- Department of Urology, The Second Affiliated Hospital, Kunming Medical University, Kunming, China
Bladder cancer is one of the most common malignancies of the urogenital system, with a high incidence and mortality. The treatment of bladder cancer is diverse, with surgical treatment being the most common approach, including transurethral resection of bladder tumor and radical cystectomy. Following radical cystectomy, patients often undergo ileal neobladder reconstruction to restore urinary storage and voiding functions. However, postoperative changes in the urinary microbiota have become a major issue for bladder cancer patients. Traditionally, urine was believed to be sterile, but an increasing body of research has demonstrated the presence of a resident microbiota in urine, which is closely associated with the development of bladder diseases, postoperative complications, and patient prognosis. Dynamic changes in the urinary microbiota may lead to urinary tract infections, tumor recurrence, and other issues, severely affecting patients’ recovery and quality of life. In recent years, with the advancement of high-throughput sequencing technology, research on the urinary microbiota has deepened, particularly regarding its changes and clinical significance after bladder cancer surgery. Although studies have explored the impact of urinary microbiota on recurrence and prognosis after bladder cancer surgery, research on urinary microbiota changes following ileal neobladder reconstruction is still limited. Therefore, this review aims to summarize the latest research on the dynamic changes of urinary microbiota in bladder cancer patients postoperatively, especially focusing on changes after ileal neobladder reconstruction, providing references for clinical treatment and future research directions.
1 Introduction
Bladder cancer is a common malignant tumor of the urinary system and is one of the top ten most prevalent cancers worldwide, with approximately 550,000 new cases diagnosed each year (1, 2). According to the Global Cancer Statistics 2020, there are approximately 573,278 new cases and 212,536 deaths from bladder cancer in 2020, with the incidence in men significantly higher than in women, placing a substantial burden on global public health systems (3). The latest GLOBOCAN data reveal that bladder cancer accounts for 3% of all cancer diagnoses globally, with a particularly high prevalence in developed countries (4). In recent years, with continuous advancements in diagnostic technologies, early screening and diagnosis of bladder cancer have gradually gained attention. However, the high recurrence rate and metastatic nature of bladder cancer continue to pose significant challenges in its treatment.
Studies have shown that the high incidence of bladder cancer is closely associated with smoking habits, industrial pollution, and occupational exposure to carcinogenic substances (5–7). Smoking is considered one of the most significant risk factors for bladder cancer. Evidence suggests that smokers have a substantially higher risk of developing bladder cancer compared to non-smokers (8). Carcinogens present in cigarette smoke, such as polycyclic aromatic hydrocarbons and aromatic amines, can damage the DNA of bladder epithelial cells, thereby promoting tumorigenesis (9). Despite the increasing implementation of tobacco control policies, the global prevalence of smoking remains high, which continues to be a major contributor to the burden of bladder cancer. In addition to smoking, environmental factors also play a critical role in the pathogenesis of bladder cancer (10). Certain occupational groups, such as workers in the chemical manufacturing, petrochemical, and dye industries, are at higher risk of exposure to aromatic amines, which have been strongly linked to the development of bladder cancer (11). Moreover, the incidence of bladder cancer increases with age. The majority of patients are over 60 years old, with those aged 70 and above being particularly susceptible (12). This age-dependent incidence may be attributed to age-related declines in immune function, reduced DNA repair capacity, and prolonged exposure to environmental carcinogens.
Currently, bladder cancer treatment options are heterogeneous, with surgery being one of the most important therapeutic approaches (13). The choice of surgical treatment depends on the stage and pathological characteristics of the bladder cancer. There are two main types of bladder cancer: non-muscle invasive bladder cancer and muscle-invasive bladder cancer (14). For non-muscle invasive bladder cancer, the tumor is confined to the bladder mucosa or submucosa without invading the muscular layer of the bladder wall. The standard surgical treatment for this stage is transurethral resection of bladder tumor, followed by risk stratification-based adjuvant intravesical therapy, with an overall survival rate of 90% (15). For muscle-invasive bladder cancer, where the tumor has invaded the bladder muscular layer, the standard treatment method is radical cystectomy combined with urinary diversion. However, the cure rate remains low due to various factors (15, 16). Ileal neobladder reconstruction is a common form of urinary diversion, where the ileum is used to replace the resected bladder, thus restoring the patient’s urinary storage and voiding functions (17). In contemporary clinical practice, ileal neobladder reconstruction has gained widespread adoption, establishing itself as a cornerstone technique for bladder cancer treatment across multidisciplinary medical institutions.
Postoperative changes in the urinary microbiota represent a significant issue for bladder cancer patients (18). Traditionally, urine was believed to be sterile; however, increasing evidence suggests that a resident microbiota exists in urine and influences the development of various bladder diseases, such as overactive bladder, interstitial cystitis, neurogenic bladder, and other bladder disorders (19). Recent studies have found that the urinary microbiota is closely associated with bladder cancer and may serve as potential biomarkers and therapeutic targets for the disease (20–22). Following cystectomy and urinary diversion, the structure and function of the urinary microbiota may undergo significant changes. These alterations may be closely related to the occurrence of postoperative complications, patients’ quality of life, and long-term prognosis (23). Research indicates that changes in the urinary microbiota may lead to a range of issues, including urinary tract infections and tumor recurrence, all of which severely affect patients’ recovery and quality of life (24). In recent years, with the development of high-throughput sequencing technology, research on the urinary microbiota has deepened (23). The dynamic changes in the urinary microbiota and its interactions with the host have become a research hotspot in the field of urological surgery. However, studies on the dynamic changes of the urinary microbiota and its clinical significance in bladder cancer patients postoperatively are still insufficient, particularly regarding the changes in the urinary microbiota after ileal neobladder reconstruction and its impact on patient prognosis, which requires further exploration.
Therefore, this review aims to summarize the research progress on the dynamic changes of urinary microbiota in bladder cancer patients postoperatively, with a particular focus on the changes in the urinary microbiota after ileal neobladder reconstruction and its clinical significance. This narrative review intends to provide a theoretical basis for clinical treatment and offer insights for future research directions.
2 Characteristics of the urinary microbiota in patients with bladder cancer before surgery
2.1 The burden of bladder cancer disease and regional differences in urine microbiota
The incidence of bladder cancer varies significantly across regions, with higher incidence generally observed in the high-income regions (5). Moreover, in 204 countries and territories worldwide, the 2021 Global Burden of Disease (GBD) study for bladder cancer is displayed in Figure 1; Supplementary Table 1. Data reveal that, in 2021, the age-standardized rate (ASR) of bladder cancer-related deaths (ASDR) is the highest in Mali, with an ASDR of 8.994 per 100,000 persons (95% uncertainty interval (UI): 6.956-11.522) and estimated annual percentage change (EAPC) of 0.07 (95% confidence interval (CI): 0.01 to 0.14) (Figures 1A, B; Supplementary Table 1). The disability-adjusted life years (DALYs) of bladder cancer are the highest in Malawi, with the age-standardized DALY rate of 179.915 per 100,000 persons (95% UI: 140.229-227.226) and EAPC of 0.29 (95% CI: 0.12 to 0.47) (Figures 1C, D; Supplementary Table 1). Notably, from 1990 to 2021, the increase in bladder cancer-deaths and DALYs are the most in Cabo Verde, indicating the high disease burden in this country (Figure 1; Supplementary Table 1). Another 2021 GBD study illustrates that the ASR of bladder cancer-related incidence exhibits an upward trend in the middle and low socio-demographic index (SDI) regions, with the highest age-standardized DALY rate in Central Europe (25). The significant variation in bladder cancer-related deaths and DALYs across countries may reflect substantial disparities in the healthcare systems and prevention strategies for bladder cancer.
