- 1National Institute for Health and Care Research (NIHR) Biomedical Research Centre, School of Clinical and Experimental Sciences, Faculty of Medicine, University of Southampton, Southampton, United Kingdom
- 2Institute for Life Sciences, University of Southampton, Southampton, United Kingdom
CD1-restricted T cells constitute an unconventional arm of immunity that recognises lipid antigens, a feature particularly pertinent to Mycobacterium tuberculosis (Mtb), a pathogen with a lipid-rich cell wall. Unlike classical MHC-restricted responses, CD1-mediated lipid antigen presentation includes donor-unrestricted T cell responses, offering a promising pathway for universally protective tuberculosis (TB) vaccines. This review explores the biology of CD1 isoforms, the functional diversity of CD1-restricted T cell subsets, and their roles in TB immunity. We discuss Mtb’s lipid antigens, mechanisms of CD1 trafficking and antigen presentation, immune evasion strategies, and emerging translational insights. By highlighting key knowledge gaps and future directions, we argue that harnessing CD1-restricted T cells could unlock novel vaccine strategies against the world’s leading infectious killer.
Introduction
In 2023, 10.8 million people fell ill with tuberculosis (TB), and 1.25 million died, making it the world’s leading cause of death from an infectious disease. The causative agent, Mycobacterium tuberculosis (Mtb), is estimated to infect around a quarter of the global population (1). Although TB primarily affects the lungs, it can also manifest in other parts of the body (2). The disease is highly contagious, disproportionately affects those living in poverty, and is costly and time-consuming to treat, all of which contribute to its immense global health burden (3). Compounding these challenges, the emergence of antibiotic-resistant Mtb strains poses a growing threat to TB control efforts (2).
Host immune responses to TB have traditionally focussed on peptide antigens presented by MHC class I and class II molecules, which are well studied in the context of conventional T cell immunity. However, these molecules are encoded by some of the most polymorphic genes in the human genome (4), resulting in substantial inter-individual variability in immune responses to Mtb (5, 6).
Mtb has a complex and lipid-rich cell wall, known as the mycolyl-arabinogalactan-peptidoglycan complex (7). Around 6% of the Mtb genome is dedicated to lipid metabolism (8), and lipids constitute approximately 40% of the cell envelope by weight (9). This unusual lipid composition underpins the pathogen’s virulence and resistance to antibiotics (10–15). Indeed, some of the most effective TB drugs, such as isoniazid, act by inhibiting Mtb lipid biosynthesis (8).
Importantly, these lipid components of the Mtb cell wall can be presented by CD1 molecules, a family of non-classical antigen-presenting molecules, to activate CD1-restricted T cells and initiate antimicrobial immune responses (8). Unlike MHC molecules, CD1 proteins are virtually non-polymorphic, meaning that the responses they elicit are genetically unrestricted and shared across the population (16, 17). This feature makes CD1 an attractive target for broadly effective TB vaccines, capable of overcoming the genetic variability that hampers conventional MHC-restricted vaccine approaches (18).
Despite their potential, CD1-restricted T cells remain underexplored in TB research, in part due to the technical challenges associated with studying them. However, harnessing these unconventional T cell responses may offer a novel strategy to enhance the efficacy of future TB vaccines (19). CD1-restricted T cell activation in TB appears to occur via two distinct but potentially complementary mechanisms. The first involves direct recognition of mycobacterial lipid antigens, such as mycolic acid, glucose monomycolate (GMM), or phosphomycoketides, presented by CD1 molecules on infected antigen-presenting cells (20–22). The second involves infection- or inflammation-induced remodelling of host lipid metabolism, leading to enhanced presentation of stimulatory self-lipids by CD1 and activation of autoreactive T cells (23). For instance, Toll-like receptor (TLR) signalling has been shown to promote the presentation of endogenous lipids such as sulfatide and GM1 (23), and in the case of CD1d-restricted iNKT cells, Brennan et al. (2011) demonstrated that microbial sensing by antigen-presenting cells (APCs) can induce self-lipid switching that drives T cell activation even in the absence of microbial antigens (24). Clarifying the relative importance of these pathways is essential for rational vaccine design and for maximising the potential of CD1-targeted immunity.
Mechanisms of lipid antigen presentation by CD1 molecules
CD1 molecules are a family of non-polymorphic, non-classical MHC class I-like antigen-presenting molecules encoded on chromosome 1. They are specialised for the presentation of both self- and foreign lipid antigens to T cells. Based on sequence similarities, CD1 isoforms are classified into three groups: group 1 molecules (CD1a, CD1b, and CD1c), which are expressed exclusively by antigen-presenting cells (25) and cortical thymocytes, where they likely contribute to thymic selection of CD1-restricted T cells (26). CD1d, the sole member of group 2, is expressed across a broader range of immune cells (27). In contrast, CD1e, the only group 3 molecule, is restricted to intracellular compartments. CD1e localises to late endosomal compartments and facilitates lipid loading onto other CD1 isoforms (28). Structurally, CD1 molecules share homology with MHC class I molecules. Each CD1 molecule comprises a heavy chain with α1 and α2 domains forming the antigen-binding groove, structured as two antiparallel α-helices over a β-pleated sheet, and an immunoglobulin-like α3 domain with a transmembrane region and a short cytoplasmic tail anchoring the molecule to the membrane. Like MHC class I molecules, CD1 molecules associate non-covalently with β2-microglobulin. Lipid antigens bind within deep hydrophobic channels, with their polar headgroups exposed at the solvent interface for recognition by the T cell receptor (TCR). Differences in the size and shape of the antigen-binding grooves among CD1 isoforms facilitate the presentation of a wide range of lipid antigens (29–35).
CD1 molecules are synthesised in the endoplasmic reticulum (ER), where they associate with β2-microglobulin and undergo glycosylation, promoting interaction with ER chaperones such as calnexin, ERp57, and calreticulin (36–38). During biosynthesis, CD1 molecules bind endogenous lipids; some of these lipids stabilise the molecule, while others may be antigenic (39, 40). Following trafficking through the Golgi apparatus, CD1 molecules are transported to the plasma membrane.
Once at the cell surface, most CD1 molecules (excluding CD1a) are internalised via a clathrin-dependent pathway mediated by adaptor protein complex 2 (AP2), which recognises a tyrosine-based motif within their cytoplasmic tails (41–44). In contrast, CD1a lacks a tyrosine-based motif and is internalised independently of clathrin and AP2, through a Rab22a- and ADP-ribosylation factor 6 (ARF6)-dependent mechanism (45). After internalisation, CD1a and CD1c recycle predominantly through the early endocytic system back to the plasma membrane. CD1b and CD1d also recycle but, owing to differences in their sorting motifs, can additionally engage adaptor protein complex 3 (AP3) within sorting endosomes, facilitating trafficking through late endosomal and lysosomal compartments (46–48). CD1c can access both early and late endocytic pathways, giving it the most widespread distribution among CD1 isoforms within the endosomal system (41).
Endocytic compartments are enriched with exogenous lipids delivered via macropinocytosis (49), mannose receptors (50), langerin (51), and the low-density lipoprotein receptor (52). Within these compartments, internalised CD1 molecules encounter a variety of endogenous and exogenous lipid antigens. Lipid exchange is facilitated by CD1e and saposins, small non-enzymatic proteins found in lysosomes (28, 53–55). Thus, internalisation and trafficking through the endosomal system are crucial for the acquisition and presentation of lipid antigens (30). However, emerging evidence suggests that CD1a, possibly due to the more open structure of its binding groove, may also facilitate lipid exchange directly at the cell surface under neutral pH conditions (56, 57).
T cell responses to Mtb infection are unusual in that multiple T cell subsets recognise lipid antigens, many of which are derived from the Mtb cell wall and are presented by CD1 molecules (58). It is thought that the distinctive composition of the Mtb cell wall, together with the intracellular lifestyle of the bacilli, converges with CD1 loading pathways to promote lipid antigen presentation (26, 58, 59). Accordingly, CD1-restricted lipid presentation is considered a key element in the initiation and modulation of immune responses against Mtb.
CD1-restricted T cells in tuberculosis immunity
Both αβ and γδ T cells have been shown to recognise lipid antigens presented by CD1 molecules. Despite the non-polymorphic nature of the CD1 system, the repertoire of CD1-restricted TCRs in humans is highly diverse (60–63). Increasing evidence highlights the importance of both αβ and γδ T cell subsets in host immune responses to Mtb infection (64–67).
Although αβ T cells are far more frequent in the blood, γδ T cells have gained significant research interest, particularly in the context of infection (68). γδ T cells normally account for approximately 4% of circulating T cells (69), but during infections such as TB, they can expand dramatically, representing up to 50% of the peripheral T cell pool (70–74). In fact, γδ T cells constitute the highest frequency of Mtb-reactive T cells in human peripheral blood (75). Hoft et al. (1998) demonstrated that γδ T cells were the most dramatically expanded population following stimulation of PBMCs from Bacille Calmette-Guérin (BCG)-vaccinated individuals with mycobacterial antigens. These γδ T cells also exhibited helper functions, supporting mycobacteria-specific CD4+ and CD8+ T cell responses (76). Moreover, γδ T cells have been shown to promote dendritic cell maturation, further linking them to the orchestration of both adaptive and innate immunity (77).
Human Vδ1 T cells have been reported to recognise all CD1 isoforms (64, 65, 78–85) whereas Vδ2 T cells predominantly recognise butyrophilins due to their TCRs usually containing the canonical Vγ9 chain (86–89). While Vδ2 T cells dominate the γδ T cell compartment in the blood of healthy individuals (90), TCR sequencing studies reveal that during active TB, the proportion of Vδ1 T cells increases markedly, resulting in codominance of Vδ1 and Vδ2 populations (16). In the lungs of TB patients, the γδ T cell repertoire is often highly skewed, dominated by locally expanded Vδ1 T cell clones (16). Given their abundance, elucidating the functional roles of Vδ1 T cells could significantly enhance our understanding of protective immunity to TB. Furthermore, due to their potent cytotoxicity and ability to exhibit immunological memory, Vδ1 T cells represent an attractive target for next-generation TB vaccine strategies (16, 65, 91–95).
Pioneering studies by Porcelli et al. (1998) provided direct evidence of CD1-restricted T cell responses. From healthy donor samples, they generated two T cell lines, BK6 (expressing an αβTCR) and IDP2 (expressing a γδTCR), both of which lacked CD4 and CD8 expression. Both lines could lyse the MOLT-4 T cell line in a CD1-dependent but MHC-independent manner (64). Lysis by BK6 was blocked by anti-CD1a antibodies, while lysis by IDP2 was blocked by anti-CD1c antibodies, demonstrating restriction by CD1a and CD1c, respectively. Blocking experiments confirmed that responses were TCR-mediated through the TCR-CD3 complex. Moreover, both T cell lines lysed mouse hybridoma and rhabdomyosarcoma cell lines transduced to express CD1a or CD1c, respectively, further confirming CD1 restriction (64).
Subsequent work by Rosat et al. (1999) described the generation of two CD8+ αβTCR-expressing T cell lines, CD8–1 and CD8-2, by stimulating PBMCs with Mtb lysates. CD8–1 specifically lysed CD1c-transfected target cells pulsed with Mtb lysates, while CD8–2 specifically lysed CD1a-transfected targets, with responses inhibited by blocking antibodies against CD1c and CD1a, respectively (96). Lysis was dependent on Mtb-derived lipid antigens, as no lysis occurred when target cells were pulsed with non-mycobacterial lysates or left untreated. Additionally, CD8–1 and CD8–2 secreted IFN-γ and TNF-α in response to Mtb antigen, but not Th2 or regulatory cytokines such as IL-4 or IL-10 (96). However, it is important to note that these responses were measured against Mtb lysate-pulsed APCs rather than live Mtb-infected cells, which may present distinct antigens.
Sieling et al. (2000) generated three CD4+ αβTCR-expressing T cell lines (LCD4.1, LCD4.2, and LCD4.3) from the skin lesions of leprosy patients (97). These T cells released IFN-γ in response to Mycobacterium leprae sonicate-pulsed dendritic cells (DCs), but not untreated DCs. Blocking experiments demonstrated that LCD4.1 responses were CD1c-restricted, while LCD4.2 and LCD4.3 were CD1b-restricted. Antigen specificity studies revealed that LCD4.2 recognised phosphatidylinositol mannoside and LCD4.3 recognised mycolic acid. Importantly, anti-CD4 blocking antibodies inhibited responses of MHC class II-restricted control T cells, but not LCD4.1 or LCD4.3, indicating that CD4 co-receptor engagement is not essential for CD1-restricted T cell activation (97).
By the early 2000s, strong evidence supported a role for CD1-restricted T cells in the immune response to Mtb. However, it remained unclear whether these cells expanded following Mtb exposure or differed in frequency between healthy and TB-infected individuals. Using PBMCs from PPD-positive and PPD-negative individuals, Ulrichs et al. (2003) demonstrated that T cells from PPD-positive individuals exhibited greater proliferation and IFN-γ secretion in response to Mtb lipid extracts, and these responses were largely CD1-dependent (98). CD3+ cell depletion abrogated IFN-γ production, confirming T cell involvement. Notably, CD1-restricted T cell responses were reduced or absent in active TB patients, suggesting that effective CD1-mediated immunity may be important for controlling Mtb infection, or that CD1-restricted T cells might migrate into infected lung tissue during active disease. Immunomagnetic separation further revealed that these responses were stronger in CD4+ compared to CD8+ T cells (98).
Kawashima et al. (2003) extended these findings by demonstrating that following BCG vaccination, CD8+ but not CD4+ T cells mounted CD1-restricted IFN-γ responses against BCG-infected dendritic cells. Responses by CD4+ T cells were dependent on MHC class II and unaffected by anti-CD1 blockade (99). Given that CD4+ T cell responses are essential for effective TB immunity (100–107), optimising TB vaccines to elicit robust CD4+ CD1-restricted memory T cell responses may be critical for achieving durable protection.
One of the major obstacles to studying CD1-restricted T cells is that mice lack group 1 CD1 molecules. To address this, Felio et al. (2009) developed a transgenic mouse model expressing human CD1a, CD1b, and CD1c. In response to Mtb infection, these mice generated CD1-restricted T cell responses characterised by cytotoxicity, IFN-γ production, and memory formation (108).
