- 1School of Clinical Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 2Department of Infectious Diseases, Zigong First People’s Hospital, Zigong, Sichuan, China
- 3Department of Infectious Diseases, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
People living with HIV (PLWH) are at increased risk for depression, anxiety, and other comorbid psychiatric disorders. HIV-associated depression involves complex neurobiological disturbances, including chronic neuroinflammation. This includes microglial activation, elevated levels of pro-inflammatory cytokines and mediators, and altered brain metabolites. Additionally, there is dysregulation of monoaminergic neurotransmission, particularly impaired serotonergic signaling. Prolonged hyperactivation of the hypothalamic-pituitary-adrenal axis, indicated by abnormally high cortisol levels, is also observed. Together, these pathological processes contribute to persistent brain inflammation and metabolic imbalance. Under prolonged inflammatory conditions, activated microglia release factors such as tumor necrosis factor-alpha. These factors can induce oligodendrocyte apoptosis and demyelination, exacerbating neural injury. Psychosocial stressors—such as stigma, death-related anxiety, and internalized shame—may amplify these pathways through immune-neural crosstalk. Our primary focus, however, is on pharmacological targeting. We propose a three-tiered intervention framework: 1) Targeted neuropharmacological interventions (e.g., SSRIs and anti-inflammatory agents); 2) Optimized ART regimens; 3) Integrated psychosocial support. While further research is needed to establish long-term efficacy and personalized treatment options, this multidimensional approach may reduce the progression of HIV-associated depression and improve clinical outcomes.
1 Introduction
HIV/AIDS remains a leading global infectious disease, posing a major public health challenge. According to global HIV/AIDS statistics (1), as of 2023, approximately 39.9 million people worldwide are living with HIV, with approximately 30.7 million receiving antiretroviral therapy (ART) (1). However, funding for AIDS prevention and treatment in low- and middle-income countries reached only $19.8 billion (in 2019 constant dollars) by the end of 2023, reflecting a 7.9% decrease from 2022. Projections indicate that by 2025, these countries will require $29.3 billion (in 2019 constant dollars) for continued AIDS prevention and treatment efforts (1). This funding shortfall represents a critical barrier to eliminating AIDS as a public health threat, while also imposing a substantial economic burden on global healthcare systems.
The widespread use and improved adherence to combination antiretroviral therapy (cART) has significantly increased life expectancy among people living with HIV (PLWH) in both high- and low-income countries, bringing it closer to that of uninfected individuals (2–4). While cART has dramatically reduced HIV transmission, there is currently no cure or vaccine, and HIV infection remains incurable. Consequently, PLWH often experience significant psychological distress, which can stem from various factors, including fear of disease progression—particularly in resource-limited settings—HIV-related stigma, and concerns about long-term health outcomes (5). Comorbid mental health disorders, including depression, anxiety, and other severe psychiatric conditions, are prevalent among PLWH, with depression and anxiety being particularly common (6, 7). Depression has long been recognized as the most prevalent neuropsychiatric disorder associated with HIV infection (8). The global prevalence of depression among people living with HIV (PLWH) is estimated to be 31% (9), significantly higher than the 3.8% observed in the general population (10). This suggests that the risk of depression is approximately eight times greater in PLWH compared to HIV-negative individuals. In the United States, the depression prevalence among PLWH is nearly 30% (11), mirroring the global rate, and anxiety disorders affect 19% of this population (12). By 2030, HIV and depression are projected to become the two leading causes of global disease burden, with these conditions frequently co-occurring (13).
In the PLWH population, depression risk is influenced by gender to a significant extent. Research indicates that women with HIV are at a higher risk of developing moderate to severe depression, while men are more likely to experience moderate depression (14). Data from the Women’s Interagency HIV Study (WIHS), a multicenter cohort of 1,027 HIV-infected women in the United States, reveals a 22.1% prevalence of mood disorders and a lifetime prevalence of major depressive disorder (MDD) at 32.4%, higher than the 22.9% prevalence in the general female population (15). A meta-analysis in China found that the depression prevalence among HIV-positive men ranges from 37.9% to 71.8% (16), with 18.1% of men potentially experiencing severe depression. The severity of depression is notably higher in gay HIV-positive men compared to their heterosexual counterparts, suggesting sexual orientation plays a pivotal role in depression manifestation. Additionally, older male PLWH tend to exhibit more severe depression than younger individuals, highlighting age as a key factor in depression onset. Geographical and economic factors also influence depression prevalence, with higher rates observed in developing and underdeveloped countries. Notably, South America shows the highest prevalence at approximately 44%, while Europe has the lowest, at 22% (9). This disparity is likely linked to the uneven distribution of healthcare resources and research funding, with developed countries offering more medical support and financial resources, exacerbating the depression burden in lower-resource regions (17). In managing long-term treatment and quality of life challenges, PLWH face a heightened risk of mental health issues, particularly anxiety and depression. These psychological conditions are often closely tied to perceived stigma, social withdrawal, insomnia, and guilt (18, 19). Anxiety and depression not only impair cART adherence, increasing the risk of HIV transmission (20), but also frequently involve distressing thoughts of death, suicidal ideation, and self-harm tendencies (2). Further research has demonstrated that PLWH with depression show poorer treatment adherence, directly impacting cART efficacy. One study found that depressed patients were 1.7 times more likely to interrupt their treatment compared to non-depressed individuals, complicating disease management (21).
PLWH are at greater susceptibility for psychiatric disorders, including depression and anxiety; however, the neurobiological mechanisms underlying HIV-associated depression remain poorly understood. This study seeks to elucidate these neurobiological mechanisms and examine the influence of non-clinical factors, offering new perspectives for intervention and treatment. The research is structured around three primary objectives: First, it explores the interaction between psychosocial factors—such as social support, economic stress, and substance abuse—and neurobiological processes, investigating how these factors contribute to the onset and exacerbation of depression. Second, it examines how HIV infection impacts the central nervous system (CNS) through mechanisms such as chronic low-grade neuroinflammation and immune dysregulation, facilitating the development of depression. Third, it evaluates the effectiveness and feasibility of integrated management strategies, combining pharmacological treatments with non-clinical interventions. This study aims to clarify the complex mechanisms of HIV-associated depression and provide a solid theoretical foundation and practical guidance for clinical interventions.
2 Multidimensional factors in HIV-associated depression analysis
The pathogenesis of HIV-associated depression remains incompletely understood; however, research suggests it arises from the interplay of multiple factors, including biological, psychological, and social-environmental influences (Figure 1). Various elements contribute to the elevated prevalence of depression among PLWH (22). A study conducted across 11 low- and middle-income countries, involving 2,821 PLWH aged 40 and above, found that unhealthy alcohol consumption and depressive symptoms were most prevalent, with 14%, 9%, and 6% of participants experiencing moderate to severe depression, anxiety, and post-traumatic stress disorder (PTSD), respectively. The comorbidity rate for mental disorders was 11% (23). Behavioral risk factors, including substance abuse, alcohol misuse, and limited physical activity, are also common within this population (15). Alcohol consumption and smoking are the most frequent forms of substance abuse. Systematic reviews and meta-analyses indicate that PLWH globally exhibit higher rates of substance abuse and mental health disorders compared to the general population (24). HIV-related clinical factors, such as poor adherence to ART, are associated with an increased risk of depressive episodes. Additionally, well-established risk factors such as being female, having a history of mood disorders, and a family history of mood disorders are linked to the onset of depression (25). Another study highlighted that PLWH with depression are more likely to engage in substance abuse and risky sexual behaviors, further elevating health risks (26). This finding is supported by another study, which revealed that moderate to high-risk sexual behaviors are more prevalent among PLWH aged ≤30 years, those who are unemployed, and those who have disclosed their HIV status, particularly in individuals with moderate to severe depression (27).
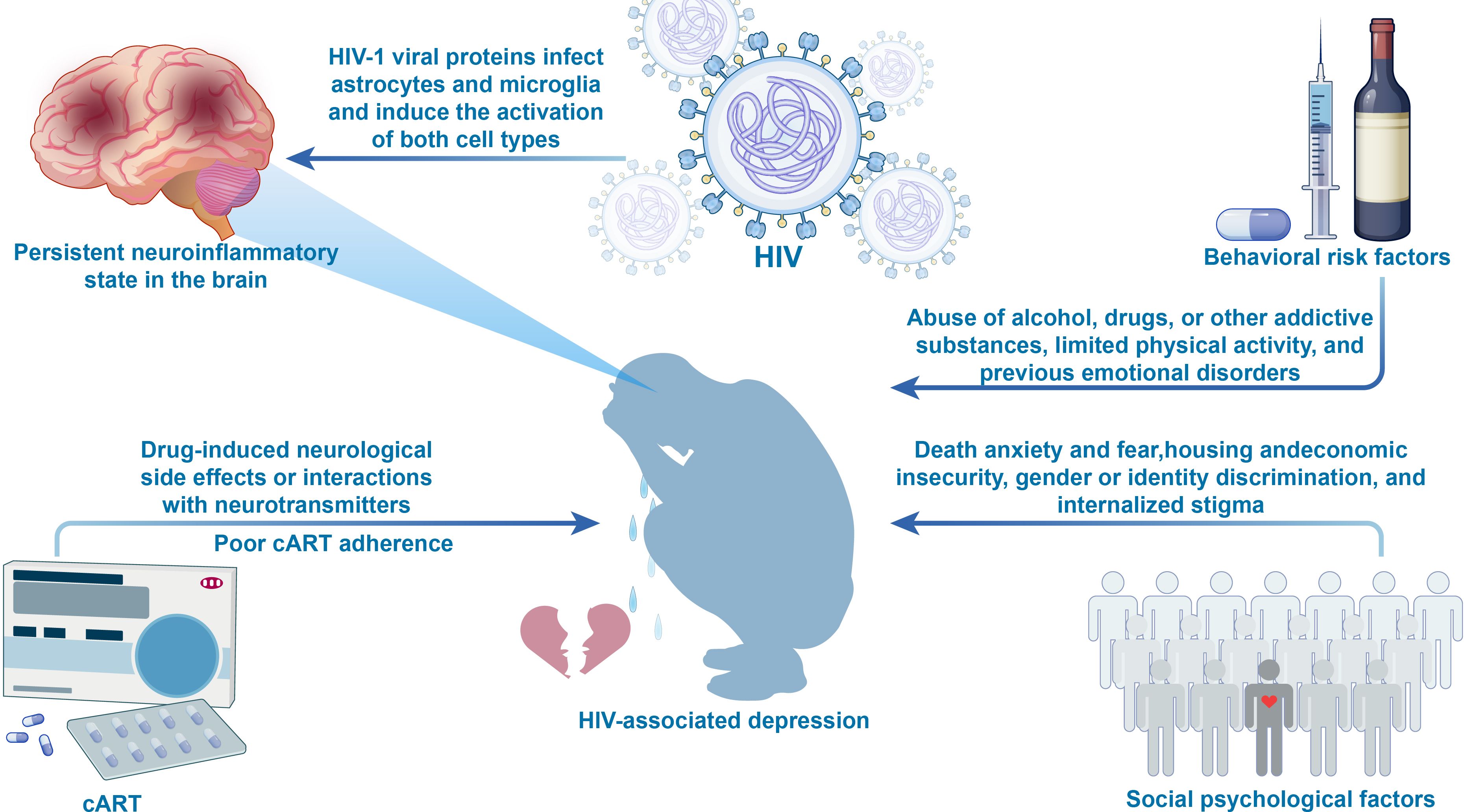
Figure 1. Figure 1 presents a multidimensional risk factor framework for HIV-associated depression. Biological factors include (1): HIV viral proteins, which activate astrocytes and microglia, leading to persistent brain inflammation (2); Poor adherence to cART, resulting in inadequate viral control and ongoing neuroinflammation (3); cART medications, which may affect neurotransmitter balance, increasing the risk of neuropsychiatric adverse events. Psychological factors include emotional stressors such as death anxiety, negative life events, and internalized stigma. Behavioral factors encompass alcohol and substance abuse, sedentary lifestyle, and a history of mood disorders. Social environmental factors include housing insecurity, economic hardship, limited access to healthcare, violence, and discrimination. The interactions between these factors collectively heighten the risk of depression, offering a comprehensive perspective on its pathogenesis. cART, Combination Antiretroviral Therapy.
