- 1School of Medicine, Wuhan University of Science and Technology, Wuhan, China
- 2Tianyou Hospital, Wuhan University of Science and Technology, Wuhan, China
- 3Department of Anesthesiology, West China Hospital, Sichuan University, Chengdu, China
- 4The Department of Clinical Research, West China Hospital, Sichuan University, Chengdu, China
Background: As one of the therapeutic modalities for treating tumors, immune checkpoint inhibitors (ICIs) have gained widespread application in clinical practice, including non-small cell lung cancer, melanoma, head and neck squamous cell carcinoma, hepatocellular carcinoma, and other types of cancers. However, the safety profile of combining ICIs remains inadequately understood, which poses limitations on the clinical utilization of this novel class of medications. To investigate the toxicity spectrum associated with combination immunotherapy, we conducted an extensive data mining and analysis of the US Food and Drug Administration Adverse Event Reporting System (FAERS) database.
Methods: By mining adverse event (AE) reports from the FAERS database covering the period from the first quarter of 2011 through the second quarter of 2024, baseline data were analyzed using Cramer’s V coefficient and p-value. Subsequently, two methods, the reporting odds ratio (ROR) and the Bayesian confidence propagation neural network, were employed to detect AE signals for single immune checkpoint inhibitors (sICIs) and dual immunotherapy group (tremelimumab plus durvalumab and ipilimumab plus nivolumab, DIG).
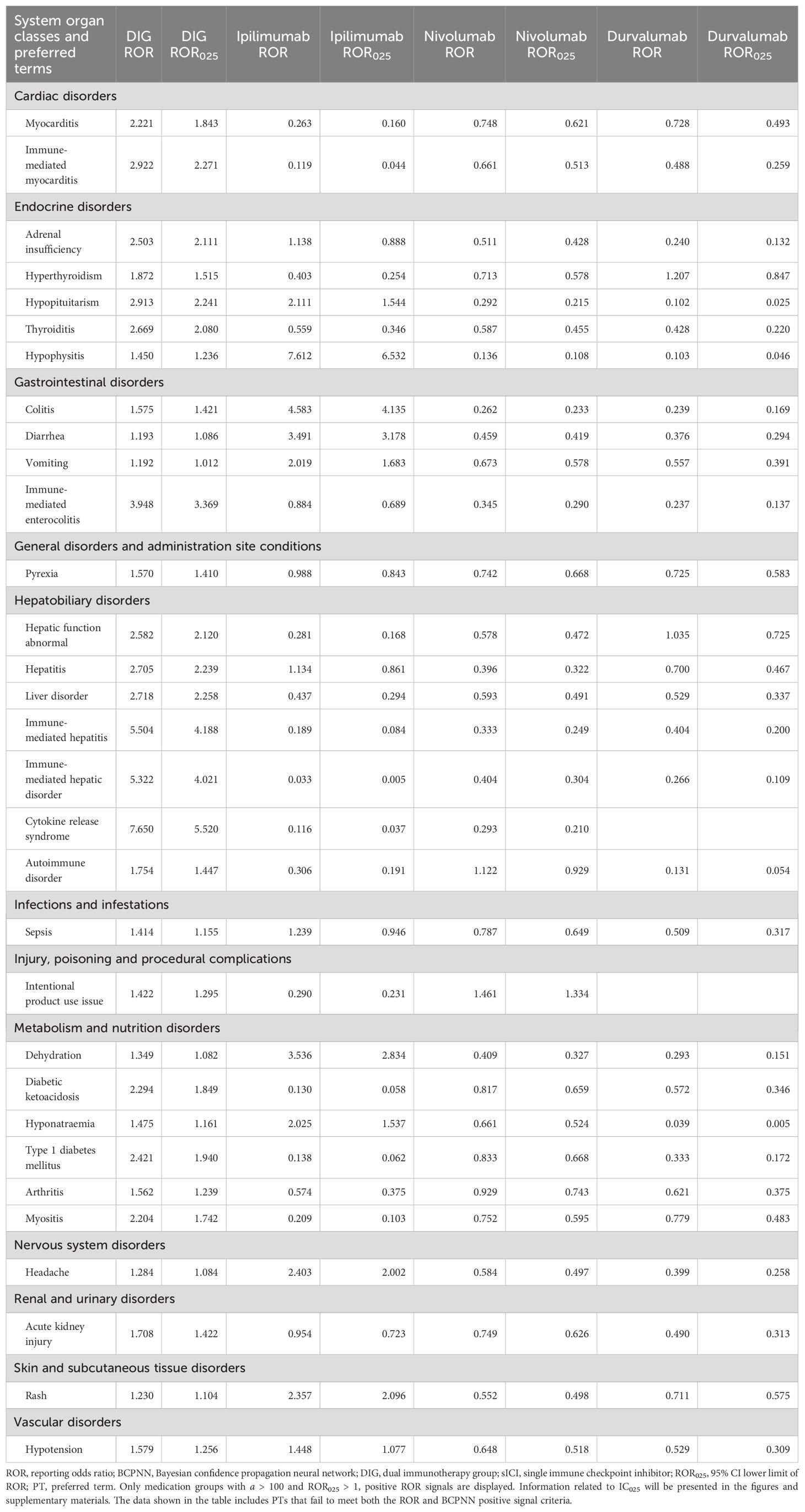
Results: A total of 55,052 patients and 118,001 AEs were selected. The DIG exhibited a higher incidence of AE signals across 14 distinct system organ class level. Moreover, DIG exhibited higher positive signal intensity compared to sICIs in the following preferred terms: myocarditis [ROR 2.221, 95% confidence interval lower limit of information component (IC025) 0.486], immune-mediated myocarditis (ROR 2.922, IC025 0.610), adrenal insufficiency (ROR 2.503, IC025 0.602), hyperthyroidism (ROR 1.872, IC025 0.305), thyroiditis (ROR 2.669, IC025 0.546), immune-mediated enterocolitis (ROR 3.948, IC025 0.937), pyrexia (ROR 1.570, IC025 0.290), hepatic function abnormality (ROR 2.582, IC025 0.591), hepatitis (ROR 2.705, IC025 0.637), liver disorder (ROR 2.718, IC025 0.646), immune-mediated hepatitis (ROR 5.504, IC025 0.994), immune-mediated liver disorder (ROR 5.322, IC025 0.966), cytokine release syndrome (ROR 7.650, IC025 1.103), autoimmune diseases (ROR 1.754, IC025 0.275), sepsis (ROR 1.414, IC025 0.062), diabetic ketoacidosis (ROR 2.294, IC025 0.472), type 1 diabetes mellitus (ROR 2.421, IC025 0.508), arthritis (ROR 1.562, IC025 0.113), myositis (ROR 2.204, IC025 0.412), and acute kidney injury (ROR 1.708, IC025 0.264).
Conclusions: Our findings indicate that the AEs associated with dual ICI predominantly originate from immune-related AEs, including myotoxicity, endocrine toxicity, and hepatotoxicity. Notably, cytokine release syndrome, a rarely reported AE with a strongly positive signal, warrants particular attention in clinical decision-making.
Introduction
As a prominent focus in tumor therapy, immune checkpoint inhibitors (ICIs) have garnered extensive attention in clinical practice and have become a crucial treatment option for various malignancies, including non-small cell lung cancer (NSCLC), melanoma, head and neck squamous cell carcinoma, hepatocellular carcinoma, and other types of cancers (1–4). In a healthy body, the immune checkpoint pathway serves as a critical regulatory mechanism to prevent immune-mediated damage. However, this pathway can also be exploited by cancer cells to evade immune surveillance. Common targets for ICIs include cytotoxic T lymphocyte-associated protein-4 (CTLA-4), programmed cell death protein-1 (PD-1) and programmed death ligand-1 (PD-L1) (5).
CTLA-4 (cluster of differentiation 152, CD152), as a competitive inhibitor of CD28, inhibits the immune activity of CD8+ T cells by blocking the co-stimulatory signals mediated by the interaction between CD28 and B7-1 (CD80) or B7-2 (CD86) on antigen-presenting cells (APCs) (6). PD-L1 (B7-H1 or CD274), expressed on the surface of tumor cells as a ligand for PD-1, can inhibit T cell activation and negatively regulate the adaptive immune response, thereby enabling tumors to evade immune surveillance (7). Antibodies directed against PD-1/PD-L1 can inhibit immune evasion and augment adaptive immune responses, leading to more effective tumor cell elimination (7, 8). Currently, ICIs approved for clinical use encompass a range of agents, including pembrolizumab, toripalimab, avelumab, and atezolizumab, among others (9–12).
Tremelimumab (Imjudo) is a human IgG2 monoclonal antibody that specifically binds to CTLA-4, blocking its interaction with the ligands, and enhances T cell activation and responses against tumor cells. In October 2022, tremelimumab received approval in the United States for use in combination with PD-L1 inhibitor durvalumab (Imfinzi) to treat unresectable hepatocellular carcinoma in adults (13). In clinical practice, the innovative strategy of initiating treatment with a low-dose CTLA-4 inhibitor followed by maintenance therapy with a PD-L1 inhibitor, referred to as the STRIDE regimen (Single Tremelimumab Regular Interval Durvalumab), provides synergistic therapeutic effects while substantially reducing immune-related toxicities, thereby demonstrating an improved safety profile (14). Ipilimumab (Yervoy), an additional CTLA-4 inhibitor, has also been evaluated in combination with PD-1 inhibitor nivolumab (Opdivo) as a first-line treatment option for metastatic NSCLC (15). It is widely recognized as the most classic treatment regimen within the combination therapies involving PD-1 inhibitors and CTLA-4 inhibitors (16).
