- 1Key Laboratory of Artificial Organs and Computational Medicine in Zhejiang Province, Shulan International Medical College, Zhejiang Shuren University, Hangzhou, China
- 2School of Basic Medicine and Forensic Medicine, Hangzhou Medical College, Hangzhou, China
- 3Ocean College, Beibu Gulf University, Qinzhou, China
Helminthic therapy, as an emerging strategy for Diabetes Mellitus (DM), demonstrates significant clinical benefits by modulating host immune and metabolic systems. Studies have shown that this approach effectively enhances insulin sensitivity, reduces chronic inflammation, and restores metabolic homeostasis through the regulation of gut microbiota. However, certain diabetic patients undergoing helminthic therapy may encounter risks such as infections or metabolic disturbances, necessitating the development of safer and more precise therapeutic methods. This review, conducted following the PRISMA guidelines, systematically retrieved and analyzed 163 high-quality studies from PubMed, Web of Science, and Scopus databases. It comprehensively evaluates the mechanisms, clinical outcomes, and safety improvement strategies associated with helminthic therapy. To ensure the safe application of this treatment, we propose strategies including genetic editing, real-time monitoring, targeted therapeutics, and helminth-derived molecules, along with a detailed clinical decision-making framework. This framework encompasses the matching of host health status with helminth species selection, guidance on dose optimization and treatment duration, and the application of modern intelligent technologies for real-time monitoring of therapeutic processes and potential adverse effects. Helminthic therapy has demonstrated success in alleviating hyperglycemia, chronic inflammation, and insulin resistance in diabetic patients, offering substantial health benefits through its immunomodulatory and metabolic regulatory effects. These findings suggest that helminthic therapy holds the potential to become a revolutionary approach in the field of DM.
Highlights
This paper examines the potential of helminthic therapy for DM treatment and the challenges it faces in clinical use. While it has gained attention for its immune-modulating benefits, studies show that diabetic patients may experience more severe side effects. The article reviews these adverse effects and how parasitic infections might speed up DM progression. It also suggests strategies to improve safety, including selecting safer parasites, using gene-editing to reduce parasite virulence, applying real-time monitoring, and developing treatments that target glucose metabolism. These innovations aim to reduce risks and improve the effectiveness of helminthic therapy. The paper further emphasizes that ethical and regulatory safeguards comparable to live biotherapeutics are prerequisites for clinical translation, with helminth-derived molecules excretory/secretory products (ESPs) offering safer and more controllable alternatives.
1 Introduction
The global prevalence of Diabetes Mellitus (DM) is alarming, particularly in countries such as China, India, and the United States, where the number of patients has surpassed 20 million (1). In other developing regions, the incidence of DM is also rising rapidly (Figure 1). This highlights the urgent need for innovative treatments to address DM as a global health crisis. In this context, helminthic therapy, as a potential treatment strategy for DM, has garnered significant attention due to its unique immunomodulatory properties.
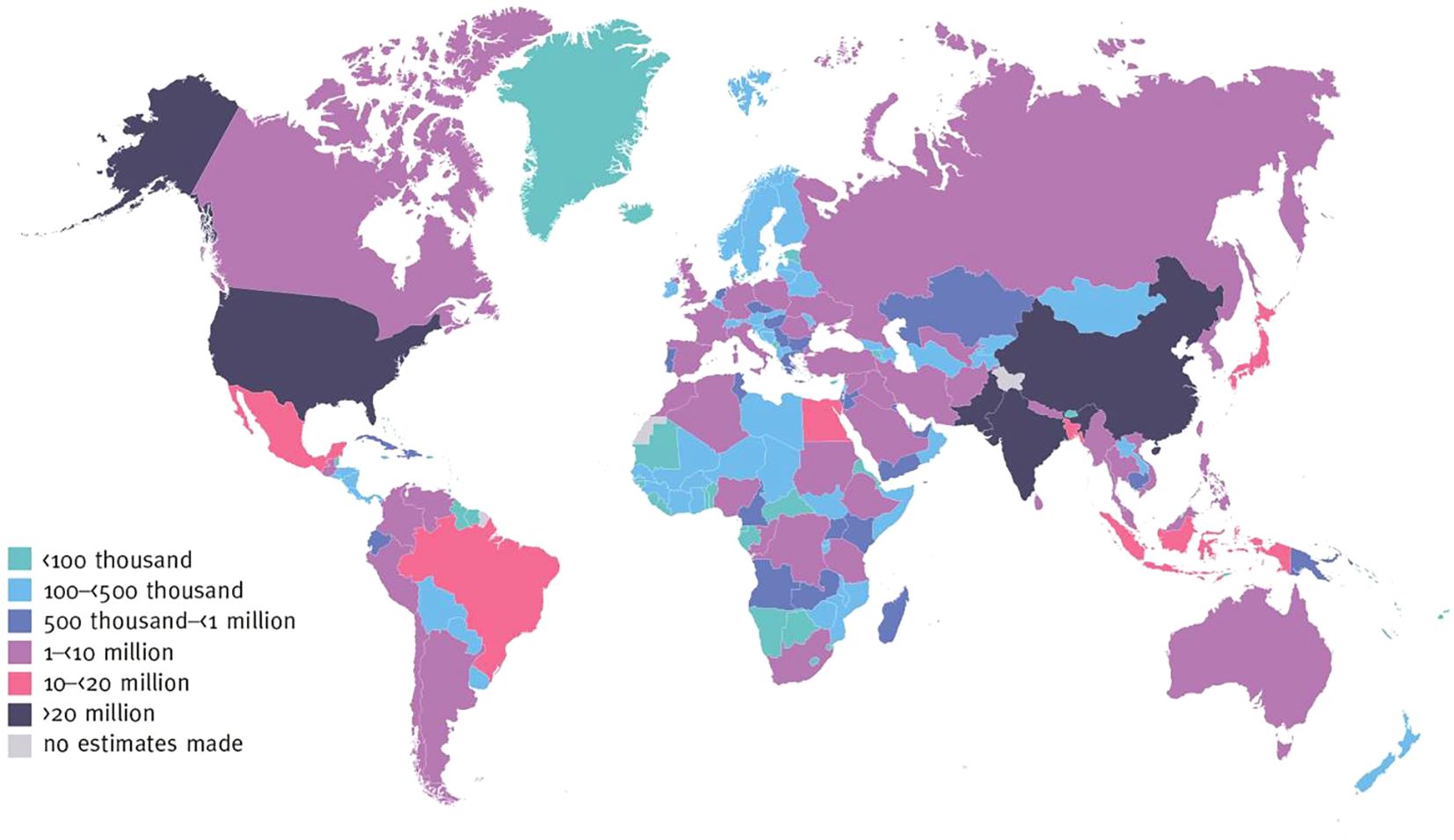
Figure 1. Number of adults with diabetes aged 20–79 years, 2021. International Diabetes Federation.IDF Diabetes Atlas, 10th edn. Brussels, Belgium: 2021. Available at: https://www.diabetesatlas.org.
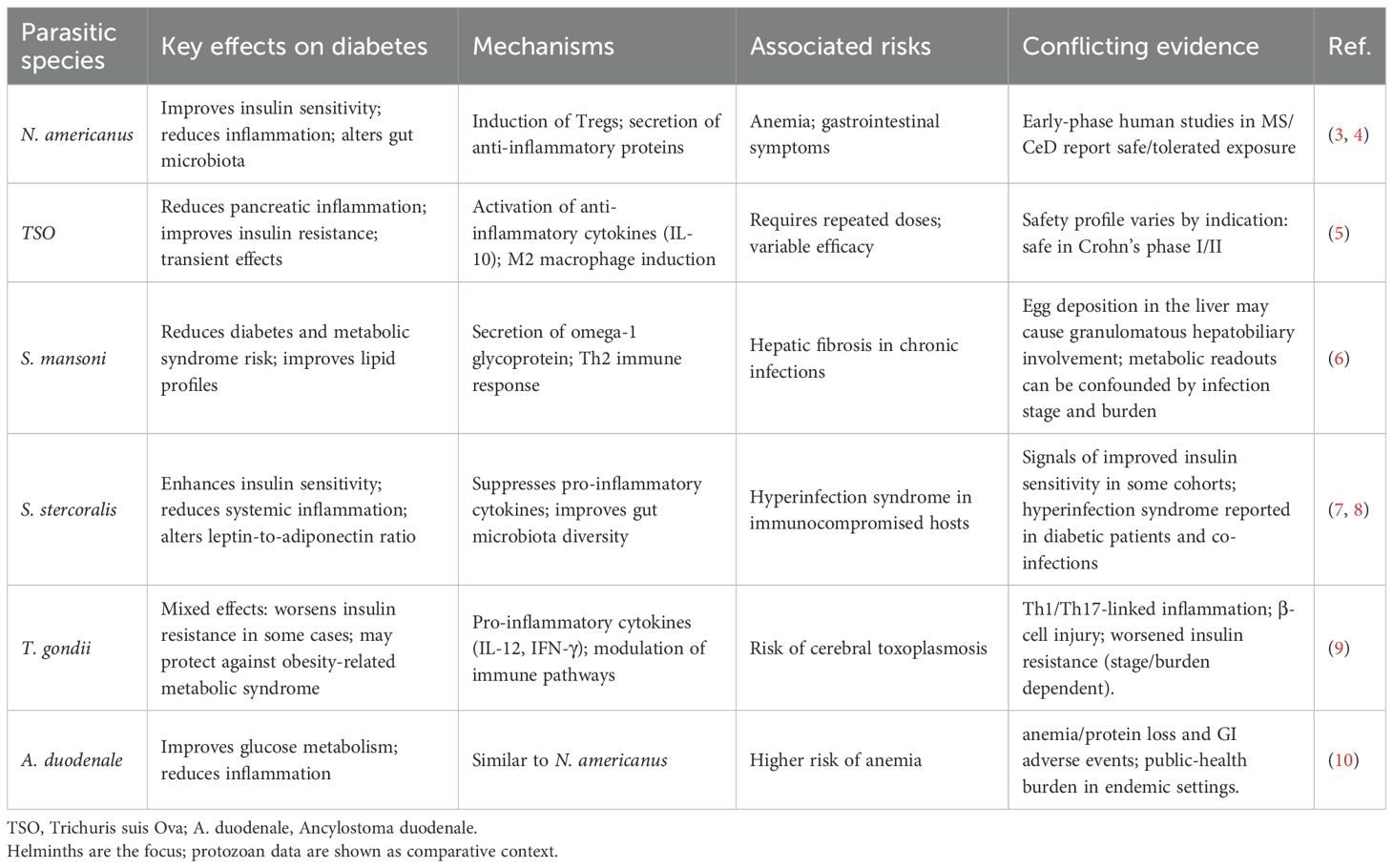
Helminthic therapy involves introducing live parasites to exploit the complex immunological interactions between the parasites and the host (2), aiming to modulate the host’s immune response to counteract the chronic inflammation and metabolic dysregulation associated with DM. A summary of the effects of different parasite species on DM is provided in Table 1. Several murine model studies have demonstrated this immunomodulatory capacity, showing that helminth infection can attenuate excessive immune responses and improve insulin resistance (2, 11). Recent clinical trials have also indicated the potential benefits of helminthic therapy, suggesting that parasite-induced immune responses may contribute to restoring immune homeostasis, thereby aiding in blood glucose regulation (3). However, the safety of helminthic therapy in diabetic patients remains a significant concern, as these individuals are particularly vulnerable to infections and metabolic disturbances. Studies have shown that inappropriate parasite selection or excessive dosing can result in serious complications, including disruptions to the gut microbiome and pancreatic function (12, 13). To mitigate these risks, several innovative strategies are critical. These include the selection of less pathogenic helminths, the use of genetic modifications to reduce pathogenicity, and the implementation of real-time biomarker analysis to monitor therapeutic outcomes (7, 14). Additionally, the development of anti-parasitic drugs that work synergistically with helminthic therapy, particularly those targeting glucose metabolism, could further enhance the safety and effectiveness of this approach. Not only live helminth but also helminth-derived products are being used as therapeutics including in DM. Numerous studies have demonstrated that parasite-associated molecules exhibit regulatory effects on the host, akin to those observed in vivo, and these findings have been comprehensively summarized in various reviews (15–17). Furthermore, based on our analysis and synthesis of high-quality literature, we have initially summarized key aspects of risk assessment, dosage adjustment, and precautions in the clinical application of helminth therapy. By employing these strategies, the safety of helminthic therapy can be better assured, facilitating its potential application in the treatment of DM and other chronic diseases.
2 Methods
2.1 Search strategy
This study utilized a systematic review methodology, adhering to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for literature screening and inclusion. The literature search was conducted up to January 2025, with data sources including PubMed, Web of Science, Scopus. The search strategy incorporated both keywords and subject terms, focusing on parasites (Parasite*, Helminth*, Parasitic therapy, Helminthic therapy, Worm therapy), diabetes (Diabetes, Type 1 diabetes, Type 2 diabetes, Diabetes mellitus), and related immunological and metabolic indicators (e.g., Immune, Inflammation, Cytokines, Insulin sensitivity, Glucose metabolism, homeostatic model assessment of insulin resistance(HOMA-IR), Gut microbiota, Adverse effects, Toxicity, Infection risk, Monitoring, Surveillance, Biomarkers, Diagnostics, Detection, Targets, Molecules, Proteins, Assessment, Evaluation). The search terms were combined as follows: [(Parasite*) OR (Helminth*) OR (Parasitic therapy) OR (Helminthic therapy) OR (Worm therapy)] AND [(diabetes) OR (Type 1 diabetes) OR (Type 2 diabetes) OR (diabetes mellitus)] AND [(immune) OR (inflammation) OR (cytokines) OR (insulin sensitivity) OR (glucose metabolism) OR (HOMA-IR) OR (gut microbiota) OR (adverse) OR (toxicity) OR (infection risk) OR (Monitoring) OR (Surveillance) OR (Biomarkers) OR (Diagnostics) OR (Detection) OR (Targets) OR (Molecules) OR (Proteins) OR (Assessment) OR (Evaluation)]. Additionally, citation searching and manual screening of references from relevant review articles were performed to identify further studies that met the inclusion criteria.
2.2 Eligibility criteria
The inclusion criteria for this study were as follows: (i) Research examining the effects of parasitic infections or helminthic therapy on DM (Type 1 or Type 2); (ii) Studies investigating biological mechanisms, including immune responses, inflammation, cytokine levels, insulin sensitivity, or glucose metabolism; (iii) Original research designs such as randomized controlled trials (RCTs), cohort studies, or case-control studies; (iv) Articles published in English; (v) Studies providing complete experimental data, clearly defined methodologies, and comprehensive results. The exclusion criteria included: (i) Articles unrelated to the research topic, such as those not addressing DM or parasitic infections; (ii) Non-original research, including review articles, conference abstracts, opinion pieces, or case reports; (iii) Studies limited to animal experiments without clinical or human data; (iv) Non-English publications without available translations; (v) Research with outdated findings that no longer align with the objectives of this study.
2.3 Screening results
This study followed the PRISMA framework for literature screening (Figure 2). Two authors independently conducted database searches, initially retrieving 1,002 articles. After removing 482 duplicates, an automated screening tool (EndnoteX20) excluded 88 articles unrelated to the research topic. Additionally, 13 articles were removed for being outside the scope of the study, leaving 419 articles for initial screening. Titles and abstracts were reviewed independently by both authors, resulting in the exclusion of 86 irrelevant studies. This process left 333 articles for full-text review. Of these, 11 articles could not be accessed and were excluded. A detailed eligibility assessment was then carried out independently on 322 articles, leading to the removal of 139 non-research articles, 3 non-English studies, and 63 studies excluded due to outdated findings or insufficient methodologies. Citation tracking, performed independently by each author, identified 53 potentially relevant articles. Of these, 2 articles were excluded due to access issues, and 4 unrelated studies were removed after further evaluation. Ultimately, 163 studies meeting the inclusion criteria were included, along with 1 newly published research report.
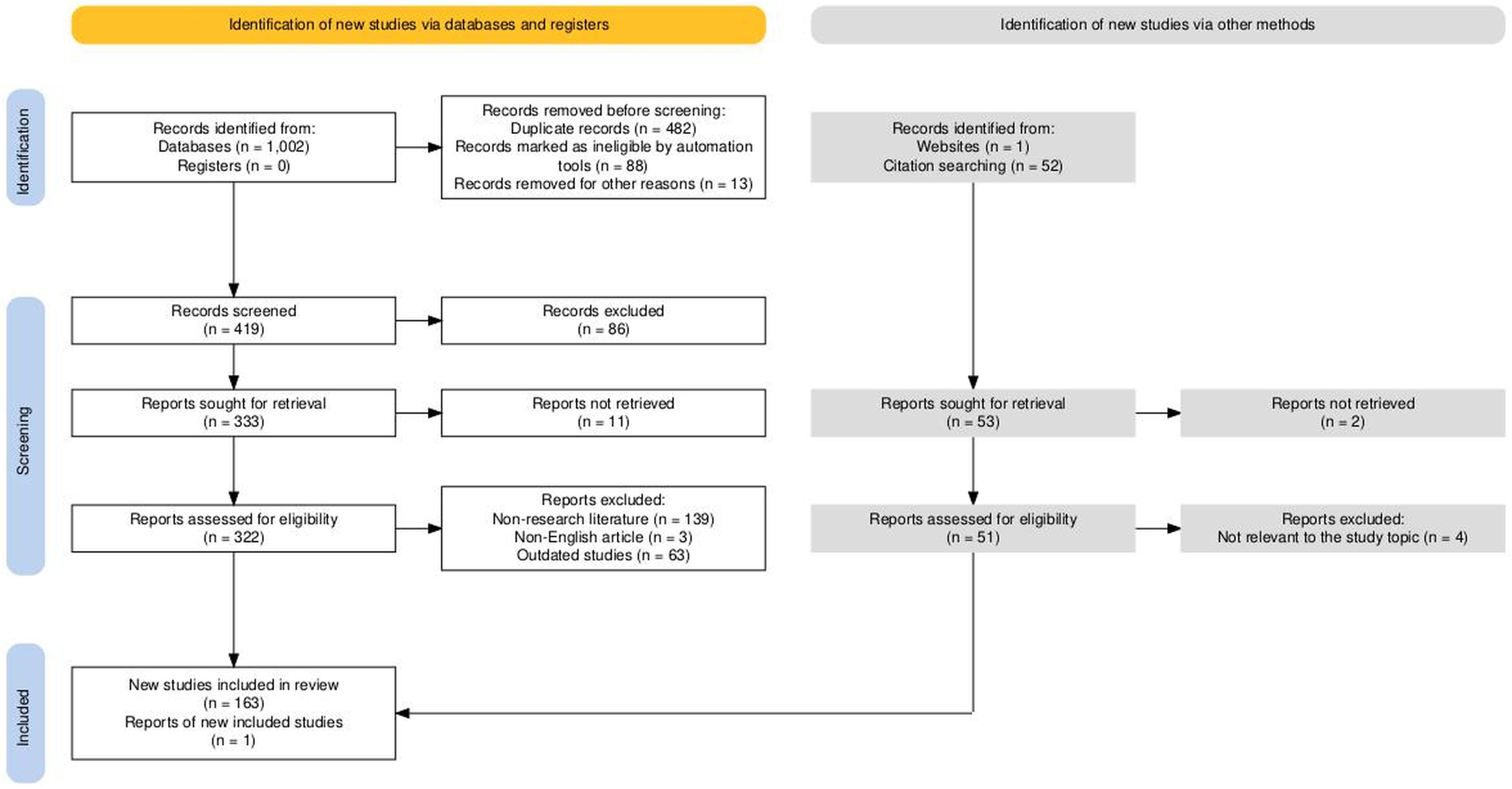
Figure 2. PRISMA flowchart for literature search. Haddaway, N. R., Page, M. J., Pritchard, C. C., & McGuinness, L. A. (2022). PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimized digital transparency and Open Synthesis Campbell Systematic Reviews, 18, e1230. https://doi.org/10.1002/cl2.1230.
3 The mechanisms of helminthic therapy
3.1 Helminths affect host metabolism
Helminths remodel host metabolism through two interconnected routes. A direct route targets epithelial and neuro-endocrine pathways—driven by IL-4/IL-13/STAT6 signaling and helminth excretory/secretory products (ESPs)—to reprogram glucose transport and local inflammation. An indirect route operates via the gut microbiota, increasing short-chain fatty acids (SCFAs) and barrier integrity. The following Sections 3.1.1 (direct) and 3.1.2 (microbiota-mediated) detail these complementary mechanisms.
3.1.1 Direct metabolic modulation by helminths
Helminth infection engages IL-4/IL-13–STAT6 signaling to tune intestinal epithelial glucose transport, thereby rebalancing luminal handling and dampening nutrient-driven inflammation.
Helminth infections significantly impact host energy metabolism by altering glucose absorption and metabolism. Initial research has demonstrated that these changes may occur through modifications in intestinal glucose transport receptors or Ach-induced contraction responses, mediated by IL-4 or IL-13 stimulated signal transducer and activator of STAT6 signaling within the enteric nervous system (18, 19). It has been shown in subsequent studies that helminths also modify intestinal M2 cells, decreasing sodium-glucose cotransporter 1 (SGLT1) expression while concurrently downregulating GLUT2 and upregulating GLUT1, thus shifting glucose absorption pathways (20, 21). In vivo, experiments by Koehler et al. in mice have demonstrated significant transcriptional reductions in GLUT1, STAT6, hypoxia-inducible factor 1-alpha (HIF-1α), and peptide transporter 1 (PepT1) in the jejunum, and in GLUT2 and PepT1 expression in the ileum, indicating a detrimental effect on physiological transport mechanisms (22).
