- Department of Orthopedics, The Fourth Hospital of Hebei Medical University, 12 Health Road, Shijiazhuang, Hebei, China
Programmed death-1 (PD-1) and its ligand PD-L1 inhibitors have become pivotal agents in cancer immunotherapy, demonstrating significant efficacy across multiple malignancies. However, beyond regulating T cell activation, the PD-1/PD-L1 axis also exerts complex and critical effects on bone metabolism. Notably, both clinical observations and mechanistic studies have revealed a paradox: on one hand, PD-1/PD-L1 blockade appears to confer bone-protective benefits; on the other hand, it has been associated with bone-related adverse events (AEs) in up to 69% of patients, including pathological fractures and vertebral compression fractures. This review comprehensively explores the bidirectional regulatory effects of the PD-1/PD-L1 pathway on bone metabolism and investigates the underlying mechanisms contributing to these contradictory findings. The discrepancies may be attributed to a combination of clinical variables, microenvironmental conditions, cell-specific responses, and intricate interactions among multiple signaling pathways, including the Wnt/β-Catenin pathway and the PD-L1–PKM2 axis. We further examine the pathophysiological basis of osteoporosis and fragility fractures occurring during PD-1/PD-L1 inhibitor therapy, and argue for their recognition as a subclass of immune-related adverse events (irAEs). Finally, we propose a framework for bone health surveillance and stratified prevention strategies aimed at preserving antitumor efficacy while improving skeletal health and quality of life—offering novel insights into osteoporosis prevention and management in the context of immune checkpoint inhibition.
1 Introduction
Inhibitors targeting programmed death-1 (PD-1) and its ligand PD-L1 have profoundly reshaped cancer therapy. Since the first PD-1 inhibitor received regulatory approval in 2014, these agents have significantly improved overall survival (OS) and progression-free survival (PFS) rates (1), becoming standard treatments for a wide range of malignancies, including non-small cell lung cancer (NSCLC), melanoma, head and neck squamous cell carcinoma, and renal cell carcinoma (2).
Beyond their immunomodulatory effects on T cells, the PD-1/PD-L1 axis plays a multifaceted role in bone metabolism. Murine models with PD-1 or PD-L1 gene knockout exhibit pronounced osteoporotic phenotypes, including decreased trabecular bone volume, disrupted microarchitecture, elevated osteoclastogenesis, and increased RANKL/OPG ratios (3). These outcomes are mediated via multiple signaling cascades that finely regulate bone-resorbing and bone-forming cells (4–6). However, the complexity of these mechanisms has led to conflicting results. While some clinical studies suggest that PD-1/PD-L1 inhibitors exert bone-protective effects (4), others report the opposite, documenting bone-related adverse events in patients receiving immune checkpoint inhibitors (ICIs) (7, 8), even as some individuals maintain stable bone mineral density (9).
At the molecular level, similarly inconsistent findings are observed. Some investigations describe a pro-osteogenic role for PD-1/PD-L1 blockade (10), while others note inhibitory effects on osteoblast differentiation (6). These contradictions are likely influenced by a convergence of factors, including patient-specific clinical features, the immune microenvironment, cell-type-specific responses, soluble PD-1/PD-L2 activity (11), and the bidirectional nature of the Wnt/β-Catenin pathway (5, 12) and PD-L1–PKM2 metabolic signaling (13).
Furthermore, clinical evidence links PD-1/PD-L1 inhibitors to a heightened risk of pathological fractures, vertebral compression fractures, and femoral neck fractures (14), contributing to a cumulative incidence of bone-related adverse events as high as 69% (15). Despite their prevalence and clinical significance, conditions such as osteoporosis and fragility fractures are not formally recognized as immune-related adverse events (irAEs) (16), underscoring a gap in clinical classification and management.
Figure 1 outlines the opportunities and challenges associated with PD-1/PD-L1 blockade in bone metabolism, emphasizing its potential protective effects alongside its regulatory complexity and the under-recognition of skeletal irAEs such as osteoporosis.
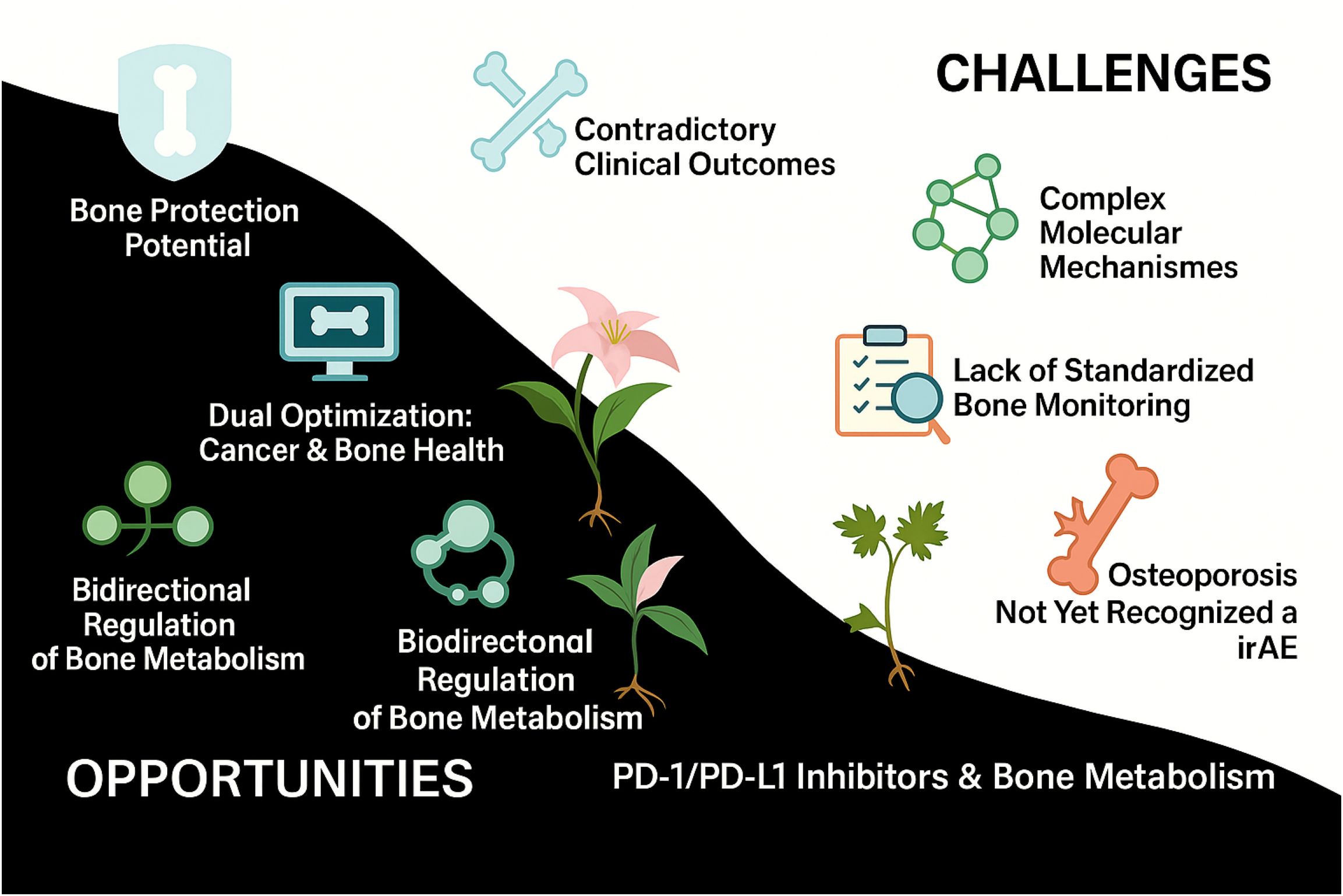
Figure 1. Opportunities and challenges of PD-1/PD-L1 inhibitors in bone metabolism. This schematic illustrates the dual impact of PD-1/PD-L1 inhibitors on bone metabolism. The left panel highlights the potential benefits, including bone-protective effects, bidirectional immuno-skeletal regulation, and dual optimization of tumor control and bone health. In contrast, the right panel outlines key challenges, such as inconsistent clinical outcomes, complex molecular mechanisms, the absence of standardized bone surveillance protocols, and the underrecognition of osteoporosis as an immune-related adverse event (irAE).
To elucidate the dual role of PD-1/PD-L1 inhibitors in bone metabolism and explore their implications in osteoporosis prevention, this review addresses the following key questions:
1. What accounts for the contradictory findings regarding the effects of PD-1/PD-L1 inhibitors on bone metabolism? Why do some studies report bone-protective outcomes (e.g., reduced resorption markers and preserved BMD), while others observe increased bone-related AEs such as vertebral fractures? What clinical factors (e.g., baseline patient characteristics, cancer type, treatment regimens) may underlie these discrepancies?
