- 1Department of Dermatology, Shanghai Skin Disease Hospital, Tongji University School of Medicine, Shanghai, China
- 2Institute of Psoriasis, Tongji University School of Medicine, Shanghai, China
Background: Patients with psoriasis have an increased risk of developing cardiovascular disease(CVD), yet reliable potential markers for cardiovascular risk assessment remain insufficient. The triglyceride-glucose (TyG) index and TyG-body mass index (TyG-BMI) have been identified as potential indicators of metabolic and cardiovascular risk in the general population. However, their specific role in psoriasis-related CVD has not been systematically evaluated.
Objective: This study aimed to investigate the relationship between TyG index, TyG-BMI index and CVD in patients with psoriasis.
Methods: This cross-sectional study utilized data from the Shanghai Psoriasis Efficacy Evaluation Cohort (SPEECH), which includes Chinese patients with moderate to severe psoriasis. Defined by strict inclusion and exclusion criteria, 1112 psoriasis patients were included. CVD included any cerebrovascular events and coronary atherosclerotic heart disease. Multivariate logistic regression and restricted cubic spline analysis (RCS) were used to examine the relationship between TyG index, TyG-BMI index, and CVD risk. Subgroup and sensitivity analyses were conducted to validate the robustness of the findings.
Results: Among the study participants, 223 (20.1%) had CVD. Compared to non-CVD patients, those with CVD were older, had a higher BMI, a higher prevalence of hypertension and diabetes, a longer course of psoriasis, higher levels of blood glucose, triglycerides, and higher levels of TyG index and TyG-BMI index. Subsequent multivariate logistic regression analysis showed a significant positive association between TyG index, TyG-BMI index with CVD risk in psoriasis patients. RCS analysis showed that there was a dose-response relationship between TyG index and TyG-BMI index with CVD risk. Subgroup analysis showed that TyG index and TyG-BMI index were significantly associated with CVD in most subgroups. Patients who were excluded from systematic therapy were subjected to sensitivity analysis, and the results were consistent with the main analysis, suggesting a robust correlation between TyG index and TyG-BMI index and CVD.
Conclusion: Elevated TyG and TyG-BMI indices are independently associated with CVD in psoriasis patients, suggesting their potential as practical markers for cardiovascular risk stratification in this population.
Introduction
Psoriasis is a chronic inflammatory skin disease affects approximately 2-3% of the global population. Its impact on the body has gone beyond the skin. Existing epidemiologic studies reveal patients with psoriasis have an increased risk for cardiovascular disease (CVD), which are common causes of morbidity and mortality in psoriasis (1–5). The early intervention of cardiovascular abnormalities is therefore of utmost importance for improving the prognosis of these patients. The link between psoriasis and CVD is thought to be related to the systemic inflammation and metabolic disturbances seen in psoriatic patients (1). In recent years, interest has grown in identifying biomarkers that may help evaluate CVD burden in psoriasis patients.
Insulin resistance(IR) is a key driver of both metabolic dysfunction and CVD. Several lipid- and glucose-derived indices have emerged as reliable surrogate markers for insulin resistance (IR), including the triglyceride-glucose (TyG) index, TyG-body mass index(TyG-BMI), Triglycerides to High-Density Lipoprotein Cholesterol ratio (TG/HDL-C), the metabolic score for insulin resistance (METS-IR) and homeostatic model assessment of IR (HOMA-IR) (6, 7). Among them, the TyG index combines two key metabolic parameters, fasting triglycerides (TG) and fasting plasma glucose (FPG), and can sensitively reflect the IR status. It is simple to calculate, cost-effective, and widely accessible (8). Recent study have shown that the combination of TyG index and BMI can significantly enhance the effectiveness of IR (9). Several observational studies have suggested an association between elevated TyG and TyG-BMI indices and an increased risk of CVD in the general population (10, 11). However, the specific impact of these index on CVD risk in patients with psoriasis remains unclear and has not been extensively studied.
Therefore, the aim of this study is to investigate the association between TyG index, TyG-BMI index and CVD in patients with psoriasis. Understanding the association between these index and CVD risk in psoriasis patients may provide insights into potential biomarkers for identifying those at increased risk of cardiovascular complications.
Methods
Study design
This cross-sectional study is based on data from the Shanghai Psoriasis Effectiveness Evaluation Cohort (SPEECH). SPEECH is a multicenter observational cohort study of psoriasis in China, which includes psoriasis patients treated with acitretin, methotrexate, phototherapy, and various biologics. The aim of SPEECH is to investigate the characteristics of psoriasis in the Chinese population and to identify an appropriate treatment protocol for psoriasis that takes into account the specific needs of the Chinese population and national conditions. The Ethics Committee of the Shanghai Skin Disease Hospital (#2020-36) thoroughly reviewed this prospective cohort study. All participants provided informed consent.
