- Department of Neurology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
The COVID-19 pandemic has unveiled the pivotal role of systemic inflammatory responses in neurological complications, particularly parainfectious encephalopathy. Accumulating evidence has established innate immune overreaction—distinct from direct viral neuro-invasion or autoantibody-mediated reaction—as the fundamental mechanism. The clinical manifestations of parainfectious encephalopathy are highly diverse, spanning from mild cases, such as mild encephalopathy with or without a reversible splenial lesion (MERS or ME), to catastrophic syndromes like acute necrotizing encephalopathy (ANE) and febrile infection-related epilepsy syndrome with or without a claustrum lesion (FIRES-C or FIRES). In this article, we summarize the phenotypes, diagnosis, and treatment strategies for parainfectious encephalopathy to enhance clinical recognition and understanding of this re-emerging disorder.
1 Introduction
During the COVID-19 pandemic in the past few years, parainfectious encephalopathy has become a key focus in neuroinfectious and neuroimmunological research. For neurologists, the initial clinical issue is whether SARS-CoV-2 causes viral encephalitis or possesses neuroinvasive properties. Accumulating evidence from systematic and in-depth research has progressively clarified our understanding of the underlying mechanisms.
2 COVID-19-associated encephalopathy: a parainfectious encephalopathy
COVID-19-associated Encephalopathy is driven primarily by systemic inflammatory cascades rather than direct viral invasion of the central nervous system (CNS). Zamani et al. (1) analyzed 182 COVID-19 cases with CNS involvement, finding no clinical or ancillary evidence of direct neural infection. Neuropathological studies support this conclusion: autopsies from the United States and Germany detected SARS-CoV-2 only in vascular endothelial cells in the brain, with no evidence of viral invasion into neurons or glial cells. Other pathological findings included glial activation, microglial nodules, and mild inflammatory infiltration (predominantly by monocytes and T lymphocytes) (2, 3). A Chinese cohort study demonstrated blood-brain barrier disruption (reflected by reduced GFAP immunoreactivity) without neuronal viral RNA detection (4). These changes resemble influenza virus-associated encephalopathy, where GFAP staining shows clasmatodendrosis—fragmentation and loss of astrocytic processes. Additionally, cerebral edema, necrosis, and hemorrhage were prominent, yet inflammatory cell infiltration was minimal (5).
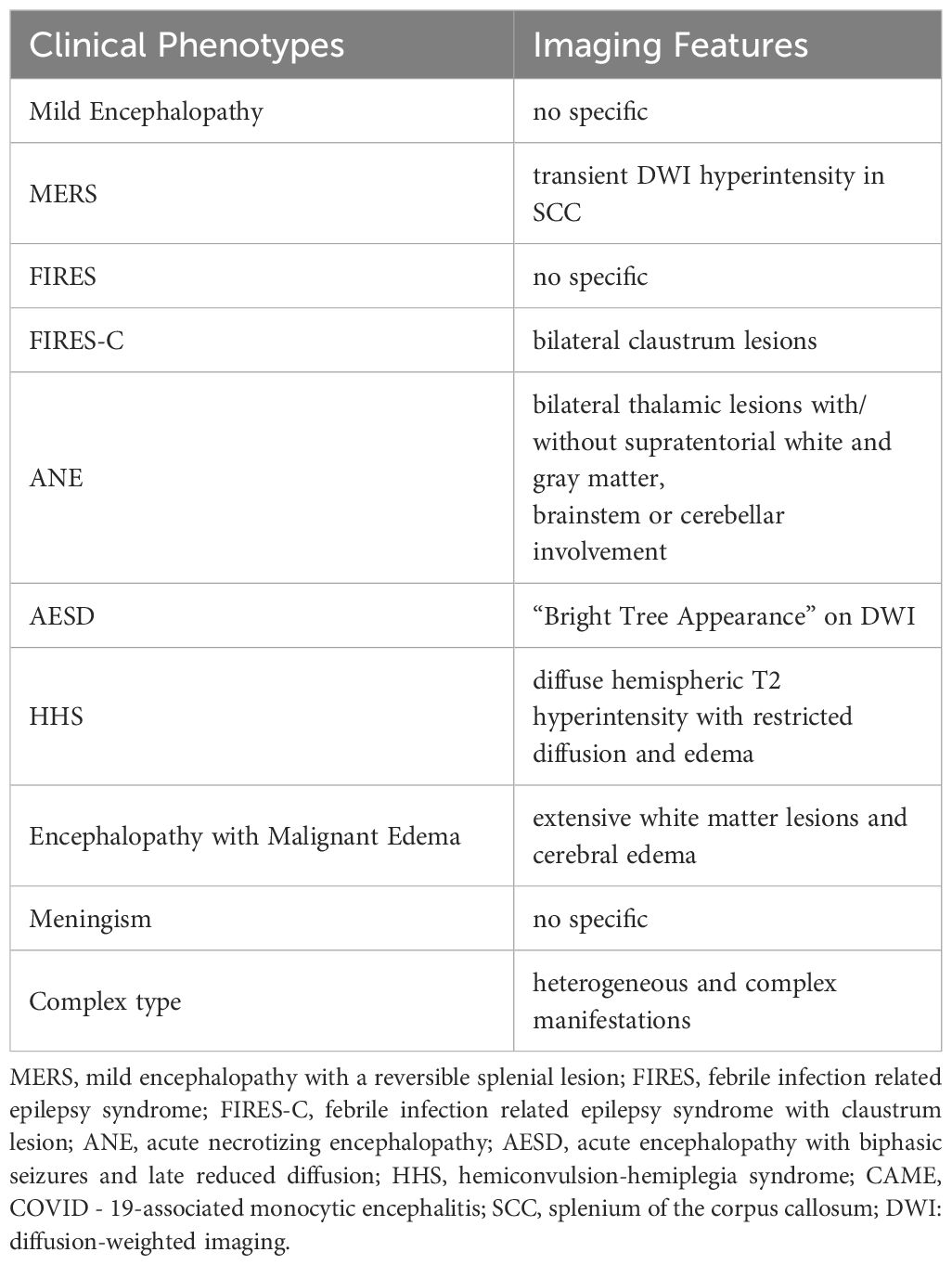
Encephalopathy caused by respiratory viruses such as SARS-CoV-2 is distinct from viral encephalitis caused by neurotropic viruses or autoimmune encephalitis mediated by specific antibodies. Instead, it is best categorized as a distinct third type—parainfectious encephalopathy. Respiratory viral infections can trigger a rapid innate immune response, leading to cytokine storms and systemic inflammatory syndrome. These conditions reflect CNS involvement in systemic inflammatory responses (6) (Figure 1). In the 1990s, Japan reported a series of influenza A–associated parainfectious encephalopathies, including acute necrotizing encephalopathy (ANE), which showed similarities to COVID - 19-related parainfectious encephalopathy in clinical