- 1Zoology Department, Faculty of Science, Menoufia University, Shibin El Kom, Egypt
- 2Natural Resources Department, Faculty of African Postgraduate Studies, Cairo University, Giza, Egypt
- 3Clinical Pathology Department, National Liver Institute Hospital, Menoufia University, Shibin El Kom, Egypt
- 4Phytochemistry and Plant Systematics Department, National Research Centre, Giza, Egypt
- 5Department of Biology, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
- 6Zoology and Entomology Department, Faculty of Science, Helwan University, Cairo, Egypt
- 7Al-Ayen Scientific Research Center, Al-Ayen Iraqi University, AUIQ, An Nasiriyah, ThiQar, Iraq
Objective: The existing study sought to highlight the modulatory effect of co-treatment based on grape seed extract (GSE) and L. ascorbic acid (AA) on tumor microenvironment and immune response in murine solid Ehrlich carcinoma (SEC).
Methods: GSE (200 mg / kg; orally) and AA (50 mg/ kg; orally) were given either separately or in a combination for 14 days. GSE active metabolites were identified using GC-MS and LC-MS/MS. Tumor size, Ki-67, Caspase-3, intratumoral infiltrated CD4+, CD8+ and FOXP3+ cells were detected immunohistochemically. Oxidative stress of tumor cells was determined. Serum levels of IL-12, IFN-γ, IL-4 and IL-10 were detected using ELISA.
Results and discussion: The results revealed treatment with GSE and/or AA markedly diminished tumor size, intensified intratumoral oxidative stress, downregulated tumor cell proliferation along with upregulated tumor cells’ apoptosis. GSE and AA enhanced tumor immune microenvironment through increasing CD8+ and CD4+ T cells accompanied by decreasing FOXP3+ Treg cells infiltrated in tumors. GSE and/ or AA moved Th1/Th2 balance in favor of Th1 as evidenced by increased serum levels of IFN-γ and IL-12 accompanied with decreased serum levels of IL-4 and IL-10. These findings may be attributed to the presence of different chemical scaffolds of phenolic acids, Flavan-3-ols and its glycosides, glycerolipids and its glycosides, glycosylated seco-iridoids, dihydrochalcone, stilbenoid, flavone, dihydroxyflavone, and methylated flavone, sugars, and fatty acids. In conclusion, results suggested that dual treatment based on GSE & AA are promising anticancer therapeutics, through their potency to control proliferation, induce apoptosis, intratumoral oxidative stress, modulate tumor immune microenvironment and shifting Th1/Th2 response toward Th1
1 Introduction
Cancer is considered one of the leading causes of death across various communities (1). In the USA alone, it is projected that there will be approximately 2,041,910 new cancer cases and 618,120 cancer-related deaths in 2025 (2). For decades, radiotherapy, chemotherapy, and surgery have remained the standard treatment modalities for cancer (3). However, the effectiveness of chemotherapeutic agents is limited by several factors, including the emergence of drug-resistant cancer cells, low drug sensitivity, and severe side effects (4, 5). These challenges highlight the need to develop novel supplemental or alternative therapeutic strategies to eradicate tumor cells (6, 7).
New anticancer entities are often found in natural materials. Recently, more than 60% of commercial drugs have been derived from natural sources, including bacteria, fungi, plants, and animals (4, 8–11). Food offers additional bioactive substances for promoting health and preventing disease, in addition to the vital nutrients required for life. Consumption of grains, fruits, and vegetables has been strongly linked to a lower risk of numerous serious illnesses, such as cancer (12–14). Combination treatment has shown the best outcomes regarding anticancer properties; its superiority lies in its capacity to target multiple pathways, which effectively decreases drug resistance, as cancer cells often cannot adjust to the concurrent harmful effects of two therapeutic drugs (15). By using treatment combinations to target different pathways, the likelihood of disease control can be increased, and the likelihood of cancer cells becoming more aggressive and incurable reduced. In many instances, combination treatment can also lower the dose requirements for each medication, which lessens adverse effects when compared to monotherapy, although some treatment combinations have been demonstrated to enhance toxicity (16, 17). Combination therapy also has the benefit of targeting all cancer cells that might contribute to drug resistance and cancer recurrence after remission in later years (18, 19).
The grape (Vitis vinifera L.) is one of the most well-known fruit crops worldwide (20), and it has long been valued as a traditional remedy with important uses in curing a range of human ailments. Grapes are among the most commonly cultivated fruit crops and are considered to be the most consumed fruit globally; they can be found growing in many regions (21). Grape seeds, a byproduct of the wine and juice industry, are an excellent source of biologically active substances (22). Approximately, seeds account for 5% of the whole grape weight, representing 40%–50% of the solid waste in grape industries. Grape seeds contain about 60%–70% of the polyphenols, compared to 28%–35% in peels and 10% in fruits (22–24). Antioxidants found in high concentrations in grape seeds, such as phenolic compounds, can reduce the risk of chronic illness by preventing damage caused by free radicals. Epicatechin, epicatechin-3-O-gallate, and catechin, often known as proanthocyanidins (PAs), are abundant in grape seeds. PAs found in grape seeds have anti-inflammatory, antiallergic, and antiarthritic qualities. They also scavenge free oxygen radicals, prevent skin aging, and reduce peroxidation activity linked to UV radiation (25, 26). Improved antioxidant, cardiovascular, anti-inflammatory, antiviral, and anticancer properties have all been attributed to polyphenols (27–29).
Ascorbic acid (vitamin C) is a fundamental micronutrient that humans cannot synthesize on their own due to mutations in the gene that encodes the final enzyme in its biosynthesis route (30, 31). As an essential component of enzymes involved in processes and outcomes critical to cancer development, vitamin C contributes to a variety of processes, including antioxidant defense, transcription, and epigenetic regulation of gene expression (32). Vitamin C has also been found to positively affect the immune system and inflammatory responses, which is important for the host’s ability to fight off precancerous and cancerous cells (33). The over-the-counter medication vitamin C is heavily promoted as a health supplement. It is accessible to the general population in dosages higher than the current Dietary Reference Intake, which is 75 mg for women and 90 mg for men daily. Although there has long been no solid medical evidence supporting high dosages, they have been employed to achieve certain therapeutic outcomes (34).
In this context, this research was performed to explore the potential antitumor and immunomodulatory roles of dual treatment based on red grape seed extract plus vitamin C in a solid Ehrlich carcinoma murine model. To the best of our knowledge, no previous study has addressed the efficacy of dual treatment based on grape seed extract and vitamin C, considering the tumor immune microenvironment and Th1/Th2 response.
2 Materials and methods
2.1 Plant materials and extract production
Grapes were obtained from the local market and identified and authenticated by a professor of Plant Taxonomy, Botany Department, Faculty of Science, Menoufia University. Grape seeds (Vitis vinifera L.) were isolated, left to dry, and ground into a fine powder. A total of 37 g of the powder was macerated and extracted using water. The resulting solution was filtered and evaporated using Rotavapor® (Heidolph, Germany), yielding 2.4763 g of dry extract (~ 6.69%).
2.2 Phytochemical studies
2.2.1 Total phenolic and flavonoid content
The content of phenolic compounds was estimated using gallic acid as a standard at concentrations of 3.125–300 μg mL−1 to construct a calibration curve, with an average R2 = 0.9941, as shown in Figure 1A. The method employed was comparable to that described by Zhang et al. (35). In addition, rutin was used as a standard at concentrations of 50–300 μg mL−1 to construct a calibration curve for determining flavonoid content, with average R2 = 0.9916 (Figure 1B) (36).
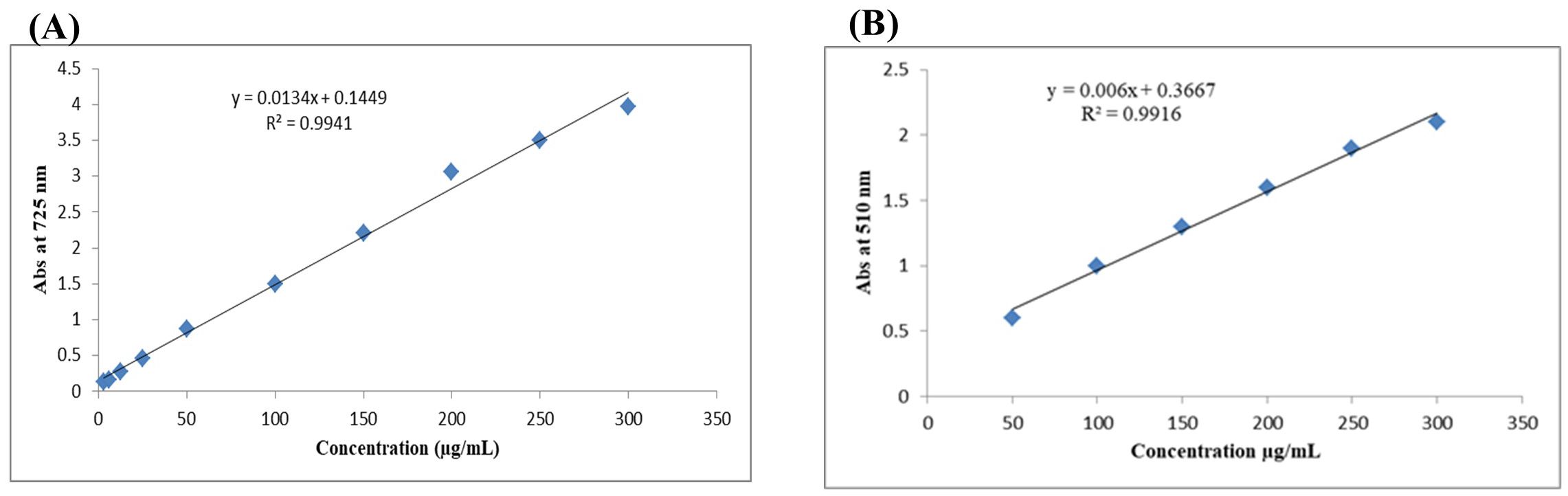
Figure 1. Calibration curves for total phenolic content (TPC) using gallic acid (A) and total flavonoid content (TFC) using rutin (B).
2.2.2 Gas chromatography-mass spectrometry sample derivatization and analysis
Before application to gas chromatography-mass spectrometry (GC-MS), the grape seed extract (GSE) sample was extracted, prepared, and treated with a silylation reagent to derivatize the functional groups into trimethylsilyl (TMS) derivatives (37). A 1-µL aliquot of the sample was then injected into the GC-MS system (Agilent Technologies; GC: 7890B with MS detector; 5977A) at the Central Laboratories Network, National Research Centre, Cairo, Egypt, funded through project number 13060131. The column specifications, carrier gas, flow rate, mass ionization energy (70 eV), temperature, and spectral range were previously described in detail. Constituents were identified by comparing the fragmentation patterns with data from the Wiley and NIST Mass Spectral Libraries.
2.2.3 LC/MS/MS conditions and parameters (instrument, ionization mode)
The GSE was dissolved and analyzed using liquid chromatography–electrospray ionization–tandem mass spectrometry (LC-ESI-MS/MS, SCIEX Triple Quad 5500+ MS/MS). The separation column type, mobile phase gradient, flow rate, ionization mode (negative), ion spray voltage, source temperature, collision energy, and ion source settings were previously described (38, 39).
2.3 Animals
Female Swiss CD1 mice (Mus musculus), aged 6–8 weeks (weighing 26–30 g), were obtained from the National Research Center in Giza, Egypt. All mice were housed under controlled laboratory conditions with a 12-h light/dark cycle. Standard rodent chow and water were made available ad libitum. Prior to the start of the experiments, mice were acclimated to the laboratory environment for 12 days. All animal handling procedures were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Menoufia University, Egypt. The study protocol was approved by the IACUC ethics review board, Faculty of Science (ID: MUFS/F/IM/3/22).
2.4 Reagents and antibodies
Doxorubicin, isoflurane, and l-ascorbic acid were obtained from Sigma (St Louis, MO, USA). ELISA kits for interleukin (IL)-10, IL-4, IL-12, and interferon gamma (IFN-γ) were purchased from CUSABIO (Houston, TX, USA). Monoclonal anti-Ki-67 (Abcam (Cambridge, UK) Cat. No. ab16667, RRID: AB_302459, clone: SP6) and anticaspase-3 (Abcam Cat. No. ab184787, RRID: AB_2827742, clone: EPR18297), antimouse CD4 mAb (BD Biosciences (USA) Cat. No. 553729, RRID: AB_395013, clone: GK1.5), antimouse CD8 mAb (BD Biosciences Cat. No. 550372, RRID: AB_393643), and antimouse FOXP3 mAb (Abcam Cat. No. ab22510, RRID: AB_447114). All other compounds used in this study were of the highest grade.
2.5 Tumor challenge and treatment protocol
Ehrlich ascites carcinoma (EAC) cells were obtained from the National Cancer Institute’s Research Unit at Cairo University, Egypt. The cells were maintained alive by repeated intraperitoneal implantation of 0.5 × 106 viable tumor cells suspended in 0.2 mL of saline into female Swiss CD1 mice. EAC cells were authenticated based on morphological characteristics, and cell viability was assessed using the trypan blue exclusion assay prior to inoculation. Solid Ehrlich carcinoma (SEC) was induced on day 0 by intramuscular (i.m.) injection of EAC cells (2.5 × l06) into the right thigh of the lower limb of each mouse (7). Cell transfers were conducted under fully sterile conditions. Seventy-two apparently healthy female Swiss CD1 mice were randomly divided into eight groups, each containing nine mice, and categorized as follows:
Group I (Naïve): Mice were not given any treatment or tumors.
Group II (AA): Nontumorized mice received 50 mg/kg l-ascorbic acid (40) by oral gavage for 14 successive days.
Group III (GSE): Nontumorized mice were given 200 mg/kg GSE (41) by oral gavage for 14 consecutive days.
Group IV (SEC): SEC-bearing nontreated mice were inoculated i.m. with 2.5 × l06 EAC cells on day 0, as previously mentioned.
Group V (DOX): SEC-bearing mice were injected i.p. with doxorubicin (4 mg/kg b.wt) every 4 days (10th, 14th, 18th, and 22nd days), according to Khedr and Khalil (42).
Group VI (SEC + AA): SEC-bearing mice were administered AA by oral gavage (50 mg/kg b.wt) for 14 successive days from the 10th day till the 24th day as the same as in Group II.
Group VII (SEC +GSE): SEC-bearing mice were administered GSE by oral gavage (200 mg/kg b.wt) for 16 successive days from the 10th day until the 24th day as the same as in Group III.
Group VIII (SEC+GSE+AA): SEC-bearing mice were co-administered with AA (50 mg/kg bwt, orally) and GSE (200 mg/kg b.wt, orally) for 14 successive days from the 10th day till the 24th day as the same as in Groups II and III.
Mice in good general health without prior illness that developed a measurable solid tumor following SEC inoculation were included in the subsequent investigations. Mice that did not develop a measurable solid Ehrlich tumor after cell injection were excluded. Random assignment to treatment groups was performed to ensure unbiased group allocation. To minimize experimental bias, blinding was implemented during data collection and analysis. Investigators responsible for measuring tumor volumes, conducting immunological assays, and performing histopathological evaluations were blinded to the treatment groups. Group identities were revealed only after the completion of all experimental procedures and data analyses.
2.6 Tumor size assessment
A two-end electronic digital caliper (Switzerland) was used to measure the dimensions of the right thigh twice weekly, starting 7 days after tumor induction. Tumor size changes were calculated using the following formula, as described by Goto et al. (43):
Tumor size (mm3) = (Tumor’s higher diameter × Tumor’s lower diameter2)/2.
Furthermore, tumor volume reduction rate(TVRR%) was computed using the formula: TVRR% = the tumor volume reduction rate [(tumor volume in control − tumor volume in treated group)/tumor volume in control group) × 100] (44).
2.7 Sample collection
On day 25, mice were anesthetized with isoflurane, and blood was collected via orbital bleeding. Tumors were excised and divided into two portions. One portion was dissected, weighed, and homogenized in 0.9% normal saline for antioxidant and oxidative stress biomarker determination. The other portion was fixed in 10% neutral formaldehyde for histological and immunohistochemical analyses.
