- 1Department of General Surgery, The Chenggong Hospital (the 73rd Group Military Hospital of PLA), Xiamen University, Xiamen, Fujian, China
- 2Fifth Department of Hepatobiliary Surgery, The Eastern Hepatobiliary Surgery Hospital, Second Military Medical University (Navy Medical University), Shanghai, China
Background: The TOPAZ-1 study results represented significant advancement in the treatment of advanced biliary tract cancer (BTC) by combining durvalumab with gemcitabine–cisplatin (DGC). However, the highly selected patient population may not reflect the real-world scenarios. To gain deeper insights into this combination regimen, we conducted an evidence collection and a mimic survival comparative analysis.
Methods: Records were identified through a formal search of PubMed and Web of Science. Six retrospective cohort studies with real-world evidence were definitively included. The individual patient data for OS and PFS were reconstructed and analyzed. The outcomes different from TOPAZ-1 were summarized and compared.
Results: Whether Asia or non-Asia group, the mOS was similar to the TOPAZ-1 (Asian group: 12.57 months vs. TOPAZ-1, HR = 0.91, 95% CI: 0.69-1.21, log rank P = 0.53; non-Asian group: 13.61 months vs. TOPAZ-1, HR = 1.10, 95% CI: 0.91-1.31, log rank P = 0.323). The mPFS for the Asian group did not show significant differences compared with TOPAZ-1 (5.63 months vs. TOPAZ-1, HR = 1.09, 95% CI: 0.88-1.35, log rank P = 0.422), whereas for the non-Asian group differences exist (6.58 months vs. TOPAZ-1, HR = 0.80, 95% CI: 0.70-0.92, log rank P = 0.002), but potentially influenced by patient ethnicity. The disease control rate in the real world was not so favorable as that in TOPAZ-1. The most common adverse events (AEs) in real-world scenarios were fatigue (26.01%), leukopenia (24.64%), anemia (24.30%), and thrombocytopenia (21.14%). The incidence of immune-related AEs of grades 3–4 was slightly higher in the real world compared with TOPAZ-1 (4.0% vs. 2.4%). Factors such as ECOG-PS, age, alternative doses of durvalumab, neutrophil-to-lymphocyte ratio (NLR), baseline CEA levels, baseline CA19–9 levels, and metastatic disease could be prognostic factors under DGC regimen, with NLR showing a potential as a predictive marker for survival benefit.
Conclusions: The efficacy and safety of the DGC regimen for patients with advanced BTC are confirmed through a comparative analysis and aggregation of real-world evidence in this study. Further real-world investigations are still warranted to determine if the DGC regimen has a broader therapeutic indication and to identify predictive markers for survival benefit. Efforts are required to improve the cost-effectiveness of the DGC regimen to facilitate its wider and standardized use.
1 Introduction
Biliary tract cancer (BTC) encompasses a group of highly aggressive malignant tumors originating from the biliary tree, including intrahepatic duct, extrahepatic bile duct, and gallbladder carcinoma (GBC) (1). Extrahepatic CCAs (eCCAs) are further classified into perihilar and distal CCAs according to anatomical location (2). Recent data indicate a rise in the global incidence (3–5), accompanied by a poor prognosis that 5-year overall survival rates range from 5% to 15% (6, 7). There is significant geographic variation in the incidence of BTC and its subtypes. In the Western world, intrahepatic cholangiocarcinoma (iCC) is the most common subtype of BTC, whereas in India, GBC is the most prevalent subtype, accounting for 10% of the global GBC burden (8). The only curative option is surgery followed by adjuvant chemotherapy, but only 20% patients with BTC are eligible for surgery at the time of diagnosis (9, 10). For the treatment for advanced BTC, gemcitabine was first established as a valid option in 1996. Then in 2010, the phase III study ABC-02 trial investigated gemcitabine in combination with cisplatin (GC) against gemcitabine alone, demonstrating a statistically significant improvement in median overall survival (11.7 vs. 8.1 months). This established a new standard of care for advanced BTC. Nevertheless, the predicted 24-month survival rate was only 15% (11).
In the past decade, treatment for advanced BTC remained unchanged. Until 2022, the TOPAZ-1 study results introduced a breakthrough. The combination of durvalumab with chemotherapy (durvalumab plus gemcitabine–cisplatin, DGC) first achieved a median overall survival exceeding 1 year, along with improved progression-free survival (PFS) and higher objective response rates (ORR) to therapy (12). The overall survival (OS) benefit with DGC was seen across all clinically relevant subgroups, and the 24-month overall survival rate with DGC was approximately doubled compared with placebo plus GC in the participants with a complete or partial response and for those with stable disease (13). Then, the European Medicines Agency (EMA) and the United States (US) Food and Drug Administration (FDA) approved DGC as the new first-line standard of care for patients with untreated, metastatic, or unresectable BTC.
However, due to the highly selected patient population enrolled in the TOPAZ-1 trial, it may not reflect the real-world clinical scenarios. During or after the TOPAZ-1 study, multiple centers worldwide actively attempted to implement this treatment regimen. Following the release of the TOPAZ-1 results, various real-world clinical outcomes were reported and compared with those from TOPAZ-1. However, international collaboration in this field remains limited. Therefore, this study gathered results from recent global real-world clinical studies on durvalumab plus chemotherapy, reconstructed the patient survival data by utilizing their KM curves, and conducted simulation comparative analysis. The aim is to provide more real-world insights into the effectiveness of durvalumab in the treatment of advanced BTC.
2 Methods
Study selection
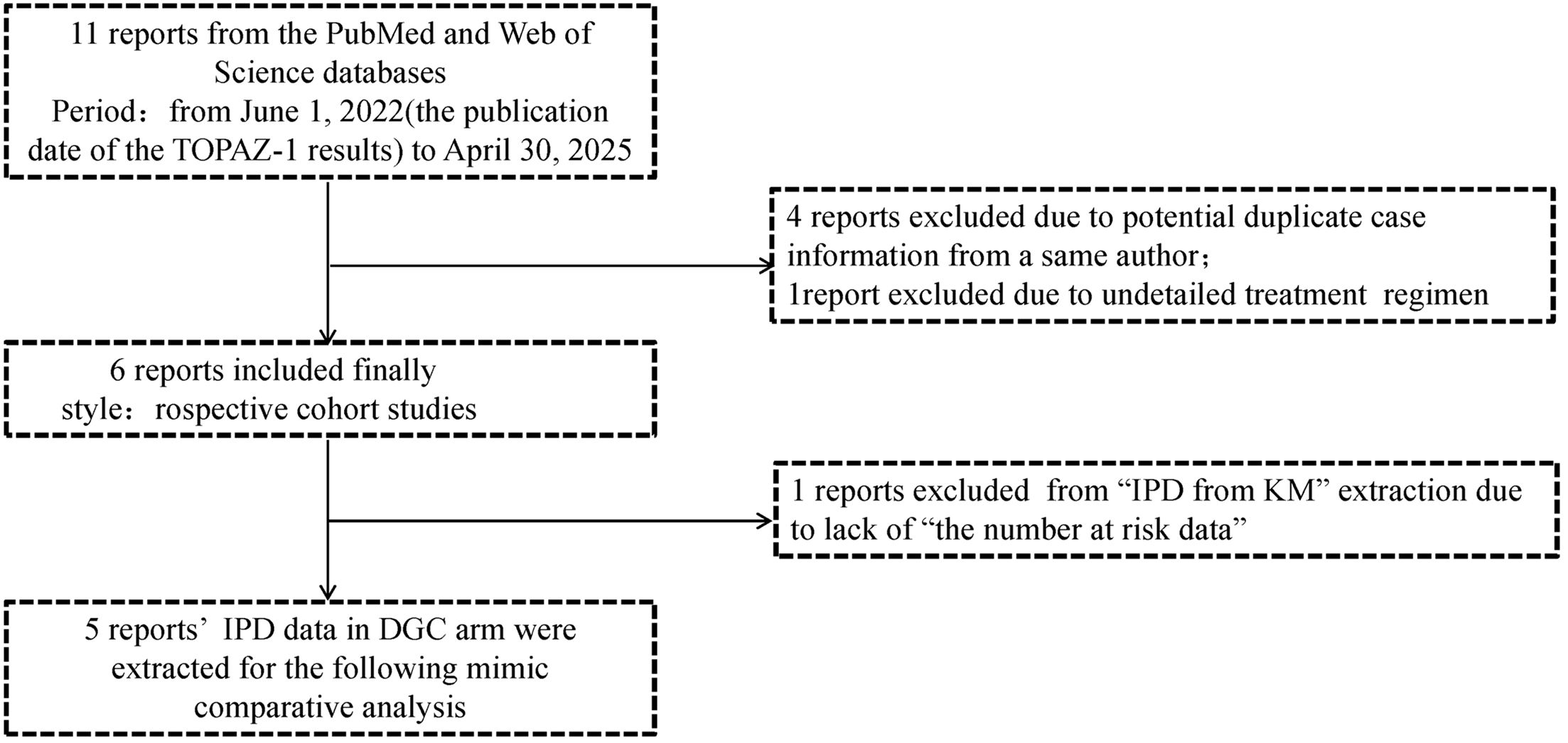
In this mimic comparative analysis by survival data reconstruction, a total of 11 studies were initially collected. A systematic search was conducted on PubMed and Web of Science from June 1, 2022 (the publication date of the TOPAZ-1 results), to April 30, 2025. Search terms included “durvalumab”, “real-world or real life”, “biliary tract cancer or BTC or cholangiocarcinoma”, “gallbladder cancer”, “unresectable or metastatic or advanced”. Each record was screened by two authors (Hong-xiang Ji and Ma-Hui Si). Considering the duplicate case information, four previous reports from a same author (Rimini M) were excluded. Another report was excluded due to an unclear treatment regimen, resulting in the final inclusion of 6 reports. This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline for individual patient data (IPD). (14, Supplementary 1).
