- 1Department of Anesthesiology, The First Affiliated Hospital of Ningbo University, Ningbo, China
- 2Health Science Center, Ningbo University, Ningbo, China
Chronic pain remains a significant global health challenge. Current anti-nociceptive therapies often fail to provide adequate relief and are associated with adverse side effects, underscoring the need for novel therapeutic approaches. Specialized pro-resolving mediators (SPMs)—bioactive lipid compounds derived from omega-3 and omega-6 fatty acids—have recently garnered attention as potential agents for pain management due to their dual anti-inflammatory and inflammation-resolving properties. This review explores the multifaceted anti-nociceptive effects of SPMs, focusing on their mechanisms of action in diverse pain models, including neuropathic, inflammatory, cancer-induced, postoperative, and spontaneous pain. We highlight the distinct roles of specific SPMs, such as Resolvin D1 (RvD1), Resolvin E1 (RvE1), and Maresin 1 (MaR1), in modulating pain pathways through mechanisms such as suppression of inflammatory cytokines, modulation of transient receptor potential (TRP) channels, and interactions with immune cells to resolve inflammation. Additionally, we discuss the implications of sexual dimorphism in SPM efficacy, endogenous SPM biosynthesis, and therapeutic strategies involving omega-3 fatty acid supplementation. While preclinical studies demonstrate the therapeutic promise of SPMs, critical gaps persist in understanding their precise mechanisms, long-term safety, and translational potential. This review emphasizes the need for rigorous preclinical and clinical research to elucidate SPMs’ role in managing recalcitrant pain conditions, with the aim of advancing targeted, non-opioid pain therapies.
1 Introduction
Pain is defined as “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” (1). Chronic pain, in particular, represents a major global health challenge, severely impacting quality of life and imposing substantial socioeconomic burdens (2). For instance, A meta-analysis estimated that the prevalence of chronic pain in the general population of developing countries is 18%, while in Germany it is 18.4%, 21.5% in Hong Kong, 24.4% in Norway, 19% in Denmark, and 19% and 20.4% in the United States (3). The economic burden of chronic pain is equally staggering. In the United States, approximately one-third of the population is affected, with annual costs estimated at US$560–635 billion (4). In Australia, individuals with chronic pain incur average annual costs of AU$22,588–42,979 per person when non-financial costs are considered (4). However, managing this pervasive condition remains profoundly challenging. First-line treatments, including antidepressants (e.g., duloxetine, amitriptyline) and anticonvulsants (e.g., gabapentin, pregabalin) for neuropathic pain, and non-steroidal anti-inflammatory drugs (NSAIDs)/cyclooxygenase-2 (COX-2) inhibitors for inflammatory pain, often yield incomplete relief and are plagued by significant side effects, ranging from sedation and gastrointestinal toxicity to the risks of dependence on opioids. These limitations of conventional analgesics underscore the urgent, unmet need for novel, mechanism-driven therapies that not only effectively suppress pain but also promote its active resolution with improved safety profiles.
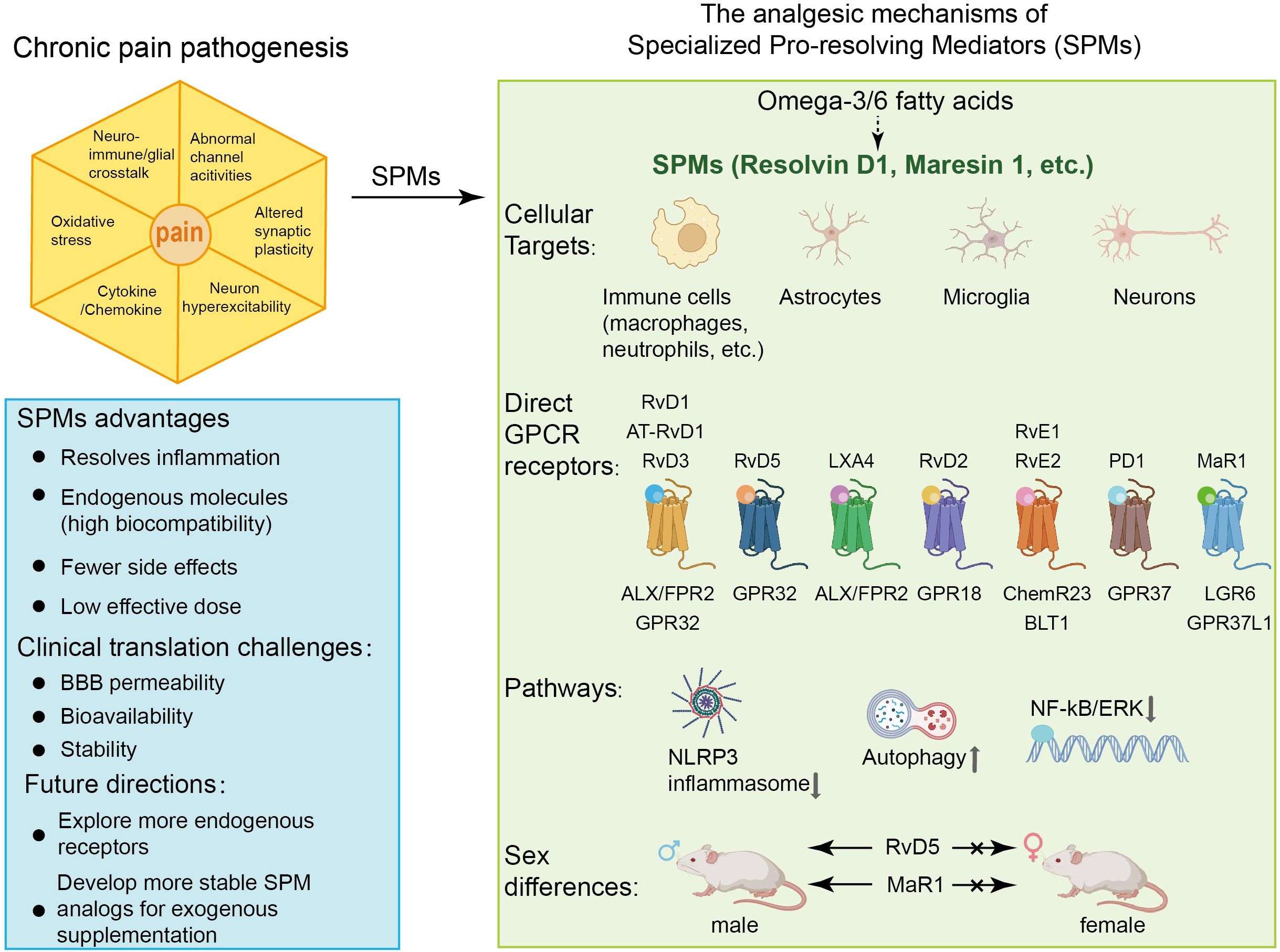
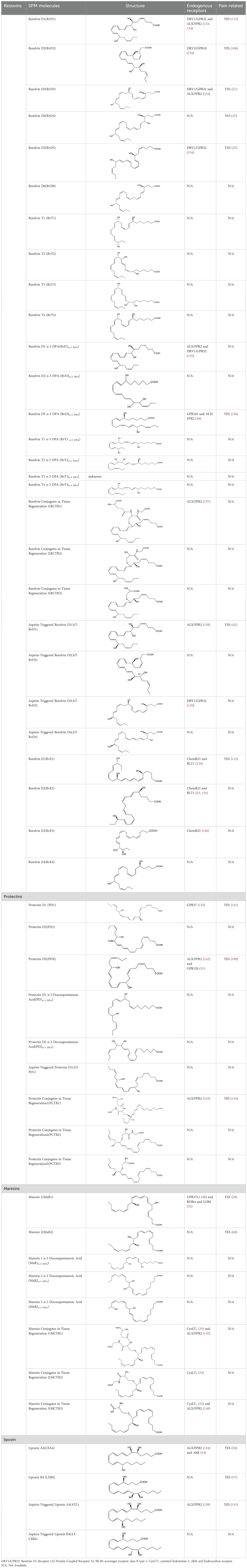
Specialized pro-resolving mediators (SPMs) are endogenous bioactive compounds biosynthesized from omega-3 [e.g., eicosapentaenoic (EPA), docosahexaenoic (DHA)] and omega-6 (e.g., arachidonic acid) polyunsaturated fatty acids. This family includes resolvins, protectins, maresins, and lipoxins (5). Unlike conventional anti-inflammatory agents, SPMs do not suppress immune function; instead, they orchestrate inflammatory resolution by actively promoting homeostasis through receptor-mediated pathways (6). Their multifaceted roles include resolving inflammation (7, 8), facilitating tissue and organ repair (9), modifying lipidome (8), providing anti-infection properties (10, 11), alleviating depressive symptoms (12), modulating tumor progression (13), preventing atherosclerosis (14), and enhancing the healing response following vascular injury (15). To date, 43 distinct SPMs have been identified. Though the quantity is relatively small, their potent pro-resolving and anti-inflammatory actions form the basis of resolution pharmacology—a therapeutic strategy focused on restoring physiological balance rather than broadly inhibiting inflammation (16). SPMs are widely distributed throughout the human body. They have been detected in multiple tissues, including the brain, adipose tissue, placenta, lymph nodes, and spleen. Notably, the brain exhibits the highest recorded tissue concentrations (380–1,800 pg per mg protein). SPMs are also present in various biological fluids—such as exhaled breath condensate, synovial fluid, serum, plasma, urine, cerebrospinal fluid, breast milk, and sputum (particularly in the context of cystic fibrosis). Among these fluids, breast milk (10–27,000 pM), serum (14–6,000 pM), and exhaled breath condensate (6,000 pM) contain the highest reported concentrations (17). Notably, their concentrations in serum rise during acute inflammatory responses, suggesting a dynamic role in resolving inflammation (18).
Emerging evidence suggests that SPMs exert anti-nociceptive effects within the nervous system by targeting specific receptors. Direct receptors currently identified include: lipoxin A receptor/formyl-peptide receptor 2 (ALX/FPR2), activated by RvD1, RvD3, AT-RvD1, and lipoxins (19–21); Chemerin receptor 23 (ChemR23) and Leukotriene B4 receptor 1, both modulated by RvE1 and RvE2 (22, 23); Resolvin D1 Receptor 1/G-protein-coupled receptor 32 (DRV1/GPR32), engaged by RvD1, RvD3, RvD5, and AT-RvD1 (24–26); GPR18 influenced by RvD2 (27); MaR1 acts on G protein-coupled receptor 37 like 1 (GPR37L1) and LGR6 as its specific receptor (26, 28); PD1 enhances macrophage phagocytosis through GPR37 (29). Beyond the nervous system, SPMs also demonstrate therapeutic potential in modulating cancer progression, suppressing inflammation, accelerating tissue repair and regeneration, and providing antioxidant protection through interactions with GPR101, GPR120, RORα, LGR6, CysLT1, and AhR (30–34) (See Table 1 and graphic abstract).
The exploration of SPMs for pain relief is in its early stages, yet preclinical trials already indicate these compounds may become valuable therapeutic options. Notably, resolvins—unlike conventional analgesics such as morphine (35)—do not alter baseline pain sensitivity in healthy rodents, suggesting a favorable safety profile due to their targeted activity in pathological states (36–39). In preclinical models, SPMs demonstrate promising efficacy compared with traditional pain medicine. Resolvins outperformed gabapentin in alleviating neuropathic pain in the chronic constriction injury (CCI) model (40). In the formalin-induced inflammatory pain model, their effective doses were substantially lower than those required for morphine or the Cyclooxygenase-2 (COX-2) inhibitor NS-398 (36). Compared with standard anti-inflammatory drugs, only DEXA alleviated pain evoked by both 500 μg and 100 μg carrageenan, whereas INDO and CX were effective only against the high-dose stimulus. In contrast, RvD1 and RvE1 reduced nociception at both concentrations, demonstrating that the resolvins possess a broader therapeutic spectrum of anti-inflammatory and analgesic potential than traditional analgesics (41). Administration of 17(R)-HDoHE attenuates Complete Freund’s Adjuvant (CFA)-induced nociception. This analgesic potency is superior to that of clinically standard analgesics, including indomethacin, morphine, gabapentin, and dexamethasone (42) in the rat model. These findings suggest that SPMs may offer improved therapeutic efficacy compared to traditional analgesics, with the added advantage of reduced off-target effects. However, the clinical translation of SPMs is challenged by their metabolic instability, as they can be rapidly inactivated and degraded in vivo. Consequently, developing more stable SPM analogs or advanced delivery systems is crucial to realizing their therapeutic potential (26, 43). Studies have demonstrated that the metabolically stable RvE1 analog, 19-(p-fluorophenoxy)-RvE1 (19-pf-RvE1), produces potent and sustained antinociceptive effects by resisting rapid inactivation, a limitation associated with native RvE1 (36). This review synthesizes current evidence on the anti-nociceptive effects of SPMs and examines their mechanisms of action, aiming to inform future translational research and therapeutic development.
2 Anti-nociceptive effects of SPMs
2.1 Neuropathic pain
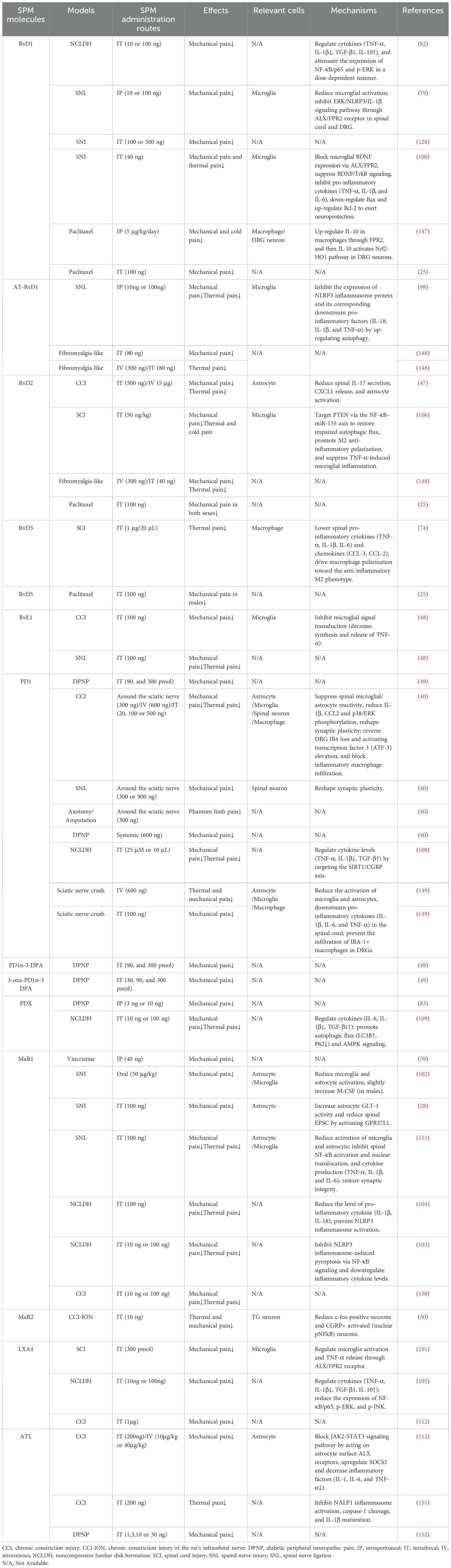
Neuropathic pain arises from lesions or diseases affecting the somatosensory nervous system (44), and is clinically characterized by spontaneous pain, hyperalgesia (heightened pain sensitivity to noxious stimuli), and paresthesia (abnormal sensations such as tingling or numbness). These symptoms often manifest as heterogeneous sensory abnormalities, complicating both diagnosis and management. Current treatment options for neuropathic pain—including antidepressants, anticonvulsants, and opioids—are limited by variable efficacy, adverse side effects, and high healthcare costs. While these pharmacological interventions may transiently alleviate symptoms, they often fail to modify the underlying pathophysiology driving pain chronification (45). This gap underscores the urgent need for therapies that target disease mechanisms rather than merely suppressing symptoms. Case series studies suggest that omega-3 fatty acids may benefit the management of patients with neuropathic pain (46).