2.8 Oxidative stress and antioxidant markers
Tumor tissue homogenates were used to assess oxidative stress and antioxidant biomarkers. Superoxide dismutase (SOD) activity was determined by measuring superoxide anion levels via nitroblue tetrazoluim formazan color development, according to Masayasu and Hiroshi (45). One unit of SOD activity (defined as the enzyme concentration causing 50% inhibition) corresponds to 7.47 g mL−1 in the reaction mixture. Reduced glutathione (GSH) levels in tumor tissue were measured following the method of Beutler et al. (46). Catalase (CAT) was determined according to Sinha (47). Lipid peroxidation (LPO) content was assessed by measuring thiobarbituric acid-reactive substances, following the method of Yang et al. (48). To quantify the concentration of nitric oxide (NO), the Griess reagent was utilized, as described by Green et al. (49).
2.9 Detection of tumor cell apoptosis, proliferation, and infiltrated immune cells
Tumor tissues fixed in 10% neutral formalin were processed and immunostained for nuclear proliferating protein Ki-67, caspase-3, CD4, CD8, and FOXP3 mAbs, following the manufacturer’s instructions. Immunohistochemical (IHC) staining was performed according to Arriazu et al. (50). Digital IHC images were analyzed using a semiquantitative counting method (Fiji-Image J software, Java-based application for image analysis) (http://imagej.nih.gov/ij/). Six randomly selected high-power fields (× 40) were evaluated to calculate the area fraction, representing the proportion of the immunoreactive area (51).
2.10 Cytokine serum levels
Blood samples were centrifuged at 3,000 × g for 15 min at 4°C. Serum was then isolated and stored at − 80°C. Cytokine levels of IL-4, IL-10, IL-12, and IFN-γ were detected using the ELISA method. Optical density was recorded at a wavelength of 450 nm, and cytokine concentrations were calculated by comparing the optical density to a standard curve.
2.11 Statistical analysis
One-way analysis of variance (ANOVA) followed by Tukey’s post-hoc testing, and two-way ANOVA for tumor size, were used to evaluate the results. When ANOVA assumptions were violated, the Kruskal–Wallis test was applied, with p-values < 0.05 considered statistically significant. Data were plotted using GraphPad Prism version 8.4 for Windows (GraphPad Software, San Diego, CA, USA).
3 Results
3.1 Phytochemical results
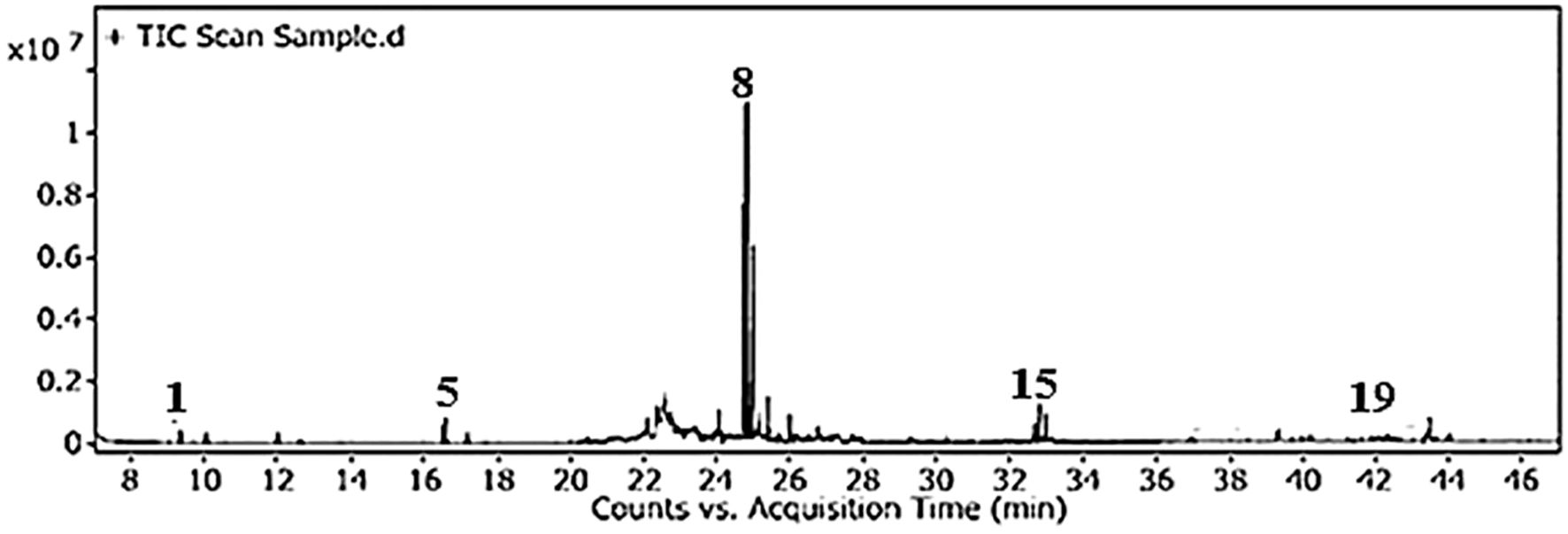
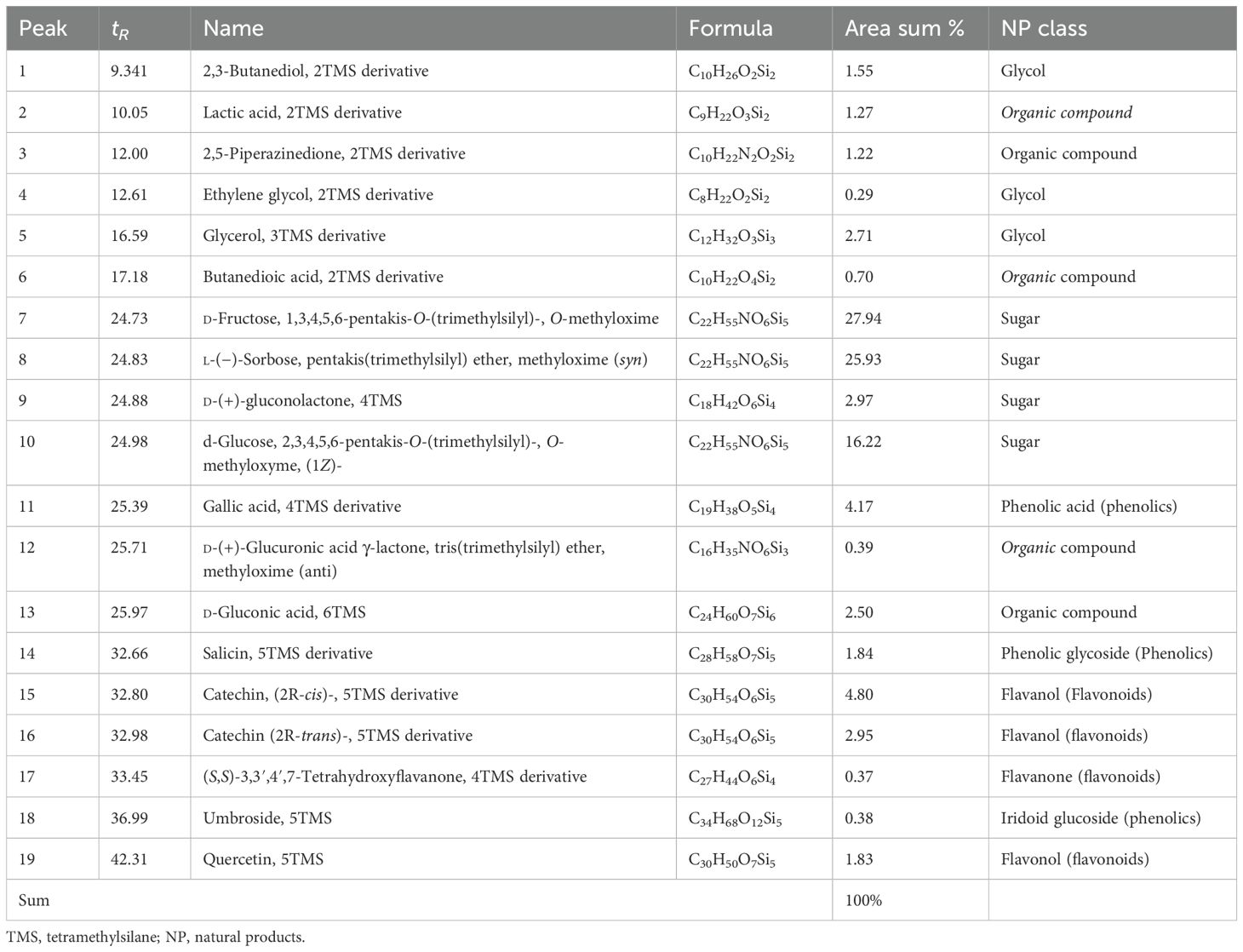
The total phenolic content (TPC) and total flavonoid content (TFC) of GSE were 105.5 mg ± 2.81 mg of gallic acid equivalent/g dry material and 252.1 mg ± 13.63 mg of rutin equivalent/g dry material, respectively. The silylation method was followed by the GC-MS identification of 19 chemicals (Figures 2, 3, Table 1). As shown in Table 1, sugars represented the most abundant group, accounting for 73.05%, while glycol structures were the least abundant at 4.55%. The percentages of organic compounds, phenolics, and flavonoids were 5.68%, 6.78%, and 9.94%, respectively.
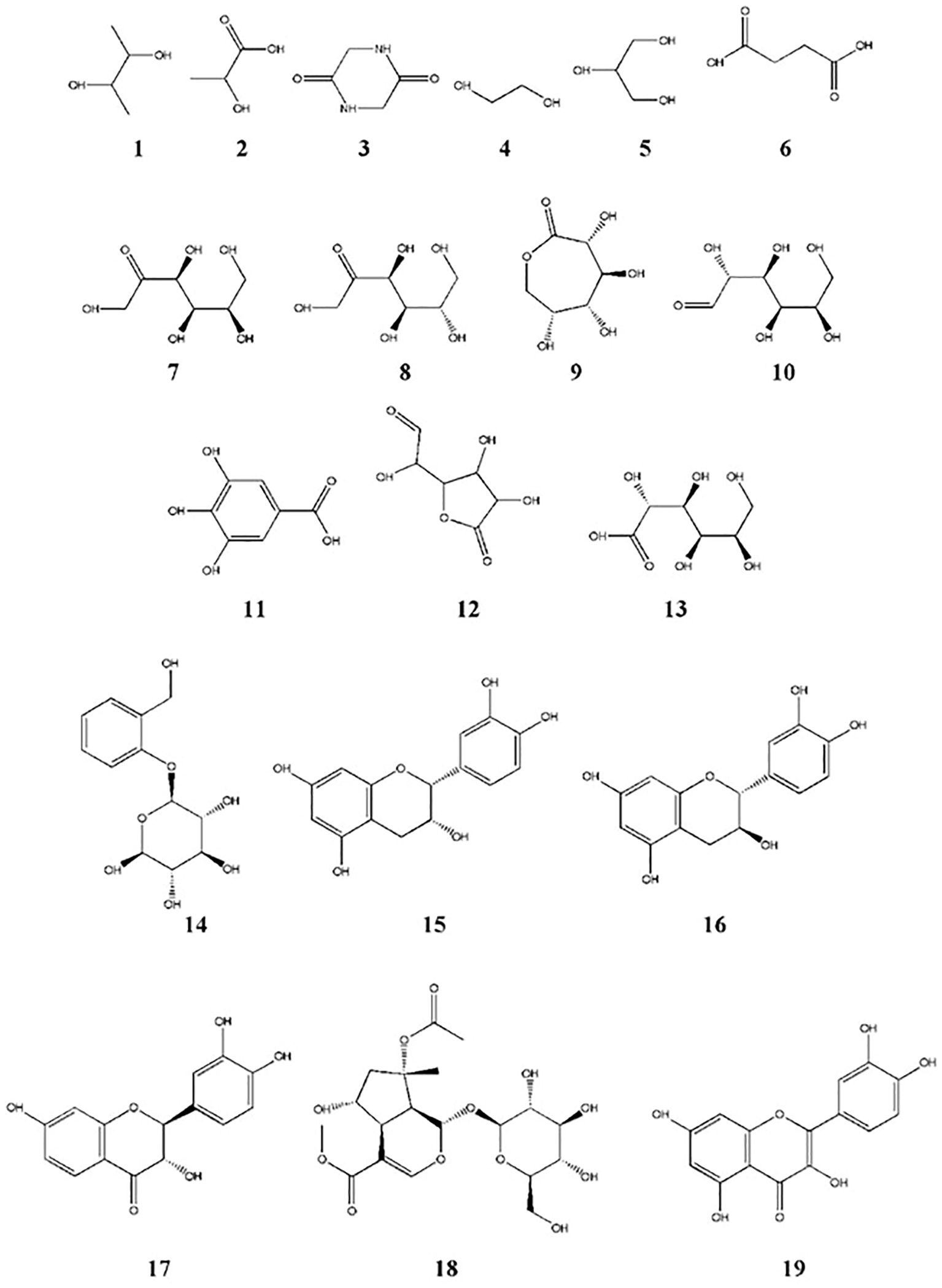
Figure 3. Chemical structures of compounds identified in GSE by GC-MS. Structure numbers correspond to those listed in Table 1.
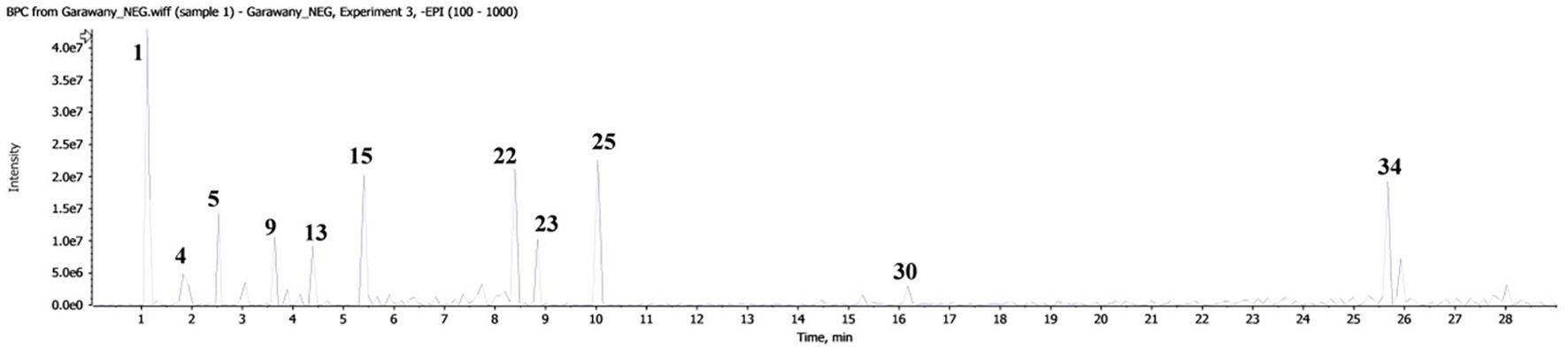
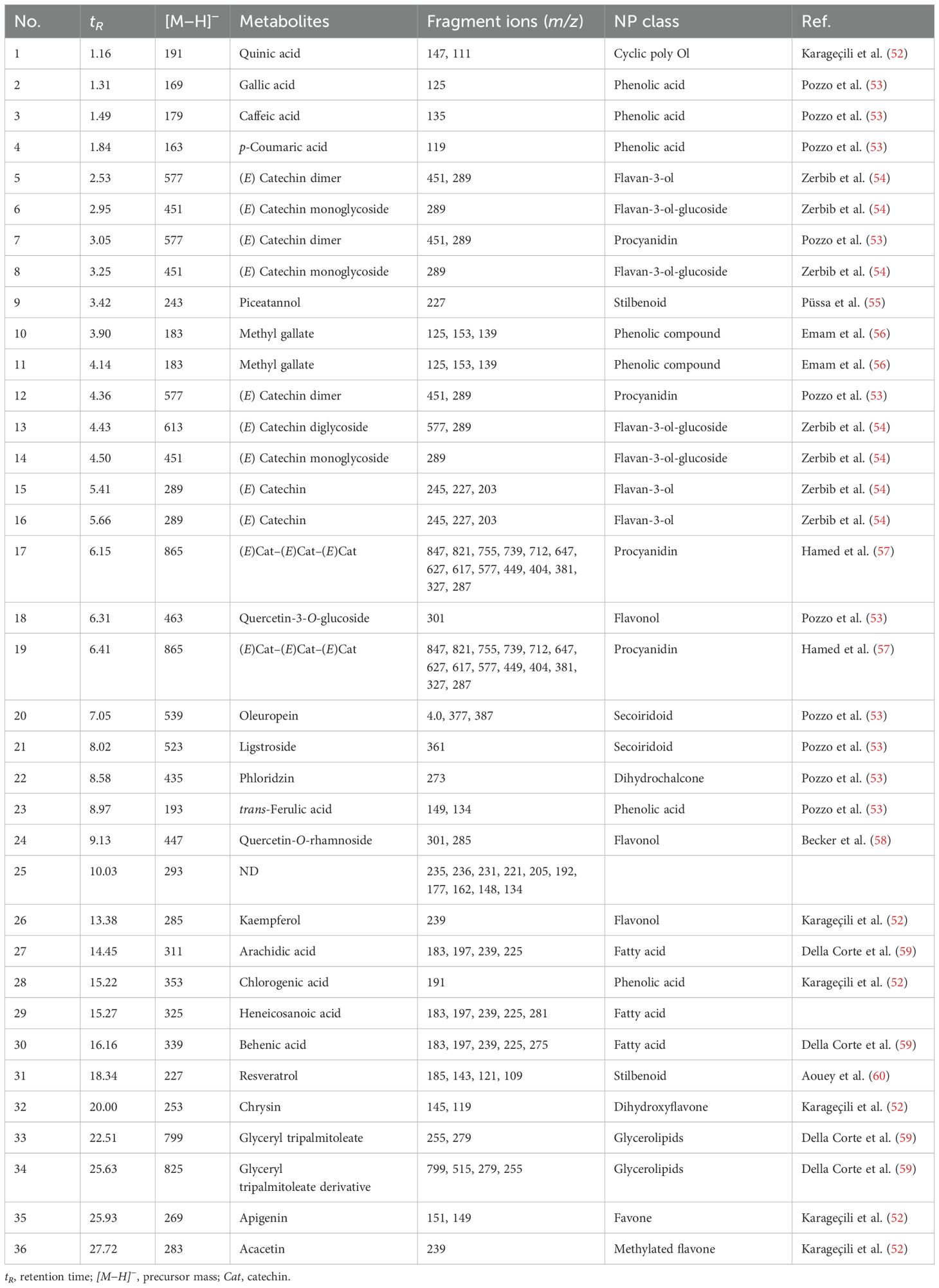
Liquid chromatography coupled with mass spectrometry (Figure 4, Table 2) revealed the presence of various phenolic structures as major secondary metabolites, along with a few fatty acid and glycerolipid structures. Among the phenolics, phenolic acids such as caffeic acid, gallic acid, trans-ferulic acid, and p-coumaric acid were characterized by the loss of 44 Daltons of CO2 moiety. In addition, chlorogenic acid was identified by a fragment peak at m/z 191, corresponding to the quinic moiety. Flavan-3-ol structures—including aglycones, dimers, trimers, and their mono- and diglycosides—were characteristic components of GSE. Flavonol structures were represented by quercetin glycosides and kaempferol aglycone. Glycosylated seco-iridoids, such as oleuropein and ligstroside, were also observed, with precursor masses of m/z 539 and 523, respectively. In addition, different structures of phenolics—such as dihydrochalcone, stilbenoid, flavone, dihydroxyflavone, and methylated flavone—were tentatively identified based on their molecular masses and characteristic fragment ions.
