- 1Department of Radiotherapy, Shanxi Province Cancer Hospital/Shanxi Hospital Affiliated to Cancer Hospital, Chinese Academy of Medical Sciences/Cancer Hospital Affiliated to Shanxi Medical University, Taiyuan, Shanxi, China
- 2Department of Ultrasound, Shanxi Province Cancer Hospital/Shanxi Hospital Affiliated to Cancer Hospital, Chinese Academy of Medical Sciences/Cancer Hospital Affiliated to Shanxi Medical University, Taiyuan, Shanxi, China
- 3Department of Geriatric Medicine, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Third Hospital of Shanxi Medical University, Tongji Shanxi Hospital, Taiyuan, China
- 4Department of Radiotherapy, Shanxi Provincial People’s Hospital, Taiyuan, Shanxi, China
- 5Department of General Medicine, Xinzhou People’s Hospital, Xinzhou, China
- 6Department of Gastrointestinal Oncology, Shanxi Province Cancer Hospital/Shanxi Hospital Affiliated to Cancer Hospital, Chinese Academy of Medical Sciences/Cancer Hospital Affiliated to Shanxi Medical University, Taiyuan, Shanxi, China
Objectives: To examine whether habitual whole-grain intake is associated with lower patient-reported systemic inflammatory distress among ambulatory survivors of esophageal squamous-cell carcinoma (ESCC).
Methods: We conducted a cross-sectional questionnaire study (May 2023–July 2025) at four tertiary hospitals in Shanxi Province among adults with stage I–IIIA ESCC (n = 392). A validated semi-quantitative food frequency questionnaire quantified five whole-grain categories. Exposures were modeled as grams/day-¹ (sex-specific quartiles; continuous per 10 g), an energy-adjusted density metric (g/1,000 kcal), and a diversity score (0–5 categories consumed ≥ once/week). Systemic inflammatory symptoms were measured with the seven-item Inflammation Distress Index (IDI). Multivariable logistic models estimated adjusted odds ratios (aORs) for elevated IDI (≥ 10); γ-log generalized linear models analyzed continuous IDI; restricted cubic splines assessed dose–response. Models adjusted for sociodemographic, clinical, and behavioral covariates, with total energy included when grams were exposed.
Results: Median whole-grain intake was 35.4 g/day-¹ (IQR 22.1–58.7); 28.1% had elevated IDI. Prevalence declined across quartiles (39.8%, 34.7%, 25.5%, 12.2%). Fully adjusted aORs (vs. Q1) were 0.95 (0.62–1.47), 0.49 (0.31–0.76), and 0.19 (0.11–0.33) for Q2–Q4 (p-trend < 0.001). Each 10 g/day-¹ increment corresponded to a 6% lower mean IDI (mean ratio 0.94; 0.92–0.96). Splines showed a steep inverse slope up to ~60 g/day-¹ with a plateau (p-nonlinearity = 0.031). Findings were consistent by stage (interaction p = 0.59) and smoking status (p = 0.67), robust in sensitivity analyses, and supported by density (Q4 vs. Q1 aOR 0.21; per +5 g/1,000 kcal-¹ aOR 0.93) and diversity (per +1 category aOR 0.86; ≥ 3 vs. 0–1 aOR 0.48) metrics.
Conclusion: In Shanxi ESCC survivorship care, higher whole-grain intake—particularly ~50 g/day-¹ and with greater variety—aligns with substantially lower systemic inflammatory distress, supporting grain-centered dietary counseling.
1 Introduction
Whole-grain consumption in mid-to-late adulthood is a layered, diet-derived exposure that combines ingestion of cereal kernels retaining bran, germ, and endosperm with the slow-release fermentation of complex polysaccharides and downstream biochemical effects accompanying sustained fiber intake (1–5). In northern China—and particularly in Shanxi—wheat-based staples dominate daily fare. Within this context, our focus on brown rice, whole-wheat noodles, millet, cornmeal products, and multigrain breads captures the main whole-grain foods realistically accessible to esophageal squamous-cell carcinoma (ESCC) survivors. Framed against widening dietary disparities, a habitual pattern of whole-grain eating may function as a nutrition-centered “protective–vulnerability” axis that buffers glycemic excursions, endocrine tone, and host–microbiota crosstalk (6–9).
Mechanistic and epidemiologic evidence converge on an anti-inflammatory potential inherent in the whole-grain matrix. β-Glucans and arabinoxylans augment short-chain fatty acid (SCFA) generation; magnesium and trace antioxidants support redox homeostasis; and phenolic acids dampen toll-like receptor/MyD88 and NF-κB signaling, thereby curbing downstream cytokine release (10–14). SCFAs—chiefly butyrate and propionate—bind G-protein–coupled receptors on peripheral immune cells and inhibit histone deacetylase activity, offering a biologically coherent path from dietary structure to immune tone (15–18). While these pathways are well described in cardiometabolic cohorts, their symptom-level implications for ESCC survivorship remain insufficiently characterized.
Patient-reported inflammatory distress provides a pragmatic lens for real-world oncology. The Inflammation Distress Index (IDI) distills seven common symptoms—night sweats, low-grade feverishness, unexplained fatigue, myalgia/arthralgia, mucosal soreness, ocular dryness, and anorexia—into a reliable, internally consistent scale validated in Chinese oncology populations (2, 19–22). Unlike solitary biomarkers that fluctuate with acute illness or medications, the IDI reflects the lived experience of systemic inflammation and aligns with quality of life and treatment adherence.
Yet the diet–symptom nexus rarely operates in isolation. Age, sex, tumor stage, smoking status, comorbidity burden, and regional foodways can shape both inflammatory tone and the opportunity to consume whole grains (23–26). Smoking-related oxidative stress may magnify diet-derived antioxidant gains, whereas more advanced disease could blunt perceived benefits through tumor-driven cytokine release (27–31). Parsing such effect modification is essential for tailoring counseling to heterogeneous survivorship profiles.
