- 1State Key Laboratory of Mariculture Biobreeding and Sustainable Goods, Key Laboratory of South China Sea Fishery Resources Exploitation & Utilization, Ministry of Agriculture and Rural Affairs, South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Guangzhou, China
- 2Key Laboratory of Efficient Utilization and Processing of Marine Fishery Resources of Hainan Province, Sanya Tropical Fisheries Research Institute, Sanya, China
- 3Shenzhen Base of South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Shenzhen, China
Sudden fluctuations in environmental temperatures are primarily caused by climate change. Aquatic organisms such as shrimp are poikilothermic animals, making them highly vulnerable to rising water temperatures, which can trigger stress responses and reduce aquaculture productivity. Hepatopancreas is of vital importance to the immunity, metabolism and detoxification of shrimp. In this study, the shrimp Litopenaeus vannamei were continuously exposed to high temperature (HT) stress at 33 °C for 7 days, and the hepatopancreatic histopathology, immune-related indexes, and metabolite patterns were explored. The results showed that HT stress caused abnormal morphological changes in the hepatopancreas of the shrimp, with the hepatic tubules becoming twisted, atrophied, and even ruptured and autolyzed. At the molecular level, stress-related indexes, such as Nrf2, GPx, and HSP70 genes expression were increased, while SOD and HSP90 genes were decreased; immune-related indexes, such as ALF, Crus, and proPO genes expression were increased, whereas Pen3 gene was decreased; inflammation-related genes (JNK and TNFα) and apoptosis-related genes (Casp9 and Casp3) expression were increased; autophagy-related indexes, such as Atg3, Atg16, and Beclin1 genes expression were increased. Furthermore, HT stress caused the alterations in the metabolic patterns of the hepatopancreas, such as amino acid biosynthesis and metabolism, pentose and glucuronate interconversions, pantothenate and CoA biosynthesis, pyrimidine metabolism, and glycerophospholipid metabolism. Functional metabolites, such as tryptophan, arachidonic acid, cinnamic acid derivatives, vitamins, etc., were identified as biomarker candidates. The results revealed that HT stress induced comprehensive histomorphological and functional impairments in the hepatopancreas of L. vannamei through a cascade of oxidative damage, immune dysregulation, and metabolic disturbance.
1 Introduction
Pacific white shrimp Litopenaeus vannamei is one of the most widely cultivated shrimp species globally, which is critical for securing high-quality animal protein supplies and promoting the fishery economy (1). The global aquaculture production of L. vannamei exceeded 4.5 million tons in 2023, with China accounting for over 2.2 million tons. Shrimp are poikilothermic animals, highly susceptible to water temperature fluctuations. Both elevated and reduced temperatures can have significant impacts on shrimp, reducing their survival rate. Under the background of global warming, extreme high temperature (HT) events are becoming increasingly frequent, posing a huge challenge to the aquaculture industry (2, 3). In tropical and subtropical shrimp farming areas, the water temperature in summer usually exceeds 32 °C in shrimp ponds (4–6), sometimes reaching as high as 34 °C (7–9). HT stress can disrupt the physiological balance of shrimp as ectothermic animals, leading to metabolic disorders, weakened immunity, stunted growth, and even mass mortality, which seriously threatens shrimp farming and causes economic losses (10–14). Therefore, exploring the physiological responses of shrimp to HT stress is conducive to formulating anti-stress strategies.
HT stress has been shown to adversely impact shrimp, mainly focusing on inducing stress responses and disrupting immune homeostasis. For instance, HT stress can trigger oxidative stress responses in shrimp, leading to the accumulation of reactive oxygen species (ROS) and altering the activity of antioxidant enzymes (15, 16). HT stress can induce the up-regulation of heat shock protein (HSPs) gene expression in L. vannamei, including HSP60, HSP70, and HSP90 (17, 18). Furthermore, HT stress can also disrupt the immune homeostasis of L. vannamei by affecting hemolymph osmolality, total hemocyte count (THC), and phenoloxidase activity (19). Additionally, HT stress compromises intestinal health of L. vannamei by damaging mucosal morphology, altering immune parameters, and inducing microbial community variations (1, 20).
HT stress also exerts notable impacts on shrimp metabolic processes. For instance, previous studies have demonstrated that HT stress influences glucose metabolism in the gills of L. vannamei, promoting a shift toward anaerobic carbohydrate utilization (21). Additionally, HT stress alters hemolymph glucose levels in L. vannamei, while having no significant impact on cholesterol, acylglycerol, or total protein contents (21). Metabolomic analyses further reveal that acute HT stress (33 °C) induces substantial metabolic alterations in the hemolymph of L. vannamei, particularly in the metabolism of “arachidonic acid”, “phenylalanine” and “alanine, aspartate and glutamate”, as well as in the biosynthesis of “phenylalanine, tyrosine and tryptophan” (1). Although numerous studies have explored the negative impacts of HT stress on shrimp health, the research remains insufficiently in-depth, lacking investigations into the mechanisms at different biological levels. A more comprehensive investigation is needed to elucidate the underlying mechanisms across multiple biological levels.
The hepatopancreas, as an important immune and metabolic organ of the shrimp, plays a key role in responding to environmental stress. In this study, we aim to systematically explore the effects of HT stress on the physiological functions in the hepatopancreas of L. vannamei by integrating immune indicators and metabonomics. Firstly, the morphological changes of the hepatopancreas were explored. Then, the characteristics of immune responses were analyzed based on the indicators related to stress, antibacterial, inflammation, apoptosis and autophagy. Finally, the metabolic pathways and potential metabolite markers were identified based on metabonomics methods. These results indicated that HT stress damaged the hepatopancreas structure and function of the shrimp via oxidative, immune, and metabolic disruption, which can provide insights for understanding HT stress adaptation and developing HT-resistant aquaculture strategies in shrimp.
2 Materials and methods
2.1 Shrimp and their rearing conditions
The shrimp L. vannamei used in this study were procured from an indoor shrimp pond in Shenzhen (China), with an average body weight of 6.3 ± 0.5 g. These shrimp had undergone strict pathogen detection, were specific pathogen-free, and had normal appearances without clinical symptoms of diseases. Before the HT stress exposure experiment, the shrimp individuals were acclimated for 7 days in tanks holding 300 L of aerated seawater. The rearing conditions were maintained through continuous water aeration and daily water exchange to ensure optimal water quality for shrimp culture, including a stable temperature of 28 ± 0.2 °C, pH 8.1-8.2, and salinity 30. The shrimp were fed commercial compound feed twice daily, with the feces and uneaten residues promptly removed from the tanks to maintain water cleanliness.
2.2 HT stress experiment and sampling
In this study, 33 °C was selected as the experimental temperature for HT stress exposure, based on actual shrimp farming practices in tropical and subtropical regions, as well as the previous research reports on high-temperature stress in shrimp (1, 20). After a 7-day acclimation period in tanks, the shrimp were randomly divided into two groups: a control (CK) group and a HT group. Each group consisted of three replicate tanks, with each tank containing 300 L of seawater and 50 shrimp. The CK group was maintained in normal seawater at a constant temperature of 28 ± 0.2 °C. For the HT group, the water temperature was set at 33 °C. A constant-temperature heater was used to gradually increase the temperature from 28 °C to 33 °C at a rate of 1 °C per hour, then maintained at constant temperature. Each tank’s water was changed daily. Prior to water exchange, we preheated the water to 33 °C in advance and then replaced the water in all the tanks of the HT group, thus preventing fluctuations in water temperature. All the rearing conditions except temperature were maintained consistently between the acclimation and experimental phases, with stable parameters of pH 8.1-8.2 and salinity 30. During the stress experiment, the shrimp were fed twice daily, with the feces and uneaten feed removed from the tank promptly.
After 7 days of stress, the hepatopancreas samples were collected randomly for analysis. Since shrimp are aquatic invertebrates with considerable individual variations, to reduce the differences among individuals, the hepatopancreas from five shrimp per tank were pooled and stored in RNAFollow solution for the mRNA expression analysis. For metabolomics analysis, two samples were collected from each tank, with each sample consisting of a mixture of the hepatopancreases from five shrimp, meaning there were six samples per group. Additionally, the hepatopancreas from three shrimp per tank was sampled for the histomorphological analysis.
2.3 Histomorphological analysis
Hepatopancreas samples were fixed in 4% paraformaldehyde for 24 h. After rinsing with running water for 30 min, the tissues were dehydrated through a series of ethanol solutions (70%, 80%, 90%, and 100%), washed with xylene, embedded in paraffin, and sectioned into 4 μm slices using a microtome (Leica RM2016, Shanghai). Following Hematoxylin and Eosin (H&E) staining, the sections were examined under a microscope (Nikon, Tokyo, Japan).
2.4 Gene expression analysis
Total RNA was isolated from the hepatopancreas samples using TRIzol reagent (Invitrogen, USA). Following the removal of genomic DNA and the purification of the RNA, cDNA synthesis was performed from the RNA using the Servicebio RT First-Strand cDNA Synthesis Kit (Wuhan, China). Real-time quantitative PCR (qPCR) was carried out with the SGExcel Fast SYBR qPCR Mix Kit (Sangon Biotech, China) on a Heal Force CG-02 qPCR system (Shanghai, China). The β-actin served as the reference gene, and the specific qPCR primer sequences are listed in Supplementary Table S1. Each sample underwent four technical replicates in the qPCR analysis. Relative mRNA expression levels of the target genes were calculated according to the method described by Livak and Schmittgen (22), presented as fold-changes relative to the CK group.
