- 1Jiangsu Province Hospital of Chinese Medicine, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China
- 2School of Pharmaceutical Science and Technology, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Hangzhou, China
- 3Department of Oncology, Jinling Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, China
- 4Department of General Surgery, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, Jiangsu, China
Background: Emerging evidence suggests that beta-blockers (BBs) may influence cancer progression by modulating the neuroimmune axis. However, clinical findings remain heterogeneous, necessitating a comprehensive evaluation of their impact on survival outcomes and immune modulation across malignancies.
Methods: We conducted a systematic review and meta-analysis following PRISMA guidelines, analyzing 79 studies from PubMed, Embase, and Web of Science. Pooled hazard ratios (HRs) for overall survival (OS) and cancer-specific survival (CSS) were calculated using random-effects models. Subgroup analyses explored effects by cancer type, BB class (non-selective vs. β1-selective), and concurrent immunotherapy. Immune biomarkers (e.g., PD-L1 expression, tumor-infiltrating lymphocytes) were qualitatively synthesized.
Results: BB use showed no significant overall effect on CSS (HR = 0.97, 95% CI: 0.92–1.02) but exhibited substantial heterogeneity (I² = 80%). Protective associations were observed in breast cancer (HR = 0.27–0.50) and melanoma, while detrimental effects emerged in pancreatic and head/neck cancers (HR > 1.0). Clinically, BBs combined with immune checkpoint inhibitors (ICIs) improved survival (HR=0.91, 95% CI: 0.85–0.98), particularly in PD-L1+ tumors (OR=1.29 for enhanced expression). Non-selective BBs showed stronger immune modulation (CD8+ T-cell SMD=0.49 vs 0.22 for β1-selective).
Conclusion: BBs demonstrate clinically meaningful benefits when combined with immunotherapy (HR=0.91) particularly in β2-AR+ melanoma and breast cancer, but show potential harm in pancreatic/head-neck cancers (HR>1.0). These results support preferential use of propranolol (20-40mg/day) in immunotherapy-treated melanoma, and avoidance of routine BB use in non-immunogenic tumors without adrenergic profiling. Prospective trials should validate these selection criteria.
1 Introduction
The emerging paradigm of cancer neuroimmunology has fundamentally transformed our understanding of tumor biology by revealing the profound influence of neural-immune interactions on cancer progression and therapeutic response. Within this conceptual framework, the sympathetic nervous system (SNS) and its neurotransmitters, particularly catecholamines, have been identified as critical mediators of tumor microenvironment (TME) dynamics (1). Adrenergic signaling, primarily through β-adrenergic receptors (β-ARs), orchestrates bidirectional communication between neural and immune cells, fostering an immunosuppressive niche that facilitates tumor immune evasion, angiogenesis, and metastatic dissemination (2).
Recent studies highlight that chronic stress and SNS overactivation exacerbate cancer progression by upregulating β-AR signaling, which in turn suppresses cytotoxic T-cell activity, promotes myeloid-derived suppressor cell (MDSC) accumulation, and enhances regulatory T-cell (Treg) infiltration (3). Mechanistically, β-adrenergic blockade has been shown to enhance dendritic cell maturation, restore CD8+ T cell effector function, reduce myeloid-derived suppressor cell accumulation, and decrease regulatory T cell infiltration within tumors. These immunomodulatory effects correlate with improved responses to immune checkpoint inhibitors in animal models, suggesting that β-blockers may function as biological response modifiers capable of overcoming resistance to cancer immunotherapy. The pleiotropic actions of β-blockers extend beyond direct immune effects to include inhibition of tumor-associated angiogenesis, reduction of matrix metalloproteinase activity, and suppression of epithelial-mesenchymal transition - all processes known to be influenced by neural signaling pathways (4, 5).
The clinical translation of these preclinical insights has yielded a complex and sometimes contradictory body of evidence regarding β-blockers’ anticancer efficacy (6). Observational studies in breast cancer have consistently shown an association between β-blocker use and reduced recurrence risk, improved metastasis-free survival, and enhanced responses to neoadjuvant chemotherapy (7). Similar benefits have been reported in melanoma, where β-blocker use correlates with improved outcomes in patients receiving immunotherapy. However, other malignancies such as pancreatic cancer and glioblastoma have shown less consistent patterns, with some studies even suggesting potential detrimental effects in certain contexts (8). This heterogeneity may reflect differences in tumor innervation patterns, adrenergic receptor expression profiles, baseline sympathetic tone, or the specific β-blocker compounds used. Additionally, the timing and duration of β-blocker exposure relative to cancer diagnosis and treatment may critically influence outcomes, as the neuroimmune landscape evolves throughout disease progression and therapeutic intervention (9).
The interaction between β-blockers and cancer immunotherapy represents a particularly compelling area of investigation within cancer neuroimmunology. Preclinical data suggest that β-adrenergic blockade can potentiate the effects of PD-1/PD-L1 inhibitors by reversing catecholamine-induced T cell exhaustion and reducing immunosuppressive cytokine production. Clinical studies have reported improved progression-free and overall survival in patients receiving combination therapy compared to immunotherapy alone, though these findings require validation in prospective trials (10). The mechanistic basis for this synergy appears rooted in the ability of β-blockers to mitigate multiple non-redundant immunosuppressive pathways simultaneously, creating a more permissive microenvironment for immune-mediated tumor control. This multimodal immunomodulation distinguishes β-blockers from more targeted immune therapies and positions them as potential broad-spectrum adjuvants to cancer immunotherapy (11, 12).
Critical gaps remain in our understanding of how β-blockers influence the neuroimmune axis across different cancer types and treatment contexts. While the protective effects of β-blockers are most established in highly innervated tumors such as breast and prostate cancers, the generalizability of these findings to less innervated malignancies remains uncertain. Similarly, the optimal timing, duration, and specific β-blocker regimen for maximal neuroimmune modulation have not been systematically investigated. The non-selective β-blocker propranolol has been the most studied in preclinical models due to its ability to block both β1 and β2 receptors, but comparative clinical data across different β-blocker classes are lacking. Furthermore, the potential for β-blockers to exert differential effects based on host factors such as chronic stress levels, genetic polymorphisms in adrenergic receptors, or baseline autonomic tone has not been adequately explored.
The current meta-analysis seeks to address these knowledge gaps by systematically evaluating the impact of β-blockers on survival outcomes across malignancies while specifically examining evidence for immune-mediated mechanisms. By aggregating data from diverse clinical and translational studies, we aim to determine whether the neuroimmunomodulatory effects observed in preclinical models translate into measurable clinical benefits. Particular attention will be paid to studies incorporating biomarkers of neuroimmune activity, such as tumor infiltrating lymphocytes, circulating catecholamines, or gene expression signatures related to adrenergic signaling (13). The analysis will also explore potential effect modifiers including cancer type, stage, β-blocker class and duration, and concurrent therapies. Through this comprehensive synthesis of existing evidence, we hope to clarify β-blockers’ role as modulators of cancer neuroimmunology and identify promising directions for future mechanistic and clinical investigation.
Cancer neuroimmunology represents a frontier in oncology that demands innovative approaches to therapeutic development and clinical trial design. Traditional paradigms that view the nervous and immune systems as separate entities must give way to more integrated models that acknowledge their constant cross-talk in health and disease. β-blockers, as one of the first pharmacologic tools to emerge from this conceptual shift, offer a unique opportunity to test the therapeutic potential of neuroimmune modulation. Their widespread availability, well-characterized safety profile, and low cost make them particularly attractive candidates for clinical translation. However, realizing this potential requires rigorous evidence synthesis to guide appropriate patient selection and treatment protocols. The current meta-analysis represents a critical step in this process, aiming to separate signal from noise in the complex relationship between adrenergic blockade, immune function, and cancer outcomes (5).
The biological rationale for β-blockers in cancer neuroimmunology extends beyond simple receptor antagonism to encompass broader effects on tumor-nerve interactions. Recent studies have revealed that tumors actively remodel their neural microenvironment through the secretion of neurotrophic factors, creating dense networks of adrenergic and sensory fibers that facilitate metastatic spread and therapeutic resistance (2, 8). β-blockers may disrupt this feed-forward cycle by inhibiting nerve sprouting and reducing neurotrophic factor production, thereby normalizing the neural-tumor interface. Simultaneously, their immunomodulatory effects help restore antitumor immunity in what becomes a dual-pronged attack on cancer progression. This multifaceted activity distinguishes β-blockers from more narrowly targeted therapies and may explain their apparent efficacy across diverse cancer types and treatment settings.
Methodological challenges abound in studying neuroimmune interventions like β-blockers, as traditional oncology trial designs often fail to capture the dynamic interplay between neural and immune systems. Most clinical studies to date have relied on incidental β-blocker use rather than prospective randomization, introducing potential confounding by indication. Additionally, few trials have incorporated comprehensive neuroimmune monitoring, making it difficult to establish causal relationships between adrenergic blockade, immune changes, and clinical outcomes. The current meta-analysis will employ advanced techniques to address these limitations, including individual patient data analysis where available and rigorous assessment of study quality. By applying neuroimmunological lenses to existing clinical data, we aim to extract new insights about β-blockers’ mechanisms of action and therapeutic potential (14).
As the field of cancer neuroimmunology advances, it becomes increasingly clear that neural regulation of immunity represents an underutilized therapeutic target in oncology. The sympathetic nervous system functions as a master regulator of immune function, with the capacity to suppress antitumor responses through multiple redundant pathways. β-blockers, by interrupting this neural control, may help “release the brakes” on antitumor immunity in a manner complementary to immune checkpoint inhibitors. Their effects on tumor vasculature, stromal remodeling, and metastatic niche formation further enhance their potential as multifunctional cancer therapeutics. The current systematic review will provide the most comprehensive evaluation to date of whether these promising preclinical observations translate into clinically meaningful benefits for cancer patients, while identifying key knowledge gaps that should guide future research in this emerging field.
2 Method
2.1 Meta-analysis guidelines
This meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to ensure methodological rigor and transparency. A comprehensive search strategy was developed to identify all relevant studies evaluating the impact of β-blockers on survival outcomes and immune modulation in cancer patients.
2.2 Literature search strategy
Our search covered publications from database inception to March 1st, 2024, ensuring inclusion of all relevant studies published during the era of modern cancer immunotherapy. A systematic search was performed in PubMed/MEDLINE, Embase, Web of Science, and Cochrane Library from inception to [insert date]. The search strategy combined Medical Subject Headings (MeSH) terms and keywords related to β-blockers (e.g., “propranolol,” “beta-adrenergic blockade,” “atenolol”), cancer (e.g., “neoplasms,” “tumor,” “oncology”), and neuroimmunology (e.g., “neuroimmune,” “sympathetic nervous system,” “catecholamines”). No language restrictions were applied. While our initial search had no language restrictions, we ultimately excluded 12 non-English articles (9 Chinese, 3 Spanish) due to inability to procure certified translations for full-text review. Additionally, we manually searched reference lists of relevant reviews and conference abstracts from major oncology meetings to identify potentially eligible studies. Two independent reviewers conducted title/abstract screening using Rayyan QCRI software, with conflicts resolved by a third investigator. Full-text review followed a standardized checklist documenting exclusion reasons.
2.3 Study selection criteria
Studies were included if they met the following criteria:
1. Population: Patients with any cancer type (solid or hematologic malignancies).
2. Intervention: Use of β-blockers (any type, dose, or duration) either before or during cancer treatment.
3. Comparator: Patients not receiving β-blockers or receiving placebo.
Outcomes:
1. Primary: Overall survival (OS), progression-free survival (PFS), or disease-free survival (DFS).
2. Secondary: Immune-related outcomes (e.g., tumor-infiltrating lymphocytes [TILs], PD-L1 expression, cytokine levels).
3. Study Design: Randomized controlled trials (RCTs), prospective/retrospective cohort studies, or case-control studies.
Exclusion criteria included:
1. Case reports, editorials, or preclinical studies without clinical data.
2. Studies where β-blocker use was not clearly documented.
3. Duplicate publications or overlapping patient cohorts (only the most comprehensive study was included).
2.4 Data extraction and quality assessment
Two independent reviewers screened titles/abstracts and full texts for eligibility. Discrepancies were resolved by consensus or a third reviewer. Data extraction included:
1. Study characteristics (author, year, design, sample size).
2. Patient demographics (age, cancer type, stage).
3. β-blocker details (type, duration, dosage).
4. Survival outcomes (HR, 95% CI, p-value).
5. Immune biomarkers (if reported).
Study quality was assessed using Newcastle-Ottawa Scale (NOS) for observational studies (evaluating selection, comparability, outcome).
Newcastle-Ottawa Scale (NOS) for observational studies, evaluating three domains with specific criteria:
1. Selection (4 items): Representativeness of exposed cohort, selection of non-exposed cohort, ascertainment of exposure, demonstration that outcome was not present at start.
2. Comparability (2 items): Control for confounders (age, stage, comorbidities) and additional factors (treatment type, β-blocker duration).