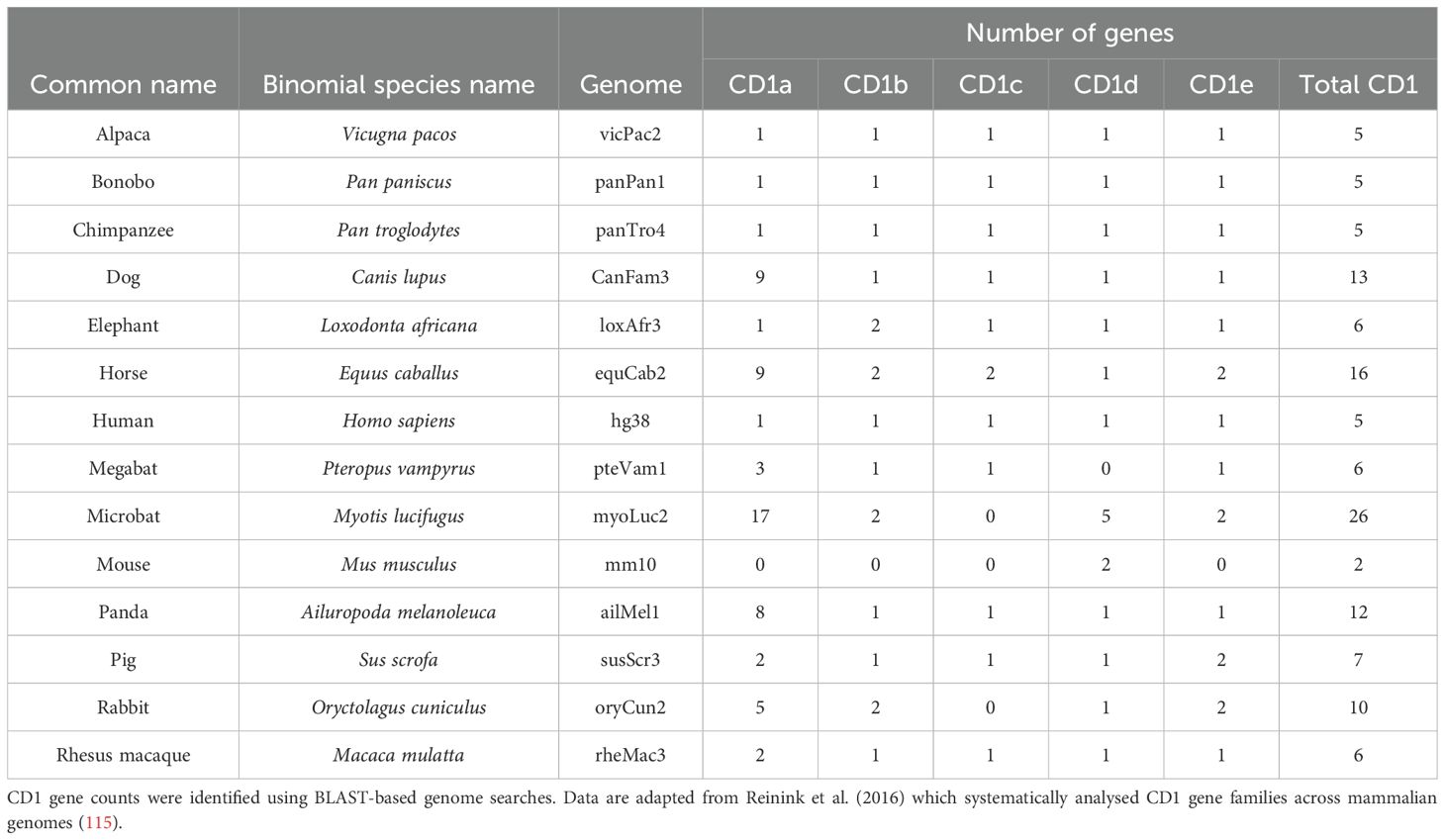
Although most mammals possess group 1 CD1 genes, muroid rodents (mice and rats) are an exception (109–112). Thus, guinea pigs, which express CD1b and CD1c, have also been used as an alternative model. Hiromatsu et al. (2002) showed that guinea pigs immunised with Mtb lipids generated CD1-restricted T cell responses that were cytotoxic and exhibited immunological memory (113). In subsequent studies it was shown that immunised guinea pigs had reduced lung pathology as well as reduced bacterial burden in the lungs and spleen following Mtb infection (114). Together, these findings highlight the importance of CD1-restricted T cells in immunity to Mtb and underscore their potential as targets for future TB vaccine development. Additional details on CD1 genes across various mammalian species are provided in Table 1.
CD1a-restricted responses to infection
CD1a is highly expressed by Langerhans cells (LCs) and plays an important role in generating T cell responses in the skin and at other mucosal sites (51, 116). Unlike other CD1 isoforms, CD1a may be capable of lipid exchange at the plasma membrane under neutral pH conditions, possibly due to the more open structure of its binding groove. CD1a molecules are also stabilised by exogenous lipids present in serum (56, 57).
LCs are a specialised subset of dendritic cells critical for initiating and regulating immune responses in the skin. Hunger et al. (2004) compared the expression of dendritic cell markers on LCs and conventional DCs. LCs exhibited higher expression of langerin (CD207), CD58, and CD1a, whereas DCs expressed higher levels of CD86, CD11c, CD1b, and HLA-DR; expression of CD14, CD80, CD83, and CD1c was similar between the two populations (51). Using CD1a+ LC-like DCs derived from leprosy patients, two CD1a-restricted αβTCR-expressing T cell clones, B2.1 and B2.11, were generated. Both clones were double-negative (DN) for CD4 and CD8, and they proliferated in response to CD1a+ LC-like DCs pulsed with Mycobacterium leprae extracts. Their responses were specifically inhibited by anti-CD1a, but not anti-CD1b or anti-CD1c, blocking antibodies, confirming CD1a restriction (51).
Interestingly, B2.1 and B2.11 also responded to extracts from M. tuberculosis, Mycobacterium smegmatis, and Mycobacterium phlei, but not to extracts from Mycobacterium avium, Nocardia, Aspergillus, or Rhodococcus species, suggesting recognition of a specific exogenous lipid antigen present in a subset of bacterial species. These clones expanded and secreted IFN-γ when co-cultured with LC-like DCs but showed only limited responses to monocyte-derived DCs (MoDCs), highlighting the superior ability of LCs to stimulate CD1a-restricted T cell responses (51). Langerin is involved in pathogen sensing (117) and in the formation of Birbeck granules (118). Given the differences in langerin expression between LCs and DCs, Hunger et al. investigated its role in CD1a-restricted responses. Pre-treatment of LC-like DCs with anti-langerin antibodies before, but not after, pulsing with M. leprae extracts inhibited T cell proliferation, suggesting that langerin is involved in the uptake, processing, or presentation of lipid antigens (51).
In parallel, Moody et al. (2004) used a CD1a-restricted αβTCR transfected J.RT3-T3.5 T cell reporter line to screen Mtb lipid fractions for antigens (119). High-performance liquid chromatography and mass spectrometry identified a series of related stimulatory lipids. Further structural analysis using nuclear magnetic resonance and mass spectrometry revealed the antigen as a lipopeptide, named didehydroxymycobactin, likely an intermediate in the mycobactin biosynthetic pathway of the Mtb cell wall. Importantly, Mtb-infected MoDCs, but not uninfected cells, could present this antigen to activate the CD1a-restricted reporter line, confirming that didehydroxymycobactin is naturally processed and presented during infection (119).
CD1b-restricted responses to infection
Among the group 1 CD1 molecules, CD1b is unique in its ability to present lipid antigens with very long acyl chains, such as mycolic acid (120). CD1b-restricted T cells are the best characterised of all group 1 CD1-restricted populations, and extensive studies have established their role in responses to Mtb infection.
The first evidence of CD1 antigen presentation came from Porcelli et al. (1992), who generated a CD1b-restricted T cell line from αβTCR-expressing double-negative (DN) T cells cultured with Mtb extract-pulsed MoDCs. These T cells lysed Mtb-infected, CD1b-transfected C1R cells, but not cells transfected with CD1a, CD1c, or empty vectors, demonstrating CD1b restriction (121).
Building on this, Beckman et al. (1994) identified mycolic acid as a lipid antigen presented by CD1b using organic phase separation and T cell proliferation assays. A CD1b-restricted T cell clone, DN1, specifically recognised mycolic acid derivatives, including 6,6’-trehalosedimycolate, but not irrelevant lipids, suggesting TCR-mediated recognition (21). Further studies expanded the catalogue of CD1b-presented antigens. Sieling et al. (1995) identified lipoarabinomannan as a mycobacterial lipid recognised in a CD1b-dependent manner by DN αβ T cells derived from leprosy patients and healthy donors, with these T cells capable of lysing antigen-pulsed monocytes and secreting IFN-γ (122).
Similarly, Stenger et al. (1997) showed that CD1b-restricted T cells from TB patients and healthy donors could lyse Mtb-infected macrophages in a CD1-dependent manner. Distinct cytotoxic mechanisms were observed: DN T cells relied on Fas-FasL interactions, while CD8+ T cells used granule-mediated killing. Importantly, CD8+ T cells, but not DN T cells, significantly reduced intracellular Mtb growth, likely via granulysin secretion (123, 124).
The identification of specific mycobacterial lipid antigens continued with Moody et al. (1997), who demonstrated that the LDN5 T cell clone recognised GMM presented by CD1b (22). Gilleron et al. (2004) later characterised Ac2SGL, a sulfoglycolipid, as a potent CD1b-restricted antigen stimulating IFN-γ and granulysin secretion by CD8+ T cells, leading to reduced Mtb growth. Responses to Ac2SGL required endosomal processing and were absent in PBMCs from PPD-negative individuals, suggesting selective expansion with prior Mtb exposure (125). Layre et al. (2009) identified glycerol monomycolate (GroMM) as another CD1b-presented antigen using the Z5B71 T cell clone. IFN-γ responses to GroMM were observed in BCG-vaccinated and latent TB individuals, but absent in active TB, suggesting defective memory responses during disease (126).
Montamat-Sicotte et al. (2011) further demonstrated that mycolic acid-specific CD1b-restricted T cells were enriched in TB patients, including at the site of infection (bronchoalveolar lavage fluid), and persisted long after treatment, indicating durable memory responses. Interestingly, BCG vaccination alone did not generate strong mycolic acid-specific memory T cells, possibly due to differences in mycolic acid structure between Mtb and BCG strains (127–129).
The development of CD1b tetramers revolutionised the study of CD1b-restricted T cells. Kasmar et al. (2011) showed that GMM-loaded CD1b tetramers specifically stained the LDN5 T cell clone and rare T cells in TB patient PBMCs, which were predominantly CD4+ (130). Rhijn et al. (2013) used tetramers to isolate and characterise CD1b-GMM specific T cell clones. High-affinity clones, called germline-encoded mycolyl lipid-reactive (GEM) T cells, all shared a TRAV1-2–TRAJ9 α-chain signature and expressed predominantly CD4. TCR sequencing confirmed that both α- and β-chains contributed to antigen specificity. GEM T cells expanded in TB patients, supporting their role in immune responses to Mtb (131). In contrast, LDN5-like T cells, expressing TRAV17 and TRBV4-1, represented a second group of GMM-specific T cells with more diverse TCR usage and coreceptor expression (132).
Functional evidence for CD1b-mediated protection came from Busch et al. (2016), who showed that lipoarabinomannan-specific CD1b-restricted T cells from latent TB individuals inhibited Mtb growth in MoDCs, and that these T cells produced granulysin, a molecule essential for direct killing of Mtb (124, 133).
Most recently, Sakai et al. (2024) identified trehalose monomycolate (TMM) as a novel CD1b-presented antigen. Using CD1b tetramers and single-cell RNA and TCR sequencing, they showed that TMM-specific T cells upregulate cytotoxic molecules such as granzyme B, perforin, and granulysin. These T cells expanded in TB patients and recognised TMM from multiple mycobacterial species but required the trehalose headgroup for TCR recognition (134). Cryo-electron microscopy revealed the ternary structure of the CD1b-TMM-TCR complex, providing detailed molecular insights into lipid antigen recognition.
Finally, Zhao et al. (2015) generated a transgenic mouse model expressing human CD1a, CD1b, CD1c, and a DN1 TCR specific for mycolic acid. In this model, DN1 T cells reduced Mtb burden after adoptive transfer, highlighting the protective capacity of CD1b-restricted T cells during TB infection (135). Together, these findings establish CD1b-restricted T cells as key contributors to host defence against Mtb and highlight their potential as targets for next-generation TB vaccines.
CD1c-restricted responses to infection
Among the group 1 CD1 molecules, CD1c is the most widely expressed and exhibits the broadest distribution throughout the endocytic system (25, 27, 41, 60, 66, 136, 137). This extensive trafficking enables CD1c to survey a diverse range of lipid antigens. Moreover, unlike other CD1 isoforms, CD1c lipid loading is independent of compartment acidification, a process that Mtb actively inhibits to evade phagocytic destruction, giving CD1c a potential advantage in infection settings (41, 138).
Both αβ and γδ T cells can recognise lipid antigens presented by CD1c. Despite the non-polymorphic nature of the CD1 system, the repertoire of CD1c-restricted TCRs is highly diverse (60–67).
Moody et al. (2000) first demonstrated that lymphocytes from individuals with prior Mtb exposure and positive PPD skin tests showed significantly greater proliferation and activation in response to synthetic isoprenoid glycolipids, structurally similar to Mtb antigens, in a CD1c-dependent manner. These findings provided the first evidence of CD1c-mediated lipid-specific memory T cell responses in infectious disease (66).
Building on this, Matsunaga et al. (2004) investigated the CD1c-restricted T cell line CD8-1 [previously described by Rosat et al. (96)]. They demonstrated that CD8–1 cells proliferated in response to CD1c-transfected C1R cells pulsed with Mtb or BCG whole lipid extracts, as well as with mannosyl-β-1-phosphoisoprenoids, a family of Mtb lipid antigens presented by CD1c (66). Disruption of the pks12 gene in Mtb abrogated the synthesis of mannosyl-β-1-phosphoisoprenoids, and lipid extracts from pks12 knockout strains failed to activate CD8–1 T cells. Mass spectrometry confirmed the absence of mannosyl-β-1-phosphoisoprenoids in the mutant strains, establishing pks12 as essential for their biosynthesis (8). Among these antigens, mannosyl-β1-phosphomycoketide (MPM) is now recognised as a major target for CD1c-restricted T cell responses in Mtb-exposed individuals (20).
Further work by Ly et al. (2013) expanded the repertoire of known CD1c-presented Mtb lipids. Using fractionated lipid extracts and the DN6 CD1c-restricted T cell line as a reporter, they identified a novel antigen, C32 phosphomycoketide (PM), a fully saturated C32 alkylphosphate structurally related to MPM (20). DN6 T cells were strongly activated by PM-pulsed MoDCs, but not by extracts from pks12-deficient Mtb, confirming PM as a natural mycoketide antigen. Notably, DN6 responded to both PM and deglycosylated forms of MPM, suggesting that antigen processing by APCs, involving removal of β-linked mannose units, can influence CD1c-restricted T cell recognition. Plate-bound CD1c experiments further confirmed distinct modes of recognition by different T cell clones (20).