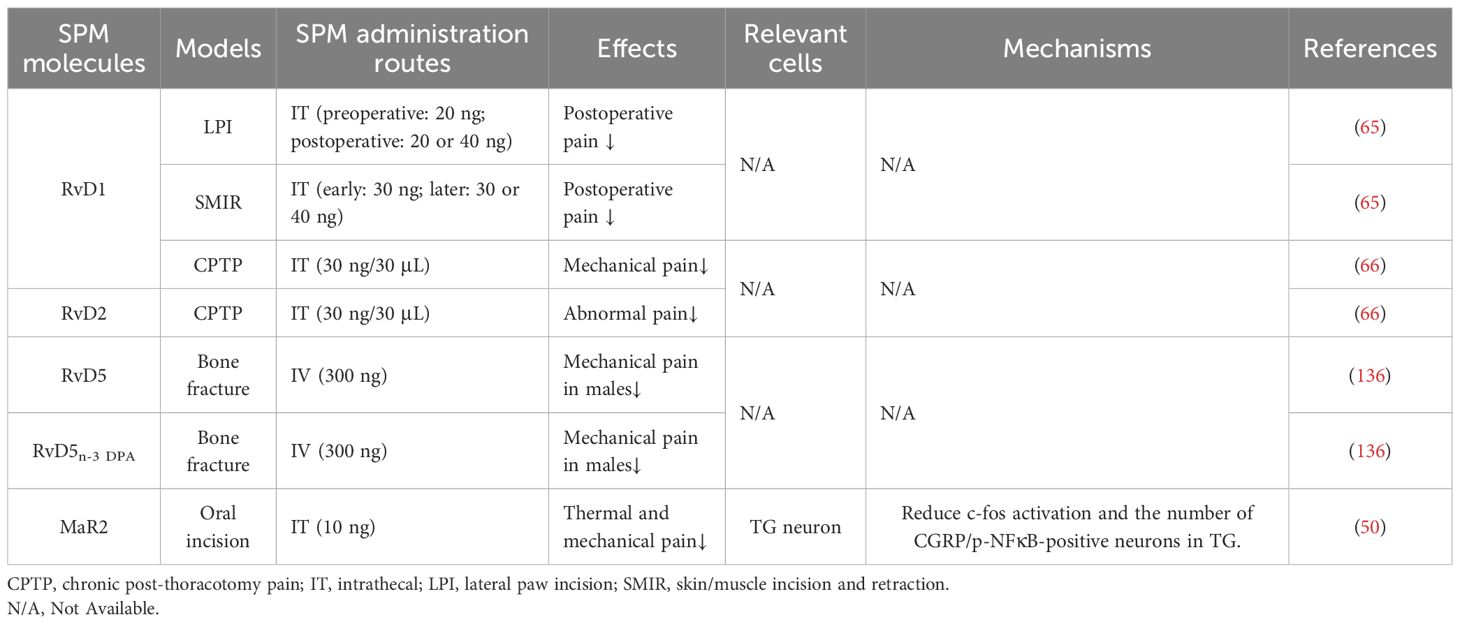
Preclinical studies highlight the importance of timing in SPM administration for sustained anti-nociceptive efficacy. Timing-dependent effects are also observed in the CCI model: early post-surgical administration of RvD2 for 3 days induced robust analgesia lasting ≥4 days, whereas delayed RvD2 treatment yielded only transient relief (47). In the spinal nerve ligation model, intrathecal RvE1 administered 3 weeks post-injury rapidly reduced mechanical hypersensitivity within 1 hour, though effects waned by 3 hours (48). Notably, prophylactic PD1 administered during surgery completely prevented CCI-induced mechanical hyperalgesia for 4 weeks. When delivered at 3, 24, and 48 hours post-surgery, PD1 blocked neuropathic pain development, while injections initiated 2 weeks post-surgery alleviated established neuropathic pain for >3 hours (40). PD1 alleviates mechanical and thermal hyperalgesia in the CCI model. In contrast, its precursor DHA fails to produce significant analgesia, even at high doses, highlighting the necessity of SPM biosynthesis for therapeutic efficacy in neuropathic pain (40). In diabetic neuropathic pain models, 3-oxa-PD1n-3 DPA exerts remarkable anti-nociceptive effects within just one hour. Moreover, it has a lower effective dose than PD1 and PD1n-3 DPA. However, at a high dose of 300 pmol (i.t.), its anti-nociceptive effect duration is shorter than that of PD1n-3 DPA (49). In a rat postoperative pain model, daily intrathecal MaR2 (10 ng) administered on days 10, 12, 14, and 16 post-surgery reversed mechanical allodynia, with effects persisting until day 18 before diminishing by day 20 (50). Similarly, in mice, the same MaR2 dosing regimen (days 3, 5, 7, and 9 post-surgery) produced analgesia lasting until day 11, followed by a decline on days 12–13 (50). These findings underscore that SPMs exert time-sensitive therapeutic effects, with early intervention favoring prolonged efficacy (See Table 2).
2.2 Inflammatory pain
Inflammatory pain arises as a consequence of immune system activation, a protective physiological response to tissue injury or infection. However, this response can paradoxically amplify pain through the release of pro-inflammatory mediators such as cytokines, chemokines, and prostaglandins (51). These mediators act directly on nociceptors (pain-sensing neurons), lowering their activation thresholds and increasing spontaneous firing rates. This process drives peripheral sensitization—heightened sensitivity at the injury site—and central sensitization, a maladaptive plasticity in the spinal cord and brain that sustains pain even after tissue healing (52). Together, these mechanisms transform acute protective inflammation into chronic, pathological pain states.
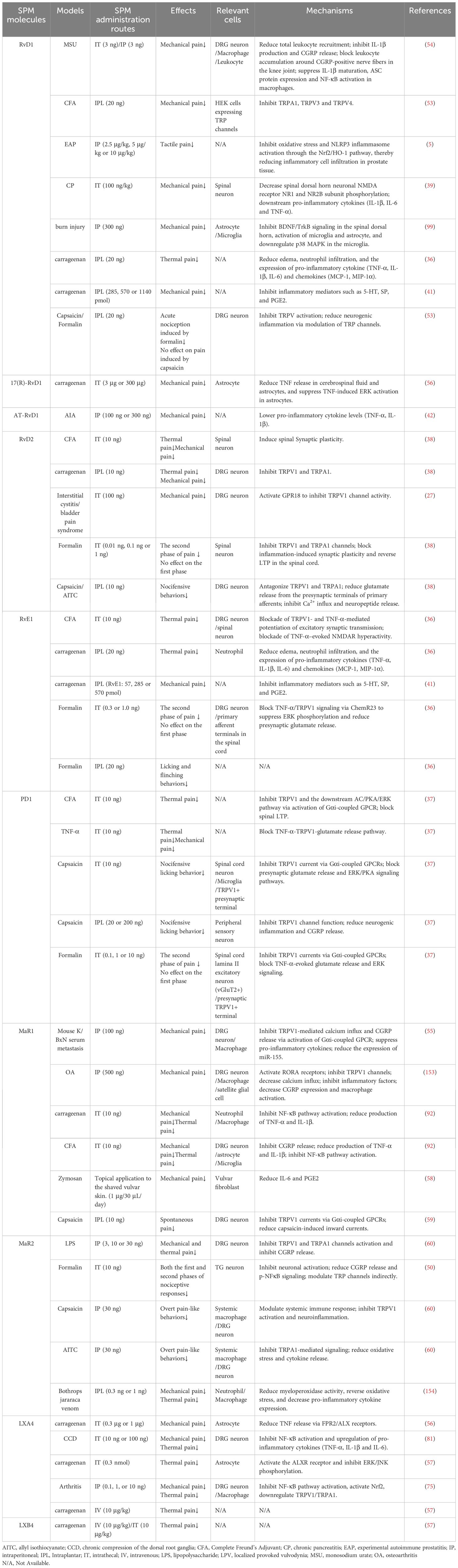
Multiple lines of evidence demonstrate that SPMs attenuate CFA-induced nociception (Table 3). RvD1 reduced mechanical hypersensitivity with efficacy similar to AP18, a transient receptor potential ankyrin 1 (TRPA1) channel antagonist (53). Repeated AT-RvD1 administration (twice daily for 4 days) further suppressed the development of CFA-induced mechanical hyperalgesia, suggesting cumulative benefits with prolonged treatment (42). RvD2, administered 3 days post-CFA, diminished thermal hyperalgesia and mechanical allodynia for >3 hours (38). RvE1, delivered on day 3 post-CFA, attenuated thermal hyperalgesia within 15 minutes, though effects were transient (36). In CFA-induced thermal hyperalgesia, RvE1 achieves comparable pain relief at doses 10,000-fold lower than those required for DHA and EPA, underscoring the superior potency of SPMs in inflammatory settings (36). Both RvD1 and RvE1 can alleviate nociception induced by CFA, but RvE1 is twice as potent as RvD1 in reducing paw withdrawal responses to mechanical stimuli at 3 h and 2 h post-treatment (41). 10 ng of PD1 induced a rapid reduction in thermal hyperalgesia within 20 minutes, lasting for 2 hours, while 1 ng of PD1 sustained its anti-nociceptive effect for 40 minutes (37).
SPMs also alleviate nociception resulting from arthritis (Table 3). In mice with gouty arthritis, both intrathecal and intraperitoneal pretreatment with RvD1 (administered 72 hours before disease induction) alleviated mechanical hyperalgesia, with the 72-hour pretreatment window showing maximal efficacy. Dose-response studies revealed comparable effects at 3 ng and 30 ng RvD1, both superior to the 0.3 ng dose (54). A single dose of AT-RvD1 administered on day 3 post-induction alleviated mechanical hyperalgesia for 6 hours (42). Notably, MaR1 demonstrated delayed yet sustained efficacy: when administered every other day during peak joint inflammation (days 5–11 post-CFA), the first dose lacked acute anti-nociceptive effects. However, subsequent doses progressively reversed mechanical hypersensitivity, with analgesia persisting until day 25—well beyond the resolution of joint swelling and despite ongoing hyperalgesia (55).
SPMs play a role in carrageenan-induced nociception (Table 3). Both RvD1 and RvE1 were effective in preventing carrageenan-induced nociception; however, their combined use showed no additive or synergistic effects compared to monotherapy (41). Intrathecal pretreatment with LXA4 or 17(R)-RvD1 reduced the hyperalgesia index, with effects observed within the first 6 hours post-administration (56). Similar to LXA4, intravenous or intrathecal injection of ATLa (a more stable ATL analog) exerts comparable efficacy in alleviating carrageenan-induced hyperalgesia. However, 8,9-aLXB4 [(8,9)-acetylenic LXB4, an LXB4 analog] requires a higher dose to achieve anti-nociceptive effects similar to those of LXB4 (57).
Additionally, SPMs have been shown to exert effects in other models of nociception. RvD1 also exhibited prolonged efficacy in a trinitrobenzene sulfonic acid-induced visceral pain model, alleviating mechanical allodynia in a dose-dependent manner. Anti-nociceptive effects emerged 2 hours post-administration, peaked at 4 hours, and persisted for ≥12 hours before resolving by 24 hours (39). Both MaR1 and its precursor DHA markedly accelerate pain recovery in a mouse model of vulvodynia (58). RvD1, RvD2, PD1, MaR1, and MaR2 all inhibit capsaicin-evoked pain (37, 38, 53, 59, 60); RvD1, RvD2, RvE1, PD1, and MaR2 reduce formalin-evoked pain (36–38, 50, 53); and RvD2 and MaR2 attenuate AITC-evoked pain (38, 60).
2.3 Cancer pain
Pain is a prevalent and debilitating symptom among cancer patients, with approximately 40% experiencing inadequate pain relief despite current therapeutic regimens (61). Opioids remain the cornerstone of cancer pain management due to their potent anti-nociceptive efficacy. However, their use is frequently limited by adverse effects—including sedation, constipation, respiratory depression, and risk of dependence—that compromise both quality of life and, in some cases, overall survival (61). These challenges highlight the urgent need for novel analgesics that target pain mechanisms with greater specificity, thereby minimizing off-target effects while maintaining therapeutic efficacy.
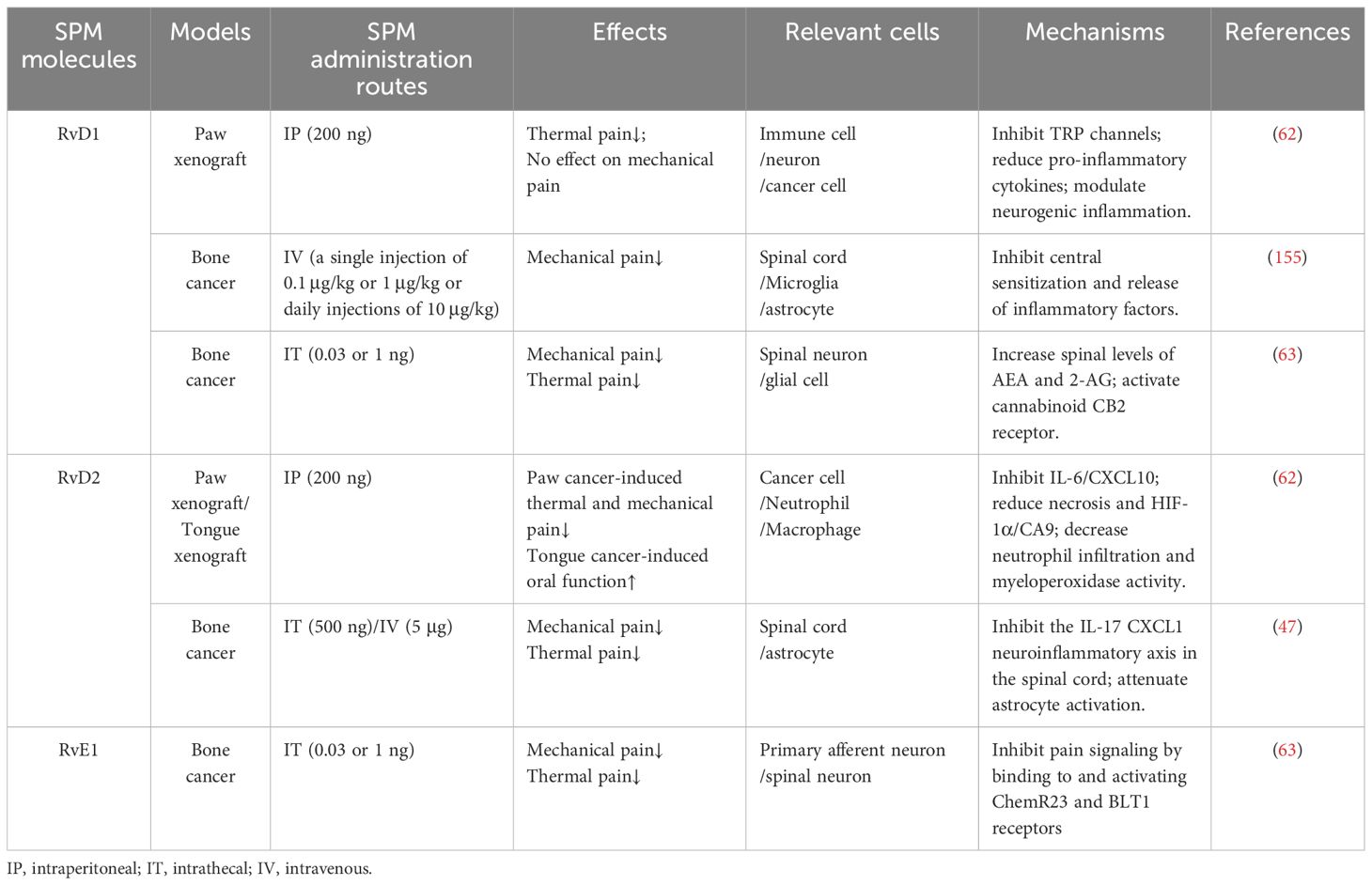
SPMs exhibit divergent efficacy profiles in cancer pain models depending on the compound and disease context (Table 4). In the claw tumor model, RvD1 alleviated thermal hyperalgesia but had no significant effect on mechanical hypersensitivity. In contrast, RvD2 treatment reduced both mechanical and thermal hyperalgesia by day 21 post-tumor induction, though this effect declined by day 28 (62). Similarly, in a tongue tumor model, RvD1 failed to attenuate pain sensitivity, while RvD2 provided moderate analgesia; however, its effects were transient and lacked long-term sustainability (29). Notably, intrathecal RvD2 administration in a sarcoma model (NCTC cells injected into the distal femoral condyle) suppressed early-stage mechanical allodynia and thermal hyperalgesia. However, this anti-nociceptive effect was short-lived during later disease stages, suggesting time-dependent limitations in monotherapy (47). Additionally, while both RvD1 and RvE1 resolve bone cancer-induced mechanical pain comparably, RvD1 shows superior efficacy in alleviating thermal hyperalgesia at higher doses (63).
2.4 Postoperative pain
Postoperative pain is a clinically significant challenge affecting the majority of patients following surgical procedures. Its intensity and duration are shaped by multifactorial determinants, including surgical invasiveness, patient-specific variables (e.g., age, sex, psychological state, comorbidities), and the adequacy of perioperative anti-nociceptive strategies (64). Despite advances in pain management, over 80% of patients report postoperative pain, with many experiencing inadequate relief due to suboptimal prevention or treatment protocols (64). This high prevalence underscores the urgent need for mechanism-driven, personalized approaches to improve both preoperative risk mitigation and postoperative pain control.
