- 1National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, China
- 2First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
Migraines are among the most common neurological disorders, disabling nearly one in seven people worldwide, whereas glioblastoma (GBM) is the most aggressive primary brain tumour, with median survival scarcely beyond 15 months. Historically considered distinct, these conditions are increasingly linked by trigeminal nerve-driven neurogenic inflammation. Activation of trigeminovascular afferents provokes antidromic release of calcitonin gene-related peptide (CGRP), substance P (SP), and pituitary adenylate cyclase-activating polypeptide (PACAP); beyond mediating migraine pain, these peptides remodel vasculature, immune infiltrates, and extracellular matrix to facilitate GBM invasion. Pre-clinical studies show CGRP and SP up-regulate matrix-metalloproteinases and integrins, while PACAP modulates cAMP–MAPK signalling, collectively promoting perivascular migration and temozolomide resistance. Epidemiological analyses report higher migraine antecedents in patients later diagnosed with brain tumours, and high-resolution MRI frequently localises GBM spread along trigeminal pathways, underscoring anatomical plausibility. Emerging therapeutics mirror these insights: aprepitant (an NK1-receptor antagonist) triggers GBM apoptosis, gepant-class CGRP blockers curb invasive phenotypes, and radiolabelled SP analogues deliver focal alpha-therapy. These discoveries facilitate more precise pathogenetic characterisation, reduce diagnostic uncertainty, and expedite translational drug development. This review synthesises current evidence on trigeminal neurogenic inflammation as a mechanistic conduit between migraine biology and GBM progression, mapping cellular circuits, molecular crosstalk, and translational interventions. By integrating neurobiology, oncology, and pharmacology, we aim to delineate diagnostic blind spots, spotlight drug-repurposing opportunities, and chart a roadmap toward personalised strategies that simultaneously alleviate migraine burden and restrain glioblastoma aggressiveness.
1 Introduction
Both migraine and glioma contribute substantially to global neurological disability (1, 2). Migraine alone afflicts roughly one in seven individuals and stands among the leading contributors to neurological years-lived-with-disability globally (3–5). Glioblastoma (GBM), the most common lethal brain tumour, still carries a median survival of only ~15 months despite multimodal therapy (6, 7). Ipsilateral tumour-headache and incidental benefit of anti-migraine drugs in GBM therapy highlight neuron–tumour crosstalk unique to cranial, not peripheral, cancers (8–10).
Central to migraine pathogenesis is trigeminovascular neurogenic inflammation. Electrical or chemical activation of trigeminal nociceptors provokes a rapid antidromic release of vasoactive neuropeptides—chiefly calcitonin gene-related peptide (CGRP), substance P (SP), and pituitary adenylate cyclase-activating peptide (PACAP)—from perivascular afferents innervating the dura and cortical vessels (11, 12). CGRP’s importance is underscored by the dense expression of its canonical receptor components, calcitonin receptor-like receptor (CLR) and receptor activity-modifying protein-1 (RAMP1), on human trigeminal ganglion neurons, satellite glia, and vascular smooth-muscle cells (13–15). These peptides collectively induce vasodilatation, plasma protein extravasation, mast-cell degranulation, and leukocyte chemotaxis—hallmarks of the sterile inflammatory milieu that sensitises meningeal nociceptors and drives migraine pain (16–18).
Intriguingly, each of these neuropeptides also possesses documented oncological bio-activity within the glioma micro-environment (19–21). PACAP and vasoactive intestinal peptide (VIP) modulate cyclic-AMP signalling and proliferation in C6 and T98G glioma models, revealing cell-context–dependent pro- and anti-growth actions. Substance P engages the neurokinin-1 receptor (NK1-R), which is consistently over-expressed across the glioma malignancy spectrum and has become a target for radionuclide therapy in recurrent GBM (22–24). Beyond direct mitogenic effects, neuropeptide stimulation enhances extracellular-matrix remodelling: CGRP and SP up-regulate matrix-metalloproteinases (MMP-2, MMP-9) and membrane-type MMPs that are indispensable for GBM cell invasion along white-matter tracts (25–27). These observations point to a molecular convergence whereby trigeminal-derived mediators, originally evolved for host defence and vasoregulation, inadvertently cultivate a micro-environment permissive to glioma infiltration.
Recognising this overlap, the present review interrogates the hypothesis that trigeminal nerve-driven neurogenic inflammation constitutes a mechanistic bridge between migraine biology and GBM progression. We first dissect the cellular and molecular architecture of trigeminal neurogenic inflammation in migraine, then map how the same mediators and signalling nodes orchestrate GBM invasion. By integrating otherwise disparate literatures, we aim to illuminate novel pathophysiological cross-talk and identify therapeutic targets capable of attenuating both migraine burden and glioblastoma aggressiveness.
2 Trigeminal neurogenic inflammation in migraine
As shown in Figure 1, the trigeminovascular system (TVS) forms an anatomically discrete, yet functionally expansive, pain-signalling network in which pseudo-unipolar neurons of the ophthalmic branch of the trigeminal nerve project collaterals to both cranial vessels and the spinal trigeminal nucleus caudalis (28, 29). These perivascular afferents densely invest the dura mater, middle-meningeal artery and cortical arterioles, positioning the TVS to couple vascular status with nociceptive traffic. Electrical, mechanical or metabolite-driven activation of meningeal C- and Aδ-fibres—whether secondary to cortical spreading depolarisation, meningeal stretch or nitrosative stress—initiates a robust antidromic secretory reflex that is now recognised as the biochemical fulcrum of migraine pain (30–32).
