- 1Department of Ophthalmology, The Second Hospital of Jilin University, Changchun, Jilin, China
- 2Department of Clinical Laboratory, The Second Hospital of Jilin University, Changchun, Jilin, China
Glaucoma, a leading cause of irreversible blindness, is characterized by retinal ganglion cell (RGC) degeneration and optic nerve damage. While elevated intraocular pressure (IOP) is a major risk factor, emerging evidence highlights neuroinflammation as a critical driver of disease progression. Glial cells, particularly microglia, astrocytes, and Müller cells, are central to this inflammatory process, orchestrating immune responses through the release of cytokines, chemokines, and complement proteins. Microglia and astrocytes contribute to early inflammatory amplification through tumor necrosis factor-alpha (TNF-α), complement, and Toll-like receptor 4 (TLR4) pathways, while Müller cells further promote tissue damage via ATP/P2X7R signaling and senescence-associated mechanisms. Leukocyte infiltration, triggered by glial-derived chemokines and matrix metalloproteinases (MMPs), underscores the intersection of innate and adaptive immunity in glaucoma. Importantly, preclinical studies demonstrate that targeting neuroinflammatory pathways confers RGC protection, thus modulating glial activation and immune signaling represents a promising therapeutic strategy for glaucoma, particularly in IOP-refractory cases. This review synthesizes current knowledge on the role of glial cells in initiating and perpetuating immune responses that exacerbate RGC loss, and details how activated microglia and astrocytes release pro-inflammatory mediators and upregulate pathogenic signaling pathways.
1 Introduction
Glaucoma, a progressive optic neuropathy, involves retinal ganglion cell (RGC) apoptosis and axonal degeneration (1). Its pathogenesis is multifactorial, driven by elevated intraocular pressure (IOP), aging, oxidative stress, and genetic predisposition, yet mounting evidence identifies neuroinflammation and immune dysregulation as pivotal contributors to disease progression (2, 3). The lamina cribrosa, the principal site of injury, exhibits marked glial activation and inflammatory remodeling in both human and experimental models, where inhibition of glial activation and cytokine signaling preserves RGC integrity and optic nerve structure (4, 5). Clinically, optic disc hemorrhages and peripapillary chorioretinal atrophy may reflect secondary manifestations of glia-driven neuroinflammation (6, 7).
Studies have shown that activation of resident glial cells in the retina, including microglia, astrocytes, and Müller cells, and infiltration of peripheral immune cells such as T lymphocytes, B lymphocytes, and regulatory T cells (Tregs), play pathogenic roles and are closely associated with RGC loss (8–10). Activated microglia release tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, and complement components that propagate inflammatory cascades and sensitize RGCs to injury (8, 9). Astrocytes amplify these immune responses through Toll-like receptor (TLR) activation, NF-κB signaling, and chemokine secretion, whereas Müller cells contribute to retinal immune modulation by releasing ATP, IL-6, and matrix metalloproteinases (MMPs), which facilitate leukocyte infiltration and tissue remodeling. This coordinated glia—immune axis establishes a self-perpetuating inflammatory loop that drives chronic neurodegeneration (8, 11). Notably, pharmacological modulation of these pathways demonstrates therapeutic promise. For example, rapamycin exerts neuroprotective effects not solely through inhibition of microglia activation but also by suppressing mTOR-dependent immune activation and cytokine release, underscoring the immunoregulatory dimension of glial targeting (12–14). The convergence of glial activation and immune signaling thus represents a central mechanism linking ocular hypertension to neurodegenerative pathology.
In summary, this review synthesizes current advances on how glial cells—microglia, astrocytes, and Müller cells—mediate immune modulation in glaucomatous neurodegeneration. It further highlights the molecular pathways by which glial-derived cytokines, chemokines, and complement proteins orchestrate retinal immune responses, contributing to RGC loss and optic nerve damage.
2 Central nervous system immune cells and glaucoma
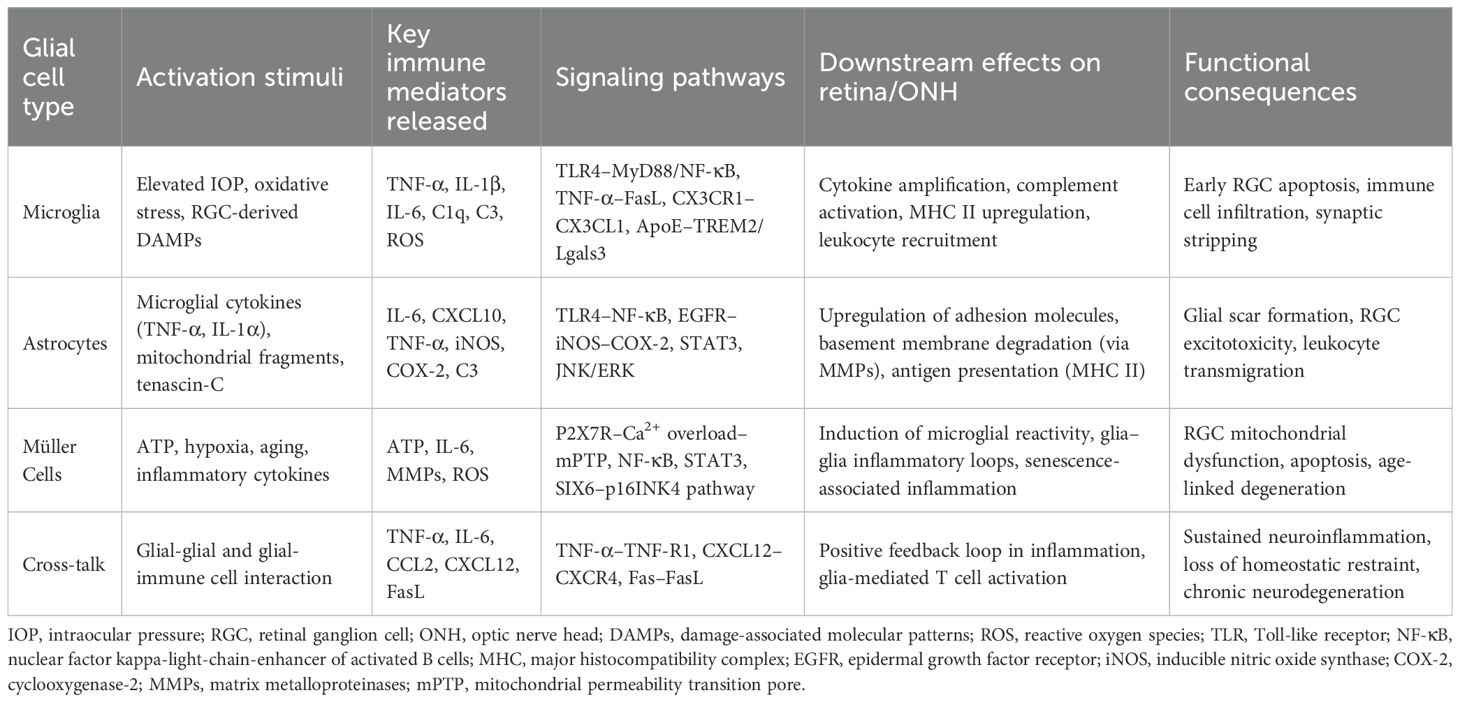
Glial cells in the retina and optic nerve head (ONH) are broadly classified into microglia (the resident immune cells of the central nervous system (CNS) and macroglia, which include astrocytes and Müller cells (15, 16). Microglia continuously survey the microenvironment, respond rapidly to injury, and orchestrate immune responses. In contrast, astrocytes and Müller cells maintain structural integrity, regulate extracellular ion homeostasis, and provide metabolic support to neurons (17). Under glaucomatous stress, these glial cells undergo activation and phenotypic shifts that transform them into neurotoxic effectors, contributing to progressive RGC loss (11).
