- 1School of Medicine, University of Pittsburgh, Pittsburgh, PA, United States
- 2Department of Oral and Maxillofacial Surgery, Charité – Universitätsmedizin Berlin, Berlin, Germany
- 3Department of Otolaryngology, Head and Neck Surgery, Technical University of Munich (TUM) School of Medicine and Health, Technical University of Munich, Munich, Germany
- 4College of Medicine, California Northstate University, Elk Grove, CA, United States
- 5Department of Hand, Plastic and Reconstructive Surgery, Burn Center, BG Trauma Center Ludwigshafen, University of Heidelberg, Ludwigshafen, Germany
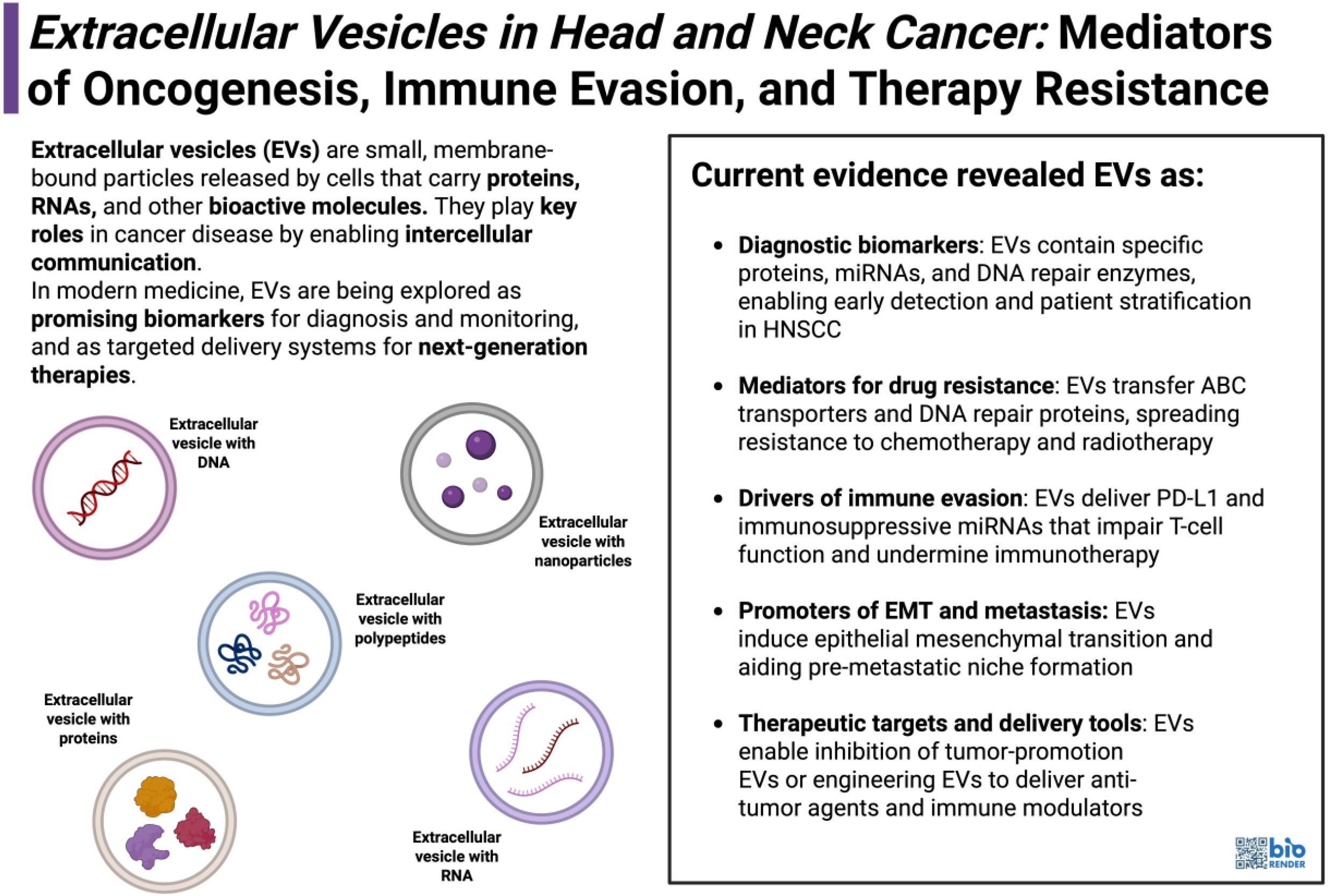
Head and neck squamous cell carcinoma (HNSCC) remains a clinically challenging malignancy due to its intratumoral heterogeneity, aggressive progression, and resistance to multimodal treatment. Extracellular vesicles (EVs)—including exosomes and microvesicles—have gained attention as active contributors to these phenotypes by mediating intercellular signaling and molecular cargo transfer. HNSCC-derived EVs carry oncogenic and drug resistance proteins, along with microRNAs that promote immune evasion and EMT. Enrichment of microRNAs including miR-21, miR-214, and miR-221/222 within EVs supports angiogenesis, apoptosis evasion, and immune suppression. EV-associated PD-L1 impairs antigen presentation and T cell activity, contributing to resistance to checkpoint blockade. Additionally, EVs promote epithelial-to-mesenchymal transition and extracellular matrix remodeling, facilitating invasion and pre-metastatic niche formation. Through modulation of T cell function, macrophage polarization, and stromal recruitment, EVs help establish an immune-tolerant microenvironment. This review synthesizes current knowledge on the mechanistic roles of EVs in HNSCC and discusses their potential as diagnostic biomarkers and therapeutic targets.
1 Introduction
Therapeutic resistance is a pervasive challenge in oncology, accounting for the vast majority of treatment failures in metastatic cancers (1, 2). This resistance emerges from a multifactorial network involving genetic mutations, epigenetic alterations, and microenvironmental influences. Increasingly, extracellular vesicles (EVs) have gained attention as critical mediators of this process (3, 4). These lipid bilayer-enclosed vesicles—secreted by diverse cell types including tumor, stromal, immune, and platelet-derived cells—serve as carriers of bioactive molecules such as proteins, microRNAs, mRNAs, long non-coding RNAs, and fragments of oncogenic DNA (5, 6). By transferring this molecular cargo, EVs facilitate both autocrine and paracrine communication, reprogramming recipient cells and reshaping the tumor microenvironment (TME) to support angiogenesis, immune evasion, metabolic adaptation, and metastasis.
Of particular interest is the role of EVs in undermining anti-tumor immunity and promoting resistance to immunotherapy. In this context, EV-associated PD-L1 can be delivered to T cells and antigen-presenting cells, inducing T cell exhaustion and functional impairment (7–9). Additionally, EVs transport resistance-associated molecules such as ATP-binding cassette (ABC) transporters, anti-apoptotic proteins like Bcl-2, and regulatory microRNAs that collectively suppress apoptosis, enhance DNA repair mechanisms, and facilitate drug efflux (3, 10, 11). These vesicles can also impair antigen presentation and downregulate MHC expression, contributing to the development of an immunologically “cold” tumor microenvironment, even in the presence of immune checkpoint blockade (6, 12, 13).
Head and neck squamous cell carcinoma (HNSCC), a malignancy originating from the mucosal surfaces of the oral cavity, pharynx, and larynx, exemplifies the clinical impact of EV-mediated resistance. Despite aggressive, multimodal treatment strategies—including surgery, radiation, chemotherapy, and immunotherapy—survival rates remain poor for patients with advanced disease (14–16). HNSCC is characterized by a highly immunosuppressive and heterogeneous TME, displaying a spectrum from immune-inflamed to immune-excluded or desert phenotypes (17, 18). These features, coupled with the tumor’s inherent genomic instability and adaptability, make HNSCC a particularly valuable model for studying the functional and translational implications of EVs (17, 19).
This narrative review aims to synthesize current knowledge on the role of extracellular vesicles in HNSCC, with a focus on their contribution to immune modulation and therapy resistance (Figure 1). We begin by outlining EV biogenesis, classification, and cargo profiles (Table 1). We then explore the mechanisms by which HNSCC-derived EVs influence tumor progression, immune escape, and resistance to treatment. Finally, we examine emerging therapeutic strategies targeting EVs and discuss future directions for leveraging EV biology to improve outcomes in HNSCC.