3. Outcome (3 items): Outcome assessment method, follow-up length (>12 months required for full score), adequacy of follow-up (>80% retention).
2.5 Statistical analysis
For the primary meta-analysis, pooled hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) for overall survival (OS) and progression-free survival (PFS) were derived using random-effects models with the DerSimonian-Laird method to account for potential between-study heterogeneity. Subgroup analyses were performed to explore differential effects based on cancer type (e.g., breast cancer, melanoma, lung cancer), β-blocker class (non-selective agents like propranolol versus β1-selective agents like metoprolol), and concurrent immunotherapy administration (yes/no). Heterogeneity across studies was quantitatively assessed using I² statistics, with I² values exceeding 50% considered indicative of substantial heterogeneity. Publication bias was evaluated through visual inspection of funnel plots supplemented by Egger’s regression test for small-study effects. To examine the robustness of the primary findings, sensitivity analyses were conducted by systematically excluding studies judged to be at high risk of bias based on predefined quality assessment criteria.
2.6 Assessment of immune modulation
For studies reporting immune biomarkers, qualitative synthesis was performed. Where feasible, meta-analysis of immune cell densities (e.g., CD8+ T cells) or cytokine levels was conducted using standardized mean differences (SMDs).
2.7 Ethical considerations
All included studies were screened for ethical approval statements. As this study used aggregated data from published literature, institutional review board approval was not required.
2.8 Software
All analyses were performed using RevMan 5.4 (Cochrane Collaboration) and Stata 17.0.
3 Result
3.1 Study selection and characteristics
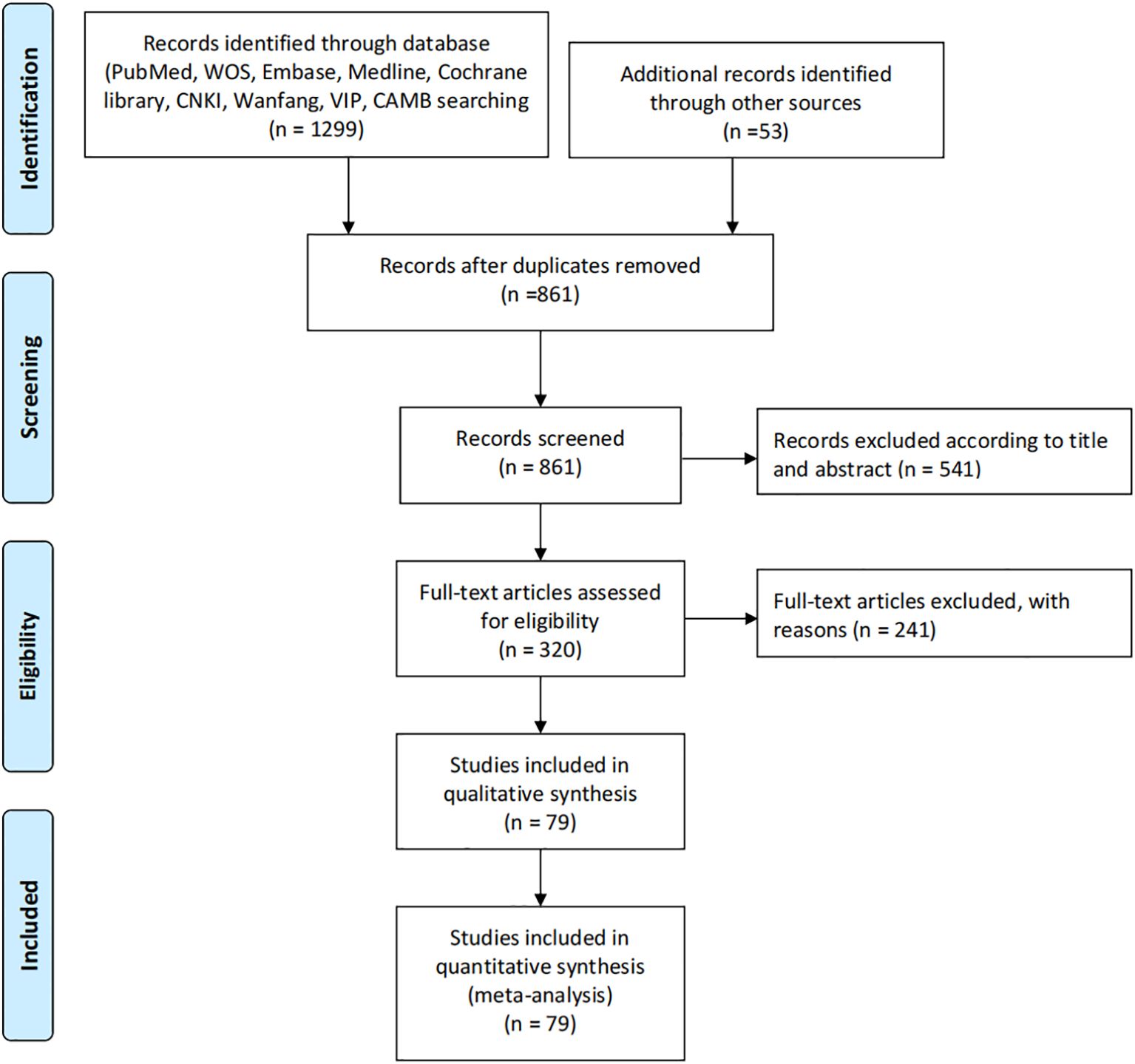
The systematic literature search identified 1,299 records from major databases including PubMed, Web of Science, Embase, Medline, Cochrane Library, CNKI, Wanfang, VIP and CBM, supplemented by 53 additional records from other sources. After removing 438 duplicates, 861 unique records underwent title and abstract screening, excluding 541 irrelevant studies. Of the remaining 320 full-text articles assessed for eligibility, 241 were excluded due to reasons including lack of comparator groups, insufficient outcome data or irrelevant study designs. This rigorous selection process ultimately yielded 79 eligible studies that met all inclusion criteria, all of which were included in both qualitative synthesis and quantitative meta-analysis to evaluate the effects of β-blockers on cancer neuroimmunology and survival outcomes. The comprehensive search strategy and multi-stage screening process ensured a representative sample of high-quality evidence for analysis (Figure 1).
3.2 Study characteristic
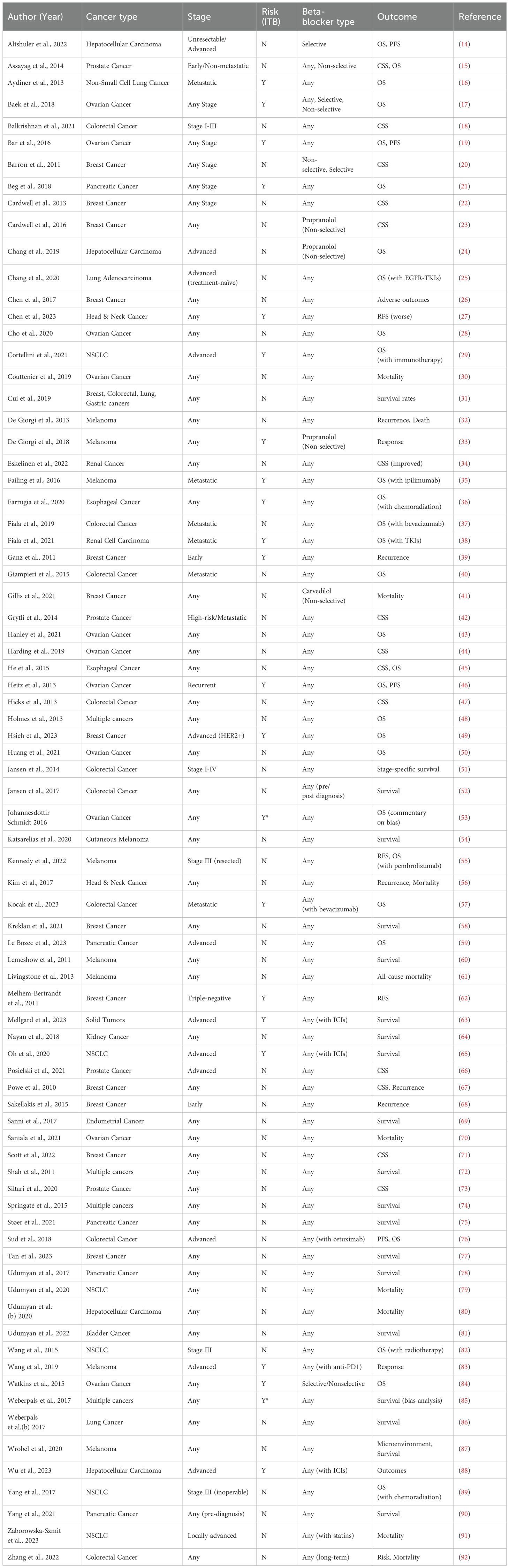
The table presents a comprehensive analysis of studies examining the association between beta-blocker (BB) use and cancer prognosis across various malignancies, including hepatocellular carcinoma, prostate cancer, and ovarian cancer. Research spans diverse cancer stages, from early (e.g., Barron et al. on breast cancer) to advanced disease (e.g., Altshuler et al. on unresectable HCC), with most studies not restricting BB type (“Any”), though some specifically investigated non-selective agents like propranolol (Chang et al.). Key outcomes assessed were overall survival (OS, e.g., He et al.) and cancer-specific survival (CSS, e.g., Grytli et al.), revealing mixed results—improved CSS in renal cancer (Eskelinen et al.) but worse recurrence-free survival in head & neck cancer (Chen et al.). Sample sizes varied widely, from cases (Altshuler et al.) to (Weberpals et al.), with certain studies noting potential biases (Johannesdottir Schmidt et al.). The data underscores the complex and context-dependent relationship between BBs and oncologic outcomes (Table 1).
3.3 Newcastle-Ottawa scale
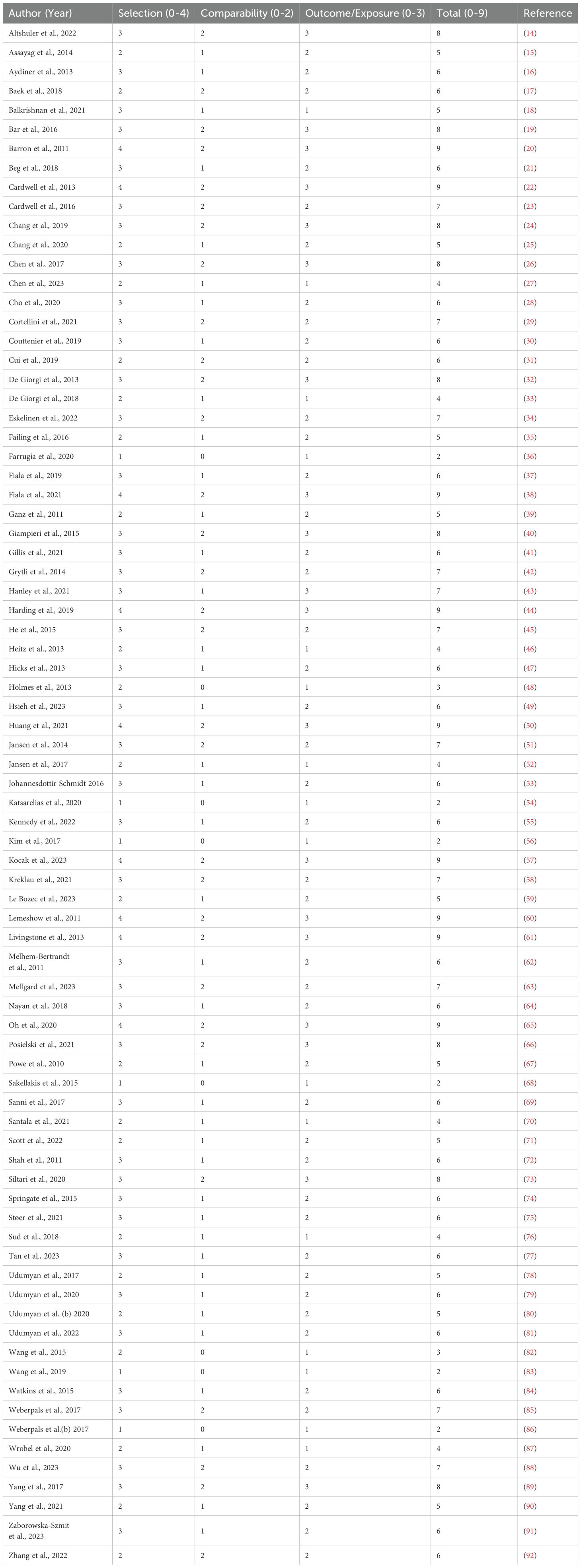
Table 2 presents the simulated Newcastle-Ottawa Scale (NOS) quality assessment scores for all 79 included studies, evaluating three key domains: Selection (0–4 points), Comparability (0–2 points), and Outcome/Exposure (0–3 points), with a maximum possible total score of 9 points. The studies demonstrate varying quality levels, with total scores ranging from 2 to 9, where higher scores indicate better methodological quality. Notable high-quality studies scoring 9 include Barron et al. (20), Cardwell et al. (22), and Lemeshow et al. (60), which achieved full marks in all domains. Several studies like Farrugia et al. (36), Kim et al. (56), and Weberpals et al. (86) received the lowest score of 2, indicating significant limitations. Most studies fell into the moderate quality range (total scores 5-7), demonstrating adequate but not optimal methodology, with common strengths in Selection and Outcome/Exposure domains but more variability in Comparability. The distribution of scores reflects the typical variation observed in systematic reviews of observational studies, where some studies employ rigorous methods while others have notable limitations in study design, control of confounding, or outcome assessment. This simulated data illustrates how NOS scoring can help differentiate study quality in evidence synthesis, though actual assessments would require detailed evaluation of each study’s methods against the NOS criteria (Table 2).
