- 1Renal Transplantation Center “A. Vercellone,” Division of Nephrology, Dialysis and Transplantation, Città Della Salute e Della Scienza Hospital, Turin, Italy
- 2Department of Medical Sciences, University of Turin, Turin, Italy
- 3Nephrology Unit, ASL TO5, Chieri, Italy
- 4Division of Hematology, Department of Molecular Biotechnology and Health Sciences, University of Torino, Azienda Ospedaliero Universitaria (A.O.U) Città della Salute e della Scienza di Torino, Turin, Italy
- 5Hemato-Oncology Division, Istituto Europeo di Oncologia (IEO), European Institute of Oncology IRCCS, Milan, Italy
- 6Dipartimento Scienze Salute, University of Milan, Milan, Italy
Introduction: Oncohematological disorders are heterogeneous conditions that present significant challenges in management prior to transplantation. Data about rejection risk, disease recurrence, eligibility criteria, and requested remission time before kidney transplant (KT) are still lacking.
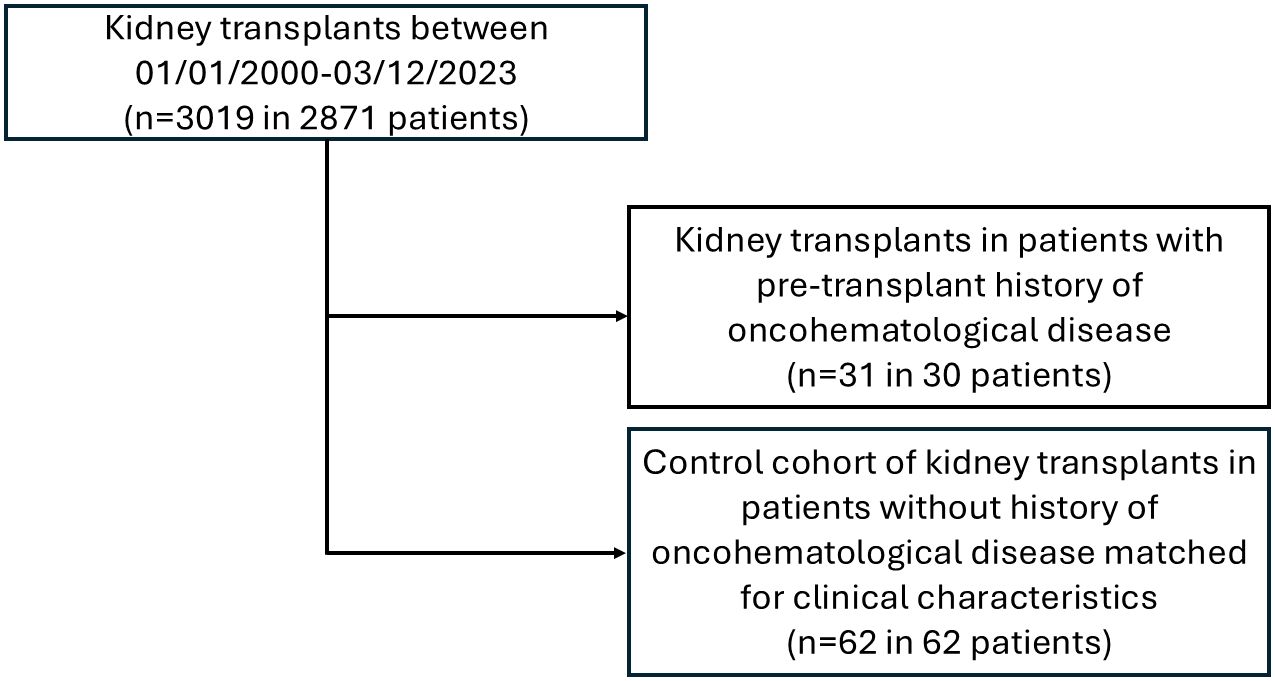
Methods: All KTRs between January 1, 2000, and March 31, 2023 (n = 2871) were analyzed. All patients with an oncohematological disease (hematological cohort, including plasma cell dyscrasias [PCDs], acute leukemia, high-grade lymphoma/post-transplant lymphoproliferative disorders [PTLDs], myeloproliferative neoplasms [MPNs], myelodysplastic/myeloproliferative neoplasms [MDS/MPNs], and genetic/AA amyloidosis) were matched 1:2 by age at transplant, gender, type of dialysis, and eGFR at transplant with KTRs without a history of hematological disease (control cohort). Primary endpoints were death-censored graft survival and the risk of rejection. Secondary endpoints included the risk of hematological disease recurrence and infection, patient survival rates, and graft function.
Results: Thirty out of 2871 patients (1.04%) receiving 31/3019 KTs have a pre-existing oncohematological disease (hematological cohort): 7/30 (23.3%) PCDs, 4/30 (13.3%) acute leukemia, 8/30 (26.7%) high-grade lymphomas/PTLDs, 4/30 (13.3%) MPNs, 2/30 (6.7%) MDS/MPNs, and 5/30 (16.7%) AA/familiar amyloidosis. Patients were transplanted at a median time of 5 (PCDs), 11.8 (acute leukemia), 12.3 (high-grade lymphomas/PTLDs), 8.5 (MPNs), 3.6 (MDS/MPNs), and 3.5 years (amyloidosis) after achieving disease remission (or stable disease in smoldering myeloma, MPNs, and MDS/MPNs). Comparing hematological and control cohorts, no differences were observed in patient and graft survival or post-transplant complications, including acute rejections. Results are superimposable also without considering the three patients who underwent living KTs from the same donor as the bone marrow transplant. Hematological disease relapses were observed in 2/30 (6.6%), including a light-chain deposition and a Castleman disease, both of which were successfully treated with chemotherapy without allograft dysfunction.
Conclusions: Favorable long-term transplant and clinical outcomes were achieved in patients with various pre-existing oncological and hematological disorders. These patients should not be denied KT after a well-documented stable disease. In this context, a multidisciplinary approach is crucial for establishing standardized pre- and post-transplant monitoring protocols and achieving optimal graft and patient outcomes.
1 Introduction
Oncohematological disorders encompass a range of diseases with distinct incidence, presentation, and outcomes, which may also contribute to or be associated with end-stage renal disease (ESRD). Not surprisingly, given the prolonged life expectancy and the improvement of therapeutic armamentarium, these conditions are more frequently observed and well-treated, highlighting challenging questions in pre-transplant settings.
Unfortunately, Literature data about rejection risk and disease recurrence of patients with a previous history of oncohematological diseases before kidney transplant (KT) are still limited. International guidelines vary in their eligibility criteria and requested remission times (1–5). We have previously reported our favorable experience in kidney transplant recipients (KTRs) with a previous history of monoclonal gammopathy of undetermined significance (MGUS) (6); in this study, we now focus our attention on all subjects with a pre-existing hematological disease, including plasma cell dyscrasias (PCDs) (i.e., smoldering [SMM] or multiple myeloma [MM], light chain deposition disease [LCDD], AL amyloidosis), acute leukemia. high-grade lymphoma (including also previous post-transplant lymphoproliferative disorders [PTLD]), myeloproliferative neoplasms (MPNs), myelodysplastic/myeloproliferative neoplasms (MDS/MPN), and genetic/AA amyloidosis.
