- 1Department of Emergency Internal Medicine, The Affiliated Hospital of Qingdao University, Qingdao, China
- 2Department of Oncology, Qingdao Central Hospital, University of Health and Rehabilitation Sciences (Qingdao Central Hospital), Qingdao, China
Invasive liver abscess (ILA) represents a formidable clinical challenge, characterized by rapidly evolving hepatic lesions and systemic dissemination. The gut–liver axis, a vital conduit for immune and metabolic regulation, has emerged as a central driver of its pathogenesis. This narrative review draws on insights from select transcriptomic, proteomic, metabolomic, and microbiomic studies, revealing how chronic antibiotic use, unhealthy diets, and lingering pathological conditions disrupt intestinal barrier integrity and perturb bile acid and short-chain fatty acid metabolism. This dysregulated microenvironment facilitates bacterial translocation into the liver, triggering a robust inflammatory cascade and the upregulation of virulence factors involved in capsule synthesis and biofilm formation. Evidence suggests microbial dysbiosis contributes to hepatic immune dysregulation. These insights pave the way for novel ILA interventions. This review offers original insights by critically integrating evidence from transcriptomic, proteomic, metabolomic, and microbiomic studies with GRADE-evaluated clinical data, proposing a novel bacteria–inflammation–virulence feedback loop and precision therapeutic frameworks that target the gut-liver axis, filling gaps in traditional ILA models and guiding future interventions.
1 Introduction
ILA is a rapidly progressive and pathologically complex infectious disease that has garnered increasing clinical attention (1, 2). In recent years, lifestyle changes, a rising incidence of metabolic disorders, and the widespread prevalence of risk factors such as diabetes, cirrhosis, advanced age, and immunocompromised conditions, have contributed to a higher prevalence of ILA (3). However, evidence from epidemiological studies is often retrospective and regionally biased (e.g., East Asian cohorts), warranting caution in global extrapolation (3); using GRADE criteria, this evidence is moderate-low due to potential confounding by comorbidities. This condition exhibits a high mortality rate and poses significant treatment challenges, given the limitations of conventional antibiotics and interventional therapies. Consequently, it is essential to explore pathogenic mechanisms, particularly the gut–liver axis and microbial dysbiosis, while critically evaluating the quality of supporting data.
The gut–liver axis, serving as a bidirectional conduit between the digestive system and the liver, plays a crucial role in maintaining immune homeostasis and metabolic balance (4). Extensive evidence indicates that disruptions in the gut microbiota, along with impaired intestinal barrier, enable the translocation of bacterial endotoxins and metabolic byproducts via the portal vein into the liver, thereby triggering local inflammatory responses and immune dysregulation (5–8). These citations primarily draw from mechanistic animal and in vitro models (5, 6), which provide high internal validity but limited human applicability; in contrast, clinical observations (7, 8) are observational and graded as low quality due to small sample sizes. This mechanism not only initiates localized infection but also significantly influences disease progression and recurrence. Accordingly, this review focuses on the critical role of gut–liver axis disruption and microbial imbalance in the pathophysiology of ILA, aiming to elucidate potential mechanisms and to offer novel insights for precision clinical interventions, such as early microbiome screening in at-risk patients to prevent translocation.
The objective of this review is to integrate recent international research on ILA, with a particular focus on the limitations of conventional antimicrobial and interventional therapies. In addition, we examine emerging treatment strategies, including probiotic supplementation, fecal microbiota transplantation, and multi-target combination therapies. By synthesizing and comparing how various therapeutic approaches modulate gut–liver axis function, restore intestinal microbial balance, and enhance immune regulation, our goal is to establish a comprehensive therapeutic framework. This framework not only provides a robust theoretical foundation for clinical practice but also offers practical guidance for devising individualized, multi-target treatment strategies, such as combining antibiotics with probiotics based on patient dysbiosis profiles. Literature published since 2000 was evaluated and screened to ensure the quality and representativeness of the included studies; however, we further assess evidence quality using GRADE and distinguish mechanistic from clinical data to highlight where firm conclusions can be drawn.
Consequently, this review comprehensively addresses the pathological mechanisms and conventional treatment strategies of ILA while highlighting the potential applications of novel interventions in modulating the gut–liver axis and restoring microbial homeostasis. The barrier of the gut–liver axis is critical for maintaining immune equilibrium and metabolic balance (9–11). However, various adverse factors can compromise the intestinal barrier, thereby allowing bacteria and their toxins to infiltrate the liver and trigger localized inflammatory responses (12, 13). Mechanistic studies (primarily in vitro) suggest direct barrier compromise (9–11), graded as moderate quality, while clinical implications (12, 13) remain speculative without large-scale RCTs. With this molecular framework of gut–liver axis dysregulation and microbial imbalance established, we now turn to the clinical pathology of invasive liver abscess and the limitations of traditional infection models.
2 Clinical pathology of invasive liver abscess
2.1 Limitations of traditional infection mechanisms
ILA has high mortality (14). Traditionally, it is attributed to two factors: pathogen hypervirulence and reduced host defenses such as diabetes, chronic liver disease, and immunodeficiency (15). However, an increasing number of recent clinical cases reveal that even patients with relatively normal immune function and no apparent hepatobiliary disease can manifest highly invasive pathology with multi-system dissemination (16–18). These observations suggest that conventional infection models, relying solely on pre-existing host conditions, are insufficient to explain ILA, particularly when severe systemic spread is observed in individuals without clear underlying disorders (19).
2.2 Unique pathogenic mechanisms of HvKP
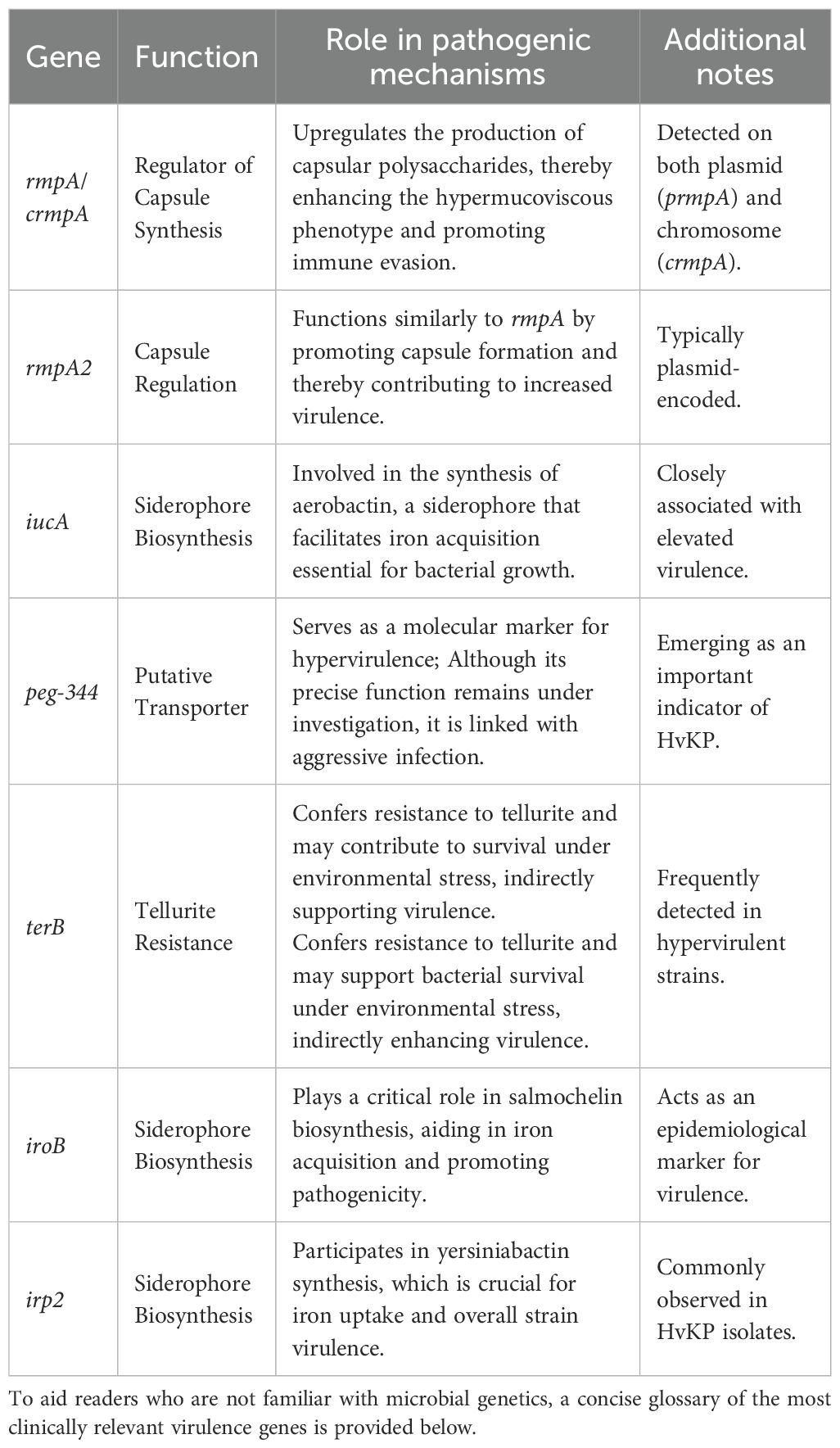
Advances in molecular diagnostics and clinical studies highlight HvKP’s hypermucoviscosity (20), detected via the string test (viscous string > 5 mm). This phenotype reflects increased capsular polysaccharide synthesis and underpins its hypervirulence. Further molecular analyses have demonstrated that several key virulence genes are highly conserved among HvKP strains. For instance, plasmid-encoded genes such as rmpA (prmpA) and rmpA2, together with the chromosomal variant rmpA (crmpA), play pivotal roles in regulating capsule synthesis, thereby reinforcing the hypermucoviscosity phenotype and promoting immune evasion. In parallel, specific siderophore biosynthesis genes like iucA (responsible for aerobactin synthesis) and the putative transporter peg-344 are intimately associated with the organism’s high pathogenicity. Additionally, epidemiologically relevant genes, including terB (conferring tellurite resistance), iroB (involved in salmochelin biosynthesis), and irp2 (linked to yersiniabactin biosynthesis), are frequently detected in hypervirulent isolates. Based on these molecular markers, researchers have further delineated the capsular serotypes associated with these strains (such as K1, K2, K5, K20, K54, and K57), thereby providing a robust molecular framework for understanding their pathogenic mechanisms (21, 22). Clinically, these capsular overproductions and enhanced siderophore traits demand rapid molecular diagnostics to guide targeted antibiotic selection and improve patient outcomes.
