- 1Department of Pulmonary Medicine, Sanjay Gandhi Post Graduate Institute of Medical Sciences (SGPGIMS), Lucknow, India
- 2Department of Respiratory Medicine, King George’s Medical University, Lucknow, India
Introduction
Macrophages constitute a heterogenous population of innate immunity cells that exhibit dynamic plasticity and maintain tissue homeostasis. A dichotomous classification of macrophages exists, i.e., the classically activated proinflammatory M1 subtype and alternatively activated reparative M2 cells that are modulated by the tissue microenvironment, however, it is being realised that a continuum of phenotypes exists based on different stimulation factors, receptor profile and cytokine expression and a certain subtype dominates at a certain stage in the disease process based on signal from the surrounding tissue (1, 2). These phenotypes play an important role in pathogenesis of autoimmune diseases that are characterised by dysregulated innate immunity, activation of T-lymphocytes, autoantibody formation and development of interstitial lung disease due to ongoing abnormal inflammation as well as uncontrolled stimulation of fibrotic pathways. Targeted therapy directed at these pathways can be a supplicating strategy to avoid and limit pulmonary fibrosis in autoimmune disorders (3, 4).
Macrophage origin, function and homeostasis
Macrophages are either yolk sac/fetal liver in origin or derived from bone marrow monocyte lineage (4). Most tissue-resident are embryonic in origin while brain microglia, dermal, intestinal macrophages and those recruited in inflammation arise from blood monocytes. Pulmonary alveolar and interstitial macrophages originate from yolk sac, though the latter can also be recruited (5–12).
Human alveolar macrophages are identified by the presence of sialoadhesin CD169, scavenger MARCO and interstitial macrophages by CD36, CX3CR1; both share the antigens HLA-DR, CD11b, CD11c, CD 14low, CD16+ and mannose receptor CD206. In resting conditions, the renewal of cells is dependent on CSFR-1, MCSF and IL-34 (13).
Macrophages exhibit TLRs, C-type lectin, dectin and NOD-like receptors on their surface (14). Alveolar macrophages are chiefly concerned with phagocytosis while interstitial macrophages are primarily involved in tissue homeostasis and immune regulation. Endogenous or exogenous signals such as apoptotic cells or microorganisms/irritants lead to recruitment of bone marrow derived monocytes to the lungs (13). Upon recognition of such triggers by surface receptors, macrophages engulf the particles incorporating it into phagolysome under influence of PI3K/Akt and mTOR pathways respectively, leading to its digestion (15).
Similarly, the dysfunctional cytoplasmic organelles are cleared through autophagy. Efferocytosis is accomplished through scavenger receptors and MERTK (13–16). This effective clearance of damaged and apoptotic cells is necessary to avoid exposure of autoantigens and thus development of autoimmunity. Concurrently there is release inflammatory cytokines (TNF-α, IL-1β, -6, -12, -18, -23) and chemokines (CXCL-1, CXCL-2) that recruit inflammatory cells and upregulate MHC-I expression on surrounding tissue cells thereby promoting autoantigens presentation to T-cells. They also exhibit CD86 and MHC II molecules to present antigens to T-lymphocytes thereby linking innate and acquired immunity. The macrophage produced degrade extracellular matrix releasing sequestered vascular endothelial growth factor promoting angiogenesis further amplifying inflammation (17, 18) (Figure 1).
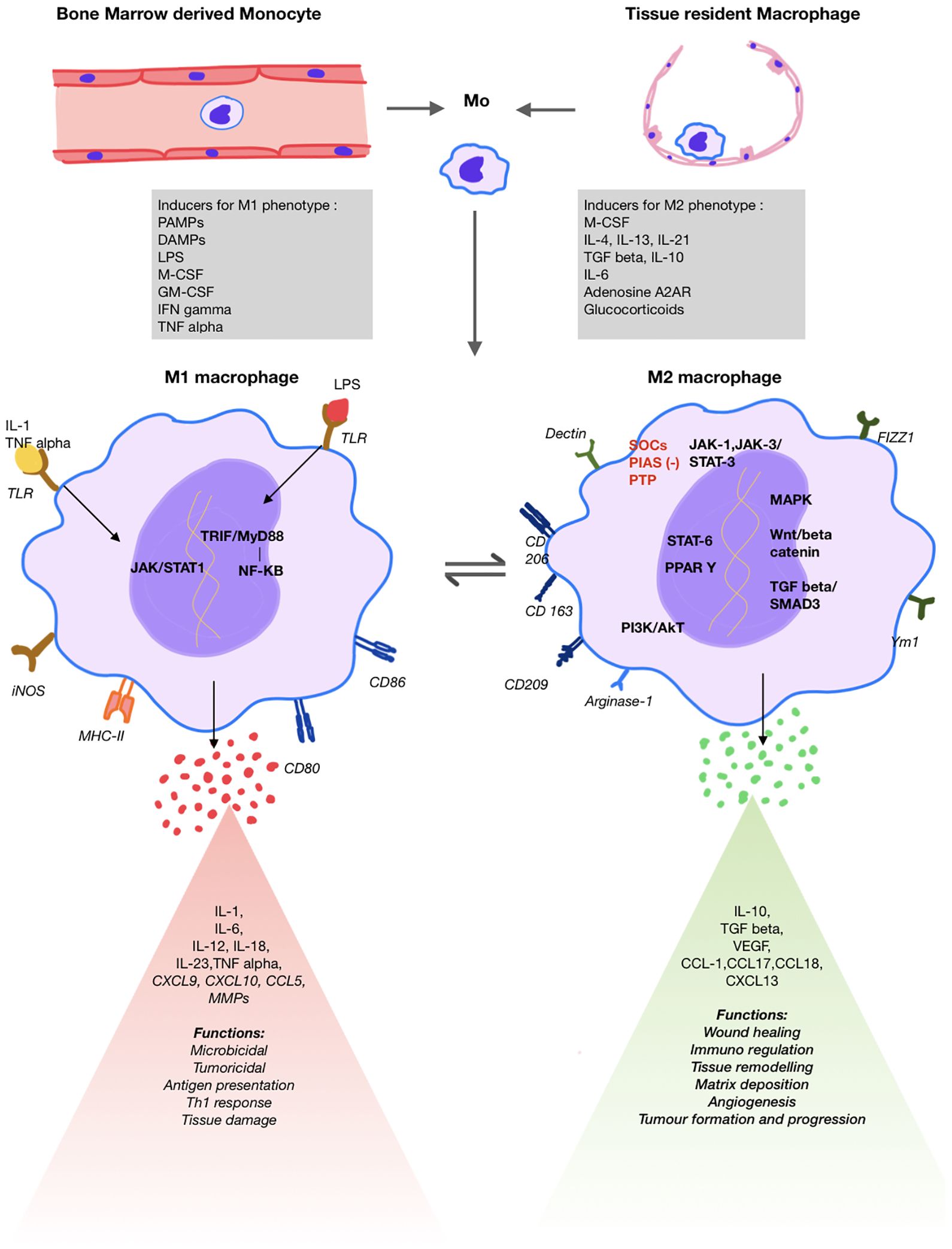
Figure 1. Schematic diagram showing macrophage origin, differentiation, key surface markers, signalling pathways, products released and respective functions of M1 and M2 macrophages.