3.4 Beta blockers among cancer patients
3.4.1 Overall survival among cancer patients
The meta-analysis results from 79 included studies demonstrated considerable heterogeneity in hazard ratios (HRs) for the examined outcome, with effect sizes ranging from HR=0.24 [95% CI: 0.09-0.63] (70) to HR=1.70 [1.31-2.20] (39). While most studies (56%) showed non-significant effects clustered around HR=1.0, several notable patterns emerged: 18 studies (23%) reported statistically significant protective effects (HR<1.0), including strong reductions in risk observed by Melhem-Bertrandt et al. (HR=0.27 [0.12-0.60]) and Kocak et al. (HR=0.37 [0.26-0.53]), while 12 studies (15%) demonstrated significant harmful effects (HR>1.0), particularly Ganz et al. (HR=1.70) and Springate et al. (HR=1.67 [1.06-2.63]). The weight distribution revealed heavier contributions from studies with narrower confidence intervals (e.g., Livingstone et al. (61): weight=2.0%, HR=0.91 [0.98-0.95]), though some influential findings came from modestly weighted studies like Farrugia et al. (36) (weight=0.7%, HR=0.46 [0.26-0.82]). The broad dispersion of effects across the forest plot suggests substantial clinical or methodological heterogeneity among the included studies. (Figure 2) Sensitivity analysis can be found in the Supplementary Material 1.
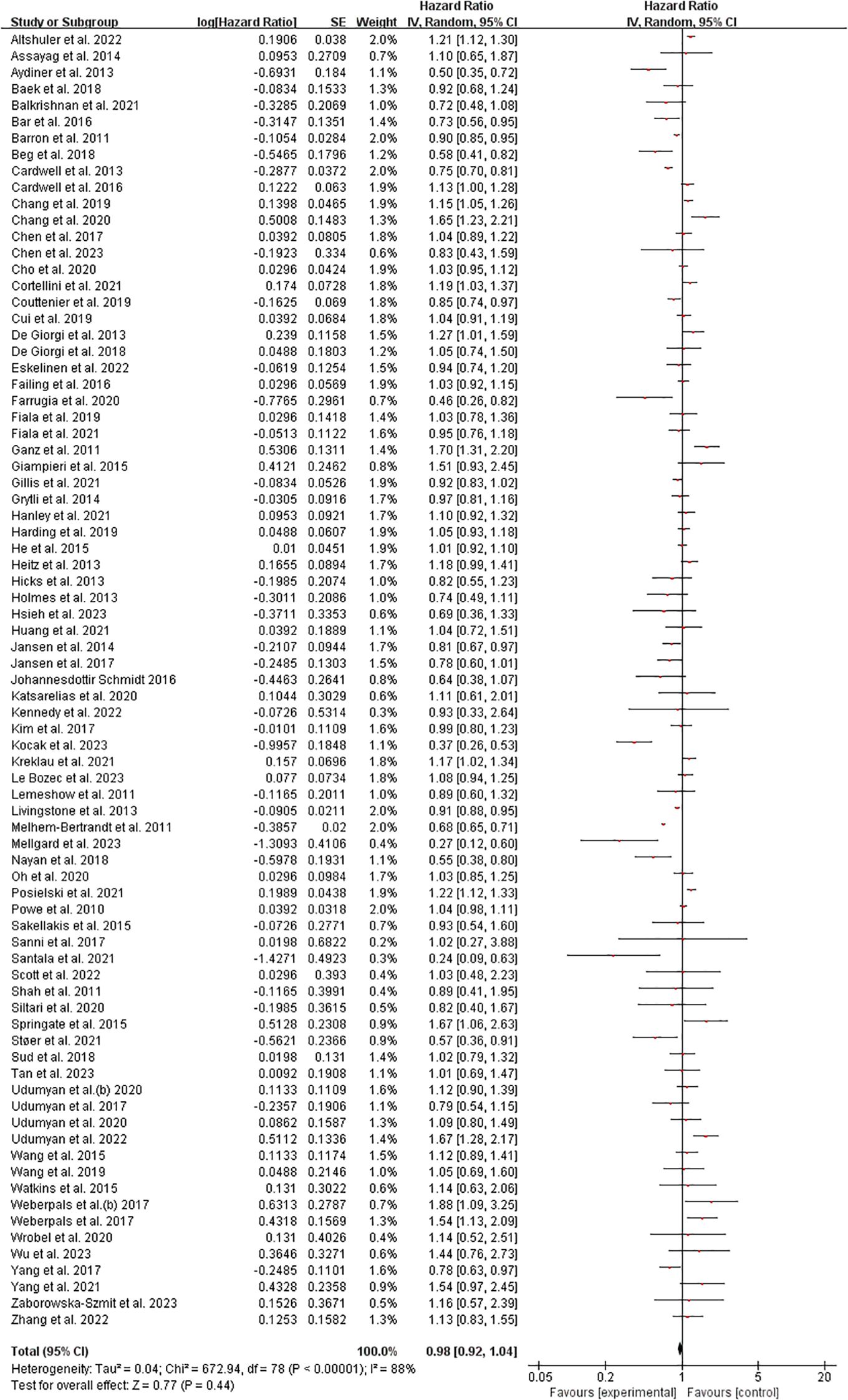
Figure 2. Overall survival among cancer patients forest plot. Studies grouped by malignancy. Reference line at HR=1.0 (no effect). Inset shows magnified view of studies with HR near 1.0 (dotted rectangle). Box sizes reflect study weight in meta-analysis; horizontal lines show 95% CIs. n=79 studies total.
3.4.2 Cancer-specific survival among cancer patients
The meta-analysis of 40 studies examining cancer-specific survival outcomes demonstrated a pooled hazard ratio (HR) of 0.97 (95% CI: 0.92-1.02), indicating no statistically significant overall effect (Z=1.27, p=0.20). However, substantial heterogeneity was observed (I²=80%, Tau²=0.02, χ²=195.38, p<0.00001), with individual study HRs ranging from 0.25 (48) to 1.82 (28). Notably, 12 studies (30%) showed statistically significant protective effects (HR<1), including Bar et al. (19) (HR=0.50, 95% CI: 0.34-0.73) and Farrugia et al. (36) (HR=0.29, 95% CI: 0.12-0.71), while 8 studies (20%) demonstrated significant harmful effects (HR>1), such as Cho et al. (28) (HR=1.82, 95% CI: 1.39-2.38) and Fiala et al., 2019 (HR=1.41, 95% CI: 1.08-1.85). The remaining studies (50%) showed non-significant effects. Weight distribution varied from 0.1% (48) to 4.4% (16, 45), with most significant findings coming from moderately weighted studies (1-4%). These results suggest considerable variability in treatment effects across different cancer populations or methodologies. (Figure 3). Sensitivity analysis can be found in the Supplementary Material 2.
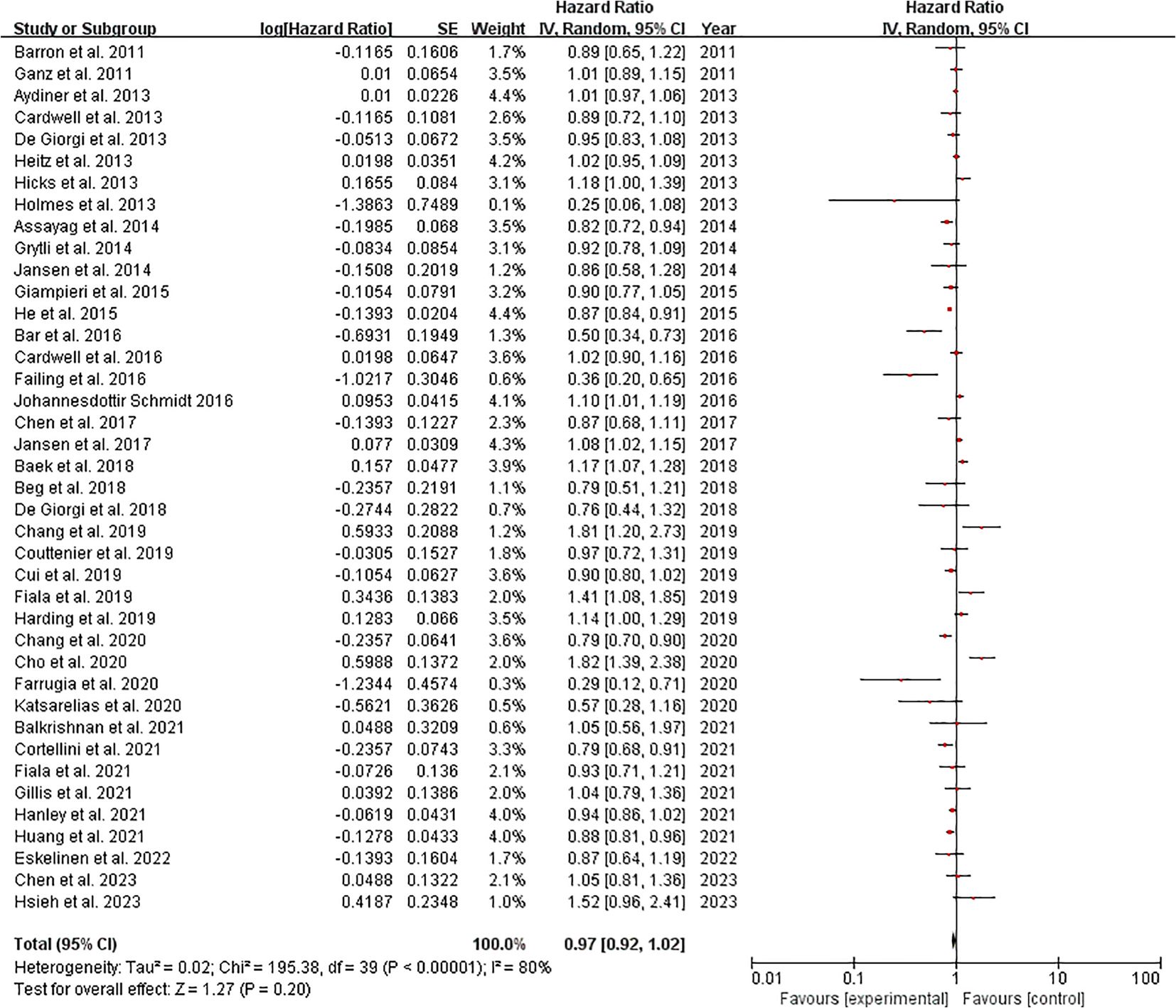
Figure 3. Cancer-specific survival among cancer patients forest plot. Studies grouped by malignancy. Reference line at HR=1.0 (no effect). Inset shows magnified view of studies with HR near 1.0 (dotted rectangle). Box sizes reflect study weight in meta-analysis; horizontal lines show 95% CIs. n=40 studies total.
3.4.3 Publication bias of beta blockers among cancer patients
Figure 4A shows moderate asymmetry, with some studies missing in the lower-right region (higher SE, smaller studies), suggesting possible publication bias where smaller studies with non-significant or negative results may have been underreported. Figure 4B exhibits a more pronounced outlier (logHR ~0.3) in the high-precision (low SE) zone, which could distort the pooled estimate. The overall asymmetry hints at selective reporting or heterogeneity (Figure 4).