3.2 Grape seed extract and/or ascorbic acid reduced tumor size
SEC-bearing mice that received GSE and AA as single or dual treatments exhibited a significant (p < 0.05) shrinkage in tumor size (Figure 5), accompanied by a marked decrease in mean tumor volume. Tumor growth reduction reached 63.40% (mean tumor volume to 269.14 mm3 ± 13.69 mm3) in SEC-bearing mice mono-treated with GSE and 56.94% in SEC-bearing mice treated with vitamin C only (316.69 mm3 ± 8.74 mm3). Dual treatment in tumor-bearing mice with GSE plus AA resulted in a 76.61% reduction (171.977 mm3 ± 4.151 mm3), which was comparable to the 68.82% reduction in the DOX group (229.27 mm3 ± 6.898 mm3). In contrast, untreated SEC-bearing mice showed no tumor growth reduction, with a mean tumor volume of 735.40 mm³ ± 11.89 mm³.
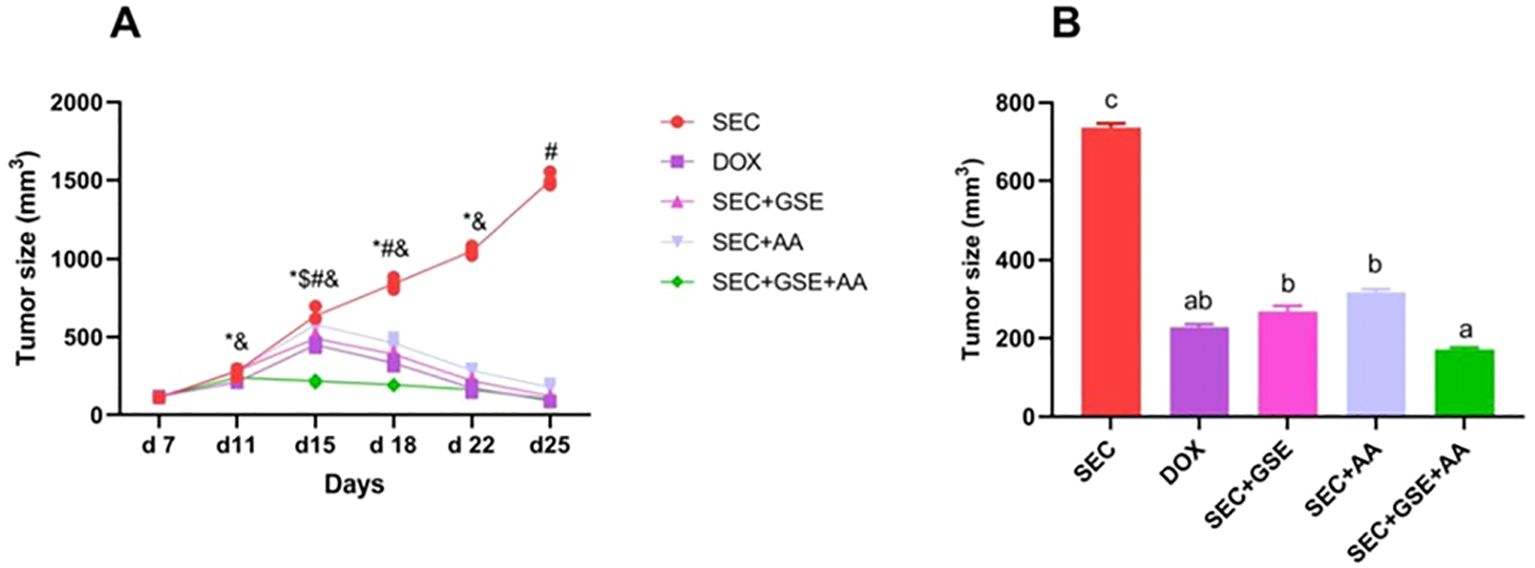
Figure 5. Effect of single and dual treatments with GSE and AA on tumor growth in SEC-bearing mice. (A) Tumor size was measured from days 7 to 25. (B) Final tumor size on day 25. Data are presented as mean ± SD (n = 5). Significant differences are indicated by an asterisk (*) compared to day 7, a dollar sign ($) compared to day 11, a number sign (#) compared to day 22, and an ampersand (&) compared to day 25 (p < 0.05). Different letters indicated importance (p < 0.05). SEC, solid Ehrlich carcinoma; DOX, doxorubicin; GSE, grape seed extract; AA, ascorbic acid.
3.3 Effect of the GSE and/or AA on the oxidative status in tumor tissues of mice with SEC
The effects of GSE and AA, administered as single or dual treatments, on tumor LPO, NO, SOD, CAT, and GSH levels were assessed in treated and control mice (Figure 6). The results showed that dual treatment with GSE and AA induced oxidative stress in solid Ehrlich tumor, as evidenced by a significant (p < 0.05) increase in LPO and NO by 60.8% and 29.44%, respectively. This was accompanied by a significant (p < 0.05) reduction in the enzymatic “CAT and SOD” by 64.899% and 79.697%, respectively, and the nonenzymatic antioxidant “GSH” by 69.29%, compared to the SEC group. Notably, monotherapy with either GSE or AA—particularly GSE—also intensified oxidative stress in tumor cells. GSE monotherapy increased LPO levels by 71.58%, while SOD, CAT, NO, and GSH levels declined by 52.85%, 60.84%, 42.322%, and 58.7%, respectively, compared to the SEC group.
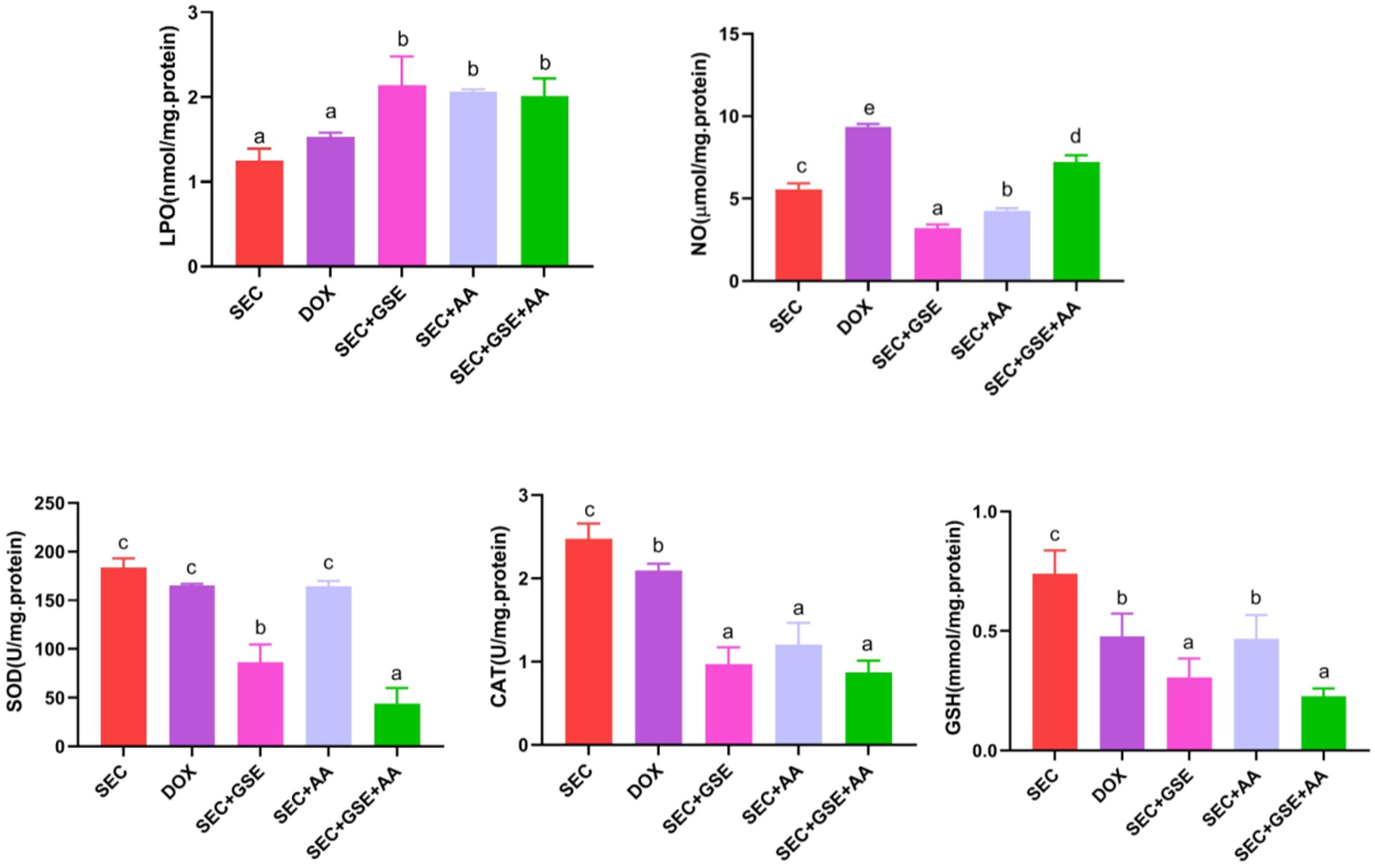
Figure 6. Effect of single and dual treatments with GSE and AA on tumor LPO, CAT, SOD activity, GSH, and NO concentration in SEC-bearing mice. Data are displayed as mean ± SD (n = 5). Different letters indicate statistically significant differences (p < 0.05). NO, nitric oxide; LPO, lipid peroxidase; SOD, superoxide dismutase; CAT, catalase; GSH, reduced glutathione; SEC, solid Ehrlich carcinoma; DOX, doxorubicin; GSE, grape seed extract; AA, ascorbic acid.
3.4 Histopathological alterations
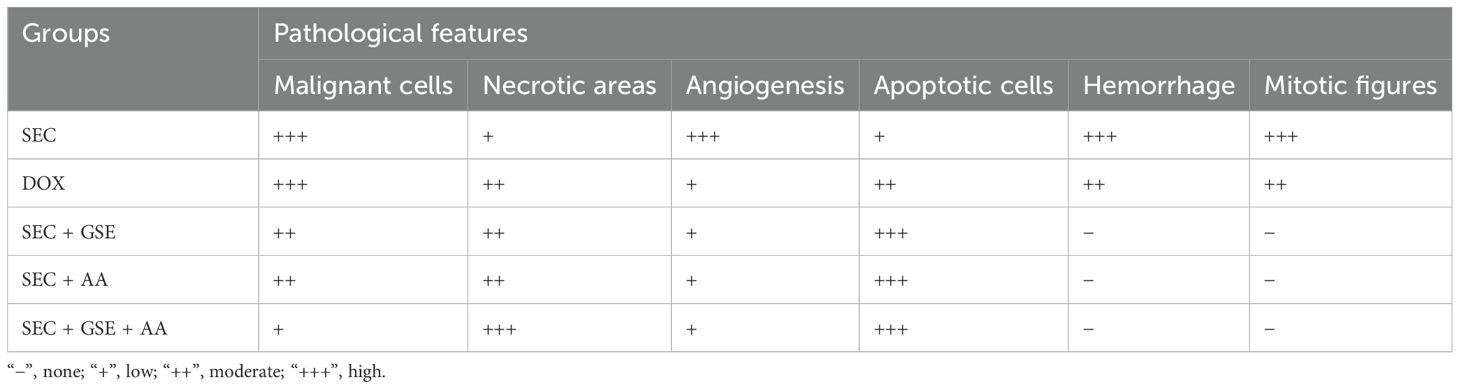
Histopathological examination of the SEC group revealed numerous intramuscular masses of solid tumors in the thigh regions of mice. Tumor sections showed sheets of malignant cells with pleomorphic, hyperchromatic nuclei infiltrating between muscle bundles, necrotic areas, hemorrhage, angiogenesis, and mitotic figures. Mice treated with DOX exhibited necrotic regions and a noticeable presence of apoptotic cells within the tumor muscles. Tumors treated with ascorbic acid showed features of coagulative necrosis and apoptotic cells. In contrast, tumors exposed to GSE demonstrated extensive areas of apoptosis, hemorrhage, necrosis surrounded by fibrous tissue, and the presence of adipocytes. The combined treatment with AA and GSE demonstrated a potent antitumor effect against solid tumors, characterized by widespread areas of complete necrosis, residual tumor cells, cystic structures surrounded by fibrous tissue, and a markedly reduced number of viable tumor cells (Figure 7, Table 3).
