- 1Department of General Surgery, Gansu Provincial Hospital, Lanzhou, China
- 2The Second Clinical School of Medicine, Lanzhou University, Lanzhou, China
- 3NHC Key Laboratory of Diagnosis and Therapy of Gastrointestinal Tumor, Gansu Provincial Hospital, Lanzhou, China
- 4Key Laboratory of Molecular Diagnostics and Precision Medicine for Surgical Oncology in Gansu Province, Gansu Provincial Hospital, Lanzhou, China
Long-term inflammatory reaction may promote gastric cancer initiation and development through multiple mechanisms. Recent studies have demonstrated that inflammatory mediators play a crucial role in the transition from gastritis to gastric cancer. Pro-inflammatory cytokines, chemokines, and other signaling molecules interact and synergistically regulate gastric epithelial cell proliferation, apoptosis, migration, and invasiveness, thereby promoting tumorigenesis. Specifically, interleukins activate immune cells, induce the secretion of inflammatory mediators, and maintain local immune responses; however, in the context of cancer, they exhibit a dual role by both enhancing anti-tumor immunity and driving tumor progression. Tumor necrosis factor amplifies immune responses by stimulating the production of pro-inflammatory cytokines, yet excessive or chronic Tumor necrosis factor activity is a hallmark of autoimmune diseases. Interferons initiate antiviral responses, modulate immune cell functions, and influence the inflammatory cascade. Chemokines primarily mediate the recruitment of immune cells to sites of infection, inflammation, or injury, but also play key roles in immune evasion and tumor immune regulation. This review summarizes the cooperative roles of these inflammatory mediators in the progression from gastritis to gastric cancer and discusses their potential as therapeutic targets. A better understanding of these mechanisms may facilitate the development of novel strategies for the prevention and treatment of gastric cancer.
1 Introduction
1.1 Inflammation and tumorigenesis
In 1863, Rudolf Virchow first proposed the connection between inflammation and cancer, suggesting that certain stimuli, along with the tissue damage and inflammation they induce, can drive cell proliferation (1, 2). The inflammatory cells and cytokines present in the TME play a crucial role in promoting tumor growth, metastasis, and modulating immune responses (3). This concept has evolved over time, and decades of research have provided further validation of this link. Chronic inflammation is considered a marker of cancer (4). Mutations contribute to tumorigenesis; however, in the majority of cases (>90%), cancer development is closely linked to chronic inflammation in some form (5).
The relationship between inflammation and tumorigenesis is both complex and deeply interconnected, with chronic inflammation widely regarded as a key factor driving tumor initiation and progression across various cancers. Inflammatory processes, whether infectious—such as H. pylori-induced gastritis (6) or hepatitis B virus-related chronic hepatitis (7)—or non-infectious (8), including autoimmune diseases and chronic tissue damage caused by environmental factors, contribute to tumorigenesis through multifaceted mechanisms (9–12). Chronic inflammation is often characterized by repeated cycles of tissue injury and repair, leading to accelerated cell proliferation, genetic mutation accumulation, disrupted signaling pathways, and diminished immune surveillance, collectively creating a conducive environment for tumor development (9, 13).
In the context of chronic inflammation, inflammatory cells such as macrophages, neutrophils, and lymphocytes release significant amounts of pro-inflammatory cytokines (e.g., IL-6, TNF-α, IL-1β), chemokines, and reactive oxygen species (ROS) or reactive nitrogen species (RNS). These mediators not only induce direct DNA damage (14) but also lead to epigenetic alterations (15, 16) that silence tumor suppressor genes or activate oncogenes. Additionally, pro-inflammatory signals activate critical intracellular pathways such as NF-κB and STAT3, which drive abnormal cell proliferation, inhibit apoptosis, and enhance the invasive and metastatic capabilities of cells (17, 18). Accumulated ROS and RNS further impair DNA repair mechanisms, heightening genomic instability and fostering conditions that facilitate the emergence of cancer cells (19, 20).
Beyond cellular effects, inflammation profoundly influences tumorigenesis by shaping the TME (21). Chronic inflammation drives ECM remodeling paving the way for tumor cell invasion and metastasis (22). Furthermore, pro-angiogenic factors like VEGF (23) secreted within the inflammatory milieu significantly promote angiogenesis, supplying tumors with essential nutrients and oxygen while enabling cancer cells to enter the circulatory system (22, 24). Chronic inflammation also weakens immune surveillance. For instance, TAMs (25) and MDSCs (26), which accumulate in inflammatory conditions, secrete immunosuppressive cytokine that dampen the activity of effector T cells, thereby aiding tumor cells in evading immune responses.
The effects of inflammation on tumorigenesis vary across tissue types and inflammation forms. Chronic inflammation is notably linked to specific cancers, such as colorectal cancer associated with chronic ulcerative colitis (27) and hepatocellular carcinoma linked to chronic hepatitis (28). Compared to acute inflammation, which may transiently activate immune defenses, chronic inflammation exerts more subtle yet persistent effects, including genomic instability, localized immune suppression, and profound alterations to the TME, thereby amplifying tumorigenic potential.In fact, not all chronic inflammatory diseases increase the risk of cancer. Some of these diseases, such as psoriasis, can even reduce the risk of cancer (29).
In conclusion, inflammation serves as a “double-edged sword” in tumorigenesis. While acute inflammation may bolster immune surveillance and eliminate abnormal cells, chronic inflammation promotes genetic mutations, activates oncogenic pathways, suppresses immune defenses, and reconfigures the TME, thereby facilitating cancer initiation and progression. Elucidating the mechanisms linking chronic inflammation to tumorigenesis will deepen our understanding of cancer biology and support the development of innovative anti-inflammatory and anticancer therapies, paving the way for more effective and personalized treatment strategies.
1.2 Inflammation and tumorigenesis
Inflammatory factors are a class of cytokines, chemical substances, or small molecules secreted by immune cells, epithelial cells, and other tissue cells during the inflammatory response (30). These factors play a critical role in regulating the immune system, promoting tissue repair, and maintaining homeostasis. However, the excessive or prolonged activation of inflammatory factors may lead to chronic inflammation, which can trigger a variety of diseases, including autoimmune diseases (31), cardiovascular diseases (32), and cancer (33).
Based on their function and chemical properties, inflammatory factors can be classified into several categories: Pro-inflammatory factors (34) enhance the inflammatory response by activating pro-inflammatory signaling pathways, resulting in tissue damage and abnormal cell proliferation. Second, anti-inflammatory factors (34) play a key role in maintaining the balance of the inflammatory response by inhibiting the production of pro-inflammatory factors and reducing tissue damage. In addition, chemokines (34) primarily function to recruit immune cells to the site of inflammation, thereby expanding the scope of the inflammatory response. The functions of inflammatory factors and their communication network are shown in Table 1 and Figure 1.

Figure 1. Schematic representation of the dynamic regulatory network of inflammatory factor secretion, cellular targeting effects, and associated molecular mechanisms. The figure was adapted from Thermo Fisher (https://www.thermofisher.cn/). Red text indicates system-related pathologies, green text denotes biological or pathological processes, and black text represents structural or molecular entities.
Inflammatory factors play a central role in the link between inflammation and cancer through various mechanisms. In GC, inflammatory factors contribute to tumorigenesis by activating signaling pathways, reshaping the TME, and suppressing immune surveillance, thus driving the entire process from early tumor formation to late-stage metastasis (98). A comprehensive understanding of the function and regulatory mechanisms of inflammatory factors will not only help elucidate the pathogenesis of GC but also provide novel insights into the development of targeted anti-inflammatory cancer therapies, laying a theoretical foundation for personalized treatment strategies.
1.3 Gastritis and GC
Gastritis broadly refers to inflammatory or reactive injury of the gastric mucosa with diverse etiologies (e.g., H. pylori, autoimmune atrophic gastritis, bile-reflux/chemical injury, eosinophilic or lymphocytic gastritis). Clinically, it is important to distinguish reactive/chemical injury from leukocyte-predominant inflammatory gastritis and to record acute versus chronic patterns and anatomic distribution (antrum-predominant, corpus-predominant, or pangastritis), which in turn influence mechanisms and risks of progression from chronic inflammation to cancer (99, 100). GC is a significant global health issue, often resulting from a multifactorial process involving genetic, environmental, and microbial factors (101, 102).
When gastritis becomes chronic, it can lead to progressive damage of the stomach lining, starting with atrophy (thinning of the gastric mucosa), followed by metaplasia (the transformation of normal cells into abnormal ones) and dysplasia (abnormal cell growth) (99). These changes are considered precursors to GC. Persistent inflammation can also lead to the accumulation of genetic mutations, disruption of normal cell signaling pathways, and the activation of pro-inflammatory factors, all of which contribute to the development of cancer. If left untreated, this chronic inflammatory process can eventually promote the transformation of normal gastric cells into malignant cancer cells, resulting in GC.
2 ILs in inflammation and cancer
ILs play a central role in inflammation by regulating the immune response and the inflammatory response (103, 104). By promoting the activation of immune cells, secreting pro-inflammatory factors, and maintaining local immune responses, they are involved in acute and chronic inflammatory processes. However, persistent or excess expression of ILs can lead to chronic inflammation and increase the risk of diseases like infectious diseases (105), cardiovascular diseases (106) and cancer (107).
In cancer, the role of ILs is even more complex. ILs can both enhance tumor immunity by modulating immune cell function in the TME (108) and drive tumor progression by promoting immune escape and tumor cell growth (109). Thus, the role of ILs in cancer is a dual one, both protective and potentially aggravating. The specific mechanism of IL in GC and gastritis is detailed in Table 2.
3 ILs
3.1 IL-1
IL-1 is a pivotal cytokine produced by various cell types, including monocytes, macrophages, and fibroblasts, primarily in two isoforms: IL-1α and IL-1β (174). We will focus primarily on IL-1β, IL-1α, and IL-1β, although the IL-1 family also includes the disease-associated cytokines IL-18, IL-33, and IL-36 (175). It serves as a central mediator in the immune and inflammatory responses, regulating immune activity (176), enhancing inflammation (177), and influencing cellular proliferation and tissue repair through the activation of multiple signaling pathways (178, 179). The involvement of IL-1 in gastritis (180), GC (115), and the TME (181) is extensive and multifaceted, playing a significant role in the pathogenesis and progression of these conditions.
3.1.1 Role of IL-1 in gastritis and GC
IL-1 is a critical mediator in the onset and progression of gastritis, especially in chronic forms, where elevated IL-1 levels amplify inflammation (182). Through activation of NF-κB, IL-1 induces the release of pro-inflammatory cytokines such as TNF-α and IL-6, exacerbating the inflammatory response (106, 183). During H. pylori infection, IL-1 promotes immune cell infiltration and gastric epithelium injury, which may exacerbate lesions and contribute to disease progression (184).
IL-1 promotes tumor growth and metastasis through a variety of mechanisms and plays an important role in GC. Such as NF-κB pathway, thereby promoting cell proliferation, survival, and metastasis (185). IL-1 also alters the TME by upregulating immune suppressive cells like T cells (186) and M2 macrophages (187), which reduces the immune response against tumors and promotes tumor growth.
3.1.2 The role of IL-1 in the TME
In both gastritis and GC, IL-1 plays a key role in the tolerance of the immune system. In gastritis, IL-1 promotes immune responses, but if dysregulated, can impair immune tolerance, leading to chronic inflammation and tissue damage. In GC, IL-1 promotes immune escape by establishing an immunosuppressive microenvironment which enables tumor cells to escape immune surveillance, making immunotherapeutic approaches difficult.
IL-1 enhances the immune response in gastritis by promoting antigen presentation through the activation of dendritic cells (188) and macrophages (189). However, excessive IL-1 can damage the gastric mucosa (114). In GC, tumors manipulate IL-1 to interfere with the presentation of antigens, weaken the immune response, and facilitate immune escape (190).
In GC in particular, IL-1 is a promising target for immunotherapy. Inhibitors of IL-1 have shown the potential to reduce the immune escape of the tumor and to increase the activity of T cells (191). However, to develop effective treatments for gastritis and GC, it is critical to balance its pro-inflammatory and immunosuppressive effects.
3.1.3 The future of IL-1
Going forward, targeted therapies targeting IL-1 are poised to become a key strategy in treating GC. Novel IL-1 inhibitors or combination therapies with other immunotherapies could be developed to more effectively regulate the TME and restore the anti-tumor function of the immune system by gaining a deeper understanding of the mechanisms by which IL-1 modulates the TME. Optimizing the efficacy of IL-1 inhibitors, improving their selectivity and exploring their potential synergistic effects with other immunotherapeutic agents are expected to be the focus of future research. For GC and other cancers associated with chronic inflammation, these advances may provide new therapeutic options.
3.2 IL-2
IL-2 plays a key role in the TME and is an important immunomodulatory factor. IL-2 maintains the immune response mainly by promoting T-cell proliferation, activation and survival, and also has a major influence on immune tolerance and immunosuppression mechanisms (192). The most important are the high affinity IL-2Rα, IL-2Rβ and IL-2Rγ (193).
3.2.1 Role of IL-2 in gastritis and GC
IL-2 helps activate T cells and NK cells, leading to effective pathogen clearance in H. pylori-infected gastritis (194). However, excess IL-2 also promotes the expansion of regulatory T cells, which interfere with the resolution of inflammation and contribute to a pro-tumor environment (195), highlighting the dual role of IL-2 in immunomodulation.
By promoting both anti-tumor immunity and immune tolerance, IL-2 plays a key role in GC. Early on, IL-2 promotes activation of effector T and NK cells, which are essential for targeting and eliminating tumor cells (117). IL-2 also stimulates T cells to proliferate, contributing to immune tolerance and cancer progression (117). This dual role of IL-2 highlights the need for a balanced immune response to effectively fight cancer and avoid immune suppression.
3.2.2 The role of IL-2 in the TME
The function of IL-2 in the TME is twofold. Especially in tumor immunotherapy, where the use of IL-2 sometimes significantly increases the therapeutic effect, IL-2 promotes the proliferation and activation of effector T cells (196) and enhances anti-tumor (197), antiviral (198) and antibacterial immune responses (199). IL-2 is also important for the expansion of regulatory T cells that maintain immune tolerance (196) and prevent autoimmune reactions by secreting immunosuppressive cytokines (192, 200) (eg, TGF-β, IL-10). Therefore, to avoid excessive immune response or immune escape, the level and role of IL-2 in the TME must be maintained at an appropriate balance.
However, immunosuppressive factors in the TME such as TGF-β and PD-L1 may block the effect of IL-2 (201). For this reason, IL-2directed immunotherapy strategies often need to be combined with other immune checkpoint inhibiting or immune-enhancing agents to optimize therapeutic efficacy. In addition, an in-depth understanding of the complex mechanisms of IL-2 action in the TME is important to improve immunotherapy, as the effects of IL-2 on the TME are also regulated by its interactions with different immune cells.
3.2.3 The future of IL-2
IL-2 has a promising future in immunotherapy, particularly for cancer, autoimmune and infectious disease. Optimizing IL-2 delivery methods to enhance its anti-tumor effects while minimizing side effects through adjustments in dosage and delivery strategies will likely be the focus of future studies. In addition, by regulating T-cell function, restoring the balance of the immune system and alleviating disease symptoms, IL-2’s role in immune tolerance represents a novel approach to the treatment of autoimmune diseases. In addition, by enhancing local immune responses and improving therapeutic outcomes, IL-2 is expected to contribute to the development of vaccines and the treatment of infectious diseases. Therefore, to pave the way for more targeted and effective immunotherapy strategies, a deeper understanding of the mechanisms of IL-2 will be critical.
3.3 IL-4
IL-4 is a key cytokine secreted by immune cells such as Th2 cells, mast cells, and eosinophils, and it plays a crucial role in regulating the TME (202). Its primary function is to drive a Th2-type immune response by promoting B cell differentiation into plasma cells, which secrete antibodies, while simultaneously suppressing Th1-type immune responses. IL-4 also has significant roles in anti-inflammatory processes (203), fostering immune tolerance (204), and facilitating immune escape mechanisms (205).
3.3.1 Role of IL-4 in gastritis and GC
Through modulation of the Th1/Th2 balance, IL-4 is a regulator of the TME in gastritis (206). In H. pylori infection, it promotes a Th2 response, reduces inflammatory cytokines such as IFN-γ, and limits gastric damage (118). IL-4 also supports B cell differentiation (207) and eosinophil recruitment (208). However, chronic expression of IL-4 can perpetuate inflammation, facilitate the persistence of H. pylori, and increase the risk of progression to GC (194).
In GC, IL-4 promotes an immunosuppressive microenvironment by polarizing M2 macrophages and promoting Treg expansion (209). This suppresses effector T and NK cell activity. IL-4 also upregulates PD-L1 in tumor cells, which impairs antigen presentation and promotes the escape of the immune system. In addition, tumor proliferation, invasion and metastasis are enhanced by IL-4-activated (210). For GC immunotherapy, targeting the IL-4 signaling pathway offers potential.
3.3.2 The role of IL-4 in the TME
IL-4 secreted by Th2 cells not only promotes the activation, proliferation, and secretion of antibodies but also suppresses the cytotoxic immune response by Th1 cells (211). In allergic diseases (212), parasitic infections (213) and the TME of tumors (214), this effect is particularly pronounced.
Stimulated by IL-4, M2 macrophages secrete immunosuppressive factors to reduce inflammatory responses while supporting tissue repair by remodeling the ECM and enhancing neovascularization (215, 216). In the TME, however, M2-type macrophages can have pro-tumorigenic effects by promoting tumor cell growth, promoting immune escape, and inhibiting the immune response (209).
IL-4 affects not only immune cells but also nonimmune cells such as fibroblasts, epithelial and endothelial cells. In chronic inflammatory and fibrotic diseases, IL-4 promotes the fibrotic process through stimulation of fibroblast proliferation and collagen secretion (217, 218).
In the TME, IL-4 has a dual role to play. On the one hand, it has a pro-tumorigenic effect by promoting the escape of the immune system and supporting the proliferation of tumor cells (219). On the other hand, IL-4 can also exert an inhibitory effect on certain tumors by modulating the activity of immune cells (220). Therapeutic strategies targeting IL-4 or its pathway have potential in antitumor immunotherapy.
3.3.3 The future of IL-4
As a key regulator of the immune system, the dual role of IL-4 in the regulation of inflammation and tumor immunity provides a broad perspective for future research and treatment. Further exploration of the IL-4 pathway, especially its interaction with other signal transduction networks, will help to elucidate its complex functions in the immune milieu. At the same time, new avenues for regulating inflammation and restoring anti-tumor immunity may be explored through the development of therapeutic strategies targeting IL-4 or its receptors, such as IL-4 antagonists, ADCs or small molecule inhibitors. Furthermore, combining IL-4 blockade strategies with existing immunotherapeutic approaches [e.g. immune checkpoint inhibitors (221) or CAR-T therapy (222)] may improve therapeutic efficacy and advance clinical intervention for gastritis, GC and other related diseases.
3.4 IL-6
IL-6 is a multifunctional inflammatory cytokine secreted by a variety of cells including macrophages, monocytes, fibroblasts and tumor cells (223). It promotes the production of acute phase proteins and the recruitment of immune cells in acute inflammation, while in chronic inflammation it can be a trigger for tissue damage and disease progression. In cancer development and progression (224), IL-6 can promote tumor cell proliferation, anti-apoptosis and angiogenesis by activating JAK/STAT3 and other signaling pathways (225, 226). At the same time, IL-6 can inhibit anti-tumor immune responses.
3.4.1 Role of IL-6 in gastritis and GC
IL-6 is a pro-inflammatory cytokine that is central to the immune response to H. pylori infection, the most common cause of gastritis (227). It promotes the recruitment of immune cells such as macrophages and neutrophils to the gastric mucosa and contributes to the activation of inflammatory pathways (227). This exacerbates tissue damage and inflammation through the release of additional inflammatory mediators. Prolonged IL-6 signaling may lead to chronic inflammation that impairs mucosal healing and promotes progression of gastritis to pre-cancerous states such as atrophic gastritis or intestinal metaplasia (228).
In GC, IL-6 plays a dual role in tumor progression and in the modulation of the immune system. It promotes cancer growth through activation of the STAT3 pathway, enhancing cell proliferation, survival, angiogenesis and metastasis (229). In addition, IL-6 contributes to immune evasion by promoting the expansion of MDSCs (230) and regulatory T cells (231). This attenuates anti-tumor immune responses. Chronic elevation of IL-6 in the TME also maintains the inflammatory state and creates a niche that is favorable for the progression of cancer.
3.4.2 The role of IL-6 in the TME
IL-6 can not only participate in inflammatory response, but also promote tumorigenesis and development in the TME. In gastritis, IL-6 mainly affects the damage and repair process of gastric mucosa by activating the JAK/STAT3 signaling pathway, regulating inflammatory response and immune cell differentiation (225). In GC, IL-6 enhances the proliferation and anti-apoptosis of tumor cells by reshaping the TME, helping them evade the clearance of the immune system (232). Therefore, IL-6 plays a crucial role in the TME of gastritis and GC.
H. pylori infection induces IL-6 secretion, which protects the gastric mucosa from acute inflammation, but long-term IL-6 signaling can lead to chronic inflammation and increase the risk of GC (233). In GC, IL-6 promotes the activation of TAMs and CAFs, which further enhance the inflammatory response by secreting IL-6 and other factors, creating a vicious cycle (227, 234). In addition, IL-6 directly promotes the proliferation, survival, and invasion of tumor cells by activating STAT3 signaling (223).
IL-6 impairs immune surveillance of tumors by inducing T cells differentiation and inhibiting the activity of effector T cells (235). In addition, IL-6 can also inhibit the maturation and antigen presentation function of dendritic cells, further reducing the immune system’s ability to respond to pathogens or tumor cells (223). High levels of IL-6 in chronic gastritis may lead to the immune system’s tolerance to H. pylori, creating the conditions for the persistence of inflammation and the development of GC. In addition, IL-6 can help tumor cells achieve immune escape through a variety of pathways (236).
In conclusion, IL-6 has an important dual role in the TME of gastritis and GC.
3.4.3 The future of IL-6
Although IL-6 has a role in fighting inflammation and supporting immune defense, its tumori-promoting effect in GC makes it an important target for immunotherapy. In the future, it is expected that the treatment strategies for gastritis and GC will be optimized by precisely regulating the IL-6 signaling pathway, combined with immune checkpoint inhibitors or other treatments, and providing patients with more effective clinical interventions.
3.5 IL-10
IL-10 is an anti-inflammatory cytokine that is mainly secreted by regulatory T cells, B cells, monocytes, and TAMs, and plays an important role in maintaining immune homeostasis and inhibiting excessive inflammation (237).
3.5.1 Role of IL-10 in gastritis and GC
In the early stage of H. pylori-induced gastritis or gastritis caused by other stimuli, immune cells such as macrophages and Th1 cells release large amounts of pro-inflammatory factors, including TNF-α, IL-1β, and IFN-γ. IL-10 downregulates the expression of these factors by activating the STAT3 pathway. This effectively alleviates the mucosal inflammatory response and reduces tissue damage. Meanwhile, IL-10 inhibits the antigen-presenting function of DCs and macrophages. It also reduces CD4+ T cell activation and decreases chemokine expression. Thus, IL-10 controls the excessive infiltration of immune cells into the gastric mucosa and prevents the spread of inflammatory responses (118, 124). However, persistent expression of IL-10 allows H. pylori to evade the immune system, maintain infection and create a microenvironment conducive to GC progression (238). Elevated levels of IL-10 may reduce bacterial immune clearance and increase cancer risk in chronic H. pylori gastritis. IL-10 from B cells has been associated with an accelerated rate of progression of GC.
3.5.2 The role of IL-10 in the TME
Within the complex milieu of the TME in cancer, IL-10 can exhibit a dichotomous role, exhibiting antagonistic and stimulatory properties in distinct contexts. Specifically, IL-10 has been shown to reduce chronic inflammation, thereby lowering the risk of tumorigenesis. Conversely, elevated levels of IL-10 within the TME can impede effective anti-tumoral immune responses, thus facilitating immune evasion and tumor progression (239).
3.5.3 The future of IL-10
Due to its potent anti-inflammatory properties, IL-10 holds great promise for therapeutic applications in inflammation, cancer and autoimmune diseases. Strategies are being developed to improve the stability and delivery of IL-10 derivatives to effectively modulate the immune balance in autoimmune diseases such as rheumatoid arthritis (240) and inflammatory bowel disease (241). In cancer, IL-10’s dual role is being intensively studied, particularly its potential to enhance antitumor responses with immune checkpoint inhibitors. Targeting IL-10 therapeutics to improve efficacy and minimize side effects is possible through advances in (242) and precision delivery systems (243). Personalized therapies for immune-related diseases may emerge from further research into the signaling pathways and regulatory mechanisms of IL-10.
3.6 IL-12
IL-12 is a key pro-inflammatory cytokine that regulates immune responses and is secreted by antigen-presenting cells such as dendritic cells and macrophages (244). It promotes the differentiation of CD4+ T cells into Th1 cells (245). It drives the production of IFN-γ and enhances cell-mediated immunity (246). In addition, bridging innate and adaptive immunity, IL-12 activates NK cells and enhances their cytotoxic and antitumor functions (247). In the TME, IL-12 inhibits tumoral growth and supports anti-tumoral immunity. However, underscoring the need for balanced IL-12 expression, excessive IL-12 can lead to harmful inflammation and has been linked to autoimmune diseases (248).
3.6.1 Role of IL-12 in gastritis and GC
It has been established that IL-12 plays a crucial role in the immune response associated with gastritis, particularly in cases of H. pylori -induced gastritis. As a pro-inflammatory cytokine, IL-12 facilitates the differentiation of CD4+ T cells into Th1 cells, thereby enhancing the production of IFN-γ, which, in turn, accelerates the eradication of H. pylori (249). However, the predominance of this Th1-type immune response can also intensify gastric inflammation, thereby contributing to mucosal damage (250). The persistent inflammation that is driven by IL-12 has been demonstrated to heighten the risk of progression from gastritis to gastric atrophy, and eventually, GC, thereby underscoring its dualistic role in both protecting against infection and contributing to disease progression.
3.6.2 The role of IL-12 in the TME
Within the TME, IL-12 has been shown to regulate immune cell function, activate effector T and NK cells, and augment anti-tumor immune responses. By inducing a Th1-type immune response, IL-12 contributes to enhancing cell-mediated immune responses and impeding the growth and metastasis of tumor cells (251). Furthermore, IL-12 has been observed to enhance antigen presentation via its modulation of dendritic cells (252), thereby contributing to the initiation and sustenance of immune surveillance within tumors. Nevertheless, immunosuppressive factors in the TME have the potential to impede the effects of IL-12 and curtail its therapeutic potential (253).
Notwithstanding the capacity of IL-12 to augment the immune response, tumor cells have the capacity to inhibit the action of IL-12 through a variety of mechanisms, thereby leading to immune evasion. Immunosuppressive cells within the TME, such as regulatory T cells (254) and M2 macrophages (255), may hinder the pro-inflammatory effects of IL-12 by secreting cytokines like IL-10 (256), thereby diminishing the strength of the immune response. Furthermore, prolonged IL-12 activation has been shown to induce immune tolerance, a process that can impede the immune system’s capacity to recognize and combat tumor cells, thus creating a favorable environment for tumor cell proliferation and immune evasion (257).
3.6.3 The future of IL-12
It is reasonable to hypothesize that in the future, immunotherapy strategies that target IL-12 will become more sophisticated. Research is anticipated to prioritize optimizing targeted delivery of IL-12 through genetic engineering, reducing systemic adverse effects, and enhancing its efficacy in the TME. A promising avenue for advancement in GC and other tumors may lie in the combination of IL-12 with other immunotherapy methods, such as immune checkpoint inhibitors (258) and CAR-T cell therapy (259). The significance of IL-12 in the realm of tumor immunotherapy is anticipated to be further underscored by advancements in precision medicine and targeted delivery methodologies.
3.7 IL-17
The IL-17 class of pro-inflammatory cytokines is secreted by Th17 cells and their derivatives, including gamma delta T cells and natural killer T cells (260). These cytokines play a pivotal role in regulating inflammatory responses. The IL-17 family comprises IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F (260). Among them, IL-17A is regarded as the most representative and the most extensively studied member. By binding to its receptor, designated as IL-17R, IL-17 triggers the activation of multiple signaling pathways, resulting in the promotion of downstream cytokine production and leukocyte recruitment. This phenomenon manifests a dual effect on both immune response and tissue damage (261).