2.1 Microglia
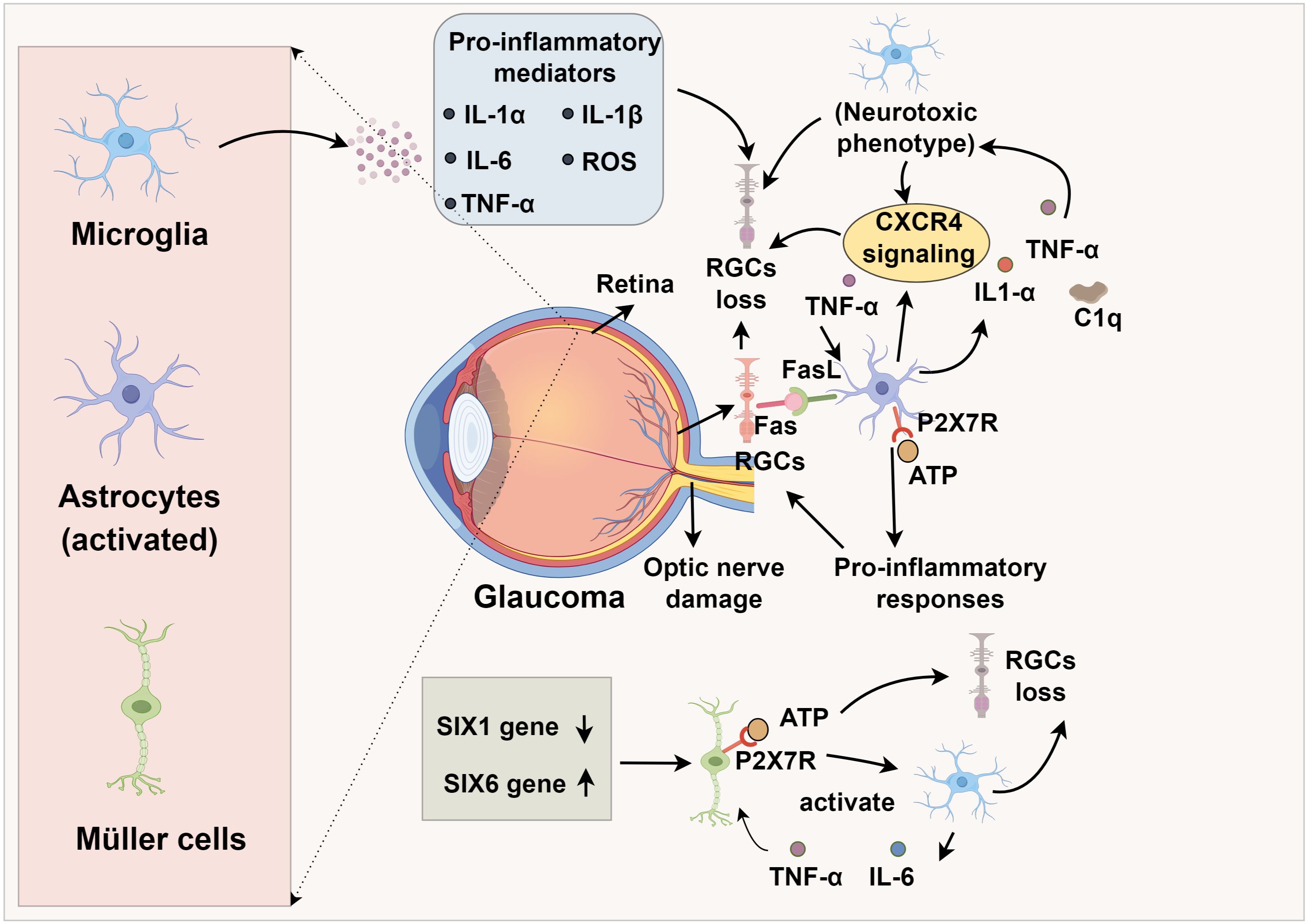
Microglia are resident immune cells within the retina that enter through the pars plana of the ciliary body and the optic nerve head (18, 19). Under physiological conditions, these cells play a crucial role in preserving retinal equilibrium by clearing cellular waste through phagocytic mechanisms (20, 21). Progressive loss of RGCs is a pathological hallmark of glaucoma, with microglia actively participating in RGC damage and immune-inflammatory responses (22). Importantly, alterations in microglial morphology and gene expression profiles emerge prior to observable RGC degeneration and measurable declines in visual function loss in glaucoma (23). As the disease advances, microglia transition into an activated state, adopting a neurodegenerative phenotype that promotes neuronal toxicity, ultimately exacerbating RGC injury and apoptosis (24, 25). Since the optic nerve constitutes a critical component of the CNS, research by Liu et al. (26) demonstrated that infrared stimulation of the CNS results in microglial activation, enhanced phagocytic activity, and release of multiple pro-inflammatory factors, including IL-1α, IL-1β, IL-6, reactive oxygen species (ROS), and TNF-α (27). Such modifications foster a pro-inflammatory CNS microenvironment that hastens RGC depletion. Retinal gap junctions (GJs) serve as vital neuroprotective structures for RGCs. In a microbead-induced ocular hypertension (OHT) murine glaucoma model, Kumar et al. (28) observed that GJ inhibition occurs concurrently with microglial activation and RGC degeneration, implying a causative relationship between microglial reactivity and RGC loss.
2.2 Astrocytes
Astrocytes, the predominant non-neuronal glial population within the retinal nerve fiber layer, ganglion cell layer, and optic nerve, are indispensable for maintaining retinal homeostasis by restraining RGC and axonal degeneration (29). Astrocytic injury has been shown to induce degeneration of RGCs and their axons, contributing to glaucomatous vision loss (30). Additionally, deformation and remodeling of the lamina cribrosa (LC), the principal structural component of the optic nerve head, can damage both the traversing optic nerve fibers and capillaries, thus acting as a critical pathological factor in glaucoma progression (31). Disruption of astrocytic integrity precipitates RGC loss and axonal damage, thereby driving glaucomatous vision decline (32, 33), indicating that astrocytic dysfunction has direct consequences for optic nerve stability and may initiate or exacerbate glaucomatous pathology. Under pathological stress, astrocytes can undergo a phenotypic shift toward a neurotoxic, pro-inflammatory state (34). This phenotypic transition is driven by microglia-derived mediators, notably interleukin (IL)-1α, tumor necrosis factor (TNF)-α, and complement component C1q (35). Given the central role of activated microglia in glaucoma pathogenesis, microglia-driven astrocytic polarization is likely a key driver of RGC apoptosis and optic nerve injury (36). Joshi et al. (37) demonstrated that mitochondrial fragments released by microglia or in vitro preconditioning with IL-18 induces a neurotoxic astrocytic phenotype detrimental to RGC viability.
2.3 Müller cells
Müller cells, the principal macroglial cells of the retina extending across its entire thickness, are indispensable for maintaining retinal homeostasis but exhibit profound dysfunction in glaucomatous pathology, with their presence and activation confirmed in human and experimental glaucoma models (38, 39). A hallmark of their reactive state is the upregulation of glial fibrillary acidic protein (GFAP), prominently observed in the glaucomatous optic nerve head (40). Pathological accumulation of extracellular ATP, a common feature in glaucoma, activates Müller cells via purinergic P2 receptors (P2Rs), initiating a feed-forward loop of additional ATP release. Given that RGCs express the high-threshold purinergic receptor P2X7R (32, 41), Müller cell–derived ATP can bind to RGC P2X7R, eliciting sustained calcium influx that perturbs intracellular calcium homeostasis (42, 43). This calcium overload promotes the opening of the mitochondrial permeability transition pore (mPTP), leading to mitochondrial depolarization, cytochrome c release, and the activation of calcium-dependent proteases such as calpains, ultimately culminating in caspase activation and apoptotic RGC death (44, 45). Müller-derived ATP promotes RGC injury via P2X7R-mediated calcium overload and apoptosis (41, 46). Müller–microglia crosstalk further amplifies retinal inflammation. In chronic ocular hypertension models, reactive Müller cells activate microglia through ATP/P2X7R signaling, stimulating the production of pro-inflammatory cytokines such as TNF-α and IL-6 (8). These cytokines, in turn, act on Müller cells to intensify inflammatory responses, with NF-κB signaling serving as a central mediator (47). The anti-inflammatory SIX1 gene is downregulated in glaucomatous Müller cells (48), suggesting its loss potentiates inflammatory cascades. Age-related mechanisms further link Müller cells to glaucoma. Epidemiological data associate glaucoma prevalence with aging (49), while the glaucoma-risk gene SIX6, overexpressed in glaucomatous Müller cells and astrocytes, drives senescence via p16INK4 upregulation (50, 51). These converging inflammatory, neurotoxic, and senescence-associated pathways underscore Müller cells as central effectors in glaucoma pathogenesis, irrespective of whether the initiating insult arises from RGCs, the optic nerve, or systemic aging processes (52, 53). Notably, glial cells operate not in isolation but through dynamic crosstalk. These interactions exemplify how glial communication drives a feed-forward neuroinflammatory circuit that accelerates RGC injury and optic nerve damage (54, 55). Recognizing this network-level integration is key to identifying therapeutic strategies that target glial synergy rather than isolated cell types.
3 Mechanistic role of glial cells in glaucoma pathogenesis
3.1 Activation of astrocytes, microglia, and Müller cells
Astrocytes and microglia, the principal glial cell populations within the retina and ONH, undergo rapid activation during the earliest stages of glaucomatous pathology (56, 57). Astrocytes, concentrated within the retinal nerve fiber layer and ganglion cell layer, are especially abundant at the lamina cribrosa, where they provide essential structural and metabolic support for RCGs (58). However, it remains debated whether glial activation serves as an initiating insult or a downstream effector in glaucoma pathogenesis. Evidence suggests that glial reactivity is not sufficient by itself to induce glaucomatous neurodegeneration, as substantial glial activation is also observed in certain ocular inflammatory models without subsequent RGC loss or optic nerve damage. Instead, elevated IOP and aging—both established glaucoma risk factors—appear to be critical upstream modulators that sensitize glial cells toward a pathogenic phenotype (11, 59). Notably, prior to detectable RGC axon damage, the expression of genes and proteins related to astrocytic activation, including pattern recognition receptor (PRR)–associated adaptor proteins and effector molecules, is markedly upregulated (60, 61). This includes enhanced complement deposition, epidermal growth factor receptor (EGFR) expression, and increased levels of pro-inflammatory mediators such as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) (62). Activated microglia similarly exhibit heightened expression of inflammatory cytokines, complement components, and major histocompatibility complex (MHC) molecules (63), while facilitating the recruitment of circulating immune cells into the ONH, thereby amplifying neuroinflammatory cascades (64). Müller cells, which span the entire retinal thickness, are also activated in response to intraocular pressure elevation and neurodegenerative stress, contributing to the early pro-inflammatory milieu through ATP release and P2X7R signaling cascades. Their activation promotes microglial reactivity and amplifies retinal inflammation via NF-κB–dependent pathways (8). Neuroinflammation driven by glial cells activation in the glaucomatous ONH may lower neuronal stress thresholds and promote neuronal injury (65, 66), culminating in glial scar formation and inhibition of RGC axonal regeneration (50). Although transient or moderate glial activation can confer neuroprotection, through trophic factor release and metabolic support, prolonged or excessive activation transitions these cells into chronic inflammatory phenotypes, thereby accelerating progressive neurodegeneration (67).
3.2 Inflammatory signaling pathways activated by glial cells
3.2.1 TLR-mediated neuroinflammatory signaling
Toll-like receptors (TLRs), a pivotal subset of pattern recognition receptors (PRRs), serve as sentinels of the innate immune system by recognizing pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) (54, 68). Distinct TLR family members display ligand specificity; for example, TLR3 is activated by viral double-stranded RNA, whereas TLR4 senses endogenous stress signals such as heat shock proteins (HSPs) (69, 70). In the central nervous system, microglia express the full complement of TLRs, while astrocytes selectively express TLR2, TLR3, TLR4, TLR5, and TLR9, each attuned to discrete PAMP or DAMP signatures (71, 72). In glaucomatous retina, TLR expression is markedly elevated (73). In vitro studies reveal that both HSPs and oxidative stress act as potent inducers of TLR expression, thereby amplifying the release of pro-inflammatory cytokines and immunostimulatory mediators that activate both innate and adaptive immune pathways (74, 75). Notably, tenascin-C, an endogenous ligand for TLR4, is upregulated in glaucomatous ONH and has been shown to activate TLR4 signaling in arthritis (76, 77). It is hypothesized that tenascin-C may initiate inflammation via TLR4 prior to DAMP release by injured RGCs (78, 79). TLR activation converges on two principal signaling axes. The myeloid differentiation primary response 88 (MyD88)-dependent pathway activates nuclear factor-κB (NF-κB) and activator protein-1 (AP-1), driving transcriptional upregulation of TNF-α, IL-1, IL-6, and a spectrum of chemokines (80, 81). Alternatively, the Toll/IL-1 receptor (TIR)-domain-containing adaptor-inducing interferon-β (TRIF)-dependent pathway predominantly engages interferon regulatory factors (IRFs) (82). Caffeic acid phenethyl ester suppresses glial activation and migration, inhibits NF-κB-mediated inflammation, and protects RGCs from degeneration (12).
