- 1Vita e Salute San Raffaele University, Medicine and Surgery, Milan, Italy
- 2Department of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy
- 3Department of Gastroenterology, Azienda Socio Sanitaria Territoriale (ASST) Grande Ospedale Metropolitano Niguarda, Milano, Italy
- 4Division of Gastroenterology, Center for Autoimmune Liver Diseases, European Reference Network of Hepatological Diseases (ERN RARE-LIVER), IRCCS Fondazione San Gerardo dei Tintori, Monza, Italy
- 5Department of Internal Medicine and Medical Therapeutics, University of Pavia, Pavia, Italy
- 6First Department of Internal Medicine, Fondazione Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Policlinico San Matteo, Pavia, Italy
- 7Gastroenterology and Endoscopy, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) San Raffaele Hospital, Milan, Italy
Background: Autoimmune gastritis (AIG) and autoimmune liver diseases (AILDs)—including autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), and primary sclerosing cholangitis (PSC)—are chronic organ-specific immune-mediated disorders. While both conditions frequently co-occur with other autoimmune diseases, the prevalence, clinical overlap, and immunological associations between AIG and AILDs remain underexplored.
Objective: To investigate the prevalence of AIG in patients with AILD and characterize the clinical, serological, and histopathological features of this overlap, to improve early detection and guide integrated management strategies.
Methods: We conducted a prospective study on 104 patients with a confirmed diagnosis of AILD. All participants were screened for anti-parietal cell antibodies (APCA); those testing positive underwent upper gastrointestinal endoscopy and gastric biopsies. Histological assessment was based on the updated Sydney System, with evaluation of mucosal inflammation, glandular atrophy, and intestinal metaplasia.
Results: APCA positivity was observed in 22.1% of AILD patients, with a female predominance (78.3%). The median age of AIG diagnosis in APCA-positive patients was 58 years. Among APCA-positive individuals, histological confirmation of AIG was achieved in 91.3%, with a high rate of intestinal metaplasia (95.7%) and variable OLGA stages of gastric atrophy. Comorbid autoimmune conditions were common, with 43.5% of APCA-positive patients also presenting with autoimmune thyroiditis. Notably, PBC was disproportionately represented in the APCA-positive subgroup (47.8%) compared to the overall cohort (39.0%).
Conclusion: This study highlights a clinically significant association between AIG and AILDs, particularly in patients with PBC and concurrent autoimmune conditions. Given the elevated risk of gastric mucosal atrophy and potential neoplastic transformation, targeted screening for AIG in AILD patients—especially those with APCA positivity or thyroid autoimmunity—should be considered. These findings underscore the importance of cross-specialty surveillance and open new avenues for research into shared immunopathogenic mechanisms.
Lay Summary: This study found that a significant number of patients with autoimmune liver diseases, especially those with primary biliary cholangitis, also show signs of autoimmune gastritis. These results support the consideration of targeted screening for gastric involvement in selected patients to improve early detection and clinical management of associated complications.
Introduction
Autoimmune diseases constitute a heterogeneous group of conditions defined by a loss of immunological tolerance to self-antigens, resulting in targeted immune-mediated destruction of host tissues. Among these, autoimmune gastritis (AIG) and autoimmune liver diseases (AILDs)—including autoimmune hepatitis (AIH), primary biliary cholangitis (PBC), and primary sclerosing cholangitis (PSC)—represent typical forms of organ-specific autoimmunity, distinguished by chronic progressive inflammation and a substantial risk of long-term morbidity (1–4). Although each disease entity demonstrates distinct pathophysiological features, these conditions may share overlapping immunological mechanisms, genetic predispositions, and serological profiles, suggesting a potential pathophysiological link (4–7). AILD represents a major clinical challenge due to its progressive nature, often culminating in cirrhosis, liver failure, or the need for liver transplantation (8). These conditions frequently coexist with other autoimmune disorders, reflecting the systemic nature of immune dysregulation in affected patients (9).
AIG is a chronic inflammatory condition characterized by immune-mediated destruction of the gastric parietal cells, leading to atrophy of the oxyntic mucosa, decreased acid secretion, and ultimately to malabsorption and vitamin B12 deficiency (10–14). It is frequently associated with other autoimmune conditions, particularly autoimmune thyroiditis, type 1 diabetes, and vitiligo—forming part of the autoimmune polyendocrine syndrome (APS) spectrum (15). Moreover, AIG can result in several complications, including intestinal metaplasia, gastric atrophy, and neoplastic conditions (16). Even if the risk of developing gastric cancer remains debatable (17, 18), it is recognized as a condition of possible long-term neoplastic complications such as neuroendocrine neoplasms (gNENs) (19). Due to these risks, AIG is a condition that requires individualized endoscopic surveillance to monitor the progression of atrophy and the development of neoplastic changes (20–22).
Although the link between AILD and AIG, is not completely clarified and the prevalence of AIG in AILD patients has not been extensively studied, the literature suggests significant clinical overlap (10, 23–25). Shared features include similar autoantibody profiles and HLA-associated genetic susceptibility factors (26), as demonstrated by insights from genome-wide association studies (27). Furthermore, the frequent coexistence of autoimmune conditions, such as thyroid disease, Sjögren’s syndrome, and type 1 diabetes, for both AILD and AIG, highlights the systemic nature of these disorders (28, 29). This underscores the systemic nature of autoimmunity and the need for an integrated diagnostic and therapeutic approach.
This study aims to address this gap by investigating the prevalence and characteristics of AIG in a cohort of patients with AIH, PSC, and PBC, assessing whether patients with AILD are at increased risk of developing AIG and offering novel insights that may refine clinical surveillance protocols, facilitate earlier diagnosis, and inform the development of more targeted therapeutic strategies.
Methods
Study design, setting, and participants
This prospective cohort study was conducted at the IRCCS San Gerardo Dei Tintori, a tertiary care Center specializing in autoimmune diseases. All consecutive adult patients with an existing or new diagnosis of AILD, including AIH, PBC, and PSC, between January 2019 and December 2022 were considered for inclusion, provided they gave informed consent and had complete data for AIG evaluation. As part of the study protocol, all patients were tested for the presence of anti-parietal cell antibodies (APCA), which were measured using a standardized ELISA method. In our institution, APCA are not part of the routine autoantibody screening panel for AILD and was specifically tested as part of this prospective study protocol. Those who tested positive for APCA were proposed to undergo esophagogastroduodenoscopy (EGD) with biopsy to assess for the presence of AIG and gastric complications.
Patients were included if they gave their consent to participate in the study. The study complied with the Declaration of Helsinki and was approved by the Institutional Review Board (ID 4940).
