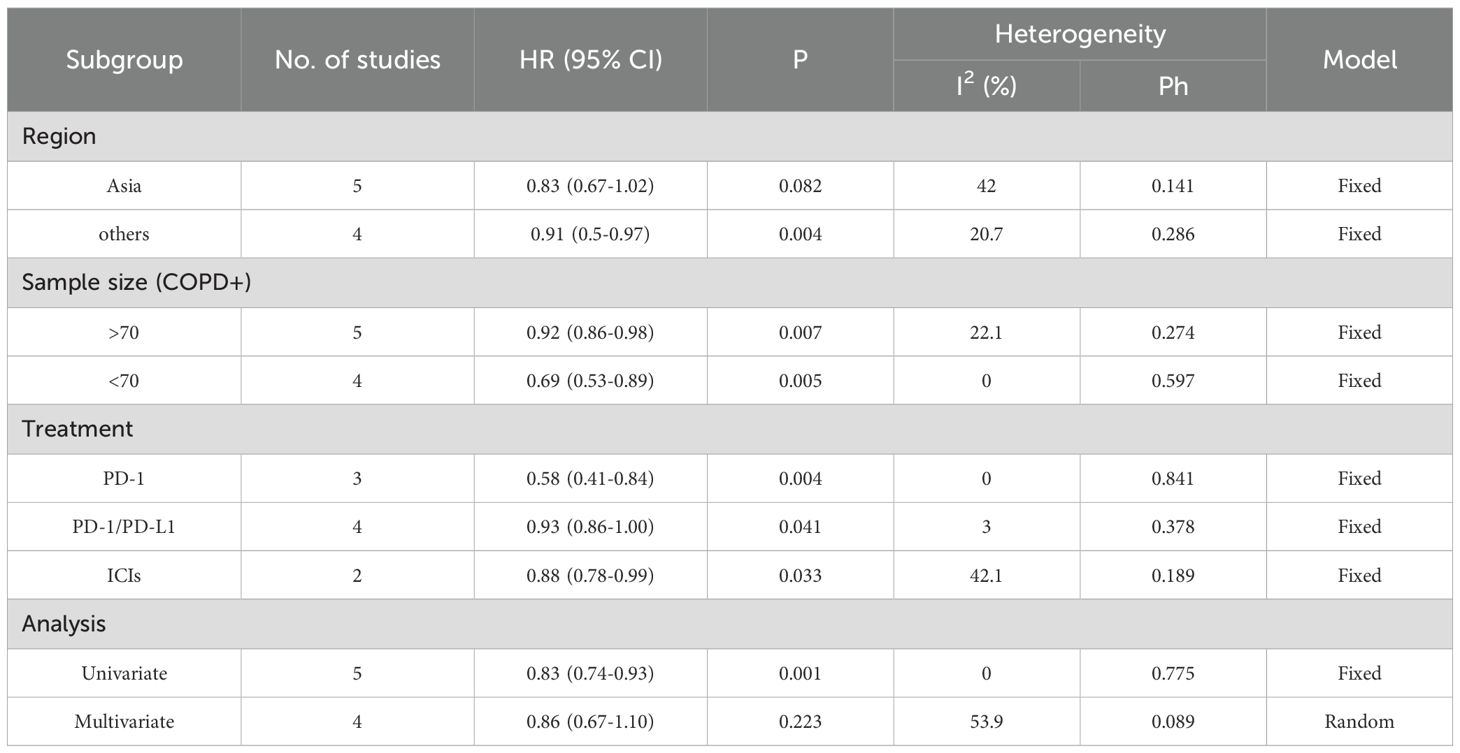
Table 3. Subgroup analysis of OS in the COPD-lung cancer immunoaxis meta-analysis of patients treated with ICIs.