presentation, neuroimaging features, and neuropathology (7). In recent years, we have encountered a range of parainfectious encephalopathy cases in clinical practice. Based on accumulating experience and literature review, we propose a preliminary classification of parainfectious encephalopathy subtypes.
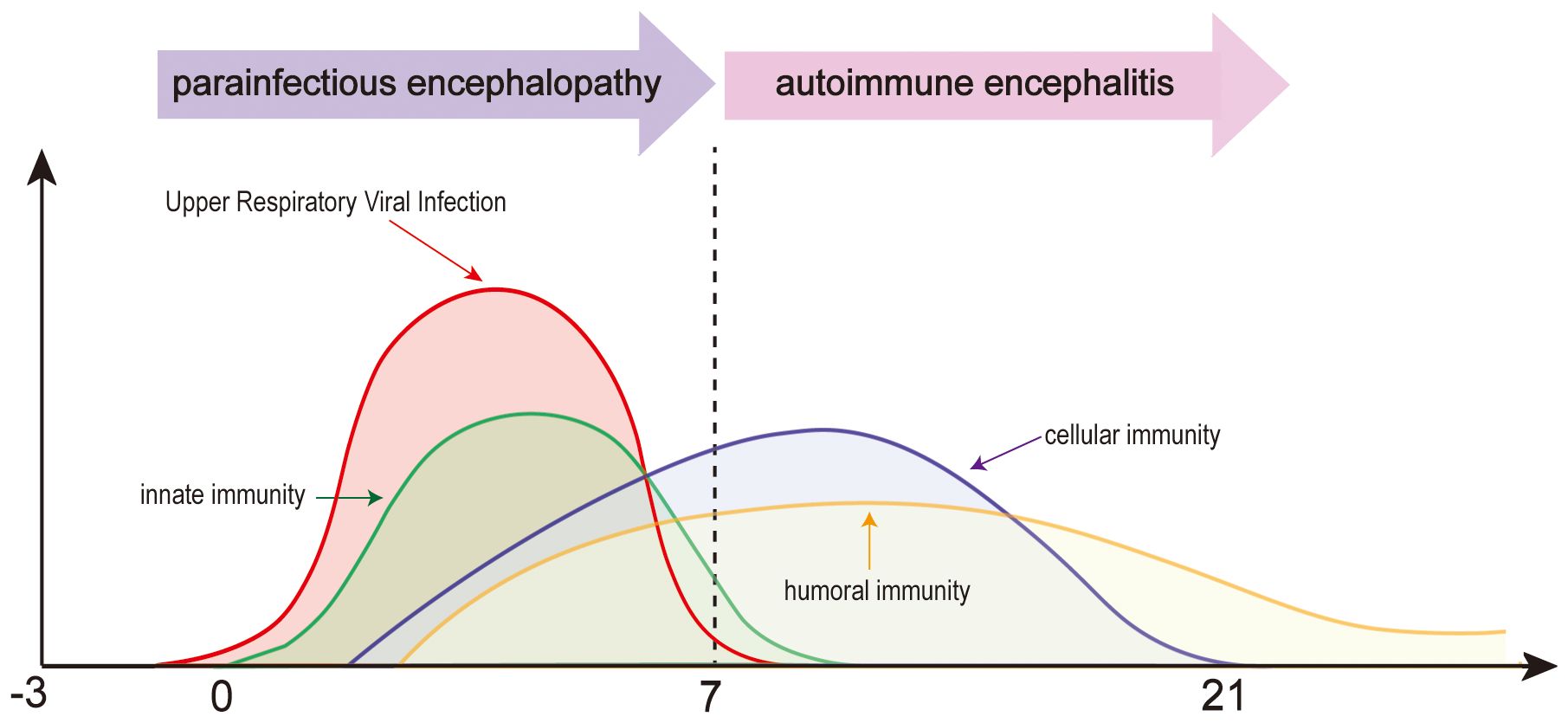
Figure 1. Temporal dynamics of parainfectious and autoimmune encephalitis following upper respiratory viral infection.
3 Clinical phenotypes of parainfectious encephalopathy
3.1 Mild encephalopathy
During the acute phase of respiratory viral infections, some patients may experience transient confusion or delirium accompanied by fever, with normal neuroimaging, electroencephalography (EEG), and cerebrospinal fluid (CSF) analysis. After excluding potential causes such as hypoxia, ischemia, and metabolic disturbances, this self-limiting mild encephalopathy can be classified as reversible mild encephalopathy. Some researchers have proposed the term “benign variant of encephalopathy”, distinguishing it from other severe subtypes of parainfectious encephalopathy (8).
3.2 Mild encephalopathy with a reversible splenial lesion
The neuroimaging hallmark of this condition is reversible corpus callosum splenium lesions, manifesting as diffusion-weighted imaging (DWI) hyperintensity with restricted diffusion (Figure 2A). First described by Tada et al. (9) in 2004, MERS was initially identified in a case series predominantly involving Japanese children and adolescents. The etiology of MERS is multifactorial. While early cases were primarily associated with influenza and rotavirus, recent evidence implicates SARS-CoV-2 as an additional trigger (10).
