- 1Department of Gerontology and Geriatrics, The First Hospital of China Medical University, Shenyang, Liaoning, China
- 2Department of Medical Oncology, The First Hospital of China Medical University, Shenyang, Liaoning, China
Background: This clinical study aims to investigate the incidence of cerebral small vessel disease (CSVD) in lung cancer patients treated with ICIs and to analyze its risk factors by comparing the clinical features and laboratory tests in ICIs-treated lung cancer patients with or without CSVD.
Methods: This retrospective study included 400 hospitalized patients from January 2018 to May 2024. All patients had confirmed lung cancer, received at least one cycle of ICIs, and underwent cranial MR imaging before and after ICIs treatment. Information from the medical records, including clinical features, MR imaging findings, laboratory tests, complications, treatment, and clinical outcomes, was extracted for analysis.
Results: 104 (26%) patients with CSVD were confirmed and 53.25% were aged≥65 years. Risk factors identified as independent predictors of CSVD included age (OR, 1.03), stage IV (OR, 2.87), and hyperlipidemia (OR, 1.02). In the CSVD group, FT4 levels decreased significantly between baseline and at the time of CSVD diagnosis, from 13.21 ± 4.56 pmol/L to 11.01 ± 2.11 pmol/L. TSH levels increased from 4.12 ± 0.46 pmol/L to 4.78 ± 1.13 pmol/L, cysteine C levels increased from 1.01 ± 0.98 mg/L to 1.29 ± 0.86 mg/L, PLR increased from 164.93 ± 27.86 to 171.27 ± 32.29 and SII rose from 774.28 ± 53.57 to 790.65 ± 68.34. All of them had no significance in the Non-CSVD group. Further Cox regression analysis showed that hypothyroidism (HR=2.38; 95% CI:1.89-5.04, P=0.005) was independent risk factors for CSVD. The incidence of hypothyroidism was 19.5% (78/400), and 43.6% (34/78) among them had CSVD. As predictors of CSVD, the cut point for FT4 was 11.84 pmol/L, and for TSH, it was 4.23 pmol/L. In Survival Analysis, CSVD did not show a significant impact on the median progression-free survival (PFS) and overall survival (OS) of lung cancer patients.
Conclusion: This study found that CSVD may be a related adverse event of immunotherapy in lung cancer patients. In addition to age≥65 years, hyperlipidemia and stage IV, hypothyroidism, elevated cysteine C levels, and elevated systemic inflammatory markers such as PLR and SII were further associated with an increased risk of CSVD.
1 Introduction
Lung cancer is a malignant tumor with the highest incidence and mortality rates globally. Due to the 40% global tobacco consumption rate, and the high levels of ambient particulate matter pollution in developing countries, China is among the countries with a high incidence of lung cancer (1). Lung cancer has always been the leading cause of cancer mortality in China for both men and women (2). In recent years, immunotherapy, as an emerging strategy for cancer treatment, has gradually played a significant role in the treatment of lung cancer. Immune checkpoint inhibitors (ICIs) block immune checkpoints on the surface of tumor cells, such as Programmed Death Protein 1 (PD-1) and its ligand PD-L1, thereby enhancing the body’s anti-tumor immune response. Immunotherapy has achieved significant clinical outcomes in real-world studies, greatly improving patient survival rates (3, 4). Currently, there are 18 types of ICIs listed in China, 11 of which have been independently developed by China. Sintilimab, Tislelizumab, Toripalimab, and others have achieved good therapeutic effects in the treatment of lung cancer, thereby reducing the medical burden as part of their expenses can be covered by medical insurance (5–7). However, the widespread clinical application of ICIs is accompanied by a series of immune-related adverse events (irAEs). Neurologic irAEs include irMeningitis, irEncephalitis, irDemyelinating disease, irVasculitis, irNeuropathy, irNeuromuscular junction disorders and irMyopathy (8, 9). Previous studies suggest that the rate of progression of total aortic plaque volume was more than threefold higher with ICIs (10), and ICIs-related acute cerebrovascular events have been reported (11, 12).
The cerebral small vessels include arterioles, venules, and capillaries, which are important components of the cerebral vascular system. Cerebral small vessel disease (CSVD) is a class of diseases that affect the cerebral small vessels, which can manifest as white matter hyperintensities, lacunar infarcts, and other imaging features. Vascular endothelial dysfunction may be the underlying pathological alteration in CSVD (13). CSVD is explicitly age-related, once thought to be innocuous, but now recognized as the most important vascular contributor to dementia, and associated with clinical manifestations such as cognitive impairment and difficulty walking (14). Current research has identified that CSVD is related to a variety of risk factors, including hypertension, diabetes, and hyperlipidemia, among others (15). Cognitive dysfunction associated with ICIs has also attracted attention. Cancer-related cognitive decline is caused by multiple factors, including concomitant co-morbidities and various cancer treatments (16). A longitudinal study showed that among 240 non-small cell lung cancer patients treated with ICIs, significant deterioration was observed in TMT (psychomotor speed, executive function), HVLTi (verbal memory), and HVLTd (delayed recall) scores after 6 and 12 months of treatment (17). However, there is still a lack of systematic research on whether immunotherapy increases the risk of CSVD in lung cancer patients, which provides an important background for this study.
To evaluate the correlation between ICIs and CSVD, this study adopts a retrospective observational research method, analyzing large-scale clinical data to investigate the incidence of CSVD in lung cancer patients after receiving ICIs treatment, and conducts an in-depth analysis of its related risk factors.
2 Methods
2.1 Study design and data sources
608 lung cancer patients who received at least one cycle of ICIs therapy were collected between January 2018 and May 2024 at the First Hospital of China Medical University. Data was extracted from 12 months after the use of ICIs. All patients underwent cranial MRI imaging examination before receiving ICIs treatment, and we subsequently excluded cases meeting the following criteria: (a) age under 18 years, (b) a history of hematologic, (c) a history of primary brain cancer and cerebrovascular disease, (d) absence of brain MRI data (At least two cranial MRI images for analysis: pre-ICI and within 12 months post-ICI). 419 patients were included with cranial MR imaging before and after ICIs treatment; then, 19 patients were excluded for lack of response assessment. Ultimately, 400 patients were analyzed in this study. For further analysis, all patients were divided into two groups: those with CSVD and those without CSVD, based on cranial MR imaging. The flow chat of our study design is shown in Figure 1. Demographic, clinical, and survival data were retrieved from electronic medical records. All procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of the First Hospital of China Medical University (Project number: 2023-544-2).
Figure 1. The flow chat of study design and patient inclusion. ICIs, immune checkpoint inhibitors; CSVD, cerebral small vessel disease.
2.2 Diagnosis and grouping of CSVD
In this study, CSVD was diagnosed using cranial MR imaging (3.0T) to obtain axial T1, T2-weighted, fluid-attenuated inversion recovery (FLAIR), and T2-weighted gradient echo (GRE) images. All MRI images were independently reviewed by two vascular neurologists; a third vascular neurologist adjudicated discordant findings. The imaging features of CSVD are white matter hyperintensity (WMH), lacunar infarction (LI), enlarged perivascular space (EPVS), cerebral atrophy (CA) and cerebral microbleed (CMB) (18). By comparing with pre-immunotherapy cranial MRI images, patients who had no baseline CSVD but developed new CSVD after treatment, as well as patients with baseline CSVD who exhibited new CSVD lesions or significant worsening of existing lesions post-treatment, were included in the CSVD group, while the remaining patients were assigned to the non-CSVD group.
2.3 Data collection
The demographic and clinical characteristics of lung cancer patients were collected from patient’s electronic medical records, including age (at initiation of ICIs), gender, body mass index (BMI), tumor histology type, initial cancer stage, immunotherapy regimens, line of ICIs therapy (e.g., first line, second line), cranial MR imaging history, and vascular risk factors (smoking, drinking, hypertension, diabetes, coronary heart disease and hyperlipidemia). Peripheral blood parameters included white blood cell count (WBC), neutrophilic count (NE), lymphocyte count (LY), hemoglobin count (HGB), platelet count (PLT), systemic immune-inflammation index ratio (SII: neutrophil count × platelet count)/lymphocyte count absolute monocyte count), neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte Ratio (PLR), creatinine (Cr), cystatin C (Cys-c), estimated glomerular filtration rate (eGFR), uric acid (UA), C-reactive protein (CRP), lymphocyte subsets (CD4/CD8), free triiodothyronine (FT3), free thyroxine (FT4), thyroid stimulating hormone (TSH), cortisol (Cor) and adrenocorticotropic hormone (ACTH), fibrinogen (Fg) and D-dimer (D-D).