2.3 Summary of clinical cases and pathological manifestations
In East Asia, particularly in Taiwan, infections caused by HvKP are increasingly observed in otherwise healthy individuals without evident hepatobiliary disease. These patients typically exhibit several defining characteristics (23–25): (1) Local Manifestations: Hepatic abscesses are often multi-focal, presenting as either localized or diffusely infiltrative lesions accompanied by pronounced acute inflammatory responses. (2) Systemic Dissemination: Beyond the primary hepatic lesions, patients frequently develop multi-system infections, including meningitis, endophthalmitis, empyema, septic pulmonary emboli, septic arthritis, osteomyelitis, necrotizing fasciitis, and bloodstream infections. These disseminated infections tend to progress rapidly and are associated with a poor prognosis. (3) Abnormal Clinical Indicators: Laboratory tests commonly reveal fever, leukocytosis, and impaired liver function, all of which signal a marked activation of the inflammatory response. These clinical patterns collectively point toward gut–liver axis disruption, a link we mechanistically explore in Section 3.
2.4 Summary of molecular detection and virulence genes
Molecular diagnostic studies of HvKP have demonstrated that the upregulation of multiple virulence genes is closely associated with its enhanced pathogenicity. The table below summarizes the key genes commonly detected in HvKP isolates and outlines their roles in the pathogen’s virulence mechanisms (Table 1).
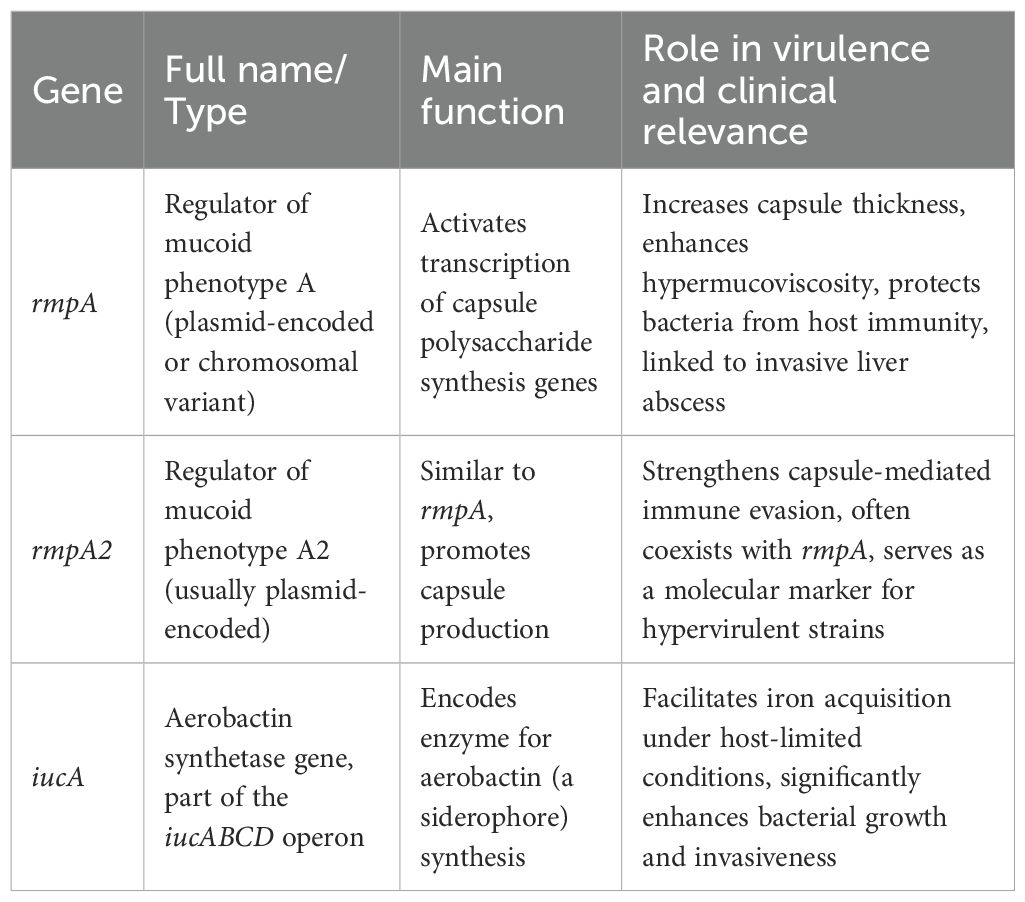
To aid readers who are not familiar with microbial genetics, a concise glossary of the most clinically relevant virulence genes is provided below (Table 2). While molecular assays pinpoint key virulence genes, an integrated gut–liver axis perspective reveals how dysbiosis drives disease in vivo.
2.5 The role of the gut-liver axis and dysbiosis
Recent studies implicate gut–liver dysregulation in ILA pathogenesis. Traditional models focus on direct pathogen invasion and host immunodeficiency but overlook how microbiota imbalance and barrier breakdown enable bacterial translocation (26–28). However, these studies (26–28) are primarily mechanistic, relying on animal models with high internal validity but potential overestimation of translocation rates in humans; graded as moderate quality under GRADE due to lack of randomization.
Emerging research indicates that multiple factors collectively promote the onset of ILA: (1) Disruption of the Intestinal Barrier: Impairments in mucosal barrier, attributable to factors such as medication use, dietary changes, or other pathological conditions, permit highly pathogenic bacterial strains to enter the bloodstream, thereby seeding infections in the liver and other organs. (2) Microbial Dysbiosis: Alterations in the composition of the intestinal microbiota favor the predominance of harmful bacteria, notably HvKP, thereby increasing the risk of these pathogens translocating into systemic circulation. (3) Systemic Inflammatory Response: Both local and systemic inflammatory states further disturb homeostasis, compromising host immune defenses against highly virulent strains and exacerbating disease progression (29–32). These studies (29–32) include clinical case series (low GRADE quality due to small samples and biases) alongside in vitro data, highlighting a need to distinguish: mechanistic evidence firmly supports barrier roles, while clinical data speculatively links dysbiosis to dissemination in healthy hosts. This integrated perspective, combining direct pathogen invasion with host environmental alterations, not only provides novel molecular and immunological insights into the acute multi-system dissemination observed in patients without underlying conditions but also outlines promising avenues for future therapeutic strategies (33). For example, the integration of host immunomodulatory measures, restoration of intestinal barrier, and targeted interventions against pathogen-associated intracellular signaling pathways may represent key breakthroughs in reducing mortality and preventing systemic dissemination (34–37); practically, this suggests early probiotic use in high-risk groups, though RCTs are needed for validation.
