- 1Senior Department of Hematology, The Fifth Medical Center of Chinese PLA General Hospital, Beijing, China
- 2Translational Medicine Research Center, Medical Innovation Research Division of The Fourth Medical Center of Chinese PLA General Hospital, Beijing, China
- 3Department of Emergency Medicine, The Second Medical Center of Chinese PLA General Hospital, Beijing, China
- 4Department of Internal Medicine, Medical School of Chinese PLA, Beijing, China
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative therapy for hematologic malignancies. However, the initial clinical experience with allo-HSCT revealed a concerning prevalence of severe graft-versus-host disease (GVHD) and graft failure. Subsequent randomized studies highlighted the role of anti-thymocyte globulin (ATG) in reducing acute and chronic GVHD and graft failure, although it did not improve overall survival. Pharmacodynamic studies have established an association between ATG concentration and the incidence of GVHD and life-threatening infections. However, ATG concentration at designated timepoints showed no such correlations with non-relapse mortality and overall survival in allo-HSCT. There is a delicate balance between ATG exposure and the outcomes of allo-HSCT. More specifically, insufficient ATG exposure may diminish its function on GVHD prophylaxis, while excessive ATG may delay immune reconstitution and increase risk of disease relapse and infection. Considering the significant inter-individual heterogeneity in ATG pharmacokinetics, individualized ATG dosing could potentially increase the proportion of transplant recipients attaining the optimal ATG exposure. Recent studies have shown that individualized ATG dosing, guided by absolute lymphocyte count or therapeutic drug monitoring, can improve optimal exposure attainment rate. Which indicated a potential approach to achieve superior transplant outcomes. This review summarizes the advances and the challenges of individualized ATG dosing in allo-HSCT.
1 Introduction
Despite advances in chemotherapy and novel cellular therapies, allogeneic hematopoietic stem cell transplantation (allo-HSCT) remains a well-established curative therapy for defined subsets of hematologic malignancies (1, 2). In its initial historical application decades ago, allo-HSCT was associated with substantial risks, including graft-versus-host disease (GVHD) and graft failure (GF) (3, 4). Multiple randomized trials demonstrated that anti-thymocyte globulin (ATG) could reduce the incidence of both severe acute (aGVHD) and chronic GVHD (cGVHD) post-transplant (5–7), while some randomized studies reported no significant improvement in cGVHD-free survival in specific cohorts with ATG (8, 9). Collectively, these findings provide a compelling rationale for the incorporation of ATG into allo-HSCT to prevent GVHD.
ATG is a polyclonal antibody that could deplete a variety of immune cells, while the primary mechanism of GVHD prophylaxis is T-cell depletion (10). Historically, three main types of ATG products have been available for clinical use. The first ATG formulation was horse-derived ATG (ATGAM®, Pfizer, USA) (11). ATGAM® is not typically used for the indication of allo-HSCT, as two prospective trials failed to demonstrate its efficacy in prophylaxis of aGVHD (12, 13). The other two ATGs, Thymoglobulin® (ATG-T, Sanofi, France) and Grafalon® (formerly known as ATG-Fresenius, ATG-F, Neovii, Germany), are both derived from rabbits. Although most of these products are commercially available, ATG-T remains the most commonly used ATG preparation in clinical practice (14, 15). Consequently, this review will focus on the investigations into optimizing the dosage of ATG-T. It is important to note that there is no universally accepted bioequivalent dosing between ATG-T and ATG-F, special caution should be exercised when switching between the two ATG preparations in clinical practice (16–18).
Pharmacological studies of ATG found that the immunological effects of ATG are critically influenced by its concentration (19–26). Therefore, optimizing ATG dose in allo-HSCT to maximize its GVHD prophylaxis effect and minimize its potential side effects is crucial for improving transplant outcomes (14, 27, 28). Early studies explored the optimal ATG dose using body weight-adjusted dosing strategy (6, 8, 29). However, due to ATG pharmacokinetics being influenced by body weight of recipients, lymphocyte count and timing of ATG administration, the inter-individual heterogeneity is considerable (30–32). As such, the optimal ATG dose in allo-HSCT has not yet been determined. Given the ATG pharmacokinetic heterogeneity among transplant recipients, individualized ATG dosing may be a potential solution and has garnered significant research interest. Recent pharmacological studies have found that optimal ATG exposure is associated with lower incidence of GVHD and virus reactivation, and may even lead to improved non-relapse mortality (NRM) and overall survival (OS) (33–35). Importantly, achieving optimal ATG exposure through individualized dosing can reduce adverse events in allo-HSCT and improve health-related quality of life (19, 36, 37).
This review aims to provide a comprehensive summary of the advances of individualized ATG dosing in allo-HSCT and its effect on transplant outcomes.
2 Immunomodulatory effects and concentration detection of ATG
ATG-T is a heterologous polyclonal immunoglobulin G (IgG) that targets over 40 antigens (14, 15). These antigens are classified into two categories based on their biological function: immune cell response antigens and adhesion/cell-trafficking molecules (Figure 1) (14, 15, 38, 39). ATG-T mediates its immunomodulatory effects primarily by targeting T cells and other immune effector cells. It targets key T-cell antigens, including CD2, CD3, CD4, CD5, CD6, CD8, CD28 and HLA class I molecules, leading to T-cell depletion via complement-dependent lysis and T-cell activation-induced apoptosis (38, 40). Additionally, ATG-T also contains antibodies against B-cell surface proteins CD5, CD19, CD20, CD30, CD38, CD40, CD80, CD95, CD138 and HLA-DR, triggering caspase- and cathepsin-dependent B cell apoptosis (38, 40, 41). Furthermore, ATG-T could inhibit dendritic cell (DC) maturation and migration by targeting CD1a, CD4, CD11a, CD11b, CD29, CD32, CD51/61, CD86, MHC I and MHC II (38, 42). In vitro studies have demonstrated its capacity to expand CD4+ CD25+ regulatory T cells (Tregs) by targeting CTLA-4, FOXP3, GITR (43, 44). Finally, ATG modulates leukocyte-endothelial interactions by targeting integrins (VLA-4, LPAM-1), chemokine receptors (CXCR4, CCR5, CCR7), and leukocyte adhesion molecules (ICAM-1, ICAM-2, ICAM-3), thereby disrupting leukocyte adhesion to endothelia (38, 45).
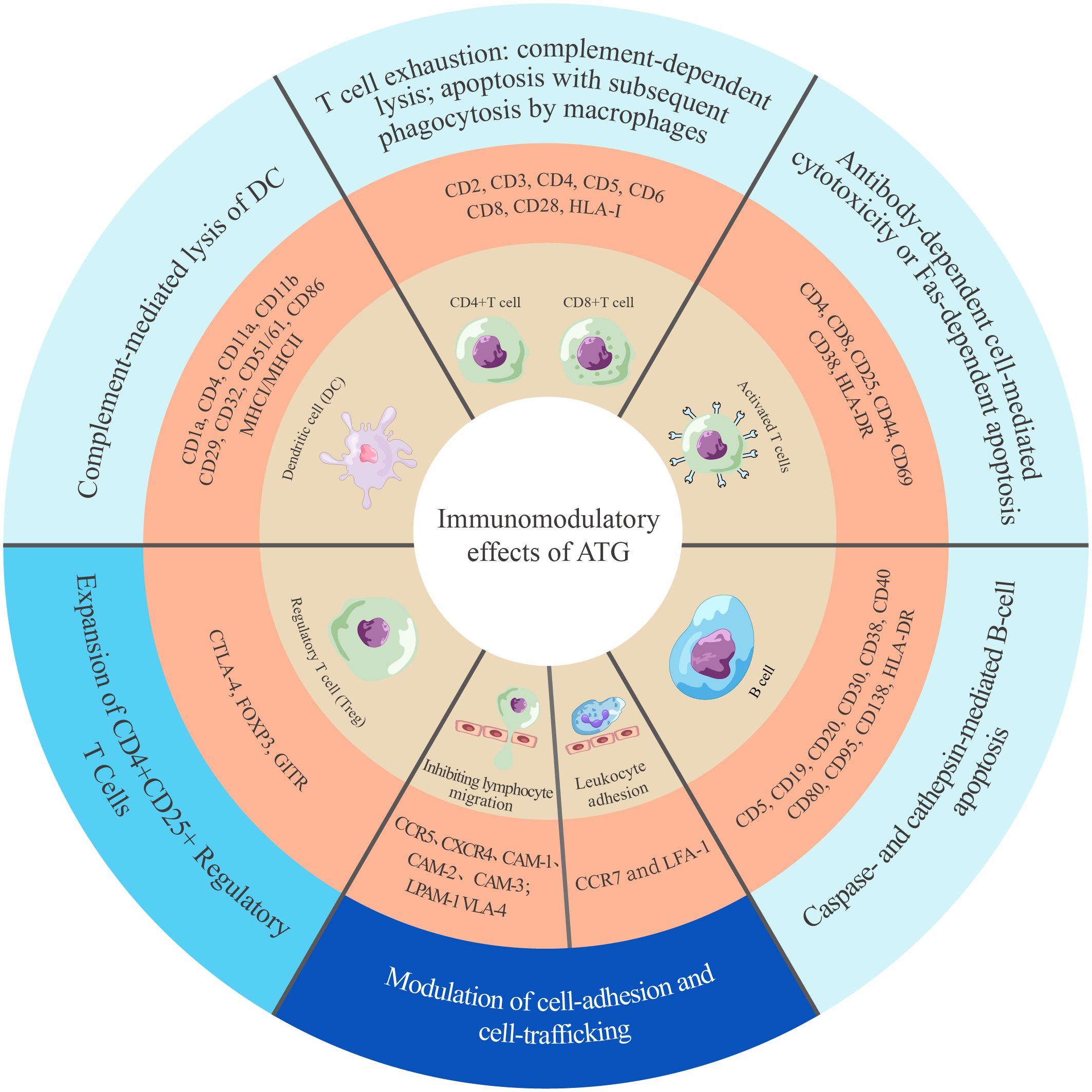
Figure 1. Landscape of ATG-induced immunomodulation mechanisms. The mechanisms are categorized into three groups, indicated by colors in the outermost circle: cell clearance and apoptosis (light blue) (14, 38, 41, 42), cell expansion (lake blue) (43), and cell adhesion and trafficking (dark blue) (45). CAM, cell adhesion molecule; CCR, C-C chemokine receptor; CD, cluster of differentiation; CTLA, cytotoxic T-lymphocyte antigen; CXCR, C-X-C chemokine receptor; DC, dentritic cell; FOXP3, forkhead box P3; GITR, glucocorticoid-induced tumor necrosis factor receptor family-related protein; HLA-DR, human leukocyte antigen-DR isotype; HLA-I/II, human leukocyte antigen class I/II; LFA, lymphocyte function-associated antigen; LPAM, lymphocyte Peyer’s patch adhesion molecule; VLA, very late antigen.
