- 1College of Basic Medical Sciences, Heilongjiang University of Chinese Medicine, Harbin, China
- 2School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, Singapore, Singapore
- 3Experimental Teaching & Practical Training Center, Heilongjiang University of Chinese Medicine, Harbin, China
- 4Department of Dermatology, Chongqing Traditional Chinese Medicine Hospital, Chongqing, China
- 5National Skin Centre, Singapore, Singapore
- 6Skin Research Institute of Singapore, Singapore, Singapore
- 7Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore
Chronic pruritus is a defining and therapeutically challenging symptom of atopic dermatitis (AD). Recent advances highlight the mast cell–neuron axis as a central neuroimmune interface orchestrating bidirectional crosstalk between the immune and peripheral nervous systems. Skin mast cells located in close proximity to sensory nerve endings release pruritogenic and neuroregulatory mediators, including histamine, tryptase, and nerve growth factor (NGF), and also modulate IL-31 signaling pathways. These mediators act on neuronal receptors such as IL-31RA, protease-activated receptors 1/2 (PAR-1/2), TrkA, and the adenosine triphosphate (ATP)-gated P2X3 receptor, thereby enhancing neuronal excitability and sensitizing transient receptor potential (TRP) channels (TRPV1, TRPA1). Conversely, sensory neurons release neuropeptides, among which substance P (SP) has been clearly demonstrated to activate Mas-related G protein–coupled receptor X2 (MRGPRX2) on mast cells, inducing non-IgE-mediated degranulation, whereas calcitonin gene-related peptide (CGRP) primarily regulates vascular tone and inflammation, with its direct role in MRGPRX2 activation remaining under investigation. This bidirectional interaction drives a feed-forward itch–inflammation loop. This circuit is further amplified by epidermal barrier dysfunction, microbial dysbiosis, type 2 immune polarization, and neurovascular remodeling. Structural adaptations–including intraepidermal nerve fiber branching and synapse-like mast cell–neuron junctions–provide anatomical substrates for chronic peripheral sensitization. While IL-31RA antagonists such as nemolizumab have demonstrated clinical efficacy, emerging targets like MRGPRX2 and TRPV1/TRPA1 channels offer additional therapeutic avenues but face challenges in translation and safety. Moreover, the P2X3 receptor has been proposed as a potential target for neurogenic itch in AD, but current research remains at an early stage and lacks direct clinical validation, highlighting limitations in its therapeutic development. This review provides a comprehensive mechanistic synthesis of the mast cell–neuron axis in AD-associated pruritus, critically evaluates current and investigational therapies, and explores the potential of multi-target interventions, including traditional Chinese medicine (TCM), for axis-level modulation. These efforts support the advancement of precision therapies targeting neuroimmune circuits in chronic inflammatory dermatoses.
1 Introduction
Atopic dermatitis (AD) is a prevalent chronic relapsing inflammatory skin disorder, clinically characterized by intense pruritus, eczematous lesions, and impaired skin barrier function, all of which significantly diminish patients’ quality of life (1, 2). Globally, AD affects more than 200 million individuals, with a rising prevalence observed over the past decade, particularly among children and women (3). Pruritus, the hallmark symptom of AD, serves as a major driver of scratching behavior, local inflammation, and disease chronicity. In moderate to severe cases, particularly in pediatric populations, it often manifests as persistent and treatment-refractory itch that contributes to emotional dysregulation, learning difficulties, and substantial impairments in quality of life (4–6). In the United Kingdom alone, itch-associated sleep disturbance and loss of productivity in AD patients have led to an estimated economic loss exceeding £3.8 billion over a 5-year period (7). Although notable progress has been made in alleviating inflammation through targeted immunomodulatory therapies, the effective control of chronic pruritus remains an unmet clinical challenge (8).
Recent evidence suggests that pruritus in AD is not merely a unidirectional response of sensory neurons to external stimuli, but rather arises from complex neuroimmune interactions that sustain pathological signaling (9). Among these, the mast cell–neuron axis has emerged as a pivotal mechanism that links immune activation with sensory hypersensitivity, providing both anatomical and functional integration between the immune and nervous systems (10). mast cells (MCs), densely distributed in the dermis, are frequently located adjacent to sensory nerve endings, establishing a structural foundation for bidirectional communication within this axis (11). Increasing evidence indicates that through the release of inflammatory mediators and neuropeptides, this axis initiates, amplifies, and maintains itch signaling, forming a self-perpetuating itch–inflammation loop that persists even in the absence of continued external triggers.
This review provides a comprehensive overview of the mast cell–neuron axis in AD-related chronic pruritus, with emphasis on its structural basis, molecular pathways, and translational relevance for targeted therapeutic development.
2 Mast cell–neuron axis: a pathological nexus of chronic pruritus
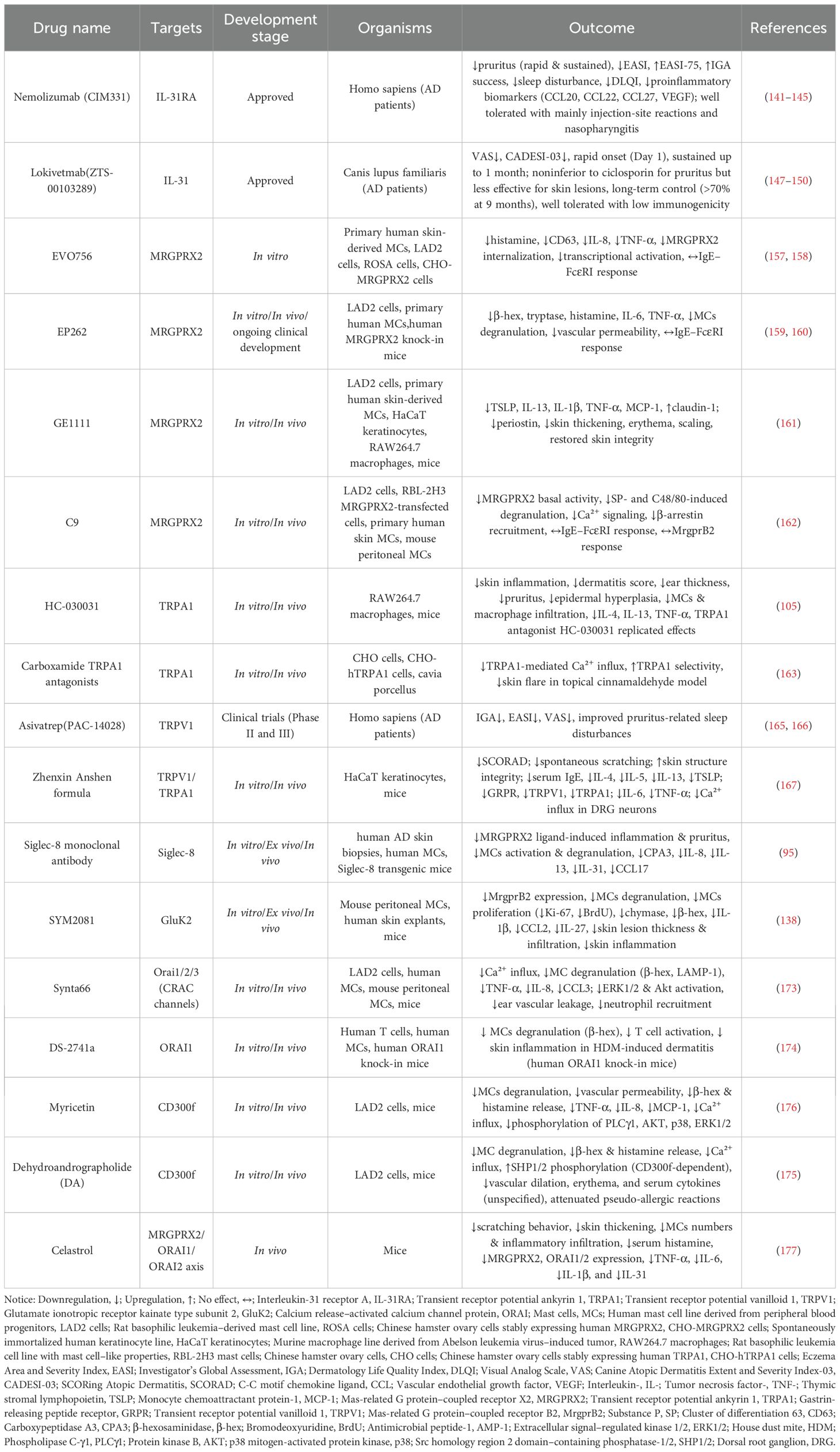
Chronic pruritus is the hallmark symptom of AD, arising from a multifactorial interplay involving epidermal barrier disruption, microbial dysbiosis, inflammatory mediator accumulation, neuronal sensitization, vascular abnormalities, and neuroplastic remodeling (12). These mechanisms form a dynamic feedback network, in which the mast cell–neuron axis has emerged as a central integrative hub. Bridging neuroimmune signaling pathways, this axis not only initiates itch responses independently but also amplifies and sustains pruritus by integrating inputs from barrier dysfunction, microbial imbalance, and immune activation (13). The overall pathophysiological framework of this axis is illustrated in Figure 1.
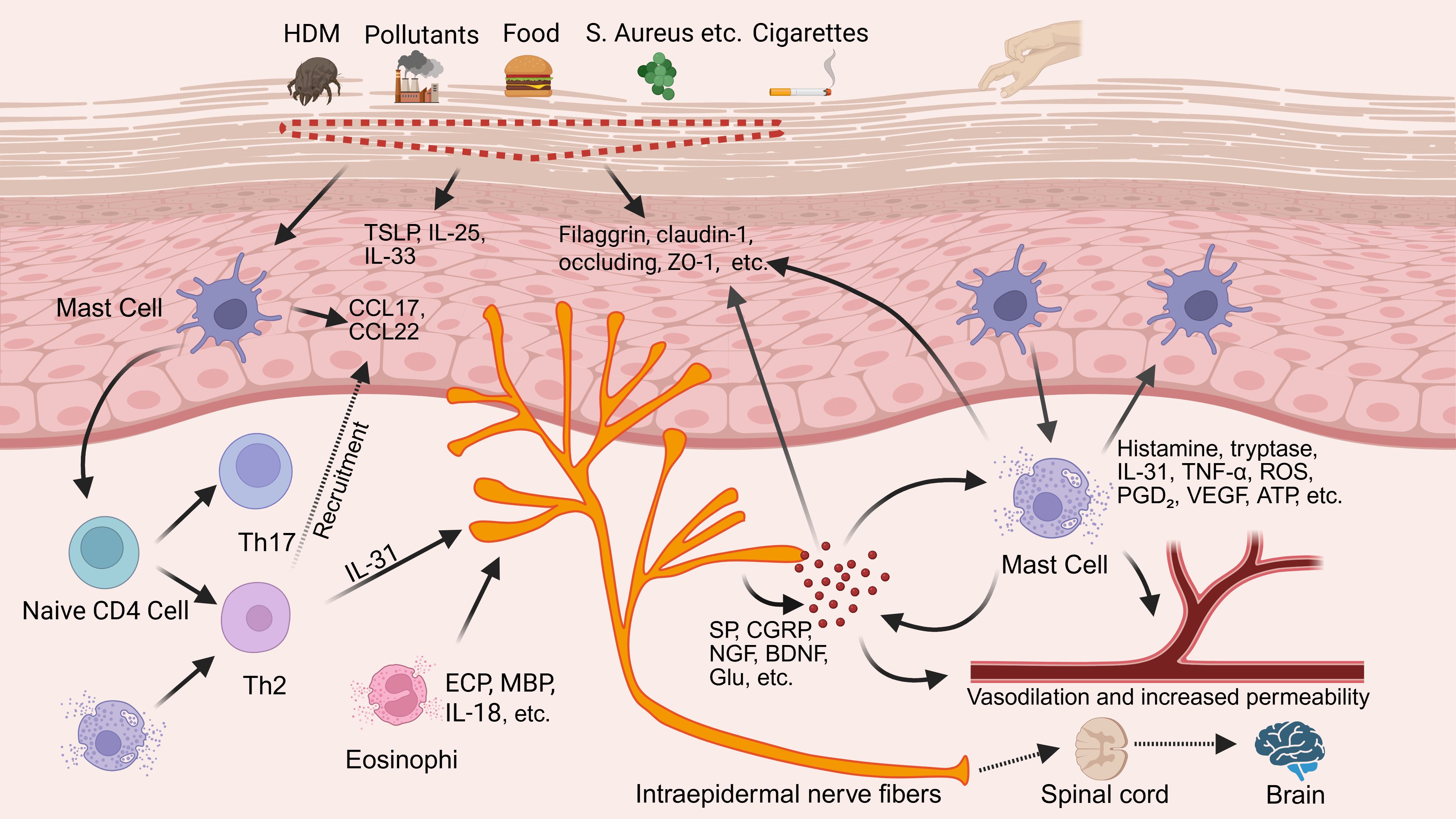
Figure 1. Core role of mast cell–neuron axis in chronic pruritus underlying AD. By BioRender. Note: Tumor necrosis factor alpha, TNF-α; Reactive oxygen species, ROS; Prostaglandin D2, PGD2; Vascular endothelial growth factor, VEGF; Adenosine triphosphate, ATP; Eosinophil cationic protein, ECP; Major basic protein, MBP; T helper type, Th; Thymic stromal lymphopoietin, TSLP; Interleukin-, IL-; C-C motif chemokine ligand, CCL; Zonula occludens-1, ZO-1; House dust mite, HDM; Substance P, SP; Calcitonin gene-related peptide, CGRP; Nerve growth factor, NGF; Brain-derived neurotrophic factor, BDNF; Glutamate, Glu.
2.1 Crosstalk with epidermal barrier dysfunction
Epidermal barrier dysfunction is a critical initiating factor in the pathogenesis of chronic pruritus in AD, and the mast cell–neuron axis plays an active role not only in responding to external insults but also in perpetuating barrier disruption and impaired repair (14, 15).
On one hand, barrier impairment enhances the skin’s susceptibility to environmental stimuli. Hallmark features include lipid layer depletion and the downregulation of key structural proteins such as filaggrin, claudin-1, loricrin, and Zonula Occludens-1 (ZO-1) (16, 17), thereby facilitating the deeper penetration and immunoneuronal activation by allergens (e.g., house dust mite antigens), bacterial toxins, and other exogenous agents (18). In response to such stimuli, keratinocytes release a series of epithelial alarmins, including thymic stromal lymphopoietin (TSLP), interleukin (IL)-33, and IL-25 (19), which directly activate resident MCs and stimulate sensory neurons, initiating a local neuroimmune cascade (20, 21). On the other hand, once activated, the mast cell–neuron axis exacerbates barrier injury. Mast cell–derived inflammatory mediators such as IL-31 (22, 23) not only enhance neuronal excitability but also act on keratinocytes (24, 25). Through activation of protease-activated receptor 2 (PAR-2), keratinocytes upregulate TSLP, nerve growth factor (NGF), and endothelin-1, thereby facilitating inflammatory cell infiltration, barrier impairment, and aberrant neuro–epidermal interactions that contribute to local inflammation and epidermal abnormalities (26).
Furthermore, neuropeptides released from sensory nerves, such as substance P (SP) and calcitonin gene-related peptide (CGRP), stimulate keratinocytes to produce proinflammatory cytokines including IL-1, IL-6, and TNF (27), and their effects are mediated in part through Ca²+ influx and the activation of transient receptor potential (TRP) channels (28), which play important roles in cutaneous inflammation (28, 29).
2.2 Crosstalk with cutaneous microbial dysbiosis
As a barrier-associated ecological niche, the skin microbiota plays a pivotal role in maintaining host–microbial homeostasis. In AD, this balance is frequently disrupted, leading to microbial dysbiosis that not only exacerbates local inflammation but also serves as a potential trigger for aberrant activation of the mast cell–neuron axis (12).