Data collection
Prospective data were collected through an electronic database, which compiled comprehensive clinical, laboratory, and diagnostic information. This included demographic data (age, sex, date of birth), detailed records of liver and other autoimmune diseases, and results of autoantibody panels including APCA. Patients who tested positive for APCA underwent EGD with biopsies.
Inclusion and exclusion criteria
All adult patients (aged 18 and above) with confirmed diagnoses of AIH, PSC, or PBC based on appropriate clinical, biochemical, and histological criteria were eligible for inclusion. Patients were excluded if they did not consent to participate in the study or had incomplete data regarding the status of AIG.
Endoscopic examination
EGD was performed in all patients who tested positive for APCA, as part of the diagnostic work-up for AIG. EGD procedures were conducted using high-definition endoscopes, operated by experienced endoscopists at IRCCS San Gerardo dei Tintori.
During the examination, the gastric mucosa was carefully inspected, with particular attention to the body and fundus regions—areas typically affected in AIG. The presence of mucosal abnormalities, including pallor, visible submucosal vessels, or nodularity, was recorded. Any evidence suggestive of atrophic changes or neoplastic lesions was photo-documented.
Standardized biopsy sampling was performed according to the updated Sydney System protocol (30, 31). A minimum of five gastric biopsies were obtained from predefined sites: two from the antrum (lesser and greater curvature, approximately 3 cm from the pylorus), two from the corpus (lesser and greater curvature, approximately 8 cm from the cardia), and one from the incisura angularis (30). Additional targeted biopsies were taken in the presence of suspicious lesions or mucosal abnormalities.
Biopsy samples were fixed in formalin, embedded in paraffin, and stained with hematoxylin-eosin. Histological evaluation focused on confirming features of chronic atrophic gastritis, assessing for the presence of intestinal metaplasia, and excluding dysplasia or neoplastic transformation. H. pylori status was assessed using Giemsa staining and/or immunohistochemistry as needed.
Endoscopic findings were integrated with serological and histological data to support the diagnosis of AIG. In patients with moderate to severe atrophy or intestinal metaplasia, follow-up EGD was recommended according to current international guidelines to monitor for progression and assess neoplastic risk (20).
Histological examination
Gastric biopsies obtained during EGD from APCA-positive patients were subjected to comprehensive pathological examination using the Sydney System (30). This system standardizes the assessment of gastritis by grading and classifying the gastric mucosa based on several histological criteria: i) Inflammation: The presence and degree of inflammation were evaluated, noting the density of inflammatory cells (lymphocytes and plasma cells) in both the antral and corpus mucosa. ii) Atrophy: Atrophy was assessed by examining the loss of glandular structures in the gastric mucosa. This involves determining whether the glandular loss is focal, multifocal, or diffuse, and noting the regions (antrum, body) most affected. iii) Activity: This refers to the presence of neutrophilic activity within the glandular and surface epithelium, indicative of active inflammation. iv) H Pylori infection. v) Intestinal Metaplasia: The presence of intestinal metaplasia was recorded, characterized by the replacement of the native gastric epithelial cells with intestinal-type cells, including goblet cells. Each of these parameters was graded on a scale from 0 (absent) to 3 (severe), allowing for a quantitative assessment of the gastric mucosal status. The sum of these grades provided a comprehensive score, which could be used to classify the severity of gastritis. The results of the biopsies examination were integrated with clinical data and serological markers to aid in the comprehensive diagnosis of AIG in the context of other AILD.
Outcome measures
The primary outcome was the diagnosis of AIG, confirmed through histological examination of gastric biopsies and positive EGD findings. Secondary outcomes included the detection of gastric complications associated with AIG, including intestinal metaplasia, gastric atrophy, gNENs, and gastric cancer.
Statistical analysis
Descriptive statistics summarized the cohort characteristics. The prevalence of AIG was calculated and compared across different liver disease types using the Chi-square test.
Comparisons between patients with and without AIG were performed using chi-square tests or Fisher’s exact tests for categorical variables, and t-tests or Mann-Whitney U tests for continuous variables.
A p-value of less than 0.05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism version 6.00 for Windows, GraphPad Software, California USA, www.graphpad.com.
Results
Overall AILD cohort
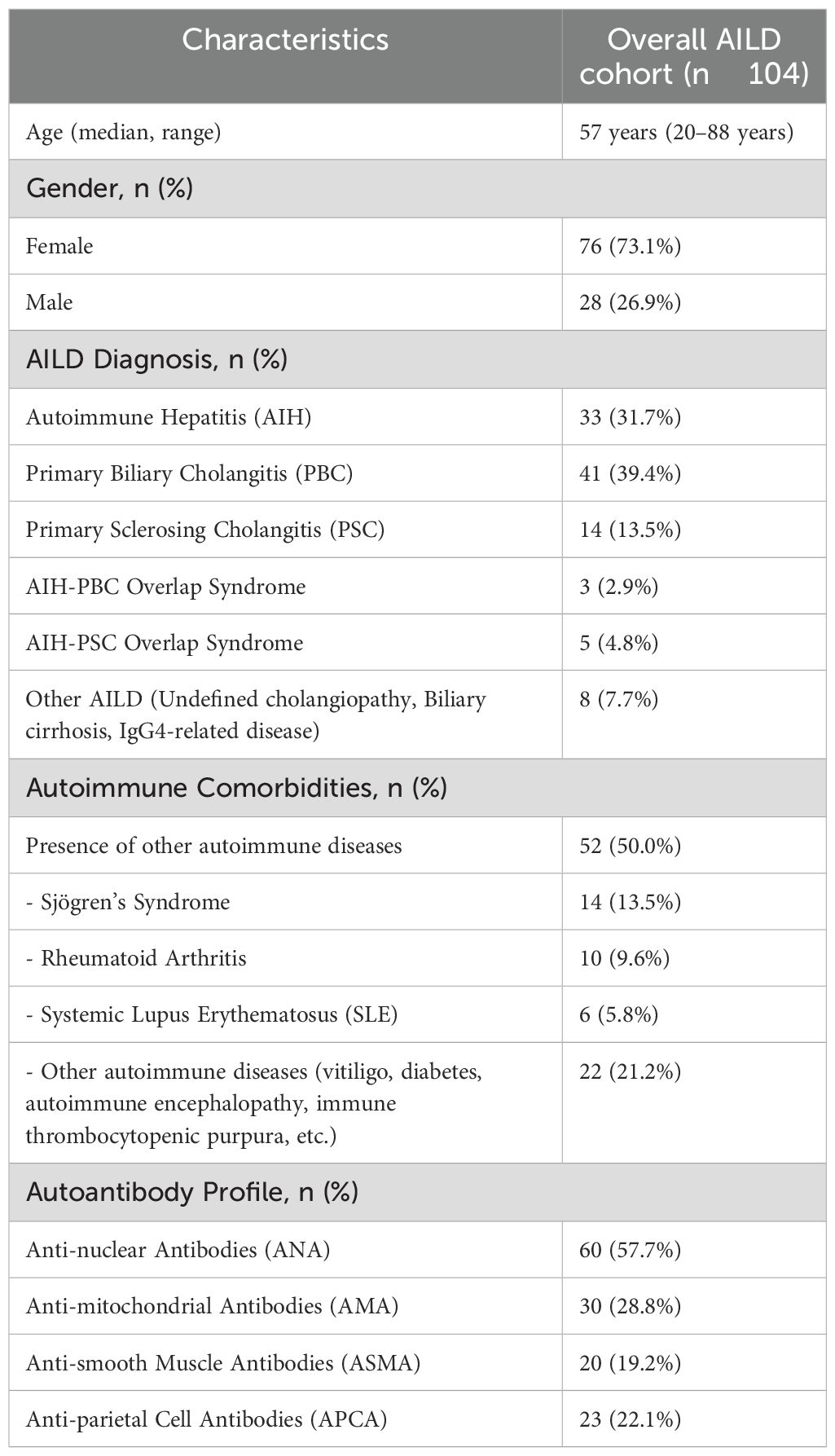
A total of 104 patients with AILD who were consecutively evaluated at IRCCS San Gerardo Dei Tintori, Monza, Italy, were enrolled in the study.