It is important to note that the immunomodulatory effects of ATG depended critically on its concentration. Specifically, a low dose of ATG (e.g., 1 mg/kg) is sufficient to induce antibody-dependent cell-mediated cytotoxicity (ADCC) against activated T cells in blood circulation. However, this ATG concentration is inadequate for depleting lymphocytes (T cells, B cells and NK cells) and antigen-presenting cells residing within secondary lymphoid tissues (22, 46). Additionally, B cells (CD20+) and NK cells (CD16+/CD56+) may only be affected at higher doses (> 5mg/kg) of ATG-T (22). Lower-dose ATG selectively depleted activated T cells while preserving the function of B and NK cells, thereby mitigating systemic immunosuppression (14). Although the effects of ATG are dose-dependent on various cell types, it needs special caution to adjust the dose of ATG for individuals to improve the efficacy of HSCT.
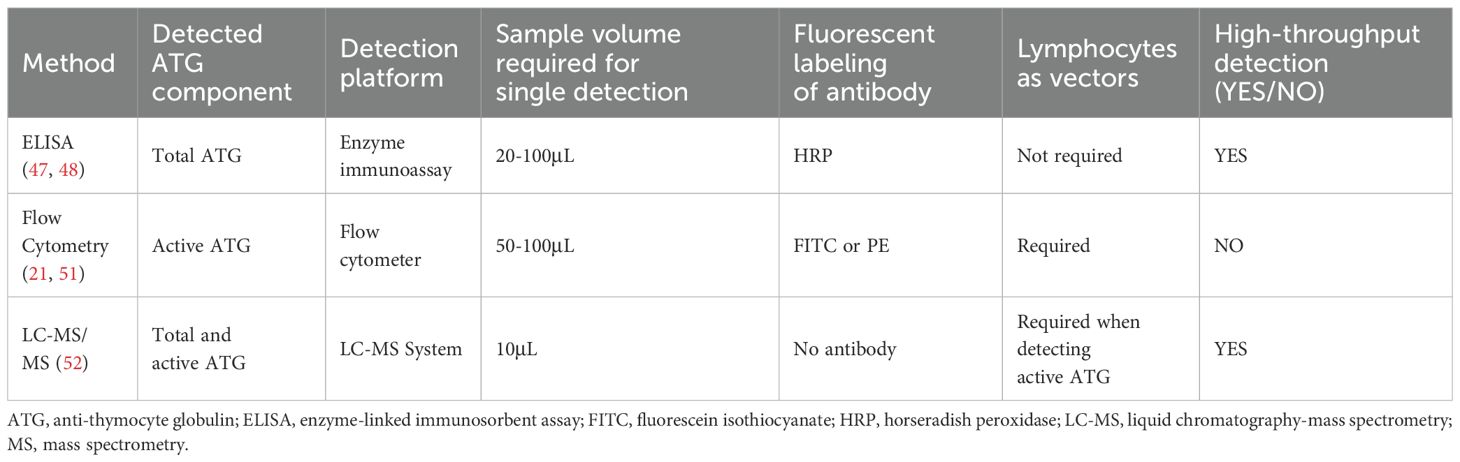
The concentration of ATG, often labeled on the vial, generally refers to the total ATG. Total ATG levels in patient samples could be quantified by enzyme-linked immunosorbent assay (ELISA) (47, 48). The component capable of binding to human lymphocytes was defined as active ATG. Despite comprising only 10% of total ATG, active ATG significantly affects aGVHD, immune reconstitution and post-transplant lymphoproliferative disorder (PTLD) (19–21, 49). The quantification of active ATG remains challenging (50, 51), with flow cytometry being the most widely utilized method for its detection (21, 51). In 2020, liquid chromatography-mass spectrometry (LC-MS) was employed for the first time to quantify the active fraction of ATG in plasma (52). This technique offers superior precision; however, its application remains limited due to restricted accessibility. The establishment of ATG detecting methods enables its pharmacokinetic and pharmacodynamic evaluation in allo-HSCT (Table 1).
3 Interindividual heterogeneity in ATG pharmacokinetics
The complex immunomodulatory mechanisms of ATG underlies the significant interindividual heterogeneity in its pharmacokinetics (27, 48, 51, 53–55). Waller, et al. (48) reported that the clearance of ATG was relatively slow, and serum total ATG remained detectable up to 90 days post-transplant. The calculated half-life of active and total ATG were 7 days and 14 days, respectively. The study further demonstrated that the time for active ATG levels decreasing to sub-therapeutic levels (1 μg/mL) in the 6 mg/kg group (17 days) was significantly shorter than 10 mg/kg group (45 days; P = 0.002). Similarly, when using 16-20mg/kg ATG-T, the median time for active ATG level to decline to less than 2.0μg/ml was 45.5 days (51). The clearance time of the 16–20 mg/kg ATG group was not significantly longer than that of the 10 mg/kg group, suggesting that a higher dose (> 10 mg/kg) of ATG-T may not be necessary. An Austrian study by Seidel, et al. found that the half-life of ATG-T was consistent when the ATG-T dose within the range of 7.5-20mg/kg, with a linear correlation between the dose and maximum serum concentration (Cmax). However, when the ATG-T dose was 30–40 mg/kg, the active fraction of ATG-T accumulated in the body, leading to a sharp increase in Cmax and resulting in ATG overexposure (55).
Weight-based ATG dosing induces marked interindividual variability in ATG exposure, arising from recipient-specific and regimen-related determinants (Figure 2). Body weight and absolute lymphocyte count (ALC) constitute principal recipient-specific determinants of ATG clearance. Pharmacokinetic analyses demonstrate that pediatric HSCT recipients with higher body weight and lower ALC exhibit over exposure to active ATG (31, 56).
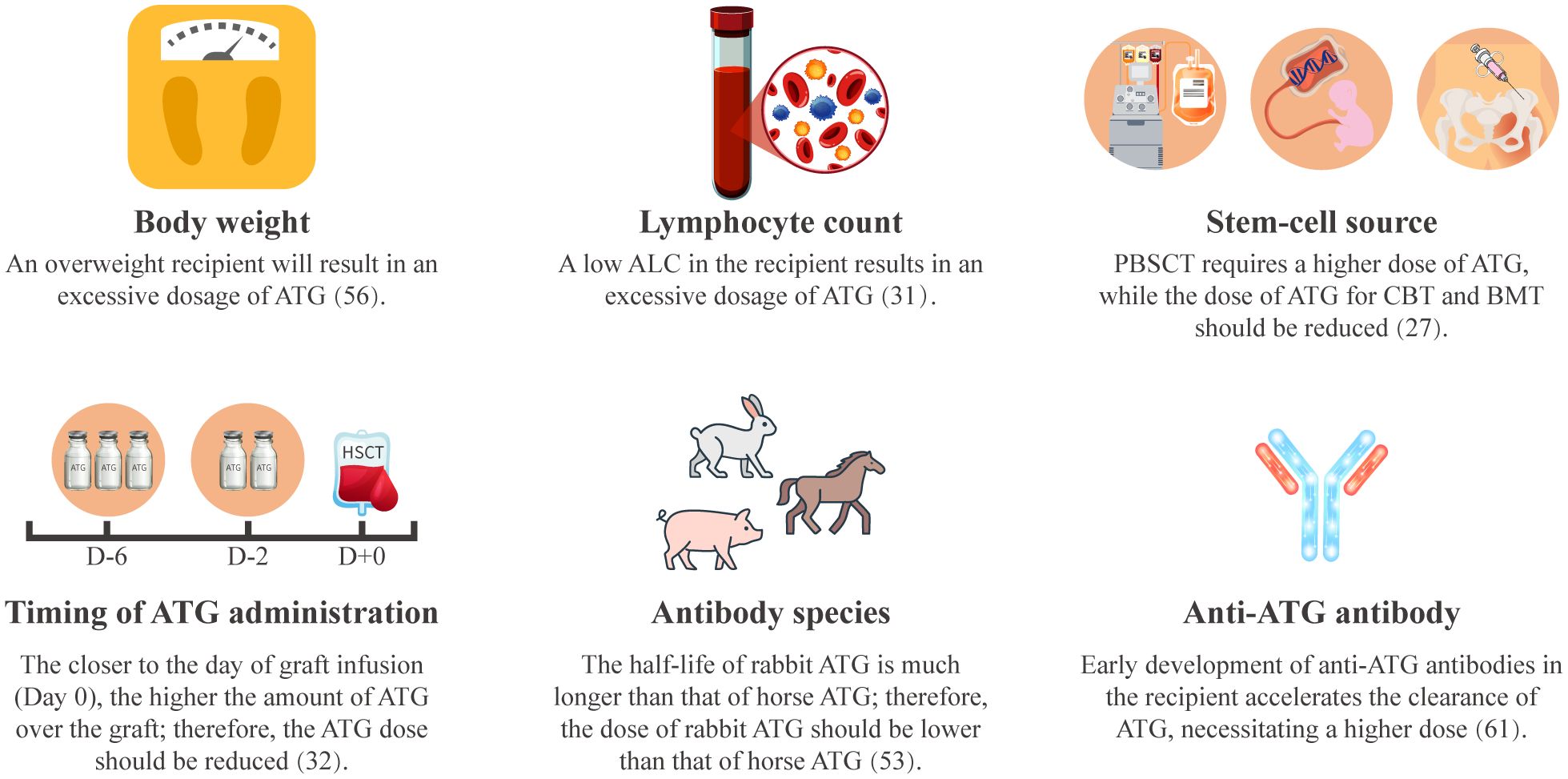
Figure 2. Factors influencing ATG pharmacokinetics. ALC, absolute lymphocyte count; ATG, anti-thymocyte globulin; BMT, bone marrow transplantation; CBT, cord blood transplantation; PBSCT, peripheral blood stem cell transplantation.
Both graft source and timing of ATG administration significantly modulate ATG exposure (27, 32). Compared to G-CSF-mobilized peripheral blood stem cells (G-PBSC), bone marrow and cord blood grafts contain fewer memory T cells and more naïve T cells, contributing to delayed post-transplant T-cell reconstitution. This necessitates ATG dose reduction in bone marrow or cord blood HSCT to promote T-cell recovery (32, 57–59). The timing of ATG administration is also important. Early ATG administration (between days -9 and -5) demonstrated reduced ATG exposure and accelerate T-cell reconstitution compared to later administration (between days -5 and 0) (32, 60).
Furthermore, ATG pharmacokinetics differ between preparations. Rabbit-ATG (Thymoglobulin®) exhibits a longer half-life, with detectable plasma active ATG persisting for one month, whereas active horse-ATG (ATGAM®) components decline within two weeks (53). As xenogeneic proteins, ATG preparations can induce anti-ATG antibodies. Early antibody formation (before day +22) mediates accelerated ATG clearance, substantially reducing post-transplant exposure (61).