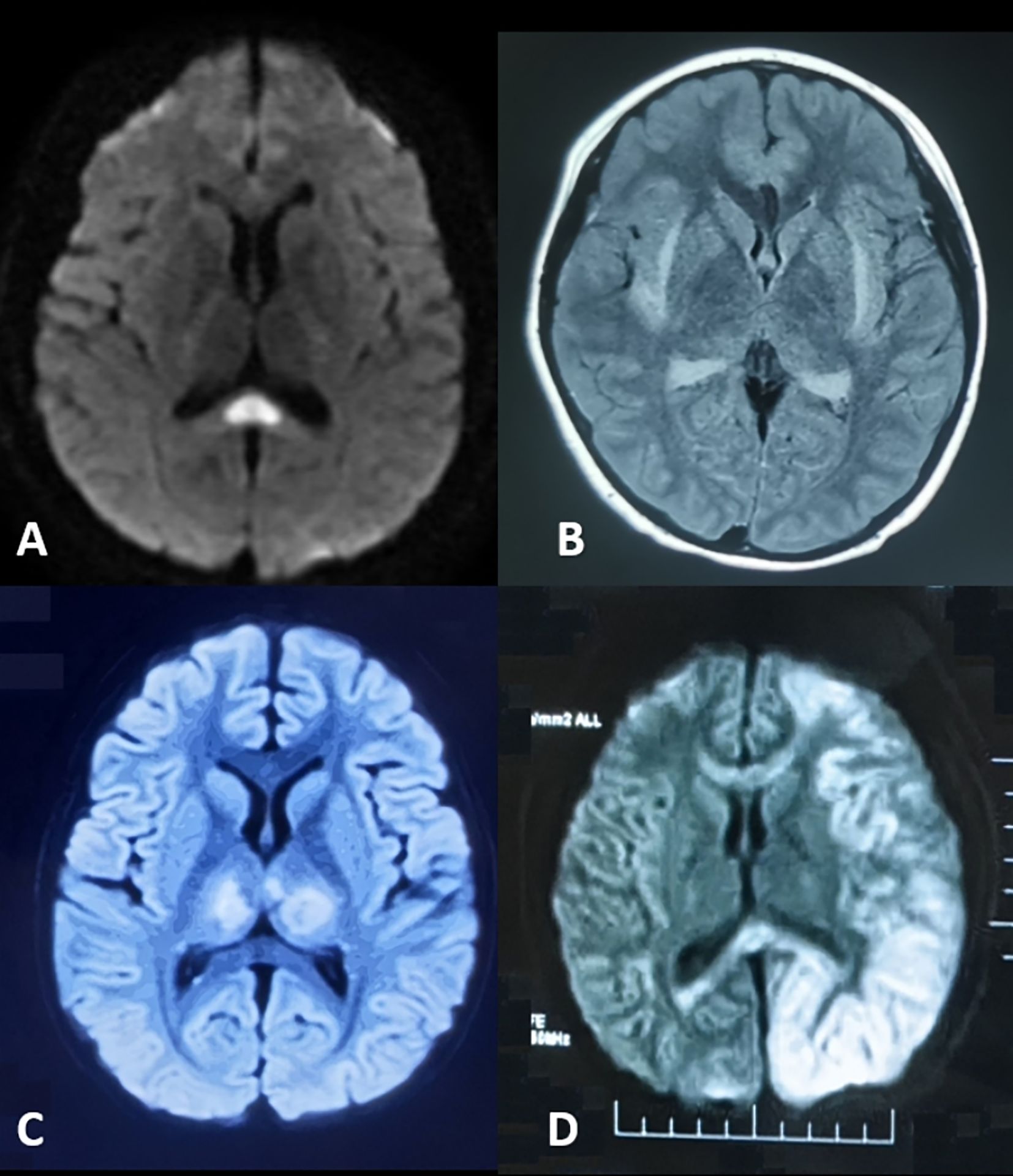
Figure 2. Neuroimaging of parainfectious encephalopathies. (A) Mild Encephalopathy with Reversible Splenial Lesion: splenium of the corpus callosum hyperintensity on diffusion-weighted imaging (DWI). (B) Febrile Infection-Related Epilepsy Syndrome with Claustrum Lesion: bilateral claustrum hyperintensity on T2-weighted and fluid-attenuated inversion recovery (FLAIR) imaging. (C) Acute Necrotizing Encephalopathy: bilateral thalamic hyperintensity on T2/FLAIR imaging. (D) Hemiconvulsion-Hemiplegia Syndrome: left cerebral cortex and subcortical hyperintensity on DWI with corpus callosum involvement and associated edema.
3.3 Febrile infection-related epilepsy syndrome
Previously termed Febrile Illness-Related Epilepsy Syndrome. FIRES predominantly affects children, manifesting as seizure onset within 24 hours to several days after a prodromal infection (typically respiratory viral infections), with rapid progression to status epilepticus. The acute-phase mortality rate reaches up to 30%, with survivors frequently developing long-term sequelae such as drug-resistant epilepsy and cognitive impairment (11). The acronym “FIRES” aptly captures the disease’s devastating and rapid course. However, the absence of specific diagnostic biomarkers poses significant challenges to timely diagnosis and management.
