- 1Molecular Biology and Biochemistry Department, Molecular Genetics Research Team (MGRT), Faculty of Biotechnology, German International University (GIU), Cairo, Egypt
- 2Clinical Sciences Department, College of Medicine, University of Sharjah, Sharjah, United Arab Emirates
- 3Research Institute for Medical and Health Sciences, University of Sharjah, Sharjah, United Arab Emirates
- 4Biotechnology School, Nile University, Giza, Egypt
- 5Department of Chemistry (Biochemistry Division), Faculty of Science, Cairo University, Giza, Egypt
- 6Proteomics and Metabolomics Research Program, Basic Research Unit, Research Department, Children’s Cancer Hospital Egypt, Cairo, Egypt
- 7Department of Pharmacology and Toxicology, Heliopolis University for Sustainable Development (HU), Cairo, Egypt
Macrophage migration inhibitory factor (MIF) is a pleiotropic cytokine with a pivotal role in immune regulation, inflammation, and tumorigenesis. Originally identified as a T cell-derived factor inhibiting macrophage migration, MIF has since been recognized as a key player in the progression of a wide range of solid tumors. This comprehensive review traces the historical discovery and evolving understanding of MIF, highlighting its structural features, receptor interactions, and intracellular signaling mechanisms. The review also explores the molecular mechanisms of MIF involvement in tumor pathogenesis through promoting proliferation, angiogenesis, immune evasion, and metastasis. Special focus is given to MIF interplay with several oncogenic pathways, modulation of the tumor microenvironment, and its dual role in both autocrine and paracrine signaling within tumors. The review also discusses emerging insights into MIF’s involvement in therapeutic resistance and its potential as a diagnostic biomarker and therapeutic target. By consolidating current knowledge, the authors aim to provide a detailed perspective on MIF’s multifaceted role in solid tumors and to outline future directions for research and clinical intervention.
1 Introduction
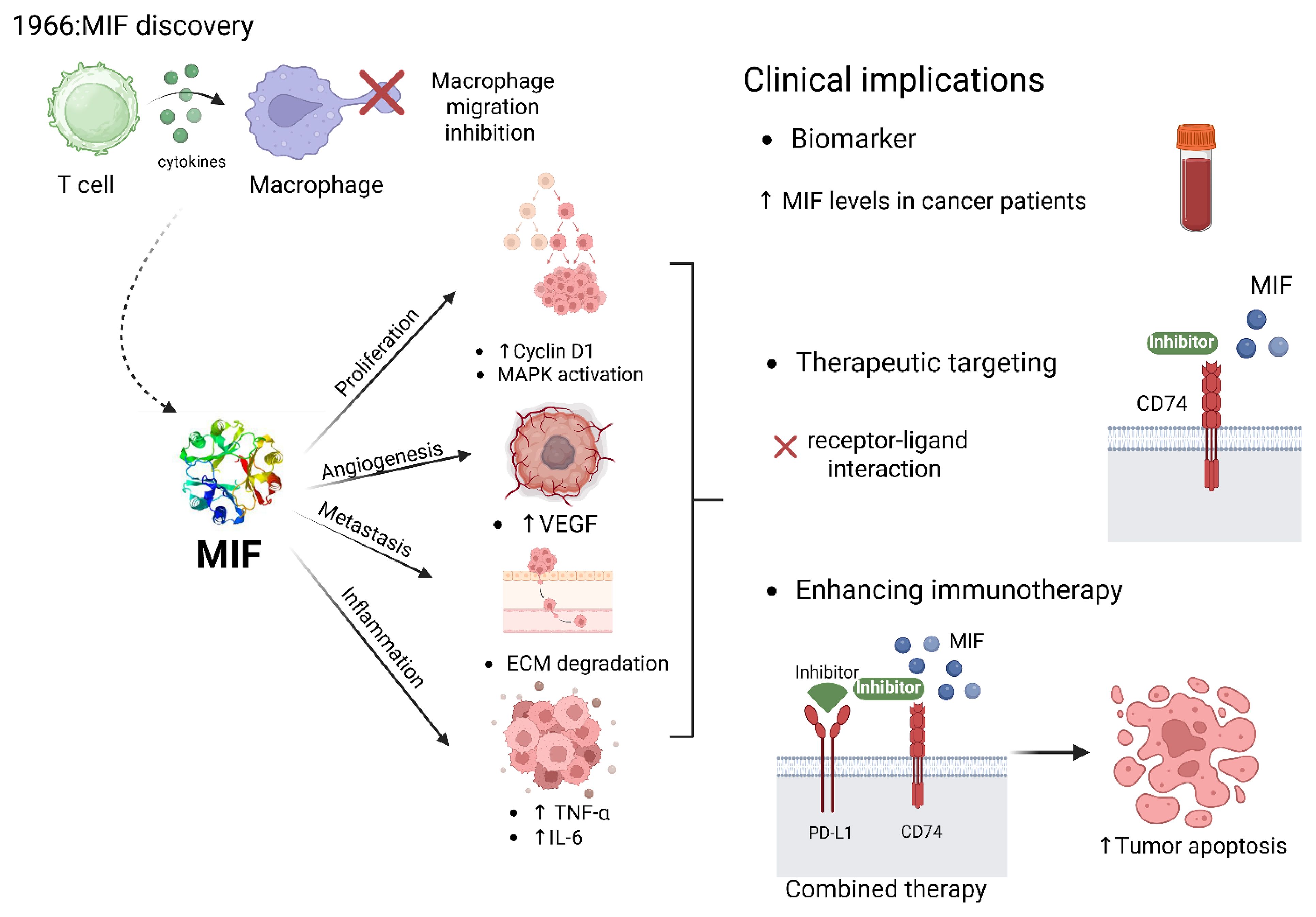
Macrophage migration inhibitory factor (MIF) is a multi-functional cytokine that exerts a crucial role in immune and inflammatory regulation (1). It is recognized as a pro-inflammatory cytokine and hormone that performs vital roles in stress responses. MIF is primarily produced in the anterior pituitary gland; its secretion is induced by corticotrophin-releasing hormone in response to stress (2). MIF is secreted by various cells including monocytes, macrophages, B cells, T cells, in addition to endocrine, endothelial, and epithelial cells (3) and is released by binding of toll-like receptors or pattern recognition receptors to small molecular motifs such as pathogen-associated molecular patterns (4). On the functional level, MIF is involved in a variety of biological functions including the production of inflammatory cytokines, such as tumor necrosis factor, interleukin-6, interferon-γ, and interleukin-1β; hormone immunomodulation; muscle glucose catabolism regulation; tumor growth promotion; and pathology of diseases such as rheumatoid arthritis, asthma, lupus, and atherosclerosis (5). Moreover, MIF exhibits chemokine-like activity besides stimulating target cell migration and recruiting leukocytes to infectious and inflammatory areas (6). The MIF journey, starting from its discovery to its current role as one of the pivotal cytokines in immunology, represents a remarkable odyssey that traces the evolution of concepts on immune regulation as illustrated in Figure 1. In 1932, the Bulletin of the Johns Hopkins Hospital described a formerly unrecognized biological activity attributing to Mycobacteria-sensitized lymphocytes to arrest the migration of tissue macrophages in vitro (7). In the late 1950s, MIF was first identified as a product derived from activated T cells that inhibited the random migration of macrophages which was related to delayed-type hypersensitivity reactions (8). In 1962, MIF was identified to quantitate the migration of peritoneal cells in capillary tubes (9). Independently, in 1966, John David and Barry Bloom reported that MIF is indeed a protein produced by activated lymphocytes further establishing MIF as a unique cytokine (10). Cloning of the human MIF gene in 1989 provided information about its structure, biological roles, and functional features (11).
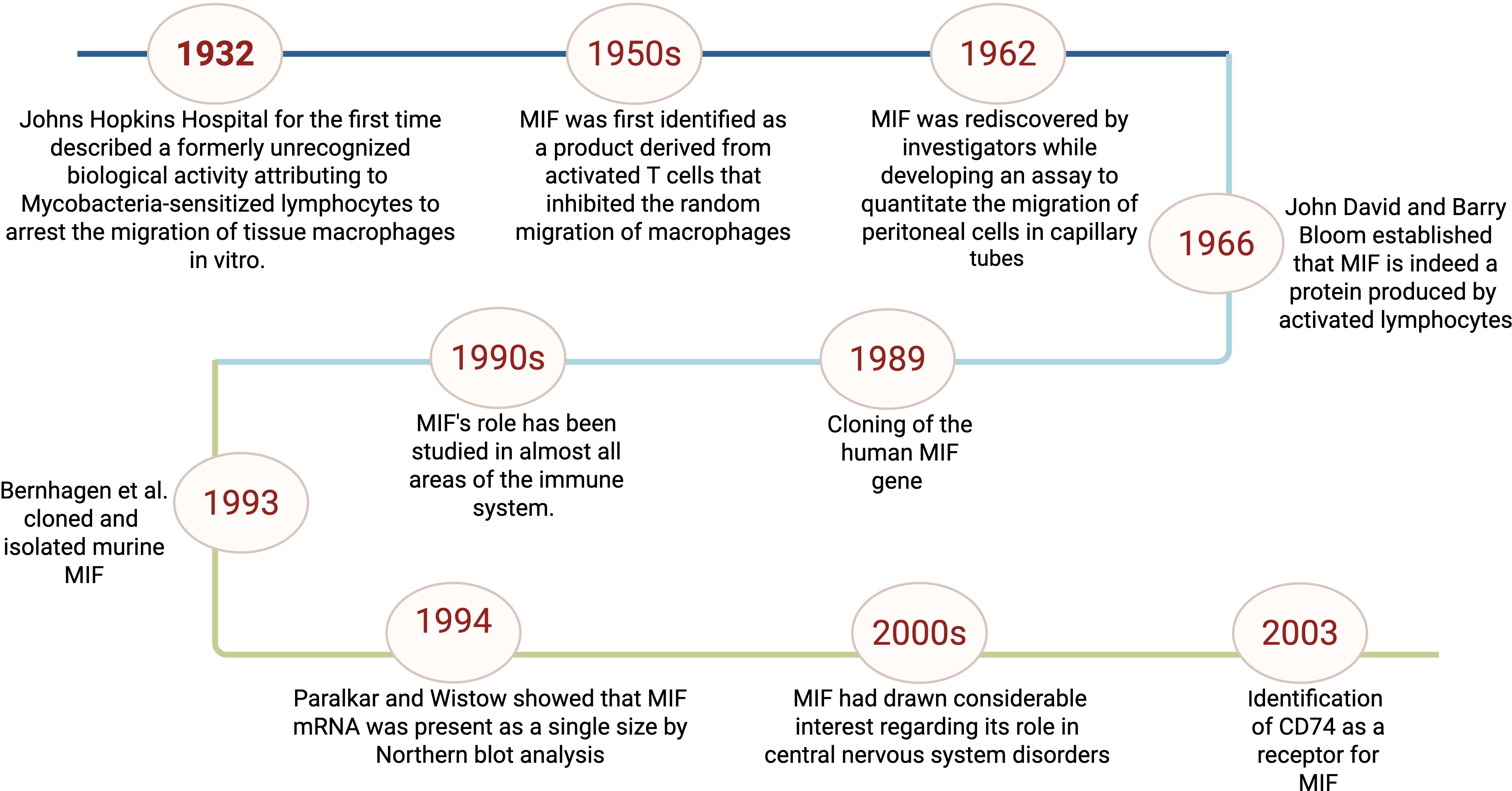
Figure 1. Timeline of key discoveries related to macrophage migration inhibitory factor (MIF) This figure represents a schematic representation for the timeline of MIF initial identification to the discovery of its receptor and roles in immunity.
In 1996, the three-dimensional crystal structure of MIF was identified unraveling a novel protein fold and nominating MIF as a new superfamily. This structural insight suggested that the natural form of MIF is a homotrimer with a molecular weight of about 37.5 kDa, thus providing a structural basis for understanding its functional properties (12).
Human MIF depicts 90% sequence similarity with mouse MIF. MIF gene is located on chromosome 22 (22q11.2) and consists of three short exons and two introns. Besides, two promoter polymorphisms -CATT5–8 and G/C- are located at positions –794 and –173, respectively (Figure 2) (13). MIF encodes an evolutionarily conserved protein with a relative molecular mass of ~12.5 kDa, composed of 114–115 amino acids folded into an enzymatically active homotrimer with four β-strands and two α-helices (14). The catalytic function of MIF is attributed to a distinctive N-terminal proline that enables the keto-enol tautomerization of substrates such as D-dopachrome and L-dopachrome methyl ester into their corresponding indole derivatives (15). CD74 is a type II transmembrane protein with an apparent molecular weight of 31–41 kDa (16). MIF interacts with the extracellular domain of CD74 transmembrane receptor, resulting in the activation of various signaling pathways such as Extracellular Signal-Regulated Kinase (ERK), Phosphatidylinositol 3-Kinase (PI3K)-Akt, and Nuclear Factor-kappa B (NF-κB) (16). The MIF-induced cellular functions are mostly mediated by mitogen-activated protein kinases (MAPKs) (16). MIF-dependent phosphorylation of extracellular signal-regulated kinases (ERK-1/2), synthesis of prostaglandin E2, and cell proliferation rely on the cell surface expression of CD74 (6). The cytoplasmic tail of CD74 lacks a signal transduction domain; however, CD44 constitutes an essential part of the CD74 receptor complex through which MIF signal transduction occurs. The interaction of MIF with this receptor complex phosphorylates CD74 and CD44 in the intracytoplasmic domain (17). CD74 is essential for MIF-mediated protection against apoptosis, which requires the presence of its receptor counterpart, CD44. The polymorphic transmembrane protein CD44 has demonstrated tyrosine kinase activation characteristics and activates Src-family tyrosine kinases which in turn phosphorylate ERK (Figure 2) (17). In addition, MIF directly interacts with the chemokine receptors CXCR2 and CXCR4, modulating cell migration and inflammation by activating the ERK1/2 and PI3K/Akt signaling pathways. MIF rapidly associates with CXCR2 and competes with its ligands (18), which suggests that MIF signaling pathway is mediated by the complex CD74/CXCR2 receptor (Figure 2) (19).
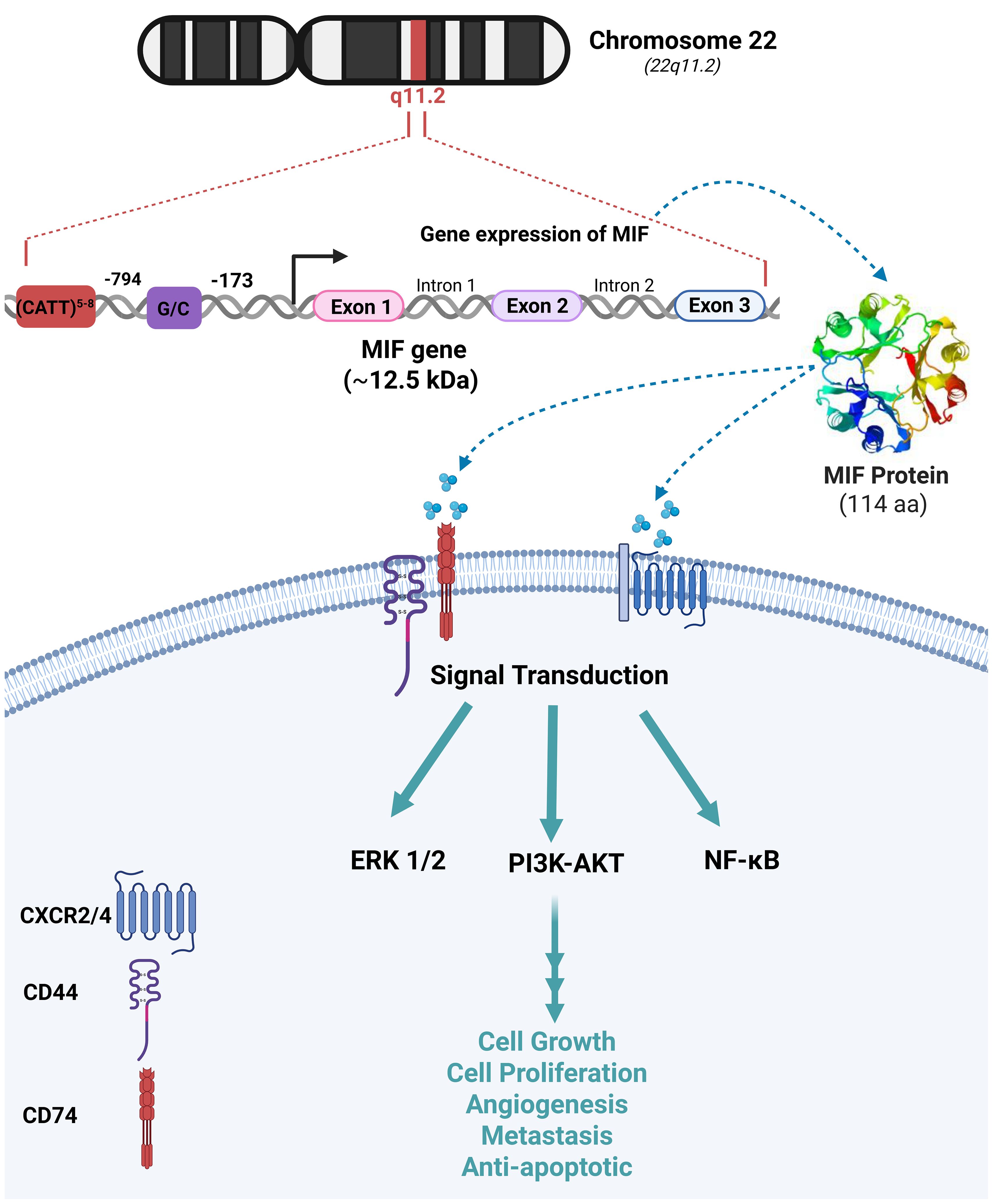
Figure 2. MIF Chromosomal location, genetic dissection and biological functions The migration inhibitory factor (MIF) gene is located on chromosome 22q11.2 and consists of three exons separated by two introns. Its transcription is regulated by two distinct promoter elements, CATT5–8 and G/C, positioned at nucleotides 794 and 173, respectively. MIF exerts its biological functions through interactions with CD74, CD44, and chemokine receptors CXCR2/4 on the cell surface. These interactions activate downstream signaling cascades, including the extracellular signal-regulated kinase 1/2 (ERK1/2), phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT), and nuclear factor kappa B (NF-κB) pathways. These pathways regulate key cellular processes such as proliferation, growth, angiogenesis, metastasis, and anti-apoptosis.
2 Intracellular MIF mechanisms in innate immunity, inflammation, and autoimmunity
MIF interacts with intracellular proteins such as c-Jun activation domain-binding protein 1 (JAB1) which is a coactivator of the Activator Protein 1 (AP-1) transcription factor (20). The MIF–JAB1 interaction inhibits the degradation of cyclin-dependent kinase inhibitor p27Kip1 (21), which in turn results in cell cycle arrest (22). MIF exerts control over its functions through catalytic activities (23), where it exhibits both enzymatic thiol-protein oxidoreductase and tautomerase/isomerase activities (24), which are implicated in the catecholamine-converting activity of MIF (25). We will further discuss the critical roles of MIF in modulating the immune system.
MIF has a crucial role in the regulation of innate immunity (26, 27). The cytokine is constitutively expressed by most immune cells, including macrophages, and is rapidly released in response to stress and microbial stimulation (28). MIF induces the expression of pro-inflammatory cytokines and up-regulates TLR4, thus facilitating the recognition of bacterial infections such as Salmonella typhimurium (29). MIF triggers several signal pathways, including the activation of the mitogen-activated protein kinase-analyzed ERK1/ERK2 pathway, necessary for macrophage activation and function (17). It further suppresses p53-dependent apoptosis in macrophages to allow continuous execution of pro-inflammatory functions as shown in Figure 3 (30). MIF is implicated in numerous inflammatory and autoimmune conditions, such as rheumatoid arthritis, septic shock, and inflammatory bowel disease. Its dysregulation can lead to exacerbation of the inflammatory response and perpetuation of diseases (31). Furthermore, it plays a pivotal role in the modulation of inflammatory response. By activating immune cells through its receptor CD74, MIF modulates the secretion of pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6 (32). This pro-inflammatory activity is crucial in response to infection and injury, helping to coordinate defense mechanisms (33). However, in chronic inflammatory conditions, sustained MIF expression can contribute to pathological inflammation, leading to tissue damage and autoimmune diseases.
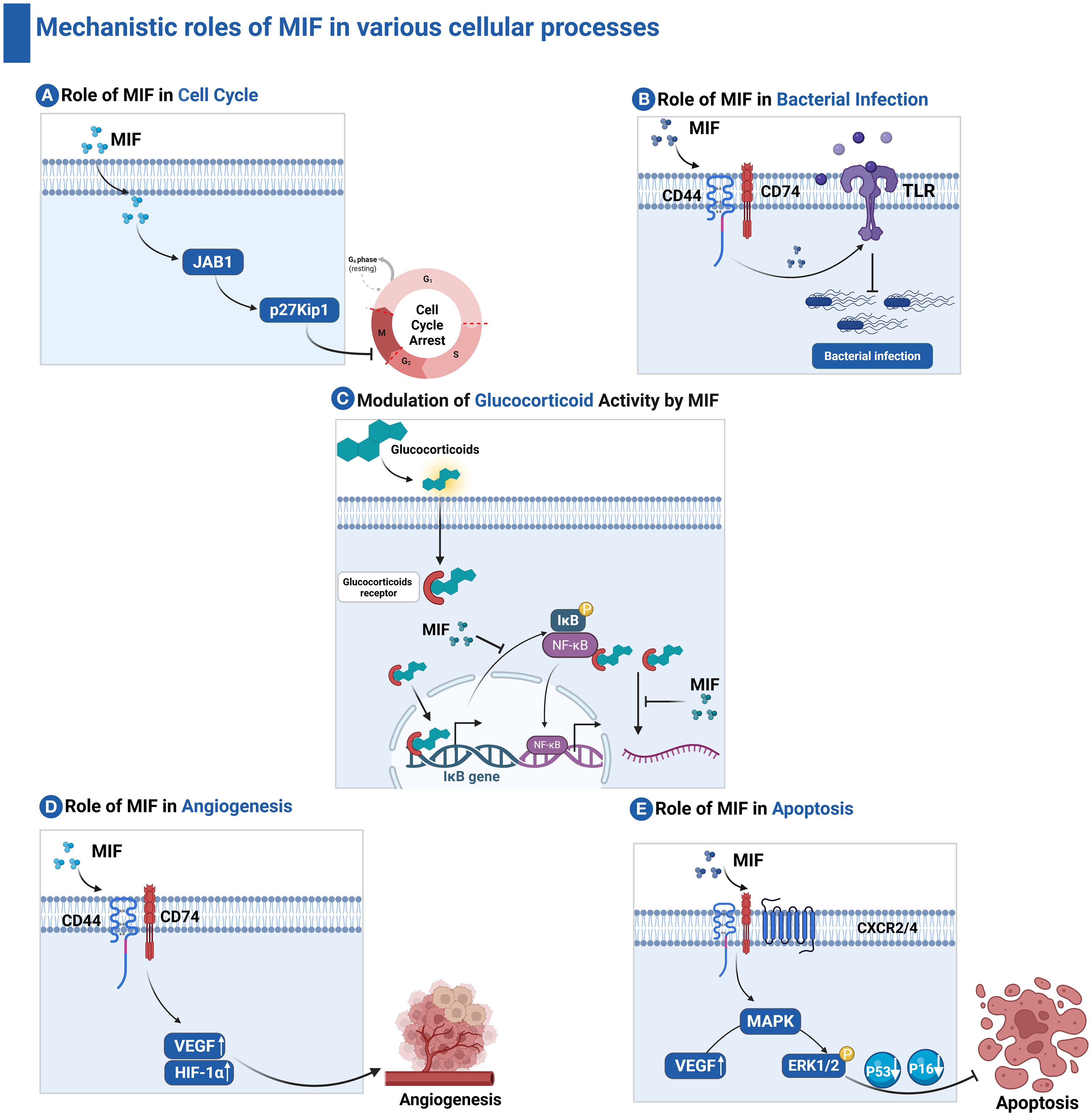
Figure 3. Mechanistic roles of MIF in various cellular processes. (A) Role of MIF in Cell Cycle: MIF interacts with JAB1, preventing the degradation of the cyclin-dependent kinase inhibitor p27Kip1, thereby inducing cell cycle arrest. (B) Role of MIF in bacterial infection: MIF promotes the expression of pro-inflammatory cytokines and upregulates TLR4, enhancing bacterial recognition. (C) Modulation of Glucocorticoid Activity by MIF: MIF antagonizes the immunosuppressive effects of glucocorticoids by inhibiting glucocorticoid-induced IκB synthesis and mRNA destabilization. (D) Role of MIF in Angiogenesis: MIF engages CD74 and CD44 receptors to promote the release of vascular endothelial growth factor (VEGF), thereby facilitating angiogenesis. (E) Role of MIF in apoptosis: MIF activates ERK1/2 signaling, leading to suppression of pro-apoptotic proteins such as p53 and p16, ultimately inhibiting apoptosis.