Accordingly, we conducted a single-province questionnaire study at four hospitals in Shanxi (May 2023– July 2025) among ambulatory adults with stage I–IIIA ESCC. Habitual whole-grain intake over three months was assessed with a validated semi-quantitative food frequency questionnaire (FFQ) that queried five whole-grain categories. Exposures were operationalized as grams/day, an energy-adjusted density metric (g/1,000 kcal), and a qualitative diversity score (number of categories consumed ≥ once/week; range, 0–5). Systemic inflammatory symptom burden was measured with the IDI. Multivariable logistic and gamma-regression models, restricted cubic splines, and prespecified subgroup analyses by tumor stage and smoking status were used to evaluate dose–response relations while adjusting for sociodemographic, clinical, and behavioral covariates. Restricting recruitment to Shanxi was intended to maximize internal validity by minimizing cross-province ecological heterogeneity and reducing region-specific portion-size misclassification inherent to FFQ-based assessment. We hypothesized that higher whole-grain quantity—and greater variety—would be associated with a lower likelihood and intensity of inflammatory distress, following a nonlinear inverse trajectory that plateaus once fermentative capacity is approached.
2 Methods
2.1 Participants and setting
Between 15 May 2023 and 29 July 2025, consecutive adults (≥ 18 y) attending routine follow-up or peri-treatment counseling for histologically confirmed esophageal squamous-cell carcinoma (ESCC) at four tertiary hospitals in Shanxi Province were screened through daily registry checks. We restricted enrollment to stage I–IIIA outpatients because later-stage (IIIB–IV) or hospitalized survivors frequently require enteral feeding, experience cachexia, and receive high-dose corticosteroids—factors that profoundly alter grain exposure and symptom perception—so their diet–symptom profile could differ qualitatively from that of ambulatory early-stage patients. TNM stages I, II, or IIIA (AJCC 8th edition) were eligible. Exclusion criteria were stage IIIB/IV disease, concurrent enrollment in interventional trials, conditions that precluded informed consent, enteral or parenteral nutrition dependence, or physician-documented dietary restrictions unrelated to cancer. Of those screened, eligible outpatients who completed both the dietary and symptom instruments comprised the analytic sample (n = 392). This study was approved by Xinzhou People’s Hospital Biomedical Research Ethics Committee (2025-LLSC-06-19). All participants gave written informed consent in Mandarin; data were anonymized before analysis.
2.2 Assessment of habitual whole-grain consumption
Dietary intake during the previous three months was assessed with a semi-quantitative, 26-item food-frequency questionnaire (FFQ) adapted from the China Health and Nutrition Survey. In a reproducibility study of 120 ESCC patients, the instrument showed 2-week test–retest intraclass correlation coefficients of 0.82 for whole-grain intake and 0.78–0.89 across other food groups, while relative-validity correlations against three nonconsecutive 24-h recalls ranged from 0.54 to 0.71. In a biomarker subsample (n = 60), plasma DHPPA—a validated alkylresorcinol metabolite of wheat/rye—correlated with FFQ-estimated whole-grain consumption (Spearman r = 0.47, p < 0.001), supporting construct validity in an ESCC context. Five whole-grain categories—brown rice, whole-wheat noodles, millet, cornmeal products, and multigrain breads—were queried. Frequency options ranged from “never” to “≥ 3 times/day”; three portion photographs plus common household measures aided quantification. Daily grams were calculated by multiplying reported frequency by the median portion weight and summing across categories. To enhance comparability across participants, total energy intake (kcal/day) was estimated from the FFQ using the Chinese Food Composition Tables, and an energy-adjusted density metric for whole grains (g/1,000 kcal) was derived and reported in descriptive tables as well as evaluated as an alternative exposure in regression models. Because the FFQ distinguished five whole-grain categories, we also constructed a diversity score reflecting variety of intake (count of categories consumed ≥ once/week; range, 0–5). Sex-specific quartiles of whole-grain intake were generated separately for men and women; intake was additionally modeled continuously (per 10 g/day) and in sensitivity analyses as sex-specific quintiles. Focusing on a single province (Shanxi)—where wheat-based whole-grain foods predominate—reduced potential province-level measurement error stemming from region-specific portion norms.
2.3 Measurement of systemic inflammatory symptom burden
The seven-item Inflammation Distress Index (IDI) was administered concurrently. Items assessed low-grade feverishness, night sweats, unexplained fatigue, myalgia/arthralgia, mucosal soreness, ocular dryness, and anorexia on a 0–4 Likert frequency scale anchored at “never” and “almost every day.” Scores ranged from 0 to 28 (higher = worse); internal consistency in prior work was excellent (Cronbach’s α = 0.87). Guided by prior psychometric work in Chinese oncology populations, the upper tertile (IDI ≥ 10) denoted “elevated inflammatory symptomatology” for logistic models, while the continuous score served as the dependent variable in gamma-regression analyses.
2.4 Covariates
Sociodemographic factors captured were age (years), sex, educational attainment (≤ primary, middle school, ≥ high school), and monthly household income (center-specific quintiles). Clinical variables included time since diagnosis (< 6, 6–24, > 24 months), current treatment modality (surgery only, chemoradiotherapy, multimodal), BMI (kg/m², calibrated scale), Charlson comorbidity index (0, 1, ≥ 2), and use of systemic corticosteroids in the preceding four weeks. Behavioral covariates comprised smoking status (never, former, current), alcohol consumption (none, ≤ 2 times/week, > 2 times/week), average daily fruit/vegetable servings, and weekly minutes of moderate-to-vigorous physical activity estimated with abbreviated China Kadoorie Biobank instruments. Total energy intake (kcal/day) was included to support energy-adjustment strategies when modeling absolute whole-grain grams; refined-grain intake (white-rice equivalents) was available for confounding checks. Missingness for covariates was modest; assuming data were missing at random conditional on observed sociodemographic and clinical variables, we performed multiple imputations by chained equations (m = 20) after verifying monotone missing-data patterns.