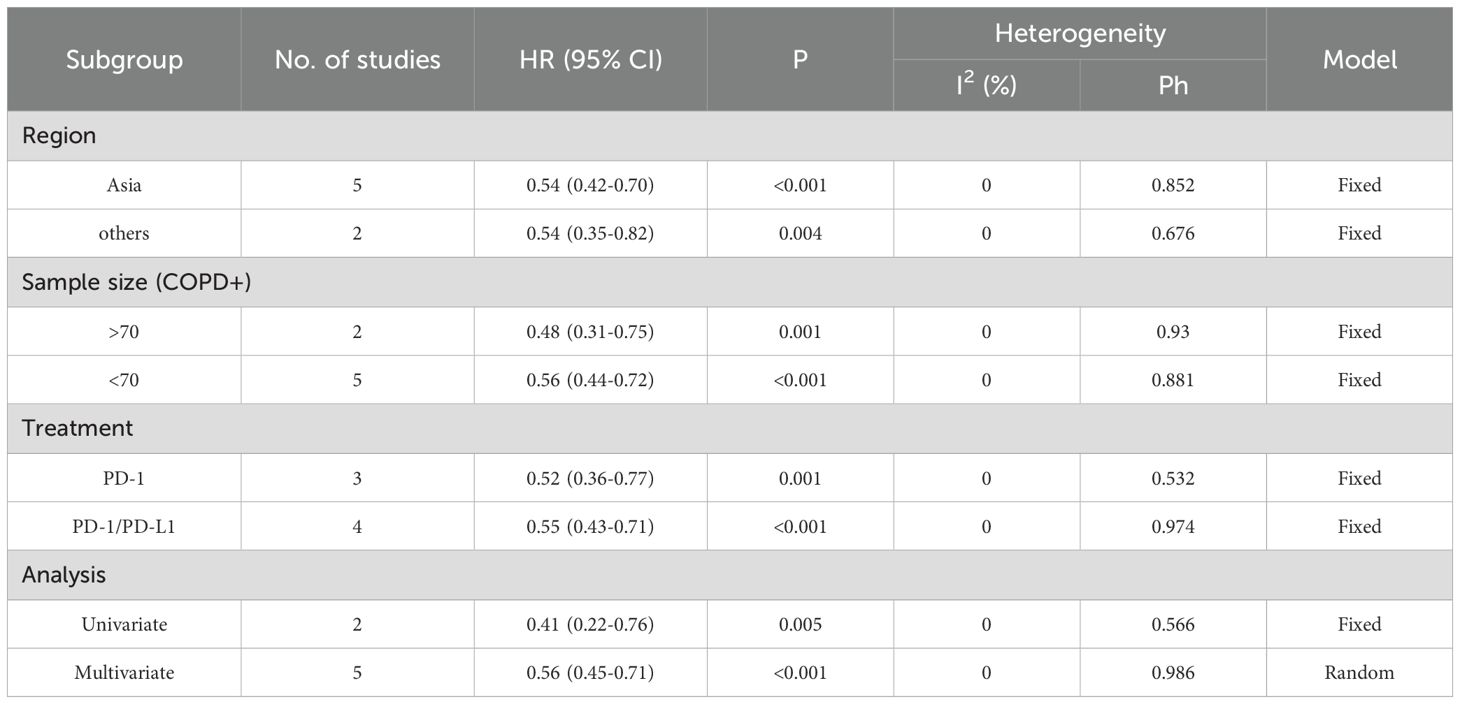
Table 4. Subgroup analysis of PFS in the COPD-lung cancer immunoaxis meta-analysis of patients treated with ICIs.
3.3 Publication bias
Publication bias was rigorously assessed using funnel plots, Egger’s test, and Begg’s test. The funnel plots for both OS and PFS demonstrated high symmetry, indicating that the robustness of the pooled results was not significantly affected by bias. For OS (Figure 3A), the funnel plot showed a symmetric distribution of effect sizes around the pooled estimates, suggesting no substantial publication bias. Similarly, the PFS funnel plot (Figure 3B) exhibited balanced dispersion of data points, further supporting the absence of significant bias.
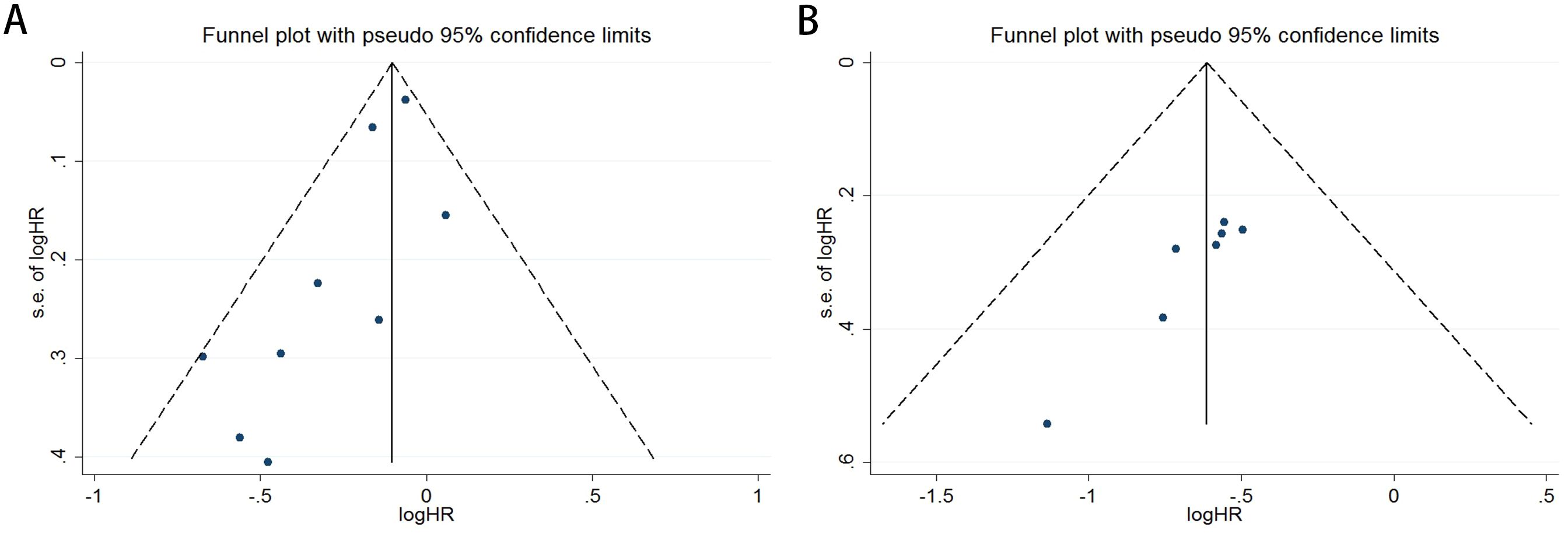
Figure 3. Funnel plots illustrate the assessment of publication bias for the association between COPD and survival outcomes in lung cancer patients receiving ICIs therapy [OS: (A); PFS: (B)].
