- 1Department of Dermatology, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 2Department of Dermatology, The Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 3Guizhou Hospital of Guangdong Provincial Hospital of Traditional Chinese Medicine, Guiyang, Guizhou, China
Atopic dermatitis (AD) and alopecia areata (AA) have traditionally been regarded as inflammatory dermatoses with independent pathogenic mechanisms, with the former mostly categorized as a type 2 inflammatory disease and the latter as a type 1 inflammatory disease. However, immunologic studies have shown that the immunologic properties of AD and AA do not strictly follow the traditional classification. Both diseases are associated with systemic Th1, Th2, Th17, and Th22 cytokine imbalances, shared genetic susceptibility loci, overlapping immune pathways, and microbiome-mediated modulation of skin pathology. This review systematically investigates the intricate interactions between AD and AA, focusing on shared pathophysiologic mechanisms such as immune network crosstalk, metabolic dysregulation, and microbial influences. Furthermore, it critically evaluates current therapeutic strategies for overlapping disease manifestations, with a detailed analysis of emerging targeted therapies and their implications for clinical practice. By integrating existing evidence and identifying research gaps, this article aims to provide new perspectives on the understanding of the mechanisms of AD-AA interactions and to inform clinical decision-making and future research directions.
1 Introduction
Atopic dermatitis (AD) is a chronic, relapsing, multifactorial inflammatory skin disorder characterized by eczematous lesions such as erythema, papules, exudation, xerosis, and pruritus. The Global Burden of Disease Study shows that AD is the most burdensome of the dermatologic diseases and ranks among the top non-fatals (1). Alopecia areata (AA) is a chronic tissue-specific autoimmune disease characterized by non-scarring alopecia affecting approximately 2% of the population (2, 3). Although traditionally viewed as distinct entities, emerging evidence highlights a bidirectional epidemiologic link between AD and AA, underpinned by shared immunopathogenic pathways. The convergence of genetic susceptibility, environmental triggers, epidermal barrier dysfunction, microbiome dysbiosis, and immune dysregulation collectively drive the pathogenesis of both conditions (4–6). Notably, atopic predisposition—particularly a history of AD—is significantly overrepresented in AA cohorts and serves as a key risk factor for AA development (7). Conversely, AD patients exhibit a markedly elevated risk of AA onset (8). However, the existing literature suffers from an insufficient understanding of the comorbidity mechanism and a lack of consistency in treatment protocols, etc. This review explains this association from the perspectives of the interactions between AD and AA, clinical characteristics, and treatment strategies, with the aim of providing references for an in-depth understanding of the mechanisms of the comorbidity between the two and for the development of effective interventions.
2 Epidemiology
In a retrospective study of 51,561 patients with AA, Kridin et al. identified a robust bidirectional association between AA and AD (9), showing that AD and AA are most frequently comorbid compared to other atopic diseases, and that AA patients with comorbid AD presented an earlier disease onset and a higher prevalence of female patients. In addition, a key observation was that the risk of AA in atopic disease patients correlated with the type of comorbid atopy. However, it is worth noting that the database did not include information on the severity of AA versus AD, so it was not possible to delve into the specific association between disease severity and increased risk. A Korean retrospective study of 871 patients with early-onset (prepubertal) AA further supports these findings (10), highlighting AD as the most prevalent comorbidity in this population. Similarly, Conic et al. observed a high co-prevalence of AD of 17.4% in 3,510 AA patients under 18 years of age by analyzing data from 26 major healthcare networks in the United States (covering more than 360 hospitals) (11), suggesting that this bi-directional correlation is also present in pre-pubertal patients. Notably, a Taiwanese cohort study involving 12,022 AA patients and 40,307 AD patients (8), not only reiterated the bidirectional increased risk between AD and AA, but also demonstrated that AD patients carrying Filaggrin gene (FLG) mutations exhibited exacerbated AA manifestations compared to those without genetic predisposition, further strengthening the further reinforcing the complex and multidimensional association between AD and AA.
3 Complex immune networks
AD is mainly mediated by Th2-driven immune responses, and key cytokines such as interleukin (IL)-4 and IL-13 play a central role in skin barrier disruption, promoting immunoglobulin (Ig)E production, and modulating the inflammatory process (12). Based on the concentration of IgE and the status of the skin barrier, AD is further classified into two distinct subtypes: exogenous and endogenous. Specifically, exogenous AD is characterized by high serum total IgE levels, significantly increased expression of Th2-type cytokines, and impairment of skin barrier function. In contrast, endogenous AD exhibits normal serum total IgE concentrations, low expression levels of Th2-type cytokines, and relatively intact skin barrier function (13). Further research indicates that in the presence of AA, AA is more inclined to exhibit a skewed Th1-type immune response when combined with endogenous AD. In contrast, it is more likely to present a skewed Th2-type immune response when combined with exogenous AD (14). This immune dynamic manifests temporally in AD progression: Th2 predominance characterizes the acute phase, while Th1 dominance emerges during chronic stages (15, 16). Critically, cross-regulation between Th1-derived cytokines (e.g., IFN-γ) and Th2-associated IL-4/IL-13 orchestrates the evolving immune microenvironment (17). This mechanism of immune switching from Th2 to Th1 may contribute to the chronic progression of AD, exacerbating the complexity and intractability of the disease. AA pathogenesis is predominantly mediated by Th1 cell-derived interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α), which elicit immune responses to environmental stressors (e.g., psychological stress, viral infection, trauma), resulting in follicular immune dyshomeostasis and compromised hair growth (18). Interestingly, the pattern of immune response in AA patients is equally complex and diverse, also involving Th2 cytokines (IL-4, IL-5, IL-10), IgE, and eosinophils (3, 19). Collectively, these findings challenge the traditional Th1/Th2 dichotomy, revealing a continuum of immune activation in AD and AA. The concurrent activation of Th1, Th2, and Th17/Th22 axes—interconnected through comorbid crosstalk—drives disease pathogenesis and progression (20, 21). These observations indicate that immune responses in AD and AA form a dynamic continuum rather than distinct binary classifications, with Th1, Th2, and Th17/Th22 pathways interwoven under comorbid states. This interplay jointly influences disease pathogenesis and progression, while the simultaneous activation of multiple T-helper (Th) cell subsets underscores the necessity to understand immune equilibrium in diverse dermatoses for therapeutic optimization. Immunopathogenesis of AD, AA, and their overlap (Figure 1).
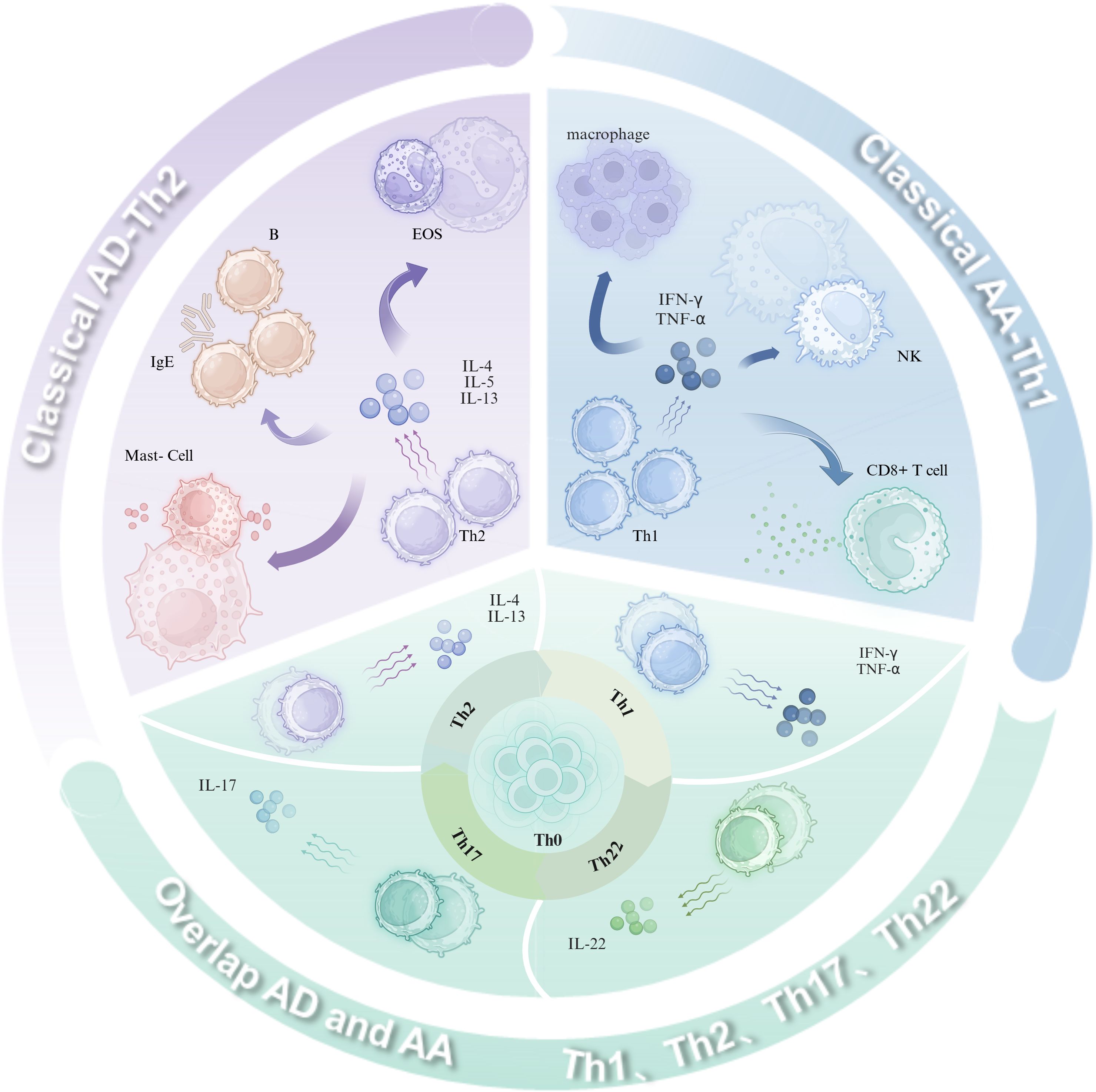
Figure 1. Immunopathogenesis of AD, AA, and their overlap. Classical AD is characterized by a Th2-predominant immune-inflammatory response, where cytokines such as IL-4, IL-13, and IL-31 stimulate B-cell production of IgE, promote mast cell degranulation, and recruit Eos. In contrast, classical AA is driven by a Th1-dominant immune-inflammatory response, with cytokines like TNF-α and IFN-γ activating macrophages, CD8+ T cells, and inducing NK cell activation. In overlapping AD and AA, Th1, Th2, Th17, and Th22 cells collectively contribute to disease progression through the secretion of IL-4, IL-13, IL-17, IL-22, TNF-α, and IFN-γ. (Figure created with BioRender.com). AD, Atopic dermatitis; AA, Alopecia areata; Th, T helper cells; IL, Interleukin; IFN, Interferon; TNF, Tumor necrosis factor; Eos, Eosinophils; NK, Natural killer cells; CD8+ T cells, CD8-positive cytotoxic T cells.
3.1 Th1-type immunity
The core physiological function of IFN-γ, as a central cytokine of the Th1-type immune response, focuses on recognizing and defending against intracellularly parasitized viruses and malignant cells (22, 23) and has been established as a key mediator in the pathogenesis of AA. In the serum and lesion sites of AA patients, the upregulation of IFN-γ and TNF expression was positively correlated with disease severity and duration, especially in total/purple baldness and active AA (21, 24–26), which effectively promotes immune cell recruitment and exacerbates immune responses mediated by Th1 and natural killer (NK) cells by inducing the enhancement of chemokine expression (27), a cascade of biological events that severely disrupts the natural cycle of hair growth and the normal function of the hair follicle. Emerging evidence highlights IFN-γ’s critical involvement in AD. Stratification of AD patients according to IFNG expression levels and IFN-γ-secreting T cell capacity reveals two distinct subgroups: IFN-γ-high AD (exhibiting predominant endogenous AD characteristics) and IFN-γ-low AD (manifesting classical exogenous AD features) (28), which is consistent with previous studies (29, 30). Pathway analysis revealed that significant enrichment of gene sets associated with innate immunity, lymphocyte activation, inflammatory signaling, and immune system processes in the IFN-γ-high AD subgroup. IFN-γ and TNF-α bind to keratinocytes, induce apoptosis, and create a pro-inflammatory environment, leading to inflammatory skin lesions in AD. Meanwhile, IFN-γ also disrupts the skin barrier homeostasis by down-regulating the expression of FLG and mitogenin-1 and ceramide synthesis (31, 32). This barrier dysfunction facilitates inflammatory mediator release, creating a self-perpetuating cycle of immune activation and tissue damage.