One of the hallmarks and unique characteristics of MIF is its ability to counteract the immunosuppressive action of glucocorticoids, thus preserving the immunological responses during conditions of inflammation and stress and allowing a much more effective immune response against pathogens (34). Furthermore, MIF promotes the migration and recruitment of immune cells by inducing the expression of chemokines and adhesion molecules as shown in Figure 3. These functions are crucial for appropriate immune surveillance and infection responses (35). Recently, MIF has been implicated in tumor immunity; it can establish both pro-inflammatory and immunosuppressive environments depending on the context. MIF has a dual role in tumor development through modulating tumor growth and angiogenesis (36). It promotes angiogenesis, which is critical for tumor growth and metastasis, through enhancing the release of vascular endothelial growth factor, a key mediator of angiogenesis as shown in Figure 3. This role is essential not only in the context of wound healing and tissue regeneration but also in enabling tumors to establish their blood supply, facilitating their growth and metastasis (37–39).
3 Interactions of MIF with immune cells in the tumor microenvironment
MIF acts on a wide array of immune cells, including macrophages, T cells, dendritic cells, Tumor-Associated Neutrophils (TANs), Myeloid-Derived Suppressor Cells (MDSCs), and natural killers’ cells (40). The interaction with all these elements has a wide-ranging effect on tumor development, and immune evasion.
3.1 Macrophages
In the context of TME, MIF plays a very critical role in polarizing macrophages toward an M2 immunosuppressive and tissue repair phenotype (27, 41). Polarization mediates tumor growth due to enhanced tumor angiogenesis and suppression of anti-tumor immunity (42). MIF acts mainly through its receptor CD74 together with co-receptors, including CD44 and CXCR4. These complexes, upon binding with MIF, trigger the signaling pathways that culminate in the release of various cytokines and chemokines to promote an immunosuppressive milieu (43). The activation of NF-κB mediates transcription of various pro-inflammatory cytokines, including IL-10. MIF is able to increase the secretion of IL-10 from macrophages and offers a more permissive immunological milieu. Besides IL-10, MIF can induce the release of other pro-inflammatory cytokines like TNF-α and IL-1β. This dual role of stimulating pro-inflammatory cytokines while enhancing anti-inflammatory signals underlines the very complex nature of MIF influence on macrophage function within the TME (Figure 4) (43).
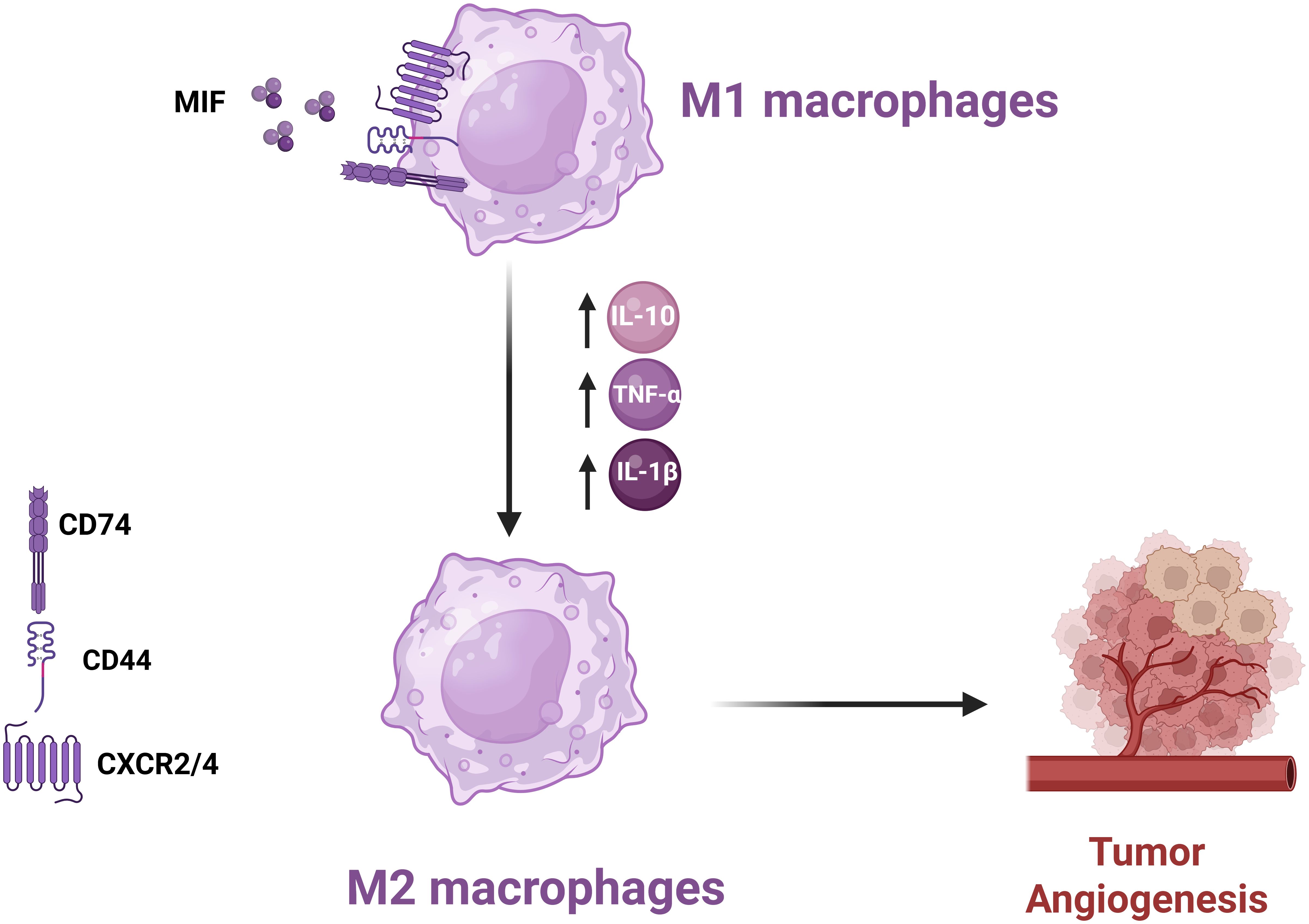
Figure 4. Mechanism of MIF-Induced Polarization of M1 Macrophages to M2 Phenotype MIF promotes M2 macrophage polarization in the TME, enhancing angiogenesis and suppressing anti-tumor immune responses, thereby facilitating tumor progression.
3.2 T cells
MIF has a substantial impact on T cell functionality by influencing the activation and differentiation of numerous T cell subsets, including Th1, Th2, and Tregs (41, 44). High levels of MIF can cause immunosuppressive phenotypes, which prevent efficient anti-tumor responses. Previous studies have demonstrated that MIF stimulates the development of Tregs by modulating IL-2 production, hence increasing tumor growth by generating an environment permissive to immunological tolerance (45). CD74 is expressed on the surface of activated T lymphocytes, and its binding to MIF causes important intracellular signaling events. This involves the stimulation of pathways involved in T cell migration 3and proliferation. MIF’s interaction with CD74 increases Th17 differentiation, supporting a more aggressive immune response in some circumstances and contributing to T cell exhaustion in others. Of note, MIF increases Treg activities; thus, MIF increases the formation of tumor-associated Tregs that are crucial in maintaining immunological tolerance in the TME. Notably, MIF knockout models demonstrated a high reduction in Treg levels and increased anti-tumor immunity (Figure 5) (19, 45).
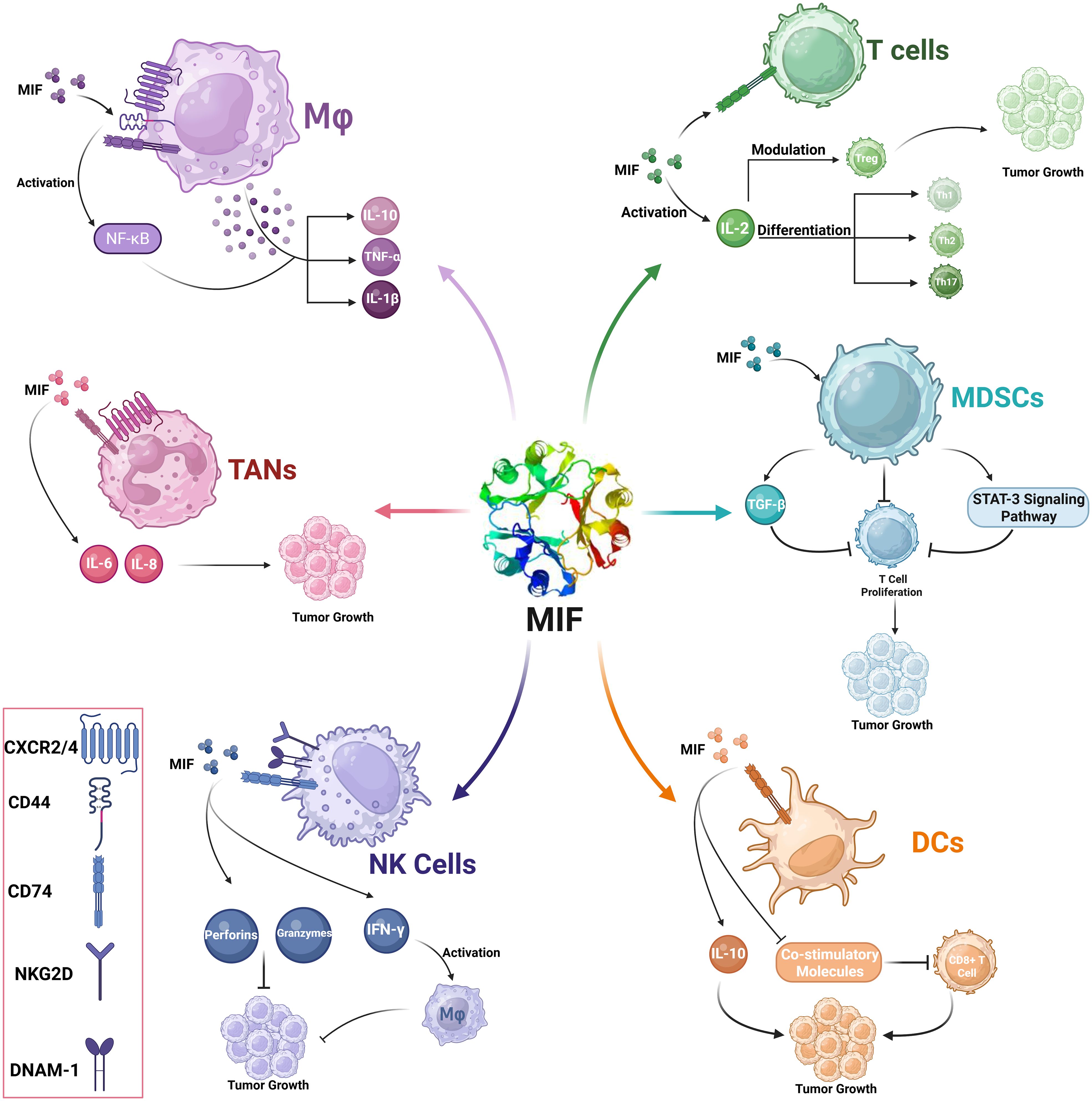
Figure 5. MIF-mediated modulation of immune cells in the tumor microenvironment (TME). This schematic illustration represents the role of MIF in modulating several immune cells at the TME.
3.3 Dendritic cells
MIF controls the function and maturation of DCs, which represent one of the most powerful antigen-presenting cells of the immune system. Through controlling the interaction of DC with T cells, MIF alters the adaptive immune response toward tumors. The major receptor through which MIF acts on DCs is the CD74 receptor, which represents the invariant chain of MHC class II molecules (46). The interaction plays an important role in triggering downstream signaling pathways that control DC activity. MIF binding to CD74 suppresses the expression of key co-stimulatory molecules on DCs. This downregulation inhibits T-cell activation and proliferation. MIF acts through manipulating the cytokine profile that is produced by DCs. Instead of secreting pro-inflammatory cytokines such as IL-12, which induce Th1 responses, MIF-exposed DCs may secrete immunosuppressive cytokines such as IL-10. This further contributes to the tolerogenic environment that allows tumor development (Figure 3) (47).
MIF interaction with CD74 triggers a number of intracellular signaling pathways, including the PI3K/Akt pathway facilitates cell survival, but it may also have immunosuppressive implications when triggered in DCs. Activated ERK1/2 can lead to long-term inflammatory reactions, but upon being influenced by MIF, it also expresses the capability to induce tolerance. Binding of MIF to CD74 downregulates key co-stimulatory molecule expression in DCs. This results in impaired efficient T cell activation and proliferation (Figure 5) (48).
The elevated levels of MIF may result in poor maturation and functioning of DCs, thereby affecting their capability for activation of CD8+ cytotoxic T lymphocytes (49). Mature DCs generally deliver antigens in an efficient manner and provide the co-stimulatory cues necessary for the activation of CTLs. However, where maturation of DCs is impeded by MIF, owing to the inability to effectively present tumor antigens, the activation of CTL cells is lowered, reducing anti-tumor immunity. Poor effector function combined with increased expression of inhibitory receptors, such as PD-1, is characteristic of T cell exhaustion driven by chronic exposure to an immunosuppressive environment. If T cell responses can be downregulated and blocked, then tumors may escape immune surveillance eventually leading to tumor progression and spread. In conclusion, MIF regulates the production of immune suppressive factors by DCs and other immune cells that promote tumor growth (48, 49).
3.4 Natural killer cells
MIF modulates the complex activities of NK cells, one of the primary elements of the innate immune response that directly kill tumor cells (50, 51). Most of MIF functions are mediated with CD74, which is expressed on NK cells (52). The interaction induces downstream signaling that includes PI3K/Akt and MAPK/ERK cascades, which are required for activation and proliferation of NK cells (51, 53, 54). These pathways result in the increased synthesis of cytotoxic chemicals such as perforins and granzymes, which enhance the lysing capability of NK cells against tumor cells (55). MIF can induce NK cells to produce pro-inflammatory cytokines, including IFN-γ. IFN-γ, in turn, activates macrophages and enhances Th1 responses, further augmenting the antitumor immune response. MIF interacting with its receptors on NK cells upregulates the cytotoxic activity of NK cells. This includes the overexpression of activating receptors such as natural killer group 2D (NKG2D) and DNAX accessory molecule-1 (DNAM-1), which is needed for recognition and killing of tumor cells (Figure 5). The high levels of MIF in TME may evoke an immunosuppressive form leading to lower NK cell cytotoxicity, as high levels of MIF inhibit the NK cell-activating receptors, thus limiting its tumor cell recognition and killing capability (40). Furthermore, high levels of MIF have been associated with transcriptional down-regulation of NKG2D on the NK cells, hence impeding its cytotoxic activity against the tumor cells (56).
3.5 Myeloid-derived suppressor cells
Myeloid-Derived Suppressor Cells (MDSCs) are generally divided into two subsets, namely monocytic (M-MDSCs) and granulocytic or polymorphonuclear (PMN-MDSCs). They are characterized by their capability of inhibiting T cell proliferation and promoting Tregs expansion, thus contributing to an immune suppressive environment that favors tumor growth (57). MIF significantly influences the recruitment and expansion of MDSCs in the TME. Clinical evidence has shown that high levels of MIF are accompanied by higher levels and activity of MDSCs, an integral aspect of tumor immune survival. MIF promotes the expansion of myeloid cells into MDSCs (58). Several reports have identified that tumor-derived MIF can drive the expansion of both monocytic and granulocytic populations, thus enhancing the overall immunosuppressive capability of the tumor. MIF also enhances the production of key immunosuppressive molecules by MDSCs including Arginase-1 which lowers the levels of L-arginine in the microenvironment, thus directly inhibiting T cell activation (Figure 5) (59).
MIF triggers the STAT3 signaling pathway, which is involved in MDSC differentiation and function. Activation of STAT3 upregulates the production of various immunosuppressive molecules and promotes the capability to impede T cell responses from MDSCs. This mechanism also maintains the immunosuppressive phenotype in these cells, which enables their survival within the TME (60). MIF also acts by triggering the production of TGF-β by MDSCs. TGF-β is an immunosuppressive cytokine. It suppresses the function of effector T cells while promoting the development and proliferation of Tregs in parallel. TGF-β is present in the TME, maintaining or enhancing the immunosuppressive environment that enables tumors to escape immune recognition. The interaction between MIF and its receptor CXCR2 on MDSCs induces the migration of MDSCs toward the tumor sites. This causes a chemotactic effect that can increase the concentration of suppressor cells within the TME (Figure 3) (61).
3.6 Tumor-associated neutrophils
Upon infiltrating the TME, Tumor-Associated Neutrophils (TANs) represent one subpopulation of neutrophils and express either pro- or anti-tumor activities. The interaction between TANs and MIF influences TAN activities and tumor progression, structuring the immune compartment of TME (62). MIF promotes neutrophil infiltration into tumors as a chemotactic factor. This is further facilitated by the interaction with receptors like CXCR2 on TANs, thereby favoring recruitment of the latter within the TME (18).
MIF and TANs communicate through several signaling pathways, which comodulate their functions. MIF binds to CD74 on neutrophils, thereby activating downstream signaling cascades that promote cell survival, proliferation, and activation. The activation of pathways such as ERK1/2 and AKT due to this interaction leads to an increased expression of pro-tumoral genes. MIF may change the cytokine secretion profile of TAN. It could induce the release of IL-6 and IL-8, which would attract more immune cells to the TME and accelerate inflammatory responses that support tumor growth (Figure 5) (46, 63).
4 Role of MIF in immune cells and inflammation
MIF plays a role in various biological functions, such as leukocyte recruitment, inflammation, immune responses, cell proliferation, tumor development, and the counter-regulation of glucocorticoids in both physiological and pathological processes. Previous studies reported MIF’s role in inflammatory and immune mediated diseases such as rheumatoid arthritis, cancer, multiple sclerosis, systemic lupus erythematosus (SLE), and psoriasis (64–68). A possible pathogenic mechanism is through the enhanced production of inflammatory molecules such as TNF-α, nitric oxide, IL-1, IL-6, IL-8, and cyclo-oxygenase (COX) (69).
MIF was initially identified as a product of T lymphocytes; however, studies has shown that endotoxin-induced T cell-deficient mice still exhibit circulating MIF (69). MIF is a pro-inflammatory cytokine and immunomodulator that is rapidly released by both immune cells such as monocytes/macrophages, B cells, and T cells as well as non-immune cells including endocrine, endothelial, and epithelial cells in response to various stimuli as shown in Figure 6 (3). Growing evidence has revealed that MIF is constitutively expressed in a variety of immune cell types and its over secretion was associated with various pathological conditions. MIF opposes the anti-inflammatory and immunosuppressive effects of glucocorticoids. Normally, glucocorticoids activate MAP kinase phosphatase-1 (MKP-1), which inhibits inflammatory mediators, thereby exerting anti-inflammatory effects (69).
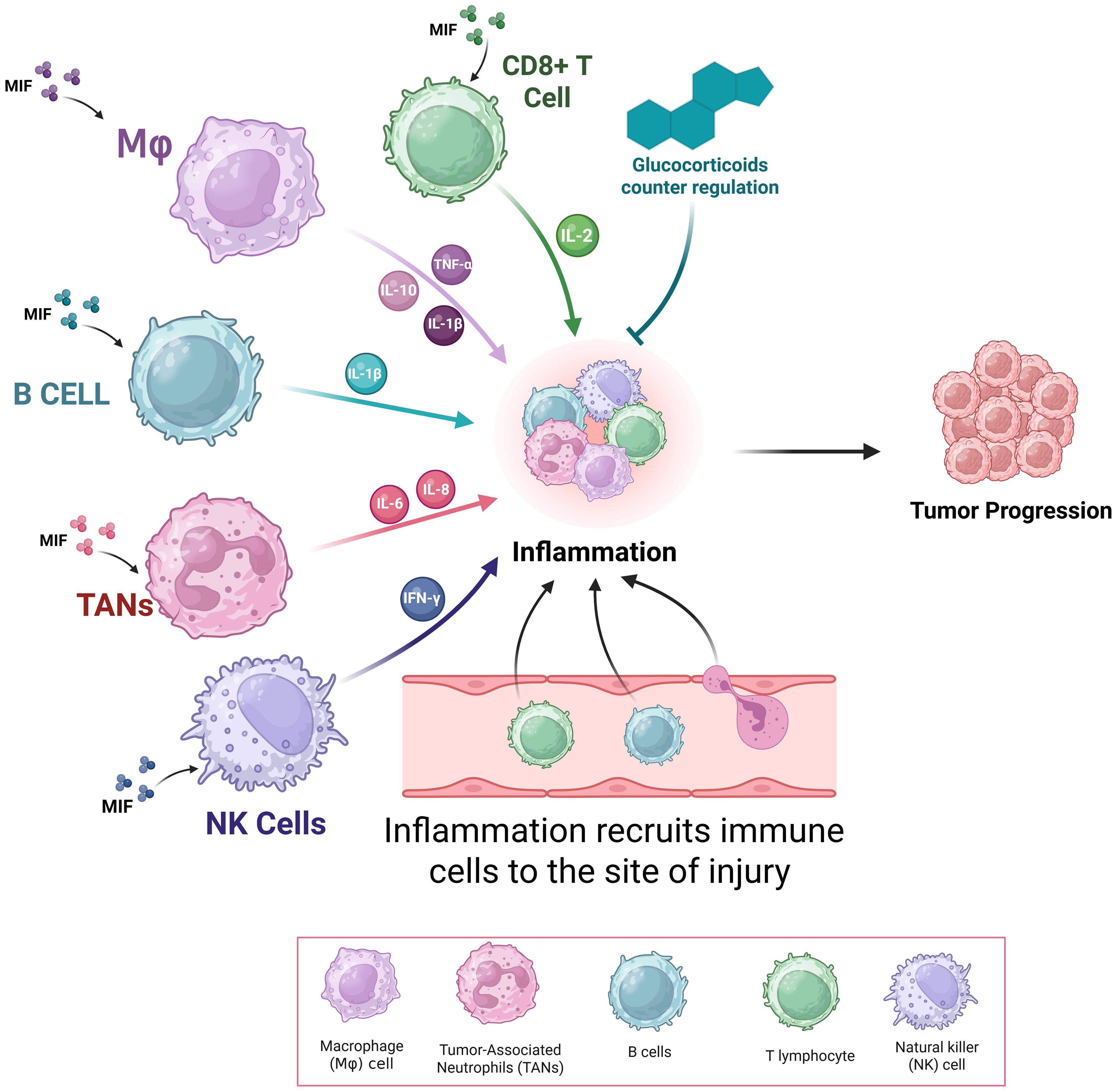
Figure 6. Role of Macrophage Migration Inhibitory Factor (MIF) in Inflammation. This figure shows the pivotal role played by MIF in the regulation of immune cell function and inflammation. MIF, secreted from immune (T cells, B cells, macrophages, and neutrophils) and non-immune cells, enhances production of pro-inflammatory mediators like TNF-α, IL-1β, IL-6, IL-8, nitric oxide, and COX-2—mainly by macrophage MIF..
MIF interacts with four membrane receptors: CD74, chemokine receptor with CXC motif 2 (CXCR2), CXCR4, and CXCR7. Depending on the inflammatory context, MIF could bind to individual receptors or receptor complexes, which determine its functional activity (70, 71). This might explain the chemokine-like properties of MIF, thus facilitating the migration and recruitment of leukocytes to sites of infection and inflammation (18, 72).