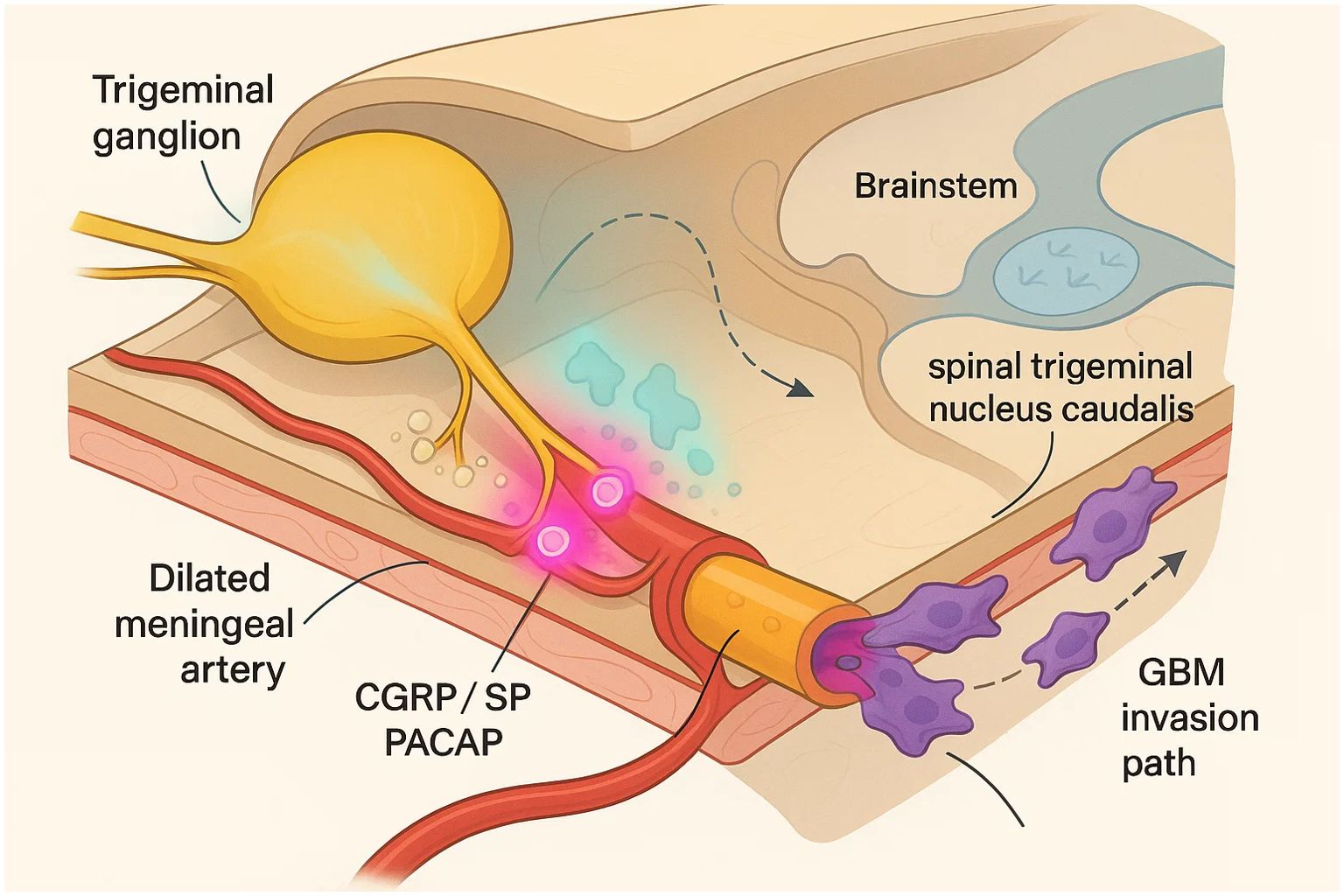
Figure 1. Trigeminovascular neurogenic inflammation as a bridge between migraine and glioblastoma invasion.
Within milliseconds of depolarisation, large-dense-core vesicles at the peripheral terminals fuse and discharge a stereotyped cocktail of neuropeptides (33). Approximately two-thirds of human trigeminal ganglion neurons co-express calcitonin gene-related peptide (CGRP) and substance P (SP), while 15–20% harbour pituitary adenylate cyclase-activating polypeptide-38 (PACAP-38) or its splice variants (34, 35); immunofluorescence and in-situ hybridisation in rodent and human tissues confirm parallel expression of their canonical receptors (CLR/RAMP1 for CGRP, NK1-R for SP, PAC1-R/VIP-R1 for PACAP/VIP) on satellite glia, vascular smooth-muscle cells and meningeal fibroblasts (36, 37). PACAP-38 itself can provoke secondary CGRP release from both ganglion and central terminals, illustrating hierarchical neuropeptide crosstalk that amplifies the inflammatory signal. CGRP, together with nitric-oxide–cGMP signalling, is the principal effector in this cascade. Nanomolar concentrations dilate cranial arteries via cyclic-AMP–dependent protein kinase and endothelial nitric-oxide synthase, while concurrently lowering nociceptor firing thresholds through CaV3.2 T-type channel modulation (38, 39). Genomic data show higher RAMP1/CALCA expression in migraineurs; oestrogen-responsive enhancers and X-chromosome copy-number gains, together with Y-linked TSPY loss, amplify CGRP output from hormone-primed trigeminal and GBM cells (40, 41). SP complements these actions by engaging endothelial NK1-receptors to increase post-capillary venular permeability and provoke plasma-protein extravasation, a hallmark of sterile neurogenic inflammation visualised in rodent dura and attenuated by triptans or NK1 antagonists.
The inflammatory milieu that ensues is not neuron-restricted. SP and CGRP trigger rapid degranulation of dural mast cells; histamine, tumour-necrosis factor-α and tryptase released thereby further sensitise afferents and recruit neutrophils and macrophages, perpetuating the cycle (42, 43). Parallel activation of meningeal fibroblasts and endothelial cells leads to interleukin-6 and prostanoid synthesis, creating a cytokine gradient that diffuses centrally and primes second-order neurons (44, 45).
Central amplification follows. Persistent primary-afferent barrage phosphorylates NMDA receptors and ERK1/2 within the spinal trigeminal nucleus caudalis; activated microglia and astrocytes liberate brain-derived neurotrophic factor and nitric oxide, sustaining long-term potentiation of nociceptive neurons and manifesting clinically as cutaneous allodynia (13, 46). Functional imaging corroborates these findings, and both attacks and CGRP/PACAP release peak in the early morning, hinting at circadian control (29, 47).