3.2 Th2-type immunity
In both AD and AA, a Th2-skewed immune response is observed (33), characterized by elevated levels of type 2 inflammatory cytokines, predominantly IL-4 and IL-13. IL-4/IL-13 can combine with type 1 cytokines (e.g., IFN-γ) to upregulate eosinophil-activating chemokines, including eotaxin-1 (CCL11) and eotaxin-3 (CCL26). These chemokines demonstrate significantly elevated expression levels in lesional AD skin (34), driving the recruitment of T lymphocytes, eosinophils, and basophils to the skin lesions. Furthermore, IL-4/IL-13 signaling downregulates key epidermal barrier molecules including FLG and involucrin, compromising tight junction integrity in the skin, whereas FLG gene mutations constitute the primary genetic determinant of epidermal barrier dysfunction (35), and also reduces ceramide synthesis and impairs corneal cohesion (20, 36, 37). The Th2 cytokine interleukin-4 disrupts normal stratum corneum cohesion in mice, providing implications for AD pathogenesis. Th2 cells are also shown to have a skewed cytokine homeostasis in patients with AA state. Specifically, IgE levels are significantly elevated in AA patients and this elevation is not dependent on the atopic state (38). At the same time, Th2-associated cytokines (IL-4, IL-13) also showed increased levels (39), and these cytokines may further promote T-cell infiltration into the skin and hair follicles, thereby triggering inflammatory responses and follicular damage (33, 40), and in the group of patients with AA combined with AD, the infiltration of Th2 cells around the diseased follicles was more prevalent. prevalent (14), and there was a significant correlation with IL-13 levels (41). In addition, it was further noted that enhanced activation of both skin-homing and circulating Th2 cells in AA patients versus healthy controls, with activation intensity directly correlating with clinical severity scores. Conversely, IFN-γ expression demonstrates stronger association with chronic disease progression (42).This observation aligns with the established immune trajectory of AD: The acute phase is predominantly characterized by a Th2 response, where IL-4/IL-13 signaling via the STAT6 (Signal Transducer and Activator of Transcription 6) pathway suppresses IFN-γ signaling. In contrast, the chronic phase exhibits significantly upregulated IFN-γ (43). This elevated IFN-γ can stimulate keratinocytes to produce CXCL10, which subsequently recruits cytotoxic CD8+ T cells to infiltrate hair follicles, ultimately triggering the autoimmune attack characteristic of AA. Thus, the Th2-to-Th1 immune shift occurring during the AD chronicity phase serves as a pivotal link connecting prolonged AD to AA pathogenesis.
3.3 Th17/Th22-type immunity
Imbalances in the immune axis play a central role in the development of AD. Specifically, the increase in Th1/IFN-γ-associated products may not only signal a pro-inflammatory activation state of Th17/Th22 cells, but may also serve as a mechanism of counter-regulation of Th2 and Th17 activation (44, 45). Gittler et al. emphasized the strong association between the enhancement of this immune axis and the increase of immune activation in the progression of AD to the chronic phase, and this immune imbalance is already evident in the acute lesion stage (16), especially with a significant increase in Th22, Th2 and Th1-related products. Critically, IL-17 and IL-22, as key pro-inflammatory cytokines for Th17 and Th22 cells, exert pivotal roles in AD pathogenesis through distinct mechanisms. IL-22 upregulates gastrin-releasing peptide receptor expression in keratinocytes of the skin (46), which is key in mediating both non-histaminergic and pathologic itch (47, 48), thus affecting the itch symptoms in AD itch symptoms in patients. Simultaneously, Th17 cell activation in AD facilitates the generation of pro-inflammatory cytokines, which consequently might intensify inflammation and lead to alopecia (49, 50). In AA, perifollicular dermal infiltration by Th17 cells coincides with significantly elevated serum IL-17 levels compared to healthy controls, which is strongly correlated with the severity of AA (51). In addition, higher serum IL-17A levels in young AA patients may be associated with their increased susceptibility to psychological stress (52), and this susceptibility may stem from chronic stress-induced conversion of lymphocytes to Th17 responses (53). Therefore, adolescent-onset AA patients may portend a poor prognosis due to high IL-17A levels compared to those with advanced age onse (54). On the other hand, Atwa et al. observed that AA patients displayed elevated serum IL-22 levels compared to healthy controls, with a positive correlation with AA and depression duration (51, 55). These findings further support the critical role of immune imbalance in the chronic pathology of AD and AA.
3.4 JAK-STAT signaling pathway
The JAK (Janus Kinase) -STAT signaling pathway, as a core intracellular transduction mechanism for cytokines, critically regulating organism development, maintaining homeostasis, promoting cell proliferation and mediating immune responses. In the pathological process of AD, this pathway significantly affects keratinocyte function through JAK/STAT-dependent signaling of IL-4 and IL-13 (56). This dysregulation is demonstrated by suppressed FLG and endocannabinoid production, subsequently compromising skin barrier integrity (57, 58). Notably, STAT6 protein plays a central regulatory role in this signaling pathway, which not only upregulates the expression of the Th2-specific transcription factor GATA3 and regulates T cell proliferation and Th2 cell differentiation, but also promotes the conversion of immunoglobulin classes to IgE and IgG1 in B cells (59), which correlates with the fact that multiple STAT6 polymorphisms are associated with high levels of IgE and an increased AD susceptibility is closely associated with increased susceptibility (60). In addition, the STAT3 component of the JAK-STAT signaling pathway plays an amplifier role in the development of chronic itch (61), and its activation is directly associated with sustained itch signaling. Genome-wide association studies (GWAS) in AA patients have identified JAK-STAT pathway components—including STAT5A/B, STAT3, JAK1, and JAK3—as critical regulators of follicular cycling (62, 63). The up-regulated expression of these genes during the regression and resting phases, as well as their down-regulation during the early anagen phase, implies that JAK-STAT signaling may inhibit hair re-entry into the anagen phase (64). In contrast, JAK inhibitors have indeed demonstrated a significant ability to promote hair regrowth in clinical studies (65), as evidenced by elevated post-treatment hair keratin content, a reduction in perifollicular T-lymphocyte infiltration, and a significant down-regulation of inflammatory markers in the gene expression profile (66, 67).
3.5 OX40-OX40L signaling pathway
OX40 and its ligand OX40L are both key members of the TNF superfamily. OX40 is primarily expressed on enhanced effector T cells (including Th1, Th2, Th17, and Th22 subpopulations) and regulatory T cells (Treg), while OX40L is primarily expressed on activated antigen-presenting cells (68). Ilves et al. found that OX40+ T cells were increased by 10-fold in the skin of AD patients compared to non-lesional skin (69). In the pathophysiology of AD, the OX40-OX40L signaling pathway plays a crucial role, promoting Th2 cell differentiation, These activated Th2 cells not only express OX40 but also release cytokines, further exacerbating damage to the epidermal barrier function. Preclinical studies in skin inflammation and asthma models further support that the OX40-OX40L signaling interaction is critical for the efficiency of regulatory responses in memory Th2 cells (70, 71). Additionally, this signaling pathway promotes the recruitment and proliferation of Th1, Th17, and Th22 cell subsets, which mediate keratinocyte proliferation, epidermal thickening, and T cell recruitment by upregulating the production of cytokines such as IFN-γ, IL-17, and IL-22 (72, 73). A study on mechanistic biomarkers demonstrated that blocking OX40 signaling not only regulates Th2 characteristics but also simultaneously suppresses Th1 and Th17/Th22-related immune activation (74). However, OX40 and OX40L are not significantly correlated with the clinical severity of AD. Additionally, Xiao et al. found that OX40 signaling significantly inhibits IL-17A production and Th17 differentiation in an experimental autoimmune encephalomyelitis model (75). The role of the OX40L/OX40 pathway in influencing Th cell fate remains to be further elucidated. Nevertheless, the activation of the OX40L/OX40 pathway, which promotes abnormal infiltration of effector T cells and cytokine release, may still be a key trigger for the disruption of follicular immune privilege. It is well known that mast cells (MCs) play a key role in various allergic diseases, including AD. Overactivation of the OX40L/OX40 pathway promotes Th2 cell proliferation, and the IL-4 secreted by these cells can induce B cells to differentiate into IgE-secreting cells. IgE binds to the high-affinity IgE receptor (FcϵRI) on MCs, leading to the release of histamine and IL-6, among other chemokines, which further recruit Th2 cells and eosinophils, exacerbating the immune inflammatory response (76). Additionally, mast cells upregulate OX40L expression due to IgE-FcϵRI cross-linking (77). Recent studies have further demonstrated that MCs are also involved in the pathogenesis of AA (78, 79). Specifically, compared with healthy control skin, the density of MCs in the dermis around lesions and hair follicles in AA patients is significantly increased. By releasing inflammatory mediators such as TNF-α and IL-6, they exacerbate autoimmune reactions, a phenomenon also observed in HF mesenchyme. This may indicate that Th2 cells act as an important bridge between AD and AA through the OX40L/OX40 pathway.
Meanwhile, Tregs also occupy a central position in the pathogenesis of AA (80), while OX40L on the MC surface can effectively weaken the immunosuppressive function of Tregs by interacting with OX40 on Tregs. This interaction leads to CD8+ T-cell hyperactivation, characterized by excessive IFN-γ and TNF-α production, alongside inflammatory cell recruitment (81, 82), while promoting the production of IgE by B cells as well as the release of Th2 cytokines (77), an interaction that disrupts the homeostasis between Tregs and CD8+ T cells and exacerbates the breakdown of immune privilege in AA hair follicles. Recent experimental studies have provided new evidence. Kim et al. found in a large-scale study of moderate-to-severe AA patients that the OX40L/OX40 axis was significantly upregulated in both the follicular surroundings and the circulatory system of patients, and this phenomenon was unrelated to atopic background. Additionally, the study observed an increase in OX40L+ antigen-presenting cells (APC) in the circulation, and non-skin-homing Tregs exhibited high OX40 expression, suggesting that at least part of Treg dysfunction may originate in the peripheral blood circulation prior to their migration to the skin (83). Therefore, the OX40/OX40L axis plays a key role in regulating inflammatory responses by modulating the interaction between Tregs and MCs. Abnormal activation of the OX40/OX40L pathway is not only a core driver of chronic inflammation in AD but also a critical link in the susceptibility of AD patients to AA. In AD, the sustained activation of this pathway sensitizes effector T cells and impairs Treg function, leading to an immune imbalance that directly disrupts the microenvironment necessary for maintaining follicular health. Thus, the activation of the OX40/OX40L pathway in AD is an important initiating factor in triggering the autoimmune process of AA. The OX40/OX40L axis, as a potential novel therapeutic target, may offer broad therapeutic benefits for patients with AA and AD comorbidity (Figure 2).