The Begg’s rank correlation test demonstrated no significant publication bias in the OS analysis of lung cancer patients with comorbid COPD receiving ICIs (p = 0.118 > 0.05; Figure 4A). However, the Begg’s test results for PFS revealed significant publication bias in the PFS analysis (p= 0.016 < 0.05; Figure 4B). For OS, the results of Egger’s regression test aligned with Begg’s rank correlation test (p=0.504>0.05), further supporting the absence of significant publication bias (Figure 5A). In contrast, for PFS, Egger’s test corroborated Begg’s test findings (p=0.003<0.05), confirming the presence of statistically significant publication bias (Figure 5B).
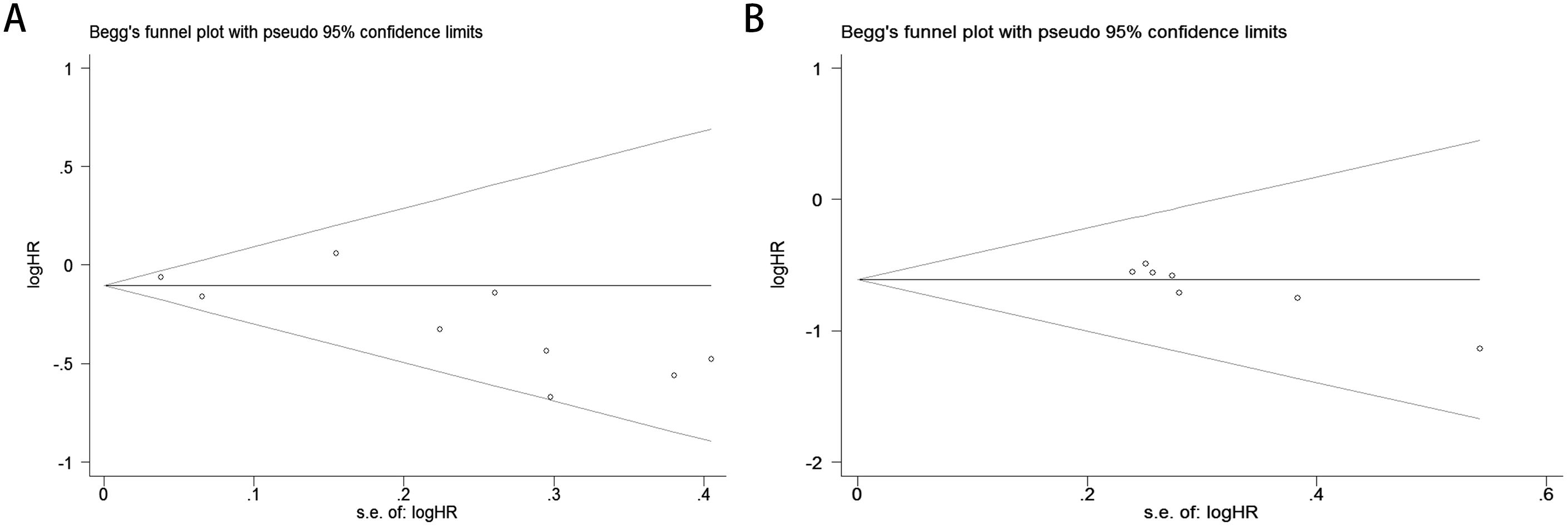
Figure 4. Publication bias assessment. (A) Begg’s test for OS (p = 0.118); (B) Begg’s test for PFS (p = 0.016).