4 ATG dose adjustment guided by ATG concentration at designated timepoints or GVHD biomarkers
4.1 ATG dose adjustment guided by ATG concentration at designated timepoints
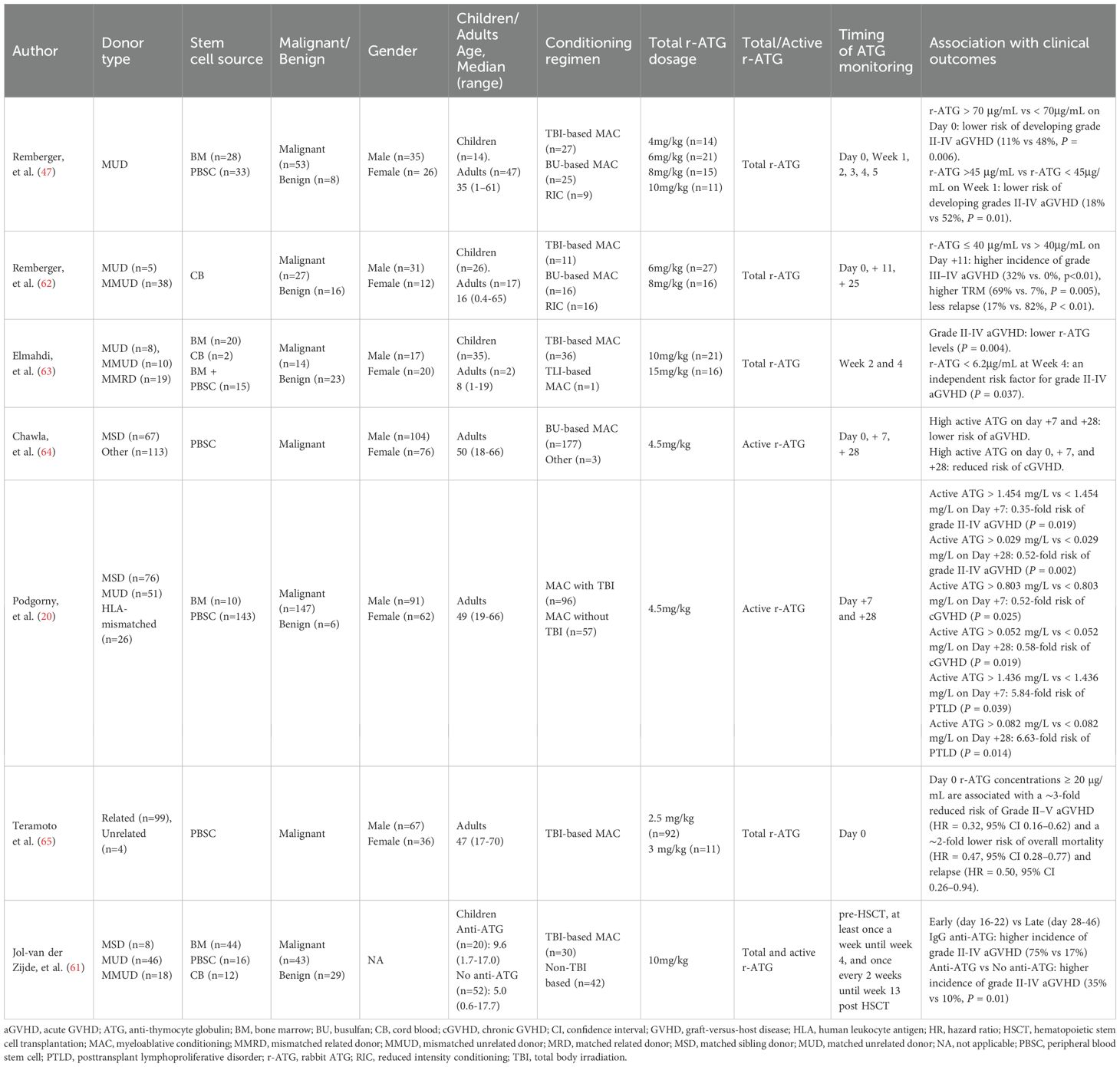
Several studies have highlighted the association between ATG concentrations at designated timepoints of allo-HSCT and transplant outcomes (Table 2). Generally, increased ATG concentrations reduce the risk of GVHD, while most studies suggest that ATG concentrations at designated timepoints do not affect the incidence of relapse, death, or infection. A study conducted by Remberger, et al. in Sweden reported that patients with serum ATG-T levels >70 μg/mL on day 0 had lower risk of grades II-IV aGVHD compared to those with ATG-T levels <70 μg/mL (11% vs 48%, P = 0.006) (47). In another study by the same group, patients received ATG-T at a total dose of 6 or 8 mg/kg as part of GVHD prophylaxis. The results revealed that patients with total ATG-T levels ≤ 40 μg/mL on day +11 had a higher incidence of grades III-IV aGVHD (32% vs. 0%, P < 0.01). However, their OS and relapse-free survival (RFS) at 5 years were similar (62). Elmahdi, et al. from Japan reported that a lower total ATG-T concentration in week 4 post-transplant was an independent risk factor for grade II-IV aGVHD, but no correlation was found between total ATG-T concentration at week 2 or 4 and recurrence (63). Chawla, et al. evaluated the relationship between active ATG concentration and GVHD in 180 allo-HSCT recipients receiving 4.5 mg/kg ATG-T. Higher concentrations at days +7 and +28 correlated with a reduced risk of aGVHD, while elevated levels at days 0, + 7, and +28 were associated with lower cGVHD incidence (64). Similarly, Podgorny, et al. from Canada found that both higher ATG-T levels on day +7 and +28 were associated with lower risks of grade II-IV aGVHD and cGVHD, but not with relapse, death, or infection (20). Teramoto et al. identified ATG concentration on day 0 (Cday_0) as the strongest predictor for grade II-IV aGVHD. They found Cday_0 ≥ 20μg/mL correlated with an approximately 3-fold reduced risk of aGVHD and 2-fold decrease in overall mortality and relapse. Their population pharmacokinetic modeling indicated a total ATG dose of 3 mg/kg (1.5 mg/kg per dose on days -2 and -1) to achieve target Cday_0 with 80% probability (65).
To investigate the reason why ATG concentration at designated timepoints did not affect transplant outcomes, Jol-van der Zijde, et al. measured concentrations of ATG-T and anti-ATG antibodies in pediatric HSCT recipients. They found that 28% of the recipients developed anti-ATG antibodies. Early production of these antibodies (before day +22 of HSCT) led to a rapid decrease in ATG concentration and swift recovery of T cells (61). These findings suggest that overall ATG exposure is more important than concentration at a designated timepoint.
4.2 ATG dose adjustment guided by GVHD biomarkers
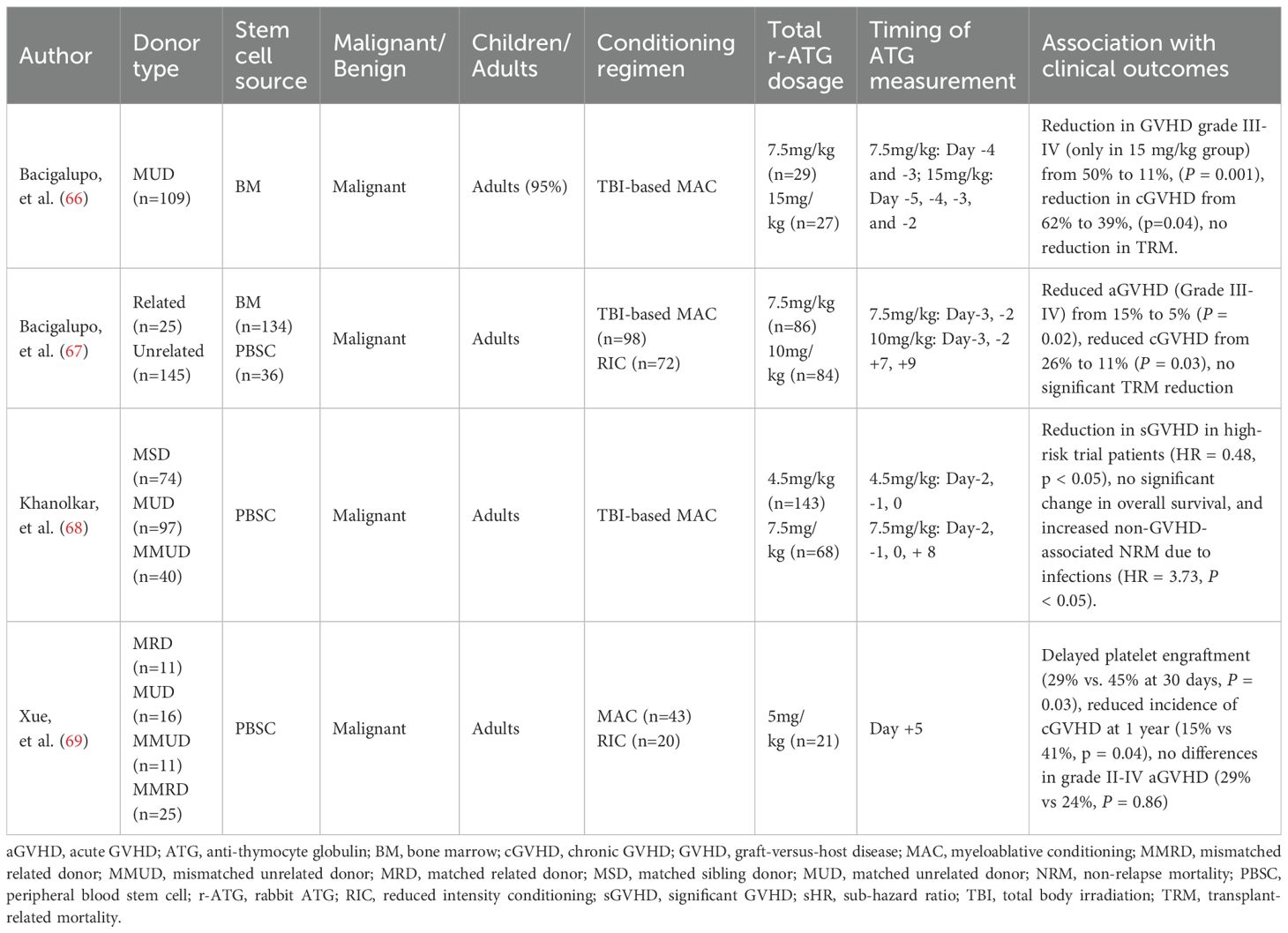
Several studies have investigated biomarker-guided individualized ATG dosing to optimize allo-HSCT outcomes (Table 3) (66–69). As early as 2001, Bacigalupo et al. demonstrated the efficacy of this approach in alternative donor bone marrow transplantation. Patients with serum bilirubin levels ≥ 0.9 mg/dl and blood urea nitrogen (BUN) ≥ 21 mg/dl on day +7 were defined as a high-risk group. An additional dose of 3.75 mg/kg ATG-T (1.25 mg/kg on days +7, +9, and +11) was added to high-risk patients. This intervention significantly reduced severe GVHD from 55% to 27% and 1-year transplant-related mortality (TRM) from 60% to 40% (66). A subsequent multicenter randomized trial confirmed these findings: the same ATG regimen significantly reduced grade III–IV aGVHD (15% to 5%) and cGVHD (26% to 11%) in high-risk recipients, though it demonstrated no significant benefit for TRM or OS (67).
