Abstract
Objective:
This study evaluated trends in population immunity against measles, rubella, mumps, and varicella in Suqian City (2005–2024) using serological surveys (2019–2024) and incidence data, incorporating participants with known/unknown vaccination statuses to explore immunity dynamics amid evolving policies, and inform public health planning.
Methods:
Serum samples from 541 (2019) and 506 (2024) healthy participants were analyzed for virus-specific IgG antibodies using ELISA. Disease incidence data were obtained from China’s National Notifiable Disease Reporting System. Statistical analyses compared seroprevalence, geometric mean concentrations (GMCs), and incidence trends before and after policy adjustments.
Results:
Optimized two-dose strategies significantly improved seropositivity rates for rubella (65.1% to 70.8%), mumps (77.6% to 85.8%), and varicella (76.9% to 79.5%), with corresponding GMC increases. Incidence declines were notable: varicella (183.3/100,000 to 59.3/100,000), rubella (2.8/100,000 to 0.04/100,000), and mumps (24.4/100,000 to <5.0/100,000). However, measles seropositivity declined from 85.9% to 79.3% (p<0.05). GMC analysis showed increases for rubella (31.5 to 42.2 IU/ml), mumps (277.8 to 350.6 IU/ml), and varicella (295.9 to 309.8 IU/ml), but a decrease for measles (577.9 to 499.2 mIU/ml, p<0.001). Preschool children (2–5 years) exhibited the highest immunity levels in 2024.
Conclusion:
Population immunity against rubella, mumps, and varicella improved (2019–2024) with reduced incidence, while measles immunity declined, revealing vulnerabilities. Targeted strategies (e.g., catch-up campaigns for adolescents/adults, optimized infant vaccination) are needed to strengthen protection, considering interactions of policies, public health measures, and demographics.
1 Introduction
Vaccination remains the most effective tool for preventing infectious diseases and protecting public health (1). However, viral infections such as measles, rubella, mumps, and varicella continue to impose significant health burdens worldwide (2–5). Taking measles as an example, although global mortality rates have dramatically declined since 2000 through mass vaccination campaigns, persistent immunization gaps remain. According to the latest WHO report, global measles cases in 2023 reached an estimated 10.3 million, marking a 20% increase from 2022 and a notable resurgence compared to previous low incidence levels. Several countries experienced large-scale outbreaks, underscoring the risks associated with insufficient vaccine coverage (6, 7). Furthermore, the COVID-19 pandemic significantly disrupted routine immunization services (8, 9). Lockdown measures and healthcare resource reallocation during the pandemic led to substantial declines in vaccination rates across multiple nations (10). This reduction in immunization coverage directly exacerbated existing immunity gaps, triggering post-pandemic resurgences of vaccine-preventable diseases. Notably, upward trends in measles, rubella, mumps, and varicella cases have been observed not only in low-income countries but also in developed nations (11).
China has progressively optimized its vaccination strategies since the introduction of measles vaccine in 1965. The single-dose measles vaccination implemented in 1978 was replaced by a two- dose regimen (8 months and 7 years) in 1986, with the timing of the second dose moved earlier, to 18 months of age, in 2005. The national immunization program incorporated measles-rubella (MR) and measles-mumps-rubella (MMR) combination vaccines in 2007, and recommended two MMR doses at 8 and 18 months in 2020 (12, 13). Through five major immunization strategy adjustments from 1965 to 2020, China achieved remarkable progress in measles control, with incidence rates plummeting from 1432.4 per 100,000 in 1959 to 0.06 per 100,000 in 2020 (13). However, regional and temporal disparities in policy implementation persist. The 2019 measles outbreaks in certain areas revealed vulnerabilities in national immunity barriers. While varicella vaccine remained voluntary and self-funded with single-dose recommendation for decades, over half of Jiangsu Province’s cities adopted two-dose varicella vaccination in regional immunization programs starting 2020 (14), expanding province-wide by January 2023. Rubella, although largely controlled, remains a concern due to its ability to cause congenital rubella syndrome (CRS) in infants born to susceptible women. While rubella-containing vaccines have been integrated into China’s routine immunization schedule since the mid-2000s, immunity gaps persist among adolescents and adults born before its inclusion (15). Mumps is generally a self-limiting disease, but can result in complications such as orchitis, meningitis, and hearing loss. Outbreaks have been documented among school-age children and young adults, even among those with a history of one or two doses of mumps-containing vaccines, suggesting waning immunity or suboptimal vaccine effectiveness (16). Compared with countries employing MMRV (measles-mumps-rubella-varicella) quadrivalent vaccine strategies (17), China’s current single-vaccine approach may present limitations in enhancing compliance and achieving cross-protective immunity.
This study aimed to evaluate trends in population immunity levels against measles, rubella, mumps, and varicella in Suqian City, Jiangsu Province, China, across a 10-year period (2005–2024), using a dual cross-sectional serological survey (2019–2024) and disease incidence analysis. By incorporating participants with both known and unknown vaccination statuses, this study examines overall immunity dynamics in the context of evolving immunization policies, providing insights for future public health planning while recognizing the constraints of incomplete vaccination status data.
2 Materials and methods
2.1 Surveillance of measles, rubella, mumps, and varicella
In China, measles, rubella, and mumps are notifiable diseases monitored through the National Notifiable Disease Reporting System (NNDRS), a web-based platform that requires reporting of both clinically diagnosed and laboratory-confirmed cases. This surveillance system provided the data used to determine the incidence rates of measles, rubella, mumps, and varicella in Jiangsu Province analyzed in this study. Although varicella is not officially classified as a statutory notifiable disease, it is routinely surveilled and reported using the same protocols in practice in Jiangsu province. All reported cases are recorded by date of symptom onset and classified based on clinical diagnosis, laboratory confirmation, or epidemiological linkage, in accordance with national guidelines. Incidence rates are calculated per 100,000 population using demographic data from the National Bureau of Statistics. Since 1965, China has introduced a series of measles vaccination policy adjustments. These include the implementation of a single−dose schedule in 1978; a two−dose measles or measles–rubella schedule at 8 months and 7 years in 1986; rescheduling of the second measles−only dose to 18 months in 2005; and, in 2020, optimizing the program to a two−dose measles–mumps–rubella (MMR) schedule at 8 and 18 months, thereby introducing the more advantageous combined vaccine. For varicella, Jiangsu Province began piloting a two-dose schedule in select cities in 2020, with province-wide implementation by January 2023. These policy changes aim to improve population immunity and reduce the incidence of vaccine-preventable diseases.