2.5 Non-targeted metabolomics analysis
Six hepatopancreas biological replicate samples per group were subjected to metabolomics analysis. After the pre-treatment of the hepatopancreas samples, the metabolites were extracted using a solution of methanol/chloroform and 2-chlorophenylalanine. Subsequently, the samples were detected by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The liquid chromatography analysis was carried out on a Thermo Ultimate 3000 system, employing an ACQUITY UPLC HSS T3 (150 × 2.1 mm, 1.8 μm, Waters) chromatographic column. Mass spectrometry analysis was performed using a Thermo Q Exactive mass spectrometer. Data-dependent acquisition (DDA) MS/MS experiments were conducted using high-energy collision dissociation scans. To enhance data quality, dynamic exclusion was applied to filter out redundant information from the MS/MS spectra, ensuring the acquisition of highly relevant and accurate data.
Following the quality control, the metabonomic data were analyzed via partial least squares discriminant analysis (PLS-DA) to identify differential metabolites between the HT vs CK groups. Significance criteria were set as P < 0.05 and variable importance in projection (VIP) > 1.0. Agglomerative hierarchical clustering of the differential metabolites was performed using the pheatmap package in R software (v3.3.2). KEGG pathway annotation of differential metabolites was conducted using MetaboAnalyst software (www.metaboanalyst.ca), with subsequent analysis of metabolic pathways and interaction networks. Based on existing literature reports, we focused specifically on the differential metabolites with physiological and health-regulating functions, regarding them as potential biomarkers, and systematically analyzed their variation characteristics.
2.6 Statistical analysis
All the gene expression data were expressed as mean ± standard error (SE), and subjected to statistical analysis using one-way ANOVA with SPSS 27.0 software. A P-value < 0.05 was considered to denote statistical significance.
3 Results
3.1 Histomorphological changes of the hepatopancreas
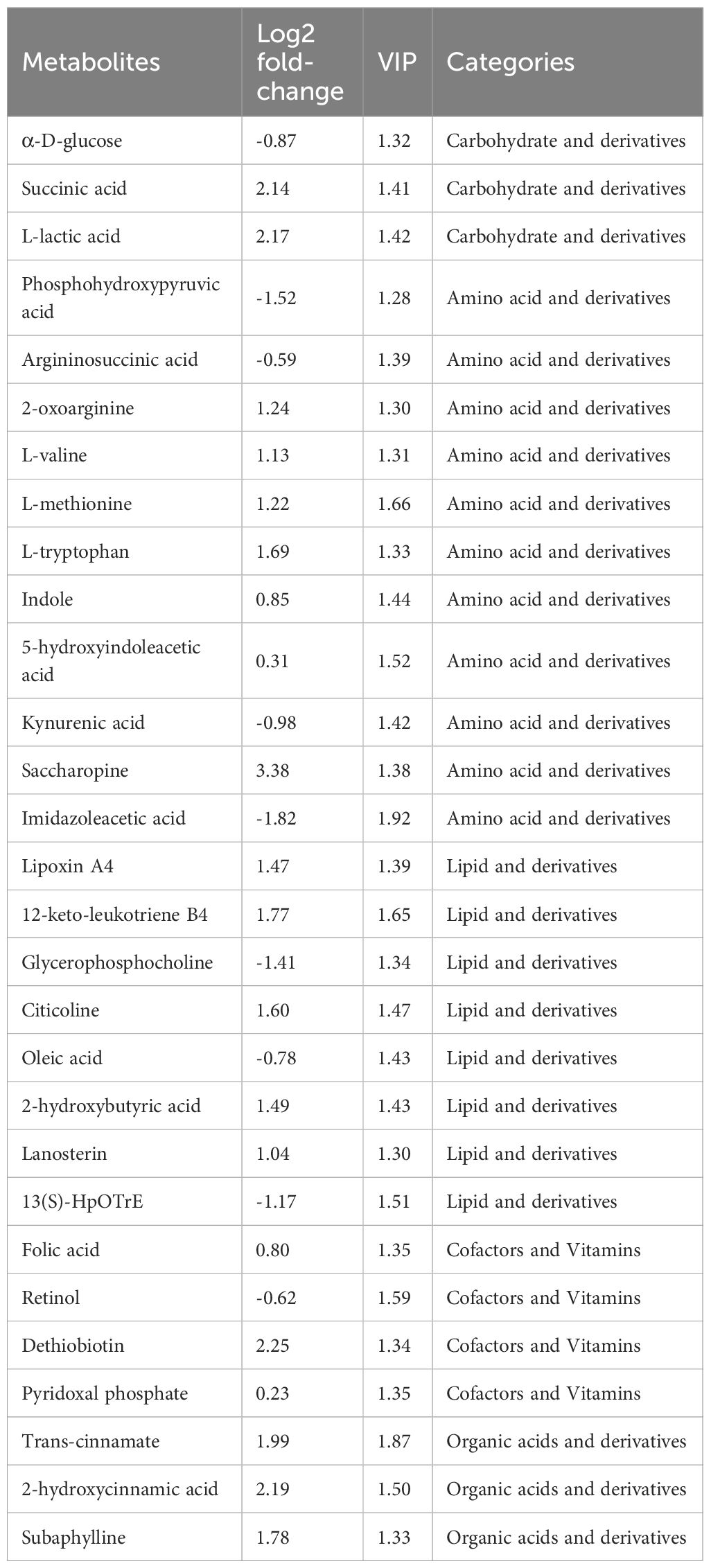
In the CK group, the hepatopancreatic tubules of the shrimp hepatopancreas showed relatively normal morphology, with tight connections and distinct star-shaped lumens (Figures 1a, b). However, in the HT group, the hepatopancreas exhibited abnormal morphological changes, such as irregular star-shaped structures of the hepatopancreatic tubules, which were twisted, atrophied, and detached from the basement membrane; some hepatopancreatic tubules even showed rupture and autolysis (Figures 1c, d). In the HT group, the diameter of hepatic tubules was significantly higher than that in the CK group (P < 0.05), while the lumen diameter showed a slight increase with no significant difference (P > 0.05) (Supplementary Figure S1), and the degeneration index reaching over 63%.
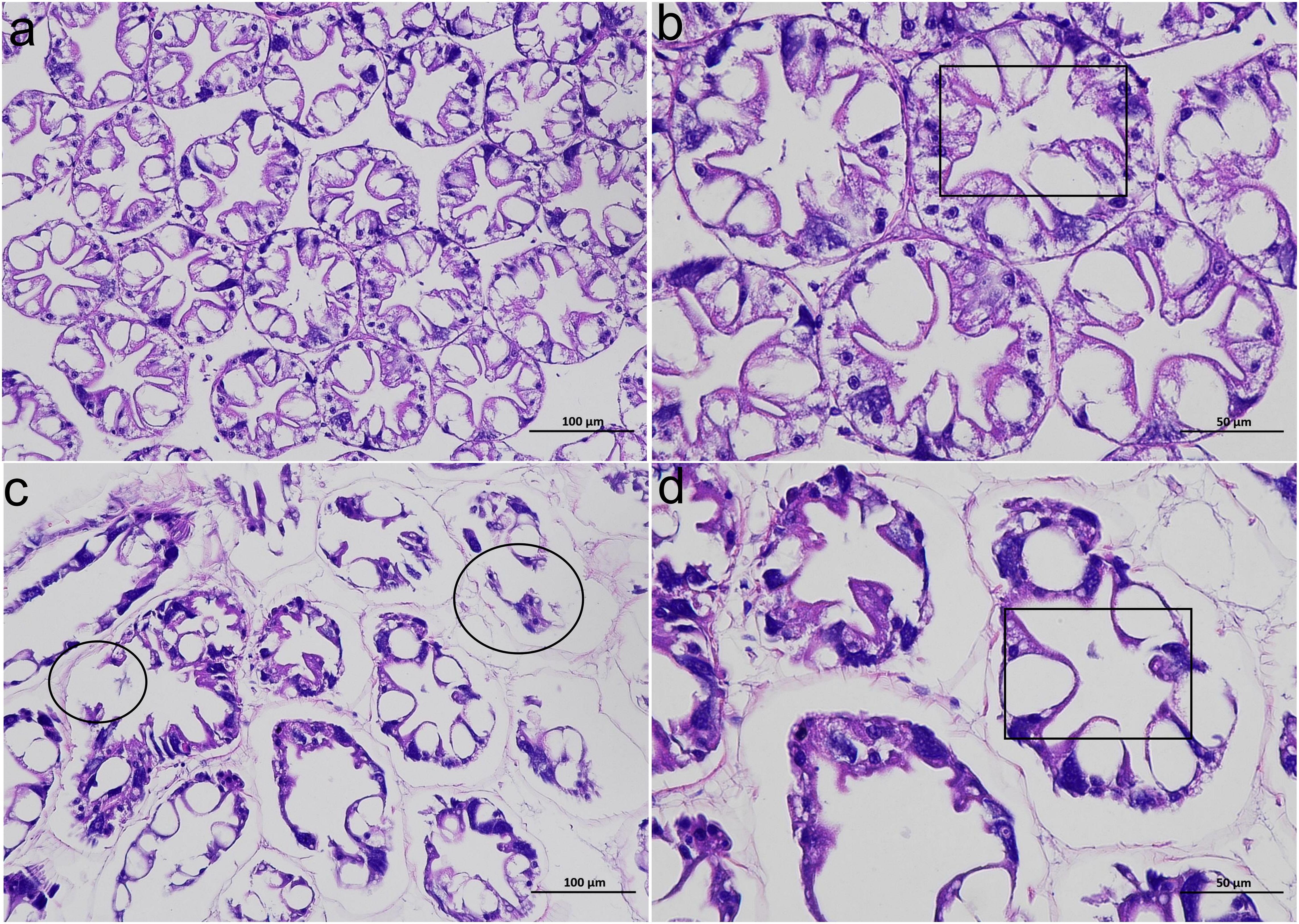
Figure 1. The alterations of the histological structure of the L. vannamei hepatopancreas after HT stress. (a, b) the CK group; (c, d) the HT group. (a, c) 200 ×; (b, d) 400 ×. A black box indicates the lumen; a black circle indicates a damaged hepatic tubule.