Helminth excretory/secretory products (ESPs) act directly on epithelial transport and local cytokine milieus (hereafter “ESPs”). Recent findings have revealed the suppression of the transporter protein GLUT8 during Opisthorchis viverrini (O. viverrini) infections and alterations in glucose metabolism around host cells due to the total excretory-secretory (ES) antigens of Ascaris suum (A. suum) (23, 24). Functionally, ESP-triggered signaling yields rapid, microbiota-independent improvements in epithelial glucose handling and local inflammatory tone.
Direct reinforcement of mucosal/tissue repair programs and alternatively activated M2 macrophages reduces contact-dependent inflammation and supports metabolic recovery. Adult Schistosoma mansoni (S. mansoni) worms reside in host veins, and their eggs accumulate in the liver, affecting both adipose and liver metabolism (2). Cytokines such as IL-4 and IL-13 are critical for adipose tissue homeostasis (25). In obese mice, the injection of S. mansoni antigens has been found to stimulate IL-33 release from adipocytes, activating group 2 innate lymphoid cells (ILC2s) and promoting the infiltration of M2 cells and eosinophils into white adipose tissue, thereby enhancing metabolic functions (26). Additionally, Ni et al. discovered that Schistosoma japonicum (S. japonicum) infections regulate lipid metabolism via miRNAs pathways: the interaction of Sjp40 with CD36 on hepatocytes inhibits miR-802, enhancing the expression of Prkab1 or Prkaa1 and increasing phosphorylated AMP-activated protein kinase (AMPK) levels, leading to reduced hepatic lipid synthesis (27). Notably, Heligmosomoides polygyrus (H. polygyrus) infection in mice fed a high-fat diet (HFD) has been shown to prevent obesity, dyslipidemia, and glucose intolerance (28). These studies illuminate the complex interactions between parasitic infections and host metabolic processes, offering innovative approaches for managing metabolic disorders. By stabilizing local inflammatory tone and hepatic/lipid pathways, these direct mechanisms help lower peripheral and hepatic insulin resistance and consolidate early glycemic benefits.
Taken together, these direct epithelial and neuro-endocrine mechanisms initiate early improvements in glucose handling that are further amplified by microbiota-mediated processes (Section 3.1.2).
3.1.2 Microbiota-mediated metabolic effects
Since the initial germ-free mouse experiments, the role of the gut microbiota in regulating energy metabolism has been extensively studied (29). It has been established that microbial populations are crucial for energy extraction, lipid storage, and vitamin synthesis. Additionally, infections with helminths such as H. polygyrus, Nippostrongylus brasiliensis (N. brasiliensis), and Trichuris muris (T. muris), have been shown to increase the population of Lactobacillaceae, recognized for its probiotic potential through immune response regulation (30, 31). Research has found that parasites depend on the host’s gut microbiota for nutrient acquisition and survival, suggesting that helminths may modulate the host’s metabolic state by altering the gut microbiome (32).
Further investigations have explored the interactions between parasites and the host’s gut bacterial communities, particularly noting the diversity of Blastocystis sp (33)., which is associated with an increased risk of type 2 diabetes (T2D), IR, and metabolic syndrome (MetS). It has been shown that parasites secrete substances that inhibit specific bacterial populations while promoting the production of short-chain fatty acids (SCFAs) (34), such as butyrate, propionate, and acetate, by the gut microbiome. Butyrate and propionate are particularly noted for enhancing insulin sensitivity in muscle and fat tissues, reducing blood glucose levels, and aiding obesity prevention and T2D risk reduction (35–38). Significant reductions in weight gain under a high-fat diet in mother rats infected with H. polygyrus and their offspring have been attributed to changes in the gut microbiome and elevated SCFA levels (39).
Microbiota-dependent strengthening of tight junctions and immunoglobulin A (IgA)-mediated containment reduces endotoxin leakage and systemic inflammatory tone. SCFAs also play a positive role by strengthening the intestinal barrier, reducing inflammation and metabolic endotoxemia, thereby mitigating systemic inflammation and IR in diabetics (40). Recent research has linked improvements in insulin sensitivity in diet-induced obesity (DIO) mice and Venezuelan hookworm infections to microbial community alterations, notably an increase in lactobacilli and decreased intestinal permeability. These microbiota alterations enhance host metabolic functions by increasing levels of anti-inflammatory cytokines, transitioning adipose tissue MΦ from an M1 to an M2 phenotype, boosting the expression of tight junction proteins in intestinal cells, and lowering serum lipopolysaccharides (LPS) (41). Consequently, diminished microbial translocation lowers systemic TNF-α/IL-6 and supports restoration of insulin signaling in metabolic tissues. Experimental evidence indicates that increases in SCFAs in helminth-infected mice strongly correlate with shifts in microbial populations. Studies have observed a rise in the Clostridiales order, known for its efficient SCFA production, across various infection models, highlighting the potential of SCFAs in modifying obesity and enhancing insulin sensitivity (35, 42–45).
Notably, associations with Blastocystis sp. remain heterogeneous; we treat these findings as comparative context rather than core evidence for helminthic therapy. Further investigations have explored the interactions between parasites and the host’s gut bacterial communities, particularly noting the diversity of Blastocystis sp (33)., which is associated with an increased risk of type 2 diabetes (T2D), insulin resistance(IR), and metabolic syndrome (MetS). Accordingly, non-helminth species are summarized in Table 1 and discussed in Section 4.2 to explain apparent contradictions without conflating pathogen classes.
Further studies indicate that H. polygyrus infection may offer protective effects against obesity by indirectly modulating norepinephrine (NE) concentrations through microbial community changes (46), with Th2 cells significantly facilitating metabolic enhancements by influencing microbial shifts (47).
3.2 Helminths regulate host immune responses
Many parasites must deploy evasion strategies against the mammalian immune system to ensure long-term survival within their definitive hosts. These parasites can cause chronic infections in host tissues and typically induce a shift in the host immune response toward an anti-inflammatory phenotype while suppressing pro-inflammatory responses (Figure 3) (48).
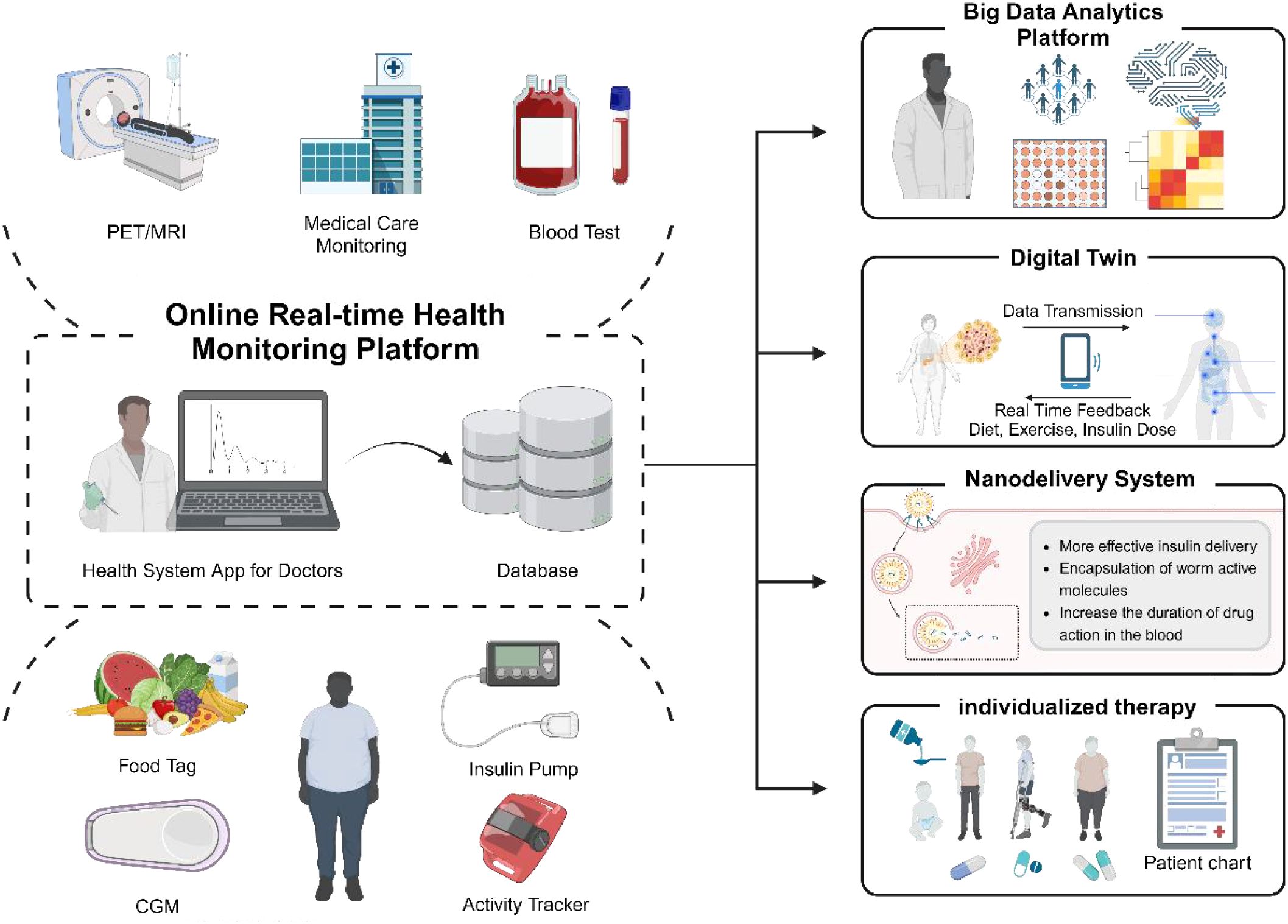
Figure 3. Anticipated future health monitoring platform used for treating T2D with parasitic therapy. This platform seamlessly integrates a range of data sources, including portable medical devices, nutritional tracking, and monitoring of physiological parameters, facilitating the real-time upload of health information to a cloud-based database. Leveraging big data analytics and digital twin technology, it can accurately simulate and dynamically adjust to individual health conditions based on the acquired data. Additionally, applying nano-delivery technology, particularly encapsulating active molecules derived from parasites, significantly improves medications’ bioavailability and circulation duration. By synthesizing these advanced technologies, the platform crafts personalized treatment plans that enhance the therapeutic outcomes for patients.
Inflammation triggered by macrophages (MΦ) plays a crucial role in the dysfunction of pancreatic β-cells. Within the islets, MΦ regulates the survival and metabolic activity of β-cells by acting as sensors, engaging in bi-directional exchanges of signals such as cytokines, hormones, and growth factors (49). However, in the progression of T2D, inflammatory signals disrupt this physiological equilibrium, resulting in interactions between MΦ and β-cells that lead to β-cell dysfunction and apoptosis. Specifically, early-stage T2D is characterized by pancreatic hypoxia that induces cellular stress and necrosis, releasing fatty acids and intracellular substances that activate the transcription inhibitor basic helix-loop-helix family member e40 (BHLHE40) (50), impairing insulin secretion. These conditions foster the transformation of MΦ into a pro-inflammatory state (M1 type), with increased levels of IL-12 and decreased IL-10 exacerbating the inflammation (51, 52). Furthermore, the upregulation of miR-212-5p in M1 macrophage-derived exosomes (M1-Exos) and high-fat diet-derived exosomes (HFD-Exos) influences β-cell insulin secretion by modulating the sirtuin two gene and the Akt/GSK-3β/β-catenin pathway (53), also generating pro-inflammatory mediators such as nitric oxide (NO) and reactive oxygen species (ROS), and expressing chemokine receptors like C-C motif CCR5, C-X-C motif CXCR3, and CCR8, which recruit pro-inflammatory cells (54). Parasitic infections often induce a Th2 immune response in the host, promoting intestinal “clearance and excretion” processes like increased mucus production and intestinal contractions, thus altering the gut microbiota (43, 55). Mishra et al. observed that in obese mice infected with S. mansoni, MΦ differentiation in white adipose tissue shifted towards an anti-inflammatory M2 phenotype, benefiting reductions in body weight and fat mass (FM) and enhancing glucose tolerance and insulin sensitivity (56). This was further supported by a study by Kang, where mice on a high-fat diet (HFD) infected with whipworms showed significantly lower increases in body weight, fat content, total cholesterol, and food efficiency ratio levels compared to uninfected controls (57). This protective effect is closely associated with the parasite’s ability to synergistically induce M2 through anti-inflammatory mediators such as IL-4 (58), promoting their proliferation and inhibiting inflammatory responses.
Numerous studies have confirmed that several worms can modulate human T helper 1 (Th1)/T helper 2 (Th2) cells and cytokines, steering inflammatory responses towards a Th2-dominated immune reaction and ameliorating conditions like obesity or malnutrition (59). Research involving subjects infected with worms has shown varying improvements in metabolic health. Typically, worm infections increase Th2 cytokines, such as IL-4 and IL-13, while decreasing Th1 cytokines like IL-12 and IFN-γ (60). These cytokines further orchestrate the immune response, facilitating the recruitment of eosinophils, the production of B-cell-like entities, and the activation of alternative MΦ (61, 62). The equilibrium between T helper 17 (Th17) cells and Treg cells, both critical T cell subsets, is essential for maintaining immune homeostasis. Clinical data reveal that diabetic patients exhibit significantly elevated levels of Th17 and Th1 cells and reduced Treg cells (P < 0.01) (63). Furthermore, regulatory T cells(Treg) levels inversely correlate with high glucose levels, and diabetic patients experience more significant fluctuations in average glucose levels compared to those with normal albuminuria (P < 0.05) (64). It is hypothesized that elevated blood glucose may increase Th1 and Th17 cells and inflammatory cytokines, impeding improvements in T2D (65). Experimental evidence suggests that infection with the H. polygyrus parasite increases the expression of IL-10 and adiponectin, reduces levels of leptin and anti-insulin, and enhances the infiltration of Th2 cells and eosinophils in adipose tissue—particularly enhancing the functionality and expression of activation markers, such as latency-associated peptide and CD134 in Treg cells (66).
The proliferation of eosinophils (EOS) is a distinguishing feature of host immune responses, enabling the differentiation of parasitic infections from other pathogenic challenges. In T2D development, eosinophils are pivotal in mediating Th2-type immune responses. IL-10, secreted by eosinophils, orchestrates these responses and limits local NO production. Parasites dependent on chronic infections may leverage this adaptive mechanism to sustain their presence and manipulate host immune responses (67, 68). Research indicates that eosinophils are the primary cells expressing IL-4 in the adipose tissue of mice. When subjected to an HFD, mice lacking eosinophils exhibit increased adiposity, diminished glucose tolerance, and heightened IR. Therefore, eosinophils are essential for maintaining glucose equilibrium and countering metabolic disturbances induced by HFD (69). In experimental models of diet-induced T2D in mice, administering the parasitic nematode N. brasiliensis for prevention and treatment significantly ameliorated fasting glucose levels, oral glucose tolerance, and weight gain, a process linked to increased EOS counts in mesenteric lymph nodes, liver, and adipose tissue (70).
Additionally, Mast cells (MCs) have been shown to influence immune regulation significantly. During infections with Hymenolepis diminuta (H. diminuta), activated MCs are instrumental in modulating the production of Th2 cytokines, contributing to a robust Th2-skewed immune response and expedited recovery in mice (71, 72).
3.3 Clinical researches
To balance mechanistic insights with human evidence, we synthesize observational and interventional data linking helminth exposure to metabolic outcomes, then summarize current trial status and the outstanding gaps that should guide subsequent Phase II/III designs.
Observational cohorts. Population studies in helminth-endemic settings suggest a protective association with metabolic traits. In Flores Island, Indonesia, soil-transmitted helminth (STH) infection correlated with lower insulin resistance (HOMA-IR), with a dose-response pattern whereby each additional STH species was associated with further HOMA-IR reduction (73). In rural China, previous schistosome infection was linked to lower fasting and postprandial glucose, lower HbA1c and HOMA-IR, and lower prevalence of diabetes and metabolic syndrome (adjusted OR for diabetes 0.51; 95% CI 0.34–0.77) (74). In Northern Australia, Strongyloides stercoralis seropositivity showed an inverse association with type 2 diabetes in Aboriginal adults, reinforcing the epidemiological signal across distinct host and parasite contexts (75). Collectively, these cohorts indicate that helminth exposure may align with improved glycemic indices, while acknowledging residual confounding and the cross-sectional nature of many datasets.
Early-phase trials. The first randomized, double-blind, placebo-controlled Phase Ib trial of Necator americanus in adults at risk of type 2 diabetes (Australia; n=40) prioritized safety and assessed metabolic secondary outcomes: participants inoculated with 20 or 40 L3 larvae showed lower HOMA-IR and fasting glucose at 12 months, with body mass reduction observed in the 20-larvae group at 24 months; adverse events were mainly mild-to-moderate gastrointestinal symptoms, and completion rates were comparable to placebo (76). The published trial protocol details dose, follow-up (24 months), and predefined metabolic endpoints (HOMA-IR, body composition) (3). By contrast, multiple RCTs of Trichuris suis ova (TSO) in non-metabolic indications (e.g., allergic rhinitis, Crohn’s disease) demonstrate acceptable safety but inconsistent efficacy, underscoring feasibility while highlighting the need for indication-specific endpoints and adequately powered designs before translation to diabetes care (77, 78).
Limitations and next steps. Despite encouraging signals, heterogeneity in host background, parasite species/dose, endpoints (HOMA-IR vs OGTT vs HbA1c), and follow-up limits inference. Importantly, a cluster-randomized trial in Indonesia showed community-level deworming did not change insulin resistance overall but significantly increased insulin resistance in the subgroup with microscopy-confirmed helminth infection, suggesting reverse-causality-consistent effects and emphasizing the value of stratification and baseline phenotyping in future trials (79). Mechanistic analyses from the same program linked deworming to adipokine shifts (↑ leptin/adiponectin ratio) that may partially mediate metabolic changes (80). Future studies should power for standardized metabolic endpoints (HOMA-IR, HbA1c, OGTT), prespecify safety monitoring (including anemia and GI AEs), and consider comparators such as helminth-derived molecules/ESPs to mitigate risks while preserving immunometabolic benefits.
Studies have observed an inverse relationship between parasitic infections and metabolic diseases such as DM and atherosclerosis. For example, the prevalence of metabolic syndrome was significantly lower (18.28%) in individuals with schistosome infections compared to those without (34.01%) (17, 81). This aligns with the “hygiene hypothesis,” which associates lower DM incidence in low-income countries, where parasitic infections are more common, with reduced immune-mediated metabolic disorders (74, 82–84) (Figure 4).
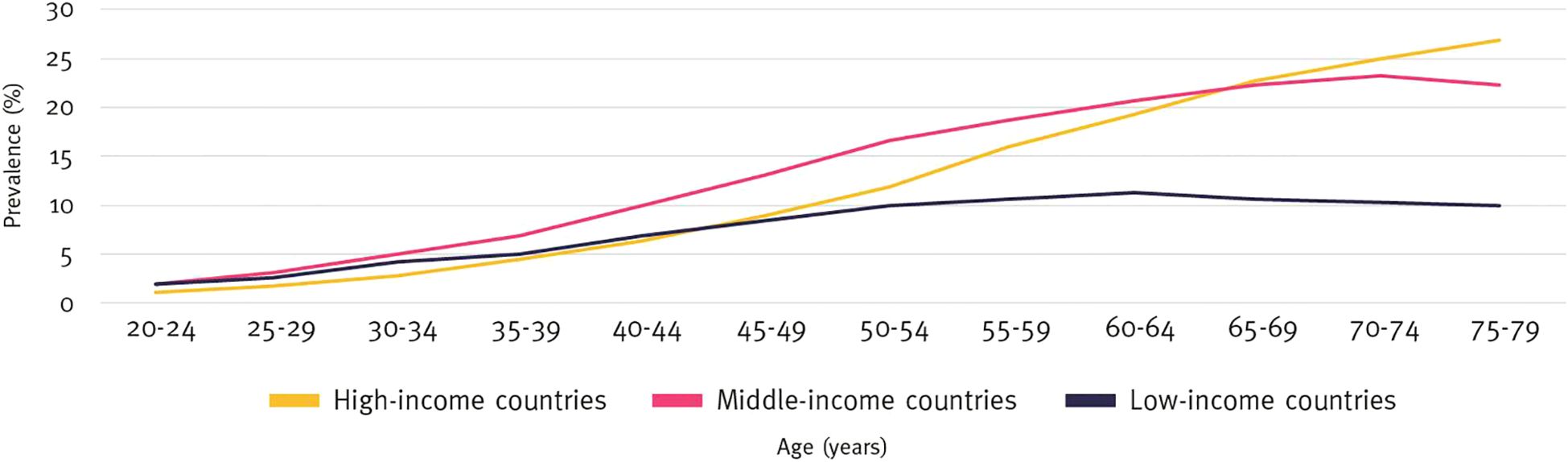
Figure 4. Prevalence of diabetes by age and income group (%), 2021. International Diabetes Federation.IDF Diabetes Atlas, 10th edn. Brussels, Belgium: 2021. Available at: https://www.diabetesatlas.org.
