- 1School of Dentistry, Hainan Medical University, Haikou, China
- 2Department of Health Management Center, The First Affiliated Hospital of Hainan Medical University, Haikou, China
- 3Department of Implantology, Stomatological Hospital of Xiamen Medical College, Xiamen, China
Advanced Glycation End Products (AGEs) are key pathogenic drivers in periodontitis, a chronic inflammatory disease leading to destruction of tooth-supporting tissues. This review synthesizes evidence on the multi-dimensional roles of AGEs, focusing on three core areas: direct modification and degradation of the periodontal extracellular matrix (ECM), induction of a self-perpetuating inflammatory cycle via the Receptor for AGEs (RAGE), and dysregulation of the local neuro-immune axis, an emerging pathogenic frontier. AGEs, which accumulate with age and in metabolic diseases like diabetes, trigger pro-inflammatory signaling (e.g., NF-κB, MAPKs), leading to oxidative stress, cytokine release, and matrix metalloproteinase (MMP) activation. This disrupts ECM homeostasis by suppressing collagen synthesis while promoting its degradation. Notably, specific AGEs like Nϵ-(carboxymethyl)lysine (CML) directly induce osteoblast apoptosis, contributing to alveolar bone loss. A crucial, and increasingly recognized, aspect of AGE pathology is their ability to modulate neuro-immune crosstalk by activating both immune cells and sensory neurons. This creates a complex inflammatory network that exacerbates tissue damage and may contribute to clinical manifestations such as pain and chronic disease. The interplay between systemic AGE load and local production within inflamed periodontal tissues establishes a vicious cycle, making periodontitis a compelling model for studying AGE-driven pathology. Understanding this integrated network reveals novel therapeutic targets aimed at inhibiting AGE formation, blocking RAGE signaling, and modulating downstream inflammatory and neuro-immune pathways to improve periodontal and potentially systemic health.
1 Introduction: the converging paths of advanced glycation end products and periodontal destruction
Periodontitis is a chronic inflammatory disease defined by the progressive destruction of tooth-supporting tissues, including the gingiva, periodontal ligament, and alveolar bone (1). Primarily initiated by dysbiotic microbial biofilms, the disease leads to periodontal pocket formation, attachment loss, and ultimately, tooth loss, posing a significant global health burden (2). While microbes are the trigger, the host’s own immune-inflammatory response is the primary engine of tissue destruction (3). In this context, Advanced Glycation End Products (AGEs)—a diverse group of molecules formed non-enzymatically from sugars and proteins—are emerging as critical pathogenic mediators (4). AGEs accumulate with age, are exacerbated by conditions like diabetes mellitus, and can be introduced exogenously through diet and tobacco smoke (5). Their roles in promoting oxidative stress and inflammation are well-established in numerous chronic diseases (6).
The link between diabetes and periodontitis is strong, with periodontitis recognized as a major diabetic complication (7). AGEs serve as a key molecular bridge in this bidirectional relationship (8). Elevated AGE levels are found in the gingival crevicular fluid (GCF) and serum of periodontitis patients, even those without diabetes, pointing to their direct role in periodontal pathology (9, 10). This inherent connection is particularly concerning because both periodontitis and AGE accumulation are considered largely “irreversible” processes (1, 4). This shared feature suggests a compounding effect where persistent molecular damage fuels progressive tissue loss, making established disease exceptionally difficult to manage and underscoring the need for early intervention.
While the roles of AGEs in matrix remodeling and immune activation have been extensively reviewed, their impact on the periodontal neuro-immune axis represents an underexplored, emerging mechanism that deserves focused attention. This review aims to provide a multi-dimensional overview of the pathogenic network orchestrated by AGEs in periodontitis. We will delve into how AGEs: (1) directly alter the periodontal extracellular matrix (ECM); (2) fuel a cycle of inflammation and oxidative stress through their primary receptor, RAGE; and (3) dysregulate local neuro-immune crosstalk, a novel mechanism that may explain key clinical features of periodontitis, such as pain and chronicity. By framing the discussion around this emerging concept, we highlight how periodontitis serves as a model for the complex interplay between systemic factors, local inflammation, and neuro-immune responses, reinforcing the principles of periodontal medicine.
2 Advanced glycation end products: formation, diversity, and systemic impact
2.1 Definition and formation
Advanced Glycation End Products (AGEs) are a diverse group of molecules formed through a non-enzymatic process known as the Maillard reaction. This reaction begins when a reducing sugar, such as glucose, reacts with the free amino group of a protein, lipid, or nucleic acid to form an unstable Schiff base. This base then rearranges into a more stable, yet still reversible, ketoamine structure known as an Amadori product (e.g., fructoselysine) (4). These early-stage products can then undergo a series of further irreversible reactions—including oxidation, dehydration, and condensation—to form a wide range of irreversible, heterogeneous compounds collectively known as AGEs. This process is significantly accelerated by hyperglycemia and oxidative stress (5). The formation of AGEs can also be driven by highly reactive dicarbonyl compounds like glyoxal, methylglyoxal (MGO), and 3-deoxyglucosone, which are byproducts of glucose metabolism (4). Beyond endogenous formation, the body’s total AGE pool is supplemented by exogenous AGEs from sources like tobacco smoke and thermally processed foods (11).
2.2 Chemical diversity and classification
The term “AGEs” encompasses a wide array of structurally varied compounds, with more than 20 distinct AGEs having been identified in human tissues (12). They are broadly classified based on their fluorescent properties. Fluorescent, cross-linking AGEs include well-characterized examples like pentosidine, while non-fluorescent AGEs, which are often more abundant, include Nϵ-(carboxymethyl)lysine (CML) and Nϵ-(carboxyethyl)lysine (CEL) (6). This heterogeneity implies that different AGE species may possess distinct biological activities and pathogenic potentials. Therefore, understanding the specific profile of AGEs in a given condition is crucial for elucidating precise disease mechanisms (13).
2.3 Systemic accumulation and pathological consequences
Under normal physiological conditions, a balance exists between the formation of AGEs and their detoxification and excretion, primarily via the kidneys (6). When this balance is disrupted—due to increased formation (e.g., in diabetes), increased intake, or impaired clearance (e.g., in renal insufficiency)—AGEs accumulate in tissues and circulation (14). This accumulation is implicated in a wide range of chronic conditions, contributing to cellular dysfunction and tissue damage by promoting sustained inflammation, inducing oxidative stress, and directly altering protein structure and function by forming cross-links (15). Consequently, higher circulating levels of AGEs are associated with an increased risk of all-cause and cardiovascular mortality (16).
2.4 The receptor for AGEs and other AGE receptors
The biological effects of AGEs are largely mediated through their interaction with specific cell surface receptors. The most extensively studied of these is the Receptor for Advanced Glycation End Products (RAGE), a multi-ligand member of the immunoglobulin superfamily expressed on various cells, including endothelial cells, macrophages, lymphocytes, and osteoblasts (17). While RAGE expression is typically low in health, its levels are significantly upregulated in environments rich in its ligands, such as in diabetes and chronic inflammation (5).
The engagement of RAGE by AGEs initiates a cascade of intracellular signaling events that perpetuate cellular dysfunction. Key pathways activated downstream of RAGE include nuclear factor-kappa B (NF-κB) and various mitogen-activated protein kinases (MAPKs) (5). Activation of NF-κB upregulates numerous pro-inflammatory genes, resulting in the production of cytokines (e.g., TNF-α, IL-1β, IL-6) that fuel inflammation (18). Furthermore, AGE-RAGE interaction stimulates NADPH oxidase, leading to increased production of reactive oxygen species (ROS) and heightened oxidative stress (15). This upregulation of RAGE in pathological states creates a detrimental positive feedback loop: AGE accumulation promotes RAGE expression, which in turn sensitizes cells to further AGE-mediated damage, thus amplifying and sustaining inflammatory responses (Figure 1).