3.4 Febrile infection-related epilepsy syndrome with claustrum lesion
FIRES-C is a distinct subtype of FIRES, marked by claustrum abnormalities on neuroimaging (Figure 2B), which may serve as a diagnostic biomarker. In a cohort reported by Lin Bai et al. (12), 65% of patients were female, with a median age of 20.5 years. The median interval from fever onset to seizures was 5 days. Most presented with generalized tonic-clonic seizures; 45% experienced transient psychiatric or behavioral symptoms, and 50% required ICU admission. MRI typically revealed bilateral claustral lesions, sometimes accompanied by hippocampal signal abnormalities, usually detected around 12.5 days after symptom onset. CSF showed normal or mildly elevated white cell counts. Despite intensive immunotherapy, prognosis was poor, with 58% developing refractory epilepsy at one-year follow-up.
3.5 Acute necrotizing encephalopathy
ANE is characterized by rapid-onset encephalopathy, seizures, and focal neurological deficits, often following a febrile illness. Geng Chang et al. reported that in the Chinese population, symptoms typically develop 1~6 days (median 2 days) after respiratory viral infections, presenting as rapidly progressive consciousness disturbances and seizures (13). The hallmark imaging feature is bilateral thalamic lesions (Figure 2C), often accompanied by involvement of supratentorial gray and white matt, brainstem, and cerebellum. Elevated CSF IL - 6 levels and RANBP2 gene abnormalities are key diagnostic markers. ANE is associated with high mortality and disability rates. However, early aggressive immunotherapy may improve outcomes. As a prototypical parainfectious encephalopathy, ANE has been extensively studied in China, especially during COVID-19, advancing our understanding of its mechanisms and management (14–16).
3.6 Acute encephalopathy with biphasic seizures and late reduced diffusion
AESD was first described by Takanashi et al. in 2008 (17). This East Asia-prevalent disorder predominantly affects infants and young children. Zhang Meijiao et al. reported 21 pediatric cases presenting biphasic progression: initial seizures within 24 hours post-febrile onset, a quiescent period of 3~5 days, followed by recurrent seizures or altered consciousness from days 3 to 7. Characteristic DWI findings (days 3~14) demonstrate subcortical/cortical hyperintensity (“bright tree appearance”), while follow-up MRI reveals frontal/frontoparietal atrophy or residual lesions. Atypical monophasic cases have also been documented (18).
3.7 Hemiconvulsion-hemiplegia syndrome
HHS typically occurs in infants and young children. It is characterized by prolonged unilateral clonic seizures during febrile illnesses, followed by persistent hemiplegia. MRI reveals diffuse T2 hyperintensity and restricted diffusion in the affected hemisphere, often with severe edema (Figure 2D). Within one month, the affected hemisphere may show atrophy and hippocampal sclerosis. Approximately 80% of patients develop focal epilepsy.
3.8 Encephalopathy with malignant edema
It presents with acute psychiatric symptoms, refractory seizures, impaired consciousness, and coma. CT and MRI reveal extensive white matter lesions and cerebral edema. Cases have been reported in both influenza A and COVID-19 infections, with a very poor prognosis (8, 19, 20).
3.9 Meningism (Pseudomeningitis)
Meningism manifests meningeal irritation during acute febrile illness. In 1938, Merritt et al. reported 70 cases (21), mostly in individuals under 20, characterized by acute headache, neck stiffness, and a positive Kernig’s sign. CSF findings typically showed normal leukocyte counts with elevated intracranial pressure. The condition was self-limiting. Fishman later described meningismus as a diagnosis of exclusion, requiring differentiation from viral meningitis (22).
3.10 Complex type of parainfectious encephalopathy
Multiple clinical phenotypes may coexist in a single patient. In some cases, patients present with diffuse lesions resembling acute disseminated encephalomyelitis (ADEM); however, pathological findings often reveal minimal perivascular lymphocytic infiltration and absence of classic perivenous inflammation, distinguishing them from typical ADEM or other demyelinating diseases of the CNS (23).
