- 1Department of Biochemistry, School of Life Sciences, University of Sussex, Brighton, United Kingdom
- 2SwipeBiome, Loos, France
- 3Department of Pathology, Gomel State Medical University, Gomel, Belarus
Endometriosis (EMS) is an oestrogen-dependent condition characterised by ectopic endometrial-like tissue growth with a chronic and inflammatory nature leading to severe symptoms and reduced quality of life. Emerging evidence implicates gut microbiome dysbiosis in EMS pathogenesis, driving chronic inflammation, immune dysfunction, and altered bacterial taxa within patient gut microbiome. This review examines the intricate relationship between gut dysbiosis and EMS, with a focus on immunomodulatory mechanisms and the downstream consequences of the bacterial contamination theory. It evaluates recent findings regarding microbial imbalances and microbial diversity, pinpointing gaps in current research that mandate further understanding. For example, while microbial markers like Lactobacillus depletion and elevated Escherichia coli have been observed in patients, their diagnostic potential remains poorly defined. Additionally, it addresses the broader implications of EMS, including its physical, mental and healthcare burdens. Simultaneously, critiquing current drawbacks in diagnostic and therapeutic strategies such as their invasiveness and limited efficacy. The review further evaluates novel microbiome-based strategies namely Lactobacillus-based probiotics and faecal microbiota transplantation (FMT), assessing their potential in modulating immune responses and alleviating EMS symptoms while considering associated challenges. Lastly, it highlights the emerging role of metabolomics in identifying non-invasive and diagnostic biomarkers like short-chain fatty acids (SCFAs), implicated in the interplay between microbial metabolites and immune signalling pathways in EMS.
1 Introduction to the epidemiology, pathophysiology and management of endometriosis
Endometriosis is a chronic, inflammatory, oestrogen-dependent gynaecological condition characterised by the ectopic growth of endometrial-like tissue outside the uterine cavity, leading to a range of debilitating symptoms (1). The term “Endometriosis” is derived from Greek: “endo” (within), “metra” (uterus), and “osis” (disease), with pelvic pain as the primary symptom. Risk factors include a shorter menstrual cycle, alcohol use, caffeine intake and earlier age at menarche (2). The condition is thought to affect approximately 10% of people assigned female at birth (3), with 6 - 10% of individuals of childbearing age affected (4) however, prevalence may vary depending on the population studied. Regardless, EMS remains a significant cause of infertility and reduced quality of life (5, 6) for an estimated 176 million women globally (7).
The most widely recognised theory for EMS development is the ‘Retrograde Menstruation Theory’, where endometrial fragments shed during menstruation flow back through the fallopian tubes. These fragments can then implant in the pelvis (ovaries, fallopian tubes, peritoneal surfaces, bowel, bladder), proliferating into invasive lesions that bleed and grow in a manner similar to the uterine lining. This process results in the development of adhesions, fibrosis, and ultimately localised inflammation (8–10). While this theory provides some groundwork, it fails to explain the clinical heterogeneity of the disease or why many women experience retrograde menstruation without developing EMS. This indicates that other underlying mechanisms are implicated, with the gut-immune axis and the balance of the microbiome gaining significant awareness. Dysbiosis in the gut can drive systemic inflammation and immune aberrations, creating an environment conducive to the survival and proliferation of ectopic endometrial cells. This critically influences the susceptibility to and progression of endometriosis beyond the initial cellular translocation.
Beyond immune dysregulation, imbalances and alterations in gut microbiota such as an altered Bacillota/Bacteroidota ratio have also been implicated (11). This dysbiosis is postulated to compromise pelvic stability by disrupting local immunomodulation, leading to a cycle of inflammation, pain, and tissue damage. The resulting immune and microbial imbalance is at the core of the diverse symptomatology of EMS, including dysmenorrhea, dyspareunia, infertility and chronic pelvic pain (12, 13). Recognising and leveraging the immunomodulatory role of the gut and vaginal microbiome should be prioritised due to the vast drawbacks of current therapeutic and diagnostic approaches such as side effects, high recurrence rates and limited detection of lesions in asymptomatic women (4).
2 The role of gut microbiome in driving dysbiosis in endometriosis
2.1 Role of the gut microbiome in health and disease
The gut microbiome comprises all microorganisms residing in the gastrointestinal tract, including their genes and metabolites, within a specific anatomical site. In contrast, the microbiota refers solely to the community of microorganisms such as bacteria, viruses, fungi, archaea, and protozoa (14). Amid the body’s microbiomes, the gut bacteria is the most extensively studied due to its critical roles in nutrient absorption, synthesis, immune system development, mucosal health, and host defence (15). Despite their smaller size, bacterial cells are as numerous as human cells, with their microbiome encoding over three million genes; 150 times more than the human genome (16). The importance of the gut in health was recognised as early as 400 B.C., when Hippocrates stated, “Death sits in the bowels” (17). Beyond gastrointestinal function, a fair amount of research is discussing the role of microbiome as a major regulator and biomarker for numerous inflammatory and proliferative diseases (18–20). For example, Long et al. (21) identified 11 microbiota-related causal links to cancers, including breast cancer (22). Research continues to explore the microbiome’s vast genetic potential in modulating immune responses, nutrient metabolism, neuromodulation, and barrier integrity.
The gut microbiome exists in two primary states. The eubiotic state supports homeostasis through immune and endocrine regulation, nutrient absorption, and protection against pathogens (23). Conversely, dysbiosis, marked by alterations in microbiota composition, compromises these and is linked to impaired intestinal barrier function, inflammation, and diseases such as obesity, hypertension, cardiovascular and neurological disorders, diabetes, and inflammatory bowel disease (24–26). Qin et al. (27) metagenomically analysed 650 bacterial and archaeal genomes to identify a ‘common core’ microbiome in eubiotic and dysbiotic environments. Eubiotic microbiota were dominated by Bacillota and Bacteroidota (>90%), with low levels of Pseudomonadota. By contrast, dysbiotic microbiota, displayed an altered Firmicutes/Bacteroidetes (F/B) ratio and changes in Lactobacillus abundance. Investigations into the transition to a dysbiotic state have primarily employed measures of alpha diversity, taxa abundance, and the Bacillota/Bacteroidota ratio.
Expanding the scope beyond the extensively studied gut microbiome, the vaginal microbial ecosystem represents a critical, yet often underappreciated, facet of women’s health, especially relevant in the context of EMS. The vaginal microbiome is a complex community of microorganisms, with its composition significantly influencing local immunity and susceptibility to gynaecological conditions (28) (Figure 1). In healthy women, the vaginal environment is usually dominated by species of the genus Lactobacillus, which play a pivotal role in maintaining homeostasis (29). Focusing more on the maintenance and modulation of immunity, recent evidence continues to elucidate the intricate relationship between the vaginal microbiome and inflammation. For instance, Yichan et al.’s (30) study on Chinese women demonstrated a negative correlation between the presence of Lactobacillus crispatus and Lactobacillus iners and pro-inflammatory cytokines IL - 1α and IL - 1β, while conversely, non-Lactobacillus species like Gardnerella vaginalis and Escherichia coli showed positive associations (30). This aligns with another recent finding which highlights that vaginal dysbiosis, marked by a reduction in Lactobacillus dominance and increased microbial diversity, is linked to an elevated risk of adverse genital tract diseases, pregnancy complications and can trigger pro-inflammatory responses by impairing the vaginal mucosal barrier (31). Given the anatomical proximity to the pelvic cavity and the potential for systemic immune modulation, understanding the functions and dynamics of the vaginal microbiome is increasingly recognised as crucial for a holistic understanding of gynaecological health and conditions such as EMS.
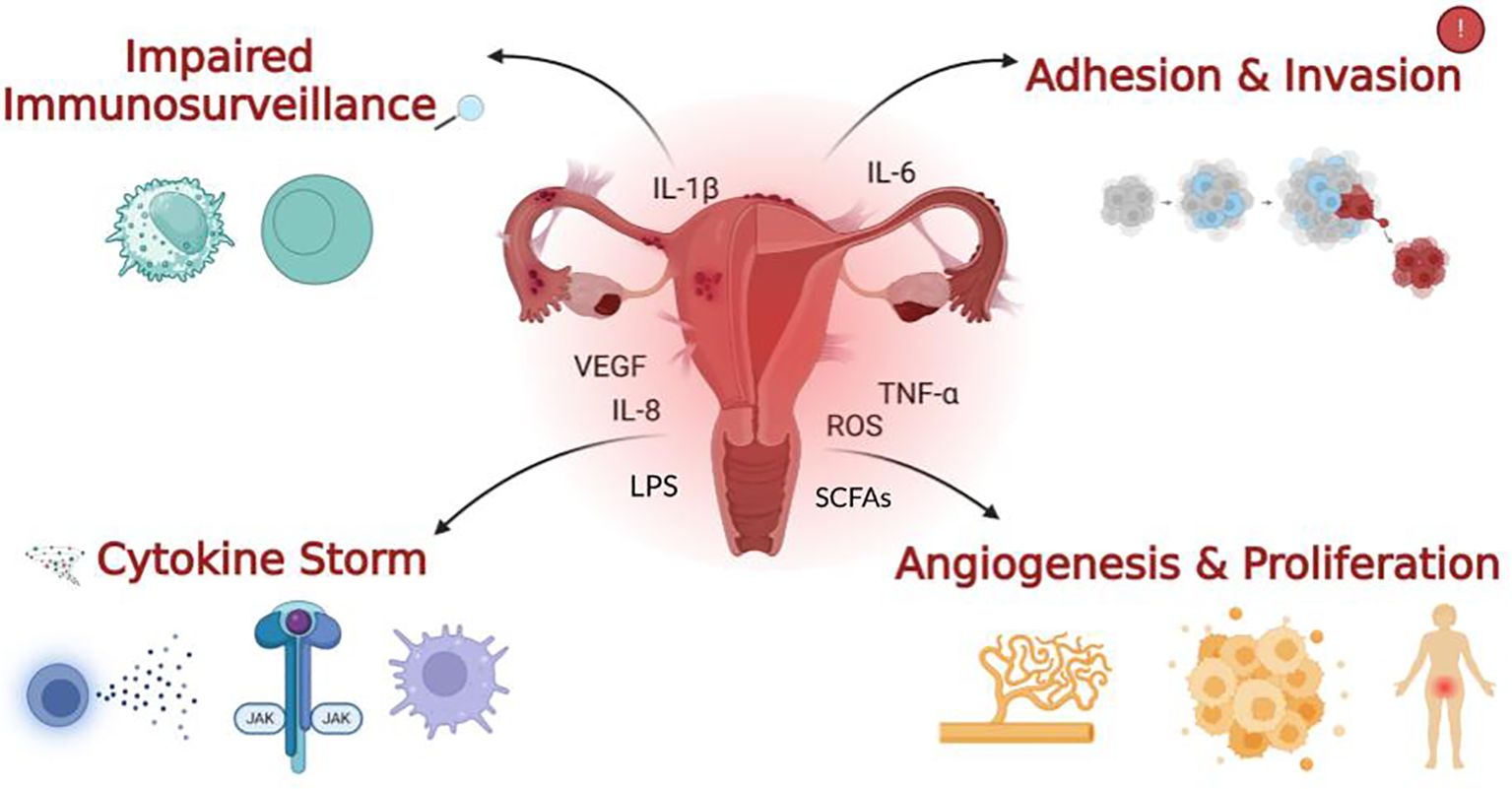
Figure 1. The four main outcomes of immune dysregulation in endometriosis. Impaired immune surveillance is characterised by a reduction in natural killer (NK) cell activity observed in EMS patients. A cytokine storm reflects the excessive recruitment and over-activation of pro-inflammatory cytokines and immune cells, which heavily contribute to chronic and severe inflammation. Within the context of gut dysbiosis, an increase in gut permeability can lead to elevated levels of pro-inflammatory bacterial metabolites like lipopolysaccharide (LPS) entering systemic circulation, exacerbating this immune dysregulation. Contrarily, a reduction in beneficial Short-Chain Fatty Acids (SCFAs), produced by a healthy gut microbiota, diminishes their anti-inflammatory and immune-modulating effects, further contributing to the inflammatory cascade. The immune cells, cytokines and pro-inflammatory factors depicted in the centre of the image, along with adhesion, invasion, angiogenesis and proliferation on the right, arise as downstream effects of diminished immune surveillance and increased inflammation as illustrated on the left. Together, these processes promote lesion survival and further exacerbate the inflammatory nature of the condition playing a major role in the pathogenesis of EMS.
