- 1Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Graduate School, Hebei University of Chinese Medicine, Shijiazhuang, China
- 3Graduate School, Beijing University of Chinese Medicine, Beijing, China
In order to preserve homeostasis, macrophages—phagocytic innate immune cells—interact with different tissue types, modulating immunological responses and secreting a variety of cytokines. They are extensively dispersed throughout the body’s tissues and organs. Based on their developmental origins, tissue-resident macrophages (TRMs) in humans can be classified into those of embryonic origin and those derived from bone marrow-derived monocytes (BMDMs); embryonically derived macrophages emerge during early development, possess self-renewal capacity, and persist into adulthood in specific tissues such as microglia in the brain and Kupffer cells in the liver, whereas BMDMs originate from hematopoietic stem cells in the bone marrow via monocytic differentiation, infiltrate tissues during inflammation or injury, and differentiate into macrophages that transiently reside in tissues but lack self-renewal capability, thus requiring continuous replenishment. Because of their flexibility and diversity, macrophages participate in a variety of physiological and pathological processes by changing phenotypically and functionally in response to microenvironmental stimuli. This process is known as macrophage polarization. As a consequence, macrophage cultivation in vitro has emerged as a crucial biological technique for mimicking the microenvironment of different disease models. Primary macrophage models and immortalized macrophage models are two distinct types of macrophage models, each with unique origins, functions, benefits, and drawbacks. The features, advantages, disadvantages, isolation procedures, and differentiation induction techniques of primary and immortalized macrophage models are compiled in this review. It also works at the differences between various macrophage cell lines in an effort to shed light on the pathophysiology of inflammatory disorders, viral infection processes, and macrophage immunoregulatory roles.
1 Introduction
Elie Metchnikoff identified and named macrophages in the late 1800s (1). In addition to aiding in tissue repair, macrophages, one of the body’s primary immune cells, control the physiological and pathological aspects of immunological and inflammatory responses by presenting antigens, polarizing, and phagocytosing. Before animal studies showed that some macrophages could have evolved from red pulp progenitors in the yolk sac around 1990, it was generally accepted that all macrophages were derived from bone marrow monocytes (2, 3). These macrophages can live in adult tissues and are capable of self-renewal (4). Macrophages living in various tissues show considerable heterogeneity, depending on niche distinctions and the tissue microenvironment. The term “macrophage polarization” describes the temporary and reversible process by which macrophages acquire certain phenotypic and functional responses to signals and stimuli from the microenvironment (5). M0 macrophages differentiate into M1/M2 subtypes according to variations in signaling. Inflammatory reactions begin with M0 macrophages, which are in an inactivated condition. Pro-inflammatory M1 macrophages mainly contribute to inflammatory reactions by phagocytosing and eliminating pathogens. Anti-inflammatory M2 macrophages primarily work to reduce inflammation and encourage tissue regeneration. Immune and inflammatory responses can be modulated by controlling the M1/M2 macrophage ratio (6). The M1/M2 distinction, considered helpful for understanding macrophage functional polarization, oversimplifies the complex continuum of states in vivo and ignores cellular origin and tissue milieu, according to recent findings (7). For example, regulatory macrophages (Mregs) are a unique subset of macrophages that are not dependent on M1/M2 functional states. They have been found to have angiogenic and immunosuppressive properties (8) and have been effectively used in clinical trials to lessen immunosuppressive therapy and rejection after kidney transplantation (9, 10). Similarly, there are specialized populations like tumor-associated macrophages (TAMs) (11) and heme-handling macrophages (Mhem) (12). The general M1 and M2 classifications, however, continue to be helpful recommendations for examining macrophage polarization and function, improving our comprehension of macrophage functional states in vitro, and enabling the investigation of new treatment approaches (7).
Serious developmental disorders, such as neurodevelopmental delays, skeletal abnormalities, impaired tissue repair/remodeling, and functional disorders in the liver, spleen, reproductive system, lungs, and cardiovascular system, are facilitated by genetic abnormalities that lead to macrophage dysfunction in both humans and mice (13). Macrophages, an essential part of the human immune system, are involved in many physiological and pathological processes and are strongly linked to a number of disorders, including infections, atherosclerosis, obesity, malignancies, and asthma (14). Therefore, studying the polarization states of macrophages has become essential for treating inflammatory diseases. Thus, the development of in vitro cell lines through genetic manipulation, the establishment of primary cell culture models for immunological and cell biological research, and the creation of single-source immortalized cell lines with distinct backgrounds and retained functionality constitute vital strategies for tackling today’s problems.
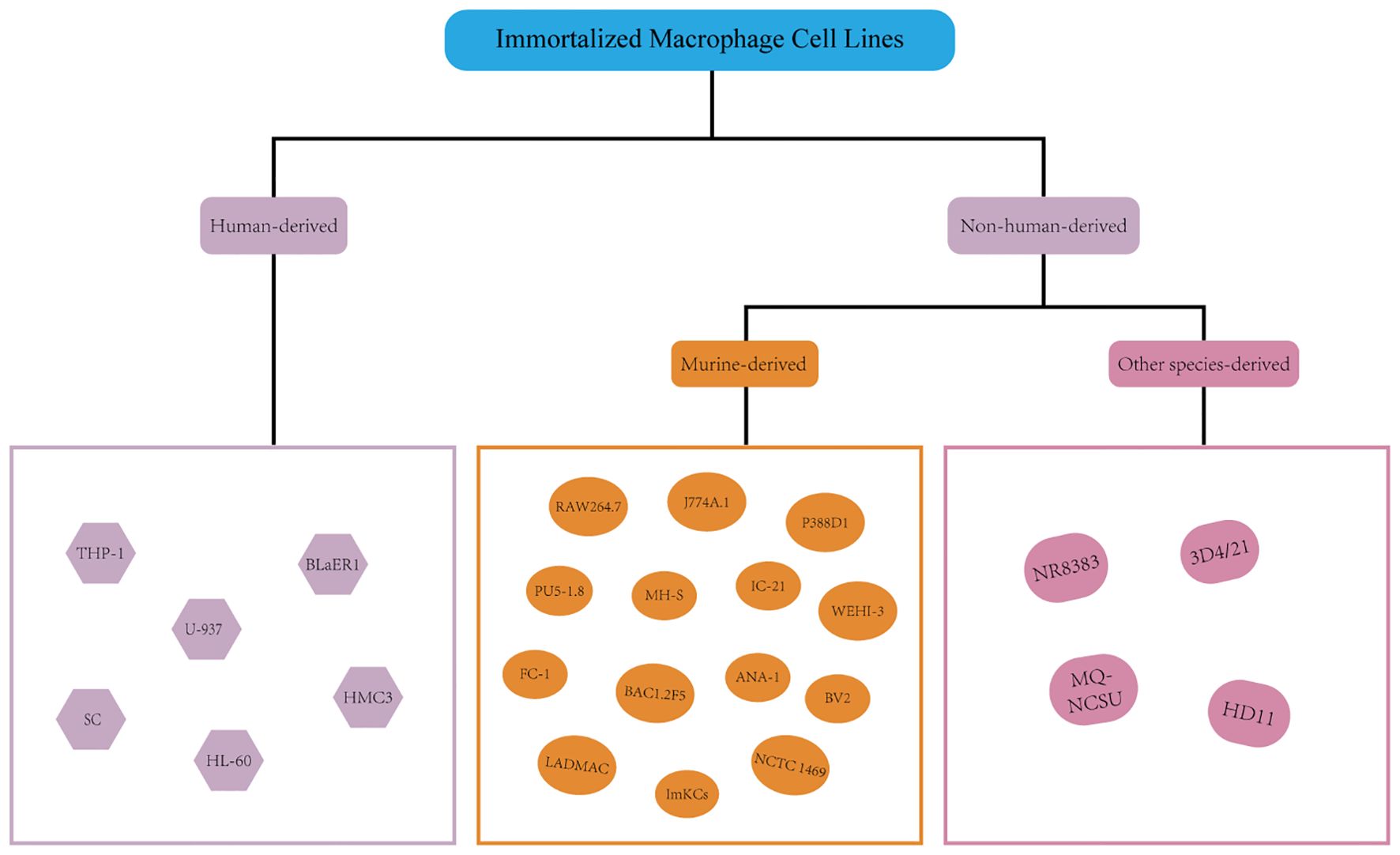
Without going through genetic alteration or immortalization, primary cells are extracted and cultivated straight from organisms, maintaining biological activity and population features. Cell lines are stable proliferative populations created by repeatedly subculturing primordial cells. These cells are derived from malignancies or normal tissues, but they typically lose their stable chromosome composition and population specificity. Through genetic modification (e.g., introduction of SV40 large T antigen (SV40 LT), telomerase activation, or viral transformation (e.g., Epstein-Barr virus)), cells may prevent normal senescence and death during in vitro culture, allowing for long-term subculture and unrestricted proliferation. This process is recognized as cellular immortalization. The same is true with macrophages. Primary macrophages and immortalized murine macrophage cell lines are the two basic groups into which macrophages can be divided according to their origin. They share the following traits: (1) Primary macrophages. The limited proliferative capability, great functional heterogeneity, and closer resemblance to in vivo physiological states are characteristics of these cells, which are directly collected from either humans or animals. Their manipulation and culture are rather complicated, though, and they have limited stability, short survival periods, and no capacity for long-term subculture. As a result, during use, rigorous control of the experimental conditions is necessary. Furthermore, differences in the main macrophages’ source, separation, and culture methods could affect the results of experiments (15). Animals (like peritoneal macrophages [PMs] and bone marrow-derived macrophages [BMDMs]) or humans (such as peripheral blood mononuclear cells [PBMCs] and induced pluripotent stem cells [iPSCs]) can serve as primary macrophage models. (2) Immortalized macrophage cell lines. Large-scale investigations can benefit from these cell lines’ rapid growth, stability, reproducibility, independence from conditioned medium, lack of other cell types, and ease of culture and passage (16). However, immortalized macrophage cell lines are frequently created by viral infection or derived from malignant single cells/tumors, which causes genotypic and phenotypic drift throughout culture and subculture (17–19). Genetic drift compromises the reliability and reproducibility of findings, particularly in long-term studies involving multiple passages. Resultant phenotypic alterations, including those in polarization, cytokine secretion, and phagocytosis, can lead to misleading conclusions in disease modeling. Rigorous cell line authentication, use of low-passage stocks, and reporting of passage numbers are therefore essential to mitigate risks and enhance cross-study data comparability (17–19). This is in contrast to primary cells. As a consequence, macrophage cell lines may develop molecular phenotypes different from those of primary isolated cells or macrophage-specific functions. Additionally, they depart from the primary cells’ inherent functional properties by not being able to accurately mimic the intricate in vivo milieu. Animal-derived lines (such as RAW264.7, J774A.1, and P388D1) and human-derived lines (including THP-1, U-937, and BlaER1) are an instance of immortalized macrophage cell lines. Research on macrophages will be addressed in more detail in the sections that follow.
2 Primary macrophages
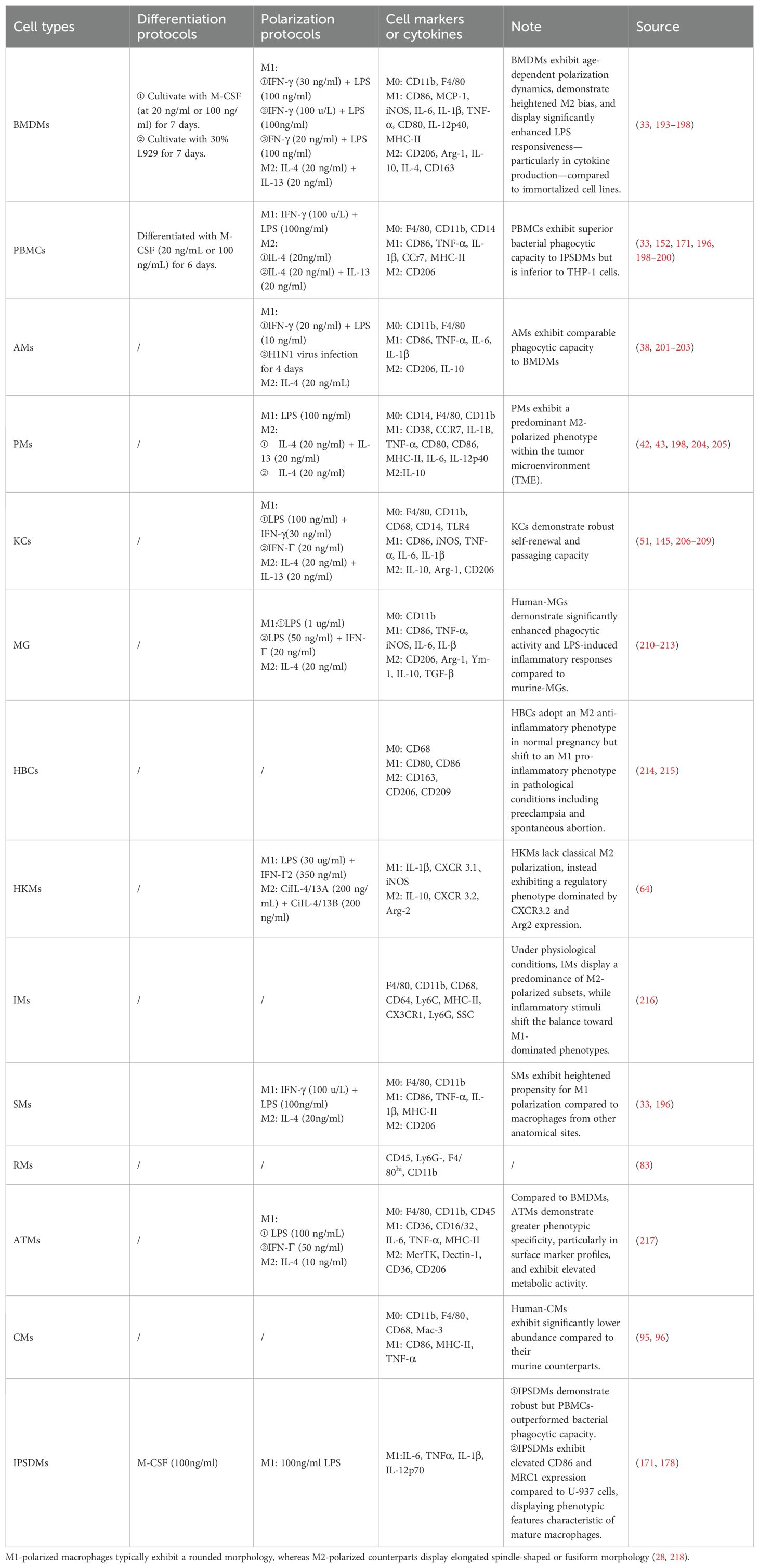
Initially, it was generally believed that bone marrow monocytes were the only source of macrophages (20). Around 1990, however, research on animals showed that certain macrophages could originate from the yolk sac during the development of the embryo (2, 3). These macrophages from the yolk sac can remain tissue-resident cells in adult organs and are capable of self-renewal (4). Bone marrow, peripheral blood, and tissues (e.g., as the lungs, peritoneal cavity, spleen, and placenta) are among the various sites from which primary macrophages can be isolated (Figure 1). Adherence selection, density gradient centrifugation, magnetic bead sorting, enzymatic digestion (collagenase/trypsin), and flow cytometry sorting are the main isolation techniques used today (21).