Subsequent studies have demonstrated that helminth infections may protect against type 2 diabetes (T2D) and metabolic syndrome by modulating metabolic processes. For instance, a randomized controlled trial in Indonesia revealed that participants infected with helminths had an insulin resistance index (HOMA-IR) approximately 16% lower than uninfected individuals; however, this improvement diminished by about 10% following deworming treatment (85). In a large cross-sectional study in rural China, the prevalence of DM was 14.9% among individuals who had previously been infected with schistosomes, compared to 25.4% among those who had never been infected. This study also reported that the prevalence of metabolic syndrome was 14.0% in those with a history of infection, compared to 35.0% in those without (74). Similarly, an Australian study observed that the incidence of T2D was about 13% lower in individuals infected with Strongyloides stercoralis (S. stercoralis) compared to uninfected individuals, with the risk of DM increasing by approximately 10% after deworming treatment (86). A randomized controlled trial in Uganda indicated that individuals infected with S. mansoni had low-density lipoprotein cholesterol (LDL-c) levels approximately 0.26 mmol/L lower than uninfected individuals (2.37 mmol/L vs. 2.63 mmol/L), which could potentially lower their cardiovascular disease risk (87). These findings suggest that helminth infections play a protective role against T2D and metabolic syndrome through intricate metabolic mechanisms. Research by Pierce et al. found that low-dose hookworm infections are safe for managing inflammatory diseases and obesity-related metabolic syndrome (3, 88–90). Although these studies did not directly assess metabolic health, hookworm infections have been shown to elicit immune responses (91) and alter the gut microbiome (92), which might be advantageous for T2D. An Australian study further examined the effects of hookworms on T2D risk, where 40 participants were randomly assigned to receive either a placebo or varying doses of hookworm larvae. After two inoculations, participants were evaluated every six months over two years. The study primarily assessed safety and changes in insulin resistance. Results indicated that, although adverse events were more frequent in the hookworm group, the completion rate was similar, and this group exhibited improvements in fasting blood glucose and insulin resistance after one year, with a reduction in body weight observed after two years. These findings suggest that hookworm infection may be a safe and effective intervention for individuals at risk for T2D (76). Helminth infections have also been shown to significantly impact fat metabolism-related hormones. Studies indicate that leptin levels decrease significantly in infected individuals, while adiponectin levels increase, leading to a reduced leptin-to-adiponectin ratio (LAR). This hormonal adjustment enhances insulin sensitivity, further supporting the beneficial effects of helminth infections on metabolic health (93, 94). Additionally, helminth infections may affect the progression of T2D by inhibiting angiogenic pathways. For example, during infection, serum levels of vascular endothelial growth factor (VEGF) significantly decrease, with levels returning to baseline following deworming treatment. This suggests that helminth infections might modulate DM progression by inhibiting angiogenesis (95).
Patients with hypoglycemia and complicated T2D exhibit significantly elevated levels of total white blood cells, neutrophils, monocytes, the neutrophil-to-lymphocyte ratio (NLR), the monocyte-to-lymphocyte ratio (MLR), the mean platelet volume-to-lymphocyte ratio (MPVLR), and the platelet-to-lymphocyte ratio (PLR). In contrast, red blood cell parameters and indices are significantly decreased in cases of hypoglycemia and complicated T2D (96). Consequently, the relationship between parasitic infections and T2D can also be understood through immune regulation mechanisms. Multiple studies have demonstrated that infection with S. stercoralis can significantly reduce the levels of pro-inflammatory cytokines in patients with T2D, including key inflammatory mediators such as IL-1β, IL-6, and TNF-α. This inhibitory effect diminishes following antiparasitic treatment, with cytokine levels returning to near-normal levels comparable to uninfected individuals, underscoring the crucial role of helminth infections in modulating inflammatory responses (97). Furthermore, hookworm infections have been shown to alter the composition of the gut microbiota, thereby influencing the host’s immune response. Following infection, there is a significant increase in the proportion of anaerobic bacteria such as Lactobacillus and Bifidobacterium in the gut. This increase is closely associated with reduced inflammation and improved insulin sensitivity (98). Continuing research into 2024 has further examined the effects of S. stercoralis infection on the complement system, a key component of immune regulation. The findings indicate that in T2D patients infected with S. stercoralis, the levels of complement proteins [(e.g., C1q, C4b, and MBL (lectin))] and complement regulatory factors (e.g., factor B and factor D) partially recover after antiparasitic treatment, suggesting that S. stercoralis may reduce inflammation by modulating complement activation (7). Taken together, helminth infections appear to offer significant protective effects against metabolic diseases such as T2D through mechanisms that include the inhibition of inflammation, regulation of lipid metabolism, modulation of angiogenesis, and enhancement of gut microbiota composition.
4 Adverse effects of helminthic therapy
4.1 Patients with diabetes exhibit an increased susceptibility to infections by parasites
Currently, parasites are widely recognized as a significant pathogenic factor associated with various health issues, including malnutrition (99), anemia (100), and intestinal obstruction (101). However, their connection with DM is not universal. Salvador, for example, identified no correlation between trichostrongyliasis and DM or other metabolic disorders (102).
Meta-analytical studies indicate that diabetic patients have a higher overall prevalence of intestinal parasitic infections (IPIs) than healthy controls, with rates of 25.7% compared to 15.5%. Infections by Cryptosporidium, Entamoeba histolytica, and hookworms are notably more common in these patients, with odds ratios (ORs) of 3.30, 1.57, and 6.09, respectively (103). These findings highlight the critical need for targeted health education and preventative strategies for this population. Most experts agree that patients with T2D should be shielded from parasitic infections due to their compromised glucose regulation (104, 105), which could exacerbate susceptibility to infections like those caused by acarids, potentially accelerating parasite growth and enhancing virulence (106, 107). Abdelhamid et al., using glial fibrillary acidic protein (GFAP) as a diagnostic marker, observed that mice resistant to cerebral toxoplasmosis exhibited more severe encephalitis when infected with Toxoplasma gondii (T. gondii) post-DM onset (108). Li’s study in China revealed significantly higher seropositivity rates for T. gondii in patients with type 1 diabetes (T1D) and T2D, as well as gestational DM, compared to controls (109). However, a key concern is the potential for exaggerated immune responses in certain individuals. Some parasites can trigger excessive IgE-mediated immune reactions, leading to allergic responses such as asthma or eczema, particularly in genetically susceptible populations (110, 111). Additionally, patients with autoimmune disorders such as rheumatoid arthritis or systemic lupus erythematosus (SLE) may experience worsened disease activity due to helminth-induced immune modulation (112).
Contrarily, not all parasitic infections confer protection against DM; some may trigger its onset. The prevalence of IPIs in such case studies was higher in the affected population than in controls, with a noted correlation (OR, 1.80) (103). Additional meta-analytical research is required to explore the interplay between T1D-induced immunological changes and the risk of T. gondii infection, and vice versa (113–115). Moreover, a case report highlighted the death of a 40-year-old diabetic patient with a history of alcoholism due to severe S. stercoralis infection (116). It remains prudent for most individuals, especially those at high risk for DM, to maintain environmental cleanliness and minimize exposure to parasitic infections until the benefits of such infections on DM are definitively understood.
4.2 Parasitic infection exacerbates diabetes
Furthermore, not all parasites have beneficial effects on the health of individuals with DM; indeed, harmful parasitic infections can worsen their condition. Chronic parasitic infections can lead to long-term complications, including organ damage, fibrosis, and metabolic disruptions (117). T. gondii has garnered considerable attention recently. Initial studies using mouse models demonstrated that acute T. gondii infection significantly swells and reduces the number of pancreatic Langerhans cells, indicating severe damage (114). In Australia, a study found prevalent T. gondii infections in Western Australia but no serological evidence linking these infections to T2D (118). Ashraf reported that the high incidence of chronic toxoplasmosis in Bangladesh might be associated with elevated secretion of the pro-inflammatory cytokine IL-12 (119). Salem et al. found that T. gondii infection could induce immune-metabolic responses; in the group with positive obesity and MetS, measurements such as trunk FM, HOMA-IR, chemerin, and IFN-γ were significantly higher than in the opposing group. There was a strong correlation between serum levels of chemerin and IFN-γ (P<0.001), which were positively associated with BMI, waist circumference (WC), total and trunk FM, HOMA-IR, cholesterol, and triglycerides, and negatively with high-density lipoprotein cholesterol (HDL-C) (120). Diabetic patients with positive anti-Toxoplasma IgG antibodies experienced a longer duration of DM compared to those with negative antibodies (7.14 ± 2.962 years versus 3.26 ± 1.583 years, P < 0.001) (121), suggesting that T. gondii may also be a factor in hypertension among T2D patients (122). Parasitic infections can also contribute to severe nutrient depletion, exacerbating metabolic disorders in diabetic patients. Chronic helminth infections may lead to anemia, protein deficiency, and micronutrient deficiencies, all of which can negatively impact glycemic control and overall metabolic function (14, 123). Yingklan noted that participants with S. stercoralis infection estimated glomerular filtration rate (eGFR) and higher levels of alanine aminotransferase (ALT) and urine albumin-to-creatinine ratio (UACR), indicating potential severe renal complications (124). Furthermore, co-infections with opportunistic pathogens pose a significant risk in patients receiving helminth therapy. Immunocompromised individuals, such as those with HIV/AIDS or undergoing immunosuppressive treatments, are more susceptible to severe opportunistic infections, including Cryptosporidium, cytomegalovirus (CMV), and bacterial superinfections (125, 126).
Parasitic therapy remains experimental. Although S. stercoralis infection negatively correlated with T2D in Northeast Thailand, infected individuals exhibited lower eGFR and higher ALT and UACR levels. In immunocompromised patients, inoculation with S. stercoralis could lead to co-infections with cytomegalovirus CMV, and worsening renal parameters associated with complications (127). The impact of parasites on gut microbiota composition also remains a key area of concern (128). While some helminths have been shown to promote beneficial microbiota shifts, others may increase gut permeability, leading to bacterial translocation and systemic inflammation, which can worsen metabolic diseases (129, 130). Demodex folliculorum (D. folliculorum), a mite that causes demodicosis, is noted for facial damage and may be linked to altered immune responses in conditions such as T2D (104, 131). A 45-year-old woman with interstitial lung disease and localized scleroderma-related pulmonary arterial hypertension (PAH) began treatment with Necator americanus (N. americanus) as an alternative therapy. Despite treatment, her respiratory symptoms worsened after an increase in eosinophils and elevated IgE levels, with detected S. stercoralis IgG antibodies, highlighting the complex interplay between parasitic infections and chronic diseases (132).
Explaining Divergent Findings. Apparent inconsistencies across studies largely reflect (i) pathogen class—protozoa commonly drive Th1/Th17-polarized inflammation linked to β-cell stress, whereas helminths elicit Th2/Treg-leaning programs that promote tissue repair and metabolic tolerance; (ii) host context—genetic background, baseline inflammation, and metabolic comorbidities; (iii) infection features—stage (acute vs. chronic), parasite burden, and tissue tropism; and (iv) study design and endpoints (e.g., HOMA-IR, HbA1c, clamp, cytokines). Protozoan data are therefore treated as comparative context rather than counter-examples to helminthic therapy.
5 Control living therapeutic parasites
5.1 Improve safety and tolerance
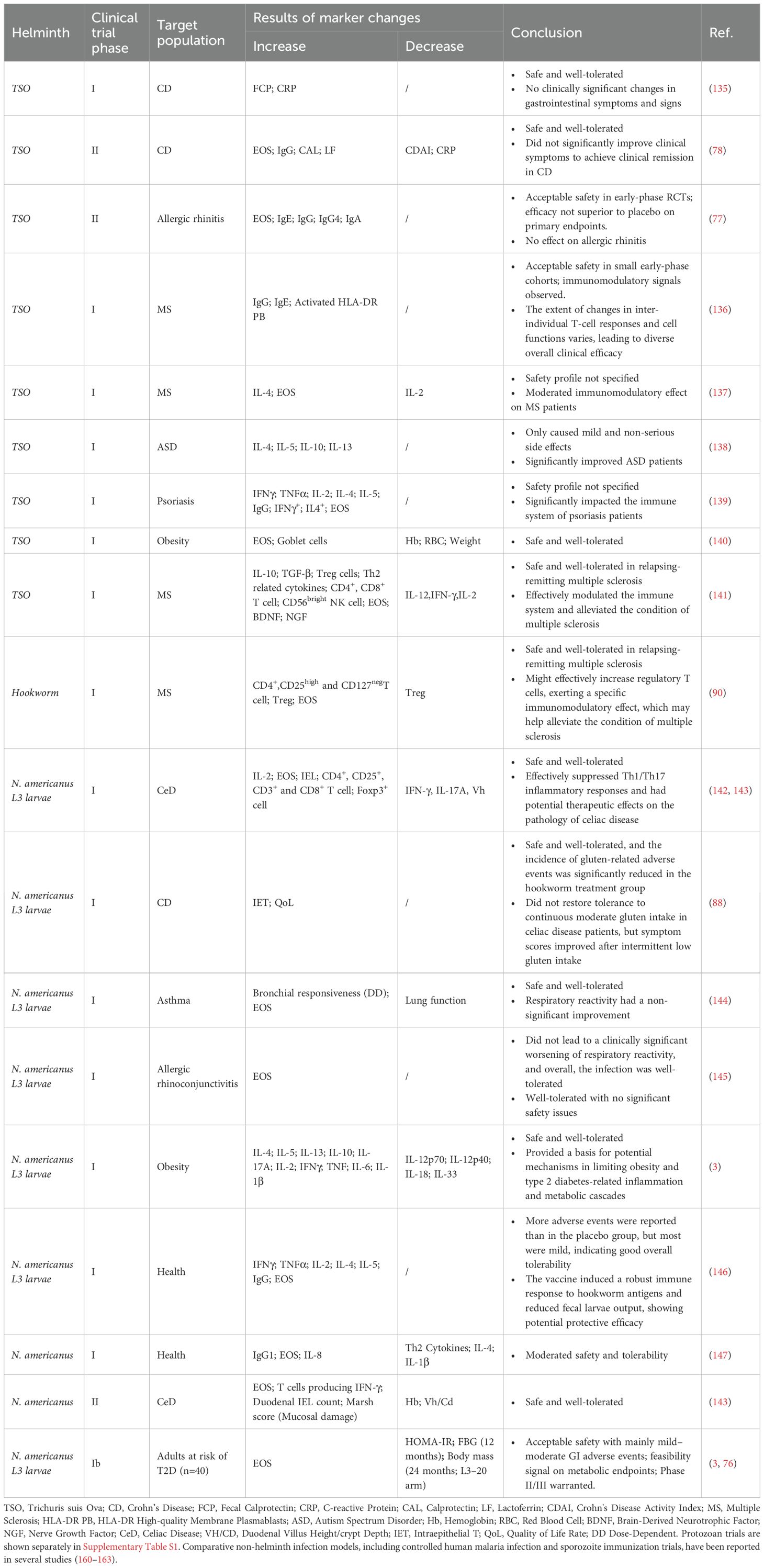
Firstly, the selection of parasites with lower toxicity is paramount. For instance, among the various species of Plasmodium, Plasmodium vivax (P. vivax) demonstrates superior infection and replication abilities in the spleen and bone marrow compared to Plasmodium falciparum (P. falciparum), which exhibits a relatively mild impact (133). Regarding therapeutic applications, Taenia saginata (T. saginata) is preferred over Trichuris suis ova (TSO), which is known to cause neurocysticercosis (134). Numerous clinical trials have highlighted the safety of certain parasites, including T. solium and Ancylostoma duodenale, as indicated in Table 2. However, the available data do not allow the determination of a safe dosage free of safety concerns. The group has therefore concluded that the safety of novel food remains unconfirmed (148). Additionally, by controlling the reproductive capabilities of parasites, both the population of parasites and the complications arising from their eggs can be reduced. For instance, manipulating hTNF-α to influence the development, metabolism, and egg-laying of parasites has significantly lessened the burden in mice infected with unisexual schistosomes (149, 150). Moreover, short interfering RNA (siRNA) targeting of SjGT and SjNCSTN can induce minor morphological changes in the testes of male nematodes, substantially diminishing their vitality and fertility (151). However, it is crucial to consider the variations in the biological traits and host interactions of parasites when implementing these strategies (152).
We can also reduce the virulence of parasites through artificial methods. For instance, adding gentamicin during in vitro culture can maintain the long-term low virulence of wild-type parasites (153). Similarly, the application of genetic engineering techniques, such as irradiation or gene editing, can effectively lessen the virulence of parasites (154, 155). These methods have demonstrated potential in experimental studies with hookworms, aimed at treating conditions such as asthma (155), cancer (156), ulcerative colitis (157), and anemia (158). The rapid advancements in bioengineering have ushered in next-generation CRISPR gene editing tools like CRISPR-Cas13 (159), enabling the modification of parasites to attain specific traits such as reduced life cycles, inhibited proliferation, or increased drug sensitivity. RNA interference techniques have been utilized to silence target mRNA transcription, aiding in elucidating gene functions in nematodes, with proven efficacy in Caenorhabditis elegans. The CRISPR/Cas9 system facilitates the knockout or deletion of specific genes in parasitic worms, though the potential for gene insertion remains largely unexplored (164). CRISPR technologies and their derivatives are poised to advance, potentially leading to novel methods for managing protozoan parasites (165).
Gene editing has been shown to effectively reduce the virulence of parasitic organisms across various species by targeting specific genes associated with pathogenicity. In the liver fluke O. viverrini, CRISPR/Cas9-mediated knockout of the OV-GRN-1 gene has been demonstrated to reduce pathological effects, as evidenced by decreased biliary hyperplasia and fibrosis in infected hosts. This finding underscores the role of OV-GRN-1 in virulence during opisthorchiasis (166). Similarly, in Toxoplasma gondii, CRISPR-Cas9 deletion of the wx2 gene significantly inhibits parasite growth and replication in vitro and leads to a reduction in virulence in vivo. This effect is primarily mediated through modulation of the host immune response via the Th1 and Th17 pathways (167). In malaria parasites, zinc-finger nucleases have been utilized to induce double-strand breaks, resulting in developmentally arrested attenuated strains. This approach presents a promising strategy for vaccine development (168). Additionally, the CRISPR-Cas9 system has been applied to Plasmodium falciparum to enable rapid gene deletion and nucleotide substitution, facilitating gene function studies and the identification of novel drug targets (169). The application of CRISPR in parasitology extends to Eimeria tenella, where gene editing has been used to investigate gene function and identify essential genes for parasite development and survival. These studies provide valuable insights into potential therapeutic targets (170). Finally, in the nematode N. brasiliensis, a novel method utilizing extracellular vesicles for CRISPR/Cas9 component delivery has successfully disrupted secreted DNase II. This disruption resulted in reduced gene expression and demonstrated the feasibility of genetic manipulation in parasitic nematodes (171). Research also indicates that CRISPR/Cas9 can enhance the safety of parasitic eggs, particularly those of schistosomes, by reducing their pathogenicity (172). Experimental results show that eliminating the omega-1 protein significantly reduces the size of pulmonary granulomas in mice (173). This protein is a soluble egg antigen known to damage tissues and modulate the Th2 immune response (174), thereby attenuating parasite toxicity (173). CRISPR/Cas9 can also alter the acetylcholinesterase (AChE) gene in parasites, diminishing their activity and eliciting a Th2 immune response, which is essential for enhancing host defenses against parasites and potentially reducing resistance to atovaquone (175, 176). Furthermore, employing electroporation has proven to improve the efficiency of CRISPR/Cas9 gene editing (177), and studies have demonstrated that Cas12a offers superior gene editing capabilities compared to Cas9 (178). These advancements are crucial for improving the efficiency of clinical products related to parasites. While gene editing efficiencies are low, exploring new delivery mechanisms and markers could optimize these methods and foster further technological developments (179).
5.2 Real-time monitoring of therapeutic efficacy
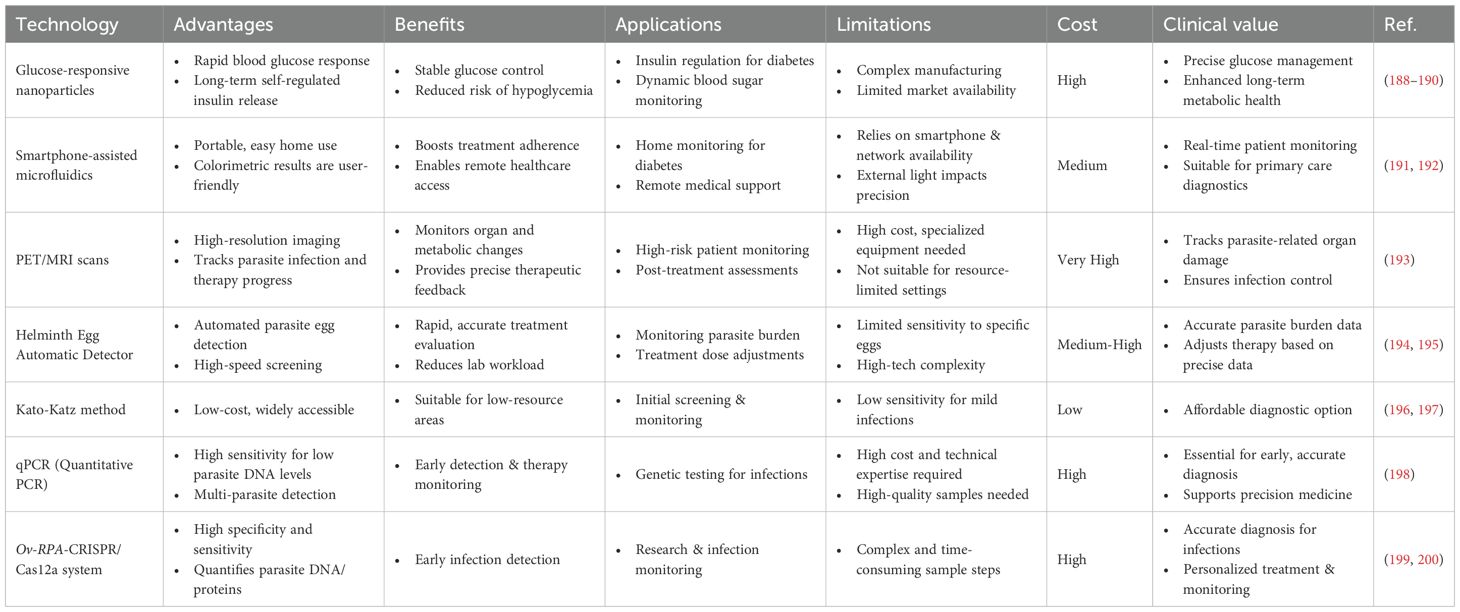
With modern medicine and information technology advancements, research into parasitic therapy is evolving. Rapid developments in intelligent detection technologies are now applied to monitoring DM-related markers and increasingly detecting parasites, revealing intricate interactions and potential therapeutic mechanisms between parasites and their hosts. For instance, glucose-responsive nanoparticles facilitate rapid and extended self-regulation of insulin delivery (180, 181). Similarly, smartphone-assisted microfluidic chemical analyzers use image-based colorimetry for comprehensive monitoring of DM and hyperlipidemia, offering real-time tracking of dynamic patient information (182).