2.2 Microbial alpha diversity alterations in endometriosis as a consequence of dysbiosis
Numerous studies highlight how altered microbial diversity and populations are present in EMS patients, however the nature of these changes is still unclear (Figure 2). Diversity alterations were represented by Shannon (represents both richness and evenness) and Simpson (focuses on evenness) biodiversity measures (Chen, 2021). Many studies reported reduced microbial richness, for instance, a study conducted by Lin et al. (32), observed reduced Shannon and Simpson measures within faecal samples from EMS patients; 10.5% (p = 0.006) and 5.7% (p = 0.013) decreases, respectively (32). In concordance, Svensson also saw a marked reduction in the alpha diversity of EMS patients (p = 4.9 × 10−5) as did Shan et al. (33, 34). For adults, a less diverse gut microbiome has been linked to reduced production of beneficial metabolites like short-chain fatty acids (SCFAs), potentially leading to impaired mucosal immunity and contributing to immune dysregulation and chronic inflammation in EMS (35). To substantiate this, a study on a murine model found that mice with endometriosis had significantly lower concentrations of SCFAs, such as n-butyrate, compared to healthy controls. This research further demonstrated that n-butyrate directly inhibited the growth of human endometriotic epithelial and stromal cells in vitro, highlighting a direct anti-proliferative effect (36). Additional pioneering evidence from a separate murine model revealed that Fecal Microbiota Transplantation (FMT) from healthy donors elevated the levels of the SCFA acetate in both the gut and ectopic lesions, which in turn activated the JAK1/STAT3 signalling pathway ultimately driving macrophages towards an anti-inflammatory M1 phenotype within the lesions. Conversely, FMT from endometriosis patients with reduced acetate production exacerbated the condition (37). The current discussion primarily centers on SCFAs, but for a wider perspective on other beneficial metabolites, readers are referred to the review by Liu et al. (38). In contrast to these findings in the gut, other research has indicated increased microbial diversity within the endometrial tissue of EMS patients, with statistically significant associations reported between higher bacterial diversity and EMS (39: p = 0.09; 40: p = 0.036).
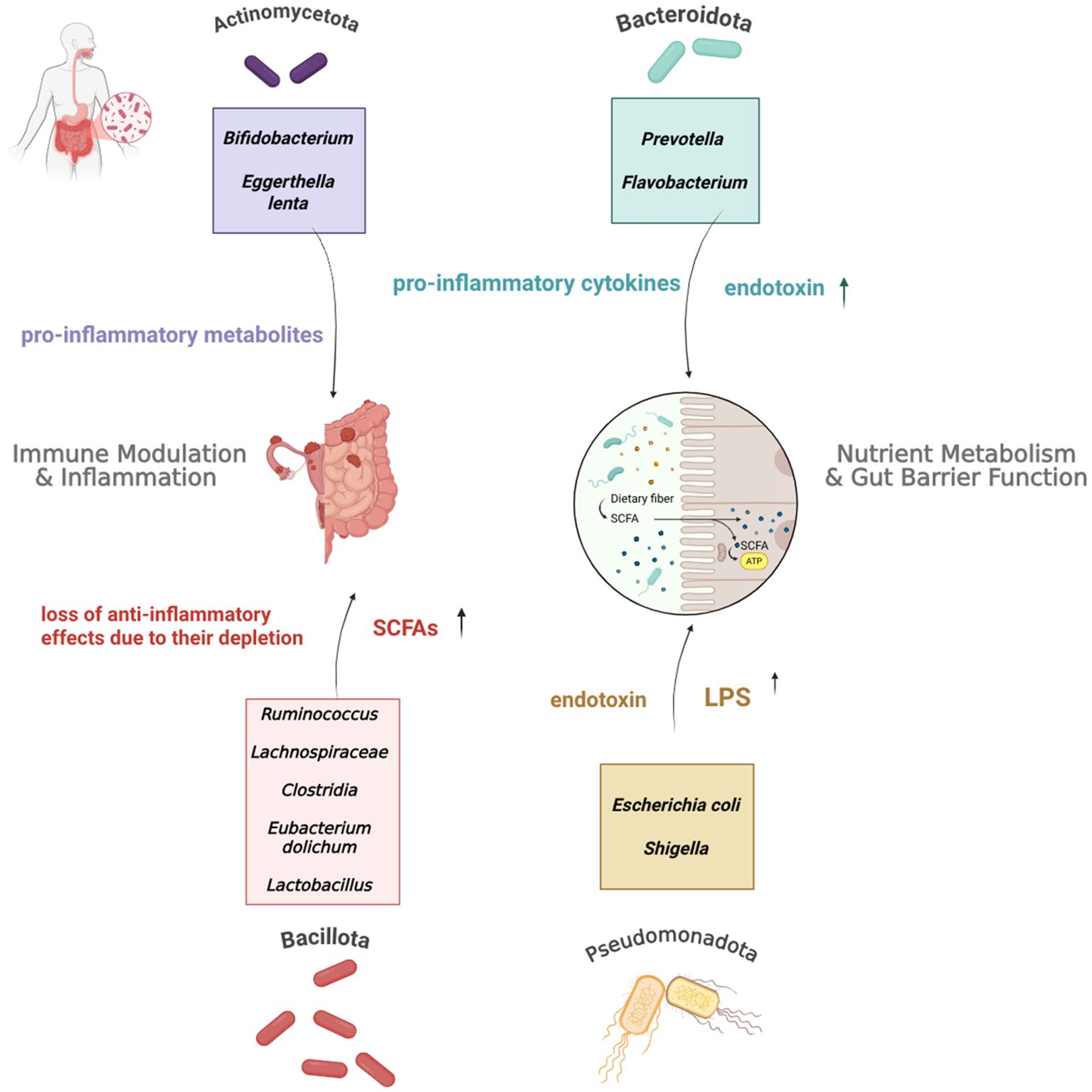
Figure 2. Interplay Between the Four Main Gut Microbiome Phyla, their Respective Taxa, and Immune Dysregulation in Endometriosis. As highlighted in the studies discussed above, these phyla and taxa exhibit altered abundances in EMS patients. This network illustrates their dysregulated immunomodulatory roles within the context of gut dysbiosis and emphasises their biological contribution to the progression of the condition.
These inconsistent findings regarding microbial diversity in EMS are likely influenced by several methodological limitations. Notably, studies by Shan et al. (33) and Wessels et al. (39) were constrained by small sample sizes and issues with control group definition. For instance, Shan et al. lacked laparoscopic confirmation to definitively exclude EMS in their controls, while Wessels et al. did not include a healthy control group to establish a baseline endometrial microbiota profile. Furthermore, Svensson et al. (34) highlighted the potential for undiagnosed EMS within their control cohort and the restricted functional insights afforded by 16S rRNA sequencing. While these results appear inconsistent, they may also reflect distinct anatomical niche-specific dynamics driven by the pathogenesis of the disease. It is hypothesised that systemic dysbiosis in the gut, characterised by an overall reduced alpha diversity, could lead to a compromised intestinal barrier. This impaired barrier could then facilitate the translocation of opportunistic bacteria from the gut to the peritoneal cavity and endometrial tissue. This translocation process presents novel microbial species to the local endometriotic environment, resulting in a paradoxical increase in local alpha diversity at the site of the lesions. While methodological limitations inarguably contribute to these discrepancies, a synthesis of the data suggests that a systemic loss of diversity can precipitate a localised increase in pro-inflammatory bacterial diversity. To achieve greater clarity and establish a consensus, future research should prioritise larger, well-controlled, multi-omics studies. These investigations must incorporate comprehensive controls for library preparation and focus on the functional roles of bacteria and their metabolites within peritoneal fluid and endometriotic lesions. This would enable a more complete understanding of their impact on local immunity and inflammation and account for the niche-specific dynamics of microbial diversity in endometriosis.
2.3 Specific taxa alterations and their relevance in endometriosis
Several studies have reported concurrent findings regarding dysbiotic shifts in bacterial taxa across the four main gut phyla. For example, Huang et al. (41) and Svensson et al. (34), identified significant abundance reductions in Bacillota, including taxa such as Clostridia and Lachnospiraceae, which are crucial for the hydrolysis of starch and other complex carbohydrates into short-chain fatty acids (SCFAs) like butyrate (42). The reduction of these taxa compromises the gut’s capacity to synthesise these beneficial metabolites, which are vital for regulating inflammation and maintaining intestinal barrier integrity. Additionally, Svensson et al. (34), highlighted the association between an increased abundance of specific genera,such as Prevotella and the manifestation of gastrointestinal symptoms, including constipation and bloating which are commonly reported in individuals with endometriosis (34). This suggests that shifts in microbial composition may directly impact gut motility and function, contributing to disease pathophysiology.
Concurrently, an increase in Actinomycetota, particularly Eggerthella lenta, was observed by Svensson et al. (34). This bacterium has been implicated in the activation of pro-inflammatory Th17 cells and is enriched in other inflammatory conditions, including irritable bowel disease (IBD) (43). Furthermore, studies by Wessels et al. (39) and Ata et al. (44) also reported an enrichment of Actinomycetota, specifically in the species Oxalobacteraceae, Streptococcaceae, Bifidobacterium, and Parasutterella. Ata’s study, in particular, revealed overlapping patterns of dysbiosis across vaginal, cervical, and gut microbial profiles. This multi-site comparison stresses the interconnectedness of microbial communities across the gut and reproductive tract, suggesting that dysbiosis may contribute to immune dysregulation beyond a single anatomical site. Specifically, the enrichment of Actinobacteria has been linked to impaired immunomodulation and the persistence of low-grade inflammation (45, 46), potentially sustaining inflammatory responses within the pelvic environment and promoting disease progression.