2.2 Study participants
Serum samples were obtained from the Jiangsu Provincial Surveillance Program on Immunization Levels for Vaccine-Preventable Diseases in Healthy Populations. Suqian City served as the single-center study site. Residual serum samples collected from healthy individuals in 2019 (n = 541) and 2024 (n = 506) were analyzed, yielding a total of 1,047 samples. To ensure that seroprevalence reflected vaccine-induced immunity, individuals with recent vaccination (within six months) or a confirmed history of measles, rubella, mumps, or varicella infection were excluded. A stratified random sampling approach was used to enhance representativeness, with stratification based on age, sex, and geographic distribution across Suqian’s districts and counties. Age groups were defined as follows: 0–11 months, 12–23 months, 2–5 years, 6–18 years, 19–29 years, and ≥30 years. These corresponded to birth cohorts of approximately 2018-2019, 2017-2018, 2014-2017, 2001-2013, 1990-2000, and ≤1989 for the 2019 sample; and 2023-2024, 2022-2023, 2019-2022, 2006-2018, 1995-2005, and ≤1994 for the 2024 sample. Within each age group, samples were stratified by sex to ensure an approximately equal male-to-female ratio. Geographic stratification was conducted according to population distributions derived from the National Bureau of Statistics of China, with sample sizes allocated proportionally across administrative divisions. Random sampling was then performed within each stratum. Identical sampling procedures were used in both years to ensure comparability. For participants under 14 years of age, vaccination records were verified using official immunization documents or the Jiangsu Provincial Vaccination Integrated Service Management Information System. For individuals aged 14 years and older, vaccination history was primarily obtained through self-report, and participants with missing or unverified records were categorized as the “unknown” group. These participants were retained in the analysis to reflect real-world immunity levels, as their exclusion could introduce selection bias, especially in adult populations where vaccination records are often incomplete. Disease history was collected via participant recall.
2.3 Laboratory testing
Blood samples were collected from all participants. Serum was separated and stored at –20°C until analysis. IgG antibodies specific to measles, mumps, rubella, and varicella were measured using enzyme-linked immunosorbent assay (ELISA) kits (SERION ELISA classic, Institut Virion/Serion GmbH, Würzburg, Germany). All testing was performed at the central laboratory of the Affiliated Suqian First People’s Hospital of Nanjing Medical University. Quantitative analysis was conducted to determine seroprotection levels. Antibody concentrations were interpreted according to the manufacturer’s guidelines, which are based on internal validation and referenced literature. The cutoff values for seroprotection were as follows: Measles: ≥200 mIU/mL, Mumps: ≥100 IU/mL, Rubella: ≥20 IU/mL, Varicella: ≥50 mIU/mL.
2.4 Statistical analysis
Data were analyzed using Microsoft Excel and R software. Seroprevalence estimates were calculated with 95% confidence intervals (CIs) using the Clopper–Pearson exact binomial method. Geometric mean concentrations (GMCs) of measles- and rubella-specific IgG antibodies were summarized using medians and interquartile ranges [M (IQR)]. For comparisons of seroprevalence across ordered categories, the trend chi-square test was applied; for comparisons among unordered groups, the standard chi-square test was used. The primary dependent variables included binary IgG serostatus (positive/negative) for each virus and GMCs of virus-specific IgG antibodies. Independent variables were age group, sex, vaccination history, and survey year. Because antibody concentrations are often skewed or contain outliers, non-parametric tests (Wilcoxon rank-sum and Kruskal–Wallis) were used to compare GMCs, ensuring robustness and validity without assuming normality. Overall comparisons of seroprevalence and GMCs between 2019 and 2024 were conducted. Subgroup comparisons by age, sex, or vaccination history were not performed due to limited sample sizes and the need to reduce multiple testing bias. However, subgroup results are presented descriptively for transparency. All statistical tests were two-sided, and p-values <0.05 were considered statistically significant.
3 Results
3.1 Reported cases and incidence rates of measles, rubella, mumps, and varicella
As illustrated in Figure 1, Jiangsu Province exhibited significant declines in reported cases and incidence rates of varicella, measles, rubella, and mumps (2005–2024). Varicella incidence decreased from 183.3 per 100,000 in 2019 to 59.3 per 100,000 in 2024 (67.6% reduction). Measles incidence declined from 11.2 per 100,000 to 0.01 per 100,000 (99.9% reduction). Rubella incidence dropped from 2.8 to 0.04 per 100,000 (98.6% reduction), and mumps incidence fell from 24.4 to less than 5.0 per 100,000 (79.5% reduction).
Figure 1

Reported cases and incidence of measles (A), rubella (B), mumps (C) and varicella (D) from 2005–2024 in Jiangsu Province, China.
3.2 Baseline characteristics of study participants
A total of 541 samples were collected in 2019 and 506 in 2024. The sex distribution was balanced in both years, with males and females each accounting for about half of the participants. In both years, participants were distributed across all age groups. The proportion of children aged 6–18 years was higher in 2024 (30.4%) than in 2019 (22.2%), while the proportion of those aged 0–5 years was slightly lower in 2024. MMR vaccination history improved over time. In 2019, 10.5% of participants had not received any MMR dose, while in 2024, all participants had received at least one dose. The proportion of participants with two or more MMR doses increased from 36.4% in 2019 to 48.0% in 2024 (Table 1).