3.7.1 Role of IL-17 in gastritis and GC
By promoting an inflammatory response that recruits and activates immune cells such as neutrophils and macrophages, IL-17 plays a central role in H. pylori-induced gastritis. IL-17 is critical for the elimination of H. pylori (156). However, its overactivity can lead to chronic inflammation, creating an environment conducive to the development of GC. Particularly in individuals with gastritis, elevated levels of IL-17 correlate with an increased risk of GC. IL-17 plays a dual function in the development of gastritis and cancer: in the early stages, IL-17 can contribute to tumor cell killing, but in the tumor environment, IL-17 supports immune evasion and promotes tumor cell survival and growth through modulation of immune cell function (262, 263).
Studies have shown that by promoting inflammatory responses, activating immune cells and inducing the release of pro-inflammatory factors, IL-17 is able to drive GC development and progression (158). The dual role of IL-17 in GC makes it a potential target for research and therapy.
3.7.2 The role of IL-17 in the TME
IL-17, produced by Th17 cells, γδ T cells and other immune cells, is central to inflammation, immunity and tissue repair through binding to its receptor, IL-17R, and activation of downstream pathways. It enhances local immune defense against pathogens by inducing the secretion of pro-inflammatory cytokines. Sustained IL-17 activity may drive chronic inflammation (264) and contribute to cancer (156), autoimmunity (265) and fibrotic disorders (266). In addition, IL-17 regulates immune cell interactions by influencing the balance of Th17 and Treg and promoting immune suppression via MDSCs (267). This facilitates immune escape in tumors.
By stimulating fibroblasts, collagen synthesis and ECM remodeling, IL-17 also supports tissue repair (268). These processes can exacerbate pathological fibrosis and tissue damage in chronic conditions such as cancer and fibrosis (266). While the role of IL-17 is protective, its dysregulation poses challenges. Therapeutic approaches that target the IL-17 pathway are promising but require careful management to balance benefits and risks.
3.7.3 The future of IL-17
Hitherto, research on IL-17 has focused on its role in immune modulation. By leveraging an enhanced comprehension of the IL-17 signaling pathway, the development of more precise treatment methodologies can be facilitated. These methodologies hold promise in reducing adverse effects and enhancing the precision of treatment, thus improving patient outcomes. Moreover, the potential synergistic effect of IL-17 when employed in conjunction with other immunotherapy modalities, such as with immune checkpoint inhibitors (269), warrants further exploration. Consequently, the therapeutic potential of IL-17 in tumor immunotherapy merits further investigation, as it could offer novel concepts and strategies for the management of GC, among other types of tumors.
3.8 IL-23
IL-23 is a pro-inflammatory cytokine that plays a pivotal role in the TME, primarily through the regulation of Th17 cell differentiation and function (270). Its function includes the maintenance of Th17 cell expansion through the activation of the JAK-STAT pathway, the promotion of inflammatory factor production (e.g., IL-17 and IL-22), and, consequently, the enhancement of mucosal barrier defense and pathogen clearance (271). However, uncontrolled activation of IL-23 has been associated with the pathogenesis of various autoinflammatory conditions, including psoriasis (272) and inflammatory bowel disease (248). Within the TME, IL-23 exhibits a dual role, functioning both to enhance anti-tumor immunity and to promote tumor progression through the mechanisms of chronic inflammation and immune escape (273). Consequently, IL-23 represents a significant target for the therapeutic management of inflammatory diseases and demonstrates potential value in the context of tumor immunotherapy.
3.8.1 Role of IL-23 in gastritis and GC
In H. pylori -induced gastritis, IL-23 drives chronic inflammation by promoting the differentiation of Th17 cells, which in turn produce pro-inflammatory cytokines such as IL-17 (263). This cytokine cascade damages the gastric mucosa and impedes healing. This contributes to chronic gastritis. Persistent IL-23 activation is a potential target for therapeutic intervention because it exacerbates inflammation and may perpetuate H. pylori infection.
In GC, IL-23 has a dual role. Through Th17-mediated tumor surveillance, it can enhance antitumor immunity. Chronic IL-23 activation promotes a proinflammatory milieu that is conducive to angiogenesis (274), and cancer progression (170). The complex role of IL-23 in GC is underscored by the interplay between its protective and tumor-promoting effects.
3.8.2 The role of IL-23 in the TME
In the TME, IL-23 is a key player in chronic inflammatory conditions and autoimmune diseases. It maintains the inflammatory milieu and immune cell activation. IL-23 has been shown to cause tissue damage and chronic inflammation, making people more prone to cancer.
In the context of cancer, the role of IL-23 is more complex. On the one hand, by activating Th17 cells and NK cells that can recognize and kill cancer cells, it can enhance the immune system’s ability to fight tumors (247, 275). On the other hand, persistent IL-23 activity can contribute to a chronic inflammatory environment that is conducive to tumor growth and progression through the promotion of angiogenesis (276) and immune evasion (277). Thus, depending on the specific context and balance of immune responses, IL-23 is a double-edged sword in the TME.
3.8.3 The future of IL-23
Particularly in the treatment of autoimmune diseases, chronic inflammation and cancer, the future of IL-23 research holds significant therapeutic potential. Given its critical role in driving Th17 cell differentiation and perpetuating inflammation, IL-23 is a target for therapeutic intervention in diseases like psoriasis. In clinical trials, monoclonal antibodies that inhibit IL-23 signaling have shown promise. In cancer, the pro-inflammatory effects of IL-23 can also promote tumor growth, although IL-23 may stimulate anti-tumor immunity. The refinement of IL-23 modulation strategies to exploit its therapeutic benefits while minimizing its potential to promote chronic inflammation or immune evasion in cancer will likely be the focus of future research.
4 TNF
TNF is a master regulator of inflammatory responses, produced primarily by macrophages, dendritic cells and T cells (278). TNF binds to TNFR1 and TNFR2 to mediate its effects (279). It plays a critical role in acute inflammation by promoting the activation of the endothelium and the adhesion and migration of leukocytes to the sites of inflammation (280). TNF stimulates the production of pro-inflammatory cytokines, and thus amplifies the immune response. However, excessive or chronic TNF activity is characteristic in autoimmune diseases, such as rheumatoid arthritis (281) and inflammatory bowel disease (282), where it drives tissue damage and systemic inflammation. The specific mechanism of TNF in GC and gastritis is detailed in Table 3.
4.1 Role of TNF in gastritis and GC
TNF drives inflammation in H. pylori -induced gastritis by activating the NF-κB pathway, stimulating the release of other inflammatory cytokines (288, 290). This results in infiltration of immune cells and damage to the stomach lining. Chronic elevated TNF contributes to persistent inflammation and compromises mucosal repair, laying the foundation for GC (278).
In GC, TNF drives tumor progression through NF-κB and MAPK pathways, promotes angiogenesis, cell proliferation and metastasis, and suppresses antitumor immunity (291). Reflecting its dual function, despite its pro-tumor role, TNF also has apoptotic effects on tumor cells (278).
4.2 The role of TNF in the TME
In the TME of a tumor, TNF-α plays a complex dual role, both as an inhibitor of tumorigenesis and, under certain conditions, as a potential promoter of tumor progression.
By activating cytotoxic T cells and NK cells, TNF-α enhances its anti-tumor effects. In addition, TNF-α induces tumor cell expression of death receptors (e.g., Fas (292) and TNFR1 (293)), which initiates apoptosis through extracellular pathways and inhibits tumor growth. On the one hand, TNF-α plays a key role in enhancing the antigen-presenting function and promoting the release of inflammatory factors, thereby providing the body with an effective anti-tumor immune environment (294).
Under conditions of chronic inflammation, TNF-α supports tumor development and proliferation through multiple mechanisms. First, TNF-α is able to promote angiogenesis and tumor invasion through the up-regulation of VEGF (295) and MMPs (296). Second, TNF-α suppresses the activity of effector T cells by recruiting immunosuppressive cells such as regulatory T cells (235) and MDSCs (297), creating an immune escape environment. In addition, TNF-α activates the M2-type polarization of TAMs (298) and secretes inhibitory factors such as IL-10 and TGF-β, further suppressing anti-tumor immune responses.
In summary, depending on its concentration, local environment and regulatory status of signaling pathways, the role of TNF-α in the TME varies. Therapeutic strategies based on TNF-α need to enhance its anti-tumor ability while at the same time avoiding its tumor-promoting effects. In recent years, new ideas for optimizing tumor immunotherapy have emerged, such as combination therapy targeting TNF-α signaling (299).
4.3 The future of TNF
The future of TNF research is aimed at optimizing its therapeutic potential, particularly in autoimmune diseases and cancer treatment. Efforts are focused on refining TNF-targeted therapies to minimize side effects and improve outcomes. In cancer, the combination of TNF modulation with immune checkpoint inhibitors is being explored to boost anti-tumor immunity while addressing its role in chronic inflammation and immune tolerance. Understanding the dual role of TNF in disease progression is essential for the development of more effective, targeted therapies.
5 IFN
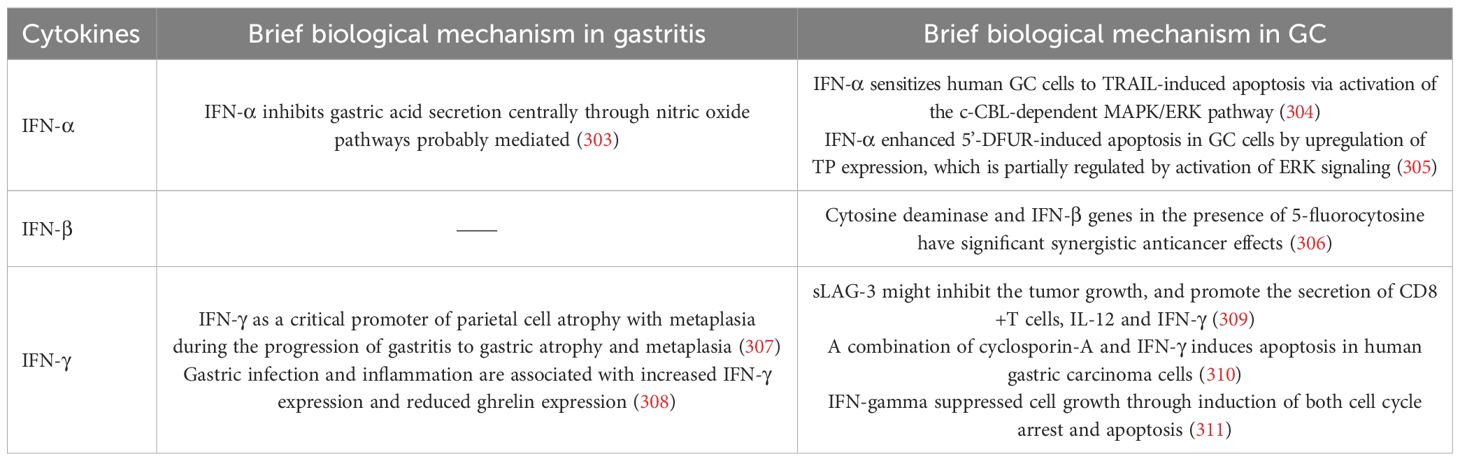
IFNs are a family of cytokines that play a key role in the regulation of the immune system and are divided into three types: Type I (e.g., IFN-α, IFN-β), Type II (IFN-γ), and Type III (IFN-λ) (300). In response to infection, stress and malignancy, these cytokines are produced (301, 302). Their primary role in inflammation is to initiate an antiviral response, to modulate the function of immune cells, and to influence the inflammatory cascade. The specific mechanism of IFNs in GC and gastritis is detailed in Table 4.
5.1 Role of IFN in gastritis and GC
In gastritis caused by H. pylori, IFNs play an important role in the immune response. The inflammatory response induces the production of these cytokines, which increase local inflammation and recruit other immune cells (T cells, NK cells) through macrophage/dendritic cell activation (312). In H. pylori-induced gastritis, the expression of IFN-γ is elevated, enhancing the antimicrobial immune response. IFN-γ induces the expression of PD-L1, which contributes to limiting persistent inflammation and alleviating gastric mucosal tissue damage. However, PD-L1 binds to PD-1 on T cells, leading to T cell exhaustion and suppression of the immune response. This ultimately results in an immunosuppressive microenvironment that promotes tumor cell survival, metastasis, and therapeutic resistance (313). Recombinant forms of IFN-α have been used to treat melanoma (314), renal cell carcinoma (315), and GC (316) because of their ability to induce tumor cell apoptosis and enhance immune activation. However, chronic IFN signaling in the GC microenvironment may enhance tumor progression by promoting vascularization and tumor survival via pathways including VEGF and TGF-β (317, 318).
5.2 The role of IFN in the TME
IFN activate immune responses by modulating the activity of immune cells and influencing the TME. Type I IFNs (IFN-α/β) activate antigen presentation, enhance NK cell and macrophage function, and stimulate the expression of ISGs to establish an antiviral state. They play an essential role in early immune responses to infections and tumors (319). Type II IFN (IFN-γ), produced mainly by T and NK cells, promotes Th1 differentiation, macrophage activation and antigen presentation, which are critical for controlling infection and tumor growth (320). However, excessive or prolonged IFN signaling can induce chronic inflammation, tissue damage and immune dysregulation (321). In the TME, prolonged IFN exposure can upregulate immune checkpoint molecules such as PD-L1, leading to immune tolerance and facilitating immune escape (322). In addition, prolonged IFN signaling may promote tumor cell survival, angiogenesis, and metastasis, complicating its therapeutic use. The balance between immune activation and suppression driven by IFNs is critical in cancer and chronic inflammatory diseases.
5.3 The future of IFN
Improving their therapeutic applications, particularly in cancer, viral infections and autoimmune diseases, is the future of IFNs in medical research. New approaches aim to refine the use of IFNs to enhance immune responses against tumors. Combinations of IFNs and immune checkpoint inhibitors show promise in boosting anti-tumor immunity. Researchers are also investigating strategies to minimize the adverse effects of prolonged IFN signaling, which can contribute to chronic inflammation and immune tolerance. Future therapies may offer more effective and targeted solutions for a variety of immune-related diseases through a better understanding of the mechanisms of IFN signaling in the TME and autoimmune contexts.
6 Chemokines
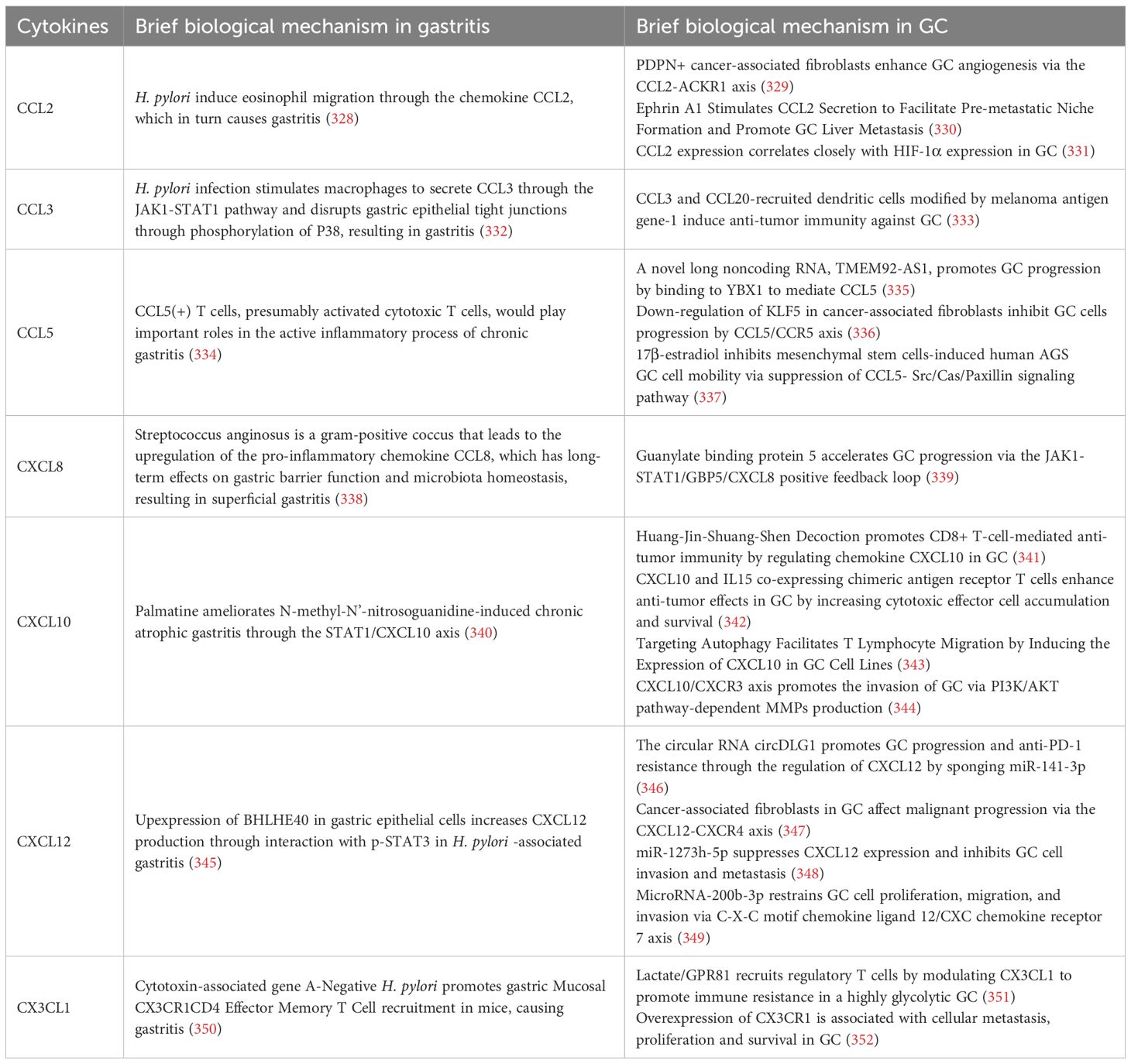
Chemokines are a class of small signaling proteins that play important roles in the immune response, primarily by directing immune cell migration to sites of infection, inflammation, or injury (323). They play a critical role in immune surveillance (324), tissue homeostasis (325), and development of the immune system (326) and receptor signaling in cancer (327). The specific mechanism of Chemokines in GC and gastritis is detailed in Table 5.
6.1 CCL2
CCL2 also known as MCP-1, is an important chemokine (353). It is a member of the C-C motif chemokine family. It promotes the chemotaxis of immune cells, in particular monocytes, macrophages and dendritic cells, by binding to its receptor CCR2.CCL2 (354) plays an important role in a wide variety of physiological and pathological processes, including inflammation, the immune response, the TME and immune escape (354).
6.1.1 Role of CCL2 in gastritis and GC
In gastritis, especially chronic gastritis caused by H. pylori, the role of CCL2 is particularly prominent. Infection with H. pylori stimulates the gastric mucosa to produce CCL2, which in turn attracts monocytes and macrophages to the site of inflammation (355, 356). Macrophages promote gastric mucosal injury and repair by secreting inflammatory factors such as IL-8 and JAK, which enhance the local immune response. Although CCL2 contributes to the antimicrobial immune response, its overexpression can also lead to chronic inflammation and immune dysregulation (135, 357). This may increase the risk of precancerous lesions such as GC. CCL2 plays a role in promoting the recruitment of immune cells, particularly monocytes and macrophages, in the TME of GC (358). By secreting CCL2, tumor cells induce immune cells into the TME. These immune cells, particularly TAMs, can promote tumor growth and metastasis by secreting various cytokines (e.g., IL-10, TGF-β, etc.) to maintain an immunosuppressive status in the TME (358). Macrophages not only play a role in immune escape from tumors, but also exacerbate tumor progression through promotion of angiogenesis and suppression of effector T cell function (359). Therefore, the role of CCL2 in GC may be both to initiate the immune response and to be part of the immune escape mechanism of tumors.
6.1.2 The role of CCL2 in the TME
By promoting the recruitment of immunosuppressive immune cells such as TAMs and Treg cells, CCL2 contributes to tumor immune escape. Tumor cells and CAFs recruit macrophages into the TME by secreting CCL2, and these macrophages are usually M2type with immunosuppressive function (354).
Researchers are exploring immunotherapeutic strategies that target the CCL2/CCR2 pathway because of the important role of CCL2 in immune escape (360). Inhibition of the binding of CCL2 to CCR2 or blocking the production of CCL2 may decrease the accumulation of immunosuppressive cells, such as M2 macrophages, in the TME and increase effector T-cell clearance (361, 362). This targeted therapy may represent a new direction for immunotherapy of tumors such as GC, as it has shown good results in preclinical studies in several tumor types.
6.1.3 The future of CCL2
In order to reduce the immunosuppressive effects in the TME and enhance the anti-tumor immune response, future studies may focus on fine-tuning the CCL2/CCR2 pathway. Furthermore, combining CCL2 with other immunotherapeutic strategies (e.g. immune checkpoint inhibitors, CAR-T cell therapies, etc.) can significantly improve immunotherapy efficacy (363). New opportunities for the treatment of GC and other tumors will be opened by optimizing the targeting of CCL2 and better understanding its complex role in the TME.
6.2 CCL3
CCL3 also known as MIP-1α, is an important chemokine belonging to the C-C motif chemokine family. CCL3 plays an important role in inflammation, immunomodulation, infectious diseases, and tumors (364).
6.2.1 Role of CCL3 in gastritis and GC
H. pylori infection was found to activate immune cells in the gastric lining, leading to the production of CCL3, which promotes a local immune response by binding to CCR-1 and CCR-5 receptors and recruits immune cells including monocytes, macrophages and T cells to the site of inflammation (332). However, prolonged overexpression of CCL3 can lead to chronic inflammation, providing a permissive environment for precancerous lesions such as GC to develop (365).
CCL3 plays an important role in the TME of GC. Tumor cells recruit immune cells, particularly macrophages and T cells, into the TME through the secretion of CCL3 (333, 366). However, tumor cells can suppress anti-tumor immune responses by altering the function of immune cells. In addition, CCL3 has a role in the promotion of angiogenesis, which may increase the supply of oxygen and nutrients to tumors and promote tumor growth and metastasis (367).
6.2.2 The role of CCL3 in the TME
By recruiting immunosuppressive cells such as M2-type macrophages and Treg cells, CCL3 can promote immune evasion during tumor immune escape (368). Although CCL3 can enhance local immune evasion, its recruitment of these suppressive cells can diminish effector T cell function and impair tumor cell recognition and clearance, thereby promoting tumor growth and metastasis (369). As a result, the role of CCL3 in the TME can be both supportive of the immune response and contribute to immune escape through immunosuppressive mechanisms. Targeting CCL3 with immunotherapeutic strategies, such as blocking the CCL3/CCR1/CCR5 interaction, could reduce the accumulation of immunosuppressive cells and enhance anti-tumor immune responses (369, 370). The CCL3/CCR5 pathway is a promising target for overcoming immune escape in GC, as studies suggest that CCR5 antagonists may improve the efficacy of immunotherapy in various cancers.
6.2.3 The future of CCL3
Future studies targeting the CCL3/CCR5 signaling pathway may aim to enhance immunotherapy efficacy, particularly when combined with immune checkpoint inhibitors or CAR-T cell therapy (371, 372). Inhibiting CCL3 activity or its receptor could reduce immunosuppressive effects in the TME, restoring anti-tumor immune responses. Additionally, precise regulation of CCL3 expression in the TME may offer new therapeutic strategies for immunotherapy in GC and other malignancies.
6.3 CCL5
CCL5 also known as RANTES, is an important chemokine that belongs to the C-C motif chemokine family (373). It is a chemokine secreted mainly by T cells, macrophages, dendritic cells, endothelial cells and tumor cells (374). It regulates the migration of immune cells, especially immune cells such as T cells, macrophages and eosinophils, by binding to CCR1, CCR3 and CCR5 receptors (375–377). CCL5 not only promotes the aggregation of immune cells, but also enhances cell-cell interactions, thereby strengthening the immune response. In addition, CCL5 is involved in the regulation of immune cell activation, proliferation, differentiation and cytokine secretion (378).
6.3.1 Role of CCL5 in gastritis and GC
The expression of CCL5 is normally increased when the gastric mucosa is infected or injured, which recruits immune cells such as T cells and macrophages to the site of inflammation and enhances the immune response (379). However, excessive CCL5 activity can lead to a persistent activation of the immune response, which can induce chronic inflammation and increase the damage to the gastric mucosa, thus providing favorable conditions for pre-cancerous lesions such as GC (379). In the TME of GC, CCL5 plays a complex dual role. On the one hand, CCL5 enhances the anti-tumor immune response by promoting the recruitment of T cells and NK cells. Studies show that high CCL5 expression has been linked to stronger anti-tumor immune responses, particularly effector T-cell and NK cell recruitment (380–382). On the other hand, by binding to the CCR5 receptor, CCL5 can recruit immunosuppressive cells such as TAMs and inhibit the function of tumor-specific T cells, thereby exacerbating tumor immune escape (336, 379). In addition, CCL5 may also support tumor growth through the promotion of angiogenesis and the enhancement of tumor cell migration and metastasis (383).
6.3.2 The role of CCL5 in the TME
CCL5 can promote the immune response against tumors through the recruitment of effector cells such as T cells and NK cells, but in some TMEs it can also promote immune escape through the recruitment of immunosuppressive cells such as M2 macrophages and Treg cells (384). CCL5 recruits M2 macrophages via the CCR5 receptor. M2 macrophages secrete anti-inflammatory factors (e.g., IL-10, TGF-β) that suppress tumor-specific immunity and promote tumor survival and metastasis (385). Because of its role in immune escape, targeting the CCL5/CCR5 pathway has become a focus of immunotherapy research.
6.3.3 The future of CCL5
The accumulation of suppressive cells can be reduced and anti-tumor immune responses can be enhanced by inhibiting CCL5/CCR5 binding (379). Studies have shown that CCR5 antagonists, especially when combined with immune checkpoint inhibitors or CAR-T cell therapy, can improve immunotherapy outcomes in various cancers, making the CCL5/CCR5 pathway a promising strategy for the treatment of GC (386).
6.4 CXCL8
CXCL8 is an important chemokine belonging to the C-X-C motif chemokine family, also known as IL-8 (387). It is predominantly secreted by various cell types including neutrophils, macrophages, endothelial cells, fibroblasts, tumor cells and others (388). By binding to its receptors CXCR1 and CXCR2, CXCL8 exerts chemotactic effects on immune cells, in particular neutrophil recruitment and activation (387). Furthermore, CXCL8 plays important roles in physiological and pathological processes including inflammation, immune response and TME (387).
6.4.1 Role of CXCL8 in gastritis and GC
In the gastric mucosa, CXCL8 enhances local immune responses by promoting neutrophil chemotaxis and activation, thereby contributing to the resolution of infection (389). However, prolonged high expression of CXCL8 and excessive neutrophil recruitment can lead to chronic inflammation and damage to the gastric mucosal lining, creating conditions conducive to the development of diseases like GC (390). In addition to enhancing local inflammatory responses in the tumor by recruiting immune cells, CXCL8 may also promote tumor development by promoting tumor cell growth, angiogenesis and metastasis (391). CXCL8 recruitment and activation of neutrophils by binding to CXCR1 and CXCR2 has been shown to enhance tumor growth and proliferation through secretion of a variety of cytokines and angiogenic factor release (392, 393). In addition, by inducing the accumulation of TAMs, CXCL8 may promote immune escape from the TME (394).
6.4.2 The role of CXCL8 in the TME
By regulating the migration and function of immune cells in the TME, CXCL8 may support immune escape of tumor cells (394). Targeting CXCL8 or its receptors (CXCR1 and CXCR2) has emerged as a potential immunotherapeutic strategy due to the important role of CXCL8 in immune escape. By inhibiting the binding of CXCL8 and CXCR1/2, the aggregation of immunosuppressive cells (e.g., neutrophils, TAMs, etc.) in the TME can be reduced, thereby promoting the anti-tumor activity of effector T cells (392, 393). Studies have shown that inhibition of the CXCL8 pathway has the potential to enhance the effectiveness of immunotherapy, especially when combined with immune checkpoint inhibitors or other immunotherapy (395).
6.4.3 The future of CXCL8
Therapeutic strategies that precisely target the CXCL8 receptor to reduce immunosuppression in the TME and restore anti-tumor immune responses are likely to be the focus of future CXCL8 research. New ideas and therapeutic approaches for the treatment of GC and other malignancies may be provided by optimizing the role of CXCL8 in the TME.
6.5 CXCL12
CXCL12 also known as stromal cell-derived factor 1α, is an important chemokine (396). It belongs to the C-X-C motif chemokine family. CXCL12 can be secreted by various cell types including fibroblasts, endothelial cells, macrophages, and tumor cells (397). CXCL12 binds to the CXCR4 and CXCR7 receptors and is involved in many physiological and pathologic processes, including immune response, cell migration and tumor metastasis (397, 398).