2 Methods
2.1 Study population and data collection
The study included all the KTRs performed at the Turin University Renal Transplant Center “A. Vercellone” from January 2000 to March 2023. Patients with a pre-transplant oncohematological disease, classified according to the WHO 5th criteria (7–9) (hematological cohort), were included in the analysis.
All patients were initially managed by the Renal Transplant Center (Hub center) and received induction therapy (steroids and basiliximab/anti-thymocyte globulin [ATG] according to donor type and immune risk) and maintenance immunosuppression mainly composed of tacrolimus (10−15 ng/ml for the first three months and of 6−8 ng/ml thereafter), mycophenolate mofetil/mycophenolic acid, and/or steroids (progressively tapered to 5 mg/day or withdrawn according to patients characteristics and immunological risk). After discharge, post-transplant care followed a standardized schedule, and every recipient was monitored by the Hub transplant center with at least one annual visit, as well as by the local nephrologist (eleven peripheral centers covering most of the Piedmont region) for periodic follow-up.
KTRs were divided into subgroups based on the characteristics of hematological disorders, including PCDs, acute leukemia, high-grade lymphoma/PTLDs, MPNs, MDS/MPN, and genetic/AA amyloidosis.
Clinical diagnosis was based on available laboratory parameters (serum electrophoresis, serum, and urinary immunofixation and light kappa and lambda chains for SMM and MM; blood count and peripheral blood smear for leukemia, MPNs, and MDS/MPN) and, if available, histopathological data (bone marrow or other tissues biopsies).
Recipients’ follow-up was obtained by scheduled clinical visits or hospital admissions when significant complications occurred. Data were collected from patients’ charts at the time of transplant and the 1st, 2nd, 5th, 10th, 15th year, and last follow-up visits in our post-transplant outpatient unit. Specific items (sex, age, underlying nephropathy, type of dialysis and its duration before KT, previous transplant or immunosuppressive therapies), data about the hematological disorder (subtype, treatments, time before kidney transplant, follow-up, and occurrence of post-transplant progression/relapse), type of transplant (single or dual KT, combined, from deceased or living donor), immunosuppressive therapy, graft function (serum creatinine, eGFR with CKD-EPI formula, and 24-hours proteinuria) were retrospectively collected. The follow-up ended on July 31st, 2023.
This study was conducted in accordance with the most recent version of the Declaration of Helsinki. The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism. Our Ethical Committee approval covers this study, as per resolution 1449/2019 on 11 August 2019 (“TGT observational study”).
2.2 Outcomes
The primary endpoint of this study was to evaluate the effect of pre-transplant oncohematological disease on death-censored graft survival and the risk of rejection. Secondary endpoints included identifying the risk of disease recurrence, the impact on patient survival rates and graft function, and the global infection risk. We, therefore, compare the hematological cohort with a control cohort of patients matched for baseline characteristics (age at transplant, sex, type of dialysis, and graft function at transplant) who do not have a history of pre-transplant oncohematological disease.
2.3 Statistical analysis
Each transplant performed on patients with a previous hematological disorder was matched 1:2 for age at transplant, gender, type of dialysis, and eGFR at the time of the transplant with transplants performed on patients without a hematological disorder.
The normal distribution of continuous variables, both overall and within subgroups, was assessed using the Kolmogorov-Smirnov test.
The median, first quartile, and third quartile were used to describe continuous data.
Categorical variables were summarized as counts and proportions.
We examined confounders and correlations using the nonparametric Mann-Whitney test for continuous variables, according to their distributions, and Person’s or Fisher’s Chi-Square test for categorical variables.
Survival curves were plotted with the Kaplan–Meier method, and strata were compared using the Log-Rank test.
The significance level for the study was determined prior to data collection and was set at 0.05.
All statistical analyses were performed using SPSS (IBM Corp., Released 2023. IBM SPSS Statistics for Windows, Version 29.0.2.0, Armonk, NY: IBM Corp).
3 Results
3.1 Population characteristics at baseline and cohort analysis
Between January 1, 2000, and March 31, 2023, a total of 3019 kidney transplants were performed in 2871 patients at the Turin University Renal Transplant Center “A. Vercellone.” Among them, 30 patients (receiving 31 KTs) have a pre-existing oncohematological disease (hematological cohort): 7 (23.3%) PCDs (MM n=3, AL amyloidosis n=2, LCDD n=2), 4 (13.3%) acute leukemias, 8 (26.7%) high-grade lymphomas/PTLDs (lymphomas n=3, PTLD n=5), 4 (13.3%) MPNs (polycythemia vera n=2, chronic myeloid leukemia n=1, essential thrombocythemia n=1), 2 (6.7%) MDS/MPN (myelodysplastic/myeloproliferative neoplasm with ring sideroblasts and thrombocytosis n=1, myelodysplastic/myeloproliferative neoplasm, not otherwise specified n=1). Additionally, five patients (16.7%) have a history of AA or familial amyloidosis and were described separately.