2.1 Quality assessment and IPD extraction from reported Kaplan–Meier curves
The risk of bias among the trials was evaluated by Hong-xiang Ji and Ma-Hui Si using the Cochrane Risk of Bias Tool and Newcastle–Ottawa Scale (NOS) (15). Disagreements were discussed and resolved by a third reviewer (Ning Yang). The OS and PFS Kaplan–Meier curves and the number at risk data from the durvalumab plus chemotherapy arm were extracted from the eligible trials. Since one report (16) has not provided the number at risk data, which was important for survival data reconstruction, we decided to exclude it from “IPD from KM” extraction but still collected its positive outcomes.
2.2 Reconstruction of survival data and mimic comparative analysis
The methods for data reconstruction referred to the report from Liu et al. and utilized the Shiny application (https://www.trialdesign.org/one-page-shell.html# IPDfromKM) following its user guide (17). The quality of the reconstructed patient-level data was assessed by inspecting the shape of the survival curves, survival outcomes, survival rates, and hazard ratios (HRs). The IPD data reconstruction process was repeated more than three times by two different authors (Hong-xiang Ji and Ma-Hui Si) for each report to obtain a dataset which can best reproduce the original results. The final version of the dataset was determined by a third author (Zhe Sun) based on quality assessment, and then the necessary data synthesis for the study was performed. Mimic survival comparative analyses were also conducted using the Shiny application. A P value < 0.05 was considered statistically significant.
3 Results
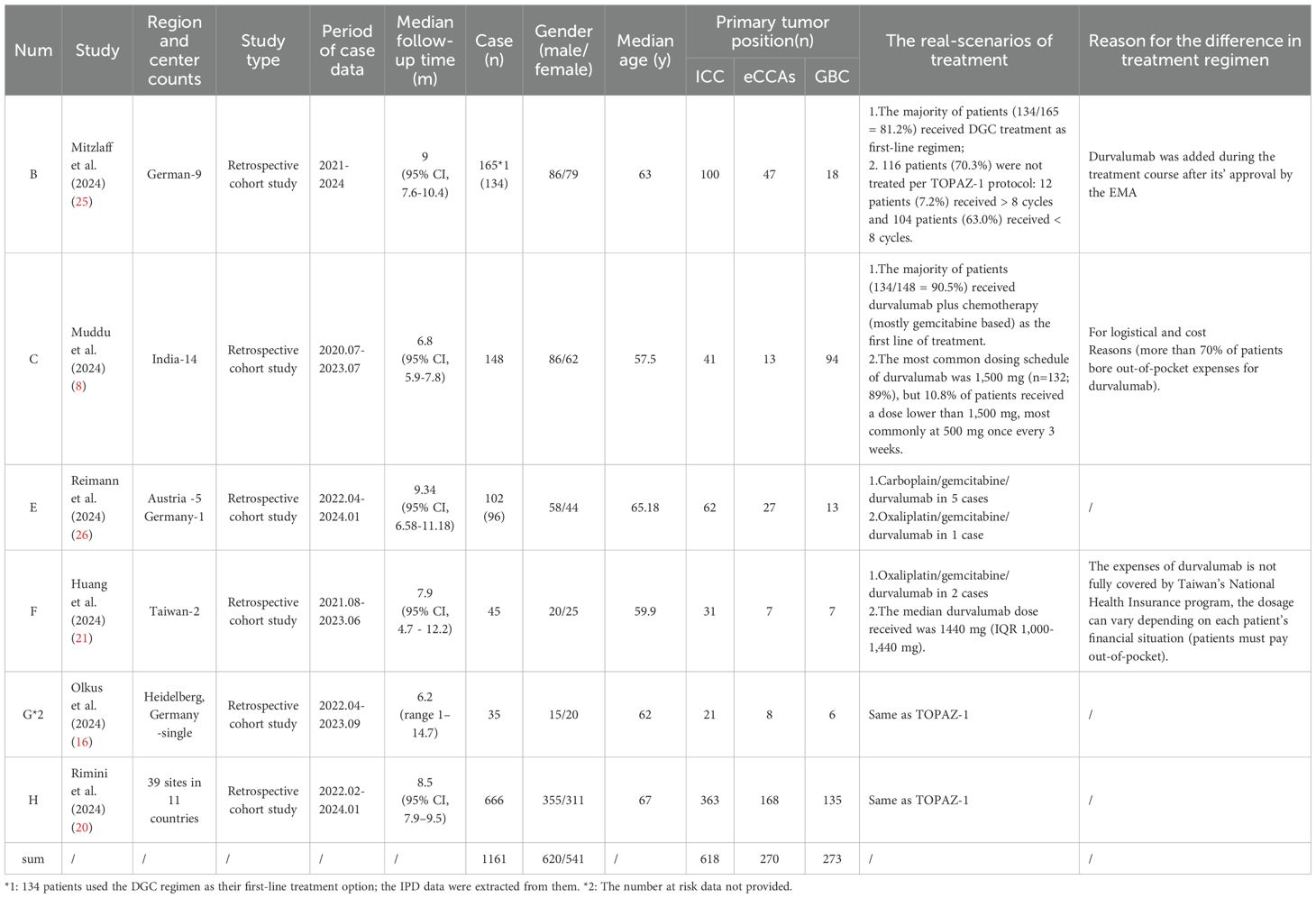
Through systematic searching, 11 reports (8, 16, 18–26) were initially collected from the PubMed and Web of Science databases, but we found that five in these reports were authored by a same researcher from Rimini M, Italy. After careful screening, we excluded four (18, 19, 22, 23) of these five reports due to potential duplicate case information, retaining the one (20) with the largest case records and the most recent publication date. One more report (24) was excluded due to undetailed treatment regimen. Ultimately, six reports were included in this study; the basic characteristics of these reports are listed in Table 1. All of them were retrospective cohort studies and published in 2024. Within them, five reports rated as high-quality (≥7 stars) (NOS scores ranged from 5 to 9) and all clearly defined exposure (Supplementary 2). During the IPD data reconstruction process, we found one additional report (Olkus A et al., 2024) that did not provide the number at risk data, which led us to decide to exclude it from “IPD from KM” extraction but still collected its positive outcomes. The strategy of report collection is shown in Figure 1.