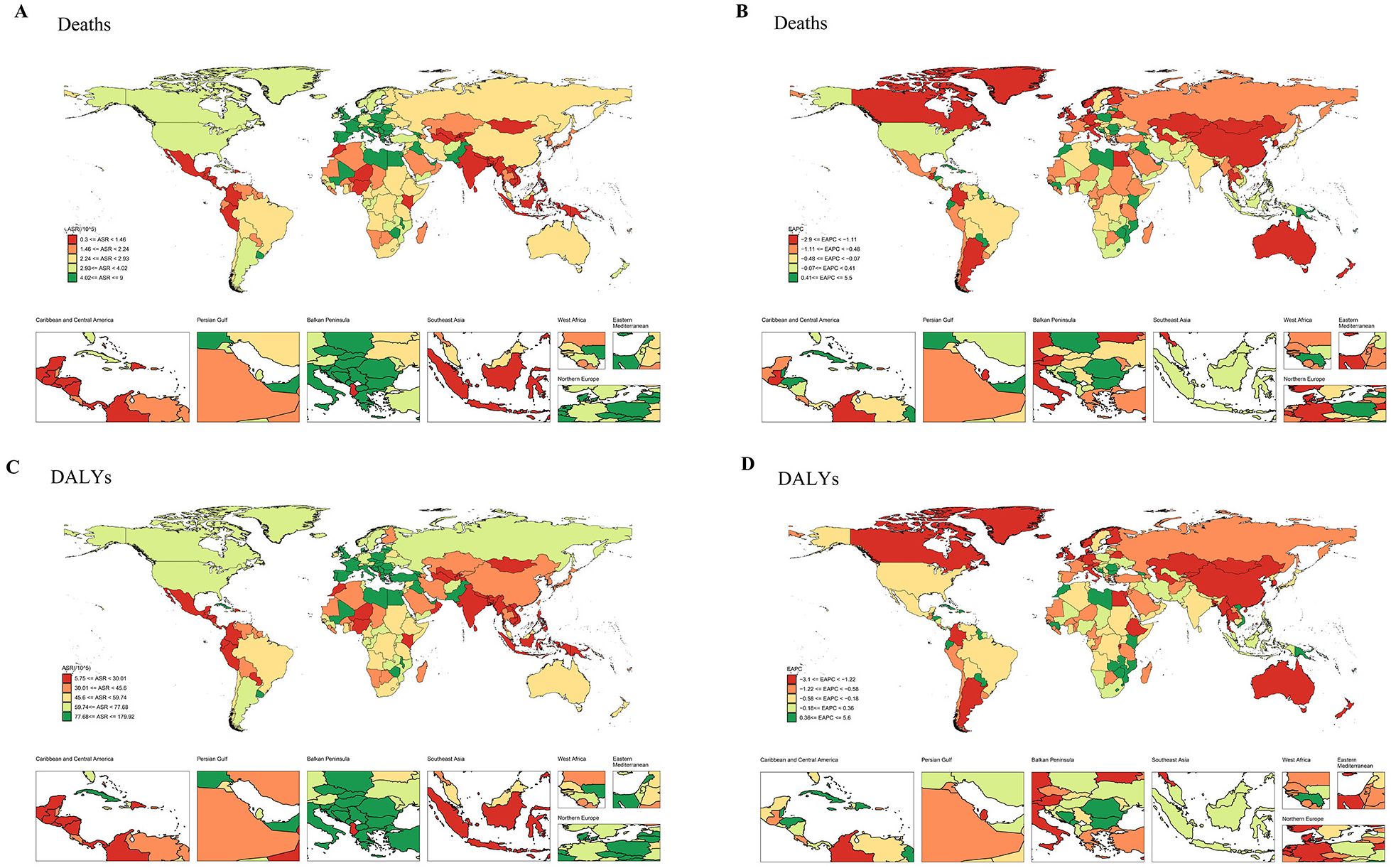
Figure 1. The ASR and EAPC maps of bladder cancer in 204 countries and territories. (A) ASR map for deaths. (B) EAPC map for deaths. (C) ASR map for DALYs. (D) EAPC map for DALYs. ASR, age-standardized rate; EAPC, estimated annual percentage change; DALYs, disability-adjusted life years.
The differences in bladder cancer incidence across different regions may be closely related to environmental factors, tobacco exposure, and other factors (8). These factors can influence the composition for the urinary microbiota, which in turn affects the formulation of disease intervention strategies. Furthermore, dietary habits, can have a profound effect on the urinary microbiome (26). Diet risk may alter the structure of the urinary microbiome, thereby influencing the development of bladder cancer (27). Therefore, when addressing bladder cancer patients in different regions, it is essential to consider local environment and microbiome characteristics to develop more targeted intervention strategies.
2.2 Differences in urinary microbiota between healthy individuals and bladder cancer patients
Compared to healthy individuals, there are significant differences in the urinary microbiome composition of patients with urogenital cancers. Lactobacillus is enriched in the urine of adult women and may be valuable in enhancing the response of Bacillus Calmette-Guerin treatment for bladder cancer (28). For instance, Lactobacillus helps regulate urinary pH, creating an acidic environment that inhibits the growth of harmful pathogenic bacteria. Lactobacillus has antimicrobial properties, which further protect the urinary tract from infections (29). Additionally, the immune system in healthy individuals effectively controls the population of these microorganisms, preventing overgrowth. However, significant differences exist between the urinary microbiota of bladder cancer patients and healthy individuals (30, 31) (Table 1). The urine of bladder cancer patients often contains higher levels of pathogenic microorganisms compared to healthy individuals, including Escherichia coli, Klebsiella spp., and intestinal anaerobes (30). In bladder cancer patients, the abundance of polycyclic aromatic hydrocarbons-degrading bacteria such as Sphingomonas, Acinetobacter, Micrococcus, Pseudomonas and Ralstonia are increased (31). These bacteria typically enter the urine via the urethra, but bladder cancer patients are unable to effectively eliminate these pathogens due to immune system suppression. Moreover, the urinary microbiota is further altered by antibiotic treatment and additional immunosuppression caused by chemotherapy/radiotherapy. Studies have shown a high prevalence of antibiotic-resistant bacteria in the urine of bladder cancer patients, particularly in those undergoing long-term treatment. These resistant bacteria include Klebsiella oxytoca, Morganella morganii, Salmonella enterica, and Trabulsiella farmeri (32). The colonization of these antibiotic-resistant bacteria not only increases the risk of infections but may also worsen disease prognosis.
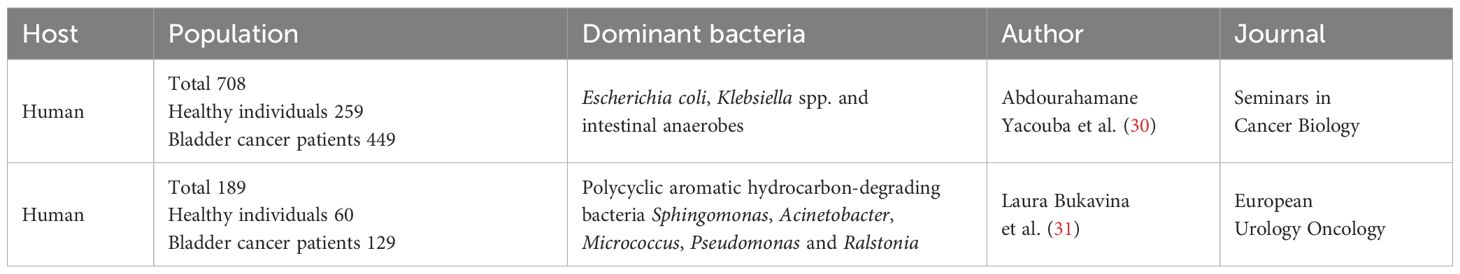
Table 1. Characteristics of urinary microbiota between healthy individuals and bladder cancer patients.
2.3 Bladder cancer subtypes and microbiota characteristics
Bladder cancer is classified into non-muscle invasive bladder cancer and muscle-invasive bladder cancer based on the depth of invasion into the bladder wall. These two subtypes of bladder cancer differ in clinical presentation, treatment approaches, and prognosis. Recent studies have suggested that the different subtypes of bladder cancer are also associated with variations in the urinary microbiota of patients (33). Non-muscle invasive bladder cancer is typically diagnosed at an earlier stage, with tumors confined to the epithelial layer of the bladder and not extending into the muscle layer. Since this type of cancer is diagnosed early, the immune system of the patient has not been severely compromised, and as a result, the urinary microbiota retains a certain level of diversity (34). In contrast, muscle-invasive bladder cancer, characterized by tumor invasion into the muscle layer of the bladder, places a greater burden on the patient’s immune system (35). A clinical study shows that the content of Cupriavidus in patients with NMIBC is increased significantly (36). Moreover, the proportions of Haemophilus and Veillonella in the urine of muscle-invasive bladder cancer patients are significantly higher than those in non-muscle invasive bladder cancer patients (36). This dysbiosis may be closely related to the immunosuppressive state of bladder cancer patients.
