- 1Division of Pulmonary, Allergy, Critical Care, and Sleep Medicine, Department of Medicine, Emory University, Atlanta, GA, United States
- 2Department of Hematology and Medical Oncology, Winship Cancer Institute, Emory University, Atlanta, GA, United States
- 3Division of Rheumatology, Department of Medicine, Emory University, Atlanta, GA, United States
- 4Lowance Center for Human Immunology, Emory University, Atlanta, GA, United States
Plasma cells are known antibody-secreting factories with immunoglobulin (Ig) transcripts that increase as the cell matures into a long-lived plasma cell (LLPC) in the bone marrow (BM). Whether the Ig secretion rates among human antibody-secreting cells (ASC) are homogeneous or BM LLPC are capable of secreting more antibodies per cell compared to early-minted blood ASC remain unclear. Here, we use bulk and single cell cultures in a novel in vitro BM mimetic survival system to measure the IgG secretion rates of human ASC. We find that the mature BM ASC produce more IgG molecules per cell compared to immature, early-minted blood ASC. Furthermore, these blood ASC can mature into LLPC phenotypes in culture, and we show that ASC on day 7 secrete more IgG per cell than the input ASC from day 0. Thus, as human ASC mature, they increase the number of Ig transcripts and result in higher Ig secretion. These results also demonstrate that the mature ASC in the BM have higher Ig secretion rates compared to early-minted blood ASC.
Introduction
Human antibody-secreting cells (ASC) are now well recognized to be heterogeneous, consisting of early-minted ASC in the blood and intermediate and mature plasma cells in the bone marrow (BM) (1–3). In the blood, immature ASC can also be quite heterogeneous (4) while in the BM, ASC can be transcriptionally classified as short-lived, intermediate, and long-lived plasma cells (LLPC) that have the potential to survive indefinitely (5–7). Phenotypically, human BM ASC are heterogeneous with PopA exemplifying an immature subset, PopD (LLPC) representing the most mature one, and PopB as a diverse group that consists of intermediate phenotypes (5, 8, 9). Although difficult to study in vivo, the model of human ASC maturation into a LLPC was initially described with the use of a novel in vitro BM mimetic system (10, 11). This study (11) illustrates that maturation takes place upon arrival to the BM niche and that early-minted ASC from the blood can undergo further maturation in the BM niche conditions, which is mimicked, at least in part, by our culture system (4, 5, 8, 10). For clarity, the term ASC is used to refer to all Ig-secreting cells, which include early-minted blood ASC (plasmablasts) (3) and mature ASC known as plasma cells that can contain the LLPC subset.
As a protein secreting factory, a human ASC can produce massive amounts of immunoglobulins (Ig): 100-10,000 molecules per cell per second or 2–220 pg/cell/day (pg/c/d) (12–16). Studies reported a wide range of Ig secretion rates with some as high as 3,334 pg/c/d (17), while ASC differentiated in vitro from human B cells secrete less at 20–140 pg/c/d (18, 19), and malignant myeloma cells have even lower rates (20). These Ig secretion rates were thought to be influenced by both extrinsic and intrinsic factors (11, 21) since mature ASC have expansion of subcellular secretory network (the Golgi and ER) and organelles important in metabolism (mitochondrial mass) which prepare the cells to specialize in Ig secretion (11, 21). In addition, we found increasing Ig transcripts from blood to BM ASC, with the most mature LLPC subset containing the highest number of Ig transcripts (8). Whether these differences ultimately translate into higher Ig secretion rates remain unexplored.
Since ASC rapidly die ex vivo, we use a cell-free specialized culture system derived from factors within the human BM microniche that enables survival of ASC to measure Ig secretion rates (10). Since this system also mimics the BM microniche, we can follow the maturation process of blood ASC into a mature BM ASC phenotype (11). Here, we show that mature BM ASC secrete more Ig per cell compared to early-minted blood ASC, illustrating the heterogeneity of Ig secretion rates. As blood ASC mature in this BM mimetic system, they begin to secrete more Ig over time, demonstrating the importance of the maturation process for increased Ig secretion.
Materials and methods
Human subjects
We enrolled 69 healthy adults (aged 23–65 years) for peripheral blood samples collected 6–7 days after vaccination with influenza, Tdap (tetanus, diphtheria, and acellular pertussis), hepatitis A, hepatitis B, shingles, HPV, or COVID-19. We also obtained 32 BM aspirates from healthy adults (aged 21–68 years, with 13 men (41%) and 19 women (59%)). Samples were collected during July 2014-March 2024. All samples were fresh and no frozen samples were used. All research was approved by the Emory University Institutional Review Board and written informed consent was obtained from all subjects.
ASC purification and a human myeloma cell line
Mononuclear cells from peripheral blood and BM aspirate samples were isolated, enriched, and stained as previously described (5, 10). Cells were sorted on a BD FACSAria II as (3, 5, 10): blood ASC (IgD-CD27hiCD38hi) (Supplementary Figure 1a), BM PopA (CD19+IgD-CD38hiCD138-), BM PopB (CD19+IgD-CD38hiCD138+), and BM PopD (CD19-IgD-CD38hiCD138+) (Supplementary Figure 1b). The human myeloma cell line, ARH-77, was purchased (ATCC).
In vitro BM mimetic cultures
ASC were cultured for one day or up to 7–8 days in the BM mimetic system, which consists of the BM mesenchymal stromal cell (MSC) survival medium and in hypoxia (e.g., 2.5% O2 and 5% CO2) at 37°C supplemented with 200 ng/mL APRIL (R&D Systems) (10). ARH-77 cells were handled as per recommendations (3).