3.2.2 TNF-α mediated neuroinflammatory signaling
TNF-α, a central pro-inflammatory cytokine in neurodegeneration, is secreted by both astrocytes and microglia, with astrocytes constituting the predominant source in the ONH (83, 84). In glaucomatous retina and ONH, TNF-α and its primary receptor TNF receptor 1 (TNF-R1) are markedly upregulated, with elevated expression detected in RGCs and their axons (85). Despite some evidence suggests that TNF-α may exert transient neuroprotective effects during the initial stages of optic nerve injury, the preponderance of experimental data implicates it in promoting RGC death through TNF-R–dependent caspase activation, mitochondrial dysfunction, and oxidative stress (86). Binding of TNF-α to TNF-R1 activates the TNF receptor-associated death domain (TRADD), the TNF receptor-associated factor (TRAF) superfamily, and various kinases, culminating in caspase-mediated apoptosis in RGCs (87, 88). Additionally, soluble TNF-α may promote CP-AMPAR (Ca2+-permeable AMPA receptor) expression in RGCs, exacerbating excitotoxicity (89, 90). TNF-α can also induce RGC death via the FasL pathway, as intraocular injection of TNF-α leads to RGC loss, which is attenuated by FasL inhibition (91). Pharmacological or genetic TNF-α inhibition mitigates microglial activation, axonal degeneration, and RGC loss (92, 93). In corneal chemical injury models, TNF-α blockade reduces monocyte infiltration into the retina and microglial activation, thereby decreasing the incidence of secondary glaucoma and RGC death (94). TNF-α also activates c-Jun N-terminal kinase (JNK), NF-κB, and extracellular signal-regulated kinase (ERK) pathways, further potentiating glia-mediated inflammation via IL-1 upregulation (95, 96) (Figure 1).
3.2.3 Complement activation
Complement activation constitutes another key early inflammatory event (97). Elevated complement levels are detected in glaucomatous retinas, particularly at the ONH and inner retinal layers (98, 99). Activation of retinal astrocytes correlates with increased C1q expression in RGCs (100). In a genetic model of glaucoma, Shinozaki et al. (30) reported that elevated IOP was accompanied by retinal C1q upregulation and RGC apoptosis. RGCs can sense damage and activate C1, triggering a cascade that activates C3 and C5 and recruits immune cells to the injury site (101, 102). Importantly, the role of C3 in glaucoma appears to be context dependent, showing a duality between early neuroprotection and late-stage neurotoxicity (103). Complement factors have dual roles in glaucoma (104). C1qa is expressed in ONH microglia and RGC dendrites; its inhibition reduces dendritic and synaptic loss in glaucoma (105). Moreover, membrane attack complexes (MACs) accumulate in the ONH and RGCs, and MAC inhibition reduces RGC apoptosis (104). Recent evidence suggests that in the early phase of disease, astrocyte-derived C3 can signal through the epidermal growth factor receptor (EGFR) pathway, promoting astrocytic survival responses and metabolic support to stressed axons (106). This transient EGFR–C3 interaction may act as a compensatory mechanism, particularly under acute intraocular pressure (IOP) elevation, to preserve tissue integrity (107, 108). However, with sustained or chronic activation, persistent C3 cleavage leads to excessive production of downstream complement fragments (C3b, C5b-9) and ultimately the formation of MAC (109, 110). MAC deposition on RGC somas and axons contributes to neurodegeneration by inducing membrane pore formation and triggering inflammatory cell recruitment (111). Although Harder et al. (106) suggest that early astrocytic C3/EGFR signaling can be beneficial, other studies indicate that in chronic glaucoma, prolonged complement activity becomes detrimental. This switch depends on the timing of activation, the cellular source (astrocyte-derived C3 and microglia-driven complement cascade), and the local inflammatory milieu, which collectively determine whether complement exerts neuroprotective or neurotoxic effects (112–114). Ischemia-reperfusion increases retinal complement expression and deposition, while C3 knockout reduces optic nerve damage and increases RGC survival (115, 116). Bosco et al. (117) demonstrated that intravitreal AAV2.CR2-Crry injection reduces retinal C3d deposition and protects RGC axons and soma under chronic ocular hypertension.
3.2.4 EGFR/iNOS/COX-2 pathways
EGFR expression and tyrosine phosphorylation are elevated in activated ONH astrocytes, promoting the production of iNOS, COX-2, and prostaglandins (PGs), thereby affecting RGC survival and ONH structure (118, 119). In high IOP mouse models, both neuronal nitric oxide synthase (nNOS) and iNOS are elevated in astrocytes at the ONH and retinal layers (120). Neufeld et al. (121) linked axonal damage to excessive nitric oxide (NO) production. While eNOS and nNOS are constitutively expressed in normal ONH astrocytes and vasculature, iNOS is upregulated by day 4 of elevated IOP and persists for months. Aminoguanidine, an iNOS inhibitor, significantly reduces RGC loss and may offer neuroprotection in glaucoma (122). Zhang et al. (119, 123) showed that while COX-2 is undetectable in normal ONH, it becomes detectable within 24 hours in cultured explants and peaks at 3 days. EGF induces COX-2 expression and PGE2 synthesis in astrocytes in a time-dependent manner, which is blocked by EGFR inhibitor AG1478. Downstream, ERK and p38 pathways also regulate COX-2/PGE2 production via EGFR signaling. COX-2 oxidizes arachidonic acid to produce PGs; PGD2, PGE2, and PGI2 may exert neuroprotective effects via DP1, EP2/EP4, and IP receptors, whereas PGE2 and PGF2α can induce neurotoxicity via EP1 and FP receptors (123, 124). Although substantial evidence implicates the EGFR/iNOS/COX-2 axis in glaucomatous neuroinflammation, the temporal dynamics and context-dependent roles of individual inflammatory mediators across disease stages remain incompletely understood, warranting further investigation.
3.2.5 JAK/STAT-mediated inflammatory signaling
The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway is a key regulator of glial-mediated neuroinflammation and has been increasingly implicated in glaucomatous neurodegeneration (125). Upon cytokine binding—particularly IL-6, IL-10, and interferons—glial cell–expressed receptors activate JAK kinases, leading to phosphorylation and nuclear translocation of STAT proteins, which then drive transcription of pro- or anti-inflammatory genes depending on the cellular context (126). In glaucomatous retina, STAT3 is the most prominently activated STAT protein in astrocytes and Müller cells and is responsible for upregulating genes involved in gliosis (GFAP), cellular stress responses, and cytokine amplification (IL-6, SOCS3). Notably, elevated STAT3 phosphorylation has been documented in the optic nerve head of both rodent models and human glaucoma tissues, suggesting a conserved role in glial activation and RGC injury (127, 128). Inhibition of JAK2 or STAT3 pharmacologically (with AG490 or Stattic) mitigates gliosis, preserves RGC function, and reduces optic nerve damage in experimental models of chronic ocular hypertension (127). However, the JAK/STAT pathway exhibits dual roles: transient STAT3 activation may promote glial neuroprotective programs, while sustained or excessive activation promotes gliosis and neurotoxicity (11, 126). Importantly, crosstalk with NF-κB and PI3K/AKT pathways further integrates STAT3 into a complex signaling hub that fine-tunes glial responses under stress conditions (129). Thus, therapeutic targeting of the JAK/STAT pathway requires precise temporal and cell-specific modulation to avoid disrupting beneficial glial responses.
3.3 Leukocyte infiltration and RGC death triggered by glial activation
3.3.1 Immune cells infiltration in glaucoma
Astrocyte-derived matrix metalloproteinases (MMPs) may degrade the basement membrane and compromise the glial lamina at the glaucomatous ONH (130). Leukocyte transendothelial migration is among the earliest detectable changes in DBA/2J glaucoma mouse models (131). In healthy optic nerves, CD163+ macrophages are sparsely distributed along axonal septa, whereas both early- and late-stage glaucoma show increased infiltration of CD163+ macrophages into the nerve bundles (132). The accumulation and activation of macrophages and microglia within the optic nerve have been documented across multiple glaucoma models and are considered pivotal contributors to early disease pathogenesis (103, 133, 134). Transcriptomic profiling by Howell and Johnson revealed early-stage upregulation of selectins, adhesion molecules, and chemokines in glaucomatous ONH, which collectively facilitate leukocyte recruitment prior to overt axonal injury. Experimental depletion of monocytes via targeted irradiation prevents optic nerve damage, whereas restoration of injury following endothelin-2 (ET-2) administration underscores the causal role of immune cell infiltration in glaucomatous neurodegeneration (135). Glycosylation-dependent cell adhesion molecule 1 (GlyCAM-1) may facilitate monocyte trafficking to the ONH (136). In addition to macrophages, T lymphocytes have emerged as critical contributors to glaucomatous immune pathology (137). Glial cells upregulate MHC class II molecules in glaucomatous human and experimental retinas, enabling antigen presentation and promoting T-cell activation (11, 137, 138). Glia-derived chemokines such as CCL2 and CXCL10 recruit T cells to the ONH, where infiltrating T cells release IFN-γ, TNF-α, and other pro-inflammatory mediators that further activate resident glial populations. This reciprocal amplification loop—where glial cytokines attract T cells, and T cells in turn enhance glial reactivity—forms a self-sustaining inflammatory circuit that exacerbates RGC injury (120, 137). Within the CNS, TNF-α activates endothelial cells, promoting integrin-dependent leukocyte migration (139). Astrocytes, which express multiple integrin isoforms, exhibit elevated perivascular integrin expression in response to elevated IOP. This upregulation facilitates extracellular matrix (ECM)–cytoskeleton coupling and promotes cellular migration, adhesion, differentiation, and pro-inflammatory signaling (140, 141).