Under conditions of barrier disruption or dysbiosis, opportunistic pathogens, most notably Staphylococcus aureus, can overcolonize the skin (30). In mouse bone marrow–derived and fetal skin–derived MCs, the δ-toxin secreted by Staphylococcus aureus induces non–IgE-dependent degranulation via a Ca²+ influx–PI3K signaling pathway, and in vivo provokes vascular leakage and eczematous skin lesions; these effects are absent in mast cell–deficient mice but restored upon MCs reconstitution (31). In human LAD2 MCs and HEK293 cells stably transfected with Mas-related G protein–coupled receptor X2 (MRGPRX2), δ-toxin likewise directly engages the MRGPRX2 receptor to trigger degranulation, an effect that can be effectively blocked by the antagonist QWF (32). Collectively, these findings suggest that the δ-toxin–MRGPRX2 axis represents a critical link between cutaneous microbial colonization and MCs activation, and may contribute to Th2-skewed inflammation and pruritus in AD. Thus, microbial dysbiosis acts not only as a passive consequence of barrier dysfunction but also as an active driver of mast cell–neuron axis activation, reinforcing chronic pruritic inflammation in AD.
2.3 Crosstalk with immune response networks
In addition to transmitting pruritic signals, the mast cell–neuron axis contributes to immune activation and polarization in AD by modulating the function of various immune cells, thereby participating in the amplification of type 2 inflammation (33).
Upon activation, MCs release a variety of proinflammatory mediators, including IL-4, IL-13, tumor necrosis factor-α (TNF-α), and tryptase. IL-4 and IL-13 promote the differentiation of naïve CD4+ T helper cells (Th0) into T helper 2 cells (Th2) cells and maintain their effector function (34, 35). Tryptase and tumor necrosis factor-alpha (TNF-α) have been shown to activate immune signaling pathways through protease-activated receptor 2 (PAR-2) and tumor necrosis factor receptor 2 (TNFR2), respectively (13). Tryptase activates PAR-2 on endothelial and immune cells, leading to the engagement of downstream ERK/NF-κB signaling cascades and the subsequent release of pro-inflammatory mediators (36). Meanwhile, TNF-α signals through TNFR2 on dendritic cells (DCs), promoting their maturation and enhancing the production of interleukin-12 (IL-12), thereby facilitating Th1 polarization (37). Recent studies also highlight the immunomodulatory role of natural compounds such as diarylheptanoids derived from Curcuma kwangsiensis, which have been found to suppress DC maturation, antigen uptake, and the secretion of IL-6 and IL-12, ultimately inhibiting Th1 and Th17 differentiation. These findings collectively underscore the critical role of DC signaling pathways in shaping adaptive immune responses and maintaining inflammatory homeostasis (38). Moreover, mast cell–derived IL-4 can also activate the STAT6 signaling pathway in dendritic cells (DCs), leading to the upregulation of the chemokines CCL17 and CCL22. These chemokines are crucial for the recruitment of Th2 cells, thereby promoting the establishment of a Th2-skewed immune microenvironment in inflamed skin lesions (39). IL-31 secreted by activated Th2 cells binds to IL-31RA on sensory neurons, activating STAT3 signaling and inducing the release of neuropeptides such as CGRP, which contributes to neurogenic inflammation and modulation of local immune responses (24, 40). In addition, upon recruitment to inflamed skin, eosinophils release cytotoxic granules containing major basic protein (MBP) and eosinophil cationic protein (ECP) (41), which can directly act on peripheral sensory nerve terminals, alter membrane potential, and induce neuronal hyperexcitability, contributing to pruritus and neurogenic inflammation (42). Although IL-4 and IL-13 do not directly activate sensory neurons, they upregulate the expression of transient receptor potential (TRP) channels and related gene programs, thereby enhancing neuronal sensitivity to pruritogens and promoting peripheral sensitization (43, 44).
2.4 Crosstalk with the neurovascular system
A bidirectional regulatory relationship exists between the mast cell–neuron axis and the local cutaneous vasculature, forming a key conduit for inflammatory spread and peripheral neural sensitization (45). Under chronic inflammatory conditions, the neurovascular unit undergoes structural remodeling, characterized by increased sensory nerve fiber density and impaired endothelial barrier function, which strengthens neuro–immune–vascular coupling and provides a histopathological foundation for the persistence and therapeutic resistance of chronic pruritus in AD (15, 26).
Upon activation, MCs rapidly release a repertoire of vasoactive mediators, including histamine, ATP, prostaglandin D2 (PGD2), leukotrienes (e.g., LTC4), vascular endothelial growth factor (VEGF), and TNF-α (46, 47). These factors act on endothelial cells to induce vasodilation, increase vascular permeability, and trigger neurogenic vasoreflexes (48, 49). Experimental evidence demonstrates that mast cell–derived mediators can directly act on receptors of sensory neurons, enhancing the sensitivity of peripheral nerve endings to inflammatory signals and thereby sustaining the persistent transmission of itch (50, 51).Conversely, sensory neurons, upon activation, release neuropeptides such as SP and CGRP, which act directly on vascular smooth muscle cells and endothelial cells to induce neurogenic vasodilation and plasma extravasation (52, 53). This process reinforces the establishment of a neurovascular–immune coupling network. Notably, activation of transient receptor potential vanilloid 1 (TRPV1) and transient receptor potential ankyrin 1 (TRPA1) channels enhances CGRP release from sensory nerve endings (54), which has been shown to promote capillary dilation, sustain neurogenic inflammation, and activate MCs, thereby amplifying local itch and inflammation (52, 55).
Importantly, the synergistic interaction between vascular-derived factors such as VEGF and neuropeptides has been widely observed in various dermatological disease models. These findings suggest that cutaneous vasculature is not merely a passive route for immune cell trafficking but also serves as an active amplifier of pruritic signaling (56, 57).
3 Bidirectional crosstalk between MCs and intraepidermal nerve fibers
The bidirectional communication between mast cells and sensory neurons and their signaling pathways are illustrated in Figure 2.
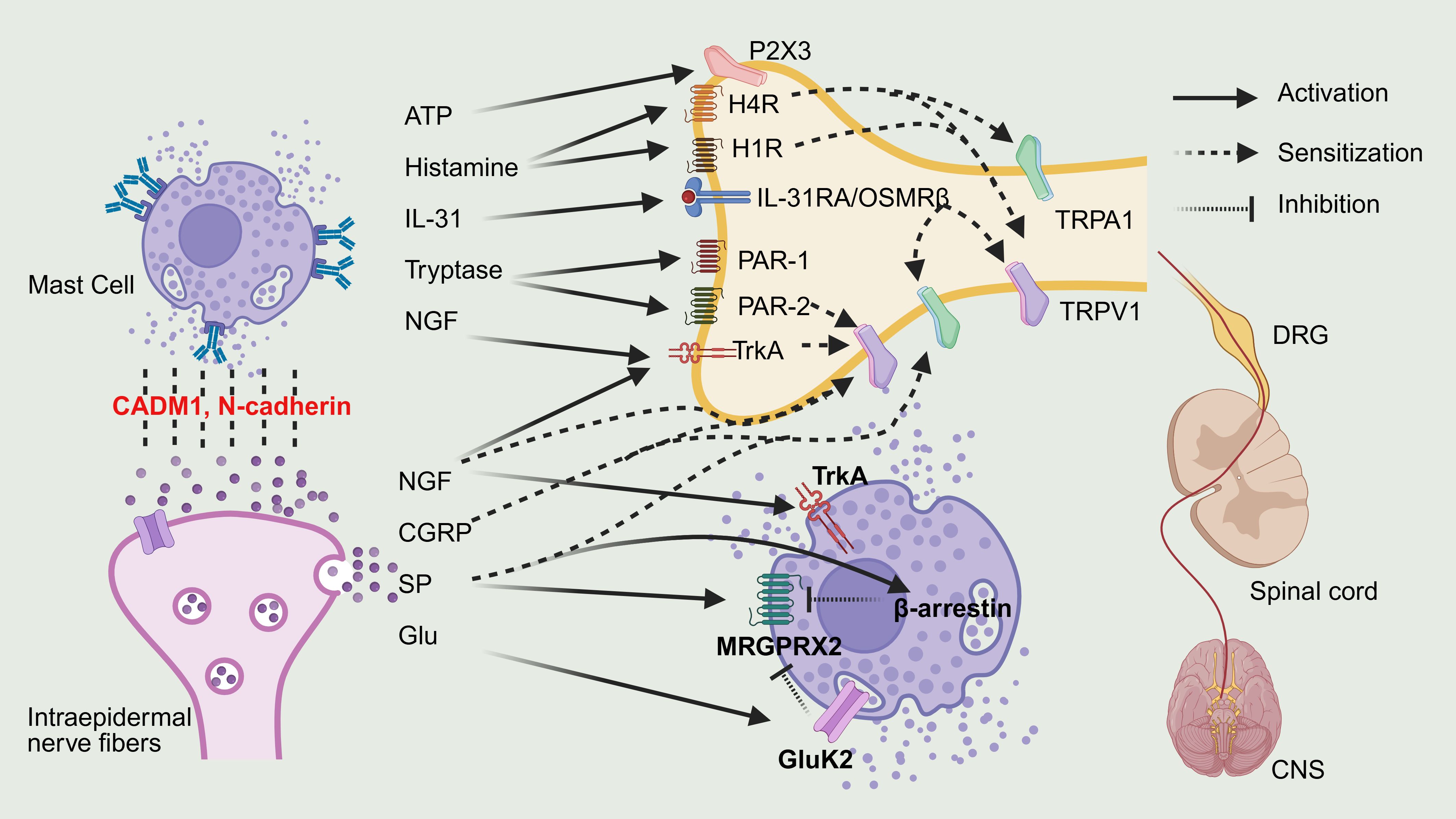
Figure 2. Bidirectional crosstalk between MCs and sensory neurons. By BioRender. Note: Interleukin-31, IL-31; Interleukin-31 Receptor A, IL-31RA; Oncostatin M receptor beta, OSMRβ; Nerve growth factor, NGF;Tropomyosin receptor kinase A, TrkA; Substance P, SP; Calcitonin gene-related peptide, CGRP; Glutamate, Glu; Glutamate Ionotropic receptor kainate type subunit 2, GluK2; Mas-related G protein-coupled receptor X2, MRGPRX2; Protease-activated receptor-2/-1, PAR-2/1; Transient receptor potential ankyrin 1, TRPA1; Transient receptor potential vanilloid 1, TRPV1; Histamine receptor H1/H4, H1R/H4R; Central nervous system, CNS; Dorsal root ganglion, DRG.
3.1 Structural basis of MCs and intraepidermal nerve fibers
3.1.1 MCs
MCs are tissue-resident immune cells derived from hematopoietic stem cells in the bone marrow. They are broadly distributed across barrier tissues such as the skin, respiratory tract, and gastrointestinal tract, with particularly high densities in the dermis (58). Traditionally, skin MCs are classified as the connective tissue type (MCTC), characterized by the presence of both tryptase and chymase in their secretory granules, in contrast to the mucosal type (MCT), which contains tryptase but lacks chymase (59). Recent advances in single-cell transcriptomic profiling, however, have revealed that skin MCs are not a homogeneous MCTC population. Instead, they comprise transcriptionally diverse subsets. In mice, skin MCs are almost exclusively MrgprB2+ connective tissue–type cells of embryonic origin that persist independently of bone marrow renewal (60), whereas in humans, at least four distinct clusters (MC1–MC4) are enriched in the skin, including MC1 as a dominant subset with distinct effector programs and spatial associations (61). These findings highlight that skin MC heterogeneity extends far beyond the classical protease-based dichotomy, reflecting developmental origin, tissue niche, and disease-specific adaptations. In the skin, MCs are commonly localized near blood vessels, lymphatic vessels, and peripheral sensory nerve endings, forming a highly dynamic neuroimmune interface that anatomically supports local interactions between inflammatory and neural signals (62, 63). In active lesions of AD, the number of skin MCs is significantly elevated (10, 64). Mechanistically, this focal accumulation is thought to result from the synergistic effects of cytokines such as IL-4, IL-33, and TSLP, along with chemokines such as CCL17 and CCL22 within the inflamed tissue microenvironment (65, 66).
In terms of receptor expression, MCs are traditionally characterized by high levels of FcϵRI, the high-affinity Immunoglobulin E (IgE) receptor that mediates type I hypersensitivity responses and plays a key role in initiating allergic inflammation in AD (67, 68). In addition, skin MCs selectively express MRGPRX2 (69), which recognizes neurogenic ligands such as SP, CGRP, and neurokinin A (33, 70). This dual receptor repertoire enables MCs to respond not only to IgE-mediated stimuli but also to neuropeptide signaling, thereby contributing to both allergic and non-IgE-dependent neurogenic inflammation (71). Together, these properties establish MCs as a central component of the neuroimmune interaction network in AD.
3.1.2 Intraepidermal nerve fibers
On the neuronal side, the skin is primarily innervated by unmyelinated C-fibers and thinly myelinated Aδ-fibers, which play essential roles in itch perception and transmission (72, 73).
Early investigations using 2-dimensional methods to study the histological and ultrastructural features of AD have yielded conflicting results. Some studies have reported marked neural remodeling in lesional skin, including axonal hypertrophy, increased axon numbers, and a significant elevation in intraepidermal nerve fiber density (IENFD) (74, 75). However, other studies have arrived at opposite conclusions (76, 77). With the implementation of advanced 3-dimensional imaging modalities, subsequent research has increasingly demonstrated that chronic mechanical injury, barrier dysfunction, and local inflammation contribute to a paradoxical reduction in IENFD within AD lesions (78, 79). Although an increased IENFD is commonly observed in acute or subacute lesions of AD, this phenomenon is thought to be closely associated with elevated levels of NGF and reduced expression of the axon-repelling molecule Semaphorin 3A (Sema3A) (80, 81). However, as the disease progresses into a chronic phase, the expression of Sema3A gradually increases, potentially inhibiting further neurite outgrowth and contributing to a reduction in cutaneous nerve fiber density (82).Recent evidence highlights that, rather than total nerve fiber density alone, qualitative alterations in specific subsets of sensory fibers such as increased branching, convoluted trajectories, and terminal swellings represent key morphological features of peripheral sensitization (82, 83). These structural alterations decrease the activation threshold of sensory neurons and bring them into closer proximity to environmental stimuli, thereby increasing susceptibility to light touch, allergens, and chemical irritants and facilitating itch responses (5). Notably, neural remodeling in AD appears to be at least partially reversible. This pathological feature is further substantiated by the reversal of nerve fiber alterations in lesional skin observed after dupilumab therapy (84).
Intraepidermal nerve fibers in AD can be subdivided into functionally distinct subtypes based on their molecular markers, which define their roles in histamine-independent pruritic signaling and the chronicity of itch. According to the expression patterns of the Mas-related G protein–coupled receptor (Mrgpr) family, intraepidermal nerve fibers in AD can be categorized into MrgprA3+ (85), MrgprX1+ (86), and MrgprX2+ (33, 87)subtypes. These fibers primarily mediate non-histaminergic itch by responding to exogenous stimuli or activating immune cells such as MCs, thereby inducing both acute and chronic pruritic responses and contributing to neuroimmune interactions within the AD microenvironment (88).
From the perspective of transient receptor potential (TRP) channel expression, TRPV1+ and TRPA1+ fibers represent key pathways for itch transmission in AD (89, 90). These ion channels detect thermal, mechanical, and chemical stimuli and are sensitized by inflammatory mediators such as IL-31, thereby increasing neuronal excitability and amplifying pruritic signaling (91).
In addition to TRP channels, ATP-gated P2X3 receptors represent another important class of ion channels expressed by specific subsets of intraepidermal sensory fibers. Notably, MrgprA3+ pruriceptive neurons, which are enriched in lesional skin of AD, exhibit high levels of P2X3 expression as revealed by transcriptional profiling (92). These receptors mediate rapid depolarization in response to extracellular ATP and are further sensitized by neurotrophic factors such as NGF and CGRP (93, 94).
3.2 Mast cell–mediated neuronal excitation and sensitization
In lesional skin of patients with AD, MCs release a repertoire of bioactive mediators, including histamine, IL-31, tryptase, and NGF, at markedly elevated levels, reflecting an active state of the MC–neuron axis in the pathogenesis of pruritus (95, 96).