2. How does the PD-1/PD-L1 pathway achieve bidirectional regulation of bone metabolism? Through which signaling networks does it affect osteoclast differentiation/function and osteoblast activity/mineralization? What roles do the Wnt/β-Catenin pathway and the PD-L1–PKM2 axis play in this context, and how do these mechanisms reconcile clinical inconsistencies?
3. How can the dual regulatory features of PD-1/PD-L1 blockade be translated into osteoporosis prevention strategies? What are the evidence-based approaches for integrating bone health monitoring and stratified prevention during ICI therapy? What justifies the inclusion of osteoporosis and fragility fractures as irAEs, and how can this reclassification enhance both cancer and skeletal outcomes?
2 Effects of PD-1/PD-L1 inhibitors on bone metabolism
PD-1/PD-L1 immune checkpoint inhibitors have become a cornerstone of modern cancer immunotherapy. However, their effects on bone metabolism remain underrecognized and exhibit contradictory findings across studies. Gassner et al. reported that in cancer patients without bone metastases, treatment with PD-1/PD-L1 inhibitors led to a significant early reduction in the bone resorption marker CTX (from a baseline mean of 0.51 ng/ml to 0.42 ng/ml at week 3), while bone formation markers such as PINP and osteocalcin (OCN) increased after 4 months of treatment, suggesting a bone-protective effect (4). Conversely, Pantano et al. observed a marked increase in CTX-I levels and a downward trend in PINP levels after 3 months of immune checkpoint inhibitor (ICI) therapy, which correlated with poor treatment response and decreased survival (7).
Moreover, some studies have documented adverse skeletal events during ICI treatment, including vertebral compression fractures and osteolytic lesions (8). In contrast, others have reported relatively stable bone mineral density (BMD) in ICI-treated patients compared to non-ICI counterparts, suggesting a long-term bone-preserving effect (9). These conflicting outcomes underscore the complex and multifactorial nature of PD-1/PD-L1 blockade on bone metabolism, likely influenced by clinical heterogeneity such as age, sex, tumor type, treatment regimen (monotherapy vs. combination therapy), and baseline skeletal health (17).
Importantly, there remains a lack of standardized bone health monitoring protocols for patients receiving ICIs, potentially delaying the detection and intervention of subclinical bone metabolic disorders (18). This gap may contribute to skeletal-related adverse events (SREs), ultimately impairing therapeutic efficacy and quality of life. Therefore, the impact of PD-1/PD-L1 inhibition on bone health warrants greater clinical attention and systematic evaluation.
3 Regulatory effects of PD-1/PD-L1 pathway and inhibitors on osteoclasts
While the PD-1/PD-L1 axis plays a pivotal role in immune regulation, it also significantly influences bone metabolism, particularly osteoclast differentiation and function. Genetic deletion of PD-1 or PD-L1 leads to osteoporotic phenotypes, implicating this pathway in the maintenance of bone homeostasis (3). Within the tumor microenvironment (TME), upregulation of PD-L1 and CCL2 facilitates osteoclastogenesis by activating the JNK pathway and enhancing CCL2-mediated RANKL signaling, thereby promoting bone resorption (5). Additionally, soluble PD-1 (sPD-1), which is elevated in inflammatory settings, stimulates IL-17A production—a key mediator of osteoclast activation—resulting in accelerated bone destruction. In contrast, PD-L2 expression under inflammatory conditions appears to suppress osteoclastogenesis and confer bone-protective effects (11).
Osteoclasts, particularly in their activated state, can upregulate PD-L1 expression to inhibit T cell proliferation and cytotoxicity, contributing to an immunosuppressive microenvironment. This PD-L1 upregulation is itself modulated by pro-inflammatory cytokines such as IFN-γ and IL-6, forming a feedback regulatory loop (19, 20).
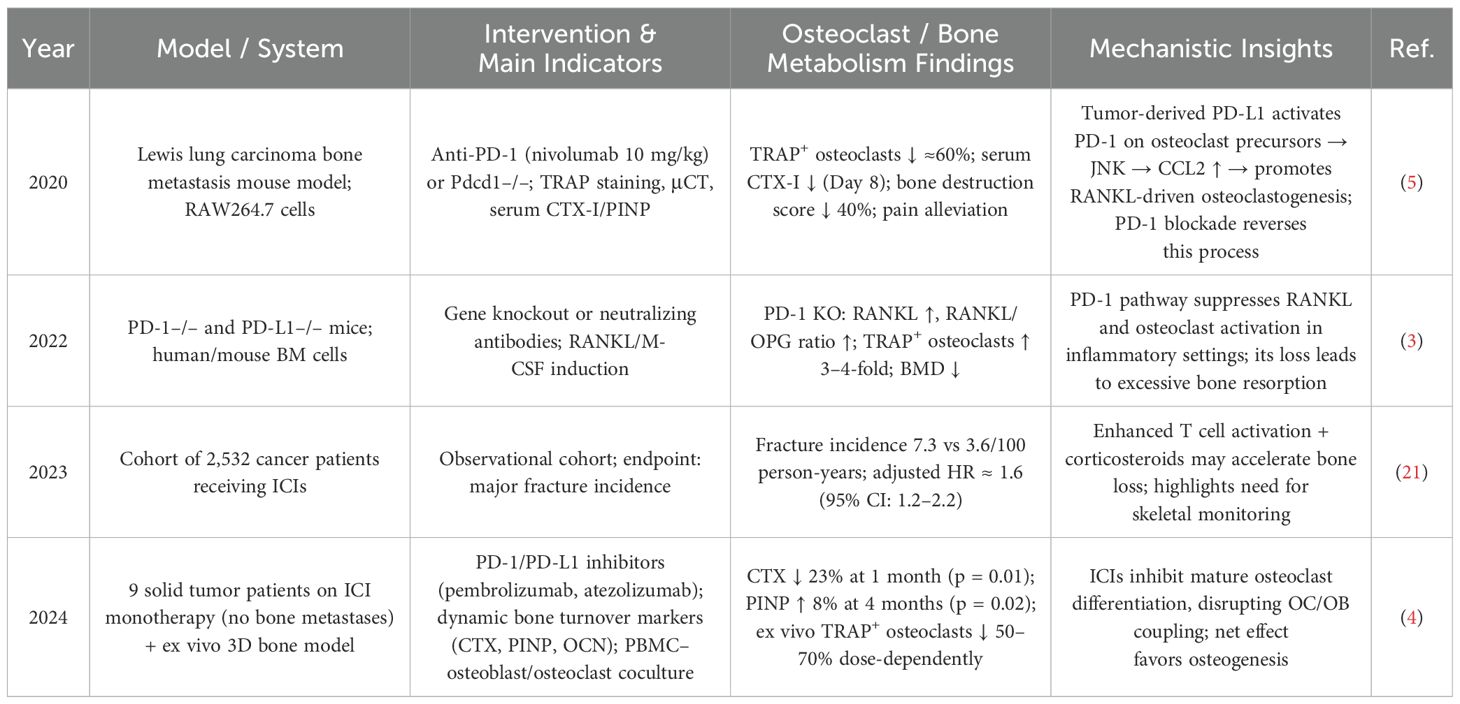
PD-1/PD-L1 inhibitors interrupt this axis and exert both direct and indirect effects on osteoclast activity. By inhibiting the JNK pathway, these agents reduce osteoclast proliferation and resorptive capacity, leading to decreased CTX levels (4, 5). They can also interfere with the STAT3/NFATc1 signaling cascade, impeding pre-osteoclast maturation and reversing osteoclast-mediated immunosuppression (20). This dual mechanism results in a bidirectional modulation of bone remodeling (4), with short-term treatment reducing TRAP+ osteoclasts by approximately 60%, lowering bone destruction scores by 40%, and decreasing CTX by 23%, while increasing PINP levels by 8% at 4 months (p = 0.02) (4, 5). For a detailed summary of these findings across preclinical and clinical contexts, see Table 1.
However, prolonged ICI therapy has been associated with increased fracture risk, with adjusted hazard ratios nearing 1.6 (21), suggesting a shift toward net bone loss over time. Chronic inflammation during extended PD-1 blockade can enhance RANKL expression and osteoclast activation, further contributing to skeletal damage (3).
