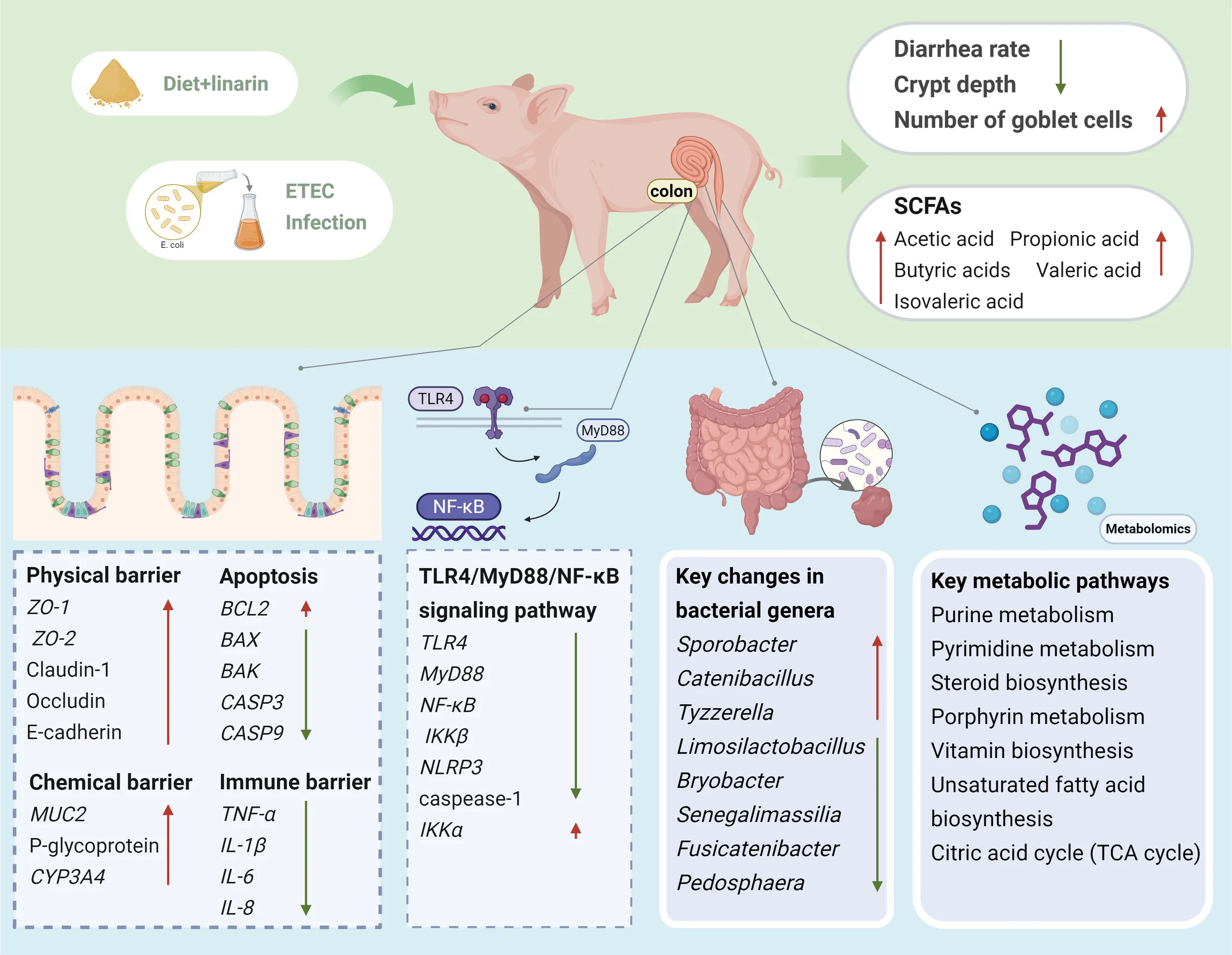
Abstract
Introduction:
Enterotoxigenic Escherichia coli (ETEC) is a globally recognized gastrointestinal pathogen and a major cause of diarrhea in neonatal and post‐weaning animals, leading to significant economic losses in pig production. Premature weaning disrupts colonic morphology and barrier integrity, resulting in diarrhea, dehydration, growth retardation, and increased mortality. Linarin, a natural flavonoid derived from wild chrysanthemum, exhibits antioxidant, sedative, and anti‐osteoporotic properties, demonstrating potential as a therapeutic agent and functional food ingredient.
Methods:
24 healthy 21‐day‐old weaned piglets (Duroc × Landrace × Large Yorkshire) were randomly assigned to four groups fed a basal diet (BD) or linarin-supplemented diet (LN) with oral infusion of 10 mL nutrient broth (NB) or 10⁹ colony-forming units/mL ETEC. Following a 3‐day acclimation period, piglets were fed the corresponding diet for 21 days; infusion with ETEC or NB was performed for 3 days on days 8 and 18. Colonic morphology, diarrhea incidence, gene expression, short-chain fatty acids (SCFAs), microbiota composition, and metabolomic profiles were assessed.
Results:
Linarin supplementation significantly ameliorated colonic crypt hyperplasia, increased goblet cell numbers, and decreased diarrhea incidence following ETEC infusion. It downregulated pro‐apoptotic and pro‐inflammatory gene expression while upregulating barrier‐associated genes. Linarin also significantly increased the concentrations of short-chain fatty acids (acetic, propionic, valeric, and isovaleric acids) in the colon. Integrated analysis of 16S rRNA gene sequencing and non-targeted metabolomics revealed that linarin modulated the intestinal microbiota by altering the relative abundance of key bacterial taxa (Pedosphaera, Fusicatenibacteria, Tyzerella, Sporobacteria, Limosilactobacillus, Senegalimassilia, Catenibacillus, and Bryobacteria), and associated metabolic pathways, including purine and pyrimidine metabolism; steroid, porphyrin, and vitamin biosynthesis; various amino acid and nucleotide metabolic processes; unsaturated fatty acid biosynthesis; and the citric acid cycle.
Discussion:
These findings indicate that linarin restores colonic barrier function and intestinal microbiota homeostasis, enhancing resistance to ETEC infection along with the development and well-being of piglets after weaning. This study offers a new mechanistic understanding of how linarin confers protection against ETEC, which can promote its widespread application as a natural feed additive to replace antibiotics.
1 Introduction
The premature weaning of piglets often disrupts the colonic morphology and impairs intestinal barrier function, leading to diarrhea, dehydration, growth retardation, and increased mortality (1). In particular, post-weaning diarrhea (PWD) causes slow growth and a decreased feed conversion rate in piglets, resulting in serious economic losses and reduced production efficiency in the swine industry (1). Enterotoxigenic Escherichia coli (ETEC) is a major gastrointestinal pathogen that impairs intestinal function, which is a leading cause of diarrhea in neonatal and post-weaning animals. ETEC infection can lead to oxidative stress, disruption of intestinal barrier integrity (2), and cell death and tissue damage (3). ETEC adheres to intestinal epithelial cells using a colonization factor to colonize the small intestine of humans and animals, especially piglets and calves (4). As ETEC infection is one of the major causes of economic losses in pig production worldwide, safe and alternative treatment methods are needed.
ETEC K88 has F4 pili, facilitating binding to specific receptors on the surface of the pig intestine (5). The intestinal microbiota consists of a wide range of bacteria in the intestinal tract, mainly in the colon (6). The intestinal microbiota contribute to formation of the intestinal barrier; thus regulating the equilibrium of colonic microbial communities can help maintains the balance of nutrient metabolism, prevents disease, and achieve healthy growth (7). The health status of the gut determines the health status of the animal and is closely related to its production level and efficiency. Therefore, it is important to discover effective functional nutrients to mitigate intestinal damage in piglets.
Medicinal and dietary homology is a foundational concept in traditional Chinese medicine, which refers to the dual functionality of natural substances that serve both therapeutic and nutritional purposes (8). Linarin, a natural flavonoid abundant in wild chrysanthemums (9), has received increasing attention for its pharmacological activities. Linarin glycosides possess antioxidant properties, sleep-promoting and sedative effects, and anti-osteoporotic activity (10). One study showed that linarin had anti-inflammatory effects by inhibiting activation of the TXNIP/NLRP3 inflammasome, NF-κB pathway, and the release of various pro-inflammatory cytokines such as interleukin (IL)-1β, tumor necrosis factor (TNF)-α, and IL-6 (11). Another study showed that linarin reduced the expression level of Toll-like receptor 4 (TLR4) and its downstream factors MyD88, IRAK1, and TRAF6, thereby reversing the excessive phosphorylation of ERK, p38, and JNK to ultimately inhibit the inflammatory signaling cascade (12). Notably, linarin alleviated dextran sulfate sodium-induced colitis in mice (13). Our previous studies showed that ETEC infection disrupts the gut microbiota and activates TLR4/MyD88/NF-κB signaling, which causes gut inflammation in piglets (14). In addition, ETEC exposure may activate the Nrf2 antioxidant pathway as a compensatory response to infection-driven oxidative stress, potentially mitigating intestinal damage (14). To our knowledge, no study has directly assessed the effects of linarin on the intestinal barrier integrity of weaned piglets. To fill this gap, the aim of this study was to explore the potential of linarin to inhibit ETEC infection, consequently improving the intestinal health and breeding benefits of weaned piglets. Toward this end, we used a weaned piglet model infected with ETEC to investigate the effects of linarin on the diarrhea rate, histomorphology, barrier function, apoptosis, intestinal microbiota, and metabolic pathways, offering a potential alternative to conventional antibiotic-based treatments for ETEC-induced diarrhea.
