- 1Department of Oncology, The Second People’s Hospital of Guiyang, Guiyang, China
- 2Department of Intensive Care Unit, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Sun Yat-sen University, Guangzhou, China
- 3Department of Oncology, Guizhou Provincial People’s Hospital, Guiyang, China
- 4Cordeliers Research Center, Sorbonne Université, Inserm, Université Paris Cité, Paris, France
- 5Department of Neurology, Zhenyuan County Hospital, Zhenyuan, China
- 6Department of Neurosurgery, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Sun Yat-sen University, Guangzhou, China
Background: Primary central nervous system (CNS) germ cell tumors (GCTs) are common neoplasms in the CNS of pediatric and adolescent patients. This study aimed to identify prognostic factors associated with CNS GCTs and establish an effective nomogram for predicting overall survival (OS) in patients with CNS GCTs.
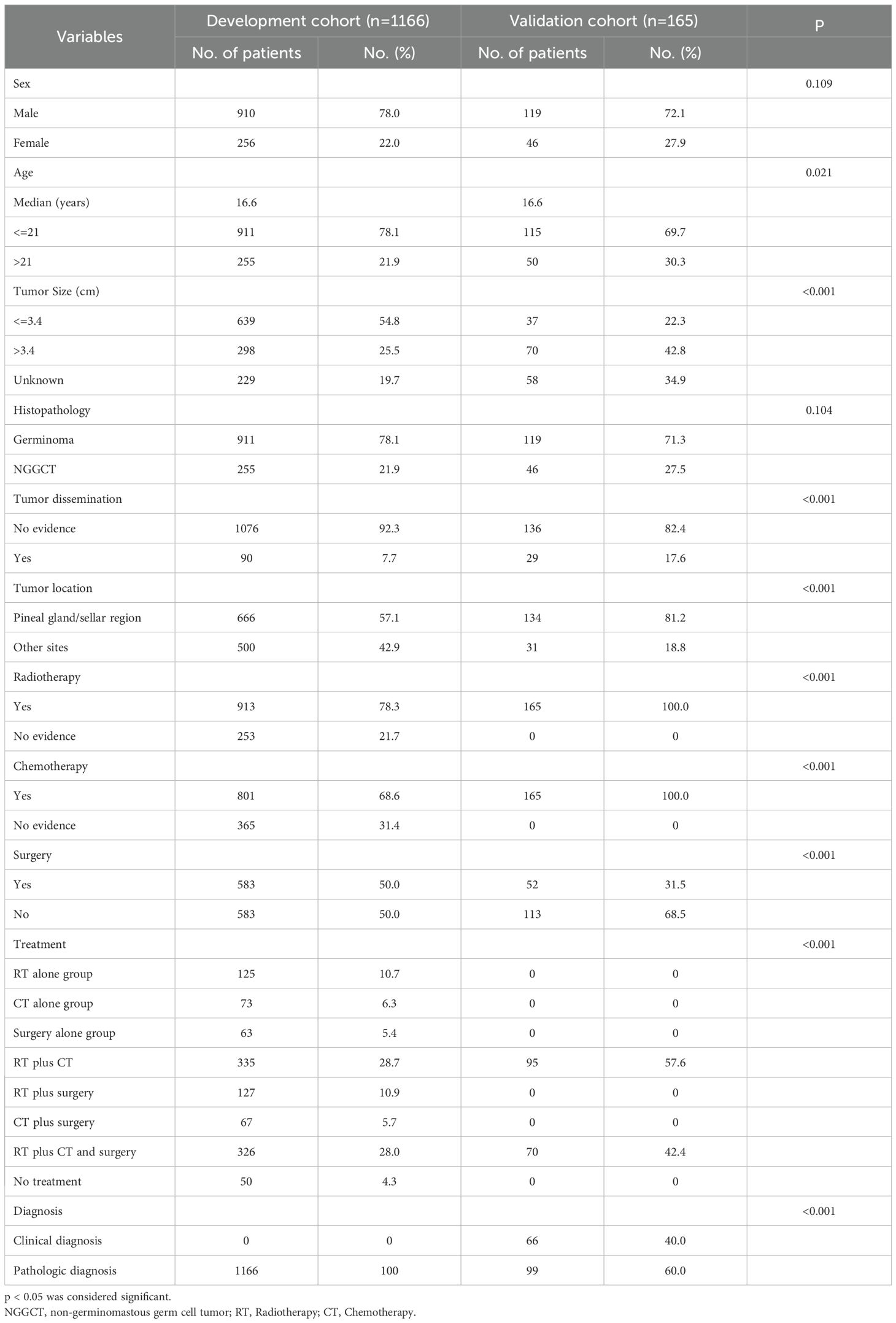
Methods: The development cohort including 1166 CNS GCTs patients was selected from Surveillance, Epidemiology, and End Results (SEER) program between 2000 and 2021. An additional 165 CNS GCTs patients treated at the Sun Yat-sen University Cancer Center (SYSUCC) between 1997 and 2019 were included as validation cohort.
Results: The nomogram incorporated the variables screened by multivariate Cox regression analysis, which included age, sex, histopathology, dissemination, tumor size, radiotherapy, and chemotherapy. The model demonstrated good discriminative performance, with C-index of 0.773 (95% CI, 0.734 - 0.812) and 0.712 (95% CI, 0.599- 0.825) in the development and validation cohorts, respectively. Calibration curves and area under the time-dependent receiver operating characteristic curve (time-dependent AUC) verified the superiority of our nomogram for clinical usefulness. Decision curve analysis (DCA) further illustrated the potential clinical value of the nomogram for treatment decision-making. Additionally, we established a comprehensive risk grouping system that effectively categorized patients into distinct prognostic groups based on their predicted outcomes.
Conclusion: A precise prognostic nomogram was developed for patients with CNS GCTs, utilizing seven independent prognostic factors. It demonstrated satisfactory performance and clinical usability, aiding clinicians in accurately estimating prognosis and guiding the treatment and long-term management of patients with CNS GCTs.
1 Introduction
Primary central nervous system (CNS) germ cell tumors (GCTs) are prevalent neoplasms in the pediatric and adolescent central nervous system, representing about 3% of malignant brain tumors in Western countries (1, 2). In contrast, in Asia, particularly in countries such as South Korea and Japan, the incidence ranges from 9% to 15% (3, 4). CNS GCTs are categorized as germinomas or non-germinomatous germ cell tumors (NGGCTs) according to their specific pathological characteristics. The NGGCTs category encompasses teratoma, embryonal carcinoma, endodermal sinus tumor, choriocarcinoma, mixed germ cell tumor, as well as other tumor varieties. Currently, radiotherapy is widely acknowledged for its effectiveness in treating CNS GCTs. With the exception of teratomas, the primary treatment for CNS GCTs involves a combination of chemotherapy along with either whole-brain irradiation (WBI) or craniospinal irradiation (CSI) (5). The rarity of CNS GCTs poses challenges in conducting extensive prospective and retrospective studies, leading to ongoing debates on distinct prognostic factors, such as surgery, tumor dissemination, and sex, and the lack of established models for accurate long-term survival predictions in CNS GCTs. Hence, conducting further research on prognostic factors of CNS GCTs and developing a reliable prognostic model become imperative in order to predict long-term survival outcomes and facilitate individualized treatment and clinical decision-making.
The nomogram was first introduced by Kattan et al. from Memorial Sloan-Kettering Cancer Center in 1998 for predicting the recurrence rate of prostate cancer post-radical resection (6). In contrast to the conventional staging system, the nomogram can incorporate a wider range of prognostic factors to enable personalized predictions. It offers improved predictive accuracy, discrimination, and user-friendliness, making it a more beneficial and convenient tool for predicting survival and stratifying risks. Presently, the nomogram is increasingly employed in predicting the prognosis of different types of tumors (7–13).
In this study, we aimed to identify prognostic factors for CNS GCTs and construct a prognostic nomogram for predicting treatment outcomes based on the Surveillance, Epidemiology, and End Result (SEER) database. Additionally, the nomogram was validated using Sun Yat-sen University Cancer Center (SYSUCC) database.
2 Materials and methods
2.1 Patient and study variable
The data of CNS GCTs patients from the SEER database (https://seer.cancer.gov/data-software/) were divided into the development cohort. The data were extracted using the following specifications: “Incidence -SEER Research Data,17 Registries, Nov 2023 Sub (2000-2021). The inclusion criteria for patient selection were: (1) histologically proven diagnosis of CNS GCTs; (2) ICD-O-3 codes for histopathology from the 3rd edition International Classification of Diseases for Oncology (ICD-O-3) were utilized to identify cases of CNS GCTs, with the specific codes as follows: 9060/3: Dysgerminomas, 9061/3: Seminoma, NOS, 9062/3: Seminoma, anaplastic, 9064/3: Germinomas, 9065/3: Germ cell tumor, nonseminomatous, 9070/3: Embryonal carcinoma, NOS, 9071/3: Yolk sac tumor, 9080/3: Teratoma, malignant, NOS, 9081/3: Teratocarcinoma, 9082/3: Malignant teratoma, undifferentiated, 9084/3: Teratoma with malignant transformation, 9085/3: Mixed germ cell tumor; (3) Among these codes, 9060/3, 9061/3, 9062/3, and 9064/3 were classified as germinomas, whereas 9065/3, 9070/3, 9071/3, 9080/3, 9081/3, 9082/3, 9084/3, and 9085/3 were classified as NGGCTs; (4) documented survival status and survival time.