The concern is that the use of these medications is not exclusively associated with a favorable prognosis, even though they are considered safer and more conventional treatment options. Single immune checkpoint inhibitors (sICIs) are frequently reported to cause adverse events (AEs) such as rash, jaundice, hypothyroidism, pneumonitis, neutropenia, and in some cases, necessitate medication discontinuation (17–21). We can also identified reports of AEs associated with dual immune checkpoint inhibitors (ipilimumab plus nivolumab or tremelimumab plus durvalumab, dICIs), including nausea, fatigue, pneumonia, neutropenia, leukopenia, and instances of treatment discontinuation (13, 15). However, limited specific comparisons of these AEs have been conducted between sICIs and dICIs.
Although dICIs are currently approved for various cancer indications and have demonstrated superior efficacy compared to sICI therapy, their safety profile and limitations warrant careful consideration (22). Given the inherent limitations of clinical trials, the US Food and Drug Administration Adverse Event Reporting System (FAERS) database serves as a spontaneous reporting system for pharmacovigilance to assess the safety of suspected AEs. To gain a deeper understanding of AEs associated with dICI, refine knowledge of its toxicity spectrum, and provide valuable clinical information, we conducted an in-depth analysis of the FAERS database.
Materials and methods
Data source
The original data utilized for our data mining were sourced exclusively from the FAERS database available on the official FDA website. The raw ASCII data files were downloaded for subsequent data mining and statistical analysis. SAS 9.4 software, recommended by the FDA for FAERS database analysis, was employed for this purpose. It is important to note that the FAERS database contains self-reported data, which may include duplicate or withdrawn/deleted reports. In accordance with the FDA’s official guidance documents, we conducted data cleaning as follows: We selected the PRIMARYID, CASEID, and FDA_DT fields from the DEMO table, sorted them by CASEID, FDA_DT, and PRIMARYID, and retained the maximum values in sequence. Since the first quarter of 2019, each quarterly data package has included a list of deleted reports. After deduplication, we removed the deleted reports based on the CASEID provided in the deletion list. The latest version of the Medical Dictionary for Regulatory Activities (MedDRA 27.0), which includes system organ class (SOC) and preferred term (PT), was systematically utilized to encode the AE terminology within the FAERS database.
Statistical analysis
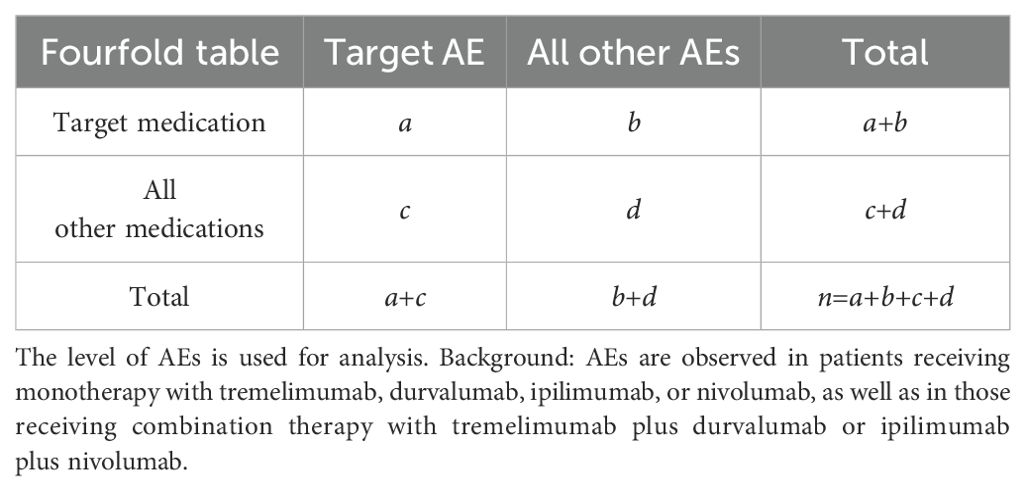
For the analysis of baseline data, we employed Cramer’s V coefficient in conjunction with the p-value to evaluate both the strength and statistical significance of associations between categorical variables, particularly for those with larger sample sizes. A p-value more than 0.05 accompanied by a V value close to zero suggests a weak association between the variables. Such a weak association may arise by chance and is therefore considered to have limited reliability. And we employed a case-control study design, specifically a case versus non-case approach, utilizing a fourfold table for disproportionality analysis, as illustrated in Table 1, to investigate the AEs associated with the target medications. In the database, each patient (represented by an individual report) is associated with a unique “Primary Suspect (PS)” medication. When defining the target medication user population, only the patient’s PS medication is taken into account. If the PS medication recorded in the background database matches the target medication of the study, the patient is included in the target medication population; otherwise, the patient is classified into the other medication population.
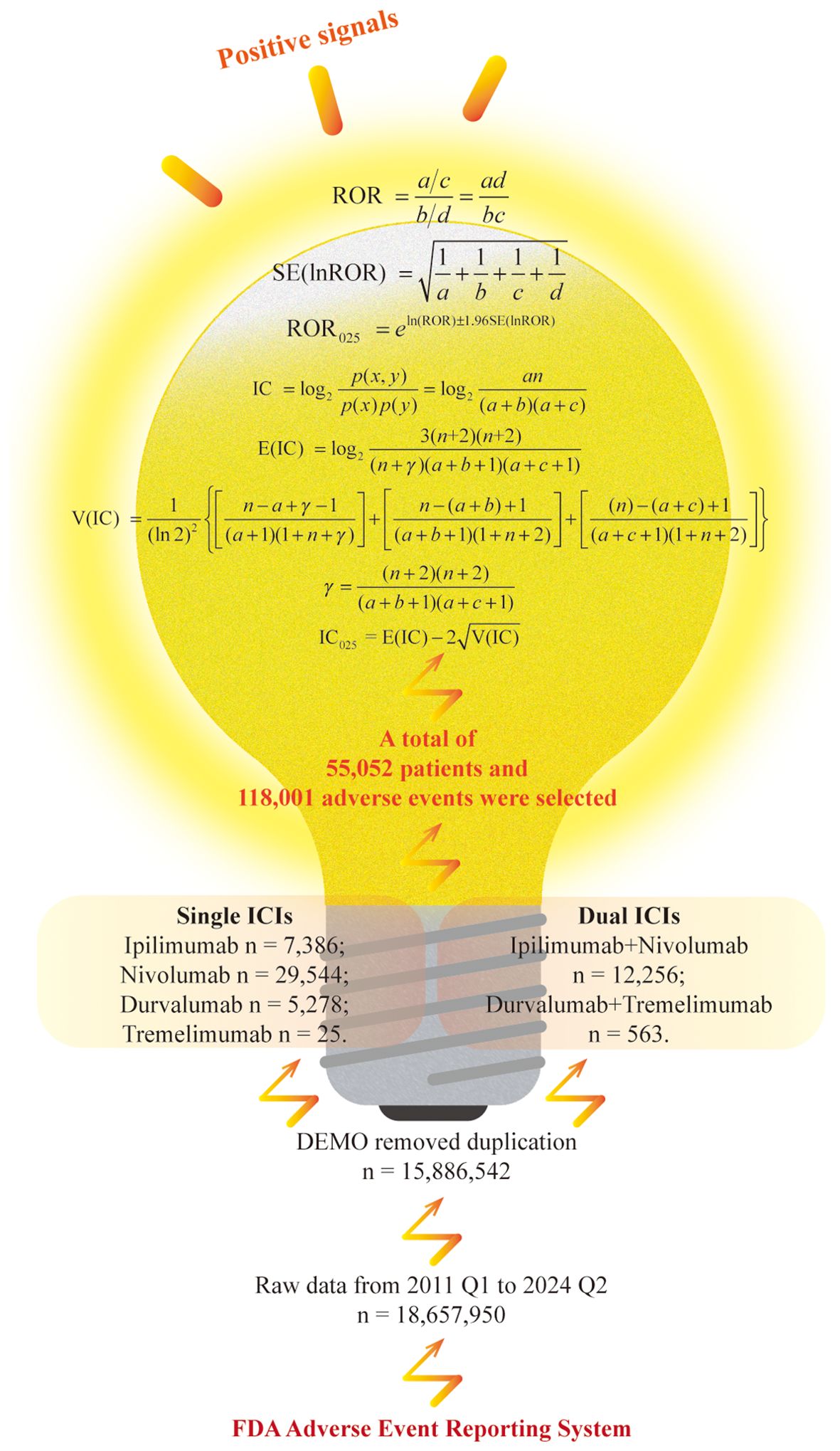
In this study, we employed widely recognized methods to detect adverse medication event signals. Specifically, positive signals were identified using the reporting odds ratio (ROR) and the Bayesian confidence propagation neural network (BCPNN) (23, 24). For the ROR method, a signal is triggered when the analysis data satisfies a ≥ 3 and 95% confidence interval (CI) lower limit of ROR (ROR025) > 1. For the BCPNN method, a signal is generated if 95% CI lower limit of the information component (IC025) > 0. As illustrated in Figure 1, a positive signal is produced when the AE included in the analysis meets the criteria for both methods.