6.5.1 Role of CXCL12 in gastritis and GC
When infected by H. pylori, the stomach produces CXCL12, which recruits immune cells such as T cells and macrophages to the inflamed area (399). CXCL12 helps resolve the infection by regulating immune cell localization and activation through binding to CXCR4 and CXCR7 receptors (400). However, excessive expression of CXCL12 can lead to chronic inflammation, which can damage the lining of the stomach and increase the risk of pre-cancerous lesions such as GC (401). In the TME of GC, CXCL12 plays a dual role. First, by recruiting immune cells to the TME, CXCL12 enhances the immune response (347). In some cases, CXCL12 expression may enhance effector T cells, NK cells, and other antitumor immune function (402). However, CXCL12 can also promote tumor metastasis by facilitating the migration and invasion of tumor cells. Tumor cells, CAFs, and others may secrete CXCL12 and activate the CXCR4 receptor, which directs tumor cells to specific sites and promotes metastatic and neovascular growth (403, 404).
6.5.2 The role of CXCL12 in the TME
By recruiting immunosuppressive cells such as Treg cells and M2 macrophages, the CXCL12/CXCR4 signaling pathway plays a critical role in tumor immune escape (400). High CXCL12 expression has been implicated in immune escape, metastasis and drug resistance in several tumor types, including GC (405). CXCL12 promotes immunosuppression by recruiting CAFs and reducing effector T-cell and NK-cell function (406).
6.5.3 The future of CXCL12
Targeting the CXCL12/CXCR4 signaling pathway by inhibiting their binding or by blocking the expression of CXCL12 can reduce the accumulation of immunosuppressive cells and enhance the anti-tumor immunity. This pathway is a promising therapeutic target as studies have shown that CXCR4 antagonists can improve immune responses and slow tumor progression.
6.6 CXCL10
CXCL10, also known as IP-10 (IFN-γ-induced protein 10), is an important chemokine that belongs to the family of chemokines with a C-X-C motif (407). CXCL10 has been shown to be secreted by various cell types including macrophages, endothelial cells, fibroblasts and tumor cells (408). The expression of CXCL10 is significantly increased by the chemotaxis induced by IFN-γ and is involved in the chemotaxis of immune cells, the modulation of immune responses, and the immune surveillance of the TME (409).
6.6.1 Role of CXCL10 in gastritis and GC
In chronic gastritis, CXCL10 enhances the immune response by recruiting CD4+ T cells and CD8+ T cells for infection control (341). CXCL10 modulates immune cell function and the intensity of local immune responses by binding to the CXCR3 receptor (410). CXCL10 potentiates the immune response against tumors and reduces tumor growth and metastasis, mainly by regulating immune cell migration and activation. The role of CXCL10 is to recruit immunosuppressive cells (such as Treg cells) to the tumor, and these cells suppress the activity of effector T cells (411).
6.6.2 The role of CXCL10 in the TME
CXCL10, through its receptor CXCR3, plays a dual role in tumor immune escape (412). On the one hand, it recruits anti-tumor immune cells such as effector T cells and NK cells to the tumor site. This enhances the immune response and promotes tumor elimination (413). On the other hand, prolonged high expression of CXCL10 can lead to an overaccumulation of immunosuppressive cells, particularly Treg cells. Treg cells suppress effector T cell function and contribute to immune escape (414). Thus, its ability to direct immune cell recruitment, as well as the local immune status and cell types present, determine the impact of CXCL10 in the TME.
6.6.3 The future of CXCL10
Because of its role in the modulation of immune responses, CXCL10 has emerged as a promising target for immunotherapy. Strategies that increase CXCL10 expression or activate its CXCR3 receptor could enhance anti-tumor immunity by promoting effector cell recruitment to the tumor site. The combination of CXCL10 modulation with immune checkpoint inhibitors (e.g. PD-1/PD-L1 inhibitors) (415), cancer vaccines or CAR T-cell therapies may improve overall therapeutic efficacy through synergistic enhancement of the immune response (416). As a result, the CXCL10/CXCR3 pathway is a valuable target for the development of novel immunotherapeutic strategies in cancers such as GC.
6.7 CX3CL1
CX3CL1, also known as fractalkine, is a unique chemokine. It belongs to the C-X3-C motif chemokine family (417). Unlike other chemokines, CX3CL1 can be expressed on the cell surface in either soluble or membrane-associated forms and plays important roles in the immune response, particularly in immune cell migration, inflammatory responses, tissue repair and the TME (418).
6.7.1 Role of CX3CL1 in gastritis and GC
In gastritis, CX3CL1 regulates the migration of immune cells (particularly monocytes and macrophages) by binding to the CX3CR1 receptor and helps to direct immune cells toward the site of inflammation, thereby maintaining local immune responses and preventing the spread of pathogens (350). However, overexpression of CX3CL1 can lead to chronic inflammation that damages the lining of the stomach and increases the risk of GC, and can direct immunosuppressive cells, such as Treg cells, to accumulate at the site of inflammation, thereby supporting immune escape (419).
In GC, through increased recruitment of immune cells such as effector T cells and NK cells, CX3CL1 enhances the anti-tumor immune response and limits tumor growth and metastasis (352, 420).
6.7.2 The role of CX3CL1 in the TME
By interacting with the CX3CR1 receptor, CX3CL1 recruits immunosuppressive cells (e.g., Treg cells, M2-type macrophages) to help tumors evade immune surveillance during immune escape in tumors (421, 422). At the same time, CX3CL1 enhances the secretion of immunosuppressive factors, inhibits the anti-tumor activity of effector T cells and NK cells, and promotes immune escape and tumor growth (423).
6.7.3 The future of CX3CL1
By understanding the role of CX3CL1 in immune escape and tumor immune modulation, new targeted therapeutic strategies have been developed. In particular, new breakthroughs in the treatment of malignancies such as GC may be achieved through combination with immune checkpoint inhibitors, cytokine therapy and CAR T-cell therapy (424, 425).
7 Targeted agents against inflammatory cytokines
Targeted agents against inflammatory cytokines have been widely applied in various diseases, including hematological disorders, autoimmune diseases, and chronic inflammatory conditions, with their efficacy and safety well established (426–428). However, in inflammation-driven tumors—particularly in the context of GC—the therapeutic effectiveness and safety profile of these agents remain to be fully elucidated. The research progress of several targeted agents is summarized in Table 6. IL-6, TNF-α, and CXCL8 are three key pro-inflammatory cytokines extensively involved in remodeling the TME, thereby promoting tumor cell proliferation, metastasis, and immune evasion. Targeted interventions against these cytokines have entered preclinical or early-phase clinical research in various inflammation-associated diseases and selected malignancies, demonstrating considerable therapeutic potential.
In the IL-6 signaling pathway, the IL-6 receptor antagonist Tocilizumab has been approved by the FDA for the treatment of rheumatoid arthritis and giant cell arteritis, and its potential application in solid tumors is gaining increasing attention. Related studies also indicate that Bempegaldesleukin, an IL-2 pathway agonist, significantly enhances the anti-tumor efficacy of radiotherapy through a T cell–dependent mechanism (429). Furthermore, Bazedoxifene inhibits IL-11–dependent STAT3 signaling, thereby blocking gastrointestinal tumor growth (431).
In the CXCL8 pathway, Reparixin, a CXCR1/2 receptor inhibitor, has been shown to markedly suppress the malignant behavior of GC MKN45 cells in vitro and in vivo. When combined with first- and second-line chemotherapy, it reduces toxicity and prolongs survival (438). Reparixin also diminishes the protective effect of CAFs on CD8+ T cells and improves the efficacy of anti-PD-L1 antibodies, thereby enhancing cytotoxic immune responses (142).
Plerixafor, a small-molecule CXCR4 antagonist, is a leading candidate in gastrointestinal cancer therapy targeting the CXCL12–CXCR4/CXCR7 axis (400). Studies demonstrate that Plerixafor modulates TAMs, suppresses GC progression, and enhances immune recognition and T cell activation (439).
In the TNF-α pathway, inhibitors such as Infliximab and Adalimumab are widely used in the clinical management of inflammatory bowel disease. Research suggests that Infliximab can suppress H. pylori–induced upregulation of CXCR4 by inhibiting TNF-α signaling, thereby reducing GC cell migration and exhibiting anti-tumor potential (434).
Additionally, the highly selective CCR5 antagonist Maraviroc, when combined with cisplatin, significantly inhibits the growth of GC organoids and shows promising anti- GC activity (436). Its mechanism may involve blocking the CCR5 pathway, thereby reducing GC cell migration induced by MIP-1α, MIP-1β, and RANTES (437).
Although the above targeted strategies have shown good safety profiles in approved disease settings, their application in the context of cancer still requires cautious evaluation. Inflammatory cytokines play essential roles in maintaining immune homeostasis; thus, long-term or systemic inhibition may lead to immune imbalance and an increased risk of infection. In addition, the presence of complex bidirectional regulatory mechanisms among different signaling pathways may result in unexpected immunosuppressive effects. In the future, it will be necessary to integrate tumor molecular subtypes, immune cell infiltration patterns, and peripheral pro-inflammatory cytokine levels to accurately identify patient populations most likely to benefit from cytokine-targeted therapies. A systematic assessment of the synergistic effects between cytokine inhibitors and immune checkpoint inhibitors, conventional chemotherapy, and anti-angiogenic therapies is needed to improve overall therapeutic efficacy and overcome resistance to monotherapy. With the aid of these technologies, the cellular sources and target sites of inflammatory cytokines can be precisely identified at single-cell resolution, thus providing a basis for individualized and precise therapeutic interventions.
8 miRNA-driven inflammatory persistence in gastric
MicroRNAs (miRNAs) regulate the intensity and persistence of inflammatory signaling by targeting multiple signaling components, acting as molecular adaptive mechanisms that facilitate immune evasion (440). In the context of H. pylori infection, key immunoregulatory miRNAs—particularly miR-155 and miR-146a—are significantly upregulated, thereby reprogramming TLR/NF-κB and associated downstream pathways (441). miR-155 is typically upregulated during infection and chronic inflammation, promoting or sustaining Th1/Th17 responses and functional remodeling of myeloid cells. However, its excessive or sustained expression may also indirectly promote immune evasion and pro-tumor microenvironment formation by modulating antigen presentation, suppressing certain inhibitory factors, or affecting immune checkpoint pathways. Conversely, miR-146a is often induced by NF-κB as a negative feedback regulator, targeting upstream adaptors like IRAK1/TRAF6 to reduce excessive inflammatory output and protect tissues (442). However, altered miR-146a expression (or functional imbalance) during chronic infection and carcinogenesis may contribute to dysregulated inflammation and influence tumor-associated NF-κB activity and cell proliferation signaling (443). Collectively, the dynamic regulation of miRNAs transforms pathogen-induced initial NF-κB/TLR signaling into a more persistent and individualized inflammatory state (444). This not only explains how inflammation-repair imbalance is sustained long-term to promote genomic instability and tumor progression but also reveals the value of miRNA regulatory axes as potential biomarkers or intervention targets.
9 Challenge and future perspective
In this review, we primarily focused on the inflammatory mechanisms underlying H. pylori–induced chronic gastritis and its progression to gastric cancer. However, relatively limited discussion was devoted to other well-defined etiologies of gastritis, such as autoimmune atrophic gastritis, bile reflux–related chemical injury, eosinophilic/lymphocytic or granulomatous gastritis, portal hypertensive gastropathy, and gastric mucosal injury caused by non–H. pylori infections (e.g., certain viruses or bacteria). Moreover, the prevalence of H. pylori infection varies across different geographic regions, which may influence the risk assessment and mechanistic understanding of gastric carcinogenesis. Future studies should place greater emphasis on the inflammatory characteristics of these distinct gastritis subtypes and their potential roles in gastric cancer development, thereby contributing to a more comprehensive understanding of the underlying pathogenic network.
9.1 CagPAI-mediated signaling cascades and pro-inflammatory responses
Among the various triggers of chronic gastritis, H. pylori infection represents the most well-characterized and potent inducer of gastric tumorigenesis. Persistent infection initiates and sustains mucosal inflammation through continuous activation of epithelial and immune signaling networks, ultimately transforming the gastric microenvironment into a pro-tumor niche. The cag pathogenicity island (cagPAI), a major virulence determinant, encodes the complete Cag type IV secretion system (Cag-T4SS) together with a set of structural and effector proteins that directly remodel host signaling at multiple levels (445, 446).
During intimate bacterial–epithelial contact, the Cag-T4SS assembles into transmembrane secretion and adhesion complexes, including the outer membrane core complex (OMCC) and sheath/axon-like structures (447). Structural components such as CagY, CagX, CagT, and CagM form the OMCC and determine the system’s material transport capacity, while effector proteins including CagA and the adhesion molecule CagL mediate host cell engagement and downstream signaling (448). CagL binds integrins (α5β1, αVβ6, etc.) with high affinity, activating the FAK/Src axis and receptor tyrosine kinase cascades (e.g., EGFR), leading to MAPK (ERK, JNK, p38) activation (449). This cascade induces AP-1 and NF-κB–dependent transcription of pro-inflammatory cytokines such as IL-8 and IL-6, establishing a strong chemokine gradient that recruits neutrophils and macrophages (450).
Concurrently, the Cag-T4SS delivers bacterial peptidoglycan (PGN) and CagA into host cytoplasm. Intracellular PGN is recognized by NOD1, triggering the canonical NF-κB and MAPK pathways that further amplify inflammatory gene expression (451). Once translocated, CagA undergoes phosphorylation at its EPIYA motifs by Src/Abl kinases; phosphorylated CagA aberrantly activates SHP2, leading to dysregulated growth factor signaling, enhanced proliferation, and motility (452, 453). Non-phosphorylated CagA binds the polarity regulator PAR1b, disrupting epithelial cell polarity and promoting epithelial–mesenchymal transition (EMT)-like changes (454). Additionally, CagA impairs DNA damage repair (e.g., BRCA1-dependent pathways), induces mitochondrial dysfunction and ROS accumulation, and increases genomic instability—all hallmarks of malignant transformation (455).
Chronic infection with cagPAI-positive H. pylori strains therefore promotes gastric carcinogenesis through sustained cytokine and chemokine secretion (IL-8, IL-6, TNF-α, IL-1β), which recruit and activate neutrophils and macrophages to produce reactive oxygen and nitrogen species (445). In parallel, persistent activation of IL-6/STAT3 and NF-κB signaling sustains epithelial survival and proliferation, while simultaneously inducing an immunomodulatory milieu characterized by the recruitment and polarization of MDSCs, regulatory T cells, and TAMs (456). These processes collectively establish a microenvironment with both pro-inflammatory and immunosuppressive features, fostering tumor initiation and progression.
With respect to inflammasome activation, studies suggest cell type– and strain-dependent variability. In macrophages and dendritic cells, H. pylori can “prime” NLRP3 via TLR2/NOD2 signaling, allowing pro–IL-1β synthesis and its Caspase-1–mediated maturation under specific stimuli. Conversely, other studies indicate weak or inhibitory effects on canonical NLRP3 activation, implying that H. pylori may fine-tune inflammasome responses to balance persistent inflammation and immune evasion (457).
9.2 HLA and inflammatory heterogeneity
HLA class I/II molecules form the core immunogenetic locus that regulates antigen presentation and determines the types of peptides presented to CD4+ and CD8+ T cells. This influences Th1/Th2/Th17 cell polarisation and the secretion of corresponding cytokine profiles (e.g. IFN-γ, IL-10, IL-1β and TNF-α) (458). Numerous studies have shown that the frequency of HLA-II alleles (particularly HLA-DQA1, HLA-DQB1 and HLA-DRB1) correlates with mucosal inflammation phenotypes and cytokine expression following H. pylori infection (459). In certain populations, specific HLA-II alleles have been found to correlate with either increased IL-10 expression or a heightened risk of pro-inflammatory factor production (e.g. IL-1β and TNF-α). This suggests that immunogenetic variation is a critical factor in explaining the differences observed in the intensity of the inflammatory response and disease susceptibility between individuals (460). Failing to consider HLA and antigen presentation polymorphisms restricts discussions of inflammatory responses to the ‘commonality’ level of pathogen-signalling pathways. This approach is unable to explain why different hosts exhibit markedly divergent inflammatory profiles and disease courses despite similar pathogen exposures.
9.3 Synergistic and antagonistic interactions of inflammatory cytokines and their signaling pathways in GC and gastritis
In the relationship between gastritis and GC, inflammatory factors play a crucial role (461, 462). A long-term chronic inflammatory response lays the foundation for the development of GC in chronic gastritis, especially that caused by H. pylori (463). The specific mechanisms of evolution are shown in Figure 2. This figure systematically illustrates how chronic gastric mucosal inflammation, induced by H. pylori infection or other high-risk factors, drives the progression from gastritis to GC. It highlights the cascade of inflammatory mediators and signaling pathways involved, along with their positive feedback regulation mechanisms.

Figure 2. Progressive transition from chronic inflammation to GC: a multi-stage mechanism initiated by H. pylori infection and mediated by inflammatory signaling.
Inflammatory factors (464) such as cytokines like IL-1, IL-6, TNF-α, IL-17 and chemokines like CXCL8 and CCL2 play an important role in this process. By activating multiple oncogenic signaling pathways (464) (e.g., NF-κB, JAK-STAT, MAPK, etc.), they promote tumor cell proliferation, survival, immune escape, and enhance tumor invasiveness and metastasis.
A. In the initial phase, H. pylori infection or other risk factors compromise the gastric mucosal barrier, leading to immune cell infiltration (e.g., neutrophils, macrophages, T cells). These cells release large amounts of pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, and CXCL8, marking the onset of the gastritis response.
B. In the second phase, inflammatory cytokines activate multiple signaling pathways, primarily NF-κB and JAK/STAT axes, which regulate immune amplification, cell survival, angiogenesis, and epithelial proliferation. These pathways engage in positive feedback loops that sustain and amplify the chronic inflammatory state. “Other signal pathways” may include MAPK, PI3K/AKT, and TLRs, which cross-regulate each other to enhance stress and injury responses in the gastric mucosa.
C. In the third phase, inflammatory mediators further promote immune cell recruitment and activation—e.g., CCL2-mediated monocyte/macrophage infiltration—forming a tripartite cycle of immune cells, cytokines, and signaling pathways that reinforce local inflammation.
D. In the fourth phase, sustained inflammation induces genetic mutations, stem cell damage, and epigenetic reprogramming in the gastric epithelium, leading to precancerous lesions such as intestinal metaplasia, atrophic gastritis, and dysplasia.
E. In the final phase, chronic inflammation promotes tumorigenesis by enhancing immune evasion, inducing EMT, and facilitating angiogenesis and stromal remodeling, ultimately driving the development of GC.
Inflammatory cytokines such as IL-1β, TNF-α, and IL-6 synergistically amplify immune responses during the early stage of gastritis via classical signaling pathways including NF-κB, JAK/STAT3, and MAPK, promoting mucosal hyperplasia, angiogenesis, and immune cell infiltration. Meanwhile, negative feedback regulators such as IL-10, TGF-β, SOCS3]/, and A20 maintain mucosal homeostasis by inhibiting these signaling axes and restrict excessive inflammation during the precancerous phase. However, when these antagonistic mechanisms become dysregulated or are hijacked by tumor cells, pro-inflammatory and pro-tumorigenic signals remain persistently active, while anti-inflammatory factors paradoxically facilitate immune evasion and microenvironment remodeling, thereby driving gastric carcinogenesis. This network exhibits marked heterogeneity both temporally (from early inflammation to precancerous lesions to advanced tumors) and spatially (across different mucosal regions and tumor core versus invasive margin). Only by constructing a multidimensional systems model integrating factors, pathways, disease stages, and spatial context can the dual regulatory roles and dynamic balance of inflammation in gastritis-to-GC progression be comprehensively elucidated. Detailed mechanisms are shown in Tables 7 and 8.
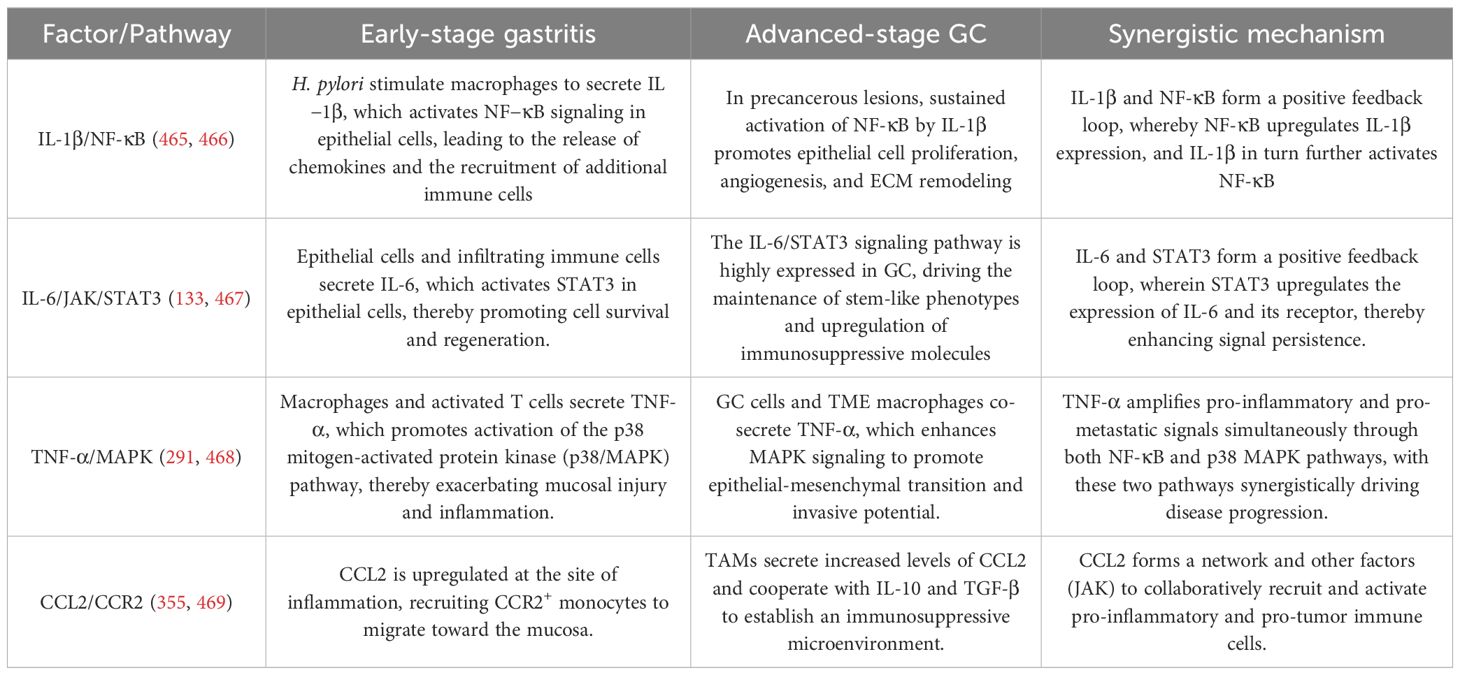
Table 7. Synergistic roles of inflammatory cytokines and their signaling pathways in GC and gastritis.

Table 8. Antagonistic roles of inflammatory cytokines and their signaling pathways in GC and gastritis.
Immune cells such as Treg cells, MDSCs and M2-type macrophages infiltrate the TME and form an immunosuppressive microenvironment as the inflammatory response continues (475). These immunosuppressive cells inhibit an effective anti-tumor immune response through the secretion of immunosuppressive cytokines, thus allowing tumor cells to escape from immune surveillance (475). The development of immune escape mechanisms, which allow tumors to continue to grow under the pressure of the immune system, is an important feature of GC progression (476).
Inflammatory factors play an important role in immune escape in GC (477). Factors such as TNF-α and IL-1 exacerbate immune escape by promoting infiltration of immunosuppressive cells, upregulating immune checkpoint molecules such as PD-L1, and promoting tumor cell survival through pathways such as NF-κB (478–480). Thus, under the watchful eye of the immune system, GC cells can continue to grow and metastasize.
9.4 The neuroinflammation–tumor triangular interaction network
Within the TME, the nervous system, immune inflammation, and tumor cells form a dynamically intertwined “third space” network. Neural signaling can regulate inflammatory responses, while inflammatory mediators, in turn, influence neuronal function. In parallel, both inflammation and neural activity jointly modulate tumor cell proliferation, migration, and invasion. Conversely, tumor cells can secrete various factors to remodel both the neural and immune landscape. These three components interact reciprocally and causally, constituting a “neuroinflammation–tumor” triangular interaction network (Figure 3A). The dysregulation of this network is a critical driving force behind tumor initiation, progression, and metastasis (372). Furthermore, the neuroimmune axis regulates immune responses through the vagus nerve and other neural pathways, maintaining immune homeostasis. This complex interplay acts as a double-edged sword in both inflammation and cancer. Inflammatory factors play a dual role in gastritis and GC, as shown in Figure 3B. Future research should focus on this crosstalk phenomenon, laying an important foundation for subsequent studies.

Figure 3. (A) Activated immune cells release pro-inflammatory cytokines such as TNF-α and IL-6, which enhance neuronal activity. The activated neurons then secrete neurotransmitters including VIP, SP, CGRP, NE, and ACh, which stimulate tumor cells to produce neurotrophic factors and chemokines. This reciprocal interaction sustains the neuro–inflammation–cancer signaling loop. (B) Relevant neurotransmitters promote an immunosuppressive microenvironment and tumor progression via the STAT3/NF-κB signaling pathway. This process facilitates the development of chronic inflammation and drives the polarization of immune cells from the M1 (anti-tumor) to the M2 (pro-tumor) phenotype, thereby shifting gastric tissue responses from inflammation repair toward gastric carcinogenesis. Meanwhile, tumor cells release neurotrophic factors that induce neural remodeling, further enhancing tumor growth and immune evasion.
Within this network, the inflammatory response typically serves as the initiating event. Immune cells such as macrophages, dendritic cells, T cells, and microglia become activated within the TME and release a wide array of pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6, and CXCL1. These cytokines not only directly promote tumor cell growth and metastasis but also act on local nerve endings, leading to increased neuronal excitability and neural remodeling.
Neural signaling regulates immune cells via adrenergic and cholinergic receptors. The sympathetic nervous system releases norepinephrine, which binds to β2-adrenergic receptors on macrophages, dendritic cells, and T cells, promoting M2 polarization and suppressing Th1 responses. This modulation influences cytokine production, cell migration, and overall immune function. Conversely, the parasympathetic nervous system regulates neural architecture through acetylcholine or modulates immune cell recruitment, polarization, and function via neuropeptides. Simultaneously, aberrant neural fiber growth within tumors—referred to as neoneurogenesis—can enhance tumor malignancy by transferring miRNAs and lncRNAs to tumor cells via exosomal pathways.
Tumor cells also play an active role in this interactive network. They can secrete neurotrophic factors (e.g., NGF, BDNF), chemokines (e.g., CXCL12), and extracellular vesicles to induce neural regeneration or remodeling, thereby establishing a more complex “tumor–nerve” axis. Some tumors even acquire neuronal-like properties through transcriptional reprogramming—a phenomenon known as neuronal mimicry—which enhances their responsiveness to neural signals. In addition, tumor-derived factors can reshape the inflammatory microenvironment by promoting the recruitment of immunosuppressive cells such as regulatory T cells and MDSCs, thus enabling immune evasion (475, 476).
This triangular interaction network can ultimately form a positive feedback loop: inflammation promotes neural activation; neural signals regulate immune responses; immune activity further facilitates tumor progression; and tumor cells, in turn, reactivate both inflammatory and neural pathways. Therefore, targeting the “neuro–inflammation–tumor” interaction network has emerged as a promising therapeutic strategy in cancer treatment. Potential approaches include blocking neurotransmitter signaling, inhibiting neurotrophic factors, modulating immune cell polarization, or applying denervation techniques to suppress tumor progression.
9.5 Application of emerging technologies
In recent years, emerging high-throughput technologies such as single-cell RNA sequencing (481, 482) and spatial transcriptomics (483, 484) have been widely applied in the study of gastrointestinal diseases, offering unprecedented resolution in elucidating the relationship between inflammatory factors and gastric pathologies. These techniques enable the dissection of transcriptional heterogeneity among different cell types—such as epithelial cells, immune cells, and fibroblasts—within the gastric mucosa at single-cell resolution, allowing for precise identification of the sources and targets of inflammatory mediators. For example, in models of chronic gastritis and H. pylori infection, single-cell analysis has revealed that pro-inflammatory cytokines such as IL-6 is primarily secreted by activated macrophages and mucosa-associated T cells, and can further influence the proliferation and differentiation trajectories of gastric epithelial stem cells (234, 485). Moreover, spatial transcriptomics enables the visualization of inflammatory factor expression across distinct anatomical regions of the gastric mucosa, thereby shedding light on the spatial relationship between localized inflammation and tumor progression. These advances are reshaping our understanding of gastric disease pathogenesis from the perspectives of cellular ecology and microenvironmental remodeling, and offer more precise strategies for early diagnosis and therapeutic intervention.