Histamine induces acute itch by activating H1 and H4 receptors on sensory neurons (97, 98). IL-31 signals through a heterodimeric receptor complex composed of IL-31RA and oncostatin M receptor β (OSMRβ), eliciting chronic itch and enhancing TRPV1 activity (91). Notably, IL-31RA expression is regulated by STAT3 and may persist even after the resolution of inflammation, suggesting the existence of a ‘neuronal itch memory’ (40). Tryptase primarily activates PAR-2, and to a lesser extent PAR-1, on sensory neurons, inducing intracellular calcium influx and triggering downstream signaling cascades that amplify neuronal excitability and promote local inflammation (99, 100). NGF binds to its high-affinity receptor TrkA, promoting axonal growth cone formation and epidermal neurite extension, thereby increasing intraepidermal nerve fiber density, enhancing peripheral sensitivity, and aggravating itch perception (101, 102).
In addition to direct neuronal activation, MC-derived mediators further contribute to neuronal sensitization by increasing the activity of membrane-bound TRP channels, particularly TRPA1 and TRPV1, which lower the activation threshold of neurons and establish a state of peripheral hyperexcitability (40, 91, 103). Among these, TRPA1 is particularly active in inflamed skin and has been implicated in both pruriceptive transduction and immune amplification (104, 105). TRPV1, on the other hand, has been demonstrated to mediate MC-dependent itch in AD models, and its pharmacological blockade significantly reduces scratching behavior and alleviates local inflammation (90).
Beyond the well-established roles of histamine, IL-31, and neuropeptides in sensory modulation, purinergic signaling mediated by ATP has recently emerged as a critical complementary pathway in the sensitization of the mast cell–neuron axis in AD, with particular attention to the P2X3 receptor. Single-cell transcriptomic analyses of dorsal root ganglia have identified pruriceptive neuron subsets co-expressing P2rx3 and itch-related markers, supporting P2X3 as a molecular signature in sensory neurons involved in inflammatory skin diseases (106). Experimental studies have demonstrated that P2X3 regulates scratching behavior in murine models of itch, and pharmacological inhibition of P2X3 significantly attenuates symptoms, highlighting its pivotal role in pruritic signal transduction, despite the absence of direct evidence in AD-specific models (92). Furthermore, P2X3 activation has been shown to potentiate IgE-dependent MCs degranulation, thereby amplifying immune-mediated inflammation and supporting its involvement in allergic disease contexts (107).
P2X3 receptors are also highly responsive to neurotrophic factors such as NGF and CGRP, both of which are elevated in inflamed skin and capable of enhancing P2X3 expression while lowering the threshold for neuronal activation, thereby promoting a state of hyperexcitability (108). Given that P2X3 is also expressed in MCs, this receptor may contribute to a bidirectional amplification loop between immune and sensory cells, driving sustained neuroinflammation and chronic itch (109). Although direct experimental validation in AD remains limited, these findings collectively provide important mechanistic insight into the potential involvement of P2X3 in the regulation of the mast cell–neuron axis.
3.3 Neuronal feedback loops driving MCs reactivation
Sensory neurons not only mediate itch perception but also play a pivotal role in amplifying skin inflammation through active neuroimmune interactions. Neuropeptide-expressing fibers, including those positive for SP and CGRP, interact with immune cells such as MCs to initiate neurogenic inflammation and promote the release of type 2 cytokines like IL-4 and IL-13, thereby exacerbating both cutaneous inflammation and pruritus (24, 29). In parallel, fibers expressing the cytokine receptor IL-31RA respond to IL-31 secreted by T cells by driving neurite outgrowth and branching, a structural remodeling process that increases cutaneous sensitivity to mild stimuli and represents a key mechanism underlying chronic itch in AD (40, 110).
A central feature of chronic itch in AD is sustained MCs activation mediated through MRGPRX2, which is significantly upregulated in MCs within active lesions (35). SP released from activated intraepidermal nerve fibers binds to MRGPRX2 on MCs, triggering non-IgE-mediated degranulation and initiating a positive neuroimmune feedback loop (24, 111). At the signaling level, SP–MRGPRX2 engagement activates calcium-dependent degranulation pathways and drives nuclear translocation of lysyl-tRNA synthetase (LysRS), leading to activation of the MITF signaling axis and enhanced inflammatory mediator production (112). Neurotrophic factors further strengthen this loop. NGF and BDNF promote MCs migration toward the superficial dermis and enhance their responsiveness through TrkA-mediated signaling (29, 113). Concurrently, NGF binds to TrkA on sensory neurons, promoting axonal outgrowth, intraepidermal extension, and increased branching complexity, ultimately enhancing cutaneous neuroresponsiveness and contributing to chronic itch (82, 101, 114).
Importantly, neuronal regulation of MCs is not exclusively proinflammatory. Recent findings suggest that certain non-peptidergic sensory neurons release glutamate, which acts on GluK2 receptors expressed by MCs to downregulate mice MrgprB2 expression and suppress degranulation (115). In addition, SP exhibits biased agonism by recruiting β-arrestin, which modulates MRGPRX2 internalization and signal regulation (116). These mechanisms highlight the existence of a potential negative feedback circuit that fine-tunes neuroimmune activation in AD.
4 Structural and functional remodeling in chronic pruritus
4.1 Plasticity of mast cell–neuron axis
Chronic pruritus in AD is not merely the result of prolonged immune activation but also arises from sustained remodeling of the peripheral neuroimmune architecture (117). The mast cell–neuron axis, located within the dermis as a critical amplification unit, plays a central role in initiating and maintaining itch signaling (50). Studies have shown that MCs tend to accumulate around peripheral sensory nerve terminals (118), and their density is markedly increased in lesional AD skin (58), where they frequently remain in a highly degranulated state (35, 119).
A potential anatomical basis may underlie the amplification of chronic itch. In addition to molecular amplification, structural adaptations further reinforce this pathway. Studies have shown that under inflammatory conditions, MCs can undergo remodeling, forming tighter synapse-like structures with sensory neurons and thereby contributing to peripheral neural sensitization (45, 119). In AD lesions, these synapse-like junctions are mediated by adhesion molecules such as cell adhesion molecule 1 (CADM1) and N-cadherin, facilitating highly efficient local signal transmission (118). Cryo-electron microscopy and confocal imaging have further confirmed the presence of such structures, demonstrating that they enable efficient, short-range signal exchange between cells (29, 119). These structural interactions were initially described in models of asthma and neuroinflammation (120), and similar patterns have recently been observed in the lesional skin of murine AD models, where MCs tend to cluster near nerve terminals (121, 122). Recent experimental data further indicate that pharmacological or genetic disruption of these synapse-like MC–neuron contacts alleviates itch behavior and reduces peripheral sensitization in murine AD models, underscoring their functional relevance in chronic neuroinflammation (93).
From the neuronal perspective, intraepidermal nerve fibers in AD can be subdivided into functionally distinct subsets that mediate histamine-independent itch by responding to external stimuli or by engaging immune cells such as MCs, thereby contributing to both acute and chronic pruritus and shaping neuro–immune interactions in the lesional microenvironment (123). In addition, fibers expressing IL-31RA respond to IL-31 secreted by T cells by promoting neurite outgrowth and branching, a structural remodeling process that enhances cutaneous sensitivity to mild stimuli and represents a key mechanism underlying chronic itch (33, 90) (124).
4.2 Peripheral sensitization
A defining feature of peripheral sensitization is the lowering of the neuronal activation threshold (125), which refers to the minimal membrane depolarization required to initiate an action potential in sensory neurons. Under homeostatic conditions, this threshold prevents excessive neuronal firing in response to innocuous stimuli (126). However, in the context of chronic inflammation, pruritogenic mediators and neurotrophic factors can significantly reduce this threshold, rendering sensory fibers hyperexcitable and responsive to otherwise non-pruritic inputs (5, 127).
Among the molecular determinants of this lowered threshold, TRP channels have been extensively studied. Transient receptor potential (TRP) channels further contribute to this sensitization process. TRPV1+ and TRPA1+ fibers detect thermal, mechanical, and chemical stimuli and are sensitized by inflammatory mediators such as IL-31 (91), resulting in heightened neuronal excitability and amplification of itch signaling (128). Under inflammatory conditions, TRPV1 expression is significantly upregulated (91), and its activation enhances neuronal responsiveness to pruritogens while promoting the release of neuropeptides such as SP and CGRP (124, 129, 130). These neuropeptides interact with MCs to initiate neurogenic inflammation and stimulate the release of type 2 cytokines such as IL-4 and IL-13, thereby exacerbating cutaneous inflammation and pruritus (35) (131).
In addition to TRP channels, purinergic signaling via P2X3 receptors also plays a critical role in neuronal sensitization under inflammatory conditions (132). P2X3 is an ATP-gated cation channel highly expressed in itch-selective neurons and has been shown to mediate scratching behavior in murine models of pruritus (133). In AD models, upregulation of P2X3 receptors has been demonstrated to mediate itch-associated scratching (133). Moreover, NGF, which is elevated in AD skin, has been reported to enhance neuronal sensitization partly by potentiating P2X3 receptor function via rapid PKC activation (134, 135), a mechanism highlighted in recent reviews on peripheral itch sensitization in AD (5). NGF has been shown to upregulate P2X3 receptor transcription and enhance its trafficking to the neuronal membrane, thereby increasing surface availability and amplifying ATP-evoked responses (136). These molecular changes heighten neuronal responsiveness to extracellular ATP. Moreover, NGF stimulation induces spontaneous membrane potential fluctuations and intracellular calcium influx—hallmarks of increased excitability—which further amplify P2X3-mediated signaling (137). Together, these effects position P2X3 as a central mediator of NGF-driven peripheral sensitization and hyperexcitability in sensory neurons.
4.3 neuroimmune feedback driving chronic itch persistence
Chronic itch in AD is not merely a symptom but a dynamic driver of disease progression. Sensory neurons, particularly C-type fibers, play an active role in sustaining inflammation. Upon exposure to mechanical or allergen-derived stimuli as well as proinflammatory cytokines, these neurons release neuropeptides, such as SP and CGRP (69), which accumulate in lesional skin and amplify local immune responses (119).
Scratching, a behavioral consequence of itch, further perpetuates this cycle. Mechanical stimulation enhances TRPV1 activation in peripheral sensory neurons, leading to the release of neuropeptides such as SP and CGRP, thereby perpetuating the itch-scratch cycle through mechanical stimulation (90). In human MCs, SP, particularly in the presence of interleukin-33 (IL-33), enhances IL-31 gene expression and secretion via MRGPRX2 activation, independent of classical degranulation pathways (22). In parallel, TRPV1+ nociceptive neurons release neuropeptides such as SP which activate MrgprB2 the murine ortholog of human MRGPRX2 on adjacent MCs leading to non-IgE-mediated degranulation and the release of inflammatory mediators including tryptase thereby promoting type 2 inflammation (35, 119). These mediators, in turn, act via IL-31RA and TRPV1 to sustain neuronal hyperexcitability, creating a self-reinforcing loop of scratching–release–reactivation (40, 91).
At the molecular level, this cycle forms a closed mediator–receptor–remediator feedback circuit. Neuropeptide-induced MCs degranulation leads to release of pruritogenic mediators, which further sensitize neurons and promote additional neuropeptide release (22, 24). Non-peptidergic sensory neurons, particularly MrgprD+ subsets, have been shown to tonically release glutamate, thereby restraining MCs hyperresponsiveness through regulation of Mrgprb2 expression (115). Building on this finding, recent evidence demonstrated that glutamate acts via GluK2 receptors on MCs to suppress Mrgprb2/MRGPRX2 expression and inhibit degranulation (138). Together, these studies delineate a previously unrecognized neuroimmune feedback circuit in which neuronal glutamate provides a negative feedback brake to restrain MCs hyperactivation in cutaneous inflammation.
5 Therapeutic targets in the mast cell–neuron axis
An overview of the major molecular targets and related therapeutic agents is summarized in Table 1.
5.1 IL-31/IL-31RA: a clinically validated target
IL-31, a prototypical neuroimmune cytokine secreted by Th2 cells, MCs, and eosinophils, is one of the most extensively validated itch mediators within the mast cell–neuron axis (24). It exerts its pruritogenic effects through a heterodimeric receptor complex composed of IL-31RA and OSMRβ, which is expressed on cutaneous sensory neurons. Receptor activation has been shown to upregulate TRPV1 and TRPA1 channels, thereby inducing a state of neuronal hyperexcitability and establishing a chronic pruritus circuit characterized by a low activation threshold and high firing frequency (91).
In lesional skin of patients with AD, IL-31RA expression is significantly elevated and correlates positively with subjective pruritus scores, such as the Peak Pruritus Numerical Rating Scale (PP-NRS) (91). Notably, IL-31RA expression is regulated by STAT3 within sensory neurons and remains elevated even after clinical resolution of inflammation, suggesting a pruritic memory mechanism that enables sustained neuronal activation independent of primary inflammatory cues (40). Mechanistic studies further indicate that IL-31 not only acts on neurons but also modulates the immune microenvironment, for example, by inducing CGRP release to negatively regulate Th2 responses. These findings imply that blockade of IL-31RA may carry the risk of immune rebound (24, 139).
Nemolizumab, a humanized immunoglobulin G2(IgG2) monoclonal antibody targeting IL-31RA, was specifically developed to inhibit IL-31 signaling within the mast cell–neuron axis and entered clinical development in 2016 (140). Subsequent clinical studies have consistently demonstrated its efficacy in alleviating moderate-to-severe AD–associated pruritus.
In a phase III randomized controlled trials (RCT) in adults with moderate-to-severe AD, nemolizumab with topical therapy significantly improved pruritus and skin lesions. At 16 weeks, the nemolizumab group had a 42.8% reduction in pruritus VAS score vs. 21.4% in the placebo group. The eczema area and severity index (EASI) score decreased by 45.9% in the nemolizumab group versus 33.2% in the placebo group. Nemolizumab also led to significant improvements in sleep disturbances (55% of patients achieved ISI ≤7 vs. 21% in the placebo group) and daily functioning (40% achieved DLQI ≤4 vs. 22% in the placebo group), indicating enhanced quality of life. Additionally, some patients in the nemolizumab group reported significant pruritus relief as early as day 2 of treatment (141). Long-term follow-up studies have shown that these benefits are durable, with efficacy maintained up to 68 weeks, EASI improvement exceeding 70%, and continued decline in pruritus scores, supporting its potential for chronic disease control (142). In the recent Phase III RCTs ARCADIA 1 and ARCADIA 2, nemolizumab plus standard topical therapy achieved a ≥4-point reduction in PP-NRS scores at week 16 in 43–46% of patients versus 18–19% with placebo. Moreover, 28–31% of nemolizumab-treated patients reached a PP-NRS score <2 (indicative of near-complete itch relief), compared with 11% in the placebo arm. Notably, by week 1, 5–7% of patients receiving nemolizumab had already achieved a ≥4-point decrease in PP-NRS, versus <1% with placebo. Improvements were also noted in sleep quality, EASI-75 response, and Investigator’s Global Assessment (IGA) scores, supporting its broad therapeutic benefit (143). These improvements were accompanied by downregulation of inflammatory biomarkers (CCL20, CCL22, CCL27, VEGF), suggesting systemic modulation of the neuroimmune axis (144). The Nemolizumab-JP04 trial extended these findings to pediatric patients aged 6–12 years, showing significant reductions in pruritus NRS and improvements in EASI at week 16, along with better quality of life outcomes including sleep and caregiver burden (145).
With respect to safety, nemolizumab has demonstrated a favorable profile across age groups. Common short-term adverse events included injection site reactions, mild conjunctivitis, and upper respiratory tract infections, all occurring at rates below 5%. No serious drug-related adverse events were reported. Long-term follow-up data confirm that nemolizumab is not associated with cumulative toxicity, immunosuppression, or neurological adverse effects over a 68-week treatment period (142, 146). In adolescents and children, the adverse event profile was consistent with that observed in adults, predominantly mild to moderate in severity and not affecting treatment adherence (144, 145).
Lokivetmab, a monoclonal antibody against IL-31 approved for the treatment of canine AD, provides interspecies validation of the IL-31–neural axis in pruritus. Clinical studies have shown that lokivetmab can significantly relieve itch within 24 hours of administration, with a 2 mg/kg dose maintaining efficacy for approximately 1 month and reducing pruritus visual analog scale (PVAS) scores by more than 50% (147, 148). Its antipruritic efficacy was comparable to that of cyclosporine, although it was less effective for skin lesion resolution, and it was associated with fewer adverse effects (149). Long-term safety studies have not identified any significant immunosuppressive or organ-specific toxicity (150).