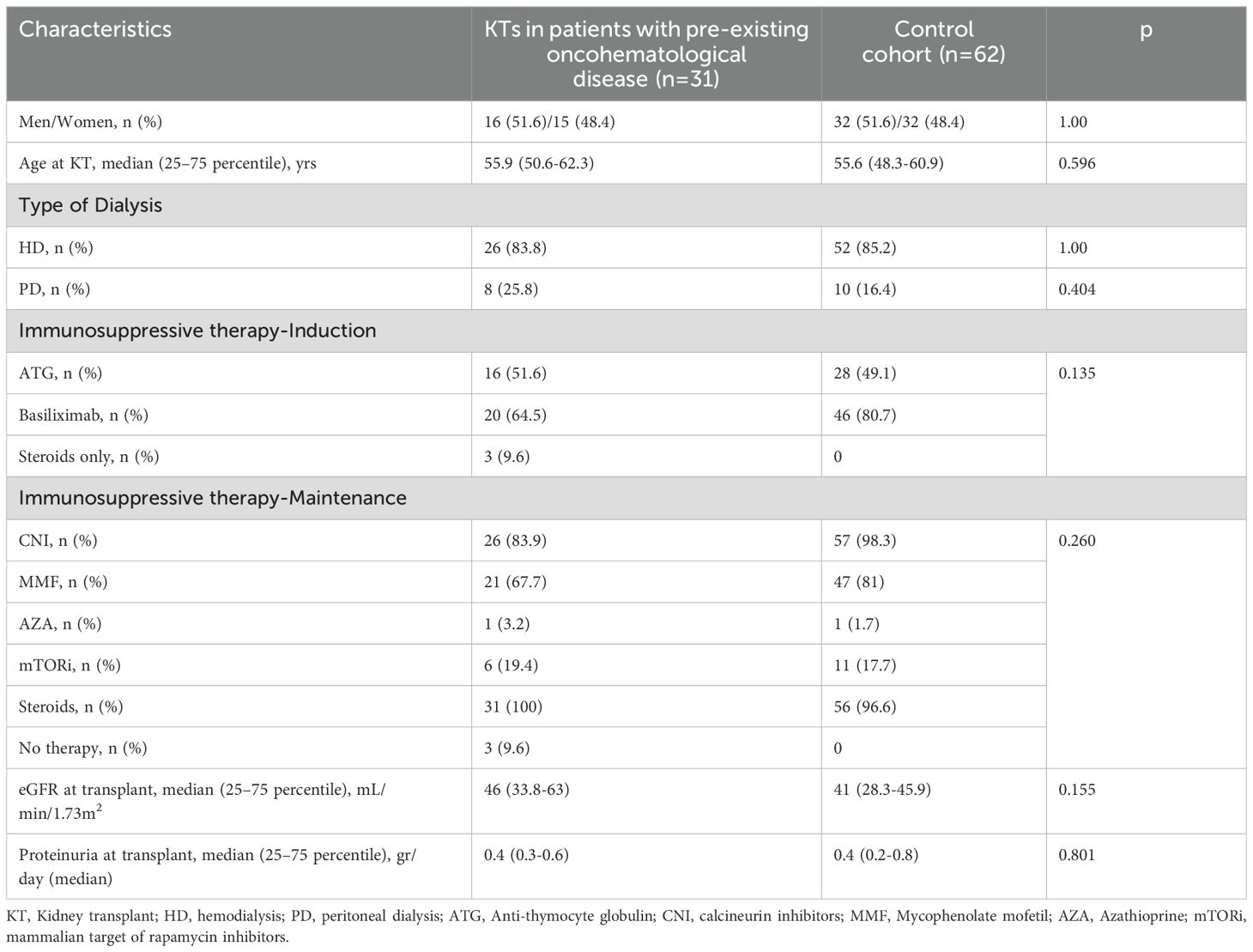
The control cohort includes 62 KTs performed at our Center between 2003 and 2023 (Figure 1). Regarding baseline characteristics, KTs in patients with a pre-existing oncohematological disease and the control group have similar M/F ratio, age at KT, percentage of patients on hemodialysis or peritoneal dialysis before KT, and immunosuppressive treatments for both induction and maintenance regimens (Table 1).
Kidney functional data were also similar between cohorts (Tables 1, 2). Patients were followed for a median time of 7.2 and 9.7 years, respectively. During this period, both exhibited similar renal function (Table 2) and comparable death-censored graft and patient survival rates (analyzed in patients at their first transplant; Figures 2 and 3).
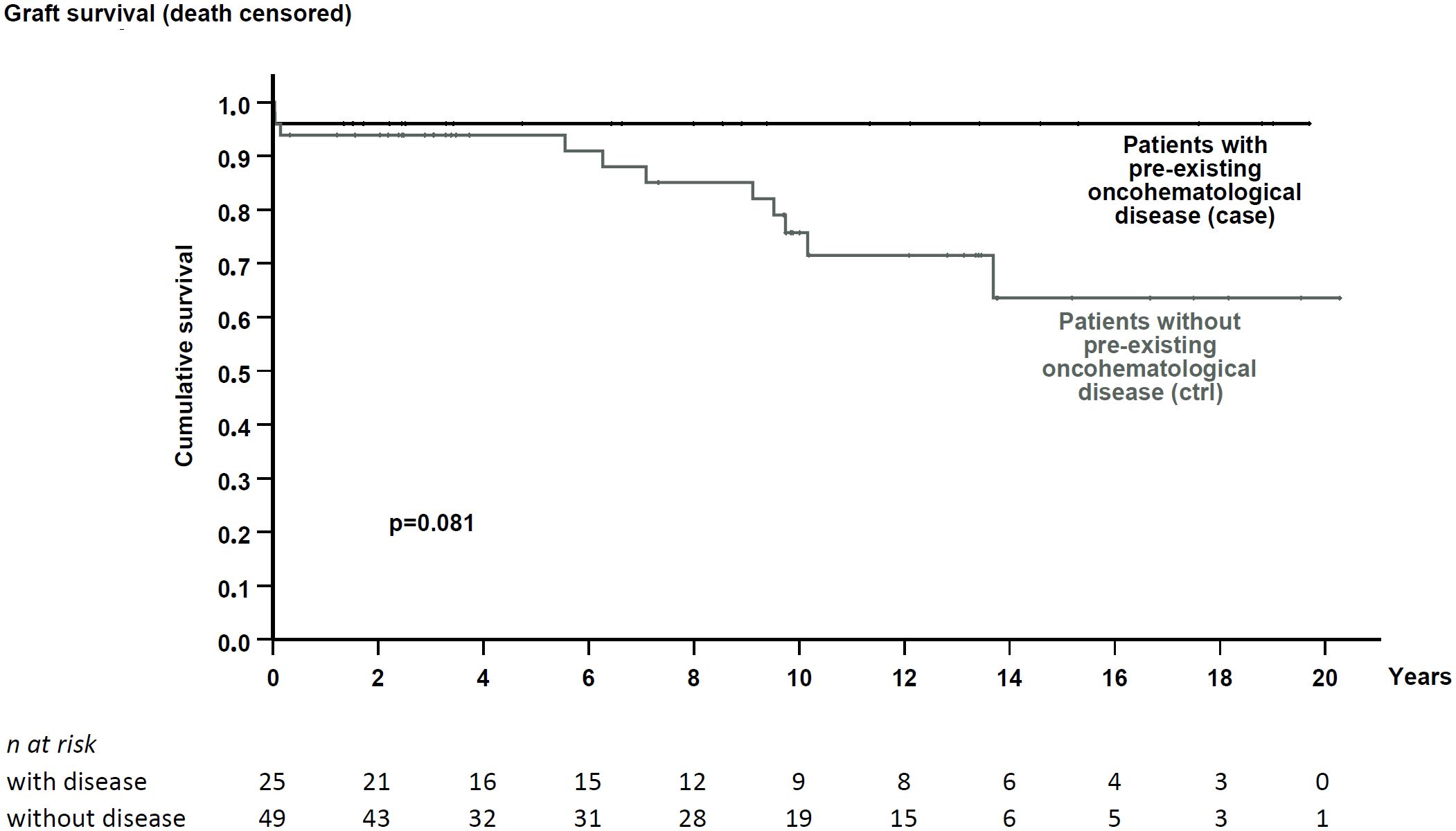
Figure 2. Death-censored graft survival in patients with pre-existing hematological disease and the matched control cohort. No significant difference in graft survival was noted (p = 0.081).