The action of SPMS in postoperative pain is summarized in Table 5. In the lateral paw incision model, prophylactic administration of RvD1 (20 ng, 30 minutes pre-surgery) produced sustained analgesia lasting 10 days postoperatively. Postoperative administration (20 ng or 40 ng on day 1) rapidly normalized pain thresholds to presurgical levels within 1 hour (65). In the skin/muscle incision and retraction model, early RvD1 treatment (day 2 post-surgery) prevented the development of skin/muscle incision and retraction-associated pain; delayed administration (day 9 or 17 post-surgery) provided only short-term relief, highlighting the critical window for therapeutic intervention (65). Similarly, in a thoracotomy model, RvD1 administered preoperatively or on postoperative day 4 attenuated pathological pain. Treatment initiated on or after day 14 showed no efficacy, further underscoring the importance of timing in SPM-based analgesia (66). In a postoperative pain mouse model induced by femoral fracture, MaR1 and RvD5 proved to be more effective than RvD1 and PD1 in providing pain relief, suggesting context-specific advantages (67). While DHA may transiently reduce perioperative pain through partial conversion to SPMs, its effects are markedly less potent than those of preformed SPMs, underscoring the limitations of relying on endogenous biosynthesis for pain therapy (67).
3 Mechanism of action of SPMs
3.1 Peripheral mechanism
Peripheral immune and glial cells contribute to pain signaling through neuro-immune crosstalk and inflammatory reactions, which play a role in pain sensitization and chronic pain (68–71). Research suggests that in the peripheral nervous system (PNS), SPMs show activity in the dorsal root ganglion (DRG) and trigeminal ganglion (TG). Current evidence indicates these effects may involve modulation of inflammatory cytokine signaling, TRP channels, G protein-coupled receptor (GPCR) activity, macrophage function, calcitonin gene-related peptide (CGRP) signaling, COX activity, and oxidative stress pathways. The mechanisms in DRG and TG are shown in Figures 1 and 2, respectively.
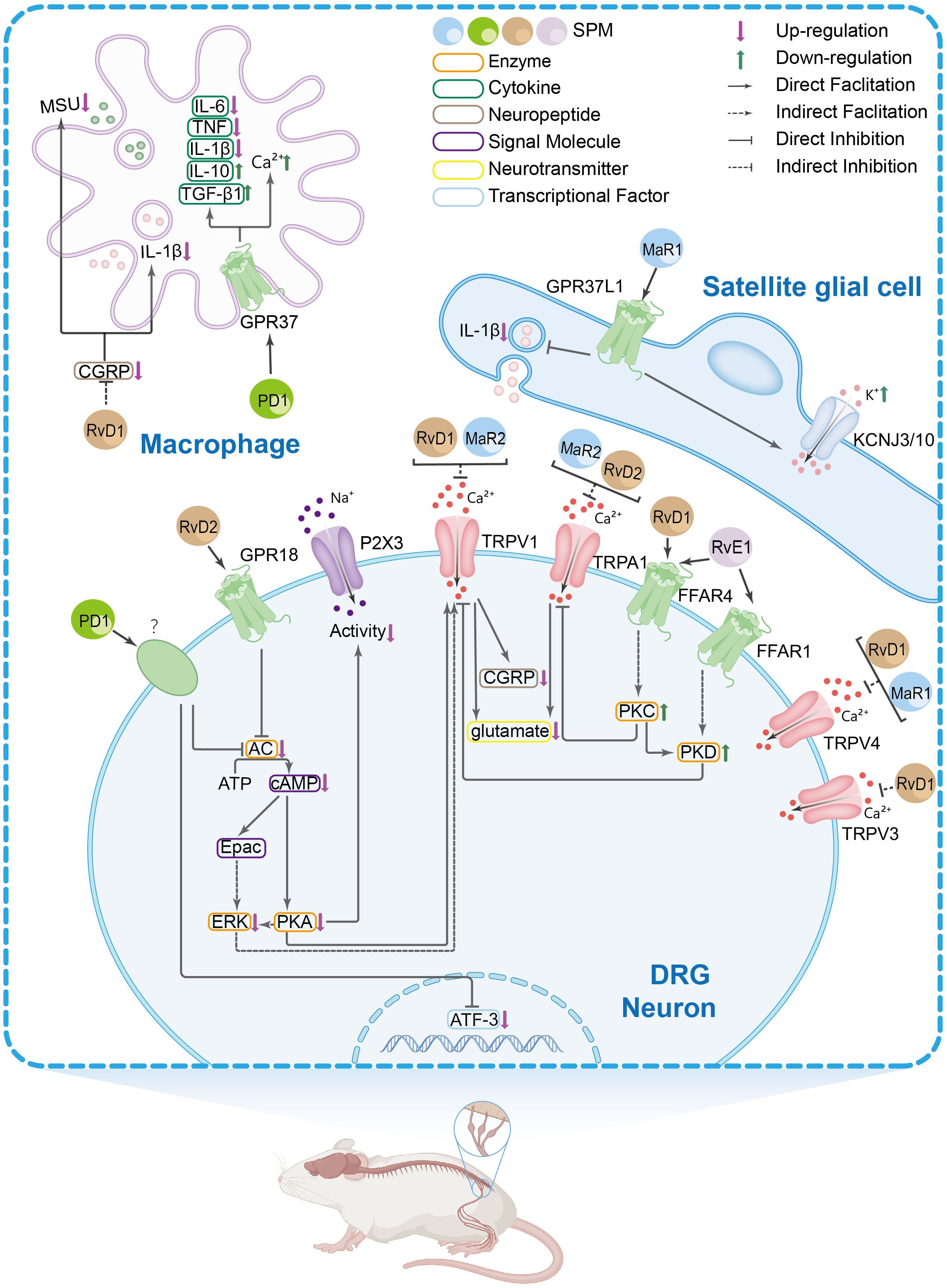
Figure 1. The main peripheral mechanisms of SPM’s anti-nociceptive effect in the dorsal root ganglia (DRGs) and macrophages. Mechanism of action of SPM at the DRG and macrophages level: 1. In DRG neurons: SPMs suppress transient receptor potential vanilloid subtype 1 (TRPV1) and TRPA1 activity, thereby reducing spinal dorsal horn release of CGRP and glutamate. This inhibition attenuates CGRP-induced interleukin-1β (IL-1β) release and monosodium urate (MSU) in macrophages. Evidence also suggests that SPMs may block TRPV1 signaling by suppressing AC-PKA-extracellular signal-regulated kinase (ERK) pathway activity and inhibiting the FFAR/protein kinase C (PKC) signaling axis and its effector PKD. The inhibition of TRPA1 by SPM is, rather, exerted by suppressing the free fatty acid receptor 4 (FFAR4)-PKC pathway. SPM targets GPR18 to inhibit the AC/cyclic adenosine monophosphate (cAMP)/PKA signaling pathway, thus inhibiting the P2X3 receptor. 2. In satellite glial cells: SPM acts on GPR37L1 to suppress IL-1β and promote KCNJ10-mediated potassium influx. 3. In macrophages: In the knee joint, RvD1 reduces macrophage phagocytosis of MSU crystals and the maturation and release of IL-1β. In natural macrophages, SPMs activate GPR37, elevating intracellular calcium concentrations to enhance phagocytic capacity. Concurrently, they inhibit the release of pro-inflammatory cytokines [e.g., IL-6, IL-1β, tumor necrosis factor alpha (TNF-α)] from peritoneal macrophages while promoting anti-inflammatory mediators such as IL-10 and transforming growth factor-β 1(TGF-β1).
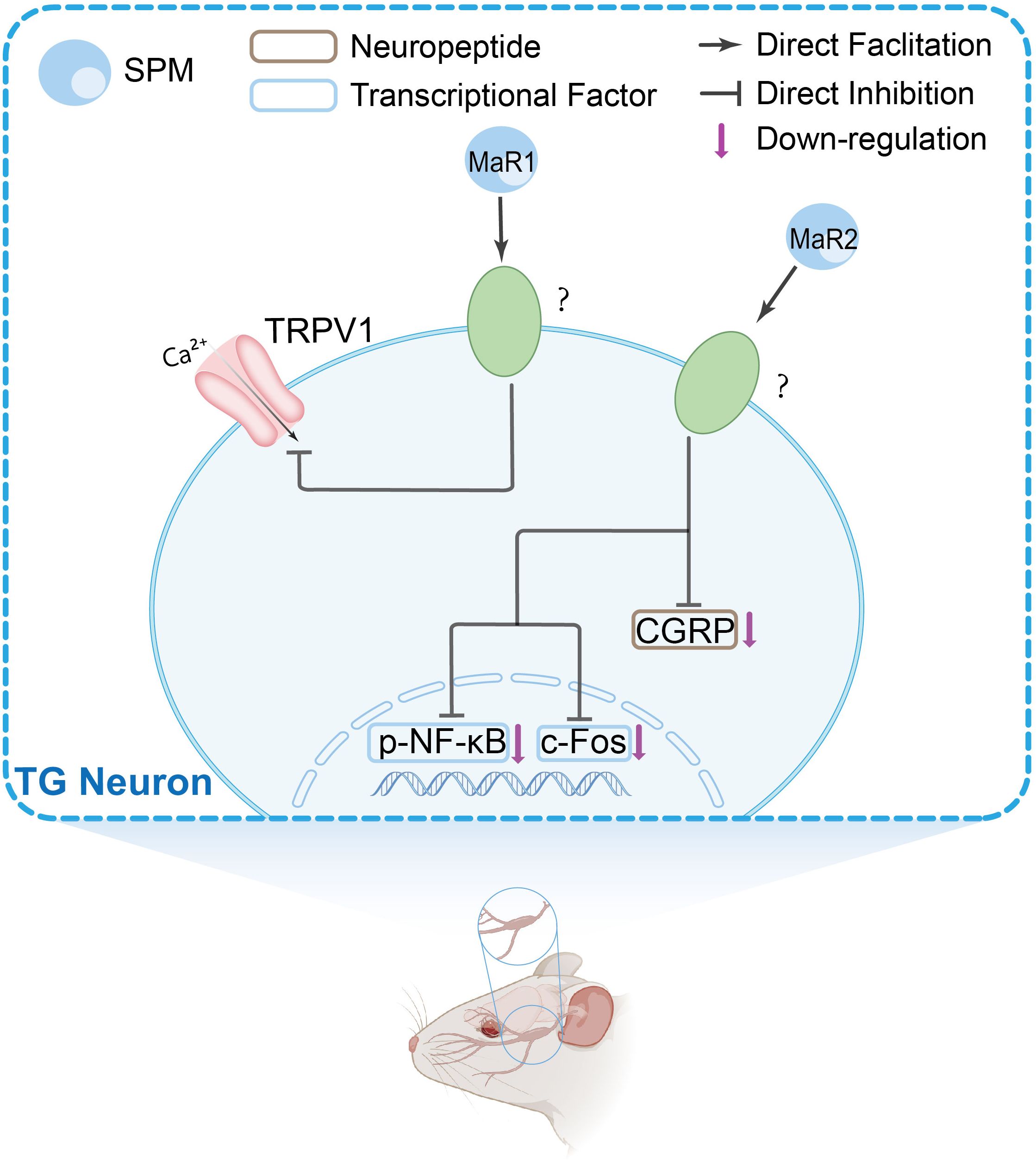
Figure 2. The main peripheral mechanisms of SPM’s anti-nociceptive effect in the trigeminal ganglia (TGs). Mechanism of action of SPMs in TG: SPMs inhibit TRPV1 channel activity, reduce CGRP levels, and decrease phosphorylated nuclear factor-κB (NF-κB) and c-fos expression, suggesting a role in modulating trigeminal nociceptive signaling.
3.1.1 Cell-specific effects in PNS
3.1.1.1 Neuron-immune crosstalk in PNS
SPMs regulate pain by modulating immune cell function, particularly macrophage polarization (Figure 1). The classification of macrophage phenotypes traditionally follows the M1-M2 dichotomy (72), wherein M1 macrophages are pro-inflammatory, and M2 macrophages play anti-inflammatory roles and assist in tissue repair (73). At seven days post-spinal cord injury (SCI), cluster of differentiation 68 (CD68) (a marker for M1 macrophages) was up-regulated, while NT-3 (a marker associated with the M2 phenotype) was notably down-regulated. Intrathecal administration of RvD3 completely reversed this pattern, demonstrating that RvD3 promotes macrophage polarization toward the M2 phenotype (74). By favoring M2 polarization, these SPMs reduce pro-inflammatory cytokine release (e.g., TNF-α, IL-6) while amplifying anti-inflammatory mediators (e.g., IL-10, TGF-β). This rebalancing of macrophage activity not only mitigates inflammatory pain but also addresses the underlying tissue pathology driving nociceptive sensitization. LXA4 therapy reduces titanium dioxide-induced recruitment of total white blood cells, monocytes, and polymorphonuclear cells. In TiO2-driven inflammation, CD45+F4/80+ macrophages—the dominant monocyte-derived population—are the primary cellular target of LXA4. Specifically, LXA4 suppresses their recruitment and inhibits NF-κB activation within this macrophage subset, demonstrating a dual anti-inflammatory mechanism focused on this key cell type (75).
GPR37 exerts anti-inflammatory and anti-nociceptive effects primarily through its activity in macrophages. Upon activation by PD1, GPR37 signaling induces intracellular Ca2+ influx in macrophages, enhancing their phagocytic capacity. PD1 also promotes M2 macrophage differentiation via GPR37 activation, reducing pro-inflammatory cytokines (IL-1β, TNF, IL-6) and increasing anti-inflammatory ones (IL-10, TGF-β1). Preclinical studies indicate that PD1-GPR37 signaling augments macrophage-mediated pathogen clearance, which may contribute to its therapeutic potential in bacterial infections, sepsis, and malaria-associated complications. These mechanisms also correlate with alleviation of infection-related acute and chronic pain (29).
In the formalin-induced inflammatory pain model, RvE1’s anti-nociceptive effect is mediated by Gi-coupled GPCR ChemR23, which is expressed in DRG and spinal neurons (36). RvE1 directly activates ChemR23, and knockdown of ChemR23 abolishes RvE1’s anti-nociceptive action, linking ChemR23 to RvE1’s anti-inflammatory and anti-nociceptive effects (36). In human peripheral blood monocytes, RvE1 binds ChemR23 to activate the ERK/MAPK signaling pathway. In dendritic cells, RvE1 blocks IL-12 production (76).
3.1.1.2 Neuron-glial crosstalk in PNS
In the peripheral nervous system, SPMs also exert their resolution action via glial cells. Through the MaR1/GPR37L1 signaling axis, PDX enhances surface expression and functional activity of KCNJ10 (Kir4.1) in satellite glial cells. Additionally, PDX inhibits PTX-induced interleukin-1β release in satellite glial cells-neuron co-cultures, suggesting dual mechanisms of action: potentiating Kir4.1-mediated homeostasis and suppressing neuroinflammatory signaling (77).
3.1.2 Molecular mechanisms in PNS
3.1.2.1 Inflammasome-, cytokine/chemokine- and Prostaglandin regulation in PNS
The NLR family pyrin domain containing 3 (NLRP3) inflammasome, a key driver of chronic pain pathogenesis, represents a promising therapeutic target for resolving persistent inflammation (78). SPMs modulate NLRP3 activity through distinct mechanisms. RvD1 inhibits NLRP3 inflammasome activation in mouse prostate tissue and alleviates pelvic pain and inflammation in preclinical models (5). In the spinal nerve ligation model, RvD1 alleviates mechanical allodynia by upregulating ALX/FPR2 receptor expression and concurrently inhibiting NLRP3 inflammasome assembly (79). RvD1 inhibits the phosphorylation of the ERK signaling pathway, consequently reducing the expression of NLRP3 inflammasome components and downstream cytokines (IL-1β and IL-18) mediated by phosphorylated ERK. This indicates anti-nociceptive action through the ERK/NLRP3/IL-1β inflammatory pathway (79). RvD2 promotes NLRP3 degradation via autophagy-dependent pathways, as evidenced by the reversal of its anti-IL-1β effects with autophagy inhibitors (bafilomycin A, 3-MA) but not proteasome inhibitors (MG-132) (80).
Notably, 17(R)-hydroxy-docosahexaenoic acid diminishes phosphorylation of the p65 subunit of NF-κB in the DRG three days post-adjuvant-induced arthritis induction (42). In the CCI-ION model, MaR2 reduces the number of TG neurons exhibiting phosphorylated NF-κB (50). Both RvD1 and LXA4 treatment inhibit the expression of NF-κB/p65 in the DRG (81, 82) (Figure 2).