Among these patients, 76 (73.1%) were female and 28 (26.9%) were male, with a mean age of 52 years (range 18–75 years) (Table 1).
PBC was the most prevalent condition, diagnosed in 41 patients (39.4%), followed by AIH in 33 patients (31.7%), while PSC was present in 14 patients (13.5%). Three patients (2.9%) had an overlap picture of AIH-PBC. Five patients (4.8%) had an overlap syndrome of AIH-PSC.
Eight patients (7.7%) had other AILDs, further classified as: Undefined cholangiopathy in 4 patients (3.8%); Biliary cirrhosis in 1 patient (1.0%); Undefined primary/secondary sclerosing cholangitis in 1 patient (1.0%); IgG4-related disease in 2 patients (1.9%).
52 patients (50% of the cohort) had comorbid autoimmune conditions beyond their liver disease. The most frequently reported were Sjögren’s Syndrome in 14 patients, Rheumatoid Arthritis in 10 patients, and Systemic Lupus Erythematosus (SLE) in 6 patients. Other specific autoimmune conditions (such as vitiligo, diabetes, autoimmune encephalopathy, and immune thrombocytopenic purpura) were documented in 22 patients. The presence of other autoimmune markers varied, with antinuclear antibodies (ANA) found in 60 patients (57.7%), anti-mitochondrial antibodies (AMA) in 30 (28.8%), and anti-smooth muscle antibodies (ASMA) in 20 (19.2%).
All patients were tested for APCA.
APCA-positive population
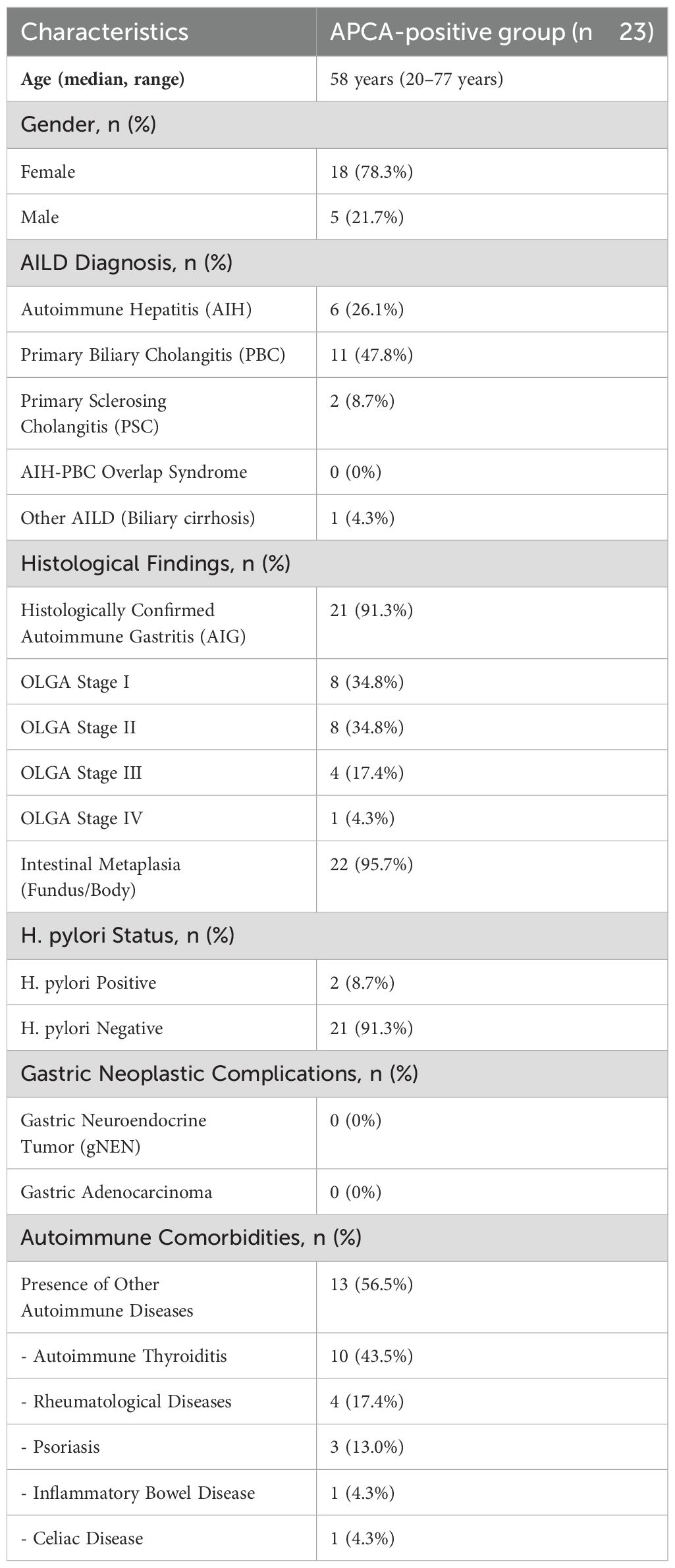
Out of the 104 patients, 23 (22.1%) tested positive for APCA (Table 2). The majority of these patients were female, accounting for 78.3% (18/23), while 21.7% (5/23) were male. The mean age of the cohort was 55.5 years (range: 20–77 years).