To validate the nomogram, we retrieved data from the SYSUCC database for patients diagnosed with CNS GCTs between January 1997 and January 2019, who were assigned to the validation cohort. The inclusion criteria for case selection were: (1) histologically or clinically confirmed diagnosis of CNS GCTs; (2) availability of detailed clinicopathological profiles, complete treatment records, and comprehensive long-term follow-up information. All histopathological diagnoses in this study were verified by neuropathologists at Sun Yat-sen University Cancer Center. The clinical diagnostic criteria utilized in this study are as follows: (1) Typical imaging features along with serum/cerebrospinal fluid levels of AFP (alpha-fetoprotein) ≤ 25 ng/mL and/or HCG(human chorionic gonadotropin) ≤ 50 IU/L indicate germinomas; (2) Typical imaging features along with serum/cerebrospinal fluid levels of AFP > 25 ng/mL and/or HCG > 50 IU/L indicate NGGCTs (14). The tumor markers in blood serum of clinically diagnosed patients in SYSUCC cohort are presented in Supplementary Table 1. The primary endpoint of this study was overall survival (OS).
2.2 Definition of variables
We utilized the X-tile software to establish the optimal cutoff values for age and tumor size. Age was categorized into two groups: ≤ 21 years and > 21 years, and tumor size was divided into three categories based on the tumor’s largest diameter: ≤ 3.4 cm, > 3.4 cm, and/or unknown. Moreover, the presence of tumor dissemination was identified by either distant metastasis visible in radiographic images or the detection of tumor cells in the cerebrospinal fluid (CSF) at the time of initial diagnosis. In terms of tumor location, the pineal gland and sellar region were merged into a single variable labeled “pineal gland/sellar region,” while all other intracranial sites were grouped as “other sites”.
2.3 Statistical analysis
We conducted univariate and multivariate Cox regression analysis in the development cohort to identify independent prognostic factors. Factors with a significance level of p<0.05 in the univariate analysis were incorporated into the multivariate regression model for additional investigation. Then, factors demonstrating significance with p<0.05 in the multivariate analysis were chosen for inclusion in the nomogram development. Based on the contribution degree of each factor for the outcome in the model, the nomogram can express the relationship between various variables in the model according to drawing a line segment with certain proportion on the same plane, which transformed the complex regression equation into graphic visualization, and made the results of the prediction model more reifiable and convenient for clinician.
The predictive performance of the nomogram was assessed using multiple validation metrics, including C-index, calibration curves and area under the time-dependent receiver operating characteristic curve (time-dependent AUC). Following established methodological standards, C-index and AUC values exceeding 0.7 were considered indicative of satisfactory predictive accuracy (15). Furthermore, we implemented decision curve analysis (DCA) to evaluate the clinical utility of the nomogram by quantifying net benefits across a range of threshold probabilities for clinical decision-making.
This study utilized a risk grouping system to evaluate the prognosis of patients with CNS GCTs based on the nomogram. The risk grouping system classified patients from the SEER cohort into four prognostic groups based on their total point scores using the X-tile software. Furthermore, Kaplan-Meier curves were employed to compare the prognosis of patients across the four nomogroups.
Statistical analyses were conducted using SPSS 24.0, incorporating methods such as the chi-square test, Kaplan–Meier method, log-rank test, univariate and multivariate analyses. Significance was determined at a two-tailed P-value of <0.05. R Studio 4.4.1 was utilized for designing and validating the nomogram. The chi-square test was used to assess the correlation between the development and validation cohorts. Survivorship curves were created using GraphPad Prism 7 and compared through the Kaplan–Meier method and log-rank test. The impact of the treatment regimen on CNS GCTs was examined using a Cox proportional hazards model. This study was a retrospective analysis approved by the Institutional Review Board of SYSUCC.