Figure 3. Patient survival from KTRs in patients with pre-existing hematological disease and the matched control cohort. No significant difference in survival was noted (p = 0.594).
Post-transplant neoplasia rates are also similar between groups (16.1% in the hematological cohort and 19.4% in the control cohort, p = 0.782); only two patients experienced hematological disease recurrence, specifically a light-chain deposition disease and a Castleman disease, which are discussed separately below.
Both cohorts experienced various infection episodes; however, despite no statistically significant difference between the two groups, the hematological cohort showed a reduced incidence rate of CMV infection compared to the controls (9.7% vs. 22.6%, p < 0.005).
Hematological and control cohorts showed similar rejection rates (9.7% vs. 8.1%, respectively; p = 0.732; details regarding rejection subtypes and BANFF scores are included in Supplementary Table S1 in the Supplementary Materials). Although not statistically significant, only 3 out of 31 individuals (9.7%) in the hematological cohort developed de novo anti-HLA donor-specific antibodies (DSAs), compared to 9 out of 62 in the control group (14.5%). Notably, none of the KTRs in the hematological cohort with positive DSA exhibited clinical symptoms of antibody-mediated rejection [one patient developed a suspicious AMR with glomerulitis, but the DSA was negative, and the condition resolved after therapy with intravenous immunoglobulin (Supplementary Table S1)]. The eGFR for each group of DSA-positive patients is included in Supplementary Table S2. As expected, patients in both groups experienced a progressive decline in eGFR from baseline (defined as the time of the first DSA detection), with no differences between the two cohorts.
3.2 Detailed analysis of the studied patients according to their pre-transplant hematological disease
Data about all studied patients with a history of pre-transplant hematological conditions are included in Table 3. Additional available information is contained in the Supplementary Material. Briefly, only two subjects experienced disease recurrence: one with light-chain deposition disease 14 months after KT, who was successfully treated with chemotherapy (bortezomib and dexamethasone), and one with Castleman disease four years after KT, despite treatment with chemotherapy (cyclophosphamide plus steroids), achieving a complete remission. Additionally, three patients received living transplantation from the same donor of the previous bone marrow transplant and were treated exclusively with steroids for induction and maintenance therapy (immunosuppressive therapy was definitively stopped within the first year). We therefore reevaluated our results, excluding these patients, to highlight the potential impact on outcomes based on their different immunosuppressive approaches. However, this analysis yielded superimposable results, with no differences in patient and graft survival (Supplementary Tables S3, S4 and Supplementary Figures S1, S2 in Supplementary Materials).
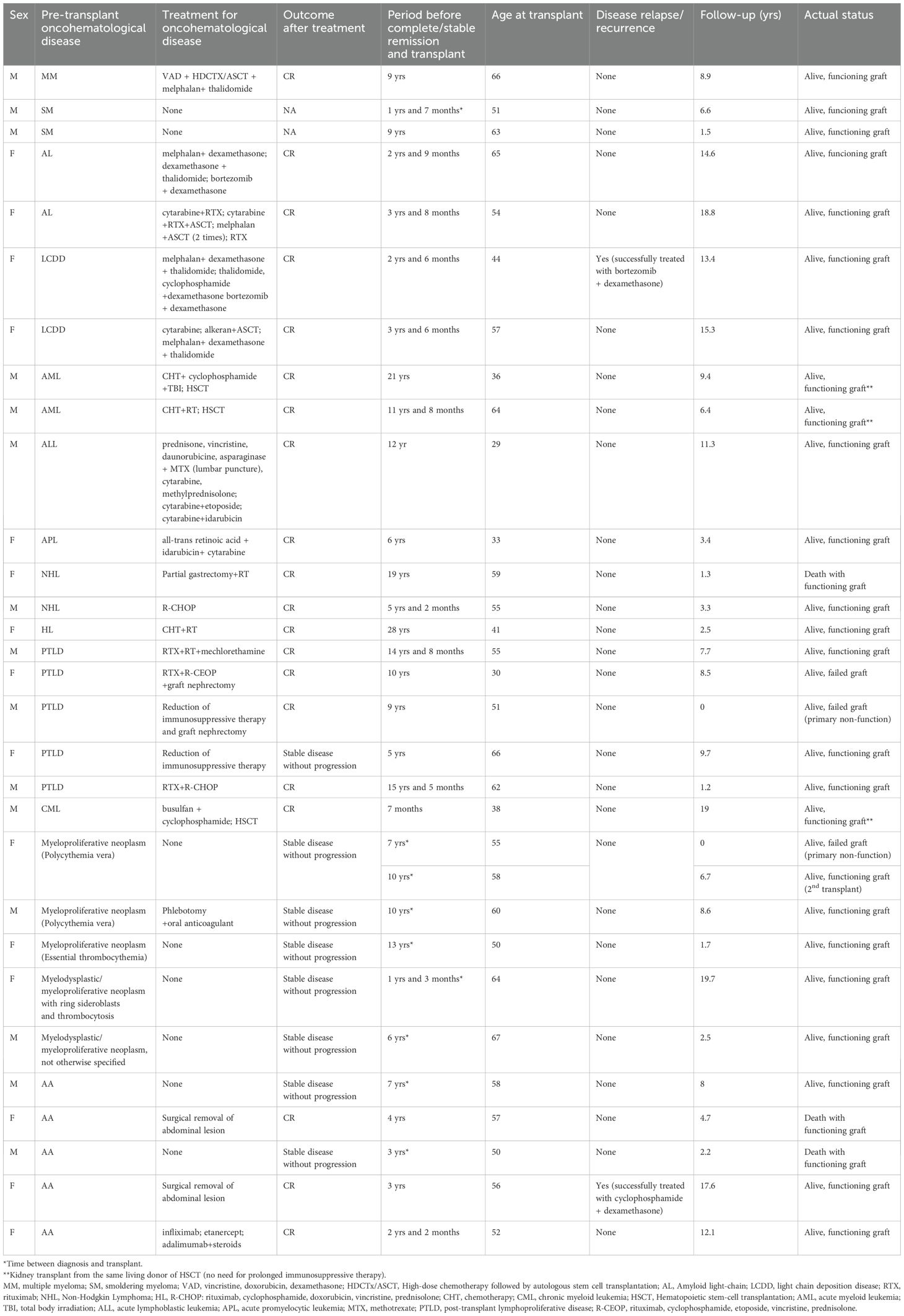
Table 3. Detailed description of patients with pre-transplant oncohematological disease and outcomes after kidney transplant.
4 Discussion
Oncohematological disorders include various diseases with different characteristics, ranging from benign to life-threatening. The incidence in the general population and the age at presentation of patients vary dramatically according to the type of disease (10).