2.5 Statistical analysis
Descriptive statistics are reported as mean ± SD, median (interquartile range), or frequency (percentage). Interquartile comparisons employed one-way ANOVA, Kruskal–Wallis, or χ² tests, as appropriate. We retained the prespecified modeling plan from the protocol, acknowledging that precision would be reduced in the single-province analytic sample (n = 392). Multivariable logistic regression estimated odds ratios (ORs) and 95% confidence intervals (CIs) for elevated IDI across whole-grain–intake quartiles, with Q1 as the referent. Models were sequentially adjusted: (1) age and sex; (2) plus education and household income; (3) plus clinical factors, BMI, and Charlson index; (4) plus smoking, alcohol, fruit/vegetable intake, and weekly physical activity. When the exposure was absolute whole-grain grams, total energy intake (kcal/day) was additionally included to address confounding by energy intake. When the exposure was whole-grain density (g/1,000 kcal), energy was not entered to avoid overadjustment. The whole-grain diversity score (0–5) was analyzed descriptively and, in exploratory models, as an alternative qualitative exposure. Linearity of the logit for continuous covariates was inspected with fractional polynomials, and variance inflation factors were monitored to exclude problematic multicollinearity. For continuous IDI scores, generalized linear models with a gamma distribution and log link accounted for right skewness. Dose–response relations were explored with restricted cubic splines (knots at the 5th, 35th, 65th and 95th percentiles of intake); departure from linearity was evaluated with a Wald χ² test of the joint spline coefficients. Prespecified effect modification by tumor stage and smoking status was examined through multiplicative interaction terms. Sensitivity analyses entailed complete-case restriction, multiply imputed datasets, exclusion of participants with BMI > 30 kg/m², alternative IDI thresholds (≥ 9, ≥ 11), intake modeled in quintiles and replacing absolute grams with the energy-density metric. To account for clustering by hospital, mixed-effects logistic models with center-level random intercepts were also fitted as a robustness check. Analyses were conducted in R version 4.3. A two-sided p < 0.05 signified statistical significance.
3 Results
3.1 Participant characteristics
Among 392 adults with stage I–IIIA ESCC enrolled across four tertiary hospitals in Shanxi Province, the mean age was 59.1 ± 8.6 years, and 116 (29.6%) were women. Center contributions were balanced (204 vs. 188 participants), and baseline characteristics were similar by hospital (all p > 0.20). Median habitual whole-grain intake was 35.4 g/day-¹ (IQR 22.1–58.7), corresponding to a median energy-adjusted density of 19.8 g/1,000 kcal-¹ (12.2–31.0). The median IDI score was 8.2 (6.2–10.0); 110 participants (28.1%) met the a priori threshold for elevated inflammatory symptomatology (IDI ≥ 10). Stage distribution was 27.0% stage I (n = 106), 49.0% stage II (n = 192), and 24.0% stage IIIA (n = 94). Smoking status was 50.5% never (n = 198), 19.6% former (n = 77), and 29.8% current (n = 117). Participants with elevated IDI were marginally older (60.3 ± 8.9 vs. 58.6 ± 8.4 years; p = 0.041), reported lower whole-grain intake (median 25.1 vs. 40.2 g/day; p < 0.001) and lower energy-adjusted density (14.1 vs. 22.5 g/1,000 kcal; p < 0.001), and were more often stage IIIA (29.1% vs. 22.0%; p = 0.044). Current smoking was somewhat more prevalent among those with elevated IDI (35.5% vs. 27.7%; p = 0.096). Fruit/vegetable intake (median 3.7 vs. 4.4 servings/day; p = 0.008) and weekly MVPA (93 vs. 105 min/week; p = 0.038) were lower with elevated IDI, whereas BMI and Charlson comorbidity counts differed little (both p > 0.10) (Table 1).
3.2 Whole-grain intake and baseline profiles
Sex-specific quartiles of whole-grain intake (Q1 < 22.0; Q2 22.0–35.3; Q3 35.4–58.6; Q4 ≥ 58.7 g/day; n = 98 each) showed expected gradients in related behaviors (Table 2). Compared with Q1, participants in Q4 reported higher fruit/vegetable intake (median 5.0 vs. 3.2 servings/day) and more MVPA (125 vs. 80 min/week), alongside a lower prevalence of current smoking (22.4% vs. 36.7%). BMI and comorbidity profiles were broadly comparable across quartiles (all p > 0.10). The energy-adjusted whole-grain density metric rose monotonically—median (IQR) 9.7 (7.4–12.0), 15.5 (13.0–18.0), 24.2 (20.1–28.6), and 34.8 (30.2–41.5) g/1,000 kcal-¹ across Q1–Q4—while total energy intake varied minimally (median ~1,940 vs. 1,880 kcal/day-¹ in Q1 vs. Q4; p = 0.042). Diversity of whole-grain categories (0–5) also increased by quartile (median 1, 2, 3, and 4, respectively; p < 0.001).

Table 2. Baseline characteristics across sex-specific quartiles of whole-grain intake (n = 98 per quartile).
3.3 Prevalence of elevated inflammatory symptomatology
The prevalence of IDI ≥ 10 declined across increasing whole-grain quartiles: 39.8% (39/98) in Q1, 34.7% (34/98) in Q2, 25.5% (25/98) in Q3, and 12.2% (12/98) in Q4 (linear-trend χ² = 20.5; p < 0.001). Median IDI scores mirrored this pattern (9.7, 9.1, 7.7, and 6.4 across Q1–Q4) (Table 3).

Table 3. Distribution of elevated inflammatory symptomatology (IDI ≥ 10) across sex-specific quartiles of whole-grain intake (N = 392; n = 98 per quartile).
3.4 Multivariable logistic regression
In multivariable models using absolute whole-grain grams as the exposure (with total energy intake included per protocol), higher intake was associated with lower odds of elevated IDI (Table 4; Figure 1). Using Q1 as referent, age- and sex-adjusted aORs were 0.92 (95% CI 0.61–1.37) for Q2, 0.52 (0.34–0.78) for Q3, and 0.22 (0.13–0.37) for Q4 (p-trend < 0.001). Sequential adjustment produced minimal attenuation: Model 2 (education, income) aORs 0.93, 0.53, and 0.22; Model 3 (plus clinical factors, BMI, Charlson) 0.94, 0.50, and 0.20; and fully adjusted Model 4 (plus smoking, alcohol, fruit/vegetables, MVPA) 0.95 (0.62–1.47), 0.49 (0.31–0.76), and 0.19 (0.11–0.33), respectively (p-trend < 0.001). Goodness-of-fit was acceptable (Hosmer–Lemeshow p = 0.61), and multicollinearity was limited (all VIF < 2.0).

Table 4. Multivariable logistic regression for elevated inflammatory symptomatology (IDI ≥ 10) according to quartiles of whole-grain intake (g·day-¹).