3 Results
3.1 Patient characteristics
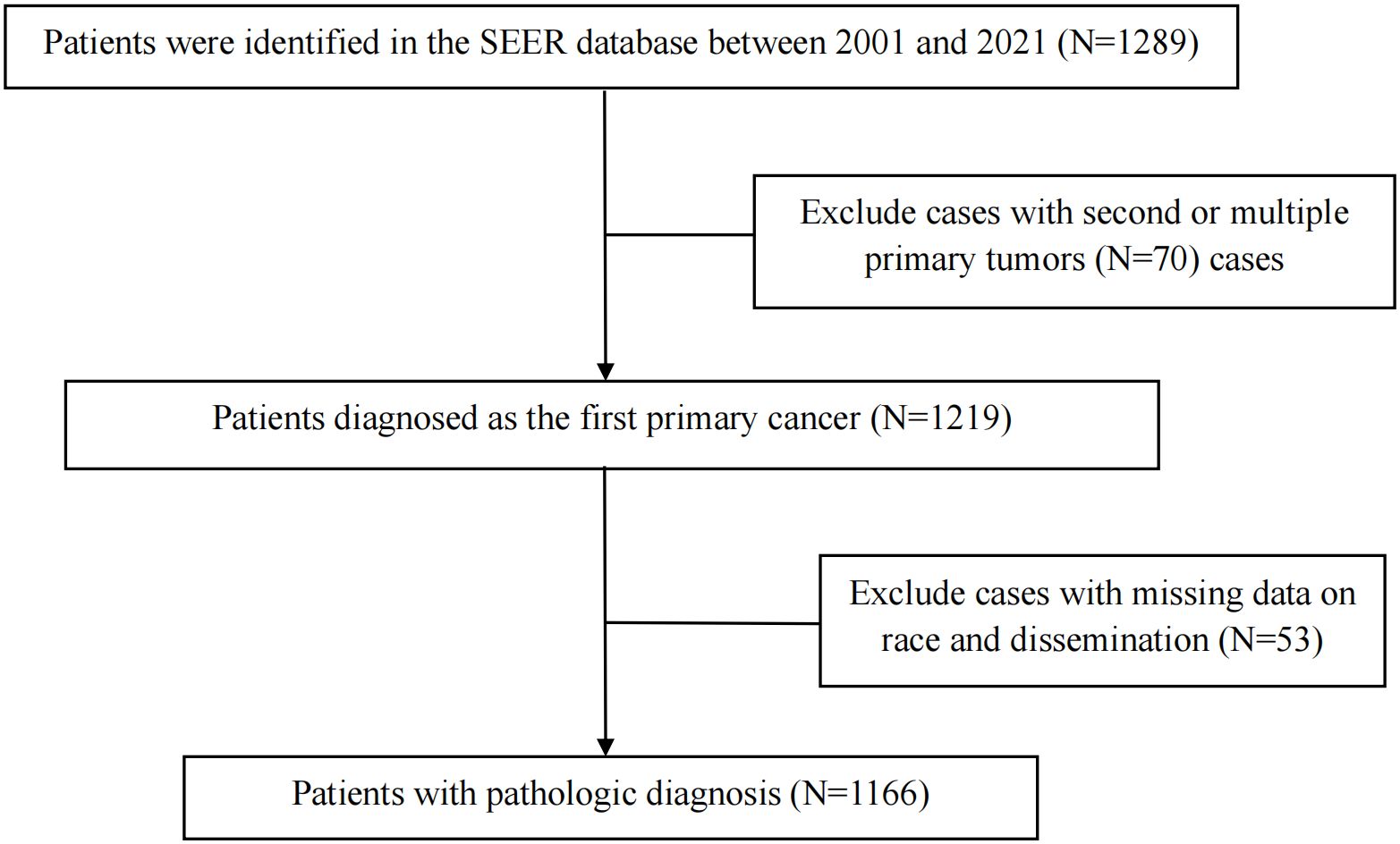
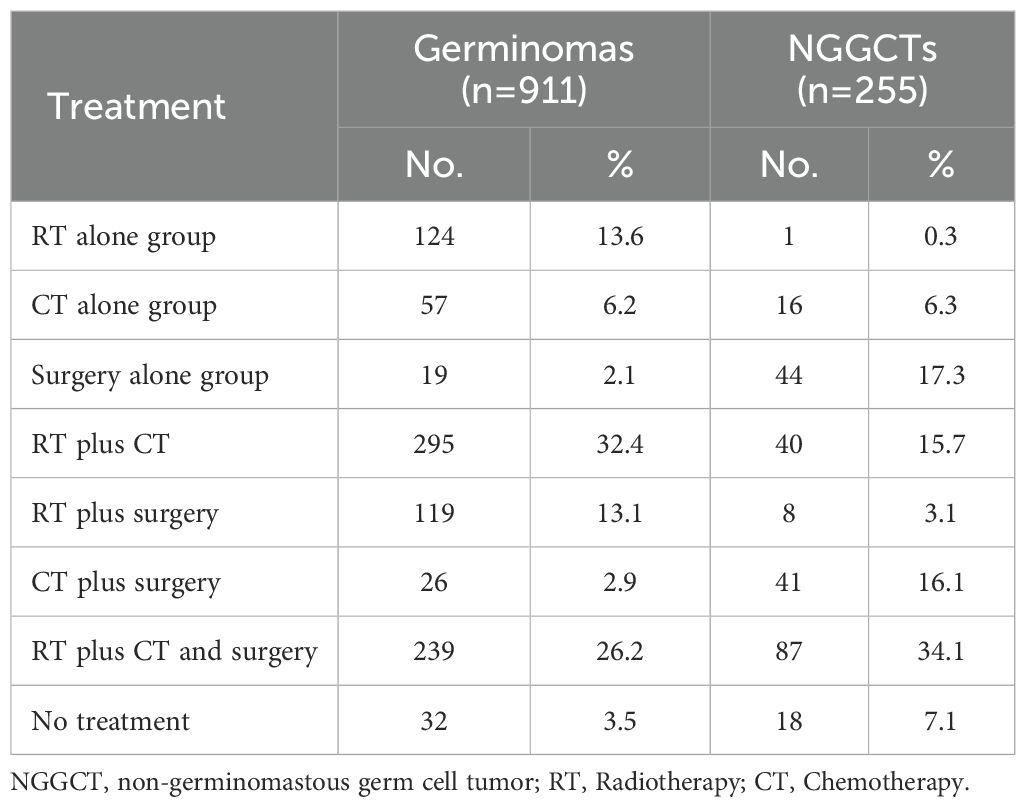
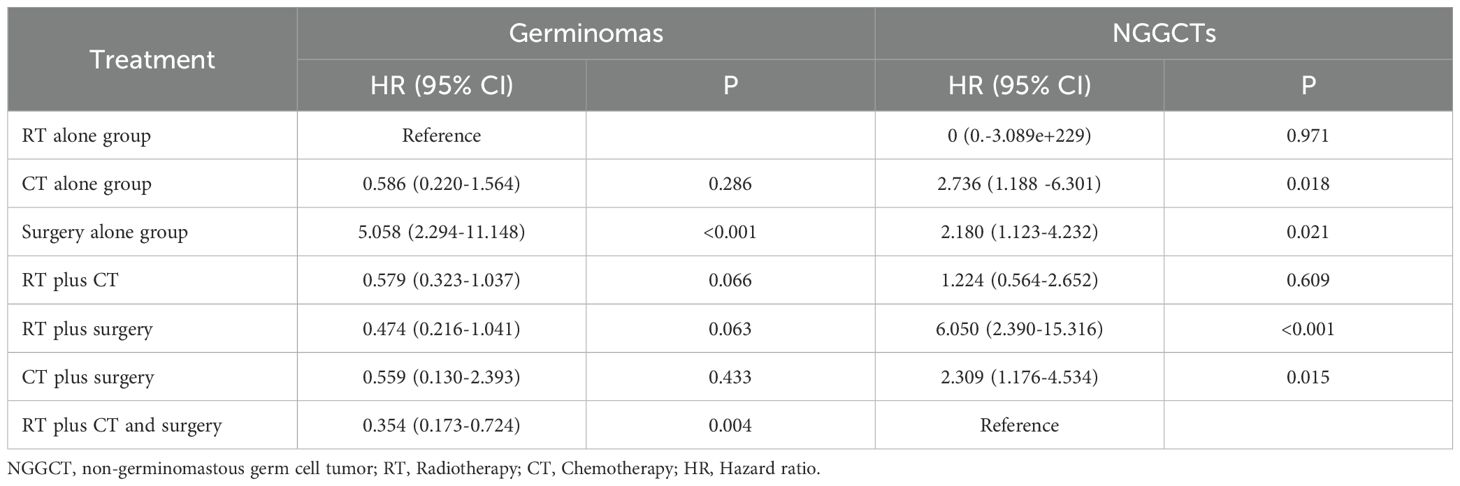
The clinical data of 1289 CNS GCTs patients were extracted from the SEER database from 2001 to 2021. A total of 1166 eligible patients who met the eligibility criteria for CNS GCTs were included (Figure 1). Detailed patient demographics and clinicopathological information can be found in Table 1. Among these, 911 were diagnosed with germinomas, and 255 with NGGCTs. The 5-year OS rate among patients with germinomas was 93.0%, while for those with NGGCTs, it was 73.3% (Supplementary Figure 1). Treatment modalities varied, as shown in Table 2, with 28.7% receiving chemoradiotherapy, 10.7% receiving radiotherapy alone, and 6.3% receiving chemotherapy alone, resulting in 5-year OS rates of 94.9%, 91.2%, and 86.3%, respectively (Supplementary Figure 2). Tumor dissemination occurred in 90 patients (7.7%), comprising 63 germinoma cases and 27 NGGCTs cases, with corresponding 5-year OS rates of 90.0% and 44.4% (Supplementary Figure 3). Within the SEER cohort, the median OS was 109 months (ranging from 0 to 263), with 5- and 10-year OS rates of 88.7% and 87.1%, respectively. The Cox proportional hazards model analysis indicated no significant difference in survival outcomes between germinoma patients treated with chemotherapy versus radiotherapy (HR = 0.586, p=0.286) (Table 3). For NGGCTs patients, a more favorable prognosis was observed in those undergoing a combination of surgery and chemoradiotherapy compared to other treatment combinations. However, no significant difference was noted when compared to patients receiving chemoradiotherapy (HR = 1.224, p = 0.609) (Table 3).
A validation cohort comprising 165 patients with CNS GCTs from SYSUCC was established, consisting of 119 germinomas and 46 NGGCTs. The demographic and clinicopathological data of the patients are detailed in Table 1. Among the diagnostic approaches, 99 patients (60.0%) received a pathological diagnosis, while 66 patients (40.0%) were diagnosed clinically. Patients with clinically diagnosed germinomas and NGGCTs have 5-year survival rates of 92.8% and 77.7%, whereas patients with pathologically diagnosed germinomas and NGGCTs have respective 5-year survival rates of 93.6% and 72.8%. As of January 1, 2019, the SYSUCC cohort had a median follow-up time of 70.7 months, during which 21 patients (12.7%) had passed away. The median OS in the SYSUCC cohort was 68 months (ranging from 6 to 197), with 5- and 10-year survival rates standing at 89.7% and 87.8%, respectively.
3.2 Kaplan-Meier survival analysis of germinomas and NGGCTs in the development cohort
Within the SEER database, CNS GCTs patients underwent varied treatment regimens, as illustrated in Table 2. The survival analysis of patients with germinomas and NGGCTs undergoing differing treatment regimens is illustrated in Figures 2A, C. Notably, among germinoma patients, those undergoing only surgical intervention displayed significantly worse prognostic outcomes compared to alternative treatments (P<0.001). We further investigated prognostic differences between patients opting for single treatment modes (radiotherapy, chemotherapy, or surgery) versus those choosing combined modality therapy (radiotherapy plus chemotherapy, radiotherapy plus surgery, chemotherapy plus surgery, or trimodal therapy combining chemoradiotherapy with surgery), visually depicted in Figures 2B, D. Additionally, in patients with NGGCTs, age over 21, tumor size larger than 3.4 cm, tumor dissemination, and female gender are associated with an adverse prognosis (Figure 3). Conversely, in those with germinomas, only female patients demonstrate a less favorable prognosis, as indicated in Figure 4.
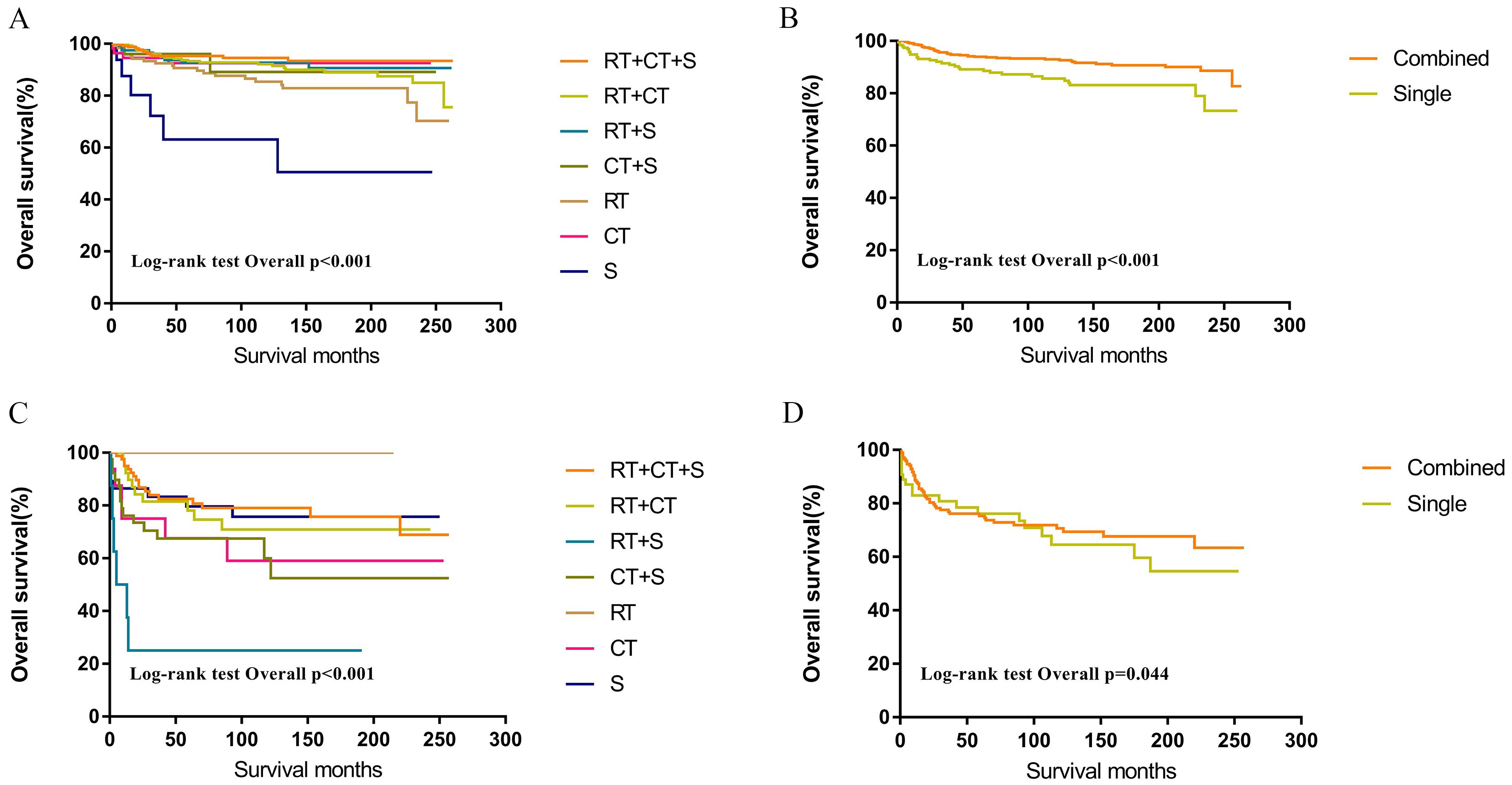
Figure 2. Survival curves of overall survival in germinomas (A, B) and NGGCTs (C, D) according to treatment regimen. RT, Radiotherapy; CT, Chemotherapy; S, Surgery; Single = RT or CT or S; Combined = RT+CT or RT+S or CT+S or RT+CT+S.