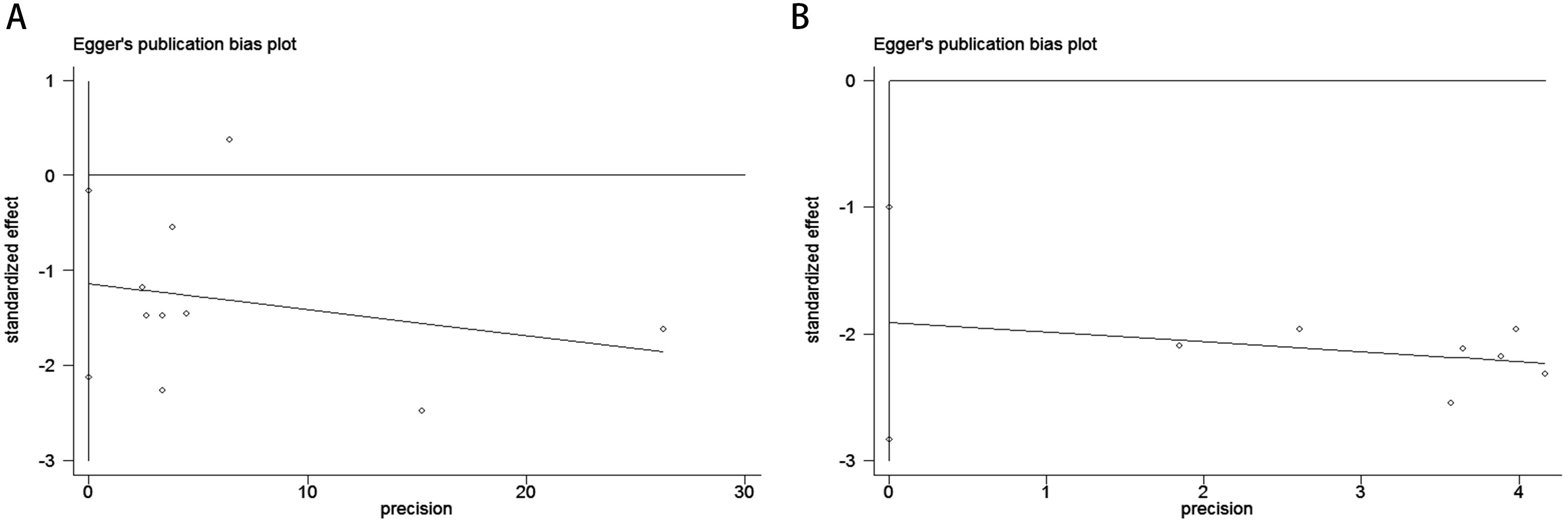
Figure 5. Publication bias assessment. (A) Egger’s test for OS (p = 0.504); (B) Egger’s test for PFS (p = 0.003).
3.4 Sensitivity analysis
The sensitivity analysis revealed that omitting specific studies (e.g., Chan 2025 or Greib 2025 with HR = 0.98) might significantly reduce the pooled HR for OS, suggesting a dilution effect of these studies on the overall results and potentially weakening the survival benefit conclusion of ICIs in the current meta-analysis (Figure 6A). In contrast, for PFS, even after excluding outliers (e.g., Mark 2017 with HR = 0.42), the pooled HR remained stable within the 0.44–0.70 range without crossing the null threshold (HR = 1). All studies consistently demonstrated significant PFS improvement, validating the robust and uniform efficacy of ICIs in delaying disease progression (Figure 6B).