2.4 Possible mechanisms of dysbiosis
The dysbiosis of the urinary microbiota in bladder cancer patients is not incidental, but rather influenced by multiple factors, primarily including inflammation, immune suppression, and changes in metabolic products (37). Bladder cancer is a malignant tumor, and the onset and progression of the tumor lead to chronic inflammatory responses in the bladder. Research has shown that microbiota-induced inflammation is one of significant factors contributing to the deterioration caused by the bladder cancer (38). The persistent local inflammatory response in bladder cancer patients may provide a favorable growth environment for pathogenic microorganisms. Inflammation alters the pH, oxygen levels, and nutritional components of urine through the secretion of cytokines, recruitment of immune cells, and changes in the local microenvironment, thereby influencing the growth and colonization of microorganisms (39). Moreover, after undergoing treatments such as chemotherapy, radiotherapy, or cystectomy, the immune function of bladder cancer patients is significantly suppressed. The immunosuppressive state can further promote the immune escape of cancer cells (40). Additionally, the metabolic products within the bodies of bladder cancer patients can affect the composition of the urinary microbiota (41). The metabolic activity of cancer cells differs from that of normal cells. Lactate and other organic acids produced during tumor cell metabolism may create a favorable environment for the growth of anaerobic bacteria (42).
3 Dynamic changes of urinary microbiota in patients with bladder cancer after different surgeries
With the continuous advancement of surgical treatments, some complex issues faced by bladder cancer patients during their postoperative recovery process have gradually gained attention, especially the dynamic changes in the urinary microbiota following bladder cancer surgery and its relationship with patient recovery. In recent years, an increasing number of studies have shown that the structure, function, and diversity of the urinary microbiota undergo significant changes in bladder cancer patients postoperatively, which may be closely related to postoperative infections, immune system reconstruction, and disease recurrence (18). The urinary microbiota of bladder cancer patients experiences dynamic changes after surgery, and these changes are not only closely associated with the patient’s immune response, treatment methods (antibiotic use and chemotherapy), and postoperative complications, but also influence the patient’s postoperative recovery and survival prognosis to some extent. After bladder cancer surgery, patients may experience urinary tract infections, alterations in antibiotic-resistant bacterial populations, and dysbiosis of beneficial microbiota in the urine (43, 44).
3.1 Impact of surgical methods on urinary microbiota
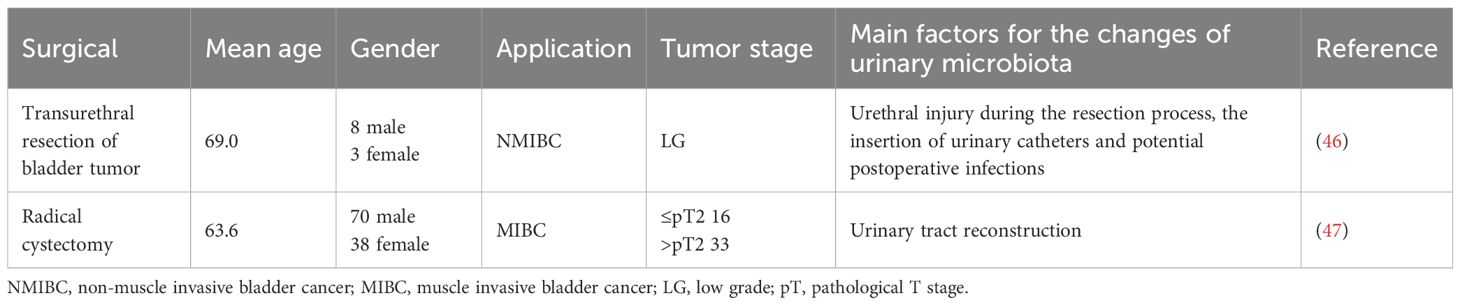
The surgical management of bladder cancer primarily involves two key procedures: transurethral resection of bladder tumor and radical cystectomy (45). Transurethral resection of bladder tumor is typically used for non-muscle invasive bladder cancer, whereas radical cystectomy is often required for more advanced or muscle-invasive cases. Research has indicated that these different surgical approaches can significantly affect the urinary microbiota, with each method having distinct influences on the composition and diversity of microbial communities present in the urine (46, 47) (Table 2). After undergoing transurethral resection of bladder tumor, patients may experience structural shifts in their urinary microbiota. These changes can be attributed to various factors such as urethral injury during the resection process, the insertion of urinary catheters, and potential postoperative infections. These factors can introduce disturbances in the natural balance of the microbial environment in the urinary system, potentially leading to an altered microbiome (46). On the other hand, radical cystectomy, which involves the complete removal of the bladder, can lead to even more substantial alterations in the urinary microbiota (47) (Table 3). This is due to the complex procedures involved in urinary tract reconstruction following cystectomy. Different techniques for reconstructing the urinary tract, such as the creation of an ileal conduit or an ileal neobladder, can significantly impact the physiological characteristics of the urine. These changes may include variations in the pH level, the ionic composition, and other aspects of urine chemistry, all of which can affect the microbial ecosystem within the urinary system. Under normal conditions, the pH of urine ranges from 4.5 to 8.0. However, the pH of the ileum is typically alkaline. When using an ileal conduit or ileal neobladder, the mucosa of the ileum comes into contact with urine, leading to the increase of urine pH, but it is still acidic (48). Additionally, the storage of urine in intestinal mucosa will lead to the reabsorption of urea, potassium and chloride, and the excretion of sodium and bicarbonate, which will lead to the increase of acid load (49). Beyond pH and ionic composition, the level of urinary components may also change (48). The increase in ammonia in the urine may lead to a higher pH, further altering the ecosystem of the microbiome. As a result, the type of surgical procedure performed plays an important role in shaping the urinary microbiota, which may have implications for the patient’s recovery and overall health (46).
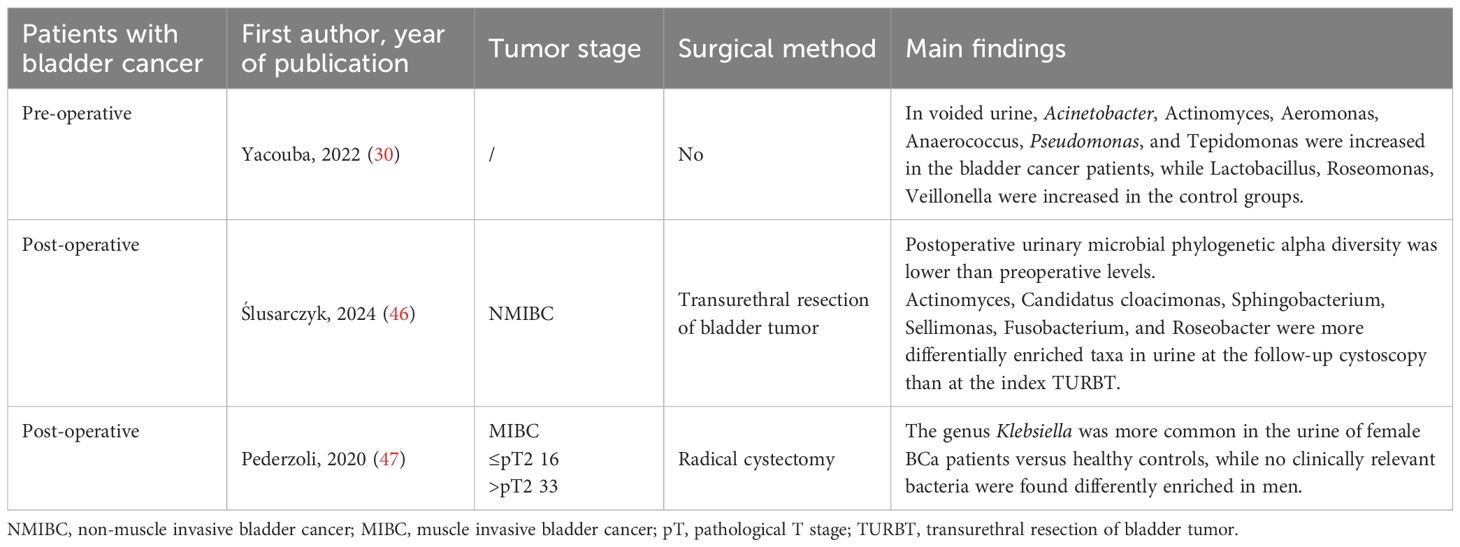
Table 3. Comparison of urinary microbiota profiles across patient subtypes: pre-operative vs. post-operative and NMIBC vs. MIBC.