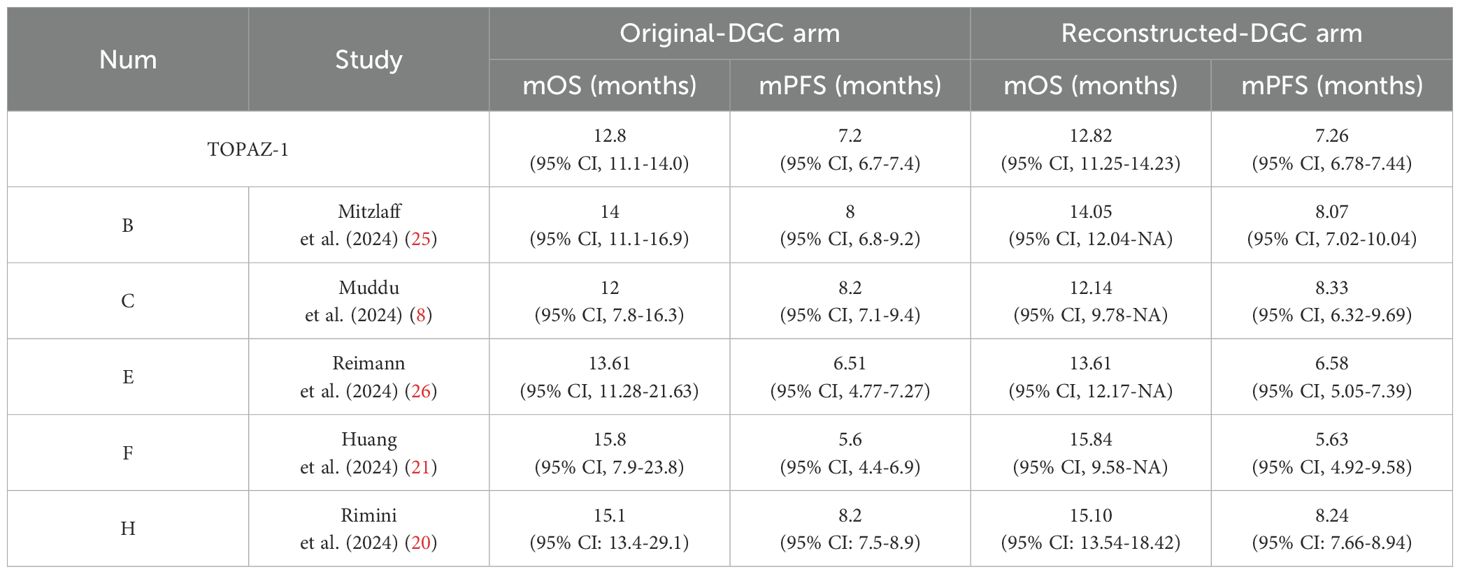
Before conducting the mimic survival comparisons, the patient-level data in the durvalumab plus chemotherapy arm from the TOPAZ-1 and the five included reports were reconstructed first to confirm the feasibility of the methods used in this analysis. For TOPAZ-1, the reconstructed median OS was 12.82 months (95% CI, 11.25-14.23) (original: 12.8 months [95% CI, 11.1-14.0]) and median PFS 7.26 months (95% CI, 6.78-7.44) (original: 7.2 months [95% CI, 6.7-7.4]). The comparison of the reconstructed data with the original outcomes for the five included reports were shown in Table 2. Based on this results, our reconstructed Kaplan–Meier survival curves demonstrated satisfactory repeatability compared with the original curves and datasets.
3.1 Mimic comparative analysis by survival data reconstruction
Among the studies included, the cases reported in B, E, and H primarily comes from non-Asian regions (Germany, Austria, Italy), and those in C and F are mainly from Asian regions (India, Taiwan), and the “number at risk time” intervals differ, being 5 and 6 months, respectively. At the same time, the etiological and genomic differences between Asian and non-Asian BTC patients were considered. For example, hepatitis B and biliary stones are the high-risk factors for Asian patients whereas metabolic syndrome, hepatitis C, and alcoholism are the high-risk factors for European and American patients. The IDH1 mutation in European and American patients with iCC was higher than that in Asian patients (37, 38). Ultimately, this study combined B, E, and H into one group (non-Asian group), and C and F into another (Asian group), for data integration.
The two group’s survival data in DGC arm were reconstructed and then compared with the DGC arm from the TOPAZ-1 study. The results showed that the reconstructed median overall survival (mOS) for the non-Asian group was 13.61 months (95% CI: 13.54-14.04), whereas for the Asian group it was 12.57 months (95% CI: 9.78-NA). There was no significant difference between the two groups compared with the TOPAZ-1 study regarding mOS (non-Asian group mOS vs. TOPAZ-1 mOS: HR = 1.10, 95% CI: 0.91-1.31, log rank P = 0.323; Asian group mOS vs. TOPAZ-1 mOS: HR = 0.91, 95% CI: 0.69-1.21, log rank P = 0.53) (Figures 2A, B). The reconstructed median progression-free survival (mPFS) for the non-Asian group was 6.58 months (95% CI: 6.41-7.04), which was statistically different from the DGC arm in the TOPAZ-1 study (non-Asian group mPFS vs. TOPAZ-1 mPFS: HR = 0.80, 95% CI:0.70-0.92, log rank P = 0.002). For the Asian group, the mPFS was 5.63 months (95% CI: 5.20-7.22), but there was no statistical difference compared with the DGC arm in the TOPAZ-1 study (Asian group mPFS vs. TOPAZ-1 mPFS: HR = 1.09, 95% CI: 0.88-1.35, log rank P = 0.422) (Figures 2C, D).
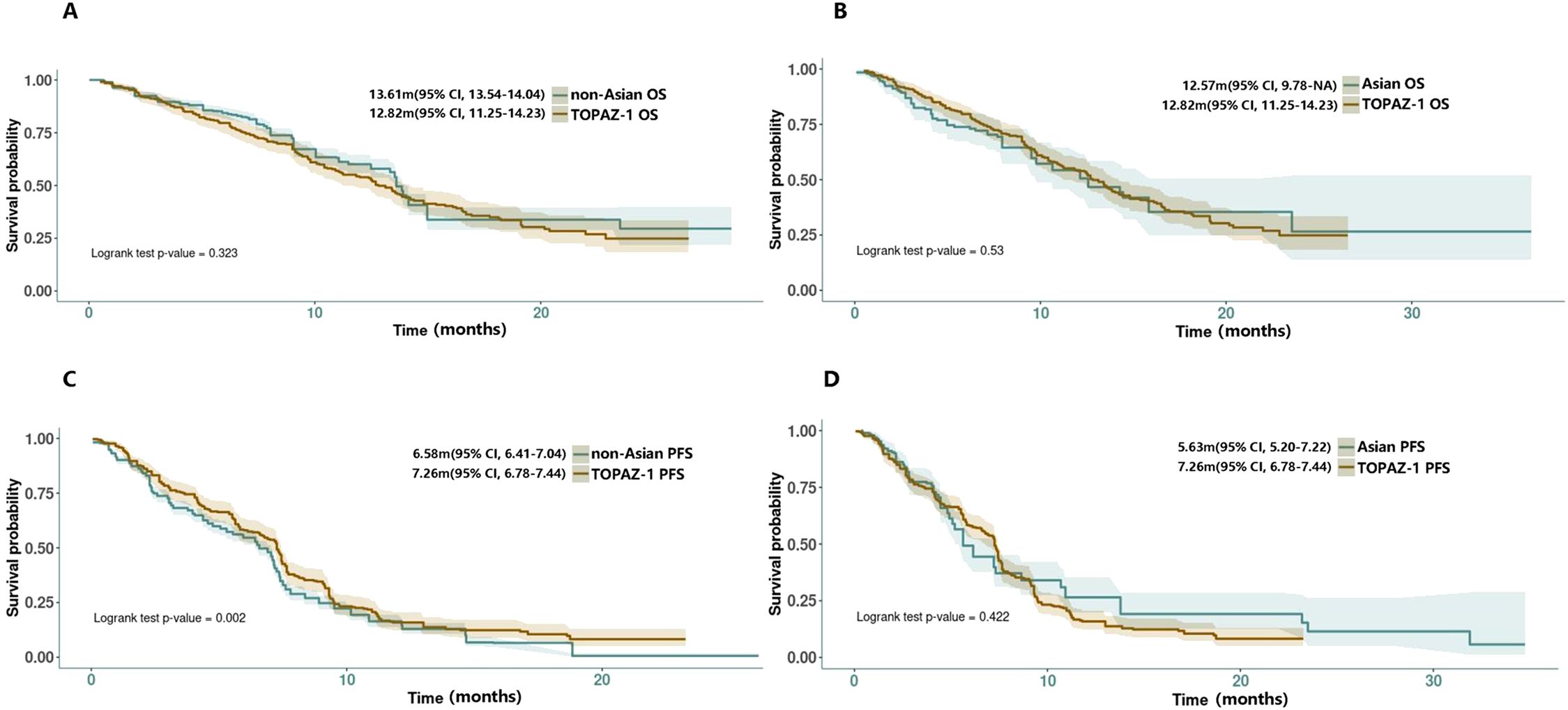
Figure 2. (A, B) Reconstructed OS curves for comparison between non-Asian/Asian group with TOPAZ-1 DGC arm. (C, D) Reconstructed PFS curves for comparison between non-Asian/Asian groups with the TOPAZ-1 DGC arm.
In the TOPAZ-1 study, the observed benefits in overall survival and progression-free survival with DGC remained generally consistent across relevant subgroups based on primary tumor location (iCCAs, eCCAs, GBC). However, some real-world findings have shown different results. In Mitzlaff et al. (2024) (25), they found that survival was significantly shorter for GBC patients with a median OS of 9 months (95% CI, 5.5-12.4; p=0.02) in comparison with patients with iCC and/or eCCAs (mOS not reached). The mPFS for patients with GBC was 6 months (95% CI, 4.6-7.4), also shorter than that for patients with iCC at 7 months (95% CI, 5.8-8.1) and/or eCCAs at 13 months (95% CI, 10.5-15.5; p=0.015). In Muddu et al. (2024) (8), they also observed a trend of lower mOS in the GBC subgroup compared with the remaining subgroups (mOS: iCC 14.2months [95% CI, 9.8-18.6] vs. eCCAs [not reached] vs. GBC 12.1 [95% CI, 8.8-15.3]), although not statistically significant (p=0.381).