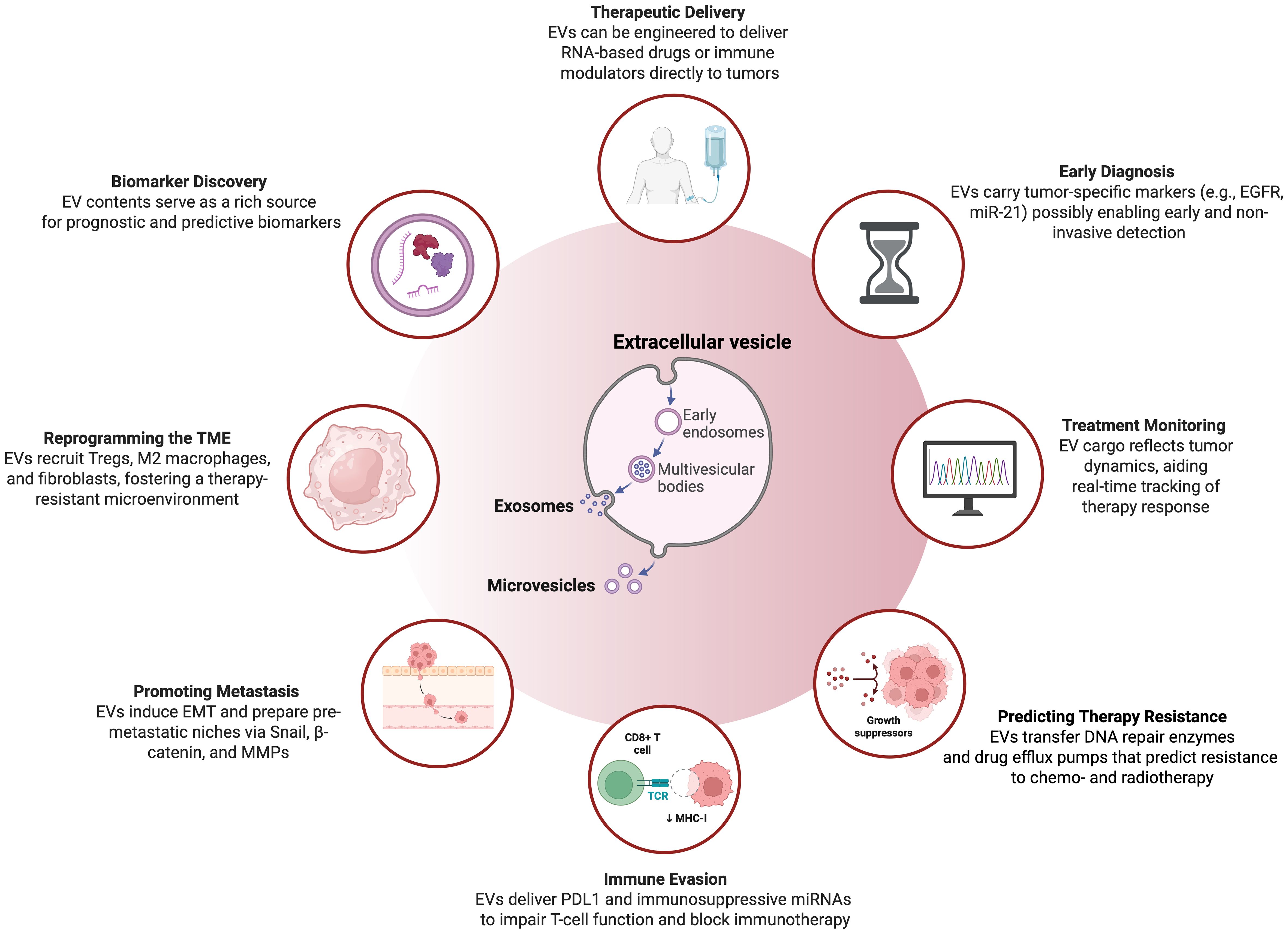
Figure 1. Overview on the multifaceted application and characteristics of extracellular vesicles in head and neck squamous cell carcinoma. This illustration highlights the diverse functions and clinical potential of EVs—primarily exosomes and microvesicles—in HNSCC. Originating from early endosomes and multivesicular bodies, EVs contribute to tumor progression, immune modulation, and therapeutic response. Clinically, they enable early diagnosis via tumor-specific cargo such as EGFR and miR-21, and support treatment monitoring by reflecting dynamic tumor changes. EVs also promote resistance to therapy by transferring DNA repair enzymes and drug efflux pumps, and aid immune evasion through delivery of PD-L1 and immunosuppressive miRNAs. In the tumor microenvironment, they recruit regulatory T cells, M2 macrophages, and fibroblasts, fostering immune suppression and treatment resistance. EVs facilitate metastasis by inducing epithelial-to-mesenchymal transition (EMT) and establishing pre-metastatic niches via Snail, β-catenin, and MMPs. Additionally, they are emerging as delivery platforms for RNA-based drugs and immune modulators, and serve as valuable sources of predictive and prognostic biomarkers. Figure was created using BioRender Premium.
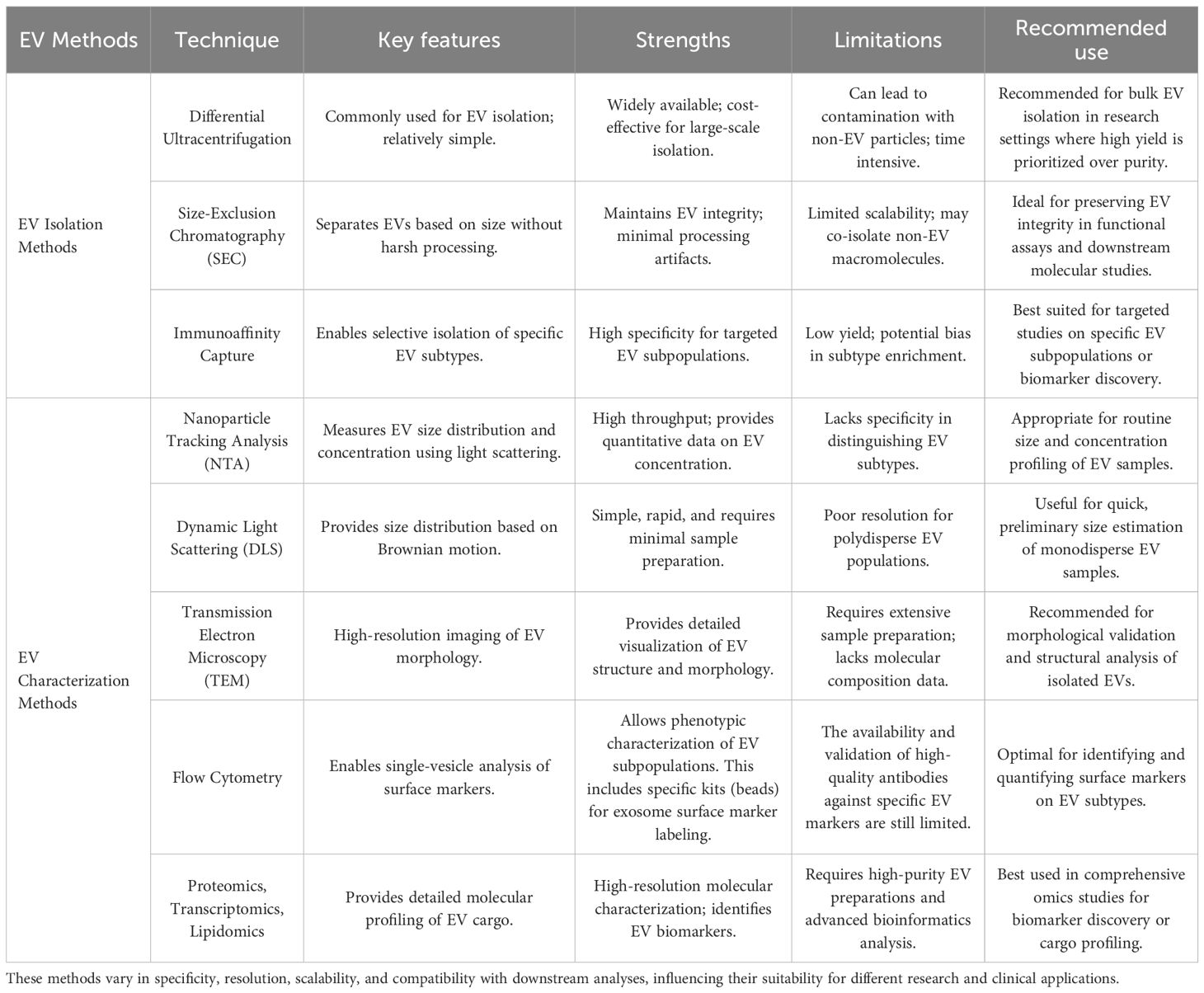
Table 1. Summary of commonly used techniques for EV isolation and characterization, highlighting key features, strengths, and limitations.