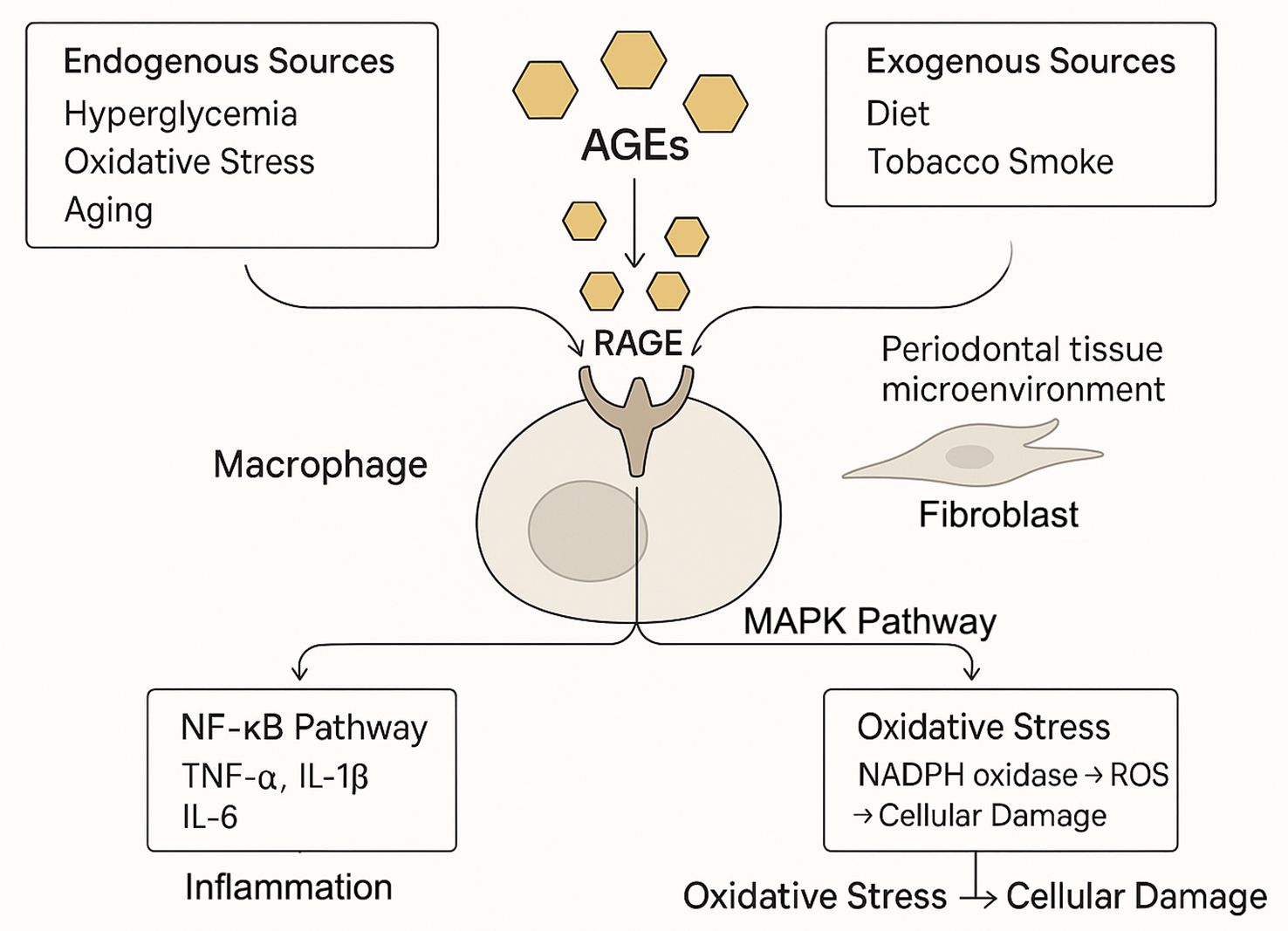
Figure 1. The AGE-RAGE axis as the central engine of periodontal inflammation. Advanced glycation end products (AGEs) accumulate in periodontal tissues from systemic sources (e.g., diabetes, aging) and local production. The binding of AGEs to their receptor (RAGE) on cells like macrophages triggers downstream signaling, including NF-κB and MAPK pathways. This leads to a surge in pro-inflammatory cytokines (e.g., TNF-α, IL-6) and reactive oxygen species (ROS), creating a self-amplifying cycle of inflammation and oxidative stress that drives tissue damage.
Beyond the pro-inflammatory RAGE, other receptors are involved in AGE biology. Receptors such as AGE-R1 (OST-48) and galectin-3 are thought to play protective roles by mediating the clearance and detoxification of AGEs (5). Additionally, scavenger receptors like CD36 can bind specific AGEs such as CML, contributing to cellular responses distinct from RAGE signaling (19). The balance between pathogenic signaling via RAGE and the protective functions of these alternative receptors likely plays a critical role in determining susceptibility to AGE-related pathologies.
3 Periodontitis: a complex inflammatory disease of the tooth-supporting tissues
3.1 Definition and etiology
Periodontitis is an inflammatory disease affecting the supporting structures of the teeth, primarily initiated by dysbiotic dental plaque biofilms (2). Key pathogens frequently implicated include Porphyromonas gingivalis, Tannerella forsythia, and Aggregatibacter actinomycetemcomitans (25). However, the critical determinant of tissue destruction is not the microbial burden alone, but the host’s dysregulated immune-inflammatory response to it (26). This response, modulated by genetic and environmental factors (e.g., smoking, diet), can become overly aggressive, leading to collateral damage to the host’s own tissues (27). As potent pro-inflammatory molecules, AGEs are perfectly positioned to fuel this dysregulated host response, tipping the balance towards a destructive phenotype (28).
3.2 Pathological features
Clinically, periodontitis is characterized by the progressive destruction of the periodontal ligament and alveolar bone, leading to the formation of periodontal pockets and/or gingival recession (2). If untreated, this can result in tooth mobility and loss (28). Beyond its local effects, periodontitis contributes to systemic inflammation, evidenced by elevated systemic markers like C-reactive protein (CRP) (29). The disease’s prevalence and severity increase with age, a phenomenon linked to “inflammaging”—a chronic, low-grade systemic inflammation that renders tissues more susceptible to damage (30). This complexity explains why management often requires a multi-faceted approach targeting both the microbial insult and the host response (31).
4 The AGE-periodontitis nexus: evidence for a pathogenic partnership
4.1 Elevated AGEs in periodontal disease: local and systemic evidence
A strong association between AGEs and periodontitis is confirmed by their accumulation in affected individuals. Locally, within the gingival crevicular fluid (GCF)—the inflammatory exudate of the periodontal pocket—AGE levels are significantly elevated in periodontitis patients, with the highest concentrations found in those who also have diabetes (8). More importantly, the impact of periodontitis extends to the systemic AGE load. A key clinical study demonstrated that even in normoglycemic (non-diabetic) individuals, the presence of chronic periodontitis is associated with significantly higher serum AGE levels compared to periodontally healthy controls (10). This crucial finding suggests that the chronic inflammation of periodontitis itself may contribute to the systemic AGE burden, independent of diabetes. This is further supported by immunohistochemical analyses of gingival tissues, which show increased deposition of AGEs and upregulation of their receptor, RAGE, in periodontitis patients regardless of their diabetic status (8). Several studies also report a positive correlation between the severity of periodontal destruction and systemic AGE levels, reinforcing the pathogenic link (37).
4.2 Periodontitis as an endogenous source of AGEs: a vicious cycle
The AGE-periodontitis relationship is bidirectional; not only do systemic AGEs worsen periodontitis, but the disease process itself generates AGEs, creating a vicious cycle. This local production is driven by both microbial and host factors.
A key breakthrough in this area was the discovery that the periodontal pathogen Tannerella forsythia can directly produce methylglyoxal (MGO), a potent AGE precursor. Mechanistic studies using a genetically engineered MGO-deficient mutant of T. forsythia proved that this metabolic capability is a key virulence factor; the mutant strain failed to cause significant AGE accumulation or bone loss in a mouse model, unlike its wild-type counterpart (45). The clinical relevance of this finding was confirmed in humans, where MGO levels in GCF were found to be nearly 20 times higher at diseased periodontal sites compared to healthy sites (46). This locally produced MGO can then modify host proteins like collagen. Subsequent research has shown that this MGO-modified collagen specifically activates human monocytes through RAGE, triggering the secretion of pro-inflammatory and pro-osteoclastogenic cytokines, thus directly linking a bacterial product to host-mediated tissue destruction (47).
Furthermore, the host inflammatory response itself fuels AGE formation. Activated immune cells in the periodontal lesion release vast amounts of reactive oxygen species (ROS) during the oxidative burst (48). This state of intense oxidative stress creates a highly conducive environment for the chemical reactions that convert Amadori products and other precursors into mature AGEs (8). This evidence paints a clear picture of a self-perpetuating pathogenic loop: systemic AGEs and microbial triggers initiate inflammation, which in turn generates more AGEs locally, amplifying tissue destruction and potentially contributing to the systemic AGE pool (49).
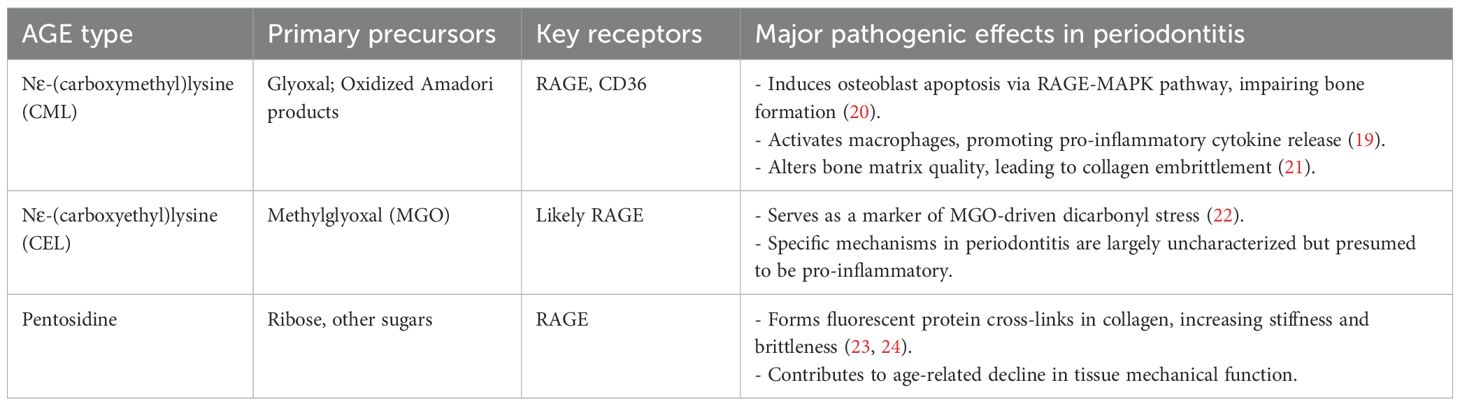
5 Molecular specificity of AGEs in periodontal pathogenesis
While often discussed collectively, the chemical diversity of AGEs means that specific molecules may exert distinct biological effects. Understanding this molecular specificity is crucial for developing targeted therapies. Key AGEs implicated in periodontitis include Nϵ-(carboxymethyl)lysine (CML), Nϵ-(carboxyethyl)lysine (CEL), and pentosidine (Table 1).