Once secreted, MIF can act in both autocrine and paracrine manners by binding to these transmembrane receptors, triggering intracellular signaling cascades (73, 74). When MIF binds to CD74, the invariant chain of major histocompatibility complex II (MHC II), it activates signaling through the extracellular signal-regulated kinase (ERK)/MAP kinase pathway and CD44, promoting cellular proliferation and prostaglandin E2 production (16, 75). When MIF binds to CXCR2, it triggers chemotactic responses, driving the recruitment and migration of immune cells, such as monocytes and neutrophils, to sites of inflammation, potentially contributing to the early immune response against infections (18). Similarly, MIF interaction with CXCR4 promotes the directed migration of T cells, playing a role in the adaptive immune response to infections (18). Additionally, the MIF-CXCR7 axis has been implicated in inflammatory processes, particularly in B cell chemotaxis (70).
MIF is primarily secreted by macrophages. A study by Lee et al. demonstrated that autophagy deficient monocytes and macrophages release significantly high levels of MIF when stimulated with LPS (76). Furthermore, MIF plays a crucial role in regulating macrophage responses during infection. It promotes the production of pro-inflammatory cytokines and other inflammatory mediators, including TNF-α, IFN-γ, IL-1β, IL-2, IL-6, IL-8, nitric oxide, and COX2, to facilitate pathogen clearance (3, 77). Macrophage-derived MIF is both sufficient and necessary for driving the angiogenic role of bone marrow-derived macrophages in teratoma formation in mice, highlighting its critical role in promoting M2 macrophage polarization (78). These findings were later validated in mouse models of both primary and metastatic melanoma, where macrophage-derived MIF was essential for the maximal expression of angiogenic growth factors in M2-polarized macrophages and was required for the T-cell immunosuppressive function of melanoma-associated TAMs. Notably, TAMs deficient in MIF or treated with the small molecule MIF inhibitor, 4-IPP, spontaneously shifted to an M1-like polarization profile (79, 80). Collectively, these results demonstrate that loss or inhibition of MIF in solid tumors effectively reprograms intra-tumoral TAMs from an immunosuppressive, pro-angiogenic, tumor-promoting phenotype to an immunostimulatory, non-angiogenic, anti-tumor phenotype (79). Moreover, MIF plays a crucial role in the early innate immune response to parasitic infections, as it facilitates the expression of proinflammatory cytokines and their receptors, promotes parasite recognition, supports macrophage-mediated microbial clearance, and enhances effective antigen presentation (81).
Studies suggest that MIF contributes to immunosuppression by inhibiting cross presentation of tumor antigens by DCs to CD8+ T cells via MHC class I (82). Furthermore, MIF downregulates MHC class II, implying that it also hinders the presentation of tumor antigens to CD4+ T cells (83, 84). Such findings highlight MIF’s role in promoting tumor-associated immune evasion by affecting DC function and consequently CD4+ and CD8+ T cell responses.
Granulocytes such as unstimulated human circulating eosinophils have performed cytosolic MIF. Upon stimulation with phorbol myristate acetate, C5a or IL-5, eosinophils could secrete MIF in a concentration and time-dependent manners (85). Similarly, human neutrophils store preformed MIF, which could be released following secondary necrosis (86). Chen et al. demonstrated that mast cells constitutively express MIF and secrete it during degranulation to modulate innate immune responses, drive fibrogenic activities, and contribute to non-scleroderma-related fibrosis (3, 87). Human eosinophils also possess significant amounts of stored MIF, and elevated MIF levels were detected in the airways of individuals with asthma. In experimental asthma models, mice lacking MIF exhibit reduced pulmonary inflammation and decreased airway hyperresponsiveness compared to their wild-type counterparts (85, 88).
Secreted MIF mediates evasion from NK cell-mediated cytolysis in uveal melanoma, the most common intraocular cancer in adults (89). On the other hand, MIF overexpression was reported to facilitate immune evasion by downregulating NKG2D expression on tumor cells, a key receptor involved in triggering NK-mediated tumor cell cytolysis in ovarian cancer cell lines (90). Other studies reported that MIF may inhibit NK cell function by competing with NK-associated MHC class I molecules (40). Similarly, CTL-derived MIF directly suppressed the anti-tumor activity of primed cytotoxic T lymphocytes (CTLs). Additionally, tumor-derived MIF inhibits T cell activation by inducing T cell death in an IFN-γ-dependent pathway (91).
Another reported mechanism of MIF is the generation and expansion of CD4+CD25+FOXP3+ regulatory T cells by enhancing IL-2 expression in activated splenocytes in mouse models of mammary and colon carcinoma (92). Remarkably, a subset of MIF−/− mice exhibited complete tumor rejection with a significant decrease in Tregs and an increase in CD4+ and CD8+ lymphocytes. Also, tumor-derived MIF enhances the expansion and migration of Th17 cells through CXCR4 signaling. This increase in Th17 cells was associated with improved clinical outcomes (93). Moreover, MIF expression was elevated in advanced stages and more aggressive molecular subtypes of breast cancer, showing a strong correlation with IL-17 levels (94). Beyond cancer, MIF has been linked to IL-17 expression in various autoimmune disorders, such as Hashimoto’s thyroiditis and rheumatoid arthritis, where Th17-mediated responses are known to drive pro-inflammatory processes (95, 96).
The role of MIF in the immune response to microbial pathogens have been extensively studied, revealing that it could either protect the host or exacerbate tissue damage, depending on the specific microorganism involved (29, 97–100). MIF could be protective by activating immune cells, particularly macrophages, and promoting the release of pro-inflammatory cytokines, thus facilitating the efficient clearance of invading pathogens (101–103). Conversely, MIF’s overactivity can exacerbate inflammation, leading to tissue damage. This dual role is particularly evident in chronic inflammatory and autoimmune diseases, where MIF is implicated in sustaining inflammation, resulting in persistent tissue injury and dysfunction (104). In certain viral infections, such as HIV, MIF can create a microenvironment favorable to viral survival and replication, allowing the pathogen to evade immune defenses and establish persistent infections. This detrimental effect may stem from MIF’s ability to dysregulate immune responses, alter cytokine profiles, and increase immune cell susceptibility to viral invasion (105). The impact of MIF is highly context-dependent, influenced by factors such as the specific pathogen, stage of infection, overall immunological environment, and affected organ. Notably, MIF may exhibit organ-specific protective effects in certain diseases, further underscoring the complexity of its role in host-pathogen interactions (106).
Beyond its involvement in infectious diseases, MIF plays a significant role in various inflammatory and pathological conditions, including asthma, atherosclerosis, cancer, autoimmune diseases, burn injuries, and wound healing (107, 108). MIF is implicated in a wide range of autoimmune diseases, reflecting its diverse role in immune regulation (107, 109). High levels of MIF have been observed in patients with SLE, systemic sclerosis, Wegener’s granulomatosis, and relapsing polychondritis (110–112). Elevated levels of MIF have been detected within inflamed synovial tissue of rheumatoid arthritis patients, highlighting its involvement in localized inflammation (113, 114). By counter-regulating the immunosuppressive effects of glucocorticoids, MIF might also contribute to the development of steroid resistance in conditions such as asthma and autoimmune diseases (115).
MIF plays a critical role in amplifying inflammation during carcinogenesis and the development of early-stage hyperplasia or carcinoma as shown in Figure 6 (116). This is particularly evident in inflammatory colitis, a contributing factor to the progression of colorectal adenomas and adenocarcinomas (117). For instance, in head and neck cancer, elevated MIF levels were associated with increased expression of CD66b, a granulocyte/neutrophil marker, as well as lymph node metastasis, and poorer overall survival (118).
Mechanistically, tumor-derived MIF drives neutrophil chemotaxis via CXCR2 signaling and enhances neutrophil production of CCL4 and MMP9 (118). These MIF-induced factors would promote lymphangiogenesis and tumor-stromal remodeling, thus contributing to tumor progression (119–121). On the other hand, CCL4 can recruit various immune cells, including T lymphocytes, into the TME through CCR5 (121). Thus, the composition and activation state of infiltrating immune cells within the tumor microenvironment would influence whether the response to MIF is pro- or anti-tumorigenic.
5 MIF in solid tumors
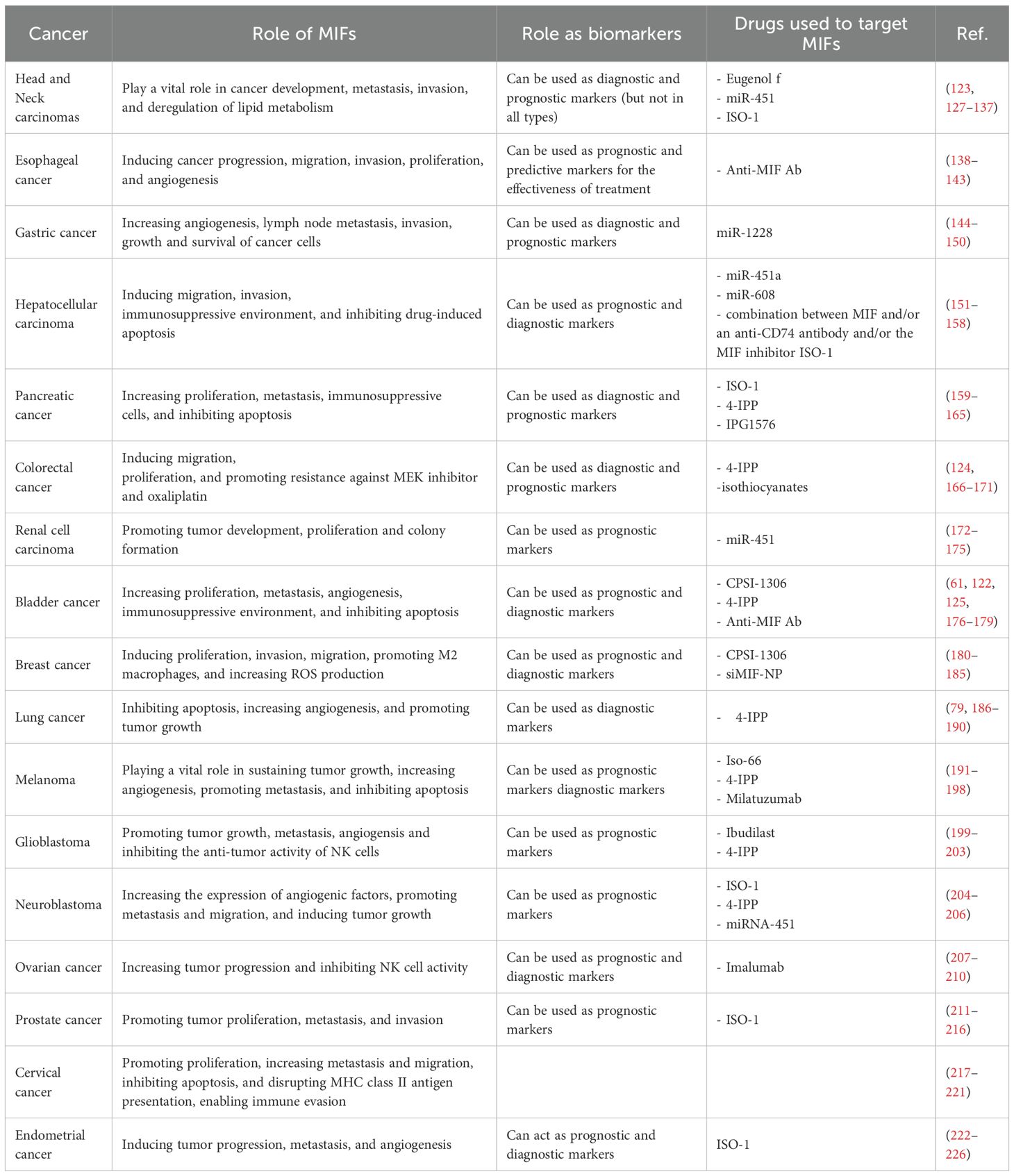
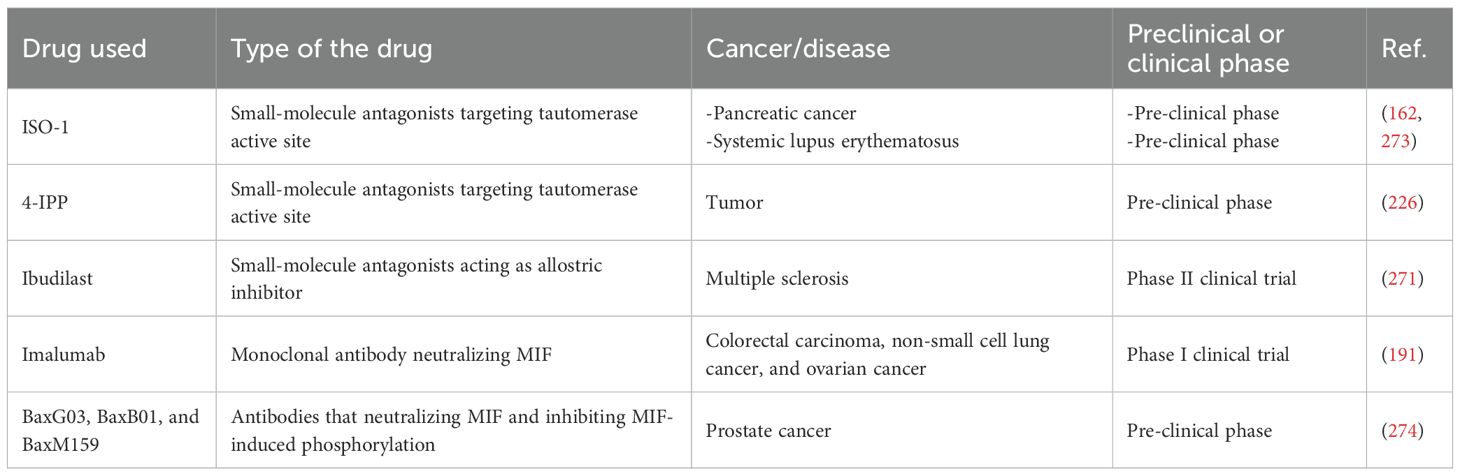
Over the last two decades, MIF has been linked to carcinogenesis (122), invasion (123), metastasis (124), tumor-induced angiogenesis (125), and disease prognosis and diagnosis (126, 127) of several solid malignancies. The function of MIF in various solid tumors, their diagnostic and prognostic significance, and their potential as a therapeutic target are summarized in Table 1 and discussed in this section.
5.1 Breast cancer
Breast cancer (BC) is among the most frequently diagnosed cancers in women (227, 228). MIF plays a crucial role in breast cancer progression and has been the subject of multiple investigations (229, 230). Studies have demonstrated that MIF is significantly overexpressed in non-invasive BC cell lines, such as MDA-MB-468 and ZR75-1, when compared to benign cell lines like MCF-12A. However, MIF expression is notably lower in highly invasive BC cells (MDA-MB-231) even though the MIF receptor CD74 is highly expressed. This suggests that MIF likely targets invasive BC cells through its interaction with CD74. Blocking CD74 has been shown to inhibit MDA-MB-231 cell proliferation, highlighting the role of MIF/CD74 signaling in driving the growth of both non-invasive and invasive cancer cells. Moreover, MDA-MB-468 cells had a 2.1 times higher proliferation rate than MCF-12A cells, yet MDA-MB-231 cells had the highest baseline proliferation rate (approximately 7.8 times higher than that of benign cells). When treated with recombinant MIF (rMIF) at doses in the range of 0–150 ng/ml, MCF-12A and both tumor cell lines demonstrated increased proliferation, with the strongest effect observed in cells with the highest CD74 expression which are MDA-MB-231. MIF not only supports cell growth but also promotes the migration and invasion of cancer cells in a dose-dependent manner. In MDA-MB-231 cells, MIF acts in a chemokine-like manner to facilitate movement through basement membrane-like layers, suggesting that MIF produced in the TME contributes to cancer invasiveness (231). Interestingly, in mouse models, when comparing 67NR non-metastatic cells to both a non-tumorigenic control and the metastatic 4T1 cells, researchers observed higher basal levels of MIF secretion in the 67NR cells. However, this basal MIF secretion was not linked to the invasive phenotype in the studied mouse breast cancer cell lines. Despite the differences in MIF secretion levels, no clear relationship was established between MIF secretion and invasiveness in these models (180). Inhibition of autophagy in 66cl4 triple negative breast cancer cell line led to increased intracellular ROS levels, which further upregulated MIF expression, potentially through ROS-dependent transcription factors. This mechanism underscores MIF’s involvement in macrophage polarization, particularly in promoting the M2 subtype. Research involving MIF-deficient mouse macrophages revealed reduced M1 polarization, suggesting that MIF is essential for macrophage function and amplifies both extracellular and intracellular signaling (181). Additionally, MIF has been identified as a novel 3’ flap nuclease that helps remove unpaired 3’ flaps in DNA during replication. It cooperates with nuclease-deficient polymerases, such as Pol α, to ensure proper DNA elongation and fidelity. In cancer cells, the absence of MIF’s nuclease activity results in increased mutations, slower DNA replication, and cell cycle delays, highlighting its role in helping cancer cells survive DNA replication stress. Moreover, MIF’s recruitment to DNA replication sites by PARP1(Poly(ADP-ribose) polymerase 1) during the S phase further emphasizes its involvement in maintaining cancer cell proliferation (182).
As a biomarker, MIF also has diagnostic and prognostic implications in BC. Immunohistochemical studies have shown that higher MIF levels correlate with larger tumors and more advanced stages of the disease (227). Although MIF expression alone does not significantly affect overall survival, CD163 expression—on tumor-associated macrophages—has been linked to disease-free survival, suggesting that MIF influences the TME in patients with triple-negative BC (183). Furthermore, elevated mRNA levels of CXCR7, an extracellular MIF receptor, have been associated with decreased overall survival, indicating that extracellular MIF signaling plays a critical role in BC progression (181).
Given its wide-ranging physiological and pathological roles, MIF represents a promising therapeutic target for the development of small-molecule inhibitors and antibody-based therapies. In the context of BC treatment, the small molecule CPSI-1306 (isoxazoline drug), which has demonstrated the ability to suppress MIF’s oncogenic activity (184), holds promise as a non-toxic, well-tolerated therapy. CPSI-1306 induces apoptosis in triple-negative BC cells by increasing ROS levels and inhibiting key signaling pathways such as Akt, PDK, and RAF. Its ability to interfere with MIF’s negative regulation of apoptosis further supports its potential use in cancer treatment by promoting cell death while inhibiting proliferation pathways (232). Moreover, a previous study demonstrated the effects of CPSI-1306 treatment and MIF reduction in human TNBC grafts, showing a decrease in the infiltration of MDSCs in the tumor. Additionally, CPSI-1306 treatment appeared to enhance the infiltration of CD8+ T cells, while reducing the levels of granulocyte colony-stimulating factor (GCSF), granulocyte-macrophage colony-stimulating factor (GMCSF), IL-2, and IL-4, when compared to the control group (232). On the other hand, MIF knockdown using siRNA (siMIF-NP) has proven to be an effective method for reducing immunosuppression in tumors. Accordingly, MIF, which is overexpressed in many solid tumors, is a suitable target for siRNA-mediated knockdown. MIF knockdown not only decreases the levels of CD206, a marker for M2 macrophages, but also increases the expression of MHCII, which is crucial for antigen presentation to CD8+ T cells. Accordingly, tumors treated with siMIF-NP exhibit an elevated infiltration of CD8+ T cells, enhancing the immune response and potentially improving therapeutic outcomes (185). Consequently, the therapeutic efficacy of immune checkpoint blockade, a strategy that aids the body’s immune system in attacking tumor cells, was enhanced by targeting MIF (233).
5.2 Lung cancer
MIF plays a pivotal role in inflammatory signaling and tumor growth. Its enzymatic function, particularly at the tautomerase active site, is essential for processes like ERK phosphorylation, COX-2 induction, and p53 inhibition, which are crucial for tumor development. Mutation of this active site or inhibition by chemical compounds has been shown to dampen these critical pathways (234). To further understand MIF’s role in lung cancer, researchers explored its interaction with its receptor, CD74, which was found to be widely expressed in lung cancers. In some tumors, CD74 was primarily located in stromal cells, suggesting that MIF might influence the TME. In other cases, CD74 expression was shared between stromal and malignant epithelial cells, hinting at MIF’s involvement in autocrine regulation of angiogenic factors or inhibition of apoptosis in cancer cells. Co-expression of MIF and CD74, detected using factor VII staining, correlated with elevated levels of angiogenic CXC chemokines and greater tumor vascularity, which reinforces MIF’s role in promoting tumor angiogenesis. Inhibition of either MIF or CD74 in vitro led to a reduction in the production of these chemokines, further supporting the previous findings (187).
As a biomarker, MIF demonstrates diagnostic potential due to its consistent overexpression innon-small cell lung cancer (NSCLC) tissues compared to normal lung samples. These expression patterns, confirmed by western blotting and immunohistochemistry (IHC), highlight MIF’s overexpression in lung tumors, and its absence in healthy tissues (188). However, its prognostic utility is limited: elevated MIF levels do not correlate with chemotherapy response or overall survival in advanced-stage patients (190). Nonetheless, elevated MIF levels have been consistently associated with increased proliferation and migration of lung cancer cells, reinforcing its potential as part of a diagnostic biomarker panel for lung cancer (189).
On the therapeutic front, MIF inhibition has shown promise. Lungs from MIF-deficient mice showed a noticeable reduction in metastatic tumor burden, both visually and in terms of total lung mass. To assess changes in macrophage polarization, F4/80+ cells were isolated from the lungs of metastatic tumor-bearing MIF-deficient and wild-type mice. Macrophages in MIF-deficient mice exhibited a shift in polarization. The expression of M2 markers, including ARG-1 and IL-10, was significantly elevated, while the expression of M1 markers such as TNF-α was reduced in lung-associated TAMs. Additionally, when 4-IPP was applied ex vivo to TAMs from wild-type mice, it caused a similar shift, converting TAMs from an M1-like profile to a more pro-inflammatory M2 profile, further supporting the role of MIF in regulating macrophage polarization (235).
5.3 Melanoma
In melanoma cell lines, MIF knockdown led to reduction in the cell number and viability over five days with a notable decline after three days. Western blot analysis confirmed MIF knockdown across six melanoma cell lines, with substantial reductions in Akt phosphorylation in MelCV, Me1007, and MelRMu cells (40–70% decrease), which corresponded with significant cell proliferation inhibition. Notably, melanoma lines resistant to MIF depletion (MelRM and MM200) displayed minimal changes in Akt activity, while the most sensitive lines exhibited the greatest reduction in Akt activity, underscoring a positive correlation between MIF knockdown and Akt signaling (198). These findings demonstrate that MIF plays a significant role in sustaining tumor growth and survival. On the other hand, MIF–CD74 signaling plays a critical role in enhancing melanoma cell survival by promoting a TME favorable to malignancy. Activation of CD74 by MIF contributes to melanoma progression (48), as presented by its influence on immune responses like TNF-α signaling and apoptosis in the TME (197). Additional in vivo studies have linked MIF with angiogenesis in melanoma, where anti-MIF antibodies in mice reduced angiogenesis (236). Depletion of MIF also protected mice from lung metastasis by modulating the immune response and reprogramming TAMs, indicating that MIF’s primary role in metastasis is linked to immune system modification (235). Furthermore, high CD74 and MIF ratios correlated with increased pro-inflammatory markers and immune cell infiltration, suggesting that these ratios could serve as important indicators of immune response in melanoma (47).
As biomarkers, MIF and CD74 were identified as key proteins associated with melanoma prognosis. High CD74 and low MIF expression correlated with improved survival outcomes and progression-free survival supporting the idea that the MIF−/CD74+ signature could serve as a prognostic marker in stage III melanoma. This finding is particularly intriguing because these two circulating proteins were able to sort patient outcomes as effectively as tumor-based markers (79). Although results suggest that serum sCD74 may not be a suitable diagnostic marker for early-stage melanoma, its elevated levels in advanced disease suggest a potential role in tumor progression. The increased sCD74 levels could be attributed to secretion by tumor cells themselves or by surrounding cells stimulated by the tumor (196). In addition, poor response to immune checkpoint drugs is substantially correlated with high MIF expression; this is supported by patient data on melanoma that were derived from the TCGA database (198).
Therapeutically, the MIF–CD74 axis presents a promising target in melanoma treatment due to its role in promoting tumor growth and survival. Several compounds have been developed to inhibit MIF signaling (48). Iso-66, a fluorinated oxazoline derivative that has great chemical stability and non-toxic characteristics, was shown to reduce tumor burden in in vivo mouse models of melanoma by enhancing antitumor immune responses through the growth of antitumor-specific effector cells. It was also observed in ex vivo studies to be associated with the restoration of MIF activity. MIF inhibition suppresses the ability of CTLs and NK cells to target tumors, but reactivating MIF can enhance their antitumor activity (237).