ELISpots, ELISA, and multiplex bead binding assays
We quantified total and vaccine-specific IgG ASC using ELISpots, as previously described (3, 9, 10) (Supplementary Figure 2). We measured secreted IgG by MBBA (9) or by ELISA. For the ELISA, 96-well flat-bottom ELISA microplates (Nunc/Corning) were pre-coated overnight at 4°C with goat anti-human IgG or specific vaccine. Plates were then washed using a microplate washer (Biotek) and nonspecific binding was blocked with SuperBlock Blocking Buffer (Thermo Fisher), followed by washing. Culture supernatants and the standard protein molecules or the monoclonal antibody (mAb) standards were then loaded and plates were incubated, followed by washing. Alkaline phosphatase–conjugated secondary antibody was then added and plates were incubated, followed by washing. The amount of bound secondary antibody was visualized with an enzymatic color reaction using BluePhos Microwell Phosphatase Substrate System (KPL). Light absorbance was measured at 650 nm using a microplate reader (BioTek). The sensitivity of the assay was ~0.17-0.34 ng/mL. The intra-assay variability of the assay was <12%, as determined by measuring multiple sets of supernatants in two separate runs.
For vaccine-specific IgG capturing, tetanus toxoid Clostridium tetani (Calbiochem) or quadrivalent influenza vaccine 2015-2016 (Fluarix Quadrivalent Influenza Vaccine 2015–2016 Formula; GSK Biologicals) was used. For relative quantitation of the concentrations of total IgG or antigen-specific IgG, standard curves were generated using purified human IgG (ChromePure human IgG, JacksonImmuno Research Laboratories) or mAb standards of anti-tetanus toxin mAb (clone #TetE3; The Native Antigen Company).
Calculation of the IgG secretion rates
As the amount of total IgG secreted by a single ASC in one day was below the detection limit by our assays, we maintained single-sorted ASC in vitro for 7–8 days. For bulk cultures, average IgG concentrations were calculated based on the number of spots and the concentrations of secreted IgG (assuming equal secretion rates both per cell and per day). For single cell cultures, although blood ASC could increase Ig secretion rates with maturation in the cultures, we assumed equal secretion per day and measured the cumulative total IgG amount in the cultures and divided by number of days in culture. Except for day 0, the secretion rates for bulk cultures were calculated as follows: Secretion rates (pg/cell/day) = Amount (pg) of IgG secreted in culture supernatants/(Quantity of IgG spots x Number of days in culture). For single cell cultures, the secretion rates were calculated as follows: Secretion rates (pg/cell/day) = Amount (pg) of IgG secreted in culture supernatants/Number of days in culture.
On-chip single-cell culture, in-channel IgG capture, and fluorescence semi-quantitation
To overcome apoptosis and ongoing maturation in the 7-day single ASC cultures, we directly assess single ASC secretion rates using a novel microfluidic system to visualize IgG secretion ex vivo with high precision (3). Single ASC visualization was performed using the Lightning system (Bruker Cellular Analysis) (3). The instrument captures individual ASC in the act of Ig secretion (3) which develops as fluorescent halos (“blooms”) generated by accumulation of fluorescence from secondary antibodies on the IgG-coated bead upon secreted IgG binding in the channels adjacent to the pens. We loaded blood ASC and BM ASC on the Lightning platform and directly visualized IgG production from a single primary ASC ex vivo within 30 minutes (3, 10). Semi-quantitation of relative fluorescent signal intensity of blooms was performed using ImageJ (NIH) (3, 22). Specifically, we calculated the relative corrected total in-channel fluorescence intensity (CTiCF) across an in-channel region of interest by subtracting out the mean fluorescence of background (Reference) readings as follows: CTiCF = Integrated density – (Area of selected region x Mean fluorescence of background readings) (22).
Statistics
Statistical differences and correlation were evaluated using Excel (Microsoft) and GraphPad Prism (GraphPad Software). p values (calculated with Student’s t-test (two-tailed unpaired t-test)) of ≤0.05 were considered significance.
Results
Mature ASC have higher IgG secretion rates
We first interrogated production of total IgG in healthy human ASC ex vivo from the blood and the BM. In one-day bulk cultures of blood ASC, the average IgG secretion rate is 37 ± 19 pg/c/d, which is similar to PopA (34 ± 17 pg/c/d), but significantly lower than PopB (55 ± 26 pg/c/d) or PopD (63 ± 30 pg/c/d) (Figure 1a). While secretion rates by blood ASC or PopA are significantly lower than PopB or PopD, no significant differences exist between PopB vs PopD (Supplementary Table 1). Thus, IgG secretion rates increase from blood ASC and early BM ASC (PopA) to mature BM ASC (PopB and PopD). This finding is consistent with high Ig transcript abundance in PopD, the most mature BM ASC subset (8).