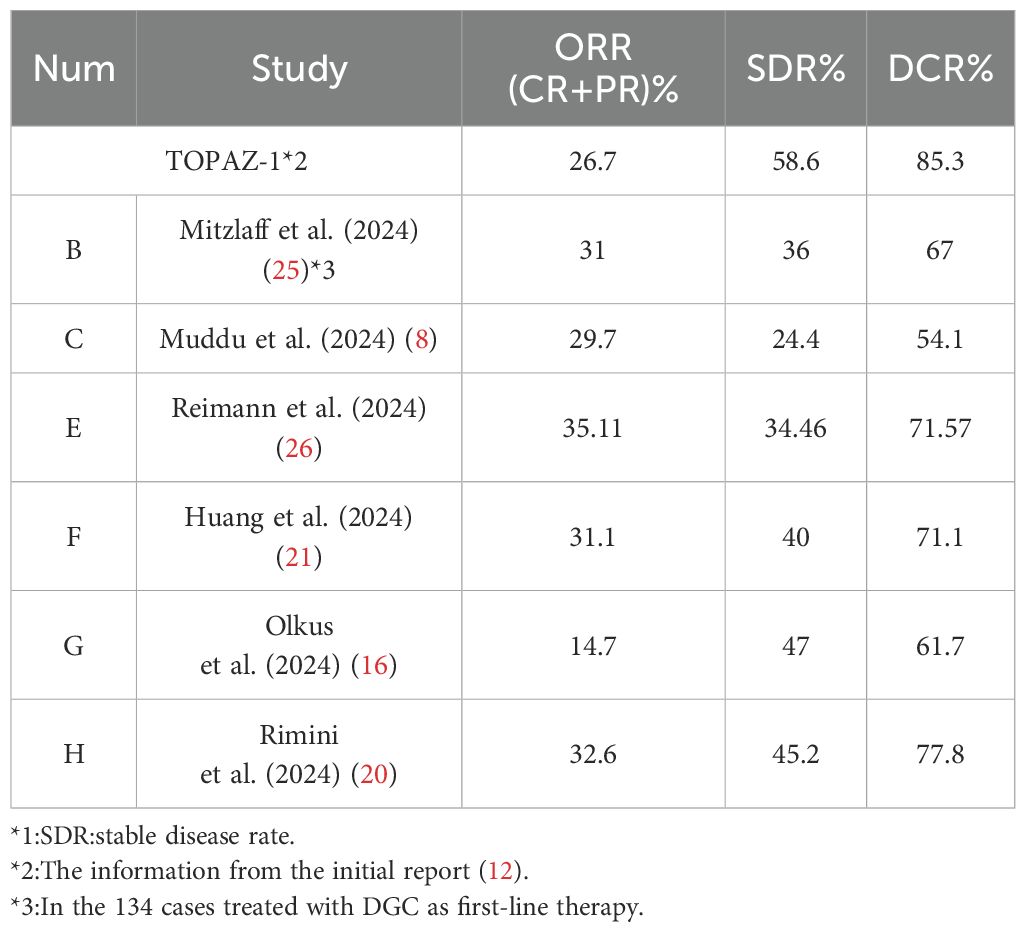
3.2 The ORR, SDR, and DCR of the DGC treatment
Table 3 illustrates the ORR (overall response rate), SDR (stable disease rate), and DCR (disease control rate) of the DGC arm in real-world reports. It is evident that in real-world clinical scenarios, the DCR was not so favorable as it is in the TOPAZ-1 trial. However, in the TOPAZ-1 trial, the overall survival, including long-term survival, improved for participants who achieved disease control in the DGC arm, whether CR (complete response) or PR (partial response) or SD (stable disease). The results from real-world reports are largely consistent with this and even showed more pronounced outcomes. In Muddu et al. (2024) (8), the mOS in patients who achieved clinical benefit was 23.1 months (95% CI, 10.2-36), obviously longer than 7.6 months (95% CI, 1.6-13.5) in patients who had PD (progressive disease) during treatment (p=0.002). In Reimann et al. (2024) (26), the patients who achieved at least a PR got a mOS of 14.14 months (95% CI, 13.55-NR). For the patients with SD, the mOS was 12.20 months (95% CI, 10.52-NR), and for those with PD, it was 8.52 months (95% CI, 5.46-NR). Patients with CR or PR, demonstrated significantly better overall survival compared with non-responders (HR: 0.29; 95% CI: 0.12-0.72; p=0.007). In Huang et al. (2024) (21), the responders, who had at least a PR or SD≧6 months, had a mOS of 15.8 months, whereas the non-responders, who had SD <6 months or PD, had a mOS of only 3.3 months.
Additionally, in Mitzlaff et al. (2024) (25), the ORR and DCR of patients who received DGC regimen as the first‐line treatment (n=134, ORR = 31%, DCR = 67%) was significantly better in comparison with patients who received it as a second- or later‐line treatment, supporting its upfront use. In a previous report from Rimini, they highlighted a correlation between baseline ALT levels (within normal ranges) and NLR (NLR <3) with better ORR (NLR<3 50.0% vs. NLR≧3 31.0%; normal ALT 40.7% vs. elevated ALT 20.4%) (18). Then, in Rimini et al.’s (2024) (20) latest research, the ECOG-PS, disease status, and absence of drainage or stent were reported also associated with higher ORR to treatment (ECOG-PS 0 39.2% vs. ECOG-PS >0 25.6%; locally advanced disease 39.2% vs. metastatic disease 31.8%; absence of drainage or stent 35.2% vs. with drainage or stent 29.9%), but the primary tumor site had no relationship with ORR.
3.3 The AEs in DGC treatment
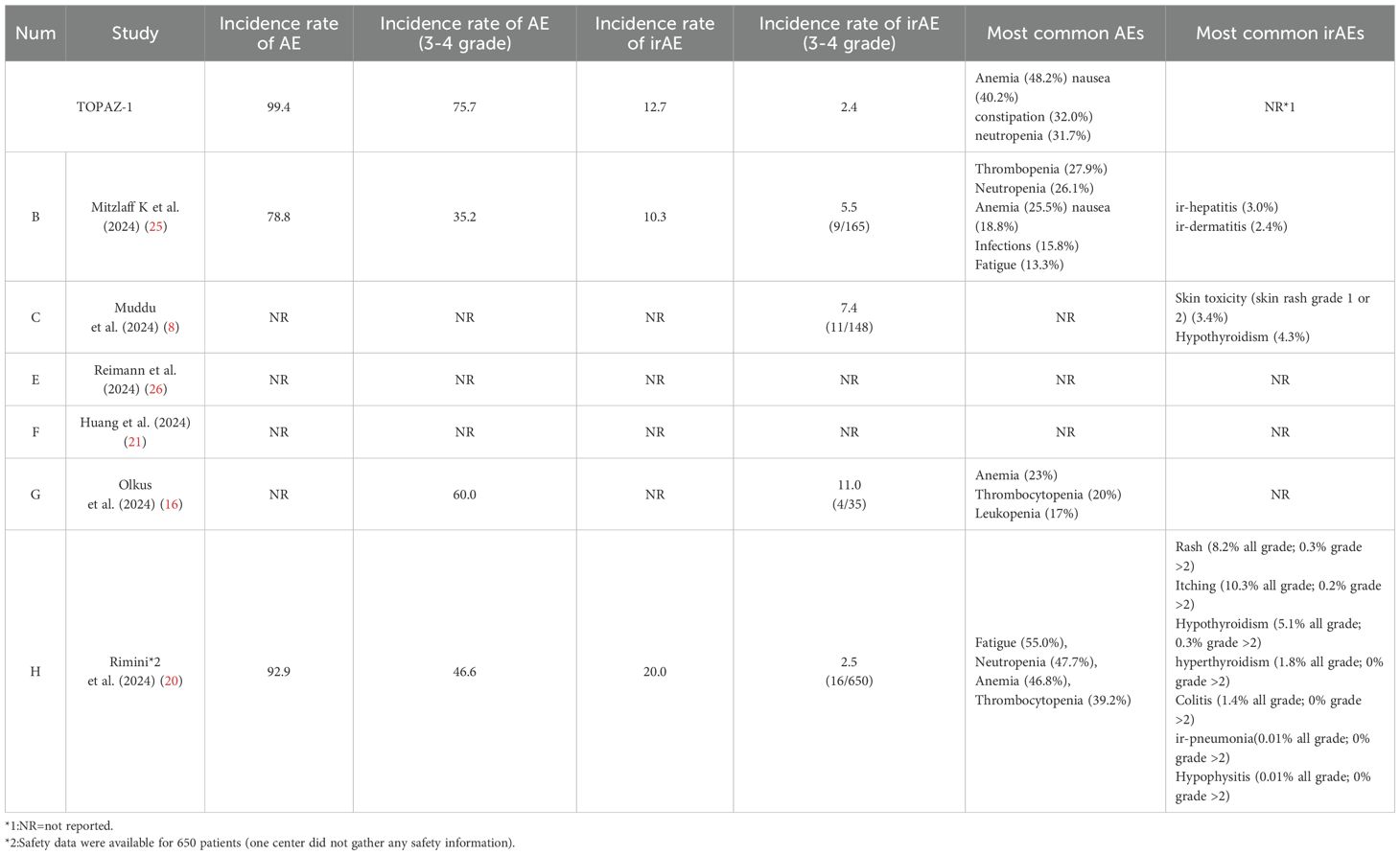
In TOPAZ-1, compared with placebo plus chemotherapy, the DGC treatment was associated with a similar rate of discontinuations due to adverse events. The observed toxicities with DGC were similar to those with chemotherapy or immunotherapy alone. The most common adverse events seen in the DGC arm were anemia (48.2%), nausea (40.2%), constipation (32.0%), and neutropenia (31.7%). The updated report showed that the rate of immune therapy-related adverse effects (irAEs) in long-term survivors was higher in the DGC arm, but grade 3 or 4 irAEs only occurred in one (1%) of 88 participants.