Currently, PM and MPM remain the only two natural CD1c-presented Mtb lipid antigens that have been clearly identified (20, 60, 66, 139, 140). Further antigen discovery will likely be important for optimising TB vaccines aimed at targeting CD1c-restricted T cell responses.
To address antigen stability, Reijneveld et al. (2021) synthesised an MPM analogue, MPM-3, designed to resist enzymatic hydrolysis during antigen processing. In vitro immunisation with MPM-3 expanded MPM-specific T cells that demonstrated dual reactivity towards both MPM and MPM-3. These findings suggest that MPM-3 could serve as a more stable vaccine component for inducing robust CD1c-restricted T cell responses (141).
CD1d-mediated responses to mycobacteria
CD1d presents lipid antigens to natural killer T (NKT) cells, including invariant NKT (iNKT) cells and some Vδ1 T cells (79, 142). NKT cells are a distinct population of αβTCR-expressing T cells that co-express natural killer (NK) markers such as CD94 and CD161 (143). In humans, iNKT cells, also referred to as type 1 NKT cells, are defined by expression of a semi-invariant Vα24-Jα18 TCR (144, 145), whereas type 2 NKT cells possess a more diverse TCR repertoire (146). The synthetic glycosphingolipid α-galactosylceramide (α-GalCer), originally isolated from a marine sponge, binds CD1d and strongly activates iNKT cells by engaging their TCR with high affinity (147–149). The development of CD1d-α-GalCer tetramers enabled detailed characterisation of iNKT cells in both mice and humans (150, 151). Importantly, α-GalCer does not activate type 2 NKT cells, providing a selective tool for studying iNKT biology. Much of our understanding of CD1d-restricted immunity stems from iNKT cell research, largely because both CD1d and iNKT cells are conserved across mice and humans (152), unlike group 1 CD1 molecules, which are absent in murine models.
While iNKT cells were first investigated in the context of cancer, where α-GalCer treatment reduced tumour metastases and improved survival in mouse models (153), they have also been implicated in protection against Mtb infection. Chackerian et al. (2002) showed that α-GalCer administration prolonged survival and reduced lung bacterial burden in Mtb-infected CD1d-sufficient, but not CD1d-deficient mice, demonstrating a CD1d-dependent protective effect (154).
Subsequent studies identified microbial lipid antigens presented by CD1d. Fisher et al. (2004) demonstrated that phosphatidylinositol mannoside (PIM), a lipid isolated from BCG, could stimulate murine iNKT cells via CD1d presentation. Using CD1d-transfected B cell lymphoma cells pulsed with PIM, they observed IFN-γ secretion from Vα14-Jα281 transgenic mouse splenic T cells, a response abrogated by anti-CD1d blocking antibodies. These responses were absent with untransfected B cells, confirming CD1d restriction. Moreover, CD1d-PIM tetramers could stain murine iNKT cells similarly to CD1d-α-GalCer tetramers (155).
Investigations in humans revealed that iNKT cell clones stained by both CD1d-PIM and CD1d-α-GalCer tetramers also secreted IFN-γ and lysed CD1d-transfected HeLa cells pulsed with PIM. No lysis was observed when untransfected HeLa cells were used, confirming CD1d-restricted recognition. These findings identify PIM as a mycobacterial lipid antigen capable of activating human iNKT cells (155). Beyond recognition of foreign lipids, iNKT cells can also be activated by stress-induced self-lipid antigens. Brennan et al. (2011) showed that TLR stimulation of dendritic cells triggers lipid remodelling, promoting presentation of endogenous agonists on CD1d and enhancing iNKT activation in the absence of microbial lipid antigens. This ‘self-lipid switching’ provides a key mechanism by which innate immune cues can modulate CD1d-restricted T cell responses during infection (24). Functional studies further demonstrated a role for iNKT cells in controlling Mtb infection. Sada-Ovalle et al. (2008) showed that murine iNKT cells upregulated the activation marker CD69 upon contact with Mtb-infected macrophages, but not uninfected controls. Splenocytes from wild-type, but not iNKT-deficient, mice were able to reduce Mtb growth in infected macrophages. Furthermore, splenocytes failed to control Mtb growth when infected macrophages lacked CD1d, demonstrating the necessity of CD1d-mediated presentation. Pure iNKT cell lines were sufficient to inhibit Mtb growth when cultured with infected macrophages, and adoptive transfer of iNKT cells into irradiated, Mtb-infected mice significantly reduced bacterial burden in both lungs and spleen (156).
Collectively, these findings suggest that CD1d-restricted iNKT cells can contribute to anti-Mtb immunity. However, conflicting results exist. One study found no significant difference in survival between wild-type and CD1d-deficient mice infected with Mtb, suggesting that CD1d-restricted responses may not be essential for protection (157). Thus, while evidence supports a role for iNKT cells in immunity to Mtb, their contribution may vary depending on the infection model and experimental conditions.
Autoreactivity is an intrinsic feature of CD1 biology
An unusual feature of CD1-restricted T cells is their frequent autoreactivity. Although thymic selection minimises self-reactivity in conventional T cells, CD1-autoreactive T cells are nevertheless abundant in healthy individuals (26).
De Jong et al. (2010) showed that T cells from all 14 healthy donors tested exhibited reactivity towards CD1a-expressing K562 cells, whereas responses to CD1b, CD1c, or CD1d were less common. Blocking with anti-CD1a antibodies confirmed CD1a restriction. CD1a-autoreactive T cells, comprising approximately 2% of circulating T cells, were predominantly CD4+, produced IFN-γ, IL-22, and sometimes IL-13, but often lacked IL-2 production. Many expressed cutaneous lymphocyte antigen (CLA), suggesting skin homing. T cells isolated from skin biopsies similarly showed CD1a reactivity, with stronger responses when stimulated by Langerhans cells (158).
De Lalla et al. (2011) independently confirmed that CD1 autoreactivity is relatively frequent. Single-cell cloning revealed that around 10% of both CD4+ and DN αβT cells were self-reactive to CD1 molecules, predominantly CD1a and CD1c. TCR repertoire analysis showed high diversity among self-reactive clones, contrasting with the invariant TCRs of iNKT cells (159). CD1c-autoreactive T cells were functionally heterogeneous: CD4+ clones were more likely to secrete TNF-α, DN clones secreted GM-CSF, and some clones produced both Th1 and Th2 cytokines. Importantly, CD1a- and CD1c-autoreactive clones demonstrated cytotoxicity against target cells expressing their cognate CD1 isoforms without exogenous antigen, indicating intrinsic autoreactive killing potential (159). Beyond classical Th1 cytokines, CD1-autoreactive T cells can secrete a broad range of effector molecules, including GM-CSF, IL-13, IL-22, and IL-5 (158–160). For example, CD1c- and CD1b-autoreactive T cell clones have been shown to produce polyfunctional responses that include both Th1 and Th2 cytokines, and in some cases, GM-CSF and IL-22, which can enhance antigen presentation, promote monocyte recruitment, and contribute to mucosal barrier integrity (159, 160). IL-13 and IL-5, though traditionally associated with Th2 responses, may modulate inflammation or tissue repair in TB lesions. These findings suggest that CD1-restricted T cells may play diverse immunomodulatory roles during TB infection, beyond direct cytotoxicity or classical macrophage activation.
Despite their prevalence, CD1-restricted self-reactive T cells rarely cause pathology, suggesting regulatory mechanisms are in place. Nevertheless, associations with autoimmune diseases have been reported. CD1c+ antigen-presenting cells infiltrate lesions in Graves’ disease and Hashimoto’s thyroiditis, and T cells capable of lysing CD1c+ targets have been isolated from thyroid tissue (161). In systemic lupus erythematosus (SLE), DN T cells reactive to CD1c can produce IL-4 and IFN-γ, and may support IgG production by CD1c+ B cells (162). In rheumatoid arthritis, synovial fluid contains increased numbers of activated CD1c+ dendritic cells that stimulate CD4+ T cells, although CD1c restriction was not definitively proven (163). Autoreactivity has also been implicated in multiple sclerosis (MS). Shamshiev et al. (1999) found increased frequencies of T cells reactive to brain-derived glycolipids in MS patients. Two T cell clones recognised monosialo-ganglioside GM1 presented by CD1b, and responses were blocked by anti-CD1b antibodies, implicating CD1b in autoreactive responses against myelin components (164).
Recent mechanistic studies have further clarified how CD1 autoreactivity may be regulated. De Jong et al. (2014) demonstrated that CD1a-autoreactive T cells, such as clone BC2, recognised CD1a loaded with endogenous lipids from the epidermis, including squalene from sebaceous glands. These findings suggest that spatial separation of self-lipids, for example, lipids located beyond T cell access in healthy skin, helps prevent inappropriate activation (165). Moreover, Betts et al. (2017) showed that the contact dermatitis agent 2,4-dinitrochlorobenzene (DNCB) activates CD1a-autoreactive T cells, suggesting environmental exposures can trigger pathological autoreactive responses. In functional studies, DNCB-treated CD1a+ APCs stimulated a polyfunctional cytokine response from autoreactive T cells (166).
Guo et al. (2018) engineered K562 cells to express high levels of CD1c and used them to stimulate peripheral blood T cells without exogenous antigen (167). Activated T cells upregulated CD154 and showed enrichment of TRBV4+ TCRs, specifically TRBV4-1. Responses were blocked by anti-CD1c antibodies. When TRBV4-1+ TCRs were transduced onto Jurkat cells, they responded to CD1c-expressing targets, confirming CD1c autoreactivity (167).
Further mechanistic insights have come from studies of lipid antigen structure. Cotton et al. (2021) found that sphingomyelins with long unsaturated acyl chains (e.g., 42:2 sphingomyelin) inhibit CD1a-TCR interactions by protruding from the antigen-binding groove and sterically blocking TCR engagement, whereas shorter chain sphingomyelins are permissive. Thus, specific endogenous lipids can negatively regulate CD1a autoreactivity (23).
Structural studies also support a model of direct CD1 recognition without lipid co-recognition. Wun et al. (2018) solved the structure of an autoreactive CD1c-restricted TCR bound to CD1c presenting a fully sequestered endogenous lipid. The TCR contacted CD1c itself rather than the presented lipid, consistent with “CD1-as-antigen” recognition. These findings explain how high frequencies of autoreactive CD1c-restricted T cells can exist without constant activation, provided regulatory mechanisms are intact (136).
Several broader mechanisms are also thought to limit autoreactive responses:
● Inhibitory lipid loading: Endogenous lipids with bulky head groups, such as phosphatidylcholine or sphingomyelin, may block TCR access to CD1 molecules (23).
● Tissue-specific CD1 expression: Although CD1c is expressed on B-cells, group 1 CD1 molecules are expressed relatively sparsely in the peripheral blood, restricting opportunities for autoreactive encounters (136, 168).
● TCR internalisation: In CD1b-transgenic mice, autoreactive CD1b-restricted T cells showed reduced surface TCR expression compared to wild-type mice, suggesting that downregulation of TCR levels may suppress autoreactivity in vivo (169).
Together, these mechanisms contribute to immune tolerance, preventing frequent autoreactivity from manifesting as autoimmune disease. Importantly, autoreactivity does not necessarily equate to autoimmunity, and controlled self-reactivity may even have physiological roles yet to be fully defined.
CD1-autoreactive T cells: are they really so evil?
Despite their association with autoimmune diseases, CD1-autoreactive T cells have been conserved throughout human evolution, suggesting they may play beneficial roles in immunity.
CD1c is expressed on several haematological malignancies, including B cell acute lymphoblastic leukaemia (B-ALL) and acute myeloid leukaemia (AML) in both adults and children (170). Lepore et al. (2014) isolated CD1c-autoreactive T cell clones from healthy donors and found that these clones secreted GM-CSF and IFN-γ in response to CD1c-transfected THP1 and C1R cells in a CD1c-dependent manner. Lipid extraction and fractionation of THP1 cells identified a stimulatory lipid, later determined by mass spectrometry as methyl-lysophosphatidic acid (mLPA). Synthetic mLPA analogues loaded onto recombinant CD1c similarly activated CD1c-autoreactive T cells, confirming mLPA as an endogenous immunogenic ligand.
Functionally, mLPA-specific T cell clones secreted IFN-γ in response to co-culture with CD1c+ AML cells, but not with healthy monocytes. Despite lower CD1c expression on some AML cells compared to monocytes, stronger T cell activation was observed against the leukaemic cells, suggesting increased mLPA presentation. Similarly, B-ALL cells induced greater IFN-γ secretion compared to normal B cells despite similar CD1c expression levels. Direct quantification showed mLPA accumulation was significantly higher in leukaemic cells than in healthy cells.
mLPA-specific T cells preferentially killed B-ALL and AML cells while sparing most normal B cells and monocytes. Killing was CD1c-dependent, as blocking antibodies abrogated cytotoxicity. In vivo, mLPA-specific T cells prolonged survival in an immunodeficient mouse model grafted with CD1c+ MOLT-4 leukaemia cells. Furthermore, healthy donor T cells transduced with mLPA-specific TCRs acquired the ability to recognise and respond to CD1c+ target cells, demonstrating the therapeutic potential of CD1c-autoreactive TCRs (170). Beyond cancer, CD1-autoreactive T cells have also been shown to contribute to antimicrobial immunity. Vincent et al. (2005) generated 15 group 1 CD1-restricted T cell clones by stimulating CD4-depleted T cells with CD1-expressing MoDCs and lipid extracts from Mtb, E. coli, or Yersinia enterocolitica. All clones were CD8+ αβTCR+ T cells that proliferated in response to CD1-expressing MoDCs without additional stimulation, demonstrating autoreactivity. These T cells were highly cytotoxic against CD1-transfected HeLa and C1R cells, as well as MoDCs. Cytokine profiling showed expression of IFN-γ, GM-CSF, IL-5, and IL-13, and functional responses were abrogated by CD1-blocking antibodies. Moreover, CD1a- and CD1b-restricted TCRs transduced into Jurkat cells conferred both self-reactivity and enhanced responses to microbial lipids, highlighting dual specificity for self- and foreign antigens (160).
Similarly, Roy et al. (2016) identified CD1c-restricted γδ T cells through CD1c-PM tetramer staining of human PBMCs. Sorted lines predominantly expressed the Vδ1 TCR chain, confirming that Vδ1 cells are the main γδ T cell population recognising CD1c (65, 78). Upon transduction of Vδ1+ TCRs into Jurkat cells, spontaneous activation was observed, indicating low-level autoreactivity towards endogenous CD1c ligands. These TCR-transduced Jurkat cells responded more strongly when stimulated with PM, demonstrating dual reactivity to both endogenous and microbial lipid antigens. Binding studies confirmed that different lipids modulated TCR engagement: some TCRs bound more strongly to CD1c presenting self-lipids, while others preferred microbial lipids (78).