Table 1
| Characteristic | 2019 (N=541) | 2024 (N=506) | P-value |
|---|---|---|---|
| Sex | 0.531 | ||
| Male | 273 (50.5%) | 247 (48.8%) | |
| Female | 268 (49.5%) | 259 (51.2%) | |
| Age Group | 0.032* | ||
| 0-11 months | 10 (1.8%) | 5 (1.0%) | |
| 12-23 months | 62 (11.5%) | 43 (8.5%) | |
| 2-5 years | 113 (20.9%) | 88 (17.4%) | |
| 6-18 years | 120 (22.2%) | 154 (30.4%) | |
| 19-29 years | 101 (18.7%) | 82 (16.2%) | |
| ≥30 years | 135 (25.0%) | 134 (26.5%) | |
| Vaccination History(MMR) | <0.001* | ||
| 0 doses | 57 (10.5%) | 0 (0%) | |
| 1 dose | 37 (6.8%) | 24 (4.7%) | |
| ≥2 doses | 197 (36.4%) | 243 (48.0%) | |
| Unknown | 250 (46.2%) | 239 (47.2%) |
Sample Distribution by Sex, Age, and Vaccination History in 2019 and 2024.
*a statistically significant difference between 2019 and 2024 (p < 0.05).
3.3 Seropositivity rates of measles, rubella, mumps, and varicella across different populations
In 2019, the seropositivity rates for measles, rubella, mumps, and varicella were 85.9%, 65.1%, 77.6%, and 76.9%, respectively. By 2024, significant increases were observed in rubella (70.8%) and mumps (85.8%) seropositivity (p<0.05), while varicella also rose to 79.5%(p>0.05), indicating improved population immunity against these three diseases. Notably, measles seropositivity declined from 85.9% in 2019 to 79.3% in 2024 (p<0.05), suggesting potential immunity gaps in measles protection. Gender analysis revealed no significant differences in seropositivity rates between males and females in either 2019 or 2024 (P>0.05), indicating minimal gender-based variations in immune responses to these four diseases. Age-stratified analysis showed significant differences among age groups (P<0.05), with children aged 2–5 years demonstrating higher seropositivity rates for all four diseases in 2024, while those under 2 years (particularly 0–11 months) exhibited lower overall seropositivity. Regarding vaccination history, following the immunization strategy adjustment, the measles seropositivity rate among recipients of two doses of measles-containing vaccine decreased from 94.4% in 2019 to 88.1% in 2024, while significant improvements were observed for rubella (84.8%), mumps (91.4%), and varicella (77.9%) seropositivity rates (Tables 2A, B, Figure 2).
Table 2
| Characteristics | Measles | Rubella | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Survey year | 2019 N positive, % (95%CI) | 2024 N positive, % (95%CI) | P-value | 2019 N positive, % (95%CI) | 2024 N positive, % (95%CI) | P-value | ||||
| Sex | ||||||||||
| Male | 227 | 83.1 (78.3-87.1) | 188 | 76.1 (70.4-81.0) | 0.049* | 182 | 66.7 (60.9-72.0) | 172 | 69.6 (63.6-75.0) | 0.468 |
| Female | 238 | 88.8 (84.5-92.0) | 213 | 82.2 (77.1-86.4) | 0.035* | 170 | 63.4 (57.5-68.9) | 186 | 71.8 (66.0-76.9) | 0.043* |
| P value | 0.058 | 0.090 | 0.430 | 0.591 | ||||||
| Age | ||||||||||
| 0-11 months | 1 | 10.0 (1.8-40.4) | 2 | 40.0 (11.8-76.9) | 0.494 | 2 | 20.0 (5.7-50.9) | 2 | 40.0 (11.8-76.9) | 0.494 |
| 12-23 months | 50 | 80.7 (69.1-88.6) | 16 | 37.2 (24.4-52.1) | <0.001* | 44 | 70.9 (58.7-80.8) | 12 | 27.9 (16.8-42.7) | <0.001* |
| 2-5 years | 99 | 87.6)80.3-92.5) | 88 | 100.0 | 0.002* | 81 | 71.7 (62.8-79.2) | 84 | 95.5 (88.9-98.2) | <0.001* |
| 6-18 years | 108 | 90.0)83.3-94.2) | 124 | 80.5 (73.6-86.0) | 0.031* | 75 | 62.5)53.6-70.6) | 123 | 79.9 (72.8-85.4) | <0.001* |
| 19-29 years | 88 | 87.1 (79.2-92.3) | 53 | 64.6 (53.8-74.1) | <0.001* | 60 | 59.4 (49.7-68.5) | 40 | 48.8 (38.3-59.4) | 0.150 |
| ≥30 | 119 | 88.2 (81.6-92.6) | 118 | 88.1 (81.5-92.5) | 0.961 | 90 | 66.7 (58.4-74.1) | 97 | 72.4 (64.3-79.3) | 0.308 |
| aP value | 0.000 | 0.003 | 0.864 | 0.651 | ||||||
| Vaccination history | ||||||||||
| 0 dose | 37 | 64.9 (51.9-76.0) | – | – | (N/A) | 32 | 56.1 (43.2-68.2) | – | – | (N/A) |
| 1 dose | 35 | 94.6 (82.3-98.5) | 0 | – | <0.001* | 27 | 72.9 (57.0-84.6) | 2 | 8.3 (2.3-25.8) | <0.001* |
| ≥ 2 doses | 186 | 94.4 (90.3-96.9) | 214 | 88.1 (83.4-91.6) | 0.01* | 135 | 68.5 (61.7-74.6) | 206 | 84.8 (79.7-88.7) | <0.001* |
| unknown | 207 | 82.8 (77.6-86.9) | 187 | 78.2 (72.6-83.0) | 0.186 | 158 | 63.2 (57.1-68.9) | 150 | 62.8 (56.5-68.6) | 0.950 |
| aP value | 0.263 | 0.946 | 0.547 | 0.001 | ||||||
| Total | 465 | 85.9 (82.8-88.6) | 401 | 79.3 (75.5-82.6) | <0.05* | 352 | 65.1 (60.9-68.9) | 358 | 70.8 (66.6-74.6) | <0.05* |
(A) Seroprevalence of measles, rubella, mumps in different groups in 2019 and 2024.
aLinear-by-linear association in the chi-square test; *a statistically significant difference between 2019 and 2024 (p < 0.05).