Furthermore, the two other major gut microbiome phyla Pseudomonadota and Bacteroidota, exhibited the highest relative abundance in stool samples, as reported by Huang et al. (41) and Svensson et al. (34), who observed increased levels of Bacteroidota and Parabacteroidota in EMS patients (34, 41). Enterotoxigenic species within these phyla, such as Escherichia coli (Pseudomonadota) and Bacteroides fragilis (Bacteroidota) are associated with chronic tissue inflammation and the release of carcinogenic and pro-inflammatory mediators (47). Supporting this, additional studies have identified an enrichment of Pseudomonadota including Escherichia and Shigella (44, 48). These taxa are known to disrupt immune homeostasis and activate pro-inflammatory cytokine pathways ultimately, increasing intestinal susceptibility to chronic inflammation (49).
2.4 Altered Bacillota/Bacteroidota ratio and dysbiosis in endometriosis
As previously mentioned, measures of biodiversity, alongside the abundances of specific taxa, serve as key indicators of gut dysbiosis. Another commonly utilised parameter for assessing microbial dysbiosis is the ratio of the two predominant phyla in the gut, Bacillota and Bacteroidota (27). This ratio has been extensively used in research and has been observed to be elevated in various pathological conditions, including obesity, Alzheimer’s disease, Parkinson’s disease, and type 1 diabetes (50–52). In the context of EMS, evidence also points to an altered Bacillota/Bacteroidota ratio indicative of disrupted microbial homeostasis. Shan et al. (33) and Ni et al. (48) reported increases in the ratio amongst EMS patients. Notably, Ni et al. (48) observed a significant two-fold increase of the ratio in an EMS-induced mouse model, strongly suggesting a dysbiotic shift within the gut microbiome. However, the findings are not entirely consistent across studies. For example, Li et al. (53), reported only an incremental increase in the ratio and emphasised fluctuations in specific microbial genera, perhaps reflecting experimental variability or disease progression. These inconsistencies question whether the Bacillota/Bacteroidota ratio is a sufficiently specific or sensitive marker of dysbiosis in endometriosis. Further research is necessary to determine whether this metric is robust or if alternative measures of dysbiosis may offer more reliable insights for diagnosis or prognosis.
Overall, there appears to be a consistent shift towards a pro-inflammatory, disrupted gut microbial composition and function in EMS patients. This shift is mainly characterised by an enrichment of proinflammatory Actinomycetota genera, a reduction in SCFA-producing Firmicutes, and a marked increase in enterotoxic and pathogenic taxa within the Pseudomonadota and Bacteroidota phyla. However, it’s uncertain whether the observed variability in these findings is attributable to EMS heterogeneity or the influence of external factors such as antibiotic usage, diet, geographical location, age, severity of EMS or hormonal therapies; all of which have a suspected implication in microbial composition (54). Researching these microbial fluctuations further, could help elucidate a mechanism connecting dysbiosis to systemic and localised inflammation but also clarify the biological relevance of the Bacillota/Bacteroidota ratio and other aforementioned phyla and taxa. Ultimately, this could provide a plausible link between immunomodulation of gut dysbiosis and EMS pathogenesis. Understanding this in greater detail could strongly support the development of targeted and personalised microbiome-based immunomodulatory interventions.
3 Examining the disruption of immunomodulation in endometriosis
3.1 Bacterial contamination, immune dysfunction and chronic inflammation
Emerging evidence suggests a significant association between gut microbiome dysbiosis and immune system dysregulation, contributing to the chronic inflammation that is the hallmark of endometriosis. Khan’s theory highlights how disruptions in gastrointestinal tract maintenance, mucosal integrity, and barrier function promote intestinal permeability, leakage of metabolites, and inflammatory changes (Figure 3) (9, 55, 56). In a series of studies, Khan explored the role of lipopolysaccharide (LPS), a bacterial cell wall endotoxin, in initiating and propagating endometriosis when present in the intrauterine environment (57, 58). The research revealed that LPS concentrations in the menstrual fluid of patients with endometriosis were four to six times higher than in controls. Specifically, menstrual fluid endotoxin levels averaged 285.5 ± 64.5 pg/mL in patients compared to 114.9 ± 17.0 pg/mL in controls (p < 0.01). Furthermore, menstrual blood samples from patients with endometriosis were highly contaminated with Gram-negative Pseudomonadota, such as Escherichia coli, with a median concentration of 4.5 Log10 CFU/mL (IQR 1.4 – 7.2), compared to 1.2 Log10 CFU/mL (IQR 0.8 – 1.9) in controls (p < 0.01) (58). This observation is supported by evidence that a compromised intestinal barrier may facilitate the translocation of E. coli from the gut to the pelvic cavity via enterocytes (44). The inflammatory cascade is pivotal in understanding this process. Supporting this link between gut and pelvic dysbiosis, Ata et al. (44) previously demonstrated that patients with moderate-to-severe endometriosis (n = 14) exhibited a higher Shigella/Escherichia ratio in their colonic microbiota compared to healthy controls (n = 14).
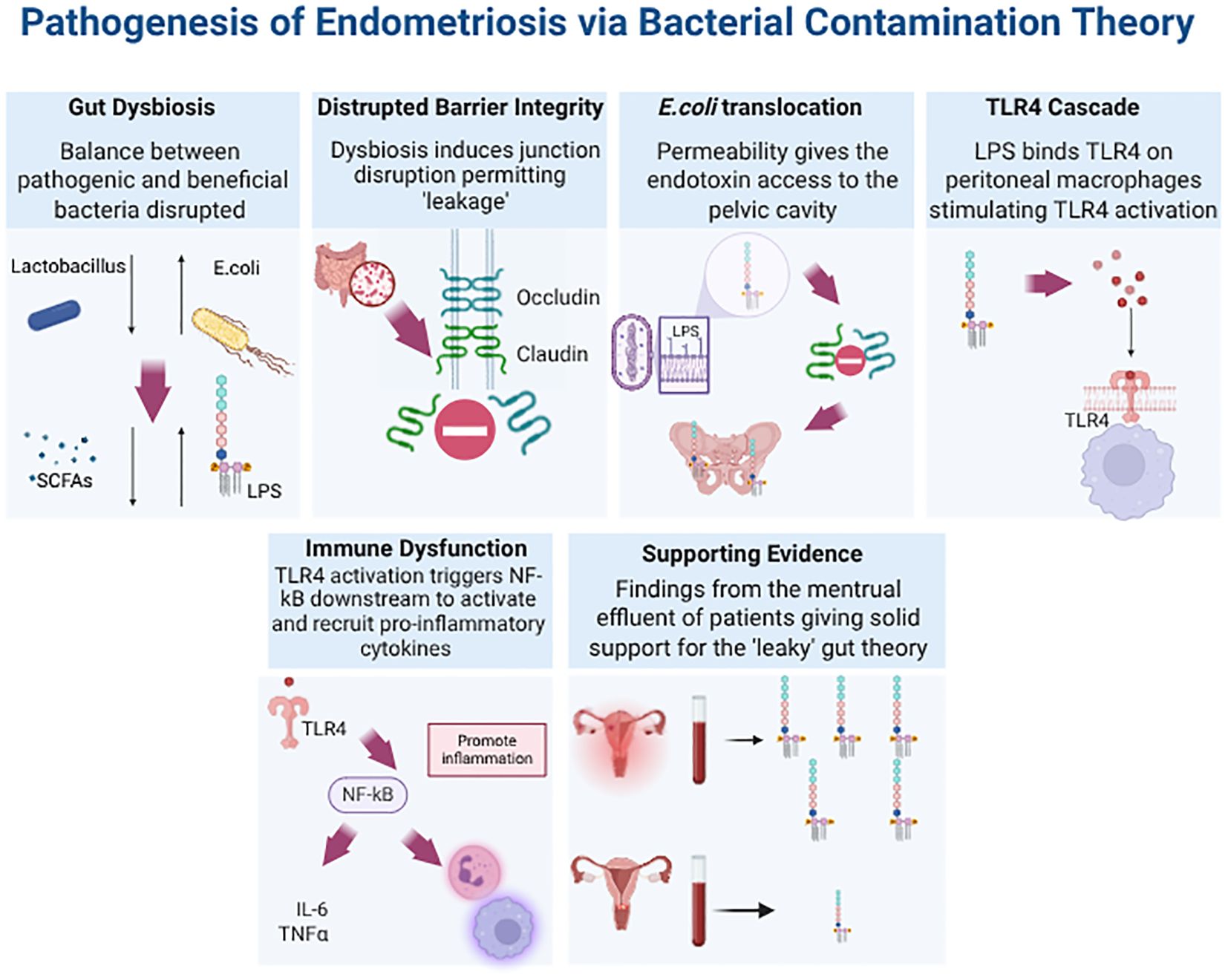
Figure 3. Downstream Effects of Disrupted Epithelial Barrier Integrity Due to Gut Dysbiosis in Endometriosis. The figure illustrates the cascade of signalling events, collectively known as the “Bacterial Contamination Theory.” This theory provides a mechanism explaining how gut dysbiosis in EMS leads to increased concentrations of endotoxins, such as lipopolysaccharide (LPS), in the menstrual effluent of patients. Furthermore, it links the presence of LPS to immune dysregulation and inflammation. In the bottom right corner, a spider diagram summarises how further research into the cellular and molecular components of this signalling cascade could aid in the development of microbiome-based diagnostics and therapeutics.
A compromised barrier may facilitate the translocation of Escherichia coli (E. coli) from the gut to the pelvic cavity via enterocytes (44). Once in the uterine and peritoneal cavities, LPS binds to TLR4 upon entering the peritoneal fluid. This initiates signalling cascades critical for host immune responses (58). To illustrate, the activation of TLR4 triggers the NF-κB pathway, driving the expression of pro-inflammatory cytokines, including interleukin-6 (IL - 6), tumour necrosis factor-alpha (TNF-α), and IL - 1β, and increasing COX - 2 mediated PGE2 production. This ultimately results in elevated oestrogen synthesis (discussed in section 3.2), ultimately generating a positive feedback loop that further supports lesion survival and growth. A pivotal study by Shan et al, demonstrated that dysbiosis heightened pathways that promoted NF-kB and therefore interleukin-8 (IL - 8) and TNF-α expression; all contributing to an inflammatory response (33). All these play a significant role in inducing endometrial tissue adhesion and angiogenesis along with promoting the formation, infiltration of these endometriosis peritoneal nodules (59–62). Additionally, LPS-TLR4 binding significantly increases immune cell recruitment and alters their functionality, particularly macrophages. This altered macrophage phenotype impairs their phagocytic ability, reducing their capacity to clear newly implanted endometriotic lesions, thereby promoting lesion survival (9, 63, 64). In summary, microbial dysbiosis fosters immune dysfunction in EMS through impaired immune surveillance and increased bacterial proliferation within an inflammatory environment. Insights into these mechanisms through microbial and metabolomics profiling could accelerate the identification of microbial and immune cell biomarkers, enabling the development of non-invasive diagnostic tools and advancing personalised therapeutic strategies.