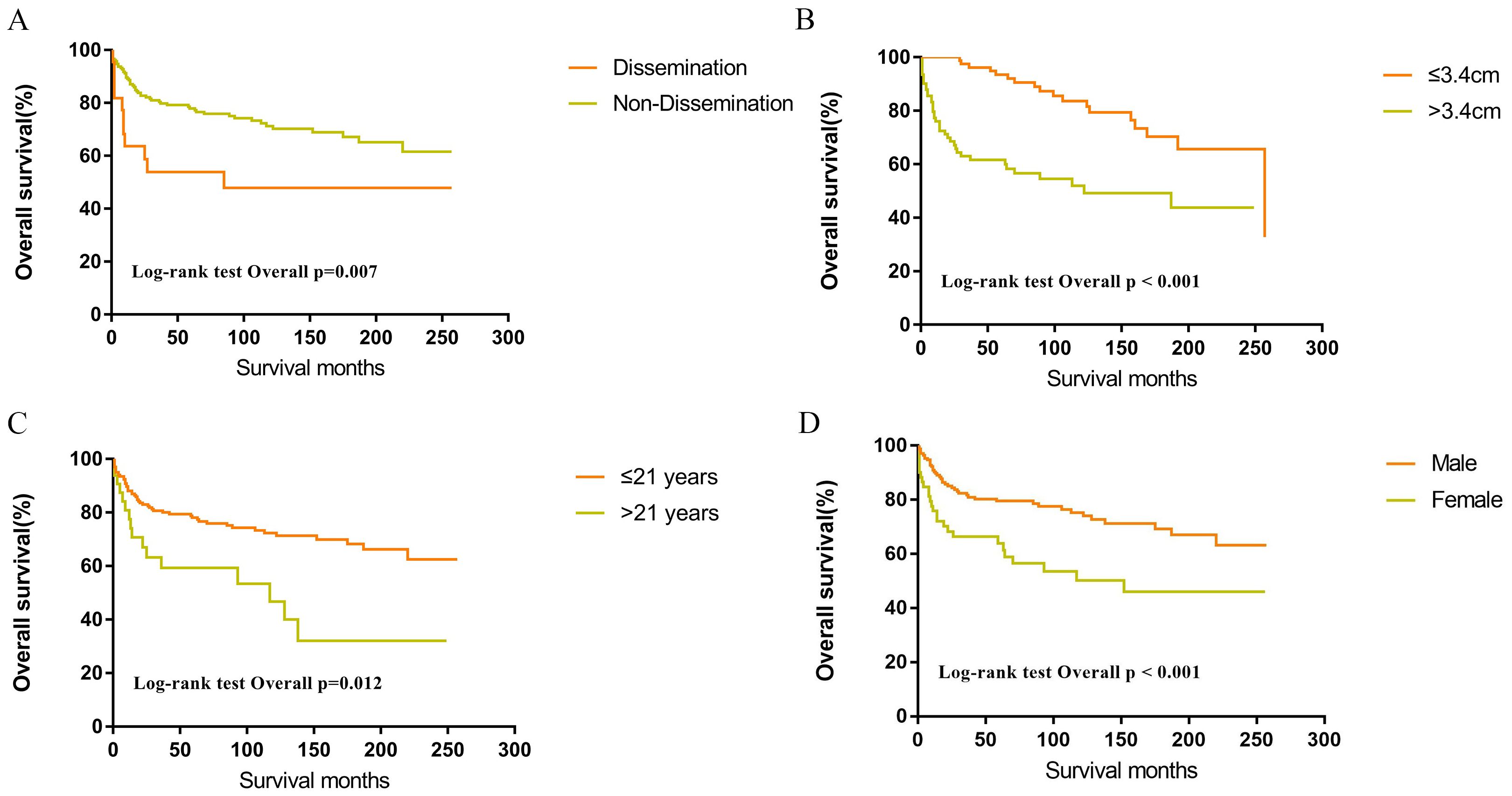
Figure 3. Survival curves of overall survival for dissemination (A), tumor size (B), age (C), sex (D) in patients with NGGCTs.
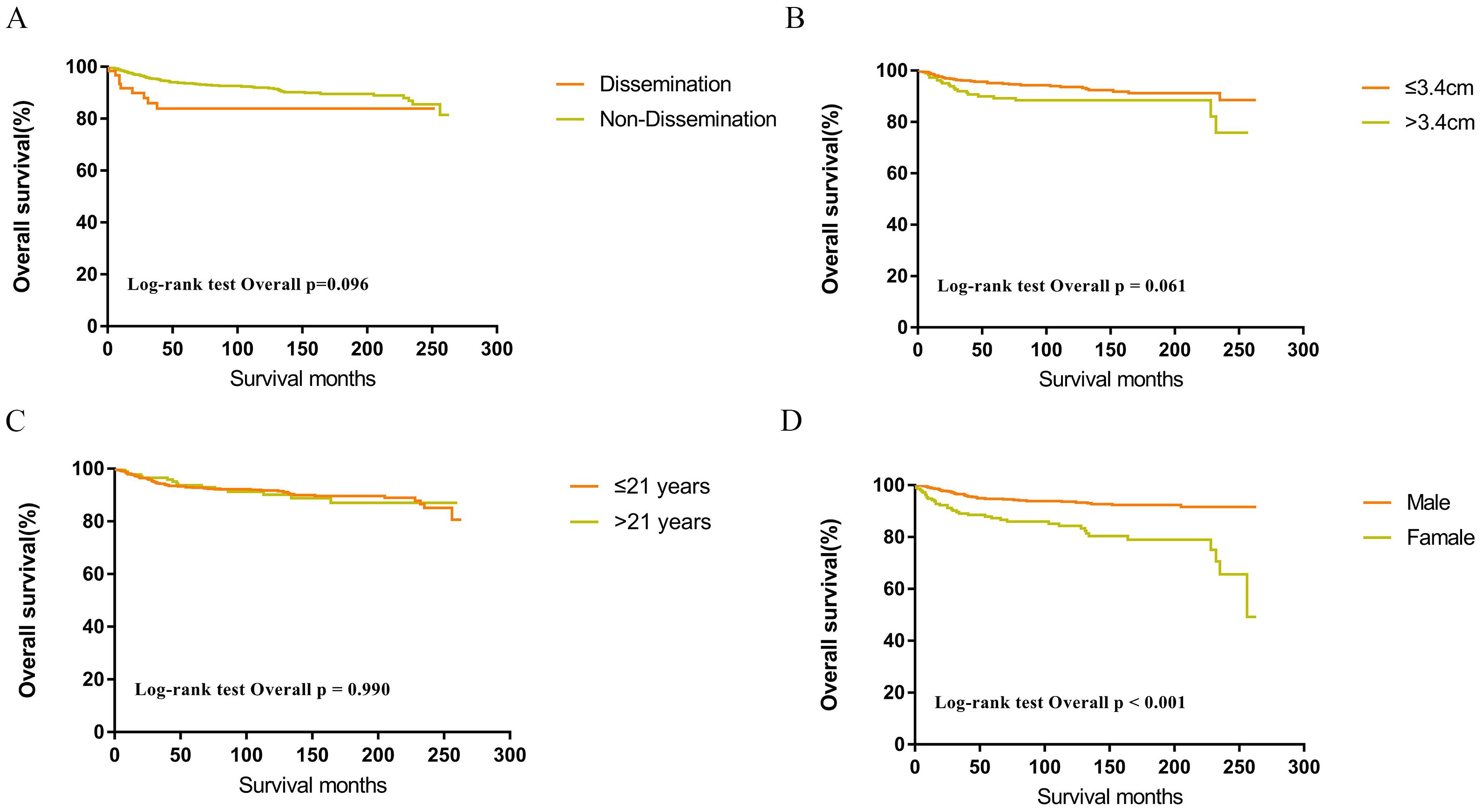
Figure 4. Survival curves of overall survival for dissemination (A), tumor size (B), age (C), sex (D) in patients with germinomas.