HIV-associated depression is closely associated with socio-psychological risk factors and shows significant temporal and regional variations. In the pre-cART era, death anxiety and PTSD were the primary risk factors (28). However, after the widespread use of cART, structural stressors (such as housing insecurity and disparities in healthcare access) and the internalization of stigma have become increasingly significant (29, 30). This shift manifests differently across regions with varying resources: in low- and middle-income countries, external community discrimination, barriers to treatment accessibility, and internalized stigma are predominant (31), while in high-income countries, factors such as employment discrimination, intimate partner violence, and housing instability are more prominent (32). Notably, social isolation and the lack of a social support system remain important mechanisms across all periods and regions (33, 34). Additionally, PLWH frequently experience substantial stress due to factors such as stigma, discrimination, financial hardship, ART side effects, and unemployment, all of which can lead to depression, anxiety, and suicidal tendencies (35). Intimate partner violence, particularly prevalent among HIV-infected men who have sex with men, is associated with negative behavioral and mental health outcomes (36). Death anxiety and fear are critical factors impacting the quality of life in PLWH. As individuals become aware of the potentially fatal nature of their illness, they may consciously or unconsciously dwell on death, which can trigger anxiety and fear (37). To mitigate these emotions, some PLWH may resort to high-risk behaviors, such as substance or alcohol abuse, as a form of self-soothing. This coping strategy can introduce additional health complications. Furthermore, the presence of death anxiety may distort the interpretation of disease symptoms, increasing the risk of PTSD (38). Death anxiety may also prompt engagement in high-risk sexual behaviors, exacerbating HIV transmission risk (39). Additionally, intense death-related fear and anxiety can lead to social withdrawal, causing PLWH to avoid new social interactions. This not only impacts their social well-being but may create a detrimental cycle of isolation. The intricate relationship between death anxiety, anxiety disorders, and depression is noteworthy, as previous studies have identified high levels of depression as a key determinant of death anxiety (40). In summary, psychological factors such as death anxiety, negative life events, and PTSD—often exacerbated by depression—can drive PLWH to engage in high-risk sexual behaviors, further fueling the spread of HIV. Notably, some individuals living with infectious diseases, including PLWH, may experience improvements in mental health and a reduction in stigma through non-pharmacological interventions, such as psychotherapy and social support (41, 42). These findings underscore the significant role socio-psychological factors play in the onset and progression of HIV-associated depression.
The risk of depression in PLWH is associated not only with medication adherence but also with the neurotoxic effects of certain ART drugs. Studies have shown a negative correlation between depression and ART adherence in PLWH (43, 44). It is important to note that, in addition to the impact of adherence behaviors, the neurotoxic effects of ART medications themselves also represent a significant mechanism. Specifically, ART medications may influence neurotransmitter synthesis, metabolism, or receptor function, thereby indirectly affecting CNS function and elevating the risk of depression. Certain ART drugs, particularly non-nucleoside reverse transcriptase inhibitors (NNRTIs) like Efavirenz (EFV), have been shown to impact the CNS, potentially increasing the risk of depression and other psychiatric disorders by disrupting neurotransmitter equilibrium, interfering with metabolic pathways, and altering neurophysiological processes (45, 46). In EFV users, the incidence of neuropsychiatric adverse drug reactions (ADRs) approaches 50%, with common side effects including dizziness, sleep disturbances, vivid dreams, mood disorders, severe depression, and anxiety disorders (47). Research suggests EFV may induce neurotoxicity by impairing mitochondrial function and energy metabolism in the brain, processes linked to cognitive decline and depression (48). However, further clinical and experimental validation is required to confirm these effects. ART medications may also induce neurotoxicity through mechanisms such as dendritic spine damage, mitochondrial dysfunction, oxidative stress, endoplasmic reticulum stress, and alterations in neuronal growth and synaptogenesis in vitro. Collectively, these mechanisms contribute to an elevated risk of depression and other ADRs (49, 50). Compared to monotherapy, ART regimens may lead to a higher incidence of CNS-related adverse reactions and discontinuation. For example, the risk of discontinuation due to CNS adverse effects is 1.92 times higher when dolutegravir (DTG) is combined with abacavir (ABC) compared to DTG monotherapy (51).
HIV activates the NLRP3 inflammasome in microglial cells upon infection, leading to the activation of both microglial cells and astrocytes, which fosters chronic inflammation in the brain and heightens the risk of depression. HIV-1 viral proteins trigger the NLRP3 inflammasome in brain microglia via the TLR2-NF-κB signaling pathway, initiating microglial activation (52–54), intensifying the inflammatory response, and contributing to neuronal dysfunction or neurotoxicity (55, 56). Furthermore, research has linked NLRP3 inflammasome activation with HIV-associated neuropathological changes, suggesting that HIV infection can induce harmful neuroinflammatory responses in the CNS (57). It is important to note that the aforementioned HIV-induced neuroinflammatory mechanisms are primarily observed during the untreated viremia phase. However, in the context of cART treatment, residual inflammation may persist even after viral suppression is achieved. Clinically, an early study measuring plasma inflammatory cytokines in 23 PLWH revealed that, compared to non-depressed controls, patients with depression exhibited elevated levels of IL-15, IP-10, IL-12, and granulocyte colony-stimulating factor (G-CSF) (58). Subsequent research, including a multicenter cohort study involving 1,727 participants, demonstrated a strong interaction between chronic depressive symptoms and elevated inflammation levels (59–61). A large meta-analysis supports the bidirectional relationship between inflammation and depression (62). Notably, C-reactive protein (CRP), a peripheral inflammation marker, has been extensively studied for its association with both the incidence and severity of depression (63). Even after adjusting for clinical and psychosocial factors, CRP levels remain significantly correlated with depression (64). Further studies suggest that elevated CRP may be a consequence of depression, whereas elevated IL-6 levels are more likely to be a risk factor, implicating the IL-6/IL-6R signaling pathway in depression pathophysiology (65, 66). It should be emphasized that the detection of CRP and IL-6 in these studies is aimed at assessing the chronic neuroinflammatory burden in PLWH, and these nonspecific pro-inflammatory cytokines and other inflammatory markers may not be suitable to be considered as diagnostic indicators of depression.
Moreover, HIV infection has been shown to induce alterations in brain metabolites, which reflect ongoing CNS inflammation and suggest continuous neural damage (67, 68). Neurofilament light chain protein (NfL) has emerged as a novel biomarker for neuronal injury and neuroinflammation. Studies indicate that NfL concentrations are significantly elevated in the blood and cerebrospinal fluid (CSF) of PLWH, with levels correlating to the severity of depressive symptoms (69–71). As aging progresses, the inflammatory state in the brain intensifies, and microglial dysfunction and neuronal damage worsen (72). These findings suggest that HIV infection not only triggers neuroimmune activation but may also exacerbate age-related neural damage. In summary, the long-term effects of HIV infection on the nervous system may involve a complex interplay between chronic neuroinflammatory responses and age-related neuronal damage, ultimately impacting brain health.
3 Neuropathological changes in HIV-associated depression
3.1 Inflammatory and metabolic dysregulation in HIV-associated depression
Neuroinflammatory responses likely play a central role in the neuropathology of HIV-associated depression (Figure 2). The complex interplay between immune suppression and activation, elevated peripheral pro-inflammatory cytokines and other inflammatory markers, and cytokine involvement in neurophysiology suggests that inflammation is a key pathological mechanism underlying depression (73–76). Research has shown that low-grade inflammation and excessive cytokine secretion in peripheral blood are closely linked to HIV-associated depression (77). The “cytokine hypothesis of depression” proposes that inflammatory cytokines act as neuromodulators, influencing behavior, neuroendocrine function, and neurochemical characteristics associated with depression (78, 79). Clinical studies consistently report elevated levels of pro-inflammatory cytokines and other inflammatory markers, such as IL-1β, IL-6, CRP, and TNF-α, in individuals with depressive symptoms (80–82). Additionally, exogenous inflammation induction can lead to the development of core depressive features (83).

Figure 2. Figure 2 illustrates the complex interaction between inflammation and metabolic dysregulation in HIV-associated depression, emphasizing the central role of microglial activation in driving both inflammatory and metabolic disturbances. HIV viral proteins activate NLRP3 inflammasomes in microglial cells, which triggers the activation of both microglia and astrocytes. This activation promotes the release of pro-inflammatory factors (such as IL-1β, IL-6, CRP, and TNF-α) and inflammatory mediators, while also increasing the production of ROS and RNS. IL-1β and TNF-α modulate the expression of glutamate receptors and enhance Glu release, leading to excessive accumulation of Glu in the synaptic cleft. This, in turn, stimulates glial cells to secrete additional pro-inflammatory cytokines, amplifying the inflammatory signals within the CNS. Moreover, these inflammatory cytokines activate IDO via signaling pathways such as STAT1α, IRF-1, p38 MAPK, and NF-κB, resulting in a metabolic shift from TRP to KYN. This shift not only decreases 5-HT synthesis but also promotes the overproduction of QUIN. By activating NMDA receptors on the postsynaptic membrane, QUIN promotes glutamate release, resulting in the excessive accumulation of neurotransmitters and subsequent neurotoxicity. Concurrently, chronic inflammation and excessive glial cell activation release TNF-α, further exacerbating neuroinflammation by inducing oligodendrocyte apoptosis and demyelination, thereby damaging the nervous system. Additionally, alterations in brain metabolites, such as reduced NAA and elevated NfL, mI, and Cho, indicate ongoing neuroinflammation and potential axonal damage. Together, these mechanisms of inflammation and metabolic dysregulation sustain chronic inflammation within the CNS, increasing the susceptibility to depression in HIV-infected individuals. CNS, central nervous system; Glu, glutamate; ROS, reactive oxygen species; RNS, reactive nitrogen species; IDO, indoleamine 2,3-dioxygenase; TRP, tryptophan; KYN, kynurenine; QUIN, quinolinic acid; 5-HT, serotonin; NMDA, N-methyl-D-aspartate; mI, myo-inositol; NAA, N-acetylaspartate; Cho, choline; NfL, neurofilament light chain; NTFs, neurotrophic factors; CRP, C-reactive protein; TNFα, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-18, interleukin-18; gp120, human immunodeficiency virus envelope glycoprotein 120; Tat, trans-activator of transcription; Vpr, viral protein R.
In HIV-infected individuals, the brain is a primary target for the virus, predominantly affecting perivascular macrophages, microglia, and astrocytes (84, 85). For instance, HIV-1 envelope glycoprotein 120 (gp120) activates microglial cells via the TLR2-NF-κB signaling pathway, triggering NLRP3 inflammasome activation and pro-inflammatory cytokine secretion (53, 55). Other viral proteins, such as HIV-Tat and Vpr, further activate microglial cells through multiple signaling pathways, exacerbating inflammatory responses and neurotoxicity, which leads to neuronal dysfunction and severe CNS damage (52, 54, 56). The synergistic effects of these viral proteins highlight the complexity of HIV-induced CNS damage, involving intricate inflammatory and neurotoxic pathways.
In an inflammatory state, microglia and astrocytes further amplify the secretion of pro-inflammatory cytokines such as IL-1β, IL-6, IL-18, and TNF-α (86). IL-1β and TNF-α regulate the expression of glutamate (Glu) receptors and enhance Glu release, causing excessive accumulation of Glu in the synaptic cleft. This not only promotes further cytokine release from glial cells but also amplifies inflammatory signaling within the CNS. Additionally, these cytokines trigger the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), directly damaging neurons and inducing oligodendrocyte apoptosis (87, 88). Chronic inflammation and TNF-α release by hyperactivated glial cells exacerbate neuroimmune activation by promoting oligodendrocyte apoptosis and demyelination, thereby contributing to progressive CNS damage (89, 90).
Metabolic dysregulation plays a critical role in HIV-associated depression. Depression itself is closely linked to impaired serotonin (5-HT) function, and HIV infection may exacerbate this through several pathways, including inflammation and stress. One such pathway is the metabolic shift from tryptophan (TRP) to kynurenine (KYN), which has been strongly associated with depression development (91, 92). As a precursor to 5-HT, the diversion of TRP towards the KYN pathway reduces 5-HT synthesis while promoting the overproduction of the neurotoxic metabolite quinolinic acid (QUIN), further amplifying neuroinflammation and neurotoxicity (93, 94). Inflammatory cytokines activate indoleamine 2,3-dioxygenase (IDO) via signaling pathways such as STAT1α, IRF-1, p38 MAPK, and NF-κB, driving this metabolic shift and contributing to neurotransmitter dysfunction linked to depression (95–97).
Although cART enables most PLWH to achieve viral suppression, some patients still exhibit residual neuronal dysfunction and abnormalities in neuroinflammatory biomarkers. In untreated HIV-1-infected individuals, CSF levels of sTREM2 not only increase with disease progression (as indicated by CD4+ T cell depletion) but are also closely associated with NfL levels (98). While PLWH who achieve viral suppression show an overall decrease in sTREM2 levels, neurodamage biomarkers, such as NfL, can still be detected, suggesting the presence of irreversible damage or low-level persistent inflammation (69–71). These changes are not only closely linked to the severity of depression but may also exacerbate HIV-related cognitive and emotional impairments.
HIV infection and inflammatory states also disrupt the balance of brain metabolites. Elevated levels of neurometabolites, such as myo-inositol (mI), have been linked to both the incidence and severity of depression (99). While cART partially mitigates HIV-induced neuroinflammation and metabolite changes (67, 100), neuronal dysfunction (evidenced by reduced N-acetylaspartate [NAA]) and neuroinflammation (indicated by increased mI and choline [Cho]) persist in chronically infected individuals, often accompanied by axonal damage (68, 100).
In summary, HIV disrupts the balance between neuroprotection and neurotoxicity within the CNS through neurotransmitter dysfunction, metabolic dysregulation, and the sustained elevation of pro-inflammatory cytokines. The excessive activation of microglia and astrocytes, driven by inflammation, amplifies these processes, ultimately contributing to the onset of depression.