3.2 Antibiotic use and changes in the urinary microbiota
Bladder cancer patients often require antibiotic therapy following surgical procedures to prevent or treat infections that may arise as a result of the surgery or subsequent postoperative complications. Antibiotics play an essential role in managing infections by targeting and eliminating harmful pathogens that could lead to more severe health issues. However, the prolonged or excessive use of antibiotics, especially broad-spectrum antibiotics, can have unintended consequences (50). One such consequence is the disruption of the natural balance of the urinary microbiota, a condition known as dysbiosis (51). Dysbiosis refers to an imbalance in the microbial community, where the beneficial bacteria that help maintain a healthy microbiome are reduced in number, while harmful or pathogenic bacteria proliferate. This imbalance can have serious consequences for the urinary tract health (52). The overuse of antibiotics can result in the overgrowth of pathogenic microorganisms such as Escherichia coli and Klebsiella spp., which are known to be harmful to the urinary tract. Escherichia coli may evade immune surveillance by altering the function of host immune cells. It can suppress T cell function by secreting immunosuppressive factors, thereby reducing the body’s immune response (53). Meanwhile, the populations of beneficial bacteria, like Lactobacillus and Bifidobacterium spp., that play a crucial role in supporting a healthy microbiome, are diminished. This microbial imbalance creates an environment more prone to infections, particularly urinary tract infections, which are common in bladder cancer patients, particularly those who have undergone surgical interventions (21). Infections caused by antibiotic-related dysbiosis may delay the healing process, impair immune function, and exacerbate other health issues, which can ultimately lead to poorer treatment outcomes (54). This highlights the importance of carefully managing antibiotic use, not only to treat infections but also to preserve the integrity of the urinary microbiota. By balancing the need for antibiotic intervention with efforts to minimize disruptions to the microbiota, clinicians can help ensure better recovery outcomes and reduce the risks associated with dysbiosis. Therefore, while antibiotics are a crucial component of infection management, close monitoring and appropriate prescribing practices are necessary to avoid their adverse effects on the patient’s recovery and long-term survival.
3.3 Changes in dominant microbial communities
The structure of the urinary microbiota in bladder cancer patients often experiences significant changes after surgery, with alterations in the dominant microbial communities and an increase in certain pathogenic bacteria (55). The dominant microbial communities refer to the bacterial species that occupy a substantial proportion of the microbiota in a particular environment. In bladder cancer patients, especially following surgery, the urinary microbiota undergoes changes (56). After transurethral resection of bladder tumor, the quantities of Virongella and Bifidobacterium in the urine of patients with bladder cancer are increased (56). Research has shown that after procedures like cystectomy, there is often an increase in the abundance of these pathogenic bacteria in the urine. This phenomenon is closely linked to the use of postoperative antibiotics and the trauma caused by surgery. These changes can contribute to urinary tract infections and other complications, further complicating the recovery process for bladder cancer patients (57).
3.4 Proliferation of pathogenic bacteria
Postoperatively, especially following radical cystectomy, bladder cancer patients may experience excessive proliferation of certain pathogenic bacteria. The most common pathogenic bacteria include Escherichia coli, Enterococcus spp., and Staphylococcus spp. (50). The overgrowth of these pathogenic bacteria can lead to postoperative urinary tract infections, urosepsis, and other complications, thereby affecting the patient’s recovery (57). Specifically, in patients following cystectomy, alterations in urine pH, ion concentration, and urinary flow dynamics may create a favorable environment for the growth of certain pathogenic bacteria.
3.5 Changes in urinary microbiota diversity
The diversity of the urinary microbiota is an important indicator of the health of microbial communities and is typically assessed through α-diversity and β-diversity (55). α-diversity reflects the species richness and evenness within a single environment, while β-diversity indicates the differences in microbial communities between different environments. A study has shown that the α-diversity of the urinary microbiota in bladder cancer patients typically decreases after transurethral resection of the bladder tumor (46). This reduction in diversity may increase the risk of urinary tract infections and impact the patient’s postoperative immune recovery. The β-diversity of the urinary microbiota in bladder cancer patients postoperatively generally shows significant variation. An increase in β-diversity indicates greater differences in the urinary microbiota between different patients (46). After transurethral resection of bladder tumor, the β-diversity of the urinary microbiota in bladder cancer patients changes significantly over time (46). This variation may be closely related to the physiological changes brought about by postoperative urinary tract reconstruction.
3.6 Functional changes in the urinary microbiota
The functionality of the urinary microbiota in bladder cancer patients also undergoes changes after Bacillus Calmette-Guerin treatment, particularly in terms of metabolic functions and resistance (32). These functional changes in the urinary microbiota can directly influence the occurrence of postoperative infections, the recovery of the patient’s immune function, and cancer recurrence. Changes in the metabolic functions of the urinary microbiota post-surgery may affect the patient’s postoperative recovery. One key area of research in bladder cancer postoperative care is the immune-regulatory role of the urinary microbiota. The urinary microbiota interacts with the host immune system, influencing the immune response (58). Beneficial bacteria, such as Lactobacillus, help maintain immune balance in the urine through mechanisms like promoting immune cell activation, increasing the secretion of anti-inflammatory factors, and suppressing inflammatory responses. However, dysbiosis in the urinary microbiota may lead to immune dysfunction, which can negatively affect the patient’s recovery and contribute to immune escape phenomena (58). With the widespread use of antibiotics, bladder cancer patients may face the challenge of antibiotic-resistant bacterial populations postoperatively. Recent studies have shown that the dysbacteriosis caused by antibiotic treatment will increase the risk of failure of immunoscreen inhibitor treatment (59). The emergence of antibiotic-resistant bacteria complicates treatment regimens, increases the risk of infections, and adversely affects the patient’s survival prognosis.
3.7 Urinary microbiota characteristics related to postoperative infections
Bladder cancer patients often face a range of complications following surgical treatment, with postoperative infections, particularly urinary tract infections, being among the most common issues (43, 44). In some cases, urinary tract infections are not merely short-term clinical manifestations; they can also lead to the colonization of antibiotic-resistant bacteria, further complicating and making treatment more difficult. The occurrence of urinary tract infections may stem from either postoperative infections or pre-existing chronic bacterial infections, especially in immunocompromised patients (58). Moreover, bladder cancer patients often receive broad-spectrum antibiotics postoperatively, which, while effectively preventing infections, may also lead to dysbiosis of the normal microbiota, providing an opportunity for the proliferation of resistant bacteria (32). The occurrence of postoperative infections not only impacts the patient’s recovery process but can also increase hospitalization time, treatment costs, and in some cases, lead to the complications of urinary tract infections and surgical site infections (60). The presence of these resistant bacteria may affect the patient’s prognosis and treatment strategies.
The prolonged use of antibiotics severely impacts the normal microbiota in the urine, inhibiting the growth of beneficial bacteria while selectively promoting the proliferation of resistant bacteria, leading to the colonization of resistant strains. When bladder cancer patients receive postoperative antibiotic treatment, bacteria that were originally sensitive to antibiotics may be suppressed, while resistant bacteria survive and establish colonization due to their resistance (44). One study found that, in patients undergoing radical cystectomy, the bacterial pathogens in greatest proportions are Enterococcus (42.0%), Escherichia coli (21.70%), and Candida (13.0%) (61). Additionally, bladder cancer patients often require the insertion of a urinary catheter postoperatively to maintain urinary tract patency. Prolonged catheterization can lead to mechanical irritation of the urethra, increasing the risk of urinary tract infections, particularly when catheters are not replaced in a timely manner (62). The presence of a catheter provides a habitat for bacterial colonization, thereby facilitating the invasion of antibiotic-resistant bacteria. A study has shown that the cultures from discharge catheters are closely associated with postoperative urinary tract infections (63). Therefore, timely replacement of catheters and strict adherence to sterile techniques are critical to reducing colonization by antibiotic-resistant bacteria.
3.8 Association between urinary microbiota and LUTS and recurrence
Changes in the urinary microbiome are closely related to patients’ lower urinary tract symptoms (LUTS). A spearman’s correlation analysis revealed that urine samples showing the presence of the bacterial genera Haemophilus, Staphylococcus, Listeria, Dolosigranulum, Phascolarctobacterium, Enhydrobacter, Bacillus, [Ruminococcus] torques, Faecalibacterium, and Finegoldia correlated with a high international prostate symptom score (IPSS), and severe storage and voiding symptoms (64). A study showed that compared with patients with primary bladder cancer, patients with recurrent bladder cancer had lower diversity in their urinary microbiota or were dominated by specific microbial groups. The relative abundance of Firmicutes was significantly higher and Bacteroidetes significantly lower in patients with recurrent bladder cancer (34).
4 Characteristics of the urinary microbiota after ileal neobladder reconstruction
Ileal neobladder reconstruction is a common urinary tract reconstruction surgery performed after bladder cancer surgery. It is typically used following radical cystectomy, where a portion of the ileum is utilized to replace the function of the bladder (65). In ileal neobladder reconstruction, part of the ileum is reconfigured into a urinary pouch and connected to the urethra, allowing patients to void. Postoperatively, patients typically need to urinate through the urethra, an artificial urethra, or a catheter (65). Ileal neobladder reconstruction is associated with a high rate of improvement in quality of life and urinary function recovery, making it an effective treatment option for bladder cancer patients (66, 67). However, the risk of urinary tract infections increases following ileal neobladder reconstruction, and changes in the urinary microbiota play an important role in postoperative recovery. Recent studies have demonstrated that ileal neobladder reconstruction not only alters the histological architecture but also causes substantial modifications of both the compositional profile and functional dynamics of the urinary microbiota (57).