2 Materials and methods
2.1 Animal ethics
The experimental animals (piglets) used in this study were privately owned. Animal protocols received approval from Experimental Animal Ethics Committee of Anhui Science and Technology University (approval no AHSTU2023006), and were strictly followed local regulations and relevant institutional norms. Animals participating in this study have obtained written informed consent from their owners.
2.2 Bacterial strains
Linarin (Chengdu Zhibiaohua Pure Biotechnology Co., Ltd., Chengdu, China; CAS No. 480-36-4, HPLC ≥ 90%). The E. coli F4 (K88 ac) strain was obtained from CVCC1500 (China Veterinary Culture Collection Center).
2.3 Animal experiment design
Twenty-four healthy (Duroc × Landrace × Large Yorkshire; average initial body weight: 6.45 ± 0.18 kg) weaned piglets at 21 days of age were selected and randomly divided into four treatment groups, each with 6 piglets (6 replicates, half male and half female), and reared in separate pens, while there was no significant difference in the initial body weight of the piglets among the treatment groups. The treatment groups were set up as follows: (i) Control group (BD+NB), basal diet with oral nutrient broth for piglets; (ii) linarin group (LN+NB), basal diet with 150 mg/kg linarin; (iii) ETEC group (BD+ETEC), basal diet with oral ETEC solution; (iv) linarin + ETEC group (LN+ETEC), basal diet with 150 mg/kg linarin, with oral ETEC solution. The experimental design included a 3-day acclimation phase preceding a 21-day trial period. During the experimental phase, piglets in the BD+ETEC and LN+ETEC groups received oral administration of 10 mL ETEC suspension (109 CFU/mL) on days 8 and 18, while those in the BD+NB group were given 10 mL nutrient broth daily for three consecutive days (Figure 1). The linarin dosage was determined based on previous studies in mouse colitis models, with conversion to piglet-equivalent doses using surface area normalization (13, 15). The ETEC challenge dose and duration was established according to our previous research (16). Feed and water were available for piglets to consume freely during the test period. After the test ended, colon samples from the piglets were collected and maintained for later investigation. The experimental diet was provided in powdered form, diet composition and nutrients are detailed in Supplementary Table S1.
Figure 1

Design of animal experiments. BD, basal diet; LN, basal diet with linarin (150 mg/kg); NB, nutrient broth.
2.4 Diarrhea rate of piglets
Pre feeding period was 3 days, and the feeding trial was 21 days, the number of piglets with diarrhea was observed and the diarrhea score was recorded every day (0 = normal, hard feces; 1 = soft, probably mild diarrhea; 2 = apparently unformed, moderate diarrhea; 3 = feces very much watery and foamy, severe diarrhea), via the formula below. Refer to previous articles published in our laboratory (16),
2.5 Sample collection and processing
On the 25th day, piglets were treated humanely through the administration of sodium pentobarbital (40 mg per kilogram of body weight), after which dissection was performed. Blood was collected and allowed to clot at room temperature, then spun at high speed (3500 rpm) for 15 minutes. Centrifugation was carried out to yield serum, which was stored at -20°C. In addition, colonic tissue was collected from all piglets, and segments approximately 3 cm from the center of each colon were immediately isolated, rinsed with saline, and fixed in 4% paraformaldehyde solution for morphological examination and immunohistochemical analysis.
2.6 Morphological analysis of the colon
Colon specimens were fixed in 4% paraformaldehyde (pH 7.4) for 24 h, paraffin-embedded, and sectioned at 4 μm thickness. Sequential staining with hematoxylin & eosin (H&E) and Alcian blue periodic acid-Schiff staining (AB-PAS) was performed to evaluate crypt architecture and goblet cell density. For quantitative analysis, four representative fields per section meeting selection criteria (intact mucosal surface, vertically oriented crypts) were imaged at 200× magnification. Using ImageJ software (version 1.53; National Institutes of Health, Bethesda, MD, USA, crypt depth (measured from crypt base to luminal opening) and goblet cell counts (quantified as AB-PAS+ cells per crypt) were assessed in 30 intact crypts per sample.
2.7 Real-time PCR
Colon tissue RNA was extracted using the RNAprep Pure Tissue Kit (Tiangen Biotech, Beijing, China; Cat. No. DP431), followed by genomic DNA elimination, and 4 μL (1000 ng) of total RNA was reverse transcribed with HiScript III RT SuperMix (Vazyme Biotech, Nanjing, China; Cat. No. R323-01). QuantStudio 5 system (Applied Biosystems, USA) equipped with ChamQ SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd., Nanjing, China; Cat. No. Q311-02) was used to conduct qPCR amplification. Primers were designed with reference to previously published studies from our research team (14, 16), utilizing NCBI Primer−BLAST, the designed primers were validated for specificity using BLAST. Standard-curve efficiency analysis was not performed in this study. Instead, amplification specificity and efficiency consistency were verified through melt-curve and Ct analyses. Each primer pair produced a single, well-defined melt-curve peak without nonspecific amplification or primer-dimer formation. The Ct values for individual genes remained within a narrow and consistent range across biological replicates, supporting the assumption of comparable amplification efficiency among reactions. Therefore, Relative mRNA expression was calculated using the 2−ΔΔCt method, with the control group serving as the calibrator and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin as endogenous controls.
2.8 Determination of SCFAs
Colonic chyme samples were analyzed for short-chain fatty acid (SCFAs) encompassing acetate, propionate, butyrate, isovaleric, and valeric acids. Fresh samples were homogenized with ddH2O, centrifuged (10,000 rpm, 10 min), and supernatants were acidified with 25% metaphosphoric acid (4°C, 2 h), followed by centrifugation (20,000 × g, 10 min, 4°C). The 0.45 μm filtered supernatants were measured employing the Agilent 6890 Gas Chromatography system (manufactured by Agilent Technologies, United States), with concentrations expressed as mg/g.
2.9 Intestinal microbiota analysis
The intestinal microbiota was analyzed following the methods described in previous studies (17, 18). Microbial DNA was extracted from colonic samples using the E.Z.N.A.® Stool DNA Kit (Omega Bio-tek, Norcross, GA, U.S.; Cat. No. D4015-02) according to manufacturer’s protocols. The V4-V5 regions of bacterial 16S rRNA genes were amplified using primers 341F (CCTACGGGNGGCWGCAG) and 806R (GGACTACHVGGGTATCTAAT), with unique 8-bp barcodes for each sample. Paired-end sequencing (2×250 bp) was conducted on an Illumina MiSeq platform. Raw sequencing data are available in the NCBI SRA database (BioProject: PRJNA1263251; BioSample: SAMN48516502; SRA: SRR33580208). Microbial diversity was evaluated using Chao, ACE, and Shannon indices.
2.10 LC-MS-based untargeted metabolomics
Metabolites were extracted from colon tissues, serum, microbial cultures using prechilled 80% methanol with 0.1% formic acid (19, 20). Tissue samples (100 mg) were homogenized in liquid nitrogen, while liquid samples (100 μL) and cell/bacterial pellets were mixed with methanol, vortexed, and centrifuged (15,000 rpm, 4°C) (21). Supernatants were diluted to 53% methanol, filtered, and analyzed via UHPLC-MS/MS (Vanquish system; Hypersil Gold C18 column) under a 17-min gradient (22). Mass spectrometry (Orbitrap Q Exactive™ HF) operated in positive/negative modes with a spray voltage of 3.2 kV and a capillary temperature of 320°C was employed, and a m/z 100–1500 scan range.
Raw data were processed using Compound Discoverer 3.1 for peak alignment (mass tolerance: 5 ppm; S/N ≥ 3), annotated via mzCloud, HMDB, and LIPID Maps databases. Significant metabolic markers (VIP: ≥ 1, P-value: < 0.05, at least a 2-fold increase or a 0.5-fold decrease) were identified using PCA/PLS-DA (metaX) and enriched in KEGG pathways (P < 0.05).
2.11 Statistical analysis
Data were first tested for normality and transformed as needed. Two-way ANOVA was used to assess group differences. For non-normally distributed data (assessed by Shapiro-Wilk test, P < 0.05), including gut microbiota relative abundance, we applied the Kruskal-Wallis test. For the Pearson correlation analysis between microbial taxa and metabolites (or other variables), multiple testing correction was applied using the False Discovery Rate (FDR) method (Benjamini-Hochberg procedure), with significance thresholds set at P < 0.05 (statistically significant) and P < 0.01 (extremely statistically significant). Data analysis used SPSS 26.0, while GraphPad Prism 10.0 created visualizations.
3 Result
3.1 Effects of linarin on the ETEC-induced diarrhea rate
As shown in Table 1, piglets in the LN+ETEC group exhibited significantly lower diarrhea rates than those in the BD+ETEC group at 1–10 d, 11–20 d, and 1–20 d (P < 0.01), whereas no significant difference in diarrhea rate was observed between the LN+NB and BD+NB groups (P > 0.05).
Table 1
| Measure (%) | Experimental diet | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| BD+NB | LN+NB | BD+ETEC | LN+ETEC | Diet | ETEC | Interaction | ||
| 1-10d | 5.25b | 3.70b | 10.49a | 4.94b | 0.77 | 0.36 | <0.01 | 0.10 |
| 11-20d | 2.99b | 3.09b | 9.26a | 5.56b | 0.68 | 0.94 | <0.01 | 0.05 |
| 1-20d | 4.14bc | 3.40c | 9.88a | 5.24b | 0.59 | 0.39 | <0.01 | <0.01 |
Effect of linarin on diarrhea rate in weaned piglets after ETEC infection.
a,b,cValues with different superscripts in the row indicate significant differences (P < 0.05).