3.3.2 RGC death triggered by glial activation
The membrane-bound form of Fas ligand (FasL) has been identified as a key effector in RGC apoptosis in glaucomatous mouse models (91). Krishnan et al. (21) found that activated microglia release TNF-α, which upregulates FasL expression on microglia in glaucomatous retinas, enhancing FasL-Fas binding to RGCs and thereby directly triggering apoptosis and exacerbating glaucoma progression. Moreover, microglial activation is associated with upregulation of the apolipoprotein E (ApoE) gene and the galectin-3 (Lgals3) gene (142). Knockout of ApoE in glaucomatous mouse models prevented RGC loss and suppressed the expression of neurodegenerative genes including Lgals3, indicating that ApoE-related microglial activation contributes to disease progression (142). The ApoE–Lgals3 signaling axis thus represents a potential therapeutic target for glaucoma. Importantly, ApoE also serves as an endogenous ligand for the microglial receptor TREM2, activating the DAP12–SYK signaling cascade that drives metabolic reprogramming, proliferation, and a disease-associated microglial (DAM) phenotype (143–145). In this context, upregulated Lgals3 reinforces the TREM2-ApoE axis, amplifying pro-inflammatory gene expression and promoting phagocytic activity that, while initially protective, becomes maladaptive and neurotoxic (146). Concurrently, these changes are associated with the downregulation of the homeostatic CX3CR1–CX3CL1 axis, which ordinarily restrains microglial overactivation (147, 148). The combined effect is a shift toward chronic microglial activation and synaptic toxicity, accelerating RGC degeneration. These intersecting pathways highlight a mechanistic bridge between ApoE-Lgals3 and canonical TREM2/CX3CR1 signaling in shaping microglial responses during glaucomatous neurodegeneration (149).
Extracellular ATP serves as a potent astrocytic activator (150, 151). At high concentrations, ATP activates purinergic receptor P2X7R, a high-threshold subtype of the P2 receptor (P2R) family, which induces pro-inflammatory responses in astrocytes (152, 153). P2X7R activation triggers the release of chemokines, cytokines, and ROS, thereby amplifying RGC injury, and also stimulates cytokine production in Müller cells (154, 155). Furthermore, chemokine signaling pathways contribute to astrocyte-mediated neurotoxicity. Enhanced CXCR4 activation in astrocytes or microglia facilitates glutamate release from astrocytes, provoking excitotoxic injury and promoting RGC degeneration and necrosis (30). In normal-tension glaucoma (NTG) mouse models, retinal astrocytes exhibit upregulation of CXCL-12, the endogenous agonist of CXCR4 (156). Collectively, these findings underscore astrocytes as both immune effectors and direct mediators of neuronal injury, positioning astrocytic activation and phenotypic modulation as central mechanisms in glaucoma pathogenesis (Table 1).
4 Conclusion
Glial activation and immune modulation are central drivers of glaucomatous neurodegeneration, operating through tightly interlinked inflammatory networks within the optic nerve head. Activated microglia, astrocytes, and Müller cells release cytokines, chemokines, complement components, and ROS which lead to RGC injury and recruit peripheral immune cells, further amplifying tissue damage. These responses are further shaped by key signaling pathways, including TLR4–NF-κB, TNF-α–FasL, complement C3/C5–MAC formation, and EGFR/iNOS/COX-2 cascades, as well as age-related senescence programs. Chronic or excessive activation transforms initially protective glial responses into maladaptive, neurotoxic states, driving progressive RGC apoptosis, lamina cribrosa remodeling, and irreversible vision loss.
Future therapeutic strategies should prioritize temporally targeted modulation of glial activation, inhibition of pathogenic inflammatory pathways, and disruption of maladaptive glia–immune interactions. Integrating longitudinal biomarker profiling with advanced imaging could enable stage-specific interventions, particularly in early glaucoma when neuroprotection is most feasible. Moreover, the convergence of neuroinflammatory mechanisms in glaucoma with other central nervous system disorders suggests that repurposing or co-developing glia-targeted agents may accelerate translational progress. Such approaches hold the potential to expand treatment paradigms beyond intraocular pressure control, offering new avenues for preserving vision.
Author contributions
FZ: Writing – original draft. JY: Writing – original draft. HW: Writing – original draft, Writing – review & editing. XW: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang X, Sun L, Han X, Li Z, Xing Y, Chen X, et al. The molecular mechanisms underlying retinal ganglion cell apoptosis and optic nerve regeneration in glaucoma (Review). Int J Mol Med. (2025) 55:63. doi: 10.3892/ijmm.2025.5504
2. Tribble JR, Hui F, Quintero H, El Hajji S, Bell K, Di Polo A, et al. Neuroprotection in glaucoma: Mechanisms beyond intraocular pressure lowering. Mol Aspects Med. (2023) 92:101193. doi: 10.1016/j.mam.2023.101193
3. Ishikawa M, Izumi Y, Sato K, Sato T, Zorumski CF, Kunikata H, et al. Glaucoma and microglia-induced neuroinflammation. Front Ophthalmol (Lausanne). (2023) 3:1132011. doi: 10.3389/fopht.2023.1132011
4. Fekrazad S, Gouravani M, Hassanzadeh G, Mafhoumi A, Mirzad M, S SV, et al. Optical coherence tomography alteration of the choroid and lamina cribrosa in pseudoexfoliation syndrome and glaucoma. J Glaucoma. (2025) 34:507–19. doi: 10.1097/IJG.0000000000002571
5. Kurysheva NI, Kim VY, Kim VE, and Plieva KM. The role of the structure of the lamina cribrosa in the diagnosis and treatment of glaucoma. Structural and circulatory changes in the lamina cribrosa with aging and elevated intraocular pressure. Vestn Oftalmol. (2025) 141:76–82. doi: 10.17116/oftalma202514101176
6. Song MK, Lee Y, Shin JW, Lee JY, Hong JW, Kook MS, et al. Comparative analysis of the optic nerve microvasculature between different optic disc phenotypes of normal-tension glaucoma patients. BMC Ophthalmol. (2025) 25:152. doi: 10.1186/s12886-025-03987-z
7. Huang J, Luo N, Ye L, Cheng L, Xiang Y, Yang Y, et al. Peripapillary intrachoroidal cavitation in myopic eyes with open-angle glaucoma: association with myopic fundus changes. Graefes Arch Clin Exp Ophthalmol. (2025) 263:2619–29. doi: 10.1007/s00417-025-06822-9
8. Hu X, Zhao GL, Xu MX, Zhou H, Li F, Miao Y, et al. Interplay between Müller cells and microglia aggravates retinal inflammatory response in experimental glaucoma. J Neuroinflamm. (2021) 18:303. doi: 10.1186/s12974-021-02366-x
9. Llorián-Salvador M, de la Fuente AG, McMurran CE, Dashwood A, Dooley J, Liston A, et al. Regulatory T cells limit age-associated retinal inflammation and neurodegeneration. Mol Neurodegener. (2024) 19:32. doi: 10.1186/s13024-024-00724-w
10. Salkar A, Palanivel V, Basavarajappa D, Mirzaei M, Schulz A, Yan P, et al. Glial and immune dysregulation in glaucoma independent of retinal ganglion cell loss: a human post-mortem histopathology study. Acta Neuropathol Commun. (2025) 13:141. doi: 10.1186/s40478-025-02066-0
11. Fernández-Albarral JA, Ramírez AI, de Hoz R, Matamoros JA, Salobrar-García E, Elvira-Hurtado L, et al. Glaucoma: from pathogenic mechanisms to retinal glial cell response to damage. Front Cell Neurosci. (2024) 18:1354569. doi: 10.3389/fncel.2024.1354569
12. Jia Y, Jiang S, Chen C, Lu G, Xie Y, Sun X, et al. Caffeic acid phenethyl ester attenuates nuclear factor−κB−mediated inflammatory responses in Müller cells and protects against retinal ganglion cell death. Mol Med Rep. (2019) 19:4863–71. doi: 10.3892/mmr.2019.10151
13. Echevarria FD, Formichella CR, and Sappington RM. Interleukin-6 deficiency attenuates retinal ganglion cell axonopathy and glaucoma-related vision loss. Front Neurosci. (2017) 11:318. doi: 10.3389/fnins.2017.00318
14. Wang Y, Fung NSK, Lam W-C, and Lo ACYJA. mTOR signalling pathway: a potential therapeutic target for ocular neurodegenerative diseases. Antioxidants (Basel). (2022) 11:1304. doi: 10.3390/antiox11071304
15. Reichenbach A and Bringmann AJG. Glia of the human retina. Glia. (2020) 68:768–96. doi: 10.1002/glia.23727
16. Rodrigues MF. Influence of Elevated Hydrostatic Pressure on Müller Cells Phenotype: Do Microglia-Derived Microvesicles Play a Role?Portugal: Universidade de Coimbra (2021).