In terms of liver disease diagnoses, PBC was the most prevalent condition, identified in 11/23 patients (47.8%). AIH was diagnosed in 6/23 (26.1%) of the APCA-positive group, while PSC was present in 2/23 patients (8.7%) of the cohort. No patients presented with an overlap syndrome of AIH and PBC or PSC-PBC. One patient (4.3%) was diagnosed with advanced undefined biliary cirrhosis.
In the APCA-positive cohort, 21 patients (91.3%) were confirmed to have histological atrophic gastritis, primarily affecting the body and fundus of the stomach. The severity of atrophic changes was assessed using the OLGA staging system, with the following distribution: Stage I: 8 patients; Stage II: 8 patients; Stage III: 4 patients; Stage IV: 1 patient (Figure 1).
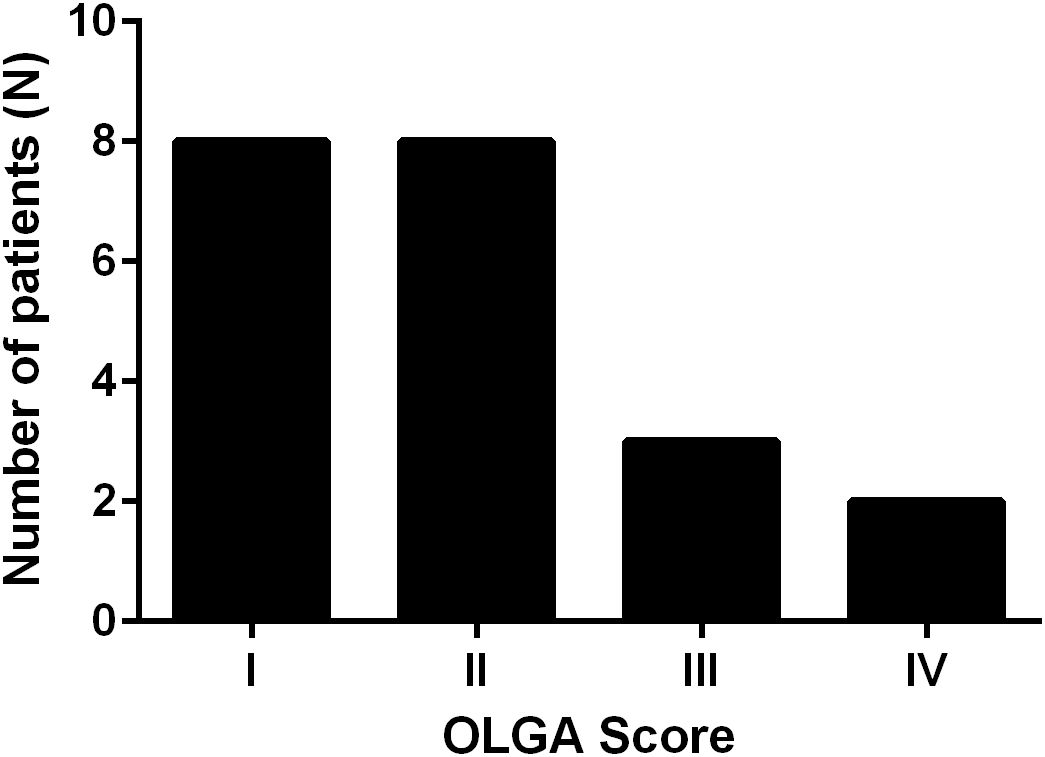
Figure 1. Distribution of OLGA (Operative Link on Gastritis Assessment) stages among APCA-positive patients. Most patients presented with early-stage atrophic gastritis (Stages I–II), while a smaller proportion exhibited advanced stages (III–IV).
Intestinal metaplasia was detected in 22 patients (95.7%) in the fundus/body and in none of the patients in the antrum. Two patients (8.7%) were found to be H. pylori-positive at the time of histological evaluation, while the remaining 21 patients (91.3%) were H. pylori-negative. Both H. pylori-positive patients underwent successful eradication therapy.
In the APCA-positive cohort, 13 patients (56.5%) exhibited comorbid autoimmune conditions.
The most commonly reported comorbidities were thyroid disease in 10 patients (43.5%), rheumatological diseases in four patients (17.4%), psoriasis in three (13.0%), inflammatory bowel disease in one (4.3%), and one patient (4.3%) had celiac disease.
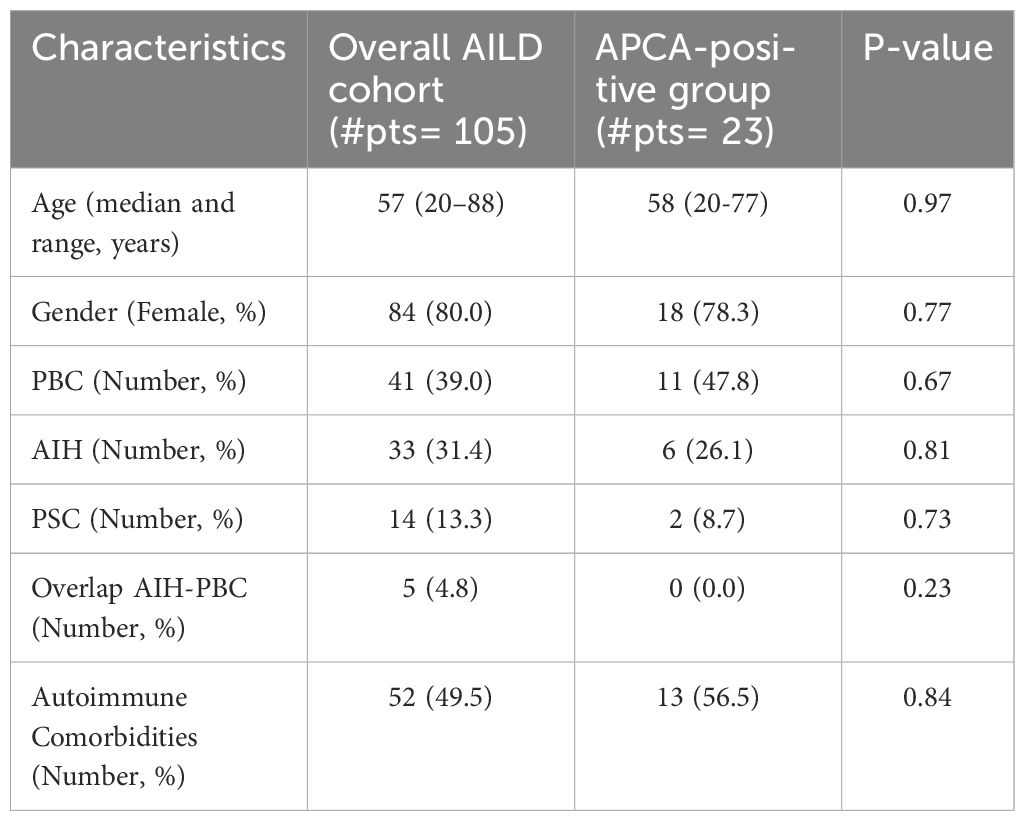
When comparing the APCA-positive group to the overall AILD cohort, there were no statistically significant differences in age, gender, or autoimmune comorbidities. There was a slight difference, even if not statistically significant, in the distribution of AILD etiologies: PBC was more prevalent in the APCA-positive group (47.8%) compared to the overall AILD cohort (39.0%), while PSC was less prevalent in the APCA-positive group (8.7%) compared to the overall AILD cohort (13.3%) (Table 3).