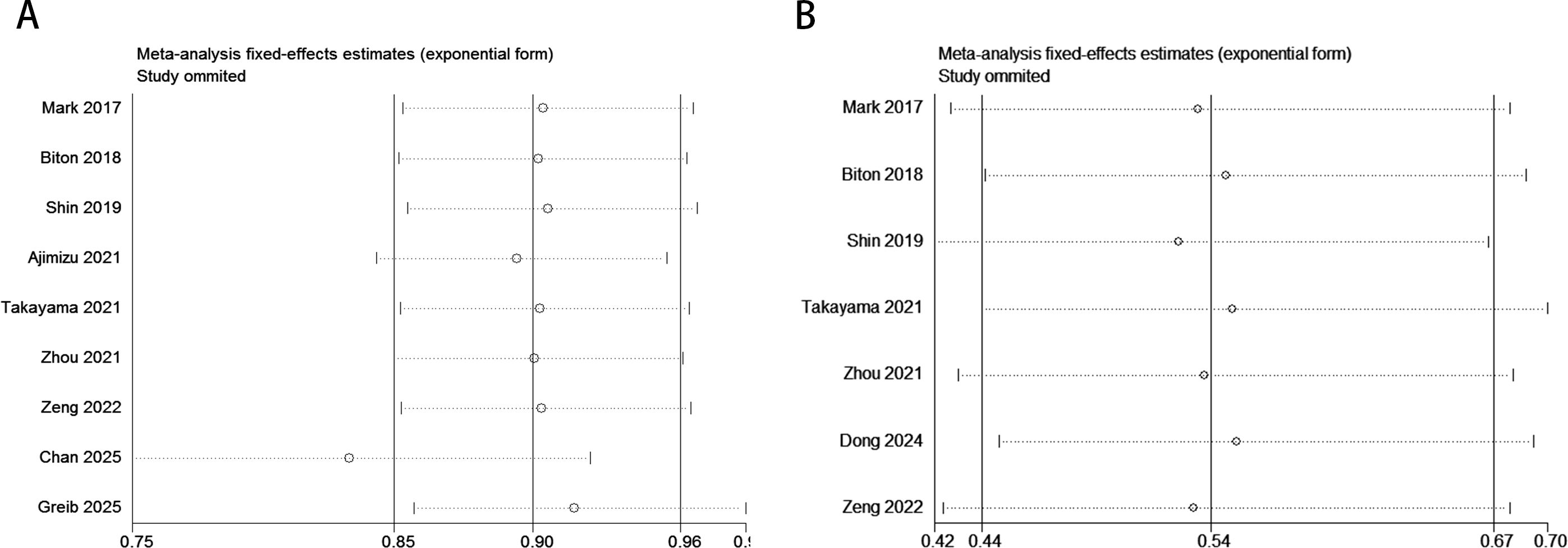
Figure 6. Sensitivity analysis evaluating the robustness of pooled survival outcomes (OS, PFS) in the COPD-lung cancer immunoaxis meta-analysis for patients treated with ICIs. [(A): OS; (B): PFS].
4 Discussion
Lung cancer and COPD are two of the most prevalent and closely related respiratory diseases worldwide (34, 35). COPD, characterized by persistent airflow limitation and chronic inflammation, is an independent risk factor for lung cancer, with both sharing pathogenic pathways such as smoking exposure and dysregulated immune responses (36). Notably, patients concurrently suffering from COPD and lung cancer exhibit extremely poor prognosis, underscoring the urgency to elucidate their biological interplay. Immune checkpoint inhibitors, which target PD-1/PD-L1 or CTLA-4 pathways to harness the host immune system against malignancies, have revolutionized lung cancer treatment (25, 37). However, significant heterogeneity in clinical responses to ICIs has driven researchers to explore potential biomarkers and comorbidities influencing therapeutic efficacy.
Meta-analysis of OS revealed that lung cancer patients with comorbid COPD had a 10% reduced mortality risk after receiving ICIs (HR = 0.90, 95% CI: 0.85–0.96, p<0.05), with the confidence interval not crossing the null value (HR = 1), indicating statistical significance. PFS analysis showed COPD patients had a remarkable 46% reduction in disease progression risk (HR = 0.54, 95% CI: 0.44–0.67, p<0.001), with the confidence interval far from 1 and minimal heterogeneity (I²=0%, p=0.957), suggesting high consistency across studies. Subgroup analysis further demonstrated that PD-1 monotherapy achieved optimal outcomes for both OS (HR = 0.58) and PFS (HR = 0.52), while PD-1/PD-L1 combination regimens showed weaker benefits, implying monotherapy may be more suitable for COPD populations. Geographical analysis indicated significant PFS benefits in Asia (HR = 0.54), whereas OS did not reach statistical significance (HR = 0.83), potentially due to differences in subsequent therapies or insufficient follow-up. Univariate analysis overestimated COPD’s effect (e.g., OS univariate HR = 0.83 vs. multivariate HR = 0.86), emphasizing the need to control confounders like PD-L1 expression and smoking history. Despite publication bias risks (particularly for PFS), results support that COPD may enhance ICI efficacy through chronic inflammatory microenvironments, though further validation of true effects and optimization of stratification strategies (e.g., COPD subtypes, combined biomarker detection) are needed for personalized treatment. Publication bias analysis revealed critical limitations: OS bias paradox: Begg’s test showed no significant bias (adjusted P = 0.118), but Egger’s test intercept was statistically significant (P = 0.029), suggesting small-sample studies (e.g., Shin 2019 HR = 0.51) might overreport survival benefits, slightly inflating effect estimates. PFS bias significance: Both Begg’s (adjusted P = 0.016) and Egger’s tests (P = 0.003) confirmed substantial publication bias, implying the pronounced PFS benefit (HR = 0.54) may be amplified by selective reporting of positive outcomes. Potential explanations include: (1) Selective publication: PFS, as a secondary endpoint, is more susceptible to “positive result prioritization,” while OS, influenced by subsequent therapies, tolerates negative outcomes better. (2) Endpoint characteristics: OS, confounded by survival status and later-line treatments, may dilute bias effects, whereas PFS, as an early endpoint with subjective measurements (e.g., imaging assessments) and short-term effects, is prone to selective reporting. (3) COPD heterogeneity: Unadjusted confounders (e.g., COPD severity, PD-L1 levels, smoking history) may skew effect estimates. For instance, Ajimizu 2021 (HR = 1.06) suggested certain COPD subgroups (e.g., severe emphysema) might respond poorly