SPMs modulate the production of inflammatory and anti-inflammatory cytokines/chemokines in pain states. RvD1 suppresses pro-inflammatory cytokines, including TNF-α, IL-18, and IL-1β, while enhancing anti-inflammatory mediators such as IL-10 and TGF-β1 (79, 82). AT-RvD1 reduces TNF-α and IL-1β levels in the ipsilateral hind paw, with greater efficacy in attenuating TNF-α (42). RvD2 alleviates cancer-induced hyperalgesia by suppressing IL-6 production in tumor and immune cells (62). LXA4 inhibits the overexpression of TNF-α, IL-1β, and IL-6 in the DRG and knee joint washes, and it can also enhance the production of IL-10 in knee joint washes (75, 81). MaR1 suppresses pro-inflammatory mediators, including TNF-α, inducible nitric oxide synthase (NOS2), and microRNA 155 (miR-155) (55). MaR2 downregulates pro-inflammatory cytokines (IL-1β, TNF-α, IL-6), chemokines [C-C motif chemokine ligand 2 (CCL2), CCL3, CCL17], and vascular endothelial growth factor by inhibiting neutrophil and monocyte infiltration into inflamed tissues, thereby disrupting immune-mediated hyperalgesia (60).
COX-2 is a key mediator of inflammatory pain, driving prostaglandin synthesis that sensitizes nociceptors and amplifies spinal nociceptive transmission (42). 17(R)-HDoHE, a docosahexaenoic acid-derived SPM, suppresses COX-2 overexpression in DRG neurons (42).
3.1.2.2 Oxidative stress in PNS
RvD1 activates the nuclear factor erythroid 2-related factor 2/heme oxygenase-1 signaling axis, a critical regulator of cellular redox balance. By enhancing HO-1 expression, RvD1 reduces oxidative stress via scavenging of reactive oxygen species (ROS) while suppressing ROS-induced nociceptor sensitization and neuronal hyperexcitability (5). PDX treatment reduced ROS in serum (83). LXA4 restores antioxidant capacity through three interconnected mechanisms: it directly scavenges free radicals, including the model radical 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonate); upregulates the critical endogenous antioxidant glutathione by enhancing nuclear factor erythroid 2-related factor 2 (Nrf2) mRNA expression to drive antioxidant gene transcription; and reduces ROS levels, thereby mitigating oxidative stress and reestablishing redox homeostasis (75).
3.1.2.3 Pain-related ion channels and neuropeptides in PNS
SPMs can inhibit the activation and upregulated expression of nociceptive TRP and purinergic P2X channels, which are key drivers of chronic pain (84, 85). Their modulation of ion channels and associated neuropeptides represents a significant mechanism for resolving pain.
Among resolvins, RvD1 exerts broad analgesic effects by inhibiting TRPA1, TRPV3, and TRPV4 at nanomolar to micromolar concentrations. Its peripheral administration attenuates agonist-evoked acute pain, while pretreatment reverses inflammatory mechanical and thermal hypersensitivity (53). RvD1 appears to inhibit TRPA1 channel activity via the FFAR4-PKC signaling axis (86). A metabolically stable RvD1 analog, 17R-RvD1, selectively inhibits TRPV3 channels, highlighting its potential for pain related to TRPV3 hyperactivity (87). In contrast, RvD2 indirectly suppresses TRPV1 and TRPA1 currents (38). Similarly, RvD3’s inhibition of TRPV1 in DRG neurons is mediated through the ALX/FPR2 receptor (21). RvE1 exhibits a multimodal action, alleviating both TRPV1-dependent and independent pain. Its effects are dose-dependent (at low concentrations it suppresses TRPV1 activity, while at high concentrations it further modulates TRPA1), and involve multiple receptors (FFAR1/FFAR4) and pathways (PKD/PKC) (36, 86). In cultured small-diameter DRG neurons, RvE1 also inhibits substance P-mediated TRPV1 activity (88). Beyond resolvins, other SPMs also inhibit TRP channels. PD1 and MaR1 specifically inhibit TRPV1 via Gαi-coupled GPCRs, without affecting TRPA1, highlighting their selectivity (37, 55, 59, 89). MaR1 also rapidly terminates TRPV4 signaling, broadening its antinociceptive scope (90). In contrast, MaR2 and LXA4 exhibit a broader inhibitory profile, suppressing both TRPV1 and TRPA1 (60). In addition, RvD2 also mediates analgesia by activating GPR18, which couples to pertussis toxin-sensitive Gαi/o proteins. RvD2/GPR18 signaling cascade further inhibits ionotropic P2X3 receptor activity by suppressing the cAMP/PKA pathway (91).
SPMs also alleviate pain by modulating key neuropeptides like CGRP. MaR1 suppresses neutrophil/macrophage recruitment near CGRP+ fibers in the hind paw skin and reduces CGRP release from DRG neurons (92). MaR2 concentration-dependently reduces CGRP release evoked by TRPV1/TRPA1 agonists from sensory neurons, and attenuates TRPV1/TRPA1-mediated nociception (60). Similarly, MaR2 reverses activation of CGRP+ neurons in TG in a model of trigeminal neuropathic pain (CCI-ION) (50).
3.2 Central mechanism
The spinal cord serves as a critical hub for nociceptive signal transmission and processing. Current research suggests that SPMs exert antinociceptive effects in the central nervous system through several potential mechanisms, including: modulation of spinal synaptic plasticity, regulation of inflammatory cytokine signaling, interaction with glial cells, influence on GPCR-mediated pathways, alteration of CGRP signaling, effects on autophagy processes, and modulation of COX and oxidative stress pathways. These proposed mechanisms are summarized in Figure 3, Figure 4, and Figure 5.
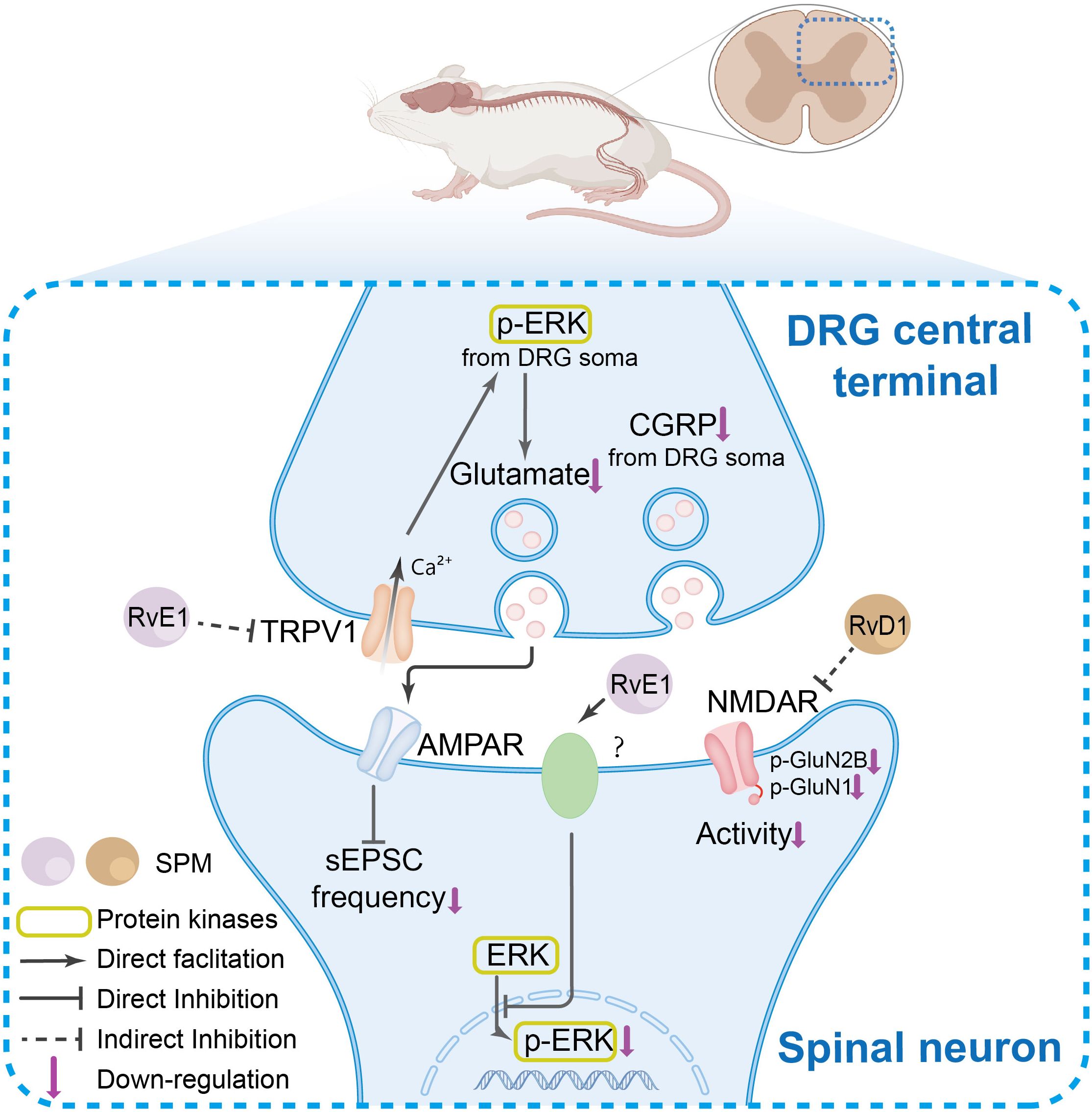
Figure 3. The SPM’s anti-nociceptive mechanisms in spinal neurons and synaptic transmission. In the DRG central terminals, under pain conditions, TRPV1 channel activation and ERK phosphorylation, which collectively enhance spinal synaptic plasticity and central sensitization. SPMs suppress TRPV1 activation, thereby inhibiting ERK phosphorylation in DRG central terminals. This reduction in phosphorylated ERK diminishes its facilitatory effect on glutamate release from presynaptic terminals in the spinal cord, ultimately attenuating α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)-mediated synaptic plasticity in spinal neurons. Furthermore, SPMs inhibit N-methyl-D-aspartate receptor (NMDAR) hyperactivity and ERK phosphorylation, modulating nociceptive signal transmission.
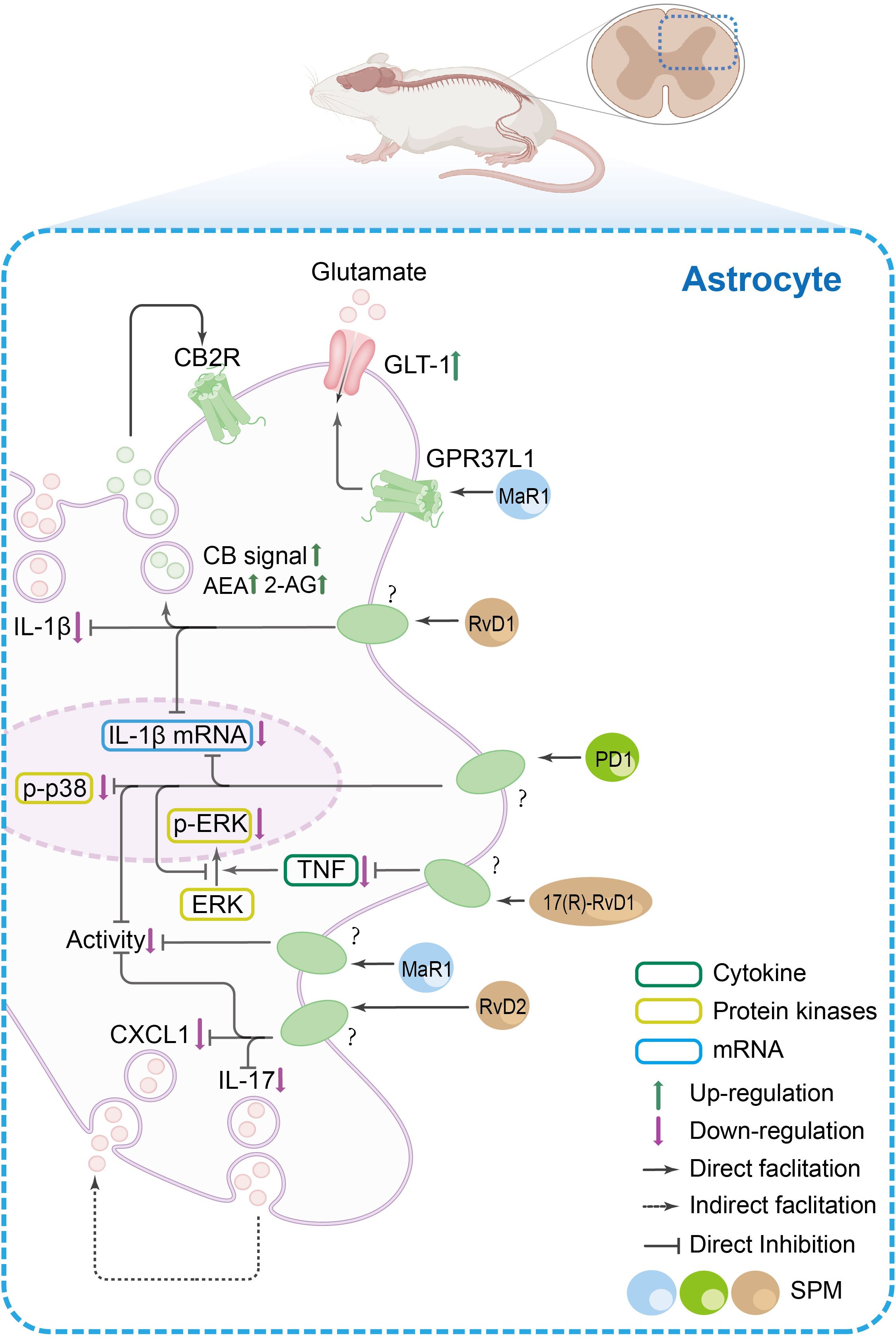
Figure 4. The SPM’s anti-nociceptive mechanisms in spinal astrocytes. In spinal astrocytes, inflammatory pain states are characterized by increased release of pro-inflammatory cytokines (e.g., IL-1β, IL-17), chemokines [e.g., C-X-C motif chemokine ligand 1(CXCL1)], and glutamate, alongside reduced endocannabinoid levels and elevated p38 mitogen-activated protein kinase/ERK phosphorylation. SPMs exert dual regulatory effects: (1) they promote the release of anti-inflammatory mediators such as endocannabinoids, which act on cannabinoid 2 receptor (CB2R) in glial cells to amplify anti-nociceptive signaling, and (2) they suppress pro-inflammatory mechanisms by inhibiting IL-1β production, p38/ERK phosphorylation, and CXCL1/IL-17 release. Additionally, SPMs enhance glutamate clearance via GPR37L1-mediated activation of glutamate transporter 1 (GLT-1).
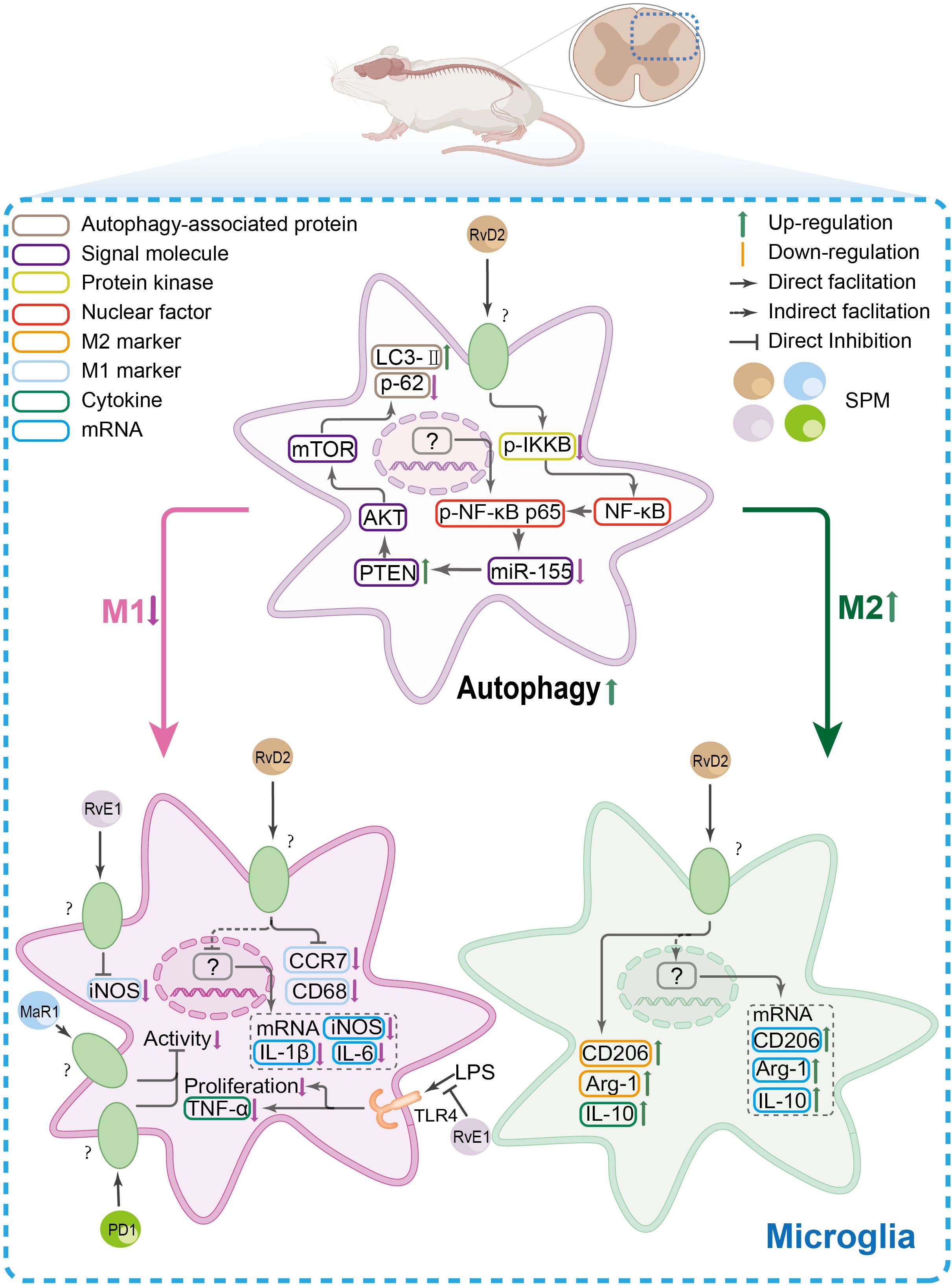
Figure 5. The SPM’s anti-nociceptive mechanisms in spinal microglia. In spinal microglia, inflammatory conditions shift their polarization toward a pro-inflammatory M1 phenotype [marked by elevated CC-chemokine receptor 7(CCR7), CD68, and inducible nitric oxide synthase (iNOS)] while suppressing the anti-inflammatory M2 phenotype [CD206, arginase-1(Arg-1), and IL-10]. Concomitantly, autophagy is downregulated. SPMs reverse this imbalance by promoting M2 polarization, suppressing TNF-α-driven microglial inflammation, and restoring autophagy via the NF-κB/miR-155 axis, which targets the phosphatase and tensin homolog (PTEN)/protein kinase B/mammalian target of rapamycin (mTOR) pathway.