Wnt/β-Catenin signaling also plays a complex, bidirectional role in mediating the skeletal effects of PD-1/PD-L1 inhibition (Table 2). The canonical Wnt/β-Catenin pathway synergizes with short-term PD-1 blockade to suppress osteoclastogenesis and increase bone mass (5). In contrast, the non-canonical Wnt5a–Ror2 axis promotes osteoclast formation and reduces BMD, with Wnt5a overexpression in the TME antagonizing the bone-preserving effects of PD-1 inhibitors (12). Moreover, chronic PD-1 deficiency can suppress canonical Wnt signaling through sustained inflammation, leading to a 72% increase in osteoclast numbers and a 12% decrease in bone density (22).
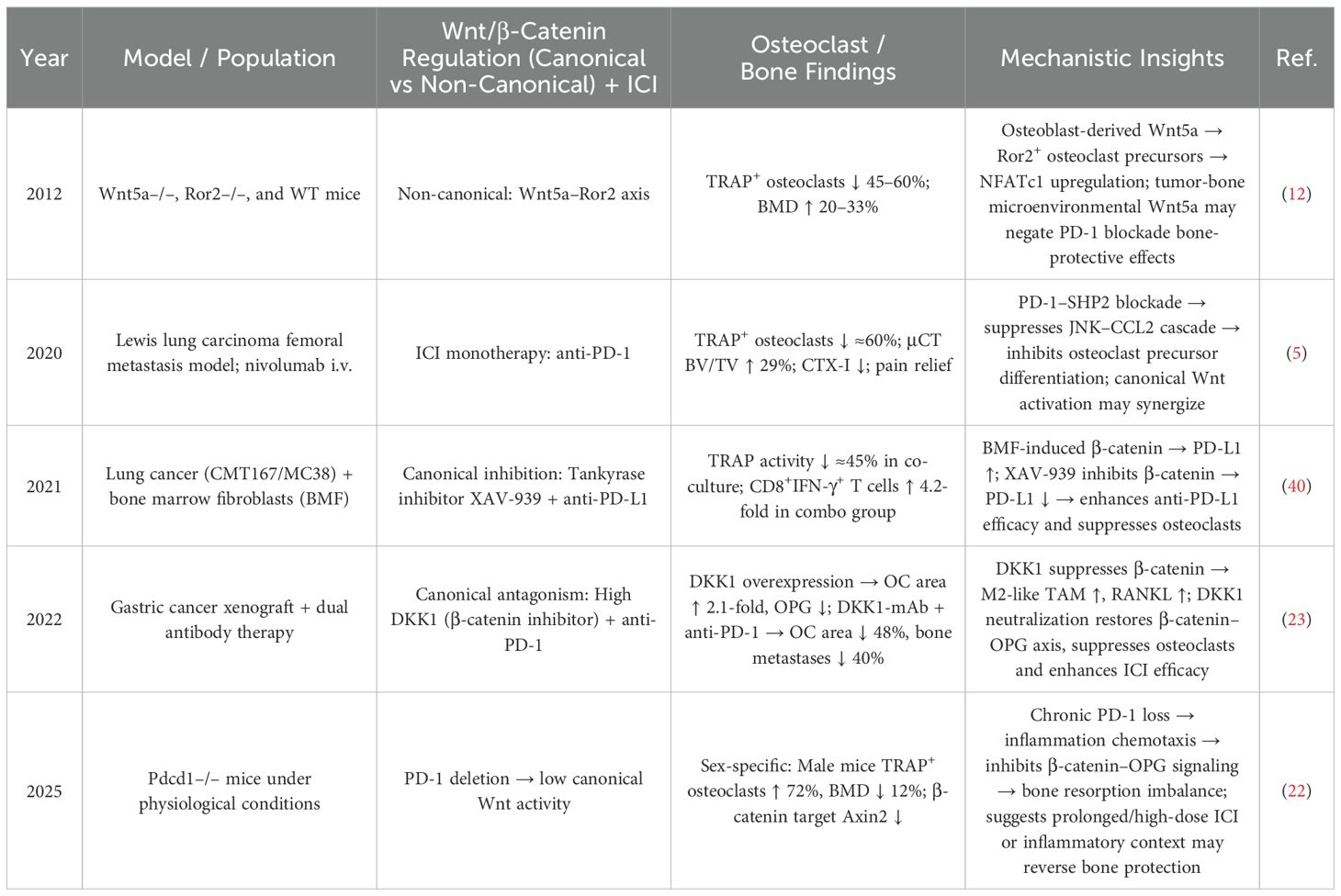
Table 2. Bidirectional modulation by the Wnt/β-catenin pathway in ICI-treated patients: effects on osteoclasts and bone metabolism.
To mitigate this deleterious effect, concurrent administration of anti-DKK1 antibodies—targeting inhibitors of canonical Wnt signaling—has shown promise in reducing skeletal damage and restoring bone homeostasis in the context of long-term ICI therapy (23).
4 Regulation of osteoblasts by the PD-1/PD-L1 pathway
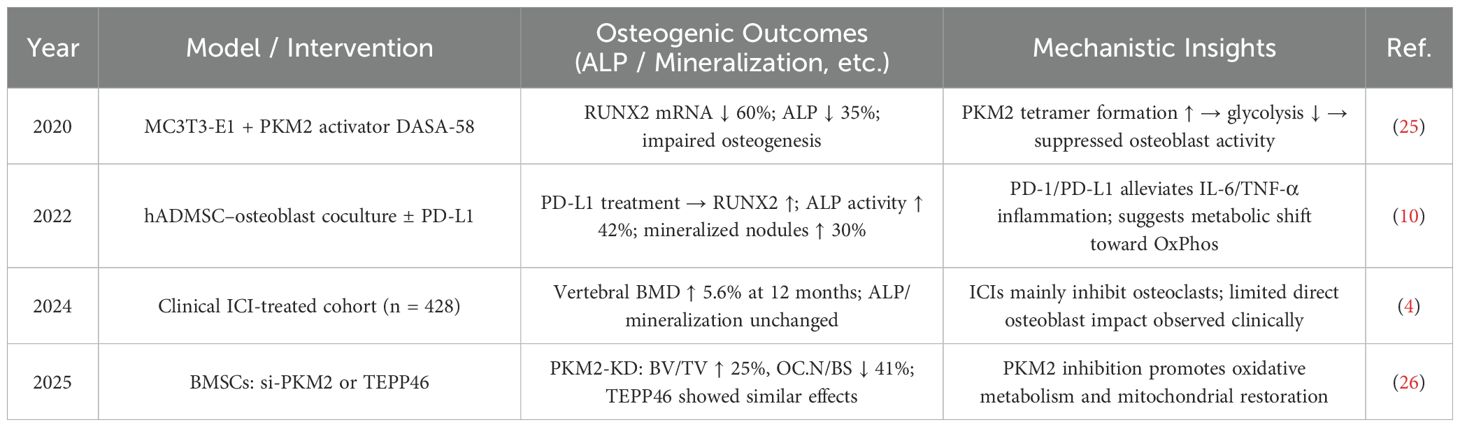
Although the PD-1/PD-L1 pathway has been shown to promote osteogenic gene expression and calcium deposition (10), it can also inhibit osteogenic differentiation (6). This inhibition may occur through suppression of the SHP2 signaling pathway, which relieves its inhibitory effect on NF-κB activation, thereby enhancing osteoblast differentiation and bone formation (24). These seemingly contradictory effects may be related to the PD-1/PD-L1–PKM2 axis (13) (see Table 3). The balance within this axis plays a critical role: PD-L1 promotes osteogenic differentiation by upregulating RUNX2 expression, increasing ALP activity by 42%, enhancing mineralization by 30%, reducing inflammation, and promoting oxidative phosphorylation metabolism (10). In contrast, activation of PKM2 leads to increased tetramer formation, inhibition of glycolysis, a 60% reduction in RUNX2 expression, and a 35% decrease in ALP activity, collectively impairing osteoblast differentiation (25). Notably, PKM2 inhibition can reduce osteoclast numbers by 41%, improve oxidative metabolism and mitochondrial function, and enhance bone volume/tissue volume (BV/TV) ratio by 25%, thus promoting osteogenesis (26).