Participants
Patients were screened from the SPEECH database for inclusion in this study. The inclusion criteria are as follows: 1. Adults diagnosed with moderate-to-severe plaque psoriasis; 2. Routine blood tests, fasting glucose, and triglyceride tests require venous blood samples to be collected early in the morning. The main exclusion criteria include a lack of baseline information, venous blood samples or other important data.
Data acquisition
All analysis data were directly extracted from the database and included variables such as age, gender, Body Mass Index(BMI), smoking history, drinking history, hypertension history, diabetes history, duration of psoriasis, psoriatic arthritis (PSA), psoriasis area and severity index (PASI), and family history of psoriasis. In addition, blood samples were collected from all participants in the early morning to analyze fasting blood glucose and triglyceride levels. All clinical and laboratory data were collected at the time of cohort entry (baseline), prior to initiation or change of systemic therapy, as part of the standardized SPEECH baseline assessment. Smoking and drinking were categorized as “Never” or “Past or current”. Psoriasis-related inquiries, assessment of PASI, and diagnosis of PSA were independently performed by a specialized dermatologist. The diagnosis of PSA was based on the CASPAR diagnostic criteria. Comorbidity data for psoriasis were obtained through self-reported information on a health questionnaire or from past medical records. Participants were asked, “Have you ever been diagnosed with hypertension/diabetes/MAFLD by a doctor?” A response of “yes” indicated a case of hypertension/diabetes/MAFLD. If the patient had a clear diagnosis of hypertension, diabetes, or MAFLD in their medical record, it was considered as a case of hypertension, diabetes, or MAFLD.
Data on CVD were also obtained through questionnaires and past medical records. Participants were asked, “Has a doctor or other healthcare professional ever told you that you have congestive heart failure/coronary heart disease/angina pectoris/myocardial infarction/stroke?” Any response of “yes” to these questions indicated a case of CVD. If the patient had a clear diagnosis of any of these diseases in their previous medical record, it was considered as a case of CVD. TyG is defined as Ln [triglyceride (mg/dL)×fasting blood glucose (mg/dL)/2]; and TyG-BMI is calculated as TyG x BMI (kg/m2).
Statistical analysis
The primary method of analysis in this study is cross-sectional analysis. The group was divided into two categories based on the presence or absence of CVD. Continuous variables that exhibit a normal distribution are reported as mean ± standard deviation (SD) and evaluated using the student t-test. Non-normally distributed continuous variables were presented with the median (interquartile range [IQR]) and compared using the Mann-Whitney U test. Categorical variables are expressed as frequency (%) and evaluated using Chi-square tests or Fisher’s exact tests.
The patients were divided into three groups according to the tertiles level of the TyG and TyG-BMI in ascending order of Q1, Q2, and Q3. Covariates included age, gender, BMI, smoking history, drinking history, hypertension, diabetes, PSA, duration of psoriasis, family history and PASI score based on previous literature. Three models were developed to investigate the correlation: Model 1 did not include any covariates, Model 2 only adjusted for age, gender, smoking history, drinking history, and BMI, and Model 3 included all covariates for analysis. When using TyG-BMI as the target variable, BMI was not used as a covariate. Odds ratios (OR) and 95% confidence intervals (95% CI) for different groups of IR indictors were calculated using univariate logistic regression and multivariate logistic regression. And the trend test was also carried out. Multiple linear regression describes the relationship between the IR indicator and CVD when TyG and TyG-BMI indices considered as a numerical variable. Subgroup analysis was then conducted to further explore potential interactions and influencing factors. Due to the potential impact of psoriasis systemic treatment on CVD, patients who had previously received systemic therapy were excluded from the sensitivity analysis. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using R version 4.2.1.