3.2.1 Cell-specific effects in the spinal cord
3.2.1.1 Neuron-glial crosstalk in the spinal cord
Spinal astrocytes and microglia are central regulators of neuroinflammation and pain pathogenesis (93–95). In astrocytes, which contribute to pain maintenance through metabolic coupling with neurons (96), RvD2 exerts its effect by inhibiting interleukin-17 secretion and the subsequent release of the chemokine CXCL1, thereby attenuating astrocyte-mediated neuroinflammation (47). In microglia, key amplifiers of nociception via synaptic remodeling and cytokine production (97), various SPMs demonstrate inhibitory effects. AT-RvD1 reduces SNL-induced expression of the microglial marker Iba1 (98). In primary microglial cultures, RvE1 alleviates neuropathic pain by inhibiting lipopolysaccharide (LPS)-induced proliferation and the synthesis/release of TNF-α (48). RvD1 suppresses microglial activation and reduces phosphorylated p38 levels; it further exerts anti-inflammatory effects by modulating the brain-derived neurotrophic factor (BDNF)/tropomyosin-related kinase B (TrkB) signaling pathway, which participates in a positive feedback loop with p38 to amplify pro-inflammatory responses (99, 100). Lipoxin A4 (LXA4), while not affecting spinal cord injury (SCI)-induced astrocytosis, specifically reduces microglial reactivity and suppresses interferon-gamma-induced TNF-α release (101).
Notably, several SPMs exhibit broad-spectrum inhibition across glial cell types. For instance, pretreatment with protectin D1 (PD1) inhibits the robust microglial and astrocytic activation triggered by nerve injury (40). Similarly, MaR1 reduces the activation of both cell types in the spared nerve injury (SNI) model, as indicated by decreased expression of Iba1 and glial fibrillary acidic protein (GFAP), an effect that is sex-independent (102).
3.2.2 Molecular mechanisms in the brain and spinal cord
3.2.2.1 Inflammasome-, cytokine/chemokine- and prostaglandin regulation in the spinal cord
Within the spinal cord, SPMs orchestrate the resolution of inflammation by targeting key signaling hubs, such as the NLRP3 inflammasome, the NF-κB and MAPK pathways, and the downstream cytokine and COX networks, in order to alleviate pain.
In the spinal cord, RvD1 inhibits the SNL-induced upregulation of the NLRP3 inflammasome complex and associated nociceptive behavior by suppressing the ERK/NLRP3/IL-1β signaling axis (79). Similarly, MaR1 inhibits NLRP3 inflammasome activation and pyroptosis in inflammatory pain models (103, 104). SPMs also target MAPK and NF-κB pathways, which regulate inflammasome assembly and pro-inflammatory gene transcription. LXA4 attenuates phosphorylated ERK expression in a dose-dependent manner (105), while PD1 inhibits the phosphorylation of both p38 and ERK. Concurrently, multiple SPMs, including MaR1, 17(R)-HDHA, RvD1, and LXA4, suppress the activation or phosphorylation of NF-κB in various pain models (38, 82, 92, 105). RvD2 further enhances autophagic flux through an NF-κB-regulated miR-155/PTEN axis, promoting a microglial phenotypic switch to the anti-inflammatory M2 state (106).
By targeting these signaling hubs, SPMs comprehensively reprogram the spinal cytokine synthesis and release. They effectively suppress a broad spectrum of pro-inflammatory mediators (e.g., IL-1β, IL-6, IL-18, TNF-α, CCL2) while promoting the production of anti-inflammatory cytokines (e.g., IL-10, TGF-β). RvD1, AT-RvD1, and RvD5 suppress IL-6, IL-18, TNF-α, and IL-1β, while enhancing IL-10 and TGF-β1 (39, 79, 82, 98, 107). RvD2 specifically inhibits IL-17 secretion (47). PD1/PDX downregulates TNF-α, IL-1β, and CCL2, while upregulating TGF-β. PDX reverses non-compressive lumbar disc herniation (NCLDH)-induced increases in IL-6/IL-1β and decreases in TGF-β mRNA (40, 108, 109). MaR1 inhibits the production of multiple chemokines and cytokines (e.g., CXC12, CXCL1, CCL3/4, IL-1β, IL-6, IL-18, TNF-α), likely via STAT and MAPK pathways (92, 103, 110, 111). LXA4 and its analog ATL inhibit TNF-α and IL-1β while upregulating TGF-β1 and IL-10, with ATL also suppressing IL-1β, IL-6, and TNF-α mRNA levels (105, 112).
Beyond cytokine networks, SPMs also target enzymes critical for prostaglandin synthesis. For instance, 17(R)-HDHA downregulates spinal COX-2 mRNA, thereby interrupting prostaglandin-driven hyperalgesia (42).
3.2.2.2 Pain-related channels and neuropeptides in the spinal cord
CB2 receptors are predominantly expressed on neuroglial cells. RvD1 has been shown to elevate spinal levels of the endocannabinoids anandamide (AEA) and 2-arachidonoylglycerol (2-AG). Given that the receptors for RvD1 are also primarily localized on spinal glial cells, these cells are likely the principal sites through which RvD1 induces the upregulation of AEA and 2-AG. Subsequently, these endocannabinoids activate CB2 receptors, leading to the inhibition of pro-inflammatory signaling and reduced sensitization of nociceptors (63).
SPMs modulate pain by targeting the interplay between CGRP and sirtuin 1 (SIRT1), key regulators of neuroinflammation and nociception. In the NCLDH model, PD1 downregulates CGRP (a potent pro-nociceptive neuropeptide) while upregulating SIRT1 (a deacetylase with anti-inflammatory and neuroprotective roles) in the NCLDH model (108). CGRP exacerbates pain by enhancing TNF-α/IL-1β (pro-inflammatory) and suppressing TGF-β (anti-inflammatory). Conversely, SIRT1 counteracts this by reducing TNF-α/IL-1β and elevating TGF-β (108). Additionally, intrathecal injection of EX-527 (a classic SIRT1 enzyme activity inhibitor) results in increased activation of CGRP, while administration of SIRT1720 (a specific agonist of SIRT1) reduces CGRP expression in the spinal cord (108). Furthermore, administration of CGRP and a CGRP antagonist influences SIRT1 release, with CGRP reducing and the antagonist promoting SIRT1 release (108). This reciprocal inhibition highlights a CGRP-SIRT1 axis central to NCLDH pain (108). These findings indicate an interaction between CGRP and SIRT1, suggesting that PD1 alleviates pain by restoring SIRT1-mediated suppression of CGRP signaling, rebalancing inflammatory and reparative pathways (108). These findings establish SPMs as master regulators of the CGRP-SIRT1 axis, providing dual therapeutic benefits: anti-inflammatory effects by suppressing pro-nociceptive cytokines (such as TNF-α and IL-1β), and pro-resolution effects by enhancing anti-inflammatory mediators (like TGF-β) and the neuroprotective SIRT1.
3.2.2.3 Oxidative stress in the brain
In a rat model of diabetic pain, PDX treatment reduced lipid peroxidation in pain-related brain regions, including the prefrontal cortex and hippocampus, with the hippocampus showing greater sensitivity to this effect. Furthermore, PDX potently suppressed ROS levels specifically in the hippocampus (83).
3.2.2.4 Spinal synaptic plasticity/neurotransmitter regulation
A hallmark of chronic pain is central sensitization, characterized by enhanced spinal synaptic plasticity [e.g., long-term potentiation (LTP)] and neuronal hyperexcitability (38, 113). SPMs counteract this process through regulation of key ion channels and receptors essential in synaptic plasticity and neurotransmitter (e.g., glutamate) signaling.
SPMs effectively normalize maladaptive spinal synaptic plasticity. RvD2 inhibits inflammation-induced spinal synaptic plasticity by suppressing TRPV1/TRPA1 signaling. A comparative study showed that RvD1, RvE1, and RvD2 all attenuate TRPV1/TRPA1-mediated plasticity in a dose-dependent manner, with efficacy following RvD1 > RvE1 > RvD2 (38). Particularly, PD1 not only reverses neuropathy-induced synaptic plasticity but also prevents the induction of spinal LTP. PD1 also reduces TNF-α- and TRPV1-driven increases in spontaneous excitatory postsynaptic current (sEPSC) frequency without affecting baseline transmission (37). Notably, PD1 is more effective than equimolar doses of RvE1 in blocking LTP development (37, 40).
Beyond synaptic plasticity, SPMs modulate critical postsynaptic receptors. The NMDA receptor, particularly those containing the NR2B subunit, is pivotal for central sensitization. RvD1 suppresses NMDA receptor signaling by inhibiting the phosphorylation of NR1 and NR2B subunits in spinal dorsal horn neurons, thereby reducing receptor excitability (39). This NR2B phosphorylation can propagate pain via spinal astrocytes by activating c-Jun N-terminal kinase (JNK) and driving pro-inflammatory cytokine production (114). RvE1 may modulate nociceptive processing presynaptically by inhibiting TRPV1/TNF-α signaling and postsynaptically by suppressing ERK-dependent NMDA receptor activation, contributing to attenuated central sensitization (36).
SPMs dampen the pain-evoked neuron hyperexcitability. AT-RvD1, acting through the ALX/FPR2 receptor, reduces the hyperactivity of spinal wide-dynamic-range (WDR) neurons evoked by peripheral inflammation. Crucially, it selectively dampens pathological pain transmission while preserving baseline nociceptive function (20).
To note, SPMs can simultaneously regulate synaptic transmission through presynaptic, postsynaptic, and astrocyte-mediated mechanisms. MaR1 reverses TRPV1-mediated presynaptic hyperactivity (reflected in sEPSC frequency) and normalizes postsynaptic receptor sensitization (reflected in sEPSC amplitude), offering a comprehensive strategy to restore synaptic homeostasis (89). MaR1 also activates astrocytic GPR37L1, promoting its association with the glutamate transporter GLT-1. This enhances glutamate reuptake, reduces synaptic glutamate levels, and mitigates neuropathic pain in rodent models (28).
3.2.2.5 Autophagy in the spinal cord
Autophagy, a crucial process for maintaining cellular homeostasis, plays a stage-dependent role in neuropathic pain. Its transient inhibition may initially delay neuroinflammation, but chronic impairment of autophagic flux sustains inflammatory responses and exacerbates pain (115). The AMP-activated protein kinase (AMPK) pathway serves as a key regulator linking cellular energy status to autophagic activity (116). AT-RvD1 promotes autophagy, as indicated by an increased LC3B-II/I ratio and accumulation of autophagy-related proteins, thereby attenuating NLRP3 inflammasome activation (98). In an NCLDH pain model, where pain is associated with suppressed AMPK signaling and impaired autophagy, the SPM analog PDX reverses this deficit. It restores phosphorylated AMPK/AMPK activity, normalizes autophagic flux, and ultimately alleviates neuropathic pain (109).
4 Summary and future directions
4.1 Therapeutic advantages of SPMs over conventional analgesics
SPMs offer a targeted and physiological approach to pain resolution by engaging natural resolution pathways, which distinguishes them from conventional analgesics. A key advantage is their ability to promote active inflammation resolution rather than merely suppressing inflammatory signals, leading to more complete tissue recovery and potentially improved long-term outcomes. Unlike non-steroidal anti-inflammatory drugs that globally inhibit inflammation, SPMs function in a context-dependent manner, resolving inflammation without compromising host defense mechanisms. Their multi-modal mechanism of action—simultaneously targeting ion channels on neurons, modulating glial cell activity, and reprogramming immune responses—allows for synergistic effects that address both peripheral and central sensitization processes. This comprehensive approach may explain their efficacy in diverse pain conditions while demonstrating a favorable safety profile in preclinical models. Notably, SPMs exhibit selective action on pathological pain states while preserving physiological nociceptive function, suggesting a reduced risk of side effects compared to broad-spectrum analgesics. Their ability to resolve neuroinflammation and reverse maladaptive plasticity further positions them as promising candidates for preventing the transition from acute to chronic pain. These inherent advantages, combined with emerging strategies to enhance their stability and bioavailability, underscore the potential of SPMs as a novel class of therapeutics that could complement or reduce reliance on current analgesics, particularly opioids.
4.2 Current gaps and mechanistic limitations
The current understanding of SPM mechanisms in pain resolution remains incomplete, constrained by several key limitations. Most research has focused on a narrow subset of SPMs—such as RvD1 and PD1—while the bioactivities and therapeutic potential of other members remain underexplored. This restricted focus not only limits a systems-level view of the resolvome but also leads to a related methodological gap: the general lack of head-to-head comparisons between different SPM subtypes within the same study. Without such direct comparative controls, it remains challenging to establish a clear hierarchy of efficacy or delineate unique versus shared functions among SPMs, thereby limiting the interpretation of their therapeutic potential. Furthermore, although SPMs are known to modulate immune cells and promote inflammatory resolution, their direct molecular targets and receptor-specific mechanisms are not fully elucidated. Receptors such as BLT1, ALX/FPR2, GPR32, ChemR23/CMKLR1, GPR18, GPR37, and LRG6 have been identified (see graphic abstract), yet a comprehensive mapping of SPM-receptor interactions across relevant cell types in pain pathways is still lacking (26). Moreover, the majority of mechanistic insights derive from macrophages and microglia, with far less attention paid to other types of cells. The roles of SPMs in regulating synaptic plasticity, ion channel activity, and neurotransmitter clearance within neural circuits therefore, require deeper investigation. Critically, the feedback effect of pain itself on SPM metabolism remains poorly understood. Chronic pain conditions may alter the expression of SPM biosynthetic enzymes or enhance degradation pathways, yet such adaptive mechanisms have not been systematically studied. The dynamic changes in endogenous SPM levels throughout different phases of pain progression also remain poorly characterized. For instance, one clinical study observed that migraine patients had significantly lower LXA4 levels than healthy controls, both during and between attacks, and those with attacks lasting longer than 12 hours showed even lower LXA4 levels than those with shorter attacks, suggesting that LXA4 depletion may accompany pain persistence (117). This clinical observation underscores the dynamic nature of the SPM system in vivo, further highlighting the importance of study designs that include appropriate controls—such as precursor molecules (e.g., DHA/EPA) or different SPMs—to distinguish specific pharmacological effects from broader, condition-dependent alterations in resolution pathways.
4.3 Sexual dimorphism and consideration for individualized therapy
Emerging evidence highlights sexual dimorphism in the anti-nociceptive effects of SPMs, with males and females exhibiting divergent responses across preclinical models. Specifically, intrathecal administration of RvD5 attenuates paclitaxel-induced mechanical allodynia (25), and it selectively inhibits the inflammatory phase of formalin-induced spontaneous pain in males (118). However, RvD5 shows no significant effect in female mice in either of these pain models. In male trigeminal neuralgia models, RvD5 downregulates IL-6 production specifically in male models of trigeminal neuralgia (118). Post-MaR1 treatment in the spared nerve injury model, males exhibit elevated macrophage colony-stimulating factor levels compared to females (102). The implications of sexual dimorphism in pain modulation highlight the importance of considering sex-specific mechanisms and therapeutic strategies. Specifically, sex hormones (e.g., testosterone, estrogen) likely interact to modulate the efficacy of SPMs, although the exact mechanisms remain unclear. These findings underscore the necessity for sex-balanced preclinical studies and tailored therapeutic approaches to optimize SPM-based pain management.