Additionally, the bidirectional modulation of the Wnt/β-Catenin pathway influences osteoblast function and bone metabolism in patients receiving immune checkpoint inhibitors (ICIs) (see Table 4). Suppression of Wnt signaling significantly reduces bone mass (BV/TV by approximately 60%) and bone strength (by 33%), suggesting the need for bone-protective interventions during ICI therapy (27). Conversely, DKK1 can relieve Wnt inhibition, increasing osteoblast density (2.4-fold), osteoblast surface coverage (by 65%), and bone mineral density (by 9%). When combined with anti-PD-1 therapy, it further enhances antitumor immunity (28, 29), as β-catenin not only promotes osteogenesis but also induces PD-L1 expression (3–4-fold increase), contributing to a “self-limiting” immunosuppressive feedback loop. PD-1/PD-L1 blockade can reverse this suppression (30). From an ICI-centered perspective, PD-1 receptor activation promotes osteogenic markers via the ERK/β-catenin signaling pathway (ALP ↑ 61%, OCN ↑ 54%, mineralization ↑ 83%), whereas PD-1 blockade may weaken this osteogenic effect (10). Therefore, in cancer patients receiving ICIs, bone metabolism assessment must take into account the net outcome of these bidirectional regulatory mechanisms.
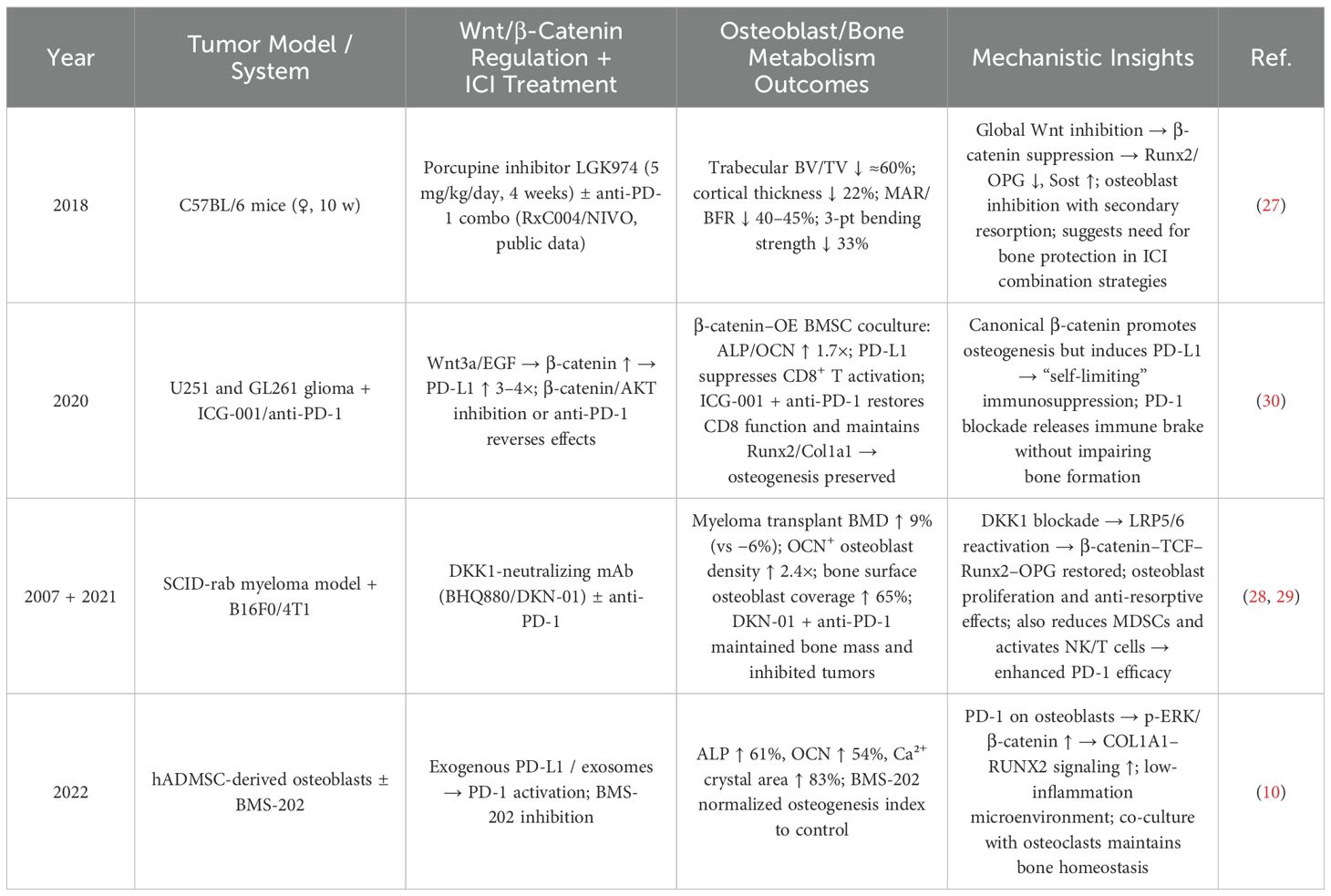
Table 4. Bidirectional effects of the Wnt/β-catenin pathway on osteoblast function in ICI-treated conditions.
Nevertheless, clinical evidence suggests that the primary bone-related effects of ICIs stem from inhibition of osteoclast activity and indirect promotion of bone formation, rather than direct osteoblast activation (4). These findings offer important implications for future osteoporosis management strategies.
Figure 2 provides an integrated overview of the bidirectional regulatory mechanisms of the PD-1/PD-L1 axis in bone metabolism and the effects of its inhibition. It illustrates how the PD-1/PD-L1 pathway interacts with multiple signaling axes to regulate both osteoclasts and osteoblasts, and highlights the temporal differences in short- vs. long-term blockade, the influence of the bone microenvironment, and crosstalk with the Wnt/β-Catenin pathway.
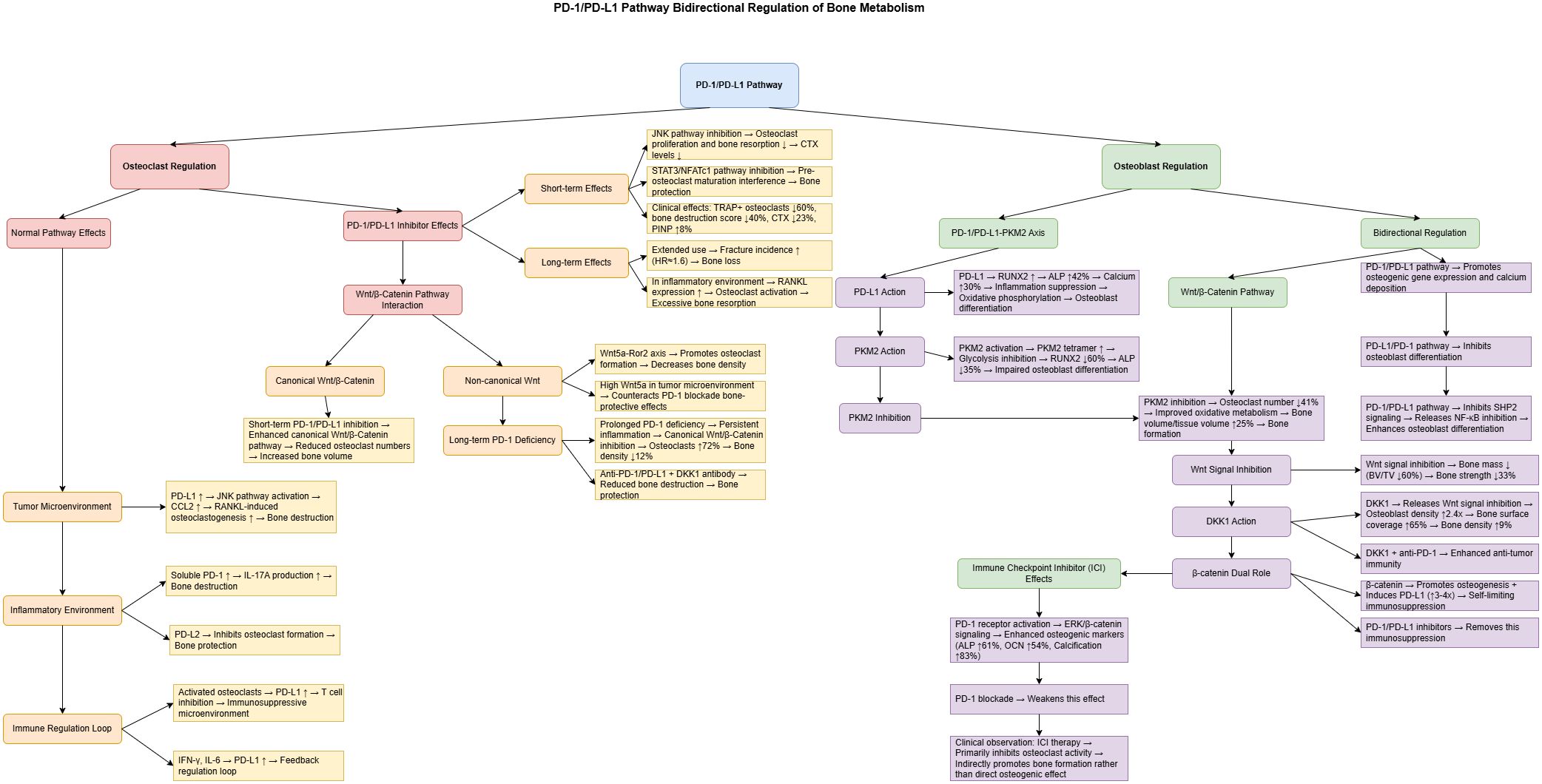
Figure 2. Bidirectional regulation of bone metabolism by the PD-1/PD-L1 pathway and the mechanisms of its inhibitors. This figure illustrates the regulatory network of the PD-1/PD-L1 pathway in bone metabolism. The left panel represents osteoclast regulation, while the right panel shows osteoblast regulation. Short-term administration of PD-1/PD-L1 inhibitors reduces osteoclast activity by approximately 60% via inhibition of the JNK signaling pathway, whereas long-term use is associated with an increased risk of fractures (hazard ratio ≈ 1.6). The PD-1/PD-L1–PKM2 axis plays a pivotal role in osteoblast differentiation: PD-L1 enhances osteogenesis by upregulating RUNX2 expression, while PKM2 activation suppresses osteoblast function. The Wnt signaling pathway exerts dual effects—its canonical branch inhibits osteoclastogenesis.