Trigeminal neurogenic inflammation represents a dynamic, multi-cellular feed-forward loop in which neuropeptide release, vascular dysfunction, immune cell mobilisation and central sensitisation operate in concert. This finely tuned yet pathologically labile circuitry not only underpins migraine pain but also generates a repertoire of cytokines, proteases and growth factors that reshape the local extracellular matrix. Many of these same mediators—CGRP-driven matrix metalloproteinase induction, SP/NK1-R–mediated mitogenic signalling and PACAP-dependent cyclic-AMP modulation—are co-opted by glioblastoma cells to infiltrate neural parenchyma. Understanding the bidirectional dialogue between trigeminal afferents and their vascular–immune partners therefore offers a conceptual bridge between episodic migraine and malignant glioma invasion.
3 Molecular convergence driving glioblastoma invasion
The same neuropeptide circuits that ignite trigeminal neurogenic inflammation are increasingly recognised as oncogenic drivers within the glioblastoma (GBM) micro-environment. Transcriptomic and single-cell atlases show that high-grade gliomas over-express both tachykinin and calcitonin-family G-protein-coupled receptors (GPCRs); notably, neurokinin-1 receptor (NK1-R) and the calcitonin receptor-like receptor (CLR, encoded by CALCRL) track with mesenchymal programmes and shortened survival, underscoring their functional relevance to tumour spread (12, 48).
Substance P (SP) signalling exemplifies this hijacking. Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases that cleave basement-membrane type IV collagen, laminin and proteoglycans; the gelatinases MMP-2 and MMP-9 become fully active when surface-trimmed by the membrane-type protease MT1-MMP (MMP-14) and are opposed by tissue inhibitors of metalloproteinases (TIMP-1/-2). This triad is indispensable for GBM cells to tunnel along myelinated white-matter tracts. GBM cells form an autocrine loop in which neuronal or tumour-derived SP engages NK1-R to activate ERK1/2 and PI3K–Akt, culminating in β-arrestin-1 recruitment, cyclin‐dependent kinase activation and accelerated cell-cycle transit. Pharmacologic or genetic blockade of NK1-R curtails SP-driven chemotaxis, while the clinically approved antagonist aprepitant suppresses lamellipodia dynamics and reduces orthotopic tumour burden in vivo (49–51). Mechanistically, NK1-R stimulation up-regulates matrix-metalloproteinase-2 (MMP-2) and its membrane activator MT1-MMP, thereby enabling perivascular and white-matter tract infiltration (52).
A parallel axis operates through CGRP and its receptor family. Glioblastoma specimens and cell lines display heightened CLR/RAMP2–3 expression, a configuration better known as the adrenomedullin (ADM) receptor. ADM and CGRP ligation elevates intracellular cAMP, trans-activates JNK, and drives cyclin-D1–dependent proliferation; neutralising ADM antibodies or silencing CLR attenuate spheroid outgrowth and invasion in xenograft models (53–55). Moreover, CALCRL co-expresses with SERPINE1 and MMP-14 on invasive edge populations, linking CGRP-family signalling to extracellular-matrix turnover (56).
Pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP) complete this convergence. High-affinity PAC1/VIP receptors are present on patient-derived GBM cultures; PACAP/VIP exposure modulates cAMP-EPAC-Rap1 and MAPK cascades, finely balancing proliferation and motility in a context-dependent manner. In hypoxia (pO2 ≤ 1%), VIP-VPAC1 signalling curtails EGFR transcription and suppresses Rac1-dependent motility, yet the same pathway under normoxic conditions activates a cAMP-EPAC1-Rap1 cascade that loosens cadherin adhesion and accelerates migration—underscoring a hypoxia-to-normoxia flip from anti- to pro-migratory behaviour (57).
Downstream, these receptors converge on a protease-rich programme that sculpts the peritumoural matrix. NK1-R and CLR activation heighten transcription and surface trafficking of gelatinases (MMP-2/-9) and MT-MMPs, while concurrently up-regulating αvβ3 integrin and focal-adhesion kinase phosphorylation, which together enable traction through dense parenchyma (52, 56, 58). Targeting this redox-sensitive MT1-MMP hub—activated by migraine-associated ROS—offers combined anti-invasive and anti-angiogenic benefit (58).
Neuropeptide signalling also remodels the immune and vascular niches that guide glioma dispersal. SP induces astrocytoma and microglial release of IL-6, IL-8 and GM-CSF, cytokines that polarise tumour-associated macrophages toward an M2-like, invasion-supportive phenotype and stimulate endothelial VEGF secretion. Single-cell RNA-seq atlases nevertheless identify KIT+ TPSAB1+ mast-cells in < 2% of immune cells at the invasive edge, hinting that a sparse but responsive mast-cell niche may still release histamine and VEGF in reaction to SP or CGRP. In parallel, CGRP-family peptides relax peri-tumoural arterioles, augmenting shear stress and facilitating perivascular migration of tumour cells (59, 60). The resulting feedback between nociceptive neuropeptides, immunocytes and vasculature mirrors the sterile inflammation that sensitises meningeal nociceptors during migraine, but in GBM it is repurposed to carve permissive migratory tracks. Recent connectomes in adult GBM and diffuse midline/paediatric high-grade gliomas show CGRP-positive trigeminal afferents forming AMPA-like synapses that drive calcium-dependent invasion (7, 16).
4 Bridging evidence between migraine and glioblastoma
The epidemiological intersection between episodic migraine and malignant glioma, while subtle, is increasingly discernible. A nationwide, population-based case-control analysis of more than 22–000 adults showed that patients subsequently diagnosed with brain tumours were 2.45-times more likely to have carried a prior migraine diagnosis; the association remained significant after excluding migraines recorded within three years of tumour detection and was especially pronounced in men (odds ratio 3.04) (59). By contrast, a 39 534-participant prospective cohort of female health professionals that relied on self-reported headache phenotypes failed to detect a higher long-term incidence of brain tumours among migraineurs—a discrepancy the authors ascribed to mis-classification and the small number of high-grade tumours captured over 15 years of follow-up (60). Clinically, headache itself is a sentinel manifestation of glioma; a systematic review encompassing 32 studies reported a weighted mean prevalence of 27% across the disease trajectory, ranking it just behind seizures and cognitive change (61). These data—despite potential confounders such as glucocorticoids, anticonvulsants or anti-VEGF therapy—suggest trigeminovascular activation may precede glioma expansion.