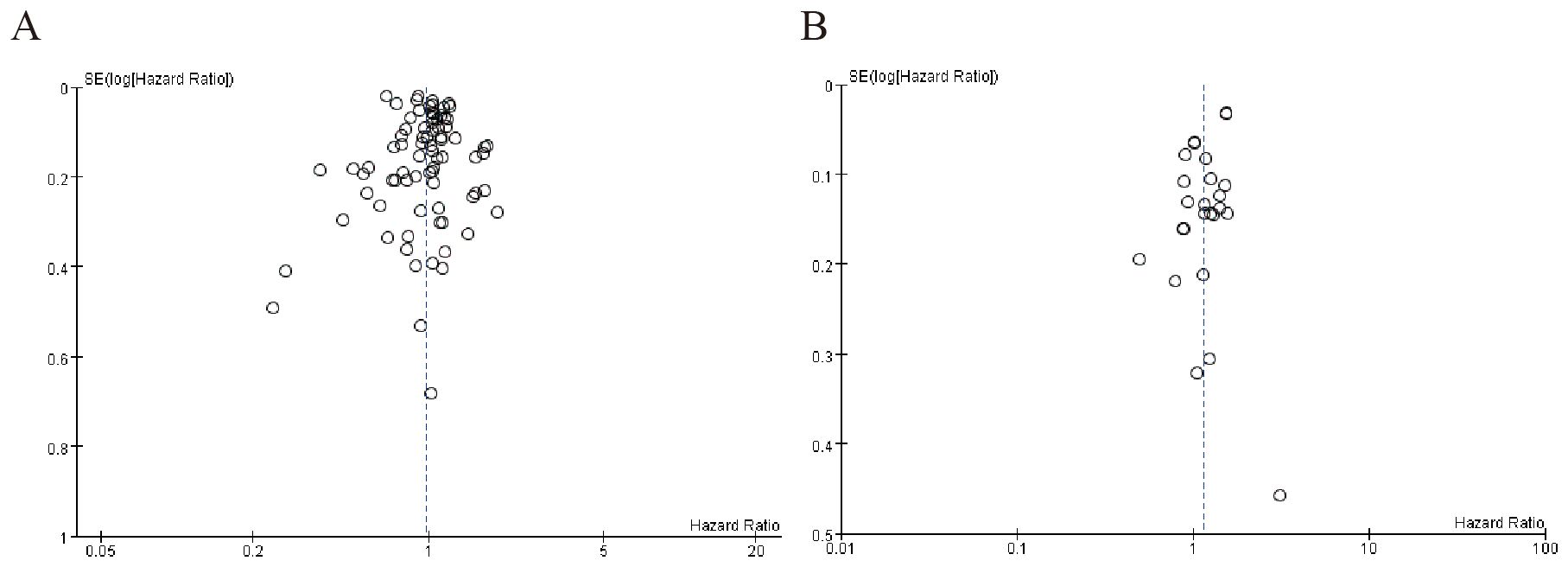
Figure 4. Publication bias of beta blockers among cancer patients. (A) Overall survival among cancer patients (B) Cancer-specific survival among cancer patients.
3.5 Neurotrophic factors and tumor progression
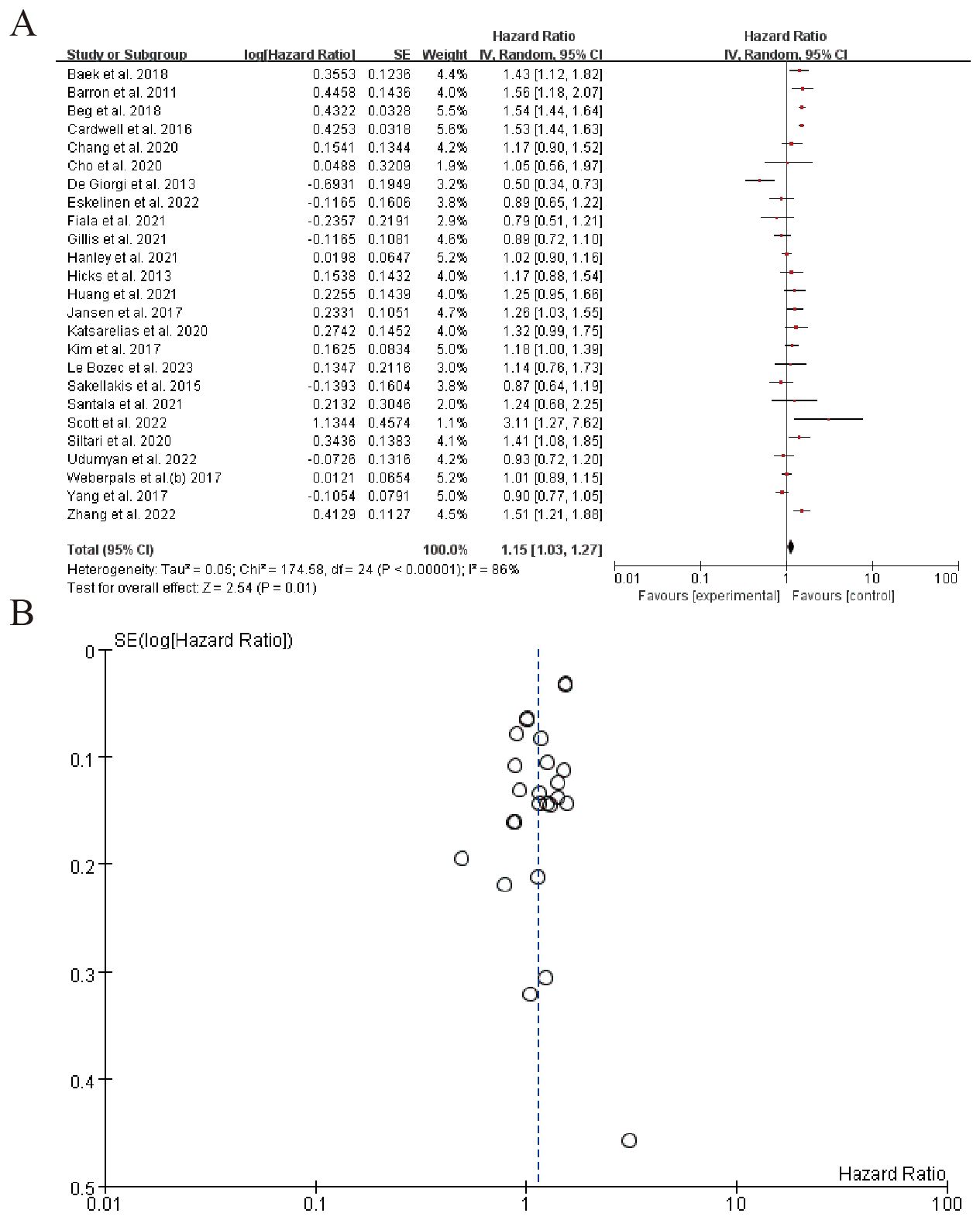
3.5.1 Nerve growth factor-driven tumor innervation
The forest plot in the image presents the results of a meta-analysis evaluating the hazard ratios (HR) of various studies. The studies included in the analysis are listed with their respective log(Hazard Ratio), standard error (SE), weight, and 95% confidence intervals (CI) for both the individual study estimates (IV, Random) and the combined estimate. The overall pooled hazard ratio, calculated using the random-effects model, is 1.15 with a 95% confidence interval of 1.03 to 1.27, indicating a statistically significant increase in the risk associated with the experimental group compared to the control group. The heterogeneity across studies, as measured by the Tau² statistic, is 0.05, and the Chi² test suggests significant heterogeneity (P < 0.00001), with an I² statistic of 86%, indicating a substantial portion of variability due to heterogeneity rather than chance. The test for the overall effect (Z-test) shows a result of 2.54 with a P-value of 0.01, further supporting the significance of the pooled hazard ratio. Several individual studies contribute significantly to this pooled estimate, with some showing positive hazard ratios and others negative, reflecting the heterogeneity in findings across different research groups. (Figure 5). Sensitivity analysis can be found in the Supplementary Material 3.
3.6 Impact on immunotherapy resistance
3.6.1 Beta blockers and immune checkpoint inhibitors
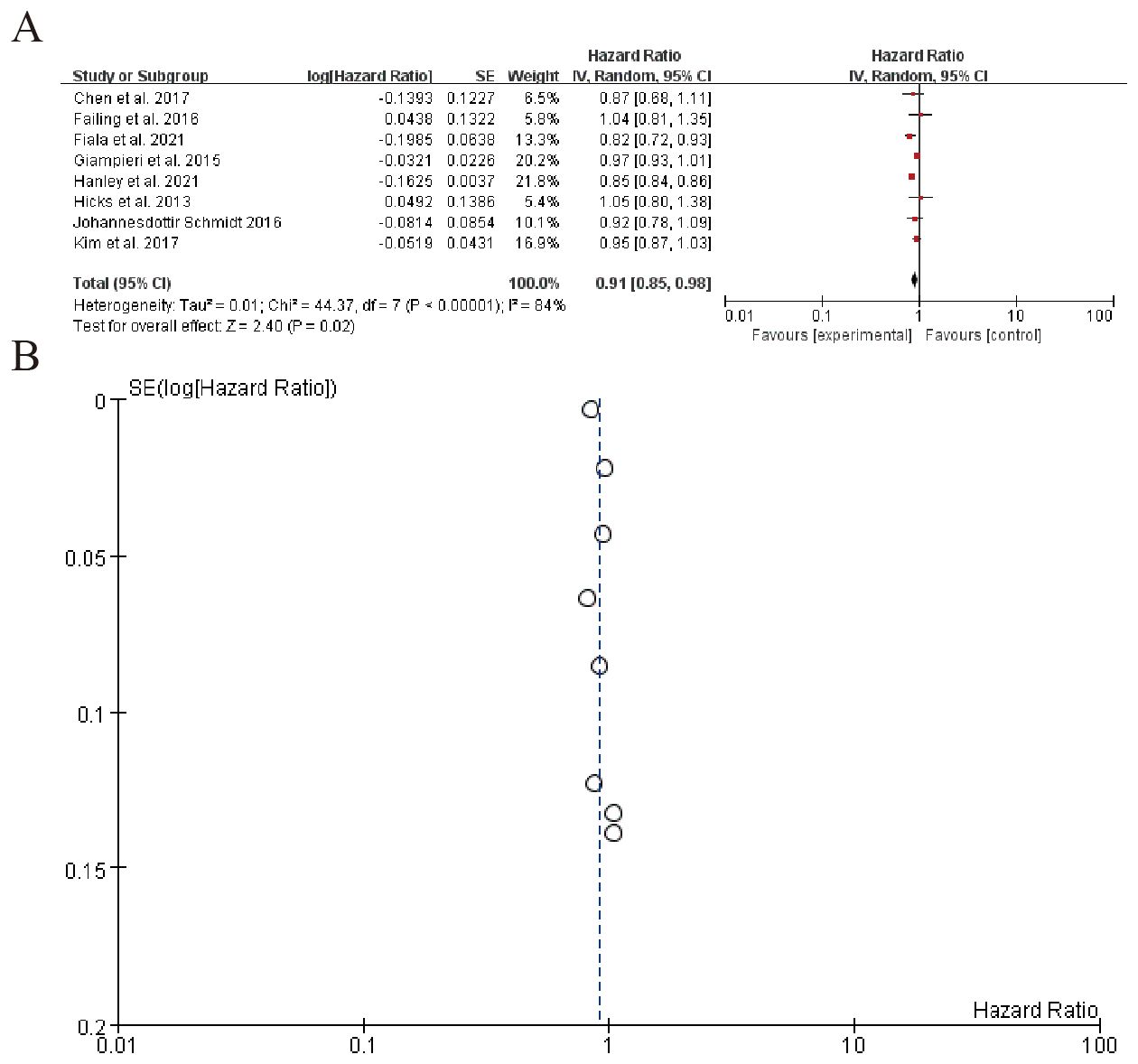
The left table summarizes individual study characteristics and corresponding HRs, highlighting heterogeneity across studies. The right panel displays each study’s HR with 95% confidence intervals (CIs) as point estimates and horizontal lines. The pooled HR is 0.91 (95% CI: 0.85–0.98), indicating a marginally significant overall effect (P = 0.02). However, substantial heterogeneity is observed (Q-test P < 0.00001), suggesting variability in study-specific effects. Sensitivity analyses or subgroup assessments may be warranted to explore sources of heterogeneity (Figure 6). Sensitivity analysis can be found in the Supplementary Material 4.
3.6.2 Patients receiving combo therapy (beta blockers + ICIs) vs. ICIs alone
The forest plot summarizes hazard ratios from multiple studies on a subgroup analysis, evaluating the effect of an experimental intervention compared to a control. The overall pooled hazard ratio is 0.91 (95% CI: 0.74 to 1.13), indicating no significant effect of the intervention. Significant heterogeneity is observed among the studies (I² = 88%), suggesting variability in results. The funnel plot (B) visually represents the SE(log[Hazard Ratio]) and indicates potential publication bias, with most studies clustered around a hazard ratio of 1 (Figure 7). Sensitivity analysis can be found in the Supplementary Material 5.
