- 1Department of Rheumatology and Immunology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2College of Basic Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 3Sichuan Provincial Engineering Technology Research Center of Natural Small Molecule Drug, Tianfu Traditional Chinese Medicine (TCM) Innovation Harbor, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 4Traditional Chinese Medicine (TCM) Regulating Metabolic Diseases Key Laboratory of Sichuan Province, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 5Sichuan Provincial Engineering Research Center of Innovative Re-development of Famous Classical Formulas, Tianfu Traditional Chinese Medicine (TCM) Innovation Harbour, Chengdu University of Traditional Chinese Medicine, Chengdu, China
Colon cancer persists as a major global health burden due to therapy resistance and metastasis, with tumor-associated macrophages (TAMs) in the microenvironment driving progression through immune evasion and angiogenesis. This review highlights plant-derived therapeutics targeting TAMs to disrupt protumor signaling. Key phytochemicals (e.g., Curcumin, Cucurbitacin B, Astragaloside IV) suppress M2 polarization via NF-κB/STAT3 inhibition, block VEGF/HIF-1α-mediated angiogenesis, and enhance antitumor immunity by downregulating PD-L1. Cannabidiol, Hydroxygenkwanin regulate TAM metabolism. Dietary agents like sulforaphane and β-glucans modulate TAM-gut microbiome crosstalk. Nanoparticle-encapsulated phytochemicals enhance TAM-targeted delivery, while clinical translation requires standardized phytopreparations and biomarker-guided trials. We propose integrating validated botanical adjuvants (e.g., Fucoidan for TLR4 inhibition, dihydroisotanshinone I for CCL2 suppression) with immunotherapies to remodel immunosuppressive niches. Phytotherapy offers a multifaceted strategy to overcome TAM-driven therapeutic barriers in colon cancer, emphasizing plant-based precision medicine to augment conventional treatments.
1 Introduction
Colon cancer is the fourth most lethal malignancy worldwide. It is estimated that there will be 2 million new cases of colorectal cancer globally in 2020 By 2040, it is projected to reach 3.2 million new cases, with the greatest relative increase occurring in countries in transition and in younger age groups, resulting in a significant burden of disease (1). Despite efforts to lower the screening age and implement early interventions in certain countries, the high fatality rate of colon cancer remains a daunting challenge.
Currently, treatments for colon cancer include surgery, radiotherapy, chemotherapy, immunotherapy, and targeted therapy, however, the efficacy is limited and there are many adverse effects (2, 3). For instance, although chemotherapeutic drugs like 5-Fluorouracil (5-FU) demonstrate evident anticancer efficacy, their detrimental side effects including neurological symptoms, bone marrow suppression, and gastrointestinal reactions intensify patients’ discomfort. Moreover, the development of drug resistance has further compounded the constraints of their clinical utilization (4). Immunotherapy has shown unique advantages in current cancer therapies, especially immune checkpoint inhibitors. The US Food and Drug Administration (FDA) approved anti-PD-1 (Pembrolizumab and Nivolumab) as monotherapy or in combination with cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) inhibitor (Ipilimumab) for the treatment of colon cancer with high microsatellite instability (MSI-H) or mismatch repair deficiency (DMMR), unfortunately, only 5-15% of colon cancer patients benefit (5, 6). New drug development against colon cancer is challenging given the limited efficacy of current treatments and the inevitable off-target effect (7).
Natural products are widely sourced and structurally diverse, with excellent pharmacological activity, demonstrating significant advantages in the treatment of colorectal cancer. Several synthetic derivatives derived from natural products have been used in clinical anti-cancer treatments, such as the semi-synthetic water-soluble camptothecin derivative, irinotecan (8). Many botanical active compounds have shown anti-colon cancer activity, including improving the tumor inflammatory microenvironment, inhibiting tumor cell proliferation, migration, and angiogenesis, as well as regulating the gut microbiota (9–11).
It is known that tumor microenvironment (TME) is critical for cancer progression, of which immune cell subsets are key components. Macrophages, as important members of the immune system, are involved in inflammatory responses and immune regulation, while dynamically changing in response to various signals in TME (12, 13). Studies have shown that in mouse models of colon cancer, macrophages may lose their ability to phagocytose tumor cells and even be used by tumor cells to promote their growth and proliferation (14, 15). Targeted regulation of the activity of macrophages, promotion of its phagocytosis of tumor cells and immunomodulatory function has become a new research hotspot in current cancer therapy. Moreover, because of the stability of the genome of macrophages, they are relatively less likely to develop drug resistance compared to tumor cells (16). Existing reports have found that natural compounds such as Astragaloside, Curcumin can exert anti-colorectal cancer effects by modulating macrophage activity (17, 18). Further investigation into the immune regulatory roles of phytochemicals will help provide more effective treatment options for colon cancer patients. Therefore, this review summarizes the mechanisms by which macrophages are involved in the development of colon cancer and the potential strategies of targeting macrophages with natural products for the treatment of colon cancer.
2 Overview of macrophages
2.1 Basic biological characteristics of macrophages
Macrophages are innate immune cells of bone marrow and embryonic origin that phagocytose foreign substances or their own components and present them to T cells to activate adaptive immune responses. In addition, macrophages also participate in tissue homeostasis, repair and remodeling by expressing a variety of receptors, cytokines, chemokines, and protein hydrolases (19).
Depending on the tissue-specific microenvironmental signals macrophages acquire different phenotypes, mainly including M1 phenotype (classical activation) and M2 phenotype (alternative activation) (20, 21). M1 macrophages can be activated by bacterial lipopolysaccharide (LPS) and type 1 helper T (Th1) cytokines such as interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), releasing pro-inflammatory cytokines e.g., interleukin(IL)-1β, IL-6, IL-12, and TNF-α, as well as chemokines e.g., C-X-C Motif Chemokine Ligand (CXCL) 9 and CXCL10, and it is capable of presenting antigens via MHC class II molecules (20, 22). M1 macrophage surface markers mainly include CD80, CD86 and CD16/32. M1 macrophages are often considered to have proinflammatory, antitumor, and adaptive immune activation capabilities (23, 24).
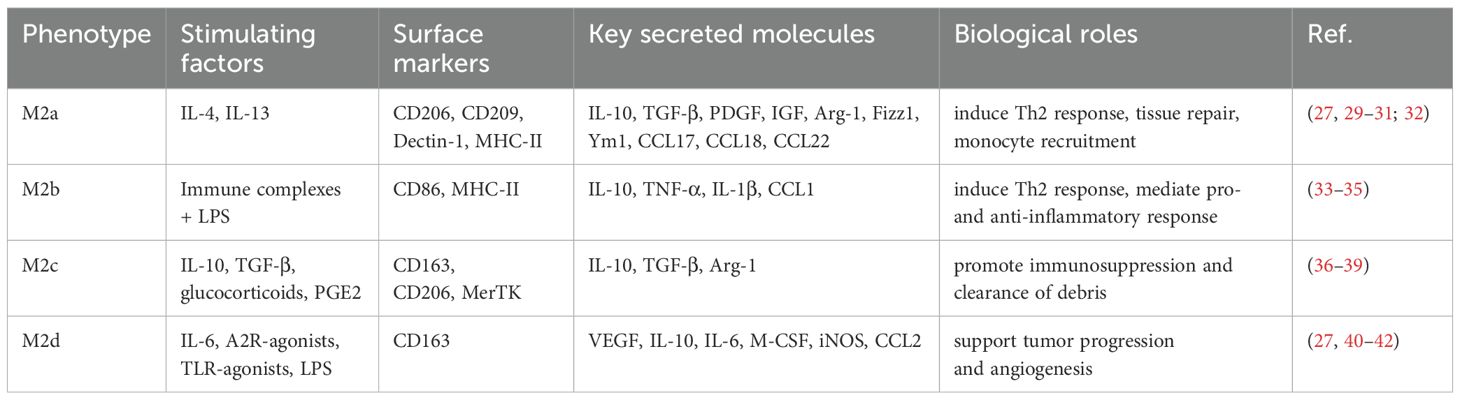
M2 macrophages can be activated by Th2-type cytokines IL-4 and IL-13, producing anti-inflammatory factors such as IL-10 and Transforming growth factor beta (TGF-β), as well as chemokines e.g., C-C Motif Chemokine Ligand (CCL)1, CCL17, CCL18, CCL22, and CCL24, with surface markers CD163, CD206, and CD209 (25). M2 macrophages have the function of promoting the resolution of inflammation and participate in tissue repair through angiogenesis at the later stages of inflammation (26–28). Polarized M2 macrophages are divided into M2a, M2b, M2c and M2d subpopulations. The characteristics of the different subtypes of M2 macrophages are listed in Table 1. M2a and M2b types are involved in immune regulation by promoting the Th2 cell response, while the M2c type is associated with immune response suppression and tissue remodeling (43). The M2d type is activated mainly by tumor cells, tumor-associated factors, or chemotherapeutic agents, and is sometimes referred to as TAMs. This type of macrophage has immunosuppressive and tumor-promoting functions (44, 45) and specifically expresses IL-10. and vascular endothelial growth factor (VEGF) to participate in tumor progression and angiogenesis (44, 46) (Figure 1A).
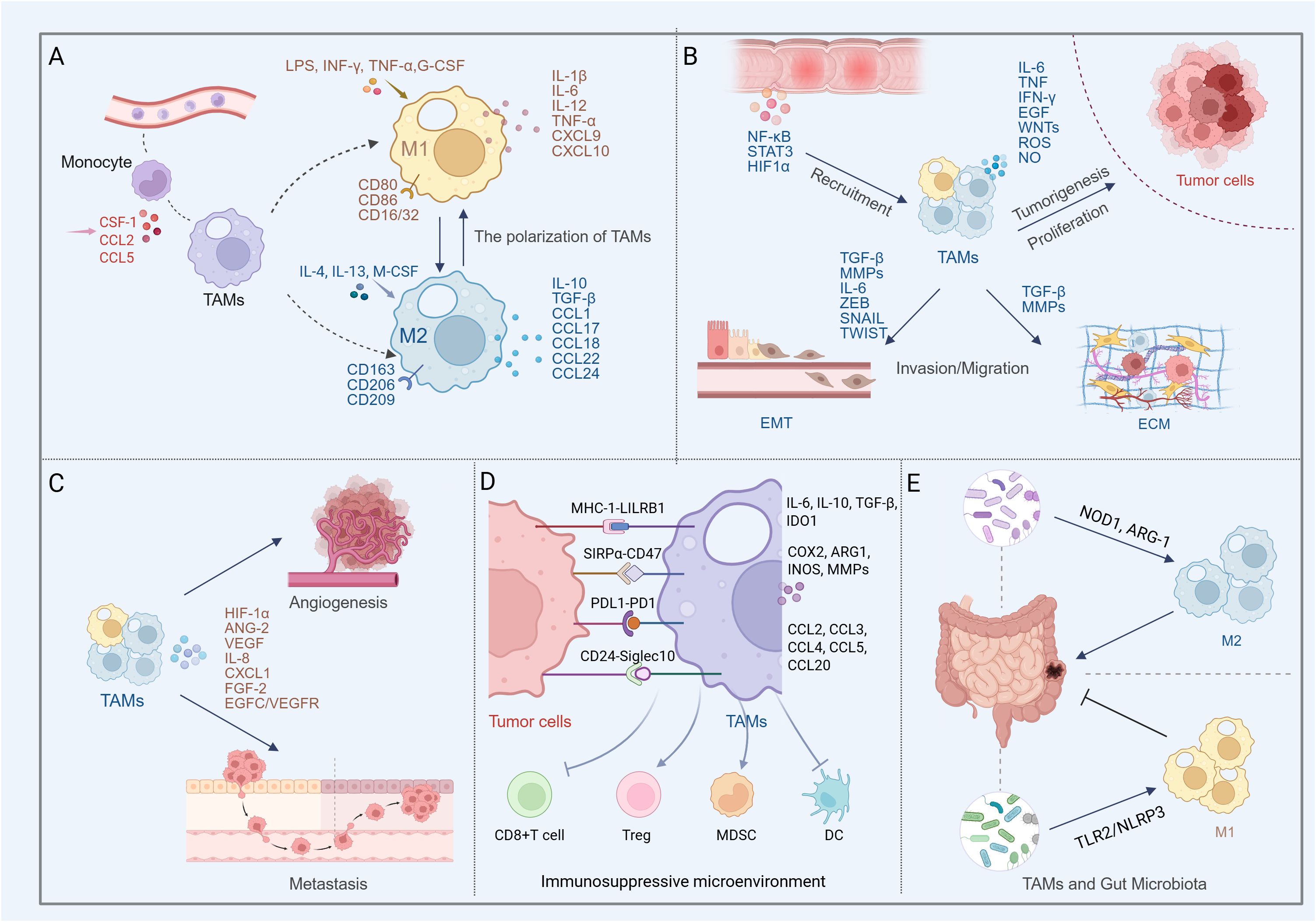
Figure 1. Multifaceted roles of TAMs in colon cancer progression and microenvironment regulation (Created in BioRender. qingman, H. (2025) https://BioRender.com/t5uzu98). (A) Peripheral monocytes are recruited by chemokines and differentiate into TAMs. Depending on external stimuli, TAMs polarize into the M1 phenotype, which releases pro-inflammatory factors with anti-tumor and immune-activating functions, or the M2 phenotype, which secretes anti-inflammatory mediators that promote tumor growth and angiogenesis. (B) In colon cancer, TAMs predominantly exhibit the M2 phenotype. The TAMs contribute to shaping the inflammatory milieu, promoting EMT, remodeling the ECM, and secreting cytokines, chemokines, and proteases that enhance tumor cell proliferation, invasion, and migration. (C) TAM-mediated angiogenesis and metastasis in colon cancer. (D) TAMs shape an immunosuppressive microenvironment by secreting inhibitory factors, recruiting Tregs and MDSCs, and suppressing the functions of cytotoxic T cells and dendritic cells. Meanwhile, colon cancer cells evade macrophage-mediated clearance through phagocytosis checkpoints such as CD47-SIRPα, CD24-Siglec-10, MHC-I-LILRB1, and PD1-PD-L1. (E) Gut microbiota modulates TAM phenotypes, exerting dual roles in both promoting and suppressing colon cancer progression.
2.2 Polarization of tumor-associated macrophages
TME serves as the internal environment in which tumor cells are produced and live and regulates tumor initiation and progression. It consists of non-cancerous cells (a variety of immune cells, cancer-associated fibroblasts, endothelial cells), as well as non-cellular components (cytokines, chemokines, proteins, extracellular vesicles, etc.) (47), and the network formed by these elements builds the tumor-promoting environment (48, 49). Monocytes in the peripheral blood are recruited into the highly structured TME and differentiate into TAMs under the influence of chemokines secreted by tumors, such as colony stimulating factor 1 (CSF-1), CCL2, and CCL5 (50, 51). Significant macrophage accumulation has been found in both tumor mouse models and tumor patient tissue samples (46, 52).
TAMs polarization is usually influenced by complex environmental factors in the TME, such as hypoxia, low pH and variable extracellular matrix (ECM) composition (53, 54). In fact, in the early stage of tumor, the immune system promotes macrophages and T cells to clear tumor cells to play anti-tumor and pro-immune response roles (55), and the majority of infiltrating macrophages at this stage are of the M1 phenotype and secrete pro-inflammatory factors (56). For example, granulocyte colony-stimulating factor (G-CSF) polarizes macrophages to a pro-inflammatory phenotype by promoting IL-10 production and reducing IL-12 in colon cancer TME (57). However, as the tumor progresses and the tumor cells continue to interact with the TME, most TAMs are “educated” to become “bad” macrophages that promote tumor growth and metastasis, and then the TAMs take on a predominantly M2 phenotype, creating an immunosuppressive environment and mediating the Th2 immune response (58). Although the M1/M2 polarization model offers a foundational framework for classifying macrophages, it falls short in capturing the phenotypic diversity and plasticity of TAMs. In recent years, researchers have employed surface markers such as F4/80+CD68+ and F4/80+CD206+ to delineate distinct TAM subsets (59). Complementing this approach, clustering analyses derived from single-cell RNA sequencing have identified four novel TAM subpopulations across various solid tumors: FCN1+, SPP1+, C1Q+, and CCL18+ macrophages. (60, 61). These advancements contribute to a more nuanced understanding of TAM lineage hierarchies and their functional heterogeneity within the tumor microenvironment. TAMs as one of the abundantly infiltrating immune cells in TME, affect tumor angiogenesis and lymphangiogenesis, tumor cell immune escape, immune regulation, and tumor invasion and metastasis (50, 62) (Figure 1A).
3 Core roles of TAMs in colon cancer progression
As described in the previous section, TAMs play an important role in the development of tumors. Current studies consider TAMs to be a diverse macrophage population with characteristics of both M1 and M2 subpopulations, rather than being categorized solely by M1/M2 phenotype. In different tumors, the plasticity of macrophages may enable them to play different roles. Several studies have confirmed that macrophage infiltration in the early stage of tumor invasion has a positive impact on the prognosis of colon cancer (63, 64). However, many studies now support the opposite view that TAMs promote malignant progression of colon cancer and play an immunosuppressive role in the TME of colon cancer, as we will describe below.
3.1 Promotion of tumor proliferation, invasion, and epithelial-mesenchymal transition
The inflammatory micro-environment involved and mediated by TAMs is closely related to the occurrence and development of colon cancer (65). Activation of cytokines and chemokines produced by chronic inflammation of tissues, as well as transcription factors such as nuclear factor kappa-B (NF-κB), signal transducer and activator of transcription 3 (STAT3), hypoxia-inducible factor 1α (HIF1α), promotes macrophage recruitment and initiates macrophage host responses (66–68).Macrophages subsequently produce inflammatory mediators (IL-6, TNF, IFN-γ), growth factors such as epidermal growth factor (EGF) and WNTs, proteases, reactive oxygen species (ROS), as well as nitric oxide (NO) that may activate proto-oncogenes to promote tissue carcinogenesis (69–71). Not only that, these substances produced by TAMs can also promote colon cancer growth. Studies have found that CCL2, IL-1α and IL-6 secreted by TAMs can promote the proliferation of colon cancer cells (72), and the release of the matrix metalloproteinase (MMP) 1 accelerates the transition of the tumor cell cycle from the G0/G1 phase to the S phase and G2/M phase (73).CSF1 is an important cytokine involved in macrophage proliferation and differentiation, and CSF1 produced by colon cancer cells contributes to the recruitment and infiltration of TAMs. In turn, IL-8 produced by TAMs can activate the PKC pathway on which CSF1 production depends (74).
Epithelial-mesenchymal transition (EMT) is an important development process that is activated during tumor cell migration and invasion (75). Activation of EMT prompts colon tumor cells to lose cell polarity and intercellular adhesion, and to acquire a mesenchymal appearance and greater infiltration and migration capabilities (76). In TME, several EMT-associated transcription factors (ZEB, SNAIL, and TWIST) synergize with signaling pathways in tumor cells (77–79) to induce EMT in response to stimulation by TGF-β, WNT, NOTCH, hypoxia (80–82), etc. TAMs have been demonstrated to have a key role in EMT in colon cancer. M2 macrophages overexpress MMP2 and MMP9, which can induce EMT (83). TGF-β is an important factor in EMT (84). M2 macrophages secrete a large amount of TGF-β, which promotes the expression of mesenchymal marker proteins (Vimentin) and reduces the expression of epithelial marker proteins (E-cadherin) during EMT in colon cancer cells (85). In addition, Wei et al. reported that TAM-derived IL-6 induced the EMT program by regulating the JAK2/STAT3/miR-506-3p/FoxQ1 axis to promote colon cancer cell invasion and migration (86). In colorectal cancer, the number of infiltrating TAMs is positively correlated with the expression of the activating transcription factor SANIL in cancer cells (76). TAMs also promote tumor invasion by up-regulating the expression of S100A8 and S100A9 in cancer cells (87).