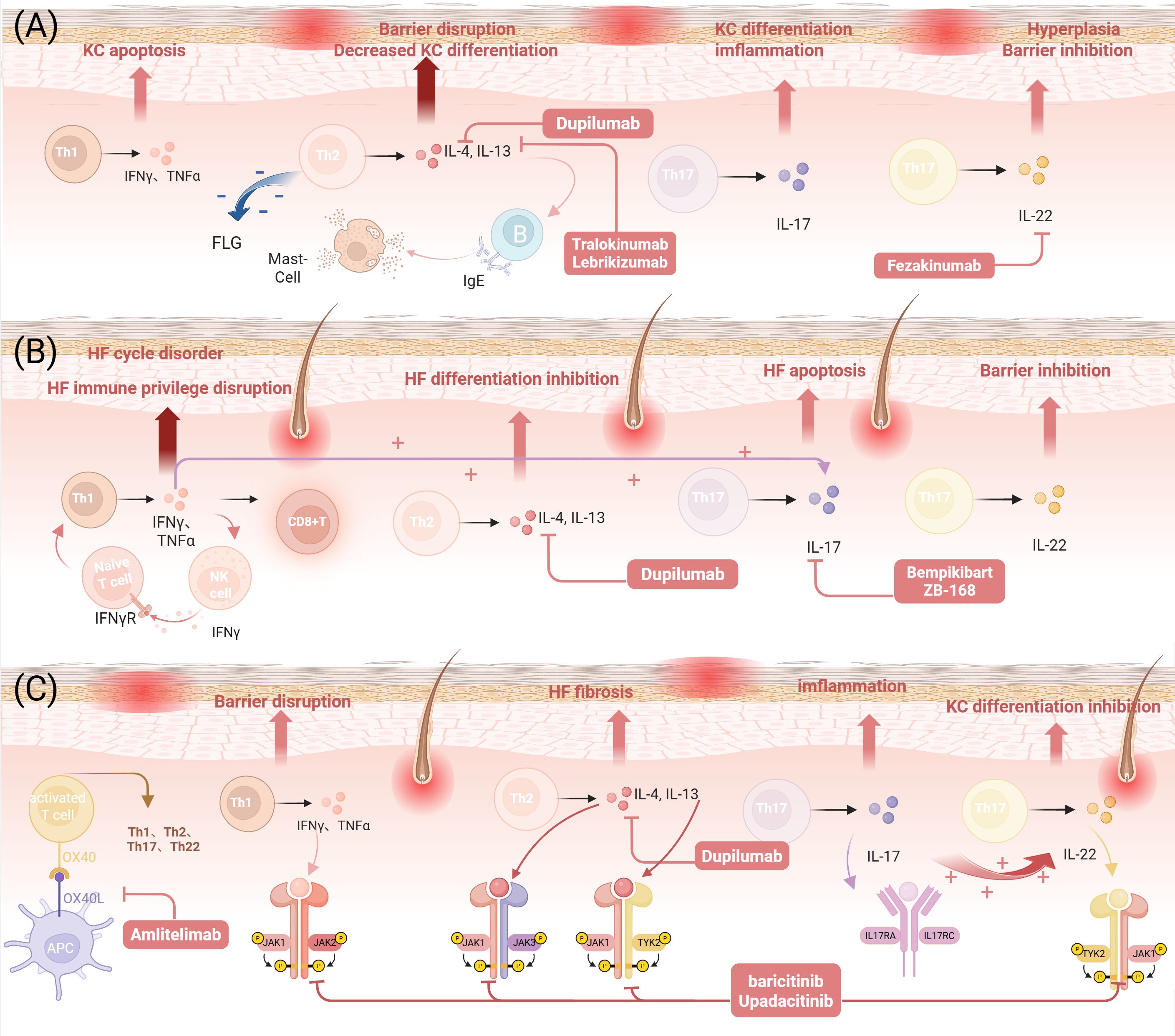
Figure 2. AD, AA, and overlapping immune responses and therapeutic targets. (A) The role of Th1/Th2/Th17/Th22 immunity in AD and therapeutic targets. (B) The role of Th1/Th2/Th17/Th22 immune responses in AA and therapeutic targets. (C) The role of Th1/Th2/Th17/Th22 immune responses, the JAK-STAT signaling pathway, and the OX40-OX40L signaling pathway in AD and AA and therapeutic targets. (Figure created with BioRender.com). AD, Atopic dermatitis; AA, Alopecia areata; Th, T helper cells; IL, Interleukin; IFN, Interferon; TNF, Tumor necrosis factor; KC, Keratinocyte;CD8+ T cells, CD8-positive cytotoxic T cells; NK, Natural killer cells; HF, Hair Follicle; APC, Antigen-Presenting Cell; TYK2, Tyrosine Kinase 2; FLG, Filaggrin gene; IgE, Immunoglobulin E; OX40, Tumor Necrosis Factor Receptor Superfamily Member 4; OX40L, Tumor Necrosis Factor Superfamily Member 4 Ligand; JAK, Janus kinase; STAT, Signal Transducer and Activator of Transcription.
4 Genetic factors
The contribution of genetic factors to elucidating the pathogenesis of AD and AA has received much attention in recent years. This surge in investigation has been most pronounced in the field of AD research, with genetics being a key word in up to 11% of AD studies over the past decade (84). Similarly, positive family history, a direct reflection of genetic influence, significantly elevates the risk of developing AA and AD. Specifically, up to 48% of people with AA cases exhibit disease manifestation in a relative (85), and children with a history of atopic disease in both parents are five times more likely to develop early-onset AD and persistent phenotypes (86). GWAS studies have revealed significant associations between susceptibility loci in AA and AD, particularly in genomic regions encoding epidermal structural proteins (e.g., FLG) and immune-related genes involved in innate and adaptive immunity (87–89). Epidermal barrier dysfunction caused by FLG mutations is considered a major genetic factor for AD, not only increasing the risk of AD, but also strongly associated with early-onset disease and severe clinical phenotypes (90). Notably, FLG mutations also elevated the risk of developing AA and exacerbate disease severity in patients with a history of AD (91). The underlying mechanism may involve FLG mutation-induced stratum corneum dysfunction, which compromises the skin barrier in AD. This barrier defect can subsequently disrupt the hair follicle’s immune privilege microenvironment, facilitating the penetration of environmental antigens. The ensuing activation of local immune responses may ultimately trigger AA. Concurrently, chronic inflammation in AA can spread to the skin, inhibiting keratinocyte proliferation and downregulating FLG expression, thereby further aggravating AD-associated barrier dysfunction and establishing a vicious cycle. These findings underscore the pivotal role of FLG mutations as a shared genetic underpinning in mediating the complex comorbid relationship between AD and AA. In addition, another GWAS showed multiple IL-4 promoter polymorphisms in AD patients, suggesting that abnormal IL-4 production is associated with AD susceptibility (92, 93). Similarly, intronic tandem repeat polymorphisms in IL-4 (specifically within intron 3) were identified as AA risk alleles in a Turkish cohort (94). On the other hand, IL-13 gene polymorphisms are linked to both the allergic phenotype of AD and AA susceptibility, with this Th2 cytokine serving as a shared genetic risk factor (93, 95). Meanwhile, IL-13 and KIAA0350/CLEC16A loci—previously associated with autoimmune diseases—mediate genetic overlap between AA and atopy (e.g., allergic rhinitis, asthma) (96), thereby reinforcing the shared etiological framework linking AA, AD, and atopic disorders.
5 Microbiome
Emerging evidence from expanding human microbiome studies has established the central involvement of both gut and cutaneous microbiota in the pathogenesis of AD and AA (97, 98). The strong association between gut flora imbalance and AA is partly attributed to the fact that they share a genetic basis capable of inducing a Th1 response and promoting the production of IFN-γ. As a principal immunomodulatory mediator, IFN-γ exerts its biological effects primarily through JAK-STAT pathway activation (99), driving dysregulated follicular keratinocyte proliferation that culminates in hair cycle disruption. Disruption of the gut microbiota not only affects local intestinal homeostasis but may also compromise the integrity of the intestinal epithelial barrier, leading to “leaky gut syndrome”(LGS). This immune homeostasis disruption triggered by microbiota imbalance is considered one of the potential mechanisms exacerbating AA: Rafik et al.’s case-control study showed a positive correlation between intestinal permeability biomarkers and AA severity (100); However, Hacınecipoğlu et al. reached the opposite conclusion, finding no significant association between LGS and AA (101), so the direct causal relationship between increased intestinal permeability and AA still needs further verification. After intestinal barrier disruption, bacterial metabolites can spread through the bloodstream, thereby regulating systemic immune responses and affecting the function of distant organs, including the skin, which forms the core of the “gut-skin axis” theory (102). Specifically, LGS leads to the leakage of intestinal bacteria and their products, which interact with skin receptors to induce Th2-type immune responses, exacerbating skin inflammation in AD (49), and may even trigger systemic pathological processes, including autoimmune diseases (103).
Although the skin microbiota dysbiosis characteristics of AD and AA differ, AD is primarily characterized by reduced skin microbiota diversity and increased Staphylococcus aureus abundance (104), while AA is primarily characterized by increased Propionibacterium acnes abundance, with no significant changes in the relative abundance of Staphylococcus aureus (105). However, skin microbiome imbalance may weaken the immune privilege state of hair follicles, and the skin barrier dysfunction caused by AD may promote bacterial antigen invasion into hair follicle structures, jointly exacerbating the pathological progression of AA (106). When gut microbiota ferment undigested substrates such as dietary fiber, they produce a class of metabolites known as short-chain fatty acids (SCFA), whose main components are butyrate, propionate, and acetate. These substances exert anti-inflammatory effects through various mechanisms, including maintaining the integrity of the mucus layer and epithelial cells (107). Studies have shown that SCFAs can regulate immune cell activity by activating G protein-coupled receptors, not only inhibiting the release of pro-inflammatory cytokines but also promoting the differentiation and function of Treg cells (108). Treg cells induce and maintain the body’s tolerance to self-antigens by secreting TGF-β and IL-10, playing a key role in preventing autoimmune reactions and maintaining immune homeostasis (109).
Conversely, gut microbiota dysbiosis leads to a significant reduction in SCFA production, which may disrupt the balance between pro-inflammatory CD4+IL-17+ T cells and anti-inflammatory CD4+ FOXP3+ Treg cells in the gut, promoting abnormal differentiation of Th17 cells and the release of pro-inflammatory factors such as IL-17 and IL-22, thereby inducing tissue inflammation (110), potentially contributing to the onset of AD and AA. Han et al. also confirmed that AA patients exhibit significantly elevated Th17 cell counts and reduced Treg cell counts (111). Therefore, the reduction in SCFAs caused by gut microbiota dysbiosis may be a potential underlying cause of intestinal barrier damage, increased permeability, and exacerbated inflammation in AD patients. While no microbiome-targeted therapies have achieved clinical translation to date, emerging microbiota-modulating strategies show therapeutic promise for inflammatory autoimmune disorders. Importantly, the pathophysiological interplay between AD and AA remains underexplored, necessitating systematic multi-omics investigations to delineate shared microbial etiologies and identify novel therapeutic targets (Figure 3).
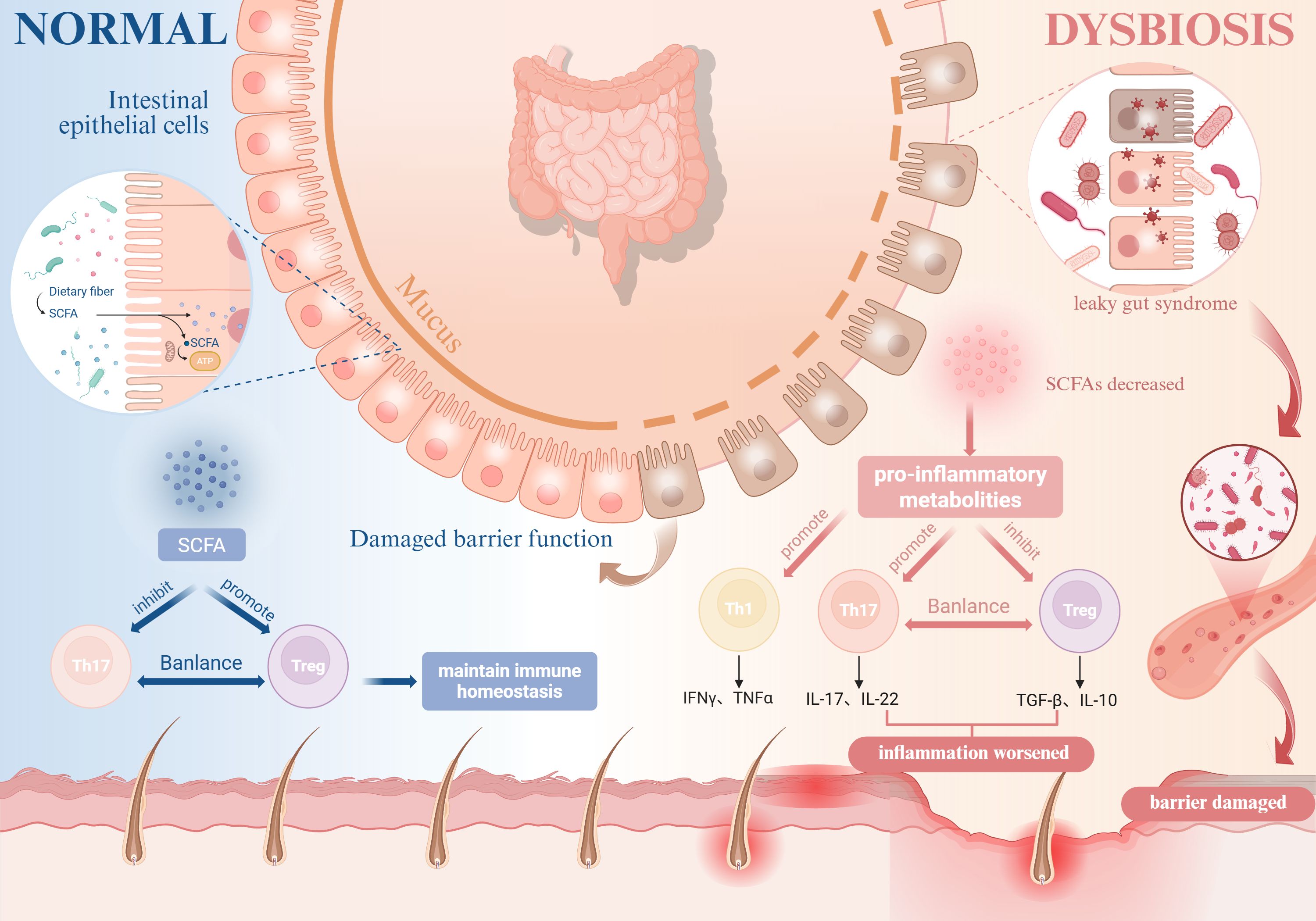
Figure 3. Impact of gut microbiota dysbiosis on AD and AA. Gut microbiota dysbiosis impairs intestinal epithelial barrier integrity, leading to “leaky gut syndrome” and reduced SCFA production. This disruption shifts the balance between pro-inflammatory CD4+IL-17+ T cells and anti-inflammatory regulatory Tregs, suppresses Tregs functionality and their secretion of anti-inflammatory cytokines (e.g., IL-10), and stimulates the release of pro-inflammatory cytokines such as TNF-α and IFN-γ, thereby exacerbating the pathogenesis of both AD and AA. (Figure created with BioRender.com). AD, Atopic dermatitis; AA, Alopecia areata; SCFA, Short-chain fatty acids; Tregs, Regulatory T cells; IL, Interleukin; TNF-α, Tumor necrosis factor-alpha; IFN-γ, Interferon-gamma.