ROR is a disproportionality analysis method based on the fourfold contingency table, characterized by high sensitivity in detecting typical AEs associated with commonly used medications. It considers the total number of reports and allows for adjustment of potential confounding factors (25, 26). And BCPNN is an advanced algorithm grounded in Bayesian theory, designed to evaluate the likelihood of a causal association between medications and AEs. This method demonstrates superior performance in handling sparse data compared to conventional approaches and tends to produce fewer false-positive signals. Furthermore, the Bayesian framework of BCPNN inherently accounts for reporting bias by incorporating prior distributions to mitigate spurious associations (27, 28). Therefore, this study employs both the ROR and BCPNN methods, which together can help minimize bias resulting from proportional imbalances and are widely recognized for their effectiveness in mining and analyzing similar pharmacovigilance databases such as FAERS (29, 30).
Results
Descriptive results
The data screening and analysis process is illustrated in Figure 1. A total of 54 quarters of ASCII packets were downloaded from the FAERS database, covering the period from the first quarter of 2011 to the second quarter of 2024, resulting in the acquisition of 18,657,950 records. After eliminating duplicate reports, 15,886,542 unique reports remained. Subsequently, we screened for reports where the target medication was listed as the PS medication, selecting a total of 118,001 AEs involving 55,052 individuals as the background dataset. Specifically, among sICIs, there were 7,386 reports for ipilimumab, 29,544 for nivolumab, 5,278 for durvalumab, and 25 for tremelimumab. For dICIs, 12,256 participants reported receiving ipilimumab combined with nivolumab, while 563 people received durvalumab and tremelimumab.
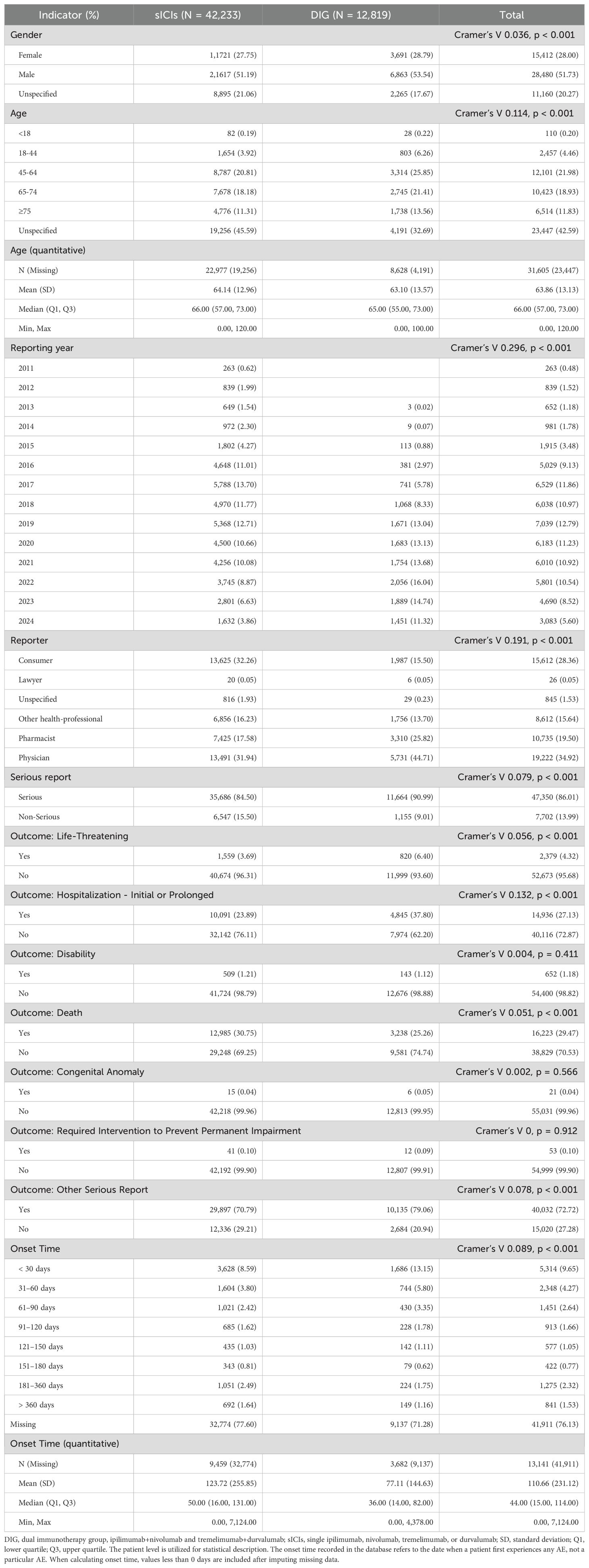
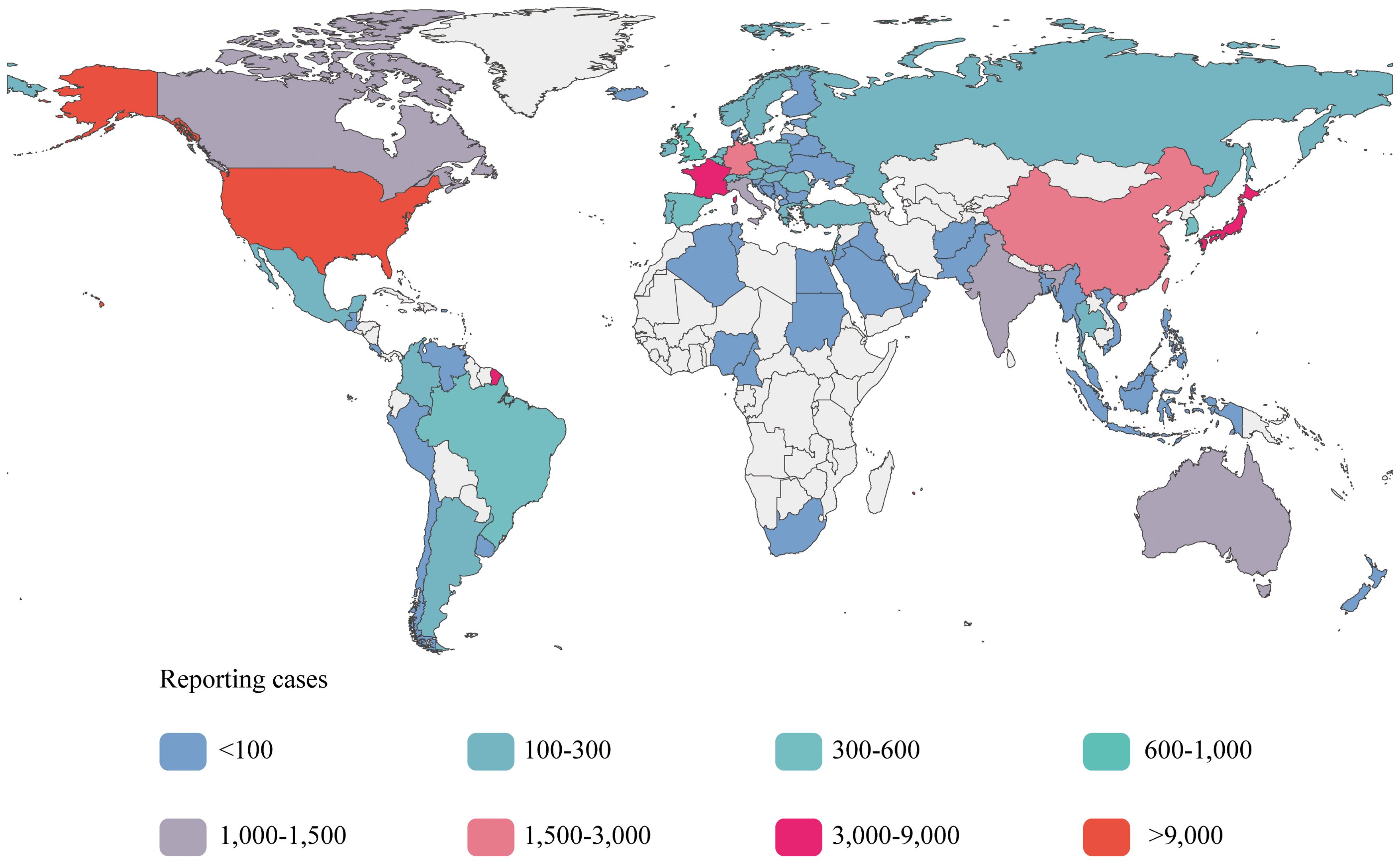
Among the six subgroups included in the study, patients who received ipilimumab, nivolumab, tremelimumab, or durvalumab as monotherapies were classified as sICIs. Patients who received combination therapies of ipilimumab plus nivolumab or tremelimumab plus durvalumab were classified into the dual immunotherapy group (DIG). We have presented the baseline data for the sICIs and DIG in Table 2, and provided detailed data pertaining to the six subgroups in Additional File 1. After excluding unspecified cases, 51.73% of patients were male, with 51.19% in the sICIs and 53.54% in the DIG, both proportions exceeding those of female patients. The highest frequency of AEs was observed in the age group of 45–64 years, accounting for 20.81% in the sICIs and 25.58% in the DIG. In the DIG, the majority of AE reports were submitted by physicians (44.71%), whereas in the sICIs, consumers (32.26%) and physicians (31.94%) were the primary reporters. Regarding the sources of the reports, Figure 2 presents a choropleth map illustrating the countries contributing to the reports. The majority of the reports originate from the United States (25,788, 46.85%) and Japan (8,794, 15.98%), followed by France (4,031, 7.32%) and China (2,697, 4.90%). Notably, each of these four countries has contributed more than 2,000 reports, whereas the others have not. Serious reports in the FAERS database are categorized at the patient level rather than at the level of specific AEs. Reports that include outcome results are classified as serious (47,350, 86.01%), whereas those lacking outcome information are deemed non-serious (7,702, 13.99%).