4.1 Changes in the urinary microbiota after ileal neobladder reconstruction
The urinary microbiota refers to the various bacteria, fungi, and other microorganisms present in the urine (68). Under normal conditions, urine is considered sterile; however, because ileal neobladder reconstruction involves connecting intestinal tissue to the urinary system, different microbial communities may be present in the urine postoperatively. Recent studies have shown that the characteristics of the urinary microbiota in bladder cancer patients undergoing ileal neobladder reconstruction undergo significant changes (57). Due to the incorporation of the ileum, intestinal microbiota may colonize the urine, including species such as Escherichia coli, Klebsiella spp., and Enterococcus spp. These microbiota differ markedly from the microbiota typically found in the urine of healthy individuals (57). Additionally, endogenous bacteria from the ileal tissue may interact with the external environment through the urinary tract, leading to dysbiosis of the urinary microbiota. The incidence of urinary tract infections is higher in bladder cancer patients following ileal neobladder reconstruction (69). The colonization of antibiotic-resistant bacteria in the urine is one of the primary causes by postoperative infections. Research has shown that following ileal neobladder reconstruction, the urine of patients contains higher levels of E. coli, Klebsiella spp., Enterococcus spp., and other bacteria with higher resistance to commonly used antibiotics such as amoxicillin and ampicillin (70). The colonization by these antibiotic-resistant bacteria may lead to chronic urinary tract infections, and their resistance to traditional antibiotic treatments complicates the treatment process.
4.2 Clinical significance of ileal neobladder reconstruction on the urinary microbiota of bladder cancer patients
The impact of ileal neobladder reconstruction on the urinary microbiota of bladder cancer patients holds significant clinical implications. Firstly, this procedure alters the composition of the urinary microbiota, potentially leading to the colonization by antibiotic-resistant bacteria and an increased risk of urinary tract infections (69). Secondly, postoperative infections may result in extended hospitalization, increased treatment costs, and could even affect the patient’s long-term survival prognosis. Therefore, understanding the effects of ileal neobladder reconstruction on the urinary microbiota and implementing appropriate infection prevention and treatment measures are crucial for improving patient outcomes. To reduce the occurrence of urinary tract infections after ileal neobladder reconstruction, patients should undergo strict infection control protocols. Tailoring personalized antibiotic treatment plans based on the changes in the urinary microbiota post-surgery is essential. Additionally, as patients undergoing this procedure often have weakened immune systems, enhancing immune function regulation is also critical for infection prevention. Further research has revealed that the occurrence of LUTS in patients after ileal neobladder surgery is closely related to specific changes in the microbiota. Kim et al. found that Enterococcus was significantly enriched in patients with postoperative febrile urinary tract infections, and these patients had an increased incidence of urinary urgency and nocturia (57). So, microbiota-targeted interventions may offer novel therapeutic strategies for preventing recurrence and improving patient outcomes.
5 Research prospects
The study of urinary microbiota as a biomarker for prognostic evaluation in bladder cancer patients has emerged as a growing focus in the field of oncology (71). With the continuous development of microbiomics, research on the urinary microbiota offers new directions for cancer diagnosis, prognostic assessment, and treatment strategies (23). Bladder cancer, as a common urological malignancy, has long relied on traditional imaging techniques and histopathological examinations for treatment and prognostic evaluation. However, with the continuous advancements in molecular biology techniques, an increasing number of studies have shown that the urinary microbiota not only plays a significant role in the occurrence and development of bladder cancer but can also serve as a non-invasive biomarker, providing strong support for early diagnosis, prognostic evaluation, and treatment of bladder cancer.
5.1 Relationship between the urinary microbiota and bladder cancer
Recent studies have shown that the development of bladder cancer is influenced not only by environmental factors, genetic background, and lifestyle but also by dysbiosis of the local bladder microbiota (22, 23). As an important organ of the urinary system, the bladder is in prolonged contact with microorganisms in urine, and changes in the urinary microbiota may directly or indirectly affect the immune environment of the bladder, tumor-related signaling pathways, and the growth and metastasis of cancer cells (72). Research has found significant differences in the urinary microbiota composition between bladder cancer patients and healthy individuals (73). Specific bacterial communities, such as Akkermansia and Bacteroidetes, are often found in the urine of bladder cancer patients, with notable changes in their abundance and diversity (74). In addition to the early onset of bladder cancer, the urinary microbiota is also associated with cancer progression and metastasis. A study has shown that certain bacterial communities may promote tumor growth, invasion, and metastasis in the bladder cancer microenvironment (35). Therefore, the urinary microbiota not only holds potential diagnostic value in the early stages of bladder cancer but also plays a crucial role in the progression of the disease.
5.2 Potential of the urinary microbiota as a prognostic biomarker for bladder cancer patients
The urinary microbiota holds significant potential as a biomarker for the prognostic evaluation of bladder cancer. Traditional methods for assessing bladder cancer prognosis primarily rely on tumor staging, histological grading, imaging studies, and blood tests (75). However, these methods often have certain limitations and may not accurately reflect a patient’s actual prognosis. In contrast, the urinary microbiota offers advantages such as non-invasiveness, ease of collection, and high-throughput detection, providing valuable information for early screening, disease monitoring, and prognostic assessment in bladder cancer. A decrease in the diversity and abundance of the urinary microbiota may be associated with higher risk, higher recurrence rates, and lower survival rates in bladder cancer (76). Additionally, the abundance of Veillonella, Brevundimonas, and Methylobacterium genera in the urine of bladder cancer patients is significantly elevated, and changes in the abundance of these bacteria are closely linked to clinical staging, pathological grading, and the patient’s survival time (76). Therefore, the diversity and composition of the urinary microbiota may serve as important indicators for prognostic assessment in bladder cancer patients. Moreover, the high recurrence rate of bladder cancer is a critical factor impacting patient quality of life and prognosis. Research has found that the increase in certain pathogenic bacteria (Veillonella and Bifidobacterium) in urine may lead to the recurrence of bladder cancer (56). Thus, the urinary microbiota could serve as an early warning signal, helping to identify high-risk bladder cancer patients and providing a basis for early intervention in recurrence.
5.3 Future research directions
Although preliminary research on the relationship between the urinary microbiota and bladder cancer has yielded important initial findings, many unanswered questions remain. Therefore, future research should delve deeper in the following directions:
5.3.1 Multi-omics integration analysis
Changes in the urinary microbiota are increasingly being recognized as potentially important factors in various diseases, including bladder cancer (30). These changes may be influenced by several biological processes, including the body’s metabolic and genetic pathways. In particular, factors such as metabolomics and transcriptomics are thought to play a significant role in shaping the urinary microbiota and influencing its impact on the health (77). Metabolomics involves the study of metabolites, the small molecules produced during metabolism, while transcriptomics focuses on gene expression profiles, revealing how genes are activated or deactivated in response to various biological conditions. Together, these fields offer deep insights into how changes in the urinary microbiota might contribute to disease processes like bladder cancer. To further explore how the microbiota interacts with other biological systems, future research could aim to examine the relationships between the urinary microbiota, the metabolome, and the transcriptome through the integration of multi-omics data. Multi-omics integration analysis allows researchers to gather data from various omics layers, such as metagenomics, which studies the microbial communities, metabolomics, and transcriptomics. By combining these techniques, researchers can generate a more holistic understanding of how the microbiota, metabolites, and gene expression patterns are interconnected and how they contribute to bladder cancer.
5.3.2 Targeted microbiota intervention strategies
The urinary microbiota has emerged as a potential biomarker for the prognostic assessment of bladder cancer, offering new opportunities for personalized treatment approaches (22). As research into the role of the microbiota in various diseases advances, its significance in bladder cancer prognosis is becoming increasingly recognized. The unique microbial composition of the urinary tract could provide valuable insights into the course of the disease and help predict patient outcomes. Moreover, the urinary microbiota could serve as a new target for therapeutic intervention, opening up novel avenues for treatment that focus on modulating the microbial environment. These strategies, which include probiotics, are gaining popularity as potential methods to improve patient outcomes by influencing the microbial communities within the body (27, 78). Probiotics, which are live beneficial microorganisms, have the potential to restore a healthy balance between the intestinal microbiota and the urinary microbiota. By reintroducing beneficial bacteria, probiotics could help to modulate the immune system, potentially enhancing the body’s ability to fight bladder cancer (78).