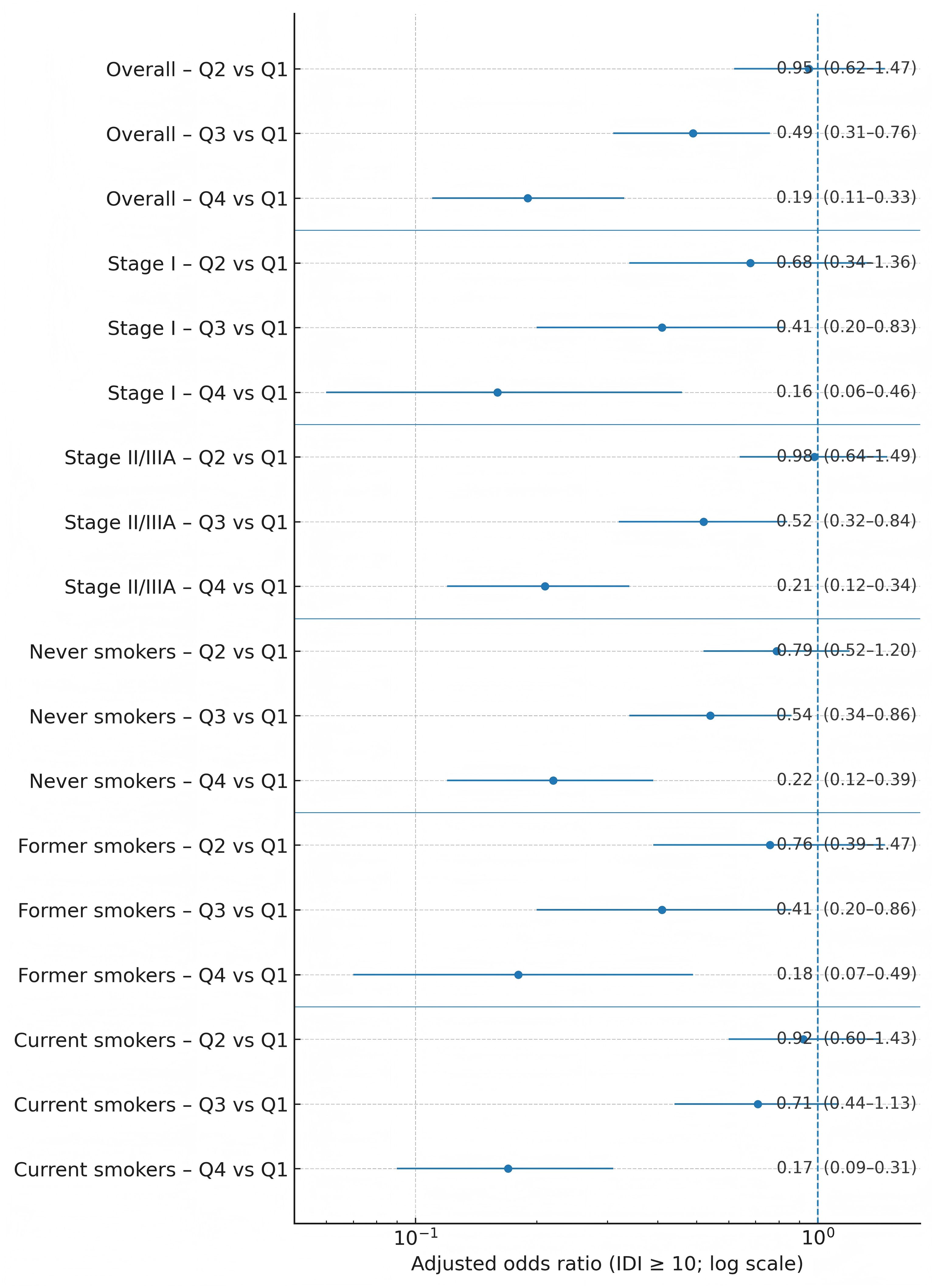
Figure 1. Forest plot of adjusted odds ratios for elevated inflammatory symptomatology by whole-grain-intake quartile, overall and within stage and smoking strata.
3.5 Continuous IDI score analyses
Generalized linear models (gamma distribution with log link) indicated that each 10 g/day-¹ increment in whole-grain intake corresponded to a 6.1% lower expected IDI score (β = −0.063 ± 0.013; 95% CI −0.088 to −0.038; p < 0.001), equivalent to a mean ratio of 0.94 (0.92–0.96). Predicted mean IDI values declined from 9.9 at the 10th percentile of intake (~14 g/day) to 8.1 at the median (~35 g/day) and 6.5 at the 90th percentile (~85 g/day) (Table 5).

Table 5. Association between whole-grain intake and continuous IDI (γ-log GLMs) with restricted cubic-spline (RCS) predictions.
3.6 Dose–response relationship
Restricted cubic spline models (knots at the 5th, 35th, 65th, and 95th percentiles) supported nonlinearity (p-nonlinearity = 0.031), with a steep inverse slope up to ~60 g/day-¹ and a plateau thereafter (Figure 2; Table 5).