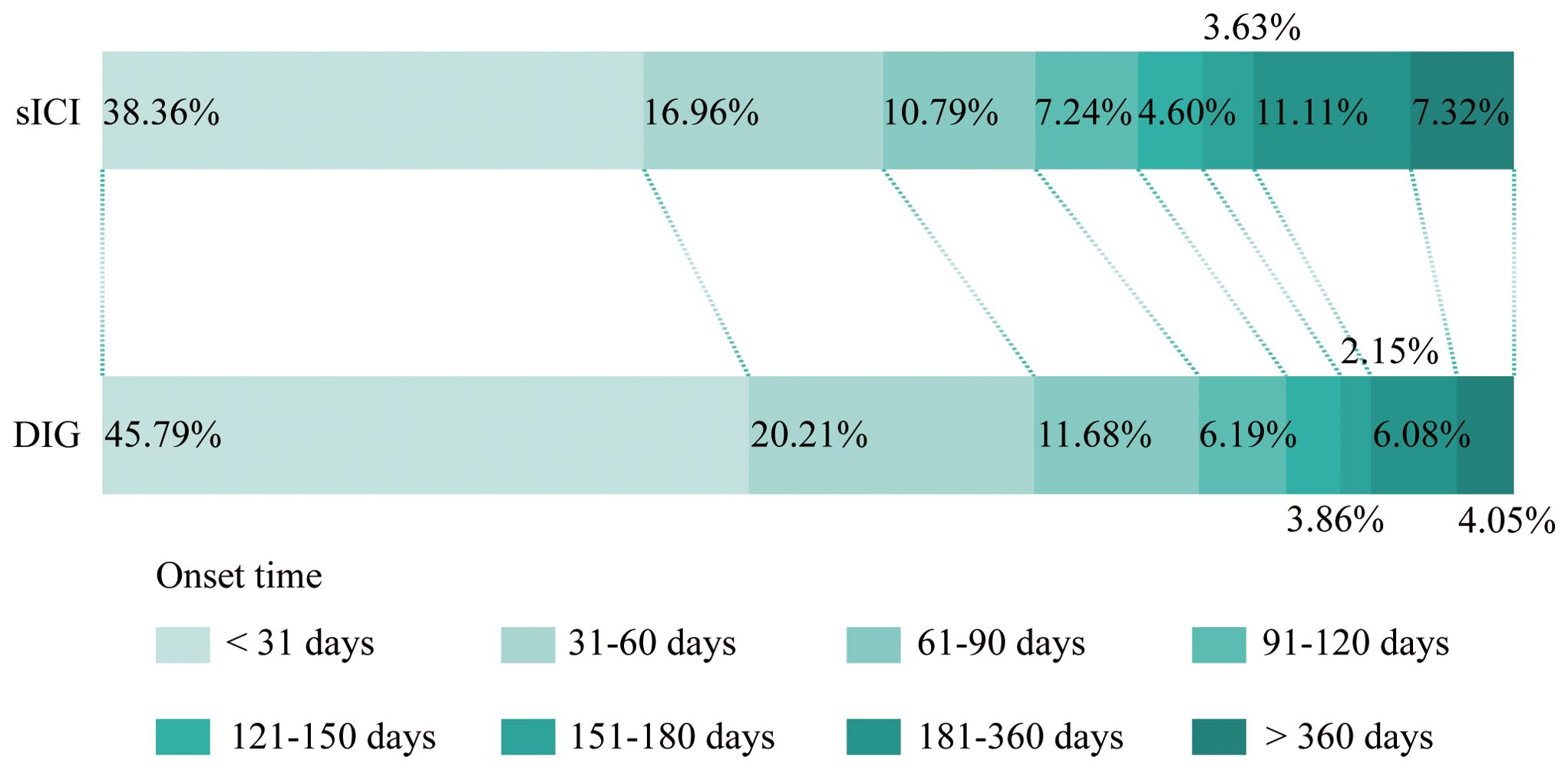
Onset time refers to the duration from the initiation of medication until the occurrence of AEs. As shown in Figure 3, after excluding reports with missing or abnormal onset times, DIG exhibits an earlier first AE compared to sICIs. For this processed dataset, we focus primarily on the median values (Table 2). The median onset time for DIG is 36 days (interquartile range 14–82 days), which is notably shorter than that of sICIs at 50 days (interquartile range 16–131 days). It is important to note that a substantial proportion of onset time data is missing (76.13%), which may potentially introduce significant bias into the interpretation and reporting of onset time results.
Positive signals of DIG at SOC level
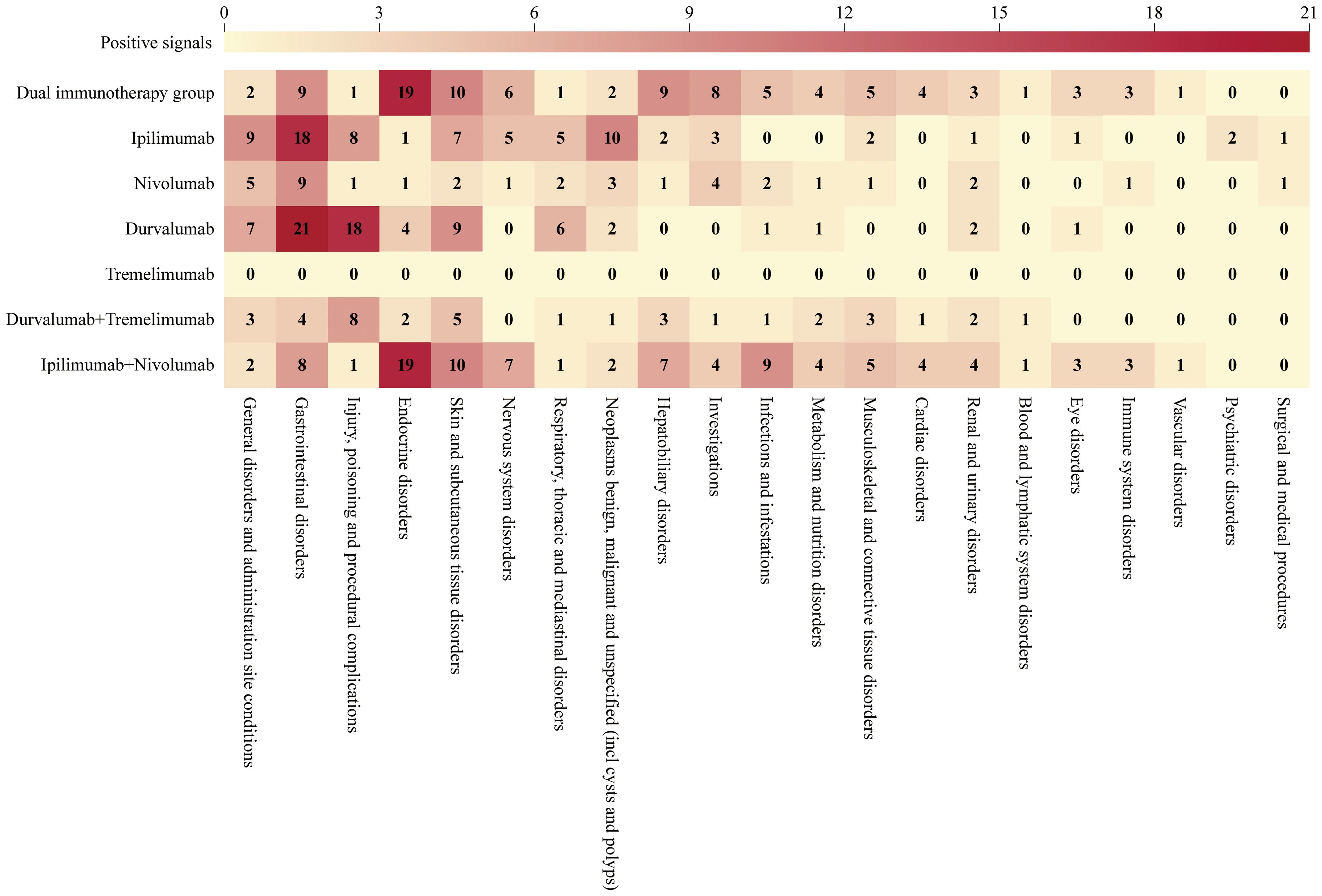
We analyzed the target AEs using two algorithms, ROR and BCPNN. An AE positive signal was generated only when both algorithms met their respective signal generation conditions simultaneously. As illustrated in Figure 4, at the SOC level, DIG (primarily derived from ipilimumab plus nivolumab) demonstrated significant AEs in detecting endocrine disorders (SOC: 10014698), with 19 distinct types of positive signals identified. In contrast, sICIs exhibited fewer types of positive signals. Moreover, DIG detected a higher number of AE types across multiple SOCs, including skin and subcutaneous tissue disorders (10 PT types, SOC: 10040785), nervous system disorders (6 PT types, SOC: 10029205), hepatobiliary disorders (9 PT types, SOC: 10019805), investigations (8 PT types, SOC: 10022891), infections and infestations (5 PT types, SOC: 10021881), metabolism and nutrition disorders (4 PT types, SOC: 10027433), musculoskeletal and connective tissue disorders (5 PT types, SOC: 10028395), cardiac disorders (4 PT types, SOC: 10007541), renal and urinary disorders (3 PT types, SOC: 10038359), blood and lymphatic system disorders (1 PT type, SOC: 10005329), eye disorders (3 PT types, SOC: 10015919), immune system disorders (3 PT types, SOC: 10021428), and vascular disorders (1 PT type, SOC: 10047065) compared to sICIs.