6 Targeted therapy
6.1 IL-4/IL-13 inhibitors
Tralokinumab, the first-in-class monoclonal antibody targeting IL-13, was developed for AD. The drug modulates the expression levels of key AD biomarkers in the skin, restoring them to a near-nondiseased state, and effectively reduces the levels of systemic markers of Type 2 inflammation. In addition, a published case study showed that tralokinumab demonstrated significant efficacy in patients with severe AD accompanied by moderate to mild AA baseline severity AA scores (SALT of 22) (112), thus revealing its potential application in the treatment of comorbidities. Dupilumab, a monoclonal antibody targeting IL-4/IL-13 receptor signaling, has demonstrated significant efficacy in AD and AA, particularly in patients with an atopic backgrounds and elevated IgE levels (113–116). However, its therapeutic response in AA patients presents complexity. On the one hand, dupilumab is effective in improving symptoms in some AA patients; on the other hand, it has been reported that the drug may lead to worsening or new onset of AA (117). This two-sided response may be related to the immune skewed state of patients. In patients with Th2-skewed AA, dupilumab may bring positive efficacy through its inhibitory effect. Although dupilumab may ameliorate symptoms in AA patients with a Th2-skewed immune profile, it can paradoxically exacerbate hair loss in those with Th1-dominant AA, particularly among patients with low IgE levels (118–120). This differential efficacy stems from dupilumab’s dual immunomodulatory effects mediated through IL-4Rα blockade. In comorbid AD/AA characterized by Th2 skewing, dupilumab inhibits Th2 responses, effectively counteracting Th2-driven IgE elevation and barrier damage in AD, while also suppressing Th2 cell-mediated assistance to pathogenic CD8+ T cells in AA. Conversely, in Th1-skewed AA lacking significant Th2 activity (e.g., low IgE), dupilumab’s suppression of the Th2 pathway lifts the brake on the key negative feedback control normally exerted over the Th1/IFN-γ axis. This results in excessive amplification of IFN-γ signaling, which in turn promotes CD8+ T cell-mediated autoimmune attack on hair follicles, ultimately manifesting as worsened alopecia. In addition, gender differences also affect the efficacy of dupilumab in patients with AA. Studies have shown that female patients are more inclined to Th2 skewed disease, whereas males are more often associated with Th1 skewed disease. Thus, during treatment, female patients (Th2 skewed) are more likely to benefit from dupliyumab therapy, whereas male patients (Th1 skewed) may be at higher risk of deterioration (118). Stratified treatment strategy based on Th1/Th2 skewed status and IgE levels: preferred dopplerizumab for Th2 skewed (high IgE) patients; combined with JAK inhibitors or OX40L antagonists for Th1 skewed (low IgE) patients. Notably, although the prognosis of patients with dupilumab-induced AA worsening is usually favorable, and some patients even remit spontaneously, early recognition and intervention of AA worsening symptoms are crucial for timely adjustment of treatment regimens. Therefore, in clinical practice, physicians should fully consider patients’ immune skewed status and gender differences in order to develop individualized treatment plans and closely monitor changes in disease.
6.2 JAK inhibitors
The JAK-STAT pathway is a key signaling pathway in the development of AD and AA, and JAK inhibitors have shown promising therapeutic efficacy by blocking the signaling of key cytokines in this pathway and suppressing inflammatory responses (121, 122). Compared with first-generation JAK inhibitors (e.g., ruxolitinib and baricitinib), second-generation JAK inhibitors (e.g., upadacitinib and abrocitinib) have demonstrated a significant increase in therapeutic efficacy and have been noted for their higher selectivity and excellent safety profile. In particular, upadacitinib has shown significant efficacy and favorable tolerability in managing comorbid AA and AD comorbidities (123, 124), and its therapeutic potential is particularly promising in AD patients who have failed to respond to or experienced side effects from dupilumab (125). In clinical practice, upadacitinib has not only brought new therapeutic hope to adolescent AA patients with mild AD, but also enabled certain patients with moderate-to-severe AD comorbid with AA, who demonstrated response to baricitinib but discontinued the drug due to side effects, to achieve skin lesion regression and scalp hair regrowth. Real-world evidence documents upadacitinib’s dual efficacy: adolescent AA-AD patients (12–17 years) achieved 80% hair regrowth by 6 months (Child-SALT ≤20), while baricitinib-intolerant adults showed 2.3-fold greater body surface area(BSA) improvement versus baseline (126, 127). Similarly, upadacitinib has shown positive therapeutic effects in adolescent cases of severe refractory AD combined with AA that failed to respond to treatment with dupilumab, as well as in AD patients with refractory AA (128, 129). Despite reports that AA may be induced after upadacitinib treatment for AD (130), the exact mechanism of this complication is not yet clear, and although the efficacy of JAK inhibitors as novel oral small molecule drugs, including baricitinib, upatinib, and abrutinib, is significant, the non-specificity of the mechanism of action raises additional safety consideration (131, 132). In summary, Overall, these findings imply that, as with AD, distinct clinical phenotypes and linked endotypes may characterize patients with AA. Emerging evidence demonstrates the therapeutic promise of second-generation JAK inhibitors (e.g., deuruxolitinib, brepocitinib) in AA-AD comorbidities, yet critical gaps persist in delineating their tissue-specific JAK-STAT pathway modulation and optimal dosing schedules, requiring validation through multicenter Phase IIIb trials coupled with longitudinal biomarker profiling (Figure 4).
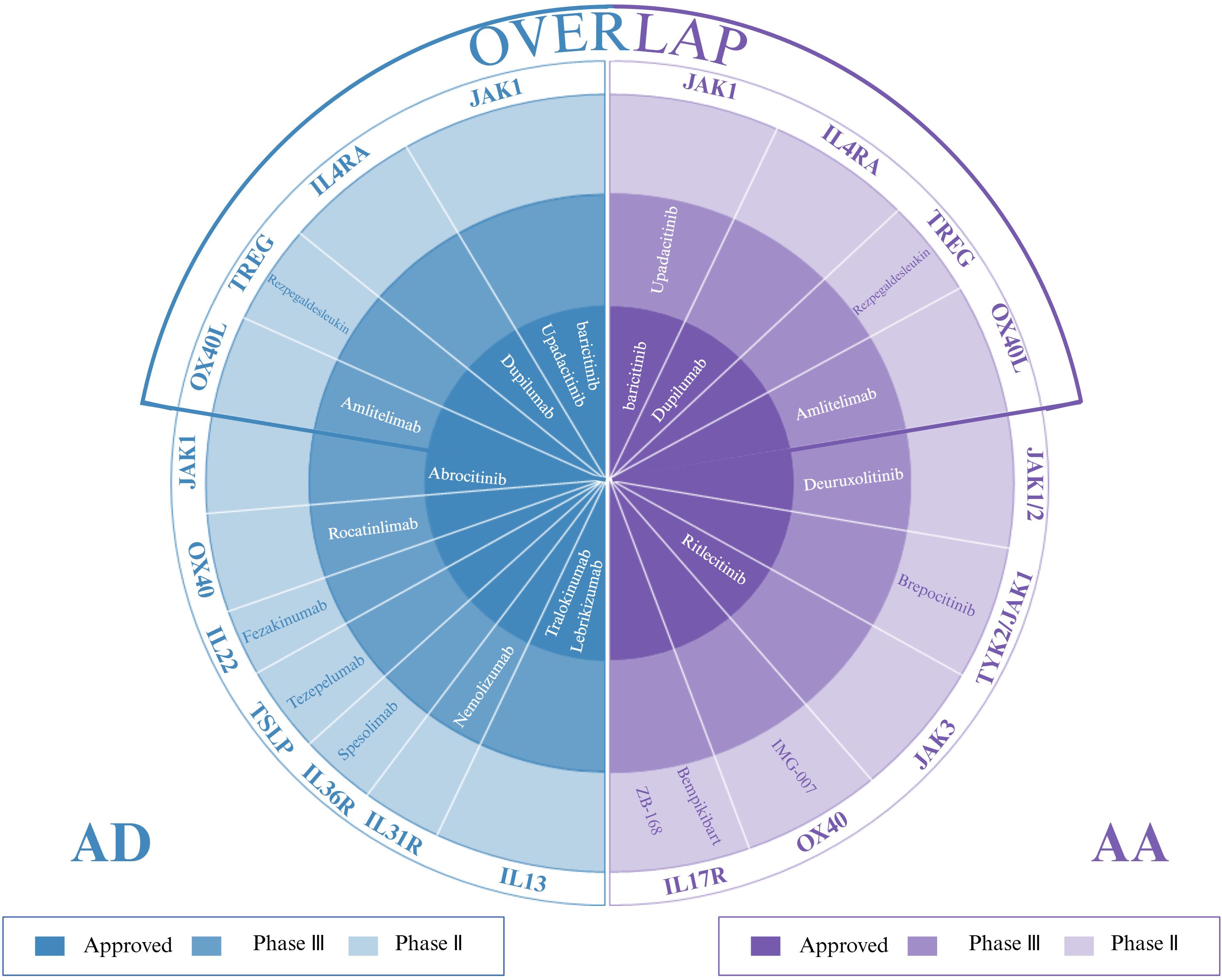
Figure 4. Targeted therapies for AD, AA, and their overlap: approved agents and investigational drugs in phase II/III trials. (Figure created with BioRender.com). AD, atopic dermatitis; AA, alopecia areata.
6.3 Other biological agents
Rezpegaldesleukin, a novel biotherapeutic drug in clinical trials, works by specifically targeting the IL-2R complex to stimulate the proliferation of Tregs, thereby inhibiting the aberrant activation of pathogenic T-cell subsets and cytokine storm-mediated excessive immune responses. The drug is currently advancing into clinical studies in several immune-related disease areas and is currently in pivotal phase 2b trials for moderately severe AD and severe to very severe AA (133, 134). These studies will systematically evaluate the therapeutic potential of drugs in different immune disorder scenarios and provide evidence-based medical support for subsequent indication expansion. However, there are still challenges in the field of immunotherapy where efficacy has not met expectations, for example, a phase II study showed that for the treatment of AD, strategies targeting IL-17A in isolation had limited efficacy even in the hyper-activated state of Th17 (135), and that there was no significant difference in the treatment of AD for the anti-IL-22 monoclonal antibody compared to placebo (136). Similarly, no significant efficacy was observed for the anti-IL-17A drug suxinumab in patients with AA (137). In contrast, a variety of drugs targeting the OX40L/OX40 pathway are in phase II/III clinical development for AD treatment and show promise. For example, rocatilinimab has shown good efficacy at all four doses evaluated in its phase 2b trial, with sustained improvement up to 36 weeks post-treatment and efficacy maintained for 5 months after discontinuation. In addition, amlitelimab, by combining with OX40L and blocking its interaction with OX40, has shown rapid improvement in patients with moderate-to-severe AD, with a favorable safety profile, and efficacy was maintained for 6 months after discontinuation, both of which have demonstrated promise for AD treatment. Both demonstrate durable improvement in AD (138). OX40/OX40L inhibitors demonstrate compelling efficacy and favorable safety in AD treatment. Targeting this pathway concurrently depletes multiple pathogenic T-cell subsets (including Th1, Th2, Th17, Th22) and their memory counterparts. Given that aberrant interactions between OX40+ T cells and OX40L+ antigen-presenting cells (APC) also drive AA progression—a systemic inflammatory disease that may benefit from systemic OX40 inhibition regardless of atopic status—this approach holds therapeutic promise for AA. Although OX40L/OX40-targeted research in AA remains nascent, its potential warrants rigorous clinical validation. In light of these findings, future multicenter cohort studies are needed to clarify biomarkers of AD and AA comorbidity (e.g., OX40L serum levels, FLG mutation status) and to explore the synergistic effects and long-term safety of combination targeted therapies (e.g., JAK inhibitors + IL-4/IL-13 blockers) (Table 1).