3.2 Microglial activation in the metabolic and inflammatory mechanisms of HIV-associated depression
Microglial activation linked to depression primarily occurs in the prefrontal cortex, insula, and anterior cingulate cortex, with neuronal and synaptic damage resulting from this activation serving as a key trigger for depressive-like behaviors. This activation is strongly associated with neuropsychiatric disorders, including depression and anxiety (101, 102). The process typically involves the secretion of pro-inflammatory cytokines, which initiate both peripheral and central inflammation. In HIV infection, glucose metabolism is significantly upregulated, as evidenced by increased glucose transport and metabolic flux (103, 104). This metabolic alteration is closely tied to heightened production of pro-inflammatory cytokines, such as CRP, TNF-α, and IL-6 (105, 106). These cytokines can cross the blood-brain barrier and affect key brain regions like the amygdala, hippocampus, hypothalamus, and cortex, influencing pathophysiological processes associated with depression, including neurotransmitter metabolism, neuroendocrine function, and neuroplasticity (107). These disruptions further impair mood and cognitive function, providing a critical pathological basis for depression.
3.3 Metabolic dysregulation and inflammation in ART-treated PLWH
Although the neurotoxicity of the latest generation of ART has been significantly reduced, treated individuals still exhibit metabolic abnormalities. These metabolic shifts may perpetuate inflammation and immune dysfunction, thereby increasing the risk of depression (108). Notably, while ART partially normalizes certain depression-related inflammatory cytokines in HIV-infected individuals, others, such as IL-6 and CRP, remain elevated (109, 110). Further research has highlighted significant changes in CNS metabolites in ART-treated PLWH through CSF metabolomics, including alterations in neurotransmitters (e.g., Glu and NAA), microglial activation markers (e.g., mI), and ketone bodies (e.g., β-hydroxybutyrate and 1,2-propanediol) (111). These alterations suggest ongoing inflammation, microglial activation, and glutamate-mediated neurotoxicity (111). Despite improvements in viral control and immune function, studies indicate that HIV-associated cognitive impairments and brain inflammatory changes persist in PLWH (67). These findings suggest that ART does not fully resolve CNS pathological changes induced by HIV infection, with underlying mechanisms likely related to persistent inflammation and metabolic dysregulation.
3.4 HPA axis dysregulation in HIV-associated depression
The hypothalamic-pituitary-adrenal (HPA) axis plays a critical role in regulating the stress response through the coordinated interaction of the hypothalamus, pituitary gland, and adrenal glands. Under stress, the hypothalamus releases corticotropin-releasing hormone (CRH), which stimulates the pituitary to secrete adrenocorticotropic hormone (ACTH), ultimately prompting the adrenal glands to release cortisol. Cortisol, in turn, modulates the secretion of CRH and ACTH through a negative feedback mechanism to maintain homeostasis. In PLWH, prolonged activation of the HPA axis can occur due to various stressors, such as emotional stress (e.g., death anxiety, stigma, and discrimination) and economic pressure. These factors exacerbate psychological burden and disrupt neuroendocrine function through abnormal cortisol levels (112). Research has shown that PLWH are particularly susceptible to HPA axis dysfunction and fatigue, heightening the risk of HIV-associated mood disorders (113, 114). For instance, the HIV-Tat protein, in response to stress, can induce neurodegeneration and impair HPA axis function, leading to excessive activation and elevated cortisol levels—key mechanisms driving HIV-related neuroendocrine dysfunction and mood symptoms (115, 116). However, studies on HPA axis alterations in PLWH remain scarce, warranting further investigation into the underlying mechanisms and their impacts.
4 Potential clinical interventions and challenges in HIV-associated depression
In the management of depression in the context of HIV infection, three primary interventions are typically employed (1): Antidepressant medications and adjunctive treatments with antidepressant effects (2); Non-pharmacological approaches, including exercise therapy and psychosocial interventions, as outlined in Figure 3; and (3) Optimization of ART to mitigate the risk of HIV-associated psychiatric disorders. Each intervention presents distinct advantages and limitations, necessitating a tailored approach based on the unique characteristics of the individual PLWH. The specific implementation and suitability of these strategies will be explored further in the subsequent sections.
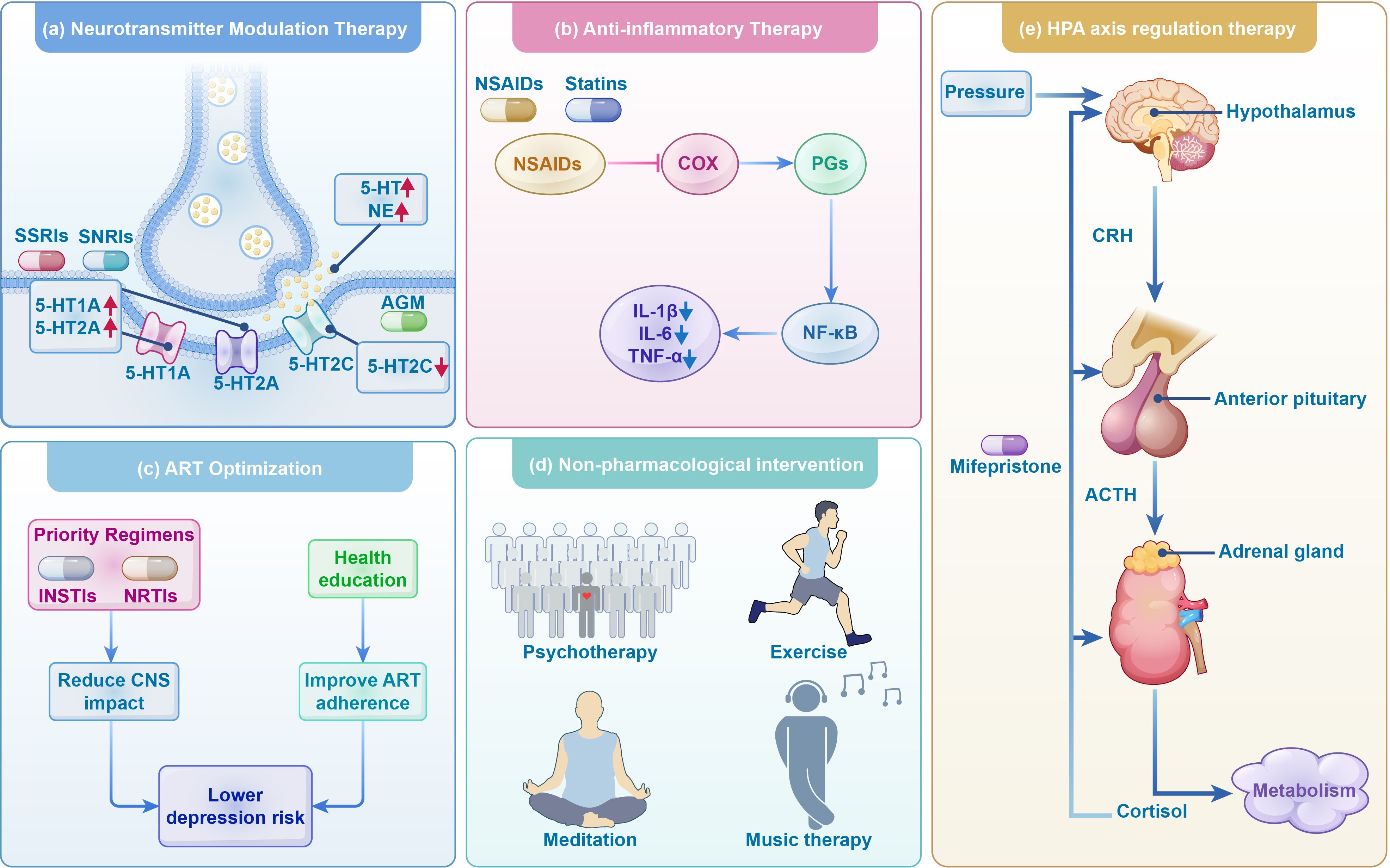
Figure 3. Treatment strategies for depression in HIV infection. This figure delineates treatment approaches for depression in individuals with HIV, encompassing antidepressant therapies such as neurotransmitter modulation, anti-inflammatory treatments, ART optimization, and HPA axis regulation, alongside the use of non-pharmacological interventions as adjunctive therapies. SSRIs, Selective serotonin reuptake inhibitors; SNRIs, Selective norepinephrine-serotonin reuptake inhibitors; AGM, agomelatine; 5-HT, serotonin; 5-HT1A, 5-Hydroxytryptamine 1A receptors; 5-HT2A, 5-Hydroxytryptamine 2A receptor; 5-HT2C, 5-hydroxytryptamine 2C receptors; NSAIDs, Non-steroidal anti-inflammatory drugs; COX, cyclooxygenase; PGs, prostaglandin; CRH,corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone; INSTIs, integrase strand transfer inhibitors; NRTIs, nucleoside reverse transcriptase inhibitors. ART, antiretroviral therapy.
4.1 Neurotransmitter modulation in depression therapy
Selective serotonin reuptake inhibitors (SSRIs) represent an important treatment strategy for managing HIV-associated depression by improving mood symptoms while also modulating neuroinflammation and neurodegeneration. SSRIs exert their therapeutic effects primarily by inhibiting the reuptake of 5-HT into presynaptic neurons, which results in increased 5-HT concentrations in the synaptic cleft. This boost in serotonin levels enhances binding to postsynaptic receptors such as 5-HT1A and 5-HT2A, thereby facilitating improved signal transmission (117, 118). With prolonged use, the enhanced 5-HT signaling helps regulate neural network function, leading to clinical improvements in mood disorders like depression and anxiety. In addition to their mood-regulating effects, SSRIs may also have anti-inflammatory and neuroprotective properties, making them a promising adjunctive therapy for depression in PLWH through their anti-inflammatory and neuroprotective effects (119). Studies have shown that SSRIs can reduce CNS inflammation and neuronal damage. For example, escitalopram has demonstrated the ability to repair HIV-1-mediated neuronal damage and improve neurological function (120). SSRIs may also downregulate the expression of CD4 receptors and chemokine receptors, reducing immune cell susceptibility to HIV infection, further supporting their potential as adjunctive therapies (121). However, despite the benefits, long-term SSRI use can lead to alterations in 5-HT receptor sensitivity, which may trigger side effects such as sexual dysfunction, mood fluctuations, weight changes, and even drug resistance (122). These potential adverse effects require careful monitoring and consideration in clinical practice.
Selective norepinephrine-serotonin reuptake inhibitors (SNRIs) enhance neurotransmitter concentrations in the synaptic cleft by inhibiting the reuptake of 5-HT and norepinephrine (NE), thus improving mood and alleviating depressive symptoms (123). Compared to SSRIs, SNRIs may offer superior efficacy in treating depression and anxiety, particularly in cases linked to NE deficiency. Mirtazapine, for example, due to its sedative and appetite-stimulating properties, coupled with relatively mild gastrointestinal side effects, may be preferred over SSRIs in certain patient populations (124). Additionally, SNRIs exert beneficial effects by reducing neuroinflammation through inhibition of pro-inflammatory cytokine release and modulation of inflammatory pathways, demonstrating significant efficacy in patients with major depressive disorder (125–127). However, prolonged SNRI use in HIV-associated depression treatment may lead to adverse reactions, such as drug interactions, liver toxicity, and withdrawal symptoms. Venlafaxine, for instance, has been associated with increased risk of sexual dysfunction, anorexia, nausea, and insomnia (128). Withdrawal reactions, including dizziness, anxiety, and emotional instability, have also been reported with SSRIs and SNRIs (e.g., venlafaxine and paroxetine) (129, 130), underscoring the importance of a gradual tapering process to prevent abrupt discontinuation. In conclusion, while SNRIs present distinct advantages in the treatment of depression, their potential adverse effects and drug interactions must be meticulously managed to ensure clinical safety and efficacy.
The concomitant use of SSRIs or SNRIs with ART drugs may lead to altered plasma concentrations and clinical adverse reactions. Most antidepressants are substrates of CYP2D6, CYP2B6, or CYP3A4, and interactions between certain ART drugs and antidepressants are primarily mediated through the CYP450 enzyme system. Protease inhibitors (PIs) and NNRTIs can significantly alter antidepressant concentrations by inhibiting or inducing these isoenzymes. Studies have shown that high-dose ritonavir can cause significant changes in the area under the curve (AUC) and maximum plasma concentration of desipramine and trazodone (131). Additionally, the combination of fosamprenavir-ritonavir may reduce the AUC of paroxetine by up to 55% (132). Furthermore, case reports suggest that co-administration of darunavir/ritonavir with duloxetine and aripiprazole may increase duloxetine exposure due to slowed metabolism and elevate the risk of aripiprazole toxicity (133). Therefore, for HIV-infected individuals with impaired liver function, the following measures are recommended: 1) avoid the concomitant use of potent CYP inhibitors (e.g., high-dose ritonavir combined with fluoxetine may trigger serotonin syndrome (134)); 2) regularly monitor liver function and drug concentrations; 3) closely monitor patients for drug-related adverse events when initiating antidepressant therapy and adjust the antiretroviral treatment regimen dosage based on individual responses.
Agomelatine (AGM) is an innovative antidepressant with a distinct mechanism of action, involving the activation of melatonin receptors (MT1/MT2) and inhibition of 5-hydroxytryptamine 2C receptors (5-HT2C). This dual action effectively regulates circadian rhythms while enhancing mood and sleep functions, making AGM particularly suitable for patients with depression accompanied by sleep disturbances or circadian rhythm disruptions (135). Additionally, AGM promotes neuroplasticity by activating melatonin receptors and upregulating brain-derived neurotrophic factor (BDNF) expression, demonstrating notable therapeutic effects in patients with depression linked to sleep disorders and neurofunctional impairments (136). Research indicates that AGM is generally well-tolerated, although it may lead to elevated liver enzyme levels; therefore, regular liver function monitoring is advised during the early stages of treatment (137). However, the use of AGM presents certain limitations (138): first, its efficacy depends on the extent of circadian rhythm disruption and the patient’s specific condition, with more pronounced effects seen in those with significant circadian disturbances; second, while its sleep-enhancing effects are rapid, antidepressant effects typically take several weeks to manifest, similar to other antidepressants.