Tremelimumab exhibited a very low number of positive signals, likely attributable to the limited number of reports. Besides tremelimumab, fewer types of positive signals were observed in several other categories: general disorders and administration site conditions (2 PT types, SOC: 10018065), injury, poisoning and procedural complications (1 PT type, SOC: 10022117), respiratory, thoracic and mediastinal disorders (1 PT type, SOC: 10038738), as well as neoplasms benign, malignant and unspecified (incl cysts and polyps) (2 PT types, SOC: 10029104).
Positive signals of DIG at PT level
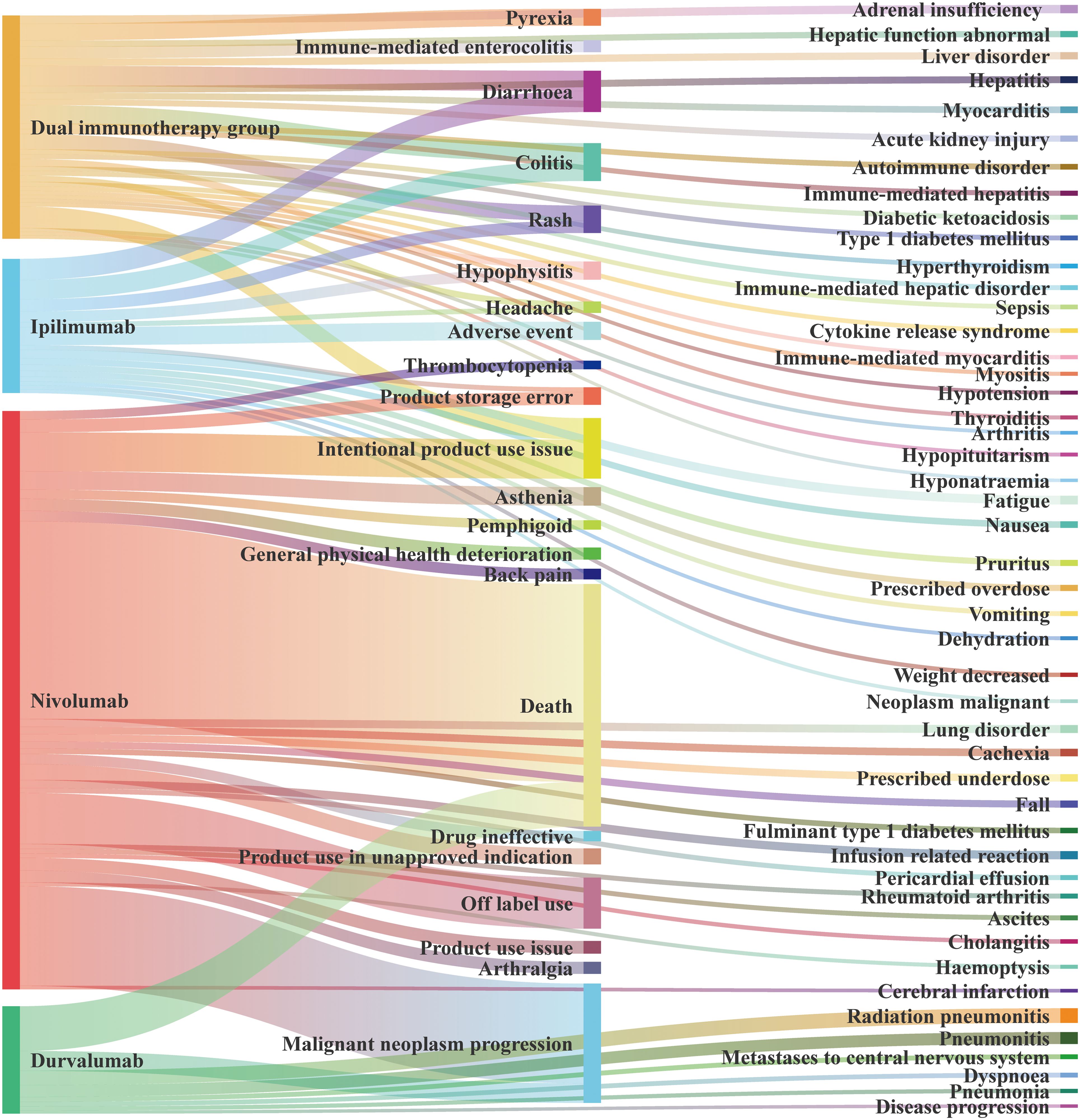
We present the AEs with a > 100, summarized in Figure 5 according to the source of the positive signal. In DIG, the most frequently reported AE was intentional product use issue (a = 679), followed by diarrhea (a = 633), colitis (a = 581), pyrexia (a = 530), and others. In sICI, the top three AEs associated with ipilimumab were diarrhea (a = 665), colitis (a = 607), and adverse event (a = 566, PT: 10060933). For nivolumab, the leading AEs were death (a = 6,216), malignant neoplasm progression (a = 3,129), and off-label use (a = 1,605). Durvalumab was associated with death (a = 1,405), malignant neoplasm progression (a = 619), and radiation pneumonitis (a = 456).
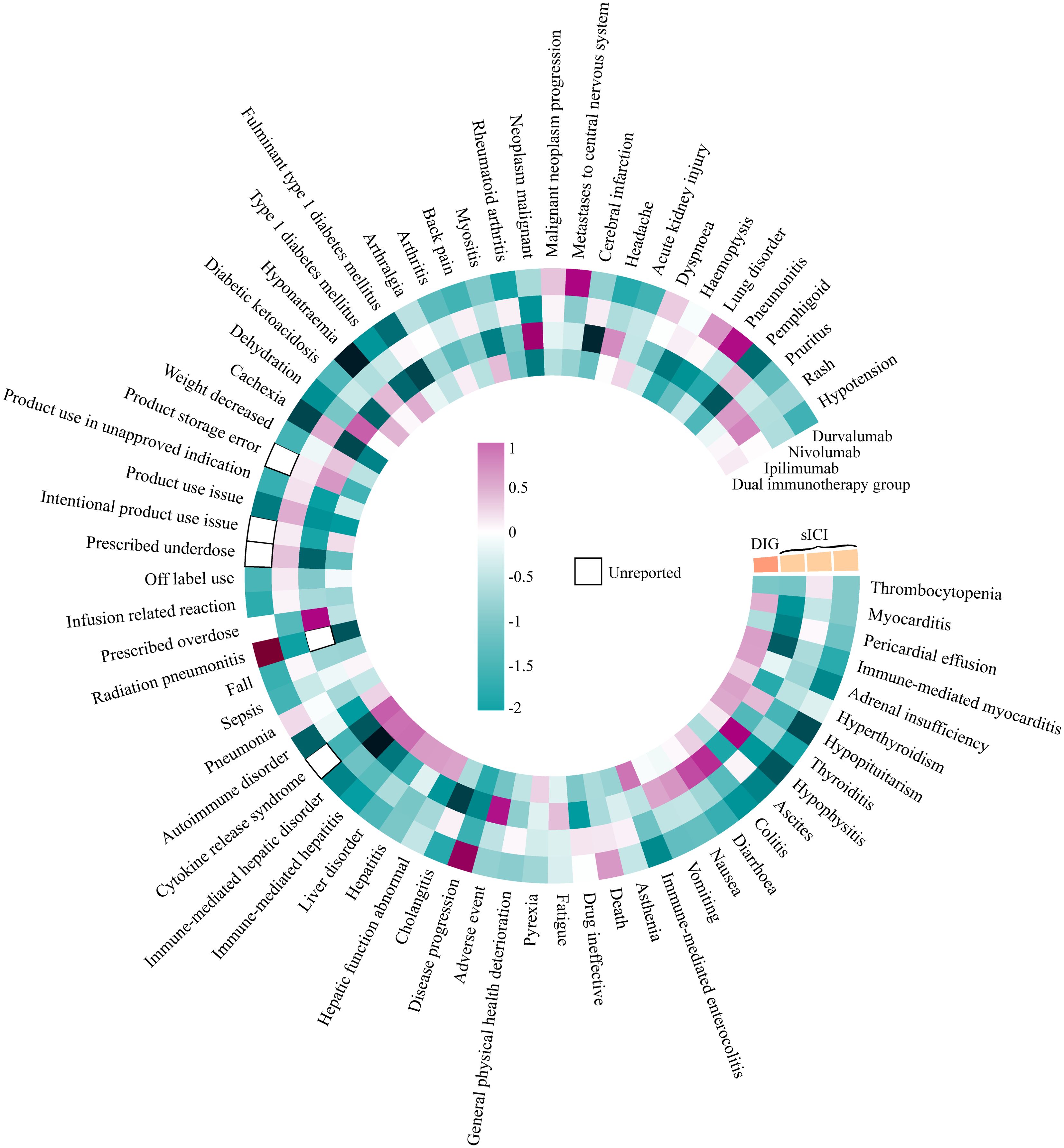
The data were analyzed using two methods, ROR and BCPNN, with detailed results provided in Additional File 2. In Figure 6, we summarize the IC025 of positive signals with a > 100 obtained by the BCPNN method for DIG and sICIs (including non-positive signals). DIG exhibited higher positive signal intensity compared to sICIs across the following PTs: myocarditis (ROR 2.221, IC025 0.486, PT: 10028606), immune-mediated myocarditis (ROR 2.922, IC025 0.610, PT: 10082606), adrenal insufficiency (ROR 2.503, IC025 0.602, PT: 10001367), hyperthyroidism (ROR 1.872, IC025 0.305, PT: 10020850), thyroiditis (ROR 2.669, IC025 0.546, PT: 10043778), immune-mediated enterocolitis (ROR 3.948, IC025 0.937, PT: 10078961), pyrexia (ROR 1.570, IC025 0.290, PT: 10037660), hepatic function abnormality (ROR 2.582, IC025 0.591, PT: 10019670), hepatitis (ROR 2.705, IC025 0.637, PT: 10019717), liver disorder (ROR 2.718, IC025 0.646, PT: 10024670), immune-mediated hepatitis (ROR 5.504, IC025 0.994, PT: 10078962), immune-mediated liver disorder (ROR 5.322, IC025 0.966, PT: 10083521), cytokine release syndrome (ROR 7.650, IC025 1.103, PT: 10052015), autoimmune diseases (ROR 1.754, IC025 0.275, PT: 10061664), sepsis (ROR 1.414, IC025 0.062, PT: 10040047), diabetic ketoacidosis (ROR 2.294, IC025 0.472, PT: 10012671), type 1 diabetes mellitus (ROR 2.421, IC025 0.508, PT: 10067584), arthritis (ROR 1.562, IC025 0.113, PT: 10003246), myositis (ROR 2.204, IC025 0.412, PT: 10028653), and acute kidney injury (ROR 1.708, IC025 0.264, PT: 10069339). In contrast, the occurrence of death (PT: 10011906), product storage error (PT: 10079843), malignant neoplasm progression (PT: 10051398), and lung disorder (PT: 10025082) is lower in DIG compared to sICIs. The relevant ROR analysis results (only DIG ROR025 > 1) are presented in Table 3.