3.2 Changes in hepatopancreatic stress response indices
Compared with the CK group, oxidative stress related indices, such as the relative mRNA expression levels of nuclear factor erythroid-derived 2-like 2 (Nrf2) and glutathione peroxidase (GPx) genes were significantly increased in the HT group (P < 0.05), while the expression of copper zinc superoxide dismutase (SOD) gene was significantly decreased (P < 0.05); stress related proteins, such as the relative mRNA expression levels of HSP70 gene was significantly increased in the HT group (P < 0.05), while the expression of HSP90 gene was slightly decreased with no statistical significance (P > 0.05) (Figure 2).
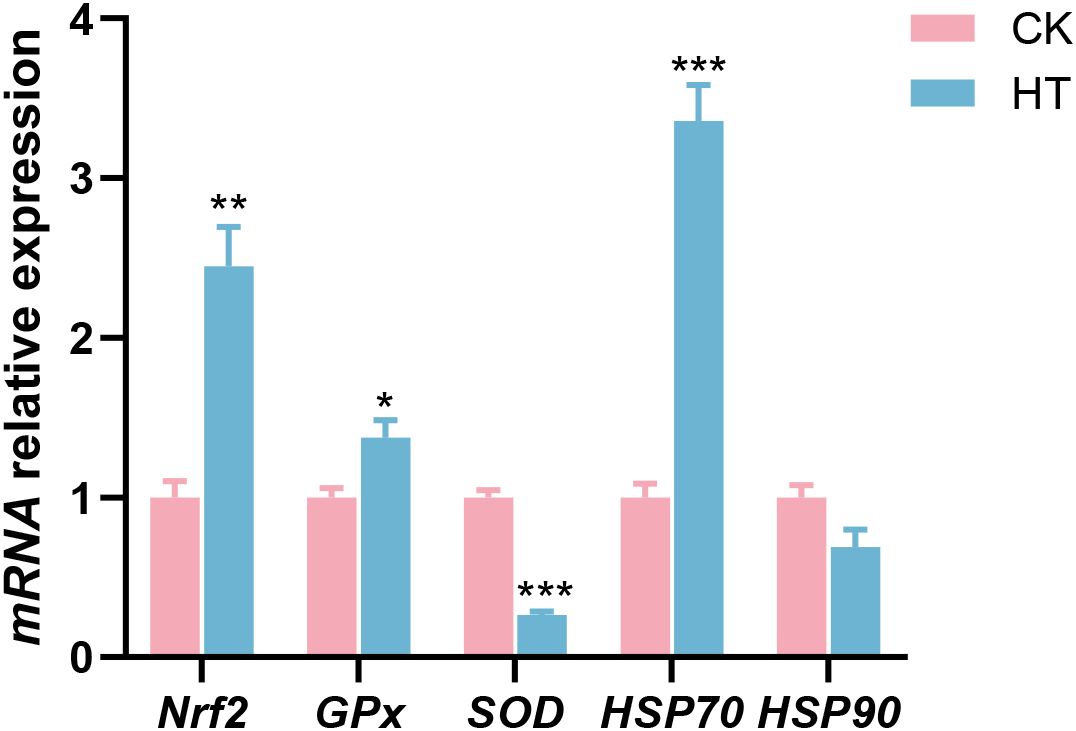
Figure 2. The alterations of the stress-related genes expression in the L. vannamei hepatopancreas after HT stress. The asterisk on the error bar show significant differences (*P < 0.05, **P < 0.01, ***P < 0.001).
3.3 Changes in hepatopancreatic immunological indices
Compared with the CK group, immune related indices, such as the relative mRNA expression levels of anti-lipopolysaccharide factor AV-K (ALF), crustin (Crus), and prophenoloxidase (proPO) genes were significantly increased in the HT group (P < 0.05), while the expression of penaeidin 3a (Pen3) gene was significantly decreased (P < 0.05); the expression of lysozyme (Lys) gene was slightly decreased with no statistical significance (P > 0.05) (Figure 3).
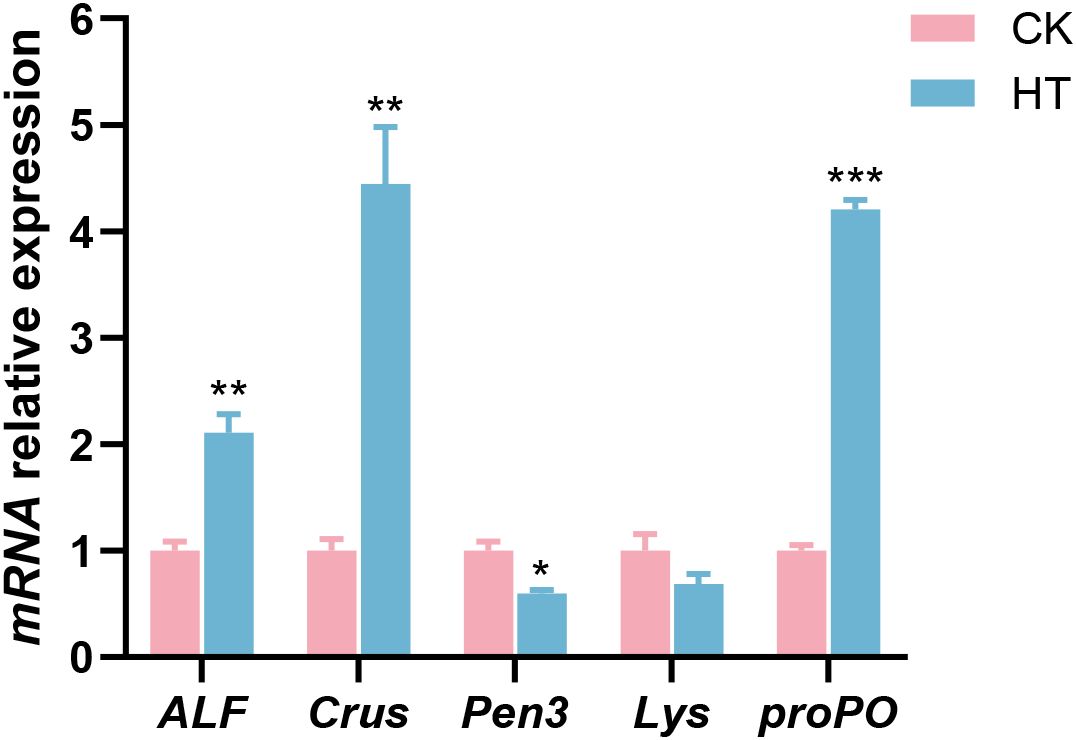
Figure 3. The alterations of the antibacterial-related genes expression in the L. vannamei hepatopancreas after HT stress. The asterisk on the error bar show significant differences (*P < 0.05, **P < 0.01, ***P < 0.001).
3.4 Changes in hepatopancreatic inflammatory and apoptotic indices
Compared with the CK group, inflammatory related indices, such as the relative mRNA expression levels of c-Jun amino-terminal kinase (JNK) and tumor necrosis factor-α (TNFα) genes were significantly increased in the HT group (P < 0.05), while the expression of nuclear factor kappa-B (NF-κB) gene was slightly increased with no statistical significance (P > 0.05); apoptosis related indices, such as the relative mRNA expression levels of caspase-9 (Casp9) and caspase-3 (Casp3) genes were significantly increased (P < 0.05) in the HT group (Figure 4).