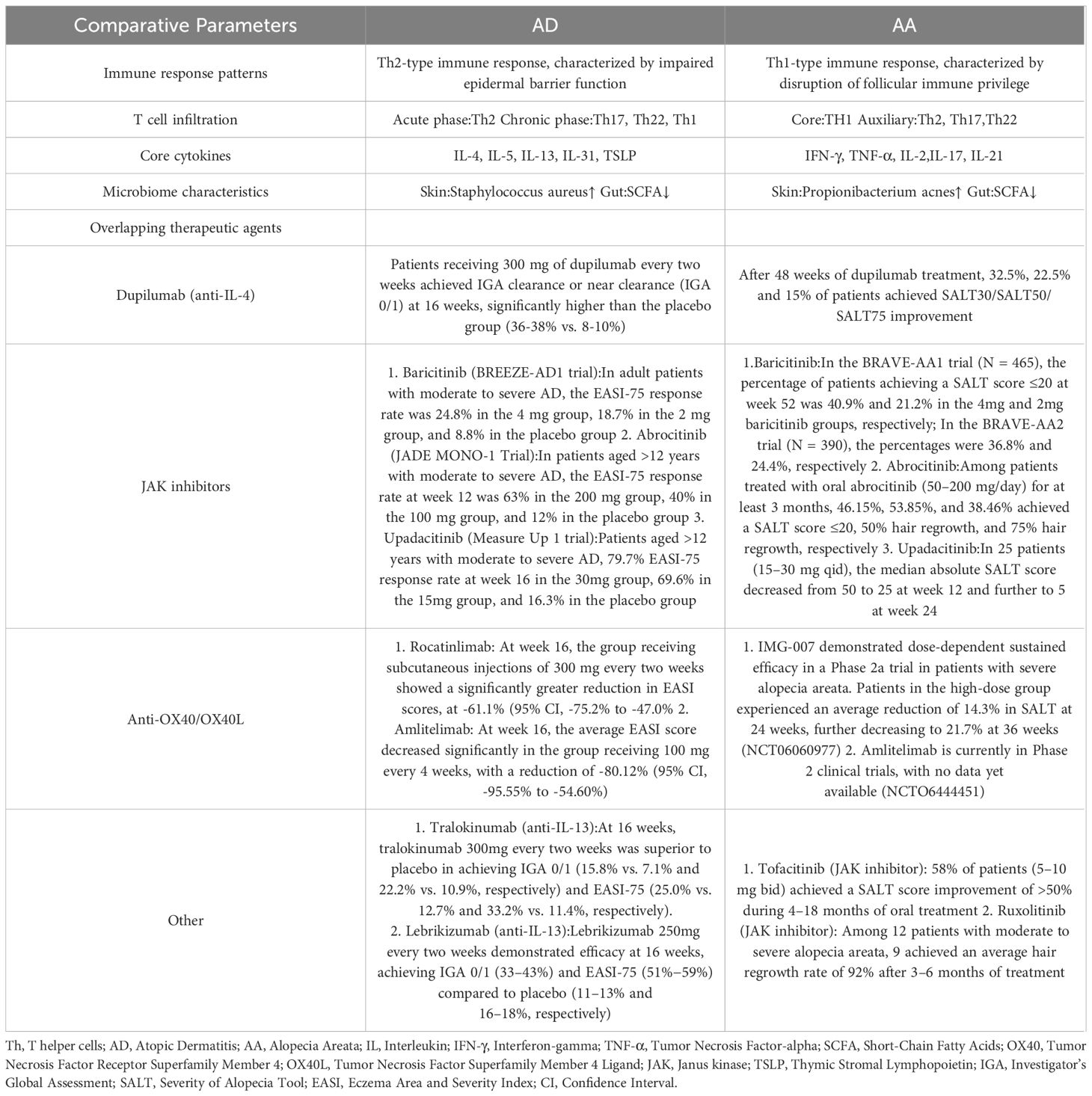
Table 1. Systematic comparison of the immunopathological mechanisms and treatment responses of AD and AA. Modified from (116, 121, 122).
7 Probiotic therapy
A study by Enomoto et al. (139) showed that prenatal and six-month postnatal administration of Bifidobacterium shortum M-16V and Bifidobacterium longum BB536 to mothers in combination with their newborns significantly reduced the risk of AD in infants during the first 18 months of life. In addition, specific strains such as Lactobacillus paracasei KBL382 and Lactobacillus sinensis CAU 28(T) showed potential in alleviating AD symptoms and modulating the structure of gut flora (140, 141). Iemoli et al. demonstrated that probiotics balance Th1/Th2 immunity and enhance Treg activity via interactions with dendritic cells (142). However, a randomized controlled trial by Allen et al. found no significant reduction in AD incidence among 2-year-olds receiving probiotic supplementation compared to placebo (143). Subsequent systematic reviews confirm considerable heterogeneity in probiotic efficacy across pediatric, adult, and prenatal populations with AD (144). Although considered a potential therapeutic option, the clinical value of probiotics in AD remains inconclusive with conflicting evidence (145). In a recent randomized, double-blind, placebo-controlled clinical trial, Liu et al. found that FMT therapy could effectively improve the Eczema Area and Severity Index (EASI) scores of patients with AD by regulating the Th2/Th17 ratio, serum TNF-α, and total IgE levels, with good safety, and may serve as a new therapeutic approach for inflammatory diseases (146). Similarly, the long-term hair growth cases observed after FMT provide additional support for the involvement of the gut microbiota in the pathogenesis of alopecia areata (147), but such studies have small sample sizes and lack controls. Based on the limited studies identified in the literature, a clear association between gut microbiota dysbiosis and alopecia areata has not yet been established. It is worth noting that AA is strongly associated with nutritional factors, especially vitamin D deficiency and its receptor low expression are associated with AA (148, 149). Given that vitamin D receptor expression is regulated by the gut microbiota (150) and that the gut microbiome influences nutrient absorption, it has been hypothesized that reversal of gut dysbiosis may improve the absorption of hair-growth-friendly nutrients such as vitamin D (151). Recent studies have shown that exploratory therapies such as fecal microbial transplantation (FMT) exhibit potential in reducing AD severity (152), although these findings are largely based on small experimental studies. Similarly, cases of long-term hair growth observed after FMT provide additional support for the involvement of the gut microbiome in AA pathogenesis (153), although the statistical significance of the results of tests for changes in gut flora in some AA patients was not significant (154).
8 Conclusions and perspectives
This review has systematically examined the intricate relationship between AD and AA, focusing on their overlapping epidemiological links, shared pathophysiological mechanisms, and implications for therapeutic strategies. We first delineated the robust bidirectional epidemiological association between these conditions, highlighting AD as a significant risk factor for AA development and vice versa. Subsequently, we dissected the complex immune networks underpinning both diseases, moving beyond the traditional Th1/Th2 dichotomy to emphasize the concurrent dysregulation and crosstalk among multiple axes, including Th1 (IFN-γ, TNF-α), Th2 (IL-4, IL-13, IgE), Th17 (IL-17), and Th22 (IL-22). Key signaling pathways, notably JAK-STAT and OX40-OX40L, were identified as critical convergent hubs driving inflammation, barrier dysfunction, and follicular damage in both AD and AA. Shared genetic susceptibility loci, particularly involving the FLG gene and Th2 cytokines (IL-4, IL-13), further solidify their common etiological framework. The review also explored the emerging role of gut and skin microbiome dysbiosis, characterized by reduced SCFA production and increased intestinal permeability (“leaky gut”), in modulating systemic and local immune responses that exacerbate both conditions.
Collectively, the convergence of genetic predisposition, immune dysregulation across multiple axes, and microbiome-mediated modulation creates a shared pathophysiological landscape for AD and AA. We propose that the active inflammatory state in one disease may predispose to or exacerbate the other, reflecting their mechanistic interplay. Critically, this mechanistic overlap holds significant therapeutic implications. Emerging evidence suggests that targeted therapies effective for one condition may exert beneficial effects on the other, as exemplified by the potential efficacy of certain IL-4/IL-13 inhibitors and JAK inhibitors in comorbid presentations (Figure 3). However, the bidirectional response observed with agents like dupilumab underscores the complexity and the need for patient stratification based on immune endotypes (e.g., Th1 vs. Th2 skew, IgE levels).
Despite these advances, critical knowledge gaps persist. The precise pathophysiological interplay mediated by the microbiome in the AD-AA dyad requires further elucidation through integrated multi-omics approaches. The long-term efficacy and safety profiles of novel biologics targeting pathways like OX40-OX40L in AA, either alone or in combination with existing agents (e.g., JAK inhibitors), warrant rigorous investigation in large-scale clinical trials. Furthermore, reliable biomarkers predictive of comorbid risk, disease severity, and therapeutic response (e.g., serum OX40L levels, specific microbial signatures, FLG mutation status) remain to be robustly defined. Future research should prioritize elucidating these unresolved questions and optimizing personalized therapeutic strategies, including exploring synergistic combinations of targeted agents and microbiota-modulating interventions (e.g., defined probiotics, FMT), to effectively manage the challenging clinical scenario of overlapping AD and AA.
Author contributions
JC: Writing – original draft. YJ: Writing – original draft, Visualization. QC: Writing – review & editing. MX: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Th, T helper cell; AD, Atopic Dermatitis; AA, Alopecia Areata; IL, Interleukin; IgE, Immunoglobulin E; IFN-γ, Interferon-gamma; TNF-α, Tumor Necrosis Factor-alpha; NK, Natural Killer cell; IFNG, Interferon-gamma gene; FLG, Filaggrin gene; CCL11, C-C Motif Chemokine Ligand 11; T cell, T Lymphocyte; JAK, Janus Kinase; STAT, Signal Transducer and Activator of Transcription; B cell, B lymphocyte; IgG, Immunoglobulin G; OX40, Tumor Necrosis Factor Receptor Superfamily Member 4; OX40L, Tumor Necrosis Factor Superfamily Member 4 Ligand; Treg, Regulatory T cell; FcϵRI, High-affinity Immunoglobulin E Receptor; CD8+ T, CD8-positive T cell; GWAS, Genome-Wide Association Study; SCFA, Short-Chain Fatty Acid; TGF, Transforming Growth Factor; SALT, Severity of Alopecia Tool; BSA, Body Surface Area; FMT, Fecal Microbiota Transplantation; LGS, Leaky Gut Syndrome; APC, Antigen-Presenting Cell; EASI, Eczema Area and Severity Index.
References
1. Laughter MR, Maymone MBC, Mashayekhi S, Arents BWM, Karimkhani C, Langan SM, et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990-2017. Br J Dermatol. (2021) 184:304–9. doi: 10.1111/bjd.19580
2. Lee HH, Gwillim E, Patel KR, Hua T, Rastogi S, Ibler E, et al. Epidemiology of alopecia areata, ophiasis, totalis, and universalis: A systematic review and meta-analysis. J Am Acad Dermatol. (2020) 82:675–82. doi: 10.1016/j.jaad.2019.08.032
3. Strazzulla LC, Wang EHC, Avila L, Lo Sicco K, Brinster N, Christiano AM, et al. Alopecia areata: Disease characteristics, clinical evaluation, and new perspectives on pathogenesis. J Am Acad Dermatol. (2018) 78:1–12. doi: 10.1016/j.jaad.2017.04.1141
5. Šutić Udović I, Hlača N, Massari LP, Brajac I, Kaštelan M, and Vičić M. Deciphering the complex immunopathogenesis of alopecia areata. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25115652
6. Carrington AE, Maloh J, Nong Y, Agbai ON, Bodemer AA, and Sivamani RK. The gut and skin microbiome in alopecia: associations and interventions. J Clin Aesthet Dermatol. (2023) 16:59–64.