to ICIs, though limited sample sizes prevented significant impacts on pooled results. Comprehensive analysis indicates that despite bias risks, both outcomes support COPD’s potential to enhance ICI efficacy via immune modulation: OS: Pooled HR = 0.90 suggests modest survival benefits, though true effects may be slightly weaker, requiring long-term follow-up validation. PFS: HR = 0.54 reflects significant disease control advantages, but publication bias-induced overestimation warrants caution, necessitating expanded datasets to verify robustness. However, this study has limitations: First, COPD diagnosis in included cohorts predominantly relied on pulmonary function rather than imaging parameters, potentially missing emphysema-dominant subgroups. Second, unadjusted confounders like pre-treatment corticosteroid use may overestimate COPD’s independent effects. Finally, correlations between peripheral immune markers and tissue microenvironments require validation via spatial transcriptomics and novel technologies.
In conclusion, the immune interaction between COPD and lung cancer transcends mere pathogenetic linkage and may harbor therapeutic response clues. Redefining COPD as an “immune response modulator” rather than a mere comorbidity could unlock new dimensions in precision immunotherapy. Clinically, COPD may serve as a predictive biomarker for ICI efficacy, informing treatment decisions. Future research should prioritize dose-optimization studies for COPD-lung cancer populations, balancing immune activation and adverse event risks.
5 Conclusions
This meta-analysis demonstrates that lung cancer patients with comorbid COPD treated with ICIs exhibit significant clinical advantages in both OS and PFS. Although bias analysis suggests potential publication bias in some results, the overall trends in OS and PFS still support the core conclusion that COPD enhances ICI efficacy through immune-modulatory mechanisms. Future large-scale studies are needed to further validate the robustness of these findings and optimize individualized treatment strategies tailored to COPD subtypes (e.g., emphysema, chronic bronchitis) to balance therapeutic efficacy and safety risks.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
LC: Conceptualization, Data curation, FormalAnalysis, Methodology, Project administration, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. DS: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SH: Data curation, Funding acquisition, Project administration, Resources, Software, Validation, Writing – original draft, Writing –review & editing. AL: Conceptualization, Data curation, Project administration, Resources, Software, Visualization, Writing –original draft, Writing – review & editing. JG: Conceptualization,Data curation, Formal Analysis, Funding acquisition, Methodology,Project administration, Resources, Software, Writing – originaldraft, Writing – review & editing. LW: Data curation, Methodology, Supervision, Conceptualization, Formal Analysis, Project administration, Validation, Investigation, Funding acquisition, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Wang M, Herbst RS, and Boshoff C. Toward personalized treatment approaches for non-small-cell lung cancer. Nat Med. (2021) 27:1345–56. doi: 10.1038/s41591-021-01450-2
3. Uramoto H and Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res. (2014) 3:242–9. doi: 10.3978/j.issn.2218-6751.2013.12.05
4. Torrente M, Sousa PA, Franco F, Guerreiro G, Sousa A, Parejo C, et al. Understanding prognosis and survival outcomes in patients with early-stage non-small-cell lung cancer. Clin Med (Lond). (2022) 22:38–40. doi: 10.7861/clinmed.22-4-s38
5. Bessa CM, Silva LMD, Zamboni MM, Costa GJ, Bergmann A, Thuler LCS, et al. Bone metastasis after stage IIIA non-small cell lung cancer: risks and prognosis. J Bras Pneumol. (2022) 48:e20220211. doi: 10.36416/1806-3756/e20220211