Following on from previous work (169), Bagchi et al. (2016) demonstrated that a CD1b-autoreactive T cell line, which recognises phospholipids, secreted IL-2 in response to plate-bound CD1b protein loaded with lipids extracted from normal cells, indicating autoreactivity. However, IL-2 secretion was significantly higher when CD1b was loaded with lipids extracted from the T lymphoblast cell line MOLT-4, suggesting that cancer cell-derived lipids are more immunogenic. Furthermore, these T cells were able to lyse CD1b-transfected, but not wild-type, murine RMA-S T cell lymphoma cells, confirming CD1b-restricted recognition and cytotoxicity (171).
In follow-up experiments, mice were inoculated with either wild-type or CD1b-transfected RMA-S tumour cells, alongside CD1b-autoreactive T cells. On day 14, mice were sacrificed, and tumour size was measured. Tumour growth was significantly reduced in mice that received CD1b-transfected RMA-S cells and CD1b-autoreactive T cells, suggesting that these T cells can mediate anti-tumour immunity in a CD1b-dependent manner. In contrast, no reduction in tumour size was observed in mice inoculated with wild-type RMA-S cells, confirming that the protective effect was specifically mediated by CD1b recognition (171). Collectively, these findings suggest that CD1-autoreactive T cells, rather than being solely pathogenic, may have beneficial roles in immune surveillance. Based on this evidence, CD1-autoreactive T cells could contribute to host defence against both tumours and infections and may play a previously underappreciated role in the immune response to infectious diseases such as tuberculosis (Figure 1).
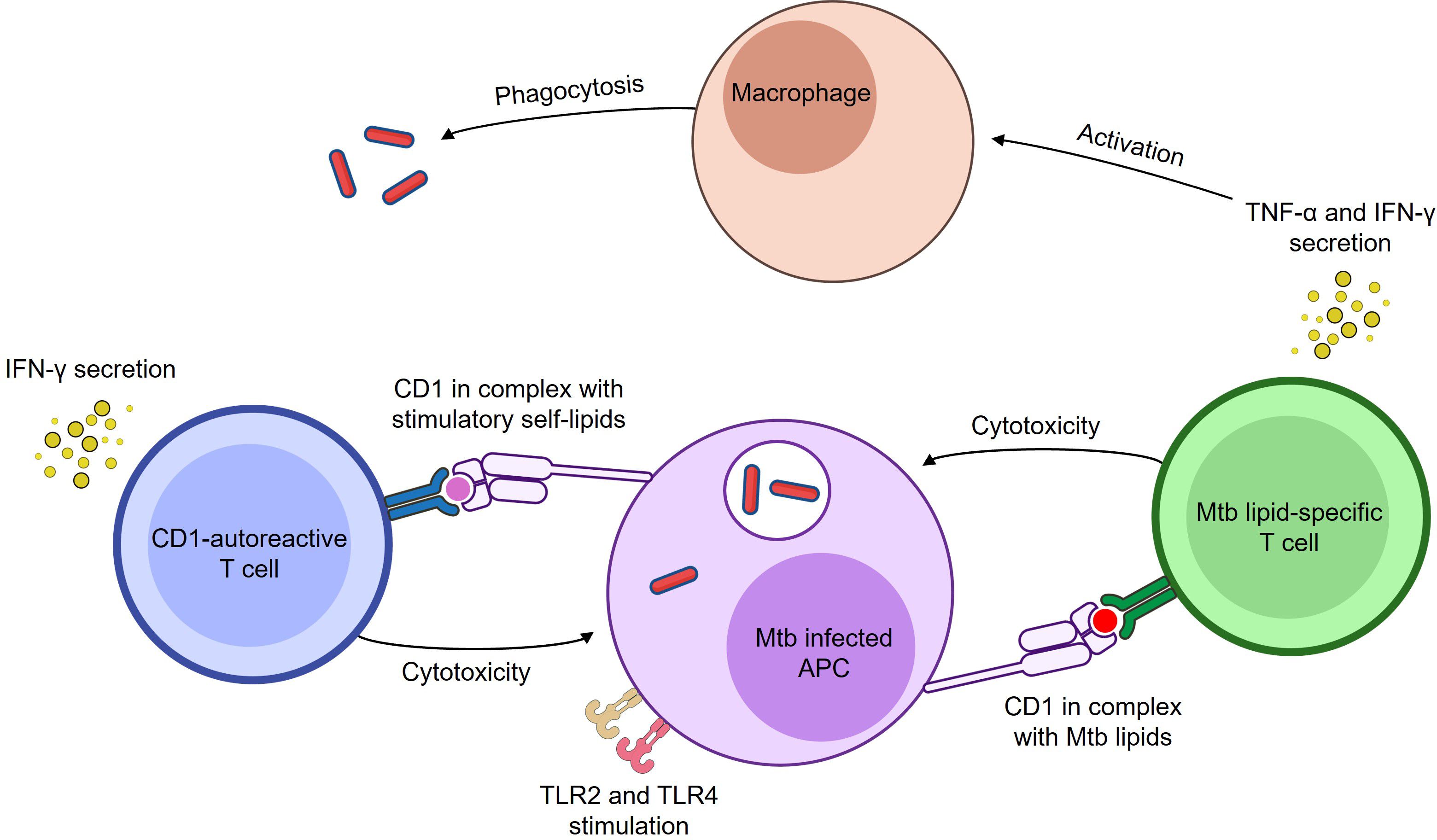
Figure 1. Both Mtb lipid-specific and CD1-autoreactive T cells respond to Mtb infection. Both Mtb lipid-specific and CD1-autoreactive T cells respond to Mtb infection. Mtb lipid-specific T cells become cytotoxic and secrete IFN-γ and TNF-α (96, 113, 123, 134) in response to Mtb lipid antigens presented by CD1 molecules on infected APCs. CD1-autoreactive T cells can also become cytotoxic and secrete IFN-γ in response to either Mtb lipid or stress-induced self-lipid antigens presented by CD1 molecules on infected APCs (78, 160). While Mtb can gain access to the cytosol, it predominantly resides in the phagosome, where it is sensed by innate receptors and processed for antigen presentation. TLR signalling enhances these responses by upregulating CD1 expression, costimulatory molecules, and presentation of stimulatory self-lipids. Specifically, TLR2 and TLR4, which recognise Mtb lipoproteins and cell wall components, are shown in the figure. These receptors bridge innate sensing of Mtb with adaptive CD1-restricted T cell responses. Secreted IFN-γ and TNF-α can stimulate macrophages to enhance antimicrobial functions (172, 173). Image created using BioArt.
TLR signalling modulates CD1-autoreactive T cell responses during infection
TLR stimulation can influence the functional responses of CD1-autoreactive T cells, linking innate immune sensing to adaptive lipid-specific immunity. De Libero et al. (2005) investigated two CD1a-restricted T cell clones specific for sulfatide and two CD1b-restricted clones specific for monosialo-ganglioside GM1 (172). When co-cultured with immature DCs infected with E. coli, B. subtilis, S. aureus, or BCG, all four clones secreted IFN-γ. Similarly, stimulation of DCs or CD1-transfected THP1 cells with the TLR4 agonist LPS or the TLR2 agonist Pam3Cys significantly enhanced IFN-γ production by these CD1-autoreactive clones. Responses were CD1-dependent, as blocking antibodies abrogated T cell activation, and were not observed in MHC class II-restricted or γδ T cell clones under identical conditions (172).
Mechanistically, LPS and Pam3Cys stimulation modestly increased CD1 and co-stimulatory molecule expression (B7.1, CD40) on DCs and THP1 cells. More strikingly, infection or TLR stimulation induced increased synthesis of the self-lipid antigens sulfatide and monosialo-ganglioside GM1, suggesting that infection-driven changes in lipid metabolism enhance CD1-restricted T cell activation by elevating the abundance of stimulatory self-lipids (172). This mechanism is mirrored in the CD1d–iNKT cell axis, where TLR activation of dendritic cells drives lipidome remodelling and presentation of stimulatory self-lipids, enabling iNKT activation in the absence of microbial antigens (24). Earlier work from the same group showed that microbial infection can activate iNKT cells via CD1d-mediated presentation of endogenous lipids, further supporting TLR-induced self-lipid switching as a general mechanism of CD1-restricted immunity (174). Zeissig et al. (2012) similarly demonstrated that hepatitis B virus infection alters hepatocyte lipid composition to generate CD1d-presented lysophospholipids, triggering NKT cell activation (175). Together, these findings highlight a broader mechanism by which pathogen sensing promotes autoreactive T cell responses through enhanced self-lipid presentation.
Li et al. (2011) further explored this phenomenon using a transgenic mouse model expressing group 1 CD1 molecules and a CD1b-autoreactive TCR (169). Treatment of bone marrow-derived DCs with Pam3Cys, LPS, or Listeria monocytogenes infection resulted in heightened secretion of IFN-γ and IL-17A from CD1b-autoreactive T cells compared to untreated controls. Following in vivo challenge with Listeria monocytogenes, CD1b-autoreactive T cells upregulated the activation marker CD69 and contributed to a reduced bacterial burden in the liver and spleen, compared to non-transferred controls. These findings suggest that TLR2 and TLR4 signalling can amplify CD1b-autoreactive T cell responses during infection, promoting pathogen clearance (169). This highlights a model in which inflammation-driven upregulation of CD1 expression and stress lipid synthesis can activate CD1-autoreactive T cells, even in the absence of strong pathogen-derived lipid presentation. For a more extensive discussion on how TLR pathways intersect with CD1-restricted T cell immunity, we refer readers to the comprehensive review by Moody et al. (2006) (176).
Mtb infection influences CD1 expression
During the late 1990s and early 2000s, a series of studies investigated how mycobacterial infection affects CD1 molecule expression by APCs.
Stenger et al. (1998) infected human adherent mononuclear cells (AMNCs) treated with GM-CSF and IL-4 with live Mtb and measured group 1 CD1 expression by flow cytometry. No significant changes were observed at 4 hours post-infection; however, by 24 hours, reduced staining of all group 1 CD1 isoforms was evident, and by 48 hours, expression was undetectable (177). Quantitative RT-PCR confirmed a substantial decrease in group 1 CD1 mRNA levels in infected cells compared to controls. Notably, infection with heat-killed Mtb did not reduce CD1 expression, indicating that live bacilli are necessary for this effect. Using a transwell system, the authors demonstrated that soluble factors alone were insufficient to mediate CD1 downregulation, suggesting that direct interactions between live Mtb and host cells are required (177).
Giuliani et al. (2001) further explored the effects of mycobacteria on CD1 expression. They found that infection with live BCG inhibited the GM-CSF-induced upregulation of group 1 CD1 molecules, particularly CD1b. In contrast to Stenger et al., heat-killed BCG also suppressed CD1b expression, attributed to alternative mRNA splicing mechanisms. Interestingly, using a transwell system, they observed that soluble factors secreted by BCG-infected AMNCs could reduce CD1b expression in adjacent uninfected cells, suggesting a mechanism of bystander suppression not observed with Mtb (178).
Wen et al. (2013) later demonstrated that CD1c mRNA levels are reduced in PBMCs from TB patients compared to healthy controls (179). An inverse correlation between CD1c expression and miR-381-3p levels was identified, suggesting post-transcriptional regulation. Binding of miR-381-3p to the 3’ untranslated region of CD1c was confirmed using a luciferase reporter assay. Overexpression of miR-381-3p in DCs decreased CD1c expression, while inhibition of miR-381-3p restored it. Furthermore, BCG infection increased miR-381-3p levels and decreased CD1c expression, effects reversible with miR-381-3p inhibition. Importantly, blocking miR-381-3p enhanced CD1c-restricted T cell responses to BCG, suggesting that miR-381-3p inhibitors could be therapeutically useful to improve vaccine-induced CD1-mediated immunity (179).
Given that mycobacteria have coevolved with innate immune systems (180), it is plausible that downregulating group 1 CD1 molecule expression represents an immune evasion strategy, limiting recognition by CD1-restricted T cells. These findings collectively suggest that modulation of CD1 expression by mycobacteria could impair host immunity, and that targeting these pathways could lead to improved vaccine strategies capable of eliciting more robust CD1-restricted memory responses (177–179). Given Mtb’s ability to modulate CD1 expression, it is essential that future vaccine strategies account for these evasion mechanisms.
In the following section, we review the current landscape of TB vaccine development and explore how targeting CD1-restricted immunity could offer new opportunities for protection.
TB vaccine development
Developing a more effective vaccine remains one of the most promising strategies to control TB and limit the rise of antibiotic-resistant strains. The only currently available TB vaccine, BCG, contains an attenuated form of Mycobacterium bovis (181). Although BCG is widely administered and offers relatively high protection against childhood TB meningitis and miliary TB, it provides limited protection against pulmonary TB in adults and adolescents, the major drivers of Mtb transmission (18, 182–184). Furthermore, BCG is contraindicated in immunocompromised individuals, including those with untreated HIV infection, due to the risk of disseminated BCG infection (185).
Several new TB vaccine candidates have entered clinical trials in recent years. One strategy involves viral-vectored vaccines such as MVA85A, based on a modified vaccinia Ankara virus expressing Mtb antigen 85A. Despite encouraging preclinical data, MVA85A failed to provide protection in Phase IIb clinical trials in infants and adults, marking a major disappointment as the first new TB vaccine candidate to undergo an efficacy trial in over 80 years (186–188).
Subunit vaccines have also been explored. H56:IC31 is a fusion protein combining Ag85B, ESAT-6, and Rv2660c, the latter preferentially expressed during Mtb latency (189). In mice, a BCG prime followed by an H56:IC31 boost reduced lung bacterial burden after Mtb challenge (190). In cynomolgus macaques, H56:IC31 boosting after BCG vaccination delayed progression to active TB, extended survival, and reduced pathology (191). Early-phase human trials showed that H56:IC31 was well tolerated and induced robust IgG and antigen-specific CD4+ T cell responses in both Mtb-infected and uninfected individuals (192, 193). However, in a Phase IIb trial, although immunogenic, H56:IC31 unexpectedly showed higher TB incidence among vaccinees (5.8%) compared to placebo (3.4%) (194).