Drawing on the concepts used in glucose-responsive materials and intelligent detection technologies, it is possible to continuously monitor the effectiveness of parasitic formulations and the patient’s status, enhancing the safety and efficacy of parasitic therapy. Patients receiving helminthic therapy are often subjected to regular medical monitoring, such as PET/MRI scans (133), to evaluate morphological and metabolic changes in organs affected by infection, ensuring that parasite levels remain safe. The incorporation of state-of-the-art monitoring technologies like the Helminth Egg Automatic Detector (HEAD) (183), Kato-Katz method (184), Mini-FLOTAC technique (185), qPCR (186), and Ov-RPA-CRISPR/Cas12a system (187) enables regular medical examinations during treatment, monitoring parasite numbers and activity to maintain control of the infection. The advantages and disadvantages of different new monitoring technologies are summarized in Table 3.
Furthermore, leveraging big data to explore new protein molecules, analyze parasite-human interactions, and acquire proteomics data from H. contortus through bioinformatics (201) are critical for harnessing parasites for human benefit. We also introduce a convolutional neural network (CNN) for the classification and automated detection of O. viverrini eggs from digital images (202). Digital imaging systems for identifying and quantifying pathogenic helminth eggs (203), together with the Helminth Egg Analysis Platform (HEAP) — an open platform integrating deep learning architecture for microscopically identifying and quantifying helminth eggs (204) — represent the forefront of this research area (Figure 3).
In conclusion, by integrating intelligent nanomaterial delivery systems with sophisticated monitoring technologies such as image-based colorimetry, microfluidic chemical analysis, and automatic helminth egg detection, we can achieve real-time surveillance of biochemical markers in diabetic patients and treatment outcomes in those undergoing parasitic therapy. This improves the precision and timeliness of treatments and opens new avenues for future medical interventions.
5.3 Anthelmintic drugs targeting glucose metabolism
In cases where patients do not achieve the anticipated therapeutic effects during parasite therapy or experience severe complications or immune responses, the immediate administration of anthelmintic drugs represents the most direct strategy to prevent the worsening of adverse reactions. Currently, pyrroloquinoline class drugs are the first choice for clinical antiparasitic treatments (205). However, due to growing concerns about resistance to these drugs (206), new anthelmintics targeting alternative biological pathways have been introduced. Notably, drugs targeting the glycometabolic pathways of parasites offer significant advantages for treating patients with T2D.
Parasites rely heavily on glucose as an energy source. Their metabolic competition for glucose results in enhanced uptake and metabolism by host cells to offset energy deficits (207). Research indicates that individuals with DM are more prone to parasitic infections, possibly due to higher glucose levels in the blood, which can directly influence blood sugar changes, particularly affecting men more significantly (208). Schistosomes, highly dependent on glucose for survival and reproduction, suffer in their ability to live and reproduce when glucose availability is inhibited (209). Targeted pharmaceutical research has identified vital glucose transport proteins (SGTPs) and enzymes within the glycolytic pathway in S. mansoni and S. japonicum as potential drug targets. Four compounds—pyrroquinoline, licochalcone A, licarin, and harmonine—have effectively inhibited SGTP4 (210), which is crucial for extracting glucose from the host’s bloodstream. Recent studies suggest that protein kinase B (Akt) is vital for SGTP4 expression (211), and inhibiting Akt reduces glucose uptake by the parasites (212). Moreover, deficiencies in glucose-6-phosphate dehydrogenase (G6PD) and pyruvate kinase (PK) in Plasmodium have been shown to protect against the severe effects of malaria, underscoring the enzymes’ critical role in the parasite’s lifecycle, thereby making PfGluPho a promising target for antimalarial drugs (213). Additionally, the fusion enzyme G6PD::6PGL in Giardia lamblia, with its increased glycometabolic efficiency and structural differences from human G6PD, represents a novel target for developing anti-Giardia medications (214, 215).
By targeting the glycometabolic processes essential for parasite survival and reproduction, we can precisely regulate their population within the host and mitigate their impact on host glucose metabolism (Figure 5). Furthermore, the inhibition of parasite-specific insulin receptors and human TNF-α has significantly decreased parasite glucose consumption, thus mitigating the adverse effects of parasite therapy (149, 216).
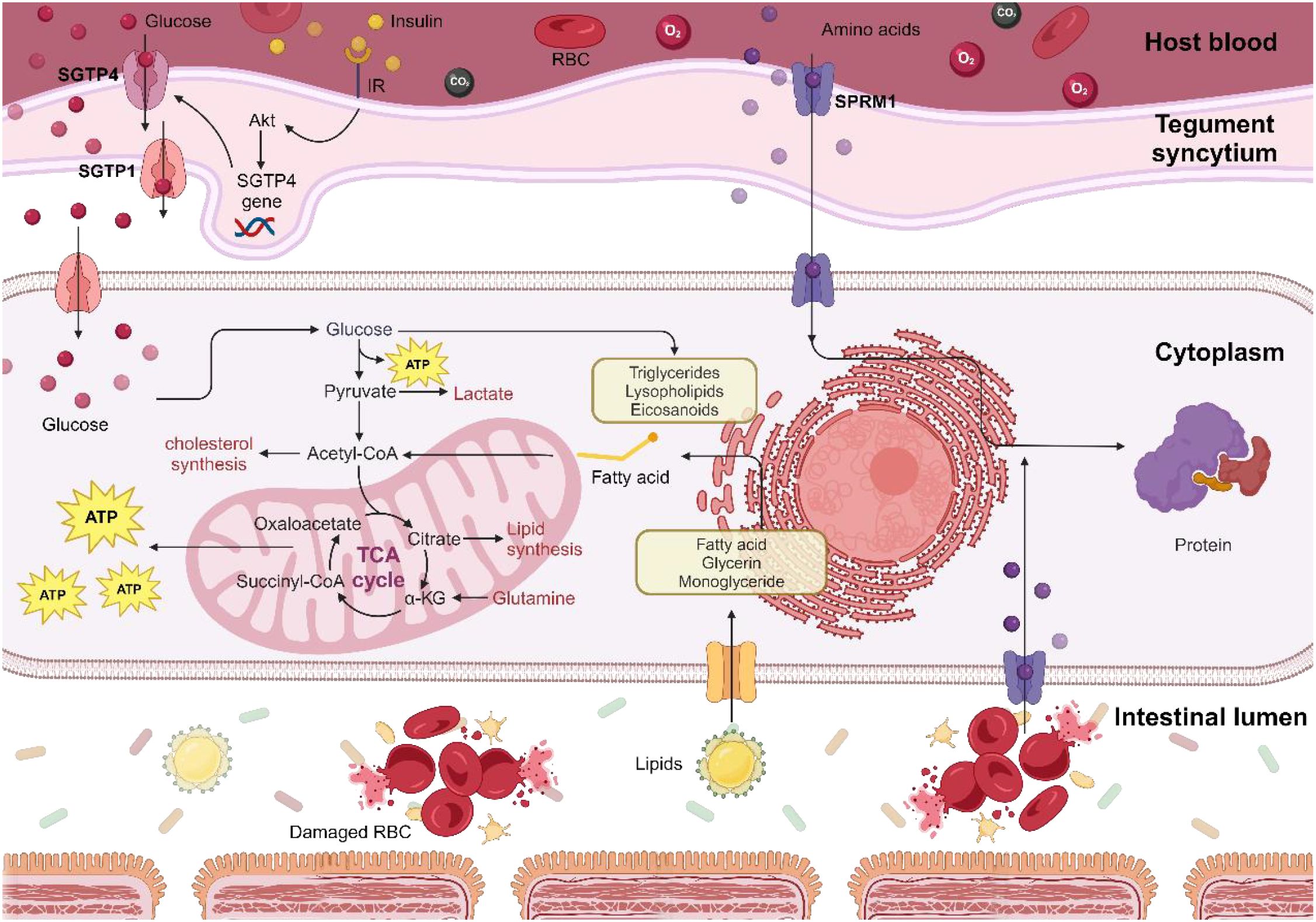
Figure 5. Mechanisms of energy metabolism in schistosomes. Schistosomes exploit host-derived glucose, lipids, proteins, and amino acids for metabolic energy production and as precursors for synthesizing their essential biomolecules, which are crucial for their growth, reproduction, and egg-laying activities. Consequently, targeting glucose uptake by schistosomes could offer heightened therapeutic sensitivity in patients with T2D.
5.4 The therapeutic application of molecules derived from helminths
Parasitic infections typically lead to pathological conditions; however, the therapeutic application of helminth-derived molecules in DM treatment has gained increasing interest in recent years. These molecules regulate the host immune response through multiple mechanisms and have shown potential therapeutic effects in both T1D and T2D.
Recent studies have highlighted the significant immunomodulatory properties of Fasciola hepatica secretions, which have been shown to prevent T1D in NOD mice. These secretions induce M2 macrophages and regulatory T cells, thereby suppressing pancreatic inflammation, reducing autoimmune destruction, and effectively halting DM progression (217). Similarly, the Fasciola hepatica-derived protein FhHDM-1 has demonstrated the ability to activate the PI3K/Akt signaling pathway and preserve β-cell mass in NOD mice, protecting against cytokine-induced apoptosis and enhancing insulin secretion (102). In addition to trematode-derived molecules, nematode-derived proteins from Wuchereria bancrofti (W. bancrofti) and Brugia malayi (B. malayi) have exhibited promising therapeutic potential in diabetic mouse models. These proteins lower blood glucose levels and reduce islet inflammation by inhibiting pro-inflammatory cytokines such as TNF-α and IFN-γ, while simultaneously promoting anti-inflammatory cytokines, including IL-4, IL-5, and IL-10 (218). This immunoregulatory mechanism further supports the potential of helminth-derived molecules in DM treatment. Beyond their effects on T1D, helminth infections have also shown benefits in T2D models. For instance, S. mansoni infection has been found to enhance Th2-type immune responses, leading to improved insulin sensitivity in obese mice and reduced inflammation in adipose tissue. This suggests that helminth infections may alleviate metabolic disturbances associated with T2D by modulating systemic immune responses (6). Similarly, S. mansoni-derived soluble egg antigens (SEA) and ω1 have been shown to enhance metabolic homeostasis and insulin sensitivity through immune modulation, particularly by promoting Th2 cells, eosinophils, and M2 macrophages in adipose tissue (208, 209). Additional research has demonstrated that antigens from Litomosoides sigmodontis (L. sigmodontis) can enhance the efficacy of insulin-specific therapy. When combined with insulin treatment in NOD mice, these antigens effectively prevent the onset of T1D, even in the presence of early pancreatic inflammation (P > 0.05) (219). Likewise, hookworm-derived secretions have exhibited potential in improving T2D-related metabolic disorders. Studies indicate that these secretions enhance glucose tolerance in diabetic mouse models and reduce systemic inflammation by modulating gut microbiota and local immune responses, ultimately improving insulin sensitivity (220). Furthermore, excretory-secretory products (ESPs) from N. brasiliensis, including those from adult and third-stage larvae, have been shown to significantly lower fasting blood glucose levels and improve glucose metabolism in HFD-fed C57BL/6 mice. These effects are accompanied by immune modulation, characterized by increased eosinophil and IL-5 levels and decreased IL-6 levels in adipose tissue (207). These results highlight the therapeutic potential of worm-derived molecules, which modulate both metabolic and immune pathways, offering new strategies for managing DM and related metabolic conditions.
5.5 Challenges in parasite engineering and monitoring
Delivery and editing efficiency. Genome engineering in multicellular helminths remains limited by stage-specific physical barriers (egg shell, cuticle) and low transformation/editing rates across life stages. Proof-of-concept CRISPR/Cas9 editing in Schistosoma mansoni and the recent generation of stable transgenic lines in Strongyloides stercoralis demonstrate feasibility, yet frequent mosaicism and limited heritability still complicate phenotype attribution and scale-up, indicating platform-level optimization is required before translation (173, 221).
Off-target risk and validation. Translational applications require genome-wide, unbiased off-target surveillance beyond in silico predictions. CHANGE-seq provides sensitive and scalable maps of nuclease activity and should be coupled with amplicon deep sequencing or whole-genome sequencing across relevant life stages to minimize false attribution of engineered phenotypes (222).
Monitoring readouts and clinically meaningful endpoints. For parasite burden, circulating antigen assays (e.g., CCA/CAA) outperform microscopy at low intensities in schistosomiasis and are increasingly recommended for surveillance and clinical evaluations, although implementation remains heterogeneous across settings. For host metabolism, helminth–metabolism trials should pre-specify HOMA-IR, fasting plasma glucose, HbA1c and—when feasible—CGM-derived time-in-range (TIR) per contemporary diabetes standards to enable cross-study comparability (223, 224).
Regulatory and biosafety expectations. Clinical development of live or engineered helminths should meet LBP-like Chemistry, Manufacturing, and Controls (CMC) expectations (identity/potency, sterility, genomic stability) under U.S. Food and Drug Administration (FDA) guidance and comply with the updated National Institutes of Health (NIH) Guidelines for recombinant/synthetic nucleic acid research (Institutional Biosafety Committee (IBC) oversight; appropriate containment for gene-drive-related constructs), with jurisdiction-specific alignment. Protocols must include rescue therapy, environmental containment/kill-switch plans, and post-trial surveillance.
6 Proposal for clinical decision-making
6.1 Risk assessment
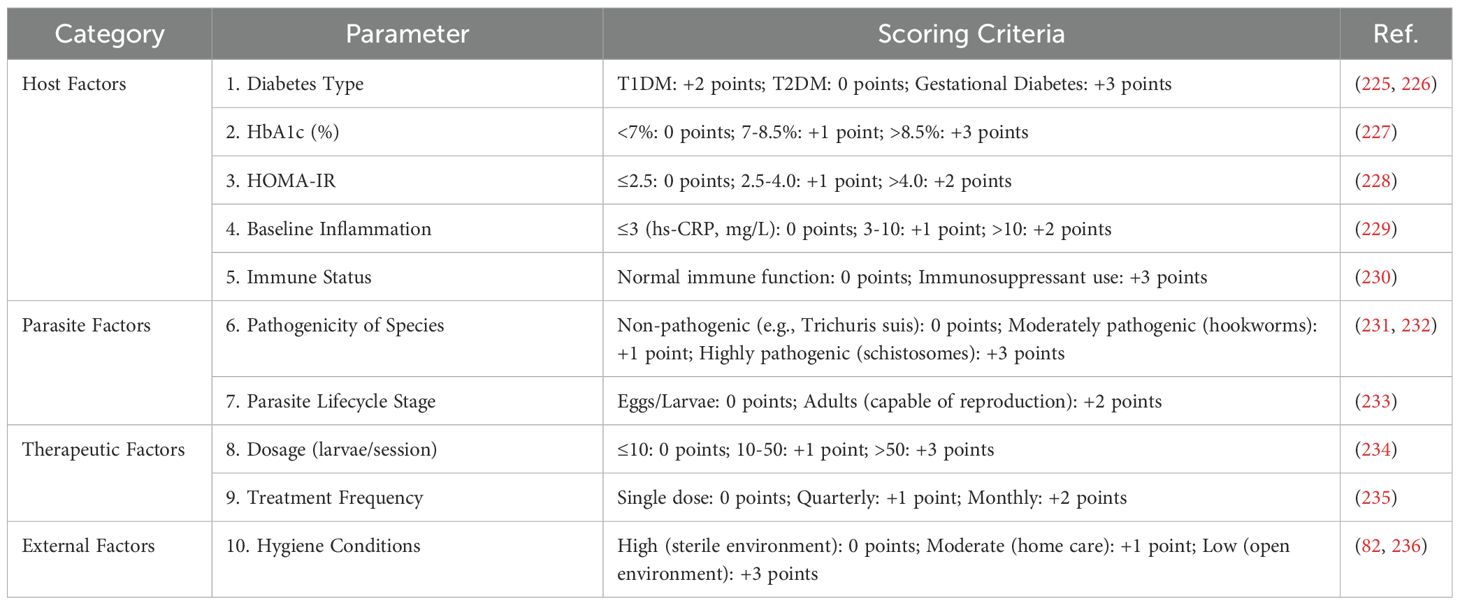
In the application of parasitic therapy for DM treatment, establishing appropriate patient selection criteria is essential for ensuring both its adaptability and safety. To address this need, we have developed a comprehensive risk assessment framework that integrates host factors, parasitic factors, treatment parameters, and environmental considerations to evaluate the suitability and potential risks of parasitic therapy for individual patients. (i) Host Factors: These include DM type (225, 226), glycated hemoglobin (HbA1c) levels (227), homeostatic model assessment of insulin resistance (HOMA-IR) (228), baseline inflammation levels (229), and immune status (230). These indicators provide critical insights into the patient’s metabolic condition and inflammatory response, serving as key criteria for determining their eligibility for parasitic therapy. (ii) Parasitic Factors: Given that different parasite species and their life cycle stages exert varying effects on the host, we assess their pathogenicity and developmental stage to determine the most appropriate and safe parasite type for therapeutic use (231–233). (iii) Treatment Parameters: Factors such as treatment dosage and frequency are tailored to each patient based on prior risk assessment results, optimizing therapeutic efficacy while minimizing potential adverse effects (234, 235). (iv) Environmental Factors: Considerations such as hygiene conditions are crucial in mitigating the risk of infections that may occur during treatment (82, 236). The risk scoring table and risk classification are shown in Table 4a and Table 4b, respectively.
Based on these variables, we have developed a quantitative risk scoring system to systematically assess each patient’s overall risk profile. Patients are categorized into three risk levels—low, medium, or high—each corresponding to specific treatment recommendations. This stratified approach enables a more personalized treatment strategy while maintaining safety and efficacy. By implementing this risk assessment framework, we provide clinicians with a structured decision-making tool to determine patient eligibility for parasitic therapy, identify those requiring specialized treatment strategies, and recognize cases where the therapy should be avoided altogether. This systematic approach not only enhances the precision and personalization of treatment but also improves patient safety and overall treatment satisfaction.
6.2 Dosage and duration of treatment
The appropriate selection of parasite species, dosing regimen, and treatment duration is critical for ensuring the safety and efficacy of helminth therapy in patients with T2D. Based on current clinical trials and experimental studies, N. americanus (American hookworm) and Trichuris suis ova (TSO, pig whipworm eggs) are the most extensively studied helminths for T2D treatment. The typical dosages are as follows.
N. americanus: In a randomized, double-blind, placebo-controlled Phase Ib trial of 40 adults at risk of type 2 diabetes, a single percutaneous inoculation of 20 or 40 L3 larvae was evaluated; at 12 months, both doses showed signals of improvement in HOMA-IR and fasting glucose, and the 20-larvae arm exhibited body mass reduction at 24 months. Adverse events were predominantly mild–moderate gastrointestinal symptoms with acceptable overall tolerability; dose, follow-up schedule, and predefined metabolic endpoints are detailed in the published protocol. Accordingly, 20 or 40 L3 is recommended as starting doses in clinical studies with rigorous metabolic and safety monitoring (3, 76).
TSO: Clinical studies recommend an initial dose of 2,500 eggs, administered orally every two weeks, with potential escalation to 7,500 eggs based on patient tolerance and therapeutic response. The standard treatment duration is 12–24 weeks, during which immune and metabolic parameters—such as cytokine levels (IL-10, TNF-α), homeostatic model assessment of insulin resistance (HOMA-IR), and fasting blood glucose—should be closely monitored (135, 239).
Dose titration is essential for minimizing adverse effects and optimizing therapeutic outcomes. Factors such as body weight, baseline glycemic control, and especially HbA1c should be considered (Table 5). Patients with lower body weight or elevated baseline inflammatory markers may require lower initial doses to prevent excessive immune activation (245, 246). Routine assessment of key biomarkers—including CRP, eosinophil count, and gastrointestinal symptoms—is advisable to guide gradual dose escalation or reduction (246, 247). The duration of therapy should be tailored to individual patient needs and treatment goals. For patients with well-controlled blood glucose, a shorter treatment course (e.g., 12 weeks) may suffice, whereas those with poor glycemic control or severe insulin resistance may require an extended course of up to 24 weeks (248). Long-term follow-up is essential for evaluating the durability of therapeutic benefits and detecting delayed adverse effects, such as gut microbiota dysbiosis or residual parasite burden (249).