Neuro-imaging observations lend anatomical plausibility to this overlap. High-resolution MRI increasingly delineates glioblastoma growth along cisternal and cavernous segments of the trigeminal nerve as well as Meckel’s cave, underscoring the tumour’s capacity to exploit perineural corridors innervated by migraine-relevant afferents (62). Such tracking brings glioma cells into juxtaposition with meningeal vessels and dural mast cells—the very structures targeted during neurogenic inflammation—creating a micro-environment saturated with vasoactive peptides and proteases. Microdialysis and immuno-electron microscopy indicate that CGRP released from perivascular trigeminal endings at the tumour rim dominates this milieu, whereas intraparenchymal cortical fibres contribute less but may facilitate newly discovered neuron-to-tumour synapses.
At the molecular interface, glioblastoma co-opts virtually every neuropeptide axis canonically implicated in migraine. Transcriptomic and proteomic surveys confirm over-expression of tachykinin and calcitonin-family G-protein-coupled receptors, with full-length neurokinin-1 receptor (NK1-R), PAC1, VPAC1/2 and CLR–RAMP isoforms enriched at the invasive margin (63, 64). Functional studies demonstrate that exogenous substance P accelerates GBM chemotaxis, while radiolabelled SP analogues accumulate selectively within tumour parenchyma (65). Selective NK1-R antagonists (e.g., MEN 11467, aprepitant) produce dose-dependent suppression of U373-MG xenograft growth and trigger apoptotic signalling in vitro, directly implicating the SP–NK1-R axis as a driver of invasion rather than a mere epiphenomenon (66). Parallel pathways operate through the CGRP/adrenomedullin family: glioma specimens up-regulate adrenomedullin-2, whose ligation of CLR–RAMP2 boosts ERK1/2 activation, enhances filopodia formation and potentiates temozolomide resistance (49). PACAP and its high-affinity PAC1 receptor are likewise detectable in astrocytic tumours; PACAP modulates cyclic-AMP and MAPK cascades to yield context-dependent trophic or anti-proliferative effects (67, 68).
Pharmacological experience from the migraine field further strengthens this bridge. Aprepitant—licensed for chemotherapy-induced emesis and explored for refractory migraine—induces apoptosis in NK1-R-positive glioma cultures and diminishes Akt phosphorylation in vivo (66, 69, 70). Although gepant-class CGRP antagonists have yet to enter neuro-oncology trials, the demonstrable dependence of ADM/CGRP-responsive glioma sub-populations on CLR signalling nominates these agents as credible dual-purpose therapeutics. The emerging discipline of cancer neurobiology recognises sensory neurons as active architects of tumour behaviour, providing a conceptual scaffold that unifies migraine neurogenic inflammation with glioblastoma invasion (71).
Taken together, epidemiological signals, radiological patterns and convergent neuropeptide circuitry converge on a common narrative: the trigeminovascular system and its inflammatory mediators do not merely coexist with glioblastoma but actively facilitate its permeation through neural and perivascular channels.
5 Therapeutic perspectives and future directions
A growing body of translational evidence positions the trigeminal neuropeptide axis as more than an epiphenomenon of tumour-induced pain; instead, it represents a tractable vulnerability that can be co-targeted to blunt glioblastoma (GBM) infiltration while simultaneously alleviating migraine-like symptomatology. The convergence of CGRP, substance P, PACAP and adrenomedullin signalling on matrix-remodelling, angiogenesis and immune polarisation suggests that pharmacological or radiopharmaceutical interruption of these cues could deliver a double therapeutic dividend.
Repurposing clinically approved antagonists is the most immediate path to bedside impact. Yet BBB pharmacokinetics diverge sharply: anti-CGRP monoclonal antibodies (~150 kDa) cross an intact barrier at < 0.1% ID, restricting action to regions of contrast enhancement, whereas the lipophilic NK1-antagonist aprepitant (MW ≈ 534 Da, logP ≈ 3.5) reaches CSF-to-plasma ratios of ~0.05—adequate for receptor occupancy but still sub-therapeutic in deeply infiltrated zones. Moreover, chronic CGRP blockade erodes vasodilatory reserve, slows dermal repair, and may blunt dendritic priming—risks that must be balanced during multimodal therapy. Aprepitant, an oral neurokinin-1 receptor (NK1-R) blocker widely used for chemotherapy-induced nausea, induces apoptosis, suppresses lamellipodial dynamics and synergises with 5-aminolevulinic acid in patient-derived GBM cultures (72, 73). Compassionate-use series and the multi-drug CUSP9 protocol already exploit this agent in recurrent disease, providing a safety dossier that greatly exceeds that of de-novo anticancer compounds. Parallel interest surrounds gepant CGRP antagonists now used for migraine; however, migraine doses yield sub-micromolar intratumour exposure, so carrier-mediated delivery or escalation will be necessary to exploit CLR/RAMP2–3 dependency (74, 75).
Where systemic small molecules may falter against the blood-brain barrier, peptide-based radiotheranostics offer focal, high-linear-energy transfer delivery. Targeted α-therapy with NK1-directed peptides has yielded encouraging but still preliminary data: the largest 225Ac-DOTA-Substance P dose-escalation study (n = 21, median 3 cycles) reported grade-3 thrombocytopenia in 1/21 patients and a median overall survival (OS) of 9.0 months from first dose, with RANO responses limited to one partial response and seven cases of stable disease. An earlier 213Bi-DOTA-Substance P trial (n = 20, cumulative activity ≤ 11.2 GBq) recorded only transient grade 1/2 toxicities and a 7.5-month median OS from therapy start, again with low objective-response rates (76, 77). Updated European Association of Nuclear Medicine guidelines now list NK1-R–targeted alpha therapy among the most mature investigational options for infiltrative glioma (78, 79), while complementary β-emitting constructs are entering first-in-human evaluation. Such vector-agnostic platforms could, in principle, be adapted to CGRP or PACAP receptors as radioligand scaffolds once high-affinity ligands become available.
Adrenomedullin, a close CGRP family member, exemplifies the broader potential of neuropeptide blockade. Neutralising antibodies or small-molecule antagonists down-regulate JNK–cyclin-D1 signalling, curb spheroid expansion and enhance temozolomide sensitivity in xenografts (80, 81). Given its additional roles in vascular permeability, dual adrenomedullin/VEGF inhibition could synergistically normalise aberrant tumour vessels, thereby improving immune-cell ingress and drug delivery.