Another prominent candidate is M72/AS01E, a recombinant fusion protein vaccine combining the Mtb antigens PepA and PPE18. PepA is thought to function as a serine protease (195), while PPE18 may interact with TLR2, inducing immunosuppressive IL-10 responses (196–198), and promoting Mtb survival (199). However, PPE18 exhibits substantial structural variability across strains (200), raising concerns about antigenic consistency. The Phase IIb trial of M72/AS01E was hailed as a breakthrough by the WHO, demonstrating approximately 50% protection against progression to active TB three years post-vaccination (201). Nevertheless, injection-site reactogenicity led to delayed recruitment in some Phase II trials (202), and the moderate efficacy suggests further vaccine improvements are still needed.
One limitation of viral-vectored, subunit, and recombinant fusion vaccines is their narrow antigenic focus. None of MVA85A, H56:IC31, or M72/AS01E can generate CD1-restricted memory responses to Mtb lipids. However, future formulations could incorporate immunogenic lipid antigens to elicit CD1-restricted immunity. Morgun et al. (2023) developed a nanoparticle-based TB vaccine containing both mycolic acid and the protein antigen Ag85B. In mice, this formulation activated adoptively transferred DN1 T cells (specific for mycolic acid) and Ag85B-specific T cells in vivo. Additionally, human mycolic acid-specific T cells responded to the same nanoparticles in vitro, highlighting the potential of subunit vaccine platforms that combine lipid and protein antigens to elicit broad CD1- and MHC-restricted T cell responses (203).
Whole-cell vaccines offer broader antigen presentation, including lipid antigens (99). MTBVAC, a live attenuated Mtb strain with deletions in phoP and fadD26, genes essential for virulence lipid synthesis, is currently in Phase III trials (204–209). Another candidate, VPM1002, is a recombinant BCG expressing listeriolysin O from Listeria monocytogenes and lacking urease C. This enables phagosome acidification and cytosolic antigen release, enhancing immunogenicity (187, 210–216).
However, because both Mtb and BCG have been shown to downregulate CD1 expression and impair CD1-restricted T cell responses (177–179), MTBVAC and VPM1002 may still have limited capacity to induce optimal CD1-restricted memory. Identifying and reversing the mechanisms by which mycobacteria suppress CD1 expression could offer a route to improving future vaccines. It also remains unclear whether optimal CD1-restricted responses should be elicited by including defined mycobacterial lipids as vaccine immunogens, or by leveraging innate activation to drive self-lipid presentation and autoreactive T cell activation. Both approaches merit investigation.
Importantly, CD1 molecules are non-polymorphic, meaning immune responses to CD1-presented antigens are shared across genetically diverse human populations. Thus, targeting CD1-restricted responses may enable broader and more universal vaccine coverage (18). Furthermore, lipid antigens are less prone to mutational escape compared to peptides presented by classical MHC molecules, as alterations to essential lipid biosynthetic pathways often compromise bacterial viability (170).
Given the major advances in TB vaccine development in recent years, there is real hope that new immunisation tools capable of providing better global protection are within reach. Targeting CD1-restricted T cell responses offers a promising strategy to boost future vaccine efficacy against TB, the world’s leading infectious killer (1, 19).
Conclusions
CD1-restricted T cells offer a compelling yet underutilised opportunity to transform TB vaccine development. Their capacity to recognise lipid antigens via non-polymorphic CD1 molecules allows for genetically unrestricted, population-wide immune responses (17, 18). While pathogen-specific (20–22, 66, 97, 119, 122, 125, 126, 129–134, 155) and autoreactive (78, 160, 169, 172) CD1-restricted T cells can both contribute to antimicrobial defence, Mtb’s ability to downregulate CD1 expression presents a key challenge (177–179). Current vaccines fail to engage lipid-specific memory responses, revealing a critical gap. Future strategies that incorporate immunogenic lipid antigens, restore CD1 expression (179), and selectively expand protective CD1-restricted T cell subsets may deliver the next leap in TB vaccine efficacy. Integrating CD1-targeted immunity could move us closer to durable, universal protection against TB.
Author contributions
SM: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing – original draft, Writing – review & editing. MM: Conceptualization, Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. MM was supported by a UKRI MRC DTP studentship award (MR/W007045/1). SM was supported by a UKRI/MRC award (MR/S024220/1).
Acknowledgments
All figures were prepared using bioart.niaid.nih.gov.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Houben RMGJ and Dodd PJ. The global burden of latent tuberculosis infection: A re-estimation using mathematical modelling. PloS Med. (2016) 13:e1002152. doi: 10.1371/journal.pmed.1002152
2. Global Tuberculosis Report 2024. Available online at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2024. (Accessed April 29, 2025).
4. Jin Y, Wang J, Bachtiar M, Chong SS, and Lee CGL. Architecture of polymorphisms in the human genome reveals functionally important and positively selected variants in immune response and drug transporter genes. Hum Genomics. (2018) 12:43. doi: 10.1186/s40246-018-0175-1
5. Trowsdale J and Knight JC. Major histocompatibility complex genomics and human disease. Annu Rev Genomics Hum Genet. (2013) 14:301–23. doi: 10.1146/annurev-genom-091212-153455
6. D’Souza MP, Adams E, Altman JD, Birnbaum ME, Boggiano C, Casorati G, et al. Casting a wider net: Immunosurveillance by nonclassical MHC molecules. PloS Pathog. (2019) 15:e1007567. doi: 10.1371/journal.ppat.1007567
7. Fu LM and Fu-Liu CS. Is Mycobacterium tuberculosis a closer relative to Gram-positive or Gram-negative bacterial pathogens? Tuberc Edinb Scotl. (2002) 82:85–90. doi: 10.1054/tube.2002.0328
8. Matsunaga I, Bhatt A, Young DC, Cheng TY, Eyles SJ, Besra GS, et al. Mycobacterium tuberculosis pks12 Produces a Novel Polyketide Presented by CD1c to T Cells. J Exp Med. (2004) 200:1559–69. doi: 10.1084/jem.20041429
9. Brennan PJ. Structure, function, and biogenesis of the cell wall of Mycobacterium tuberculosis. Tuberc Edinb Scotl. (2003) 83:91–7. doi: 10.1016/S1472-9792(02)00089-6
10. Zuber B, Chami M, Houssin C, Dubochet J, Griffiths G, and Daffé M. Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J Bacteriol. (2008) 190:5672–80. doi: 10.1128/JB.01919-07
11. Jankute M, Cox JAG, Harrison J, and Besra GS. Assembly of the mycobacterial cell wall. Annu Rev Microbiol. (2015) 69:405–23. doi: 10.1146/annurev-micro-091014-104121
12. Forrellad MA, Klepp LI, Gioffré A, Sabio y García J, Morbidoni HR, de la Paz Santangelo M, et al. Virulence factors of the Mycobacterium tuberculosis complex. Virulence. (2013) 4:3–66. doi: 10.4161/viru.22329
13. Becker K and Sander P. Mycobacterium tuberculosis lipoproteins in virulence and immunity – fighting with a double-edged sword. FEBS Lett. (2016) 590:3800–19. doi: 10.1002/1873-3468.12273
14. Delogu G, Sali M, and Fadda G. The biology of mycobacterium tuberculosis infection. Mediterr J Hematol Infect Dis. (2013) 5:e2013070. doi: 10.4084/mjhid.2013.070
15. Hoffmann C, Leis A, Niederweis M, Plitzko JM, and Engelhardt H. Disclosure of the mycobacterial outer membrane: Cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc Natl Acad Sci. (2008) 105:3963–7. doi: 10.1073/pnas.0709530105
16. Ogongo P, Steyn AJC, Karim F, Dullabh KJ, Awala I, Madansein R, et al. Differential skewing of donor-unrestricted and γδ T cell repertoires in tuberculosis-infected human lungs. J Clin Invest. (2020) 130:214–30. doi: 10.1172/JCI130711
17. Rhijn IV and Moody DB. Donor unrestricted T cells: A shared human T cell response. J Immunol. (2015) 195:1927–32. doi: 10.4049/jimmunol.1500943
18. Joosten SA, Ottenhoff THM, Lewinsohn DM, Hoft DF, Moody DB, and Seshadri C. Harnessing donor unrestricted T-cells for new vaccines against tuberculosis. Vaccine. (2019) 37:3022–30. doi: 10.1016/j.vaccine.2019.04.050
19. Schaible UE, Linnemann L, Redinger N, Patin EC, and Dallenga T. Strategies to improve vaccine efficacy against tuberculosis by targeting innate immunity. Front Immunol. (2017) 8:1755. doi: 10.3389/fimmu.2017.01755
20. Ly D, Kasmar AG, Cheng TY, de Jong A, Huang S, Roy S, et al. CD1c tetramers detect ex vivo T cell responses to processed phosphomycoketide antigens. J Exp Med. (2013) 210:729–41. doi: 10.1084/jem.20120624
21. Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, and Brenner MB. Recognition of a lipid antigen by CD1-restricted αβ+ T cells. Nature. (1994) 372:691–4. doi: 10.1038/372691a0
22. Moody DB, Reinhold BB, Guy MR, Beckman EM, Frederique DE, Furlong ST, et al. Structural requirements for glycolipid antigen recognition by CD1b-restricted T cells. Science. (1997) 278:283–6. doi: 10.1126/science.278.5336.283
23. Cotton RN, Wegrecki M, Cheng TY, Chen YL, Veerapen N, Le Nours J, et al. CD1a selectively captures endogenous cellular lipids that broadly block T cell response. J Exp Med. (2021) 218:e20202699. doi: 10.1084/jem.20202699
24. Brennan PJ, Tatituri RVV, Brigl M, Kim EY, Tuli A, Sanderson JP, et al. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. (2011) 12:1202–11. doi: 10.1038/ni.2143
25. Siddiqui S, Visvabharathy L, and Wang CR. Role of group 1 CD1-restricted T cells in infectious disease. Front Immunol. (2015) 6:337. doi: 10.3389/fimmu.2015.00337
26. Brigl M and Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. (2004) 22:817–90. doi: 10.1146/annurev.immunol.22.012703.104608
27. Gourapura RJ, Khan MA, Gallo RM, Shaji D, Liu J, and Brutkiewicz RR. Forming a complex with MHC class I molecules interferes with mouse CD1d functional expression. PloS One. (2013) 8:e72867. doi: 10.1371/journal.pone.0072867
28. Facciotti F, Cavallari M, Angénieux C, Garcia-Alles LF, Signorino-Gelo F, Angman L, et al. Fine tuning by human CD1e of lipid-specific immune responses. Proc Natl Acad Sci. (2011) 108:14228–33. doi: 10.1073/pnas.1108809108
29. Koch M, Stronge VS, Shepherd D, Gadola SD, Mathew B, Ritter G, et al. The crystal structure of human CD1d with and without α-galactosylceramide. Nat Immunol. (2005) 6:819–26. doi: 10.1038/ni1225
30. Barral DC and Brenner MB. CD1 antigen presentation: how it works. Nat Rev Immunol. (2007) 7:929–41. doi: 10.1038/nri2191
31. Zeng Z, Castaño AR, Segelke BW, Stura EA, Peterson PA, and Wilson IA. Crystal structure of mouse CD1: An MHC-like fold with a large hydrophobic binding groove. Science. (1997) 277:339–45. doi: 10.1126/science.277.5324.339
32. Chancellor A, Gadola SD, and Mansour S. The versatility of the CD1 lipid antigen presentation pathway. Immunology. (2018) 154:196–203. doi: 10.1111/imm.12912
33. Zajonc DM, Crispin MDM, Bowden TA, Young DC, Cheng TY, Hu J, et al. Molecular mechanism of lipopeptide presentation by CD1a. Immunity. (2005) 22:209–19. doi: 10.1016/j.immuni.2004.12.009
34. Gadola SD, Zaccai NR, Harlos K, Shepherd D, Castro-Palomino JC, Ritter G, et al. Structure of human CD1b with bound ligands at 2.3 Å, a maze for alkyl chains. Nat Immunol. (2002) 3:721–6. doi: 10.1038/ni821
35. Mansour S, Tocheva AS, Cave-Ayland C, Machelett MM, Sander B, Lissin NM, et al. Cholesteryl esters stabilize human CD1c conformations for recognition by self-reactive T cells. Proc Natl Acad Sci. (2016) 113:E1266–75. doi: 10.1073/pnas.1519246113
36. Kang SJ and Cresswell P. Calnexin, calreticulin, and ERp57 cooperate in disulfide bond formation in human CD1d heavy chain. J Biol Chem. (2002) 277:44838–44. doi: 10.1074/jbc.M207831200
37. Sugita M, Porcelli SA, and Brenner MB. Assembly and retention of CD1b heavy chains in the endoplasmic reticulum. J Immunol Baltim Md 1950. (1997) 159:2358–65. doi: 10.4049/jimmunol.159.5.2358
38. Hüttinger R, Staffler G, Majdic O, and Stockinger H. Analysis of the early biogenesis of CD1b: involvement of the chaperones calnexin and calreticulin, the proteasome and beta(2)-microglobulin. Int Immunol. (1999) 11:1615–23. doi: 10.1093/intimm/11.10.1615
39. Garcia-Alles LF, Versluis K, Maveyraud L, Vallina AT, Sansano S, Bello NF, et al. Endogenous phosphatidylcholine and a long spacer ligand stabilize the lipid-binding groove of CD1b. EMBO J. (2006) 25:3684–92. doi: 10.1038/sj.emboj.7601244