Another inhibitor, 4-iodo-6-phenylpyrimidine (4-IPP), reduced tumor cell proliferation and motility (238), improved survival in melanoma-bearing mice (193) and enhanced the effectiveness of anti-CTLA-4 therapy by increasing CD8+ T-cell infiltration and metabolic reprogramming (194).
MIF-CD74 interaction has been identified as a major factor in preserving a favorable tumor microenvironment for tumor cells and as a regulator of PD-L1 expression. Therefore, a potential target for the efficient treatment of melanoma patients could be the MIF-CD74 interaction (192).
Furthermore, CD74-inhibiting antibodies such as the FDA approved monoclonal antibody milatuzumab can suppress MIF; thus, they can be employed in treatment approaches (191).
5.4 Glioblastoma
Glioblastoma (GBM) is an extremely aggressive brain tumor known for its resistance to treatment (239). GBM exhibits marked upregulation of MIF expression compared to lower-grade gliomas (240). This upregulation is accompanied by significantly higher levels of CD74, the MIF receptor, and its co-receptor CD44, as well as non-cognate receptors like CXCR2 and CXCR4. These receptors are notably overexpressed in GBM compared to lower-grade gliomas (241). The pro-tumorigenic role of MIF in GBM is evident through its promotion of proliferation, migration, angiogenesis, and contribution to the immunosuppressive phenotype of glioma cells. High levels of MIF expression are observed in WHO grade IV glioblastomas and high-grade WHO grade III tumors, with the highest levels in the most malignant variants of GBM. MIF is expressed variably in GBM cells, localized primarily on the poles of the nuclei in early-stage cells, and its expression varies with cell passage. Interestingly, while MIF expression ranges between 5.12 and 82.04 ng/ml, it does not correlate with cell proliferation rates, growth behavior, or cell morphology (200). Additionally, MIF signaling through the CD74/CD44 receptor complex activates the ERK MAP kinase pathway, further promoting tumor cell proliferation (203).
MIF negatively regulates wild type p53 signaling in glioblastoma cells, with higher MIF levels associated with functional p53, further implicating MIF in gliomagenesis. Moreover, MIF inhibits the anti-tumor activity of NK cells, reinforcing its role in immune evasion (200).
As a biomarker, MIF shows a complex relationship with glioma prognosis. While treatment groups with elevated MIF expression exhibit poorer prognosis than those with low MIF expression levels (203). However, there is an observed paradox where higher MIF expression does not correlate with reduced overall survival, instead showing a trend toward improved OS. Despite this, neoadjuvant therapy significantly increases MIF expression, further complicating its role in prognosis (241).
Therapeutically, targeting MIF has shown promise in inhibiting pro-tumorigenic traits and restoring immune sensitivity, enhancing the efficacy of radiation and chemotherapy (202). Ibudilast, a drug that penetrates the blood-brain barrier, has been identified as an agent that targets the MIF-CD74 interaction on MDSCs. In preclinical studies, treatment with Ibudilast decreased CD74 expression and increased CD8+ T cell infiltration within the tumor, suggesting its potential in treating brain malignancies (242).
5.5 Neuroblastoma
By studying AS-MIF-transfected cells, researchers have gained insights into how MIF blockade may affect the expression of molecules related to neuroblastoma. Western blot analysis revealed that AS-MIF transfection significantly reduced N-Myc expression, a gene crucial for neuroblastoma progression. MIF was found in all 21 neuroblastoma samples studied, with both MIF and c-Met (tumor progression related receptors) detected in the cytoplasm of tumor cells. Double immunohistochemistry staining demonstrated that neuroblastoma cells highly expressed MIF and c-Met, with a strong positive correlation between their expressions. Where MIF likely promotes neuroblastoma development by regulating N-Myc and increasing the expression of angiogenic factors. However, transfection of SK-N-DZ neuroblastoma cells with an AS-MIF expression vector reduced MIF expression, leading to a decrease in pro-tumor genes such as N-Myc, TrkB, Ras, and IL-8, while tumor-suppressing genes like EPHB6 and BLU were elevated. In in vitro studies and nude mice models, downregulation of MIF significantly reduced neuroblastoma cell proliferation, tumor formation, and metastasis (204).
The role of miR-451 in regulating MIF expression was also studied. Transfection of miR-451 mimics into SK-N-SH and GI-LA-N neuroblastoma cells reduced MIF protein levels, as confirmed by Western blot analysis. A luciferase reporter assay identified a direct interaction between miR-451 and the 3’UTR of MIF transcript, leading to reduced luciferase activity in cells co-transfected with miR-451. This demonstrated that miR-451 negatively regulated MIF expression by directly targeting its transcript. Moreover, the suppression of neuroblastoma cell invasion and migration by miR-451 was reversed when MIF was re-expressed, further supporting MIF’s role as a direct target of miR-451 (243).
As prognostic markers, MIF and its associated receptors play a significant role in neuroblastoma. Analysis of public gene expression datasets revealed that both MIF and CXCR4 were highly expressed in primary neuroblastoma tumors and BM-derived disseminated neuroblastoma tumor cells. An RNA-seq dataset (GSE62564) of 498 primary neuroblastoma samples, was used to evaluate the expression profiles of MIF, CXCL12, and the receptors CXCR4, CXCR2, CXCR7, CD74, and CD44, showed that MIF expression was highest in stage 4 high-risk tumors, while CXCR4 expression was lower in low-risk stage 4S tumor (206).
The biological hallmark of neuroblastomas is the complexity of the genetic abnormalities acquired by the tumor cells. Some of these abnormalities are powerful prognostic markers that are independent of the clinical features (204). Higher MIF or CXCR4 expression was associated with worse overall survival, while elevated levels of CD44, CXCL12, and CD74 were linked to better outcomes. Patients were categorized based on their MIF expression levels, and a survival curve was analyzed for overall survival; patients with lower MIF expression exhibited improved overall survival (206).
Therapeutically, targeting MIF has shown promise in cancer treatment. MIF antagonists, such as ISO-1 and 4-IPP, have been explored as potential therapeutic agents for cancer. They can bind to MIF’s catalytic active site and reduce its activity, leading to decreased tumor growth and extended survival in animal models. However, the cytotoxic effects of these antagonists vary, with ISO-1 showing higher toxicity compared to 4-IPP (206). Moreover a study confirmed that tumor associated MIF suppresses T-cell activation through suppressing three mechanisms of T-cell activation: cytokine, solid-phase anti-CD3,and allogeneic MHC (91). MIF can be targeted by knocking out or inhibitory molecules to restore T cell activation; thus, it can be used as an immunotherapeutic target.
5.6 Ovarian cancer
Studies have reported elevated levels of MIF in ovarian cancer (OC) cells, leading to the hypothesis that higher MIF levels may characterize patients with ovarian cancer. Serum analysis from patients with newly diagnosed OC, showed significantly increased MIF levels in the cancer group compared to those in healthy age-matched controls, suggesting a possible link between abnormal MIF expression and ovarian cancer pathogenesis (207). MIF plays a role in immune suppression by inhibiting NK cell function. Specifically, MIF reduces the expression of NKG2D, an activating receptor on NK cells, impairing their ability to lyse ovarian cancer cells. Blocking MIF using anti-MIF antibodies resulted in enhanced lytic activity of NK cells against ovarian cancer cells from ascites. This effect was further confirmed in vitro, where NK cells pretreated with supernatant from MIF-depleted ovarian cancer cells showed improved activity against SK-OV-3 cells. In contrast, MIF-rich supernatant reduced NK cell activity.
As a potential biomarker, MIF has emerged as a valuable tool in the prognosis and diagnosis of ovarian cancer. Although MIF levels were not significantly different between patients with early (Stage I/II) and late-stage (Stage III/IV) ovarian cancer (207), higher MIF levels were associated with poor overall survival in recurrent cases, even though MIF had no correlation with progression-free survival (208). These findings suggest that MIF might serve as a valuable biomarker for prognosis in patients with ovarian cancer (209). Furthermore, a proteomic analysis indicated the utility of MIF in diagnostic screening, and a multiplex test combining MIF with other biomarkers, including CA-125 and prolactin, demonstrated high sensitivity (95.3%) and specificity (99.4%) for ovarian cancer detection. In addition, recently a unique multiplex test for six blood biomarkers—prolactin, MIF, insulin-like growth factor II, osteopontin, and CA-125—was developed. This combination of biomarkers appears to exhibit great sensitivity (95.3%) and specificity (99.4%) for the identification of ovarian cancer (209).
Therapeutically, MIF represents a promising target for enhancing anti-tumor immunity. Quantitative real-time PCR and flow cytometry analysis also demonstrated that MIF significantly downregulates NKG2D expression on both NK and CD8+ T cells, supporting the hypothesis that MIF contributes to immune evasion by modulating activating and inhibitory signals in these immune cells (52). Thus, MIF could be a potential immunotherapeutic target by reversing its anti-immune effect on NK and T cells. Furthermore, MIF has been targeted therapeutically with monoclonal antibodies like imalumab, which specifically inhibits oxidized MIF. Early clinical trials have shown promising results, with imalumab being well-tolerated and demonstrating stable disease in some patients. Ongoing studies are further exploring its efficacy in patients with ovarian cancer (244).
5.7 Prostate cancer
Previous studies highlight the critical role of MIF and its interactions with chemokine receptors CXCR7 and CXCL12 in tumor growth, prognosis, and treatment of prostate cancer. The median MIF serum levels were significantly increased in patients with PCa compared to those in non- PCa individuals (211). This supports previous findings of elevated MIF in patients with CaP (212). In situ hybridization of 20 matched benign and malignant prostate tissues showed that MIF mRNA levels were low in benign epithelial cells and confined to the cytoplasm but increased in malignant tissues (211).
Furthermore, the inhibition of the CXCR7 receptor blocked MIF-induced proliferation, highlighting CXCR7’s role in cancer progression. Reducing endogenous MIF levels using siRNA significantly decreased C4-2B cell growth and migration, which could be further suppressed by inhibiting CXCR7 with CCX771. CXCL12 had no effect on cell migration in this PCa model (213).
As a prognostic biomarker, MIF has been implicated in cancer progression and recurrence, particularly in castration-resistant prostate cancer (CRPC). A study of 42 patients with PCa revealed that those with high-expression MIF polymorphisms had a higher recurrence rate (46.2%) compared to those with lower expression (10.3%) within 5 years (214). Additionally, CXCR7 expression was linked to poor prognosis, as patients with high CXCR7 expression had shorter disease-free survival than those with low expression. The related chemokine receptor, CXCR4, did not show a similar correlation with biochemical recurrence, suggesting that CXCR7 plays a more pivotal role in castration-resistant prostate cancer (CRPC) progression (213).
In terms of therapy, MIF raises the concentration of MDSCs in the bloodstream of individuals with diverse malignancies and employs multiple strategies to obstruct NK and T-cell activities, hence reducing anti-tumor immunity (215). Moreover, the malignant cells stimulated by MIF in PCa develop the capacity to eliminate DC by apoptosis and suppress their generation, preventing these cells from acting as antitumor activators. The CXCR7 antagonist, CCX771, and MIF inhibitor, ISO-1, inhibited CaP cell proliferation (213). ISO-1 preferentially targeted DU-145 cells, suggesting that targeting MIF could be a promising therapeutic approach (216).
5.8 Cervical cancer
Using immunohistochemistry, MIF expression was examined in surgical biopsy specimens from patients with CC and control subjects. MIF expression was predominantly present in malignant specimens, with most of the expression localized to the cytoplasm of tumor cells (217). similarly, CD74 expression was mainly observed in the cytoplasm (245). Functional assays demonstrate that MIF knockdown (via shRNA) inhibits HeLa cell proliferation and induces apoptosis at early and late stages (218, 219). MIF-CD74 signaling activates oncogenic pathways (e.g., Src, ERK1/2) while dephosphorylating p53 to block apoptosis. CD74 overexpression further disrupts MHC class II antigen presentation, enabling immune evasion and metastasis (220).
As a biomarker, MIF shows limited standalone prognostic utility in CC. Despite its overexpression in malignant tissues, no significant differences in MIF levels or promoter allele frequencies (-794CATT5–7) correlate with tumor differentiation grade (well/moderate/poor) or clinical stage (I/II vs. III/IV) (217, 222). These findings suggest MIF alone is insufficient for risk stratification but may complement multi-marker panels to improve diagnostic sensitivity.
Therapeutically, inhibiting the MIF-CD74 axis holds promise for CC treatment. Preclinical studies show that MIF knockdown reduces tumor cell viability and apoptosis resistance (178, 179). Targeting CD74, overexpressed in CC, could restore MHC class II-mediated antigen presentation to enhance CD4+ T cell activation and block oncogenic signaling (e.g., ERK1/2, p53 suppression) (220). Future strategies should prioritize CD74-directed immunotherapies to counteract immune evasion and tumor survival mechanisms.
5.9 Endometrial cancer
MIF plays a significant role in tumor growth and progression by promoting tumor-associated angiogenesis. However, this association has not been clearly observed in endometrial cancer (EC). Research reported high expression of MIF in malignant ascites, and in ovarian cancer cells. MIF enhances the release of cytokines, chemokines, and angiogenesis factors, contributing to tumor vascularization and angiogenesis (246). In EC, MIF mRNA and protein were detectable in all endometrial samples, with upregulation of MIF being associated with early FIGO stages, absence of lymphovascular invasion, and low histological grade. This suggests that MIF overexpression in EC may be linked to the suppression of metastatic spread (247).
In terms of prognosis and diagnosis, research has shown a correlation between MIF concentrations and the prognosis of patients with EC (247). The mean MIF concentrations in the serum of patients with EC (5.871 ± 3.37 ng/mL) were significantly higher than those in healthy individuals (4.825 ± 1.05 ng/mL). ROC curves demonstrated that serum MIF levels could effectively distinguish between EC patients and healthy controls, with an area under the curve of 0.664 (248).
Therapeutically, targeting MIF has shown promising potential in EC. RNAi-mediated knockdown of MIF reduced proliferation and migration in EC cells (224). Additionally, the MIF inhibitor, (S,R)-3-(4-hydroxyphenyl) 4,5-dihydro-5-isoxazole acetic acid methyl ester demonstrated antiangiogenic effects (225). These findings suggest that targeting MIF could be a potential therapeutic strategy in EC (228).
To date, the immunotherapeutic role of MIF in EC is yet to be stated. However, the progression of endometrial tumor cells are thought to be the result of defective NK cells as well as reduced T cell cytotoxicity (249). Previous studies reported the role of MIF on suppressing the anti-tumor effect of NKs (52, 237) and inactivation of T cells (91) in different solid tumors.
5.10 Head and neck cancers
Head and neck cancer (HNC) is one of the deadliest cancers worldwide (250, 251). Recent research has illuminated the role of MIF molecules in the progression of this malignancy (126, 252). Increased MIF expression has been observed in head and neck squamous cell carcinomas (HNSCC), specifically in the nasopharygeal carcinoma (NPC) (127), hypopharynx carcinoma (132), laryngopharyngeal carcinoma (253), and oral squamous cell carcinoma (OSCC) (129).
In OSCC, it has been reported that MIF increases the cancer invasion and metastasis via the activation of MMP-2 and MMP-9 (129). Another study found a positive correlation between high MIF expression in OSCC and second primary tumor recurrence (128). Additionally, MIF played role in NPC, the most common malignant tumor of the head and neck carcinomas (130). It was reported that, exosomal MIF derived from NPC promotes metastasis to lung through decreasing and repressing macrophages ferroptosis (130). In another study it was found that, NPC increased in growth and proliferation via MIF/CXCL8 (C-X-C motif chemokine ligand-8)/CXCR2 (C-X-C motif chemokine receptor-2) axis (254).
Moreover, some investigations regarding the role of MIF laryngopharyngeal tumor progression have been conducted (131, 253, 255). A study revealed that signaling axis of MIF-CD44-β1 integrin enhances the spread of laryngeal cancer (255). Another research discovered that hypoxia-induced MIF causes deregulation of lipid metabolism in Hep2 laryngocarcinoma via the IL-6/JAK-STAT (signal transducer and activator of transcription) axis leading to tumor progression (131).
On the other hand, hypopharyngeal squamous cell carcinoma has received very little investigation (132). For instance, it has been reported that increased expression of MIF influences the development and growth of hypopharyngeal squamous cell carcinoma (132).
As a biomarker, MIFs have showed promise as both diagnostic and prognostic biomarkers for head and neck malignancies, with higher levels related with disease presence and severity (126, 134, 256). Its ability to correlate with tumor aggressiveness and patient outcomes demonstrates its value in influencing clinical decision-making and improving patient care (126, 127). For instance, in NPC cells, it was reported that MIF represents a potential non-EBV (Epstein-Barr virus) plasma marker for the diagnosis of NPC (134). In addition, the combination of MIF and the EBV viral capsid antigen antibody (VCA-IgA) has been shown to enhance the specificity and predictive value of detecting NPC and to improve the diagnostic accuracy of NPC in high-risk individuals (134). Furthermore, in a different study, it was found that MIF overexpression in tumor cells is substantially linked to lymph node metastases, advanced clinical stage, and a poor prognosis and so increases its potential to be prognostic biomarker (256). However, a different study found that having a high serum MIF level was associated with a better prognosis and overall survival rate (133). This appears controversial, so further research is necessary.
Additionally, studies showed that MIF could be a potential biomarker in OSCC (135, 257). According to a study, it was revealed that MIF overexpression in OSCC tissues is linked to perineural and deeper tumor invasion as well as lymph node invasion, resulting in a higher pathological stage and low overall survival rate and so it may be a potential marker for poor prognosis (257). Similarly, in another investigation, MIFs high levels have been linked to a worse overall survival rate in OSCC patients, suggesting that they may be a useful prognostic marker for the disease (135).
According to some studies, MIF may also be a useful biomarker for laryngopharyngeal cancers, providing information on the existence and course of the tumor (137). For instance, it was found that patients with laryngeal SCC overexpressing MIF were positively correlated with poor survival and poor prognosis (137).
Current investigations into MIF as a biomarker in hypopharyngeal carcinoma remain limited, highlighting a significant scarce in the existing research and so need more research and investigations.
Therapeutically, there is substantial data regarding MIF as a target in head and neck cancers (131, 136). For instance, Eugenol inhibits the malignant processes of OSCC cells by binding to MIF and decreasing its level (136). In another study it was revealed that ectopic production of miR-451 inhibited NPC proliferation and invasion by targeting MIF mRNA and reducing its expression (123). Moreover, in laryngocarcinomas, it was showed that the MIF inhibitor ISO-1 reduced proliferation and invasion (131). However, there is still a large information gap regarding the use of MIFs as targets in hypopharyngeal cancer, and more investigations are required.
5.11 Esophageal cancer
Esophageal cancer is one of most commonly diagnosed cancer and one of the leading causes of cancer death worldwide (258, 259). Similarly, like head and neck cancers, MIFs were found to participate in promoting esophageal squamous cell carcinoma (ESCC) progression (138, 139). According to an investigation, it was found that in ESCC cell lines MIF promotes VEGF and IL-8 production, stimulating the angiogenesis process (143). In another study it was reported that, MIF has been linked to esophageal cancer growth through activating the Akt (Protein kinase B), MEK/ERK (MAPK kinase/extracellular signal–regulated kinase), and NF-κB (Nuclear factor kappa B) pathways, as well as decreasing the expression of the tumor suppressor gene GSK3β (glycogen synthase kinase 3 beta) (139). Moreover, it was found that MIF bind to ACKR3 (atypical chemokine receptor 3) and increases esophageal cancer migration and invasion (138).
As a biomarker, MIF demonstrates clinical relevance for esophageal cancer (140, 142). In a study it was reported that the expression of MIF and its receptor CXCR4 (C-X-C motif chemokine receptor-4) in ESCC was positively correlated with low overall survival rate as so can be used as a prognostic biomarker (140). Another study revealed that MIF expression was negatively correlated with the efficiency of anti-PD-l combined with chemotherapy as a neoadjuvant therapy, suggesting that it could not only be a biomarker for a bad prognosis in ESCC but also a predictive biomarker for the effectiveness of the treatment for ESCC (142).
Therapeutically, targeting MIF remains understudied but shows early promise for esophageal cancer (141). For example, anti-macrophage inhibitory factor 1 antibody has been shown to suppress the growth, proliferation and establishment of the neovascularization lumen of esophageal squamous cell carcinoma in nude mice (141). There is still a great lack of information concerning the use of MIF as a target in esophageal cancer, which calls for more studies and research.
5.12 Gastric cancer
Many investigations highlighted the role of MIF in gastric cancer, which considered as one of the most leading causes of cancer-related fatalities (147, 260, 261). For instance, it was found that elevated MIF levels in gastric cancer boosted and improved the angiogenesis process (147). In another study it was reported that, overexpressed MIF in gastric adenocarcinoma increased lymph node metastasis (261). Similarly, it was shown that MIF in gastric cancer increase invasion and lymph node metastasis (148). Another investigation found that the overexpression of ZFPM2-AS1 (ZFPM2 Antisense RNA 1) resulted in upregulation of MIF which led to suppressing the activation and nuclear translocation of the p53 protein which in turn enhanced growth and survival of the tumor cells (146).
As a biomarker, MIF demonstrates diagnostic and prognostic utility for gastric cancer (144, 150). In a study, it was shown that the transcriptome data from CagA+ (cytotoxin-associated gene A protein) gastric carcinomas showed that MIF released in the TME (tumor microenvironment) causes TAM (tumor associated macrophages) polarization, epithelial-mesenchymal transition, and inhibition of MAPK4 pathways—all of which are associated with poor prognosis (144). Another study reported that, elevated levels of MIF were significantly associated with advanced tumor stage, increased lymph node invasion, and poor survival and prognosis, making it a possible prognostic biomarker (150). According to another investigation, it was discovered that high serum MIFs are more sensitive and selective than CEA and hence a better diagnostic marker for gastric cancer (149).
Therapeutically, targeting MIF remains underexplored but promising. In an investigation it was found that, gastric cancer cells’ pro-angiogenic activity is inhibited by microRNA-1228 via binding to MIF and decreasing its expression (145). Further research and investigations are needed to fill up the large knowledge scarce regarding MIF as a therapeutic target in gastric cancer.
5.13 Hepatocellular carcinoma
MIFs were also found to have a role in hepatocellular carcinoma (HCC) development, the predominant form of liver cancer (151, 152, 262). According to a study, MIF was found to regulate secreted phosphoprotein 1 (SPP1) and increase TAM migration to HCC cells, and resulted in increased cancer metastasis and invasion (151). In another study, it was shown that MIF suppressed therapy-induced HCC apoptosis (diethylnitrosamine/carbon tetrachloride) and carried out CD74-mediated carcinogenic effects during hepatocarcinogenesis (152). In a single-cell transcriptome study, it was found that CD36+ HCC-associated fibroblasts released MIF through enhanced intratumor lipid oxidation, which improved the immunosuppressive environment by increasing the number of monocytic MDSCs (Myeloid-derived suppressor cells) within tumors (154).
As a biomarker, MIF demonstrates clinical relevance in HCC (153, 157). According to a study, it was found that MIF 755622–2 polymorphism is linked to HCC metastasis and that the GC and CC genotypes were positively correlated with decreased overall survival rate and so can be a predictive marker for poor prognosis (153). In another investigation, it was shown that patients with MIF-794CATT high repetitive-sequence genotypes showed increased metastasis, lower differentiation, and lower survival rate and so it could be a biomarker for poor prognosis (157). Another study reported that plasma MIF was inversely correlated with overall survival rate and had a better value for distinguishing HCC patients from controls (liver cirrhosis, benign lesions, and healthy people). As a result, it may be used as a diagnostic and prognostic marker (155).
Therapeutically, a number of studies suggested that MIFs could be targets in HCC (152, 158). For instance, it was reported that miR-608 decreased HCC progression through directly binding and decreasing MIF expression (158). In another study, it was shown that miR-451a bound to MIF and decreased its expression resulted in inhibiting HCC progression (156). According to another investigation it was found that combination between MIF and/or an anti-CD74 antibody and/or the MIF inhibitor ISO-1 resulted in decreased HCC development and growth (152).