In a study of adult peripheral blood stem cell transplantation (PBSCT), Khanolkar et al. defined patients with day +7 serum sIL-2Rα levels >4500 ng/L or IL-15 levels <31 ng/L as being at high risk for GVHD. These high-risk patients received an additional dose of 3 mg/kg ATG on day +8, following a conditioning regimen with 4.5 mg/kg ATG. Compared with controls, this strategy significantly reduced the risk of clinically significant GVHD (hazard ratio, 0.48, P = 0.045), without increasing relapse. However, the OS benefit was offset by a higher rate of infections in the intervention group, resulting in no improvement in OS (68). More recently, in a study of post-transplant cyclophosphamide (PT-Cy)-based allogeneic PBSCT, Xue et al. administered an additional 5 mg/kg anti-T-lymphocyte globulin (ATLG) on day +5 to patients receiving grafts with CD3+ counts > 3 × 108/kg. Compared with historical controls, the addition of ATLG significantly reduced 1-year cGVHD (41% vs. 15%, P = 0.04) but did not impact grade II-IV aGVHD, NRM, or OS (69). Consistent with these data, the biomarker-guided personalized ATG dosing strategy ultimately failed to improve patient survival across studies.
5 Timing of ATG administration and its impact on transplant outcomes
The timing of ATG administration significantly influences allo-HSCT outcomes. Late ATG administration (closer to day 0) more effectively depletes donor T cells in the graft, while its effect on recipient T cells and antigen-presenting cells remain comparable with earlier dosing. Consequently, late ATG administration is often associated with reduced GVHD but carries an increased risk of viral reactivation compared to early dosing (before day -5) (70).
These timing-dependent effects are further supported by clinical studies. In severe aplastic anemia patients undergoing haplo-PBSCT, Wu, et al. demonstrated that shifting ATG dosing from early (days -9 to -7) to late (days -5 to -3) effectively controlled GVHD but led to increased rates of CMV reactivation and EBV-associated post-transplant lymphoproliferative disorder (EBV-PTLD) (71). Conversely, early ATG administration appears to facilitate T-cell reconstitution. Lindemans, et al. observed accelerated reconstitution of CD3+, CD4+, and naïve T cells in cord blood transplant recipients receiving early ATG (days -9 to -5) compared to later ATG (days -5 to 0) (32). Similarly, a Japanese study in adult PBSCT found that early ATG administration (1.25mg/kg on day -4), rather than the standard schedule (1.25 mg/kg, days -2 and -1), reduced post-transplant ATG exposure and accelerated CD4+ T-cell recovery (60). These findings indicate that ATG dosing should be adjusted according to timing of administration. A relatively increased dose may be necessary with early ATG dosing, whereas the dose could be reduced if ATG administered closer to day 0.
6 Impact of ATG exposure on transplant outcomes
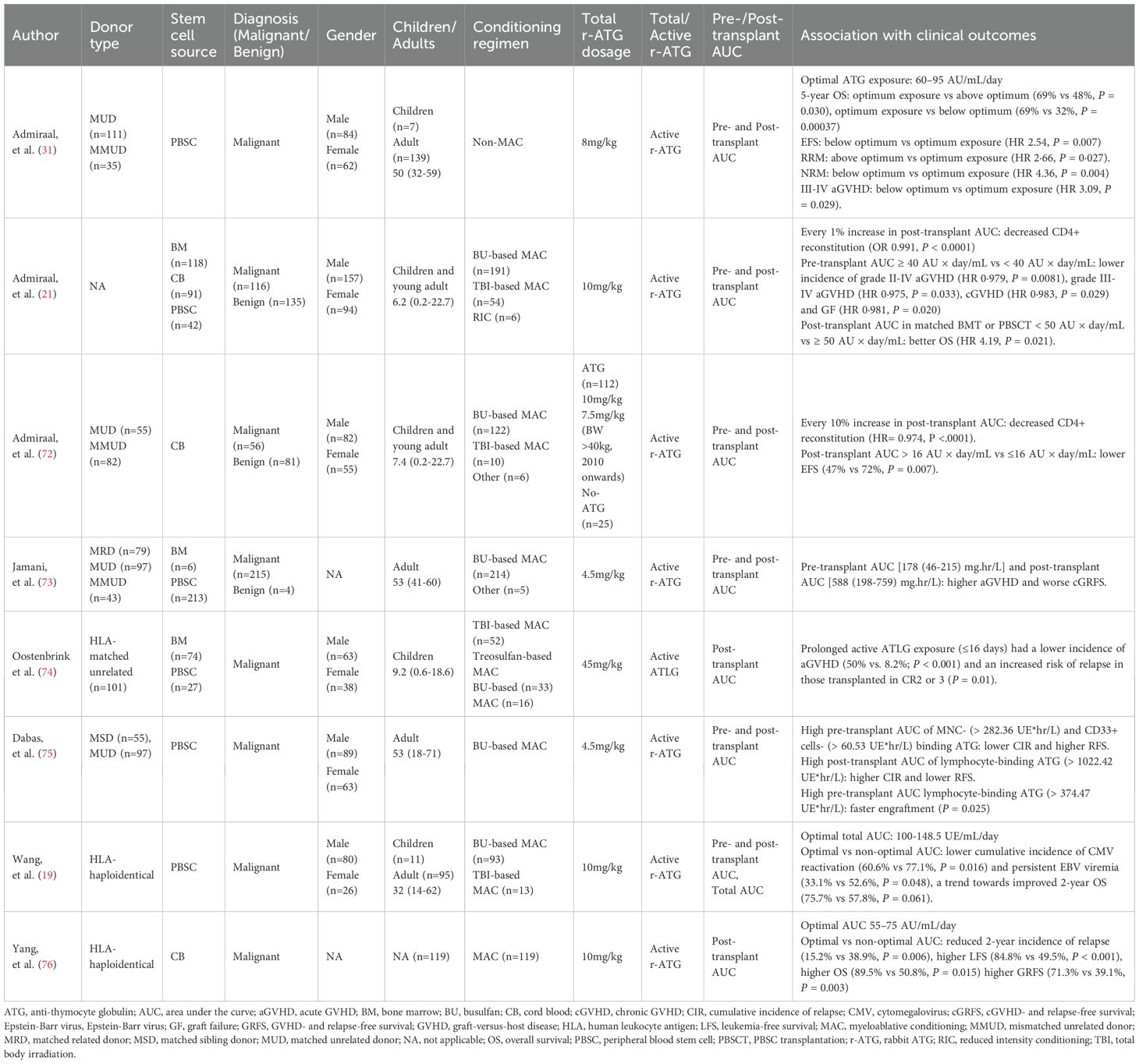
ATG exposure was quantified using the area under the concentration-time curve (AUC). Total ATG exposure was divided into pre- and post-transplant exposure using day 0 (graft infusion) as the reference point. ATG exposure better predicts outcomes in allo-HSCT than the concentration at designated timepoints. Several studies have assessed the association between ATG exposure and transplant outcomes, including GVHD, immune reconstitution, relapse, and survival (Table 4) (19, 21, 31, 72–76).
Admiraal, et al. discovered that excessive exposure to active ATG post-transplant significantly decreases the rate of successful immune reconstitution (21). In subsequent study, they revealed that for every 10% increase in the post-transplant AUC of active ATG-T, the likelihood of successful CD4+ T cell immune reconstitution decreased by 26%. Lower post-transplant active ATG exposure (< 16 AU × day/mL) and successful CD4+ immune reconstitution were both associated with improved event-free survival (72). Additionally, they found that pre-transplant active ATG exposure ≥ 40 AU × day/mL significantly reduced the incidence of grade II-IV aGVHD, cGVHD, and graft failure (21). Similarly, Jamani, et al. (73) from Canada discovered that the lowest quintile of pre-transplant AUC and post-transplant AUC of active ATG were associated with higher aGVHD and worse cGVHD- and relapse-free survival (cGRFS) in myeloablative allo-HSCT. A multinational prospective study by Oostenbrink, et al. reported that prolonged ATLG exposure (active ATLG ≥ 1 AU/mL on day +16) significantly reduced the incidence of grade II-IV aGVHD (from 50% to 8.2%) (74). A study by Dabas, et al. from Canada showed that high pre-transplant active ATG exposure of MNC-binding (> 282.36 UE*hr/L) and CD33+ cells- binding (> 60.53 UE*hr/L) were associated with a lower risk of relapse and better RFS. Whereas higher post-transplant exposure of lymphocyte-binding (> 1022.42 UE*hr/L) was associated with higher risk of relapse and lower RFS (75).
These studies highlight the importance of maintaining pre- or post-transplant ATG exposure within an optimal range, as both excessive and insufficient exposure compromise transplant outcomes. A Dutch retrospective analysis identified an optimal post-transplant active ATG exposure of 60–95 AU/mL/day. Sub-optimal exposure (< 60 AU/mL/day) increased grade III-IV aGVHD and NRM, while over-optimal exposure (> 95 AU/mL/day) increased relapse-related mortality (RRM). Only patients within the optimal range achieved the best 5-year event-free survival (EFS) and OS (31). A single-center prospective study from China found an optimal total active ATG exposure of 100 to 148.5 UE/mL/day in haplo-HSCT following Beijing Protocol. Interestingly, the optimal AUC group showed a significantly lower incidence of cytomegalovirus (CMV) reactivation and persistent CMV viremia compared to the non-optimal AUC group (total AUC < 100 or >148.5 UE/mL/day). While no significant difference in NRM and recurrence were observed between two groups, optimal AUC group showed a trend toward better 2-year OS (75.7% vs. 57.8%, P = 0.061) (19). A recent phase IV trial established an optimal post-transplant ATG exposure (55–75 AU/mL/day) for acute leukemia patients undergoing myeloablative haplo-cord HSCT. Compared to non-optimal range, patients within optimal range have lower 2-year relapse (38.9% vs. 15.2%), higher leukemia-free survival (LFS) (49.5% vs. 84.8%), superior OS (50.8% vs. 89.5%) and GRFS (39.1% vs. 71.3%), and reduced grade II-IV aGVHD (37.8% vs. 20.5%) (76).