3.2 Effects of linarin and ETEC on colonic histomorphology and goblet cells
There was no significant difference in crypt depth between the BD+NB and LN+NB groups (P > 0.05) (Figure 2C). However, the crypt depth in the LN+ETEC group was decreased compared to that of the BD+ETEC group (P < 0.01) (Figure 2C). In addition, compared to the BD+NB group, goblet cells numbers in the LN+NB group were increased (P < 0.01) (Figure 2D). Similarly, goblet cell numbers in the BD+ETEC group were increased compared to those of the BD+ETEC and LN+ETEC groups (P < 0.01) (Figure 2D).
Figure 2

Effects of dietary linarin supplementation on colonic histomorphology and goblet cells of weaned piglets after ETEC infection. (A) The colon tissue was subjected to representative hematoxylin and eosin (HE) staining. (C) Crypt depth, 100 μm. (D) Goblet cell count. BD+NB, piglets received the basal diet + oral nutrient broth; LN+NB, piglets received the basal diet supplemented with 150 mg/kg linarin + oral nutrient broth; BD+ETEC, ETEC-challenged piglets fed the basal diet; LN+ETEC, piglets with ETEC infection fed the basal diet + 150 mg/kg linarin. Significance thresholds are set at P < 0.05 (statistically significant) and P < 0.01 (extremely statistically significant). *: P < 0.05 (statistically significant), **: P < 0.01 (highly statistically significant).
3.3 Effects of linarin and ETEC on the colonic physical barrier
The mRNA expression levels of ZO-1, ZO-2, claudin-1, occludin, and E-cadherin were increased in the LN+NB group compared to those of the BD+NB group (P < 0.01). Similarly, compared to those of the BD+ETEC group, the mRNA expression levels of ZO-1, ZO-2, E-cadherin (P < 0.01), and occludin (P < 0.05) were increased in the LN+ETEC group (Figure 3A).
Figure 3

Effects of dietary linarin supplementation on colonic barrier integrity and inflammatory signaling in weaned piglets after ETEC challenge. The mRNA expresssion levels of (A) physical barrier-related genes (ZO-1, ZO-2, claudin-1, occludin, E-cadherin), (B) apoptosis regulators (BCL2, BAX, CASP3, CASP9), (C) chemical barrier-related genes (MUC2, P-glycoprotein, CYP3A4), (D) immune barrier-related genes (TNF-α, IL-1β, IL-6, IL-8), and (E) TLR4/MyD88/NF-κB pathway genes (TLR4, MyD88, NF-κB, NLRP3, IκBα, IKKα, IKKβ, caspase-1). BD+NB, piglets received the basal diet + oral nutrient broth; LN+NB, piglets received the basal diet supplemented with 150 mg/kg linarin + oral nutrient broth; BD+ETEC, ETEC-challenged piglets fed the basal diet; LN+ETEC, piglets with ETEC infection fed the basal diet + 150 mg/kg linarin. Significance thresholds are set at P < 0.05 (statistically significant) and P < 0.01 (extremely statistically significant). *: P < 0.05 (statistically significant), **: P < 0.01 (highly statistically significant).
3.4 Effects of linarin and ETEC on apoptosis gene expression in colonic epithelial cells
Compared to that of the BD+NB group, the mRNA expression level of BCL2 (P < 0.01) was increased, whereas the levels of BAX (P < 0.01), BAK, CASP3, and CASP9 (P < 0.05) were significantly decreased in the LN+NB group. Similarly, compared to that of the BD+ETEC group, the mRNA expression level of BCL2 was increased (P < 0.01), whereas the levels of BAX, CASP3, CASP9 (all P < 0.01), and BAK (P < 0.05) were significantly decreased in the LN+ETEC group (Figure 3B).
3.5 Effects of linarin and ETEC on the colonic chemical barrier
The mRNA expression levels of MUC2, P-glycoprotein, and CYP3A4 were significantly higher in the LN+NB group than in the BD+NB group (P < 0.01). The mRNA expression levels of MUC2 (P < 0.01), P-glycoprotein, and CYP3A4 (P < 0.05) were also significantly higher in the BD+ETEC group compared to those of the LN+ETEC group (Figure 3C).
3.6 Effects of linarin and ETEC on the colonic immune barrier
Compared with those of the BD+NB group, the mRNA expression levels of TNF-α, IL-1β (P < 0.01), IL-6, and IL-8 (P < 0.05) were decreased in the LN+NB group. Similarly, compared to those of the BD+ETEC group, the LN+ETEC group had decreased levels of IL-1β, TNF-α, IL-6, and IL-8 (P < 0.05 or P < 0.01) (Figure 3D).
3.7 Effects of linarin and ETEC on genes involved in the TLR4/MyD88/NF-κB signaling pathway
Compared with those of the BD+NB group, the mRNA expression levels of TLR4, MyD88, NF-κB, NLRP3, IKKα, and caspase-1 were significantly decreased (P < 0.05), whereas the level of IκBα was increased (P < 0.01) in the LN+NB group. Compared with those of the BD+ETEC group, the mRNA expression levels of MyD88, NF-κB, IKKα, IKKβ (P < 0.01), TLR4, NLRP3, and caspase-1 (P < 0.05) were decreased, whereas the level of IκBα was increased (P < 0.01) in the LN+ETEC group (Figure 3E).
3.8 Effects of linarin and ETEC on colonic short-chain fatty acids
As shown in Table 2, relative to the BD+NB group, the LN+NB group exhibited a highly significant (P < 0.01) increase in the levels of acetic, propionic, and butyric acids, along with a significant (P < 0.05) increase in the level of isovaleric acid. However, the concentration of valeric acid did not differ between the two groups. Compared with those of the BD+ETEC group, the LN+ETEC group showed increased levels of acetic, propionic, butyric, valeric (P < 0.01), and isovaleric (P < 0.05) acids.
Table 2
| Measure | Experimental diet | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| BD+NB | LN+NB | BD+ETEC | LN+ETEC | Diet | ETEC | Interaction | ||
| Acetate acid | 50.41b | 58.94a | 43.05c | 55.01b | 1.29 | <0.01 | <0.01 | 0.05 |
| Propionic acid | 19.50b | 22.50a | 16.36c | 19.78b | 0.57 | <0.01 | <0.01 | 0.77 |
| Butyric acid | 5.59b | 9.10a | 4.38c | 7.52b | 0.40 | <0.01 | <0.01 | 0.56 |
| Isovaleric acid | 1.66b | 2.16 a | 1.18c | 1.42b | 0.10 | 0.01 | 0.04 | 0.70 |
| Valeric acid | 1.23 b | 1.28b | 1.16b | 2.30a | 0.12 | 0.8 | <0.01 | <0.01 |
Effects of linarin on colonic short-chain fatty acid levels in piglets after ETEC infection.
a,b,cValues with different superscripts in the row indicate significant differences (P < 0.05).
3.9 Effects of linarin and ETEC on the colonic biological barrier
The ACE index for the LN+NB group was significantly (P < 0.05) reduced compared to that of the BD+NB group, whereas no notable alterations were observed in the observed species, Chao1, Shannon, and Simpson indices between these two groups (P > 0.05) (Figure 4A). Principal Coordinates Analysis (PCoA) and Non-metric Multidimensional Scaling (NMDS) analysis indicated significant differences in the structural features of the gut microbiota composition between the BD+NB and LN+NB groups (Figures 4B, C). Firmicutes and Bacteroidota were the dominant phyla, followed by Euryarchaeota, Proteobacteria, and Spirochaetota (Figure 4D). A total of 20 families (Figure 4E) and 26 genera (Figure 4F) had a major contribution to the observed differences, based on a relative abundance exceeding 1%. At the genus level, the relative abundances of Sarcina, Roseimarinus, Fibrobacter, and Mesoplasma were higher in the LN+NB group than in the BD+NB group (P < 0.05), whereas Tyzzerella, Ralstonia, Anaerofilum, Bose (P < 0.01), Catenibacillus, and Faecalibacterium decreased in abundance with linarin supplementation (P < 0.05) (Figure 4G).
Figure 4

Effects of dietary linarin on weaned piglet microbiota and colonic barrier gene correlations. (A) Alpha diversity (ACE, Chao1, Shannon, and Simpson indices). (B) Principal coordinate analysis (PCoA). (C) Nonmetric multidimensional scaling (NMDS) analysis. Composition of the gut microbiome (>1% relative abundance) at the (D) phylum, (E) family, and (F) genus levels. (G) Intergroup genus-level abundance differences. BD+NB, piglets received the basal diet + oral nutrient broth; LN+NB, piglets received the basal diet supplemented with 150 mg/kg linarin + oral nutrient broth; BD+ETEC, ETEC-challenged piglets fed the basal diet; LN+ETEC, piglets with ETEC infection fed the basal diet + 150 mg/kg linarin. Significance thresholds are set at P < 0.05 (statistically significant) and P < 0.01 (extremely statistically significant). *: P < 0.05 (statistically significant), **: P < 0.01 (highly statistically significant).