Due to subsequent requirements, the timeline of hypothyroidism in all patients and the progression of CSVD in those with hypothyroidism were also documented. Among patients with CSVD, we collected peripheral blood parameters at two time points: baseline (before ICI treatment) and at the time of CSVD diagnosis. In the non-CSVD group, these parameters were recorded at two time points: baseline data prior to the initiation of ICI therapy, and the final data within 12 months after ICIs treatment. The progression free survival (PFS) was calculated from the date of first administration of the ICIs until the progression of disease. The overall survival (OS) was calculated from the date of first administration of the ICIs until death or the last follow-up date (31 May 2025).
2.4 Statistical analysis
All analyses were performed using SPSS 26.0 (IBM, Armonk, NY, USA) and GraphPad Prism 9.0 (GraphPad Software, La Jolla, CA, USA). Two-side P values <0.05 were considered statistically significant. To describe general baseline characteristics, continuous variables data were expressed as mean ± standard deviation, and categorical variable data were summarized by frequency (%). The T-test, nonparametric test, or chi-square test were used to compare the baseline characteristics between groups, as appropriate. Logistic regression was performed to analyze the risk factors of CSVD. Selection of covariates in the multivariable models was based on univariate associations and biological relevance. An odds ratio (OR) with 95% confidence interval (CI) was reported for each covariate of interest. To address time-to-event outcomes, Cox proportional hazards regression was employed to identify CSVD risk factors. Univariable analyses of age, cancer stage, vascular risk factors and hypothyroidism yielded hazard ratio (HR) with 95% CI; significant predictors (P < 0.05) were retained in the final multivariable model with covariate adjustment. Proportional hazards assumptions were validated via Schoenfeld residuals (global P > 0.05), and multicollinearity was excluded (VIF < 2.0). The receiver operating characteristic (ROC) curve was performed to evaluate the diagnostic efficacy of data related to the occurrence of CSVD. The survival rates between the different groups were compared using the Kaplan-Meier method.
3 Results
3.1 MR image characters of CSVD
The representative MR imaging examples were demonstrated in Figure 2A. Figure 2B showed the percentages of each lesion when one lesion was present: WMH (67/104, 64.42%), LI (18/104, 17.31%), EPVS (12/104, 11.54%), CA (6/104, 5.77%), and CMB (1/104, 0.96%). Figure 2C showed the percentages of the combinations when two lesions were present. We could see the most common combinations was WMH and LI (51.43%). Secondly, WMH and CA (17.14%), LI and EPVS (17.14%) with same proportion. Then, EPVS and CA (5.71%), WML and CMB (5.71%) also with same proportion. A total of 11 cases were diagnosed with CSVD within 0–3 months after ICIs treatment, peaking at 6–9 months with 38 cases. Figure 2D clearly demonstrates the temporal distribution of CSVD following immunotherapy.
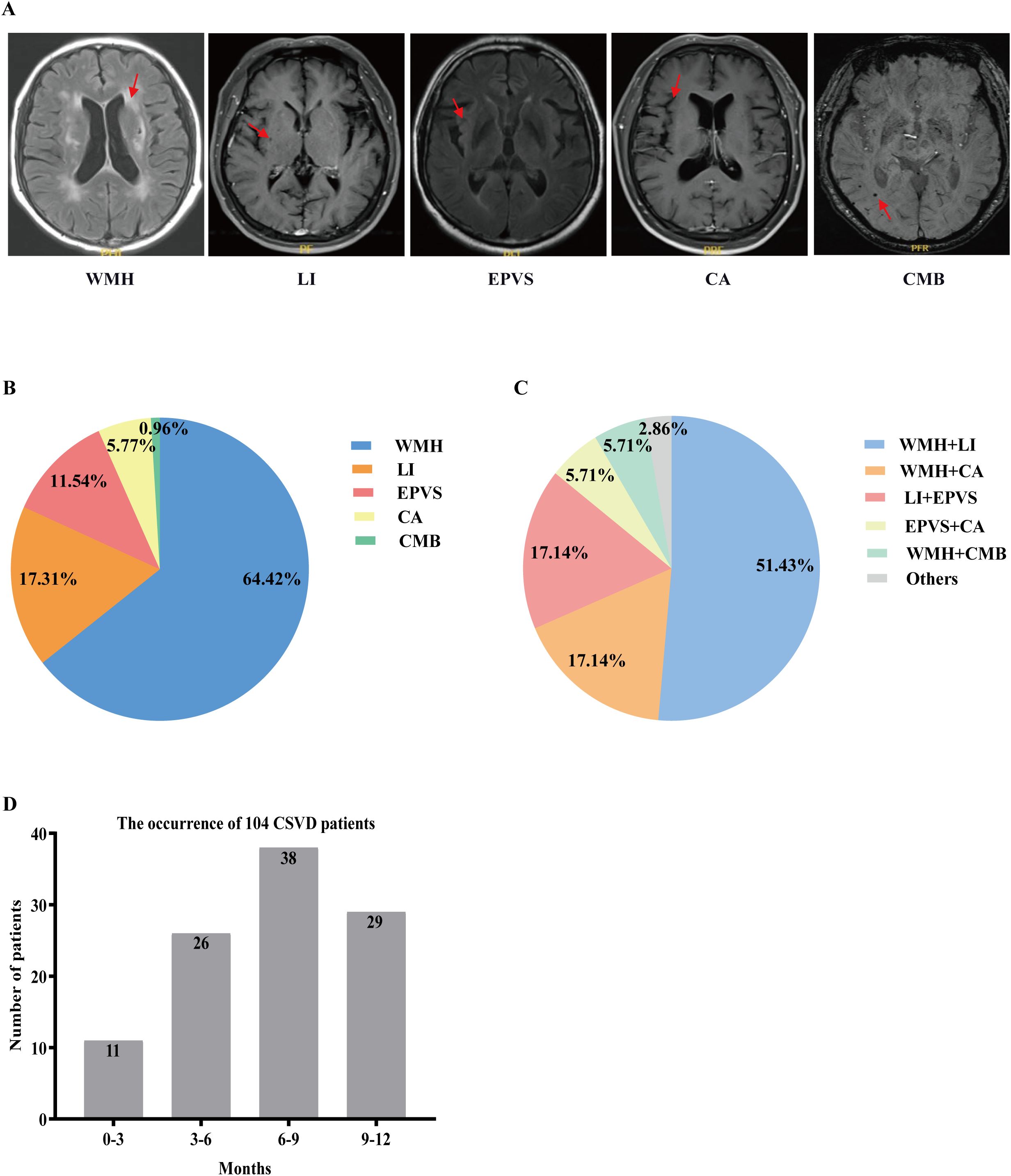
Figure 2. Imaging features of CSVD and distribution of (combinations of) CSVD manifestations. (A) Key imaging characteristics of CSVD. (B) Percentages of each lesion when one lesion is present. (C) Percentages of the combinations when two lesions are present. (D) The temporal distribution of CSVD following immunotherapy. CSVD, cerebral small vessel disease; WMH, white matter hyperintensity; LI, lacunar infarction; EPVS, enlarged perivascular space; CA, cerebral atrophy; CMB, cerebral microbleed.