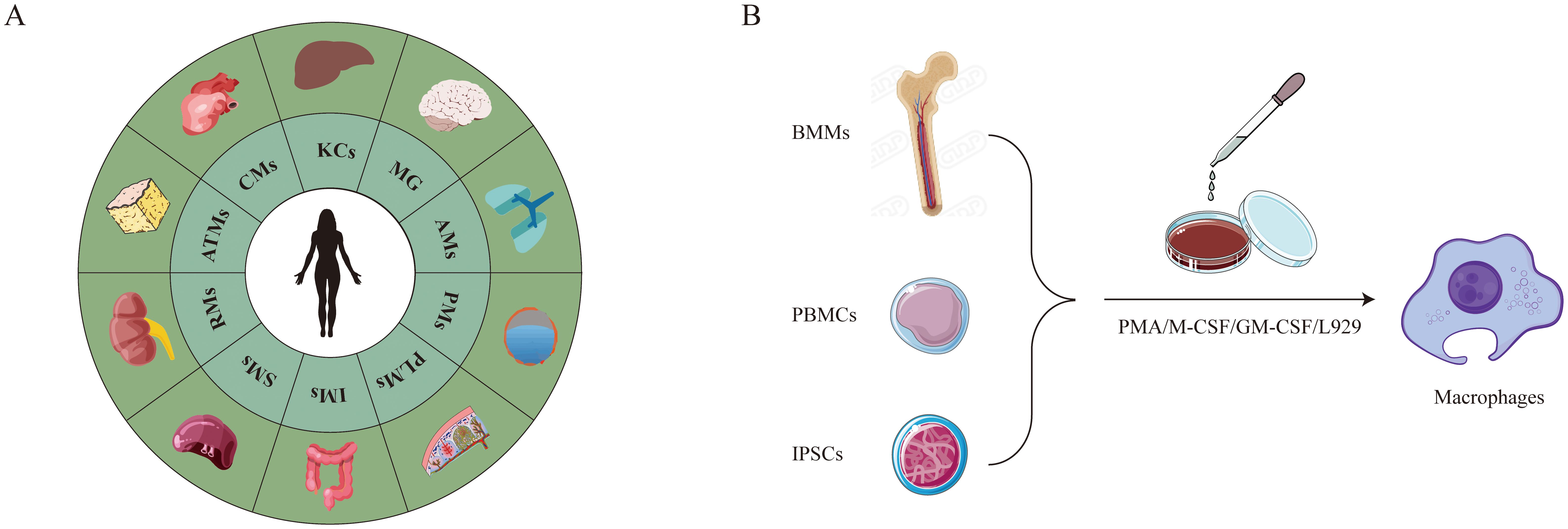
Figure 1. Primary macrophages. (A) Undergoes spontaneous differentiation without artificial induction. (B) Requires artificial induction of differentiation.
2.1 BMDMs
The primary reason for the irreplaceable core value of BMDMs is their physiological relevance. They are ideal for metabolic studies (such as glycolysis and oxidative phosphorylation assays) and validation of genetic knockout models because of their high migratory capacity, potent secretory activity (e.g., IL-1β, lysozyme), strong phagocytic capability (though they cannot trigger LC3-associated phagocytosis (LAP) (22, 23)), pronounced polarization plasticity (different M1/M2 phenotypes), and comprehensive expression of membrane proteins, including Toll-like receptors (TLRs) and scavenger receptors (24–27). The morphology of BMDMs is significantly influenced by polarization status; stimulation with LPS and IFN-γ (M1-polarizing) leads to flattened pancake-like morphology within 24 hours, whereas elongated cellular shape is promoted by IL-4 and IL-13 (M2-polarizing) (28). A Study demonstrating significantly higher numbers of aged murine BMDMs (CD11b, MHC-II, F4/80) with a roughly 2.5-fold higher percentage of M1 macrophages compared to young controls suggest that the polarization status of BMDMs may be age-dependent, while the M2 macrophage percentage did not significantly increase in aged mice (29).
2.1.1 Animal-derived
In order to ensure experimental reproducibility, BMDMs closely resemble native in vivo macrophage characteristics and functions, thereby avoiding culture-induced variations inherent to cell lines.
BMDMs were isolated from mouse femurs and tibias using a standard bone marrow harvest and erythrocyte lysis protocol (28, 29). BMDMs differentiate from bone marrow monocytes (BMMs); however, the isolation of bone marrow monocytes is technically challenging and requires fresh preparation for each experiment. Mature BMDMs can only be obtained after a 5–7 day induction period with M-CSF or equivalent factors (e.g., secretory factors from L929 cells) in the extracellular milieu, which significantly extends experimental timelines (30, 31).
2.1.2 Human-derived
Macrophages produced in human bone marrow are terminally differentiated, non-proliferative cells that resemble macrophages but are unable to passage. However, because of ethical limitations, a lack of therapeutic necessity, and logistical difficulties in procurement, their usage in research is still restricted.
2.2 PBMCs
Peripheral blood mononuclear cells, or PBMCs, are a mixed population that includes stem cells (0.1–0.2%), natural killer cells (5–10%), dendritic cells (1–2%), T lymphocytes (70–90%), and monocytes (10–30%) (32). They are among the primary sources of immune cells and can be further refined. Different from granulocytes and anucleate erythrocytes, peripheral blood mononuclear cells (PBMCs) have a single round or reniform nucleus and express surface markers involving MHC-II, CD14, and CD11b that facilitate antigen presentation. Yet, they do not have the myeloid lineage markers CD66b and CD15 (33, 34). The biggest leukocytes in blood, monocytes are immature when they enter circulation and are derived from bone marrow hematopoietic stem cells. They undergo directed differentiation into monocyte-derived macrophages (MDMs) when cultivated with cytokines.
2.2.1 Animal-derived
PBMCs can be isolated from porcine, bovine, rabbit, or piscine sources using standardized density gradient centrifugation protocols. For bovine PBMCs isolation, representative methods involve heparinized blood collection, density-based fractionation with species-specific separation media, and adherence-based purification under controlled culture conditions (35).
2.2.2 Human-derived
It is challenging to directly isolate sufficient macrophages from human tissues. Since human blood monocytes are easily obtained in high quantities and have the capacity to develop into macrophages in vitro, MDMs offer a great substitute. Procedure for MDMs in humans: Use density gradient centrifugation to separate PBMCs. PBMCs should be resuspended in full macrophage culture media. Adherent MDMs remain after non-adherent cells are removed.
2.3 Tissue-derived macrophages
2.3.1 Alveolar macrophages
The pulmonary microenvironment contains at least three different types of macrophages: AMs, interstitial lung macrophages, and bronchial macrophages (36). The majority of the innate immune cells in distal lung tissues are alveolar macrophages AMs that reside on the alveolar luminal surface and have cytoplasm that is enriched with lysosomes and lamellar bodies. These cells constitutively express core surface markers such as CD206, CD169, and F4/80, and they are involved in the phagocytosis of pathogens and the efferocytosis of apoptotic cells (37, 38). AMs are a great model for researching bacterial infections since they are the first line of defense against lung pathogens. Their sources, which include humans and animals, make their isolation quite simple.
2.3.1.1 Animal-derived
Bronchoalveolar lavage (BAL) is commonly used to isolate AMs from rodents (e.g., rats, mice) or mammalian species (e.g., porcine, caprine). Caprine alveolar macrophages can be isolated using standardized bronchoalveolar lavage procedures combined with density gradient centrifugation and selective adhesion protocols (39). Representative methods involve lung lavage, mononuclear cell fractionation, and adherence-based purification under controlled culture conditions. SV40 large T antigen transfection was used in a Chinese investigation (39) to accomplish caprine AMs immortalization; however, the immortalized cells showed dendritiform transformation with pseudopodial extension, which deviated morphologically from primary AMs phenotypes and compromised physiological relevance.
2.3.1.2 Human-derived
The BAL fluid of patients with pulmonary diseases (such as asthma, Mycoplasma pneumoniae pneumonia, and COPD) is the primary source of human AMs, and BAL cytology is utilized as a biomarker of disease severity. AMs were isolated using a standardized clinical protocol (40) based on bronchoalveolar lavage of affected segments, processing of the lavage fluid (including centrifugation and washing), and short-term adherence culture. Cells adhering after this culture period were definitively identified as AMs by their CD68+ immunophenotype.
2.3.2 PMs
Compared to other macrophage subtypes, PMs possess significant immunoregulatory functions, improved functional/phenotypic stability, and superior cytokine expression (41). PMs are ideal for short-term phagocytosis functional tests because they exhibit high expression of surface markers such as F4/80, CD11b, and CD14 (42, 43).
2.3.2.1 Animal-derived
Direct or induced peritoneal lavage is a common method used to separate PMs from mammalian species (murine, rat, rabbit, pig, and caprine) or non-mammalian models (e.g., Oreochromis niloticus, Danio rerio).
1) Direct peritoneal lavage
PMs are commonly isolated from mice using a standard peritoneal lavage protocol (33). Following euthanasia, sterile PBS is introduced into the peritoneal cavity, the abdomen is gently manipulated, and the lavage fluid is collected. PMs are then purified by centrifugation, resuspension in culture medium, and adherence-based separation, where non-adherent cells are removed after a brief incubation period. Low yield (2–3 × 106 peritoneal cells/mouse, PMs purity ~35% by F4/80+ flow cytometry (33)) is the main drawback. 2) Induced Peritoneal Lavage.
To create sterile inflammation and encourage PMs recruitment, inject stimulants (including thioglycollate broth (33)) into the mouse peritoneal cavity for three days in a row. Then, undertake adherent culture after obtaining these cells by peritoneal lavage. Treatment with thioglycollate enhances PMs yield by a factor of 10 per mouse (44). Even while PMs produced under inflammatory stimulation are more prevalent, the particular inflammatory conditions may cause their biological properties to differ from those of PMs in physiologically normal states. For example, thioglycollate injection-induced macrophages in mice show increased anti-inflammatory (M2) phenotypic bias (45). As a result, the choice of method should be in line with the goals and specifications of the experiment.
2.3.2.2 Human-derived
Typically, human PMs are extracted from the ascites of patients suffering from serious illnesses (cirrhosis, ovarian cancer, post-dialysis uremia, etc.). Based on a study (46), there are plenty of mature, resident, and inflammatory PMs in cirrhotic ascites. Activated PMs from cirrhotic ascites release considerably higher levels of IL-6, IL-10, and TNF-α but lower levels of IL-1β and IL-12 once compared to PBMCs from healthy donors.
2.3.3 Kupffer cells
KCs are tissue-resident macrophages (TRMs) that are exclusive to the liver and are identified by the surface expression of CD11b, F4/80, TIM4, and CLEC4F. These cells, which make up more than 80% of the body’s resident macrophage pool, are produced from blood monocytes that adhere to hepatic sinusoids. They support hepatic repair and regeneration and preserve inflammatory homeostasis (47). Through scavenger receptor-dependent pathways, KCs eliminate gut-derived bacterial products, senescent blood cells, apoptotic hepatocytes, and immunological complexes, reducing the severity of viral infections in the liver (48, 49). Through a variety of mechanisms, KCs are activated upon hepatic damage and are essential in a number of liver disorders. Research has shown that in mice fed a methionine/choline-deficient (MCD) diet, KCs are depleted during the early stages of non-alcoholic steatohepatitis (NASH), and that Ly6C monocyte-derived macrophages subsequently take their position as the predominant population (50). KCs have a strong capacity for self-renewal and passaging in vitro (51).
Primary KCs were isolated from rodent liver using established enzymatic digestion and density gradient centrifugation methods (51), with purification via selective adhesion. Cell identity was confirmed by immunophenotyping using macrophage-specific markers (CD68、CD163、CK18、CD31、α-SMA).
2.3.4 Microglia
Under physiological conditions, the blood-brain barrier (BBB) restricts cellular and molecular transit into the brain, limiting macrophage infiltration. Microglia, however, serve as the resident macrophages of the central nervous system (CNS), ubiquitously distributed throughout the brain and spinal cord. They constitute 5-20% of all glial cells in the CNS, with their physiological density and function being essential for maintaining neural homeostasis (7). Beyond phagocytic capabilities, microglia play pivotal roles in CNS development and homeostasis through immune surveillance, neurogenesis support, synaptic refinement, axonal growth guidance, injury repair, and neurotrophic factor secretion (52, 53). Microglial polarization shares similarities with but exhibits greater specificity than the classical M1/M2 dichotomy: M1-polarized microglia predominantly exert pro-inflammatory and neurotoxic effects, while their M2-polarized counterparts demonstrate anti-inflammatory and neuroprotective functions. The M2 phenotype is further subclassified into three distinct subsets (M2a, M2b, and M2c), each possessing unique molecular signatures and biological functions (54, 55).
Primary microglia exhibit a complicated morphology with cytoplasmic vacuolization and short processes, and they proliferate slowly. Myeloid markers CD68, CD11b, and CD14 are first expressed by them, but with prolonged in vitro culture, marker expression gradually declines (56). Neonatal microglia were isolated from brain tissue through mechanical dissociation and orbital shaking separation of mixed glial cultures (57), followed by phenotypic validation with standard markers.
2.3.5 Placental macrophages
A special immune system found in the placenta strikes a balance between the mother’s protection against infections and the fetus’s immunological tolerance. Decidual macrophages and Hofbauer cells are two different groups of placental macrophages (58). As demonstrated by immunohistochemical research, the placenta’s macrophage proportions fluctuate dynamically throughout pregnancy, from around 50% to 20% prior to birth (59, 60). Typically, isolation procedures include density gradient centrifugation (Ficoll or Percoll), enzymatic digestion (collagenase, DNase, and/or trypsin), and positive/negative selection employing antibodies that target CD68, CD10, or CD14 or take advantage of adhesion qualities (61). However, the translational applicability of mouse models is restricted due to the phenotypic differences between human and rat placental macrophages (62), which in turn limits the functional characterization of human placental macrophages. In view of this, the majority of research on human placental macrophages focuses on tissue section immunohistochemical analysis, with functional studies being comparatively rare (61).
2.3.6 Head kidney macrophages
In teleost fish, the head kidney’s primary purpose in the early stages of life is excretion. As an adult, it develops into a vital hematological and immunological organ that functions similarly to human bone marrow and is the genesis of macrophages and phagocytic activity (63). HKMs have a distinct polarization profile from the mammalian M1/M2 paradigm. M1-polarized HKMs express CXCR3.1 and iNOS, but they do not have traditional M2 polarization; instead, they have a regulatory phenotype that is dominated by CXCR3.2 and Arg2 (64). Numerous fish species, including salmonids, Atlantic cod, and southern bluefin tuna, have well-established macrophage isolation and culture methods, which have made it possible to conduct in-depth study in fish immunology (65–68).
2.3.7 Intestinal macrophages
In order to maintain intestinal microenvironmental equilibrium, IMs are essential for differentiating between dangerous microorganisms and innocuous antigens, such as dietary proteins and resident commensal flora (69). Lamina propria macrophages (LPMs) and muscularis macrophages (MMs) are two types of IMs. LPMs perform essential innate immune effector functions and act as the main sentinels against pathogens that penetrate the epithelial barrier. Living close to the enteric nervous system, MMs modulate peristalsis, protect tissue during inflammation and stress, and maintain enteric neurons by providing trophic and neuroprotective support (70, 71). With the expression of specific receptors such as Toll-like receptors (TLR3-TLR9), CD36, NOD-like receptors, and TREM2, LpMs have strong phagocytic and bactericidal action against pathogens (70). The boosted expression of tissue-protective genes (including Retnla, Mrc1, and CD163) distinguishes MMs from their LPMs in terms of transcription and morphology (70). Weigmann et al. used traditional enzymatic digestion to create an isolated procedure for mouse intestine lamina propria mononuclear cells (72). In order to isolate rat intestinal LPMs, Ana et al. improved this method using mechanically aided enzymatic dissociation, leading to increased purity and viability with less tissue input (73).
2.3.8 Macrophages in other tissues
2.3.8.1 Splenic macrophages
The red pulp, white pulp, and marginal zone are the three main sections of the spleen, an essential immunological organ. Red pulp macrophages (RPMs), marginal zone macrophages (MZMs), and metallophilic macrophages (MMs) are the three subsets of SMs that are distinguished by their distribution and function. RPMs phagocytose senescent erythrocytes to release heme and IL-33, promote iron recycling, and have low levels of CD169 and high levels of F4/80, CD68, and CD206 (74, 75). MZMs acquire bloodborne pathogens and control B-cell responses while not expressing F4/80 (76). Without F4/80, MMs exhibit strong CD169 expression, eliminate pathogens and apoptotic cells (76), and indirectly stimulate T lymphocytes (77). Splenic macrophages were obtained using antibody-based enrichment techniques from dissociated spleen tissue, with purity assessed by flow cytometry (78–81).
2.3.8.2 Renal macrophages
Renal macrophages are necessary for immune monitoring, help maintain homeostasis, and take part in tissue damage and repair (82). These macrophages fall into two subgroups: kidney-resident macrophages and infiltrating macrophages, which are further split into Ly6Chi and Ly6Clo cells (83). Research shows that pro-inflammatory M1-polarized macrophages are drawn to the kidney during the early stages of ischemia/reperfusion injury (≤48 hours), where they release inflammatory mediators like TNF-α and iNOS that worsen tissue damage; genetically deleting M1 macrophages reduces renal damage (84). Effective techniques for separating murine renal macrophage populations and carrying out flow cytometry analysis are described in a work by Sarah et al. (83).