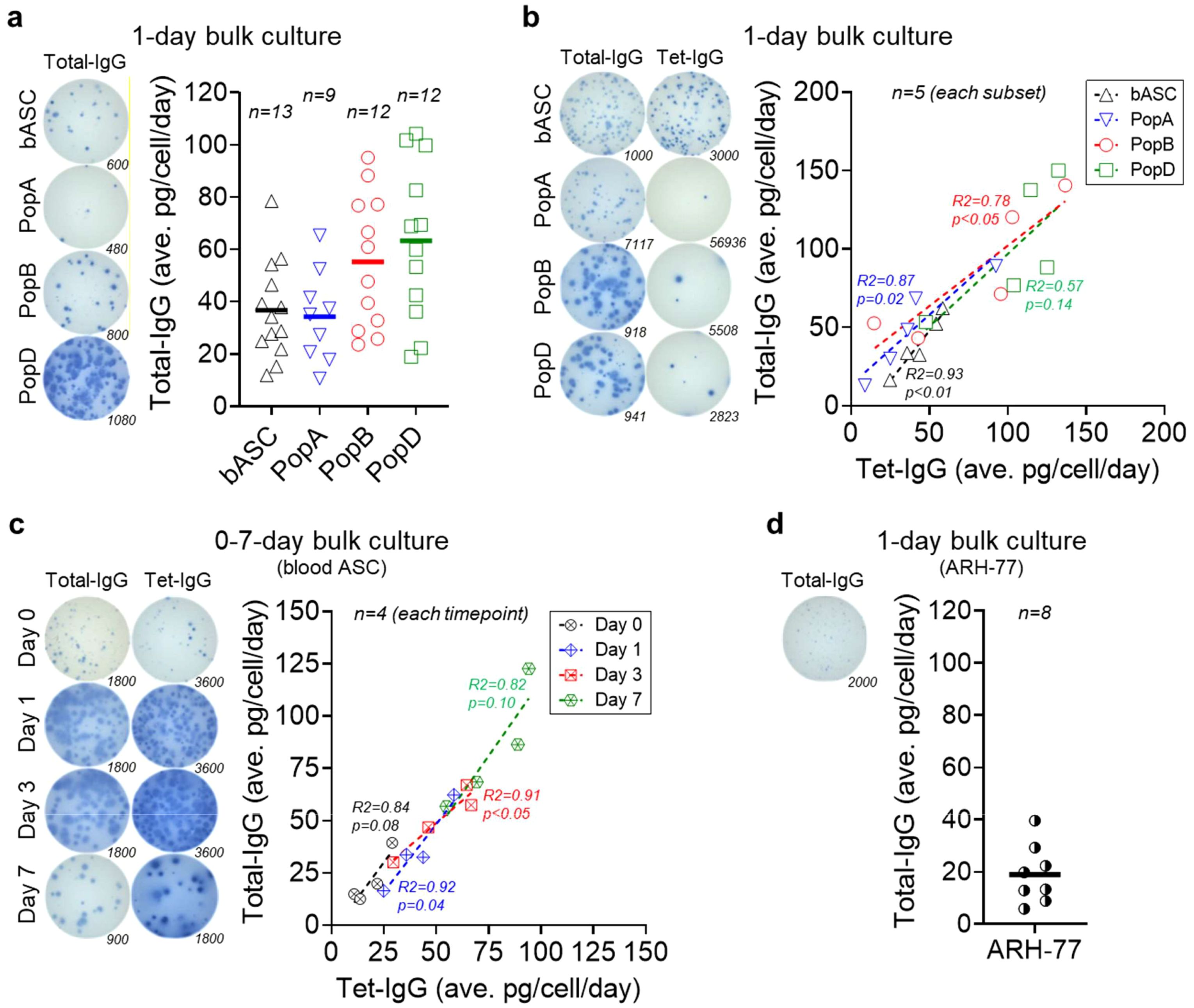
Figure 1. Mature ASC have higher IgG secretion rates by bulk culture interrogation. (a) Average total IgG secretion rates by blood ASC and BM ASC maintained in the BM mimetic bulk cultures for 1 day. (Left) Representative ELISpot scanned images; numbers indicate the quantity of the input ASC (seeded at day 0). (Right) Each symbol represents one experiment. Numbers (n) indicate the quantity of independent biological experiments. (b) Average total IgG and Tet-IgG secretion rates by blood ASC and BM ASC maintained in the BM mimetic bulk cultures for 1 day. (Left) Representative ELISpot scanned images; numbers indicate the quantity of the input ASC (seeded at day 0). (Right) Each symbol represents one experiment (per each subset). Numbers (n) indicate the quantity of independent biological experiments. (c) Average total IgG and Tet-IgG secretion rates by blood ASC maintained in the BM mimetic bulk cultures for up to 7 days. (Left) Representative ELISpot scanned images; numbers indicate the quantity of the input ASC (seeded at day 0). (Right) Each symbol represents one experiment (per each timepoint). Numbers (n) indicate the quantity of independent biological experiments. (d) Average total IgG secretion rates by ARH-77 cells maintained in the in vitro BM mimetic bulk cultures for one day. (Left) A representative ELISpot scanned image; the number indicates the quantity of the input ARH-77 cells (seeded at day 0). (Right) Each symbol represents one experiment. The number (n) indicates the quantity of independent biological experiments. For statistical differences among groups (ASC subsets or timepoints) in (a-c), see Supplementary Tables 1, 2, and 4, respectively. In (b, c), R and p values calculated from simple linear regression analysis in GraphPad Prism (GraphPad Software). In (c), blood ASC day 1 data reproduced from four out of five blood ASC experiments shown in (b). bASC, early-minted blood ASC; Tet, tetanus.
Mature ASC have higher vaccine-specific IgG secretion rates
Single cell transcriptomics of the human BM ASC show tremendous heterogeneity (8). Thus, we next examined the blood ASC from adults on day 6–7 after Tdap vaccination, which is the peak of the vaccine responses, compared to the BM ASC from adults 5–10 years after the Tdap vaccine. We measured if average Tdap vaccine-specific (Tet-) IgG secretion rates differ from total IgG secretion rates in blood and BM ASC in one-day bulk cultures (9). As expected, the frequencies of Tet-IgG ASC are 35 ± 15% of the total IgG ASC in the blood days after vaccination, compared to the Tet-IgG ASC frequences of 0.3 ± 0.2%, 1.2 ± 0.6%, and 2.4 ± 1.1% of total IgG PopA, PopB, and PopD, respectively, in the BM years after the vaccination. We also observe blood ASC and PopA are the lowest secretors: average total IgG of 39 ± 18 pg/c/d or 50 ± 30 pg/c/d, and average Tet-IgG of 43 ± 14 pg/c/d or 41 ± 31 pg/c/d for blood ASC or PopA, respectively (Figure 1b). For mature BM ASC, PopB and PopD secrete substantially higher total IgG (average of 86 ± 43 pg/c/d and 101 ± 41 pg/c/d, respectively) and Tet-IgG (average of 78 ± 49 pg/c/d and 105 ± 34 pg/c/d, respectively). In all, there are differences between average total IgG secretion rates of blood ASC vs PopB and blood ASC vs PopD, and between average antigen-specific (Tet-) IgG secretion rates of blood ASC vs PopD and PopA vs PopD (Supplementary Table 2). The similar total IgG and Tet-specific IgG secretion rates among the ASC subsets suggest that differences in secretion rates are likely influenced by the maturity of the ASC.