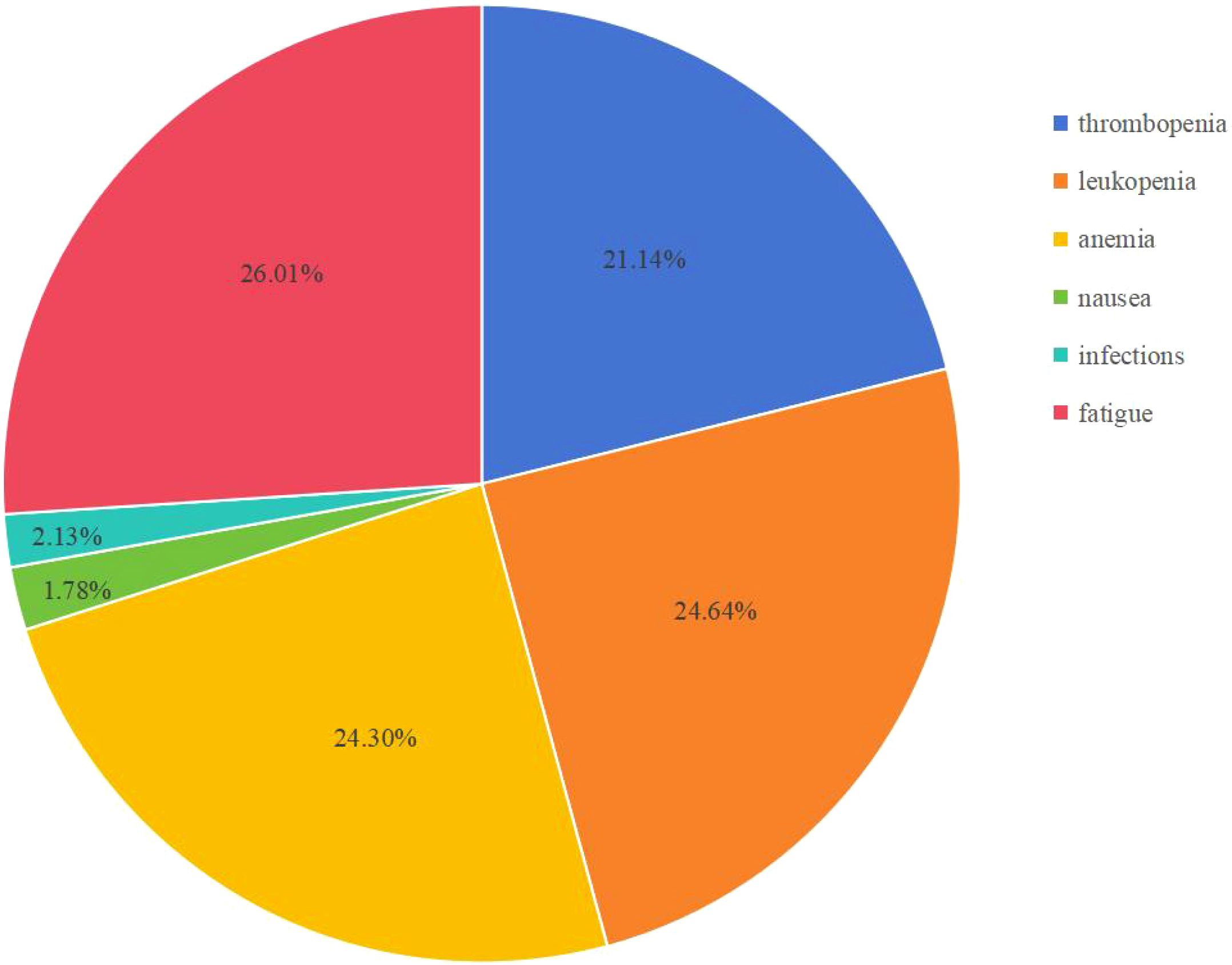
However, in real-world clinical scenarios, some differences may exist, especially in irAEs (Table 4, Figure 3). The most common adverse events seen in the DGC arm were fatigue (26.01%), leukopenia (24.64%), anemia (24.30%), and thrombocytopenia (21.14%) calculated based on the reported real-world data. The incidence rate of irAEs (3–4 Grade) calculated based on the reported data in our study is 4.0% (40/998) in the DGC arm. Among included reports, the most common irAEs were skin or thyroid related toxicity, and all 3–4 grade irAEs can be well managed with corticosteroids or immunosuppressants. Considering that the incidence of irAEs was more likely associated with the addition of durvalumab, researchers have also paid attention to the correlation between irAEs and survival outcomes. In Mitzlaff et al. (2024) (25), the occurrence of irAEs was found to be associated with a slight trend of better outcome with a mOS not reached versus 12 months (95% CI, 9.3-14.7; p=0.35) and mPFS of 11 months (95% CI, 3.4-18.5) versus 8 months (95% CI, 7.1-8.9; p=0.401). However, the response rates was not significantly different between patients with irAEs or not.
3.4 The prognostic factors for OS and PFS
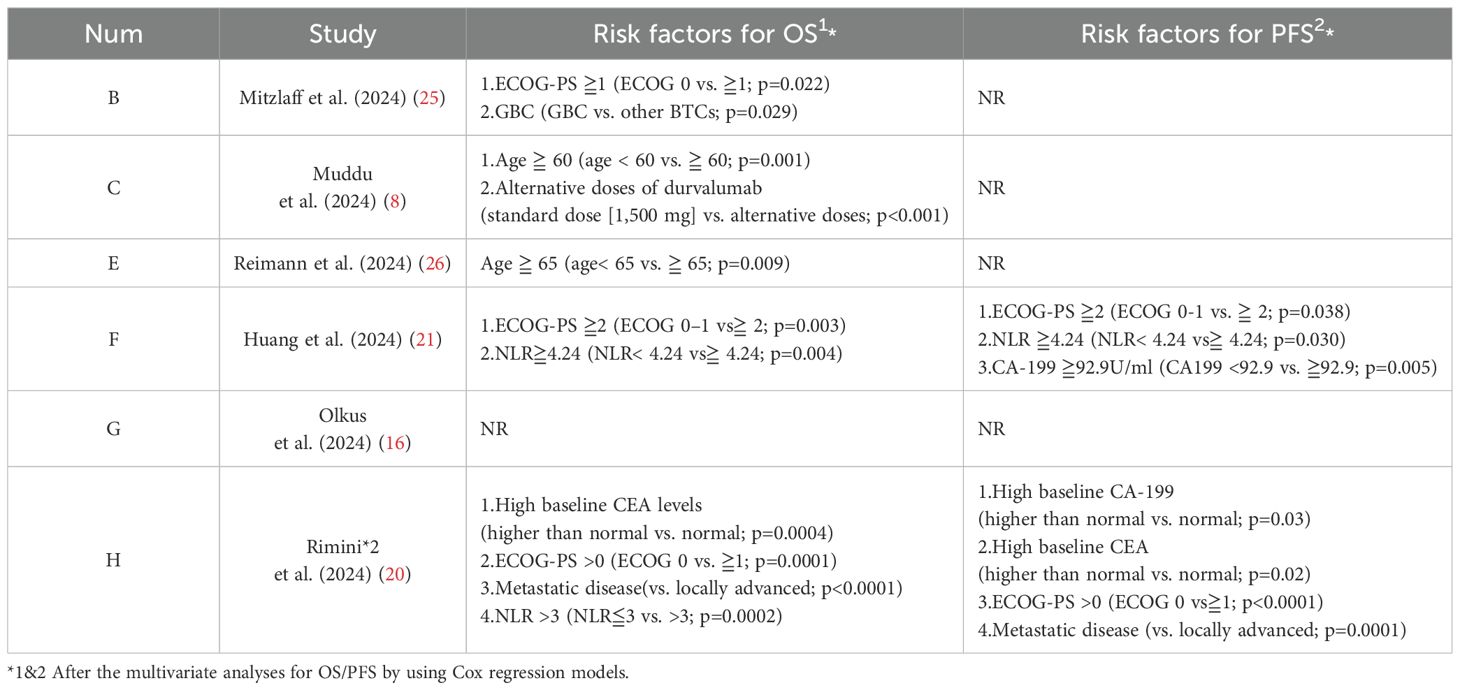
In TOPAZ-1, the PD-L1 status (patients with a PD-L1 tumor area positivity [TAP] score of 1% or greater) has not emerged as a significant indicator for clinical prognosis. In the updated result, it was found that among long-term survivors in the DGC arm, more patients (≧10%) had recurrent disease at baseline, an NLR (neutrophil to lymphocyte ratio)≦3, a CA-199 (cancer antigen 199)≦500U/mL, or a CEA (carcinoembryonic antigen)≦5 ng/mL.