New avenues for the treatment of GC are emerging, including immunotherapy, particularly suppression of immune checkpoints such as PD-1/PD-L1 antibodies (486, 487), and targeted therapies against inflammatory factors (477, 488). Through the reversal of immune suppression and the reactivation of anti-tumor immune responses, these therapies are expected to be more effective in the treatment of GC patients. However, the challenge remains how to effectively control pro-inflammatory and escape mechanisms to improve patient prognosis.
10 Conclusions
This review highlights the central role of inflammatory factors in the transition from chronic gastritis to gastric cancer, emphasizing their interactions within the tumor microenvironment that promote both tumorigenesis and immune evasion. Inflammatory mediators establish a dynamic pro-tumor network through multiple signaling cascades. On one hand, they induce epithelial injury, stimulate aberrant proliferation, and foster genomic instability, thereby driving chronic inflammation toward malignant transformation. On the other hand, the same inflammatory signals sculpt an immunosuppressive microenvironment that dampens anti-tumor immunity and facilitates tumor immune escape. Thus, carcinogenesis and immune evasion represent interdependent processes—two facets of a single pathological continuum—linked by temporal and spatial feedback loops orchestrated by inflammatory signaling.
From this integrative perspective, targeting a single inflammatory pathway only offers limited and transient therapeutic benefit. A combinatorial strategy that suppresses pro-inflammatory signalling, reprograms immunosuppressive cells and activates anti-tumour immunity offers greater therapeutic potential. Biomarkers reflecting inflammatory network dynamics and the status of the TME are essential for patient stratification, combination therapy design and treatment response monitoring.
Furthermore, additional longitudinal clinical samples and mechanistic studies are required in order to identify biomarkers that can predict treatment response and guide stratified therapy. In summary, unravelling the interactive networks of inflammatory factors within the TME will provide a theoretical foundation for developing combined, personalised therapeutic strategies, ultimately improving clinical outcomes for gastric cancer patients.
Author contributions
MZ: Conceptualization, Writing – review & editing, Writing – original draft. AS: Conceptualization, Writing – review & editing, Writing – original draft. HS: Conceptualization, Writing – review & editing, Writing – original draft. YD: Writing – review & editing, Validation. WJ: Project administration, Conceptualization, Writing – review & editing. JG: Investigation, Validation, Writing – review & editing. WZ: Investigation, Writing – review & editing, Validation. YM: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. MH: Conceptualization, Writing – review & editing, Supervision. SZ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by Gansu Provincial Science and Technology Plan (Joint Scientific Research Fund) Project (24JRRA885); Natural Science Foundation of Gansu Province funding project (22JR5RA663); Research Project of Gansu Provincial People’s Hospital (2024KYQDJ-A-14). The APC was funded by Gansu Provincial Science and Technology Plan (Joint Scientific Research Fund) Project (24JRRA885).
Acknowledgments
The authors thank all the members of Department of General Surgery of Gansu Provincial People’s Hospital for the discussions. All figures were drawn BioGDP (https://biogdp.com/).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer LLin declared a shared affiliation with the author AS to the handling editor at the time of the review.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
GC: Gastric Cancer
H. pylori: Helicobacter pylori
IL: Interleukin
TNF: Tumor Necrosis Factor
IFN: Interferon
TME: Tumor microenvironment
ECM: Extracellular matrix
VEGF: Vascular endothelial growth factor
TAMs: Tumor-associated macrophages
MDSCs: Myeloid-derived suppressor cells
NSAIDs: Nonsteroidal anti-inflammatory drugs
TGF: Transforming Growth Factor
ROS: Reactive oxygen species
ADCs: Antibody-drug conjugates
PD-L1: Programmed death-ligand 1
PD-1: Programmed death-1
miRNAs: MicroRNAs
CagPAI: Cag pathogenicity island
Cag-T4SS: Cag type IV secretion system
PGN: Peptidoglycan
EMT: Epithelial–mesenchymal transition
CAFs: Cancer-associated fibroblasts
OMCC: Outer membrane core complex
TNFR: Tumor Necrosis Factor Receptor
MMPs: Matrix metalloproteinases
ISGs: Interferon-stimulated genes
MCP-1: Monocyte chemotactic protein-1
MIP-1α: Macrophage inflammatory protein-1α
RANTES: Regulated on Activation, Normal T Expressed and Secreted
MIP: Macrophage inflammatory proteins
SOCS: Suppressor of Cytokine Signaling
CAPS: Cryopyrin-Associated Periodic Syndromes
TRAPS: Tumor Necrosis Factor Receptor Associated Periodic Syndrome
HIDS: Hyperimmunoglobulin D Syndrome
MKD: Mevalonate Kinase Deficiency
FMF: Familial Mediterranean Fever
AOSD: Active Adult-Onset Still’s Disease
SJIA: Systemic Juvenile Idiopathic Arthritis
RA: Rheumatoid Arthritis
BD: Behçet’s Disease
CHD: Coronary Heart Disease
DMARDs: Disease modifying antirheumatic drugs
NOMID: Neonatal Onset Multi-System Inflammatory Disease
DIRA: Interleukin-1 Receptor Antagonist
RCC: Renal Cell Carcinoma
AOR: Acute organ rejection
NSCLC: Non-Small Cell Lung Cancer
HNSCC: Head and Neck Squamous Cell Carcinoma
AD: Atopic Dermatitis
CRSwNP: Chronic rhinosinusitis with nasal polyposis
EoE: Eosinophilic Esophagitis
CRS: Cytokine Release Syndrome
MCD: Multicentric Castleman Disease
NMOSD: Neuromyelitis Optica Spectrum Disorder
KTR: Kidney Transplant Rejection
BC: Breast Cancer
HCC: Hepatocellular Carcinoma
PsO: Psoriasis
AS: Ankylosing Spondylitis
PsA: Psoriatic Arthritis
AoSD: Adult -Onset Still’s Disease
CD: Crohn’s Disease
UC: Ulcerative Colitis
STS: Soft Tissue Sarcoma
HCL: Hairy Cell Leukemia
KS: Kaposi Sarcoma
CHB: Chronic Hepatitis B
CHC: Chronic Hepatitis C
RRMS: Relapsing Multiple Sclerosis
SPMS: Secondary Progressive Multiple Sclerosis
CGD: Chronic Granulomatous Disease
HIV: Human Immunodeficiency Virus
PCa: Prostate Cancer
CRC: Colorectal Cancer
MM: Multiple Myeloma
NHL: Non-Hodgkin Lymphoma
GBM: Glioblastoma
References
1. Balkwill F and Mantovani A. Inflammation and cancer: back to Virchow? Lancet. (2001) 357:539–45. doi: 10.1016/S0140-6736(00)04046-0
2. Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. (2006) 441:431–6. doi: 10.1038/nature04870
3. Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. (2009) 15:103–13. doi: 10.1016/j.ccr.2009.01.001
4. Fan Y, Mao R, and Yang J. NF-kappaB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell. (2013) 4:176–85. doi: 10.1007/s13238-013-2084-3
5. Aggarwal BB, Vijayalekshmi RV, and Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. (2009) 15:425–30. doi: 10.1158/1078-0432.CCR-08-0149
6. Wang X, Zhao G, Shao S, and Yao Y. Helicobacter pylori triggers inflammation and oncogenic transformation by perturbing the immune microenvironment. Biochim Biophys Acta Rev Cancer. (2024) 1879:189139. doi: 10.1016/j.bbcan.2024.189139
7. Broholm M, Mathiasen AS, Apol AD, and Weis N. The adaptive immune response in hepatitis B virus-associated hepatocellular carcinoma is characterized by dysfunctional and exhausted HBV-specific T cells. Viruses. (2024) 16. doi: 10.3390/v16050707
8. Todorovic N, Martinelli S, Nannini G, Weiskirchen R, and Amedei A. Etiology-dependent microbiome differences in hepatocellular carcinoma development. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms252413510
9. Tezcan G, Yakar N, Hasturk H, Van Dyke TE, and Kantarci A. Resolution of chronic inflammation and cancer. Periodontol 2000. (2024) 96:229–49. doi: 10.1111/prd.12603
10. Kiely M, Lord B, and Ambs S. Immune response and inflammation in cancer health disparities. Trends Cancer. (2022) 8:316–27. doi: 10.1016/j.trecan.2021.11.010
11. Tong Y, Gao H, Qi Q, Liu X, Li J, Gao J, et al. High fat diet, gut microbiome and gastrointestinal cancer. Theranostics. (2021) 11:5889–910. doi: 10.7150/thno.56157
12. Maiorino L, Dassler-Plenker J, Sun L, and Egeblad M. Innate immunity and cancer pathophysiology. Annu Rev Pathol. (2022) 17:425–57. doi: 10.1146/annurev-pathmechdis-032221-115501
13. Fernandes Q, Inchakalody VP, Bedhiafi T, Mestiri S, Taib N, Uddin S, et al. Chronic inflammation and cancer; the two sides of a coin. Life Sci. (2024) 338:122390. doi: 10.1016/j.lfs.2023.122390
14. Klapp V, Alvarez-Abril B, Leuzzi G, Kroemer G, Ciccia A, and Galluzzi L. The DNA damage response and inflammation in cancer. Cancer Discov. (2023) 13:1521–45. doi: 10.1158/2159-8290.CD-22-1220
15. Vasquez Martinez IP, Perez-Campos E, Perez-Campos Mayoral L, Cruz Luis HI, Pina Canseco MDS, Zenteno E, et al. O-glcNAcylation: crosstalk between hemostasis, inflammation, and cancer. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25189896
16. Lopez-Moyado IF, Ko M, Hogan PG, and Rao A. TET enzymes in the immune system: from DNA demethylation to immunotherapy, inflammation, and cancer. Annu Rev Immunol. (2024) 42:455–88. doi: 10.1146/annurev-immunol-080223-044610
17. Yu H, Lin L, Zhang Z, Zhang H, and Hu H. Targeting NF-kappaB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. (2020) 5:209. doi: 10.1038/s41392-020-00312-6
18. Zou S, Tong Q, Liu B, Huang W, Tian Y, and Fu X. Targeting STAT3 in cancer immunotherapy. Mol Cancer. (2020) 19:145. doi: 10.1186/s12943-020-01258-7
19. Wang P, Gong Q, Hu J, Li X, and Zhang X. Reactive oxygen species (ROS)-responsive prodrugs, probes, and theranostic prodrugs: applications in the ROS-related diseases. J Med Chem. (2021) 64:298–325. doi: 10.1021/acs.jmedchem.0c01704
20. Caliri AW, Tommasi S, and Besaratinia A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat Res Rev Mutat Res. (2021) 787:108365. doi: 10.1016/j.mrrev.2021.108365
21. Grivennikov SI, Greten FR, and Karin M. Immunity, inflammation, and cancer. Cell. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
22. Poh AR and Ernst M. Functional roles of SRC signaling in pancreatic cancer: Recent insights provide novel therapeutic opportunities. Oncogene. (2023) 42:1786–801. doi: 10.1038/s41388-023-02701-x
23. Lee HJ, Hong YJ, and Kim M. Angiogenesis in chronic inflammatory skin disorders. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms222112035
24. Cao Y, Xia H, Tan X, Shi C, Ma Y, Meng D, et al. Intratumoural microbiota: a new frontier in cancer development and therapy. Signal Transduct Target Ther. (2024) 9:15. doi: 10.1038/s41392-023-01693-0
25. Vago JP, Amaral FA, and Van De Loo FAJ. Resolving inflammation by TAM receptor activation. Pharmacol Ther. (2021) 227:107893. doi: 10.1016/j.pharmthera.2021.107893
26. Lasser SA, Ozbay Kurt FG, Arkhypov I, Utikal J, and Umansky V. Myeloid-derived suppressor cells in cancer and cancer therapy. Nat Rev Clin Oncol. (2024) 21:147–64. doi: 10.1038/s41571-023-00846-y
27. Waldner MJ and Neurath MF. Colitis-associated cancer: the role of T cells in tumor development. Semin Immunopathol. (2009) 31:249–56. doi: 10.1007/s00281-009-0161-8
28. Waldum H and Fossmark R. Inflammation and digestive cancer. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms241713503
29. Nickoloff BJ, Ben-Neriah Y, and Pikarsky E. Inflammation and cancer: is the link as simple as we think? J Invest Dermatol. (2005) 124:x–xiv. doi: 10.1111/j.0022-202X.2005.23724.x
30. Fu C, Chen J, Lu J, Yi L, Tong X, Kang L, et al. Roles of inflammation factors in melanogenesis (Review). Mol Med Rep. (2020) 21:1421–30. doi: 10.3892/mmr.2020.10950
31. Benucci M, Bernardini P, Coccia C, De Luca R, Levani J, Economou A, et al. JAK inhibitors and autoimmune rheumatic diseases. Autoimmun Rev. (2023) 22:103276. doi: 10.1016/j.autrev.2023.103276
32. Henein MY, Vancheri S, Longo G, and Vancheri F. The role of inflammation in cardiovascular disease. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms232112906
33. Shah SC and Itzkowitz SH. Colorectal cancer in inflammatory bowel disease: mechanisms and management. Gastroenterology. (2022) 162:715–730.e3. doi: 10.1053/j.gastro.2021.10.035
34. Turner MD, Nedjai B, Hurst T, and Pennington DJ. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. (2014) 1843:2563–82. doi: 10.1016/j.bbamcr.2014.05.014
35. Khawkhiaw K, Panaampon J, Imemkamon T, and Saengboonmee C. Interleukin-1beta: Friend or foe for gastrointestinal cancers. World J Gastrointest Oncol. (2024) 16:1676–82. doi: 10.4251/wjgo.v16.i5.1676
36. Tremoulet AH, Jain S, Kim S, Newburger J, Arditi M, Franco A, et al. Rationale and study design for a phase I/IIa trial of anakinra in children with Kawasaki disease and early coronary artery abnormalities (the ANAKID trial). Contemp Clin Trials. (2016) 48:70–5. doi: 10.1016/j.cct.2016.04.002
37. Zhang F, Si M, Wang H, Mekhemar MK, Dorfer CE, and Fawzy El-Sayed KM. IL-1/TNF-alpha inflammatory and anti-inflammatory synchronization affects gingival stem/progenitor cells’ Regenerative attributes. Stem Cells Int. (2017) 2017:1349481. doi: 10.1155/2017/1349481
38. Bick F, Brenis Gomez CM, Lammens I, Van Moorleghem J, De Wolf C, Dupont S, et al. IL-2 family cytokines IL-9 and IL-21 differentially regulate innate and adaptive type 2 immunity in asthma. J Allergy Clin Immunol. (2024) 154:1129–45. doi: 10.1016/j.jaci.2024.07.024
39. Fukushima K, Hara-Kuge S, Ideo H, and Yamashita K. Carbohydrate recognition site of interleukin-2 in relation to cell proliferation. J Biol Chem. (2001) 276:31202–8. doi: 10.1074/jbc.M102789200
40. Xie Z, Zheng J, Wang Y, Li D, Maermaer T, Li Y, et al. Deficient IL-2 produced by activated CD56(+) T cells contributes to impaired NK cell-mediated ADCC function in chronic HIV-1 infection. Front Immunol. (2019) 10:1647. doi: 10.3389/fimmu.2019.01647
41. Runnstrom MC, Lamothe PA, Faliti CE, Cheedarla N, Moreno A, Suthar MS, et al. Patients taking benralizumab, dupilumab, or mepolizumab have lower postvaccination SARS-CoV-2 immunity. J Allergy Clin Immunol. (2024) 154:435–46. doi: 10.1016/j.jaci.2024.03.029
42. da Silva GM, De Figueiredo CS, Da Rocha Oliveira AC, Raony I, De Araujo Miranda RA, De Mello Silva E, et al. Interleukin-4 activates divergent cell-intrinsic signals to regulate retinal cell proliferation induced by classical growth factors. Mol Cell Neurosci. (2022) 123:103780. doi: 10.1016/j.mcn.2022.103780
43. Hart PH, Bonder CS, Jones CA, and Finlay-Jones JJ. Control of major histocompatibility complex class II expression on human monocytes by interleukin-4: regulatory effect of lipopolysaccharide. Immunology. (1996) 89:599–605. doi: 10.1046/j.1365-2567.1996.d01-779.x
44. Hils M, Hoffard N, Iuliano C, Kreft L, Chakrapani N, Swiontek K, et al. IgE and anaphylaxis specific to the carbohydrate alpha-gal depend on IL-4. J Allergy Clin Immunol. (2024) 153:1050–1062 e6. doi: 10.1016/j.jaci.2023.12.003
45. Padro M, Mejias-Luque R, Cobler L, Garrido M, Perez-Garay M, Puig S, et al. Regulation of glycosyltransferases and Lewis antigens expression by IL-1beta and IL-6 in human gastric cancer cells. Glycoconj J. (2011) 28:99–110. doi: 10.1007/s10719-011-9327-4
46. Adelman DC, Matsuda T, Hirano T, Kishimoto T, and Saxon A. Elevated serum interleukin-6 associated with a failure in B cell differentiation in common variable immunodeficiency. J Allergy Clin Immunol. (1990) 86:512–21. doi: 10.1016/S0091-6749(05)80207-6
47. Nabil G, Ahmed YH, Ahmed O, Milad SS, Hisham M, Rafat M, et al. Argel’s stemmoside C as a novel natural remedy for mice with alcohol-induced gastric ulcer based on its molecular mechanistic pathways. J Ethnopharmacol. (2024) 327:117970. doi: 10.1016/j.jep.2024.117970
48. Roring RJ, Scognamiglio F, De Jong LC, Groh LA, Matzaraki V, Koeken V, et al. Interleukin-10 inhibits important components of trained immunity in human monocytes. J Leukoc Biol. (2024), 117. doi: 10.1093/jleuko/qiae240
49. Garcia-Torre A, Bueno-Garcia E, Moro-Garcia MA, Lopez-Martinez R, Rioseras B, Diaz-Molina B, et al. IL-10 indirectly modulates functional activity of CD4(+)CD28(null) T-lymphocytes through LFA-3 and HLA class II inhibition. Immunology. (2024) 173:296–309. doi: 10.1111/imm.13824
50. Liu Z, Gao M, Yan F, Zhang H, Wang L, Zhao Y, et al. Cucurbitacin IIb mitigates concanavalin A-induced acute liver injury by suppressing M1 macrophage polarization. Int Immunopharmacol. (2025) 147:113964. doi: 10.1016/j.intimp.2024.113964
51. Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. (1995) 13:251–76. doi: 10.1146/annurev.iy.13.040195.001343
52. Luo WJ, Yu SL, Chang CC, Chien MH, Chang YL, Liao KM, et al. HLJ1 amplifies endotoxin-induced sepsis severity by promoting IL-12 heterodimerization in macrophages. Elife. (2022) 11. doi: 10.7554/eLife.76094.sa2
53. Schwarz E, Savardekar H, Zelinskas S, Mouse A, Lapurga G, Lyberger J, et al. Trabectedin enhances the antitumor effects of IL-12 in triple-negative breast cancer. Cancer Immunol Res. (2025) 13:560–76. doi: 10.1158/2326-6066.CIR-24-0775
54. Gagnon B, Murphy J, Simonyan D, Penafuerte CA, Sirois J, Chasen M, et al. Cancer anorexia-cachexia syndrome is characterized by more than one inflammatory pathway. J Cachexia Sarcopenia Muscle. (2024) 15:1041–53. doi: 10.1002/jcsm.13430
55. Balakumar A, Cox A, and Thangamani S. Cell aggregation mediated by ACE2 deletion in Candida auris modulates fungal colonization and host immune responses in the skin. mSphere. (2024) 9:e0073424. doi: 10.1128/msphere.00734-24
56. Zhao G, Zhang H, Zhu S, Wang S, Zhu K, Zhao Y, et al. Interleukin-18 accelerates cardiac inflammation and dysfunction during ischemia/reperfusion injury by transcriptional activation of CXCL16. Cell Signal. (2021) 87:110141. doi: 10.1016/j.cellsig.2021.110141
57. Poznanski SM, Lee AJ, Nham T, Lusty E, Larche MJ, Lee DA, et al. Combined stimulation with interleukin-18 and interleukin-12 potently induces interleukin-8 production by natural killer cells. J Innate Immun. (2017) 9:511–25. doi: 10.1159/000477172
58. Jaldeep L, Lipi B, and Prakash P. Neurotrophomodulatory effect of TNF-alpha through NF-kappaB in rat cortical astrocytes. Cytotechnology. (2025) 77:37. doi: 10.1007/s10616-025-00743-5
59. Kitaura H, Marahleh A, Ohori F, Noguchi T, Nara Y, Pramusita A, et al. Role of the interaction of tumor necrosis factor-alpha and tumor necrosis factor receptors 1 and 2 in bone-related cells. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23031481
60. Zhao X, Wang M, Zhang Y, Zhang Y, Tang H, Yue H, et al. Macrophages in the inflammatory response to endotoxic shock. Immun Inflammation Dis. (2024) 12:e70027. doi: 10.1002/iid3.70027
61. Saleh AA, Mohamed AZ, Elnesr SS, Khafaga AF, Elwan H, Abdel-Aziz MF, et al. Expression and Immune Response Profiles in Nile Tilapia (Oreochromis niloticus) and European Sea Bass (Dicentrarchus labrax) During Pathogen Challenge and Infection. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms252312829
62. Yao Z, Guo F, Tan Y, Zhang Y, Geng Y, Yang G, et al. Causal relationship between inflammatory cytokines and autoimmune thyroid disease: a bidirectional two-sample Mendelian randomization analysis. Front Immunol. (2024) 15:1334772. doi: 10.3389/fimmu.2024.1334772
63. Harris AR, Wang T, Heng YJ, Baker GM, Le PA, Wang J, et al. Association of early menarche with breast tumor molecular features and recurrence. Breast Cancer Res. (2024) 26:102. doi: 10.1186/s13058-024-01839-0
64. Li T, Yang X, Li W, Song J, Li Z, Zhu X, et al. ADAR1 stimulation by IFN-alpha downregulates the expression of MAVS via RNA editing to regulate the anti-HBV response. Mol Ther. (2021) 29:1335–48. doi: 10.1016/j.ymthe.2020.11.031
65. Guo Y, Qian R, Li Z, Lv T, Yang C, Li W, et al. Tumor-derived nanovesicles enhance cancer synergistic chemo-immunotherapy by promoting cGAS/STING pathway activation and immunogenetic cell death. Life Sci. (2024) 348:122687. doi: 10.1016/j.lfs.2024.122687
66. Gao D, Ciancanelli MJ, Zhang P, Harschnitz O, Bondet V, Hasek M, et al. TLR3 controls constitutive IFN-beta antiviral immunity in human fibroblasts and cortical neurons. J Clin Invest. (2021) 131. doi: 10.1172/JCI134529
67. Hiebinger F, Kudulyte A, Chi H, Burbano De Lara S, Ilic D, Helm B, et al. Tumour cells can escape antiproliferative pressure by interferon-beta through immunoediting of interferon receptor expression. Cancer Cell Int. (2023) 23:315. doi: 10.1186/s12935-023-03150-y
68. Yang S, Chen R, Wu Y, Song X, Peng X, and Chen M. Fluorinated polyethyleneimine vectors with serum resistance and adjuvant effect to deliver LMP2 mRNA vaccine for nasopharyngeal carcinoma therapy. Acta Biomater. (2024) 192:340–52. doi: 10.1016/j.actbio.2024.12.022
69. Bordi L, D’auria A, Frasca F, Mazzotta V, Mazzetti P, Fracella M, et al. MPXV infection impairs IFN response but is partially sensitive to IFN-gamma antiviral effect. Med Microbiol Immunol. (2024) 213:25. doi: 10.1007/s00430-024-00808-w
70. Huang W, O’keefe RJ, and Schwarz EM. Exposure to receptor-activator of NFkappaB ligand renders pre-osteoclasts resistant to IFN-gamma by inducing terminal differentiation. Arthritis Res Ther. (2003) 5:R49–59. doi: 10.1186/ar612
71. Fraccarollo D, Neuser J, Moller J, Riehle C, Galuppo P, and Bauersachs J. Expansion of CD10(neg) neutrophils and CD14(+)HLA-DR(neg/low) monocytes driving proinflammatory responses in patients with acute myocardial infarction. Elife. (2021) 10. doi: 10.7554/eLife.66808
72. Bhanpattanakul S, Tharasanit T, Buranapraditkun S, Sailasuta A, Nakagawa T, and Kaewamatawong T. Modulation of MHC expression by interferon-gamma and its influence on PBMC-mediated cytotoxicity in canine mast cell tumour cells. Sci Rep. (2024) 14:17837. doi: 10.1038/s41598-024-68789-7
73. Sagar D, Lamontagne A, Foss CA, Khan ZK, Pomper MG, and Jain P. Dendritic cell CNS recruitment correlates with disease severity in EAE via CCL2 chemotaxis at the blood-brain barrier through paracellular transmigration and ERK activation. J Neuroinflamm. (2012) 9:245. doi: 10.1186/1742-2094-9-245
74. Wang X, Lin L, Zhang X, Zhang M, Sun Z, Yang Y, et al. Single-cell Atlas reveals core function of CPVL/MSR1 expressing macrophages in the prognosis of triple-negative breast cancer. Front Immunol. (2024) 15:1501009. doi: 10.3389/fimmu.2024.1501009
75. Hao Q, Vadgama JV, and Wang P. CCL2/CCR2 signaling in cancer pathogenesis. Cell Commun Signal. (2020) 18:82. doi: 10.1186/s12964-020-00589-8
76. Zhao X, Gu M, Xu X, Wen X, Yang G, Li L, et al. CCL3/CCR1 mediates CD14(+)CD16(-) circulating monocyte recruitment in knee osteoarthritis progression. Osteoarthritis Cartilage. (2020) 28:613–25. doi: 10.1016/j.joca.2020.01.009
77. Zhang G, Liu HB, Zhou L, Cui XQ, and Fan XH. CCL3 participates in the development of rheumatoid arthritis by activating AKT. Eur Rev Med Pharmacol Sci. (2018) 22:6625–32. doi: 10.26355/eurrev20181016137
78. Zhao N, Zhang C, Wu Y, Ding J, Wang F, Cheng W, et al. ROS-CCL5 axis recruits CD8(+) T lymphocytes promoting the apoptosis of granulosa cells in diminished ovary reserve. J Reprod Immunol. (2023) 155:103789. doi: 10.1016/j.jri.2022.103789
79. Tachizaki M, Kobori Y, Kawaguchi S, Seya K, Tanaka H, and Imaizumi T. Cylindromatosis lysine 63 deubiquitinase (CYLD) suppress TLR3-mediated CCL5 expression in human renal proximal tubular epithelial cells. Mol Biol Rep. (2024) 51:974. doi: 10.1007/s11033-024-09904-9
80. Tian Y, Zhang L, Ping Y, Zhang Z, Yao C, Shen C, et al. CCR5 and IL-12 co-expression in CAR T cells improves antitumor efficacy by reprogramming tumor microenvironment in solid tumors. Cancer Immunol Immunother. (2025) 74:55. doi: 10.1007/s00262-024-03909-w
81. Zeng Z, Lan T, Wei Y, and Wei X. CCL5/CCR5 axis in human diseases and related treatments. Genes Dis. (2022) 9:12–27. doi: 10.1016/j.gendis.2021.08.004
82. Gunasekara S, Tamil Selvan M, Murphy CL, Shatnawi S, Cowan S, More S, et al. Characterization of neutrophil functional responses to SARS-CoV-2 infection in a translational feline model for COVID-19. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms251810054
83. Ishimoto N, Park JH, Kawakami K, Tajiri M, Mizutani K, Akashi S, et al. Structural basis of CXC chemokine receptor 1 ligand binding and activation. Nat Commun. (2023) 14:4107. doi: 10.1038/s41467-023-39799-2
84. Yang X, Chen Z, Qiu T, Liu Y, Ren H, Luo W, et al. Lichong decoction improves inflammatory microenvironment and alleviates fibrosis in uterine leiomyoma via targeting CXCL8. J Ethnopharmacol. (2024) 340:119276. doi: 10.1016/j.jep.2024.119276
85. Qiu J, Xu Q, Panah T, Morshed A, Wang X, Zhou F, et al. Reactive oxygen species mediate ovarian cancer development, platinum resistance, and angiogenesis via CXCL8 and GSK-3beta/p70S6K1 axis. Genes Dis. (2025) 12:101378. doi: 10.1016/j.gendis.2024.101378
86. Song N, Cui K, Zeng L, Li M, Fan Y, Shi P, et al. Advance in the role of chemokines/chemokine receptors in carcinogenesis: Focus on pancreatic cancer. Eur J Pharmacol. (2024) 967:176357. doi: 10.1016/j.ejphar.2024.176357
87. Hirani DV, Thielen F, Mansouri S, Danopoulos S, Vohlen C, Haznedar-Karakaya P, et al. CXCL10 deficiency limits macrophage infiltration, preserves lung matrix, and enables lung growth in bronchopulmonary dysplasia. Inflammation Regener. (2023) 43:52. doi: 10.1186/s41232-023-00301-6