Blood ASC after in vitro maturation have higher IgG secretion rates
Unlike the mouse models, human ASC in vivo timestamping is difficult in order to follow early-minted blood ASC into the BM microniches. Therefore, we developed an in vitro maturation culture system to observe step-wise programs of early-minted blood ASC in long-lived phenotypes (8, 23). Using this system, we next evaluated if the same immature blood ASC could increase IgG secretion rates after in vitro maturation. We measured blood ASC total IgG and Tet-IgG secretion rates at day 0, 1, 3, and 7 in culture. Total IgG and Tet-IgG gradually accumulated in the supernatants (Supplementary Figure 3 and Supplementary Table 3), suggesting cultured blood ASC maintain functionality overtime. Assuming individual blood ASC produce the same Ig amount each day and have equal survival rates within the assessing period, we observe a progressive increase in average total IgG secretion rates: from 22 ± 12 pg/c/d at day 0 to 36 ± 19 pg/c/d at day 1, then 50 ± 16 pg/c/d at day 3, and 84 ± 29 pg/c/d at day 7 (Figure 1c). While no significant difference exists between average total IgG secretion rates for day 0 vs day 1, day 1 vs day 3, and day 3 vs day 7, there is significance between day 0 vs day 3, day 0 vs day 7, and day 1 vs day 7 (Supplementary Table 4). We also see a similar pattern of progressive increase in average Tet-IgG secretion rates: 19 ± 8 pg/c/d, 41 ± 14 pg/c/d, 52 ± 17 pg/c/d, and 77 ± 18 pg/c/d at day 0, day 1, day 3, and day 7, respectively. Thus, the same blood ASC has higher average total IgG and Tet-IgG secretion rates following in vitro maturation. These kinetics experiments of human blood ASC maturation are the first to clearly illustrate these differences.
ARH-77 myeloma cells have lower IgG secretion rates relative to healthy blood ASC
To understand secretion rates of healthy vs malignant plasma cells, we evaluated a particular myeloma cell line, ARH-77. As shown previously, the size of the IgG ELISpots of this myeloma cell line was much smaller compared to that of healthy blood ASC or BM ASC, suggesting lower secretion rates (3). Corroborating this finding, we show ARH-77 secrete on average 19 ± 11 pg/c/d (Figure 1d) which is less than the rates from blood or BM ASC. Appreciating that primary myeloma cells and myeloma cell lines are also very heterogeneous, it is likely myeloma cell secretion rates may be quite variable. Nonetheless, for this particular established myeloma cell line, secretion rates are lower than those of healthy human ASC.
Mature ASC have higher IgG secretion rates on a single cell basis
We next studied if total IgG secretion rates generated from bulk cultures are corroborated by single cell cultures after 7–8 days. We see that on a single cell basis, in blood ASC, total IgG secretion rate is 56 ± 39 pg/c/d, while for BM ASC, total IgG secretion rates are the lowest in PopA (37 ± 30 pg/c/d) and higher in PopB (71 ± 48 pg/c/d) and PopD (81 ± 64 pg/c/d), with no significant difference between PopB vs PopD (Figure 2a and Supplementary Table 5). Thus, similar to bulk cultures, mature ASC have higher total IgG secretion rates on a single cell basis. Although not statistically significant, single blood ASC surviving in 7-day single cell cultures have a greater variability of IgG secretion rates compared to 1-day bulk cultures, most likely due to ongoing maturation in the cultures even at the single cell level.
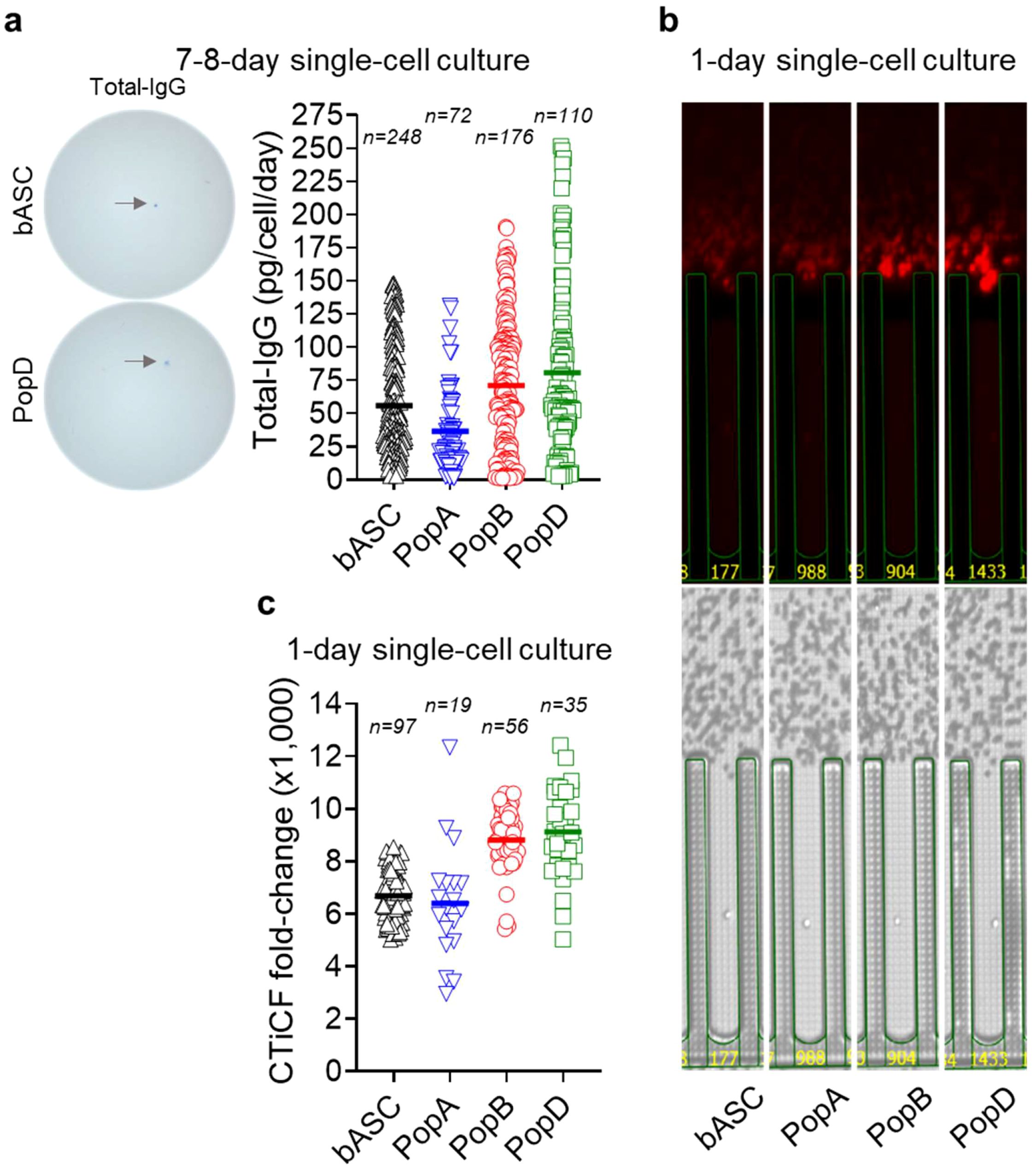
