- 1Department of Respiratory Medicine, The Second Affiliated Hospital of Jiaxing University, Jiaxing, Zhejiang, China
- 2Department of Surgery, The Second Affiliated Hospital of Jiaxing University, Jiaxing, Zhejiang, China
Palmitoylation, a reversible post-translational modification involving the attachment of palmitic acid to cysteine residues of proteins, plays a critical role in the regulation of protein localization, stability, and function. Recent studies have revealed its significant involvement in various oncogenic processes, including tumor initiation, progression, metastasis, and immune evasion. This review comprehensively explores the molecular mechanisms of palmitoylation and its functional implications in different types of tumors. We discuss how palmitoylation modulates key signaling pathways such as Ras and Wnt/β-catenin, influencing tumor cell behavior and the tumor microenvironment. Additionally, we examine the impact of palmitoylation on anti-tumor immunity and its potential as a therapeutic target. Understanding the intricate roles of palmitoylation in cancer biology not only advances our knowledge of tumor pathogenesis but also opens new avenues for targeted cancer therapies. Future research directions and clinical applications are also highlighted to guide the development of novel interventions.
1 Introduction
Palmitoylation, a reversible post-translational lipid modification, involves the covalent attachment of the 16-carbon saturated fatty acid palmitic acid to cysteine residues on target proteins via a thioester bond (1, 2). This process is catalyzed by specific palmitoyl acyltransferases (PATs), particularly the DHHC (Asp-His-His-Cys) family, named after its conserved catalytic motif (3, 4). The reversibility of palmitoylation stems from palmitoyl-protein thioesterases (PPTs), which cleave the thioester bond, enabling proteins to cycle between palmitoylated and depalmitoylated states (5). Unlike other lipid modifications such as myristoylation or prenylation, palmitoylation is dynamic, allowing precise temporal and spatial regulation of protein function in response to cellular signals (6).
Palmitoylation profoundly influences protein localization, trafficking, stability, and function (1, 7–9). By anchoring proteins to membranes, it critically determines the subcellular distribution of diverse proteins, including receptors, kinases, G-proteins, and ion channels (10, 11). Furthermore, this modification regulates protein-protein interactions, thereby modulating the assembly of signaling complexes and intracellular signal propagation (5, 12). The dynamic nature of palmitoylation enables cells to rapidly adapt to environmental cues and maintain homeostasis, establishing it as a crucial regulatory mechanism in various physiological processes.
In cancer, palmitoylation has emerged as a key player in tumorigenesis, impacting cancer initiation, progression, metastasis, and therapy resistance. Tumorigenesis is a multifaceted process driven by genetic mutations, epigenetic alterations, and aberrant signaling pathways that promote uncontrolled cell proliferation, survival, and invasion (13–15). Recent studies highlight palmitoylation’s role in modulating oncogene and tumor suppressor activity, influencing the balance between oncogenic and tumor-suppressive signaling pathways (16, 17). For example, the Ras family GTPases—frequently mutated in various cancers—undergo palmitoylation. This modification is essential for their proper plasma membrane localization and subsequent activation of downstream pathways such as MAPK/ERK and PI3K/AKT (18, 19). Palmitoylation dysregulation can lead to aberrant Ras localization and hyperactivation, promoting oncogenic transformation and tumor progression (20). Similarly, the Wnt/β-catenin signaling pathway—central to many cancers—is also regulated by palmitoylation. Palmitoylation of Wnt proteins is essential for their secretion and interaction with cell surface receptors, modulating β-catenin activation and downstream transcriptional programs that drive proliferation and differentiation (21).
Beyond regulating oncogenic signaling, palmitoylation shapes the tumor microenvironment and modulates anti-tumor immune responses (22). Tumors exploit palmitoylation to evade immune surveillance—for instance, by altering immune checkpoint proteins or cytokine receptor function. This immune modulation contributes to immune resistance, significantly challenging the efficacy of immunotherapies such as checkpoint inhibitors (17, 23). Understanding how palmitoylation regulates these processes could yield novel insights into overcoming immune resistance and improving cancer treatments.
Given the growing recognition of palmitoylation’s role in cancer, targeting this modification represents a promising therapeutic strategy. Small molecule inhibitors of PATs have shown efficacy in preclinical models, highlighting their potential as novel anticancer agents. This review comprehensively overviews the current understanding of palmitoylation in tumor biology, exploring its molecular mechanisms, functional impacts, and therapeutic potential. We also discuss challenges and future directions in studying cancer-associated palmitoylation, emphasizing opportunities for translating these findings into clinical applications.
2 Molecular mechanism of palmitoylation
Palmitoylation, a reversible post-translational lipid modification, involves the covalent attachment of palmitic acid (a 16-carbon saturated fatty acid) to cysteine residues on target proteins via a thioester bond (24, 25). This modification is catalyzed by palmitoyl acyltransferases (PATs), with the DHHC family (defined by its conserved Asp-His-His-Cys catalytic motif) being the most studied group (9, 26). The approximately 23 human DHHC enzymes, integral membrane proteins typically localized to the Golgi apparatus, endoplasmic reticulum (ER), and plasma membrane, exhibit distinct tissue distribution, subcellular localization, and substrate specificity (Table 1) (26). At these locations, DHHC PATs palmitoylate diverse protein substrates (27).
DHHC enzymes share common structural features, including multiple transmembrane domains (typically four to six) and a cytosolic catalytic domain containing the critical DHHC motif (58, 59). Their catalytic mechanism involves two key steps: autoacylation and substrate acylation (60–62). First, the enzyme undergoes autoacylation: a cysteine residue within the DHHC motif forms a thioester bond with palmitoyl-CoA, transferring the palmitoyl group to the enzyme itself. Subsequently, the palmitoyl group is transferred from the autoacylated DHHC enzyme to a cysteine residue on the substrate protein, facilitated by the close proximity of the substrate cysteine to the enzyme’s catalytic site, resulting in substrate palmitoylation.
Substrate recognition by DHHC enzymes is highly specific and governed by multiple factors: the amino acid sequence context surrounding the target cysteine, substrate localization, palmitoylation consensus motifs, and regulation by accessory proteins (37, 63–65). Specifically, the local amino acid sequence near the target cysteine critically determines recognition. Particular DHHC enzymes preferentially recognize substrates containing specific sequence motifs, often found near transmembrane domains or lipid-binding regions. Furthermore, the subcellular localization of both the enzyme and substrate is crucial for efficient palmitoylation. For example, substrates localized to the Golgi apparatus or plasma membrane are primarily palmitoylated by DHHC enzymes residing in those compartments (27). Although no universal palmitoylation consensus sequence exists, some DHHC enzymes exhibit preferences for substrates with specific motifs or structural features, such as a hydrophobic region preceding the cysteine or proximity to other lipid modifications like myristoylation or prenylation (27, 65). Finally, the activity and substrate specificity of DHHC enzymes can be regulated by accessory proteins or cofactors, which may recruit specific substrates or modulate enzyme localization and activity.
Protein palmitoylation is a reversible process enabling dynamic regulation of protein function. This reversibility occurs through palmitoyl-protein thioesterases (PPTs), such as PPT1 and PPT2, which hydrolyze the thioester bond to remove palmitoyl groups from substrate proteins (5, 66, 67). Depalmitoylation can occur in various cellular compartments, providing a mechanism for spatiotemporal control of palmitoylation. This dynamic nature allows cells to precisely modulate proteins involved in critical processes like signal transduction, membrane trafficking, and cell-cell communication (2, 68, 69). By regulating protein palmitoylation status, cells rapidly adapt to environmental changes, fine-tune signaling pathways, and maintain homeostasis (2, 70, 71). Such regulation is particularly vital for synaptic plasticity in neurons and immune responses across cell types.
Aberrant palmitoylation is implicated in diverse diseases, including cancer, neurodegenerative disorders, and infectious diseases (1, 72, 73). Dysregulation of DHHC enzymes or PPTs can cause mislocalization or dysfunction of key regulatory proteins, thereby driving disease pathogenesis. Given palmitoylation’s critical cellular roles, targeting its regulatory enzymes represents a promising therapeutic strategy. Small-molecule inhibitors against specific DHHC enzymes show potential in preclinical models, highlighting novel avenues for anticancer drug development.
In the following sections, we will further explore the role of palmitoylation in tumor progression.
3 Role of palmitoylation in tumors
This section examines how DHHC family members regulate tumor-associated proteins through palmitoylation. As key enzymes governing this modification, DHHC proteins control the palmitoylation status of critical oncoproteins and tumor suppressors, thereby dictating their activity, localization, and stability in cancer cells. We analyze how these regulatory mechanisms contribute to tumor initiation, progression, invasion, and metastasis. Table 2 systematically summarizes current research findings on palmitoylated tumor-associated proteins, their functional roles, and associated DHHC enzymes.
3.1 Pro-oncogenic ZDHHCs
A subset of ZDHHC enzymes, such as ZDHHC3, ZDHHC5, ZDHHC9, and ZDHHC20, function as potent oncogenic amplifiers by strategically palmitoylating key effector proteins that anchor to the plasma membrane. This spatial redistribution constitutively activates Ras/MAPK, Wnt/β-catenin, and PI3K/AKT pathways—core signaling cascades implicated in tumor proliferation, invasion, and therapy resistance. Critically, their overexpression or genetic amplification correlates with advanced TNM staging, metastatic recurrence, and poor overall survival (OS). Targeting these enzymes thus represents a promising strategy to dismantle oncogenic signaling scaffolds at their membrane-centric origins, particularly in cancers with defined pathway dependencies.
IIn metastatic breast cancer, DHHC3 overexpression correlates with reduced patient survival. Elimination of DHHC3 in xenograft models reduces primary tumor growth and lung metastasis while increasing oxidative stress, cellular senescence, and recruitment of anti-tumor immune cells (e.g., macrophages, NK cells). DHHC3 depletion also downregulates the oxidative stress regulator TXNIP, indicating DHHC3 promotes tumorigenesis by modulating oxidative stress and senescence pathways (Figure 1A) (119). Notably, curcumin selectively inhibits DHHC3 auto-palmitoylation, reducing ITGβ4 palmitoylation and suppressing breast cancer invasion without broadly disrupting cysteine modifications (120). Contrastingly, zDHHC3 is downregulated in kidney renal clear cell carcinoma (KIRC), where high expression correlates with favorable prognosis. In KIRC, zDHHC3 regulates apoptosis through SLC9A2 palmitoylation and expression (Figure 1A) (121). ZDHHC5 demonstrates tissue-specific oncogenicity: In lung adenocarcinoma (LUAD), ZDHHC5 overexpression correlates with tumor progression and INCENP expression. Nuclear-localized ZDHHC5 modulates cancer stem cells (CSCs) via INCENP palmitoylation at Cys15 (122). In pancreatic cancer, ZDHHC5-mediated palmitoylation of SSTR5 enables cancer cell proliferation. The inhibitor lomitapide blocks this process, enhancing anti-tumor responses (123). In gliomas, mutant p53 cooperates with NF-Y to upregulate ZDHHC5, promoting stemness and tumorigenicity through EZH2 palmitoylation/phosphorylation (124). In NSCLC, DHHC5 silencing inhibits proliferation, colony formation, invasion, and xenograft growth without affecting normal bronchial cells (38). In triple-negative breast cancer (TNBC), ZDHHC5 palmitoylates Flotillin-1, stabilizing it to drive metastasis. Flotillin-1 palmitoylation deficiency or pharmacological inhibition suppresses tumor progression and lung metastasis (125).
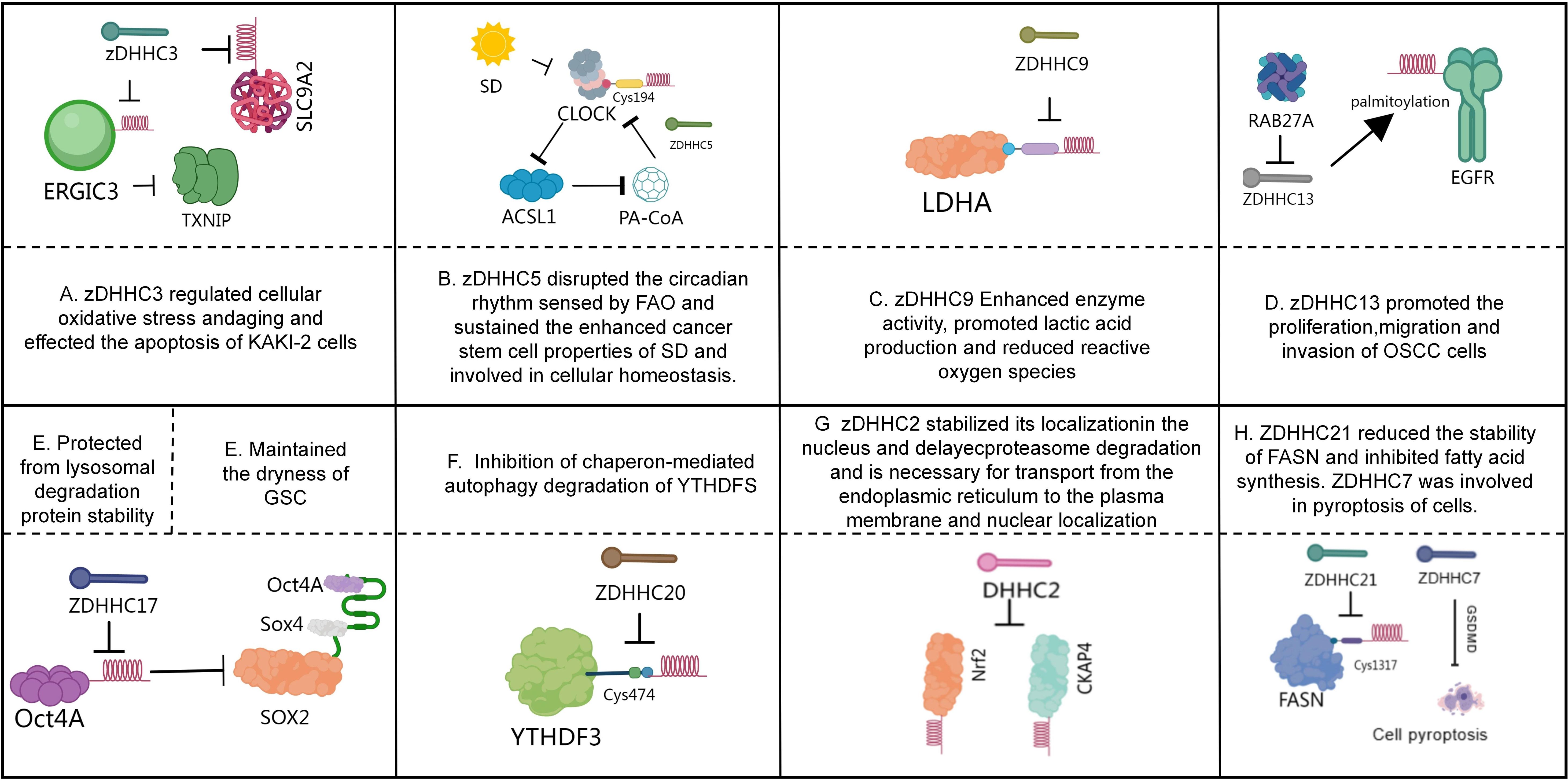
Figure 1. Role of palmitoylation in tumors. (A) The reduced DHHC3-dependent palmitoylation of ERGIC3 protein may play a crucial role in the upregulation of TXNIP (119). zDHHC3 influences the apoptosis process of Caki-2 cells by regulating the S-palmitoylation and expression levels of SLC9A2 (121). (B) CLOCK, dysregulated by SD, hyperactivates ACSL1 to produce PA-CoA, which promotes the S-palmitoylation of CLOCK-Cys194 in a ZDHHC5-dependent manner. This forms a positive feedback loop, preventing CLOCK degradation, leading to FAO-sensing circadian rhythm disruption, and maintaining SD-enhanced cancer stemness (126). (C) LDHA undergoes post-translational palmitoylation at Cysteine 163 by ZDHHC9, thereby enhancing its enzymatic activity, promoting lactate production, and reducing reactive oxygen species (ROS) production (128). (D) RAB27A may influence disease progression by regulating the palmitoylation of epidermal growth factor receptor (EGFR) mediated by zinc-finger DHHC-type 13 (ZDHHC13) (131). (E) ZDHHC17-mediated palmitoylation of Oct4A maintains GSC function by protecting Oct4A from lysosomal degradation and promoting its integration with Sox4 at the SOX2 enhancer region (132). (F) ZDHHC20 inhibits the autophagic degradation of YTHDF3 through S-palmitoylation at Cys474, leading to abnormal accumulation of MYC and promoting pancreatic cancer progression (136). (G) DHHC2 mediates the palmitoylation of Nrf2 in the cytoplasm, stabilizing its nuclear localization and delaying its proteasomal degradation (140). DHHC2-mediated palmitoylation of CKAP4 is essential for its transport from the endoplasmic reticulum to the plasma membrane and nuclear localization (142). (H) ZDHHC21 reduced the stability of FASN and inhibited fatty acid synthesis. ZDHHC7 was involved in pyroptosis of cells (145).
Interestingly, sleep deprivation (SD) disrupts circadian rhythms by increasing palmitoyl-CoA (PA-CoA) synthesis catalyzed by ACSL1. SD-induced CLOCK hyperactivation elevates PA-CoA levels via ACSL1 and promotes ZDHHC5-dependent palmitoylation of CLOCK at Cys194. This forms a positive feedback loop that stabilizes CLOCK, preventing its degradation and perpetuating circadian disruption while maintaining SD-enhanced cancer stemness. Timely β-endorphin supplementation resets circadian rhythms and suppresses Acsl1 expression, mitigating SD-driven tumorigenesis. Clinically, poor sleep quality and low serum β-endorphin correlate with tumor progression and elevated CLOCK/ACSL1 expression, suggesting β-endorphin as a potential therapeutic for SD-associated cancers (Figure 1B) (126). Additionally, DHHC5 binds and palmitoylates the auxiliary protein Golga7b, stabilizing it at the plasma membrane and blocking clathrin-mediated endocytosis. The DHHC5/Golga7b complex recruits adhesion proteins (e.g., plakophilin-3, desmoplakin-2) to desmosomes, with desmoplakin-2 localization being critically dependent on this complex. Disruption of DHHC5/Golga7b impairs cell adhesion, underscoring their essential role in intercellular cohesion (127). Collectively, these findings position DHHC5 as a novel therapeutic target in cancer, providing a mechanistic foundation for developing targeted anticancer strategies.