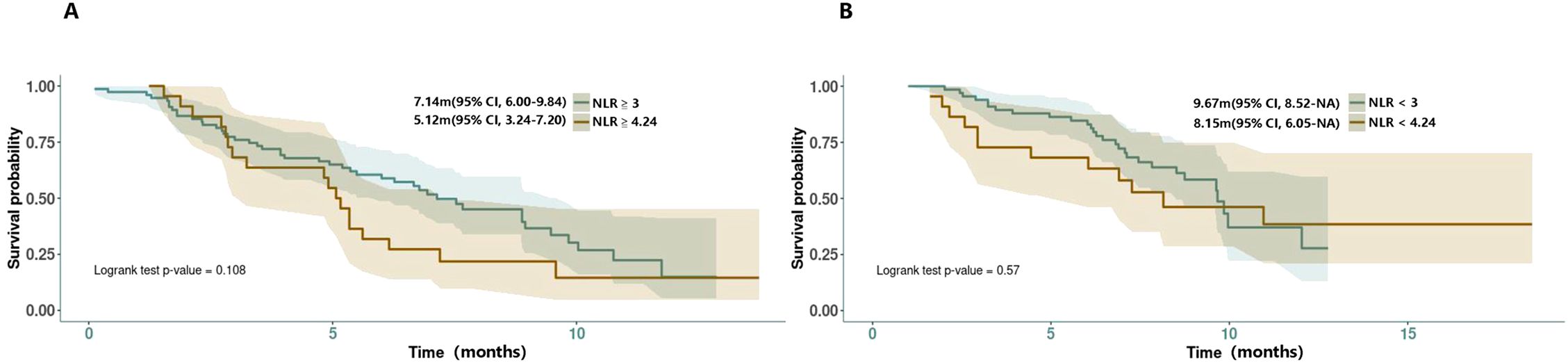
In the real-world clinical scenarios, various factors have been reported as valuable references for prognosis (Table 5). According to Muddu et al. (2024) (8), factors such as sex, ECOG-PS, BTC subtype, tumor differentiation, durvalumab cycle frequency, chemotherapy dose interruptions, and immunotherapy dose interruptions all did not affect the overall survival, although a lower mOS was observed in the GBC subset. Its multivariable analysis showed that age <60 years and standard dose of durvalumab were associated with improved OS. Patients <60 years had a mOS of 23.5 months compared with 9.5 months for those age ≧ 60 years (P = 0.002). In the study, 10.8% of patients received durvalumab at a dose lower than 1,500 mg (most at 500 mg/3 weeks) due to logistical and cost reasons. The mOS for patients on the standard dose was 12.1 months, compared with 7.5 months for those on alternative doses. In Reimann et al. (2024) (26), age <65 years was significantly associated with improved OS. In Huang et al. (2024) (21), a restricted cubic spline (RCS) analysis suggested a similar efficacy between doses of 1,000 and 1500 mg but potentially worsened at doses less than 1,000 mg, although not statistically significant. They also found a high NLR (≧4.24) to be associated with poor survival outcomes, a finding supported by Rimini et al.’s previous research (18), in which 25.4% of the patients with NLR≧3 had PD compared with 4.5% of patients with NLR<3 (p=0.002), and 23.2% of patients with ECOG PS > 0 had progression versus 8.2% of patients with ECOG PS = 0 (p=0.02) at the first CT scan. As the NLR-related PFS KM curves were provided by Rimini et al. (18) and Huang et al. (21) and with the attempt to find a more credible threshold, we also conducted a reconstruction of NLR-related survival data for comparison (NLR≧4.24 vs. ≧3; NLR≦4.24 vs. ≦3). However, the result showed no statistical significance between them (NLR≧4.24 vs. ≧3: 5.12m vs. 7.14m, HR = 1.56, 95% CI:0.90-2.70, log rank P = 0.108; NLR≦4.24 vs. ≦3: 8.15m vs. 9.67m, HR = 1.22, 95% CI: 0.61-2.44, log rank P = 0.57) (Figure 4). In the latest research from Rimini et al. (2024) (20), factors such as high baseline CA-199, high baseline CEA, and metastatic disease at initial were also found to affect the survival outcomes with statistical significance.
4 Discussion
In TOPAZ-1, the chemoimmunotherapy-DGC significantly prolonged overall survival and progression-free survival with the addition of durvalumab, a human IgG1 monoclonal antibody, and demonstrated a 24% decrease in risk of death at 2 years. A growing body of evidence indicated that cytotoxic chemotherapy has immunomodulatory effects, providing a rationale for chemoimmunotherapy (27, 28). The KEYNOTE-966 study also showed similar results, with the combination of pembrolizumab and chemotherapy (PGC) yielding a mOS of 12.7 months and mPFS of 6.5 months. There is one difference between the two trials; in KEYNOTE‐966, cisplatin was stopped after eight cycles of combined therapy and the duration of gemcitabine was not limited, whereas in the TOPAZ‐1 trial, both cisplatin and gemcitabine were stopped after eight cycles. The DCR in KEYNOTE-966 was lower compared with the TOPAZ-1 trial (54.1% vs. 85.3%), but the ORR was comparable (29.7% vs. 26.7%) (29). Recently, the results from the IMbrave151 trial showed that the effect of atezolizumab, another PD-L1 drug, combined with GC in the treatment of BTC was largely comparable with that of DGC or PGC with respect to mPFS (7.9 months), OS(14.6 months), and ORR (26.5%) (36).
However, the highly selected patient population in TOPAZ-1 may have reduced the representativeness of the real-world scenarios, whereas the international collaboration in this field is still limited. Based on our mimic comparative analysis utilizing available limited real-world data, the efficacy of the DGC regimen in treating advanced BTC is commendable. The survival outcomes from our analysis are similar to those of the TOPAZ-1 study, except for the median mPFS in the non-Asian group (6.58 months), which showed a statistically significant difference compared with the DGC group in the TOPAZ-1 study. This discrepancy may be attributed to the higher proportion of Caucasian patients, which was emphasized in Reimann et al. (2024) (26). Compared with TOPAZ-1, the inclusion criteria for real-world clinical trials were more complex and broad due to some objective reasons, but the reported outcomes still supported the therapeutic value of DGC regimen in advanced BTC. In the early report from Rimini et al. (23), no significant differences were observed between early and late relapse groups (disease recurrence occurring ≦6 months or >6 months after surgery) in OS or PFS, under DGC therapy. In Olkus et al. (2024) (16), 51% patients met the inclusion criteria of the TOPAZ-1 trial (TOPAZ-1 IN group), whereas the remaining 49% did not (TOPAZ-1 OUT group). The most common reason for the TOPAZ-1 OUT group was preexisting autoimmune-associated disorders, followed by ECOG PS > 1. Then, the comparison between the two groups showed no significant difference in OS and PFS (mOS:10.3 vs. 9.7 months; mPFS:5.3 vs. 5 months); the DCR was also similar in both groups (61.1% vs. 58.8%), although the ORR in the TOPAZ-1 IN subgroup was higher (22.2% vs. 5.8%). The inclusion of patients beyond the TOPAZ-1 criteria even did not lead to an increase in AEs or alteration of the DGC treatment. Both Mitzlaff et al. and Rimini et al. found that patients with chronic liver diseases (including chronic viral hepatitis B or C or liver cirrhosis) could get similar outcomes and safety profiles. In the earlier research from Rimini et al., they even noted that the absence of viral infection correlated with a worse PFS (18, 25).