17. Chong RS and Martin KR. Glial cell interactions and glaucoma. Curr Opin Ophthalmol. (2015) 26:73–7. doi: 10.1097/ICU.0000000000000125
18. MacDowell Kaswan ZA, Brooks AK, Hurtado M, Chen EY, Steelman AJ, McCusker RH, et al. Microglia-specific Ido2 deficiency attenuates ictogenesis in the TMEV model of viral encephalitis. Brain Behav Immun. (2025) 129:839–56. doi: 10.1016/j.bbi.2025.07.017
19. Pitha I, Du L, Nguyen TD, and Quigley H. IOP and glaucoma damage: The essential role of optic nerve head and retinal mechanosensors. Prog Retin Eye Res. (2024) 99:101232. doi: 10.1016/j.preteyeres.2023.101232
20. Li H, Li B, and Zheng Y. Role of microglia/macrophage polarisation in intraocular diseases (Review). Int J Mol Med. (2024) 53:45. doi: 10.3892/ijmm.2024.5446
21. Krishnan A, Kocab AJ, Zacks DN, Marshak-Rothstein A, and Gregory-Ksander M. A small peptide antagonist of the Fas receptor inhibits neuroinflammation and prevents axon degeneration and retinal ganglion cell death in an inducible mouse model of glaucoma. J Neuroinflamm. (2019) 16:184. doi: 10.1186/s12974-019-1576-3
22. Bourauel L and Mercieca K. Retinal nerve fibre layer OCT for glaucoma diagnosis - gold standard or just a piece of the puzzle? Klin Monbl Augenheilkd. (2025) 242:726–34. doi: 10.1055/a-2501-8538
23. Tan Z, Guo Y, Shrestha M, Sun D, Gregory-Ksander M, Jakobs TC, et al. Microglia depletion exacerbates retinal ganglion cell loss in a mouse model of glaucoma. Exp Eye Res. (2022) 225:109273. doi: 10.1016/j.exer.2022.109273
24. Choi S, Guo L, and Cordeiro MF. Retinal and brain microglia in multiple sclerosis and neurodegeneration. Cells. (2021) 10:1507. doi: 10.3390/cells10061507
25. Zou J, Yang J, Chen B, Jiang J, Liu J, Wang C, et al. Melatonin protects against NMDA-induced retinal ganglion cell injury by regulating the microglia-TNFα-RGC p38 MAPK pathway. Int Immunopharmacol. (2023) 118:109976. doi: 10.1016/j.intimp.2023.109976
26. Liu Q, Huang Y, Duan M, Yang Q, Ren B, Tang F, et al. Microglia as therapeutic target for radiation-induced brain injury. Int J Mol Sci. (2022) 23:8286. doi: 10.3390/ijms23158286
27. Xu Y, Li W, Zhang J, Gao W, Zhang T, Chen Q, et al. Inhibition of P2Y6 receptor-mediated microglia phagocytosis aggravates brain injury in mice of intracerebral hemorrhage. Cell Mol Neurobiol. (2025) 45:67. doi: 10.1007/s10571-025-01573-x
28. Korimerla N and Wahl DR. A complementary strategy to mitigate radiation-induced cognitive decline. Cancer Res. (2021) 81:1635–6. doi: 10.1158/0008-5472.CAN-20-4277
29. Petty RM, Rangan RS, Curry S, Brooks CD, Sabnis N, Clark AF, et al. Biodistribution of reconstituted high-density lipoprotein nanoparticles for targeted delivery to retinal ganglion cells. J Ocul Pharmacol Ther. (2025) 41:281–9. doi: 10.1089/jop.2024.0191
30. Shinozaki Y, Kashiwagi K, and Koizumi S. Astrocyte immune functions and glaucoma. Int J Mol Sci. (2023) 24:2747. doi: 10.3390/ijms24032747
31. Tang Y, Chen Y, and Chen D. The heterogeneity of astrocytes in glaucoma. Front Neuroanat. (2022) 16:995369. doi: 10.3389/fnana.2022.995369
32. Shinozaki Y and Koizumi S. Potential roles of astrocytes and Müller cells in the pathogenesis of glaucoma. J Pharmacol Sci. (2021) 145:262–7. doi: 10.1016/j.jphs.2020.12.009
33. Lee EJ, Park DY, Han JC, and Kee C. Understanding glaucoma as astrocyte-driven neurodegeneration in the optic nerve head: an integrative clinicopathological perspective. Prog Retin Eye Res. (2025) 107:101379. doi: 10.1016/j.preteyeres.2025.101379
34. Han X, Cao X, Ju Q, Ge C, Lin Y, Shi J, et al. Microglial TAK1 promotes neurotoxic astrocytes and cognitive impairment in LPS-induced hippocampal neuroinflammation. J Biol Chem. (2025) 301:110225. doi: 10.1016/j.jbc.2025.110225
35. Liu YX, Sun H, and Guo WY. Astrocyte polarization in glaucoma: a new opportunity. Neural Regener Res. (2022) 17:2582–8. doi: 10.4103/1673-5374.339470
36. Zhu A, Cui H, Su W, Liu C, Yu X, Huang Y, et al. C3aR in astrocytes mediates post-thoracotomy pain by inducing A1 astrocytes in male rats. Biochim Biophys Acta Mol Basis Dis. (2023) 1869:166672. doi: 10.1016/j.bbadis.2023.166672
37. Joshi AU, Minhas PS, Liddelow SA, Haileselassie B, Andreasson KI, Dorn GW, et al. Author Correction: Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nat Neurosci. (2021) 24:289. doi: 10.1038/s41593-020-00774-5
38. Redruello-Guerrero P, Gómez-Tomás M, Rechi-Sierra T, Molinero-Sicilia L, Galindo-Cabello N, Usategui-Martín R, et al. Inflammatory mechanisms in the management and treatment of retinal detachment. Metabolites. (2025) 15:442. doi: 10.3390/metabo15070442
39. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med. (2021) 384:1191–203. doi: 10.1056/NEJMoa2032125
40. Strat AN, Kirschner A, Yoo H, Singh A, Bagué T, Li H, et al. Engineering a 3D hydrogel system to study optic nerve head astrocyte morphology and behavior. Exp Eye Res. (2022) 220:109102. doi: 10.1016/j.exer.2022.109102
41. Ye SS, Tang Y, and Song JT. ATP and adenosine in the retina and retinal diseases. Front Pharmacol. (2021) 12:654445. doi: 10.3389/fphar.2021.654445
42. Križaj D, Ryskamp DA, Tian N, Tezel G, Mitchell CH, Slepak VZ, et al. From mechanosensitivity to inflammatory responses: new players in the pathology of glaucoma. Curr Eye Res. (2014) 39:105–19. doi: 10.3109/02713683.2013.836541
43. Wei M, Zhang G, Huang Z, Ding X, Sun Q, Zhang Y, et al. ATP-P2X(7)R-mediated microglia senescence aggravates retinal ganglion cell injury in chronic ocular hypertension. J Neuroinflamm. (2023) 20:180. doi: 10.1186/s12974-023-02855-1
44. Dostál Z, Zholobenko AV, Přichystalová H, Gottschalk B, Valentová K, Malli R, et al. Quercetin protects cardiomyoblasts against hypertonic cytotoxicity by abolishing intracellular Ca(2+) elevations and mitochondrial depolarisation. Biochem Pharmacol. (2024) 222:116094. doi: 10.1016/j.bcp.2024.116094
45. Xing L, Venables MJ, and Trudeau VL. Role of aromatase and radial glial cells in neurotoxin-induced dopamine neuron degeneration and regeneration. Gen Comp Endocrinol. (2017) 241:69–79. doi: 10.1016/j.ygcen.2016.02.011
46. Shigetomi E, Saito K, Sano F, and Koizumi S. Aberrant calcium signals in reactive astrocytes: A key process in neurological disorders. Int J Mol Sci. (2019) 20:996. doi: 10.3390/ijms20040996
47. Liu Z, Mar KB, Hanners NW, Perelman SS, Kanchwala M, Xing C, et al. A NIK-SIX signalling axis controls inflammation by targeted silencing of non-canonical NF-κB. Nature. (2019) 568:249–53. doi: 10.1038/s41586-019-1041-6
48. Lukowski SW, Lo CY, Sharov AA, Nguyen Q, Fang L, Hung SS, et al. A single-cell transcriptome atlas of the adult human retina. EMBO J. (2019) 38:e100811. doi: 10.15252/embj.2018100811
49. Tashtitova L and Aldasheva N. Study of the prevalence of glaucoma in Kazakhstan. Klin Monbl Augenheilkd. (2022) 239:202–7. doi: 10.1055/a-1327-3999
50. Skowronska-Krawczyk D, Zhao L, Zhu J, Weinreb RN, Cao G, Luo J, et al. P16INK4a upregulation mediated by SIX6 defines retinal ganglion cell pathogenesis in glaucoma. Mol Cell. (2015) 59:931–40. doi: 10.1016/j.molcel.2015.07.027
51. Diacou R, Zhao Y, Zheng D, Cvekl A, and Liu W. Six3 and six6 are jointly required for the maintenance of multipotent retinal progenitors through both positive and negative regulation. Cell Rep. (2018) 25:2510–2523.e4. doi: 10.1016/j.celrep.2018.10.106
52. Choi S, Choi SH, Bastola T, Kim KY, Park S, Weinreb RN, et al. AIBP protects müller glial cells against oxidative stress-induced mitochondrial dysfunction and reduces retinal neuroinflammation. Antioxidants (Basel). (2024) 13:1252. doi: 10.3390/antiox13101252
53. Kashihara T, Morita Y, Hatta M, Inoue S, Suzuki Y, Morita A, et al. YAP activation in Müller cells protects against NMDA-induced retinal ganglion cell injury by regulating Bcl-xL expression. Front Pharmacol. (2024) 15:1446521. doi: 10.3389/fphar.2024.1446521
54. Geiduschek EK and McDowell CM. The fibro-inflammatory response in the glaucomatous optic nerve head. J.I.J.O.M.S. (2023) 24:13240. doi: 10.3390/ijms241713240
55. Zhao X, Sun R, Luo X, Wang F, and Sun X. The interaction between microglia and macroglia in glaucoma. Front Neurosci. (2021) 15:610788. doi: 10.3389/fnins.2021.610788