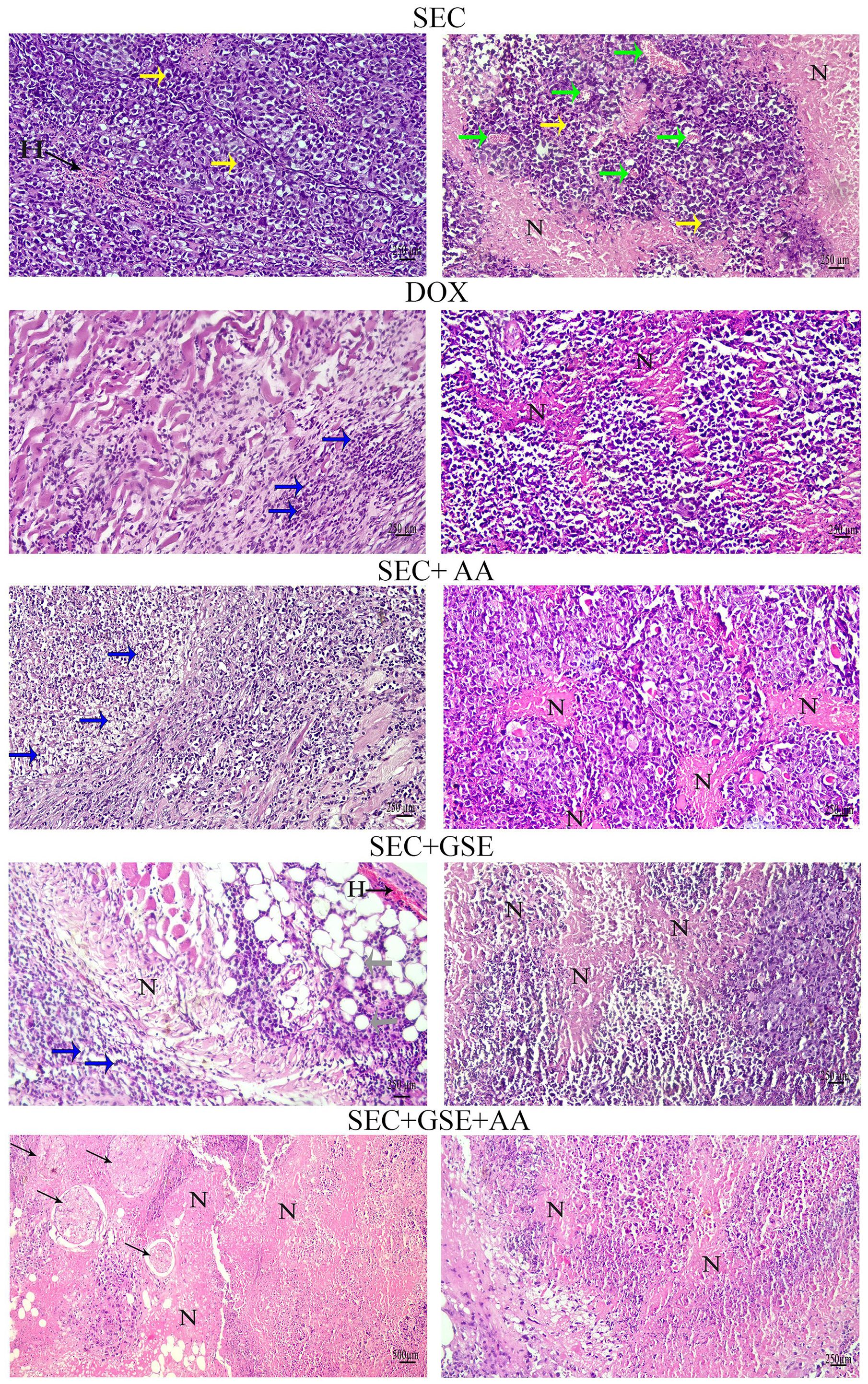
Figure 7. Photomicrographs of tumor sections from mice. Tumors from the SEC group showed sheets of pleomorphic, hyperchromatic malignant cells (yellow arrows), hemorrhage (H), angiogenesis (green arrows), and necrotic areas (N). Tumors from the DOX group showed necrotic areas (N) and apoptotic cells (blue arrows). Tumors from the AA group exhibited necrotic areas (N) and apoptotic cells (blue arrows). Tumor sections from the GSE group displayed extensive apoptotic cells (blue arrows), hemorrhage (H), necrotic areas (N), and adipocytes (gray arrows). The combination group (AA and GSE) showed widespread complete necrosis and cystic cells surrounded by fibers (thin arrows). (H&E stain; scale bar = 500 and 250 µm at × 10 and × 20 magnification).
3.5 Grape seed extract and/or ascorbic acid induced tumor cell apoptosis
Caspase-3 is a critical mediator of apoptosis, activated in the cytoplasm of apoptotic cells via both intrinsic and extrinsic pathways. Herein, caspase-3 expression levels in solid Ehrlich tumor tissues were assessed using IHC staining (Figure 8A). The results showed that single treatment with either GSE or AA significantly (p < 0.05) increased caspase-3 expression by 33.6% and 29.31%, respectively, compared to the SEC group (Figure 9B). Furthermore, dual treatment with GSE and AA resulted in a significant (p < 0.05) elevation in caspase-3 expression by 39.93% and 7.18% relative to the SEC and DOX groups, respectively (Figure 8B).
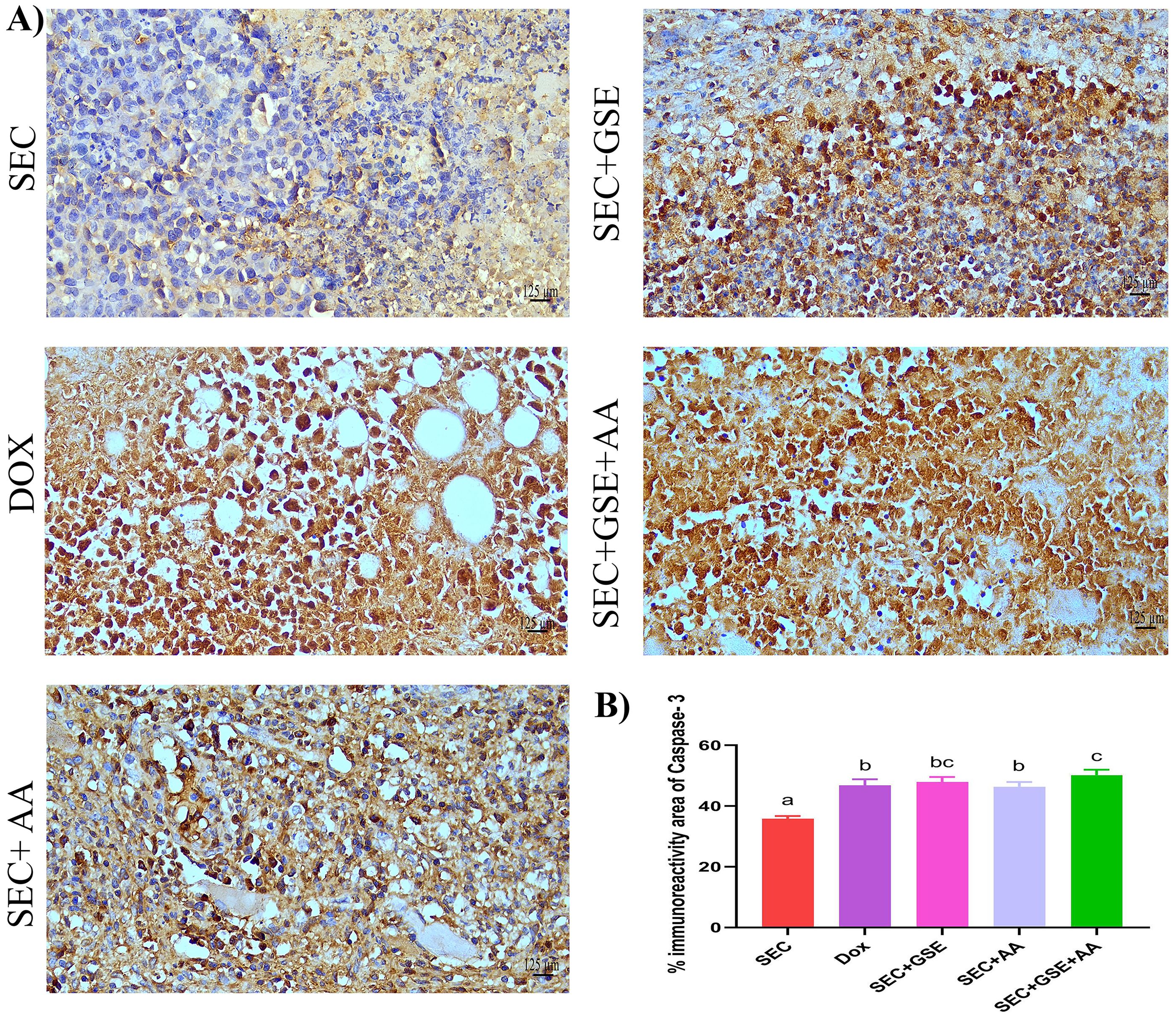
Figure 8. Effect of single and dual treatments with GSE and AA on tumor cell apoptosis in SEC-bearing mice. (A) Representative images of caspase-3 immunoreactivity in tumor tissue. (B) Percentage of caspase-3-positive cells across several experimental groups. Bar graphs represent the mean of six readings, expressed as mean ± SD (n = 5). Different letters indicate statistically significant differences (p < 0.05). Scale bar = 125 µm at × 40 magnification. SEC, solid Ehrlich carcinoma; DOX, doxorubicin; GSE, grape seed extract; AA, ascorbic acid.
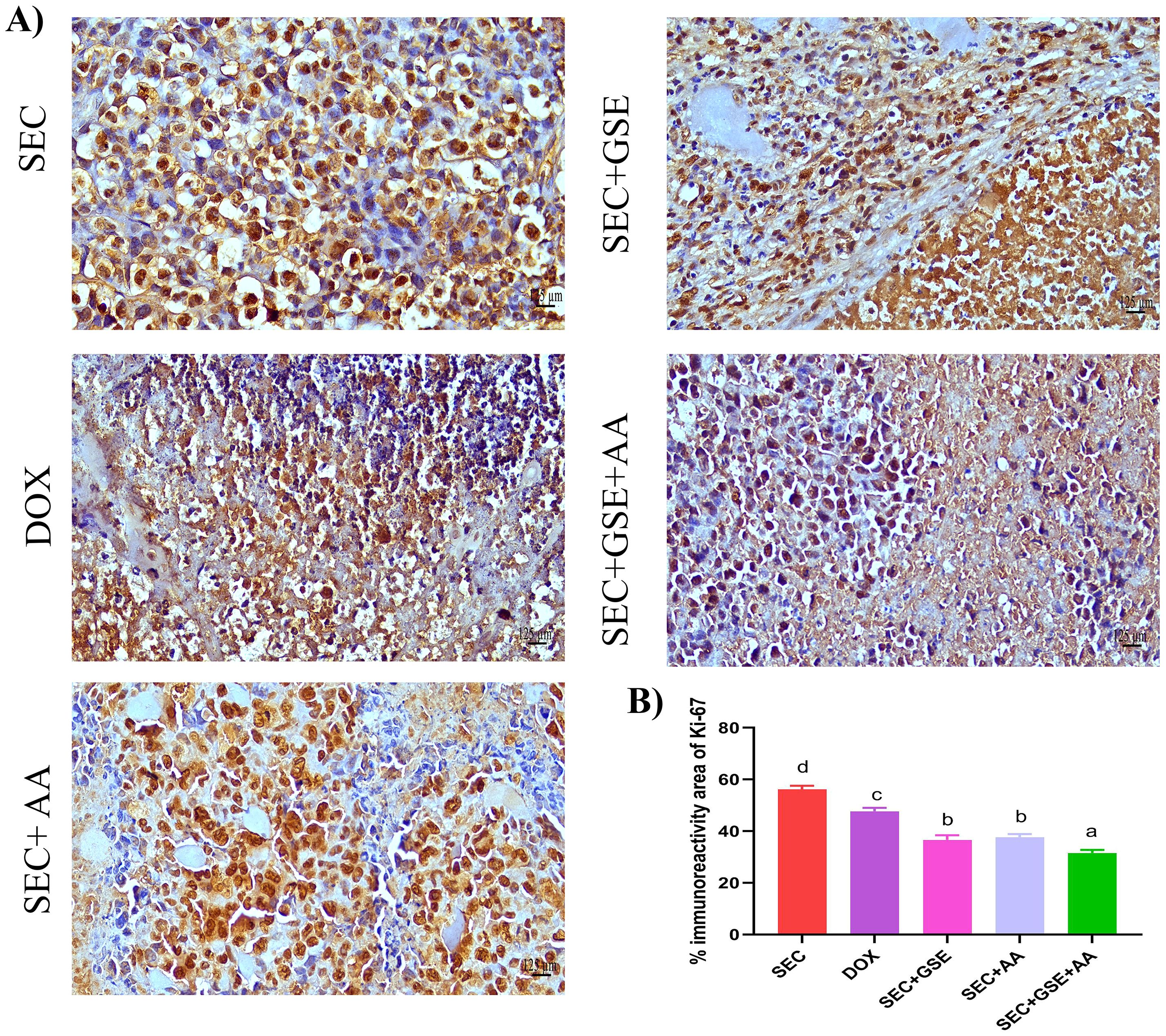
Figure 9. Effect of single and dual treatments with GSE and AA on tumor cell proliferation in SEC-bearing mice. (A) Representative Ki-67 immunoreactivity in tumor tissue. (B) Proportion of Ki-67-positive cells across several experimental groups. Bars represent the mean of six readings, expressed as mean ± SD (n = 5). Different letters indicate statistically significant differences (p < 0.05). Scale bar = 125 µm at × 40 magnification. SEC, solid Ehrlich carcinoma; DOX, doxorubicin; GSE, grape seed extract; AA, ascorbic acid.
3.6 Grape seed extract and ascorbic acid dampened tumor cells’ proliferative capabilities
Ki-67 is a nuclear proliferating marker, and its overexpression is associated with poor prognosis in solid tumors. In this study, Ki-67 expression in tumor tissues was detected using IHC staining (Figure 9A). The results revealed that the highest Ki-67 immunoreactivity was observed in the tumor tissues of mice in the SEC group. Single treatment of SEC-bearing mice with either GSE or AA significantly (p < 0.05) reduced Ki-67 expression by 34.86% and 32.96%, respectively, compared to the SEC group, and by 23.21% and 20.97% compared to the DOX group. Moreover, dual treatment with GSE plus AA led to a significant (p < 0.05) reduction in Ki-67 immunoreactivity by 43.8% and 33.75%, compared to the SEC and DOX groups, respectively (Figure 9B).
3.7 Grape seed extract and vitamin C modulated the immune cell infiltration in tumors
The immunoreactivity of various lymphocyte subsets infiltrating the tumors showed membranous, cytoplasmic, and peritumor staining for FOXP3, CD4, and CD8 surface markers. CD4+ staining was primarily observed on the membranes of helper T lymphocytes (Figure 10A). The results demonstrated that the number of infiltrated CD4+ cells in tumor tissue was significantly increased (p < 0.05) following treatment with GSE, showing a 15.79% rise compared to the SEC group (Figure 10B). CD8+ staining was predominantly located on the membranes of cytotoxic T lymphocytes (Figure 11A). Similar to CD4+ cells, intratumoral CD8+ cell infiltration significantly increased (p < 0.05) after the treatment with GSE and AA by 11.8% and 10.89%, respectively, compared to tumor sections from the SEC group (Figure 11B). FOXP3+ staining was primarily localized in the nuclei of regulatory T lymphocytes (Figure 12A). The results indicated that the number of intratumoral FOXP3+ Treg cells was significantly (p < 0.05) decreased following treatment with GSE and AA by 26.29% and 21.86%, respectively, compared to tumor sections from SEC-bearing mice (Figure 12B). Overall, cotreatment with GSE and AA enhanced the immune competence of the tumor microenvironment. Compared to the SEC group, the combined treatment significantly increased CD4+ and CD8+ cell infiltration by 18.45% and 34.85%, respectively, while significantly decreasing FOXP3+ cell expression by 26.43%.