2.3.8.3 Adipose tissue macrophages
ATMs have been found in humans, primates, rodents, an array of non-rodent animals, and amphibians. They appear in the stromal vascular fraction (SVF) of adipose tissue (85). Adipose tissue macrophages have the ability to either directly or indirectly contribute to the body’s energy storage (86). Although human adipose tissue can be isolated to produce primary ATMs for in vitro culture, mouse studies seldom use primary ATMs directly; instead, BMDMs or immortalized macrophage cell lines are used as stand-ins (87, 88). For instance, chemokines and inflammatory cues stimulate the membrane receptor CCR2, which attracts bone marrow-derived mononuclear macrophages to adipose tissue. By releasing inflammatory chemicals like TNF, these cells control the activity of adipose tissue (89, 90). However, a 3D in vitro system for producing and cultivating functional ATM-resident macrophages that had better metabolic rates and stronger surface marker specificity than BMDMs was described by Adele et al. (2024) (91). This 3D approach exemplifies how advanced culture systems can enhance physiological relevance compared to traditional 2D models or immortalized lines.
2.3.8.4 Cardiac macrophages
CMs are the most prevalent immune cell group in the heart, making up 6–10% of all cardiac cells. They are essential for preserving cardiac homeostasis and controlling the heart’s reactions to stress (92–94). In 2007, Nahrendorf et al. published the first methodology for making single-cell suspensions from mouse hearts (95). Geetika et al.’s later improvements (96) made it possible to successfully isolate macrophages from human heart tissue. More readily available cell lines, like RAW 264.7, are frequently used in place of primary cells in experiments (97).
2.3.9 iPSC-derived macrophages
Numerous techniques for producing iPSCs from somatic cells have surfaced since Yamanaka et al. converted mouse fibroblasts into iPSCs by adding the OSKM transcription factors (OCT4, SOX2, c-MYC, and KLF4) (98). Through reprogramming, human-iPSCs are created from terminally differentiated somatic cells while maintaining the ability to self-renew (99). iPSCs function and develop similarly to embryonic stem cells (ESCs); they can differentiate into any type of cell, including macrophages, and have an infinite capacity for proliferation (100). Furthermore, iPSCs synthesis does not require embryonic material, in contrast to ESCs. By making it possible to derive stable, uniform, and genetically defined macrophages from pre-engineered genotype-specific iPSCs, IPSDMs solve important issues in macrophage procurement (101). Investigating immune response mechanisms, activation/polarization dynamics, macrophage-specific functions, and pathologies linked to macrophages (such as inflammatory disorders and cancer immunotherapy) is made possible by this platform.
3 Immortalized macrophage cell lines
Beginning in the middle to late 20th century, methods for cell immortalization were developed in order to get around the drawbacks of primary cells, such as their limited lifespan and rigorous culture needs (Figure 2). These strategies include activating telomerase via the TERT gene to achieve unrestricted proliferation or introducing viral oncogenes (such as the SV40 LT antigen or human papillomavirus E6/E7 proteins) (102–104).
3.1 Animal-derived
3.1.1 RAW264.7
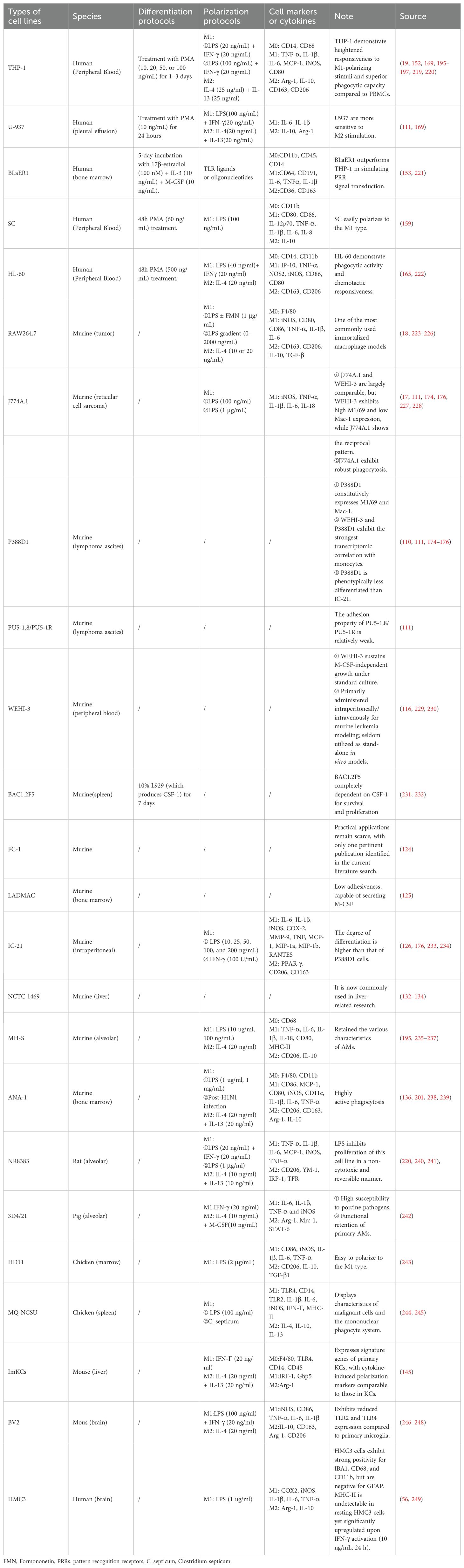
The RAW264.7 cell line was first established in 1976 by Raschke et al., isolated from a male BALB/c mouse injected with Abelson murine leukemia virus (MuLV) (18). These cells exhibit a round or oval morphology with dark pigmentation, strong adherence, pseudopod extension, enhanced migratory capacity, and robust phagocytic activity. Due to their capabilities in pinocytosis and phagocytosis, RAW264.7 cells are widely utilized as a macrophage model in inflammation, immunology, apoptosis, and cancer research (105). While this cell line serves as a practical tool for preliminary screening of macrophage-related factors and functions, prolonged passaging may lead to progressive depletion of macrophage-specific gene/protein expression and impaired immune functionality compared to primary macrophages or in vivo models. Research indicates that RAW264.7 macrophages exhibit increased expression of genes including HIF1α, ITGAL, and CD86 after 50 passages, whereas changes in ARG1, TRF2, and IRF8 expression start to occur as early as 15 passages (106).
3.1.2 J774A.1
In 1968, a BALB/c mouse’s reticulum cell sarcoma gave rise to the J774A.1 cell line (17). In addition to retaining many of the functional characteristics of normal macrophages, such as phagocytosis (e.g., engulfing bacteria, apoptotic cells, or fluorescent-labeled particles), activation (inducible to M1/M2 polarization in vitro), and secretory activity (e.g., production of IL-1β and high levels of lysozyme), J774A.1 also demonstrates relative stability and ease of proliferation in culture (17, 107). LPS, dextran sulfate, pure protein derivative (PPD), and other substances suppress its proliferation (108). It is noteworthy that during in vitro culture, this cell line does not require external addition of M-CSF or other growth hormones. It is unclear, nevertheless, if J774A.1 makes M-CSF on its own to get around this requirement or if its tumor-derived origin gives it intrinsic proliferative ability that is not dependent on M-CSF signaling (109).
3.1.3 P388D1
After methylcholanthrene-induced lymphoma development, DBA/2 mice were employed to create the murine monocyte/macrophage-like cell line P388D1 (110). IL-1 and lysozyme production in response to lipopolysaccharide (LPS) and phorbol myristate acetate (PMA) stimulation (111), phagocytosis of zymosan and latex microspheres (112), and surface immunoglobulin (sIg) negativity (112, 113) are among the macrophage-associated traits displayed by P388D1.
3.1.4 PU5-1.8 (PU5-1R)
Originating from a spontaneous tumor in BALB/c mice, the PU5-1.8 cell line is a leukemia cell line that resembles macrophages. It has been extensively employed as an in vitro model for the investigation of monocyte differentiation, apoptosis, and cellular proliferation (114). PU5-1.8 cells are non-adherent and non-aggregating under conventional culture conditions (RPMI-1640 + 5% FBS + 1% penicillin/streptomycin; 5% CO2; 37°C), which is compatible with undifferentiated macrophage characteristics. M-CSF induction can differentiate the cells (115).
3.1.5 WEHI-3
WEHI-3, first reported by Warner et al. in 1969, is a granulocytic leukemia cell line with macrophage-like properties derived from the peripheral blood of tumor-induced BALB/c mice. It exhibits sustained secretion of IL-3, lysozyme, and granulocyte colony-stimulating activity (CSA), along with phagocytic capability and expression of complement C3 receptor (C3R) (116). In intervertebral disc degeneration research, the WEHI-3 model may simulate mechanisms where M1 macrophages exacerbate inflammation through IL-1β and IL-6 secretion, while M2 macrophages promote tissue repair via TGF-β production (117). In hepatocellular carcinoma studies, WEHI-3 mimics tumor-associated macrophages (TAMs) to investigate how M2 polarization supports tumor progression through IL-1β secretion and angiogenesis promotion (118). WEHI-3 proliferates under conventional culture conditions without exogenous M-CSF, in contrast to the majority of established macrophage-like cell lines that require M-CSF for proliferation (119). It most likely depends on self-secreted IL-3 and components of the basal medium (120, 121).
3.1.6 BAC1.2F5
By transforming a hybrid strain of BALB/c and A.CA mice with the SV40 virus, the murine macrophage cell line BAC1.2F5 was created (119). It retains functions including Fc-mediated phagocytosis, Ia antigen expression, IL-1 secretion, and the synthesis of lysozyme, collagenase, and esterase, but it is entirely dependent on M-CSF for survival and proliferation, mimicking the behavior of primary macrophages (122). Securioside B’s growth-inhibitory action and capacity to trigger macrophage apoptosis through the mitochondrial pathway in the presence of L-cell-conditioned media (LCCM) were examined by Satoru et al. (123) in their investigation of the drug’s effects on BAC1.2F5.
3.1.7 FC-1
Yet there is limited information on FC-1. In a 1983 study, FC-1 was compared to thioglycollate-induced peritoneal macrophages in the generation of plasminogen activator and six other macrophage cell lines (WEHI-3, J774A.1, RAW264, P388D1, PU5-R, and PU5-1.8). The findings indicated that FC-1 secretes non-plasminogen-dependent proteases in addition to plasminogen activators (124).
3.1.8 LADMAC
The bone marrow of BALB/c mice was utilized to create the murine monocyte/macrophage cell line LADMAC. It has poor adhesion and grows in suspension (125). The research of macrophage biology, in particular growth factor interactions and M-CSF-dependent mechanisms, has benefited greatly from the use of this cell line. In order to promote the survival and proliferation of BAC1.2F5, Wong et al. (125) showed that LADMAC cells release macrophage colony-stimulating factor (M-CSF), which can be employed in place of recombinant human CSF-1.
3.1.9 IC-21
By transforming peritoneal macrophages from C57BL/6 mice with the SV40 virus, IC-21 was created (126). It maintains the phenotypic and functional characteristics of normal peritoneal macrophages, including phagocytic activity, lysozyme synthesis, particular receptors (TLR4, CD14, C3R), and antigen-presenting molecules (MHC-II). Because IC-21 is naturally adherent, it reduces experimental variability and does not require extra reagents like PMA (127). Nevertheless, IC-21 and primary peritoneal macrophages differ in the following ways: ①Enhanced erythrocyte-targeted phagocytosis is demonstrated by IC-21 (128). ②IC-21 has a more homogeneous tumor-binding capacity and binds tumor cells four times more efficiently than primary macrophages. Because of its easy cultivation and persistent, uniform tumor-binding characteristics, IC-21 is an advantageous model for understanding the molecular mechanisms behind activated macrophage subsets’ selective, high-affinity tumor contacts (129).
3.1.10 NCTC 1469
In 1952, the NCTC 1469 cell line was created using the liver tissue of healthy C3H/An mice. Studies on its chemotactic activity in the 1980s showed that this cell line, which resembles macrophages, responds consistently to different chemotactic substances (such as casein) in chemotaxis tests (130). Although NCTC 1469 CB, a mutant subline of NCTC 1469, lacks phagocytic activity toward erythrocytes coated with complement and IgG, it nonetheless possesses the functional and morphological characteristics of normal macrophages (131). Since more specialized macrophage models have been established, NCTC 1469 is now mostly used in hepatic research, such as investigations on liver regeneration, lipid metabolism regulation, and antioxidant capability (132–134).
3.1.11 MH-S
SV40 viral transformation of alveolar macrophages collected from male BALB/c mice aged 7 weeks resulted in the establishment of the MH-S cell line (135). This cell line maintains several characteristic traits of alveolar macrophages, such as (1) typical macrophage morphology with adhesive properties; (2) phagocytic capacity; (3) enzymatic profiles that are esterase-positive and peroxidase-negative; (4) positive expression of surface membrane antigens Mac-1 and I-A; and (5) Fc receptor-dependent phagocytosis of sheep red blood cells (SRBCs) coated with immunoglobulin G. Additionally, LPS stimulation markedly increased constitutive IL-1 production.
3.1.12 ANA-1
ANA-1 is a macrophage cell line that is generated by immortalizing bone marrow cells from C57BL/6 (H-2b) mice through the J2 recombinant retrovirus, and it makes up the myeloid-monocytic lineage (136). These cells have dual adherent/suspended growth patterns, reveal morphology akin to that of macrophages, with prominent vacuolization and eccentric nuclei, and have high phagocytic activity against pathogens (e.g., Mycobacterium tuberculosis, Penicillium marneffei, and others) and particulate matter. ANA-1 expresses surface markers associated with macrophages, such as FcγR (Ly-17), Mac-1, and Ly-5, and may be involved in immune and inflammatory processes (137, 138).
3.1.13 NR8383
In 1983, AMs from normal Sprague-Dawley (SD) rats collected through lung lavage served as a means to create the NR8383 cell line. NR8383 acquires an unlimited proliferative capability while maintaining normal macrophage activities through serial cloning and spontaneous immortalization (139). For in vitro research, especially in pulmonary inflammation, these semi-suspension cells, which have an oval or spherical shape, offer a consistent supply of highly sensitive alveolar macrophages (140). NR8383 expresses TGF-β precursor, upregulates its mRNA, and secretes pro-inflammatory cytokines (such as IL-1, TNF-β, and IL-6) when stimulated by bleomycin. The cells are extremely susceptible to endotoxins; 50% of proliferation is suppressed by 10 ng/mL LPS.
3.1.14 3D4/21
In 1998, the swine alveolar macrophages that gave rise to the pig alveolar macrophage cell line 3D4/21 were immortalized through SV40 transformation. It has persistent phagocytic capacity, nonspecific esterase activity, and morphology analogous to macrophages (141). Only a small proportion of 3D4/21 cells demonstrate the capacity to phagocytose latex beads following extended incubation (3 hours to overnight), as determined by Weingartl et al. (142). These results imply that while cytokine secretion functions are unaffected by immortalization, effective phagocytosis is somewhat lost.
3.1.15 HD11 and MQ-NCSU
MQ-NCSU and HD11 are immortalized cell lines of chicken macrophages. The avian myelocytomatosis virus MC29 strain has been applied to turn chicken bone marrow into the HD11 cell line, which has unmistakable macrophage-like characteristics. These include elevated generation of reactive oxygen species (ROS) and distinctive morphological characteristics (143). Derived from the spleen of chickens infected with the Marek’s disease virus, MQ-NCSU demonstrates mononuclear phagocyte lineage traits and malignant characteristics (109). According to Ahmed-Hassan et al. (144), LPS causes MQ-NCSU to undergo a type I interferon (IFN) response, which in turn causes the creation of nitric oxide (NO) and IFN-β.
3.1.16 Immortalized Kupffer cells
ImKCs are isolated from hepatic tissue of male H-2Kb-tsA58 transgenic mice expressing the thermolabile mutant tsA58 of SV40 large T antigen and purified via F4/80 marker expression. These cells exhibit detectable p53 expression, elevated telomerase activity, and retained functional characteristics of primary KCs, with cytokine-induced polarization marker profiles comparable to primary counterparts. ImKCs may thus serve as functional substitutes for primary KCs in experimental systems (145).
3.1.17 BV2
In 1990, E. Braschi et al. created the BV2 cell line, a commonly used immortalized murine microglial model, by transfecting primary C57BL/6 mouse microglia with v-raf/v-myc oncogenes using a retroviral vector (146). BV2 cells lack peroxidase activity but have phagocytic and nonspecific esterase activity. In addition to constitutively secreting lysozyme, they also generate TNF and IL-1 when stimulated appropriately. Using MAC3 immunoreactivity, immunohistochemical profiling validates MAC1 and MAC2 expression (146).