Table 2
| Characteristics | Mumps | Varicella | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Survey year | 2019 N positive, % (95%CI) | 2024 N positive, % (95%CI) | P-value | 2019 N positive, % (95%CI) | 2024 N positive, % (95%CI) | P-value | ||||
| Sex | ||||||||||
| Male | 212 | 77.6 (72.4-82.2) | 207 | 83.8 (78.7-87.9) | 0.099 | 206 | 75.5 (70.0-80.2) | 200 | 80.9 (75.6-85.4) | 0.189 |
| Female | 208 | 77.6 (72.4-82.2) | 227 | 87.6 (83.1-91.1) | 0.011* | 210 | 78.4 (73.1-82.9) | 202 | 77.9 (72.6-82.6) | 0.734 |
| P value | 0.990 | 0.217 | 0.424 | 0.408 | ||||||
| Age | ||||||||||
| 0-11 months | 3 | 30.0 (10.8-60.3) | 2 | 40.0 (11.8-76.9) | 0.595 | 6 | 60.0 (31.3-83.1) | 4 | 80.0 (37.6-96.4) | 0.320 |
| 12-23 months | 37 | 59.7 (47.3-70.9) | 19 | 44.2 (30.4-58.9) | 0.112 | 43 | 69.4 (57.0-79.4) | 20 | 46.5 (32.5-61.1) | 0.015* |
| 2-5 years | 85 | 75.2 (66.5-82.3) | 80 | 90.9 (83.1-95.3) | 0.001* | 69 | 61.1 (51.9-69.6) | 63 | 71.6 (61.4-79.9) | 0.094 |
| 6-18 years | 102 | 85.0 (77.5-90.3) | 148 | 96.1 (91.7-98.2) | <0.001* | 85 | 70.8 (62.2-78.2) | 119 | 77.3 (70.0-83.2) | 0.135 |
| 19-29 years | 76 | 75.3 (66.0-82.6) | 52 | 63.4 (52.6-73.0) | 0.026* | 86 | 85.2 (76.9-90.8) | 72 | 87.8 (78.9-93.2) | 0.601 |
| ≥30 | 117 | 86.7 (79.9-91.4) | 127 | 94.8 (89.6-97.5) | 0.017* | 127 | 94.1 (88.7-96.9) | 114 | 85.1 (78.1-90.1) | 0.013* |
| aP value | 0.000* | 0.000* | 0.000* | 0.000* | ||||||
| Vaccination history | ||||||||||
| 0 dose | 35 | 61.4 (48.4-72.9) | – | – | (N/A)) | 23 | 51.1 (37.0-65.0) | 6 | 60.0 (31.3-83.2) | 0.637 |
| 1 dose | 25 | 67.6 (51.5-80.4) | 6 | 25.0 (12.0-44.9) | 0.001* | 134 | 69.8 (62.9-75.8) | 48 | 56.5, (5.9-66.5) | 0.047* |
| ≥ 2 doses | 164 | 83.3 (77.4-87.8) | 222 | 91.4 (87.2-94.3) | 0.006* | 1 | 100.0 | 134 | 77.9 (71.1-83.5) | <0.001* |
| unknown | 196 | 78.4 (72.9-83.1) | 206 | 86.2 (81.2-89.9) | <0.001* | 258 | 85.2 (80.7-88.7) | 214 | 89.5 (85.0-92.8) | 0.057 |
| aP value | 0.333 | 0.438 | 0.000 | 0.001 | ||||||
| Total | 420 | 77.6 (73.9-80.9) | 434 | 85.8 (82.5-88.5) | <0.05* | 416 | 76.9 (73.2-80.3) | 402 | 79.5 (75.7-82.7) | >0.05 |
(B) Seroprevalence of mumps and Varicella in different groups in 2019 and 2024.
aLinear-by-linear association in the chi-square test; *a statistically significant difference between 2019 and 2024 (p < 0.05).
Figure 2
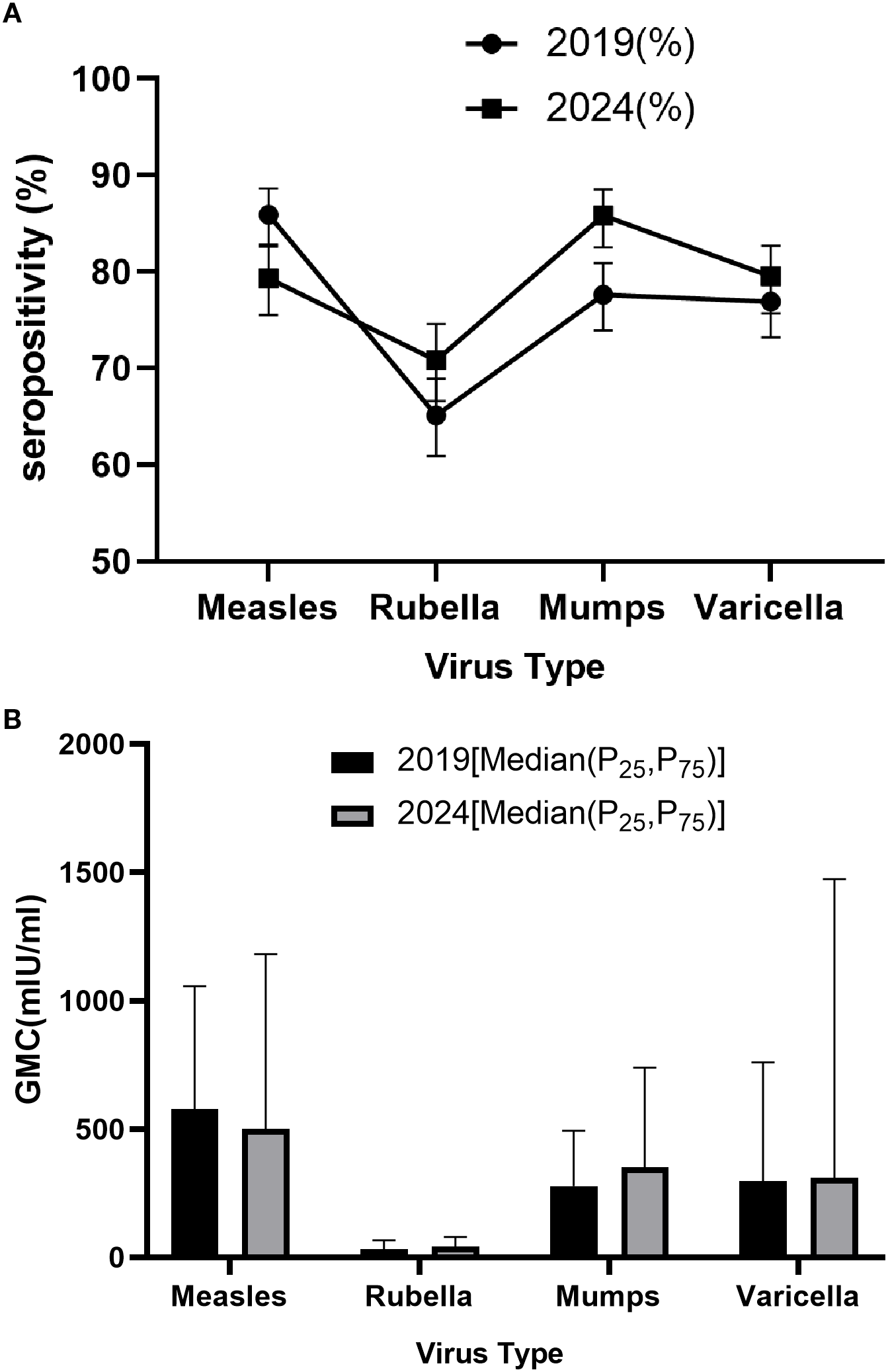
Seropositivity (A) and GMCs (B) of measles, rubella, mumps and varicella, 2019 and 2024.