3.3 Correlations among variables
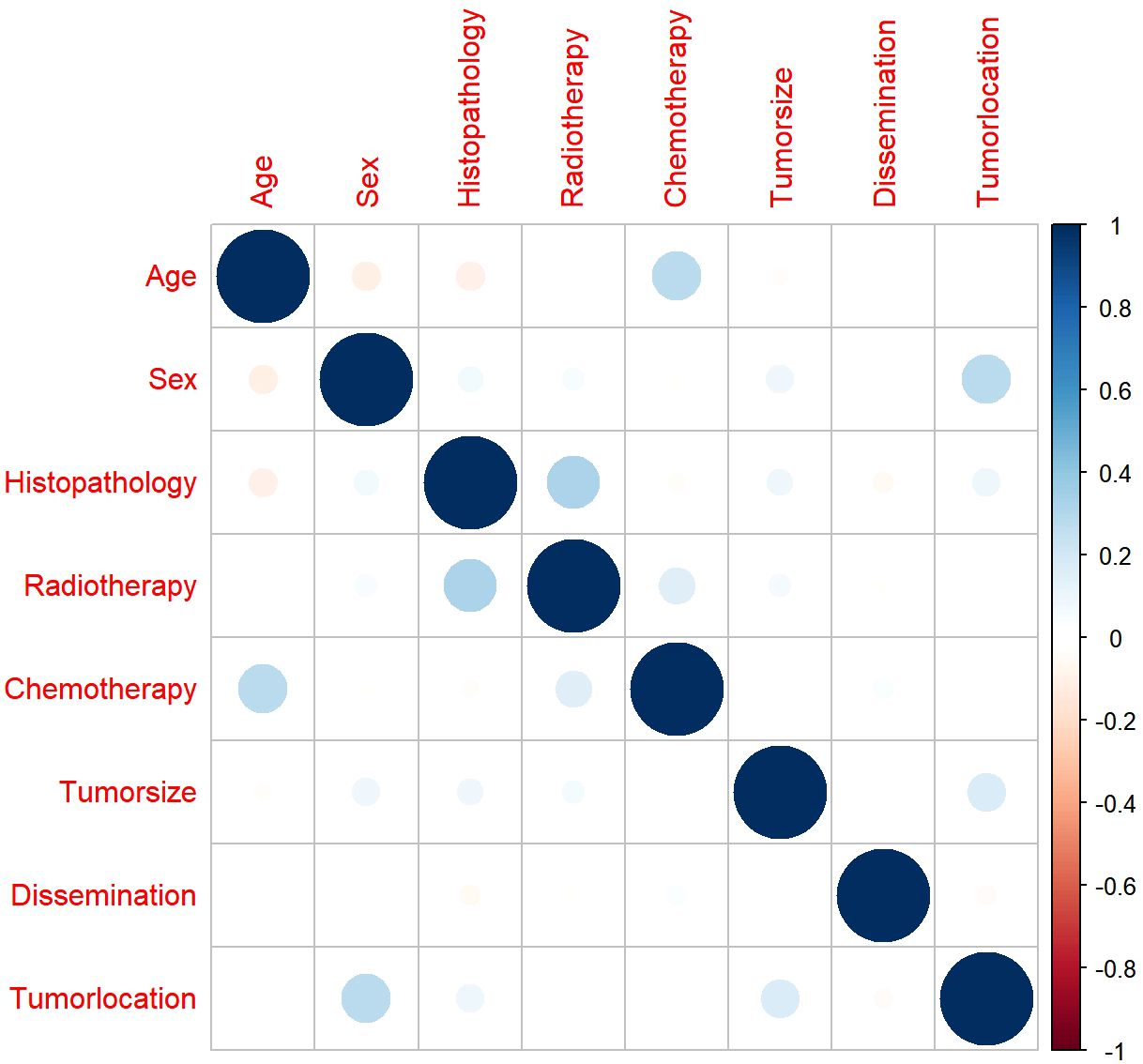
Before we performed the Cox regression analysis, Spearman’s correlation analysis was used to ensure that there was no collinearity existed between screened variables. The results demonstrated that all pairwise correlation coefficients were below 0.5, indicating only weak correlations between variables and confirming the absence of significant collinearity (Figure 5).
3.4 Univariate and multivariate Cox regression analysis in the development cohort
In the development cohort, a range of prognostic factors was assessed, comprising sex, age, race, histopathology, tumor dissemination, location, tumor size, radiotherapy, chemotherapy and surgery. Through univariate Cox regression analysis, all these variables, except for race and surgery, were identified as potential prognostic factors. Subsequently, the multivariate Cox regression analysis identified seven factors as independent prognostic factors for OS: age, sex, histopathology, dissemination, tumor size, radiotherapy, and chemotherapy (Table 4). A forest plot was utilized to depict the impact and level of contribution of each independent prognostic factor in relation to the hazard ratio (HR) (Figure 6A).
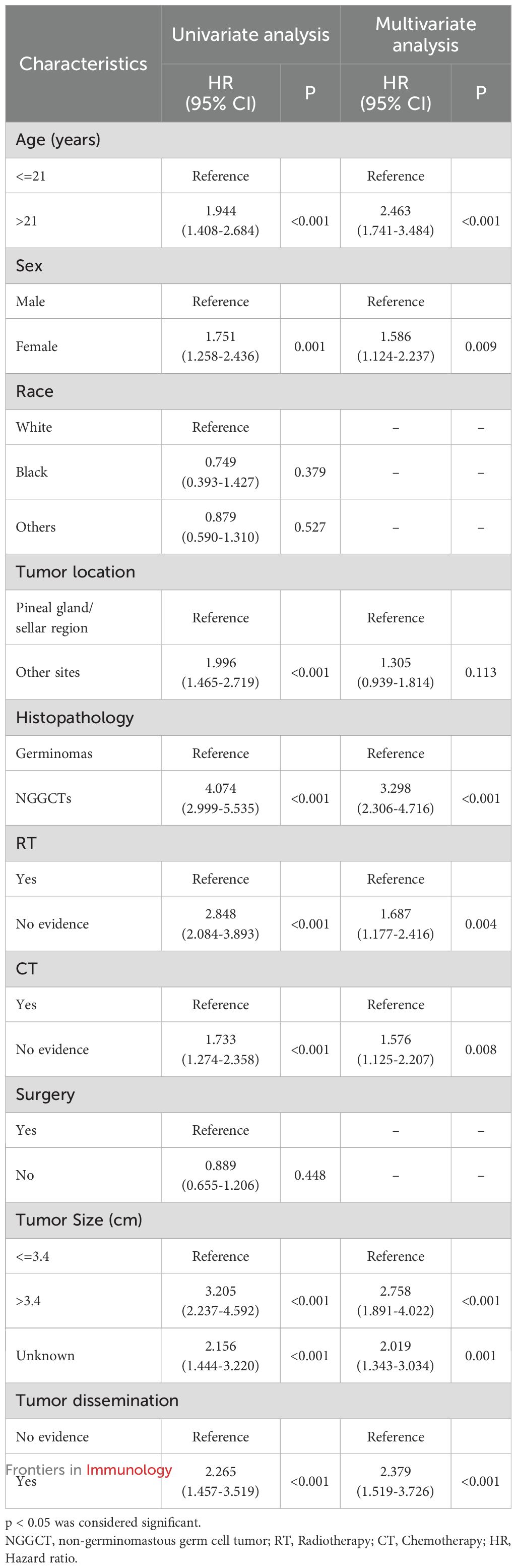
Table 4. Univariate and multivariate cox regression analysis for overall survival in CNS GCTs patients in the development cohort.
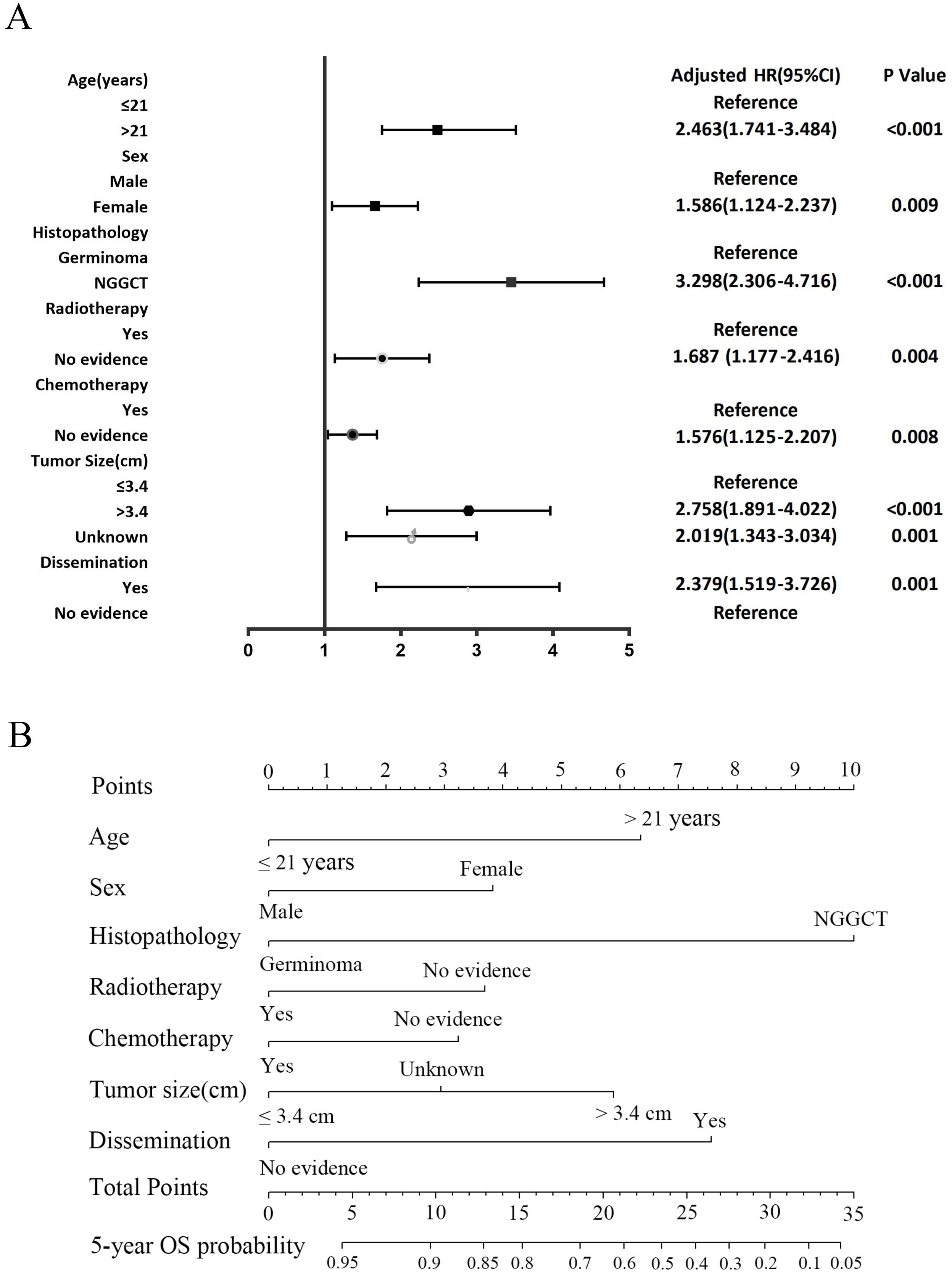
Figure 6. The forest plot of Cox proportional hazard ratio (HR) and 95% confidence intervals (CI) for overall survival of CNS GCTs patients (A). Nomogram predicting the 5-year overall survival rate for CNS GCTs patients (B).