6. Schoenfeld AJ and Hellmann MD. Acquired resistance to immune checkpoint inhibitors. Cancer Cell. (2020) 37:443–55. doi: 10.1016/j.ccell.2020.03.017
7. Reda M, Ngamcherdtrakul W, Nelson MA, Siriwon N, Wang R, Zaidan HY, et al. Development of a nanoparticle-based immunotherapy targeting PD-L1 and PLK1 for lung cancer treatment. Nat Commun. (2022) 13:4261. doi: 10.1038/s41467-022-31926-9
8. Hou S, Song D, Hao R, Li L, Zhang Y, and Zhu J. Prognostic relevance of prognostic nutritional indices in gastric or gastro-esophageal junction cancer patients receiving immune checkpoint inhibitors: a systematic review and meta-analysis. Front Immunol. (2024) 15:1382417. doi: 10.3389/fimmu.2024.1382417
9. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. (2015) 373:1627–39. doi: 10.1056/NEJMoa1507643
10. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. (2015) 373:123–35. doi: 10.1056/NEJMoa1504627
11. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. (2018) 24:541–50. doi: 10.1038/s41591-018-0014-x
12. Zhu Y, Yu X, Thamphiwatana SD, Zheng Y, and Pang Z. Nanomedicines modulating tumor immunosuppressive cells to enhance cancer immunotherapy. Acta Pharm Sin B. (2020) 10:2054–74. doi: 10.1016/j.apsb.2020.08.010
13. Narayanapillai SC, Han YH, Song JM, Kebede ME, Upadhyaya P, and Kassie F. Modulation of the PD-1/PD-L1 immune checkpoint axis during inflammation-associated lung tumorigenesis. Carcinogenesis. (2020) 41:1518–28. doi: 10.1093/carcin/bgaa059
14. Biton J, Ouakrim H, Dechartres A, Alifano M, Mansuet-Lupo A, Si H, et al. Impaired tumor-infiltrating T cells in patients with chronic obstructive pulmonary disease impact lung cancer response to PD-1 blockade. Am J Respir Crit Care Med. (2018) 198:928–40. doi: 10.1164/rccm.201706-1110OC
15. Wang X, Wu B, Yan Z, Wang G, Chen S, Zeng J, et al. Association of PTPRD/PTPRT mutation with better clinical outcomes in NSCLC patients treated with immune checkpoint blockades. Front Oncol. (2021) 11:650122. doi: 10.3389/fonc.2021.650122
16. Tang T, Huang X, Zhang G, Hong Z, Bai X, and Liang T. Advantages of targeting the tumor immune microenvironment over blocking immune checkpoint in cancer immunotherapy. Signal Transduct Target Ther. (2021) 6:72. doi: 10.1038/s41392-020-00449-4
17. Petitprez F, Meylan M, de Reyniès A, Sautès-Fridman C, and Fridman WH. The tumor microenvironment in the response to immune checkpoint blockade therapies. Front Immunol. (2020) 11:784. doi: 10.3389/fimmu.2020.00784
18. Liu CH, Chen Z, Chen K, Liao FT, Chung CE, Liu X, et al. Lipopolysaccharide-mediated chronic inflammation promotes tobacco carcinogen-induced lung cancer and determines the efficacy of immunotherapy. Cancer Res. (2021) 81:144–57. doi: 10.1158/0008-5472.CAN-20-1994
19. Calderon AA, Dimond C, Choy DF, Pappu R, Grimbaldeston MA, Mohan D, et al. Targeting interleukin-33 and thymic stromal lymphopoietin pathways for novel pulmonary therapeutics in asthma and COPD. Eur Respir Rev. (2023) 32:220144. doi: 10.1183/16000617.0144-2022
20. Shin SH, Park HY, Im Y, Jung HA, Sun JM, Ahn JS, et al. Improved treatment outcome of pembrolizumab in patients with nonsmall cell lung cancer and chronic obstructive pulmonary disease. Int J Cancer. (2019) 145:2433–9. doi: 10.1002/ijc.32235
21. Ajimizu H, Ozasa H, Sato S, Funazo T, Sakamori Y, Nomizo T, et al. Survival impact of treatment for chronic obstructive pulmonary disease in patients with advanced non-small-cell lung cancer. Sci Rep. (2021) 11:23677. doi: 10.1038/s41598-021-03139-5
22. Qi C, Sun SW, and Xiong XZ. From COPD to lung cancer: mechanisms linking, diagnosis, treatment, and prognosis. Int J Chron Obstruct Pulmon Dis. (2022) 17:2603–21. doi: 10.2147/COPD.S380732
23. Forder A, Zhuang R, Souza VGP, Brockley LJ, Pewarchuk ME, Telkar N, et al. Mechanisms contributing to the comorbidity of COPD and lung cancer. Int J Mol Sci. (2023) 24:2859. doi: 10.3390/ijms24032859
24. Moher D, Liberati A, Tetzlaff J, Altman DG, and Group P. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. J Clin Epidemiol. (2009) 62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005