5.14 Pancreatic cancer
Investigations have found that MIF also played role in pancreatic cancer development (159, 165). According to a study, it was reported that increased MIF in pancreatic cancer resulted in increased proliferation and metastasis via decreasing P53 expression and translocation which led to decreased low-densitylipoprotein receptor–related protein 1 (LRP1) (165). It was discovered, in a separate research, that MIF tautomerase activity controlled the expression of genes necessary for the recruitment, differentiation, and activation of MDSCs, which led to the build-up of immunosuppressive cells in TME of the pancreatic cancer (160). Moreover, it was shown that MIF helped pancreatic adenocarcinoma cells to evade apoptosis (163). In another study it was found that MIF increased pancreatic cancer progression and metastasis via AKT/ERK (Extracellular signal-regulated protein kinases)/CCND1 (Cyclin D1)/MMP-2 (Matrix metalloproteinase-9) axis (159).
As a biomarker, it was shown that MIFs may have the potential to be biomarkers in pancreatic cancer (159, 161). In a study, it was found that high expression of MIF with correlated with decreased overall survival rate and so associated with poor prognosis (159). Another investigations reported that, serum MIF showed higher levels in pancreatic cancer cells than normal cells and also correlated with poor survival and poor prognosis and so can be a non-invasive diagnostic and prognostic biomarker for pancreatic cancer (161).
Therapeutically, studies showed that MIFs can be a therapeutic target in pancreatic cancer (160, 162, 164). For instance, a study found that MIF inhibitor, ISO-1, decrease pancreatic ductal adenocarcinoma cells proliferation, migration and invasion in vitro and in vivo (162). Furthermore, in another investigation, it was reported that tautomerase inhibitor, IPG1576 (inhibitor for tautomerase activity of MIF) resulted in decreased exosome-induced MDSCs activation and differentiation and pancreatic tumor growth (160). Moreover, it was found that MIF inhibitor (4-iodo-6-phenylpyrimidine; 4IPP) decreased PANC-1 (pancreatic ductal cancer cells) cells growth and colony formation (164).
5.15 Colorectal cancer
MIFs have been frequently described in colorectal carcinoma (CRC), the third most prevalent malignancy and the second-leading cause of cancer death globally (124, 167, 263). In a study it was reported that MIF induces metastasis and growth of CRC cells by targeting SLC3A2 (solute carrier family 3 member 2) and regulating the AKT/GSK- 3β axis (124). Similarly, in another research, it was found that MIF increases proliferation and inhibits apoptosis of colorectal cancer cells (168). Furthermore, it was discovered that KRAS mutant CRC cells treated with refametinib, a MEK inhibitor, stimulated MIF production and resulted in the activation of STAT3 (signal transducer and activator of transcription 3) and MAPK, which promotes resistance to this medication (167). Another study showed that MIF increased oxaliplatin resistance in colorectal cancer by upregulating CXCR7 chemokine (169).
As a biomarker, MIF demonstrates significant clinical utility for CRC (166, 171). MIF (-173 GC/CC) polymorphism, for example, has been demonstrated to be associated with tumor dedifferentiation, advanced illness and poor survival, suggesting that it may be a useful prognostic biomarker for colorectal cancer (171). Another study found that elevated MIF expression in colorectal cancer was associated with an excellent AUC value of 0.933, suggesting that it may be a useful diagnostic marker (166). Lee et al. (170), reported that MIF is a more sensitive but less specific diagnostic biomarker than CEA in early colorectal cancer detection so combination of these two biomarker could be a very useful early diagnostic biomarker.
Therapeutically, targeting MIF shows promise in overcoming resistance and suppressing tumor growth in CRC (167, 264). The combination of refametinib with 4-IPP, significantly lowered STAT3 and MAPK activity compared to single-agent therapy. As a consequence, combination treatment was revealed to have a synergistic growth inhibitory impact against refametinib-resistant colorectal cancer cells via inhibiting MIF activation (167). In a different research, it was demonstrated that isothiocyanates decreased the proliferation of colorectal cell lines via inhibiting MIF tautomerase activity via covalent bonding to the N-terminal proline (264).
5.16 Renal cell carcinoma
Renal cell carcinoma (RCC) is the most common type of kidney cancer in adults, representing about 90% of all cases. Its incidence has steadily increased over recent decades, making it a major global health concern (265). Recent studies demonstrate that MIFs were also found to contribute to renal cell carcinoma (RCC) progression (172, 174). According to a study, it was found that MIF knockdown led to decreased proliferation and colon forming ability of renal cell carcinoma (172). Another study found that high MIF expression in the kidney increases RCC development via interacting with HIF1α (hypoxia-inducible factor-1 alpha) and HIF2α (hypoxia-inducible factor-2 alpha) (174).
In terms of diagnostic and prognostic potential, MIF shows promise as a biomarker RCC (173). For instance, high expression in clear cell renal cell carcinoma (one of the most common renal cell carcinomas) was positively correlated with low survival rate and poor prognosis (173). MIF’s potential as a biomarker in renal cell cancer remains largely unexplored and so needs more investigation.
Therapeutically, targeting MIF in RCC has shown early experimental promise (175). According to an investigation, it was found that miRNA-451 decreased and suppressed renal cell carcinoma proliferation, migration and invasion by inhibiting MIF expression via direct binding, highlighting MIF’s potential as a therapeutic vulnerability (175). Despite these findings, the development of MIF-targeted therapies for RCC is still in its infancy, needs more mechanistic studies.
5.17 Bladder cancer
In many studies, it was found that MIFs could contribute to bladder cancer progression (122, 179). According to a study, it was reported that MIF can increase proliferation and growth of bladder cancer cells via ERK pathway and also enhances angiogenesis via increasing the expression of VEGF (Vascular endothelial growth factor) (125). In another study it was shown that chimeric transcript SLC2A11 (solute carrier family 2 member 11)–MIF promoted metastasis, proliferation and inhibited apoptosis in bladder cancer cells via PTBP1 (Polypyrimidine tract binding protein)-dependent mechanism (122). Furthermore, it was reported that MIF was increased in muscle invasive bladder cancer and this indicates that MIF could be involved in bladder cancer invasion and migration (179). Another investigation found that, CXCL2/MIF-CXCR2 pathway increased and enhanced recruitment of MDSCs in bladder cancer which produces high immunosuppressive molecules including Arg1 (arginase 1), iNOS (inducible nitric oxide synthase), PD-L1 (Programmed death-ligand 1) and P-STAT3 (266).
In terms of diagnostic and prognostic utility, MIF demonstrates potential as a biomarker for bladder cancer (177, 266). For instance, it was found that Urinary bladder cancer (UBC) was shown to have considerably higher serum MIF levels than urinary bladder disease (UBD) and higher than normal patients indicating that MIF could be a potential diagnostic marker for bladder cancer (177). Another study reported that MIF was negatively correlated with overall survival rate in bladder cancer, highlighting its prognostic relevance (266).
Additionally, MIFs could be potential targets in bladder cancer (176, 178). Anti-MIF antibody reduced the proliferation in HT-1376 cell (human bladder cancer cell) (176). In another study it was reported that CPSI-1306 decreased both tumor development and neovascularization via blocking the enzymatic portion of MIF (125). Furthermore, 4-IPP inhibits and blocks MIF-2 leading to decreased tumor stage and tumor growth (178).
Collectively, these findings establish MIF not only as a driver of tumor progression but also as a promising, though still underexploited, target for therapeutic intervention. Having established the widespread involvement of MIF in the biology of solid tumors, it is essential to consider its potential as a clinical target. The following sections will explore how MIF can be leveraged as a biomarker and a therapeutic target, highlighting both current progress and future opportunities in translational oncology.
6 Clinical implications of targeting MIF
MIF has emerged as a promising biomarker across multiple solid tumor types as extensively discussed in the current review. Elevated MIF expression in tumor tissue, serum, or plasma often correlates with advanced disease stage, poor prognosis, and resistance to therapy (155, 267–269). In BC, high MIF levels are associated with more aggressive subtypes such as TNBC (183). Similarly, MIF expression predicts poor survival and increased metastatic potential in colorectal cancer as discussed earlier (270). As a dynamic marker of tumor-immune interactions, MIF levels may also guide patient selection for immunotherapy or combination regimens. Efforts to therapeutically target MIF are ongoing, with several classes of inhibitors under preclinical and early clinical evaluation (271, 272). Strategies include direct enzymatic inhibitors, neutralizing antibodies, and agents that interfere with MIF-receptor interactions as summarized in Table 2. Despite promising preclinical results, translation into clinical efficacy remains a challenge, highlighting the need for refined targeting strategies and combination approaches.
Small-molecule antagonists targeting MIF have been developed by exploiting its tautomerase active site, which overlaps with the CD74 binding domain (275, 276). Small molecule inhibitors such as ISO-1 and 4-IPP target the tautomerase active site of MIF, disrupting its interaction with receptors and downstream signaling (Table 2). 4-IPP forms a covalent bond with MIF, leading to irreversible inactivation (276). While these agents show efficacy in reducing tumor growth in experimental models, issues with specificity and off-target effects have limited their clinical progression. Other small molecule inhibitors block MIF–CD74 binding and show therapeutic activity in mouse models of systemic lupus erythematosus (273). The most advanced MIF antagonist in clinical trials is ibudilast, originally a phosphodiesterase inhibitor, which acts as an allosteric inhibitor of MIF. Ibudilast binds to a dynamic site on MIF that is revealed only upon binding, inducing conformational changes that inactivate MIF (277). It has shown efficacy in a Phase II trial for multiple sclerosis, where high-expression MIF genotypes increase disease risk (271). MIF’s dynamic properties also enable the discovery of agonists, which could be useful in conditions where low-expression MIF alleles are unfavorable.
Developing antibody-based therapies such as monoclonal antibodies that neutralize extracellular MIF or block CD74–MIF interaction is still under investigation (278). A number of monoclonal antibodies targeting MIF have been developed; BaxB01, BaxG03, and BaxM159 induced apoptosis in prostate cancer cells in-vitro by inhibiting ERK1/2 signaling, leading to reduced tumor growth in a xenograft mouse model (274, 279). Additionally, Imalumab (Bax69) was evaluated in a Phase 1 clinical trial, which demonstrated promising efficacy against solid tumors such as colorectal carcinoma, non-small cell lung cancer, and ovarian cancer as shown in Table 2. Approximately one-third of the patients treated with Imalumab showed stable disease (191). Since anti-MIF antibodies have demonstrated the ability to reduce tumor burden and enhance immune cell infiltration in preclinical studies, such approaches offer improved specificity compared to small molecule inhibitors. However, anti-MIF antibodies still face challenges related to tumor penetration and immunogenicity. Given MIF’s role in immune suppression, combining MIF inhibitors with immune checkpoint blockades such as anti-PD-1/PD-L1 is a compelling strategy. Preclinical studies suggest that MIF inhibition can sensitize tumors to immunotherapy by altering the tumor microenvironment (280, 281). Future clinical trials will be critical to validate these synergistic effects. Despite encouraging advances in targeting MIF therapeutically, significant challenges remain. MIF’s complex and sometimes paradoxical roles in tumor biology, as well as the limitations of current therapeutic approaches, present important hurdles that must be addressed to fully realize its clinical potential.
7 Challenges and controversies
Although predominantly characterized as a tumor promoter, MIF may exert context-dependent tumor-suppressive effects in certain context (282). These dual roles complicate therapeutic targeting and necessitate a nuanced understanding of tumor-specific biology (283). Furthermore, MIF’s role varies significantly across tumor types, stages, and even subclones within the same tumor. Factors such as the local immune contexture, presence of specific co-receptors, and tumor mutational burden influence whether MIF acts primarily as a promoter or modulator of cancer progression (284). Thus, personalized approaches to MIF targeting will be necessary to overcome this heterogeneity. In addition, most MIF inhibitors lack tumor specificity and can impact normal physiological processes, leading to potential toxicity. Additionally, compensatory mechanisms, such as upregulation of MIF homologs such as D-DT/MIF-2, may reduce the effectiveness of MIF-targeted therapies (285). Accordingly, there is a pressing need for next-generation inhibitors with improved specificity and for strategies to block redundancy in MIF family signaling. Addressing these challenges requires innovative strategies that account for tumor heterogeneity and the intricate functions of MIF. Emerging technologies and combination therapies offer promising avenues to overcome current barriers, paving the way for more effective and personalized interventions.
8 Future perspectives and conclusion
Integrating MIF expressions and signaling profiles into molecular diagnostic panels could help identify patients who are most likely to benefit from MIF-targeted therapies. Genomic, transcriptomic, and proteomic approaches may reveal actionable MIF-driven tumor subtypes, enabling more precision medicine tailored interventions. In addition, combining MIF inhibition with immune checkpoint inhibitors, cancer vaccines, or T-cell therapies holds great promise. MIF blockade may overcome resistance mechanisms that currently limit the success of immunotherapies in “cold” tumors, converting them into “hot” immunologically active tumors. Finally, advanced delivery systems, such as nanoparticle-based carriers, could enhance the precision and efficacy of MIF-targeted therapies. Encapsulation of MIF inhibitors within tumor-targeting nanoparticles may improve biodistribution, minimize off-target effects, and allow combination payloads, such as immunomodulators or chemotherapeutics. As the field continues to evolve, the integration of MIF-targeted therapies into broader cancer treatment paradigms appears increasingly feasible. A deeper understanding of MIF’s biology, coupled with technological innovation, will be critical to translating research advances into tangible clinical benefits for patients. In conclusion, MIF plays a multifaceted role in the progression of solid tumors by promoting inflammation, immune evasion, proliferation, angiogenesis, and metastasis. Its expression correlates with poor clinical outcomes across several major cancers, making it a critical focus for diagnostic, prognostic, and therapeutic research. While challenges remain, including tumor heterogeneity and therapeutic specificity, the future for MIF-targeted interventions is promising. Advances in precision medicine, immunotherapy, and drug delivery technologies may unlock the potential of MIF inhibition as a cornerstone of solid tumor therapy. Continued preclinical exploration and carefully designed clinical trials will be key to translating these insights into meaningful patient benefit.
Author contributions
RY: Conceptualization, Writing – original draft, Writing – review & editing, Project administration, Supervision, Funding acquisition. NE: Conceptualization, Writing – original draft, Writing – review & editing, Project administration, Supervision, Funding acquisition. AA: Writing – original draft, Writing – review & editing, Visualization. AM: Writing – original draft, Writing – review & editing. LE: Writing – original draft, Writing – review & editing. AR: Writing – original draft, Writing – review & editing, Visualization. RA: Conceptualization, Writing – original draft, Writing – review & editing, Project administration, Supervision, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. RY and NE are recipients of the L’Oréal-UNESCO For Women in Science Egypt and Middle East Regional Young Talents program grant, respectively. The funders had no role in the study design, analysis, writing, or decision to publish the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gu Y-L, Xiao L-L, Li D-J, Liu Y-N, Zhu C-J, and Zhang S-J. Gene knockout or inhibition of macrophage migration inhibitory factor alleviates lipopolysaccharide-induced liver injury via inhibiting inflammatory response. Hepatobiliary Pancreatic Dis Int. (2021) 20:469–77. doi: 10.1016/j.hbpd.2021.07.002
2. Skurk T, Herder C, Krauft I, Muller-Scholze S, Hauner H, and Kolb H. Production and release of macrophage migration inhibitory factor from human adipocytes. Endocrinology. (2005) 146:1006–11. doi: 10.1210/en.2004-0924
3. Calandra T and Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. (2003) 3:791–800. doi: 10.1038/nri1200
4. Arizza V, Bonura A, La Paglia L, Urso A, Pinsino A, and Vizzini A. Transcriptional and in silico analyses of MIF cytokine and TLR signalling interplay in the LPS inflammatory response of Ciona robusta. Sci Rep. (2020) 10:11339. doi: 10.1038/s41598-020-68339-x
5. Rodriguez-Sosa M, Cabellos-Avelar T, Sanchez-Zamora Y, Juárez-Avelar I, García-Reyes E, Lira-León A, et al. Proinflammatory cytokine MIF plays a role in the pathogenesis of type-2 diabetes mellitus, but does not affect hepatic mitochondrial function. Cytokine. (2017) 99:214–24. doi: 10.1016/j.cyto.2017.07.012
6. Mitchell RA, Metz CN, Peng T, and Bucala R. Sustained mitogen-activated protein kinase (MAPK) and cytoplasmic phospholipase A2 activation by macrophage migration inhibitory factor (MIF). Regul role Cell proliferation glucocorticoid action. J Biol Chem. (1999) 274:18100–6. doi: 10.1074/jbc.274.25.18100
7. Rich AR and Lewis MR. The nature of allergy in tuberculosis as revealed by tissue culture studies. Bull Johns Hopkins Hosp. (1932) 50:115.
8. Bloom B and Bennett B. Relation of the migration inhibitory factor (MIF) to delayed-type hypersensitivity reactions. Ann New York Acad Sci. (2006) 169:258–65. doi: 10.1111/j.1749-6632.1970.tb55994.x
9. George M and Vaughan JH. In vitro cell migration as a model for delayed hypersensitivity. Proc Soc Exp Biol Med. (1962) 111:514–21. doi: 10.3181/00379727-111-27841
10. David JR. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. (1966) 56:72–7. doi: 10.1073/pnas.56.1.72
11. Weiser WY, Temple PA, Witek-Giannotti JS, Remold HG, Clark SC, and David JR. Molecular cloning of a cDNA encoding a human macrophage migration inhibitory factor. Proc Natl Acad Sci U S A. (1989) 86:7522–6. doi: 10.1073/pnas.86.19.7522
12. Sugimoto H, Suzuki M, Nakagawa A, Tanaka I, and Nishihira J. Crystal structure of macrophage migration inhibitory factor from human Å lymphocyte at 2.1 Å resolution. FEBS Lett. (1996) 389:145–8. doi: 10.1016/0014-5793(96)00553-4
13. Khezrian A, Shojaeian A, Khaghani Boroujeni A, and Amini R. Therapeutic opportunities in breast cancer by targeting macrophage migration inhibitory factor as a pleiotropic cytokine. Breast Cancer (Auckl). (2024) 18:11782234241276310. doi: 10.1177/11782234241276310
14. Cui D, Peng Y, Zhang C, Li Z, Su Y, Qi Y, et al. Macrophage migration inhibitory factor mediates metabolic dysfunction induced by atypical antipsychotic therapy. J Clin Invest. (2018) 128:4997–5007. doi: 10.1172/JCI93090
15. Sanchez-Niño MD, Sanz AB, Ruiz-Andres O, Poveda J, Izquierdo MC, Selgas R, et al. MIF, CD74 and other partners in kidney disease: tales of a promiscuous couple. Cytokine Growth Factor Rev. (2013) 24:23–40. doi: 10.1016/j.cytogfr.2012.08.001
16. Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, et al. MIF signal transduction initiated by binding to CD74. J Exp Med. (2003) 197:1467–76. doi: 10.1084/jem.20030286
17. Shi X, Leng L, Wang T, Wang W, Du X, Li J, et al. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity. (2006) 25:595–606. doi: 10.1016/j.immuni.2006.08.020
18. Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. (2007) 13:587–96. doi: 10.1038/nm1567
19. Farr L, Ghosh S, and Moonah S. Role of MIF cytokine/CD74 receptor pathway in protecting against injury and promoting repair. Front Immunol. (2020) 11:1273. doi: 10.3389/fimmu.2020.01273
20. Kleemann R, Hausser A, Geiger G, Mischke R, Burger-Kentischer A, Flieger O, et al. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature. (2000) 408:211–6. doi: 10.1038/35041591
21. Lue H, Thiele M, Franz J, Dahl E, Speckgens S, Leng L, et al. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene. (2007) 26:5046–59. doi: 10.1038/sj.onc.1210318
22. Young-Hoon P, Mi Suk J, Ki-Tae H, Hak Sun Y, and Se Bok J. Structural characterization of As-MIF and hJAB1 during the inhibition of cell-cycle regulation. BMB Rep. (2017) 50:269–74. doi: 10.5483/BMBRep.2017.50.5.201
23. Bai F, Asojo OA, Cirillo P, Ciustea M, Ledizet M, Aristoff PA, et al. A novel allosteric inhibitor of macrophage migration inhibitory factor (MIF)*. J Biol Chem. (2012) 287:30653–63. doi: 10.1074/jbc.M112.385583
24. Lubetsky JB, Dios A, Han J, Aljabari B, Ruzsicska B, Mitchell R, et al. The tautomerase active site of macrophage migration inhibitory factor is a potential target for discovery of novel anti-inflammatory agents. J Biol Chem. (2002) 277:24976–82. doi: 10.1074/jbc.M203220200
25. Alam A, Haldar S, Thulasiram HV, Kumar R, Goyal M, Iqbal MS, et al. Novel anti-inflammatory activity of epoxyazadiradione against macrophage migration inhibitory factor: inhibition of tautomerase and proinflammatory activities of macrophage migration inhibitory factor. J Biol Chem. (2012) 287:24844–61. doi: 10.1074/jbc.M112.341321
26. Ramzy A, Abdel-Halim M, Manie T, Elemam NM, Mansour S, Youness RA, et al. In-vitro immune-modulation of triple-negative breast cancer through targeting miR-30a-5p/MALAT1 axis using nano-PDT combinational approach. Transl Oncol. (2025) 55:102365. doi: 10.1016/j.tranon.2025.102365
27. Ramzy A, ElSafy S, Elshoky HA, Soliman A, Youness R, Mansour S, et al. Drugless nanoparticles tune-up an array of intertwined pathways contributing to immune checkpoint signaling and metabolic reprogramming in triple-negative breast cancer. BioMed Mater. (2022) 18:015023. doi: 10.1088/1748-605X/aca85d
28. Mariscalco MM. Chapter 84 - infection and the host response. In: Fuhrman BP and Zimmerman JJ, editors. Pediatric critical care, 3rd ed.Mosby, Philadelphia (2006). p. 1299–319.
29. Koebernick H, Grode L, David JR, Rohde W, Rolph MS, Mittrücker HW, et al. Macrophage migration inhibitory factor (MIF) plays a pivotal role in immunity against Salmonella typhimurium. Proc Natl Acad Sci U S A. (2002) 99:13681–6. doi: 10.1073/pnas.212488699
30. Brock SE, Rendon BE, Xin D, Yaddanapudi K, and Mitchell RA. MIF family members cooperatively inhibit p53 expression and activity. PloS One. (2014) 9:e99795. doi: 10.1371/journal.pone.0099795
31. Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. (2018) 9:7204–18. doi: 10.18632/oncotarget.23208
32. Emara H, Abdelnaser A, Manie T, Motaal A, Allam N, and Youness RA. 44P Potential combination of anti-CD-47 and natural compounds to harness calreticulin/CD47 immune checkpoint in triple-negative breast cancer patients. ESMO Open. (2025) 10:104203. doi: 10.1016/j.esmoop.2025.104203
33. Cooke G, Armstrong ME, and Donnelly SC. Macrophage migration inhibitory factor (MIF), enzymatic activity and the inflammatory response. Biofactors. (2009) 35:165–8. doi: 10.1002/biof.27
34. Strehl C, Ehlers L, Gaber T, and Buttgereit F. Glucocorticoids-all-rounders tackling the versatile players of the immune system. Front Immunol. (2019) 10:1744. doi: 10.3389/fimmu.2019.01744
35. Kasama T, Ohtsuka K, Sato M, Takahashi R, Wakabayashi K, and Kobayashi K. Macrophage migration inhibitory factor: a multifunctional cytokine in rheumatic diseases. Arthritis. (2010) 2010:106202. doi: 10.1155/2010/106202
36. Soumoy L, Kindt N, Ghanem G, Saussez S, and Journe F. Role of macrophage migration inhibitory factor (MIF) in melanoma. Cancers. (2019) 11:529. doi: 10.3390/cancers11040529
37. Asare Y, Schmitt M, and Bernhagen J. The vascular biology of macrophage migration inhibitory factor (MIF). Expression and effects in inflammation, atherogenesis and angiogenesis. Thromb Haemost. (2013) 109:391–8. doi: 10.1160/TH12-11-0831
38. Klemke L, De Oliveira T, Witt D, Winkler N, Bohnenberger H, Bucala R, et al. Hsp90-stabilized MIF supports tumor progression via macrophage recruitment and angiogenesis in colorectal cancer. Cell Death Dis. (2021) 12:155. doi: 10.1038/s41419-021-03426-z
39. Youness RA, Gohar A, Kiriacos CJ, and El-Shazly M. Heat shock proteins: central players in oncological and immuno-oncological tracks. In: Turksen K, editor. Cell biology and translational medicine, volume 18: tissue differentiation, repair in health and disease. Springer Nature Switzerland, Cham (2023). p. 193–203.