3.2 Demographic and clinical characteristics
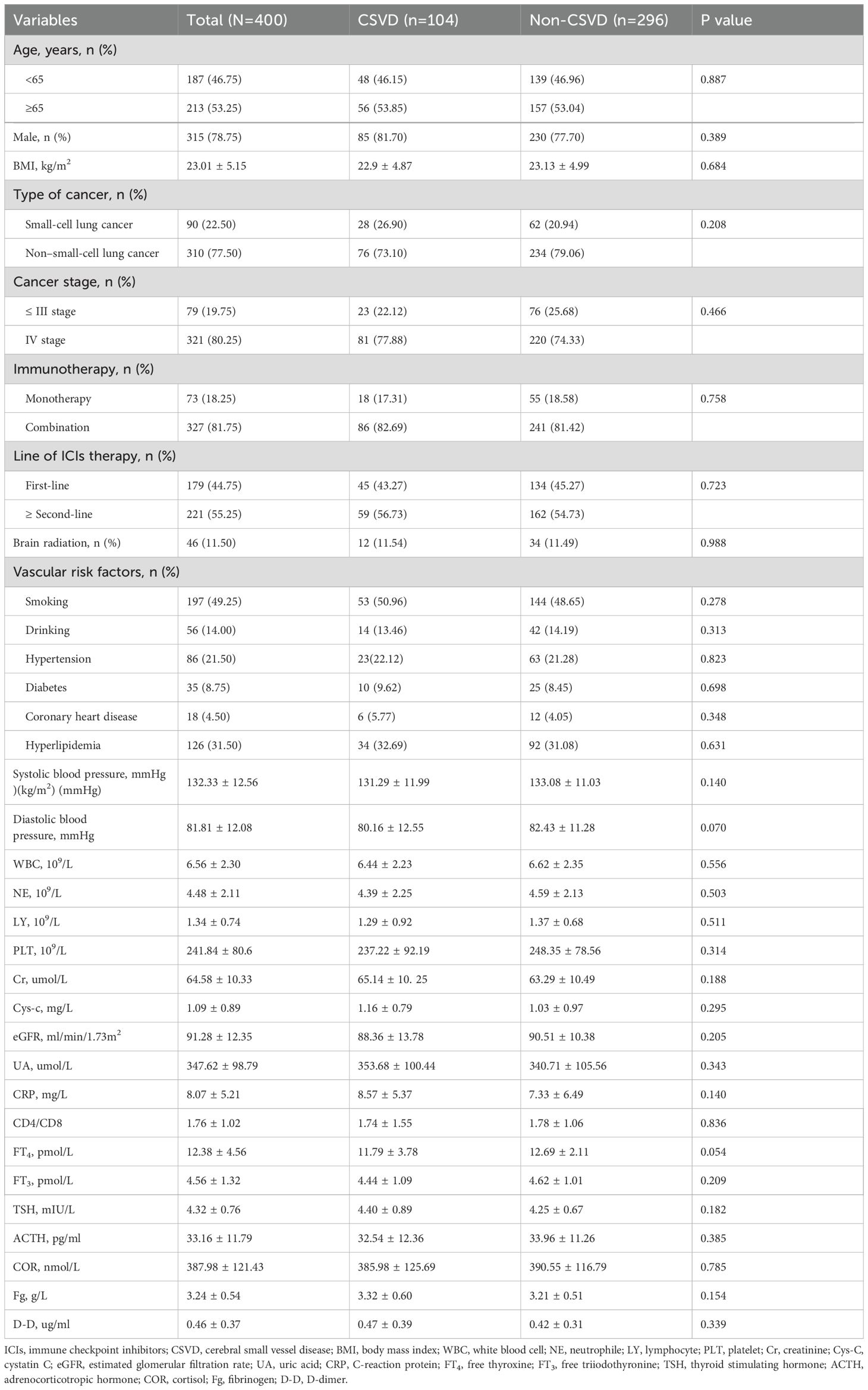
400 patients were included in this study and the incidence of CSVD in lung cancer patients treated with ICIs was 26% (104/400 patients). Slightly more than half were aged≥65 years, and 81.7% were male (Table 1). In China, the incidence of lung cancer caused by smoking is significantly higher in male patients than in females (19). The demographic and clinical characteristics of the enrolled patients were shown in Table 1. CSVD and Non-CSVD patients consisted of 26% (n = 104) and 74% (n = 296) of the entire lung cancer patients. 53.25% were aged≥65 years and 78.75% were male. There was no significant difference between groups with/without CSVD for age, gender, BMI, tumor histology, tumor stage, treatment data, vascular risk factors, baseline blood cell count, baseline eGFR and baseline TSH/FT4.
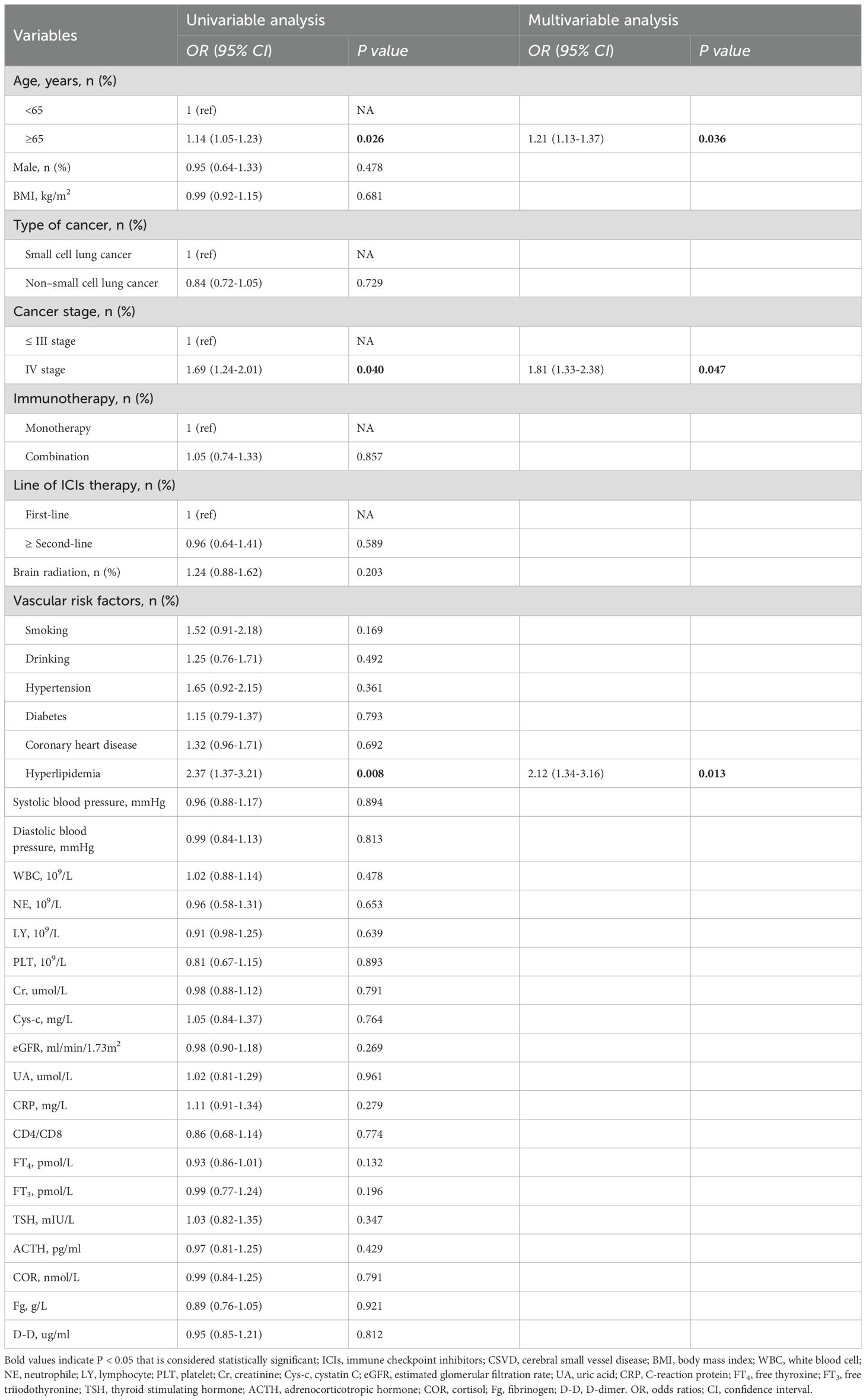
In the univariate logistic regression analysis, the results indicated that age (OR, 1.97; 95% CI: 1.16-2.79, P=0.025), stage IV (OR, 1.69; 95% CI: 1.24-2.01, P=0.040), and hyperlipidemia (OR, 2.37; 95% CI: 1.37-3.21, P=0.008) were associated with an increased risk of CSVD. Variables with a P-value ≤ 0.05 from the univariate logistic regression analysis were included in the multivariate logistic regression analysis. The results indicated that age (OR, 1.86; 95% CI: 1.22-2.81, P=0.036), cancer stage IV (OR, 1.81; 95% CI: 1.33-2.38, P=0.047), and hyperlipidemia (OR, 2.12; 95% CI: 1.34-3.16, P=0.013) were significantly and independently associated with the risk of CSVD (Table 2). Numerous studies have shown that age is a recognized independent risk factor for CSVD. Moreover, the severity and progression of CSVD increase with age (20, 21). Hyperlipidemia is a fatal risk factor for the development of stroke. Studies have shown that several common lipid abnormalities have a causal relationship with CSVD subtype infarction (22, 23). In this study, the results suggest that hyperlipidemia is an independent risk factor for CSVD in lung cancer patients undergoing immunotherapy.