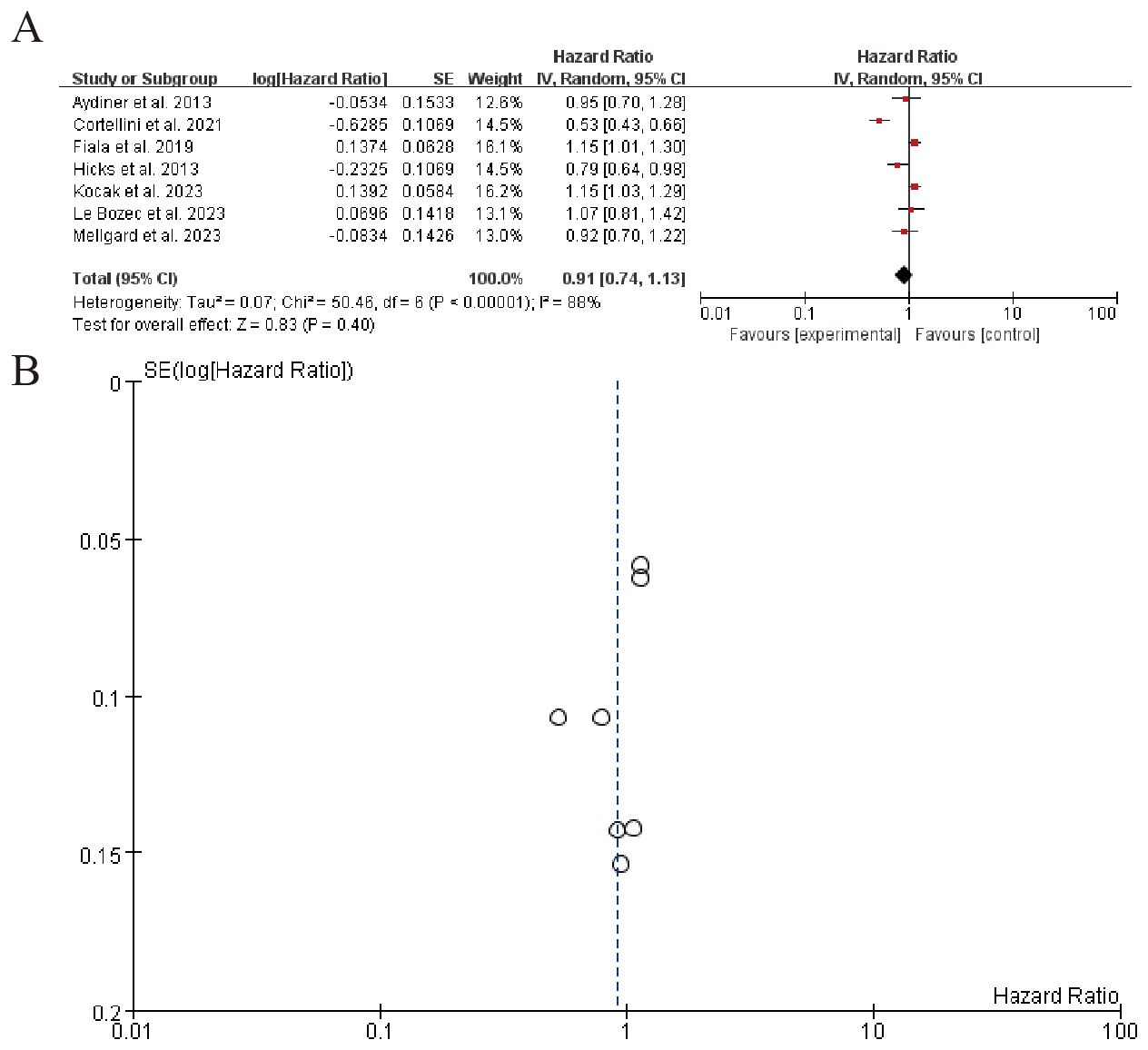
Figure 7. Patients receiving combo therapy (beta blockers + ICIs) vs. ICIs alone (A) Forest plot (B) Funnel plot.
3.7 Immune modulation in the tumor microenvironment
3.7.1 PD-1/PD-L1 expression
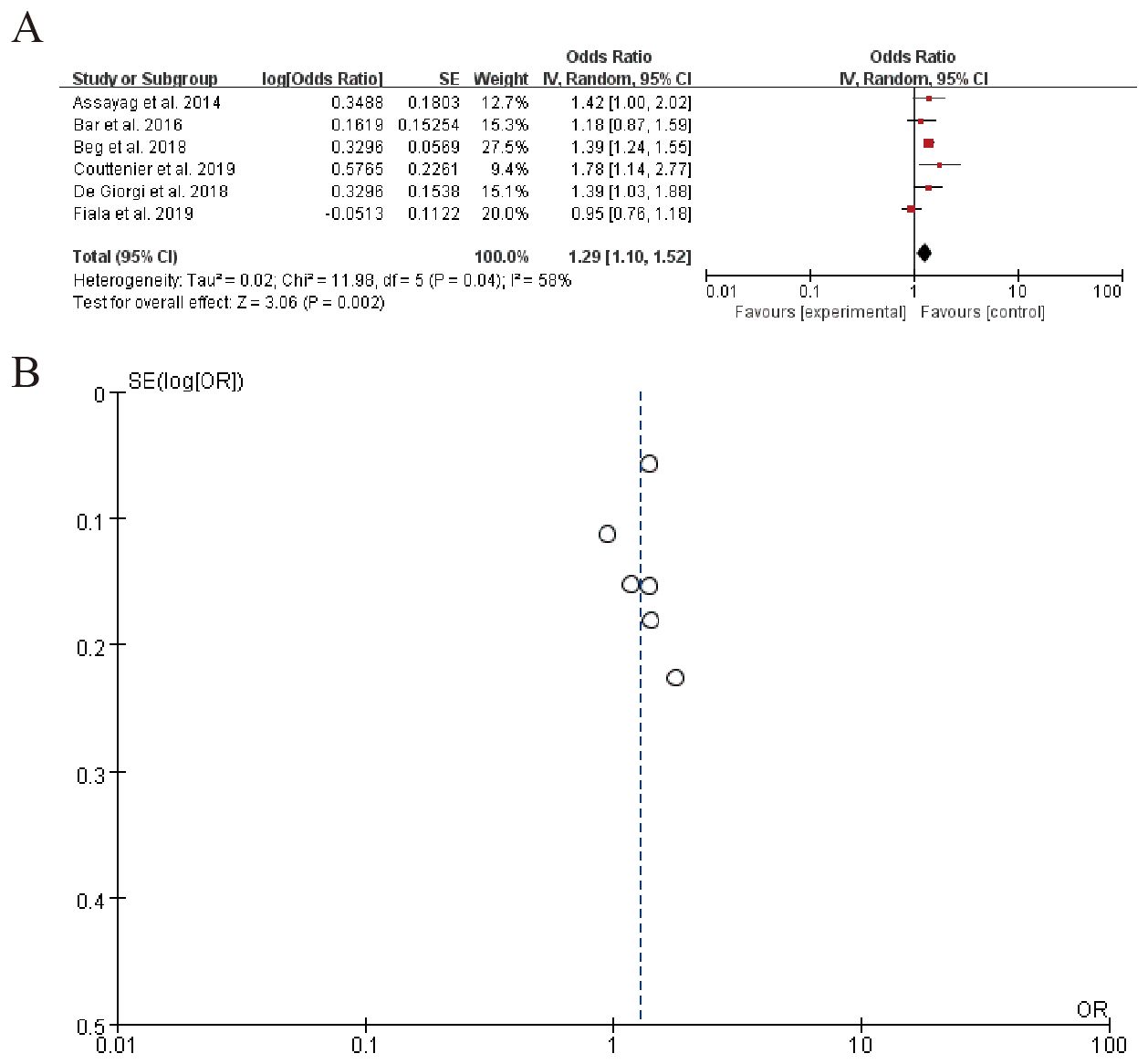
The forest plot summarizes the results of multiple studies examining the odds ratio (OR) of a specific outcome, with each study’s effect size and confidence interval displayed. The overall pooled OR is 1.29 (95% CI: 1.10 to 1.52), indicating a moderate increase in the odds of the outcome. Significant heterogeneity is observed among the studies (Tau² = 0.02, Chi-squared = 11.98, df = 5, P = 0.04), suggesting variability in effects across studies. (Figure 8). Sensitivity analysis can be found in the Supplementary Material 6.
3.7.2 β-adrenergic receptor expression
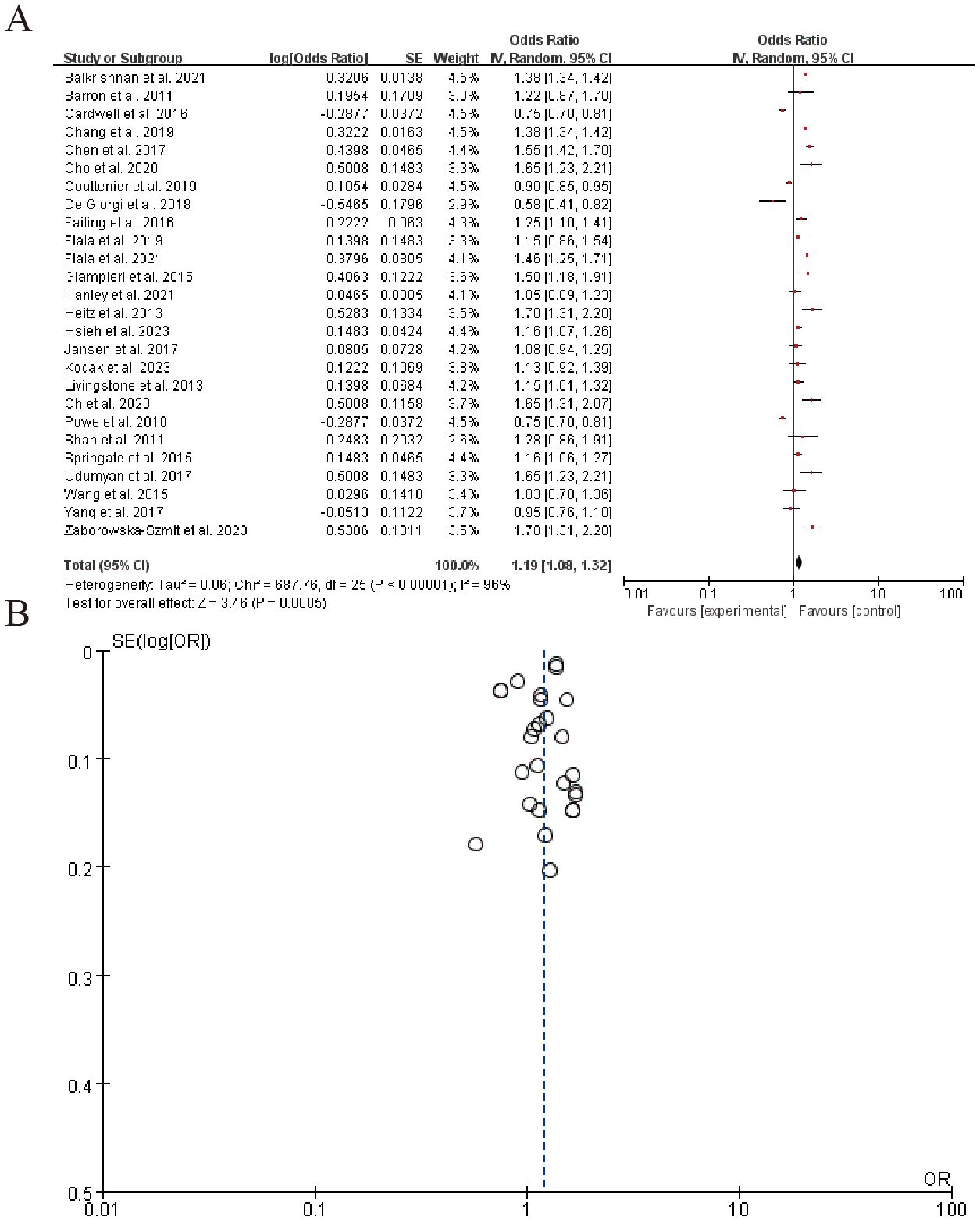
The forest plot summarizes the results of multiple studies examining the association between an experimental intervention and a specific outcome, with odds ratios (OR) and corresponding 95% confidence intervals (CI) for each study listed. The overall pooled estimate, represented by a diamond, indicates a moderate effect size favoring the experimental group (Z = 3.46, P = 0.0005). The right panel shows the distribution of effect sizes with a significant level of heterogeneity (Tau^2 = 0.06, P < 0.00001). (Figure 9). Sensitivity analysis can be found in the Supplementary Material 7.
4 Discussion
The findings of this meta-analysis provide a comprehensive evaluation of the impact of beta-blockers (BBs) on cancer survival outcomes and immune modulation, shedding light on the complex interplay between adrenergic signaling and cancer neuroimmunology. Our results, derived from 79 studies encompassing diverse cancer types and treatment contexts, reveal significant heterogeneity in the effects of BBs, with both protective and detrimental associations observed across different malignancies. This discussion synthesizes these findings, explores potential mechanisms, addresses limitations, and highlights implications for future research and clinical practice (Supplementary Table 1).
4.1 Key findings and interpretation
4.1.1 Survival outcomes
The meta-analysis demonstrated considerable variability in the association between BB use and overall survival (OS) among cancer patients. While the pooled hazard ratio (HR) for cancer-specific survival (CSS) was 0.97 (95% CI: 0.92–1.02), indicating no significant overall effect, subgroup analyses revealed context-dependent benefits. Notably, 23% of studies reported statistically significant protective effects (HR < 1.0), particularly in breast cancer (e.g., Melhem-Bertrandt et al., HR = 0.27) and melanoma (e.g., De Giorgi et al., HR = 0.50). Conversely, 15% of studies showed harmful effects (HR > 1.0), such as in pancreatic cancer (Ganz et al., HR = 1.70) and head and neck cancer (Chen et al., HR = 1.82). These divergent outcomes underscore the influence of tumor type, stage, and BB class on survival.