7 Individualized ATG dosing strategies in allo-HSCT
7.1 Individualized ATG dosing guided by absolute lymphocyte count
A retrospective pharmacokinetic-pharmacodynamic study demonstrated that patients who had optimal post-transplant active ATG exposure (60–95 AU/mL/day) achieved the best 5-year OS. Further analysis of the pharmacokinetic model identified recipient’s body weight (< 50 kg) and ALC as significant covariates influencing ATG clearance. In adult allo-HSCT, conventional weight-based ATG dosing regimen achieved optimal exposure only in 30%-53% of patients (when body weight > 50 kg), whereas ALC-based dosing regimen achieved optimal exposure in 95%, thereby enhancing survival outcomes (31). Subsequently, the same team conducted a prospective single-arm Phase II study to explore the efficacy and safety of individualized ATG dosing based on ALC (36). The study identified three key parameters (recipient’s body weight, ALC before the first dose of ATG, and source of graft) to guide individualized ATG dosing (ranging from 2 to 10mg/kg). Of the 51 evaluable patients, 41 (80%) met CD4+ immune reconstitution criteria, defined as two consecutive CD4+ T cell counts > 0.05 × 109/L within 100 days post-transplantation. Their previous studies have shown that patients who achieved CD4+ immune reconstitution early after transplantation had better OS, lower NRM, and fewer virus reactivations. These findings indicate that individualized ATG dosing may improve transplant outcomes by increasing the proportion of patients attaining optimal AUC (21, 72). Seo, et al. found that the weight-based dosing regimen in unrelated donor transplantation with reduced-intensity conditioning could cause overexposure to ATG-T in adult recipient with an ALC < 500/μl at day -7. This overexposure resulted in severe T-cell depletion, increasing the risk of life-threatening infections, and impairing OS (77). Similarly, Woo, et al. demonstrated in a study of adult matched sibling donor transplantation that those with an ALC < 500/μl at day -7 had a higher mortality, primarily due to infection-related complications (78). These results support adjusting the ATG dose based on ALC to avoid overexposure to ATG in patients with low ALC.
However, ALC-based individualized ATG dosing regimen may not be universally applicable. A French study enrolled 116 adult patients undergoing matched sibling or unrelated donor transplantation investigated the association between ALC before ATG administration and transplant outcomes. The study revealed that whether the ALC was higher than the median value did not affect survival (79). In a retrospective study of adult unrelated donor transplantation, Heelan, et al. compared weight-based dosing strategy versus ALC-guided individualized dosing strategy. The study revealed substantial dose variation between the two regimens: conventional weight-based ATG dosing yielded a median total dose of 201 mg, whereas ALC (day -2) - guided individualized dosing required a significantly higher dose with a mean of 1205 mg, representing a 5-fold increase over conventional weight-based dosing strategy. They assumed that when the administration of ATG is close to graft infusion, the lymphocytes are depleted by myeloablative conditioning, resulting in an overestimation of the ATG dose when calculated based on ALC (80).
7.2 Individualized ATG dosing guided by therapeutic drug monitoring
Therapeutic drug monitoring (TDM) of calcineurin inhibitors (CNIs) has been used in allo-HSCT for many years, which correlated with improved transplant outcomes (81, 82). However, current evidence regarding TDM-guided individualized ATG dosing in allo-HSCT remains limited. In a Phase II study, Wang, et al. developed a machine learning-based, TDM-guided individualized ATG dosing model for haplo-PBSCT. ATG was administered for 4 days (days -5 to -2) during conditioning. Active ATG concentration was detected on day -5 and -4 via flow cytometry, and the adjusted ATG doses on day -3 and -2 were calculated according to the individualized dosing model. This adjustment aimed to maintain total active ATG exposure within the optimal range of 100-148.5 UE/mL/day, a range previously identified by the same group to effectively reduce CMV/EBV reactivation in haplo-PBSCT without increasing GVHD or relapse (19, 37, 83). Additionally, researchers from the Netherlands and the United States have theoretically verified the feasibility of TDM-guided ATG dosing strategy using population pharmacokinetic model. Their TDM-guided ATG dosing framework is as follows: the total dose of ATG is administered over 4 days, and on the third day after ATG administration, the peak and trough concentrations of active ATG are measured. ATG dose on the fourth day is then adjusted according to the model-predicted AUC. If the adjustment exceeds 25% of the total ATG dose, the administration of ATG needs to be extended to the fifth day. The investigators assumed that TDM-guided ATG dosing was more accurate than ALC-guided dosing for patients presenting with immune deficiencies and/or hyperinflammation (84). A randomized phase III multicenter trial evaluated targeted ATG dosing strategy (Total ATG dose calculated based on pharmacokinetic parameters, range: 6–13 mg/kg) against a fixed dose of 10 mg/kg in adults haplo-PBSCT. Compared to fixed dosing, targeted dosing reduced CMV reactivation (54.9% vs. 31.0%), improved GRFS (48.0% vs. 63.4%), and enhanced CD4+ T-cell reconstitution (72.7% vs. 91.0%) (85).
7.3 Challenges in individualized ATG dosing in allo-HSCT
It should be noted that individualized ATG dosing in allo-HSCT faces significant challenges. First, detecting active ATG is complex and difficult to standardize. Flow cytometry, the predominant detection method for active ATG, demonstrates an inter-laboratory variability due to heterogeneity in flow cytometer models and biological materials (e.g., cells and antibody clones). Second, the clinical assessment of optimal ATG exposure lacks consensus criteria. Different optimal ATG exposure ranges were reported across centers due to inconsistent optimal exposure definitions [(e.g., successful CD4+ T cell reconstitution (31, 86), or reduction of virus reactivation (83)]. Heterogeneity in the timing and dosing of ATG administration further complicates this issue, and collaborative efforts are needed to establish a consensus-defined optimal active ATG exposure in allo-HSCT. Third, current personalized dosing strategies including ALC-guided and TDM-guided approaches, have population-specific limitations (14, 84, 87, 88) (Figure 3). It is necessary to conduct further research to establish a universally applicable individualized dosing regimen using population pharmacokinetic modeling.
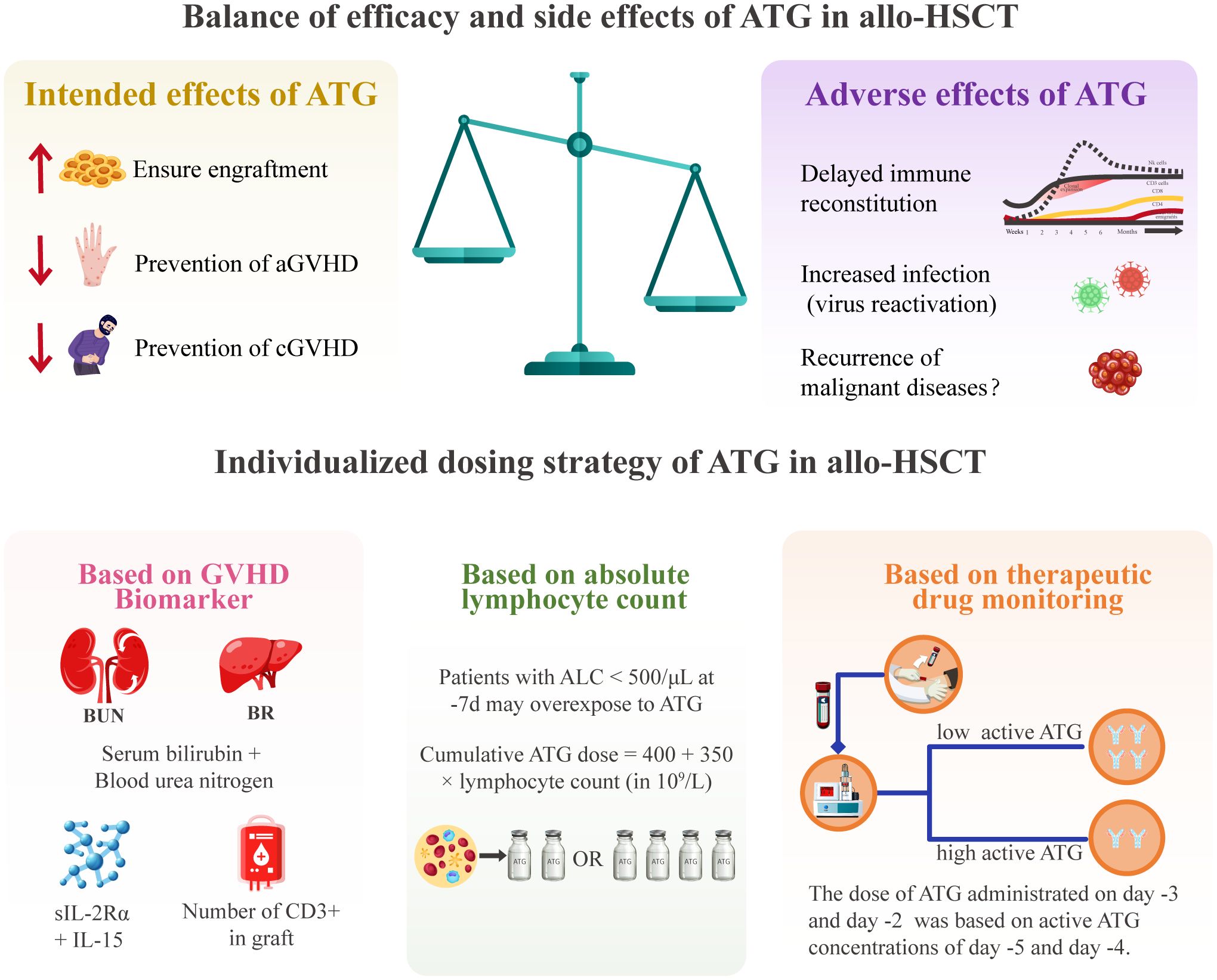
Figure 3. The balance of efficacy and toxicity of ATG and individualized dosing strategies in allo-HSCT. aGVHD, acute graft-versus-host disease; allo-HSCT, allogeneic hematopoietic stem cell transplantation; ALC, absolute lymphocyte count; ATG, anti-thymocyte globulin; BUN, blood urea nitrogen; CD, cluster of differentiation; cGVHD, chronic graft-versus-host disease; GVHD, graft-versus-host disease; IL, interleukin; slL-2Ra, soluble interleukin-2 receptor alpha.
8 Discussion
This review discussed the challenges of optimizing ATG dosing in allo-HSCT to balance GVHD prophylaxis with immune reconstitution, while minimizing malignant disease recurrence and life-threatening infections. Extensive research has focused on weight-based ATG dosing regimens in allo-HSCT, yet this approach remains suboptimal in addressing the pharmacokinetic variability mediated by multiple parameters, including genetic polymorphisms (e.g., HLA compatibility and Fcγ receptor genotypes), timing of ATG, anti-ATG antibody development, and comorbidities. The weight-based ATG dosing approach failed to address the substantial pharmacokinetic variability among patients, thus attempting to establish body weight based optimal ATG dosing regimen will continue to prove futile. Pharmacodynamic studies demonstrated that lower ATG concentration was associated with increased risks of aGVHD and cGVHD, although its impact on TRM and relapse remains unclear (47, 62, 63). Notably, emerging evidence highlights the association between ATG exposure and transplant outcomes such as GVHD incidence, immune reconstitution, relapse, and OS (21, 31, 72, 75). Importantly, an optimal ATG exposure range has been identified, associated with reduced viral reactivation, accelerated immune reconstitution, and improved OS (19, 31).