Figure 2. Mature ASC have higher IgG secretion rates by single cell culture interrogation. (a) Total IgG secretion rates by blood ASC and BM ASC maintained in the BM mimetic single-cell cultures for 7–8 days. (Left) Representative ELISpot scanned images. (Right) Each symbol represents one cell. Numbers (n) indicate the quantity of individual cells. (b) Representative image series displaying ongoing IgG secretion by a single blood ASC and BM ASC maintained in the BM mimetic single-cell cultures on-chip for one day. (Lower) Bright-field image series for direct visualization of a single cell in a (nano)pen. (Upper) The act of ongoing IgG secretion into the channel developed as fluorescent halos (“bloom”; captured at the end of an 80-minute cycle). (c) CTiCF fold-changes as an indirect comparison of total IgG secretion rates among the four ASC subsets. Each symbol represents one in-pen cell. Numbers (n) indicate the quantity of individual in-pen cells. For statistical differences among ASC subsets in (a, c), see Supplementary Tables 5 and 6, respectively. All the four ASC subsets were run on the same chips (for CTiCF comparison) and by all the same capture assays. bASC, early-minted blood ASC.
We then investigated total IgG and influenza-specific (Flu-) IgG secretion rates in single-sorted blood ASC maintained in vitro. Of 192 single-cells, total IgG and Flu-IgG are detected in 165 (~86%) and 114 (~59%), respectively, indicating high plating efficiency. We show a wide range of total IgG and Flu-IgG secretion rates (3–148 pg/c/d and 12–142 pg/c/d, respectively), with average total IgG secretion rates of 48 ± 42 pg/c/d and Flu-IgG secretion rates of 35 ± 40 pg/c/d (Supplementary Figure 4). Similar to total IgG and Tet-IgG (Figure 1b), we also note similar secretion rates for total IgG and Flu-IgG. Thus, significant differences in secretion rates may be based on the maturity of the ASC and not necessarily the antigen of interest.
Mature ASC have higher IgG secretion rates by direct single cell visualization
To overcome apoptosis and ongoing maturation in 7-day single cultures, we directly interrogated single ASC secretion rates. Total IgG blooms are captured (Figure 2b) and semi-quantitatively calculated as CTiCF fold-changes from the background (3). We show PopB and PopD secrete more IgG per cell compared to blood ASC or PopA, with average CTiCF fold-change by blood ASC, PopA, PopB, or PopD is 6,687, 6,397, 8,809, or 9,120, respectively (Figure 2c). Significant fold-change differences (p<0.01) exist between blood ASC vs PopB, blood ASC vs PopD, PopA vs PopB, and PopA vs PopD, but not (p>0.29) between blood ASC vs PopA or PopB vs PopD (Supplementary Table 6). Thus, blood ASC or PopA consistently produces less IgG per cell than PopB or PopD, suggesting increased Ig transcripts (8) lead to increased Ig secretion.
Discussion
In this study, we show that total IgG and vaccine-specific IgG secretion rates are higher in mature BM plasma cells (PopB and PopD) compared to immature blood ASC and BM PopA, the latter may include new arrivals and cells near death. As a fraction of blood ASC migrate to BM (23–25), the majority of these new arrivals die while some undergo progressive maturation (5, 11, 23, 25). Early studies showed transcriptional differences between BM plasma cells compared to earlier non-BM plasma cells (26, 27). Furthermore, single-cell RNAseq revealed heterogeneity of the human BM plasma cell pool and offered a trajectory analysis with a step-wise maturation process from new arrivals to a bona fide LLPC (8). The findings of this study provide significant insights into the functional heterogeneity of human ASC and their maturation-dependent Ig secretion capacities.
A wide range in the IgG secretion rates of human ASC have been previously documented. However, many of the previous studies used in vitro differentiated ASC from stimulated memory B cells (18, 28, 29), which may not reflect human ex vivo ASC from the blood and BM (3). Other studies were limited to blood ASC ex vivo (14, 17, 30) but did not show a comparison of BM ASC with the same methods. Furthermore, none of the studies compare differences of early-minted blood ASC found with the mature BM ASC that contain the LLPC compartments. Finally, no previous studies show a direct comparison of the IgG secretion rates from human blood and BM ASC at a single cell level. In this study, we provide definitive evidence of IgG secretion rates in early-minted ASC and mature BM ASC as well as early-minted ASC that have undergone in vitro maturation.