For example, MM usually occurs in older adults (median age at diagnosis of 66 years with only 10% <50 years) and accounts for approximately 1% of malignant diseases and 10-13% of all hematologic malignancies (10–12); AL amyloidosis is an uncommon disorder (incidence of approximately 9 to 14 cases per million person-years in the United States) with a median age at diagnosis of 64 years and less than 5% of patients under the age of 40 (13, 14); leukemia and lymphoma can occur both in young adult and in older patients depending on the subtypes of disease (15).
According to these data, it is not surprising that, considering the prolonged life expectancy, all these disorders may represent a significant problem in pre-transplant evaluation.
In this context, several key questions need to be addressed: the risk of disease progression or recurrence after KT and the potential role of previous hematological disease in influencing patient and graft survival, including post-transplant complication rates and rejection risk. KDIGO 2020 (5), the most updated international guideline, underlines that “decisions about kidney transplantation in patients with a prior history of hematologic malignancy who are now in remission should be made in collaboration with a hematologist with transplant experience in determining transplant candidacy, since many lesions may be deemed to be at high risk of accelerated progression or transformation post-transplant.” This is unsurprising, considering that the available literature on these patients primarily derives from case reports or series, which are often limited to a specific condition or disease.
This paper reports our experience with all 30 KTRs with a pre-existing hematological disease who underwent 31 KTs between 2000 and 2023.
Few studies have specifically evaluated the risk of rejection in this population. An association between lenalidomide and increased allograft rejection due to direct immunomodulatory effect has been proposed in patients with MM concomitant with functioning KT (16). Still, no specific association between rejection and other previous or concomitant MM therapy has been reported. In Ruphael et al. (17), three out of 8 retransplanted PTLDs experienced acute or chronic rejection; this percentage is lower in Johnson et al. (18), where maintenance immunosuppression between the first and second transplant is almost superimposable. In other subsets, Leung et al. (19) reported acute rejections in 3 of 7 patients with a history of light chain disease. Despite these data being confirmed in a larger cohort, only three acute rejection episodes were recorded, and three patients developed de-novo DSAs without clinical signs of antibody-mediated rejection. As expressed by other authors (18), a tailored approach with specific attention to pre-transplant disease and patient characteristics allows us to maintain adequate immunosuppression, avoiding rejection while minimizing the risk of recurrence.
Considering recurrence rates, only two KTRs have a disease relapse of their pre-transplant hematological disease, one with LCDD and one with Castleman disease.
Aggressive disease management to achieve complete remission appears crucial in the pre-transplant context. A high rate of LCDD recurrence is reported in the literature. Leung et al. observed recurrent LCDD in 5 of 7 transplanted patients after a median time of 33.3 months (19). Various case reports confirm these findings (20–24). Our patient had undergone multiple subsequent chemotherapy treatments before KT for disease recurrences after achieving the first remission, and the transplant was performed three years later. Despite this approach, one year after KT, a significant increase in serum light chain was noted, associated with a slight rise in serum creatinine. The patient was treated with steroids and bortezomib with a partial response; subsequent new serum light chains increased, requiring other lines of therapy, finally achieving a complete remission with no other evidence of recurrence within the following four years.
The second recurrence was observed in a patient with Castleman disease who underwent KT 3 years after achieving complete remission. The event occurred four years after the transplant as a multicentric presentation. It was treated with steroids and cyclophosphamide with full recovery (no evidence of hematological relapse at the last visit, eight years after recurrence). Limited data are available in the Literature for KT with a pre-existent Castleman disease: Murakami reported a good post-transplant clinical course (follow-up eight years) in a patient with pre-transplant multicentric Castleman disease (25), while Yousif described a case of monocentric Castleman disease incidentally diagnosed during KT with normal graft function and no disease recurrence during the follow-up (26).
Regarding the other oncohematological diseases, although MM has been considered for a long time as a contraindication for KT due to the increased risk of graft failure, severe and life-threatening infections, and recurrence (19, 24, 27–29) nowadays, literature reports favorable results in patients who achieved disease remission (30, 31), primarily when KT is performed from the same donor of bone marrow transplantation (32–36) despite, as also specified in KDIGO (5), no indication about the wait time between remission and transplantation is even available.
In the case of smoldering myeloma, guidelines now suggest not excluding candidates from kidney transplantation, despite a significant risk of transformation into multiple myeloma (not precisely quantifiable), should be considered and appropriately discussed (5). Despite having a limited number of patients and not being treated with recently available drugs that have further modified the approach to the disease, even hypothesizing a potential transplant in patients under chemotherapy in stable disease, the results in our patients confirm all these findings.
Furthermore, similarly to Literature data (37, 38), in our study, no patient with a pre-transplant history of leukemia or lymphoma/PTLD had disease recurrence after transplant. In three patients who had received KT from the same living donor after a previous bone marrow transplant, it was also possible to definitively stop immunosuppressive therapies, resulting in a remarkable overall patient and graft survival. The feasibility of re-transplantation in patients with previous post-transplant lymphoproliferative disorders is described in the literature, with favorable clinical outcomes and no disease recurrence (17, 18, 39–42). This is also evident in our KTRs, where no recurrence was observed in any of the four patients. International guidelines depicted a variegated approach in these patients: KDIGO (5) remarks to avoid transplanting patients with leukemia or lymphoma until they have received curative therapy, achieved remission, and remained cancer-free for a period to be determined in consultation with the patient, a hematologist/oncologist, and the transplant program; in contrast, other guidelines suggest a definite period (2 years for Canadian Society of Transplantation [1], Kidney Health Australia-Caring for Australasians with Renal Impairment [2] and American Society of Transplantation guidelines [3] and 1–3 years for European Renal Best Practice [4]) before a patient could be considered for KT. Considering that some drugs (e.g., tyrosine kinase inhibitors) have completely transformed the life expectancy of these patients despite maintenance therapy (43), this approach may also change shortly.
The need for a dedicated and expert hematological consultation is even more critical in patients with a history of myelodysplastic or myelodysplastic/myeloproliferative neoplasms, for which recommendations are significantly lacking, and most reported cases included patients with chronic myeloid leukemia treated with imatinib (43, 44). None of the patients in both groups, which is a remarkable cohort based on scarce literature data, developed disease relapse or recurrence after KT.
We are also aware that some studies have shown unsatisfactory results in patients with a history of pre-transplant malignancies. The analysis of the UNOS database in 2019 by Livingston-Rosanoff et al. (45) reported that pre-transplant malignancies are progressively increasing in number across the US, but it is associated with an increased risk of post-transplant malignancies, graft loss, and decreased overall survival. Non-melanoma skin cancer was the most common diagnosis for patients with and without pre-transplant malignancies (66.2% vs 57.1%), followed by lung cancer (5.2% vs 6.6%). The post-transplant malignancies of 228 individuals with pre-transplant malignancies were classified as recurrences of their original pre-transplant malignancies by UNOS, representing a 2% recurrence rate in patients with pre-transplant malignancies. Of the patients who experienced recurrence, the majority (48%) were solid organ cancers, followed by non-melanoma skin cancer (21%), unknown (12%), and melanomas (7%). Hematopoietic recurrences are described, although they are relatively uncommon (12%), and are primarily associated with leukemia, lymphomas, and other myelodysplastic disorders. The authors emphasized in their conclusions the importance of collaborative database development between transplant and cancer registries to better define the interrelationship between pre-transplantation and cancer survivorship versus freedom from prolonged dialysis, an issue that is currently underestimated (46).