3.2 Human-derived
3.2.1 THP-1
In 1980, Tsuchiya et al. developed the THP-1 cell line from the peripheral blood of a kid with acute monocytic leukemia who was barely one year old. It is frequently employed in monocyte/macrophage studies and demonstrates the capacity to differentiate into different macrophage subtypes (19). Using retinoic acid (147), vitamin D3 (148), or phorbol myristate acetate (PMA) (149), THP-1 cells can be separated into resting M0 macrophages based on metabolic and morphological similarities. Under certain triggers, these differentiated cells polarize into different subtypes and exhibit high expression of markers characteristic of macrophages, among them CD14 and CD68. For instance: M1 polarization: Pro-inflammatory cytokines (e.g., TNF-α and IL-6) are secreted by PMA-induced M0 macrophages stimulated with LPS and IFN-γ. M2 polarization: the production of anti-inflammatory cytokines, such as TGF-β and IL-10, is triggered by IL-4 and IL-13. However, since THP-1 lacks a defined differentiation strategy, PMA concentration must be optimized for certain experimental objectives (109).
3.2.2 U-937
The pleural effusion of a male patient with generalized diffuse histiocytic lymphoma was used by Sundstrom and Nilsson in 1974 to generate the U-937 cell line (111). U-937 is regarded as a relatively young monocytic population and exhibits monocyte-like characteristics. The following factors can cause it to terminally differentiate into macrophages: M0 macrophage differentiation is induced by PMA (150). Vitamin D3: Encourages the development of monocytes into macrophages (151). Cytokine-driven polarization: M1 polarization is induced by IFN-γ, whereas M2 polarization is induced by IL-4 (152). U-937 allows for the functional diversity of macrophages in inflammatory and immunological responses thanks to these differentiation mechanisms.
3.2.3 BLaER1
The Seraphina Burkitt lymphoma cell line was transfected to produce the human B-cell precursor leukemia cell line BLaER1. The bone marrow of a female patient with acute lymphoblastic leukemia, trisomy 8, and chromosomal rearrangement t(1;19) served as the source of its progenitor line (153). Research shows that BLaER1 cells can be effectively reprogrammed to resemble macrophages, with a transcriptome similar to that of normal macrophages and improved adhesion, phagocytosis, and quiescent characteristics (154). When compared to other monocyte models with limited editing capabilities, BLaER1 offers improved genetic manipulability owing to its compatibility with CRISPR-Cas9 gene editing in the undifferentiated B-cell state (155–157).
3.2.4 SC
The only documented human monocytic cell line that emerges from healthy peripheral blood is SC, which has been used in research pertaining to macrophages (158). Using PMA induction, a Chinese research team created a model of SC-derived macrophages. Among the main conclusions are (159): Phenotypic maturation: Compared to undifferentiated SC monocytes, SC macrophages exhibited a marked elevation of CD11b and CD14. Changes in morphology: LPS treatment resulted in the production of distinct pseudopodia and an elongated spindle-shaped morphology. M1 polarization is highlighted by increased secretion of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, and IL-8) and increased expression of M1 surface markers (CD80, CD86). These findings verify that SC macrophages replicate the functional and phenotypic characteristics of typical M1-polarized macrophages.
3.2.5 HL-60
A 36-year-old Caucasian woman with acute myelomonocytic leukemia gave origin to the promyelocytic cell population characterized as the HL-60 cell line (160). Using substances like PMA (161) and vitamin D3 (162), HL-60 cells can develop into macrophage-like cells, show phagocytic activity, and react to chemotactic stimuli. This cell line provides a special paradigm for examining how lipoprotein receptor expression and regulation are affected by macrophage development. Increased production of platelet-activating factors (PAF) is linked to HL-60 differentiation into macrophages (163). Further research showed that increased amounts of ethanolamine plasmalogens and phospholipids within arachidonic acid metabolism are correlated with macrophage-like differentiation in HL-60 (161).
3.2.6 HMC3
Tardieu et al. (1995) immortalized human fetal brain-derived primary microglial cultures SV40-dependently to create the HMC3 cell line (164). Although transformed HMC3 cells retain important primary microglia physical and phenotypic characteristics, they proliferate more quickly. The astrocytic marker GFAP is negative in resting HMC3 cells, but they show significant positivity for the microglial/macrophage markers IBA1, CD68, and CD11b. When HMC3 cells are at rest, the activated microglia marker MHC-II is not visible; nevertheless, after IFN-γ treatment (10 ng/mL, 24 h), it is markedly elevated (56, 164).
4 Experimental applications of primary macrophages versus immortalized macrophage cell lines
4.1 Macrophage differentiation protocols
The study of macrophage adaptability is made possible by the capacity to induce immortalized macrophage cell lines and primary macrophages to adopt particular functional states under a range of pathophysiological circumstances. The main components of differentiation techniques include exogenous signals, endogenous signals, and particular elements or components: 1. Endogenous Signals PMA is an activator of protein kinase C (PKC) that drives monocyte-to-macrophage differentiation by activating downstream signaling cascades (e.g., NF-κB, MAPK pathways) (165). 2 Exogenous Signals: To encourage macrophage conversion, cyclosporin A causes genetic reprogramming and changes to surface receptors (166). 3 Particular Elements/Components ts (109): ① Co-culturing monocytes with macrophage-stimulating factors (typically M-CSF or GM-CSF) for five to seven days causes the monocytes to differentiate into macrophages.GM-CSF stimulates differentiation into the pro-inflammatory M1 phenotype, whereas M-CSF encourages polarization toward M2-type macrophages. Parts of Bacteria: Macrophages differentiate into M1-polarized macrophages in response to LPS. ③ Cytokines: IFN-γ stimulates M1 polarization, and when combined with LPS, it works much better. The differentiation of macrophages into M2-polarized macrophages is triggered by IL-4 and IL-13.Different cell lines have their own specific induction protocols. PMA is primarily used to induce monocytic cell lines (such as THP-1 and U937) to differentiate into macrophage-like cells. When inducing primary monocytes (e.g., PBMCs), M-CSF is typically required in combination, as PMA alone may only induce partial functional changes (such as enhanced adhesion). M-CSF serves as the main regulator for macrophage survival, proliferation, and differentiation.
Different macrophages/cell lines require different induction procedures. The main purpose of PMA is to cause monocytic cell lines (such as THP-1 and U937) to undergo differentiation into cells that resemble macrophages. Since PMA alone may only partially elicit functional changes (such as improved adhesion), M-CSF is usually needed in conjunction with PMA when producing primary monocytes (e.g., PBMCs). The primary modulator of macrophage survival, proliferation, and differentiation is M-CSF. M-CSF, which is consistently present in mouse plasma at a concentration of around 10 ng/ml, is essential for monocyte recruitment and differentiation (167). M-CSF is frequently used in in vitro research to encourage the differentiation of immature PBMCs and BMDMs into macrophages while preserving cell viability. These cells barely live for two to three days without M-CSF treatment (168). However, mature cell lines such as PMs, RAW264.7, and J774A.1 still need bacterial components or cytokines to induce M1/M2 polarization, even if they do not require M-CSF or other growth factors to survive in vitro. Macrophages are the main immune cells that react to LPS and IFN-γ in classical immunological responses. Prolonged exposure to high quantities of LPS and IFN-γ may allow the body to produce pro-inflammatory substances that can hyperactivate macrophages toward M1 polarization, escalating inflammatory responses and deteriorating disorders linked to inflammation. As a result, M1-polarizing agents for macrophages are frequently LPS and IFN-γ. On the other hand, the anti-inflammatory cytokines IL-4 and IL-13 help resolve inflammation-related illnesses, decrease inflammatory responses, and encourage macrophage M2 polarization.
The responses of THP-1 and U937 macrophage cell lines to M1/M2 polarization conditions were compared by Nascimento et al. (169). The findings demonstrated that PMA stimulation could differentiate THP-1 and U937 into M0 macrophages. While U937 demonstrated stronger responses to M2-polarizing stimuli, favoring the M2 phenotype, THP-1 demonstrated stronger responses to M1-polarizing stimuli, with a considerable tilt toward the M1 phenotype. THP-1-derived macrophages showed more phagocytic activity and ROS generation than U937-derived macrophages. Both THP-1 and U937 showed decreased phagocytic activity and increased ROS production in response to M1-polarizing stimulation. The study also showed that although M-CSF doses and treatment times were adequate to promote differentiation in primary monocytes, neither THP-1 nor U937 cells could be made to develop into macrophages by M-CSF. Similarly, after M-CSF stimulation, Aldo et al. (170) found no alterations in CD14 surface expression in THP-1 cells. Results are greatly impacted by the concentrations and lengths of treatment with induction factors, even after they are discovered. For instance, the ideal PMA concentration is still being studied, and THP-1 cells lack a reliable PMA differentiation technique (109). Systematic research on the variations in biological response among cell lines is still lacking, despite the wide range of macrophage differentiation techniques.
4.2 Comparison between macrophage models
In conclusion, it is critical to examine the similarities and differences among the various macrophage models that are available.
4.2.1 Comparison among primary macrophages
Three primary murine macrophages—SMs, PMs, and BMDMs—were compared in terms of their properties by Zhao et al. (33). The study found that 48 hours of LPS+IFN-γ stimulation could polarize all three types of macrophages into an M1 state, while 48 hours of IL-4 stimulation could polarize them into an M2 state. While there were no appreciable variations in M2-polarizing capacity, SMs showed a greater M1-polarizing capability than the others. The most homogeneous macrophages are produced via adherent culture of BMDMs; however, their phenotype leans more toward M2. It was determined that although BMDMs offer a large number of uniform macrophages, TRMs cannot be completely replaced by them. IPSDMs showed a considerable but lesser bacterial phagocytic ability than PBMCs, according to Monkley et al. (171), while their transcriptome profiles and pro-inflammatory responses were similar. Conflicting research, however, indicates that IPSDMs tend to have an M2 phenotype, which lowers phagocytic activity (172, 173).
4.2.2 Comparison among immortalized macrophage cell lines
In order to assess how J774A.1, WEHI-3, P388D1, IC-21, NCTC 1469, and U937 responded to several chemotactic agents (casein, an N-formyl tetrapeptide, and culture supernatants of murine SL2 lymphoma cells), Terheggen et al. (130) performed chemotactic activity experiments on these cells. The findings showed that the cell lines’ susceptibility to various chemoattractants varied significantly. The most potent chemotactic activity was demonstrated by J774A.1 and WEHI-3 macrophage-like cells toward casein and N-formyl tetrapeptide, respectively. Van et al. (174) examined the levels of surface antigen expression, M-CSF secretion, and peroxidase activity in five cell lines: NCTC 1469, J774A.1, WEHI-3, IC21, and P388D1. According to the study, M-CSF secretion was unique to WEHI-3 cells, whereas peroxidase activity was present in all five cell lines. M1/69 and Mac-1 antigens were significantly expressed by P388D1 cells, while they were faintly expressed by NCTC 1469 and IC21 cells. J774A.1 cells showed the reverse pattern, with strong M1/69 and low Mac-1 expression in WEHI-3 cells. Nibbering et al. (175) compared quantitative data from four cell lines (WEHI-3, P388D1, J774A.1, and PU5-1.8) on surface antigen expression (e.g., F4/80, CDb11) with data from different mononuclear phagocytes. The four macrophage-like cell lines showed clear phenotypic differences, with WEHI-3 and P388D1 exhibiting the highest association with monocytes, according to the data. None of the macrophage-like cell lines closely matched any particular mononuclear phagocyte population, even though they shared several characteristics with mature mononuclear phagocytes. In comparison to RAW 264.7 and J774A.1 cells, IC-21 cells had a higher degree of differentiation than P388D1 cells (176) and more filopodia and membrane ruffles (176, 177).
4.2.3 Comparison between immortalized macrophage cell lines and primary macrophages
The phagocytic activity and polarization capacity of THP-1 and PBMCs were contrasted by Shiratori et al. (152). According to the study, THP-1 has higher phagocytic activity and a stronger propensity toward M1 polarization than PBMCs. IPSDMs exhibit more distinctive macrophage surface characteristics and express larger amounts of CD86 and MRC1 than U-937 (178). The immunobiological profile of BMDMs can only be partially replicated by THP-1 cells, according to Gaidt et al. (179). Using four macrophage cell lines (J774A.1, PU5-1.8, WEHI-3, and RAW 264.1) and newly obtained primary macrophages from C3H/He mice under standard culture conditions, Nibbering et al. (180) examined the production of N-nitrosamine during immunological stimulation in vitro. The findings showed that when LPS was stimulated, all cell types produced nitrite and N-nitrosomorpholine, and that IFN-γ amplified this action. According to Bodel et al. (181), J774A.1, PU5-1.8, P388D1, and WEHI-3 were still able to manufacture lysozyme and pyrogens on their own in vitro. Via et al. (182) assessed the ability of two human macrophage/monocyte cell lines and four murine macrophage cell lines to degrade unmodified LDL (native LDL) and acetylated low-density lipoprotein (AcLDL). According to the findings, PU5-1.8, HL-60, and U-937 are not appropriate models for researching scavenger pathways; however, P388D1, J774A.1, and RAW 264.7 are. The morphology and quantitative surface expression of C3b antibodies in primary macrophages, J774A.1, PU5-1.8, WEHI-3, and P388D1 were compared by Furth et al. (183). According to morphological data, J774A.1 and WEHI-3 cell lines were almost the same in most ways, while P388D1 was the most different from them but most comparable to primary macrophages. Furthermore, WEHI-3 and P388D1 cell lines exhibited significantly reduced surface C3b receptor expression in comparison to the other two cell lines.
Plasmid DNA, siRNA, and/or shRNA transfection has been portrayed to be possible in RAW264.7 cells, but it is very difficult in primary macrophages, where it commonly results in cellular death (184–187). Interestingly, J774.1 cells share functional similarities with primary macrophages, yet exhibit distinct responses from RAW264.7 cells: transfection with plasmid DNA induces significant cell death, whereas mRNA transfection maintains cellular viability (188). For eukaryotic cells, common transfection methods include electroporation, mechanical methods (such as gene guns), and viral vectors. Yet, since almost all known transfection processes severely impair cellular survival or obstruct their differentiation and polarization tendencies, macrophages are especially resistant to transfection techniques (188). In order to overcome these constraints, optimal transfection techniques have been suggested: Michael et al. (189) created a modified nucleofection-based method that effectively transfects THP-1 cells and allows them to differentiate into mature macrophages while maintaining cellular viability and functionality. Similarly, using an improved electroporation-based, non-viral nucleofection technique that preserved cellular integrity and physiological functioning, Maeß et al. (190) successfully transfected human THP-1 macrophages. Moreover, recent studies demonstrate that engineered nanoparticles (191) and the macrophage-specific editing (MAGE) system (192) enable highly efficient transfection of plasmid DNA/RNA in primary macrophages, surpassing conventional approaches in both delivery efficacy and specificity (Tables 1, 2).
5 Conclusion
The development of macrophage research models has been fueled by the critical functions that macrophages play in a variety of physiological and pathological processes, such as innate immunity, tissue homeostasis, disease etiology, and tissue repair. BMDMs, MDMs, or TRMs customized for particular research goals are the most common experimental procedures used in primary macrophage models to mimic normal macrophage populations due to they accurately reflect in vivo features. Nevertheless, procurement and scalability issues are common to all primary macrophage types, severely restricting their experimental uses. Several immortalized monocyte/macrophage cell line-based models, including THP-1, U-937, RAW264.7, and J774A.1, have been developed to overcome these material limitations. Nevertheless, these cell lines are primarily derived from cancerous origins, which naturally limits their ability to accurately replicate normal macrophage physiology. Notably, primary models are preferred in cancer immunotherapy to retain patient-specific TAMs heterogeneity, whereas immortalized cells such as THP-1 serve better for mechanistic dissection in autoimmune disease remodeling due to their genetic tractability. When choosing models for certain experimental objectives, it is necessary to carefully weigh functional fidelity against operational practicality because these two paradigms show complementary strengths in current research. Emerging 3D in vitro models such as organoids and scaffold-based co-cultures offer promising avenues to overcome key limitations. These systems provide physiologically relevant microenvironments that recapitulate tissue architecture, cellular interactions, and spatial constraints essential for macrophage studies, as demonstrated in recent investigations.
6 Limitation
① Although macrophages are found in many tissues, the main subsets of macrophages discussed here are still quite small. Despite their being outside the purview of this analysis, emerging immortalized models (such as the canine DH82 macrophage line) still merit thorough functional profiling.
② This work acknowledges that the inherent complexity and context-dependent characteristics of macrophage populations hinder in-depth comparative investigations of phenotypic and functional variability among populations.
③ Distinct molecular fingerprints and signaling network dysregulations—mechanisms not mechanistically examined in this study—are probably the cause of phenotypic differences.