88. Wang X, Huang W, Sun H, Wang H, Wang D, and Wang Y. Tomatidine relieves neuronal damage in spinal cord injury by inhibiting the inflammatory responses and apoptosis through blocking the NF-kappaB/CXCL10 pathway activation. Front Pharmacol. (2024) 15:1503925. doi: 10.3389/fphar.2024.1503925
89. Zhang X, Huang J, Wang J, Li Y, Hu G, and Li H. Circ_0001667 accelerates breast cancer proliferation and angiogenesis through regulating CXCL10 expression by sponging miR-6838-5p. Thorac Cancer. (2023) 14:881–92. doi: 10.1111/1759-7714.14820
90. Ghadge SK, Muhlstedt S, Ozcelik C, and Bader M. SDF-1alpha as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol Ther. (2011) 129:97–108. doi: 10.1016/j.pharmthera.2010.09.011
91. Meng Z, Feng G, Hu X, Yang L, Yang X, and Jin Q. SDF factor-1alpha promotes the migration, proliferation, and osteogenic differentiation of mouse bone marrow mesenchymal stem cells through the wnt/beta-catenin pathway. Stem Cells Dev. (2021) 30:106–17. doi: 10.1089/scd.2020.0165
92. Sun Y, Mo Y, Jiang S, Shang C, Feng Y, and Zeng X. CXC chemokine ligand-10 promotes the accumulation of monocyte-like myeloid-derived suppressor cells by activating p38 MAPK signaling under tumor conditions. Cancer Sci. (2023) 114:142–51. doi: 10.1111/cas.15598
93. Chen CB, Hung SI, Chang JW, Yang CK, Ma DH, Teng YC, et al. Immune checkpoint inhibitor-induced severe epidermal necrolysis mediated by macrophage-derived CXCL10 and abated by TNF blockade. Nat Commun. (2024) 15:10733. doi: 10.1038/s41467-024-54180-7
94. Sun X, Cao Y, Wang L, Chen H, and Zhang F. CCL26 in primary biliary cholangitis - Is it a novel disease mediator? Int J Rheum Dis. (2023) 26:648–56. doi: 10.1111/1756-185X.14578
95. Zi R, Zhao X, Liu L, Wang Y, Zhang R, Bian Z, et al. Metabolic-immune suppression mediated by the SIRT1-CX3CL1 axis induces functional enhancement of regulatory T cells in colorectal carcinoma. Adv Sci (Weinh). (2025) 12:e2404734. doi: 10.1002/advs.202404734
96. Liu PP, Liu XH, Ren MJ, Liu XT, Shi XQ, Li ML, et al. Neuronal cathepsin S increases neuroinflammation and causes cognitive decline via CX3CL1-CX3CR1 axis and JAK2-STAT3 pathway in aging and Alzheimer’s disease. Aging Cell. (2024) 24:e14393. doi: 10.1111/acel.14393
97. Pezeshkian F, Shahriarirad R, and Mahram H. An overview of the role of chemokine CX3CL1 (Fractalkine) and CX3C chemokine receptor 1 in systemic sclerosis. Immun Inflammation Dis. (2024) 12:e70034. doi: 10.1002/iid3.70034
98. Ma J, Shi Y, Lu Q, and Huang D. Inflammation-related gene ADH1A regulates the polarization of macrophage M1 and influences the Malignant progression of gastric cancer. J Inflammation Res. (2024) 17:4647–65. doi: 10.2147/JIR.S452670
99. Waldum H and Fossmark R. Gastritis, gastric polyps and gastric cancer. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22126548
100. Yang H, Yang WJ, and Hu B. Gastric epithelial histology and precancerous conditions. World J Gastrointest Oncol. (2022) 14:396–412. doi: 10.4251/wjgo.v14.i2.396
101. Smyth EC, Nilsson M, Grabsch HI, Van Grieken NC, and Lordick F. Gastric cancer. Lancet. (2020) 396:635–48. doi: 10.1016/S0140-6736(20)31288-5
102. Song H, Zhang M, Guo C, Guo X, Ma Y, and Ma Y. Implication of protein post translational modifications in gastric cancer. Front Cell Dev Biol. (2025) 13:1523958. doi: 10.3389/fcell.2025.1523958
103. Briukhovetska D, Suarez-Gosalvez J, Voigt C, Markota A, Giannou AD, Schubel M, et al. T cell-derived interleukin-22 drives the expression of CD155 by cancer cells to suppress NK cell function and promote metastasis. Immunity. (2023) 56:143–161 e11. doi: 10.1016/j.immuni.2022.12.010
104. Zhao N, Liu C, Li N, Zhou S, Guo Y, Yang S, et al. Role of Interleukin-22 in ulcerative colitis. BioMed Pharmacother. (2023) 159:114273. doi: 10.1016/j.biopha.2023.114273
105. McNab F, Mayer-Barber K, Sher A, Wack A, and O’garra A. Type I interferons in infectious disease. Nat Rev Immunol. (2015) 15:87–103. doi: 10.1038/nri3787
106. Weber BN, Giles JT, and Liao KP. Shared inflammatory pathways of rheumatoid arthritis and atherosclerotic cardiovascular disease. Nat Rev Rheumatol. (2023) 19:417–28. doi: 10.1038/s41584-023-00969-7
107. Briukhovetska D, Dorr J, Endres S, Libby P, Dinarello CA, and Kobold S. Interleukins in cancer: from biology to therapy. Nat Rev Cancer. (2021) 21:481–99. doi: 10.1038/s41568-021-00363-z
108. Soler MF, Abaurrea A, Azcoaga P, Araujo AM, and Caffarel MM. New perspectives in cancer immunotherapy: targeting IL-6 cytokine family. J Immunother Cancer. (2023) 11. doi: 10.1136/jitc-2023-007530
109. Li M, Jin S, Zhang Z, Ma H, and Yang X. Interleukin-6 facilitates tumor progression by inducing ferroptosis resistance in head and neck squamous cell carcinoma. Cancer Lett. (2022) 527:28–40. doi: 10.1016/j.canlet.2021.12.011
110. Ding L, Sontz EA, Saqui-Salces M, and Merchant JL. Interleukin-1beta suppresses gastrin via primary cilia and induces antral hyperplasia. Cell Mol Gastroenterol Hepatol. (2021) 11:1251–66. doi: 10.1016/j.jcmgh.2020.12.008
111. Rech TF, Mazzoleni LE, Mazzoleni F, Francesconi CFM, Sander GB, Michita RT, et al. Analysis of the influence of interleukin-1beta gene polymorphism on gastric inflammatory response and precancerous lesions development in patients with functional dyspepsia. Immunol Invest. (2020) 49:585–96. doi: 10.1080/08820139.2019.1710532
112. Teng Y, Cang B, Mao F, Chen W, Cheng P, Peng L, et al. Expression of ETS1 in gastric epithelial cells positively regulate inflammatory response in Helicobacter pylori-associated gastritis. Cell Death Dis. (2020) 11:498. doi: 10.1038/s41419-020-2705-8
113. Yuan XY, Zhang Y, Zhao X, Chen A, and Liu P. IL-1beta, an important cytokine affecting Helicobacter pylori-mediated gastric carcinogenesis. Microb Pathog. (2023) 174:105933. doi: 10.1016/j.micpath.2022.105933
114. Han TS, Voon DC, Oshima H, Nakayama M, Echizen K, Sakai E, et al. Interleukin 1 up-regulates microRNA 135b to promote inflammation-associated gastric carcinogenesis in mice. Gastroenterology. (2019) 156:1140–1155 e4. doi: 10.1053/j.gastro.2018.11.059
115. Han Y, Zhang YY, Pan YQ, Zheng XJ, Liao K, Mo HY, et al. IL-1beta-associated NNT acetylation orchestrates iron-sulfur cluster maintenance and cancer immunotherapy resistance. Mol Cell. (2023) 83:1887–1902 e8. doi: 10.1016/j.molcel.2023.05.011
116. Davinelli S, Melvang HM, Andersen LP, Scapagnini G, and Nielsen ME. Astaxanthin from shrimp cephalothorax stimulates the immune response by enhancing IFN-gamma, IL-10, and IL-2 secretion in splenocytes of helicobacter pylori-infected mice. Mar Drugs. (2019) 17. doi: 10.3390/md17070382
117. Lv Y, Tian W, Teng Y, Wang P, Zhao Y, Li Z, et al. Tumor-infiltrating mast cells stimulate ICOS(+) regulatory T cells through an IL-33 and IL-2 axis to promote gastric cancer progression. J Adv Res. (2024) 57:149–62. doi: 10.1016/j.jare.2023.04.013
118. Yang T, Wang R, Liu H, Wang L, Li J, Wu S, et al. Berberine regulates macrophage polarization through IL-4-STAT6 signaling pathway in Helicobacter pylori-induced chronic atrophic gastritis. Life Sci. (2021) 266:118903. doi: 10.1016/j.lfs.2020.118903
119. Zavros Y, Rathinavelu S, Kao JY, Todisco A, Del Valle J, Weinstock JV, et al. Treatment of Helicobacter gastritis with IL-4 requires somatostatin. Proc Natl Acad Sci U.S.A. (2003) 100:12944–9. doi: 10.1073/pnas.2135193100
120. Xiang ZD, Guan HD, Zhao X, Xie Q, Cai FJ, Xie ZJ, et al. Protoberberine alkaloids: A review of the gastroprotective effects, pharmacokinetics, and toxicity. Phytomedicine. (2024) 126:155444. doi: 10.1016/j.phymed.2024.155444
121. Song X, Traub B, Shi J, and Kornmann M. Possible roles of interleukin-4 and -13 and their receptors in gastric and colon cancer. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22020727
122. Chen Y, Wang X, Yu Y, Xiao Y, Huang J, Yao Z, et al. Serum exosomes of chronic gastritis patients infected with Helicobacter pylori mediate IL-1alpha expression via IL-6 trans-signalling in gastric epithelial cells. Clin Exp Immunol. (2018) 194:339–49. doi: 10.1111/cei.13200
123. Zhou Q, Xue B, Gu R, Li P, and Gu Q. Lactobacillus plantarum ZJ316 Attenuates Helicobacter pylori-Induced Gastritis in C57BL/6 Mice. J Agric Food Chem. (2021) 69:6510–23. doi: 10.1021/acs.jafc.1c01070
124. Han L, Li T, Wang Y, Lai W, Zhou H, Niu Z, et al. Weierning, a Chinese patent medicine, improves chronic atrophic gastritis with intestinal metaplasia. J Ethnopharmacol. (2023) 309:116345. doi: 10.1016/j.jep.2023.116345
125. Zhou P, Zheng ZH, Wan T, Liao CW, and Wu J. Yiqi Jiedu Huayu decoction inhibits precancerous lesions of chronic atrophic gastritis by inhibiting NLRP3 inflammasome-mediated pyroptosis. World J Gastrointest Oncol. (2024) 16:3158–68. doi: 10.4251/wjgo.v16.i7.3158
126. Crabtree JE, Shallcross TM, Heatley RV, and Wyatt JI. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. (1991) 32:1473–7. doi: 10.1136/gut.32.12.1473
127. Basso D, Scrigner M, Toma A, Navaglia F, Di Mario F, Rugge M, et al. Helicobacter pylori infection enhances mucosal interleukin-1 beta, interleukin-6, and the soluble receptor of interleukin-2. Int J Clin Lab Res. (1996) 26:207–10. doi: 10.1007/BF02592984
128. Piao JY, Lee HG, Kim SJ, Kim DH, Han HJ, Ngo HK, et al. Helicobacter pylori activates IL-6-STAT3 signaling in human gastric cancer cells: potential roles for reactive oxygen species. Helicobacter. (2016) 21:405–16. doi: 10.1111/hel.12298
129. Kinoshita H, Hirata Y, Nakagawa H, Sakamoto K, Hayakawa Y, Takahashi R, et al. Interleukin-6 mediates epithelial-stromal interactions and promotes gastric tumorigenesis. PloS One. (2013) 8:e60914. doi: 10.1371/journal.pone.0060914
130. Liu M, Li H, Zhang H, Zhou H, Jiao T, Feng M, et al. RBMS1 promotes gastric cancer metastasis through autocrine IL-6/JAK2/STAT3 signaling. Cell Death Dis. (2022) 13:287. doi: 10.1038/s41419-022-04747-3
131. Xu M, Ren L, Fan J, Huang L, Zhou L, Li X, et al. Berberine inhibits gastric cancer development and progression by regulating the JAK2/STAT3 pathway and downregulating IL-6. Life Sci. (2022) 290:120266. doi: 10.1016/j.lfs.2021.120266
132. Zhou Q, Qi F, Zhou C, Ji J, Jiang J, Wang C, et al. VPS35 promotes gastric cancer progression through integrin/FAK/SRC signalling-mediated IL-6/STAT3 pathway activation in a YAP-dependent manner. Oncogene. (2024) 43:106–22. doi: 10.1038/s41388-023-02885-2
133. Ding LL, Zhang M, Zhang T, Liu H, and Liu PF. MFGE8 promotes gastric cancer progression by activating the IL-6/JAK/STAT3 signaling. Cell Signal. (2025) 125:111486. doi: 10.1016/j.cellsig.2024.111486
134. Bartchewsky W,JR, Martini MR, Masiero M, Squassoni AC, Alvarez MC, Ladeira MS, et al. Effect of Helicobacter pylori infection on IL-8, IL-1beta and COX-2 expression in patients with chronic gastritis and gastric cancer. Scand J Gastroenterol. (2009) 44:153–61. doi: 10.1080/00365520802530853
135. Eck M, Schmausser B, Scheller K, Toksoy A, Kraus M, Menzel T, et al. CXC chemokines Gro(alpha)/IL-8 and IP-10/MIG in Helicobacter pylori gastritis. Clin Exp Immunol. (2000) 122:192–9. doi: 10.1046/j.1365-2249.2000.01374.x
136. Kim SH, Lim JW, and Kim H. Astaxanthin inhibits mitochondrial dysfunction and interleukin-8 expression in helicobacter pylori-infected gastric epithelial cells. Nutrients. (2018) 10. doi: 10.3390/nu10091320
137. Choi MS, Ze EY, Park JY, Shin TS, and Kim JG. Helicobacter pylori-derived outer membrane vesicles stimulate interleukin 8 secretion through nuclear factor kappa B activation. Korean J Intern Med. (2021) 36:854–67. doi: 10.3904/kjim.2019.432
138. Kyung S, Lim JW, and Kim H. alpha-lipoic acid inhibits IL-8 expression by activating nrf2 signaling in helicobacter pylori-infected gastric epithelial cells. Nutrients. (2019) 11. doi: 10.3390/nu11102524
139. Guan X, Ning J, Fu W, Wang Y, Zhang J, and Ding S. Helicobacter pylori with trx1 high expression promotes gastric diseases via upregulating the IL23A/NF-kappaB/IL8 pathway. Helicobacter. (2024) 29:e13072. doi: 10.1111/hel.13072
140. Li X, Xie G, Chen J, Wang Y, Zhai J, and Shen L. Tumour cell-derived serglycin promotes IL-8 secretion of CAFs in gastric cancer. Br J Cancer. (2024) 131:271–82. doi: 10.1038/s41416-024-02735-2
141. Ma Y, Fu Y, Fan X, Ji Q, Duan X, Wang Y, et al. FAK/IL-8 axis promotes the proliferation and migration of gastric cancer cells. Gastric Cancer. (2023) 26:528–41. doi: 10.1007/s10120-023-01384-3
142. Lou M, Iwatsuki M, Wu X, Zhang W, Matsumoto C, and Baba H. Cancer-associated fibroblast-derived IL-8 upregulates PD-L1 expression in gastric cancer through the NF-kappaB pathway. Ann Surg Oncol. (2024) 31:2983–95. doi: 10.1245/s10434-023-14586-x
143. Li X, Zhai J, Shen Y, Zhang T, Wang Y, He Y, et al. Tumor-derived IL-8 facilitates lymph node metastasis of gastric cancer via PD-1 up-regulation in CD8(+) T cells. Cancer Immunol Immunother. (2022) 71:3057–70. doi: 10.1007/s00262-022-03223-3
144. Xie SS, Zhi Y, Shao CM, and Zeng BF. Yangyin Huowei mixture alleviates chronic atrophic gastritis by inhibiting the IL-10/JAK1/STAT3 pathway. World J Gastrointest Surg. (2024) 16:2296–307. doi: 10.4240/wjgs.v16.i7.2296
145. Pachathundikandi SK and Backert S. Helicobacter pylori controls NLRP3 expression by regulating hsa-miR-223-3p and IL-10 in cultured and primary human immune cells. Innate Immun. (2018) 24:11–23. doi: 10.1177/1753425917738043
146. Torisu M, Murakami H, Akbar F, Matsui H, Hiasa Y, Matsuura B, et al. Protective role of interleukin-10-producing regulatory dendritic cells against murine autoimmune gastritis. J Gastroenterol. (2008) 43:100–7. doi: 10.1007/s00535-007-2133-x
147. Aziz F, Chakraborty A, Liu K, Zhang T, Li X, Du R, et al. Gastric tumorigenesis induced by combining Helicobacter pylori infection and chronic alcohol through IL-10 inhibition. Carcinogenesis. (2022) 43:126–39. doi: 10.1093/carcin/bgab114
148. Lee SY, Jhun J, Woo JS, Lee KH, Hwang SH, Moon J, et al. Gut microbiome-derived butyrate inhibits the immunosuppressive factors PD-L1 and IL-10 in tumor-associated macrophages in gastric cancer. Gut Microbes. (2024) 16:2300846. doi: 10.1080/19490976.2023.2300846
149. Chen L, Shi Y, Zhu X, Guo W, Zhang M, Che Y, et al. IL−10 secreted by cancer−associated macrophages regulates proliferation and invasion in gastric cancer cells via c−Met/STAT3 signaling. Oncol Rep. (2019) 42:595–604. doi: 10.3892/or.2019.7206
150. Chionh YT, Ng GZ, Ong L, Arulmuruganar A, Stent A, Saeed MA, et al. Protease-activated receptor 1 suppresses Helicobacter pylori gastritis via the inhibition of macrophage cytokine secretion and interferon regulatory factor 5. Mucosal Immunol. (2015) 8:68–79. doi: 10.1038/mi.2014.43
151. Dellalibera-Joviliano R, Garcia ME, Marins M, Fachin ALU, Couto LB, Mesquita E, et al. Interleukin-12 treatment reduces tumor growth and modulates the expression of CASKA and MIR-203 in athymic mice bearing tumors induced by the HGC-27 gastric cancer cell line. Pathol Res Pract. (2024) 263:155625. doi: 10.1016/j.prp.2024.155625
152. Guo Y, Chen J, Huang Y, Ke S, Xie F, Li D, et al. Increased infiltration of CD4(+) IL-17A(+) FOXP3(+) T cells in Helicobacter pylori-induced gastritis. Eur J Immunol. (2024) 54:e2350662. doi: 10.1002/eji.202350662
153. Tu E, Ang DK, Bellingham SA, Hogan TV, Teng MW, Smyth MJ, et al. Both IFN-gamma and IL-17 are required for the development of severe autoimmune gastritis. Eur J Immunol. (2012) 42:2574–83. doi: 10.1002/eji.201142341
154. Arachchi PS, Fernando N, Weerasekera MM, Senevirathna B, Weerasekera DD, and Gunasekara CP. Proinflammatory cytokine IL-17 shows a significant association with helicobacter pylori infection and disease severity. Gastroenterol Res Pract. (2017) 2017:6265150. doi: 10.1155/2017/6265150
155. Kabir S. The role of interleukin-17 in the Helicobacter pylori induced infection and immunity. Helicobacter. (2011) 16:1–8. doi: 10.1111/j.1523-5378.2010.00812.x
156. Brackman LC, Jung MS, Green EH, Joshi N, Revetta FL, Mcclain MS, et al. IL-17 signaling protects against Helicobacter pylori-induced gastric cancer. Gut Microbes. (2024) 16:2430421. doi: 10.1080/19490976.2024.2430421
157. Li S, Cong X, Gao H, Lan X, Li Z, Wang W, et al. Correction to: Tumor-associated neutrophils induce EMT by IL-17a to promote migration and invasion in gastric cancer cells. J Exp Clin Cancer Res. (2019) 38:177. doi: 10.1186/s13046-019-1168-1
158. Bastid J, Dejou C, Docquier A, and Bonnefoy N. The emerging role of the IL-17B/IL-17RB pathway in cancer. Front Immunol. (2020) 11:718. doi: 10.3389/fimmu.2020.00718
159. Xu J, Lv S, Meng W, and Zuo F. LCN2 mediated by IL-17 affects the proliferation, migration, invasion and cell cycle of gastric cancer cells by targeting SLPI. Cancer Manag Res. (2020) 12:12841–9. doi: 10.2147/CMAR.S278902
160. Dzierzanowska-Fangrat K, Michalkiewicz J, Cielecka-Kuszyk J, Nowak M, Celinska-Cedro D, Rozynek E, et al. Enhanced gastric IL-18 mRNA expression in Helicobacter pylori-infected children is associated with macrophage infiltration, IL-8, and IL-1 beta mRNA expression. Eur J Gastroenterol Hepatol. (2008) 20:314–9. doi: 10.1097/MEG.0b013e3282f340da
161. Tomita T, Jackson AM, Hida N, Hayat M, Dixon MF, Shimoyama T, et al. Expression of Interleukin-18, a Th1 cytokine, in human gastric mucosa is increased in Helicobacter pylori infection. J Infect Dis. (2001) 183:620–7. doi: 10.1086/318541
162. Day AS, Su B, Ceponis PJ, Jones NL, Yau E, Sieveking D, et al. Helicobacter pylori infection induces interleukin-18 production in gastric epithelial (AGS) cells. Dig Dis Sci. (2004) 49:1830–5. doi: 10.1007/s10620-004-9579-y
163. Yan H, Wang P, Zhou Q, Dong X, Wang Q, Yuan Z, et al. Eupafolin hinders cross-talk between gastric cancer cells and cancer-associated fibroblasts by abrogating the IL18/IL18RAP signaling axis. Phytomedicine. (2024) 134:155984. doi: 10.1016/j.phymed.2024.155984
164. Deswaerte V, Nguyen P, West A, Browning AF, Yu L, Ruwanpura SM, et al. Inflammasome adaptor ASC suppresses apoptosis of gastric cancer cells by an IL18-mediated inflammation-independent mechanism. Cancer Res. (2018) 78:1293–307. doi: 10.1158/0008-5472.CAN-17-1887
165. Zhao X, Tong D, and Ferrero RL. Interleukin-18 produced by gastric epithelial cells protects against pre-neoplastic lesions in Helicobacter pylori infection in mice. Genes Immun. (2024) 25:346–7. doi: 10.1038/s41435-024-00253-y
166. Koussoulas V, Vassiliou S, Giamarellos-Bourboulis EJ, Tassias G, Kotsaki A, Barbatzas C, et al. Implications for a role of interleukin-23 in the pathogenesis of chronic gastritis and of peptic ulcer disease. Clin Exp Immunol. (2009) 156:97–101. doi: 10.1111/j.1365-2249.2008.03859.x
167. Horvath DJ, JR., Washington MK, Cope VA, and Algood HM. IL-23 contributes to control of chronic helicobacter pylori infection and the development of T helper responses in a mouse model. Front Immunol. (2012) 3:56. doi: 10.3389/fimmu.2012.00056
168. Vivas JR, Regnault B, Michel V, Bussiere FI, Ave P, Huerre M, et al. Interferon gamma-signature transcript profiling and IL-23 upregulation in response to Helicobacter pylori infection. Int J Immunopathol Pharmacol. (2008) 21:515–26. doi: 10.1177/039463200802100305
169. Hor YT, Voon DC, Koo JK, Wang H, Lau WM, Ashktorab H, et al. A role for RUNX3 in inflammation-induced expression of IL23A in gastric epithelial cells. Cell Rep. (2014) 8:50–8. doi: 10.1016/j.celrep.2014.06.003
170. Xu X, Yang C, Chen J, Liu J, Li P, Shi Y, et al. Interleukin-23 promotes the migration and invasion of gastric cancer cells by inducing epithelial-to-mesenchymal transition via the STAT3 pathway. Biochem Biophys Res Commun. (2018) 499:273–8. doi: 10.1016/j.bbrc.2018.03.144
171. Liu C, Zhang Y, Zhan J, Zhao Y, Wan Q, Peng H, et al. Interleukin-23A is associated with tumor growth in Helicobacter-pylori-related human gastric cancer. Cancer Cell Int. (2014) 14:104. doi: 10.1186/s12935-014-0104-x
172. Chen B, Zeng Z, Xu L, Wu X, Yu J, Xue L, et al. IL23R + 2199A/C polymorphism is associated with decreased risk of certain subtypes of gastric cancer in Chinese: a case-control study. Cancer Epidemiol. (2011) 35:165–9. doi: 10.1016/j.canep.2010.08.006
173. Wang J, Yao Y, Zhang Q, Li S, and Tang L. Inflammatory responses induced by Helicobacter pylori on the carcinogenesis of gastric epithelial GES−1 cells. Int J Oncol. (2019) 54:2200–10. doi: 10.3892/ijo.2019.4775
174. Auron PE, Webb AC, Rosenwasser LJ, Mucci SF, Rich A, Wolff SM, et al. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U.S.A. (1984) 81:7907–11. doi: 10.1073/pnas.81.24.7907
175. Mantovani A, Dinarello CA, Molgora M, and Garlanda C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity. (2019) 50:778–95. doi: 10.1016/j.immuni.2019.03.012
176. Matarazzo L, Hernandez Santana YE, Walsh PT, and Fallon PG. The IL-1 cytokine family as custodians of barrier immunity. Cytokine. (2022) 154:155890. doi: 10.1016/j.cyto.2022.155890
177. Broderick L and Hoffman HM. IL-1 and autoinflammatory disease: biology, pathogenesis and therapeutic targeting. Nat Rev Rheumatol. (2022) 18:448–63. doi: 10.1038/s41584-022-00797-1
178. Huang J, Kuang W, and Zhou Z. IL-1 signaling pathway, an important target for inflammation surrounding in myocardial infarction. Inflammopharmacology. (2024) 32:2235–52. doi: 10.1007/s10787-024-01481-4
179. Xiao Y, Cong M, Li J, He D, Wu Q, Tian P, et al. Cathepsin C promotes breast cancer lung metastasis by modulating neutrophil infiltration and neutrophil extracellular trap formation. Cancer Cell. (2021) 39:423–437 e7. doi: 10.1016/j.ccell.2020.12.012
180. Ahmadnia Z, Ranaee M, Mohammadi Abandansari R, Bagheri N, and Shirzad H. Evaluating the microRNA expression of IL-35 and IL-37 in helicobacter pylori-infected patients with gastritis and gastric ulcer. Iran J Allergy Asthma Immunol. (2022) 21:20–6. doi: 10.18502/ijaai.v21i1.8609
181. Chen Y, Jia K, Chong X, Xie Y, Jiang L, Peng H, et al. Implications of PD-L1 expression on the immune microenvironment in HER2-positive gastric cancer. Mol Cancer. (2024) 23:169. doi: 10.1186/s12943-024-02085-w
182. Outlioua A, Badre W, Desterke C, Echarki Z, El Hammani N, Rabhi M, et al. Gastric IL-1beta, IL-8, and IL-17A expression in Moroccan patients infected with Helicobacter pylori may be a predictive signature of severe pathological stages. Cytokine. (2020) 126:154893. doi: 10.1016/j.cyto.2019.154893
183. Zhou Q, Wu X, Wang X, Yu Z, Pan T, Li Z, et al. The reciprocal interaction between tumor cells and activated fibroblasts mediated by TNF-alpha/IL-33/ST2L signaling promotes gastric cancer metastasis. Oncogene. (2020) 39:1414–28. doi: 10.1038/s41388-019-1078-x
184. El Filaly H, Outlioua A, Desterke C, Echarki Z, Badre W, Rabhi M, et al. IL-1 polymorphism and helicobacter pylori infection features: highlighting VNTR’s potential in predicting the susceptibility to infection-associated disease development. Microorganisms. (2023) 11. doi: 10.3390/microorganisms11020353
185. Diep S, Maddukuri M, Yamauchi S, Geshow G, and Delk NA. Interleukin-1 and nuclear factor kappa B signaling promote breast cancer progression and treatment resistance. Cells. (2022) 11. doi: 10.3390/cells11101673
186. Lei S, Jin J, Zhao X, Zhou L, Qi G, and Yang J. The role of IL-33/ST2 signaling in the tumor microenvironment and Treg immunotherapy. Exp Biol Med (Maywood). (2022) 247:1810–8. doi: 10.1177/15353702221102094
187. Dan H, Liu S, Liu J, Liu D, Yin F, Wei Z, et al. RACK1 promotes cancer progression by increasing the M2/M1 macrophage ratio via the NF-kappaB pathway in oral squamous cell carcinoma. Mol Oncol. (2020) 14:795–807. doi: 10.1002/1878-0261.12644
188. Chen Y, Huang J, and Xu C. Lipopolysaccharide-induced DC-SIGN/TLR4 crosstalk activates NLRP3 inflammasomes via MyD88-independent signaling in gastric epithelial cells. Exp Cell Res. (2020) 396:112292. doi: 10.1016/j.yexcr.2020.112292
189. Petersen CP, Meyer AR, De Salvo C, Choi E, Schlegel C, Petersen A, et al. A signalling cascade of IL-33 to IL-13 regulates metaplasia in the mouse stomach. Gut. (2018) 67:805–17. doi: 10.1136/gutjnl-2016-312779
190. Lu B, Yang M, and Wang Q. Interleukin-33 in tumorigenesis, tumor immune evasion, and cancer immunotherapy. J Mol Med (Berl). (2016) 94:535–43. doi: 10.1007/s00109-016-1397-0
191. Kwon JW, Seok SH, Kim S, An HW, Choudhury AD, Woo SH, et al. A synergistic partnership between IL-33/ST2 and Wnt pathway through Bcl-xL drives gastric cancer stemness and metastasis. Oncogene. (2023) 42:501–15. doi: 10.1038/s41388-022-02575-5
192. Shouse AN, Laporte KM, and Malek TR. Interleukin-2 signaling in the regulation of T cell biology in autoimmunity and cancer. Immunity. (2024) 57:414–28. doi: 10.1016/j.immuni.2024.02.001
193. Malek TR. The biology of interleukin-2. Annu Rev Immunol. (2008) 26:453–79. doi: 10.1146/annurev.immunol.26.021607.090357
194. Fan XG, Yakoob J, Fan XJ, and Keeling PW. A change of IL-2 and IL-4 production in patients with Helicobactor pylori infection. Mediators Inflammation. (1995) 4:289–92. doi: 10.1155/S0962935195000469
195. Tauber PA, Kratzer B, Schatzlmaier P, Smole U, Kohler C, Rausch L, et al. The small molecule inhibitor BX-795 uncouples IL-2 production from inhibition of Th2 inflammation and induces CD4(+) T cells resembling iTreg. Front Immunol. (2023) 14:1094694. doi: 10.3389/fimmu.2023.1094694
196. Harris F, Berdugo YA, and Tree T. IL-2-based approaches to Treg enhancement. Clin Exp Immunol. (2023) 211:149–63. doi: 10.1093/cei/uxac105