25. Guo X, Chen S, Wang X, and Liu X. Immune-related pulmonary toxicities of checkpoint inhibitors in non-small cell lung cancer: Diagnosis, mechanism, and treatment strategies. Front Immunol. (2023) 14:1138483. doi: 10.3389/fimmu.2023.1138483
26. Moskalewicz A and Oremus M. No clear choice between Newcastle-Ottawa scale and appraisal tool for cross-sectional studies to assess methodological quality in crosssectional studies of health-related quality of life and breast cancer. J Clin Epidemiol. (2020) 120:94–103. doi: 10.1016/j.jclinepi.2019.12.013
27. Mark NM, Kargl J, Busch SE, Yang GHY, Metz HE, Zhang H, et al. Chronic obstructive pulmonary disease alters immune cell composition and immune checkpoint inhibitor efficacy in non-small cell lung cancer. Am J Respir Crit Care Med. (2018) 197:325–36. doi: 10.1164/rccm.201704-0795OC
28. Takayama Y, Nakamura T, Fukushiro Y, Mishima S, Masuda K, and Shoda H. Coexistence of emphysema with non-small-cell lung cancer predicts the therapeutic efficacy of immune checkpoint inhibitors. In Vivo. (2021) 35:467–74. doi: 10.21873/invivo.12280
29. Zhou J, Chao Y, Yao D, Ding N, Li J, Gao L, et al. Impact of chronic obstructive pulmonary disease on immune checkpoint inhibitor efficacy in advanced lung cancer and the potential prognostic factors. Transl Lung Cancer Res. (2021) 10:2148–62. doi: 10.21037/tlcr-21-214
30. Zeng Z, Qu J, Yao Y, Xu F, Lu S, Zhang P, et al. Clinical outcomes and risk factor of immune checkpoint inhibitors-related pneumonitis in non-small cell lung cancer patients with chronic obstructive pulmonary disease. BMC Pulm Med. (2022) 22:458. doi: 10.1186/s12890-022-02190-w
31. Dong W, Yin Y, Yang S, Liu B, Chen X, Wang L, et al. Impact of chronic obstructive pulmonary disease on the efficacy and safety of neoadjuvant immune checkpoint inhibitors combined with chemotherapy for resectable non-small cell lung cancer: a retrospective cohort study. BMC Cancer. (2024) 24:153. doi: 10.1186/s12885-024-11902-w
32. Chan SWS, Pond GR, and Goffin JR. The impact of chronic obstructive pulmonary disease on immune checkpoint inhibitor effectiveness in non-small cell lung cancer: A population health study. J Immunother. (2025) 48:138–46. doi: 10.1097/CJI.0000000000000551
33. Greib A, Zhao S, Ploch M, Henricks J, Easterling R, Moodabagil M, et al. Evaluating the effect of immune checkpoint inhibitor treatment on chronic obstructive pulmonary disease in lung cancer patients. Oncoimmunology. (2025) 14:2469375. doi: 10.1080/2162402X.2025.2469375
34. Schabath MB and Cote ML. Cancer progress and priorities: lung cancer. Cancer Epidemiol Biomarkers Prev. (2019) 28:1563–79. doi: 10.1158/1055-9965.EPI-19-0221
35. Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. (2017) 389:299–311. doi: 10.1016/S0140-6736(16)30958-8
36. Sekine Y, Hata A, Koh E, and Hiroshima K. Lung carcinogenesis from chronic obstructive pulmonary disease: characteristics of lung cancer from COPD and contribution of signal transducers and lung stem cells in the inflammatory microenvironment. Gen Thorac Cardiovasc Surg. (2014) 62:415–21. doi: 10.1007/s11748-014-0386-x
Keywords: lung cancer, non-small cell lung cancer, immune checkpoint inhibitors, overall survival, progression-free survival, meta-analysis
Citation: Chen L, Song D, Hou S, Liu A, Gao J and Wang L (2025) The impact of comorbid COPD on survival outcomes in lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Front. Immunol. 16:1627557. doi: 10.3389/fimmu.2025.1627557
Received: 13 May 2025; Accepted: 27 October 2025;
Published: 10 November 2025.
Edited by:
Christian Rolfo, The Ohio State University, United StatesCopyright © 2025 Chen, Song, Hou, Liu, Gao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Wang, d2FuZ2xlaTk5OXFxQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Lin Chen1,2†
Lin Chen1,2† Dandan Song
Dandan Song Shufu Hou
Shufu Hou