3.3 Correlation of laboratory findings with CSVD
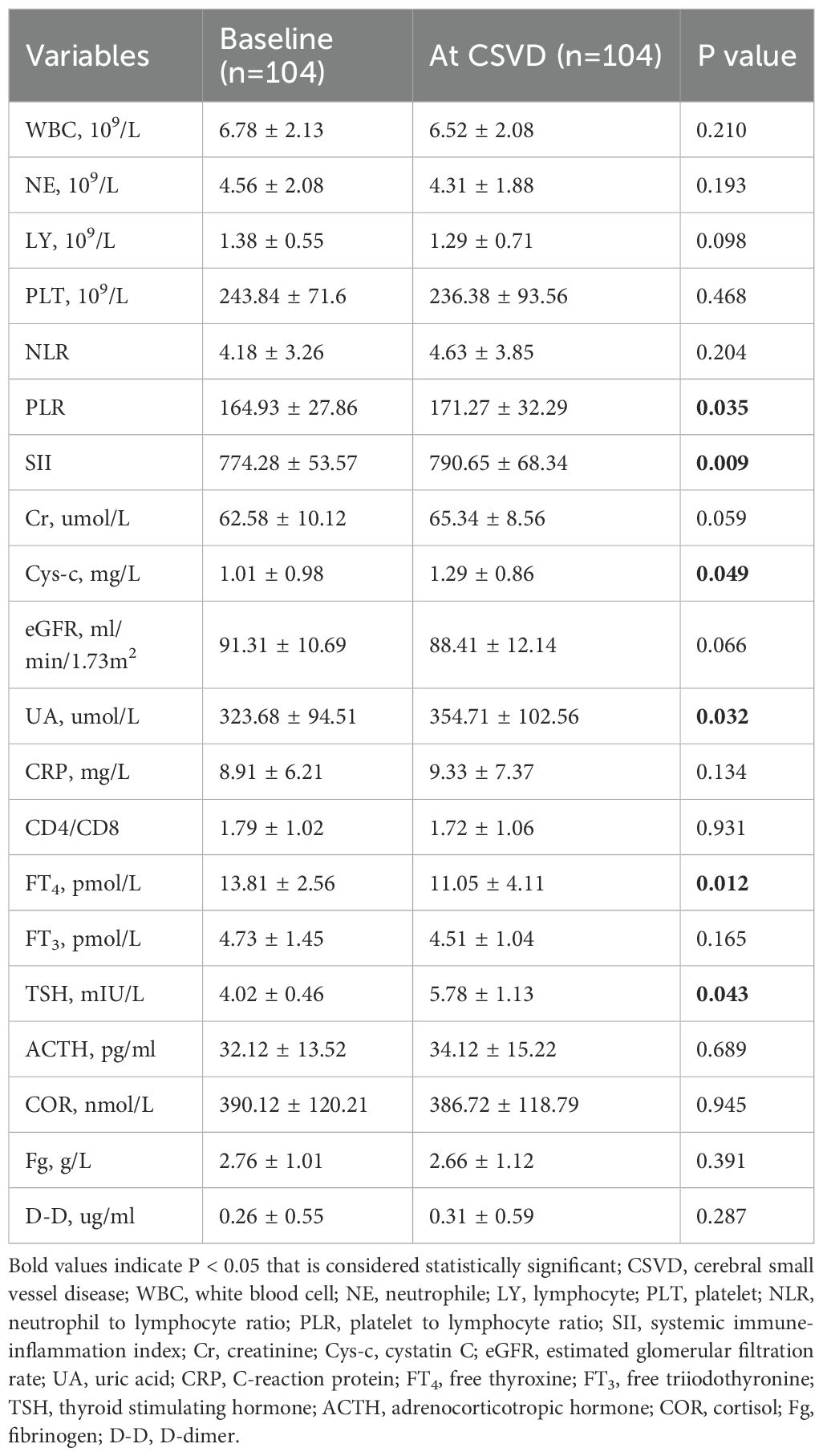
To clarify the specific changes in laboratory indicators during the occurrence of CSVD, we extracted laboratory data at the time of CSVD diagnosis and conducted a comparative analysis with baseline data. The results indicated no significant alterations in WBC, NE, LY, HGB, PLT, NLR, Cr levels, eGFR, CRP, CD4/CD8 ratio, FT3, Cor, ACTH, Fg, or D-D. However, there were notable changes in PLR, SII, Cys-c, UA, FT4, and TSH levels from baseline to the onset of CSVD, as shown in Table 3. A comparison of these six biomarkers was made between the CSVD group and the non-CSVD group. All six indicators showed no statistically significant differences when compared to baseline and final medication data (within 12 months after ICIs treatment) in the non-CSVD group (Figure 3). Apart from UA, the remaining five data points in the non-CSVD group did not show any statistically significant differences when comparing the baseline data with the final data (12 months after initiating immune checkpoint inhibitor therapy) (Figure 3).
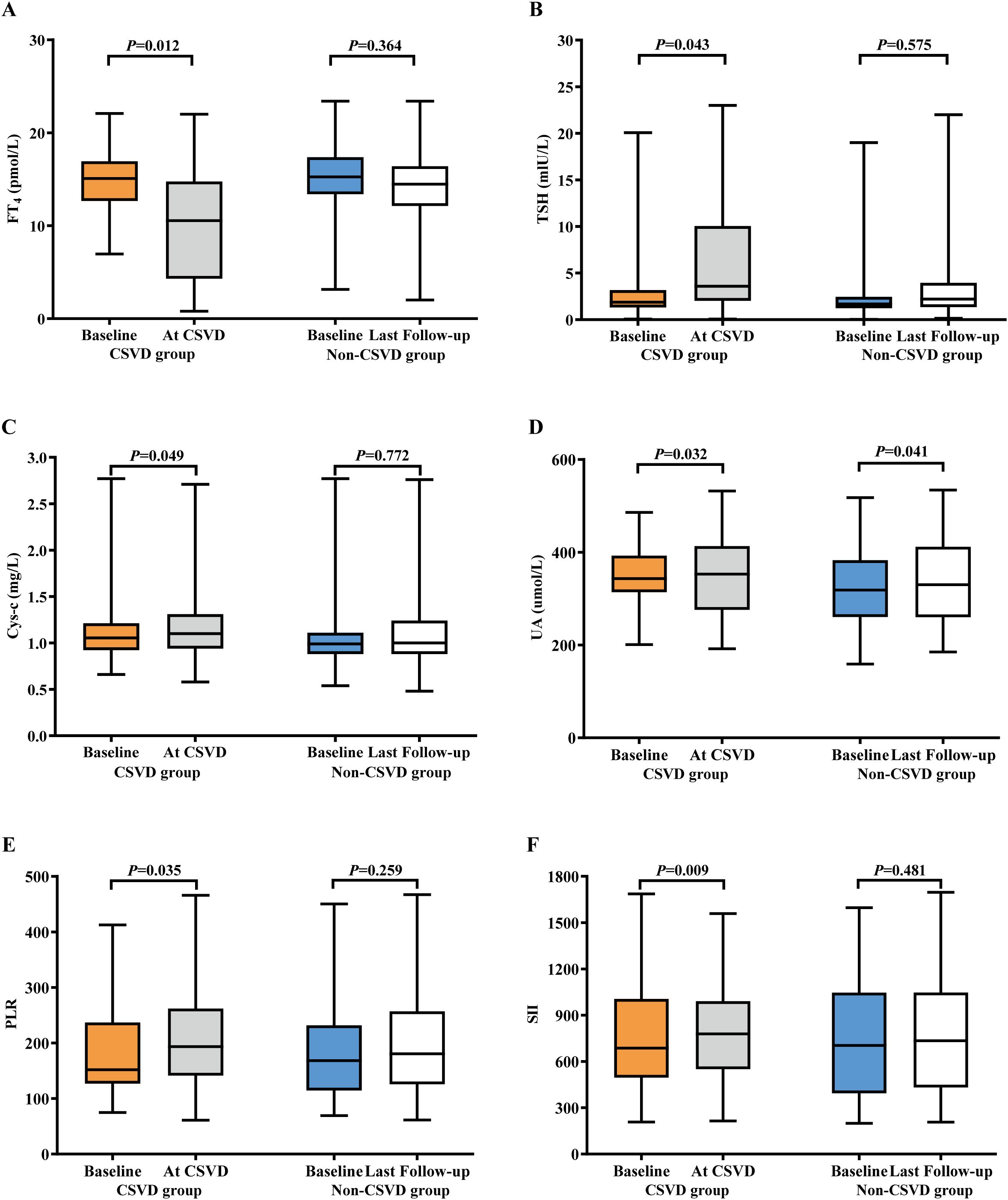
Figure 3. Bar plots of laboratory indicators in lung cancer patients with CSVD and non-CSVD at different times. (A) FT4. (B) TSH. (C) Cys-c. (D) UA. (E) PLR. (F) SII. CSVD, cerebral small vessel disease; UA, uric acid; Cys-c, cystatin C; FT4, free thyroxine; TSH, thyroid stimulating hormone; PLR, platelet to lymphocyte ratio; SII, systemic immune-inflammation index.