Results
Characteristics of the study participants
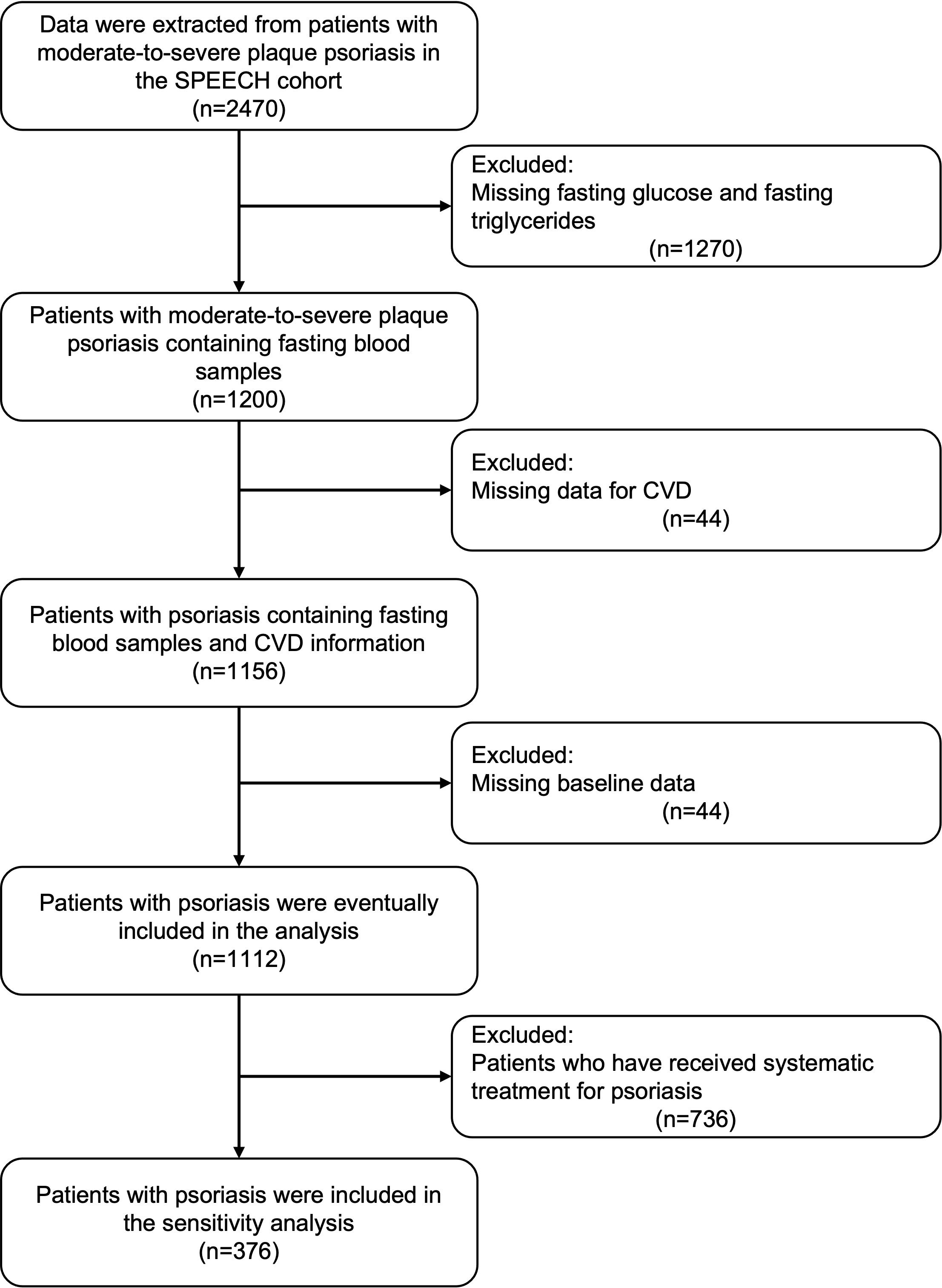
After screening the patient information of the SPEECH cohort according to the exclusion criteria, a total of 1112 patients were included in the present study (Figure 1). Among them, 223 patients (20.1%) were found to have CVD. Consistent with previous studies, patients with CVD had a higher BMI, higher prevalence of hypertension and Type 2 diabetes mellitus (T2DM), and higher levels of fasting blood glucose, triglycerides, TyG, and TyG-BMI. Surprisingly, patients with CVD had a shorter disease duration and milder PASI (Table 1).
Association between TyG index and CVD in psoriasis
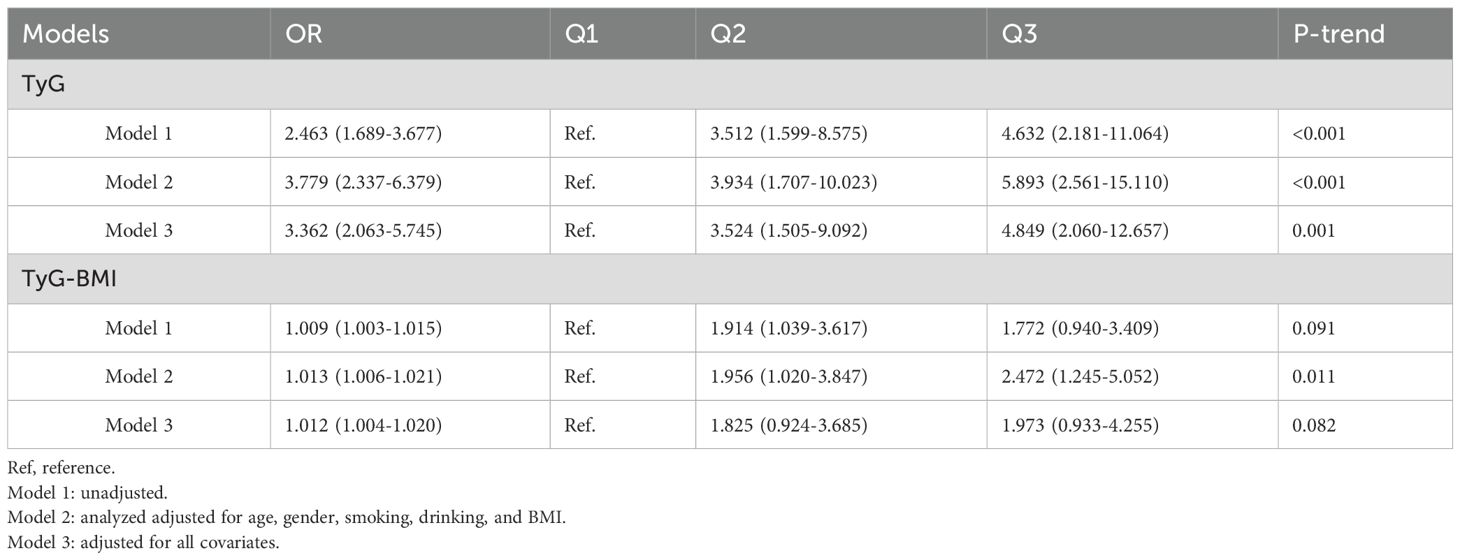
The relationships between TyG-related index and CVD are presented in Table 2. We found that TyG and TyG-BMI were significantly associated with CVD in psoriasis, with a dose-dependent correlation observed in Q2 and Q3 compared to Q1.