Beyond direct immune activation, dysbiosis may also disrupt mucosal tolerance mechanisms fundamental to immune homeostasis. The gut-associated lymphoid tissue (GALT), which includes Peyer’s patches, isolated lymphoid follicles and mesenteric lymph nodes; serves as a key component for mucosal immunity. These structures help maintain a delicate balance between immune tolerance towards commensal microbes and dietary antigens, and activation against potential pathogens (65). The gut microbiota plays a pivotal role in educating the immune system by informing T cell development, promoting Treg induction, and guiding pattern recognition receptor (PRR) responsiveness (15). Specific taxa, such as Bacteroides fragilis and Clostridia clusters, have been shown to hijack the Treg differentiation process in the gut to promote mucosal tolerance and dampen inflammation (66, 67). In conditions like EMS, dysbiosis may disturb this delicate “training” process, leading to excessive immune activation or impaired immune regulation. Moreover, the reduction in Firmicutes (discussed in Section 2.4), key producers of SCFAs, could also impair Treg induction. Since SCFAs are essential for Treg differentiation and immune tolerance, their loss may disrupt Treg function, impairing the immune response, potentially favouring an inflammatory environment which would ultimately contribute to disease progression (68).
3.2 The estrobolome: hormonal crosstalk between the microbiome and immune regulation
While bacterial contamination and endotoxin-induced immune responses form one axis of immunomodulatory disruption in endometriosis, another critical but often underexplored pathway lies in the interplay between the gut microbiome and oestrogen metabolism; referred to as the estrobolome (69). This microbial-hormonal interface provides an additional mechanism through which dysbiosis perpetuates immune dysregulation and chronic inflammation in endometriosis. Throughout a woman’s lifetime, the gut microbiota significantly influences the reproductive endocrine system by interacting with hormones such as oestrogen, which are crucial in immune and metabolic regulation (70). Disequilibrium of oestrogen-modulated pathways has been implicated in the pathophysiology of various female reproductive disorders, including endometriosis (71). Notably, the relationship between oestrogen and the microbiome is bidirectional; while oestrogen levels can shape microbial composition, microbial alterations can, in turn, influence systemic oestrogen levels. For example, GnRH-agonist suppression of oestrogen has been shown to alter uterine microbiota, while oestrogen supplementation promoted Lactobacillus dominance in the genital microbiota (72) (Figure 4).
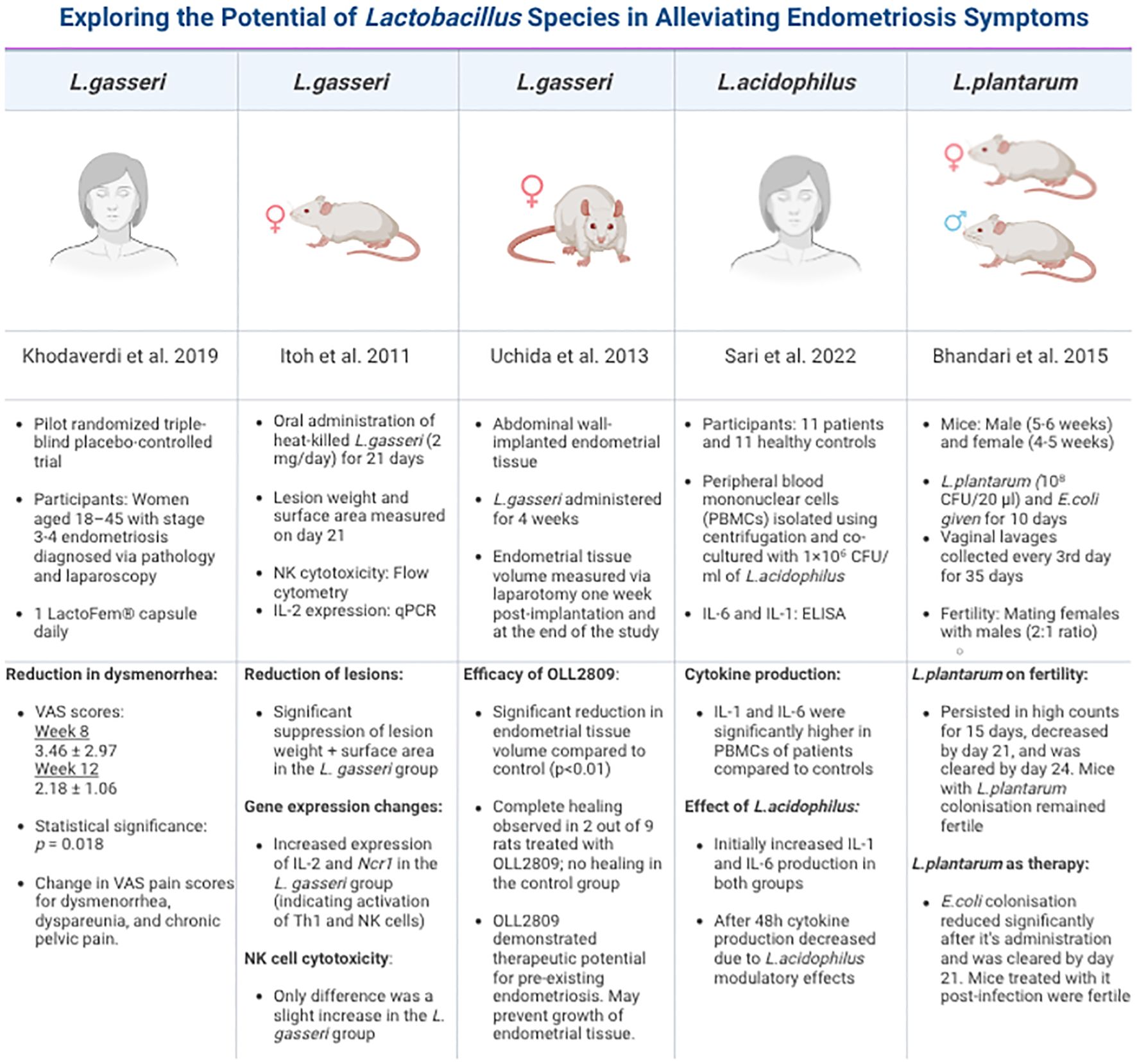
Figure 4. Summary of studies on different Lactobacillus species in endometriosis. This table is compiled of pivotal, recent and groundbreaking research that has been conducted using diverse models, strategies and Lactobacillus strains. Each study reports the beneficial effects of these strains focusing on their immunomodulatory impact on EMS.
The gut microbiome houses the genetic inventory to produce oestrogen-metabolising enzymes; particularly β-glucuronidase and β-glucosidase, through a subset of microbial genes collectively known as the estrobolome (69). Bacterial genera such as Bacteroides, Bifidobacterium, Escherichia, and Lactobacillus contribute to this enzymatic activity, promoting the deconjugation and reabsorption of oestrogens, thus influencing circulating hormone levels (73, 74). In states of dysbiosis, an increased Bacillota/Bacteroidota ratio enhances the abundance of β-glucuronidase-producing bacteria, which in turn raises levels of free oestrogens. This results in amplified oestrogen receptors (ERα and ERβ) signalling and a hyperoestrogenic state; an established feature of endometriosis (75).
The downstream effects of an overactive estrobolome contribute to hallmark features of endometriosis, including altered cell proliferation, resistance to apoptosis, increased angiogenesis, and heightened oxidative stress. These changes not only worsen local inflammation but also create an immune environment that supports lesion survival and persistence. Thus, the estrobolome represents a key intersection where microbial dysbiosis and hormonal imbalance converge to disrupt immune homeostasis, driving the progression of endometriosis (76).
3.3 Impaired immune surveillance and its role in lesion survival in endometriosis
The immune microenvironment of endometriotic lesions is profoundly influenced by chronic exposure to bacterial products, which impairs immune surveillance and promotes lesion survival. This exposure, particularly through LPS-TLR4 signalling, drives a shift in macrophage polarisation toward an immunosuppressive, M2-like phenotype. These pathogenic M2 macrophages, a major cellular component within endometriotic lesions, are found in significantly greater numbers in patients with EMS. Elevated levels of IL - 17A in both plasma and lesions have been shown to stimulate this pathogenic M2 polarisation (77). These alternatively activated (M2) macrophages secrete key immunosuppressive cytokines like IL - 10 and TGF-β, which collectively promote angiogenesis, fibrosis, and immune tolerance (78). This immune deregulation facilitates the escape of ectopic lesions and impairs normal clearance mechanisms, such as NK cell cytotoxicity. While this shift in macrophage phenotype provides a permissive environment for lesion survival and progression, the specific factors influencing M2 polarisation within endometriotic lesions are not yet fully understood. However, M2 macrophage infiltration in ectopic endometrial tissues positively correlates with the expression of markers such as CD47, PDPK1, and LDHA (79). Therefore, designated studies are crucial to fully elucidate the precise molecular mechanisms driving M2 polarisation in endometriosis.
The permissive immune environment of endometriosis is not only defined by macrophage polarisation; it also involves significant alterations in other immune cell populations. To demonstrate, data from a study on EMS patients (n=6-8) showed significantly reduced levels of uterine natural killer (uNK) cells in menstrual effluent (5 – 10%) compared to control subjects (10 – 40%) (p=0.01) (80). Furthermore, in EMS-induced olive baboons (n=8), a notable decrease of approximately 20% in peripheral natural T regulatory cells (nTregs) was detected at 3-month and 9-month intervals, alongside an increase of induced T regulatory cells (iTregs) at the 3-months mark (81). The diminished count of nTregs compromises the system’s capacity to suppress excessive inflammatory responses (82). Considering that the increase in iTregs was generated peripherally, it can be influenced by the inflammatory environment (83). This reflects a shift towards a pro-inflammatory state which is allowed to persist within endometrial lesions due to less effective regulation.
Alongside their altered phenotypes, dysfunctional macrophages in EMS patients are found in significantly greater numbers, facilitating the development of a distinct pro-inflammatory cytokine profile. This includes elevated levels of tumour necrosis factor-alpha (TNF-α), interleukin-8 (IL - 8), interleukin-1 receptor (IL - 1R), vascular endothelial growth factor (VEGF), interleukin-6 (IL - 6), and interleukin-17 (IL - 17). This profile may not only contribute to local and systemic inflammation but also holds promise as an immunological biomarker. In this case, integrating cytokine profiling with metabolomic analyses (discussed in Section 5) could help identify predictive readouts of disease activity or therapeutic responsiveness. A pivotal study by Shan et al, demonstrated that dysbiosis heightened pathways that promoted NF-kB and therefore IL - 8 and TNF-α expression; all contributing to an inflammatory response (33). All these play a significant role in inducing endometrial tissue adhesion and angiogenesis along with promoting the formation, infiltration of these endometriosis peritoneal nodules (59–62). The overall dysregulated immune response is essentially generating an immunosuppressive and inflamed environment; hallmark features of endometriosis facilitating the spread and growth of escaped ectopic endometrial cells outside the uterus (60).