Structural support for tumor development is provided by the ECM, including its main components collagen, glycoproteins and proteoglycans (88–90). Afik et al. found that in an orthotopic colorectal cancer model, TAMs promote the deposition, cross-linking, and linearization of collagen fibers to remodel tumor ECM composition and structure and promote tumor development (91).
Colon cancer cells can also activate matrix degradation-related signals to induce TAMs to produce tissue proteases, MMPs and serine proteases, etc., to promote ECM degradation and accelerate the invasion and migration of colon cancer cells (76, 88) (Figure 1B).
3.2 Mediation of angiogenesis and metastasis
Tumor growth and metastasis require increased intratumoral blood supply, which is usually triggered by tumor hypoxia (92). TAMs infiltrating the TME of colon tumors express HIF1α as an “angiogenic switch”, and respond to hypoxia signals by producing pro-angiogenic cytokines and growth factors, such as Angiopoietin-2 (Ang-2), VEGF, IL-8, CXCL1, and Fibroblast growth factor 2 (FGF-2), to induce endothelial cell recruitment, proliferation, and maturation, forming new blood vessels to promote tumor growth (67, 93, 94). GPR35 on macrophages in colon cancer promotes new angiogenesis and increases MMPs activity through Na/K-ATPase-dependent ion pumps and Src activation (94). Additionally, multiple studies have shown that the number of TAMs in colon cancer correlates with vascular density and the number of lymphatic vessels (95–97). Macrophages on blood vessels emit attractive signals that assist colon cancer cells in migrating along collagen fibers toward the vessels, enabling their escape into the bloodstream. TAMs can also activate the EGFC/VEGFR3 axis, which supports primary colorectal cancer cells growth and metastasis by shaping the lymphatic vessels and providing a pathway for tumor cells to invade the lymphatic system (98) (Figure 1C).
3.3 Establishment of immunosuppressive microenvironment
During colon cancer progression, the body’s anti-tumor immunity is suppressed, and tumor cells effectively evade anti-tumor immunity by deriving a tumor-immunosuppressive microenvironment, which involves inducing deletion or functional impairment of tumor-reactive T-cells (99) and recruiting immunosuppressive cell populations such as regulatory T-cells (Tregs) and myeloid-derived suppressor cells (MDSCs) (100). TAMs participate in and regulate the tumor immunosuppressive microenvironment by secreting cytokines and altering their phenotype (predominantly M2 macrophages) (101).
TAMs exert immunosuppressive functions through the secretion of inhibitory factors, such as IL-10, TGF-β, and Indoleamine 2,3-Dioxygenase 1 (IDO1) (68, 102), some enzymes, such as COX2, Arginase 1 (ARG1), Inducible nitric oxide synthase (INOS), and MMPs (101), as well as the release of chemokines (e.g., CCL2, CCL3, CCL4, CCL5, and CCL20) (68, 103, 104). The release of these factors not only directly inhibits the activity of T cells (105), but also promotes the recruitment of Tregs and MDSCs in the TME. For example, TAMs promote the aggregation of CCR6+ Treg cells by secreting CCL20 (106), and secreted CCL5 inhibits the killing of HT29 cells by T cells by stabilizing PD-L1, which in turn promotes immune escape (107). In tissue samples from colon cancer patients, the number of Treg cells and macrophages was significantly higher in cancerous tissues than in precancerous tissues (108). The infiltration of a large number of M2 macrophages is associated with poor prognosis, while the infiltration of CD8+ T cells is associated with improved patient survival (109). It was found that cytotoxic T cell responses could be enhanced to prevent colon cancer metastasis by inhibiting TGF-β produced by M2 macrophages (110). In addition, infiltration of high-density CXCR6+ TAMs promote the overexpression of IL-6, which would inhibit the activation of CD8+ T cells, thus contributing to immunosuppression (111). Ding et al. demonstrated that targeted inhibition of MDSCs could improve the immune microenvironment of colon cancer, not only promoting the maturation of dendritic cells and tumor infiltration of CD8+ T cells, but also reducing the number of Tregs and M2 macrophages (112) (Figure 1D).
3.4 Phagocytosis checkpoints and tumor immune evasion
Macrophages, as “professional” phagocytes in the innate immune system, play the role of scavengers. However, to evade clearance, tumor cells usually overexpress anti-phagocytic membrane proteins with “don’t eat me” signals, such as CD47, CD24, MHC-I, and PD-L1. These antiphagocytic proteins may interact with receptors on macrophages (e.g., SIRPα, Siglec-10, LILRB1, and PD-1) to evade phagocytosis. The anti-phagocytic signals generated by these binding are known as “Phagocytosis checkpoints”. Targeting phagocytosis checkpoints such as CD47-SIRPα, CD24-Siglec-10, MHC-LILRB1, PD1-PDL1 and thereby modulating immune escape in colon cancer cells has also been demonstrated.
Studies have found that using blocking antibodies targeting CD47 overexpressed on colon cancer cells and SIRPα on TAMs can inhibit the migration of colon cancer cells (113–115). In addition, phagocytic activity of macrophages was enhanced after anti-SIRPα antibody treatment (116). Interestingly, under hypoxic conditions, TAMs showed decreased SIRPα expression accompanied by increased phagocytic activity, whereas colon cancer cells showed elevated expression of CD47 and exhibited greater invasiveness (117). Therefore, it can be hypothesized that the regulation of the CD47-SIRPα axis in colon cancer may be affected by hypoxia.
CD24 serves as a critical “don’t eat me” signal molecule expressed by tumor cells. Several studies have indicated that its overexpression in colorectal cancer cells is associated with early-stage tumor metastasis (118). In colon cancer xenograft mouse models, treatment with anti-CD24 antibodies effectively suppressed the tumor growth (119, 120). Interestingly, Nersisyan et al. reported that reduced CD24 expression or gene loss in colon cancer patients was linked to shorter overall survival (121). These findings suggest that CD24 may function as a stage-specific driver during colorectal tumorigenesis—upregulated in early disease and potentially regulated later by shifts in immune cell activity. Zhao et al. further found a negative correlation between CD24 expression and multiple immune-related pathways, including TNF-α, NF-κB, and IFN-γ signaling (122). Siglec-10 has also been identified as an early driver gene in colorectal malignancies, with mutations observed in both multiple colonic adenomas and colorectal tumors (123). Single-cell RNA sequencing data revealed an enrichment of FOLR2+ LYVE1+ macrophages in KRAS/TP53 double-mutant colon cancer, where tumor cells appear to engage in immunosuppressive crosstalk with macrophages via the CD24–Siglec-10 axis. (124). Blocking the CD24–Siglec-10 anti-phagocytic axis effectively activates macrophage-mediated phagocytosis in the MC38 colon cancer model (125).
In colorectal cancer DLD1 cells, MHC-I expression suppresses macrophage-mediated phagocytosis and promotes immune evasion, primarily through its interaction with LILRB1 on TAMs. In vivo studies have shown that CRISPR-mediated knockout of MHC-I molecules in tumor cells suppresses tumor growth in a macrophage-dependent manner (126). However, in tumor tissues from patients with colon cancer liver metastases, high expression of MHC-I (β2m) on tumor cells has been associated with improved overall survival and increased T cell infiltration (126). The dual role of MHC-I in tumor immune evasion may stem from its capacity to engage both innate and adaptive immune responses. When functioning as an antigen-presenting molecule to cytotoxic T lymphocytes, MHC-I may no longer interact effectively with LILRB1. Additionally, some studies have reported that reduced MHC expression on tumor cell surfaces impairs antigen presentation, which may, in turn, enhance macrophage-mediated phagocytosis (127).
PD-1/PD-L1 inhibitors have been shown to effectively reverse T cell immune tolerance. In addition to T cells, PD-1 is also expressed on other immune cells, including macrophages, B cells, NK cells, and dendritic cells. Notably, increasing attention has been directed toward the functional role of PD-1/PD-L1 signaling in TAMs (128). Studies have reported that colon cancers with MSI-H tend to exhibit elevated PD-L1 expression, and that PD-1 levels on TAMs increase as the disease progresses (14, 129). PD-1 expression on macrophages has been implicated in promoting polarization toward the immunosuppressive M2 phenotype. In murine models of colorectal cancer, PD-1 blockade has been shown to reduce the infiltration of M2 macrophages within the TME (130). The PD-1/PD-L1 axis is considered part of an anti-phagocytic signaling pathway. In both colon cancer patients and mouse models, high PD-1 expression on TAMs has been associated with reduced phagocytic activity against tumor cells. Disruption of this axis can induce M1 polarization of macrophages and promote CD8+ T cell activation, thereby enhancing antitumor immunity (14). (Figure 1D).
3.5 The metabolic plasticity of TAMs
In pro-inflammatory or anti-inflammatory TME, TAMs exhibit notable metabolic plasticity. They comprehensively reprogram their energy sources, metabolic pathways, and metabolites to adapt to tumor progression. In colon cancer, tumor cells undergoing rapid proliferation produce lactate through the Warburg effect. The resulting acidic environment facilitates TAM recruitment and induces their immunosuppressive functions.
Similar to tumor cells, M1 macrophages rely on aerobic glycolysis and the pentose phosphate pathway (PPP), with upregulation of HIF-1α-related signaling, promoting the production of lactate and succinate (131). In contrast, M2 macrophages exhibit lower glucose consumption and primarily depend on fatty acid oxidation (FAO) and oxidative phosphorylation (OXPHOS) for energy supply. They express high levels of CD36, facilitating fatty acid uptake and β-oxidation. Additionally, FAO-induced mitochondrial stress and elevated ROS levels contribute to M2 polarization (132). It has been shown that the STING agonist GB2 can induce metabolic reprogramming of TAMs in colorectal cancer by upregulating the glycolysis–ROS–HIF-1α axis and downregulating OXPHOS, thereby promoting M1 polarization and enhancing phagocytic activity (133).
Increased glucose uptake leads to the accumulation of lactate in the TME. Zhang et al. were the first to reveal the role of histone lysine lactylation in the modification of macrophages, demonstrating that the level of lysine lactylation is positively correlated with intracellular lactate concentration (54). In colon cancer, elevated lactate levels promote M2 polarization and the secretion of signaling molecules that facilitate tumor progression. (76, 133). For example, lactate in the tumor microenvironment epigenetically upregulates the expression of VSIG4 in macrophages, which subsequently activates the JAK2/STAT3 pathway, enhances fatty acid oxidation and PPAR-γ expression, and drives polarization toward an immunosuppressive M2 phenotype (134).
In terms of amino acid metabolism, M1 macrophages primarily utilize arginine metabolism by upregulating iNOS to produce citrulline and NO, thereby enhancing antimicrobial and antitumor activity. In contrast, M2 macrophages upregulate ARG1, converting arginine into ornithine and polyamines, which contribute to immunosuppression (16). Increased expression of ARG1 has been identified as a key factor in rapid tumor growth and progression (16). Additionally, M2 macrophages metabolize tryptophan via IDO1 to generate kynurenine, which suppresses T cell function and promotes immune tolerance. It has been shown that inhibiting ARG1 while activating iNOS can effectively increase the number of M1 macrophages and suppress colon tumor growth (135).
3.6 Exosomal crosstalk with colon cancer cells
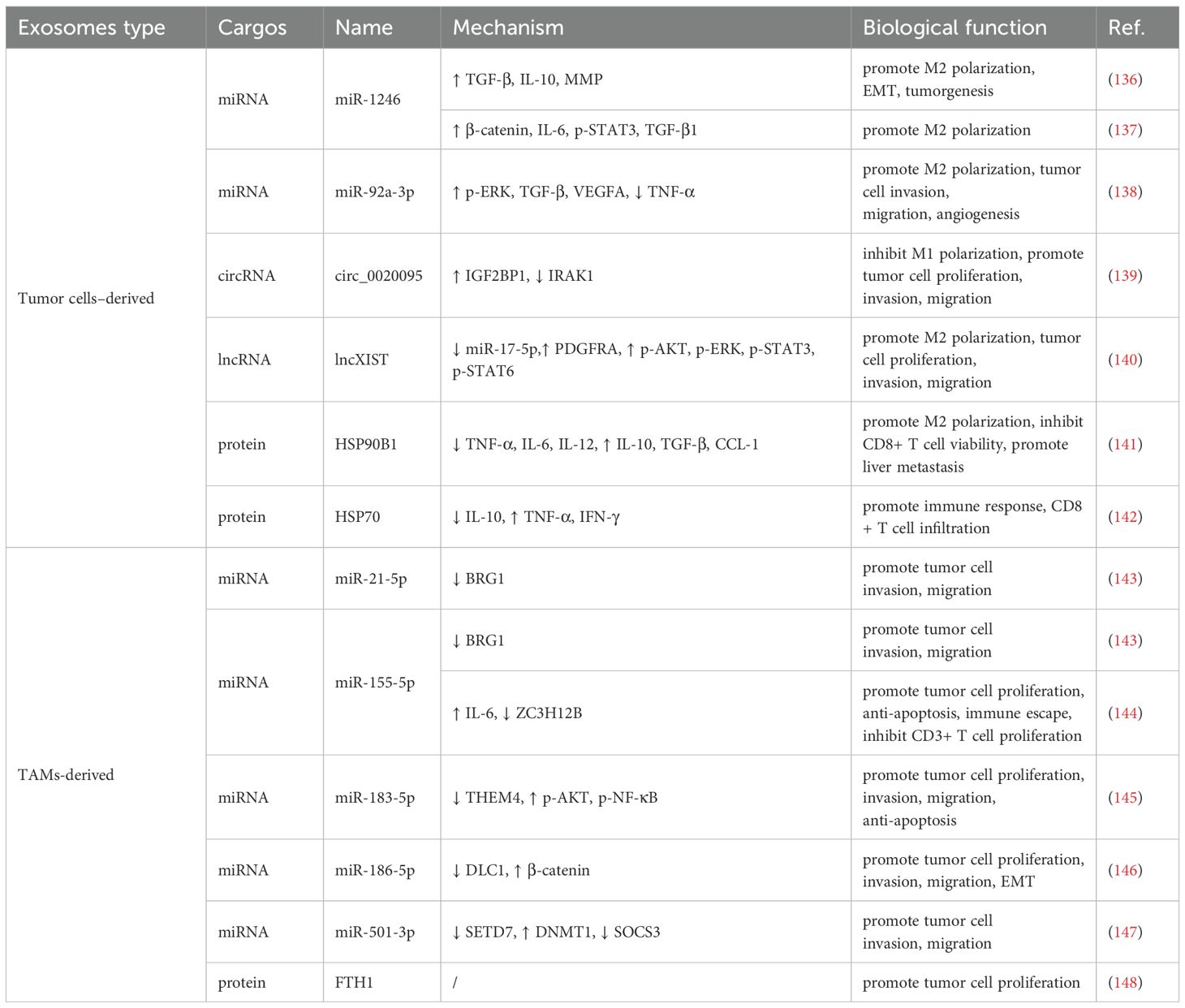
Autocrine and paracrine signaling mediated by cytokines and chemokines plays a pivotal role in the communication between tumor cells and TAMs. Both tumor cells and TAMs are capable of internalizing exosomes secreted by one another, thereby releasing their endosomal contents. This exchange contributes to the regulation of macrophage polarization and influences key tumor behaviors, including invasion, metastasis, and immune evasion (Table 2) (Figure 2).
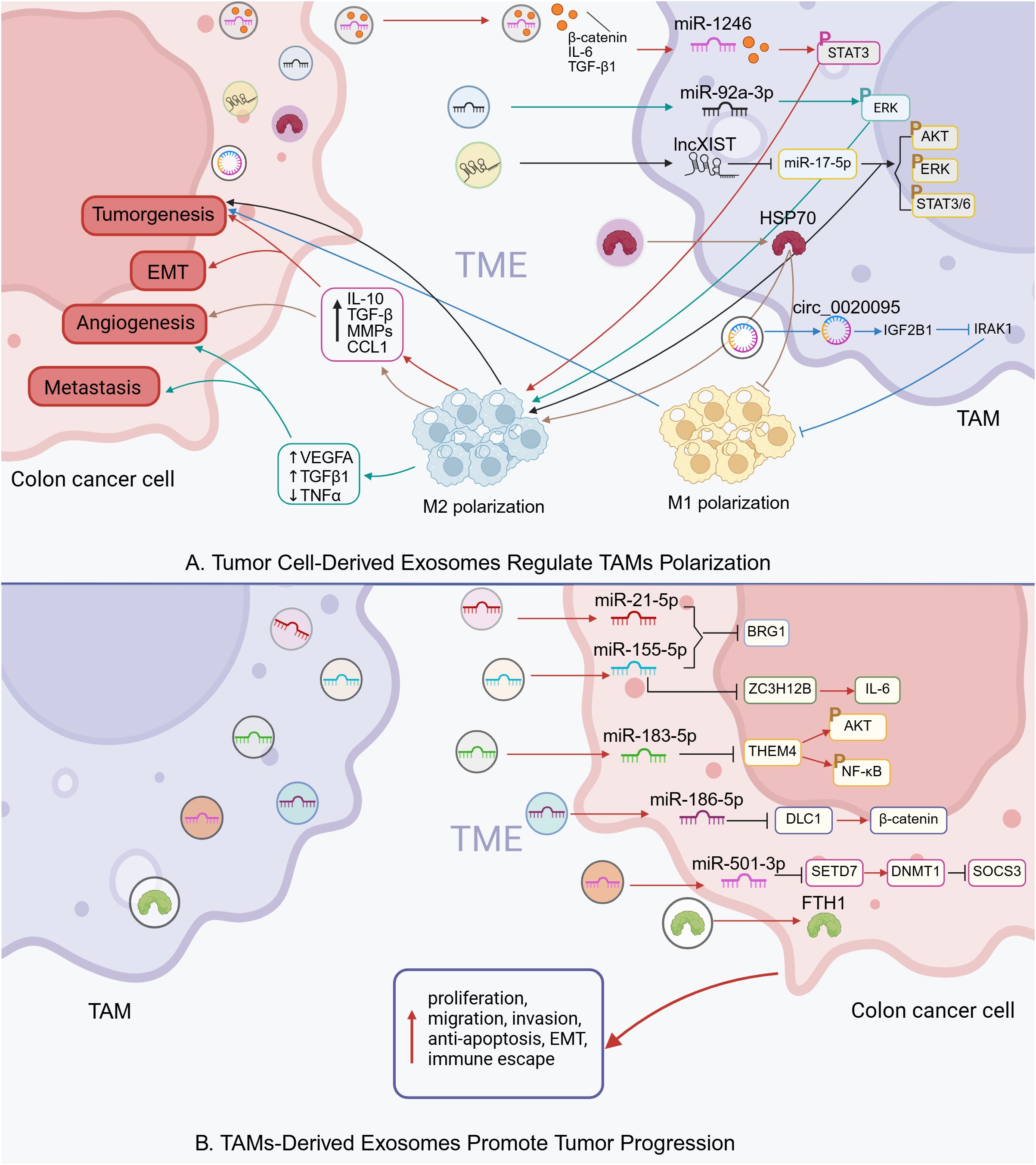
Figure 2. Intercellular communication via exosomes in colon cancer TME (Created in BioRender. qingman, H. (2025) https://BioRender.com/ijd8zht). (A) Tumor cell-derived exosomes regulate TAMs polarization. (B) TAMs-derived exosomes promote tumor progression.