3.5 Construction and evaluation of nomogram
The nomogram for the 5-year OS rate was developed by integrating all prognostic factors identified through multivariate Cox regression analysis (Figure 6B). The nomogram was validated through bootstrap analyses with 1000 resamples. The internal and external validation cohorts exhibited favorable discrimination of the nomogram, with C-index values of 0.773 (95% CI, 0.734 - 0.812) and 0.712 (95% CI, 0.599-0.825), respectively. The calibration curves illustrated a high agreement between the observed 5-year OS probability in the development and validation cohorts and the predicted OS from the nomogram (Figures 7A, B). Furthermore, the time-dependent area under the curve (AUC) was > 0.7 for predicting 5-year OS in both the development and validation cohorts (Figures 7C, D). The 5-year DCA further supported the reliability of our nomogram as a predictor of survival in patients with CNS GCTs (Figures 7E, F). These results offer strong evidence supporting the practical application of the nomogram.
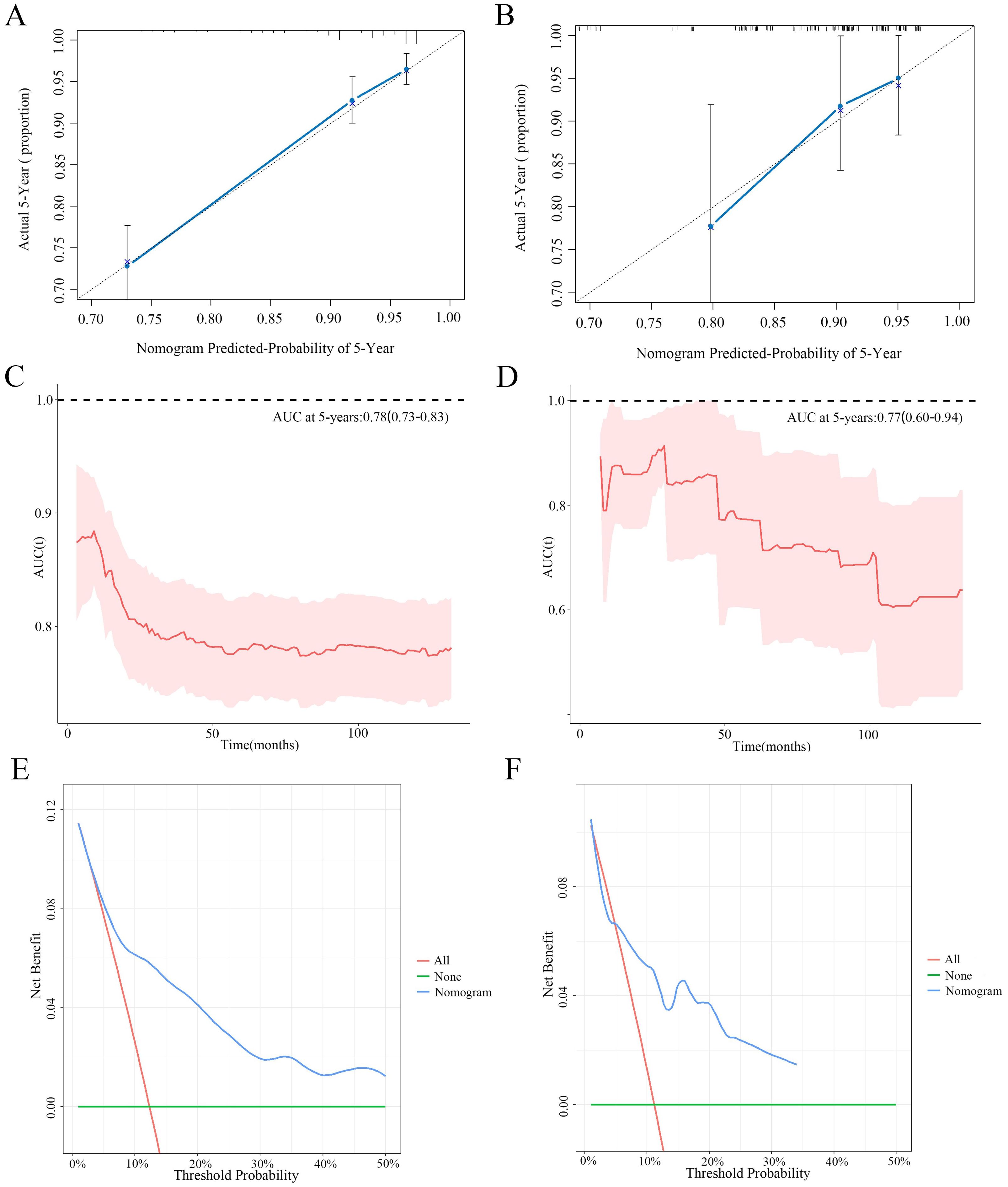
Figure 7. The calibration curves for predictions of 5-year overall survival rate in the development cohort (A) and validation cohort (B). Time-dependent AUC of using the nomogram to predict overall survival probability in the development cohort (C) and validation cohort (D). Prognostic decision curve analysis (DCA) of CNS GCTs patients within 5-year survival in the development cohort (E) and validation cohort (F).
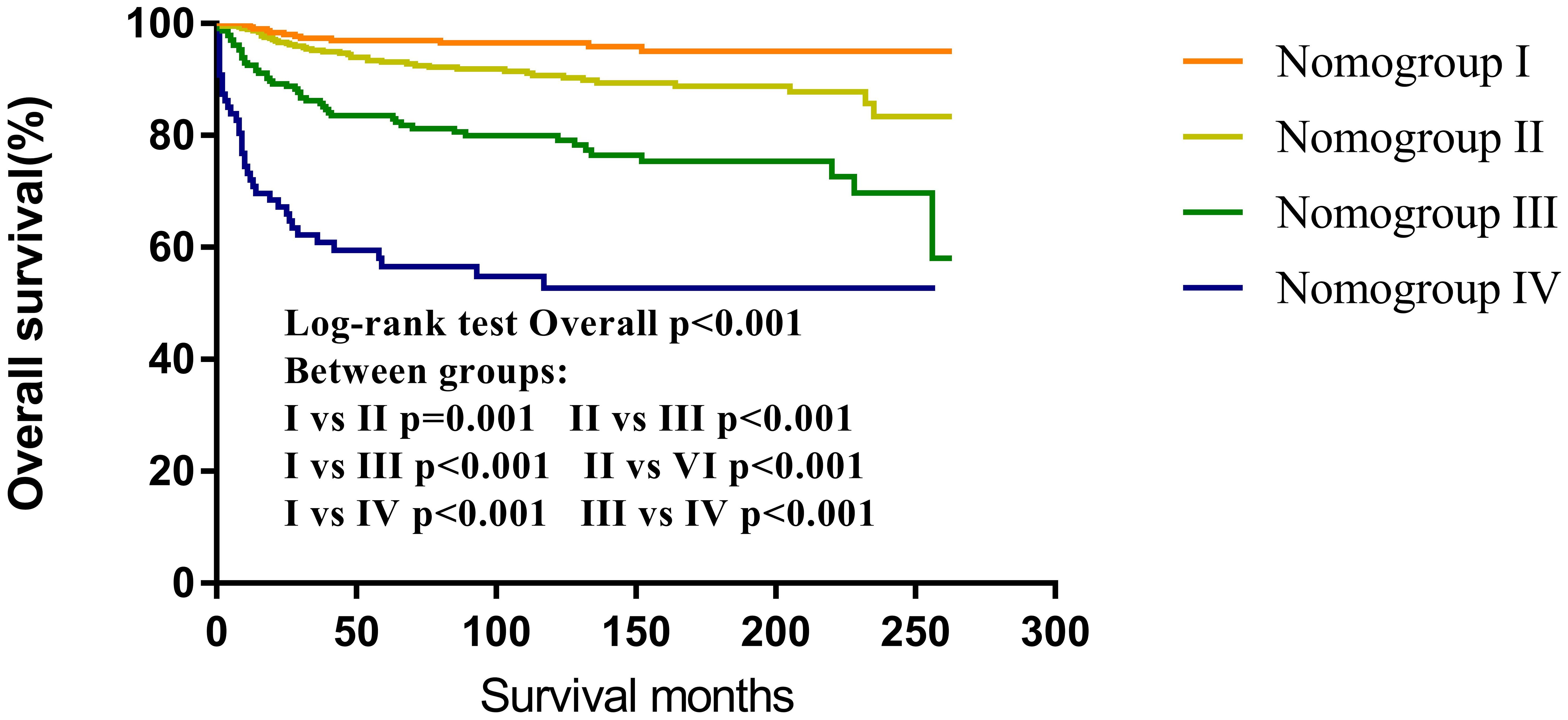
3.6 Nomogroups of risk grouping system
According to the nomogram, CNS GCTs patients from the SEER cohort were classified into four prognostic groups based on their total point scores utilizing X-tile software. The risk nomogroups were as follows: nomogroup I (0–3 points), nomogroup II (4–11 points), nomogroup III (13–19 points), and nomogroup IV (≥20 points). Notably, Kaplan-Meier analysis revealed statistically significant differences in prognosis among the four risk nomogroups (Figure 8).