3.4 GMC of antibodies against measles, rubella, mumps, and varicella in different populations
GMC analysis revealed that from 2019 to 2024, measles antibody levels decreased from 577.9 mIU/ml (IQR: 298.2-1056.3) to 499.2 mIU/ml (IQR: 220.6-1181.1) (p<0.001), while antibody levels increased for rubella (31.5 to 42.2 IU/ml), mumps (277.8 to 350.6 IU/ml), and varicella (295.9 to 309.8 IU/ml). Gender analysis showed no significant differences in GMC for measles, rubella, mumps, and varicella between males and females in both 2019 and 2024 (Wilcoxon rank-sum test, p>0.05). Age group analysis indicated significant differences in GMC across different age groups for all four diseases (Kruskal-Wallis H test, p<0.001). Specifically, in 2024, children aged 2–5 years achieved the highest GMC values: 1367.2 mIU/ml (IQR: 649.8-2869.5) for measles, 74.9 IU/ml (IQR: 41.4-122.6) for rubella, 570.6 IU/ml (IQR: 288.7-1039.3) for mumps, and 133.8 mIU/ml (IQR: 35.4-262.5) for varicella. Vaccination history analysis also showed significant differences in GMC among different vaccination dose groups for the four diseases (Kruskal-Wallis H test, p<0.001). The GMC trends aligned with seropositivity patterns, showing noticeable increases for all three diseases except measles, which demonstrated a slight decline (Tables 3A, B, Figure 2).
Table 3
| Characteristics | Measles | Rubella | ||||
|---|---|---|---|---|---|---|
| Survey year | 2019 Median (IQR:P25-P75) (mIU/ml, IU/ml) |
2024 Median (IQR:P25-P75) (mIU/ml, IU/ml) |
P-value | 2019 Median (IQR:P25-P75) (mIU/ml, IU/ml) |
2024 Median (IQR:P25-P75) (mIU/ml, IU/ml) |
P-value |
| Sex | ||||||
| Male | 605.3 (286.5-1141.5) | 552.9 (270.5-1338.0) | 0.444 | 32.5 (13.2-66.0) | 40.7 (14.5-72.1) | 0.004* |
| Female | 548.8 (308.7-996.1) | 431.6 (206.5-1013.2) | 0.045* | 29.9 (13.2-70.2) | 42.8 (17.9-84.4) | 0.125 |
| aP value | 0.872 | 0.013 | 0.617 | 0.334 | ||
| Age | ||||||
| 0-11 months | 49.9 (35.2-141.9) | 192.4 (77.6-490.4) | 0.129 | 4.3 (2.4-14.7) | 16.3 (12.4-60.1) | 0.040* |
| 12-23 months | 569.7 (159.2-1155.5) | 97.3 (29.5-1585.9) | 0.018* | 46.1 (13.2-106.4) | 7.2 (2.7-71.1) | 0.001* |
| 2-5 years | 689.1 (347.9-1190.2) | 1367.2(649.8-2869.5) | <0.001* | 41.1 (14.1-88.3) | 74.9 (41.4-122.6) | <0.001* |
| 6-18 years | 533.0 (313.7-1001.4) | 392.7 (228.2-688.2) | 0.002* | 25.4 (15.4-49.6) | 45.6 (23.1-71.7) | <0.001* |
| 19-29 years | 544.3 (298.9-857.0) | 247.3 (162.8-408.9) | <0.001* | 25.7 (8.8-58.3) | 19.1 (6.9-46.9) | 0.183 |
| ≥30 | 646.3 (314.5-1176.4) | 749.3 (389.6-1359.5) | 0.183 | 32.4 (13.6-64.5) | 44.5 (18.1-91.4) | 0.011* |
| bP value | 0.000 | 0.000 | 0.000 | 0.000 | ||
| Vaccination history | ||||||
| 0 dose | 286.9 (124.5-627.3) | – | – | 24.4 (3.7-49.4) | – | – |
| 1 dose | 980.9 (440.0-1621.5) | 32.7 (24.0-91.6) | 0.265 | 58.3 (18.9-142.7) | 2.9 (2.4-7.2) | 0.132 |
| ≥ 2 doses | 690.6 (373.8-1233.8) | 644.7 (353.5-1491.4) | 0.278 | 33.9 (16.2-78.5) | 54.1 (28.1-91.2) | 0.172 |
| unknown | 538.9 (257.5-992.6) | 429.5 (215.1-934.3) | 0.213 | 29.6 (10.3-57.9) | 34.4 (12.6-62.7) | 0.118 |
| bP value | 0.000 | 0.000 | 0.000 | 0.001 | ||
| Total | 577.9 (298.2-1056.3) | 499.2 (220.6-1181.1) | >0.05 | 31.5 (13.2-66.7) | 42.2 (16.4-79.5) | <0.05* |
(A) GMC of measles, rubella, mumps in different groups in 2019 and 2024.
aWilcoxon rank-sum test;bKruskal–Wallis H test; *a statistically significant difference between 2019 and 2024 (p < 0.05).