In colon cancer, exosomes released by TAMs and colon cancer cells jointly contribute to the regulation of tumor immunity. Specifically, colon cancer cells can secrete exosomes containing various non-coding RNAs—including microRNAs (miRNAs), long non-coding RNAs, and circular RNAs, which influence TAM polarization. For instance, exosomal miR-1246 and miR-92a-3p derived from tumor cells have been shown to activate STAT3 and ERK signaling pathways in TAMs, promoting their polarization toward an immunosuppressive M2 phenotype (137, 149). Similarly, circ_0020095 facilitates tumor cell invasion and migration by downregulating IRAK1 expression and suppressing M1 polarization (139). In addition to RNA cargo, tumor-derived exosomes can also transport specific proteins that modulate the immune response. HSP90B1, delivered via exosomes from colorectal cancer cells, has been associated with poor prognosis in advanced colorectal cancer and contributes to liver metastasis by promoting the conversion of M1 to M2 macrophages (141). Notably, treatment targeting HSP70-positive exosomes produced by colorectal cancer cells has been reported to enhance antitumor immune responses, suggesting that TAMs possess functional plasticity (142).
On the other hand, TAMs can also influence tumor progression through the transfer of RNA and protein molecules encapsulated within exosomes. For example, M2 macrophages have been shown to promote colon cancer cell proliferation by delivering ferritin heavy chain (FTH1) protein via exosomes (148). Additionally, TAM-derived exosomes exhibit high levels of miR-21-5p and miR-155-5p, which target and suppress the expression of BRG1 in tumor cells, thereby enhancing their malignant properties and facilitating immune evasion (143).
3.7 Interaction with gut microbiota
Evidence suggests that chronic low-grade inflammation due to gut microbial dysbiosis as well as impaired intestinal barriers are important factors driving colon cancer development (150–152). Infiltrating bacterial products can construct an immunosuppressive TME by shaping TAMs (153). Fewer macrophages were found in the intestinal wall of germ-free mice compared to SPF mice (154, 155). Enrichment of Fusobacterium nucleatum, Bacteroides fragilis, Escherichia coli, and Enterococcus faecalis has been observed in the feces or tumor tissues of colorectal cancer patients (156–159). The establishment of chronic inflammation promotes colorectal cancer development. In APCmin/+ mice, Fusobacterium nucleatum facilitates the recruitment and infiltration of specific myeloid cell subsets into tumor tissue, producing NF-kB-related pro-inflammatory characteristics (160, 161). In a mouse model infected with Citrobacter rodentium, macrophage-derived ornithine decarboxylase promotes M1 macrophages activation, leading to increased mucosal inflammation in the colon (162). Gut microbes also promote colon carcinogenesis by modulating macrophage immunoreactivity. In Fusobacterium nucleatum-fed mouse models with intestinal tumors, M2-like macrophage accumulation has been observed within tumor tissues, showing significant suppressive activity against CD4+ T cells (160). Additionally, bacterial products such as muramyl peptides activate NOD1, maintaining ARG-1 overexpression and promoting colorectal tumor formation by modulating the immunosuppressive activity of MDSCs and TAMs (153). However, most current studies have focused on the crosstalk between tumorigenic bacteria and TAMs, and the role of some beneficial bacterial species is easily overlooked. For example, Akkermansia muciniphila, a probiotic with strong adhesion to the intestinal epithelial cell surface, can mediate M1 macrophages accumulation in a TLR2/NLRP3-dependent manner to inhibit colorectal tumorigenesis (163) (Figure 1E).
4 Therapeutic strategies targeting TAMs in colon cancer
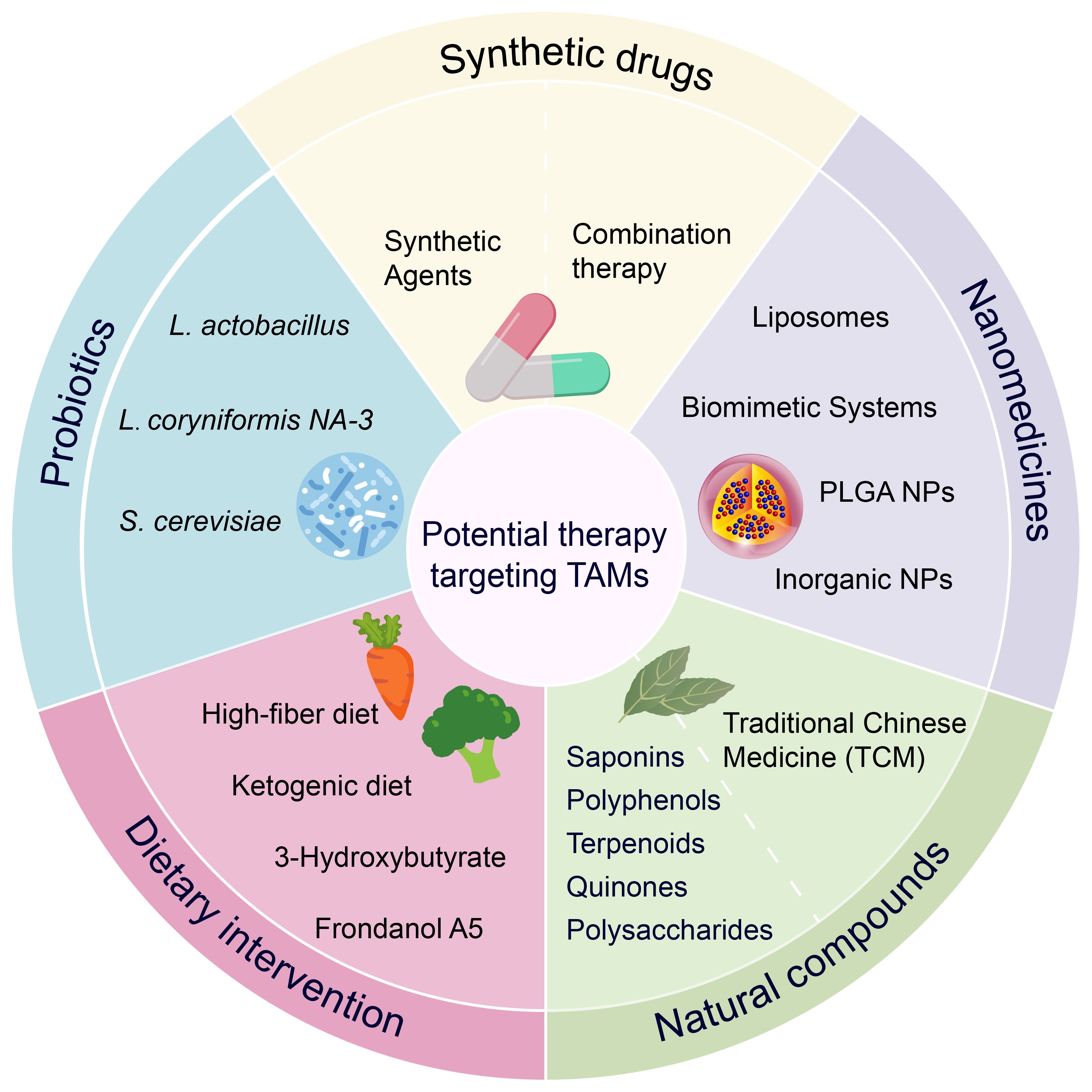
Historically, natural products have provided many active molecules with novel structures for new drug development. Many anticancer compounds derived from natural products or their derivatives, such as paclitaxel and vincristine, have played an important role in the clinical treatment of cancer. Recent preclinical studies have further confirmed that natural products can effectively prevent and treat colon cancer by targeting TAMs. We summarize various natural products, including monomeric compounds, synthetic formulations based on natural products, combination therapies of natural products with existing anti-cancer drugs, nanoparticle-based drugs, traditional Chinese medicine, and dietary interventions. The primary mechanisms by which these natural products target TAMs include: inhibition of the inflammatory tumor microenvironment, regulation of macrophage polarization (such as inducing M1 polarization and suppressing M2 polarization) (Figure 3), modulation of metabolism, enhancement of macrophage phagocytic function, and modulation of other immune cells through TAMs, thereby effectively inhibiting immunosuppressive TME. These aspects will be discussed in detail in the following sections.
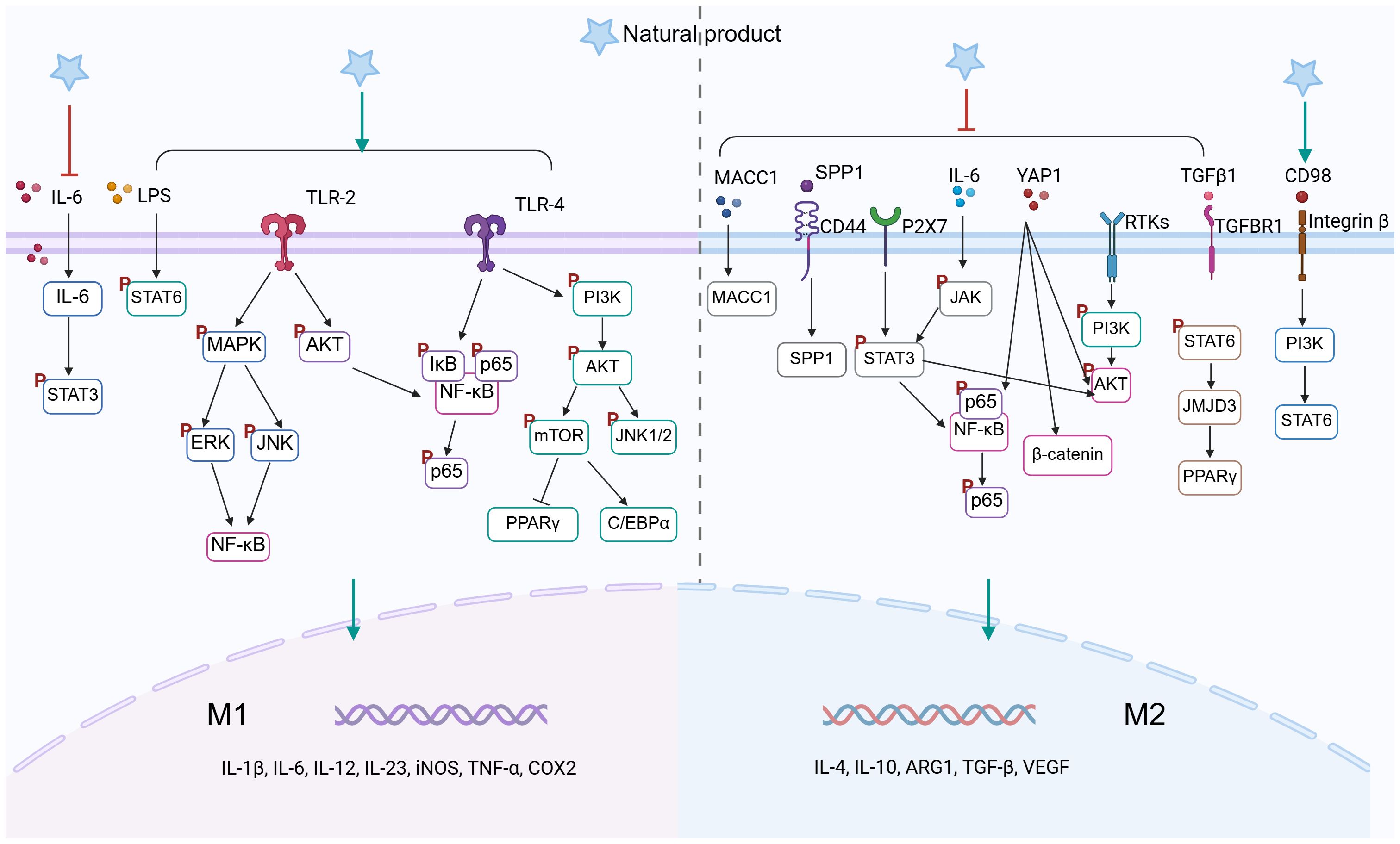
Figure 3. Natural product–mediated signaling pathways in TAMs polarization (Created in BioRender. qingman, H. (2025) https://BioRender.com/3g8lx62).
4.1 Botanical compounds and medicinal plants
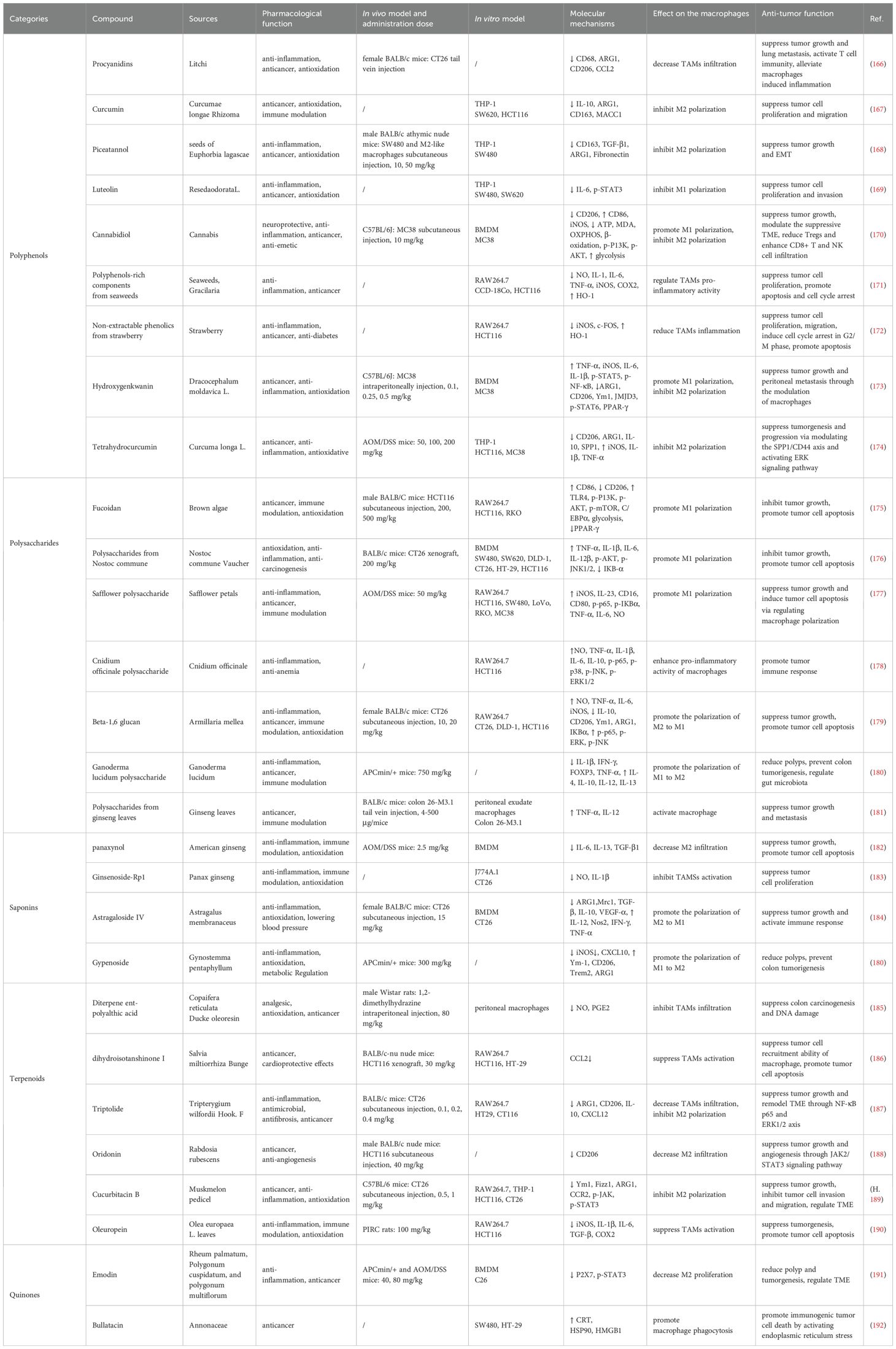
Studies have confirmed that botanical compounds exhibit significant pharmacological and biological activities in the treatment of colon cancer (164, 165). Among these, polysaccharides, polyphenols, saponins, quinones, and terpenoids have been found to exert anti-tumor effects by modulating TAMs. The specific mechanisms of action are summarized in the table below (Table 3).
4.1.1 Polyphenols: remodeling immune microenvironment and inhibiting inflammation
Polyphenols are the most common plant-based bioactive constituents of the diet and are widely found in fruits, vegetables, cereals, coffee and green tea. Numerous experimental studies have demonstrated that polyphenols (e.g., procyanidins (166), curcumin (167), piceatannol (168), luteolin (169)) have significant anti-inflammatory, anti-tumor, pro-apoptotic, and immunomodulatory effects in colon cancer. Mechanistic studies indicate that these effects are primarily achieved through the regulation of TAM polarization, downregulation of inflammatory signaling, enhancement of immune responses, modulation of macrophage metabolism, and regulation of the gut microbiota. For example, single-cell sequencing analysis has shown that cannabidiol, a non-psychoactive compound from cannabis, promotes TAM glycolysis while inhibiting FAO and oxidative phosphorylation OXPHOS. It also regulates the PI3K/AKT pathway, promoting M1 polarization of TAMs to exert anti-colon cancer effects (170). Meanwhile, some polyphenol-rich extracts/non-extractable polyphenolic substances are also of interest. For example, polyphenol-rich components from edible seaweeds (171) and non-extractable phenolic from strawberries (172) were able to inhibit the pro-inflammatory activity of LPS-stimulated macrophages, induce cell cycle arrest in HCT116 human colon cancer cells, and promote their apoptosis.
4.1.2 Polysaccharides: activating M1 polarization and modulating signaling pathways
Natural polysaccharides have a wide range of biological activities, with anti-tumor, antioxidant, liver protection, immunomodulation and other functions. Because of their good therapeutic effects and mild adverse reactions, they have received extensive attention in the field of biomedicine. Many natural polysaccharides, such as fucoidan, safflower polysaccharides, cnidium officinale polysaccharides, and ganoderma lucidum polysaccharide, are effective in preventing and controlling colon cancer by regulating the signaling pathway, influencing the macrophage phenotype and function, enhancing immune response, and maintaining intestinal homeostasis.
Polysaccharides from algae have been extensively studied, for example, fucoidan extracted from brown algae activates the TLR4-mediated PI3K/AKT/mTOR signaling axis, promoting M1 polarization and infiltration of TAMs, thereby altering the immunosuppressive TME (175). Polysaccharides from Nostoc commune Vaucher can inhibit colon cancer cell proliferation by activating NF-κB, AKT/JNK1/2 signaling pathway, upregulating the expression of inflammatory factors, and enhancing the phagocytic activity of TAMs (176). Studies have shown that safflower polysaccharide (108), Cnidium officinale polysaccharide (178) and Beta-1,6 glucan (179) can activate the NF-κB and MAPK signaling pathways in colon cancer, promote the activation of M1 macrophages in the TME to generate immune responses. Shin et al. found that treatment of colon cancer model mice with the polysaccharides from ginseng leaves exhibited antitumor activity, and it promoted the secretion of TNF-α and IL-12 from macrophages in peritoneal exudates (181). Adenomatous polyps are considered precancerous lesions of sporadic colon cancer, and Ganoderma lucidum polysaccharide were effective in improving the inflammatory intestinal barrier in APCmin/+ mice reducing the formation of adenomatous polyps by decreasing the infiltration of inflammatory TAMs (180).