Table 2. Association of the TyG index, TyG-BMI index with cardiovascular disease in patients with psoriasis.
To investigate the presence of subgroup differences in terms of the association between continuous TyG-related index and CVD, we conducted subgroup analyses according to the potential atherosclerosis risk factors, including age, gender, family history of psoriasis, smoking/drinking history, BMI, and comorbidities (Figure 2). Overall, there were no significant interactions detected in most categories (P > 0.05), except age, hypertension, T2DM for the outcomes of CVD. However, these variables appear to have minimal impact on the robustness of the results, as TyG-related index remain significantly associated with CVD in psoriasis across all subgroups (P < 0.05). It is worth noting that in patients with elevated triglycerides, the association between TyG and CVD was still maintained a good correlation.
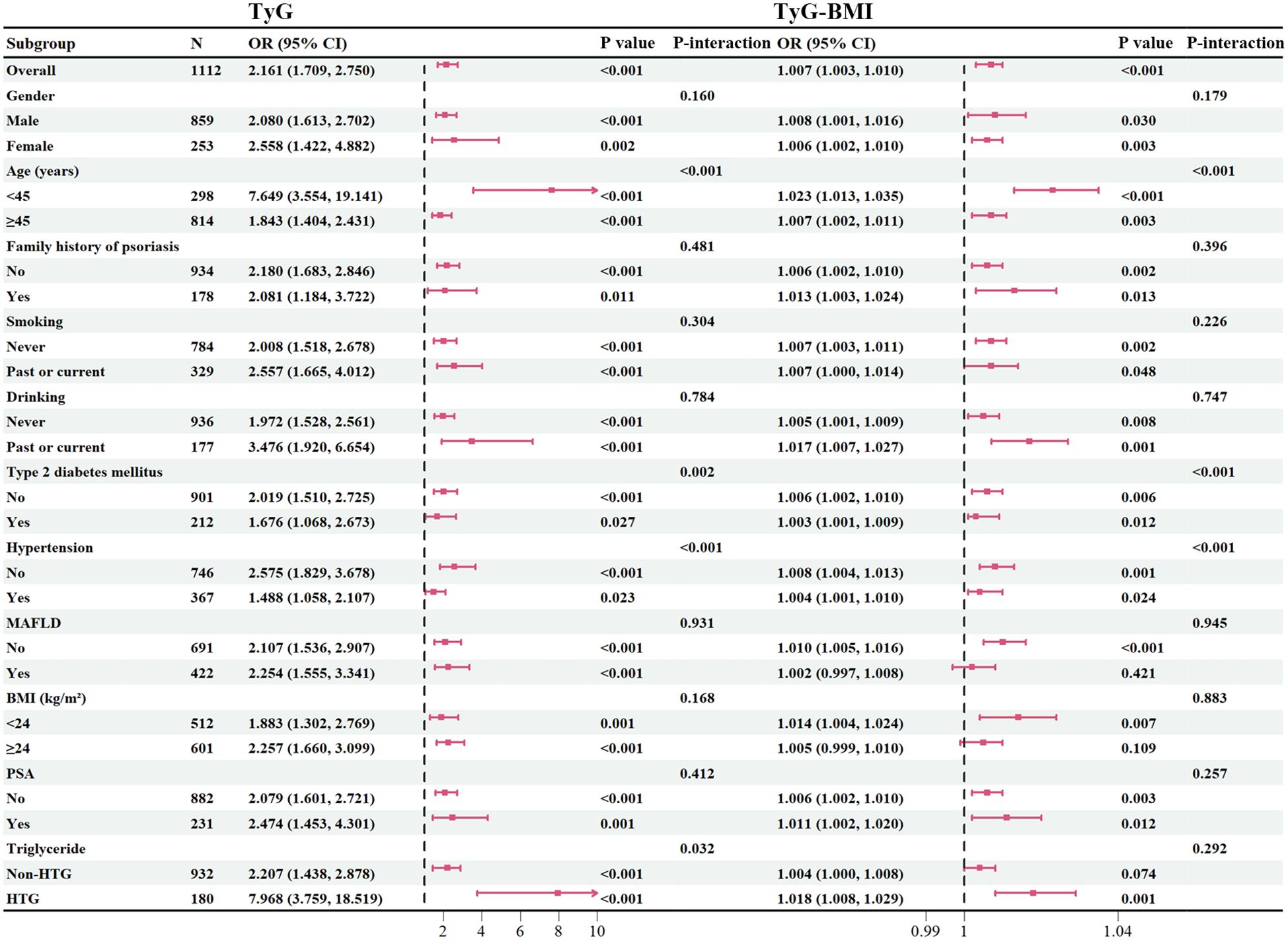
Figure 2. Subgroup analysis. BMI, body mass index; PSA, psoriatic arthritis; TyG, triglyceride-glucose; TyG-BMI, triglyceride glucose-body mass index; HTG, hypertriglyceridemia.