Positive signals of Durvalumab + Tremelimumab and Durvalumab at PT level
Due to the promotion of the STRIDE regimen, the combination of Durvalumab and Tremelimumab has been more widely adopted in clinical practice compared to Tremelimumab monotherapy, largely due to its improved safety profile. Comparing the safety signals between the Durvalumab + Tremelimumab group and the Durvalumab monotherapy group can more clearly illustrate the differences in their safety profiles. We present the AEs with positive and strongly positive IC signal intensities in Additional File 3. The results indicate that the use of Durvalumab alone is strongly associated with AEs such as radiation pneumonitis (PT: 10037765), recurrence of non-small cell lung cancer (PT: 10029515), and disease progression (PT: 10061818), whereas these associations are not observed in the Durvalumab + Tremelimumab group. However, it should be noted that the use of this dICI regimen is strongly linked to AEs including cytokine release syndrome (PT: 10052015) and ruptured liver carcinoma (PT: 10050842).
Discussion
Previous studies have reported that the most frequently observed AEs associated with dICIs were predominantly dermatological (34% for any grade and 4.2% for grade 3 or higher), endocrine (23.8% for any grade and 4.2% for grade 3 or higher), gastrointestinal (18.2% for any grade and 2.4% for grade 3 or higher), and hepatic (15.8% for any grade and 8.2% for grade 3 or higher) (22). Through deep mining and signal analysis of the FAERS database, we identified a higher incidence of immune-related adverse events (irAEs) associated with DIG, including immune-mediated myocarditis, immune-mediated enterocolitis, immune-mediated hepatitis, and immune-mediated liver disorders. Moreover, the pronounced signal intensity associated with cytokine release syndrome (CRS) merits thorough investigation. For pyrexia, sepsis, arthritis, and other AEs with low signal intensity, this does not imply that the risks and challenges they pose in clinical practice can be overlooked.
Khoja et al. summarized in their review that the risk of irAEs varies depending on the type of ICI. Specifically, PD-1/PD-L1 inhibitors have a lower incidence of irAEs compared to CTLA-4 inhibitors, and the gastrointestinal tract, liver, skin, and thyroid are the most commonly affected organs (31). Furthermore, the toxicity associated with CTLA-4 inhibitors exhibits a dose-dependent relationship (32–34). The administration of dICI can enhance CTLA-4-induced expansion of CD4+/CD8+ T cells and reduce regulatory T cells (Tregs) in tumors, this phenomenon is not observed with sICI alone (35).
In all irAEs reporting positive signals, immune-mediated myotoxicit is a rare but potentially fatal complication (36, 37). Current understanding of immune-mediated myocarditis pathogenesis includes the absence of central tolerance due to the lack of specific cardiac antigen expression in thymic epithelial cells, impaired peripheral tolerance, activation of autoreactive T cells, interaction between T cells and cardiac APCs to recognize cardiac peptides, and a positive feedback loop involving cytokines and effector T cells that leads to cardiac tissue infiltration (38). The current diagnostic criteria for ICI-related myocarditis encompass elevated serum troponin and natriuretic peptide levels, prolonged PR intervals on electrocardiography, atrioventricular block, ventricular arrhythmias, ST-segment depression, or T-wave inversion. Additionally, echocardiography and cardiac magnetic resonance imaging can provide supportive evidence of myocardial injury. Endomyocardial biopsy serves as the gold standard for diagnosing myocarditis when necessary. Upon diagnosis of ICI-related myocarditis, ICIs should be discontinued even in cases of mild toxicity. High-dose glucocorticoids are recommended for a minimum duration of 4 to 6 weeks, with continuous monitoring of troponin levels. For patients who exhibit an inadequate response to glucocorticoids, other immunomodulatory agents and conventional cardiac therapies may be considered (39).
Compared with sICI, dICI was associated with a significantly higher incidence of immune-mediated enterocolitis (OR 3.53, 95% CI 3.4-4.4) (40). Firstly, the administration of dICI can disrupt the balance of the intestinal microbiota, leading to the activation of T helper 1 and 17 cells, which subsequently results in inflammation of the intestinal mucosa (41, 42). Secondly, patients with immune-mediated enterocolitis exhibited significantly higher levels of CD4+ T cells and lower levels of Tregs compared to those without colitis, thereby markedly reducing the colitis-free interval (43). Fecal microbiota transplantation has been proposed as a therapeutic approach to alleviate inflammation in patients with ICI-related enteritis who are refractory to immunosuppressive therapy (44). Following fecal transplantation, the gut microbiota of recipients becomes more similar to that of donors, characterized by an expansion of certain bacteria such as Akkermansia and Bifidobacterium, which have been associated with reduced inflammation (45). Reducing the use of antibiotics that target anaerobic bacteria when necessary has been shown to mitigate the recurrence of ICI-associated enteritis and improve patient survival (45).
The mechanism of immune-related hepatotoxicity encompasses multiple facets. The disruption of immune tolerance leads to aberrant expression of major histocompatibility complex molecules, co-stimulatory molecules, and anti-inflammatory cytokines in the liver, thereby mediating hepatic injury (46). Concurrently, dICI administration induces tumor cell lysis, resulting in epitope spreading and subsequent uptake by APCs (47). Furthermore, CTLA-4 inhibitors enhance the diversity of CD4+ and CD8+ T cells while diminishing the suppressive function of Tregs, as evidenced by biopsy findings from patients experiencing immune-related hepatotoxicity (48–50). CD4+/CD8+ T cells play a crucial role in the digestive system-related AEs mentioned above. Kawashima et al. proposed that CD4+ T cells are essential for supporting both B cells and CD8+ T cells (51). Moreover, CD4+/CD8+ T cells exhibit a significant association with CTLA-4 inhibitors in dICI (35). The application of CTLA-4 inhibitors may serve as a critical factor contributing to dICI-related AEs in clinical settings, which requires further investigation to better understand the balance between PD-1/PD-L1 inhibitors and CTLA-4 inhibitors in clinical practice.