4.1.3 Saponins and terpenoids: modulation TAMs recruitment and suppressing M2 polarization
Studies on the regulation of TAMs in colon cancer by saponin compounds are mainly focused on ginsenosides. Ginsenosides have a variety of pharmacological activities and biological effects, such as anti-inflammatory, antioxidant, immunomodulatory, anti-tumor, and pro-apoptotic. Panaxynol, isolated from ginsenosides, promotes tumor cell apoptosis by effectively inhibiting M2 polarization in colon cancer TME (182). Furthermore, ginsenoside-Rp1 inhibits phenotypic variation in CT26 colon cancer cells by suppressing LPS-stimulated macrophage activation (183). Astragaloside IV (184) and jiaogulan saponins (180) extracted from traditional Chinese medicine, were able to reduce the formation of intestinal polyps and tumors by modulating the ratio of pro-inflammatory to anti-inflammatory macrophages in the TME.
From the current reports, it has been found that terpenoids improve colon cancer outcomes by interfering with TAMs including 1) prevention of colon precancerous lesions, 2) inhibition of tumor cell recruitment, proliferation, migration, and angiogenesis, 3) promotion of apoptosis, and 4) activation of immune response. Copaifera reticulata Ducke oleoresin, widely used in Brazilian folk medicine, has wound healing-promoting and anti-inflammatory effects. Its main chemical component, diterpene ent-polyalthic acid, reduces DNA damage and preneoplastic lesions induced by the carcinogen, also reduces NO and PGE2 production in mouse TAMs, demonstrating the potential for preventing the development of colon cancer (185). Dihydroisotanshinone I, a bioactive compound in Salvia miltiorrhiza Bunge can hinder the recruitment of TAMs to colon cancer cells by inhibiting the secretion of CCL2 from TAMs (186). Jiang et al. found that tretinoin inhibited NF-κB and ERK1/2 signaling and reduced M2 macrophage polarization remodeling TME by downregulating CXCL12 expression (187). Oridonin has excellent anti-angiogenic effects in colon cancer, reduces M2 macrophage infiltration, and also improves the hypoxic environment (188).
4.1.4 Quinones: inducing immunogenic cell death
Quinones are widely distributed in nature and are categorized into four major subclasses, including benzoquinone, naphthoquinone, phenanthrenequinone and anthraquinone. There are limited reports on whether quinones can prevent colon cancer by affecting TAMs, which have only been reported in Bullatacin. Emodin is a naturally occurring anthraquinone compound isolated from herbs such as rhubarb, Tigen stick as well as Tuber Fleeceflower Root, and has a variety of biological activities including anti-inflammatory, antioxidant, and antitumor. Studies have confirmed the role of Emodin in regulating colonic TME, not only by decreasing the proportion of M2 macrophages, but also by reducing inflammatory activation through inhibition of the P2X7 receptor (191). Bullatacin, a natural quinone compound isolated from the plant Lycopersiconaceae, induces immunogenic cell death (ICD) in tumor cells. Fan et al. demonstrated that treatment with Bullatacin promotes the accumulation of the “eat me” signaling proteins, CRT and HSP90, on the surface of colorectal tumor cells. It triggers ICD by activating the endoplasmic reticulum stress (ERS) signaling pathway and promotes phagocytosis of tumor cells by macrophages (192).
4.2 Natural product–based synthetic agents
Natural compounds face limitations in clinical applications, such as unstable efficacy, poor solubility, and challenges in standardization. To overcome these issues, synthetic modifications can be employed to create novel drug formulations by knocking out or altering certain unstable structures of the compounds. We summarize the role and mechanisms of existing synthetic formulations based on natural products targeting TAMs in colon cancer (Table 4).
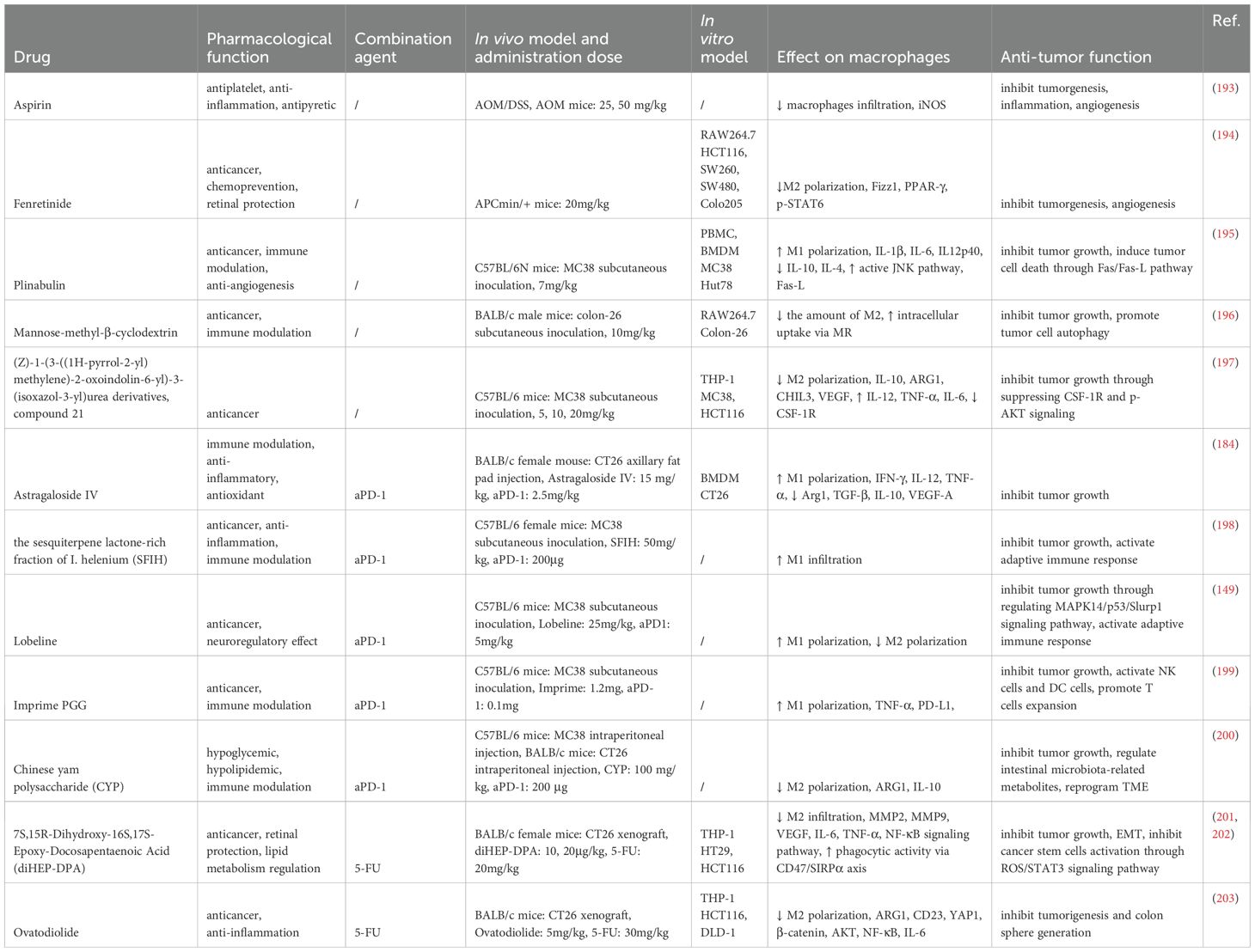
Table 4. Synthetic agents derived from natural products and combination strategies targeting TAMs in colon cancer.
Aspirin, derived from salicylic acid, is a classic non-steroidal anti-inflammatory drug. Long-term low-dose administration of aspirin (25 mg/kg daily in healthy mice, equivalent to 100 mg in human subjects) significantly reduces macrophage infiltration in colon tumors, shrinks tumor size, and decreases the formation of prostaglandin E2 (PGE2) in the colon (193).Modified mannose β-cyclodextrin (Man-MβCD) can be recognized by mannose receptors (MR) on TAMs, enhancing the cytotoxic activity of M2 macrophages while promoting tumor cell autophagy (196).Plinabulin, a microtubule inhibitor synthesized from a natural product isolated from marine Fusarium fungi through structural optimization, is undergoing global Phase III clinical trials for tumor treatment. In vivo studies have shown that Plinabulin (7 mg/kg) induces M1 polarization in macrophages through the JNK signaling pathway, effectively inhibiting tumor growth in MC38 colon cancer-bearing mice (195).The synthetic retinoid derivative fenretinide demonstrates advantages in chemoprevention, significantly reducing tumor occurrence in APCmin/+ mice by inhibiting STAT6 signaling and decreasing M2 macrophage-driven angiogenesis in tumor tissues (194).Blocking CSF-1/CSF-1R signaling to regulate TAM polarization is a promising therapeutic approach. A synthesized compound 21 from a series of (Z)-1-(3-((1H-pyrrol-2-yl)methylene)-2-oxoindolin-6-yl)-3-(isoxazol-3-yl)urea derivatives exhibits excellent CSF-1R inhibitory activity (IC50 = 2.1nM), promoting M1 macrophage infiltration and enhancing anti-tumor immune responses (197).
While synthetic formulations effectively improve the efficacy and stability of natural products, the high research and development costs, along with complex clinical trial processes, remain significant obstacles to their widespread application. Improving the production methods of synthetic formulations, such as utilizing advanced techniques like computer-aided drug design, can aid in the development of synthetic formulations based on natural products.
4.3 Combination therapy: immune checkpoint inhibitors and multitarget synergy
Currently, immune checkpoint inhibitors (ICIs, immune checkpoint inhibitors) are working in microsatellite instable/mismatch repair deficient patients that account for about 5-10% of CRC cases. Optimized combination therapy strategies could expand the use of anti-PD-1/PD-L1-based immunotherapy in colon cancer. Although current immunotherapies are mostly centered on T cells, more and more reports support that the effector function of innate immunity is also important for cancer treatment (Table 4).
Several studies have explored the synergistic effects of natural products combined with anti-PD-1 (aPD-1) immunotherapy in colon cancer, primarily through the inhibition of TAMs’ M2 polarization and the activation of T-cell immune responses. For example, Astragaloside IV (AS-IV) from Astragalus membranaceus, sesquiterpene lactones from Arctium lappa, and the natural Chinese yam polysaccharide (CYP) have been shown to shift TAMs from the immune-suppressive M2 phenotype to the anti-tumor M1 phenotype. When used in combination with aPD-1, these natural products enhance the tumor immune response, increase the infiltration of CD8+ T cells, and inhibit tumor growth (184, 198, 200). Additionally, CYP has been found to effectively regulate the disruption of gut microbiota and metabolic products associated with aPD-1 treatment (200).
Another promising strategy involves combining natural compounds with conventional chemotherapy agents such as 5-FU. While 5-FU is a cornerstone in the treatment of colon cancer, its efficacy is limited due to the development of chemoresistance. 7S,15R-Dihydroxy-16S,17S-Epoxy-Docosapentaenoic Acid (diHEP-DPA), a DHA derivative, has been shown to overcome 5-FU resistance when used in combination, inhibiting TAM infiltration, reducing the accumulation of cancer stem cells (CSCs), and suppressing EMT (201, 202). Similarly, Ovatodiolide enhances anti-tumor effects when combined with 5-FU by inhibiting the YAP1 oncogenic pathway and preventing M2 TAM polarization (203).
The combination of natural products with existing cancer therapies (such as ICIs and chemotherapy) offers multiple advantages, particularly in enhancing current therapeutic efficacy, overcoming resistance, and reducing side effects. However, current research has primarily focused on the combination of natural products with anti-PD-1 immunotherapy, with limited studies on the use of other immunotherapies in combination with natural products for colon cancer treatment. Future research should explore the synergistic effects of different immune checkpoint inhibitors with natural products to provide more treatment options for clinical practice.
4.4 Nanomedicine strategies involving natural products
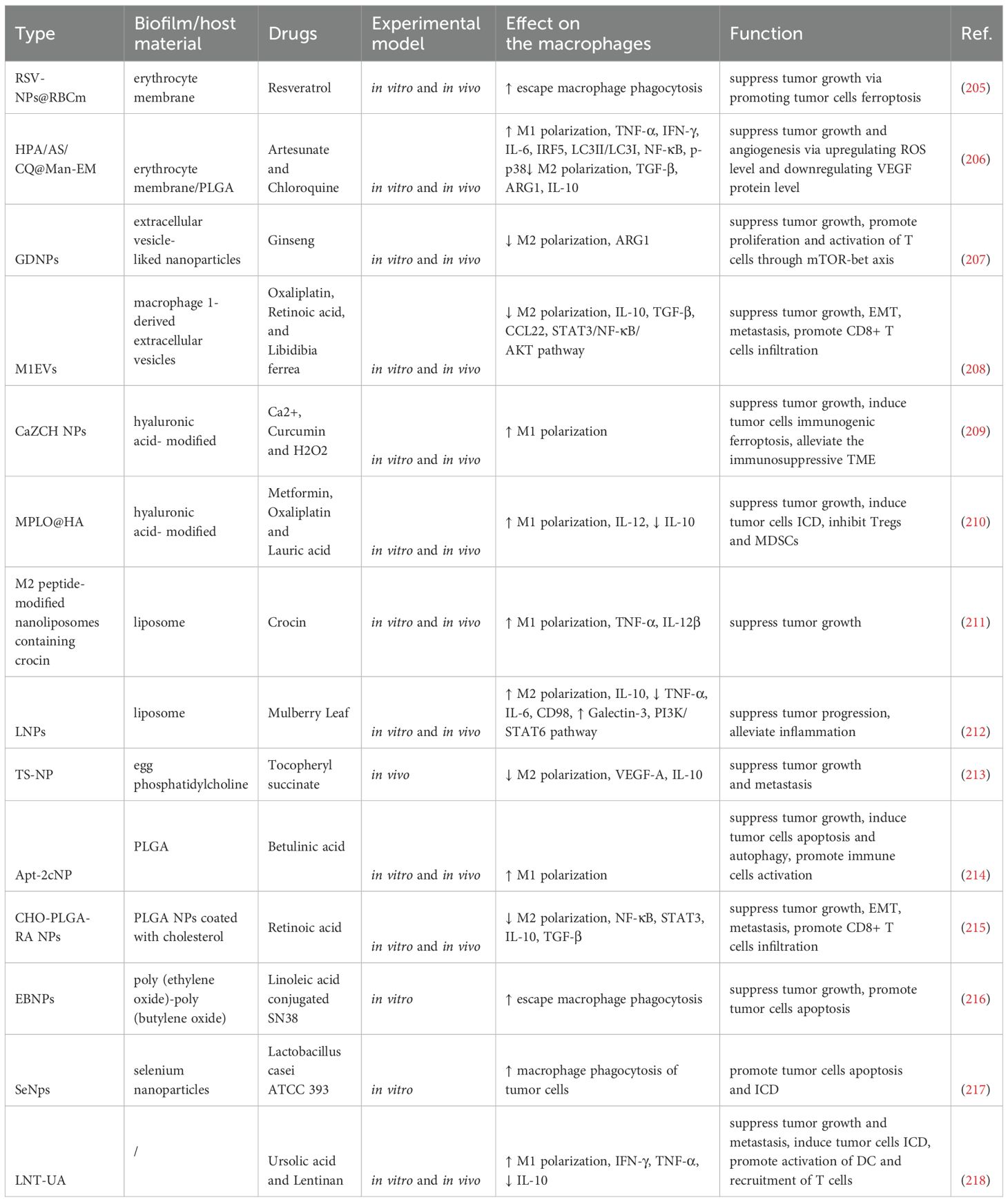
Nanomedicines have shown great potential in cancer treatment and have become a promising strategy to overcome the pharmacokinetic limitations of natural compounds. Despite substantial evidence supporting the superior antitumor effects of natural products, their clinical application remains limited due to issues such as poor solubility, low bioavailability, and insufficient systemic stability. The rapid development of functional nanomaterials has enabled nanomedicines to effectively address these challenges by facilitating accurate drug release, enhancing tumor penetration, and improving retention through the enhanced permeability and retention (EPR) effect, all while reducing toxicity and side effects (204). Common carriers include biomimetic delivery platforms (e.g., erythrocyte membranes, leukocyte membranes, stem cell membranes, and extracellular vesicles), liposomes, polymeric particles, and inorganic nanoparticles. Preclinical studies suggest that TAM-targeting nanomedicines exhibit good safety, enhanced stability, and improved anticancer performance in colon cancer treatment (Table 5).
4.4.1 Biomimetic delivery systems
Cell membrane-coated nanoparticles, which mimic the structure of cell membranes, can enhance biocompatibility and improve drug targeting. For example, erythrocyte membrane-coated nanoparticles (RSV-NPs@RBCm) effectively evade macrophage phagocytosis, extend the circulation time of drugs in the bloodstream, and improve tumor-targeted delivery through tumor-penetrating peptides (205). Extracellular vesicle-like nanoparticles derived from fresh ginseng can inhibit M2 polarization, modulating arginine metabolism to affect the mTOR-T-bet axis, thereby effectively alleviating T cell exhaustion (207). Furthermore, macrophage-derived extracellular vesicle nanoparticles possess excellent biocompatibility, enabling efficient drug loading and inhibition of colorectal cancer cell proliferation, migration, and resistance (208).
4.4.2 Liposomes
Liposomes, due to their excellent biocompatibility and biodegradability, are widely used as classic nanocarriers. Their structure consists of an outer lipid bilayer and an internal hydrophilic core. For example, saffron extract-loaded liposomes modified with M2 peptides specifically target M2 macrophages in the colorectal TME, inducing their polarization to the M1 phenotype, thereby exerting antitumor effects (211). Additionally, tocopheryl succinate succinate nanoparticles synthesized with egg phosphatidylcholine prevent peritoneal dissemination caused by colon cancer through intraperitoneal injection. This mechanism may involve the suppression of M2 macrophages, reducing the release of IL-10 and VEGF, and improving the TME (213).
4.4.3 Inorganic nanomaterials
Inorganic nanomaterials, such as metal nanoparticles, metal oxides, and carbon-based nanomaterials, are widely utilized in tumor therapy due to their biological stability and targeting capabilities. For instance, CaZCH NPs, which respond to the acidic conditions of the tumor microenvironment, release active substances that promote the infiltration of M1 macrophages and enhance antitumor immune responses by inducing ICD (209).
4.4.4 Polymeric nanomaterials
Poly(lactic-co-glycolic acid) (PLGA) is a biodegradable and controlled-release drug delivery system approved by the FDA for sustained drug formulations. Natural product-based formulations, such as Apt-2cNP and CHO-PLGA-RA NPs, utilize PLGA as a carrier to modulate TAM polarization within the colorectal TME, inhibiting the NF-κB/STAT3 signaling pathway associated with M2 polarization and reshaping the immunosuppressive microenvironment (214, 215). Moreover, EBNPs avoid macrophage phagocytosis, effectively promoting cancer cell uptake, and achieving targeted inhibition of colon cancer cells (216).