197. Thiolat A, Pilon C, Caudana P, Moatti A, To NH, Sedlik C, et al. Treg-targeted IL-2/anti-IL-2 complex controls graft-versus-host disease and supports anti-tumor effect in allogeneic hematopoietic stem cell transplantation. Haematologica. (2024) 109:129–42. doi: 10.3324/haematol.2022.282653
198. Sledzinska A, Vila De Mucha M, Bergerhoff K, Hotblack A, Demane DF, Ghorani E, et al. Regulatory T cells restrain interleukin-2- and blimp-1-dependent acquisition of cytotoxic function by CD4(+) T cells. Immunity. (2020) 52:151–166 e6. doi: 10.1016/j.immuni.2019.12.007
199. Chen J, Peng J, Ma C, Zhang L, Wu X, Wei H, et al. Co-expression of pig IL-2 and fusion bovine cathelicidin gene by recombinant plasmids in yeast and their promotion of mouse antibacterial defense. Biol (Basel). (2022) 11. doi: 10.3390/biology11101491
200. Hernandez R, Poder J, Laporte KM, and Malek TR. Engineering IL-2 for immunotherapy of autoimmunity and cancer. Nat Rev Immunol. (2022) 22:614–28. doi: 10.1038/s41577-022-00680-w
201. Park YJ, Kim S, Bang H, Kang SC, Cho S, Park JE, et al. MB2033, an anti-PD-L1 x IL-2 variant fusion protein, demonstrates robust anti-tumor efficacy with minimal peripheral toxicity. Cancer Immunol Immunother. (2024) 73:157. doi: 10.1007/s00262-024-03742-1
202. Allen JE. IL-4 and IL-13: regulators and effectors of wound repair. Annu Rev Immunol. (2023) 41:229–54. doi: 10.1146/annurev-immunol-101921-041206
203. Cutolo M, Campitiello R, Gotelli E, and Soldano S. The role of M1/M2 macrophage polarization in rheumatoid arthritis synovitis. Front Immunol. (2022) 13:867260. doi: 10.3389/fimmu.2022.867260
204. Borelli A, Santamaria JC, Zamit C, Apert C, Chevallier J, Pierre P, et al. Lymphotoxin limits Foxp3(+) regulatory T cell development from Foxp3(lo) precursors via IL-4 signaling. Nat Commun. (2024) 15:6976. doi: 10.1038/s41467-024-51164-5
205. Zheng Y, Ren S, Zhang Y, Liu S, Meng L, Liu F, et al. Circular RNA circWWC3 augments breast cancer progression through promoting M2 macrophage polarization and tumor immune escape via regulating the expression and secretion of IL-4. Cancer Cell Int. (2022) 22:264. doi: 10.1186/s12935-022-02686-9
206. Liu Q, Wang Y, and Harpaz N. Coexisting Th1 and Th2 cytokines in patients with collagenous gastritis and implications for its pathogenesis. J Pediatr Gastroenterol Nutr. (2024) 78:231–40. doi: 10.1002/jpn3.12109
207. Duan L, Liu D, Chen H, Mintz MA, Chou MY, Kotov DI, et al. Follicular dendritic cells restrict interleukin-4 availability in germinal centers and foster memory B cell generation. Immunity. (2021) 54:2256–2272 e6. doi: 10.1016/j.immuni.2021.08.028
208. Kurup VP, Murali PS, Guo J, Choi H, Banerjee B, Fink JN, et al. Anti-interleukin (IL)-4 and -IL-5 antibodies downregulate IgE and eosinophilia in mice exposed to Aspergillus antigens. Allergy. (1997) 52:1215–21. doi: 10.1111/j.1398-9995.1997.tb02526.x
209. Zhang J, Dong Y, Yu S, Hu K, Zhang L, Xiong M, et al. IL-4/IL-4R axis signaling drives resistance to immunotherapy by inducing the upregulation of Fcgamma receptor IIB in M2 macrophages. Cell Death Dis. (2024) 15:500. doi: 10.1038/s41419-024-06875-4
210. Zhang C, Wei S, Dai S, Li X, Wang H, Zhang H, et al. The NR_109/FUBP1/c-Myc axis regulates TAM polarization and remodels the tumor microenvironment to promote cancer development. J Immunother Cancer. (2023) 11. doi: 10.1136/jitc-2022-006230
211. Li S, Liu M, Do MH, Chou C, Stamatiades EG, Nixon BG, et al. Cancer immunotherapy via targeted TGF-beta signalling blockade in T(H) cells. Nature. (2020) 587:121–5. doi: 10.1038/s41586-020-2850-3
212. Wang J, Zhou Y, Zhang H, Hu L, Liu J, Wang L, et al. Pathogenesis of allergic diseases and implications for therapeutic interventions. Signal Transduct Target Ther. (2023) 8:138. doi: 10.1038/s41392-023-01344-4
213. Afshan K, Sarfraz K, Kayani T, and Firasat S. IL-4 gene polymorphisms and their association with nematodes infection in Pakistani population. Afr Health Sci. (2022) 22:216–28. doi: 10.4314/ahs.v22i2.25
214. Mollaoglu G, Tepper A, Falcomata C, Potak HT, Pia L, Amabile A, et al. Ovarian cancer-derived IL-4 promotes immunotherapy resistance. Cell. (2024) 187:7492–7510 e22. doi: 10.1016/j.cell.2024.10.006
215. Ou K, Li Y, Wang Y, Liu J, Luo Y, Jiang J, et al. Marine bromophenols suppressed choroidal neovascularization by targeting HUWE1 through NF-kappab signaling pathway. Int J Biol Macromol. (2024) 257:128620. doi: 10.1016/j.ijbiomac.2023.128620
216. Gao L, Jiang W, Liu H, Chen Z, and Lin Y. Receptor-selective interleukin-4 mutein attenuates laser-induced choroidal neovascularization through the regulation of macrophage polarization in mice. Exp Ther Med. (2021) 22:1367. doi: 10.3892/etm.2021.10801
217. Melo-Cardenas J, Bezavada L, Crawford JC, Gurbuxani S, Cotton A, Kang G, et al. IL-13/IL-4 signaling contributes to fibrotic progression of the myeloproliferative neoplasms. Blood. (2022) 140:2805–17. doi: 10.1182/blood.2022017326
218. Carsuzaa F, Bequignon E, Bainaud M, Jegou JF, Dufour X, Lecron JC, et al. Oncostatin M counteracts the fibrotic effects of TGF-beta1 and IL-4 on nasal-polyp-derived fibroblasts: A control of fibrosis in chronic rhinosinusitis with nasal polyps? Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23116308
219. LaMarche NM, Hegde S, Park MD, Maier BB, Troncoso L, Le Berichel J, et al. An IL-4 signalling axis in bone marrow drives pro-tumorigenic myelopoiesis. Nature. (2024) 625:166–74. doi: 10.1038/s41586-023-06797-9
220. Gunassekaran GR, Poongkavithai Vadevoo SM, Baek MC, and Lee B. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials. (2021) 278:121137. doi: 10.1016/j.biomaterials.2021.121137
221. Sadik A, Somarribas Patterson LF, Ozturk S, Mohapatra SR, Panitz V, Secker PF, et al. IL4I1 is a metabolic immune checkpoint that activates the AHR and promotes tumor progression. Cell. (2020) 182:1252–1270 e34. doi: 10.1016/j.cell.2020.07.038
222. Stewart CM, Siegler EL, Sakemura RL, Cox MJ, Huynh T, Kimball B, et al. IL-4 drives exhaustion of CD8(+) CART cells. Nat Commun. (2024) 15:7921. doi: 10.1038/s41467-024-51978-3
223. Kang S, Narazaki M, Metwally H, and Kishimoto T. Historical overview of the interleukin-6 family cytokine. J Exp Med. (2020) 217. doi: 10.1084/jem.20190347
224. Jourdan M, Bataille R, Seguin J, Zhang XG, Chaptal PA, and Klein B. Constitutive production of interleukin-6 and immunologic features in cardiac myxomas. Arthritis Rheum. (1990) 33:398–402. doi: 10.1002/art.1780330313
225. Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, and Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. (2003) 374:1–20. doi: 10.1042/bj20030407
226. Taniguchi K, Wu LW, Grivennikov SI, De Jong PR, Lian I, Yu FX, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. (2015) 519:57–62. doi: 10.1038/nature14228
227. Yu B, De Vos D, Guo X, Peng S, Xie W, Peppelenbosch MP, et al. IL-6 facilitates cross-talk between epithelial cells and tumor- associated macrophages in Helicobacter pylori-linked gastric carcinogenesis. Neoplasia. (2024) 50:100981. doi: 10.1016/j.neo.2024.100981
228. Zhang Z, Chen S, Fan M, Ruan G, Xi T, Zheng L, et al. Helicobacter pylori induces gastric cancer via down-regulating miR-375 to inhibit dendritic cell maturation. Helicobacter. (2021) 26:e12813. doi: 10.1111/hel.12813
229. Ma Z, Sun Q, Zhang C, Zheng Q, Liu Y, Xu H, et al. RHOJ induces epithelial-to-mesenchymal transition by IL-6/STAT3 to promote invasion and metastasis in gastric cancer. Int J Biol Sci. (2023) 19:4411–26. doi: 10.7150/ijbs.81972
230. Daneshmandi S, Yan Q, Choi JE, Katsuta E, Macdonald CR, Goruganthu M, et al. Exportin 1 governs the immunosuppressive functions of myeloid-derived suppressor cells in tumors through ERK1/2 nuclear export. Cell Mol Immunol. (2024) 21:873–91. doi: 10.1038/s41423-024-01187-1
231. Hajimoradi M, Rezalotfi A, Esmaeilnejad-Ahranjani P, Mohammad Hassan Z, and Ebrahimi M. STAT3 inactivation suppresses stemness properties in gastric cancer stem cells and promotes Th17 in Treg/Th17 balance. Int Immunopharmacol. (2022) 111:109048. doi: 10.1016/j.intimp.2022.109048
232. Wei R, Song J, Pan H, Liu X, and Gao J. CPT1C-positive cancer-associated fibroblast facilitates immunosuppression through promoting IL-6-induced M2-like phenotype of macrophage. Oncoimmunology. (2024) 13:2352179. doi: 10.1080/2162402X.2024.2352179
233. Rasool KH, Mahmood Alubadi AE, and Al-Bayati IFI. The role of Serum Interleukin-4 and Interleukin-6 in Helicobacter pylori-infected patients. Microb Pathog. (2022) 162:105362. doi: 10.1016/j.micpath.2021.105362
234. Li X, Sun Z, Peng G, Xiao Y, Guo J, Wu B, et al. Single-cell RNA sequencing reveals a pro-invasive cancer-associated fibroblast subgroup associated with poor clinical outcomes in patients with gastric cancer. Theranostics. (2022) 12:620–38. doi: 10.7150/thno.60540
235. Zong Y, Deng K, and Chong WP. Regulation of Treg cells by cytokine signaling and co-stimulatory molecules. Front Immunol. (2024) 15:1387975. doi: 10.3389/fimmu.2024.1387975
236. Pradhan R, Paul S, Acharya SS, Sinha S, Dash SR, and Kundu CN. Nano formulated Resveratrol inhibits PD-L1 in oral cancer cells by deregulating the association between tumor associated macrophages and cancer associated fibroblasts through IL-6/JAK2/STAT3 signaling axis. J Nutr Biochem. (2024) 125:109568. doi: 10.1016/j.jnutbio.2024.109568
237. York AG, Skadow MH, Oh J, Qu R, Zhou QD, Hsieh WY, et al. IL-10 constrains sphingolipid metabolism to limit inflammation. Nature. (2024) 627:628–35. doi: 10.1038/s41586-024-07098-5
238. Hussain K, Letley DP, Greenaway AB, Kenefeck R, Winter JA, Tomlinson W, et al. Helicobacter pylori-mediated protection from allergy is associated with IL-10-secreting peripheral blood regulatory T cells. Front Immunol. (2016) 7:71. doi: 10.3389/fimmu.2016.00071
239. Zhang P and Hill GR. Interleukin-10 mediated immune regulation after stem cell transplantation: Mechanisms and implications for therapeutic intervention. Semin Immunol. (2019) 44:101322. doi: 10.1016/j.smim.2019.101322
240. Ummarino D. Rheumatoid arthritis: Defective IL-10-producing B(reg) cells. Nat Rev Rheumatol. (2017) 13:132. doi: 10.1038/nrrheum.2017.10
241. Kelsall B. Interleukin-10 in inflammatory bowel disease. N Engl J Med. (2009) 361:2091–3. doi: 10.1056/NEJMe0909225
242. Hosseinikhah SM, Barani M, Rahdar A, Madry H, Arshad R, Mohammadzadeh V, et al. Nanomaterials for the diagnosis and treatment of inflammatory arthritis. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22063092
243. Zhang X, Yue L, Cao L, Liu K, Yang S, Liang S, et al. Tumor microenvironment-responsive macrophage-mediated immunotherapeutic drug delivery. Acta Biomater. (2024) 186:369–82. doi: 10.1016/j.actbio.2024.07.042
244. Teng MW, Bowman EP, Mcelwee JJ, Smyth MJ, Casanova JL, Cooper AM, et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. (2015) 21:719–29. doi: 10.1038/nm.3895
245. Vignali DA and Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol. (2012) 13:722–8. doi: 10.1038/ni.2366
246. Howell I, Yang F, Brown V, Cane J, Marchi E, Azim A, et al. Airway proteomics reveals broad residual anti-inflammatory effects of prednisolone in mepolizumab-treated asthma. J Allergy Clin Immunol. (2024) 154:1146–58. doi: 10.1016/j.jaci.2024.07.020
247. Glassman CR, Mathiharan YK, Jude KM, Su L, Panova O, Lupardus PJ, et al. Structural basis for IL-12 and IL-23 receptor sharing reveals a gateway for shaping actions on T versus NK cells. Cell. (2021) 184:983–999 e24. doi: 10.1016/j.cell.2021.01.018
248. Verstockt B, Salas A, Sands BE, Abraham C, Leibovitzh H, Neurath MF, et al. IL-12 and IL-23 pathway inhibition in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. (2023) 20:433–46. doi: 10.1038/s41575-023-00768-1
249. Kong H, You N, Chen H, Teng YS, Liu YG, Lv YP, et al. Helicobacter pylori-induced adrenomedullin modulates IFN-gamma-producing T-cell responses and contributes to gastritis. Cell Death Dis. (2020) 11:189. doi: 10.1038/s41419-020-2391-6
250. Ghosh N, Ghosh P, Kesh K, Mukhopadhyay AK, and Swarnakar S. Attenuation of Helicobacter pylori-induced gastric inflammation by prior cag(-) strain (AM1) infection in C57BL/6 mice. Gut Pathog. (2017) 9:14. doi: 10.1186/s13099-017-0161-5
251. Mirlekar B and Pylayeva-Gupta Y. IL-12 family cytokines in cancer and immunotherapy. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13020167
252. Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C, et al. Successful anti-PD-1 cancer immunotherapy requires T cell-dendritic cell crosstalk involving the cytokines IFN-gamma and IL-12. Immunity. (2018) 49:1148–1161 e7. doi: 10.1016/j.immuni.2018.09.024
253. Wu L, Hong X, Yang C, Yang Y, Li W, Lu L, et al. Noncanonical MAVS signaling restrains dendritic cell-driven antitumor immunity by inhibiting IL-12. Sci Immunol. (2023) 8:eadf4919. doi: 10.1126/sciimmunol.adf4919
254. Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin Cancer Biol. (2015) 35 Suppl:S185–98. doi: 10.1016/j.semcancer.2015.03.004
255. Hu Y, Nie W, Lyu L, Zhang X, Wang W, Zhang Y, et al. Tumor-microenvironment-activatable nanoparticle mediating immunogene therapy and M2 macrophage-targeted inhibitor for synergistic cancer immunotherapy. ACS Nano. (2024) 18:3295–312. doi: 10.1021/acsnano.3c10037
256. Hurtubise R, Audiger C, Dominguez-Punaro MC, Chabot-Roy G, Chognard G, Raymond-Marchand L, et al. Induced and spontaneous colitis mouse models reveal complex interactions between IL-10 and IL-12/IL-23 pathways. Cytokine. (2019) 121:154738. doi: 10.1016/j.cyto.2019.154738
257. Fabian KP, Santiago-Sanchez G, Padget MR, Lassoued W, Allen CT, Battula S, et al. Alum-anchored IL-12 combined with cytotoxic chemotherapy and immune checkpoint blockade enhanced antitumor immune responses in head and neck cancer models. J Immunother Cancer. (2024) 12. doi: 10.1136/jitc-2024-009712
258. Minnar CM, Chariou PL, Horn LA, Hicks KC, Palena C, Schlom J, et al. Tumor-targeted interleukin-12 synergizes with entinostat to overcome PD-1/PD-L1 blockade-resistant tumors harboring MHC-I and APM deficiencies. J Immunother Cancer. (2022) 10. doi: 10.1136/jitc-2022-004561
259. Li F, Zhao S, Wei C, Hu Y, Xu T, Xin X, et al. Development of Nectin4/FAP-targeted CAR-T cells secreting IL-7, CCL19, and IL-12 for Malignant solid tumors. Front Immunol. (2022) 13:958082. doi: 10.3389/fimmu.2022.958082
260. Huangfu L, Li R, Huang Y, and Wang S. The IL-17 family in diseases: from bench to bedside. Signal Transduct Target Ther. (2023) 8:402. doi: 10.1038/s41392-023-01620-3
261. Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. (2015) 522:345–8. doi: 10.1038/nature14282
262. Kang JH, Park S, Rho J, Hong EJ, Cho YE, Won YS, et al. IL-17A promotes Helicobacter pylori-induced gastric carcinogenesis via interactions with IL-17RC. Gastric Cancer. (2023) 26:82–94. doi: 10.1007/s10120-022-01342-5
263. Dewayani A, Fauzia KA, Alfaray RI, Waskito LA, Doohan D, Rezkitha YAA, et al. The roles of IL-17, IL-21, and IL-23 in the helicobacter pylori infection and gastrointestinal inflammation: A review. Toxins (Basel). (2021) 13. doi: 10.3390/toxins13050315
264. Mills KHG. IL-17 and IL-17-producing cells in protection versus pathology. Nat Rev Immunol. (2023) 23:38–54. doi: 10.1038/s41577-022-00746-9
265. Regen T, Isaac S, Amorim A, Nunez NG, Hauptmann J, Shanmugavadivu A, et al. IL-17 controls central nervous system autoimmunity through the intestinal microbiome. Sci Immunol. (2021) 6. doi: 10.1126/sciimmunol.aaz6563
266. Lurje I, Gaisa NT, Weiskirchen R, and Tacke F. Mechanisms of organ fibrosis: Emerging concepts and implications for novel treatment strategies. Mol Aspects Med. (2023) 92:101191. doi: 10.1016/j.mam.2023.101191
267. Chang SH. T helper 17 (Th17) cells and interleukin-17 (IL-17) in cancer. Arch Pharm Res. (2019) 42:549–59. doi: 10.1007/s12272-019-01146-9
268. Mu X, Gu R, Tang M, Wu X, He W, and Nie X. IL-17 in wound repair: bridging acute and chronic responses. Cell Commun Signal. (2024) 22:288. doi: 10.1186/s12964-024-01668-w
269. Zhang Y, Chandra V, Riquelme Sanchez E, Dutta P, Quesada PR, Rakoski A, et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J Exp Med. (2020) 217. doi: 10.1084/jem.20190354
270. Krueger JG, Eyerich K, Kuchroo VK, Ritchlin CT, Abreu MT, Elloso MM, et al. IL-23 past, present, and future: a roadmap to advancing IL-23 science and therapy. Front Immunol. (2024) 15:1331217. doi: 10.3389/fimmu.2024.1331217
271. Yan J, Smyth MJ, and Teng MWL. Interleukin (IL)-12 and IL-23 and their conflicting roles in cancer. Cold Spring Harb Perspect Biol. (2018) 10. doi: 10.1101/cshperspect.a028530
272. Ghoreschi K, Balato A, Enerback C, and Sabat R. Therapeutics targeting the IL-23 and IL-17 pathway in psoriasis. Lancet. (2021) 397:754–66. doi: 10.1016/S0140-6736(21)00184-7
273. Neurath MF. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor Rev. (2019) 45:1–8. doi: 10.1016/j.cytogfr.2018.12.002
274. Iida T, Iwahashi M, Katsuda M, Ishida K, Nakamori M, Nakamura M, et al. Tumor-infiltrating CD4+ Th17 cells produce IL-17 in tumor microenvironment and promote tumor progression in human gastric cancer. Oncol Rep. (2011) 25:1271–7. doi: 10.3892/or.2011.1201
275. Liu XC, Zhai A, Li JQ, and Qi HZ. Interleukin-23 promotes natural killer T-cell production of IL-17 during rat liver transplantation. Transplant Proc. (2011) 43:1962–6. doi: 10.1016/j.transproceed.2011.01.175
276. Habanjar O, Bingula R, Decombat C, Diab-Assaf M, Caldefie-Chezet F, and Delort L. Crosstalk of inflammatory cytokines within the breast tumor microenvironment. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24044002
277. Fu Q, Xu L, Wang Y, Jiang Q, Liu Z, Zhang J, et al. Tumor-associated macrophage-derived interleukin-23 interlinks kidney cancer glutamine addiction with immune evasion. Eur Urol. (2019) 75:752–63. doi: 10.1016/j.eururo.2018.09.030
278. van Loo G and Bertrand MJM. Death by TNF: a road to inflammation. Nat Rev Immunol. (2023) 23:289–303. doi: 10.1038/s41577-022-00792-3
279. Freeman AJ, Kearney CJ, Silke J, and Oliaro J. Unleashing TNF cytotoxicity to enhance cancer immunotherapy. Trends Immunol. (2021) 42:1128–42. doi: 10.1016/j.it.2021.10.003
280. Siegmund D and Wajant H. TNF and TNF receptors as therapeutic targets for rheumatic diseases and beyond. Nat Rev Rheumatol. (2023) 19:576–91. doi: 10.1038/s41584-023-01002-7
281. Suto T, Tosevska A, Dalwigk K, Kugler M, Dellinger M, Stanic I, et al. TNFR2 is critical for TNF-induced rheumatoid arthritis fibroblast-like synoviocyte inflammation. Rheumatol (Oxford). (2022) 61:4535–46. doi: 10.1093/rheumatology/keac124
282. Wang F, Schwarz BT, Graham WV, Wang Y, Su L, Clayburgh DR, et al. IFN-gamma-induced TNFR2 expression is required for TNF-dependent intestinal epithelial barrier dysfunction. Gastroenterology. (2006) 131:1153–63. doi: 10.1053/j.gastro.2006.08.022
283. Fei X, Chen S, Li L, Xu X, Wang H, Ke H, et al. Helicobacter pylori infection promotes M1 macrophage polarization and gastric inflammation by activation of NLRP3 inflammasome via TNF/TNFR1 axis. Cell Commun Signal. (2025) 23:6. doi: 10.1186/s12964-024-02017-7
284. Yu J, Chen Z, Zhou Q, Li P, Wu S, Zhou T, et al. Exopolysaccharide from Lacticaseibacillus paracasei alleviates gastritis in Helicobacter pylori-infected mice by regulating gastric microbiota. Front Nutr. (2024) 11:1426358. doi: 10.3389/fnut.2024.1426358
285. Hasegawa S, Nishikawa S, Miura T, Saito Y, Madarame H, Sekikawa K, et al. Tumor necrosis factor-alpha is required for gastritis induced by Helicobacter felis infection in mice. Microb Pathog. (2004) 37:119–24. doi: 10.1016/j.micpath.2004.06.004
286. Suganuma M, Watanabe T, Sueoka E, Lim IK, and Fujiki H. Role of TNF-alpha-inducing protein secreted by helicobacter pylori as a tumor promoter in gastric cancer and emerging preventive strategies. Toxins (Basel). (2021) 13. doi: 10.3390/toxins13030181
287. Oriuchi M, Lee S, Uno K, Sudo K, Kusano K, Asano N, et al. Porphyromonas gingivalis lipopolysaccharide damages mucosal barrier to promote gastritis-associated carcinogenesis. Dig Dis Sci. (2024) 69:95–111. doi: 10.1007/s10620-023-08142-6
288. Qu Y, Wang X, Bai S, Niu L, Zhao G, Yao Y, et al. The effects of TNF-alpha/TNFR2 in regulatory T cells on the microenvironment and progression of gastric cancer. Int J Cancer. (2022) 150:1373–91. doi: 10.1002/ijc.33873
289. Gao S, Tan H, and Li D. Oridonin suppresses gastric cancer SGC-7901 cell proliferation by targeting the TNF-alpha/androgen receptor/TGF-beta signalling pathway axis. J Cell Mol Med. (2023) 27:2661–74. doi: 10.1111/jcmm.17841
290. Han N, Jiang W, Li G, Lu L, Shan J, Feng L, et al. Low-intensity pulsed ultrasound at ST36 improves the gastric motility by TNF-alpha/IKKbeta/NF-kappaB signaling pathway in diabetic rats. J Gastroenterol Hepatol. (2023) 38:2018–26. doi: 10.1111/jgh.16321
291. Huang Y, Chen S, Yao Y, Wu N, Xu M, Du H, et al. Ovotransferrin inhibits TNF-alpha induced inflammatory response in gastric epithelial cells via MAPK and NF-kappaB pathway. J Agric Food Chem. (2023) 71:12474–86. doi: 10.1021/acs.jafc.3c00950
292. Shimizu S, Yamada Y, Okuno M, Ohnishi H, Osawa Y, Seishima M, et al. Liver injury induced by lipopolysaccharide is mediated by TNFR-1 but not by TNFR-2 or Fas in mice. Hepatol Res. (2005) 31:136–42. doi: 10.1016/j.hepres.2004.11.012
293. Weinelt N, Karathanasis C, Smith S, Medler J, Malkusch S, Fulda S, et al. Quantitative single-molecule imaging of TNFR1 reveals zafirlukast as antagonist of TNFR1 clustering and TNFalpha-induced NF-kB signaling. J Leukoc Biol. (2021) 109:363–71. doi: 10.1002/JLB.2AB0420-572RR
294. Li X, Korner H, and Liu X. Susceptibility to intracellular infections: contributions of TNF to immune defense. Front Microbiol. (2020) 11:1643. doi: 10.3389/fmicb.2020.01643
295. Bakshi HA, Quinn GA, Nasef MM, Mishra V, Aljabali AAA, El-Tanani M, et al. Crocin inhibits angiogenesis and metastasis in colon cancer via TNF-alpha/NF-kB/VEGF pathways. Cells. (2022) 11. doi: 10.3390/cells11091502
296. Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, and Saparov A. Immunology of acute and chronic wound healing. Biomolecules. (2021) 11. doi: 10.3390/biom11050700
297. Li M, Tang Z, Shu R, Wu H, Wang Y, Chen Z, et al. Polymorphonuclear myeloid-derived suppressor cells play a proinflammatory role via TNF-alpha(+) B cells through BAFF/BTK/NF-kappaB signalling pathway in the pathogenesis of collagen-induced arthritis mice. Immunology. (2023) 170:286–300. doi: 10.1111/imm.13668
298. Muendlein HI, Connolly WM, Cameron J, Jetton D, Magri Z, Smirnova I, et al. Neutrophils and macrophages drive TNF-induced lethality via TRIF/CD14-mediated responses. Sci Immunol. (2022) 7:eadd0665. doi: 10.1126/sciimmunol.add0665
299. Leone GM, Mangano K, Petralia MC, Nicoletti F, and Fagone P. Past, present and (Foreseeable) future of biological anti-TNF alpha therapy. J Clin Med. (2023) 12. doi: 10.3390/jcm12041630
300. Siebeler R, De Winther MPJ, and Hoeksema MA. The regulatory landscape of macrophage interferon signaling in inflammation. J Allergy Clin Immunol. (2023) 152:326–37. doi: 10.1016/j.jaci.2023.04.022
301. Goel RR, Kotenko SV, and Kaplan MJ. Interferon lambda in inflammation and autoimmune rheumatic diseases. Nat Rev Rheumatol. (2021) 17:349–62. doi: 10.1038/s41584-021-00606-1
302. Jones SA and Morand EF. Targeting interferon signalling in systemic lupus erythematosus: lessons learned. Drugs. (2024) 84:625–35. doi: 10.1007/s40265-024-02043-2
303. Czimmer J, Kiraly A, Szabo IL, Mozsik G, and Suto G. Role of nitric oxide in the central interferon-alpha-induced inhibition of gastric acid secretion in rats. Curr Pharm Des. (2013) 19:11–6. doi: 10.2174/13816128130104
304. Qu J, Zhao M, Teng Y, Zhang Y, Hou K, Jiang Y, et al. Interferon-alpha sensitizes human gastric cancer cells to TRAIL-induced apoptosis via activation of the c-CBL-dependent MAPK/ERK pathway. Cancer Biol Ther. (2011) 12:494–502. doi: 10.4161/cbt.12.6.15973
305. Zhu Y, Xu L, Fan Y, Li C, Zhang Y, Zheng H, et al. Interferon-alpha enhances 5’-deoxy-5-fluorouridine-induced apoptosis by ERK-dependant upregulation of thymidine phosphorylase. BioMed Res Int. (2013) 2013:132793. doi: 10.1155/2013/132793
306. Kim KY, Yi BR, Lee HR, Kang NH, Jeung EB, Kim SU, et al. Stem cells with fused gene expression of cytosine deaminase and interferon-beta migrate to human gastric cancer cells and result in synergistic growth inhibition for potential therapeutic use. Int J Oncol. (2012) 40:1097–104. doi: 10.3892/ijo.2011.1288
307. Osaki LH, Bockerstett KA, Wong CF, Ford EL, Madison BB, Dipaolo RJ, et al. Interferon-gamma directly induces gastric epithelial cell death and is required for progression to metaplasia. J Pathol. (2019) 247:513–23. doi: 10.1002/path.5214
308. Strickertsson JA, Dossing KB, Aabakke AJ, Nilsson HO, Hansen TV, Knigge U, et al. Interferon-gamma inhibits ghrelin expression and secretion via a somatostatin-mediated mechanism. World J Gastroenterol. (2011) 17:3117–25. doi: 10.3748/wjg.v17.i26.3117
309. Li N, Jilisihan B, Wang W, Tang Y, and Keyoumu S. Soluble LAG3 acts as a potential prognostic marker of gastric cancer and its positive correlation with CD8+T cell frequency and secretion of IL-12 and INF-gamma in peripheral blood. Cancer biomark. (2018) 23:341–51. doi: 10.3233/CBM-181278
310. Morisaki T, Matsunaga H, Beppu K, Ihara E, Hirano K, Kanaide H, et al. A combination of cyclosporin-A (CsA) and interferon-gamma (INF-gamma) induces apoptosis in human gastric carcinoma cells. Anticancer Res. (2000) 20:3363–73.