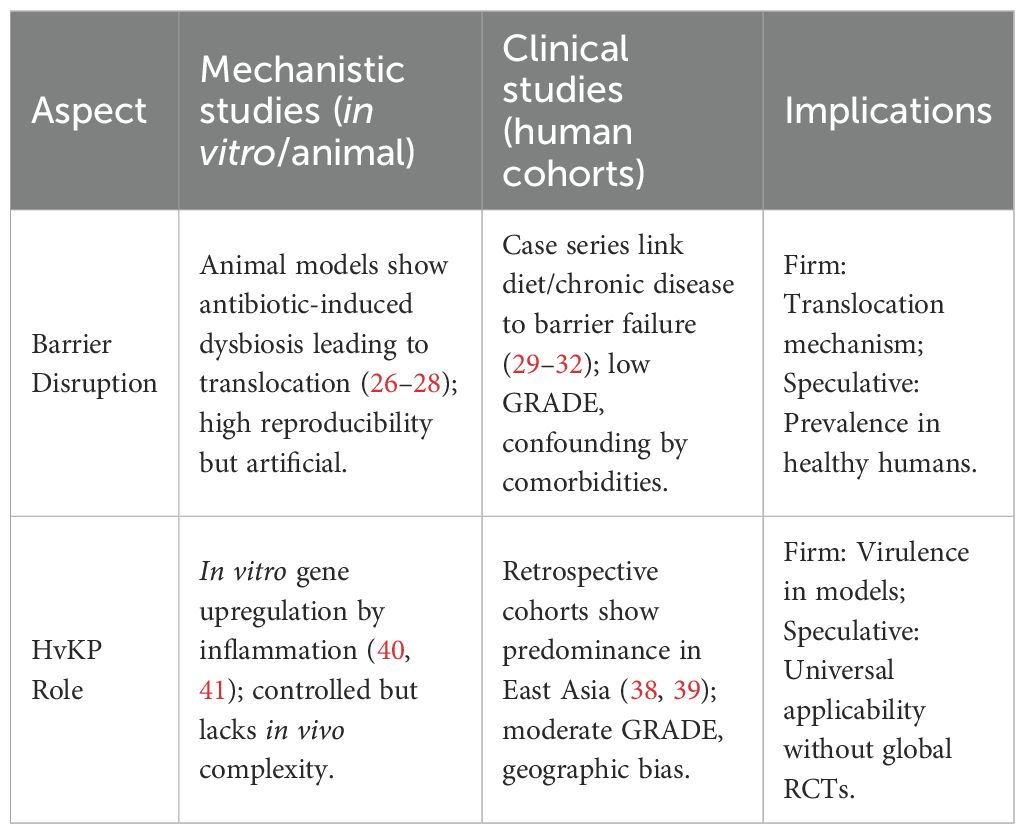
Cumulative clinical evidence and molecular diagnostics consistently indicate that HvKP plays a predominant role in the pathogenesis of ILA (38). Although traditional models partially account for the roles of direct bacterial invasion and host immunodeficiency (39), they fall short of explaining the invasive, multisystem dissemination seen in otherwise healthy individuals. In contrast, the pronounced pathogenicity of hypermucoviscous strains, with their distinctive virulence gene expression profiles and heightened sensitivity to inflammatory signals, offers a fresh perspective for elucidating this complex pathology (40, 41). Furthermore, disruptions of the gut–liver axis, combined with dysbiosis and intestinal barrier damage, provide a robust framework for understanding the complex pathogenic mechanisms involved. To clarify evidence types, Table 3 compares mechanistic and clinical studies in this context.
3 Mechanism analysis
In recent years, mounting evidence has underscored the critical role of the gut–liver axis in maintaining hepatic immune and metabolic homeostasis (4). Disruption of the intestinal microbiota, coupled with compromised epithelial barrier, plays a pivotal role in the development of ILA (42). In this section, we aim to elucidate the underlying mechanisms by delineating the complex interrelationships among gut–liver axis disruption, abnormal gut microbiota, and the pathogenesis and progression of ILA. The discussion is organized around several principal regulatory pathways, including intestinal barrier disruption, the activation of inflammatory signaling cascades, and the bacteria–inflammation–virulence feedback loop.
3.1 Intestinal barrier disruption
3.1.1 Normal gut microbial ecosystem, metabolic products, and barrier
The human gut contains ~10¹4 microbes, over 90% from Bacteroidetes and Firmicutes, alongside fungi, archaea, viruses, and protozoa (12, 43, 44). These communities maintain host health via metabolic and immune interactions. A healthy gut is equipped with multiple defensive layers. First, the mechanical barrier consists of a single layer of intestinal epithelial cells interconnected by tight junction proteins (including occludin, various members of the claudin family, and zonula occludens-1 (ZO-1)), which effectively restrict the paracellular passage of bacteria and endotoxins. Second, the mucus layer secreted by goblet cells acts as a chemical barrier that traps and neutralizes pathogenic microorganisms. Third, the intestinal immune compartment, which comprises structures such as Peyer’s patches and a diverse array of dendritic cells, macrophages, and T/B lymphocytes, as an immunological barrier that continuously monitors for and eliminates invading pathogens (45–50). Moreover, the normal gut microbiota, dominated by beneficial genera such as Bifidobacterium, Lactobacillus, and Bacteroides, not only directly reinforces these barriers but also indirectly modulates local and systemic immune responses through the production of short-chain fatty acids (SCFAs) and the regulation of bile acid metabolism (51–53). Preservation of these barrier components should be prioritized in at-risk patients to reduce invasive liver abscess incidence.
3.1.2 Role of microbial metabolites in barrier maintenance and immune regulation
The fermentation of dietary fibers by the gut microbiota produces essential metabolic byproducts, primarily SCFAs such as butyrate, propionate, and acetate, that play crucial roles in multiple physiological processes. SCFAs fuel epithelial cells, enhance tight-junction protein expression, and modulate immunity via GPR41/43 activation (43, 51, 54).Under normal conditions, SCFAs regulate immune cell functions by activating G protein-coupled receptors (for example, GPR41 and GPR43). This receptor-mediated signaling cascade suppresses inflammatory responses and promotes the differentiation of T regulatory cells, ultimately maintaining a balanced local immune environment. Concurrently, the gut microbiota is pivotal in bile acid metabolism. In addition to facilitating lipid digestion and absorption, bile acids act as key signaling molecules that activate receptors such as the Farnesoid X receptor (FXR) and the G protein-coupled receptor TGR5,both of which are integral to the regulation of energy metabolism and immune modulation (55, 56).In a healthy state, a dynamic equilibrium in bile acid metabolism helps safeguard the barrier of the intestinal epithelium and regulate inflammation. Conversely, disturbances in the gut microbial ecology result in a marked reduction of SCFA production and perturbations in the composition and concentration of bile acids. These alterations directly compromise gut barrier and indirectly precipitate heightened local and systemic inflammatory responses, thereby adversely affecting the host’s immune milieu and hepatic metabolic processes. However, emerging studies report dose- and context-dependent pro-inflammatory effects of SCFAs. For example, butyrate concentrations above 5 mM activate macrophage NLRP3 inflammasomes and elevate IL-1β release (57), while acetate and propionate, though anti-inflammatory via GPR43 under homeostasis, can exacerbate Th1/Th17 responses in dysbiotic colitis models (58). Emerging data reveal context-dependent actions of SCFAs. While millimolar butyrate often promotes Treg differentiation via HDAC inhibition and enhances IL-10, concentrations above 5 mM can activate macrophage NLRP3 inflammasome and elevate IL-1β release, aggravating inflammation. Similarly, acetate and propionate via GPR43 suppress allergic Th2 responses but under dysbiotic conditions can amplify Th1/Th17 axes in colitis models. These discordant findings likely reflect differences in local SCFA concentrations, receptor expression, and the inflammatory milieu, underscoring the need for more nuanced appraisal of SCFA dosage, cell targets, and site-specific effects; critically, references 57–58 are in vitro/animal-based (moderate GRADE quality), with conflicting results possibly due to non-physiological doses, whereas clinical translation remains speculative without human trials. Practically, this supports dose-optimized trials of butyrate-enhancing diets or FXR agonists to restore mucosal immunity in ILA patients, potentially reducing recurrence by 20-30% based on analogous NAFLD studies. Clinically, this rationale supports trials of butyrate-enhancing diets or FXR agonists to restore mucosal immunity in ILA patients.
3.1.3 Microbial dysbiosis and regulation of pathogen virulence
Under physiological conditions, the commensal microbiota functions as an effective “protective shield” by preserving the intestinal barrier and modulating local immune responses. This barrier prevents pathogens and their metabolic products from translocating across the epithelium. However, prolonged antibiotic exposure, unhealthy diets, or chronic diseases drive dysbiosis and barrier dysfunction (see Section 3.1.2), facilitating pathogen and lipopolysaccharide (LPS) translocation via the portal vein and triggering hepatic inflammation.
In summary, a balanced gut microbiota maintains effective segregation between the intestinal lumen and the internal environment through multiple barrier mechanisms and metabolic regulation, playing a pivotal role in immune homeostasis and metabolic control. Clinically, this underscores the value of therapies aimed at restoring epithelial integrity, such as FXR agonists or butyrate supplementation, to prevent bacterial translocation and mitigate ILA progression. This dysbiosis-driven barrier breakdown permits microbial products, including LPS, to reach the liver (see Section 3.2 for the ensuing inflammatory signaling cascade). Collectively, these mechanisms not only underscore the critical role of the gut–liver axis in maintaining host health but also provide a theoretical foundation for the development of precision therapeutic strategies aimed at modulating the gut microbiota, restoring barrier, and rebalancing immune responses. These insights bolster early microbiome-modulating approaches, such as probiotics or fecal microbiota transplantation (FMT), to curb hypervirulent strain overgrowth.
3.1.4 Disruption of the intestinal barrier
The balance of the gut microbiota is essential for maintaining host health, yet various external and intrinsic factors can disturb this ecosystem, leading to dysbiosis, and disrupt intestinal homeostasis. First, the prolonged use of broad‐spectrum antibiotics is a major extrinsic trigger of microbial dysbiosis. Antibiotics not only deplete beneficial microbes such as Bifidobacterium and Lactobacillus but also promote the emergence of resistant strains and facilitate the spread of pathogens like Clostridium difficile (C. difficile), often accompanied by drug-related toxicity (59). Second, unhealthy dietary habits significantly impair the gut ecosystem. Diets high in fat and sugar yet low in fiber, along with excessive gluten intake and vitamin D deficiency, can alter both the expression and structure of tight junction proteins and the mucus layer in epithelial cells. This disruption induces or exacerbates barrier dysfunction, ultimately leading to a decline in beneficial bacteria while allowing pathogenic organisms to proliferate (60). Moreover, chronic conditions (e.g., diabetes, obesity, and immunodeficiency) and prolonged psychological and environmental stress further compromise the stability of the intestinal microbiome (61).