With ongoing inflammation, there is activation of the profibrotic M2 phenotype to accomplish tissue healing and restore homeostasis (1, 2, 19). These cells produce anti-inflammatory cytokines such as IL-10 and TGF-β. The inflammatory cytokines IL-6 and IL-23 along with TGF-β promote TH17 differentiation of T-cells that play a crucial role in the pathogenesis autoimmune inflammation (20–22). Production of TGF-β is associated with increased fibroblast differentiation and fibrosis that plays an important role in development of ILD (23) (Figure 1).
Macrophages also carry out key metabolic roles. M1 macrophages convert arginine to nitric oxide via iNOS, promoting inflammation, while M2 macrophages turn arginine into proline and polyamines, aiding tissue repair (24). They also respond differently to hypoxia: M1 cells favor glycolysis, causing succinate accumulation, fatty acid synthesis, and inflammation through HIF-1α/IL-1β activation; M2 cells rely on oxidative phosphorylation, enhancing PDL-1 expression and TREG differentiation while reducing IL-1β (24, 25). M2 macrophages also perform fatty acid oxidation, relevant in lipid-driven diseases like lupus, rheumatoid arthritis, and psoriasis.
Additionally, macrophages regulate iron metabolism by phagocytosing senescent RBCs. M1 cells retain iron promoting bacteriostasis, whereas M2 cells release it to support proliferation and matrix remodeling. High glutathione promotes M2 polarization, while low glutathione favors M1 activation for parasite defense (26–28).
Pathways in macrophage activation and polarisation
Macrophage phenotypes have different gene expression, receptor and chemokine profile serving as markers for early identification. The M1 macrophages are activated by GM-CSF, Th-1 cytokines such as TNF-α, IFN-γ, IL-1, bacterial lipopolysaccharide, PAMPs and DAMPS. This triggers JAK/STAT1, nuclear factor kappa-β and IRF pathways resulting in expression of iNOS, MHC-II and SOCs-1 and proinflammatory cytokines such as IL-1,6,12, TNF-α and chemokines (3, 13, 18, 29) (Figure 1).
The anti-inflammatory M2 phenotype can be studied in four subgroups (2a, 2b, 2c, 2d) as studied in vitro. M2a cells play an important role in lung fibrosis. Triggered by Th2 cytokines (IL-4, IL-13) and M-CSF, they express innate scavenger receptor CD206, CD163, proteins like TGF-β, FIZZ1, arginase1 and chitinase-3 promoting fibroblast activation and CCL18 promoting collagen deposition. Insulin like growth factor-1 is also released that prevents myofibroblast apoptosis. M2b, stimulated by TLR and IL1R ligands, performs immunoregulatory function by producing high level of anti-inflammatory cytokine IL-10; along with proinflammatory cytokines such as IL-1β, IL-6, TNF-α, and low IL-12—making it the only M2 subtype to produce both anti- and pro-inflammatory cytokines. M2c, induced by TGF-β, IL-10 and glucocorticoids, is responsible for efferocytosis. M2d subtype is triggered by TLR, adenosine A2AR ligands and IL-6 and induces angiogenesis by promoting VEGF production (18, 30).
The M2 subtypes gene expression is controlled by the JAK1/JAK3 signalling pathways via STAT-3 activation which induces expression of anti-inflammatory genes. The STAT-3 pathway also cross talks with other key pathways including NF-kB, PI3K/Akt, Notch, Hedgehog, Wnt signalling and MAPK pathway. This pathway has negative regulators such as SOCs, PIAS and PTPs that are being explored for therapeutic use (30–32). Additionally, hydrogen peroxide generation by Cu/Zn superoxide dismutase leads to STAT-6 activation that triggers PPAR γ and δ transcription that promote fatty acid oxidation, mitochondrial biosynthesis and arginase-1 transcription respectively, hence, sustaining M2 phenotype (33, 34). PI3K/Akt, specific subtypes of interferon regulatory factors (IRFs) also modulate polarisation (35–37). Additionally, under hypoxia, HIF-1α promotes the production of profibrotic factors by upregulation of adenosine A2B receptor on M2 macrophages (3, 38).
Epigenetic regulation of polarisation also occurs as histone acetylation and methylation promote expression of fibrotic genes like IL1RA, MMP9, SPP1, CHI3L1, MARCK5 and PLA2G7 (39, 40).
There is increased recognition that a clear M1 and M2 differentiation does not exist in vivo rather there is a continuum of phenotypes. Single cell RNA sequence study of the healthy and fibrotic lung revealed several stages including monocytes (CD14+CD206neg/loCD68neg/lo), intermittent transitional macrophages (CD14+ CD206neg/loCD68mid/hi) and alveolar macrophages (CD14neg/lo CD206mid/hiCD68mid/hi). PDGF-AA+ transitional and SPPhi monocyte derived macrophages have been identified in fibrotic lungs. PDGF-AA promotes fibroblast proliferation and migration while osteopontin (SPP) promotes ECM deposition (41–43).