4.4 Challenges in clinical translation
The translation of SPMs from preclinical promise to clinical application remains at an early stage. Human studies have predominantly used SPM precursors (e.g., DHA, EPA) or intermediates (e.g., 17-HDHA and 18-HEPE) rather than pre-formed SPMs due to the latter’s poor bioavailability and metabolic instability (119–121). The reason may relate to the inherent instability of SPMs, which are sensitive to oxygen, light, and heat, raising concerns about shelf-life. However, precursor efficacy is inherently limited because PUFAs require enzymatic conversion to active SPMs via rate-limiting steps involving lipoxygenases and cyclooxygenases (26), explaining their reduced potency (43). Even excess PUFAs show limited therapeutic impact due to this metabolic bottleneck. This limitation is compounded in disease states where PUFA metabolism is impaired. Obesity disrupts 17-HDHA and PD1 biosynthesis (122); S. aureus infection in macrophages elevates COX-2/mPGES-1 but suppresses 15-LOX-1, impairing SPM production (123); and fatty acid desaturase 1 deficiency alters hepatic PUFA metabolism, limiting SPM synthesis (124). Thus, simply increasing precursor supply is ineffective when biosynthetic pathways are compromised, underscoring the rationale for using pre-formed SPMs. Clinical progress is further hindered by sexual dimorphism in SPM responses (26), heterogeneous trial designs, small cohorts, and varied control groups (120, 121, 125, 126). No large-scale trials have directly assessed fully synthesized SPMs, leaving a major translational gap.
4.5 Future research directions
Future strategies include both enhancing endogenous SPM production and developing exogenous supplementation. Elevating endogenous SPM biosynthesis through interventions is a promising strategy. For example, by increasing the activity of key enzymes such as lipoxygenases to improve precursor conversion efficiency. Several interventions—including high-intensity swimming (127), spinal cord stimulation (128), vagus nerve stimulation (129), the caspase-1 inhibitor VX-765 (104), soluble epoxide hydrolase (sEH) inhibition (130), and specific inhibitors such as SAFit2 (131) —have shown promise in elevating SPM levels in preclinical models. Given that particular pathological conditions like obesity may impair SPM production (122–124), exogenous supplementation with stabilized SPM analogs represents a more direct approach in several circumstances. Critical translational barriers include inherent SPM instability, necessitating advanced formulations (e.g., nano-encapsulation, structural modifications, prodrug designs) to improve shelf-life, half-life, and bioavailability (26, 132). Scalable synthetic production and rigorous safety profiling are essential. Developing non-invasive delivery routes (oral, transdermal) is crucial for clinical feasibility beyond intrathecal administration. Finally, exploring SPMs in combination therapy with conventional analgesics may yield synergistic effects, supporting a multi-mechanistic approach to pain management. Future clinical efforts must incorporate sex as a biological variable to ensure therapies are effective for all patients.
Author contributions
YC: Visualization, Writing – original draft, Investigation, Writing – review & editing. XW: Writing – review & editing. JL: Writing – review & editing. YR: Writing – review & editing. HM: Writing – review & editing. XZ: Writing – review & editing. CH: Writing – review & editing. XC: Funding acquisition, Writing – review & editing, Conceptualization, Investigation, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by National Natural Science Foundation of China (82071239), Ningbo Top Medical and Health Research Program (2024010317), and Ningbo Natural Science Foundation (2022J070).
Acknowledgments
We thank the technical support of the Core Facilities, Health Science Center, Ningbo University. Parts of cartoons in figures were created with BioRender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
2-AG: 2-Arachidonoylglycerol
AEA: anandamide
ALX: lipoxin A receptor
AMPAR: α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
AMPK: AMP-activated protein kinase
Arg-1: arginase-1
ATF-3: activating transcription factor 3
cAMP: cyclic adenosine monophosphate
CB2R: cannabinoid 2 receptor
CCI: chronic constriction injury
CCL2: C-C motif chemokine ligand 2
CCR7: C-C chemokine receptor 7
CD68: cluster of differentiation 68
CFA: Complete Freund’s Adjuvant
CGRP: calcitonin gene-related peptide
ChemR23: chemerin receptor 23
COX-2: cyclooxygenase-2
CXCL1: C-X-C motif chemokine ligand 1
DHA: docosahexaenoic
DRG: dorsal root ganglion
DRV1: Resolvin D1 receptor 1
EPA: eicosapentaenoic
Epac: exchange protein activated by cAMP
ERK: extracellular signal-regulated kinase
FFAR4: free fatty acid receptor 4
FPR2: formyl-peptide receptor 2
GLT-1: glutamate transporter 1
GPCR: G protein-coupled receptor
GPR32: G protein-coupled receptor 32
IKKβ: IκB kinase β
IL-1β: interleukin-1β
iNOS: inducible nitric oxide synthase
LC3-II: microtubule-associated protein 1 light chain 3 II
LTP: long-term potentiation
miR-155: microRNA 155
MSU: monosodium urate
mTOR: mammalian target of rapamycin
NCLDH: non-compressive lumbar disc herniation
NF-κB: nuclear factor-κB
NLRP3: NLR family pyrin domain containing 3
NMDAR: N-methyl-D-aspartate receptor
p-62: sequestosome 1
PKC: protein kinase C
PNS: peripheral nervous system
PTEN: phosphatase and tensin homolog
sEPSC: spontaneous excitatory postsynaptic current
SIRT1: sirtuin 1
SPMs: specialized pro-resolving mediators
TG: trigeminal ganglion
TGF-β1: transforming growth factor-β
TNF-α: tumor necrosis factor alpha
TRPA1: transient receptor potential ankyrin subtype 1
TRPV1: transient receptor potential vanilloid subtype 1.
References
1. Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain. (2020) 161:1976–82. doi: 10.1097/j.pain.0000000000001939
2. Andrejeva N, Baumeister D, Eich W, and Tesarz J. Psychosocial factors in the prevention of pain. Schmerz. (2021) 35:21–9. doi: 10.1007/s00482-020-00523-4
3. Sá KN, Moreira L, Baptista AF, Yeng LT, Teixeira MJ, Galhardoni R, et al. Prevalence of chronic pain in developing countries: systematic review and meta-analysis. Pain Rep. (2019) 4:e779. doi: 10.1097/PR9.0000000000000779
4. Cohen SP, Vase L, and Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. (2021) 397:2082–97. doi: 10.1016/S0140-6736(21)00393-7
5. Zhang J, Chen J, Jiang Q, Feng R, Zhao X, Li H, et al. Resolvin D1 attenuates inflammation and pelvic pain associated with EAP by inhibiting oxidative stress and NLRP3 inflammasome activation via the nrf2/HO-1 pathway. J Inflammation Res. (2023) 16:3365–79. doi: 10.2147/JIR.S408111
6. Serhan CN, Chiang N, and Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. (2008) 8:349–61. doi: 10.1038/nri2294
7. Brennan E, Kantharidis P, Cooper ME, and Godson C. Pro-resolving lipid mediators: regulators of inflammation, metabolism and kidney function. Nat Rev Nephrol. (2021) 17:725–39. doi: 10.1038/s41581-021-00454-y
8. Derada Troletti C, Enzmann G, Chiurchiù V, Kamermans A, Tietz SM, Norris PC, et al. Pro-resolving lipid mediator lipoxin A(4) attenuates neuro-inflammation by modulating T cell responses and modifies the spinal cord lipidome. Cell Rep. (2021) 35:109201. doi: 10.1016/j.celrep.2021.109201
9. Romano M, Patruno S, Pomilio A, and Recchiuti A. Proresolving lipid mediators and receptors in stem cell biology: concise review. Stem Cells Transl Med. (2019) 8:992–8. doi: 10.1002/sctm.19-0078
10. Basil MC and Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol. (2016) 16:51–67. doi: 10.1038/nri.2015.4
11. Tam VC, Quehenberger O, Oshansky CM, Suen R, Armando AM, Treuting PM, et al. Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation. Cell. (2013) 154:213–27. doi: 10.1016/j.cell.2013.05.052
12. Deyama S, Minami M, and Kaneda K. Resolvin E1 as a potential lead for the treatment of depression. Nihon Yakurigaku Zasshi. (2024) 159:210–3. doi: 10.1254/fpj.23008
13. Lavy M, Gauttier V, Poirier N, Barillé-Nion S, and Blanquart C. Specialized pro-resolving mediators mitigate cancer-related inflammation: role of tumor-associated macrophages and therapeutic opportunities. Front Immunol. (2021) 12:702785. doi: 10.3389/fimmu.2021.702785
14. Viola JR, Lemnitzer P, Jansen Y, Csaba G, Winter C, Neideck C, et al. Resolving lipid mediators maresin 1 and resolvin D2 prevent atheroprogression in mice. Circ Res. (2016) 119:1030–8. doi: 10.1161/CIRCRESAHA.116.309492
15. Wu B, Mottola G, Chatterjee A, Lance KD, Chen M, Siguenza IO, et al. Perivascular delivery of resolvin D1 inhibits neointimal hyperplasia in a rat model of arterial injury. J Vasc Surg. (2017) 65:207–217.e203. doi: 10.1016/j.jvs.2016.01.030
16. Vidar Hansen T and Serhan CN. Protectins: Their biosynthesis, metabolism and structure-functions. Biochem Pharmacol. (2022) 206:115330. doi: 10.1016/j.bcp.2022.115330
17. Serhan CN. Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology. Mol Aspects Med. (2017) 58:1–11. doi: 10.1016/j.mam.2017.03.001
18. Turnbull J, Jha RR, Ortori CA, Lunt E, Tighe PJ, Irving WL, et al. Serum levels of proinflammatory lipid mediators and specialized proresolving molecules are increased in patients with severe acute respiratory syndrome coronavirus 2 and correlate with markers of the adaptive immune response. J Infect Dis. (2022) 225:2142–54. doi: 10.1093/infdis/jiab632
19. Chandrasekharan JA and Sharma-Walia N. Lipoxins: nature's way to resolve inflammation. J Inflammation Res. (2015) 8:181–92. doi: 10.2147/JIR.S90380
20. Meesawatsom P, Burston J, Hathway G, Bennett A, and Chapman V. Inhibitory effects of aspirin-triggered resolvin D1 on spinal nociceptive processing in rat pain models. J Neuroinflammation. (2016) 13:233. doi: 10.1186/s12974-016-0676-6
21. Lee SH, Tonello R, Im ST, Jeon H, Park J, Ford Z, et al. Resolvin D3 controls mouse and human TRPV1-positive neurons and preclinical progression of psoriasis. Theranostics. (2020) 10:12111–26. doi: 10.7150/thno.52135
22. Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, and Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. (2007) 178:3912–7. doi: 10.4049/jimmunol.178.6.3912
23. Oh SF, Dona M, Fredman G, Krishnamoorthy S, Irimia D, and Serhan CN. Resolvin E2 formation and impact in inflammation resolution. J Immunol. (2012) 188:4527–34. doi: 10.4049/jimmunol.1103652
24. Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc Natl Acad Sci U S A. (2010) 107:1660–5. doi: 10.1073/pnas.0907342107
25. Luo X, Gu Y, Tao X, Serhan CN, and Ji RR. Resolvin D5 inhibits neuropathic and inflammatory pain in male but not female mice: distinct actions of D-series resolvins in chemotherapy-induced peripheral neuropathy. Front Pharmacol. (2019) 10:745. doi: 10.3389/fphar.2019.00745
26. Ji RR. Specialized pro-resolving mediators as resolution pharmacology for the control of pain and itch. Annu Rev Pharmacol Toxicol. (2023) 63:273–93. doi: 10.1146/annurev-pharmtox-051921-084047
27. Lu Q, Yang Y, Zhang H, Chen C, Zhao J, Yang Z, et al. Activation of GPR18 by resolvin D2 relieves pain and improves bladder function in cyclophosphamide-induced cystitis through inhibiting TRPV1. Drug Des Devel Ther. (2021) 15:4687–99. doi: 10.2147/DDDT.S329507
28. Xu J, Yan Z, Bang S, Velmeshev D, and Ji RR. GPR37L1 identifies spinal cord astrocytes and protects neuropathic pain after nerve injury. Neuron. (2025) 113:1206–22.e6. doi: 10.1016/j.neuron.2025.01.012
29. Zhang Q, Bang S, Chandra S, and Ji RR. Inflammation and infection in pain and the role of GPR37. Int J Mol Sci. (2022) 23:14426. doi: 10.3390/ijms232214426
30. Lei J, Zhou Y, Zhao H, Chen Y, Yan G, Wu L, et al. Dabigatran activates inflammation resolution by promoting fibrinogen-like protein 2 shedding and RvD5(n-3 DPA) production. Theranostics. (2021) 11:4251–61. doi: 10.7150/thno.50182
31. Hwang HJ, Jung TW, Kim JW, Kim JA, Lee YB, Hong SH, et al. Protectin DX prevents H(2)O(2)-mediated oxidative stress in vascular endothelial cells via an AMPK-dependent mechanism. Cell Signal. (2019) 53:14–21. doi: 10.1016/j.cellsig.2018.09.011
32. Zhao M, Li C, Zhang J, Yin Z, Zheng Z, Wan J, et al. Maresin-1 and its receptors RORα/LGR6 as potential therapeutic target for respiratory diseases. Pharmacol Res. (2022) 182:106337. doi: 10.1016/j.phrs.2022.106337
33. Chiang N, Riley IR, Dalli J, Rodriguez AR, Spur BW, and Serhan CN. New maresin conjugates in tissue regeneration pathway counters leukotriene D(4)-stimulated vascular responses. FASEB J. (2018) 32:4043–52. doi: 10.1096/fj.201701493R
34. Huang ZX, He XR, Ding XY, Chen JH, Lei YH, Bai JB, et al. Lipoxin A4 depresses inflammation and promotes autophagy via AhR/mTOR/AKT pathway to suppress endometriosis. Am J Reprod Immunol. (2023) 89:e13659. doi: 10.1111/aji.13659
35. Yaksh TL and Rudy TA. Analgesia mediated by a direct spinal action of narcotics. Science. (1976) 192:1357–8. doi: 10.1126/science.1273597
36. Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. (2010) 16:592–7. doi: 10.1038/nm.2123
37. Park CK, Lü N, Xu ZZ, Liu T, Serhan CN, and Ji RR. Resolving TRPV1- and TNF-α-mediated spinal cord synaptic plasticity and inflammatory pain with neuroprotectin D1. J Neurosci. (2011) 31:15072–85. doi: 10.1523/JNEUROSCI.2443-11.2011
38. Park CK, Xu ZZ, Liu T, Lü N, Serhan CN, and Ji RR. Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J Neurosci. (2011) 31:18433–8. doi: 10.1523/JNEUROSCI.4192-11.2011
39. Quan-Xin F, Fan F, Xiang-Ying F, Shu-Jun L, Shi-Qi W, Zhao-Xu L, et al. Resolvin D1 reverses chronic pancreatitis-induced mechanical allodynia, phosphorylation of NMDA receptors, and cytokines expression in the thoracic spinal dorsal horn. BMC Gastroenterol. (2012) 12:148. doi: 10.1186/1471-230X-12-148
40. Xu ZZ, Liu XJ, Berta T, Park CK, Lü N, Serhan CN, et al. Neuroprotectin/protectin D1 protects against neuropathic pain in mice after nerve trauma. Ann Neurol. (2013) 74:490–5. doi: 10.1002/ana.23928
41. Fonseca FC, Orlando RM, Turchetti-Maia RM, and de Francischi JN. Comparative effects of the ω3 polyunsaturated fatty acid derivatives resolvins E1 and D1 and protectin DX in models of inflammation and pain. J Inflammation Res. (2017) 10:119–33. doi: 10.2147/JIR.S142424