2 Extracellular vesicles in HNSCC: mechanisms of tumor progression, immune evasion, and therapeutic resistance
2.1 Biogenesis and classification of EVs in HNSCC
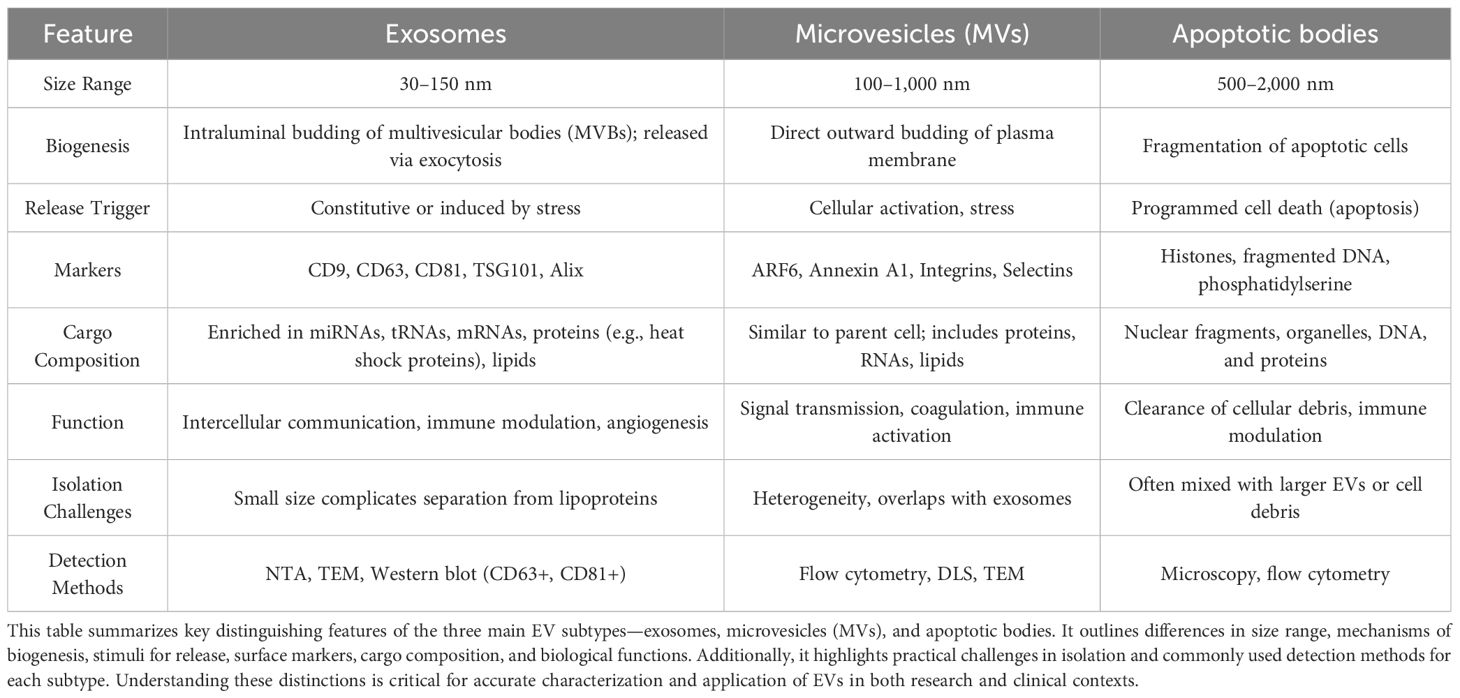
EVs in HNSCC include exosomes, microvesicles, and apoptotic bodies, which differ in biogenesis and molecular signature but collectively act as powerful modulators of tumor progression. Exosomes (30–150 nm), originating from multivesicular bodies via ESCRT-dependent and independent pathways, are enriched in HNSCC with markers like ALIX, TSG101, and tetraspanins, and play a central role in immune suppression through PD-L1 delivery (5, 20, 21). Microvesicles (100–1000 nm), formed by membrane budding and cytoskeletal rearrangement, are particularly enriched in chemoresistant HNSCC cells and carry functional efflux pumps such as P-glycoprotein and DNA repair proteins like ERCC1 (3, 4, 17). Apoptotic bodies (1000–5000 nm), once thought to be inert debris, are now recognized as potential vectors for DAMPs and oncogenic DNA, contributing to the evolution of resistance and intratumoral heterogeneity in HNSCC (4, 5, 22–24). In summary, the classification and cellular origin of EVs in HNSCC directly shape their diverse functional roles in therapy resistance, immune modulation, and tumor adaptation (Table 2).
2.2 Molecular cargo and oncogenic signatures of HNSCC-derived EVs
HNSCC-derived EVs contain a distinctive and functionally potent cargo profile that drives tumor growth and immune modulation. Proteomic analyses confirm enrichment of receptor tyrosine kinases (e.g., EGFR), heat shock proteins (HSP70, HSP90), and ATP-binding cassette transporters (MRP1, ABCG2), all of which support survival signaling and resistance to targeted and cytotoxic therapies (10, 11, 17). In addition, EVs from HNSCC tumors frequently carry DNA repair enzymes such as ERCC1 and XRCC1, enabling recipient cells to better withstand platinum-based chemotherapy (15, 25, 26). Regulatory microRNAs are another key cargo class; miR-21, miR-214, and miR-221/222 are consistently enriched in tumor-derived EVs and modulate gene expression to suppress apoptosis, enhance angiogenesis, and promote invasion (19, 27, 28). These miRNAs exert their effects through well-characterized signaling axes: for example, miR-21 suppresses PTEN, leading to activation of the PI3K-AKT pathway and enhanced cell survival, while miR-221/222 target both PTEN and TIMP3 to promote migration, EMT, and matrix remodeling. In support of this, plasma-derived small EVs (sEVs) from HNSCC patients have been shown to strongly enhance angiogenic potential, underscoring their systemic bioactivity and functional relevance beyond the local tumor microenvironment (29).
2.3 EV-mediated mechanisms of resistance in HNSCC
In HNSCC, EVs serve as mobile vectors of resistance, particularly in the context of cisplatin therapy. Exosomes and microvesicles from resistant HNSCC cells are enriched with ERCC1 and XRCC1, which facilitate nucleotide and base excision repair, thereby neutralizing the cytotoxic effects of DNA-damaging agents (15, 17, 30, 31). These vesicles also carry high levels of HSP70 and HSP90, which stabilize DNA repair proteins and stress-response effectors, further enhancing survival under chemotherapeutic pressure (17). Notably, field cancerization in HNSCC allows EVs to transmit resistance phenotypes across spatially distinct tumor foci, fostering a functionally resistant network (4, 5, 26). Moreover, the presence of anti-apoptotic proteins like Bcl-2 in radioresistant HNSCC-derived EVs confirms their role in shielding cells from radiation-induced cell death (32). Overall, EVs in HNSCC orchestrate a multifaceted resistance network that undermines therapeutic efficacy across both clonal populations and anatomical compartments.