56. Batsuuri K, Toychiev AH, Viswanathan S, Wohl SG, and Srinivas M. Targeting connexin 43 in retinal astrocytes promotes neuronal survival in glaucomatous injury. Glia. (2025) 73:1398–419. doi: 10.1002/glia.70013
57. Ma B, Ren J, and Qian X. Study on the polarization of astrocytes in the optic nerve head of rats under high intraocular pressure: in vitro. Bioengineering (Basel). (2025) 12:104. doi: 10.3390/bioengineering12020104
58. Waxman S, Schilpp H, Linton A, Jakobs TC, and Sigal IA. Morphological comparison of astrocytes in the lamina cribrosa and glial lamina. Invest Ophthalmol Vis Sci. (2025) 66:1. doi: 10.1167/iovs.66.3.1
59. Au NPB and Ma CHE. Neuroinflammation, microglia and implications for retinal ganglion cell survival and axon regeneration in traumatic optic neuropathy. Front Immunol. (2022) 13:860070. doi: 10.3389/fimmu.2022.860070
60. Li P, Shi X, and Prokosch V. Dual role of microglia in glaucoma: Regulation of neuroinflammation and neuroregeneration. Neural Regener Res. (2025). doi: 10.4103/NRR.NRR-D-24-00876
61. Williams PA, Marsh-Armstrong N, and Howell GR. Neuroinflammation in glaucoma: A new opportunity. Exp Eye Res. (2017) 157:20–7. doi: 10.1016/j.exer.2017.02.014
62. Lee IT, Lin HC, Huang TH, Tseng CN, Cheng HT, Huang WC, et al. Anti-Inflammatory Effect of Resveratrol Derivatives via the Downregulation of Oxidative-Stress-Dependent and c-Src Transactivation EGFR Pathways on Rat Mesangial Cells. Antioxidants (Basel). (2022) 11:835. doi: 10.3390/antiox11050835
63. Shirahama S, Okunuki Y, Lee MY, Karg MM, Refaian N, Krasniqi D, et al. Preventing the antigen-presenting function of retinal microglia blocks autoimmune neuroinflammation by dendritic cell-primed CD4(+) T cells. J Autoimmun. (2025) 153:103417. doi: 10.1016/j.jaut.2025.103417
64. Çalışkan G, French T, Enrile Lacalle S, Del Angel M, Steffen J, Heimesaat MM, et al. Antibiotic-induced gut dysbiosis leads to activation of microglia and impairment of cholinergic gamma oscillations in the hippocampus. Brain Behav Immun. (2022) 99:203–17. doi: 10.1016/j.bbi.2021.10.007
65. Gaviglio EA, Peralta Ramos JM, Arroyo DS, Bussi C, Iribarren P, Rodriguez-Galan MC, et al. Systemic sterile induced-co-expression of IL-12 and IL-18 drive IFN-γ-dependent activation of microglia and recruitment of MHC-II-expressing inflammatory monocytes into the brain. Int Immunopharmacol. (2022) 105:108546. doi: 10.1016/j.intimp.2022.108546
66. Porte R, Belloy M, Audibert A, Bassot E, Aïda A, Alis M, et al. Protective function and differentiation cues of brain-resident CD8+ T cells during surveillance of latent Toxoplasma gondii infection. Proc Natl Acad Sci U.S.A. (2024) 121:e2403054121. doi: 10.1073/pnas.2403054121
67. Wang N, Sun C, Yang Y, Zhang D, Huang L, Xu C, et al. Gut microbiota-derived indoleacetic acid attenuates neuroinflammation and neurodegeneration in glaucoma through ahr/rage pathway. J Neuroinflamm. (2025) 22:179. doi: 10.1186/s12974-025-03505-4
68. Choi S, Choi SH, Bastola T, Park Y, Oh J, Kim KY, et al. AIBP: A new safeguard against glaucomatous neuroinflammation. Cells. (2024) 13:198. doi: 10.3390/cells13020198
69. Zhuang S, Li F, Wang L, Lai Z, Li D, Wu H, et al. Neutrophil extracellular trap-derived double-stranded RNA aggravates PANoptosis in renal ischemia reperfusion injury. Cell Commun Signal. (2025) 23:140. doi: 10.1186/s12964-025-02145-8
70. Shi Y, Li J, Zhong Q, Chen H, Ma X, Hu Y, et al. Genetic deletion of histone deacetylase 6 prevents peritoneal fibrosis via suppression of heat shock protein 90 deacetylation. J Pathol. (2025) 266:447–64. doi: 10.1002/path.6436
71. Augusto-Oliveira M, Tremblay M, and Verkhratsky A. Receptors on microglia. Adv Neurobiol. (2024) 37:83–121. doi: 10.1007/978-3-031-55529-9_6
72. Fischer S, Nasyrov E, Brosien M, Preissner KT, Marti HH, Kunze R, et al. Self-extracellular RNA promotes pro-inflammatory response of astrocytes to exogenous and endogenous danger signals. J Neuroinflamm. (2021) 18:252. doi: 10.1186/s12974-021-02286-w
73. Shevchenko AV, Prokofiev VF, Konenkov VI, Chernykh VV, and Trunov AN. Features of toll-like receptor genes (TLR-2, TLR-3, TLR-4 and TLR-6) polymorphism in open-angle glaucoma patients. Vavilovskii Zhurnal Genet Selektsii. (2025) 29:128–34. doi: 10.18699/vjgb-25-15
74. Hosseindoust A, Ha S, Lokhande A, Mun J, Kim YI, Kim J, et al. The targeted anti-Salmonella bacteriophage attenuated the inflammatory response of laying hens challenged with Salmonella Gallinarum. Poult Sci. (2023) 102:102296. doi: 10.1016/j.psj.2022.102296
75. Luo C, Yang X, Kain AD, Powell DW, Kuehn MH, Tezel G, et al. Glaucomatous tissue stress and the regulation of immune response through glial Toll-like receptor signaling. Invest Ophthalmol Vis Sci. (2010) 51:5697–707. doi: 10.1167/iovs.10-5407
76. Wiemann S, Reinhard J, Reinehr S, Cibir Z, Joachim SC, Faissner A, et al. Loss of the extracellular matrix molecule tenascin-C leads to absence of reactive gliosis and promotes anti-inflammatory cytokine expression in an autoimmune glaucoma mouse model. Front Immunol. (2020) 11:66279. doi: 10.3389/fimmu.2020.566279
77. Hongu T, Pein M, Insua-Rodríguez J, Gutjahr E, Mattavelli G, Meier J, et al. Perivascular tenascin C triggers sequential activation of macrophages and endothelial cells to generate a pro-metastatic vascular niche in the lungs. Nat Cancer. (2022) 3:486–504. doi: 10.1038/s43018-022-00353-6
78. Gong T, Zhang X, Liu X, Ye Y, Tian Z, Yin S, et al. Exosomal Tenascin-C primes macrophage pyroptosis amplifying aberrant inflammation during sepsis-induced acute lung injury. Transl Res. (2024) 270:66–80. doi: 10.1016/j.trsl.2024.04.001
79. Soto I and Howell GR. The complex role of neuroinflammation in glaucoma. Cold Spring Harb Perspect Med. (2014) 4:a017269. doi: 10.1101/cshperspect.a017269
80. Wagner N, Reinehr S, Palmhof M, Schuschel D, Tsai T, Sommer E, et al. Microglia activation in retinal ischemia triggers cytokine and toll-like receptor response. J Mol Neurosci. (2021) 71:527–44. doi: 10.1007/s12031-020-01674-w
81. Dawuti A, Sun S, Wang R, Gong D, Liu R, Kong D, et al. Salvianolic acid A alleviates heart failure with preserved ejection fraction via regulating TLR/Myd88/TRAF/NF-κB and p38MAPK/CREB signaling pathways. BioMed Pharmacother. (2023) 168:115837. doi: 10.1016/j.biopha.2023.115837
82. Fathy Mohamed Y and Fernandez RC. Programming Bordetella pertussis lipid A to promote adjuvanticity. Microb Cell Fact. (2024) 23:250. doi: 10.1186/s12934-024-02518-7
83. Lahiri A, Sims SG, Herstine JA, Meyer A, Marshall MJ, Jahan I, et al. Endoplasmic reticulum stress amplifies cytokine responses in astrocytes via a PERK/eIF2α/JAK1 signaling axis. Glia. (2025) 73:2273–88. doi: 10.1002/glia.70067
84. Thougaard E, Nielsen PV, Raffaele S, Nielsen AN, Larsen LL, Corradini S, et al. Systemic inhibition of soluble TNF significantly changes glial cell populations leading to improved myelin integrity and better functional outcome after experimental stroke. BioMed Pharmacother. (2025) 189:118334. doi: 10.1016/j.biopha.2025.118334
85. Fuchs C, Forster V, Balse E, Sahel JA, Picaud S, Tessier LH, et al. Retinal-cell-conditioned medium prevents TNF-alpha-induced apoptosis of purified ganglion cells. Invest Ophthalmol Vis Sci. (2005) 46:2983–91. doi: 10.1167/iovs.04-1177
86. Malpetti M, Swann P, Tsvetanov KA, Chouliaras L, Strauss A, Chikaura T, et al. Blood inflammation relates to neuroinflammation and survival in frontotemporal lobar degeneration. Brain. (2025) 148:493–505. doi: 10.1093/brain/awae269
87. Sun Z, Ye J, Sun W, Jiang L, Shan B, Zhang M, et al. Cooperation of TRADD- and RIPK1-dependent cell death pathways in maintaining intestinal homeostasis. Nat Commun. (2025) 16:1890. doi: 10.1038/s41467-025-57211-z
88. Tian W, Li Y, Liu F, Liu H, Li C, Bao L, et al. Strychni Semen and two alkaloidal components cause apoptosis in HK-2 cells through TRADD-MAPK/NF-κB pathway. Toxicon. (2025) 256:108224. doi: 10.1016/j.toxicon.2024.108224
89. Wen X, Cahill AL, Barta C, Thoreson WB, and Nawy S. Elevated pressure increases ca(2+) influx through AMPA receptors in select populations of retinal ganglion cells. Front Cell Neurosci. (2018) 12:162. doi: 10.3389/fncel.2018.00162
90. Cueva Vargas JL, Osswald IK, Unsain N, Aurousseau MR, Barker PA, Bowie D, et al. Soluble tumor necrosis factor alpha promotes retinal ganglion cell death in glaucoma via calcium-permeable AMPA receptor activation. J Neurosci. (2015) 35:12088–102. doi: 10.1523/JNEUROSCI.1273-15.2015