Author contributions
DT: Writing – review & editing, Writing – original draft. DY: Data curation, Writing – review & editing. YB: Methodology, Writing – review & editing. TW: Data curation, Writing – original draft. LJ: Data curation, Writing – original draft. XJ: Resources, Writing – review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was funded by Xiyuan Hospital, China Academy of Chinese Medical Sciences (No. XYXZ0303-14).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tauber AI. Metchnikoff and the phagocytosis theory. Nat Rev Mol Cell Biol. (2003) 4:897–901. doi: 10.1038/nrm1244
2. Cuadros MA, Coltey P, Carmen Nieto M, and Martin C. Demonstration of a phagocytic cell system belonging to the hemopoietic lineage and originating from the yolk sac in the early avian embryo. Development. (1992) 115:157–68. doi: 10.1242/dev.115.1.157
3. Naito M, Yamamura F, Nishikawa S, and Takahashi K. Development, differentiation, and maturation of fetal mouse yolk sac macrophages in cultures. J Leukoc Biol. (1989) 46:1–10. doi: 10.1002/jlb.46.1.1
4. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. (2018) 233:6425–40. doi: 10.1002/jcp.26429
5. Sica A and Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. (2012) 122:787–95. doi: 10.1172/JCI59643
6. Peng Y, Zhou M, Yang H, Qu R, Qiu Y, Hao J, et al. Regulatory mechanism of M1/M2 macrophage polarization in the development of autoimmune diseases. Mediators Inflammation. (2023) 2023:8821610. doi: 10.1155/2023/8821610
7. Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, et al. Microglial and macrophage polarization—new prospects for brain repair. Nat Rev Neurol. (2015) 11:56–64. doi: 10.1038/nrneurol.2014.207
8. Zitta K, Berndt R, Hess K, Fändrich F, Gurvich O, Sirviö K, et al. Transcriptomic characterization of GMP-compliant regulatory macrophages (TRI-001) under inflammatory and hypoxic conditions: a comparative analysis across macrophage subtypes. J Transl Med. (2025) 23:551. doi: 10.1186/s12967-025-06548-6
9. Sawitzki B, Harden PN, Reinke P, Moreau A, Hutchinson JA, Game DS, et al. Regulatory cell therapy in kidney transplantation (The ONE Study): a harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet. (2020) 395:1627–39. doi: 10.1016/S0140-6736(20)30167-7
10. Hutchinson JA, Riquelme P, Sawitzki B, Tomiuk S, Miqueu P, Zuhayra M, et al. Cutting Edge: Immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J Immunol. (2011) 187:2072–8. doi: 10.4049/jimmunol.1100762
11. Pan Y, Yu Y, Wang X, and Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. (2020) 11:583084. doi: 10.3389/fimmu.2020.583084
12. Nakai K. Multiple roles of macrophage in skin. J Dermatol Sci. (2021) 104:2–10. doi: 10.1016/j.jdermsci.2021.08.008
13. Lazarov T, Juarez-Carreño S, Cox N, and Geissmann F. Physiology and diseases of tissue-resident macrophages. Nature. (2023) 618:698–707. doi: 10.1038/s41586-023-06002-x
14. Luo M, Zhao F, Cheng H, Su M, and Wang Y. Macrophage polarization: an important role in inflammatory diseases. Front Immunol. (2024) 15:1352946. doi: 10.3389/fimmu.2024.1352946
15. Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. (2014) 41:14–20. doi: 10.1016/j.immuni.2014.06.008
16. Salo RJ, Bleam DK, Greer VL, and Ortega AP. Interferon production in murine macrophage-like cell lines. J Leukoc Biol. (1985) 37:395–406. doi: 10.1002/jlb.37.4.395
17. Ralph P and Nakoinz I. Phagocytosis and cytolysis by a macrophage tumour and its cloned cell line. Nature. (1975) 257:393–4. doi: 10.1038/257393a0
18. Raschke WC, Baird S, Ralph P, and Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. (1978) 15:261–7. doi: 10.1016/0092-8674(78)90101-0
19. Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K, et al. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. (1980) 26:171–6. doi: 10.1002/ijc.2910260208
20. van Furth R, Cohn Z A, Hirsch JG, Humphrey JH, Spector WG, Langevoort HL, et al. The mononuclear phagocyte system: a new classification of macrophages, monocytes, and their precursor cells. Bull World Health Organ. (1972) 46:845–52.
21. Ścieżyńska A, Nogowska A, Sikorska M, Konys J, Karpińska A, Komorowski M, et al. Isolation and culture of human primary keratinocytes-a methods review. Exp Dermatol. (2019) 28:107–12. doi: 10.1111/exd.13860
22. Heckmann BL and Green DR. LC3-associated phagocytosis at a glance. J Cell Sci. (2019) 132:1–6. doi: 10.1242/jcs.222984
23. Herb M, Gluschko A, and Schramm M. LC3-associated phagocytosis - The highway to hell for phagocytosed microbes. Semin Cell Dev Biol. (2020) 101:68–76. doi: 10.1016/j.semcdb.2019.04.016
24. Misra S, Lee TJ, Sebastian A, McGuigan J, Liao C, Koo I, et al. Loss of selenoprotein W in murine macrophages alters the hierarchy of selenoprotein expression, redox tone, and mitochondrial functions during inflammation. Redox Biol. (2023) 59:102571. doi: 10.1016/j.redox.2022.102571
25. Wang L, Zhang X, Cao Y, Ma Q, Mao X, Xu J, et al. Mice with a specific deficiency of Pfkfb3 in myeloid cells are protected from hypoxia-induced pulmonary hypertension. Br J Pharmacol. (2021) 178:1055–72. doi: 10.1111/bph.15339
26. Wang A, Kang X, Wang J, and Zhang S. IFIH1/IRF1/STAT1 promotes sepsis associated inflammatory lung injury via activating macrophage M1 polarization. Int Immunopharmacol. (2023) 114:109478. doi: 10.1016/j.intimp.2022.109478
27. Chen Z, Ross JL, and Hambardzumyan D. Intravital 2-photon imaging reveals distinct morphology and infiltrative properties of glioblastoma-associated macrophages. Proc Natl Acad Sci U S A. (2019) 116:14254–9. doi: 10.1073/pnas.1902366116
28. McWhorter F Y, Wang T, Nguyen P, Chung T, and Liu WF. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci U S A. (2013) 110:17253–8. doi: 10.1073/pnas.1308887110
29. Kim OH, Kim H, Kang J, Yang D, Kang YH, Lee DH, et al. Impaired phagocytosis of apoptotic cells causes accumulation of bone marrow-derived macrophages in aged mice. BMB Rep. (2017) 50:43–8. doi: 10.5483/BMBRep.2017.50.1.167
30. Assouvie A, Daley-Bauer LP, and Rousselet G. Growing murine bone marrow-derived macrophages. Methods Mol Biol. (2018) 1784:29–33. doi: 10.1007/978-1-4939-7837-3_3
31. Mendoza R, Banerjee I, Manna D, Reghupaty SC, Yetirajam R, Sarkar D, et al. Mouse bone marrow cell isolation and macrophage differentiation. Methods Mol Biol. (2022) 2455:85–91.
32. Yakin K, Oktem O, and Urman B. Intrauterine administration of peripheral mononuclear cells in recurrent implantation failure: a systematic review and meta-analysis. Sci Rep. (2019) 9:3897. doi: 10.1038/s41598-019-40521-w
33. Zhao YL, Tian PX, Han F, Zheng J, Xia XX, Xue WJ, et al. Comparison of the characteristics of macrophages derived from murine spleen, peritoneal cavity, and bone marrow. J Zhejiang Univ Sci B. (2017) 18:1055–63. doi: 10.1631/jzus.B1700003
34. Kadić E, Moniz RJ, Huo Y, Chi A, and Kariv I. Effect of cryopreservation on delineation of immune cell subpopulations in tumor specimens as determinated by multiparametric single cell mass cytometry analysis. BMC Immunol. (2017) 18:6. doi: 10.1186/s12865-017-0192-1
35. Chen YH, Zhou WG, Gao J, Gao L, Ji XL, Xi NNG, et al. Isolation, culture and identification of bovine peripheral blood monocyte macrophages. Xumu Yu Siliao Kexue. (2013) 34:21–22 + 25.
36. Schyns J, Bai Q, Ruscitti C, Radermecker C, De Schepper S, Chakarov S, et al. Non-classical tissue monocytes and two functionally distinct populations of interstitial macrophages populate the mouse lung. Nat Commun. (2019) 10:3964. doi: 10.1038/s41467-019-11843-0
37. Yu YR, Hotten DF, Malakhau Y, Volker E, Ghio AJ, Noble PW, et al. Flow cytometric analysis of myeloid cells in human blood, bronchoalveolar lavage, and lung tissues. Am J Respir Cell Mol Biol. (2016) 54:13–24. doi: 10.1165/rcmb.2015-0146OC
38. Becerra-Díaz M, Lerner AD, Yu DH, Thiboutot JP, Liu MC, Yarmus LB, et al. Sex differences in M2 polarization, chemokine and IL-4 receptors in monocytes and macrophages from asthmatics. Cell Immunol. (2021) 360:104252. doi: 10.1016/j.cellimm.2020.104252
39. Zhang WX, Ding YG, Sun JY, Wang XH, Liu Z, Ding JB, et al. Establishment and phenotypic characterization of an immortalizedgoat alveolar macrophage line. Microbiol China. (2025) 52:822–32.
40. Momeni MK, Taghipour H, Ghayebzadeh M, Mohammadi M, and Keikhaee R. Isolation and characterization of microplastics from the human respiratory system: Sputum, broncho-alveolar lavage fluid, and pleural fluid simultaneously. Environ pollut. (2025) 365:125389. doi: 10.1016/j.envpol.2024.125389
41. Beyer K, Menges P, Keßler W, and Heidecke CD. Pathophysiology of peritonitis. Chirurg. (2016) 87:5–12. doi: 10.1007/s00104-015-0117-6
42. Chen C, Wang R, Feng W, Yang F, Wang L, Yang X, et al. Peritoneal resident macrophages in mice with MLL-AF9-induced acute myeloid leukemia show an M2-like phenotype. Ann Transl Med. (2021) 9:266. doi: 10.21037/atm-21-139
43. Ghalavand M, Moradi-Chaleshtori M, Dorostkar R, Mohammadi-Yeganeh S, and Hashemi SM. Exosomes derived from rapamycin-treated 4T1 breast cancer cells induced polarization of macrophages to M1 phenotype. Biotechnol Appl Biochem. (2023) 70:1754–71. doi: 10.1002/bab.2473
44. Layoun A, Samba M, and Santos MM. Isolation of murine peritoneal macrophages to carry out gene expression analysis upon Toll-like receptors stimulation. J Vis Exp. (2015) 98):e52749. doi: 10.3791/52749
45. Zubkova ES, Dergilev KV, Beloglazova IB, Molokotina YD, Boldyreva MA, Tsokolaeva ZI, et al. Features of the population of mouse peritoneal macrophages isolated after stimulation with concanavalin A and thioglycolate. Bull Exp Biol Med. (2021) 171:532–40. doi: 10.1007/s10517-021-05265-6
46. García-Peñarrubia P, Ruiz-Alcaraz AJ, Ruiz-Ballester M, Ramírez-Pávez TN, and Martínez-Esparza M. Recent insights into the characteristics and role of peritoneal macrophages from ascites of cirrhotic patients. World J Gastroenterol. (2021) 27:7014–24. doi: 10.3748/wjg.v27.i41.7014
47. Chen J, Deng X, Liu Y, Tan Q, Huang G, Che Q, et al. Kupffer cells in non-alcoholic fatty liver disease: friend or foe? Int J Biol Sci. (2020) 16:2367–78. doi: 10.7150/ijbs.47143
48. Sitia G, Iannacone M, Aiolfi R, Isogawa M, van Rooijen N, Scozzesi C, et al. Kupffer cells hasten resolution of liver immunopathology in mouse models of viral hepatitis. PloS Pathog. (2011) 7:e1002061. doi: 10.1371/journal.ppat.1002061
49. Vollmar B and Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. (2009) 89:1269–339. doi: 10.1152/physrev.00027.2008
50. Reid DT, Reyes JL, McDonald BA, Vo T, Reimer RA, Eksteen B, et al. Kupffer cells undergo fundamental changes during the development of experimental NASH and are critical in initiating liver damage and inflammation. PloS One. (2016) 11:e0159524. doi: 10.1371/journal.pone.0159524
51. Zeng WQ, Zhang JQ, Li Y, Yang K, Chen YP, Liu ZJ, et al. A new method to isolate and culture rat kupffer cells. PLoS One. (2013) 8:e70832. doi: 10.1371/journal.pone.0070832
52. Borst K, Dumas AA, and Prinz M. Microglia: Immune and non-immune functions. Immunity. (2021) 54:2194–208. doi: 10.1016/j.immuni.2021.09.014
53. Krishna S, Choudhury A, Keough MB, Seo K, Ni L, Kakaizada S, et al. Glioblastoma remodelling of human neural circuits decreases survival. Nature. (2023) 617:599–607. doi: 10.1038/s41586-023-06036-1
54. Kisucká A, Bimbová K, Bačová M, Gálik J, and Lukáčová N. Activation of Neuroprotective Microglia and Astrocytes at the Lesion Site and in the Adjacent Segments Is Crucial for Spontaneous Locomotor Recovery after Spinal Cord Injury. Cells. (2021) 10:1–20. doi: 10.3390/cells10081943
55. Gensel JC and Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. (2015) 1619:1–11. doi: 10.1016/j.brainres.2014.12.045
56. Dello Russo C, Cappoli N, Coletta I, Mezzogori D, Paciello F, Pozzoli G, et al. The human microglial HMC3 cell line: where do we stand? A systematic literature review. J Neuroinflamm. (2018) 15:259. doi: 10.1186/s12974-018-1288-0
57. Li L, Jiang W, Yu B, Liang H, Mao S, Hu X, et al. Quercetin improves cerebral ischemia/reperfusion injury by promoting microglia/macrophages M2 polarization via regulating PI3K/Akt/NF-κB signaling pathway. BioMed Pharmacother. (2023) 168:115653. doi: 10.1016/j.biopha.2023.115653
58. Mor G and Abrahams VM. Potential role of macrophages as immunoregulators of pregnancy. Reprod Biol Endocrinol. (2003) 1:119. doi: 10.1186/1477-7827-1-119
59. Williams PJ, Searle RF, Robson SC, Innes BA, and Bulmer JN. Decidual leucocyte populations in early to late gestation normal human pregnancy. J Reprod Immunol. (2009) 82:24–31. doi: 10.1016/j.jri.2009.08.001
60. Yang F, Zheng Q, and Jin L. Dynamic function and composition changes of immune cells during normal and pathological pregnancy at the maternal-fetal interface. Front Immunol. (2019) 10:2317. doi: 10.3389/fimmu.2019.02317
61. Mezouar S, Katsogiannou M, Ben Amara A, Bretelle F, and Mege JL. Placental macrophages: Origin, heterogeneity, function and role in pregnancy-associated infections. Placenta. (2021) 103:94–103. doi: 10.1016/j.placenta.2020.10.017
62. Cox B, Kotlyar M, Evangelou AI, Ignatchenko V, Ignatchenko A, Whiteley K, et al. Comparative systems biology of human and mouse as a tool to guide the modeling of human placental pathology. Mol Syst Biol. (2009) 5:279. doi: 10.1038/msb.2009.37
63. Joerink M, Ribeiro CM, Stet RJ, Hermsen T, Savelkoul HF, Wiegertjes GF, et al. Head kidney-derived macrophages of common carp (Cyprinus carpio L.) show plasticity and functional polarization upon differential stimulation. J Immunol. (2006) 177:61–9. doi: 10.4049/jimmunol.177.1.61
64. Xia N, Zhang Y, Zhu W, and Su J. GCRV-II invades monocytes/macrophages and induces macrophage polarization and apoptosis in tissues to facilitate viral replication and dissemination. J Virol. (2024) 98:e0146923. doi: 10.1128/jvi.01469-23
65. Braun-Nesje R, Kaplan G, and Seljelid R. Rainbow trout macrophages in vitro: morphology and phagocytic activity. Dev Comp Immunol. (1982) 6:281–91. doi: 10.1016/S0145-305X(82)80011-6