40. Noe JT and Mitchell RA. MIF-dependent control of tumor immunity. Front Immunol. (2020) 11:609948. doi: 10.3389/fimmu.2020.609948
41. Selem NA, Nafae H, Manie T, Youness RA, and Gad MZ. Let-7a/cMyc/CCAT1/miR-17–5p circuit re-sensitizes atezolizumab resistance in triple negative breast cancer through modulating PD-L1. Pathol - Res Practice. (2023) 248:154579. doi: 10.1016/j.prp.2023.154579
42. Kerneur C, Cano CE, and Olive D. Major pathways involved in macrophage polarization in cancer. Front Immunol. (2022) 13:1026954. doi: 10.3389/fimmu.2022.1026954
43. Munir MT, Kay MK, Kang MH, Rahman MM, Al-Harrasi A, Choudhury M, et al. Tumor-associated macrophages as multifaceted regulators of breast tumor growth. Int J Mol Sci. (2021) 22:6526. doi: 10.3390/ijms22126526
44. Elemam NM, Youness RA, Abdelhamid AM, and Talaat IM. CAR-NK/CAR-T cells: emerging immunotherapy of cancer. Cham: Springer International Publishing (2024) p. 1–37. doi: 10.1007/16833_2024_429
45. Ryan N, Lamenza F, Shrestha S, Upadhaya P, Springer A, Jordanides P, et al. Host derived macrophage migration inhibitory factor expression attenuates anti-tumoral immune cell accumulation and promotes immunosuppression in the tumor microenvironment of head and neck squamous cell carcinoma. Biochim Biophys Acta (BBA) - Mol Basis Disease. (2024) 1870:167345. doi: 10.1016/j.bbadis.2024.167345
46. Zhang Z, Zhou X, Guo J, Zhang F, Qian Y, Wang G, et al. TA-MSCs, TA-MSCs-EVs, MIF: their crosstalk in immunosuppressive tumor microenvironment. J Transl Med. (2022) 20:320. doi: 10.1186/s12967-022-03528-y
47. Figueiredo CR, Azevedo RA, Mousdell S, Resende-Lara PT, Ireland L, Santos A, et al. Blockade of MIF-CD74 signalling on macrophages and dendritic cells restores the antitumour immune response against metastatic melanoma. Front Immunol. (2018) 9:1132. doi: 10.3389/fimmu.2018.01132
48. Tanese K and Ogata D. The role of macrophage migration inhibitory factor family and CD74 in the pathogenesis of melanoma. Exp Dermatol. (2024) 33:e15122. doi: 10.1111/exd.15122
49. Dayakar A, Chandrasekaran S, Kuchipudi SV, and Kalangi SK. Cytokines: key determinants of resistance or disease progression in visceral leishmaniasis: opportunities for novel diagnostics and immunotherapy. Front Immunol. (2019) 10. doi: 10.3389/fimmu.2019.00670
50. Abdelhamid AM, Zeinelabdeen Y, Manie T, Khallaf E, Assal RA, and Youness RA. miR-17–5p/STAT3/H19: A novel regulatory axis tuning ULBP2 expression in young breast cancer patients. Pathol - Res Practice. (2024) 263:155638. doi: 10.1016/j.prp.2024.155638
51. Abdel-Latif M and Youness RA. Why natural killer cells in triple negative breast cancer? World J Clin Oncol. (2020) 11:464–76. doi: 10.5306/wjco.v11.i7.464
52. Krockenberger M, Dombrowski Y, Weidler C, Ossadnik M, Hönig A, Häusler S, et al. Macrophage migration inhibitory factor contributes to the immune escape of ovarian cancer by down-regulating NKG2D. J Immunol. (2008) 180:7338–48. doi: 10.4049/jimmunol.180.11.7338
53. Rahmoon MA, Ahmed YR, Ibrahim GA, Tarif HM, Imam W, Mohamed ETH, et al. MiR-615-5p depresses natural killer cells cytotoxicity through repressing IGF-1R in hepatocellular carcinoma patients. Growth Factors. (2017) 35:76–87. doi: 10.1080/08977194.2017.1354859
54. Youness RA, Atef RM, Amr AR, Ibrahim GA, Tarif HM, Imam W, et al. Contradicting interplay between insulin-like growth factor-1 and miR-486-5p in primary NK cells and hepatoma cell lines with a contemporary inhibitory impact on HCC tumor progression. Growth Factors. (2016) 34:128–40. doi: 10.1080/08977194.2016.1200571
55. Fallone L, Walzer T, and Marçais A. Signaling pathways leading to mTOR activation downstream cytokine receptors in lymphocytes in health and disease. Int J Mol Sci. (2023) 24:12736. doi: 10.3390/ijms241612736
56. Duan S, Guo W, Xu Z, He Y, Liang C, Mo Y, et al. Natural killer group 2D receptor and its ligands in cancer immune escape. Mol Cancer. (2019) 18:29. doi: 10.1186/s12943-019-0956-8
57. Veglia F, Sanseviero E, and Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol. (2021) 21:485–98. doi: 10.1038/s41577-020-00490-y
58. Simpson KD and Cross JV. MIF: metastasis/MDSC-inducing factor? Oncoimmunology. (2013) 2:e23337. doi: 10.4161/onci.23337
59. Lu J, Luo Y, Rao D, Wang T, Lei Z, Chen X, et al. Myeloid-derived suppressor cells in cancer: therapeutic targets to overcome tumor immune evasion. Exp Hematol Oncol. (2024) 13:39. doi: 10.1186/s40164-024-00505-7
60. Cui H, Lan Z, Zou KL, Zhao YY, and Yu GT. STAT3 promotes differentiation of monocytes to MDSCs via CD39/CD73-adenosine signal pathway in oral squamous cell carcinoma. Cancer Immunol Immunother. (2023) 72:1315–26. doi: 10.1007/s00262-022-03336-9
61. Zhang H, Ye YL, Li MX, Ye SB, Huang WR, Cai TT, et al. CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer. Oncogene. (2017) 36:2095–104. doi: 10.1038/onc.2016.367
62. Masucci MT, Minopoli M, and Carriero MV. Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. Front Oncol. (2019) 9:1146. doi: 10.3389/fonc.2019.01146
63. Mora Barthelmess R, Stijlemans B, and Van Ginderachter JA. Hallmarks of cancer affected by the MIF cytokine family. Cancers. (2023) 15:395. doi: 10.3390/cancers15020395
64. Kim H-R, Kim K-W, Jung HG, Yoon K-S, Oh H-J, Cho M-L, et al. Macrophage migration inhibitory factor enhances osteoclastogenesis through upregulation of RANKL expression from fibroblast-like synoviocytes in patients with rheumatoid arthritis. Arthritis Res Ther. (2011) 13:1–13. doi: 10.1186/ar3279
65. Nobre CCG, de Araújo JMG, TAAdM F, Cobucci RNO, Lanza DCF, VS A, et al. Macrophage migration inhibitory factor (MIF): biological activities and relation with cancer. Pathol Oncol Res. (2017) 23:235–44. doi: 10.1007/s12253-016-0138-6
66. Benedek G, Meza-Romero R, Jordan K, Zhang Y, Nguyen H, Kent G, et al. MIF and D-DT are potential disease severity modifiers in male MS subjects. Proc Natl Acad Sci. (2017) 114:E8421–E9. doi: 10.1073/pnas.1712288114
67. Bezdek S, Leng L, Busch H, Mousavi S, Rades D, Dahlke M, et al. Macrophage migration inhibitory factor (MIF) drives murine psoriasiform dermatitis. Front Immunol. (2018) 9:2262. doi: 10.3389/fimmu.2018.02262
68. Tu Y, Guo R, Li J, Wang S, Leng L, Deng J, et al. MiRNA regulation of MIF in SLE and attenuation of murine lupus nephritis with miR-654. Front Immunol. (2019) 10:2229. doi: 10.3389/fimmu.2019.02229
69. Sumaiya K, Langford D, Natarajaseenivasan K, and Shanmughapriya S. Macrophage migration inhibitory factor (MIF): A multifaceted cytokine regulated by genetic and physiological strategies. Pharmacol Ther. (2022) 233:108024. doi: 10.1016/j.pharmthera.2021.108024
70. Alampour-Rajabi S, El Bounkari O, Rot A, Müller-Newen G, Bachelerie F, Gawaz M, et al. MIF interacts with CXCR7 to promote receptor internalization, ERK1/2 and ZAP-70 signaling, and lymphocyte chemotaxis. FASEB J. (2015) 29:4497–511. doi: 10.1096/fj.15-273904
71. Schwartz V, Lue H, Kraemer S, Korbiel J, Krohn R, Ohl K, et al. A functional heteromeric MIF receptor formed by CD74 and CXCR4. FEBS Letters. (2009) 583:2749–57. doi: 10.1016/j.febslet.2009.07.058
72. Song S, Xiao Z, Dekker FJ, Poelarends GJ, and Melgert BN. Macrophage migration inhibitory factor family proteins are multitasking cytokines in tissue injury. Cell Mol Life Sci. (2022) 79:105. doi: 10.1007/s00018-021-04038-8
73. Fukuzawa J, Nishihira J, Hasebe N, Haneda T, Osaki J, Saito T, et al. Contribution of macrophage migration inhibitory factor to extracellular signal-regulated kinase activation by oxidative stress in cardiomyocytes *. J Biol Chem. (2002) 277:24889–95. doi: 10.1074/jbc.M112054200
74. Mitchell RA, Metz CN, Peng T, and Bucala R. Sustained mitogen-activated protein kinase (MAPK) and cytoplasmic phospholipase A2 activation by macrophage migration inhibitory factor (MIF): REGULATORY ROLE IN CELL PROLIFERATION AND GLUCOCORTICOID ACTION *. J Biol Chem. (1999) 274:18100–6. doi: 10.1074/jbc.274.25.18100
75. Farr L, Ghosh S, and Moonah S. Role of MIF cytokine/CD74 receptor pathway in protecting against injury and promoting repair. Front Immunol. (2020) 11. doi: 10.3389/fimmu.2020.01273
76. Lee JPW, Foote A, Fan H, Peral de Castro C, Lang T, Jones SA, et al. Loss of autophagy enhances MIF/macrophage migration inhibitory factor release by macrophages. Autophagy. (2016) 12:907–16. doi: 10.1080/15548627.2016.1164358
77. Das R, LaRose MI, Hergott CB, Leng L, Bucala R, and Weiser JN. Macrophage migration inhibitory factor promotes clearance of pneumococcal colonization. J Immunol. (2014) 193:764–72. doi: 10.4049/jimmunol.1400133
78. Wang X, Chen T, Leng L, Fan J, Cao K, Duan Z, et al. MIF produced by bone marrow–derived macrophages contributes to teratoma progression after embryonic stem cell transplantation. Cancer Res. (2012) 72:2867–78. doi: 10.1158/0008-5472.CAN-11-3247
79. Yaddanapudi K, Putty K, Rendon BE, Lamont GJ, Faughn JD, Satoskar A, et al. Control of tumor-associated macrophage alternative activation by macrophage migration inhibitory factor. J Immunol. (2013) 190:2984–93. doi: 10.4049/jimmunol.1201650
80. Barbosa de Souza Rizzo M, Brasilino de Carvalho M, Kim EJ, Rendon BE, Noe JT, Darlene Wise A, et al. Oral squamous carcinoma cells promote macrophage polarization in an MIF-dependent manner. QJM: Int J Med. (2018) 111:769–78. doi: 10.1093/qjmed/hcy163
81. de Dios Rosado J and Rodriguez-Sosa M. Macrophage migration inhibitory factor (MIF): a key player in protozoan infections. Int J Biol Sci. (2011) 7:1239. doi: 10.7150/ijbs.7.1239
82. Gutiérrez-Martínez E, Planès R, Anselmi G, Reynolds M, Menezes S, Adiko AC, et al. Cross-presentation of cell-associated antigens by MHC class I in dendritic cell subsets. Front Immunol. (2015) 6. doi: 10.3389/fimmu.2015.00363
83. Figueiredo CR, Azevedo RA, Mousdell S, Resende-Lara PT, Ireland L, Santos A, et al. Blockade of MIF–CD74 signalling on macrophages and dendritic cells restores the antitumour immune response against metastatic melanoma. Front Immunol. (2018) 9. doi: 10.3389/fimmu.2018.01132
84. Balogh KN, Templeton DJ, and Cross JV. Macrophage Migration Inhibitory Factor protects cancer cells from immunogenic cell death and impairs anti-tumor immune responses. PloS One. (2018) 13:e0197702. doi: 10.1371/journal.pone.0197702
85. Rossi AG, Haslett C, Hirani N, Greening AP, Rahman I, Metz CN, et al. Human circulating eosinophils secrete macrophage migration inhibitory factor (MIF). Potential role asthma. J Clin Invest. (1998) 101:2869–74. doi: 10.1172/JCI1524
86. Roth S, Solbach W, and Laskay T. IL-16 and MIF: messengers beyond neutrophil cell death. Cell Death Disease. (2016) 7:e2049–e. doi: 10.1038/cddis.2015.388
87. Ningyan G, Xu Y, Hongfei S, Jingjing C, and Min C. The role of macrophage migration inhibitory factor in mast cell-stimulated fibroblast proliferation and collagen production. PloS One. (2015) 10:e0122482. doi: 10.1371/journal.pone.0122482
88. Mizue Y, Ghani S, Leng L, McDonald C, Kong P, Baugh J, et al. Role for macrophage migration inhibitory factor in asthma. Proc Natl Acad Sci U S A. (2005) 102:14410–5. doi: 10.1073/pnas.0507189102
89. Repp AC, Mayhew ES, Apte S, and Niederkorn JY. Human uveal melanoma cells produce macrophage migration-inhibitory factor to prevent lysis by NK cells1. J Immunol. (2000) 165:710–5. doi: 10.4049/jimmunol.165.2.710
90. Krockenberger M, Dombrowski Y, Weidler C, Ossadnik M, Höunig A, Häuusler S, et al. Macrophage migration inhibitory factor contributes to the immune escape of ovarian cancer by down-regulating NKG2D1. T. he J Immunol. (2008) 180:7338–48. doi: 10.4049/jimmunol.180.11.7338
91. Yan X, Orentas RJ, and Johnson BD. Tumor-derived macrophage migration inhibitory factor (MIF) inhibits T lymphocyte activation. Cytokine. (2006) 33:188–98. doi: 10.1016/j.cyto.2006.01.006
92. Choi S, Kim H-R, Leng L, Kang I, Jorgensen WL, Cho C-S, et al. Role of macrophage migration inhibitory factor in the regulatory T cell response of tumor-bearing mice. J Immunol. (2012) 189:3905–13. doi: 10.4049/jimmunol.1102152
93. Li J, Mo H-Y, Xiong G, Zhang L, He J, Huang Z-F, et al. Tumor microenvironment macrophage inhibitory factor directs the accumulation of interleukin-17-producing tumor-infiltrating lymphocytes and predicts favorable survival in nasopharyngeal carcinoma patients *. J Biol Chem. (2012) 287:35484–95. doi: 10.1074/jbc.M112.367532
94. Avalos-Navarro G, Muñoz-Valle JF, Daneri-Navarro A, Quintero-Ramos A, Franco-Topete RA, Morán-Mendoza ADJ, et al. Circulating soluble levels of MIF in women with breast cancer in the molecular subtypes: relationship with Th17 cytokine profile. Clin Exp Med. (2019) 19:385–91. doi: 10.1007/s10238-019-00559-6
95. Xue H, Yang Y, Zhang Y, Song S, Zhang L, Ma L, et al. Macrophage migration inhibitory factor interacting with th17 cells may be involved in the pathogenesis of autoimmune damage in hashimoto’s thyroiditis. Mediators Inflammation. (2015) 2015:621072. doi: 10.1155/2015/621072
96. Hernández-Palma LA, García-Arellano S, Bucala R, Llamas-Covarrubias MA, de la Cruz-Mosso U, Oregon-Romero E, et al. Functional MIF promoter haplotypes modulate Th17-related cytokine expression in peripheral blood mononuclear cells from control subjects and rheumatoid arthritis patients. Cytokine. (2019) 115:89–96. doi: 10.1016/j.cyto.2018.11.014
97. Bozza M, Satoskar AR, Lin G, Lu B, Humbles AA, Gerard C, et al. Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis. J Exp Med. (1999) 189:341–6. doi: 10.1084/jem.189.2.341
98. Flores M, Saavedra R, Bautista R, Viedma R, Tenorio EP, Leng L, et al. Macrophage migration inhibitory factor (MIF) is critical for the host resistance against Toxoplasma gondii. FASEB J. (2008) 22:3661–71. doi: 10.1096/fj.08-111666
99. Das R, Koo MS, Kim BH, Jacob ST, Subbian S, Yao J, et al. Macrophage migration inhibitory factor (MIF) is a critical mediator of the innate immune response to Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. (2013) 110:E2997–3006. doi: 10.1073/pnas.1301128110
100. Satoskar Abhay R, Bozza M, Rodriguez Sosa M, Lin G, and David John R. Migration-inhibitory factor gene-deficient mice are susceptible to cutaneous leishmania major infection. Infection Immunity. (2001) 69:906–11. doi: 10.1128/IAI.69.2.906-911.2001
101. Reyes José L, Terrazas Luis I, Espinoza B, Cruz-Robles D, Soto V, Rivera-Montoya I, et al. Macrophage Migration Inhibitory Factor Contributes to Host Defense against Acute Trypanosoma cruzi Infection. Infection Immunity. (2006) 74:3170–9. doi: 10.1128/IAI.01648-05
102. Tilstam PV, Schulte W, Holowka T, Kim BS, Nouws J, Sauler M, et al. MIF but not MIF-2 recruits inflammatory macrophages in an experimental polymicrobial sepsis model. J Clin Invest. (2021) 131:e127171. doi: 10.1172/JCI127171
103. Trifone C, Salido J, Ruiz MJ, Leng L, Quiroga MF, Salomón H, et al. Interaction between macrophage migration inhibitory factor and CD74 in human immunodeficiency virus type I infected primary monocyte-derived macrophages triggers the production of proinflammatory mediators and enhances infection of unactivated CD4(+) T cells. Front Immunol. (2018) 9:1494. doi: 10.3389/fimmu.2018.01494
104. Baugh JA and Donnelly SC. Macrophage migration inhibitory factor: a neuroendocrine modulator of chronic inflammation. J Endocrinol. (2003) 179:15–23. doi: 10.1677/joe.0.1790015
105. Trifone C, Baquero L, Czernikier A, Benencio P, Leng L, Laufer N, et al. Macrophage migration inhibitory factor (MIF) promotes increased proportions of the highly permissive th17-like cell profile during HIV infection. Viruses. (2022) 14:2218. doi: 10.3390/v14102218
106. Jankauskas SS, Wong DWL, Bucala R, Djudjaj S, and Boor P. Evolving complexity of MIF signaling. Cell Signalling. (2019) 57:76–88. doi: 10.1016/j.cellsig.2019.01.006
107. Bucala R and Donnelly SC. Macrophage migration inhibitory factor: A probable link between inflammation and cancer. Immunity. (2007) 26:281–5. doi: 10.1016/j.immuni.2007.03.005
108. Breidung D, Megas I-F, Freytag DL, Bernhagen J, and Grieb G. The role of macrophage migration inhibitory factor (MIF) and D-dopachrome tautomerase (D-DT/MIF-2) in infections: A clinical perspective. Biomedicines. (2024) 12:2. doi: 10.3390/biomedicines12010002
109. Grieb G, Merk M, Bernhagen J, and Bucala R. Macrophage migration inhibitory factor (MIF): a promising biomarker. Drug News Perspect. (2010) 23:257–64. doi: 10.1358/dnp.2010.23.4.1453629
110. Foote A, Briganti EM, Kipen Y, Santos L, Leech M, and Morand EF. Macrophage migration inhibitory factor in systemic lupus erythematosus. J Rheumatol. (2004) 31:268–73.
111. Wu S-P, Leng L, Feng Z, Liu N, Zhao H, McDonald C, et al. Macrophage migration inhibitory factor promoter polymorphisms and the clinical expression of scleroderma. Arthritis Rheumatism. (2006) 54:3661–9. doi: 10.1002/art.22179
112. Ohwatari R, Inuyama Y, Fukuda S, Onoé K, Iwabuchi K, and Nishihira J. Serum level of macrophage migration inhibitory factor as a useful parameter of clinical course in patients with wegener’s granulomatosis and relapsing polychondritis. Ann Otology Rhinology Laryngology. (2001) 110:1035–40. doi: 10.1177/000348940111001108
113. Leech M, Metz C, Hall P, Hutchinson P, Gianis K, Smith M, et al. Macrophage migration inhibitory factor in rheumatoid arthritis: Evidence of proinflammatory function and regulation by glucocorticoids. Arthritis Rheumatism. (1999) 42:1601–8. doi: 10.1002/1529-0131(199908)42:8<1601::AID-ANR6>3.0.CO;2-B
114. Kim HR, Park MK, Cho ML, Yoon CH, Lee SH, Park SH, et al. Macrophage migration inhibitory factor upregulates angiogenic factors and correlates with clinical measures in rheumatoid arthritis. J Rheumatol. (2007) 34:927–36.
115. Flaster H, Bernhagen, Calandra T, and Bucala R. The macrophage migration inhibitory factor-glucocorticoid dyad: regulation of inflammation and immunity. Mol Endocrinology. (2007) 21:1267–80. doi: 10.1210/me.2007-0065
116. Bach J-P, Rinn B, Meyer B, Dodel R, and Bacher M. Role of MIF in inflammation and tumorigenesis. Oncology. (2008) 75:127–33. doi: 10.1159/000155223
117. Shah YM, Ito S, Morimura K, Chen C, Yim SH, Haase VH, et al. Hypoxia-inducible factor augments experimental colitis through an MIF&x2013;Dependent inflammatory signaling cascade. Gastroenterology. (2008) 134:2036–48.e3. doi: 10.1053/j.gastro.2008.03.009
118. Dumitru CA, Gholaman H, Trellakis S, Bruderek K, Dominas N, Gu X, et al. Tumor-derived macrophage migration inhibitory factor modulates the biology of head and neck cancer cells via neutrophil activation. Int J Cancer. (2011) 129:859–69. doi: 10.1002/ijc.25991
119. Lien M-Y, Tsai H-C, Chang A-C, Tsai M-H, Hua C-H, Wang S-W, et al. Chemokine CCL4 induces vascular endothelial growth factor C expression and lymphangiogenesis by miR-195-3p in oral squamous cell carcinoma. Front Immunol. (2018) 9. doi: 10.3389/fimmu.2018.00412
120. Quintero-Fabián S, Arreola R, Becerril-Villanueva E, Torres-Romero JC, Arana-Argáez V, Lara-Riegos J, et al. Role Matrix Metalloproteinases Angiogenesis Cancer. Front Oncol. (2019) 9:1370. doi: 10.3389/fonc.2019.01370
121. Vilgelm AE and Richmond A. Chemokines modulate immune surveillance in tumorigenesis, metastasis, and response to immunotherapy. Front Immunol. (2019) 10. doi: 10.3389/fimmu.2019.00333
122. Cheng L, Yang C, Lu J, Huang M, Xie R, Lynch S, et al. Oncogenic SLC2A11–MIF fusion protein interacts with polypyrimidine tract binding protein 1 to facilitate bladder cancer proliferation and metastasis by regulating mRNA stability. MedComm. (2024) 5:e685. doi: 10.1002/mco2.685
123. Liu N, Jiang N, Guo R, Jiang W, He Q-M, Xu Y-F, et al. MiR-451 inhibits cell growth and invasion by targeting MIF and is associated with survival in nasopharyngeal carcinoma. Mol cancer. (2013) 12:1–8. doi: 10.1186/1476-4598-12-123
124. Huang G, Ma L, Shen L, Lei Y, Guo L, Deng Y, et al. MIF/SCL3A2 depletion inhibits the proliferation and metastasis of colorectal cancer cells via the AKT/GSK-3β pathway and cell iron death. J Cell Mol Med. (2022) 26:3410–22. doi: 10.1111/jcmm.17352
125. Choudhary S, Hegde P, Pruitt JR, Sielecki TM, Choudhary D, Scarpato K, et al. Macrophage migratory inhibitory factor promotes bladder cancer progression via increasing proliferation and angiogenesis. Carcinogenesis. (2013) 34:2891–9. doi: 10.1093/carcin/bgt239
126. Lechien JR, Nassri A, Kindt N, Brown DN, Journe F, and Saussez S. Role of macrophage migration inhibitory factor in head and neck cancer and novel therapeutic targets: A systematic review. Head neck. (2017) 39:2573–84. doi: 10.1002/hed.24939
127. Wang SS, Cen X, Liang XH, and Tang YL. Macrophage migration inhibitory factor: a potential driver and biomarker for head and neck squamous cell carcinoma. Oncotarget. (2017) 8:10650. doi: 10.18632/oncotarget.12890
128. Kindt N, Lechien J, Decaestecker C, Rodriguez A, Chantrain G, Remmelink M, et al. Expression of macrophage migration-inhibitory factor is correlated with progression in oral cavity carcinomas. Anticancer Res. (2012) 32:4499–505.