A number of patents have been filed for anti-IL-31 antibodies, including IgG4 variants specifically designed to block IL-31 binding to its receptor complex (151). However, aside from nemolizumab, no human IL-31–targeting antibodies have yet completed phase II clinical trials.
5.2 MRGPRX2: an emerging pruritogenic target
MRGPRX2 is a member of the G protein-coupled receptor (GPCR) superfamily and functions as a critical signaling hub that links extracellular stimuli to intracellular responses (152, 153). Evidence from both clinical samples and animal models indicates that MRGPRX2 in humans and its murine ortholog MrgprB2 are positively correlated with inflammation severity (35), contribute to sensory neuron activation and scratching behavior (154), and mediate non-IgE-dependent inflammatory responses in AD models (155), highlighting their potential as functional therapeutic targets. In recent years, drug discovery efforts have focused on small-molecule antagonists that inhibit MRGPRX2-mediated, non-IgE-dependent MCs degranulation as a novel strategy for treating chronic pruritus in AD (156).
EVO756, a selective MRGPRX2 antagonist, potently inhibits SP and compound 48/80-induced histamine release, CD63 upregulation, and cytokine secretion (IL-8, TNF-α) in human LAD2 cells and primary cutaneous MCs, without affecting IgE–FcϵRI-dependent activation (157, 158). It also reprograms MCs transcriptional profiles from an activated to a quiescent state (158) and reduces vascular permeability in humanized mouse models (157), suggesting both anti-inflammatory and barrier-protective effects relevant to AD. EP262, another MRGPRX2 antagonist, has demonstrated both in vitro and in vivo inhibition of MCs degranulation and vascular permeability by suppressing the release of β-hexosaminidase, tryptase, histamine, IL-6, TNF-α, and other proinflammatory mediators (159, 160). Another lead compound, GE1111, demonstrated efficacy in a DNFB-induced mouse model of AD, ameliorating erythema, scaling, and skin thickening confirmed by histological analysis.
This antagonist also downregulated inflammatory mediators such as Il-1β, Il-13, and TSLP, while upregulating barrier proteins including claudin-1. In a human three-cell co-culture system consisting of MCs, keratinocytes, and macrophages, GE1111 effectively inhibited mast cell–mediated cytokine release, underscoring its dual anti-inflammatory and barrier-restorative potential as an MRGPRX2-targeted therapy (161).
By contrast, C9 represents a novel inverse agonist that stabilizes the inactive conformation of MRGPRX2, thereby reducing its constitutive activity. C9 potently inhibits SP- and compound 48/80-induced degranulation without affecting IgE–FcϵRI-mediated responses and having no effect on murine MrgprB2-expressing MCs, indicating high pathway specificity and cross-species selectivity (162). Although in vivo validation and clinical translation of C9 remain pending, its mechanism offers a promising approach to selectively target non-histaminergic itch pathways.
5.3 TRPV1 and TRPA1: mechanistically validated targets
TRPV1 and TRPA1 channels are pivotal mediators in itch signal transduction within the mast cell–neuron axis (124). A growing body of experimental and clinical evidence has demonstrated the therapeutic potential of TRP channel antagonists in modulating both pruritus and inflammation in AD. These agents represent a promising class of precision therapeutics that directly target neuroimmune pathways and may offer a breakthrough in AD management.
The TRPA1 antagonist HC-030031 has been shown to significantly reduce spontaneous scratching behavior, epidermal hyperplasia, and dermal thickening in a DNCB-induced mouse model of AD. Treatment also led to decreased MCs infiltration and downregulation of inflammatory cytokines such as IL-4 and IL-13, indicating its potential to alleviate chronic skin inflammation and itch, possibly via non-histaminergic pathways (105). Carboxamide-based TRPA1 antagonists represent a novel class of topical agents with favorable skin permeability and pharmacokinetic properties. In guinea pig models, these compounds significantly reduced cinnamaldehyde-induced neurogenic skin flare, supporting their potential for further development as TRPA1-targeted therapies (163). While TRPA1 antagonists have shown consistent anti-inflammatory and antipruritic effects in preclinical studies, the current pipeline lacks candidates with both high selectivity and optimal pharmacokinetics, and clinical development remains in its early stages (164).
For TRPV1, Asivatrep (PAC-14028) is currently the most clinically advanced small-molecule antagonist. It is the first topical TRPV1-targeting compound of its class to reach phase III trials. In a phase IIb study, Asivatrep significantly reduced pruritus with a 2.3-point decrease in visual analog scale (VAS) scores (165). In the subsequent phase III trial, it achieved a 44.3% mean improvement in EASI and a 36.0% IGA 0/1 response rate, indicating both antipruritic and anti-inflammatory efficacy compared to placebo (166).In parallel, traditional herbal formulations such as Zhenxin Anshen formula have been shown in both AD mouse models and in vitro systems to suppress TRPV1 and TRPA1 expression, reduce scratching behavior, and downregulate inflammatory mediators including IgE, IL-4, IL-5, IL-13, and TSLP. These findings highlight its potential as a multi-targeted therapeutic strategy through integrated modulation of neural and immune pathways (167). Although TRPV1 antagonists show promising antipruritic efficacy, their further development has been limited by safety concerns. As TRPV1 is also involved in thermoregulation, early-phase clinical trials have reported adverse events including hyperthermia and hypothermia, raising challenges for the long-term use of this target (168).
5.4 Other targets
Beyond direct antagonism of MRGPRX2, several studies have explored indirect strategies to suppress its activity through upstream or parallel regulatory mechanisms.
Siglec-8 is an inhibitory receptor selectively expressed on human MCs and eosinophils. It exerts broad immunomodulatory effects by interfering with downstream signaling pathways of FcϵRI, including Syk, ERK, and Akt (169). In transgenic mice expressing human Siglec-8, treatment with an anti-Siglec-8 monoclonal antibody significantly suppressed IgE-mediated MCs degranulation and effectively blocked MRGPRX2-mediated, non-IgE-dependent MCs activation and pruritus (95). These findings suggest that Siglec-8 may serve as a dual-pathway immunoregulatory target with broad therapeutic potential in neuroimmune-mediated disorders. GluK2, a kainate-type glutamate receptor subunit predominantly expressed in MCs, forms functional complexes with kainate receptor subunit 2 (KA2) subunits. This receptor senses glutamate released from peripheral neurons and negatively regulates MrgprB2-mediated MCs activation, thereby serving as a key suppressor of neuroimmune hyperactivation and maintaining local immune homeostasis (115). In vitro mouse MCs, ex vivo human skin explants, and in vivo murine models have demonstrated that pharmacological activation of GluK2 significantly inhibits MCs degranulation as well as cutaneous inflammatory and pruritic responses, supporting its potential as a negative regulatory therapeutic target (138).
The calcium release–activated calcium (CRAC) channels formed by calcium release-activated calcium channel protein (ORAI) 1 and ORAI2 also play essential roles in the non-IgE-dependent activation of MCs within the neuroimmune axis, and they are increasingly being investigated as novel pharmacological targets (170, 171). In vitro studies have shown that activation of MRGPRX2 or its murine ortholog MrgprB2 induces MCs degranulation via Orai channels, with Orai1 and Orai2 being the predominant contributors. Importantly, Orai2 also acts as a negative regulator, as its genetic deletion augments CRAC currents, enhances MCs degranulation, and exacerbates anaphylaxis severity (172). In contrast, silencing ORAI1/2 with shRNA or pharmacological inhibition using the CRAC channel blocker Synta66 markedly suppressed SP-induced Ca²+ influx, CD63 expression, β-hex release, and production of TNF-α, IL-8, and CCL3, while reducing ERK1/2 and Akt phosphorylation. In vivo, Synta66 attenuated SP-induced vascular permeability and neutrophil recruitment, highlighting the central role of ORAI channels in MRGPRX2/MrgprB2-driven neuroimmune amplification (173). Further development led to the generation of DS-2741a, a monoclonal antibody that selectively targets ORAI1. In human ORAI1 knock-in mice, DS-2741a inhibited T cell activation and MCs degranulation, and alleviated house dust mite–induced dermatitis, supporting the therapeutic potential of ORAI1 blockade in allergic diseases (174).
In addition to synthetic compounds, several natural products have gained attention for their ability to regulate MCs function. Flavonoids such as myricetin and the diterpenoid dehydroandrographolide (DA), both derived from medicinal plants, have been shown to activate the inhibitory receptor CD300f and its downstream Src homology region 2 domain-containing phosphatase (SHP)1/2 signaling cascade. These pathways negatively regulate MRGPRX2-mediated MCs degranulation, resulting in reduced histamine release, suppression of proinflammatory cytokines, and attenuation of vascular permeability and pseudo-allergic skin reactions in LAD2 human MCs and murine models (175, 176). These findings underscore their potential as non-IgE-targeting anti-inflammatory candidates. Celastrol has also been shown to suppress the expression of MRGPRX2, ORAI1, and ORAI2. In DNFB-induced mouse models of AD, Celastrol reduced MCs activation, skin inflammation, and histamine release, while in compound 48/80-induced models it alleviated pruritic behavior. Its anti-inflammatory and antipruritic effects were significantly diminished in MRGPRX2-overexpressing mice, indicating that its primary mechanism involves modulation of the MRGPRX2/ORAI1/ORAI2 axis (177).
6 Therapeutic challenges and perspectives
Although the mast cell–neuron axis has been increasingly recognized as a central integrative hub that orchestrates neuronal excitation, immune amplification, and sustained inflammation. However, therapeutic strategies targeting this axis continue to face multiple challenges, including limited availability of validated targets, substantial disease heterogeneity, fragmentation of current intervention approaches, and the pressing need to shift from localized symptom control toward comprehensive restoration of systemic immune and neurophysiological homeostasis.
6.1 Limitations of current targeted strategies
The IL-31/IL-31RA axis represents one of the most clinically advanced antipruritic targets. Agents such as nemolizumab have demonstrated significant efficacy in alleviating chronic itch in patients with AD (178, 179). However, their primary mechanism of action involves inhibition of neuronal signal transmission at the peripheral nerve terminals, with limited direct effects on skin barrier restoration and local immune modulation. Consequently, these therapies may fall short in addressing the multifaceted pathogenesis of AD, including epidermal repair, microbiome rebalancing, and immune reprogramming, thereby limiting their capacity to achieve sustained disease control throughout the entire disease course (25, 180). In addition, the IL-31/IL-31RA axis contributes not only to pruritus signaling but also to the immunoregulatory balance by inducing the neurogenic release of CGRP, which negatively regulates Th2 responses. Complete blockade of this pathway may provide effective relief from itch but could potentially disrupt immune homeostasis, raising concerns about immune rebound and proinflammatory flare-ups (24).
MRGPRX2, a receptor selectively expressed in primates, presents further challenges due to the absence of a natural ortholog in rodents, which complicates the development of translational animal models. Although MrgprB2 is widely used as the murine homolog, it differs from MRGPRX2 in ligand specificity, signaling potency, and regulatory control (88, 181). As a result, most current evidence is derived from in vitro or rodent studies, and preclinical data remain limited. Pharmacological agents targeting MRGPRX2 are still in early stages of development and lack robust translational validation (33).
TRP channels such as TRPV1 and TRPA1 have shown promising antipruritic effects in preclinical models and are considered key targets for neurogenic itch. However, their essential physiological roles in thermoregulation and nociception pose significant safety concerns. Systemic modulation of TRP channels is often associated with adverse effects such as thermal dysregulation and burning sensations, which substantially limit their clinical utility and broader therapeutic application (182, 183).
In addition, the P2X3 receptor, an ATP-gated ion channel expressed on sensory neurons, has attracted growing attention in the context of neurogenic itch. Several novel antagonists have demonstrated potent inhibitory activity in sensory neurons (184). Among them, the representative agent gefapixant has undergone extensive clinical evaluation in chronic cough: a phase IIb trial demonstrated that oral administration of 50 mg twice daily significantly reduced cough frequency (185), and subsequent global phase III studies further confirmed the efficacy and safety of the 45 mg twice-daily regimen (186). Although P2X3 antagonists have shown success in other neurogenic disorders, their investigation in AD remains at an early exploratory stage, and further studies are required to validate their therapeutic potential.
6.2 Molecular heterogeneity–driven precision stratification
Although treatment of moderate-to-severe AD has entered a new era centered on biologic agents, interindividual differences in the expression of key neuroimmune genes such as IL-31, MRGPRX2, TRPA1, NGF, and CGRP contribute to molecular heterogeneity that is closely associated with the intensity of pruritus and disease chronicity, and these differences significantly affect patients’ responses to therapy (187). In current clinical practice, the use of biologic agents largely relies on empirical judgment rather than stratification based on immunological characteristics, resulting in a trial-and-error approach to treatment that often delays optimal intervention (188). This, to some extent, accounts for the substantial variability in patient responses to different targeted therapeutic strategies (189, 190). Therefore, there is an urgent need to establish a more refined and mechanism-driven classification system to guide individualized treatment and inform drug development strategies, ultimately improving therapeutic efficacy.
Although several studies have attempted to classify patients with AD based on clinical features and immunological endotypes, these classification frameworks have not yet evolved into standardized tools widely applicable in clinical practice (191).
To improve stratification, a two-dimensional model incorporating pruritus intensity and lesional severity has been proposed by some researchers, with the subtype characterized by severe itch and mild-to-moderate lesions (SI-ML) capturing the discordance between subjective symptoms and objective signs and supporting more targeted and individualized treatment approaches (192). To further address the challenges of disease stratification and mechanistic heterogeneity, cutting-edge technologies such as single-cell RNA sequencing (scRNA-seq), spatial transcriptomics, artificial intelligence algorithms, and integrative multi-omics analysis have enabled the high-resolution identification of key cellular populations and molecular markers that are closely associated with disease severity and therapeutic response (189, 193–195). These approaches facilitate the prediction of individual responsiveness to specific targeted therapies, including IL-4Ra, IL-13, and IL-31 inhibitors. In clinical practice for psoriasis, a study established an in vitro model that combines peripheral blood mononuclear cells (PBMCs) from patients with streptococcal stimulation to mimic immune activation. The results demonstrated that levels of IFN-γ, IL-17A, and their ratios to IL-4/IL-13 could effectively predict the therapeutic efficacy of multiple biologic agents (196). These findings offer valuable insights that may inform immunological stratification and targeted treatment strategies in AD.
In summary, the application of high-resolution technologies to systematically delineate immunophenotypic differences among patients and to develop classification models that are closely associated with therapeutic outcomes, particularly those focusing on the functional activity of critical pathways such as the mast cell–neuron axis, will facilitate the implementation of more efficient, individualized, and low-toxicity targeted therapies, while also advancing drug development and clinical translation.
6.3 Theoretical and practical insights into multi-target synergistic interventions
Most current therapeutic strategies targeting the mast cell–neuron axis remain focused on single-pathway interventions, primarily involving blockade of specific ligand–receptor interactions. However, the persistence of chronic pruritus in AD is not driven by isolated signaling events but rather results from a complex interplay of neuronal sensitization, structural coupling, and immune dysregulation. Both clinical and experimental studies indicate that although single-target approaches may offer short-term symptomatic relief, they often fail to interrupt the dynamic feedback loop of degranulation, neuronal excitation, and inflammatory amplification. These findings underscore the necessity of coordinated, multi-target intervention strategies to achieve sustained disease control (197).
Therefore, the concept of synergistic blockade across multiple pathways is gradually replacing the traditional single-target paradigm. Simultaneous targeting of inflammatory mediators and neuronal signaling components enables cascade-level inhibition across different layers of the neuroimmune axis, including receptor-level, ion channel-level, and structural domains. This multi-tiered approach facilitates integrated modulation from local barrier function to systemic immune response and represents a conceptual shift from discrete ‘point inhibition’ to coordinated ‘axis regulation’ in the treatment of chronic pruritus (197, 198).