311. Shyu RY, Su HL, Yu JC, and Jiang SY. Direct growth suppressive activity of interferon-alpha and -gamma on human gastric cancer cells. J Surg Oncol. (2000) 75:122–30. doi: 10.1002/1096-9098(200010)75:2<122::AID-JSO9>3.0.CO;2-4
312. Cao LL, Lu H, Soutto M, Bhat N, Chen Z, Peng D, et al. Multivalent tyrosine kinase inhibition promotes T cell recruitment to immune-desert gastric cancers by restricting epithelial-mesenchymal transition via tumour-intrinsic IFN-gamma signalling. Gut. (2023) 72:2038–50. doi: 10.1136/gutjnl-2022-329134
313. Jorgovanovic D, Song M, Wang L, and Zhang Y. Roles of IFN-gamma in tumor progression and regression: a review. biomark Res. (2020) 8:49. doi: 10.1186/s40364-020-00228-x
314. Grasso CS, Tsoi J, Onyshchenko M, Abril-Rodriguez G, Ross-Macdonald P, Wind-Rotolo M, et al. Conserved interferon-gamma signaling drives clinical response to immune checkpoint blockade therapy in melanoma. Cancer Cell. (2020) 38:500–515 e3. doi: 10.1016/j.ccell.2020.08.005
315. Hoefflin R, Harlander S, Schafer S, Metzger P, Kuo F, Schonenberger D, et al. HIF-1alpha and HIF-2alpha differently regulate tumour development and inflammation of clear cell renal cell carcinoma in mice. Nat Commun. (2020) 11:4111. doi: 10.1038/s41467-020-17873-3
316. An M, Mehta A, Min BH, Heo YJ, Wright SJ, Parikh M, et al. Early immune remodeling steers clinical response to first-line chemoimmunotherapy in advanced gastric cancer. Cancer Discov. (2024) 14:766–85. doi: 10.1158/2159-8290.CD-23-0857
317. Luo J, Lu C, Chen Y, Wu X, Zhu C, Cui W, et al. Nuclear translocation of cGAS orchestrates VEGF-A-mediated angiogenesis. Cell Rep. (2023) 42:112328. doi: 10.1016/j.celrep.2023.112328
318. Takagaki Y, Lee SM, Dongqing Z, Kitada M, Kanasaki K, and Koya D. Endothelial autophagy deficiency induces IL6 - dependent endothelial mesenchymal transition and organ fibrosis. Autophagy. (2020) 16:1905–14. doi: 10.1080/15548627.2020.1713641
319. Neulinger-Munoz M, Schaack D, Grekova SP, Bauer AS, Giese T, Salg GA, et al. Human retrotransposons and the global shutdown of homeostatic innate immunity by oncolytic parvovirus H-1PV in pancreatic cancer. Viruses. (2021) 13. doi: 10.3390/v13061019
320. Fu B, Xiong Y, Sha Z, Xue W, Xu B, Tan S, et al. SEPTIN2 suppresses an IFN-gamma-independent, proinflammatory macrophage activation pathway. Nat Commun. (2023) 14:7441. doi: 10.1038/s41467-023-43283-2
321. Qiu J, Xu B, Ye D, Ren D, Wang S, Benci JL, et al. Cancer cells resistant to immune checkpoint blockade acquire interferon-associated epigenetic memory to sustain T cell dysfunction. Nat Cancer. (2023) 4:43–61. doi: 10.1038/s43018-022-00490-y
322. Balasubramaniam A and Srinivasan S. Role of stimulator of interferon genes (STING) in the enteric nervous system in health and disease. Neurogastroenterol Motil. (2023) 35:e14603. doi: 10.1111/nmo.14603
323. Ozga AJ, Chow MT, and Luster AD. Chemokines and the immune response to cancer. Immunity. (2021) 54:859–74. doi: 10.1016/j.immuni.2021.01.012
324. Ullah A, Ud Din A, Ding W, Shi Z, Pervaz S, and Shen B. A narrative review: CXC chemokines influence immune surveillance in obesity and obesity-related diseases: Type 2 diabetes and nonalcoholic fatty liver disease. Rev Endocr Metab Disord. (2023) 24:611–31. doi: 10.1007/s11154-023-09800-w
325. Mempel TR, Lill JK, and Altenburger LM. How chemokines organize the tumour microenvironment. Nat Rev Cancer. (2024) 24:28–50. doi: 10.1038/s41568-023-00635-w
326. Comerford I and McColl SR. Atypical chemokine receptors in the immune system. Nat Rev Immunol. (2024) 24:753–69. doi: 10.1038/s41577-024-01025-5
327. Korbecki J, Kupnicka P, Chlubek M, Goracy J, Gutowska I, and Baranowska-Bosiacka I. CXCR2 receptor: regulation of expression, signal transduction, and involvement in cancer. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23042168
328. Nagy TA, Allen SS, Wroblewski LE, Flaherty DK, Slaughter JC, Perez-Perez G, et al. Helicobacter pylori induction of eosinophil migration is mediated by the cag pathogenicity island via microbial-epithelial interactions. Am J Pathol. (2011) 178:1448–52. doi: 10.1016/j.ajpath.2010.12.018
329. Zhao Z, Sun H, Liu Y, Zhang Y, Wang X, Wang X, et al. PDPN+ cancer-associated fibroblasts enhance gastric cancer angiogenesis via AKT/NF-kappaB activation and the CCL2-ACKR1 axis. MedComm (2020). (2025) 6:e70037. doi: 10.1002/mco2.70037
330. Cui Y, Chang Y, Ma X, Sun M, Huang Y, Yang F, et al. Ephrin A1 stimulates CCL2 secretion to facilitate premetastatic niche formation and promote gastric cancer liver metastasis. Cancer Res. (2025) 85:263–76. doi: 10.1158/0008-5472.CAN-24-1254
331. Tao LL, Shi SJ, Chen LB, and Huang GC. Expression of monocyte chemotactic protein-1/CCL2 in gastric cancer and its relationship with tumor hypoxia. World J Gastroenterol. (2014) 20:4421–7. doi: 10.3748/wjg.v20.i15.4421
332. Wei YF, Li X, Zhao MR, Liu S, Min L, Zhu ST, et al. Helicobacter pylori disrupts gastric mucosal homeostasis by stimulating macrophages to secrete CCL3. Cell Commun Signal. (2024) 22:263. doi: 10.1186/s12964-024-01627-5
333. He S, Wang L, Wu Y, Li D, and Zhang Y. CCL3 and CCL20-recruited dendritic cells modified by melanoma antigen gene-1 induce anti-tumor immunity against gastric cancer ex vivo and in vivo. J Exp Clin Cancer Res. (2010) 29:37. doi: 10.1186/1756-9966-29-37
334. Ohtani N, Ohtani H, Nakayama T, Naganuma H, Sato E, Imai T, et al. Infiltration of CD8+ T cells containing RANTES/CCL5+ cytoplasmic granules in actively inflammatory lesions of human chronic gastritis. Lab Invest. (2004) 84:368–75. doi: 10.1038/labinvest.3700039
335. Song S, He X, Wang J, Song H, Wang Y, Liu Y, et al. A novel long noncoding RNA, TMEM92-AS1, promotes gastric cancer progression by binding to YBX1 to mediate CCL5. Mol Oncol. (2021) 15:1256–73. doi: 10.1002/1878-0261.12863
336. Yang T, Chen M, Yang X, Zhang X, Zhang Z, Sun Y, et al. Down-regulation of KLF5 in cancer-associated fibroblasts inhibit gastric cancer cells progression by CCL5/CCR5 axis. Cancer Biol Ther. (2017) 18:806–15. doi: 10.1080/15384047.2017.1373219
337. Kuo CH, Liu CJ, Lu CY, Hu HM, Kuo FC, Liou YS, et al. 17beta-estradiol inhibits mesenchymal stem cells-induced human AGS gastric cancer cell mobility via suppression of CCL5- Src/Cas/Paxillin signaling pathway. Int J Med Sci. (2014) 11:7–16. doi: 10.7150/ijms.6851
338. Guo F, Li L, and Li L. Streptococcus anginosus: A new pathogen of superficial gastritis, atrophic gastritis and gastric cancer. Biomol BioMed. (2024) 24:1040–3. doi: 10.17305/bb.2024.10705
339. Cao FY, Wang CH, Li X, Ma MZ, Tao GC, Yang C, et al. Guanylate binding protein 5 accelerates gastric cancer progression via the JAK1-STAT1/GBP5/CXCL8 positive feedback loop. Am J Cancer Res. (2023) 13:1310–28.
340. Zhou Y, Wang Q, Tang W, Ma Z, Yang Z, Li X, et al. Palmatine ameliorates N-methyl-N’-nitrosoguanidine-induced chronic atrophic gastritis through the STAT1/CXCL10 axis. FASEB J. (2024) 38:e70037. doi: 10.1096/fj.202401624R
341. Yang C, Xu X, Wu M, Zhao Z, Feng Y, Liang W, et al. Huang-Jin-Shuang-Shen Decoction promotes CD8+ T-cell-mediated anti-tumor immunity by regulating chemokine CXCL10 in gastric cancer. Phytomedicine. (2024) 135:156065. doi: 10.1016/j.phymed.2024.156065
342. Nie S, Song Y, Hu K, Zu W, Zhang F, Chen L, et al. CXCL10 and IL15 co-expressing chimeric antigen receptor T cells enhance anti-tumor effects in gastric cancer by increasing cytotoxic effector cell accumulation and survival. Oncoimmunology. (2024) 13:2358590. doi: 10.1080/2162402X.2024.2358590
343. Meng Q, Zhang Y, and Hu LG. Targeting autophagy facilitates T lymphocyte migration by inducing the expression of CXCL10 in gastric cancer cell lines. Front Oncol. (2020) 10:886. doi: 10.3389/fonc.2020.00886
344. Zhou H, Wu J, Wang T, Zhang X, and Liu D. CXCL10/CXCR3 axis promotes the invasion of gastric cancer via PI3K/AKT pathway-dependent MMPs production. BioMed Pharmacother. (2016) 82:479–88. doi: 10.1016/j.biopha.2016.04.069
345. Teng YS, Zhao YL, Li MS, Liu YG, Cheng P, Lv YP, et al. Upexpression of BHLHE40 in gastric epithelial cells increases CXCL12 production through interaction with p-STAT3 in Helicobacter pylori-associated gastritis. FASEB J. (2020) 34:1169–81. doi: 10.1096/fj.201900464RR
346. Chen DL, Sheng H, Zhang DS, Jin Y, Zhao BT, Chen N, et al. The circular RNA circDLG1 promotes gastric cancer progression and anti-PD-1 resistance through the regulation of CXCL12 by sponging miR-141-3p. Mol Cancer. (2021) 20:166. doi: 10.1186/s12943-021-01475-8
347. Qin Y, Wang F, Ni H, Liu Y, Yin Y, Zhou X, et al. Cancer-associated fibroblasts in gastric cancer affect Malignant progression via the CXCL12-CXCR4 axis. J Cancer. (2021) 12:3011–23. doi: 10.7150/jca.49707
348. Wang YC, Lu S, Zhou XJ, Yang L, Liu P, Zhang L, et al. miR-1273h-5p suppresses CXCL12 expression and inhibits gastric cancer cell invasion and metastasis. Open Med (Wars). (2022) 17:930–46. doi: 10.1515/med-2022-0486
349. Li D and Li Q. MicroRNA-200b-3p restrains gastric cancer cell proliferation, migration, and invasion via C-X-C motif chemokine ligand 12/CXC chemokine receptor 7 axis. Bioengineered. (2022) 13:6509–20. doi: 10.1080/21655979.2022.2034585
350. Sun H, He T, Wu Y, Yuan H, Ning J, Zhang Z, et al. Cytotoxin-associated gene A-negative helicobacter pylori promotes gastric mucosal CX3CR1(+)CD4(+) effector memory T cell recruitment in mice. Front Microbiol. (2022) 13:813774. doi: 10.3389/fmicb.2022.813774
351. Su J, Mao X, Wang L, Chen Z, Wang W, Zhao C, et al. Lactate/GPR81 recruits regulatory T cells by modulating CX3CL1 to promote immune resistance in a highly glycolytic gastric cancer. Oncoimmunology. (2024) 13:2320951. doi: 10.1080/2162402X.2024.2320951
352. Wei LM, Cao S, Yu WD, Liu YL, and Wang JT. Overexpression of CX3CR1 is associated with cellular metastasis, proliferation and survival in gastric cancer. Oncol Rep. (2015) 33:615–24. doi: 10.3892/or.2014.3645
353. Zlotnik A and Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. (2000) 12:121–7. doi: 10.1016/S1074-7613(00)80165-X
354. Xu M, Wang Y, Xia R, Wei Y, and Wei X. Role of the CCL2-CCR2 signalling axis in cancer: Mechanisms and therapeutic targeting. Cell Prolif. (2021) 54:e13115. doi: 10.1111/cpr.13115
355. Teng Y, Xie R, Xu J, Wang P, Chen W, Shan Z, et al. Tubulointerstitial nephritis antigen-like 1 is a novel matricellular protein that promotes gastric bacterial colonization and gastritis in the setting of Helicobacter pylori infection. Cell Mol Immunol. (2023) 20:924–40. doi: 10.1038/s41423-023-01055-4
356. Bliss CM, JR, Golenbock DT, Keates S, Linevsky JK, and Kelly CP. Helicobacter pylori lipopolysaccharide binds to CD14 and stimulates release of interleukin-8, epithelial neutrophil-activating peptide 78, and monocyte chemotactic protein 1 by human monocytes. Infect Immun. (1998) 66:5357–63. doi: 10.1128/IAI.66.11.5357-5363.1998
357. Cho SO, Lim JW, and Kim H. Red ginseng extract inhibits the expression of MCP-1 and iNOS in Helicobacter pylori-infected gastric epithelial cells by suppressing the activation of NADPH oxidase and Jak2/Stat3. J Ethnopharmacol. (2013) 150:761–4. doi: 10.1016/j.jep.2013.09.013
358. Jung HC, Kim JM, Song IS, and Kim CY. Helicobacter pylori induces an array of pro-inflammatory cytokines in human gastric epithelial cells: quantification of mRNA for interleukin-8, -1 alpha/beta, granulocyte-macrophage colony-stimulating factor, monocyte chemoattractant protein-1 and tumour necrosis factor-alpha. J Gastroenterol Hepatol. (1997) 12:473–80. doi: 10.1111/j.1440-1746.1997.tb00469.x
359. Chiba Y, Mizoguchi I, Furusawa J, Hasegawa H, Ohashi M, Xu M, et al. Interleukin-27 exerts its antitumor effects by promoting differentiation of hematopoietic stem cells to M1 macrophages. Cancer Res. (2018) 78:182–94. doi: 10.1158/0008-5472.CAN-17-0960
360. Wolf MJ, Hoos A, Bauer J, Boettcher S, Knust M, Weber A, et al. Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer Cell. (2012) 22:91–105. doi: 10.1016/j.ccr.2012.05.023
361. Li C, Xu X, Wei S, Jiang P, Xue L, Wang J, et al. Tumor-associated macrophages: potential therapeutic strategies and future prospects in cancer. J Immunother Cancer. (2021) 9. doi: 10.1136/jitc-2020-001341
362. Chen C, He W, Huang J, Wang B, Li H, Cai Q, et al. LNMAT1 promotes lymphatic metastasis of bladder cancer via CCL2 dependent macrophage recruitment. Nat Commun. (2018) 9:3826. doi: 10.1038/s41467-018-06152-x
363. Li X, He G, Liu J, Yan M, Shen M, Xu L, et al. CCL2-mediated monocytes regulate immune checkpoint blockade resistance in pancreatic cancer. Int Immunopharmacol. (2022) 106:108598. doi: 10.1016/j.intimp.2022.108598
364. Vidyarthi A, Agnihotri T, Khan N, Singh S, Tewari MK, Radotra BD, et al. Predominance of M2 macrophages in gliomas leads to the suppression of local and systemic immunity. Cancer Immunol Immunother. (2019) 68:1995–2004. doi: 10.1007/s00262-019-02423-8
365. Shimizu T, Kusugami K, Ina K, Imada A, Nishio Y, Hosokawa T, et al. Helicobacter pylori-associated gastric ulcer exhibits enhanced mucosal chemokine activity at the ulcer site. Digestion. (2000) 62:87–94. doi: 10.1159/000007800
366. Eum HH, Kwon M, Ryu D, Jo A, Chung W, Kim N, et al. Tumor-promoting macrophages prevail in Malignant ascites of advanced gastric cancer. Exp Mol Med. (2020) 52:1976–88. doi: 10.1038/s12276-020-00538-y
367. To SKY, Tang MKS, Tong Y, Zhang J, Chan KKL, Ip PPC, et al. A Selective beta-Catenin-Metadherin/CEACAM1-CCL3 Axis Mediates Metastatic Heterogeneity upon Tumor-Macrophage Interaction. Adv Sci (Weinh). (2022) 9:e2103230. doi: 10.1002/advs.202103230
368. Wang X, Zhang L, Zhou Y, Wang Y, Wang X, Zhang Y, et al. Chronic stress exacerbates the immunosuppressive microenvironment and progression of gliomas by reducing secretion of CCL3. Cancer Immunol Res. (2024) 12:516–29. doi: 10.1158/2326-6066.CIR-23-0378
369. Guan B, Li H, Yao J, Guo J, Yu F, Li G, et al. CCL3-CCR5 axis promotes cell migration and invasion of colon adenocarcinoma via Akt signaling pathway. Environ Toxicol. (2023) 38:172–84. doi: 10.1002/tox.23675
370. Kodama T, Koma YI, Arai N, Kido A, Urakawa N, Nishio M, et al. CCL3-CCR5 axis contributes to progression of esophageal squamous cell carcinoma by promoting cell migration and invasion via Akt and ERK pathways. Lab Invest. (2020) 100:1140–57. doi: 10.1038/s41374-020-0441-4
371. Kang TG, Park HJ, Moon J, Lee JH, and Ha SJ. Enriching CCL3 in the tumor microenvironment facilitates T cell responses and improves the efficacy of anti-PD-1 therapy. Immune Netw. (2021) 21:e23. doi: 10.4110/in.2021.21.e23
372. Wang W, Chu HY, Zhong ZM, Qi X, Cheng R, Qin RJ, et al. Platelet-secreted CCL3 and its receptor CCR5 promote invasive and migratory abilities of anaplastic thyroid carcinoma cells via MMP-1. Cell Signal. (2019) 63:109363. doi: 10.1016/j.cellsig.2019.109363
373. Marra F and Tacke F. Roles for chemokines in liver disease. Gastroenterology. (2014) 147:577–594 e1. doi: 10.1053/j.gastro.2014.06.043
374. Marques RE, Guabiraba R, Russo RC, and Teixeira MM. Targeting CCL5 in inflammation. Expert Opin Ther Targets. (2013) 17:1439–60. doi: 10.1517/14728222.2013.837886
375. Wang SW, Wu HH, Liu SC, Wang PC, Ou WC, Chou WY, et al. CCL5 and CCR5 interaction promotes cell motility in human osteosarcoma. PloS One. (2012) 7:e35101. doi: 10.1371/journal.pone.0035101
376. Long H, Xie R, Xiang T, Zhao Z, Lin S, Liang Z, et al. Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem-like cells via NF-kappaB-mediated MMP-9 upregulation. Stem Cells. (2012) 30:2309–19. doi: 10.1002/stem.1194
377. Kim JE, Kim HS, Shin YJ, Lee CS, Won C, Lee SA, et al. LYR71, a derivative of trimeric resveratrol, inhibits tumorigenesis by blocking STAT3-mediated matrix metalloproteinase 9 expression. Exp Mol Med. (2008) 40:514–22. doi: 10.3858/emm.2008.40.5.514
378. Aldinucci D, Borghese C, and Casagrande N. The CCL5/CCR5 axis in cancer progression. Cancers (Basel). (2020) 12. doi: 10.3390/cancers12071765
379. Aldinucci D and Casagrande N. Inhibition of the CCL5/CCR5 axis against the progression of gastric cancer. Int J Mol Sci. (2018) 19. doi: 10.3390/ijms19051477
380. Mills CD, Lenz LL, and Harris RA. A breakthrough: macrophage-directed cancer immunotherapy. Cancer Res. (2016) 76:513–6. doi: 10.1158/0008-5472.CAN-15-1737
381. Shiao SL, Chu GC, and Chung LW. Regulation of prostate cancer progression by the tumor microenvironment. Cancer Lett. (2016) 380:340–8. doi: 10.1016/j.canlet.2015.12.022
382. Nagase H, Takeoka T, Urakawa S, Morimoto-Okazawa A, Kawashima A, Iwahori K, et al. ICOS(+) Foxp3(+) TILs in gastric cancer are prognostic markers and effector regulatory T cells associated with Helicobacter pylori. Int J Cancer. (2017) 140:686–95. doi: 10.1002/ijc.30475
383. Macedo F, Ladeira K, Longatto-Filho A, and Martins SF. Gastric cancer and angiogenesis: is VEGF a useful biomarker to assess progression and remission? J Gastric Cancer. (2017) 17:1–10. doi: 10.5230/jgc.2017.17.e1
384. Xu J, Shi Q, Lou J, Wang B, Wang W, Niu J, et al. Chordoma recruits and polarizes tumor-associated macrophages via secreting CCL5 to promote Malignant progression. J Immunother Cancer. (2023) 11. doi: 10.1136/jitc-2023-006808
385. Yu-Ju Wu C, Chen CH, Lin CY, Feng LY, Lin YC, Wei KC, et al. CCL5 of glioma-associated microglia/macrophages regulates glioma migration and invasion via calcium-dependent matrix metalloproteinase 2. Neuro Oncol. (2020) 22:253–66. doi: 10.1093/neuonc/noz189
386. Bronte V and Bria E. Interfering with CCL5/CCR5 at the tumor-stroma interface. Cancer Cell. (2016) 29:437–9. doi: 10.1016/j.ccell.2016.03.019
387. Waugh DJ and Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. (2008) 14:6735–41. doi: 10.1158/1078-0432.CCR-07-4843
388. Brat DJ, Bellail AC, and Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro Oncol. (2005) 7:122–33. doi: 10.1215/S1152851704001061
389. Lagisetty KH, Mcewen DP, Nancarrow DJ, Schiebel JG, Ferrer-Torres D, Ray D, et al. Immune determinants of Barrett’s progression to esophageal adenocarcinoma. JCI Insight. (2021) 6. doi: 10.1172/jci.insight.143888
390. Lee KE, Khoi PN, Xia Y, Park JS, Joo YE, Kim KK, et al. Helicobacter pylori and interleukin-8 in gastric cancer. World J Gastroenterol. (2013) 19:8192–202. doi: 10.3748/wjg.v19.i45.8192
391. Piao H, Fu L, Wang Y, Liu Y, Wang Y, Meng X, et al. A positive feedback loop between gastric cancer cells and tumor-associated macrophage induces Malignancy progression. J Exp Clin Cancer Res. (2022) 41:174. doi: 10.1186/s13046-022-02366-6
392. Ji HZ, Chen L, Ren M, Li S, Liu TY, Chen HJ, et al. CXCL8 promotes endothelial-to-mesenchymal transition of endothelial cells and protects cells from erastin-induced ferroptosis via CXCR2-mediated activation of the NF-kappaB signaling pathway. Pharm (Basel). (2023) 16. doi: 10.3390/ph16091210
393. Han ZJ, Li YB, Yang LX, Cheng HJ, Liu X, and Chen H. Roles of the CXCL8-CXCR1/2 axis in the tumor microenvironment and immunotherapy. Molecules. (2021) 27. doi: 10.3390/molecules27010137
394. Lin C, He H, Liu H, Li R, Chen Y, Qi Y, et al. Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut. (2019) 68:1764–73. doi: 10.1136/gutjnl-2018-316324
395. Hou Y and Huttenlocher A. Advancing chemokine research: the molecular function of CXCL8. J Clin Invest. (2024) 134. doi: 10.1172/JCI180984
396. Janssens R, Struyf S, and Proost P. The unique structural and functional features of CXCL12. Cell Mol Immunol. (2018) 15:299–311. doi: 10.1038/cmi.2017.107
397. Yang Y, Li J, Lei W, Wang H, Ni Y, Liu Y, et al. CXCL12-CXCR4/CXCR7 axis in cancer: from mechanisms to clinical applications. Int J Biol Sci. (2023) 19:3341–59. doi: 10.7150/ijbs.82317
398. Mortezaee K. CXCL12/CXCR4 axis in the microenvironment of solid tumors: A critical mediator of metastasis. Life Sci. (2020) 249:117534. doi: 10.1016/j.lfs.2020.117534
399. Lee HJ and Jo DY. The role of the CXCR4/CXCL12 axis and its clinical implications in gastric cancer. Histol Histopathol. (2012) 27:1155–61. doi: 10.14670/HH-27.1155
400. Daniel SK, Seo YD, and Pillarisetty VG. The CXCL12-CXCR4/CXCR7 axis as a mechanism of immune resistance in gastrointestinal Malignancies. Semin Cancer Biol. (2020) 65:176–88. doi: 10.1016/j.semcancer.2019.12.007
401. Xue LJ, Mao XB, Ren LL, and Chu XY. Inhibition of CXCL12/CXCR4 axis as a potential targeted therapy of advanced gastric carcinoma. Cancer Med. (2017) 6:1424–36. doi: 10.1002/cam4.1085
402. Ishigami S, Natsugoe S, Okumura H, Matsumoto M, Nakajo A, Uenosono Y, et al. Clinical implication of CXCL12 expression in gastric cancer. Ann Surg Oncol. (2007) 14:3154–8. doi: 10.1245/s10434-007-9521-6
403. Xin Q, Zhang N, Yu HB, Zhang Q, Cui YF, Zhang CS, et al. CXCR7/CXCL12 axis is involved in lymph node and liver metastasis of gastric carcinoma. World J Gastroenterol. (2017) 23:3053–65. doi: 10.3748/wjg.v23.i17.3053
