- 1Department of Biosciences and Biomedical Engineering (BSBE), Indian Institute of Technology Indore (IITI), Indore, India
- 2Division of Medical Microbiology, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa
- 3National Institute of Animal Biotechnology (NIAB), Hyderabad, India
- 4School of Life Sciences, Devi Ahilya Vishwavidyalaya (DAVV), Indore, India
Editorial on the Research Topic
Community series in targeting signalling pathways in inflammatory diseases, volume II
The Toll-interleukin-1 Receptor (TIR) domain-containing adaptor protein (TIRAP) is a critical intracellular facilitator in immune surveillance that coordinates different signaling pathways. Its utility as a bridging entity, and its versatility in binding with diverse components of Toll-like Receptor (TLR) pathway, has been the subject of several investigations (1). During signal transmission, TIRAP undergoes distinct binding mechanisms and conformational changes leading to differential binding with various intracellular mediators thereby contributing to diverse effects in immunological responses. Among its notable interactions, TIRAP engages with proteins such as MyD88, TRAF6, and IRAK-2, facilitating downstream activation of NF-κB and AP-1 (2). Hence, a convoluted mesh of protein-protein interactions forms the foundation of TIRAP signaling, which is regulated through its TIR domain (2) (Figure 1A).
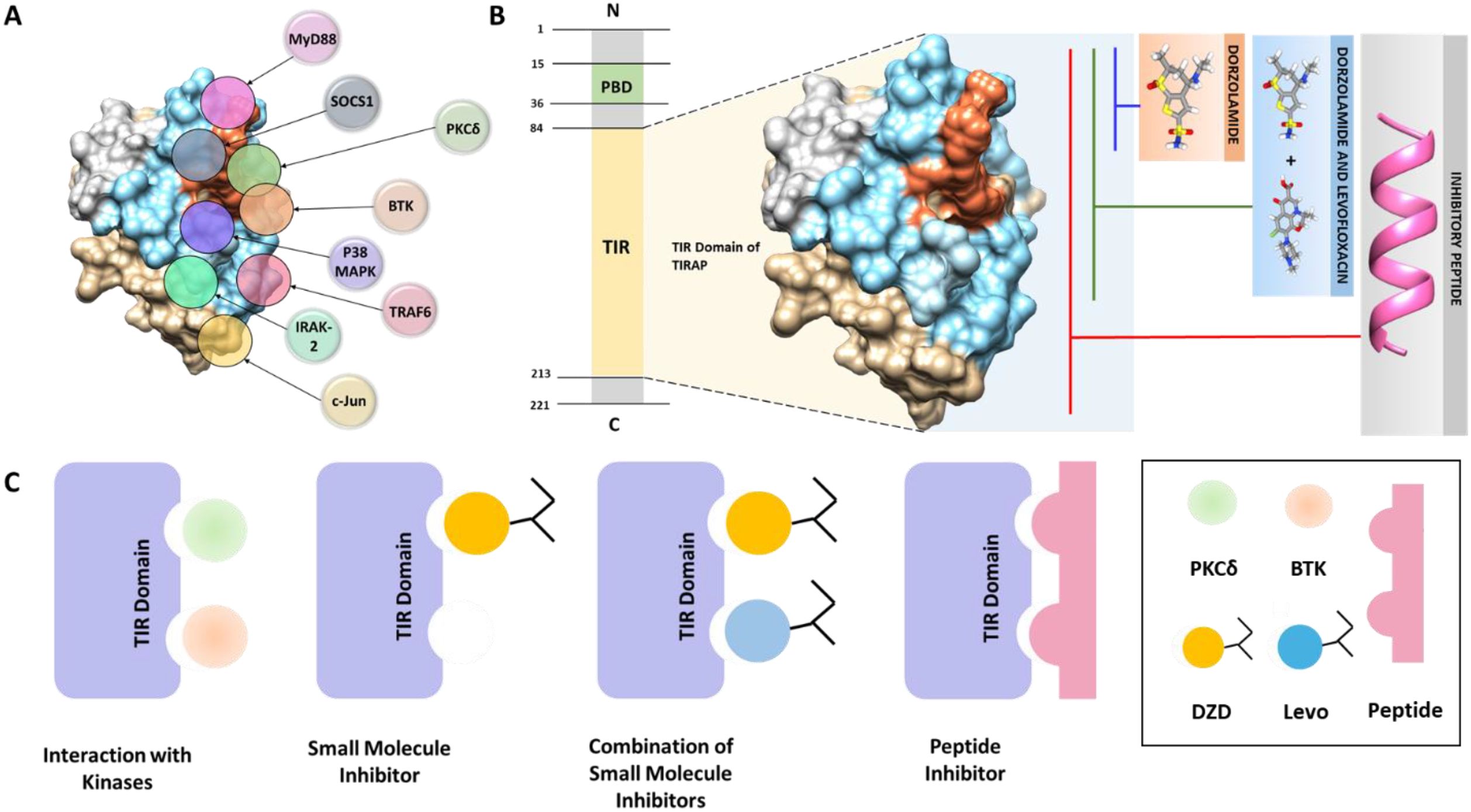
Figure 1. (A) Interaction of the TIR domain of TIRAP with various proteins involved in TLR-mediated signaling. (B) Strategies for inhibiting the TIR domain of TIRAP, including small molecules, combination therapies, and peptide-based approaches. (C) Diagrammatic representation showing inhibition of TIR domain interactions between TIRAP and BTK or PKCδ through targeted small molecules, combination treatments, or peptides.