4 Discussion
In this study, given that most patients with CNS GCTs survive for over 5 years, the primary endpoint of the nomogram was chosen as the 5-year OS rate. the primary endpoint of the nomogram was chosen as the 5-year OS rate. It has been reported that germinoma patients can achieve a 5-year OS rate exceeding 90% due to their high sensitivity to radiotherapy and chemotherapy (16–18). In contrast, NGGCTs often show reduced response to radiotherapy and chemotherapy, resulting in a 5-year OS rate ranging from 60% to 70% after receiving chemoradiotherapy (19, 20). Consistent with published data, our analysis of the SEER cohort unveiled a 5-year OS rate of 93.0% for patients with germinomas, whereas the 5-year OS rate for NGGCTs stands at 73.3%. Thus, the prognosis of CNS GCT patients is primarily influenced by the histopathological type. Challenges related to surgery or patients’ reluctance for biopsy hinder the use of pathological diagnosis in clinical settings. Hence, clinical diagnosis is performed for patients who do not have pathological specimens available. Patients in the SYSUCC cohort without accessible pathological specimens had their clinical diagnosis determined in accordance with the consensus guidelines for CNS GCTs management (14). Our analysis revealed similar 5-year OS rates in clinically diagnosed and pathologically confirmed instances of germinomas and NGGCTs. These findings support the reliability of clinical diagnosis in this study, justifying the inclusion of clinically diagnosed patients in the validation cohort. This inclusion not only augmented the sample size but also improved the predictive precision of our assessment model.
It is generally believed that the outcome of CNS GCTs is not associated with tumor size, while several studies have suggested that a larger tumor size is a poorer prognostic factor for CNS GCTs (21–24). In the current study, multivariate Cox regression revealed that a tumor diameter greater than 3.4 cm was an independent risk factor for overall survival. Interestingly, subgroup analysis found that the negative impact of a large tumor size on prognosis was observed only in NGGCTs and not in germinomas. This variation in the effect of tumor size on prognosis could be due to the heightened sensitivity of germinomas to chemoradiotherapy. Consequently, the influence of tumor size on prognosis might be confined to patients with NGGCTs, showing no discernible impact on patients with germinomas.
Chen et al. reported that survival rates were comparable between patients with disseminated germinomas and those without dissemination (25). Tumor dissemination in this study served as a significant independent prognostic factor for patients with CNS GCTs in the SEER cohort. Notably, subsequent subgroup analysis revealed no statistically significant variance in survival rates between patients with disseminated germinomas and those without dissemination. Furthermore, for CNS GCTs patients with dissemination, germinomas exhibited a more favorable prognosis than NGGCTs. These findings can be attributed to the higher radiosensitivity of germinomas.
Radiotherapy plays a vital role in CNS GCTs management. However, considering the neurological side effects linked to radiotherapy, the current strategy emphasizes shrinking tumor size using chemotherapy prior to commencing radiation therapy (16, 18, 26). Analysis within the SEER cohort revealed that CNS GCTs patients receiving chemoradiotherapy and radiotherapy experienced extended survival compared to those treated solely with chemotherapy. Typically, patients receiving chemotherapy alone are thought to encounter elevated recurrence rates and inferior prognoses compared to those receiving radiotherapy (27, 28). Nevertheless, the Cox proportional hazards model analysis conducted in this study revealed that the prognosis does not vary among patients with germinomas undergoing radiotherapy alone and chemotherapy alone, this observation could be attributed to certain patients who underwent chemotherapy alone also receiving extra treatments, like radiotherapy, not recorded in the SEER database. Furthermore, our discovery indicated that standalone surgery yielded the poorest prognosis for germinoma, emphasizing the limitation of relying solely on surgical intervention, aligning with present recommendations (29).
NGGCTs exhibit inherent resistance to radiation therapy, resulting in the infrequency of using radiotherapy as a monotherapy in current clinical practices (1, 16, 30). Present therapeutic strategies for NGGCTs emphasize the multidisciplinary approach of integrating radiotherapy with chemotherapy (5, 31). Controversy exists regarding the effectiveness of incorporating neurosurgical procedures into the core of chemoradiotherapy for CNS GCTs, mainly because of the notable responses of the majority of histological subtypes of CNS GCTs to radiotherapy and chemotherapy. Aoyama et al. studied 33 pediatric cases of CNS GCTs, illustrating that surgical excision, in conjunction with combined chemotherapy and limited radiotherapy, resulted in a 5-year overall survival rate surpassing 69% (32). In this research, no distinction in prognosis was found between the combined treatment of surgery with chemoradiotherapy and chemoradiotherapy, suggesting that the advantage of adding surgery to chemoradiotherapy is not conclusively proven. Presently, surgical intervention is deemed suitable for suspected teratomas, residual lesions after chemoradiotherapy, and for alleviating life-threatening space-occupying manifestations (14, 33). However, due to the limited sample sizes of NGGCTs in this study, further prospective clinical trials must be done to assess the efficacy of combined surgery and chemoradiotherapy treatment for NGGCTs. Furthermore, our findings indicate that combination therapy offers substantial benefits in enhancing the survival outcomes of CNS GCTs patients, whether germinomas or NGGCTs, which indicated that future therapeutic approaches for CNS GCTs favor a multidisciplinary integrated therapy.
Gender variations are evident in CNS GCTs, with a higher prevalence among male patients compared to female patients (29), aligning with our research findings. Additionally, Acharya et al. found an increased mortality risk in female germinoma patients compared to their male counterparts (34). Nevertheless, a study by Steven et al. revealed comparable prognoses among male and female germinoma patients (35). Remarkably, our study detected survival differences based on gender in CNS GCTs. Although the underlying reason remains unclear, we propose that the gender disparities in CNS GCTs incidence and outcomes stem from complex interactions encompassing DNA methylation discrepancies, epigenetic alterations, and brain sexual differentiation influenced by exposure to sex hormones (36). Accordingly, additional prospective investigations are imperative to explore the influence of gender on prognosis.
Emphasizing the strengths of our nomogram is crucial. Firstly, our nomogram demonstrates extensive generalizability by integrating databases from both Western and Eastern populations, rendering it suitable for diverse regions. Secondly, most research on CNS GCTs faces limitations with small sample sizes. In contrast, our dataset is sourced from the SEER database, encompassing 18 cancer registries and reflecting around 34.6% of the US population. This extensive dataset greatly enhances the reliability and accuracy of our research. Lastly, by utilizing the risk grouping system, clinicians can also identify different risk stratification of CNS GCTs patients, which assists decision making about the risk and benefit of treatment or care options.
However, being a retrospective study, our study has limitations. Firstly, the recently updated SEER database categorizes “No/Unknown” as a single group, with regards to chemotherapy and radiotherapy. Secondly, detailed data on chemotherapy and radiotherapy therapy such as chemotherapy regimen, radiotherapy dose and radiotherapy methods were not accessible in the SEER database. Thirdly, information about several important clinicopathological parameters such as tumor size was not complete. In order to mitigate potential biases, we retained this data despite its classification as “unknown”. Finally, limited by the constraints on case numbers of the SEER database, the NGGCTs group had only one patient who received radiotherapy exclusively, underscoring the need for extensive studies to explore the impact of radiotherapy on NGGCTs prognosis with larger sample sizes.
5 Conclusion
In conclusion, we have successfully identified prognostic factors for CNS GCTs and developed a nomogram that integrates seven clinicopathological and treatment-related variables. This nomogram accurately predicts the prognosis of patients with CNS GCTs and provides clinicians with personalized treatment options and guidance for long-term management.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of Sun Yat-sen University Cancer Center. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
DCY: Methodology, Conceptualization, Writing – review & editing, Supervision, Writing – original draft. BY: Writing – original draft, Investigation. HZ: Methodology, Writing – original draft. LP: Resources, Writing – original draft. DJY: Writing – original draft, Software. XL: Conceptualization, Writing – original draft, Supervision. CG: Writing – original draft, Supervision, Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. The research is supported with fund from the National Science Foundation of China (82373440), and the Grant of Beijing Science and Technology Innovation Medical Development Foundation (KC2021-JX-0186-115).
Acknowledgments
We are all grateful to the Sun-Yat-Sen University Cancer Center for statistics work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1630061/full#supplementary-material
Supplementary Figure 1 | Survival curves with the log-rank tests of overall survival according to histological subtype.
Supplementary Figure 2 | Survival curves with the log-rank tests of overall survival according to treatment regimen. RT, radiotherapy; CT, chemotherapy.
Supplementary Figure 3 | Survival curves with the log-rank tests of overall survival in patients with disseminated according to histological subtype.