66. Teles M, Mackenzie S, Boltaña S, Callol A, and Tort L. Gene expression and TNF-alpha secretion profile in rainbow trout macrophages following exposures to copper and bacterial lipopolysaccharide. Fish Shellfish Immunol. (2011) 30:340–6. doi: 10.1016/j.fsi.2010.11.006
67. MacKenzie S, Planas JV, and Goetz FW. LPS-stimulated expression of a tumor necrosis factor-alpha mRNA in primary trout monocytes and in vitro differentiated macrophages. Dev Comp Immunol. (2003) 27:393–400. doi: 10.1016/S0145-305X(02)00135-0
68. Nowak BF, Dang M, Webber C, Neumann L, Bridle A, Bermudez R, et al. Changes in the splenic melanomacrophage centre surface area in southern bluefin tuna (Thunnus maccoyii) are associated with blood fluke infections. Pathogens. (2021) 10:1–8. doi: 10.3390/pathogens10010079
69. Na YR, Stakenborg M, Seok SH, and Matteoli G. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat Rev Gastroenterol Hepatol. (2019) 16:531–43. doi: 10.1038/s41575-019-0172-4
70. Viola MF and Boeckxstaens G. Intestinal resident macrophages: Multitaskers of the gut. Neurogastroenterol Motil. (2020) 32:e13843. doi: 10.1111/nmo.13843
71. Ning S, Zhang Z, Zhou C, Wang B, Liu Z, Feng B, et al. Cross-talk between macrophages and gut microbiota in inflammatory bowel disease: a dynamic interplay influencing pathogenesis and therapy. Front Med (Lausanne). (2024) 11:1457218. doi: 10.3389/fmed.2024.1457218
72. Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF, et al. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protoc. (2007) 2:2307–11. doi: 10.1038/nprot.2007.315
73. Bargalló A, Abad L, Odena G, Planas R, and Bartolí R. New method for isolation of rat lamina propria macrophages in colonic tissue. J Immunol Methods. (2014) 408:132–6. doi: 10.1016/j.jim.2014.05.002
74. Gordon S, Plüddemann A, and Mukhopadhyay S. Sinusoidal immunity: macrophages at the lymphohematopoietic interface. Cold Spring Harb Perspect Biol. (2014) 7:a016378. doi: 10.1101/cshperspect.a016378
75. Lu Y, Basatemur G, Scott IC, Chiarugi D, Clement M, Harrison J, et al. Interleukin-33 signaling controls the development of iron-recycling macrophages. Immunity. (2020) 52:782–93.e785. doi: 10.1016/j.immuni.2020.03.006
76. den Haan JM and Kraal G. Innate immune functions of macrophage subpopulations in the spleen. J Innate Immun. (2012) 4:437–45. doi: 10.1159/000335216
77. Bellomo A, Gentek R, Golub R, and Bajénoff M. Macrophage-fibroblast circuits in the spleen. Immunol Rev. (2021) 302:104–25. doi: 10.1111/imr.12979
78. Rund CR, Christiansen JL, and Johnson JC. In vitro culture of melanomacrophages from the spleen and liver of turtles: comments on melanomacrophage morphology. Pigment Cell Res. (1998) 11:114–9. doi: 10.1111/j.1600-0749.1998.tb00720.x
79. Hwang JS, Chung HK, Bae EK, Lee AY, Ji HJ, Park DW, et al. The polysaccharide fraction AIP1 from Artemisia iwayomogi suppresses apoptotic death of the mouse spleen cells in culture. Arch Pharm Res. (2003) 26:294–300. doi: 10.1007/BF02976958
80. Song JH, Kwak S, Kim H, Jun W, Lee J, Yoon HG, et al. Dendropanax morbifera branch water extract increases the immunostimulatory activity of RAW264.7 macrophages and primary mouse splenocytes. J Med Food. (2019) 22:1136–45. doi: 10.1089/jmf.2019.4424
81. Xu Y, Wang Y, and Li Y. Expression of spleen macrophages in a mouse model of alveolar bone resorption periodontitis. Cell Mol Biol (Noisy-le-grand). (2023) 69:125–31. doi: 10.14715/cmb/2023.69.6.19
82. Huen SC and Cantley LG. Macrophages in renal injury and repair. Annu Rev Physiol. (2017) 79:449–69. doi: 10.1146/annurev-physiol-022516-034219
83. Miller SJ, Yashchenko A, and Zimmerman KA. Isolation and flow cytometry analysis of macrophages from the kidney. Methods Mol Biol. (2024) 2713:171–81. doi: 10.1007/978-1-0716-3437-0_12
84. Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. (2011) 22:317–26. doi: 10.1681/ASN.2009060615
85. Ampem G and Röszer T. Isolation and characterization of adipose tissue macrophages. Methods Mol Biol. (2019) 1966:225–36. doi: 10.1007/978-1-4939-9195-2_18
86. Cox N, Crozet L, Holtman IR, Loyher PL, Lazarov T, White JB, et al. Diet-regulated production of PDGFcc by macrophages controls energy storage. Science. (2021) 373:1–29. doi: 10.1126/science.abe9383
87. Zhang D, Yao X, Teng Y, Zhao T, Lin L, Li Y, et al. Adipocytes-Derived Exosomal microRNA-1224 Inhibits M2 Macrophage Polarization in Obesity-Induced Adipose Tissue Inflammation via MSI2-Mediated Wnt/β-Catenin Axis. Mol Nutr Food Res. (2022) 66:e2100889. doi: 10.1002/mnfr.202100889
88. Caldari-Torres C and Beck J. Effects of co-incubation of LPS-stimulated RAW 264.7 macrophages on leptin production by 3T3-L1 adipocytes: a method for co-incubating distinct adipose tissue cell lines. Bull Natl Res Cent. (2022) 46:57. doi: 10.1186/s42269-022-00747-7
89. Ito A, Suganami T, Yamauchi A, Degawa-Yamauchi M, Tanaka M, Kouyama R, et al. Role of CC chemokine receptor 2 in bone marrow cells in the recruitment of macrophages into obese adipose tissue. J Biol Chem. (2008) 283:35715–23. doi: 10.1074/jbc.M804220200
90. Kim J, Chung K, Choi C, Beloor J, Ullah I, Kim N, et al. Silencing CCR2 in macrophages alleviates adipose tissue inflammation and the associated metabolic syndrome in dietary obese mice. Mol Ther Nucleic Acids. (2016) 5:e280. doi: 10.1038/mtna.2015.51
91. Arlat A, Renoud ML, Nakhle J, Thomas M, Fontaine J, Arnaud E, et al. Generation of functionally active resident macrophages from adipose tissue by 3D cultures. Front Immunol. (2024) 15:1356397. doi: 10.3389/fimmu.2024.1356397
92. Kopecky BJ and Lavine KJ. Cardiac macrophage metabolism in health and disease. Trends Endocrinol Metab. (2024) 35:249–62. doi: 10.1016/j.tem.2023.10.011
93. Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D'Antoni ML, Debuque R, et al. Revisiting cardiac cellular composition. Circ Res. (2016) 118:400–9. doi: 10.1161/CIRCRESAHA.115.307778
94. Swirski FK and Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol. (2018) 18:733–44. doi: 10.1038/s41577-018-0065-8
95. Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. (2007) 204:3037–47. doi: 10.1084/jem.20070885
96. Bajpai G and Lavine KJ. Isolation of macrophage subsets and stromal cells from human and mouse myocardial specimens. J Vis Exp. (2019) 154):1–2. doi: 10.3791/60015
97. Cevey Á C, Mascolo PD, Penas FN, Pieralisi AV, Sequeyra AS, Mirkin GA, et al. Benznidazole anti-inflammatory effects in murine cardiomyocytes and macrophages are mediated by class I PI3Kδ. Front Immunol. (2021) 12:782891. doi: 10.3389/fimmu.2021.782891
98. Yamanaka S and Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. (2010) 465:704–12. doi: 10.1038/nature09229
99. Malik N and Rao MS. A review of the methods for human iPSC derivation. Methods Mol Biol. (2013) 997:23–33. doi: 10.1007/978-1-62703-348-0_3
100. Abdin SM, Paasch D, Kloos A, Oliveira MC, Jang MS, Ackermann M, et al. Scalable generation of functional human iPSC-derived CAR-macrophages that efficiently eradicate CD19-positive leukemia. J Immunother Cancer. (2023) 11:1–8. doi: 10.1136/jitc-2023-007705
101. Cao X, van den Hil FE, Mummery CL, and Orlova VV. Generation and functional characterization of monocytes and macrophages derived from human induced pluripotent stem cells. Curr Protoc Stem Cell Biol. (2020) 52:e108. doi: 10.1002/cpsc.108
102. Meng X, Han X, Lin X, Li G, Wang J, Sun A, et al. Construction and identification of a human colorectal adenoma epithelial cell line by SV40 large T-antigen transfection. Recent Pat Anticancer Drug Discov. (2024). doi: 10.2174/0115748928297500240522080820
103. Shao Y, Shah PT, Su Q, Li S, Huang F, Wang J, et al. Recombinant adenoviruses expressing HPV16/18 E7 upregulate the HDAC6 and DNMT3B genes in C33A cells. Front Cell Infect Microbiol. (2024) 14:1459572. doi: 10.3389/fcimb.2024.1459572
104. Yang J, Dong Y, Hu L, Wang W, Li Y, Wang S, et al. Immortalization of mesenchymal stem cell lines from sheep umbilical cord tissue. Biol (Basel). (2024) 13:1–15. doi: 10.3390/biology13070551
105. Li P, Hao Z, Wu J, Ma C, Xu Y, Li J, et al. Comparative proteomic analysis of polarized human THP-1 and mouse RAW264.7 macrophages. Front Immunol. (2021) 12:700009. doi: 10.3389/fimmu.2021.700009
106. Taciak B, Białasek M, Braniewska A, Sas Z, Sawicka P, Kiraga Ł, et al. Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages. PLoS One. (2018) 13:e0198943. doi: 10.1371/journal.pone.0198943
107. Abdi H, Roshanravan M, Mirzavi F, Hosseinzadeh H, and Mosaffa F. Crocin’s effect on phenotype switching of J774A.1 macrophages depends on their polarization state during exposure. Iran J Basic Med Sci. (2023) 26:1431–7. doi: 10.22038/IJBMS.2023.70859.15392
108. Ralph P and Nakoinz I. Direct toxic effects of immunopotentiators on monocytic, myelomonocytic, and histiocytic or macrophage tumor cells in culture. Cancer Res. (1977) 37:546–50.
109. Herb M, Schatz V, Hadrian K, Hos D, Holoborodko B, Jantsch J, et al. Macrophage variants in laboratory research: most are well done, but some are RAW. Front Cell Infect Microbiol. (2024) 14:1457323. doi: 10.3389/fcimb.2024.1457323
110. Snyderman R, Pike MC, Fischer DG, and Koren HS. Biologic and biochemical activities of continuous macrophage cell lines P388D1 and J774.1. J Immunol. (1977) 119:2060–6. doi: 10.4049/jimmunol.119.6.2060
111. Ralph P, Moore MA, and Nilsson K. Lysozyme synthesis by established human and murine histiocytic lymphoma cell lines. J Exp Med. (1976) 143:1528–33. doi: 10.1084/jem.143.6.1528
112. Koren HS, Handwerger BS, and Wunderlich JR. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. (1975) 114:894–7. doi: 10.4049/jimmunol.114.2_Part_2.894
113. De Weger RA, Van Loveren H, Van Basten CD, Oskam R, Van Der Zeijst BA, Den Otter W, et al. Functional activities of the NCTC 1469 macrophage-like cell line: comparison of the NCTC 1469 cell line with various other macrophage-like cell lines. J Reticuloendothel Soc. (1983) 33:55–66.
114. Liu WN and Leung KN. Jacaric acid inhibits the growth of murine macrophage-like leukemia PU5-1.8 cells by inducing cell cycle arrest and apoptosis. Cancer Cell Int. (2015) 15:90. doi: 10.1186/s12935-015-0246-5
115. Keeling S, Deashinta N, Howard KM, Vigil S, Moonie S, and Schneider BS. Macrophage colony stimulating factor-induced macrophage differentiation influences myotube elongation. Biol Res Nurs. (2013) 15:62–70. doi: 10.1177/1099800411414871
116. Warner NL, Moore MA, and Metcalf D. A transplantable myelomonocytic leukemia in BALB-c mice: cytology, karyotype, and muramidase content. J Natl Cancer Inst. (1969) 43:963–82.
117. Li XC, Luo SJ, Fan W, Zhou TL, Tan DQ, Tan RX, et al. Macrophage polarization regulates intervertebral disc degeneration by modulating cell proliferation, inflammation mediator secretion, and extracellular matrix metabolism. Front Immunol. (2022) 13:922173. doi: 10.3389/fimmu.2022.922173
118. Qiu X, Zhou T, Li S, Wu J, Tang J, Ma G, et al. Spatial single-cell protein landscape reveals vimentin(high) macrophages as immune-suppressive in the microenvironment of hepatocellular carcinoma. Nat Cancer. (2024) 5:1557–78. doi: 10.1038/s43018-024-00824-y
119. Johnson CR, Kitz D, and Little JR. A method for the derivation and continuous propagation of cloned murine bone marrow macrophages. J Immunol Methods. (1983) 65:319–32. doi: 10.1016/0022-1759(83)90127-8
120. Dexter TM, Garland J, Scott D, Scolnick E, and Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. (1980) 152:1036–47. doi: 10.1084/jem.152.4.1036
121. Lee JC, Hapel AJ, and Ihle JN. Constitutive production of a unique lymphokine (IL 3) by the WEHI-3 cell line. J Immunol. (1982) 128:2393–8. doi: 10.4049/jimmunol.128.6.2393
122. Schwarzbaum S, Halpern R, and Diamond B. The generation of macrophage-like cell lines by transfection with SV40 origin defective DNA. J Immunol. (1984) 132:1158–62. doi: 10.4049/jimmunol.132.3.1158
123. Yui S, Kudo T, Hodono K, Mimaki Y, Kuroda M, Sashida Y, et al. Characterization of the growth-inhibitory and apoptosis-inducing activities of a triterpene saponin, securioside B against BAC1.2F5 macrophages. Mediators Inflammation. (2003) 12:157–66. doi: 10.1080/0962935031000134879
124. Jones CM, Goldfarb RH, and Holden HT. Macrophage cell lines behave as activated macrophages in the production and regulation of plasminogen activator. Cancer Invest. (1983) 1:207–13. doi: 10.3109/07357908309041360
125. Wong AP, Perez-Castillejos R, Christopher Love J, and Whitesides GM. Partitioning microfluidic channels with hydrogel to construct tunable 3-D cellular microenvironments. Biomaterials. (2008) 29:1853–61. doi: 10.1016/j.biomaterials.2007.12.044
126. Traber MG, Defendi V, and Kayden HJ. Receptor activities for low-density lipoprotein and acetylated low-density lipoprotein in a mouse macrophage cell line (IC21) and in human monocyte-derived macrophages. J Exp Med. (1981) 154:1852–67. doi: 10.1084/jem.154.6.1852
127. Alvarez-Domínguez C, Carrasco-Marín E, López-Mato P, and Leyva-Cobián F. The contribution of both oxygen and nitrogen intermediates to the intracellular killing mechanisms of C1q-opsonized Listeria monocytogenes by the macrophage-like IC-21 cell line. Immunology. (2000) 101:83–9. doi: 10.1046/j.1365-2567.2000.00083.x
128. Duraisamy P, Ravi S, Martin LC, Kumaresan M, Manikandan B, and Ramar M. Differential phagocytic expression of IC-21 macrophages and their scavenging receptors during inflammatory induction by oxysterol: A microscopic approach. Microsc Res Tech. (2024) 87:2745–56. doi: 10.1002/jemt.24647
129. Crawford EK, Latham PS, Shah EM, and Hasday JD. Characterization of tumor binding by the IC-21 macrophage cell line. Cancer Res. (1990) 50:4578–83.
130. Terheggen P, Van Loveren H, and Den Otter W. Macrophage-like tumor cells as tools to study chemoattractive activity. J Leukoc Biol. (1985) 38:747–52. doi: 10.1002/jlb.38.6.747
131. Van Basten CD, De Weger RA, and Van Loveren H. NCTC 1469 CB, a subline of the macrophage-like cell line NCTC 1469 with reduced phagocytic activity. J Reticuloendothel Soc. (1983) 33:47–53.