Macrophages in autoimmune ILDs
Autoimmune disorders are characterized by formation of antibodies against self-antigens and dysregulated innate immunity. Although the exact mechanisms have not been elucidated in pathogenesis of autoimmune ILDs, macrophages being the chief cells of innate immunity, are involved in exaggerated inflammatory response, defective efferocytosis, presenting self antigens, and releasing profibrotic cytokines and chemokines.
Blood transcriptomic studies of fibrotic ILD patients have revealed overexpression of CD14+ monocytes that predict disease severity as well as mortality. The lineage of alveolar macrophages (MoAMs) is crucial as evidence shows their deletion markedly reduces the severity of bleomycin induced lung fibrosis in mice models while deletion of tissue resident macrophage has no such impact (44, 45).
General pathogenesis of autoimmune disorders is depicted in Figure 2. Key pathways of CTDs with high ILD prevalence are specified here.
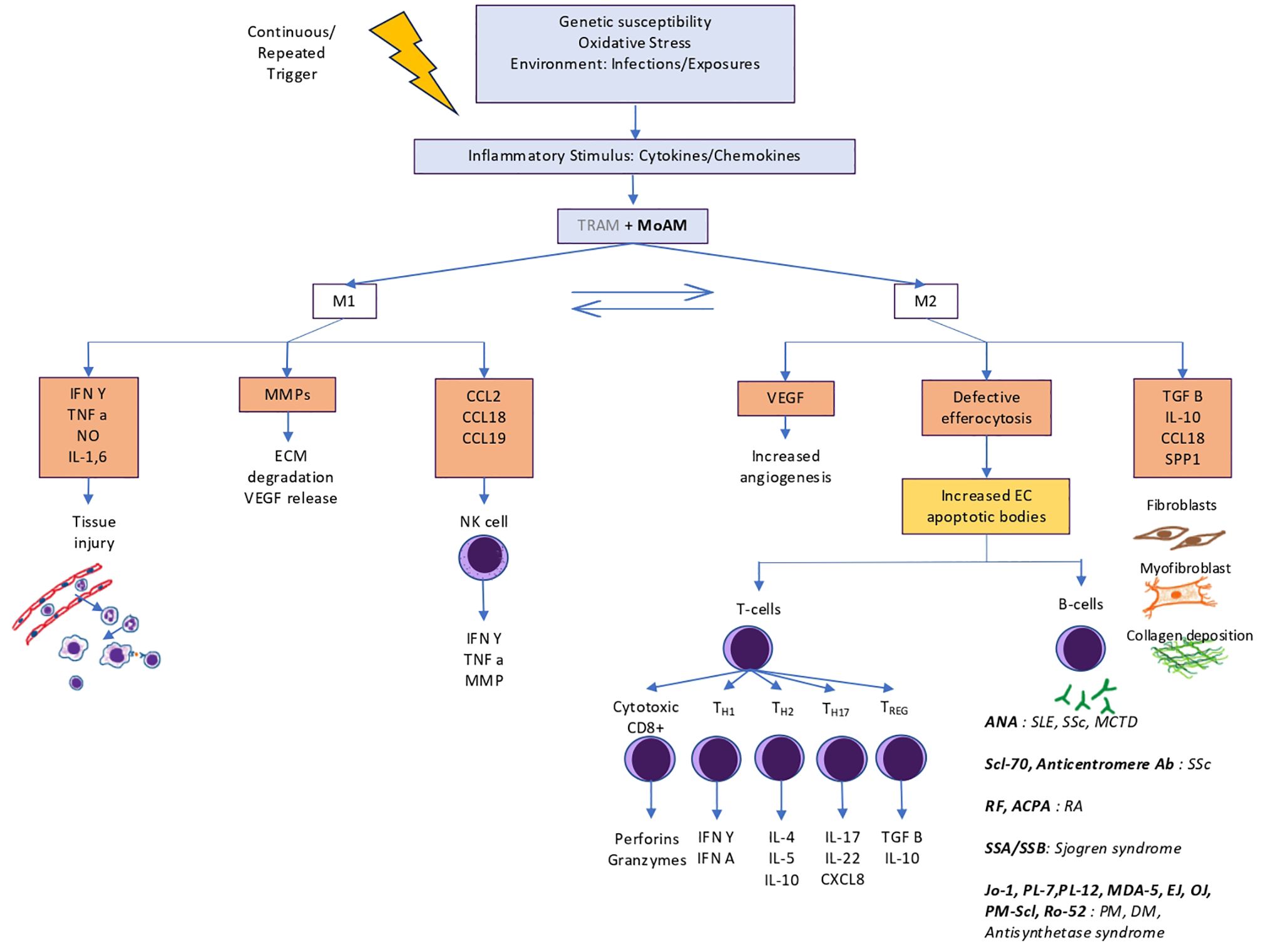
Figure 2. Role of macrophages in pathogenesis of autoimmune interstitial lung diseases. TRAMs play a limited role in initial inflammation and are mostly overtaken by MoAMs that convert into profibrotic macrophages. Failed efferocytosis leads to development of autoantibodies against intranuclear and antra cytoplasmic antigens that further perpetuates inflammation and dysregulated fibrosis.
The prevalence of ILD in rheumatoid arthritis patients is about 11% (46). In patients with RA-ILD, high peripheral monocyte count is associated with increased mortality (47, 48). The gene expression of these cells shows increased M1 polarisation in transcriptomics studies. There is upregulation of Dectin-1, IL-27, SOCs, IRF7 and JAK/STAT pathways. Epigenetically, glycotransferase guanylate binding protein 5 is overexpressed that promotes IFN-γ induced M1 typing (49–58). The enzyme peptidylarginine deiminases which mediates citrullination is regulated by PI3K/Akt signalling. Interestingly, this pathway is inhibited by syndecan-2 (SDC-2) an M2-associated CD148 ligand with antifibrotic effect. Also, in alveolar epithelium SDC-2 promotes caveolin mediated TGF-β receptor 1 degradation thereby preventing TGF-β mediated apoptosis (59–61).
ILD can be seen in roughly half the patients with systemic sclerosis (46). RNA sequencing in lung fibrosis mice models have demonstrated markedly increased M2 population (62). IL-4,13 and 10 promote M2 polarisation via STAT3 pathway activation. Additionally, CpG-binding domain 2 and MMP28 are significantly upregulated in SSc that promote PI3K/Akt signalling for M2 differentiation. Redox enzymes such as Sart-1 and Cu/Zn-SOD promote M2 phenotype by enhancing STAT-6 signalling (63–67).