6.3 Monitoring during the treatment period
In the application of parasitic therapy, continuous and precise patient monitoring is of primary importance (250). Advances in modern medicine and information technology, particularly the integration of intelligent detection systems and nanomaterials, have the potential to enhance the monitoring capabilities of parasitic therapy for DM treatment. The proposed monitoring protocol is designed to comprehensively assess therapeutic outcomes and their physiological effects on patients, ensuring both safety and efficacy. Once treatment begins, the monitoring plan should include routine blood glucose and HbA1c tests every three months, which help evaluate DM management and assess the therapy’s impact on insulin resistance (251, 252). Additionally, inflammatory markers and lipid levels should be assessed every six months, as this is crucial for monitoring chronic inflammation and cardiovascular health (253). These periodic evaluations facilitate timely adjustments to the treatment regimen, ensuring optimal therapeutic outcomes (254). Following the completion of treatment, annual follow-up examinations will focus on the long-term control of DM and changes in gut microbiota—key factors in assessing the sustained efficacy and broader physiological effects of parasitic therapy (3). This comprehensive monitoring protocol provides physicians with a complete dataset on treatment progression, allowing for more precise adjustments to personalized treatment plans (see detailed Table 5).
6.4 Ethical and regulatory considerations
Clinical translation of helminth-based interventions raises substantial ethical and regulatory challenges.
Ethical oversight and informed consent. Participants must be provided with clear and comprehensive information about potential risks (e.g., acute infection, immunopathology, allergic reactions), uncertain benefits, and long-term unknowns, and they must retain the right to withdraw at any time. Vulnerable groups such as pregnant individuals, children, and immunocompromised persons require special protection. Long-term follow-up (≥12–24 months where feasible) is ethically essential to capture delayed or chronic adverse effects (255).
Regulatory classification and quality control. Live helminth therapies are likely to be classified as Live Biotherapeutic Products (LBPs) or biologics under authorities such as the FDA (US) and European Medicines Agency (EMA) (EU). Regulatory guidance emphasizes stringent requirements for identity, purity, potency, viability, and stability, with well-defined Chemistry, Manufacturing, and Controls (CMC) procedures to ensure batch-to-batch consistency, traceability, and freedom from adventitious agents (256, 257).
Safety monitoring and risk mitigation. Clinical protocols should guarantee immediate availability of rescue anthelmintics to terminate infection in case of adverse events. Independent Data Safety Monitoring Board (DSMB) oversight and standardized adverse event (AE) reporting are required. Predefined stopping rules should be implemented to halt trials in response to serious safety concerns (256).
Environmental and biosafety considerations. Trials involving genetically modified helminths or non-endemic species must include robust biosafety and containment strategies to prevent accidental release. Risk assessments should evaluate persistence, transmissibility, and ecological consequences.
Alternatives and translational pathways. Given the ethical and regulatory complexity of live infection, many groups advocate prioritizing helminth-derived molecules or excretory/secretory products (ESPs) as lower-risk alternatives. These may reproduce immunomodulatory benefits while avoiding risks of live organism administration (258, 259).
7 Conclusion
Helminthic therapy presents a promising yet complex approach for DM management due to its immunomodulatory and metabolic regulatory effects. However, its broader clinical application remains constrained by significant challenges, including infection risks, patient-specific variability in therapeutic response, and the need for long-term safety data. Addressing these concerns requires a more refined understanding of host-parasite interactions, strategic risk mitigation, and the integration of intelligent monitoring technologies to optimize patient outcomes.
Future research should focus on elucidating the precise molecular and immunological mechanisms underlying the therapeutic effects of helminths. While studies have demonstrated improvements in insulin sensitivity and metabolic homeostasis, the specific pathways through which helminths modulate glucose metabolism remain insufficiently explored (260). Additionally, there is a pressing need for longitudinal clinical trials to assess the long-term safety and efficacy of helminthic therapy in diverse patient populations. Large-scale studies incorporating real-world data will be essential to determine the sustainability of treatment benefits and to identify potential delayed adverse effects (261, 262). Furthermore, epidemiological investigations into the inverse correlation between helminthic infections and DM prevalence across different geographic regions could provide valuable insights into population-level metabolic trends (263). From a clinical perspective, developing practical guidelines for patient selection and risk stratification is crucial to ensuring treatment safety and efficacy. Not all diabetic patients are suitable candidates for helminthic therapy, and those with compromised immune function or chronic infections may be at greater risk for adverse effects (264). A structured framework should be established to categorize patients based on their metabolic profile, immune response, and infection susceptibility, allowing for more personalized and controlled therapeutic interventions (265). Treatment regimens should also be optimized to determine the most effective dosing strategies and duration of therapy. The integration of helminthic therapy with conventional anti-diabetic treatments, such as metformin or Glucagon-Like Peptide-1 (GLP-1) receptor agonists, may enhance therapeutic outcomes while mitigating risks associated with live parasite exposure (14). Advancements in monitoring technologies will play a pivotal role in improving the safety and precision of helminthic therapy. Real-time tracking of treatment responses using AI-driven biomarker analysis, wearable biosensors, and digital health tools could provide continuous feedback on patient health status (266). Machine learning algorithms analyzing individualized patient data may further enable predictive modeling, allowing clinicians to tailor treatment regimens dynamically (267). Additionally, developing genetically modified helminths with reduced pathogenicity and reproductive potential may minimize adverse effects while preserving immunotherapeutic benefits. Risk mitigation strategies must also incorporate contingency measures for potential complications (268). Patients undergoing helminthic therapy should have access to immediate anthelmintic interventions if unexpected side effects arise. Moreover, targeted anthelmintic drugs that selectively suppress helminth survival without disrupting their beneficial immune effects could improve treatment safety (269, 270). In addition, clinical translation will also require strict adherence to ethical and regulatory standards, including frameworks governing live biotherapeutic products, to ensure patient safety, infection control, and public acceptance (256, 257).
Despite these challenges, helminthic therapy remains a compelling avenue for future metabolic disease management. With robust ethical and regulatory safeguards in place, together with biotechnological and personalized strategies, this therapy has the potential to evolve into a safe, effective, and accessible therapeutic option. Continued interdisciplinary research and innovation will be key to unlocking the full therapeutic potential of helminths and translating their immunomodulatory properties into viable clinical applications. Overall, therapeutic signals appear species-specific and context-dependent, emphasizing careful stratification by pathogen class, host background, and infection dynamics.
Author contributions
YuZ: Writing – review & editing, Software, Conceptualization, Writing – original draft, Methodology. XF: Writing – review & editing, Methodology. RW: Formal Analysis, Data curation, Validation, Writing – review & editing. JW: Writing – original draft, Methodology, Validation, Writing – review & editing. XL: Methodology, Writing – review & editing. YiZ: Conceptualization, Writing – review & editing. JX: Visualization, Writing – review & editing. QZ: Formal Analysis, Writing – review & editing. KC: Writing – review & editing. XZ: Writing – review & editing. HL: Conceptualization, Writing – review & editing, Validation, Supervision, Methodology, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was funded by the National Natural Science Foundation of China (Grant No: 32000293); Guangxi Natural Science Foundation (Grant Nos: 2020JJA130077 and 2018JJB140423); the University Level Scientific Research Project of Zhejiang Shuren University (Grant No: 2022R064); Zhejiang Shuren University Basic Scientific Research Special Funds (Grant No: 2024XZ014); Zhejiang Shuren University School-Level Research Project (Grant No: 2023A11001); and the National Innovation and Entrepreneurship Training Program for College Students in 2023(Grant No: 202311842052X).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1642707/full#supplementary-material
References
1. IDF diabetes atlas (2021). Available online at: https://diabetesatlas.org/ (Accessed August 2024).
2. Ozir-Fazalalikhan A, Berbee JFP, van Dijk KW, van Harmelen V, Yazdanbakhsh M, Guigas B, et al. Chronic helminth infection and helminth-derived egg antigens promote adipose tissue M2 macrophages and improve insulin sensitivity in obese mice. FASEB J. (2015) 29:3027–39. doi: 10.1096/fj.14-266239, PMID: 25852044
3. Pierce D, Merone L, Lewis C, Rahman T, Croese J, Loukas A, et al. Safety and tolerability of experimental hookworm infection in humans with metabolic disease: study protocol for a phase 1b randomised controlled clinical trial. BMC Endocr Disord. (2019) 19:136. doi: 10.1186/s12902-019-0461-5, PMID: 31829172
4. Pierce DR, McDonald M, Merone L, Becker L, Thompson F, Lewis C, et al. Effect of experimental hookworm infection on insulin resistance in people at risk of Type 2 Diabetes: a randomized, placebo-controlled trial. medrxiv. (2023). doi: 10.1101/2023.03.16.23287372
5. Xing H, Li-Rong Z, Feng-Song C, Jing-Ping Z, and Meng-Hua Z. Trichuris suis ova therapy in inflammatory bowel disease: a meta-analysis. Medicine. (2018) 97:e12087. doi: 10.1097/MD.0000000000012087, PMID: 30142867
6. Amer AS, Othman AA, Dawood LM, El-Nouby KA, Gobert GN, Abou Rayia DM, et al. The interaction of Schistosoma mansoni infection with diabetes mellitus and obesity in mice. Sci Rep. (2023) 13:9417. doi: 10.1038/s41598-023-36112-5, PMID: 37296126
7. Rajamanickam A, Dasan B, Munisankar S, Nott S, Menon PA, Shaik FA, et al. Impact of Strongyloides stercoralis infection on complement activation in Type 2 diabetes mellitus: Insights from a clinical and anthelmintic intervention study. PloS Negl Trop Dis. (2024) 18:e0012048. doi: 10.1371/journal.pntd.0012048, PMID: 38564496
8. Rajamanickam A, Munisankar S, Bhootra Y, Dolla C, Thiruvengadam K, Nutman TB, et al. Metabolic consequences of concomitant Strongyloides stercoralis infection in patients with type 2 diabetes mellitus. Clin Infect Dis. (2019) 69:697–704. doi: 10.1093/cid/ciy935, PMID: 30407548
9. Molan A, Nosaka K, Hunter M, and Wang W. The association between Toxoplasma gondii and type 2 diabetes mellitus: a systematic review and meta-analysis of human case-control studies. Bull Natl Res Centre. (2020) 44:1–9. doi: 10.1186/s42269-019-0256-x
10. Htun NSN, Odermatt P, Paboriboune P, Sayasone S, Vongsakid M, Phimolsarn-Nusith V, et al. Association between helminth infections and diabetes mellitus in adults from the Lao People’s Democratic Republic: a cross-sectional study. Infect Dis poverty. (2018) 7:1–11. doi: 10.1186/s40249-018-0488-2, PMID: 30396368
11. Geach T. Diabetes: Helminths improve insulin sensitivity and enhance M2 macrophage numbers in WAT of obese mice. Nat Rev Endocrinol. (2015) 11:316. doi: 10.1038/nrendo.2015.68, PMID: 25917360
12. Chandi DH and Lakhani SJ. Prevalence of intestinal parasites among diabetes mellitus patients in tertiary care hospital. J Pure Appl Microbiol. (2024) 18(2):1051–6. doi: 10.22207/JPAM.18.2.20
13. Morsy SM, Ghallab MMI, Ismail MAM, and Ismail AR. OPPORTUNISTIC PROTOZOA COINFECTED WITH HELICOBACTER PYLORI AMONG EGYPTIAN DIABETIC PATIENTS. J Egyptian Soc Parasitol. (2024) 54:103–8. doi: 10.21608/jesp.2024.351361
14. Sikder S, Pierce D, Sarkar ER, McHugh C, Quinlan KGR, Giacomin P, et al. Regulation of host metabolic health by parasitic helminths. Trends Parasitol. (2024) 40:386–400. doi: 10.1016/j.pt.2024.03.006, PMID: 38609741
15. Chakraborty P, Aravindhan V, and Mukherjee S. Helminth-derived biomacromolecules as therapeutic agents for treating inflammatory and infectious diseases: What lessons do we get from recent findings? Int J Biol Macromolecules. (2023) 241:124649. doi: 10.1016/j.ijbiomac.2023.124649, PMID: 37119907
16. Yeshi K, Ruscher R, Loukas A, and Wangchuk P. Immunomodulatory and biological properties of helminth-derived small molecules: Potential applications in diagnostics and therapeutics. Front Parasitol. (2022) 1:984152. doi: 10.3389/fpara.2022.984152, PMID: 39816468
17. Mughal MAS, Khan MK, Abbas Z, Abbas RZ, Bajwa HR, Chatha AK, et al. Helminth protection against type-1 diabetes: An insight into immunomodulatory effect of helminth-induced infection. Mol Biol Rep. (2021) 48:6581–8. doi: 10.1007/s11033-021-06663-9, PMID: 34432219
18. Madden KB, Whitman L, Sullivan C, Gause WC, Urban JF, Katona IM, et al. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J Immunol. (2002) 169:4417–22. doi: 10.4049/jimmunol.169.8.4417, PMID: 12370375
19. Zhao A, McDermott J, Urban JF, Gause W, Madden KB, Au Yeung K, et al. Dependence of IL-4, IL-13, and nematode-induced alterations in murine small intestinal smooth muscle contractility on Stat6 and enteric nerves. J Immunol. (2003) 171:948–54. doi: 10.4049/jimmunol.171.2.948, PMID: 12847266
20. Notari L, Riera DC, Sun R, Bohl JA, McLean LP, Madden KB, et al. Role of macrophages in the altered epithelial function during a type 2 immune response induced by enteric nematode infection. PloS One. (2014) 9:e84763. doi: 10.1371/journal.pone.0084763, PMID: 24465430
21. Worthington JJ, Samuelson LC, Grencis RK, and McLaughlin JT. Adaptive immunity alters distinct host feeding pathways during nematode induced inflammation, a novel mechanism in parasite expulsion. PloS Pathog. (2013) 9:e1003122. doi: 10.1371/journal.ppat.1003122, PMID: 23349631
22. Koehler S, Springer A, Issel N, Klinger S, Wendt M, Breves G, et al. Effects of adult Ascaris suum and their antigens (total and trans-cuticular excretory-secretory antigen, cuticular somatic antigen) on intestinal nutrient transport in vivo. Parasitology. (2022) 150:1–34. doi: 10.1017/S0031182022001512, PMID: 36274629
23. Kha S, Chaiyadet S, Saichua P, Tangkawatana S, Sripa B, Suttiprapa S, et al. Opisthorchis viverrini excretory-secretory products suppress GLUT8 of cholangiocytes. Parasitol Res. (2024) 123:161. doi: 10.1007/s00436-024-08184-3, PMID: 38491300
24. Koehler S, Springer A, Issel N, Klinger S, Wendt M, Breves G, et al. Ascaris suum nutrient uptake and metabolic release, and modulation of host intestinal nutrient transport by excretory-secretory and cuticle antigens in vitro. Pathogens. (2021) 10:1419. doi: 10.3390/pathogens10111419, PMID: 34832575
25. Su CW, Chen CY, Li Y, Long SR, Massey W, Kumar DV, et al. Helminth infection protects against high fat diet-induced obesity via induction of alternatively activated macrophages. Sci Rep. (2018) 8:4607. doi: 10.1038/s41598-018-22920-7, PMID: 29545532
26. Hams E, Bermingham R, Wurlod FA, Hogan AE, O'Shea D, Preston RJ, et al. The helminth T2 RNase ω1 promotes metabolic homeostasis in an IL-33- and group 2 innate lymphoid cell-dependent mechanism. FASEB J. (2016) 30:824–35. doi: 10.1096/fj.15-277822, PMID: 26490658
27. Ni Y, Xu Z, Li C, Zhu Y, Liu R, Zhang F, et al. Therapeutic inhibition of miR-802 protects against obesity through AMPK-mediated regulation of hepatic lipid metabolism. Theranostics. (2021) 11:1079–99. doi: 10.7150/thno.49354, PMID: 33391522
28. Obi S, Shimokawa C, Katsuura M, Olia A, Imai T, Suzue K, et al. IL-33 is essential to prevent high-fat diet-induced obesity in mice infected with an intestinal helminth. Parasite Immunol. (2020) 42:e12700. doi: 10.1111/pim.12700, PMID: 32027755
29. Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U.S.A. (2004) 101:15718–23. doi: 10.1073/pnas.0407076101, PMID: 15505215
30. Blander JM, Longman RS, Iliev ID, Sonnenberg GF, and Artis D. Regulation of inflammation by microbiota interactions with the host. Nat Immunol. (2017) 18:851–60. doi: 10.1038/ni.3780, PMID: 28722709
31. Fricke WF, Song Y, Wang AJ, Smith A, Grinchuk V, Pei C, et al. Type 2 immunity-dependent reduction of segmented filamentous bacteria in mice infected with the helminthic parasite Nippostrongylus brasiliensis. Microbiome. (2015) 3:40. doi: 10.1186/s40168-015-0103-8, PMID: 26377648
32. Zhou S, Lu Y, Chen J, Pan Z, Pang L, Wang Y, et al. Parasite reliance on its host gut microbiota for nutrition and survival. Isme J. (2022) 16:2574–86. doi: 10.1038/s41396-022-01301-z, PMID: 35941172
33. Caudet J, Trelis M, Cifre S, Soriano JM, and Merino-Torres JF. Presence and significance of intestinal unicellular parasites in a morbidly obese population. Int J Obes (Lond). (2022) 46:220–7. doi: 10.1038/s41366-021-00980-6, PMID: 34650200
34. Rausch S, Affinass N, Midha A, Radonic A, Kuhring M, Kühl AA, et al. Parasitic nematodes exert antimicrobial activity and benefit from microbiota-driven support for host immune regulation. Front Immunol. (2018) 9:2282. doi: 10.3389/fimmu.2018.02282, PMID: 30349532
35. Canfora EE, Jocken JW, and Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. (2015) 11:577–91. doi: 10.1038/nrendo.2015.128, PMID: 26260141
36. May KS and den Hartigh LJ. Gut microbial-derived short chain fatty acids: impact on adipose tissue physiology. Nutrients. (2023) 15:272. doi: 10.3390/nu15020272, PMID: 36678142
37. Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. (2009) 58:1509–17. doi: 10.2337/db08-1637, PMID: 19366864
38. Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci U.S.A. (2011) 108:8030–5. doi: 10.1073/pnas.1016088108, PMID: 21518883
39. Su CW, Chen CY, Mao T, Chen N, Steudel N, Jiao L, et al. Maternal helminth infection protects offspring from high-fat-diet-induced obesity through altered microbiota and SCFAs. Cell Mol Immunol. (2023) 20:389–403. doi: 10.1038/s41423-023-00979-1, PMID: 36788341
40. Peng L, Li ZR, Green RS, Holzman IR, and Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. (2009) 139:1619–25. doi: 10.3945/jn.109.104638, PMID: 19625695
41. Pace F, Carvalho BM, Zanotto TM, Santos A, Guadagnini D, Silva KLC, et al. Helminth infection in mice improves insulin sensitivity via modulation of gut microbiota and fatty acid metabolism. Pharmacol Res. (2018) 132:33–46. doi: 10.1016/j.phrs.2018.04.008, PMID: 29653264
42. Singh AK, Kumar P, Mishra SK, Rajput VD, Tiwari KN, Singh AK, et al. A dual therapeutic approach to diabetes mellitus via bioactive phytochemicals found in a poly herbal extract by restoration of favorable gut flora and related short-chain fatty acids. Appl Biochem Biotechnol. (2024) 196:6690–715. doi: 10.1007/s12010-024-04879-6, PMID: 38393580
43. Ramanan D, Bowcutt R, Lee SC, Tang MS, Kurtz ZD, Ding Y, et al. Helminth infection promotes colonization resistance via type 2 immunity. Science. (2016) 352:608–12. doi: 10.1126/science.aaf3229, PMID: 27080105
44. Losol P, Wolska M, Wypych TP, Yao L, O'Mahony L, Sokolowska M, et al. A cross talk between microbial metabolites and host immunity: Its relevance for allergic diseases. Clin Transl Allergy. (2024) 14:e12339. doi: 10.1002/clt2.12339, PMID: 38342758
45. Sun XM, Hao CY, Wu AQ, Luo ZN, El-Ashram S, Alouffi A, et al. Trichinella spiralis -induced immunomodulation signatures on gut microbiota and metabolic pathways in mice. PloS Pathog. (2024) 20:e1011893. doi: 10.1371/journal.ppat.1011893, PMID: 38166140
46. Shimokawa C, Obi S, Shibata M, Olia A, Imai T, Suzue K, et al. Suppression of obesity by an intestinal helminth through interactions with intestinal microbiota. Infect Immun. (2019) 87:e00042-19. doi: 10.1128/IAI.00042-19, PMID: 30962398
47. Su CW, Chen CY, Jiao L, Long SR, Mao T, Ji Q, et al. Helminth-induced and th2-dependent alterations of the gut microbiota attenuate obesity caused by high-fat diet. Cell Mol Gastroenterol Hepatol. (2020) 10:763–78. doi: 10.1016/j.jcmgh.2020.06.010, PMID: 32629118
48. Loke P and Harris NL. Networking between helminths, microbes, and mammals. Cell Host Microbe. (2023) 31:464–71. doi: 10.1016/j.chom.2023.02.008, PMID: 37054669
49. Cosentino C and Regazzi R. Crosstalk between macrophages and pancreatic β-cells in islet development, homeostasis and disease. Int J Mol Sci. (2021) 22:1765. doi: 10.3390/ijms22041765, PMID: 33578952
50. Tsuyama T, Sato Y, Yoshizawa T, Matsuoka T, and Yamagata K. Hypoxia causes pancreatic β-cell dysfunction and impairs insulin secretion by activating the transcriptional repressor BHLHE40. EMBO Rep. (2023) 24:e56227. doi: 10.15252/embr.202256227, PMID: 37341148
51. Yang S, Zhao M, and Jia S. Macrophage: Key player in the pathogenesis of autoimmune diseases. Front Immunol. (2023) 14:1080310. doi: 10.3389/fimmu.2023.1080310, PMID: 36865559
52. Ward MG, Li G, and Hao M. Apoptotic β-cells induce macrophage reprogramming under diabetic conditions. J Biol Chem. (2018) 293:16160–73. doi: 10.1074/jbc.RA118.004565, PMID: 30213857
53. Qian B, Yang Y, Tang N, Wang J, Sun P, Yang N, et al. M1 macrophage-derived exosomes impair beta cell insulin secretion via miR-212-5p by targeting SIRT2 and inhibiting Akt/GSK-3β/β-catenin pathway in mice. Diabetologia. (2021) 64:2037–51. doi: 10.1007/s00125-021-05489-1, PMID: 34117507
54. Dludla PV, Mabhida SE, Ziqubu K, Nkambule BB, Mazibuko-Mbeje SE, Hanser S, et al. Pancreatic β-cell dysfunction in type 2 diabetes: Implications of inflammation and oxidative stress. World J Diabetes. (2023) 14:130–46. doi: 10.4239/wjd.v14.i3.130, PMID: 37035220
55. Dickson I. IBD: Parasites promote protective microbiota. Nat Rev Gastroenterol Hepatol. (2016) 13:316. doi: 10.1038/nrgastro.2016.73, PMID: 27147492
56. Mishra PK, Palma M, Bleich D, Loke P, and Gause WC. Systemic impact of intestinal helminth infections. Mucosal Immunol. (2014) 7:753–62. doi: 10.1038/mi.2014.23, PMID: 24736234
57. Kang SA, Choi JH, Baek KW, Lee DI, Jeong MJ, Yu HS, et al. Trichinella spiralis infection ameliorated diet-induced obesity model in mice. Int J Parasitol. (2021) 51:63–71. doi: 10.1016/j.ijpara.2020.07.012, PMID: 32966835
58. Jensen DM, Hendricks KV, Mason AT, and Tessem JS. Good cop, bad cop: the opposing effects of macrophage activation state on maintaining or damaging functional β-cell mass. Metabolites. (2020) 10:485. doi: 10.3390/metabo10120485, PMID: 33256225
59. Schmidt V, Hogan AE, Fallon PG, and Schwartz C. Obesity-mediated immune modulation: one step forward, (Th)2 steps back. Front Immunol. (2022) 13:932893. doi: 10.3389/fimmu.2022.932893, PMID: 35844529
60. Vacca F and Le Gros G. Tissue-specific immunity in helminth infections. Mucosal Immunol. (2022) 15:1212–23. doi: 10.1038/s41385-022-00531-w, PMID: 35680972
61. Maizels RM and McSorley HJ. Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol. (2016) 138:666–75. doi: 10.1016/j.jaci.2016.07.007, PMID: 27476889
62. Allen JE and Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. (2011) 11:375–88. doi: 10.1038/nri2992, PMID: 21610741
63. Li J, Wang J, Song X, Li Z, Zhang Y, Lu H, et al. Effect of type 2 diabetes mellitus and periodontitis on the Th1/Th2 and Th17/Treg paradigm. Am J Dent. (2022) 35:55–60., PMID: 35316594
64. Gu QW, Sun Q, Wang J, Gu WS, Wang W, Mao XM, et al. Effects of glycemic variability on regulatory T cells in patients with type 2 diabetes and kidney disease. Diabetes Metab Syndr Obes. (2023) 16:2365–75. doi: 10.2147/DMSO.S413407, PMID: 37577044
65. Varghese RT and Jialal I. Diabetic nephropathy. In: StatPearls. StatPearls Publishing LLC, Treasure Island (FL. (2025).