In TOPAZ-1, no clear indication of prognostic risk factors were identified. However, in real-world clinical settings, this issue cannot be overlooked. Among those real-world reports, ECOG-PS, age, alternative doses of durvalumab, NLR, baseline CEA level, baseline CA-199 level, and metastatic disease were found to be factors affecting patients’ OS or PFS. It is noteworthy that NLR has been mentioned in multiple reports, indicating its potential as a predictive marker for patients’ survival benefit under DGC regimen. Previous studies have shown that NLR plays a prognostic role in various solid tumors treated with systemic therapy, particularly ICIs (20). Now, NLR has been widely recognized as a possible surrogate of systemic inflammatory status. Tanaka et al. demonstrated a negative correlation between NLR and CD8+T cells which are crucial for the antitumor immune response (30). More investigations are needed for the confirmation of its predictive role.
In TOPAZ-1, all subgroups could benefitted from the DGC treatment regardless of the primary tumor location. There is also no clear evidence supporting that a specific gene, type of genetic mutation, or TMB (tumor mutation burden) or MSI-H/dMMR status can serve as a therapeutic benefit predictive indicator under a DGC regimen. Unfortunately, in the included real-world studies in our research, no prognostic-related gene markers were identified either. In Mitzlaff et al. (2024) (25), the detected molecular profiles of the cohort matched the known distribution of molecular alterations including FGFR2 alterations (14.6%) and IDH1 or two mutations (14.6%) in iCC, KRAS (44.4%), and TP53 (36.1%) in eCCAs and Her2 (13.3%) in GBC. However, no specific gene mutation among them was found to be a prognostic marker for OS, as well as the microsatellite instability (MSI). In Reimann Pet al. (2024) (26), comprehensive molecular profiling was conducted in 90 patients to explore whether mutations in specific genes or pathways (including insertions, deletions, frameshift alterations, splice site, nonsense, and missense mutations) influenced the response and efficacy of the combined therapy. However, no individual gene or mutation pathway demonstrated a significant association with improved outcome. In Olkus et al. (2024) (16), loss of TP53 (28%), deletion mutations in BRCA1-associated protein 1 (BAP1; 24%), activating mutations of KRAS (21%), deletion mutation of ARID1A (17%), and the TMB of patients were not associated with the response to treatment. A study by Rimini et al. clustered patients based on molecular and genomic alterations, finding that those with alterations in the RTK/RAS pathway and cell-cycle apoptosis had worse outcomes compared with patients with chromatin modification pathway alterations (31), but the sample size was low (n = 51) and further validation on larger and external cohorts are needed.
In our study, DGC treatment-related AEs appeared slightly higher than that in TOPAZ-1 but most seems related to chemotherapy, indicating that durvalumab is indeed a relatively safe medication. Regarding the implementation of the DGC regimen, Olkus et al. found that the frequency of durvalumab cycles did not affect the survival (8), whereas Mitzlaff et al. found that patients who completed eight cycles of DGC regimen (n = 49) showed a similar outcome compared with patients who received more than eight cycles (n = 12) but was significantly better than those received less than eight cycles (n = 71) (25). In the USA and EU, the recommended dosage of durvalumab is 1,500 mg without reduction or escalation. In patients who weigh <30 kg (USA) or ≦36 kg (EU), the recommended dosage is 20 mg/kg. The terminal half-life of durvalumab, based on clearance at baseline, is 18–21 days. There were no clinically significant differences in durvalumab pharmacokinetics based on age (18–96 years), body weight (31–175 kg), gender, or race. Serum levels of albumin (4-57g/L), lactate dehydrogenase (18-15,800 U/L), soluble PD-L1 (67-3,470 pg/mL), tumor type (non-small cell lung, small cell lung, biliary tract, and hepatocellular cancers), or ECOG-PS also did not affect the pharmacokinetics of durvalumab (32) (one possible reason for the inability to identify prognostic markers for durvalumab). On the other hand, in Huang et al. (2024) (21), the pharmacodynamic evidence showed that a 10-mg/kg Q2W dose of durvalumab leads to approximately 93.3% of patients achieving complete suppression of sPD-L1. Moreover, the pharmacokinetic analyses revealed a linear dose–response relationship at doses over 3 mg/kg Q2W, which can ensure the consistent drug exposure. Transitioning to a flat dosing regimen, this corresponds to approximately 15 mg/kg Q3W, or around 900–975 mg. These findings highlight the importance of maintaining a dosage of at least 1,000mg Q3W for clinical efficacy.
For advanced BTC, the cost-effectiveness of durvalumab may hinder its wider and standardized use in real-world scenarios. Zhao et al. conducted a cost-effectiveness analysis for the DGC regimen and found that it was not cost-effective for advanced BTC regardless of receiving or not receiving charitable assistance, unless the price of durvalumab fell by more than 94.2% to less than $0.33/mg (33). Kashiwa et al. found in their analysis that both DGC and PGC (pembrolizumab with gemcitabine–cisplatin) were not cost-effective for Japanese patients compared with the combined chemotherapy regimen-GCS (gemcitabine, cisplatin plus S-1) (35). Ye et al. also found that even for the US patients, the DGC regimen did not offer a cost-effective advantage at current prices (for US:11,730 dollar per cycle), only when the price of durvalumab fell by 67.4% or more (34).
Of course, in addition to chemical immunotherapy, many therapeutic strategies are under active research, for example, the optimized systemic chemotherapy, targeted drugs, ICI-targeted drug combination strategy, double-ICI combination strategy, CAR-T, and tumor vaccine. It is believed that BTC patients will have more effective treatment options in the future.
5 Limitations
Several limitations exist in the analysis. Firstly, due to the recent time of release of TOPAZ-1 results, the number of included studies is limited. Then, although there was a low risk of bias among the enrolled trials (Supplementary 2, 3), due to the nature of real-world evidence (RWE), all included studies were all retrospective cohort studies, and inherent heterogeneity may be inevitable, for example, differences in treatment implementation due to real-world objective factors and the expansion of patient inclusion criteria. Due to the incomplete availability of KM curves and no at-risk data provided by included studies, there is a pity that further reconstruction and comparison cannot be conducted between subgroups based on points of interest, such as primary tumor location or region/race. Because of the inability to reconstruct response and AE data, only comparison by outcome summarize was conducted in the relevant sections.
6 Conclusions
Based on the mimic comparative analysis results and the aggregation of real-world evidence in this study, the efficacy and safety of the DGC regimen for patients with advanced BTC are reaffirmed. Further real-world investigations are still warranted to determine if the DGC regimen has a broader therapeutic indication. Currently, there are no clear indicators for predicting the treatment benefits of DGC regimen. NLR has the potential to become a useful and convenient indicator. In real-world clinical scenarios, the improved cost-effectiveness of the DGC regimen will facilitate its wider application.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
H-XJ: Methodology, Writing – review & editing, Formal analysis, Data curation, Investigation, Writing – original draft, Funding acquisition. M-HS: Writing – original draft, Data curation, Methodology, Formal analysis, Writing – review & editing. ZS: Data curation, Formal analysis, Writing – original draft, Supervision. NY: Writing – review & editing, Supervision, Validation, Methodology, Conceptualization. ZC: Methodology, Funding acquisition, Validation, Conceptualization, Writing – review & editing, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of Xiamen, China (No. 3502Z20227125).