In non-tumor diseases, studies have shown that TNF-induced palmitoylation of RIPK1 (mediated by DHHC5 and dependent on its K63 ubiquitination) is a key mechanism that activates RIPK1 kinase activity and bypasses the cell death checkpoint, thereby inducing downstream apoptosis/necroptosis. This modification enhances the activity by promoting homophilic interactions in the kinase domain of RIPK1. Under pathological conditions such as MASH, fatty acid-driven DHHC5 increase promotes RIPK1 palmitoylation and cytotoxicity, highlighting the importance of this pathway in inflammatory diseases and providing new target ideas for therapeutic intervention (40). In addition, palmitoylation (especially the Beclin 1 modification mediated by DHHC5) plays a crucial role in regulating autophagy initiation and maintaining cellular protein homeostasis. DHHC5-mediated Beclin 1 S-palmitoylation drives the assembly and activation of the functional PI3KC3-C1 complex by promoting its hydrophobic interaction with ATG14L and VPS15. The downregulation of DHHC5 expression during aging leads to a reduction in Beclin 1 palmitoylation, becoming an important driving factor for the decline in autophagy function. This decline is manifested as protein homeostasis collapse within neurons and exacerbation of neurodegeneration in mouse models of Alzheimer’s disease, emphasizing the importance of the DHHC5-Beclin1 pathway in maintaining cellular homeostasis in aging-related diseases (especially neurodegenerative diseases), and providing potential targets for intervention (such as targeting DHHC5 or Beclin1 palmitoylation) (39).
ZDHHC9 overexpression in pancreatic cancer correlates with poor prognosis. It palmitoylates lactate dehydrogenase A (LDHA)—a key glycolytic enzyme regulating the Warburg effect—at Cys163. This modification enhances LDHA enzymatic activity, promoting lactate production and reducing reactive oxygen species (ROS). Substituting endogenous LDHA with a palmitoylation-deficient mutant suppresses pancreatic cancer proliferation, increases tumor-infiltrating T cells, limits tumor growth, and alters chemotherapy response. Notably, ZDHHC9-mediated LDHA palmitoylation is upregulated in gemcitabine-resistant pancreatic cancer (Figure 1C) (128). Additionally, ZDHHC9 palmitoylates glucose transporter GLUT1 at Cys207, maintaining its plasma membrane localization. This modification sustains glycolytic flux, proliferation, colony formation, and tumorigenesis in glioblastoma (129). ZDHHC12 mediates CLDN3 palmitoylation at three juxtamembrane cysteine residues, critical for ovarian cancer progression. Palmitoylation stabilizes CLDN3 and ensures its proper membrane localization. Loss of palmitoylation abolishes CLDN3’s oncogenic effects. Knocking down ZDHHC12 disrupts CLDN3 membrane targeting/stability and impairs ovarian cancer tumorigenicity, highlighting ZDHHC12 as a therapeutic target (130). In oral squamous cell carcinoma (OSCC), RAB27A overexpression correlates with metastasis and poor survival. RAB27A regulates ZDHHC13-mediated EGFR palmitoylation. Silencing RAB27A significantly inhibits OSCC proliferation, migration, and invasion (Figure 1D) (131).
ZDHHC17 palmitoylates Oct4A in glioblastoma stem cells (GSCs), maintaining its stability and stemness. Palmitoylation prevents Oct4A lysosomal degradation and facilitates its interaction with Sox4 at the SOX2 enhancer. Oct4A palmitoylation inhibitors effectively suppress GSC self-renewal and tumorigenicity, revealing a promising therapeutic strategy (Figure 1E) (132). Palmoylation precisely and hierarchically regulates the NLRP3 inflammasome at different activation stages by targeting multiple conserved cysteine sites on the NLRP3 protein (such as Cys130/901/958, Cys837/838, Cys419, Cys844, Cys8). In the priming stage (initiation), the palmitoylation catalyzed by DHHC1/3/5/7 (and DHHC1 catalyzing Cys958) promotes the localization of NLRP3 to the Golgi network (TGN), laying a spatial foundation for subsequent activation, and this process is negatively regulated by the thioesterase APT2. In the activation stage, the palmitoylation of Cys130 and Cys901 drives the translocation of NLRP3 to the dispersed Golgi (dTGN), while the palmitoylation mediated by DHHC5 (regulated by ABHD17A de-palmitoylation) and DHHC17-mediated palmitoylation of Cys419 enhance the initial binding of NLRP3 to NEK7’s LRR domain and the secondary binding of NACHT domain to NEK7, significantly strengthening the NLRP3-NEK7 interaction, thereby directly promoting the assembly of the inflammasome. In the termination stage, the palmitoylation catalyzed by DHHC12 (mediated by Cys844) promotes the binding of NLRP3 to the molecular chaperone HSC70, guiding its degradation through the molecular chaperone-mediated autophagy (CMA) pathway, and the palmitoylation mediated by PPT1 (regulated by Cys8 de-palmitoylation) also reduces the stability of NLRP3. Both of these processes jointly negatively regulate the duration of the inflammatory signal. Therefore, the different sites of palmitoylation precisely coordinate the subcellular localization, protein interaction, complex assembly, and protein stability of NLRP3 through spatiotemporal specificity and enzyme specificity mechanisms, ultimately achieving precise control over the activation of the inflammasome and the intensity of the inflammatory response (133). Additionally, ZDHHC17 mediates the palmitoylation of NLRP3 at the Cys419 residue. ZDHHC17 interacts with NLRP3 and facilitates its binding with NIMA-related kinase 7 (NEK7), thereby enhancing NLRP3 activity. The palmitoylation inhibitor 2-bromopalmitate can block NLRP3 palmitoylation and effectively inhibit NLRP3 activation in vitro. In a mouse colitis model, treatment with 2-bromopalmitate alleviated weight loss, improved survival, and ameliorated colonic pathological changes (134).
Epithelial ovarian cancer (EOC) utilizes the tricarboxylic acid (TCA) cycle and oxidative phosphorylation to sustain anabolic metabolism. In high-grade serous ovarian cancer (HGSOC) patient samples, ZDHHC18-mediated palmitoylation of malate dehydrogenase 2 (MDH2) at Cys138 enhances its enzymatic activity. This modification sustains mitochondrial respiration and accelerates malignant progression. Silencing MDH2 suppresses mitochondrial respiration and ovarian cancer cell proliferation, indicating that targeting ZDHHC18-mediated MDH2 palmitoylation represents a potential therapeutic strategy for EOC (135). In pancreatic ductal adenocarcinoma (PDAC), KRAS signaling upregulates ZDHHC20 expression in KPC mouse models, with its overexpression correlating with poor patient prognosis. ZDHHC20 palmitoylates YTHDF3 at Cys474, inhibiting its autophagic degradation. This leads to MYC accumulation and promotes pancreatic cancer progression. YTHDF3-derived peptide inhibitors competitively block ZDHHC20-mediated palmitoylation, downregulating MYC expression and inhibiting KRAS-mutant PDAC progression (Figure 1F) (136). Furthermore, in vivo shRNA screening identifies ZDHHC20 as essential for PDAC metastatic growth, though it does not affect in vitro proliferation or primary tumor development. This pro-metastatic function depends on tumor cell interactions with innate immunity, as evidenced by abolished effects in immunocompromised and NK-depleted models. Chemical genetic approaches have identified specific ZDHHC20 substrates that drive PDAC metastasis (137). Notably, DHHC20 inhibition sensitizes KRAS/EGFR-mutant cells—but not KRAS-wild-type cells—to the EGFR inhibitor gefitinib. This occurs through loss of EGFR palmitoylation at C-terminal cysteines. The palmitoylation-deficient mutant EGFRC1025A requires activated KRAS to confer gefitinib sensitivity. Moreover, DHHC20 inhibition overcomes resistance in EGFRT790M-mutant lung cancer cells. Combined treatment with 2-bromopalmitate and gefitinib induces cell death in gefitinib-resistant lines like NCI-H1975 (138).
3.2 Tumor-suppressive ZDHHCs
The hypermethylated gene ZDHHC1 inhibits tumor growth upon restored expression. Its substrate p53 undergoes palmitoylation at Cys135, Cys176, and Cys275—a novel modification essential for p53 nuclear translocation and tumor suppressor function. Notably, p53 recruits DNMT3A to the ZDHHC1 promoter, inducing hypermethylation and establishing an epigenetic feedback loop that inactivates p53 independently of genetic mutations (139). DHHC2 catalyzes Nrf2 palmitoylation, stabilizing its nuclear localization by inhibiting ubiquitination and delaying proteasomal degradation (Figure 1G). Inhibiting Nrf2 palmitoylation via 2-bromopalmitate (2-BP) or DHHC2 knockdown enhances Nrf2’s tumor-suppressive effects in gastric cancer (140). Separately, 2-BP inhibits colorectal cancer growth by targeting β-catenin palmitoylation (141). DHHC2 also palmitoylates cytoskeleton-associated protein 4 (CKAP4), enabling its ER-to-plasma membrane trafficking and nuclear localization (Figure 1G). CKAP4—a receptor for the antiproliferative factor (APF)—requires DHHC2-mediated palmitoylation to regulate E-cadherin, filamin, and ZO-1 expression. DHHC2 knockdown disrupts APF-mediated suppression of tumor proliferation, confirming DHHC2’s tumor suppressor role (142). zDHHC4 palmitoylates pericyte-expressed KAI1, enabling membrane localization. KAI1 induces LIF release via Src/p53 signaling and directly binds VEGF/PDGF to inhibit angiogenesis. In vivo, KAI1 supplementation suppresses tumor angiogenesis and growth (143). DHHC7 and DHHC21 palmitoylate steroid receptors (ER, PR, AR), enabling membrane localization and signaling in cancer cells. Their knockdown disrupts receptor function, highlighting their potential as therapeutic targets (144). In diffuse large B-cell lymphoma (DLBCL), ZDHHC21 acts as a tumor suppressor by palmitoylating FASN at Cys1317, reducing FASN stability and fatty acid synthesis. ZDHHC21 downregulation correlates with poor prognosis. Mahuangoside C (FDA-approved) stabilizes ZDHHC21 and suppresses DLBCL growth (Figure 1H) (145). In prostate cancer, ZDHHC7 downregulation correlates with poor outcomes. ZDHHC7 suppresses androgen receptor (AR) transcription, inhibiting proliferation and invasion. ZDHHC7 restoration reverses oncogenic phenotypes in vitro and in vivo (146). In non-tumor diseases, there are also studies reporting that DHHC7 is involved in the process of pyroptosis. Pyroptosis is a destructive and programmed form of cell death mediated by pyroptosis proteins (GSDMs), among which GSDMD plays a significant role in innate immunity and pathological processes (147). The palmitoylation mediated by DHHC7 is necessary for the cleavage of GSDMD by caspase and the activation of its active fragment GSDMD-NT, which targets the cell membrane. Subsequently, the de-palmitoylation mediated by APT2 is a key step in the oligomerization of GSDMD-NT on the membrane to form pores. This “palmitoylation-de-palmitoylation relay” mechanism precisely controls the temporal and spatial sequence of the activation of the pyroptosis execution protein GSDMD, and is crucial for the body’s resistance to infection and regulation of inflammatory responses. Disrupting this regulatory pathway will significantly affect the occurrence of pyroptosis and the outcome of infectious diseases in the body (44).
3.2.1 Depalmitoylation
Insulin-like growth factor-1 (IGF-1) signaling through IGF-1 receptor (IGF-1R) induces palmitoylation turnover of Flotillin-1 (Flot-1) at the plasma membrane, promoting cell proliferation. Mechanistically, acyl protein thioesterase-1 (APT-1) catalyzes Flot-1 depalmitoylation while zDHHC19 mediates its repalmitoylation—a cycle facilitating cervical cancer transformation. This dynamic modification prevents IGF-1R endocytosis and lysosomal degradation, causing receptor overactivation. In malignant cervical tissues, elevated FLOT1, LYPLA1, and ZDHHC19 cooperatively upregulate TIAM1 and GREM1 to induce epithelial-mesenchymal transition (EMT). Blocking this palmitoylation cycle inhibits EMT, migration, and invasion (Figure 2A) (148). In chronic lymphocytic leukemia (CLL), downregulated miR-138 and miR-424 cause APT1/2 overexpression. These key depalmitoylases significantly reduce membrane protein palmitoylation in CLL. APT1/2 directly interact with CD95, promoting its depalmitoylation and inhibiting CD95-mediated apoptosis. Restoring apoptosis through APT inhibition, miR-138/-424 supplementation, or pharmacological intervention highlights APT’s critical role in regulating CD95 death signaling (Figure 2B) (149). Hypoxia induces nitric oxide production, triggering S-nitrosylation of H-Ras at its C-terminal cysteine. This modification promotes H-Ras depalmitoylation and mislocalization. In PC12 cells, hypoxia/nitric oxide significantly reduces H-Ras palmitoylation, altering ERK phosphorylation and metabolic pathways—revealing new therapeutic opportunities (Figure 2C) (150). Naringenin (Nar), a bioactive flavonoid, exerts anticancer effects through dual estrogenic/anti-estrogenic activities. Nar induces rapid ERα depalmitoylation, dissociating it from caveolin-1 and disrupting interactions with c-Src signaling complexes. Concurrently, Nar activates p38 kinase via palmitoylation-independent ER mechanisms, collectively inhibiting cancer proliferation (Figure 2D) (151).
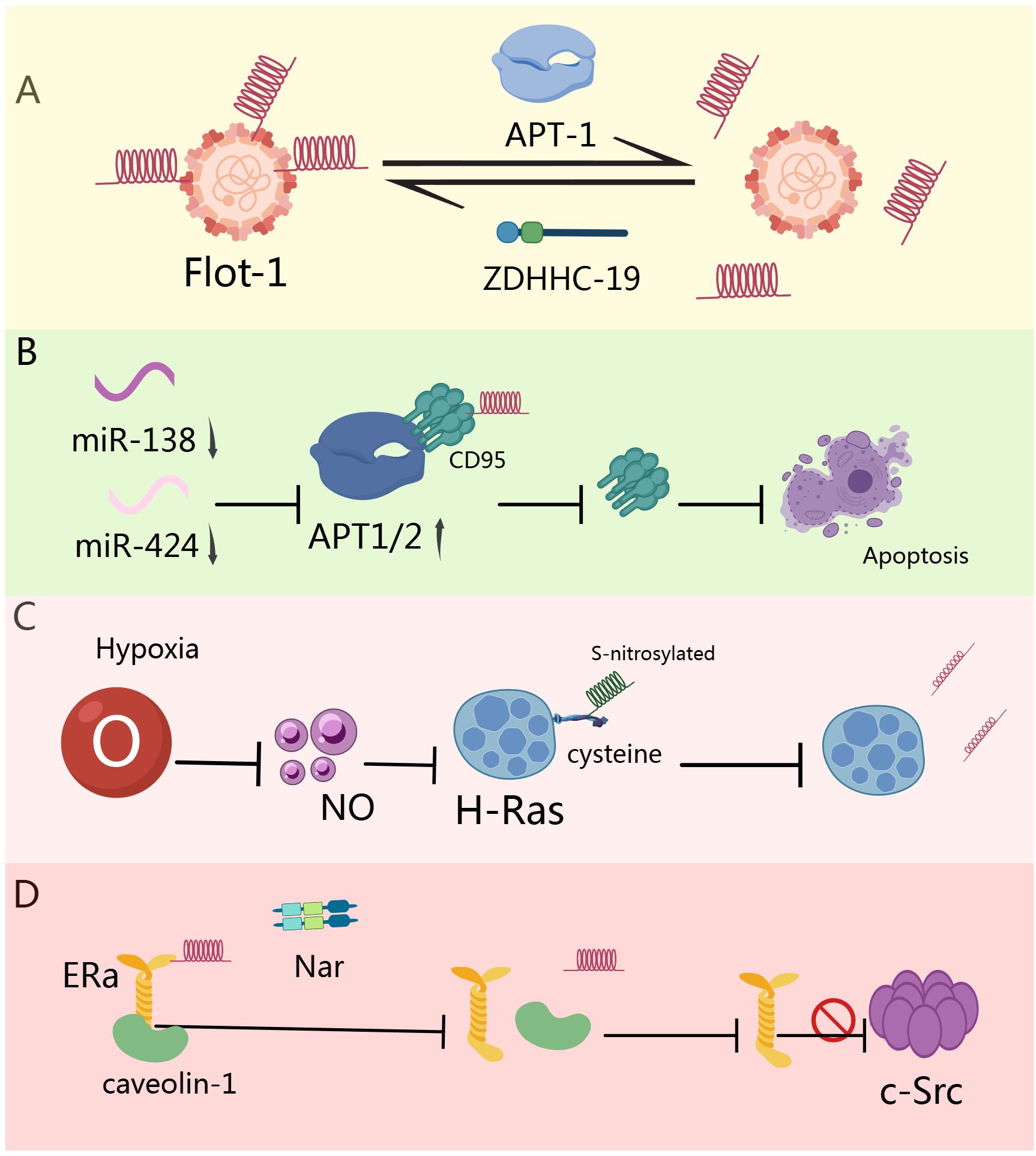
Figure 2. Role of depalmitoylation in tumors. (A) miR-138 and miR-424 are downregulated in CLL cells, leading to the overexpression of APT1 and 2. APT directly interacts with CD95, promoting its depalmitoylation, thereby inhibiting CD95-mediated apoptosis (149). (B) High concentrations of hypoxia induce nitric oxide production, resulting in the S-nitrosylation of terminal cysteine in H-Ras, which triggers H-Ras depalmitoylation and mislocalization (150). (C) Nar rapidly depalmitoylates ERα, leading to its dissociation from caveolin-1, thereby hindering the binding of ERα with adaptor proteins and signaling proteins (c-Src) involved in the mitogenic signaling cascade (151). (D) APT-1 catalyzes the depalmitoylation of Flot-1, while ZDHHC-19 catalyzes the repalmitoylation of Flot-1 after depalmitoylation, thereby promoting the transformation of cervical cancer cells (148).
4 Palmitoylation and tumor signaling pathways
4.1 Hippo-associated signaling pathways
The Hippo pathway critically regulates tissue growth, organ size, and cancer suppression. Its dysregulation drives uncontrolled proliferation and tumorigenesis, while also governing stem cell maintenance, regeneration, and development. Central to this pathway are TEAD transcription factors and their co-activators YAP/TAZ, which orchestrate gene expression programs essential for cell growth, differentiation, and organ size control. TEAD palmitoylation is indispensable for protein stability and activity, modulating core domain hydrophobicity through lipid tail extensions. Biochemical and structural studies reveal that palmitoylation occurs within conserved hydrophobic cavities in TEAD2 and TEAD3, ensuring proper protein folding and stability (153). This modification regulates TEAD protein levels and transcriptional activity, with dysregulation severely impairing function (152). When Hippo signaling is inactivated, nuclear-translocated YAP/TAZ bind palmitoylated TEAD to drive pro-growth and anti-apoptotic gene expression, promoting cancer proliferation, metastasis, and therapy resistance. Critically, TEAD palmitoylation facilitates YAP/TAZ-TEAD interactions (Figure 3A) (154). Recent studies identified JM7—a novel small-molecule inhibitor that binds TEAD’s lipid pocket, disrupts palmitoylation, and induces destabilization. JM7 suppresses YAP target gene expression, inhibits cancer cell proliferation, colony formation, and migration across multiple lines, positioning it as a promising therapeutic lead (155). Loss of TEAD palmitoylation also prevents chromatin binding, inactivating downstream Hippo target genes. Virtual screening identified potent TEAD2 palmitoylation inhibitors (ChEBML196567, ZINC000013942794) with superior binding affinity and drug-like properties (156).