We demonstrate that ASC maturation is accompanied by both elevated Ig transcript levels (8) and increased Ig secretion capacity, highlighting the enhanced antibody-producing potential of mature BM plasma cells. Our results contradict previous thoughts that blood ASC had required rapid Ig production with high Ig secretion rates in response to acute infections, and that mature BM plasma cells have lower and slower secretion rates to sustain long-term immunity. Our findings align with the model that in the BM niche, ASC maturation undergo transcriptional and/or post-transcriptional adaptations such as elevated Ig transcript levels in LLPC (8) to optimize their role as antibody-secreting factories. Moreover, our findings show that the ASC maturation process provides an explanation for the wide range of secretion rates by human ASC (12, 14, 17, 18, 28–30).
A key contribution of this study is the use of a novel in vitro BM mimetic survival system (10), which allowed us to track the maturation of ASC (8, 11) and quantify their IgG secretion dynamics over time. The finding that early-minted blood ASC cultured in this system for 7 days secreted more IgG per cell than their day 0 counterparts suggests that the factors in the BM microenvironment play a critical role in driving functional maturation. This increase in Ig secretory capacity may reflect upregulation of Ig transcripts, enhanced ER function, or improved secretory pathway efficiency as ASC mature (31). These results underscore the importance of the BM niche in supporting not only plasma cell survival but also their functional specialization. This in vitro BM mimetic survival system offers a useful tool for studying human plasma cell biology, including ASC maturation in vitro.
The observed differences in IgG secretion rates between early-minted blood ASC and mature BM plasma cells have implications for understanding humoral immunity. Higher secretion rates in BM plasma cells likely contribute to the sustained antibody production required for long-term immune protection in response to vaccination or infection (7, 9). This efficiency of BM plasma cells to secrete nearly twice as much antibody per cell suggests prioritizing higher efficiency and fewer LLPC needed to achieve serologic protection. Conversely, the lower secretion rates of early-minted blood ASC may reflect their transient role in acute immune responses. These findings raise questions about the molecular mechanisms underlying the transition from early ASC to mature plasma cells, especially LLPC, including potential epigenetic or signaling pathways that regulate Ig secretion capacity.
While our study provides robust evidence for maturation-dependent increases in IgG secretion, certain limitations warrant consideration. First, the in vitro BM mimetic system, although effective in supporting ASC maturation, may not fully recapitulate the complexity of the in vivo BM niche, including interactions with cytokines or other immune cells. Second, our findings about lower IgG secretion rates in one particular myeloma cell line are specific to the ARH-77 cell line since no other myeloma cell lines or malignant human ASC were investigated. It is likely that secretion rates of other myeloma cell lines or primary myeloma cells are heterogeneous and vary significantly; and thus, one cannot conclude that myeloma cell lines or primary myeloma cells inherently secrete less antibody per cell compared to healthy ASC. Third, due to the limited cell numbers for evaluating phenotypic changes by flow cytometry, the frequencies of each BM ASC subset in cultures over time remain to be determined. Fourth, our focus on IgG secretion leaves open questions about whether similar trends apply to other Ig isotypes, such as IgA or IgM, which may be relevant in specific immune contexts. Finally, to address the detection limit of single-day bulk cultures and estimate the average secretion rates during ASC maturation, we calculated the total Ig secreted per cell per day over multiple days in bulk cultures. Although our 1-day single cell on-chip cultures confirm mature ASC have higher IgG secretion rates, this calculation does not account for variations in Ig production among different ASC over the course of multiple days. Future studies are needed to investigate the translational potential of modulating ASC maturation to enhance antibody responses in clinical settings, such as vaccine or immunotherapy.
In conclusion, our study highlights the functional specialization of human ASC as they mature into BM LLPC, with significant increases in per-cell IgG secretion rates. Our results are consistent with increased Ig transcripts of mature BM plasma cells (8) which results in higher Ig secretion rates. These findings advance our understanding of plasma cell biology and emphasize the critical role of the BM niche in optimizing antibody production. As LLPC are the basis of a successful vaccine (7), our findings emphasize the role of ongoing ASC maturation in the BM and highlight the importance of filling the BM LLPC compartment (9) for more stable and durable immunity. Further exploration of the molecular and environmental factors driving these changes will be essential for harnessing the full potential of ASC in therapeutic applications.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Emory University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DN: Writing – review & editing, Supervision, Writing – original draft, Investigation, Formal analysis, Conceptualization, Methodology, Validation, Visualization. IH: Methodology, Writing – review & editing. MC: Writing – review & editing, Methodology. SK: Writing – review & editing, Methodology. AM: Writing – review & editing, Methodology. NH: Writing – review & editing, Methodology. JA: Writing – review & editing, Resources. DR: Writing – review & editing, Resources. SL: Resources, Writing – review & editing. IS: Funding acquisition, Supervision, Writing – review & editing. FL: Writing – review & editing, Supervision, Investigation, Conceptualization, Methodology, Resources, Funding acquisition, Writing – original draft.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the following grants: NIH/NIAID 1R01 AI121252, 1P01 AI125180, R01 AI172254, U01 AI141993, U54 CA260563, U19 AI110483, and the Bill & Melinda Gates Foundation Grant INV-002351.
Acknowledgments
We thank Vivien Warren and Celia Saney (Emory University), and Drs. Eugene Gibbs and Jonathan Didier (Berkeley Lights, Inc.; now Bruker Cellular Analysis, Inc.), for technical assistance. We thank Robert Karaffa, Kametha Fife, Sommer Durham, Aaron Rae, Bridget Neary, Wayne Harris, and Ernestine Mahar (Emory University’s Flow Cytometry Cores) for technical support. We thank the clinical coordinators and donors who made this study possible.