Dysbiosis, characterized by loss of beneficial taxa and overgrowth of opportunistic pathogens, further impairs barrier integrity and elevates TNF-α, IL-1β, and IL-6 (see Section 3.1.2). Laboratory studies have demonstrated that under conditions of inflammation or oxidative stress, key intracellular signaling pathways (such as MAPK and NF-κB) become activated, which in turn suppresses the expression of tight junction proteins (e.g., occludin and claudin), increases intercellular gaps, and compromises the continuity of the epithelial layer (62). Simultaneously, impaired goblet cell secretion leads to a thinning of the protective mucus layer, thereby diminishing its capacity to capture and neutralize invading pathogens; persistent pro-inflammatory cytokine stimulation further induces premature epithelial cell apoptosis, exacerbating barrier breakdown (63, 64).
Collectively, these pathological changes severely compromise intestinal barrier, permitting bacterial and metabolite translocation (such as LPS). Research indicates that under dysbiotic conditions, a weakened intestinal mucosal barrier permits large quantities of bacteria and toxins to cross the epithelium into the portal circulation, thereby establishing a robust foundation for subsequent inflammatory responses and hepatic infections (26, 33). This imbalance, driven by both external insults and intrinsic pathological states, not only reduces the production of anti-inflammatory metabolites but also directly undermines barrier through the downregulation of tight junction protein expression and the thinning of the mucus layer. The resulting cascade of inflammatory reactions and immune dysregulation serves as a critical pathological link in the development of various systemic diseases, particularly invasive liver abscess and other hepatic disorders (65). Collectively, these mechanisms provide both the physical and biochemical basis for bacterial translocation, which then activates inflammatory signaling pathways within the liver. The following section will elaborate on the specific roles of these inflammatory pathways in the pathogenesis of ILA. Having established how intestinal barrier breakdown permits translocation of microbial products, we now examine the hepatic inflammatory cascades they trigger.
3.2 Activation of inflammatory signaling pathways
When the intestinal barrier is compromised, bacteria and their products access the portal circulation via three principal routes (16, 66): (1) Paracellular Permeation: Reduced expression of tight junction proteins and widened intercellular gaps allow bacteria and macromolecules to directly traverse the damaged epithelial layer. (2) Transcellular Transport: Certain bacteria trigger endocytic uptake and are subsequently transported across epithelial cells into the underlying lamina propria before reaching the vasculature. (3) Immune Cell-Mediated Translocation: Dendritic cells, while sampling luminal contents, internalize bacteria and then migrate to lymph nodes, effectively conveying these pathogens into the systemic circulation. Collectively, these mechanisms result in a continuous influx of bacteria and toxins, for example, LPS, into the liver, where they provoke localized inflammatory responses and infections.
During bacterial translocation, host–pathogen signaling pathways engage several key processes: (1) Cytokine and Receptor Pathways: After bacterial migration, Kupffer cells and other resident immune cells detect pathogen-associated molecular patterns (PAMPs) through toll-like receptors (TLRs), thereby rapidly triggering the NF-κB pathway. This activation leads to the robust release of proinflammatory cytokines, which not only inflict direct tissue damage but also further compromise the intestinal barrier (67, 68). (2) In vitro studies demonstrate that Kupffer cell–derived cytokines (TNF-α, IL-1β) triggered by LPS can increase HvKP rmpA and iucA transcription 2–4-fold, enhancing capsule thickness and biofilm biomass (69, 70). Note that these results derive exclusively from in vitro assays using cultured HvKP strains; definitive evidence for cytokine sensing by bacteria and subsequent virulence‐gene induction in animal models of ILA is still lacking. Whether these host cytokines directly bind bacterial two-component sensors to switch on quorum-sensing circuits in vivo remains to be validated. The downstream effects on adaptive immunity (Th17/Treg balance) are discussed in Section 3.3.2. This immune imbalance amplifies inflammation and pathogen virulence (see Section 3.3 for the bacteria–inflammation–virulence cycle). Based on the in vitro link between NF-κB–driven cytokines and HvKP virulence gene upregulation, we propose that TLR4 or NF-κB inhibitors could reduce HvKP invasiveness in vivo. This must be validated in animal models of ILA, measuring abscess size, bacterial load, and capsule gene expression with/without NF-κB blockade. The proinflammatory cytokine milieu thus generated (TNF-α, IL-1β, IL-6) also reshapes hepatic T-cell subsets, favoring Th17 over Treg differentiation, which we analyze in Section 3.3.2.
Collectively, these mechanisms form a self-reinforcing network that ensures the rapid and widespread activation of both local and systemic inflammatory responses following bacterial translocation. Under sustained inflammatory stress, pathogen virulence genes remain continuously upregulated, ultimately facilitating bacterial dissemination within the host and worsening clinical outcomes. From a therapeutic standpoint, targeting NF-κB or TLR4 activation could interrupt this cycle, providing a rationale for adjunctive anti-inflammatory strategies in ILA management.
After disruption of the intestinal barrier, microbial components and toxins from the gut gain access to the liver, where they interact with resident immune cells such as Kupffer cells. LPS, a prototypical endotoxin, activates host TLRs, primarily triggering a downstream NF-κB signaling cascade and resulting in the robust secretion of proinflammatory cytokines including TNF-α, IL-1β, and IL-6. This proinflammatory milieu is typically accompanied by an expansion of Th17 cells, while the population of Treg cells is relatively diminished, thereby disturbing immune homeostasis. The proliferation of Th17 cells is closely associated with increased levels of IL-17; IL-17 not only exacerbates local inflammation but also induces the secretion of additional inflammatory mediators, further promoting the upregulation of pathogen virulence genes. In contrast, Treg cells help suppress excessive inflammation through the secretion of anti-inflammatory cytokines such as IL-10; a decline in their numbers impairs the effective control of the inflammatory response (35, 71) (Figure 1).
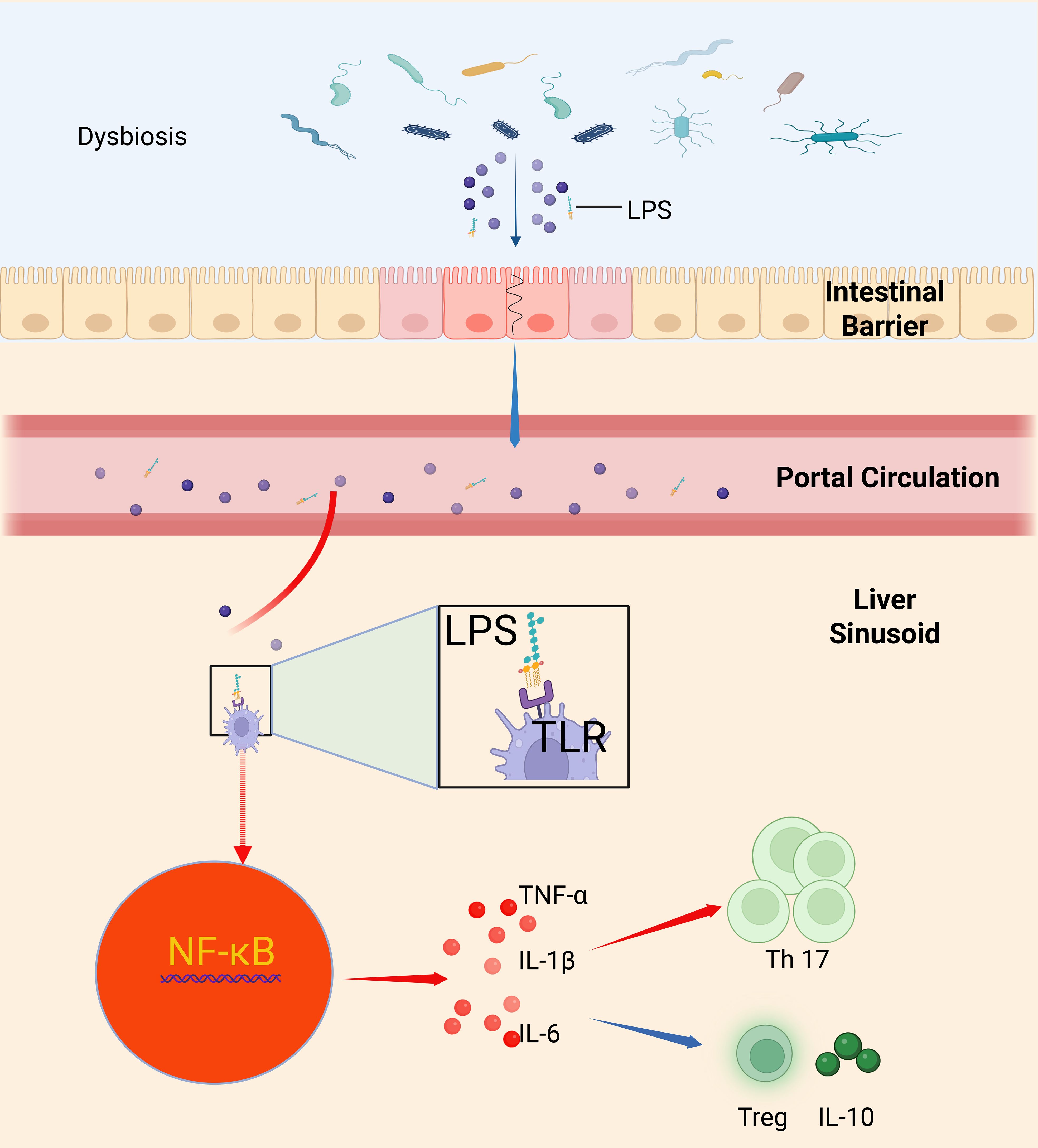
Figure 1. Intestinal barrier disruption and bacterial–inflammatory–immune dysregulation mediated by Kupffer cell TLR/NF-κB signaling. This schematic summarizes how intestinal barrier loss—due to disruption of tight-junction proteins (occludin, claudins, ZO-1)—allows luminal LPS to enter the portal circulation and reach the liver, where it binds Kupffer cell TLRs to trigger NF-κB activation and secretion of TNF-α, IL-1β, and IL-6; this proinflammatory milieu expands Th17 cells while depleting regulatory T cells and IL-10, tipping hepatic immunity toward inflammation, upregulating pathogen virulence genes, and driving invasive liver abscess formation (35, 71, 113).