In support, numerous studies have noted evidence of bacterial contamination and elevated inflammatory markers. For example, IL - 17A levels have been shown to positively correlate with the abundance of Bacteroides (r = 0.89, p < 0.05) and inversely with Streptococcus and Bifidobacterium (r = –0.89, p < 0.05) (33). Additional bacterial taxa that exhibited positive correlations include Actinobacteria, Euryarchaeota, Fusobacteria, Lentisphaerae, Spirochaetes, and Synergistetes (81). These microbial shifts are accompanied by immunological changes, including a significant decrease in peripheral natural T regulatory cells (nTregs) at the 3- and 9-month intervals. Conversely, an increase in induced T regulatory cells (iTregs) was observed at 3 months, which showed a negative association with Porphyromonas and Prevotella. In conjunction, these findings support the presence of a distinct microbiota-immune interaction profile in EMS and suggest that changes in microbial diversity and T cell populations could profoundly affect immune regulation. This strengthens the case for developing non-invasive diagnostic biomarkers based on microbiota and cytokine signatures. However, larger, longitudinal studies are needed to validate these associations and unravel the underlying mechanisms linking the microbiome, immune function, and EMS pathology.
4 Current endometriosis landscape: burden, diagnostics, and treatment obstacles
Finding a non-invasive diagnostic biomarker for endometriosis (EMS) is critical due to its profound impact on patients and healthcare systems. EMS significantly impairs physical and mental well-being, leading to higher rates of depression (18.9% vs. 9.3%) and anxiety (29.7% vs. 7.0%) compared to healthy controls (84). Sufferers experience severe menstrual and chronic pelvic pain, alongside common gastrointestinal symptoms like nausea and bloating, affecting 90% of confirmed cases (85–88). Additionally, the economic burden is substantial, with indirect costs in the EU reaching an estimated €54 million annually due to lost workdays (89). Socially, women frequently face minimisation or dismissal of their pain, contributing to an alarming diagnostic delay of 7 to 10 years (90–92). Current diagnostic tools, primarily ultrasound and MRI, can only suggest EMS, not definitively diagnose it (93, 94). Surgical validation is the sole definitive method, yet it’s often inaccessible due to cost and availability, and carries high recurrence rates, with about half of patients needing another surgery within five years, potentially leading to organ deterioration (95, 96). Even after diagnosis, first-line pharmacological treatments like progestins, while versatile, have significant side effects including irregular spotting, mood swings, and weight gain (97). Given the extensive diagnostic delays and adverse treatment effects, there’s a clear, unmet need for personalised diagnostic and clinical approaches to address the heterogeneity of EMS.
5 Future directions of endometriosis management
Building on the urgent need for improved strategies, future directions in EMS management are exploring novel, personalised approaches. Emerging research within the field of the gut microbiome suggests that addressing gut dysbiosis and its role in immunomodulation is the key to uncovering the mechanisms underlying the bidirectional relationship between the microbiome and endometriosis. Relevant and significant approaches include the utilisation of probiotics and faecal microbiota transplantation (FMT) which aim to restore eubiosis alongside omics analyses of metabolic derivatives associated with inflammation. These nuanced strategies show great promise in identifying microbial biomarkers for diagnosis and in the provision of personalised therapeutics aimed at alleviating pain, thus paving the way for tailored clinical approaches in the management of endometriosis.
5.1 Lactobacillus-based probiotics in the treatment of endometriosis
Many researchers have turned to the microbiome in search of answers regarding EMS diagnosis and treatment, spurred by recent findings regarding probiotics’ potential to address a range of diverse health issues from infections and rare genetic disorders to cancer (98, 99). Considering Lactobacilli is the most extensively studied probiotic bacteria (100) it was employed by many researchers. For example, both Khodaverdi et al. (101) and Itoh et al. (102) explored the benefits of orally administered Lactobacillus (LactoFem®) on pain severity in EMS patients through pilot and double-blind placebo-controlled studies; respectively. Their findings concluded that Lactobacillus gasseri (OLL2809) was able to ameliorate EMS-associated pain and dysmenorrhea in stage 3 and 4 EMS without any side-effects over an 8-week period. Building on this clinical evidence, Uchida and Kobayashi (103) conducted preclinical trials using a rat model to investigate the biological effects of L. gasseri on lesion progression. Their study revealed a statistically significant reduction in EMS lesion size within the abdominal cavity (p < 0.01) and suggested the probiotic’s potential not only for treatment but also for prevention of disease progression, with two rats even demonstrating signs of complete healing (103).
Regarding the immunomodulatory effects of Lactobacillus species, a study by Sari et al. (104) investigated this by demonstrating that L.acidophilus post 48h of administration, lowered both pro-inflammatory cytokine IL - 6 and IL - 1 concentrations by 29% (104).This suggests probiotics may work by enhancing immunomodulation by increasing both NK cell activity and IL - 12 levels which may counteract the immune dysregulation caused by the gut-dysbiosis (102, 103, 105). Beyond gynaecological pain, probiotics may address EMS-related infertility, with L.plantarum showing potential as an infertility therapeutic agent (106). Given EMS patients have a 50% increased risk of developing inflammatory bowel disease (IBD) (107), there is also a fair amount of research suggesting probiotics can alleviate EMS and IBD-related GI symptoms. For instance, a randomised, double-blind study by Weizman et al. (108), looked at 101 paediatric patients with irritable-bowel syndrome (IBS) and revealed that a supplementation of L. reuteri (DSM 17938) reduced abdominal pain frequency and intensity in the span of a month (108).
Overall, these findings are impressive however they also highlight the inadequacy of a “one-size-fits-all” approach, as individual efficacy likely varies widely due to the complex interplay of host and microbial factors. Standardised methodologies and large-scale studies encompassing ethnically diverse cohorts are essential for establishing reliable microbial biomarkers. Given the observed variation in EMS immunomodulation across ethnicities, with a higher prevalence reported within Asian women (109), future studies should prioritise underrepresented populations to enhance the generalisability of microbial biomarkers and address health disparities. Beyond ethnicity, factors such as genetics, diet and lifestyle significantly influence microbial profiles suggesting that interventions like probiotics or dietary strategies may require individual tailoring for optimal efficacy. In turn, precision medicine frameworks that integrate host–microbiome interactions offer a promising avenue for developing more targeted and effective treatment strategies for endometriosis.
5.2 Conventional and autologous faecal microbiota transplantation in restoring eubiosis
An alternative technology to probiotics, is faecal microbiota transplantation (FMT). It involves delivering stool from a healthy donor to a patient via either enema, colonoscopy or upper GI routes (endoscopy, nasogastric or nasoenteric tubes or oral capsules) (110). FMT’s mechanism of action has been linked to competing with pathogenic bacteria, stimulating the intestinal immune system and protecting the intestinal barrier (111). Maintaining eubiosis is imperative for human health and therefore, could be a valuable therapeutic target. Currently, FMT represents the leading innovative technique for accomplishing this (112). The clinical efficacy of FMT has been validated in numerous diseases, to illustrate, a well-documented example is FMT’s use as for recurrent Clostridioides difficile infections. Over the last decade, FMT has had a success rate of around 90%, by restoring healthy colonic flora; surpassing the effectiveness of vancomycin (113).
To date, there are currently no clinical reports that outline FMT application in gynaecological disorders except in mouse models. However, laboratory research data provides a solid foundation to encourage further studies involving human models. For instance, a study conducted by Kim et al. (114) displayed that administering NK49 (B.longum) and NK3 (L.plantarum) individually and combined, reduced GV-induced BV in mice. Supporting this observation was a decrease in TNF-a levels and Pseudomonadota alongside an increase in IL - 10 and Bacteroidota. These bacterial-induced changes inhibited LPS production by the gut microbiota, partially “reversing” inflammation through induced immunomodulation (114).These findings suggest a novel and effective approach for treating endometriosis or at least reducing its symptoms by targeting microbiome restoration.
However, FMT’s risks and challenges must be accounted for as well. A successful FMT requires strict donor selection that excludes immunocompromised or comorbid patients, fresh treatment preparation and pathogen screening. Satisfying all these criteria proves challenging in both logistic and financial aspects (115). Continuing, FMT also poses some clinical risks. For instance, transplantation of disease-associated microbiota has been seen to trigger pathology such as diarrhoea, abdominal cramping and nausea (116). The possibility of long-term adverse effects to patients due to the alteration of their gut microbiota is another concern. In an effort to overcome conventional FMT’s limitations, autologous FMT (aFMT) has emerged. In aFMT, a patient’s own microbiome is collected during a healthy state and later reintroduced when illness occurs. This could eliminate extremely selective processes and enhance long-term sustainability (117). Despite its encouraging prospects, further extensive research is necessary to validate the safety, efficacy, long-term outcomes, cost-effectiveness and affordability of this microbiome-targeted intervention in EMS, before it’s implemented in a clinical setting.
5.3 Metabolomics profiling in the development of microbial biomarkers
In parallel with mechanistic-focused research, the incorporation of advanced omics techniques, particularly metabolomics should be a priority in future investigations. Metabolomics refers to the analysis of small-molecule metabolites in tissues or biofluids, providing insights into the physiological or pathological state of a system (118). Its recent application in cancer and chronic inflammatory diseases has proven valuable in identifying biomarkers, such as specific levels and types of short-chain fatty acids (SCFAs), which are microbial-derived metabolites that modulate host immunity (119–121). In the context of EMS, metabolomic approaches have begun to uncover characteristic signatures. For instance, Ni et al. (48) reported altered faecal metabolites related to secondary bile acid biosynthesis in murine models, with decreased levels of alpha-linoleic acid (ALA); a compound known for its intestinal protective and anti-inflammatory roles (122). Similarly, a systematic review by Adamyan et al. (123) highlighted increased levels of succinate, β-hydroxybutyric acid, and ketone bodies in EMS patient biofluids, linking metabolic dysregulation to disease pathology and oxidative stress (123).
To complement these metabolic markers, emerging evidence supports the use of immune cell-derived cytokines such as IL - 17A, IL - 6, and TNF-α as immunological biomarkers of dysbiosis. These pro-inflammatory cytokines are elevated in EMS and are closely tied to microbial imbalances, especially in the gut and reproductive tract. IL - 17A, for example, shows strong correlations with specific microbial taxa such as Bacteroides and is known to drive inflammatory tissue responses (33). When analysed alongside metabolomic profiles, such immune mediators could serve as functional readouts of microbial activity and host response, strengthening biomarker precision and interpretability. Together, the integration of metabolomics with immunological biomarkers offers a powerful, non-invasive approach for monitoring disease progression, stratifying patients by inflammatory or metabolic subtypes, and enabling more personalised therapeutic strategies. Future research should prioritise the co-analysis of microbiota composition, metabolite signatures, and cytokine profiles, ideally using longitudinal cohort designs and high-resolution multi-omics platforms.