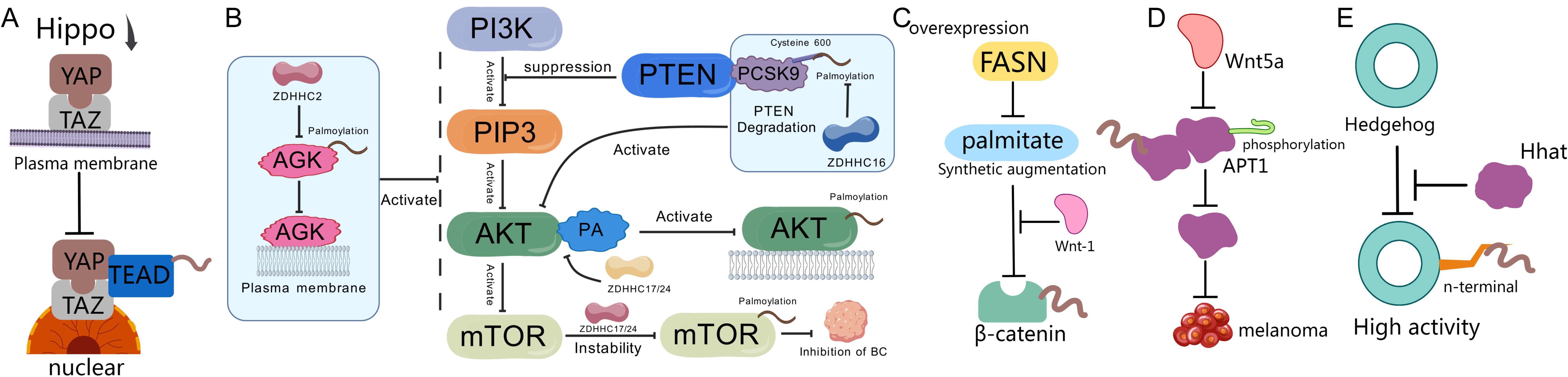
Figure 3. Palmitoylation and tumor signaling pathways. (A) When the Hippo pathway is inactivated, YAP/TAZ proteins translocate from the cytoplasm to the nucleus, where they bind to TEAD transcription factors, promoting the transcription of pro-growth and anti-apoptotic genes, thereby enhancing cancer cell proliferation, metastasis, and therapeutic resistance (154). (B) PA can promote the palmitoylation and activation of AKT via ZDHHC17/24, anchoring it to the cell membrane (160). After being palmitoylated at Cysteine 600 by ZDHHC16, PCSK9 shows increased affinity for binding to PTEN, inducing PTEN degradation and activating AKT (19). ZDHHC22 reduces mTOR stability through palmitoylation, decreasing the activation of the AKT signaling pathway, thereby inhibiting breast cancer cell proliferation both in vitro and in vivo (167). ZDHHC2 promotes AGK translocation to the plasma membrane and activates the PI3K-AKT-mTOR signaling pathway by mediating AGK S-palmitoylation, thereby modulating sensitivity to sunitinib (162). (C) Overexpression of FASN leads to increased synthesis of palmitate, which stabilizes the accumulation and activation of β-catenin in the cytoplasm through Wnt-1-mediated palmitoylation (170). (D) Wnt5a signaling increases melanoma invasiveness by promoting APT1-mediated depalmitoylation of pro-metastatic cell adhesion molecules CD44 and MCAM (172). (E) The N-terminal of Hedgehog protein requires palmitoylation by the MBOAT family multi-transmembrane enzyme Hedgehog acyltransferase (Hhat) to achieve high activity (179).
4.2 Palmitoylation-mediated cross-pathway regulation in cancer
Palmitoylation serves as a critical nexus for oncogenic signaling crosstalk, particularly between EGFR and PI3K-AKT pathways. In non-small cell lung cancer (NSCLC), EGFR palmitoylation regulates the interaction between PI3K regulatory subunit PIK3R1 (p85) and EGFR, enhancing recruitment of PI3K heterodimers to the plasma membrane and amplifying downstream AKT activation. Knocking down palmitoyltransferase DHHC20 or expressing palmitoylation-resistant EGFR mutants reduces PI3K/MYC signaling and cell proliferation (157). Therapeutically, targeting this axis improves EGFR tyrosine kinase inhibitor (TKI) sensitivity: DHHC20 inhibition increases cancer cell dependence on EGFR signaling, sensitizing tumors to TKIs (158). This cross-pathway crosstalk drives treatment resistance. In TKI-resistant NSCLC, palmitoylation sustains kinase-inactive EGFR dimerization, maintaining persistent signaling. Disrupting palmitoylation (via cysteine mutations or DHHC20 inhibition) eliminates aberrant dimerization and overcomes resistance (159). Similarly, in hepatocellular carcinoma (HCC), PCSK9 palmitoylation at Cys600 by ZDHHC16 enhances PTEN degradation, activating AKT-S473 phosphorylation and conferring sorafenib resistance (19). Clinically relevant solutions: PCSK9-derived peptides competitively inhibit palmitoylation, suppress AKT activation, and restore sorafenib efficacy (19). Beyond EGFR crosstalk, palmitoylation directly regulates AKT activation. High-fat-diet-induced HCC promotes palmitic acid (PA)-driven AKT palmitoylation via ZDHHC17/24, anchoring AKT to membranes in a PIP3-independent manner and preventing inactive polymerization (160). Therapeutic interventions: Orlistat (FASN inhibitor) limits PA synthesis, reducing AKT palmitoylation and suppressing liver tumors (160, 161). In clear cell renal cell carcinoma (ccRCC), ZDHHC2-mediated S-palmitoylation of AGK activates PI3K-AKT-mTOR signaling, driving sunitinib resistance (162). Palmitoylation critically modulates EGFR stability and trafficking. In colorectal cancer, lipid-rich microenvironments upregulate FASN, enhancing EGFR palmitoylation and plasma membrane stabilization (163). Conversely, CD82 palmitoylation at Cys5/Cys74 promotes EGFR internalization in breast cancer (164). Notably, Orlistat inhibits FASN, inducing EGFR ubiquitination and eliminating oncogenic signaling in NSCLC (161). Cross-pathway integration extends to transcriptional regulation. In EGFR-activated cancers, AKT phosphorylates TSPAN8 (Ser129), enabling its palmitoylation/cholesterol-dependent nuclear translocation with 14-3-3θ/importin-β. Nuclear TSPAN8 stabilizes STAT3 chromatin binding, upregulating MYC/BCL2/MMP9 and driving aggressiveness (165). Therapeutic relevance: Targeting the EGFR-AKT-TSPAN8-STAT3 axis may benefit refractory cancers. Combination therapies show synergy: Co-inhibition of TEAD palmitoylation and AKT induces cancer cell death (166). Palmitoyltransferase inhibitors (e.g., against ZDHHC16 or DHHC20) sensitize tumors to targeted therapies (19, 158). Metabolic interventions (Orlistat, FASN blockers) disrupt palmitate supply for oncogenic palmitoylation (160, 161). Palmitoylation-resistant mutants provide templates for peptide-based therapeutics (19, 158). Furthermore, there are studies indicating that in breast cancer cases, ZDHHC22 palmitoylates mTOR, suppressing AKT signaling and restoring tamoxifen sensitivity (167). Prostate cancer progression involves Cav-1 palmitoylation, which modulates Src/AKT/EGFR interactions (168). DKK1-induced CKAP4/LRP6 de-palmitoylation relocates them to non-DRM membranes via PI3K-AKT activation (169). We summarize palmitoylation-mediated crosstalk in the PI3K/PTEN/AKT/mTOR pathway in Figure 3B.
4.3 Wnt-associated signaling pathways
In prostate cancer, FASN overexpression increases palmitate ester synthesis and stabilizes β-catenin accumulation through Wnt-1-mediated palmitoylation, driving oncogenesis (Figure 3C) (170). The novel PORCN inhibitor WHN-88—featuring a unique diiodopyridinone structure—effectively blocks Wnt ligand palmitoylation, inhibiting their secretion and downstream signaling to provide a therapeutic strategy for Wnt-driven cancers (171). In melanoma, Wnt5a signaling phosphorylates APT1, enhancing its depalmitoylation activity while reducing dimerization. This APT1 phosphorylation promotes melanoma invasion in vitro and correlates with advanced tumor grade and metastasis, suggesting APT1 inhibition as a therapeutic strategy for Wnt5a-driven cancers (Figure 3D) (172). Wnt5a further regulates cell polarity by depalmitoylating melanoma cell adhesion molecule (MCAM) at Cys590. Mutation of Cys590 to glycine mimics Wnt5a-induced MCAM polarity. APT1 inhibition blocks Wnt5a-mediated MCAM depalmitoylation, asymmetric localization, and invasion. Direct manipulation of basal palmitoylation mechanisms enhances invasion, while cancer-associated palmitoyltransferase mutations reduce MCAM palmitoylation and weaken its invasion-suppressing function. These findings establish Wnt5a-induced depalmitoylation as critical for protein polarity and invasion (173). APT1-mediated depalmitoylation also maintains dynamics of Notch/Wnt proteins, gene expression, and asymmetric cell division (174).
Porcupine (PORCN), a membrane-bound O-acyltransferase, is essential for Wnt palmitoylation, secretion, and bioactivity. Studies evaluating the PORCN inhibitor Wnt-C59 (C59) demonstrate its nanomolar in vitro efficacy and oral bioavailability in mice. C59 inhibits mammary tumor development in MMTV-WNT1 transgenic mice by downregulating Wnt/β-catenin targets without significant toxicity (175). Wnt signaling inhibitors (IWPs) antagonize Wnt pathways by blocking PORCN-mediated palmitoylation. IWPs are ATP-competitive inhibitors of wild-type CK1δ and M82FCK1δ, with IWP-2 showing specificity among 320 kinases and broad anticancer activity. Improved IWP-derived CK1 inhibitors suggest effects beyond PORCN to CK1δ/ϵ pathways (175). In colorectal cancer, Wnt secretion requires PORCN palmitoylation. PORCN inhibitor IWP2 blocks epithelial transition in mesenchymal LIM1863-Mph cells, upregulating Wnt genes—particularly Wnt2B. Recombinant Wnt2B overcomes IWP2 inhibition by collaborating with Frizzled7 to mediate mesenchymal-epithelial transition (MET) (176). Scaffold hybridization strategies yielded lead compound 62, exhibiting sub-nanomolar Wnt inhibition (IC50 = 0.11 nM) in reporter assays. Compound 62 suppresses Wnt protein secretion, confirming direct PORCN targeting, while demonstrating favorable chemical and metabolic stability—supporting further development of potent Wnt inhibitors (177).
4.4 Hedgehog-associated signaling pathways
Research demonstrates that Hedgehog family protein overexpression critically drives oncogenesis in multiple cancers. Functional Sonic Hedgehog (Shh) signaling requires N-terminal palmitoylation catalyzed by Hedgehog acyltransferase (Hhat). To precisely quantify this process, a novel Microfluidic Mobility Shift Assay (MSA) was developed. MSA enables real-time quantitative analysis of palmitoylated Shh, facilitating studies of Hhat catalytic mechanisms, kinetics, and small-molecule inhibitor efficacy. This technique overcomes limitations of traditional methods by providing direct measurement of lipid modifications (Figure 3E) (178).
Hedgehog proteins require N-terminal palmitoylation by the MBOAT-family multipass transmembrane enzyme Hhat for full activity. In PDAC cell line PANC-1 and transfected HEK293a cells, Hhat localizes to the endoplasmic reticulum (ER). Hhat is essential for Shh palmitoylation, formation of high-molecular-weight extracellular complexes, and functional activity. Hhat knockout inhibits autocrine/juxtacrine Hh signaling and suppresses PDAC cell growth and invasion in vitro. In Shh-expressing HEK293a and A549 NSCLC cells, Hhat knockdown impairs juxtacrine/paracrine signaling to C3H10T1/2 and Shh-Light2 reporter cells, underscoring Hhat’s critical role in Hh-dependent tumorigenesis (179).
Hedgehog protein maturation involves N-terminal palmitate addition and C-terminal cholesterol modification—both essential for function and localization. Structural studies reveal HHAT possesses 10 transmembrane domains with two reentrant loops, positioning key His/Asp residues across the ER membrane. HHAT undergoes palmitoylation at multiple cytoplasmic cysteines, crucial for its membrane stability. Critically, mutations in conserved catalytic-domain His residues abolish HHAT’s ability to palmitoylate Hedgehog proteins, revealing new insights into its intracellular mechanism (180).
4.5 Others
Beyond classical pathways, palmitoylation regulates additional oncogenic signaling cascades.
The small GTPase RAB27B activates NRAS signaling by facilitating NRAS palmitoylation and membrane trafficking. RAB27B overexpression in myeloid malignancies with CBL or JAK2 mutations correlates with poor acute myeloid leukemia (AML) prognosis. RAB27B loss inhibits growth in CBL-deficient or NRAS-mutant cell lines. In vivo, Rab27b deletion abrogates mutation-driven progenitor expansion, ERK signaling, and NRAS palmitoylation, suppressing myelomonocytic leukemogenesis. Mechanistically, RAB27B governs NRAS palmitoylation via ZDHHC9 interaction, thereby modulating c-RAF/MEK/ERK signaling to impact leukemia progression. Critically, RAB27B depletion suppresses oncogenic NRAS signaling and leukemic growth in primary human AML, while RAB27B expression predicts MEK inhibitor sensitivity (181).
Baicalin modulates PLSCR1 and N-RAS palmitoylation in primary AML cells, promoting their nuclear translocation or Golgi trafficking. These alterations inactivate the N-RAS/RAF1 pathway (182). In hypopharyngeal squamous cell carcinoma (HPSCC), elevated DHHC9/DHHC15 expression drives tumorigenesis. The palmitoylation inhibitor 2-bromopalmitate (2BP) reduces proliferation, invasion, and migration without inducing apoptosis. 2BP decreases Ras palmitoylation, membrane localization, and FGF/ERK signaling (183).
In hepatocellular carcinoma (HCC), ZDHHC7 overexpression correlates with poor outcomes. DHHC7 mediates reversible STAT3 palmitoylation at Cys108, enhancing its transcriptional activity. Palmitoylated STAT3 upregulates HIF1A, increasing HIF1α protein. DHHC7 inhibition reduces STAT3 palmitoylation and HIF1α abundance. Cyclin-dependent kinase 5 (CDK5) stabilizes HIF1α, promoting ZDHHC7 expression and forming a DHHC7-STAT3-HIF1α positive feedback loop. Disrupting this axis suppresses HCC growth in vivo (184).
In glioblastoma stem cells (GSCs), local anesthetics suppress IL-6/STAT3 signaling by reducing ZDHHC15 transcription, GP130 palmitoylation, and membrane localization (52). In neuropathic cancer pain (NCP), spinal dorsal horn astrocyte activation progresses with pain severity, upregulating palmitoyltransferase ZDHHC23 and GFAP palmitoylation. This enhances secretion of CXCL-10, IL-6, and GM-CSF, further activating astrocytes via STAT3 signaling (185).
5 Palmitoylation and anti-tumor immunity
In the tumor microenvironment (TME), metabolic reprogramming of tumor-associated macrophages (TAMs) serves as a key driver of immunosuppression. Studies demonstrate that in hepatocellular carcinoma (HCC), TAMs regulate the activity of serine palmitoyltransferase (SPT) via the kinase NEK2, thereby promoting the biosynthesis of sphingosine-1-phosphate (S1P). S1P acts as an immunosuppressive factor derived from TAMs, accelerating tumor progression and conferring resistance to immunotherapy by promoting the expansion of regulatory T cells (Tregs) while suppressing effector T cell function. Targeting NEK2 or S1P reverses the immunosuppressive phenotype of TAMs and enhances the efficacy of immune checkpoint blockade therapy (186). Furthermore, palmitoylated lipopeptides (e.g., the di-palmitoylated peptide Pam2IDG) promote dendritic cell maturation through activation of TLR2/6 signaling; however, their antitumor efficacy is hampered by TAM-mediated suppression. Depletion of TAMs or blockade of IL-10 and COX-2 significantly augments the immunotherapeutic effect of palmitoylated lipopeptides (187). The accumulation of myeloid-derived suppressor cells (MDSCs) represents a critical mechanism for tumor immune evasion. In acute myeloid leukemia (AML), tumor-derived extracellular vesicles (EVs) activate TLR2 signaling on monocytes via their surface palmitoylated proteins, inducing their differentiation into immunosuppressive CD14+HLA-DRlow MDSCs. This process is dependent on Akt/mTOR pathway activation and glycolytic metabolic reprogramming, accompanied by upregulation of immunosuppressive factors such as IDO and S100A8/9. Targeting protein palmitoylation in AML cells blocks EV-mediated MDSC differentiation and restores antitumor immune responses (188). In pancreatic ductal adenocarcinoma (PDAC), the palmitoyltransferase ZDHHC20 promotes metastatic outgrowth by palmitoylating unidentified substrate(s), an effect dependent on NK cell-mediated immune evasion mechanisms (137). Additionally, chemotherapeutic agents (e.g., cisplatin) induce tumor release of oxidized phospholipids (oxPAPC), which recruit MDSCs via palmitoylation-dependent MCP-1/CCL2 and LTB4/LTB4R pathways, ultimately leading to chemoresistance (189). Palmitoylation directly suppresses NK cell function by regulating immune checkpoint molecules and metabolites. TIM-3, a key inhibitory receptor on NK cells, undergoes palmitoylation mediated by DHHC9 at cysteine residue 296 (Cys296). This modification prevents binding of the E3 ubiquitin ligase HRD1, thereby inhibiting TIM-3 ubiquitination and degradation, consequently promoting NK cell exhaustion. Targeting DHHC9 or employing palmitoylation inhibitors accelerates TIM-3 degradation and restores the antitumor activity of NK cells (190). In breast cancer, ablation of the palmitoyltransferase DHHC3 enhances NK cell-mediated tumor clearance by inducing tumor cell oxidative stress and senescence (119). Furthermore, the accumulation of long-chain acylcarnitines (e.g., palmitoyl-carnitine) in the HCC microenvironment indirectly impairs NK cell immunosurveillance by inducing iNKT cell senescence and diminishing their cytotoxicity (191). Clinical translation strategies targeting these mechanisms have been proposed. PPT1 inhibitors (e.g., GNS561) activate the cGAS-STING pathway to induce type I interferon secretion, promote macrophage polarization towards the M1 phenotype, reduce MDSC infiltration, and enhance the efficacy of anti-PD-1 therapy (192, 193). DHHC3/DHHC9 inhibitors hold potential for restoring T cell and NK cell function, warranting exploration in clinical trials combined with immune checkpoint blockade (119, 190). Targeting lipid metabolism (e.g., clearance of palmitoyl-carnitine) or combining with IL-2 may reverse iNKT cell senescence and augment NK cell activity (191, 194).