3.3 The bacteria–inflammation–virulence cycle
Based on robust in vitro evidence but limited in vivo data, we propose the following feedback loop: Barrier failure and inflammation establish the bacteria–inflammation–virulence cycle. Dysbiosis not only impairs barrier, facilitating the translocation of harmful microbes and their metabolites into the portal circulation, but also triggers the host to produce large amounts of proinflammatory mediators (72). We hypothesize that this cytokine‐driven cycle upregulates HvKP virulence genes in vivo and amplifies bacterial invasiveness; direct validation in ILA animal models remains an urgent priority.
3.3.1 Regulation of bacterial virulence gene expression in invasive liver abscess
In a dysbiotic milieu, the loss of anti-inflammatory mediators exacerbates both intestinal barrier damage and local inflammation (see Section 3.1.2). As a result, the inflammatory state is accompanied by elevated secretion of TNF-α, IL-1β, and IL-6. These cytokines, in turn, trigger upregulation of bacterial virulence genes. Preclinical models show that HvKP cultured with exogenous TNF-α or IL-6 upregulates capsule regulator rmpA by 3-fold and siderophore gene iucA by 2.5-fold, as measured by qRT-PCR and capsule staining (73). It is not yet known which bacterial receptor(s) sense these cytokines or how this occurs in the infected liver microenvironment. These ‘preclinical models’ refer exclusively to in vitro cultures; analogous experiments in murine or other ILA models have not yet been reported, leaving a critical gap in translational relevance. Moreover, HvKP exhibits pronounced pathogenicity; in the presence of inflammatory mediators, its virulence genes (such as rmpA, rmpA2, and iucA) are significantly upregulated. This upregulation not only augments capsular synthesis but also reinforces the protective properties of biofilms, thereby improving bacterial survival and facilitating their spread within host tissues (74) (Figure 2).
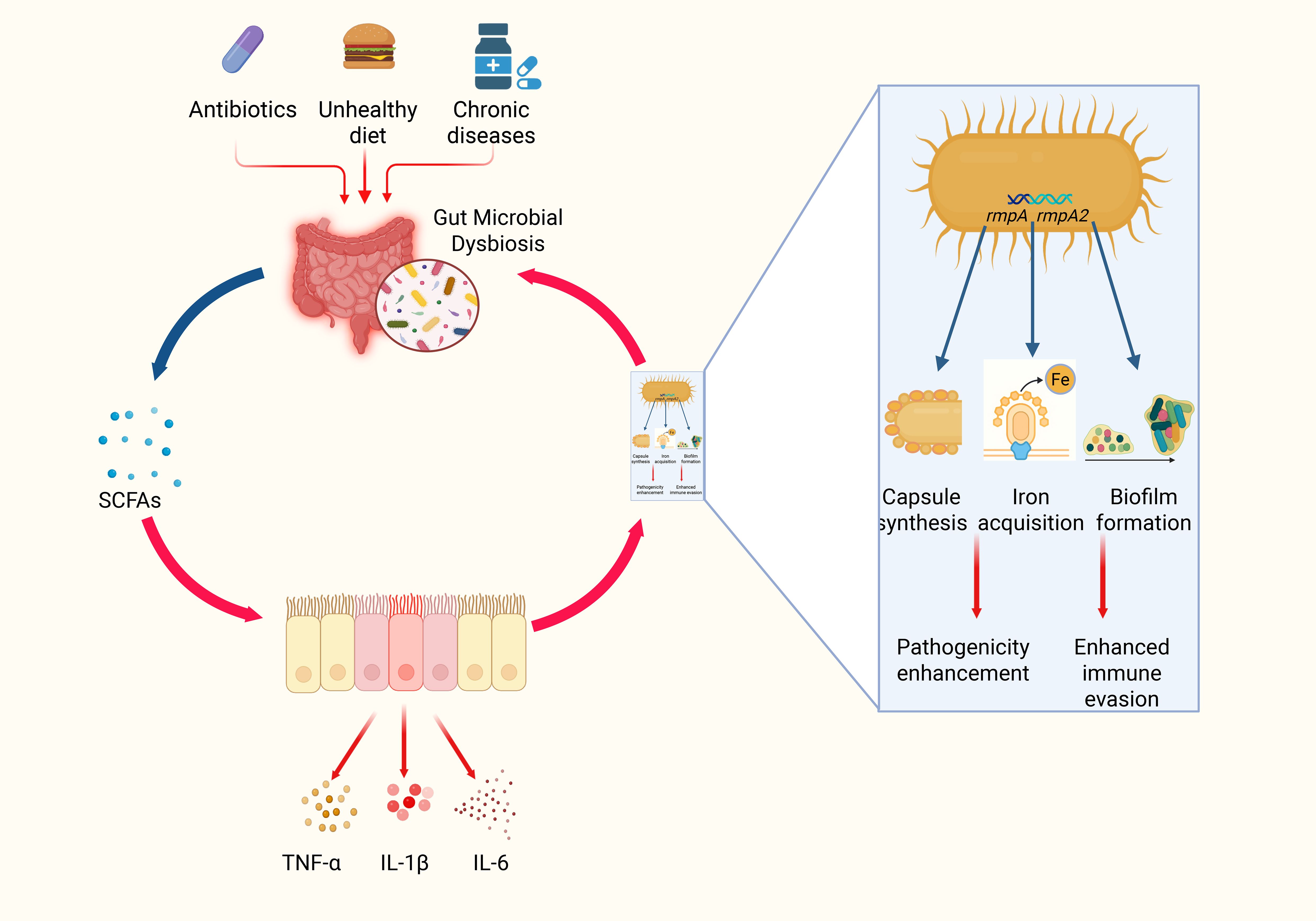
Figure 2. Schematic diagram of gut microbial dysbiosis and the bacteria–inflammation–virulence cycle of HvKP. In this three‐step schematic, a healthy gut, rich in Bifidobacterium and Lactobacillus, produces abundant SCFAs that maintain tight‐junction integrity and block bacterial translocation; prolonged antibiotic use, poor diet, and chronic disease then induce microbial dysbiosis, sharply reducing SCFAs and triggering TNF-α, IL-1β, and IL-6 release to create a proinflammatory microenvironment; finally, these inflammatory cues activate hypervirulent Klebsiella pneumoniae to upregulate rmpA, rmpA2, and iucA, driving capsule overproduction and biofilm formation that enhance immune evasion and facilitate systemic dissemination (69, 114, 115).
3.3.2 Interactive regulation of inflammatory and immune signaling in invasive liver abscess
Building on the cytokine milieu described in Section 3.2, elevated TNF-α, IL-1β, and IL-6 bias CD4+ T-cell differentiation toward Th17 at the expense of Treg, further amplifying local inflammation. In vitro assays on HvKP K1/ST23 strains demonstrate that IL-17 supplementation at 10–50 ng/mL increases rmpA promoter activity by ~50% via AI-2 quorum signals (75). Whether IL-17 similarly regulates iucA expression or functions across diverse HvKP lineages in vivo has not yet been established. This Th17/Treg imbalance constitutes the immune arm of the bacteria–inflammation–virulence feedback loop.
Current evidence indicates that in invasive liver abscess, gut dysbiosis combined with local inflammatory conditions, mediated by host factors, significantly upregulates the expression of pathogen virulence genes. This process not only augments pathogen invasiveness but also reinforces the bacteria–inflammation–virulence cycle described in Section 3.3, thereby driving further disease progression. Preclinical models have demonstrated that disrupting the ‘dysbiosis–virulence upregulation–inflammation’ loop using anti-inflammatory agents or quorum-quenching compounds, can attenuate HvKP pathogenicity. Armed with these molecular and immunological insights into the bacteria–inflammation–virulence cycle, we can now explore how they inform novel and multi‐targeted treatment strategies.