5 Challenges
5.1 Contradictory clinical findings and incomplete mechanistic understanding
Current clinical observations on the impact of PD-1/PD-L1 inhibition on bone metabolism yield conflicting results, and the underlying mechanisms remain poorly elucidated. Differences in clinical characteristics may partly explain these inconsistencies (see Table 5). Age and sex are major determinants: elderly female patients treated with ICIs have a significantly increased risk of fractures (21, 31), and baseline bone mineral density (BMD) has been identified as a critical predictor—patients with lower BMD derive less benefit from PD-1 blockade (32). Additionally, treatment regimens (monotherapy vs. combination therapy), tumor type, and tumor burden also influence skeletal outcomes, sometimes resulting in opposing bone metabolic responses (4, 5). These contradictions highlight the complexity of PD-1/PD-L1-mediated regulation of bone metabolism and underscore the urgent need for further research.
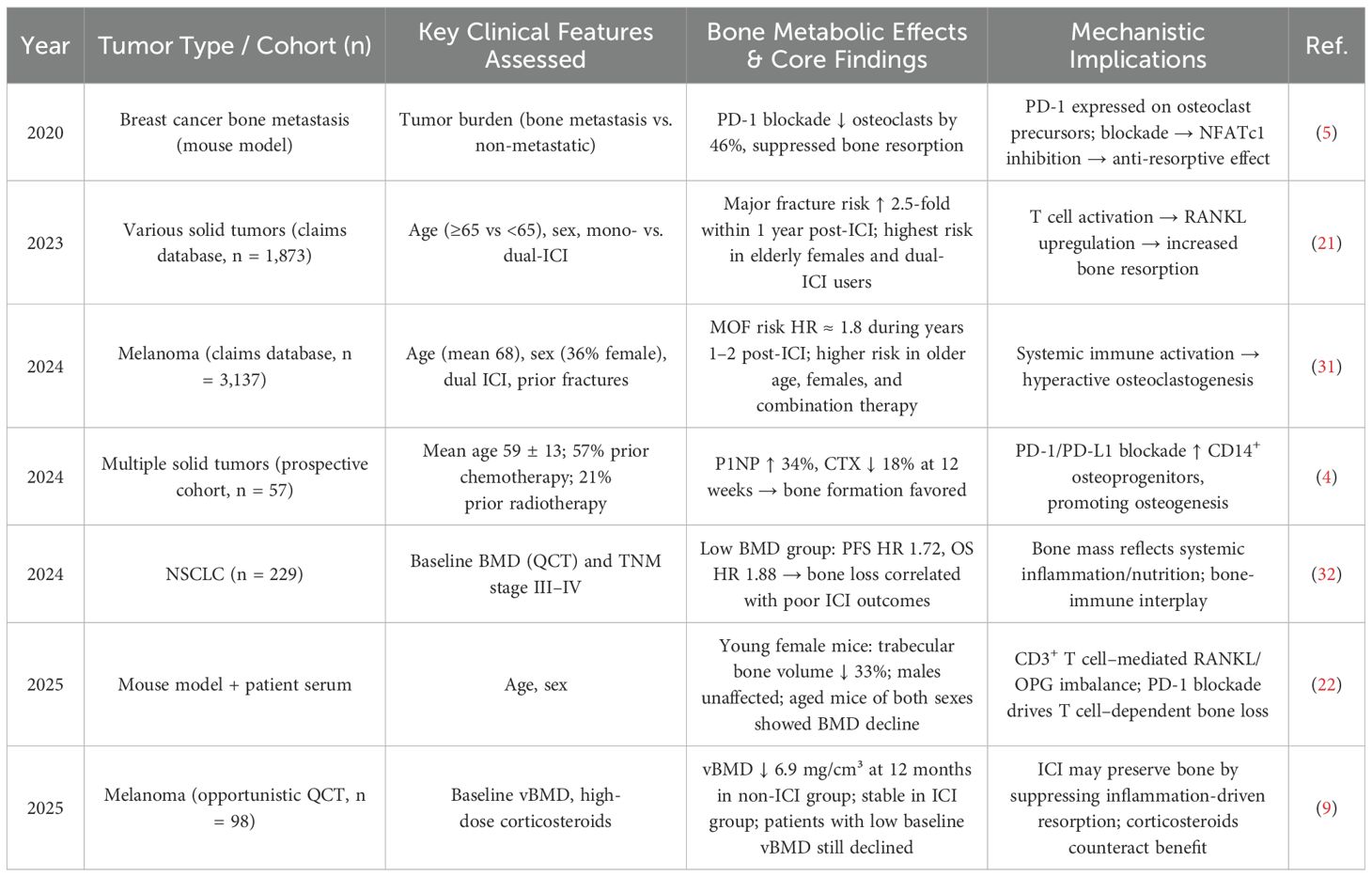
Table 5. Impact of clinical characteristics on bone metabolism in patients receiving PD-1/PD-L1 inhibitors.
5.2 Lack of clinical monitoring and interventions targeting bone metabolism
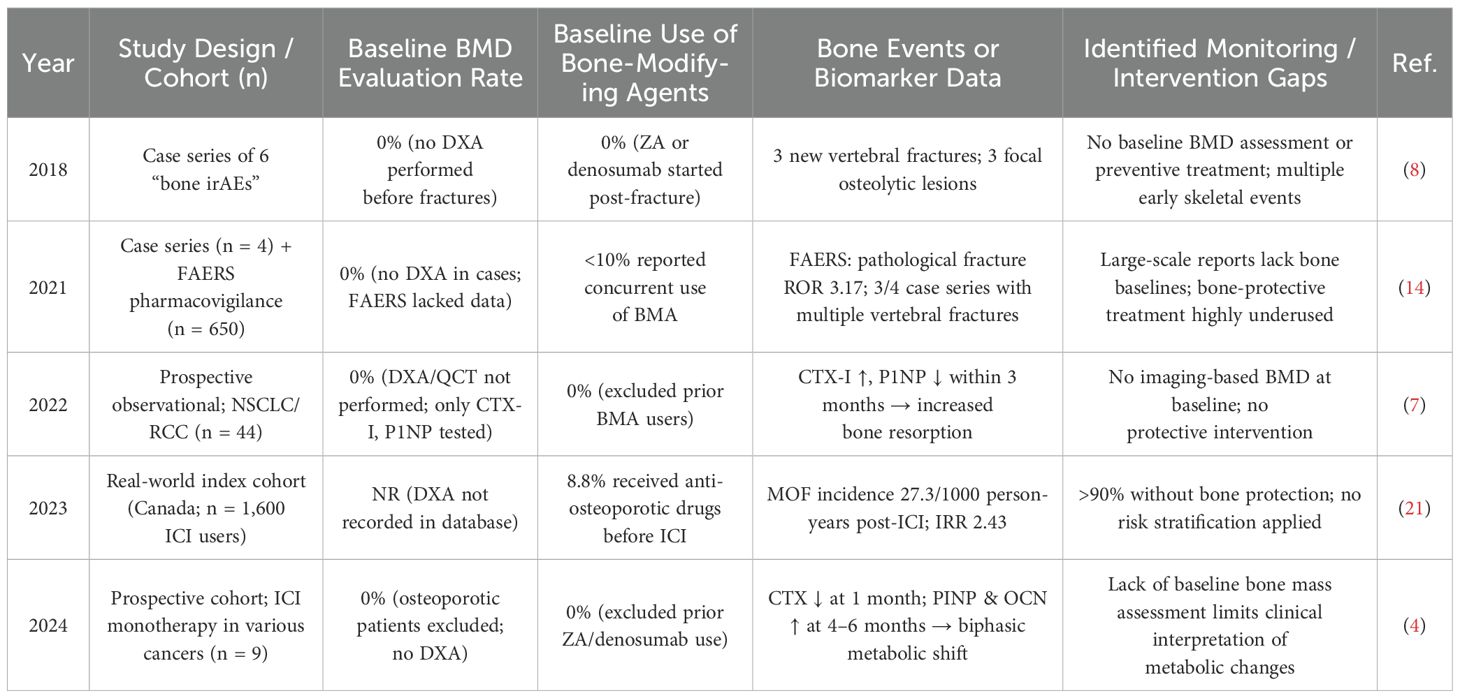
Although ICIs are significantly associated with adverse skeletal events—including pathological fractures, vertebral compression fractures, and femoral neck fractures (14)—these complications are often under-recognized in clinical practice. Reports indicate that up to 69% of patients may experience bone-related adverse events, with some requiring extended treatment intervals or premature discontinuation of cancer therapy (15). These are not isolated cases but rather reflect a widespread oversight in clinical monitoring (see Table 6). Multiple studies (4, 7) reveal a lack of baseline BMD evaluation and exceedingly low usage of bone-protective agents—fewer than 10% of patients receive treatment for osteoporosis (14, 21). Such gaps in screening and intervention may directly contribute to the occurrence of multiple fractures and bone resorptive lesions (8). Nevertheless, osteoporosis and fragility fractures are not yet formally recognized as immune-related adverse events (irAEs) (16). Baseline and longitudinal assessments of skeletal health are essential and should be systematically implemented in patients undergoing ICI therapy (33).