66. Queiroz-Glauss CP, Vieira MS, Gonçalves-Pereira MH, Almeida SS, Freire RH, Gomes MA, et al. Helminth infection modulates number and function of adipose tissue Tregs in high fat diet-induced obesity. PloS Negl Trop Dis. (2022) 16:e0010105. doi: 10.1371/journal.pntd.0010105, PMID: 35499991
67. Huang L, Gebreselassie NG, Gagliardo LF, Ruyechan MC, Lee NA, Lee JJ, et al. Eosinophil-derived IL-10 supports chronic nematode infection. J Immunol. (2014) 193:4178–87. doi: 10.4049/jimmunol.1400852, PMID: 25210122
68. Fabre V, Beiting DP, Bliss SK, Gebreselassie NG, Gagliardo LF, Lee NA, et al. Eosinophil deficiency compromises parasite survival in chronic nematode infection. J Immunol. (2009) 182:1577–83. doi: 10.4049/jimmunol.182.3.1577, PMID: 19155506
69. Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. (2011) 332:243–7. doi: 10.1126/science.1201475, PMID: 21436399
70. Khudhair Z, Alhallaf R, Eichenberger RM, Whan J, Kupz A, Field M, et al. Gastrointestinal helminth infection improves insulin sensitivity, decreases systemic inflammation, and alters the composition of gut microbiota in distinct mouse models of type 2 diabetes. Front Endocrinol (Lausanne). (2020) 11:606530. doi: 10.3389/fendo.2020.606530, PMID: 33613446
71. da Silva EZ, Jamur MC, and Oliver C. Mast cell function: a new vision of an old cell. J Histochem Cytochem. (2014) 62:698–738. doi: 10.1369/0022155414545334, PMID: 25062998
72. McKay DM. The immune response to and immunomodulation by Hymenolepis diminuta. Parasitology. (2010) 137:385–94. doi: 10.1017/S0031182009990886, PMID: 19691904
73. Wiria AE, Hamid F, Wammes LJ, Prasetyani MA, Dekkers OM, May L, et al. Infection with soil-transmitted helminths is associated with increased insulin sensitivity. PloS One. (2015) 10:e0127746. doi: 10.1371/journal.pone.0127746, PMID: 26061042
74. Chen Y, Lu J, Huang Y, Wang T, Xu Y, Xu M, et al. Association of previous schistosome infection with diabetes and metabolic syndrome: a cross-sectional study in rural China. J Clin Endocrinol Metab. (2013) 98:E283–7. doi: 10.1210/jc.2012-2517, PMID: 23275524
75. Hays R, Esterman A, Giacomin P, Loukas A, and McDermott R. Does Strongyloides stercoralis infection protect against type 2 diabetes in humans? Evidence from Australian Aboriginal adults. Diabetes Res Clin Pract. (2015) 107:355–61. doi: 10.1016/j.diabres.2015.01.012, PMID: 25656764
76. Pierce DR, McDonald M, Merone L, Becker L, Thompson F, Lewis C, et al. Effect of experimental hookworm infection on insulin resistance in people at risk of type 2 diabetes. Nat Commun. (2023) 14:4503. doi: 10.1038/s41467-023-40263-4, PMID: 37495576
77. Bager P, Arnved J, Rønborg S, Wohlfahrt J, Poulsen LK, Westergaard T, et al. Trichuris suis ova therapy for allergic rhinitis: a randomized, double-blind, placebo-controlled clinical trial. J Allergy Clin Immunol. (2010) 125:123–30.e1-3. doi: 10.1016/j.jaci.2009.08.006, PMID: 19800680
78. Schölmerich J, Fellermann K, Seibold FW, Rogler G, Langhorst J, Howaldt S, et al. A Randomised, Double-blind, Placebo-controlled Trial of Trichuris suis ova in Active Crohn’s Disease. J Crohns Colitis. (2017) 11:390–9. doi: 10.1093/ecco-jcc/jjw184, PMID: 27707789
79. Tahapary DL, de Ruiter K, Martin I, Brienen EAT, van Lieshout L, Cobbaert CM, et al. Effect of anthelmintic treatment on insulin resistance: A cluster-randomized, placebo-controlled trial in Indonesia. Clin Infect Dis. (2017) 65:764–71. doi: 10.1093/cid/cix416, PMID: 28472383
80. Tahapary DL, de Ruiter K, Martin I, Brienen EAT, van Lieshout L, Djuardi Y, et al. Effect of anthelmintic treatment on leptin, adiponectin and leptin to adiponectin ratio: a randomized-controlled trial. Nutr Diabetes. (2017) 7:e289. doi: 10.1038/nutd.2017.37, PMID: 29035384
81. Shen SW, Lu Y, Li F, Shen ZH, Xu M, Yao WF, et al. Potential long-term effects of previous schistosome infection may reduce the atherogenic index of plasma in Chinese men. Int J Parasitol. (2015) 45:289–94. doi: 10.1016/j.ijpara.2015.01.001, PMID: 25683374
82. Shen SW, Lu Y, Li F, Shen ZH, Xu M, Yao WF, et al. The potential long-term effect of previous schistosome infection reduces the risk of metabolic syndrome among Chinese men. Parasite Immunol. (2015) 37:333–9. doi: 10.1111/pim.12187, PMID: 25809087
83. Shen SW, Lu Y, Li F, Shen ZH, Xu M, Yao WF, et al. Parasitic helminths and their beneficial impact on type 1 and type 2 diabetes. Diabetes/metabolism Res Rev. (2016) 32:238–50. doi: 10.1002/dmrr.2673, PMID: 26119261
84. Zaccone P and Hall SW. Helminth infection and type 1 diabetes. Rev Diabetic studies: RDS. (2012) 9:272. doi: 10.1900/RDS.2012.9.272, PMID: 23804266
85. Tahapary DL, de Ruiter K, Martin I, van Lieshout L, Guigas B, Soewondo P, et al. Helminth infections and type 2 diabetes: a cluster-randomized placebo controlled SUGARSPIN trial in Nangapanda, Flores, Indonesia. BMC Infect Dis. (2015) 15:133. doi: 10.1186/s12879-015-0873-4, PMID: 25888525
86. Hays RJ. Helminth infection and metabolic disease: Strongyloides stercoralis infection and type 2 diabetes mellitus in an Aboriginal community. (Townsville, Queensland, Australia: James Cook University) (2018).
87. Sanya RE, Webb EL, Zziwa C, Kizindo R, Sewankambo M, Tumusiime J, et al. The effect of helminth infections and their treatment on metabolic outcomes: results of a cluster-randomized trial. Clin Infect Diseases: Off Publ Infect Dis Soc America. (2019) 71:601–613. doi: 10.1093/cid/ciz859, PMID: 31504336
88. Croese J, Miller GC, Marquart L, and Llewellyn S. Randomized, placebo controlled trial of experimental hookworm infection for improving gluten tolerance in celiac disease. Clin Transl Gastroenterol. (2020) 11:e00274. doi: 10.14309/ctg.0000000000000274, PMID: 33512796
89. Croese J, Giacomin P, Navarro S, Clouston A, McCann L, Dougall A, et al. Experimental hookworm infection and gluten microchallenge promote tolerance in celiac disease. J Allergy Clin Immunol. (2015) 135:508–16. doi: 10.1016/j.jaci.2014.07.022, PMID: 25248819
90. Tanasescu R, Tench CR, and Constantinescu CS. Hookworm treatment for relapsing multiple sclerosis: A randomized double-blinded placebo-controlled trial. JAMA Neurol. (2020) 77:1089–98. doi: 10.1001/jamaneurol.2020.1118, PMID: 32539079
91. Croese J, Gaze ST, and Loukas A. Changed gluten immunity in celiac disease by Necator americanus provides new insights into autoimmunity. Int J Parasitol. (2013) 43:275–82. doi: 10.1016/j.ijpara.2012.12.005, PMID: 23291460
92. Cantacessi C, Giacomin P, Croese J, Zakrzewski M, Sotillo J, McCann L, et al. Impact of experimental hookworm infection on the human gut microbiota. J Infect Dis. (2014) 210:1431–4. doi: 10.1093/infdis/jiu256, PMID: 24795483
93. Alimpolos JA, Martinez CRA, Morris NMG, and Solano RNP. Potential Association of Strongyloides stercoralis and its effectivity against Type 2 Diabetes Mellitus: A Systematic Review of Scientific Attestation. Int J Multidisciplinary: Appl Business Educ Res. (2023) 4:4496–503. doi: 10.11594/ijmaber.04.12.25
94. Dasan B, Rajamanickam A, Munisankar S, Menon PA, Ahamed SF, Nott S, et al. Hookworm infection induces glycometabolic modulation in South Indian individuals with type 2 diabetes. IJID regions. (2023) 9:18–24. doi: 10.1016/j.ijregi.2023.08.009, PMID: 37745942
95. Brignole M. Establishing the efficacy of midodrine to prevent vasovagal syncope. Ann Intern Med. (2021) 174:1460–1. doi: 10.7326/M21-2859, PMID: 34339220
96. Regassa DA, Kiya GT, Kebede RA, and Beyene W. Assessment of hematological profiles and prognostic role of hemogram-derived novel markers for diabetes mellitus and its complications among type 2 diabetes mellitus adult patients attending bishoftu general hospital, central, Ethiopia: A comparative cross-sectional study. J Blood Med. (2023) 14:681–99. doi: 10.2147/JBM.S435452, PMID: 38164459
97. Rajamanickam A, Munisankar S, Dolla C, Menon PA, Thiruvengadam K, Nutman TB, et al. Helminth infection modulates systemic pro-inflammatory cytokines and chemokines implicated in type 2 diabetes mellitus pathogenesis. PloS Negl Trop Dis. (2020) 14:e0008101. doi: 10.1371/journal.pntd.0008101, PMID: 32126084
98. Mgbere O, Bell TK, Rodriguez-Barradas MC, Essien EJ, and Singh M. System and patient barriers to care among people living with HIV/AIDS in houston/harris county, texas: HIV medical care providers’ Perspectives. J Int Assoc Provid AIDS Care. (2015) 14:505–15. doi: 10.1177/2325957414539045, PMID: 24943655
99. Nolan MS, Murray KO, Mejia R, Hotez PJ, Villar Mondragon MJ, Rodriguez S, et al. Elevated pediatric chagas disease burden complicated by concomitant intestinal parasites and malnutrition in El Salvador. Trop Med Infect Dis. (2021) 6:72. doi: 10.3390/tropicalmed6020072, PMID: 34067079
100. Apaza C, Cuna W, Brañez F, Passera R, and Rodriguez C. Frequency of gastrointestinal parasites, anemia, and nutritional status among children from different geographical regions of Bolivia. J Trop Med. (2023) 2023:5020490. doi: 10.1155/2023/5020490, PMID: 38107388
101. Ali AY, Mohamed Abdi A, and Mambet E. Small bowel obstruction caused by massive ascariasis: two case reports. Ann Med Surg (Lond). (2023) 85:486–9. doi: 10.1097/MS9.0000000000000224, PMID: 36923774
102. Salvador F, Galvis D, Treviño B, Sulleiro E, Sánchez-Montalvá A, Serre-Delcor N, et al. Imported Strongyloides stercoralis infection and diabetes mellitus and other metabolic diseases: Is there any association? Trop Med Int Health. (2023) 28:232–6. doi: 10.1111/tmi.13853, PMID: 36651761
103. Taghipour A, Javanmard E, Mohammad Rahimi H, Abdoli A, Matin S, Haghbin M, et al. Prevalence of intestinal parasitic infections in patients with diabetes: a systematic review and meta-analysis. Int Health. (2024) 16:23–34. doi: 10.1093/inthealth/ihad027, PMID: 37052134
104. Gökçe C, Aycan-Kaya Ö, Yula E, Üstün İ, Yengil E, Sefil F, et al. The effect of blood glucose regulation on the presence of opportunistic Demodex folliculorum mites in patients with type 2 diabetes mellitus. J Int Med Res. (2013) 41:1752–8. doi: 10.1177/0300060513494730, PMID: 23934047
105. Zhang N, Wen K, Liu Y, Huang W, Liang X, Liang L, et al. High prevalence of demodex infestation is associated with poor blood glucose control in type 2 diabetes mellitus: A cross-sectional study in the guangzhou diabetic eye study. Cornea. (2023) 42:670–4. doi: 10.1097/ICO.0000000000003116, PMID: 36729706
106. Abidha CA, Amoako YA, Nyamekye RK, Bedu-Addo G, Grziwotz F, Mockenhaupt FP, et al. Fasting blood glucose in a Ghanaian adult is causally affected by malaria parasite load: a mechanistic case study using convergent cross mapping. Malar J. (2022) 21:93. doi: 10.1186/s12936-022-04076-y, PMID: 35303892
107. Ch’ng JH, Moll K, Wyss K, Hammar U, Rydén M, Kämpe O, et al. Enhanced virulence of Plasmodium falciparum in blood of diabetic patients. PloS One. (2021) 16:e0249666. doi: 10.1371/journal.pone.0249666, PMID: 34138868
108. Abdelhamid GA, Abdelaal AA, Shalaby MA, Fahmy MAA, Badawi MA, Afife AA, et al. Type-1 diabetes mellitus down-regulated local cerebral glial fibrillary acidic protein expression in experimental toxoplasmosis. J Parasit Dis. (2023) 47:319–28. doi: 10.1007/s12639-023-01573-y, PMID: 37193484
109. Li YX, Han ZM, Xiao C, Xie S, Bai HL, Zhang YL, et al. Toxoplasma gondii infection in diabetes mellitus patients in China: seroprevalence, risk factors, and case-control studies. BioMed Res Int. (2018) 2018:4723739. doi: 10.1155/2018/4723739, PMID: 30662909
110. Caraballo L and Llinás-Caballero K. The relationship of parasite allergens to allergic diseases. Curr Allergy Asthma Rep. (2023) 23:363–73. doi: 10.1007/s11882-023-01089-8, PMID: 37269427
111. Mehrdana F, Lavilla M, Kania PW, Pardo MÁ, Audicana MT, Longo N, et al. Evidence of IgE-mediated cross-reactions between Anisakis simplex and Contracaecum osculatum proteins. Pathogens. (2021) 10:950. doi: 10.3390/pathogens10080950, PMID: 34451414
112. Shimokawa C. The gut microbiome–helminth–immune axis in autoimmune diseases. Parasitol Int. (2024) 104:102985. doi: 10.1016/j.parint.2024.102985, PMID: 39491642
113. Camaya I, O’Brien B, and Donnelly S. How do parasitic worms prevent diabetes? An exploration of their influence on macrophage and β-cell crosstalk. Front Endocrinol (Lausanne). (2023) 14:1205219. doi: 10.3389/fendo.2023.1205219, PMID: 37564976
114. El-Kady AM, Alzahrani AM, Elshazly H, Alshehri EA, Wakid MH, Gattan HS, et al. Pancreatic pathological changes in murine toxoplasmosis and possible association with diabetes mellitus. Biomedicines. (2022) 11:18. doi: 10.3390/biomedicines11010018, PMID: 36672526
115. Catchpole A, Zabriskie BN, Bassett P, Embley B, White D, Gale SD, et al. Association between toxoplasma gondii infection and type-1 diabetes mellitus: A systematic review and meta-analysis. Int J Environ Res Public Health. (2023) 20:4436. doi: 10.3390/ijerph20054436, PMID: 36901457
116. Rodríguez-Pérez EG, Arce-Mendoza AY, Saldívar-Palacios R, and Escandón-Vargas K. Fatal Strongyloides stercoralis hyperinfection syndrome in an alcoholic diabetic patient from México. Biomedica. (2020) 40:32–6. doi: 10.7705/biomedica.5071, PMID: 32463606
117. Oliveira FMS, Cruz RE, Pinheiro GRG, and Caliari MV. Comorbidities involving parasitic diseases: A look at the benefits and complications. Exp Biol Med. (2022) 247:1819–26. doi: 10.1177/15353702221108387, PMID: 35876147
118. Molan A, Nosaka K, Hunter M, Zhang J, Meng X, Song M, et al. First age- and gender-matched case-control study in Australia examining the possible association between toxoplasma gondii infection and type 2 diabetes mellitus: the busselton health study. J Parasitol Res. (2020) 2020:3142918. doi: 10.1155/2020/3142918, PMID: 32257421
119. Ashraf T, Sarker PK, Hosen MI, Rahman A, Hasan AKMM, Rahman T, et al. Association of chronic toxoplasma gondii infection with pro-inflamatory cytokine interleukin (IL)-12 responses in type-2 diabetes mellitus patients of Bangladesh. J Parasitol Res. (2023) 2023:3885160. doi: 10.1155/2023/3885160, PMID: 37197738
120. Salem DA, Salem NA, and Hendawy SR. Association between Toxoplasma gondii infection and metabolic syndrome in obese adolescents: A possible immune-metabolic link. Parasitol Int. (2021) 83:102343. doi: 10.1016/j.parint.2021.102343, PMID: 33831579
121. Mohamed GA, Ez Eldeen MES, Elossily NA, Gaber M, Hassan TM, Mahran ZG, et al. Anti-toxoplasma igG level in type 2 diabetic patients: does it affect glycemic control? Egypt J Immunol. (2020) 27:119–27., PMID: 33180394
122. Han Y, Nie L, Ye X, Zhou Z, Huang S, Zeng C, et al. The association between Toxoplasma gondii infection and hypertensive disorders in T2DM patients: a case-control study in the Han Chinese population. Parasitol Res. (2018) 117:689–95. doi: 10.1007/s00436-017-5737-y, PMID: 29349623
123. Alkholy UM, et al. The impact of parasitic infestation on nutritional status and micronutrients among children. J Parasitol Res. (2024) 2024:6996968. doi: 10.1155/2024/6996968, PMID: 38576864
124. Yingklang M, Chaidee A, Dangtakot R, Jantawong C, Haonon O, Sitthirach C, et al. Association of Strongyloides stercoralis infection and type 2 diabetes mellitus in northeastern Thailand: Impact on diabetic complication-related renal biochemical parameters. PloS One. (2022) 17:e0269080. doi: 10.1371/journal.pone.0269080, PMID: 35639713
125. Ying Y, Bytyci J, and Lee L. Immunocompromised individuals are at increased risk of COVID-19 breakthrough infection, hospitalisation and death in the post vaccination era: A systematic review. Authorea Preprints. (2024). doi: 10.22541/au.170663752.23533095/v1, PMID: 38661301
126. de Oliveira Reis AB, Gomes SM, and Carmo ES. Opportunistic infections in individuals living with HIV/AIDS: what is the situation found in a specialized care service located in northeastern Brazil? Rev Prevenção Infecção e Saúde. (2023) 9:e-location only. doi: 10.26694/repis.v9i1.3659
127. Wang Z, Guo J, and Liu J. Strongyloides hyperinfection syndrome and cytomegalovirus infection in a patient with type II diabetes mellitus. New Microbiol. (2023) 46:86–9., PMID: 36853825
128. Ramírez JD, Castañeda S, Weatherhead J, and Poveda C. Parasite-microbiota interactions: a pathway to innovative interventions for Chagas disease, leishmaniasis, and ascariasis. Future Microbiol. (2024) p:1–13. doi: 10.1080/17460913.2024.2431417, PMID: 39574234
129. Suryowati T. Metabolic shifts induced by helminth infections and their contribution to stunting in vulnerable populations. Int J Trop Dis Health. (2024) 45:33–45. doi: 10.9734/ijtdh/2024/v45i101596
130. Mules TC, Tang JS, Vacca F, Yumnam B, Schmidt A, Lavender B, et al. Modulation of intestinal epithelial permeability by chronic small intestinal helminth infections. Immunol Cell Biol. (2024) 102:396–406. doi: 10.1111/imcb.12749, PMID: 38648862
131. Bendezu-Quispe G, Rojas-Zevallos J, and Rosales-Rimache J. Type 2 diabetes mellitus and demodex folliculorum infestation: A cross-sectional study in Peruvian patients. Int J Environ Res Public Health. (2022) 19:13582. doi: 10.3390/ijerph192013582, PMID: 36294163
132. Zeynalyan AA, Kolasani B, Naik C, Sigakis CJG, Silhan L, Mathai SK, et al. Rapidly progressive respiratory failure after helminth larvae ingestion. BMC Pulm Med. (2021) 21:422. doi: 10.1186/s12890-021-01788-w, PMID: 34930198
133. Woodford J, Gillman A, Jenvey P, Roberts J, Woolley S, Barber BE, et al. Positron emission tomography and magnetic resonance imaging in experimental human malaria to identify organ-specific changes in morphology and glucose metabolism: A prospective cohort study. PloS Med. (2021) 18:e1003567. doi: 10.1371/journal.pmed.1003567, PMID: 34038421