404. Liu Y, Li Q, Tang D, Li M, Zhao P, Yang W, et al. SNHG17 promotes the proliferation and migration of colorectal adenocarcinoma cells by modulating CXCL12-mediated angiogenesis. Cancer Cell Int. (2020) 20:566. doi: 10.1186/s12935-020-01621-0
405. Zhou W, Guo S, Liu M, Burow ME, and Wang G. Targeting CXCL12/CXCR4 axis in tumor immunotherapy. Curr Med Chem. (2019) 26:3026–41. doi: 10.2174/0929867324666170830111531
406. Zhong T, Li X, Lei K, Tang R, Zhou Z, Zhao B, et al. CXCL12-CXCR4 mediates CD57(+) CD8(+) T cell responses in the progression of type 1 diabetes. J Autoimmun. (2024) 143:103171. doi: 10.1016/j.jaut.2024.103171
407. Gao J, Wu L, Wang S, and Chen X. Role of chemokine (C-X-C motif) ligand 10 (CXCL10) in renal diseases. Mediators Inflammation. (2020) 2020:6194864. doi: 10.1155/2020/6194864
408. Karin N and Razon H. Chemokines beyond chemo-attraction: CXCL10 and its significant role in cancer and autoimmunity. Cytokine. (2018) 109:24–8. doi: 10.1016/j.cyto.2018.02.012
409. Nie Y, Liu C, Liu Q, and Zhu X. CXCL10 is a prognostic marker for pancreatic adenocarcinoma and tumor microenvironment remodeling. BMC Cancer. (2023) 23:150. doi: 10.1186/s12885-023-10615-w
410. Ye Y, Li L, Kang H, Wan Z, Zhang M, Gang B, et al. LAMP1 controls CXCL10-CXCR3 axis mediated inflammatory regulation of macrophage polarization during inflammatory stimulation. Int Immunopharmacol. (2024) 132:111929. doi: 10.1016/j.intimp.2024.111929
411. Kumagai S, Togashi Y, Sakai C, Kawazoe A, Kawazu M, Ueno T, et al. An oncogenic alteration creates a microenvironment that promotes tumor progression by conferring a metabolic advantage to regulatory T cells. Immunity. (2020) 53:187–203.e8. doi: 10.1016/j.immuni.2020.06.016
412. Karin N. Chemokines and cancer: new immune checkpoints for cancer therapy. Curr Opin Immunol. (2018) 51:140–5. doi: 10.1016/j.coi.2018.03.004
413. Zhang MJ, Lin WP, Wang Q, Wang S, Song A, Wang YY, et al. Oncolytic herpes simplex virus propagates tertiary lymphoid structure formation via CXCL10/CXCR3 to boost antitumor immunity. Cell Prolif. (2025) 58:e13740. doi: 10.1111/cpr.13740
414. Torphy RJ, Sun Y, Lin R, Caffrey-Carr A, Fujiwara Y, Ho F, et al. GPR182 limits antitumor immunity via chemokine scavenging in mouse melanoma models. Nat Commun. (2022) 13:97. doi: 10.1038/s41467-021-27658-x
415. Limagne E, Nuttin L, Thibaudin M, Jacquin E, Aucagne R, Bon M, et al. MEK inhibition overcomes chemoimmunotherapy resistance by inducing CXCL10 in cancer cells. Cancer Cell. (2022) 40:136–152 e12. doi: 10.1016/j.ccell.2021.12.009
416. Liu C, Zheng S, Wang Z, Wang S, Wang X, Yang L, et al. KRAS-G12D mutation drives immune suppression and the primary resistance of anti-PD-1/PD-L1 immunotherapy in non-small cell lung cancer. Cancer Commun (Lond). (2022) 42:828–47. doi: 10.1002/cac2.12327
417. Korbecki J, Siminska D, Kojder K, Grochans S, Gutowska I, Chlubek D, et al. Fractalkine/CX3CL1 in neoplastic processes. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21103723
418. Zhang C, Zhang Y, Zhuang R, Yang K, Chen L, Jin B, et al. Alterations in CX3CL1 levels and its role in viral pathogenesis. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25084451
419. Wu F, Chen C, and Peng F. Potential association between asthma, helicobacter pylori infection, and gastric cancer. Front Oncol. (2021) 11:630235. doi: 10.3389/fonc.2021.630235
420. Lv CY, Zhou T, Chen W, Yin XD, Yao JH, and Zhang YF. Preliminary study correlating CX3CL1/CX3CR1 expression with gastric carcinoma and gastric carcinoma perineural invasion. World J Gastroenterol. (2014) 20:4428–32. doi: 10.3748/wjg.v20.i15.4428
421. Helmke A, Nordlohne J, Balzer MS, Dong L, Rong S, Hiss M, et al. CX3CL1-CX3CR1 interaction mediates macrophage-mesothelial cross talk and promotes peritoneal fibrosis. Kidney Int. (2019) 95:1405–17. doi: 10.1016/j.kint.2018.12.030
422. Ni Y, Zhuge F, Ni L, Nagata N, Yamashita T, Mukaida N, et al. CX3CL1/CX3CR1 interaction protects against lipotoxicity-induced nonalcoholic steatohepatitis by regulating macrophage migration and M1/M2 status. Metabolism. (2022) 136:155272. doi: 10.1016/j.metabol.2022.155272
423. Ma J, Wu Y, Wu S, Fang Z, Chen L, Jiang J, et al. CX3CR1(+)CD8(+) T cells: Key players in antitumor immunity. Cancer Sci. (2024) 115:3838–45. doi: 10.1111/cas.16359
424. Chaudhri A, Bu X, Wang Y, Gomez M, Torchia JA, Hua P, et al. The CX3CL1-CX3CR1 chemokine axis can contribute to tumor immune evasion and blockade with a novel CX3CR1 monoclonal antibody enhances response to anti-PD-1 immunotherapy. Front Immunol. (2023) 14:1237715. doi: 10.3389/fimmu.2023.1237715
425. Trinh T, Adams WA, Calescibetta A, Tu N, Dalton R, So T, et al. CX3CR1 deficiency-induced TIL tumor restriction as a novel addition for CAR-T design in solid Malignancies. iScience. (2023) 26:106443. doi: 10.1016/j.isci.2023.106443
426. Korbecki J, Bosiacki M, Kupnicka P, Barczak K, Chlubek D, and Baranowska-Bosiacka I. CXCR4 as a therapeutic target in acute myeloid leukemia. Leukemia. (2024) 38:2303–17. doi: 10.1038/s41375-024-02326-3
427. Wu W, Zhou Z, Pang C, Wen X, Ye S, Quan JH, et al. IGF2BP2 regulates inflammation in ulcerative colitis through N6-methyladenosine-dependent modulation of CBR1. Int Immunopharmacol. (2025) 161:115072. doi: 10.1016/j.intimp.2025.115072
428. Liu T, Wang L, Zhang X, Chen L, Liu Y, Jiang Z, et al. Tocilizumab monotherapy or combined with methotrexate for rheumatoid arthritis: A randomized clinical trial. JAMA Netw Open. (2025) 8:e2511095. doi: 10.1001/jamanetworkopen.2025.11095
429. Rolig AS, Rose DC, Mcgee GH, Rubas W, Kivimae S, and Redmond WL. Combining bempegaldesleukin (CD122-preferential IL-2 pathway agonist) and NKTR-262 (TLR7/8 agonist) improves systemic antitumor CD8(+) T cell cytotoxicity over BEMPEG+RT. J Immunother Cancer. (2022) 10. doi: 10.1136/jitc-2021-004218
430. Sia T, Bacchus L, Tanaka R, Khuda R, Mallik S, and Leung J. Dupilumab can induce remission of eosinophilic gastritis and duodenitis: A retrospective case series. Clin Transl Gastroenterol. (2024) 15:e00646. doi: 10.14309/ctg.0000000000000646
431. Thilakasiri P, Huynh J, Poh AR, Tan CW, Nero TL, Tran K, et al. Repurposing the selective estrogen receptor modulator bazedoxifene to suppress gastrointestinal cancer growth. EMBO Mol Med. (2019) 11. doi: 10.15252/emmm.201809539
432. Burkhardt C, Buhler L, Tihy M, Morel P, and Forni M. Bazedoxifene as a novel strategy for treatment of pancreatic and gastric adenocarcinoma. Oncotarget. (2019) 10:3198–202. doi: 10.18632/oncotarget.26833
433. Song M, Liang J, Wang L, Li W, Jiang S, Xu S, et al. IL-17A functions and the therapeutic use of IL-17A and IL-17RA targeted antibodies for cancer treatment. Int Immunopharmacol. (2023) 123:110757. doi: 10.1016/j.intimp.2023.110757
434. Zhao C, Lu X, Bu X, Zhang N, and Wang W. Involvement of tumor necrosis factor-alpha in the upregulation of CXCR4 expression in gastric cancer induced by Helicobacter pylori. BMC Cancer. (2010) 10:419. doi: 10.1186/1471-2407-10-419
435. Rossi EA, Rossi DL, Cardillo TM, Chang CH, and Goldenberg DM. Redirected T-cell killing of solid cancers targeted with an anti-CD3/Trop-2-bispecific antibody is enhanced in combination with interferon-alpha. Mol Cancer Ther. (2014) 13:2341–51. doi: 10.1158/1535-7163.MCT-14-0345
436. Mora-Lagos B, Reyes ME, Lobos-Gonzalez L, Del Campo M, Buchegger K, Zanella L, et al. Maraviroc/cisplatin combination inhibits gastric cancer tumoroid growth and improves mice survival. Biol Res. (2025) 58:4. doi: 10.1186/s40659-024-00581-3
437. Mencarelli A, Graziosi L, Renga B, Cipriani S, D’amore C, Francisci D, et al. CCR5 antagonism by maraviroc reduces the potential for gastric cancer cell dissemination. Transl Oncol. (2013) 6:784–93. doi: 10.1593/tlo.13499
438. Wang J, Hu W, Wang K, Yu J, Luo B, Luo G, et al. Repertaxin, an inhibitor of the chemokine receptors CXCR1 and CXCR2, inhibits Malignant behavior of human gastric cancer MKN45 cells in vitro and in vivo and enhances efficacy of 5-fluorouracil. Int J Oncol. (2016) 48:1341–52. doi: 10.3892/ijo.2016.3371
439. Cao Q, Cheng X, Lv R, Sun D, Wang J, Fu R, et al. Nanoparticle-mediated CXCL12-CXCR4 inhibition reprograms macrophages and suppresses gastric carcinoma. Adv Sci (Weinh). (2025) 12:e00225. doi: 10.1002/advs.202500225
440. Libanio D, Dinis-Ribeiro M, and Pimentel-Nunes P. Helicobacter pylori and microRNAs: Relation with innate immunity and progression of preneoplastic conditions. World J Clin Oncol. (2015) 6:111–32. doi: 10.5306/wjco.v6.i5.111
441. Xiao B, Liu Z, Li BS, Tang B, Li W, Guo G, et al. Induction of microRNA-155 during Helicobacter pylori infection and its negative regulatory role in the inflammatory response. J Infect Dis. (2009) 200:916–25. doi: 10.1086/605443
442. Liu Z, Xiao B, Tang B, Li B, Li N, Zhu E, et al. Up-regulated microRNA-146a negatively modulate Helicobacter pylori-induced inflammatory response in human gastric epithelial cells. Microbes Infect. (2010) 12:854–63. doi: 10.1016/j.micinf.2010.06.002
443. Crone SG, Jacobsen A, Federspiel B, Bardram L, Krogh A, Lund AH, et al. microRNA-146a inhibits G protein-coupled receptor-mediated activation of NF-kappaB by targeting CARD10 and COPS8 in gastric cancer. Mol Cancer. (2012) 11:71. doi: 10.1186/1476-4598-11-71
444. Blosse A, Levy M, Robe C, Staedel C, Copie-Bergman C, and Lehours P. Deregulation of miRNA in Helicobacter pylori-Induced Gastric MALT Lymphoma: From Mice to Human. J Clin Med. (2019) 8. doi: 10.3390/jcm8060845
445. Sgouras DN, Trang TT, and Yamaoka Y. Pathogenesis of helicobacter pylori infection. Helicobacter. (2015) 20 Suppl 1:8–16. doi: 10.1111/hel.12251
446. Reyes VE. Helicobacter pylori and its role in gastric cancer. Microorganisms. (2023) 11. doi: 10.3390/microorganisms11051312
447. Roberts JR, Tran SC, Frick-Cheng AE, Bryant KN, Okoye CD, Mcdonald WH, et al. Subdomains of the Helicobacter pylori Cag T4SS outer membrane core complex exhibit structural independence. Life Sci Alliance. (2024) 7. doi: 10.26508/lsa.202302560
448. Mok CY, Chu HY, Lam WWL, and Au SWN. Structural insights into the assembly pathway of the Helicobacter pylori CagT4SS outer membrane core complex. Structure. (2024) 32:1725–1736 e4. doi: 10.1016/j.str.2024.06.019
449. Gorrell RJ, Guan J, Xin Y, Tafreshi MA, Hutton ML, Mcguckin MA, et al. A novel NOD1- and CagA-independent pathway of interleukin-8 induction mediated by the Helicobacter pylori type IV secretion system. Cell Microbiol. (2013) 15:554–70. doi: 10.1111/cmi.12055
450. Smolka AJ and Backert S. How Helicobacter pylori infection controls gastric acid secretion. J Gastroenterol. (2012) 47:609–18. doi: 10.1007/s00535-012-0592-1
451. Liu X, Wang D, Wei X, Yang D, Ma Y, and Liu G. Selectively antagonizing the NOD1-mediated inflammatory signaling pathway mitigates the gastric inflammation induced by helicobacter pylori infection. J Med Chem. (2024) 67:22145–67. doi: 10.1021/acs.jmedchem.4c02139
452. Lind J, Backert S, Hoffmann R, Eichler J, Yamaoka Y, Perez-Perez GI, et al. Systematic analysis of phosphotyrosine antibodies recognizing single phosphorylated EPIYA-motifs in CagA of East Asian-type Helicobacter pylori strains. BMC Microbiol. (2016) 16:201. doi: 10.1186/s12866-016-0820-6
453. Kocazeybek BS, Caliskan R, Erdamar Cetin S, Ergin S, Kuskucu M, Kepil N, et al. Patterns of EPIYA motifs among cagA-positive Helicobacter pylori strains: a case-control study in a Turkish population with Eurasian geographical features. J Med Microbiol. (2015) 64:1117–23. doi: 10.1099/jmm.0.000141
454. Yamahashi Y and Hatakeyama M. PAR1b takes the stage in the morphogenetic and motogenetic activity of Helicobacter pylori CagA oncoprotein. Cell Adh Migr. (2013) 7:11–8. doi: 10.4161/cam.21936
455. Salvatori S, Marafini I, Laudisi F, Monteleone G, and Stolfi C. Helicobacter pylori and gastric cancer: pathogenetic mechanisms. Int J Mol Sci. (2023) 24. doi: 10.3390/ijms24032895
456. Sokolova O and Naumann M. NF-kappaB signaling in gastric cancer. Toxins (Basel). (2017) 9. doi: 10.3390/toxins9040119
457. Kim DJ, Park JH, Franchi L, Backert S, and Nunez G. The Cag pathogenicity island and interaction between TLR2/NOD2 and NLRP3 regulate IL-1beta production in Helicobacter pylori infected dendritic cells. Eur J Immunol. (2013) 43:2650–8. doi: 10.1002/eji.201243281
458. Datta De D and Roychoudhury S. To be or not to be: The host genetic factor and beyond in Helicobacter pylori mediated gastro-duodenal diseases. World J Gastroenterol. (2015) 21:2883–95. doi: 10.3748/wjg.v21.i10.2883
459. Herrera-Goepfert R, Yamamoto-Furusho JK, Onate-Ocana LF, Camorlinga-Ponce M, Munoz L, Ruiz-Morales JA, et al. Role of the HLA-DQ locus in the development of chronic gastritis and gastric carcinoma in Mexican patients. World J Gastroenterol. (2006) 12:7762–7. doi: 10.3748/wjg.v12.i48.7762
460. Ando T, Ishikawa T, Kato H, Yoshida N, Naito Y, Kokura S, et al. Synergistic effect of HLA class II loci and cytokine gene polymorphisms on the risk of gastric cancer in Japanese patients with Helicobacter pylori infection. Int J Cancer. (2009) 125:2595–602. doi: 10.1002/ijc.24666
461. Malfertheiner P, Camargo MC, El-Omar E, Liou JM, Peek R, Schulz C, et al. Helicobacter pylori infection. Nat Rev Dis Primers. (2023) 9:19. doi: 10.1038/s41572-023-00431-8
462. Santos MP, Pereira JN, Delabio RW, Smith MAC, Payao SLM, Carneiro LC, et al. Increased expression of interleukin-6 gene in gastritis and gastric cancer. Braz J Med Biol Res. (2021) 54:e10687. doi: 10.1590/1414-431x2020e10687
463. Wang F, Meng W, Wang B, and Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. (2014) 345:196–202. doi: 10.1016/j.canlet.2013.08.016
464. Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther. (2021) 6:263. doi: 10.1038/s41392-021-00658-5
465. Ma L, Zeng J, Guo Q, Liang X, Shen L, Li S, et al. Mutual amplification of HNF4alpha and IL-1R1 composes an inflammatory circuit in Helicobacter pylori associated gastric carcinogenesis. Oncotarget. (2016) 7:11349–63. doi: 10.18632/oncotarget.7239
466. Wang X, Wang B, Xie J, Hou D, Zhang H, and Huang H. Melatonin inhibits epithelial−to−mesenchymal transition in gastric cancer cells via attenuation of IL−1beta/NF−kappaB/MMP2/MMP9 signaling. Int J Mol Med. (2018) 42:2221–8. doi: 10.3892/ijmm.2018.3788
467. Xu W, Wang L, Yang L, Li X, Li C, and Liu B. Vitamin D3 alleviates the gastritis that associated with Helicobacter pylori infection in mice with hypercholesterolemia by enhancing the activity of vitamin D receptors in the liver tissue and blocking the signaling pathway of JAK/STAT3. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. (2024) 40:520–6.
468. Cao J, Yao M, Wang K, Qin L, Zhang Q, Zhang H, et al. Sea Cucumber Fucoidan Inhibits Helicobacter pylori Gastritis via MAPK/NF-kappaB Signaling and Gut Microbiota Modulation. J Agric Food Chem. (2025) 73:14333–52. doi: 10.1021/acs.jafc.5c02190
469. Li D, Gao Z, Zhang Z, Chen H, Tang R, Zhou L, et al. Suprabasin promotes gastric cancer liver metastasis via hepatic stellate cells-mediated EGF/CCL2/JAK2 intercellular signaling pathways. Oncogene. (2025) 44:1975–89. doi: 10.1038/s41388-025-03370-8
470. Wu D and Wang Z. Gastric cancer cell-derived kynurenines hyperactive regulatory T cells to promote chemoresistance via the IL-10/STAT3/BCL2 signaling pathway. DNA Cell Biol. (2022) 41:447–55. doi: 10.1089/dna.2021.0936
471. Sarajlic M, Neuper T, Vetter J, Schaller S, Klicznik MM, Gratz IK, et al. H. pylori modulates DC functions via T4SS/TNFalpha/p38-dependent SOCS3 expression. Cell Commun Signal. (2020) 18:160. doi: 10.1186/s12964-020-00655-1
472. Jafarzadeh A, Jafarzadeh Z, Nemati M, and Yoshimura A. The interplay between helicobacter pylori and suppressors of cytokine signaling (SOCS) molecules in the development of gastric cancer and induction of immune response. Helicobacter. (2024) 29:e13105. doi: 10.1111/hel.13105
473. Du B, Liu M, Li C, Geng X, Zhang X, Ning D, et al. The potential role of TNFAIP3 in Malignant transformation of gastric carcinoma. Pathol Res Pract. (2019) 215:152471. doi: 10.1016/j.prp.2019.152471
474. Lim MCC, Maubach G, Sokolova O, Feige MH, Diezko R, Buchbinder J, et al. Pathogen-induced ubiquitin-editing enzyme A20 bifunctionally shuts off NF-kappaB and caspase-8-dependent apoptotic cell death. Cell Death Differ. (2017) 24:1621–31. doi: 10.1038/cdd.2017.89
475. Denk D and Greten FR. Inflammation: the incubator of the tumor microenvironment. Trends Cancer. (2022) 8:901–14. doi: 10.1016/j.trecan.2022.07.002
476. Miao Z, Li J, Wang Y, Shi M, Gu X, Zhang X, et al. Hsa_circ_0136666 stimulates gastric cancer progression and tumor immune escape by regulating the miR-375/PRKDC Axis and PD-L1 phosphorylation. Mol Cancer. (2023) 22:205. doi: 10.1186/s12943-023-01883-y
477. Li X, Pan K, Vieth M, Gerhard M, Li W, and Mejias-Luque R. JAK-STAT1 signaling pathway is an early response to helicobacter pylori infection and contributes to immune escape and gastric carcinogenesis. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23084147
478. He Q, Liu M, Huang W, Chen X, Zhang B, Zhang T, et al. IL-1beta-induced elevation of solute carrier family 7 member 11 promotes hepatocellular carcinoma metastasis through up-regulating programmed death ligand 1 and colony-stimulating factor 1. Hepatology. (2021) 74:3174–93. doi: 10.1002/hep.32062
479. Efferth T and Oesch F. The immunosuppressive activity of artemisinin-type drugs towards inflammatory and autoimmune diseases. Med Res Rev. (2021) 41:3023–61. doi: 10.1002/med.21842
480. Chen Z, Giotti B, Kaluzova M, Vallcorba MP, Rawat K, Price G, et al. A paracrine circuit of IL-1beta/IL-1R1 between myeloid and tumor cells drives genotype-dependent glioblastoma progression. J Clin Invest. (2023) 133. doi: 10.1172/JCI163802
481. Zhang M, Hu S, Min M, Ni Y, Lu Z, Sun X, et al. Dissecting transcriptional heterogeneity in primary gastric adenocarcinoma by single cell RNA sequencing. Gut. (2021) 70:464–75. doi: 10.1136/gutjnl-2019-320368
482. Li N, Chen S, Xu X, Wang H, Zheng P, Fei X, et al. Single-cell transcriptomic profiling uncovers cellular complexity and microenvironment in gastric tumorigenesis associated with Helicobacter pylori. J Adv Res. (2024) 74:471–91. doi: 10.21203/rs.3.rs-3641851/v1
483. Lee SH, Lee D, Choi J, Oh HJ, Ham IH, Ryu D, et al. Spatial dissection of tumour microenvironments in gastric cancers reveals the immunosuppressive crosstalk between CCL2+ fibroblasts and STAT3-activated macrophages. Gut. (2025) 74:714–27. doi: 10.1136/gutjnl-2024-332901
484. Tang J, Wei W, Xu Y, Chen K, Miao Y, Fan W, et al. CXC chemokine receptor 4 - mediated immune modulation and tumor microenvironment heterogeneity in gastric cancer: Utilizing multi-omics approaches to identify potential therapeutic targets. Biofactors. (2025) 51:e2130. doi: 10.1002/biof.2130
485. Verona F, Di Bella S, Schirano R, Manfredi C, Angeloro F, Bozzari G, et al. Cancer stem cells and tumor-associated macrophages as mates in tumor progression: mechanisms of crosstalk and advanced bioinformatic tools to dissect their phenotypes and interaction. Front Immunol. (2025) 16:1529847. doi: 10.3389/fimmu.2025.1529847
486. Ma X, Jia S, Wang G, Liang M, Guo T, Du H, et al. TRIM28 promotes the escape of gastric cancer cells from immune surveillance by increasing PD-L1 abundance. Signal Transduct Target Ther. (2023) 8:246. doi: 10.1038/s41392-023-01450-3
487. Shen DD, Pang JR, Bi YP, Zhao LF, Li YR, Zhao LJ, et al. LSD1 deletion decreases exosomal PD-L1 and restores T-cell response in gastric cancer. Mol Cancer. (2022) 21:75. doi: 10.1186/s12943-022-01557-1
488. Low JT, Christie M, Ernst M, Dumoutier L, Preaudet A, Ni Y, et al. Loss of NFKB1 results in expression of tumor necrosis factor and activation of signal transducer and activator of transcription 1 to promote gastric tumorigenesis in mice. Gastroenterology. (2020) 159:1444–1458 e15. doi: 10.1053/j.gastro.2020.06.039
Keywords: gastritis, gastric cancer, inflammatory factors, tumor micro environment, Helicobacter pylori
Citation: Zhang M, Su A, Song H, Zhang S, Deng Y, Jing W, Guo J, Zhan W, Ma Y and Hu M (2025) Inflammatory factors collaboratively link Helicobacter pylori-induced gastritis to gastric cancer. Front. Immunol. 16:1628543. doi: 10.3389/fimmu.2025.1628543
Received: 14 May 2025; Accepted: 31 October 2025;
Published: 25 November 2025.
Edited by:
Leandro J. Carreno, University of Chile, ChileReviewed by:
Bekir Kocazeybek, Istanbul University-Cerrahpasa, TürkiyeLin Liu, Xiyuan Hospital of China Academy of Chinese Medical Sciences, China
Li Lin, Lanzhou University, China
Copyright © 2025 Zhang, Su, Song, Zhang, Deng, Jing, Guo, Zhan, Ma and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuntao Ma, MzU3NTUxNTY2NUBxcS5jb20=; Ming Hu, MzA5MTk5NzRAcXEuY29t
†These authors have contributed equally to this work and share first authorship
 Mingze Zhang
Mingze Zhang Ade Su2†
Ade Su2† Houji Song
Houji Song