Inflammatory myopathies have a high prevalence of ILDs ranging between 50% to 90% (68). This group is notorious for rapidly progressive ILDs and thus require urgent measures to combat inflammation and fibrosis (46, 69). Soluble CD206, a marker for M2 macrophages is highly elevated in patients of dermatomyositis ILD (69). Additionally, there is increased expression of IFN-γ and TNF- α suggestive of increased M1 polarisation early on in the disease (70–72). Ergo, there is possible involvement of different subtypes at different stages of the disease.
Potential targets
The following aspects of macrophage physiology may be targeted to prevent pulmonary fibrosis in autoimmune disorders.
i. Inflammatory cytokines: Methyl palmitate is a promising drug that inhibits macrophage activation, reduces TNF-α level and reduces fibrosis (73, 74). Anti-IL-6 Tocilizumab has been demonstrated to preserve lung function in patients with systemic sclerosis (75). IL-27 inhibitors are currently are undergoing trial for cancer immunotherapy and may be utilised for ILD (50).
ii. Recruitment: Nintedanib is an existing triple kinase inhibitor that has inhibitory action on CSF-1R as well and blocks M2 polarisation (76).
iii. Signalling pathways: Tacrolimus, Ruxolitinib inhibit the JAK/STAT pathways supressing the profibrotic M2 pathway (63, 65, 75). JAK inhibitor Tofacitinib has demonstrated positive results in IIM-ILD (77). Akt-1 pathway promotes ROS and TGF-β. Its deletion is associated with apoptosis of alveolar macrophages and prevention of lung fibrosis. Clevudine is a purine analog that can block Akt signalling (78). Syndecan is another potential agent inhibiting Akt pathway with demonstrated antifibrotic effect in human lung homogenates (59, 60).
iv. Fibroblast activation: Traditional Chinese medicine like Schisandra chinensis and Resveratrol have anti-TGF-β activity and hold excellent potential (79, 80). Pirfenidone has anti-TGF-β1 action and prevents M2 polarisation (81). PRI-724 reduces TGF-β gene expression by inhibiting B catenin signalling thus inhibition collagen deposition (82). Niclosamide, an antiparasitic drug shows promise in inhibiting Wnt/β catenin and TGF-β pathways (83). Another potential target for inhibition is S100A4, a M2 macrophage produced calcium binding protein involved in fibroblast proliferation (84). Fresolimumab, anti-TGF antibody has been beneficial in SSc (85). Microcystin-leucine arginine is another agent that prevents epithelial to mesenchymal transition (86). Imatinib loaded gold particles have been shown to inhibit macrophage and fibroblast activation (87).
v. Macrophage apoptosis: BCL-2 inhibitors promote apoptosis and have demonstrated resolution of fibrosis in mice (88).
Discussion
The first step in the development of lung fibrosis in most autoimmune diseases is damage of alveolar epithelium and inflammation followed by continuous TGF-β signalling resulting in epithelial to mesenchymal transformation (87). Macrophages are involved in pathogenesis right from excessive monocyte recruitment, MHC-I upregulation, T-lymphocytes activation, TH17 differentiation, impaired autophagy and apoptosis to activation of profibrotic pathways. These are highly plastic cells with potential to switch from one phenotype to another at different developmental stage of the disease process depending on the stimulus. Exaggerated response at any stage can contribute to chronic disease. The bivalent macrophage classification does not justify their heterogenous genetic and functional spectrum. With advent of scRNA sequencing transcriptomics, macrophages have been genetically characterised in fibrotic ILDs, their lineage traced to blood monocytes and prognostic markers identified. More importantly, the high risk genes have been identified from peripheral blood monocytes also. Similarly, genetic studies in autoimmune diseases can help identify the high risk biomarkers and better understanding of pathogenesis.
Several immunomodulatory and antifibrotic drugs are under study. Rather than upstream targeting like CSFR-1 that poses risks of widespread immune dysregulation, specific signalling pathways may be halted. The signalling pathways have complex interactions and different isoforms of molecules perform different actions thus inhibition of complimenting pathways simultaneously may yield better results in terms of fibrosis containment (63, 65, 75, 76). JAK/STAT kinase inhibitors not only prevent pulmonary fibrosis but also fibrosis in other organs expanding benefits (83). Anti-TGF-β agents as well as negative regulators of profibrogenic pathways are already under study (79–83). Targeting autophagy is a promising area for drug development to regulate the tissue microenvironment (89). Concomitant impact of metabolic and oxidative state needs to be studied in further detail to develop therapy directed at lipid mediators such as prostaglandins (24–28, 38).
Our knowledge has only recently grown in understanding the diverse landscape of macrophages. As MoAMs are the culprit cells responsible for promoting lung fibrosis, genetic/epigenetic modulation holds promise for precise targeting while sparing the homeostatic function of the resident macrophages. Novel agents targeting profibrotic macrophage are still in experimental stage. Application of scRNA sequencing to lung lavage/tissue/blood samples of different autoimmune disorders holds great promise for improving the understanding, prevention and better management of lung fibrosis.