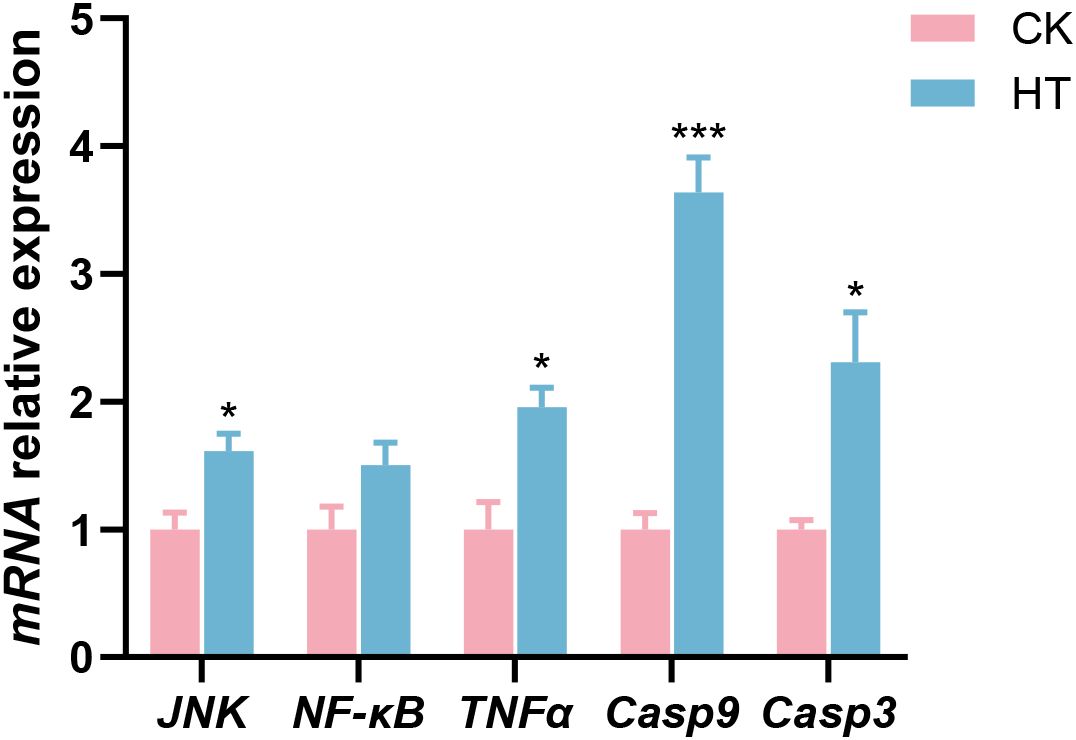
Figure 4. The alterations of the inflammation and apoptosis-related genes expression in the L. vannamei hepatopancreas after HT stress. The asterisk on the error bar show significant differences (*P < 0.05, ***P < 0.001).
3.5 Changes in hepatopancreatic autophagic indices
Compared with the CK group, autophagic related indices, such as the relative mRNA expression levels of autophagy-related protein 3 (Atg3), autophagy-related protein 16 (Atg16), and Beclin1 genes were significantly increased in the HT group (P < 0.05), while the expression of autophagy-related protein 12 (Atg12) and heat shock cognate 70 (Hsc70) genes was slightly increased with no statistical significance (P > 0.05) (Figure 5).
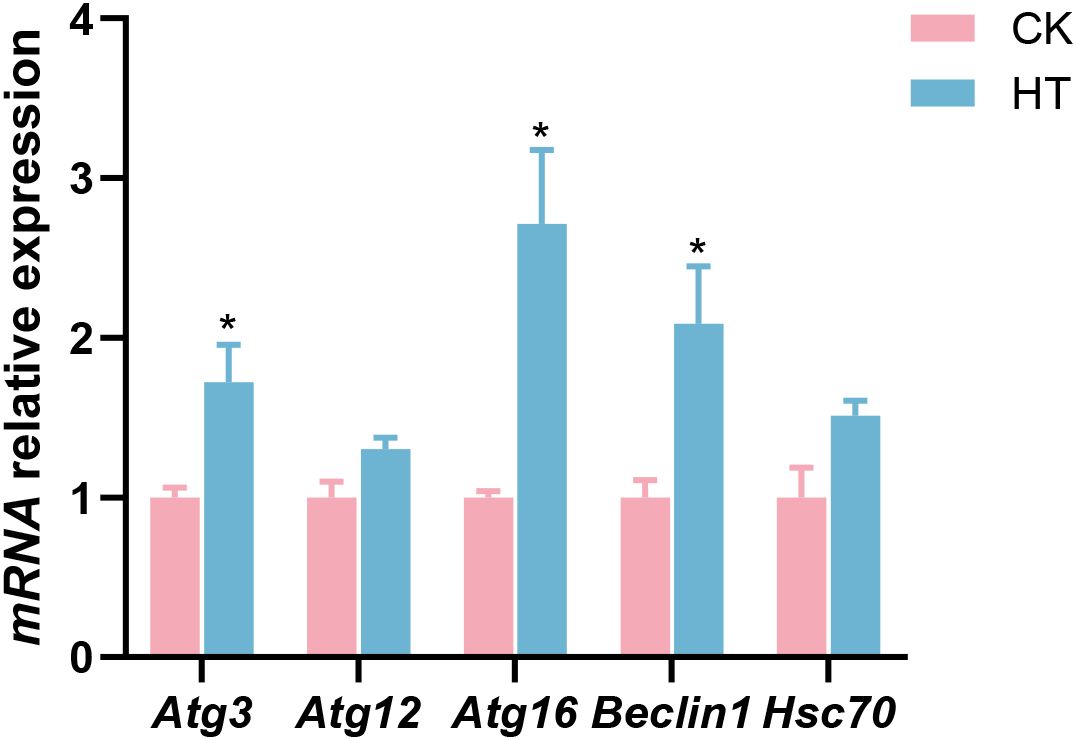
Figure 5. The alterations of the autophagy-related genes expression in the L. vannamei hepatopancreas after HT stress. The asterisk on the error bar show significant differences (*P < 0.05).
3.6 Changes in hepatopancreatic metabolic patterns
3.6.1 Functional analysis of differential metabolites
The changes of metabolites in the hepatopancreas under HT stress were further analyzed (Figure 6a). Based on the multivariate statistical analysis of PLS-DA, there were obvious differences in the metabolic patterns between the HT and CK group (Figures 6b–d). Compared with the CK group, a total of 65 differential metabolites were identified in the HT group, including 52 up-regulated metabolites and 13 down-regulated metabolites (Supplementary Figure S2).
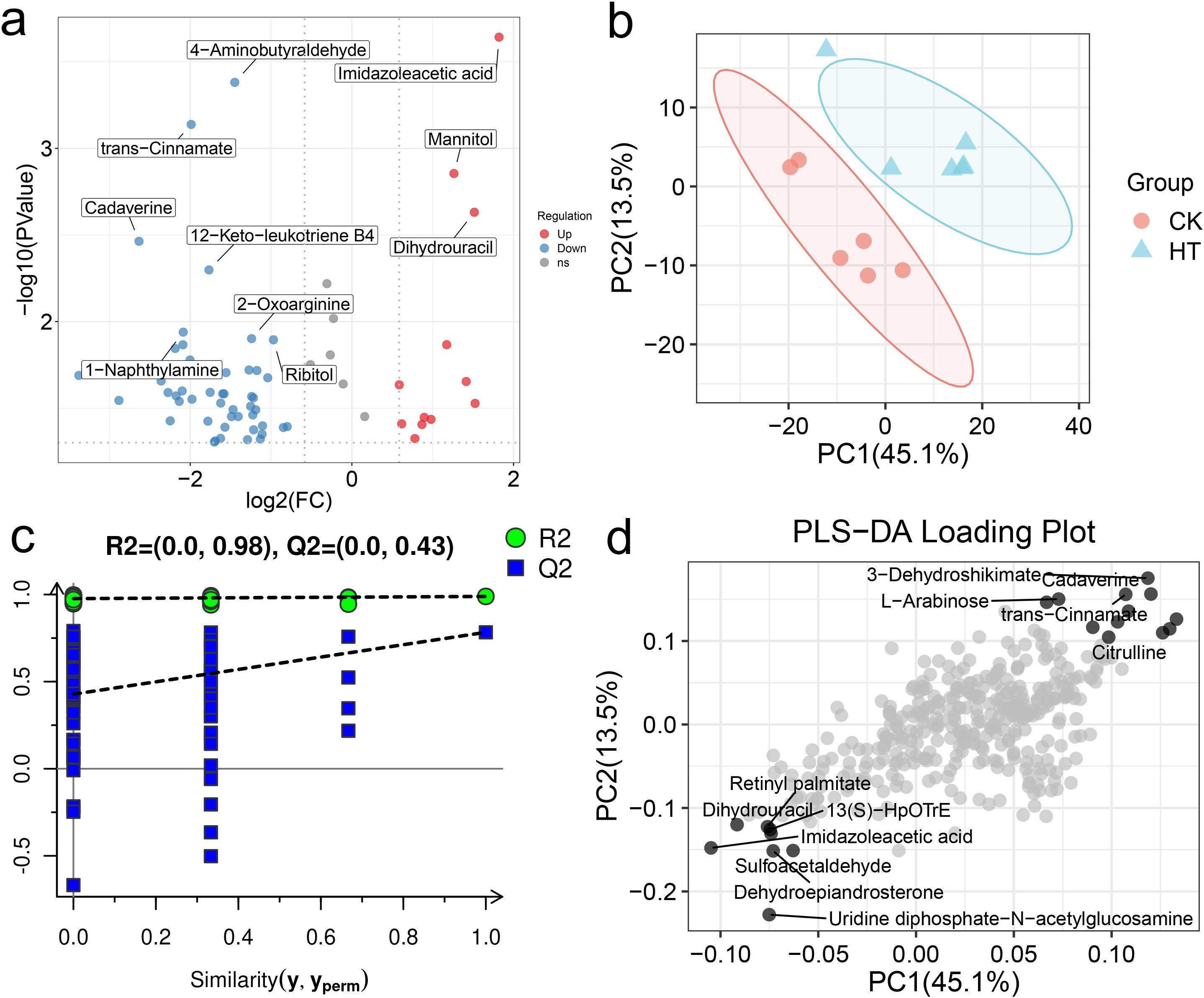
Figure 6. The alterations of the metabolite patterns in the L. vannamei hepatopancreas after HT stress. (a) the volcano plot of the metabolites; (b) the PLS-DA multivariate statistics of the metabolites; (c) the PLS-DA permutation test of the metabolites; (d) the PLS-DA loading plot of the metabolites.