7. Drucker AM, Thompson JM, Li WQ, Cho E, Li T, Guttman-Yassky E, et al. Incident alopecia areata and vitiligo in adult women with atopic dermatitis: Nurses’ Health Study 2. Allergy. (2017) 72:831–4. doi: 10.1111/all.13128
8. Wei YH, Tai YH, Dai YX, Chang YT, Chen TJ, and Chen MH. Bidirectional association between alopecia areata and atopic dermatitis: A population-based cohort study in Taiwan. Clin Exp Allergy. (2020) 50:1406–14. doi: 10.1111/cea.13729
9. Kridin K, Renert-Yuval Y, Guttman-Yassky E, and Cohen AD. Alopecia areata is associated with atopic diathesis: results from a population-based study of 51,561 patients. J Allergy Clin Immunol Pract. (2020) 8:1323–8.e1. doi: 10.1016/j.jaip.2020.01.052
10. Lee NR, Kim BK, Yoon NY, Lee SY, Ahn SY, and Lee WS. Differences in comorbidity profiles between early-onset and late-onset alopecia areata patients: A retrospective study of 871 Korean patients. Ann Dermatol. (2014) 26:722–6. doi: 10.5021/ad.2014.26.6.722
11. Conic RZ, Tamashunas NL, Damiani G, Fabbrocini G, Cantelli M, and Bergfeld WF. Comorbidities in pediatric alopecia areata. J Eur Acad Dermatol Venereol. (2020) 34:2898–901. doi: 10.1111/jdv.16727
12. Weidinger S and Novak N. Atopic dermatitis. Lancet. (2016) 387:1109–22. doi: 10.1016/S0140-6736(15)00149-X
13. Tokura Y. Extrinsic and intrinsic types of atopic dermatitis. J Dermatol Sci. (2010) 58:1–7. doi: 10.1016/j.jdermsci.2010.02.008
14. Kageyama R, Ito T, Hanai S, Morishita N, Nakazawa S, Fujiyama T, et al. Immunological properties of atopic dermatitis-associated alopecia areata. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22052618
15. Leung DY, Boguniewicz M, Howell MD, Nomura I, and Hamid QA. New insights into atopic dermatitis. J Clin Invest. (2004) 113:651–7. doi: 10.1172/JCI21060
16. Gittler JK, Shemer A, Suárez-Fariñas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. (2012) 130:1344–54. doi: 10.1016/j.jaci.2012.07.012
17. David Boothe W, Tarbox JA, and Tarbox MB. Atopic dermatitis: pathophysiology. Adv Exp Med Biol. (2017) 1027:21–37. doi: 10.1007/978-3-319-64804-0_3
18. Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. (2014) 20:1043–9. doi: 10.1038/nm.3645
19. Juárez-Rendón KJ, Rivera Sánchez G, Reyes-López M, García-Ortiz JE, Bocanegra-García V, Guardiola-Avila I, et al. Alopecia Areata. Current situation and perspectives. Arch Argent Pediatr. (2017) 115:e404–e11. doi: 10.5546/aap.2017.eng.e404
20. Furue M. Regulation of filaggrin, loricrin, and involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: pathogenic implications in atopic dermatitis. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21155382
21. Waśkiel-Burnat A, Osińska M, Salińska A, Blicharz L, Goldust M, Olszewska M, et al. The role of serum th1, th2, and th17 cytokines in patients with alopecia areata: clinical implications. Cells. (2021) 10. doi: 10.3390/cells10123397
22. Brar K and Leung DY. Recent considerations in the use of recombinant interferon gamma for biological therapy of atopic dermatitis. Expert Opin Biol Ther. (2016) 16:507–14. doi: 10.1517/14712598.2016.1135898
23. Kak G, Raza M, and Tiwari BK. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol Concepts. (2018) 9:64–79. doi: 10.1515/bmc-2018-0007
24. Hong JW, Lee CY, Ha SM, Choi SH, Kim TH, Song KH, et al. The contributory roles of th17 lymphocyte and cytotoxic T lymphocyte at the hair bulge region as well as the hair bulb area in the chronic alopecia areata patients. Ann Dermatol. (2017) 29:156–66. doi: 10.5021/ad.2017.29.2.156
25. Ma X, Chen S, Jin W, and Gao Y. Th1/Th2 PB balance and CD200 expression of patients with active severe alopecia areata. Exp Ther Med. (2017) 13:2883–7. doi: 10.3892/etm.2017.4312
26. Omar SI, Hamza AM, Eldabah N, and Habiba DA. IFN-α and TNF-α serum levels and their association with disease severity in Egyptian children and adults with alopecia areata. Int J Dermatol. (2021) 60:1397–404. doi: 10.1111/ijd.15658
27. Rajabi F, Drake LA, Senna MM, and Rezaei N. Alopecia areata: a review of disease pathogenesis. Br J Dermatol. (2018) 179:1033–48. doi: 10.1111/bjd.16808
28. Wasserer S, Jargosch M, Mayer KE, Eigemann J, Raunegger T, Aydin G, et al. Characterization of high and low IFNG-expressing subgroups in atopic dermatitis. Int J Mol Sci. (2024) 25. doi: 10.3390/ijms25116158
29. Fania L, Moretta G, Antonelli F, Scala E, Abeni D, Albanesi C, et al. Multiple roles for cytokines in atopic dermatitis: from pathogenic mediators to endotype-specific biomarkers to therapeutic targets. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23052684
30. Biedermann T, Skabytska Y, Kaesler S, and Volz T. Regulation of T cell immunity in atopic dermatitis by microbes: the yin and yang of cutaneous inflammation. Front Immunol. (2015) 6:353. doi: 10.3389/fimmu.2015.00353
31. Mizutani Y, Takagi N, Nagata H, and Inoue S. Interferon-γ downregulates tight junction function, which is rescued by interleukin-17A. Exp Dermatol. (2021) 30:1754–63. doi: 10.1111/exd.14425
32. Tawada C, Kanoh H, Nakamura M, Mizutani Y, Fujisawa T, Banno Y, et al. Interferon-γ decreases ceramides with long-chain fatty acids: possible involvement in atopic dermatitis and psoriasis. J Invest Dermatol. (2014) 134:712–8. doi: 10.1038/jid.2013.364
33. Ito T, Kageyama R, Nakazawa S, and Honda T. Understanding the significance of cytokines and chemokines in the pathogenesis of alopecia areata. Exp Dermatol. (2020) 29:726–32. doi: 10.1111/exd.14129
34. Chieosilapatham P, Kiatsurayanon C, Umehara Y, Trujillo-Paez JV, Peng G, Yue H, et al. Keratinocytes: innate immune cells in atopic dermatitis. Clin Exp Immunol. (2021) 204:296–309. doi: 10.1111/cei.13575
35. Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. (2006) 38:441–6. doi: 10.1038/ng1767
36. Hatano Y, Katagiri K, Arakawa S, and Fujiwara S. Interleukin-4 depresses levels of transcripts for acid-sphingomyelinase and glucocerebrosidase and the amount of ceramide in acetone-wounded epidermis, as demonstrated in a living skin equivalent. J Dermatol Sci. (2007) 47:45–7. doi: 10.1016/j.jdermsci.2007.02.010
37. Hatano Y, Adachi Y, Elias PM, Crumrine D, Sakai T, Kurahashi R, et al. The Th2 cytokine, interleukin-4, abrogates the cohesion of normal stratum corneum in mice: implications for pathogenesis of atopic dermatitis. Exp Dermatol. (2013) 22:30–5. doi: 10.1111/exd.12047
38. Inui S, Noguchi F, Nakajima T, and Itami S. Serum thymus and activation-regulated chemokine as disease activity and response biomarker in alopecia areata. J Dermatol. (2013) 40:881–5. doi: 10.1111/1346-8138.12273
39. Tembhre MK and Sharma VK. T-helper and regulatory T-cell cytokines in the peripheral blood of patients with active alopecia areata. Br J Dermatol. (2013) 169:543–8. doi: 10.1111/bjd.12396
40. Meng J, Li Y, Fischer MJM, Steinhoff M, Chen W, and Wang J. Th2 modulation of transient receptor potential channels: an unmet therapeutic intervention for atopic dermatitis. Front Immunol. (2021) 12:696784. doi: 10.3389/fimmu.2021.696784
41. Chu SY, Chen YJ, Tseng WC, Lin MW, Chen TJ, Hwang CY, et al. Comorbidity profiles among patients with alopecia areata: the importance of onset age, a nationwide population-based study. J Am Acad Dermatol. (2011) 65:949–56. doi: 10.1016/j.jaad.2010.08.032
42. Czarnowicki T, He HY, Wen HC, Hashim PW, Nia JK, Malik K, et al. Alopecia areata is characterized by expansion of circulating Th2/Tc2/Th22, within the skin-homing and systemic T-cell populations. Allergy. (2018) 73:713–23. doi: 10.1111/all.13346
43. Sroka-Tomaszewska J and Trzeciak M. Molecular mechanisms of atopic dermatitis pathogenesis. Int J Mol Sci. (2021) 22. doi: 10.3390/ijms22084130
44. Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol. (2008) 181:4733–41. doi: 10.4049/jimmunol.181.7.4733
45. Kalinke U and Prinz M. Endogenous, or therapeutically induced, type I interferon responses differentially modulate Th1/Th17-mediated autoimmunity in the CNS. Immunol Cell Biol. (2012) 90:505–9. doi: 10.1038/icb.2012.8
46. Lou H, Lu J, Choi EB, Oh MH, Jeong M, Barmettler S, et al. Expression of IL-22 in the skin causes th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial th2 cytokines and the GRP pathway. J Immunol. (2017) 198:2543–55. doi: 10.4049/jimmunol.1600126
47. Barry DM, Liu XT, Liu B, Liu XY, Gao F, Zeng X, et al. Exploration of sensory and spinal neurons expressing gastrin-releasing peptide in itch and pain related behaviors. Nat Commun. (2020) 11:1397. doi: 10.1038/s41467-020-15230-y
48. Peng S, Zhan Y, Zhang D, Ren L, Chen A, Chen ZF, et al. Structures of human gastrin-releasing peptide receptors bound to antagonist and agonist for cancer and itch therapy. Proc Natl Acad Sci U S A. (2023) 120:e2216230120. doi: 10.1073/pnas.2216230120
49. Kim J, Kim BE, and Leung DYM. Pathophysiology of atopic dermatitis: Clinical implications. Allergy Asthma Proc. (2019) 40:84–92. doi: 10.2500/aap.2019.40.4202
50. Tanemura A, Oiso N, Nakano M, Itoi S, Kawada A, and Katayama I. Alopecia areata: infiltration of Th17 cells in the dermis, particularly around hair follicles. Dermatology. (2013) 226:333–6. doi: 10.1159/000350933
51. Atwa MA, Youssef N, and Bayoumy NM. T-helper 17 cytokines (interleukins 17, 21, 22, and 6, and tumor necrosis factor-α) in patients with alopecia areata: association with clinical type and severity. Int J Dermatol. (2016) 55:666–72. doi: 10.1111/ijd.12808
52. El-Morsy EH, Eid AA, Ghoneim H, and Al-Tameemi KA. Serum level of interleukin-17A in patients with alopecia areata and its relationship to age. Int J Dermatol. (2016) 55:869–74. doi: 10.1111/ijd.12994
53. Harpaz I, Abutbul S, Nemirovsky A, Gal R, Cohen H, and Monsonego A. Chronic exposure to stress predisposes to higher autoimmune susceptibility in C57BL/6 mice: glucocorticoids as a double-edged sword. Eur J Immunol. (2013) 43:758–69. doi: 10.1002/eji.201242613
54. Shreberk-Hassidim R, Ramot Y, Gilula Z, and Zlotogorski A. A systematic review of pulse steroid therapy for alopecia areata. J Am Acad Dermatol. (2016) 74:372–4.e1-5. doi: 10.1016/j.jaad.2015.09.045
55. Bain KA, McDonald E, Moffat F, Tutino M, Castelino M, Barton A, et al. Alopecia areata is characterized by dysregulation in systemic type 17 and type 2 cytokines, which may contribute to disease-associated psychological morbidity. Br J Dermatol. (2020) 182:130–7. doi: 10.1111/bjd.18008
56. Bao L, Zhang H, and Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. Jakstat. (2013) 2:e24137. doi: 10.4161/jkst.24137
57. Albanesi C, Fairchild HR, Madonna S, Scarponi C, De Pità O, Leung DY, et al. IL-4 and IL-13 negatively regulate TNF-alpha- and IFN-gamma-induced beta-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J Immunol. (2007) 179:984–92. doi: 10.4049/jimmunol.179.2.984
58. Sehra S, Yao Y, Howell MD, Nguyen ET, Kansas GS, Leung DY, et al. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. (2010) 184:3186–90. doi: 10.4049/jimmunol.0901860