Metabolomics holds vast potential for non-invasive and precise detection of immunomodulatory disruptions caused by EMS. This innovative approach could be leveraged to monitor disease progression, stratify patients, and enable personalised treatments tailored to individual metabolomic profiles.
6 Conclusion
Gut dysbiosis is increasingly recognised as a key factor in the pathogenesis of EMS, particularly through its dysregulation of key processes within the female reproductive system. These include the modulation of oestrogen pathways, metabolic derivatives, oxidative stress, and immune-mediated inflammation. This paper specifically focuses on the dysregulation of immunomodulation and its role in driving immune dysfunction and perpetuating chronic inflammation. Underlying this immune imbalance is the disruption of mucosal immune structures (e.g GALT and Peyer’s patches), which normally support immune tolerance by mediating the microbiome’s regulation of innate and adaptive immunity. Although microbiome-based therapies, such as probiotics and FMT hold considerable promise, their clinical implementation remains halted by unresolved questions regarding microbial diversity, safety, and efficacy. Progress in this field will require the integration of advanced omics technologies, large-scale cohort studies, and efforts to address ethical, genetic, diversity-related, and economic barriers. An extensive understanding of the connection between gut microbiota alterations to endometriosis is necessary. It could comprehensively map these pathways and tackle gaps in interpreting and translating gut microbiota findings, informing early diagnosis and targeted interventions in a non-invasive manner. Moreover, it could support the development of personalised therapeutic approaches, tailored to unique microbiome profiles and genetic predispositions of individual patients. However, achieving this will require collaborative research efforts to bridge the gap between scientific discovery and clinical application; this represents the critical key to unlocking the untapped potential of the gut microbiome in the care and management of endometriosis.
Author contributions
EK: Investigation, Writing – original draft. AZ: Validation, Writing – review & editing. DZ: Writing – review & editing. MB: Writing – review & editing. CW: Writing – review & editing. ZP: Conceptualization, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Acknowledgments
The figures were created in biorender.com.
Conflict of interest
Author AZ was employed by company SwipeBiome.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Maybin JA and Critchley HOD. Menstrual physiology: implications for endometrial pathology and beyond. Hum Reprod Update. (2015) 21:748–61. doi: 10.1093/humupd/dmv038
2. Petroianu A. Endometriosis: an improper name for two different disorders. Rev Assoc Med Bras. (2023) 69:1–1. doi: 10.1590/1806-9282.20221484
3. Taylor HS and Polimanti R. Epidemiologic and genetic associations of endometriosis with depression, anxiety, and eating disorders. JAMA Netw Open. (2023) 6:e2251214. doi: 10.1001/jamanetworkopen.2022.51214
4. Brennan C, Chan K, Kumar T, Maissy E, Brubaker L, Dothard MI, et al. Harnessing the power within: engineering the microbiome for enhanced gynecologic health. Reprod Fertil. (2024) 5:e230060. doi: 10.1530/RAF-23-0060
5. Smolarz B, Szyłło K, and Romanowicz H. Endometriosis: epidemiology, classification, pathogenesis, treatment and genetics (Review of literature). IJMS. (2021) 22:10554. doi: 10.3390/ijms221910554
6. Horne AW and Saunders PTK. SnapShot: endometriosis. Cell. (2019) 179:1677–1677.e1. doi: 10.1016/j.cell.2019.11.033
7. Soliman AM, Surrey ES, Bonafede M, Nelson JK, Vora JB, and Agarwal SK. Health care utilization and costs associated with endometriosis among women with Medicaid insurance. J Managed Care Specialty Pharm. (2019) 25:566–72. doi: 10.18553/jmcp.2019.25.5.566
8. Yovich JL, Rowlands PK, Lingham S, Sillender M, and Srinivasan S. Pathogenesis of endometriosis: Look no further than John Sampson. Reprod BioMed Online. (2020) 40:7–11. doi: 10.1016/j.rbmo.2019.10.007
9. Laschke MW and Menger MD. The gut microbiota: a puppet master in the pathogenesis of endometriosis? Am J Obstet Gynecol. (2016) 215:68.e1–4. doi: 10.1016/j.ajog.2016.02.036
10. Cousins FL, McKinnon BD, Mortlock S, Fitzgerald HC, Zhang C, Montgomery GW, et al. New concepts on the etiology of endometriosis. J Obstet Gynaecol. (2023) 49:1090–105. doi: 10.1111/jog.15549
11. Maciel-Fiuza MF, Muller GC, Campos DMS, Do Socorro Silva Costa P, Peruzzo J, Bonamigo RR, et al. Role of gut microbiota in infectious and inflammatory diseases. Front Microbiol. (2023) 14:1098386. doi: 10.3389/fmicb.2023.1098386
12. Armour M, Sinclair J, Ng CHM, Hyman MS, Lawson K, Smith CA, et al. Endometriosis and chronic pelvic pain have similar impact on women, but time to diagnosis is decreasing: an Australian survey. Sci Rep. (2020) 10:16253. doi: 10.1038/s41598-020-73389-2
13. Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie van Leeuwenhoek. (2020) 113:2019–40. doi: 10.1007/s10482-020-01474-7
14. Gilbert JA, Blaser MJ, Caporaso JG, Jansson JK, Lynch SV, and Knight R. Current understanding of the human microbiome. Nat Med. (2018) 24:392–400. doi: 10.1038/nm.4517
15. Hooper LV, Littman DR, and Macpherson AJ. Interactions between the microbiota and the immune system. Science. (2012) 336:1268–73. doi: 10.1126/science.1223490
16. Sender R, Fuchs S, and Milo R. Revised estimates for the number of human and bacteria cells in the body. PloS Biol. (2016) 14:e1002533. doi: 10.1371/journal.pbio.1002533
17. Afzaal M, Saeed F, Shah YA, Hussain M, Rabail R, Socol CT, et al. Human gut microbiota in health and disease: Unveiling the relationship. Front Microbiol. (2022) 13:999001. doi: 10.3389/fmicb.2022.999001
18. Forbes JD, Chen C, Knox NC, Marrie R-A, El-Gabalawy H, De Kievit T, et al. A comparative study of the gut microbiota in immune-mediated inflammatory diseases—does a common dysbiosis exist? Microbiome. (2018) 6:221. doi: 10.1186/s40168-018-0603-4
19. West CE, Renz H, Jenmalm MC, Kozyrskyj AL, Allen KJ, Vuillermin P, et al. The gut microbiota and inflammatory noncommunicable diseases: Associations and potentials for gut microbiota therapies. J Allergy Clin Immunol. (2015) 135:3–13. doi: 10.1016/j.jaci.2014.11.012
20. Pickard JM, Zeng MY, Caruso R, and Núñez G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. (2017) 279:70–89. doi: 10.1111/imr.12567
21. Long Y, Tang L, Zhou Y, Zhao S, and Zhu H. Causal relationship between gut microbiota and cancers: a two-sample Mendelian randomisation study. BMC Med. (2023) 21:66. doi: 10.1186/s12916-023-02761-6
22. Pös O, Styk J, Buglyó G, Zeman M, Lukyova L, Bernatova K, et al. Cross-kingdom interaction of miRNAs and gut microbiota with non-invasive diagnostic and therapeutic implications in colorectal cancer. IJMS. (2023) 24:10520. doi: 10.3390/ijms241310520
23. Wu HJ and Wu E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes. (2012) 3:4–14. doi: 10.4161/gmic.19320
24. von Martels JZ, Sadabad MS, Bourgonje AR, Blokzijl T, Dijkstra G, Faber KN, et al. The role of gut microbiota in health and disease: In vitro modeling of host-microbe interactions at the aerobe-anaerobe interphase of the human gut. Anaerobe. (2017) 44:3–12. doi: 10.1016/j.anaerobe.2017.01.001
25. Kho ZY and Lal SK. The human gut microbiome–A potential controller of wellness and disease. Front Microbiol. (2018) 9:1835. doi: 10.3389/fmicb.2018.01835
26. Szablewski L. Human gut microbiota in health and Alzheimer’s disease. J Alzheimer’s Dis. (2018) 62:549–60. doi: 10.3233/JAD-170908
27. Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. (2010) 464:59–65. doi: 10.1038/nature08821
28. Zheng Q, Sun T, Li X, and Zhu L. Reproductive tract microbiome dysbiosis associated with gynecological diseases. Front Cell Infect Microbiol. (2025) 15:1519690. doi: 10.3389/fcimb.2025.1519690
29. Chen Z, Radjabzadeh D, Chen L, Kurilshikov A, Kavousi M, Ahmadizar F, et al. Association of insulin resistance and type 2 diabetes with gut microbial diversity: A microbiome-wide analysis from population studies. JAMA Netw Open. (2021) 4:e2118811. doi: 10.1001/jamanetworkopen.2021.18811
30. Yichan Z, Ina SK, Huarui W, Vicente PG, Xiangyang T, Wei-Hua C, et al. Vaginal microbiome and inflammation among Chinese women. bioRxiv. (2024) 2024-11.