2.4 EV-induced EMT and metastatic reprogramming in HNSCC
EVs in HNSCC actively drive epithelial-mesenchymal transition (EMT), a key program in metastasis and immune evasion. Tumor-derived EVs transport transcriptional repressors such as Snail and β-catenin, which suppress epithelial markers (e.g., E-cadherin) and upregulate mesenchymal traits (e.g., vimentin, N-cadherin), promoting migratory and invasive capacities (33–36). These EVs also deliver MMP1, MMP3, and integrins (ITGA6, ITGB1), which degrade the extracellular matrix and enable pre-metastatic niche formation—processes particularly relevant in high-grade HNSCC (23, 37). Recent findings have shown that plasma-derived sEVs can reprogram macrophages to facilitate pre-metastatic niche formation in HNSCC, emphasizing their role in priming distant sites for metastatic colonization (38). Furthermore, EVs contain annexins and galectin-3-binding protein (LGALS3BP), which contribute to stromal reprogramming and immune cell dysfunction (39–42). Thereby, EV-driven EMT in HNSCC fosters a dual threat of immune escape and enhanced invasiveness, positioning EVs as central regulators of metastatic evolution.
2.5 Immunosuppressive functions of EVs in the HNSCC microenvironment
HNSCC-derived EVs are potent immunosuppressive agents that sculpt a TME conducive to tumor persistence and immune escape. Additionally, miR-27a within these vesicles targets immune-activating genes, suppressing co-stimulatory molecule expression in dendritic cells and macrophages (43, 44). EVs also act as antigen decoys, shedding tumor-associated antigens (TAAs) to divert immune recognition while displaying surface CD47 to inhibit macrophage-mediated clearance (45, 46). Moreover, HNSCC EVs secrete TGF-β and IL-10, driving Treg expansion and M2 macrophage polarization, both of which contribute to a tolerogenic and therapy-resistant microenvironment (6, 13). In summary, EVs play a pivotal role in HNSCC immune evasion by blunting anti-tumor immunity and engineering an immune landscape hostile to therapeutic response. The diverse immunosuppressive, pro-metastatic, and resistance-promoting functions of EVs in HNSCC are summarized in Figure 2.
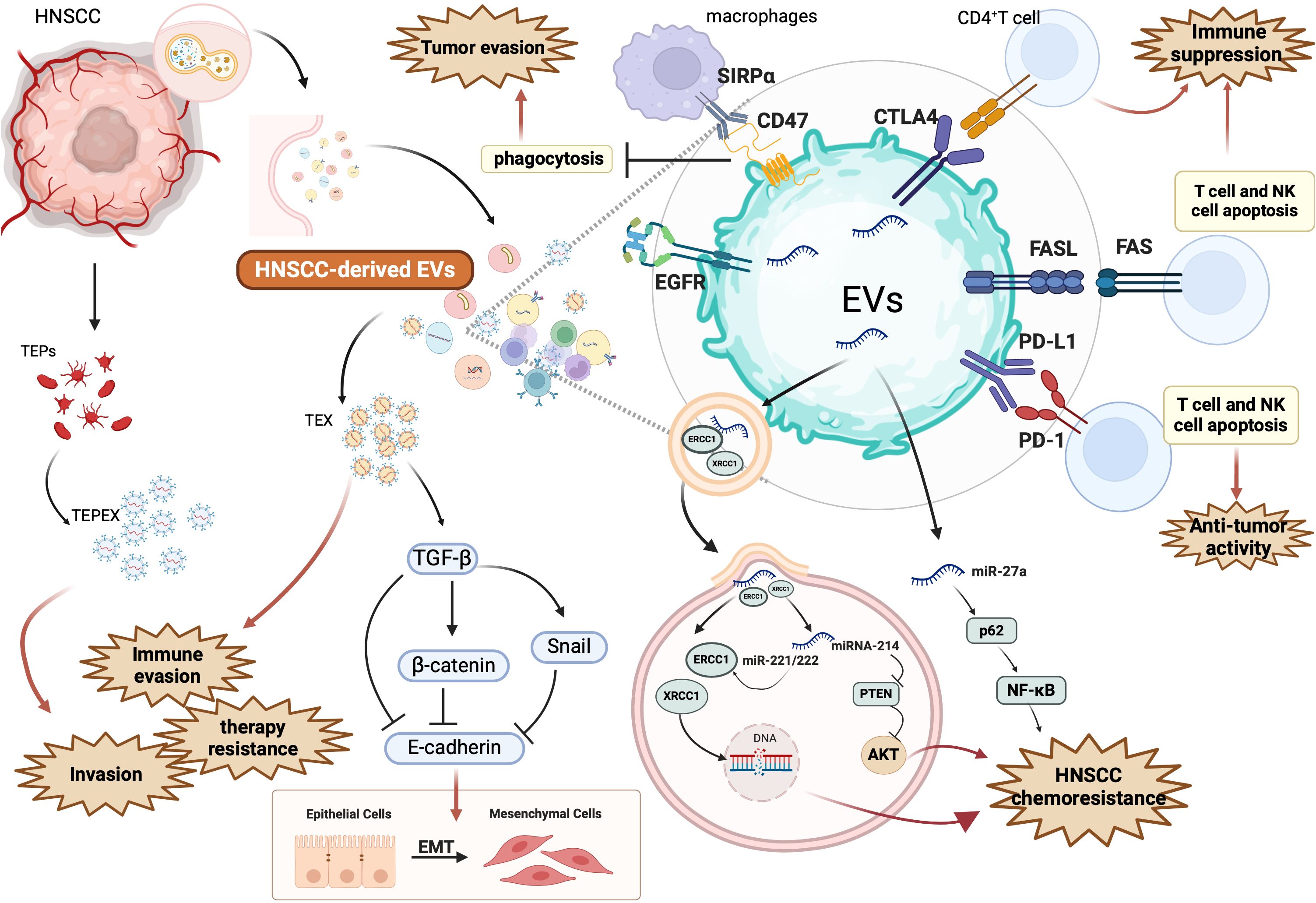
Figure 2. Mechanisms by which HNSCC-derived EV promote tumor progression, immune suppression, and therapeutic resistance. This figure illustrates the multifaceted role of EVs secreted by head and neck squamous cell carcinoma (HNSCC) cells in shaping a pro-tumorigenic microenvironment. HNSCC-derived EVs carry a range of immunosuppressive ligands, signaling molecules, and non-coding RNAs that disrupt immune surveillance and enhance cancer cell survival. Specifically, EV-associated PD-L1 and FasL bind to PD-1 and FAS on T cells and NK cells, triggering apoptosis and weakening anti-tumor immunity. They also express CTLA4 and CD47, which inhibit CD4+ T cell activation and macrophage-mediated phagocytosis via SIRPα signaling. These EVs also reprogram immune cells and tumor-associated endothelial precursors (TEX, TEPs), contributing to immune evasion and expansion of TEPEX populations. Through cargoes such as TGF-β, β-catenin, and Snail, EVs induce epithelial-to-mesenchymal transition (EMT), characterized by E-cadherin downregulation, thereby promoting invasion and metastasis. EVs further enhance chemoresistance by transferring DNA repair enzymes ERCC1 and XRCC1, and miRNAs such as miR-221/222, miR-214, and miR-27a, which regulate PTEN/AKT and NF-κB pathways. For example, miR-27a suppresses p62, activating NF-κB and supporting resistance. These alterations bolster cell survival and therapy resistance. Additionally, EGFR-enriched EVs may influence receptor-mediated signaling and modulate downstream oncogenic pathways. Collectively, this figure underscores how HNSCC-derived EVs modulate the immune microenvironment, facilitate EMT and invasion, and transmit resistance mechanisms to both immune checkpoint inhibitors and chemotherapies. Figure was created using BioRender Premium.
2.6 Integrative role of EVs in HNSCC progression and therapeutic resistance
Extracellular vesicles in HNSCC serve as multifaceted conduits of tumor adaptation, orchestrating molecular, cellular, and systemic changes that reinforce malignancy. By transferring oncogenic and resistance-associated cargo, EVs orchestrate interactions between tumor, stromal, and immune compartments that reinforce therapeutic failure (2, 3, 5, 17). Moreover, the capacity of EVs to cross anatomical and histological boundaries through lymphatic and circulatory systems makes them ideal vehicles for intercellular influence across the entire tumor landscape (4, 23, 47). In the vascular compartment, HNSCC-derived EVs have been shown to activate and aggregate platelets through tissue factor in a calcium-dependent manner, potentially facilitating hematogenous metastasis and immune cloaking (48). Strategies to disrupt EV-mediated signaling networks include pharmacologic inhibitors of vesicle secretion, antibodies targeting surface ligands, CRISPR-Cas9-based gene editing, and engineered EVs designed to deliver immunomodulatory or gene-silencing cargo, as illustrated in Figure 3.