40. Park JJ, Kang SJ, De Silva AD, Stanic AK, Casorati G, Hachey DL, et al. Lipid–protein interactions: Biosynthetic assembly of CD1 with lipids in the endoplasmic reticulum is evolutionarily conserved. Proc Natl Acad Sci. (2004) 101:1022–6. doi: 10.1073/pnas.0307847100
41. Sugita M, van der Wel N, Rogers RA, Peters PJ, and Brenner MB. CD1c molecules broadly survey the endocytic system. Proc Natl Acad Sci. (2000) 97:8445–50. doi: 10.1073/pnas.150236797
42. Briken V, Jackman RM, Dasgupta S, Hoening S, and Porcelli SA. Intracellular trafficking pathway of newly synthesized CD1b molecules. EMBO J. (2002) 21:825–34. doi: 10.1093/emboj/21.4.825
43. Bonifacino JS and Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. (2003) 72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800
44. Sugita M, Jackman RM, van Donselaar E, Behar SM, Rogers RA, Peters PJ, et al. Cytoplasmic tail-dependent localization of CD1b antigen-presenting molecules to MIICs. Science. (1996) 273:349–52. doi: 10.1126/science.273.5273.349
45. Barral DC, Cavallari M, McCormick PJ, Garg S, Magee AI, Bonifacino JS, et al. CD1a and MHC class I follow a similar endocytic recycling pathway. Traffic Cph Den. (2008) 9:1446–57. doi: 10.1111/j.1600-0854.2008.00781.x
46. Elewaut D, Lawton AP, Nagarajan NA, Maverakis E, Khurana A, Höning S, et al. The adaptor protein AP-3 is required for CD1d-mediated antigen presentation of glycosphingolipids and development of Vα14i NKT cells. J Exp Med. (2003) 198:1133–46. doi: 10.1084/jem.20030143
47. Sugita M, Cao X, Watts GFM, Rogers RA, Bonifacino JS, and Brenner MB. Failure of trafficking and antigen presentation by CD1 in AP-3-deficient cells. Immunity. (2002) 16:697–706. doi: 10.1016/S1074-7613(02)00311-4
48. Lawton AP, Prigozy TI, Brossay L, Pei B, Khurana A, Martin D, et al. The mouse CD1d cytoplasmic tail mediates CD1d trafficking and antigen presentation by adaptor protein 3-dependent and -independent mechanisms. J Immunol Baltim Md 1950. (2005) 174:3179–86. doi: 10.4049/jimmunol.174.6.3179
49. Roura-Mir C, Wang L, Cheng TY, Matsunaga I, Dascher CC, Peng SL, et al. Mycobacterium tuberculosis Regulates CD1 Antigen Presentation Pathways through TLR-2. J Immunol. (2005) 175:1758–66. doi: 10.4049/jimmunol.175.3.1758
50. Prigozy TI, Sieling PA, Clemens D, Stewart PL, Behar SM, Porcelli SA, et al. The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity. (1997) 6:187–97. doi: 10.1016/S1074-7613(00)80425-2
51. Hunger RE, Sieling PA, Ochoa MT, Sugaya M, Burdick AE, Rea TH, et al. Langerhans cells utilize CD1a and langerin to efficiently present nonpeptide antigens to T cells. J Clin Invest. (2004) 113:701–8. doi: 10.1172/JCI200419655
52. van den Elzen P, Garg S, León L, Brigl M, Leadbetter EA, Gumperz JE, et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. (2005) 437:906–10. doi: 10.1038/nature04001
53. Zhou D, Cantu C, Sagiv Y, Schrantz N, Kulkarni AB, Qi X, et al. Editing of CD1d-bound lipid antigens by endosomal lipid transfer proteins. Science. (2004) 303:523–7. doi: 10.1126/science.1092009
54. Winau F, Schwierzeck V, Hurwitz R, Remmel N, Sieling PA, Modlin RL, et al. Saposin C is required for lipid presentation by human CD1b. Nat Immunol. (2004) 5:169–74. doi: 10.1038/ni1035
55. Kang SJ and Cresswell P. Saposins facilitate CD1d-restricted presentation of an exogenous lipid antigen to T cells. Nat Immunol. (2004) 5:175–81. doi: 10.1038/ni1034
56. Manolova V, Kistowska M, Paoletti S, Baltariu GM, Bausinger H, Hanau D, et al. Functional CD1a is stabilized by exogenous lipids. Eur J Immunol. (2006) 36:1083–92. doi: 10.1002/eji.200535544
57. Shamshiev A, Gober HJ, Donda A, Mazorra Z, Mori L, and De Libero G. Presentation of the same glycolipid by different CD1 molecules. J Exp Med. (2002) 195:1013–21. doi: 10.1084/jem.20011963
58. O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, and Berry MPR. The immune response in tuberculosis. Annu Rev Immunol. (2013) 31:475–527. doi: 10.1146/annurev-immunol-032712-095939
59. De Libero G and Mori L. Recognition of lipid antigens by T cells. Nat Rev Immunol. (2005) 5:485–96. doi: 10.1038/nri1631
60. Roy S, Ly D, Li NS, Altman JD, Piccirilli JA, Moody DB, et al. Molecular basis of mycobacterial lipid antigen presentation by CD1c and its recognition by αβ T cells. Proc Natl Acad Sci. (2014) 111(42):E4648–57. doi: 10.1073/pnas.1408549111
61. Beckman EM, Melián A, Behar SM, Sieling PA, Chatterjee D, Furlong ST, et al. CD1c restricts responses of mycobacteria-specific T cells. Evidence for antigen presentation by a second member of the human CD1 family. J Immunol. (1996) 157(7):2795–803. doi: 10.4049/jimmunol.157.7.2795
62. Van Rhijn I, Ly D, and Moody DB. CD1a, CD1b, and CD1c in immunity against mycobacteria. Adv Exp Med Biol. (2013) 783:181–97. doi: 10.1007/978-1-4614-6111-1_10
63. Reijneveld JF, Ocampo TA, Shahine A, Gully BS, Vantourout P, Hayday AC, et al. Human γδ T cells recognize CD1b by two distinct mechanisms. Proc Natl Acad Sci. (2020) 117(37):22944–52. doi: 10.1073/pnas.2010545117
64. Porcelli S, Brenner MB, Greenstein JL, Terhorst C, Balk SP, and Bleicher PA. Recognition of cluster of differentiation 1 antigens by human CD4–CD8>– cytolytic T lymphocyte. Nature. (1989) 341(6241):447–50. doi: 10.1038/341447a0
65. Spada FM, Grant EP, Peters PJ, Sugita M, Melián A, Leslie DS, et al. Self-recognition of cd1 by γ/δ T cells: implications for innate immunity. J Exp Med. (2000) 191(6):937–48. doi: 10.1084/jem.191.6.937
66. Moody DB, Ulrichs T, Mühlecker W, Young DC, Gurcha SS, Grant E, et al. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature. (2000) 404(6780):884–8. doi: 10.1038/35009119
67. Van Rhijn I, Young DC, De Jong A, Vazquez J, Cheng TY, Talekar R, et al. CD1c bypasses lysosomes to present a lipopeptide antigen with 12 amino acids. J Exp Med. (2009) 206:1409–22. doi: 10.1084/jem.20082480
68. Zhao Y, Lin L, Xiao Z, Li M, Wu X, Li W, et al. Protective role of γδ T cells in different pathogen infections and its potential clinical application. J Immunol Res. (2018) 2018:5081634. doi: 10.1155/2018/5081634
69. Groh V, Porcelli S, Fabbi M, Lanier LL, Picker LJ, Anderson T, et al. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. (1989) 169:1277–94. doi: 10.1084/jem.169.4.1277
70. Gay L, Mezouar S, Cano C, Frohna P, Madakamutil L, Mège JL, et al. Role of Vγ9vδ2 T lymphocytes in infectious diseases. Front Immunol. (2022) 13:928441. doi: 10.3389/fimmu.2022.928441
71. Ito M, Kojiro N, Ikeda T, Ito T, Funada J, and Kokubu T. Increased proportions of peripheral blood γδ T cells in patients with pulmonary tuberculosis. Chest. (1992) 102:195–7. doi: 10.1378/chest.102.1.195
72. Chen ZW. Immune biology of Ag-specific γδ T cells in infections. Cell Mol Life Sci CMLS. (2011) 68:2409–17. doi: 10.1007/s00018-011-0703-9
73. Morita CT, Jin C, Sarikonda G, and Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. (2007) 215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x
74. Poccia F, Agrati C, Martini F, Capobianchi MR, Wallace M, and Malkovsky M. Antiviral reactivities of γδ T cells. Microbes Infect. (2005) 7:518–28. doi: 10.1016/j.micinf.2004.12.009
75. Kabelitz D, Bender A, Schondelmaier S, Schoel B, and Kaufmann SH. A large fraction of human peripheral blood gamma/delta + T cells is activated by Mycobacterium tuberculosis but not by its 65-kD heat shock protein. J Exp Med. (1990) 171:667–79. doi: 10.1084/jem.171.3.667
76. Hoft DF, Brown RM, and Roodman ST. Bacille calmette-guérin vaccination enhances human γδ T cell responsiveness to mycobacteria suggestive of a memory-like phenotype1. J Immunol. (1998) 161:1045–54. doi: 10.4049/jimmunol.161.2.1045
77. Ismaili J, Olislagers V, Poupot R, Fournié JJ, and Goldman M. Human γδ T cells induce dendritic cell maturation. Clin Immunol. (2002) 103:296–302. doi: 10.1006/clim.2002.5218
78. Roy S, Ly D, Castro CD, Li NS, Hawk AJ, Altman JD, et al. Molecular analysis of lipid-reactive Vδ1 γδ T cells identified by CD1c tetramers. J Immunol. (2016) 196:1933–42. doi: 10.4049/jimmunol.1502202
79. Uldrich AP, Le Nours J, Pellicci DG, Gherardin NA, McPherson KG, Lim RT, et al. CD1d-lipid antigen recognition by the γδ TCR. Nat Immunol. (2013) 14:1137–45. doi: 10.1038/ni.2713
80. Willcox BE and Willcox CR. γδ TCR ligands: the quest to solve a 500-million-year-old mystery. Nat Immunol. (2019) 20:121–8. doi: 10.1038/s41590-018-0304-y
81. Zeng X, Wei YL, Huang J, Newell EW, Yu H, Kidd BA, et al. γδ T cells recognize a microbial encoded B cell antigen to initiate a rapid antigen-specific interleukin-17 response. Immunity. (2012) 37:524–34. doi: 10.1016/j.immuni.2012.06.011
82. Willcox CR, Pitard V, Netzer S, Couzi L, Salim M, Silberzahn T, et al. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat Immunol. (2012) 13:872–9. doi: 10.1038/ni.2394
83. Bai L, Picard D, Anderson B, Chaudhary V, Luoma A, Jabri B, et al. The majority of CD1d-sulfatide-specific T cells in human blood use a semiinvariant Vδ1 TCR. Eur J Immunol. (2012) 42:2505–10. doi: 10.1002/eji.201242531
84. Luoma AM, Castro CD, Mayassi T, Bembinster LA, Bai L, Picard D, et al. Crystal structure of Vδ1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human γδ T cells. Immunity. (2013) 39:1032–42. doi: 10.1016/j.immuni.2013.11.001
85. Wegrecki M, Ocampo TA, Gunasinghe SD, von Borstel A, Tin SY, Reijneveld JF, et al. Atypical sideways recognition of CD1a by autoreactive γδ T cell receptors. Nat Commun. (2022) 13:3872. doi: 10.1038/s41467-022-31443-9
86. Vavassori S, Kumar A, Wan GS, Ramanjaneyulu GS, Cavallari M, El Daker S, et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nat Immunol. (2013) 14:908–16. doi: 10.1038/ni.2665
87. Karunakaran MM, Willcox CR, Salim M, Paletta D, Fichtner AS, Noll A, et al. Butyrophilin-2A1 directly binds germline-encoded regions of the Vγ9Vδ2 TCR and is essential for phosphoantigen sensing. Immunity. (2020) 52:487–498.e6. doi: 10.1016/j.immuni.2020.02.014
88. Rigau M, Ostrouska S, Fulford TS, Johnson DN, Woods K, Ruan Z, et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science. (2020) 367:eaay5516. doi: 10.1126/science.aay5516
89. Dimova T, Brouwer M, Gosselin F, Tassignon J, Leo O, Donner C, et al. Effector Vγ9Vδ2 T cells dominate the human fetal γδ T-cell repertoire. Proc Natl Acad Sci. (2015) 112:E556–65. doi: 10.1073/pnas.1412058112
90. Willcox CR, Davey MS, and Willcox BE. Development and selection of the human Vγ9Vδ2+ T-cell repertoire. Front Immunol. (2018) 9:1501. doi: 10.3389/fimmu.2018.01501
91. Lalor SJ and McLoughlin RM. Memory γδ T cells–newly appreciated protagonists in infection and immunity. Trends Immunol. (2016) 37:690–702. doi: 10.1016/j.it.2016.07.006
92. Davey MS, Willcox CR, Baker AT, Hunter S, and Willcox BE. Recasting human Vδ1 lymphocytes in an adaptive role. Trends Immunol. (2018) 39:446–59. doi: 10.1016/j.it.2018.03.003
93. Paul S, Singh AK, Shilpi, and Lal G. Phenotypic and functional plasticity of gamma-delta (γδ) T cells in inflammation and tolerance. Int Rev Immunol. (2014) 33:537–58. doi: 10.3109/08830185.2013.863306
94. Carding SR and Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. (2002) 2:336–45. doi: 10.1038/nri797
95. Khairallah C, Chu TH, and Sheridan BS. Tissue adaptations of memory and tissue-resident gamma delta T cells. Front Immunol. (2018) 9:2636. doi: 10.3389/fimmu.2018.02636
96. Rosat JP, Grant EP, Beckman EM, Dascher CC, Sieling PA, Frederique D, et al. CD1-restricted microbial lipid antigen-specific recognition found in the CD8+ alpha beta T cell pool. J Immunol Baltim Md 1950. (1999) 162:366–71. doi: 10.4049/jimmunol.162.1.366
97. Sieling PA, Ochoa MT, Jullien D, Leslie DS, Sabet S, Rosat JP, et al. Evidence for human CD4+ T cells in the CD1-restricted repertoire: derivation of mycobacteria-reactive T cells from leprosy lesions1. J Immunol. (2000) 164:4790–6. doi: 10.4049/jimmunol.164.9.4790
98. Ulrichs T, Moody DB, Grant E, Kaufmann SHE, and Porcelli SA. T-cell responses to CD1-presented lipid antigens in humans with mycobacterium tuberculosis infection. Infect Immun. (2003) 71:3076–87. doi: 10.1128/IAI.71.6.3076-3087.2003
99. Kawashima T, Norose Y, Watanabe Y, Enomoto Y, Narazaki H, Watari E, et al. Cutting edge: major CD8 T cell response to live bacillus Calmette-Guérin is mediated by CD1 molecules. J Immunol Baltim Md 1950. (2003) 170:5345–8. doi: 10.4049/jimmunol.170.11.5345
100. Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, and Flynn JL. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol Baltim Md 1950. (1999) 162:5407–16.