Sensitivity analysis
A sensitivity analysis was performed excluding patients who had previously received systemic treatments that might influence cardiovascular risk profiles. Acitretin and Tumor Necrosis Factor-α(TNF-α) inhibitors were selected because of their potential metabolic and inflammatory effects that could confound associations between TyG-related indices and CVD. Although acitretin is not typically associated with an increased risk of major cardiovascular events, it has been linked to alterations in lipid metabolism, which might affect TyG index values. TNF-α inhibitors may improve cardiovascular outcomes via systemic inflammation reduction, potentially attenuating observed associations. We further assessed the predictive ability of TyG-related index for CVD beyond established risk factors in basic Models 1, 2 and 3. We found that TyG were significantly associated with CVD in psoriasis, with a dose-dependent correlation observed in Q2 and Q3 compared to Q1 (Table 3) in Model 1 to 3. But TyG-BMI was only associated with CVD in psoriasis in Model 2 in sensitivity analysis.
Discussion
Psoriasis has been increasingly characterized as a systemic inflammatory disorder that contributes to heightened cardiovascular disease (CVD) risk. Identifying potential biomarkers of CVD risk in psoriasis patients is therefore critical to facilitate early intervention and improve long-term outcomes. In the present study, we found that an increase in TyG related-index was significantly associated with CVD in psoriasis patients. These associations persisted across various subgroups and remained robust in sensitivity analyses. Our finding demonstrated that TyG related-index is associated with the occurrence of CVD in psoriasis and can be used as a simple and effective cardiovascular risk assessment tool in daily practice.
A convincing deal of evidence supports that psoriasis is associated with CVD (12–14). A prospective, population-based cohort study of 130,000 patients with psoriasis and 500,000 controls reported an overall 50% increased risk of myocardial infarction(MI) in patients with psoriasis (15). Compared to controls, severe psoriasis confers the highest CV risk, including up to a 3-fold increased odds of MI, 60% higher odds of stroke, and 40% higher odds of CV death (15). A meta-analysis including 75 studies with 503,686 psoriasis patients reported up to a 50% increased odds of CVD in psoriasis compared to controls without psoriasis (16). However, some studies have shown no correlation between psoriasis and CVD risk (17–19). The conflicting results may be related to region and race, disease severity, disease course, follow-up time, confounding factors, etc.
The precise underlying mechanisms linking psoriasis and CVD are not well defined. Psoriasis shares common pathophysiologic mechanisms with atherosclerosis and cardiovascular risk factors. The Polymorphism of interleukin (IL)-23R and IL-23 genes, as well as other genes involved in lipid and fatty-acid metabolism, renin-angiotensin system and endothelial function, have been described in patients with psoriasis and with cardiovascular risk factors. Moreover, systemic inflammation in patients with psoriasis, including elevated serum proinflammatory cytokines (e.g.,TNF-α, IL-17, and IL-23) may contribute to an increased risk of atherosclerosis, hypertension, alteration of serum lipid composition, and IR (20). Platelets overactivation may contribute to initiation and progression of immune responses in lesional skin and blood vessels, ultimately leading to psoriasis and its comorbidities such as atherosclerosis, ischemic heart disease, stroke, MI, and other CVD (21). An clinical randomized trial (RCT) indicates that psoriatic patients treated with TNF inhibitors have a significantly lower risk of MI (22).
The TyG index is a surrogate marker of IR, a central feature of metabolic syndrome. The prevalence of metabolic syndrome is increased in psoriasis and has been linked to higher coronary atherosclerosis in this population (23, 24). The TyG index relates to cardiometabolic risk factors, IR, and subclinical atherosclerosis in psoriasis (25). The link between psoriasis and metabolic syndrome is partly attributed to systemic inflammation. However, other chronic inflammatory skin diseases such as atopic dermatitis also involve systemic inflammation, but the association with metabolic syndrome is less clear (26). Differences in immune activation may contribute to this discrepancy. Nonetheless, CVD has also been reported in atopic dermatitis, even in subclinical stages (27, 28). Several studies have identified a link between hidradenitis suppurativa (HS) and CVD (29–31). Therefore, the TyG index also has a potential role in detecting CVD in other chronic inflammations such as atopic dermatitis and HS. Although the metabolic participation in these chronic inflammations is relatively small, the cardiovascular risk is relatively high.