Figure 3. Therapeutic strategies for targeting EVs in HNSCC. This figure summarizes new and established strategies aimed at modulating EV biogenesis, release, and function in head and neck squamous cell carcinoma (HNSCC). Pharmacologic agents such as GW4869 (a neutral sphingomyelinase inhibitor) and PX-478 (a hypoxia-inducible factor [HIF] inhibitor) suppress EV production and cargo release under stress conditions, including hypoxia. Spastin and VPS4a, key mediators of multivesicular body (MVB) formation, and the ESCRT machinery, are involved in EV biogenesis and can be therapeutically targeted to reduce vesicle secretion. These approaches remain in the preclinical stage. EV-mediated immune suppression via PD-L1 can be blocked using anti-PD-L1 antibodies (clinically approved), thereby restoring T cell activation through CD28 and reducing tumor immune evasion. Inhibition of miR-214, which regulates PTEN and promotes PI3K/AKT-mediated survival and drug resistance, is another preclinical strategy. Engineered EVs are being developed to deliver therapeutic RNAs (siRNAs, miRNAs, mRNAs) and immune ligands to tumors, aiming to enhance anti-tumor responses and suppress proliferation. Clinically approved agents such as Cetuximab, an anti-EGFR monoclonal antibody, and EGFR tyrosine kinase inhibitors (Gefitinib, Erlotinib) block EGFR-positive EV signaling and downstream cancer growth. TGF-β signaling, which promotes EV-facilitated immunosuppression and cancer-associated fibroblast (CAF) activation, can be targeted with the TGF-β receptor I inhibitor LY2157299 (preclinical). Additional therapies like Marimastat (MMP inhibitor), Heparin/Annexin V (inhibiting EV uptake), and Cilengitide (integrin antagonist) are being explored in preclinical studies to limit metastasis and drug resistance by disrupting EV-ECM and immune interactions. Finally, CRISPR-Cas9 is under investigation as a tool to knock out resistance-related genes or oncogenic miRNAs (e.g., miR-214), offering precision EV-based therapies. Together, these interventions aim to block EV-mediated oncogenic communication, reduce tumor proliferation, enhance immune responses, and overcome chemoresistance. Figure was created using BioRender Premium.
3 Discussion
EVs have emerged as central regulators of tumor biology, playing multifaceted roles in driving oncogenesis, modulating immune responses, and promoting resistance to therapy. In HNSCC, a highly heterogeneous and aggressive malignancy, EVs enable dynamic intercellular communication that reinforces tumor progression and therapeutic failure. This review synthesizes current findings on the contributions of EVs to immune evasion, drug resistance, and metastasis in HNSCC. By elucidating these mechanisms, new avenues may emerge for leveraging EVs as diagnostic biomarkers and therapeutic targets in this challenging disease.
Our review found EVs in HNSCC to play a pivotal role in tumor progression by transferring oncogenic proteins, drug resistance factors, and immunosuppressive molecules, thereby promoting chemoresistance, immune evasion, and metastasis. Their ability to drive epithelial-mesenchymal transition, remodel the tumor microenvironment, and disseminate resistance traits across tumor sites highlighted their integrative function in maintaining malignant phenotypes.
However, despite the growing understanding of EV-mediated oncogenesis, literature revealed several challenges in studying and therapeutically targeting EVs. The heterogeneity of EV populations, the dynamic nature of their biogenesis, and the diversity in their molecular cargo present substantial obstacles in precisely defining their functional roles (12, 47). Additionally, the lack of standardized methods for EV isolation, characterization, and functional analysis continues to hinder the translation of laboratory findings into clinical applications (49, 50). Several recent advances are helping to overcome the technical challenges posed by EV heterogeneity and lack of standardized workflows. Updated protocols from the International Society for Extracellular Vesicles (MISEV) now provide widely adopted guidelines for EV nomenclature, isolation, and characterization, helping to reduce methodological variability across studies (51). On the technical front, bead-based multiplex assays and immunocapture platforms now allow for parallel profiling of distinct EV subpopulations from patient plasma or cell culture media (52). In addition, tools like tangential flow filtration (TFF), size-exclusion chromatography, and the exoRNeasy system have improved reproducibility and purity across isolation protocols (53). Single-EV profiling using nano-flow cytometry or super-resolution microscopy is also increasingly accessible, allowing researchers to resolve cargo heterogeneity within individual vesicles (54).
The immunomodulatory effects of EVs, particularly through PD-L1 delivery, further complicate the evolving landscape of immunotherapy in HNSCC. EV-mediated PD-L1 transfer enhances immune evasion by suppressing T-cell activity, creating an immunosuppressive microenvironment that favors tumor persistence and therapeutic resistance (7–9). The challenge of intercepting EV-driven immune escape necessitates novel strategies that restore immune surveillance and enhance the efficacy of immune checkpoint inhibitors.
Despite these challenges, targeting EV biogenesis, release, and uptake presents critical focus of ongoing translational research. Engineered EVs that selectively deliver anti-tumor agents, inhibitors of oncogenic pathways, or immune-modulating molecules offer a precision medicine approach to counteract EV-mediated disease progression, although therapeutic specificity remains complicated by EV heterogeneity (3, 4). Additionally, small-molecule inhibitors that block EV formation or disrupt their interactions with recipient cells could enhance the efficacy of existing treatments by mitigating drug resistance and limiting metastatic spread (5, 49). Heterogeneity presents a translational challenge because differences in vesicle origin, cargo, and surface markers complicate efforts to selectively target tumor-promoting subsets while preserving normal intercellular signaling. New technologies such as single-vesicle flow cytometry, high-resolution proteomics, and droplet-based microfluidics are enhancing the sensitivity and resolution of EV profiling, offering new avenues for dissecting EV subtypes and improving biomarker discovery (55–57). As research advances, the development of EV-targeted therapeutics and EV-based biomarkers has the potential to revolutionize cancer diagnostics and treatment paradigms (3, 5, 13).
This review underscores EVs as mediators of immune evasion, therapeutic resistance, and metastatic behavior in HNSCC. For clinicians, the translational potential lies in leveraging EVs as diagnostic biomarkers, such as for PD-L1 or drug-resistance markers and as novel therapeutic targets, including strategies that block EV secretion, uptake, or immunosuppressive cargo. Arginase-1 enrichment in plasma-derived EVs has been identified as a potential biomarker for metastatic disease in HNSCC patients, alongside other clinically investigated EV markers such as PD-L1, EGFR, and miR-21, offering minimally invasive tools for risk stratification and therapy response monitoring (58).
In addition to Arginase-1, several other EV-based applications are entering clinical workflows. PD-L1–expressing EVs have been proposed as predictive biomarkers to stratify patients for immune checkpoint inhibitor therapy, particularly in non-responders with low tumor cell PD-L1 expression but high EV-PD-L1 burden (59). Moreover, liquid biopsies leveraging EV cargo such as miR-21 and EGFR have shown promise in early detection and recurrence monitoring, with prospective trials exploring their integration into routine follow-up protocols (60). Therapeutically, engineered EVs are being developed to deliver targeted siRNAs, CRISPR-Cas9 systems, or immune ligands to tumor sites, enabling cell-type–specific modulation with minimal systemic toxicity (34). For example, miR-34a has shown potent anti-tumor effects in HNSCC xenograft models when delivered using chemically stabilized mimics, and its therapeutic delivery via engineered EVs is currently under investigation in other cancer types, supporting its potential for EV-based applications in HNSCC (61).