91. Krishnan A, Fei F, Jones A, Busto P, Marshak-Rothstein A, Ksander BR, et al. Overexpression of soluble fas ligand following adeno-associated virus gene therapy prevents retinal ganglion cell death in chronic and acute murine models of glaucoma. J Immunol. (2016) 197:4626–38. doi: 10.4049/jimmunol.1601488
92. Wang Q, Dong J, Du M, Liu X, Zhang S, Zhang D, et al. Chitosan-rapamycin carbon dots alleviate glaucomatous retinal injury by inducing autophagy to promote M2 microglial polarization. Int J Nanomedicine. (2024) 19:2265–84. doi: 10.2147/IJN.S440025
93. Roh M, Zhang Y, Murakami Y, Thanos A, Lee SC, Vavvas DG, et al. Etanercept, a widely used inhibitor of tumor necrosis factor-α (TNF-α), prevents retinal ganglion cell loss in a rat model of glaucoma. PloS One. (2012) 7:e40065. doi: 10.1371/journal.pone.0040065
94. Paschalis EI, Lei F, Zhou C, Kapoulea V, Thanos A, Dana R, et al. The role of microglia and peripheral monocytes in retinal damage after corneal chemical injury. Am J Pathol. (2018) 188:1580–96. doi: 10.1016/j.ajpath.2018.03.005
95. Shen Y, Zhang ZJ, Zhu MD, Jiang BC, Yang T, Gao YJ, et al. Exogenous induction of HO-1 alleviates vincristine-induced neuropathic pain by reducing spinal glial activation in mice. Neurobiol Dis. (2015) 79:100–10. doi: 10.1016/j.nbd.2015.04.012
96. Chistyakov DV, Astakhova AA, Goriainov SV, and Sergeeva MG. Comparison of PPAR ligands as modulators of resolution of inflammation, via their influence on cytokines and oxylipins release in astrocytes. Int J Mol Sci. (2020) 21:9577. doi: 10.3390/ijms21249577
97. Gao J, Wang W, and Mo Y. Retinal degenerative diseases: complement system-microglia crosstalk. Surv Ophthalmol. (2025) 9577. doi: 10.1016/j.survophthal.2025.08.005
98. Chan ASY, Tun SBB, Lynn MN, Ho C, Tun TA, Girard MJA, et al. Intravitreal neuroglobin mitigates primate experimental glaucomatous structural damage in association with reduced optic nerve microglial and complement 3-astrocyte activation. Biomolecules. (2023) 13:961. doi: 10.3390/biom13060961
99. Reinehr S, Doerner JD, Mueller-Buehl AM, Koch D, Fuchshofer R, Dick HB, et al. Cytokine and complement response in the glaucomatous βB1-CTGF mouse model. Front Cell Neurosci. (2021) 15:718087. doi: 10.3389/fncel.2021.718087
100. Sterling JK, Adetunji MO, Guttha S, Bargoud AR, Uyhazi KE, Ross AG, et al. GLP-1 receptor agonist NLY01 reduces retinal inflammation and neuron death secondary to ocular hypertension. Cell Rep. (2020) 33:108271. doi: 10.1016/j.celrep.2020.108271
101. Peterson SL, Li Y, Sun CJ, Wong KA, Leung KS, de Lima S, et al. Retinal ganglion cell axon regeneration requires complement and myeloid cell activity within the optic nerve. J Neurosci. (2021) 41:8508–31. doi: 10.1523/JNEUROSCI.0555-21.2021
102. Stasi K, Nagel D, Yang X, Wang RF, Ren L, Podos SM, et al. Complement component 1Q (C1Q) upregulation in retina of murine, primate, and human glaucomatous eyes. Invest Ophthalmol Vis Sci. (2006) 47:1024–9. doi: 10.1167/iovs.05-0830
103. Reinehr S, Wulf J, Theile J, Schulte KK, Peters M, Fuchshofer R, et al. In a novel autoimmune and high-pressure glaucoma model a complex immune response is induced. Front Immunol. (2024) 15:1296178. doi: 10.3389/fimmu.2024.1296178
104. Hoppe C and Gregory-Ksander M. The role of complement dysregulation in glaucoma. Int J Mol Sci. (2024) 25:2307. doi: 10.3390/ijms25042307
105. Williams PA, Tribble JR, Pepper KW, Cross SD, Morgan BP, Morgan JE, et al. Inhibition of the classical pathway of the complement cascade prevents early dendritic and synaptic degeneration in glaucoma. Mol Neurodegener. (2016) 11:26. doi: 10.1186/s13024-016-0091-6
106. Harder JM, Braine CE, Williams PA, Zhu X, MacNicoll KH, Sousa GL, et al. Early immune responses are independent of RGC dysfunction in glaucoma with complement component C3 being protective. Proc Natl Acad Sci U.S.A. (2017) 114:E3839–e3848. doi: 10.1073/pnas.1608769114
107. Chen J, Jiang C, Huang Q, Lin X, Wu W, Li J, et al. Detection of plasma complement and immune globulin in different sorts of glaucoma. Eur J Ophthalmol. (2022) 32:2907–12. doi: 10.1177/11206721221074202
108. Meng S, Wen D, Xiao J, Zhang Q, Fang W, Xue X, et al. Age of rats affects the degree of retinal neuroinflammatory response induced by high acute intraocular pressure. Dis Markers. (2022) 2022:9404977. doi: 10.1155/2022/9404977
109. Cooke RS, Spicer BA, Harrison RA, Dunstone MA, Morgan BP, Zelek WM, et al. CD59, disulphide-locked human C9 and horse C9 inhibit human membrane attack complex assembly by similar mechanisms. Immunology. (2025) 176:363–72. doi: 10.1111/imm.70008
110. Liu Y, Zhao W, Huang Q, Wan L, Ren Z, Zhang B, et al. Advances in Research on the Release of von Willebrand Factor from Endothelial Cells through the Membrane Attack Complex C5b-9 in Sepsis. J Inflammation Res. (2025) 18:6719–33. doi: 10.2147/JIR.S520726
111. Becker S, Reinehr S, Dick HB, and Joachim SC. Complement activation after induction of ocular hypertension in an animal model. Ophthalmologe. (2015) 112:41–8. doi: 10.1007/s00347-014-3100-6
112. Lish AM, Grogan EFL, Benoit CR, Pearse RV, Heuer SE, Luquez T, et al. CLU alleviates Alzheimer's disease-relevant processes by modulating astrocyte reactivity and microglia-dependent synaptic density. Neuron. (2025) 113:1925–1946.e11. doi: 10.1016/j.neuron.2025.03.034
113. Zhang H, Jin Q, Li J, Wang J, Li M, Yin Q, et al. Astrocyte-derived complement C3 facilitated microglial phagocytosis of synapses in Staphylococcus aureus-associated neurocognitive deficits. PloS Pathog. (2025) 21:e1013126. doi: 10.1371/journal.ppat.1013126
114. Fang Y, Li Y, Wang S, Deng J, Liang J, Li S, et al. Corydalis yanhusuo Polysaccharides regulates HPA-axis mediated microglia activation and inhibits astrocyte A1 transformation to improve depression-like behavior. Brain Res. (2025) 1864:149780. doi: 10.1016/j.brainres.2025.149780
115. Silverman SM, Kim BJ, Howell GR, Miller J, John SW, Wordinger RJ, et al. C1q propagates microglial activation and neurodegeneration in the visual axis following retinal ischemia/reperfusion injury. Mol Neurodegener. (2016) 11:24. doi: 10.1186/s13024-016-0089-0
116. Kuehn MH, Kim CY, Jiang B, Dumitrescu AV, and Kwon YH. Disruption of the complement cascade delays retinal ganglion cell death following retinal ischemia-reperfusion. Exp Eye Res. (2008) 87:89–95. doi: 10.1016/j.exer.2008.04.012
117. Bosco A, Anderson SR, Breen KT, Romero CO, Steele MR, Chiodo VA, et al. Complement C3-targeted gene therapy restricts onset and progression of neurodegeneration in chronic mouse glaucoma. Mol Ther. (2018) 26:2379–96. doi: 10.1016/j.ymthe.2018.08.017
118. Liu B and Neufeld AH. Activation of epidermal growth factor receptor signals induction of nitric oxide synthase-2 in human optic nerve head astrocytes in glaucomatous optic neuropathy. Neurobiol Dis. (2003) 13:109–23. doi: 10.1016/S0969-9961(03)00010-X
119. Zhang X and Neufeld AH. Activation of the epidermal growth factor receptor in optic nerve astrocytes leads to early and transient induction of cyclooxygenase-2. Invest Ophthalmol Vis Sci. (2005) 46:2035–41. doi: 10.1167/iovs.04-1473
120. García-Bermúdez MY, Freude KK, Mouhammad ZA, van Wijngaarden P, Martin KK, Kolko M, et al. Glial cells in glaucoma: friends, foes, and potential therapeutic targets. Front Neurol. (2021) 12:624983. doi: 10.3389/fneur.2021.624983
121. Shareef S, Sawada A, and Neufeld AH. Isoforms of nitric oxide synthase in the optic nerves of rat eyes with chronic moderately elevated intraocular pressure. Invest Ophthalmol Vis Sci. (1999) 40:2884–91.
122. Husain S, Abdul Y, Singh S, Ahmad A, and Husain M. Regulation of nitric oxide production by δ-opioid receptors during glaucomatous injury. PloS One. (2014) 9:e110397. doi: 10.1371/journal.pone.0110397
123. Zhang X and Neufeld AH. Signal transduction pathways for epidermal growth factor stimulated cyclooxygenase-2 induction in astrocytes. Exp Eye Res. (2007) 85:280–8. doi: 10.1016/j.exer.2007.05.002
124. Deng C, Chen S, Li X, Luo H, Zhang Q, Hu P, et al. Role of the PGE2 receptor in ischemia-reperfusion injury of the rat retina. Mol Vis. (2020) 26:36–47.