Acknowledgments
We truly appreciate the efforts contributed by the authors/researchers of those included clinical trials, for the providing of valuable real-world evidences.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1643844/full#supplementary-material
References
1. Valle JW, Kelley RK, Nervi B, Oh DY, and Zhu AX. Biliary tract cancer. Lancet (London England). (2021) 397:428–44. doi: 10.1016/S0140-6736(21)00153-7
2. Khan SA, Tavolari S, and Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver international: Off J Int Assoc Study Liver. (2019) 39 Suppl 1:19–31. doi: 10.1111/liv.14095
3. Vogel A, Bridgewater J, Edeline J, Kelley RK, Klümpen HJ, Malka D, et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol. (2023) 34:127–40. doi: 10.1016/j.annonc.2022.10.506
4. Siegel RL, Giaquinto AN, and Jemal A. Cancer statistics, 2024. CA: Cancer J Clin. (2024) 74:12–49. doi: 10.3322/caac.21820
5. Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. (2024) 74:229–63. doi: 10.3322/caac.21834
6. Anderson C and Kim R. Adjuvant therapy for resected extrahepatic cholangiocarcinoma: a review of the literature and future directions. Cancer Treat Rev. (2009) 35:322–7. doi: 10.1016/j.ctrv.2008.11.009
7. Harrison JM and Visser BC. Cholangiocarcinoma. Surg Clinics North America. (2024) 104:1281–93. doi: 10.1016/j.suc.2024.04.003
8. Muddu VK, Shah A, John A, Raj A, Bahl A, Rajappa SJ, et al. Gemcitabine cisplatin and durvalumab experience in advanced biliary tract cancers: A real-world, multicentric data from India. JCO Global Oncol. (2024) 10:e2400216. doi: 10.1200/GO.24.00216
9. Wu J, Yang S, Xu K, Ding C, Zhou Y, Fu X, et al. Patterns and trends of liver cancer incidence rates in eastern and southeastern asian countries (1983-2007) and predictions to 2030. Gastroenterology. (2018) 154:1719–1728.e5. doi: 10.1053/j.gastro.2018.01.033
10. Mazzaferro V, Gorgen A, Roayaie S, Droz Dit Busset M, and Sapisochin G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol. (2020) 72:364–77. doi: 10.1016/j.jhep.2019.11.020
11. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. New Engl J Med. (2010) 362:1273–81. doi: 10.1056/NEJMoa0908721
12. Oh DY, Ruth He A, Qin S, Chen LT, Okusaka T, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM evidence. (2022) 1:EVIDoa2200015. doi: 10.1056/EVIDoa2200015
13. Oh DY, He AR, Bouattour M, Okusaka T, Qin S, Chen LT, et al. Durvalumab or placebo plus gemcitabine and cisplatin in participants with advanced biliary tract cancer (TOPAZ-1): updated overall survival from a randomised phase 3 study. Lancet Gastroenterol Hepatol. (2024) 9:694–704. doi: 10.1016/S2468-1253(24)00095-5
14. Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, et al. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. JAMA. (2015) 313:1657–65. doi: 10.1001/jama.2015.3656
15. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
16. Olkus A, Tomczak A, Berger AK, Rauber C, Puchas P, Wehling C, et al. Durvalumab plus gemcitabine and cisplatin in patients with advanced biliary tract cancer: an exploratory analysis of real-world data. Targeted Oncol. (2024) 19:213–21. doi: 10.1007/s11523-024-01044-1
17. Liu N, Zhou Y, and Lee JJ. IPDfromKM: reconstruct individual patient data from published Kaplan-Meier survival curves. BMC Med Res Method. (2021) 21:111. doi: 10.1186/s12874-021-01308-8
18. Rimini M, Fornaro L, Lonardi S, Niger M, Lavacchi D, Pressiani T, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer: An early exploratory analysis of real-world data. Liver international: Off J Int Assoc Study Liver. (2023) 43:1803–12. doi: 10.1111/liv.15641
19. Rimini M, Masi G, Lonardi S, Nichetti F, Pressiani T, Lavacchi D, et al. Durvalumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin in biliary tract cancer: a real-world retrospective, multicenter study. Targeted Oncol. (2024) 19:359–70. doi: 10.1007/s11523-024-01060-1
20. Rimini M, Fornaro L, Rizzato MD, Antonuzzo L, Rossari F, Satake T, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer: A large real-life worldwide population. Eur J Cancer (Oxford England: 1990). (2024) 208:114199. doi: 10.1016/j.ejca.2024.114199
21. Huang WK, Tang YJ, Wu CE, Hou MM, Hsu HC, Su PJ, et al. Real-world effectiveness and prognostic factors of durvalumab plus chemotherapy in a multicentric cohort with advanced biliary tract cancer. oncologist. (2024) 30:oyae306. doi: 10.1093/oncolo/oyae306
22. Rimini M, Foti S, Camera S, Rossari F, Vitiello F, Lo Prinzi F, et al. Survival outcomes of durvalumab in combination with cisplatin and gemcitabine in advanced biliary tract cancer: real-world results from a single italian institution. Oncology. (2024) 103:677–698. doi: 10.1159/000541891
23. Lo Prinzi F, Salani F, Rimini M, Rizzato MD, Antonuzzo L, Camera S, et al. Efficacy of cisplatin-gemcitabine-durvalumab in patients with advanced biliary tract cancer experiencing early vs late disease relapse after surgery: a large real-life worldwide population. oncologist. (2025) 30:oyae256. doi: 10.1093/oncolo/oyae256
24. Lu Y, Jin Y, Liu F, Wang Z, Zhou W, Zhang Y, et al. Efficacy of durvalumab plus chemotherapy in advanced biliary duct cancer and biomarkers exploration. Cancer immunology immunotherapy: CII. (2024) 73:220. doi: 10.1007/s00262-024-03796-1
25. Mitzlaff K, Kirstein MM, Müller C, Venerito M, Olkus A, Dill MT, et al. Efficacy, safety and differential outcomes of immune-chemotherapy with gemcitabine, cisplatin and durvalumab in patients with biliary tract cancers: A multicenter real world cohort. United Eur Gastroenterol J. (2024) 12:1230–42. doi: 10.1002/ueg2.12656
26. Reimann P, Mavroeidi IA, Burghofer J, Taghizadeh H, Webersinke G, Kasper S, et al. Exploring the impact of durvalumab on biliary tract cancer: insights from real-world clinical data. Cancer immunology immunotherapy: CII. (2024) 73:251. doi: 10.1007/s00262-024-03842-y
27. Galluzzi L, Buqué A, Kepp O, Zitvogel L, and Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. (2015) 28:690–714. doi: 10.1016/j.ccell.2015.10.012
28. Wang Q, Ju X, Wang J, Fan Y, Ren M, and Zhang H. Immunogenic cell death in anticancer chemotherapy and its impact on clinical studies. Cancer Lett. (2018) 438:17–23. doi: 10.1016/j.canlet.2018.08.028
29. Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London England). (2023) 401:1853–65. doi: 10.1016/S0140-6736(23)00727-4
30. Tanaka R, Kimura K, Eguchi S, Tauchi J, Shibutani M, Shinkawa H, et al. Preoperative neutrophil-to-lymphocyte ratio predicts tumor-infiltrating CD8+ T cells in biliary tract cancer. Anticancer Res. (2020) 40:2881–7. doi: 10.21873/anticanres.14264
31. Rimini M, Loi E, Rizzato MD, Pressiani T, Vivaldi C, Gusmaroli E, et al. Different genomic clusters impact on responses in advanced biliary tract cancer treated with cisplatin plus gemcitabine plus durvalumab. Targeted Oncol. (2024) 19:223–35. doi: 10.1007/s11523-024-01032-5
32. Fung S and Syed YY. Durvalumab: A review in advanced biliary tract cancer. Targeted Oncol. (2023) 18:965–72. doi: 10.1007/s11523-023-01007-y
33. Zhao Q, Xie R, Zhong W, Liu W, Chen T, Qiu X, et al. Cost-effectiveness analysis of adding durvalumab to chemotherapy as first-line treatment for advanced biliary tract cancer based on the TOPAZ-1 trial. Cost effectiveness resource allocation: C/E. (2023) 21:19. doi: 10.1186/s12962-023-00429-9
34. Ye ZM, Xu Z, Li H, and Li Q. Cost-effectiveness analysis of durvalumab plus chemotherapy as first-line treatment for biliary tract cancer. Front Public Health. (2023) 11:1046424. doi: 10.3389/fpubh.2023.1046424
35. Kashiwa M and Maeda H. Comparative cost-effectiveness of gemcitabine and cisplatin in combination with S-1, durvalumab, or pembrolizumab as first-line triple treatment for advanced biliary tract cancer. J gastrointestinal Cancer. (2024) 55:1569–80. doi: 10.1007/s12029-024-01106-7
36. Macarulla T, Ren Z, Chon HJ, Park JO, Kim JW, Pressiani T, et al. Atezolizumab plus chemotherapy with or without bevacizumab in advanced biliary tract cancer: clinical and biomarker data from the randomized phase II IMbrave151 trial. J Clin Oncol Off J Am Soc Clin Oncol. (2025) 43:545–57. doi: 10.1200/JCO.24.00337
37. Jain A, Kwong LN, and Javle M. Genomic profiling of biliary tract cancers and implications for clinical practice. Curr Treat options Oncol. (2016) 17:58. doi: 10.1007/s11864-016-0432-2
Keywords: biliary tract cancer, durvalumab, immunotherapy, gemcitabine-cis platin, real-world
Citation: Ji H-x, Si M-H, Sun Z, Yang N and Chen Z (2025) The real-world clinical effectiveness of durvalumab in advanced biliary tract cancer: a mimic comparative analysis through survival data reconstruction. Front. Immunol. 16:1643844. doi: 10.3389/fimmu.2025.1643844
Received: 09 June 2025; Accepted: 30 October 2025;
Published: 18 November 2025.
Edited by:
Vamshi Muddu, AIG Hospitals, IndiaReviewed by:
Shuofeng Li, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaSumit Goyal, Rajiv Gandhi Cancer Institute and Research Center, India
Copyright © 2025 Ji, Si, Sun, Yang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Yang, bGFuY2V0MDBAMTYzLmNvbQ==; Zhan Chen, Y2hlbnpoYW4xOTc1QDE2My5jb20=
†These authors contributed equally to this work and share first authorship
 Hong-xiang Ji1†
Hong-xiang Ji1† Ning Yang
Ning Yang Zhan Chen
Zhan Chen