Conflict of interest
FL is the founder of Micro-Bplex, Inc. and BeaconDx, Inc., serves on the scientific board of Be Biopharma, is a recipient of grants from the BMGF and Genentech, Inc., and has served as a consultant for Astra Zeneca. IS has consulted for GSK, Pfizer, Kayverna, Johnson & Johnson, Celgene, Bristol Myer Squibb, and Visterra. FEL, DCN, and IS are inventors of the patents concerning the plasma cell survival media related to this work including US11124766B2, US11125757B2, US12163965B2, and US12173315B2.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1644102/full#supplementary-material
Supplementary Figure 1 | General FACS gating strategy used for sorting blood ASC and BM ASC. (a) PBMC or (b) BMMC were first gated for lymphocytes, singlets, and viable cells (based on FSC/SSC and Live/Death properties). CD3 and CD14 were then used as dump markers to capture CD19+ and CD19- B cell populations. (a) Subsequent sub-gating using CD38 vs CD27 on the IgD- fraction (of CD19+ population) allows for sorting for blood ASC (IgD-CD27hiCD38hi). (b) Subsequent sub-gating from CD19+ population on the IgD- fraction (vs CD27) and using CD138 versus CD38 allowed for breaking down BM ASC populations into three subsets of interest: PopA (CD19+CD38hiCD138-), PopB (CD19+CD38hiCD138+), and PopD (LLPC; CD19-CD38hiCD138+).
Supplementary Figure 2 | Summary of the techniques and experimental designs for detection of total IgG, Tet-IgG, and Flu-IgG ASC, as well as IgG secreted in the culture supernatants by ELISpots and the Lightning platform, as well as by ELISA and MBBA, respectively. Tet, tetanus. Flu, influenza.
Supplementary Figure 3 | Cumulative total IgG and Tet-IgG by early-minted blood ASC maintained in bulk cultures for up to 7 days. Each symbol represents one experiment (per each timepoint). Numbers (n) indicate the quantity of independent biological experiments. For statistical differences among timepoints, see Supplementary Table 3.
Supplementary Figure 4 | Total IgG and Flu-IgG secretion rates by blood ASC maintained in single-cell cultures for 7–8 days. Each symbol represents one cell. The number (n) indicates the quantity of individual cells. R and p values calculated from simple linear regression analysis in GraphPad Prism (GraphPad Software) of data generated from 114 cells (out of individual 192 cells) positive for both total IgG and Flu-IgG. Flu, influenza.
References
1. Tarlinton DM, Ding Z, Tellier J, and Nutt SL. Making sense of plasma cell heterogeneity. Curr Opin Immunol. (2023) 81:102297. doi: 10.1016/j.coi.2023.102297
2. Fooksman DR, Jing Z, and Park R. New insights into the ontogeny, diversity, maturation and survival of long-lived plasma cells. Nat Rev Immunol. (2024) 24:461–70. doi: 10.1038/s41577-024-00991-0
3. Nguyen DC, Saney C, Hentenaar IT, Cabrera-Mora M, Capric V, Woodruff MC, et al. Majority of human circulating IgG plasmablasts stop blasting in a cell-free pro-survival culture. Sci Rep. (2024) 14:3616. doi: 10.1038/s41598-024-53977-2
4. Garimalla S, Nguyen DC, Halliley JL, Tipton C, Rosenberg AF, Fucile CF, et al. Differential transcriptome and development of human peripheral plasma cell subsets. JCI Insight. (2019) 4(9):e126732. doi: 10.1172/jci.insight.126732
5. Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV, et al. Long-lived plasma cells are contained within the CD19(-)CD38(hi)CD138(+) subset in human bone marrow. Immunity. (2015) 43:132–45. doi: 10.1016/j.immuni.2015.06.016
6. Slifka MK, Antia R, Whitmire JK, and Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. (1998) 8:363–72. doi: 10.1016/S1074-7613(00)80541-5
7. Amanna IJ, Carlson NE, and Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. (2007) 357:1903–15. doi: 10.1056/NEJMoa066092
8. Duan M, Nguyen DC, Joyner CJ, Saney CL, Tipton CM, Andrews J, et al. Understanding heterogeneity of human bone marrow plasma cell maturation and survival pathways by single-cell analyses. Cell Rep. (2023) 42:112682. doi: 10.1016/j.celrep.2023.112682
9. Nguyen DC, Hentenaar IT, Morrison-Porter A, Solano D, Haddad NS, Castrillon C, et al. SARS-CoV-2-specific plasma cells are not durably established in the bone marrow long-lived compartment after mRNA vaccination. Nat Med. (2024) 31(1):235–244. doi: 10.1101/2024.03.02.24303242
10. Nguyen DC, Garimalla S, Xiao H, Kyu S, Albizua I, Galipeau J, et al. Factors of the bone marrow microniche that support human plasma cell survival and immunoglobulin secretion. Nat Commun. (2018) 9:3698. doi: 10.1038/s41467-018-05853-7
11. Joyner CJ, Ley AM, Nguyen DC, Ali M, Corrado A, Tipton C, et al. Generation of human long-lived plasma cells by developmentally regulated epigenetic imprinting. Life Sci Alliance. (2022) 5(3):e202101285. doi: 10.26508/lsa.202101285
12. Hibi T and Dosch HM. Limiting dilution analysis of the B cell compartment in human bone marrow. Eur J Immunol. (1986) 16:139–45. doi: 10.1002/eji.1830160206