Within this context, traditional Chinese medicine (TCM) offers unique advantages due to its intrinsic multi-target and low-toxicity properties. Herbal formulas and their active constituents frequently modulate multiple molecular targets and signaling cascades associated with AD pathogenesis, thereby embodying a systems-level therapeutic approach (199, 200). For instance, natural compounds such as myricetin and dehydroandrographolide have been shown to negatively regulate MRGPRX2-mediated MCs degranulation, and may hold promise for further exploration into additional neuroimmune regulatory mechanisms (175, 176). Moreover, myricetin can also effectively alleviates erythema, edema, pruritus, and epidermal thickening by suppressing proinflammatory cytokines, modulating Th1/Th2/Th17 immune balance, inhibiting MCs infiltration and the release of histamine and IgE, and restoring skin barrier function through the inhibition of NF-κB and STAT1 signaling pathways in vivo/in vitro (201, 202). In addition, the TCM Jiu-Wei-Yong-An Formula has been shown to reduce IL-31 expression in the skin of AD mice, while also alleviating cutaneous inflammation, pruritic behavior, epidermal hyperplasia, MCs infiltration, and dysregulated Th1/Th2 cytokine expression, exerting synergistic anti-inflammatory and immunomodulatory effects primarily through inhibition of the JAK1/STAT3 and MAPK signaling pathways (203).
Taken together, these insights underscore the necessity and feasibility of adopting multi-target, systems-oriented strategies for the treatment of chronic pruritus in AD. Such approaches offer clear advantages in disrupting complex neuroimmune networks, and align closely with the intrinsic therapeutic potential of traditional Chinese medicine, which operates through coordinated, network-based mechanisms.
7 Conclusion
The mast cell–neuron axis functions as a key neuroimmune interface that contributes to the initiation, progression, and persistence of chronic pruritus in AD. It mediates both sensory neuronal activation and immune amplification and establishes a self-sustaining itch–inflammation loop through complex signaling pathways and intercellular interactions. This dynamic network makes chronic itch in AD difficult to effectively control.
Current therapeutic approaches targeting this axis, particularly IL-31RA inhibitors, have demonstrated partial success. These treatments modulate specific signaling pathways, leading to relief of itch symptoms and improved quality of life. However, the development of drugs targeting other key components, including MRGPRX2, TRPV1/TRPA1, ORAI1/2/3 and P2X3 receptors, is still constrained by species specificity, safety concerns, and the lack of validated translational models. As a result, their clinical application remains limited, and no breakthrough has yet been achieved.
The complexity of AD, including its immunological heterogeneity, multilayered mast cell–neuron interactions, and the finely regulated nature of the neuroimmune axis, presents further challenges for therapeutic translation. Clinical limitations such as suboptimal efficacy, variability in patient response, risk of immune imbalance, and concerns regarding long-term safety continue to hinder progress. Future research should focus on elucidating the precise mechanisms through which the mast cell–neuron axis contributes to chronic pruritus and identifying effective multi-target strategies that can comprehensively regulate this axis. Advances in technologies such as single-cell sequencing, spatial transcriptomics, and integrative multi-omics are expected to facilitate the identification of pathogenic pathways and functional patient subtypes, supporting the development of personalized therapeutic strategies. In parallel, traditional medicine, particularly Chinese herbal medicine, may offer additional therapeutic value due to its multi-target and systemic regulatory properties. Investigating its modulatory effects on the mast cell–neuron axis may help establish safe and effective treatment options tailored to individual patient profiles.
In conclusion, continued research into the mast cell–neuron axis will provide a strong foundation for the precision management of chronic pruritus in AD and may contribute to long-term symptom control and improved patient outcomes.
Author contributions
DL: Formal Analysis, Data curation, Writing – original draft, Conceptualization, Visualization, Funding acquisition, Methodology. YH: Formal Analysis, Conceptualization, Writing – original draft, Methodology. JZ: Writing – original draft, Formal Analysis, Conceptualization. HY: Formal Analysis, Writing – original draft. JC: Conceptualization, Writing – review & editing, Supervision, Project administration, Data curation, Methodology, Formal Analysis. HT: Methodology, Conceptualization, Project administration, Supervision, Formal Analysis, Writing – review & editing. TT: Supervision, Methodology, Conceptualization, Writing – review & editing, Formal Analysis, Project administration.
Funding
The authors declare financial support was received for the research and/or publication of this article. This study was supported by the Heilongjiang Provincial National Natural Science Foundation of China (Grant No. LH2021H085), the Key Laboratory of Basic Theory of Traditional Chinese Medicine in Heilongjiang Province, and the China Scholarship Council (Grant No. 202308230308). No commercial funding was involved.
Acknowledgments
Figure 1, 2 were created by BioRender (https://BioRender.com), for which we are grateful.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Luger T, Amagai M, Dreno B, Dagnelie M-A, Liao W, Kabashima K, et al. Atopic dermatitis: role of the skin barrier, environment, microbiome, and therapeutic agents. J Dermatol Sci. (2021) 102:142–57. doi: 10.1016/j.jdermsci.2021.04.007
2. Guttman-Yassky E, Renert-Yuval Y, and Brunner PM. Atopic dermatitis. Lancet. (2025) 405:583–96. doi: 10.1016/S0140-6736(24)02519-4
3. Tian J, Zhang D, Yang Y, Huang Y, Wang L, Yao X, et al. Global epidemiology of atopic dermatitis: a comprehensive systematic analysis and modelling study. Br J Dermatol. (2024) 190:55–61. doi: 10.1093/bjd/ljad339
4. Weidinger S, Simpson EL, Silverberg JI, Barbarot S, Eckert L, Mina-Osorio P, et al. Burden of atopic dermatitis in paediatric patients: an international cross-sectional study. Br J Dermatol. (2024) 190:846–57. doi: 10.1093/bjd/ljad449
5. Tominaga M and Takamori K. Peripheral itch sensitization in atopic dermatitis. Allergol Int. (2022) 71:265–77. doi: 10.1016/j.alit.2022.04.003
6. Ansbro B and Silverberg JI. 688 - the heterogeneous characteristics of itch vary by atopic dermatitis severity and differentially impact quality of life in children and adults: a prospective cohort study. Br J Dermatol. (2024) 191:ljae266.62. doi: 10.1093/bjd/ljae266.062
7. Pierce EJ, Burge RT, Hirst AJ, Fox AM, Suokas AK, and Yi Y. Economic burden of itch-related sleep loss in moderate-to-severe atopic dermatitis in the United Kingdom. Dermatol Ther. (2024) 14:1103–14. doi: 10.1007/s13555-024-01153-9
8. Labib A, Ju T, and Yosipovitch G. Emerging treatments for itch in atopic dermatitis: a review. J Am Acad Dermatol. (2023) 89:338–44. doi: 10.1016/j.jaad.2023.04.057
9. Liu AW, Gillis JE, Sumpter TL, and Kaplan DH. Neuroimmune interactions in atopic and allergic contact dermatitis. J Allergy Clin Immunol. (2023) 151:1169–77. doi: 10.1016/j.jaci.2023.03.013
10. Zuo W, Yue Z, Xu S, Sun C-H, Zou X-F, Ma J, et al. Emerging trends and research hotspots in the relationship between mast cells and atopic dermatitis based on the literature from 2001 to 2024: a bibliometric and visualized analysis. Skin Res Technol Off J Int Soc Bioeng Skin ISBS Int Soc Digit Imaging Skin ISDIS Int Soc Skin Imaging ISSI. (2024) 30:e70053. doi: 10.1111/srt.70053
11. Toyoshima S and Okayama Y. Neuro-allergology: mast cell–nerve cross-talk. Allergol Int. (2022) 71:288–93. doi: 10.1016/j.alit.2022.04.002
12. Misery L, Pierre O, Gall-Ianotto CL, Lebonvallet N, Chernyshov PV, Garrec RL, et al. Basic mechanisms of itch. J Allergy Clin Immunol. (2023) 152:11–23. doi: 10.1016/j.jaci.2023.05.004
13. Plum T, Feyerabend TB, and Rodewald H-R. Beyond classical immunity: mast cells as signal converters between tissues and neurons. Immunity. (2024) 57:2723–36. doi: 10.1016/j.immuni.2024.11.016
14. Liu R, Buttaci DR, and Sokol CL. Neurogenic inflammation and itch in barrier tissues. Semin Immunol. (2025) 77:101928. doi: 10.1016/j.smim.2024.101928
15. Ständer S, Luger T, Kim B, Lerner E, Metz M, Adiri R, et al. Cutaneous components leading to pruritus, pain, and neurosensitivity in atopic dermatitis: a narrative review. Dermatol Ther. (2024) 14:45–57. doi: 10.1007/s13555-023-01081-0
16. Furue M. Regulation of filaggrin, loricrin, and involucrin by IL-4, IL-13, IL-17A, IL-22, AHR, and NRF2: pathogenic implications in atopic dermatitis. Int J Mol Sci. (2020) 21:5382. doi: 10.3390/ijms21155382
17. Dai X, Utsunomiya R, Shiraishi K, Mori H, Muto J, Murakami M, et al. Nuclear IL-33 plays an important role in the suppression of FLG, LOR, keratin 1, and keratin 10 by IL-4 and IL-13 in human keratinocytes. J Invest Dermatol. (2021) 141:2646–2655.e6. doi: 10.1016/j.jid.2021.04.002
18. Kabashima K and Izuhara K. Barrier dysfunction in allergy. Allergol Int. (2018) 67:1–2. doi: 10.1016/j.alit.2017.12.001
19. Yi L, Cheng D, Zhang K, Huo X, Mo Y, Shi H, et al. Intelectin contributes to allergen-induced IL-25, IL-33, and TSLP expression and type 2 response in asthma and atopic dermatitis. Mucosal Immunol. (2017) 10:1491–503. doi: 10.1038/mi.2017.10
20. Wang Z-Y, Zheng Y-X, Xu F, Cui Y-Z, Chen X-Y, Chen S-Q, et al. Epidermal keratinocyte-specific STAT3 deficiency aggravated atopic dermatitis-like skin inflammation in mice through TSLP upregulation. Front Immunol. (2023) 14:1273182. doi: 10.3389/fimmu.2023.1273182
21. Marques-Mejias A, Bartha I, Ciaccio CE, Chinthrajah RS, Chan S, Hershey GKK, et al. Skin as the target for allergy prevention and treatment. Ann Allergy Asthma Immunol. (2024) 133:133–43. doi: 10.1016/j.anai.2023.12.030
22. Petra AI, Tsilioni I, Taracanova A, Katsarou-Katsari A, and Theoharides TC. Interleukin 33 and interleukin 4 regulate interleukin 31 gene expression and secretion from human laboratory of allergic diseases 2 mast cells stimulated by substance P and/or immunoglobulin E. Allergy Asthma Proc. (2018) 39:153–60. doi: 10.2500/aap.2018.38.4105
23. Che DN, Shin JY, Kang HJ, Cho BO, Kim Y-S, and Jang SI. Luteolin suppresses IL-31 production in IL-33-stimulated mast cells through MAPK and NF-κB signaling pathways. Int Immunopharmacol. (2020) 83:106403. doi: 10.1016/j.intimp.2020.106403
24. Fassett MS, Braz JM, Castellanos CA, Salvatierra JJ, Sadeghi M, Yu X, et al. IL-31-dependent neurogenic inflammation restrains cutaneous type 2 immune cell accumulation and cytokine production in allergic dermatitis. Sci Immunol. (2023) 8:eabi6887. doi: 10.1126/sciimmunol.abi6887
25. Orfali RL and Aoki V. Blockage of the IL-31 pathway as a potential target therapy for atopic dermatitis. Pharmaceutics. (2023) 15:577. doi: 10.3390/pharmaceutics15020577
26. Buhl T, Ikoma A, Kempkes C, Cevikbas F, Sulk M, Buddenkotte J, et al. Protease-activated receptor-2 regulates neuro-epidermal communication in atopic dermatitis. Front Immunol. (2020) 11:1740. doi: 10.3389/fimmu.2020.01740
27. Shi X, Wang L, Clark JD, and Kingery WS. Keratinocytes express cytokines and nerve growth factor in response to neuropeptide activation of the ERK1/2 and JNK MAPK transcription pathways. Regul Pept. (2013) 186:92–103. doi: 10.1016/j.regpep.2013.08.001
28. Gouin O, L’Herondelle K, Buscaglia P, Gall-Ianotto CL, Philippe R, Legoux N, et al. Major role for TRPV1 and InsP3R in PAR2-elicited inflammatory mediator production in differentiated human keratinocytes. J Invest Dermatol. (2018) 138:1564–72. doi: 10.1016/j.jid.2018.01.034
29. Choi JE and Di Nardo A. Skin neurogenic inflammation. Semin Immunopathol. (2018) 40:249–59. doi: 10.1007/s00281-018-0675-z
30. Moriwaki M, Iwamoto K, Niitsu Y, Matsushima A, Yanase Y, Hisatsune J, et al. Staphylococcus aureus from atopic dermatitis skin accumulates in the lysosomes of keratinocytes with induction of IL -1α secretion via TLR 9. Allergy. (2019) 74:560–71. doi: 10.1111/all.13622
31. Nakamura Y, Oscherwitz J, Cease KB, Chan SM, Muñoz-Planillo R, Hasegawa M, et al. Staphylococcus δ-toxin promotes mouse allergic skin disease by inducing mast cell degranulation. Nature. (2013) 503:397–401. doi: 10.1038/nature12655
32. Azimi E, Reddy VB, and Lerner EA. MRGPRX2, atopic dermatitis, and red man syndrome. Itch. (2017) 2:e5. doi: 10.1097/itx.0000000000000005
33. Thapaliya M, Ayudhya CCN, Amponnawarat A, Roy S, and Ali H. Mast cell-specific MRGPRX2: a key modulator of neuro-immune interaction in allergic diseases. Curr Allergy Asthma Rep. (2021) 21:3. doi: 10.1007/s11882-020-00979-5
34. Cheng W-H, Zhuang T-L, Lee M-J, Chou C-L, Chen B-C, Kuo H-P, et al. IL-33/ST2 axis mediates diesel exhaust particles-induced mast cell activation. Mol Med. (2024) 30:262. doi: 10.1186/s10020-024-01035-y
35. Jia T, Che D, Zheng Y, Zhang H, Li Y, Zhou T, et al. Mast cells initiate type 2 inflammation through tryptase released by MRGPRX2/MRGPRB2 activation in atopic dermatitis. J Invest Dermatol. (2024) 144:53–62.e2. doi: 10.1016/j.jid.2023.06.201
36. Zhou Q, Wang Y, Ni P, Chen Y, Dong H, and Qian Y. Effect of tryptase on mouse brain microvascular endothelial cells via protease-activated receptor 2. J Neuroinflamm. (2018) 15:248. doi: 10.1186/s12974-018-1287-1