Linarin supplementation did not have an influence on the microbiota diversity in the context of ETEC infection, with no significant differences in any of the diversity indices (observed species, Chao1, ACE, Shannon, Simpson) noted between the LN+ETEC and BD+ETEC groups (all P > 0.05) (Figure 5A). Nevertheless, PCoA and NMDS analysis revealed distinct gut microbiota structures between the BD+ETEC and LN+ETEC groups (Figures 5B, C). Firmicutes and Bacteroidota were the dominant phyla, followed by Euryarchaeota, Proteobacteria, and Spirochaetota (Figure 5D). A total of 21 families (Figure 5E) and 26 genera (Figure 5F) had major contributions to the differences, with relative abundances exceeding 1%. At the genus level, the abundances of Sporobacter, Catenibacillus, and Tyzzerella were significantly (P < 0.05) higher, whereas the abundances of Limonolobacillus, Bryobacter, Senecamassilia, Fusicatenibacter, and Pedosphaera were significantly (P < 0.05) lower in the LN+ETEC group compared to the BD+ETEC group (Figure 5G).
Figure 5

Effects of dietary linarin on weaned piglet microbiota and colon barrier gene correlations after ETEC infection. (A) Alpha diversity (ACE, Chao1, Shannon, and Simpson indices). (B) Principal coordinate analysis (PCoA). (C) Nonmetric multidimensional scaling (NMDS) analysis. Composition of the gut microbiome (>1% relative abundance) at the (D) phylum, (E) family, and (F) genus levels. (G) Intergroup genus-level abundance differences. BD+NB, piglets received the basal diet + oral nutrient broth; LN+NB, piglets received the basal diet supplemented with 150 mg/kg linarin + oral nutrient broth; BD+ETEC, ETEC-challenged piglets fed the basal diet; LN+ETEC, piglets with ETEC infection fed the basal diet + 150 mg/kg linarin. Significance thresholds are set at P < 0.05 (statistically significant) and P < 0.01 (extremely statistically significant). *: P < 0.05 (statistically significant), **: P < 0.01 (highly statistically significant).
3.10 Non-targeted metabolomic analysis
As shown in the volcano diagram in Figure 6A, we found 1709 differentially expressed metabolites between the BD+NB and LN+NB groups, including 17 upregulated metabolites and 26 downregulated metabolites, following linarin supplementation. Based on Bray–Curtis distances, the NMDS analysis and PLS-DA showed that the microbial community structures of the BD+NB and LN+NB groups differed substantially (Figures 6B, C). The lollipop chart in Figure 6D shows that 12 of the differentially expressed metabolites, including glycitin and lycotein, were significantly upregulated, whereas 18 of the differentially expressed metabolites, including daidzein, genistein, (R)-equol, and anacardic acid, were significantly downregulated with linarin supplementation. KEGG enrichment analysis demonstrated that dietary linarin significantly modulated 31 key metabolic pathways, including biosynthesis of cofactors, oxidative phosphorylation, carbon metabolism, and methionine metabolism (Figure 6E).
Figure 6

Effects of dietary linarin on the metabolites in the colonic chyme of weaned piglets. (A) Volcano plots indicating significant metabolites [log2 fold change (FC) ≥ 1; P < 0.05]. (B) Non-metric multidimensional scaling (NMDS) analysis. (C) Partial least-squares discriminant analysis (PLS-DA). (D) Differential metabolite lollipop chart. (E) Differential metabolite KEGG enrichment map.
The volcano diagram in Figure 7A shows the 1709 differentially expressed metabolites between the BD+ETEC and LN+ETEC groups, including 82 upregulated metabolites and 90 downregulated metabolites. According to Bray–Curtis distances, the NMDS analysis and PLS-DA identified significant variations in microbial community composition between these two groups (Figures 7B, C). The lollipop chart in Figure 7D demonstrates that 14 differentially expressed metabolites, including biochanin A, monobenzyl phthalate, and glycitein, showed significant upregulation, whereas 12 differentially expressed metabolites, including calcitriol, 2-methoxyestradiol, and ouabain, showed significant downregulation after linarin supplementation in the context of ETEC infection. KEGG enrichment analysis showed that linarin with ETEC infection significantly modulated 30 key metabolic pathways, including the glutathione and sphingolipid metabolism pathways (Figure 7E).
Figure 7

Effects of linarin on the metabolic products of the colonic chyme. (A) Volcano plots indicating significant metabolites [log2 fold change (FC) ≥ 1; P < 0.05]. (B) Non-metric multidimensional scaling (NMDS) analysis. (C) Partial least-squares discriminant analysis (PLS-DA). (D) Differential metabolite lollipop chart. (E) Differential metabolite KEGG enrichment map.
3.11 Correlation analysis
3.11.1 Correlations among the gut microbiome, intestinal barrier function, apoptosis, and TLR4 signaling pathway
Correlation analysis was performed to explore the interactions among dietary linarin-induced alterations in the gut microbiota, colonic barrier function, apoptosis, and TLR4 signaling pathway in weaned piglets. Bosea abundance was positively correlated with ZO-1 and BAX mRNA expression, whereas it was negatively correlated with ZO-2 expression (P < 0.05). The relative abundance of Catenibacillus was positively correlated with ZO-2, IL-6, NF-κB, and IκBα expression levels, whereas it was negatively correlated with BCL2, CASP3, MUC2, NLRP3, caspase-1, and IKKβ mRNA expression levels (P < 0.05). The relative abundance of Faecalibacterium was positively correlated with MUC2 expression, but was negatively correlated with ZO-1, BCL2, CASP3, P-glycoprotein, TNF-α, and IL-1β mRNA expression levels (P < 0.05). The relative abundance of Fibrobacter was positively correlated with claudin-1, MUC2, caspase-1, and IKKα mRNA expression levels and was negatively correlated with IKKβ expression (P < 0.05). The relative abundance of Mesoplasma was positively correlated with claudin-1, BAK, CASP3, CASP9, CYP3A4, and TNF-α expression, whereas it was negatively correlated with E-cadherin, BCL2, BAX, MUC2, P-glycoprotein, IL-6, and MyD88 mRNA expression levels (P < 0.05). Roseimarinus abundance was positively associated with BAX, IL-1β, and TLR4 expression, but was negatively correlated with CASP3 and TNF-α expression (P < 0.05). The relative abundance of Sarcina was positively correlated with ZO-1, IL-8, and IκBα levels, whereas it was negatively correlated with claudin-1, E-cadherin, MUC2, P-glycoprotein, CYP3A4, and MyD88 mRNA expression levels (P < 0.05). The relative abundance of Tyzzerella was positively correlated with E-cadherin, CASP9, CYP3A4, NLRP3, MyD88, and IKKα expression and was negatively correlated with MUC2, IL-1β, caspase-1, and TLR4 mRNA expression levels (P < 0.05) (Figure 8A).
Figure 8

Correlations between microbiota abundance at the genus level and intestinal barrier/apoptosis/TLR4 signaling pathway-related gene expression in weaned piglets. (A) BD+NB vs. LN+NB comparison. (B) BD+ETEC vs. LN+ETEC comparison. BD+NB, piglets received the basal diet + oral nutrient broth; LN+NB, piglets received the basal diet supplemented with 150 mg/kg linarin + oral nutrient broth; BD+ETEC, ETEC-challenged piglets fed the basal diet; LN+ETEC, piglets with ETEC infection fed the basal diet + 150 mg/kg linarin. Significance thresholds are set at P < 0.05 (statistically significant) and P < 0.01 (extremely statistically significant).
Under ETEC infection with and without linarin supplementation, Bryobacter abundance showed a positive correlation with ZO-1, ZO-2, BAX, CASP9, IL-8, and MyD88 expression, whereas it was negatively correlated with TLR4 and NF-κB expression (P < 0.05). The relative abundance of Catenibacillus was positively correlated with ZO-2, BCL2, TNF-α, NLRP3, TLR4, NF-κB, and IKKα expression, but was negatively correlated with claudin-1, CASP3, caspase-1, and MyD88 mRNA expression levels (P < 0.05). Fusicatenibacter abundance was positively correlated with CYP3A4, IL-1β, and IL-6 expression, whereas it was negatively correlated with ZO-1 expression (P < 0.05). The relative abundance of Limosillactobacillus was positively correlated with BCL2, BAK, TNF-α, NLRP3, and NF-κB expression, whereas it was negatively correlated with claudin-1, CASP3, MUC2, P-glycoprotein, caspase-1, and MyD88 mRNA expression levels (P < 0.05). The relative abundance of Pedosphaera was positively correlated with ZO-1, E-cadherin, BCL2, MUC2, CYP3A4, TNF-α, IL-8, and IKKβ mRNA levels, but was negatively correlated with CASP9, P-glycoprotein, IL-6, NLRP3, and IKKα mRNA expression levels (P < 0.05). Senegalimassilia abundance was positively correlated with BAX, MUC2, CYP3A4, and IκBα, but was negatively correlated with BAK, CASP3, P-glycoprotein, IL-6, IKKα, and IKKβ mRNA expression (P < 0.05). The relative abundance of Sporobacter was positively correlated with E-cadherin, IL-1β, IL-8, MyD88, and IKKα, whereas it was negatively correlated with ZO-2, claudin-1, BAK, CASP9, NLRP3, and TLR4 mRNA expression levels (P < 0.05). The relative abundance of Tyzzerella was positively correlated with P-glycoprotein, NLRP3, TLR4, and MyD88, but showed an inverse relationship with ZO-1, occludin, caspase-1, and IκBα mRNA expression levels (P < 0.05) (Figure 8B).