6 Discussion
Both clinical and mechanistic studies have reported contradictory findings regarding the role of the PD-1/PD-L1 pathway in bone metabolism regulation (4, 6, 10, 18). These discrepancies highlight the complexity of this pathway and the bidirectional effects of its inhibitors on bone homeostasis, suggesting a potential bone-protective role for PD-1/PD-L1 blockade. Compared with traditional bone-protective agents (Table 7), PD-1/PD-L1 inhibitors act further upstream in the RANKL axis (5), exerting a dual regulatory effect—initial inhibition of osteoclastogenesis followed by promotion of osteogenesis (4). This complements the unidirectional effects of conventional agents such as bisphosphonates and denosumab (34, 35).
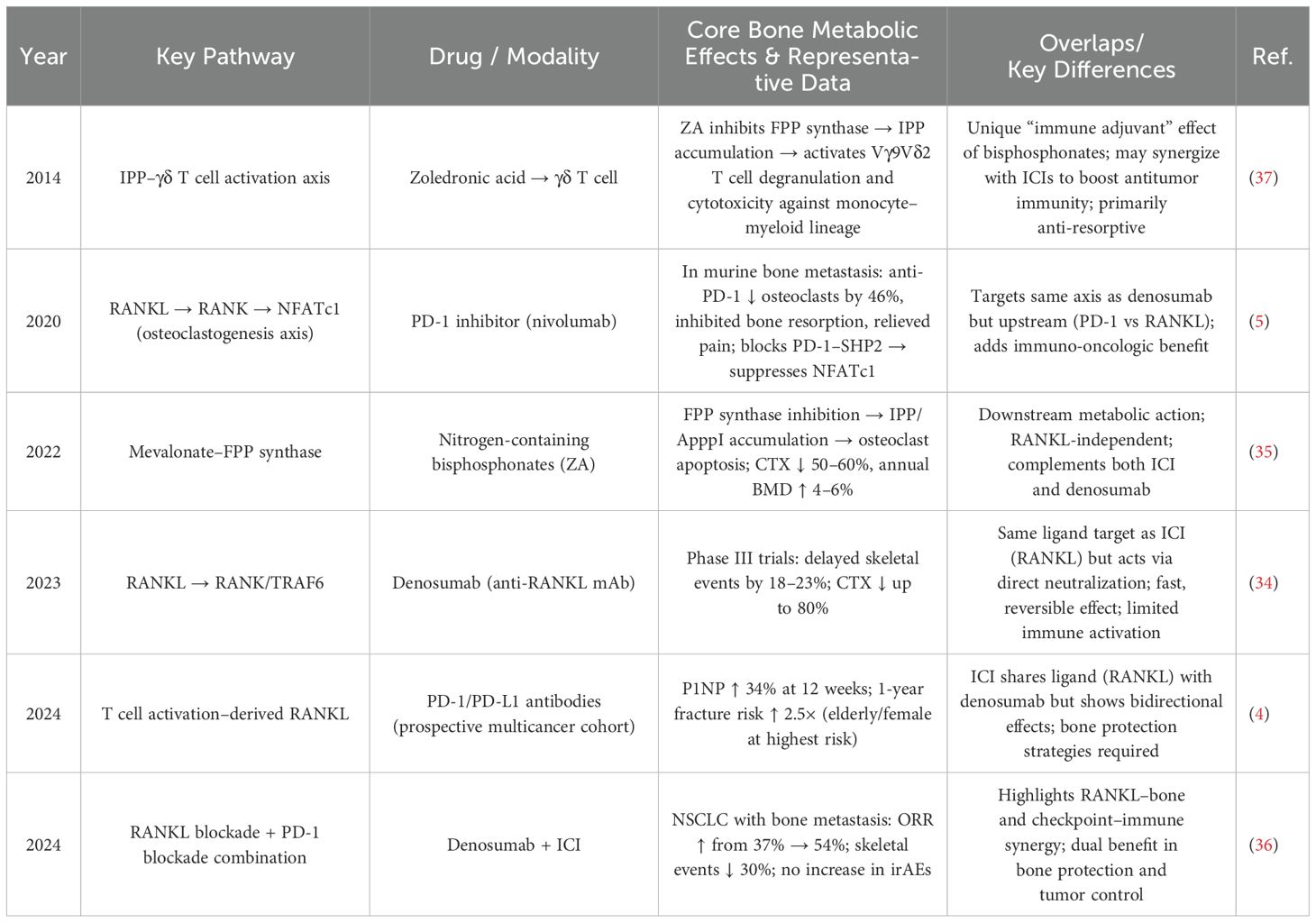
Table 7. Distinct effects of PD-1/PD-L1 inhibitors versus traditional bone-protective agents on bone metabolism.
Notably, combining PD-1 inhibitors with denosumab has been shown to increase the objective response rate in tumors (from 37% to 54%) and reduce the incidence of skeletal-related events by approximately 30% (36). Similarly, their combination with zoledronic acid can synergistically enhance anti-tumor immunity and anti-osteoporotic effects through the activation of γδ T cells (37). These findings suggest that PD-1/PD-L1 inhibitors may offer a novel therapeutic avenue for osteoporosis prevention and the management of skeletal-related adverse events (SREs) (14, 15).
As evidence continues to accumulate, it is anticipated that within the next five years, osteoporosis and fragility fractures will be increasingly recognized as part of the spectrum of immune-related adverse events (irAEs) associated with immune checkpoint inhibitors (ICIs) (16). Looking further ahead, over the next decade, the integration of bone-targeted strategies with ICI-specific bone-protective protocols may emerge (33), aiming to balance anti-tumor efficacy with bone health preservation and reduce the risk of SREs.