37. Kaur R, Harvey JM, Brambilla R, Chandrasekharan UM, and Elaine Husni ME. Targeting dendritic cell-specific TNFR2 improves skin and joint inflammation in a murine model of psoriatic arthritis. Sci Rep. (2025) 15:31574. doi: 10.1038/s41598-025-15175-6
38. Liu Q, Yin W, Han L, Lv J, Li B, Lin Y, et al. Diarylheptanoid from rhizomes of curcuma kwangsiensis (DCK) inhibited imiquimod-induced dendritic cells activation and Th1/Th17 differentiation. Int Immunopharmacol. (2018) 56:339–48. doi: 10.1016/j.intimp.2018.01.044
39. Takemura M, Nakahara T, Hashimoto-Hachiya A, Furue M, and Tsuji G. Glyteer, soybean tar, impairs IL-4/Stat6 signaling in murine bone marrow-derived dendritic cells: the basis of its therapeutic effect on atopic dermatitis. Int J Mol Sci. (2018) 19:1169. doi: 10.3390/ijms19041169
40. Takahashi S, Ochiai S, Jin J, Takahashi N, Toshima S, Ishigame H, et al. Sensory neuronal STAT3 is critical for IL-31 receptor expression and inflammatory itch. Cell Rep. (2023) 42:113433. doi: 10.1016/j.celrep.2023.113433
41. Sarbinowska J, Wiatrak B, and Waśko-Czopnik D. Searching for noninvasive predictors of the diagnosis and monitoring of eosinophilic esophagitis—the importance of biomarkers of the inflammatory reaction involving eosinophils. Biomolecules. (2021) 11:890. doi: 10.3390/biom11060890
42. Lin A-H, Athukorala A, Gleich GJ, and Lee L-Y. Cough responses to inhaled irritants are enhanced by eosinophil major basic protein in awake mice. Am J Physiol-Regul Integr Comp Physiol. (2019) 317:R93–7. doi: 10.1152/ajpregu.00081.2019
43. Uchida AM, Ro G, Qiang L, Peterson KA, Round J, Dougan M, et al. Human differentiated eosinophils release IL-13 in response to IL-33 stimulation. Front Immunol. (2022) 13:946643. doi: 10.3389/fimmu.2022.946643
44. Mack MR, Miron Y, Chen F, Miller PE, Zhang A, Korotzer A, et al. Type 2 cytokines sensitize human sensory neurons to itch-associated stimuli. Front Mol Neurosci. (2023) 16:1258823. doi: 10.3389/fnmol.2023.1258823
45. Siiskonen H and Harvima I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front Cell Neurosci. (2019) 13:422. doi: 10.3389/fncel.2019.00422
46. Marone G, Rossi FW, Pecoraro A, Pucino V, Criscuolo G, de Paulis A, et al. HIV gp120 induces the release of proinflammatory, angiogenic, and lymphangiogenic factors from human lung mast cells. Vaccines. (2020) 8:208. doi: 10.3390/vaccines8020208
47. Varricchi G, Loffredo S, Borriello F, Pecoraro A, Rivellese F, Genovese A, et al. Superantigenic activation of human cardiac mast cells. Int J Mol Sci. (2019) 20:1828. doi: 10.3390/ijms20081828
48. Numata T, Harada K, and Nakae S. Roles of mast cells in cutaneous diseases. Front Immunol. (2022) 13:923495. doi: 10.3389/fimmu.2022.923495
49. Cristinziano L, Poto R, Criscuolo G, Ferrara AL, Galdiero MR, Modestino L, et al. IL-33 and superantigenic activation of human lung mast cells induce the release of angiogenic and lymphangiogenic factors. Cells. (2021) 10:145. doi: 10.3390/cells10010145
50. Bao C and Abraham SN. Mast cell–sensory neuron crosstalk in allergic diseases. J Allergy Clin Immunol. (2024) 153:939–53. doi: 10.1016/j.jaci.2024.02.005
51. Solinski HJ, Kriegbaum MC, Tseng P-Y, Earnest TW, Gu X, Barik A, et al. Nppb neurons are sensors of mast cell-induced itch. Cell Rep. (2019) 26:3561–3573.e4. doi: 10.1016/j.celrep.2019.02.089
52. Tarighi N, Menger D, Pierre S, Kornstädt L, Thomas D, Ferreirós N, et al. Thromboxane-induced α-CGRP release from peripheral neurons is an essential positive feedback loop in capsaicin-induced neurogenic inflammation. J Invest Dermatol. (2019) 139:656–64. doi: 10.1016/j.jid.2018.10.011
53. Liu L, Dana R, and Yin J. Sensory neurons directly promote angiogenesis in response to inflammation via substance P signaling. FASEB J. (2020) 34:6229–43. doi: 10.1096/fj.201903236R
54. Lebonvallet N, Fluhr JW, Le Gall-Ianotto C, Leschiera R, Talagas M, Reux A, et al. A re-innervated in vitro skin model of non-histaminergic itch and skin neurogenic inflammation: PAR2-, TRPV1- and TRPA1-agonist induced functionality. Skin Health Dis. (2021) 1:e66. doi: 10.1002/ski2.66
55. Rivera-Mancilla E, Al-Hassany L, Marynissen H, Bamps D, Garrelds IM, Cornette J, et al. Functional analysis of TRPA1, TRPM3, and TRPV1 channels in human dermal arteries and their role in vascular modulation. Pharmaceuticals. (2024) 17:156. doi: 10.3390/ph17020156
56. Chen Y, Tai Z, Zhu C, Yu Q, Zhu Q, and Chen Z. Vascular endothelial growth factor a VEGFA inhibition: an effective treatment strategy for psoriasis. Int J Mol Sci. (2023) 25:59. doi: 10.3390/ijms25010059
57. Liu Q-Y, Liu H-F, Ye L-Q, Li T, Chen Z-M, Wang Y, et al. Vascular endothelial growth factor a promotes chronic itch via VEGFA-VEGFR2-PI3K-TRPV1 axis in allergic contact dermatitis. J Inflammation Res. (2024) 17:7423–39. doi: 10.2147/JIR.S470094
58. Keith YH, Honda T, Ono S, Lee B, Shibuya R, Hanakawa S, et al. Infiltration and local differentiation of bone marrow–derived integrinβ7-positive mast cell progenitors in atopic dermatitis–like skin. J Allergy Clin Immunol. (2023) 151:159–171.e8. doi: 10.1016/j.jaci.2022.09.011
59. Liu F-T, Goodarzi H, and Chen H-Y. IgE, mast cells, and eosinophils in atopic dermatitis. Clin Rev Allergy Immunol. (2011) 41:298–310. doi: 10.1007/s12016-011-8252-4
60. Derakhshan T, Boyce JA, and Dwyer DF. Defining mast cell differentiation and heterogeneity through single-cell transcriptomics analysis. J Allergy Clin Immunol. (2022) 150:739–47. doi: 10.1016/j.jaci.2022.08.011
61. Tauber M, Basso L, Martin J, Bostan L, Pinto MM, Thierry GR, et al. Landscape of mast cell populations across organs in mice and humans. J Exp Med. (2023) 220:e20230570. doi: 10.1084/jem.20230570
62. Baran J, Sobiepanek A, Mazurkiewicz-Pisarek A, Rogalska M, Gryciuk A, Kuryk L, et al. Mast cells as a target-a comprehensive review of recent therapeutic approaches. Cells. (2023) 12:1187. doi: 10.3390/cells12081187
63. Kröger M, Scheffel J, Nikolaev VV, Shirshin EA, Siebenhaar F, Schleusener J, et al. In vivo non-invasive staining-free visualization of dermal mast cells in healthy, allergy and mastocytosis humans using two-photon fluorescence lifetime imaging. Sci Rep. (2020) 10:14930. doi: 10.1038/s41598-020-71901-2
64. Luo X, Chen J, Yang H, Hu X, Alphonse MP, Shen Y, et al. Dendritic cell immunoreceptor drives atopic dermatitis by modulating oxidized CaMKII-involved mast cell activation. JCI Insight. (2022) 7:e152559. doi: 10.1172/jci.insight.152559
65. Sato M, Matsuo K, Susami Y, Yamashita A, Hayasaka H, Hara Y, et al. A CCR4 antagonist attenuates atopic dermatitis-like skin inflammation by inhibiting the recruitment and expansion of Th2 cells and Th17 cells. Int Immunol. (2023) 35:437–46. doi: 10.1093/intimm/dxad019
66. White L, Yin X, Price E, Krisna SS, Lima H, Sehmi R, et al. Mast cell expression of IL-33, ST2 and TSLPR in the skin of atopic dermatitis post-allergen exposure. J Allergy Clin Immunol. (2019) 143:AB65. doi: 10.1016/j.jaci.2018.12.197
67. Li Y, Leung PSC, Gershwin ME, and Song J. New mechanistic advances in FcϵRI-mast cell–mediated allergic signaling. Clin Rev Allergy Immunol. (2022) 63:431–46. doi: 10.1007/s12016-022-08955-9
68. Yanase Y, Matsuo Y, Kawaguchi T, Ishii K, Tanaka A, Iwamoto K, et al. Activation of human peripheral basophils in response to high IgE antibody concentrations without antigens. Int J Mol Sci. (2018) 20:45. doi: 10.3390/ijms20010045
69. Wang Z and Babina M. MRGPRX2 signals its importance in cutaneous mast cell biology: does MRGPRX2 connect mast cells and atopic dermatitis? Exp Dermatol. (2020) 29:1104–11. doi: 10.1111/exd.14182
70. Li Y, Zhang J, Falo LD, Larregina AT, and Sumpter TL. The pseudo-allergy receptor, MrgprB2/X2 is controlled by neurokinin a and the neurokinin 2 receptor in human and mouse skin. J Immunol. (2020) 204:66.17. doi: 10.4049/jimmunol.204.Supp.66.17
71. Sutradhar S and Ali H. Mast cell MrgprB2 in neuroimmune interaction in IgE-mediated airway inflammation and its modulation by β-arrestin2. Front Immunol. (2024) 15:1470016. doi: 10.3389/fimmu.2024.1470016
72. Liu P, Zhang X, He X, Jiang Z, Wang Q, and Lu Y. Spinal GABAergic neurons are under feed-forward inhibitory control driven by aδ and C fibers in Gad2 td-tomato mice. Mol Pain. (2021) 17:1744806921992620. doi: 10.1177/1744806921992620
73. Sutaria N, Adawi W, Goldberg R, Roh YS, Choi J, and Kwatra SG. Itch: pathogenesis and treatment. J Am Acad Dermatol. (2022) 86:17–34. doi: 10.1016/j.jaad.2021.07.078
74. Urashima R and Mihara M. Cutaneous nerves in atopic dermatitis. Virchows Arch. (1998) 432:363–70. doi: 10.1007/s004280050179
75. Tominaga M, Ogawa H, and Takamori K. Decreased production of semaphorin 3A in the lesional skin of atopic dermatitis. Br J Dermatol. (2008) 158:842–4. doi: 10.1111/j.1365-2133.2007.08410.x
76. Johansson O, Han S-W, and Enhamre A. Altered cutaneous innervation in psoriatic skin as revealed by PGP 9.5 immunohistochemistry. Arch Dermatol Res. (1991) 283:519–23. doi: 10.1007/BF00371926
77. Maddison B, Namazi MR, Samuel LS, Sanchez J, Pichardo R, Stocks J, et al. Unexpected diminished innervation of epidermis and dermoepidermal junction in lichen amyloidosus. Br J Dermatol. (2008) 159:403–6. doi: 10.1111/j.1365-2133.2008.08685.x
78. Tsutsumi M, Kitahata H, Fukuda M, Kumamoto J, Goto M, Denda S, et al. Numerical and comparative three-dimensional structural analysis of peripheral nerve fibres in epidermis of patients with atopic dermatitis. Br J Dermatol. (2016) 174:191–4. doi: 10.1111/bjd.13974
79. Tan Y, Ng WJ, Lee SZX, Lee BTK, Nattkemper LA, Yosipovitch G, et al. 3-dimensional optical clearing and imaging of pruritic atopic dermatitis and psoriasis skin reveals downregulation of epidermal innervation. J Invest Dermatol. (2019) 139:1201–4. doi: 10.1016/j.jid.2018.11.006
80. Wong L-S, Lee C-H, and Yen Y-T. Increased epidermal nerve growth factor without small-fiber neuropathy in dermatomyositis. Int J Mol Sci. (2022) 23:9030. doi: 10.3390/ijms23169030
81. Tsuji G, Yumine A, Kawamura K, Takemura M, and Nakahara T. Induction of semaphorin 3A by resveratrol and pinostilbene via activation of the AHR-NRF2 axis in human keratinocytes. Antioxidants. (2024) 13:732. doi: 10.3390/antiox13060732
82. Agelopoulos K, Renkhold L, Wiegmann H, Dugas M, Süer A, Zeidler C, et al. Transcriptomic, epigenomic, and neuroanatomic signatures differ in chronic prurigo, atopic dermatitis, and brachioradial pruritus. J Invest Dermatol. (2023) 143:264–272.e3. doi: 10.1016/j.jid.2022.08.042
83. Pogatzki-Zahn EM, Pereira MP, Cremer A, Zeidler C, Dreyer T, Riepe C, et al. Peripheral sensitization and loss of descending inhibition is a hallmark of chronic pruritus. J Invest Dermatol. (2020) 140:203–211.e4. doi: 10.1016/j.jid.2019.05.029
84. Ständer S, Agelopoulos K, Nattkemper L, Bagel J, Praestgaard A, Bologna G, et al. 99 dupilumab treatment normalizes intraepidermal nerve fiber density in patients with moderate-to-severe atopic dermatitis and improves patient-reported outcomes. J Invest Dermatol. (2023) 143:S349. doi: 10.1016/j.jid.2023.09.107
85. Fujii K, Miyagawa R, Tanaka R, Saito M, Tanaka S, Shiratori-Hayashi M, et al. MrgprA3+ primary sensory neurons mediate acute allergic itch responses in atopic dermatitis model mice. Biol Pharm Bull. (2024) 47:1624–30. doi: 10.1248/bpb.b24-00522
86. Cao C, Kang HJ, Singh I, Chen H, Zhang C, Ye W, et al. STRUCTURE, FUNCTION AND PHARMACOLOGY OF HUMAN ITCH GPCRs. Nature. (2021) 600:170–5. doi: 10.1038/s41586-021-04126-6
87. Meixiong J, Anderson M, Limjunyawong N, Sabbagh MF, Hu E, Mack MR, et al. Activation of mast-cell-expressed mas-related G-protein-coupled receptors drives non-histaminergic itch. Immunity. (2019) 50:1163–1171.e5. doi: 10.1016/j.immuni.2019.03.013
88. Al Hamwi G, Riedel YK, Clemens S, Namasivayam V, Thimm D, and Müller CE. MAS-related G protein-coupled receptors X (MRGPRX): orphan GPCRs with potential as targets for future drugs. Pharmacol Ther. (2022) 238:108259. doi: 10.1016/j.pharmthera.2022.108259
89. Yang N, Shao H, Deng J, Yang Y, Tang Z, Wu G, et al. Dictamnine ameliorates chronic itch in DNFB-induced atopic dermatitis mice via inhibiting MrgprA3. Biochem Pharmacol. (2023) 208:115368. doi: 10.1016/j.bcp.2022.115368
90. Tang L, Gao J, Cao X, Chen L, Wang H, and Ding H. TRPV1 mediates itch-associated scratching and skin barrier dysfunction in DNFB-induced atopic dermatitis mice. Exp Dermatol. (2022) 31:398–405. doi: 10.1111/exd.14464
91. Lee S, Lim NY, Kang MS, Jeong Y, Ahn J-O, Choi JH, et al. IL-31RA and TRPV1 expression in atopic dermatitis induced with trinitrochlorobenzene in nc/nga mice. Int J Mol Sci. (2023) 24:13521. doi: 10.3390/ijms241713521
92. Shiratori-Hayashi M, Hasegawa A, Toyonaga H, Andoh T, Nakahara T, Kido-Nakahara M, et al. Role of P2X3 receptors in scratching behavior in mouse models. J Allergy Clin Immunol. (2019) 143:1252–1254.e8. doi: 10.1016/j.jaci.2018.10.053
93. Xu G-Y and Huang L-YM. Peripheral inflammation sensitizes P2X receptor-mediated responses in rat dorsal root ganglion neurons. J Neurosci. (2002) 22:93–102. doi: 10.1523/JNEUROSCI.22-01-00093.2002
94. He X, Wei J, Shou S, Fang J, and Jiang Y. Effects of electroacupuncture at 2 and 100 hz on rat type 2 diabetic neuropathic pain and hyperalgesia-related protein expression in the dorsal root ganglion. J Zhejiang Univ-Sci B. (2017) 18:239–48. doi: 10.1631/jzus.B1600247