Looking ahead, future research should focus on understanding how targeted modulation of the urinary microbiota could influence the development and progression of bladder cancer. By investigating the effects of probiotics and other microbiota-targeted interventions, scientists could uncover new ways to enhance cancer treatment and improve patient prognosis. Additionally, studies could explore the potential for personalized therapy based on the unique microbiota composition of each patient, offering a more tailored and effective approach to bladder cancer management. Further research is needed to explore the relationship between urinary microbiota and LUTS as well as cancer recurrence, and to establish a predictive model that includes microbial parameters, LUTS scores, and recurrence indicators. Ultimately, these innovative strategies could significantly improve treatment outcomes and the quality of life for bladder cancer patients, leading to better long-term survival rates and enhanced overall health.
5.3.3 Clinical translation challenges
Although the urinary microbiota has shown considerable promise as a potential biomarker for prognostic evaluation in bladder cancer, its successful clinical application is still hindered by a number of challenges. One of the primary obstacles is the unclear causal relationship between the urinary microbiota and the development of bladder cancer. While several studies have suggested a potential association, the exact mechanisms through which the microbiota may influence cancer progression are not well understood. To address this gap, more extensive and carefully designed prospective studies are needed to establish whether there is a direct causative link between changes in the urinary microbiota and the onset or progression of bladder cancer. Additionally, designing well-structured, large-scale clinical trials to assess the accuracy, reliability, and overall clinical utility of the urinary microbiota as a prognostic biomarker remains a critical issue. These trials must account for various factors such as patient demographics, disease stages, and treatment regimens, which could impact the microbiota. Moreover, the standardization of methods for analyzing and interpreting urinary microbiota data is essential for ensuring consistency across different studies. Overcoming these challenges will be key to unlocking the full potential of the urinary microbiota as a useful tool for predicting outcomes and guiding treatment decisions in bladder cancer patients.
6 Conclusion
In conclusion, this article reviewed the dynamic changes in the urinary microbiota of bladder cancer patients after surgery and their clinical significance. It summarized the role of different urinary microbiota in the postoperative recovery process of the disease and their impact on patient prognosis, while also discussing the challenges and limitations present in current research. It is hoped that further research on the changes in the urinary microbiota will provide new clinical insights for postoperative management of bladder cancer and lay the foundation for deeper exploration and innovative development in this field in the future.
Author contributions
YL: Writing – original draft, Writing – review & editing. PL: Writing – original draft, Writing – review & editing. RH: Data curation, Formal analysis, Methodology, Software, Visualization, Writing – review & editing. BZ: Formal analysis, Writing – review & editing. GW: Data curation, Supervision, Project administration, Funding acquisition, Writing – review & editing. JL: Conceptualization, Investigation, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Yunnan International Joint R&D Center of Key Technologies in Urological Diagnosis and Treatment (202403AP140016); Yunnan Provincial Science and Technology Department Yaoguang Zhang Expert’s Workstation (202405AF140058); Zhou Li-qun Expert Workstation of the Yunnan Provincial Department of Science and Technology (202505AF350061).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1638628/full#supplementary-material
References
1. Richters A, Aben KKH, and Kiemeney L. The global burden of urinary bladder cancer: an update. World J Urol. (2020) 38:1895–904. doi: 10.1007/s00345-019-02984-4
2. van Hoogstraten LMC, Vrieling A, van der Heijden AG, Kogevinas M, Richters A, and Kiemeney LA. Global trends in the epidemiology of bladder cancer: challenges for public health and clinical practice. Nat Rev Clin Oncol. (2023) 20:287–304. doi: 10.1038/s41571-023-00744-3
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
4. Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, and Barsouk A. Epidemiology of bladder cancer. Med Sci (Basel Switzerland). (2020) 8:15. doi: 10.3390/medsci8010015
5. Tian YQ, Yang JC, Hu JJ, Ding R, Ye DW, and Shang JW. Trends and risk factors of global incidence, mortality, and disability of genitourinary cancers from 1990 to 2019: systematic analysis for the global burden of disease study 2019. Front Public Health. (2023) 11:1119374. doi: 10.3389/fpubh.2023.1119374
6. Chen J, Rodopoulou S, Strak M, de Hoogh K, Taj T, Poulsen AH, et al. Long-term exposure to ambient air pollution and bladder cancer incidence in a pooled european cohort: the elapse project. Br J Cancer. (2022) 126:1499–507. doi: 10.1038/s41416-022-01735-4
7. Jubber I, Ong S, Bukavina L, Black PC, Compérat E, Kamat AM, et al. Epidemiology of bladder cancer in 2023: A systematic review of risk factors. Eur Urol. (2023) 84:176–90. doi: 10.1016/j.eururo.2023.03.029
8. Gaffney CD, Katims A, D’Souza N, Bjurlin MA, and Matulewicz RS. Bladder cancer carcinogens: opportunities for risk reduction. Eur Urol Focus. (2023) 9:575–8. doi: 10.1016/j.euf.2023.03.017
9. Bellamri M, Walmsley SJ, Brown C, Brandt K, Konorev D, Day A, et al. DNA damage and oxidative stress of tobacco smoke condensate in human bladder epithelial cells. Chem Res Toxicol. (2022) 35:1863–80. doi: 10.1021/acs.chemrestox.2c00153
10. Lobo N, Afferi L, Moschini M, Mostafid H, Porten S, Psutka SP, et al. Epidemiology, screening, and prevention of bladder cancer. Eur Urol Oncol. (2022) 5:628–39. doi: 10.1016/j.euo.2022.10.003
11. Nakano M, Gi M, Toyooka T, Suzuki S, Wanibuchi H, and Takebayashi T. Occupational health topics series on the effects of chemicals: epidemiological and toxicological risk assessments of ortho-toluidine for bladder cancer. J Occup Health. (2025) 67:uiaf005. doi: 10.1093/joccuh/uiaf005
12. Contieri R, Grajales V, Tan WS, Martini A, Sood A, Hensley P, et al. Impact of age >70 Years on oncological outcomes in patients with non-muscle-invasive bladder cancer treated with bacillus calmette-guérin. BJU Int. (2024) 133:63–70. doi: 10.1111/bju.16127
13. Dobruch J and Oszczudłowski M. Bladder cancer: current challenges and future directions. Med (Kaunas Lithuania). (2021) 57:749. doi: 10.3390/medicina57080749
14. Winnicka A, Brzeszczyńska J, Saluk J, and Wigner-Jeziorska P. Nanomedicine in bladder cancer therapy. Int J Mol Sci. (2024) 25:10388. doi: 10.3390/ijms251910388
15. Lopez-Beltran A, Cookson MS, Guercio BJ, and Cheng L. Advances in diagnosis and treatment of bladder cancer. BMJ (Clinical Res ed). (2024) 384:e076743. doi: 10.1136/bmj-2023-076743
16. Neuzillet Y, Audenet F, Loriot Y, Allory Y, Masson-Lecomte A, Leon P, et al. French afu cancer committee guidelines - update 2022-2024: muscle-invasive bladder cancer (Mibc). Progres en Urol. (2022) 32:1141–63. doi: 10.1016/j.purol.2022.07.145
17. Alfred Witjes J, Max Bruins H, Carrión A, Cathomas R, Compérat E, Efstathiou JA, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2023 guidelines. Eur Urol. (2024) 85:17–31. doi: 10.1016/j.eururo.2023.08.016