132. Xie J, Ye H, Du M, Yu Q, Chen Y, and Shen M. Mung bean protein hydrolysates protect mouse liver cell line nctc-1469 cell from hydrogen peroxide-induced cell injury. Foods. (2019) 9:1–13. doi: 10.3390/foods9010014
133. Dou Z, Lu F, Hu J, Li B, and Li X. CBX7 silencing promoted liver regeneration by interacting with BMI1 and activating the Nrf2/ARE signaling pathway. Sci Rep. (2024) 14:11008. doi: 10.1038/s41598-024-58248-8
134. Liu Y, Xu W, Zhai T, You J, and Chen Y. Silibinin ameliorates hepatic lipid accumulation and oxidative stress in mice with non-alcoholic steatohepatitis by regulating CFLAR-JNK pathway. Acta Pharm Sin B. (2019) 9:745–57. doi: 10.1016/j.apsb.2019.02.006
135. Mbawuike IN and Herscowitz HB. MH-S, a murine alveolar macrophage cell line: morphological, cytochemical, and functional characteristics. J Leukoc Biol. (1989) 46:119–27. doi: 10.1002/jlb.46.2.119
136. Cox GW, Mathieson BJ, Giardina SL, and Varesio L. Characterization of IL-2 receptor expression and function on murine macrophages. J Immunol. (1990) 145:1719–26. doi: 10.4049/jimmunol.145.6.1719
137. May AE, Redecke V, Grüner S, Schmidt R, Massberg S, Miethke T, et al. Recruitment of Chlamydia pneumoniae-infected macrophages to the carotid artery wall in noninfected, nonatherosclerotic mice. Arterioscler Thromb Vasc Biol. (2003) 23:789–94. doi: 10.1161/01.ATV.0000068645.60805.7C
138. Liu L, Zhai K, Chen Y, Chen X, Wang G, and Wu L. Effect and mechanism of mycobacterium tuberculosis lipoprotein lpqH in NLRP3 inflammasome activation in mouse ana-1 macrophage. BioMed Res Int. (2021) 2021:8239135. doi: 10.1155/2021/8239135
139. Helmke RJ, German VF, and Mangos JA. A continuous alveolar macrophage cell line: comparisons with freshly derived alveolar macrophages. In Vitro Cell Dev Biol. (1989) 25:44–8. doi: 10.1007/BF02624409
140. Diabaté S, Mülhopt S, Paur HR, Wottrich R, and Krug HF. In vitro effects of incinerator fly ash on pulmonary macrophages and epithelial cells. Int J Hyg Environ Health. (2002) 204:323–6. doi: 10.1078/1438-4639-00109
141. Li X, Zhang X, Luo Y, Liu R, Sun Y, Zhao S, et al. Large fragment inDels reshape genome structure of porcine alveolar macrophage 3D4/21 cells. Genes (Basel). (2022) 13:1–12. doi: 10.3390/genes13091515
142. Weingartl HM, Sabara M, Pasick J, van Moorlehem E, and Babiuk L. Continuous porcine cell lines developed from alveolar macrophages: partial characterization and virus susceptibility. J Virol Methods. (2002) 104:203–16. doi: 10.1016/S0166-0934(02)00085-X
143. Sung YJ, Hotchkiss JH, and Dietert RR. 2,4-Diamino-6-hydroxypyrimidine, an inhibitor of GTP cyclohydrolase I, suppresses nitric oxide production by chicken macrophages. Int J Immunopharmacol. (1994) 16:101–8. doi: 10.1016/0192-0561(94)90065-5
144. Ahmed-Hassan H, Abdul-Cader MS, Sabry MA, Hamza E, and Abdul-Careem MF. Toll-like receptor (TLR)4 signalling induces myeloid differentiation primary response gene (MYD) 88 independent pathway in avian species leading to type I interferon production and antiviral response. Virus Res. (2018) 256:107–16. doi: 10.1016/j.virusres.2018.08.008
145. Aguilar-Briseño JA, Elliff JM, Patten JJ, Wilson LR, Davey RA, Bailey AL, et al. Effect of interferon gamma on ebola virus infection of primary Kupffer cells and a Kupffer cell line. Viruses. (2023) 15:1–17. doi: 10.3390/v15102077
146. Blasi E, Barluzzi R, Bocchini V, Mazzolla R, and Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. (1990) 27:229–37. doi: 10.1016/0165-5728(90)90073-V
147. Chen Q and Ross AC. Retinoic acid regulates cell cycle progression and cell differentiation in human monocytic THP-1 cells. Exp Cell Res. (2004) 297:68–81. doi: 10.1016/j.yexcr.2004.02.017
148. Daigneault M, Preston JA, Marriott HM, Whyte MK, and Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PloS One. (2010) 5:e8668. doi: 10.1371/journal.pone.0008668
149. Molaaghaee-Rouzbahani S, Asri N, Sapone A, Baghaei K, Yadegar A, Amani D, et al. Akkermansia muciniphila exerts immunomodulatory and anti-inflammatory effects on gliadin-stimulated THP-1 derived macrophages. Sci Rep. (2023) 13:3237. doi: 10.1038/s41598-023-30266-y
150. Oberg F, Hult N, Bjare U, Ivhed I, Kivi S, Bergh J, et al. Characterization of a U-937 subline which can be induced to differentiate in serum-free medium. Int J Cancer. (1992) 50:153–60. doi: 10.1002/ijc.2910500130
151. Oberg F, Botling J, and Nilsson K. Functional antagonism between vitamin D3 and retinoic acid in the regulation of CD14 and CD23 expression during monocytic differentiation of U-937 cells. J Immunol. (1993) 150:3487–95. doi: 10.4049/jimmunol.150.8.3487
152. Shiratori H, Feinweber C, Luckhardt S, Linke B, Resch E, Geisslinger G, et al. THP-1 and human peripheral blood mononuclear cell-derived macrophages differ in their capacity to polarize in vitro. Mol Immunol. (2017) 88:58–68. doi: 10.1016/j.molimm.2017.05.027
153. Jack I, Seshadri R, Garson M, Michael P, Callen D, Zola H, et al. RCH-ACV: a lymphoblastic leukemia cell line with chromosome translocation 1;19 and trisomy 8. Cancer Genet Cytogenet. (1986) 19:261–9. doi: 10.1016/0165-4608(86)90055-5
154. Rapino F, Robles EF, Richter-Larrea JA, Kallin EM, Martinez-Climent JA, Graf T, et al. C/EBPα induces highly efficient macrophage transdifferentiation of B lymphoma and leukemia cell lines and impairs their tumorigenicity. Cell Rep. (2013) 3:1153–63. doi: 10.1016/j.celrep.2013.03.003
155. Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, et al. Human monocytes engage an alternative inflammasome pathway. Immunity. (2016) 44:833–46. doi: 10.1016/j.immuni.2016.01.012
156. Schüssler M, Schott K, Fuchs NV, Oo A, Zahadi M, Rauch P, et al. Gene editing of SAMHD1 in macrophage-like cells reveals complex relationships between SAMHD1 phospho-regulation, HIV-1 restriction, and cellular dNTP levels. mBio. (2023) 14:e0225223. doi: 10.1128/mbio.02252-23
157. Volkmar K, Jaedtka M, Baars I, Walber B, Philipp MS, Bagola K, et al. Investigating pyroptosis as a mechanism of L. major cell-to-cell spread in the human BLaER1 infection model. Mol Microbiol. (2024) 121:453–69. doi: 10.1111/mmi.15142
158. Hultgren EM, Patrick ME, Evans RL, Stoos CT, and Egland KA. SUSD2 promotes tumor-associated macrophage recruitment by increasing levels of MCP-1 in breast cancer. PloS One. (2017) 12:e0177089. doi: 10.1371/journal.pone.0177089
159. Tan ZH, Huang JY, Guo JH, Yan ZQ, Cui YZ, Wang T, et al. Characterization of M1 macrophage model prepared from normal human monocyte SC cells. Chin J Cell Biol (2010-). (2018) 40:1905–14.
160. Gallagher R, Collins S, Trujillo J, McCredie K, Ahearn M, Tsai S, et al. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. (1979) 54:713–33. doi: 10.1182/blood.V54.3.713.713
161. Manning R, Fallani A, and Ruggieri S. Lipid changes in HL-60 cells on differentiation into macrophages by treatment with a phorbol ester. Lipids. (1995) 30:811–5. doi: 10.1007/BF02533956
162. Jouni ZE, Winzerling JJ, and McNamara DJ. 1,25-Dihydroxyvitamin D3-induced HL-60 macrophages: regulation of cholesterol and LDL metabolism. Atherosclerosis. (1995) 117:125–38. doi: 10.1016/0021-9150(95)05569-I
163. Camussi G, Bussolino F, Ghezzo F, and Pegoraro L. Release of platelet-activating factor from HL-60 human leukemic cells following macrophage-like differentiation. Blood. (1982) 59:16–22. doi: 10.1182/blood.V59.1.16.16
164. Janabi N, Peudenier S, Héron B, Ng KH, and Tardieu M. Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV40 large T antigen. Neurosci Lett. (1995) 195:105–8. doi: 10.1016/0304-3940(94)11792-H
165. Mandic M, Misirkic Marjanovic M, Vucicevic L, Jovanovic M, Bosnjak M, Perovic V, et al. MAP kinase-dependent autophagy controls phorbol myristate acetate-induced macrophage differentiation of HL-60 leukemia cells. Life Sci. (2022) 297:120481. doi: 10.1016/j.lfs.2022.120481
166. Bai X, Yang W, Li H, Zhao Y, Fan W, Zhang H, et al. Cyclosporine A regulates influenza A virus-induced macrophages polarization and inflammatory responses by targeting cyclophilin A. Front Immunol. (2022) 13:861292. doi: 10.3389/fimmu.2022.861292
167. Hamilton JA and Achuthan A. Colony stimulating factors and myeloid cell biology in health and disease. Trends Immunol. (2013) 34:81–9. doi: 10.1016/j.it.2012.08.006
168. Wang C, Yu X, Cao Q, Wang Y, Zheng G, Tan TK, et al. Characterization of murine macrophages from bone marrow, spleen and peritoneum. BMC Immunol. (2013) 14:6. doi: 10.1186/1471-2172-14-6
169. Nascimento CR, Rodrigues Fernandes NA, Gonzalez Maldonado LA, and Rossa Junior C. Comparison of monocytic cell lines U937 and THP-1 as macrophage models for in vitro studies. Biochem Biophys Rep. (2022) 32:101383. doi: 10.1016/j.bbrep.2022.101383
170. Aldo PB, Craveiro V, Guller S, and Mor G. Effect of culture conditions on the phenotype of THP-1 monocyte cell line. Am J Reprod Immunol. (2013) 70:80–6. doi: 10.1111/aji.12129
171. Monkley S, Krishnaswamy JK, Göransson M, Clausen M, Meuller J, Thörn K, et al. Optimised generation of iPSC-derived macrophages and dendritic cells that are functionally and transcriptionally similar to their primary counterparts. PloS One. (2020) 15:e0243807. doi: 10.1371/journal.pone.0243807
172. Buchrieser J, James W, and Moore MD. Human induced pluripotent stem cell-derived macrophages share ontogeny with MYB-independent tissue-resident macrophages. Stem Cell Rep. (2017) 8:334–45. doi: 10.1016/j.stemcr.2016.12.020
173. Haideri SS, McKinnon AC, Taylor AH, Kirkwood P, Starkey Lewis PJ, O'Duibhir E, et al. Injection of embryonic stem cell derived macrophages ameliorates fibrosis in a murine model of liver injury. NPJ Regener Med. (2017) 2:14. doi: 10.1038/s41536-017-0017-0
174. Van Loveren H, Hilgers J, De Bakker JM, De Weger RA, Brederoo P, Den Otter W, et al. Macrophage-like cell lines: endogenous peroxidatic activity, cell surface antigens, and colony-stimulating factor production. J Reticuloendothel Soc. (1983) 33:221–9.
175. Nibbering PH and van Furth R. Quantitative immunocytochemical characterization of four murine macrophage-like cell lines. Immunobiology. (1988) 176:432–9. doi: 10.1016/S0171-2985(88)80024-X
176. Walker WS and Gandour DM. Detection and functional assessment of complement receptors on two murine macrophage-like cell lines. Exp Cell Res. (1980) 129:15–21. doi: 10.1016/0014-4827(80)90326-2
177. Godek ML, Sampson JA, Duchsherer NL, McElwee Q, and Grainger DW. Rho GTPase protein expression and activation in murine monocytes/macrophages is not modulated by model biomaterial surfaces in serum-containing in vitro cultures. J Biomater Sci Polym Ed. (2006) 17:1141–58. doi: 10.1163/156856206778530731
178. Zhang YT, Hou GJ, and Shen N. Comparison of human-induced pluripotent stem cell-derived macrophages with peripheral blood-derived macrophages using single-cell genomics. J Shanghai Jiaotong Univ. Med Sci. (2024) 44:1477–89.
179. Gaidt MM, Rapino F, Graf T, and Hornung V. Modeling primary human monocytes with the trans-differentiation cell line BLaER1. Methods Mol Biol. (2018) 1714:57–66. doi: 10.1007/978-1-4939-7519-8_4
180. Miwa M, Stuehr DJ, Marletta MA, Wishnok JS, and Tannenbaum SR. Nitrosation of amines by stimulated macrophages. Carcinogenesis. (1987) 8:955–8. doi: 10.1093/carcin/8.7.955
181. Bodel P. Spontaneous pyrogen production by mouse histiocytic and myelomonocytic tumor cell lines in vitro. J Exp Med. (1978) 147:1503–l1506.
182. Via DP, Plant AL, Craig IF, Gotto Jr AM, and Smith LC. Metabolism of normal and modified low-density lipoproteins by macrophage cell lines of murine and human origin. Biochim Biophys Acta. (1985) 833:417–28. doi: 10.1016/0005-2760(85)90099-2
183. van Furth R, van Schadewijk-Nieuwstad M, Elzenga-Claasen I, Cornelisse C, and Nibbering P. Morphological, cytochemical, functional, and proliferative characteristics of four murine macrophage-like cell lines. Cell Immunol. (1985) 90:339–57. doi: 10.1016/0008-8749(85)90199-6
184. Jiang W, Reich IC, and Pisetsky DS. Mechanisms of activation of the RAW264.7 macrophage cell line by transfected mammalian DNA. Cell Immunol. (2004) 229:31–40. doi: 10.1016/j.cellimm.2004.06.003
185. Xu F, Su C, Wu T, Chen H, Zhang P, Liu Y, et al. CRISPR/Cas9-based knockout of GPR43 gene in RAW264.7 cells inhibits their phagocytosis to Klebsiella pneumoniae. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. (2020) 36:481–6.