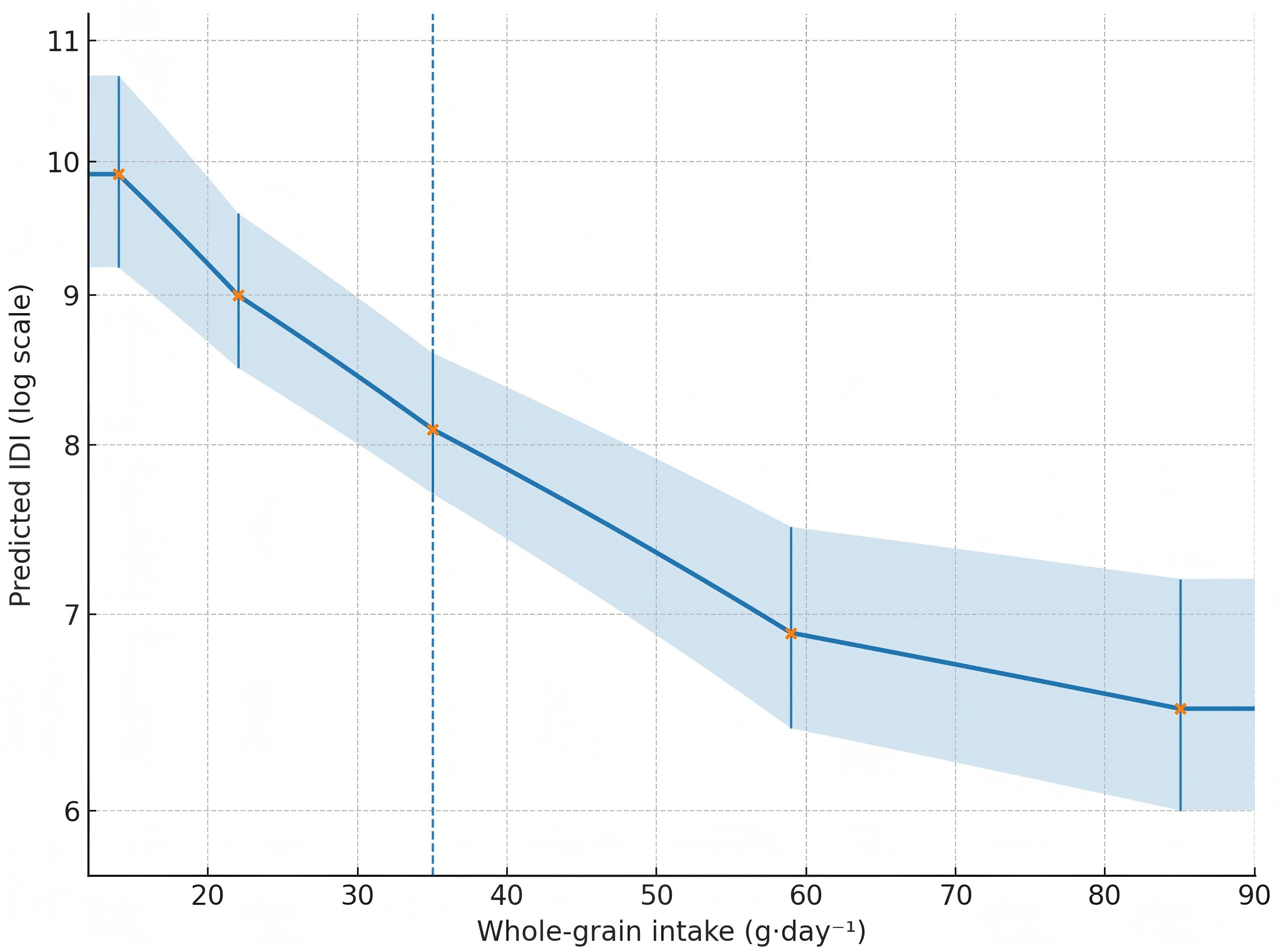
Figure 2. Restricted cubic spline curve for whole-grain intake (g·day-¹) vs predicted IDI (log-scale), showing a steep inverse slope up to ~60 g·day-¹ and a plateau thereafter.
3.7 Subgroup analyses
Prespecified strata yielded consistent estimates (Figure 1; Tables 6, 7). For Q4 vs. Q1, aORs were 0.16 (0.06–0.46) in stage I and 0.21 (0.12–0.34) in stage II/IIIA (interaction p = 0.59). By smoking status, aORs were 0.22 (0.12–0.39) in never-smokers, 0.18 (0.07–0.49) in former smokers, and 0.17 (0.09–0.31) in current smokers (interaction p = 0.67). Patterns within each stratum were monotonic across quartiles.

Table 6. Fully adjusted (Model 4) odds ratios for elevated inflammatory symptomatology (IDI ≥ 10) across whole-grain quartiles, stratified by TNM stage.

Table 7. Fully adjusted (Model 4) odds ratios for elevated inflammatory symptomatology (IDI ≥ 10) across whole-grain quartiles, stratified by smoking status.
3.8 Sensitivity and ancillary analyses
Results were robust to alternative specifications (Table 8). Complete-case analyses (n = 382) gave aOR = 0.20 (0.12–0.34) for Q4 vs. Q1; multiple imputation (m = 20) reproduced the primary estimate (0.19; 0.12–0.33). Excluding participants with BMI > 30 kg/m² (n = 15) had negligible impact (0.20; 0.12–0.34). Using alternative IDI thresholds (≥ 9 or ≥ 11) yielded aORs of 0.22 (0.15–0.34) and 0.17 (0.10–0.30), respectively. Modeling intake in sex-specific quintiles retained a graded association (Q5 ≥ 72 g/day-¹ vs. Q1 aOR = 0.17; 0.10–0.29; p-trend < 0.001). Mixed-effects logistic models with hospital-level random intercepts returned near-identical estimates (Q4 vs. Q1 aOR = 0.20; 0.12–0.35), with minimal clustering (random-intercept variance = 0.03; intraclass correlation coefficient ~0.009). As prespecified, we also evaluated the energy-adjusted density metric (g/1,000 kcal) and the whole-grain diversity score (0–5). For density, quartile cut points at ~11.0, 18.9, and 29.7 g/1,000 kcal-¹ gave fully adjusted aORs of 0.93 (0.61–1.43), 0.55 (0.35–0.85), and 0.21 (0.12–0.36) for Q2–Q4 vs. Q1 (p-trend < 0.001), and a 7% lower odds per 5 g/1,000 kcal-¹ increment (aOR = 0.93; 0.90–0.97). For diversity, the distribution was 30, 84, 106, 92, 60, and 20 participants with scores 0 through 5, respectively; elevated IDI occurred in 43.3%, 40.5%, 28.3%, 21.7%, 16.7%, and 15.0% across these categories. Each one-category increase in diversity was associated with lower odds of elevated IDI (aOR per category = 0.86; 0.78–0.95; p = 0.003), and consuming ≥ 3 distinct whole-grain categories at least weekly (vs. 0–1) corresponded to an aOR of 0.48 (0.29–0.80). Model diagnostics were satisfactory throughout: linearity in the logit for continuous covariates held under fractional polynomial checks, and all VIF values remained < 2.0.

Table 8. Sensitivity and ancillary analyses: alternative samples, models, exposure metrics and thresholds.