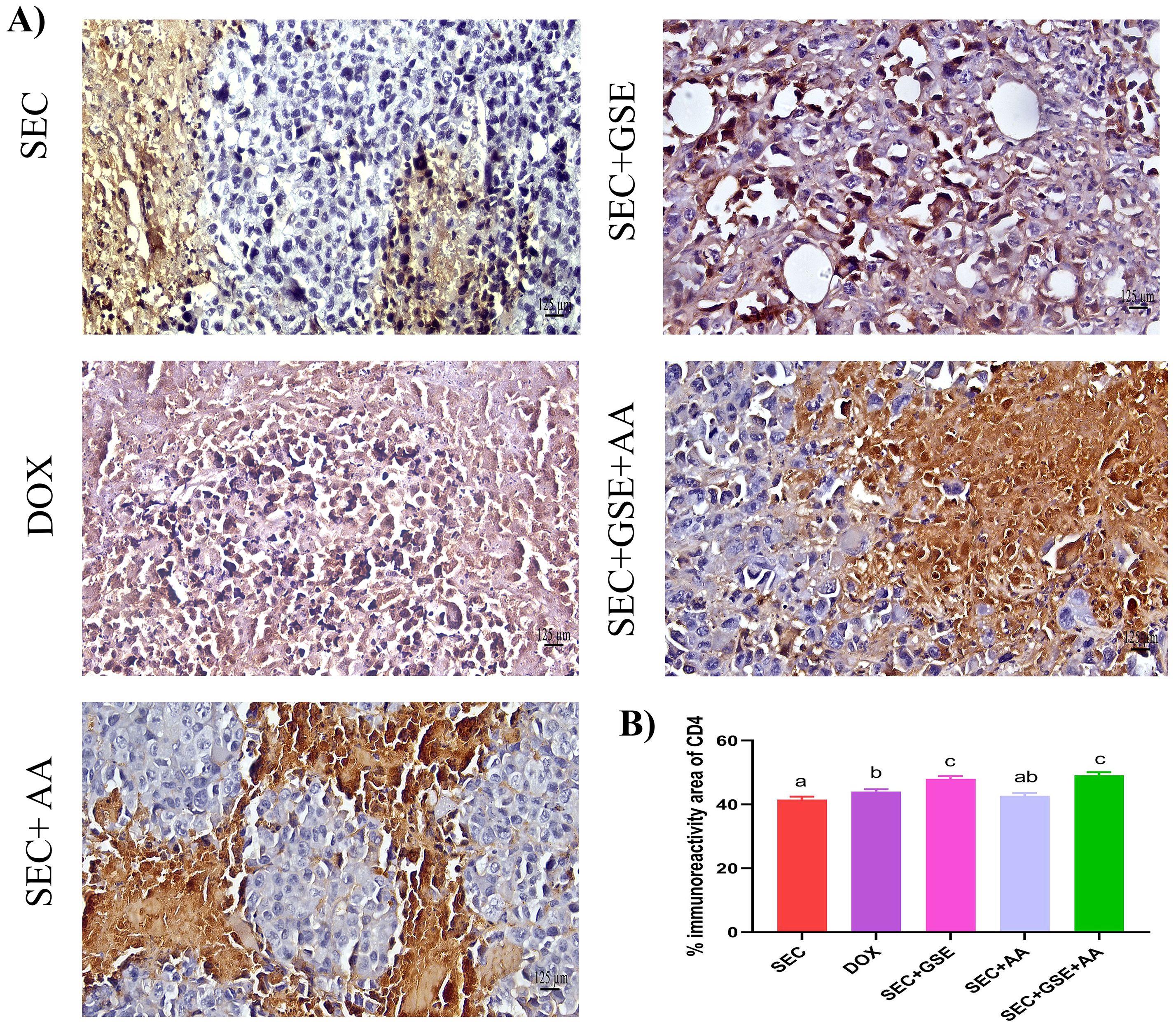
Figure 10. Effect of single and dual treatments with GSE and AA on infiltrated CD4+ cells in tumors of SEC-bearing mice. (A) Representative CD4+ immunoreactivity in tumor sections. (B) Percentage of CD4+-positive cells across several experimental groups. Bars represent the mean of six readings, expressed as mean ± SD (n = 5). Different letters indicate statistically significant differences (p < 0.05). Scale bar = 125 µm at × 40 magnification. SEC, solid Ehrlich carcinoma; DOX, doxorubicin; GSE, grape seed extract; AA, ascorbic acid.
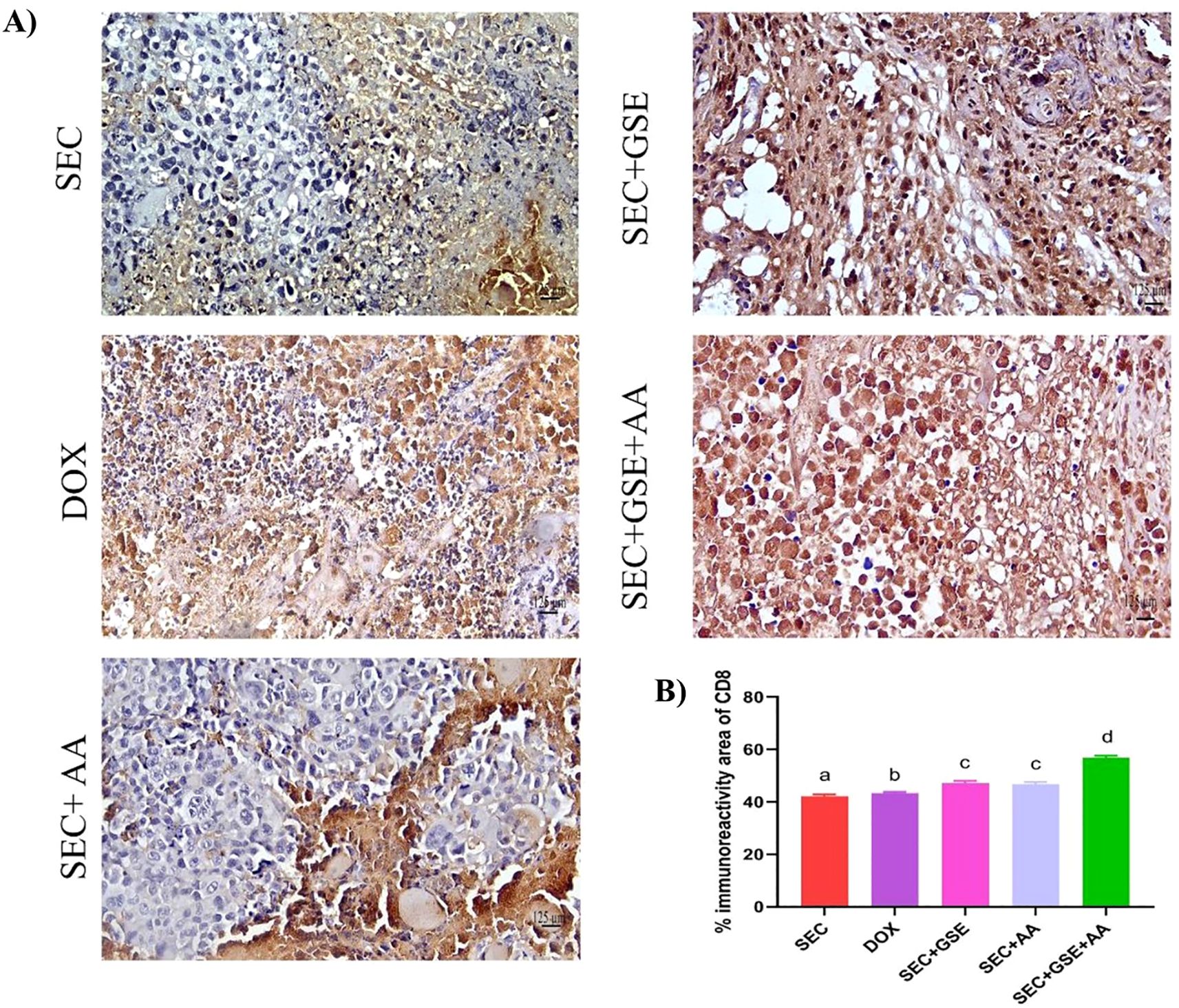
Figure 11. Effect of single and dual treatments with GSE and AA on infiltrated CD8+ cells in tumors of SEC-bearing mice. (A) Representative CD8+ immunoreactivity in tumor sections. (B) Percentage of CD8+ cells across the experimental groups. Bar graphs represent the mean of six readings, expressed as mean ± SD, with a sample size of n = 5. Different letters indicate significances (p < 0.05). Scale bar = 125 µm at × 40 magnification. SEC, solid Ehrlich carcinoma; DOX, doxorubicin; GSE, grape seed extract; AA, ascorbic acid.
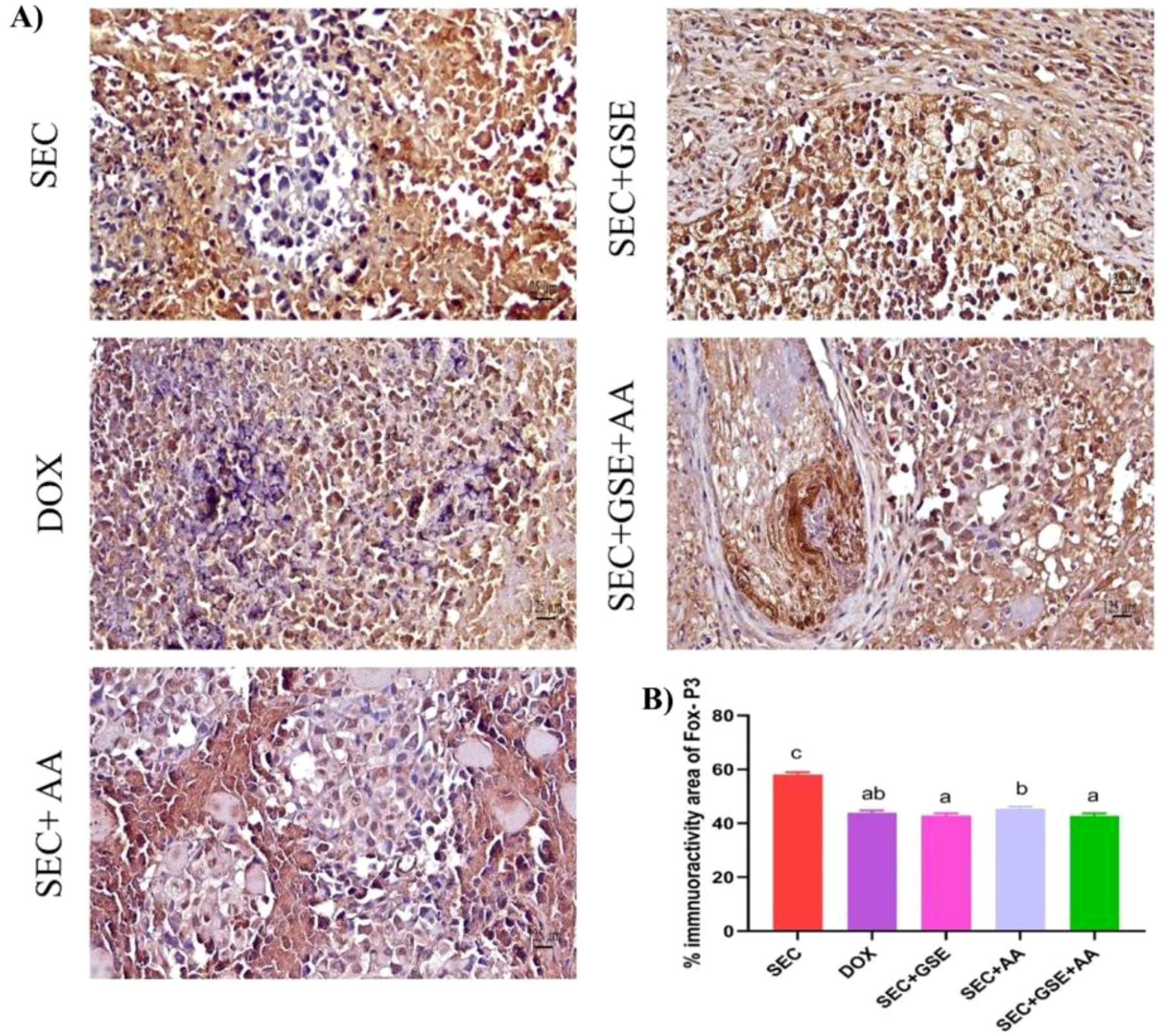
Figure 12. Effect of single and dual treatments with GSE and AA on infiltrated FOXP3+ cells in tumors of SEC-bearing mice. (A) Representative FOXP3 immunoreactivity in tumor sections. (B) Percentage of FOXP3+-positive cells across several experimental groups. Bars represent the mean of six readings, expressed as mean ± SD (n = 5). Different letters indicate statistically significant differences (p < 0.05). Scale bar = 125 µm at × 40 magnification. SEC, solid Ehrlich carcinoma; DOX, doxorubicin; GSE, grape seed extract; AA, ascorbic acid.
3.8 Grape seed extract and/or ascorbic acid boosted Th1/Th2 into a Th1 response
The effects of GSE and/or AA on the T-cell-mediated antitumor immune response were evaluated by measuring Th1 (IFN-γ and IL-12) and Th2 (IL-4 and IL-10) cytokines, as shown in Figure 13. IL-4 and IL-10 levels were significantly decreased in SEC-bearing mice treated with GSE, AA, and or their combination compared to untreated SEC-bearing mice. Specifically, IL-4 levels were 25.2 ± 0.84, 24.4 ± 2.5, and 23 ± 1.58 in mice treated with GSE, AA, or both, respectively, compared to 47.8 ± 2.28 in the untreated SEC-bearing mice. Similarly, IL-10 levels were 27.8 ± 2.17, 11.14 ± 2.05, and 27.4 ± 2.88 in the treated groups, versus 38 ± 2.45 in the untreated SEC-bearing mice. Additionally, increased levels of IL-12 and IFN-γ were observed in all SEC-bearing mice treated with GSE and AA, either as single or dual treatments, compared to untreated SEC-bearing mice. The DOX group showed a significant decrease in IL-4 (31 ± 1.58) and IL-10 (24 ± 2) levels compared to the untreated SEC-bearing mice. Additionally, IL-12 and IFN-γ levels in the DOX group significantly increased to 559.6 ± 22.86 and 556 ± 2.45, respectively, compared to 501.6 ± 5.27 and 443.6 ± 1.82 in the untreated SEC-bearing mice. Notably, the highest and most adverse IL-12 and IFN-γ levels were observed in the dual-treatment group, exceeding those of all other groups. The results indicated that both single and dual treatments with GSE and AA promoted the release of IL-12 and IFN-γ while suppressing the secretion of IL-10 and IL-4. These findings suggest that GSE and AA may shift the Th1/Th2 balance toward a Th1 cell-mediated immune response.
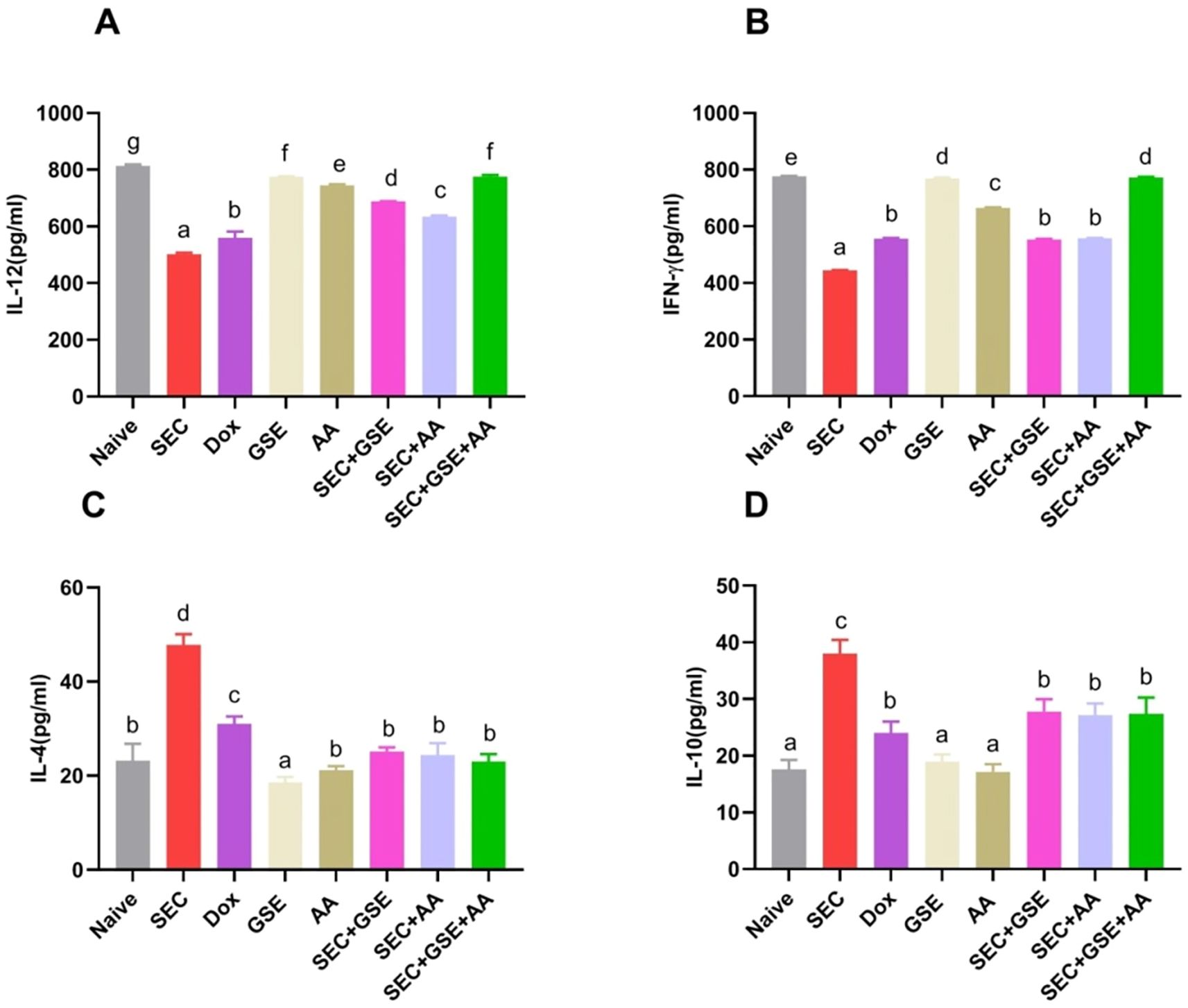
Figure 13. Effect of single and dual treatments with GSE and AA on Th1/Th2 cytokines in SEC-bearing mice. (A, B) Serum levels of Th1 cytokines IL-12 and IFN-γ; (C, D) Serum levels of Th2 cytokines IL-4 and IL-10. Data are presented as mean ± SD (n = 5). Different letters indicate statistically significant differences (p < 0.05). SEC, solid Ehrlich carcinoma; DOX, doxorubicin; GSE, grape seed extract; AA, ascorbic acid.