Future development will hinge on precise patient stratification. Receptor-specific PET tracers (e.g., 68Ga-labelled NK1-R or uPAR ligands) already delineate invasive margins with sub-centimetre resolution and could guide convection-enhanced delivery catheters or focused-ultrasound BBB modulation. Integration of these imaging biomarkers with plasma neuropeptide signatures promises real-time pharmacodynamic read-outs and early identification of escape pathways. Coupling neuropeptide blockade with immunotherapy is mechanistically attractive because CGRP- and SP-driven M2 polarisation up-regulates PD-L1; neutralising these cues may re-programme macrophages and rest.
Author contributions
XS: Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing. QZ: Data curation, Investigation, Visualization, Writing – original draft. JZ: Data curation, Investigation, Writing – original draft. JY: Visualization, Writing – original draft. XZ: Writing – original draft. QS: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Innovation Incubation Project of First Teaching Hospital of Tianjin University of Traditional Chinese Medicine (ZZ2024014); Open Project of National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion (NCRCOP2023011); Scientific Research Program of Hebei Provincial Administration of Traditional Chinese Medicine (T2025099).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Elser H, Skajaa N, Ehrenstein V, Fuglsang CH, Farkas DK, Sørensen HT, et al. Cancer risk in patients with migraine: a population-based cohort study in Denmark. Headache: J Head Face Pain. (2022) 62:57–64. doi: 10.1111/head.14251
2. Murata T, Matsuda M, Shinozaki T, and Ishiyama K. Glioblastoma, IDH-wildtype with leptomeningeal metastasis to Meckel’s cave: A case report. Acta Radiologica Open. (2022) 11:20584601221131480. doi: 10.1177/20584601221131480
3. Qin Z, Bian XW, and Shi Y. Identification of hypoxic macrophages in glioblastoma: Unveiling therapeutic insights from tumour microenvironment analysis. Clin Trans Med. (2024) 14:e70013. doi: 10.1002/ctm2.70013
4. Xiong J, Zhou X, Su L, Jiang L, Ming Z, Pang C, et al. The two-sided battlefield of tumour-associated macrophages in glioblastoma: unravelling their therapeutic potential. Discover Oncol. (2024) 15:1–13. doi: 10.1007/s12672-024-01464-5
5. Królicki L, Kunikowska J, Bruchertseifer F, Kulinski R, Pawlak D, Koziara H, et al. Locoregional treatment of glioblastoma with targeted α Therapy:[: 213: bi] bi-DOTA–substance P versus [: 225: ac] ac-DOTA–substance P—Analysis of influence parameters. Clin Nucl Med. (2023) 48:387–92.
6. Gu S, Shu L, Zhou L, Wang Y, Xue H, Jin L, et al. Interfering with CALCRL expression inhibits glioma proliferation, promotes apoptosis, and predicts prognosis in low-grade gliomas. Ann Trans Med. (2022) 10:1277. doi: 10.21037/atm-22-5154
7. Sun Y, Wang X, Zhang DY, Zhang Z, Bhattarai JP, Wang Y, et al. Brain-wide neuronal circuit connectome of human glioblastoma. Nature. (2025) 641:1–3. doi: 10.1038/s41586-025-08634-7
8. Krishna S, Choudhury A, Keough MB, Seo K, Ni L, Kakaizada S, et al. Glioblastoma remodelling of human neural circuits decreases survival. Nature. (2023) 617:599–607. doi: 10.1038/s41586-023-06036-1
9. Chen Z, Zhang S, Jiang C, et al. Integrating multi-omics data to identify the role of Aggrephagy-related genes in tumor microenvironment and key tumorigenesis factors of GB from the perspective of single-cell sequencing. Discover Oncol. (2025) 16:1–27. doi: 10.1007/s12672-025-02431-4
10. Kawamura S, Katsuki M, Kashiwagi K, and Koh A. Fremanezumab improved migraine and headache attributed to glioblastoma-case report. (2022). doi: 10.21203/rs.3.rs-2014225/v1
11. Ashina M, Phul R, Khodaie M, Löf E, and Florea I. A monoclonal antibody to PACAP for migraine prevention. New Engl J Med. (2024) 391:800–9. doi: 10.1056/NEJMoa2314577
12. Lee S, Weiss T, Bühler M, Mena J, Lottenbach Z, Wegmann R, et al. High-throughput identification of repurposable neuroactive drugs with potent anti-glioblastoma activity. Nat Med. (2024) 30:3196–208. doi: 10.1038/s41591-024-03224-y
13. Al-Karagholi MAM, Zhuang ZA, Beich S, Ashina H, and Ashina M. PACAP38-induced migraine attacks are independent of CGRP signaling: a randomized controlled trial. J Headache Pain. (2025) 26:79. doi: 10.1186/s10194-025-02022-2
14. Shen Y, Chi H, Xu K, et al. A novel classification model for lower-grade glioma patients based on pyroptosis-related genes. Brain Sci. (2022) 12:700. doi: 10.3390/brainsci12060700
15. Della Pietra A, Gómez Dabó L, Mikulenka P, Espinoza-Vinces C, Vuralli D, Baytekin I, et al. Mechanosensitive receptors in migraine: a systematic review. J Headache Pain. (2024) 25:6. doi: 10.1186/s10194-023-01710-1
16. Taylor KR, Barron T, Hui A, Spitzer A, Yalçin B, Ivec AE, et al. Glioma synapses recruit mechanisms of adaptive plasticity. Nature. (2023) 623:366–74. doi: 10.1038/s41586-023-06678-1
17. Hou Y, Lin B, Xu T, Jiang J, Luo S, Chen W, et al. The neurotransmitter calcitonin gene-related peptide shapes an immunosuppressive microenvironment in medullary thyroid cancer. Nat Commun. (2024) 15:5555. doi: 10.1038/s41467-024-49824-7
18. Barron T, Yalçın B, Su M, Byun YG, Gavish A, Shamardani K, et al. GABAergic neuron-to-glioma synapses in diffuse midline gliomas0. Nature. (2025) 639:1–9.