129. Wang SS, Zheng M, Pang X, Zhang M, Yu XH, Wu JB, et al. Macrophage migration inhibitory factor promotes the invasion and metastasis of oral squamous cell carcinoma through matrix metalloprotein-2/9. Mol carcinogenesis. (2019) 58:1809–21. doi: 10.1002/mc.23067
130. Chen W, Zuo F, Zhang K, Xia T, Lei W, Zhang Z, et al. Exosomal MIF derived from nasopharyngeal carcinoma promotes metastasis by repressing ferroptosis of macrophages. Front Cell Dev Biol. (2021) 9:791187. doi: 10.3389/fcell.2021.791187
131. Yang M, Wu S, Cai W, Ming X, Zhou Y, and Chen X. Hypoxia-induced MIF induces dysregulation of lipid metabolism in Hep2 laryngocarcinoma through the IL-6/JAK-STAT pathway. Lipids Health disease. (2022) 21:82. doi: 10.1186/s12944-022-01693-z
132. Cludts S, Decaestecker C, Johnson B, Lechien J, Leroy X, Kindt N, et al. Increased expression of macrophage migration inhibitory factor during progression to hypopharyngeal squamous cell carcinoma. Anticancer Res. (2010) 30:3313–9.
133. Liang T, Xiao D, Lu S, Ye X, and Xiao Z. Prognostic value of a serum panel of inflammatory factors in non-metastatic nasopharyngeal carcinoma patients undergoing radical radiotherapy with adjuvant chemotherapy. Cancer Manage Res. (2023) 14:2763–72. doi: 10.2147/CMAR.S371922
134. Xue N, Xing S, Ma W, Sheng J, Huang Z, and Xu Q. Combination of plasma MIF and VCA-IgA improves the diagnostic specificity for patients with nasopharyngeal carcinoma. Technol Cancer Res Treat. (2020) 19:1533033820935773. doi: 10.1177/1533033820935773
135. Kang Y, Zhang Y, and Sun Y. Macrophage migration inhibitory factor is a novel prognostic marker for human oral squamous cell carcinoma. Pathology-Research Practice. (2018) 214:1192–8. doi: 10.1016/j.prp.2018.06.020
136. Duan Y, Huang X, Qiao B, Ma R, and Li J. Eugenol inhibits the biological activities of an oral squamous cell carcinoma cell line SCC9 via targeting MIF. Anti-Cancer Agents Medicinal Chem. (2022) 22:2799–806. doi: 10.2174/1871520622666220324105435
137. Dumitru CA, Bankfalvi A, Gu X, Zeidler R, Brandau S, and Lang S. AHNAK and inflammatory markers predict poor survival in laryngeal carcinoma. PloS One. (2013) 8:e56420. doi: 10.1371/journal.pone.0056420
138. Zhang T-L, Chen W-K, Huang X-P, Zheng B-W, Wu P-F, Zheng B-Y, et al. Single-cell RNA sequencing reveals the MIF/ACKR3 receptor-ligand interaction between neutrophils and nucleus pulposus cells in intervertebral disc degeneration. Trans/ Res. (2024) 272:1–18. doi: 10.1016/j.trsl.2024.05.011
139. Liu R-M, Sun D-N, Jiao Y-L, Wang P, Zhang J, Wang M, et al. Macrophage migration inhibitory factor promotes tumor aggressiveness of esophageal squamous cell carcinoma via activation of Akt and inactivation of GSK3β. Cancer letters. (2018) 412:289–96. doi: 10.1016/j.canlet.2017.10.018
140. Zhang L, Ye S-B, Ma G, Tang X-F, Chen S-P, He J, et al. The expressions of MIF and CXCR4 protein in tumor microenvironment are adverse prognostic factors in patients with esophageal squamous cell carcinoma. J Trans Med. (2013) 11:1–10. doi: 10.1186/1479-5876-11-60
141. Wang XB, Jiang XR, Yu XY, Wang L, He S, Feng FY, et al. Macrophage inhibitory factor 1 acts as a potential biomarker in patients with esophageal squamous cell carcinoma and is a target for antibody-based therapy. Cancer Sci. (2014) 105:176–85. doi: 10.1111/cas.12331
142. Wu L, Gao Y, Xie S, Ye W, Uemura Y, Zhang R, et al. The level of macrophage migration inhibitory factor is negatively correlated with the efficacy of PD-1 blockade immunotherapy combined with chemotherapy as a neoadjuvant therapy for esophageal squamous cell carcinoma. Trans Oncol. (2023) 37:101775. doi: 10.1016/j.tranon.2023.101775
143. Ren Y, Law S, Huang X, Lee PY, Bacher M, Srivastava G, et al. Macrophage migration inhibitory factor stimulates angiogenic factor expression and correlates with differentiation and lymph node status in patients with esophageal squamous cell carcinoma. Ann Surg. (2005) 242:55–63. doi: 10.1097/01.sla.0000168555.97710.bb
144. Beswick EJ, Pinchuk IV, Suarez G, Sierra JC, and Reyes VE. Helicobacter pylori CagA-dependent macrophage migration inhibitory factor produced by gastric epithelial cells binds to CD74 and stimulates procarcinogenic events. J Immunol. (2006) 176:6794–801. doi: 10.4049/jimmunol.176.11.6794
145. Jia L, Chen J, Xie C, Shao L, Xu Z, and Zhang L. microRNA-1228 impairs the pro-angiogenic activity of gastric cancer cells by targeting macrophage migration inhibitory factor. Life Sci. (2017) 180:9–16. doi: 10.1016/j.lfs.2017.04.023
146. Kong F, Deng X, Kong X, Du Y, Li L, Zhu H, et al. Correction: ZFPM2-AS1, a novel lncRNA, attenuates the p53 pathway and promotes gastric carcinogenesis by stabilizing MIF. Oncogene. (2018) 37:6010–. doi: 10.1038/s41388-018-0412-z
147. Shun C-T, Lin J-T, Huang S-P, Lin M-T, and Wu M-S. Expression of macrophage migration inhibitory factor is associated with enhanced angiogenesis and advanced stage in gastric carcinomas. World J gastroenterology: WJG. (2005) 11:3767. doi: 10.3748/wjg.v11.i24.3767
148. Zheng Y-X, Yang M, Rong T-T, Yuan X-L, Ma Y-H, Wang Z-H, et al. CD74 and macrophage migration inhibitory factor as therapeutic targets in gastric cancer. World J gastroenterology: WJG. (2012) 18:2253. doi: 10.3748/wjg.v18.i18.2253
149. Xia HHX, Yang Y, Chu KM, Gu Q, Zhang YY, He H, et al. Serum macrophage migration-inhibitory factor as a diagnostic and prognostic biomarker for gastric cancer. Cancer. (2009) 115:5441–9. doi: 10.1002/cncr.24609
150. He L-J, Xie D, Hu P-J, Liao Y-J, Deng H-X, Kung H-F, et al. Macrophage migration inhibitory factor as a potential prognostic factor in gastric cancer. World J Gastroenterology: WJG. (2015) 21:9916. doi: 10.3748/wjg.v21.i34.9916
151. Liao Y, Wu C, Li Y, Wen J, and Zhao D. MIF is a critical regulator of mononuclear phagocytic infiltration in hepatocellular carcinoma. Iscience. (2023) 26:107273. doi: 10.1016/j.isci.2023.107273
152. Wirtz TH, Saal A, Bergmann I, Fischer P, Heinrichs D, Brandt EF, et al. Macrophage migration inhibitory factor exerts pro-proliferative and anti-apoptotic effects via CD74 in murine hepatocellular carcinoma. Br J Pharmacol. (2021) 178:4452–67. doi: 10.1111/bph.15622
153. Qin L, Qin J, Lv X, Yin C, Qe Z, and Zhang J. MIF promoter polymorphism increases peripheral blood expression levels, contributing to increased susceptibility and poor prognosis in hepatocellular carcinoma. Oncol Letters. (2021) 22:1–9. doi: 10.3892/ol.2021.12810
154. Zhu G-Q, Tang Z, Huang R, Qu W-F, Fang Y, Yang R, et al. CD36+ cancer-associated fibroblasts provide immunosuppressive microenvironment for hepatocellular carcinoma via secretion of macrophage migration inhibitory factor. Cell Discov. (2023) 9:25. doi: 10.1038/s41421-023-00529-z
155. Zhao YM, Wang L, Dai Z, Wang DD, Hei ZY, Zhang N, et al. Validity of plasma macrophage migration inhibitory factor for diagnosis and prognosis of hepatocellular carcinoma. Int J Cancer. (2011) 129:2463–72. doi: 10.1002/ijc.25918
156. Zhao J, Li H, Zhao S, Wang E, Zhu J, Feng D, et al. Epigenetic silencing of miR-144/451a cluster contributes to HCC progression via paracrine HGF/MIF-mediated TAM remodeling. Mol Cancer. (2021) 20:1–16. doi: 10.1186/s12943-021-01343-5
157. Yuan T, Tang C, Chen M, Deng S, and Chen P. Influence of the human MIF promoter polymorphism on hepatocellular carcinoma prognosis. Genet Mol Res. (2013) 12:6629–35. doi: 10.4238/2013.January.4.3
158. Wang K, Liang Q, Wei L, Zhang W, and Zhu P. MicroRNA-608 acts as a prognostic marker and inhibits the cell proliferation in hepatocellular carcinoma by macrophage migration inhibitory factor. Tumor Biol. (2016) 37:3823–30. doi: 10.1007/s13277-015-4213-5
159. Wang D, Wang R, Huang A, Fang Z, Wang K, He M, et al. Upregulation of macrophage migration inhibitory factor promotes tumor metastasis and correlates with poor prognosis of pancreatic ductal adenocarcinoma. Oncol Rep. (2018) 40:2628–36. doi: 10.3892/or.2018.6703
160. Jia X, Xi J, Tian B, Zhang Y, Wang Z, Wang F, et al. The tautomerase activity of tumor exosomal MIF promotes pancreatic cancer progression by modulating MDSC differentiation. Cancer Immunol Res. (2024) 12:72–90. doi: 10.1158/2326-6066.CIR-23-0205
161. Ahmed A, Koehler S, Klotz R, Giese N, Lasitschka F, Hackert T, et al. Peripheral blood and tissue assessment highlights differential tumor-circulatory gradients of IL2 and MIF with prognostic significance in resecta ble pancreatic ductal adenocarcinoma. OncoImmunology. (2021) 10:1962135. doi: 10.1080/2162402X.2021.1962135
162. Cheng B, Wang Q, Song Y, Liu Y, Liu Y, Yang S, et al. MIF inhibitor, ISO-1, attenuates human pancreatic cancer cell proliferation, migration and invasion in vitro, and suppresses xenograft tumour growth in vivo. Sci Rep. (2020) 10:6741. doi: 10.1038/s41598-020-63778-y
163. Denz A, Pilarsky C, Muth D, Rückert F, Saeger H-D, and Grützmann R. Inhibition of MIF leads to cell cycle arrest and apoptosis in pancreatic cancer cells. J Surg Res. (2010) 160:29–34. doi: 10.1016/j.jss.2009.03.048
164. Guo D, Guo J, Yao J, Jiang K, Hu J, Wang B, et al. D-dopachrome tautomerase is over-expressed in pancreatic ductal adenocarcinoma and acts cooperatively with macrophage migration inhibitory factor to promote cancer growth. Int J Cancer. (2016) 139:2056–67. doi: 10.1002/ijc.30278
165. Sun H, Cheng R, Zhang D, Guo Y, Li F, Li Y, et al. MIF promotes cell invasion by the LRP1-uPAR interaction in pancreatic cancer cells. Front Oncol. (2023) 12:1028070. doi: 10.3389/fonc.2022.1028070
166. Cui M, Zhao Y, Zhang Z, Zhao Y, Han S, Wang R, et al. FGF-9, ANG-2 and AgRP collection were identified for the diagnosis of colorectal cancer based on the support vector machine model. Cell Cycle. (2021) 20:781–91. doi: 10.1080/15384101.2021.1903208
167. Cheon SK, Kim HP, Park YL, Jang JE, Lim Y, Song SH, et al. Macrophage migration inhibitory factor promotes resistance to MEK blockade in KRAS mutant colorectal cancer cells. Mol Oncol. (2018) 12:1398–409. doi: 10.1002/1878-0261.12345
168. Gordon-Weeks A, Lim S, Yuzhalin A, Jones K, and Muschel R. Macrophage migration inhibitory factor: a key cytokine and therapeutic target in colon cancer. Cytokine Growth factor Rev. (2015) 26:451–61. doi: 10.1016/j.cytogfr.2015.03.002
169. Hu S, Feng J, Fu W, and Guo Y. Macrophage migration inhibitory factor (MIF) upregulates CXCR7 and contributes to chemotherapy resistance in colorectal cancer. Cell Biochem Biophysics. (2024) 82:3437–52. doi: 10.1007/s12013-024-01430-6
170. Lee H, Rhee H, Kang HJ, Kim H-S, Min BS, Kim NK, et al. Macrophage migration inhibitory factor may be used as an early diagnostic marker in colorectal carcinomas. Am J Clin Pathol. (2008) 129:772–9. doi: 10.1309/GFCLLRH8A68XKMJN
171. Ramireddy L, Chen WTL, Peng CT, Hu RM, Ke TW, Chiang HC, et al. Association between genetic polymorphism of the MIF gene and colorectal cancer in Taiwan. J Clin Lab Analysis. (2015) 29:268–74. doi: 10.1002/jcla.21763
172. Du W, Wright B, Li X, Finke J, Rini B, Zhou M, et al. Tumor-derived macrophage migration inhibitory factor promotes an autocrine loop that enhances renal cell carcinoma. Oncogene. (2013) 32:1469–74. doi: 10.1038/onc.2012.143
173. Parol-Kulczyk M, Durślewicz J, Blonkowska L, Wujec R, Gzil A, Piątkowska D, et al. Macrophage migration inhibitory factor (MIF) predicts survival in patients with clear cell renal cell carcinoma. J Pathology: Clin Res. (2024) 10:e12365. doi: 10.1002/2056-4538.12365
174. Pasupuleti V, Du W, Gupta Y, Yeh I-J, Montano M, Magi-Galuzzi C, et al. Dysregulated D-dopachrome tautomerase, a hypoxia-inducible factor-dependent gene, cooperates with macrophage migration inhibitory factor in renal tumorigenesis. J Biol Chem. (2014) 289:3713–23. doi: 10.1074/jbc.M113.500694
175. Tang Y, Wan W, Wang L, Ji S, and Zhang J. microRNA-451 inhibited cell proliferation, migration and invasion through regulation of MIF in renal cell carcinoma. Int J Clin Exp Pathol. (2015) 8:15611.
176. Meyer-Siegler KL, Leifheit EC, and Vera PL. Inhibition of macrophage migration inhibitory factor decreases proliferation and cytokine expression in bladder cancer cells. BMC cancer. (2004) 4:1–12. doi: 10.1186/1471-2407-4-34
177. Omar HO, Moeen AM, Ahmed Mohareb DA, and Abdelhamed EM. Evaluation of macrophage migration inhibitory factor in bladder cancer. J Curr Med Res Practice. (2022) 7:203–7. doi: 10.1093/noajnl/vdae142
178. Rajendran G, Woolbright BL, Abbott E, Martin A, Dennis K, and Taylor JA. MIF-2 in bladder cancer: potential therapeutic target. Cancer Res. (2020) 80:5150–. doi: 10.1158/1538-7445.AM2020-5150
179. Taylor JA, Kuchel GA, Hegde P, Voznesensky OS, Claffey K, Tsimikas J, et al. Null mutation for macrophage migration inhibitory factor (MIF) is associated with less aggressive bladder cancer in mice. BMC cancer. (2007) 7:1–8. doi: 10.1186/1471-2407-7-135
180. Cotzomi-Ortega I, Rosas-Cruz A, Ramírez-Ramírez D, Reyes-Leyva J, Rodriguez-Sosa M, Aguilar-Alonso P, et al. Autophagy inhibition induces the secretion of macrophage migration inhibitory factor (MIF) with autocrine and paracrine effects on the promotion of Malignancy in breast cancer. Biology. (2020) 9:20. doi: 10.3390/biology9010020
181. Cotzomi-Ortega I, Nieto-Yanez O, Juarez-Avelar I, Rojas-Sanchez G, Montes-Alvarado JB, Reyes-Leyva J, et al. Autophagy inhibition in breast cancer cells induces ROS-mediated MIF expression and M1 macrophage polarization. Cell signalling. (2021) 86:110075. doi: 10.1016/j.cellsig.2021.110075
182. Wang Y, Chen Y, Wang C, Yang M, Wang Y, Bao L, et al. MIF is a 3’ flap nuclease that facilitates DNA replication and promotes tumor growth. Nat Commun. (2021) 12:2954. doi: 10.1038/s41467-021-23264-z
183. Chen M, Liu H, Hong B, Xiao Y, and Qian Y. MIF as a potential diagnostic and prognostic biomarker for triple-negative breast cancer that correlates with the polarization of M2 macrophages. FASEB J. (2024) 38:e23696. doi: 10.1096/fj.202400578R
184. Nagarajan P, Tober KL, Riggenbach JA, Kusewitt DF, Lehman AM, Sielecki T, et al. MIF antagonist (CPSI-1306) protects against UVB-induced squamous cell carcinoma. Mol Cancer Res. (2014) 12:1292–302. doi: 10.1158/1541-7786.MCR-14-0255-T
185. Zhang M, Yan L, and Kim J. Modulating mammary tumor growth, metastasis and immunosuppression by siRNA-induced MIF reduction in tumor microenvironment. Cancer Gene Ther. (2015) 22:463–74. doi: 10.1038/cgt.2015.42
186. Mawhinney L, Armstrong ME, O’Reilly C, Bucala R, Leng L, Fingerle-Rowson G, et al. Macrophage migration inhibitory factor (MIF) enzymatic activity and lung cancer. Mol Med. (2014) 20:729–35. doi: 10.2119/molmed.2014.00136
187. McClelland M, Zhao L, Carskadon S, and Arenberg D. Expression of CD74, the receptor for macrophage migration inhibitory factor, in non-small cell lung cancer. Am J Pathol. (2009) 174:638–46. doi: 10.2353/ajpath.2009.080463
188. Gámez-Pozo A, Sánchez-Navarro I, Calvo E, Agulló-Ortuño MT, López-Vacas R, Díaz E, et al. PTRF/cavin-1 and MIF proteins are identified as non-small cell lung cancer biomarkers by label-free proteomics. PloS One. (2012) 7:e33752. doi: 10.1371/journal.pone.0033752
189. Florez-Sampedro L, Soto-Gamez A, Poelarends GJ, and Melgert BN. The role of MIF in chronic lung diseases: looking beyond inflammation. . Am J Physiology-Lung Cell Mol Physiol. (2020) 318:L1183–L97. doi: 10.1152/ajplung.00521.2019
190. Rupp A, Bahlmann S, Trimpop N, von Pawel J, and Holdenrieder S. Lack of clinical utility of serum macrophage migration inhibitory factor (MIF) for monitoring therapy response and estimating prognosis in advanced lung cancer. Tumor Biol. (2024) 46:S341–S53. doi: 10.3233/TUB-230006
191. Mahalingam D, Patel MR, Sachdev JC, Hart LL, Halama N, Ramanathan RK, et al. Phase I study of imalumab (BAX69), a fully human recombinant antioxidized macrophage migration inhibitory factor antibody in advanced solid tumours. Br J Clin Pharmacol. (2020) 86:1836–48. doi: 10.1111/bcp.14289
192. Imaoka M, Tanese K, Masugi Y, Hayashi M, and Sakamoto M. Macrophage migration inhibitory factor-CD 74 interaction regulates the expression of programmed cell death ligand 1 in melanoma cells. Cancer Sci. (2019) 110:2273–83. doi: 10.1111/cas.14038
193. Culp WD, Tsagozis P, Burgio M, Russell P, Pisa P, and Garland D. Interference of macrophage migration inhibitory factor expression in a mouse melanoma inhibits tumor establishment by up-regulating thrombospondin-1. Mol Cancer Res. (2007) ;5:1225–31. doi: 10.1158/1541-7786.MCR-07-0229
194. de Azevedo RA, Shoshan E, Whang S, Markel G, Jaiswal AR, Liu A, et al. MIF inhibition as a strategy for overcoming resistance to immune checkpoint blockade therapy in melanoma. Oncoimmunology. (2020) 9:1846915. doi: 10.1080/2162402X.2020.1846915
195. Ekmekcioglu S, Davies MA, Tanese K, Roszik J, Shin-Sim M, Bassett RL Jr., et al. Inflammatory marker testing identifies CD74 expression in melanoma tumor cells, and its expression associates with favorable survival for stage III melanoma. Clin Cancer Res. (2016) 22:3016–24. doi: 10.1158/1078-0432.CCR-15-2226
196. Fukuda Y, Bustos MA, Cho S-N, Roszik J, Ryu S, Lopez VM, et al. Interplay between soluble CD74 and macrophage-migration inhibitory factor drives tumor growth and influences patient survival in melanoma. Cell Death disease. (2022) 13:117. doi: 10.1038/s41419-022-04552-y
197. Valdez CN, Sánchez-Zuno GA, Osmani L, Ibrahim W, Galan A, Bacchiocchi A, et al. Prognostic and therapeutic insights into MIF, DDT, and CD74 in melanoma. Oncotarget. (2024) 15:507. doi: 10.18632/oncotarget.28615
198. Oliveira CS, de Bock CE, Molloy TJ, Sadeqzadeh E, Geng XY, Hersey P, et al. Macrophage migration inhibitory factor engages PI3K/Akt signalling and is a prognostic factor in metastatic melanoma. BMC cancer. (2014) 14:630. doi: 10.1186/1471-2407-14-630
199. Presti M, Mazzon E, Basile MS, Petralia MC, Bramanti A, Colletti G, et al. Overexpression of macrophage migration inhibitory factor and functionally−related genes, D−DT, CD74, CD44, CXCR2 and CXCR4, in glioblastoma. Oncol Letters. (2018) 16:2881–6. doi: 10.3892/ol.2018.8990
200. Mittelbronn M, Platten M, Zeiner P, Dombrowski Y, Frank B, Zachskorn C, et al. Macrophage migration inhibitory factor (MIF) expression in human Malignant gliomas contributes to immune escape and tumour progression. Acta neuropathologica. (2011) 122:353–65. doi: 10.1007/s00401-011-0858-3
201. Sanftner L, Gibbons J, Gross M, Suzuki B, Gaeta F, and Johnson K. Cross-species comparisons of the pharmacokinetics of ibudilast. Xenobiotica. (2009) 39:964–77. doi: 10.3109/00498250903254340
202. Alban TJ, Bayik D, Otvos B, Rabljenovic A, Leng L, Jia-Shiun L, et al. Glioblastoma myeloid-derived suppressor cell subsets express differential macrophage migration inhibitory factor receptor profiles that can be targeted to reduce immune suppression. Front Immunol. (2020) 11:1191. doi: 10.3389/fimmu.2020.01191
203. Lee SH, Kwon HJ, Park S, Kim CI, Ryu H, Kim SS, et al. Macrophage migration inhibitory factor (MIF) inhibitor 4-IPP downregulates stemness phenotype and mesenchymal trans-differentiation after irradiation in glioblastoma multiforme. PloS One. (2021) 16:e0257375. doi: 10.1371/journal.pone.0257375
204. Ren Y, Chan H, Fan J, Xie Y, Chen Y, Li W, et al. Inhibition of tumor growth and metastasis in vitro and in vivo by targeting macrophage migration inhibitory factor in human neuroblastoma. Oncogene. (2006) 25:3501–8. doi: 10.1038/sj.onc.1209395
205. Liu G, Xu Z, and Hao D. MicroRNA-451 inhibits neuroblastoma proliferation, invasion and migration by targeting macrophage migration inhibitory factor. Mol Med Rep. (2016) 13:2253–60. doi: 10.3892/mmr.2016.4770