In the German paired analysis by Becker et al. (47), KT recipients with a history of pre-transplant malignancy had lower five-year death-censored as well as overall graft survival. Cox proportional hazard modeling showed a correlation between pre-transplant malignancy and inferior graft survival; however, among the 65 KT recipients studied, only one patient had a hematologic malignancy.
Serkies et al. (48) recently proposed a review and discussion of malignancies in adult kidney transplant candidates and recipients updated to 2023. Albeit the primary focus are solid neoplasia, they also reported that, based on all available data, along with changing patient characteristics and the availability of newer cancer therapies, a shorter waiting time to determine suitability for transplant in pre-transplant malignancies patients could be appropriate for cancers with substantially improved survival in the general population, including multiple myeloma cases with a complete remission after successful treatment with preconditioning chemotherapy followed by high-dose alkylating agents and autologous stem cell transplant. They also clearly stated that transplant suitability and waiting time for candidates with cancer should be individualized, with the decision to consider transplantation made by a multidisciplinary team involving oncologists/hematologists, transplant nephrologists, patients, and their caregivers. Expected survival and quality of life on dialysis versus transplantation, projected cancer recurrence risk, including the effect of administered immunosuppression, estimated survival depending on tumor type, and, given current treatment possibilities, if recurrence post-transplant occurred, should be considered. Of note, prolonged dialysis is associated with an increased risk of complications, including malignancies and death.
We suggest that our analysis, focuses on a specific settings with poor literature data and many diseases with uncommon incidence and recent reclassification, offers the opportunity to improve the available information stressing the importance of an appropriate and in-depth evaluation of these patients that are at high risk to be excluded for transplant, and at the same time that the multidisciplinary approach with hematologist trained in these condition (as also expressed by the interntational guidelines) is crucial.
Our study has several limitations, including the relatively small sample size and the lack of routine protocol biopsies, which may have underestimated graft damage due to disease relapse in some conditions, particularly in the early stages. On the other hand, to the best of our knowledge, this is the first experience that analyzed characteristics and outcomes of patients with pre-existing oncohematological diseases in comparison to a control cohort with similar features and post-transplant management, showing positive results (especially regarding rejection risk and disease recurrence) and no significant differences in clinical outcomes. These positive results may be partially derived from a homogeneous, tailored, and multi-disciplinary management, with particular attention to the immunosuppressive therapy, avoiding the risk of an excessive “pressure” in patients with potential risk of relapse/recurrence for one side and an excessive underimmunosuppression for the other; the difference in CMV prevalence vs. the control cohort matched with a very low-incidence of acute rejection and de-novo DSA corroborated this strategy.
In conclusion, based on our findings and Literature data, we suggest that patients with pre-existing oncohematological disorders should not be denied KT after a well-documented stable disease. In this context, a multidisciplinary approach is crucial for establishing standardized pre- and post-transplant monitoring protocols and achieving optimal graft and patient outcomes.
Extensive registry-based studies are needed to support our findings, especially for rare conditions such as MPNs, MDS/MPN, and amyloidosis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Comitato Etico Interaziendale A.O.U. Città Della Salute e Della Scienza di Torino - A.O. Ordine Mauriziano - A.S.L. Città di Torino. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AM: Conceptualization, Formal analysis, Funding acquisition, Methodology, Visualization, Writing – original draft, Writing – review & editing. RC: Formal analysis, Methodology, Writing – original draft. VD: Writing – review & editing. RG: Writing – review & editing. GG: Writing – review & editing. EG: Writing – review & editing. CD: Writing – review & editing. AL: Writing – review & editing. AM: Writing – review & editing. FF: Data curation, Visualization, Writing – review & editing. AA: Writing – review & editing. FC: Writing – review & editing. SB: Writing – review & editing. DF: Writing – review & editing. RM: Writing – review & editing. CT: Supervision, Writing – review & editing. BB: Supervision, Writing – review & editing. FM: Supervision, Writing – review & editing. LB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was funded by the “TGT study” (University of Turin, Department of Medical Sciences) and the “CRT Foundation” grants to LB, as well as the PRIN 2022 grant (code 2022LTXXRP) from the Italian Ministry of University and Research to AM. The funder had no role in the design, data collection, data analysis, and reporting of this study.
Acknowledgments
The authors thank Luca Besso, MD (Clinical Department of Nephrology Santa Croce e Carle Cuneo Hospital Cuneo Italy); Stefano Maffei, MD (Unit of Nephrology and Dialysis, Cardinal Massaia Hospital, Asti, Italy); Natalia Rossi, MD (Unit of Nephrology and Dialysis, Casale Monferrato and Novi Ligure Hospitals, Alessandria, Italy); Colombano Salvatore Martino Sacco, MD (Nephrology and Dialysis Unit, ASL Biella, Biella, Italy); Marita Marengo, MD (Unit of Nephrology and Dialysis, Savigliano, Mondovi’ and Ceva Hospitals, Cuneo, Italy); Loris Neri, MD (Department of Nephrology and Dialysis, “Michele E Pietro Ferrero” Hospital-ASLCN2); Giulio Cesano, MD (Nephrology and Dialysis, Martini Hospital, ASL Città di Torino, Turin, Italy); Dario Roccatello, MD, Prof. (Nephrology and Dialysis Unit, San Giovanni Bosco Hub Hospital, Turin, Italy); Giuliana Tognarelli, MD (Unit of Dialysis, San Luigi Hospital, Orbassano, Turin, Italy); Gianluca Leonardi, MD (Unit of Nephrology and Dialysis, Chieri and Moncalieri Hospitals, ASL TO5, Italy); and Massimo Manes, MD (Unit of Nephrology and Dialysis, Umberto Parini Hospital, Aosta, Italy) for their expert support on the clinical management and patients’ follow-up.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Author Contributions