TDM and pharmacogenomics (PGx) are fundamental approaches for achieving personalized dosing in clinical practice. Advances in understanding ATG-PGx, including drug-metabolizing enzymes, therapeutic targets, and drug transporters, will enable optimized balancing of ATG’s efficacy against treatment-related toxicity. Integrating TDM with PGx in ATG personalized dosing represents a promising strategy to improve outcomes of allo-HSCT. Recent studies have shown promising outcomes using individualized ATG dosing strategies based on ALC or TDM (36, 37). However, current individualized ATG dosing protocols are often derived from physiologically based pharmacokinetic (PBPK) and population pharmacokinetic (popPK) models (21, 56). These protocols exhibit inherent static limitations of failing to integrate real-time patient data and dynamic health trends. Model-informed precision dosing (MIPD) provides a potential solution for optimizing ATG dosing via mathematical modeling that integrates multidimensional data, including patient characteristics, drug properties, and disease status. Collaboration across clinicians, informaticians, clinical pharmacologists, and TDM specialists will establish ethical framework for data sharing, technology accessibility, and patient privacy, thereby facilitating clinical implementation of MIPD. Artificial intelligence (AI) and machine learning (ML) represent emerging tools for advancing MIPD in personalized medicine, but their clinical application remains experimental with unproven benefits for patient care (89–91). Robust clinical validation and technological innovation are essential to overcome inherent challenges, including data privacy and algorithmic bias, thereby enabling tangible patient benefits and facilitating clinical implementation (92). In conclusion, the ongoing development and optimization of individualized ATG dosing strategies are critical for enhancing the safety and efficacy of allo-HSCT, ultimately improving transplant outcomes.
Author contributions
HW: Writing – original draft, Investigation, Writing – review & editing, Data curation. HY: Writing – original draft, Investigation, Methodology. JD: Visualization, Software, Writing – review & editing. LD: Funding acquisition, Supervision, Writing – review & editing, Investigation. DL: Project administration, Funding acquisition, Conceptualization, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Key Research and Development Program of China (grant No.2021YFA1100904) (DHL), the National Natural Science Foundation of China (grant No.82270162) (DHL), the Innovation Science Foundation of Youth Program of Chinese PLA General hospital (Grant No.22QNFC020) (HTW), the Postdoctoral Fellowship Program of CPSF (Grant No.GZC20242288) (HTW), and Medical writing was supported by Sanofi.
Acknowledgments
No honoraria were provided to the authors for their participation. The authors would like to apologize to those colleagues whose work could not be cited for reasons of space.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A, et al. Hematopoietic stem cell transplantation: A global perspective. Jama. (2010) 303:1617–24. doi: 10.1001/jama.2010.491
2. Gratwohl A, Pasquini MC, Aljurf M, Atsuta Y, Baldomero H, Foeken L, et al. One million haemopoietic stem-cell transplants: A retrospective observational study. Lancet Haematol. (2015) 2:e91–100. doi: 10.1016/s2352-3026(15)00028-9
3. Najarian JS and Simmons RL. The clinical use of antilymphocyte globulin. N Engl J Med. (1971) 285:158–66. doi: 10.1056/nejm197107152850310
4. Xu ZL and Huang XJ. Haploidentical stem cell transplantation for aplastic anemia: the current advances and future challenges. Bone Marrow Transplant. (2021) 56:779–85. doi: 10.1038/s41409-020-01169-7
5. Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant. (2006) 12:560–5. doi: 10.1016/j.bbmt.2005.12.034
6. Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: A randomised, open-label, multicentre phase 3 trial. Lancet Oncol. (2009) 10:855–64. doi: 10.1016/s1470-2045(09)70225-6
7. Kröger N, Solano C, Wolschke C, Bandini G, Patriarca F, Pini M, et al. Antilymphocyte globulin for prevention of chronic graft-versus-host disease. New Engl J Med. (2016) 374:43–53. doi: 10.1056/NEJMoa1506002
8. Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from gruppo italiano trapianti midollo osseo (Gitmo). Blood. (2001) 98:2942–7. doi: 10.1182/blood.v98.10.2942
9. Soiffer RJ, Kim HT, McGuirk J, Horwitz ME, Johnston L, Patnaik MM, et al. Prospective, randomized, double-blind, phase iii clinical trial of anti–T-lymphocyte globulin to assess impact on chronic graft-versus-host disease–free survival in patients undergoing hla-matched unrelated myeloablative hematopoietic cell transplantation. J Clin Oncol. (2017) 35:4003–11. doi: 10.1200/jco.2017.75.8177
10. Zeiser R and Blazar BR. Acute graft-versus-host disease - biologic process, prevention, and therapy. N Engl J Med. (2017) 377:2167–79. doi: 10.1056/NEJMra1609337
11. Gaber AO, Monaco AP, Russell JA, Lebranchu Y, and Mohty M. Rabbit antithymocyte globulin (Thymoglobulin): 25 years and new frontiers in solid organ transplantation and haematology. Drugs. (2010) 70:691–732. doi: 10.2165/11315940-000000000-00000
12. Atta EH, de Sousa AM, Schirmer MR, Bouzas LF, Nucci M, and Abdelhay E. Different outcomes between cyclophosphamide plus horse or rabbit antithymocyte globulin for hla-identical sibling bone marrow transplant in severe aplastic anemia. Biol Blood Marrow Transplant. (2012) 18:1876–82. doi: 10.1016/j.bbmt.2012.07.004
13. Champlin RE, Perez WS, Passweg JR, Klein JP, Camitta BM, Gluckman E, et al. Bone marrow transplantation for severe aplastic anemia: A randomized controlled study of conditioning regimens. Blood. (2007) 109:4582–5. doi: 10.1182/blood-2006-10-052308
14. Nishihori T, Al-Kadhimi Z, Hamadani M, and Kharfan-Dabaja MA. Antithymocyte globulin in allogeneic hematopoietic cell transplantation: benefits and limitations. Immunotherapy. (2016) 8:435–47. doi: 10.2217/imt.15.128
15. Siddiqui S, Cox J, Herzig R, Palaniyandi S, Hildebrandt GC, and Munker R. Anti-thymocyte globulin in haematology: recent developments. Indian J Med Res. (2019) 150:221–7. doi: 10.4103/ijmr.IJMR_752_19
16. Baron F, Mohty M, Blaise D, Socie G, Labopin M, Esteve J, et al. Anti-thymocyte globulin as graft-versus-host disease prevention in the setting of allogeneic peripheral blood stem cell transplantation: A review from the acute leukemia working party of the european society for blood and marrow transplantation. Haematologica. (2017) 102:224–34. doi: 10.3324/haematol.2016.148510
17. Popow I, Leitner J, Grabmeier-Pfistershammer K, Majdic O, Zlabinger GJ, Kundi M, et al. A comprehensive and quantitative analysis of the major specificities in rabbit antithymocyte globulin preparations. Am J Transplant. (2013) 13:3103–13. doi: 10.1111/ajt.12514
18. Notarantonio AB, Morisset S, Piucco R, Pérès M, Boulangé L, Alitcher A, et al. Differential clinical and immunological impacts of anti-T-lymphocyte globulin (Atlg) vs. Anti-thymocyte globulin (Atg) in preventing graft-versus-host disease post-allogeneic hematopoietic stem cell transplantation: A comparative study. Am J Hematol. (2025) 100:626–37. doi: 10.1002/ajh.27619
19. Wang H, Zhao Y, Fang S, Wang L, Peng B, Yang J, et al. Optimal active anti-thymocyte globulin exposure associated with minimum risk of virus reactivation and comparable acute graft-versus-host disease under adult myeloablative haploidentical peripheral blood stem cell transplantation. Transplant Cell Therapy Off Publ Am Soc Transplant Cell Ther. (2022) 28:332. doi: 10.1016/j.jtct.2022.03.018
20. Podgorny PJ, Ugarte-Torres A, Liu Y, Williamson TS, Russell JA, and Storek J. High rabbit-antihuman thymocyte globulin levels are associated with low likelihood of graft-vs-host disease and high likelihood of posttransplant lymphoproliferative disorder. Biol Blood Marrow Transplant. (2010) 16:915–26. doi: 10.1016/j.bbmt.2010.02.027
21. Admiraal R, van Kesteren C, Jol-van der Zijde CM, Lankester AC, Bierings MB, Egberts TC, et al. Association between anti-thymocyte globulin exposure and cd4+ Immune reconstitution in paediatric haemopoietic cell transplantation: A multicentre, retrospective pharmacodynamic cohort analysis. Lancet Haematol. (2015) 2:e194–203. doi: 10.1016/s2352-3026(15)00045-9
22. Préville X, Flacher M, LeMauff B, Beauchard S, Davelu P, Tiollier J, et al. Mechanisms involved in antithymocyte globulin immunosuppressive activity in a nonhuman primate model. Transplantation. (2001) 71:460–8. doi: 10.1097/00007890-200102150-00021
23. Fang L, Fehse B, Engel M, Zander A, and Kröger N. Antithymocyte globulin induces ex vivo and in vivo depletion of myeloid and plasmacytoid dendritic cells. Transplantation. (2005) 79:369–71. doi: 10.1097/01.tp.0000150210.77543.1b
24. Naujokat C, Berges C, Fuchs D, Sadeghi M, Opelz G, and Daniel V. Antithymocyte globulins suppress dendritic cell function by multiple mechanisms. Transplantation. (2007) 83:485–97. doi: 10.1097/01.tp.0000251975.81281.22
25. Shimony O, Nagler A, Gellman YN, Refaeli E, Rosenblum N, Eshkar-Sebban L, et al. Anti-T lymphocyte globulin (Atg) induces generation of regulatory T cells, at least part of them express activated cd44. J Clin Immunol. (2012) 32:173–88. doi: 10.1007/s10875-011-9599-2
26. Valdez-Ortiz R, Bestard O, Llaudó I, Franquesa M, Cerezo G, Torras J, et al. Induction of suppressive allogeneic regulatory T cells via rabbit antithymocyte polyclonal globulin during homeostatic proliferation in rat kidney transplantation. Transpl Int. (2015) 28:108–19. doi: 10.1111/tri.12448