Although no statistically significant differences were observed in inflammatory cell counts and non-specific biomarkers of inflammation (CRP and D-D), the composite biomarkers of systemic inflammation indices PLR and SII both exhibited an upward trend. Specifically, PLR increased from 164.93 ± 27.86 to 171.27 ± 32.29, with a P-value of 0.035; while SII rose from 774.28 ± 53.57 to 790.65 ± 68.34, with a P-value of 0.009. The final results indicated that FT4 levels decreased significantly from the baseline to when CSVD was diagnosed, from 13.21 ± 4.56 pmol/L to 11.01 ± 2.11 pmol/L, with a P-value of 0.012. A corresponding tendency in TSH levels was observed in the CSVD group, increasing from 4.12 ± 0.46 pmol/L to 4.78 ± 1.13 pmol/L, with a P-value of 0.043. Cys-c levels gradually increased from 1.01 ± 0.98 mg/L to 1.29 ± 0.86 mg/L (P = 0.049) in the CSVD group.
3.4 Potential risk factors for CSVD
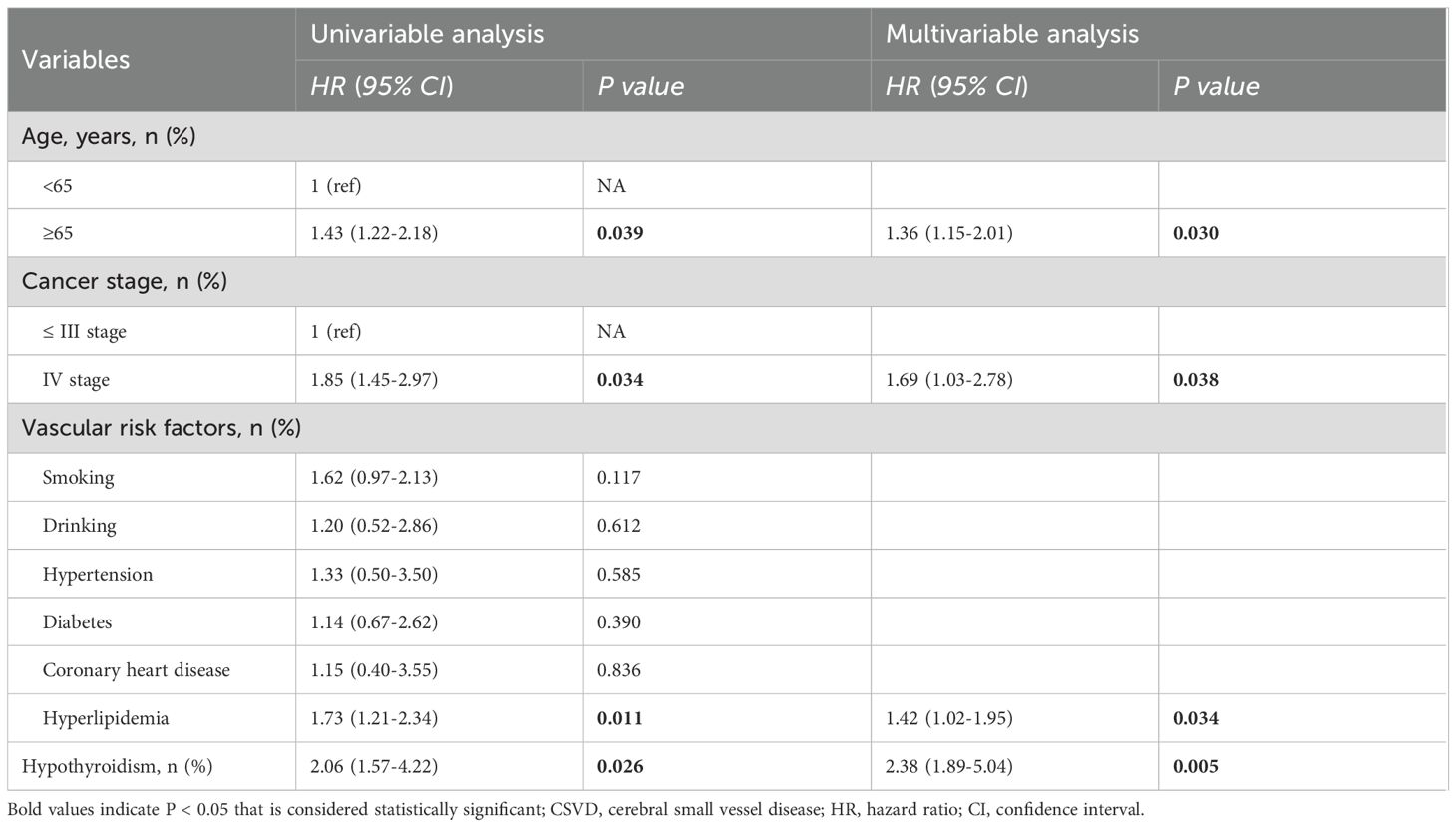
To clarify the correlation between thyroid function and CSVD, hypothyroidism was further incorporated as a time-dependent covariate for Cox regression analyses. The results demonstrated that age ≥65 years (HR=1.31; 95% confidence interval CI: 1.05-1.81, P=0.043), stage IV cancer (HR=1.82; 95% CI: 1.42-2.96, P=0.015), hyperlipidemia (HR=1.65; 95% CI: 1.20-2.25, P=0.012), and hypothyroidism (HR, 2.38; 95% CI: 1.89-5.04, P=0.005) all exhibited significant independent correlations with CSVD (Table 4).
3.5 Association between ICIs-related hypothyroidism and CSVD
Observations suggest that thyroid dysfunction may contribute to the occurrence of CSVD. Consequently, we compiled the timeline of hypothyroidism in all patients and analyzed the ROC curves to evaluate the predictive performance of FT4 and TSH levels as individual indicators. The optimal cutoff value for FT4 to distinguish the occurrence of CSVD was determined to be 11.84 pmol/L [AUC= 0.775 (95% CI 0.749-0.891), sensitivity = 70.4%, specificity = 80.5%, P = 0.028, Figure 4A]. The optimal cutoff value for TSH was determined to be 4.23 pmol/L [AUC = 0.548 (95% CI 0.672-0.805), sensitivity = 45.2%, specificity = 60.8%, P = 0.247] (Supplementary Figure S1).
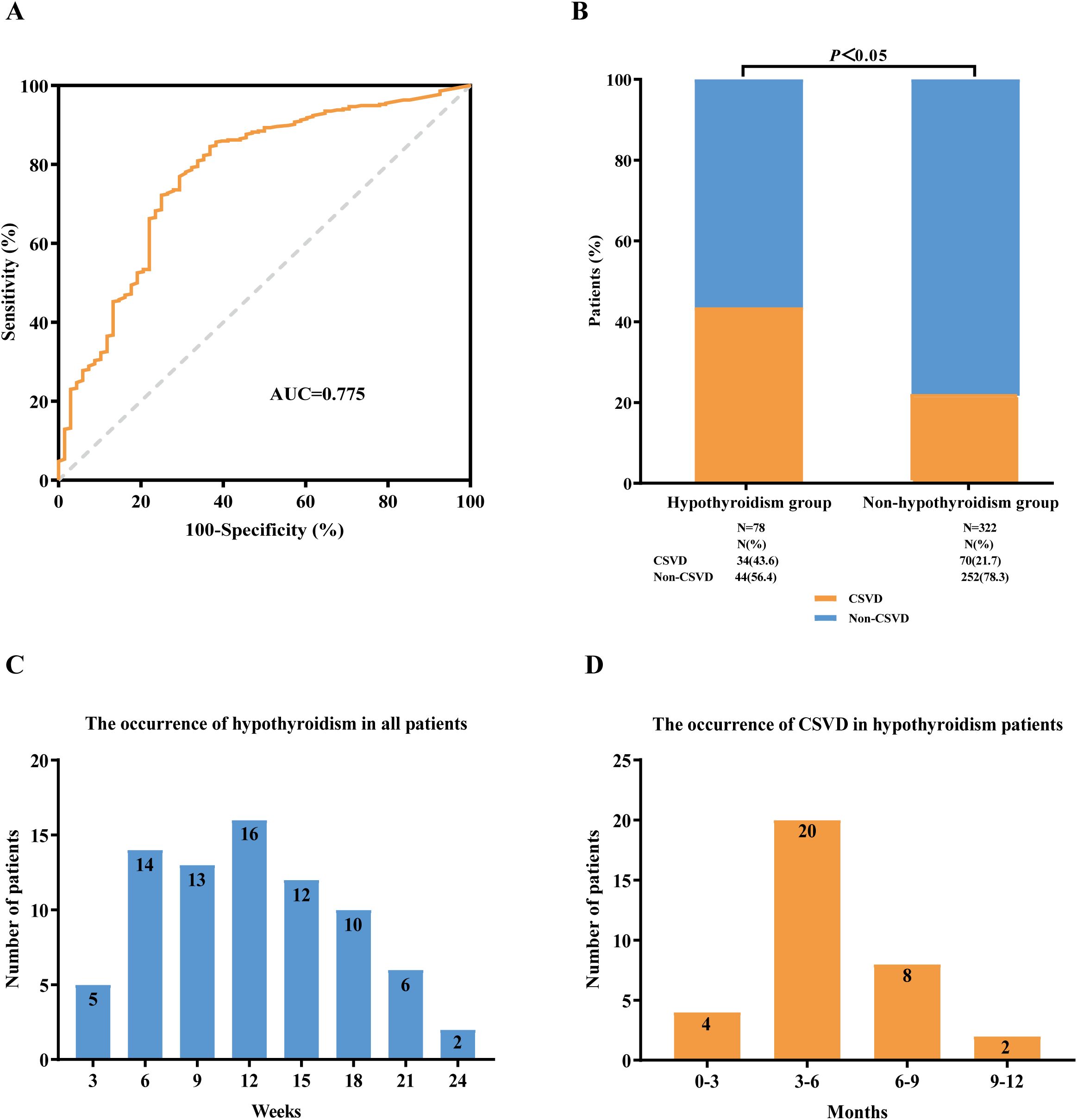
Figure 4. Association between immune checkpoint inhibitor-related hypothyroidism and CSVD. (A) ROC curve analysis of FT4. (B) The incidence of CSVD in lung cancer patients with hypothyroidism and non-hypothyroidism, respectively. (C) The occurrence of hypothyroidism in all lung cancer patients treated with ICIs therapy. (D) The occurrence of CSVD in ICIs-related hypothyroidism patients. ROC curve, receiver operating characteristic curve; AUC, area under the curve; FT4, free thyroxine; ICIs, immune checkpoint inhibitors; CSVD, cerebral small vessel disease.