3.11.2 Correlations among microorganisms and metabolic pathways
Correlation analysis was further conducted to examine the associations of the dietary linarin-induced alterations in gut microbiota with differential metabolites and metabolic pathways in weaned piglets. The relative abundance of Faecalibacterium was positively correlated with tyrosine metabolism, arginine biosynthesis, citrate cycle [i.e., the tricarboxylic acid (TCA) cycle], pyruvate metabolism, alanine/aspartate/glutamate metabolism, amino/nucleotide sugar metabolism, and cysteine/methionine metabolism. The relative abundance of Bosea was negatively correlated with arginine biosynthesis; pyruvate metabolism; alanine, aspartate, and glutamate metabolism; and tyrosine metabolism. Fibrobacter abundance showed a positive link to tyrosine metabolism; arginine biosynthesis; TCA cycle; pyruvate metabolism; alanine, aspartate, and glutamate metabolism; cysteine and methionine metabolism; and pyrimidine metabolism (Figure 9A).
Figure 9

Relationships among colonic microbiota, metabolites, and metabolic pathways. (A) BD+NB vs. LN+NB comparison. (B) BD+ETEC vs. LN+ETEC comparison. BD+NB, piglets received the basal diet + oral nutrient broth; LN+NB, piglets received the basal diet supplemented with 150 mg/kg linarin + oral nutrient broth; BD+ETEC, ETEC-challenged piglets fed the basal diet; LN+ETEC, piglets with ETEC infection fed the basal diet + 150 mg/kg linarin. Significance thresholds are set at P < 0.05 (statistically significant) and P < 0.01 (extremely statistically significant).
Under ETEC infection with and without linarin supplementation, Pedosphaera abundance showed a positive link with purine and pyrimidine metabolism, but was negatively correlated with steroid biosynthesis and steroid hormone biosynthesis. The relative abundance of Fusicatenibacter was positively correlated with porphyrin metabolism and pyrimidine metabolism. The relative abundance of Tyzzerella was positively correlated with alanine, aspartate, and glutamate metabolism; steroid biosynthesis; tryptophan metabolism; amino sugar and nucleotide sugar metabolism; and steroid hormone biosynthesis. Sporobacter abundance was positively correlated with purine, histidine, and pyrimidine metabolism. Limosilactobacillus abundance showed a positive correlation with purine, histidine, β-alanine, and pyrimidine metabolism, but was negatively correlated with tyrosine metabolism. The abundance of Senegalimassilia showed a positive association with arginine biosynthesis, glutathione metabolism, and tyrosine and proline metabolism, whereas it was negatively correlated with histidine, purine, and β-alanine metabolism. Catenibacillus abundance was positively linked to pathways involving purine and amino acid metabolism, steroid biosynthesis, tryptophan, and sugar metabolism, whereas negative correlations were found with glutathione, thiamine, taurine, pantothenate, galactose, glycine/serine/threonine, cysteine/methionine, and steroid hormone metabolism. Bryobacter abundance showed positive correlations to glutathione, arginine, and pantothenate/CoA biosynthesis; porphyrin, glycine, serine, threonine, cysteine, methionine, and fatty acids metabolism; and steroid and steroid hormone biosynthesis. However, the relative abundance of Bryobacter was negatively correlated with thiamine, taurine, and hypotaurine metabolism; the TCA cycle; β-alanine metabolism; galactose metabolism; alanine, aspartate, and glutamate metabolism; glyoxylate and dicarboxylate metabolism; pyrimidine metabolism; and tryptophan metabolism (Figure 9B).
4 Discussion
Weaning under stress-induced intestinal barrier dysfunction represents a critical constraint on piglet health and growth. This vulnerability is further compounded by ETEC infection, which exacerbates intestinal permeability, disrupts tight junction-proteins, and triggers inflammatory cascades, ultimately resulting in diarrhea and growth retardation (23, 24). Owing to their anti-inflammatory, antioxidant, and gut-modulating activities, plant-derived bioactive compounds have emerged as viable antibiotic substitutes in feed formulations. Among these, linarin, a flavonoid that is abundant in Asteraceae species, has demonstrated the ability to enhance intestinal immunity and hinder pathogen adhesion (10). In this study, combined analysis of intestinal morphology, tight-junction mRNA expression profiles, and inflammatory signaling pathways in a piglet model infected with ETEC provides the first demonstration that linarin can alleviate intestinal barrier damage through a multi-target regulatory mechanism.
ETEC invasion may disturb the balance of gut microbiota, thereby inducing diarrhea (4). The broad biological activities of flavonoids are well established, including antibacterial and inflammation-reducing effects, along with their ability to regulate the structure and composition of gut microbiota, which can help alleviate diarrhea. In line with this previous work, the present study indicated that dietary linarin significantly decreased the diarrhea rate of piglets, even in the context of ETEC infection.
Consistent with reports that ETEC challenge reduces colonic crypt depth (25), we observed similar morphological damage. Crucially, dietary linarin significantly increased crypt depth, suggesting it counteracts damage caused by ETEC and potentially enhances intestinal barrier integrity. Furthermore, our finding that linarin markedly boosted goblet cell counts under both basal conditions and following ETEC challenge aligns with the established role of these cells in mucosal protection and barrier function, and their depletion during ETEC pathogenesis (26). These findings suggest that linarin may promote intestinal repair and improve mucosal protection by supporting goblet cell function, thereby strengthening the intestinal barrier.
We further investigated the impact of dietary linarin on the physical barrier of the colon. In this study, dietary linarin significantly increased the mRNA expression levels of ZO-1, ZO-2, claudin-1, occludin, and E-cadherin, both under basal conditions and following ETEC challenge in weaned piglets. This suggests that linarin may attenuate the ETEC-induced disruption of colonic tight and adhesive junctions. Mechanistically, linarin’s barrier-fortifying effect aligns with the established role of flavonoids like quercetin and rutin, which enhance intestinal barrier function by promoting junctional protein expression during stress or infection (13), thereby improving intestinal barrier integrity and potentially mitigating ETEC-induced injury.
Intestinal pathogens such as ETEC exploit host cell apoptosis to enhance their survival and dissemination during infection (27). In this study, dietary linarin significantly upregulated the mRNA expression level of BCL2 while reducing the levels of BAX, BAK, CASP3, and CASP9 in weaned piglets. This suggests that linarin may alleviate ETEC-induced apoptosis by enhancing anti-apoptotic signals and inhibiting pro-apoptotic pathways, thereby protecting intestinal cells from pathogen-induced damage.
The intestinal chemical barrier, comprising secreted antimicrobial factors, is critical for defense against pathogens like ETEC (16). In this study, dietary linarin significantly increased MUC2, CYP3A4, and P-glycoprotein mRNA expression levels. This suggests that linarin could enhance intestinal chemical barrier function by upregulating key defense markers (MUC2, CYP3A4, and P-glycoprotein) in weaned piglets after ETEC infection.
The intestinal immune barrier regulates gut microbiota homeostasis by suppressing pathogens while preserving commensal bacteria (28). In this study, dietary linarin significantly decreased the mRNA expression levels of TNF-α, IL-1β, IL-6, and IL-8 in both healthy and ETEC-challenged weaned piglets This downregulation suggests that linarin helps maintain immune barrier integrity during ETEC infection by limiting cytokine-driven epithelial damage and inflammatory cell infiltration, thereby preserving gut homeostasis and improving resistance to enteric pathogens.
To further elucidate the mechanisms by which linarin mitigates ETEC-induced inflammation, we examined its influence on the TLR4/MyD88/NF-κB signaling pathway. ETEC-derived lipopolysaccharide binds to TLR4 on epithelial cells, initiating downstream MyD88 recruitment and NF-κB activation. This process activates NLRP3 inflammasomes and pro-inflammatory cytokines through caspase-1 activation. IκBα, a key NF-κB inhibitor, is phosphorylated and degraded upon IKK complex activation, allowing NF-κB nuclear translocation and cytokine transcription; IKKα and IKKβ subunits are central to this process (29, 30). In this study, dietary linarin significantly decreased the mRNA expression levels of TLR4, MyD88, NF-κB, NLRP3, IKKα, and caspase-1, both without and with ETEC infection. Overall, these findings indicate that dietary linarin could suppress the NF-κB/MyD88/TLR4 signaling pathway and downstream IKK complex activation in intestinal epithelial cells, block activation of the NLRP3 inflammasome and caspase-1, and significantly reduce the mRNA expression of TNF-α, IL-1β, IL-6, and IL-8, ultimately enhancing gut immune defenses and mitigating ETEC-induced inflammation.
Short-chain fatty acids (SCFAs) are metabolites derived from the fermentation of dietary fiber by commensal gut bacteria, which modulate gut health and immune cell function (31). Among the main SCFAs, butyrate is essential for immune modulation and barrier function (32); acetic acid regulates immune responses and energy metabolism by activating G protein-coupled receptors, thereby modulating immune cell function and inhibiting inflammation; and propionic acid exerts anti-inflammatory and immunomodulatory effects, reducing pro-inflammatory cytokines in inflammatory bowel disease (33). A previous study showed that ETEC infection reduces SCFAs in piglet intestines (34). In this study, dietary linarin (LN+NB) significantly elevated the concentrations of acetic, propionic, butyric, and isovaleric acids. Similarly, after ETEC infection, dietary linarin significantly increased acetic, propionic, butyric, valeric, and isovaleric acid levels. This suggests that linarin could elevate intestinal barrier function and adjust the immune response by facilitating the synthesis of SCFAs.
The gut microbiome maintains intestinal barrier function by competitively inhibiting pathogens and producing antibacterial substances. In our study, linarin supplementation significantly reduced the relative abundances of several taxa—including Faecalibacterium, Catenibacillus, Tyzzerella, Ralstonia, Bosea and Anaerofilum—that are either associated with pro−inflammatory signaling or opportunistic pathogenicity (35–39). This shift may underlie the observed inhibition of the TLR4/NF−κB pathway. In contrast, linarin promoted the growth of strict anaerobes and SCFA producers such as Sarcina, Roseimarinus, Fibrobacter and Mesoplasma (40–43). By lowering colonic pH through butyrate and other SCFAs, and by reducing oxidative stress via its antioxidant properties, linarin created an environment favorable to beneficial anaerobes while suppressing taxa that exacerbate inflammation. Together, these data reveal that linarin confers protection against ETEC‐induced dysbiosis by both weakening a pro−inflammatory microbial milieu and fostering SCFA‐mediated barrier reinforcement.