Gastric complications
Among the APCA-positive patients, no cases of gNENs or gastric adenocarcinoma were identified during the study period.
Discussion
This study provides additional insights into the association between AILD and AIG, evaluated by the prevalence of APCA in patients with AILD. We found that 22.1% of the AILD cohort tested positive for APCA, indicating a noteworthy overlap between these two autoimmune conditions.
This aligns with preliminary findings reporting a similarly high prevalence of histologically confirmed AIG in AILD patients (32). Only other few case reports are published in Literature, showing the association between AIG and AILD (33–36).
Our findings support the hypothesis that AIG and AILD may share common autoimmune mechanisms and genetic predispositions, as previously suggested by other studies (33). The observed female predominance in APCA-positive patients (78.3%) is consistent with the broader epidemiological trend in autoimmune diseases (11). Also, the median age of APCA-positive patients was 58 years, aligning with the overall AILD cohort (57 years). Interestingly, the distribution of liver diseases within the APCA-positive group differed slightly from the overall AILD cohort. We noted indeed that PBC was relatively more frequent among APCA-positive patients compared to the overall AILD cohort. Only one preliminary cohort has reported similar trends, with PBC showing stronger associations with autoimmune gastritis compared to other liver autoimmune diseases (32). For context, the prevalence of PCA positivity in the general population is approximately 2–8%, increasing with age and more common in women (37). Moreover, in unselected PCA-positive individuals, the prevalence of histologically confirmed AIG is about 50–60%, with intestinal metaplasia being considerably less common than the 95.7% observed in our APCA-positive AILD cohort (38, 39). This suggests that AILD patients with PCA positivity may represent a higher-risk group for both AIG and intestinal metaplasia.
Notably, a significant proportion (56.5%) of AILD/APCA-positive patients exhibited other autoimmune comorbidities, including thyroid disease (43.5%), rheumatological diseases (17.4%), and psoriasis (13.0%). Thyroid disease, in particular, was the most common autoimmune comorbidity in this group (43.5%), consistent with previous studies showing that up to 40–50% of patients with AIG or atrophic gastritis have coexisting autoimmune thyroid diseases (40–42). Given this strong association, clinicians should consider routine screening for thyroid dysfunction in patients with AILD who test positive for APCA, independently from which type of AILD. This is supported by multiple studies showing a significantly increased prevalence of thyroid dysfunction among patients with AILD, especially PBC (43, 44). In line with this, a study by Liaskos et al. found that 31.8% of PBC patients had gastric parietal cell antibodies, a significantly higher prevalence than in other liver diseases, suggesting a distinct immunological profile for PBC in this context (45). Similarly, in the context of autoimmune gastritis, the strong bidirectional relationship with autoimmune thyroid disease—commonly referred to as “thyrogastric syndrome”—is well documented (46, 47). These associations reinforce the rationale for implementing targeted screening strategies in APCA-positive AILD patients, particularly those with PBC or concurrent thyroid autoimmunity.
In terms of gastric pathology, a high percentage of APCA-positive patients (91.3%) had histological evidence of atrophic gastritis affecting the body and fundus of the stomach. The OLGA staging system revealed varying degrees of severity, with most patients classified as Stage I or II, though a small number exhibited more advanced atrophy (Stage III and IV). Intestinal metaplasia was detected in 95.7% of patients, a finding that underscores the need for regular endoscopic surveillance (20) to monitor for neoplastic transformation. H. pylori status was assessed in all APCA-positive patients. Two patients (8.7%) were found to be H. pylori-positive at the time of histological assessment, while the remaining 21 patients (91.3%) were H. pylori-negative. In both H. pylori-positive patients, eradication therapy was successfully offered and completed. However, these patients exhibited corpus-restricted atrophic gastritis, showed oxyntic gland loss, and were APCA-positive, supporting the hypothesis that the H. pylori infection may have represented a superinfection on a background of AIG rather than being the primary driver of gastritis. This observation aligns with emerging evidence suggesting that H. pylori infection and autoimmune gastritis can occasionally coexist, particularly in the early phases of disease (48).
Importantly, none of the patients in this cohort were found to have gastric cancer or gNENs. This finding must be interpreted with caution due to the relatively small sample size and the cross-sectional design, which inherently limits the duration of clinical follow-up. Neoplastic transformation in AIG is known to occur over a prolonged period, and its absence at a single time point does not eliminate the possibility of future malignancy. This is supported by a long-term prospective study that found that 8.5% of patients with AIG developed neoplastic complications during the observation period (16, 49). Similarly, a meta-analysis by Chen et al. calculated an annual incidence of 0.14% for gastric cancer and 0.83% for type 1 gNENs in patients with autoimmune metaplastic atrophic gastritis, emphasizing the cumulative neoplastic risk over time (50). However, the association between AIG and gastric cancer remains controversial and is not yet fully established (51). In contrast, the link between AIG and gNENs is more consistently reported (19), although these tumors typically have a favorable prognosis. Therefore, the role of routine AIG screening in AILD patients as well as the decision to pursue gastric surveillance in this setting should be carefully individualized, and universal screening cannot currently be recommended. Further longitudinal studies with extended follow-up and larger AIG/AILD patient populations are needed to accurately assess the incidence of neoplastic risk in these patients.