Signaling pathways governed by TIR-domain containing proteins have emerged as a key target for the development of anti-inflammatory therapeutic strategies (3). Dimerization is a central phenomenon required for the functionality of most TIR domains, that span over 200 amino acids and harbor a 14 residues BB loop motif. TIR-mediated signaling mainly relies on the function of the conserved BB loop responsible for signal complex assembly and stabilization (4). TIRAP is a 221 amino acid long protein, which is structured into two main domains namely an N-terminal phosphatidylinositol 4,5-bisphosphate (PIP2) binding domain (PBD) and a C-terminal Toll/interleukin-1 receptor (TIR) domain. TIRAP’s positioning is mediated by Phosphatidylinositol 4-Phosphate 5-Kinase α (PIP5Kα), which generates PIP2, a crucial lipid that serves as a docking site for TIRAP (5, 6). TIRAP’s TIR domain displays structural differences in contrast to the canonic TIR domains. It comprises an extended AB loop that links αA and αB that are developed due to the absence of αB helix classically situated between βB and βC strands (3). These unique structural characteristics of TIRAP have significant implications for immune signaling, as they influence its interactions with other signaling molecules. Consequently, strategies that target and modulate TIRAP-mediated signaling pathways hold promise for the treatment of diseases associated with dysregulated TIR-driven inflammatory responses (3, 7). For example, aberrant TIRAP signaling has been implicated in the pathogenesis of rheumatoid arthritis, where it contributes to destructive inflammation by promoting cytokine production and immune cell activation within affected joints (9).
Mechanistically, TIRAP is known to be activated via a post-translation modification i.e. phosphorylation by kinases namely BTK and PKCδ. Expanding TIR domain targeting through small molecules binding key residues, dual-molecule strategies, or peptide inhibitors spanning the domain, can enhance TIRAP inhibition and disrupt inflammatory signaling (Figure 1B). Previously, Rajpoot et al. explored the TIRAP-PKCδ axis and successfully repurposed an FDA approved compound, Dorzolamide (DZD), targeting the interface residues of PKCδ on TIRAP thereby dampening the downstream inflammatory signaling (8). Though DZD attenuated the PKCδ mediated TIRAP activation, BTK-mediated phosphorylation remains an area to explore.
Recently, Baig et al. proposed a combination therapeutic approach for TIRAP-mediated chronic inflammatory septic condition. They addressed two unique aspects of sepsis progression—the destruction of bacteria and the restoration of damaged organs through homeostasis by developing a novel combination of the broad-spectrum antibiotic Levofloxacin and the repurposed anti-inflammatory medication Dorzolamide (LeDoz) (10). Unlike individual drugs that target a single kinase binding site, we discovered that Levofloxacin and DZD interacted with a section inside the binding groove (19 residues) on the TIRAP TIR domain essential for its interaction with not only PKCδ but also with BTK, which are responsible for its activation (10).
Various such alternative modalities have been investigated to silence TIRAP signaling. In one study, molecular-docking and dynamics simulations predicted that the plant alkaloid Narciclasine binds with high affinity to the TIRAP TIR domain as well as other LPS–TLR4-pathway proteins, stabilizing the complexes and thereby suppressing pro-inflammatory signaling (11). In another investigation, Phycocyanin treatment up-regulated miR-3150a-3p, miR-6883-3p and miR-627-5p, which led to depleted TIRAP transcripts and reduced cellular proliferation, thereby establishing a post-transcriptional checkpoint on adaptor availability (12). Interestingly, one study demonstrated that synthetic decoy peptides derived from the TIRAP TIR domain competitively interrupted TIRAP–MyD88 recruitment, abolishing downstream NF-κB activation and highlighting the value of peptide-based blockade of adaptor–adaptor contacts (13). Collectively, these observations indicate that small molecules and miRNA inducers curtail TIRAP through binding or expression control, whereas peptide modalities can potentially better dismantle the protein-interaction surfaces essential for signal propagation. Based on these insights, a therapeutic peptide has been proposed which targets the entire binding pocket in the TIR domain, dampening TIRAP homo-dimerization required for its functionality. THPdb (Therapeutic Peptides and Proteins Database) was screened against the TIRAP TIR domain, identifying a top candidate peptide. Following it's optimization, the peptide exhibited strong binding to TIRAP, interacting with residues 152–193, including the dimerization pocket. Additionally, its binding outside conventional pockets induced structural conformational changes, enhancing its inhibitory effect on TIRAP.