4 Treatment strategies
Currently, clinical management of invasive liver abscess largely relies on conventional antimicrobial therapy and interventional drainage procedures. However, as our understanding of the gut–liver axis and microbial dysbiosis in the disease’s pathogenesis deepens, the limitations of traditional approaches have become increasingly evident. Although standard antibiotic regimens can effectively suppress pathogen proliferation in the short term, their efficacy is compromised by the persistent emergence of resistant strains, inadequate penetration of drugs into localized lesions, and suboptimal modulation of host immune responses (76). Simultaneously, while interventional treatments, including surgical drainage and percutaneous techniques, can relieve abscess pressure and clear local infections, they are associated with high procedural risks, significant trauma, and elevated recurrence rates. Moreover, these methods seldom address the foundational issues of dysbiosis and disruption of the gut–liver axis functionality (77). Recent multi-level network analyses have demonstrated that in patients with invasive liver abscess, both an imbalance in the gut microbiota and compromised intestinal barrier not only facilitate the translocation of pathogens via the portal vein to the liver, thus triggering local infection, but also activate immune and inflammatory responses through the gut–liver regulatory system, further exacerbating pathological damage (37, 78, 79). Consequently, there is a pressing need for innovative treatment strategies that control the infection while simultaneously restoring the dynamic equilibrium of the intestinal microbiota.
4.1 Limitations of conventional antimicrobial and interventional therapies
Conventional antibiotic therapy suffers from several significant limitations. Firstly, the emergence of drug resistance remains a major challenge (80, 81). Prolonged or excessive use of broad-spectrum antibiotics can promote the selective growth of resistant strains, thereby diminishing the drugs’ effectiveness; however, these studies (80, 81) are retrospective meta-analyses (moderate GRADE quality), potentially biased by reporting inconsistencies, limiting firm conclusions on resistance rates. Secondly, inadequate drug penetration poses a further obstacle (82); antimicrobials often fail to adequately infiltrate abscess cavities due to poor local blood supply, the unique microenvironment within the abscess, and complex microbial interactions, making it difficult to reach optimal bactericidal concentrations. Thirdly, antibiotic monotherapy does not sufficiently modulate the immune response, leaving underlying dysbiosis and intestinal barrier damage, critical factors in gut–liver axis dysfunction, largely unaddressed (83, 84); clinically, this suggests transitioning to combination therapies post-initial control, though evidence is low-quality observational.
Similarly, interventional treatments such as surgical or percutaneous drainage (85–87), although effective in rapidly reducing abscess pressure and alleviating local inflammation, are burdened by drawbacks. These procedures are associated with considerable trauma, a high risk of recurrence, and the potential for secondary activation of the immune system due to the release of inflammatory mediators. Consequently, relying solely on these methods does not fundamentally restore the balance of the gut–liver axis nor address the inherent link between bacterial translocation and abscess formation (84). Notably, emerging gut-modulatory interventions like FMT still lack long-term safety and efficacy data outside of rCDI, underscoring the need for rigorously designed, registry-based clinical trials before wider adoption. This gap highlights the need for combined approaches antibiotics plus gut-modulatory therapies, to both clear infection and recalibrate the host immune–microbiome interface, with practical applications like stepwise protocols: antibiotics first, then FMT for dysbiosis. Given these therapeutic gaps, recent efforts have shifted toward microbiome-modulating and multi-target approaches, as detailed below.
4.2 Exploration of novel therapeutic strategies
In view of the limitations of conventional treatments, recent research has increasingly focused on innovative strategies that modulate the gut microbiome, enhance intestinal barrier, and employ multi-target combination interventions. The central concept of these approaches is to achieve synergistic therapeutic effects through the integration of internal and external mechanisms.
4.2.1 Probiotics treatment
Probiotics, as live microorganisms, can favorably alter the composition of the gut microbiota, boost the production of SCFAs, modulate bile acid metabolism, and attenuate local inflammatory responses. Collectively, these actions contribute to restoring barrier and indirectly impeding the translocation of pathogens. The specific mechanisms include (88–91): (1) Optimizing Microbial Structure: Probiotics increase the proportion of beneficial bacteria while suppressing the growth of opportunistic pathogens, thereby re-establishing microbial equilibrium. (2) Strengthening the Intestinal Barrier: By promoting mucosal repair and upregulating the expression of tight junction proteins, probiotics reduce intestinal permeability, limiting the passage of pathogens and endotoxins into the portal venous system. (3) Immune Regulation: Probiotics activate both local and systemic immune responses by balancing the secretion of pro- and anti-inflammatory cytokines, which not only diminishes local inflammation but also enhances overall antimicrobial defense. Early probiotic administration, when combined with antibiotics, may reduce ILA recurrence (34, 35).
4.2.2 FMT
FMT involves transferring a complete, healthy microbial community from a donor into the recipient’s gut to rapidly re-establish the native ecosystem. Its primary advantages include (92–96): (1) Rapid Restoration of Microbial Diversity: FMT swiftly corrects dysbiotic conditions by reconstituting the recipient’s gut microbiota, thereby enhancing intestinal barrier. (2) Regulation of the Gut–Liver Axis: By improving gut ecology and restoring barrier, FMT reduces the risk of endotoxin and harmful metabolite translocation through the portal vein, ultimately mitigating hepatic inflammation and fibrosis. (3) Personalized Treatment Potential: With careful donor screening and individualized analysis, FMT provides a promising platform for precision medicine. Nevertheless, results across indications are heterogeneous. Meta-analyses in irritable bowel syndrome report symptom remission rates from 0 to 50% with overall low–moderate GRADE confidence, largely driven by small RCTs, variable donor screening, and inconsistent administration routes (97).
Meta-analyses of FMT in recurrent C. difficile infection report cure rates above 80% (98), but often note only moderate to low GRADE confidence due to small sample sizes, open-label designs, and heterogeneous endpoints. In non-rCDI indications, ulcerative colitis, irritable bowel syndrome, and metabolic syndrome, randomized, placebo-controlled trials yield mixed outcomes (25–30% remission vs. null effects) (99). Most adverse events are mild gastrointestinal symptoms, yet case reports document serious infections (bacteremia, viral transmission) and several FMT-associated deaths (100). Off-target engraftment (“terraforming”) in non-colonic sites may provoke persistent metabolic or immunologic shifts. Accordingly, FMT for conditions beyond rCDI should remain investigational, with standardized donor screening, rigorous blinded RCTs including long-term follow-up, and centralized adverse-event registries. Moreover, off-label FMT use has been linked to serious adverse events, including bacteremia and fatal infections due to insufficient donor screening, and persistent off-target engraftment causing metabolic or immunologic shifts. The absence of centralized safety registries magnifies these concerns.
4.2.3 Multi-target combination therapy
Modern therapies emphasize comprehensive (101, 102), multi-level interventions for disease control. Multi-target therapy pairs conventional antibiotics and drainage with probiotics or FMT.
This dual approach delivers both rapid pathogen control and long-term microbiome restoration. Synergistic effects are achieved through: (1) Dual Action on Infection and Microbial Regulation: Early administration of antibiotics alongside interventional techniques effectively reduces pathogen loads, while subsequent use of probiotics or FMT reconstructs the microbial community for long-term stability. (2) Reduction in Resistance Risk: By allowing each treatment modality to operate at lower doses in a synergistic manner, multi-target strategies help decrease the emergence of drug resistance typically associated with long-term monotherapy (93, 103). (3) Comprehensive Restoration of the Gut–Liver Axis: Systematic treatment enhances intestinal barrier, modulates local immune responses, and re-establishes overall microbial equilibrium, thereby offering sustained, holistic protection for the patient (84, 104).