EVs from HNSCC are increasingly studied for liquid biopsy. For example, circulating exosomal PD‐L1 levels correlate with tumor stage and can predict relapse (62). Similarly, tumor‐derived exosomal EGFR and phospho‐EGFR fall during anti‐EGFR (cetuximab) therapy, suggesting EV‐EGFR can monitor therapeutic response (63). EV‐microRNAs also show promise: an 11‐miRNA signature in serum EVs robustly detected HPV+ oropharyngeal SCC (64). On the therapy side, EVs can be engineered as drug carriers. Engineered EVs have been loaded with siRNAs, cytokines or even gene‐editing systems (e.g., CRISPR/Cas9) to modulate tumors (65, 66). For instance, exosomes displaying immunostimulatory ligands or checkpoint inhibitors have been constructed to activate T cells (7, 67). Several clinical trials now involve EV platforms. For instance, a Phase I trial (NCT03608631) is testing MSC‐derived exosomes delivering KRAS‐G12D siRNA in pancreatic cancer (68). In fact, multiple EV‐based trials (most using MSC or dendritic‐cell EVs) are registered for solid tumors (69).
However, clinical translation and translation to HNSCC in particular faces key hurdles: the heterogeneity of EV populations, lack of standardization in isolation and profiling methods, and incomplete understanding of their biological roles all limit current applicability. Furthermore, selectively targeting tumor-promoting EVs without disrupting physiological intercellular communication remains a major challenge. For patients, the promise of EV-based interventions is substantial, offering hope for more personalized and effective therapies—especially in refractory or recurrent disease—but realization of these benefits will depend on robust clinical validation. Overall, future research must focus on refining EV detection technologies, validating EV-based biomarkers in large patient cohorts, and rigorously testing EV-targeted therapies in preclinical and clinical models.
4 Conclusion
By shaping the tumor microenvironment, promoting immune escape, and facilitating metastasis, EVs serve as key drivers of therapeutic resistance and disease persistence. Their biofluid stability, tumor-derived cargo, and immunomodulatory roles make EVs promising candidates for precision diagnostics and therapeutic targeting in HNSCC. However, clinical translation is limited by the heterogeneity of EV populations, lack of standardization in isolation and characterization methods, and incomplete understanding of their context-specific functions. Future work should focus on refining EV profiling techniques, developing selective targeting strategies, and validating clinical applications in well-designed studies. As our understanding deepens, EVs may ultimately offer a novel framework for precision diagnostics and therapy in HNSCC.
Author contributions
JD: Formal Analysis, Writing – original draft, Investigation. TN: Investigation, Methodology, Writing – original draft. CH: Visualization, Conceptualization, Writing – original draft. BM: Writing – review & editing, Investigation. BW: Writing – review & editing. FM: Writing – review & editing. GH: Writing – review & editing. MR: Writing – review & editing. MH: Writing – review & editing. JV: Writing – review & editing. AP: Writing – review & editing. SK: Conceptualization, Writing – review & editing, Supervision. LK: Supervision, Writing – review & editing, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
ABC: ATP-binding cassette
ABCG2: ATP-binding cassette sub-family G member 2
ALIX: Apoptosis-linked gene 2-interacting protein X
BER: Base excision repair
Bcl-2: B-cell lymphoma 2
CAF: Cancer-associated fibroblast
DAMP: Damage-associated molecular pattern
DLS: Dynamic light scattering
EGFR: Epidermal growth factor receptor
ECM: Extracellular matrix
ESCRT: Endosomal sorting complex required for transport
EV: Extracellular vesicle
HIF: Hypoxia-inducible factor
HNSCC: Head and neck squamous cell carcinoma
HSP: Heat shock protein
IL-8: Interleukin-8
ITGA6: Integrin alpha-6
ITGB1: Integrin beta-1
LGALS3BP: Galectin-3-binding protein
lncRNA: Long non-coding RNA
LAMP1: Lysosomal-associated membrane protein 1
LAMP2: Lysosomal-associated membrane protein 2
MAPK: Mitogen-activated protein kinase
MMP: Matrix metalloproteinase
MRP1: Multidrug resistance protein 1
MVB: Multivesicular body
mRNA: Messenger RNA
NER: Nucleotide excision repair
NTA: Nanoparticle tracking analysis
PD-1: Programmed cell death protein 1
PD-L1: Programmed death-ligand 1
PDCD6IP: Programmed cell death 6-interacting protein
PI3K/AKT: Phosphoinositide 3-kinase/Protein kinase B
PS: Phosphatidylserine
PTEN: Phosphatase and tensin homolog
SEC: Size-exclusion chromatography
SSB: Single-strand break
siRNA: Small interfering RNA
TAA: Tumor-associated antigens
TGF-β: Transforming growth factor beta
TIL: Tumor-infiltrating lymphocyte
TME: Tumor microenvironment
TNM: Tumor-node-metastasis classification system
TSG101: Tumor susceptibility gene 101
VEGF: Vascular endothelial growth factor
VIM: Vimentin
XRCC1: X-ray repair cross-complementing protein 1.
References
1. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
2. Kilmister EJ, Koh SP, Weth FR, Gray C, and Tan ST. Cancer metastasis and treatment resistance: mechanistic insights and therapeutic targeting of cancer stem cells and the tumor microenvironment. Biomedicines. (2022) 10:2988. doi: 10.3390/biomedicines10112988
3. Yang Q, Xu J, Gu J, Shi H, Zhang J, Zhang J, et al. Extracellular vesicles in cancer drug resistance: roles, mechanisms, and implications. Advanced Sci. (2022) 9:2201609. doi: 10.1002/advs.202201609
4. Maacha S, Bhat AA, Jimenez L, Raza A, Haris M, Uddin S, et al. Extracellular vesicles-mediated intercellular communication: roles in the tumor microenvironment and anti-cancer drug resistance. Mol Cancer. (2019) 18:55. doi: 10.1186/s12943-019-0965-7
5. Kumar MA, Baba SK, Sadida HQ, Marzooqi SAl, Jerobin J, Altemani FH, et al. Extracellular vesicles as tools and targets in therapy for diseases. Sig Transduct Target Ther. (2024) 9:27. doi: 10.1038/s41392-024-01735-1
6. Kuang L, Wu L, and Li Y. Extracellular vesicles in tumor immunity: mechanisms and novel insights. Mol Cancer. (2025) 24:45. doi: 10.1186/s12943-025-02233-w
7. Chen J, Yang J, Wang W, Guo D, Zhang C, Wang S, et al. Tumor extracellular vesicles mediate anti-PD-L1 therapy resistance by decoying anti-PD-L1. Cell Mol Immunol. (2022) 19:1290–301. doi: 10.1038/s41423-022-00926-6
8. Liu J, Peng X, Yang S, Li X, Huang M, Wei S, et al. Extracellular vesicle PD-L1 in reshaping tumor immune microenvironment: biological function and potential therapy strategies. Cell Commun Signal. (2022) 20:14. doi: 10.1186/s12964-021-00816-w
9. Yu Z-L, Liu J-Y, and Chen G. Small extracellular vesicle PD-L1 in cancer: the knowns and unknowns. NPJ Precis Onc. (2022) 6:42. doi: 10.1038/s41698-022-00287-3
10. Rigalli JP, Gagliardi A, Diester K, Bajraktari-Sylejmani G, Blank A, Burhenne J, et al. Extracellular vesicles as surrogates for the regulation of the drug transporters ABCC2 (MRP2) and ABCG2 (BCRP). Int J Mol Sci. (2024) 25:4118. doi: 10.3390/ijms25074118
11. Cole SPC. Multidrug resistance protein 1 (MRP1, ABCC1), a ‘multitasking’ ATP-binding cassette (ABC) transporter. J Biol Chem. (2014) 289:30880–8. doi: 10.1074/jbc.R114.609248
12. Ma F, Vayalil J, Lee G, Wang Y, and Peng G. Emerging role of tumor-derived extracellular vesicles in T cell suppression and dysfunction in the tumor microenvironment. J Immunother Cancer. (2021) 9:e003217. doi: 10.1136/jitc-2021-003217
13. Mittal S, Gupta P, Chaluvally-Raghavan P, and Pradeep S. Emerging role of extracellular vesicles in immune regulation and cancer progression. Cancers (Basel). (2020) 12:3563. doi: 10.3390/cancers12123563
14. Smith CDL, McMahon AD, Purkayastha M, Creaney G, Clements K, Inman GJ, et al. Head and neck cancer incidence is rising but the sociodemographic profile is unchanging: a population epidemiological study (2001–2020). BJC Rep. (2024) 2:71. doi: 10.1038/s44276-024-00089-z
15. Vaezi, Niedernhofer L, and Feldman. ERCC1 and XRCC1 as biomarkers for lung and head and neck cancer. PGPM 47. (2011) 4:47–63. doi: 10.2147/PGPM.S20317
16. Barsouk A, Aluru JS, Rawla P, Saginala K, and Barsouk A. Epidemiology, risk factors, and prevention of head and neck squamous cell carcinoma. Med Sci. (2023) 11:42. doi: 10.3390/medsci11020042
17. Wang X, Guo J, Yu P, Guo L, Mao X, Wang J, et al. The roles of extracellular vesicles in the development, microenvironment, anticancer drug resistance, and therapy of head and neck squamous cell carcinoma. J Exp Clin Cancer Res. (2021) 40:35. doi: 10.1186/s13046-021-01840-x
18. Muijlwijk T, Nauta IH, Van Der Lee A, Grünewald KJT, Brink A, Ganzevles SH, et al. Hallmarks of a genomically distinct subclass of head and neck cancer. Nat Commun. (2024) 15:9060. doi: 10.1038/s41467-024-53390-3
19. Li L-J, Chang W-M, and Hsiao M. Aberrant expression of microRNA clusters in head and neck cancer development and progression: current and future translational impacts. Pharm (Basel). (2021) 14:194. doi: 10.3390/ph14030194
20. Kowal J, Tkach M, and Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. (2014) 29:116–25. doi: 10.1016/j.ceb.2014.05.004
21. Elsherbini A and Bieberich E. Ceramide and exosomes: A novel target in cancer biology and therapy. In: Advances in cancer research, vol. 140. Amsterdam, Netherlands: Elsevier (2018). p. 121–54.