27. Storek J, Mohty M, and Boelens JJ. Rabbit anti-T cell globulin in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. (2015) 21:959–70. doi: 10.1016/j.bbmt.2014.11.676
28. Grasso AG, Simeone R, Maestro A, Zanon D, and Maximova N. Pre-transplant total lymphocyte count determines anti-thymocyte globulin exposure, modifying graft-versus-host disease incidence and post-transplant thymic restoration: A single-center retrospective study. J Clin Med. (2023) 12:730. doi: 10.3390/jcm12020730
29. Wang Y, Fu HX, Liu DH, Xu LP, Zhang XH, Chang YJ, et al. Influence of two different doses of antithymocyte globulin in patients with standard-risk disease following haploidentical transplantation: A randomized trial. Bone Marrow Transplant. (2014) 49:426–33. doi: 10.1038/bmt.2013.191
30. Admiraal R, van Kesteren C, Jol-van der Zijde CM, van Tol MJ, Bartelink IH, Bredius RG, et al. Population pharmacokinetic modeling of thymoglobulin((R)) in children receiving allogeneic-hematopoietic cell transplantation: towards improved survival through individualized dosing. Clin Pharmacokinet. (2015) 54:435–46. doi: 10.1007/s40262-014-0214-6
31. Admiraal R, Nierkens S, de Witte MA, Petersen EJ, Fleurke GJ, Verrest L, et al. Association between anti-thymocyte globulin exposure and survival outcomes in adult unrelated haemopoietic cell transplantation: A multicentre, retrospective, pharmacodynamic cohort analysis. Lancet Haematol. (2017) 4:e183–e91. doi: 10.1016/S2352-3026(17)30029-7
32. Lindemans CA, Chiesa R, Amrolia PJ, Rao K, Nikolajeva O, de Wildt A, et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood. (2014) 123:126–32. doi: 10.1182/blood-2013-05-502385
33. Dvorak CC, Long-Boyle JR, Holbrook-Brown L, Abdel-Azim H, Bertaina A, Vatsayan A, et al. Effect of rabbit atg pk on outcomes after tcr-αβ/cd19-depleted pediatric haploidentical hct for hematologic Malignancy. Blood Adv. (2024) 8:6003–14. doi: 10.1182/bloodadvances.2024012670
34. Lakkaraja M, Mauguen A, Boulad F, Cancio MI, Curran KJ, Harris AC, et al. Impact of rabbit anti-thymocyte globulin (Atg) exposure on outcomes after ex vivo T-cell-depleted hematopoietic cell transplantation in pediatric and young adult patients. Cytotherapy. (2024) 26:351–9. doi: 10.1016/j.jcyt.2024.01.004
35. Teramoto M, Maruyama S, Tamaki H, Kaida K, Mayumi A, Fukunaga K, et al. Association between the pharmacokinetics of rabbit anti-thymocyte globulin and acute graft-versus-host disease in patients who received haploidentical hematopoietic stem cell transplantation. Int J Hematol. (2022) 116:248–57. doi: 10.1007/s12185-022-03342-8
36. Admiraal R, Nierkens S, Bierings MB, Bredius RGM, van Vliet I, Jiang Y, et al. Individualised dosing of anti-thymocyte globulin in paediatric unrelated allogeneic haematopoietic stem-cell transplantation (Parachute): A single-arm, phase 2 clinical trial. Lancet Haematol. (2022) 9:e111–e20. doi: 10.1016/s2352-3026(21)00375-6
37. Wang H, Wang N, Wang L, Du J, Li F, Shao Y, et al. Targeted dosing of anti-thymocyte globulin in adult unmanipulated haploidentical peripheral blood stem cell transplantation: A single-arm, phase 2 trial. Am J Hematol. (2023) 98:1732–41. doi: 10.1002/ajh.27068
38. Mohty M. Mechanisms of action of antithymocyte globulin: T-cell depletion and beyond. Leukemia. (2007) 21:1387–94. doi: 10.1038/sj.leu.2404683
39. Mohty M and Gaugler B. Mechanisms of action of antithymocyte globulin: old dogs with new tricks! Leuk Lymphoma. (2008) 49:1664–7. doi: 10.1080/10428190802163321
40. Rebellato LM, Gross U, Verbanac KM, and Thomas JM. A comprehensive definition of the major antibody specificities in polyclonal rabbit antithymocyte globulin. Transplantation. (1994) 57:685–94. doi: 10.1097/00007890-199403150-00010
41. Zand MS, Vo T, Huggins J, Felgar R, Liesveld J, Pellegrin T, et al. Polyclonal rabbit antithymocyte globulin triggers B-cell and plasma cell apoptosis by multiple pathways. Transplantation. (2005) 79:1507–15. doi: 10.1097/01.tp.0000164159.20075.16
42. Monti P, Allavena P, Di Carlo V, and Piemonti L. Effects of anti-lymphocytes and anti-thymocytes globulin on human dendritic cells. Int Immunopharmacol. (2003) 3:189–96. doi: 10.1016/s1567-5769(02)00253-9
43. Lopez M, Clarkson MR, Albin M, Sayegh MH, and Najafian N. A novel mechanism of action for anti-thymocyte globulin: induction of cd4+Cd25+Foxp3+ Regulatory T cells. J Am Soc Nephrol. (2006) 17:2844–53. doi: 10.1681/asn.2006050422
44. Zeng D, Lewis D, Dejbakhsh-Jones S, Lan F, García-Ojeda M, Sibley R, et al. Bone marrow nk1.1(-) and nk1.1(+) T cells reciprocally regulate acute graft versus host disease. J Exp Med. (1999) 189:1073–81. doi: 10.1084/jem.189.7.1073
45. Michallet MC, Preville X, Flacher M, Fournel S, Genestier L, and Revillard JP. Functional antibodies to leukocyte adhesion molecules in antithymocyte globulins. Transplantation. (2003) 75:657–62. doi: 10.1097/01.Tp.0000053198.99206.E6
46. Bonnefoy-Berard N and Revillard JP. Mechanisms of immunosuppression induced by antithymocyte globulins and okt3. J Heart Lung Transplant. (1996) 15:435–42.
47. Remberger M and Sundberg B. Rabbit-immunoglobulin G levels in patients receiving thymoglobulin as part of conditioning before unrelated donor stem cell transplantation. Haematologica. (2005) 90:931–8.
48. Waller EK, Langston AA, Lonial S, Cherry J, Somani J, Allen AJ, et al. Pharmacokinetics and pharmacodynamics of anti-thymocyte globulin in recipients of partially hla-matched blood hematopoietic progenitor cell transplantation. Biol Blood Marrow Transplant. (2003) 9:460–71. doi: 10.1016/s1083-8791(03)00127-7
49. Drozdov D, Kandil J, Long SE, Demorest CV, Cao Q, Lund TC, et al. Bodyweight and absolute lymphocyte count-based dosing of rabbit anti-thymocyte globulin results in early cd4(+) immune reconstitution in patients with inborn errors of metabolism undergoing umbilical cord blood transplantation. Transplant Cell Ther. (2025) 31:263. doi: 10.1016/j.jtct.2025.01.893
50. Call SK, Kasow KA, Barfield R, Madden R, Leung W, Horwitz E, et al. Total and active rabbit antithymocyte globulin (Ratg;Thymoglobulin) pharmacokinetics in pediatric patients undergoing unrelated donor bone marrow transplantation. Biol Blood Marrow Transplant. (2009) 15:274–8. doi: 10.1016/j.bbmt.2008.11.027
51. Kakhniashvili I, Filicko J, Kraft WK, and Flomenberg N. Heterogeneous clearance of antithymocyte globulin after cd34+-selected allogeneic hematopoietic progenitor cell transplantation. Biol Blood Marrow Transplant. (2005) 11:609–18. doi: 10.1016/j.bbmt.2005.05.001
52. Amrani ME, Admiraal R, Willaert L, Ebskamp-van Raaij LJC, Lacna AM, Hack CE, et al. Quantification of T cell binding polyclonal rabbit anti-thymocyte globulin in human plasma with liquid chromatography tandem-mass spectrometry. AAPS J. (2020) 22:43. doi: 10.1208/s12248-020-0419-6
53. Feng X, Scheinberg P, Biancotto A, Rios O, Donaldson S, Wu C, et al. In vivo effects of horse and rabbit antithymocyte globulin in patients with severe aplastic anemia. Haematologica. (2014) 99:1433–40. doi: 10.3324/haematol.2014.106542
54. Mohty M, Bacigalupo A, Saliba F, Zuckermann A, Morelon E, and Lebranchu Y. New directions for rabbit antithymocyte globulin (Thymoglobulin(®)) in solid organ transplants, stem cell transplants and autoimmunity. Drugs. (2014) 74:1605–34. doi: 10.1007/s40265-014-0277-6
55. Seidel MG, Fritsch G, Matthes-Martin S, Lawitschka A, Lion T, Pötschger U, et al. Antithymocyte globulin pharmacokinetics in pediatric patients after hematopoietic stem cell transplantation. J Pediatr Hematol Oncol. (2005) 27:532–6. doi: 10.1097/01.mph.0000184575.00717.25
56. Admiraal R, van Kesteren C, Jol-van der Zijde CM, van Tol MJ, Bartelink IH, Bredius RG, et al. Population pharmacokinetic modeling of thymoglobulin(®) in children receiving allogeneic-hematopoietic cell transplantation: towards improved survival through individualized dosing. Clin Pharmacokinet. (2015) 54:435–46. doi: 10.1007/s40262-014-0214-6
57. Komanduri KV, St. John LS, de Lima M, McMannis J, Rosinski S, McNiece I, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. (2007) 110:4543–51. doi: 10.1182/blood-2007-05-092130
58. Mohty M and Gaugler B. Advances in umbilical cord transplantation: the role of thymoglobulin/atg in cord blood transplantation. Best Pract Res Clin Haematol. (2010) 23:275–82. doi: 10.1016/j.beha.2010.05.004
59. Rocha V, Wagner JE, Sobocinski KA, Klein JP, Zhang M-J, Horowitz MM, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an hla-identical sibling. New Engl J Med. (2000) 342:1846–54. doi: 10.1056/NEJM200006223422501
60. Kuwano S, Terakura S, Imai K, Hirano S, Yokota H, Takeuchi Y, et al. Adjustment of low-dose atg exposure improves outcomes in allogeneic hematopoietic stem cell transplantation: A prospective multicenter study. Cytotherapy. (2025) 27:962–72. doi: 10.1016/j.jcyt.2025.05.009
61. Jol-van der Zijde CM, Bredius RG, Jansen-Hoogendijk AM, Raaijmakers S, Egeler RM, Lankester AC, et al. Igg antibodies to atg early after pediatric hematopoietic sct increase the risk of acute gvhd. Bone Marrow Transplant. (2012) 47:360–8. doi: 10.1038/bmt.2011.166
62. Remberger M, Persson M, Mattsson J, Gustafsson B, and Uhlin M. Effects of different serum-levels of atg after unrelated donor umbilical cord blood transplantation. Transpl Immunol. (2012) 27:59–62. doi: 10.1016/j.trim.2012.06.003