Author contributions
RT: Writing – original draft, Conceptualization. SK: Supervision, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CD, Cluster of Differentiation; CSF, Colony Stimulating Factor; DAMP, Damage Associated Molecular Patterns; DM, Dermatomyositis; EC, Extra Cellular; FIZZ, Found in Inflammatory Zone; HIF, Hypoxia Inducible Factor; HLA, Human Leukocyte Antigen; ILs, Interleukins; iNOS, inducible Nitric Oxide Synthase; IRFs, Interferon Regulatory Factors; JAK/STAT, Janus Kinase-Signal Transducer and Activator of Transcription; MCSF, Macrophage Colony Stimulating Factor; MCTD, Mixed Connective Tissue Disease; MARCO, Macrophage Receptor with Collagenous structure; MERTK, MER proto-oncogene tyrosine kinase; MHC, Major Histocompatibitlity Complex; MMPs, Matrix Metalloproteinases; mTOR, Mechanistic Target of Rapamycin; MyD88, Myeloid Differentiation response 88; NOD, Nucleotide-binding and Oligomerization Domain; PAMP, Pathogen Associated Molecular Patterns; PDL, Programmed Death Ligand; PI3K/Akt, Phosphoinositide 3-kinase/protein kinase B; PIAS, Protein inhibtors of activated STATS; PM, Polymyositis; PPAR, Peroxisome Proliferator-Activated Receptor; PTPs, Protein tyrosine phosphatases; RA-ILD, Rheumatoid Arthritis Interstitial Lung Disease; Sart-1, splicoeosome associated factor-1; SOD, Superoxide Dismutase; SLE, Systemic Lupus Erythematosis; SSc, Systemic Sclerosis; TGF, Transforming Growth Factor; TLRs, Toll like receptors; TNF, Tumor Necrosis Factor; TRAM, Tissue Resident Alveolar Macrophages; TRIF, TIR-domain-containing adapter-inducing interferon-β; TREG, T-regulatory cell; VEGF, Vascular Endothelial Growth Factor.
References
1. Martinez FO and Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. (2014) 6:13. doi: 10.12703/P6-13
2. Kesapragada M, Sun YH, Zlobina K, Recendez C, Fregoso D, Yang HY, et al. Deep learning classification for macrophage subtypes through cell migratory pattern analysis. Front Cell Dev Biol. (2024) 12:1259037. doi: 10.3389/fcell.2024.1259037
3. Chen S, Saeed AFUH, Liu Q, Jiang Q, Xu H, Xiao GG, et al. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. (2023) 8:207. doi: 10.1038/s41392-023-01452-1
4. Ma WT, Gao F, Gu K, and Chen DK. The role of monocytes and macrophages in autoimmune diseases: A comprehensive review. Front Immunol. (2019) 10:1140. doi: 10.3389/fimmu.2019.01140
5. Ginhoux F and Guilliams M. Tissue-resident macrophage ontogeny and homeostasis. Immunity. (2016) 44:439–49. doi: 10.1016/j.immuni.2016.02.024
6. Gordon S and Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. (2005) 5:953–64. doi: 10.1038/nri1733
7. Den Haan JM and Kraal G. Innate immune functions of macrophage subpopulations in the spleen. J Innate Immun. (2012) 4:437–45. doi: 10.1159/000335216
8. Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, et al. Adult langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. (2012) 209:1167–81. doi: 10.1084/jem.20120340
9. Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. (2010) 330:841–5. doi: 10.1126/science.1194637
10. Sheng J, Ruedl C, and Karjalainen K. Most tissue-resident macrophages except microglia are derived from fetal hematopoietic stem cells. Immunity. (2015) 43:382–93. doi: 10.1016/j.immuni.2015.07.016
11. Bain CC, Bravo-Blas A, Scott CL, Perdiguero EG, Geissmann F, Henri S, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. (2014) 15:929–37. doi: 10.1038/ni.2967
12. Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C, et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. (2013) 39:925–38. doi: 10.1016/j.immuni.2013.10.004
13. Hou F, Xiao K, Tang L, and Xie L. Diversity of macrophages in lung homeostasis and diseases. Front Immunol. (2021) 12: 753940. doi: 10.3389/fimmu.2021.753940
14. Aderem A and Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. (1999) 17:593–623. doi: 10.1146/annurev.immunol.17.1.593
15. Larson-Casey JL, Deshane JS, Ryan AJ, Thannickal VJ, and Carter AB. Macrophage akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity. (2016) 44:582–96. doi: 10.1016/j.immuni.2016.01.001
16. Dikic I and Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. (2018) 19:349–64. doi: 10.1038/s41580-018-0003-4
17. Tseng CC, Sung YW, Chen KY, Wang PY, Yen CY, Sung WY, et al. The role of macrophages in connective tissue disease-associated interstitial lung disease: focusing on molecular mechanisms and potential treatment strategies. Int J Mol Sci. (2023) 24:11995. doi: 10.3390/ijms241511995
18. Arango Duque G and Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. (2014) 5:491. doi: 10.3389/fimmu.2014.00491
19. Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediat Inflamm. (2015) 2015:816460. doi: 10.1155/2015/816460
20. Huang G, Wang Y, and Chi H. Regulation of TH17 cell differentiation by innate immune signals. Cell Mol Immunol. (2012) 9:287–95. doi: 10.1038/cmi.2012.10
21. Zambrano-Zaragoza JF, Romo-Martínez EJ, Durán-Avelar Mde J, García-Magallanes N, and Vibanco-Pérez N. Th17 cells in autoimmune and infectious diseases. Int J Inflam. (2014) 2014:651503. doi: 10.1155/2014/651503
22. Yasuda K, Takeuchi Y, and Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol. (2019) 41:283–97. doi: 10.1007/s00281-019-00733-8
23. Biernacka A, Dobaczewski M, and Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors. (2011) 29:196–202. doi: 10.3109/08977194.2011.595714