18. Bajic P, Wolfe AJ, and Gupta GN. The urinary microbiome: implications in bladder cancer pathogenesis and therapeutics. Urology. (2019) 126:10–5. doi: 10.1016/j.urology.2018.12.034
19. Whiteside SA, Razvi H, Dave S, Reid G, and Burton JP. The microbiome of the urinary tract–a role beyond infection. Nat Rev Urol. (2015) 12:81–90. doi: 10.1038/nrurol.2014.361
20. Zeng J, Zhang G, Chen C, Li K, Wen Y, Zhao J, et al. Alterations in urobiome in patients with bladder cancer and implications for clinical outcome: A single-institution study. Front Cell Infect Microbiol. (2020) 10:555508. doi: 10.3389/fcimb.2020.555508
21. Wu P, Zhang G, Zhao J, Chen J, Chen Y, Huang W, et al. Profiling the urinary microbiota in male patients with bladder cancer in China. Front Cell Infect Microbiol. (2018) 8:167. doi: 10.3389/fcimb.2018.00167
22. Bi H, Tian Y, Song C, Li J, Liu T, Chen Z, et al. Urinary microbiota - a potential biomarker and therapeutic target for bladder cancer. J Med Microbiol. (2019) 68:1471–8. doi: 10.1099/jmm.0.001058
23. Lou K, Chi J, Wu J, Ma J, Liu S, and Cui Y. Research progress on the microbiota in bladder cancer tumors. Front Cell Infect Microbiol. (2024) 14:1374944. doi: 10.3389/fcimb.2024.1374944
24. Shoemaker R and Kim J. Urobiome: an outlook on the metagenome of urological diseases. Invest Clin Urol. (2021) 62:611–22. doi: 10.4111/icu.20210312
25. Su X, Tao Y, Chen F, Han X, and Xue L. Trends in the global, regional, and national burden of bladder cancer from 1990 to 2021: an observational study from the global burden of disease study 2021. Sci Rep. (2025) 15:7655. doi: 10.1038/s41598-025-92033-5
26. Aragón IM, Herrera-Imbroda B, Queipo-Ortuño MI, Castillo E, Del Moral JS, Gómez-Millán J, et al. The urinary tract microbiome in health and disease. Eur Urol Focus. (2018) 4:128–38. doi: 10.1016/j.euf.2016.11.001
27. Colella M, Topi S, Palmirotta R, D’Agostino D, Charitos IA, Lovero R, et al. An overview of the microbiota of the human urinary tract in health and disease: current issues and perspectives. Life (Basel Switzerland). (2023) 13:1486. doi: 10.3390/life13071486
28. Pfail J, Drobner J, Doppalapudi K, Saraiya B, Packiam V, and Ghodoussipour S. The role of tumor and host microbiome on immunotherapy response in urologic cancers. J Cancer Immunol. (2024) 6:1–13. doi: 10.33696/cancerimmunol.6.078
29. Neugent ML, Hulyalkar NV, Nguyen VH, Zimmern PE, and De Nisco NJ. Advances in understanding the human urinary microbiome and its potential role in urinary tract infection. mBio. (2020) 11:e00218-20. doi: 10.1128/mBio.00218-20
30. Yacouba A, Tidjani Alou M, Lagier JC, Dubourg G, and Raoult D. Urinary microbiota and bladder cancer: A systematic review and a focus on uropathogens. Semin Cancer Biol. (2022) 86:875–84. doi: 10.1016/j.semcancer.2021.12.010
31. Bukavina L, Isali I, Ginwala R, Sindhani M, Calaway A, Magee D, et al. Global meta-analysis of urine microbiome: colonization of polycyclic aromatic hydrocarbon-degrading bacteria among bladder cancer patients. Eur Urol Oncol. (2023) 6:190–203. doi: 10.1016/j.euo.2023.02.004
32. Min K, Zheng CM, Kim S, Kim H, Lee M, Piao XM, et al. Differential urinary microbiome and its metabolic footprint in bladder cancer patients following bcg treatment. Int J Mol Sci. (2024) 25:11157. doi: 10.3390/ijms252011157
33. Sun JX, Xia QD, Zhong XY, Liu Z, and Wang SG. The bladder microbiome of nmibc and mibc patients revealed by 2brad-M. Front Cell Infect Microbiol. (2023) 13:1182322. doi: 10.3389/fcimb.2023.1182322
34. Sheng Z, Xu J, Wang M, Xu X, Zhu J, Zeng S, et al. The role of urinary microbiota in primary and recurrent bladder cancer: insights from a propensity score matching study. BMC Cancer. (2025) 25:468. doi: 10.1186/s12885-025-13817-6
35. Ghabousian A, Shafigh A, Tayebi S, Salehi-Pourmehr H, Mostafaei H, Lemberger U, et al. The potential role of urinary microbiota in bladder carcinogenesis: A systematic review. Urol J. (2024) 21:208–20. doi: 10.22037/uj.v20i.8036
36. Hussein AA, Elsayed AS, Durrani M, Jing Z, Iqbal U, Gomez EC, et al. Investigating the association between the urinary microbiome and bladder cancer: an exploratory study. Urol Oncol. (2021) 39:370.e9–.e19. doi: 10.1016/j.urolonc.2020.12.011
37. Kustrimovic N, Bilato G, Mortara L, and Baci D. The urinary microbiome in health and disease: relevance for bladder cancer. Int J Mol Sci. (2024) 25:1732. doi: 10.3390/ijms25031732
38. Li Q, Sun Y, Zhai K, Geng B, Dong Z, Ji L, et al. Microbiota-induced inflammatory responses in bladder tumors promote epithelial-mesenchymal transition and enhanced immune infiltration. Physiol Genomics. (2024) 56:544–54. doi: 10.1152/physiolgenomics.00032.2024
39. Huang X, Pan T, Yan L, Jin T, Zhang R, Chen B, et al. The inflammatory microenvironment and the urinary microbiome in the initiation and progression of bladder cancer. Genes Dis. (2021) 8:781–97. doi: 10.1016/j.gendis.2020.10.002
40. Chen C, Huang Z, Huang P, Li K, Zeng J, Wen Y, et al. Urogenital microbiota: potentially important determinant of pd-L1 expression in male patients with non-muscle invasive bladder cancer. BMC Microbiol. (2022) 22:7. doi: 10.1186/s12866-021-02407-8
41. Wu C, Wei X, Huang Z, Zheng Z, Zhang W, Chen J, et al. Urinary microbiome dysbiosis is associated with an inflammatory environment and perturbed fatty acids metabolism in the pathogenesis of bladder cancer. J Trans Med. (2024) 22:628. doi: 10.1186/s12967-024-05446-7
42. Mazzio EA, Smith B, and Soliman KF. Evaluation of endogenous acidic metabolic products associated with carbohydrate metabolism in tumor cells. Cell Biol Toxicol. (2010) 26:177–88. doi: 10.1007/s10565-009-9138-6
43. Gayarre Abril P, Subirá Ríos J, Muñiz Suárez L, Murillo Pérez C, Ramírez Fabián M, Hijazo Conejos JI, et al. Urinary tract infection as the main cause of admission in cystectomized patients. Actas Urol Espanolas. (2021) 45:247–56. doi: 10.1016/j.acuro.2020.10.001
44. Kim CJ, Kim KH, Song W, Lee DH, and Choi HJ. Impact of a change in duration of prophylactic antibiotics on infectious complications after radical cystectomy with a neobladder. Medicine. (2018) 97:e13196. doi: 10.1097/md.0000000000013196
45. Defidio L, Antonucci M, Castellani D, Civitella A, Esperto F, and Scarpa RM. Transurethral resection of bladder tumor: electrosurgical and laser. J Endourol. (2021) 35:S46–s51. doi: 10.1089/end.2020.1068
46. Ślusarczyk A, Ismail H, Zapała Ł, Piecha T, Zapała P, and Radziszewski P. Changes in the urinary microbiome after transurethral resection of non-muscle-invasive bladder cancer: insights from a prospective observational study. Ann Surg Oncol. (2024) 31:4773–86. doi: 10.1245/s10434-024-15198-9
47. Pederzoli F, Ferrarese R, Amato V, Locatelli I, Alchera E, Lucianò R, et al. Sex-specific alterations in the urinary and tissue microbiome in therapy-naïve urothelial bladder cancer patients. Eur Urol Oncol. (2020) 3:784–8. doi: 10.1016/j.euo.2020.04.002
48. El-Assmy A, Mahmoud O, Kamal M, Soliman W, Ashamallah A, El-Wakeel N, et al. Characterization of standard urine properties in noncomplicated orthotopic ileal neobladders: A prospective controlled study. Urology. (2016) 96:80–4. doi: 10.1016/j.urology.2016.06.047
49. Pyrgidis N, Sokolakis I, Haltmair G, and Hatzichristodoulou G. The effect of urinary diversion on renal function after cystectomy for bladder cancer: comparison between ileal conduit, orthotopic ileal neobladder, and heterotopic ileocecal pouch. World J Urol. (2022) 40:3091–7. doi: 10.1007/s00345-022-04211-z