The reporting of ICI-related CRS is still in its early stages, and awareness of CRS as an irAE remains insufficient, despite the compelling positive findings from this study (52–54). Currently, it is widely recognized that the mechanism underlying ICI-induced CRS involves excessive activation of the immune system. Specifically, the “dual release of immune brakes” mechanism associated with dICI serves as the foundational trigger for CRS. Activation of innate immune cells leads to the release of cytokines, which subsequently stimulate T and B cells within the adaptive immune system. Prolonged hyperactivation disrupts the normal negative feedback mechanisms that typically constrain immune-mediated damage, resulting in a positive feedback loop that amplifies the “cytokine cascade”. This self-perpetuating cycle promotes the accumulation of immune cells, sustained cytokine release, and further recruitment and activation of innate immune cells, thereby destabilizing the hot tumor microenvironment and leading to systemic immune cell infiltration, which ultimately precipitates CRS (55, 56). The specific mechanism of CRS induced by tumor treatment varies depending on the type of therapy. In this study, the combination of Durvalumab and Tremelimumab (ROR 15.811, IC025 2.55, FDR-adjusted p < 0.001) demonstrated a stronger association with the occurrence of CRS compared to the combination of Ipilimumab and Nivolumab (ROR 4.448, IC025 0.861, FDR-adjusted p < 0.001). This suggests that clinicians should remain vigilant regarding the potential development of CRS associated with dICIs in clinical practice. Further clinical trials and basic research are warranted to validate these findings and elucidate the underlying mechanisms. Notably, chimeric antigen receptor-T cell therapy has a higher incidence of CRS compared to ICI. However, this should not overshadow the fact that ICI also poses a significant risk of CRS (55, 57).
Strength and limitation
We conducted an extensive analysis of the FAERS database to investigate the real-world toxicity profile of dICI, representing the most comprehensive evaluation of AE reports for dICI since its clinical introduction. This study aims to provide detailed insights into AE occurrences for clinical practice and establish a foundation for risk assessment in clinical decision-making. However, several limitations must be acknowledged. The FAERS database relies on voluntary reporting, where reporters may not possess specialized medical knowledge, and there is no requirement to provide evidence of a causal relationship between a drug and a specific AE. Furthermore, the incidence rate cannot be accurately determined based on these reports. AEs may be associated with the underlying medical condition being treated, concomitant medications, or other external factors. It should be noted that these reports only represent the observations and opinions of the individuals who submitted them. Moreover, the US Food and Drug Administration cannot capture all adverse event or medication error reports, and the quantity and quality of submitted reports are influenced by various factors.
Conclusion
We utilized two algorithms, ROR and BCPNN, to comprehensively analyze the FAERS database. Our findings indicate that the AEs associated with dICI predominantly originate from irAEs, including myotoxicity, endocrine toxicity, and hepatotoxicity. Notably, CRS, a strongly positive signal and rarely reported AE, warrants particular attention in clinical decision-making.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
ZL: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. YX: Conceptualization, Formal analysis, Investigation, Methodology, Writing – review & editing. TW: Conceptualization, Methodology, Writing – review & editing. YL: Formal analysis, Investigation, Writing – review & editing. YT: Formal analysis, Investigation, Writing – review & editing. YH: Resources, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1631273/full#supplementary-material
Additional File 1 | Baseline information of all medications. Description of data: Presentation of baseline data for 6 medication groups.
Additional File 2 | AEs signal strength of all medications at the level of PTs (a > 100). Description of data: A demonstration of the intensity of medication-related AEs at the PT level for cases exceeding 100.
Additional File 3 | AEs signal strength of Durvalumab + Tremelimumab and Durvalumab at the level of PTs (IC025 > 1.5) Description of data: A demonstration of the intensity of medication-related AEs at the PT level for IC025 > 1.5.
Glossary
AE: Adverse event
APC: Antigen-presenting cells
BCPNN: Bayesian confidence propagation neural network
CD: Cluster of differentiation
CI: Confidence interval
CRS: Cytokine release syndrome
dICI: Dual immune checkpoint inhibitor/Ipilimumab+Nivolumab or Tremelimumab+Durvalumab
DIG: Dual immunotherapy group/Ipilimumab+Nivolumab and Tremelimumab+Durvalumab
FAERS: The US Food and Drug Administration Adverse Event Reporting System
IC025: 95% CI lower limit of information component
ICI: Immune checkpoint inhibitor
irAE: Immune-related adverse event
MedDRA: Medical Dictionary for Regulatory Activities
NSCLC: Non-small cell lung cancer
PS: Primary suspect
PT: Preferred term
Q1: Lower quartile
Q3: Upper quartile
ROR: Reporting odds ratio
ROR025: 95% CI lower limit of ROR
SD: Standard deviation
sICI: Single immune checkpoint inhibitor
SOC: System organ class
Treg: Regulatory T cell.
References
1. Tang S, Qin C, Hu H, Liu T, He Y, Guo H, et al. Immune checkpoint inhibitors in non-small cell lung cancer: progress, challenges, and prospects. Cells. (2022) 11:320. doi: 10.3390/cells11030320
2. Carlino MS, Larkin J, and Long GV. Immune checkpoint inhibitors in melanoma. Lancet. (2021) 398:1002–14. doi: 10.1016/s0140-6736(21)01206-x
3. Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, and Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. (2020) 6:92. doi: 10.1038/s41572-020-00224-3
4. Vogel A, Meyer T, Sapisochin G, Salem R, and Saborowski A. Hepatocellular carcinoma. Lancet. (2022) 400:1345–62. doi: 10.1016/S0140-6736(22)01200-4
5. Zhang Y and Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. (2020) 17:807–21. doi: 10.1038/s41423-020-0488-6
6. Burke KP, Chaudhri A, Freeman GJ, and Sharpe AH. The B7:CD28 family and friends: Unraveling coinhibitory interactions. Immunity. (2024) 57:223–44. doi: 10.1016/j.immuni.2024.01.013
7. Zhou YJ, Li G, Wang J, Liu M, Wang Z, Song Y, et al. PD-L1: expression regulation. Blood Sci. (2023) 5:77–91. doi: 10.1097/BS9.0000000000000149
8. Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. (2019) 38:255. doi: 10.1186/s13046-019-1259-z
9. Harrington KJ, Burtness B, Greil R, Soulières D, Tahara M, de Castro G, et al. Pembrolizumab with or without chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma: updated results of the phase III KEYNOTE-048 study. J Clin Oncol. (2023) 41:790–802. doi: 10.1200/jco.21.02508
10. Mai H-Q, Chen Q-Y, Chen D, Hu C, Yang K, Wen J, et al. Toripalimab plus chemotherapy for recurrent or metastatic nasopharyngeal carcinoma. Jama. (2023) 330(20):1961–70. doi: 10.1001/jama.2023.20181
11. Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. New Engl J Med. (2020) 383:1218–30. doi: 10.1056/NEJMoa2002788
12. Park S, Kim TM, Han J-Y, Lee G-W, Shim BY, Lee Y-G, et al. Randomized study of atezolizumab plus bevacizumab and chemotherapy in patients with EGFR- or ALK-rearranged or translocated non–small-cell lung cancer (ATTLAS, KCSG-LU19-04). J Clin Oncol. (2024) 42:1241–51. doi: 10.1200/jco.23.01891
14. Sangro B, Chan SL, Kelley RK, Lau G, Kudo M, Sukeepaisarnjaroen W, et al. Four-year overall survival update from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann Oncol. (2024) 35:448–57. doi: 10.1016/j.annonc.2024.02.005
15. Carbone DP, Ciuleanu TE, Schenker M, Cobo M, Bordenave S, Juan-Vidal O, et al. Four-year clinical update and treatment switching-adjusted outcomes with first-line nivolumab plus ipilimumab with chemotherapy for metastatic non-small cell lung cancer in the CheckMate 9LA randomized trial. J Immunother Cancer. (2024) 12(2):e008189. doi: 10.1136/jitc-2023-008189
16. Blank CU, Lucas MW, Scolyer RA, van de Wiel BA, Menzies AM, Lopez-Yurda M, et al. Neoadjuvant nivolumab and ipilimumab in resectable stage III melanoma. New Engl J Med. (2024) 391:1696–708. doi: 10.1056/NEJMoa2402604
17. Rimini M, Fornaro L, Rizzato MD, Antonuzzo L, Rossari F, Satake T, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer: A large real-life worldwide population. Eur J Cancer. (2024) 208:114199. doi: 10.1016/j.ejca.2024.114199
18. Girard N, Bar J, Garrido P, Garassino MC, McDonald F, Mornex F, et al. Treatment characteristics and real-world progression-free survival in patients with unresectable stage III NSCLC who received durvalumab after chemoradiotherapy: findings from the PACIFIC-R study. J Thorac Oncol. (2023) 18:181–93. doi: 10.1016/j.jtho.2022.10.003
19. Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. C. investigators: Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. (2019) 394:1929–39. doi: 10.1016/S0140-6736(19)32222-6
20. Herrera AF, LeBlanc M, Castellino SM, Li H, Rutherford SC, Evens AM, et al. Nivolumab+AVD in advanced-stage classic hodgkin’s lymphoma. N Engl J Med. (2024) 391:1379–89. doi: 10.1056/NEJMoa2405888
21. van der Heijden MS, Sonpavde G, Powles T, Necchi A, Burotto M, Schenker M, et al. I. CheckMate 901 trial: nivolumab plus gemcitabine-cisplatin in advanced urothelial carcinoma. N Engl J Med. (2023) 389:1778–89. doi: 10.1056/NEJMoa2309863
22. Cheng W, Kang K, Zhao A, and Wu Y. Dual blockade immunotherapy targeting PD-1/PD-L1 and CTLA-4 in lung cancer. J Hematol Oncol. (2024) 17:54. doi: 10.1186/s13045-024-01581-2
23. Sakaeda T, Tamon A, Kadoyama K, and Okuno Y. Data mining of the public version of the FDA Adverse Event Reporting System. Int J Med Sci. (2013) 10:796–803. doi: 10.7150/ijms.6048
24. Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. (1998) 54:315–21. doi: 10.1007/s002280050466
25. Yin Y, Shu Y, Zhu J, Li F, and Li J. A real-world pharmacovigilance study of FDA Adverse Event Reporting System (FAERS) events for osimertinib. Sci Rep. (2022) 12:19555. doi: 10.1038/s41598-022-23834-1
26. Ahdi HS, Wichelmann TA, Pandravada S, and Ehrenpreis ED. Medication-induced osteonecrosis of the jaw: a review of cases from the Food and Drug Administration Adverse Event Reporting System (FAERS). BMC Pharmacol Toxicol. (2023) 24(1):15. doi: 10.1186/s40360-023-00657-y
27. Liu W-H, Hu H-M, Li C, Shi Q, Liu C-H, Liu A-X, et al. Real-world study of adverse events associated with triptan use in migraine treatment based on the U.S. Food and Drug Administration (FDA) adverse event reporting system (FAERS) database. J Headache Pain. (2024) 25(1):206. doi: 10.1186/s10194-024-01913-0
28. Zou F, Cui Z, Lou S, Ou Y, Zhu C, Shu C, et al. Adverse drug events associated with linezolid administration: a real-world pharmacovigilance study from 2004 to 2023 using the FAERS database. Front Pharmacol. (2024) 15:1338902. doi: 10.3389/fphar.2024.1338902
29. Zhao J and Tao Y. Adverse event reporting of the IGF-1R monoclonal antibody teprotumumab: a real-world study based on the US food and drug administration adverse event reporting system. Front Pharmacol. (2024) 15:1393940. doi: 10.3389/fphar.2024.1393940
30. Wen M-T, Li J-C, Lu B-W, Shao H-R, Ling P-X, Liu F, et al. Indications and adverse events of teriparatide: based on FDA adverse event reporting system (FAERS). Front Pharmacol. (2024) 15:1391356. doi: 10.3389/fphar.2024.1391356
31. Khoja L, Day D, Wei-Wu Chen T, Siu LL, and Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. (2017) 28:2377–85. doi: 10.1093/annonc/mdx286
32. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol. (2022) 40:127–37. doi: 10.1200/JCO.21.02229
33. Tarhini AA, Lee SJ, Hodi FS, Rao UNM, Cohen GI, Hamid O, et al. Phase III study of adjuvant ipilimumab (3 or 10 mg/kg) versus high-dose interferon alfa-2b for resected high-risk melanoma: north american intergroup E1609. J Clin Oncol. (2020) 38:567–75. doi: 10.1200/JCO.19.01381
34. Lebbe C, Meyer N, Mortier L, Marquez-Rodas I, Robert C, Rutkowski P, et al. Evaluation of two dosing regimens for nivolumab in combination with ipilimumab in patients with advanced melanoma: results from the phase IIIb/IV checkMate 511 trial. J Clin Oncol. (2019) 37:867–75. doi: 10.1200/JCO.18.01998
35. Wei SC, Anang NAS, Sharma R, Andrews MC, Reuben A, Levine JH, et al. Combination anti-CTLA-4 plus anti-PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc Natl Acad Sci U.S.A. (2019) 116:22699–709. doi: 10.1073/pnas.1821218116
36. Moslehi J, Lichtman AH, Sharpe AH, Galluzzi L, and Kitsis RN. Immune checkpoint inhibitor-associated myocarditis: manifestations and mechanisms. J Clin Invest. (2021) 131(5):e145186. doi: 10.1172/JCI145186
37. Salem JE, Bretagne M, Abbar B, Leonard-Louis S, Ederhy S, Redheuil A, et al. Abatacept/ruxolitinib and screening for concomitant respiratory muscle failure to mitigate fatality of immune-checkpoint inhibitor myocarditis. Cancer Discov. (2023) 13:1100–15. doi: 10.1158/2159-8290.CD-22-1180
38. Munir AZ, Gutierrez A, Qin J, Lichtman AH, and Moslehi JJ. Immune-checkpoint inhibitor-mediated myocarditis: CTLA4, PD1 and LAG3 in the heart. Nat Rev Cancer. (2024) 24:540–53. doi: 10.1038/s41568-024-00715-5
39. Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, and Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. (2020) 9:e013757. doi: 10.1161/JAHA.119.013757
40. Gougis P, Jochum F, Abbar B, Dumas E, Bihan K, Lebrun-Vignes B, et al. Clinical spectrum and evolution of immune-checkpoint inhibitors toxicities over a decade-a worldwide perspective. EClinicalMedicine. (2024) 70:102536. doi: 10.1016/j.eclinm.2024.102536
41. Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. (2016) 7:10391. doi: 10.1038/ncomms10391
42. Anderson R and Rapoport BL. Immune dysregulation in cancer patients undergoing immune checkpoint inhibitor treatment and potential predictive strategies for future clinical practice. Front Oncol. (2018) 8:80. doi: 10.3389/fonc.2018.00080
43. Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. (2017) 28:1368–79. doi: 10.1093/annonc/mdx108
44. Fasanello MK, Robillard KT, Boland PM, Bain AJ, and Kanehira K. Use of fecal microbial transplantation for immune checkpoint inhibitor colitis. ACG Case Rep J. (2020) 7:e00360. doi: 10.14309/crj.0000000000000360
45. Abu-Sbeih H and Wang Y. Gut microbiome and immune checkpoint inhibitor-induced enterocolitis. Dig Dis Sci. (2020) 65:797–9. doi: 10.1007/s10620-020-06103-x
46. Jenne CN and Kubes P. Immune surveillance by the liver. Nat Immunol. (2013) 14:996–1006. doi: 10.1038/ni.2691
47. Cunningham M, Gupta R, and Butler M. Checkpoint inhibitor hepatotoxicity: pathogenesis and management. Hepatology. (2024) 79:198–212. doi: 10.1097/HEP.0000000000000045
48. Shojaie L, Ali M, Iorga A, and Dara L. Mechanisms of immune checkpoint inhibitor-mediated liver injury. Acta Pharm Sin B. (2021) 11:3727–39. doi: 10.1016/j.apsb.2021.10.003
49. De Martin E, Michot JM, Papouin B, Champiat S, Mateus C, Lambotte O, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. (2018) 68:1181–90. doi: 10.1016/j.jhep.2018.01.033
50. Kubo T, Sugawara T, Shinkawa T, Kurisu T, Kouzen N, Tanaka T, et al. Fatal fulminant hepatitis induced by combined ipilimumab and nivolumab therapy despite favorable histologic response and confirmed by autopsy in a patient with clear cell renal cell carcinoma. Immunol Med. (2021) 44:136–41. doi: 10.1080/25785826.2020.1788229
51. Kawashima K, Andreata F, Beccaria CG, and Iannacone M. Priming and maintenance of adaptive immunity in the liver. Annu Rev Immunol. (2024) 42:375–99. doi: 10.1146/annurev-immunol-090122-041354
52. Tay SH, Toh MMX, Thian YL, Vellayappan BA, Fairhurst AM, Chan YH, et al. Cytokine release syndrome in cancer patients receiving immune checkpoint inhibitors: A case series of 25 patients and review of the literature. Front Immunol. (2022) 13:807050. doi: 10.3389/fimmu.2022.807050
53. Yomota M, Mirokuji K, Sakaguchi M, Kitahara Y, Chin F, Setoguchi K, et al. Cytokine release syndrome induced by immune-checkpoint inhibitor therapy for non-small-cell lung cancer. Intern Med. (2021) 60:3459–62. doi: 10.2169/internalmedicine.5922-20
54. Ceschi A, Noseda R, Palin K, and Verhamme K. Immune checkpoint inhibitor-related cytokine release syndrome: analysis of WHO global pharmacovigilance database. Front Pharmacol. (2020) 11:557. doi: 10.3389/fphar.2020.00557
55. Chen P, Tang Y, He W, Yang R, Lan Z, Chen R, et al. Potential pathophysiological mechanisms underlying multiple organ dysfunction in cytokine release syndrome. Mediators Inflammation. (2022) 2022:7137900. doi: 10.1155/2022/7137900
56. Thomas BC, Staudt DE, Douglas AM, Monje M, Vitanza NA, and Dun MD. CAR T cell therapies for diffuse midline glioma. Trends Cancer. (2023) 9:791–804. doi: 10.1016/j.trecan.2023.07.007
Keywords: tremelimumab, durvalumab, ipilimumab, nivolumab, immune checkpoint inhibitor, FAERS, adverse event
Citation: Li Z, Xie Y, Wang T, Liu Y, Tian Y and Hua Y (2025) Evaluating the toxicity profile of combination immune checkpoint inhibitors: a disproportionality analysis of real-world adverse events from the FDA Adverse Event Reporting System for tremelimumab, durvalumab, ipilimumab, and nivolumab. Front. Immunol. 16:1631273. doi: 10.3389/fimmu.2025.1631273
Received: 19 May 2025; Accepted: 06 August 2025;
Published: 01 September 2025.
Edited by:
Zhenhua Ren, Changping Laboratory, ChinaCopyright © 2025 Li, Xie, Wang, Liu, Tian and Hua. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yusi Hua, eXVzaWh1YUB3Y2hzY3UuY24=
†These authors have contributed equally to this work and share first authorship
 Zhuoyang Li
Zhuoyang Li Yuxuan Xie3†
Yuxuan Xie3† Tianhong Wang
Tianhong Wang Yusi Hua
Yusi Hua