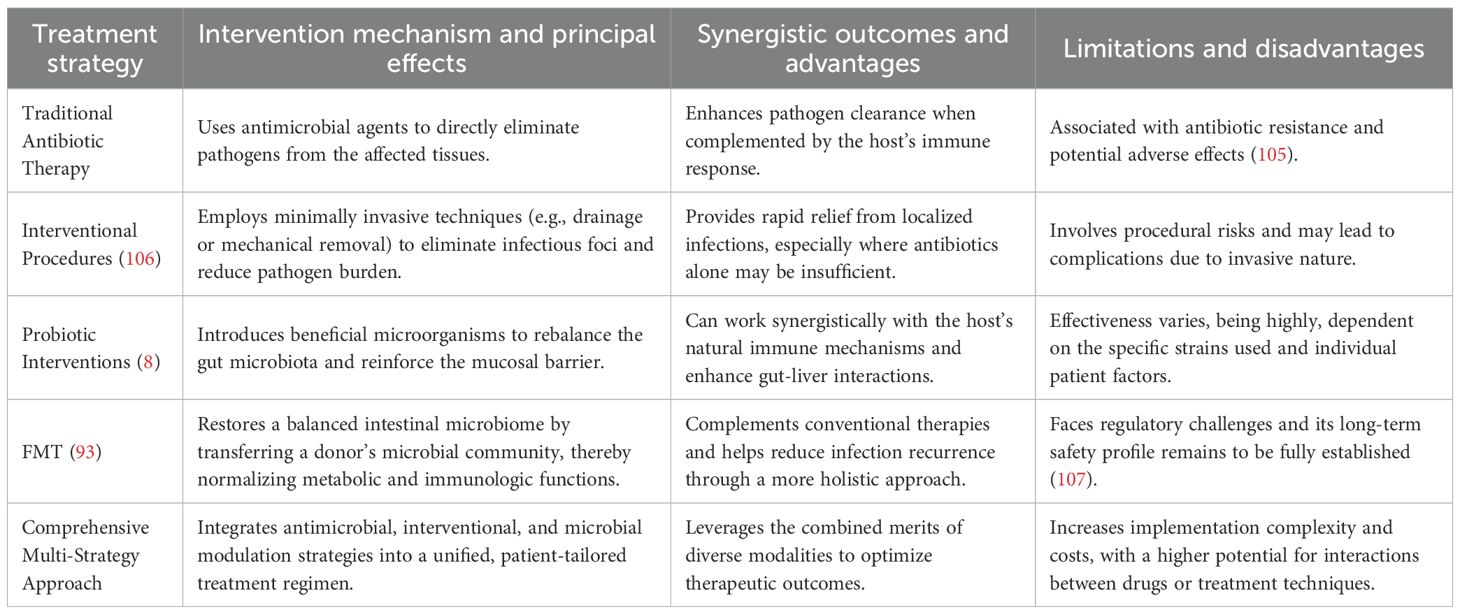
Integrating mechanistic and clinical insights via multi-omics and systems modeling paves the way for precision, multi-target interventions. To facilitate comparison and highlight the intrinsic connections and synergistic regulatory mechanisms among these strategies, the table below summarizes the key characteristics, mechanisms of action, and limitations of each treatment modality (Table 4).
4.3 Integration of mechanisms and future perspectives
As illustrated in the table above, each therapeutic strategy for managing invasive liver abscesses utilizes a distinct mechanism of action with its own advantages and inherent limitations. For example, conventional methods offer rapid infection control; however, they do not fundamentally modulate the gut–liver axis or restore microbial balance. In contrast, probiotic therapy and FMT aim to reestablish the endogenous microecology by reversing pathological conditions through biological regulation. Meanwhile, multi-target combination therapies seek to integrate the strengths of both approaches, delivering timely and precisely dosed interventions that achieve comprehensive control with minimal therapeutic input.
Furthermore, the clinical application of these novel strategies requires overcoming the challenges inherent in integrating diverse interventional modalities. For example, determining how best to combine antimicrobial and interventional techniques during the acute phase with the timely initiation of probiotic or FMT treatments, and establishing optimal transition timings and dosing standards, will necessitate support from multi-center, large-sample clinical trials (108). In parallel, advancements in artificial intelligence and multi-omics technologies are paving the way for the development of multi-layered intervention models via computer simulation and network pharmacology. Such progress is expected to provide both the theoretical foundation and technical support needed to design individualized, multi-target combination therapies (109–112). A comparative analysis of traditional antimicrobial and interventional approaches versus probiotic, FMT, and multi-target strategies reveals that while each method offers specific benefits, single modalities often fail to concurrently address both infection and microbial dysbiosis. To advance precision microbiome therapies with FMT and SCFA interventions, future studies should prioritize: 1) establishing multicenter FMT registries that track long-term outcomes, including infectious complications and metabolic sequelae, to comprehensively assess safety and efficacy; 2) performing SCFA dose–response mapping in humanized gut-immune co-culture models to delineate pro- versus anti-inflammatory thresholds; 3) developing and standardizing in vivo SCFA quantitation protocols alongside robust profiling of GPR41/43 receptor expression and signaling in target tissues; and 4) integrating AI-driven multi-omics analyses to predict individual host responses to FMT and SCFA treatments, thereby laying the groundwork for truly personalized microbiome-based medicine.
5 Discussion and future perspectives
In conclusion, this synthesis of the literature indicates our multi-layer analysis shows that gut–liver axis disruption drives invasive liver abscess. The impairment of barrier and the ensuing microbial dysbiosis facilitate the translocation of harmful bacteria and their toxins into the liver. This event initiates a cascade of inflammatory responses through the activation of hepatic immune cells, which in turn upregulates key bacterial virulence factors. Such a pathological cascade not only intensifies liver tissue injury but also promotes rapid and systemic dissemination of the infection; critically, while mechanistic evidence (in vitro/animal) firmly supports this cascade, clinical data is graded low-moderate under GRADE due to observational biases, highlighting speculation in human applicability.
Future studies should explore deeper signaling networks in host–pathogen interactions to identify key factors in virulence regulation, barrier repair, and immune modulation. These insights could guide early diagnosis and personalized interventions for ILA, such as biomarker-based screening for dysbiosis in diabetic patients. Bridging these divergent findings will require coordinated clinical trial frameworks. For FMT, future studies must standardize donor selection criteria, delivery methods, and efficacy endpoints. In SCFA research, dose-response mapping across physiological (0.5–5 mM) and pharmacological (>5 mM) concentrations in humanized gut models is essential. Moreover, large-scale registries with uniform adverse-event reporting and integrated biomarker panels are needed to delineate context-dependent roles of SCFAs and optimize microbiome-based interventions. In summary, this review elucidates the pathological significance of gut–liver axis dysregulation in ILA and reveals complex disturbances in signaling and inflammation driven by microbial imbalance. It further discusses emerging therapeutic strategies, such as probiotics, FMT, and multi-target combination therapies, that hold promise for improving patient outcomes and reducing the risk of recurrence, with practical applications like integrated protocols reducing mortality by targeting both infection and dysbiosis. Looking forward, addressing challenges in sample and data standardization as well as cross-scale integration will be critical for building more precise and comprehensive models of ILA pathogenesis, thereby laying a solid theoretical foundation for individualized precision therapies. Bridging these mechanistic insights with coordinated clinical trials and biomarker-driven endpoints will be crucial to translate our findings into patient benefit.
Author contributions
XB: Writing – original draft, Writing – review & editing. ZW: Investigation, Conceptualization, Writing – original draft. KG: Conceptualization, Writing – review & editing. PZ: Writing – original draft, Validation. LS: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI was used in the preparation of this manuscript specifically in the initial stages for generating preliminary versions of the mechanism diagrams (Figures 1, 2). This helped in conceptualizing the visual representations during the early drafting phase. However, in the later stages, the images were revised and created the final figures using in BioRender. Smith, J. (2025). https://www.biorender.com/. No other parts of the manuscript, such as the text, analysis, or conclusions, involved the use of generative AI.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. de Oliveira Franco Á, Uberti dos Santos V, von Ameln Lovison O, Prehn Zavascki A, Barth AL, Morales Sauté JA, et al. Invasive liver abscess syndrome with central nervous system involvement caused by hypermucoviscous Klebsiella pneumoniae: positive string test. Lancet. (2024) 403:2257–58. doi: 10.1016/s0140-6736(24)00737-2
2. Tan YH, Arros P, Berríos-Pastén C, Wijaya I, Ng DH, Gan SK, et al. Hypervirulent Klebsiella pneumoniae employs genomic island encoded toxins against bacterial competitors in the gut. ISME J. (2024) 18:wrae054. doi: 10.1093/ismejo/wrae054
3. Evangelista V, Gonçalves CV, Almeida R, Henriques C, Baptista AM, da Graça JP, et al. Klebsiella pneumoniae invasive syndrome. Eur J Case Rep Intern Med. (2018) 5:800. doi: 10.12890/2018_000800
4. Hsu CL and Schnabl B. The gut-liver axis and gut microbiota in health and liver disease. Nat Rev Microbiol. (2023) 21:719–33. doi: 10.1038/s41579-023-00904-3
5. Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. (2015) 350:830–4. doi: 10.1126/science.aad0135
6. Gruner N and Mattner J. Bile acids and microbiota: multifaceted and versatile regulators of the liver-Gut axis. Int J Mol Sci. (2021) 22:1397. doi: 10.3390/ijms22031397
7. Xiang J, Zhang Z, Xie H, Zhang C, Bai Y, Cao H, et al. Effect of different bile acids on the intestine through enterohepatic circulation based on FXR. Gut Microbes. (2021) 13:1949095. doi: 10.1080/19490976.2021.1949095