Studies show that macrophage-targeting nanomedicines based on natural products have significant advantages. However, challenges remain, including instability in distribution, inter-individual variations in the EPR effect, and off-target effects. Furthermore, the heterogeneity of TAMs (including different subtypes, such as resident vs. recruited, anti-inflammatory vs. pro-inflammatory) influences therapeutic outcomes. The pharmacokinetic interactions between nanomedicines, tumor cells, and macrophages require further investigation. Additionally, animal models cannot fully replicate the complexity of the human tumor microenvironment. Future research should focus on improving the selective delivery of nanoparticles, reducing off-target toxicity, and enhancing therapeutic outcomes through more accurate pharmacokinetic modeling. Furthermore, in-depth studies on TAM heterogeneity and strategies for targeting different TAM subtypes will contribute to advancing the clinical application of nanomedicines.
4.5 Traditional Chinese medicine formulas: multitarget synergistic antitumor effects
Traditional Chinese medicines effectively inhibit the development of colon cancer through various pathways, such as enhancing immune response and inhibiting tumor proliferation and metastasis. Garcinia yunnanensis, a traditional Chinese medicine, has anticancer and anti-inflammatory effects, and after Garcinia yunnanensis treatment of APCmin/+ colon cancer model mice, JNK, STAT3 and ERK signaling were inhibited, M2 macrophage infiltration in the TME was reduced, and the size of tumors was reduced (219). Yi-Yi-Fu-Zi-Bai-Jiang-San (YYFZBJS) is a commonly used traditional Chinese medicine formula for treating inflammatory diseases. YYFZBJS can alter the pro-tumor effects of M2 macrophages by inhibiting inflammation-related signaling pathways in colon cancer and delay the development of the disease by reshaping the gut microbiota (220). Si-Jun-Zi Decoction (SJZ) is a well-known Chinese herbal formula that can regulate human immunity. After 3 weeks of treatment with full dose (45 g/kg) of modified SJZ in mice with colorectal cancer, the expression level of GM-CSF in plasma and the number of splenic macrophages were significantly increased, and the nude mice had better body defenses and colorectal cancer survival rate, and the modified SJZ also reduced the hepatic metastasis of colorectal cancer by activating the innate immunity (221).
The development of malignant tumors can have a deleterious effect on the patient’s mood and even accelerate tumor metastasis, and some traditional Chinese medicines can slow down this process. Xiao-Yao-San, is widely used clinically for the relief of symptoms associated with depression. Treatment of colorectal model mice with Xiao-Yao-San reduced the recruitment of M2 macrophages and MDSCs, and inhibited the expression of TGF-β, IL-6, MMP-9 and VEGF. Not only that, Xiao-Yao-San can also effectively inhibit liver metastasis of chronic stress colon tumors (222). Hereby, the natural products, with their excellent activity, safety and novel structures, offer more possibilities for the development of novel anti-colon cancer drugs.
4.6 Probiotics and gut microbiome regulation
Probiotics are defined by the World Health Organization (WHO) as “active microorganisms that, when ingested in sufficient quantities, are beneficial to the health of the host”. Probiotics stabilize the intestinal barrier and modulate intestinal immunity. Extensive research has shown that the consumption of probiotics (e.g. Lactobacillus plantarum, Lactobacillus acidophilus, and Bacillus subtilis) can alter the distribution of cellular junctional proteins, reduce the absorption of potentially cancer-causing compounds, and improve the poor prognosis of colon cancer (223–226).
lactobacilli, a probiotic used with high frequency in the treatment of diseases. Hradicka et al. found that Lactobacillus has immunomodulatory activity, remodels macrophage polarization, and reduces colon tumor tissue volume and total tumor number (227). Lactobacillus acidophilus lysate in combination with CTLA-4 helps to enhance anti-tumor immune response in colon cancer mice by decreasing Treg and M2 macrophage infiltration, and increasing the number of CD8+ T cells in the TME (228). Saccharomyces cerevisiae (S. cerevisiae) is a conventional yeast used in food products and a probiotic that maintains intestinal homeostasis. Beta-glucan, a major component of the cell wall of S. cerevisiae, inhibits colon tumor activity by suppressing intestinal ecological dysregulation and activating macrophages (229). Loigolactobacillus coryniformis NA-3 induces NO, IL-6, TNF-α, and ROS production in colonic TAMs and significantly inhibits the proliferation of colonic tumor cells (230). Notably, appropriate dosage and sufficient intestinal residence time are crucial for probiotics to exert anticancer immune effects (231). In addition, several studies have confirmed that the use of probiotics can help alleviate gastrointestinal adverse reactions such as diarrhea and nausea caused by chemotherapeutic drugs and improve patients’ treatment tolerance (231–233).
4.7 Dietary intervention
Dietary intervention is an emerging strategy for disease prevention and treatment. A Western diet characterized by high intake of red meat and high fat has been shown to significantly increase the risk of colorectal cancer (234). In contrast, diets rich in dietary fiber have a protective effect on the gut, not only providing an energy source for gut microbes, but also helping to maintain gut homeostasis and barrier integrity. Compared to normal-diet colon cancer model mice, ketogenic diet induces oxidative stress within colon tumors, promotes conversion of M2 macrophages to M1-type, and downregulates MMP expression to inhibit colon tumor progression (235). In addition, some dietary intake can also alleviate colon cancer progression to some extent. Feeding a diet rich in Oleuropein-Rich Leaf Extract to colon cancer model rats abrogated the overexpression of iNOS in rat tumors and inhibited the pro-inflammatory behaviors of macrophages in rats with loaded tumors, suppressing tumor proliferation (190). 3-Hydroxybutyrate (3HB) has various utilities as an oral food supplement, such as energy supply, anti-inflammation, neuroprotection, and can be used to improve alcoholic fatty liver, osteoporosis, and Alzheimer’s disease. Li et al. found that 3HB could regulate colon TME, modulate the proportion of infiltrating macrophages, and reduce the level of MDSCs, as well as inhibit the NF-κB pathway that reduces inflammatory factor release (236). Sea cucumber, a marine animal rich in bioactive molecules, is a dietary nutrient with anti-inflammatory and antibacterial effects. Adding sea cucumber extract Frondanol A5 to the feed fed to APCmin/+mice significantly reduced the volume of colon tumors, increased the phagocytosis efficiency of mouse peritoneal macrophages, and provided prevention for intestinal tumorigenesis (237).
5 Challenges and future perspectives
In this review, we summarize the potential of targeted modulation of TAMs in colon cancer prevention, occurrence, metastasis and drug resistance. The main pathways include modulation of TAMs infiltration in TME, remodeling of TAMs polarization, modulation of key signaling pathways in TAMs, promotion of phagocytic activity of TAMs, and activation of immunomodulatory function of TAMs. Potential therapeutic strategies include drug treatments based on natural products and dietary interventions (Figure 4). Colon carcinogenesis is a heterogeneous process with a diverse array of somatic molecular alterations influenced by diet, environmental and microbial exposures, and host immunity. With the deepening of research on colon cancer and the emergence of new therapeutic modalities, especially targeted therapies and immunotherapies, coupled with increasing early interventions, colon cancer has made great progress in diagnosis and treatment. However, toxic side effects, drug resistance, off-target effects, high metastasis and recurrence rates resulting from treatment are still important factors threatening human health.
Based on the current challenges in colon cancer treatment, it is particularly important to improve efficacy and overcome drug resistance without exacerbating immune-related side effects. A growing body of findings suggests that targeting macrophages for colon cancer treatment is a promising therapeutic approach.
However, there are some unresolved issues in the current studies on macrophages in colon cancer, and despite the large number of studies supporting that macrophage infiltration is suggestive of a poor prognosis, there are still data supporting the opposite result, which may be related to the lack of clarity of macrophage markers as well as difficulty in identifying the phenotype. Due to the tumor heterogeneity and plasticity of macrophages, the mechanism of how to inhibit macrophages from being “educated” by TME to perform their scavenging role to phagocytose tumor cells needs to be explored in greater depth. Macrophages signaling pathways and key regulatory factors affecting macrophages in colon cancer also need to be further revealed.
We have summarized potential natural drugs for targeting TAMs in the treatment of colon cancer. These include botanical active compounds, natural product-based synthetic formulations, therapies combined with existing anticancer treatments (such as ICIs and chemotherapy), natural product-based nanomedicines, probiotics, traditional Chinese medicine, and dietary interventions. These therapeutic strategies have addressed issues such as single efficacy, off-target effects, and drug resistance to some extent. Compared with traditional chemotherapy drugs, natural products tend to have fewer adverse effects and toxicities. Additionally, some traditional medicines can inhibit the growth of colon tumors by improving the body’s stress response. However, there are several challenges regarding the clinical application of natural products. Many phytochemicals exhibit poor solubility, resulting in low bioavailability. The bioavailability of some natural compounds remains unclear, and their metabolic pathways and transformation processes in vivo are not fully understood. Although progress has been made in improving the drug delivery efficiency and bioavailability of natural product-based nanomedicines and synthetic formulations, most studies are still in the basic experimental stage, and sufficient clinical data to support widespread application is lacking.
Natural products targeting TAMs for colon cancer treatment show great potential. Future research should focus on addressing key issues such as solubility, delivery efficiency, and bioavailability of natural products, and conduct more clinical trials to verify their efficacy and safety. As cancer biology continues to advance, these challenges are expected to be overcome through further technological innovations and research, ultimately contributing to cancer treatment and human health.
Author contributions
QH: Formal Analysis, Writing – original draft, Data curation, Methodology, Visualization. LX: Data curation, Writing – original draft. YL: Methodology, Writing – original draft. RW: Writing – original draft, Formal Analysis. CZ: Writing – original draft, Supervision. YG: Writing – review & editing, Project administration. HY: Writing – review & editing, Supervision, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Sichuan Provincial Science and Technology Program Projects (Grant Number: 2024JDRC0030).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, et al. Global burden of colorectal cancer in 2020 and 2040: incidence and mortality estimates from GLOBOCAN. Gut. (2023) 72:338–44. doi: 10.1136/gutjnl-2022-327736
2. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19:329–59. doi: 10.6004/jnccn.2021.0012
3. Morton D, Seymour M, Magill L, Handley K, Glasbey J, Glimelius B, et al. Preoperative chemotherapy for operable colon cancer: mature results of an international randomized controlled trial. J Clin Oncol. (2023) 41:1541–52. doi: 10.1200/jco.22.00046
4. Panjeta A, Kaur K, Sharma R, Verma I, and Preet S. Human intestinal defensin 5 ameliorates the sensitization of colonic cancer cells to 5-fluorouracil. Arch Med Res. (2024) 55:102966. doi: 10.1016/j.arcmed.2024.102966
5. Kasi PM, Budde G, Krainock M, Aushev VN, Koyen Malashevich A, Malhotra M, et al. Circulating tumor DNA (ctDNA) serial analysis during progression on PD-1 blockade and later CTLA-4 rescue in patients with mismatch repair deficient metastatic colorectal cancer. J Immunother Cancer. (2022) 10(1):e003312. doi: 10.1136/jitc-2021-003312
6. Zhang X, Yang R, Wu T, Cai X, Li G, Yu K, et al. Efficacy and safety of neoadjuvant monoimmunotherapy with PD-1 inhibitor for dMMR/MSI⁃H locally advanced colorectal cancer: A single-center real-world study. Front Immunol. (2022) 13:913483. doi: 10.3389/fimmu.2022.913483
7. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. (2018) 36:1714–68. doi: 10.1200/jco.2017.77.6385
8. Ozawa S, Miura T, Terashima J, and Habano W. Cellular irinotecan resistance in colorectal cancer and overcoming irinotecan refractoriness through various combination trials including DNA methyltransferase inhibitors: a review. Cancer Drug Resist. (2021) 4:946–64. doi: 10.20517/cdr.2021.82
9. Chen B, Wu L, Tang X, Wang T, Wang S, Yu H, et al. Quercetin inhibits tumorigenesis of colorectal cancer through downregulation of hsa_circ_0006990. Front Pharmacol. (2022) 13:874696. doi: 10.3389/fphar.2022.874696
10. Chen Y, Wang B, Yuan X, Lu Y, Hu J, Gao J, et al. Vitexin prevents colitis-associated carcinogenesis in mice through regulating macrophage polarization. Phytomedicine. (2021) 83:153489. doi: 10.1016/j.phymed.2021.153489
11. Li Y, Lu Q, Xiao R, Ma J, Tang Y, Chen W, et al. Synthesis and anti-tumor activity of nitrogen-containing derivatives of the natural product diphyllin. Eur J Med Chem. (2022) 243:114708. doi: 10.1016/j.ejmech.2022.114708
12. Chen S, Saeed A, Liu Q, Jiang Q, Xu H, Xiao GG, et al. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. (2023) 8:207. doi: 10.1038/s41392-023-01452-1
13. Mantovani A, Allavena P, Marchesi F, and Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. (2022) 21:799–820. doi: 10.1038/s41573-022-00520-5
14. Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. (2017) 545:495–9. doi: 10.1038/nature22396
15. Li Y, Zhou H, Liu P, Lv D, Shi Y, Tang B, et al. SHP2 deneddylation mediates tumor immunosuppression in colon cancer via the CD47/SIRPα axis. J Clin Invest. (2023) 133(4):e162870. doi: 10.1172/jci162870
16. Yurdagul A Jr., Subramanian M, Wang X, Crown SB, Ilkayeva OR, Darville L, et al. Macrophage metabolism of apoptotic cell-derived arginine promotes continual efferocytosis and resolution of injury. Cell Metab. (2020) 31:518–533.e510. doi: 10.1016/j.cmet.2020.01.001
17. Güllü N, Smith J, Herrmann P, and Stein U. MACC1-dependent antitumor effect of curcumin in colorectal cancer. Nutrients. (2022) 14(22):4792. doi: 10.3390/nu14224792
18. Yuan W, Zhang J, Chen H, Zhuang Y, Zhou H, Li W, et al. Natural compounds modulate the mechanism of action of tumour-associated macrophages against colorectal cancer: a review. J Cancer Res Clin Oncol. (2024) 150:502. doi: 10.1007/s00432-024-06022-8
19. Cox N, Pokrovskii M, Vicario R, and Geissmann F. Origins, biology, and diseases of tissue macrophages. Annu Rev Immunol. (2021) 39:313–44. doi: 10.1146/annurev-immunol-093019-111748
20. Locati M, Curtale G, and Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. (2020) 15:123–47. doi: 10.1146/annurev-pathmechdis-012418-012718
21. Rodríguez-Morales P and Franklin RA. Macrophage phenotypes and functions: resolving inflammation and restoring homeostasis. Trends Immunol. (2023) 44:986–98. doi: 10.1016/j.it.2023.10.004
22. Orecchioni M, Ghosheh Y, Pramod AB, and Ley K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front Immunol. (2019) 10:1084. doi: 10.3389/fimmu.2019.01084
23. Park MD, Silvin A, Ginhoux F, and Merad M. Macrophages in health and disease. Cell. (2022) 185:4259–79. doi: 10.1016/j.cell.2022.10.007
24. Sheu KM and Hoffmann A. Functional hallmarks of healthy macrophage responses: their regulatory basis and disease relevance. Annu Rev Immunol. (2022) 40:295–321. doi: 10.1146/annurev-immunol-101320-031555
25. Lazarov T, Juarez-Carreño S, Cox N, and Geissmann F. Physiology and diseases of tissue-resident macrophages. Nature. (2023) 618:698–707. doi: 10.1038/s41586-023-06002-x
26. Sica A and Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. (2012) 122:787–95. doi: 10.1172/jci59643
27. Toledo B, Zhu Chen L, Paniagua-Sancho M, Marchal JA, Perán M, and Giovannetti E. Deciphering the performance of macrophages in tumour microenvironment: a call for precision immunotherapy. J Hematol Oncol. (2024) 17:44. doi: 10.1186/s13045-024-01559-0