63. Elmahdi S, Muramatsu H, Narita A, Torii Y, Ismael O, Kawashima N, et al. Correlation of rabbit antithymocyte globulin serum levels and clinical outcomes in children who received hematopoietic stem cell transplantation from an alternative donor. Pediatr Transplant. (2016) 20:105–13. doi: 10.1111/petr.12620
64. Chawla S, Dharmani-Khan P, Liu Y, Prokopishyn N, Amlish Munir M, Griffiths C, et al. High Serum Level of Antithymocyte Globulin Immediately before Graft Infusion Is Associated with a Low Likelihood of Chronic, but Not Acute, Graft-Versus-Host Disease. Biol Blood Marrow Transplant. (2014) 20:1156–62. doi: 10.1016/j.bbmt.2014.04.007
65. Teramoto M, Takahashi T, Matsumoto K, Jaber M, Kaida K, Tamaki H, et al. Individualized rabbit anti-thymocyte globulin dosing in adult haploidentical hematopoietic cell transplantation with high-risk hematologic Malignancy: exposure-response analysis and population pharmacokinetics simulations. Am J Hematol. (2024) 99:387–95. doi: 10.1002/ajh.27195
66. Bacigalupo A, Oneto R, Lamparelli T, Gualandi F, Bregante S, Raiola AM, et al. Pre-emptive therapy of acute graft-versus-host disease: A pilot study with antithymocyte globulin (Atg). Bone Marrow Transplant. (2001) 28:1093–6. doi: 10.1038/sj.bmt.1703306
67. Bacigalupo A, Lamparelli T, Milone G, Sormani MP, Ciceri F, Peccatori J, et al. Pre-emptive treatment of acute gvhd: A randomized multicenter trial of rabbit anti-thymocyte globulin, given on day+7 after alternative donor transplants. Bone Marrow Transplant. (2010) 45:385–91. doi: 10.1038/bmt.2009.151
68. Khanolkar RA, Kalra A, Kinzel M, Pratt LM, Dharmani-Khan P, Chaudhry A, et al. A biomarker-guided, prospective, phase 2 trial of pre-emptive graft-versus-host disease therapy using anti-thymocyte globulin. Cytotherapy. (2021) 23:1007–16. doi: 10.1016/j.jcyt.2021.06.003
69. Xue E, Lorentino F, Lupo Stanghellini MT, Giglio F, Piemontese S, Clerici DT, et al. Addition of a single low dose of anti T-lymphocyte globulin to post-transplant cyclophosphamide after allogeneic hematopoietic stem cell transplant: A pilot study. J Clin Med. (2022) 11:1106. doi: 10.3390/jcm11041106
70. Bacigalupo A. Antilymphocyte/thymocyte globulin for graft versus host disease prophylaxis: efficacy and side effects. Bone Marrow Transplant. (2005) 35:225–31. doi: 10.1038/sj.bmt.1704758
71. Wu L, Zhou M, Li Y, Chen X, Mo W, Wang C, et al. Prospective study of a modified post-transplantation cyclophosphamide regimen for severe aplastic anemia patients with hla-haploidentical transplantation. Transplant Cell Ther. (2023) 29:463.e1–.e7. doi: 10.1016/j.jtct.2023.04.015
72. Admiraal R, Lindemans CA, van Kesteren C, Bierings MB, Versluijs AB, Nierkens S, et al. Excellent T-cell reconstitution and survival depend on low atg exposure after pediatric cord blood transplantation. Blood. (2016) 128:2734–41. doi: 10.1182/blood-2016-06-721936
73. Jamani K, Dabas R, Kangarloo SB, Prokopishyn NL, Luider J, Dharmani-Khan P, et al. Rabbit antithymocyte globulin serum levels: factors impacting the levels and clinical outcomes impacted by the levels. Biol Blood Marrow Transplant. (2019) 25:639–47. doi: 10.1016/j.bbmt.2018.12.065
74. Oostenbrink LVE, von Asmuth EGJ, Jol-van der Zijde CM, Jansen-Hoogendijk AM, Vervat C, Bredius RGM, et al. Anti-T-lymphocyte globulin exposure is associated with acute graft- versus -host disease and relapse in pediatric acute lymphoblastic leukemia patients undergoing hematopoietic stem cell transplantation: A multinational prospective study. Haematologica. (2024) 109:2854–63. doi: 10.3324/haematol.2023.284632
75. Dabas R, Jamani K, Kangarloo SB, Dharmani-Khan P, Williamson TS, Ousia S, et al. Antirelapse effect of pretransplant exposure to rabbit antithymocyte globulin. Blood Adv. (2019) 3:1394–405. doi: 10.1182/bloodadvances.2018030247
76. Yang M, Huang C, Liu X, Yuan J, Cao X, Cui Q, et al. (S263) pharmacokinetic-guided atg dosing optimizes outcomes in myeloablative haplo-cord hct. HemaSphere. (2025) 9:383–4. doi: 10.1002/hem3.70151
77. Seo J, Shin DY, Koh Y, Kim I, Yoon SS, Min Byun J, et al. Association between preconditioning absolute lymphocyte count and transplant outcomes in patients undergoing matched unrelated donor allogeneic hematopoietic stem cell transplantation with reduced-intensity conditioning and anti-thymocyte globulin. Ther Adv Hematol. (2021) 12:20406207211063783. doi: 10.1177/20406207211063783
78. Woo GU, Hong J, Kim H, Byun JM, Koh Y, Shin DY, et al. Preconditioning absolute lymphocyte count and transplantation outcomes in matched related donor allogeneic hematopoietic stem cell transplantation recipients with reduced-intensity conditioning and antithymocyte globulin treatment. Biol Blood Marrow Transplant. (2020) 26:1855–60. doi: 10.1016/j.bbmt.2020.06.005
79. Jullien M, Guillaume T, Peterlin P, Garnier A, Le Bourgeois A, Debord C, et al. Antithymocyte globulin administration in patients with profound lymphopenia receiving a pbsc purine analog/busulfan-based conditioning regimen allograft. Sci Rep. (2020) 10:15399. doi: 10.1038/s41598-020-72415-7
80. Heelan F, Mallick R, Bryant A, Radhwi O, Atkins H, Huebsch L, et al. Does lymphocyte count impact dosing of anti-thymocyte globulin in unrelated donor stem cell transplantation? Biol Blood Marrow Transplant. (2020) 26:1298–302. doi: 10.1016/j.bbmt.2020.02.026
81. McCune JS and Bemer MJ. Pharmacokinetics, pharmacodynamics and pharmacogenomics of immunosuppressants in allogeneic haematopoietic cell transplantation: part I. Clin Pharmacokinet. (2016) 55:525–50. doi: 10.1007/s40262-015-0339-2
82. McCune JS, Bemer MJ, and Long-Boyle J. Pharmacokinetics, pharmacodynamics, and pharmacogenomics of immunosuppressants in allogeneic hematopoietic cell transplantation: part ii. Clin Pharmacokinet. (2016) 55:551–93. doi: 10.1007/s40262-015-0340-9
83. Wang H, Dou L, Wang L, Peng B, Wang N, Zhao Y, et al. Individualized dosing of anti-thymocyte globulin led to reduced cmv/ebv reactivation and superior overall survival in adult unmanipulated haploidentical peripheral blood stem cell transplantation: A prospective phase ii study. Blood. (2022) 140:4753–4. doi: 10.1182/blood-2022-158994
84. Meesters-Ensing JI, Admiraal R, Ebskamp L, Lacna A, Boelens JJ, Lindemans CA, et al. Therapeutic drug monitoring of anti-thymocyte globulin in allogeneic stem cell transplantation: proof of concept. Front Pharmacol. (2022) 13:828094. doi: 10.3389/fphar.2022.828094
85. Dou L, Wang N, Zhang H, Liu Q, Wang H, Zhang X, et al. (S256) targeted dosing of anti-thymocyte globulin versus fixed dosing strategy in patients undergoing unmanipulated haploidentical haematopoietic stem-cell transplantation: A randomized, multicenter, phase 3 clinical trial. HemaSphere. (2025) 9:365–6. doi: 10.1002/hem3.70151
86. Takahashi T, Teramoto M, Matsumoto K, Jaber MM, Tamaki H, Ikegame K, et al. Population pharmacokinetics of total rabbit anti-thymocyte globulin in non-obese adult patients undergoing hematopoietic cell transplantation for hematologic Malignancy. Clin Pharmacokinet. (2023) 62:1081–91. doi: 10.1007/s40262-023-01252-4
87. Kekre N and Antin JH. Atg in allogeneic stem cell transplantation: standard of care in 2017? Counterpoint. Blood Adv. (2017) 1:573–6. doi: 10.1182/bloodadvances.2016001552
88. Shichijo T, Fuji S, Nagler A, Bazarbachi A, Mohty M, and Savani BN. Personalizing rabbit anti-thymocyte globulin therapy for prevention of graft-versus-host disease after allogeneic hematopoietic cell transplantation: is there an optimal dose? Bone Marrow Transplant. (2020) 55:505–22. doi: 10.1038/s41409-019-0643-9
89. Admiraal R, Nierkens S, Bierings MB, Belderbos ME, Huitema ADR, Bredius RG, et al. Improved survival with model-based dosing of anti-thymocyte globulin in pediatric hematopoietic cell transplantation. Blood Adv. (2025) 9:2344–53. doi: 10.1182/bloodadvances.2024014836
90. Villeneuve C, Humeau A, Monchaud C, Labriffe M, Rerolle JP, Couzi L, et al. Better rejection-free survival at three years in kidney transplant recipients with model-informed precision dosing of mycophenolate mofetil. Clin Pharmacol Ther. (2024) 116:351–62. doi: 10.1002/cpt.3206
91. Poweleit EA, Vinks AA, and Mizuno T. Artificial intelligence and machine learning approaches to facilitate therapeutic drug management and model-informed precision dosing. Ther Drug Monit. (2023) 45:143–50. doi: 10.1097/ftd.0000000000001078
Keywords: antithymocyte globulin, graft-versus-host disease, hematopoietic stem cell transplantation, precision dosing, therapeutic drug monitoring, pharmacokinetics
Citation: Wang H, Yang H, Du J, Dou L and Liu D (2025) Optimizing anti-thymocyte globulin dosing in allogeneic hematopoietic stem cell transplantation: individualized approaches and clinical implications. Front. Immunol. 16:1634157. doi: 10.3389/fimmu.2025.1634157
Received: 23 May 2025; Accepted: 23 July 2025;
Published: 08 August 2025.
Edited by:
Adrian Bogdan Tigu, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaReviewed by:
Haitham Abdelhakim, University of Kansas Medical Center, United StatesMadalina-Lorena Nistor, University of Medicine and PHarmacy “Iuliu Haţieganu”, Romania
Copyright © 2025 Wang, Yang, Du, Dou and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liping Dou, bGlwaW5ncnVpcnVpQDEyNi5jb20=; Daihong Liu, ZGFpaG9uZ3JtQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Haitao Wang
Haitao Wang Hongqi Yang
Hongqi Yang Jishan Du
Jishan Du Liping Dou
Liping Dou Daihong Liu
Daihong Liu