During the period of ICIs treatment, we found 78 patients (19.5%) with confirmed hypothyroidism, and among these, 34 patients (43.6%) were confirmed to have CSVD. We observed that the incidence of CSVD was higher in the hypothyroidism group compared with the non-hypothyroidism group (43.6% versus 21.7%, P<0.05) (Figure 4B). The onset of hypothyroidism in all patients occurred from 3 to 24 weeks, with a peak at 12 weeks (Figure 4C). The incidence of CSVD in patients with hypothyroidism ranged from 0 to 12 months, peaking at 3 to 6 months (Figure 4D).
3.6 Association between CSVD and clinical outcomes
Among the 400 lung cancer patients, we compared the PFS and OS between the CSVD group and non-CSVD group. The Kaplan-Meier curve analysis revealed no significant difference in median PFS between the CSVD group and the non-CSVD group (14.52 months vs. 13.12 months, P = 0.882, Figure 5A), and similarly, no significant difference in median OS was observed (27.74 months vs. 23.69 months, P = 0.068, Figure 5B).
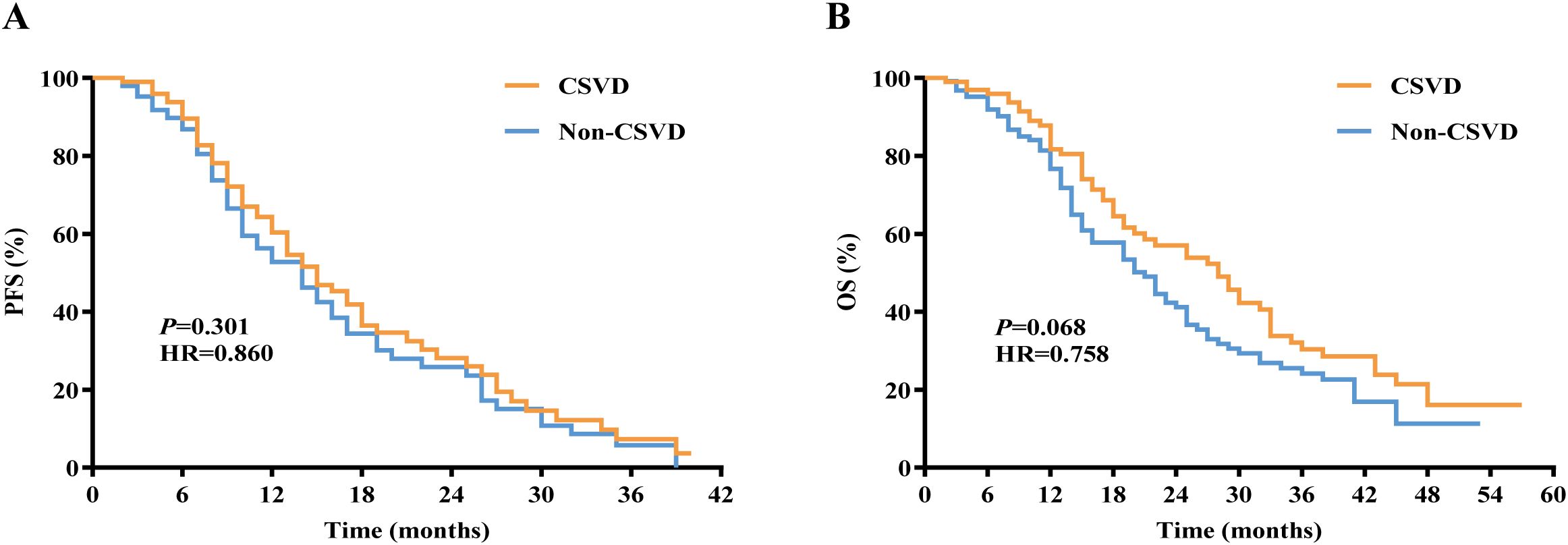
Figure 5. Kaplan-Meier curves for (A) PFS and (B) OS in lung cancer patients with or without CSVD. PFS, progression-free survival; OS, overall survival; CSVD, cerebral small vessel disease.
4 Discussion
Neurological adverse reactions to ICIs (n-irAEs) have garnered attention from neurologists and oncologists due to their severe consequences, prompting the need for early diagnosis and management (24). CSVD is a syndrome caused by various pathological changes in intracranial small blood vessels, commonly affecting the elderly. Its prevalence increasing with age (25), and often leading to cognitive dysfunction. The relationship between CSVD and ICIs is unclear. This retrospective study demonstrates, through the analysis of a large-scale sample, that lung cancer patients receiving immunotherapy exhibit a higher incidence of CSVD. Consistent with previous research findings, this study also confirms that age and hyperlipidemia are independent risk factors for CSVD. However, compared to the 20% incidence rate observed in elderly community populations, lung cancer patients undergoing immunotherapy exhibited a significantly higher incidence rate of 25% within a short-term period (12 months), despite having a younger average age (26). Poor staging may be related to cerebral small vessel damage caused by the late stage of the disease. Cystatin C is related to the severity and mortality of lung cancer, and some studies have proposed that it is also related to the prevalence of subclinical cerebral infarction (27–29). In this study, we similarly found that elevated cystatin C levels can increase the incidence of CSVD. Cystatin C is a cysteine protease inhibitor that has long been regarded as an ideal biomarker for evaluating renal function due to its nearly complete clearance by the kidneys. However, recent studies have shown that cystatin C plays a unique role in disease states such as atherosclerosis and cancer (30, 31). The specific mechanism by which cystatin C leads to CSVD remains unclear. Its potential mechanisms may involve vascular damage caused by the disruption of the balance between cystatin C and related cysteine proteases, as well as the participation of cystatin C as an inflammatory inducer in the inflammatory response process (32, 33).
Another novel finding in the present study is the level of FT4 and TSH is related to CSVD. Further data investigation and analysis revealed that a decline in thyroid function increases the incidence of CSVD. Thyroid dysfunction stands out as one of the most common endocrinopathies induced by ICIs therapy. Destructive thyroiditis is the pathophysiological basis shared by the most common patterns of thyrotoxicosis which was caused by T cell activation, alongside the involvement of various antibodies and cytokines (34–36). Thyroid hormones play a crucial role in the development of the brain and in maintaining brain function (37). Previous studies have suggested that thyroid dysfunction may accelerate the progression of CSVD (38). Hypothyroidism can lead to decreased cardiac output, which in turn causes insufficient microcirculatory perfusion in the brain, thereby affecting normal brain function (39). Other mechanisms include endothelial dysfunction and oxidative stress damage (40).The widespread application of ICIs in lung cancer patients may lead to an increased incidence of CSVD due to immune-related hypothyroidism. Although CSVD does not affect the PFS and OS of lung cancer patients, it may more significantly impact the patients’ quality of life. Maintaining stable thyroid function in patients is more conducive to treatment and reduces the incidence of CSVD. This study also provides the cut-off points for FT4 and TSH.