42. Lima-Garcia JF, Dutra RC, da Silva K, Motta EM, Campos MM, and Calixto JB. The precursor of resolvin D series and aspirin-triggered resolvin D1 display anti-hyperalgesic properties in adjuvant-induced arthritis in rats. Br J Pharmacol. (2011) 164:278–93. doi: 10.1111/j.1476-5381.2011.01345.x
43. Tao X, Lee MS, Donnelly CR, and Ji RR. Neuromodulation, specialized proresolving mediators, and resolution of pain. Neurotherapeutics. (2020) 17:886–99. doi: 10.1007/s13311-020-00892-9
44. Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, et al. Neuropathic pain. Nat Rev Dis Primers. (2017) 3:17002. doi: 10.1038/nrdp.2017.2
45. Hange N, Poudel S, Ozair S, Paul T, Nambakkam M, Shrestha R, et al. Managing chronic neuropathic pain: recent advances and new challenges. Neurol Res Int. (2022) 2022:8336561. doi: 10.1155/2022/8336561
46. Ko GD, Nowacki NB, Arseneau L, Eitel M, and Hum A. Omega-3 fatty acids for neuropathic pain: case series. Clin J Pain. (2010) 26:168–72. doi: 10.1097/AJP.0b013e3181bb8533
47. Pang J, Xin P, Kong Y, Wang Z, and Wang X. Resolvin D2 reduces chronic neuropathic pain and bone cancer pain via spinal inhibition of IL-17 secretion, CXCL1 release and astrocyte. Brain Sci. (2023) 13(1):152. doi: 10.3390/brainsci13010152
48. Xu ZZ, Berta T, and Ji RR. Resolvin E1 inhibits neuropathic pain and spinal cord microglial activation following peripheral nerve injury. J Neuroimmune Pharmacol. (2013) 8:37–41. doi: 10.1007/s11481-012-9394-8
49. Nesman JI, Chen O, Luo X, Ji RR, Serhan CN, and Hansen TV. A new synthetic protectin D1 analog 3-oxa-PD1(n-3 DPA) reduces neuropathic pain and chronic itch in mice. Org Biomol Chem. (2021) 19:2744–52. doi: 10.1039/D0OB02136A
50. Lopes RV, Baggio DF, Ferraz CR, Bertozzi MM, Saraiva-Santos T, Verri Junior WA, et al. Maresin-2 inhibits inflammatory and neuropathic trigeminal pain and reduces neuronal activation in the trigeminal ganglion. Curr Res Neurobiol. (2023) 4:100093. doi: 10.1016/j.crneur.2023.100093
51. Varrassi G, Alon E, Bagnasco M, Lanata L, Mayoral-Rojals V, Paladini A, et al. Towards an effective and safe treatment of inflammatory pain: A delphi-guided expert consensus. Adv Ther. (2019) 36:2618–37. doi: 10.1007/s12325-019-01053-x
52. Zhang YH, Adamo D, Liu H, Wang Q, Wu W, Zheng YL, et al. Editorial: Inflammatory pain: mechanisms, assessment, and intervention. Front Mol Neurosci. (2023) 16:1286215. doi: 10.3389/fnmol.2023.1286215
53. Bang S, Yoo S, Yang TJ, Cho H, Kim YG, and Hwang SW. Resolvin D1 attenuates activation of sensory transient receptor potential channels leading to multiple anti-nociception. Br J Pharmacol. (2010) 161:707–20. doi: 10.1111/j.1476-5381.2010.00909.x
54. Zaninelli TH, Fattori V, Saraiva-Santos T, Badaro-Garcia S, Staurengo-Ferrari L, Andrade KC, et al. RvD1 disrupts nociceptor neuron and macrophage activation and neuroimmune communication, reducing pain and inflammation in gouty arthritis in mice. Br J Pharmacol. (2022) 179:4500–15. doi: 10.1111/bph.15897
55. Allen BL, Montague-Cardoso K, Simeoli R, Colas RA, Oggero S, Vilar B, et al. Imbalance of proresolving lipid mediators in persistent allodynia dissociated from signs of clinical arthritis. Pain. (2020) 161:2155–66. doi: 10.1097/j.pain.0000000000001908
56. Abdelmoaty S, Wigerblad G, Bas DB, Codeluppi S, Fernandez-Zafra T, El-Awady el S, et al. Spinal actions of lipoxin A4 and 17(R)-resolvin D1 attenuate inflammation-induced mechanical hypersensitivity and spinal TNF release. PloS One. (2013) 8:e75543. doi: 10.1371/journal.pone.0075543
57. Svensson CI, Zattoni M, and Serhan CN. Lipoxins and aspirin-triggered lipoxin inhibit inflammatory pain processing. J Exp Med. (2007) 204:245–52. doi: 10.1084/jem.20061826
58. Falsetta ML, Wood RW, Linder MA, Bonham AD, Honn KV, Maddipati KR, et al. Specialized pro-resolving mediators reduce pro-nociceptive inflammatory mediator production in models of localized provoked vulvodynia. J Pain. (2021) 22:1195–209. doi: 10.1016/j.jpain.2021.03.144
59. Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, et al. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. FASEB J. (2012) 26:1755–65. doi: 10.1096/fj.11-201442
60. Fattori V, Zaninelli TH, Ferraz CR, Brasil-Silva L, Borghi SM, Cunha JM, et al. Maresin 2 is an analgesic specialized pro-resolution lipid mediator in mice by inhibiting neutrophil and monocyte recruitment, nociceptor neuron TRPV1 and TRPA1 activation, and CGRP release. Neuropharmacology. (2022) 216:109189. doi: 10.1016/j.neuropharm.2022.109189
61. Mestdagh F, Steyaert A, and Lavand'homme P. Cancer pain management: A narrative review of current concepts, strategies, and techniques. Curr Oncol. (2023) 30:6838–58. doi: 10.3390/curroncol30070500
62. Ye Y, Scheff NN, Bernabé D, Salvo E, Ono K, Liu C, et al. Anti-cancer and analgesic effects of resolvin D2 in oral squamous cell carcinoma. Neuropharmacology. (2018) 139:182–93. doi: 10.1016/j.neuropharm.2018.07.016
63. Khasabova IA, Golovko MY, Golovko SA, Simone DA, and Khasabov SG. Intrathecal administration of Resolvin D1 and E1 decreases hyperalgesia in mice with bone cancer pain: Involvement of endocannabinoid signaling. Prostaglandins Other Lipid Mediat. (2020) 151:106479. doi: 10.1016/j.prostaglandins.2020.106479
64. Niyonkuru E, Iqbal MA, Zhang X, and Ma P. Complementary approaches to postoperative pain management: A review of non-pharmacological interventions. Pain Ther. (2024) 14:121–44. doi: 10.1007/s40122-024-00688-1
65. Huang L, Wang CF, Serhan CN, and Strichartz G. Enduring prevention and transient reduction of postoperative pain by intrathecal resolvin D1. Pain. (2011) 152:557–65. doi: 10.1016/j.pain.2010.11.021
66. Wang JC and Strichartz GR. Prevention of chronic post-thoracotomy pain in rats by intrathecal resolvin D1 and D2: effectiveness of perioperative and delayed drug delivery. J Pain. (2017) 18:535–45. doi: 10.1016/j.jpain.2016.12.012
67. Zhang L, Terrando N, Xu ZZ, Bang S, Jordt SE, Maixner W, et al. Distinct analgesic actions of DHA and DHA-derived specialized pro-resolving mediators on post-operative pain after bone fracture in mice. Front Pharmacol. (2018) 9:412. doi: 10.3389/fphar.2018.00412
68. Gao Y, Mei C, Chen P, and Chen X. The contribution of neuro-immune crosstalk to pain in the peripheral nervous system and the spinal cord. Int Immunopharmacol. (2022) 107:108700. doi: 10.1016/j.intimp.2022.108700
69. Xu Y, Jiang Z, and Chen X. Mechanisms underlying paclitaxel-induced neuropathic pain: Channels, inflammation and immune regulations. Eur J Pharmacol. (2022) 933:175288. doi: 10.1016/j.ejphar.2022.175288
70. Mei C, Pan C, Xu L, Miao M, Lu Q, Yu Y, et al. Trimethoxyflavanone relieves Paclitaxel-induced neuropathic pain via inhibiting expression and activation of P2X7 and production of CGRP in mice. Neuropharmacology. (2023) 236:109584. doi: 10.1016/j.neuropharm.2023.109584
71. Pan C, Xu Y, Jiang Z, Fan C, Chi Z, Zhang Y, et al. Naringenin relieves paclitaxel-induced pain by suppressing calcitonin gene-related peptide signalling and enhances the anti-tumour action of paclitaxel. Br J Pharmacol. (2024) 181:3136–59. doi: 10.1111/bph.16397
72. Hourani T, Perez-Gonzalez A, Khoshmanesh K, Luwor R, Achuthan AA, Baratchi S, et al. Label-free macrophage phenotype classification using machine learning methods. Sci Rep. (2023) 13:5202. doi: 10.1038/s41598-023-32158-7
73. Wang J, Wu Q, Wang X, Liu H, Chen M, Xu L, et al. Targeting macrophage phenotypes and metabolism as novel therapeutic approaches in atherosclerosis and related cardiovascular diseases. Curr Atheroscler Rep. (2024) 26:573–88. doi: 10.1007/s11883-024-01229-z
74. Kim J, Joshi HP, Sheen SH, Kim KT, Kyung JW, Choi H, et al. Resolvin D3 promotes inflammatory resolution, neuroprotection, and functional recovery after spinal cord injury. Mol Neurobiol. (2021) 58:424–38. doi: 10.1007/s12035-020-02118-7
75. Saraiva-Santos T, Zaninelli TH, Manchope MF, Andrade KC, Ferraz CR, Bertozzi MM, et al. Therapeutic activity of lipoxin A(4) in TiO(2)-induced arthritis in mice: NF-κB and Nrf2 in synovial fluid leukocytes and neuronal TRPV1 mechanisms. Front Immunol. (2023) 14:949407. doi: 10.3389/fimmu.2023.949407
76. Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. (2005) 201:713–22. doi: 10.1084/jem.20042031
77. Bang S, Jiang C, Xu J, Chandra S, McGinnis A, Luo X, et al. Satellite glial GPR37L1 and its ligand maresin 1 regulate potassium channel signaling and pain homeostasis. J Clin Invest. (2024) 134:e173537. doi: 10.1172/JCI173537
78. Chen R, Yin C, Fang J, and Liu B. The NLRP3 inflammasome: an emerging therapeutic target for chronic pain. J Neuroinflammation. (2021) 18:84. doi: 10.1186/s12974-021-02131-0
79. Wang YH, Gao X, Tang YR, Chen FQ, Yu Y, Sun MJ, et al. Resolvin D1 alleviates mechanical allodynia via ALX/FPR2 receptor targeted nod-like receptor protein 3/extracellular signal-related kinase signaling in a neuropathic pain model. Neuroscience. (2022) 494:12–24. doi: 10.1016/j.neuroscience.2022.04.019
80. Cao L, Wang Y, Wang Y, Lv F, Liu L, and Li Z. Resolvin D2 suppresses NLRP3 inflammasome by promoting autophagy in macrophages. Exp Ther Med. (2021) 22:1222. doi: 10.3892/etm.2021.10656
81. Sun T, Yu E, Yu L, Luo J, Li H, and Fu Z. LipoxinA(4) induced antinociception and decreased expression of NF-κB and pro-inflammatory cytokines after chronic dorsal root ganglia compression in rats. Eur J Pain. (2012) 16:18–27. doi: 10.1016/j.ejpain.2011.05.005
82. Liu ZH, Miao GS, Wang JN, Yang CX, Fu ZJ, and Sun T. Resolvin D1 inhibits mechanical hypersensitivity in sciatica by modulating the expression of nuclear factor-κB, phospho-extracellular signal-regulated kinase, and pro- and antiinflammatory cytokines in the spinal cord and dorsal root ganglion. Anesthesiology. (2016) 124:934–44. doi: 10.1097/ALN.0000000000001010
83. Waltrick APF, Radulski DR, de Oliveira KM, Acco A, Verri WA, da Cunha JM, et al. Early evidence of beneficial and protective effects of Protectin DX treatment on behavior responses and type-1 diabetes mellitus related-parameters: A non-clinical approach. Prog Neuropsychopharmacol Biol Psychiatry. (2024) 133:111028. doi: 10.1016/j.pnpbp.2024.111028
84. Bang S, Yoo S, Oh U, and Hwang SW. Endogenous lipid-derived ligands for sensory TRP ion channels and their pain modulation. Arch Pharm Res. (2010) 33:1509–20. doi: 10.1007/s12272-010-1004-9
85. Roh J, Go EJ, Park JW, Kim YH, and Park CK. Resolvins: potent pain inhibiting lipid mediators via transient receptor potential regulation. Front Cell Dev Biol. (2020) 8:584206. doi: 10.3389/fcell.2020.584206
86. Pyo HJ, An X, and Cho H. The role of free fatty acid receptor pathways in a selective regulation of TRPA1 and TRPV1 by resolvins in primary sensory neurons. J Cell Physiol. (2022) 237:3651–60. doi: 10.1002/jcp.30826
87. Bang S, Yoo S, Yang TJ, Cho H, and Hwang SW. 17(R)-resolvin D1 specifically inhibits transient receptor potential ion channel vanilloid 3 leading to peripheral antinociception. Br J Pharmacol. (2012) 165:683–92. doi: 10.1111/j.1476-5381.2011.01568.x
88. Jo YY, Lee JY, and Park CK. Resolvin E1 inhibits substance P-induced potentiation of TRPV1 in primary sensory neurons. Mediators Inflamm. (2016) 2016:5259321. doi: 10.1155/2016/5259321
89. Park CK. Maresin 1 inhibits TRPV1 in temporomandibular joint-related trigeminal nociceptive neurons and TMJ inflammation-induced synaptic plasticity in the trigeminal nucleus. Mediators Inflamm. (2015) 2015:275126. doi: 10.1155/2015/275126
90. Bekauri T, Fischer S, Honn KV, Maddipati KR, Love T, Little C, et al. Inflammation, lipid dysregulation, and transient receptor potential cation channel subfamily V member 4 signaling perpetuate chronic vulvar pain. Pain. (2024) 165:820–37. doi: 10.1097/j.pain.0000000000003088
91. Hao JW, Liu TT, Qiu CY, Li XM, Qiao WL, Li Q, et al. Lipid mediator resolvin D2 inhibits ATP currents in rat primary sensory neurons. J Neurochem. (2024) 168:3715–26. doi: 10.1111/jnc.16009
92. Fattori V, Pinho-Ribeiro FA, Staurengo-Ferrari L, Borghi SM, Rossaneis AC, Casagrande R, et al. The specialised pro-resolving lipid mediator maresin 1 reduces inflammatory pain with a long-lasting analgesic effect. Br J Pharmacol. (2019) 176:1728–44. doi: 10.1111/bph.14647
93. Yang JX, Wang HF, Chen JZ, Li HY, Hu JC, Yu AA, et al. Potential neuroimmune interaction in chronic pain: A review on immune cells in peripheral and central sensitization. Front Pain Res (Lausanne). (2022) 3:946846. doi: 10.3389/fpain.2022.946846
94. Tiberi M and Chiurchiù V. Specialized pro-resolving lipid mediators and glial cells: emerging candidates for brain homeostasis and repair. Front Cell Neurosci. (2021) 15:673549. doi: 10.3389/fncel.2021.673549
95. Zhang J, Li Z, Fan M, and Jin W. Lipoxins in the nervous system: brighter prospects for neuroprotection. Front Pharmacol. (2022) 13:781889. doi: 10.3389/fphar.2022.781889
96. Marty-Lombardi S, Lu S, Ambroziak W, Schrenk-Siemens K, Wang J, DePaoli-Roach AA, et al. Neuron-astrocyte metabolic coupling facilitates spinal plasticity and maintenance of inflammatory pain. Nat Metab. (2024) 6:494–513. doi: 10.1038/s42255-024-01001-2
97. Chen G, Zhang YQ, Qadri YJ, Serhan CN, and Ji RR. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron. (2018) 100:1292–311. doi: 10.1016/j.neuron.2018.11.009
98. Wang Y-H, Tang Y-R, Gao X, Zhang N-N, Lv Q-Q, Liu J, et al. Aspirin-triggered Resolvin D1 ameliorates activation of the NLRP3 inflammasome via induction of autophagy in a rat model of neuropathic pain. Front Pharmacol. (2023) 14:971136. doi: 10.3389/fphar.2023.971136
99. Zhao X, Li X, Guo H, Liu P, Ma M, and Wang Y. Resolvin D1 attenuates mechanical allodynia after burn injury: Involvement of spinal glia, p38 mitogen-activated protein kinase, and brain-derived neurotrophic factor/tropomyosin-related kinase B signaling. Mol Pain. (2023) 19:17448069231159970. doi: 10.1177/17448069231159970
100. Bo C, Liu X, Liu Y, Xu L, and Huang Q. Resolvin D1 accelerates resolution of neuroinflammation by inhibiting microglia activation through the BDNF/TrkB signaling pathway. Eur J Med Res. (2025) 30:189. doi: 10.1186/s40001-025-02424-7
101. Martini AC, Berta T, Forner S, Chen G, Bento AF, Ji RR, et al. Lipoxin A4 inhibits microglial activation and reduces neuroinflammation and neuropathic pain after spinal cord hemisection. J Neuroinflammation. (2016) 13:75. doi: 10.1186/s12974-016-0540-8