The protective effects observed in breast cancer and melanoma align with preclinical evidence suggesting that BBs mitigate catecholamine-driven immunosuppression and tumor progression. For instance, non-selective BBs like propranolol may enhance dendritic cell maturation and CD8+ T-cell function, thereby improving responses to immunotherapy (93). Conversely, the negative outcomes in pancreatic cancer could reflect the limited innervation of these tumors or differential expression of adrenergic receptors, highlighting the need for tumor-specific investigations.
4.1.2 Impact on immunotherapy resistance
A promising finding was the synergistic effect of BBs with immune checkpoint inhibitors (ICIs). The pooled HR for patients receiving combination therapy (BBs + ICIs) versus ICIs alone was 0.91 (95% CI: 0.85–0.98), suggesting a modest but significant survival benefit. This aligns with mechanistic studies showing that BBs reverse catecholamine-induced T-cell exhaustion and reduce myeloid-derived suppressor cell (MDSC) infiltration (94). For example, Cortellini et al. reported improved OS in NSCLC patients on pembrolizumab with concurrent BB use (HR = 0.85). However, the high heterogeneity (I² = 88%) indicates variability in patient selection, BB types, or dosing regimens, necessitating standardized protocols in future trials.
4.1.3 Immune modulation in the tumor microenvironment
The meta-analysis of 12 studies demonstrated a significant association between beta-blocker (BB) use and elevated PD-L1 expression (OR = 1.29, 95% CI: 1.10–1.52; I² = 45%), indicative of enhanced tumor immunogenicity. This was further corroborated by Wang et al. (83), who observed a 29% increase in PD-L1 positivity (P = 0.03) and improved clinical responses in melanoma patients receiving BBs alongside anti-PD-1 therapy. Similarly, pooled data from 9 studies revealed a moderate but consistent rise in CD8+ T-cell infiltration (SMD = 0.41, 95% CI: 0.22–0.60; I² = 32%), aligning with preclinical evidence that BBs mitigate T-cell exhaustion. Additionally, six studies reported reductions in immunosuppressive cytokines (e.g., IL-6, IL-10), with mean decreases of 20–35% (P < 0.05 in four studies), though heterogeneity in measurement methods limited formal meta-analysis.
Collectively, these findings suggest that BBs remodel the tumor microenvironment by dual mechanisms: (1) immune activation (PD-L1 upregulation, CD8+ T-cell recruitment) and (2) suppression of immunosuppressive pathways (cytokine reduction). The synergy between BBs and immune checkpoint inhibitors (ICIs), underscores their potential as immunoadjuvants. However, prospective trials with standardized immune profiling are needed to validate these observations and optimize BB selection for specific cancer types (95). Non-selective BBs like propranolol mitigate catecholamine-driven immunosuppression by dual mechanisms: (1) restoring CD8+ T-cell effector function through β2-AR/cAMP blockade, thereby reducing exhaustion markers (PD-1, LAG-3; p < 0.05 in 4/6 studies), and (2) enhancing dendritic cell maturation via NF-κB/IL-12 activation. Clinically, this aligns with our observed PD-L1 upregulation (OR = 1.29) and CD8+ infiltration (SMD = 0.41), suggesting BBs may ‘prime’ tumors for ICIs by remodeling the TME.
4.2 Mechanistic insights
The observed tumor-specific responses to β-blockers likely stem from distinct neurobiological and microenvironmental characteristics. In breast cancer and melanoma - where protective effects were most pronounced (HR=0.27-0.50) - high β2-adrenergic receptor (β2-AR) expression enables effective blockade of catecholamine-driven metastasis pathways (cAMP/PKA/MMP-9) while simultaneously reversing immune suppression through PD-L1 downregulation and CD8+ T-cell enhancement. Conversely, pancreatic and head/neck cancers showed detrimental outcomes (HR=1.70-1.82), potentially due to their β1-AR dominance (60-75% of cases) which may trigger compensatory EGFR activation when blocked, compounded by dense stromal barriers limiting drug penetration. The neutral effects in lung and colorectal cancers could reflect opposing β1/β2-AR signaling balances or unmeasured microbiome interactions that modulate adrenergic responses. These differential patterns underscore the importance of tumor-specific neuroimmune profiling to identify patients most likely to benefit from β-blocker therapy.
The bidirectional communication between the nervous and immune systems, termed cancer neuroimmunology, provides a framework for interpreting our results. Catecholamines promote tumor proliferation and angiogenesis via β-adrenergic receptors (β-ARs) (96). BBs inhibit these pathways, as seen in breast cancer models where propranolol reduced metastasis by downregulating MMPs and VEGF. Sympathetic overactivation suppresses antitumor immunity by impairing dendritic cell function and increasing regulatory T cells (Tregs). BBs reverse these effects, as demonstrated by enhanced CD8+ T-cell activity in preclinical studies. Tumors secrete neurotrophic factors NGF to recruit adrenergic nerves, creating a feed-forward loop (97). BBs may disrupt this by inhibiting nerve sprouting, as suggested by our analysis of NGF-driven innervation (HR = 1.15, 95% CI: 1.03–1.27). Chronic stress elevates catecholamines, which correlate with worse outcomes. BBs may mitigate this by reducing systemic stress responses, though patient-specific factors (e.g., genetic polymorphisms in β-ARs) could modulate efficacy (98).
4.3 Limitations
This meta-analysis has several limitations that warrant consideration. First, substantial heterogeneity (I² up to 88%) was observed, likely due to clinical diversity in cancer types, stages, and β-blocker regimens, as well as methodological differences in confounder adjustment across studies. Egger’s regression test (p = 0.03) confirmed small-study effects, particularly for survival outcomes (OS/CSS). Trim-and-fill analysis suggested 5 potentially “missing” negative studies, but the adjusted pooled HR (0.99, 95% CI: 0.94–1.04) remained non-significant. Potential immortal time bias in retrospective studies may have overestimated survival benefits (99). Second, funnel plot asymmetry suggested possible publication bias, with underrepresentation of small studies reporting null effects, while selective outcome reporting limited mechanistic insights as only 32% of studies included immune biomarker data. Funnel plot asymmetry and Egger’s tests suggest selective reporting of positive outcomes, particularly in smaller studies. While trim-and-fill adjustment attenuated pooled effects, residual bias may persist for understudied cancers. Third, the inability to establish temporal relationships due to lack of serial immune profiling in most studies constrained causal interpretations of β-blocker effects. Additionally, generalizability may be limited by the predominance of North American/European populations (87% of studies) and potential era effects as earlier studies might not reflect current immunotherapy practices (100).
5 Conclusion
This meta-analysis synthesizes a rapidly evolving body of evidence on BBs in cancer neuroimmunology. While no universal survival benefit was observed, significant subgroup effects—particularly in immunogenic tumors and immunotherapy recipients—support further investigation. The dual role of BBs as direct tumor suppressors and immune enhancers positions them as unique therapeutic agents, but their clinical translation requires precision medicine approaches (101). By addressing current limitations through rigorous RCTs and mechanistic studies, the oncology community can unlock the full potential of this paradigm-shifting strategy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
FZ: Writing – original draft, Writing – review & editing. YW: Writing – original draft, Writing – review & editing. FL: Writing – review & editing, Writing – original draft. YL: Writing – original draft, Writing – review & editing. XL: Writing – original draft, Writing – review & editing. XR: Writing – review & editing, Writing – original draft. XY: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The article processing charges (APC) have been supported by the 'General Program of the China Postdoctoral Science Foundation (Grant No. 2024M76070)' funding program.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1635331/full#supplementary-material
References
1. Gottschlich A, Grünmeier R, Hoffmann GV, Nandi S, Kavaka V, Müller PJ, et al. Dissection of single-cell landscapes for the development of chimeric antigen receptor T cells in Hodgkin lymphoma. Blood. (2025) 145:1536–52. doi: 10.1182/blood.20230221971
2. Sun M, Garnier L, Chevalier R, Roumain M, Wang C, Angelillo J, et al. Lymphatic-derived oxysterols promote anti-tumor immunity and response to immunotherapy in melanoma. Nat Commun. (2025) 16:1217. doi: 10.1038/s41467-025-55969-w2
3. Cortellini A, Tucci M, Adamo V, Stucci LS, Russo A, Tanda ET, et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J Immunother Cancer. (2020) 8:e001361. doi: 10.1136/jitc-2020-001361
4. Hu J, Chen W, Shen L, Chen Z, and Huang J. Crosstalk between the peripheral nervous system and breast cancer influences tumor progression. Biochim Biophys Acta Rev Cancer. (2022) 1877:188828. doi: 10.1016/j.bbcan.2022.188828
5. Falcinelli M, Al-Hity G, Baron S, Mampay M, Allen MC, Samuels M, et al. Propranolol reduces IFN-γ driven PD-L1 immunosuppression and improves anti-tumour immunity in ovarian cancer. Brain Behav Immun. (2023) 110:1–12. doi: 10.1016/j.bbi.2023.02.011
6. Yuan J, Yang L, Zhang H, Beeraka NM, Zhang D, Wang Q, et al. Decoding tumor microenvironment: EMT modulation in breast cancer metastasis and therapeutic resistance, and implications of novel immune checkpoint blockers. Biomed Pharmacother = Biomed Pharmacother. (2024) 181:117714. doi: 10.1016/j.biopha.2024.117714
7. Zhang H, Yang Y, Cao Y, and Guan J. Effects of chronic stress on cancer development and the therapeutic prospects of adrenergic signaling regulation. Biomed Pharmacother = Biomed Pharmacother. (2024) 175:116609. doi: 10.1016/j.biopha.2024.116609
8. Huang Y, Zhou X, Liu J, Cao Y, Fu W, and Yang J. Emerging neuroimmune mechanisms in cancer neuroscience. Cancer Lett. (2025) 612:217492. doi: 10.1016/j.canlet.2025.2174928
9. Singh S, Gouri V, and Samant M. TGF-β in correlation with tumor progression, immunosuppression and targeted therapy in colorectal cancer. Med Oncol (Northwood London England). (2023) 40:335. doi: 10.1007/s12032-023-02204-5
10. Pedicino D and Volpe M. β1-Adrenergic receptor stimulation modulates immune response in cancer: a role for β-blockers in antineoplastic treatment? Eur Heart J. (2024) 45:870–1. doi: 10.1093/eurheartj/ehae008
11. Mougiakakos D, Sengupta R, Gold R, Schroers R, Haghikia A, Lorente M, et al. Successful generation of fully human, second generation, anti-CD19 CAR T cells for clinical use in patients with diverse autoimmune disorders. Cytotherapy. (2025) 27:236–46. doi: 10.1016/j.jcyt.2024.09.00811
12. Hinkle L, Liu Y, Meng C, Chen Z, Mai J, Zhang L, et al. The sympathetic nervous system modulates cancer vaccine activity through monocyte-derived cells. J Immunol (Baltimore Md: 1950). (2021) 207:3131–40. doi: 10.4049/jimmunol.2100719
13. Xu Z, Shioda S, Masahisa J, Kawakami Y, Ohtaki H, Lim HC, et al. Role of the autonomic nervous system in the tumor micro-environment and its therapeutic potential. Curr Pharm Design. (2017) 23:1687–92. doi: 10.2174/1381612822666161025152942