24. Rath M, Müller I, Kropf P, Closs EI, and Munder M. Metabolism via arginase or nitric oxide synthase: Two competing arginine pathways in macrophages. Front Immunol. (2014) 5:532. doi: 10.3389/fimmu.2014.00532
25. Viola A, Munari F, Sánchez-Rodríguez R, Scolaro T, and Castegna A. The metabolic signature of macrophage responses. Front Immunol. (2019) 10:1462. doi: 10.3389/fimmu.2019.01462
26. Cairo G, Recalcati S, Mantovani A, and Locati M. Iron trafficking and metabolism in macrophages: contribution to the polarized phenotype. Trends Immunol. (2011) 32:241–7. doi: 10.1016/j.it.2011.03.007
27. Marshall ES, Elshekiha HM, Hakimi MA, and Flynn RJ. Toxoplasma gondii peroxiredoxin promotes altered macrophage function, caspase-1-dependent IL-1β secretion enhances parasite replication. Vet Res. (2011) 42:80. doi: 10.1186/1297-9716-42-80
28. Biswas SK and Mantovani A. Orchestration of metabolism by macrophages. Cell Metab. (2012) 15:432–7. doi: 10.1016/j.cmet.2011.11.013
29. Fitzgerald KA and Kagan JC. Toll-like receptors and the control of immunity. Cell. (2020) 180:1044–66. doi: 10.1016/j.cell.2020.02.041
30. Gordon S and Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. (2010) 32:593–604. doi: 10.1016/j.immuni.2010.05.007
31. Wu M, Song D, Li H, Yang Y, Ma X, Deng S, et al. Negative regulators of STAT3 signaling pathway in cancers. Cancer Manag Res. (2019) 11:4957–69. doi: 10.2147/CMAR.S206175
32. Jiang M, Zhang WW, Liu P, Yu W, Liu T, and Yu J. Dysregulation of SOCS-mediated negative feedback of cytokine signaling in carcinogenesis and its significance in cancer treatment. Front Immunol. (2017) 8:70. doi: 10.3389/fimmu.2017.00070
33. Chawla A. Control of macrophage activation and function by PPARs. Circ Res. (2010) 106:1559–69. doi: 10.1161/CIRCRESAHA.110.216523
34. Odegaard JI and Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol. (2011) 6:275–97. doi: 10.1146/annurev-pathol-011110-130138
35. Tarassishin L, Suh HS, and Lee SC. Interferon regulatory factor 3 plays an anti-inflammatory role in microglia by activating the PI3K/Akt pathway. J Neuroinflammation. (2011) 8:187. doi: 10.1186/1742-2094-8-187
36. Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, and Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol. (2017) 198:1006–14. doi: 10.4049/jimmunol.1601515
37. Günthner R and Anders HJ. Interferon-regulatory factors determine macrophage phenotype polarization. Mediators Inflamm. (2013) 2013:731023. doi: 10.1155/2013/731023
38. Liang J, Ran Y, Hu C, Zhou J, Ye L, Su W, et al. Inhibition of HIF-1α ameliorates pulmonary fibrosis by suppressing M2 macrophage polarization through PRMT1/STAT6 signals. Int Immunopharmacol. (2025) 146:113931. doi: 10.1016/j.intimp.2024.113931
39. Juan Guardela BM, Sun J, Zhang T, Xu B, Balnis J, Huang Y, et al. 50-gene risk profiles in peripheral blood predict COVID-19 outcomes: A retrospective, multicenter cohort study. EBioMedicine. (2021) 69:103439. doi: 10.1016/j.ebiom.2021.103439
40. Tourki B, Jia M, Karampitsakos T, Vera IM, Arsenault A, Fatima Z, et al. Convergent and divergent immune aberrations in COVID-19, post-COVID-19-interstitial lung disease, and idiopathic pulmonary fibrosis. Am J Physiol Cell Physiol. (2025) 328:C199–211. doi: 10.1152/ajpcell.00528.2024
41. Perrot CY, Karampitsakos T, and Herazo-Maya JD. Monocytes and macrophages: emerging mechanisms and novel therapeutic targets in pulmonary fibrosis. Am J Physiol Cell Physiol. (2023) 325:C1046–57. doi: 10.1152/ajpcell.00302.2023
42. Aran D, Looney AP, Liu L, Wu E, Fong V, Hsu A, et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat Immunol. (2019) 20:163–72. doi: 10.1038/s41590-018-0276-y
43. Adams TS, Schupp JC, Poli S, Ayaub EA, Neumark N, Ahangari F, et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv. (2020) 6:eaba1983. doi: 10.1126/sciadv.aba1983
44. Karampitsakos T, Tourki B, Jia M, Perrot CY, Visinescu B, Zhao A, et al. The transcriptome of CD14 + CD163 - HLA-DR low monocytes predicts mortality in Idiopathic Pulmonary Fibrosis. medRxiv. (2024). doi: 10.1101/2024.08.07.24311386
45. Karampitsakos T, Tourki B, and Herazo-Maya JD. The dawn of precision medicine in fibrotic interstitial lung disease. Chest. (2025) 167:1120–32. doi: 10.1016/j.chest.2024.10.042
46. Joy GM, Arbiv OA, Wong CK, Lok SD, Adderley NA, Dobosz KM, et al. Prevalence, imaging patterns and risk factors of interstitial lung disease in connective tissue disease: a systematic review and meta-analysis. Eur Respir Rev. (2023) 32:220210. doi: 10.1183/16000617.0210-2022
47. Olson AL, Swigris JJ, Sprunger DB, Fischer A, Fernandez-Perez ER, Solomon J, et al. Rheumatoid arthritis–interstitial lung disease–associated mortality. Am J Respir Crit Care Med. (2011) 183:372–8. doi: 10.1164/rccm.201004-0622OC
48. Saku A, Fujisawa T, Nishimoto K, Yoshimura K, Hozumi H, Karayama M, et al. Prognostic significance of peripheral blood monocyte and neutrophil counts in rheumatoid arthritis-associated interstitial lung disease. Respir Med. (2021) 182:106420. doi: 10.1016/j.rmed.2021.106420
49. Erkelens MN, Goverse G, Konijn T, Molenaar R, Beijer MR, Van den Bossche J, et al. Intestinal macrophages balance inflammatory expression profiles via vitamin A and dectin-1-mediated signaling. Front Immunol. (2020) 11:551. doi: 10.3389/fimmu.2020.00551