4.2 Anti-inflammatory-based treatment strategies for depression
Recent studies have highlighted that depression is not only a mental disorder linked to neurotransmitter imbalances but also closely associated with chronic low-grade inflammation. This insight suggests that anti-inflammatory agents could serve as potential adjunctive treatments for depression. Previous research has shown that various anti-inflammatory drugs, including non-steroidal anti-inflammatory drugs (NSAIDs), statins, cytokine inhibitors, glucocorticoids, and minocycline, demonstrate therapeutic potential in alleviating depression and its associated symptoms (139). As noted, depression is strongly connected to chronic inflammation, and NSAIDs, by inhibiting cyclooxygenase (COX) activity and reducing prostaglandin (PG) synthesis, indirectly regulate the NF-κB signaling pathway, thus decreasing the release of pro-inflammatory cytokines like interleukins and TNF-α, which may mitigate the inflammatory responses involved in depression (140). While earlier studies suggested that NSAIDs might have minimal effects on depressive symptoms (141), more recent research indicates that anti-inflammatory treatments, including NSAIDs, can improve depressive symptoms in patients with MDD (139, 142). However, further studies are needed to identify specific patient subgroups who may benefit most from these treatments. Bai et al. also emphasized that NSAIDs, alongside other anti-inflammatory agents such as Omega-3 fatty acids, statins, and minocycline, exhibit antidepressant effects in both monotherapy and adjunctive therapy for MDD, with favorable safety profiles (143). Furthermore, TNF antagonists have shown promise as adjunctive therapies to enhance antidepressant efficacy in treatment-resistant depression (144), and patients with MDD may also benefit from corticosteroids and NSAIDs (145). However, the long-term use of NSAIDs is associated with gastrointestinal side effects (such as ulcers and bleeding), an increased risk of cardiovascular events, and renal dysfunction. Consequently, NSAIDs are typically regarded as adjunctive treatments rather than primary therapeutic options for depression.
Statins may play a potential role in the adjunctive treatment of depression through several mechanisms, including anti-inflammatory effects, enhancement of cerebrovascular function, and neurovascular protection (146, 147). Recent studies further support their application in the prevention and management of depression. A cohort study utilizing the UK Biobank, which involved over 360,000 participants, found that regular statin use was associated with a reduced risk of depression (HR = 0.87, 95% CI: 0.81 – 0.94), with consistent results in sensitivity analysis (148). Similarly, a Swedish registry study encompassing more than 1.14 million individuals corroborated this finding, revealing that statin use was linked to a reduced risk of depressive disorders (HR = 0.91, 95% CI: 0.87 – 0.94), a result that remained robust even after adjusting for antidepressant use (HR = 0.91, 95% CI: 0.88 – 0.94) (149). Moreover, a comparison between fluoxetine monotherapy and combination therapy with high-dose atorvastatin (80 mg/day) demonstrated a reduction in pro-inflammatory cytokines and other pro-inflammatory cytokines and other inflammatory markers such as NLRP-3 and IL-6, as well as improved Hamilton Depression Rating Scale scores (150). These findings suggest that atorvastatin may be a promising adjunctive therapy for patients with MDD by modulating the AMPK/NLRP3 and IL-6/STAT-3 signaling pathways (150). Although these studies provide compelling evidence for the potential of statins in depression prevention and intervention, the pharmacokinetic properties and drug interaction risks of statins require careful consideration. Statins such as simvastatin, lovastatin, and atorvastatin are primarily metabolized via CYP3A4. When co-administered with strong CYP3A4 inhibitors (e.g., ritonavir, saquinavir), these statins may exhibit significantly increased blood concentrations, raising the risk of severe adverse effects such as rhabdomyolysis and liver damage (151). In contrast, statins with lower drug interaction risks, such as pravastatin and rosuvastatin, are cleared via non-hepatic enzymes and may be more suitable for special populations, including PLWH. Therefore, for patients requiring concomitant use of CYP3A4 inhibitors, pravastatin or rosuvastatin is preferred, with dose adjustments and close monitoring of muscle and liver function markers to minimize the risk of adverse effects.
4.3 HPA axis-based treatment strategies for depression
As discussed in Section 3.4, PLWH who also suffer from depression often exhibit excessive activation of the HPA axis, leading to abnormally elevated cortisol levels. Consequently, interventions targeting the HPA axis are of considerable clinical and theoretical significance for treating depression, offering a scientific basis for optimizing antidepressant strategies and advancing personalized medicine. Cortisol, a central hormone in the stress response, when chronically elevated, can exert neurotoxic effects on brain regions such as the hippocampus and prefrontal cortex, which are essential for memory and emotional regulation. This prolonged exposure to elevated cortisol can lead to neuronal atrophy and functional impairment (152, 153). Mifepristone, a glucocorticoid receptor (GR) antagonist, exerts its therapeutic effects by blocking GR, thereby rectifying HPA axis overactivation, restoring negative feedback regulation, and lowering cortisol levels. This mechanism helps to reduce the stress-induced hyperactivity of the HPA axis, alleviate cortisol-induced neurotoxicity in the hippocampus and prefrontal cortex, and potentially provide neuroprotective effects in these regions (154, 155). In summary, mifepristone has shown potential efficacy in certain depression subtypes, such as treatment-resistant depression or cortisol-related depression. However, due to its antagonistic effects on progesterone receptors, its use in female patients, particularly those of reproductive age, warrants caution. While preliminary studies have shown promising results, its efficacy and safety must be confirmed through large-scale, long-term clinical trials to better define its practical application in depression treatment.
4.4 Application of non-pharmacological interventions in adjunctive treatment of depression
While pharmacological treatment remains the cornerstone of depression management in PLWH, concerns regarding its potential adverse effects—such as physical discomfort, sexual dysfunction, substance abuse, and overdose-related fatalities—along with the interactions between antidepressants and antiretroviral medications, and the risk of withdrawal and rebound phenomena, have prompted increasing interest in non-pharmacological treatments (131, 156). Non-pharmacological approaches, which include psychotherapy (e.g., cognitive-behavioral therapy) (157, 158), psychosocial support (e.g., meditation and yoga) (157–159), physical therapy (e.g., exercise) (160–162), and music therapy (163), have gained attention as complementary options. Clinical guidelines recommend psychotherapy and exercise therapy as first-line treatments, particularly for patients with mild to moderate depression (164). Studies indicate that exercise therapy offers significant benefits in addressing immune-mediated depression (IMD), particularly in patients with pronounced IMD features, and can serve as an alternative or adjunctive treatment to antidepressant medications (165). Moreover, a meta-analysis has demonstrated that resistance training provides clear antidepressant effects in patients without major physical comorbidities, highlighting its potential for clinical application in the comprehensive management of depression (166). These psychosocial intervention strategies are characterized by low technical requirements, high cost-effectiveness, and strong cultural adaptability, making them particularly suitable for implementation in resource-limited low- and middle-income countries. Overall, the increasing body of scientific evidence supports the integration of exercise therapy and psychotherapy as essential components of a comprehensive strategy for managing depression in PLWH.
4.5 Optimization of antiviral therapy regimens
Antiretroviral drugs used in the treatment of HIV infection include nucleoside reverse transcriptase inhibitors (NRTIs), NNRTIs, PIs, integrase strand transfer inhibitors (INSTIs), and fusion inhibitors. While these drugs effectively suppress HIV replication, lower viral load, enhance immune function, and significantly reduce the risk of drug resistance, certain medications, particularly NNRTIs, are associated with CNS toxicity, which may increase the risk of anxiety and depression (167, 168). These side effects can diminish treatment adherence, quality of life, and disease prognosis in PLWH, and may also lead to discontinuation of therapy. A prospective observational study involving 129 PLWH found that, on average, each participant experienced 2.4 neuropsychiatric disorders, with 89.9% of patients changing their treatment regimen due to symptoms such as sleep disturbances (75.2%), anxiety (65.1%), and depression (38.7%) induced by EFV (170). Another real-world study assessing discontinuation rates in patients on DTG-based regimens revealed that 13.7% of participants discontinued treatment due to intolerance, primarily due to neuropsychiatric symptoms such as sleep disturbances, anxiety, and depression (51).
Although CCR5 antagonists (e.g., Maraviroc), post-attachment inhibitors (e.g., Ibalizumab), and capsid inhibitors (e.g., Lenacapavir) hold significant therapeutic value in specific populations, their clinical use remains limited by targeted indication requirements and high treatment costs. Existing clinical trial data indicate that these three classes of drugs have not yet reported clinically significant neuropsychiatric side effects (169–171). However, their long-term neuropsychiatric safety in the real-world setting, particularly in individuals with comorbid mental health disorders, still requires further research for validation.
Maintaining good adherence to ART is a key factor in preventing viral rebound and associated neuroinflammation. It is important to note that depressive symptoms themselves may reduce medication adherence, highlighting the need for clinicians to closely monitor and intervene promptly when depressive symptoms occur in PLWH. For patients experiencing neuropsychiatric side effects, prompt adjustment of the treatment regimen is crucial to prevent further exacerbation of psychiatric symptoms. Studies have shown that compared to EFV(a NNRTI)-containing regimens, DTG (an INSTI)-based regimens, an integrase strand transfer inhibitor (INSTI), exhibit superior safety with a lower incidence of ADRs (172). According to the HIV Antiretroviral Therapy Guidelines for Adults and Adolescents, published by the U.S. Department of Health and Human Services (DHHS) on September 12, 2024, INSTIs such as DTG or bictegravir are recommended for ART due to their excellent virological suppression and favorable tolerability (173). Additionally, since the mechanism of action of NRTIs does not involve the CNS, they are particularly effective in minimizing neuropsychiatric symptoms. NRTIs are often combined with INSTIs, offering a lower risk of drug discontinuation due to toxicity and providing good overall treatment tolerability (174). In contrast, NNRTIs like EFV, which carry a higher risk of ADRs, should be used with caution in patients with existing neuropsychiatric symptoms. Overall, the combination of INSTIs and NRTIs is recommended to reduce the risk of psychiatric disorders, especially for patients with poor tolerance to other classes of drugs such as NNRTIs or PIs. It is particularly important to note that for PLWH presenting with unexplained cognitive impairment or neurological symptoms, even if plasma viral load is controlled, CSF HIV RNA quantification and drug resistance genotype testing should be considered to rule out the possibility of CSF viral escape (175).
5 Conclusion
PLWH face an increased risk for depression, anxiety, and other comorbid psychiatric disorders. However, the exact mechanisms underlying HIV-associated depression remain inadequately understood. This study integrates existing evidence linking HIV, depression, and chronic neuroinflammation, positing that the pathophysiological mechanisms involve intricate interactions across biological, psychological, and sociological dimensions. Biologically, factors such as neuroimmune activation, neurotransmitter dysregulation, HPA axis dysfunction, and structural and functional alterations in the brain are pivotal. Psychologically, the burden of chronic illness—encompassing stigma, social isolation, and trauma—plays a substantial role in the onset of depression. Sociologically, insufficient social support, economic stress, and a reduced quality of life further exacerbate the risk. These findings offer a novel perspective on the pathophysiology of HIV-associated depression, shedding light on its multifactorial nature. A particularly noteworthy aspect is the kynurenine pathway linking inflammation and neurotransmitter dysregulation: inflammatory signals alter the tryptophan metabolism process, which, on one hand, leads to serotonin depletion and, on the other hand, generates neurotoxic metabolites, thereby causing dysfunction in the glutamate system. Future research could focus on comparing the molecular characteristics (such as cerebrospinal fluid inflammatory marker profiles) of HIV-associated depression and primary depression, which would help further clarify the HIV-specific pathological mechanisms, particularly in terms of inflammation and neurotransmitter dysregulation related to the kynurenine pathway.
Addressing these risk factors necessitates comprehensive, targeted interventions. At the biological level, maintaining good adherence to ART is crucial for preventing viral rebound and associated neuroimmune activation. Depressive symptoms may reduce medication adherence, thus clinicians should closely monitor and intervene promptly. Optimizing ART regimens should involve prioritizing INSTIs with higher CNS safety, such as DTG, while avoiding the use of NNRTIs with significant neurotoxicity, such as EFV. Regular monitoring for neuropsychiatric adverse effects is also essential. In addition, neurotransmitter-modulating drugs (e.g., SSRIs/SNRIs), anti-inflammatory treatments, and HPA axis regulators may be used in combination, but potential interactions between these drugs and ART must be carefully evaluated. Psychologically, interventions like psychological support, stress management, and trauma-focused therapies are effective in improving mental health. Additionally, physical exercise and health education have potential in reducing depression risk. Sociologically, enhancing social support networks, mitigating economic stress, and improving patients’ quality of life can further improve therapeutic outcomes and promote adherence to treatment.