22. Samuels M, Cilibrasi C, Papanastasopoulos P, and Giamas G. Extracellular vesicles as mediators of therapy resistance in the breast cancer microenvironment. Biomolecules. (2022) 12:132. doi: 10.3390/biom12010132
23. Yang X, Zhang Y, Zhang Y, Zhang S, Qiu L, Zhuang Z, et al. The key role of exosomes on the pre-metastatic niche formation in tumors. Front Mol Biosci. (2021) 8:703640. doi: 10.3389/fmolb.2021.703640
24. Zou X, Lei Q, Luo X, Yin J, Chen S, Hao C, et al. Advances in biological functions and applications of apoptotic vesicles. Cell Commun Signal. (2023) 21:260. doi: 10.1186/s12964-023-01251-9
25. Dong X, Bai X, Ni J, Zhang H, Duan W, Graham P, et al. Exosomes and breast cancer drug resistance. Cell Death Dis. (2020) 11:987. doi: 10.1038/s41419-020-03189-z
26. Chen S-H and Chang J-Y. New insights into mechanisms of cisplatin resistance: from tumor cell to microenvironment. Int J Mol Sci. (2019) 20:4136. doi: 10.3390/ijms20174136
27. Di Martino MT, Arbitrio M, Caracciolo D, Cordua A, Cuomo O, Grillone K, et al. miR-221/222 as biomarkers and targets for therapeutic intervention on cancer and other diseases: A systematic review. Mol Ther - Nucleic Acids. (2022) 27:1191–224. doi: 10.1016/j.omtn.2022.02.005
28. Liu J, Chen W, Zhang H, Liu T, and Zhao L. miR−214 targets the PTEN−mediated PI3K/Akt signaling pathway and regulates cell proliferation and apoptosis in ovarian cancer. Oncol Lett. (2017) 14(5):5711–8. doi: 10.3892/ol.2017.6953
29. Zheng Y, Xie J, Jiang F, Li Y, Chang G, Ma H, et al. Inhibition of miR−21 promotes cell apoptosis in oral squamous cell carcinoma by upregulating PTEN. Oncol Rep. (2018) 40(5):2798–805. doi: 10.3892/or.2018.6663
30. Arora S, Kothandapani A, Tillison K, Kalman-Maltese V, and Patrick SM. Downregulation of XPF–ERCC1 enhances cisplatin efficacy in cancer cells☆. DNA Repair. (2010) 9:745–53. doi: 10.1016/j.dnarep.2010.03.010
31. Moor N and Lavrik O. Coordination of DNA base excision repair by protein-protein interactions. In: Mognato M, editor. DNA repair- an update. London, United Kingdom: IntechOpen (2019). doi: 10.5772/intechopen.82642
32. Yamana K, Inoue J, Yoshida R, Sakata J, Nakashima H, Arita H, et al. Extracellular vesicles derived from radioresistant oral squamous cell carcinoma cells contribute to the acquisition of radioresistance via the miR-503-3p-BAK axis. J Extracellular Vesicle. (2021) 10:e12169. doi: 10.1002/jev2.12169
33. Mastronikolis NS, Kyrodimos E, Spyropoulou D, Delides A, Giotakis E, Piperigkou Z, et al. The role of exosomes in epithelial–to-mesenchymal transition and cell functional properties in head and neck cancer. Cancers. (2023) 15:2156. doi: 10.3390/cancers15072156
34. Paskeh MDA, Entezari M, Mirzaei S, Zabolian A, Saleki H, Naghdi MJ, et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J Hematol Oncol. (2022) 15:83. doi: 10.1186/s13045-022-01305-4
35. Loh C-Y, Chai J, Tang T, Wong W, Sethi G, Shanmugam M, et al. The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic implications, and challenges. Cells. (2019) 8:1118. doi: 10.3390/cells8101118
36. Usman S, Waseem NH, Nguyen TKN, Mohsin S, Jamal A, Teh M-T, et al. Vimentin is at the heart of epithelial mesenchymal transition (EMT) mediated metastasis. Cancers (Basel). (2021) 13:4985. doi: 10.3390/cancers13194985
37. Grzywa TM, Klicka K, and Włodarski PK. Regulators at Every Step-How microRNAs Drive Tumor Cell Invasiveness and Metastasis. Cancers (Basel). (2020) 12:3709. doi: 10.3390/cancers12123709
38. Huber D, Kors TA, Schütt L, Hofmann L, Betzler A, Lotfi R, et al. The role of plasma-derived small extracellular vesicles in pre-metastatic niche formation through modulation of macrophages in head and neck squamous cell carcinoma. Br J Cancer. (2025) 133:121–30. doi: 10.1038/s41416-025-03001-9
39. Rana R, Chauhan K, Gautam P, Kulkarni M, Banarjee R, Chugh P, et al. Plasma-derived extracellular vesicles reveal galectin-3 binding protein as potential biomarker for early detection of glioma. Front Oncol. (2021) 11:778754. doi: 10.3389/fonc.2021.778754
40. Yang J, Pei T, Su G, Duan P, and Liu X. AnnexinA6: a potential therapeutic target gene for extracellular matrix mineralization. Front Cell Dev Biol. (2023) 11:1201200. doi: 10.3389/fcell.2023.1201200
41. Novizio N, Belvedere R, Pessolano E, Tosco A, Porta A, Perretti M, et al. Annexin A1 released in extracellular vesicles by pancreatic cancer cells activates components of the tumor microenvironment, through interaction with the formyl-peptide receptors. Cells. (2020) 9:2719. doi: 10.3390/cells9122719
42. Weijie S. Annexin A2: the feasibility of being a therapeutic target associated with cancer metastasis and drug resistance in cancer microenvironment. Discov Onc. (2024) 15:783. doi: 10.1007/s12672-024-01693-8
43. Li X, Xu M, Ding L, and Tang J. MiR-27a: A novel biomarker and potential therapeutic target in tumors. J Cancer. (2019) 10:2836–48. doi: 10.7150/jca.31361
44. Li Y, Tan J, Miao Y, and Zhang Q. MicroRNA in extracellular vesicles regulates inflammation through macrophages under hypoxia. Cell Death Discov. (2021) 7:285. doi: 10.1038/s41420-021-00670-2
45. Kato T, Fahrmann JF, Hanash SM, and Vykoukal J. Extracellular vesicles mediate B cell immune response and are a potential target for cancer therapy. Cells. (2020) 9:1518. doi: 10.3390/cells9061518
46. Koh E, Lee EJ, Nam G-H, Hong Y, Cho E, Yang Y, et al. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials. (2017) 121:121–9. doi: 10.1016/j.biomaterials.2017.01.004
47. Gurung S, Perocheau D, Touramanidou L, and Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. (2021) 19:47. doi: 10.1186/s12964-021-00730-1
48. Weiser T, Hoch CC, Petry J, Shoykhet M, Schmidl B, Yazdi M, et al. Head and neck squamous cell carcinoma-derived extracellular vesicles mediate Ca2+-dependent platelet activation and aggregation through tissue factor. Cell Commun Signal. (2025) 23:210. doi: 10.1186/s12964-025-02215-x
49. Catalano M and O’Driscoll L. Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. J Extracell Vesicles. (2020) 9:1703244. doi: 10.1080/20013078.2019.1703244