59. Karpathiou G, Papoudou-Bai A, Ferrand E, Dumollard JM, and Peoc’h M. STAT6: A review of a signaling pathway implicated in various diseases with a special emphasis in its usefulness in pathology. Pathol Res Pract. (2021) 223:153477. doi: 10.1016/j.prp.2021.153477
60. Tamura K, Arakawa H, Suzuki M, Kobayashi Y, Mochizuki H, Kato M, et al. Novel dinucleotide repeat polymorphism in the first exon of the STAT-6 gene is associated with allergic diseases. Clin Exp Allergy. (2001) 31:1509–14. doi: 10.1046/j.1365-2222.2001.01191.x
61. Shiratori-Hayashi M, Koga K, Tozaki-Saitoh H, Kohro Y, Toyonaga H, Yamaguchi C, et al. STAT3-dependent reactive astrogliosis in the spinal dorsal horn underlies chronic itch. Nat Med. (2015) 21:927–31. doi: 10.1038/nm.3912
62. Petukhova L, Duvic M, Hordinsky M, Norris D, Price V, Shimomura Y, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. (2010) 466:113–7. doi: 10.1038/nature09114
63. Betz RC, Petukhova L, Ripke S, Huang H, Menelaou A, Redler S, et al. Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nat Commun. (2015) 6:5966. doi: 10.1038/ncomms6966
64. Harel S, Higgins CA, Cerise JE, Dai Z, Chen JC, Clynes R, et al. Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. (2015) 1:e1500973. doi: 10.1126/sciadv.1500973
65. Liu LY and King BA. Response to tofacitinib therapy of eyebrows and eyelashes in alopecia areata. J Am Acad Dermatol. (2019) 80:1778–9. doi: 10.1016/j.jaad.2018.11.037
66. Guttman-Yassky E, Pavel AB, Diaz A, Zhang N, Del Duca E, Estrada Y, et al. Ritlecitinib and brepocitinib demonstrate significant improvement in scalp alopecia areata biomarkers. J Allergy Clin Immunol. (2022) 149:1318–28. doi: 10.1016/j.jaci.2021.10.036
67. Mackay-Wiggan J, Jabbari A, Nguyen N, Cerise JE, Clark C, Ulerio G, et al. Oral ruxolitinib induces hair regrowth in patients with moderate-to-severe alopecia areata. JCI Insight. (2016) 1:e89790. doi: 10.1172/jci.insight.89790
68. Croft M, So T, Duan W, and Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev. (2009) 229:173–91. doi: 10.1111/j.1600-065X.2009.00766.x
69. Ilves T and Harvima IT. OX40 ligand and OX40 are increased in atopic dermatitis lesions but do not correlate with clinical severity. J Eur Acad Dermatol Venereol. (2013) 27:e197–205. doi: 10.1111/j.1468-3083.2012.04587.x
70. Jember AG, Zuberi R, Liu FT, and Croft M. Development of allergic inflammation in a murine model of asthma is dependent on the costimulatory receptor OX40. J Exp Med. (2001) 193:387–92. doi: 10.1084/jem.193.3.387
71. Seshasayee D, Lee WP, Zhou M, Shu J, Suto E, Zhang J, et al. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest. (2007) 117:3868–78. doi: 10.1172/JCI33559
72. Guttman-Yassky E, Croft M, Geng B, Rynkiewicz N, Lucchesi D, Peakman M, et al. The role of OX40 ligand/OX40 axis signalling in atopic dermatitis. Br J Dermatol. (2024) 191:488–96. doi: 10.1093/bjd/ljae230
73. Croft M, Esfandiari E, Chong C, Hsu H, Kabashima K, Kricorian G, et al. OX40 in the pathogenesis of atopic dermatitis-A new therapeutic target. Am J Clin Dermatol. (2024) 25:447–61. doi: 10.1007/s40257-023-00838-9
74. Guttman-Yassky E, Pavel AB, Zhou L, Estrada YD, Zhang N, Xu H, et al. GBR 830, an anti-OX40, improves skin gene signatures and clinical scores in patients with atopic dermatitis. J Allergy Clin Immunol. (2019) 144:482–93.e7. doi: 10.1016/j.jaci.2018.11.053
75. Xiao X, Shi X, Fan Y, Wu C, Zhang X, Minze L, et al. The costimulatory receptor OX40 inhibits interleukin-17 expression through activation of repressive chromatin remodeling pathways. Immunity. (2016) 44:1271–83. doi: 10.1016/j.immuni.2016.05.013
76. Jia T, Che D, Zheng Y, Zhang H, Li Y, Zhou T, et al. Mast cells initiate type 2 inflammation through tryptase released by MRGPRX2/MRGPRB2 activation in atopic dermatitis. J Invest Dermatol. (2024) 144:53–62.e2. doi: 10.1016/j.jid.2023.06.201
77. Gri G, Piconese S, Frossi B, Manfroi V, Merluzzi S, Tripodo C, et al. CD4+CD25+ regulatory T cells suppress mast cell degranulation and allergic responses through OX40-OX40L interaction. Immunity. (2008) 29:771–81. doi: 10.1016/j.immuni.2008.08.018
78. Bertolini M, Zilio F, Rossi A, Kleditzsch P, Emelianov VE, Gilhar A, et al. Abnormal interactions between perifollicular mast cells and CD8+ T-cells may contribute to the pathogenesis of alopecia areata. PloS One. (2014) 9:e94260. doi: 10.1371/journal.pone.0094260
79. Zhang X, Zhao Y, Ye Y, Li S, Qi S, Yang Y, et al. Lesional infiltration of mast cells, Langerhans cells, T cells and local cytokine profiles in alopecia areata. Arch Dermatol Res. (2015) 307:319–31. doi: 10.1007/s00403-015-1539-1
80. Bertolini M, McElwee K, Gilhar A, Bulfone-Paus S, and Paus R. Hair follicle immune privilege and its collapse in alopecia areata. Exp Dermatol. (2020) 29:703–25. doi: 10.1111/exd.14155
81. Piconese S, Gri G, Tripodo C, Musio S, Gorzanelli A, Frossi B, et al. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood. (2009) 114:2639–48. doi: 10.1182/blood-2009-05-220004
82. Bulfone-Paus S and Bahri R. Mast cells as regulators of T cell responses. Front Immunol. (2015) 6:394. doi: 10.3389/fimmu.2015.00394
83. Kim M, Del Duca E, Dahabreh D, Lozano-Ojalvo D, Carroll B, Manson M, et al. Alopecia areata exhibits cutaneous and systemic OX40 activation across atopic backgrounds. Allergy. (2024) 79:3401–14. doi: 10.1111/all.16268
84. Arkwright PD and Koplin JJ. Impact of a decade of research into atopic dermatitis. J Allergy Clin Immunol Pract. (2023) 11:63–71. doi: 10.1016/j.jaip.2022.09.021
85. Biran R, Zlotogorski A, and Ramot Y. The genetics of alopecia areata: new approaches, new findings, new treatments. J Dermatol Sci. (2015) 78:11–20. doi: 10.1016/j.jdermsci.2015.01.004
86. Criado PR, Miot HA, Bueno-Filho R, Ianhez M, Criado RFJ, and de Castro CCS. Update on the pathogenesis of atopic dermatitis. Anais Brasileiros Dermatologia. (2024) 99:895–915. doi: 10.1016/j.abd.2024.06.001
87. Paternoster L, Standl M, Waage J, Baurecht H, Hotze M, Strachan DP, et al. Multi-ancestry genome-wide association study of 21,000 cases and 95,000 controls identifies new risk loci for atopic dermatitis. Nat Genet. (2015) 47:1449–56. doi: 10.1038/ng.3424
88. Nedoszytko B, Reszka E, Gutowska-Owsiak D, Trzeciak M, Lange M, Jarczak J, et al. Genetic and epigenetic aspects of atopic dermatitis. Int J Mol Sci. (2020) 21. doi: 10.3390/ijms21186484
89. Liang Y, Chang C, and Lu Q. The genetics and epigenetics of atopic dermatitis-filaggrin and other polymorphisms. Clin Rev Allergy Immunol. (2016) 51:315–28. doi: 10.1007/s12016-015-8508-5
90. Weidinger S, Illig T, Baurecht H, Irvine AD, Rodriguez E, Diaz-Lacava A, et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol. (2006) 118:214–9. doi: 10.1016/j.jaci.2006.05.004
91. Betz RC, Pforr J, Flaquer A, Redler S, Hanneken S, Eigelshoven S, et al. Loss-of-function mutations in the filaggrin gene and alopecia areata: strong risk factor for a severe course of disease in patients comorbid for atopic disease. J Invest Dermatol. (2007) 127:2539–43. doi: 10.1038/sj.jid.5700915
92. Liang J, Liu Y, Xue R, Chen L, Chen H, Shao L, et al. Interleukin 4 -590C/T (rs2243250) polymorphism is associated with increased risk of atopic dermatitis: meta-analysis of case-control studies. Dermatitis. (2017) 28:144–51. doi: 10.1097/DER.0000000000000265
93. Namkung JH, Lee JE, Kim E, Kim HJ, Seo EY, Jang HY, et al. Association of polymorphisms in genes encoding IL-4, IL-13 and their receptors with atopic dermatitis in a Korean population. Exp Dermatol. (2011) 20:915–9. doi: 10.1111/j.1600-0625.2011.01357.x
94. Kalkan G, Karakus N, Baş Y, Takçı Z, Ozuğuz P, Ateş O, et al. The association between Interleukin (IL)-4 gene intron 3 VNTR polymorphism and alopecia areata (AA) in Turkish population. Gene. (2013) 527:565–9. doi: 10.1016/j.gene.2013.05.086
95. Jagielska D, Redler S, Brockschmidt FF, Herold C, Pasternack SM, Garcia Bartels N, et al. Follow-up study of the first genome-wide association scan in alopecia areata: IL13 and KIAA0350 as susceptibility loci supported with genome-wide significance. J Invest Dermatol. (2012) 132:2192–7. doi: 10.1038/jid.2012.129
96. Magen E, Chikovani T, Waitman DA, and Kahan NR. Association of alopecia areata with atopic dermatitis and chronic spontaneous urticaria. Allergy Asthma Proc. (2018) 39:96–102. doi: 10.2500/aap.2018.39.4114
97. Mahmud MR, Akter S, Tamanna SK, Mazumder L, Esti IZ, Banerjee S, et al. Impact of gut microbiome on skin health: gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes. (2022) 14:2096995. doi: 10.1080/19490976.2022.2096995
98. Liu Z and Liu X. Gut microbiome, metabolome and alopecia areata. Front Microbiol. (2023) 14:1281660. doi: 10.3389/fmicb.2023.1281660
99. Simakou T, Butcher JP, Reid S, and Henriquez FL. Alopecia areata: A multifactorial autoimmune condition. J Autoimmun. (2019) 98:74–85. doi: 10.1016/j.jaut.2018.12.001
100. Rafik D, Younis I, Atef R, and Eid H. Claudin-3 is a novel intestinal integrity marker in patients with alopecia areata: Correlation with the disease severity. J Cosmet Dermatol. (2023) 22:1377–81. doi: 10.1111/jocd.15582
101. Hacınecipoğlu F, Gönül M, Özdemir Ş, and Demir ÖF. Is there a link between alopecia areata and gut? J Cosmet Dermatol. (2022) 21:6049–55. doi: 10.1111/jocd.15095
102. Migacz-Gruszka K, Branicki W, Obtulowicz A, Pirowska M, Gruszka K, and Wojas-Pelc A. What’s new in the pathophysiology of alopecia areata? The possible contribution of skin and gut microbiome in the pathogenesis of alopecia - big opportunities, big challenges, and novel perspectives. Int J Trichol. (2019) 11:185–8. doi: 10.4103/ijt.ijt_76_19
103. Kinashi Y and Hase K. Partners in leaky gut syndrome: intestinal dysbiosis and autoimmunity. Front Immunol. (2021) 12:673708. doi: 10.3389/fimmu.2021.673708
104. Geoghegan JA, Irvine AD, and Foster TJ. Staphylococcus aureus and atopic dermatitis: A complex and evolving relationship. Trends Microbiol. (2018) 26:484–97. doi: 10.1016/j.tim.2017.11.008
105. Pinto D, Sorbellini E, Marzani B, Rucco M, Giuliani G, and Rinaldi F. Scalp bacterial shift in Alopecia areata. PloS One. (2019) 14:e0215206. doi: 10.1371/journal.pone.0215206
106. Polak-Witka K, Rudnicka L, Blume-Peytavi U, and Vogt A. The role of the microbiome in scalp hair follicle biology and disease. Exp Dermatol. (2020) 29:286–94. doi: 10.1111/exd.13935
107. Macia L, Thorburn AN, Binge LC, Marino E, Rogers KE, Maslowski KM, et al. Microbial influences on epithelial integrity and immune function as a basis for inflammatory diseases. Immunol Rev. (2012) 245:164–76. doi: 10.1111/j.1600-065X.2011.01080.x
108. Lv J, Hao P, Zhou Y, Liu T, Wang L, Song C, et al. Role of the intestinal flora-immunity axis in the pathogenesis of rheumatoid arthritis-mechanisms regulating short-chain fatty acids and Th17/Treg homeostasis. Mol Biol Rep. (2025) 52:617. doi: 10.1007/s11033-025-10714-w