125. Lozano DC, Choe TE, Cepurna WO, Morrison JC, and Johnson EC. Early optic nerve head glial proliferation and jak-stat pathway activation in chronic experimental glaucoma. Invest Ophthalmol Vis Sci. (2019) 60:921–32. doi: 10.1167/iovs.18-25700
126. Jain M, Singh MK, Shyam H, Mishra A, Kumar S, Kumar A, et al. Role of JAK/STAT in the neuroinflammation and its association with neurological disorders. Ann Neurosci. (2021) 28:191–200. doi: 10.1177/09727531211070532
127. Li Q, Cheng Y, Zhang S, Sun X, and Wu JJJon. TRPV4-induced Müller cell gliosis and TNF-α elevation-mediated retinal ganglion cell apoptosis in glaucomatous rats via JAK2/STAT3/NF-κB pathway. J Neuroinflammation. (2021) 18:271. doi: 10.1186/s12974-021-02315-8
128. Salkar A, Wall RV, Basavarajappa D, Chitranshi N, Parilla GE, Mirzaei M, et al. Glial cell activation and immune responses in glaucoma: A systematic review of human postmortem studies of the retina and optic nerve. Aging Dis. (2024) 15:2069–83. doi: 10.14336/AD.2024.0103
129. Wawrzyniak A, Krawczyk-Marć I, Żuryń A, Walocha J, and Balawender KJIJoMS. Diversity, functional complexity, and translational potential of glial cells in the central nervous system. Int J Mol Sci. (2025) 26:9080. doi: 10.3390/ijms26189080
130. Bou Ghanem GO, Koktysh D, Baratta RO, Del Buono BJ, Schlumpf E, Wareham LK, et al. Collagen mimetic peptides promote repair of MMP-1-damaged collagen in the rodent sclera and optic nerve head. Int J Mol Sci. (2023) 24:17031. doi: 10.3390/ijms242317031
131. Williams PA, Braine CE, Kizhatil K, Foxworth NE, Tolman NG, Harder JM, et al. Inhibition of monocyte-like cell extravasation protects from neurodegeneration in DBA/2J glaucoma. Mol Neurodegener. (2019) 14:6. doi: 10.1186/s13024-018-0303-3
132. Margeta MA, Lad EM, and Proia AD. CD163+ macrophages infiltrate axon bundles of postmortem optic nerves with glaucoma. Graefes Arch Clin Exp Ophthalmol. (2018) 256:2449–56. doi: 10.1007/s00417-018-4081-y
133. Gu X, Chen X, Zhang X, Liu K, Li JJ, Lv W, et al. Macrophage-induced integrin signaling promotes Schlemm's canal formation to prevent intraocular hypertension and glaucomatous optic neuropathy. Cell Rep. (2024) 43:113799. doi: 10.1016/j.celrep.2024.113799
134. Cameron EG, Nahmou M, Toth AB, Heo L, Tanasa B, Dalal R, et al. A molecular switch for neuroprotective astrocyte reactivity. Nature. (2024) 626:574–82. doi: 10.1038/s41586-023-06935-3
135. Howell GR, Soto I, Zhu X, Ryan M, Macalinao DG, Sousa GL, et al. Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J Clin Invest. (2012) 122:1246–61. doi: 10.1172/JCI61135
136. Williams PA, Braine CE, Foxworth NE, Cochran KE, and John SWM. GlyCAM1 negatively regulates monocyte entry into the optic nerve head and contributes to radiation-based protection in glaucoma. J Neuroinflamm. (2017) 14:93. doi: 10.1186/s12974-017-0868-8
137. Wang L and Wei X. T cell-mediated autoimmunity in glaucoma neurodegeneration. J.F.I.I. (2021) 12:803485. doi: 10.3389/fimmu.2021.803485
138. Tezel G. The role of glia, mitochondria, and the immune system in glaucoma. Invest Ophthalmol Vis Sci. (2009) 50:1001–12. doi: 10.1167/iovs.08-2717
139. Chen PJ, Wang YL, Kuo LM, Lin CF, and Chen CY. Honokiol suppresses TNF-α-induced neutrophil adhesion on cerebral endothelial cells by disrupting polyubiquitination and degradation of IκBα. Sci Rep. (2016) 6:26554. doi: 10.1038/srep26554
140. Tanigami H, Okamoto T, Yasue Y, and Shimaoka M. Astroglial integrins in the development and regulation of neurovascular units. Pain Res Treat. (2012) 2012:964652. doi: 10.1155/2012/964652
141. Tehrani S, Davis L, Cepurna WO, Choe TE, Lozano DC, Monfared A, et al. Astrocyte structural and molecular response to elevated intraocular pressure occurs rapidly and precedes axonal tubulin rearrangement within the optic nerve head in a rat model. PloS One. (2016) 11:e0167364. doi: 10.1371/journal.pone.0167364
142. Margeta MA, Yin Z, Madore C, Pitts KM, Letcher SM, Tang J, et al. Apolipoprotein E4 impairs the response of neurodegenerative retinal microglia and prevents neuronal loss in glaucoma. Immunity. (2022) 55:1627–1644.e7. doi: 10.1016/j.immuni.2022.07.014
143. Margeta MA, Letcher SM, Igo RP, Cooke Bailey JN, and Pasquale LR. Association of APOE with primary open-angle glaucoma suggests a protective effect for APOE ϵ4. Invest Ophthalmol Vis Sci. (2020) 61:3. doi: 10.1167/iovs.61.8.3
144. Wu X, Pan G, Chang L, Liu Q, Liu Y, Zhang W, et al. Endoplasmic reticulum stress-induced triggering receptor expressed on myeloid cells 2 (TREM2) downregulation exacerbates platelet activation and myocardial infarction in patients with coronary artery disease. J Am Heart Assoc. (2025) 14:e041220. doi: 10.1161/JAHA.124.041220
145. Fremuth LE, Hu H, van de Vlekkert D, Annunziata I, Weesner JA, Alessandra dA, et al. Neuraminidase 1 regulates neuropathogenesis by governing the cellular state of microglia via modulation of Trem2 sialylation. Cell Rep. (2025) 44:115204. doi: 10.1016/j.celrep.2024.115204
146. Shi H, Yin Z, Koronyo Y, Fuchs DT, Sheyn J, Davis MR, et al. Regulating microglial miR-155 transcriptional phenotype alleviates Alzheimer's-induced retinal vasculopathy by limiting Clec7a/Galectin-3(+) neurodegenerative microglia. Acta Neuropathol Commun. (2022) 10:136. doi: 10.1186/s40478-022-01439-z
147. Méndez-Salcido FA, Torres-Flores MI, Ordaz B, and Peña-Ortega F. Abnormal innate and learned behavior induced by neuron-microglia miscommunication is related to CA3 reconfiguration. Glia. (2022) 70:1630–51. doi: 10.1002/glia.24185
148. Zheng L, Wang Y, Shao B, Zhou H, Li X, Zhang C, et al. Multiple mild stimulations reduce membrane distribution of CX3CR1 promoted by annexin a1 in microglia to attenuate excessive dendritic spine pruning and cognitive deficits caused by a transient ischemic attack in mice. Neurosci Bull. (2022) 38:753–68. doi: 10.1007/s12264-022-00847-4
149. Yu C, Lad EM, Mathew R, Littleton S, Chen Y, Schlepckow K, et al. Microglia at Sites of Atrophy Restrict the Progression of Retinal Degeneration via Galectin-3 and Trem2 Interactions. bioRxiv. (2023) 221:e20231011. doi: 10.1101/2023.07.19.549403
150. Won W, Bhalla M, Lee JH, and Lee CJ. Astrocytes as key regulators of neural signaling in health and disease. Annu Rev Neurosci. (2025) 48:251–76. doi: 10.1146/annurev-neuro-112723-035356
151. Yang SS, Brooks NAH, Da Silva DE, Gibon J, Islam H, Klegeris A, et al. Extracellular ATP regulates phagocytic activity, mitochondrial respiration, and cytokine secretion of human astrocytic cells. Purinergic Signal. (2025) 21:485–98. doi: 10.1007/s11302-025-10066-x
152. Bijelić DD, Milićević KD, Lazarević MN, Miljković DM, Bogdanović Pristov JJ, Savić DZ, et al. Central nervous system-infiltrated immune cells induce calcium increase in astrocytes via astroglial purinergic signaling. J Neurosci Res. (2020) 98:2317–32. doi: 10.1002/jnr.24699
153. Zheng H, Liu Q, Zhou S, Luo H, and Zhang W. Role and therapeutic targets of P2X7 receptors in neurodegenerative diseases. Front Immunol. (2024) 15:1345625. doi: 10.3389/fimmu.2024.1345625
154. Lagos-Cabré R, Alvarez A, Kong M, Burgos-Bravo F, Cárdenas A, Rojas-Mancilla E, et al. α(V)β(3) Integrin regulates astrocyte reactivity. J Neuroinflamm. (2017) 14:194. doi: 10.1186/s12974-017-0968-5
155. Miao Y, Zhao GL, Cheng S, Wang Z, and Yang XL. Activation of retinal glial cells contributes to the degeneration of ganglion cells in experimental glaucoma. Prog Retin Eye Res. (2023) 93:101169. doi: 10.1016/j.preteyeres.2023.101169
Keywords: glaucoma, neuroinflammation, microglia, astrocytes, Müller cells, TNF-α, complement, TLR4
Citation: Zong F, You J, Wu H and Wang X (2025) Glial-mediated immune modulation in glaucomatous neurodegeneration: mechanisms and therapeutic implications. Front. Immunol. 16:1640110. doi: 10.3389/fimmu.2025.1640110
Received: 03 June 2025; Accepted: 13 November 2025; Revised: 05 November 2025;
Published: 02 December 2025.
Edited by:
Danian Chen, Sichuan University, ChinaReviewed by:
Yu Lin, Shanghai Roche Pharmaceutical Co., Ltd, ChinaXiangyu Fu, Sichuan University, China
Jishuang Gu, First Affiliated Hospital of Jilin University, China
Copyright © 2025 Zong, You, Wu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuerui Wang, d2FuZ3h1ZXJ1aTEzMTRAamx1LmVkdS5jbg==; Hong Wu, d3VfaG9uZ0BqbHUuZWR1LmNu
 Fangwei Zong
Fangwei Zong Jiaxin You1
Jiaxin You1 Hong Wu
Hong Wu Xuerui Wang
Xuerui Wang