Palmitoylation critically regulates anti-tumor immunity and tumor progression. Programmed Death-Ligand 1 (PD-L1) undergoes intracellular storage and membrane redistribution, diminishing checkpoint blockade efficacy. Cytoplasmic domain palmitoylation stabilizes PD-L1 by blocking ubiquitination and lysosomal degradation (Figure 4A). ZDHHC3 is the primary palmitoyltransferase for PD-L1 modification. Inhibiting PD-L1 palmitoylation via 2-bromopalmitate or DHHC3 silencing activates anti-tumor immunity in vitro and in vivo. PD-L1 palmitoylation competitive inhibitors reduce tumor PD-L1 expression and enhance T-cell immunity (17). Parallelly, specific DHHC enzymes palmitoylate PD-1, enhancing its stability by preventing lysosomal degradation. This activates mTOR signaling and promotes tumor proliferation (Figure 4A) (195). Targeting epigenetic regulators enhances anti-PD-1 immunotherapy by activating Type I interferon (IFN-I) responses. CPT1A recruits ER-localized ZDHHC4 to palmitoylate MAVS at Cys79, stabilizing it through altered ubiquitination (inhibiting K48-linked, promoting K63-linked). Increased CPT1A amplifies MAVS palmitoylation and IFN-I responses, improving viral control and anti-tumor immunity. CPT1A inducers enhance epigenetic therapy combined with PD-1 blockade in refractory tumors (Figure 4B) (196). In HCC, FASN inhibition reduces MHC-I palmitoylation, preventing lysosomal degradation and increasing MHC-I expression (Figure 4A). This enhances antigen presentation and CD8+ T-cell activation. DHHC3 directly binds and negatively regulates MHC-I. FASN deficiency promotes CD8+ T-cell infiltration and tumor killing. Combining FASN inhibitors (orlistat/TVB-2640) with anti-PD-L1 antibodies suppresses tumor growth (197). In chemoresistant bladder cancer, FASN inhibition suppresses palmitoylated PD-L1 expression, suggesting targeted therapy potential (Figure 4A) (198). Additionally, nanoparticle-based PD-L1 inhibitors (FRS) with fluoralkylated competitive peptides disrupt endogenous PD-L1. FRS combined with doxorubicin reduces PD-L1 abundance and induces immunogenic cell death in colon cancer models (199). In pancreatic cancer, ZDHHC9 overexpression correlates with impaired immunity. ZDHHC9 knockdown converts “cold” to “hot” tumor microenvironments, inhibiting progression and extending survival. ZDHHC9 deficiency sensitizes tumors to anti-PD-L1 therapy via CD8+ T-cell involvement (200). Colorectal cancers resistant to immunotherapy show disrupted IFN/MHC signaling. Optineurin maintains pathway integrity by blocking IFNGR1 palmitoylation (Cys122)-dependent lysosomal sorting via AP3D1. Targeting IFNGR1 palmitoylation stabilizes IFNGR1, enhances tumor immunity, and sensitizes to checkpoint therapy (Figure 4A) (23). In AML, lipid transporter CD36 promotes immune evasion. CD36 senses OxLDL, initiating TLR4-LYN-MYD88-NF-κB signaling and enhancing CD36 palmitoylation via exogenous palmitate transfer. This activates MYD88-mediated pathways, and NF-κB induces immunosuppressive genes. High-fat diets or decitabine treatment amplify CD36-mediated immune suppression. Statins enhance decitabine efficacy by countering CD36-driven immunosuppression (201).
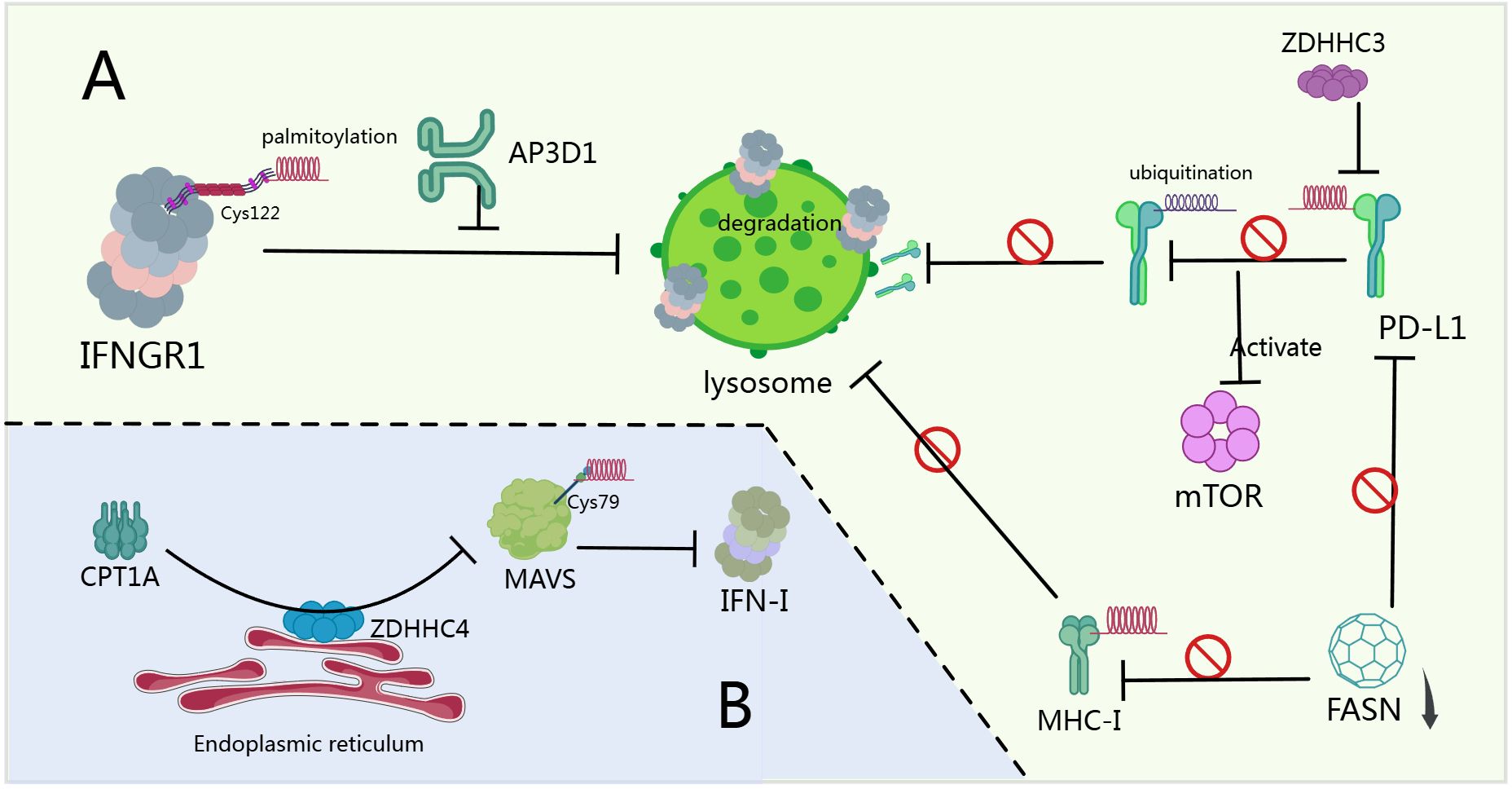
Figure 4. Palmitoylation and anti-tumor immunity. (A) After IFNGR1 is S-palmitoylated at Cys122, it is sorted by AP3D1 to the lysosome for degradation (23). ZDHHC3 mediates the palmitoylation of PD-L1 in its cytoplasmic domain, and this modification stabilizes PD-L1 by blocking its ubiquitination, thereby preventing lysosomal degradation. Palmitoylation of PD-1 promotes the activation of the mTOR signaling pathway and tumor cell proliferation (17, 195). FASN inhibition reduces MHC-I palmitoylation, preventing its lysosomal degradation (197). Pharmacological inhibition of FASN effectively suppresses PD-L1 palmitoylation and expression (198). (B) CPT1A recruits endoplasmic reticulum-localized ZDHHC4 to catalyze the palmitoylation of MAVS at Cys79, thereby stabilizing and activating MAVS. Enhanced palmitoylation of MAVS amplifies the IFN-I response (196).
Palmitoylation, as a dynamic and reversible post-translational modification, directly drives immunotherapy resistance across diverse cancers by regulating the stability, subcellular localization, and functional activity of key immune signaling proteins. Previous studies indicate that palmitoylation can disrupt the integrity of antitumor immune signaling pathways to modulate therapeutic efficacy. In colorectal cancer, optineurin deficiency promotes AP3D1-mediated lysosomal sorting and degradation of S-palmitoylated IFNGR1 (at Cys122), thereby blocking IFNγ signal transduction. Degradation of IFNGR1 directly suppresses MHC-I expression and impairs CD8+ T cell recognition capacity, ultimately leading to immune evasion. Pharmacological inhibition of IFNGR1 palmitoylation stabilizes IFNGR1, restores T cell function, and reverses treatment resistance (23). Additionally, acute myeloid leukemia (AML) cells uptake exogenous palmitate via CD36, activating ZDHHC6-mediated MYD88 palmitoylation to potentiate the TLR4-LYN-MYD88-NF-κB signaling axis. NF-κB subsequently drives the expression of immunosuppressive genes (e.g., IL-10, TGF-β), directly inhibiting CD8+ T cell activity. High-fat diet or decitabine treatment exacerbates AML immunosuppression through this mechanism, while statins can reverse resistance by blocking CD36-mediated signaling (201). Evidence further demonstrates that palmitoylation maintains the stability of immune checkpoint proteins. In tumor cells, DHHC3 (ZDHHC3) catalyzes PD-L1 palmitoylation to facilitate its plasma membrane localization and prevent lysosomal degradation. The chimera degrader cp-PCCs (e.g., PCC16) targeting DHHC3 effectively reduces PD-L1 levels and significantly suppresses tumor growth in immunotherapy-resistant models (202, 203). Disrupting PD-L1 palmitoylation with 2-bromopalmitate (2-BP) delivered via a chemotherapeutic co-loaded nanocarrier simultaneously eliminates cell-surface and exosomal PD-L1, overcoming therapeutic resistance (203). Moreover, ZDHHC9 overexpression promotes an immunosuppressive ‘cold tumor’ microenvironment. Its ablation enhances CD8+ T cell infiltration to establish ‘hot’ tumors, significantly improving anti-PD-L1 efficacy. This strategy is validated by ZDHHC9-silencing siRNA nanoparticles (200). Palmitoylation also enhances tumor cell resistance to ferroptosis. ZDHHC8-catalyzed palmitoylation of GPX4 augments its enzymatic activity and stability, protecting tumor cells from ferroptosis. Disruption of this modification restores lipid peroxidation sensitivity and promotes CD8+ T cell-induced ferroptotic death (204). In lung cancer stem cells (CSCs), CPT1A forms a positive feedback loop by inhibiting c-Myc ubiquitination and degradation, which activates the NRF2/GPX4 antioxidant pathway and downregulates ACSL4 to reduce polyunsaturated fatty acid (PUFA) accumulation, thereby conferring ferroptosis resistance. Targeting CPT1A enhances antitumor efficacy of immune checkpoint blockade (205, 206). Palmitoylation contributes to metabolism-driven T cell exhaustion. In hepatocellular carcinoma (HCC), Riplet deficiency stabilizes fatty acid synthase (FASN), increasing secretion of palmitic acid (PA/C16:0). PA induces terminal CD8+ T cell exhaustion by enhancing STAT3 palmitoylation, driving anti-PD-1 resistance. FASN inhibitors reverse T cell exhaustion and overcome this resistance (207). Tumor-associated macrophages (TAMs) in HCC employ NEK2 to regulate palmitoylation-associated sphingolipid metabolism, promoting S1P production. S1P confers immunotherapy resistance by activating regulatory T cells (Tregs) and suppressing effector T cell function. Targeting the NEK2/S1P axis restores immune responses (186). High PPT1 expression correlates with poor prognosis in HCC. Its inhibitor DC661 enhances CD8+ T cell activity by inhibiting palmitoylation-dependent autophagy pathways, reversing resistance to both sorafenib and immunotherapy (208). In summary, palmitoylation drives immunotherapy resistance through multifaceted mechanisms: direct modification of immune signaling proteins, stabilization of immune checkpoints, enhancement of anti-ferroptotic defenses, and remodeling of the metabolic microenvironment. These mechanisms provide actionable pathways for rational therapeutic combinations. Disrupting the palmitoylation regulatory network represents a promising precision strategy to overcome immunotherapy resistance.
6 Clinical applications
This section examines the clinical translational potential and future research trajectories of targeting protein palmitoylation in oncology. Palmitoylation represents a promising therapeutic frontier due to its fundamental role in tumor pathogenesis and immune regulation. We synthesize current advances in palmitoyltransferase inhibitors and depalmitoylase modulators, emphasizing their capacity to potentiate treatment efficacy and circumvent therapeutic resistance. Furthermore, we delineate emerging directions—including novel small-molecule discovery, biomarker-driven patient stratification, and rational combination strategies—that may optimize clinical outcomes in precision cancer medicine.
Current research explores diverse palmitoylation-targeting inhibitors—including 2-bromopalmitate (2-BP) and TEAD inhibitors—demonstrating significant therapeutic potential. 2-BP irreversibly inhibits palmitoyltransferase activity across all DHHC proteins (209). Building on earlier discussions of 2-BP’s anticancer properties, recent work in triple-negative breast cancer (TNBC) developed AFT/2-BP@PLGA@MD: an advanced biomimetic nanoplatform integrating targeted therapy and immunotherapy. This system encapsulates the palmitoylation inhibitor 2-BP within poly(lactic-co-glycolic acid) (PLGA) nanoparticles coated with tumor-derived membranes (MD), simultaneously enhancing afatinib’s (AFT) therapeutic efficacy against TNBC cells while blocking PD-1/PD-L1 checkpoint signaling. In vitro, 2-BP potentiates AFT’s suppression of tumor cell proliferation and migration. In murine models, AFT/2-BP@PLGA@MD nanoparticles significantly inhibit 4T1 tumor growth/metastasis, extend survival, and activate antitumor immunity—offering new therapeutic avenues for refractory TNBC (210).
The Hippo pathway critically drives tumor growth. Genetic ablation of YAP/TAZ combined with novel TEAD palmitoylation inhibitors significantly blocks and reverses schwannoma progression in vitro and in vivo (211). Building on this, SWTX-143—a covalent YAP/TAZ-TEAD inhibitor—irreversibly binds the palmitoylation pocket across all four TEAD isoforms, specifically suppressing YAP/TAZ-TEAD transcriptional activity. SWTX-143 demonstrates efficacy in Hippo-mutant tumor cells and induces substantial regression in human mesothelioma xenografts and orthotopic mouse models (212). Parallelly, the irreversible TEAD inhibitor MYF-03–69 covalently occupies TEAD’s palmitoylation site, blocking YAP-TEAD complex formation and transcriptional activity while inhibiting malignant pleural mesothelioma growth (213). Additionally, TM2—a novel TEAD inhibitor class—effectively suppresses TEAD autopalmitoylation. TM2 monotherapy or MEK inhibitor combination exerts potent antiproliferative effects against YAP-dependent cancers (214).
Beyond 2-BP and TEAD inhibitors, diverse palmitoylation-targeting agents show therapeutic promise. Ras proteins (including N-Ras) require palmitoylation/depalmitoylation cycling for subcellular trafficking and oncogenicity. While broad lipase inhibitors like Palmostatin M (Palm M) lack specificity, ABD957 selectively covalently inhibits ABHD17 depalmitoylases. In human AML cells, ABD957 blocks N-Ras depalmitoylation with higher proteome selectivity than Palm M. ABD957 synergizes with MEK inhibitors to suppress N-Ras signaling and inhibit NRAS-mutant AML growth, suggesting ABHD17 inhibitors as targeted therapies for NRAS-driven cancers (215). The marine-derived compound bengamide C (BC) enhances antitumor immunity by reducing PD-L1 abundance and boosting T-cell cytotoxicity. BC inhibits DHHC3 activity, preventing PD-L1 palmitoylation and triggering its membrane-to-cytoplasm translocation and lysosomal degradation. BC combined with anti-CTLA4 significantly enhances antitumor T-cell responses (216). Artemisinin—a clinically approved antimalarial—demonstrates anticancer activity by covalently inhibiting ER palmitoyltransferase ZDHHC6. This reduces oncogenic NRas palmitoylation, disrupting its subcellular localization and attenuating proliferative signaling. Clinical trials are evaluating artemisinin’s anticancer potential (217, 218). In neuropathic cancer pain (NCP), chronic morphine use accumulates morphine-3-glucuronide, activating microglia via ERK1/2 signaling and apelin receptor (APLNR). NCP models show ZDHHC9 upregulation palmitoylates APLNR, preventing degradation. APLNR palmitoylation inhibitors reduce inflammatory cytokine release and morphine tolerance, suggesting combination therapy potential for cancer pain (219). Additional small molecules regulate palmitoylation machinery:BI-2531, etoposide, piperlongumine; RXC004, all-trans retinoic acid (RA); MEK-PI3K dual inhibitors. These agents offer promising cancer treatment strategies (220–223). Notably, natural compounds (lutein, 5-hydroxyflavone, 6-hydroxyflavone) exhibit higher binding affinity to DHHC20 than 2-BP, supporting their development as selective DHHC20 inhibitors (224).