95. Benet Z, Wong A, Schanin J, and Youngblood BA. 287 targeting the inhibitory receptor siglec-8 on mast cells represents an attractive approach to reduce MRGPRX2-mediated mast cell activation. Br J Dermatol. (2023) 188:ljac140.3. doi: 10.1093/bjd/ljac140.003
96. Rommel FR, Tumala S, Urban A-L, Siebenhaar F, Kruse J, Gieler U, et al. Stress affects mast cell proteases in murine skin in a model of atopic dermatitis-like allergic inflammation. Int J Mol Sci. (2024) 25:5738. doi: 10.3390/ijms25115738
97. Jurcakova D, Ru F, and Undem BJ. Allergen-induced histaminergic and non-histaminergic activation of itch C-fiber nerve terminals in mouse skin. Neuroscience. (2019) 410:55–8. doi: 10.1016/j.neuroscience.2019.04.039
98. Huang K, Hu D-D, Bai D, Wu Z-Y, Chen Y-Y, Zhang Y-J, et al. Persistent extracellular signal-regulated kinase activation by the histamine H4 receptor in spinal neurons underlies chronic itch. J Invest Dermatol. (2018) 138:1843–50. doi: 10.1016/j.jid.2018.02.019
99. Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. (2003) 23:6176–80. doi: 10.1523/JNEUROSCI.23-15-06176.2003
100. Fukuoka Y, Sanchez-Munoz LB, Dellinger AL, and Schwartz LB. Expression and functional characterization of protease-activated receptor 2 (PAR2) and PAR1 on human skin mast cells. J Immunol. (2021) 206:111.17. doi: 10.4049/jimmunol.206.Supp.111.17
101. Deng J, Parthasarathy V, Marani M, Bordeaux Z, Lee K, Trinh C, et al. Extracellular matrix and dermal nerve growth factor dysregulation in prurigo nodularis compared to atopic dermatitis. Front Med. (2022) 9:1022889. doi: 10.3389/fmed.2022.1022889
102. Sung K, Ferrari LF, Yang W, Chung C, Zhao X, Gu Y, et al. Swedish nerve growth factor mutation (NGFR100W) defines a role for TrkA and p75NTR in nociception. J Neurosci. (2018) 38:3394–413. doi: 10.1523/JNEUROSCI.1686-17.2018
103. Meng J, Moriyama M, Feld M, Buddenkotte J, Buhl T, Szöllösi A, et al. New mechanism underlying IL-31–induced atopic dermatitis. J Allergy Clin Immunol. (2018) 141:1677–1689.e8. doi: 10.1016/j.jaci.2017.12.1002
104. Maglie R, Souza Monteiro de Araujo D, Antiga E, Geppetti P, Nassini R, and De Logu F. The role of TRPA1 in skin physiology and pathology. Int J Mol Sci. (2021) 22:3065. doi: 10.3390/ijms22063065
105. Zeng D, Chen C, Zhou W, Ma X, Pu X, Zeng Y, et al. TRPA1 deficiency alleviates inflammation of atopic dermatitis by reducing macrophage infiltration. Life Sci. (2021) 266:118906. doi: 10.1016/j.lfs.2020.118906
106. Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, et al. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. (2015) 18:145–53. doi: 10.1038/nn.3881
107. Burchett J and Ryan JJ. P2X3 potentiates IgE mediated mast cell function. J Immunol. (2023) 210:151.10–0. doi: 10.4049/jimmunol.210.Supp.151.10
108. Giniatullin R, Nistri A, and Fabbretti E. Molecular mechanisms of sensitization of pain-transducing P2X3 receptors by the migraine mediators CGRP and NGF. Mol Neurobiol. (2008) 37:83–90. doi: 10.1007/s12035-008-8020-5
109. Kee SA, Haque T, Dailey J, and Ryan JJ. P2X3 is a female-dominant activator of mast cells. J Immunol. (2021) 206:64. doi: 10.4049/jimmunol.206.Supp.64.16
110. Ogura M, Endo K, Suzuki T, and Homma Y. Prenylated quinolinecarboxylic acid compound-18 prevents sensory nerve fiber outgrowth through inhibition of the interleukin-31 pathway. PloS One. (2021) 16:e0246630. doi: 10.1371/journal.pone.0246630
111. Macphee CH, Dong X, Peng Q, Paone DV, Skov PS, Baumann K, et al. Pharmacological blockade of the mast cell MRGPRX2 receptor supports investigation of its relevance in skin disorders. Front Immunol. (2024) 15:1433982. doi: 10.3389/fimmu.2024.1433982
112. Guo Y, Ollé L, Proaño-Pérez E, Aparicio C, Guerrero M, Muñoz-Cano R, et al. MRGPRX2 signaling involves the lysyl-tRNA synthetase and MITF pathway. Front Immunol. (2023) 14:1154108. doi: 10.3389/fimmu.2023.1154108
113. Jiang T, Ao X, Xiang X, Zhang J, Cai J, Fu J, et al. Mast cell activation by NGF drives the formation of trauma-induced heterotopic ossification. JCI Insight. (2025) 10:e179759. doi: 10.1172/jci.insight.179759
114. Lerner EA. Is the nervous system more important than the immune system in itch and atopic dermatitis? J Investig Dermatol Symp Proc. (2018) 19:S94. doi: 10.1016/j.jisp.2018.10.002
115. Zhang S, Edwards TN, Chaudhri VK, Wu J, Cohen JA, Hirai T, et al. Nonpeptidergic neurons suppress mast cells via glutamate to maintain skin homeostasis. Cell. (2021) 184:2151–66.e16. doi: 10.1016/j.cell.2021.03.002
116. Chompunud Na Ayudhya C, Amponnawarat A, and Ali H. Substance P serves as a balanced agonist for MRGPRX2 and a single tyrosine residue is required for β-arrestin recruitment and receptor internalization. Int J Mol Sci. (2021) 22:5318. doi: 10.3390/ijms22105318
117. Yosipovitch G, Berger T, and Fassett MS. Neuroimmune interactions in chronic itch of atopic dermatitis. J Eur Acad Dermatol Venereol. (2020) 34:239–50. doi: 10.1111/jdv.15973
118. Magadmi R, Meszaros J, Damanhouri ZA, and Seward EP. Secretion of mast cell inflammatory mediators is enhanced by CADM1-dependent adhesion to sensory neurons. Front Cell Neurosci. (2019) 13:262. doi: 10.3389/fncel.2019.00262
119. Serhan N, Basso L, Sibilano R, Petitfils C, Meixiong J, Bonnart C, et al. House dust mites activate nociceptor–mast cell clusters to drive type 2 skin inflammation. Nat Immunol. (2019) 20:1435–43. doi: 10.1038/s41590-019-0493-z
120. Dragunas G, Koster CS, de Souza Xavier Costa N, Melgert BN, Munhoz CD, Gosens R, et al. Neuroplasticity and neuroimmune interactions in fatal asthma. Allergy. (2025) 80:462–73. doi: 10.1111/all.16373
121. Tripathi GM, Brandt EB, Queme LF, Ruff BP, Hoffman MC, Khurana Hershey GK, et al. Peripheral neuro-immune interactions in atopic dermatitis. J Pain. (2022) 23:13. doi: 10.1016/j.jpain.2022.03.050
122. Hagiyama M, Inoue T, Furuno T, Iino T, Itami S, Nakanishi M, et al. Increased expression of cell adhesion molecule 1 by mast cells as a cause of enhanced nerve–mast cell interaction in a hapten-induced mouse model of atopic dermatitis. Br J Dermatol. (2013) 168:771–8. doi: 10.1111/bjd.12108
123. Han L, Ma C, Liu Q, Weng H-J, Cui Y, Tang Z, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. (2013) 16:174–82. doi: 10.1038/nn.3289
124. Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, et al. A sensory neuron-expressed interleukin-31 receptor mediates T helper cell-dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. (2014) 133:448–460.e7. doi: 10.1016/j.jaci.2013.10.048
125. Hsu W-MM, Kastner DB, Baccus SA, and Sharpee TO. How inhibitory neurons increase information transmission under threshold modulation. Cell Rep. (2021) 35:109158. doi: 10.1016/j.celrep.2021.109158
126. Dunham JP, Kelly S, and Donaldson LF. Inflammation reduces mechanical thresholds in a population of transient receptor potential channel A1-expressing nociceptors in the rat. Eur J Neurosci. (2008) 27:3151–60. doi: 10.1111/j.1460-9568.2008.06256.x
127. Ahn J-W, Kim S-E, Kim D-Y, Jeong I, Kim S, Chung S, et al. Cav3.2 T-type calcium channel mediates acute itch and contributes to chronic itch and inflammation in experimental atopic dermatitis. J Invest Dermatol. (2024) 144:612–620.e6. doi: 10.1016/j.jid.2023.07.029
128. Meng J, Li Y, Fischer MJM, Steinhoff M, Chen W, and Wang J. Th2 modulation of transient receptor potential channels: an unmet therapeutic intervention for atopic dermatitis. Front Immunol. (2021) 12:696784. doi: 10.3389/fimmu.2021.696784
129. Schmidt M, Dubin AE, Petrus MJ, Earley TJ, and Patapoutian A. Nociceptive signals induce trafficking of TRPA1 to the plasma membrane. Neuron. (2009) 64:498–509. doi: 10.1016/j.neuron.2009.09.030
130. Perner C, Flayer CH, Zhu X, Aderhold PA, Dewan ZNA, Voisin T, et al. Substance P release by sensory neurons triggers dendritic cell migration and initiates the type-2 immune response to allergens. Immunity. (2020) 53:1063–1077.e7. doi: 10.1016/j.immuni.2020.10.001
131. Azimi E, Reddy VB, Pereira PJS, Talbot S, Woolf CJ, and Lerner EA. Substance P activates mas-related G protein–coupled receptors to induce itch. J Allergy Clin Immunol. (2017) 140:447–453.e3. doi: 10.1016/j.jaci.2016.12.980
132. Gu Y, Wang C, Li G, and Huang L-YM. F-actin links epac-PKC signaling to purinergic P2X3 receptor sensitization in dorsal root ganglia following inflammation. Mol Pain. (2016) 12:1744806916660557. doi: 10.1177/1744806916660557
133. Fujii M, Takeuchi K, Umehara Y, Takeuchi M, Nakayama T, Ohgami S, et al. Barbiturates enhance itch-associated scratching in atopic dermatitis mice: a possible clue to understanding nocturnal pruritus in atopic dermatitis. Eur J Pharmacol. (2018) 836:57–66. doi: 10.1016/j.ejphar.2018.08.018
134. Zhang P-A, Zhu H-Y, Xu Q-Y, Du W-J, Hu S, and Xu G-Y. Sensitization of P2X3 receptors in insular cortex contributes to visceral pain of adult rats with neonatal maternal deprivation. Mol Pain. (2018) 14:1744806918764731. doi: 10.1177/1744806918764731
135. Fabbretti E, D’Arco M, Fabbro A, Simonetti M, Nistri A, and Giniatullin R. Delayed upregulation of ATP P2X3 receptors of trigeminal sensory neurons by calcitonin gene-related peptide. J Neurosci. (2006) 26:6163–71. doi: 10.1523/JNEUROSCI.0647-06.2006
136. D’Arco M, Giniatullin R, Simonetti M, Fabbro A, Nair A, Nistri A, et al. Neutralization of nerve growth factor induces plasticity of ATP-sensitive P2X3 receptors of nociceptive trigeminal ganglion neurons. J Neurosci. (2007) 27:8190–201. doi: 10.1523/JNEUROSCI.0713-07.2007
137. Ozaki Y, Kitamura N, Tsutsumi A, Dayanithi G, and Shibuya I. NGF-induced hyperexcitability causes spontaneous fluctuations of intracellular Ca2+ in rat nociceptive dorsal root ganglion neurons. Cell Calcium. (2009) 45:209–15. doi: 10.1016/j.ceca.2008.10.002
138. Zhang YR, Keshari S, Kurihara K, Liu J, McKendrick LM, Chen C-S, et al. Agonism of the glutamate receptor GluK2 suppresses dermal mast cell activation and cutaneous inflammation. Sci Transl Med. (2024) 16:eadq9133. doi: 10.1126/scitranslmed.adq9133
139. Miake S, Tsuji G, Takemura M, Hashimoto-Hachiya A, Vu YH, Furue M, et al. IL-4 augments IL-31/IL-31 receptor alpha interaction leading to enhanced ccl 17 and ccl 22 production in dendritic cells: implications for atopic dermatitis. Int J Mol Sci. (2019) 20:4053. doi: 10.3390/ijms20164053
140. Nemoto O, Furue M, Nakagawa H, Shiramoto M, Hanada R, Matsuki S, et al. The first trial of CIM331, a humanized antihuman interleukin-31 receptor a antibody, in healthy volunteers and patients with atopic dermatitis to evaluate safety, tolerability and pharmacokinetics of a single dose in a randomized, double-blind, placebo-controlled study. Br J Dermatol. (2016) 174:296–304. doi: 10.1111/bjd.14207
141. Kabashima K, Matsumura T, Komazaki H, and Kawashima M. Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. N Engl J Med. (2020) 383:141–50. doi: 10.1056/NEJMoa1917006
142. Kabashima K, Matsumura T, Komazaki H, and Kawashima M. Nemolizumab plus topical agents in patients with atopic dermatitis (AD) and moderate-to-severe pruritus provide improvement in pruritus and signs of AD for up to 68 weeks: results from two phase III, long-term studies. Br J Dermatol. (2022) 186:642–51. doi: 10.1111/bjd.20873
143. Silverberg JI, Wollenberg A, Reich A, Thaçi D, Legat FJ, Papp KA, et al. Nemolizumab with concomitant topical therapy in adolescents and adults with moderate-to-severe atopic dermatitis (ARCADIA 1 and ARCADIA 2): results from two replicate, double-blind, randomised controlled phase 3 trials. Lancet. (2024) 404:445–60. doi: 10.1016/S0140-6736(24)01203-0
144. Sidbury R, Alpizar S, Laquer V, Dhawan S, Abramovits W, Loprete L, et al. Pharmacokinetics, safety, efficacy, and biomarker profiles during nemolizumab treatment of atopic dermatitis in adolescents. Dermatol Ther. (2022) 12:631–42. doi: 10.1007/s13555-021-00678-7
145. Igarashi A, Katsunuma T, Matsumura T, Komazaki H, and for the Nemolizumab-JP04 Study Group. Efficacy and safety of nemolizumab in paediatric patients aged 6–12 years with atopic dermatitis with moderate-to-severe pruritus: results from a phase III, randomized, double-blind, placebo-controlled, multicentre study. Br J Dermatol. (2024) 190:20–8. doi: 10.1093/bjd/ljad268
146. Keam SJ. Nemolizumab: first approval. Drugs. (2022) 82:1143–50. doi: 10.1007/s40265-022-01741-z
147. Michels GM, Ramsey DS, Walsh KF, Martinon OM, Mahabir SP, Hoevers JD, et al. A blinded, randomized, placebo-controlled, dose determination trial of lokivetmab (ZTS-00103289), a caninized, anti-canine IL-31 monoclonal antibody in client owned dogs with atopic dermatitis. Vet Dermatol. (2016) 27:478–e129. doi: 10.1111/vde.12376
148. Michels GM, Walsh KF, Kryda KA, Mahabir SP, Walters RR, Hoevers JD, et al. A blinded, randomized, placebo-controlled trial of the safety of lokivetmab (ZTS-00103289), a caninized anti-canine IL-31 monoclonal antibody in client-owned dogs with atopic dermatitis. Vet Dermatol. (2016) 27:505–e136. doi: 10.1111/vde.12364
149. Moyaert H, Van Brussel L, Borowski S, Escalada M, Mahabir SP, Walters RR, et al. A blinded, randomized clinical trial evaluating the efficacy and safety of lokivetmab compared to ciclosporin in client-owned dogs with atopic dermatitis. Vet Dermatol. (2017) 28:593–e145. doi: 10.1111/vde.12478
150. Krautmann M, Walters RR, King VL, Esch K, Mahabir SP, Gonzales A, et al. Laboratory safety evaluation of lokivetmab, a canine anti-interleukin-31 monoclonal antibody, in dogs. Vet Immunol Immunopathol. (2023) 258:110574. doi: 10.1016/j.vetimm.2023.110574
151. Hamann CR and Thyssen JP. Monoclonal antibodies against interleukin 13 and interleukin 31RA in development for atopic dermatitis. J Am Acad Dermatol. (2018) 78:S37–42. doi: 10.1016/j.jaad.2017.12.018