28. Varol C, Mildner A, and Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. (2015) 33:643–75. doi: 10.1146/annurev-immunol-032414-112220
29. Mommert S, Gregor K, Rossbach K, Schaper K, Witte T, Gutzmer R, et al. Histamine H2 receptor stimulation upregulates T(H)2 chemokine CCL17 production in human M2a macrophages. J Allergy Clin Immunol. (2018) 141:782–785.e785. doi: 10.1016/j.jaci.2017.06.023
30. Pepe G, Calderazzi G, De Maglie M, Villa AM, and Vegeto E. Heterogeneous induction of microglia M2a phenotype by central administration of interleukin-4. J Neuroinflamm. (2014) 11:211. doi: 10.1186/s12974-014-0211-6
31. Sezginer O and Unver N. Dissection of pro-tumoral macrophage subtypes and immunosuppressive cells participating in M2 polarization. Inflamm Res. (2024) 73:1411–23. doi: 10.1007/s00011-024-01907-3
32. Zhang J, Li Y, Duan Z, Kang J, Chen K, Li G, et al. The effects of the M2a macrophage-induced axonal regeneration of neurons by arginase 1. Biosci Rep. (2020) 40(2):BSR20193031. doi: 10.1042/bsr20193031
33. Asai A, Nakamura K, Kobayashi M, Herndon DN, and Suzuki F. CCL1 released from M2b macrophages is essentially required for the maintenance of their properties. J Leukoc Biol. (2012) 92:859–67. doi: 10.1189/jlb.0212107
34. Bianchini R, Roth-Walter F, Ohradanova-Repic A, Flicker S, Hufnagl K, Fischer MB, et al. IgG4 drives M2a macrophages to a regulatory M2b-like phenotype: potential implication in immune tolerance. Allergy. (2019) 74:483–94. doi: 10.1111/all.13635
35. Yang C, Zhang DM, Song ZB, Hou YQ, Bao YL, Sun LG, et al. Protumoral TSP50 regulates macrophage activities and polarization via production of TNF-α and IL-1β, and activation of the NF-κB signaling pathway. PLoS One. (2015) 10:e0145095. doi: 10.1371/journal.pone.0145095
36. Junior L, Nguyen HT, Salmanida FP, and Chang KT. MERTK(+/hi) M2c macrophages induced by baicalin alleviate non-alcoholic fatty liver disease. Int J Mol Sci. (2021) 22(19):10604. doi: 10.3390/ijms221910604
37. Miki S, Suzuki JI, Takashima M, Ishida M, Kokubo H, and Yoshizumi M. S-1-Propenylcysteine promotes IL-10-induced M2c macrophage polarization through prolonged activation of IL-10R/STAT3 signaling. Sci Rep. (2021) 11:22469. doi: 10.1038/s41598-021-01866-3
38. Mokarram N, Merchant A, Mukhatyar V, Patel G, and Bellamkonda RV. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. (2012) 33:8793–801. doi: 10.1016/j.biomaterials.2012.08.050
39. Tu GW, Shi Y, Zheng YJ, Ju MJ, He HY, Ma GG, et al. Glucocorticoid attenuates acute lung injury through induction of type 2 macrophage. J Transl Med. (2017) 15:181. doi: 10.1186/s12967-017-1284-7
40. Barilo J, Ratsimor M, Chan A, Hembruff H, and Basta S. Polarized tissue-derived macrophages display enhanced M2d phenotype after prolonged stimulation with adenosine A(2A) receptor agonist in the presence of LPS. Front Biosci (Landmark Ed). (2025) 30:27638. doi: 10.31083/fbl27638
41. Cao W, Peters JH, Nieman D, Sharma M, Watson T, and Yu J. Macrophage subtype predicts lymph node metastasis in oesophageal adenocarcinoma and promotes cancer cell invasion in vitro. Br J Cancer. (2015) 113:738–46. doi: 10.1038/bjc.2015.292
42. Ferrante CJ, Pinhal-Enfield G, Elson G, Cronstein BN, Hasko G, Outram S, et al. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Rα) signaling. Inflammation. (2013) 36:921–31. doi: 10.1007/s10753-013-9621-3
43. Murray PJ. Macrophage polarization. Annu Rev Physiol. (2017) 79:541–66. doi: 10.1146/annurev-physiol-022516-034339
44. Carrara SC, Davila-Lezama A, Cabriel C, Berenschot EJW, Krol S, Gardeniers JGE, et al. 3D topographies promote macrophage M2d-Subset differentiation. Mater Today Bio. (2024) 24:100897. doi: 10.1016/j.mtbio.2023.100897
45. Liu R, Han C, Hu J, Zhang B, Luo W, and Ling F. Infiltration of apoptotic M2 macrophage subpopulation is negatively correlated with the immunotherapy response in colorectal cancer. Int J Mol Sci. (2022) 23(19):11014. doi: 10.3390/ijms231911014
46. Haston S, Gonzalez-Gualda E, Morsli S, Ge J, Reen V, Calderwood A, et al. Clearance of senescent macrophages ameliorates tumorigenesis in KRAS-driven lung cancer. Cancer Cell. (2023) 41:1242–1260.e1246. doi: 10.1016/j.ccell.2023.05.004
47. Vitale I, Manic G, Coussens LM, Kroemer G, and Galluzzi L. Macrophages and metabolism in the tumor microenvironment. Cell Metab. (2019) 30:36–50. doi: 10.1016/j.cmet.2019.06.001
48. Nasir I, McGuinness C, Poh AR, Ernst M, Darcy PK, and Britt KL. Tumor macrophage functional heterogeneity can inform the development of novel cancer therapies. Trends Immunol. (2023) 44:971–85. doi: 10.1016/j.it.2023.10.007
49. Pitt JM, Marabelle A, Eggermont A, Soria JC, Kroemer G, and Zitvogel L. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. (2016) 27:1482–92. doi: 10.1093/annonc/mdw168
50. Cassetta L and Pollard JW. A timeline of tumour-associated macrophage biology. Nat Rev Cancer. (2023) 23:238–57. doi: 10.1038/s41568-022-00547-1
51. Mantovani A, Marchesi F, Malesci A, Laghi L, and Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. (2017) 14:399–416. doi: 10.1038/nrclinonc.2016.217
52. Chen Y, Zhang S, Wang Q, and Zhang X. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. J Hematol Oncol. (2017) 10:36. doi: 10.1186/s13045-017-0408-0
53. Bai R, Li Y, Jian L, Yang Y, Zhao L, and Wei M. The hypoxia-driven crosstalk between tumor and tumor-associated macrophages: mechanisms and clinical treatment strategies. Mol Cancer. (2022) 21:177. doi: 10.1186/s12943-022-01645-2
54. Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, et al. Metabolic regulation of gene expression by histone lactylation. Nature. (2019) 574:575–80. doi: 10.1038/s41586-019-1678-1
55. Deligne C, Murdamoothoo D, Gammage AN, Gschwandtner M, Erne W, Loustau T, et al. Matrix-targeting immunotherapy controls tumor growth and spread by switching macrophage phenotype. Cancer Immunol Res. (2020) 8:368–82. doi: 10.1158/2326-6066.Cir-19-0276
56. Mehla K and Singh PK. Metabolic regulation of macrophage polarization in cancer. Trends Cancer. (2019) 5:822–34. doi: 10.1016/j.trecan.2019.10.007
57. Li W, Chen F, Gao H, Xu Z, Zhou Y, Wang S, et al. Cytokine concentration in peripheral blood of patients with colorectal cancer. Front Immunol. (2023) 14:1175513. doi: 10.3389/fimmu.2023.1175513
58. Wang H, Wang X, Zhang X, and Xu W. The promising role of tumor-associated macrophages in the treatment of cancer. Drug Resist Update. (2024) 73:101041. doi: 10.1016/j.drup.2023.101041
59. Khanduri I, Maru DM, and Parra ER. Exploratory study of macrophage polarization and spatial distribution in colorectal cancer liver metastasis: a pilot study. Front Immunol. (2023) 14:1223864. doi: 10.3389/fimmu.2023.1223864
60. Wang J, Zhu N, Su X, Gao Y, and Yang R. Novel tumor-associated macrophage populations and subpopulations by single cell RNA sequencing. Front Immunol. (2023) 14:1264774. doi: 10.3389/fimmu.2023.1264774
61. Wu K, Lin K, Li X, Yuan X, Xu P, Ni P, et al. Redefining tumor-associated macrophage subpopulations and functions in the tumor microenvironment. Front Immunol. (2020) 11:1731. doi: 10.3389/fimmu.2020.01731
62. Gao J, Liang Y, and Wang L. Shaping polarization of tumor-associated macrophages in cancer immunotherapy. Front Immunol. (2022) 13:888713. doi: 10.3389/fimmu.2022.888713
63. Cavalleri T, Greco L, Rubbino F, Hamada T, Quaranta M, Grizzi F, et al. Tumor-associated macrophages and risk of recurrence in stage III colorectal cancer. J Pathol Clin Res. (2022) 8:307–12. doi: 10.1002/cjp2.267
64. Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, and Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. (2007) 13:1472–9. doi: 10.1158/1078-0432.Ccr-06-2073
65. Schmitt M and Greten FR. The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol. (2021) 21:653–67. doi: 10.1038/s41577-021-00534-x
66. De Simone V, Franzè E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, et al. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. (2015) 34:3493–503. doi: 10.1038/onc.2014.286
67. Glaus Garzon JF, Pastrello C, Jurisica I, Hottiger MO, Wenger RH, and Borsig L. Tumor cell endogenous HIF-1α activity induces aberrant angiogenesis and interacts with TRAF6 pathway required for colorectal cancer development. Neoplasia. (2020) 22:745–58. doi: 10.1016/j.neo.2020.10.006
68. Jia SN, Han YB, Yang R, and Yang ZC. Chemokines in colon cancer progression. Semin Cancer Biol. (2022) 86:400–7. doi: 10.1016/j.semcancer.2022.02.007
69. Cho H, Seo Y, Loke KM, Kim SW, Oh SM, Kim JH, et al. Cancer-stimulated CAFs enhance monocyte differentiation and protumoral TAM activation via IL6 and GM-CSF secretion. Clin Cancer Res. (2018) 24:5407–21. doi: 10.1158/1078-0432.Ccr-18-0125
70. Dougherty U, Mustafi R, Sadiq F, Almoghrabi A, Mustafi D, Kreisheh M, et al. The renin-angiotensin system mediates EGF receptor-vitamin d receptor cross-talk in colitis-associated colon cancer. Clin Cancer Res. (2014) 20:5848–59. doi: 10.1158/1078-0432.Ccr-14-0209
71. Li C, Xu MM, Wang K, Adler AJ, Vella AT, and Zhou B. Macrophage polarization and meta-inflammation. Transl Res. (2018) 191:29–44. doi: 10.1016/j.trsl.2017.10.004
72. Nandi B, Shapiro M, Samur MK, Pai C, Frank NY, Yoon C, et al. Stromal CCR6 drives tumor growth in a murine transplantable colon cancer through recruitment of tumor-promoting macrophages. Oncoimmunology. (2016) 5:e1189052. doi: 10.1080/2162402x.2016.1189052
73. Yu J, Xu Z, Guo J, Yang K, Zheng J, and Sun X. Tumor-associated macrophages (TAMs) depend on MMP1 for their cancer-promoting role. Cell Death Discov. (2021) 7:343. doi: 10.1038/s41420-021-00730-7
74. Wang H, Shao Q, Sun J, Ma C, Gao W, Wang Q, et al. Interactions between colon cancer cells and tumor-infiltrated macrophages depending on cancer cell-derived colony stimulating factor 1. Oncoimmunology. (2016) 5:e1122157. doi: 10.1080/2162402x.2015.1122157
75. Rigillo G, Belluti S, Campani V, Ragazzini G, Ronzio M, Miserocchi G, et al. The NF-Y splicing signature controls hybrid EMT and ECM-related pathways to promote aggressiveness of colon cancer. Cancer Lett. (2023) 567:216262. doi: 10.1016/j.canlet.2023.216262
76. Wang L, Li S, Luo H, Lu Q, and Yu S. PCSK9 promotes the progression and metastasis of colon cancer cells through regulation of EMT and PI3K/AKT signaling in tumor cells and phenotypic polarization of macrophages. J Exp Clin Cancer Res. (2022) 41:303. doi: 10.1186/s13046-022-02477-0
77. Guan B, Li H, Yao J, Guo J, Yu F, Li G, et al. CCL3-CCR5 axis promotes cell migration and invasion of colon adenocarcinoma via Akt signaling pathway. Environ Toxicol. (2023) 38:172–84. doi: 10.1002/tox.23675
78. Hu X, Li YQ, Li QG, Ma YL, Peng JJ, and Cai SJ. Osteoglycin (OGN) reverses epithelial to mesenchymal transition and invasiveness in colorectal cancer via EGFR/Akt pathway. J Exp Clin Cancer Res. (2018) 37:41. doi: 10.1186/s13046-018-0718-2
79. Shen X, Hu X, Mao J, Wu Y, Liu H, Shen J, et al. The long noncoding RNA TUG1 is required for TGF-β/TWIST1/EMT-mediated metastasis in colorectal cancer cells. Cell Death Dis. (2020) 11:65. doi: 10.1038/s41419-020-2254-1
80. Qu X, Zhao L, Wang M, Zhang R, Cheng L, Qiu L, et al. Novel functional variants in the Notch pathway and survival of Chinese colorectal cancer. Int J Cancer. (2021) 149:84–96. doi: 10.1002/ijc.33561
81. Villareal LB, Falcon DM, Xie L, and Xue X. Hypoxia-inducible factor 3α1 increases epithelial-to-mesenchymal transition and iron uptake to drive colorectal cancer liver metastasis. Br J Cancer. (2024) 130:1904–15. doi: 10.1038/s41416-024-02699-3
82. Zhao H, Ming T, Tang S, Ren S, Yang H, Liu M, et al. Wnt signaling in colorectal cancer: pathogenic role and therapeutic target. Mol Cancer. (2022) 21:144. doi: 10.1186/s12943-022-01616-7
83. Vinnakota K, Zhang Y, Selvanesan BC, Topi G, Salim T, Sand-Dejmek J, et al. M2-like macrophages induce colon cancer cell invasion via matrix metalloproteinases. J Cell Physiol. (2017) 232:3468–80. doi: 10.1002/jcp.25808
84. Saitoh M. Transcriptional regulation of EMT transcription factors in cancer. Semin Cancer Biol. (2023) 97:21–9. doi: 10.1016/j.semcancer.2023.10.001
85. Li S, Xu F, Zhang J, Wang L, Zheng Y, Wu X, et al. Tumor-associated macrophages remodeling EMT and predicting survival in colorectal carcinoma. Oncoimmunology. (2018) 7:e1380765. doi: 10.1080/2162402x.2017.1380765
86. Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. (2019) 18:64. doi: 10.1186/s12943-019-0976-4
87. Lim SY, Yuzhalin AE, Gordon-Weeks AN, and Muschel RJ. Tumor-infiltrating monocytes/macrophages promote tumor invasion and migration by upregulating S100A8 and S100A9 expression in cancer cells. Oncogene. (2016) 35:5735–45. doi: 10.1038/onc.2016.107
88. Horn LA, Chariou PL, Gameiro SR, Qin H, Iida M, Fousek K, et al. Remodeling the tumor microenvironment via blockade of LAIR-1 and TGF-β signaling enables PD-L1-mediated tumor eradication. J Clin Invest. (2022) 132(8):e155148. doi: 10.1172/jci155148
89. Loo JM, Scherl A, Nguyen A, Man FY, Weinberg E, Zeng Z, et al. Extracellular metabolic energetics can promote cancer progression. Cell. (2015) 160:393–406. doi: 10.1016/j.cell.2014.12.018
90. Yin Y, Yang X, Cheng Z, Wang H, Lei J, Wang D, et al. Identification of extracellular matrix-related biomarkers in colon adenocarcinoma by bioinformatics and experimental validation. Front Immunol. (2024) 15:1371584. doi: 10.3389/fimmu.2024.1371584
91. Afik R, Zigmond E, Vugman M, Klepfish M, Shimshoni E, Pasmanik-Chor M, et al. Tumor macrophages are pivotal constructors of tumor collagenous matrix. J Exp Med. (2016) 213:2315–31. doi: 10.1084/jem.20151193
92. Liang X, Chen M, Bhattarai P, Hameed S, and Dai Z. Perfluorocarbon@Porphyrin nanoparticles for tumor hypoxia relief to enhance photodynamic therapy against liver metastasis of colon cancer. ACS Nano. (2020) 14:13569–83. doi: 10.1021/acsnano.0c05617
93. Korbecki J, Bosiacki M, Barczak K, Łagocka R, Chlubek D, and Baranowska-Bosiacka I. The clinical significance and role of CXCL1 chemokine in gastrointestinal cancers. Cells. (2023) 12(10):1406. doi: 10.3390/cells12101406
94. Pagano E, Elias JE, Schneditz G, Saveljeva S, Holland LM, Borrelli F, et al. Activation of the GPR35 pathway drives angiogenesis in the tumour microenvironment. Gut. (2022) 71:509–20. doi: 10.1136/gutjnl-2020-323363
95. Liu X, Wang B, Li Y, Hu Y, Li X, Yu T, et al. Powerful anticolon tumor effect of targeted gene immunotherapy using folate-modified nanoparticle delivery of CCL19 to activate the immune system. ACS Cent Sci. (2019) 5:277–89. doi: 10.1021/acscentsci.8b00688
96. Tacconi C, Correale C, Gandelli A, Spinelli A, Dejana E, D’Alessio S, et al. Vascular endothelial growth factor C disrupts the endothelial lymphatic barrier to promote colorectal cancer invasion. Gastroenterology. (2015) 148:1438–1451.e1438. doi: 10.1053/j.gastro.2015.03.005
97. Wang L, Ma L, Song Z, Zhou L, Chen K, Wang X, et al. Single-cell transcriptome analysis profiling lymphatic invasion-related TME in colorectal cancer. Sci Rep. (2024) 14:8911. doi: 10.1038/s41598-024-59656-6
98. Tacconi C, Ungaro F, Correale C, Arena V, Massimino L, Detmar M, et al. Activation of the VEGFC/VEGFR3 pathway induces tumor immune escape in colorectal cancer. Cancer Res. (2019) 79:4196–210. doi: 10.1158/0008-5472.Can-18-3657
99. Trimaglio G, Tilkin-Mariamé AF, Feliu V, Lauzéral-Vizcaino F, Tosolini M, Valle C, et al. Colon-specific immune microenvironment regulates cancer progression versus rejection. Oncoimmunology. (2020) 9:1790125. doi: 10.1080/2162402x.2020.1790125
100. Jiang L, Fei H, Yang A, Zhu J, Sun J, Liu X, et al. Estrogen inhibits the growth of colon cancer in mice through reversing extracellular vesicle-mediated immunosuppressive tumor microenvironment. Cancer Lett. (2021) 520:332–43. doi: 10.1016/j.canlet.2021.08.011
101. Lee SJ, Yang H, Kim WR, Lee YS, Lee WS, Kong SJ, et al. STING activation normalizes the intraperitoneal vascular-immune microenvironment and suppresses peritoneal carcinomatosis of colon cancer. J Immunother Cancer. (2021) 9(6):e002195. doi: 10.1136/jitc-2020-002195
102. Ghonim MA, Ibba SV, Tarhuni AF, Errami Y, Luu HH, Dean MJ, et al. Targeting PARP-1 with metronomic therapy modulates MDSC suppressive function and enhances anti-PD-1 immunotherapy in colon cancer. J Immunother Cancer. (2021) 9(1):e001643. doi: 10.1136/jitc-2020-001643
103. Angell HK, Bruni D, Barrett JC, Herbst R, and Galon J. The immunoscore: colon cancer and beyond. Clin Cancer Res. (2020) 26:332–9. doi: 10.1158/1078-0432.Ccr-18-1851
104. Kadomoto S, Izumi K, and Mizokami A. The CCL20-CCR6 axis in cancer progression. Int J Mol Sci. (2020) 21(15):5186. doi: 10.3390/ijms21155186
105. Jarnicki AG, Lysaght J, Todryk S, and Mills KH. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol. (2006) 177:896–904. doi: 10.4049/jimmunol.177.2.896
106. Liu J, Zhang N, Li Q, Zhang W, Ke F, Leng Q, et al. Tumor-associated macrophages recruit CCR6+ regulatory T cells and promote the development of colorectal cancer via enhancing CCL20 production in mice. PLoS One. (2011) 6:e19495. doi: 10.1371/journal.pone.0019495