Palmitoylation, a critical mechanism of post-translational modification, drives tumor drug resistance and immune evasion by regulating protein localization, stability, and signaling pathway activation. In EGFR-TKI-resistant lung cancer, drug-tolerant persister (DTP) cells rely on fatty acid oxidation (FAO) for survival. Here, dipeptidyl peptidase 4 (DPP4) enhances fatty acid uptake via carnitine palmitoyl transferase 1a (CPT1A) activation and sustains mitochondrial antioxidant function through the DPP4-MEK-Nrf2 axis, thereby promoting resistance (225). Similarly, palmitoylation at cysteine residues C104/C107 of Claudin 4 (CLDN4) in hepatocellular carcinoma (HCC) stabilizes its lipid raft anchoring by inhibiting clathrin-mediated endocytosis, activating the Notch pathway to drive lenvatinib resistance (226). Regarding immune checkpoints, palmitoylation-dependent membrane localization of PD-L1 requires DHHC3 activity. The natural small molecule benzosceptrin C (BC) inhibits DHHC3 enzymatic activity, triggering lysosomal degradation of PD-L1 and reversing T cell exhaustion (216). Synergistic mechanisms of combination strategies are recognized as promising approaches to overcome therapeutic resistance or enhance treatment efficacy. Studies demonstrate that combining palmitoylation inhibitors with targeted/chemotherapeutic agents can overcome tumor resistance. For instance, osimertinib combined with the DPP4 inhibitor sitagliptin significantly suppresses lung cancer DTP cell survival, reduces residual tumor burden, and decreases recurrence rates (225). Doxorubicin plus the palmitoylation inhibitor 2-bromopalmitate (2-BP) enhances osteosarcoma apoptosis via the ROS/CHOP pathway (227). Furthermore, combination strategies sensitize tumors to chemo/targeted therapies: the PPT1 inhibitor DC661 reverses sorafenib adaptive resistance in HCC cells by disrupting lysosomal acidification and autophagic flux, while inducing mitochondrial apoptosis (208); salvianolic acid B, targeting CLDN4, inhibits hepatic-to-biliary transition (HBT) and restores lenvatinib sensitivity in HCC (226). Combining palmitoylation inhibitors with immunotherapy also enhances immune responses. PPT1 inhibitors (e.g., hydroxychloroquine [HCQ], DC661) induce interferon-β secretion, promote M2-to-M1 macrophage polarization, reduce myeloid-derived suppressor cells (MDSCs), and synergize with anti-PD-1 antibodies to augment T cell-mediated killing in melanoma (228). Activation of the ADH1C/PPARα axis promotes fatty acid degradation, reduces TEAD1 palmitoylation levels, suppresses the Hippo pathway, and potentiates PD-1 blockade efficacy (229). Innovative combination strategies have been proposed. FDX1 upregulation combined with elesclomol-Cu induces cuproptosis in colorectal cancer by promoting DLAT lipoylation and oligomerization, while the p53 activator CP-31398 sensitizes this process via enhanced FDXR expression (230, 231). Drug repurposing combinations demonstrate efficacy: antimalarials (e.g., artesunate) and central nervous system (CNS) drugs (e.g., fluoxetine, fluphenazine) synergize with chemotherapeutic agents by downregulating PPT1 expression, exhibiting favorable safety profiles in non-tumoral cells (232). Clinical challenges remain. Firstly, target selectivity and off-target effects: functional redundancy within the palmitoyltransferase family (e.g., DHHCs) may limit efficacy when inhibiting single isoforms (e.g., DHHC3) (216). Developing highly selective inhibitors requires structural biology-based optimization while assessing impacts on normal lipid metabolism (e.g., CPT1A inhibition may impair FAO in cardiomyocytes) (225, 227). Secondly, drug delivery and biodistribution: poor tumor targeting of small-molecule inhibitors (e.g., 2-BP, DC661) restricts efficacy and increases systemic toxicity (208, 227). Nanoparticle delivery systems (e.g., HSA-POPC/chol/AMOs liposomes) improve tumor accumulation of inhibitors or nucleic acid therapeutics, as evidenced by synergistic suppression of pancreatic cancer with miR-21 silencing plus sunitinib (233). Thirdly, tumor heterogeneity and microenvironment adaptation: heterogeneity in ADH1C deficiency or spatial distribution of CLDN4+ cells in HCC contributes to variable treatment responses (226, 229). Spatial multi-omics and dual biomarkers (e.g., ADH1C/PPARα) should guide patient stratification (229). Notably, the double-edged effect of immune microenvironment modulation: PPT1 inhibition enhances T cell responses but lysosomal membrane permeabilization may release damage-associated molecular patterns (DAMPs), provoking localized inflammatory storms (208, 228). Optimizing dosing windows or combining immunomodulators (e.g., IL-6 antagonists) is crucial to balance efficacy and safety. Future directions include: developing dual-functional molecules such as bispecific antibodies simultaneously targeting palmitoyltransferases and immune checkpoints (e.g., anti-CKAP4/PD-L1) (77); implementing dynamic monitoring technologies like mass cytometry to quantify membrane palmitoylomes for guiding treatment timing (216); and optimizing intervention timing, as palmitoylation mediates early tolerance (e.g., DTP cells), necessitating combination therapy initiation during initial treatment (208, 225).
Beyond anticancer applications of palmitoylation inhibition, studies demonstrate that palmitoylation significantly enhances intracellular delivery of therapeutic molecules—including 5-carboxyfluorescein and doxorubicin—independent of their inherent membrane permeability. Fluorescence imaging and flow cytometry validate palmitoylation’s role in promoting cellular uptake of Tat-fluorescein conjugates and doxorubicin. Notably, palmitoylated Tat-doxorubicin conjugates exhibit significantly enhanced anticancer activity in cervical cancer cell lines (234).
7 Conclusion and future directions
This review comprehensively examines the multifaceted roles of palmitoylation in tumor biology, elucidating its fundamental regulatory mechanisms and functional consequences. As a critical post-translational modification, palmitoylation exerts profound influence on tumor cell behavior by governing core oncogenic processes including tumor initiation, progression, invasion, and metastasis; modulating key signaling pathways such as Hippo and Wnt/β-catenin through regulation of effector protein activity, subcellular localization, and stability; and directing the function of tumor-associated proteins that orchestrate proliferation, apoptosis, and migration. Furthermore, we demonstrate palmitoylation’s systemic impact on tumor microenvironment interactions and immune evasion mechanisms—notably its regulation of immune cell functionality and suppression of surveillance pathways. Critical questions remain regarding tissue-specific mechanistic variations across tumor types, crosstalk with other post-translational modifications, and interactions with stromal and immune microenvironment components. While emerging palmitoylation inhibitors show preclinical promise, their clinical translation requires rigorous validation of therapeutic efficacy and safety profiles. Moving forward, research should prioritize developing novel isoform-selective inhibitors and activators, exploring combinatorial approaches integrating palmitoylation-targeted agents with conventional therapies, and advancing biomarker-driven patient stratification for precision intervention. In summary, palmitoylation represents a master regulatory node in cancer pathogenesis whose continued mechanistic dissection and therapeutic exploitation hold significant potential for advancing oncology treatment paradigms and improving patient outcomes.
Author contributions
QL: Writing – review & editing, Writing – original draft, Conceptualization, Visualization. JW: Writing – review & editing, Visualization, Writing – original draft. XY: Project administration, Writing – review & editing. JQ: Visualization, Writing – original draft, Conceptualization, Writing – review & editing. SZ: Writing – review & editing, Funding acquisition, Writing – original draft, Visualization, Conceptualization.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2022KY1246), and the Science and Technology Bureau of Jiaxing City (2023AZ31002 and 2022AZ10009).
Acknowledgments
We sincerely appreciate the potential editors and reviewers for their succinct comments on improving this manuscript. BioRender (https://www.biorender.com/) and BioGDP (https://www.biogdp.com/) were used to create the figures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
PATs: Palmitoyl acyltransferases
PPTs: Palmitoyl-protein thioesterases
ER: Endoplasmic reticulum
2-BP: 2-bromopalmitate
CRC: Colorectal cancer
CKAP4: Cytoskeleton-associated protein 4
APF: Antiproliferative factor
CSCs: Cancer stem cells
HBECs: Human bronchial epithelial cells
TNBC: Triple-negative breast cancer
SD: Sleep deprivation
PA-CoA: Palmitoyl-CoA
DLBCL: Diffuse large B-cell lymphoma
FASN: Fatty acid synthase
PCa: Prostate cancer
AR: Androgen receptor
LDHA: Lactate dehydrogenase A
ROS: Reactive oxygen species
EGFR: Epidermal growth factor receptor
OSCC: Oral squamous cell carcinoma
GSCs: Gglioblastoma stem cells
NEK7: NIMA-related kinase 7
EOC: Epithelial ovarian cancer
TCA: Tricarboxylic acid
MDH2: Malate dehydrogenase 2
PDAC: Pancreatic ductal adenocarcinoma
IGF-1: Insulin-like growth factor-1
IGF-1R: IGF-1 receptor
Flot-1: Flotillin-1
APT: Acyl protein thioesterase
EMT: Epithelial-mesenchymal transition.
CLL: Chronic lymphocytic leukemia
Nar: Naringenin
ER: Estrogen receptor
LP: Lipid pocket
HCC: Hepatocellular carcinoma
PA: Palmitic acid
CcRCC: Clear cell renal cell carcinoma
DRM: Detergent-resistant membrane
NAFLD: Non-alcoholic fatty liver disease
NSCLC: Non-small cell lung cancer
MCAM: Melanoma cell adhesion molecule
PORCN: Porcupine
MET: Mesenchymal-to-epithelial transition
Shh: Sonic Hedgehog
Hhat: Hedgehog acyltransferase
MSA: Mobility Shift Assay
AML: Acute myeloid leukemia
HPSCC: Hypopharyngeal squamous cell carcinoma
CDK5: Cyclin-dependent kinase 5
GSCs: Glioblastoma stem cells
NCP: Neuropathic cancer pain
PD-L1: Programmed Death-Ligand 1
IFN-I: Type I interferon
DOX: Doxorubicin
OxLDL: Oxidized low-density lipoprotein
AFT: Afatinib
Palm M: Palmostatin M
BC: Bengamide C
RA: Retinoic acid
PRMT1: Protein arginine methyltransferase 1
HGSOC: High-grade serous ovarian cancer
OXA: Oxaliplatin
References
1. Ko PJ and Dixon SJ. Protein palmitoylation and cancer. EMBO Rep. (2018) 19:e46666. doi: 10.15252/embr.201846666
2. Liu Z, Xiao M, Mo Y, Wang H, Han Y, Zhao X, et al. Emerging roles of protein palmitoylation and its modifying enzymes in cancer cell signal transduction and cancer therapy. Int J Biol Sci. (2022) 18:3447–57. doi: 10.7150/ijbs.72244
3. Mitchell DA, Vasudevan A, Linder ME, and Deschenes RJ. Protein palmitoylation by a family of dhhc protein S-acyltransferases. J Lipid Res. (2006) 47:1118–27. doi: 10.1194/jlr.R600007-JLR200
4. Yang X, Chatterjee V, Ma Y, Zheng E, and Yuan SY. Protein palmitoylation in leukocyte signaling and function. Front Cell Dev Biol. (2020) 8:600368. doi: 10.3389/fcell.2020.600368
5. Zhou B, Hao Q, Liang Y, and Kong E. Protein palmitoylation in cancer: molecular functions and therapeutic potential. Mol Oncol. (2023) 17:3–26. doi: 10.1002/1878-0261.13308
6. Iwanaga T, Tsutsumi R, Noritake J, Fukata Y, and Fukata M. Dynamic protein palmitoylation in cellular signaling. Prog Lipid Res. (2009) 48:117–27. doi: 10.1016/j.plipres.2009.02.001
7. Villanueva CE and Hagenbuch B. Palmitoylation of solute carriers. Biochem Pharmacol. (2023) 215:115695. doi: 10.1016/j.bcp.2023.115695
8. Smotrys JE and Linder ME. Palmitoylation of intracellular signaling proteins: regulation and function. Annu Rev Biochem. (2004) 73:559–87. doi: 10.1146/annurev.biochem.73.011303.073954
9. Jin J, Zhi X, Wang X, and Meng D. Protein palmitoylation and its pathophysiological relevance. J Cell Physiol. (2021) 236:3220–33. doi: 10.1002/jcp.30122
10. Solis GP, Kazemzadeh A, Abrami L, Valnohova J, Alvarez C, van der Goot FG, et al. Local and substrate-specific S-palmitoylation determines subcellular localization of galphao. Nat Commun. (2022) 13:2072. doi: 10.1038/s41467-022-29685-8
11. Zhao L, Zhang C, Luo X, Wang P, Zhou W, Zhong S, et al. Cd36 palmitoylation disrupts free fatty acid metabolism and promotes tissue inflammation in non-alcoholic steatohepatitis. J Hepatol. (2018) 69:705–17. doi: 10.1016/j.jhep.2018.04.006
12. Cassinelli S, Vinola-Renart C, Benavente-Garcia A, Navarro-Perez M, Capera J, and Felipe A. Palmitoylation of voltage-gated ion channels. Int J Mol Sci. (2022) 23:9357. doi: 10.3390/ijms23169357
13. Zhao Y, Mir C, Garcia-Mayea Y, Paciucci R, Kondoh H, and ME LL. Rna-binding proteins: underestimated contributors in tumorigenesis. Semin Cancer Biol. (2022) 86:431–44. doi: 10.1016/j.semcancer.2022.01.010
14. Deng L, Meng T, Chen L, Wei W, and Wang P. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct Target Ther. (2020) 5:11. doi: 10.1038/s41392-020-0107-0
15. Liu J, Peng Y, and Wei W. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. (2022) 32:30–44. doi: 10.1016/j.tcb.2021.07.001
16. Zhou B, Wang Y, Zhang L, Shi X, Kong H, Zhang M, et al. The palmitoylation of aeg-1 dynamically modulates the progression of hepatocellular carcinoma. Theranostics. (2022) 12:6898–914. doi: 10.7150/thno.78377
17. Yao H, Lan J, Li C, Shi H, Brosseau JP, Wang H, et al. Inhibiting pd-L1 palmitoylation enhances T-cell immune responses against tumours. Nat BioMed Eng. (2019) 3:306–17. doi: 10.1038/s41551-019-0375-6
18. Aomatsu K, Kato T, Fujita H, Hato F, Oshitani N, Kamata N, et al. Toll-like receptor agonists stimulate human neutrophil migration via activation of mitogen-activated protein kinases. Immunology. (2008) 123:171–80. doi: 10.1111/j.1365-2567.2007.02684.x
19. Sun Y, Zhang H, Meng J, Guo F, Ren D, Wu H, et al. S-palmitoylation of pcsk9 induces sorafenib resistance in liver cancer by activating the pi3k/akt pathway. Cell Rep. (2022) 40:111194. doi: 10.1016/j.celrep.2022.111194
20. Yu F and Qian Z. Mechanisms for regulation of ras palmitoylation and plasma membrane trafficking in hematopoietic Malignancies. J Clin Invest. (2023) 133:e171104. doi: 10.1172/JCI171104
21. Liu Y, Qi X, Donnelly L, Elghobashi-Meinhardt N, Long T, Zhou RW, et al. Mechanisms and inhibition of porcupine-mediated wnt acylation. Nature. (2022) 607:816–22. doi: 10.1038/s41586-022-04952-2
22. Lu Y, Zheng Y, Coyaud E, Zhang C, Selvabaskaran A, Yu Y, et al. Palmitoylation of nod1 and nod2 is required for bacterial sensing. Science. (2019) 366:460–7. doi: 10.1126/science.aau6391
23. Du W, Hua F, Li X, Zhang J, Li S, Wang W, et al. Loss of optineurin drives cancer immune evasion via palmitoylation-dependent ifngr1 lysosomal sorting and degradation. Cancer Discov. (2021) 11:1826–43. doi: 10.1158/2159-8290.CD-20-1571
24. Rastedt DE, Vaughan RA, and Foster JD. Palmitoylation mechanisms in dopamine transporter regulation. J Chem Neuroanat. (2017) 83-84:3–9. doi: 10.1016/j.jchemneu.2017.01.002
25. Drisdel RC, Alexander JK, Sayeed A, and Green WN. Assays of protein palmitoylation. Methods. (2006) 40:127–34. doi: 10.1016/j.ymeth.2006.04.015
26. De I and Sadhukhan S. Emerging roles of dhhc-mediated protein S-palmitoylation in physiological and pathophysiological context. Eur J Cell Biol. (2018) 97:319–38. doi: 10.1016/j.ejcb.2018.03.005
27. Aicart-Ramos C, Valero RA, and Rodriguez-Crespo I. Protein palmitoylation and subcellular trafficking. Biochim Biophys Acta. (2011) 1808:2981–94. doi: 10.1016/j.bbamem.2011.07.009
28. Le X, Mu J, Peng W, Tang J, Xiang Q, Tian S, et al. DNA methylation downregulated zdhhc1 suppresses tumor growth by altering cellular metabolism and inducing oxidative/er stress-mediated apoptosis and pyroptosis. Theranostics. (2020) 10:9495–511. doi: 10.7150/thno.45631
29. Oku S, Takahashi N, Fukata Y, and Fukata M. In silico screening for palmitoyl substrates reveals a role for dhhc1/3/10 (Zdhhc1/3/11)-mediated neurochondrin palmitoylation in its targeting to rab5-positive endosomes. J Biol Chem. (2013) 288:19816–29. doi: 10.1074/jbc.M112.431676
30. Ramanan VK, Lesnick TG, Przybelski SA, Heckman MG, Knopman DS, Graff-Radford J, et al. Coping with brain amyloid: genetic heterogeneity and cognitive resilience to alzheimer’s pathophysiology. Acta Neuropathol Commun. (2021) 9:48. doi: 10.1186/s40478-021-01154-1
31. Wang X, Qian P, Cui H, Yao L, and Yuan J. A protein palmitoylation cascade regulates microtubule cytoskeleton integrity in plasmodium. EMBO J. (2020) 39:e104168. doi: 10.15252/embj.2019104168
32. Liu L, Dai L, Xu D, Wang Y, Bai L, Chen X, et al. Astrocyte secretes il-6 to modulate psd-95 palmitoylation in basolateral amygdala and depression-like behaviors induced by peripheral nerve injury. Brain Behav Immun. (2022) 104:139–54. doi: 10.1016/j.bbi.2022.05.014
33. Ni H, Wang Y, Yao K, Wang L, Huang J, Xiao Y, et al. Cyclical palmitoylation regulates tlr9 signalling and systemic autoimmunity in mice. Nat Commun. (2024) 15:1. doi: 10.1038/s41467-023-43650-z
34. Bolland DE, Moritz AE, Stanislowski DJ, Vaughan RA, and Foster JD. Palmitoylation by multiple dhhc enzymes enhances dopamine transporter function and stability. ACS Chem Neurosci. (2019) 10:2707–17. doi: 10.1021/acschemneuro.8b00558