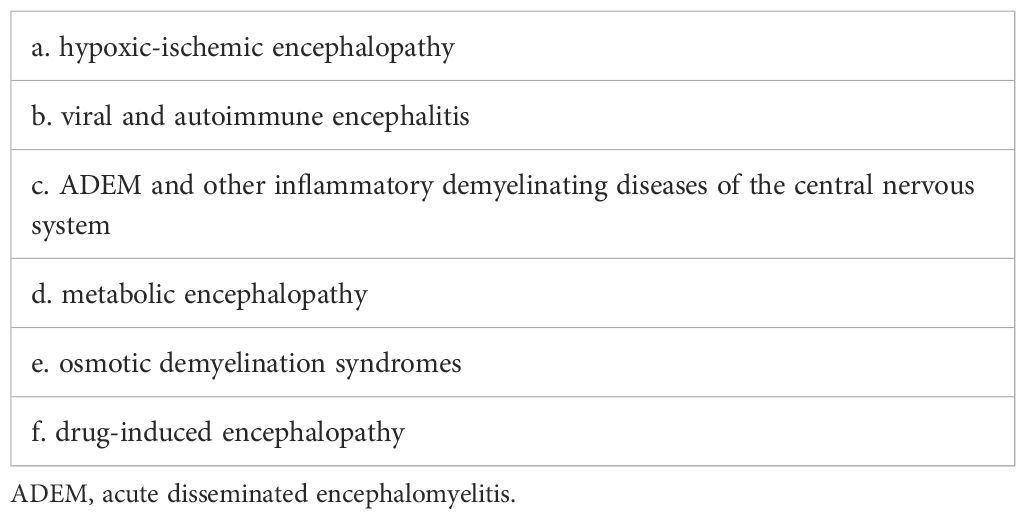
4 Diagnosis and differential diagnosis of parainfectious encephalopathy
4.1 Diagnostic key points for parainfectious encephalopathy
a. Neurological or psychiatric symptoms (e.g., encephalopathy, seizures, or altered consciousness) emerging in the acute phase (1 day to several days) of systemic infections (mainly acute respiratory viral infections).
b. CSF WBC count normal or mildly elevated, with elevated cytokines (e.g.,IL-6) in CSF and/or serum, and negative anti-neuronal antibodies.
c. Distinct imaging features for subtypes like ANE, FIRES-C, MERS, HHS, and AESD.
d. Reasonable exclusion of other causes.
4.2 Differential diagnosis of parainfectious encephalopathy
ANE, FIRES-C, MERS, AESD, and HHS exhibit distinct clinical and neuroimaging phenotypes (Table 1), making them recognizable and diagnosable. In contrast, other subtypes of parainfectious encephalopathy lack specific features and pose greater diagnostic challenges, necessitating thorough differential diagnosis (Table 2).
5 Immunotherapy for parainfectious encephalopathy
Intensive immunotherapy is crucial in severe parainfectious encephalopathies, particularly ANE and FIRES-C. First-line treatment typically includes high-dose glucocorticoid pulse therapy combined with intravenous immunoglobulin (IVIG). Our data confirm this regimen significantly improves neurological recovery in COVID - 19-associated ANE (14). Tocilizumab, an IL - 6 receptor antagonist, serves as a cornerstone therapeutic agent for COVID - 19-associated inflammatory syndromes. Given that parainfectious encephalopathy manifests as a component of systemic inflammatory response, this biologic agent demonstrates emerging therapeutic potential in severe encephalopathy subtypes, with expanding clinical applications (24). Our systematic review of tocilizumab-treated ANE cases revealed significantly improved neurological outcomes when administered within 24 hours of hospital admission (PROSPERO, ID CRD420251017817).
Parainfectious encephalopathy stems from early innate immune dysregulation and typically does not require long-term immunotherapy. Long-term therapies targeting adaptive immunity (e.g., rituximab, mycophenolate mofetil) have shown limited benefit in FIRES-C (12). Prolonged tocilizumab treatment may be effective for seizure control in some cases of FIRES-C; however, this requires further validation through cohort studies. The optimal dosing, frequency, and duration of tocilizumab remain under investigation. Based on our clinical experience, we have shortened the dosing interval from every four weeks to every two weeks to achieve a more rapid therapeutic effect.
6 Parainfectious encephalopathy and long neuro-COVID-19
The sequelae of parainfectious encephalopathy may be mechanistically associated with long neuro-COVID-19 syndrome. The long-term neurological complications of SARS-CoV-2 infection impose a substantial burden on affected patients. Proposed mechanisms include immune dysregulation, persistent inflammation, vascular dysfunction, and neurotransmitter imbalance (25). Notably, even patients with mild initial neurological symptoms demonstrate brain changes on structural MRI, particularly in white matter regions, which correlate with specific cognitive deficits (26). Recent studies by Schwabenland et al. have further demonstrated persistent neuroinflammation with distinct microglia-T-cell interactions in COVID - 19 brains (27).