The findings of this study suggest that ICIs influence the function of cerebral small vessels through hypothyroidism. However, ICIs may also contribute to CSVD through more direct factors. In this study, it was discovered that the novel inflammatory markers SII and PLR, rather than NLR, were elevated in lung cancer patients with CSVD. The rise in these inflammatory indices indicates that immune dysfunction resulting from the use of ICIs may be linked to the development of CSVD. Various meta-analyses have suggested that elevated PLR and SII could be correlated with poorer PFS and OS among cancer patients undergoing ICIs treatment (41, 42). A large number of studies have shown that elevated SII levels are closely associated with severe CSVD burden and cognitive dysfunction (43), and higher SII levels are significantly correlated with WMH volume (44). SII reflects the systemic immune inflammatory state. In CSVD, systemic inflammation has a potential role in promoting the evolution and progression of WMH and microstructural damage (45). Endothelial dysfunction, microglial activation, atherosclerosis and blood-brain barrier injury are all key mechanisms of systemic inflammation-induced CSVD (46).
In summary, the research results indicate that when applying ICIs to treat lung cancer patients, it is necessary to pay special attention to the risk assessment of CSVD, which has important guiding significance for clinicians in formulating treatment plans and monitoring patient status. With the increasing dependence of lung cancer patients on immunotherapy, understanding the neurological complications that these patients may face after treatment can provide a basis for optimizing treatment plans, thereby improving the quality of life and survival rate of patients. This study identified risk factors for CSVD development after immunotherapy. Patients aged≥65 with pre-existing hyperlipidemia and stage IV demonstrated higher risks of CSVD when receiving immunotherapy. Hypothyroidism during immunotherapy was identified as an independent risk factor for CSVD. By monitoring and managing immune-related thyroid dysfunction, we can effectively reduce the incidence of CSVD, thereby improving patients’ quality of life.
This was a retrospective and single-center study with its own limitations. In this study, a high incidence of CSVD was observed among lung cancer patients undergoing treatment with ICIs, but we could not confirm that immunotherapy was an independent risk factor for CSVD. Additionally, female patients and large sample size were necessary for future study. This study only preliminarily explored the correlation between CSVD and immunotherapy for malignant tumors, and more data will be needed in the future to determine its deeper mechanisms and to advantage in clinic.
This study has determined the incidence of CSVD and its related risk factors in lung cancer patients receiving ICIs treatment. The study found that age, stage IV, hyperlipidemia and hypothyroidism were significantly and independently related to the risk of CSVD, while also revealing a correlation between PLR、SII and elevated cystatin C levels with CSVD. Although this study has certain limitations, it has revealed CSVD is a possibly related adverse event of immunotherapy, which has a certain guiding significance for future clinical diagnosis, treatment, and research.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Hospital of China Medical University (Project number: 2023-544-2). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
NW: Software, Writing – original draft, Formal analysis, Conceptualization, Data curation, Investigation. DZ: Methodology, Data curation, Investigation, Writing – original draft, Validation, Formal analysis. XG: Conceptualization, Writing – original draft, Data curation, Investigation. JL: Writing – original draft, Software, Data curation, Conceptualization, Formal analysis. JFL: Writing – original draft, Conceptualization, Investigation, Formal analysis, Data curation. FL: Investigation, Conceptualization, Project administration, Writing – original draft. XW: Funding acquisition, Writing – original draft, Resources, Supervision, Project administration, Conceptualization, Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the Project of Science and Technology Department of Liaonin, China (2024-MS-060).
Acknowledgments
The authors express their gratitude to all study participants for their significant contributions. They also extend thanks to the contributions of colleagues from the Department of Vascular Neurology at the First Hospital of China Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2025.1645549/full#supplementary-material
Supplementary Figure 1 | ROC curve analysis of TSH.
References
1. Li C, Lei S, Ding L, Xu Y, Wu X, Wang H, et al. Global burden and trends of lung cancer incidence and mortality. Chin Med J (Engl). (2023) 136:1583–90. doi: 10.1097/CM9.0000000000002529
2. Qi J, Li M, Wang L, Hu Y, Liu W, Long Z, et al. National and subnational trends in cancer burden in China, 2005–20: an analysis of national mortality surveillance data. Lancet Public Health. (2023) 8:e943–55. doi: 10.1016/S2468-2667(23)00211-6
3. Bonanno L, Calvetti L, Dal Maso A, Pavan A, Bao LC, De Nuzzo M, et al. Real-world impact of the introduction of chemo-immunotherapy in extended small cell lung cancer: a multicentric analysis. Front Immunol. (2024) 15:1353889. doi: 10.3389/fimmu.2024.1353889
4. Pérol M, Felip E, Dafni U, Polito L, Pal N, Tsourti Z, et al. Effectiveness of PD-(L)1 inhibitors alone or in combination with platinum-doublet chemotherapy in first-line (1L) non-squamous non-small-cell lung cancer (Nsq-NSCLC) with PD-L1-high expression using real-world data. Ann Oncol. (2022) 33:511–21. doi: 10.1016/j.annonc.2022.02.008
5. Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non–small-cell lung cancer: A phase 3 randomized clinical trial. JAMA Oncol. (2021) 7:709. doi: 10.1001/jamaoncol.2021.0366
6. Wang Z, Wu L, Li B, Cheng Y, Li X, Wang X, et al. Toripalimab plus chemotherapy for patients with treatment-naive advanced non–small-cell lung cancer: A multicenter randomized phase III trial (CHOICE-01). JCO. (2023) 41:651–63. doi: 10.1200/JCO.22.00727
7. Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang S, et al. Efficacy and Safety of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC: a Randomized, Double-Blind, Phase 3 Study (Oncology pRogram by InnovENT anti-PD-1-11). J Thorac Oncol. (2020) 15:1636–46. doi: 10.1016/j.jtho.2020.07.014
8. Guidon AC, Burton LB, Chwalisz BK, Hillis J, Schaller TH, Amato AA, et al. Consensus disease definitions for neurologic immune-related adverse events of immune checkpoint inhibitors. J Immunother Cancer. (2021) 9:e002890. doi: 10.1136/jitc-2021-002890
9. Farina A, Villagrán-García M, Vogrig A, and Joubert B. Central nervous system adverse events of immune checkpoint inhibitors. Curr Opin Neurol. (2024) 37:345. doi: 10.1097/WCO.0000000000001259
10. Drobni ZD, Alvi RM, Taron J, Zafar A, Murphy SP, Rambarat PK, et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. (2020) 142:2299–311. doi: 10.1161/CIRCULATIONAHA.120.049981
11. Inoue T, Kumai T, Ohara K, and Takahara M. Cerebral infarction as a rare adverse event of immune checkpoint inhibitors in patients with head and neck squamous cell carcinoma: A case series. Cureus. (2023) 15:e47406. doi: 10.7759/cureus.47406
12. Zhu J, Chen Y, Zhang Y, Wang W, Wang Y, Lu Z, et al. Association of immune checkpoint inhibitors therapy with arterial thromboembolic events in cancer patients: A retrospective cohort study. Cancer Med. (2023) 12:18531–41. doi: 10.1002/cam4.6455
13. Wardlaw JM, Smith C, and Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. (2019) 18:684–96. doi: 10.1016/S1474-4422(19)30079-1
14. Ter Telgte A, Van Leijsen EMC, Wiegertjes K, Klijn CJM, Tuladhar AM, and De Leeuw FE. Cerebral small vessel disease: from a focal to a global perspective. Nat Rev Neurol. (2018) 14:387–98. doi: 10.1038/s41582-018-0014-y