35. Wang J, Hao JW, Wang X, Guo H, Sun HH, Lai XY, et al. Dhhc4 and dhhc5 facilitate fatty acid uptake by palmitoylating and targeting cd36 to the plasma membrane. Cell Rep. (2019) 26:209–21 e5. doi: 10.1016/j.celrep.2018.12.022
36. Gorleku OA, Barns AM, Prescott GR, Greaves J, and Chamberlain LH. Endoplasmic reticulum localization of dhhc palmitoyltransferases mediated by lysine-based sorting signals. J Biol Chem. (2011) 286:39573–84. doi: 10.1074/jbc.M111.272369
37. Howie J, Reilly L, Fraser NJ, Vlachaki Walker JM, Wypijewski KJ, Ashford ML, et al. Substrate recognition by the cell surface palmitoyl transferase dhhc5. Proc Natl Acad Sci U.S.A. (2014) 111:17534–9. doi: 10.1073/pnas.1413627111
38. Tian H, Lu JY, Shao C, Huffman KE, Carstens RM, Larsen JE, et al. Systematic sirna screen unmasks nsclc growth dependence by palmitoyltransferase dhhc5. Mol Cancer Res. (2015) 13:784–94. doi: 10.1158/1541-7786.MCR-14-0608
39. Guo R, Liu J, Min X, Zeng W, Shan B, Zhang M, et al. Reduction of dhhc5-mediated beclin 1 S-palmitoylation underlies autophagy decline in aging. Nat Struct Mol Biol. (2024) 31:232–45. doi: 10.1038/s41594-023-01163-9
40. Zhang N, Liu J, Guo R, Yan L, Yang Y, Shi C, et al. Palmitoylation licenses ripk1 kinase activity and cytotoxicity in the tnf pathway. Mol Cell. (2024) 84:4419–35 e10. doi: 10.1016/j.molcel.2024.10.002
41. Lakkaraju AK, Abrami L, Lemmin T, Blaskovic S, Kunz B, Kihara A, et al. Palmitoylated calnexin is a key component of the ribosome-translocon complex. EMBO J. (2012) 31:1823–35. doi: 10.1038/emboj.2012.15
42. Zareba-Koziol M, Bartkowiak-Kaczmarek A, Roszkowska M, Bijata K, Figiel I, Halder AK, et al. S-palmitoylation of synaptic proteins as a novel mechanism underlying sex-dependent differences in neuronal plasticity. Int J Mol Sci. (2021) 22:6253. doi: 10.3390/ijms22126253
43. Kilpatrick CL, Murakami S, Feng M, Wu X, Lal R, Chen G, et al. Dissociation of golgi-associated dhhc-type zinc finger protein (Godz)- and sertoli cell gene with a zinc finger domain-beta (Serz-beta)-mediated palmitoylation by loss of function analyses in knock-out mice. J Biol Chem. (2016) 291:27371–86. doi: 10.1074/jbc.M116.732768
44. Zhang N, Zhang J, Yang Y, Shan H, Hou S, Fang H, et al. A palmitoylation-depalmitoylation relay spatiotemporally controls gsdmd activation in pyroptosis. Nat Cell Biol. (2024) 26:757–69. doi: 10.1038/s41556-024-01397-9
45. Ho GP, Selvakumar B, Mukai J, Hester LD, Wang Y, Gogos JA, et al. S-nitrosylation and S-palmitoylation reciprocally regulate synaptic targeting of psd-95. Neuron. (2011) 71:131–41. doi: 10.1016/j.neuron.2011.05.033
46. Thomas GM, Hayashi T, Chiu SL, Chen CM, and Huganir RL. Palmitoylation by dhhc5/8 targets grip1 to dendritic endosomes to regulate ampa-R trafficking. Neuron. (2012) 73:482–96. doi: 10.1016/j.neuron.2011.11.021
47. Kouskou M, Thomson DM, Brett RR, Wheeler L, Tate RJ, Pratt JA, et al. Disruption of the zdhhc9 intellectual disability gene leads to behavioural abnormalities in a mouse model. Exp Neurol. (2018) 308:35–46. doi: 10.1016/j.expneurol.2018.06.014
48. Santos JM, Duarte N, Kehrer J, Ramesar J, Avramut MC, Koster AJ, et al. Maternally supplied S-acyl-transferase is required for crystalloid organelle formation and transmission of the malaria parasite. Proc Natl Acad Sci U.S.A. (2016) 113:7183–8. doi: 10.1073/pnas.1522381113
49. Jiang L, Wang Z, Xu T, and Zhang L. When pyro(Ptosis) meets palm(Itoylation). Cytokine Growth Factor Rev. (2024) 77:30–8. doi: 10.1016/j.cytogfr.2024.03.001
50. Guo H, Wang J, Ren S, Zheng LF, Zhuang YX, Li DL, et al. Targeting egfr-dependent tumors by disrupting an arf6-mediated sorting system. Nat Commun. (2022) 13:6004. doi: 10.1038/s41467-022-33788-7
51. Sanders SS and Hayden MR. Aberrant palmitoylation in huntington disease. Biochem Soc Trans. (2015) 43:205–10. doi: 10.1042/BST20140242
52. Fan X, Yang H, Zhao C, Hu L, Wang D, Wang R, et al. Local anesthetics impair the growth and self-renewal of glioblastoma stem cells by inhibiting zdhhc15-mediated gp130 palmitoylation. Stem Cell Res Ther. (2021) 12:107. doi: 10.1186/s13287-021-02175-2
53. Abrami L, Dallavilla T, Sandoz PA, Demir M, Kunz B, Savoglidis G, et al. Identification and dynamics of the human zdhhc16-zdhhc6 palmitoylation cascade. Elife. (2017) 6:e27826. doi: 10.7554/eLife.27826
54. Milnerwood AJ, Parsons MP, Young FB, Singaraja RR, Franciosi S, Volta M, et al. Memory and synaptic deficits in hip14/dhhc17 knockout mice. Proc Natl Acad Sci U.S.A. (2013) 110:20296–301. doi: 10.1073/pnas.1222384110
55. Huang X, Yao J, Liu L, Chen J, Mei L, Huangfu J, et al. S-acylation of P62 promotes P62 droplet recruitment into autophagosomes in mammalian autophagy. Mol Cell. (2023) 83:3485–501 e11. doi: 10.1016/j.molcel.2023.09.004
56. Carreras-Sureda A, Abrami L, Ji-Hee K, Wang WA, Henry C, Frieden M, et al. S-acylation by zdhhc20 targets orai1 channels to lipid rafts for efficient ca(2+) signaling by jurkat T cell receptors at the immune synapse. Elife. (2021) 10:e72051. doi: 10.7554/eLife.72051
57. Beard RS Jr., Yang X, Meegan JE, Overstreet JW, Yang CG, Elliott JA, et al. Palmitoyl acyltransferase dhhc21 mediates endothelial dysfunction in systemic inflammatory response syndrome. Nat Commun. (2016) 7:12823. doi: 10.1038/ncomms12823
58. Hou H, John Peter AT, Meiringer C, Subramanian K, and Ungermann C. Analysis of dhhc acyltransferases implies overlapping substrate specificity and a two-step reaction mechanism. Traffic. (2009) 10:1061–73. doi: 10.1111/j.1600-0854.2009.00925.x
59. Gonzalez Montoro A, Chumpen Ramirez S, Quiroga R, and Valdez Taubas J. Specificity of transmembrane protein palmitoylation in yeast. PloS One. (2011) 6:e16969. doi: 10.1371/journal.pone.0016969
60. Salaun C, Takizawa H, Galindo A, Munro KR, McLellan J, Sugimoto I, et al. Development of a novel high-throughput screen for the identification of new inhibitors of protein S-acylation. J Biol Chem. (2022) 298:102469. doi: 10.1016/j.jbc.2022.102469
61. Greaves J and Chamberlain LH. S-acylation by the dhhc protein family. Biochem Soc Trans. (2010) 38:522–4. doi: 10.1042/BST0380522
62. Chen JJ, Fan Y, and Boehning D. Regulation of dynamic protein S-acylation. Front Mol Biosci. (2021) 8:656440. doi: 10.3389/fmolb.2021.656440
63. Panina IS, Krylov NA, Chugunov AO, Efremov RG, and Kordyukova LV. The mechanism of selective recognition of lipid substrate by hdhhc20 enzyme. Int J Mol Sci. (2022) 23:14791. doi: 10.3390/ijms232314791
64. Verardi R, Kim JS, Ghirlando R, and Banerjee A. Structural basis for substrate recognition by the ankyrin repeat domain of human dhhc17 palmitoyltransferase. Structure. (2017) 25:1337–47 e6. doi: 10.1016/j.str.2017.06.018
65. Resh MD. Fatty acylation of proteins: the long and the short of it. Prog Lipid Res. (2016) 63:120–31. doi: 10.1016/j.plipres.2016.05.002
66. Batista CM, Saad F, Ceccoti SPC, Eger I, and Soares MJ. Subcellular localisation of flag tagged enzymes of the dynamic protein S-palmitoylation cycle of trypanosoma cruzi epimastigotes. Mem Inst Oswaldo Cruz. (2018) 113:e180086. doi: 10.1590/0074-02760180086
67. Mondal A, Appu AP, Sadhukhan T, Bagh MB, Previde RM, Sadhukhan S, et al. Ppt1-deficiency dysregulates lysosomal ca(++) homeostasis contributing to pathogenesis in a mouse model of cln1 disease. J Inherit Metab Dis. (2022) 45:635–56. doi: 10.1002/jimd.12485
68. Balasubramanian A, Hsu AY, Ghimire L, Tahir M, Devant P, Fontana P, et al. The palmitoylation of gasdermin D directs its membrane translocation and pore formation during pyroptosis. Sci Immunol. (2024) 9:eadn1452. doi: 10.1126/sciimmunol.adn1452
69. Anderson AM and Ragan MA. Palmitoylation: A protein S-acylation with implications for breast cancer. NPJ Breast Cancer. (2016) 2:16028. doi: 10.1038/npjbcancer.2016.28
70. Nelson JC, Witze E, Ma Z, Ciocco F, Frerotte A, Randlett O, et al. Acute regulation of habituation learning via posttranslational palmitoylation. Curr Biol. (2020) 30:2729–38 e4. doi: 10.1016/j.cub.2020.05.016
71. Su C, Cheng T, Huang J, Zhang T, and Yin H. 4-octyl itaconate restricts sting activation by blocking its palmitoylation. Cell Rep. (2023) 42:113040. doi: 10.1016/j.celrep.2023.113040
72. Cho E and Park M. Palmitoylation in alzheimer’s disease and other neurodegenerative diseases. Pharmacol Res. (2016) 111:133–51. doi: 10.1016/j.phrs.2016.06.008
73. Coronel Arrechea C, Giolito ML, Garcia IA, Soria G, and Valdez Taubas J. A novel yeast-based high-throughput method for the identification of protein palmitoylation inhibitors. Open Biol. (2021) 11:200415. doi: 10.1098/rsob.200415
74. Terry AR, Nogueira V, Rho H, Ramakrishnan G, Li J, Kang S, et al. Cd36 maintains lipid homeostasis via selective uptake of monounsaturated fatty acids during matrix detachment and tumor progression. Cell Metab. (2023) 35:2060–76 e9. doi: 10.1016/j.cmet.2023.09.012
75. Amendola CR, Mahaffey JP, Parker SJ, Ahearn IM, Chen WC, Zhou M, et al. Kras4a directly regulates hexokinase 1. Nature. (2019) 576:482–6. doi: 10.1038/s41586-019-1832-9
76. Tavsan Z and Ayar Kayali H. Epcam-claudin-tetraspanin-modulated ovarian cancer progression and drug resistance. Cell Adh Migr. (2020) 14:57–68. doi: 10.1080/19336918.2020.1732761
77. Nagoya A, Sada R, Kimura H, Yamamoto H, Morishita K, Miyoshi E, et al. Ckap4 is a potential exosomal biomarker and therapeutic target for lung cancer. Transl Lung Cancer Res. (2023) 12:408–26. doi: 10.21037/tlcr-22-571
78. Clayton NS, Hodge RG, Infante E, Alibhai D, Zhou F, and Ridley AJ. Rhou forms homo-oligomers to regulate cellular responses. J Cell Sci. (2024) 137:14791. doi: 10.1242/jcs.261645
79. Zhao H, Liu P, Zhang R, Wu M, Li D, Zhao X, et al. Roles of palmitoylation and the kikk membrane-targeting motif in leukemogenesis by oncogenic kras4a. J Hematol Oncol. (2015) 8:132. doi: 10.1186/s13045-015-0226-1
80. Kokabee M, Wang X, Voorand E, Alin E, Kokabee L, Khan F, et al. Palmitoylation of the alternative amino terminus of the btk-C isoform controls subcellular distribution and signaling. Cancer Genomics Proteomics. (2022) 19:415–27. doi: 10.21873/cgp.20329
81. Chong LW, Tsai CL, Yang KC, Liao CC, and Hsu YC. Targeting protein palmitoylation decreases palmitate−Induced sphere formation of human liver cancer cells. Mol Med Rep. (2020) 22:939–47. doi: 10.3892/mmr.2020.11172
82. Cao Y, Wei H, Jiang S, Lu T, Nie P, Yang C, et al. Effect of aqp4 and its palmitoylation on the permeability of exogenous reactive oxygen species: insights from computational study. Int J Biol Macromol. (2023) 253:127568. doi: 10.1016/j.ijbiomac.2023.127568
83. Kyuno D, Zhao K, Schnolzer M, Provaznik J, Hackert T, and Zoller M. Claudin7-dependent exosome-promoted reprogramming of nonmetastasizing tumor cells. Int J Cancer. (2019) 145:2182–200. doi: 10.1002/ijc.32312
84. De Piano M, Manuelli V, Zadra G, Otte J, Edqvist PD, Ponten F, et al. Lipogenic signalling modulates prostate cancer cell adhesion and migration via modification of rho gtpases. Oncogene. (2020) 39:3666–79. doi: 10.1038/s41388-020-1243-2
85. Kyuno D, Bauer N, Schnolzer M, Provaznik J, Ryschich E, Hackert T, et al. Distinct origin of claudin7 in early tumor endosomes affects exosome assembly. Int J Biol Sci. (2019) 15:2224–39. doi: 10.7150/ijbs.35347
86. Mariscal J, Vagner T, Kim M, Zhou B, Chin A, Zandian M, et al. Comprehensive palmitoyl-proteomic analysis identifies distinct protein signatures for large and small cancer-derived extracellular vesicles. J Extracell Vesicles. (2020) 9:1764192. doi: 10.1080/20013078.2020.1764192
87. Han C, Yu G, Mao Y, Song S, Li L, Zhou L, et al. Lpcat1 enhances castration resistant prostate cancer progression via increased mrna synthesis and paf production. PloS One. (2020) 15:e0240801. doi: 10.1371/journal.pone.0240801
88. Zhou B, Liu L, Reddivari M, and Zhang XA. The palmitoylation of metastasis suppressor kai1/cd82 is important for its motility- and invasiveness-inhibitory activity. Cancer Res. (2004) 64:7455–63. doi: 10.1158/0008-5472.CAN-04-1574
89. Zhang H, Chu G, Wang G, Yao M, Lu S, and Chen T. Mechanistic understanding of the palmitoylation of G(O) protein in the allosteric regulation of adhesion receptor gpr97. Pharmaceutics. (2022) 14:1856. doi: 10.3390/pharmaceutics14091856
90. Sun C, Wang P, Dong W, Liu H, Sun J, and Zhao L. Lncrna pvt1 promotes exosome secretion through ykt6, rab7, and vamp3 in pancreatic cancer. Aging (Albany NY). (2020) 12:10427–40. doi: 10.18632/aging.103268
91. Heakal Y, Woll MP, Fox T, Seaton K, Levenson R, and Kester M. Neurotensin receptor-1 inducible palmitoylation is required for efficient receptor-mediated mitogenic-signaling within structured membrane microdomains. Cancer Biol Ther. (2011) 12:427–35. doi: 10.4161/cbt.12.5.15984
92. Musial C, Knap N, Zaucha R, Bastian P, Barone G, Lo Bosco G, et al. Induction of 2-hydroxycatecholestrogens O-methylation: A missing puzzle piece in diagnostics and treatment of lung cancer. Redox Biol. (2022) 55:102395. doi: 10.1016/j.redox.2022.102395
93. Cui L, Liu M, Lai S, Hou H, Diao T, Zhang D, et al. Androgen upregulates the palmitoylation of eif3l in human prostate lncap cells. Onco Targets Ther. (2019) 12:4451–9. doi: 10.2147/OTT.S193480
94. Babina IS, McSherry EA, Donatello S, Hill AD, and Hopkins AM. A novel mechanism of regulating breast cancer cell migration via palmitoylation-dependent alterations in the lipid raft affiliation of cd44. Breast Cancer Res. (2014) 16:R19. doi: 10.1186/bcr3614
95. Harada T, Sada R, Osugi Y, Matsumoto S, Matsuda T, Hayashi-Nishino M, et al. Palmitoylated Ckap4 regulates mitochondrial functions through an interaction with Vdac2 at Er-Mitochondria contact sites. J Cell Sci. (2020) 133:jcs249045. doi: 10.1242/jcs.249045
96. Yang Z, Qin W, Chen Y, Yuan B, Song X, Wang B, et al. Cholesterol inhibits hepatocellular carcinoma invasion and metastasis by promoting cd44 localization in lipid rafts. Cancer Lett. (2018) 429:66–77. doi: 10.1016/j.canlet.2018.04.038
97. Jang D, Kwon H, Jeong K, Lee J, and Pak Y. Essential role of flotillin-1 palmitoylation in the intracellular localization and signaling function of igf-1 receptor. J Cell Sci. (2015) 128:2179–90. doi: 10.1242/jcs.169409
98. Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, et al. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. (2005) 16:231–7. doi: 10.1091/mbc.e04-07-0547
99. Yang X, Guo Z, Sun F, Li W, Alfano A, Shimelis H, et al. Novel membrane-associated androgen receptor splice variant potentiates proliferative and survival responses in prostate cancer cells. J Biol Chem. (2011) 286:36152–60. doi: 10.1074/jbc.M111.265124
100. Raturi A, Gutierrez T, Ortiz-Sandoval C, Ruangkittisakul A, Herrera-Cruz MS, Rockley JP, et al. Tmx1 determines cancer cell metabolism as a thiol-based modulator of er-mitochondria ca2+ Flux. J Cell Biol. (2016) 214:433–44. doi: 10.1083/jcb.201512077
101. Galluzzo P, Caiazza F, Moreno S, and Marino M. Role of erbeta palmitoylation in the inhibition of human colon cancer cell proliferation. Endocr Relat Cancer. (2007) 14:153–67. doi: 10.1677/ERC-06-0020
102. Liao P, Wang W, Li Y, Wang R, Jin J, Pang W, et al. Palmitoylated scp1 is targeted to the plasma membrane and negatively regulates angiogenesis. Elife. (2017) 6:e22058. doi: 10.7554/eLife.22058
103. Wu M, Huang J, Zhang J, Benes C, Jiao B, and Ren R. N-arachidonoyl dopamine inhibits nras neoplastic transformation by suppressing its plasma membrane translocation. Mol Cancer Ther. (2017) 16:57–67. doi: 10.1158/1535-7163.MCT-16-0419
104. Hasan S, White NF, Tagliatela AC, Durall RT, Brown KM, McDiarmid GR, et al. Overexpressed galpha13 activates serum response factor through stoichiometric imbalance with gbetagamma and mislocalization to the cytoplasm. Cell Signal. (2023) 102:110534. doi: 10.1016/j.cellsig.2022.110534
105. Caiazza F, Galluzzo P, Lorenzetti S, and Marino M. 17beta-estradiol induces erbeta up-regulation via P38/mapk activation in colon cancer cells. Biochem Biophys Res Commun. (2007) 359:102–7. doi: 10.1016/j.bbrc.2007.05.059
106. Behzadi M, Amininasab M, Eghtedardoost M, and Bagheri M. Turn-folded magainin lipopeptide analog induces cytoplasmic vacuoles in mda-mb-231 cells through G2-phase arrest. Biochem Biophys Res Commun. (2021) 583:199–205. doi: 10.1016/j.bbrc.2021.10.076
107. Razandi M, Pedram A, and Levin ER. Heat shock protein 27 is required for sex steroid receptor trafficking to and functioning at the plasma membrane. Mol Cell Biol. (2010) 30:3249–61. doi: 10.1128/MCB.01354-09
108. Wang CH, Shyu RY, Wu CC, Tsai TC, Wang LK, Chen ML, et al. Phospholipase a/acyltransferase enzyme activity of H-rev107 inhibits the H-ras signaling pathway. J BioMed Sci. (2014) 21:36. doi: 10.1186/1423-0127-21-36
109. Li W, Wang Q, Qi X, Lu H, Chen Y, Shi J, et al. An oncogenic viral interferon regulatory factor upregulates cub domain-containing protein 1 to promote angiogenesis by hijacking transcription factor lymphoid enhancer-binding factor 1 and metastasis suppressor cd82. Cell Death Differ. (2020) 27:3289–306. doi: 10.1038/s41418-020-0578-0
110. Dubois F, Leroy C, Simon V, Benistant C, and Roche S. Yes oncogenic activity is specified by its sh4 domain and regulates ras/mapk signaling in colon carcinoma cells. Am J Cancer Res. (2015) 5:1972–87.