134. Trevisan C, Sotiraki S, Laranjo-González M, Dermauw V, Wang Z, Kärssin A, et al. Epidemiology of taeniosis/cysticercosis in Europe, a systematic review: eastern Europe. Parasit Vectors. (2018) 11:569. doi: 10.1186/s13071-018-3153-5, PMID: 30376899
135. Sandborn WJ, Elliott DE, Weinstock J, Summers RW, Landry-Wheeler A, Silver N, et al. Randomised clinical trial: the safety and tolerability of T richuris suis ova in patients with Crohn’s disease. Alimentary Pharmacol Ther. (2013) 38:255–63. doi: 10.1111/apt.12366, PMID: 23730956
136. Yordanova IA, Ebner F, Schulz AR, Steinfelder S, Rosche B, Bolze A, et al. The worm-specific immune response in multiple sclerosis patients receiving controlled Trichuris suis ova immunotherapy. Life. (2021) 11:101. doi: 10.3390/life11020101, PMID: 33572978
137. Benzel F, Erbel C, Stuck BA, Martin K, Kamradt T, Strutz CL, et al. Immune monitoring of Trichuris suis egg therapy in multiple sclerosis patients. J helminthology. (2012) 86:339–47. doi: 10.1017/S0022149X11000460, PMID: 21838960
138. Hollander E, Haddenhorst JA, Della Rosa CR, O'Hara I, Vaeza S, Mandell P, et al. Randomized crossover feasibility trial of helminthic Trichuris suis ova versus placebo for repetitive behaviors in adult autism spectrum disorder. World J Biol Psychiatry. (2020) 21:291–9. doi: 10.1080/15622975.2018.1523561, PMID: 30230399
139. Williams AR, Dige A, Rasmussen TK, Hvas CL, Dahlerup JF, Iversen L, et al. Immune responses and parasitological observations induced during probiotic treatment with medicinal Trichuris suis ova in a healthy volunteer. Immunol Lett. (2017) 188:32–7. doi: 10.1016/j.imlet.2017.06.002, PMID: 28602842
140. Hoshina T, Shimobori T, Ariga T, Hashimoto T, Okamoto T, Sugano S, et al. Safety and tolerability of medicinal parasite ova (Trichuris suis) in healthy Japanese volunteers: A randomized, double-blind, placebo-controlled trial. Parasitol Int. (2021) 85:102441. doi: 10.1016/j.parint.2021.102441, PMID: 34425258
141. Rosche B, Schneider H, Ziemssen T, Paul F, Linker RA, Hartmann S, et al. Trichuris suis ova in relapsing-remitting multiple sclerosis and clinically isolated syndrome (TRIOMS): study protocol for a randomized controlled trial. Trials. (2013) 14:1–6. doi: 10.1186/1745-6215-14-112, PMID: 23782752
142. McSorley HJ, Giacomin PJ, Daveson AJ, Croese J, Loukas A, McCarthy JMD, et al. Suppression of inflammatory immune responses in celiac disease by experimental hookworm infection. PloS One. (2011) 6:e24092. doi: 10.1371/journal.pone.0024092, PMID: 21949691
143. Daveson AJ, Jones TJ, McCarthy JMD, Loukas A, Croese J, Giacomin PJ, et al. Effect of hookworm infection on wheat challenge in celiac disease–a randomised double-blinded placebo controlled trial. PloS One. (2011) 6:e17366. doi: 10.1371/journal.pone.0017366, PMID: 21408161
144. Feary J, Venn S, Brown K, Pritchard DA, Hall IP, Sheikh A, et al. Experimental hookworm infection: a randomized placebo-controlled trial in asthma. Clin Exp Allergy. (2010) 40:299–306. doi: 10.1111/j.1365-2222.2009.03433.x, PMID: 20030661
145. Feary J, Venn S, Brown K, Pritchard DA, Hall IP, Sheikh A, et al. Safety of hookworm infection in individuals with measurable airway responsiveness: a randomized placebo-controlled feasibility study. Clin Exp Allergy. (2009) 39:1060–8. doi: 10.1111/j.1365-2222.2009.03187.x, PMID: 19400893
146. Chapman PR, Möhrle J, Elias D, Guderian J, Coffman SR, McCarthy JS, et al. Vaccination of human participants with attenuated Necator americanus hookworm larvae and human challenge in Australia: a dose-finding study and randomised, placebo-controlled, phase 1 trial. Lancet Infect Dis. (2021) 21:1725–36. doi: 10.1016/S1473-3099(21)00153-5, PMID: 34419209
147. Hoogerwerf MA, van der Werf MAA, van Genderen MWF, van Gorp ECM, Verweij JJ, Roestenberg M, et al. Protective efficacy of short-term infection with Necator americanus hookworm larvae in healthy volunteers in the Netherlands: a single-centre, placebo-controlled, randomised, controlled, phase 1 trial. Lancet Microbe. (2023) 4:e1024–34. doi: 10.1016/S2666-5247(23)00218-5, PMID: 38042152
148. EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA), Turck D, Castenmiller J, De Henauw S, Hirsch-Ernst KI, et al. Safety of viable embryonated eggs of the whipworm Trichuris suis as a novel food pursuant to Regulation (EU) 2015/2283. Efsa J. (2019) 17:e05777. doi: 10.2903/j.efsa.2019.5777, PMID: 32626406
149. Lopes-Junior EH, Hermogenes T, Ramos-Tatagiba J, de Oliveira CT, de Jesus Louzada J, de Lima HFF, et al. Human tumor necrosis factor alpha affects the egg-laying dynamics and glucose metabolism of Schistosoma mansoni adult worms in vitro. Parasit Vectors. (2022) 15:176. doi: 10.1186/s13071-022-05278-8, PMID: 35610661
150. Casacuberta-Partal M, van Lieshout L, van Diepen A, Sijtsma JC, Ozir-Fazalalikhan A, Koopman JPR, et al. Excretion patterns of Schistosoma mansoni antigens CCA and CAA by adult male and female worms, using a mouse model and ex vivo parasite cultures. Parasitology. (2022) 149:306–13. doi: 10.1017/S0031182021001839, PMID: 34736550
151. Zhong H, Wu L, Ren Y, Qin F, and Jin Y. Comparative proteomic profiles of Schistosoma japonicum male worms derived from single-sex and bisexual infections. Int J Parasitol. (2022) 52:815–28. doi: 10.1016/j.ijpara.2022.09.005, PMID: 36265673
152. Maruszewska-Cheruiyot M, Szewczak L, Krawczak-Wójcik K, Głaczyńska M, and Donskow-Łysoniewska K. The production of excretory-secretory molecules from Heligmosomoides polygyrus bakeri fourth stage larvae varies between mixed and single sex cultures. Parasit Vectors. (2021) 14:106. doi: 10.1186/s13071-021-04613-9, PMID: 33557937
153. Daneshvar H, Coombs GH, Hagan P, and Phillips RS. Leishmania mexicana and Leishmania major: attenuation of wild-type parasites and vaccination with the attenuated lines. J Infect Dis. (2003) 187:1662–8. doi: 10.1086/374783, PMID: 12721947
154. Khan SM, Janse CJ, Kappe SHI, and Mikolajczak SA. Genetic engineering of attenuated malaria parasites for vaccination. Curr Opin Biotechnol. (2012) 23:908–16. doi: 10.1016/j.copbio.2012.04.003, PMID: 22560204
155. Vaughan AM and Kappe SHI. Vaccination using radiation- or genetically attenuated live sporozoites. Methods Mol Biol. (2013) 923:549–66. doi: 10.1007/978-1-62703-026-7_38, PMID: 22990804
156. Jacobs BA, Chetty A, Horsnell WGC, Schäfer G, Prince S, Smith KA, et al. Hookworm exposure decreases human papillomavirus uptake and cervical cancer cell migration through systemic regulation of epithelial-mesenchymal transition marker expression. Sci Rep. (2018) 8:11547. doi: 10.1038/s41598-018-30058-9, PMID: 30069018
157. Mules TC, Lavender B, Maclean K, Vacca F, Noble S-L, Yumnam B, et al. Controlled hookworm infection for medication-free maintenance in patients with ulcerative colitis: A pilot, double-blind, randomized control trial. Inflammation Bowel Dis. (2023) 30:735–45. doi: 10.1093/ibd/izad110, PMID: 37318363
158. Wu F, Xu Y, Xia M, Ying G, and Shou Z. Hookworm anemia in a peritoneal dialysis patient in China. Korean J Parasitol. (2016) 54:315–7. doi: 10.3347/kjp.2016.54.3.315, PMID: 27417086
159. Quansah E, Wang J, Chen M, Chen Y, Sun D, Yang S, et al. CRISPR-Cas13 in malaria parasite: Diagnosis and prospective gene function identification. Front Microbiol. (2023) 14:1076947. doi: 10.3389/fmicb.2023.1076947, PMID: 36760507
160. Salkeld J, Themistocleous Y, Barrett JR, Mitton CH, Rawlinson TA, Payne RO, et al. Repeat controlled human malaria infection of healthy UK adults with blood-stage Plasmodium falciparum: Safety and parasite growth dynamics. Front Immunol. (2022) 13:984323. doi: 10.3389/fimmu.2022.984323, PMID: 36072606
161. van der Boor SC, Alkema M, van Gemert G-J, Teelen K, van de Vegte-Bolmer M, Walk J, et al. Whole sporozoite immunization with Plasmodium falciparum strain NF135 in a randomized trial. BMC Med. (2023) 21:137. doi: 10.1186/s12916-023-02788-9, PMID: 37024868
162. Griffin P, Pasay C, Elliott S, Sekuloski S, Sikulu M, Hugo L, et al. Safety and reproducibility of a clinical trial system using induced blood stage plasmodium vivax infection and its potential as a model to evaluate malaria transmission. PloS Negl Trop Dis. (2016) 10:e0005139. doi: 10.1371/journal.pntd.0005139, PMID: 27930652
163. Kublin JG, Mikolajczak SA, Sack BK, Fishbaugher ME, Seilie A, Shelton L, et al. Complete attenuation of genetically engineered Plasmodium falciparum sporozoites in human subjects. Sci Transl Med. (2017) 9:eaad9099. doi: 10.1126/scitranslmed.aad9099, PMID: 28053159
164. Lalawmpuii K and Lalrinkima H. Genetic manipulations in helminth parasites. J Parasit Dis. (2023) 47:203–14. doi: 10.1007/s12639-023-01567-w, PMID: 36712591
165. Pal S and Dam S. CRISPR-Cas9: Taming protozoan parasites with bacterial scissor. J Parasit Dis. (2022) 46:1204–12. doi: 10.1007/s12639-022-01534-x, PMID: 36457766
166. Arunsan P, Ittiprasert W, Smout MJ, Cochran CJ, Mann VH, Chaiyadet S, et al. Programmed knockout mutation of liver fluke granulin attenuates virulence of infection-induced hepatobiliary morbidity. Elife. (2019) 8:e41463. doi: 10.7554/eLife.41463, PMID: 30644359
167. Ma Z, et al. A novel wx2 gene of toxoplasma gondii inhibits the parasitic invasion and proliferation in vitro and attenuates virulence in vivo via immune response modulation. Front Microbiol. (2020) 11:399. doi: 10.3389/fmicb.2020.00399, PMID: 32318029
168. Singer M, Marshall J, Heiss K, Mair GR, Grimm D, Mueller A-K, et al. Zinc finger nuclease-based double-strand breaks attenuate malaria parasites and reveal rare microhomology-mediated end joining. Genome Biol. (2015) 16:1–18. doi: 10.1186/s13059-015-0811-1, PMID: 26573820
169. Lee MC and Fidock DA. CRISPR-mediated genome editing of Plasmodium falciparum malaria parasites. Genome Med. (2014) 6:1–4. doi: 10.1186/s13073-014-0063-9, PMID: 25473431
170. Hu D, Tang X, Ben Mamoun C, Wang C, Wang S, Gu X, et al. Efficient single-gene and gene family editing in the apicomplexan parasite Eimeria tenella using CRISPR-Cas9. Front Bioengineering Biotechnol. (2020) 8:128. doi: 10.3389/fbioe.2020.00128, PMID: 32158750
171. Hagen J, Ghosh S, Sarkies P, and Selkirk ME. Gene editing in the nematode parasite Nippostrongylus brasiliensis using extracellular vesicles to deliver active Cas9/guide RNA complexes. Front Parasitol. (2023) 2:1071738. doi: 10.3389/fpara.2023.1071738, PMID: 39816841
172. Gupta D, Bhattacharjee O, Mandal D, Sen MK, Dey D, Dasgupta A, et al. CRISPR-Cas9 system: A new-fangled dawn in gene editing. Life Sci. (2019) 232:116636. doi: 10.1016/j.lfs.2019.116636, PMID: 31295471
173. Ittiprasert W, Mann VH, Arimoro GA, Skinner JP, Buddenborg JF, Rebello M, et al. Programmed genome editing of the omega-1 ribonuclease of the blood fluke, Schistosoma mansoni. Elife. (2019) 8:e41337. doi: 10.7554/eLife.41337, PMID: 30644357
174. Naidoo P and Mkhize-Kwitshana ZL. Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR associated protein 9-mediated editing of Schistosoma mansoni genes: Identifying genes for immunologically potent drug and vaccine development. Rev Soc Bras Med Trop. (2022) 55:e0131. doi: 10.1590/0037-8682-0131-2022, PMID: 35976333
175. You H, Bai X, Gobert GN, McManus DP, Li L, Liu Z-J, et al. CRISPR/Cas9-mediated genome editing of Schistosoma mansoni acetylcholinesterase. FASEB J. (2021) 35:e21205. doi: 10.1096/fj.202001745RR, PMID: 33337558
176. Sankaranarayanan G, Silva APP, Meier A, Rogers KK, Magnani DM, Gasser G, et al. Large CRISPR-Cas-induced deletions in the oxamniquine resistance locus of the human parasite Schistosoma mansoni. Wellcome Open Res. (2020) 5:178. doi: 10.12688/wellcomeopenres.16031.1, PMID: 32789192
177. Du X, Pan J, Liu R, Wang Y, Elshan AA, Zeng W, et al. Lentiviral transduction-based CRISPR/cas9 editing of schistosoma mansoni acetylcholinesterase. Curr Genomics. (2023) 24:155–70. doi: 10.2174/1389202924666230823094608, PMID: 38178986
178. Ittiprasert W, Mann VH, Arimoro GA, Skinner JP, Buddenborg JF, Rebello M, et al. RNA-guided asCas12a- and spCas9-catalyzed knockout and homology directed repair of the omega-1 locus of the human blood fluke, schistosoma mansoni. Int J Mol Sci. (2022) 23:631. doi: 10.3390/ijms23020631, PMID: 35054816
179. Du X, Pan J, Liu R, Wang Y, Elshan AA, Zeng W, et al. CRISPR/Cas9: A new tool for the study and control of helminth parasites. Bioessays. (2021) 43:e2000185. doi: 10.1002/bies.202000185, PMID: 33145822
180. Volpatti LR, Matranga MA, Cortinas AB, Delcassian D, Daniel KB, Langer R, et al. Glucose-responsive nanoparticles for rapid and extended self-regulated insulin delivery. ACS nano. (2019) 14:488–97. doi: 10.1021/acsnano.9b06395, PMID: 31765558
181. Maurya R, Ramteke S, Guru P, and Jain NK. Oral glucose-responsive nanocarrier system for management of diabetes. J Endocrinol Metab. (2022) 12:146–60. doi: 10.14740/jem747
182. Li J, Sun Y, Chen C, Sheng T, Liu P, Zhang G, et al. A smartphone-assisted microfluidic chemistry analyzer using image-based colorimetric assays for multi-index monitoring of diabetes and hyperlipidemia. Analytica chimica Acta. (2019) 1052:105–12. doi: 10.1016/j.aca.2018.11.025, PMID: 30685028
183. Jiménez B, Maya C, Velásquez G, Barrios JA, Pérez M, Román A, et al. Helminth Egg Automatic Detector (HEAD): Improvements in development for digital identification and quantification of Helminth eggs and its application online. MethodsX. (2020) 7:101158. doi: 10.1016/j.mex.2020.101158, PMID: 33318959
184. Zendejas-Heredia PA, Colella V, Hii SF, and Traub RJ. Comparison of the egg recovery rates and limit of detection for soil-transmitted helminths using the Kato-Katz thick smear, faecal flotation and quantitative real-time PCR in human stool. PloS Negl Trop Dis. (2021) 15:e0009395. doi: 10.1371/journal.pntd.0009395, PMID: 34038411
185. Lobos-Ovalle D, Navarrete C, Navedo JG, Peña-Espinoza M, and Verdugo C. Improving the sensitivity of gastrointestinal helminth detection using the Mini-FLOTAC technique in wild birds. Parasitol Res. (2021) 120:3319–24. doi: 10.1007/s00436-021-07267-9, PMID: 34347167
186. Dunn JC, Papaiakovou M, Han KT, Chooneea D, Bettis AA, Wyine NY, et al. The increased sensitivity of qPCR in comparison to Kato-Katz is required for the accurate assessment of the prevalence of soil-transmitted helminth infection in settings that have received multiple rounds of mass drug administration. Parasit Vectors. (2020) 13:324. doi: 10.1186/s13071-020-04197-w, PMID: 32580759
187. Phuphisut O, Poodeepiyasawat A, Yoonuan T, Watthanakulpanich D, Thawornkuno C, Reamtong O, et al. Ov-RPA-CRISPR/Cas12a assay for the detection of Opisthorchis viverrini infection in field-collected human feces. Parasit Vectors. (2024) 17:80. doi: 10.1186/s13071-024-06134-7, PMID: 38383404
188. Sharmah B, Barman H, Afzal NU, Akter R, Islam SA, Das BC, et al. Surface-functionalized nanoceria: dual action in diabetes management via glucose-responsive insulin delivery and oxidative stress mitigation. ACS Biomaterials Sci Eng. (2024) 10:6397–414. doi: 10.1021/acsbiomaterials.4c01368, PMID: 39324839
189. Ma Y, Yang J, Ma Y, Yang R, Han F, He M, et al. Glucose oxidase-immobilized dually-crosslinked nanogels for rapid-responsive insulin delivery. Advanced Healthcare Materials. (2024) 13:2402556. doi: 10.1002/adhm.202402556, PMID: 39319484
190. Maraues JV, Nunes R, Carvalho AM, Florindo H, Ferreira D, Sarmento B, et al. GLP-1 analogue-loaded glucose-responsive nanoparticles as allies of stem cell therapies for the treatment of type I diabetes. ACS Pharmacol Trans Sci. (2024) 7:1650–63. doi: 10.1021/acsptsci.4c00173, PMID: 38751616
191. Li Z, Li T, Wang X, Ping J, and Peng H. Smartphone-assisted fluorescent microfluidic-chip for sensitive detection of sweat glucose via dual-sensing of O2/H2O2. Talanta. (2025) 281:126883. doi: 10.1016/j.talanta.2024.126883, PMID: 39288585
192. Xing G, Ai J, Wang N, and Pu Q. Recent progress of smartphone-assisted microfluidic sensors for point of care testing. TrAC Trends Analytical Chem. (2022) 157:116792. doi: 10.1016/j.trac.2022.116792
193. Mirshahvalad SA, Farag A, Thiessen J, Wong R, and Veit-Haibach P. Current applications of PET/MR: part I: technical basics and preclinical/clinical applications. Can Assoc Radiologists J. (2024) 75:815–25. doi: 10.1177/08465371241255903, PMID: 38813998
194. He W, Zhu H, Geng J, Hu X, Li Y, Shi H, et al. Recognition of parasitic helminth eggs via a deep learning-based platform. Front Microbiol. (2024) 15:1485001. doi: 10.3389/fmicb.2024.1485001, PMID: 39633811
195. Pedraza A, Ruiz-Santaquiteria J, Deniz O, and Bueno G. (2022). Parasitic egg detection and classification with transformer-based architectures, in: 2022 IEEE International Conference on Image Processing (ICIP), IEEE. (Piscataway, New Jersey, USA: IEEE)
196. Matamoros G, Sanchez A, Cimino R, Krolewiecki A, and Mejia R. A comparison of the diagnostic capability of Kato-Katz and real-time PCR for the assessment of treatment efficacy of ivermectin and albendazole combination against T. trichiura infections. PloS Negl Trop Dis. (2024) 18:e0012677. doi: 10.1371/journal.pntd.0012677, PMID: 39561184
197. Loyo R, Gomes ECS, Pieri OS, Oliveira ECA, Nascimento WRC, Barbosa CS, et al. Kato-Katz slide preservation technique: Extension of viability and the benefits for schistosomiasis control programs. Exp Parasitol. (2024) 265:108802. doi: 10.1016/j.exppara.2024.108802, PMID: 39043325