In conclusion, the prevention and treatment of HIV-associated depression require the establishment of a three-tiered intervention system: (1) optimization of ART regimens; (2) provision of structured psychological support; and (3) enhancement of social care networks. This comprehensive strategy, encompassing pharmacological treatment, psychological support, and social dimensions, is key to reducing the risk of depression and improving overall health outcomes for patients. Future research should further explore the long-term effects and underlying mechanisms of these interventions, particularly in optimizing strategies for PLWH. Such research will establish the scientific foundation for more precise and effective intervention approaches.
Author contributions
FY: Investigation, Methodology, Resources, Software, Writing – original draft. YZ: Methodology, Resources, Software, Writing – original draft. YF: Investigation, Methodology, Software, Writing – original draft. MC: Project administration, Software, Validation, Writing – original draft. QP: Conceptualization, Methodology, Writing – original draft. SL: Methodology, Supervision, Writing – original draft. LH: Methodology, Software, Writing – original draft. FHY: Methodology, Validation, Writing – review & editing. JX: Investigation, Supervision, Writing – review & editing. XH: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The present study was supported by the Research Plan of the National Natural Science Foundation of China (No. 82575017) and the Sichuan Provincial Administration of Traditional Chinese Medicine Major Science and Technology Projects (No. 2021XYCZ004).
Acknowledgments
We thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript. We thank Home for Researchers (www.home-for-researchers.com) for guidance on the figures in the manuscript. The figures in this study (Figures 1-3) were created using the Figdraw scientific drawing platform (https://www.figdraw.com). All graphic elements were originally designed by the authors and used with permission from the platform.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PLWH, people living with HIV; ART, antiretroviral therapy; cART, combination antiretroviral therapy; WIHS, Women’s Interagency HIV Study; MDD, major depressive disorder; CNS, central nervous system; PTSD, post-traumatic stress disorder; NNRTIs, non-nucleoside reverse transcriptase inhibitors; EFV, Efavirenz; ADRs, adverse drug reactions; DTG, dolutegravir; ABC, abacavir; G-CSF, Granulocyte colony-stimulating factor; CRP, C-reactive protein; NfL, neurofilament light chain protein; CSF, cerebrospinal fluid; gp120, glycoprotein 120; Glu, glutamate; ROS, reactive oxygen species; RNS, reactive nitrogen species; 5-HT, serotonin; TRP, tryptophan; KYN, kynurenine; QUIN, quinolinic acid; IDO, indoleamine 2,3-dioxygenase; mI, myo-inositol; NAA, N-acetylaspartate; Cho, choline; HPA, hypothalamic-pituitary-adrenal; CRH, corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone; SSRIs, selective serotonin reuptake inhibitors; SNRIs, selective norepinephrine-serotonin reuptake inhibitors; NE, norepinephrine; AGM, agomelatine; 5-HT2C, 5-hydroxytryptamine 2C receptors; BDNF, brain-derived neurotrophic factor; NSAIDs, non-steroidal anti-inflammatory drugs; COX, cyclooxygenase; PG, prostaglandin; GR, glucocorticoid receptor; IMD, immune-mediated depression; NRTIs, nucleoside reverse transcriptase inhibitors; PIs, protease inhibitors; INSTIs, integrase strand transfer inhibitors; DHHS, department of Health and Human Services; CRP, C-reactive protein; TNFα, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-18, interleukin-18; gp120, human immunodeficiency virus envelope glycoprotein 120; Tat, trans-activator of transcription; Vpr, viral protein R; AUC, Area Under the Curve.
References
1. Global HIV & AIDS statistics — Fact sheet (2024). Available online at: https://www.unaids.org/en/resources/fact-sheet (Accessed December 11, 2024).
2. Rajasuriar R, Crane HM, and Semeere AS. Growing older with HIV in the treat-all era. J Int AIDS Soc. (2022) 25:e25997. doi: 10.1002/jia2.25997
3. Wandeler G, Johnson LF, and Egger M. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: comparisons with general population. Curr Opin HIV AIDS. (2016) 11:492–500. doi: 10.1097/COH.0000000000000298
4. Smiley CL, Rebeiro PF, Cesar C, Belaunzaran-Zamudio PF, Crabtree-Ramirez B, Padgett D, et al. Estimated life expectancy gains with antiretroviral therapy among adults with HIV in Latin America and the Caribbean: a multisite retrospective cohort study. Lancet HIV. (2021) 8:e266–73. doi: 10.1016/S2352-3018(20)30358-1
5. Rafiei S, Raoofi S, Pashazadeh Kan F, Masoumi M, Doustmehraban M, Biparva AJ, et al. Global prevalence of suicide in patients living with HIV/AIDS: A systematic review and meta-analysis. J Affect Disord. (2023) 323:400–8. doi: 10.1016/j.jad.2022.11.061
6. Gooden TE, Gardner M, Wang J, Chandan JS, Beane A, Haniffa R, et al. The risk of mental illness in people living with HIV in the UK: a propensity score-matched cohort study. Lancet HIV. (2022) 9:e172–81. doi: 10.1016/S2352-3018(21)00319-2
7. Lazarus JV, Safreed-Harmon K, Barton SE, Costagliola D, Dedes N, Del Amo Valero J, et al. Beyond viral suppression of HIV-the new quality of life frontier. BMC Med. (2016) 14:94. doi: 10.1186/s12916-016-0640-4
8. Arseniou S, Arvaniti A, and Samakouri M. HIV infection and depression. Psychiatry Clin Neurosci. (2014) 68:96–109. doi: 10.1111/pcn.12097
9. Rezaei S, Ahmadi S, Rahmati J, Hosseinifard H, Dehnad A, Aryankhesal A, et al. Global prevalence of depression in HIV/AIDS: a systematic review and meta-analysis. BMJ Support Palliat Care. (2019) 9:404–12. doi: 10.1136/bmjspcare-2019-001952
10. Depressive disorder (depression) (2025). Available online at: https://www.who.int/news-room/fact-sheets/detail/depression (Accessed August 10, 2025).
11. Gokhale RH, Weiser J, Sullivan PS, Luo Q, Shu F, and Bradley H. Depression prevalence, antidepressant treatment status, and association with sustained HIV viral suppression among adults living with HIV in care in the United States, 2009-2014. AIDS Behav. (2019) 23:3452–9. doi: 10.1007/s10461-019-02613-6
12. Beer L, Tie Y, Padilla M, and Shouse RL. Medical Monitoring Project. Generalized anxiety disorder symptoms among persons with diagnosed HIV in the United States. AIDS. (2019) 33:1781–7. doi: 10.1097/QAD.0000000000002286
13. Nejadghaderi SA, Bastan MM, Abdi M, Iranpour A, and Sharifi H. National and sub-national HIV/AIDS epidemiology, socioeconomic influences, and risk factors in Iran from 1990 to 2021, global burden of disease 2021 study. Sci Rep. (2025) 15:22493. doi: 10.1038/s41598-025-06499-4
14. Kelso-Chichetto NE, Okafor CN, Cook RL, Abraham AG, Bolan R, and Plankey M. Association between depressive symptom patterns and clinical profiles among persons living with HIV. AIDS Behav. (2018) 22:1411–22. doi: 10.1007/s10461-017-1822-6
15. Cook JA, Burke-Miller JK, Steigman PJ, Schwartz RM, Hessol NA, Milam J, et al. Prevalence, comorbidity, and correlates of psychiatric and substance use disorders and associations with HIV risk behaviors in a multisite cohort of women living with HIV. AIDS Behav. (2018) 22:3141–54. doi: 10.1007/s10461-018-2051-3
16. Niu L, Luo D, Liu Y, Silenzio VMB, and Xiao S. The mental health of people living with HIV in China, 1998-2014: A systematic review. PLoS One. (2016) 11:e0153489. doi: 10.1371/journal.pone.0153489
17. Evans JA, Shim JM, and Ioannidis JPA. Attention to local health burden and the global disparity of health research. PLoS One. (2014) 9:e90147. doi: 10.1371/journal.pone.0090147
18. Tao J, Wang L, Kipp AM, Qian HZ, Yin L, Ruan Y, et al. Relationship of stigma and depression among newly HIV-diagnosed chinese men who have sex with men. AIDS Behav. (2017) 21:292–9. doi: 10.1007/s10461-016-1477-8
19. Arango C, Dragioti E, Solmi M, Cortese S, Domschke K, Murray RM, et al. Risk and protective factors for mental disorders beyond genetics: an evidence-based atlas. World Psychiatry. (2021) 20:417–36. doi: 10.1002/wps.20894
20. Zhao T, Tang C, Yan H, Wang H, and Guo M. Comparative efficacy and acceptability of non-pharmacological interventions for depression among people living with HIV: A protocol for a systematic review and network meta-analysis. PLoS One. (2023) 18:e0287445. doi: 10.1371/journal.pone.0287445
21. Tucker JS, Burnam MA, Sherbourne CD, Kung FY, and Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. Am J Med. (2003) 114:573–80. doi: 10.1016/S0002-9343(03)00093-7
22. Nanni MG, Caruso R, Mitchell AJ, Meggiolaro E, and Grassi L. Depression in HIV infected patients: a review. Curr Psychiatry Rep. (2015) 17:530. doi: 10.1007/s11920-014-0530-4
23. Parcesepe AM, Stockton M, Bernard C, Kanguya T, Kwobah EK, Lopez A, et al. Prevalence and co-occurrence of symptoms of mental and substance use disorders among people with HIV age 40 and older in low- and middle-income countries: a cross-sectional study. J Int AIDS Soc. (2024) 27:e26359. doi: 10.1002/jia2.26359
24. Junaid K, Afzal S, Daood M, and Siddiqui M. Substance abuse and mental health issues among HIV/AIDS patients. J Coll Physicians Surg Pak. (2023) 33:325–34. doi: 10.29271/jcpsp.2023.03.325
25. Slot M, Sodemann M, Gabel C, Holmskov J, Laursen T, and Rodkjaer L. Factors associated with risk of depression and relevant predictors of screening for depression in clinical practice: a cross-sectional study among HIV-infected individuals in Denmark. HIV Med. (2015) 16:393–402. doi: 10.1111/hiv.12223
26. Hutton HE, Lyketsos CG, Zenilman JM, Thompson RE, and Erbelding EJ. Depression and HIV risk behaviors among patients in a sexually transmitted disease clinic. Am J Psychiatry. (2004) 161:912–4. doi: 10.1176/appi.ajp.161.5.912
27. Ross JL, Teeraananchai S, Avihingsanon A, Lee MP, Ditangco R, Rajasuriar R, et al. Brief report: depression, substance use, and factors associated with sexual risk behaviors among adults living with HIV in the asia-pacific region. J Acquir Immune Defic Syndr. (2024) 96:421–8. doi: 10.1097/QAI.0000000000003446
28. Leserman J. HIV disease progression: depression, stress, and possible mechanisms. Biol Psychiatry. (2003) 54:295–306. doi: 10.1016/S0006-3223(03)00323-8
29. Horberg M, Thompson M, Agwu A, Colasanti J, Haddad M, Jain M, et al. Primary care guidance for providers of care for persons with human immunodeficiency virus: 2024 update by the HIV medicine association of the infectious diseases society of america. Clin Infect Dis. (2024), ciae479. doi: 10.1093/cid/ciae479
30. Remien RH, Stirratt MJ, Nguyen N, Robbins RN, Pala AN, and Mellins CA. Mental health and HIV/AIDS: the need for an integrated response. AIDS (Lond Engl). (2019) 33:1411–20. doi: 10.1097/QAD.0000000000002227
31. Karamouzian M, Akbari M, Haghdoost AA, Setayesh H, and Zolala F. I am dead to them”: HIV-related stigma experienced by people living with HIV in Kerman, Iran. J Assoc Nurses AIDS Care. (2015) 26:46–56. doi: 10.1016/j.jana.2014.04.005
32. Frew PM, Parker K, Vo L, Haley D, O’Leary A, Diallo DD, et al. Socioecological factors influencing women’s HIV risk in the United States: qualitative findings from the women’s HIV SeroIncidence study (HPTN 064). BMC Public Health. (2016) 16:803. doi: 10.1186/s12889-016-3364-7
33. Ellis RJ, Iudicello J, Sun-Suslow N, Grelotti D, Cherner M, Morgan E, et al. Social isolation is linked to inflammation in aging people with HIV and uninfected individuals. J Acquir Immune Defic Syndr. (2021) 86:600–6. doi: 10.1097/QAI.0000000000002596