19. Rezaei S, Assaran Darban R, Javid H, and Hashemy SI. The therapeutic potential of aprepitant in glioblastoma cancer cells through redox modification. BioMed Res Int. (2022) 2022:8540403. doi: 10.1155/2022/8540403
20. Zhao S, Chi H, Yang Q, et al. Identification and validation of neurotrophic factor-related gene signatures in glioblastoma and Parkinson’s disease. Front Immunol. (2023) 14:1090040. doi: 10.3389/fimmu.2023.1090040
21. Królicki L, Bruchertseifer F, Kunikowska J, Koziara H, Pawlak D, Kulinski R, et al. Dose escalation study of targeted alpha therapy with [225 Ac] Ac-DOTA-substance P in recurrence glioblastoma–safety and efficacy. Eur J Nucl Med Mol Imaging. (2021) 48:3595–605.
22. Roncali L, Marionneau-Lambot S, Roy C, Eychenne R, Gouard S, Avril S, et al. Brain intratumoural astatine-211 radiotherapy targeting syndecan-1 leads to durable glioblastoma remission and immune memory in female mice. EBioMedicine. (2024) 105:105. doi: 10.1016/j.ebiom.2024.105202
23. Muñoz MF, Argüelles S, Rosso M, Medina R, Coveñas R, Ayala A, et al. The neurokinin-1 receptor is essential for the viability of human glioma cells: A possible target for treating glioblastoma. BioMed Res Int. (2022) 2022:6291504.
24. Wu Z, Yang Y, Chen M, and Zha Y. Matrix metalloproteinase 9 expression and glioblastoma survival prediction using machine learning on digital pathological images. Sci Rep. (2024) 14:15065. doi: 10.1038/s41598-024-66105-x
25. Azam A, Kurbegovic S, Carlsen EA, Andersen TL, Larsen VA, Law I, et al. Prospective phase II trial of [68Ga] Ga-NOTA-AE105 uPAR-PET/MRI in patients with primary gliomas: Prognostic value and Implications for uPAR-targeted Radionuclide Therapy. EJNMMI Res. (2024) 14:100. doi: 10.1186/s13550-024-01164-9
26. Cao Q, Wang Q, Wu X, et al. A literature review: mechanisms of antitumor pharmacological action of leonurine alkaloid. Front Pharmacol. (2023) 14:1272546. doi: 10.3389/fphar.2023.1272546
27. Wang W, Li T, Cheng Y, Li F, Qi S, Mao M, et al. Identification of hypoxic macrophages in glioblastoma with therapeutic potential for vasculature normalization. Cancer Cell. (2024) 42:815–832. e12. doi: 10.1016/j.ccell.2024.03.013
28. de Vries T, Boucherie DM, Chan KY, Rubio-Beltrán E, Labastida-Ramírez A, Labruijere S, et al. Sex differences in CGRP-induced vasodilation of human middle meningeal arteries but not human coronary arteries: implications for migraine. Cephalalgia. (2024) 44:03331024241254088. doi: 10.1177/03331024241254088
29. Thanh HD, Lee S, Nguyen TT, Huu TN, Ahn EJ, Cho SH, et al. Temozolomide promotes matrix metalloproteinase 9 expression through p38 MAPK and JNK pathways in glioblastoma cells. Sci Rep. (2024) 14:14341. doi: 10.1038/s41598-024-65398-2
30. Zhai Y, Sang W, Su L, Shen Y, Hu Y, and Zhang W. Analysis of the expression and prognostic value of MT1-MMP, β1-integrin and YAP1 in glioma. Open Med. (2022) 17:492–507. doi: 10.1515/med-2022-0449
31. Versijpt J, Paemeleire K, Reuter U, and MaassenVanDenBrink A. Calcitonin gene-related peptide-targeted therapy in migraine: current role and future perspectives. Lancet. (2025) 405:1014–26. doi: 10.1016/S0140-6736(25)00109-6
32. Mohamed MEF, Bhatnagar S, Parmentier JM, Nakasato P, and Wung P. Upadacitinib: Mechanism of action, clinical, and translational science. Clin Trans Sci. (2024) 17:e13688. doi: 10.1111/cts.13688
33. Luo Y, Liu R, Zhang H, Wang H, Yin H, Tian G, et al. Amantadine against glioma via ROS-mediated apoptosis and autophagy arrest. Cell Death Dis. (2024) 15:834. doi: 10.1038/s41419-024-07228-x
34. Russo AF and Hay DL. CGRP physiology, pharmacology, and therapeutic targets: migraine and beyond. Physiol Rev. (2023) 103:1565–644. doi: 10.1152/physrev.00059.2021
35. Mangrum R, Gerstein MT, Hall III C J, Buse DC, Houts CR, McGinley JS, et al. Priority acute and preventive migraine treatment benefits: Results of the Migraine Clinical Outcome Assessment System (MiCOAS) qualitative study of people living with migraine. Headache: J Head Face Pain. (2023) 63:953–64. doi: 10.1111/head.14521
36. Tetzlaff SK, Reyhan E, Layer N, Bengtson CP, Heuer A, Schroers J, et al. Characterizing and targeting glioblastoma neuron-tumor networks with retrograde tracing. Cell. (2025) 188:390–411. e36. doi: 10.1016/j.cell.2024.11.002
37. Guan LC, Dong X, and Green DP. Roles of mast cells and their interactions with the trigeminal nerve in migraine headache. Mol Pain. (2023) 19:17448069231181358. doi: 10.1177/17448069231181358
38. Pellesi L and Edvinsson L. Revisiting substance P in migraine: a methodological approach inspired by anti-CGRP and anti-PACAP success. J Headache Pain. (2025) 26:22. doi: 10.1186/s10194-025-01959-8
39. Gárate G, Pascual J, Pascual-Mato M, Madera J, Martín MM, and González-Quintanilla V. Untangling the mess of CGRP levels as a migraine biomarker: an in-depth literature review and analysis of our experimental experience. J Headache Pain. (2024) 25:69. doi: 10.1186/s10194-024-01769-4
40. Al-Khazali HM, Ashina H, Wiggers A, Rose K, Iljazi A, Christensen RH, et al. Calcitonin gene-related peptide causes migraine aura. J Headache Pain. (2023) 24:124. doi: 10.1186/s10194-023-01656-4
41. Thakur V, Thakur VS, Aguila B, Slepak TI, Wang M, Song W, et al. Targeting extracellular matrix remodeling sensitizes glioblastoma to ionizing radiation. Neuro-oncol Adv. (2022) 4:vdac147. doi: 10.1093/noajnl/vdac147