111. Jager D, Stockert E, Jager E, Gure AO, Scanlan MJ, Knuth A, et al. Serological cloning of a melanocyte rab guanosine 5’-triphosphate-binding protein and a chromosome condensation protein from a melanoma complementary DNA library. Cancer Res. (2000) 60:3584–91.
112. Shi Z, Li Z, Jin B, Ye W, Wang L, Zhang S, et al. Loss of lncrna duxap8 synergistically enhanced sorafenib induced ferroptosis in hepatocellular carcinoma via slc7a11 de-palmitoylation. Clin Transl Med. (2023) 13:e1300. doi: 10.1002/ctm2.1300
113. Goughnour PC, Park MC, Kim SB, Jun S, Yang WS, Chae S, et al. Extracellular vesicles derived from macrophages display glycyl-trna synthetase 1 and exhibit anti-cancer activity. J Extracell Vesicles. (2020) 10:e12029. doi: 10.1002/jev2.12029
114. Adams MN, Christensen ME, He Y, Waterhouse NJ, and Hooper JD. The role of palmitoylation in signalling, cellular trafficking and plasma membrane localization of protease-activated receptor-2. PloS One. (2011) 6:e28018. doi: 10.1371/journal.pone.0028018
115. Fan X, Fan J, Yang H, Zhao C, Niu W, Fang Z, et al. Heterogeneity of subsets in glioblastoma mediated by smad3 palmitoylation. Oncogenesis. (2021) 10:72. doi: 10.1038/s41389-021-00361-8
116. Ba Q, Zhou N, Duan J, Chen T, Hao M, Yang X, et al. Dihydroartemisinin exerts its anticancer activity through depleting cellular iron via transferrin receptor-1. PloS One. (2012) 7:e42703. doi: 10.1371/journal.pone.0042703
117. Hu L, Chen M, Chen X, Zhao C, Fang Z, Wang H, et al. Chemotherapy-induced pyroptosis is mediated by bak/bax-caspase-3-gsdme pathway and inhibited by 2-bromopalmitate. Cell Death Dis. (2020) 11:281. doi: 10.1038/s41419-020-2476-2
118. Li W, Zhang J, Zou L, Cui J, Su F, Jin J, et al. Palmitoylome profiling indicates that androgens regulate the palmitoylation of alpha−Tubulin in prostate cancer−Derived lncap cells and supernatants. Oncol Rep. (2019) 42:2788–96. doi: 10.3892/or.2019.7333
119. Sharma C, Wang HX, Li Q, Knoblich K, Reisenbichler ES, Richardson AL, et al. Protein acyltransferase dhhc3 regulates breast tumor growth, oxidative stress, and senescence. Cancer Res. (2017) 77:6880–90. doi: 10.1158/0008-5472.CAN-17-1536
120. Coleman DT, Soung YH, Surh YJ, Cardelli JA, and Chung J. Curcumin prevents palmitoylation of integrin beta4 in breast cancer cells. PloS One. (2015) 10:e0125399. doi: 10.1371/journal.pone.0125399
121. Zhang X, Hou J, Zhou G, Wang H, and Wu Z. Zdhhc3-mediated S-palmitoylation of slc9a2 regulates apoptosis in kidney clear cell carcinoma. J Cancer Res Clin Oncol. (2024) 150:194. doi: 10.1007/s00432-024-05737-y
122. Zhang Y, Li F, Fu K, Liu X, Lien IC, and Li H. Potential role of S-palmitoylation in cancer stem cells of lung adenocarcinoma. Front Cell Dev Biol. (2021) 9:734897. doi: 10.3389/fcell.2021.734897
123. Wang Y, Zhang S, He H, Luo H, Xia Y, Jiang Y, et al. Repositioning lomitapide to block zdhhc5-dependant palmitoylation on sstr5 leads to anti-proliferation effect in preclinical pancreatic cancer models. Cell Death Discov. (2023) 9:60. doi: 10.1038/s41420-023-01359-4
124. Chen X, Ma H, Wang Z, Zhang S, Yang H, and Fang Z. Ezh2 palmitoylation mediated by zdhhc5 in P53-mutant glioma drives Malignant development and progression. Cancer Res. (2017) 77:4998–5010. doi: 10.1158/0008-5472.CAN-17-1139
125. McClellan B, Wilson CN, Brenner AJ, Jolly CA, and deGraffenried L. Flotillin-1 palmitoylation is essential for its stability and subsequent tumor promoting capabilities. Oncogene. (2024) 43:1063–74. doi: 10.1038/s41388-024-02946-0
126. Peng F, Lu J, Su K, Liu X, Luo H, He B, et al. Oncogenic fatty acid oxidation senses circadian disruption in sleep-deficiency-enhanced tumorigenesis. Cell Metab. (2024) 36:1598–618 e11. doi: 10.1016/j.cmet.2024.04.018
127. Woodley KT and Collins MO. S-acylated golga7b stabilises dhhc5 at the plasma membrane to regulate cell adhesion. EMBO Rep. (2019) 20:e47472. doi: 10.15252/embr.201847472
128. Chen L, Xing X, Zhu Y, Chen Y, Pei H, Song Q, et al. Palmitoylation alters ldha activity and pancreatic cancer response to chemotherapy. Cancer Lett. (2024) 587:216696. doi: 10.1016/j.canlet.2024.216696
129. Liu C and Li X. Greasy glut1 maintains glioblastoma Malignancy. Mol Cell Oncol. (2021) 8:2009423. doi: 10.1080/23723556.2021.2009423
130. Yuan M, Chen X, Sun Y, Jiang L, Xia Z, Ye K, et al. Zdhhc12-mediated claudin-3 S-palmitoylation determines ovarian cancer progression. Acta Pharm Sin B. (2020) 10:1426–39. doi: 10.1016/j.apsb.2020.03.008
131. Huang J, Yang JG, Ren JG, Xia HF, Chen GH, Fu QY, et al. Overexpression of rab27a in oral squamous cell carcinoma promotes tumor migration and invasion via modulation of egfr membrane stability. Int J Mol Sci. (2023) 24:13103. doi: 10.3390/ijms241713103
132. Chen X, Niu W, Fan X, Yang H, Zhao C, Fan J, et al. Oct4a palmitoylation modulates tumorigenicity and stemness in human glioblastoma cells. Neuro Oncol. (2023) 25:82–96. doi: 10.1093/neuonc/noac157
133. Zhang N, Yang Y, and Xu D. Emerging roles of palmitoylation in pyroptosis. Trends Cell Biol. (2025) 35:500–14. doi: 10.1016/j.tcb.2024.10.005
134. Hu D, Li Y, Wang X, Zou H, Li Z, Chen W, et al. Palmitoylation of nlrp3 modulates inflammasome activation and inflammatory bowel disease development. J Immunol. (2024) 213:481–93. doi: 10.4049/jimmunol.2300241
135. Pei X, Li KY, Shen Y, Li JT, Lei MZ, Fang CY, et al. Palmitoylation of mdh2 by zdhhc18 activates mitochondrial respiration and accelerates ovarian cancer growth. Sci China Life Sci. (2022) 65:2017–30. doi: 10.1007/s11427-021-2048-2
136. Zhang H, Sun Y, Wang Z, Huang X, Tang L, Jiang K, et al. Zdhhc20-mediated S-palmitoylation of ythdf3 stabilizes myc mrna to promote pancreatic cancer progression. Nat Commun. (2024) 15:4642. doi: 10.1038/s41467-024-49105-3
137. Tomic G, Sheridan C, Refermat AY, Baggelaar MP, Sipthorp J, Sudarshan B, et al. Palmitoyl transferase zdhhc20 promotes pancreatic cancer metastasis. Cell Rep. (2024) 43:114224. doi: 10.1016/j.celrep.2024.114224
138. Kharbanda A, Runkle K, Wang W, and Witze ES. Induced sensitivity to egfr inhibitors is mediated by palmitoylated cysteine 1025 of egfr and requires oncogenic kras. Biochem Biophys Res Commun. (2017) 493:213–9. doi: 10.1016/j.bbrc.2017.09.044
139. Tang J, Peng W, Feng Y, Le X, Wang K, Xiang Q, et al. Cancer cells escape P53’s tumor suppression through ablation of zdhhc1-mediated P53 palmitoylation. Oncogene. (2021) 40:5416–26. doi: 10.1038/s41388-021-01949-5
140. Liu L, Wang L, Liu L, Qu X, Zhao W, Ding J, et al. Acyltransferase zinc finger dhhc-type containing 2 aggravates gastric carcinoma growth by targeting nrf2 signaling: A mechanism-based multicombination bionic nano-drug therapy. Redox Biol. (2024) 70:103051. doi: 10.1016/j.redox.2024.103051
141. Zhang Q, Yang X, Wu J, Ye S, Gong J, Cheng WM, et al. Reprogramming of palmitic acid induced by dephosphorylation of acox1 promotes beta-catenin palmitoylation to drive colorectal cancer progression. Cell Discov. (2023) 9:26. doi: 10.1038/s41421-022-00515-x
142. Planey SL, Keay SK, Zhang CO, and Zacharias DA. Palmitoylation of cytoskeleton associated protein 4 by dhhc2 regulates antiproliferative factor-mediated signaling. Mol Biol Cell. (2009) 20:1454–63. doi: 10.1091/mbc.e08-08-0849
143. Lee JW, Hur J, Kwon YW, Chae CW, Choi JI, Hwang I, et al. Kai1(Cd82) is a key molecule to control angiogenesis and switch angiogenic milieu to quiescent state. J Hematol Oncol. (2021) 14:148. doi: 10.1186/s13045-021-01147-6
144. Pedram A, Razandi M, Deschenes RJ, and Levin ER. Dhhc-7 and -21 are palmitoylacyltransferases for sex steroid receptors. Mol Biol Cell. (2012) 23:188–99. doi: 10.1091/mbc.E11-07-0638
145. Liu B, Zhao X, Zhang S, Li Q, Li X, Huang D, et al. Targeting zdhhc21/fasn axis for the treatment of diffuse large B-cell lymphoma. Leukemia. (2024) 38:351–64. doi: 10.1038/s41375-023-02130-5
146. Lin Z, Agarwal S, Tan S, Shi H, Lu X, Tao Z, et al. Palmitoyl acyltransferase zdhhc7 inhibits androgen receptor and suppresses prostate cancer. Oncogene. (2023) 42:2126–38. doi: 10.1038/s41388-023-02718-2
147. Zhang N and Xu D. Controlling pyroptosis through post-translational modifications of gasdermin D. Dev Cell. (2025) 60:994–1007. doi: 10.1016/j.devcel.2025.02.005
148. Kwon H, Choi M, Ahn Y, Jang D, and Pak Y. Flotillin-1 palmitoylation turnover by apt-1 and zdhhc-19 promotes cervical cancer progression by suppressing igf-1 receptor desensitization and proteostasis. Cancer Gene Ther. (2023) 30:302–12. doi: 10.1038/s41417-022-00546-2
149. Berg V, Rusch M, Vartak N, Jungst C, Schauss A, Waldmann H, et al. Mirs-138 and -424 control palmitoylation-dependent cd95-mediated cell death by targeting acyl protein thioesterases 1 and 2 in cll. Blood. (2015) 125:2948–57. doi: 10.1182/blood-2014-07-586511
150. Goloshvili G, Barbakadze T, and Mikeladze D. Sodium nitroprusside induces H-ras depalmitoylation and alters the cellular response to hypoxia in differentiated and undifferentiated pc12 cells. Cell Biochem Funct. (2019) 37:545–52. doi: 10.1002/cbf.3431
151. Galluzzo P, Ascenzi P, Bulzomi P, and Marino M. The nutritional flavanone naringenin triggers antiestrogenic effects by regulating estrogen receptor alpha-palmitoylation. Endocrinology. (2008) 149:2567–75. doi: 10.1210/en.2007-1173
152. Holden JK, Crawford JJ, Noland CL, Schmidt S, Zbieg JR, Lacap JA, et al. Small molecule dysregulation of tead lipidation induces a dominant-negative inhibition of hippo pathway signaling. Cell Rep. (2020) 31:107809. doi: 10.1016/j.celrep.2020.107809
153. Noland CL, Gierke S, Schnier PD, Murray J, Sandoval WN, Sagolla M, et al. Palmitoylation of tead transcription factors is required for their stability and function in hippo pathway signaling. Structure. (2016) 24:179–86. doi: 10.1016/j.str.2015.11.005
154. Mills KR, Misra J, and Torabifard H. Allosteric modulation of the yap/taz-tead interaction by palmitoylation and small-molecule inhibitors. J Phys Chem B. (2024) 128:3795–806. doi: 10.1021/acs.jpcb.3c07073
155. Gridnev A, Maity S, and Misra JR. Structure-based discovery of a novel small-molecule inhibitor of tead palmitoylation with anticancer activity. Front Oncol. (2022) 12:1021823. doi: 10.3389/fonc.2022.1021823
156. Li Y, Li Y, Ning C, Yue J, Zhang C, He X, et al. Discovering inhibitors of tead palmitate binding pocket through virtual screening and molecular dynamics simulation. Comput Biol Chem. (2022) 98:107648. doi: 10.1016/j.compbiolchem.2022.107648
157. Kharbanda A, Walter DM, Gudiel AA, Schek N, Feldser DM, and Witze ES. Blocking egfr palmitoylation suppresses pi3k signaling and mutant kras lung tumorigenesis. Sci Signal. (2020) 13:eaax2364. doi: 10.1126/scisignal.aax2364
158. Runkle KB, Kharbanda A, Stypulkowski E, Cao XJ, Wang W, Garcia BA, et al. Inhibition of dhhc20-mediated egfr palmitoylation creates a dependence on egfr signaling. Mol Cell. (2016) 62:385–96. doi: 10.1016/j.molcel.2016.04.003
159. Thomas R, Srivastava S, Katreddy RR, Sobieski J, and Weihua Z. Kinase-inactivated egfr is required for the survival of wild-type egfr-expressing cancer cells treated with tyrosine kinase inhibitors. Int J Mol Sci. (2019) 20:2515. doi: 10.3390/ijms20102515
160. Bu L, Zhang Z, Chen J, Fan Y, Guo J, Su Y, et al. High-fat diet promotes liver tumorigenesis via palmitoylation and activation of akt. Gut. (2024) 73:1156–68. doi: 10.1136/gutjnl-2023-330826
161. Ali A, Levantini E, Teo JT, Goggi J, Clohessy JG, Wu CS, et al. Fatty acid synthase mediates egfr palmitoylation in egfr mutated non-small cell lung cancer. EMBO Mol Med. (2018) 10:e8313. doi: 10.15252/emmm.201708313
162. Sun Y, Zhu L, Liu P, Zhang H, Guo F, and Jin X. Zdhhc2-mediated agk palmitoylation activates akt-mtor signaling to reduce sunitinib sensitivity in renal cell carcinoma. Cancer Res. (2023) 83:2034–51. doi: 10.1158/0008-5472.CAN-22-3105
163. Zhang C, Zhang Y, Dong Y, Zi R, Wang Y, Chen Y, et al. Non-alcoholic fatty liver disease promotes liver metastasis of colorectal cancer via fatty acid synthase dependent egfr palmitoylation. Cell Death Discov. (2024) 10:41. doi: 10.1038/s41420-023-01770-x
164. Bu J, Zhong W, Li M, He S, Zhang M, Zhang Y, et al. Cd82 Palmitoylation Site Mutations at Cys5+Cys74 Affect Egfr Internalization and Metabolism through Recycling Pathway. Acta Biochim Biophys Sin (Shanghai). (2022) 54:400–8. doi: 10.3724/abbs.2022011
165. Lu X, An L, Fan G, Zang L, Huang W, Li J, et al. Egfr signaling promotes nuclear translocation of plasma membrane protein tspan8 to enhance tumor progression via stat3-mediated transcription. Cell Res. (2022) 32:359–74. doi: 10.1038/s41422-022-00628-8
166. Sun Y, Hu L, Tao Z, Jarugumilli GK, Erb H, Singh A, et al. Pharmacological blockade of tead-yap reveals its therapeutic limitation in cancer cells. Nat Commun. (2022) 13:6744. doi: 10.1038/s41467-022-34559-0
167. Huang J, Li J, Tang J, Wu Y, Dai F, Yi Z, et al. Zdhhc22-mediated mtor palmitoylation restrains breast cancer growth and endocrine therapy resistance. Int J Biol Sci. (2022) 18:2833–50. doi: 10.7150/ijbs.70544
168. Di Vizio D, Adam RM, Kim J, Kim R, Sotgia F, Williams T, et al. Caveolin-1 interacts with a lipid raft-associated population of fatty acid synthase. Cell Cycle. (2008) 7:2257–67. doi: 10.4161/cc.7.14.6475
169. Sada R, Kimura H, Fukata Y, Fukata M, Yamamoto H, and Kikuchi A. Dynamic palmitoylation controls the microdomain localization of the dkk1 receptors ckap4 and lrp6. Sci Signal. (2019) 12:eaat9519. doi: 10.1126/scisignal.aat9519
170. Fiorentino M, Zadra G, Palescandolo E, Fedele G, Bailey D, Fiore C, et al. Overexpression of fatty acid synthase is associated with palmitoylation of wnt1 and cytoplasmic stabilization of beta-catenin in prostate cancer. Lab Invest. (2008) 88:1340–8. doi: 10.1038/labinvest.2008.97
171. Yang Q, Qin T, An T, Wu H, Xu G, Xiang J, et al. Novel porcn inhibitor whn-88 targets wnt/beta-catenin pathway and prevents the growth of wnt-driven cancers. Eur J Pharmacol. (2023) 945:175628. doi: 10.1016/j.ejphar.2023.175628
172. Sadeghi RS, Kulej K, Kathayat RS, Garcia BA, Dickinson BC, Brady DC, et al. Wnt5a signaling induced phosphorylation increases apt1 activity and promotes melanoma metastatic behavior. Elife. (2018) 7:e34362. doi: 10.7554/eLife.34362
173. Wang W, Runkle KB, Terkowski SM, Ekaireb RI, and Witze ES. Protein depalmitoylation is induced by wnt5a and promotes polarized cell behavior. J Biol Chem. (2015) 290:15707–16. doi: 10.1074/jbc.M115.639609
174. Stypulkowski E, Asangani IA, and Witze ES. The depalmitoylase apt1 directs the asymmetric partitioning of notch and wnt signaling during cell division. Sci Signal. (2018) 11:eaam8705. doi: 10.1126/scisignal.aam8705
175. Garcia-Reyes B, Witt L, Jansen B, Karasu E, Gehring T, Leban J, et al. Discovery of inhibitor of wnt production 2 (Iwp-2) and related compounds as selective atp-competitive inhibitors of casein kinase 1 (Ck1) delta/epsilon. J Med Chem. (2018) 61:4087–102. doi: 10.1021/acs.jmedchem.8b00095