31. Valeriano VD, Lahtinen E, Hwang IC, Zhang Y, Du J, and Schuppe-Koistinen I. Vaginal dysbiosis and the potential of vaginal microbiome-directed therapeutics. Front Microb. (2024) 3:1363089. doi: 10.3389/frmbi.2024.1363089
32. Lin YH, Chen YH, Chang HY, Au HK, Tzeng CR, and Huang YH. Chronic niche inflammation in endometriosis-associated infertility: current understanding and future therapeutic strategies. Int J Mol Sci. (2018) 19:2385. doi: 10.3390/ijms19082385
33. Shan J, Ni Z, Cheng W, Zhou L, Zhai D, Sun S, et al. Gut microbiota imbalance and its correlations with hormone and inflammatory factors in patients with stage 3/4 endometriosis. Arch Gynecol Obstet. (2021) 304:1363–73. doi: 10.1007/s00404-021-06057-z
34. Svensson A, Brunkwall L, Roth B, Orho-Melander M, and Ohlsson B. Associations between endometriosis and gut microbiota. Reprod Sci. (2021) 28:2367–77. doi: 10.1007/s43032-021-00506-5
35. Wiertsema SP, Van Bergenhenegouwen J, Garssen J, and Knippels LMJ. The interplay between the gut microbiome and the immune system in the context of infectious diseases throughout life and the role of nutrition in optimizing treatment strategies. Nutrients. (2021) 13:886. doi: 10.3390/nu13030886
36. Chadchan SB, Popli P, Ambati CR, Tycksen E, Han SJ, Bulun SE, et al. Gut microbiota-derived short-chain fatty acids protect against the progression of endometriosis. Life Sci Alliance. (2021) 4:e202101224. doi: 10.26508/lsa.202101224
37. Xu Y, Zhu Y, Wu X, Peng W, Zhong Y, Cai Y, et al. Gut microbiota-derived acetate ameliorates endometriosis via JAK1/STAT3-mediated M1 macrophage polarisation. Microb Biotechnol. (2025) 18:e70202. doi: 10.1111/1751-7915.70202
38. Liu J, Tan Y, Cheng H, Zhang D, Feng W, and Peng C. Functions of gut microbiota metabolites, current status and future perspectives. Aging Dis. (2022) 13:1106–26. doi: 10.14336/AD.2022.0104
39. Wessels JM, Domínguez MA, Leyland NA, Agarwal SK, and Foster WG. Endometrial microbiota is more diverse in people with endometriosis than symptomatic controls. Sci Rep. (2021) 11:18877. doi: 10.1038/s41598-021-98380-3
40. Hernandes C, Silveira P, Rodrigues Sereia AF, Christoff AP, Mendes H, Valter de Oliveira LF, et al. Microbiome profile of deep endometriosis patients: comparison of vaginal fluid, endometrium and lesion. Diagnostics. (2020) 10:163. doi: 10.3390/diagnostics10030163
41. Huang L, Liu B, Liu Z, Feng W, Liu M, Wang Y, et al. Gut microbiota exceeds cervical microbiota for early diagnosis of endometriosis. Front Cell Infect Microbiol. (2021) 11:788836. doi: 10.3389/fcimb.2021.788836
42. Biddle AS, Black SJ, and Blanchard JL. An in vitro model of the horse gut microbiome enables identification of lactate-utilizing bacteria that differentially respond to starch induction. PloS One. (2013) 8:e77599. doi: 10.1371/journal.pone.0077599
43. Dong X, Guthrie BG, Alexander M, Noecker C, Ramirez L, Glasser NR, et al. Genetic manipulation of the human gut bacterium Eggerthella lenta reveals a widespread family of transcriptional regulators. Nat Commun. (2022) 13:7624. doi: 10.1038/s41467-022-33576-3
44. Ata B, Yildiz S, Turkgeldi E, Brocal VP, Dinleyici EC, Moya A, et al. The endobiota study: comparison of vaginal, cervical and gut microbiota between women with stage 3/4 endometriosis and healthy controls. Sci Rep. (2019) 9:2204. doi: 10.1038/s41598-019-39700-6
45. Chen S, Han H, Sun X, Zhou G, Zhou Q, and Li Z. Causal effects of specific gut microbiota on musculoskeletal diseases: a bidirectional two-sample Mendelian randomization study. Front Microbiol. (2023) 14:1238800. doi: 10.3389/fmicb.2023.1238800
46. Kwong TNY, Wang X, Nakatsu G, Chow TC, Tipoe T, Dai RZW, et al. Association between bacteremia from specific microbes and subsequent diagnosis of colorectal cancer. Gastroenterology. (2018) 155:383–390.e8. doi: 10.1053/j.gastro.2018.04.028
47. Quaglio AEV, Grillo TG, De Oliveira ECS, Di Stasi LC, and Sassaki LY. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J Gastroenterol. (2022) 28:4053. doi: 10.3748/wjg.v28.i30.4053
48. Ni Z, Sun S, Bi Y, Ding J, Cheng W, Yu J, et al. Correlation of fecal metabolomics and gut microbiota in mice with endometriosis. Am J Reprod Immunol. (2020) 84:e13307. doi: 10.1111/aji.13307
49. Rinanda T, Riani C, Artarini A, and Sasongko L. Correlation between gut microbiota composition, enteric infections and linear growth impairment: a case–control study in childhood stunting in Pidie, Aceh, Indonesia. Gut Pathog. (2023) 15:54. doi: 10.1186/s13099-023-00581-w
50. Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, et al. The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients. (2020) 12:1474. doi: 10.3390/nu12051474
51. Bravo JA, Julio-Pieper M, Forsythe P, Kunze W, Dinan TG, Bienenstock J, et al. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. (2012) 12:667–72. doi: 10.1016/j.coph.2012.09.010
52. Hills R, Pontefract B, Mishcon H, Black C, Sutton S, and Theberge C. Gut microbiome: profound implications for diet and disease. Nutrients. (2019) 11:1613. doi: 10.3390/nu11071613
53. Li Y, Wang K, Ding J, Sun S, Ni Z, and Yu C. Influence of the gut microbiota on endometriosis: potential role of chenodeoxycholic acid and its derivatives. Front Pharmacol. (2022) 13:954684. doi: 10.3389/fphar.2022.954684
54. Polak G, Banaszewska B, Filip M, Radwan M, and Wdowiak A. Environmental factors and endometriosis. IJERPH. (2021) 18:11025. doi: 10.3390/ijerph182111025
55. Belkaid Y and Harrison OJ. Homeostatic immunity and the microbiota. Immunity. (2017) 46:562–76. doi: 10.1016/j.immuni.2017.04.008
56. Ogunrinola GA, Oyewale JO, Oshamika OO, and Olasehinde GI. The human microbiome and its impacts on health. Int J Microbiol. (2020) 2020:1–7. doi: 10.1155/2020/8045646
57. Khan MA, Sengupta J, Mittal S, and Ghosh D. Genome-wide expressions in autologous eutopic and ectopic endometrium of fertile women with endometriosis. Reprod Biol Endocrinol. (2012) 10:1–20. doi: 10.1186/1477-7827-10-84
58. Khan KN, Fujishita A, Hiraki K, Kitajima M, Nakashima M, Fushiki S, et al. Bacterial contamination hypothesis: a new concept in endometriosis. Reprod Med Biol. (2018) 17:pp.125–133. doi: 10.1002/rmb2.12083
59. Zhang X, Xu H, Lin J, Qian Y, and Deng L. Peritoneal fluid concentrations of interleukin-17 correlate with the severity of endometriosis and infertility of this disorder. BJOG: Int J Obstet Gynaecol. (2005) 112:1153–5. doi: 10.1111/j.1471-0528.2005.00639.x
60. Belkaid Y and Hand TW. Role of the microbiota in immunity and inflammation. Cell. (2014) 157:121–41.
61. Khan KN, Kitajima M, Hiraki K, Fujishita A, Sekine I, Ishimaru T, et al. Changes in tissue inflammation, angiogenesis and apoptosis in endometriosis, adenomyosis and uterine myoma after GnRH agonist therapy. Hum Reprod. (2010) 25:642–53. doi: 10.1093/humrep/dep437
62. Wanderley CW, Colon DF, Luiz JPM, Oliveira FF, Viacava PR, Leite CA, et al. Paclitaxel reduces tumor growth by reprogramming tumor-associated macrophages to an M1 profile in a TLR4-dependent manner. Cancer Res. (2018) 78:5891–900. doi: 10.1158/0008-5472.CAN-17-3480
63. Yuan M, Li D, Zhang Z, Sun H, An M, and Wang G. Endometriosis induces gut microbiota alterations in mice. Hum Reprod. (2018) 33:607–16. doi: 10.1093/humrep/dex372
64. Emani R, Alam C, Pekkala S, Zafar S, Emani MR, and Hänninen A. Peritoneal cavity is a route for gut-derived microbial signals to promote autoimmunity in non-obese diabetic mice. Scand J Immunol. (2015) 81:102–9. doi: 10.1111/sji.12253
65. Mörbe UM, Jørgensen PB, Fenton TM, von Burg N, Riis LB, Spencer J, et al. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. (2021) 14:793–802. doi: 10.1038/s41385-021-00389-4
66. Round JL and Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci. (2010) 107:12204–9. doi: 10.1073/pnas.0909122107
67. Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. (2011) 331:337–41. doi: 10.1126/science.1198469
68. Hu M, Alashkar Alhamwe B, Santner-Nanan B, Miethe S, Harb H, Renz H, et al. Short-chain fatty acids augment differentiation and function of human induced regulatory T cells. Int J Mol Sci. (2022) 23:5740. doi: 10.3390/ijms23105740
69. Plottel CS and Blaser MJ. Microbiome and Malignancy. Cell Host Microbe. (2011) 10:324–35. doi: 10.1016/j.chom.2011.10.003
70. Qi X, Yun C, Pang Y, and Qiao J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes. (2021) 13:1–21. doi: 10.1080/19490976.2021.1894070
71. Mauvais-Jarvis F. Estrogen and androgen receptors: regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol Metab. (2011) 22:24–33. doi: 10.1016/j.tem.2010.10.002
72. Khan I, Azhar EI, Abbas AT, Kumosani T, Barbour EK, Raoult D, et al. Metagenomic analysis of antibiotic-induced changes in gut microbiota in a pregnant rat model. Front Pharmacol. (2016) 7:104.
73. Flores R, Shi J, Gail MH, Gajer P, Ravel J, and Goedert JJ. Association of fecal microbial diversity and taxonomy with selected enzymatic functions. PloS One. (2012) 7:e39745. doi: 10.1371/journal.pone.0039745
74. Ervin SM, Li H, Lim L, Roberts LR, Liang X, Mani S, et al. Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J Biol Chem. (2019) 294:18586–99. doi: 10.1074/jbc.RA119.010950
75. Baker JM, Al-Nakkash L, and Herbst-Kralovetz MM. Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas. (2017) 103:45–53.
76. Salliss ME, Farland LV, Mahnert ND, and Herbst-Kralovetz MM. The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum Reprod Update. (2021) 28:92–131. doi: 10.1093/humupd/dmab035
77. Miller JE, Ahn SH, Marks RM, Monsanto SP, Fazleabas AT, Koti M, et al. IL-17A modulates peritoneal macrophage recruitment and M2 polarization in endometriosis. Front Immunol. (2020) 11:108. doi: 10.3389/fimmu.2020.00108
78. Ramírez-Pavez TN, Martínez-Esparza M, Ruiz-Alcaraz AJ, Marín-Sánchez P, MaChado-Linde F, and García-Peñarrubia P. The role of peritoneal macrophages in endometriosis. Int J Mol Sci. (2021) 22:10792. doi: 10.3390/ijms221910792
79. Li H and Chai X. PDPK1 governs macrophage M2 polarization via hypoxia-driven CD47/AKT-glycolytic Axis in endometriosis. Cell Signal. (2025) 134:111922. doi: 10.1016/j.cellsig.2025.111922
80. Wessels JM, Felker AM, Dupont HA, and Kaushic C. The relationship between sex hormones, the vaginal microbiome and immunity in HIV-1 susceptibility in women. Dis Models Mech. (2018) 11:dmm035147.