Table 3
| Characteristics | Mumps | Varicella | ||||
|---|---|---|---|---|---|---|
| Survey year | 2019 Median (IQR:P25-P75) (mIU/ml, IU/ml) |
2024 Median (IQR:P25-P75) (mIU/ml, IU/ml) |
P-value | 2019 Median (IQR:P25-P75) (mIU/ml, IU/ml) |
2024 Median (IQR:P25-P75) (mIU/ml, IU/ml) |
P-value |
| Sex | ||||||
| Male | 285.9 (113.6-510.0) | 310.9 (147.3-667.1) | <0.001* | 397.2 (50.6-909.2) | 438.9 (86.4-1471.6) | 0.014* |
| Female | 255.5 (115.4-474.6) | 428.1 (180.9-834.0) | 0.031* | 271.4 (62.8-660.3) | 280.8 (70.9-1527.3) | 0.006* |
| aP value | 0730 | 0.003 | 0.218 | 0.248 | ||
| Age | ||||||
| 0-11 months | 58.5 (11.6-163.6) | 74.9 (58.2-446.9) | 0.254 | 91.5 (20.5-245.9) | 600.4 (107.7-5069.4) | 0.165 |
| 12-23 months | 153.7 (32.9-402.8) | 74.8 (24.3-406.9) | 0.553 | 283.7 (31.8-711.3) | 45.8 (24.7-234.8) | 0.016* |
| 2-5 years | 304.6 (106.0-566.7) | 570.6 (288.7-1039.3) | <0.001* | 63.8 (25.9-479.1) | 133.8 (35.4-262.5) | 0.124 |
| 6-18 years | 295.6 (141.2-537.9) | 441.0 (200.3-938.9) | <0.001* | 168.1 (41.9-714.9) | 236.1 (68.3-1090.6) | 0.087 |
| 19-29 years | 236.3 (102.7-400.9) | 171.9 (95.3-393.8) | 0.424 | 464.6 (203.9-924.5) | 363.5 (119.9-1647.3) | 0.792 |
| ≥30 | 325.2 (152.4-507.5) | 366.9 (221.5-619.8) | 0.024* | 562.2 (269.0-1010.7) | 1580.5 (716.5-2932.5) | <0.001* |
| bP value | 0.000 | 0.000 | 0.000 | 0.000 | ||
| Vaccination history | ||||||
| 0 dose | 168.8 (39.5-402.5) | – | – | 51.1 (14.9-91.9) | 80.3 (36.2-1869.6) | 0.097 |
| 1 dose | 234.9 (46.2-406.9) | 28.8 (21.9-107.0) | 0.623 | 178.4 (33.5-618.5) | 91.3 (24.7-304.4) | 0.031* |
| ≥ 2 doses | 311.9 (142.9-577.3) | 526.1 (246.5-1048.6) | 0.325 | – | 203.9 (70.7-490.3) | – |
| unknown | 267.3 (121.7-453.6) | 296.5 (157.1-557.7) | 0.025* | 509.6 (198.2-913.9) | 1023.8 (280.8-2213.9) | <0.001* |
| bP value | 0.000 | 0.000 | 0.000 | 0.010 | ||
| Total | 277.8 (115.2-493.4) | 350.6 (164.7-739.2) | <0.05* | 295.9 (54.9-759.9) | 309.8 (80.7-1472.7) | <0.05* |
(B) GMC of mumps and Varicella in different groups in 2019 and 2024.
aWilcoxon rank-sum test; bKruskal–Wallis H test; *a statistically significant difference between 2019 and 2024 (p < 0.05).
4 Discussion
This study examined trends in population immunity against measles, rubella, mumps, and varicella in Suqian City, Jiangsu Province, China, between 2019 and 2024, using serological surveys and disease incidence data. Our findings reveal notable shifts in immunity levels: seropositivity rates and GMCs for rubella, mumps, and varicella increased, while measles immunity declined. These trends, accompanied by significant reductions in disease incidence, underscore the importance of maintaining robust population immunity to ensure collective protection.
The inclusion of participants with unknown vaccination status prevents us from directly attributing these immunity trends to specific vaccination strategies. Instead, the observed changes likely reflect a combination of factors, including natural exposure, overall vaccination coverage, and public health measures. For instance, varicella incidence dropped from 183.3 per 100,000 in 2019 to 59.3 per 100,000 in 2024, alongside improvements in seropositivity and GMCs. While this aligns with the implementation of a two-dose varicella vaccination strategy in Jiangsu Province, the lack of complete vaccination data limits definitive conclusions about its direct impact. Similarly, rubella and mumps seropositivity rates rose from 65.1% and 77.6% to 70.8% and 85.8%, respectively, with corresponding GMC increases and incidence reductions of 96.7% and 75.6%. These improvements coincide with global evidence on combined vaccines (18), but other factors—such as strengthened school-based prevention policies since 2019 and social distancing during the COVID-19 pandemic—may have contributed (19).
Measles presents a contrasting trend, with declines in seropositivity and GMCs. This may be linked to multiple factors: disruptions in routine immunization during the COVID-19 pandemic, waning immunity in older age groups (e.g., 19–29 years, where seropositivity fell from 87.1% to 64.6%), and reduced natural boosting due to extremely low disease prevalence (0.01/100,000 in 2024) (20). These findings suggest that unvaccinated or under-vaccinated populations remain vulnerable, highlighting the need for targeted catch-up campaigns to address age-specific immunity gaps (21).