In addition to TyG index, some other indicators such as the ratio of neutrophils to lymphocytes (NLR) and the ratio of monocytes to high-density lipoprotein (MHR) are also used to assess cardiovascular risk factors (32, 33). These indicators integrate inflammation and lipid metabolism, making up for the deficiencies of traditional risk assessment in patients with psoriasis, and is low-cost and easily accessible. With growing recognition of the systemic implications of psoriatic disease, dermatologists are increasingly engaged in cardiovascular risk management (34, 35) Incorporating simple and reliable markers like TyG, NLR, or MHR into dermatologic practice may help identify high-risk patients earlier, guide timely referrals, and improve long-term health outcomes.
One of the major strengths of this study is the use of a large, well-characterized psoriasis cohort, allowing for a comprehensive evaluation of TyG index and TyG-BMI index in relation to CVD. Additionally, the robust statistical approach, including multivariate adjustments, subgroup analyses, and sensitivity analyses, ensures the reliability of our findings. From a clinical perspective, our study highlights the need to integrate metabolic markers such as TyG index and TyG-BMI index into routine cardiovascular risk assessment for psoriasis patients. Unlike traditional markers, these indices are easily calculated from fasting blood glucose and triglyceride levels, making them accessible and cost-effective for widespread clinical application.
This study has several limitations. First, given the cross-sectional data structure, causation cannot be established regarding the association. The further accumulation of cases with a prospective cohort study are needed to prove that causal link. Second, although we adjusted for multiple confounders, residual confounding cannot be completely excluded due to the observational nature of our analysis. Third, our study cohort was derived from a single ethnic population, which may limit the generalizability of our findings to other populations. Lastly, although we have demonstrated the association among TyG index, TyG-BMI index and CVD, the role of low-density lipoprotein cholesterol (LDL-C) as a traditional cardiovascular risk factor in patients with psoriasis still cannot be ignored. There is a potential synergistic effect between TyG index and LDL-C in the risk of cardiovascular onset in psoriasis. Therefore, in the CVD risk assessment of patients with psoriasis, the combined detection of TyG index and LDL-C can provide more comprehensive risk stratification information. Future studies can further explore the interaction between TyG index and LDL-C to optimize the cardiovascular risk management strategies for patients with psoriasis.
Conclusion
In the psoriasis population, an elevated level of TyG index and TyG-BMI index is linked to cardiovascular diseases, even after adjusting for traditional cardiovascular diseases risk factors. With the advantages of being simple, accessible and reliable, the TyG index and TyG-BMI index may be a useful tool for cardiovascular risk assessment for psoriasis patients in daily practice.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the Ethical Committee of Shanghai Skin Disease Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YL: Writing – original draft. DH: Data curation, Investigation, Writing – original draft. BM: Methodology, Writing – original draft, Resources. YJ: Writing – original draft, Methodology, Investigation, Formal analysis. BY: Resources, Writing – review & editing. JZ: Investigation, Writing – review & editing. YD: Writing – review & editing, Resources. YS: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was sponsored by grants from the National Key Research and Development Program of China (No.2023YFC2508106), National Natural Science Foundation of China (No.82430101, 82273510, 82203913), Innovation Program of Shanghai Municipal Education Commission (No.2025GDZKZD06), Shanghai Dermatology Research Center(No.2023ZZ02017), Clinical Research Plan of SHDC (No. SHDC22022302), Shanghai Municipal Health Commission Health Industry Clinical Research Project(No.20224Y0375).