101. von Reyn CF. Optimal treatment of codisease due to HIV and tuberculosis. J Infect Dis. (2011) 204:817–9. doi: 10.1093/infdis/jir418
102. Leveton C, Barnass S, Champion B, Lucas S, De Souza B, Nicol M, et al. T-cell-mediated protection of mice against virulent Mycobacterium tuberculosis. Infect Immun. (1989) 57:390–5. doi: 10.1128/iai.57.2.390-395.1989
103. Mogues T, Goodrich ME, Ryan L, LaCourse R, and North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. (2001) 193:271–80. doi: 10.1084/jem.193.3.271
104. Flory CM, Hubbard RD, and Collins FM. Effects of in vivo T lymphocyte subset depletion on mycobacterial infections in mice. J Leukoc Biol. (1992) 51:225–9. doi: 10.1002/jlb.51.3.225
105. Scanga CA, Mohan VP, Yu K, Joseph H, Tanaka K, Chan J, et al. Depletion of CD4(+) T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J Exp Med. (2000) 192:347–58. doi: 10.1084/jem.192.3.347
106. Tian T, Woodworth J, Sköld M, and Behar SM. In vivo depletion of CD11c+ cells delays the CD4+ T cell response to Mycobacterium tuberculosis and exacerbates the outcome of infection. J Immunol Baltim Md 1950. (2005) 175:3268–72. doi: 10.4049/jimmunol.175.5.3268
107. Green AM, DiFazio R, and Flynn JL. IFN-γ from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection. J Immunol Baltim Md 1950. (2013) 190:270–7. doi: 10.4049/jimmunol.1200061
108. Felio K, Nguyen H, Dascher CC, Choi HJ, Li S, Zimmer MI, et al. CD1-restricted adaptive immune responses to Mycobacteria in human group 1 CD1 transgenic mice. J Exp Med. (2009) 206:2497–509. doi: 10.1084/jem.20090898
109. Dascher CC and Brenner MB. Evolutionary constraints on CD1 structure: insights from comparative genomic analysis. Trends Immunol. (2003) 24:412–8. doi: 10.1016/S1471-4906(03)00179-0
110. Looringh van Beeck FA, Zajonc DM, Moore PF, Schlotter YM, Broere F, Rutten VPMG, et al. Two canine CD1a proteins are differentially expressed in skin. Immunogenetics. (2008) 60:315–24. doi: 10.1007/s00251-008-0297-z
111. Van Rhijn I, Koets AP, Im JS, Piebes D, Reddington F, Besra GS, et al. The bovine CD1 family contains group 1 CD1 proteins, but no functional CD1d. J Immunol Baltim Md 1950. (2006) 176:4888–93. doi: 10.4049/jimmunol.176.8.4888
112. Dascher CC. Evolutionary biology of CD1. Curr Top Microbiol Immunol. (2007) 314:3–26. doi: 10.1007/978-3-540-69511-0_1
113. Hiromatsu K, Dascher CC, LeClair KP, Sugita M, Furlong ST, Brenner MB, et al. Induction of CD1-restricted immune responses in Guinea pigs by immunization with mycobacterial lipid antigens. J Immunol. (2002) 169:330–9. doi: 10.4049/jimmunol.169.1.330
114. Dascher CC, Hiromatsu K, Xiong X, Morehouse C, Watts G, Liu G, et al. Immunization with a mycobacterial lipid vaccine improves pulmonary pathology in the Guinea pig model of tuberculosis. Int Immunol. (2003) 15:915–25. doi: 10.1093/intimm/dxg091
115. Reinink P and Van Rhijn I. Mammalian CD1 and MR1 genes. Immunogenetics. (2016) 68:515–23. doi: 10.1007/s00251-016-0926-x
116. Kim S, Cho S, and Kim JH. CD1-mediated immune responses in mucosal tissues: molecular mechanisms underlying lipid antigen presentation system. Exp Mol Med. (2023) 55:1858–71. doi: 10.1038/s12276-023-01053-6
117. de Witte L, Nabatov A, Pion M, Fluitsma D, de Jong MAWP, de Gruijl T, et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med. (2007) 13:367–71. doi: 10.1038/nm1541
118. Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. (2000) 12:71–81. doi: 10.1016/S1074-7613(00)80160-0
119. Moody DB, Young DC, Cheng TY, Rosat JP, Roura-mir C, O’Connor PB, et al. T cell activation by lipopeptide antigens. Science. (2004) 303:527–31. doi: 10.1126/science.1089353
120. Batuwangala T, Shepherd D, Gadola SD, Gibson KJC, Zaccai NR, Fersht AR, et al. The crystal structure of human CD1b with a bound bacterial glycolipid. J Immunol Baltim Md 1950. (2004) 172:2382–8. doi: 10.4049/jimmunol.172.4.2382
121. Porcelli S, Morita CT, and Brenner MB. CD1b restricts the response of human CD4-8- T lymphocytes to a microbial antigen. Nature. (1992) 360:593–7. doi: 10.1038/360593a0
122. Sieling PA, Chatterjee D, Porcelli SA, Prigozy TI, Mazzaccaro RJ, Soriano T, et al. CD1-restricted T cell recognition of microbial lipoglycan antigens. Science. (1995) 269:227–30. doi: 10.1126/science.7542404
123. Stenger S, Mazzaccaro RJ, Uyemura K, Cho S, Barnes PF, Rosat JP, et al. Differential effects of cytolytic T cell subsets on intracellular infection. Science. (1997) 276:1684–7. doi: 10.1126/science.276.5319.1684
124. Ernst WA, Thoma-Uszynski S, Teitelbaum R, Ko C, Hanson DA, Clayberger C, et al. Granulysin, a T cell product, kills bacteria by altering membrane permeability. J Immunol Baltim Md 1950. (2000) 165:7102–8. doi: 10.4049/jimmunol.165.12.7102
125. Gilleron M, Stenger S, Mazorra Z, Wittke F, Mariotti S, Böhmer G, et al. Diacylated Sulfoglycolipids Are Novel Mycobacterial Antigens Stimulating CD1-restricted T Cells during Infection with Mycobacterium tuberculosis. J Exp Med. (2004) 199:649–59. doi: 10.1084/jem.20031097
126. Layre E, Collmann A, Bastian M, Mariotti S, Czaplicki J, Prandi J, et al. Mycolic acids constitute a scaffold for mycobacterial lipid antigens stimulating CD1-restricted T cells. Chem Biol. (2009) 16:82–92. doi: 10.1016/j.chembiol.2008.11.008
127. Guallar-Garrido S, Luquin M, and Julián E. Analysis of the lipid composition of mycobacteria by thin layer chromatography. JoVE J Vis Exp. (2021) 170):e62368. doi: 10.3791/62368
128. Korf J, Stoltz A, Verschoor J, De Baetselier P, and Grooten J. The Mycobacterium tuberculosis cell wall component mycolic acid elicits pathogen-associated host innate immune responses. Eur J Immunol. (2005) 35:890–900. doi: 10.1002/eji.200425332
129. Montamat-Sicotte DJ, Millington KA, Willcox CR, Hingley-Wilson S, Hackforth S, Innes J, et al. A mycolic acid–specific CD1-restricted T cell population contributes to acute and memory immune responses in human tuberculosis infection. J Clin Invest. (2011) 121:2493–503. doi: 10.1172/JCI46216
130. Kasmar AG, van Rhijn I, Cheng TY, Turner M, Seshadri C, Schiefner A, et al. CD1b tetramers bind αβ T cell receptors to identify a mycobacterial glycolipid-reactive T cell repertoire in humans. J Exp Med. (2011) 208:1741–7. doi: 10.1084/jem.20110665
131. Van Rhijn I, Kasmar A, de Jong A, Gras S, Bhati M, Doorenspleet ME, et al. A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nat Immunol. (2013) 14:706–13. doi: 10.1038/ni.2630
132. Van Rhijn I, Gherardin NA, Kasmar A, de Jager W, Pellicci DG, Kostenko L, et al. T cell receptor bias and affinity define two compartments of the CD1b-glycolipid specific T cell repertoire. J Immunol Baltim Md 1950. (2014) 192:4054–60. doi: 10.4049/jimmunol.1400158
133. Busch M, Herzmann C, Kallert S, Zimmermann A, Höfer C, Mayer D, et al. Lipoarabinomannan-responsive polycytotoxic T cells are associated with protection in human tuberculosis. Am J Respir Crit Care Med. (2016) 194:345–55. doi: 10.1164/rccm.201509-1746OC
134. Sakai Y, Asa M, Hirose M, Kusuhara W, Fujiwara N, Tamashima H, et al. A conserved human CD4+ T cell subset recognizing the mycobacterial adjuvant trehalose monomycolate. J Clin Invest. (2024) 135:e185443. doi: 10.1172/JCI185443
135. Zhao J, Siddiqui S, Shang S, Bian Y, Bagchi S, He Y, et al. Mycolic acid-specific T cells protect against Mycobacterium tuberculosis infection in a humanized transgenic mouse model. eLife. (2015) 4:e08525. doi: 10.7554/eLife.08525.013
136. Wun KS, Reijneveld JF, Cheng TY, Ladell K, Uldrich AP, Le Nours J, et al. T cell autoreactivity directed toward CD1c itself rather than toward carried self lipids. Nat Immunol. (2018) 19:397–406. doi: 10.1038/s41590-018-0065-7
137. Milne P, Bigley V, Gunawan M, Haniffa M, and Collin M. CD1c+ blood dendritic cells have Langerhans cell potential. Blood. (2015) 125:470–3. doi: 10.1182/blood-2014-08-593582
138. Briken V, Jackman RM, Watts GFM, Rogers RA, and Porcelli SA. Human cd1b and cd1c isoforms survey different intracellular compartments for the presentation of microbial lipid antigens. J Exp Med. (2000) 192:281–8. doi: 10.1084/jem.192.2.281
139. Scharf L, Li NS, Hawk AJ, Garzón D, Zhang T, Fox LM, et al. The 2.5 Å Structure of CD1c in complex with a mycobacterial lipid reveals an open groove ideally suited for diverse antigen presentation. Immunity. (2010) 33:853–62. doi: 10.1016/j.immuni.2010.11.026
140. de Jong A, Arce EC, Cheng TY, van Summeren RP, Feringa BL, Dudkin V, et al. CD1c presentation of synthetic glycolipid antigens with foreign alkyl branching motifs. Chem Biol. (2007) 14:1232–42. doi: 10.1016/j.chembiol.2007.09.010
141. Reijneveld JF, Marino L, Cao TP, Cheng TY, Dam D, Shahine A, et al. Rational design of a hydrolysis-resistant mycobacterial phosphoglycolipid antigen presented by CD1c to T cells. J Biol Chem. (2021) 297:101197. doi: 10.1016/j.jbc.2021.101197
142. Salio M, Silk JD, Jones EY, and Cerundolo V. Biology of CD1- and MR1-restricted T cells. Annu Rev Immunol. (2014) 32:323–66. doi: 10.1146/annurev-immunol-032713-120243
143. Balato A, Unutmaz D, and Gaspari AA. Natural killer T cells: an unconventional T-cell subset with diverse effector and regulatory functions. J Invest Dermatol. (2009) 129:1628–42. doi: 10.1038/jid.2009.30
144. Dellabona P, Padovan E, Casorati G, Brockhaus M, and Lanzavecchia A. An invariant V alpha 24-J alpha Q/V beta 11 T cell receptor is expressed in all individuals by clonally expanded CD4-8- T cells. J Exp Med. (1994) 180:1171–6. doi: 10.1084/jem.180.3.1171
145. Porcelli S, Yockey CE, Brenner MB, and Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. (1993) 178:1–16. doi: 10.1084/jem.178.1.1
146. Liao CM, Zimmer MI, and Wang CR. The functions of type I and type II natural killer T (NKT) cells in inflammatory bowel diseases. Inflammation Bowel Dis. (2013) 19:1330–8. doi: 10.1097/MIB.0b013e318280b1e3
147. Borg NA, Wun KS, Kjer-Nielsen L, Wilce MCJ, Pellicci DG, Koh R, et al. CD1d–lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. (2007) 448:44–9. doi: 10.1038/nature05907
148. Natori T, Koezuka Y, and Higa T. Agelasphins, novel α-galactosylceramides from the marine sponge Agelas mauritianus. Tetrahedron Lett. (1993) 34:5591–2. doi: 10.1016/S0040-4039(00)73889-5
149. Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, et al. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. (1997) 278:1626–9. doi: 10.1126/science.278.5343.1626
150. Benlagha K, Weiss A, Beavis A, Teyton L, and Bendelac A. In vivo identification of glycolipid antigen–specific T cells using fluorescent cd1d tetramers. J Exp Med. (1999) 191:1895–904. doi: 10.1084/jem.191.11.1895
151. Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. (2000) 192:741–54. doi: 10.1084/jem.192.5.741
152. Brossay L and Kronenberg M. Highly conserved antigen-presenting function of CD1d molecules. Immunogenetics. (1999) 50:146–51. doi: 10.1007/s002510050590
153. Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Sato H, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc Natl Acad Sci U S A. (1998) 95:5690–3. doi: 10.1073/pnas.95.10.5690
154. Chackerian A, Alt J, Perera V, and Behar SM. Activation of NKT cells protects mice from tuberculosis. Infect Immun. (2002) 70:6302–9. doi: 10.1128/IAI.70.11.6302-6309.2002
155. Fischer K, Scotet E, Niemeyer M, Koebernick H, Zerrahn J, Maillet S, et al. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc Natl Acad Sci U S A. (2004) 101:10685–90. doi: 10.1073/pnas.0403787101
156. Sada-Ovalle I, Chiba A, Gonzales A, Brenner MB, and Behar SM. Innate invariant NKT cells recognize mycobacterium tuberculosis–infected macrophages, produce interferon-γ, and kill intracellular bacteria. PloS Pathog. (2008) 4:e1000239. doi: 10.1371/journal.ppat.1000239
157. Behar SM, Dascher CC, Grusby MJ, Wang CR, and Brenner MB. Susceptibility of mice deficient in CD1D or TAP1 to infection with mycobacterium tuberculosis. J Exp Med. (1999) 189:1973–80. doi: 10.1084/jem.189.12.1973
158. de Jong A, Peña-Cruz V, Cheng TY, Clark RA, Van Rhijn I, and Moody DB. CD1a-autoreactive T cells are a normal component of the human αβ T cell repertoire. Nat Immunol. (2010) 11:1102–9. doi: 10.1038/ni.1956
159. de Lalla C, Lepore M, Piccolo FM, Rinaldi A, Scelfo A, Garavaglia C, et al. High-frequency and adaptive-like dynamics of human CD1 self-reactive T cells. Eur J Immunol. (2011) 41:602–10. doi: 10.1002/eji.201041211
160. Vincent MS, Xiong X, Grant EP, Peng W, and Brenner MB. CD1a-, b-, and c-restricted TCRs recognize both self and foreign antigens. J Immunol Baltim Md 1950. (2005) 175:6344–51. doi: 10.4049/jimmunol.175.10.6344
161. Roura-Mir C, Catálfamo M, Cheng TY, Marqusee E, Besra GS, Jaraquemada D, et al. CD1a and CD1c activate intrathyroidal T cells during Graves’ disease and Hashimoto’s thyroiditis. J Immunol Baltim Md 1950. (2005) 174:3773–80. doi: 10.4049/jimmunol.174.6.3773