50. Dong S, Zhang X, He Y, Xu F, Li D, Xu W, et al. Synergy of IL-27 and TNF-α in regulating CXCL10 expression in lung fibroblasts. Am J Respir Cell Mol Biol. (2013) 48:518–30. doi: 10.1165/rcmb.2012-0340OC
51. Su Y, Yao H, Wang H, Xu F, Li D, Li D, et al. IL-27 enhances innate immunity of human pulmonary fibroblasts and epithelial cells through upregulation of TLR4 expression. Am J Physiol Lung Cell Mol Physiol. (2016) 310:L133–41. doi: 10.1152/ajplung.00307.2015
52. Yoshimura A, Suzuki M, Sakaguchi R, Hanada T, and Yasukawa H. SOCS. Inflammation, and autoimmunity. Front Immunol. (2012) 3:20. doi: 10.3389/fimmu.2012.00020
53. Villanueva-Martin G, Acosta-Herrera M, Carmona EG, Kerick M, Ortego-Centeno N, Callejas-Rubio JL, et al. Non-classical circulating monocytes expressing high levels of microsomal prostaglandin E2 synthase-1 tag an aberrant IFN-response in systemic sclerosis. J Autoimmun. (2023) 140:103097. doi: 10.1016/j.jaut.2023.103097
54. Baker MC, Liu Y, Lu R, Lin J, Melehani J, and Robinson WH. Incidence of interstitial lung disease in patients with rheumatoid arthritis treated with biologic and targeted synthetic disease-modifying antirheumatic drugs. JAMA Netw Open. (2023) 6:e233640. doi: 10.1001/jamanetworkopen.2023.3640
55. Tardella M, Di Carlo M, Carotti M, Ceccarelli L, Giovagnoni A, and Salaffi F. A retrospective study of the efficacy of JAK inhibitors or abatacept on rheumatoid arthritis-interstitial lung disease. Inflammopharmacology. (2022) 30:705–12. doi: 10.1007/s10787-022-00936-w
56. Poole JA, Schwab A, Thiele GM, England BR, Nelson AJ, Gleason A, et al. Unique transcriptomic profile of peripheral blood monocytes in rheumatoid arthritis-associated interstitial lung disease. Rheumatol (Oxford). (2024), keae572. doi: 10.1093/rheumatology/keae572
57. Fujiwara Y, Hizukuri Y, Yamashiro K, Makita N, Ohnishi K, Takeya M, et al. Guanylate-binding protein 5 is a marker of interferon-γ-induced classically activated macrophages. Clin Transl Immunol. (2016) 5:e111. doi: 10.1038/cti.2016.59
58. Weiss M, Anderluh M, and Gobec M. Inhibition of O-glcNAc transferase alters the differentiation and maturation process of human monocyte derived dendritic cells. Cells. (2021) 10:3312. doi: 10.3390/cells10123312
59. Shi Y, Gochuico BR, Yu G, Tang X, Osorio JC, Fernandez IE, et al. Syndecan-2 exerts antifibrotic effects by promoting caveolin-1-mediated transforming growth factor-β receptor I internalization and inhibiting transforming growth factor-β1 signaling. Am J Respir Crit Care Med. (2013) 188:831–41. doi: 10.1164/rccm.201303-0434OC
60. Tsoyi K, Chu SG, Patino-Jaramillo NG, Wilder J, Villalba J, Doyle-Eisele M, et al. Syndecan-2 attenuates radiation-induced pulmonary fibrosis and inhibits fibroblast activation by regulating PI3K/Akt/ROCK pathway via CD148. Am J Respir Cell Mol Biol. (2018) 58:208–15. doi: 10.1165/rcmb.2017-0088OC
61. Tsoyi K, Esposito AJ, Sun B, Bowen RG, Xiong K, Poli F, et al. Syndecan-2 regulates PAD2 to exert antifibrotic effects on RA-ILD fibroblasts. Sci Rep. (2022) 12:2847. doi: 10.1038/s41598-022-06678-7
62. Hu M, Yao Z, Xu L, Peng M, Deng G, Liu L, et al. M2 macrophage polarization in systemic sclerosis fibrosis: pathogenic mechanisms and therapeutic effects. Heliyon. (2023) 9:e16206. doi: 10.1016/j.heliyon.2023.e16206
63. Lescoat A, Lelong M, Jeljeli M, Piquet-Pellorce C, Morzadec C, Ballerie A, et al. Combined anti-fibrotic and anti-inflammatory properties of JAK-inhibitors on macrophages in vitro and in vivo: Perspectives for scleroderma-associated interstitial lung disease. Biochem Pharmacol. (2020) 178:114103. doi: 10.1016/j.bcp.2020.114103
64. Silva-Carmona M, Vogel TP, Marchal S, Guesmi M, Dubus JC, Leroy S, et al. Successful treatment of interstitial lung disease in STAT3 gain-of-function using JAK inhibitors. Am J Respir Crit Care Med. (2020) 202:893–7. doi: 10.1164/rccm.201906-1204LE
65. Atschekzei F, Traidl S, Carlens J, Schütz K, von Hardenberg S, Elsayed A, et al. JAK inhibitors to treat STAT3 gain-of-function: a single-center report and literature review. Front Immunol. (2024) 15:1400348. doi: 10.3389/fimmu.2024.1400348
66. Nagai M, Hasegawa M, Takehara K, and Sato S. Novel autoantibody to Cu/Zn superoxide dismutase in patients with localized scleroderma. J Invest Dermatol. (2004) 122:594–601. doi: 10.1111/j.0022-202X.2004.22333.x
67. Pan T, Zhou Q, Miao K, Zhang L, Wu G, Yu J, et al. Suppressing Sart1 to modulate macrophage polarization by siRNA-loaded liposomes: a promising therapeutic strategy for pulmonary fibrosis. Theranostics. (2021) 11:1192. doi: 10.7150/thno.48152
68. Szodoray P, Hajas A, Kardos L, Dezso B, Soos G, Zold E, et al. Distinct phenotypes in mixed connective tissue disease: subgroups and survival. Lupus. (2012) 21:1412–22. doi: 10.1177/0961203312456751
69. Horiike Y, Suzuki Y, Fujisawa T, Yasui H, Karayama M, Hozumi H, et al. Successful classification of macrophage-mannose receptor CD206 in severity of anti-MDA5 antibody positive dermatomyositis associated ILD. Rheumatol (Oxford). (2019) 58:2143–52. doi: 10.1093/rheumatology/kez185
70. Hervier B and Uzunhan Y. Inflammatory myopathy-related interstitial lung disease: from pathophysiology to treatment. Front Med (Lausanne). (2020) 6:326. doi: 10.3389/fmed.2019.00326