42. Dana N, Dabiri A, Najafi MB, Rahimi A, Ishaghi SMM, Shariati L, et al. Advances in bioengineered CAR T/NK cell therapy for glioblastoma: Overcoming immunosuppression and nanotechnology-based strategies for enhanced CAR T/NK cell therapy. Bioengineering Trans Med. (2025) 10:e10716. doi: 10.1002/btm2.10716
43. Wu J, Lu X, and Yan C. Neuro-immune-cancer interactions: Mechanisms and therapeutic implications for tumor modulation. Brain Behav Immun Integr. (2025) 10:100119. doi: 10.1016/j.bbii.2025.100119
44. Grazzi L, Giossi R, Montisano DA, Canella M, Marcosano M, Altamura C, et al. Real-world effectiveness of Anti-CGRP monoclonal antibodies compared to OnabotulinumtoxinA (RAMO) in chronic migraine: a retrospective, observational, multicenter, cohort study. J Headache Pain. (2024) 25:14. doi: 10.1186/s10194-024-01721-6
45. Zhao W, Zhang Z, Xie M, Ding F, Zheng X, Sun S, et al. Exploring tumor-associated macrophages in glioblastoma: from diversity to therapy. NPJ Precis Oncol. (2025) 9:126. doi: 10.1038/s41698-025-00920-x
46. Yang H, Cheng J, Zhuang H, Xu H, Wang Y, Zhang T, et al. Pharmacogenomic profiling of intra-tumor heterogeneity using a large organoid biobank of liver cancer. Cancer Cell. (2024) 42:535–551. e8. doi: 10.1016/j.ccell.2024.03.004
47. Wang C, Chen S, Cheng Z, Xia S, Fei CJ, Ye L, et al. Characteristics of locus coeruleus functional connectivity network in patients with comorbid migraine and insomnia. J Headache Pain. (2024) 25:159. doi: 10.1186/s10194-024-01877-1
48. Weller M, Albert NL, Galldiks N, Bink A, Preusser M, Sulman EP, et al. Targeted radionuclide therapy for gliomas: emerging clinical trial landscape. Neuro-Oncology. (2024) 26:S208–14. doi: 10.1093/neuonc/noae125
49. He Z, Cheng M, Hu J, Liu L, Liu P, Chen L, et al. miR-1297 sensitizes glioma cells to temozolomide (TMZ) treatment through targeting adrenomedullin (ADM). J Trans Med. (2022) 20:443. doi: 10.1186/s12967-022-03647-6
50. Raswoli M, Tsang DS, Zadeh G, Gao AF, and Shultz DB. Malignant mimics of trigeminal schwannoma. Adv Radiat Oncol. (2023) 8:3196–208. doi: 10.1016/j.adro.2022.101056
51. Song KW, Lim M, and Monje M. Complex neural-immune interactions shape glioma immunotherapy. Immunity. (2025) 26(Supplement_9):S208-S214. doi: 10.1016/j.immuni.2025.04.017
52. Darragh LB, Nguyen A, Pham TT, Idlett-Ali S, Knitz MW, Gadwa J, et al. Sensory nerve release of CGRP increases tumor growth in HNSCC by suppressing TILs. Med. (2024) 5:254–270. e8. doi: 10.1016/j.medj.2024.02.002
53. Roncali L, Hindré F, Samarut E, Lacoeuille F, Rousseau A, Lemée JM, et al. Current landscape and future directions of targeted-alpha-therapy for glioblastoma treatment. Theranostics. (2025) 15:4861. doi: 10.7150/thno.106081
54. Manoharan VT, Abdelkareem A, Gill G, Brown S, Gillmor A, Hall C, et al. Spatiotemporal modeling reveals high-resolution invasion states in glioblastoma. Genome Biol. (2024) 25:264. doi: 10.1186/s13059-024-03407-3
55. Sabri ME, Moghaddasi L, Wilson P, Saran F, and Bezak E. Targeted alpha therapy for glioblastoma: review on in vitro, in vivo and clinical trials. Targeted Oncol. (2024) 19:511–31. doi: 10.1007/s11523-024-01071-y
56. Batchu S, Hanafy KA, Redjal N, Godil SS, and Thomas AJ. Single-cell analysis reveals diversity of tumor-associated macrophages and their interactions with T lymphocytes in glioblastoma. Sci Rep. (2023) 13:20874. doi: 10.1038/s41598-023-48116-2
57. Tang F, Wang Y, Zeng Y, Xiao A, Tong A, and Xu J. Tumor-associated macrophage-related strategies for glioma immunotherapy. NPJ Precis Oncol. (2023) 7:78. doi: 10.1038/s41698-023-00431-7
58. Chang M. Matrix metalloproteinase profiling and their roles in disease. RSC Adv. (2023) 13:6304–16. doi: 10.1039/D2RA07005G
59. Chen CH, Sheu JJ, Lin YC, and Lin HC. Association of migraines with brain tumors: a nationwide population-based study. J headache Pain. (2018) 19:1–7. doi: 10.1186/s10194-018-0944-1
60. Kurth T, Buring JE, and Rist PM. Headache, migraine and risk of brain tumors in women: prospective cohort study. J headache Pain. (2015) 16:1–6. doi: 10.1186/s10194-015-0501-0
61. IJzerman-Korevaar M, Snijders TJ, de Graeff A, Teunissen SCCM, and de Vos FYF. Prevalence of symptoms in glioma patients throughout the disease trajectory: a systematic review. J neuro-oncol. (2018) 140:485–96. doi: 10.1007/s11060-018-03015-9
62. Donia MM, Gamaleldin OA, Abdo AM, Desouky SED, and Helmy SAS. Intracranial neoplastic lesions of the trigeminal nerve: How MRI can help. Egyptian J Radiol Nucl Med. (2017) 48:1035–41. doi: 10.1016/j.ejrnm.2017.07.008
63. Cherry AE and Stella N. G protein-coupled receptors as oncogenic signals in glioma: emerging therapeutic avenues. Neuroscience. (2014) 278:222–36. doi: 10.1016/j.neuroscience.2014.08.015
64. Frame E, Bobba K, Gunter D, Mihailescu L, Bidkar A, Flavell R, et al. Coded aperture and Compton imaging for the development of 225Ac-based radiopharmaceuticals. Med Phys. (2023) 50:6454–68. doi: 10.1002/mp.16717
65. Królicki L, Kunikowska J, Bruchertseifer F, Koziara H, Królicki B, Jakucinski M, et al. 225Ac-and 213Bi-substance P analogues for glioma therapy[C]//Seminars in nuclear medicine. WB Saunders. (2020) 50:141–51.