81. Le N, Cregger M, Fazleabas A, and Braundmeier-Fleming A. Effects of endometriosis on immunity and mucosal microbial community dynamics in female olive baboons. Sci Rep. (2022) 12:1590. doi: 10.1038/s41598-022-05499-y
82. Rocamora-Reverte L, Melzer FL, Würzner R, and Weinberger B. The complex role of regulatory T cells in immunity and aging. Front Immunol. (2021) 11:616949. doi: 10.3389/fimmu.2020.616949
83. Lehtimäki S and Lahesmaa R. Regulatory T cells control immune responses through their non-redundant tissue specific features. Front Immunol. (2013) 4:294. doi: 10.3389/fimmu.2013.00294
84. Cavaggioni G, Lia C, Resta S, Antonielli T, Benedetti Panici P, Megiorni F, et al. Are mood and anxiety disorders and alexithymia associated with endometriosis? A preliminary study. BioMed Res Int. (2014) 2014:1–5. doi: 10.1155/2014/786830
85. DiVasta AD, Vitonis AF, Laufer MR, and Missmer SA. Spectrum of symptoms in women diagnosed with endometriosis during adolescence vs adulthood. Am J Obstet Gynecol. (2018) 218:324.e1–324.e11. doi: 10.1016/j.ajog.2017.12.007
86. Bloski T and Pierson R. Endometriosis and chronic pelvic pain: unraveling the mystery behind this complex condition. Nurs Women’s Health. (2008) 12:382–95. doi: 10.1111/j.1751-486X.2008.00362.x
87. Maroun P, Cooper MJW, Reid GD, and Keirse MJNC. Relevance of gastrointestinal symptoms in endometriosis. Aust NZ J Obst Gynaeco. (2009) 49:411–4. doi: 10.1111/j.1479-828X.2009.01030.x
88. Luscombe GM, Markham R, Judio M, Grigoriu A, and Fraser IS. Abdominal bloating: an under-recognized endometriosis symptom. J Obstet Gynaecol Canada. (2009) 31:1159–71. doi: 10.1016/S1701-2163(16)34377-8
89. Bianconi L, Hummelshoj L, Coccia ME, Vigano P, Vittori G, Veit J, et al. Recognizing endometriosis as a social disease: the European Union-encouraged Italian Senate approach. Fertil Steril. (2007) 88:1285–7. doi: 10.1016/j.fertnstert.2007.07.1324
90. Culley L, Law C, Hudson N, Denny E, Mitchell H, Baumgarten M, et al. The social and psychological impact of endometriosis on women’s lives: a critical narrative review. Hum Reprod Update. (2013) 19:625–39. doi: 10.1093/humupd/dmt027
91. Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. (2012) 27:1292–9. doi: 10.1093/humrep/des073
92. Schäfer SD and Kiesel L. Diagnosis and treatment of endometriosis according to the S2k guidelines. Der Gynäkologe. (2018) 51:49–60. doi: 10.1007/s00129-017-4148-6
93. Exacoustos C, Zupi E, and Piccione E. Ultrasound imaging for ovarian and deep infiltrating endometriosis. Semin Reprod Med. (2017) 35:005–24. doi: 10.1055/s-0036-1597127
94. Saba L, Sulcis R, Melis GB, De Cecco CN, Laghi A, Piga M, et al. Endometriosis: the role of magnetic resonance imaging. Acta Radiol. (2015) 56:355–67. doi: 10.1177/0284185114526086
95. Young K, Fisher J, and Kirkman M. Endometriosis and fertility: women’s accounts of healthcare. Hum Reprod. (2016) 31:554–62. doi: 10.1093/humrep/dev337
96. Roman H, Chanavaz-Lacheray I, Hennetier C, Tuech J-J, Dennis T, Verspyck E, et al. Long-term risk of repeated surgeries in women managed for endometriosis: a 1,092 patient-series. Fertil Steril. (2023) 120:870–9. doi: 10.1016/j.fertnstert.2023.05.156
97. Capezzuoli T, Rossi M, La Torre F, Vannuccini S, and Petraglia F. Hormonal drugs for the treatment of endometriosis. Curr Opin Pharmacol. (2022) 67:102311. doi: 10.1016/j.coph.2022.102311
98. Ma T, Shen X, Shi X, Sakandar HA, Quan K, Li Y, et al. Targeting gut microbiota and metabolism as the major probiotic mechanism-An evidence-based review. Trends Food Sci Technol. (2023) 138:178–98. doi: 10.1016/j.tifs.2023.06.013
99. Brevi A and Zarrinpar A. Live biotherapeutic products as cancer treatments. Cancer Res. (2023) 83:1929–32. doi: 10.1158/0008-5472.CAN-22-2626
100. Dempsey E and Corr SC. Lactobacillus spp. for gastrointestinal health: current and future perspectives. Front Immunol. (2022) 13:840245. doi: 10.3389/fimmu.2022.840245
101. Khodaverdi S, Mohammadbeigi R, Khaledi M, Mesdaghinia L, Sharifzadeh F, Nasiripour S, et al. Beneficial effects of oral lactobacillus on pain severity in women suffering from endometriosis: A pilot placebo-controlled randomized clinical trial. Int J Fertil Steril. (2019) 13:178–83. doi: 10.22074/ijfs.2019.5584
102. Itoh H, Uchida M, Sashihara T, Ji Z-S, Li J, Tang Q, et al. Lactobacillus gasseri OLL2809 is effective especially on the menstrual pain and dysmenorrhea in endometriosis patients: randomized, double-blind, placebo-controlled study. Cytotechnology. (2011) 63:153–61. doi: 10.1007/s10616-010-9326-5
103. Uchida M and Kobayashi O. Effects of Lactobacillus gasseri OLL2809 on the induced endometriosis in rats. Biosci Biotechnol Biochem. (2013) 77:1879–81. doi: 10.1271/bbb.130319
104. Sari FM, Mirkalantari S, Nikoo S, Sepahvand F, Allahqoli L, Asadi A, et al. Potential of Lactobacillus acidophilus to modulate cytokine production by peripheral blood monocytes in patients with endometriosis. Iranian J Microbiol. (2022) 14:698. doi: 10.18502/ijm.v14i5.10965
105. Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, et al. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. (2019) 8:92. doi: 10.3390/foods8030092
106. Bhandari P and Prabha V. Evaluation of profertility effect of probiotic Lactobacillus plantarum 2621 in a murine model. Indian J Med Res. (2015) 142:79–84. doi: 10.4103/0971-5916.162127
107. Dang Y and Zhang S. Causal relationship between endometriosis and inflammatory bowel disease: A Mendelian randomization analyses. Clin Trans Med. (2024) 14:e1496. doi: 10.1002/ctm2.1496
108. Weizman Z, Abu-Abed J, and Binsztok M. Lactobacillus reuteri DSM 17938 for the management of functional abdominal pain in childhood: a randomized, double-blind, placebo-controlled trial. J Pediatr. (2016) 174:160–4. doi: 10.1016/j.jpeds.2016.04.003
109. Bougie O, Nwosu I, and Warshafsky C. Revisiting the impact of race/ethnicity in endometriosis. Reprod Fertil. (2022) 3:R34–41. doi: 10.1530/RAF-21-0106
110. Bakken JS, Borody T, Brandt LJ, Brill JV, Demarco DC, Franzos MA, et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol. (2011) 9:1044–104.
111. Khoruts A and Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol. (2016) 13:508–16.
112. Jørgensen SM, Hansen MM, Erikstrup C, Dahlerup JF, and Hvas CL. Faecal microbiota transplantation: establishment of a clinical application framework. Eur J Gastroenterol Hepatol. (2017) 29:e36–45. doi: 10.1097/MEG.0000000000000958
113. Ianiro G, Maida M, Burisch J, Simonelli C, Hold G, Ventimiglia M, et al. Efficacy of different faecal microbiota transplantation protocols for Clostridium difficile infection: a systematic review and meta-analysis. United Eur Gastroenterol J. (2018) 6:232–1244. doi: 10.1177/2050640618780762
114. Kim DE, Kim JK, Han SK, Jang SE, Han MJ, and Kim DH. Lactobacillus plantarum NK3 and Bifidobacterium longum NK49 alleviate bacterial vaginosis and osteoporosis in mice by suppressing NF-κ B-Linked TNF-α expression. J medicinal Food. (2019) 22:1022–31. doi: 10.1089/jmf.2019.4419
115. Panchal P, Budree S, Scheeler A, Medina G, Seng M, Wong WF, et al. Scaling safe access to fecal microbiota transplantation: past, present, and future. Curr Gastroenterol Rep. (2018) 20:1–11. doi: 10.1007/s11894-018-0634-9
116. van Nood E, Speelman P, Nieuwdorp M, and Keller J. Fecal microbiota transplantation: facts and controversies. Curr Opin Gastroenterol. (2014) 30:34–9. doi: 10.1097/MOG.0000000000000024
117. Suez J, Zmora N, Zilberman-Schapira G, Mor U, Dori-Bachash M, Bashiardes S, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. (2018) 174:1406–23. doi: 10.1016/j.cell.2018.08.047
118. Goulielmos GN, Matalliotakis M, Matalliotaki C, Eliopoulos E, Matalliotakis I, and Zervou MI. Endometriosis research in the -omics era. Gene. (2020) 741:144545. doi: 10.1016/j.gene.2020.144545
119. Ning L, Zhou Y-L, Sun H, Zhang Y, Shen C, Wang Z, et al. Microbiome and metabolome features in inflammatory bowel disease via multi-omics integration analyses across cohorts. Nat Commun. (2023) 14:7135. doi: 10.1038/s41467-023-42788-0
120. Sun Y, Zhang X, Hang D, Lau HC-H, Du J, Liu C, et al. Integrative plasma and fecal metabolomics identify functional metabolites in adenoma-colorectal cancer progression and as early diagnostic biomarkers. Cancer Cell. (2024) 42:1386–1400.e8. doi: 10.1016/j.ccell.2024.07.005
121. Ney L-M, Wipplinger M, Grossmann M, Engert N, Wegner VD, and Mosig AS. Short chain fatty acids: key regulators of the local and systemic immune response in inflammatory diseases and infections. Open Biol. (2023) 13:230014. doi: 10.1098/rsob.230014
122. Cuffaro F, Russo E, and Amedei A. Endometriosis, pain, and related psychological disorders: unveiling the interplay among the microbiome, inflammation, and oxidative stress as a common thread. Int J Mol Sci. (2024) 25:6473. doi: 10.3390/ijms25126473
Keywords: endometriosis, gut microbiome, dysbiosis, immunomodulation, microbiota-based therapy, estroblome, biomarkers, probiotics
Citation: Kalopedis EA, Zorgani A, Zinovkin DA, Barri M, Wood CD and Pranjol MZI (2025) Leveraging the role of the microbiome in endometriosis: novel non-invasive and therapeutic approaches. Front. Immunol. 16:1631522. doi: 10.3389/fimmu.2025.1631522
Received: 19 May 2025; Accepted: 26 August 2025;
Published: 16 September 2025.
Edited by:
George Priya Doss C, VIT University, IndiaReviewed by:
Ata Ur Rehman, Duke University, United StatesBodil Ohlsson, Lund University, Sweden
Yulia Korneva, North-Western State Medical University named after I.I. Mechnikov, Russia
Copyright © 2025 Kalopedis, Zorgani, Zinovkin, Barri, Wood and Pranjol. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Md Zahidul I. Pranjol, Wi5QcmFuam9sQHN1c3NleC5hYy51aw==
 Eleni Andria Kalopedis
Eleni Andria Kalopedis Amine Zorgani
Amine Zorgani Dmitry A. Zinovkin
Dmitry A. Zinovkin Muruj Barri
Muruj Barri C. David Wood1
C. David Wood1 Md Zahidul I. Pranjol
Md Zahidul I. Pranjol