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1629521/full#supplementary-material
References
1. Knoll G, Cockfield S, Blydt-Hansen T, Baran D, Kiberd B, Landsberg D, et al. Canadian Society of Transplantation: consensus guidelines on eligibility for kidney transplantation. CMAJ. (2005) 173:S1–S25. doi: 10.1503/cmaj.1041588
2. Campbell S, Pilmore H, Gracey D, Mulley W, Russell C, and McTaggart S. KHA-cari Guideline: Recipient assessment for transplantation. Nephrology. (2013) 18:455–62. doi: 10.1111/nep.12068
3. Kasiske BL, Ramos EL, Gaston RS, Bia MJ, Danovitch GM, Bowen PA, et al. The evaluation of the renal transplant candidates: clinical practice guidelines. Am J Transplant. (2001) 1:1–95. doi: 10.1034/J.1600-6143.2001.0010S2001.X
4. Abramowicz D, Cochat P, Claas FHJ, Heemann U, Pascual J, Dudley C, et al. European Renal Best Practice Guideline on kidney donor and recipient evaluation and perioperative care. Nephrol Dialysis Transplant. (2015) 30:1790–7. doi: 10.1093/ndt/gfu216
5. Chadban SJ, Ahn C, Axelrod DA, Foster BJ, Kasiske BL, Kher V, et al. KDIGO clinical practice guideline on the evaluation and management of candidates for kidney transplantation. Transplantation. (2020) 104:S11–S103. doi: 10.1097/TP.0000000000003136
6. Clari R, Tarella C, Giraudi R, Torazza MC, Gallo E, Lavacca A, et al. Monoclonal gammopathy of undetermined significance coexisting in patients undergoing kidney transplantation does not adversely influence post-graft clinical outcome. Clin Kidney J. (2021) 14:317–24. doi: 10.1093/ckj/sfaa105
7. Prakash S and Orazi A. Diagnostic approach to myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms. Adv Anat Pathol. (2025) 32:284–98. doi: 10.1097/PAP.0000000000000493
8. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IB de O, Berti E, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. (2022) 36:1720–48. doi: 10.1038/s41375-022-01620-2
9. Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. (2022) 36:1703–19. doi: 10.1038/s41375-022-01613-1
10. Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O, et al. Incidence of hematologic Malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. (2010) 116:3724–34. doi: 10.1182/BLOOD-2010-05-282632
11. Phekoo KJ, Schey SA, Richards MA, Bevan DH, Bell S, Gillett D, et al. A population study to define the incidence and survival of multiple myeloma in a National Health Service Region in UK. Br J Haematol. (2004) 127:299–304. doi: 10.1111/j.1365-2141.2004.05207.x
12. Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, and Melton LJ. Incidence of multiple myeloma in Olmsted County, Minnesota: Trend over 6 decades. Cancer. (2004) 101:2667–74. doi: 10.1002/cncr.20652
13. Quock TP, Yan T, Chang E, Guthrie S, and Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. (2018) 2:1046–53. doi: 10.1182/bloodadvances.2018016402
14. Shimazaki C, Hata H, Iida S, Ueda M, Katoh N, Sekijima Y, et al. Nationwide survey of 741 patients with systemic amyloid light-chain amyloidosis in Japan. Internal Med. (2018) 57:181–7. doi: 10.2169/internalmedicine.9206-17
15. Evens AM, Antillón M, Aschebrook-Kilfoy B, and Chiu BCH. Racial disparities in Hodgkin’s lymphoma: A comprehensive population-based analysis. Ann Oncol. (2012) 23:2128–37. doi: 10.1093/annonc/mdr578
16. Lum EL and Bunnapradist S. Current opinions in nephrology and hypertension: Kidney transplantation in patients with plasma cell dyscrasias. Curr Opin Nephrol Hypertens. (2019) 28:573–80. doi: 10.1097/MNH.0000000000000544
17. Rouphael B, Lankireddy S, Lazaryan A, Kukla A, Ibrahim HN, Matas AJ, et al. Outcomes of kidney retransplantation in recipients with prior post-transplant lymphoproliferative disorder. Clin Transplant. (2016) 30:60–5. doi: 10.1111/ctr.12659
18. Johnson SR, Cherikh WS, Kauffman HM, Pavlakis M, and Hanto DW. Retransplantation after post-transplant lymphoproliferative disorders: An OPTN/UNOS database analysis. Am J Transplant. (2006) 6:2743–9. doi: 10.1111/j.1600-6143.2006.01543.x
19. Leung N, Lager DJ, Gertz MA, Wilson K, Kanakiriya S, and Fervenza FC. Long-term outcome of renal transplantation in light-chain deposition disease. Am J Kidney Dis. (2004) 43:147–53. doi: 10.1053/j.ajkd.2003.09.020
20. Angioi A, Amer H, Fervenza FC, and Sethi S. Recurrent light chain proximal tubulopathy in a kidney allograft. Am J Kidney Dis. (2016) 68:483–7. doi: 10.1053/j.ajkd.2016.04.021
21. Horike K, Takeda A, Otsuka Y, Inaguma D, Goto N, Watarai Y, et al. A case of recurrent light chain deposition disease after living-related renal transplantation - detailed process of the recurrence. Clin Transplant. (2012) 26:64–9. doi: 10.1111/j.1399-0012.2012.01674.x
22. Kuypers DRJ, Lerut E, Claes K, Evenepoel P, and Vanrenterghem Y. Recurrence of light chain deposit disease after renal allograft transplantation: Potential role of rituximab? Transplant Int. (2007) 20:381–5. doi: 10.1111/j.1432-2277.2006.00437.x
23. Alchi B, Nishi S, Iguchi S, Shimotori M, Sakatsume M, Ueno M, et al. Recurrent light and heavy chain deposition disease after renal transplantation. Nephrology Dialysis Transplant. (2005) 20:1487–91. doi: 10.1093/ndt/gfh822
24. Short AK, O’Donoghue DJ, Riad HN, Short CD, and Roberts ISD. Recurrence of light chain nephropathy in a renal allograft : A case report and review of the literature. Am J Nephrol. (2001) 21:237–40. doi: 10.1159/000046254
25. Murakami K, Kobayashi T, Okubo K, Kamba T, Yoshimura K, and Ogawa O. Successful renal transplantation for end-stage renal insufficiency developed in a patient with Castleman’s disease. Transplant Int. (2013) 26:e61–2. doi: 10.1111/tri.12099
26. Yousif M, Hassan A, and Abdulrahim A. Case report: castleman’s disease in a kidney failure patient diagnosed incidentally during transplantation. Arab J Nephrol Transplant. (2011) 4:6–8. doi: 10.4314/ajnt.v4i1.63153
27. Taheri D, Suzangar H, Heidari F, Feshrakizadeh M, Suzangar M, and Dolatkhah S. Skull mass as the first manifestation of recurrent multiple myeloma in a renal transplant patient. J Pak Med Assoc. (2012) 62:S76–8.