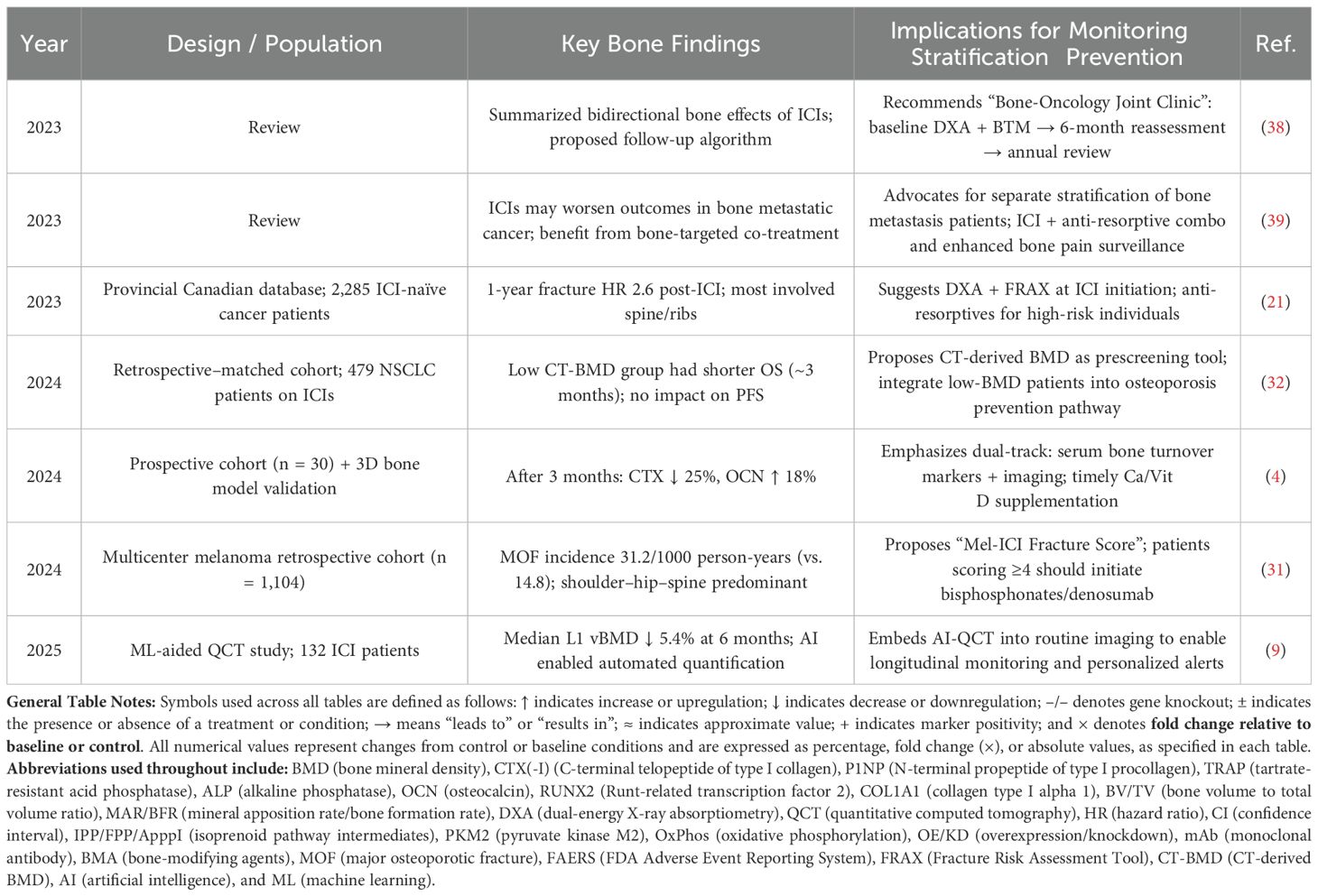
In the coming 5–10 years, an evidence-based framework for comprehensive skeletal health monitoring and stratified prevention is expected to be developed and refined. As summarized in Table 8, several emerging findings support this trend: baseline CT-derived BMD values have been shown to correlate with survival outcomes following ICI therapy (32), suggesting that CT-BMD should be incorporated into pre-treatment screening. The newly developed “Mel-ICI Fracture Score” can help identify high-risk individuals, with scores ≥4 warranting the initiation of bone-protective agents (31). Given that fracture risk increases 2.6-fold within the first year of ICI treatment, DXA assessments are recommended at therapy initiation (21). Moreover, AI-assisted QCT techniques, coupled with dual-track monitoring of BMD changes and serum bone turnover markers, can enable precise and timely surveillance of bone health (4, 9).
The establishment of a “Bone-Oncology Joint Clinic” model is also proposed, facilitating a closed-loop management approach—from baseline evaluation to regular follow-up and comprehensive intervention (38), as well as stratified care for patients with bone metastases (39). These innovations aim to address bone loss and provide continuity of care for patients who develop skeletal complications during ICI treatment (15), ultimately mitigating poor prognostic outcomes (7). Collectively, these strategies may enable dual optimization of tumor control and skeletal health during ICI therapy and significantly improve the quality of life and long-term health outcomes of cancer survivors.
whereas the non-canonical branch promotes osteoclast formation. Clinical observations suggest that immune checkpoint inhibitors primarily promote bone formation indirectly by suppressing osteoclast activity rather than by directly enhancing osteoblast differentiation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
J-WW: Writing – original draft, Investigation, Conceptualization. J-HL: Writing – review & editing, Visualization. M-WD: Project administration, Writing – review & editing, Visualization, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang BC, Cao RB, Li PD, and Fu C. The effects and safety of PD-1/PD-L1 inhibitors on head and neck cancer: A systematic review and meta-analysis. Cancer Med. (2019) 8:5969–78. doi: 10.1002/cam4.2510
2. Abaza A, Sid Idris F, Anis Shaikh H, Vahora I, Moparthi KP, Al Rushaidi MT, et al. Programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) immunotherapy: A promising breakthrough in cancer therapeutics. Cureus. (2023) 15:e44582. doi: 10.7759/cureus.44582
3. Greisen SR, Kragstrup TW, Thomsen JS, Hørslev–Pedersen K, Lund Hetland M, Stengaard–Pedersen K, et al. The programmed death-1 pathway counter-regulates inflammation-induced osteoclast activity in clinical and experimental settings. Front Immunol. (2022) 13:773946. doi: 10.3389/fimmu.2022.773946
4. Gassner T, Chittilappilly C, Pirich T, Neuditschko B, Hackner K, Lind J, et al. Favorable impact of PD1/PD-L1 antagonists on bone remodeling: an exploratory prospective clinical study and ex vivo validation. J Immunother Cancer. (2024) 12:e008669. doi: 10.1136/jitc-2023-008669
5. Wang K, Gu Y, Liao Y, Bang S, Donnelly CR, Chen O, et al. PD-1 blockade inhibits osteoclast formation and murine bone cancer pain. J Clin Invest. (2020) 130:3603–20. doi: 10.1172/JCI133334
6. Jeon SM, Lim JS, Park SH, Kim HJ, Kim HR, Lee JH, et al. Blockade of PD-L1/PD-1 signaling promotes osteo-/odontogenic differentiation through Ras activation. Int J Oral Sci. (2022) 14:18. doi: 10.1038/s41368-022-00168-2
7. Pantano F, Tramontana F, Iuliani M, Leanza G, Simonetti S, Piccoli A, et al. Changes in bone turnover markers in patients without bone metastases receiving immune checkpoint inhibitors: An exploratory analysis. J Bone Oncol. (2022) 37:100459. doi: 10.1016/j.jbo.2022.100459
8. Moseley KF, Naidoo J, Bingham CO, Carducci MA, Forde PM, Gibney GT, et al. Immune-related adverse events with immune checkpoint inhibitors affecting the skeleton: a seminal case series. J Immunother Cancer. (2018) 6:104. doi: 10.1186/s40425-018-0417-8
9. Matheson BE, Jaremko JL, Dowhanik A, Gill J, Gallant C, Walker J, et al. Assessing the effects of immune checkpoint inhibitors on bone utilizing machine learning-assisted opportunistic quantitative computed tomography. J Bone Miner Res. (2025) 40:396–403. doi: 10.1093/jbmr/zjaf009
10. Lee SC, Shin MK, Jang BY, Lee SH, Kim M, Sung JS, et al. Immunomodulatory Effect and Bone Homeostasis Regulation in Osteoblasts Differentiated from hADMSCs via the PD-1/PD-L1 Axis. Cells. (2022) 11:3152. doi: 10.3390/cells11193152
11. Rasmussen E, Østgård R, Hvid M, Greisen SR, Dahl MN, Deleuran B, et al. OP0106&x2005;SOLUBLE PD-1 PROMOTES LOCAL IL-17A PRODUCTION IN THE INFLAMED MICROENVIRONMENT IN spA. Ann OF THE RHEUMATIC Dis. (2022) 81:70. doi: 10.1136/annrheumdis-2022-eular.705
12. Maeda K, Kobayashi Y, Udagawa N, Uehara S, Ishihara A, Mizoguchi T, et al. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med. (2012) 18:405–12. doi: 10.1038/nm.2653
13. Zhu T, Wang R, Jiang H, Li Y–H, Zhao H–L, Chen J–C, et al. Fibroblast programmed cell death ligand 1 promotes osteoclastogenesis in odontogenic keratocysts. Am J Pathol. (2022) 193:286–95. doi: 10.1016/j.ajpath.2022.11.009
14. Filippini DM, Gatti M, Di Martino V, Calcaterra M, Riga S, Nasso C, et al. Bone fracture as a novel immune-related adverse event with immune checkpoint inhibitors: Case series and large-scale pharmacovigilance analysis. Int J Cancer. (2021) 149:675–83. doi: 10.1002/ijc.33592
15. Koltakova AD, Lila AM, Alekseeva OG, Kozlova OY, Smirnova SV, Ivanov AI, et al. The debut of inflammatory musculoskeletal pathology in patients receiving anticancer therapy with PD-1/PD-L1 pathway inhibitors. Sovremennaya Revmatologiya=Modern Rheumatol J. (2022) 16:46–52. doi: 10.14412/1996-7012-2022-5-46-52