107. Liu C, Yao Z, Wang J, Zhang W, Yang Y, Zhang Y, et al. Macrophage-derived CCL5 facilitates immune escape of colorectal cancer cells via the p65/STAT3-CSN5-PD-L1 pathway. Cell Death Differ. (2020) 27:1765–81. doi: 10.1038/s41418-019-0460-0
108. Wang J, Li S, Li H, Zhou X, Wen H, and Lai B. IRF4 overexpression promotes the transdifferentiation of tregs into macrophage-like cells to inhibit the development of colon cancer. Cancer Cell Int. (2021) 21:58. doi: 10.1186/s12935-021-01766-6
109. Mezheyeuski A, Micke P, Martín-Bernabé A, Backman M, Hrynchyk I, Hammarström K, et al. The immune landscape of colorectal cancer. Cancers (Basel). (2021) 13(21):5545. doi: 10.3390/cancers13215545
110. Ros XR and Vermeulen L. Turning cold tumors hot by blocking TGF-β. Trends Cancer. (2018) 4:335–7. doi: 10.1016/j.trecan.2018.03.005
111. Chang J, Lin S, Mao Y, Xu Y, Zhang Z, Wu Q, et al. CXCR6+ Tumor-associated macrophages identify immunosuppressive colon cancer patients with poor prognosis but favorable response to adjuvant chemotherapy. Cancers (Basel). (2022) 14(19):4646. doi: 10.3390/cancers14194646
112. Ding D, Zhong H, Liang R, Lan T, Zhu X, Huang S, et al. Multifunctional nanodrug mediates synergistic photodynamic therapy and MDSCs-targeting immunotherapy of colon cancer. Adv Sci (Weinh). (2021) 8:e2100712. doi: 10.1002/advs.202100712
113. Chen C, Li A, Sun P, Xu J, Du W, Zhang J, et al. Efficiently restoring the tumoricidal immunity against resistant Malignancies via an immune nanomodulator. J Control Release. (2020) 324:574–85. doi: 10.1016/j.jconrel.2020.05.039
114. Oh HH, Park YL, Park SY, Myung E, Im CM, Yu HJ, et al. CD47 mediates the progression of colorectal cancer by inducing tumor cell apoptosis and angiogenesis. Pathol Res Pract. (2022) 240:154220. doi: 10.1016/j.prp.2022.154220
115. Zhang Y, Sime W, Juhas M, and Sjölander A. Crosstalk between colon cancer cells and macrophages via inflammatory mediators and CD47 promotes tumour cell migration. Eur J Cancer. (2013) 49:3320–34. doi: 10.1016/j.ejca.2013.06.005
116. Abe T, Tanaka Y, Piao J, Tanimine N, Oue N, Hinoi T, et al. Signal regulatory protein alpha blockade potentiates tumoricidal effects of macrophages on gastroenterological neoplastic cells in syngeneic immunocompetent mice. Ann Gastroenterol Surg. (2018) 2:451–62. doi: 10.1002/ags3.12205
117. Martins F, Oliveira R, Cavadas B, Pinto F, Cardoso AP, Castro F, et al. Hypoxia and macrophages act in concert towards a beneficial outcome in colon cancer. Cancers (Basel). (2020) 12(4):818. doi: 10.3390/cancers12040818
118. Choi D, Lee HW, Hur KY, Kim JJ, Park GS, Jang SH, et al. Cancer stem cell markers CD133 and CD24 correlate with invasiveness and differentiation in colorectal adenocarcinoma. World J Gastroenterol. (2009) 15:2258–64. doi: 10.3748/wjg.15.2258
119. Sagiv E, Starr A, Rozovski U, Khosravi R, Altevogt P, Wang T, et al. Targeting CD24 for treatment of colorectal and pancreatic cancer by monoclonal antibodies or small interfering RNA. Cancer Res. (2008) 68:2803–12. doi: 10.1158/0008-5472.Can-07-6463
120. Shapira S, Shapira A, Starr A, Kazanov D, Kraus S, Benhar I, et al. An immunoconjugate of anti-CD24 and Pseudomonas exotoxin selectively kills human colorectal tumors in mice. Gastroenterology. (2011) 140:935–46. doi: 10.1053/j.gastro.2010.12.004
121. Nersisyan S, Ahlers AK, Lange T, Wicklein D, Galatenko A, Bohnenberger H, et al. Low expression of CD24 is associated with poor survival in colorectal cancer. Biochimie. (2022) 192:91–101. doi: 10.1016/j.biochi.2021.10.004
122. Zhao C, Huang Y, Zhang H, and Liu H. CD24 affects the immunosuppressive effect of tumor-infiltrating cells and tumor resistance in a variety of cancers. Discov Oncol. (2024) 15:399. doi: 10.1007/s12672-024-01284-7
123. Wolff RK, Hoffman MD, Wolff EC, Herrick JS, Sakoda LC, Samowitz WS, et al. Mutation analysis of adenomas and carcinomas of the colon: Early and late drivers. Genes Chromosomes Cancer. (2018) 57:366–76. doi: 10.1002/gcc.22539
124. Liu X, Xu X, Wu Z, Shan Q, Wang Z, Wu Z, et al. Integrated single-cell RNA-seq analysis identifies immune heterogeneity associated with KRAS/TP53 mutation status and tumor-sideness in colorectal cancers. Front Immunol. (2022) 13:961350. doi: 10.3389/fimmu.2022.961350
125. Zhang Y, He B, Zou P, Wu M, Wei M, Xu C, et al. Targeted release of a bispecific fusion protein SIRPα/Siglec-10 by oncolytic adenovirus reinvigorates tumor-associated macrophages to improve therapeutic outcomes in solid tumors. J Immunother Cancer. (2025) 13(4):e010767. doi: 10.1136/jitc-2024-010767
126. Barkal AA, Weiskopf K, Kao KS, Gordon SR, Rosental B, Yiu YY, et al. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat Immunol. (2018) 19:76–84. doi: 10.1038/s41590-017-0004-z
127. Zheng N, Wang T, Luo Q, Liu Y, Yang J, Zhou Y, et al. M2 macrophage-derived exosomes suppress tumor intrinsic immunogenicity to confer immunotherapy resistance. Oncoimmunology. (2023) 12:2210959. doi: 10.1080/2162402x.2023.2210959
128. Hutchinson L. Immunotherapy: Exploiting PD-1 on TAMs for tumour cell kill. Nat Rev Clin Oncol. (2017) 14:392–3. doi: 10.1038/nrclinonc.2017.87
129. Zhang Y, Zeng L, Wang M, Yang Z, Zhang H, Gao L, et al. RIG-I promotes immune evasion of colon cancer by modulating PD-L1 ubiquitination. J Immunother Cancer. (2023) 11(9):e007313. doi: 10.1136/jitc-2023-007313
130. Doleschel D, Hoff S, Koletnik S, Rix A, Zopf D, Kiessling F, et al. Regorafenib enhances anti-PD1 immunotherapy efficacy in murine colorectal cancers and their combination prevents tumor regrowth. J Exp Clin Cancer Res. (2021) 40:288. doi: 10.1186/s13046-021-02043-0
131. Viola A, Munari F, Sánchez-Rodríguez R, Scolaro T, and Castegna A. The metabolic signature of macrophage responses. Front Immunol. (2019) 10:1462. doi: 10.3389/fimmu.2019.01462
132. Liu Y, Xu R, Gu H, Zhang E, Qu J, Cao W, et al. Metabolic reprogramming in macrophage responses. biomark Res. (2021) 9:1. doi: 10.1186/s40364-020-00251-y
133. Deng A, Fan R, Hai Y, Zhuang J, Zhang B, Lu X, et al. A STING agonist prodrug reprograms tumor-associated macrophage to boost colorectal cancer immunotherapy. Theranostics. (2025) 15:277–99. doi: 10.7150/thno.101001
134. Liu J, Zhang W, Chen L, Wang X, Mao X, Wu Z, et al. VSIG4 promotes tumour-associated macrophage M2 polarization and immune escape in colorectal cancer via fatty acid oxidation pathway. Clin Transl Med. (2025) 15:e70340. doi: 10.1002/ctm2.70340
135. Wang J, Deng S, Cheng D, Gu J, Qin L, Mao F, et al. Engineered microparticles modulate arginine metabolism to repolarize tumor-associated macrophages for refractory colorectal cancer treatment. J Transl Med. (2024) 22:908. doi: 10.1186/s12967-024-05652-3
136. Cooks T, Pateras IS, Jenkins LM, Patel KM, Robles AI, Morris J, et al. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nat Commun. (2018) 9:771. doi: 10.1038/s41467-018-03224-w
137. Huang YJ, Huang TH, Yadav VK, Sumitra MR, Tzeng DT, Wei PL, et al. Preclinical investigation of ovatodiolide as a potential inhibitor of colon cancer stem cells via downregulating sphere-derived exosomal β-catenin/STAT3/miR-1246 cargoes. Am J Cancer Res. (2020) 10:2337–54.
138. Zhao W, Wu Y, Wang Y, Li T, Liu Q, and Hou Z. Exosomal miR-92a-3p modulates M2 macrophage polarization in colorectal cancer: implications for tumor migration and angiogenesis. Med Oncol. (2025) 42:96. doi: 10.1007/s12032-025-02635-2
139. Han Y, Zhou Z, Li R, and Wang H. Tumor-derived exosomal circ_0020095 promotes colon cancer cell proliferation and metastasis by inhibiting M1 macrophage polarization. J Biochem Mol Toxicol. (2025) 39:e70225. doi: 10.1002/jbt.70225
140. Gao B, Wang L, Wen T, Xie X, Rui X, and Chen Q. Colon cancer-derived exosomal lncRNA-XIST promotes M2-like macrophage polarization by regulating PDGFRA. Int J Mol Sci. (2024) 25(21):11433. doi: 10.3390/ijms252111433
141. Li S, Fu X, Ning D, Liu Q, Zhao J, Cheng Q, et al. Colon cancer exosome-associated HSP90B1 initiates pre-metastatic niche formation in the liver by polarizing M1 macrophage into M2 phenotype. Biol Direct. (2025) 20:52. doi: 10.1186/s13062-025-00623-0
142. Komarova EY, Suezov RV, Nikotina AD, Aksenov ND, Garaeva LA, Shtam TA, et al. Hsp70-containing extracellular vesicles are capable of activating of adaptive immunity in models of mouse melanoma and colon carcinoma. Sci Rep. (2021) 11:21314. doi: 10.1038/s41598-021-00734-4
143. Lan J, Sun L, Xu F, Liu L, Hu F, Song D, et al. M2 macrophage-derived exosomes promote cell migration and invasion in colon cancer. Cancer Res. (2019) 79:146–58. doi: 10.1158/0008-5472.Can-18-0014
144. Ma YS, Wu TM, Ling CC, Yu F, Zhang J, Cao PS, et al. M2 macrophage-derived exosomal microRNA-155-5p promotes the immune escape of colon cancer by downregulating ZC3H12B. Mol Ther Oncolytics. (2021) 20:484–98. doi: 10.1016/j.omto.2021.02.005
145. Zhang S, Li D, Zhao M, Yang F, Sang C, Yan C, et al. Exosomal miR-183-5p Shuttled by M2 Polarized Tumor-Associated Macrophage Promotes the Development of Colon Cancer via Targeting THEM4 Mediated PI3K/AKT and NF-κB Pathways. Front Oncol. (2021) 11:672684. doi: 10.3389/fonc.2021.672684
146. Guo J, Wang X, Guo Q, Zhu S, Li P, Zhang S, et al. M2 macrophage derived extracellular vesicle-mediated transfer of miR-186-5p promotes colon cancer progression by targeting DLC1. Int J Biol Sci. (2022) 18:1663–76. doi: 10.7150/ijbs.69405
147. Ding Y, Zhao H, Niu W, Zhang J, Zheng X, Liu Y, et al. M2 Macrophage-Derived Extracellular Vesicles Containing microRNA-501-3p Promote Colon Cancer Progression through the SETD7/DNMT1/SOCS3 Axis. Dis Colon Rectum. (2023) 66(12):e1234–45. doi: 10.1097/dcr.0000000000002986
148. Cui Z, Li W, Wang Y, Zhao M, Liu K, Yang Y, et al. M2 macrophage-derived exosomal ferritin heavy chain promotes colon cancer cell proliferation. Biol Trace Elem Res. (2023) 201:3717–28. doi: 10.1007/s12011-022-03488-w
149. Zhao M, Zhou L, Zhang Q, Wang M, Dong Y, Wang Y, et al. Targeting MAPK14 by lobeline upregulates slurp1-mediated inhibition of alternative activation of TAM and retards colorectal cancer growth. Adv Sci (Weinh). (2025) 12:e2407900. doi: 10.1002/advs.202407900
150. Bell HN, Rebernick RJ, Goyert J, Singhal R, Kuljanin M, Kerk SA, et al. Reuterin in the healthy gut microbiome suppresses colorectal cancer growth through altering redox balance. Cancer Cell. (2022) 40:185–200.e186. doi: 10.1016/j.ccell.2021.12.001
151. Lian Q, Yan S, Yin Q, Yan C, Zheng W, Gu W, et al. TRIM34 attenuates colon inflammation and tumorigenesis by sustaining barrier integrity. Cell Mol Immunol. (2021) 18:350–62. doi: 10.1038/s41423-020-0366-2
152. Xie Y, Zhao Y, Shi L, Li W, Chen K, Li M, et al. Gut epithelial TSC1/mTOR controls RIPK3-dependent necroptosis in intestinal inflammation and cancer. J Clin Invest. (2020) 130:2111–28. doi: 10.1172/jci133264
153. Maisonneuve C, Tsang DKL, Foerster EG, Robert LM, Mukherjee T, Prescott D, et al. Nod1 promotes colorectal carcinogenesis by regulating the immunosuppressive functions of tumor-infiltrating myeloid cells. Cell Rep. (2021) 34:108677. doi: 10.1016/j.celrep.2020.108677
154. Bader JE, Enos RT, Velázquez KT, Carson MS, Nagarkatti M, Nagarkatti PS, et al. Macrophage depletion using clodronate liposomes decreases tumorigenesis and alters gut microbiota in the AOM/DSS mouse model of colon cancer. Am J Physiol Gastrointest Liver Physiol. (2018) 314:G22–g31. doi: 10.1152/ajpgi.00229.2017
155. Kang B, Alvarado LJ, Kim T, Lehmann ML, Cho H, He J, et al. Commensal microbiota drive the functional diversification of colon macrophages. Mucosal Immunol. (2020) 13:216–29. doi: 10.1038/s41385-019-0228-3
156. Bertocchi A, Carloni S, Ravenda PS, Bertalot G, Spadoni I, Lo Cascio A, et al. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell. (2021) 39:708–724.e711. doi: 10.1016/j.ccell.2021.03.004
157. Salvucci M, Crawford N, Stott K, Bullman S, Longley DB, and Prehn JHM. Patients with mesenchymal tumours and high Fusobacteriales prevalence have worse prognosis in colorectal cancer (CRC). Gut. (2022) 71:1600–12. doi: 10.1136/gutjnl-2021-325193
158. Thiele Orberg E, Fan H, Tam AJ, Dejea CM, Destefano Shields CE, Wu S, et al. The myeloid immune signature of enterotoxigenic Bacteroides fragilis-induced murine colon tumorigenesis. Mucosal Immunol. (2017) 10:421–33. doi: 10.1038/mi.2016.53
159. Wang X, Yang Y, Moore DR, Nimmo SL, Lightfoot SA, and Huycke MM. 4-hydroxy-2-nonenal mediates genotoxicity and bystander effects caused by Enterococcus faecalis-infected macrophages. Gastroenterology. (2012) 142:543–551.e547. doi: 10.1053/j.gastro.2011.11.020
160. Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. (2013) 14:207–15. doi: 10.1016/j.chom.2013.07.007
161. Yang Y, Weng W, Peng J, Hong L, Yang L, Toiyama Y, et al. Fusobacterium nucleatum increases proliferation of colorectal cancer cells and tumor development in mice by activating toll-like receptor 4 signaling to nuclear factor-κB, and up-regulating expression of microRNA-21. Gastroenterology. (2017) 152:851–866.e824. doi: 10.1053/j.gastro.2016.11.018
162. Hardbower DM, Asim M, Luis PB, Singh K, Barry DP, Yang C, et al. Ornithine decarboxylase regulates M1 macrophage activation and mucosal inflammation via histone modifications. Proc Natl Acad Sci U S A. (2017) 114:E751–e760. doi: 10.1073/pnas.1614958114
163. Fan L, Xu C, Ge Q, Lin Y, Wong CC, Qi Y, et al. A. Muciniphila suppresses colorectal tumorigenesis by inducing TLR2/NLRP3-mediated M1-like TAMs. Cancer Immunol Res. (2021) 9:1111–24. doi: 10.1158/2326-6066.Cir-20-1019
164. Chen JF, Wu SW, Shi ZM, and Hu B. Traditional Chinese medicine for colorectal cancer treatment: potential targets and mechanisms of action. Chin Med. (2023) 18:14. doi: 10.1186/s13020-023-00719-7
165. Li XM, Yuan DY, Liu YH, Zhu L, Qin HK, Yang YB, et al. Panax notoginseng saponins prevent colitis-associated colorectal cancer via inhibition IDO1 mediated immune regulation. Chin J Nat Med. (2022) 20:258–69. doi: 10.1016/s1875-5364(22)60179-1
166. Yao Y, Feng S, Li X, Liu T, Ye S, Ma L, et al. Litchi procyanidins inhibit colon cancer proliferation and metastasis by triggering gut-lung axis immunotherapy. Cell Death Dis. (2023) 14:109. doi: 10.1038/s41419-022-05482-5
167. Ge S, Sun X, Sang L, Zhang M, Yan X, Ju Q, et al. Curcumin inhibits Malignant behavior of colorectal cancer cells by regulating M2 polarization of tumor-associated macrophages and metastasis associated in colon cancer 1 (MACC1) expression. Chem Biol Drug Des. (2023) 102:1202–12. doi: 10.1111/cbdd.14330
168. Chiou YS, Lan YM, Lee PS, Lin Q, Nagabhushanam K, Ho CT, et al. Piceatannol prevents colon cancer progression via dual-targeting to M2-polarized tumor-associated macrophages and the TGF-β1 positive feedback signaling pathway. Mol Nutr Food Res. (2022) 66:e2200248. doi: 10.1002/mnfr.202200248
169. Jiang J, Zhu F, Zhang H, Sun T, Fu F, Chen X, et al. Luteolin suppresses the growth of colon cancer cells by inhibiting the IL-6/STAT3 signaling pathway. J Gastrointest Oncol. (2022) 13:1722–32. doi: 10.21037/jgo-22-507
170. Sun X, Zhou L, Wang Y, Deng G, Cao X, Ke B, et al. Single-cell analyses reveal cannabidiol rewires tumor microenvironment via inhibiting alternative activation of macrophage and synergizes with anti-PD-1 in colon cancer. J Pharm Anal. (2023) 13:726–44. doi: 10.1016/j.jpha.2023.04.013
171. Yi L, Wang Q, Luo H, Lei D, Tang Z, Lei S, et al. Inhibitory effects of polyphenols-rich components from three edible seaweeds on inflammation and colon cancer in vitro. Front Nutr. (2022) 9:856273. doi: 10.3389/fnut.2022.856273
172. Huang M, Han Y, Li L, Rakariyatham K, Wu X, Gao Z, et al. Protective effects of non-extractable phenolics from strawberry against inflammation and colon cancer in vitro. Food Chem. (2022) 374:131759. doi: 10.1016/j.foodchem.2021.131759
173. Xun J, Hu Z, Wang M, Jiang X, Liu B, Han Y, et al. Hydroxygenkwanin suppresses peritoneal metastasis in colorectal cancer by modulating tumor-associated macrophages polarization. Chem Biol Interact. (2024) 396:111038. doi: 10.1016/j.cbi.2024.111038
174. Zhou M, Li R, Lian G, Yang M, Li L, Yin Z, et al. Tetrahydrocurcumin alleviates colorectal tumorigenesis by modulating the SPP1/CD44 axis and preventing M2 tumor-associated macrophage polarization. Phytomedicine. (2025) 141:156674. doi: 10.1016/j.phymed.2025.156674
175. Deng Z, Wu N, Suo Q, Wang J, Yue Y, Geng L, et al. Fucoidan, as an immunostimulator promotes M1 macrophage differentiation and enhances the chemotherapeutic sensitivity of capecitabine in colon cancer. Int J Biol Macromol. (2022) 222:562–72. doi: 10.1016/j.ijbiomac.2022.09.201
176. Guo M, Li Z, Huang Y, and Shi M. Polysaccharides from Nostoc commune Vaucher activate macrophages via NF-κB and AKT/JNK1/2 pathways to suppress colorectal cancer growth in vivo. Food Funct. (2019) 10:4269–79. doi: 10.1039/c9fo00595a