The lack of significant statistical differences between the APCA-positive group and the overall AILD cohort in terms of age, gender, and autoimmune comorbidities suggests that the presence of APCA is not necessarily associated with more severe liver disease or a broader autoimmune profile. This is consistent with findings in autoimmune thyroid disease (ATD), where APCA positivity does not correlate strongly with gender or disease severity (52).
This study has several limitations that should be acknowledged. First, the relatively small sample size of the APCA-positive group (n=23) limits the statistical power and generalizability of the findings. Second, the study design introduces a potential work-up bias, as only APCA-positive patients underwent upper gastrointestinal endoscopy. Consequently, cases of AIG may have gone undetected among APCA-negative individuals, potentially underestimating the true prevalence of AIG in the broader AILD population. Similar limitations have been reported in prior studies assessing the overlap between autoimmune gastritis and liver autoimmunity, where selective screening restricted the accurate estimation of disease burden (16). To overcome these limitations, future research should probably implement a standardized endoscopic screening protocol across all AILD patients, irrespective of serologic status, to enable a more accurate characterization of AIG prevalence and its clinical implications.
Moreover, the cross-sectional design of this study limits the ability to establish temporal relationships between the onset of AILD, AIG, and associated autoimmune comorbidities. As a result, causality and disease progression patterns remain unclear. Longitudinal studies are necessary to determine whether APCA-positive patients face increased long-term risks, particularly concerning gastric neoplasms or the progression of liver pathology. Prior long-term data suggest that AIG-related complications, including gastric neuroendocrine tumors and atrophic progression, may take years to manifest, reinforcing the need for prospective follow-up (16).
In addition to observational data, future research should focus on elucidating the genetic and immunological mechanisms underpinning the overlap between AIG and AILD. Genome-wide association studies (GWAS) have already identified shared susceptibility loci across multiple autoimmune diseases, including variants in HLA regions, CTLA4, and IL2RA, suggesting common immunoregulatory pathways (27). Identifying disease-specific or shared biomarkers could facilitate risk stratification, earlier diagnosis, and tailored therapeutic approaches.
Finally, it remains to be determined whether immunomodulatory treatments commonly used in AILD—such as corticosteroids, azathioprine, or ursodeoxycholic acid—have a protective, neutral, or detrimental effect on the development or progression of AIG. Clarifying these therapeutic interactions could further support an integrated management strategy for patients with overlapping autoimmune phenotypes.
Conclusion
In conclusion, this study highlights a significant overlap between AILDs and AIG, with a substantial proportion of AILD patients testing positive for APCA. The high prevalence of thyroid disease and other autoimmune comorbidities in APCA-positive patients underscores the need for a multidisciplinary approach to their management. Given the risks of gastric atrophy and intestinal metaplasia, cautious individualized evaluation for endoscopic surveillance may be considered in these patients, particularly in those with additional risk factors. Universal screening cannot currently be recommended based on our data and requires further confirmation in larger, longitudinal studies. Further research is needed to clarify the mechanisms linking AIG and AILD and to develop more targeted strategies for the diagnosis and management of these complex autoimmune conditions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by CET Lombardia 3- Telefono 039 233 6880 Email ZGlyZXppb25lLnNjaWVudGlmaWNhQGlyY2NzLXNhbmdlcmFyZG8uaXQ=. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SM: Conceptualization, Project administration, Formal Analysis, Supervision, Methodology, Writing – review & editing, Investigation, Writing – original draft. GD: Investigation, Writing – original draft, Data curation. AR: Data curation, Writing – original draft, Investigation. LC: Data curation, Investigation, Writing – original draft. ML: Conceptualization, Validation, Writing – review & editing. AG: Writing – review & editing, Investigation. AE: Investigation, Data curation, Writing – original draft. MC: Writing – review & editing, Investigation. AB: Writing – original draft, Data curation, Investigation. AD: Writing – review & editing, Conceptualization. SD: Supervision, Conceptualization, Writing – review & editing. PI: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision
The handling editor HM declared a past co-authorship with the author PI.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Trivedi PJ, Hirschfield GM, Adams DH, and Vierling JM. Immunopathogenesis of primary biliary cholangitis, primary sclerosing cholangitis and autoimmune hepatitis: themes and concepts. Gastroenterology. (2024) 166:995–1019. doi: 10.1053/j.gastro.2024.01.049
2. McNally BB and Carey EJ. Cholestatic liver diseases: modern therapeutics. Expert Rev Gastroenterol Hepatol. (2025), 1–6. doi: 10.1080/17474124.2025.2473490
3. Heneghan MA and Lohse AW. Update in clinical science: Autoimmune hepatitis. J Hepatol. (2025). doi: 10.1016/j.jhep.2024.12.041
4. Ronca V, Davies SP, Oo YH, and Lleo A. The immunological landscape of primary biliary cholangitis: Mechanisms and therapeutic prospects. Hepatology. (2025). doi: 10.1016/j.reumae.2025.501812
5. Almánzar Cortés JS, Vergara Cabra C, Uchima-Vera MP, Quintana G, and Sierra F. Rheumatological diseases in patients with autoimmune hepatitis in a fourth level hospital in Bogotá between 2013 and 2023. Reumatol Clin (Engl Ed). (2025) 501812:1225–36. doi: 10.1007/s10067-024-07273-z