66. Nizam E and Erin N. Differential consequences of neurokinin receptor 1 and 2 antagonists in metastatic breast carcinoma cells; Effects independent of Substance P. Biomed Pharmacother. (2018) 108:263–70. doi: 10.1016/j.biopha.2018.09.013
67. Tassorelli C, Nagy K, Pozo-Rosich P, Lanteri-Minet M, Sacco S, Nežádal T, et al. Safety and efficacy of atogepant for the preventive treatment of episodic migraine in adults for whom conventional oral preventive treatments have failed (ELEVATE): a randomised, placebo-controlled, phase 3b trial. Lancet Neurol. (2024) 23:382–92. doi: 10.1016/S1474-4422(24)00025-5
68. Pinto CIG, Guerreiro JF, Silva F, Mendes F, and Paulo A. Radiopharmaceuticals for molecular imaging and theranostics of glioblastoma. New Insights Into Glioblastoma. (2023) 50:667–705. doi: 10.1016/B978-0-323-99873-4.00023-2
69. Langston RG, Wardell CP, Palmer A, Scott H, Gokden M, Pait TG, et al. Primary glioblastoma of the cauda equina with molecular and histopathological characterization: case report. Neuro-Oncol Adv. (2021) 3:vdab154. doi: 10.1093/noajnl/vdab154
70. Marchesini N, Bernasconi R, Ghimenton C, and Pinna G. Glioblastoma multiforme with oculomotor nerve involvement: case report and literature review. Br J Neurosurg. (2023) 37:1228–32. doi: 10.1080/02688697.2020.1837732
71. Huang S, Zhu J, Yu L, Huang Y, and Hu Y. Cancer-nervous system crosstalk: from biological mechanism to therapeutic opportunities. Mol Cancer. (2025) 24:133. doi: 10.1186/s12943-025-02336-4
72. Ha CP, Hua TNM, Vo VTA, Om J, Han S, Cha SK, et al. Humanin activates integrin αV–TGFβ axis and leads to glioblastoma progression. Cell Death Dis. (2024) 15:464. doi: 10.1038/s41419-024-06790-8
73. Wen PY, Preusser M, and Albert NL. Design and conduct of theranostic trials in neuro-oncology: Challenges and opportunities. Neuro-Oncology. (2024) 26:S199–207. doi: 10.1093/neuonc/noae162
74. Lv W and Wang Y. Neural influences on tumor progression within the central nervous system. CNS Neurosci Ther. (2024) 30:e70097. doi: 10.1111/cns.70097
75. Knowles LM, Wolter C, Linsler S, Müller S, Urbschat S, Ketter R, et al. Clotting promotes glioma growth and infiltration through activation of focal adhesion kinase. Cancer Res Commun. (2024) 4:3124–36. doi: 10.1158/2767-9764.CRC-24-0164
76. Golchin MM, Arefian E, Fekrirad Z, and Tabar GH. miR-124-mediated temozolomide sensitivity and DNA repair modulation in Glioblastoma Multiforme. Neuroscience. (2025) 573:52–63. doi: 10.1016/j.neuroscience.2025.03.010
77. Gonzalez-Aponte MF, Damato AR, Simon T, Aripova N, Darby F, Jeon MS, et al. Daily glucocorticoids promote glioblastoma growth and circadian synchrony to the host. Cancer Cell. (2025) 43:144–160. e7. doi: 10.1016/j.ccell.2024.11.012
78. Chokshi CR, Shaikh MV, Brakel B, Rossotti MA, Tieu D, Maich W, et al. Targeting axonal guidance dependencies in glioblastoma with ROBO1 CAR T cells. Nat Med. (2024) 30:2936–46. doi: 10.1038/s41591-024-03138-9
79. Zhi X, Wu F, Qian J, Ochiai Y, Lian G, Malagola E, et al. Nociceptive neurons promote gastric tumour progression via a CGRP–RAMP1 axis. Nature. (2025) 4:1–9. doi: 10.1038/s41586-025-08591-1
80. Nelson-Maney NP, Bálint L, Beeson ALS, Serafin DS, Kistner BM, Douglas ES, et al. Meningeal lymphatic CGRP signaling governs pain via cerebrospinal fluid efflux and neuroinflammation in migraine models. J Clin Invest. (2024) 134:52–63. doi: 10.1172/JCI175616
Keywords: migraine, glioblastoma, trigeminal nerve, neurogenic inflammation, neuropeptides
Citation: Song X, Zhu Q, Zhang J, Yang J, Zhang X and Song Q (2025) Trigeminal nerve-driven neurogenic inflammation linking migraine to glioblastoma invasion: a literature review. Front. Immunol. 16:1632154. doi: 10.3389/fimmu.2025.1632154
Received: 20 May 2025; Accepted: 24 June 2025;
Published: 16 July 2025.
Edited by:
Qihang Yuan, Dalian Medical University, ChinaReviewed by:
Jinwei Li, Sichuan University, ChinaAierpati Maimaiti, First Affiliated Hospital of Xinjiang Medical University, China
Copyright © 2025 Song, Zhu, Zhang, Yang, Zhang and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qian Song, ZHJzb25nMzU4MkAxNjMuY29t
†These authors have contributed equally to this work
 Xiaoli Song
Xiaoli Song Qian Zhu
Qian Zhu Jieying Zhang
Jieying Zhang Jin Yang1,2
Jin Yang1,2 Qian Song
Qian Song