These findings highlight the potential of targeting this domain of TIRAP using small molecules, dual-molecule strategies, or peptide inhibitors as promising approaches to inhibit TIRAP function and disrupt downstream inflammatory signaling pathways. (Figure 1C). These strategies could pave the way for novel treatments for chronic inflammatory diseases, providing both structural insights and targeted interventions.
Author contributions
MB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. FS: Formal analysis, Investigation, Methodology, Writing – original draft. RA: Formal analysis, Investigation, Methodology, Writing – original draft. SP: Formal analysis, Investigation, Methodology, Writing – original draft. SF: Formal analysis, Investigation, Methodology, Writing – original draft. US: Formal analysis, Investigation, Methodology, Writing – original draft.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fitzgerald KA, Palsson-McDermott EM, Bowie AG, Jefferies CA, Mansell AS, Brady G, et al. Mal (MyD88-adapter-like) is required for Toll-like receptor-4 signal transduction. Nature. (2001) 413:78–83. doi: 10.1038/35092578
2. Rajpoot S, Wary KK, Ibbott R, Liu D, Saqib U, Thurston TLM, et al. TIRAP in the mechanism of inflammation. Front Immunol. (2021) 12:697588. doi: 10.3389/fimmu.2021.697588
3. Saqib U and Baig M. Identifying the inhibition of TIR proteins involved in TLR signalling as an anti-inflammatory strategy. SAR QSAR Environ Res. (2018) 29:295–318. doi: 10.1080/1062936X.2018.1431308
4. Gay NJ and Gangloff M. Structure and function of toll receptors and their ligands. Annu Rev Biochem. (2007) 76:141–65. doi: 10.1146/annurev.biochem.76.060305.151318
5. Nguyen TTN, Kim YM, Kim TD, Le OTT, Kim JJ, Kang HC, et al. Phosphatidylinositol 4-Phosphate 5-Kinase α facilitates toll-like receptor 4-mediated microglial inflammation through regulation of the toll/interleukin-1 receptor domain-containing adaptor protein (TIRAP) location. J Biol Chem. (2013) 288:5645–59. doi: 10.1074/jbc.M112.410126
6. Bernard NJ and O’Neill LA. Mal, more than a bridge to MyD88. IUBMB Life. (2013) 65:777–86. doi: 10.1002/iub.1201
7. Atre R, Obukhov AG, Majmudar CY, Nair K, White FA, Sharma R, et al. Dorzolamide intermediates with potential anti-inflammatory activity. Eur J Pharmacol. (2025) 987:177160. doi: 10.1016/j.ejphar.2024.177160
8. Rajpoot S, Kumar A, Gaponenko V, Thurston TLM, Mehta D, Faisal SM, et al. Dorzolamide suppresses PKCδ -TIRAP-p38 MAPK signaling axis to dampen the inflammatory response. Future Med Chem. (2023) 15:533–54. doi: 10.4155/fmc-2022-0260
9. Sacre SM, Andreakos E, Kiriakidis S, Amjadi P, Lundberg A, Giddins G, et al. The toll-like receptor adaptor proteins myD88 and Mal/TIRAP contribute to the inflammatory and destructive processes in a human model of rheumatoid arthritis. Am J Pathol. (2007) 170:518–25. doi: 10.2353/ajpath.2007.060657
11. Kingsley MK, Rao GK, and Bhat BV. Effectiveness of narciclasine in suppressing the inflammatory response in sepsis: molecular docking and in silico studies. Bioinform Biol Insights. (2024) 18:1–18. doi: 10.1177/11779322241233436
12. Hao S, Li F, Li S, Li Q, Liu Y, Yang Q, et al. miR-3150a-3p, miR-6883-3p and miR-627-5p participate in the phycocyanin-mediated growth diminishment of A549 cells, via regulating a common target toll/interleukin 1 receptor domain-containing adaptor protein. J Funct Foods. (2022) 91. doi: 10.1016/j.jff.2022.105011
Keywords: inflammation, macrophages, cytokines, anti-inflammatory drugs, TIRAP (TIR domain-containing adaptor protein), small-molecule inhibitors, peptide inhibitors
Citation: Baig MS, Siddiqi F, Atre R, Parihar SP, Faisal S and Saqib U (2025) Editorial: Community series in targeting signalling pathways in inflammatory diseases, volume II. Front. Immunol. 16:1639004. doi: 10.3389/fimmu.2025.1639004
Received: 01 June 2025; Accepted: 17 June 2025;
Published: 27 June 2025.
Edited and Reviewed by:
Pietro Ghezzi, Brighton and Sussex Medical School, United KingdomCopyright © 2025 Baig, Siddiqi, Atre, Parihar, Faisal and Saqib. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mirza S. Baig, bXNiLmlpdEBpaXRpLmFjLmlu; Uzma Saqib, dXptYXMyMDI0QGdtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Mirza S. Baig
Mirza S. Baig Faaiza Siddiqi
Faaiza Siddiqi Rajat Atre
Rajat Atre Suraj P. Parihar
Suraj P. Parihar Syed Faisal
Syed Faisal Uzma Saqib
Uzma Saqib