Conflict of interest
The author(s) declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Garshick MS, Ward NL, Krueger JG, and Berger JS. Cardiovascular risk in patients with psoriasis: JACC review topic of the week. J Am Coll Cardiol. (2021) 77:1670–80. doi: 10.1016/j.jacc.2021.02.009
2. Gelfand JM, Troxel AB, Lewis JD, Kurd SK, Shin DB, Wang X, et al. The risk of mortality in patients with psoriasis: results from a population-based study. Arch Dermatol. (2007) 143:1493–9. doi: 10.1001/archderm.143.12.1493
3. Ogdie A, Yu Y, Haynes K, Love TJ, Maliha S, Jiang Y, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. (2015) 74:326–32. doi: 10.1136/annrheumdis-2014-205675
4. Boehncke WH, Boehncke S, Tobin AM, and Kirby B. The ‘psoriatic march’: a concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol. (2011) 20:303–7. doi: 10.1111/j.1600-0625.2011.01261.x
5. Gao N, Kong M, Li X, Zhu X, Wei D, Ni M, et al. The association between psoriasis and risk of cardiovascular disease: A Mendelian randomization analysis. Front Immunol. (2022) 13:918224. doi: 10.3389/fimmu.2022.918224
6. Duan M, Zhao X, Li S, Miao G, Bai L, Zhang Q, et al. Metabolic score for insulin resistance (METS-IR) predicts all-cause and cardiovascular mortality in the general population: evidence from NHANES 2001-2018. Cardiovasc Diabetol. (2024) 23:243. doi: 10.1186/s12933-024-02334-8
7. Jiang L, Zhu T, Song W, Zhai Y, Tang Y, Ruan F, et al. Assessment of six insulin resistance surrogate indexes for predicting stroke incidence in Chinese middle-aged and elderly populations with abnormal glucose metabolism: a nationwide prospective cohort study. Cardiovasc Diabetol. (2025) 24:56. doi: 10.1186/s12933-025-02618-7
8. de la Monte SM. Therapeutic targets of brain insulin resistance in sporadic Alzheimer’s disease. Front Biosci (Elite Ed). (2012) 4:1582–605. doi: 10.2741/e482
9. Bala C, Gheorghe-Fronea O, Pop D, Pop C, Caloian B, Comsa H, et al. The association between six surrogate insulin resistance indexes and hypertension: A population-based study. Metab Syndr Relat Disord. (2019) 17:328–33. doi: 10.1089/met.2018.0122
10. Su J, Li Z, Huang M, Wang Y, Yang T, Ma M, et al. Triglyceride glucose index for the detection of the severity of coronary artery disease in different glucose metabolic states in patients with coronary heart disease: a RCSCD-TCM study in China. Cardiovasc Diabetol. (2022) 21:96. doi: 10.1186/s12933-022-01523-7
11. Wang X, Xu W, Song Q, Zhao Z, Meng X, Xia C, et al. Association between the triglyceride-glucose index and severity of coronary artery disease. Cardiovasc Diabetol. (2022) 21:168. doi: 10.1186/s12933-022-01606-5
12. Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, and Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. (2010) 31:1000–6. doi: 10.1093/eurheartj/ehp567
13. Masson W, Lobo M, and Molinero G. Psoriasis and cardiovascular risk: A comprehensive review. Adv Ther. (2020) 37:2017–33. doi: 10.1007/s12325-020-01346-6
14. Samarasekera EJ, Neilson JM, Warren RB, Parnham J, and Smith CH. Incidence of cardiovascular disease in individuals with psoriasis: a systematic review and meta-analysis. J Invest Dermatol. (2013) 133:2340–6. doi: 10.1038/jid.2013.149
15. Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, and Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. (2006) 296:1735–41. doi: 10.1001/jama.296.14.1735
16. Miller IM, Ellervik C, Yazdanyar S, and Jemec GB. Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. (2013) 69:1014–24. doi: 10.1016/j.jaad.2013.06.053
17. Dowlatshahi EA, Kavousi M, Nijsten T, Ikram MA, Hofman A, Franco OH, et al. Psoriasis is not associated with atherosclerosis and incident cardiovascular events: the Rotterdam Study. J Invest Dermatol. (2013) 133:2347–54. doi: 10.1038/jid.2013.131
18. Egeberg A, Thyssen JP, Jensen P, Gislason GH, and Skov L. Risk of myocardial infarction in patients with psoriasis and psoriatic arthritis: A nationwide cohort study. Acta Derm Venereol. (2017) 97:819–24. doi: 10.2340/00015555-2657
19. Parisi R, Rutter MK, Lunt M, Young HS, Symmons DPM, Griffiths CEM, et al. Psoriasis and the risk of major cardiovascular events: cohort study using the clinical practice research datalink. J Invest Dermatol. (2015) 135:2189–97. doi: 10.1038/jid.2015.87
20. Piaserico S, Orlando G, and Messina F. Psoriasis and cardiometabolic diseases: shared genetic and molecular pathways. Int J Mol Sci. (2022) 23. doi: 10.3390/ijms23169063
21. Jiang Z, Jiang X, Chen A, and He W. Platelet activation: a promoter for psoriasis and its comorbidity, cardiovascular disease. Front Immunol. (2023) 14:1238647. doi: 10.3389/fimmu.2023.1238647