5.1 Nϵ-(carboxymethyl)lysine: a key mediator of bone loss
CML is one of the most abundant non-fluorescent AGEs in the human body, formed from the oxidative degradation of Amadori products or the reaction of proteins with glyoxal (5). Its prevalence is particularly high in bone tissue, where it accumulates at levels 40 to 100 times greater than other AGEs like pentosidine, making it highly relevant to the alveolar bone destruction seen in periodontitis (50).
CML exerts its pathogenic effects by interacting with multiple receptors, including RAGE and the scavenger receptor CD36 (19). The mechanisms of CML are illustrated in Figure 2, and its detrimental actions in the periodontium are well-documented:
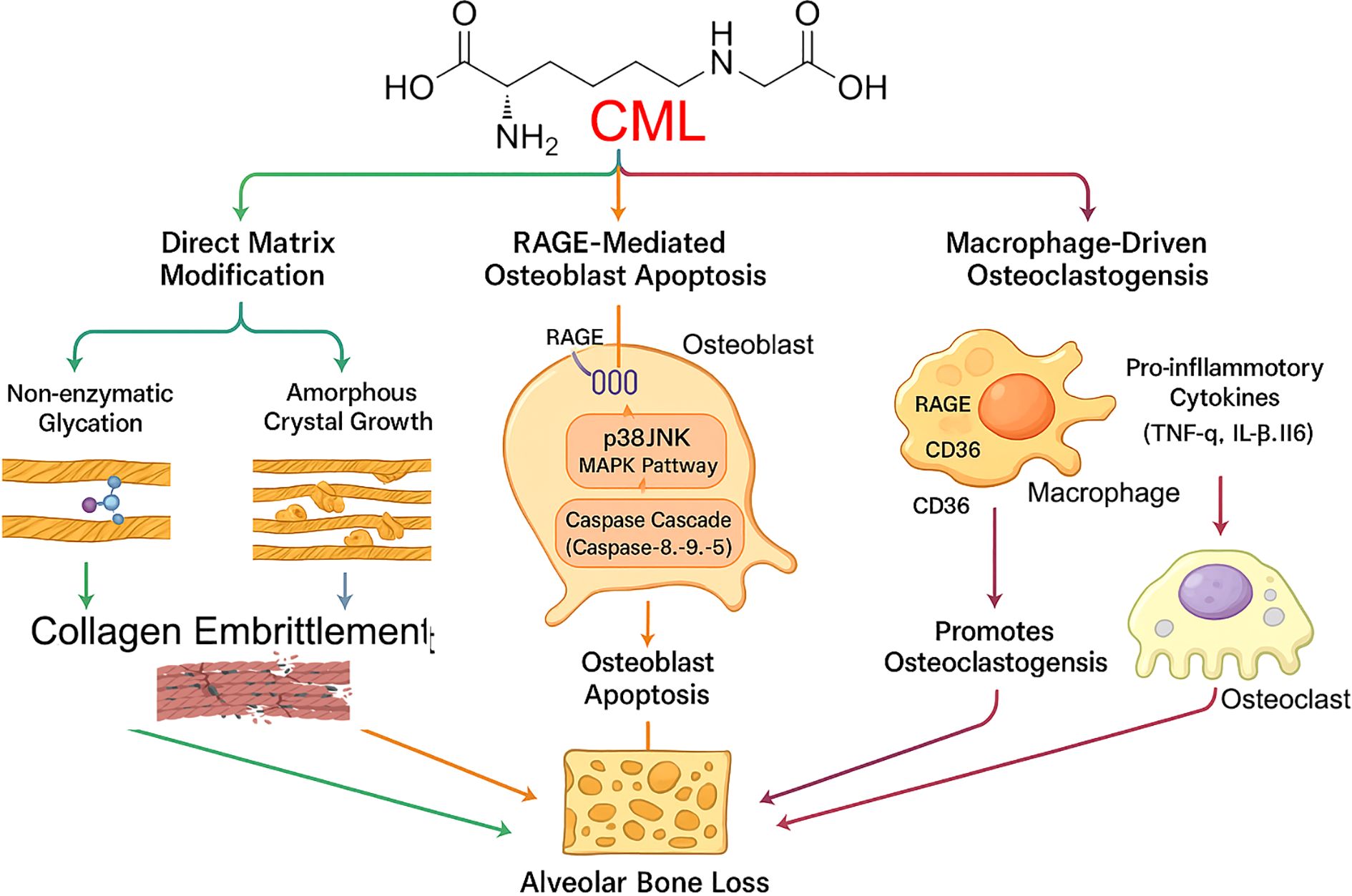
Figure 2. Pathogenic mechanisms of CML in alveolar bone loss. Nϵ-(carboxymethyl)lysine (CML), an advanced glycation end product highly prevalent in bone, orchestrates alveolar bone loss through three convergent pathways. (1) Direct Matrix Modification: CML contributes to non-enzymatic glycation and promotes amorphous crystal growth within the collagen matrix, leading to its embrittlement. (2) RAGE-Mediated Osteoblast Apoptosis: CML binds to the RAGE receptor on osteoblasts, initiating downstream signaling through the p38/JNK MAPK and Caspase Cascade pathways, ultimately inducing osteoblast apoptosis. (3) Macrophage-Driven Osteoclastogenesis: CML activates macrophages via RAGE and CD36 receptors, triggering the release of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6), which in turn promotes the formation and activation of bone-resorbing osteoclasts. All three pathways contribute to the final outcome of alveolar bone loss.
1. Osteoblast Apoptosis: CML, particularly when modifying collagen (CML-collagen), is a potent inducer of apoptosis in osteoblastic cells. This effect is mediated primarily through RAGE and involves the activation of downstream p38 and JNK MAPK pathways, leading to the activation of caspases and programmed cell death (20). By directly killing bone-forming cells, CML significantly impairs bone formation and homeostasis.
2. Macrophage Activation: CML activates macrophages via both RAGE and CD36, promoting a pro-inflammatory phenotype and enhancing lipid uptake (19). This contributes to the sustained inflammation that drives periodontal destruction.
3. Matrix Alteration: Beyond receptor-mediated effects, CML directly alters the quality of the bone matrix. Its formation on collagen changes the local charge environment, leading to aberrant, amorphous hydroxyapatite crystal growth and increased collagen embrittlement, which compromises the biomechanical integrity of alveolar bone (21).
5.2 Nϵ-(carboxyethyl)lysine and pentosidine
CEL is another non-fluorescent AGE formed from the reaction of methylglyoxal (MGO) with lysine residues (5). Its levels often parallel those of CML, suggesting it may play a significant role in AGE-related pathologies, though its specific functions in periodontitis are less characterized and represent a knowledge gap (22). Recent studies show that CEL concentrations are increased in psoriatic patients, suggesting its involvement in inflammatory conditions (51).
Pentosidine is a fluorescent, cross-linking AGE that accumulates in tissues with age and in chronic inflammatory diseases (52). Its primary pathogenic role stems from its ability to form non-enzymatic cross-links between collagen fibers (23). This cross-linking increases the stiffness and brittleness of collagenous tissues like the periodontal ligament and bone, making them more susceptible to mechanical failure and impairing normal tissue function (24).
5.3 Implications of molecular specificity
The distinct actions of CML, CEL, and pentosidine highlight the importance of molecular specificity. The high prevalence of CML in bone and its potent apoptotic effect on osteoblasts position it as a prime therapeutic target for preventing alveolar bone loss in periodontitis. In contrast, the cross-linking action of pentosidine points to a different mechanism of tissue damage related to biomechanical failure. This suggests that future diagnostic and therapeutic strategies may need to target specific AGEs rather than total AGE load.
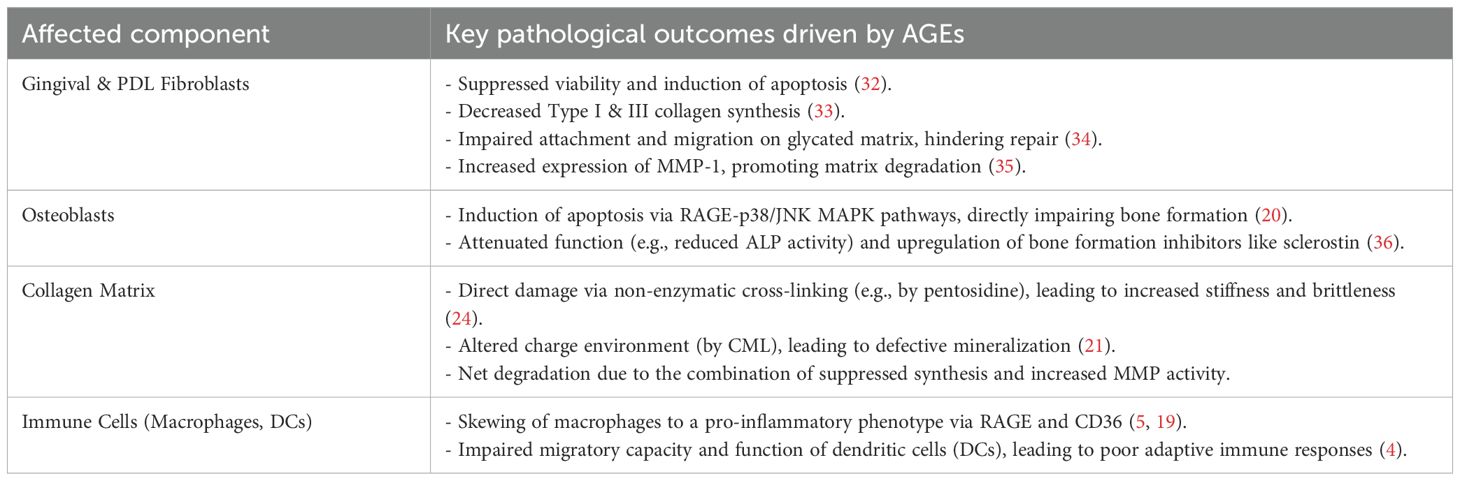
6 AGE-driven extracellular matrix remodeling in periodontitis
The periodontal ECM, rich in collagen, provides structural support and regulates cellular functions. AGEs disrupt ECM homeostasis through a multi-pronged attack that combines direct matrix modification with indirect effects on periodontal cells (Figure 3) (Table 2).