34. Smith KJ, Gavey S, RIddell NE, Kontari P, and Victor C. The association between loneliness, social isolation and inflammation: A systematic review and meta-analysis. Neurosci Biobehav Rev. (2020) 112:519–41. doi: 10.1016/j.neubiorev.2020.02.002
35. Yu Y, Luo B, Qin L, Gong H, and Chen Y. Suicidal ideation of people living with HIV and its relations to depression, anxiety and social support. BMC Psychol. (2023) 11:159. doi: 10.1186/s40359-023-01177-4
36. Yu Y, Cai H, Chen X, Xiao F, Qin K, and Li J. Intimate partner violence and its associations among HIV-infected MSM with new drug abuse in Jinan, China. BMC Public Health. (2023) 23:2517. doi: 10.1186/s12889-023-17451-4
37. Zuccala M, Menzies RE, Hunt CJ, and Abbott MJ. A systematic review of the psychometric properties of death anxiety self-report measures. Death Stud. (2022) 46:257–79. doi: 10.1080/07481187.2019.1699203
38. Safren SA, Gershuny BS, and Hendriksen E. Symptoms of posttraumatic stress and death anxiety in persons with HIV and medication adherence difficulties. AIDS Patient Care STDS. (2003) 17:657–64. doi: 10.1089/108729103771928717
39. Du P, Crook T, Whitener C, Albright P, Greenawalt D, and Zurlo J. HIV transmission risk behaviors among people living with HIV/AIDS: the need to integrate HIV prevention interventions and public health strategies into HIV care. J Public Health Manag Pract. (2015) 21:E1–10. doi: 10.1097/PHH.0000000000000038
40. Lok N, Aydın Z, Uzun G, Kayaaslan B, and Selçuk Tosun A. Relationship of depression, hopelessness and life satisfaction with death anxiety in individuals who have had COVID-19. Omega (Westport). (2023), 302228231174602. doi: 10.1177/00302228231174602
41. Anindhita M, Haniifah M, Putri AMN, Karnasih A, Agiananda F, Yani FF, et al. Community-based psychosocial support interventions to reduce stigma and improve mental health of people with infectious diseases: a scoping review. Infect Dis Poverty. (2024) 13:90. doi: 10.1186/s40249-024-01257-6
42. Crawford TN, Neilands TB, Drumright LN, Fredericksen RJ, Johnson MO, Mayer KH, et al. Internalized HIV stigma and viral suppression: examining the mediating and moderating roles of substance use and social support. AIDS. (2024) 38:2064–72. doi: 10.1097/QAD.0000000000003999
43. Sin NL and DiMatteo MR. Depression treatment enhances adherence to antiretroviral therapy: a meta-analysis. Ann Behav Med. (2014) 47:259–69. doi: 10.1007/s12160-013-9559-6
44. Uthman OA, Magidson JF, Safren SA, and Nachega JB. Depression and adherence to antiretroviral therapy in low-, middle- and high-income countries: a systematic review and meta-analysis. Curr HIV/AIDS Rep. (2014) 11:291–307. doi: 10.1007/s11904-014-0220-1
45. Cespedes MS and Aberg JA. Neuropsychiatric complications of antiretroviral therapy. Drug Saf. (2006) 29:865–74. doi: 10.2165/00002018-200629100-00004
46. McIntosh RC, Rosselli M, Uddin LQ, and Antoni M. Neuropathological sequelae of Human Immunodeficiency Virus and apathy: A review of neuropsychological and neuroimaging studies. Neurosci Biobehav Rev. (2015) 55:147–64. doi: 10.1016/j.neubiorev.2015.04.008
47. Law JKC, Butler LT, and Hamill MM. Predictors of discontinuation of efavirenz as treatment for HIV, due to neuropsychiatric side effects, in a multi-ethnic sample in the United Kingdom. AIDS Res Hum Retroviruses. (2020) 36:459–66. doi: 10.1089/aid.2019.0193
48. Apostolova N, Funes HA, Blas-Garcia A, Galindo MJ, Alvarez A, and Esplugues JV. Efavirenz and the CNS: what we already know and questions that need to be answered. J Antimicrob Chemother. (2015) 70:2693–708. doi: 10.1093/jac/dkv183
49. Shah A, Gangwani MR, Chaudhari NS, Glazyrin A, Bhat HK, and Kumar A. Neurotoxicity in the post-HAART era: caution for the antiretroviral therapeutics. Neurotox Res. (2016) 30:677–97. doi: 10.1007/s12640-016-9646-0
50. Robertson K, Liner J, and Meeker RB. Antiretroviral neurotoxicity. J Neurovirol. (2012) 18:388–99. doi: 10.1007/s13365-012-0120-3
51. de Boer MGJ, van den Berk GEL, van Holten N, Oryszcyn JE, Dorama W, Moha DA, et al. Intolerance of dolutegravir-containing combination antiretroviral therapy regimens in real-life clinical practice. AIDS. (2016) 30:2831–4. doi: 10.1097/QAD.0000000000001279
52. Mamik MK, Hui E, Branton WG, McKenzie BA, Chisholm J, Cohen EA, et al. HIV-1 viral protein R activates NLRP3 inflammasome in microglia: implications for HIV-1 associated neuroinflammation. J Neuroimmune Pharmacol. (2017) 12:233–48. doi: 10.1007/s11481-016-9708-3
53. Dutta D, Liu J, Xu E, and Xiong H. Methamphetamine enhancement of HIV-1 gp120-mediated NLRP3 inflammasome activation and resultant proinflammatory responses in rat microglial cultures. Res Sq. (2023) 25:rs.3.rs–3707515. doi: 10.21203/rs.3.rs-3707515/v1
54. King JE, Eugenin EA, Buckner CM, and Berman JW. HIV tat and neurotoxicity. Microbes Infect. (2006) 8:1347–57. doi: 10.1016/j.micinf.2005.11.014
55. Sun Y, Zhang C, Lei T, Lin F, Huang J, Hu Y, et al. HIV1 gp120 activates microglia via TLR2-nf-κb signaling to up-regulate inflammatory cytokine expression and induce neuropathic pain. Neuropharmacology. (2024) 260:110136. doi: 10.1016/j.neuropharm.2024.110136
56. Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, and Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. (2000) 879:42–9. doi: 10.1016/S0006-8993(00)02725-6
57. Walsh JG, Reinke SN, Mamik MK, McKenzie BA, Maingat F, Branton WG, et al. Rapid inflammasome activation in microglia contributes to brain disease in HIV/AIDS. Retrovirology. (2014) 11:35. doi: 10.1186/1742-4690-11-35
58. Rivera-Rivera Y, García Y, Toro V, Cappas N, López P, Yamamura Y, et al. Depression correlates with increased plasma levels of inflammatory cytokines and a dysregulated oxidant/antioxidant balance in HIV-1-infected subjects undergoing antiretroviral therapy. J Clin Cell Immunol. (2014) 5:1000276. doi: 10.4172/2155-9899.1000276
59. Lu H, Surkan PJ, Irwin MR, Treisman GJ, Breen EC, Sacktor N, et al. Inflammation and risk of depression in HIV: prospective findings from the multicenter AIDS cohort study. Am J Epidemiol. (2019) 188:1994–2003. doi: 10.1093/aje/kwz190
60. Rubin LH, Langenecker SA, Phan KL, Keating SM, Neigh GN, Weber KM, et al. Remitted depression and cognition in HIV: The role of cortisol and inflammation. Psychoneuroendocrinology. (2020) 114:104609. doi: 10.1016/j.psyneuen.2020.104609
61. Saloner R, Paolillo EW, Heaton RK, Grelotti DJ, Stein MB, Miller AH, et al. Chronically elevated depressive symptoms interact with acute increases in inflammation to predict worse neurocognition among people with HIV. J Neurovirol. (2021) 27:160–7. doi: 10.1007/s13365-020-00925-1
62. Colasanto M, Madigan S, and Korczak DJ. Depression and inflammation among children and adolescents: A meta-analysis. J Affect Disord. (2020) 277:940–8. doi: 10.1016/j.jad.2020.09.025
63. Khan A, Leonard D, Defina L, Barlow CE, Willis B, and Brown ES. Association between C reactive protein and depression in a population of healthy adults: the Cooper Center Longitudinal Study. J Investig Med. (2020) 68:1019–23. doi: 10.1136/jim-2019-001254
64. Pitharouli MC, Hagenaars SP, Glanville KP, Coleman JRI, Hotopf M, Lewis CM, et al. Elevated C-reactive protein in patients with depression, independent of genetic, health, and psychosocial factors: results from the UK biobank. Am J Psychiatry. (2021) 178:522–9. doi: 10.1176/appi.ajp.2020.20060947
65. Huang M, Su S, Goldberg J, Miller AH, Levantsevych OM, Shallenberger L, et al. Longitudinal association of inflammation with depressive symptoms: A 7-year cross-lagged twin difference study. Brain Behav Immun. (2019) 75:200–7. doi: 10.1016/j.bbi.2018.10.007
66. Milaneschi Y, Kappelmann N, Ye Z, Lamers F, Moser S, Jones PB, et al. Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts. Mol Psychiatry. (2021) 26:7393–402. doi: 10.1038/s41380-021-01188-w
67. Dahmani S, Kaliss N, VanMeter JW, Moore DJ, Ellis RJ, and Jiang X. Alterations of brain metabolites in adults with HIV: A systematic meta-analysis of magnetic resonance spectroscopy studies. Neurology. (2021) 97:e1085–96. doi: 10.1212/WNL.0000000000012394
68. van Biljon N, Robertson F, Holmes M, Cotton MF, Laughton B, van der Kouwe A, et al. Multivariate approach for longitudinal analysis of brain metabolite levels from ages 5 – 11 years in children with perinatal HIV infection. Neuroimage. (2021) 237:118101. doi: 10.1016/j.neuroimage.2021.118101
69. Chen MH, Liu YL, Kuo HW, Tsai SJ, Hsu JW, Huang KL, et al. Neurofilament light chain is a novel biomarker for major depression and related executive dysfunction. Int J Neuropsychopharmacol. (2022) 25:99–105. doi: 10.1093/ijnp/pyab068
70. Gisslen M, Keating SM, Spudich S, Arechiga V, Stephenson S, Zetterberg H, et al. Compartmentalization of cerebrospinal fluid inflammation across the spectrum of untreated HIV-1 infection, central nervous system injury and viral suppression. PLoS One. (2021) 16:e0250987. doi: 10.1371/journal.pone.0250987
71. Guha D, Mukerji SS, Chettimada S, Misra V, Lorenz DR, Morgello S, et al. Cerebrospinal fluid extracellular vesicles and neurofilament light protein as biomarkers of central nervous system injury in HIV-infected patients on antiretroviral therapy. AIDS. (2019) 33:615–25. doi: 10.1097/QAD.0000000000002121
72. Mecca C, Giambanco I, Donato R, and Arcuri C. Microglia and aging: the role of the TREM2-DAP12 and CX3CL1-CX3CR1 axes. Int J Mol Sci. (2018) 19:318. doi: 10.3390/ijms19010318
73. Beurel E, Toups M, and Nemeroff CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. (2020) 107:234–56. doi: 10.1016/j.neuron.2020.06.002
74. Green C, Shen X, Stevenson AJ, Conole ELS, Harris MA, Barbu MC, et al. Structural brain correlates of serum and epigenetic markers of inflammation in major depressive disorder. Brain Behav Immun. (2021) 92:39–48. doi: 10.1016/j.bbi.2020.11.024
75. Wohleb ES, Franklin T, Iwata M, and Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci. (2016) 17:497–511. doi: 10.1038/nrn.2016.69
76. Marrie RA and Bernstein CN. Psychiatric comorbidity in immune-mediated inflammatory diseases. World Psychiatry. (2021) 20:298–9. doi: 10.1002/wps.20873
77. Mudra Rakshasa-Loots A, Whalley HC, Vera JH, and Cox SR. Neuroinflammation in HIV-associated depression: evidence and future perspectives. Mol Psychiatry. (2022) 27:3619–32. doi: 10.1038/s41380-022-01619-2
78. Turkheimer FE, Veronese M, Mondelli V, Cash D, and Pariante CM. Sickness behaviour and depression: An updated model of peripheral-central immunity interactions. Brain Behav Immun. (2023) 111:202–10. doi: 10.1016/j.bbi.2023.03.031
79. Cooney-Quane J, Thomas DS, Nolan YM, and Dockray S. C-reactive protein is associated with pain-type somatic symptoms independent of mental health symptoms in adolescents: evidence from the ALSPAC study. Brain Behav Immun-Health. (2025) 48:101082. doi: 10.1016/j.bbih.2025.101082
80. Donnelly NA, Tsang RSM, Foley ÉM, Fraser H, Hanson AL, and Khandaker GM. Blood immuno-metabolic biomarker signatures of depression and affective symptoms in young adults. Brain Behav Immun. (2025) 128:673–84. doi: 10.1016/j.bbi.2025.05.011
81. Lee STH. Inflammation, depression, and anxiety disorder: A population-based study examining the association between Interleukin-6 and the experiencing of depressive and anxiety symptoms. Psychiatry Res. (2020) 285:112809. doi: 10.1016/j.psychres.2020.112809
82. Rossi S, Studer V, Motta C, Polidoro S, Perugini J, Macchiarulo G, et al. Neuroinflammation drives anxiety and depression in relapsing-remitting multiple sclerosis. Neurology. (2017) 89:1338–47. doi: 10.1212/WNL.0000000000004411
83. Dooley LN, Kuhlman KR, Robles TF, Eisenberger NI, Craske MG, and Bower JE. The role of inflammation in core features of depression: Insights from paradigms using exogenously-induced inflammation. Neurosci Biobehav Rev. (2018) 94:219–37. doi: 10.1016/j.neubiorev.2018.09.006