The pathways involved in these differential metabolites were further analyzed. A total of 36 pathways were enriched, among them, “arginine and proline metabolism”, “valine, leucine and isoleucine biosynthesis”, “tryptophan metabolism”, “alanine, aspartate and glutamate metabolism”, “pentose and glucuronate interconversions”, “pantothenate and CoA biosynthesis”, “pyrimidine metabolism”, “glycerophospholipid metabolism” were highly enriched functions (Figure 7a).
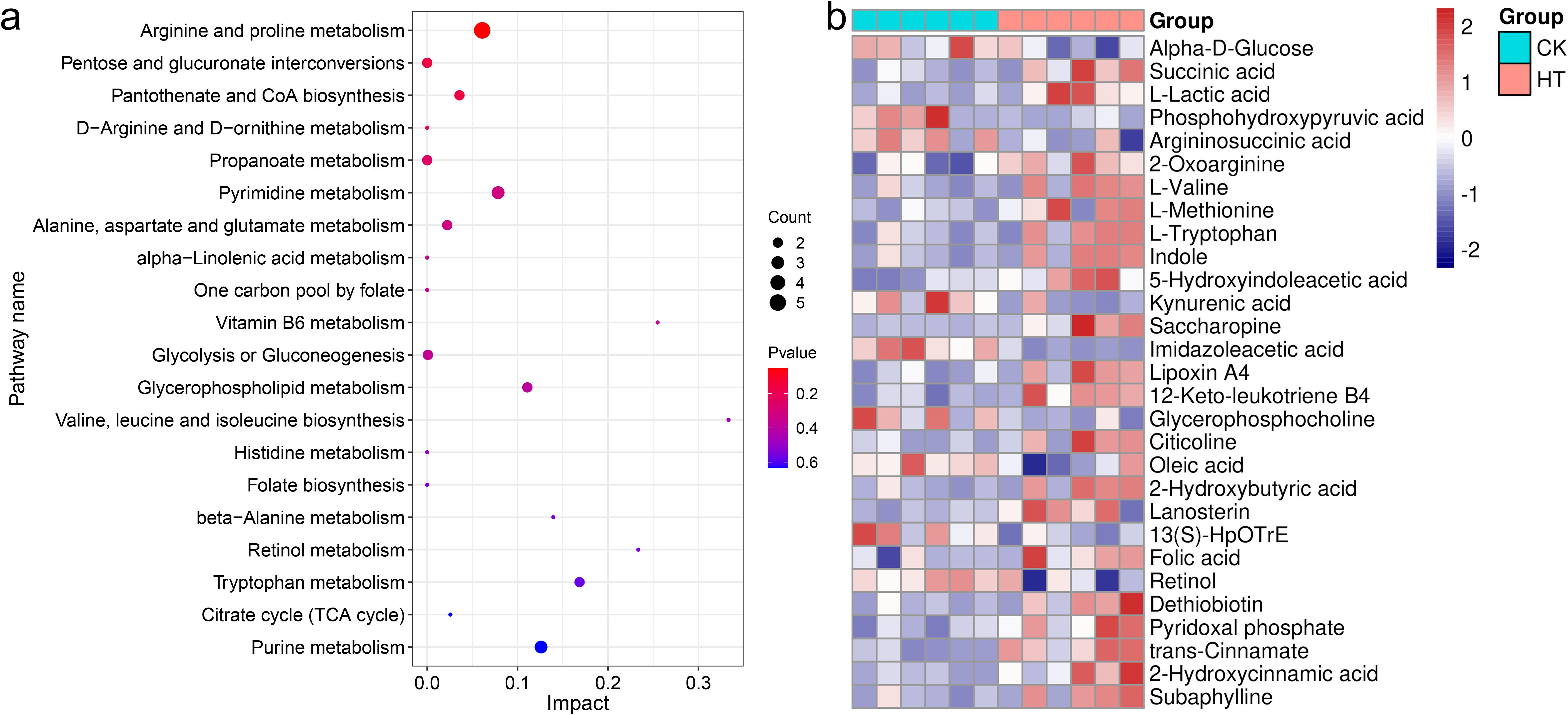
Figure 7. The enrichment pathways and candidate biomarkers of the differential metabolites in the L. vannamei hepatopancreas after HT stress. (a) the pathways of the HT vs CK group; (b) the candidate metabolite biomarkers.
The network relationships among these highly enriched pathways were explored (Figure 8a). Of these, the pathway “arginine and proline metabolism” was correlated with “alanine, aspartate and glutamate metabolism” through the metabolite argininosuccinic acid (C03406); the pathway “aminoacyl-tRNA biosynthesis” was correlated with “tryptophan metabolism” and “cysteine and methionine metabolism” through the metabolites L-tryptophan (C00078) and L-methionine (C00073) respectively; the pathway “pyrimidine metabolism” was correlated with “pantothenate and CoA biosynthesis” through the metabolite dihydrouracil (C00429); the pathway “pantothenate and CoA biosynthesis”, “valine, leucine and isoleucine biosynthesis”, “alanine, aspartate and glutamate metabolism”, and “aminoacyl-tRNA biosynthesis” were correlated with each other through the metabolite L-valine (C00183). Furthermore, Based on the metabolomics-FELLA enrichment analysis, there was a high degree of correlation among the pathways “glycine, serine and threonine metabolism”, “arginine and proline metabolism”, and “cysteine and methionine metabolism” (Figure 8b).
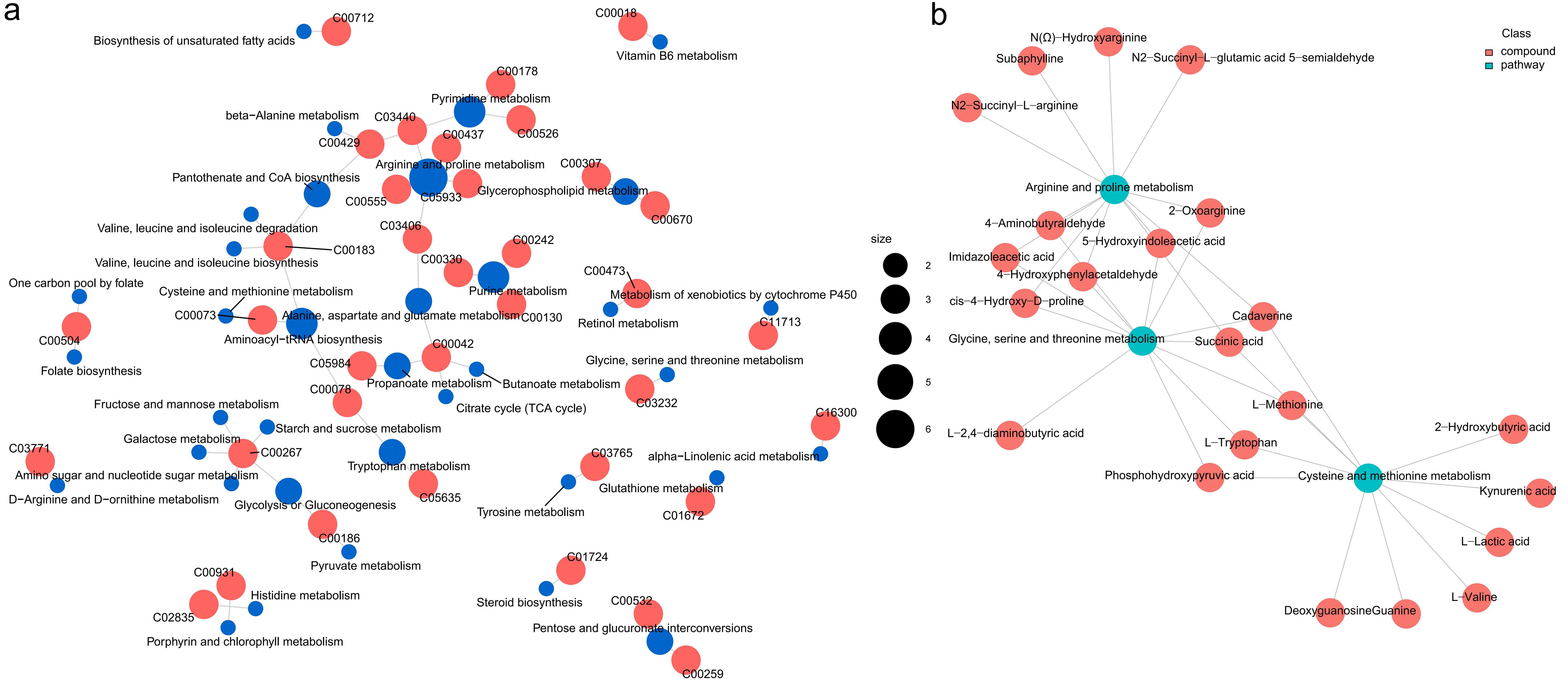
Figure 8. The relationship network between the differential metabolites and pathways in the L. vannamei hepatopancreas after HT stress. (a) the correlation network; (b) the FELLA enrichment network.