22. Wu JJ, Poon KY, Channual JC, and Shen AY. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. (2012) 148:1244–50. doi: 10.1001/archdermatol.2012.2502
23. Langan SM, Seminara NM, Shin DB, Troxel AB, Kimmel SE, Mehta NN, et al. Prevalence of metabolic syndrome in patients with psoriasis: a population-based study in the United Kingdom. J Invest Dermatol. (2012) 132:556–62. doi: 10.1038/jid.2011.365
24. Teklu M, Zhou W, Kapoor P, Patel N, Dey AK, Sorokin AV, et al. Metabolic syndrome and its factors are associated with noncalcified coronary burden in psoriasis: An observational cohort study. J Am Acad Dermatol. (2021) 84:1329–38. doi: 10.1016/j.jaad.2020.12.044
25. O’Hagan R, Gonzalez-Cantero A, Patel N, Hong CG, Berg AR, Li H, et al. Association of the triglyceride glucose index with insulin resistance and subclinical atherosclerosis in psoriasis: An observational cohort study. J Am Acad Dermatol. (2023) 88:1131–4. doi: 10.1016/j.jaad.2022.08.027
26. Shalom G, Dreiher J, Kridin K, Horev A, Khoury R, Battat E, et al. Atopic dermatitis and the metabolic syndrome: a cross-sectional study of 116–816 patients. J Eur Acad Dermatol Venereol. (2019) 33:1762–7. doi: 10.1111/jdv.15642
27. Wan J, Fuxench ZCC, Wang S, Syed MN, Shin DB, Abuabara K, et al. Incidence of cardiovascular disease and venous thromboembolism in patients with atopic dermatitis. J Allergy Clin Immunol Pract. (2023) 11:3123–32.e3. doi: 10.1016/j.jaip.2023.08.007
28. Berna-Rico E, Perez-Garcia B, Abbad-Jaime de Aragon C, Neria F, Monge D, Perez-Bootello J, et al. Subclinical atherosclerosis is increased in moderate-to-severe atopic dermatitis. J Eur Acad Dermatol Venereol. (2025). doi: 10.1111/jdv.20813
29. Egeberg A, Gislason GH, and Hansen PR. Risk of major adverse cardiovascular events and all-cause mortality in patients with hidradenitis suppurativa. JAMA Dermatol. (2016) 152:429–34. doi: 10.1001/jamadermatol.2015.6264
30. Garg A, Malviya N, Strunk A, Wright S, Alavi A, Alhusayen R, et al. Comorbidity screening in hidradenitis suppurativa: Evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. (2022) 86:1092–101. doi: 10.1016/j.jaad.2021.01.059
31. Zhou P, Jiang X, and Wang D. Hidradenitis suppurativa and cardiovascular diseases: A bidirectional Mendelian randomization study. Skin Res Technol. (2024) 30:e13853. doi: 10.1111/srt.13853
32. Dey AK, Teague HL, Adamstein NH, Rodante JA, Playford MP, Chen MY, et al. Association of neutrophil-to-lymphocyte ratio with non-calcified coronary artery burden in psoriasis: Findings from an observational cohort study. J Cardiovasc Comput Tomogr. (2021) 15:372–9. doi: 10.1016/j.jcct.2020.12.006
33. Berna-Rico E, Abbad-Jaime de Aragon C, Ballester-Martinez A, Perez-Bootello J, Solis J, Fernandez-Friera L, et al. Monocyte-to-high-density lipoprotein ratio is associated with systemic inflammation, insulin resistance, and coronary subclinical atherosclerosis in psoriasis: results from 2 observational cohorts. J Invest Dermatol. (2024) 144:2002–12.e2. doi: 10.1016/j.jid.2024.02.015
34. Barbieri JS, Beidas RS, Gondo GC, Fishman J, Williams NJ, Armstrong AW, et al. Analysis of specialist and patient perspectives on strategies to improve cardiovascular disease prevention among persons with psoriatic disease. JAMA Dermatol. (2022) 158:252–9. doi: 10.1001/jamadermatol.2021.4467
35. Berna-Rico E, Abbad-Jaime de Aragon C, Ballester-Martinez A, Perez-Bootello J, Solis J, Fernandez-Friera L, et al. Cardiovascular screening practices and statin prescription habits in patients with psoriasis among dermatologists, rheumatologists and primary care physicians. Acta Derm Venereol. (2023) 103:adv5087. doi: 10.2340/actadv.v103.5087
Keywords: psoriasis, cardiovascular disease, triglyceride-glucose, TyG-body mass index, insulin resistance
Citation: Li Y, Huang D, Ma B, Jiang Y, Yu B, Zheng J, Ding Y and Shi Y (2025) Positive associations between triglyceride-glucose (TyG) and TyG-body mass index (TyG-BMI) with cardiovascular disease in patients with psoriasis: cross-sectional results from the SPEECH. Front. Immunol. 16:1644887. doi: 10.3389/fimmu.2025.1644887
Received: 11 June 2025; Accepted: 18 August 2025;
Published: 05 September 2025.
Edited by:
Jie Li, Central South University, ChinaReviewed by:
Dongmei Shi, Jining No. 1 People’s Hospital, ChinaZhihui Yang, Peking University, China
Emilio Berna-Rico, Ramón y Cajal University Hospital, Spain
Copyright © 2025 Li, Huang, Ma, Jiang, Yu, Zheng, Ding and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianfeng Zheng, OTI1NTA1NDcxQHFxLmNvbQ==; Yangfeng Ding, ZGluZ3lhbmdmZW5nQGhvdG1haWwuY29t; Yuling Shi, c2hpeXVsaW5nMTk3M0B0b25namkuZWR1LmNu
†These authors share first authorship
 Ying Li
Ying Li Dawei Huang
Dawei Huang Biao Ma1,2†
Biao Ma1,2† Yuling Shi
Yuling Shi