84. Khan N, Halcrow PW, Afghah Z, Baral A, Geiger JD, and Chen X. HIV-1 tat endocytosis and retention in endolysosomes affects HIV-1 tat-induced LTR transactivation in astrocytes. FASEB J. (2022) 36:e22184. doi: 10.1096/fj.202101722R
85. Marino J, Maubert ME, Mele AR, Spector C, Wigdahl B, and Nonnemacher MR. Functional impact of HIV-1 tat on cells of the CNS and its role in HAND. Cell Mol Life Sci. (2020) 77:5079–99. doi: 10.1007/s00018-020-03561-4
86. Bower JE and Kuhlman KR. Psychoneuroimmunology: an introduction to immune-to-brain communication and its implications for clinical psychology. Annu Rev Clin Psychol. (2023) 19:331–59. doi: 10.1146/annurev-clinpsy-080621-045153
87. Buntinx M, Moreels M, Vandenabeele F, Lambrichts I, Raus J, Steels P, et al. Cytokine-induced cell death in human oligodendroglial cell lines: I. Synergistic effects of IFN-gamma and TNF-alpha on apoptosis. J Neurosci Res. (2004) 76:834–45. doi: 10.1002/jnr.20118
88. Dai X, Chen J, Xu F, Zhao J, Cai W, Sun Z, et al. TGFα preserves oligodendrocyte lineage cells and improves white matter integrity after cerebral ischemia. J Cereb Blood Flow Metab. (2020) 40:639–55. doi: 10.1177/0271678X19830791
89. Chai Z, Ma T, Li Y, Chen Q, Kang Y, Sun J, et al. Inhibition of inflammatory factor TNF-α by ferrostatin-1 in microglia regulates necroptosis of oligodendrocyte precursor cells. Neuroreport. (2023) 34:583–91. doi: 10.1097/WNR.0000000000001928
90. Amin R, Quispe C, Docea AO, Ydyrys A, Kulbayeva M, Durna Daştan S, et al. The role of Tumour Necrosis Factor in neuroinflammation associated with Parkinson’s disease and targeted therapies. Neurochem Int. (2022) :158:105376. doi: 10.1016/j.neuint.2022.105376
91. Almulla AF, Thipakorn Y, Vasupanrajit A, Abo Algon AA, Tunvirachaisakul C, Hashim Aljanabi AA, et al. The tryptophan catabolite or kynurenine pathway in major depressive and bipolar disorder: A systematic review and meta-analysis. Brain Behav Immun-Health. (2022) 26:100537. doi: 10.1016/j.bbih.2022.100537
92. Keegan MR, Chittiprol S, Letendre SL, Winston A, Fuchs D, Boasso A, et al. Tryptophan metabolism and its relationship with depression and cognitive impairment among HIV-infected individuals. Int J Tryptophan Res. (2016) 9:79–88. doi: 10.4137/IJTR.S36464
93. Godfrey KEM, Gardner AC, Kwon S, Chea W, and Muthukumaraswamy SD. Differences in excitatory and inhibitory neurotransmitter levels between depressed patients and healthy controls: A systematic review and meta-analysis. J Psychiatr Res. (2018) 105:33–44. doi: 10.1016/j.jpsychires.2018.08.015
94. Li Y, Wang L, Huang J, Zhang P, Zhou Y, Tong J, et al. Serum neuroactive metabolites of the tryptophan pathway in patients with acute phase of affective disorders. Front Psychiatry. (2024) 15:1357293. doi: 10.3389/fpsyt.2024.1357293
95. Dantzer R, O’Connor JC, Freund GG, Johnson RW, and Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. (2008) 9:46–56. doi: 10.1038/nrn2297
96. Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, et al. The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J Biochem. (2006) 139:655–62. doi: 10.1093/jb/mvj072
97. Liang W, Liu Y, Zhou K, Jian P, Zhang Q, Chang Z, et al. Ginsenoside Rb1 prevents lipopolysaccharide-induced depressive-like behavior by inhibiting inflammation and neural dysfunction and F2 elicits a novel antidepressant-like effect: a metabolite-based network pharmacology study. J Ethnopharmacol. (2022) 282:114655. doi: 10.1016/j.jep.2021.114655
98. Gisslén M, Heslegrave A, Veleva E, Yilmaz A, Andersson LM, Hagberg L, et al. CSF concentrations of soluble TREM2 as a marker of microglial activation in HIV-1 infection. Neurol Neuroimmunol Neuroinflamm. (2018) 6:e512. doi: 10.1212/NXI.0000000000000512
99. Lirng JF, Chen HC, Fuh JL, Tsai CF, Liang JF, and Wang SJ. Increased myo-inositol level in dorsolateral prefrontal cortex in migraine patients with major depression. Cephalalgia. (2015) 35:702–9. doi: 10.1177/0333102414557048
100. Graham AS, Holmes MJ, Little F, Dobbels E, Cotton MF, Laughton B, et al. MRS suggests multi-regional inflammation and white matter axonal damage at 11 years following perinatal HIV infection. NeuroImage Clin. (2020) 28:102505. doi: 10.1016/j.nicl.2020.102505
101. Kong Y, Zhang X, Li L, Zhao T, Huang Z, Zhang A, et al. Microglia-derived vitamin D binding protein mediates synaptic damage and induces depression by binding to the neuronal receptor megalin. Adv Sci (Weinh). (2024) 12:e2410273. doi: 10.1002/advs.202410273
102. Liu F, Bai Q, Tang W, Zhang S, Guo Y, Pan S, et al. Antioxidants in neuropsychiatric disorder prevention: neuroprotection, synaptic regulation, microglia modulation, and neurotrophic effects. Front Neurosci. (2024) 18:1505153. doi: 10.3389/fnins.2024.1505153
103. Palmer CS, Cherry CL, Sada-Ovalle I, Singh A, and Crowe SM. Glucose metabolism in T cells and monocytes: new perspectives in HIV pathogenesis. EBioMedicine. (2016) 6:31–41. doi: 10.1016/j.ebiom.2016.02.012
104. Palmer CS, Duette GA, Wagner MCE, Henstridge DC, Saleh S, Pereira C, et al. Metabolically active CD4+ T cells expressing Glut1 and OX40 preferentially harbor HIV during in vitro infection. FEBS Lett. (2017) 591:3319–32. doi: 10.1002/1873-3468.12843
105. Zwiep JC, Milaneschi Y, Giltay EJ, Vinkers CH, Penninx BWJH, and Lamers F. Depression with immuno-metabolic dysregulation: Testing pragmatic criteria to stratify patients. Brain Behav Immun. (2024) 124:115–22. doi: 10.1016/j.bbi.2024.11.033
106. Palmer CS and Crowe SM. Immunometabolism may provide new insights into novel mechanisms of HIV reservoir persistence. AIDS. (2016) 30:2895–6. doi: 10.1097/QAD.0000000000001114
107. Miller AH, Maletic V, and Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. (2009) 65:732–41. doi: 10.1016/j.biopsych.2008.11.029
108. Ahmed D, Roy D, and Cassol E. Examining relationships between metabolism and persistent inflammation in HIV patients on antiretroviral therapy. Mediators Inflamm. (2018) 2018:6238978. doi: 10.1155/2018/6238978
109. Sereti I, Krebs SJ, Phanuphak N, Fletcher JL, Slike B, Pinyakorn S, et al. Persistent, albeit reduced, chronic inflammation in persons starting antiretroviral therapy in acute HIV infection. Clin Infect Dis. (2017) 64:124–31. doi: 10.1093/cid/ciw683
110. Regidor DL, Detels R, Breen EC, Widney DP, Jacobson LP, Palella F, et al. Effect of highly active antiretroviral therapy on biomarkers of B-lymphocyte activation and inflammation. AIDS. (2011) 25:303–14. doi: 10.1097/QAD.0b013e32834273ad
111. Cassol E, Misra V, Dutta A, Morgello S, and Gabuzda D. Cerebrospinal fluid metabolomics reveals altered waste clearance and accelerated aging in HIV patients with neurocognitive impairment. AIDS. (2014) 28:1579–91. doi: 10.1097/QAD.0000000000000303
112. Antoni MH, Cruess DG, Klimas N, Carrico AW, Maher K, Cruess S, et al. Increases in a marker of immune system reconstitution are predated by decreases in 24-h urinary cortisol output and depressed mood during a 10-week stress management intervention in symptomatic HIV-infected men. J Psychosom Res. (2005) 58:3–13. doi: 10.1016/j.jpsychores.2004.05.010
113. Zhang Q, Li X, Qiao S, Liu S, Shen Z, and Zhou Y. Association between hair cortisol, hair cortisone, and fatigue in people living with HIV. Stress. (2021) 24:772–9. doi: 10.1080/10253890.2021.1919616
114. Zhang Q, Li X, Qiao S, Liu S, Shen Z, and Zhou Y. Greater pain severity is associated with higher glucocorticoid levels in hair among a cohort of people living with HIV. J Pain Res. (2021) 14:645–52. doi: 10.2147/JPR.S301651
115. Fabrazzo M, Cipolla S, Pisaturo M, Camerlengo A, Bucci P, Pezzella P, et al. Bidirectional relationship between HIV/HBV infection and comorbid depression and/or anxiety: A systematic review on shared biological mechanisms. J Pers Med. (2023) 13:1689. doi: 10.3390/jpm13121689
116. Salahuddin MF, Mahdi F, Sulochana SP, and Paris JJ. HIV-1 tat protein promotes neuroendocrine dysfunction concurrent with the potentiation of oxycodone’s psychomotor effects in female mice. Viruses. (2021) 13:813. doi: 10.3390/v13050813
117. Muraki I. Behavioral and neurochemical study on the mechanism of the anxiolytic effect of a selective serotonin reuptake inhibitor, a selective serotonin1A agonist and lithium carbonate. Hokkaido Igaku Zasshi. (2001) 76:57–70.
118. Ruiz-Santiago C, Rodríguez-Pinacho CV, Pérez-Sánchez G, and Acosta-Cruz E. Effects of selective serotonin reuptake inhibitors on endocrine system (Review). BioMed Rep. (2024) 21:128. doi: 10.3892/br.2024.1816
119. Trunfio M, Tang B, Iudicello JE, Ma Q, Franklin DR, Cookson D, et al. Distinct effects of selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors on soluble biomarkers in blood and cerebrospinal fluid of people with HIV. J Infect Dis. (2023) 229:1266–76. doi: 10.1093/infdis/jiad558
120. Denton AR, Mactutus CF, Lateef AU, Harrod SB, and Booze RM. Chronic SSRI treatment reverses HIV-1 protein-mediated synaptodendritic damage. J Neurovirol. (2021) 27:403–21. doi: 10.1007/s13365-021-00960-6
121. Greeson JM, Gettes DR, Spitsin S, Dubé B, Benton TD, Lynch KG, et al. The selective serotonin reuptake inhibitor citalopram decreases human immunodeficiency virus receptor and coreceptor expression in immune cells. Biol Psychiatry. (2016) 80:33–9. doi: 10.1016/j.biopsych.2015.11.003
122. Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord. (1998) 51:215–35. doi: 10.1016/S0165-0327(98)00221-3
123. O’Leary KB and Khan JS. Pharmacotherapy for anxiety disorders. Psychiatr Clin North Am. (2024) 47:689–709. doi: 10.1016/j.psc.2024.04.012
124. Hieronymus F, Lisinski A, and Eriksson E. Impact of sedative and appetite-increasing properties on the apparent antidepressant efficacy of mirtazapine, selective serotonin reuptake inhibitors and amitriptyline: an item-based, patient-level meta-analysis. EClinicalMedicine. (2024) 77:102904. doi: 10.1016/j.eclinm.2024.102904
125. Dionisie V, Filip GA, Manea MC, Manea M, and Riga S. The anti-inflammatory role of SSRI and SNRI in the treatment of depression: a review of human and rodent research studies. Inflammopharmacology. (2021) 29:75–90. doi: 10.1007/s10787-020-00777-5
126. Zhao K, Wang Y, Liu Q, Yu Z, and Feng W. Efficacy comparison of five antidepressants in treating anxiety and depression in cancer and non-cancer patients. Front Neurosci. (2024) 18:1485179. doi: 10.3389/fnins.2024.1485179
127. Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. (2018) 391:1357–66. doi: 10.1016/S0140-6736(17)32802-7
128. Kamp CB, Petersen JJ, Faltermeier P, Juul S, Siddiqui F, Moncrieff J, et al. The risks of adverse events with venlafaxine for adults with major depressive disorder: a systematic review of randomised clinical trials with meta-analysis and Trial Sequential Analysis. Epidemiol Psychiatr Sci. (2024) 33:e51. doi: 10.1017/S2045796024000520
129. Strawn JR, Mills JA, Poweleit EA, Ramsey LB, and Croarkin PE. Adverse effects of antidepressant medications and their management in children and adolescents. Pharmacotherapy. (2023) 43:675–90. doi: 10.1002/phar.2767
130. Fava GA, Benasi G, Lucente M, Offidani E, Cosci F, and Guidi J. Withdrawal symptoms after serotonin-noradrenaline reuptake inhibitor discontinuation: systematic review. Psychother Psychosom. (2018) 87:195–203. doi: 10.1159/000491524
131. Hughes CA, Tseng A, and Cooper R. Managing drug interactions in HIV-infected adults with comorbid illness. CMAJ. (2015) 187:36–43. doi: 10.1503/cmaj.131626