16. Elsayed M and Ye C. Osteoporotic fractures: an unrecognized adverse event of immune checkpoint inhibitors? J Immunother Cancer. (2024) 12:e009309. doi: 10.1136/jitc-2024-009309
17. Tran MT. Adverse effects of the cancer therapy on osteoclast-mediated bone loss in patients with cancers: a challenge. Asia-Pacific J Oncol. (2022) 3:10–5. doi: 10.32948/ajo.2022.12.29
18. Bedatsova L and Drake MT. The skeletal impact of cancer therapies. Br J Clin Pharmacol. (2019) 85:1161–8. doi: 10.1111/bcp.13866
19. Tai YT, Cho SF, and Anderson KC. Osteoclast immunosuppressive effects in multiple myeloma: role of programmed cell death ligand 1. Front Immunol. (2018) 9:1822. doi: 10.3389/fimmu.2018.01822
20. Tamura H, Ishibashi M, Sunakawa M, Kuroda T, Nakata H, Kuroda H, et al. Expression, functions, and treatment target of PD-L1 (B7-H1) in multiple myeloma. J Immunol Sci. (2018) 2:22–5. doi: 10.29245/2578-3009/2018/5.1162
21. Ye C, Lee K, Leslie WD, Zhao B, Zhang S–H, Yan S, et al. Fracture rate increases after immune checkpoint inhibitor treatment: a potential new immune related adverse event. Osteoporos Int. (2023) 34:735–40. doi: 10.1007/s00198-023-06690-1
22. Joseph GJ, Vecchi LA III, Uppuganti S, Rose KA, Spencer LM, Drake MT, et al. PD-1 blockade regulates skeletal remodeling in a sex- and age-dependent manner. J Bone Miner Res. (2025) 40:ezjaff055. doi: 10.1093/jbmr/zjaf055
23. Shi T, Zhang Y, Wang Y, Li Z–H, Hu C, Liu L–J, et al. DKK1 promotes tumor immune evasion and impedes anti-PD-1 treatment by inducing immunosuppressive macrophages in gastric cancer. Cancer Immunol Res. (2022) 10:1506–24. doi: 10.1158/2326-6066.CIR-22-0218
24. Li N, Li Z, Fu L, Li F–Z, Lu X–K, Wang Y–B, et al. PD-1 suppresses the osteogenic and odontogenic differentiation of stem cells from dental apical papilla via targeting SHP2/NF-κB axis. Stem Cells. (2022) 40:763–77. doi: 10.1093/stmcls/sxac037
25. Agas D, Amaroli A, Lacava G, Calabrese L, Giuliani MR, Colao A, et al. Loss of p62 impairs bone turnover and inhibits PTH-induced osteogenesis. J Cell Physiol. (2020) 235:7516–29. doi: 10.1002/jcp.29654
26. Zhu Y, Yang Y, Lan Y, Wu Q–X, Zhao L–P, Chen X–Y, et al. The role of PKM2-mediated metabolic reprogramming in the osteogenic differentiation of BMSCs under diabetic periodontitis conditions. Stem Cell Res Ther. (2025) 16:186. doi: 10.1186/s13287-025-04301-w
27. Funck–Brentano T, Nilsson KH, Brommage R, Nakashima M, Brunet J, Chamoux D, et al. Porcupine inhibitors impair trabecular and cortical bone mass and strength in mice. J Endocrinol. (2018) 238:13–23. doi: 10.1530/JOE-18-0153
28. Haas MS, Kagey MH, Heath H, Chen T, Gordon ED, Lee JC, et al. mDKN-01, a novel anti-DKK1 mAb, enhances innate immune responses in the tumor microenvironment. Mol Cancer Res. (2021) 19:717–25. doi: 10.1158/1541-7786.MCR-20-0799
29. Yaccoby S, Ling W, Zhan F, Parker DA, Hurd LF, McCarthy PL, et al. Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. (2007) 109:2106–11. doi: 10.1182/blood-2006-09-047712
30. Du L, Lee J–H, Jiang H, Wang M–Y, Li P–X, Zhang W, et al. β-Catenin induces transcriptional expression of PD-L1 to promote glioblastoma immune evasion. J Exp Med. (2020) 217:e20191115. doi: 10.1084/jem.20191115
31. Ye C, Zhao B, Leslie WD, Ruiz JI, Zhao H, Abdel-Wahab N, et al. Increase in major osteoporotic fractures after therapy with immune checkpoint inhibitors. BMJ Oncol. (2024) 3:e000398. doi: 10.1136/bmjonc-2024-000398
32. Lou J, Gong B, Li Y, Liu W, Zhang C–L, Sun H, et al. Bone mineral density as an individual prognostic biomarker in NSCLC patients treated with immune checkpoint inhibitors. Front Immunol. (2024) 15:1332303. doi: 10.3389/fimmu.2024.1332303
33. Handforth C, D’Oronzo S, Coleman R, Cooke AM, Moran M, Joel S, et al. Cancer treatment and bone health. Calcif Tissue Int. (2018) 102:251–64. doi: 10.1007/s00223-017-0369-x
34. Lu J, Hu D, Zhang Y, Peng R, Chen S–Y, Zhao X–L, et al. Current comprehensive understanding of denosumab (the RANKL neutralizing antibody) in the treatment of bone metastasis of Malignant tumors, including pharmacological mechanism and clinical trials. Front Oncol. (2023) 13:1133828. doi: 10.3389/fonc.2023.1133828
35. Wang B, Zhan Y, Yan L, Huang M–N, Tang G–H, Dong J–Q, et al. How zoledronic acid improves osteoporosis by acting on osteoclasts. Front Pharmacol. (2022) 13:961941. doi: 10.3389/fphar.2022.961941
36. Asano Y, Yamamoto N, Demura S, Takiguchi T, Hagiwara K, Ishibe H, et al. Combination therapy with immune checkpoint inhibitors and denosumab improves clinical outcomes in non-small cell lung cancer with bone metastases. Lung Cancer. (2024) 193:107858. doi: 10.1016/j.lungcan.2024.107858
37. Fowler DW, Copier J, Dalgleish AG, Spranger AM, Cook GP, Fisher RJ, et al. Zoledronic acid causes γδ T cells to target monocytes and down-modulate inflammatory homing. Immunology. (2014) 143:539–49. doi: 10.1111/imm.12331
38. Tang J. Immune checkpoint inhibitors: friend or foe for osteoporosis. Ther Adv Endocrinol Metab. (2023) 14:20420188231157194. doi: 10.1177/20420188231157194
39. Joseph GJ, Johnson DB, and Johnson RW. Immune checkpoint inhibitors in bone metastasis: Clinical challenges, toxicities, and mechanisms. J Bone Oncol. (2023) 43:100505. doi: 10.1016/j.jbo.2023.100505
Keywords: PD-1/PD-L1 inhibitors, bone metabolism, immune-related adverse events, osteoclasts, osteoblasts, RANKL, Wnt signaling, bone biomarkers
Citation: Wang J-W, Dai M-W and Liu J-H (2025) The regulatory effects of PD-1/PD-L1 inhibitors on bone metabolism: opportunities and challenges in osteoporosis management. Front. Immunol. 16:1630751. doi: 10.3389/fimmu.2025.1630751
Received: 18 May 2025; Accepted: 04 July 2025;
Published: 25 July 2025.
Edited by:
Claire J. Han, The Ohio State University, United StatesReviewed by:
Zeming Mo, Zunyi Medical University, ChinaCopyright © 2025 Wang, Dai and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mu-Wei Dai, NDg2MDE4NDlAaGVibXUuZWR1LmNu
 Jia-Wen Wang
Jia-Wen Wang Mu-Wei Dai
Mu-Wei Dai