3.6.2 The change characteristics of differential metabolite markers
Several differential metabolite markers were rigorously screened (Figure 7b, Table 1). Among the three carbohydrate and derivatives, succinic acid and L-lactic acid were increased, but α-D-glucose was decreased. Among the eleven amino acid and derivatives, 2-oxoarginine, L-valine, L-methionine, L-tryptophan, indole, 5-hydroxyindoleacetic acid and saccharopine were increased, but phosphohydroxypyruvic acid, argininosuccinic acid, kynurenic acid and imidazoleacetic acid were decreased. Among the eight lipid and derivatives, lipoxin A4, 12-keto-leukotriene B4, citicoline, 2-hydroxybutyric acid, and lanosterin were increased, but glycerophosphocholine, oleic acid, and 13(S)-HpOTrE were decreased; of the four cofactors and vitamins, folic acid, dethiobiotin and pyridoxal phosphate were increased, but retinol was decreased. Among the three organic acids and derivatives, trans-cinnamate, 2-hydroxycinnamic acid, and subaphylline were increased.
4 Discussion
During summer’s high-temperature seasons, the frequent stress problems in farmed shrimp have emerged as a critical constraint to aquaculture success. The physiological homeostasis of the shrimp organs is crucial for their defense against environmental stress, and the prerequisite for this is the integrity of histological morphology. Liao et al. reported that when L. vannamei is subjected to acute HT stress, the tissue morphology of the hepatopancreas exhibits significant damage (23). Such phenomena are also observed in this study, which will inevitably disrupt the physiological homeostasis of the shrimp hepatopancreas.
Oxidative stress serves as one of the key mechanisms contributing to the impacts of environmental stress on shrimp (24). As a key transcription factor, the Nrf2 regulates the gene expression of antioxidant enzymes (such as SOD and GPx) by binding to antioxidant response elements, thus playing a central regulatory role in safeguarding organisms against oxidative stress (25). HSP70 is a functional protein that can defend against oxidative stress (26). In this study, after HT stress, the expressions of Nrf2, GPx, and HSP70 genes were increased in the hepatopancreas of the shrimp, while the expressions of SOD and HSP90 genes were decreased. These findings indicated that HT stress triggered intracellular ROS accumulation, leading to oxidative stress in the hepatopancreas. The upregulation of the Nrf2/GPx signaling likely represented the organism’s primary defensive strategy against oxidative stress, while the downregulation of SOD might reflect stress-induced suppression of its expression. The HSP70 and HSP90 genes exhibited differential expression patterns, suggesting that the organism preferentially activated HSP70 to mount a rapid stress response, while the downregulation of HSP90 likely reflected resource reallocation under energy-limiting conditions.
Oxidative stress can induce autophagy via multiple signaling, which clears the organelles and proteins damaged by oxidative stress for intracellular stability (27). Autophagy-related genes (such as Atg3, Atg12 and Atg16) and Beclin1 play a crucial role in the autophagy process (28). Hsc70 drives chaperone-mediated autophagy by transporting substrate proteins to lysosomes for degradation (29). In this study, after HT stress, the expressions of Atg3, Atg16 and Beclin1 genes were significantly upregulated, and the expressions of Atg12 and Hsc70 genes also tended to be upregulated, indicating that the autophagy of hepatopancreatic cells in the shrimp was activated in response to the stress.
Environmental stress can affect the immune defense ability of aquatic animals. As an important component of the shrimp immune system, antimicrobial peptides can enhance the stress resistance of shrimp (30). The prophenoloxidase system participates in the melanization immune response in shrimp (31). In this study, HT stress induced the upregulation of ALF and Crus in the hepatopancreas of the shrimp, which could enhance immunity to cope with the damage caused by stress; the high expression of proPO was beneficial to tissue damage repair and the formation of a protective barrier. In contrast, the downregulation of Pen3 and Lys reflected the selective allocation of immune resources, which might be due to the adaptive inhibition of their synthesis as energy was prioritized to ensure basic activities under HT stress. TNF-α, as an inflammatory mediator, can activate the JNK and NF-κB signalings, promote the expression of inflammation-related genes, and trigger an inflammatory response (32, 33). In this study, the upregulation of JNK, NF-κB and TNFα genes indicated that HT stress induced an inflammatory response in the hepatopancreas of the shrimp. The JNK signaling can activate the apoptotic factors Casp9 and Casp3 through various mechanisms, thereby inducing apoptosis (33, 34). In this study, the upregulation of Casp9 and Casp3 genes indicated that the apoptosis of the shrimp hepatopancreas was activated to cope with HT stress.
Metabolomics can quickly identify the physiological changes occurring in an organism by analyzing the alterations of metabolites. In this study, HT stress affected the metabolic function of the shrimp hepatopancreas, especially amino acid metabolism. Among them, changes in the metabolism of amino acids such as arginine, proline, and alanine might have been involved in immunity, osmotic regulation, and energy supply, while the metabolism of branched-chain amino acids and tryptophan might have supported protein repair and stress response. In addition, pentose conversion and glycerophospholipid metabolism affected carbohydrate utilization and membrane stability; adjustments in pantothenic acid and coenzyme A biosynthesis might have influenced stress resistance through energy metabolism. Similar phenomena also exist. For example, the amino acid metabolism in the hemolymph of the shrimp under heat stress at 33 °C for 72 h was also affected (1), but the specific types of amino acids affected were not completely the same as the results of our study, which might be related to different stress durations and tissue types.
Amino acids are crucial for maintaining the normal metabolism, physiological functions, and overall health of the organism. Tryptophan and its metabolites can regulate the immune response of aquatic animals and enhance their stress resistance (35). In this study, the increased of L-tryptophan, indole and 5-hydroxyindoleacetic acid, as well as the decreased of kynurenic acid, indicated that the tryptophan metabolism was involved in the response of the shrimp hepatopancreas to HT stress. Valine participates in protein synthesis, regulates blood sugar, supplies energy, and supports nervous system function (36). Methionine has the functions of synthesizing important biomolecules, detoxification and exerting antioxidant effects (37). In this study, the increased levels of L-valine and L-methionine might be the positive response of the shrimp hepatopancreas to HT stress, which was helpful to cope with the negative effects of the stress on the physiological homeostasis.
Lipids are essential for cellular energy supply, membrane integrity, and signaling (38). In this study, the glycerophospholipid metabolism pathway in the shrimp hepatopancreas was also disrupted. Glycerophospholipids are important lipid components of cell membranes (39). In this study, the decreased level of the glycerophosphocholine indicated that HT stress affected the homeostasis of the biological membranes of the hepatopancreas cells by influencing lipid homeostasis. Arachidonic acid and its metabolites are important immunoregulatory substances (40). In this study, the increased levels of lipoxin A4 and 12-keto-leukotriene B4 implyed that HT stress might also affect the immune homeostasis of the shrimp hepatopancreas through the metabolites of arachidonic acid.
Organic acids have physiological functions such as regulating energy metabolism and enhancing immunity. Cinnamic acid exhibits effects of regulating immunity, antioxidant activity, and anti-inflammation (41); subaphylline is a derivative of hydroxycinnamic acid (42). In this study, the increased levels of trans-cinnamate, 2-hydroxycinnamic acid, and subaphylline might contribute to enhancing the ability of the shrimp hepatopancreas to defend against high-temperature stress. 2-hydroxybutyric acid is involved in energy metabolism and immune regulation, and improves drug-induced liver injury (43). In this study, the elevated level of 2-hydroxybutyric acid might be beneficial for coping with the hepatopancreatic injury in the shrimp caused by HT stress. Fluctuations in vitamin levels were also closely related to the metabolic function of the hepatopancreas. In this study, after HT stress, the increased levels of folic acid, dethiobiotin, and pyridoxal phosphate might have been involved in key processes such as one-carbon unit transfer and amino acid metabolism under stress conditions, providing coenzyme support for cell repair and the synthesis of immune molecules. In contrast, the decreased level of retinol, a precursor of vitamin A might have been related to the increased demand for epithelial cell repair that it is involved in, and the accelerated consumption might have indirectly reflected the degree of damage to the hepatopancreas tissue.
5 Conclusion
This study revealed the negative effects of 33 °C HT stress on the hepatopancreas of L. vannamei. HT stress induced the structural damage to the hepatopancreatic tubules, such as distortion, atrophy, and even rupture. At the molecular level, HT stress activated stress responses (Nrf2, GPx, HSP70), and the resulting oxidative stress further induced the upregulation of genes related to inflammation (JNK, TNFα), apoptosis (Casp3, Casp9) and autophagy (Atg3, Atg16, Beclin1), thereby causing the disordered expression of immune genes. Additionally, metabolomic profiling indicated the disturbances in crucial metabolic pathways including amino acid metabolism, pentose interconversion, and glycerophospholipid metabolism, with tryptophan and arachidonic acid identified as potential biomarkers. We inferred that HT stress induced oxidative stress, caused immune dysregulation, activated inflammatory and cell death as well as autophagy pathways, led to hepatopancreatic metabolic disorders. This eventually triggered hepatopancreatic structural damage and the impairment of functional homeostasis (Figure 9).