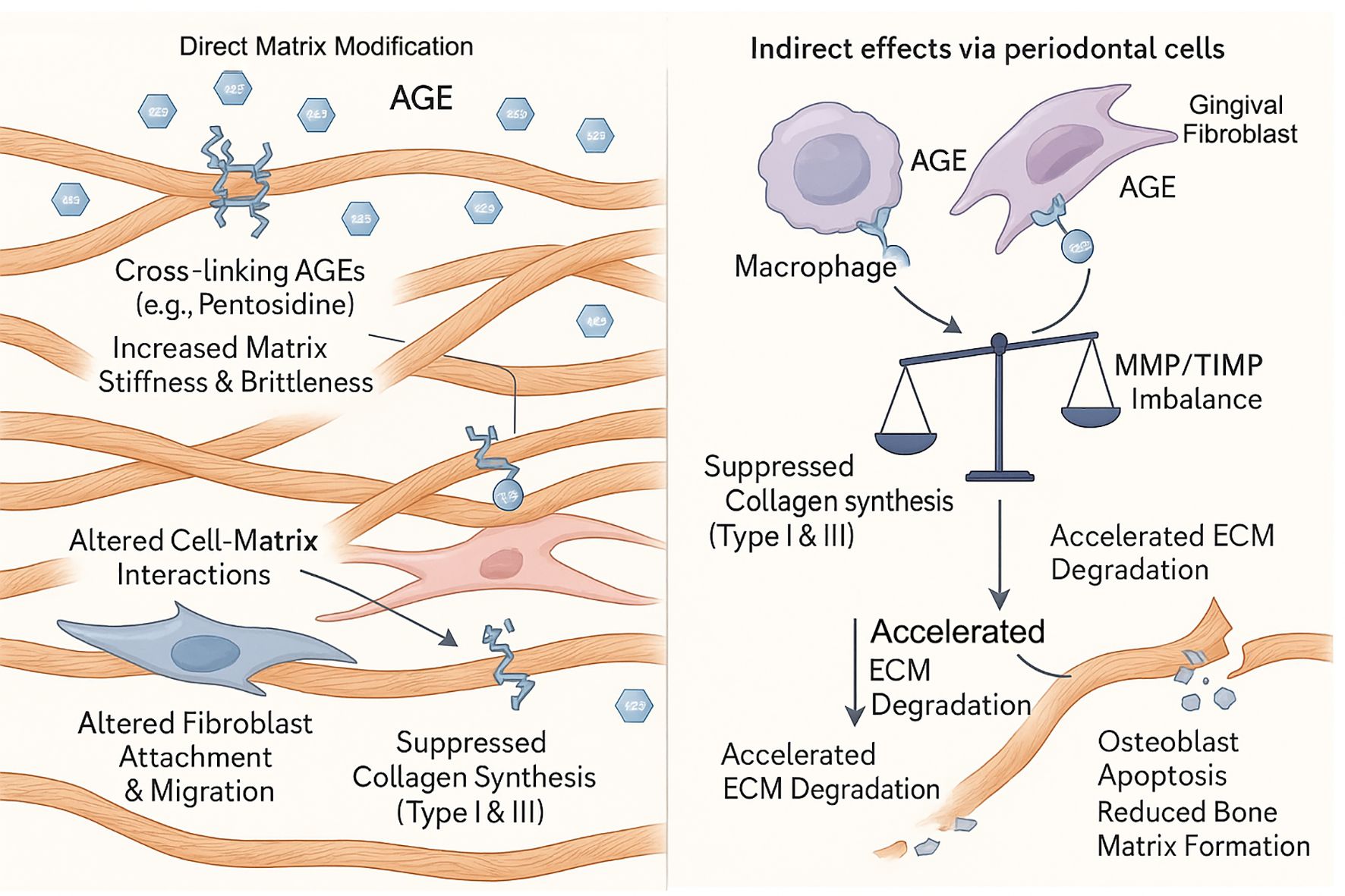
Figure 3. AGE-driven degradation of the periodontal extracellular matrix (ECM). Advanced glycation end products (AGEs) disrupt periodontal ECM homeostasis through a dual-pronged attack. (Left Panel) Direct Matrix Modification: Cross-linking AGEs, such as pentosidine, directly bind to collagen fibrils, increasing matrix stiffness and brittleness. This alteration of the matrix impairs normal cell-matrix interactions, leading to altered fibroblast attachment and migration, and can directly suppress collagen synthesis. (Right Panel) Indirect effects via periodontal cells: AGEs activate resident cells, including macrophages and gingival fibroblasts. This cellular activation creates an MMP/TIMP imbalance and suppresses collagen synthesis (Type I & III), leading to accelerated ECM degradation, osteoblast apoptosis, and reduced bone matrix formation.
6.1 Direct collagen alterations and impaired cell-matrix interactions
Collagen is a primary target of AGEs. Cross-linking AGEs like pentosidine forms covalent bonds within and between collagen molecules, increasing stiffness and brittleness (24). This compromises the biomechanical properties of the periodontal ligament and alveolar bone. Furthermore, AGE-modified matrix proteins become a dysfunctional scaffold for periodontal cells. Glycated collagen and fibronectin show a reduced capacity to support the attachment and migration of human gingival fibroblasts (HGFs) and periodontal ligament fibroblasts (HPLFs), which is critical for wound healing and tissue maintenance (34). The extracellular matrix undergoes complex changes during aging, with increased cross-linking affecting cellular functions (53).
6.2 Dysregulation of matrix synthesis and degradation
AGEs create a net-degradative environment by altering the behavior of resident periodontal cells. This is achieved through two main pathways:
1. Suppression of Synthesis: AGEs suppress the synthesis of new collagen. They have been shown to downregulate the expression of Type I and Type III collagen in HGFs (33) and induce apoptosis in both HPLFs (32) and osteoblasts (20), directly reducing the population of matrix-producing cells. This suppression is mediated through dysregulation of key signaling pathways that control ECM protein expression (54).
2. Enhancement of Degradation: AGEs simultaneously upregulate matrix-degrading enzymes. By activating RAGE, AGEs trigger signaling pathways (NF-κB, MAPKs) that increase the expression of matrix metalloproteinases (MMPs) in periodontal cells (36). For instance, AGEs have been shown to directly induce the expression of MMP-1 (a key collagenase) in HGFs (35). Elevated levels of MMPs, particularly MMP-8 and MMP-9, are consistently found in the saliva and GCF of periodontitis patients (55, 56). The aging process further complicates this matrix degradation, as aged gingival tissues show differential MMP expression profiles compared to younger tissues (37).
This dual-action—suppressing synthesis while promoting degradation—leads to a catastrophic failure of ECM homeostasis, resulting in the progressive tissue destruction characteristic of periodontitis. The importance of maintaining MMP homeostasis is underscored by the fact that MMPs play vital roles in normal tissue turnover, but become destructive when overactivated in periodontitis (57). The imbalance between tissue inhibitors of metalloproteinases (TIMPs) and MMPs contributes significantly to periodontal tissue breakdown (58).
7 Neuro-immune dysregulation: an emerging frontier in AGE-mediated pathology
The pathogenic influence of AGEs extends beyond the ECM to involve a complex dysregulation of the local neuro-immune network, representing a novel and clinically relevant frontier in our understanding of periodontitis. The AGE-RAGE axis acts as a central hub, orchestrating pro-inflammatory responses in immune cells and, importantly, modulating the function of sensory neurons within the periodontium (Figure 4).