109. Xu W, Wan S, Xie B, and Song X. Novel potential therapeutic targets of alopecia areata. Front Immunol. (2023) 14:1148359. doi: 10.3389/fimmu.2023.1148359
110. Kim HJ, Lee SH, and Hong SJ. Antibiotics-induced dysbiosis of intestinal microbiota aggravates atopic dermatitis in mice by altered short-chain fatty acids. Allergy Asthma Immunol Res. (2020) 12:137–48. doi: 10.4168/aair.2020.12.1.137
111. Han YM, Sheng YY, Xu F, Qi SS, Liu XJ, Hu RM, et al. Imbalance of T-helper 17 and regulatory T cells in patients with alopecia areata. J Dermatol. (2015) 42:981–8. doi: 10.1111/1346-8138.12978
112. Chim I, Ghiya R, Sinclair RD, and Eisman S. Novel investigational drugs for alopecia areata and future perspectives. Expert Opin Investig Drugs. (2024) 33:441–9. doi: 10.1080/13543784.2024.2348062
113. Smogorzewski J, Sierro T, Compoginis G, and Kim G. Remission of alopecia universalis in a patient with atopic dermatitis treated with dupilumab. JAAD Case Rep. (2019) 5:116–7. doi: 10.1016/j.jdcr.2018.11.007
114. Uchida H, Kamata M, Watanabe A, Agematsu A, Nagata M, Fukaya S, et al. Dupilumab improved alopecia areata in a patient with atopic dermatitis: A case report. Acta Derm Venereol. (2019) 99:675–6. doi: 10.2340/00015555-3183
115. Zhou C, Li X, Wang C, and Zhang J. Alopecia areata: an update on etiopathogenesis, diagnosis, and management. Clin Rev Allergy Immunol. (2021) 61:403–23. doi: 10.1007/s12016-021-08883-0
116. Guttman-Yassky E, Renert-Yuval Y, Bares J, Chima M, Hawkes JE, Gilleaudeau P, et al. Phase 2a randomized clinical trial of dupilumab (anti-IL-4Rα) for alopecia areata patients. Allergy. (2022) 77:897–906. doi: 10.1111/all.15071
117. Chung J, Slaught CL, and Simpson EL. Alopecia areata in 2 patients treated with dupilumab: New onset and worsening. JAAD Case Rep. (2019) 5:643–5. doi: 10.1016/j.jdcr.2019.03.019
118. Marks DH, Mesinkovska N, and Senna MM. Cause or cure? Review of dupilumab and alopecia areata. J Am Acad Dermatol. (2023) 88:651–3. doi: 10.1016/j.jaad.2019.06.010
119. McKenzie PL and Castelo-Soccio L. Dupilumab therapy for alopecia areata in pediatric patients with concomitant atopic dermatitis. J Am Acad Dermatol. (2021) 84:1691–4. doi: 10.1016/j.jaad.2021.01.046
120. Cho SK and Craiglow BG. Dupilumab for the treatment of alopecia areata in children with atopic dermatitis. JAAD Case Rep. (2021) 16:82–5. doi: 10.1016/j.jdcr.2021.07.015
121. Kobal I and Ramot Y. Janus kinase inhibitors for the treatment of alopecia areata. Hautarzt. (2022) 73:336–43. doi: 10.1007/s00105-022-04982-x
122. Butala S, Castelo-Soccio L, Seshadri R, Simpson EL, O’Shea JJ, Bieber T, et al. Biologic versus small molecule therapy for treating moderate to severe atopic dermatitis: clinical considerations. J Allergy Clin Immunol Pract. (2023) 11:1361–73. doi: 10.1016/j.jaip.2023.03.011
123. Cantelli M, Martora F, Patruno C, Nappa P, Fabbrocini G, and Napolitano M. Upadacitinib improved alopecia areata in a patient with atopic dermatitis: A case report. Dermatol Ther. (2022) 35:e15346. doi: 10.1111/dth.15346
124. Gambardella A, Licata G, Calabrese G, De Rosa A, Alfano R, and Argenziano G. Dual efficacy of upadacitinib in 2 patients with concomitant severe atopic dermatitis and alopecia areata. Dermatitis. (2021) 32:e85–e6. doi: 10.1097/DER.0000000000000780
125. Blauvelt A, Ladizinski B, Prajapati VH, Laquer V, Fischer A, Eisman S, et al. Efficacy and safety of switching from dupilumab to upadacitinib versus continuous upadacitinib in moderate-to-severe atopic dermatitis: Results from an open-label extension of the phase 3, randomized, controlled trial (Heads Up). J Am Acad Dermatol. (2023) 89:478–85. doi: 10.1016/j.jaad.2023.05.033
126. Kołcz K, Żychowska M, Sawińska E, and Reich A. Alopecia universalis in an adolescent successfully treated with upadacitinib-A case report and review of the literature on the use of JAK inhibitors in pediatric alopecia areata. Dermatol Ther (Heidelb). (2023) 13:843–56. doi: 10.1007/s13555-023-00889-0
127. Asfour L, Getsos Colla T, Moussa A, and Sinclair RD. Concurrent chronic alopecia areata and severe atopic dermatitis successfully treated with upadacitinib. Int J Dermatol. (2022) 61:e416–e7. doi: 10.1111/ijd.16316
128. Liu X, Song B, and Jin H. Abrocitinib improved dupilumab-resistant severe atopic dermatitis with comorbid mild alopecia areata in a 12-year-old boy: A case report with 1-year follow-up. J Asthma Allergy. (2024) 17:305–11. doi: 10.2147/JAA.S458684
129. Zhao J and Liu L. A case of atopic dermatitis with alopecia universalis in a patient treated with abrocitinib. JAAD Case Rep. (2022) 22:99–100. doi: 10.1016/j.jdcr.2022.02.027
130. Chang AH, Brownstone ND, and Hsu S. Drug-induced alopecia areata from upadacitinib. Cureus. (2024) 16:e66647. doi: 10.7759/cureus.66647
131. Nezamololama N, Fieldhouse K, Metzger K, and Gooderham M. Emerging systemic JAK inhibitors in the treatment of atopic dermatitis: a review of abrocitinib, baricitinib, and upadacitinib. Drugs Context. (2020) 9. doi: 10.7573/dic.2020-8-5
132. Blauvelt A, Teixeira HD, Simpson EL, Costanzo A, De Bruin-Weller M, Barbarot S, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: A randomized clinical trial. JAMA Dermatol. (2021) 157:1047–55. doi: 10.1001/jamadermatol.2021.3023
133. Therapeutics N. A phase 2b study to evaluate rezpegaldesleukin (Rezpeg) in the treatment of adult patients with moderate-to-severe atopic dermatitis (REZOLVE-AD) (2025). Available online at: https://clinicaltrials.gov/study/NCT06136741?intr=REZPEG&rank=3 (Accessed May 8, 2025).
134. Therapeutics N. A phase 2b study to evaluate rezpegaldesleukin (Rezpeg) in the treatment of severe to very severe alopecia areata in adult patients (Rezolve AA) (2025). Available online at: https://clinicaltrials.gov/study/NCT06340360 (Accessed May 8, 2025).
135. Ungar B, Pavel AB, Li R, Kimmel G, Nia J, Hashim P, et al. Phase 2 randomized, double-blind study of IL-17 targeting with secukinumab in atopic dermatitis. J Allergy Clin Immunol. (2021) 147:394–7. doi: 10.1016/j.jaci.2020.04.055
136. Guttman-Yassky E, Brunner PM, Neumann AU, Khattri S, Pavel AB, Malik K, et al. Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial. J Am Acad Dermatol. (2018) 78:872–81.e6. doi: 10.1016/j.jaad.2018.01.016
137. Guttman-Yassky E, Nia JK, Hashim PW, Mansouri Y, Alia E, Taliercio M, et al. Efficacy and safety of secukinumab treatment in adults with extensive alopecia areata. Arch Dermatol Res. (2018) 310:607–14. doi: 10.1007/s00403-018-1853-5
138. Lé AM and Torres T. OX40-OX40L inhibition for the treatment of atopic dermatitis-focus on rocatinlimab and amlitelimab. Pharmaceutics. (2022) 14. doi: 10.3390/pharmaceutics14122753
139. Enomoto T, Sowa M, Nishimori K, Shimazu S, Yoshida A, Yamada K, et al. Effects of bifidobacterial supplementation to pregnant women and infants in the prevention of allergy development in infants and on fecal microbiota. Allergol Int. (2014) 63:575–85. doi: 10.2332/allergolint.13-OA-0683
140. Yi O, Kwon HJ, Kim H, Ha M, Hong SJ, Hong YC, et al. Effect of environmental tobacco smoke on atopic dermatitis among children in Korea. Environ Res. (2012) 113:40–5. doi: 10.1016/j.envres.2011.12.012
141. Kwon HK, Lee CG, So JS, Chae CS, Hwang JS, Sahoo A, et al. Generation of regulatory dendritic cells and CD4+Foxp3+ T cells by probiotics administration suppresses immune disorders. Proc Natl Acad Sci U S A. (2010) 107:2159–64. doi: 10.1073/pnas.0904055107
142. Iemoli E, Trabattoni D, Parisotto S, Borgonovo L, Toscano M, Rizzardini G, et al. Probiotics reduce gut microbial translocation and improve adult atopic dermatitis. J Clin Gastroenterol. (2012) 46 Suppl:S33–40. doi: 10.1097/MCG.0b013e31826a8468
143. Allen SJ, Jordan S, Storey M, Thornton CA, Gravenor MB, Garaiova I, et al. Probiotics in the prevention of eczema: a randomised controlled trial. Arch Dis Child. (2014) 99:1014–9. doi: 10.1136/archdischild-2013-305799
144. Piqué N, Berlanga M, and Miñana-Galbis D. Health benefits of heat-killed (Tyndallized) probiotics: an overview. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20102534
145. Rather IA, Bajpai VK, Kumar S, Lim J, Paek WK, and Park YH. Probiotics and atopic dermatitis: an overview. Front Microbiol. (2016) 7:507. doi: 10.3389/fmicb.2016.00507
146. Liu X, Luo Y, Chen X, Wu M, Xu X, Tian J, et al. Fecal microbiota transplantation against moderate-to-severe atopic dermatitis: A randomized, double-blind controlled explorer trial. Allergy. (2025) 80:1377–88. doi: 10.1111/all.16372
147. Rebello D, Wang E, Yen E, Lio PA, and Kelly CR. Hair growth in two alopecia patients after fecal microbiota transplant. ACG Case Rep J. (2017) 4:e107. doi: 10.14309/crj.2017.107
148. Thompson JM, Mirza MA, Park MK, Qureshi AA, and Cho E. The role of micronutrients in alopecia areata: A review. Am J Clin Dermatol. (2017) 18:663–79. doi: 10.1007/s40257-017-0285-x
149. Tsai TY and Huang YC. Vitamin D deficiency in patients with alopecia areata: A systematic review and meta-analysis. J Am Acad Dermatol. (2018) 78:207–9. doi: 10.1016/j.jaad.2017.07.051
150. Waterhouse JC, Perez TH, and Albert PJ. Reversing bacteria-induced vitamin D receptor dysfunction is key to autoimmune disease. Ann N Y Acad Sci. (2009) 1173:757–65. doi: 10.1111/j.1749-6632.2009.04637.x
151. Xie WR, Yang XY, Xia HH, Wu LH, and He XX. Hair regrowth following fecal microbiota transplantation in an elderly patient with alopecia areata: A case report and review of the literature. World J Clin Cases. (2019) 7:3074–81. doi: 10.12998/wjcc.v7.i19.3074
152. Greenzaid JD, Chan LJ, Chandani BM, Kiritsis NR, and Feldman SR. Microbiome modulators for atopic eczema: a systematic review of experimental and investigational therapeutics. Expert Opin Investig Drugs. (2024) 33:415–30. doi: 10.1080/13543784.2024.2326625
153. Passeron T, King B, Seneschal J, Steinhoff M, Jabbari A, Ohyama M, et al. Inhibition of T-cell activity in alopecia areata: recent developments and new directions. Front Immunol. (2023) 14:1243556. doi: 10.3389/fimmu.2023.1243556
Keywords: alopecia areata, atopic dermatitis, overlap, cytokines, immune dysregulation
Citation: Cheng J, Jiang Y, Chen Q and Xiao M (2025) Overlapping features of atopic dermatitis and alopecia areata: from pathogenesis to treatment. Front. Immunol. 16:1641918. doi: 10.3389/fimmu.2025.1641918
Received: 05 June 2025; Accepted: 08 August 2025;
Published: 03 September 2025.
Edited by:
Ryma Toumi, Seattle Children’s Research Institute, United StatesReviewed by:
Jea-Hyun Baek, Handong Global University, Republic of KoreaMasato Tamari, National Center for Child Health and Development (NCCHD), Japan
Copyright © 2025 Cheng, Jiang, Chen and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qianqian Chen, Y3FxMjAxNDIwMzAyOEAxNjMuY29t; Min Xiao, eGlhb21pbkBjZHV0Y20uZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Jiayi Cheng
Jiayi Cheng Yugu Jiang2†
Yugu Jiang2† Min Xiao
Min Xiao