After ETEC infection, dietary linarin significantly decreased the abundance of Limosilobacillus, Bryobacter, Senecamassilia, Fusicatenibacter, and Pedosphaera—genera involved in immunomodulation, carbon metabolism and mucosal colonization (44–48). This reduction may result from linarin’s antioxidant effect lowering intestinal ROS levels, potentially inhibiting redox-signaling reliant bacteria like Bryobacter and Senecamassilia, and its upregulation of tight-junction proteins, which reduces intestinal permeability and may restrict mucosal injury-dependent colonization by genera like Senecamassilia. Conversely, dietary linarin significantly increased the abundance of Sporobacter, Catenibacillus, and Tyzzerella (49), likely through enhanced substrate availability and niche exclusion of opportunists. Collectively, these shifts suggest linarin’s anti-inflammatory and antioxidant effects help alleviate ETEC-induced intestinal damage, creating conditions favorable for the proliferation of beneficial bacteria like Sporobacter, Catenibacterium, and Tyzzerella.
Metabolomics integrates high-throughput analysis and bioinformatics to assess metabolic responses. Our metabolomics analysis demonstrates that dietary linarin profoundly reshapes colonic metabolite networks by enhancing nucleotide metabolism, TCA cycle activity, and butyrate pathways. Upregulated nucleotide turnover may curb uric acid accumulation by suppressing uricogenic bacteria such as Catenibacillus, while increased TCA cycle flux supplies ATP to meet and thereby downregulate NF−κB–driven inflammatory demands (50). Concurrently, boosted butyrate metabolism not only generates anti−inflammatory SCFAs but also promotes a microbiota environment favorable to butyrogenic taxa. This suggests that linarin may reduce the production of proinflammatory metabolites, create a more favorable growth environment for butyric acid-producing bacteria, and indirectly promote butyric acid metabolism.
After ETEC infection, dietary linarin significantly affected the characteristic distribution of metabolites in the colonic chyme of weaned piglets. Linarin also rebalanced colonic metabolite profiles by modulating sphingolipid, carbon, and arginine–proline pathways. Sphingolipid metabolism attenuation restored enzymatic/metabolite equilibrium to suppress NF-κB-driven inflammation (51), while concurrent stabilization of carbon metabolism countered ETEC toxin-induced disruption of glycolysis and TCA cycling, thereby preserving host glucose homeostasis (52). Furthermore, linarin optimized arginine-proline metabolism to enhance proline hydroxylase activity and collagen synthesis, accelerating antioxidant-dependent mucosal repair. Collectively, these shifts illustrate how linarin orchestrates a metabolomic milieu conducive to energy homeostasis, redox balance, and inflammation resolution.
To further explore the impact of linarin on the gut microbiome, we evaluated the relationships among the key alterations in the microbiome with the changes in the expression of key marker genes associated with colonic barrier function, apoptosis, and the TLR4/MyD88/NF-κB signaling pathway. The result shows that dietary linarin intervention significantly changed the abundance of Bosea, Catenibacillus, Faecalibacterium, and other flora, which was in turn significantly related to the expression of genes associated with the colonic physical barrier, chemical barrier, immune barrier, apoptosis regulation, and the TLR4/MyD88/NF-κB signaling pathway. Therefore, linarin may synergistically enhance intestinal barrier function, maintain apoptosis balance, and inhibit an excessive inflammatory reaction by regulating the flora host gene interaction network.
Similarly, after ETEC infection with dietary linarin supplementation, this study showed that after ETEC infection, dietary linarin intervention was closely related to the intestinal physical barrier, apoptosis regulation, and TLR4/MyD88/NF-κB signal pathway-related gene expression by significantly changing the abundance of Bryobacter, Catenibacillus, Limosilactobacillus, and other flora. Therefore, linarin might alleviate ETEC-induced intestinal injury by reshaping the flora–host interaction network, synergistically strengthening the integrity of the intestinal barrier, regulating cell apoptosis, and inhibiting the inflammatory cascade reaction.
By combining microbiome and metabolome analyses, we mapped how linarin reshapes microbial–metabolic interactions to support colonic barrier function. By suppressing Bosea, linarin redirects arginine toward mucosal repair via polyamine synthesis while reducing nitric oxide-mediated oxidative stress (53). Concurrent enrichment of Fibrobacter enhances fiber fermentation into short-chain fatty acids, reactivating the TCA cycle to fuel colonocyte energy demands (54). Reduction of Faecalibacterium preserves cysteine/methionine metabolism for sustained glutathione synthesis, maintaining antioxidant defenses (35). Post-ETEC infection, Fusicatenibacter-mediated porphyrin and pyrimidine metabolism further supports heme synthesis and epithelial regeneration (55). The link of this genus to pyrimidine metabolism further suggests a role in nucleotide synthesis for epithelial repair. These effects collectively mitigate ETEC-induced oxidative stress and maintain mucosal integrity.
After ETEC infection, linarin selectively suppressed genera tied to pro−inflammatory or dysregulated metabolism while enriching those supporting repair and homeostasis. By suppressing Tyzzerella, linarin attenuated corticoid-mediated inflammation and kynurenine pathway activation, while concurrently enriching Catenibacillus to convert linarin into bioactive quercetin, thereby inhibiting ETEC virulence and optimizing nitrogen metabolism for mucosal repair (56). Furthermore, linarin fine-tuned Limosilactobacillus to enhance carnosine synthesis and counter tyrosine-derived inflammatory mediators (57), and reduced Senegalimassilia to redirect arginine toward polyamine synthesis while mitigating histamine overproduction (46), Notably, Bryobacter downregulation balanced glutathione-dependent antioxidant defenses against pro-inflammatory steroid biosynthesis (58). Further illustrate how linarin orchestrates a metabolic shift toward energy provision, antioxidant defense, and reduced inflammation, thereby fostering mucosal repair.
Overall, these results show that dietary linarin maintained intestinal energy homeostasis and barrier function by changing the abundance of Bosea, Faecalibacterium, and Fibrobacter, controlling essential pathways in amino acids, the TCA cycle, and carbohydrate metabolism. After ETEC infection, linarin affected lipid metabolism, immune regulation, and nucleotide metabolism pathways by regulating the abundance of bacteria such as Fusicatonibacter and Limosilactobacillus, thereby alleviating inflammation and repairing the pathogen-induced intestinal damage.
5 Conclusion
This study demonstrated that dietary linarin significantly reduced the incidence of diarrhea in weaned piglets infected with ETEC by enhancing colonic barrier function at multiple levels. Specifically, linarin improved crypt architecture of colon, increased goblet cell numbers, upregulated the expression of key physical barrier genes, and inhibited the apoptosis of intestinal epithelial cells. At the signaling level, linarin exerted anti-inflammatory effects by regulating the TLR4/MyD88/NF-κB pathway. Moreover, linarin modulated the chemical barrier of the intestine by suppressing inflammatory cytokine overexpression and downregulating NLRP3 inflammasome and caspase-1 levels, thereby attenuating the inflammatory response. Notably, linarin elevated the concentrations of SCFAs in the colon, reshaped the microbiota structure, and influenced the interaction network between key bacterial taxa and metabolic pathways related to energy, amino acid, and nucleic acid metabolism.
To our knowledge, this is the first study to directly investigate the protective effects of linarin against ETEC infection in weaned piglets. These findings highlight the regulatory relationships between linarin supplementation and key mechanisms of infection—including colonic microbiota remodeling, intestinal barrier enhancement, apoptosis suppression, and signaling pathway modulation—alongside associated metabolite and metabolic pathway alterations. Future research will focus on characterizing the microbiota–metabolite–host cross-talk underlying linarin-mediated intestinal barrier repair. Collectively, this study offers novel mechanistic insights into how linarin mitigates ETEC-induced intestinal injury in weaned piglets, underscoring its potential as a safe alternative for improving gut health.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The animal studies were approved by Experimental Animal Ethics Committee of Anhui Science and Technology University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
QZ: Investigation, Methodology, Writing – original draft, Writing – review & editing. XL: Formal Analysis, Methodology, Writing – review & editing. CS: Formal Analysis, Investigation, Visualization, Writing – review & editing. MW: Conceptualization, Methodology, Visualization, Writing – review & editing. XJ: Methodology, Resources, Software, Writing – review & editing. SL: Conceptualization, Methodology, Resources, Writing – review & editing. EJ: Conceptualization, Investigation, Resources, Writing – review & editing. FZ: Methodology, Writing – review & editing, Conceptualization, Resources, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China (32172816, 32202728), the National Natural Science of Anhui Province (2208085MC77), the Key Disciplines Construction of Veterinary Science in Anhui Science and Technology University (XK-XJGF002), the Project of Training Outstanding Young Teachers in Higher Education Institutions of Anhui province (220052), the High-level Talents Introduction Foundation of Anhui Science and Technology University (DKYJ202101), Anhui Science and Technology University–Shouxian University-Local Cooperation Project (881214, 881215, 881988).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1631991/full#supplementary-material
Glossary
- ACE
a microbial diversity index
- BAK
BCL2-antagonist/killer 1
- BAX
BCL2-associated X protein
- BCL2
B-cell lymphoma 2
- CASP3
caspase 3
- CASP9
caspase 9
- CYP3A4
cytochrome P450 3A4
- DR
diarrhea rate
- ERK
extracellular signal-regulated kinase
- ETEC
enterotoxigenic Escherichia coli
- H&E
hematoxylin & eosin
- HPLC
high-performance liquid chromatography
- IKKα
inhibitor of nuclear factor kappa-B kinase subunit alpha
- IKKβ
inhibitor of nuclear factor kappa-B kinase subunit beta
- IκBα
nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha
- IL-1β
interleukin-1 beta
- IL-6
interleukin-6
- IL-8
interleukin-8
- IRAK1
interleukin-1 receptor associated kinase 1
- JNK
c-Jun N-terminal kinase
- KEGG
kyoto encyclopedia of genes and genomes
- MUC2
mucin 2
- MyD88
myeloid differentiation primary response 88
- NF-κB
nuclear factor kappa-B
- NLRP3
nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3
- NMDS
non-metric multidimensional scaling
- NO
nitric oxide
- P-glycoprotein
permeability glycoprotein
- PCoA
principal coordinate analysis
- PCA/PLS-DA
principal component analysis/partial least squares discriminant analysis
- PWD
post-weaning diarrhea
- qPCR
real-time quantitative polymerase chain reaction
- ROS
reactive oxygen species
- SCFA
short-chain fatty acid
- SEM
standard error of the mean
- SPSS
statistical package for the social sciences
- TCA cycle
tricarboxylic acid cycle
- TLR4
toll-like receptor 4
- TNF-α
tumor necrosis factor-alpha
- TRAF6
TNF receptor associated factor 6
- UHPLC-MS/MS
ultra-high performance liquid chromatography-tandem mass spectrometry.