4 Discussion
In this single-province cohort of ambulatory, early-stage ESCC survivors, higher habitual whole-grain intake was consistently associated with a lower burden of systemic inflammatory symptoms. Across sex-specific quartiles, the prevalence of IDI ≥ 10 declined from 39.8% in Q1 to 12.2% in Q4, with a fully adjusted odds ratio of 0.19 (95% CI 0.11–0.33) for Q4 vs. Q1 and a strong linear trend (all models p-trend < 0.001). Continuous analyses reinforced these gradients: each 10 g/day-¹ increment corresponded to a ~6% lower expected IDI (mean ratio ≈ 0.94), and restricted cubic splines showed a steep inverse slope through ~60 g/day-¹ followed by a plateau (32, 33). These symptom-level patterns are biologically plausible given the fermentable fiber, magnesium, and phenolic acids concentrated in whole grains that can strengthen SCFA-mediated immunoregulation and temper NF-κB signaling. Importantly, the IDI captures systemic, patient-perceived manifestations of inflammation rather than isolated biomarkers; thus, the observed relief likely reflects changes in whole-body immune tone that are meaningful in day-to-day survivorship.
The protective association appeared robust to potential behavioral and clinical confounding. Fully adjusted models accounted for education, income, tumor stage, time since diagnosis, current treatment, BMI, comorbidity, corticosteroid exposure, smoking, alcohol, diet quality markers, and physical activity, while also including total energy intake when grams were modeled (34, 35). The inverse associations remained monotonic after this adjustment (Q3 aOR 0.49; Q4 aOR 0.19), arguing against a generic “healthy lifestyle” artifact. Consistency across prespecified strata further strengthens inference: effect sizes were similar in stage I (Q4 aOR 0.16) and stage II/IIIA (0.21), and in never- (0.22), former- (0.18), and current-smokers (0.17), with nonsignificant interactions. The fact that current smokers retained benefit suggests grain-derived phytochemicals may partially buffer tobacco-related oxidative stress. Convergent evidence from alternative exposure metrics also supports a dose–response relation: the energy-adjusted density of whole grains (g/1,000 kcal) tracked inversely with elevated IDI (Q4 vs. Q1 aOR 0.21; 7% lower odds per +5 g/1,000 kcal), and a simple dietary-diversity indicator aligned with lower symptom risk (14% lower odds per additional whole-grain category; ≥ 3 categories vs. 0–1, aOR 0.48). Together, these findings imply that both quantity and diversity of whole-grain foods may matter for symptom relief.
The nonlinear shape of the curve deserves clinical emphasis. Spline estimates indicated rapid symptom improvement as intake rose into the 40–60 g/day-¹ range, with diminishing returns thereafter. This profile mirrors fermentative capacity constraints observed in fiber-feeding studies, wherein SCFA production and epithelial uptake approach a physiological ceiling. From a practice standpoint, aiming for ~50 g/day—a level near the upper quartile in this cohort—seems a pragmatic target for counseling, particularly because very high intakes may contribute little additional benefit while risking early satiety in patients already challenged by dysphagia or treatment-related anorexia. Notably, participants with higher whole-grain intake reported only modestly lower energy intake (~60 kcal/day), suggesting the observed associations were not simply proxies for caloric restriction.
Methodological choices likely enhanced internal validity. By restricting recruitment to four Shanxi centers and to stage I–IIIA outpatients, we reduced cross-province ecological heterogeneity and avoided profound treatment-related perturbations of diet typical of late-stage or hospitalized patients. The FFQ quantified five distinct whole-grain categories and enabled both gram-based and density-based metrics; constructing sex-specific cut points guarded against compositional differences by sex. The IDI provided a right-skewed but reliable endpoint well handled by γ-log models, and model diagnostics indicated good calibration with low multicollinearity. Sensitivity checks—including complete-case analysis, multiple imputation, exclusion of participants with BMI ≥ 30 kg/m², alternative IDI thresholds, quintiles, and mixed-effects models accounting for hospital clustering—returned estimates nearly identical to the primary models (e.g., Q4 vs. Q1 aOR 0.20 in complete cases; random-intercept ICC ≈ 0.009), underscoring robustness.
Limitations should temper interpretation. The cross-sectional design precludes causal inference and does not disentangle whether lower inflammatory distress facilitates greater whole grain eating or vice versa. Although the FFQ demonstrated acceptable reproducibility and construct validity, self-report is vulnerable to portion-size error. Focusing on Shanxi—where wheat-based staples dominate—likely reduced region-specific misclassification but cannot eliminate it. The IDI reflects perceived symptomatology rather than cytokines or CRP, and unmeasured factors such as microbiome composition, recent antibiotic use, or supplement intake could confound or modify effects. Finally, the sample comprised Mandarin-speaking outpatients in four tertiary hospitals: generalizability to other settings and to advanced-stage ESCC merits confirmation. Among Chinese ESCC survivors managed in routine outpatient care, habitual whole-grain consumption—particularly around ~50 g/day—was associated with substantially lower systemic inflammatory distress, with benefits evident across clinical strata and supported by density- and diversity-based metrics. These data, coupled with a biologically coherent dose–response that plateaus beyond ~60 g/day, position grain-centered nutrition counseling as a feasible, low-cost adjunct to symptom management in survivorship clinics. Prospective trials that integrate patient-reported outcomes with inflammatory biomarkers and microbiome readouts are now warranted to test causality and refine intake targets suited to the dietary textures of northern China’s wheat-forward food culture.
5 Conclusion
In this single-province cohort of ambulatory, early-stage ESCC survivors, higher habitual intake of whole grains was consistently linked with a lower burden of systemic inflammatory symptoms. Compared with the lowest quartile, the highest quartile of intake was associated with an adjusted 81% lower odds of elevated IDI (aOR 0.19; 95% CI 0.11–0.33), and each 10 g/day-¹ increment corresponded to a ~6% lower mean IDI. Dose–response modeling suggested rapid gains up to ~60 g/day-¹ with attenuating benefits, thereafter, identifying ~50 g/day-¹ as a pragmatic counseling target in survivorship clinics. Findings were stable after extensive adjustment and held across tumor stage and smoking strata. Converging evidence from complementary exposure metrics, the energy-adjusted density of whole grains and a simple diversity score—supported the primary results and underscores that both quantity and variety of whole-grain foods may contribute to symptom relief. By restricting recruitment to Shanxi centers and stage I–IIIA outpatients, we minimized cross-province heterogeneity and treatment-related perturbations that complicate diet–symptom inference. Nonetheless, the cross-sectional design and FFQ-based assessment preclude causal claims, and residual misclassification cannot be excluded. Taken together, these data position grain-centered, culturally consonant nutrition counseling as a low-cost, scalable adjunct for alleviating systemic inflammatory distress in ESCC survivorship. Prospective, biomarker-integrated trials—ideally incorporating microbiome readouts and patient-reported outcomes—are warranted to test causality, refine intake targets, and evaluate whether expanding both the dose and diversity of whole-grain foods can deliver durable, clinically meaningful improvements in inflammatory well-being.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by ethics committee of Xinzhou People’s Hospital (No.:2025-LLSC-06-19). The participants provided written informed consent to participate in this study.