Interestingly, no unvaccinated individuals were identified in the 2024 sample, and the proportion of one-dose MMR recipients decreased significantly since 2019. This could be explained by recruitment from routine immunization clinics, improvements in the Jiangsu Immunization Information System, post-pandemic MMR catch-up campaigns, and the younger age structure of the 2024 cohort, which likely benefited from higher two-dose coverage. Critically, by including participants with unknown vaccination status, our findings reflect real-world population immunity rather than isolated vaccine effects. Age-stratified analysis further revealed that preschoolers (2–5 years) exhibited the highest immunity levels, likely due to recent two-dose MMR completion by 18 months, reflecting policy effectiveness. While infants (0–11 months) showed low seropositivity and GMCs, indicating vulnerability before primary immunization. Conversely, adolescents and adults (born before 2005, not included in the EPI at birth) had lower rates of verified two-dose vaccination, particularly those aged 19–29 years, supporting the need for targeted catch-up strategies in these birth cohorts.
The study’s findings prompt consideration of alternative vaccination approaches, such as the MMRV quadrivalent vaccine used internationally (22, 23), which could reduce clinic visits and enhance compliance (24). However, challenges in China (25, 26), including limited domestic production capacity and regulatory hurdles, must be addressed to adopt such strategies. In the meantime, optimizing first-dose timing for infants and boosting immunity in older groups could strengthen population protection.
This study has some limitations. First, single-center design limits generalizability. Second, self-reported vaccination history risks recall bias. Third, subgroup comparisons were not statistically tested due to sample size constraints. Most importantly, by design, inclusion of participants with unknown vaccination status directs focus toward population immunity assessment rather than vaccine-specific effectiveness. Additionally, advanced methods like dynamic modeling and single-cell analyses could refine our understanding of immune response variability and inform precision immunization strategies (27–29).
5 Conclusion
Population immunity against rubella, mumps, and varicella improved from 2019 to 2024, accompanied by reduced incidence, while measles immunity declined, indicating potential vulnerabilities. These trends reflect complex interactions of immunization policies, public health measures, and demographic factors. Targeted strategies (e.g., catch-up campaigns for adolescents/adults, optimized infant vaccination timing, advanced MMRV vaccine evaluation) are needed to strengthen population protection.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Jiangsu Provincial Center for Disease Control and Prevention. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
ZG: Data curation, Formal Analysis, Writing – review & editing, Writing – original draft. WW: Funding acquisition, Data curation, Writing – review & editing, Writing – original draft. YZ: Writing – review & editing, Writing – original draft, Investigation, Software, Formal Analysis. YX: Data curation, Writing – original draft, Writing – review & editing, Investigation. QC: Methodology, Writing – review & editing, Data curation, Writing – original draft, Software. LZ: Supervision, Writing – review & editing, Data curation, Writing – original draft. JY: Methodology, Software, Writing – review & editing, Investigation, Writing – original draft. XS: Writing – original draft, Writing – review & editing. ZW: Resources, Writing – original draft, Formal Analysis, Writing – review & editing, Data curation.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Opening Foundation of Key Laboratory under Grant JSHD2022043; the Jiangsu Provincial Geriatric Health Research Project under Grant LKM2023005; Jiangsu Province Youth Science and Technology Talent Support Project under Grant JSTJ-2024-644; Research Project of Jiangsu Provincial Health Commission under Grant Z2024003; Suqian Sci&Tech Program under Grant SY202312; Jiangsu Provincial Health Commission Preventive Medicine and Hematology Prevention Research Project under Grant Ym2023099; Suqian Sci&Tech Program under Grant S202312.
Acknowledgments
The authors acknowledge the staff of SiYang CDC for their invaluable support in the collection and analysis of the data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Talbird SE Carrico J La EM Carias C Marshall GS Roberts CS et al . Impact of routine childhood immunization in reducing vaccine-preventable diseases in the United States. Pediatrics. (2022) 150:1–38. doi: 10.1542/peds.2021-056013
2
Minta AA Ferrari M Antoni S Portnoy A Sbarra A Lambert B et al . Progress toward measles elimination - worldwide, 2000-2022. Mmwr Morb Mortal Wkly Rep. (2023) 72:1262–68. doi: 10.15585/mmwr.mm7246a3
3
Hviid A Rubin S Mühlemann K . Mumps. Lancet. (2008) 371:932–44. doi: 10.1016/S0140-6736(08)60419-5
4
Winter AK Moss WJ . Rubella. Lancet. (2022) 399:1336–46. doi: 10.1016/S0140-6736(21)02691-X
5
Freer G Pistello M . Varicella-zoster virus infection: natural history, clinical manifestations, immunity and current and future vaccination strategies. New Microbiol. (2018) 41:95–105.