References
1
Tang Q Lan T Zhou C Gao J Wu L Wei H et al . Nutrition strategies to control post-weaning diarrhea of piglets: from the perspective of feeds. Anim Nutr. (2024) 17:297–311. doi: 10.1016/j.aninu.2024.03.006
2
Jin S Xu H Yang C O K . Regulation of oxidative stress in the intestine of piglets after enterotoxigenic escherichia coli (ETEC) infection. Biochim Biophys Acta (BBA) - Mol Cell Res. (2024) 1871:119711. doi: 10.1016/j.bbamcr.2024.119711
3
Cheng Y Xiao X Fu J Zong X Lu Z Wang Y . Escherichia coli K88 activates NLRP3 inflammasome-mediated pyroptosis in vitro and in vivo. Biochem Biophysics Rep. (2024) 38:101665. doi: 10.1016/j.bbrep.2024.101665
4
von Mentzer A Svennerholm A-M . Colonization factors of human and animal-specific enterotoxigenic escherichia coli (ETEC). Trends Microbiol. (2024) 32:448–64. doi: 10.1016/j.tim.2023.11.001
5
Pramudito TE Desai K Voigt C Smid EJ Schols HA . Dextran and levan exopolysaccharides from tempeh-associated lactic acid bacteria with bioactivity against enterotoxigenic escherichia coli (ETEC). Carbohydr Polymers. (2024) 328:121700. doi: 10.1016/j.carbpol.2023.121700
6
Bergstrom K Shan X Casero D Batushansky A Lagishetty V Jacobs JP et al . Proximal colon–derived O-glycosylated mucus encapsulates and modulates the microbiota. Science. (2020) 370:467–72. doi: 10.1126/science.aay7367
7
Jyoti Dey P . Mechanisms and implications of the gut microbial modulation of intestinal metabolic processes. NPJ Metab Health Dis. (2025) 3:24. doi: 10.1038/s44324-025-00066-1
8
Lv Z Chen L Ouyang H Zhu Y Ma J Zhang K et al . Development, basic information, classifications, pharmacological activities, and underlying mechanisms of medicine food homology: A review. J Funct Foods. (2024) 122:106552. doi: 10.1016/j.jff.2024.106552
9
Qi W Chen Y Sun S Xu X Zhan J Yan Z et al . Inhibiting TLR4 signaling by linarin for preventing inflammatory response in osteoarthritis. Aging. (2021) 13:5369–82. doi: 10.18632/aging.202469
10
Mottaghipisheh J Taghrir H Boveiri Dehsheikh A Zomorodian K Irajie C Mahmoodi Sourestani M et al . Linarin, a glycosylated flavonoid, with potential therapeutic attributes: A comprehensive review. Pharm (Basel Switzerland). (2021) 14. doi: 10.3390/ph14111104
11
Han X Wu YC Meng M Sun QS Gao SM Sun H . Linarin prevents LPS−Induced acute lung injury by suppressing oxidative stress and inflammation via inhibition of TXNIP/NLRP3 and NF−κB pathways. Int J Mol Med. (2018) 42:1460–72. doi: 10.3892/ijmm.2018.3710
12
Li L Lan Y Wang F Gao T . Linarin protects against CCl(4)-induced acute liver injury via activating autophagy and inhibiting the inflammatory response: involving the TLR4/MAPK/Nrf2 pathway. Drug design Dev Ther. (2023) 17:3589–604. doi: 10.2147/dddt.S433591
13
Jin C Liu J Jin R Yao Y He S Lei M et al . Linarin ameliorates dextran sulfate sodium-induced colitis in C57BL/6J mice via the improvement of intestinal barrier, suppression of inflammatory responses and modulation of gut microbiota. Food Funct. (2022) 13:10574–86. doi: 10.1039/d2fo02128e
14
Wang Y Zhang Z Du M Ji X Liu X Zhao C et al . Berberine alleviates etec-induced intestinal inflammation and oxidative stress damage by optimizing intestinal microbial composition in a weaned piglet model. Front Immunol. (2024) 15:1460127. doi: 10.3389/fimmu.2024.1460127
15
Villagómez-Estrada S Melo-Durán D van Kuijk S Pérez JF Solà-Oriol D . Specialized feed-additive blends of short- and medium-chain fatty acids improve sow and pig performance during nursery and post-weaning phase. Animals. (2024) 14:3692. doi: 10.3390/ani14243692
16
Du M Liu X Ji X Wang Y Liu X Zhao C et al . Berberine alleviates enterotoxigenic escherichia coli-induced intestinal mucosal barrier function damage in a piglet model by modulation of the intestinal microbiome. Front Nutr. (2024) 11:1494348. doi: 10.3389/fnut.2024.1494348
17
Zhang F Jin E Liu X Ji X Hu H . Effect of dietary fructus mume and scutellaria baicalensis georgi on the fecal microbiota and its correlation with apparent nutrient digestibility in weaned piglets. Animals: an Open Access J MDPI. (2022) 12. doi: 10.3390/ani12182418
18
Zhang F Zheng W Xue Y Yao W . Suhuai suckling piglet hindgut microbiome-metabolome responses to different dietary copper levels. Appl Microbiol Biotechnol. (2019) 103:853–68. doi: 10.1007/s00253-018-9533-0
19
Sellick CA Hansen R Stephens GM Goodacre R Dickson AJ . Metabolite extraction from suspension-cultured mammalian cells for global metabolite profiling. Nat Protoc. (2011) 6:1241–9. doi: 10.1038/nprot.2011.366
20
Yuan M Breitkopf SB Yang X Asara JM . A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat Protoc. (2012) 7:872–81. doi: 10.1038/nprot.2012.024
21
Barri T Dragsted LO . UPLC-ESI-QTOF/MS and multivariate data analysis for blood plasma and serum metabolomics: effect of experimental artefacts and anticoagulant. Analytica chimica Acta. (2013) 768:118–28. doi: 10.1016/j.aca.2013.01.015
22
Want EJ O’Maille G Smith CA Brandon TR Uritboonthai W Qin C et al . Solvent-dependent metabolite distribution, clustering, and protein extraction for serum profiling with mass spectrometry. Analytical Chem. (2006) 78:743–52. doi: 10.1021/ac051312t
23
Xiao K Zhou M Lv Q He P Qin X Wang D et al . Protocatechuic acid and quercetin attenuate ETEC-caused IPEC-1 cell inflammation and injury associated with inhibition of necroptosis and pyroptosis signaling pathways. J Anim Sci Biotechnol. (2023) 14:5. doi: 10.1186/s40104-022-00816-x
24
Brubaker J Zhang X Bourgeois AL Harro C Sack DA Chakraborty S . Intestinal and systemic inflammation induced by symptomatic and asymptomatic enterotoxigenic E. Coli infection and impact on intestinal colonization and etec specific immune responses in an experimental human challenge model. Gut Microbes. (2021) 13:1–13. doi: 10.1080/19490976.2021.1891852
25
Miao J Cui L Zeng H Hou M Wang J Hang S . Lactiplantibacillus plantarum L47 and inulin affect colon and liver inflammation in piglets challenged by enterotoxigenic escherichia coli through regulating gut microbiota. Front Veterinary Sci. (2024) 11:1496893. doi: 10.3389/fvets.2024.1496893
26
Xu J Jia Z Xiao S Long C Wang L . Effects of enterotoxigenic escherichia coli challenge on jejunal morphology and microbial community profiles in weaned crossbred piglets. Microorganisms. (2023) 11. doi: 10.3390/microorganisms11112646
27
Deng Y Han X Tang S Li C Xiao W Tan Z . Magnolol and honokiol attenuate apoptosis of enterotoxigenic escherichia coli-induced intestinal epithelium by maintaining secretion and absorption homeostasis and protecting mucosal integrity. Med Sci monitor: Int Med J Exp Clin Res. (2018) 24:3348–56. doi: 10.12659/msm.910350
28
Neurath MF Artis D Becker C . The intestinal barrier: A pivotal role in health, inflammation, and cancer. Lancet Gastroenterol Hepatol. (2025) 10(6):573–92. doi: 10.1016/s2468-1253(24)00390-x
29
Wan J Zhang J Xu Q Yin H Chen D Yu B et al . Alginate oligosaccharide protects against enterotoxigenic escherichia coli-induced porcine intestinal barrier injury. Carbohydr Polymers. (2021) 270:118316. doi: 10.1016/j.carbpol.2021.118316
30
Li M Zhao D Guo J Pan T Niu T Jiang Y et al . Bacillus halotolerans SW207 alleviates enterotoxigenic escherichia coli-induced inflammatory responses in weaned piglets by modulating the intestinal epithelial barrier, the TLR4/MyD88/NF-κB pathway, and intestinal microbiota. Microbiol Spectr. (2024) 12. doi: 10.1128/spectrum.03988-23