Author contributions
YR: Data curation, Writing – original draft, Visualization, Investigation, Conceptualization. YW: Data curation, Writing – original draft, Investigation. YL: Writing – original draft, Visualization, Validation. LL: Writing – original draft, Visualization, Validation. YG: Writing – original draft. N-GZ: Writing – review & editing, Supervision, Conceptualization, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by Central Guidance Fund for Local Science and Technology Development (No.YDZJSX2025D073); General Programs of Shanxi Provincial Health Commission (No.2024024); Shanxi Provincial Health Commission (approval No. 2022035).
Acknowledgments
We appreciate the efforts of the editors and reviewers in evaluating this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li C, Li Y, Wang N, Ge Z, Wang J, Ding B, et al. Comprehensive modulatory effects of whole grain consumption on immune-mediated inflammation in middle-aged and elderly community residents: A real-world randomized controlled trial. Redox Biol. (2024) 76:103337. doi: 10.1016/j.redox.2024.103337
2. Madsen MTB, Landberg R, Nielsen DS, Zhang Y, Anneberg OMR, Lauritzen L, et al. Effects of wholegrain compared to refined grain Intake on cardiometabolic risk markers, gut microbiota and gastrointestinal symptoms in children: A randomized crossover trial. Am J Clin Nutr. (2024) 119:18–28. doi: 10.1016/j.ajcnut.2023.10.025
3. Zhang SK, Jiang L, Jiang CL, Cao Q, Chen YQ, Chi H, et al. Unveiling genetic susceptibility in esophageal squamous cell carcinoma and revolutionizing pancreatic cancer diagnosis through imaging. World J Gastrointest Oncol. (2025) 17:102544. doi: 10.4251/wjgo.v17.i6.102544
4. Marshall S, Petocz P, Duve E, Abbott K, Cassettari T, Blumfield M, et al. The effect of replacing refined grains with whole grains on cardiovascular risk factors: a systematic review and meta-analysis of randomized controlled trials with GRADE clinical recommendation. J Acad Nutr Dietetics. (2020) 120:1859–1883. e31. doi: 10.1016/j.jand.2020.06.021
5. Capece D, Verzella D, Flati I, Arboretto P, Cornice J, Franzoso G, et al. NF-κB: blending metabolism, immunity, and inflammation. Trends Immunol. (2022) 43:757–75. doi: 10.1016/j.it.2022.07.004
6. Ghosh TS, Shanahan F, and O'Toole PW. The gut microbiome as a modulator of healthy ageing. Nat Rev Gastroenterol Hepatol. (2022) 19:565–84. doi: 10.1038/s41575-022-00605-x
7. Wu Z, Zhang Z, and Gu C. Prognostic and clinicopathological impact of systemic inflammation response index (SIRI) on patients with esophageal cancer: a meta-analysis. Syst Rev. (2025) 14:1–11. doi: 10.1186/s13643-025-02847-7
8. Lacourt TE, Tripathy D, Swartz MC, LaVoy EC, and Heijnen CJ. Distress and inflammation are independently associated with cancer-related symptom severity. Compr Psychoneuroendocrinol. (2024) 20:100269. doi: 10.1016/j.cpnec.2024.100269
9. Wu H, MacDonald GK, Galloway JN, Geng Y, Liu X, Zhang L, et al. A new dietary guideline balancing sustainability and nutrition for China’s rural and urban residents. Iscience. (2022) 25:105048.
10. Austin PC, Fang J, and Lee DS. Using fractional polynomials and restricted cubic splines to model non-proportional hazards or time-varying covariate effects in the Cox regression model. Stat Med. (2022) 41:612–24. doi: 10.1002/sim.9259
11. Lu J, He R, Liu Y, Zhang J, Xu H, Zhang T, et al. Exploiting cell death and tumor immunity in cancer therapy: challenges and future directions. Front Cell Dev Biol. (2024) 12:1416115. doi: 10.3389/fcell.2024.1416115
12. Kirschner SK, Engelen MP, Haas P, Bischoff SC, and Deutz NE. Short-chain fatty acid kinetics and concentrations are higher after inulin supplementation in young and older adults. A randomized trial. Am J Clin Nutr. (2025) 121(6):1224–35. doi: 10.1016/j.ajcnut.2025.04.018
13. Liu R, Liu J, Cao Q, Chu Y, Chi H, Zhang J, et al. Identification of crucial genes through WGCNA in the progression of gastric cancer. J Cancer. (2024) 15:3284. doi: 10.7150/jca.95757
14. Rai SK, Gong T, Gao Z, Qin Y, Huo M, Nakastu K, et al. Global epitranscriptomic and transcriptomic footprint revealed upregulation of methylated single minded 2-small variant (meSIM2-SV) in colorectal cancer (CRC) by NTMT1 (METTL11A) via m6A-YTHDF1-dependent manner. Cancer Res. (2024) 84:7075–5. doi: 10.1158/1538-7445.AM2024-7075
15. Zhou L, Hu S, Rong S, Mo X, Wang Q, Yin J, et al. DHPPA, a major plasma alkylresorcinol metabolite reflecting whole-grain wheat and rye intake, and risk of metabolic syndrome: a case–control study. Eur J Nutr. (2022) 61:3247–54. doi: 10.1007/s00394-022-02880-5
16. Chi H, Huang J, Yan Y, Jiang C, Zhang S, Chen H, et al. Unraveling the role of disulfidptosis-related LncRNAs in colon cancer: a prognostic indicator for immunotherapy response, chemotherapy sensitivity, and insights into cell death mechanisms. Front Mol Biosci. (2023) 10:1254232. doi: 10.3389/fmolb.2023.1254232