Although parainfectious encephalopathy typically presents as acute or transient neurological damage, its sequelae may share similarities with many symptoms of long COVID - 19, including anxiety, memory loss, and cognitive impairment (“brain fog”). In clinical practice, we have observed that some FIRES-C patients with disease courses exceeding 6 months continue to respond well to tocilizumab, suggesting that sustained inflammatory activation may persist beyond the acute phase of parainfectious encephalopathy.
In summary, parainfectious encephalopathy is a group of autoinflammatory encephalopathy closely related to innate immune response. Its pathogenic mechanism, clinical phenotype, and immunotherapy are different from those of autoimmune encephalitis mediated by adaptive immune response. Parainfectious encephalopathy comprises a spectrum of acute encephalopathy syndromes triggered by systemic infections, characterized by heterogeneous clinical manifestations. For severe parainfectious encephalopathy cases, early diagnosis followed by aggressive immunotherapy is critical to improve outcomes. As research advances, accumulating clinical evidence will pave the way for a deeper, evidence-based consensus on its diagnosis and management.
Author contributions
LB: Visualization, Writing – original draft. CG: Writing – original draft. HG: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. National High Level Hospital Clinical Research Funding (2022−PUMCH−B−120); Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2021−I2M−1−003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Solely for language polishing during manuscript preparation.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zamani R, Pouremamali R, and Rezaei N. Central neuroinflammation in Covid-19: a systematic review of 182 cases with encephalitis, acute disseminated encephalomyelitis, and necrotizing encephalopathies. Rev Neurosci. (2021) 33:397–412. doi: 10.1515/revneuro-2021-0082
2. Thakur KT, Miller EH, Glendinning MD, Al-Dalahmah O, Banu MA, Boehme AK, et al. COVID-19 neuropathology at Columbia University Irving Medical Center/New York Presbyterian Hospital. Brain. (2021) 144:2696–708. doi: 10.1093/brain/awab148
3. Matschke J, Lütgehetmann M, Hagel C, Sperhake JP, Schröder AS, Edler C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. (2020) 19:919–29. doi: 10.1016/S1474-4422(20)30308-2
4. Zhang PP, He ZC, Yao XH, Tang R, Ma J, Luo T, et al. COVID-19-associated monocytic encephalitis (CAME): histological and proteomic evidence from autopsy. Signal Transduct Target Ther. (2023) 8:24. doi: 10.1038/s41392-022-01291-6
5. Yamada S, Yasui K, Hasegawa Y, Tsuzuki T, Yoshida M, Hashidume Y, et al. An autopsy case of pandemic (H1N1) 2009 influenza virus-associated encephalopathy. Rinsho Shinkeigaku. (2012) 52:480–5. doi: 10.5692/clinicalneurol.52.480
6. Subbarao K and Mahanty S. Respiratory virus infections: understanding COVID-19. Immunity. (2020) 52:905–9. doi: 10.1016/j.immuni.2020.05.004
7. Mizuguchi M, Hayashi M, Nakano I, Kuwashima M, Yoshida K, Nakai Y, et al. Concentric structure of thalamic lesions in acute necrotizing encephalopathy. Neuroradiology. (2002) 44:489–93. doi: 10.1007/s00234-002-0773-3
8. Akins PT, Belko J, Uyeki TM, Axelrod Y, Lee KK, Silverthorn J, et al. H1N1 encephalitis with Malignant edema and review of neurologic complications from influenza. Neurocrit Care. (2010) 3:396–406. doi: 10.1007/s12028-010-9436-0
9. Tada H, Takanashi J, Barkovich AJ, Oba H, Maeda M, Tsukahara H, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology. (2004) 63:1854–8. doi: 10.1212/01.WNL.0000144274.12174.CB
10. Si W, Li Y, and Du G. Case report: Rare Guillain-Barré syndrome variants and mild encephalitis/encephalopathy with a reversible splenial lesion as the para-infectious manifestations of SARS-CoV-2 infection. Front Immunol. (2024) 15:1458231. doi: 10.3389/fimmu.2024.1458231