186. Paludan SR, Reinert LS, and Hornung V. DNA-stimulated cell death: implications for host defence, inflammatory diseases and cancer. Nat Rev Immunol. (2019) 19:141–53. doi: 10.1038/s41577-018-0117-0
187. Sato M, Shibata Y, Inoue S, Igarashi A, Tokairin Y, Yamauchi K, et al. MafB enhances efferocytosis in RAW264.7 macrophages by regulating Axl expression. Immunobiology. (2018) 223:94–100. doi: 10.1016/j.imbio.2017.10.007
188. Herb M, Farid A, Gluschko A, Krönke M, and Schramm M. Highly efficient transfection of primary macrophages with in vitro transcribed mRNA. J Vis Exp. (2019) 153):1–11. doi: 10.3791/60143
189. Schnoor M, Buers I, Sietmann A, Brodde MF, Hofnagel O, Robenek H, et al. Efficient non-viral transfection of THP-1 cells. J Immunol Methods. (2009) 344:109–15. doi: 10.1016/j.jim.2009.03.014
190. Maeß MB, Wittig B, and Lorkowski S. Highly efficient transfection of human THP-1 macrophages by nucleofection. J Vis Exp. (2014) 91):e51960. doi: 10.3791/51960
191. Zhang Y, Béland LC, Roussel S, Bertrand N, Hébert SS, Vallières L, et al. Optimization of a lipid nanoparticle-based protocol for RNA transfection into primary mononuclear phagocytes. J Leukoc Biol. (2024) 115:1165–76. doi: 10.1093/jleuko/qiae059
192. Chen Y, Chen X, Zhang Y, Wang M, Yang M, Wang R, et al. Macrophage-specific in vivo RNA editing promotes phagocytosis and antitumor immunity in mice. Sci Transl Med. (2025) 17:eadl5800. doi: 10.1126/scitranslmed.adl5800
193. Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, et al. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. (2014) 46:e70. doi: 10.1038/emm.2013.135
194. Kong M, Zhu D, Dong J, Kong L, and Luo J. Iso-seco-tanapartholide from Artemisia argyi inhibits the PFKFB3-mediated glycolytic pathway to attenuate airway inflammation in lipopolysaccharide-induced acute lung injury mice. J Ethnopharmacol. (2023) 301:115781. doi: 10.1016/j.jep.2022.115781
195. Shi R, Wang J, Zhang Z, Leng Y, and Chen AF. ASGR1 promotes liver injury in sepsis by modulating monocyte-to-macrophage differentiation via NF-κB/ATF5 pathway. Life Sci. (2023) 315:121339. doi: 10.1016/j.lfs.2022.121339
196. Cao J, Ji L, Zhan Y, Shao X, Xu P, Wu B, et al. MST4 kinase regulates immune thrombocytopenia by phosphorylating STAT1-mediated M1 polarization of macrophages. Cell Mol Immunol. (2023) 20:1413–27. doi: 10.1038/s41423-023-01089-8
197. Che K, Luo Y, Song X, Yang Z, Wang H, Shi T, et al. Macrophages reprogramming improves immunotherapy of IL-33 in peritoneal metastasis of gastric cancer. EMBO Mol Med. (2024) 16:251–66. doi: 10.1038/s44321-023-00012-y
198. Zhang W, Xu L, Zhang X, Xu J, and Jin JO. Escherichia coli adhesion portion FimH polarizes M2 macrophages to M1 macrophages in tumor microenvironment via toll-like receptor 4. Front Immunol. (2023) 14:1213467. doi: 10.3389/fimmu.2023.1213467
199. Liu X, Guo JW, Lin XC, Tuo YH, Peng WL, He SY, et al. Macrophage NFATc3 prevents foam cell formation and atherosclerosis: evidence and mechanisms. Eur Heart J. (2021) 42:4847–61. doi: 10.1093/eurheartj/ehab660
200. Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, Nonaka K, et al. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. (2008) 83:1136–44. doi: 10.1189/jlb.0907611
201. Geng P, Zhu H, Zhou W, Su C, Chen M, Huang C, et al. Baicalin inhibits influenza A virus infection via promotion of M1 macrophage polarization. Front Pharmacol. (2020) 11:01298. doi: 10.3389/fphar.2020.01298
202. Litman K, Bouch S, Litvack ML, and Post M. Therapeutic characteristics of alveolar-like macrophages in mouse models of hyperoxia and LPS-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol. (2024) 327:L269–l281. doi: 10.1152/ajplung.00270.2023
203. Yu J, Shang C, Deng X, Jia J, Shang X, Wang Z, et al. Time-resolved scRNA-seq reveals transcription dynamics of polarized macrophages with influenza A virus infection and antigen presentation to T cells. Emerg Microbes Infect. (2024) 13:2387450. doi: 10.1080/22221751.2024.2387450
204. Wang H, Yung MM, Xuan Y, Chen F, Chan W, Siu MK, et al. Polyunsaturated fatty acids promote M2-like TAM deposition via dampening RhoA-YAP1 signaling in the ovarian cancer microenvironment. Exp Hematol Oncol. (2024) 13:90. doi: 10.1186/s40164-024-00558-8
205. Azadpour M, Farajollahi MM, Dariushnejad H, Varzi AM, Varezardi A, Barati M, et al. Effects of synthetic silymarin-PLGA nanoparticles on M2 polarization and inflammatory cytokines in LPS-treated murine peritoneal macrophages. Iran J Basic Med Sci. (2021) 24:1446–54. doi: 10.22038/IJBMS.2021.59312.13161
206. Li SL, Wang ZM, Xu C, Che FH, Hu XF, Cao R, et al. Liraglutide attenuates hepatic ischemia-reperfusion injury by modulating macrophage polarization. Front Immunol. (2022) 13:869050. doi: 10.3389/fimmu.2022.869050
207. Wu H, Zhong Z, Wang A, Yuan C, Ning K, Hu H, et al. LncRNA FTX represses the progression of non-alcoholic fatty liver disease to hepatocellular carcinoma via regulating the M1/M2 polarization of Kupffer cells. Cancer Cell Int. (2020) 20:266. doi: 10.1186/s12935-020-01354-0
208. Deng X, Li Y, Jiang L, Xie X, and Wang X. 1-methylnicotinamide modulates IL-10 secretion and voriconazole metabolism. Front Immunol. (2025) 16:1529660. doi: 10.3389/fimmu.2025.1529660
209. Yang Y, Sheng J, Sheng Y, Wang J, Zhou X, Li W, et al. Lapachol treats non-alcoholic fatty liver disease by modulating the M1 polarization of Kupffer cells via PKM2. Int Immunopharmacol. (2023) 120:110380. doi: 10.1016/j.intimp.2023.110380
210. Li L, Hu H, Jiang W, Mao S, Yang Z, Lan T, et al. Artemisinin alleviates ischemic stroke injury and promotes neurogenesis through PPARγ-mediated M2 polarization of microglia. Phytomedicine. (2025) 142:156769. doi: 10.1016/j.phymed.2025.156769
211. Yang M, Bai M, Zhuang Y, Lu S, Ge Q, Li H, et al. High-dose dexamethasone regulates microglial polarization via the GR/JAK1/STAT3 signaling pathway after traumatic brain injury. Neural Regener Res. (2025) 20:2611–23. doi: 10.4103/NRR.NRR-D-23-01772
212. Li P, Zhao J, Ma Y, Wang L, Liang S, Fan F, et al. Transplantation of miR-145a-5p modified M2 type microglia promotes the tissue repair of spinal cord injury in mice. J Transl Med. (2024) 22:724. doi: 10.1186/s12967-024-05492-1
213. Woolf Z, Stevenson TJ, Lee K, Highet B, Macapagal Foliaki J, Ratiu R, et al. In vitro models of microglia: a comparative study. Sci Rep. (2025) 15:15621. doi: 10.1038/s41598-025-99867-z
214. Mercnik MH, Schliefsteiner C, Fluhr H, and Wadsack C. Placental macrophages present distinct polarization pattern and effector functions depending on clinical onset of preeclampsia. Front Immunol. (2022) 13:1095879. doi: 10.3389/fimmu.2022.1095879
215. Lasch M, Sudan K, Paul C, Schulz C, Kolben T, Dorp JV, et al. Isolation of decidual macrophages and hofbauer cells from term placenta-comparison of the expression of CD163 and CD80. Int J Mol Sci. (2022) 23:1–18. doi: 10.3390/ijms23116113
216. Wen Z, Xiong X, Chen D, Shao L, Tang X, Shen X, et al. Activating transcription factor 4 protects mice against sepsis-induced intestinal injury by regulating gut-resident macrophages differentiation. Chin Med J (Engl). (2022) 135:2585–95. doi: 10.1097/CM9.0000000000002543
217. Eslick S, Williams EJ, Berthon BS, Wright T, Karihaloo C, Gately M, et al. Weight loss and short-chain fatty acids reduce systemic inflammation in monocytes and adipose tissue macrophages from obese subjects. Nutrients. (2022) 14:1–17. doi: 10.3390/nu14040765
218. Porta C, Riboldi E, Ippolito A, and Sica A. Molecular and epigenetic basis of macrophage polarized activation. Semin Immunol. (2015) 27:237–48. doi: 10.1016/j.smim.2015.10.003
219. Louwerse M, Bila KO, van der Lienden MJC, de Beaufort AJM, Boot RG, Artola M, et al. Cultured macrophage models for the investigation of lysosomal glucocerebrosidase and gaucher disease. Int J Mol Sci. (2025) 26:1–17. doi: 10.3390/ijms26062726
220. Ding S, Guo X, Zhu L, Wang J, Li T, Yu Q, et al. Macrophage-derived netrin-1 contributes to endometriosis-associated pain. Ann Transl Med. (2021) 9:29. doi: 10.21037/atm-20-2161
221. Wegner J, Zillinger T, Schlee-Guimaraes TM, Bartok E, and Schlee M. An epigenetic GPI anchor defect impairs TLR4 signaling in the B cell transdifferentiation model for primary human monocytes BLaER1. Sci Rep. (2021) 11:14983. doi: 10.1038/s41598-021-94386-z
222. Farias JO, Pacheco DRDCG, Magalhaes YT, Russo LC, Boell VK, Hilares DJF, et al. Knockdown of dual-specificity phosphatase 3 drives differentiation and polarization of myeloid leukemia cells into macrophages with reduced proliferative and DNA repair fitness. Tissue Cell. (2025) 96:102947. doi: 10.1016/j.tice.2025.102947
223. Zhang WB, Chen ZX, Liu Z, Qian XY, Ge YZ, Zhang HY, et al. PBMC-mediated modulation of macrophage polarization in RAW264.7 cells through STAT1/STAT6 signaling cascades. Int Immunopharmacol. (2024) 138:112651. doi: 10.1016/j.intimp.2024.112651
224. Cao S, Lv B, Tai Y, Zuo HX, Xing Y, Surh YJ, et al. Formononetin ameliorates DSS-induced colitis by inhibiting the MAPK/PPAR-γ/NF-κB/ROS signaling pathways. Toxicol Appl Pharmacol. (2025) 496:117239. doi: 10.1016/j.taap.2025.117239
225. Yin J, Bao Y, Xu M, Li P, Zhang Z, Xue H, et al. Anti-inflammatory role of low-intensity pulsed ultrasound in inhibiting lipopolysaccharide-induced M1 polarization of RAW264.7 cells via Wnt2b/AXIN/β-catenin. PeerJ. (2024) 12:e18448. doi: 10.7717/peerj.18448
226. Tian L, Chen J, Yang M, Chen L, Qiu J, Jiang Y, et al. Xiezhuo Tiaozhi formula inhibits macrophage pyroptosis in the non-alcoholic fatty liver disease by targeting the SIRT1 pathway. Phytomedicine. (2024) 131:155776. doi: 10.1016/j.phymed.2024.155776
227. Sun L, Yang K, Wang L, Wu S, Wen D, Wang J, et al. LncRNA MIAT suppresses inflammation in LPS-induced J774A.1 macrophages by promoting autophagy through miR-30a-5p/SOCS1 axi. Sci Rep. (2024) 14:22608. doi: 10.1038/s41598-024-73607-1
228. Kim E, Choi DH, and Yi YS. Quercetin ameliorates acute lethal sepsis in mice by inhibiting caspase-11 noncanonical inflammasome in macrophages. Molecules. (2024) 29:1–15. doi: 10.3390/molecules29245900
229. Tong ZZ, Fang ZM, Zhang Q, Zhan Y, Zhang Y, Jiang WF, et al. Plasmodium yoelii infection inhibits murine leukaemia WEHI-3 cell proliferation in vivo by promoting immune responses. Infect Dis Poverty. (2018) 7:48. doi: 10.1186/s40249-018-0433-4
230. Yang HL, Thiyagarajan V, Liao JW, Chu YL, Chang CT, Huang PJ, et al. Toona sinensis inhibits murine leukemia WEHI-3 cells and promotes immune response in vivo. Integr Cancer Ther. (2017) 16:308–18. doi: 10.1177/1534735416642863
231. Digiacomo G, Tusa I, Bacci M, Cipolleschi MG, Dello Sbarba P, Rovida E, et al. Fibronectin induces macrophage migration through a SFK-FAK/CSF-1R pathway. Cell Adh Migr. (2017) 11:327–37. doi: 10.1080/19336918.2016.1221566
232. Morgan C, Pollard JW, and Stanley ER. Isolation and characterization of a cloned growth factor dependent macrophage cell line, BAC1.2F5. J Cell Physiol. (1987) 130:420–7. doi: 10.1002/jcp.1041300316
233. Duraisamy P, Angusamy A, Ravi S, Krishnan M, Martin LC, Manikandan B, et al. Phytol from Scoparia dulcis prevents NF-κB-mediated inflammatory responses during macrophage polarization. 3 Biotech. (2024) 14:80. doi: 10.1007/s13205-024-03924-9
234. Stevens MT, Nagaria BD, Britton WJ, and Saunders BM. Macrophages of different tissue origin exhibit distinct inflammatory responses to mycobacterial infection. Immunol Cell Biol. (2021) 99:1085–92. doi: 10.1111/imcb.12493
235. Xia L, Zhang C, Lv N, Liang Z, Ma T, Cheng H, et al. AdMSC-derived exosomes alleviate acute lung injury via transferring mitochondrial component to improve homeostasis of alveolar macrophages. Theranostics. (2022) 12:2928–47. doi: 10.7150/thno.69533
236. Yang F, Chen M, Liu Y, Hu Y, Chen Y, Yu Y, et al. ANGPTL2 knockdown induces autophagy to relieve alveolar macrophage pyroptosis by reducing LILRB2-mediated inhibition of TREM2. J Cell Mol Med. (2024) 28:e18280. doi: 10.1111/jcmm.18280
237. Wang Q, Hong L, Chen M, Shi J, Lin X, Huang L, et al. Targeting M2 Macrophages Alleviates Airway Inflammation and Remodeling in Asthmatic Mice via miR-378a-3p/GRB2 Pathway. Front Mol Biosci. (2021) 8:717969. doi: 10.3389/fmolb.2021.717969
238. Peng Y, Li Y, Yang Y, Shi T, Liu R, Luan Y, et al. The role and potential regulatory mechanism of STING modulated macrophage apoptosis and differentiation in severe acute pancreatitis-associated lung injury. J Interferon Cytokine Res. (2023) 43:455–68. doi: 10.1089/jir.2023.0077
239. Peng Y, Yang Y, Li Y, Shi T, Xu N, Liu R, et al. Mitochondrial (mt)DNA-cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) signaling promotes pyroptosis of macrophages via interferon regulatory factor (IRF)7/IRF3 activation to aggravate lung injury during severe acute pancreatitis. Cell Mol Biol Lett. (2024) 29:61. doi: 10.1186/s11658-024-00575-9
240. Kerecman J, Mustafa SB, Vasquez MM, Dixon PS, and Castro R. Immunosuppressive properties of surfactant in alveolar macrophage NR8383. Inflammation Res. (2008) 57:118–25. doi: 10.1007/s00011-007-7212-1
241. Toda M, Mizuguchi S, Minamiyama Y, Yamamoto-Oka H, Aota T, Kubo S, et al. Pirfenidone suppresses polarization to M2 phenotype macrophages and the fibrogenic activity of rat lung fibroblasts. J Clin Biochem Nutr. (2018) 63:58–65. doi: 10.3164/jcbn.17-111
242. Li YQ, Liang L, Gan ZS, Tang XY, and Du HH. Polarized activation affects iron metabolism in macrophages. Sheng Li Xue Bao. (2021) 73:244–52.
243. Xu Y, Ding X, Wang Y, Li D, Xie L, Liang S, et al. Bacterial metabolite reuterin attenuated LPS-induced oxidative stress and inflammation response in HD11 macrophages. Antioxidants (Basel). (2022) 11:1–18. doi: 10.3390/antiox11091662
244. Haddadi S, Kim DS, Jasmine H, van der Meer F, Czub M, Abdul-Careem MF, et al. Induction of Toll-like receptor 4 signaling in avian macrophages inhibits infectious laryngotracheitis virus replication in a nitric oxide dependent way. Vet Immunol Immunopathol. (2013) 155:270–5. doi: 10.1016/j.vetimm.2013.08.005
245. Criollo V, Gaghan C, John F, Orozco E, Thachil A, Crespo R, et al. Immune response evaluation in commercial Turkeys affected with clostridial dermatitis. Avian Dis. (2023) 67:80–8. doi: 10.1637/aviandiseases-D-22-00089
246. Ma C and Wang Y. BHLHE40 regulates microglia polarization after spinal cord injury via the NF-κB pathway. Brain Res Bull. (2025) 220:111139. doi: 10.1016/j.brainresbull.2024.111139
247. Ding X, Chen C, Zhao H, Dai B, Ye L, Song T, et al. Inhibiting SHP2 reduces glycolysis, promotes microglial M1 polarization, and alleviates secondary inflammation following spinal cord injury in a mouse model. Neural Regener Res. (2025) 20:858–72. doi: 10.4103/NRR.NRR-D-23-01925
248. Yu MC, Li XL, Ning ML, Yan ZZ, and Yu WT. USP22 inhibits microglial M1 polarization by regulating the PU.1/NLRP3 inflammasome pathway. Brain Res Bull. (2025) 220:111157. doi: 10.1016/j.brainresbull.2024.111157
Keywords: macrophages, primary macrophages, immortalized macrophage cell lines, cell models, cross-species
Citation: Ding T, Du Y, Yang B, Tian W, Li J and Xie J (2025) Comprehensive review of macrophage models: primary cells and immortalized lines across species. Front. Immunol. 16:1640935. doi: 10.3389/fimmu.2025.1640935
Received: 04 June 2025; Accepted: 31 July 2025;
Published: 20 August 2025.
Edited by:
Jiong Chen, Ningbo University, ChinaReviewed by:
Anto Sam Crosslee Louis Sam Titus, University of Houston, United StatesDebjeet Sur, Guru Nanak Institute of Pharmaceutical Science and Technology, India
Lei Tong, University of Chinese Academy of Sciences, China
Lexun Wang, Guangdong Pharmaceutical University, China
Copyright © 2025 Ding, Du, Yang, Tian, Li and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinghong Xie, amluZ2hvbmd4aWUyMDEyQDE2My5jb20=
 Tiansong Ding
Tiansong Ding Yuhan Du2
Yuhan Du2