6. Liang P, Huang Y, Hu Z, Zhou L, Cai S, Zhong J, et al. Clinical and laboratory characteristics of Sjögren’s syndrome-associated autoimmune liver disease: a real-world, 10-year retrospective study. Clin Rheumatol. (2025) 44:1225–36. doi: 10.1007/s10067-024-07273-z
7. Wang F, Chen L, and Tian Y. Immune traits in combination with inflammatory proteins revealing the pathogenesis of autoimmune liver diseases: A Mendelian randomization study. Cytokine. (2025) 185:156815. doi: 10.1016/j.cyto.2024.156815
8. Carbone M and Neuberger JM. Autoimmune liver disease, autoimmunity and liver transplantation. J Hepatol. (2014) 60:210–23. doi: 10.1016/j.jhep.2013.09.020
9. Anaya JM. The diagnosis and clinical significance of polyautoimmunity. Autoimmun Rev. (2014) 13:423–6. doi: 10.1016/j.autrev.2014.01.049
10. Massironi S, Zilli A, Elvevi A, and Invernizzi P. The changing face of chronic autoimmune atrophic gastritis: an updated comprehensive perspective. Autoimmun Rev. (2019) 18:215–22. doi: 10.1016/j.autrev.2018.08.011
11. Lenti MV, Rugge M, Lahner E, Miceli E, Toh BH, Genta RM, et al. Autoimmune gastritis. Nat Rev Dis Primers. (2020) 6:56. doi: 10.1038/s41572-020-0187-8
12. Toh BH. Diagnosis and classification of autoimmune gastritis. Autoimmun Rev. (2014) 13::459–62. doi: 10.1016/j.autrev.2014.01.048
13. Lenti MV, Hammer HF, Tacheci I, Burgos R, Schneider S, Foteini A, et al. European consensus on malabsorption-UEG & SIGE, LGA, SPG, SRGH, CGS, ESPCG, EAGEN, ESPEN, and ESPGHAN. Part 1: definitions, clinical phenotypes, and diagnostic testing for malabsorption. United Eur Gastroenterol J. (2025). doi: 10.1002/ueg2.70012
14. Lenti MV, Hammer HF, Tacheci I, Burgos R, Schneider S, Foteini A, et al. European consensus on malabsorption-UEG & SIGE, LGA, SPG, SRGH, CGS, ESPCG, EAGEN, ESPEN, and ESPGHAN: part 2: screening, special populations, nutritional goals, supportive care, primary care perspective. United Eur Gastroenterol J. (2025). doi: 10.1002/ueg2.70011
15. Bjørklund G, Pivin M, Hangan T, Yurkovskaya O, and Pivina L. Autoimmune polyendocrine syndrome type 1: Clinical manifestations, pathogenetic features, and management approach. Autoimmun Rev. (2022) 21:103135. doi: 10.1016/j.autrev.2022.103135
16. Miceli E, Lenti MV, Gentile A, Gambini G, Petrucci C, Pitotti L, et al. Long-term natural history of autoimmune gastritis: results from a prospective monocentric series. Am J Gastroenterol. (2024) 119:837–45. doi: 10.14309/ajg.0000000000002619
17. Rugge M, Genta RM, Malfertheiner P, and Graham DY. Gastric cancer risk in autoimmune gastritis: evidence versus opinion. Gut. (2024) 73:555–6. doi: 10.1136/gutjnl-2023-329618
18. Lenti MV, Broglio G, and Di Sabatino A. Unravelling the risk of developing gastric cancer in autoimmune gastritis. Gut. (2023) 72:1429–30. doi: 10.1136/gutjnl-2022-328345
19. Massironi S, Gallo C, Elvevi A, Stegagnini M, Coltro LA, and Invernizzi P. Incidence and prevalence of gastric neuroendocrine tumors in patients with chronic atrophic autoimmune gastritis. World J Gastrointest Oncol. (2023) 15:1451–60. doi: 10.4251/wjgo.v15.i8.1451
20. Shah SC, Piazuelo MB, Kuipers EJ, and Li D. AGA clinical practice update on the diagnosis and management of atrophic gastritis: expert review. Gastroenterology. (2021) 161:1325–32.e7. doi: 10.1053/j.gastro.2021.06.078
21. Dinis-Ribeiro M, Libânio D, Uchima H, Spaander MCW, Bornschein J, Matysiak-Budnik T, et al. Management of epithelial precancerous conditions and early neoplasia of the stomach (MAPS III): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG) and European Society of Pathology (ESP) Guideline update 2025. Endoscopy. (2025). doi: 10.1055/a-2529-5025
22. Morgan DR, Corral JE, Li D, Montgomery EA, Riquelme A, Kim JJ, et al. ACG clinical guideline: diagnosis and management of gastric premalignant conditions. Am J Gastroenterol. (2025). doi: 10.14309/ajg.0000000000003350
23. Vanderlocht J, van der Cruys M, Stals F, Bakker-Jonges L, and Damoiseaux J. Multiplex autoantibody detection for autoimmune liver diseases and autoimmune gastritis. J Immunol Methods. (2017) 448:21–5. doi: 10.1016/j.jim.2017.05.003
24. Kashiwagi Y, Hatsushika T, Tsutsumi N, Go S, Nishimata S, and Kawashima H. Gastrointestinal and liver lesions in primary childhood Sjögren syndrome. Clin Rheumatol. (2017) 36:1433–5. doi: 10.1007/s10067-017-3599-4
25. Levine J. The impact of immune dysregulation on the development of autoimmune gastrointestinal and liver disease. Curr Probl Pediatr Adolesc Health Care. (2014) 44:322–3. doi: 10.1016/j.cppeds.2014.10.001
26. Liaskou E, Hirschfield GM, and Gershwin ME. Mechanisms of tissue injury in autoimmune liver diseases. Semin Immunopathol. (2014) 36:553–68. doi: 10.1007/s00281-014-0439-3
27. Mells GF, Kaser A, and Karlsen TH. Novel insights into autoimmune liver diseases provided by genome-wide association studies. J Autoimmun. (2013) 46:41–54. doi: 10.1016/j.jaut.2013.07.004
28. Teufel A, Weinmann A, Kahaly GJ, Centner C, Piendl A, Wörns M, et al. Concurrent autoimmune diseases in patients with autoimmune hepatitis. J Clin Gastroenterol. (2010) 44:208–13. doi: 10.1097/mcg.0b013e3181c74e0d
29. Efe C, Purnak T, and Ozaslan E. Concurrent autoimmune thyroid diseases in patients with autoimmune hepatitis. J Clin Gastroenterol. (2010) 44:660–1. doi: 10.1097/mcg.0b013e3181d7b1ac
30. Dixon MF, Genta RM, Yardley JH, and Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. . Am J Surg Pathol. (1996) 20:1161–81. doi: 10.1097/00000478-199610000-00001
31. Moon RH, Puttock EJ, Chen W, Luong TQ, and Wu BU. Association of endoscopic biopsy sampling methods with detection of precursor lesions of gastric cancer. Gastrointest Endosc. (2024) 99:204–13.e5. doi: 10.1016/j.gie.2023.09.003
32. Mosciatti L, Coccia M, Palumbo C, Begini P, Annibale B, and Lahner EL. M. Autoimmune liver diseases and autoimmune atrophic gastritis: an overlooked association? In: Digestive and liver disease. Elsevier.