4 Discussion
Cancer eradication remains one of the most challenging global medical issues, highlighting the urgent need for new alternatives to supplement the limited supply of antitumoral drugs. This study aimed to explore the potential antitumor and immunomodulatory effects of a dual treatment using red grape seed extract combined with vitamin C in a murine model of solid Ehrlich carcinoma.
GC-MS and LC-MS techniques were used to determine the chemical profile of the GSE extract used in the study. This profile was established based on the spectral fragmentation patterns, molecular masses identified, and retention times observed in each chromatogram, allowing compound identification through comparison with literature data. The major compounds detected included gallic acid, catechins, quercetin, caffeic acid, kaempferol, resveratrol, and apigenin—compounds previously reported to possess antioxidant, anti-inflammatory, and antineoplastic propoerties (29, 61–73). The chemical structure of grape seed compounds can be directly linked to their tumor-inhibitory and immune-regulatory effects. Moreover, the micronutrient vitamin C (l-ascorbic acid), essential for numerous physiological functions, has demonstrated cytotoxic against various cancer types. Despite its general nontoxicity, its potential role in cancer prevention and treatment underscores its promise as a lead compound for novel anticancer therapies (74). To the best of our knowledge, this study is the first to evaluate the anticancer efficacyof ascorbic acid, grape seed extract, or their combination in tumor-bearing mice, and to investigate their impact on the tumor immune microenvironment.
In this study, GSE and AA cotreatment showed antineoplastic potencies in SEC-bearing mice by inhibiting cancer growth. A similar pattern was observed in SEC-bearing mice receiving GSE or AA as monotherapy, with no significant difference compared to DOX-treated animals from day 11 until the end of the experiment. The antineoplastic properties of GSE and AA have been previously reported (75–81).
Cancer cells are particularly susceptible to oxidative injury due to their elevated basal levels of ROS (82). Moreover, increased NO levels can be cytotoxic to cancer cells by generating peroxynitrite, a potent inducer of apoptosis and other damaging species involved in immune surveillance (83). In our study, dual treatment with GSE and AA intensified oxidative stress in Ehrlich carcinoma cells by upregulating NO levels and initiating lipid peroxidation, as evidenced by increased LPO levels. This was accompanied by a reduction in GSH content and decreased activities of SOD and CAT. A similar effect was observed with a single treatment using either GSE or AA, with GSE showing greater potency. These outcomes matched those of Shrotriya et al. (75), who reported that GSE caused accumulation of intracellular ROS in HNSCC, which was behind its tumor growth inhibition, DNA destruction, and cell death, and this was dramatically mitigated by N-acetylcysteine. Accordingly, GSE administration was previously evidenced to induce considerable superoxide radical-linked oxidative stress and a large drop in intracellular GSH levels. This suggests that GSE-induced oxidative stress plays a role in its ability to trigger apoptosis against non-small cell lung cancer (84). In context, vitamin C exerts a lethal effect on cancer cells by enhancing oxidative stress via two primary mechanisms: the production of DHA and Fe2+ during its oxidation within the tumor microenvironment (85, 86). Increased levels of labile ferric iron (Fe3+) in the tumor microenvironment can facilitate vitamin C oxidation, resulting in the generation of DHA and ferrous iron (Fe2+). H2O2 reacts with the generated Fe2+ to produce highly reactive hydroxyl radicals (·OH), which can cause direct cell membrane damage via lipid peroxidation (87).
Tumor cells are primarily characterized by uncontrolled and uncontrolled proliferation and evasion of apoptotic processes (88). To determine the mechanisms underlying the antineoplastic potency of GSE- and/or AA-based therapy, SEC-bearing mice were monitored by assessing cancer cell proliferative capacity and apoptotic profile using Ki-67 and caspase-3, respectively. Notably, tumorized mice that received dual treatment with GSE and AA exhibited a significant upregulation of caspase-3 expression, accompanied by a significant downregulation in Ki-67 expression, compared to those in the SEC and DOX groups. Consistently, ascorbic acid induced apoptosis in gastric cancer cells by disrupting mitochondrial function, including ATP consumption, ROS production, and calcium influx (89). Furthermore, vitamin C triggered apoptosis in both nonaggressive and aggressive MCF-7 breast cancer cell lines through oxidative stress generation (90). Similarly, the apoptotic and antiproliferative efficacies of GSE have been reported both in vitro and in vivo against various cancer types, including prostate cancer (91–93), colorectal cancer (41, 94), breast cancer (41, 95), human bladder carcinoma (96), and ovarian cancer (97).
Quercetin, a potent ingredient of GSE, has been reported to promote apoptosis by lowering the expression levels of antiapoptotic proteins and increasing the expression levels of proapoptotic proteins, while also inhibiting tumor cell proliferation and disrupting cell cycle progression in A375SM melanoma cells, A2780S ovarian cancer cells, HL-60 AML cells, and gastric cancer cells (65, 68, 98–101). In addition, gallic acid, another active constituent of GSE, has been shown to exert proapoptotic, antimigratory, and antiproliferative effects against NSCLC both in vivo and in vitro (64). It has been proposed that the antitumor effect of gallic acid is associated with autophagy. Autophagy can induce tumor cell apoptosis by degrading the endoplasmic reticulum, Golgi apparatus, and other cellular components, leading to protein imbalance in cancerous cells and thereby suppressing uncontrolled proliferation (64, 102, 103). Furthermore, Sarı et al. (104) reported the proapoptotic effect of gallic acid in HeLa cancer cells through activation of the p53/Bax signaling pathway. Another constituent of GSE under investigation is catechin, which has also been shown to exert antiproliferative and apoptotic effects against murine lymphoma cells LB02 by increasing Bax expression and downregulating Bcl-2 and survivin (105). Similarly, Dükel et al. (106) reported the apoptotic potency of catechin via upregulation of cell-death-related genes in human colon cancer cells.
Antitumor immunity relies heavily on lymphocytes (107). Improved clinical outcomes and survival in cancer patients are associated with tumor-infiltrating T cells (108, 109). T-cell subpopulations form a complex network within the tumor microenvironment, and the Th1/Th2 balance among these subgroups influences the immune response in malignancy. A shift from a Th1- to a Th2-dominant response is known to promote carcinogenesis (110). To examine how GSE and/or AA affect T cells in the tumor microenvironment, various tumor-infiltrating lymphocyte (TIL) subsets were identified. Both innate and adaptive immunity are essential components of antitumor defense. Cellular and humoral immunity are the two main arms of the acquired immune response. CD8+ and CD4+ T cells play pivotal roles in cellular immunity (111, 112). CD8+ cytotoxic lymphocytes mediate antitumor immunity by recognizing “foreign-looking” antigens on the surface of cancer cells (113). However, their capacity to eliminate tumor cells, secrete inflammatory mediators (such as TNF-α and IFN-γ), proliferate, and form long-term memory cells can be impaired through T-cell receptor desensitization. This desensitization is often driven by the overexpression of inhibitory receptors such as LAG-3, TIGIT, PD-1, and TIM-3 (114). Contrarily, FOXP3+ regulatory T cells contribute to a suppressive tumor microenvironment, facilitating tumor immune evasion (115, 116). The results revealed that dual treatment with GSE and AA considerably increased CD8+ and CD4+ T-cell infiltration, accompanied by a decrease in FOXP3+ Treg cells in the SEC microenvironment. Gallic acid, a constituent of GSE, was found to enhance intratumoral CD8+ T cells while suppressing tumor-infiltrating FOXP3+ Treg cells in a murine model of colorectal carcinoma (117). Notably, ascorbic acid has also been shown to modulate the tumor microenvironment by promoting T-lymphocyte infiltration (118). Moreover, Magrì et al. (119) reported that ascorbic acid can regulate immune cell infiltration into the tumor microenvironment in mice with syngeneic tumors. To further clarify the effects of GSE and/or AA on the Th1/Th2 balance, cytokines released by Th1 and Th2 cells were analyzed. The results showed that dual treatment with GSE and AA led to a remarkable increase in the Th1 cytokines IL-12 and IFN-γ, accompanied by decreased levels of Th2 cytokines IL-4 and IL-10. This indicates that dual treatment shifted the Th1/Th2 balance toward a Th1-dominant response. These findings suggest that GSE and AA inhibit tumor growth by promoting a Th1/Th2 balance in favor of the Th1 response, which is consistent with the findings of Zhao et al. (111). Accordingly, Nair et al. (120) reported that GSE stimulated the Th1 response through the induction of IFN-γ. Similarly, ascorbic acid has previously been reported to modulate Th1/Th2 balance in favor of the Th1 pole (121–125). In addition, Qin et al. (126) demonstrated that ascorbic acid enhanced Th1 immune response and dendritic cell activity in Plasmodium yoelii 17XL-infected mice. Notably, quercetin has also been shown to regulate the Th1/Th2 balance by upregulating Th1 cytokine levels and suppressing Th2 cytokine levels through modulation of T-bet and GATA-3 gene expression in an experimental asthma model (127). Consistently, quercetin and gallic acid have been shown to promote Th1/Th2 balance and regulate Treg/Th17 ratios by activating the NF-κB pathway in allergic diseases (61, 66, 128). Interestingly, GSE and vitamin C can enhance IFN-γ production, indicating a clear shift toward a Th1 response, which activates the cellular immune compartment and facilitates cancer cell destruction through mechanisms that are independent of natural killer (NK) natural killer T (NKT) cells, but dependent on CD8+ T-cell recognition via MHC class I presentation (129, 130). Moreover, IFN-γ promotes a favorable polarization of intratumoral macrophages toward the M1 phenotype, enhancing their ability to eliminate cancer cells (131).
5 Conclusion
The presented findings demonstrate that cotreatments with GSE and/or vitamin C hold promise as an anticancer strategy by reducing tumor size, modulating the intratumoral immune response, inducing oxidative stress, decreasing tumor cell proliferation, and promoting tumor cell death. The authors recommend initiating clinical trials to evaluate human pharmacokinetics, safety, and efficacy, particularly given the use of natural compounds. A key limitation of this study is its focus on short-term tumor progression and acute treatment responses in the solid Ehrlich carcinoma model. As such, long-term therapeutic durability, including tumor recurrence and potential resistance mechanisms, could not be assessed. Future investigations with extended follow-up are necessary to fully characterize the sustained efficacy and biological impact of the treatments in this model. Although the study provides compelling evidence that grape seed extract and l-ascorbic acid exert antineoplastic effects via immunomodulation and alterations in the tumor microenvironment, particularly through modulation of the Th1/Th2 balance, this focus may oversimplify the complex immune dynamics involved in tumor progression. More comprehensive immunological and molecular investigations are needed to elucidate the full spectrum of immune mechanisms underlying these effects.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by The Institutional Animal Care and Use Committee’s (IACUC) guidelines at Menoufia University in Egypt. The IACUC Faculty of Science’s ethics review board has given its approval to the study protocol (ID: MUFS/F/IM/3/22). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
DSM: Conceptualization, Methodology, Validation, Resources, Writing – review & editing. HMRH: Conceptualization, Methodology, Validation, Resources, Writing – review & editing. HSA: Methodology, Validation, Resources, Writing – review & editing. ME: Conceptualization, Investigation, Formal analysis, Visualization, Writing – review & editing. AEA: Validation, Resource acquisition, Writing – review & editing. IME: Conceptualization, Validation, Writing – review & editing. HAAQ: Resources, Formal analysis, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors extend their appreciation to the Princess Nourah bint Abdulrahman University Researchers Supporting Project (No. PNURSP2025R23), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia, for supporting this work.
Acknowledgments
This study was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2025R23), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Also, the authors thank Prof. Zaki Turki, Botany Department, Faculty of Science, Menoufia University, for his assistance with grape identification and authentication. The authors also wish to thank the National Research Centre (NRC), Egypt, for supporting the phytochemical analysis included in this work (Project No. 13060131).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lacombe J, Armstrong MEG, Wright FL, and Foster C. The impact of physical activity and an additional behavioural risk factor on cardiovascular disease, cancer and all-cause mortality: a systematic review. BMC Public Health. (2019) 19:900. doi: 10.1186/s12889-019-7030-8
2. Siegel RL, Kratzer TB, Giaquinto AN, Sung H, and Jemal A. Cancer statistic. CA: A Cancer J Clin. (2025) 75:10–45. doi: 10.3322/caac.21871
3. Abbas Z and Rehman S. An overview of cancer treatment modalities. In: Neoplasm. Great Portland Street, London, W1W 5PF, UNITED KINGDOM: InTech Open (2018) 167–9. doi: 10.5772/intechopen.76558
4. Demain AL and Vaishnav P. Natural products for cancer chemotherapy. Microbiol Technol. (2011) 4:687–99. doi: 10.1111/j.1751-7915.2010.00221.x
5. Padma. An overview of targeted cancer therapy. Biomedicine. (2015) 5:1–6. doi: 10.7603/s40681-015-0019-4
6. Salem ML, Shoukry NM, Teleb WK, Abdel-Daim MM, and Abdel-Rahman MA. In vitro and in vivo antitumor effects of the Egyptian scorpion Androctonus amoreuxi venom in an Ehrlich ascites tumor model. SpringerPlus. (2016) 5:570. doi: 10.1186/s40064-016-2269-3
7. Ibrahim HM, Mohamed AH, Salem ML, Osman GY, and Morsi DS. Anti-neoplastic and immunomodulatory potency of co-treatment based on bovine lactoferrin and/or muramyl dipeptide in tumor-bearing mice. Toxicol Res. (2020) 9:137–47. doi: 10.1093/toxres/tfaa012
8. Cragg GM and Newman DJ. Natural products: A continuing source of novel drug leads. Biochim Biophys Acta (BBA) - Gen Subj. (2013) 1830:3670–95. doi: 10.1016/j.bbagen.2013.02.008
9. Tohamy AA, El-Garawani IM, Ibrahim SR, and Moneim AEA. The apoptotic properties of Salvia aEgyptiaca and Trigonella foenumgraecum extracts on Ehrlich ascites carcinoma cells: The effectiveness of combined treatment. Res J Pharm Biol Chem Sci. (2016) 7:1872–83.