31
Tan JK Macia L Mackay CR . Dietary fiber and SCFAs in the regulation of mucosal immunity. J Allergy Clin Immunol. (2023) 151:361–70. doi: 10.1016/j.jaci.2022.11.007
32
Liu L Chen D Yu B Yin H Huang Z Luo Y et al . Fructooligosaccharides improve growth performance and intestinal epithelium function in weaned pigs exposed to enterotoxigenic. Escherichia Coli. Food Funct. (2020) 11:9599–612. doi: 10.1039/d0fo01998d
33
Mann ER Lam YK Uhlig HH . Short-chain fatty acids: linking diet, the microbiome and immunity. Nat Rev Immunol. (2024) 24:577–95. doi: 10.1038/s41577-024-01014-8
34
Xu H Gong J Lu P Azevedo P Li L Yu H et al . Functional evaluation of bacillus licheniformis PF9 for its potential in controlling enterotoxigenic escherichia coli in weaned piglets. Trans Anim Sci. (2024) 8:txae050. doi: 10.1093/tas/txae050
35
Martín R Rios-Covian D Huillet E Auger S Khazaal S Bermúdez-Humarán LG et al . Faecalibacterium: A bacterial genus with promising human health applications. FEMS Microbiol Rev. (2023) 47. doi: 10.1093/femsre/fuad039
36
Braune A Blaut M . Catenibacillus scindens gen. Nov., sp. Nov., a C-deglycosylating human intestinal representative of the lachnospiraceae. Int J systematic evolutionary Microbiol. (2018) 68:3356–61. doi: 10.1099/ijsem.0.003001
37
Cai Y Xiao C Tian B Dorthe S Meuter A Song B et al . Dietary probiotic based on a dual-strain bacillus subtilis improves immunity, intestinal health, and growth performance of broiler chickens. J Anim Sci. (2024) 102. doi: 10.1093/jas/skae183
38
Ryan MP Pembroke JT Adley CC . Ralstonia pickettii: A persistent gram-negative nosocomial infectious organism. J Hosp Infection. (2006) 62:278–84. doi: 10.1016/j.jhin.2005.08.015
39
Zellner G Stackebrandt E Nagel D Messner P Weiss N Winter J . Anaerofilum pentosovorans gen. Nov., sp. Nov., and anaerofilum agile sp. Nov., two new, strictly anaerobic, mesophilic, acidogenic bacteria from anaerobic bioreactors. Int J systematic bacteriology. (1996) 46:871–5. doi: 10.1099/00207713-46-4-871
40
Lam-Himlin D Tsiatis AC Montgomery E Pai RK Brown JA Razavi M et al . Sarcina organisms in the gastrointestinal tract: A clinicopathologic and molecular study. Am J Surg Pathol. (2011) 35:1700–5. doi: 10.1097/PAS.0b013e31822911e6
41
Thomé P Rivera-Ortega J Rodríguez J Cerqueda-García D Guzmán-Urieta E García-Maldonado J et al . Local dynamics of a white syndrome outbreak and changes in the microbial community associated with colonies of the scleractinian brain coral pseudodiploria strigosa. PeerJ. (2021) 9:e10695. doi: 10.7717/peerj.10695
42
Neumann AP McCormick CA Suen G . Fibrobacter communities in the gastrointestinal tracts of diverse hindgut-fermenting herbivores are distinct from those of the rumen. Environ Microbiol. (2017) 19:3768–83. doi: 10.1111/1462-2920.13878
43
Lo WS Gasparich GE Kuo CH . Convergent evolution among ruminant-pathogenic mycoplasma involved extensive gene content changes. Genome Biol Evol. (2018) 10:2130–9. doi: 10.1093/gbe/evy172
44
Nandi S Mandal S . Insights into the Antibacterial Activity of Limosilactobacillus Fermentum Curd Isolate against Gram-Positive and Gram-Negative Pathogenic Bacteria. Pharmacol Res - Rep. (2025) 3:100034. doi: 10.1016/j.prerep.2025.100034
45
Kulichevskaya IS Suzina NE Liesack W Dedysh SN . Bryobacter aggregatus gen. Nov., sp. Nov., a peat-inhabiting, aerobic chemo-organotroph from subdivision 3 of the acidobacteria. Int J systematic evolutionary Microbiol. (2010) 60:301–6. doi: 10.1099/ijs.0.013250-0
46
Han KI Kim JS Lee KC Eom MK Suh MK Kim HS et al . Senegalimassilia faecalis sp. Nov., an anaerobic actinobacterium isolated from human faeces, and emended description of the genus Senegalimassilia. Int J systematic evolutionary Microbiol. (2020) 70:1684–90. doi: 10.1099/ijsem.0.003958
47
Rapozo DC Bernardazzi C de Souza HS . Diet and microbiota in inflammatory bowel disease: the gut in disharmony. World J Gastroenterol. (2017) 23:2124–40. doi: 10.3748/wjg.v23.i12.2124
48
Kant R van Passel Mark WJ Sangwan P Palva A Lucas S Copeland A et al . Genome sequence of “Pedosphaera parvula” Ellin514, an aerobic verrucomicrobial isolate from pasture soil. J Bacteriology. (2011) 193:2900–1. doi: 10.1128/jb.00299-11
49
He D Liu L Zhang Z Yang X Jia Y Wen Y et al . Association between gut microbiota and longevity: A genetic correlation and mendelian randomization study. BMC Microbiol. (2022) 22:302. doi: 10.1186/s12866-022-02703-x
50
MacLean A Legendre F Appanna VD . The tricarboxylic acid (TCA) cycle: A malleable metabolic network to counter cellular stress. Crit Rev Biochem Mol Biol. (2023) 58:81–97. doi: 10.1080/10409238.2023.2201945
51
Nixon GF . Sphingolipids in inflammation: pathological implications and potential therapeutic targets. Br J Pharmacol. (2009) 158:982–93. doi: 10.1111/j.1476-5381.2009.00281.x
52
O’Keeffe S Garcia L Chen Y Law RC Liu C Park JO . Bringing carbon to life via one-carbon metabolism. Trends Biotechnol. (2025) 43:572–85. doi: 10.1016/j.tibtech.2024.08.014
53
Thomas V Casson N Greub G . New afipia and bosea strains isolated from various water sources by amoebal co-culture. Systematic Appl Microbiol. (2007) 30:572–9. doi: 10.1016/j.syapm.2007.06.004
54
Jewell KA Scott JJ Adams SM Suen G . A phylogenetic analysis of the phylum fibrobacteres. Systematic Appl Microbiol. (2013) 36:376–82. doi: 10.1016/j.syapm.2013.04.002
55
Takada T Kurakawa T Tsuji H Nomoto K . Fusicatenibacter saccharivorans gen. Nov., sp. Nov., isolated from human faeces. Int J systematic evolutionary Microbiol. (2013) 63:3691–6. doi: 10.1099/ijs.0.045823-0
56
Goris T Braune A . Genomics and physiology of catenibacillus, human gut bacteria capable of polyphenol C-deglycosylation and flavonoid degradation. Microbial Genomics. (2024) 10. doi: 10.1099/mgen.0.001245
57
Hossain TJ . Functional genomics of the lactic acid bacterium limosilactobacillus fermentum lab-1: metabolic, probiotic and biotechnological perspectives. Heliyon. (2022) 8:e11412. doi: 10.1016/j.heliyon.2022.e11412
58
Masip L Veeravalli K Georgiou G . The many faces of glutathione in bacteria. Antioxidants Redox Signaling. (2006) 8:753–62. doi: 10.1089/ars.2006.8.753
Summary
Keywords
enterotoxigenic Escherichia coli, linarin, weaned piglet, barrier function, metabonomic, correlation analysis
Citation
Zhang Q, Liu X, Sun C, Wang M, Ji X, Li S, Jin E and Zhang F (2025) Linarin alleviates colonic barrier dysfunction induced by enterotoxic Escherichia coli in weaned piglets by regulating the gut microbiota and metabolic pathways. Front. Immunol. 16:1631991. doi: 10.3389/fimmu.2025.1631991
Received
20 May 2025
Accepted
10 October 2025
Published
23 October 2025
Volume
16 - 2025
Edited by
Mohanned Alhussien, Technical University of Munich, Germany
Reviewed by
Long Cai, Beijing Academy of Agricultural and Forestry Sciences, China
Fan Wan, Shanghai Academy of Agricultural Sciences, China
Yuan Cao, Technical University of Munich, Germany
Updates
Copyright
© 2025 Zhang, Liu, Sun, Wang, Ji, Li, Jin and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Zhang, zhangfeng@ahstu.edu.cn
†These authors contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.