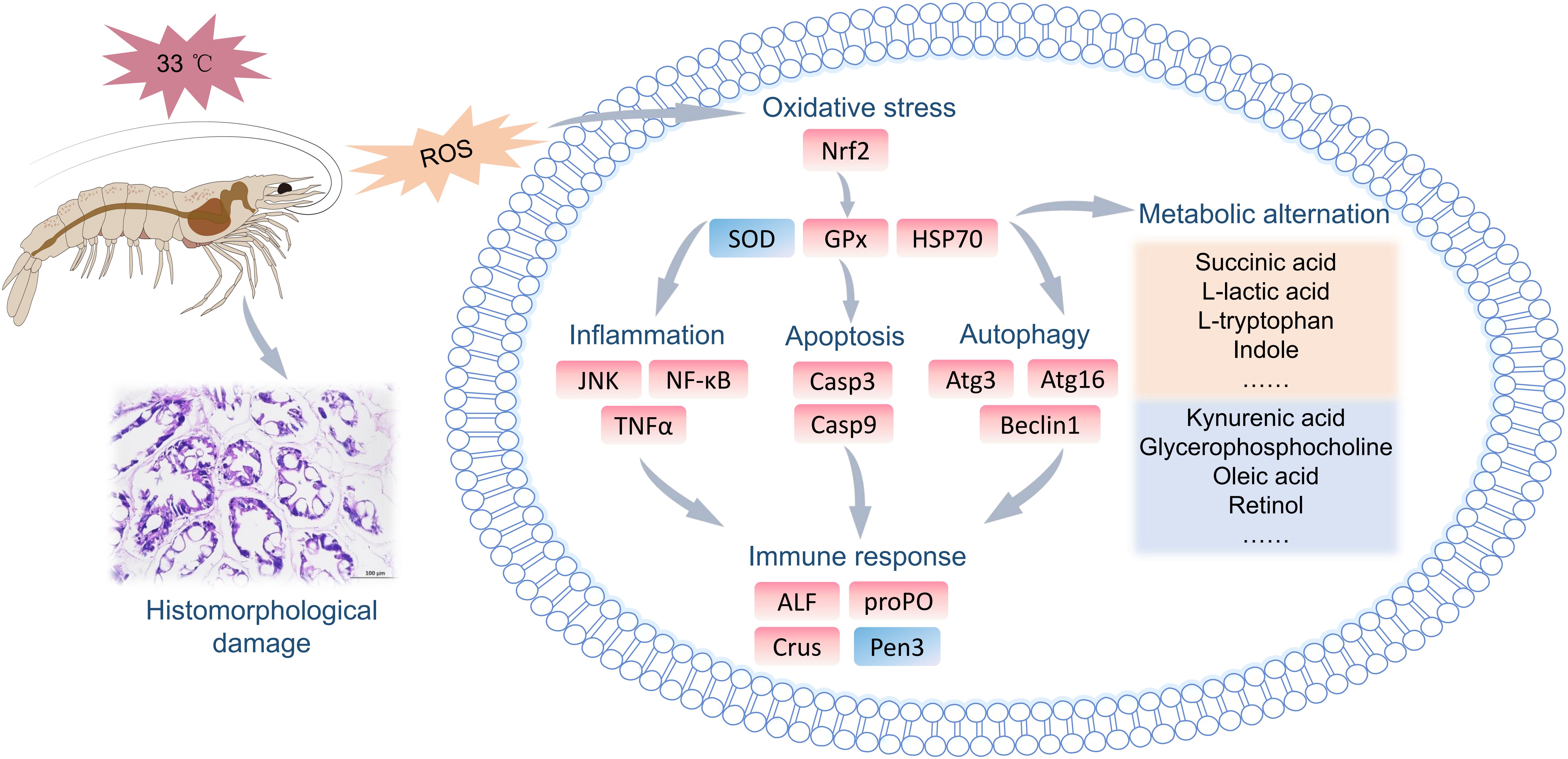
Figure 9. A mechanistic deduction of the effects of HT stress on the functional homeostasis of the hepatopancreas in L. vannamei.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Animal Care and Use Committee of the South China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YD: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MX: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – review & editing. YW: Conceptualization, Data curation, Methodology, Software, Writing – review & editing. JH: Conceptualization, Investigation, Methodology, Resources, Writing – review & editing. YY: Methodology, Resources, Software, Supervision, Writing – review & editing. HL: Conceptualization, Methodology, Resources, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the National Key Research and Development Program of China (2023YFD2401505); Guangdong Basic and Applied Basic Research Foundation (2024A1515030047); the Foundation of State Key Laboratory of Mariculture Biobreeding and Sustainable Goods (BRESG202404); Guangzhou Science and Technology Plan Project (2025D04J0016); Rural Revitalization Strategy Special Fund Seed Industry Revitalization Project of Guangdong Province (2022SPY02001, 2024SPY02001); Central Public-interest Scientific Institution Basal Research Fund, South China Sea Fisheries Research Institute, CAFS (2020KX03); Hainan Province Natural Science Foundation of China (323MS126); Agricultural Research Outstanding Talents Training Program (13210308); Central Public-interest Scientific Institution Basal Research Fund, CAFS (2023TD97). We thank the team members for their assistance in experimental analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1631655/full#supplementary-material
References
1. Duan YF, Xiong DL, Wang Y, Li H, Dong HB, and Zhang JS. Toxic effects of ammonia and thermal stress on the intestinal microbiota and transcriptomic and metabolomic responses of Litopenaeus vannamei. Sci Total Environ. (2021) 754:141867. doi: 10.1016/j.scitotenv.2020.141867
2. Zhu XY, Zheng XT, Xing YF, Huang JH, Dong HB, and Zhang JS. Study on tributyrin enhancing anti-periodic high temperature stress ability of gill tissue in Litopenaeus vannamei. South China Fish Sci. (2024) 20:66–75. doi: 10.12131/20230246
3. Bao ZM, Zou YF, Cao PH, Zhang JY, Xu Y, Xu ZQ, et al. Effect of high temperature stress on intestinal tissues morphology and transcriptome of Procambarus clarkii. South China Fish Sci. (2025) 21:105–17. doi: 10.12131/20240161
4. Rahman MM, Corteel M, Dantas-Lima JJ, Wille M, Alday-Sanz V, Pensaert MB, et al. Impact of daily fluctuations of optimum (27 °C) and high water temperature (33 °C) on Penaeus vannamei juveniles infected with white spot syndrome virus (WSSV). Aquaculture. (2007) 269:107–13. doi: 10.1016/j.aquaculture.2007.04.056
5. Dong HB, Su YQ, Mao Y, You XX, Ding SX, and Wang J. Dietary supplementation with Bacillus can improve the growth and survival of the kuruma shrimp Marsupenaeus japonicus in high-temperature environments. Aquac Int. (2014) 22:607–17. doi: 10.1007/s10499-013-9688-8
6. González-Ruiz R, Leyva-Carrillo L, Peregrino-Uriarte AB, and Yepiz-Plascencia G. The combination of hypoxia and high temperature affects heat shock, anaerobic metabolism, and pentose phosphate pathway key components responses in the white shrimp (Litopenaeus vannamei). Cell Stress Chaperones. (2023) 28:493–509. doi: 10.1007/s12192-022-01265-1
7. Hoang T, Ho HC, Le NPT, and Bui THH. Effects of high temperature on survival and feed consumption of banana shrimp Penaeus merguiensis. Aquaculture. (2020) 522:735152. doi: 10.1016/j.aquaculture.2020.735152
8. Jackson CJ and Wang YG. Modelling growth rate of Penaeus monodon Fabricius in intensively managed ponds: effects of temperature, pond age and stocking density. Aquac Res. (1998) 29:27–36. doi: 10.1111/j.1365-2109.1998.tb01358.x
9. You XX, Su YQ, Mao Y, Liu M, Wang J, Zhang M, et al. Effect of high water temperature on mortality, immune response and viral replication of WSSV infected Marsupenaeus japonicus juveniles and adults. Aquaculture. (2010) 305:133–7. doi: 10.1016/j.aquaculture.2010.04.024
10. Al-Masqari ZA, Guo H, Wang R, Yan H, Dong P, Wang G, et al. Effects of high temperature on water quality, growth performance, enzyme activity and the gut bacterial community of shrimp (Litopenaeus vannamei). Aquac Res. (2022) 53:3283–96. doi: 10.1111/are.15836
11. Cheng W, Wang LU, and Chen JC. Effect of water temperature on the immune response of white shrimp Litopenaeus vannamei to Vibrio alginolyticus. Aquaculture. (2005) 250:592–601. doi: 10.1016/j.aquaculture.2005.04.060
12. Wang FI and Chen JC. The immune response of tiger shrimp Penaeus monodon and its susceptibility to Photobacterium damselae subsp. damselae under temperature stress. Aquaculture. (2006) 258:34–41. doi: 10.1016/j.aquaculture.2006.03.043
13. Nandy T, Baag S, and Mandal S. Impact of elevated temperature on physiological energetics of Penaeus monodon post larvae: A mesocosm study. J Therm Biol. (2021) 97:102829. doi: 10.1016/j.jtherbio.2020.102829
14. Kır M, Sunar MC, Topuz M, and Sarıipek M. Thermal acclimation capacity and standard metabolism of the Pacific white shrimp Litopenaeus vannamei (Boone, 1931) at different temperature and salinity combinations. J Therm Biol. (2023) 112:103429. doi: 10.1016/j.jtherbio.2022.103429
15. Jiang S, Zhou FL, Yang QB, Huang JH, Yang LS, and Jiang SG. Impact of temperature stress on oxygen and energy metabolism in the hepatopancreas of the black tiger shrimp, Penaeus monodon (Crustacea: Decapoda: Penaeidae). Pak J Zool. (2019) 51:141–8. doi: 10.17582/journal.pjz/2019.51.1.141.148
16. Estrada-Cardenas P, Cruz-Moreno DG, Gonzalez-Ruiz R, Peregrino-Uriarte AB, Leyva-Carrillo L, Camacho-Jimenez L, et al. Combined hypoxia and high temperature affect differentially the response of antioxidant enzymes, glutathione and hydrogen peroxide in the white shrimp Litopenaeus vannamei. Comp Biochem Physiol D. (2021) 254:110909. doi: 10.1016/j.cbpa.2021.110909
17. Ulaje SA, Lluch-Cota SE, Sicard MT, Ascencio F, Cruz-Hernandez P, Racotta IS, et al. Litopenaeus vannamei oxygen consumption and HSP gene expression at cyclic conditions of hyperthermia and hypoxia. J Therm Biol. (2020) 92:102666. doi: 10.1016/j.jtherbio.2020.102666
18. Zhao Z, Liu Y, Wang B, Jiang K, Xu K, Zhong C, et al. A study on the effect of temperature training on compensatory growth and pathogen resistance of post-larval Litopenaeus vannamei. Aquac Int. (2024) 32:7387–411. doi: 10.1007/s10499-024-01520-5
19. Díaz F, Re AD, Sánchez A, Cruz H, González RA, Sánchez LN, et al. The effects of exposure to critical thermal maxima on the physiological, metabolic, and immunological responses in adult white shrimp Litopenaeus vannamei (Boone). Mar Freshw Behav Physiol. (2013) 45:365–74. doi: 10.1080/10236244.2013.771911
20. Duan YF, Wang Y, Zhang JS, and Xiong DL. Elevated temperature disrupts the mucosal structure and induces an immune response in the intestine of whiteleg shrimp Litopenaeus vannamei (Boone, 1931) (Decapoda: Dendrobranchiata: Penaeidae). J Crustacean Biol. (2018) 38:635–40. doi: 10.1093/jcbiol/ruy055
21. González-Ruiz R, Leyva-Carrillo L, Coronado Molina D, Hernández López J, and Yepiz-Plascencia G. Antioxidant stress response to fluctuations of dissolved oxygen and temperature in a semi-intensive aquaculture shrimp farm during high summer temperature. ISJ. (2023) 20:65–78.