50. Ståhl A-L, Johansson K, Mossberg M, Kahn R, and Karpman D. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatr Nephrol. (2019) 34:11–30. doi: 10.1007/s00467-017-3816-z
51. Welsh JA, Goberdhan DCI, O’Driscoll L, Buzas EI, Blenkiron C, Bussolati B, et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J Extracellular Vesicle. (2024) 13(5). doi: 10.1002/jev2.12404
52. De Sousa KP, Rossi I, Abdullahi M, Ramirez MI, Stratton D, Inal JM, et al. Isolation and characterization of extracellular vesicles and future directions in diagnosis and therapy. Wiley Interdiscip Rev Nanomed Nanobiotechnol. (2023) 15:e1835. doi: 10.1002/wnan.1835
53. Visan KS, Lobb RJ, Ham S, Lima LG, Palma C, Edna CPZ, et al. Comparative analysis of tangential flow filtration and ultracentrifugation, both combined with subsequent size exclusion chromatography, for the isolation of small extracellular vesicles. J Extracell Vesicles. (2022) 11:e12266. doi: 10.1002/jev2.12266
54. Lee Y-J, Chae S, and Choi D. Monitoring of single extracellular vesicle heterogeneity in cancer progression and therapy. Front Oncol. (2023) 13:1256585. doi: 10.3389/fonc.2023.1256585
55. Wang Z, Zhou X, Kong Q, He H, Sun J, Qiu W, et al. Extracellular vesicle preparation and analysis: A state-of-the-art review. Advanced Sci. (2024) 11. doi: 10.1002/advs.202401069
56. Wang S, Khan A, Huang R, Ye S, Di K, Xiong T, et al. Recent advances in single extracellular vesicle detection methods. Biosensors Bioelectronics. (2020) 154:112056. doi: 10.1016/j.bios.2020.112056
57. Silva TF, Hutchins E, Zhao W, Ciani Y, Kim M, Ko E, et al. Extracellular vesicle heterogeneity through the lens of multiomics. Cell Rep Med. (2025) 6:102161. doi: 10.1016/j.xcrm.2025.102161
58. Hofmann L, Harasymczuk M, Huber D, Szczepanski MJ, Dworacki G, Whiteside TL, et al. Arginase-1 in plasma-derived exosomes as marker of metastasis in patients with head and neck squamous cell carcinoma. Cancers (Basel). (2023) 15:5449. doi: 10.3390/cancers15225449
59. Schöne N, Kemper M, Menck K, Evers G, Krekeler C, Schulze AB, et al. PD-L1 on large extracellular vesicles is a predictive biomarker for therapy response in tissue PD-L1-low and -negative patients with non-small cell lung cancer. J Extracell Vesicles. (2024) 13:e12418. doi: 10.1002/jev2.12418
60. Asleh K, Dery V, Taylor C, Davey M, Djeungoue-Petga M-A, Ouellette RJ, et al. Extracellular vesicle-based liquid biopsy biomarkers and their application in precision immuno-oncology. biomark Res. (2023) 11:99. doi: 10.1186/s40364-023-00540-2
61. Wu X, Cheng Y-SL, Matthen M, Yoon A, Schwartz GK, Bala S, et al. Down-regulation of the tumor suppressor miR-34a contributes to head and neck cancer by up-regulating the MET oncogene and modulating tumor immune evasion. J Exp Clin Cancer Res. (2021) 40. doi: 10.1186/s13046-021-01865-2
62. Theodoraki M-N, Laban S, Jackson EK, Lotfi R, Schuler PJ, Brunner C, et al. Changes in circulating exosome molecular profiles following surgery/(chemo)radiotherapy: early detection of response in head and neck cancer patients. Br J Cancer. (2021) 125:1677–86. doi: 10.1038/s41416-021-01567-8
63. Van Dommelen SM, Van Der Meel R, Van Solinge WW, Coimbra M, Vader P, Schiffelers RM, et al. Cetuximab treatment alters the content of extracellular vesicles released from tumor cells. Nanomedicine (Lond.). (2016) 11:881–90. doi: 10.2217/nnm-2015-0009
64. Mayne GC, Woods CM, Dharmawardana N, Wang T, Krishnan S, Hodge JC, et al. Cross validated serum small extracellular vesicle microRNAs for the detection of oropharyngeal squamous cell carcinoma. J Transl Med. (2020) 18. doi: 10.1186/s12967-020-02446-1
65. Borup A, Sharifpour MF, Rossen LS, Whitehead B, Boysen AT, Olesen R, et al. Helminth extracellular vesicles co-opt host monocytes to drive T cell anergy. J Extracellular Vesicle. (2025) 14. doi: 10.1002/jev2.70027
66. Wan Y, Li L, Chen R, Han J, Lei Q, Chen Z, et al. Engineered extracellular vesicles efficiently deliver CRISPR-Cas9 ribonucleoprotein (RNP) to inhibit herpes simplex virus1 infection. Vitro vivo. Acta Pharm Sin B. (2024) 14:1362–79. doi: 10.1016/j.apsb.2023.10.004
67. Thakur A, Rathore R, Kondadasula SV, Uberti JP, Ratanatharathorn V, Lum L, et al. Immune T cells can transfer and boost anti-breast cancer immunity. OncoImmunology. (2018) 7:e1500672. doi: 10.1080/2162402X.2018.1500672
68. Zemanek T, Danisovic L, and Nicodemou A. Exosomes and solid cancer therapy: where are we now? Med Oncol. (2025) 42:77. doi: 10.1007/s12032-025-02626-3
Keywords: extracellular vesicles, head and neck squamous cell carcinoma, immune evasion, therapy resistance, tumor microenvironment
Citation: Dean J, Niederegger T, Hoch CC, Maheta B, Wollenberg B, Mrosk F, Hundeshagen G, Richter M, Heiland M, Voss J, Panayi AC, Koerdt S and Knoedler L (2025) Extracellular vesicles in head and neck cancer: mediators of oncogenesis, immune evasion, and therapy resistance. Front. Immunol. 16:1642639. doi: 10.3389/fimmu.2025.1642639
Received: 06 June 2025; Accepted: 28 August 2025;
Published: 19 September 2025.
Edited by:
Italia Falcone, Regina Elena National Cancer Institute (IRCCS), ItalyReviewed by:
Misba Majood, Johns Hopkins University, United StatesDr. Shilpi Gupta, Amity University Uttar Pradesh, India
Copyright © 2025 Dean, Niederegger, Hoch, Maheta, Wollenberg, Mrosk, Hundeshagen, Richter, Heiland, Voss, Panayi, Koerdt and Knoedler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leonard Knoedler, bGVvbmFyZC5rbm9lZGxlckBjaGFyaXRlLmRl
†These authors have contributed equally to this work
 Jillian Dean
Jillian Dean Tobias Niederegger
Tobias Niederegger Cosima C. Hoch
Cosima C. Hoch Bhagvat Maheta
Bhagvat Maheta Barbara Wollenberg
Barbara Wollenberg Friedrich Mrosk
Friedrich Mrosk Gabriel Hundeshagen5
Gabriel Hundeshagen5 Maximilian Richter
Maximilian Richter Max Heiland
Max Heiland Jan Voss
Jan Voss Adriana C. Panayi
Adriana C. Panayi Steffen Koerdt
Steffen Koerdt Leonard Knoedler
Leonard Knoedler