102. Teixeira-Santos L, Martins S, Sousa T, Albino-Teixeira A, and Pinho D. The pro-resolving lipid mediator Maresin 1 ameliorates pain responses and neuroinflammation in the spared nerve injury-induced neuropathic pain: A study in male and female mice. PloS One. (2023) 18:e0287392. doi: 10.1371/journal.pone.0287392
103. Wang YH, Li Y, Wang JN, Zhao QX, Jin J, Wen S, et al. Maresin 1 attenuates radicular pain through the inhibition of NLRP3 inflammasome-induced pyroptosis via NF-κB signaling. Front Neurosci. (2020) 14:831. doi: 10.3389/fnins.2020.00831
104. Wang YH, Li Y, Wang JN, Zhao QX, Wen S, Wang SC, et al. A novel mechanism of specialized proresolving lipid mediators mitigating radicular pain: the negative interaction with NLRP3 inflammasome. Neurochem Res. (2020) 45:1860–9. doi: 10.1007/s11064-020-03050-x
105. Miao GS, Liu ZH, Wei SX, Luo JG, Fu ZJ, and Sun T. Lipoxin A4 attenuates radicular pain possibly by inhibiting spinal ERK, JNK and NF-κB/p65 and cytokine signals, but not p38, in a rat model of non-compressive lumbar disc herniation. Neuroscience. (2015) 300:10–8. doi: 10.1016/j.neuroscience.2015.04.060
106. Yang L, Gao X, Tian D, Yang W, Xue S, Cao Z, et al. Resolvin D2 activates anti-inflammatory microglia via restoring autophagy flux and alleviate neuropathic pain following spinal cord injury in rats. Exp Neurol. (2023) 370:114573. doi: 10.1016/j.expneurol.2023.114573
107. Leão FF, Waltrick APF, Verri WA Jr., da Cunha JM, and Zanoveli JM. Resolvin D5 disrupts anxious- and depressive-like behaviors in a type 1 diabetes mellitus animal model. Naunyn Schmiedebergs Arch Pharmacol. (2022) 395:1269–82. doi: 10.1007/s00210-022-02274-8
108. Zhu YC, Zhang Y, Gao X, Li LX, Tang YR, and Wang YH. Protectin D1 ameliorates non-compressive lumbar disc herniation through SIRT1-mediated CGRP signaling. Mol Pain. (2024) 20:17448069241232349. doi: 10.1177/17448069241232349
109. Zhao QX, Wang YH, Wang SC, Xue S, Cao ZX, and Sun T. Protectin DX attenuates lumbar radicular pain of non-compressive disc herniation by autophagy flux stimulation via adenosine monophosphate-activated protein kinase signaling. Front Physiol. (2021) 12:784653. doi: 10.3389/fphys.2021.784653
110. Francos-Quijorna I, Santos-Nogueira E, Gronert K, Sullivan AB, Kopp MA, Brommer B, et al. Maresin 1 promotes inflammatory resolution, neuroprotection, and functional neurological recovery after spinal cord injury. J Neurosci. (2017) 37:11731–43. doi: 10.1523/JNEUROSCI.1395-17.2017
111. Gao J, Tang C, Tai LW, Ouyang Y, Li N, Hu Z, et al. Pro-resolving mediator maresin 1 ameliorates pain hypersensitivity in a rat spinal nerve ligation model of neuropathic pain. J Pain Res. (2018) 11:1511–9. doi: 10.2147/JPR.S160779
112. Wang ZF, Li Q, Liu SB, Mi WL, Hu S, Zhao J, et al. Aspirin-triggered Lipoxin A4 attenuates mechanical allodynia in association with inhibiting spinal JAK2/STAT3 signaling in neuropathic pain in rats. Neuroscience. (2014) 273:65–78. doi: 10.1016/j.neuroscience.2014.04.052
113. Ji RR, Xu ZZ, Strichartz G, and Serhan CN. Emerging roles of resolvins in the resolution of inflammation and pain. Trends Neurosci. (2011) 34:599–609. doi: 10.1016/j.tins.2011.08.005
114. Petrenko AB, Yamakura T, Baba H, and Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesth Analg. (2003) 97:1108–16. doi: 10.1213/01.ANE.0000081061.12235.55
115. Li J, Tian M, Hua T, Wang H, Yang M, Li W, et al. Combination of autophagy and NFE2L2/NRF2 activation as a treatment approach for neuropathic pain. Autophagy. (2021) 17:4062–82. doi: 10.1080/15548627.2021.1900498
116. Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. (2011) 331:456–61. doi: 10.1126/science.1196371
117. Kocaturk I, Gulten S, Ece B, and Kukul Guven FM. Exploring PGE2 and LXA4 levels in migraine patients: the potential of LXA4-based therapies. Diagnostics (Basel). (2024) 14:635. doi: 10.3390/diagnostics14060635
118. Baggio DF, da Luz FMR, Lopes RV, Ferreira LEN, Araya EI, and Chichorro JG. Sex dimorphism in resolvin D5-induced analgesia in rat models of trigeminal pain. J Pain. (2023) 24:717–29. doi: 10.1016/j.jpain.2022.12.013
119. Serhan CN, Bäck M, Chiurchiù V, Hersberger M, Mittendorfer B, Calder PC, et al. Expert consensus report on lipid mediators: Role in resolution of inflammation and muscle preservation. FASEB J. (2024) 38:e23699. doi: 10.1096/fj.202400619R
120. Möller I, Rodas G, Villalón JM, Rodas JA, Angulo F, Martínez N, et al. Randomized, double-blind, placebo-controlled study to evaluate the effect of treatment with an SPMs-enriched oil on chronic pain and inflammation, functionality, and quality of life in patients with symptomatic knee osteoarthritis: GAUDI study. J Transl Med. (2023) 21:423. doi: 10.1186/s12967-023-04283-4
121. Callan N, Hanes D, and Bradley R. Early evidence of efficacy for orally administered SPM-enriched marine lipid fraction on quality of life and pain in a sample of adults with chronic pain. J Transl Med. (2020) 18:401. doi: 10.1186/s12967-020-02569-5
122. Neuhofer A, Zeyda M, Mascher D, Itariu BK, Murano I, Leitner L, et al. Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes. (2013) 62:1945–56. doi: 10.2337/db12-0828
123. Miek L, Jordan PM, Günther K, Pace S, Beyer T, Kowalak D, et al. Staphylococcus aureus controls eicosanoid and specialized pro-resolving mediator production via lipoteichoic acid. Immunology. (2022) 166:47–67. doi: 10.1111/imm.13449
124. Gromovsky AD, Schugar RC, Brown AL, Helsley RN, Burrows AC, Ferguson D, et al. Δ-5 fatty acid desaturase FADS1 impacts metabolic disease by balancing proinflammatory and proresolving lipid mediators. Arterioscler Thromb Vasc Biol. (2018) 38:218–31. doi: 10.1161/ATVBAHA.117.309660
125. Ramsden CE, Faurot KR, Zamora D, Suchindran CM, MacIntosh BA, Gaylord S, et al. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: a randomized trial. Pain. (2013) 154:2441–51. doi: 10.1016/j.pain.2013.07.028
126. Ramsden CE, Faurot KR, Zamora D, Palsson OS, MacIntosh BA, Gaylord S, et al. Targeted alterations in dietary n-3 and n-6 fatty acids improve life functioning and reduce psychological distress among patients with chronic headache: a secondary analysis of a randomized trial. Pain. (2015) 156:587–96. doi: 10.1097/01.j.pain.0000460348.84965.47
127. Jia X, Li Z, Shen X, Zhang Y, Zhang L, and Zhang L. High-intensity swimming alleviates nociception and neuroinflammation in a mouse model of chronic post-ischemia pain by activating the resolvin E1-chemerin receptor 23 axis in the spinal cord. Neural Regener Res. (2023) 18:2535–44. doi: 10.4103/1673-5374.371373
128. Tao X, Luo X, Zhang T, Hershey B, Esteller R, and Ji RR. Spinal cord stimulation attenuates mechanical allodynia and increases central resolvin D1 levels in rats with spared nerve injury. Front Physiol. (2021) 12:687046. doi: 10.3389/fphys.2021.687046
129. Serhan CN, de la Rosa X, and Jouvene C. Novel mediators and mechanisms in the resolution of infectious inflammation: evidence for vagus regulation. J Intern Med. (2019) 286:240–58. doi: 10.1111/joim.12871
130. Abdalla HB, Alvarez C, Wu YC, Rojas P, Hammock BD, Maddipati KR, et al. Soluble epoxide hydrolase inhibition enhances production of specialized pro-resolving lipid mediator and promotes macrophage plasticity. Br J Pharmacol. (2023) 180:1597–615. doi: 10.1111/bph.16009
131. Wedel S, Hahnefeld L, Schreiber Y, Namendorf C, Heymann T, Uhr M, et al. SAFit2 ameliorates paclitaxel-induced neuropathic pain by reducing spinal gliosis and elevating pro-resolving lipid mediators. J Neuroinflammation. (2023) 20:149. doi: 10.1186/s12974-023-02835-5
132. Norling LV, Spite M, Yang R, Flower RJ, Perretti M, and Serhan CN. Cutting edge: Humanized nano-proresolving medicines mimic inflammation-resolution and enhance wound healing. J Immunol. (2011) 186:5543–7. doi: 10.4049/jimmunol.1003865
133. Liu GJ, Tao T, Wang H, Zhou Y, Gao X, Gao YY, et al. Functions of resolvin D1-ALX/FPR2 receptor interaction in the hemoglobin-induced microglial inflammatory response and neuronal injury. J Neuroinflammation. (2020) 17:239. doi: 10.1186/s12974-020-01918-x
134. Panezai J and Van Dyke TE. Resolution of inflammation: Intervention strategies and future applications. Toxicol Appl Pharmacol. (2022) 449:116089. doi: 10.1016/j.taap.2022.116089
135. Balta MG, Schreurs O, Hansen TV, Tungen JE, Vik A, Glaab E, et al. Expression and function of resolvin RvD1(n-3 DPA) receptors in oral epithelial cells. Eur J Oral Sci. (2022) 130:e12883. doi: 10.1111/eos.12883
136. Ervik K, Li YZ, Ji RR, Serhan CN, and Hansen TV. Synthesis of the methyl ester of 17(R/S)-Me-RvD5(n-3 DPA) and relief of postoperative pain in male mice. Org Biomol Chem. (2024) 22:9266–70. doi: 10.1039/D4OB01534G
137. Yang Q, Xu HR, Xiang SY, Zhang C, Ye Y, Shen CX, et al. Resolvin conjugates in tissue regeneration 1 promote alveolar fluid clearance by activating alveolar epithelial sodium channels and na, K-ATPase in lipopolysaccharide-induced acute lung injury. J Pharmacol Exp Ther. (2021) 379:156–65. doi: 10.1124/jpet.121.000712
138. Sekheri M, El Kebir D, Edner N, and Filep JG. 15-Epi-LXA(4) and 17-epi-RvD1 restore TLR9-mediated impaired neutrophil phagocytosis and accelerate resolution of lung inflammation. Proc Natl Acad Sci U S A. (2020) 117:7971–80. doi: 10.1073/pnas.1920193117
139. Dalli J, Winkler JW, Colas RA, Arnardottir H, Cheng CY, Chiang N, et al. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem Biol. (2013) 20:188–201. doi: 10.1016/j.chembiol.2012.11.010
140. Hamaguchi A, Fukuda H, Fujiwara K, Harada T, Fukushima K, Shuto S, et al. Individual resolvin E family members work distinctly and in a coordinated manner in the resolution of inflammation. Prostaglandins Other Lipid Mediat. (2023) 168:106759. doi: 10.1016/j.prostaglandins.2023.106759
141. Chávez-Castillo M, Ortega Á, Cudris-Torres L, Duran P, Rojas M, Manzano A, et al. Specialized pro-resolving lipid mediators: the future of chronic pain therapy? Int J Mol Sci. (2021) 22:10370. doi: 10.3390/ijms221910370
142. Hu X, Zhang YA, Chen B, Jin Z, Lin ML, Li M, et al. Protectin DX promotes the inflammatory resolution via activating COX-2/L-PGDS-PGD(2) and DP(1) receptor in acute respiratory distress syndrome. Int Immunopharmacol. (2022) 102:108348. doi: 10.1016/j.intimp.2021.108348
143. Lv Y, Chen D, Tian X, Xiao J, Xu C, Du L, et al. Protectin conjugates in tissue regeneration 1 alleviates sepsis-induced acute lung injury by inhibiting ferroptosis. J Transl Med. (2023) 21:293. doi: 10.1186/s12967-023-04111-9
144. Jouvene CC, Shay AE, Soens MA, Norris PC, Haeggström JZ, and Serhan CN. Biosynthetic metabolomes of cysteinyl-containing immunoresolvents. FASEB J. (2019) 33:13794–807. doi: 10.1096/fj.201902003R
145. Li H, Hao Y, Yang LL, Wang XY, Li XY, Bhandari S, et al. MCTR1 alleviates lipopolysaccharide-induced acute lung injury by protecting lung endothelial glycocalyx. J Cell Physiol. (2020) 235:7283–94. doi: 10.1002/jcp.29628
146. Zhuang R, Yang X, Cai W, Xu R, Lv L, Sun Y, et al. MCTR3 reduces LPS-induced acute lung injury in mice via the ALX/PINK1 signaling pathway. Int Immunopharmacol. (2021) 90:107142. doi: 10.1016/j.intimp.2020.107142
147. Su C-J, Zhang J-T, Zhao F-L, Xu D-L, Pan J, and Liu T. Resolvin D1/N-formyl peptide receptor 2 ameliorates paclitaxel-induced neuropathic pain through the activation of IL-10/Nrf2/HO-1 pathway in mice. Front Immunol. (2023) 14:1091753. doi: 10.3389/fimmu.2023.1091753
148. Klein CP, Sperotto ND, Maciel IS, Leite CE, Souza AH, and Campos MM. Effects of D-series resolvins on behavioral and neurochemical changes in a fibromyalgia-like model in mice. Neuropharmacology. (2014) 86:57–66. doi: 10.1016/j.neuropharm.2014.05.043
149. Tian Y, Liu Y, Liu C, and Huang S. NPD1 relieves neuropathic pain and accelerates the recovery of motor function after peripheral nerve injury. Pain Res Manage. (2024) 2024:1109287. doi: 10.1155/2024/1109287
150. Wei J, Su W, Zhao Y, Wei Z, Hua Y, Xue P, et al. Maresin 1 promotes nerve regeneration and alleviates neuropathic pain after nerve injury. J Neuroinflammation. (2022) 19:32. doi: 10.1186/s12974-022-02405-1
151. Li Q, Tian Y, Wang ZF, Liu SB, Mi WL, Ma HJ, et al. Involvement of the spinal NALP1 inflammasome in neuropathic pain and aspirin-triggered-15-epi-lipoxin A4 induced analgesia. Neuroscience. (2013) 254:230–40. doi: 10.1016/j.neuroscience.2013.09.028
152. Ferreira MV, Jesus CHA, Bonfim da Costa JP, Oliveira G, Liebl B, Verri Junior W, et al. Aspirin-triggered lipoxin A4 reduces neuropathic pain and anxiety-like behaviours in male diabetic rats: antinociceptive enhancement by cannabinoid receptor agonists. Eur J Pharmacol. (2025) 989:177254. doi: 10.1016/j.ejphar.2025.177254
153. Shih YV, Tao H, Gilpin A, Lee YW, Perikamana SM, and Varghese S. Specialized pro-resolving mediator Maresin 1 attenuates pain in a mouse model of osteoarthritis. Osteoarthritis Cartilage. (2024) 33:341–50. doi: 10.1016/j.joca.2024.10.018
154. Dantas KLS, Bianchini BHS, da Silva MDV, Piva M, da Cunha JM, Zanoveli JM, et al. Maresin 2, a specialized pro-resolution lipid mediator, reduces pain and inflammation induced by bothrops jararaca venom in mice. Toxins (Basel). (2025) 17:367. doi: 10.3390/toxins17080367
Keywords: SPM, pain, resolvin, neuro-immune interactions, resolution of inflammation
Citation: Chen Y, Wu X, Li J, Ren Y, Miao H, Zhai X, Huang C and Chen X (2025) The mechanisms of specialized pro-resolving mediators in pain relief: neuro-immune and neuroglial regulations. Front. Immunol. 16:1634724. doi: 10.3389/fimmu.2025.1634724
Received: 25 May 2025; Accepted: 14 October 2025;
Published: 30 October 2025.
Edited by:
Valerio Chiurchiù, National Research Council (CNR), ItalyReviewed by:
Paul M. Jordan, Friedrich Schiller University Jena, GermanyMegan L. Falsetta, University of Rochester, United States
Copyright © 2025 Chen, Wu, Li, Ren, Miao, Zhai, Huang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaowei Chen, Y2hlbnhpYW93ZWlAbmJ1LmVkdS5jbg==
 Yushan Chen
Yushan Chen Xuewei Wu
Xuewei Wu Jiaqi Li
Jiaqi Li Yuxuan Ren
Yuxuan Ren He Miao
He Miao Xiaojie Zhai
Xiaojie Zhai Changshun Huang
Changshun Huang Xiaowei Chen
Xiaowei Chen