22. Livak KJ and Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–ΔΔC(T)) method. Methods. (2001) 25:402–8. doi: 10.1006/meth.2001.1262
23. Liao G, Wang W, Yu J, Li J, Yan Y, Liu H, et al. Integrated analysis of intestinal microbiota and transcriptome reveals that a coordinated interaction of the endocrine, immune system and gut microbiota response to heat stress in Litopenaeus vannamei. Dev Comp Immunol. (2024) 156:105176. doi: 10.1016/j.dci.2024.105176
24. Shen CY, Zhang Z, Chen HG, Zhang LB, and Wang XF. Effects of LH crude oil and No.0 diesel oil emulsion on hepatopancreatic antioxidant enzyme activity and related functional gene expression in Litopenaeus vannamei. South China Fish Sci. (2025) 21:118–30. doi: 10.12131/20240153
25. Choi HY, Lee JH, Jegal KH, Cho IJ, Kim YW, and Kim SC. Oxyresveratrol abrogates oxidative stress by activating ERK-Nrf2 pathway in the liver. Chem-Biol Interact. (2016) 245:110–21. doi: 10.1016/j.cbi.2015.06.024
26. Zhang XX, Zhu HC, Yuan JB, Zhang XJ, Xiang JH, and Li FH. Diversity of heat shock proteins in response to various stressors in the Pacific white shrimp Litopenaeus vannamei. Aquaculture. (2024) 584:740647. doi: 10.1016/j.aquaculture.2024.740647
27. Wei YF, Ni LL, Pan JJ, Li XY, Xu B, Deng Y, et al. The roles of oxidative stress in regulating autophagy in methylmercury-induced neurotoxicity. Neuroscience. (2021) 469:175–90. doi: 10.1016/j.neuroscience.2021.06.026
28. Yang F, Liao XY, Wang LF, Xiong SL, and Wang SX. Importance and RNA interference of autophagy related genes in colorectal cancer based on medical thermal radiation imaging. Thermal Sci Eng Prog. (2025) 59:103283. doi: 10.1016/j.tsep.2025.103283
29. Dou J, Su P, Xu CC, Wen ZX, Mao ZX, and Li WM. Targeting Hsc70-based autophagy to eliminate amyloid β oligomers. Biochem Biophys Res Commun. (2020) 524:923–8. doi: 10.1016/j.bbrc.2020.02.016
30. Li HY, Li QY, Wang S, He JG, and Li CZ. Ammonia nitrogen stress increases susceptibility to bacterial infection via blocking IL-1R-Relish axis mediated antimicrobial peptides expression in shrimp. Aquaculture. (2023) 563:738934. doi: 10.1016/j.aquaculture.2022.738934
31. Sangsuriya P, Charoensapsri W, Chomwong S, Senapin S, Tassanakajon A, and Amparyup P. A shrimp pacifastin light chain-like inhibitor: molecular identification and role in the control of the prophenoloxidase system. Dev Comp Immunol. (2016) 54:32–45. doi: 10.1016/j.dci.2015.08.003
32. Wan F and Lenardo MJ. The nuclear signaling of NF-κB: current knowledge, new insights, and future perspectives. Cell Res. (2010) 20:24–33. doi: 10.1038/cr.2009.137
33. Ammendrup A, Maillard A, Nielsen K, Andersen NA, Serup P, Madsen OD, et al. The c-Jun amino-terminal kinase pathway is preferentially activated by interleukin-1 and controls apoptosis in differentiating pancreatic beta-cells. Diabetes. (2000) 49:1468–76. doi: 10.2337/diabetes.49.9.1468
34. Danial NN and Korsmeyer SJ. Cell death: critical control points. Cell. (2004) 116:205–19. doi: 10.1016/S0092-8674(04)00046-7
35. Hoseini SM, Pérez-Jiménez A, Costas B, Azeredo R, and Gesto M. Physiological roles of tryptophan in teleosts: current knowledge and perspectives for future studies. Rev Aquac. (2019) 11:3–24. doi: 10.1111/raq.12223
36. Zhao FY, Xu P, Xu GC, Huang DY, Zhang L, Ren MC, et al. Dietary valine affects growth performance, intestinal immune and antioxidant capacity in juvenile largemouth bass (Micropterus salmoides). Anim Feed Sci Technol. (2023) 295:115541. doi: 10.1016/j.anifeedsci.2022.115541
37. Liang H, Huang D, Ren M, Zhang L, Mi H, Aboseif AM, et al. A study on the function of methionine in growth, immunity, antioxidant, endoplasmic reticulum stress and apoptosis of largemouth bass (Micropterus salmoides). Aquacult Rep. (2024) 39:102531. doi: 10.1016/j.aqrep.2024.102531
38. Lee MC, Park JC, and Lee JS. Effects of environmental stressors on lipid metabolism in aquatic invertebrates. Aquat Toxicol. (2018) 200:83–92. doi: 10.1016/j.aquatox.2018.04.016
39. Duan YF, Zeng SM, Lu ZJ, Dan XM, Mo ZM, and Xing Y. Responses of lipid metabolism and lipidomics in the hepatopancreas of Pacific white shrimp Litopenaeus vannamei to microcystin-LR exposure. Sci Total Environ. (2022) 820:153245. doi: 10.1016/j.scitotenv.2022.153245
40. Sonnweber T, Pizzini A, Nairz M, Weiss G, and Tancevski I. Arachidonic acid metabolites in cardiovascular and metabolic diseases. Int J Mol Sci. (2018) 19:3285. doi: 10.3390/ijms19113285
41. Orzuna-Orzuna JF, Lara-Bueno A, Mendoza-Martínez GD, and Miranda-Romero LA. Meta-analysis of hydroxycinnamic acids into finishing lambs’ diet: growth performance, antioxidant status, and meat quality. Small Rumin Res. (2023) 223:106963. doi: 10.1016/j.smallrumres.2023.106963
42. Yang YL, Zhou G, Ding YN, Shi WJ, Chen YQ, Ge CB, et al. Microbiota dynamics and metabolic mechanisms in fermented sausages inoculated with Lactiplantibacillus plantarum and Staphylococcus xylosus. Food Res Int. (2025) 201:115680. doi: 10.1016/j.foodres.2025.115680
Keywords: shrimp, high temperature, immunity, metabolites, gene expression
Citation: Duan Y, Xiao M, Wang Y, Huang J, Yang Y and Li H (2025) The high temperature stress responses in the hepatopancreas of Litopenaeus vannamei: from immune dysfunction to metabolic remodeling cascade. Front. Immunol. 16:1631655. doi: 10.3389/fimmu.2025.1631655
Received: 20 May 2025; Accepted: 12 August 2025;
Published: 01 September 2025.
Edited by:
Magalí Rey-Campos, Spanish National Research Council (CSIC), SpainReviewed by:
Neeraj Kumar, National Institute of Abiotic Stress Management (ICAR), IndiaDoaa M. Mokhtar, Assiut University, Egypt
Copyright © 2025 Duan, Xiao, Wang, Huang, Yang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yafei Duan, ZHVhbnlhZmVpODlAMTYzLmNvbQ==
 Yafei Duan
Yafei Duan Meng Xiao1
Meng Xiao1 Yun Wang
Yun Wang Jianhua Huang
Jianhua Huang