50. Vejlgaard M, Maibom SL, Joensen UN, Moser C, and Røder A. Microbial trends in infection-related readmissions following radical cystectomy for bladder cancer. Urology. (2024) 183:134–40. doi: 10.1016/j.urology.2023.09.007
51. Rani A, Ranjan R, McGee HS, Andropolis KE, Panchal DV, Hajjiri Z, et al. Urinary microbiome of kidney transplant patients reveals dysbiosis with potential for antibiotic resistance. Trans Res. (2017) 181:59–70. doi: 10.1016/j.trsl.2016.08.008
52. Vendrell JA, Cabello-Aguilar S, Senal R, Heckendorn E, Henry S, Godreuil S, et al. Dysbiosis in human urinary microbiota may differentiate patients with a bladder cancer. Int J Mol Sci. (2024) 25:10159. doi: 10.3390/ijms251810159
53. Jirillo E, Palmirotta R, Colella M, and Santacroce L. A bird’s-eye view of the pathophysiologic role of the human urobiota in health and disease: can we modulate it? Pathophysiology. (2024) 31:52–67. doi: 10.3390/pathophysiology31010005
54. Haider M, Ladurner C, Mayr R, Tandogdu Z, Fritsche HM, Fradet V, et al. Use and duration of antibiotic prophylaxis and the rate of urinary tract infection after radical cystectomy for bladder cancer: results of a multicentric series. Urol Oncol. (2019) 37:300.e9–.e15. doi: 10.1016/j.urolonc.2019.01.017
55. Liu Y, Zhang J, Chen H, Zhang W, Ainiwaer A, Mao S, et al. Urinary microbiota signatures associated with different types of urinary diversion: A comparative study. Front Cell Infect Microbiol. (2023) 13:1302870. doi: 10.3389/fcimb.2023.1302870
56. Hussein AA, Bhat TA, Jing Z, Gomez EC, Wasay MA, Singh PK, et al. Does the urinary microbiome profile change after treatment of bladder cancer? World J Urol. (2023) 41:3593–8. doi: 10.1007/s00345-023-04627-1
57. Kim KH, Yoon HS, Yoon H, Chung WS, Sim BS, and Lee DH. Febrile urinary tract infection after radical cystectomy and ileal neobladder in patients with bladder cancer. J Korean Med Sci. (2016) 31:1100–4. doi: 10.3346/jkms.2016.31.7.1100
58. Qiu Y, Gao Y, Chen C, Xie M, Huang P, Sun Q, et al. Deciphering the influence of urinary microbiota on foxp3+ Regulatory T cell infiltration and prognosis in chinese patients with non-muscle-invasive bladder cancer. Hum Cell. (2022) 35:511–21. doi: 10.1007/s13577-021-00659-0
59. Oláh C, Váradi M, Horváth O, Nyirády P, and Szarvas T. Oncological relevance of gut and urine microbiomes. Orvosi Hetilap. (2021) 162:579–86. doi: 10.1556/650.2021.32052
60. Schneidewind L, Torabi L, Dräger DL, and Hakenberg OW. Reduction of perioperative antibiotic prophylaxis in open radical cystectomy with ileal conduit is feasible: results of a prospective clinical trial. Urol Int. (2022) 106:825–31. doi: 10.1159/000520564
61. Lu X, Jiang H, Wang D, Wang Y, Chen Q, Chen S, et al. Early warning models to predict the 90-day urinary tract infection risk after radical cystectomy and urinary diversion for patients with bladder cancer. Front Surg. (2021) 8:782029. doi: 10.3389/fsurg.2021.782029
62. Tae BS, Oh JJ, Jeong BC, and Ku JH. Catheter-associated urinary tract infections in patients who have undergone radical cystectomy for bladder cancer: A prospective randomized clinical study of two silicone catheters (Clinical benefit of antibiotic silicone material). Invest Clin Urol. (2022) 63:334–40. doi: 10.4111/icu.20210436
63. Tobia IP, Pedergrana C, Alfieri AG, Tejerizo JC, González MI, and Favre GA. Relationship between intraoperative intestinal cultures and postoperative urinary infection in radical cystectomy with ileal diversion patients. Actas Urol Espanolas. (2025) 49:501703. doi: 10.1016/j.acuroe.2025.501703
64. Lee HY, Wang JW, Juan YS, Li CC, Liu CJ, Cho SY, et al. The impact of urine microbiota in patients with lower urinary tract symptoms. Ann Clin Microbiol Antimicrob. (2021) 20:23. doi: 10.1186/s12941-021-00428-9
65. Khafagy M, Shaheed FA, and Moneim TA. Ileocaecal vs ileal neobladder after radical cystectomy in patients with bladder cancer: A comparative study. BJU Int. (2006) 97:799–804. doi: 10.1111/j.1464-410X.2006.05996.x
66. Müller G, Butea-Bocu M, Brock O, Hanske J, Pucheril D, Noldus J, et al. Association between development of metabolic acidosis and improvement of urinary continence after ileal neobladder creation. J Urol. (2020) 203:585–90. doi: 10.1097/ju.0000000000000583
67. Hautmann RE, Volkmer B, Egghart G, Frohneberg D, Gottfried HW, Gschwend J, et al. Functional outcome and complications following ileal neobladder reconstruction in male patients without tumor recurrence. More than 35 years of experience from a single center. J Urol. (2021) 205:174–82. doi: 10.1097/ju.0000000000001345
68. Qiu J, Liu J, Zhong Y, Liu W, Zhou Z, Li Y, et al. Analysis of urinary flora characteristics in urinary tumor based on 16s rrna sequence. BioMed Res Int. (2022) 2022:9368687. doi: 10.1155/2022/9368687
69. Clifford TG, Katebian B, Van Horn CM, Bazargani ST, Cai J, Miranda G, et al. Urinary tract infections following radical cystectomy and urinary diversion: A review of 1133 patients. World J Urol. (2018) 36:775–81. doi: 10.1007/s00345-018-2181-2
70. García-Rojo E, Medina-Polo J, Miranda-Utrera N, Abad-López P, Gonzalez-Padilla DA, González-Díaz A, et al. Evaluation of health care-associated infections following radical cystectomy. Actas Urol Espanolas. (2021) 45:124–31. doi: 10.1016/j.acuro.2020.06.004
71. Mai G, Chen L, Li R, Liu Q, Zhang H, and Ma Y. Common core bacterial biomarkers of bladder cancer based on multiple datasets. BioMed Res Int. (2019) 2019:4824909. doi: 10.1155/2019/4824909
72. Chorbińska J, Krajewski W, Nowak Ł, Małkiewicz B, Del Giudice F, and Szydełko T. Urinary microbiome in bladder diseases-review. Biomedicines. (2023) 11:2816. doi: 10.3390/biomedicines11102816
73. Bučević Popović V, Šitum M, Chow CT, Chan LS, Roje B, and Terzić J. The urinary microbiome associated with bladder cancer. Sci Rep. (2018) 8:12157. doi: 10.1038/s41598-018-29054-w
74. Mansour B, Monyók Á, Makra N, Gajdács M, Vadnay I, Ligeti B, et al. Bladder cancer-related microbiota: examining differences in urine and tissue samples. Sci Rep. (2020) 10:11042. doi: 10.1038/s41598-020-67443-2
75. Flaig TW, Spiess PE, Abern M, Agarwal N, Bangs R, Boorjian SA, et al. Nccn guidelines® Insights: bladder cancer, version 2.2022. J Natl Compr Cancer Netw: JNCCN. (2022) 20:866–78. doi: 10.6004/jnccn.2022.0041
76. Hrbáček J, Tláskal V, Čermák P, Hanáček V, and Zachoval R. Bladder cancer is associated with decreased urinary microbiota diversity and alterations in microbial community composition. Urol Oncol. (2023) 41:107.e15–.e22. doi: 10.1016/j.urolonc.2022.09.018
77. Chen X, Cheng Y, Tian X, Li J, Ying X, Zhao Q, et al. Urinary microbiota and metabolic signatures associated with inorganic arsenic-induced early bladder lesions. Ecotoxicol Environ Saf. (2023) 259:115010. doi: 10.1016/j.ecoenv.2023.115010
Keywords: bladder cancer, urinary microbiota, ileal neobladder reconstruction, dynamic changes, clinical significance
Citation: Lang Y, Li P, He R, Zhu B, Wang G and Li J (2025) Dynamic changes of urinary microbiota in patients with bladder cancer after surgery and its clinical significance. Front. Immunol. 16:1638628. doi: 10.3389/fimmu.2025.1638628
Received: 31 May 2025; Accepted: 11 August 2025;
Published: 01 September 2025.
Edited by:
Ramadhani Chambuso, Harvard University, United StatesReviewed by:
Zhenming Zheng, Second Affiliated Hospital of Nanchang University, ChinaSinclair Steele, Ajman University, United Arab Emirates
Copyright © 2025 Lang, Li, He, Zhu, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiongming Li, amlvbmdtaW5nbGlAYWxpeXVuLmNvbQ==; Guang Wang, d2FuZ2d1YW5na21AMTI2LmNvbQ==
†These authors have contributed equally to this work
 Ye Lang
Ye Lang Pei Li†
Pei Li† Ruixiang He
Ruixiang He