176. Schwab RHM, Amin N, Flanagan DJ, Johanson TM, Phesse TJ, and Vincan E. Wnt is necessary for mesenchymal to epithelial transition in colorectal cancer cells. Dev Dyn. (2018) 247:521–30. doi: 10.1002/dvdy.24527
177. Dong Y, Li K, Xu Z, Ma H, Zheng J, Hu Z, et al. Exploration of the linkage elements of porcupine antagonists led to potent wnt signaling pathway inhibitors. Bioorg Med Chem. (2015) 23:6855–68. doi: 10.1016/j.bmc.2015.09.048
178. Lanyon-Hogg T, Patel NV, Ritzefeld M, Boxall KJ, Burke R, Blagg J, et al. Microfluidic mobility shift assay for real-time analysis of peptide N-palmitoylation. SLAS Discov. (2017) 22:418–24. doi: 10.1177/2472555216689529
179. Konitsiotis AD, Chang SC, Jovanovic B, Ciepla P, Masumoto N, Palmer CP, et al. Attenuation of hedgehog acyltransferase-catalyzed sonic hedgehog palmitoylation causes reduced signaling, proliferation and invasiveness of human carcinoma cells. PloS One. (2014) 9:e89899. doi: 10.1371/journal.pone.0089899
180. Konitsiotis AD, Jovanovic B, Ciepla P, Spitaler M, Lanyon-Hogg T, Tate EW, et al. Topological analysis of hedgehog acyltransferase, a multipalmitoylated transmembrane protein. J Biol Chem. (2015) 290:3293–307. doi: 10.1074/jbc.M114.614578
181. Ren JG, Xing B, Lv K, O’Keefe RA, Wu M, Wang R, et al. Rab27b controls palmitoylation-dependent nras trafficking and signaling in myeloid leukemia. J Clin Invest. (2023) 133:e165510. doi: 10.1172/JCI165510
182. Li H, Yu X, Liu X, Hu P, Shen L, Zhou Y, et al. Wogonoside induces depalmitoylation and translocation of plscr1 and N-ras in primary acute myeloid leukaemia cells. J Cell Mol Med. (2018) 22:2117–30. doi: 10.1111/jcmm.13481
183. Wang C, Cui ZY, Chang HY, Wu CZ, Yu ZY, Wang XT, et al. 2-bromopalmitate inhibits Malignant behaviors of hpscc cells by hindering the membrane location of ras protein. Exp Biol Med (Maywood). (2023) 248:2393–407. doi: 10.1177/15353702231220671
184. Jiang Y, Xu Y, Zhu C, Xu G, Xu L, Rao Z, et al. Stat3 palmitoylation initiates a positive feedback loop that promotes the Malignancy of hepatocellular carcinoma cells in mice. Sci Signal. (2023) 16:eadd2282. doi: 10.1126/scisignal.add2282
185. Fan X, Zhang S, Sun S, Bi W, Li S, Wang W, et al. Gfap palmitoylcation mediated by zdhhc23 in spinal astrocytes contributes to the development of neuropathic pain. Reg Anesth Pain Med. (2024) 49:821–30. doi: 10.1136/rapm-2023-104980
186. Zhang X, Lao M, Sun K, Yang H, He L, Liu X, et al. Sphingolipid synthesis in tumor-associated macrophages confers immunotherapy resistance in hepatocellular carcinoma. Sci Adv. (2025) 11:eadv0558. doi: 10.1126/sciadv.adv0558
187. Shen KY, Song YC, Chen IH, Chong P, and Liu SJ. Depletion of tumor-associated macrophages enhances the anti-tumor immunity induced by a toll-like receptor agonist-conjugated peptide. Hum Vaccin Immunother. (2014) 10:3241–50. doi: 10.4161/hv.29275
188. Tohumeken S, Baur R, Bottcher M, Stoll A, Loschinski R, Panagiotidis K, et al. Palmitoylated proteins on aml-derived extracellular vesicles promote myeloid-derived suppressor cell differentiation via tlr2/akt/mtor signaling. Cancer Res. (2020) 80:3663–76. doi: 10.1158/0008-5472.CAN-20-0024
189. Nie J, Ai J, Hong W, Bai Z, Wang B, Yang J, et al. Cisplatin-Induced Oxpapc Release Enhances Mdscs Infiltration into Ll2 Tumour Tissues through Mcp-1/Ccl2 and Ltb4/Ltb4r Pathways. Cell Prolif. (2024) 57:e13570. doi: 10.1111/cpr.13570
190. Zhang Z, Ren C, Xiao R, Ma S, Liu H, Dou Y, et al. Palmitoylation of tim-3 promotes immune exhaustion and restrains antitumor immunity. Sci Immunol. (2024) 9:eadp7302. doi: 10.1126/sciimmunol.adp7302
191. Cheng X, Tan X, Wang W, Zhang Z, Zhu R, Wu M, et al. Long-chain acylcarnitines induce senescence of invariant natural killer T cells in hepatocellular carcinoma. Cancer Res. (2023) 83:582–94. doi: 10.1158/0008-5472.CAN-22-2273
192. Harding JJ, Awada A, Roth G, Decaens T, Merle P, Kotecki N, et al. First-in-human effects of ppt1 inhibition using the oral treatment with gns561/ezurpimtrostat in patients with primary and secondary liver cancers. Liver Cancer. (2022) 11:268–77. doi: 10.1159/000522418
193. Sharma G, Ojha R, Noguera-Ortega E, Rebecca VW, Attanasio J, Liu S, et al. Ppt1 inhibition enhances the antitumor activity of anti-pd-1 antibody in melanoma. JCI Insight. (2022) 7:e133225. doi: 10.1172/jci.insight.165688
194. Lv X, Liu J, Ruan J, Chen P, He C, Zhao X, et al. Targeting the disrupted hippo signaling to prevent neoplastic renal epithelial cell immune evasion. Nat Commun. (2025) 16:2858. doi: 10.1038/s41467-025-57697-7
195. Yao H, Li C, He F, Song T, Brosseau JP, Wang H, et al. A peptidic inhibitor for pd-1 palmitoylation targets its expression and functions. RSC Chem Biol. (2021) 2:192–205. doi: 10.1039/d0cb00157k
196. Zhang G, Jiang P, Tang W, Wang Y, Qiu F, An J, et al. Cpt1a induction following epigenetic perturbation promotes mavs palmitoylation and activation to potentiate antitumor immunity. Mol Cell. (2023) 83:4370–85 e9. doi: 10.1016/j.molcel.2023.10.043
197. Huang J, Tsang WY, Fang XN, Zhang Y, Luo J, Gong LQ, et al. Fasn inhibition decreases mhc-I degradation and synergizes with pd-L1 checkpoint blockade in hepatocellular carcinoma. Cancer Res. (2024) 84:855–71. doi: 10.1158/0008-5472.CAN-23-0966
198. Shahid M, Kim M, Jin P, Zhou B, Wang Y, Yang W, et al. S-palmitoylation as a functional regulator of proteins associated with cisplatin resistance in bladder cancer. Int J Biol Sci. (2020) 16:2490–505. doi: 10.7150/ijbs.45640
199. Xie F, Tang S, Zhang Y, Zhao Y, Lin Y, Yao Y, et al. Designing peptide-based nanoinhibitors of programmed cell death ligand 1 (Pd-L1) for enhanced chemo-immunotherapy. ACS Nano. (2024) 18:1690–701. doi: 10.1021/acsnano.3c09968
200. Lin Z, Huang K, Guo H, Jia M, Sun Q, Chen X, et al. Targeting zdhhc9 potentiates anti-programmed death-ligand 1 immunotherapy of pancreatic cancer by modifying the tumor microenvironment. BioMed Pharmacother. (2023) 161:114567. doi: 10.1016/j.biopha.2023.114567
201. Guo HZ, Feng RX, Zhang YJ, Yu YH, Lu W, Liu JJ, et al. A cd36-dependent non-canonical lipid metabolism program promotes immune escape and resistance to hypomethylating agent therapy in aml. Cell Rep Med. (2024) 5:101592. doi: 10.1016/j.xcrm.2024.101592
202. Shi YY, Fan G, Tan R, Li S, Sun HB, Li R, et al. Correction: treating icb-resistant cancer by inhibiting pd-L1 via dhhc3 degradation induced by cell penetrating peptide-induced chimera conjugates. Cell Death Dis. (2024) 15:839. doi: 10.1038/s41419-024-07182-8
203. Shi X, Zhao X, He Y, Zhang L, Zheng X, Qin X, et al. Posttranslational remodeling micelle reverses cell-surface and exosomal pd-L1 immunosuppression in tumors resistant to pd-L1 antibody therapy. J Control Release. (2025) 384:113961. doi: 10.1016/j.jconrel.2025.113961
204. Hwang D and Henry WS. Targeting gpx4 palmitoylation to boost antitumor immunity. Trends Cancer. (2025) 11:491–2. doi: 10.1016/j.trecan.2025.05.001
205. Ma L, Chen C, Zhao C, Li T, Ma L, Jiang J, et al. Targeting carnitine palmitoyl transferase 1a (Cpt1a) induces ferroptosis and synergizes with immunotherapy in lung cancer. Signal Transduct Target Ther. (2024) 9:64. doi: 10.1038/s41392-024-01772-w
206. Nandi I, Ji L, Smith HW, Avizonis D, Papavasiliou V, Lavoie C, et al. Targeting fatty acid oxidation enhances response to her2-targeted therapy. Nat Commun. (2024) 15:6587. doi: 10.1038/s41467-024-50998-3
207. Liang J, Liao J, Chang R, Jia W, Li G, Chen Z, et al. Riplet promotes lipid metabolism changes associated with cd8 T cell exhaustion and anti-pd-1 resistance in hepatocellular carcinoma. Sci Immunol. (2025) 10:eado3485. doi: 10.1126/sciimmunol.ado3485
208. Xu J, Su Z, Cheng X, Hu S, Wang W, Zou T, et al. High ppt1 expression predicts poor clinical outcome and ppt1 inhibitor dc661 enhances sorafenib sensitivity in hepatocellular carcinoma. Cancer Cell Int. (2022) 22:115. doi: 10.1186/s12935-022-02508-y
209. Jennings BC, Nadolski MJ, Ling Y, Baker MB, Harrison ML, Deschenes RJ, et al. 2-bromopalmitate and 2-(2-hydroxy-5-nitro-benzylidene)-benzo[B]Thiophen-3-one inhibit dhhc-mediated palmitoylation in vitro. J Lipid Res. (2009) 50:233–42. doi: 10.1194/jlr.M800270-JLR200
210. Wang X, Zhu X, Li B, Wei X, Chen Y, Zhang Y, et al. Intelligent biomimetic nanoplatform for systemic treatment of metastatic triple-negative breast cancer via enhanced egfr-targeted therapy and immunotherapy. ACS Appl Mater Interfaces. (2022) 14:23152–63. doi: 10.1021/acsami.2c02925
211. Laraba L, Hillson L, de Guibert JG, Hewitt A, Jaques MR, Tang TT, et al. Inhibition of yap/taz-driven tead activity prevents growth of nf2-null schwannoma and meningioma. Brain. (2023) 146:1697–713. doi: 10.1093/brain/awac342
212. Hillen H, Candi A, Vanderhoydonck B, Kowalczyk W, Sansores-Garcia L, Kesikiadou EC, et al. A novel irreversible tead inhibitor, swtx-143, blocks hippo pathway transcriptional output and causes tumor regression in preclinical mesothelioma models. Mol Cancer Ther. (2024) 23:3–13. doi: 10.1158/1535-7163.MCT-22-0681
213. Fan M, Lu W, Che J, Kwiatkowski NP, Gao Y, Seo HS, et al. Covalent disruptor of yap-tead association suppresses defective hippo signaling. Elife. (2022) 11:e78810. doi: 10.7554/eLife.78810
214. Hu L, Sun Y, Liu S, Erb H, Singh A, Mao J, et al. Discovery of a new class of reversible tea domain transcription factor inhibitors with a novel binding mode. Elife. (2022) 11:e80210. doi: 10.7554/eLife.80210
215. Remsberg JR, Suciu RM, Zambetti NA, Hanigan TW, Firestone AJ, Inguva A, et al. Abhd17 regulation of plasma membrane palmitoylation and N-ras-dependent cancer growth. Nat Chem Biol. (2021) 17:856–64. doi: 10.1038/s41589-021-00785-8
216. Wang Q, Wang J, Yu D, Zhang Q, Hu H, Xu M, et al. Benzosceptrin C induces lysosomal degradation of pd-L1 and promotes antitumor immunity by targeting dhhc3. Cell Rep Med. (2024) 5:101357. doi: 10.1016/j.xcrm.2023.101357
217. Sun G, Zhao S, Fan Z, Wang Y, Liu H, Cao H, et al. Chsy1 promotes cd8(+) T cell exhaustion through activation of succinate metabolism pathway leading to colorectal cancer liver metastasis based on crispr/cas9 screening. J Exp Clin Cancer Res. (2023) 42:248. doi: 10.1186/s13046-023-02803-0
218. Qiu N, Abegg D, Guidi M, Gilmore K, Seeberger PH, and Adibekian A. Artemisinin inhibits nras palmitoylation by targeting the protein acyltransferase zdhhc6. Cell Chem Biol. (2022) 29:530–7 e7. doi: 10.1016/j.chembiol.2021.07.012
219. Fan X, Gong M, Zhang S, Niu W, Sun S, Yu H, et al. Blocking palmitoylation of apelin receptor alleviates morphine tolerance in neuropathic cancer pain. Int J Biol Sci. (2024) 20:47–60. doi: 10.7150/ijbs.86888
220. Kong Y, Liu Y, Li X, Rao M, Li D, Ruan X, et al. Palmitoylation landscapes across human cancers reveal a role of palmitoylation in tumorigenesis. J Transl Med. (2023) 21:826. doi: 10.1186/s12967-023-04611-8
221. Dilly A, Honick BD, Lee YJ, Bartlett DL, and Choudry HA. Rational application of targeted therapeutics in mucinous colon/appendix cancers with positive predictive factors. Cancer Med. (2020) 9:1753–67. doi: 10.1002/cam4.2847
222. Phillips C, Bhamra I, Eagle C, Flanagan E, Armer R, Jones CD, et al. The wnt pathway inhibitor rxc004 blocks tumor growth and reverses immune evasion in wnt ligand-dependent cancer models. Cancer Res Commun. (2022) 2:914–28. doi: 10.1158/2767-9764.CRC-21-0095
223. Takahashi N, Iwahori A, Breitman TR, and Fukui T. Tunicamycin in combination with retinoic acid synergistically inhibits cell growth while decreasing palmitoylation and enhancing retinoylation of proteins in the human breast cancer cell line mcf-7. Oncol Res. (1997) 9:527–33.
224. Chaturvedi S, Pandya N, Sadhukhan S, and Sonawane A. Identification of selective plant-derived natural carotenoid and flavonoids as the potential inhibitors of dhhc-mediated protein S-palmitoylation: an in silico study. J Biomol Struct Dyn. (2024) 43:5110–23. doi: 10.1080/07391102.2024.2306502
225. Zhang Y, Zhang X, Yang X, Chen X, Wang Y, Hu J, et al. Egfr-tkis induced dpp4 drives metabolic reprogramming of persister cells in lung cancer. Adv Sci (Weinh). (2025) 12:e06950. doi: 10.1002/advs.202506950
226. Xu M, Zheng Y, Chen J, Gao C, Zhu M, Ma A, et al. Cldn4 palmitoylation promotes hepatic-to-biliary lineage transition and lenvatinib resistance in hepatocellular carcinoma. Cell Rep Med. (2025) 6:102208. doi: 10.1016/j.xcrm.2025.102208
227. Xu T, Huang C, Qi XT, Yang XC, Zhang N, Cao J, et al. 2-bromopalmitate sensitizes osteosarcoma cells to adriamycin-induced apoptosis via the modulation of chop. Eur J Pharmacol. (2019) 844:204–15. doi: 10.1016/j.ejphar.2018.12.019
228. Sharma G, Ojha R, Noguera-Ortega E, Rebecca VW, Attanasio J, Liu S, et al. Ppt1 inhibition enhances the antitumor activity of anti-pd-1 antibody in melanoma. JCI Insight. (2020) 5:e133225. doi: 10.1172/jci.insight.133225
229. Xu K, Wu T, Li X, Zhang X, Liu X, Ma S, et al. Adh1c maintains the homeostasis of metabolic microenvironment to inhibit steatotic hepatocellular carcinoma. Metabolism. (2025) 168:156267. doi: 10.1016/j.metabol.2025.156267
230. Ye Z, Song Y, Zhu M, Zheng F, Qin W, Li X, et al. Assessing the prognostic and therapeutic value of cuproptosis-related genes in colon adenocarcinoma patients. Front Cell Dev Biol. (2025) 13:1550982. doi: 10.3389/fcell.2025.1550982
231. Liu X, Qu H, Li J, Sun X, Wang Z, Wang D, et al. P53 enhances elesclomol-cu-induced cuproptosis in hepatocellular carcinoma via fdxr-mediated fdx1 upregulation. Front Oncol. (2025) 15:1584811. doi: 10.3389/fonc.2025.1584811
232. Duarte D, Nunes M, Ricardo S, and Vale N. Combination of antimalarial and cns drugs with antineoplastic agents in mcf-7 breast and ht-29 colon cancer cells: biosafety evaluation and mechanism of action. Biomolecules. (2022) 12:1490. doi: 10.3390/biom12101490
233. Passadouro M, Pedroso de Lima MC, and Faneca H. Microrna modulation combined with sunitinib as a novel therapeutic strategy for pancreatic cancer. Int J Nanomed. (2014) 9:3203–17. doi: 10.2147/IJN.S64456
Keywords: palmitoylation, tumorigenesis, signal transduction, cancer therapy, post-translational modification
Citation: Lu Q, Wu J, Yu X, Qian J and Song Z (2025) Palmitoylation in cancer: decoding its roles in signal transduction, tumor immunity, and emerging therapeutic opportunities. Front. Immunol. 16:1640016. doi: 10.3389/fimmu.2025.1640016
Received: 03 June 2025; Accepted: 05 August 2025;
Published: 04 September 2025.
Edited by:
Zebo Jiang, Zhuhai Hospital of Integrated Traditional Chinese & Western Medicine, ChinaReviewed by:
Linjiang Song, Chengdu University of Traditional Chinese Medicine, ChinaDaichao Xu, Interdisciplinary Research Center on Biology and Chemistry (CAS), China
Copyright © 2025 Lu, Wu, Yu, Qian and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juanjuan Qian, cWlhbmpqanh1QDE2My5jb20=; Zhengwei Song, ZG9jdG9yc29uZ3p3QHpqeHUuZWR1LmNu
†These authors have contributed equally to this work
 Qiguang Lu1†
Qiguang Lu1† Zhengwei Song
Zhengwei Song