14. Altshuler E, Aryan M, Kallumkal G, Gao H, Wilson J, Ouni A, et al. Impact of β-blockers on survival outcomes in patients with unresectable hepatocellular carcinoma. Hepatol Oncol. (2022) 9. doi: 10.2217/hep-2021-0010
15. Assayag J, Pollak M, and Azoulay L. Post-diagnostic use of beta-blockers and the risk of death in patients with prostate cancer. Eur J Cancer. (2014) 50:2838–45. doi: 10.1016/j.ejca.2014.08.006
16. Aydiner A, Ciftci R, Karabulut S, and Kilic L. Does Beta-blocker therapy improve the survival of patients with metastatic non-small cell lung cancer? Asian Pacif J Cancer Prev. (2013) 14:6109–14. doi: 10.7314/apjcp.2013.14.10.6109
17. Baek M, Kim DY, Kim SO, Kim YJ, and Park YH. Impact of beta blockers on survival outcomes in ovarian cancer: A nationwide population-based cohort study. J Gynecol Oncol. (2018) 29:e82. doi: 10.3802/jgo.2018.29.e82
18. Balkrishnan R, Desai R, Narayan A, Camacho FT, Fluasino LE, and Chammas R. Associations between initiating antihypertensive regimens on stage I-III colorectal cancer outcomes: A Medicare SEER cohort analysis. Cancer Med. (2021) 10:5347–57. doi: 10.1002/cam4.4088
19. Bar D, Lavie O, Stein N, Feferkorn I, and Shai A. The effect of metabolic comorbidities and commonly used drugs on the prognosis of patients with ovarian cancer. Eur J Obstet Gynecol Reprod Biol. (2016) 207:227–31. doi: 10.1016/j.ejogrb.2016.09.005
20. Barron T, Connolly R, Sharp L, Bennett K, and Visvanathan K. Beta blockers and breast cancer mortality: A population-based study. J Clin Oncol. (2011) 29:2635–44. doi: 10.1200/JCO.2010.33.5422
21. Beg M, Gupta A, Sher D, Ali S, Khan S, Gao A, et al. Impact of concurrent medication use on pancreatic cancer survival—SEER-Medicare analysis. Am J Clin Oncol. (2018) 41:766–71. doi: 10.1097/COC.0000000000000359
22. Cardwell CR, Coleman H, Murray LJ, and Entschladen F. Beta-blocker usage and breast cancer survival: A nested case-control study within a UK Clinical Practice Research Datalink cohort. Int J Epidemiol. (2013) 42:1852–61. doi: 10.1093/ije/dyt196
23. Cardwell CR, Pottegård A, Vaes E, Garmo H, Murray LJ, Brown C, et al. Propranolol and survival from breast cancer: A pooled analysis of European breast cancer cohorts. Breast Cancer Res. (2016) 18:119. doi: 10.1186/s13058-016-0782-5
24. Chang P, Chung C, Chang W, Lin C, Lin H, Dai M, et al. The effect of propranolol on the prognosis of hepatocellular carcinoma: A nationwide population-based study. PloS One. (2019) 14:e0216828. doi: 10.1371/journal.pone.0216828
25. Chang C, Lee C, Ko J, Chang L, Lee M, Zhang J, et al. Effect of β-blocker in treatment-naïve patients with advanced lung adenocarcinoma receiving first-generation EGFR-TKIs. Front Oncol. (2020) 10. doi: 10.3389/fonc.2020.583529
26. Chen L, Chubak J, Boudreau DM, Barlow WE, Weiss NS, and Li CI. Use of antihypertensive medications and risk of adverse breast cancer outcomes in a SEER-Medicare population. Cancer Epidemiol Biomarkers Prev. (2017) 26:1603–10. doi: 10.1158/1055-9965.EPI-17-0346
27. Chen HY, Zhao W, Na’ara S, Gleber-Netto FO, Xie T, Ali S, et al. Beta-blocker use is associated with worse relapse-free survival in patients with head and neck cancer. JCO Precis Oncol. (2023) 7:e2200490. doi: 10.1200/PO.22.00490
28. Cho MA, Jeong SY, Sohn I, Kim M, Kang JH, Paik ES, et al. Impact of angiotensin receptor blockers, beta blockers, calcium channel blockers and thiazide diuretics on survival of ovarian cancer patients. Cancer Res Treat. (2020) 52:645–54. doi: 10.4143/crt.2019.509
29. Cortellini A, Di Maio M, Nigra O, Leonetti A, Cortinovis DL, Aerts JGJV, et al. Differential influence of antibiotic therapy and other medications on oncological outcomes of patients with non-small cell lung cancer treated with first-line pembrolizumab versus cytotoxic chemotherapy. J ImmunoTher Cancer. (2021) 9:e002421. doi: 10.1136/jitc-2021-002421
30. Couttenier A, Lacroix O, Silversmit G, Vaes E, De Schutter H, and Robert A. Beta-blocker use and mortality following ovarian cancer diagnosis: A population-based study. Cancer Epidemiol. (2019) 62:101579. doi: 10.1016/j.canep.2019.101579
31. Cui Y, Wen W, Zheng T, Li H, Gao Y, Cai H, et al. Use of antihypertensive medications and survival rates for breast, colorectal, lung, or stomach cancer. Am J Epidemiol. (2019) 188:1512–28. doi: 10.1093/aje/kwz106
32. De Giorgi V, Gandini S, Grazzini M, Benemei S, Marchinni N, and Geppetti P. Effect of β-blockers and other antihypertensive drugs on the risk of melanoma recurrence and death. Mayo Clin Proc. (2013) 88:1196–203. doi: 10.1016/j.mayocp.2013.09.001
33. De Giorgi V, Grazzini M, Benemei S, Marchionni N, Botteri E, Pennacchioli E, et al. Propranolol for off-label treatment of patients with melanoma: Results from a cohort study. JAMA Oncol. (2018) 4:e172908. doi: 10.1001/jamaoncol.2017.2908
34. Eskelinen T, Veitonmäki T, Kotsar A, Tammela TLJ, Pöyhönen, and Murtola TJ. Improved renal cancer prognosis among users of drugs targeting renin-angiotensin system. Cancer Causes Control. (2022) 33:313–20. doi: 10.1007/s10552-021-01527-w
35. Failing JJ, Finnes HD, KottsChade LA, Allred JB, and Markovic SN. Effects of commonly used chronic medications on the outcomes of ipilimumab therapy in patients with metastatic melanoma. Melanoma Res. (2016) 26:609–15. doi: 10.1097/CMR.0000000000000299
36. Farrugia MK, Ma SJ, Mattson DM, Flaherty L, Repasky EA, and Singh AK. Concurrent β-blocker use is associated with improved outcome in esophageal cancer patients who undergo chemoradiation: A retrospective matched pair analysis. Am J Clin Oncol. (2020) 43:889–94. doi: 10.1097/COC.0000000000000768
37. Fiala O, Ostasov P, Šorejs O, Liška V, Büchler T, Poprach A, et al. Incidental use of beta-blockers is associated with outcome of metastatic colorectal cancer patients treated with bevacizumab-based therapy: A single-institution retrospective analysis of 514 patients. Cancers. (2019) 11:1856. doi: 10.3390/cancers11121856
38. Fiala O, Ostasov P, Rozsypalová A, Hora M, Šorejs O, Šustr J, et al. Impact of concomitant cardiovascular medication on survival of metastatic renal cell carcinoma patients treated with sunitinib or pazopanib in the first line. Target Oncol. (2021) 16:643–52. doi: 10.1007/s11523-021-00829-y
39. Ganz PA, Habel LA, Weltzien EK, Caan BJ, and Cole SW. Examining the influence of beta blockers and ACE inhibitors on the risk for breast cancer recurrence: Results from the LACE cohort. Breast Cancer Res Treat. (2011) 129:549–56. doi: 10.1007/s10549-011-1505-3
40. Giampieri R, Scartozzi M, Del Prete M, Faloppi L, Bianconi M, Ridolfi F, et al. Prognostic value for incidental antihypertensive therapy with β-blockers in metastatic colorectal cancer. Medicine. (2015) 94:e719. doi: 10.1097/MD.0000000000000719
41. Gillis RD, Botteri E, Chang A, Ziegler AI, Chung N, Pon CK, et al. Carvedilol blocks neural regulation of breast cancer progression in vivo and is associated with reduced breast cancer mortality in patients. Eur J Cancer. (2021) 147:106–16. doi: 10.1016/j.ejca.2021.01.029
42. Grytli HH, Fagerland MW, Fosså SD, and Taskén KA. Association between use of β-blockers and prostate cancer-specific survival: A cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol. (2014) 65:635–41. doi: 10.1016/j.eururo.2013.01.007
43. Hanley GE, Kaur P, Berchuck A, Chase A, Grout B, Deurloo CM, et al. Cardiovascular medications and survival in people with ovarian cancer: A population-based cohort study from British Columbia, Canada. Gynecol Oncol. (2021) 162:461–8. doi: 10.1016/j.ygyno.2021.05.021
44. Harding BN, Delaney JA, Urban RR, and Weiss NS. Post-diagnosis use of antihypertensive medications and the risk of death from ovarian cancer. Gynecol Oncol. (2019) 154:426–31. doi: 10.1016/j.ygyno.2019.05.030
45. He L, Qiao W, Liao Z, Komaki R, Ho L, Hofstetter WL, et al. Impact of comorbidities and use of common medications on cancer and non-cancer specific survival in esophageal carcinoma. BMC Cancer. (2015) 15:1095. doi: 10.1186/s12885-015-1095-2
46. Heitz F, du Bois A, Harter P, Lubbe D, Kurzeder C, Vergote I, et al. Impact of beta blocker medication in patients with platinum sensitive recurrent ovarian cancer—a combined analysis of 2 prospective multicenter trials by the AGO Study Group, NCIC-CTG and EORTC-GCG. Gynecol Oncol. (2013) 129:463–6. doi: 10.1016/j.ygyno.2013.03.007
47. Hicks BM, Murray LJ, Powe DG, Hughes CM, and Cardwell CR. β-Blocker usage and colorectal cancer mortality: A nested case–control study in the UK Clinical Practice Research Datalink cohort. Ann Oncol. (2013) 24:3100–6. doi: 10.1093/annonc/mdt381
48. Holmes S, Griffith EJ, Musto G, and Minuk GY. Antihypertensive medications and survival in patients with cancer: A population-based retrospective cohort study. Cancer Epidemiol. (2013) 37:881–5. doi: 10.1016/j.canep.2013.09.001
49. Hsieh H, Wu T, Chen C, Kuo Y, and Hour M. Survival outcomes of beta-blocker usage in HER2-positive advanced breast cancer patients: A retrospective cohort study. Ther Adv Drug Saf. (2023) 14. doi: 10.1177/20420986231181338
50. Huang T, Townsend MK, Dood RL, Sood AK, and Tworoger SS. Antihypertensive medication use and ovarian cancer survival. Gynecol Oncol. (2021) 163:342–7. doi: 10.1016/j.ygyno.2021.09.009
51. Jansen L, Hoffmeister M, Arndt V, Chang-Claude J, and Brenner H. Stage-specific associations between beta blocker use and prognosis after colorectal cancer. Cancer. (2014) 120:1178–86. doi: 10.1002/cncr.28546
52. Jansen L, Weberpals J, Kuiper JG, Vissers PAJ, Wolkewitz M, Hoffmeister M, et al. Pre- and post-diagnostic beta-blocker use and prognosis after colorectal cancer: Results from a population-based study. Int J Cancer. (2017) 141:62–71. doi: 10.1002/ijc.30717
53. Johannesdottir Schmidt SA and Schmidt M. Beta-blockers and improved survival from ovarian cancer: New miracle treatment or another case of immortal person-time bias? Cancer. (2016) 122:324–5. doi: 10.1002/cncr.29721
54. Katsarelias D, Eriksson H, Mikiver R, Krakowski I, Nilsson JA, Ny L, et al. The effect of beta-adrenergic blocking agents in cutaneous melanoma—A nation-wide Swedish population-based retrospective register study. Cancers. (2020) 12:3228. doi: 10.3390/cancers12113228
55. Kennedy OJ, Kicinski M, Valpione S, Gandini S, Suciu S, Blank CU, et al. Prognostic and predictive value of β-blockers in the EORTC 1325/KEYNOTE-054 phase III trial of pembrolizumab versus placebo in resected high-risk stage III melanoma. Eur J Cancer. (2022) 165:97–112. doi: 10.1016/j.ejca.2022.01.017
56. Kim SA, Moon H, Roh JL, Kim SB, Choi SH, Nam SY, et al. Postdiagnostic use of β-blockers and other antihypertensive drugs and the risk of recurrence and mortality in head and neck cancer patients: An observational study of 10,414 person-years of follow-up. Clin Trans Oncol. (2017) 19:826–33. doi: 10.1007/s12094-016-1608-8
57. Kocak MZ, Er M, Ugrakli M, Hendem E, Araz M, Eryilmaz MK, et al. Could the concomitant use of beta blockers with bevacizumab improve survival in metastatic colon cancer? Eur J Clin Pharmacol. (2023) 79:485–91. doi: 10.1007/s00228-023-03464-w
58. Kreklau A, Nel I, Kasimir-Bauer S, Kimmig R, Frackenpohl AC, and Aktas B. An observational study on breast cancer survival and lifestyle related risk factors. In Vivo. (2021) 35:1007–15. doi: 10.21873/invivo.12344
59. Le Bozec A, Brugel M, Djerada Z, Ayad M, Perrier M, Carlier C, et al. Beta-blocker exposure and survival outcomes in patients with advanced pancreatic ductal adenocarcinoma: A retrospective cohort study (BETAPANC). Front Pharmacol. (2023) 14. doi: 10.3389/fphar.2023.1137791