177. Wang Q, Huang Y, Jia M, Lu D, Zhang HW, Huang D, et al. Safflower polysaccharide inhibits AOM/DSS-induced mice colorectal cancer through the regulation of macrophage polarization. Front Pharmacol. (2021) 12:761641. doi: 10.3389/fphar.2021.761641
178. Yang KM, Ge Y, Palanisamy S, Zhang Y, Kou F, Yelithao K, et al. Cnidium officinale polysaccharide enhanced RAW 264.7 cells activation and NK-92 cells cytotoxicity against colon cancer via NF-κB and MAPKs signaling pathways. Int J Biol Macromol. (2023) 253:127605. doi: 10.1016/j.ijbiomac.2023.127605
179. Cheng H, Sun L, Shen D, Ren A, Ma F, Tai G, et al. Beta-1,6 glucan converts tumor-associated macrophages into an M1-like phenotype. Carbohydr Polym. (2020) 247:116715. doi: 10.1016/j.carbpol.2020.116715
180. Khan I, Huang G, Li XA, Liao W, Leong WK, Xia W, et al. Mushroom polysaccharides and jiaogulan saponins exert cancer preventive effects by shaping the gut microbiota and microenvironment in Apc(Min/+) mice. Pharmacol Res. (2019) 148:104448. doi: 10.1016/j.phrs.2019.104448
181. Shin MS, Hwang SH, Yoon TJ, Kim SH, and Shin KS. Polysaccharides from ginseng leaves inhibit tumor metastasis via macrophage and NK cell activation. Int J Biol Macromol. (2017) 103:1327–33. doi: 10.1016/j.ijbiomac.2017.05.055
182. McDonald SJ, Bullard BM, VanderVeen BN, Cardaci TD, Huss AR, Fan D, et al. Panaxynol alleviates colorectal cancer in a murine model via suppressing macrophages and inflammation. Am J Physiol Gastrointest Liver Physiol. (2023) 325:G318–g333. doi: 10.1152/ajpgi.00119.2023
183. Baik JS, Seo YN, Yi JM, Rhee MH, Park MT, and Kim SD. Ginsenoside-Rp1 inhibits radiation-induced effects in lipopolysaccharide-stimulated J774A.1 macrophages and suppresses phenotypic variation in CT26 colon cancer cells. J Ginseng Res. (2020) 44:843–8. doi: 10.1016/j.jgr.2020.01.006
184. Liu F, Ran F, He H, and Chen L. Astragaloside IV exerts anti-tumor effect on murine colorectal cancer by re-educating tumor-associated macrophage. Arch Immunol Ther Exp (Warsz). (2020) 68:33. doi: 10.1007/s00005-020-00598-y
185. Senedese JM, Rinaldi-Neto F, Furtado RA, Nicollela HD, de Souza LDR, Ribeiro AB, et al. Chemopreventive role of Copaifera reticulata Ducke oleoresin in colon carcinogenesis. BioMed Pharmacother. (2019) 111:331–7. doi: 10.1016/j.biopha.2018.12.091
186. Lin YY, Lee IY, Huang WS, Lin YS, Kuan FC, Shu LH, et al. Danshen improves survival of patients with colon cancer and dihydroisotanshinone I inhibit the proliferation of colon cancer cells via apoptosis and skp2 signaling pathway. J Ethnopharmacol. (2017) 209:305–16. doi: 10.1016/j.jep.2017.08.011
187. Jiang X, Cao G, Gao G, Wang W, Zhao J, and Gao C. Triptolide decreases tumor-associated macrophages infiltration and M2 polarization to remodel colon cancer immune microenvironment via inhibiting tumor-derived CXCL12. J Cell Physiol. (2021) 236:193–204. doi: 10.1002/jcp.29833
188. Zhou J, Li Y, Shi X, Hao S, Zhang F, Guo Z, et al. Oridonin inhibits tumor angiogenesis and induces vessel normalization in experimental colon cancer. J Cancer. (2021) 12:3257–64. doi: 10.7150/jca.55929
189. Zhang H, Zhao B, Wei H, Zeng H, Sheng D, and Zhang Y. Cucurbitacin B controls M2 macrophage polarization to suppresses metastasis via targeting JAK-2/STAT3 signalling pathway in colorectal cancer. J Ethnopharmacol. (2022) 287:114915. doi: 10.1016/j.jep.2021.114915
190. Ruzzolini J, Chioccioli S, Monaco N, Peppicelli S, Andreucci E, Urciuoli S, et al. Oleuropein-rich leaf extract as a broad inhibitor of tumour and macrophage iNOS in an apc mutant rat model. Antioxid (Basel). (2021) 10(10):1577. doi: 10.3390/antiox10101577
191. Sougiannis AT, VanderVeen B, Chatzistamou I, Kubinak JL, Nagarkatti M, Fan D, et al. Emodin reduces tumor burden by diminishing M2-like macrophages in colorectal cancer. Am J Physiol Gastrointest Liver Physiol. (2022) 322:G383–g395. doi: 10.1152/ajpgi.00303.2021
192. Fan F, Shen P, Ma Y, Ma W, Wu H, Liu H, et al. Bullatacin triggers immunogenic cell death of colon cancer cells by activating endoplasmic reticulum chaperones. J Inflammation (Lond). (2021) 18:23. doi: 10.1186/s12950-021-00289-1
193. Rohwer N, Kühl AA, Ostermann AI, Hartung NM, Schebb NH, Zopf D, et al. Effects of chronic low-dose aspirin treatment on tumor prevention in three mouse models of intestinal tumorigenesis. Cancer Med. (2020) 9:2535–50. doi: 10.1002/cam4.2881
194. Dong R, Gong Y, Meng W, Yuan M, Zhu H, Ying M, et al. The involvement of M2 macrophage polarization inhibition in fenretinide-mediated chemopreventive effects on colon cancer. Cancer Lett. (2017)388:43–53. doi: 10.1016/j.canlet.2016.11.029
195. Natoli M, Herzig P, Pishali Bejestani E, Buchi M, Ritschard R, Lloyd GK, et al. Plinabulin, a distinct microtubule-targeting chemotherapy, promotes M1-like macrophage polarization and anti-tumor immunity. Front Oncol. (2021) 11:644608. doi: 10.3389/fonc.2021.644608
196. Ohno Y, Toshino M, Mohammed AFA, Fujiwara Y, Komohara Y, Onodera R, et al. Mannose-methyl-β-cyclodextrin suppresses tumor growth by targeting both colon cancer cells and tumor-associated macrophages. Carbohydr Polym. (2023) 305:120551. doi: 10.1016/j.carbpol.2023.120551
197. Lv Q, Pan X, Wang D, Rong Q, Ma B, Xie X, et al. Discovery of (Z)-1-(3-((1H-pyrrol-2-yl)methylene)-2-oxoindolin-6-yl)-3-(isoxazol-3-yl)urea derivatives as novel and orally highly effective CSF-1R inhibitors for potential colorectal cancer immunotherapy. J Med Chem. (2021) 64:17184–208. doi: 10.1021/acs.jmedchem.1c01184
198. Chun J, Park SM, Lee M, Ha IJ, and Jeong MK. The sesquiterpene lactone-rich fraction of inula helenium L. Enhances the antitumor effect of anti-PD-1 antibody in colorectal cancer: integrative phytochemical, transcriptomic, and experimental analyses. Cancers (Basel). (2023) 15(3):653. doi: 10.3390/cancers15030653
199. Chan ASH, Kangas TO, Qiu X, Uhlik MT, Fulton RB, Ottoson NR, et al. Imprime PGG enhances anti-tumor effects of tumor-targeting, anti-angiogenic, and immune checkpoint inhibitor antibodies. Front Oncol. (2022) 12:869078. doi: 10.3389/fonc.2022.869078
200. Zhang G, Pan J, Xu X, Nie S, Lu L, Jing Y, et al. Chinese yam polysaccharide enhances anti-PD-1 immunotherapy in colorectal cancer through alterations in the gut microbiota and metabolites. Int J Biol Macromol. (2025) 310:143323. doi: 10.1016/j.ijbiomac.2025.143323
201. Su Y, Choi HS, Choi JH, Kim HS, Jang YS, and Seo JW. 7S,15R-dihydroxy-16S,17S-epoxy-docosapentaenoic acid overcomes chemoresistance of 5-fluorouracil by suppressing the infiltration of tumor-associated macrophages and inhibiting the activation of cancer stem cells in a colorectal cancer xenograft model. Mar Drugs. (2023) 21(2):80. doi: 10.3390/md21020080
202. Wang L, Choi HS, Su Y, Lee B, Song JJ, Jang YS, et al. 7S,15R-dihydroxy-16S,17S-epoxy-docosapentaenoic acid, a novel DHA epoxy derivative, inhibits colorectal cancer stemness through repolarization of tumor-associated macrophage functions and the ROS/STAT3 signaling pathway. Antioxid (Basel). (2021) 10(9):1459. doi: 10.3390/antiox10091459
203. Huang YJ, Yang CK, Wei PL, Huynh TT, Whang-Peng J, Meng TC, et al. Ovatodiolide suppresses colon tumorigenesis and prevents polarization of M2 tumor-associated macrophages through YAP oncogenic pathways. J Hematol Oncol. (2017) 10:60. doi: 10.1186/s13045-017-0421-3
204. Guo P, Huang J, and Moses MA. Cancer nanomedicines in an evolving oncology landscape. Trends Pharmacol Sci. (2020) 41:730–42. doi: 10.1016/j.tips.2020.08.001
205. Zhang Z, Ji Y, Hu N, Yu Q, Zhang X, Li J, et al. Ferroptosis-induced anticancer effect of resveratrol with a biomimetic nano-delivery system in colorectal cancer treatment. Asian J Pharm Sci. (2022) 17:751–66. doi: 10.1016/j.ajps.2022.07.006
206. Peng J, Zhou J, Sun R, Chen Y, Pan D, Wang Q, et al. Dual-targeting of artesunate and chloroquine to tumor cells and tumor-associated macrophages by a biomimetic PLGA nanoparticle for colorectal cancer treatment. Int J Biol Macromol. (2023) 244:125163. doi: 10.1016/j.ijbiomac.2023.125163
207. Lv Y, Li M, Weng L, Huang H, Mao Y, Yang DA, et al. Ginseng-derived nanoparticles reprogram macrophages to regulate arginase-1 release for ameliorating T cell exhaustion in tumor microenvironment. J Exp Clin Cancer Res. (2023) 42:322. doi: 10.1186/s13046-023-02888-7
208. de Carvalho TG, Lara P, Jorquera-Cordero C, Aragão CFS, de Santana Oliveira A, Garcia VB, et al. Inhibition of murine colorectal cancer metastasis by targeting M2-TAM through STAT3/NF-kB/AKT signaling using macrophage 1-derived extracellular vesicles loaded with oxaliplatin, retinoic acid, and Libidibia ferrea. BioMed Pharmacother. (2023) 168:115663. doi: 10.1016/j.biopha.2023.115663
209. Cheng F, He L, Wang J, Lai L, Ma L, Qu K, et al. Synergistic immunotherapy with a calcium-based nanoinducer: evoking pyroptosis and remodeling tumor-associated macrophages for enhanced antitumor immune response. Nanoscale. (2024) 16:18570–83. doi: 10.1039/d4nr01497a
210. Li X, Wu M, Wu Y, Xin Y, Gao L, Elsabahy M, et al. Multifunctional nanodrug for simultaneously combating chemoresistance and immunosuppression in Fusobacterium nucleatum-associated colorectal cancer. Acta Biomater. (2025) 195:406–20. doi: 10.1016/j.actbio.2025.02.013
211. Abdi H, Arabi L, Montazer M, Askarizadeh A, Zamani P, Hosseinzadeh H, et al. The effect of m2 peptide targeted nanoliposomes containing crocin on induction of phenotypic change in tumor macrophages to M1 state. Life Sci. (2023) 330:121992. doi: 10.1016/j.lfs.2023.121992
212. Ma L, Ma Y, Gao Q, Liu S, Zhu Z, Shi X, et al. Mulberry leaf lipid nanoparticles: a naturally targeted CRISPR/cas9 oral delivery platform for alleviation of colon diseases. Small. (2024) 20:e2307247. doi: 10.1002/smll.202307247
213. Hama S, Nishi T, Isono E, Itakura S, Yoshikawa Y, Nishimoto A, et al. Intraperitoneal administration of nanoparticles containing tocopheryl succinate prevents peritoneal dissemination. Cancer Sci. (2022) 113:1779–88. doi: 10.1111/cas.15321
214. Dutta D, Al Hoque A, Paul B, Park JH, Chowdhury C, Quadir M, et al. EpCAM-targeted betulinic acid analogue nanotherapy improves therapeutic efficacy and induces anti-tumorigenic immune response in colorectal cancer tumor microenvironment. J BioMed Sci. (2024) 31:81. doi: 10.1186/s12929-024-01069-8
215. Júnior RFA, Lira GA, Schomann T, Cavalcante RS, Vilar NF, de Paula RCM, et al. Retinoic acid-loaded PLGA nanocarriers targeting cell cholesterol potentialize the antitumour effect of PD-L1 antibody by preventing epithelial-mesenchymal transition mediated by M2-TAM in colorectal cancer. Transl Oncol. (2023) 31:101647. doi: 10.1016/j.tranon.2023.101647
216. Cheng G, Zhang X, Chen Y, Lee RJ, Wang J, Yao J, et al. Anticancer activity of polymeric nanoparticles containing linoleic acid-SN38 (LA-SN38) conjugate in a murine model of colorectal cancer. Colloids Surf B Biointerfaces. (2019) 181:822–9. doi: 10.1016/j.colsurfb.2019.06.020
217. Spyridopoulou K, Aindelis G, Pappa A, and Chlichlia K. Anticancer activity of biogenic selenium nanoparticles: apoptotic and immunogenic cell death markers in colon cancer cells. Cancers (Basel). (2021) 13(21):5335. doi: 10.3390/cancers13215335
218. Mao Q, Min J, Zeng R, Liu H, Li H, Zhang C, et al. Self-assembled traditional Chinese nanomedicine modulating tumor immunosuppressive microenvironment for colorectal cancer immunotherapy. Theranostics. (2022) 12:6088–105. doi: 10.7150/thno.72509
219. Sui H, Tan H, Fu J, Song Q, Jia R, Han L, et al. The active fraction of Garcinia yunnanensis suppresses the progression of colorectal carcinoma by interfering with tumorassociated macrophage-associated M2 macrophage polarization in vivo and in vitro. FASEB J. (2020) 34:7387–403. doi: 10.1096/fj.201903011R
220. Chai N, Xiong Y, Zhang Y, Cheng Y, Shi W, Yao Y, et al. YYFZBJS inhibits colorectal tumorigenesis by remodeling gut microbiota and influence on M2 macrophage polarization in vivo and in vitro. Am J Cancer Res. (2021) 11:5338–57. doi: 10.2139/ssrn.3837225
221. Zhou JY, Chen M, Wu CE, Zhuang YW, Chen YG, and Liu SL. The modified Si-Jun-Zi Decoction attenuates colon cancer liver metastasis by increasing macrophage cells. BMC Complement Altern Med. (2019) 19:86. doi: 10.1186/s12906-019-2498-4
222. Zhao L, Zhu X, Ni Y, You J, and Li A. Xiaoyaosan, a traditional Chinese medicine, inhibits the chronic restraint stress-induced liver metastasis of colon cancer in vivo. Pharm Biol. (2020) 58:1085–91. doi: 10.1080/13880209.2020.1839513
223. Ma EL, Choi YJ, Choi J, Pothoulakis C, Rhee SH, and Im E. The anticancer effect of probiotic Bacillus polyfermenticus on human colon cancer cells is mediated through ErbB2 and ErbB3 inhibition. Int J Cancer. (2010) 127:780–90. doi: 10.1002/ijc.25011
224. Sun M, Liu W, Song Y, Tuo Y, Mu G, and Ma F. The effects of lactobacillus plantarum-12 crude exopolysaccharides on the cell proliferation and apoptosis of human colon cancer (HT-29) cells. Probiotics Antimicrob Proteins. (2021) 13:413–21. doi: 10.1007/s12602-020-09699-8
225. Yue Y, Wang S, Shi J, Xie Q, Li N, Guan J, et al. Effects of lactobacillus acidophilus KLDS1.0901 on proliferation and apoptosis of colon cancer cells. Front Microbiol. (2021) 12:788040. doi: 10.3389/fmicb.2021.788040
226. Yue Y, Ye K, Lu J, Wang X, Zhang S, Liu L, et al. Probiotic strain Lactobacillus plantarum YYC-3 prevents colon cancer in mice by regulating the tumour microenvironment. BioMed Pharmacother. (2020) 127:110159. doi: 10.1016/j.biopha.2020.110159
227. Hradicka P, Beal J, Kassayova M, Foey A, and Demeckova V. A novel lactic acid bacteria mixture: macrophage-targeted prophylactic intervention in colorectal cancer management. Microorganisms. (2020) 8(3):387. doi: 10.3390/microorganisms8030387
228. Zhuo Q, Yu B, Zhou J, Zhang J, Zhang R, Xie J, et al. Lysates of Lactobacillus acidophilus combined with CTLA-4-blocking antibodies enhance antitumor immunity in a mouse colon cancer model. Sci Rep. (2019) 9:20128. doi: 10.1038/s41598-019-56661-y
229. Binmama S, Dang CP, Visitchanakun P, Hiengrach P, Somboonna N, Cheibchalard T, et al. Beta-Glucan from S. cerevisiae Protected AOM-Induced Colon Cancer in cGAS-Deficient Mice Partly through Dectin-1-Manipulated Macrophage Cell Energy. Int J Mol Sci. (2022) 23(18):10951. doi: 10.3390/ijms231810951
230. Xu X, Qiao Y, Peng Q, and Shi B. Probiotic properties of loigolactobacillus coryniformis NA-3 and in vitro comparative evaluation of live and heat-killed cells for antioxidant, anticancer and immunoregulatory activities. Foods. (2023) 12(5):1118. doi: 10.3390/foods12051118
231. Drago L. Probiotics and colon cancer. Microorganisms. (2019) 7(3):66. doi: 10.3390/microorganisms7030066
232. Jakubauskas M, Jakubauskiene L, Leber B, Horvath A, Strupas K, Stiegler P, et al. Probiotic supplementation attenuates chemotherapy-induced intestinal mucositis in an experimental colorectal cancer liver metastasis rat model. Nutrients. (2023) 15(5):1117. doi: 10.3390/nu15051117
233. Mahdy MS, Azmy AF, Dishisha T, Mohamed WR, Ahmed KA, Hassan A, et al. Irinotecan-gut microbiota interactions and the capability of probiotics to mitigate Irinotecan-associated toxicity. BMC Microbiol. (2023) 23:53. doi: 10.1186/s12866-023-02791-3
234. Yang J, Wei H, Zhou Y, Szeto CH, Li C, Lin Y, et al. High-fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology. (2022) 162:135–149.e132. doi: 10.1053/j.gastro.2021.08.041
235. Zhang N, Liu C, Jin L, Zhang R, Wang T, Wang Q, et al. Ketogenic diet elicits antitumor properties through inducing oxidative stress, inhibiting MMP-9 expression, and rebalancing M1/M2 tumor-associated macrophage phenotype in a mouse model of colon cancer. J Agric Food Chem. (2020) 68:11182–96. doi: 10.1021/acs.jafc.0c04041
236. Li Z, Zhang S, Zhang Y, Chen J, Wu F, Liu G, et al. Applications and mechanism of 3-hydroxybutyrate (3HB) for prevention of colonic inflammation and carcinogenesis as a food supplement. Mol Nutr Food Res. (2021) 65:e2100533. doi: 10.1002/mnfr.202100533
Keywords: tumor-associated macrophages, colon cancer, phytochemicals, immune checkpoint, nanoparticle delivery
Citation: He Q, Xiang L, Luo Y, Wang R, Zheng C, Gao Y and Yao H (2025) Tumor-associated macrophages in colon cancer immunotherapy: mechanisms, natural product interventions, and microenvironment remodeling. Front. Immunol. 16:1642091. doi: 10.3389/fimmu.2025.1642091
Received: 06 June 2025; Accepted: 23 July 2025;
Published: 12 August 2025.
Edited by:
Yinshen Wee, Taipei Medical University, TaiwanReviewed by:
Heidi Braumüller, University of Freiburg Medical Center, GermanyGaofei Wei, Northwestern Polytechnical University, China
Copyright © 2025 He, Xiang, Luo, Wang, Zheng, Gao and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuan Zheng, emhlbmdjaHVhbkBjZHV0Y20uZWR1LmNu; Yongxiang Gao, ZHJnYW95eEBjZHV0Y20uZWR1LmNu; Huan Yao, SHVhbnlhbzY2MjBAaG90bWFpbC5jb20=; SHVhbnlhb0BjZHV0Y20uZWR1LmNu
 Qingman He
Qingman He Li Xiang
Li Xiang Yuanyuan Luo1
Yuanyuan Luo1 Huan Yao
Huan Yao