71. Zhang H, He F, Shi M, Wang W, Tian X, Kang J, et al. Toll-like receptor 4-Myeloid differentiation primary response gene 88 pathway is involved in the Inflammatory Development of Polymyositis by Mediating Interferon-γ and Interleukin-17A in humans and experimental autoimmune Myositis Mouse Model. Front Neurol. (2017) 8:132. doi: 10.3389/fneur.2017.00132
72. Torres-Ruiz J, Carrillo-Vazquez DA, Padilla-Ortiz DM, Vazquez-Rodriguez R, Nuñez-Alvarez C, Juarez-Vega G, et al. TLR expression in peripheral monocyte subsets of patients with idiopathic inflammatory myopathies: association with clinicaland immunological features. J Transl Med. (2020) 18:125. doi: 10.1186/s12967-020-02290-3
73. El-Demerdash E. Anti-inflammatory and antifibrotic effects of methyl palmitate. Toxicol Appl Pharmacol. (2011) 254:238–44. doi: 10.1016/j.taap.2011.04.016
74. Sharawy MH, El-Agamy DS, Shalaby AA, and Ammar e-S. Protective effects of methyl palmitate against silica-induced pulmonary fibrosis in rats. Int Immunopharmacol. (2013) 16:191–8. doi: 10.1016/j.intimp.2013.04.007
75. Khanna D, Lin CJF, Furst DE, Wagner B, Zucchetto M, Raghu G, et al. Long-term safety and efficacy of tocilizumab in early systemic sclerosis-interstitial lung disease: open-label extension of a phase 3 randomized controlled trial. Am J Respir Crit Care Med. (2022) 205:674–84. doi: 10.1164/rccm.202103-0714OC
76. Huang J, Maier C, Zhang Y, Soare A, Dees C, Beyer C, et al. Nintedanib inhibits macrophage activation and ameliorates vascular and fibrotic manifestations in the Fra2 mouse model of systemic sclerosis. Ann rheumatic diseases. (2017) 76:1941–8. doi: 10.1136/annrheumdis-2016-210823
77. Kurasawa K, Arai S, Namiki Y, Tanaka A, Takamura Y, Owada T, et al. Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatol (Oxford). (2018) 57:2114–9. doi: 10.1093/rheumatology/key188
78. Li S, Gao S, Jiang Q, Liang Q, Luan J, Zhang R, et al. Clevudine attenuates bleomycin-induced early pulmonary fibrosis via regulating M2 macrophage polarization. Int Immunopharmacol. (2021) 101:108271. doi: 10.1016/j.intimp.2021.108271
79. Guo Z, Li S, Zhang N, Kang Q, and Zhai H. Schisandra inhibit bleomycin-induced idiopathic pulmonary fibrosis in rats via suppressing M2 macrophage polarization. BioMed Res Int. (2020) 2020:5137349. doi: 10.1155/2020/5137349
80. Xu H, Qu J, Wang J, Han K, Li Q, Bi W, et al. Discovery of pulmonary fibrosis inhibitor targeting TGF-β RI in Polygonum cuspidatum by high resolution mass spectrometry with in silico strategy. J Pharm Anal. (2022) 12:860–8. doi: 10.1016/j.jpha.2020.05.007
81. Toda M, Mizuguchi S, Minamiyama Y, Yamamoto-Oka H, Aota T, Kubo S, et al. Pirfenidone suppresses polarization to M2 phenotype macrophages and the fibrogenic activity of rat lung fibroblasts. J Clin Biochem Nutr. (2018) 63:58–65. doi: 10.3164/jcbn.17-111
82. Okazaki H, Sato S, Koyama K, Morizumi S, Abe S, Azuma M, et al. The novel inhibitor PRI-724 for Wnt/β-catenin/CBP signaling ameliorates bleomycin-induced pulmonary fibrosis in mice. Exp Lung Res. (2019) 45:188–99. doi: 10.1080/01902148.2019.1638466
83. Spathakis M, Tarapatzi G, Filidou E, Kandilogiannakis L, Karatzas E, Steiropoulos P, et al. Niclosamide attenuates inflammation-associated profibrotic responses in human subepithelial lung myofibroblasts. Biomedicines. (2023) 11:2032. doi: 10.3390/biomedicines11072032
84. Zhang W, Ohno S, Steer B, Klee S, Staab-Weijnitz CA, Wagner D, et al. S100a4 is secreted by alternatively activated alveolar macrophages and promotes activation of lung fibroblasts in pulmonary fibrosis. Front Immunol. (2018) 9:1216. doi: 10.3389/fimmu.2018.01216
85. Rice LM, Padilla CM, McLaughlin SR, Mathes A, Ziemek J, Goummih S, et al. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest. (2015) 125:2795–807. doi: 10.1172/JCI77958
86. Wang J, Xu L, Xiang Z, Ren Y, Zheng X, Zhao Q, et al. Microcystin-LR ameliorates pulmonary fibrosis via modulating CD206+ M2-like macrophage polarization. Cell Death disease. (2020) 11:136. doi: 10.1038/s41419-020-2329-z
87. Codullo V, Cova E, Pandolfi L, Breda S, Morosini M, Frangipane V, et al. Imatinib-loaded gold nanoparticles inhibit proliferation of fibroblasts and macrophages from systemic sclerosis patients and ameliorate experimental bleomycin-induced lung fibrosis. J Controlled Release. (2019) :310:198–208. doi: 10.1016/j.jconrel.2019.08.015
88. Jourdan Le Saux C, Ho TC, Brumwell AM, Kathiriya JJ, Wei Y, Hughes JB, et al. BCL-2 modulates IRE1α activation to attenuate endoplasmic reticulum stress and pulmonary fibrosis. Am J Respir Cell Mol Biol. (2024) 70:247–58. doi: 10.1165/rcmb.2023-0109OC
Keywords: connective tissue disease, interstitial lung disease, macrophage polarisation, monocyte derived alveolar macrophage, antifibrotic agents, profibrotic macrophages, single cell RNA and transcriptome sequencing, macrophage genetic characteristics
Citation: Tyagi R and Kant S (2025) Macrophage subtypes and pathways in autoimmune interstitial lung diseases: potential therapeutic targets. Front. Immunol. 16:1638345. doi: 10.3389/fimmu.2025.1638345
Received: 02 June 2025; Accepted: 15 September 2025;
Published: 01 October 2025.
Edited by:
Betty Diamond, Feinstein Institute for Medical Research, United StatesReviewed by:
Theodoros Karampitsakos, National and Kapodistrian University of Athens, GreeceCopyright © 2025 Tyagi and Kant. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richa Tyagi, ZHIucmljaGFwdWxtb0BnbWFpbC5jb20=
 Richa Tyagi
Richa Tyagi Surya Kant
Surya Kant