15. Liu Y, Dong YH, Lyu PY, Chen WH, and Li R. Hypertension-induced cerebral small vessel disease leading to cognitive impairment. Chin Med J. (2018) 131:615–9. doi: 10.4103/0366-6999.226069
16. Hodgson KD, Hutchinson AD, Wilson CJ, and Nettelbeck T. A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev. (2013) 39:297–304. doi: 10.1016/j.ctrv.2012.11.001
17. Ma Y, Liu N, Wang Y, Zhang A, Zhu Z, Zhang Z, et al. Cognitive adverse events in patients with lung cancer treated with checkpoint inhibitor monotherapy: a propensity score-matched analysis. eClinicalMedicine. (2023) 59:101987. doi: 10.1016/j.eclinm.2023.101987
18. Wardlaw J and Smith C. Mechanisms underlying sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. (2013) 12:483–97. doi: 10.1016/S1474-4422(13)70060-7
19. Li D, Shi J, Liang D, Ren M, and He Y. Lung cancer risk and exposure to air pollution: a multicenter North China case–control study involving 14604 subjects. BMC Pulm Med. (2023) 23:182. doi: 10.1186/s12890-023-02480-x
20. Poels MMF, Vernooij MW, Ikram MA, Hofman A, Krestin GP, van der Lugt A, et al. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke. (2010) 41:S103–106. doi: 10.1161/STROKEAHA.110.595181
21. Simoni M, Li L, Paul NLM, Gruter BE, Schulz UG, Küker W, et al. Age- and sex-specific rates of leukoaraiosis in TIA and stroke patients: Population-based study. Neurology. (2012) 79:1215–22. doi: 10.1212/WNL.0b013e31826b951e
22. Diener HC and Hankey GJ. Primary and secondary prevention of ischemic stroke and cerebral hemorrhage. J Am Coll Cardiol. (2020) 75:1804–18. doi: 10.1016/j.jacc.2019.12.072
23. Kraft P, Schuhmann MK, Garz C, Jandke S, Urlaub D, Mencl S, et al. Hypercholesterolemia induced cerebral small vessel disease. PloS One. (2017) 12:e0182822. doi: 10.1371/journal.pone.0182822
24. Vogrig A, Muñiz-Castrillo S, Farina A, Honnorat J, and Joubert B. How to diagnose and manage neurological toxicities of immune checkpoint inhibitors: an update. J Neurol. (2022) 269:1701–14. doi: 10.1007/s00415-021-10870-6
25. Prins ND and Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Rev Neurol. (2015) 11:157–65. doi: 10.1038/nrneurol.2015.10
26. Lam BYK, Cai Y, Akinyemi R, Biessels GJ, van den Brink H, Chen C, et al. The global burden of cerebral small vessel disease in low- and middle-income countries: A systematic review and meta-analysis. Int J Stroke. (2023) 18:15–27. doi: 10.1177/17474930221137019
27. Ashton E, Arrondeau J, Jouinot A, Boudou-Rouquette P, Hirsch L, Huillard O, et al. Impact of sarcopenia indexes on survival and severe immune acute toxicity in metastatic non-small cell lung cancer patients treated with PD-1 immune checkpoint inhibitors. Clin Nutr. (2023) 42:944–53. doi: 10.1016/j.clnu.2023.03.023
28. Ji H, Liu B, Jin P, Li Y, Cui L, Jin S, et al. Creatinine-to-cystatin C ratio and body composition predict response to PD-1 inhibitors-based combination treatment in metastatic gastric cancer. Front Immunol. (2024) 15:1364728. doi: 10.3389/fimmu.2024.1364728
29. Seliger SL, Longstreth WT, Katz R, Manolio T, Fried LF, Shlipak M, et al. Cystatin C and subclinical brain infarction. J Am Soc Nephrol. (2005) 16:3721–7. doi: 10.1681/ASN.2005010006
30. Mirzai S, Bancks MP, Brinkley TE, Carbone S, Tang WHW, Allison MA, et al. Association of creatinine-to-cystatin C ratio with computed tomography measures of skeletal muscle quantity and quality: The multi-ethnic study of atherosclerosis. Clin Nutr. (2025) 45:61–5. doi: 10.1016/j.clnu.2024.12.026
31. Leto G, Crescimanno M, and Flandina C. On the role of cystatin C in cancer progression. Life Sci. (2018) 202:152–60. doi: 10.1016/j.lfs.2018.04.013
32. Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, et al. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. (2004) 65:1416–21. doi: 10.1111/j.1523-1755.2004.00517.x
33. Bengtsson E, To F, Grubb A, Håkansson K, Wittgren L, Nilsson J, et al. Absence of the protease inhibitor cystatin C in inflammatory cells results in larger plaque area in plaque regression of apoE-deficient mice. Atherosclerosis. (2005) 180:45–53. doi: 10.1016/j.atherosclerosis.2004.12.025
34. Kotwal A, Gustafson MP, Bornschlegl S, KottsChade L, Delivanis DA, Dietz AB, et al. Immune checkpoint inhibitor-induced thyroiditis is associated with increased intrathyroidal T lymphocyte subpopulations. Thyroid. (2020) 30:1440–50. doi: 10.1089/thy.2020.0075
35. Koyama J, Horiike A, Yoshizawa T, Dotsu Y, Ariyasu R, Saiki M, et al. Correlation between thyroid transcription factor-1 expression, immune-related thyroid dysfunction, and efficacy of anti-programmed cell death protein-1 treatment in non-small cell lung cancer. J Thorac Dis. (2019) 11:1919–28. doi: 10.21037/jtd.2019.04.102
36. Okura N, Asano M, Uchino J, Morimoto Y, Iwasaku M, Kaneko Y, et al. Endocrinopathies associated with immune checkpoint inhibitor cancer treatment: A review. JCM. (2020) 9:2033. doi: 10.3390/jcm9072033
37. Sawicka-Gutaj N, Zawalna N, Gut P, and Ruchała M. Relationship between thyroid hormones and central nervous system metabolism in physiological and pathological conditions. Pharmacol Rep. (2022) 74:847–58. doi: 10.1007/s43440-022-00377-w
38. Tian Y, Yao D, Jin A, Wang M, Pan Y, Wang Y, et al. Thyroid function in causal relation to MRI markers of cerebral small vessel disease: A Mendelian randomization analysis. J Clin Endocrinol Metab. (2023) 108:2290–8. doi: 10.1210/clinem/dgad114
39. Fani L, Roa Dueñas O, Bos D, Vernooij MW, Klaver CCW, Ikram MK, et al. Thyroid status and brain circulation: the Rotterdam study. J Clin Endocrinol Metab. (2022) 107:e1293–302. doi: 10.1210/clinem/dgab744
40. Lu M, Yang CB, Gao L, and Zhao JJ. Mechanism of subclinical hypothyroidism accelerating endothelial dysfunction (Review). Exp Ther Med. (2015) 9:3–10. doi: 10.3892/etm.2014.2037
41. Xu H, He A, Liu A, Tong W, and Cao D. Evaluation of the prognostic role of platelet-lymphocyte ratio in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Int Immunopharmacol. (2019) 77:105957. doi: 10.1016/j.intimp.2019.105957
42. Zhang Y, Chen Y, Guo C, Li S, and Huang C. Systemic immune-inflammation index as a predictor of survival in non-small cell lung cancer patients undergoing immune checkpoint inhibition: A systematic review and meta-analysis. Crit Rev Oncol Hematol. (2025) 210:104669. doi: 10.1016/j.critrevonc.2025.104669
43. Xiao Y, Teng Z, Xu J, Qi Q, Guan T, Jiang X, et al. Systemic immune-inflammation index is associated with cerebral small vessel disease burden and cognitive impairment. Neuropsychiatr Dis Treat. (2023) 19:403–13. doi: 10.2147/NDT.S401098
44. Nam KW, Kwon HM, Jeong HY, Park JH, and Kwon H. Systemic immune-inflammation index is associated with white matter hyperintensity volume. Sci Rep. (2022) 12:7379. doi: 10.1038/s41598-022-11575-0
45. Walker KA, Power MC, Hoogeveen RC, Folsom AR, Ballantyne CM, Knopman DS, et al. Midlife systemic inflammation, late-life white matter integrity, and cerebral small vessel disease: the atherosclerosis risk in communities study. Stroke. (2017) 48:3196–202. doi: 10.1161/STROKEAHA.117.018675
Keywords: lung cancer, immune checkpoint inhibitors, cancer immunotherapy, neurological adverse event, cerebral small vessel disease
Citation: Wu N, Zhou D, Guo X, Liu J, Liu J, Liu F and Wang X (2025) Cerebral small vessel disease as a possibly immune-related adverse event of immunotherapy in lung cancer patients: a retrospective study. Front. Immunol. 16:1645549. doi: 10.3389/fimmu.2025.1645549
Received: 12 June 2025; Accepted: 07 August 2025;
Published: 26 August 2025.
Edited by:
Sergio Muñiz-Castrillo, Hospital Universitario 12 de Octubre, SpainReviewed by:
Romulo G A Galvani, National Laboratory for Scientific Computing (LNCC), BrazilJinghua Sun, Second Affiliated Hospital of Dalian Medical University, China
Zhitu Zhu, First Affiliated Hospital of Jinzhou Medical University, China
Copyright © 2025 Wu, Zhou, Guo, Liu, Liu, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaonan Wang, eGlhb19uYW45OUBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Na Wu1†
Na Wu1† Xiaoyu Guo
Xiaoyu Guo Xiaonan Wang
Xiaonan Wang