162. Sieling PA, Porcelli SA, Duong BT, Spada F, Bloom BR, Diamond B, et al. Human double-negative T cells in systemic lupus erythematosus provide help for IgG and are restricted by CD1c. J Immunol Baltim Md 1950. (2000) 165:5338–44. doi: 10.4049/jimmunol.165.9.5338
163. Moret FM, Hack CE, van der Wurff-Jacobs KMG, de Jager W, Radstake TRDJ, Lafeber FPJG, et al. Intra-articular CD1c-expressing myeloid dendritic cells from rheumatoid arthritis patients express a unique set of T cell-attracting chemokines and spontaneously induce Th1, Th17 and Th2 cell activity. Arthritis Res Ther. (2013) 15:R155. doi: 10.1186/ar4338
164. Shamshiev A, Donda A, Carena I, Mori L, Kappos L, and De Libero G. Self glycolipids as T-cell autoantigens. Eur J Immunol. (1999) 29:1667–75. doi: 10.1002/(SICI)1521-4141(199905)29:05<1667::AID-IMMU1667>3.0.CO;2-U
165. de Jong A, Cheng TY, Huang S, Gras S, Birkinshaw RW, Kasmar A, et al. CD1a autoreactive T cells recognize natural skin oils that function as headless antigens. Nat Immunol. (2014) 15:177–85. doi: 10.1038/ni.2790
166. Betts RJ, Perkovic A, Mahapatra S, Del Bufalo A, Camara K, Howell AR, et al. Contact sensitizers trigger human CD1-autoreactive T-cell responses. Eur J Immunol. (2017) 47:1171–80. doi: 10.1002/eji.201746939
167. Guo T, Koo MY, Kagoya Y, Anczurowski M, Wang CH, Saso K, et al. A subset of human autoreactive CD1c-restricted T cells preferentially expresses TRBV4-1+ TCRs. J Immunol. (2018) 200:500–11. doi: 10.4049/jimmunol.1700677
168. Dougan SK, Kaser A, and Blumberg RS. CD1 expression on antigen-presenting cells. Curr Top Microbiol Immunol. (2007) 314:113–41. doi: 10.1007/978-3-540-69511-0_5
169. Li S, Choi HJ, Felio K, and Wang CR. Autoreactive CD1b-restricted T cells: a new innate-like T-cell population that contributes to immunity against infection. Blood. (2011) 118:3870–8. doi: 10.1182/blood-2011-03-341941
170. Lepore M, de Lalla C, Gundimeda SR, Gsellinger H, Consonni M, Garavaglia C, et al. A novel self-lipid antigen targets human T cells against CD1c+ leukemias. J Exp Med. (2014) 211:1363–77. doi: 10.1084/jem.20140410
171. Bagchi S, Li S, and Wang CR. CD1b-autoreactive T cells recognize phospholipid antigens and contribute to antitumor immunity against a CD1b+ T cell lymphoma. Oncoimmunology. (2016) 5:e1213932. doi: 10.1080/2162402X.2016.1213932
172. Libero GD, Moran AP, Gober HJ, Rossy E, Shamshiev A, Chelnokova O, et al. Bacterial infections promote T cell recognition of self-glycolipids. Immunity. (2005) 22:763–72. doi: 10.1016/j.immuni.2005.04.013
173. Mosser DM and Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. (2008) 8:958–69. doi: 10.1038/nri2448
174. Brigl M, Bry L, Kent SC, Gumperz JE, and Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. (2003) 4:1230–7. doi: 10.1038/ni1002
175. Zeissig S, Murata K, Sweet L, Publicover J, Hu Z, Kaser A, et al. Hepatitis B virus-induced alterations in hepatocyte cd1d lipid antigens activate natural killer T cells and contribute to protective immunity. Nat Med. (2012) 18:1060–8. doi: 10.1038/nm.2811
177. Stenger S, Niazi KR, and Modlin RL. Down-regulation of CD1 on antigen-presenting cells by infection with Mycobacterium tuberculosis. J Immunol Baltim Md 1950. (1998) 161:3582–8. doi: 10.4049/jimmunol.161.7.3582
178. Giuliani A, Prete SP, Graziani G, Aquino A, Balduzzi A, Sugita M, et al. Influence of Mycobacterium bovis bacillus Calmette Guérin on in vitro induction of CD1 molecules in human adherent mononuclear cells. Infect Immun. (2001) 69:7461–70. doi: 10.1128/IAI.69.12.7461-7470.2001
179. Wen Q, Zhou C, Xiong W, Su J, He J, Zhang S, et al. MiR-381-3p regulates the antigen-presenting capability of dendritic cells and represses antituberculosis cellular immune responses by targeting CD1c. J Immunol Baltim Md 1950. (2016) 197:580–9. doi: 10.4049/jimmunol.1500481
180. Comas I, Chakravartti J, Small PM, Galagan J, Niemann S, Kremer K, et al. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat Genet. (2010) 42:498–503. doi: 10.1038/ng.590
181. Andersen P and Doherty TM. The success and failure of BCG — implications for a novel tuberculosis vaccine. Nat Rev Microbiol. (2005) 3:656–62. doi: 10.1038/nrmicro1211
182. Trunz BB, Fine P, and Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. (2006) 367:1173–80. doi: 10.1016/S0140-6736(06)68507-3
183. Rodrigues LC, Diwan VK, and Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: A meta-analysis. Int J Epidemiol. (1993) 22:1154–8. doi: 10.1093/ije/22.6.1154
184. Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, et al. The efficacy of bacillus Calmette-Guérin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. (1995) 96:29–35. doi: 10.1542/peds.96.1.29
185. Talbot EA, Perkins MD, Silva SF, and Frothingham R. Disseminated bacille Calmette-Guérin disease after vaccination: case report and review. Clin Infect Dis Off Publ Infect Dis Soc Am. (1997) 24:1139–46. doi: 10.1086/513642
186. Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. (2013) 381:1021–8. doi: 10.1016/S0140-6736(13)60177-4
187. Kaufmann SHE. Vaccination against tuberculosis: revamping BCG by molecular genetics guided by immunology. Front Immunol. (2020) 11:316. doi: 10.3389/fimmu.2020.00316
188. Ndiaye BP, Thienemann F, Ota M, Landry BS, Camara M, Dièye S, et al. Safety, immunogenicity, and efficacy of the candidate tuberculosis vaccine MVA85A in healthy adults infected with HIV-1: a randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. (2015) 3:190–200. doi: 10.1016/S2213-2600(15)00037-5
189. Yihao D, Hongyun H, and Maodan T. Latency-associated protein Rv2660c of Mycobacterium tuberculosis augments expression of proinflammatory cytokines in human macrophages by interacting with TLR2. Infect Dis Lond Engl. (2015) 47:168–77. doi: 10.3109/00365548.2014.982167
190. Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, Dolganov G, et al. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med. (2011) 17:189–94. doi: 10.1038/nm.2285
191. Lin PL, Dietrich J, Tan E, Abalos RM, Burgos J, Bigbee C, et al. The multistage vaccine H56 boosts the effects of BCG to protect cynomolgus macaques against active tuberculosis and reactivation of latent Mycobacterium tuberculosis infection. J Clin Invest. (2012) 122:303–14. doi: 10.1172/JCI46252
192. Jenum S, Tonby K, Rueegg CS, Rühwald M, Kristiansen MP, Bang P, et al. A Phase I/II randomized trial of H56:IC31 vaccination and adjunctive cyclooxygenase-2-inhibitor treatment in tuberculosis patients. Nat Commun. (2021) 12:6774. doi: 10.1038/s41467-021-27029-6
193. Luabeya AKK, Kagina BMN, Tameris MD, Geldenhuys H, Hoff ST, Shi Z, et al. First-in-human trial of the post-exposure tuberculosis vaccine H56:IC31 in Mycobacterium tuberculosis infected and non-infected healthy adults. Vaccine. (2015) 33:4130–40. doi: 10.1016/j.vaccine.2015.06.051
194. Development of the candidate tuberculosis vaccine H56:IC31 ended based on early data from the Prevention of Recurrence TB Consortium. Copenhagen, Denmark: IAVI. (2023) Available at: https://www.iavi.org/features/development-of-the-candidate-tuberculosis-vaccine-h56ic31-ended-based-on-early-data-from-the-prevention-of-recurrence-tb-consortium/. (Accessed April 29, 2025).
195. Skeiky YA, Lodes MJ, Guderian JA, Mohamath R, Bement T, Alderson MR, et al. Cloning, expression, and immunological evaluation of two putative secreted serine protease antigens of Mycobacterium tuberculosis. Infect Immun. (1999) 67:3998–4007. doi: 10.1128/IAI.67.8.3998-4007.1999
196. Qian J, Chen R, Wang H, and Zhang X. Role of the PE/PPE family in host-pathogen interactions and prospects for anti-tuberculosis vaccine and diagnostic tool design. Front Cell Infect Microbiol. (2020) 10:594288. doi: 10.3389/fcimb.2020.594288
197. Nair S, Ramaswamy PA, Ghosh S, Joshi DC, Pathak N, Siddiqui I, et al. The PPE18 of mycobacterium tuberculosis interacts with TLR2 and activates IL-10 induction in macrophage. J Immunol. (2009) 183:6269–81. doi: 10.4049/jimmunol.0901367
198. Urdahl KB, Shafiani S, and Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol. (2011) 4:288–93. doi: 10.1038/mi.2011.10
199. Bhat KH, Ahmed A, Kumar S, Sharma P, and Mukhopadhyay S. Role of PPE18 protein in intracellular survival and pathogenicity of mycobacterium tuberculosis in mice. PloS One. (2012) 7:e52601. doi: 10.1371/journal.pone.0052601
200. Hakim JMC and Yang Z. Predicted structural variability of mycobacterium tuberculosis PPE18 protein with immunological implications among clinical strains. Front Microbiol. (2020) 11:595312. doi: 10.3389/fmicb.2020.595312
201. Tait DR, Hatherill M, van der Meeren O, Ginsberg AM, Van Brakel E, Salaun B, et al. Final analysis of a trial of M72/AS01E vaccine to prevent tuberculosis. N Engl J Med. (2019) 381:2429–39. doi: 10.1056/NEJMoa1909953
202. Gillard P, Yang PC, Danilovits M, Su WJ, Cheng SL, Pehme L, et al. Safety and immunogenicity of the M72/AS01E candidate tuberculosis vaccine in adults with tuberculosis: A phase II randomised study. Tuberc Edinb Scotl. (2016) 100:100:118–27. doi: 10.1016/j.tube.2016.07.005
203. Morgun E, Zhu J, Almunif S, Bobbala S, Aguilar MS, Wang J, et al. Vaccination with mycobacterial lipid loaded nanoparticle leads to lipid antigen persistence and memory differentiation of antigen-specific T cells. BioRxiv Prepr Serv Biol. (2023) 12:RP87431. doi: 10.7554/eLife.87431.1
204. Cox JS, Chen B, McNeil M, and Jacobs WR. Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature. (1999) 402:79–83. doi: 10.1038/47042
205. Camacho LR, Ensergueix D, Perez E, Gicquel B, and Guilhot C. Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol Microbiol. (1999) 34:257–67. doi: 10.1046/j.1365-2958.1999.01593.x
206. Walters SB, Dubnau E, Kolesnikova I, Laval F, Daffe M, and Smith I. The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol Microbiol. (2006) 60:312–30. doi: 10.1111/j.1365-2958.2006.05102.x
207. Asensio JG, Maia C, Ferrer NL, Barilone N, Laval F, Soto CY, et al. The virulence-associated two-component phoP-phoR system controls the biosynthesis of polyketide-derived lipids in mycobacterium tuberculosis*. J Biol Chem. (2006) 281:1313–6. doi: 10.1074/jbc.C500388200
208. Gonzalo-Asensio J, Soto CY, Arbués A, Sancho J, del Carmen Menéndez M, García MJ, et al. The Mycobacterium tuberculosis phoPR Operon Is Positively Autoregulated in the Virulent Strain H37Rv. J Bacteriol. (2008) 190:7068–78. doi: 10.1128/JB.00712-08
209. Frigui W, Bottai D, Majlessi L, Monot M, Josselin E, Brodin P, et al. Control of M. tuberculosis ESAT-6 secretion and specific T cell recognition by phoP. PloS Pathog. (2008) 4:e33. doi: 10.1371/journal.ppat.0040033
210. Loxton AG, Knaul JK, Grode L, Gutschmidt A, Meller C, Eisele B, et al. Safety and immunogenicity of the recombinant mycobacterium bovis BCG vaccine VPM1002 in HIV-unexposed newborn infants in South Africa. Clin Vaccine Immunol CVI. (2017) 24:e00439–16. doi: 10.1128/CVI.00439-16
211. Nieuwenhuizen NE, Kulkarni PS, Shaligram U, Cotton MF, Rentsch CA, Eisele B, et al. The recombinant bacille calmette–guérin vaccine VPM1002: ready for clinical efficacy testing. Front Immunol. (2017) 8:1147. doi: 10.3389/fimmu.2017.01147
212. Grode L, Ganoza CA, Brohm C, Weiner J, Eisele B, and Kaufmann SHE. Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine. (2013) 31:1340–8. doi: 10.1016/j.vaccine.2012.12.053
213. Gordon AH, Hart PD, and Young MR. Ammonia inhibits phagosome-lysosome fusion in macrophages. Nature. (1980) 286:79–80. doi: 10.1038/286079a0
214. Reyrat JM, Berthet FX, and Gicquel B. The urease locus of Mycobacterium tuberculosis and its utilization for the demonstration of allelic exchange in Mycobacterium bovis bacillus Calmette-Guérin. Proc Natl Acad Sci U S A. (1995) 92:8768–72. doi: 10.1073/pnas.92.19.8768
215. Shaughnessy LM, Hoppe AD, Christensen KA, and Swanson JA. Membrane perforations inhibit lysosome fusion by altering pH and calcium in Listeria monocytogenes vacuoles. Cell Microbiol. (2006) 8:781–92. doi: 10.1111/j.1462-5822.2005.00665.x
Keywords: CD1, T cells, infection, tuberculosis, unconventional T cells, lipids, TB vaccine
Citation: Milton M and Mansour S (2025) CD1-restricted T cells: are unconventional allies the key to future TB vaccines? Front. Immunol. 16:1629466. doi: 10.3389/fimmu.2025.1629466
Received: 15 May 2025; Accepted: 18 June 2025;
Published: 10 July 2025.
Edited by:
Luc Van Kaer, Vanderbilt University Medical Center, United StatesReviewed by:
Bingdong Zhu, Lanzhou University, ChinaChyung-Ru Wang, Northwestern University Feinberg School of Medicine, United States
Ji Hyung Kim, Korea University, Republic of Korea
Copyright © 2025 Milton and Mansour. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Salah Mansour, cy5tYW5zb3VyQHNvdG9uLmFjLnVr
 Matthew Milton
Matthew Milton Salah Mansour
Salah Mansour