11. Wang X and Jiang W. New onset refractory status epilepticus/febrile infection-related epilepsy syndrome: the new challenge in the diagnosis and treatment of status epilepticus. Chin J Neurol. (2024) 57:309–14. doi: 10.3760/cma.j.cn113694-20231030-00280
12. Bai L, Di W, Xu Z, Liu B, Lin N, Fan S, et al. Febrile infection-related epilepsy syndrome with claustrum lesion: an underdiagnosed inflammatory encephalopathy. Neurol Sci. (2024) 45:3411–9. doi: 10.1007/s10072-024-07363-5
13. Geng C, Gong L, Zhang W, Yang X, Zhao W, Wang Q, et al. Clinical characteristics and prognosis of acute necrotizing encephalopathy. Chin J Neurol. (2025) 58: 494–500. doi: 10.3760/cma.j.cn113694-20250101-00001
14. Geng C, Zhao W, Wang Z, Wu J, Jiang D, Jiang N, et al. Acute necrotizing encephalopathy associated with COVID-19: case series and systematic review. J Neurol. (2023) 270:5171–81. doi: 10.1007/s00415-023-11915-8
15. Wu J, Hao X, Guan H, and Guo Y. SARS-CoV-2 related to parainfectious encephalopathy: three cases report. Chin J Modern Neurol. (2024) 24:238–44. doi: 10.3969/j.issn.1672-6731.2024.04.007
16. Zhao L, Li X, Yan L, Li L, Wang X, Yang X, et al. One case of acute necrotizing encephalopathy associated with novel coronavirus infection. Chin J Neuropsychiatr Dis. (2024) 50:156–8. doi: 10.3969/j.issn.1002-0152.2024.03.006
17. Tada H, Takanashi JI, Terada H, and Tajima K. Severe form of acute influenza encephalopathy with biphasic seizures and late reduced diffusion. Neuropediatrics. (2008) 39:134–6. doi: 10.1055/s-2008-1081459
18. Zhang M, Lin L, Wang W, Wei C, Xie H, Zhang Q, et al. Clinical and imaging characteristics of acute encephalopathy in children with biphasic onset and late stage diffusion reduction. Chin J Pediatrics. (2023) 61:989–94. doi: 10.3760/cma.j.cn112140-20230809-00094
19. Zhang T, Zhang QF, Yang HM, Liu P, Sun P, Li YM, et al. Children with severe neurological symptoms associated with SARS-CoV-2 infection during Omicron pandemic in China. Pediatr Res. (2024) 95:1088–94. doi: 10.1038/s41390-023-02904-8
20. Ninan S, Thompson P, Gershon T, Ford N, Mills W, Jewells V, et al. Fatal pediatric COVID - 19 case with seizures and fulminant cerebral edema. Child Neurol Open. (2021) 8:2329048X211022532. doi: 10.1177/2329048X211022532
22. Fishman RA. Cerebrospinal Fluid in Diseases of the Nervous System. Philadelphia: WB. Saunders (1980).
23. Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF, et al. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. (2020) 140:1–6. doi: 10.1007/s00401-020-02166-2
24. Liu C, Hu Q, Wang N, Li X, Zhou M, Guan H, et al. Tocilizumab in the treatment of COVID - 19 related encephalopathy with Claustrum lesion: A case report. Radiol Case Rep. (2025) 20:101–4. doi: 10.1016/j.radcr.2024.09.092
25. Slama Schwok A and Henri J. Long neuro-COVID-19: current mechanistic views and therapeutic perspectives. Biomolecules. (2024) 14:1081. doi: 10.3390/biom14091081
26. Serrano Del Pueblo VM, Serrano-Heras G, Romero Sánchez CM, and Landete PP. Brain and cognitive changes in patients with long COVID compared with infection-recovered control subjects. Brain. (2024) 147:3611–23. doi: 10.1093/brain/awae101
Keywords: parainfectious encephalopathy, COVID-19, cytokines, autoinflammatory, innate immunity
Citation: Bai L, Geng C and Guan H (2025) Rediscovering parainfectious encephalopathy in the post-COVID-19 era. Front. Immunol. 16:1634383. doi: 10.3389/fimmu.2025.1634383
Received: 24 May 2025; Accepted: 26 August 2025;
Published: 25 September 2025.
Edited by:
Paolo Immovilli, Guglielmo da Saliceto Hospital, ItalyReviewed by:
Alexandru Tatomir, University of Maryland, United StatesCopyright © 2025 Bai, Geng and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongzhi Guan, cHVtY2hnaHpAMTI2LmNvbQ==
 Lin Bai
Lin Bai Chang Geng
Chang Geng Hongzhi Guan
Hongzhi Guan