13. Kometani K, Nakagawa R, Shinnakasu R, Kaji T, Rybouchkin A, Moriyama S, et al. Repression of the transcription factor Bach2 contributes to predisposition of IgG1 memory B cells toward plasma cell differentiation. Immunity. (2013) 39:136–47. doi: 10.1016/j.immuni.2013.06.011
14. Corti D and Lanzavecchia A. Efficient methods to isolate human monoclonal antibodies from memory B cells and plasma cells. Microbiol Spectr. (2014) 2(5). doi: 10.1128/microbiolspec.AID-0018-2014
15. Eyer K, Doineau RCL, Castrillon CE, Briseno-Roa L, Menrath V, Mottet G, et al. Single-cell deep phenotyping of IgG-secreting cells for high-resolution immune monitoring. Nat Biotechnol. (2017) 35:977–82. doi: 10.1038/nbt.3964
16. Lanzavecchia A. Dissecting human antibody responses: useful, basic and surprising findings. EMBO Mol Med. (2018) 10(3):e8879. doi: 10.15252/emmm.201808879
17. Bromage E, Stephens R, and Hassoun L. The third dimension of ELISPOTs: quantifying antibody secretion from individual plasma cells. J Immunol Methods. (2009) 346:75–9. doi: 10.1016/j.jim.2009.05.005
18. Pinna D, Corti D, Jarrossay D, Sallusto F, and Lanzavecchia A. Clonal dissection of the human memory B-cell repertoire following infection and vaccination. Eur J Immunol. (2009) 39:1260–70. doi: 10.1002/eji.200839129
19. Werner-Favre C, Matthes T, Barnet M, and Zubler RH. High IgE secretion capacity of human plasma cells. Eur J Immunol. (1993) 23:2038–40. doi: 10.1002/eji.1830230849
20. Salmon SE and Smith BA. Immunoglobulin synthesis and total body tumor cell number in IgG multiple myeloma. J Clin Invest. (1970) 49:1114–21. doi: 10.1172/JCI106327
21. Nguyen DC, Duan M, Ali M, Ley A, Sanz I, and Lee FE. Plasma cell survival: The intrinsic drivers, migratory signals, and extrinsic regulators. Immunol Rev. (2021) 303:138–53. doi: 10.1111/imr.13013
22. Shihan MH, Novo SG, Le Marchand SJ, Wang Y, and Duncan MK. A simple method for quantitating confocal fluorescent images. Biochem Biophys Rep. (2021) 25:100916. doi: 10.1016/j.bbrep.2021.100916
23. Liu X, Yao J, Zhao Y, Wang J, and Qi H. Heterogeneous plasma cells and long-lived subsets in response to immunization, autoantigen and microbiota. Nat Immunol. (2022) 23:1564–76. doi: 10.1038/s41590-022-01345-5
24. Koike T, Fujii K, Kometani K, Butler NS, Funakoshi K, Yari S, et al. Progressive differentiation toward the long-lived plasma cell compartment in the bone marrow. J Exp Med. (2023) 220(2):e20221717. doi: 10.1084/jem.20221717
25. Robinson MJ, Ding Z, Dowling MR, Hill DL, Webster RH, McKenzie C, et al. Intrinsically determined turnover underlies broad heterogeneity in plasma-cell lifespan. Immunity. (2023) 56:1596–1612 e4. doi: 10.1016/j.immuni.2023.04.015
26. Underhill GH, George D, Bremer EG, and Kansas GS. Gene expression profiling reveals a highly specialized genetic program of plasma cells. Blood. (2003) 101:4013–21. doi: 10.1182/blood-2002-08-2673
27. Tarte K, Zhan F, De Vos J, Klein B, and Shaughnessy J Jr. Gene expression profiling of plasma cells and plasmablasts: toward a better understanding of the late stages of B-cell differentiation. Blood. (2003) 102:592–600. doi: 10.1182/blood-2002-10-3161
28. Henn AD, Rebhahn J, Brown MA, Murphy AJ, Coca MN, Hyrien O, et al. Modulation of single-cell IgG secretion frequency and rates in human memory B cells by CpG DNA, CD40L, IL-21, and cell division. J Immunol. (2009) 183:3177–87. doi: 10.4049/jimmunol.0804233
29. Cheng RY, Hung KL, Zhang T, Stoffers CM, Ott AR, Suchland ER, et al. Ex vivo engineered human plasma cells exhibit robust protein secretion and long-term engraftment in vivo. Nat Commun. (2022) 13:6110. doi: 10.1038/s41467-022-33787-8
30. Corti D, Voss J, Gamblin SJ, Codoni G, Macagno A, Jarrossay D, et al. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. (2011) 333:850–6. doi: 10.1126/science.1205669
Keywords: immunoglobulin, antibody-secreting cell, plasma cell, blood, bone marrow, secretion, survival, maturation
Citation: Nguyen DC, Hentenaar IT, Cabrera-Mora M, Kyu S, Morrison-Porter A, Haddad NS, Andrews J, Roberts D, Lonial S, Sanz I and Lee FE-H (2025) Maturation of human early-minted blood antibody-secreting cells is coupled with increased IgG secretion rates. Front. Immunol. 16:1644102. doi: 10.3389/fimmu.2025.1644102
Received: 09 June 2025; Accepted: 07 August 2025;
Published: 02 September 2025.
Edited by:
Manabu Sugai, University of Fukui, JapanReviewed by:
Tobit Steinmetz, University Medical Center Groningen, NetherlandsAkihiko Muto, Tohoku University, Japan
Copyright © 2025 Nguyen, Hentenaar, Cabrera-Mora, Kyu, Morrison-Porter, Haddad, Andrews, Roberts, Lonial, Sanz and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Doan C. Nguyen, ZG9hbi5jLm5ndXllbkBlbW9yeS5lZHU=
†ORCID: Doan C. Nguyen, orcid.org/0000-0002-5389-6731
Ignacio Sanz, orcid.org/0000-0003-4182-587X
F. Eun-Hyung Lee, orcid.org/0000-0002-6133-5942
 Doan C. Nguyen
Doan C. Nguyen Ian T. Hentenaar1
Ian T. Hentenaar1 Natalie S. Haddad
Natalie S. Haddad Ignacio Sanz
Ignacio Sanz F. Eun-Hyung Lee
F. Eun-Hyung Lee