33. Katsumata R, Kamada T, Murao T, Sunago A, Suehiro M, Monobe Y, et al. A case of autoimmune gastritis and hepatitis with enlarging gastric polyps after reducing the dose of prednisolone. Case Rep Gastroenterol. (2023) 17:117–23. doi: 10.1159/000529151
34. De Block CE, De Leeuw IH, Pelckmans PA, Michielsen PP, Bogers JJ, Van Marck EA, et al. Autoimmune hepatitis, autoimmune gastritis, and gastric carcinoid in a type 1 diabetic patient: a case report. J Diabetes Complications. (2000) 14:116–20. doi: 10.1016/s1056-8727(00)00059-3
35. Toyoda M, Ichikawa M, Nakamura K, Kishikawa H, and Nishida J. Autoimmune gastritis complicated by primary biliary cholangitis: A report of two cases and literature review. Cureus. (2025) 17:e80208. doi: 10.7759/cureus.80208
36. Mabuchi S, Mabuchi H, and Watari T. Autoimmune polyglandular syndrome type 3b: A key to diagnosing autoimmune gastritis and asymptomatic primary biliary cholangitis. Eur J Case Rep Intern Med. (2025) 12:005376. doi: 10.12890/2025_005376
37. Cabrera de León A, Almeida González D, Almeida AA, González Hernández A, Carretero Pérez M, Rodríguez Pérez Mdel C, et al. Factors associated with parietal cell autoantibodies in the general population. Immunol Lett. (2012) 147:63–6. doi: 10.1016/j.imlet.2012.06.004
38. Guo X, Schreurs MWJ, Marijnissen FE, Mommersteeg MC, Nieuwenburg SAV, Doukas M, et al. Increased prevalence of autoimmune gastritis in patients with a gastric precancerous lesion. J Clin Med. (2023) 12:6152. doi: 10.3390/jcm12196152
39. De Block CE, De Leeuw IH, Bogers JJ, Pelckmans PA, Ieven MM, Van Marck EA, et al. Autoimmune gastropathy in type 1 diabetic patients with parietal cell antibodies: histological and clinical findings. Diabetes Care. (2003) 26:82–8. doi: 10.2337/diacare.26.1.82
40. Kalkan Ç and Soykan I. Polyautoimmunity in autoimmune gastritis. Eur J Intern Med. (2016) 31:79–83. doi: 10.1016/j.ejim.2016.03.025
41. Lahner E, Centanni M, Agnello G, Gargano L, Vannella L, Iannoni C, et al. Occurrence and risk factors for autoimmune thyroid disease in patients with atrophic body gastritis. Am J Med. (2008) 121:136–41. doi: 10.1016/j.amjmed.2007.09.025
42. Tozzoli R, Kodermaz G, Perosa AR, Tampoia M, Zucano A, Antico A, et al. Autoantibodies to parietal cells as predictors of atrophic body gastritis: a five-year prospective study in patients with autoimmune thyroid diseases. Autoimmun Rev. (2010) 10:80–3. doi: 10.1016/j.autrev.2010.08.006
43. Efe C, Purnak T, and Tunca H. The possible link between the thyroid and autoimmune liver diseases. Liver Int. (2010) . 30:1240. doi: 10.1111/j.1478-3231.2010.02281.x
44. Zeng Q, Zhao L, Wang C, Gao M, Han X, Chen C, et al. Relationship between autoimmune liver disease and autoimmune thyroid disease: a cross-sectional study. Scand J Gastroenterol. (2020) 55:216–21. doi: 10.1080/00365521.2019.1710766
45. Liaskos C, Norman GL, Moulas A, Garagounis A, Goulis I, Rigopoulou EI, et al. Prevalence of gastric parietal cell antibodies and intrinsic factor antibodies in primary biliary cirrhosis. Clin Chim Acta. (2010) 411:411–5. doi: 10.1016/j.cca.2009.12.012
46. Lahner E, Conti L, Cicone F, Capriello S, Cazzato M, Centanni M, et al. Thyro-entero-gastric autoimmunity: Pathophysiology and implications for patient management. Best Pract Res Clin Endocrinol Metab. (2020) 34:101373. doi: 10.1016/j.beem.2019.101373
47. Cellini M, Santaguida MG, Virili C, Capriello S, Brusca N, Gargano L, et al. Hashimoto’s thyroiditis and autoimmune gastritis. Front Endocrinol (Lausanne). (2017) 8:92. doi: 10.3389/fendo.2017.00092
48. Choudhuri J, Hall S, Castrodad-Rodriguez CA, Westerhoff M, El Jabbour T, Jain S, et al. Features that aid identification of autoimmune gastritis in a background of active helicobacter pylori infection. Arch Pathol Lab Med. (2021) 145:1536–43. doi: 10.5858/arpa.2020-0615-oa
49. Hong J, Liu Z, and Zhai H. Long-term natural history of autoimmune gastritis: results from a prospective, monocentric series. Am J Gastroenterol. (2025) 119:837–45. doi: 10.14309/ajg.0000000000002619
50. Chen C, Yang Y, Li P, and Hu H. Incidence of gastric neoplasms arising from autoimmune metaplastic atrophic gastritis: A systematic review and case reports. J Clin Med. (2023) 12:1062. doi: 10.3390/jcm12031062
51. Lahner E, Annibale B, Dilaghi E, Luciano CM, Lenti MV, Di Sabatino A, et al. Clinical and endoscopic-histological features of multifocal and corpus-restricted atrophic gastritis patients with non-cardia gastric cancer or dysplasia: A multicenter, cross-sectional study. Clin Transl Gastroenterol. (2025). doi: 10.14309/ctg.0000000000000862
Keywords: autoimmune gastritis, autoimmune liver disease, primary biliary cholangitis, anti-parietal cell antibodies, intestinal metaplasia, gastric autoimmunity
Citation: Massironi S, Dispinzieri G, Rossi A, Cristoferi L, Lenti MV, Gerussi A, Elvevi A, Carbone M, Bonfichi A, Di Sabatino A, Danese S and Invernizzi P (2025) Immunological and clinical overlap between autoimmune gastritis and autoimmune liver diseases: a prospective cohort study. Front. Immunol. 16:1628478. doi: 10.3389/fimmu.2025.1628478
Received: 14 May 2025; Accepted: 04 July 2025;
Published: 25 July 2025.
Edited by:
Hani S. Mousa, University of Cambridge, United KingdomReviewed by:
Naoki Asano, Miyagi Cancer Center, JapanDermot Gleeson, Sheffield Teaching Hospitals NHS Foundation Trust, United Kingdom
Copyright © 2025 Massironi, Dispinzieri, Rossi, Cristoferi, Lenti, Gerussi, Elvevi, Carbone, Bonfichi, Di Sabatino, Danese and Invernizzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Massironi, c2FyYS5tYXNzaXJvbmlAbGliZXJvLml0
†These authors share last authorship
 Sara Massironi
Sara Massironi Giulia Dispinzieri2,3
Giulia Dispinzieri2,3 Marco Vincenzo Lenti
Marco Vincenzo Lenti Alessio Gerussi
Alessio Gerussi Marco Carbone
Marco Carbone Silvio Danese
Silvio Danese Pietro Invernizzi
Pietro Invernizzi