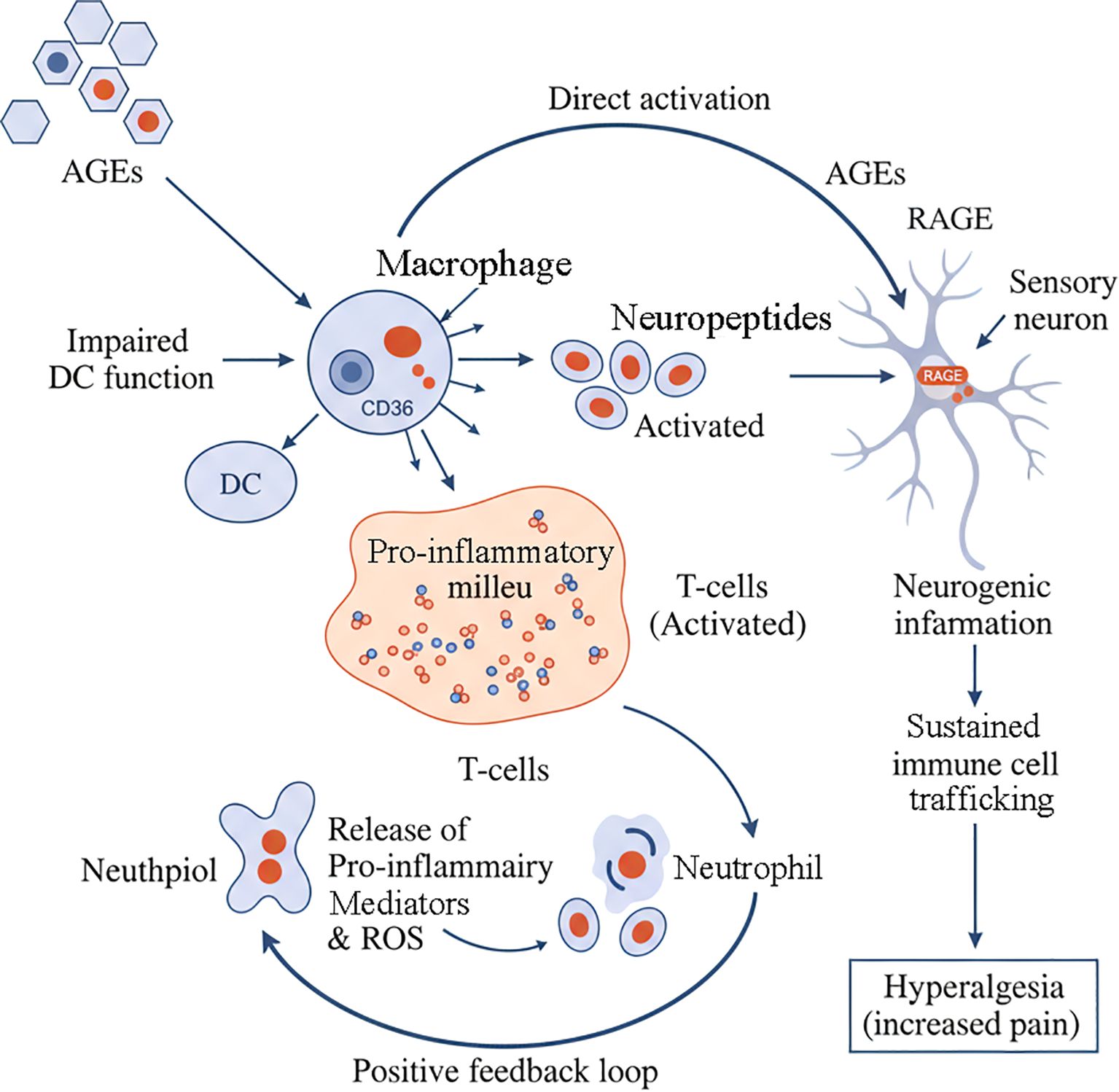
Figure 4. AGE-mediated neuro-immune dysregulation in periodontitis. AGEs orchestrate a complex neuro-immune network that drives chronic inflammation and pain. AGEs directly cause impaired dendritic cell (DC) function and activate macrophages. Macrophages contribute to a pro-inflammatory milieu that activates T-cells. These activated T-cells release neuropeptides, which in turn act on sensory neurons to cause neurogenic inflammation. Concurrently, AGEs can also directly activate RAGE receptors on sensory neurons. This sustained neuronal activation promotes immune cell trafficking and leads to hyperalgesia (increased pain). The pro-inflammatory milieu also activates neutrophils, which release pro-inflammatory mediators and ROS. These mediators feedback to promote the activation of T-cells, establishing a positive feedback loop that perpetuates the inflammatory cycle.
7.1 AGE-RAGE signaling: the engine of inflammation and oxidative stress
The binding of AGEs to RAGE on cells like macrophages, fibroblasts, and endothelial cells triggers the activation of NF-κB and MAPK signaling pathways (5). This leads to a surge in the production of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6), chemokines that recruit more immune cells, and ROS that cause oxidative damage (15, 18). This process creates a self-sustaining inflammatory loop that contributes to the chronicity of periodontitis. The aging process further amplifies this inflammatory state, as periodontal tissues become more susceptible to damage with advancing age (59, 60).
7.2 Complex immune cell dynamics in periodontitis
The immune response in periodontitis involves multiple cell types that are differentially affected by AGEs:
Macrophages are key players in periodontitis and are highly responsive to AGEs. AGE-RAGE interaction drives them into a pro-inflammatory state, characterized by the secretion of destructive cytokines (5). Specific AGEs like CML can also engage other receptors like CD36 on macrophages, further promoting inflammation (19). The role of macrophages in both periodontal tissue destruction and restoration is complex and age-dependent (48).
Dendritic Cells (DCs) function as professional antigen-presenting cells, and AGEs can impair their function by inhibiting their migration and maturation (4). This dysfunction can lead to an inadequate or inappropriate immune response to periodontal pathogens, increasing disease susceptibility. Aging further compromises DC function, contributing to increased disease susceptibility in older individuals (61).
T Helper Cells, particularly Th17 cells, play crucial roles in periodontitis pathogenesis. These cells are regulated by various factors, including the A20 protein, and their dysregulation contributes to the chronic inflammatory state (62).
B Cells also contribute to periodontal pathogenesis, with their subset distribution being altered in patients with severe periodontitis (63). B cells can promote obesity-associated periodontitis and oral pathogen-associated inflammation (64).
Neutrophils serve both protective and destructive functions in periodontal tissues. While essential for initial pathogen control, their prolonged activation and impaired resolution contribute to tissue damage. The aging process significantly impacts neutrophil functions, contributing to periodontitis progression in elderly patients (65, 66).
7.3 Chemokines and age-related changes
The complex network of chemokines plays a crucial role in recruiting and activating immune cells in periodontal tissues. Age-related changes in chemokine expression and function contribute to the increased susceptibility to periodontal disease in older adults (67). Bioinformatics analyses have identified specific periodontitis-related genes and immune cells that are altered in the disease state (68).
7.4 Neurogenic inflammation: The AGE-neuron connection
A critical and emerging concept is the role of AGEs in neurogenic inflammation. Periodontal tissues are richly innervated by sensory nerves that not only transmit pain but also actively participate in inflammation by releasing neuropeptides like Substance P (SP) and Calcitonin Gene-Related Peptide (CGRP) (69). The relationship between nociceptor mechanisms and bone remodeling represents an important area of research, particularly in understanding how pain and tissue destruction are interconnected (70).
Crucially, RAGE is expressed on these sensory neurons (71). Emerging evidence indicates that AGE-RAGE interaction can directly activate and sensitize these neurons, potentially contributing to the hyperalgesia (increased pain) often associated with inflammatory conditions (72). This neuronal activation triggers the release of pro-inflammatory mediators from the neurons themselves, which then act on surrounding immune cells, creating a vicious neuro-immune amplification loop (73). This mechanism provides a compelling molecular link between the accumulation of AGEs and key clinical symptoms of periodontitis, such as pain and persistent inflammation, that are often challenging to manage, particularly in patients with high AGE levels, such as those with diabetes (74).
The concept of neuroinflammation as a distal consequence of periodontitis is gaining recognition, highlighting the systemic implications of local periodontal inflammation (75). This neuro-immune crosstalk represents a fundamental aspect of how periodontal inflammation can contribute to systemic diseases (76).
8 Integrating the network: a multi-dimensional view of AGEs in periodontitis
The pathogenic role of AGEs in periodontitis is not a linear process but a complex, interconnected network. A central theme is the bidirectional relationship between systemic AGE load and local periodontal pathology. Systemic conditions like diabetes and aging increase the accumulation of AGEs in periodontal tissues (5), where they exacerbate the host response to microbial plaque (8). Conversely, the chronic inflammation of periodontitis, along with the metabolic activity of pathogens like Tannerella forsythia, creates an endogenous source of new AGEs, establishing a vicious cycle (45).
This integrated network demonstrates how AGEs serve as a crucial molecular bridge connecting systemic health factors with localized destructive processes. The core of this network is the AGE-RAGE axis, which, upon activation, triggers a common set of detrimental outcomes across multiple periodontal cell types: increased oxidative stress, heightened pro-inflammatory cytokine production, upregulation of matrix-degrading enzymes (MMPs), and cellular dysfunction or apoptosis (see Figures 1-4).
The impact of AGEs is significantly amplified in the presence of bacterial components like lipopolysaccharide (LPS) (36), meaning that disease progression is likely to be most aggressive when both high AGE levels and significant bacterial load are present. Furthermore, the emerging neuro-immune component reveals that AGEs can directly activate sensory neurons via RAGE, triggering neurogenic inflammation that crosstalks with the immune system (72). This amplifies local inflammation and may contribute to clinical symptoms like pain and chronicity, offering a more complete picture of how AGEs orchestrate a multi-pronged attack on the periodontium.
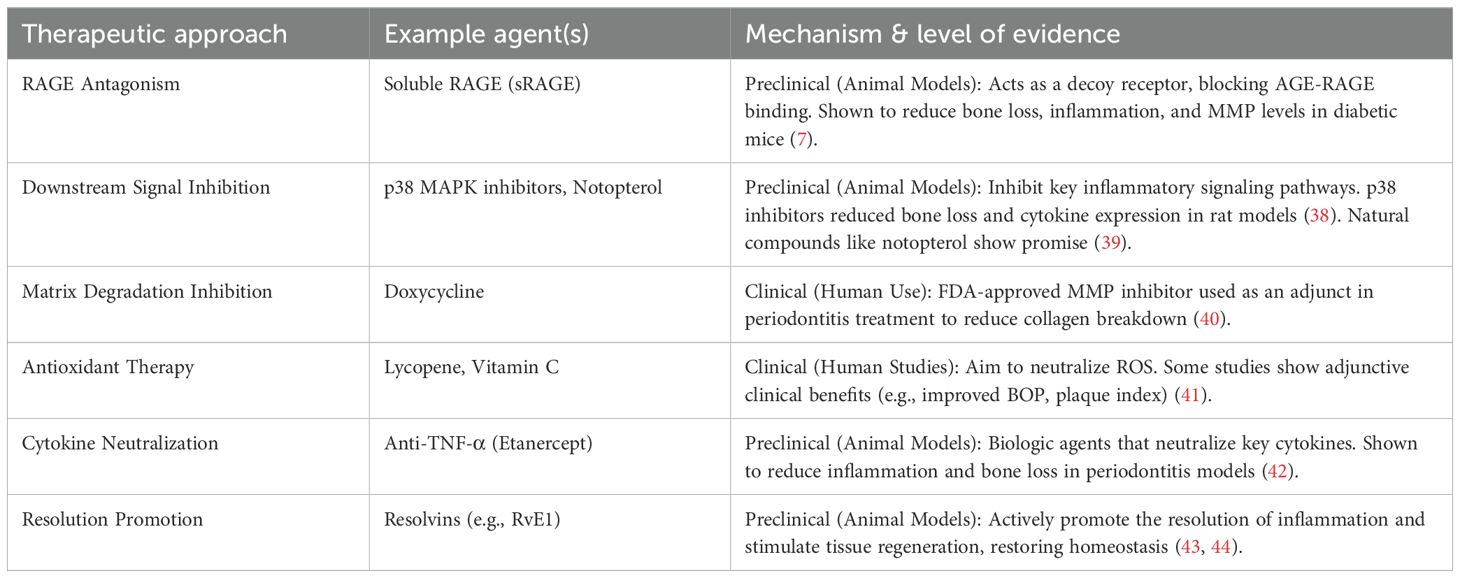
9 Therapeutic perspectives: targeting the AGE pathogenic network
The multi-dimensional involvement of AGEs in periodontitis offers several potential avenues for therapeutic intervention, from inhibiting their formation to promoting the resolution of inflammation (Table 3).