198. Islam M, Awan FR, and Baig SM. Development of ARMS-PCR assay for genotyping of Pro12Ala SNP of PPARG gene: a cost effective way for case–control studies of type 2 diabetes in developing countries. Mol Biol Rep. (2014) 41:5585–91. doi: 10.1007/s11033-014-3213-7, PMID: 25063576
199. Phuphisut O, Poodeepiyasawat A, Yoonuan T, Watthanakulpanich D, and Thawornkuno C. Ov-RPA–CRISPR/Cas12a assay for the detection of Opisthorchis viverrini infection in field-collected human feces. Parasites Vectors. (2024) 17:80. doi: 10.1186/s13071-024-06134-7, PMID: 38383404
200. Ittiprasert W and Brindley PJ. CRISPR-based functional genomics for schistosomes and related flatworms. Trends Parasitol. (2024) 40:1016–28. doi: 10.1016/j.pt.2024.09.010, PMID: 39426911
201. Liu G, Liu Q, Han Z, Wang P, and Li Y. Comparative proteomics analysis of adult Haemonchus contortus isolates from Ovis ammon. Front Cell Infect Microbiol. (2023) 13:1087210. doi: 10.3389/fcimb.2023.1087210, PMID: 37009511
202. Thanchomnang T, Chaibutr N, Maleewong W, and Janwan P. Automatic detection of Opisthorchis viverrini egg in stool examination using convolutional-based neural networks. PeerJ. (2024) 12:e16773. doi: 10.7717/peerj.16773, PMID: 38313031
203. Jiménez B, Maya C, Velásquez G, Torner F, Arambula F, Barrios JA, et al. Identification and quantification of pathogenic helminth eggs using a digital image system. Exp Parasitol. (2016) 166:164–72. doi: 10.1016/j.exppara.2016.04.016, PMID: 27113138
204. Lee C-C, Huang P-J, Yeh Y-M, Li P-H, Chiu C-H, Cheng W-H, et al. Helminth egg analysis platform (HEAP): An opened platform for microscopic helminth egg identification and quantification based on the integration of deep learning architectures. J Microbiol Immunol Infect. (2022) 55:395–404. doi: 10.1016/j.jmii.2021.07.014, PMID: 34511389
205. Waechtler A, Cezanne B, Maillard D, Sun R, Wang S, Wang J, et al. Praziquantel - 50 years of research. ChemMedChem. (2023) 18:e202300154. doi: 10.1002/cmdc.202300154, PMID: 37009677
206. Azevedo CM, Meira CS, da Silva JW, Moura DMN, de Oliveira SA, da Costa CJ, et al. Therapeutic potential of natural products in the treatment of schistosomiasis. Molecules. (2023) 28:6807. doi: 10.3390/molecules28196807, PMID: 37836650
207. Dengler F, Hammon HM, Liermann W, Görs S, Bachmann L, Helm C, et al. Cryptosporidium parvumcompetes with the intestinal epithelial cells for glucose and impairs systemic glucose supply in neonatal calves. Vet Res. (2023) 54:40. doi: 10.1186/s13567-023-01172-y, PMID: 37138353
208. Rodrigues JGM, Araújo CSA, Barbosa ES, Vieira LB, de Carvalho MNCR, Sevá AP, et al. Alterations in blood glucose concentration in wild rodents, Holochilus sciureus, naturally infected with Schistosoma mansoni. Rev Bras Parasitol Vet. (2022) 31:e021921. doi: 10.1590/s1984-29612022019, PMID: 35352759
209. Krautz-Peterson G, Simoes M, Faghiri Z, Ndegwa D, Oliveira G, Shoemaker CB, et al. Suppressing glucose transporter gene expression in schistosomes impairs parasite feeding and decreases survival in the mammalian host. PloS Pathog. (2010) 6:e1000932. doi: 10.1371/journal.ppat.1000932, PMID: 20532163
210. Zhong C, Skelly PJ, Leaffer D, Cohn RG, Caulfield JP, Shoemaker CB, et al. Immunolocalization of a Schistosoma mansoni facilitated diffusion glucose transporter to the basal, but not the apical, membranes of the surface syncytium. Parasitology. (1995) 110:383–94. doi: 10.1017/S0031182000064726, PMID: 7753579
211. Adekiya TA, Aruleba RT, Klein A, and Fadaka AO. In silico inhibition of SGTP4 as a therapeutic target for the treatment of schistosomiasis. J Biomol Struct Dyn. (2022) 40:3697–705. doi: 10.1080/07391102.2020.1850363, PMID: 33225839
212. McKenzie M, Kirk RS, and Walker AJ. Glucose uptake in the human pathogen schistosoma mansoni is regulated through akt/protein kinase B signaling. J Infect Dis. (2018) 218:152–64. doi: 10.1093/infdis/jix654, PMID: 29309602
213. Divya M, Prabhu SR, Satyamoorthy K, and Saadi AV. Therapeutics through glycobiology: an approach for targeted elimination of malaria. Biol (Bratisl). (2023) 78:1–5. doi: 10.1007/s11756-023-01312-x, PMID: 36643690
214. Berneburg I, Peddibhotla S, Heimsch KC, Haeussler K, Maloney P, Gosalia P, et al. An Optimized Dihydrodibenzothiazepine Lead Compound (SBI-0797750) as a Potent and Selective Inhibitor of Plasmodium falciparum and P. vivax Glucose 6-Phosphate Dehydrogenase 6-Phosphogluconolactonase. Antimicrob Agents Chemother. (2022) 66:e0210921. doi: 10.1128/aac.02109-21, PMID: 35266827
215. Morales-Luna L, González-Valdez A, Hernández-Ochoa B, Arreguin-Espinosa R, Ortega-Cuellar D, Castillo-Rodríguez RA, et al. Glucose-6-phosphate dehydrogenase::6-phosphogluconolactonase from the parasite giardia lamblia. A molecular and biochemical perspective of a fused enzyme. Microorganisms. (2021) 9:1678. doi: 10.3390/microorganisms9081678, PMID: 34442758
216. You H, Gobert GN, Cai P, Mou R, Nawaratna S, Fang G, et al. Suppression of the insulin receptors in adult schistosoma japonicum impacts on parasite growth and development: further evidence of vaccine potential. PloS Negl Trop Dis. (2015) 9:e0003730. doi: 10.1371/journal.pntd.0003730, PMID: 25961574
217. Lund ME, O'Brien BA, Hutchinson AT, Robinson MW, Simpson AM, Dalton JP, et al. Secreted proteins from the helminth fasciola hepatica inhibit the initiation of autoreactive T cell responses and prevent diabetes in the NOD mouse. PloS One. (2014) 9:e86289. doi: 10.1371/journal.pone.0086289, PMID: 24466007
218. Amdare NP, Khatri VK, Yadav RSP, Tarnekar A, Goswami K, Reddy MVR, et al. Therapeutic potential of the immunomodulatory proteins Wuchereria bancrofti L2 and Brugia malayi abundant larval transcript 2 against streptozotocin-induced type 1 diabetes in mice. J Helminthology. (2016) 91:539–48. doi: 10.1017/S0022149X1600064X, PMID: 27667321
219. Ajendra J, Berbudi A, Hoerauf A, and Hübner MP. Combination of worm antigen and proinsulin prevents type 1 diabetes in NOD mice after the onset of insulitis. Clin Immunol. (2016) 164:119–22. doi: 10.1016/j.clim.2016.02.005, PMID: 26898311
220. Khudhair Z, Alhallaf R, Eichenberger RM, Field M, Krause L, Sotillo J, et al. Administration of hookworm excretory/secretory proteins improves glucose tolerance in a mouse model of type 2 diabetes. Biomolecules. (2022) 12:637. doi: 10.3390/biom12050637, PMID: 35625566
221. Patel R, Bryant AS, Castelletto ML, Walsh B, Akimori D, Hallem EA, et al. The generation of stable transgenic lines in the human-infective nematode Strongyloides stercoralis. G3 (Bethesda). (2024) 14:jkae122. doi: 10.1093/g3journal/jkae122, PMID: 38839055
222. Lazzarotto CR, Malinin NL, Li Y, Zhang R, Yang Y, Lee G, et al. CHANGE-seq reveals genetic and epigenetic effects on CRISPR-Cas9 genome-wide activity. Nat Biotechnol. (2020) 38:1317–27. doi: 10.1038/s41587-020-0555-7, PMID: 32541958
223. Vaillant MT, Philippy F, Neven A, Barré J, Bulaev D, Olliaro PL, et al. Diagnostic tests for human Schistosoma mansoni and Schistosoma haematobium infection: a systematic review and meta-analysis. Lancet Microbe. (2024) 5:e366–78. doi: 10.1016/S2666-5247(23)00377-4, PMID: 38467130
224. 7. Diabetes technology: standards of care in diabetes-2024. Diabetes Care. (2024) 47:S126–s144. doi: 10.2337/dc24-S007, PMID: 38078575
225. Committee, A.D.A.P.P. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care. (2022) 45:S17–38. doi: 10.2337/dc22-S012, PMID: 34964875
226. Hossain P, Kawar B, and El Nahas M. Obesity and diabetes in the developing world—a growing challenge. New Engl J Med. (2007) 356:213–5. doi: 10.1056/NEJMp068177, PMID: 17229948
227. Stratton IM, Adler AI, Neil HAW, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. bmj. (2000) 321:405–12. doi: 10.1136/bmj.321.7258.405, PMID: 10938048
228. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC, et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. diabetologia. (1985) 28:412–9. doi: 10.1007/BF00280883, PMID: 3899825
229. Pradhan AD, Manson JE, and Rifai N. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. Jama. (2001) 286:327–34. doi: 10.1001/jama.286.3.327, PMID: 11466099
230. Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. (2015) 17:690–703. doi: 10.1016/j.chom.2015.04.004, PMID: 25974306
231. Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. (2004) 4:787–99. doi: 10.1038/nri1460, PMID: 15459670
232. Wammes LJ, Mpairwe H, Elliott AM, and Yazdanbakhsh M. Helminth therapy or elimination: epidemiological, immunological, and clinical considerations. Lancet Infect Dis. (2014) 14:1150–62. doi: 10.1016/S1473-3099(14)70771-6, PMID: 24981042
233. Hewitson JP, Grainger JR, and Maizels RM. Helminth immunoregulation: the role of parasite secreted proteins in modulating host immunity. Mol Biochem Parasitol. (2009) 167:1–11. doi: 10.1016/j.molbiopara.2009.04.008, PMID: 19406170
234. Summers RW, Elliott DE, Urban Jr JF, Thompson R, and Weinstock JV. Trichuris suis therapy in Crohn’s disease. Gut. (2005) 54:87–90. doi: 10.1136/gut.2004.041749, PMID: 15591509
235. Kisand K and Peterson P. Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy: known and novel aspects of the syndrome. Ann New York Acad Sci. (2011) 1246:77–91. doi: 10.1111/j.1749-6632.2011.06308.x, PMID: 22236432
236. Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J, et al. Helminth infections: the great neglected tropical diseases. J Clin Invest. (2008) 118:1311–21. doi: 10.1172/JCI34261, PMID: 18382743
237. Committee, A.D.A.P.P. 5. Facilitating behavior change and well-being to improve health outcomes: Standards of Medical Care in Diabetes—2022. Diabetes Care. (2022) 45:S60–82. doi: 10.2337/dc22-S005, PMID: 34964866
238. WHO. Helminth control in school-age children: a guide for managers of control programmes (2011). Available online at: https://www.who.int/publications/i/item/9789241548267 (Accessed August 2024).
239. Vejzagić N, et al. Dose-dependent establishment of Trichuris suis larvae in Göttingen minipigs. Veterinary Parasitol. (2015) 208:211–7. doi: 10.1016/j.vetpar.2015.01.018, PMID: 25700937
240. Adler AI, Stratton IM, Neil HAW, Yudkin JS, Matthews DR, Cull CA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. Bmj. (2000) 321:412–9. doi: 10.1136/bmj.321.7258.412, PMID: 10938049
241. Summers RW, Elliott DE, Urban JF, Thompson RA, and Weinstock JV. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. (2005) 128:825–32. doi: 10.1053/j.gastro.2005.01.005, PMID: 15825065
242. Lustigman S, Prichard RK, Gazzinelli A, Grant WN, Boatin BA, McCarthy JS, et al. A research agenda for helminth diseases of humans: the problem of helminthiases. PloS Negl Trop Dis. (2012) 6:e1582. doi: 10.1371/journal.pntd.0001582, PMID: 22545164
243. Abraham SN and St. John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. (2010) 10:440–52. doi: 10.1038/nri2782, PMID: 20498670
244. Jourdan PM, Lamberton PHL, Fenwick A, and Addiss DG. Soil-transmitted helminth infections. Lancet. (2018) 391:252–65. doi: 10.1016/S0140-6736(17)31930-X, PMID: 28882382
245. Chapman PR, Llewellyn S, Jennings H, Becker L, Giacomin P, McDougall R, et al. The production of Necator americanus larvae for use in experimental human infection. Parasites Vectors. (2022) 15:242. doi: 10.1186/s13071-022-05371-y, PMID: 35804460
246. Hamad H, Kyasaram RK, and Mangla A. Toxicity of weight-based vs. flat dosing for immune checkpoint inhibitors in patients with low weight: Retrospective analysis from a tertiary care center. Am Soc Clin Oncol. (2024) 42:e14616. doi: 10.1200/JCO.2024.42.16_suppl.e14616
247. Ziv-Baran T, Wasserman A, Goldiner I, Stark M, Shenhar-Tsarfaty S, Shapira I, et al. The association between C-reactive protein and common blood tests in apparently healthy individuals undergoing a routine health examination. Clinica Chimica Acta. (2020) 501:33–41. doi: 10.1016/j.cca.2019.12.002, PMID: 31816288
248. Hoogerwerf MA, Koopman JPR, Janse JJ, Langenberg MCC, van Schuijlenburg R, Kruize YCM, et al. A randomized controlled trial to investigate safety and variability of egg excretion after repeated controlled human hookworm infection. J Infect Dis. (2021) 223:905–13. doi: 10.1093/infdis/jiaa414, PMID: 32645714
249. Logan J, Pearson MS, Manda SS, Choi Y-J, Field M, Eichenberger RM, et al. Comprehensive analysis of the secreted proteome of adult Necator americanus hookworms. PloS Negl Trop Dis. (2020) 14:e0008237. doi: 10.1371/journal.pntd.0008237, PMID: 32453752
250. Mostafavi H and Povzner S. Systems and methods for determining a state of a patient. (2020), Google Patents. [Alexandria, VA: U.S. Patent and Trademark Office (USPTO)].
251. Weinstock RS, Aleppo G, Bailey TS, Bergenstal RM, Fisher WA, Greenwood DA, et al. The role of blood glucose monitoring in diabetes management. ADA Clinical Compendia (American Diabetes Association) (2020). doi: 10.2337/db2020-31, PMID: 33411424
252. Delgado JA and Bauça JM. Monitoring of diabetic patients with poor glycemic control. Are international recommendations met? EJIFCC. (2021) 32:78. doi: 10.2337/db2020-31, PMID: 33753977
253. Mukonda E, Lesosky M, Sithole S, van der Westhuizen DJ, Rusch JA, Levitt NS, et al. Comparing the effectiveness and cost-effectiveness of alternative type 2 diabetes monitoring intervals in resource limited settings. Health Policy Plann. (2024) 39:946–55. doi: 10.1093/heapol/czae072, PMID: 39096519
254. Radovnická L, Hásková A, Do QD, Horová E, Navrátilová V, Mikeš O, et al. Lower HbA1c with real-time Continuous Glucose Monitoring (rtCGM) than with intermittently scanned Continuous Glucose Monitoring (isCGM) after 1 year: the CORRIDA LIFE study. Diabetes Technol Ther. (2022) 24:859–67. doi: 10.1089/dia.2022.0152, PMID: 36037056
256. . <Early-Clinical-Trials-With-Live-Biotherapeutic-Products–Chemistry–Manufacturing–and-Control-Information–Guidance-for-Industry.pdf>.
257. Cordaillat-Simmons M, Rouanet A, and Pot B. Live biotherapeutic products: the importance of a defined regulatory framework. Exp Mol Med. (2020) 52:1397–406. doi: 10.1038/s12276-020-0437-6, PMID: 32908212
258. Smallwood TB, Giacomin PR, Loukas A, Mulvenna JP, Clark RJ, Miles JJ, et al. Helminth immunomodulation in autoimmune disease. Front Immunol. (2017) 8:453. doi: 10.3389/fimmu.2017.00453, PMID: 28484453
259. Maizels RM, Smits HH, and McSorley HJ. Modulation of host immunity by helminths: the expanding repertoire of parasite effector molecules. Immunity. (2018) 49:801–18. doi: 10.1016/j.immuni.2018.10.016, PMID: 30462997
260. McSorley HJ, Hewitson JP, and Maizels RM. Immunomodulation by helminth parasites: defining mechanisms and mediators. Int J Parasitol. (2013) 43:301–10. doi: 10.1016/j.ijpara.2012.11.011, PMID: 23291463
261. Saikia G. Helminthic infection and anthelmintic drugs: synthetic and herbal approaches. Pharmacotherapy. 66:252–6.
262. Rehman A and Abidi S. Health and helminths: revisiting the paradigm of host-parasite relationship. In: Biodiversity. (Boca Raton, Florida, USA: CRC Press) (2022). 381–97.
263. Zibaei M, Bahadory S, Saadati H, Pourrostami K, Firoozeh F, Foroutan M, et al. Intestinal parasites and diabetes: A systematic review and meta-analysis. New Microbes New Infections. (2023) 51:101065. doi: 10.1016/j.nmni.2022.101065, PMID: 36654940
264. Pham K, Pham K, Mertelsmann A, Mages K, Kingery JR, Mazigo HD, et al. Effects of helminths and anthelmintic treatment on cardiometabolic diseases and risk factors: A systematic review. PloS Negl Trop Dis. (2023) 17:e0011022. doi: 10.1371/journal.pntd.0011022, PMID: 36827239
265. Aderinto N, Abdulbasit MO, Tangmi ADJ, Okesanya JO, and Mubarak JM. Unveiling the growing significance of metabolism in modulating immune cell function: exploring mechanisms and implications; a review. Ann Med Surg. (2023) 85:5511–22. doi: 10.1097/MS9.0000000000001308, PMID: 37915697
266. Zhao H, Xing C, Zhang J, and He B. Comparative efficacy of oral insulin sensitizers metformin, thiazolidinediones, inositol, and berberine in improving endocrine and metabolic profiles in women with PCOS: a network meta-analysis. Reprod Health. (2021) 18:171. doi: 10.1186/s12978-021-01207-7, PMID: 34407851
267. Uddin MM, Islam A, Saha R, and Goswami D. The role of machine learning in transforming healthcare: A systematic review. J Business Intell Manage Inf Syst Res. (2024) 1:01–16. doi: 10.70008/jbimisr.v1i01.45
268. Shnaydman V. Efficient risk mitigation planning for a clinical trial. Ther Innovation Regul Sci. (2023) 57:717–27. doi: 10.1007/s43441-023-00521-5, PMID: 37067681
269. Finkelman FD. Worming their way into the pharmacy: use of worms and worm products to treat inflammatory diseases. Arthritis Rheum. (2012) 64:3068–71. doi: 10.1002/art.34635, PMID: 22886766
Keywords: helminthic therapy, diabetes mellitus, adverse effects, helminths-derived molecules, risk assessment, clinical monitor
Citation: Zhu Y, Fei X, Wang R, Wang J, Li X, Zhang Y, Xu J, Zhao Q, Chen K, Zhang X and Li H (2025) Clinical challenges and technological breakthroughs in helminthic therapy for diabetes. Front. Immunol. 16:1642707. doi: 10.3389/fimmu.2025.1642707
Received: 07 June 2025; Accepted: 16 October 2025;
Published: 05 November 2025.
Edited by:
Liang Wu, Jiangsu University, ChinaReviewed by:
Zhipeng Xu, Nanjing Medical University, ChinaTrini Suryowati, Universitas Kristen Indonesia, Indonesia
Copyright © 2025 Zhu, Fei, Wang, Wang, Li, Zhang, Xu, Zhao, Chen, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyu Li, aG9uZ3l1ODg5MjZAempzcnUuZWR1LmNu
†These authors have contributed equally to this work
 Yunhuan Zhu1†
Yunhuan Zhu1† Yijie Zhang
Yijie Zhang Keda Chen
Keda Chen Hongyu Li
Hongyu Li