References
1. Al-Naeeb AB, Murray M, Horan G, Harris F, Kortmann R-D, Nicholson J, et al. Current management of intracranial germ cell tumours. Clin Oncol. (2018) 30:204–14. doi: 10.1016/j.clon.2018.01.009
2. Yoko TU and Roger JP. Pediatric brain tumors. Neurol Clin. (2018) 36:533–56. doi: 10.1016/j.ncl.2018.04.009
3. Arai H, Kayama T, Saito N, Fujii Y, lihara K, Kawahara N, et al. Report of Japan neurosurgery registry (2015 - 2017). Neurol Med Chir (Tokyo). (2019) 6(3):533–56. doi: 10.2176/nmc.si.2019-0001
4. Lee SH, Jung KW, Ha J, Oh CM, Kim H, Park HJ, et al. Nationwide population-based incidence and survival rates of Malignant central nervous system germ cell tumors in Korea, 2005-2012. Cancer Res Treat. (2016) 49:494–50. doi: 10.4143/crt.2016.129
5. Kong Z, Wang Y, Dai C, Yao Y, Ma W, and Wang Y. Central nervous system germ cell tumors: A review of the literature. J Child neurology. (2018) 33:610–20. doi: 10.1177/0883073818772470
6. Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, and Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. (1998) 90:766–71. doi: 10.1093/jnci/90.10.766
7. Valentini V, van Stiphout RGPM, Lammering G, Gambacorta MA, Barba MC, Bebenek M, et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol. (2011) 29:3163–72. doi: 10.1200/JCO.2010.33.1595
8. Mittendorf EA, Hunt KK, Boughey JC, Bassett R, Degnim AC, Harrell R, et al. Incorporation of sentinel lymph node metastasis size into a nomogram predicting non-sentinel lymph node involvement in breast cancer patients with a positive sentinel lymph node. Ann surgery. (2012) 255:109. doi: 10.1097/SLA.0b013e318238f461
9. Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. (2013) 31:1188–95. doi: 10.1200/JCO.2012.41.5984
10. Gold JS, Gönen M, Gutiérrez A, Broto JM, García-del-Muro X, Smyrk TC, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. (2009) 10:1045–52. doi: 10.1016/S1470-2045(09)70242-6
11. Mariani L, Miceli R, Kattan MW, Brennan MF, Colecchia M, Fiore M, et al. Validation and adaptation of a nomogram for predicting the survival of patients with extremity soft tissue sarcoma using a three-grade system. Cancer. (2005) 103:402–8. doi: 10.1002/cncr.20778
12. Guan TW, Li YF, Qiu ZC, Zhang YC, Lin WR, Lai YX, et al. Nomograms and risk classification systems predicting overall and cancer-specific survival in primary Malignant cardiac tumor. J Card Surg. (2019) 34:1540–9. doi: 10.1111/jocs.14299
13. Zheng WP, Huang YP, Guan TW, Lu SF, Yao LQ, Wu SR, et al. Application of nomograms to predict overall and cancer-specific survival in patients with chordoma. J Bone Oncol. (2019) 18. doi: 10.1016/j.jbo.2019.100247
14. Murray MJ, Bartels U, Nishikawa R, Fangusaro J, Matsutani M, and Nicholson JC. Consensus on the management of intracranial germ-cell tumours. Lancet Oncol. (2015) 16:e470–e7. doi: 10.1016/S1470-2045(15)00244-2
15. Harrell FE, Lee KL, and Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. (1996) 15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4
16. Claude L, Faure-Conter C, Frappaz D, Mottolese C, and Carrie C. Radiation therapy in pediatric pineal tumors. Neurochirurgie. (2015) 61:212–5. doi: 10.1016/j.neuchi.2014.11.002
17. Wong K, Opimo AB, Olch AJ, All S, Waxer JF, Clark D, et al. Re-irradiation of recurrent pineal germ cell tumors with radiosurgery: report of two cases and review of literature. Cureus. (2016) 8:e585. doi: 10.7759/cureus.585
18. Frappaz D, Conter CF, Szathmari A, Valsijevic A, and Mottolese C. The management of pineal tumors as a model for a multidisciplinary approach in neuro-oncology. Neurochirurgie. (2015) 61:208–11. doi: 10.1016/j.neuchi.2014.03.003
19. Echevarría ME, Fangusaro J, and Goldman S. Pediatric central nervous system germ cell tumors: a review. Oncologist. (2008) 13:1460–9. doi: 10.1634/theoncologist.2008-0037
20. Calaminus G, Kortmann R, Worch J, Nicholson C, Alapetite C, Garrè ML, et al. SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol. (2013) 15:788–96. doi: 10.1093/neuonc/not019
21. Shirato H, Nishio M, Sawamura Y, Myohjin M, Kitahara T, Nishioka T, et al. Analysis of long-term treatment of intracranial germinoma. Int J Radiat Oncol Biol Physics. (1997) 37:511–5. doi: 10.1016/S0360-3016(96)00607-4
22. Shibamoto Y, Sasai K, Oya N, and Hiraoka M. Intracranial germinoma: radiation therapy with tumor volume-based dose selection. Radiology. (2001) 218:452–6. doi: 10.1148/radiology.218.2.r01ja08452
23. Shibamoto Y, Takahashi M, and Abe M. Reduction of the radiation dose for intracranial germinoma: a prospective study. Br J cancer. (1994) 70:984. doi: 10.1038/bjc.1994.434
24. Zhang Y, Zhu H, Deng K, Ma W, Wang Y, Sun J, et al. Results of biopsy-proven sellar germ cell tumors: nine years’ Experience in a single center. World neurosurgery. (2018) 112:e229. doi: 10.1016/j.wneu.2018.01.028
25. Chen YW, Huang PI, Hu YW, Ho DMT, Chang KP, Guo WY, et al. Treatment strategies for initially disseminated intracranial germinomas. Child’s Nervous System. (2012) 28:557–63. doi: 10.1007/s00381-012-1683-2
26. Fu H, Guo X, Li R, and Xing B. Radiotherapy and chemotherapy plus radiation in the treatment of patients with pure intracranial germinoma: A meta-analysis. J Clin Neurosci. (2017) 43:32–8. doi: 10.1016/j.jocn.2017.05.024
27. da Silva NS, Cappellano AM, Diez B, Cavalheiro S, Gardner S, Wisoff J, et al. Primary chemotherapy for intracranial germ cell tumors: results of the third international CNS germ cell tumor study. Pediatr Blood cancer. (2010) 54:377–83. doi: 10.1002/pbc.22381
28. Balmaceda C, Heller G, Rosenblum M, Diez B, Villablanca J, Kellie S, et al. Chemotherapy without irradiation–a novel approach for newly diagnosed CNS germ cell tumors: results of an international cooperative trial. The First International Central Nervous System Germ Cell Tumor Study. J Clin Oncol. (1996) 14:2908–15. doi: 10.1200/JCO.1996.14.11.2908
29. Nakamura H, Takami H, Yanagisawa T, Kumabe T, Fujimaki T, Arakawa Y, et al. The Japan Society for Neuro-Oncology guideline on the diagnosis and treatment of central nervous system germ cell tumors. Neuro-oncology. (2022) 24:503–15. doi: 10.1093/neuonc/noab242
30. Akinori T, Noriko I, Masahiro H, Hidemi T, Toshio M, Yutaka T, et al. Long-term follow-up of intensive chemotherapy followed by reduced-dose and reduced-field irradiation for intracranial germ cell tumor. J Neurosurg Pediatr. (2018) 23:503–15. doi: 10.1093/neuonc/noab242
31. Ashley WJ, Nadia N Issa L, Jan CB, Paula JS, Cynthia JW, and Paul DB. Long-term follow-up of dose-adapted and reduced-field radiotherapy with or without chemotherapy for central nervous system germinoma. Int J Radiat Oncol Biol Phys. (2010) 77:317–24. doi: 10.3171/2018.9.PEDS18181
32. Hidefumi A, Hiroki S, Jun I, Kenji F, Kazuo M, and Yutaka S. Induction chemotherapy followed by low-dose involved-field radiotherapy for intracranial germ cell tumors. J Clin Oncol. (2002) 20:1449–56. doi: 10.1016/j.ijrobp.2009.06.077
33. Kanamori M, Kumabe T, Watanabe M, Chonan M, Saito R, Yamashita Y, et al. Indications for salvage surgery during treatment for intracranial germ cell tumors. J neuro-oncology. (2018) 138:601–7. doi: 10.1007/s11060-018-2827-3
34. Sahaja A, Todd D, Eric TS, and Stephanie MP. Long-term outcomes and late effects for childhood and young adulthood intracranial germinomas. Neuro Oncol. (2014) 17:857–65. doi: 10.1200/JCO.2002.20.3.857
35. Steven D, Abhiraj DB, Shashank NP, Andrew M, Mandana B, and Ankit IM. Treatment and survival of primary intracranial germ cell tumors: a population-based study using SEER database. J Cancer Res Clin Oncol. (2019) 146:601–7. doi: 10.1007/s11060-018-2827-3
Keywords: primary central nervous system (CNS) germ cell tumors (GCTs), overall survival (OS), nomogram, risk grouping system, Surveillance, Epidemiology, and End Results (SEER)
Citation: Yao D, Ye B, Zhang H, Pan L, Yao D, Li X and Guo C (2025) Development and validation of a nomogram for predicting overall survival in patients with primary central nervous system germ cell tumors. Front. Immunol. 16:1630061. doi: 10.3389/fimmu.2025.1630061
Received: 16 May 2025; Accepted: 04 August 2025;
Published: 20 August 2025.
Edited by:
Vera Rebmann, University of Duisburg-Essen, GermanyReviewed by:
Tiantian Cui, City of Hope National Medical Center, United StatesJiapeng Miao, Central South University, China
Copyright © 2025 Yao, Ye, Zhang, Pan, Yao, Li and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Li, NDM1MjYxNDQwQHFxLmNvbQ==; Chengcheng Guo, Z3VvY2hjaEBzeXN1Y2Mub3JnLmNu
†These authors have contributed equally to this work
 Dunchen Yao
Dunchen Yao Baokui Ye
Baokui Ye Hongli Zhang3†
Hongli Zhang3† Chengcheng Guo
Chengcheng Guo