9.1 Inhibition of AGE formation and RAGE blockade
Strategies to disrupt the AGE network include inhibiting AGE formation (e.g., with aminoguanidine) or breaking existing cross-links (e.g., with alagebrium), though their specific efficacy in periodontitis requires dedicated research. A more promising approach is targeting the AGE-RAGE axis. Soluble RAGE (sRAGE), which acts as a decoy receptor, has shown significant therapeutic potential. In a preclinical diabetic mouse model, administration of sRAGE dose-dependently reduced alveolar bone loss, decreased pro-inflammatory cytokine and MMP levels in gingival tissues, and lowered gingival AGE accumulation (7, 80). This suggests sRAGE can break the vicious cycle of inflammation and AGE generation. Clinically, serum sRAGE levels negatively correlate with the Bleeding on Probing (BOP) index, a key clinical indicator of periodontal inflammation where a periodontal probe is used to assess bleeding from the gingival sulcus (77). However, translating sRAGE therapy to clinical use faces challenges, including the need to establish optimal dosing, delivery methods (systemic vs. local), and long-term safety. Additional research has shown that RAGE and its ligands play important roles in inflammatory and accelerated periodontal disease associated with diabetes, providing mechanistic insights into therapeutic modalities (78).
9.2 Modulation of downstream pathways
Targeting the signaling pathways activated by AGE-RAGE interaction offers another layer of intervention:
NF-κB and MAPK Inhibitors: Since NF-κB and MAPKs are key mediators of AGE-induced inflammation, their inhibition is a logical approach. Natural compounds like notopterol have shown promise in inhibiting the NF-κB pathway while activating protective PI3K/AKT/Nrf2 pathways in periodontal tissue (39). In preclinical models, inhibitors of p38 MAPK have been shown to reduce periodontal bone loss and inflammation (38). However, the broad and essential roles of these pathways in normal cellular function raise concerns about the potential for off-target effects with systemic, long-term use.
MMP Inhibitors: Sub-antimicrobial doses of doxycycline, an MMP inhibitor, are already FDA-approved for periodontitis treatment, validating this approach (40).
Antioxidants and Cytokine Inhibitors: The role of antioxidant enzymes in periodontitis is well-established (79), and antioxidants like lycopene have shown some adjunctive benefit in clinical studies (41). Biologic agents that neutralize specific cytokines like TNF-α or IL-6 have demonstrated efficacy in preclinical periodontitis models but carry the risk and cost associated with systemic immunosuppressive therapies (42).
9.3 Promoting resolution of inflammation
An emerging paradigm shift is to move beyond simply inhibiting inflammation and instead actively promoting its resolution. Specialized pro-resolving mediators (SPMs), such as resolvins and lipoxins, orchestrate the termination of inflammation and stimulate tissue regeneration (81). In preclinical studies, topical application of SPMs has been shown to control inflammation, promote bone regeneration, and even reverse microbial dysbiosis (43). This approach is highly promising as it aims to restore homeostasis rather than simply blocking a single pathway, potentially offering a more holistic and safer therapeutic strategy. The resolution of inflammation represents a novel approach to treating periodontal diseases, with significant clinical potential (44).
10 Conclusion and future directions
Advanced Glycation End Products are pivotal players in the complex pathogenesis of periodontitis. Their influence is multi-dimensional, spanning direct matrix destruction, profound immune dysregulation, and intricate neuro-immune interactions. The AGE-RAGE axis acts as a central engine, fueling a vicious cycle of chronic inflammation and tissue degradation that is particularly devastating in high-risk populations, such as individuals with diabetes and the elderly.
A key message of this review is the need to look beyond the classical understanding of AGEs and focus on the emerging frontier of neuro-immune crosstalk. The ability of AGEs to directly activate sensory neurons provides a compelling molecular basis for clinical features like pain and chronicity, bridging the gap between molecular pathology and patient experience. This positions periodontitis as an important clinical model for studying the systemic consequences of AGE accumulation and local neuro-inflammatory processes.
The elucidation of this complex network has paved the way for novel host-modulatory therapies. While strategies targeting RAGE, downstream signaling, and oxidative stress have shown promise in preclinical models, significant hurdles remain for clinical translation, including specificity and long-term safety. In this context, therapies based on specialized pro-resolving mediators, which aim to restore homeostasis, represent a particularly exciting path forward.
Future research should focus on: (1) elucidating the specific roles of different AGE species in neuro-immune interactions; (2) conducting robust clinical trials to translate promising preclinical findings into effective human therapies; and (3) developing reliable biomarkers to identify patients who would most benefit from AGE-targeted interventions. A holistic approach that considers the systemic and local impact of AGEs is imperative for future therapeutic innovation in periodontal medicine.
Author contributions
Y-LD: Conceptualization, Investigation, Methodology, Visualization, Software, Writing – original draft. HL: Writing – review & editing, Software, Validation, Visualization. TW: Conceptualization, Investigation, Writing – review & editing. Z-LG: Project administration, Supervision, Writing – review & editing, Funding acquisition, Resources.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (Grant No. 82201080), the High-level Talents Project of Hainan Natural Science Foundation (Grant No. 821RC687), and the Academic Enhancement Support Program of Hainan Medical University (Grant No. XSTS2025027).
Acknowledgments
The authors sincerely thank the institutional support provided by Hainan Medical University and its affiliated hospitals.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AGE, Advanced Glycation End Product; BOP, Bleeding on Probing; CEL, Nϵ-(carboxyethyl)lysine; CGRP, Calcitonin Gene-Related Peptide; CML, Nϵ-(carboxymethyl)lysine; DC, Dendritic Cell; ECM, Extracellular Matrix; GCF, Gingival Crevicular Fluid; HGF, Human Gingival Fibroblast; HPLF, Human Periodontal Ligament Fibroblast; LPS, Lipopolysaccharide; MAPK, Mitogen-Activated Protein Kinase; MGO, Methylglyoxal; MMP, Matrix Metalloproteinase; NF-κB, Nuclear Factor Kappa-light-chain-enhancer of Activated B cells; PDL, Periodontal Ligament; RAGE, Receptor for Advanced Glycation End Products; ROS, Reactive Oxygen Species; SP, Substance P; SPM, Specialized Pro-resolving Mediator; sRAGE, Soluble Receptor for Advanced Glycation End Products; TIMP, Tissue Inhibitor of Metalloproteinase.