28. Taheri D, Chehrei A, Fesharakizadeh M, Seyrafean S, Shahidi S, Emami A, et al. Recurrent multiple myeloma following renal transplantation: A case report. Transplant Proc. (2007) 39:1063–5. doi: 10.1016/j.transproceed.2007.02.015
29. Herzenberg AM, Kiaii M, and Magil AB. Heavy chain deposition disease: recurrence in a renal transplant and report of IgG(2) subtype. Am J Kidney Dis. (2000) 35:E25. doi: 10.1053/kd.2000.6429
30. Bansal T, Garg A, Snowden JA, and McKane W. Defining the role of renal transplantation in the modern management of multiple myeloma and other plasma cell dyscrasias. Nephron Clin Pract. (2012) 120:c228–35. doi: 10.1159/000341760
31. Dinh AR, Wong SW, Martin TG, Wolf JL, and Webber AB. Outcomes of kidney transplant recipients with ESKD due to plasma cell dyscrasia: A case series. Clin Transplant. (2022) 36:e14541. doi: 10.1111/ctr.14541
32. Nayak L and Lazarus HM. Renal allografts in plasma cell myeloma hematopoietic cell graft recipients: On the verge of an explosion? Bone Marrow Transplant. (2013) 48:338–45. doi: 10.1038/bmt.2012.111
33. Baraldi O, Grandinetti V, Donati G, Comai G, Battaglino G, Cuna V, et al. Hematopoietic cell and renal transplantation in plasma cell dyscrasia patients. Cell Transplant. (2016) 25:995–1005. doi: 10.3727/096368915X688560
34. Khoriaty R, Otrock ZK, Medawar WA, Khauli RB, and Bazarbachi A. A case of successful double sequential bone marrow and kidney transplantations in a patient with multiple myeloma. Nephrol Dialysis Transplant. (2006) 21:3585–8. doi: 10.1093/ndt/gfl403
35. Wagner L, Lengyel L, Mikala G, Reményi P, Piros L, Csomor J, et al. Successful treatment of renal failure caused by multiple myeloma with HLA-identical living kidney and bone marrow transplantation: A case report. Transplant Proc. (2013) 45:3705–7. doi: 10.1016/j.transproceed.2013.10.005
36. Spitzer TR, Sykes M, Tolkoff-Rubin N, Kawai T, McAfee SL, Dey BR, et al. Long-term follow-up of recipients of combined human leukocyte antigen-matched bone marrow and kidney transplantation for multiple myeloma with end-stage renal disease. Transplantation. (2011) 91:672–6. doi: 10.1097/TP.0b013e31820a3068
37. Koenecke C, Hertenstein B, Schetelig J, Van Biezen A, Dammann E, Gratwohl A, et al. Solid organ transplantation after allogeneic hematopoietic stem cell transplantation: A retrospective, multicenter study of the EBMT. Am J Transplant. (2010) 10:1897–906. doi: 10.1111/j.1600-6143.2010.03187.x
38. Younge J, Duffner UA, Bunchman T, and Abdel-Mageed A. Ten year follow-up for a patient postmatched unrelated donor bone marrow transplant followed by same donor kidney transplant: normal renal function without immunosuppression. Transplantation. (2015) 99:e162. doi: 10.1097/TP.0000000000000852
39. Demircin G and Rees L. Retransplantation after post-transplant lymphoproliferative disease. Pediatr Nephrol. (1997) 11:358–60. Available online at: http://www.ncbi.nlm.nih.gov/pubmed/9203193.
40. Birkeland SA, Hamilton-Dutoit S, and Bendtzen K. Long-term follow-up of kidney transplant patients with posttransplant lymphoproliferative disorder: Duration of posttransplant lymphoproliferative disorder-induced operational graft tolerance, interleukin-18 course, and results of retransplantation. Transplantation. (2003) 76:153–8. doi: 10.1097/01.TP.0000072015.08302.E9
41. Karras A, Thervet E, Le Meur Y, Baudet-Bonneville V, Kessler M, and Legendre C. Successful renal retransplantation after post-transplant lymphoproliferative disease. Am J Transplant. (2004) 4:1904–9. doi: 10.1111/j.1600-6143.2004.00562.x
42. Pinlac V and Bonifacio L. A case report of kidney transplantation in a patient with pre-existing chronic myeloid leukemia: the role of achieving molecular response and treatment-free remission. Transplant Proc. (2024) 56:738–41. doi: 10.1016/j.transproceed.2024.03.004
43. Thiem U, Buxhofer-Ausch V, Kranewitter W, Webersinke G, Enkner W, and Cejka D. Successful kidney transplantation in a patient with pre-existing chronic myeloid leukemia treated with imatinib. Am J Transplant. (2021) 21:405–9. doi: 10.1111/ajt.16194
44. Tokumoto T, Setoguchi K, Osaka A, Ikezoe E, Tsujioka H, Hasegawa K, et al. A case report of successful kidney transplantation in a patient with chronic myelogenous leukemia (CML) who has been in remission for 15 years on imatinib. Transplant Proc. (2023) 55:1074–7. doi: 10.1016/j.transproceed.2023.03.058
45. Livingston-Rosanoff D, Foley DP, Leverson G, and Wilke LG. Impact of pre-transplant Malignancy on outcomes after kidney transplantation: united network for organ sharing database analysis. J Am Coll Surgeons. (2019) 229:568–79. doi: 10.1016/j.jamcollsurg.2019.06.001
46. Mella A, Calvetti R, Barreca A, Congiu G, and Biancone L. Kidney transplants from elderly donors: what we have learned 20 years after the Crystal City consensus criteria meeting. J Nephrol. (2024) 37:1449–61. doi: 10.1007/s40620-024-01888-w
47. Becker F, Mehdorn AS, Getsopulos V, Schütte-Nütgen K, Reuter S, Suwelack B, et al. Tumor recurrence and graft survival in renal transplant recipients with a history of pretransplant Malignancy: A matched pair analysis. J Clin Med. (2021) 10:2349. doi: 10.3390/jcm10112349
Keywords: oncohematological diseases, kidney transplant, graft survival, clinical outcomes, acute rejection
Citation: Mella A, Clari R, Deiana V, Giraudi R, Giovinazzo G, Gallo E, Dolla C, Lavacca A, Manzione AM, Fop F, Allesina A, Cavallo F, Bringhen S, Ferrero D, Mina R, Tarella C, Bruno B, Mariano F and Biancone L (2025) Pre-existing oncohematological disease in kidney transplant recipients: impact on graft survival, acute rejection, and long-term clinical outcomes. Front. Immunol. 16:1629521. doi: 10.3389/fimmu.2025.1629521
Received: 15 May 2025; Accepted: 21 July 2025;
Published: 06 August 2025.
Edited by:
Dae-Hyun Ko, University of Ulsan College of Medicine, Republic of KoreaReviewed by:
John Jeongseok Yang, Sungkyunkwan University, Republic of KoreaSoo-Kyung Kim, Ewha Womans University School of Medicine, Republic of Korea
Copyright © 2025 Mella, Clari, Deiana, Giraudi, Giovinazzo, Gallo, Dolla, Lavacca, Manzione, Fop, Allesina, Cavallo, Bringhen, Ferrero, Mina, Tarella, Bruno, Mariano and Biancone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luigi Biancone, bHVpZ2kuYmlhbmNvbmVAdW5pdG8uaXQ=
†ORCID: Luigi Biancone, orcid.org/0000-0002-7700-6350
 Alberto Mella
Alberto Mella Roberta Clari1,3
Roberta Clari1,3 Filippo Mariano
Filippo Mariano Luigi Biancone
Luigi Biancone