6
Bidari S Yang W . Global resurgence of measles in the vaccination era and influencing factors. Int J Infect Dis. (2024) 147:107189. doi: 10.1016/j.ijid.2024.107189
7
Al-Tawfiq JA Jain N Tanasov A Schlagenhauf P . Measles matter: Recent outbreaks highlight the need for catch-up vaccination in Europe and around the globe. New Microbes New Infect. (2024) 58:101238. doi: 10.1016/j.nmni.2024.101238
8
Ota M Badur S Romano-Mazzotti L Friedland LR . Impact of COVID-19 pandemic on routine immunization. Ann Med. (2021) 53:2286–97. doi: 10.1080/07853890.2021.2009128
9
Bramer CA Kimmins LM Swanson R Kuo J Vranesich P Jacques-Carroll LA et al . Decline in child vaccination coverage during the COVID-19 pandemic - Michigan Care Improvement Registry, May 2016-May 2020. Am J Transplant. (2020) 20:1930–31. doi: 10.1111/ajt.16112
10
Shet A Carr K Danovaro-Holliday MC Sodha SV Prosperi C Wunderlich J et al . Impact of the SARS-CoV-2 pandemic on routine immunisation services: evidence of disruption and recovery from 170 countries and territories. Lancet Glob Health. (2022) 10:e186–94. doi: 10.1016/S2214-109X(21)00512-X
11
Liu Q Bi Q Liu S Xiu Y Wang F Yin Z et al . Vaccination status and incidences of measles, mumps, and rubella - worldwide, 2014-2023. China Cdc Wkly. (2025) 7:561–67. doi: 10.46234/ccdcw2025.094
12
Yan R He H Deng X Zhou Y Tang X Zhu Y et al . A serological survey of measles and rubella antibodies among different age groups in eastern China. Vaccines (Basel). (2024) 12:1–12. doi: 10.3390/vaccines12080842
13
Wang Q Cheng X Liu D Chen C Yao K . One single-center serological survey on measles, rubella and mumps antibody levels of people in Youyang, China. Hum Vaccin Immunother. (2021) 17:4203–09. doi: 10.1080/21645515.2021.1924522
14
Sun X Zhu Y Sun H Xu Y Zhang L Wang Z . Comparison of varicella outbreaks in schools in China during different vaccination periods. Hum Vaccin Immunother. (2022) 18:2114255. doi: 10.1080/21645515.2022.2114255
15
Zhuo Y Lu Z Zhang X Zhang X Yang Y Han J et al . Rubella antibody levels in the healthy Chinese population: a meta-analysis. Front Immunol. (2024) 15:1472189. doi: 10.3389/fimmu.2024.1472189
16
Melgar M Yockey B Marlow MA . Impact of vaccine effectiveness and coverage on preventing large mumps outbreaks on college campuses: Implications for vaccination strategy. Epidemics. (2022) 40:100594. doi: 10.1016/j.epidem.2022.100594
17
Macartney K Gidding HF Trinh L Wang H Dey A Hull B et al . Evaluation of combination measles-mumps-rubella-varicella vaccine introduction in Australia. JAMA Pediatr. (2017) 171:992–98. doi: 10.1001/jamapediatrics.2017.1965
18
Davidkin I Jokinen S Broman M Leinikki P Peltola H . Persistence of measles, mumps, and rubella antibodies in an MMR-vaccinated cohort: a 20-year follow-up. J Infect Dis. (2008) 197:950–56. doi: 10.1086/528993
19
Sun X Xu Y Zhu Y Tang F . Impact of non-pharmaceutical interventions on the incidences of vaccine-preventable diseases during the COVID-19 pandemic in the eastern of China. Hum Vaccin Immunother. (2021) 17:4083–89. doi: 10.1080/21645515.2021.1956227
20
Khampanisong P Pauly M Nouanthong P Vickers MA Virachith S Xaydalasouk K et al . Waning of maternal antibodies against measles suggests a large window of susceptibility in infants in lao people’s democratic republic. Pathogens. (2021) 10:1–12. doi: 10.3390/pathogens10101316
21
Ma C Su Q Hao L Wen N Fan C Cao L et al . Measles epidemiology characteristics and progress toward measles elimination in China, 2012-2013. Chin J Vaccines Immun. (2014) 20:193–99. doi: 10.19914/j.cjvi.2014.03.001
22
Lebo EJ Kruszon-Moran DM Marin M Bellini WJ Schmid S Bialek SR et al . Seroprevalence of measles, mumps, rubella and varicella antibodies in the United States population, 2009-2010. Open Forum Infect Dis. (2015) 2:ofv6. doi: 10.1093/ofid/ofv006
23
Graf W Bertram F Dost K Brennecke A Kowalski V van Rüth V et al . Immunity against measles, mumps, rubella, and varicella among homeless individuals in Germany - A nationwide multi-center cross-sectional study. Front Public Health. (2024) 12:1375151. doi: 10.3389/fpubh.2024.1375151
24
Vesikari T Sadzot-Delvaux C Rentier B Gershon A . Increasing coverage and efficiency of measles, mumps, and rubella vaccine and introducing universal varicella vaccination in Europe: a role for the combined vaccine. Pediatr Infect Dis J. (2007) 26:632–38. doi: 10.1097/INF.0b013e3180616c8f
25
Casabona G Berton O Singh T Knuf M Bonanni P . Combined measles-mumps-rubella-varicella vaccine and febrile convulsions: the risk considered in the broad context. Expert Rev Vaccines. (2023) 22:764–76. doi: 10.1080/14760584.2023.2252065
26
Leung JH Hirai HW Tsoi KK . Immunogenicity and reactogenicity of tetravalent vaccine for measles, mumps, rubella and varicella (MMRV) in healthy children: a meta-analysis of randomized controlled trials. Expert Rev Vaccines. (2015) 14:1149–57. doi: 10.1586/14760584.2015.1057572
27
Ma S Zhang X Dang D Wang W Wang Y Liu L et al . Dynamic characterization of single cells based on temporal cellular mechanical properties. IEEE Trans Nanobiosci. (2023) 22:19–27. doi: 10.1109/TNB.2021.3136198
28
Ma S Wu J Liu Z He R Wang Y Liu L et al . Quantitative characterization of cell physiological state based on dynamical cell mechanics for drug efficacy indication. J Pharm Anal. (2023) 13:388–402. doi: 10.1016/j.jpha.2023.03.002
29
Ma S Dang D Wang W Wang Y Liu L . Concentration optimization of combinatorial drugs using Markov chain-based models. BMC Bioinf. (2021) 22:451. doi: 10.1186/s12859-021-04364-5
Summary
Keywords
vaccination strategy, seroprevalence, measles, rubella, mumps, varicella
Citation
Geng Z, Wang W, Zhu Y, Xu Y, Chen Q, Zhang L, Yu J, Sun X and Wang Z (2025) Impact of vaccination strategy adjustments on antibody levels against measles, rubella, mumps, and varicella in China: a single-center serological survey. Front. Immunol. 16:1630442. doi: 10.3389/fimmu.2025.1630442
Received
17 May 2025
Accepted
25 July 2025
Published
14 August 2025
Volume
16 - 2025
Edited by
T. Mark Doherty, GlaxoSmithKline (Belgium), Belgium
Reviewed by
João Almeida Santos, National Health Institute Doutor Ricardo Jorge (INSA), Portugal
Jana Zibolenova, Comenius University, Slovakia
Shuang Ma, Shenyang Ligong University, China
Updates
Copyright
© 2025 Geng, Wang, Zhu, Xu, Chen, Zhang, Yu, Sun and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Sun, sunstone1@163.com; Zhiguo Wang, 120714991@qq.com; Jing Yu, 390943826@qq.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.