References
1. Gasner NS and Schure RS. Periodontal disease. In: Statpearls. StatPearls Publishing LLC, Treasure Island (FL (2024).
2. Saini R, Marawar PP, Shete S, and Saini S. Periodontitis, a true infection. J Glob Infect Dis. (2009) 1:149–50. doi: 10.4103/0974-777x.56251
3. Könönen E, Gursoy M, and Gursoy UK. Periodontitis: A multifaceted disease of tooth-supporting tissues. J Clin Med. (2019) 8:1135. doi: 10.3390/jcm8081135
4. Price CL, Sharp PS, North ME, Rainbow SJ, and Knight SC. Advanced glycation end products modulate the maturation and function of peripheral blood dendritic cells. Diabetes. (2004) 53:1452–8. doi: 10.2337/diabetes.53.6.1452
5. Jin X, Yao T, Zhou Z, Zhu J, Zhang S, Hu W, et al. Advanced Glycation End Products Enhance Macrophages Polarization into M1 Phenotype through Activating RAGE/NF-κB Pathway. BioMed Res Int. (2015) 2015:732450. doi: 10.1155/2015/732450
6. Uribarri J, del Castillo MD, de la Maza MP, Filip R, Gugliucci A, and Luevano-Contreras C Dietary advanced glycation end products and their role in health and disease. Adv Nutr. (2015) 6:461–73. doi: 10.3945/an.115.008433
7. Lalla E, Lamster I B, Feit M, Huang L, Spessot A, Qu W, et al. Blockade of rage suppresses periodontitis-associated bone loss in diabetic mice. J Clin Invest. (2000) 105:1117–24. doi: 10.1172/jci8942
8. Chopra A, Jayasinghe TN, and Eberhard J. Are inflamed periodontal tissues endogenous source of advanced glycation end-products (ages) in individuals with and without diabetes mellitus? A systematic review. Biomolecules. (2022) 12:642. doi: 10.3390/biom12050642
9. Schmidt AM, Weidman E, Lalla E, Yan SD, Hori O, and Cao R Advanced glycation endproducts (ages) induce oxidant stress in the gingiva: A potential mechanism underlying accelerated periodontal disease associated with diabetes. J Periodontal Res. (1996) 31:508–15. doi: 10.1111/j.1600-0765.1996.tb01417.x
10. Hussein LK and Mohammed AN. Assessment of serum advanced glycation end-product level and its effect on periodontal health status in type 2 diabetic patients with chronic periodontitis. Indian J Forensic Med Toxicology. (2020) 14:609–15. doi: 10.37506/ijfmt.v14i1.117
11. Inan-Eroglu E, Ayaz A, and Buyuktuncer Z. Formation of advanced glycation endproducts in foods during cooking process and underlying mechanisms: A comprehensive review of experimental studies. Nutr Res Rev. (2020) 33:77–89. doi: 10.1017/S0954422419000209
12. Nowotny K, Schröter D, Schreiner M, and Grune T. Dietary advanced glycation end products and their relevance for human health. Ageing Res Rev. (2018) 47:55–66. doi: 10.1016/j.arr.2018.06.005
13. Santos AL and Lindner AB. Protein posttranslational modifications: Roles in aging and age-related disease. Oxid Med Cell Longevity. (2017) 2017:5716409. doi: 10.1155/2017/5716409
14. Garay-Sevilla ME, Rojas A, Portero-Otin M, and Uribarri J. Dietary ages as exogenous boosters of inflammation. Nutrients. (2021) 13:2802. doi: 10.3390/nu13082802
15. Nowotny K, Jung T, Höhn A, Weber D, and Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. (2015) 5:194–222. doi: 10.3390/biom5010194
16. Sharifi-Zahabi E, Sharafabad FH, Abdollahzad H, Malekahmadi M, and Rad NB. Circulating advanced glycation end products and their soluble receptors in relation to all-cause and cardiovascular mortality: A systematic review and meta-analysis of prospective observational studies. Adv Nutr. (2021) 12:2157–71. doi: 10.1093/advances/nmab072
17. Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, and Simm A Role of advanced glycation end products in cellular signaling. Redox Biol. (2014) 2:411–29. doi: 10.1016/j.redox.2013.12.016
18. Origlia N, Arancio O, Domenici L, and Yan SS. Mapk, beta-amyloid and synaptic dysfunction: The role of rage. Expert Rev Neurother. (2009) 9:1635–45. doi: 10.1586/ern.09.107
19. Wang ZQ, Yao HP, and Sun Z. N(ϵ)-(carboxymethyl)lysine promotes lipid uptake of macrophage via cluster of differentiation 36 and receptor for advanced glycation end products. World J Diabetes. (2023) 14:222–33. doi: 10.4239/wjd.v14.i3.222
20. Alikhani M, Alikhani Z, Boyd C, MacLellan CM, Raptis M, and Liu R Advanced glycation end products stimulate osteoblast apoptosis via the map kinase and cytosolic apoptotic pathways. Bone. (2007) 40:345–53. doi: 10.1016/j.bone.2006.09.011
21. Ahmed MU, Brinkmann Frye E, Degenhardt TP, Thorpe SR, and Baynes JW. N-epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem J. (1997) 324:565–70. doi: 10.1042/bj3240565
22. Lin YY, Huang SF, Liao KW, Ho CT, and Hung WL. Quantitation of α-dicarbonyls, lysine- and arginine-derived advanced glycation end products, in commercial canned meat and seafood products. J Agric Food Chem. (2023) 71:6727–37. doi: 10.1021/acs.jafc.3c01205
23. Damrath JG, Creecy A, Wallace JM, and Moe SM. The impact of advanced glycation end products on bone properties in chronic kidney disease. Curr Opin Nephrol Hypertens. (2021) 30:411–7. doi: 10.1097/MNH.0000000000000713
24. Ren L, Fu Y, Deng Y, Qi L, and Jin L. Advanced glycation end products inhibit the expression of collagens type i and iii by human gingival fibroblasts. J Periodontol. (2009) 80:1166–73. doi: 10.1902/jop.2009.080669
25. Sharma A. Virulence mechanisms of tannerella forsythia. Periodontol. (2010) 54:106–16. doi: 10.1111/j.1600-0757.2009.00332.x
26. Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: Keystones, pathobionts, and host response. Trends Immunol. (2014) 35:3–11. doi: 10.1016/j.it.2013.09.001
27. Bertolini M and Clark D. Periodontal disease as a model to study chronic inflammation in aging. Geroscience. (2023) 46:3695–709. doi: 10.1007/s11357-023-00835-0
28. Amezcua-Castillo E, González-Pacheco H, Sáenz-San Martín A, Méndez-Ocampo P, Gutierrez-Moctezuma I, and Massó F C-reactive protein: The quintessential marker of systemic inflammation in coronary artery disease-advancing toward precision medicine. Biomedicines. (2023) 11:360–5. doi: 10.3390/biomedicines11092444
29. Nazir M, Al-Ansari A, Al-Khalifa K, Alhareky M, Gaffar B, and Almas K. Global prevalence of periodontal disease and lack of its surveillance. Sci World J. (2020) 2020:2146160. doi: 10.1155/2020/2146160
30. Seyedmoalemi MA and Saied-Moallemi Z. Association between periodontitis and alzheimer’s disease: A narrative review. IBRO Neurosci Rep. (2024) 18:360–5. doi: 10.1016/j.ibneur.2024.12.004
31. Verhulst MJL, Loos BG, Gerdes VEA, and Teeuw WJ. Evaluating all potential oral complications of diabetes mellitus. Front Endocrinol (Lausanne). (2019) 10:56. doi: 10.3389/fendo.2019.00056
32. Cheng R, Choudhury D, Liu C, Billet S, Hu T, and Bhowmick NA. Gingival fibroblasts resist apoptosis in response to oxidative stress in a model of periodontal diseases. Cell Death Discov. (2015) 1:15046. doi: 10.1038/cddiscovery.2015.46
33. Murillo J, Wang Y, Xu X, Klebe RJ, Chen Z, and Zardeneta G Advanced glycation of type i collagen and fibronectin modifies periodontal cell behavior. J Periodontol. (2008) 79:2190–9. doi: 10.1902/jop.2008.080210
34. Lee H, Lim J, Oh JH, Cho S, and Chung JH. IGF-1 upregulates biglycan and decorin by increasing translation and reducing ADAMTS5 expression. Int J Mol Sci. (2021) 22:1403. doi: 10.3390/ijms22031403
35. Kim S, Ahn SH, Lee JS, Song JE, Cho SH, Jung S, et al. Differential matrix metalloprotease (mmp) expression profiles found in aged gingiva. PloS One. (2016) 11:e0158777. doi: 10.1371/journal.pone.0158777
36. Sroga GE and Vashishth D. In vivo glycation—interplay between oxidant and carbonyl stress in bone. JBMR Plus. (2024) 8:ziae110. doi: 10.1093/jbmrpl/ziae110
37. Settem RP and Sharma A. Oral bacterium contributes to periodontal inflammation by forming advanced glycation end products. Infection Immunity. (2023) 91:e00560–22. doi: 10.1128/iai.00560-22
38. Boelen GJ, Boute L, d’Hoop J, EzEldeen M, Lambrichts I, and Opdenakker G. Matrix metalloproteinases and inhibitors in dentistry. Clin Oral Investigations. (2019) 23:2823–35. doi: 10.1007/s00784-019-02915-y
39. Li Q, Valerio MS, and Kirkwood KL. MAPK usage in periodontal disease progression. J Signal Transduction. (2012) 2012:308943. doi: 10.1155/2012/308943
40. Lee HM, Ciancio SG, Tüter G, Ryan ME, Komaroff E, and Golub LM. Subantimicrobial dose doxycycline efficacy as a matrix metalloproteinase inhibitor in chronic periodontitis patients is enhanced when combined with a non-steroidal anti-inflammatory drug. J Periodontol. (2004) 75:453–63. doi: 10.1902/jop.2004.75.3.453
41. Mazurek-Mochol M, Bonsmann T, Mochol M, Poniewierska-Baran A, and Pawlik A. The role of interleukin 6 in periodontitis and its complications. Int J Mol Sci. (2024) 25:2146. doi: 10.3390/ijms25042146
42. Van Dyke TE. Pro-resolving mediators in the regulation of periodontal disease. Mol Aspects Med. (2017) 58:21–36. doi: 10.1016/j.mam.2017.04.006
43. Neurath N and Kesting M. Cytokines in gingivitis and periodontitis: From pathogenesis to therapeutic targets. Front Immunol. (2024) 15:1435054. doi: 10.3389/fimmu.2024.1435054