206. Garcia-Gerique L, García M, Garrido-Garcia A, Gómez-González S, Torrebadell M, Prada E, et al. MIF/CXCR4 signaling axis contributes to survival, invasion, and drug resistance of metastatic neuroblastoma cells in the bone marrow microenvironment. BMC cancer. (2022) 22:669. doi: 10.1186/s12885-022-09725-8
207. Agarwal R, Whang DH, Alvero AB, Visintin I, Lai Y, Segal EA, et al. Macrophage migration inhibitory factor expression in ovarian cancer. Am J obstetrics gynecology. (2007) 196:348. e1–.e5. doi: 10.1016/j.ajog.2006.12.030
208. Tas F, Karabulut S, Serilmez M, Ciftci R, and Duranyildiz D. Serum levels of macrophage migration-inhibitory factor (MIF) have diagnostic, predictive and prognostic roles in epithelial ovarian cancer patients. Tumor Biol. (2014) 35:3327–31. doi: 10.1007/s13277-013-1438-z
209. Mor G, Visintin I, Lai Y, Zhao H, Schwartz P, Rutherford T, et al. Serum protein markers for early detection of ovarian cancer. Proc Natl Acad Sci. (2005) 102:7677–82. doi: 10.1073/pnas.0502178102
210. Bax HJ, Josephs DH, Pellizzari G, Spicer JF, Montes A, and Karagiannis SN. Therapeutic targets and new directions for antibodies developed for ovarian cancer. mAbs (2016) 8:1437–55. doi: 10.1080/19420862.2016.1219005
211. Meyer-Siegler KL, Iczkowski KA, and Vera PL. Further evidence for increased macrophage migration inhibitory factor expression in prostate cancer. BMC cancer. (2005) 5:1–12. doi: 10.1186/1471-2407-5-73
212. Meyer-Siegler KL, Bellino MA, and Tannenbaum M. Macrophage migration inhibitory factor evaluation compared with prostate specific antigen as a biomarker in patients with prostate carcinoma. Cancer. (2002) 94:1449–56. doi: 10.1002/cncr.10354
213. Rafiei S, Gui B, Wu J, Liu XS, Kibel AS, and Jia L. Targeting the MIF/CXCR7/AKT signaling pathway in castration-resistant prostate cancer. Mol Cancer Res. (2019) 17:263–76. doi: 10.1158/1541-7786.MCR-18-0412
214. Meyer-Siegler K, Vera P, Iczkowski K, Bifulco C, Lee A, Gregersen P, et al. Macrophage migration inhibitory factor (MIF) gene polymorphisms are associated with increased prostate cancer incidence. Genes Immunity. (2007) 8:646–52. doi: 10.1038/sj.gene.6364427
215. Simpson KD, Templeton DJ, and Cross JV. Macrophage migration inhibitory factor promotes tumor growth and metastasis by inducing myeloid-derived suppressor cells in the tumor microenvironment. J Immunol. (2012) 189:5533–40. doi: 10.4049/jimmunol.1201161
216. Meyer-Siegler KL, Iczkowski KA, Leng L, Bucala R, and Vera PL. Inhibition of macrophage migration inhibitory factor or its receptor (CD74) attenuates growth and invasion of DU-145 prostate cancer cells. J Immunol. (2006) 177:8730–9. doi: 10.4049/jimmunol.177.12.8730
217. Krockenberger M, Engel JB, Kolb J, Dombrowsky Y, Häusler SF, Kohrenhagen N, et al. Macrophage migration inhibitory factor expression in cervical cancer. J Cancer Res Clin Oncol. (2010) 136:651–7. doi: 10.1007/s00432-009-0702-5
218. Xiao D, Dai B, Chen J, Luo Q, Liu X, Lin Q, et al. Loss of macrophage migration inhibitory factor impairs the growth properties of human HeLa cervical cancer cells. Cell Proliferation. (2011) 44:582–90. doi: 10.1111/j.1365-2184.2011.00787.x
219. Guo P, Wang J, Liu J, Xia M, Li W, and He M. Macrophage immigration inhibitory factor promotes cell proliferation and inhibits apoptosis of cervical adenocarcinoma. Tumor Biol. (2015) 36:5095–102. doi: 10.1007/s13277-015-3161-4
220. Balakrishnan CK, Tye GJ, Balasubramaniam SD, and Kaur G. CD74 and HLA-DRA in cervical carcinogenesis: potential targets for antitumour therapy. Medicina. (2022) 58:190. doi: 10.3390/medicina58020190
221. Wu S, Sun J, Lian J, Shang H, Tao H, Xie J, et al. Macrophage migration inhibitory factor promoter polymorphisms (– 794CATT5–7) as potential biomarker for early-stage cervical cancer. J Obstetrics Gynaecology Res. (2017) 43:571–9. doi: 10.1111/jog.13233
222. Xiao W, Dong X, Zhao H, Han S, Nie R, Zhang X, et al. Expression of MIF and c-erbB-2 in endometrial cancer. Mol Med Rep. (2016) 13:3828–34. doi: 10.3892/mmr.2016.4992
223. Cui Z, Zhou L, An X, Liu W, Li J, Zhang Y, et al. The combination of circEPSTI1 and MIF offers diagnostic value for endometrial cancer. I. nternational J Gen Med. (2024) 17:1395–403. doi: 10.2147/IJGM.S441861
224. Fuzi AAM, Omar SZ, Mohamed Z, Adenan NAM, and Mokhtar NM. High throughput silencing identifies novel genes in endometrioid endometrial cancer. Taiwanese J Obstetrics Gynecology. (2018) 57:217–26. doi: 10.1016/j.tjog.2018.02.009
225. Veillat VR, Carli CD, Metz CN, Al-Abed Y, Naccache PH, and Akoum A. Macrophage migration inhibitory factor elicits an angiogenic phenotype in human ectopic endometrial cells and triggers the production of major angiogenic factors via CD44, CD74, and MAPK signaling pathways. J Clin Endocrinol Metab. (2010) 95:E403–E12. doi: 10.1210/jc.2010-0417
226. Kapoor R, Stratopoulou CA, and Dolmans M-M. Pathogenesis of endometriosis: New insights into prospective therapies. Int J Mol Sci. (2021) 22:11700. doi: 10.3390/ijms222111700
227. Sedky NK, Braoudaki M, Mahdy NK, Amin K, Fawzy IM, Efthimiadou EK, et al. Box-Behnken design of thermo-responsive nano-liposomes loaded with a platinum(iv) anticancer complex: evaluation of cytotoxicity and apoptotic pathways in triple negative breast cancer cells. Nanoscale Adv. (2023) 5:5399–413. doi: 10.1039/D3NA00368J
228. ZeinElAbdeen YA, AbdAlSeed A, and Youness RA. Decoding insulin-like growth factor signaling pathway from a non-coding RNAs perspective: A step towards precision oncology in breast cancer. J Mammary Gland Biol Neoplasia. (2022) 27:79–99. doi: 10.1007/s10911-022-09511-z
229. El Din GS, Youness RA, Assal RA, and Gad MZ. miRNA-506-3p directly regulates rs10754339 (A/G) in the immune checkpoint protein B7-H4 in breast cancer. Microrna. (2020) 9:346–53. doi: 10.2174/2211536609666201209152949
230. Soliman RA, Youness RA, Manie TM, Khallaf E, El-Shazly M, Abdelmohsen M, et al. Uncoupling tumor necrosis factor-α and interleukin-10 at tumor immune microenvironment of breast cancer through miR-17-5p/MALAT-1/H19 circuit. BIOCELL. (2022) 46:769–83. doi: 10.32604/biocell.2022.016636
231. Bando H, Matsumoto G, Bando M, Muta M, Ogawa T, Funata N, et al. Expression of macrophage migration inhibitory factor in human breast cancer: association with nodal spread. Jpn J Cancer Res. (2002) 93:389–96. doi: 10.1111/j.1349-7006.2002.tb01269.x
232. Charan M, Das S, Mishra S, Chatterjee N, Varikuti S, Kaul K, et al. Macrophage migration inhibitory factor inhibition as a novel therapeutic approach against triple-negative breast cancer. Cell Death Dis. (2020) 11:774. doi: 10.1038/s41419-020-02992-y
233. Yan L, Wu M, Wang T, Yuan H, Zhang X, Zhang H, et al. Breast cancer stem cells secrete MIF to mediate tumor metabolic reprogramming that drives immune evasion. Cancer Res. (2024) 84:1270–85. doi: 10.1158/0008-5472.CAN-23-2390
234. Mawhinney L, Armstrong ME, O' Reilly C, Bucala R, Leng L, Fingerle-Rowson G, et al. Macrophage migration inhibitory factor (MIF) enzymatic activity and lung cancer. Mol Med. (2015) 20:729–35. doi: 10.2119/molmed.2014.00136
235. Yaddanapudi K, Putty K, Rendon BE, Lamont GJ, Faughn JD, Satoskar A, et al. Control of tumor-associated macrophage alternative activation by macrophage migration inhibitory factor. J Immunol. (2013) 190:2984–93. doi: 10.4049/jimmunol.1201650
236. Shimizu T, Abe R, Nakamura H, Ohkawara A, Suzuki M, and Nishihira J. High expression of macrophage migration inhibitory factor in human melanoma cells and its role in tumor cell growth and angiogenesis. Biochem Biophys Res Commun. (1999) 264:751–8. doi: 10.1006/bbrc.1999.1584
237. O’Reilly C, Doroudian M, Mawhinney L, and Donnelly SC. Targeting MIF in cancer: therapeutic strategies, current developments, and future opportunities. Med Res Rev. (2016) 36:440–60. doi: 10.1002/med.21385
238. Winner M, Meier J, Zierow S, Rendon BE, Crichlow GV, Riggs R, et al. A novel, macrophage migration inhibitory factor suicide substrate inhibits motility and growth of lung cancer cells. Cancer Res. (2008) 68:7253–7. doi: 10.1158/0008-5472.CAN-07-6227
239. Doghish AS, Mahmoud A, Abd-Elmawla MA, Zaki MB, Aborehab NM, Hatawsh A, et al. Innovative perspectives on glioblastoma: the emerging role of long non-coding RNAs. Funct Integr Genomics. (2025) 25:43. doi: 10.1007/s10142-025-01557-6
240. Fahmy SA, Dawoud A, Zeinelabdeen YA, Kiriacos CJ, Daniel KA, Eltahtawy O, et al. Molecular engines, therapeutic targets, and challenges in pediatric brain tumors: A special emphasis on hydrogen sulfide and RNA-based nano-delivery. Cancers. (2022) 14:5244. doi: 10.3390/cancers14215244
241. Presti M, Mazzon E, Basile MS, Petralia MC, Bramanti A, Colletti G, et al. Overexpression of macrophage migration inhibitory factor and functionally-related genes, D-DT, CD74, CD44, CXCR2 and CXCR4, in glioblastoma. Oncol Lett. (2018) 16:2881–6. doi: 10.3892/ol.2018.8990
242. Sanftner LM, Gibbons JA, Gross MI, Suzuki BM, Gaeta FC, and Johnson KW. Cross-species comparisons of the pharmacokinetics of ibudilast. Xenobiotica. (2009) 39:964–77. doi: 10.3109/00498250903254340
243. Liu G, Xu Z, and Hao D. MicroRNA−451 inhibits neuroblastoma proliferation, invasion and migration by targeting macrophage migration inhibitory factor. Mol Med Rep. (2016) 13:2253–60. doi: 10.3892/mmr.2016.4770
244. Bax HJ, Josephs DH, Pellizzari G, Spicer JF, Montes A, and Karagiannis SN. Therapeutic targets and new directions for antibodies developed for ovarian cancer. MAbs. (2016) 8:1437–55. doi: 10.1080/19420862.2016.1219005
245. Cheng RJ, Deng WG, Niu CB, Li YY, and Fu Y. Expression of macrophage migration inhibitory factor and CD74 in cervical squamous cell carcinoma. Int J Gynecol Cancer. (2011) 21:1004–12. doi: 10.1097/IGC.0b013e31821c45b7
246. Hagemann T, Robinson SC, Thompson RG, Charles K, Kulbe H, and Balkwill FR. Ovarian cancer cell-derived migration inhibitory factor enhances tumor growth, progression, and angiogenesis. Mol Cancer Ther. (2007) 6:1993–2002. doi: 10.1158/1535-7163.MCT-07-0118
247. Xiao W, Dong X, Zhao H, Han S, Nie R, Zhang X, et al. Expression of MIF and c-erbB-2 in endometrial cancer. Mol Med Rep. (2016) 13:3828–34. doi: 10.3892/mmr.2016.4992
248. Cui Z, Zhou L, An X, Liu W, Li J, Zhang Y, et al. The combination of circEPSTI1 and MIF offers diagnostic value for endometrial cancer. Int J Gen Med. (2024) 17:1395–403. doi: 10.2147/IJGM.S441861
249. Kyama CM, Debrock S, Mwenda JM, and D’Hooghe TM. Potential involvement of the immune system in the development of endometriosis. Reprod Biol Endocrinol. (2003) 1:123. doi: 10.1186/1477-7827-1-123
250. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
251. Tasoulas J, Farquhar DR, Sheth S, Hackman T, Yarbrough WG, Agala CB, et al. Poor oral health influences head and neck cancer patient survival: an International Head and Neck Cancer Epidemiology Consortium pooled analysis. JNCI: J Natl Cancer Institute. (2024) 116:105–14. doi: 10.1093/jnci/djad156
252. Lechien J, Kindt N, Chantrain G, Preillon J, Laurent G, and Saussez S. MIF in head and neck cancer: a new therapeutic target? Rev Laryngologie-Otologie-Rhinologie. (2013) 134:67–74.
253. Kindt N, Preillon J, Kaltner H, Gabius H-J, Chevalier D, Rodriguez A, et al. Macrophage migration inhibitory factor in head and neck squamous cell carcinoma: clinical and experimental studies. J Cancer Res Clin Oncol. (2013) 139:727–37. doi: 10.1007/s00432-013-1375-7
254. Lo M-C, Yip T-C, Ngan K-C, Cheng W-W, Law C-K, Chan P-S, et al. Role of MIF/CXCL8/CXCR2 signaling in the growth of nasopharyngeal carcinoma tumor spheres. Cancer letters. (2013) 335:81–92. doi: 10.1016/j.canlet.2013.01.052
255. Klobučar M, Sedić M, Gehrig P, Grossmann J, Bilić M, Kovač-Bilić L, et al. Basement membrane protein ladinin-1 and the MIF-CD44-β1 integrin signaling axis are implicated in laryngeal cancer metastasis. Biochim Biophys Acta (BBA)-Molecular Basis Disease. (2016) 1862:1938–54. doi: 10.1016/j.bbadis.2016.07.014
256. Pei XJ, Wu TT, Bin L, Tian XY, Zhi L, and Yang QX. Increased expression of macrophage migration inhibitory factor and DJ-1 contribute to cell invasion and metastasis of nasopharyngeal carcinoma. Int J Med Sci. (2014) 11:106. doi: 10.7150/ijms.7264
257. Chang K-P, Lin S-J, Liu S-C, Yi J-S, Chien K-Y, Chi L-M, et al. Low-molecular-mass secretome profiling identifies HMGA2 and MIF as prognostic biomarkers for oral cavity squamous cell carcinoma. Sci Rep. (2015) 5:11689. doi: 10.1038/srep11689
258. Lagergren J, Smyth E, Cunningham D, and Lagergren P. Oesophageal cancer. Lancet. (2017) 390:2383–96. doi: 10.1016/S0140-6736(17)31462-9
259. Liu CQ, Ma YL, Qin Q, Wang PH, Luo Y, Xu PF, et al. Epidemiology of esophageal cancer in 2020 and projections to 2030 and 2040. Thorac cancer. (2023) 14:3–11. doi: 10.1111/1759-7714.14745
260. Iwu CD and Iwu-Jaja CJ. Gastric cancer epidemiology: Current trend and future direction. Hygiene. (2023) 3:256–68. doi: 10.3390/hygiene3030019
261. Yu H, Yu X, and Zhang S. Expression and significance of macrophage migration inhibitory factor in gastric adenocarcinoma. Zhonghua yi xue za zhi. (2010) 90:2625–8.
262. Mak L-Y, Liu K, Chirapongsathorn S, Yew KC, Tamaki N, Rajaram RB, et al. Liver diseases and hepatocellular carcinoma in the Asia-Pacific region: burden, trends, challenges and future directions. Nat Rev Gastroenterol hepatology. (2024) 21:834–51. doi: 10.1038/s41575-024-00967-4
263. Roshandel G, Ghasemi-Kebria F, and Malekzadeh R. Colorectal cancer: epidemiology, risk factors, and prevention. Cancers. (2024) 16:1530. doi: 10.3390/cancers16081530
264. Tyndall J, Putha L, Kok LK, Fellner M, Rutledge MT, Gamble AB, et al. Covalent isothiocyanate inhibitors of macrophage migration inhibitory factor as potential colorectal cancer treatments. ChemMedChem. (2024) 19:e202400394. doi: 10.1002/cmdc.202400394
265. Assal RA, Rashwan HH, Zakaria ZI, Sweillam JH, Fouda YM, Abdelhamid AM, et al. Deciphering the mysteries of MEG3 LncRNA and its implications in genitourinary cancers. Front Oncol. (2025) 15:1519103. doi: 10.3389/fonc.2025.1519103
266. Zhang H, Ye Y, Li M, Ye S, Huang W, Cai T, et al. CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer. Oncogene. (2017) 36:2095–104. doi: 10.1038/onc.2016.367
267. Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol. (2013) 14:e218–28. doi: 10.1016/S1470-2045(12)70582-X
268. Koh HM and Kim DC. Prognostic significance of macrophage migration inhibitory factor expression in cancer patients: A systematic review and meta-analysis. Med (Baltimore). (2020) 99:e21575. doi: 10.1097/MD.0000000000021575
269. Olsson L, Lindmark G, Hammarström ML, Hammarström S, and Sitohy B. Evaluating macrophage migration inhibitory factor 1 expression as a prognostic biomarker in colon cancer. Tumour Biol. (2020) 42:1010428320924524. doi: 10.1177/1010428320924524
270. Mamoori A, Gopalan V, Lu CT, Chua TC, Morris DL, Smith RA, et al. Expression pattern of miR-451 and its target MIF (macrophage migration inhibitory factor) in colorectal cancer. J Clin Pathol. (2017) 70:308–12. doi: 10.1136/jclinpath-2016-203972
271. Fox RJ, Coffey CS, Conwit R, Cudkowicz ME, Gleason T, Goodman A, et al. Phase 2 trial of ibudilast in progressive multiple sclerosis. N Engl J Med. (2018) 379:846–55. doi: 10.1056/NEJMoa1803583
272. Bilsborrow JB, Doherty E, Tilstam PV, and Bucala R. Macrophage migration inhibitory factor (MIF) as a therapeutic target for rheumatoid arthritis and systemic lupus erythematosus. Expert Opin Ther Targets. (2019) 23:733–44. doi: 10.1080/14728222.2019.1656718
273. Leng L, Chen L, Fan J, Greven D, Arjona A, Du X, et al. A small-molecule macrophage migration inhibitory factor antagonist protects against glomerulonephritis in lupus-prone NZB/NZW F1 and MRL/lpr mice. J Immunol. (2011) 186:527–38. doi: 10.4049/jimmunol.1001767
274. Hussain F, Freissmuth M, Völkel D, Thiele M, Douillard P, Antoine G, et al. Human anti-macrophage migration inhibitory factor antibodies inhibit growth of human prostate cancer cells in vitro and in vivo. Mol Cancer Ther. (2013) 12:1223–34. doi: 10.1158/1535-7163.MCT-12-0988
275. Pantouris G, Syed MA, Fan C, Rajasekaran D, Cho TY, Rosenberg EM Jr., et al. An analysis of MIF structural features that control functional activation of CD74. Chem Biol. (2015) 22:1197–205. doi: 10.1016/j.chembiol.2015.08.006
276. Fingerle-Rowson G, Kaleswarapu DR, Schlander C, Kabgani N, Brocks T, Reinart N, et al. A tautomerase-null macrophage migration-inhibitory factor (MIF) gene knock-in mouse model reveals that protein interactions and not enzymatic activity mediate MIF-dependent growth regulation. Mol Cell Biol. (2009) 29:1922–32. doi: 10.1128/MCB.01907-08
277. Cho Y, Crichlow GV, Vermeire JJ, Leng L, Du X, Hodsdon ME, et al. Allosteric inhibition of macrophage migration inhibitory factor revealed by ibudilast. Proc Natl Acad Sci U S A. (2010) 107:11313–8. doi: 10.1073/pnas.1002716107
278. Wallace DJ, Figueras F, Wegener WA, and Goldenberg DM. Experience with milatuzumab, an anti-CD74 antibody against immunomodulatory macrophage migration inhibitory factor (MIF) receptor, for systemic lupus erythematosus (SLE). Ann Rheum Dis. (2021) 80:954–5. doi: 10.1136/annrheumdis-2020-219803
279. Kerschbaumer RJ, Rieger M, Völkel D, Le Roy D, Roger T, Garbaraviciene J, et al. Neutralization of macrophage migration inhibitory factor (MIF) by fully human antibodies correlates with their specificity for the β-sheet structure of MIF. J Biol Chem. (2012) 287:7446–55. doi: 10.1074/jbc.M111.329664
280. Zhu M, Li N, Fan L, Wu R, Cao L, Ren Y, et al. Single-cell transcriptomic and spatial analysis reveal the immunosuppressive microenvironment in relapsed/refractory angioimmunoblastic T-cell lymphoma. Blood Cancer J. (2024) 14:218. doi: 10.1038/s41408-024-01199-0
281. Wang Q, Zhao D, Xian M, Wang Z, Bi E, Su P, et al. MIF as a biomarker and therapeutic target for overcoming resistance to proteasome inhibitors in human myeloma. Blood. (2020) 136:2557–73. doi: 10.1182/blood.2020005795
282. Verjans E, Noetzel E, Bektas N, Schütz AK, Lue H, Lennartz B, et al. Dual role of macrophage migration inhibitory factor (MIF) in human breast cancer. BMC Cancer. (2009) 9:230. doi: 10.1186/1471-2407-9-230
283. Elemam NM, Mekky RY, Rashid G, Braoudaki M, and Youness RA. Pharmacogenomic and epigenomic approaches to untangle the enigma of IL-10 blockade in oncology. Expert Rev Mol Med. (2024) 26:e1. doi: 10.1017/erm.2023.26
284. Savaskan NE, Fingerle-Rowson G, Buchfelder M, and Eyüpoglu IY. Brain miffed by macrophage migration inhibitory factor. Int J Cell Biol. (2012) 2012:139573. doi: 10.1155/2012/139573
Keywords: macrophage migration inhibitory factor (MIF), immune regulation, solid tumors, inflammatory mediator, tumor microenvironment
Citation: Youness RA, Elemam NM, Abdelhamid AM, Mohamed AH, Elsherbiny LM, Ramzy A and Assal RA (2025) Macrophage migration inhibitory factor (MIF) and the tumor ecosystem: a tale of inflammation, immune escape, and tumor growth. Front. Immunol. 16:1636839. doi: 10.3389/fimmu.2025.1636839
Received: 28 May 2025; Accepted: 19 September 2025;
Published: 13 October 2025.
Edited by:
Gabriela Schneider, University of Louisville, United StatesCopyright © 2025 Youness, Elemam, Abdelhamid, Mohamed, Elsherbiny, Ramzy and Assal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rana A. Youness, cmFuYS55b3VuZXNzMjFAZ21haWwuY29t; Reem A. Assal, cmVlbS5hc3NhbEBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Rana A. Youness
Rana A. Youness Noha M. Elemam
Noha M. Elemam Abdelhamid M. Abdelhamid
Abdelhamid M. Abdelhamid Adham H. Mohamed
Adham H. Mohamed Lolowa M. Elsherbiny1
Lolowa M. Elsherbiny1 Reem A. Assal
Reem A. Assal