132. van der Lee MJ, Blenke AAM, Rongen GA, Verwey-van Wissen CPWGM, Koopmans PP, Pharo C, et al. Interaction study of the combined use of paroxetine and fosamprenavir-ritonavir in healthy subjects. Antimicrob Agents Chemother. (2007) 51:4098–104. doi: 10.1128/AAC.01243-06
133. Aung GL, O’Brien JG, Tien PG, and Kawamoto LS. Increased aripiprazole concentrations in an HIV-positive male concurrently taking duloxetine, darunavir, and ritonavir. Ann Pharmacother. (2010) 44:1850–4. doi: 10.1345/aph.1P139
134. DeSilva KE, Le Flore DB, Marston BJ, and Rimland D. Serotonin syndrome in HIV-infected individuals receiving antiretroviral therapy and fluoxetine. AIDS (Lond Engl). (2001) 15:1281–5. doi: 10.1097/00002030-200107060-00010
135. Kasper S and Hamon M. Beyond the monoaminergic hypothesis: agomelatine, a new antidepressant with an innovative mechanism of action. World J Biol Psychiatry. (2009) 10:117–26. doi: 10.1080/15622970902717024
136. Savino R, Polito AN, Marsala G, Ventriglio A, Di Salvatore M, De Stefano MI, et al. Agomelatine: A potential multitarget compound for neurodevelopmental disorders. Brain Sci. (2023) 13:734. doi: 10.3390/brainsci13050734
137. Voican CS, Corruble E, Naveau S, and Perlemuter G. Antidepressant-induced liver injury: a review for clinicians. Am J Psychiatry. (2014) 171:404–15. doi: 10.1176/appi.ajp.2013.13050709
138. Gorwood P, Benichou J, Moore N, Álvarez Martínez E, Mertens J, Aguglia E, et al. The safety of agomelatine in standard medical practice in depressed patients: A 26-week international multicentre cohort study. Hum Psychopharmacol. (2021) 36:1–11. doi: 10.1002/hup.2759
139. Köhler-Forsberg O, Lydholm C N, Hjorthøj C, Nordentoft M, Mors O, and Benros ME. Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatr Scand. (2019) 139:404–19. doi: 10.1111/acps.13016
140. Yin Y, Ju T, Zeng D, Duan F, Zhu Y, Liu J, et al. Inflamed” depression: A review of the interactions between depression and inflammation and current anti-inflammatory strategies for depression. Pharmacol Res. (2024) :207:107322. doi: 10.1016/j.phrs.2024.107322
141. Eyre HA, Air T, Proctor S, Rositano S, and Baune BT. A critical review of the efficacy of non-steroidal anti-inflammatory drugs in depression. Prog Neuropsychopharmacol Biol Psychiatry. (2015) 57:11–6. doi: 10.1016/j.pnpbp.2014.10.003
142. Du Y, Dou Y, Wang M, Wang Y, Yan Y, Fan H, et al. Efficacy and acceptability of anti-inflammatory agents in major depressive disorder: a systematic review and meta-analysis. Front Psychiatry. (2024) 15:1407529. doi: 10.3389/fpsyt.2024.1407529
143. Bai S, Guo W, Feng Y, Deng H, Li G, Nie H, et al. Efficacy and safety of anti-inflammatory agents for the treatment of major depressive disorder: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry. (2020) 91:21–32. doi: 10.1136/jnnp-2019-320912
144. Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. (2013) 70:31–41. doi: 10.1001/2013.jamapsychiatry.4
145. Hang X, Zhang Y, Li J, Li Z, Zhang Y, Ye X, et al. Comparative efficacy and acceptability of anti-inflammatory agents on major depressive disorder: A network meta-analysis. Front Pharmacol. (2021) 12:691200. doi: 10.3389/fphar.2021.691200
146. Cui C, Yin Q, and Li J. Statins reduce the recurrence of spontaneous intracerebral hemorrhage: A systematic review and meta-analysis. Clin Neurol Neurosurg. (2025) 249:108723. doi: 10.1016/j.clineuro.2025.108723
147. Liu JC, Lei SY, Zhang DH, He QY, Sun YY, Zhu HJ, et al. The pleiotropic effects of statins: a comprehensive exploration of neurovascular unit modulation and blood-brain barrier protection. Mol Med. (2024) 30:256. doi: 10.1186/s10020-024-01025-0
148. Yang Q, Yang Z, Zeng B, Jia J, and Sun F. Association of statin use with risk of depression and anxiety: A prospective large cohort study. Gen Hosp Psychiatry. (2024) 90:108–15. doi: 10.1016/j.genhosppsych.2024.07.015
149. Molero Y, Cipriani A, Larsson H, Lichtenstein P, D’Onofrio BM, and Fazel S. Associations between statin use and suicidality, depression, anxiety, and seizures: a Swedish total-population cohort study. Lancet Psychiatry. (2020) 7:982–90. doi: 10.1016/S2215-0366(20)30311-4
150. Aldossary KM, Ali LS, Abdallah MS, Bahaa MM, Elmasry TA, Elberri EI, et al. Effect of a high dose atorvastatin as added-on therapy on symptoms and serum AMPK/NLRP3 inflammasome and IL-6/STAT3 axes in patients with major depressive disorder: randomized controlled clinical study. Front Pharmacol. (2024) 15:1381523. doi: 10.3389/fphar.2024.1381523
151. Hendrick V, Pohorylo E, Merchant L, Gerhart J, Arham IN, Draica F, et al. Pharmacovigilance of drug-drug interactions with nirmatrelvir/ritonavir. Infect Dis Ther. (2024) 13:2545–61. doi: 10.1007/s40121-024-01050-w
152. Shang G, Zhou T, Yan X, He K, Liu B, Feng Z, et al. Multi-scale analysis reveals hippocampal subfield vulnerabilities to chronic cortisol overexposure: evidence from cushing’s disease. Biol Psychiatry Cognit Neurosci Neuroimaging. (2025) 10:S2451–9022(25)00014-X. doi: 10.1016/j.bpsc.2024.12.015
153. Piasecka M, Papakokkinou E, Valassi E, Santos A, Webb SM, de Vries F, et al. Psychiatric and neurocognitive consequences of endogenous hypercortisolism. J Intern Med. (2020) 288:168–82. doi: 10.1111/joim.13056
154. Wulsin AC, Herman JP, and Solomon MB. Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology. (2010) 35:1100–12. doi: 10.1016/j.psyneuen.2010.01.011
155. Mailliet F, Qi H, Rocher C, Spedding M, Svenningsson P, and Jay TM. Protection of stress-induced impairment of hippocampal/prefrontal LTP through blockade of glucocorticoid receptors: implication of MEK signaling. Exp Neurol. (2008) 211:593–6. doi: 10.1016/j.expneurol.2008.02.030
156. Jørgensen CK, Juul S, Siddiqui F, Horowitz MA, Moncrieff J, Munkholm K, et al. The risks of adverse events with venlafaxine and mirtazapine versus “active placebo”, placebo, or no intervention for adults with major depressive disorder: a protocol for two separate systematic reviews with meta-analysis and Trial Sequential Analysis. Syst Rev. (2023) 12:57. doi: 10.1186/s13643-023-02221-5
157. Kuloor A, Kumari S, and Metri K. Impact of yoga on psychopathologies and quality of life in persons with HIV: A randomized controlled study. J Bodyw Mov Ther. (2019) 23:278–83. doi: 10.1016/j.jbmt.2018.10.005
158. Jiang T, Hou J, Sun R, Dai L, Wang W, Wu H, et al. Immunological and psychological efficacy of meditation/yoga intervention among people living with HIV (PLWH): A systematic review and meta-analyses of 19 randomized controlled trials. Ann Behav Med. (2021) 55:505–19. doi: 10.1093/abm/kaaa084
159. Scott-Sheldon LAJ, Balletto BL, Donahue ML, Feulner MM, Cruess DG, Salmoirago-Blotcher E, et al. Mindfulness-based interventions for adults living with HIV/AIDS: A systematic review and meta-analysis. AIDS Behav. (2019) 23:60–75. doi: 10.1007/s10461-018-2236-9
160. O’Brien K, Nixon S, Tynan AM, and Glazier R. Aerobic exercise interventions for adults living with HIV/AIDS. Cochrane Database Syst Rev. (2010) 2010:CD001796. doi: 10.1002/14651858.CD001796.pub3
161. Heissel A, Zech P, Rapp MA, Schuch FB, Lawrence JB, Kangas M, et al. Effects of exercise on depression and anxiety in persons living with HIV: A meta-analysis. J Psychosom Res. (2019) 126:109823. doi: 10.1016/j.jpsychores.2019.109823
162. Oliveira VHF, Rosa FT, Santos JC, Wiechmann SL, Narciso AMS, Franzoi de Moraes SM, et al. Effects of a combined exercise training program on health indicators and quality of life of people living with HIV: A randomized clinical trial. AIDS Behav. (2020) 24:1531–41. doi: 10.1007/s10461-019-02678-3
163. Aalbers S, Fusar-Poli L, Freeman RE, Spreen M, Ket JC, Vink AC, et al. Music therapy for depression. Cochrane Database Syst Rev. (2017) 11:CD004517. doi: 10.1002/14651858.CD004517.pub3
164. Cleare A, Pariante CM, Young AH, Anderson IM, Christmas D, Cowen PJ, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol. (2015) 29:459–525. doi: 10.1177/0269881115581093
165. Vreijling SR, Penninx BWJH, Verhoeven JE, Teunissen CE, Blujdea ER, Beekman ATF, et al. Running therapy or antidepressants as treatments for immunometabolic depression in patients with depressive and anxiety disorders: A secondary analysis of the MOTAR study. Brain Behav Immun. (2025) 123:876–83. doi: 10.1016/j.bbi.2024.10.033
166. Rossi FE, Dos Santos GG, Rossi PAQ, Stubbs B, Barreto Schuch F, and Neves LM. Strength training has antidepressant effects in people with depression or depressive symptoms but no other severe diseases: A systematic review with meta-analysis. Psychiatry Res. (2024) 334:115805. doi: 10.1016/j.psychres.2024.115805
167. Ranzani A, Castelli F, Di Biagio A, d’Arminio Monforte A, D’Avolio A, Soria A, et al. Influence of efavirenz and 8-hydroxy-efavirenz plasma levels on cognition and central nervous system side effects. HIV Med. (2024) 25:491–7. doi: 10.1111/hiv.13600
168. Nasreddine R, Florence E, Yombi JC, Henrard S, Darcis G, Van Praet J, et al. Efficacy, durability, and tolerability of bictegravir/emtricitabine/tenofovir alafenamide for the treatment of HIV in a real-world setting in Belgium. HIV Med. (2023) 24:914–24. doi: 10.1111/hiv.13493
169. De La Torre Tarazona E, Moraga E, Vaquer R, Sánchez-Palomino S, de Lazzari E, Luna L, et al. Impact of the initial administration of an antiretroviral drug with latency reversal properties on the HIV reservoir size. Sci Rep. (2025) 15:25306. doi: 10.1038/s41598-025-09474-1
170. Binkley A, Zimmerman M, and Maguire C. Expanding treatment opportunities: reviewing the current state of injectable antiretrovirals for treatment of HIV. Infect Dis Ther. (2024) 13:2475–88. doi: 10.1007/s40121-024-01062-6
171. Lynch S, Cohen RM, Kavanagh M, Sharma A, Raphael Y, Pillay Y, et al. Lessons for long-acting lenacapavir: catalysing equitable PrEP access in low-income and middle-income countries. Lancet HIV. (2025), S2352–3018(25)161-4. doi: 10.1016/S2352-3018(25)00161-4
172. Mendes JC, Braga M das G, Reis AMM, and Silveira MR. Neuropsychiatric adverse drug reactions and associated factors in a cohort of individuals starting dolutegravir-based or efavirenz-based antiretroviral therapy in Belo Horizonte, Brazil. Curr Med Res Opin. (2023) 39:523–31. doi: 10.1080/03007995.2023.2189855
173. What’s new: adult and adolescent ARV HIV clinical guidelines | NIH . Available online at: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/whats-new (Accessed January 14, 2025).
174. Rocabert A, Borjabad B, Berrocal L, Blanch J, Inciarte A, Chivite I, et al. Tolerability of bictegravir/tenofovir alafenamide/emtricitabine versus dolutegravir/lamivudine as maintenance therapy in a real-life setting. J Antimicrob Chemother. (2023) 78:2961–7. doi: 10.1093/jac/dkad338
Keywords: HIV, depression, chronic neuroinflammation, inflammatory cytokines, antiretroviral therapy
Citation: Yu F, Zhu Y, Fan Y, Chen M, Peng Q, Li S, Hao L, Ye F, Xia J and Hu X (2025) HIV-associated depression: a translational framework targeting neuroimmune inflammation and psychosocial stress modulation. Front. Immunol. 16:1645991. doi: 10.3389/fimmu.2025.1645991
Received: 12 June 2025; Accepted: 19 August 2025;
Published: 04 September 2025.
Edited by:
Amit Kumar Madeshiya, The University of Texas Health Science Center at San Antonio, United StatesReviewed by:
Paraskevas Filippidis, Yale School of Medicine, United StatesShalini Saggu, Augusta University, United States
Copyright © 2025 Yu, Zhu, Fan, Chen, Peng, Li, Hao, Ye, Xia and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyu Hu, eGlhb3l1aHUyMDIzMTBAMTYzLmNvbQ==
 Fei Yu
Fei Yu Yue Zhu
Yue Zhu Yiran Fan
Yiran Fan Mingqi Chen
Mingqi Chen Qing Peng
Qing Peng Shenghao Li
Shenghao Li Liyuan Hao
Liyuan Hao Fanghang Ye
Fanghang Ye Jiajun Xia
Jiajun Xia Xiaoyu Hu
Xiaoyu Hu