44. Van Dyke TE. Resolution of inflammation in periodontitis. Semin Immunol. (2018) 40:140–7. doi: 10.1016/j.smim.2018.06.007
45. Kashket S, Maiden MFJ, Haffajee AD, and Kashket ER. Accumulation of methylglyoxal in the gingival crevicular fluid of chronic periodontitis patients. J Clin Periodontology. (2003) 30:364–7. doi: 10.1034/j.1600-051x.2003.00322.x
46. Settem RP, Honma K, Shankar M, Li M, LaMonte M, Xu D, et al. Tannerella forsythia-produced methylglyoxal causes accumulation of advanced glycation endproducts to trigger cytokine secretion in human monocytes. Mol Oral Microbiol. (2018) 33:292–9. doi: 10.1111/omi.12224
47. Mo K, Wang Y, Lu C, and Li Z. Insight into the role of macrophages in periodontitis restoration and development. Virulence. (2024) 15:2427234. doi: 10.1080/21505594.2024.2427234
48. Caturano A, D’Angelo M, Mormone A, Pafundi PC, Galiero R, Rinaldi L, et al. Oxidative stress in type 2 diabetes: Impacts from pathogenesis to lifestyle modifications. Curr Issues Mol Biol. (2023) 45:6651–66. doi: 10.3390/cimb45080420
49. Thomas CJ, Cleland TP, Sroga GE, and Vashishth D. Accumulation of carboxymethyl-lysine (cml) in human cortical bone. Bone. (2018) 110:128–33. doi: 10.1016/j.bone.2018.01.028
50. Cresci-Anne CC, Croes C, Chrysanthou M, Hoppenbrouwers T, Wichers H, Keijer J, et al. Diabetic glycation of human serum albumin affects its immunogenicity. Biomolecules. (2024) 14:1–30. doi: 10.3390/biom14121492
51. Yoshida K, Yoneda T, Fujimoto K, Hirao Y, and Konishi N. Pentosidine and its deposition in renal tissue in renal transplantation. Transplant Proc. (2005) 37:4266–72. doi: 10.1016/j.transproceed.2005.11.018
52. Mirnic J, Djuric M, Brkic S, Andric M, Cekic J, Vujovic A, et al. Pathogenic mechanisms that may link periodontal disease and type 2 diabetes mellitus-the role of oxidative stress. Int J Mol Sci. (2024) 25:9806. doi: 10.3390/ijms25189806
53. Tenchov R, Sasso JM, Wang X, and Zhou QA. Aging hallmarks and progression and age-related diseases: A landscape view of research advancement. ACS Chem Neurosci. (2024) 15:1–30. doi: 10.1021/acschemneuro.3c00531
54. Nędzi-Góra M, Kostrzewa-Janicka J, and Górska R. Elastase and metalloproteinase-9 concentrations in saliva in patients with chronic periodontitis. Cent Eur J Immunol. (2014) 39:357–64. doi: 10.5114/ceji.2014.45948
55. Boynes SG, Sofiyeva N, Saw T, Nieto V, and Palomo L. Assessment of salivary matrix metalloproteinase (MMP8) and activated salivary matrix metalloproteinase (ammp8) in periodontitis patients: A systematic review and meta-analysis. Front Oral Health. (2023) 4:1254183. doi: 10.3389/froh.2023.1254183
56. Yu S, Li H, Ma Y, and Fu Y. Matrix metalloproteinase-1 of gingival fibroblasts influenced by advanced glycation end products (AGEs) and their association with receptor for AGEs and nuclear factor-κB in gingival connective tissue. J Periodontology. (2012) 83:119–26. doi: 10.1902/jop.2011.100754
57. Naiff P, Carneiro V, and Guimarães MDC. Importance of mechanical periodontal therapy in patients with diabetes type 2 and periodontitis. Int J Dent. (2018) 2018:6924631. doi: 10.1155/2018/6924631
58. Li DX, Deng TZ, Lv J, and Ke J. Advanced glycation end products (AGEs) and their receptor (RAGE) induce apoptosis of periodontal ligament fibroblasts. Braz J Med Biol Res. (2014) 47:1036–43. doi: 10.1590/1414-431x20143996
59. Cheng R, Choudhury D, Liu C, Billet S, Hu T, and Bhowmick NA Effect of aging on periodontal inflammation, microbial colonization, and disease susceptibility. J Dent Res. (2016) 95:460–6. doi: 10.1177/0022034515625962
60. Agrawal A and Gupta S. Impact of aging on dendritic cell functions in humans. Ageing Res Rev. (2011) 10:336–45. doi: 10.1016/j.arr.2010.06.004
61. Huang N, Dong H, Luo Y, and Shao B. Th17 cells in periodontitis and its regulation by a20. Front Immunol. (2021) 12:766614. doi: 10.3389/fimmu.2021.766614
62. Demoersman J, Pochard P, Framery C, Simon Q, Boisramé S, and Soueidan A B cell subset distribution is altered in patients with severe periodontitis. PloS One. (2018) 13:e0192986. doi: 10.1371/journal.pone.0192986
63. Zhu M, Belkina AC, DeFuria J, Carr JD, Van Dyke TE, and Gyurko R B cells promote obesity-associated periodontitis and oral pathogen-associated inflammation. J Leukoc Biol. (2014) 96:349–57. doi: 10.1189/jlb.4A0214-095R
64. Hajishengallis G, Moutsopoulos NM, Hajishengallis E, and Chavakis T. Immune and regulatory functions of neutrophils in inflammatory bone loss. Semin Immunol. (2016) 28:146–58. doi: 10.1016/j.smim.2016.02.002
65. Wang Z, Saxena A, Yan W, Uriarte SM, Siqueira R, and Li X The impact of aging on neutrophil functions and the contribution to periodontitis. Int J Oral Sci. (2024) 17:10. doi: 10.1038/s41368-024-00332-w
66. Chaudhary JK, Danga AK, Kumari A, Bhardwaj A, and Rath PC. Role of chemokines in aging and age-related diseases. Mech Ageing Dev. (2024) 223:112009. doi: 10.1016/j.mad.2024.112009
67. Ban Q, Ling X, Ding H, Meng J, Li Y, and Zhang J Screening of periodontitis-related genes and immune cells based on an integrated bioinformatics analysis. Ann Transl Med. (2022) 10:558. doi: 10.21037/atm-22-1592
68. Wang S, Ko CC, and Chung MK. Nociceptor mechanisms underlying pain and bone remodeling via orthodontic forces: Toward no pain, big gain. Front Pain Res. (2024) 5:1365194. doi: 10.3389/fpain.2024.1365194
69. Juranek J, Mukherjee K, Kordas B, Dhawan P, Manivannan E, Sas AR, et al. Role of RAGE in the pathogenesis of neurological disorders. Neurosci Bulletin. (2022) 38:1248–62. doi: 10.1007/s12264-022-00878-x
70. Caviedes-Bucheli J, Lopez-Moncayo LF, Muñoz-Alvear HD, Gomez-Sosa JF, Diaz-Barrera LE, and Curtidor H Expression of substance p, calcitonin gene-related peptide and vascular endothelial growth factor in human dental pulp under different clinical stimuli. BMC Oral Health. (2021) 21:152. doi: 10.1186/s12903-021-01519-x
71. Nair M, Jagadeeshan S, Katselis G, Chakraborty J, Bhat A, Ghosh A, et al. Lipopolysaccharides induce a RAGE-mediated sensitization of sensory neurons and fluid hypersecretion in the upper airways. Sci Rep. (2021) 11:8336. doi: 10.1038/s41598-021-86069-6
72. Begum R, Thota S, Abdulkadir A, Ahanger SH, Kain V, Rani R, et al. NADPH oxidase family proteins: Signaling dynamics to disease management. Cell Mol Immunol. (2022) 19:660–86. doi: 10.1038/s41423-022-00858-1
73. Al-Maskari AY, Al-Maskari MY, and Al-Sudairy S. Oral manifestations and complications of diabetes mellitus: A review. Sultan Qaboos Univ Med J. (2011) 11:179–86. doi: 10.18295/2075-0528.1261
74. Li X, Kiprowska M, Kansara T, Kansara P, and Li P. Neuroinflammation: A distal consequence of periodontitis. J Dental Res. (2022) 101:1441–9. doi: 10.1177/00220345221102084
75. Martínez-García M and Hernández-Lemus E. Periodontal inflammation and systemic diseases: An overview. Front Physiol. (2021) 12:709438. doi: 10.3389/fphys.2021.709438
76. Toraman A, Arabaci T, Aytekin Z, Albayrak M, and Bayir Y. Effects of vitamin C local application on ligature-induced periodontitis in diabetic rats. J Appl Oral Science. (2020) 28:e20200444. doi: 10.1590/1678-7757-2020-0444
77. Lalla E, Lamster IB, Stern DM, and Schmidt AM. Receptor for advanced glycation end products, inflammation, and accelerated periodontal disease in diabetes: Mechanisms and insights into therapeutic modalities. Ann Periodontol. (2001) 6:113–8. doi: 10.1902/annals.2001.6.1.113
78. Zhou J, Shi P, Ma R, Zheng C, Zhang H, Tang K, et al. Notopterol inhibits the NF-κB pathway and activates the PI3K/AKT/Nrf2 pathway in periodontal tissue. J Immunol. (2023) 211:1516–25. doi: 10.4049/jimmunol.2200727
79. López-Valverde N, López-Valverde A, Macedo de Sousa B, and Blanco Rueda JA. Systematic review and meta-analysis of the antioxidant capacity of lycopene in the treatment of periodontal disease. Front Bioengineering Biotechnol. (2023) 11:1309851. doi: 10.3389/fbioe.2023.1309851
80. Ruby J and Barbeau J. The buccale puzzle: The symbiotic nature of endogenous infections of the oral cavity. Can J Infect Diseases. (2002) 13:34–41. doi: 10.1155/2002/492656
Keywords: advanced glycation end products (AGEs), periodontitis, extracellular matrix remodeling, neuro-immune regulation, oxidative stress and inflammation
Citation: Dong Y-L, Lin H, Wen T and Guo Z-L (2025) Multi-dimensional role of AGEs in periodontitis: from matrix remodeling to neuro-immune crosstalk. Front. Immunol. 16:1643685. doi: 10.3389/fimmu.2025.1643685
Received: 09 June 2025; Accepted: 26 September 2025;
Published: 17 November 2025.
Edited by:
Zejian Li, First Affiliated Hospital of Jinan University, ChinaReviewed by:
Cristina Florescu, University of Medicine and Pharmacy of Craiova, RomaniaVerónica Villalobos, University of Chile, Chile
Chenghu Yin, Sun Yat-sen University, China
Copyright © 2025 Dong, Lin, Wen and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhu-Ling Guo, emxndW9AeW5wZnBoLm9yZw==
†These authors have contributed equally to this work
 Yu-Lei Dong1,2†
Yu-Lei Dong1,2† Zhu-Ling Guo
Zhu-Ling Guo