152. Calebiro D, Koszegi Z, Lanoiselée Y, Miljus T, and O’Brien S. G protein-coupled receptor-G protein interactions: a single-molecule perspective. Physiol Rev. (2021) 101:857–906. doi: 10.1152/physrev.00021.2020
153. Zhang M, Lan X, Li X, and Lu S. Pharmacologically targeting intracellular allosteric sites of GPCRs for drug discovery. Drug Discov Today. (2023) 28:103803. doi: 10.1016/j.drudis.2023.103803
154. Wolf K, Kühn H, Boehm F, Gebhardt L, Glaudo M, Agelopoulos K, et al. A group of cationic amphiphilic drugs activates MRGPRX2 and induces scratching behavior in mice. J Allergy Clin Immunol. (2021) 148:506–522.e8. doi: 10.1016/j.jaci.2020.12.655
155. Dondalska A, Rönnberg E, Ma H, Pålsson SA, Magnusdottir E, Gao T, et al. Amelioration of compound 48/80-mediated itch and LL-37-induced inflammation by a single-stranded oligonucleotide. Front Immunol. (2020) 11:559589. doi: 10.3389/fimmu.2020.559589
156. Baldo BA. MRGPRX2, drug pseudoallergies, inflammatory diseases, mechanisms and distinguishing MRGPRX2- and IgE/FcϵRI-mediated events. Br J Clin Pharmacol. (2023) 89:3232–46. doi: 10.1111/bcp.15845
157. Bagchi S, Patel J, and Harden J. Transcriptional profiling of primary human-skin derived mast cell activation by various MRGPRX2 agonists and inhibition with EVO756. J Immunol. (2024) 212:721_7011. doi: 10.4049/jimmunol.212.supp.0721.7011
158. Harden J, Folkerts J, Tam S-Y, Frischbutter S, Babina M, Hofland HE, et al. 68 EVO756 is a novel MRGPRX2 antagonist that potently inhibits human mast cell degranulation in response to multiple agonists – potential treatment for CSU and beyond. J Invest Dermatol. (2023) 143:S344. doi: 10.1016/j.jid.2023.09.076
159. Wollam J, Solomon M, Villescaz C, Lanier M, Brooks J, Kim A, et al. MRGPRX2 antagonist EP262 potently inhibits agonist-induced mast cell degranulation in vitro and in vivo. J Allergy Clin Immunol. (2023) 151:AB203. doi: 10.1016/j.jaci.2022.12.634
160. Wollam J, Solomon M, Villescaz C, Lanier M, Evans S, Bacon C, et al. Inhibition of mast cell degranulation by novel small molecule MRGPRX2 antagonists. J Allergy Clin Immunol. (2024) 154:1033–43. doi: 10.1016/j.jaci.2024.07.002
161. Wong TK, Choi YG, Li PH, Chow BKC, and Kumar M. MRGPRX2 antagonist GE1111 attenuated DNFB-induced atopic dermatitis in mice by reducing inflammatory cytokines and restoring skin integrity. Front Immunol. (2024) 15:1406438. doi: 10.3389/fimmu.2024.1406438
162. Bawazir M, Amponnawarat A, Hui Y, Oskeritzian CA, and Ali H. Inhibition of MRGPRX2 but not FcϵRI or MrgprB2-mediated mast cell degranulation by a small molecule inverse receptor agonist. Front Immunol. (2022) 13:1033794. doi: 10.3389/fimmu.2022.1033794
163. Pryde DC, Marron B, West CG, Reister S, Amato G, Yoger K, et al. The discovery of a potent series of carboxamide TRPA1 antagonists. MedChemComm. (2016) 7:2145–58. doi: 10.1039/C6MD00387G
164. Chen H and Terrett JA. Transient receptor potential ankyrin 1 (TRPA1) antagonists: a patent review (2015–2019). Expert Opin Ther Pat. (2020) 30:643–57. doi: 10.1080/13543776.2020.1797679
165. Lee YW, Won C -H., Jung K, Nam H -J., Choi G, Park Y -H., et al. Efficacy and safety of PAC-14028 cream – a novel, topical, nonsteroidal, selective TRPV1 antagonist in patients with mild-to-moderate atopic dermatitis: a phase IIb randomized trial. Br J Dermatol. (2019) 180:1030–8. doi: 10.1111/bjd.17455
166. Park CW, Kim BJ, Lee YW, Won C, Park CO, Chung BY, et al. Asivatrep, a TRPV1 antagonist, for the topical treatment of atopic dermatitis: phase 3, randomized, vehicle-controlled study (CAPTAIN-AD). J Allergy Clin Immunol. (2022) 149:1340–1347.e4. doi: 10.1016/j.jaci.2021.09.024
167. Zhao Y, Yan X, Jiang S, Liu Y, Dong C, Chi H, et al. Zhenxin anshen formula ameliorates atopic der-matitis-like skin dysfunction in mice and in vitro via regulation of transient receptor potential vanilloid 1 and transient receptor potential ankyrin 1 in neural pathways. J Tradit Chin Med. (2023) 43:887–96. doi: 10.19852/j.cnki.jtcm.20230802.003
168. Damann N, Bahrenberg G, Stockhausen H, Habermann CJ, Lesch B, Frank-Foltyn R, et al. In vitro characterization of the thermoneutral transient receptor potential vanilloid-1 (TRPV1) inhibitor GRTE16523. Eur J Pharmacol. (2020) 871:172934. doi: 10.1016/j.ejphar.2020.172934
169. Korver W, Wong A, Gebremeskel S, Negri GL, Schanin J, Chang K, et al. The inhibitory receptor siglec-8 interacts with FcϵRI and globally inhibits intracellular signaling in primary mast cells upon activation. Front Immunol. (2022) 13:833728. doi: 10.3389/fimmu.2022.833728
170. Stauderman KA. CRAC channels as targets for drug discovery and development. Cell Calcium. (2018) 74:147–59. doi: 10.1016/j.ceca.2018.07.005
171. Rubaiy HN. ORAI calcium channels: regulation, function, pharmacology, and therapeutic targets. Pharmaceuticals. (2023) 16:162. doi: 10.3390/ph16020162
172. Tsvilovskyy V, Solís-López A, Schumacher D, Medert R, Roers A, Kriebs U, et al. Deletion of Orai2 augments endogenous CRAC currents and degranulation in mast cells leading to enhanced anaphylaxis. Cell Calcium. (2018) 71:24–33. doi: 10.1016/j.ceca.2017.11.004
173. Chaki S, Alkanfari I, Roy S, Amponnawarat A, Hui Y, Oskeritzian CA, et al. Inhibition of orai channel function regulates mas-related G protein-coupled receptor-mediated responses in mast cells. Front Immunol. (2022) 12:803335. doi: 10.3389/fimmu.2021.803335
174. Aki A, Tanaka K, Nagaoka N, Kimura T, Baba D, Onodera Y, et al. Anti-ORAI1 antibody DS-2741a, a specific CRAC channel blocker, shows ideal therapeutic profiles for allergic disease via suppression of aberrant T-cell and mast cell activation. FASEB Bioadvances. (2020) 2:478–88. doi: 10.1096/fba.2020-00008
175. Che D, Zheng Y, Hou Y, Li T, Du X, and Geng S. Dehydroandrographolide targets CD300f and negatively regulated MRGPRX2-induced pseudo-allergic reaction. Phytother Res. (2022) 36:2173–85. doi: 10.1002/ptr.7445
176. Dang B, Hu S, Zhang Y, Huang Y, Zhang T, and An H. Myricetin served as antagonist for negatively regulate MRGPRX2 mediated pseudo-allergic reactions through CD300f/SHP1/SHP2 phosphorylation. Int Immunopharmacol. (2023) 118:110034. doi: 10.1016/j.intimp.2023.110034
177. Yao C, Ye W, and Chen M. Inhibition of mast cell degranulation in atopic dermatitis by celastrol through suppressing MRGPRX2. Dis Markers. (2023) 2023:9049256. doi: 10.1155/2023/9049256
178. Feld M, Garcia R, Buddenkotte J, Katayama S, Lewis K, Muirhead G, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. (2016) 138:500–508.e24. doi: 10.1016/j.jaci.2016.02.020
179. Labib A, Vander Does A, and Yosipovitch G. Nemolizumab for atopic dermatitis. Drugs Today. (2022) 58:159–73. doi: 10.1358/dot.2022.58.4.3378056
180. Yamamura Y, Nakashima C, and Otsuka A. Interplay of cytokines in the pathophysiology of atopic dermatitis: insights from murin models and human. Front Med. (2024) 11:1342176. doi: 10.3389/fmed.2024.1342176
181. Roy S, Ayudhya CCN, Thapaliya M, Deepak V, and Ali H. Multifaceted MRGPRX2: new insight into the role of mast cells in health and disease. J Allergy Clin Immunol. (2021) 148:293–308. doi: 10.1016/j.jaci.2021.03.049
182. Song PI and Armstrong CA. Novel therapeutic approach with PAC-14028 cream, a TRPV1 antagonist, for patients with mild-to-moderate atopic dermatitis. Br J Dermatol. (2019) 180:971–2. doi: 10.1111/bjd.17777
183. Nikolaeva-Koleva M, Butron L, González-Rodríguez S, Devesa I, Valente P, Serafini M, et al. A capsaicinoid-based soft drug, AG1529, for attenuating TRPV1-mediated histaminergic and inflammatory sensory neuron excitability. Sci Rep. (2021) 11:246. doi: 10.1038/s41598-020-80725-z
184. Ben DD, Buccioni M, Lambertucci C, Marucci G, Spinaci A, Marchenkova A, et al. Investigation on 2’,3’-O-substituted ATP derivatives and analogs as novel P2X3 receptor antagonists. ACS Med Chem Lett. (2018) 10 4:493–8. doi: 10.1021/ACSMEDCHEMLETT.8B00524
185. Smith J, Kitt M, Morice A, Birring S, Mcgarvey L, Sher M, et al. Gefapixant, a P2X3 receptor antagonist, for the treatment of refractory or unexplained chronic cough: a randomised, double-blind, controlled, parallel-group, phase 2b trial. Lancet Respir Med. (2020) 8:775–85. doi: 10.1016/s2213-2600(19)30471-0
186. McGarvey LP, Birring S, Morice A, Dicpinigaitis P, Pavord I, Schelfhout J, et al. Efficacy and safety of gefapixant, a P2X3 receptor antagonist, in refractory chronic cough and unexplained chronic cough (COUGH-1 and COUGH-2): results from two double-blind, randomised, parallel-group, placebo-controlled, phase 3 trials. Lancet. (2022) 399:909–23. doi: 10.1016/S0140-6736(21)02348-5
187. Steinhoff M, Ahmad F, Pandey A, Datsi A, AlHammadi A, Al-Khawaga S, et al. Neuroimmune communication regulating pruritus in atopic dermatitis. J Allergy Clin Immunol. (2022) 149:1875–98. doi: 10.1016/j.jaci.2022.03.010
188. Muraro A, Lemanske RF, Hellings PW, Akdis CA, Bieber T, Casale TB, et al. Precision medicine in patients with allergic diseases: airway diseases and atopic dermatitis—PRACTALL document of the european academy of allergy and clinical immunology and the american academy of allergy, asthma & immunology. J Allergy Clin Immunol. (2016) 137:1347–58. doi: 10.1016/j.jaci.2016.03.010
189. Schäbitz A, Hillig C, Mubarak M, Jargosch M, Farnoud A, Scala E, et al. Spatial transcriptomics landscape of lesions from non-communicable inflammatory skin diseases. Nat Commun. (2022) 13:7729. doi: 10.1038/s41467-022-35319-w
190. Rothenberg-Lausell C, Bar J, and Del Duca E. Diversity of atopic dermatitis and selection of immune targets. Ann Allergy Asthma Immunol. (2024) 132:177–86. doi: 10.1016/j.anai.2023.11.020
191. Mesjasz A, Kołkowski K, Wollenberg A, and Trzeciak M. How to understand personalized medicine in atopic dermatitis nowadays? Int J Mol Sci. (2023) 24:7557. doi: 10.3390/ijms24087557
192. Chovatiya R, Lei D, Ahmed A, Chavda R, Gabriel S, and Silverberg JI. Clinical phenotyping of atopic dermatitis using combined itch and lesional severity. Ann Allergy Asthma Immunol. (2021) 127:83–90.e2. doi: 10.1016/j.anai.2021.03.019
193. Mitamura Y, Reiger M, Kim J, Xiao Y, Zhakparov D, Bärenfaller K, et al. Spatial and single-cell transcriptomics provide insights into the complex inflammatory cell network in atopic dermatitis. J Allergy Clin Immunol. (2023) 151:AB338. doi: 10.1016/j.jaci.2022.12.790
194. He H, Suryawanshi H, Morozov P, Gay-Mimbrera J, Duca ED, Kim HJ, et al. Single-cell transcriptome analysis of human skin identifies novel fibroblast subpopulation and enrichment of immune subsets in atopic dermatitis. J Allergy Clin Immunol. (2020) 145:1615–28. doi: 10.1016/j.jaci.2020.01.042
195. Mortlock RD, Ma EC, Cohen JM, and Damsky W. Assessment of treatment-relevant immune biomarkers in psoriasis and atopic dermatitis: toward personalized medicine in dermatology. J Invest Dermatol. (2023) 143:1412–22. doi: 10.1016/j.jid.2023.04.005
196. Hsieh C-L, Yu S-J, Lai K-L, Chao W-T, and Yen C-Y. IFN-γ, IL-17A, IL-4, and IL-13: potential biomarkers for prediction of the effectiveness of biologics in psoriasis patients. Biomedicines. (2024) 12:1115. doi: 10.3390/biomedicines12051115
197. Pavlis J and Yosipovitch G. Management of itch in atopic dermatitis. Am J Clin Dermatol. (2018) 19:319–32. doi: 10.1007/s40257-017-0335-4
198. Erickson S, Heul AV, and Kim BS. New and emerging treatments for inflammatory itch. Ann Allergy Asthma Immunol. (2021) 126:13–20. doi: 10.1016/j.anai.2020.05.028
199. Yan F, Li F, Liu J, Ye S, Zhang Y, Jia J, et al. The formulae and biologically active ingredients of chinese herbal medicines for the treatment of atopic dermatitis. BioMed Pharmacother. (2020) 127:110142. doi: 10.1016/j.biopha.2020.110142
200. Nie W, Fu H, Zhang Y, Yang H, and Liu B. Chinese herbal medicine and their active ingredients involved in the treatment of atopic dermatitis related signaling pathways. Phytother Res. (2025) 39:1190–237. doi: 10.1002/ptr.8409
201. Gao J-F, Tang L, Luo F, Chen L, Zhang Y-Y, and Ding H. Myricetin treatment has ameliorative effects in DNFB-induced atopic dermatitis mice under high-fat conditions. Toxicol Sci. (2023) 191:308–20. doi: 10.1093/toxsci/kfac138
202. Hou D-D, Gu Y-J, Wang D-C, Niu Y, Xu Z-R, Jin Z-Q, et al. Therapeutic effects of myricetin on atopic dermatitis in vivo and in vitro. Phytomedicine. (2022) 102:154200. doi: 10.1016/j.phymed.2022.154200
Keywords: atopic dermatitis, chronic pruritus, mast cell-neuron axis, neuroimmune crosstalk, IL-31 signaling, MRGPRX2, targeted therapy atopic dermatitis, targeted therapy
Citation: Li D, Han Y, Zhou J, Yang H, Chen J, Tey HL and Tan TTY (2025) Mast cell–neuron axis as a core mechanism in chronic pruritus of atopic dermatitis: from mechanistic insights to therapeutic targets. Front. Immunol. 16:1645095. doi: 10.3389/fimmu.2025.1645095
Received: 11 June 2025; Accepted: 17 October 2025;
Published: 29 October 2025.
Edited by:
Martijn van Griensven, Maastricht University, NetherlandsReviewed by:
Hydar Ali, University of Pennsylvania, United StatesElsa Fabbretti, Independent researcher, Italy
Copyright © 2025 Li, Han, Zhou, Yang, Chen, Tey and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Chen, Y2hlbmppbmc2Mzg1QDE2My5jb20=; Hong Liang Tey, dGV5aG9uZ2xpYW5nQG50dS5lZHUuc2c=; Timothy T. Y. Tan, dHl0YW5AbnR1LmVkdS5zZw==
†These authors have contributed equally to this work
 Dongdong Li
Dongdong Li Yusheng Han3
Yusheng Han3 Huasen Yang
Huasen Yang Jing Chen
Jing Chen Hong Liang Tey
Hong Liang Tey