10. El-Seedi HR, Yosri N, El-Aarag B, Mahmoud SH, Zayed A, Du M, et al. Chemistry and the Potential Antiviral, Anticancer, and Anti-Inflammatory Activities of Cardiotonic Steroids Derived from Toads. Molecules. (2022) 27(19):6586. doi: 10.3390/MOLECULES27196586
11. El-Wahed AA, Yosri N, Sakr HH, Du M, Algethami AFM, Zhao C, et al. Wasp Venom Biochemical Components and Their Potential in Biological Applications and Nanotechnological Interventions. Toxins. (2021) 13(3):206. doi: 10.3390/TOXINS13030206
12. Willett WC. Diet and health: what should we eat? Science. (1994) 264:532–7. doi: 10.1126/science.8160011
13. Willett WC. Diet, nutrition, and avoidable cancer. Environ Health Perspect. (1995) 103:165–70. doi: 10.1289/ehp.95103s8165
14. Temple NJ. Antioxidants and disease: More questions than answers. Nutr Res. (2000) 20:449–59. doi: 10.1016/S0271-5317(00)00138-X
15. Zimmermann GR, Lehár J, and Keith CT. Multi-target therapeutics: when the whole is greater than the sum of the parts. Drug Discov Today. (2007) 12:34–42. doi: 10.1016/j.drudis.2006.11.008
16. Delbaldo C, Michiels S, Syz N, Soria J-C, Le Chevalier T, and Pignon J-P. Benefits of adding a drug to a single-agent or a 2-agent chemotherapy regimen in advanced non–small-cell lung cancer. JAMA. (2004) 292:470. doi: 10.1001/jama.292.4.470
17. Parhi P, Mohanty C, and Sahoo SK. Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Discov Today. (2012) 17:1044–52. doi: 10.1016/j.drudis.2012.05.010
18. Mali SB. Cancer treatment: Role of natural products. Time to have a serious rethink. Oral Oncol Rep. (2023) 6:100040. doi: 10.1016/j.oor.2023.100040
19. Wang T, Narayanaswamy R, Ren H, and Torchilin VP. Combination therapy targeting both cancer stem-like cells and bulk tumor cells for improved efficacy of breast cancer treatment. Cancer Biol Ther. (2016) 17:698–707. doi: 10.1080/15384047.2016.1190488
20. FAO statistical database. Available online at: http://www.fao.org/faostat/en/data/QC (Accessed November 20, 2024).
21. Abu-Serie MM and Habashy NH. Vitis vinifera polyphenols from seedless black fruit act synergistically to suppress hepatotoxicity by targeting necroptosis and pro-fibrotic mediators. Sci Rep. (2020) 10:2452. doi: 10.1038/s41598-020-59489-z
22. Abu Hafsa SH and Ibrahim SA. Effect of dietary polyphenol-rich grape seed on growth performance, antioxidant capacity and ileal microflora in broiler chicks. J Anim Physiol Anim Nutr. (2018) 102:268–75. doi: 10.1111/jpn.12688
23. García-Marino M, Rivas-Gonzalo JC, Ibáñez E, and García-Moreno C. Recovery of catechins and proanthocyanidins from winery by-products using subcritical water extraction. Analytica Chimica Acta. (2006) 563:44–50. doi: 10.1016/j.aca.2005.10.054
24. Libera J, Latoch A, and Wójciak KM. Utilization of grape seed extract as a natural antioxidant in the technology of meat products inoculated with a probiotic strain of LAB. Foods. (2020) 9:103. doi: 10.3390/foods9010103
25. Zhao J, Wang J, Chen Y, and Agarwal R. Anti-tumor-promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation–promotion protocol and identification of procyanidin B5-3′-gallate as the most effective antioxidant constituent. Carcinogenesis. (1999) 20:1737–45. doi: 10.1093/carcin/20.9.1737
26. Mojiri-Forushani H, Hemmati A, Khanzadeh A, and Zahedi A. Effectiveness of grape seed extract in patients with nonalcoholic fatty liver: A randomized double-blind clinical study. Hepatitis Monthly. (2022) 22. doi: 10.5812/hepatmon-132309
27. Choi C-S, Chung H-K, Choi M-K, and Kang M-H. Effects of grape pomace on the antioxidant defense system in diet-induced hypercholesterolemic rabbits. Nutr Res Pract. (2010) 4:114. doi: 10.4162/nrp.2010.4.2.114
28. Galanakis CM, Aldawoud TMS, Rizou M, Rowan NJ, and Ibrahim SA. Food ingredients and active compounds against the coronavirus disease (COVID-19) pandemic: A comprehensive review. Foods. (2020) 9:1701. doi: 10.3390/foods9111701
29. Kamah F, Basli A, Erenler R, Bouzana A, Bensouici C, Richard T, et al. Phenolic compounds and biological activities of grape (Vitis vinifera L.) seeds at different ripening stages: insights from Algerian varieties. Arquivo Brasileiro Medicina Veterinária e Zootecnia. (2025) 77. doi: 10.1590/1678-4162-13361
30. Nishikimi M, Fukuyama R, Minoshima S, Shimizu N, and Yagi K. Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. J Biol Chem. (1994) 269:13685–8. doi: 10.1016/S0021-9258(17)36884-9
31. Ara Z, Waliullah S, Rastogi D, Lafi Al-Otaibi M, Pant S, Nawati M, et al. Potential health benefits of vitamin C: an update. IntechOpen. (2025). doi: 10.5772/intechopen.1007674
32. Granger M and Eck P. Dietary Vitamin C in Human Health. Editor(s): Eskin Michael NA Advances in Food and Nutrition Research, Academic Press, (2018) 83:281–310. doi: 10.1016/bs.afnr.2017.11.006.
33. Ang A, Pullar JM, Currie MJ, and Vissers MCM. Vitamin C and immune cell function in inflammation and cancer. Biochem Soc Trans. (2018) 46:1147–59. doi: 10.1042/BST20180169
34. Abdul-Razzak KK, Alzoubi KH, Abdo SA, and Hananeh WM. High-dose vitamin C: Does it exacerbate the effect of psychosocial stress on liver? Biochemical and histological study. Exp Toxicologic Pathol. (2012) 64:367–71. doi: 10.1016/j.etp.2010.09.011
35. Zhang Q, Zhang J, Shen J, Silva A, Dennis DA, and Barrow CJ. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J Appl Phycology. (2006) 18:445–50. doi: 10.1007/s10811-006-9048-4
36. Herald TJ, Gadgil P, and Tilley M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J Sci Food Agric. (2012) 92:2326–31. doi: 10.1002/jsfa.5633
37. Seif M, Aati H, Amer M, Ragauskas AJ, Seif A, El-Sappah AH, et al. Mitigation of Hepatotoxicity via Boosting Antioxidants and Reducing Oxidative Stress and Inflammation in Carbendazim-Treated Rats Using Adiantum Capillus-Veneris L. Extract. Molecules. (2023) 28(12):4720. doi: 10.3390/molecules28124720
38. Asmaey MA. Chemical constituents from Colchicum palaestinum (Baker) C. Archer with the assessment of its antioxidant, wound scratch, and tyrosinase repressive potential. South African Journal of Botany. (2023) 157:209–8.
39. Emam M, El-Newary SA, Aati HY, Wei B, Seif M, and Ibrahim AY. Anti-Alzheimer’s Potency of Rich Phenylethanoid Glycosides Extract from Marrubium vulgare L.: In Vitro and In Silico Studies. Pharmaceuticals. (2024) 17(10):1282. doi: 10.3390/ph17101282
40. Siddique YH, Beg T, and Afzal M. Antigenotoxic effects of ascorbic acid against megestrol acetate-induced genotoxicity in mice. Hum Exp Toxicol. (2005) 24:121–7. doi: 10.1191/0960327104ht508oa
41. Kaur M, Singh RP, Gu M, Agarwal R, and Agarwal C. Grape Seed Extract Inhibits In vitro and In vivo Growth of Human Colorectal Carcinoma Cells. Clin Cancer Res. (2006) 12:6194–202. doi: 10.1158/1078-0432.CCR-06-1465
42. Khedr NF and Khalil RM. Effect of hesperidin on mice bearing Ehrlich solid carcinoma maintained on doxorubicin. Tumor Biol. (2015) 36:9267–75. doi: 10.1007/s13277-015-3655-0
43. Goto T, Nishi T, Tamura T, Dev SB, Takeshima H, Kochi M, et al. Highly efficient electro-gene therapy of solid tumor by using an expression plasmid for the herpes simplex virus thymidine kinase gene. Proceedings of the National Academy of Sciences. (2000) 97(1):354–9. doi: 10.1073/pnas.97.1.354
44. Yeo S-G, Kim DY, Park JW, Oh JH, Kim SY, Chang HJ, et al. Tumor volume reduction rate after preoperative chemoradiotherapy as a prognostic factor in locally advanced rectal cancer. Int J Radiat OncologyBiologyPhysics. (2012) 82:e193–9. doi: 10.1016/j.ijrobp.2011.03.022
45. Masayasu M and Hiroshi Y. A simplified assay method of superoxide dismutase activity for clinical use. Clinica Chimica Acta. (1979) 92:337–42. doi: 10.1016/0009-8981(79)90211-0
46. Beutler E, Duron O, and Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. (1963) 61:882–8.
47. Sinha AK. Colorimetric assay of catalase. Analytical Biochem. (1972) 47:389–94. doi: 10.1016/0003-2697(72)90132-7
48. Yang Y, Cheng J-Z, Singhal SS, Saini M, Pandya U, Awasthi S, et al. Role of glutathione S-transferases in protection against lipid peroxidation. J Biol Chem. (2001) 276:19220–30. doi: 10.1074/jbc.M100551200
49. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, and Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Analytical Biochem. (1982) 126:131–8. doi: 10.1016/0003-2697(82)90118-X
50. Arriazu R, Pozuelo JM, Henriques-Gil N, Perucho T, Martín R, Rodríguez R, et al. Immunohistochemical study of cell proliferation, Bcl-2, p53, and Caspase-3 expression on preneoplastic changes induced by cadmium and zinc chloride in the ventral rat prostate. J Histochem Cytochem. (2006) 54:981–90. doi: 10.1369/jhc.5A6733.2006
51. Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. (2012) 9(7):676–82.
52. Karageçili H, et al. Antioxidant, antidiabetic, antiglaucoma, and anticholinergic effects of Tayfi grape (Vitis vinifera): A phytochemical screening by LC-MS/MS analysis. Open Chemistry. (2023) 21(1):20230120.
53. Pozzo L, et al. Characterization of Antioxidant and Antimicrobial Activity and Phenolic Compound Profile of Extracts from Seeds of Different Vitis Species. Molecules. (2023) 28(13):4924.
54. Zerbib M, et al. Identification and quantification of flavanol glycosides in Vitis vinifera grape seeds and skins during ripening. Molecules. (2018) 23(11):2745.
55. Püssa T, et al. Survey of grapevine Vitis vinifera stem polyphenols by liquid chromatography− diode array detection− tandem mass spectrometry. Journal of Agricultural and Food Chemistry. (2006) 54(20):7488–94.
56. Emam M, et al. Larvicidal Activity of Pentagalloyl Glucose and Mangiferin Isolated from the Waste of Mango Kernel Against Culex pipiens L. Waste and Biomass Valorization. (2022) 13(1):83–93.
57. Hamed AI, et al. Profiles analysis of proanthocyanidins in the argun nut (Medemia argun—an ancient Egyptian palm) by LC–ESI–MS/MS. Journal of Mass Spectrometry. (2014) 49(4):306–15.
58. Becker L, et al. Metabolic study of grapevine leaves infected by downy mildew using negative ion electrospray–Fourier transform ion cyclotron resonance mass spectrometry. Analytica chimica acta. (2013) 795:44–51.
59. Della Corte A, et al. A rapid LC–MS/MS method for quantitative profiling of fatty acids, sterols, glycerolipids, glycerophospholipids and sphingolipids in grapes. Talanta. (2015) 140:52–61.
60. Aouey B, et al. Anti-oxidant, anti-inflammatory, analgesic and antipyretic activities of grapevine leaf extract (Vitis vinifera) in mice and identification of its active constituents by LC–MS/MS analyses. Biomedicine & pharmacotherapy. (2016) 84:1088–98.