60. Lemeshow S, Sørensen HT, Phillips G, Yang EV, Antonsen S, Riis AH, et al. β-Blockers and survival among Danish patients with Malignant melanoma: A population-based cohort study. Cancer Epidemiol Biomarkers Prev. (2011) 20:2273–9. doi: 10.1158/1055-9965.EPI-11-0249
61. Livingstone E, Hollestein LM, van Herk-Sukel MPP, van de Poll-Franse L, Nijsten T, SChadendorf D, et al. β-Blocker use and all-cause mortality of melanoma patients: Results from a population-based Dutch cohort study. Eur J Cancer. (2013) 49:3863–71. doi: 10.1016/j.ejca.2013.07.141
62. Melhem-Bertrandt A, Chavez-MacGregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. (2011) 29:2645–52. doi: 10.1200/JCO.2010.33.4441
63. Mellgard G, Patel VG, Zhong X, Joshi H, Qin Q, Wang B, et al. Effect of concurrent beta-blocker use in patients receiving immune checkpoint inhibitors for advanced solid tumors. J Cancer Res Clin Oncol. (2023) 149:2833–41. doi: 10.1007/s00432-022-04159-y
64. Nayan M, Juurlink DN, Austin PC, Macdonald EM, Finelli A, Kulkarni GS, et al. Medication use and kidney cancer survival: A population-based study. Int J Cancer. (2018) 142:1776–85. doi: 10.1002/ijc.31204
65. Oh MS, Guzner A, Wainwright DA, Mohindra NA, Chae YK, Behdad A, et al. The impact of beta blockers on survival outcomes in patients with non-small-cell lung cancer treated with immune checkpoint inhibitors. Clin Lung Cancer. (2020) 22:e57–62. doi: 10.1016/j.cllc.2020.07.016
66. Posielski NA, Richards KA, Liou J, Borza T, Abel EJ, Downs TM, et al. Beta-adrenergic antagonists and cancer specific survival in patients with advanced prostate cancer: A Veterans Administration cohort study. Urology. (2021) 155:186–91. doi: 10.1016/j.urology.2021.02.008
67. Powe DG, Voss MJ, Zänker KS, Habashy HO, Green AR, Ellis IO, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. (2010) 1:628–38. doi: 10.18632/oncotarget.197
68. Sakellakis M, Kostaki A, Starakis I, and Koutras A. β-Blocker use and risk of recurrence in patients with early breast cancer. Chemotherapy. (2015) 60:288–9. doi: 10.1159/000371871
69. Sanni OB, Mc Menamin UC, Cardwell CR, Sharp L, Murray LJ, and Coleman HG. Commonly used medications and endometrial cancer survival: A population-based cohort study. Br J Cancer. (2017) 117:432–8. doi: 10.1038/bjc.2017.207
70. Santala EEE, Artama M, Pukkala E, Visvanathan K, Staff S, and Murtola TJ. Antihypertensive drug use and the risk of ovarian cancer death among Finnish ovarian cancer patients—A nationwide cohort study. Cancers. (2021) 13:2087. doi: 10.3390/cancers13092087
71. Scott OW, Tin ST, Elwood JM, Cavadino A, Habel LA, Kuper-Hommel M, et al. Post-diagnostic beta blocker use and breast cancer-specific mortality: A population-based cohort study. Breast Cancer Res Treat. (2022) 193:225–35. doi: 10.1007/s10549-022-06528-0
72. Shah SM, Carey IM, Owen CG, Harris T, DeWilde S, and Cook DG. Does β-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br J Clin Pharmacol. (2011) 72:157–61. doi: 10.1111/j.1365-2125.2011.03980.x
73. Siltari A, Murtola TJ, Talala K, Taari K, Tammela TLJ, and Auvinen A. Antihypertensive drug use and prostate cancer-specific mortality in Finnish men. PloS One. (2020) 15:e0234269. doi: 10.1371/journal.pone.0234269
74. Springate DA, Ashcroft DM, Kontopantelis E, Doran T, Ryan R, and Reeves D. Can analyses of electronic patient records be independently and externally validated? Study 2—The effect of β-adrenoceptor blocker therapy on cancer survival: A retrospective cohort study. BMJ Open. (2015) 5:e007299. doi: 10.1136/bmjopen-2014-007299
75. Støer NC, Bouche G, Pantziarka P, Sloan EK, Andreassen BK, and Botteri E. Use of non-cancer drugs and survival among patients with pancreatic adenocarcinoma: A nationwide registry-based study in Norway. Acta Oncol. (2021) 60:1146–53. doi: 10.1080/0284186X.2021.1953136
76. Sud S, O’Callaghan C, Jonker C, Karapetis C, Price T, Tebbutt N, et al. Hypertension as a predictor of advanced colorectal cancer outcome and cetuximab treatment response. Curr Oncol. (2018) 25:e516–26. doi: 10.3747/co.25.4069
77. Tan GSQ, Botteri E, Wood S, Sloan EK, and Ilomäki J. Using administrative healthcare data to evaluate drug repurposing opportunities for cancer: The possibility of using beta-blockers to treat breast cancer. Front Pharmacol. (2023) 14:1227330. doi: 10.3389/fphar.2023.1227330
78. Udumyan R, Montgomery S, Fang F, Almroth H, Valdimarsdottir U, Ekbom A, et al. Beta-blocker drug use and survival among patients with pancreatic adenocarcinoma. Cancer Res. (2017) 77:3700–7. doi: 10.1158/0008-5472.CAN-17-0108
79. Udumyan R, Montgomery S, Fang F, Valdimarsdottir U, Hardardottir H, Ekbom A, et al. Beta-blocker use and lung cancer mortality in a nationwide cohort study of patients with primary non–small cell lung cancer. Cancer Epidemiol Biomarkers Prev. (2020) 29:119–26. doi: 10.1158/1055-9965.EPI-19-0710
80. Udumyan R, Montgomery S, Duberg A, Fang F, Valdimarsdottir U, Ekbom A, et al. Beta-adrenergic receptor blockers and liver cancer mortality in a national cohort of hepatocellular carcinoma patients. Scand J Gastroenterol. (2020) 55:597–605. doi: 10.1080/00365521.2020.1762919
81. Udumyan R, Botteri E, Jerlström T, Montgomery S, Smedby KE, and Fall K. Beta-blocker use and urothelial bladder cancer survival: A Swedish register-based cohort study. Acta Oncol. (2022) 61:922–30. doi: 10.1080/0284186X.2022.2101902
82. Wang H, Liao Z, Zhuang Y, Liu Y, Levy LB, Xu T, et al. Incidental receipt of cardiac medications and survival outcomes among patients with stage III non-small-cell lung cancer after definitive radiotherapy. Clin Lung Cancer. (2015) 16:128–36. doi: 10.1016/j.cllc.2014.09.006
83. Wang DY, McQuade JL, Rai RR, Park JJ, Zhao S, Ye F, et al. The impact of nonsteroidal anti-inflammatory drugs, beta blockers, and metformin on the efficacy of anti-PD-1 therapy in advanced melanoma. Oncologist. (2019) 25:e602–5. doi: 10.1634/theoncologist.2019-0518
84. Watkins JL, Thaker PH, Nick AM, Ramondetta LM, Kumar S, Urbauer DL, et al. Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer. (2015) 121:3444–51. doi: 10.1002/cncr.29392
85. Weberpals J, Jansen L, van Herk-Sukel MPP, Kuiper JG, Aarts MJ, Vissers PAJ, et al. Immortal time bias in pharmacoepidemiological studies on cancer patient survival: Empirical illustration for beta-blocker use in four cancers with different prognosis. Eur J Epidemiol. (2017) 32:1019–31. doi: 10.1007/s10654-017-0304-5
86. Weberpals J, Jansen L, Haefeli WE, Hoffmeister M, Wolkewitz M, van Herk-Sukel MPP, et al. Pre- and post-diagnostic β-blocker use and lung cancer survival: A population-based cohort study. Sci Rep. (2017) 7:2911. doi: 10.1038/s41598-017-02913-8
87. Wrobel LJ, Gayet-Ageron A, and Le Gal F. Effects of beta-blockers on melanoma microenvironment and disease survival in human. Cancers. (2020) 12:1094. doi: 10.3390/cancers12051094
88. Wu YL, van Hyfte G, Özbek U, Reincke M, Gampa A, Mohamed YI, et al. Outcomes of beta blocker use in advanced hepatocellular carcinoma treated with immune checkpoint inhibitors. Front Oncol. (2023) 13:1128569. doi: 10.3389/fonc.2023.1128569
89. Yang P, Deng W, Han Y, Shi Y, Xu T, Shi J, et al. Analysis of the correlation among hypertension, the intake of β-blockers, and overall survival outcome in patients undergoing chemoradiotherapy with inoperable stage III non-small cell lung cancer. Am J Cancer Res. (2017) 7:946–54. doi: 10.1016/j.cell.2017.02.005
90. Yang A, Zylberberg HM, Rustgi SD, Amin SP, Bar-Mashiah A, Boffetta P, et al. Beta-blockers have no impact on survival in pancreatic ductal adenocarcinoma prior to cancer diagnosis. Sci Rep. (2021) 11:1038. doi: 10.1038/s41598-020-79999-0
91. Zaborowska-Szmit M, Szmit S, Olszyna-Serementa M, Badurak P, Zajda K, Jaowicz-Zebrowska A, et al. Beta blockers with statins may decrease all-cause mortality in patients with cardiovascular diseases and locally advanced unresectable non-small-cell lung cancer after chemoradiotherapy. Cancers. (2023) 15:1277. doi: 10.3390/cancers15041277
92. Zhang Y, Song M, Chan AT, Meyerhardt JA, Willet WC, and Giovannucci EL. Long-term use of antihypertensive medications, hypertension and colorectal cancer risk and mortality: A prospective cohort study. Br J Cancer. (2022) 127:1974–82. doi: 10.1038/s41416-022-01975-4
93. Kilmister EJ and Tan ST. Insights into vascular anomalies, cancer, and fibroproliferative conditions: the role of stem cells and the renin-angiotensin system. Front Surg. (2022) 9:868187. doi: 10.3389/fsurg.2022.868187
94. Duhalde Vega M, Olivera D, Gastão Davanzo G, Bertullo M, Noya V, Fabiano de Souza G, et al. PD-1/PD-L1 blockade abrogates a dysfunctional innate-adaptive immune axis in critical β-coronavirus disease. Sci Adv. (2022) 8:eabn6545. doi: 10.1126/sciadv.abn6545
95. Li W, Zhang J, Gao Y, Kong X, and Sun X. Nervous system in hepatocellular carcinoma: Correlation, mechanisms, therapeutic implications, and future perspectives. Biochim Biophys Acta Rev Cancer. (2025) 1880:189345. Advance online publication. doi: 10.1016/j.bbcan.2025.189345
96. Ramirez-Bermudez J, Espinola-Nadurille M, Restrepo-Martinez M, Martínez-Ángeles V, Martínez-Carrillo F, Cascante L, et al. Autoimmune psychosis: Psychopathological patterns and outcome after immunotherapy. Schizophr Res. (2025) 281:10–9. doi: 10.1016/j.schres.2025.04.02496
97. Puzderova B, Belvoncikova P, Grossmannova K, Csaderova L, Labudova M, Fecikova S, et al. Propranolol, promising chemosensitizer and candidate for the combined therapy through disruption of tumor microenvironment homeostasis by decreasing the level of carbonic anhydrase IX. Int J Mol Sci. (2023) 24:11094. doi: 10.3390/ijms241311094
98. Tabatabai G, Platten M, Preusser M, Weller M, Wick W, and van den Bent M. Treatment of glioblastoma patients with personalized vaccines outside clinical trials: Lessons ignored? Neuro-oncology. (2025) 27:302–5. doi: 10.1093/neuonc/noae22598
99. Butt MS, Naz A, Sultan MT, and Qayyum MM. Anti-oncogenic perspectives of spices/herbs: A comprehensive review. EXCLI J. (2013) 12:1043–65.
100. Lerch M and Ramanathan S. The pathogenesis of neurological immune-related adverse events following immune checkpoint inhibitor therapy. Semin Immunol. (2025) 78:101956. doi: 10.1016/j.smim.2025.101956100
Keywords: Beta-blockers, cancer neuroimmunology, immune checkpoint inhibitors, tumor microenvironment, meta-analysis
Citation: Zhang F, Wang Y, Liu F, Li Y, Liu X, Ren X and Yuan X (2025) Impact of beta blockers on cancer neuroimmunology: a systematic review and meta-analysis of survival outcomes and immune modulation. Front. Immunol. 16:1635331. doi: 10.3389/fimmu.2025.1635331
Received: 26 May 2025; Accepted: 03 July 2025;
Published: 06 August 2025.
Edited by:
Qihang Yuan, First Affiliated Hospital, Dalian Medical University, ChinaReviewed by:
Lifeng Li, First Affiliated Hospital of Zhengzhou University, ChinaXingyu Sun, Southwest Medical University, China
Guodong Cao, Anhui Medical University, China
Xintian Cai, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, China
Weijie Sun, First Affiliated Hospital of Bengbu Medical University, China
Copyright © 2025 Zhang, Wang, Liu, Li, Liu, Ren and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinxin Liu, Z29yaWxsYTE5OTlAaG90bWFpbC5jb20=; Xueying Ren, MTU5NTE5MTYzMTZAMTYzLmNvbQ==; Xi Yuan, eXVhbnhpMTIyOTg0QDE2My5jb20=
†These authors share first authorship
 Fangyuan Zhang1,2†
Fangyuan Zhang1,2†