17. Miao Y, Nie X, He WW, Luo CY, Xia Y, Zhou AR, et al. Longitudinal patient-reported outcomes after minimally invasive McKeown esophagectomy for patients with esophageal squamous cell carcinoma. Supportive Care Cancer. (2024) 32:237. doi: 10.1007/s00520-024-08428-z
18. Liu F, Wang D, Rong Y, Wei S, SiTu Y, Wang M, et al. Safety and efficacy of neoadjuvant toripalimab plus chemotherapy followed by chemoradiotherapy for locally advanced esophageal squamous cell carcinoma in China (GASTO 1071): a non-randomised, two-cohort, phase 2 trial. EClinicalMedicine. (2025) 82:103184. doi: 10.1016/j.eclinm.2025.103184
19. Taskinen RE, Hantunen S, Tuomainen TP, and Virtanen JK. The associations between whole grain and refined grain intakes and serum C-reactive protein. Eur J Clin Nutr. (2022) 76:544–50. doi: 10.1038/s41430-021-00996-1
20. You Y, Chen Y, You Y, Zhang Q, and Cao Q. Evolutionary game analysis of artificial intelligence such as the generative pre-trained transformer in future education. Sustainability. (2023) 15:9355. doi: 10.3390/su15129355
21. Feitelson MA, Arzumanyan A, Medhat A, and Spector I. Short-chain fatty acids in cancer pathogenesis. Cancer Metastasis Rev. (2023) 42:677–98. doi: 10.1007/s10555-023-10117-y
22. Sun J, Chen S, Zang D, Sun H, Sun Y, and Chen J. Butyrate as a promising therapeutic target in cancer: From pathogenesis to clinic. Int J Oncol. (2024) 64:44. doi: 10.3892/ijo.2024.5632
23. Zhao Q, Wang L, Yang X, Feng J, and Chen Q. Preoperative inflammatory burden index for prognostication in esophageal squamous cell carcinoma undergoing radical resection. Sci Rep. (2024) 14:30811. doi: 10.1038/s41598-024-81237-w
24. Wang Y, Bai G, Huang M, and Chen W. CT-radiomics combined with inflammatory indicators for prediction of progression free survival of resectable esophageal squamous cell carcinoma. Sci Rep. (2025) 15:1–11. doi: 10.1038/s41598-025-01240-7
25. Chen Y, You Y, Wei M, Yang P, Zhang Q, Li X, et al. Exploration of physical activity, sedentary behavior and insulin level among short sleepers. Front Endocrinol. (2024) 15:1371682. doi: 10.3389/fendo.2024.1371682
26. Mann ER, Lam YK, and Uhlig HH. Short-chain fatty acids: linking diet, the microbiome and immunity. Nat Rev Immunol. (2024) 24:577–95. doi: 10.1038/s41577-024-01014-8
27. Cao S, Pierson JT, Bond AH, Zhang S, Gold A, Zhang H, et al. Intestinal-level anti-inflammatory bioactivities of whole wheat: Rationale, design, and methods of a randomized, controlled, crossover dietary trial in adults with prediabetes. Nutr Res. (2024) 131:83–95. doi: 10.1016/j.nutres.2024.09.010
28. Schild T, Wallisch P, Zhao Y, Wang YT, Haughton L, Chirayil R, et al. Metabolic engineering to facilitate anti-tumor immunity. Cancer Cell. (2025) 43:552–562. e9. doi: 10.1016/j.ccell.2025.02.004
29. Chen Y, Gao Z, Mohd-Ibrahim I, Yang H, Wu L, Fu Y, et al. Pan-cancer analyses of bromodomain containing 9 as a novel therapeutic target reveals its diagnostic, prognostic potential and biological mechanism in human tumours. Clin Trans Med. (2024) 14:e1543. doi: 10.1002/ctm2.1543
30. Deehan EC, Yang C, Perez-Muñoz ME, Nguyen NK, Cheng CC, Triador L, et al. Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty acid production. Cell Host Microbe. (2020) 27:389–404. e6. doi: 10.1016/j.chom.2020.01.006
31. Lancaster SM, Lee-McMullen B, Abbott CW, Quijada JV, Hornburg D, Park H, et al. Global, distinctive, and personal changes in molecular and microbial profiles by specific fibers in humans. Cell Host Microbe. (2022) 30:848–862. e7. doi: 10.1016/j.chom.2022.03.036
32. Müller J, Wiesenberger R, Kaufmann M, Weiß C, Ghezel-Ahmadi D, Hardt J, et al. Motivational Interviewing improves postoperative nutrition goals within the Enhanced Recovery after Surgery (ERAS®) pathway in elective bowel surgery–A randomized clinical pilot trial. Clin Nutr ESPEN. (2024) 61:181–8.
33. He S, Su L, Hu H, Liu H, Xiong J, Gong X, et al. Immunoregulatory functions and therapeutic potential of natural killer cell-derived extracellular vesicles in chronic diseases. Front Immunol. (2024) 14:1328094. doi: 10.3389/fimmu.2023.1328094
34. Cao Q, Wang Q, Wu X, Zhang Q, Huang J, Chen Y, et al. A literature review: mechanisms of antitumor pharmacological action of leonurine alkaloid. Front Pharmacol. (2023) 14:1272546. doi: 10.3389/fphar.2023.1272546
Keywords: whole grains, esophageal squamous-cell carcinoma, systemic inflammation, inflammation distress index, dietary fiber
Citation: Ren Y-Q, Wang Y, Li Y, Li L, Guo Y and Zhang N-G (2025) Whole grains temper immune-mediated inflammation in Chinese esophageal squamous-cell carcinoma survivors: a multicenter questionnaire study. Front. Immunol. 16:1643374. doi: 10.3389/fimmu.2025.1643374
Received: 08 June 2025; Accepted: 26 August 2025;
Published: 22 September 2025.
Edited by:
Biao Zhang, Dalian Medical University, ChinaCopyright © 2025 Ren, Wang, Li, Li, Guo and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning-Gang Zhang, em5nMTEyMEAxNjMuY29t
 Ya-Qiong Ren1
Ya-Qiong Ren1 Yang Li
Yang Li Ning-Gang Zhang
Ning-Gang Zhang