61. Fan Y, Piao CH, Hyeon E, Jung SY, Eom J-E, Shin HS, et al. Gallic acid alleviates nasal inflammation via activation of Th1 and inhibition of Th2 and Th17 in a mouse model of allergic rhinitis. Int Immunopharmacol. (2019) 70:512–9. doi: 10.1016/j.intimp.2019.02.025
62. Salehi B, Venditti A, Sharifi-Rad M, Kręgiel D, Sharifi-Rad J, Durazzo A, et al. The therapeutic potential of apigenin. Int J Mol Sci. (2019) 20:1305. doi: 10.3390/ijms20061305
63. Imran M, Salehi B, Sharifi-Rad J, Aslam Gondal T, Saeed F, Imran A, et al. Kaempferol: A key emphasis to its anticancer potential. Molecules. (2019) 24:2277. doi: 10.3390/molecules24122277
64. Tian X, Xu J, Ye Y, Xiao X, Yan L, Yu S, et al. Gallic acid in theabrownin suppresses cell proliferation and migration in non−small cell lung carcinoma via autophagy inhibition. Oncol Lett. (2023) 26:294. doi: 10.3892/ol.2023.13880
65. Chou C-C, Yang J-S, Lu H-F, Ip S-W, Lo C, Wu C-C, et al. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch Pharmacal Res. (2010) 33:1181–91. doi: 10.1007/s12272-010-0808-y
66. Ke X, Chen Z, Wang X, Kang H, and Hong S. Quercetin improves the imbalance of Th1/Th2 cells and Treg/Th17 cells to attenuate allergic rhinitis. Autoimmunity. (2023) 56. doi: 10.1080/08916934.2023.2189133
67. Anwar MJ, Altaf A, Imran M, Amir M, Alsagaby SA, Al Abdulmonem W, et al. Anti-cancer perspectives of resveratrol: a comprehensive review. Food Agric Immunol. (2023) 34. doi: 10.1080/09540105.2023.2265686
68. Kim S-H, Yoo E-S, Woo J-S, Han S-H, Lee J-H, Jung S-H, et al. Antitumor and apoptotic effects of quercetin on human melanoma cells involving JNK/P38 MAPK signaling activation. Eur J Pharmacol. (2019) 860:172568. doi: 10.1016/j.ejphar.2019.172568
69. Mansour MA, Nagi MN, El-Khatib AS, and Al-Bekairi AM. Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and DT-diaphorase in different tissues of mice: a possible mechanism of action. Cell Biochem Funct. (2002) 20:143–51. doi: 10.1002/cbf.968
70. Al-Khayri JM, Sahana GR, Nagella P, Joseph BV, Alessa FM, and Al-Mssallem MQ. Flavonoids as potential anti-inflammatory molecules: A review. Molecules. (2022) 27:2901. doi: 10.3390/molecules27092901
71. Tian C, Liu X, Chang Y, Wang R, Lv T, Cui C, et al. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. South Afr J Bot. (2021) 137:257–64. doi: 10.1016/j.sajb.2020.10.022
72. Intharuksa A, Kuljarusnont S, Sasaki Y, and Tungmunnithum D. Flavonoids and other polyphenols: bioactive molecules from traditional medicine recipes/medicinal plants and their potential for phytopharmaceutical and medical application. Molecules. (2024) 29:5760. doi: 10.3390/molecules29235760
73. Yu X, Jia Y, and Ren F. Multidimensional biological activities of resveratrol and its prospects and challenges in the health field. Front Nutr. (2024) 11:1408651. doi: 10.3389/fnut.2024.1408651
74. Deshmukh SR, Nalkar AS, Sarkate AP, Tiwari SV, Lokwani DK, and Thopate SR. Design, synthesis, and biological evaluation of novel 2,3-Di-O-Aryl/Alkyl sulfonate derivatives of l-ascorbic acid: Efficient access to novel anticancer agents via in vitro screening, tubulin polymerization inhibition, molecular docking study and ADME pre. Bioorganic Chem. (2024) 147:107402. doi: 10.1016/j.bioorg.2024.107402
75. Shrotriya S, Deep G, Gu M, Kaur M, Jain AK, Inturi S, et al. Generation of reactive oxygen species by grape seed extract causes irreparable DNA damage leading to G2/M arrest and apoptosis selectively in head and neck squamous cell carcinoma cells. Carcinogenesis. (2012) 33:848–58. doi: 10.1093/carcin/bgs019
76. Dinicola S, Mariggiò MA, Morabito C, Guarnieri S, Cucina A, Pasqualato A, et al. Grape seed extract triggers apoptosis in Caco-2 human colon cancer cells through reactive oxygen species and calcium increase: extracellular signal-regulated kinase involvement. Br J Nutr. (2013) 110:797–809. doi: 10.1017/S0007114512006095
77. Mahmoud EA. Anticarcinogenic effect of grape seeds extract against ehrlich ascites tumour in mice. Global Veterinaria. (2015) 15:207–14. doi: 10.5829/idosi.gv.2015.15.02.96200
78. Longchar A and Prasad SB. Biochemical changes associated with ascorbic acid–cisplatin combination therapeutic efficacy and protective effect on cisplatin-induced toxicity in tumor-bearing mice. Toxicol Rep. (2015) 2:489–503. doi: 10.1016/j.toxrep.2015.01.017
79. Böttger F, Vallés-Martí A, Cahn L, and Jimenez CR. High-dose intravenous vitamin C, a promising multi-targeting agent in the treatment of cancer. J Exp Clin Cancer Res. (2021) 40:343. doi: 10.1186/s13046-021-02134-y
80. Maekawa T, Miyake T, Tani M, and Uemoto S. Diverse antitumor effects of ascorbic acid on cancer cells and the tumor microenvironment. Front Oncol. (2022) 12:981547. doi: 10.3389/fonc.2022.981547
81. Guo D, Liao Y, Na J, Wu L, Yin Y, Mi Z, et al. The involvement of ascorbic acid in cancer treatment. Molecules. (2024) 29:2295. doi: 10.3390/molecules29102295
82. Arfin S, Jha NK, Jha SK, Kesari KK, Ruokolainen J, Roychoudhury S, et al. Oxidative stress in cancer cell metabolism. Antioxidants. (2021) 10:642. doi: 10.3390/antiox10050642
83. Lee SY, Rim Y, McPherson DD, Huang S-L, and Kim H. A novel liposomal nanomedicine for nitric oxide delivery and breast cancer treatment. Bio-Medical Materials Eng. (2014) 24:61–7. doi: 10.3233/BME-130784
84. Tyagi A, Raina K, Gangar S, Kaur M, Agarwal R, and Agarwal C. Differential effect of grape seed extract against human non-small-cell lung cancer cells: the role of reactive oxygen species and apoptosis induction. Nutr Cancer. (2013) 65:44–53. doi: 10.1080/01635581.2013.785003
85. Ngo B, Van Riper JM, Cantley LC, and Yun J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat Rev Cancer. (2019) 19:271–82. doi: 10.1038/s41568-019-0135-7
86. Bedhiafi T, Inchakalody VP, Fernandes Q, Mestiri S, Billa N, Uddin S, et al. The potential role of vitamin C in empowering cancer immunotherapy. Biomedicine Pharmacotherapy. (2022) 146:112553. doi: 10.1016/j.biopha.2021.112553
87. Torti SV and Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer. (2013) 13:342–55. doi: 10.1038/nrc3495
88. Hanahan D and Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
89. Lim JY, Kim D, Kim BR, Jun JS, Yeom JS, Park JS, et al. Vitamin C induces apoptosis in AGS cells via production of ROS of mitochondria. Oncol Lett. (2016) 12:4270–6. doi: 10.3892/ol.2016.5212
90. Mussa A, Hamid M, Hajissa K, Murtadha AH, Al-Hatamleh MAI, Mokhtar NF, et al. Pharmacological Vitamin C-induced high H2O2 generation mediates apoptotic cell death by caspase 3/7 activation in breast cancer tumor spheroids. J Trans Med. (2025) 23:31. doi: 10.1186/s12967-024-06016-7
91. Dhanalakshmi S, Agarwal R, and Agarwal C. Inhibition of NF-kappaB pathway in grape seed extract-induced apoptotic death of human prostate carcinoma DU145 cells. Int J Oncol. (2003) 23:721–7. doi: 10.3892/ijo.23.3.721
92. Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, and Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int J Cancer. (2004) 108:733–40. doi: 10.1002/ijc.11620
93. Raina K, Singh RP, Agarwal R, and Agarwal C. Oral grape seed extract inhibits prostate tumor growth and progression in TRAMP mice. Cancer Res. (2007) 67:5976–82. doi: 10.1158/0008-5472.CAN-07-0295
94. Engelbrecht A-M, Mattheyse M, Ellis B, Loos B, Thomas M, Smith R, et al. Proanthocyanidin from grape seeds inactivates the PI3-kinase/PKB pathway and induces apoptosis in a colon cancer cell line. Cancer Lett. (2007) 258:144–53. doi: 10.1016/j.canlet.2007.08.020
95. Leone A, Longo C, Gerardi C, and Trosko JE. Pro-apoptotic effect of grape seed extract on MCF-7 involves transient increase of gap junction intercellular communication and cx43 up-regulation: A mechanism of chemoprevention. Int J Mol Sci. (2019) 20:3244. doi: 10.3390/ijms20133244
96. Raina K, Tyagi A, Kumar D, Agarwal R, and A. C. Role of oxidative stress in cytotoxicity of grape seed extract in human bladder cancer cells. Food Chem Toxicol. (2013) 61:187–95. doi: 10.1016/j.fct.2013.06.039
97. Homayoun M, Ghasemnezhad Targhi R, and Soleimani M. Anti-proliferative and anti-apoptotic effects of grape seed extract on chemo-resistant OVCAR-3 ovarian cancer cells. Res Pharm Sci. (2020) 15:390. doi: 10.4103/1735-5362.293517
98. Gao X, Wang B, Wei X, Men K, Zheng F, Zhou Y, et al. Anticancer effect and mechanism of polymer micelle-encapsulated quercetin on ovarian cancer. Nanoscale. (2012) 4:7021. doi: 10.1039/c2nr32181e
99. Catanzaro D, Ragazzi E, Vianello C, Caparrotta L, and M. M. Effect of quercetin on cell cycle and cyclin expression in ovarian carcinoma and osteosarcoma cell lines. Natural Product Commun. (2015) 10:1365–8. doi: 10.1177/1934578X1501000813
100. Lee W-J, Hsiao M, Chang J-L, Yang S-F, Tseng T-H, Cheng C-W, et al. Quercetin induces mitochondrial-derived apoptosis via reactive oxygen species-mediated ERK activation in HL-60 leukemia cells and xenograft. Arch Toxicol. (2015) 89:1103–17. doi: 10.1007/s00204-014-1300-0
101. Zhu Y, Jiang Y, Shi L, Du L, Xu X, Wang E, et al. 7-O-Geranylquercetin induces apoptosis in gastric cancer cells via ROS-MAPK mediated mitochondrial signaling pathway activation. Biomedicine Pharmacotherapy. (2017) 87:527–38. doi: 10.1016/j.biopha.2016.12.095
102. Hait WN, Jin S, and Yang J-M. A matter of life or death (or both): understanding autophagy in cancer. Clin Cancer Res. (2006) 12:1961–5. doi: 10.1158/1078-0432.CCR-06-0011
103. Liu H, Liu S, Qiu X, Yang X, Bao L, Pu F, et al. Donor MSCs release apoptotic bodies to improve myocardial infarction via autophagy regulation in recipient cells. Autophagy. (2020) 16:2140–55. doi: 10.1080/15548627.2020.1717128
104. Sarı U, Zaman F, Özdemir İ, Öztürk Ş, and Tuncer MC. Gallic acid induces heLa cell lines apoptosis via the P53/Bax signaling pathway. Biomedicines. (2024) 12:2632. doi: 10.3390/biomedicines12112632
105. Papademetrio DL, Trabucchi A, Cavaliere V, Ricco R, Costantino S, Wagner ML, et al. The catechin flavonoid reduces proliferation and induces apoptosis of murine lymphoma cells LB02 through modulation of antiapoptotic proteins. Rev Bras Farmacognosia. (2013) 23:455–63. doi: 10.1590/S0102-695X2013005000025
106. Dükel M, Tavsan Z, and Kayali HA. Flavonoids regulate cell death-related cellular signaling via ROS in human colon cancer cells. Process Biochem. (2021) 101:11–25. doi: 10.1016/j.procbio.2020.10.002
107. Mohme M, Riethdorf S, and Pantel K. Circulating and disseminated tumour cells — mechanisms of immune surveillance and escape. Nat Rev Clin Oncol. (2017) 14:155–67. doi: 10.1038/nrclinonc.2016.144
108. Criscitiello C, Esposito A, Trapani D, and Curigliano G. Prognostic and predictive value of tumor infiltrating lymphocytes in early breast cancer. Cancer Treat Rev. (2016) 50:205–7. doi: 10.1016/j.ctrv.2016.09.019
109. de Melo Gagliato D, Cortes J, Curigliano G, Loi S, Denkert C, Perez-Garcia J, et al. Tumor-infiltrating lymphocytes in Breast Cancer and implications for clinical practice. Biochim Biophys Acta (BBA) - Rev Cancer. (2017) 1868:527–37. doi: 10.1016/j.bbcan.2017.10.003
110. Kiyomi A, Makita M, Ozeki T, Li N, Satomura A, Tanaka S, et al. Characterization and clinical implication of Th1/Th2/Th17 cytokines produced from three-dimensionally cultured tumor tissues resected from breast cancer patients. Trans Oncol. (2015) 8:318–26. doi: 10.1016/j.tranon.2015.06.004
111. Zhao X, Liu J, Ge S, Chen C, Li S, Wu X, et al. Saikosaponin A inhibits breast cancer by regulating Th1/Th2 balance. Front Pharmacol. (2019) 10:624. doi: 10.3389/fphar.2019.00624
112. Balta E, Wabnitz GH, and Samstag Y. Hijacked immune cells in the tumor microenvironment: molecular mechanisms of immunosuppression and cues to improve T cell-based immunotherapy of solid tumors. Int J Mol Sci. (2021) 22:5736. doi: 10.3390/ijms22115736
113. Melief CJ and Kast WM. Efficacy of cytotoxic T lymphocytes against virus-induced tumors. Cancer Cells (Cold Spring Harbor N.Y. : 1989). (1990) 2:116–20.
114. Scott AC, Dündar F, Zumbo P, Chandran SS, Klebanoff CA, Shakiba M, et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature. (2019) 571:270–4. doi: 10.1038/s41586-019-1324-y
115. Salmaninejad A, Valilou SF, Shabgah AG, Aslani S, Alimardani M, Pasdar A, et al. PD-1/PD-L1 pathway: Basic biology and role in cancer immunotherapy. J Cell Physiol. (2019) 234:16824–37. doi: 10.1002/jcp.28358
116. Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, and Ohkura N. Regulatory T cells and human disease. Annu Rev Immunol. (2020) 38:541–66. doi: 10.1146/annurev-immunol-042718-041717
117. Deng B, Yang B, Chen J, Wang S, Zhang W, Guo Y, et al. Gallic acid induces T-helper-1-like T reg cells and strengthens immune checkpoint blockade efficacy. J ImmunoTherapy Cancer. (2022) 10:e004037. doi: 10.1136/jitc-2021-004037
118. Luchtel RA, Bhagat T, Pradhan K, Jacobs WR, Levine M, Verma A, et al. High-dose ascorbic acid synergizes with anti-PD1 in a lymphoma mouse model. Proc Natl Acad Sci. (2020) 117:1666–77. doi: 10.1073/pnas.1908158117
119. Magrì A, Germano G, Lorenzato A, Lamba S, Chilà R, Montone M, et al. High-dose vitamin C enhances cancer immunotherapy. Sci Trans Med. (2020) 12. doi: 10.1126/scitranslmed.aay8707
120. Nair N, Mahajan S, Chawda R, Kandaswami C, Shanahan TC, and Schwartz SA. Grape seed extract activates Th1 cells in vitro. Clin Vaccine Immunol. (2002) 9:470–6. doi: 10.1128/CDLI.9.2.470-476.2002
121. Chang H-H, Chen C-S, and Lin J-Y. High dose vitamin C supplementation increases the Th1/Th2 cytokine secretion ratio, but decreases eosinophilic infiltration in bronchoalveolar lavage fluid of ovalbumin-sensitized and challenged mice. J Agric Food Chem. (2009) 57:10471–6. doi: 10.1021/jf902403p
122. Jeong Y-J, Hong S-W, Kim J-H, Jin D-H, Kang JS, Lee WJ, et al. Vitamin C-treated murine bone marrow-derived dendritic cells preferentially drive naïve T cells into Th1 cells by increased IL-12 secretions. Cell Immunol. (2011) 266:192–9. doi: 10.1016/j.cellimm.2010.10.005
123. Ghalibaf MHE, Kianian F, Beigoli S, Behrouz S, Marefati N, Boskabady M, et al. The effects of vitamin C on respiratory, allergic and immunological diseases: an experimental and clinical-based review. Inflammopharmacology. (2023) 31:653–72. doi: 10.1007/s10787-023-01169-1
124. S. N. Evaluation of the efficacy of vitamin C on the immune response after rabies virus vaccine in BALB/c mice. Eur Rev Med Pharmacol Sci. (2023) 27:1808–15. doi: 10.26355/eurrev_202303_31542
125. Sasidharan Nair V and Huehn J. Impact of vitamin C on the development, differentiation and functional properties of T cells. Eur J Microbiol Immunol. (2024) 14:67–74. doi: 10.1556/1886.2024.00017
126. Qin X, Liu J, Du Y, Li Y, Zheng L, Chen G, et al. Different doses of vitamin C supplementation enhances the Th1 immune response to early Plasmodium yoelii 17XL infection in BALB/c mice. Int Immunopharmacol. (2019) 70:387–95. doi: 10.1016/j.intimp.2019.02.031
127. Park H, Lee C-M, Jung ID, Lee JS, Jeong Y, Chang JH, et al. Quercetin regulates Th1/Th2 balance in a murine model of asthma. Int Immunopharmacol. (2009) 9:261–7. doi: 10.1016/j.intimp.2008.10.021
128. Tanaka Y, Furuta A, Asano K, and Kobayashi H. Modulation of Th1/Th2 cytokine balance by quercetin in vitro. Medicines. (2020) 7:46. doi: 10.3390/medicines7080046
129. Lakshmi Narendra B, Eshvendar Reddy K, Shantikumar S, and Ramakrishna S. Immune system: a double-edged sword in cancer. Inflammation Res. (2013) 62:823–34. doi: 10.1007/s00011-013-0645-9
130. Jacobs N, Langers, Renoux, Thiry, and Delvenne. Natural killer cells: role in local tumor growth and metastasis. Biologics: Targets Ther. (2012) 73. doi: 10.2147/BTT.S23976
Keywords: grape seed, vitamin C, solid ehrlich carcinoma, tumor immune microenvironment, Th/Th2 balance
Citation: Morsi DS, Hathout HMR, AboShabaan HS, Emam M, El-khadragy M, Abdel Moneim AE, El-Garawani IM and Abu Quora HA (2025) Grape seed extract and L-ascorbic acid exert antineoplastic effects against solid Ehrlich carcinoma in vivo by modulating the tumor microenvironment and Th1/Th2 balance. Front. Immunol. 16:1635071. doi: 10.3389/fimmu.2025.1635071
Received: 25 May 2025; Accepted: 26 June 2025;
Published: 06 August 2025.
Edited by:
Subhadeep Roy, Mesra, IndiaReviewed by:
Amit Kumar, Albert Einstein College of Medicine, United StatesSapna Jain, Translational Health Science and Technology Institute (THSTI), India
Copyright © 2025 Morsi, Hathout, AboShabaan, Emam, El-khadragy, Abdel Moneim, El-Garawani and Abu Quora. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dalia S. Morsi, ZGFsaWFzYW1pODhAb3V0bG9vay5jb20=; ZGFsaWEuc2FtaUBzY2llbmNlLm1lbm9maWEuZWR1LmVn; Ahmed E. Abdel Moneim, YWVzdDE5NzdAaG90bWFpbC5jb20=; YWhtZWRfYWJkZWxtb25laW1Ac2NpZW5jZS5oZWx3YW4uZWR1LmVn
†These authors have contributed equally to this work
‡ORCID: Heba M. R. Hathout, orcid.org/0000-0002-5084-8603
Hind S. AboShabaan, orcid.org/0009-0007-0390-9454
Islam M El-Garawani, orcid.org/0000-0002-7241-4910
Hagar A. Abu Quora, orcid.org/0000-0003-2613-4210
 Dalia S. Morsi
Dalia S. Morsi Heba M. R. Hathout2‡
Heba M. R. Hathout2‡ Mahmoud Emam
Mahmoud Emam Manal El-khadragy
Manal El-khadragy Ahmed E. Abdel Moneim
Ahmed E. Abdel Moneim