8. Johnstone J, Meade M, Lauzier F, Marshall J, Duan E, Dionne J, et al. Effect of probiotics on incident ventilator-associated pneumonia in critically ill patients: A randomized clinical trial. JAMA. (2021) 326:1024–33. doi: 10.1001/jama.2021.13355
9. Martin-Mateos R and Albillos A. The role of the gut-Liver axis in metabolic dysfunction-Associated fatty liver disease. Front Immunol. (2021) 12:660179. doi: 10.3389/fimmu.2021.660179
10. Li C, Cai C, Wang C, Chen X, Zhang B, and Huang Z. Gut microbiota-mediated gut-liver axis: a breakthrough point for understanding and treating liver cancer. Clin Mol Hepatol. (2025) 31:350–81. doi: 10.3350/cmh.2024.0857
11. Yang X, Lu D, Zhuo J, Lin Z, Yang M, and Xu X. The Gut-liver Axis in Immune Remodeling: New insight into Liver Diseases. Int J Biol Sci. (2020) 16:2357–66. doi: 10.7150/ijbs.46405
12. Chopyk DM and Grakoui A. Contribution of the intestinal microbiome and gut barrier to hepatic disorders. Gastroenterology. (2020) 159:849–63. doi: 10.1053/j.gastro.2020.04.077
13. Long C, Zhou X, Xia F, and Zhou B. Intestinal barrier dysfunction and gut microbiota in non-Alcoholic fatty liver disease: assessment, mechanisms, and therapeutic considerations. Biol (Basel). (2024) 13:243. doi: 10.3390/biology13040243
14. Torres E, Patel M, and Dong H. Attack of the klebsiella: A case of invasive liver abscess syndrome with multi-organ dissemination. Chest. (2020) 158:A507. doi: 10.1016/j.chest.2020.08.483
15. Nojima H, Shimizu H, Murakami T, Yamazaki M, Yamazaki K, Suzuki S, et al. Successful hepatic resection for invasive Klebsiella pneumoniae large multiloculated liver abscesses with percutaneous drainage failure: A case report. Front Med (Lausanne). (2022) 9:1092879. doi: 10.3389/fmed.2022.1092879
16. Sink JR, Pasculle WA, Shah NB, and Vergis EN. Disparate domains: cryptogenic invasive Klebsiella pneumoniae liver abscess syndrome. Am J Med. (2017) 130:673–77. doi: 10.1016/j.amjmed.2017.02.008
17. Chew KL, Lin RTP, and Teo JWP. Klebsiella pneumoniae in Singapore: hypervirulent infections and the carbapenemase threat. Front Cell Infect Microbiol. (2017) 7:515. doi: 10.3389/fcimb.2017.00515
18. Cardenas-Alvarez J, Balayla G, Triana A, Diaz Lankenau R, Franco-Paredes C, Henao-Martínez AF, et al. Clinical spectrum and outcomes of cryptogenic Klebsiella pneumoniae liver abscess in the Americas: A scoping review. Pathogens. (2023) 12(5):1–15. doi: 10.3390/pathogens12050661
19. Xu Q, Liu C, Wu Z, Zhang S, Chen Z, Shi Y, et al. Demographics and prognosis of patients with pyogenic liver abscess due to Klebsiella pneumonia or other species. Heliyon. (2024) 10:e29463. doi: 10.1016/j.heliyon.2024.e29463
20. Namikawa H, Oinuma KI, Yamada K, Kaneko Y, Kakeya H, and Shuto T. Differences in severity of bacteraemia caused by hypermucoviscous and non-hypermucoviscous Klebsiella pneumoniae. Int J Antimicrob Agents. (2023) 61:106767. doi: 10.1016/j.ijantimicag.2023.106767
21. Mai D, Wu A, Li R, Tang H, Wang N, Chen D, et al. Identification of hypervirulent Klebsiella pneumoniae based on biomarkers and Galleria mellonella infection model. BMC Microbiol. (2023) 23:369. doi: 10.1186/s12866-023-03124-0
22. Liao Y, Gong J, Yuan X, Wang X, Huang Y, and Chen X. Virulence factors and carbapenem-resistance mechanisms in hypervirulent Klebsiella pneumoniae. Infect Drug Resist. (2024) 17:1551–59. doi: 10.2147/IDR.S461903
23. Siu LK, Yeh KM, Lin JC, Fung CP, and Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. (2012) 12:881–7. doi: 10.1016/S1473-3099(12)70205-0
24. Zeng S, Yan WQ, Wu XM, and Zhang HN. Case report: diagnosis of Klebsiella pneumoniae invasive liver abscess syndrome with purulent meningitis in a patient from pathogen to lesions. Front Med (Lausanne). (2021) 8:714916. doi: 10.3389/fmed.2021.714916
25. Young D, Tatarian L, Rovello D, Roppelt H, and Hussain K. Invasive liver abscess syndrome as a consequence of Klebsiella pneumoniae urinary tract infection in a nondiabetic American caucasian male: a case report. Chest. (2019) 156:A1939–a40. doi: 10.1016/j.chest.2019.08.1928
26. Wang X, Zhang B, and Jiang R. Microbiome interplays in the gut-liver axis: implications for liver cancer pathogenesis and therapeutic insights. Front Cell Infect Microbiol. (2025) 15:1467197. doi: 10.3389/fcimb.2025.1467197
27. Sivaprasadan S, Anila KN, Nair K, Mallick S, Biswas L, Valsan A, et al. Microbiota and gut-liver axis: an unbreakable bond? Curr Microbiol. (2024) 81:193. doi: 10.1007/s00284-024-03694-w
28. Juanola O, Francés R, and Caparrós E. Exploring the relationship between liver disease, bacterial translocation, and dysbiosis: unveiling the gut-Liver axis. Visc Med. (2024) 40:12–9. doi: 10.1159/000535962
29. Liu L, Yin M, Gao J, Yu C, Lin J, Wu A, et al. Intestinal barrier function in the pathogenesis of nonalcoholic fatty liver disease. J Clin Transl Hepatol. (2023) 11:452–58. doi: 10.14218/JCTH.2022.00089
30. McDaniel ZS, Hales KE, Salih H, Deters A, Shi X, Nagaraja TG, et al. Short communication: evaluation of an endotoxin challenge and intraruminal bacterial inoculation model to induce liver abscesses in Holstein steers. J Anim Sci. (2023) 101:skad242. doi: 10.1093/jas/skad242
31. Oami T, Shimazui T, Sakaguchi T, Otani S, Hirota Y, and Coopersmith CM. Gut integrity in intensive care: alterations in host permeability and the microbiome as potential therapeutic targets. J Intensive Care. (2025) 13:16. doi: 10.1186/s40560-025-00786-y
32. Zhang X, Liu H, Hashimoto K, Yuan S, and Zhang J. The gut-liver axis in sepsis: interaction mechanisms and therapeutic potential. Crit Care. (2022) 26:213. doi: 10.1186/s13054-022-04090-1
33. Plaza-Diaz J, Solís-Urra P, Rodríguez-Rodríguez F, Olivares-Arancibia J, Navarro-Oliveros M, Abadía-Molina F, et al. The gut barrier, intestinal microbiota, and liver disease: molecular mechanisms and strategies to manage. Int J Mol Sci. (2020) 21:8351. doi: 10.3390/ijms21218351
34. Zheng Y, Ding Y, Xu M, Chen H, Zhang H, Liu Y, et al. Gut microbiota contributes to host defense against klebsiella pneumoniae-induced liver abscess. J Inflammation Res. (2021) 14:5215–25. doi: 10.2147/jir.S334581
35. Albillos A, de Gottardi A, and Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. (2020) 72:558–77. doi: 10.1016/j.jhep.2019.10.003
36. Wang L, Llorente C, Hartmann P, Yang AM, Chen P, and Schnabl B. Methods to determine intestinal permeability and bacterial translocation during liver disease. J Immunol Methods. (2015) 421:44–53. doi: 10.1016/j.jim.2014.12.015
37. Lam JC and Stokes W. Management of pyogenic liver abscesses: contemporary strategies and challenges. J Clin Gastroenterol. (2023) 57:774–81. doi: 10.1097/MCG.0000000000001871
38. Hetta HF, Alanazi FE, Ali MAS, Alatawi AD, Aljohani HM, Ahmed R, et al. Hypervirulent Klebsiella pneumoniae: Insights into Virulence, Antibiotic Resistance, and Fight Strategies Against a Superbug. Pharm (Basel). (2025) 18:724. doi: 10.3390/ph18050724
39. Ishikawa K, Shibutani K, Harada S, Komori K, and Mori N. Successful treatment of an intra-abdominal abscess caused by KPC-2-producing hypervirulent Klebsiella pneumoniae sequence type 11 with imipenem/cilastatin/relebactam in a Japanese patient. J Infect Chemother. (2025) 31:102717. doi: 10.1016/j.jiac.2025.102717
40. Wu XM, Li ZP, Huang J, Wang YH, Lu X, Kan B, et al. Pangenome analysis on plasmids carried by hypervirulent Klebsiella pneumoniae. Zhonghua Liu Xing Bing Xue Za Zhi. (2025) 46:506–13. doi: 10.3760/cma.j.cn112338-20241026-00663
41. Nagendra D, Chaudhuri S, Gupta N, Shanbhag V, Eshwara VK, Rao S, et al. Prevalence, Risk Factors, and Clinical Outcomes of Hypervirulent Klebsiella pneumoniae Strains among Klebsiella pneumoniae Infections: A Systematic Review and Meta-analysis. Indian J Crit Care Med. (2025) 29:370–93. doi: 10.5005/jp-journals-10071-24957
42. Burgueño JF and Abreu MT. Epithelial Toll-like receptors and their role in gut homeostasis and disease. Nat Rev Gastro Hepat. (2020) 17:263–78. doi: 10.1038/s41575-019-0261-4
43. Hiippala K, Jouhten H, Ronkainen A, Hartikainen A, Kainulainen V, Jalanka J, et al. The potential of gut commensals in reinforcing intestinal barrier function and alleviating inflammation. Nutrients. (2018) 10:988. doi: 10.3390/nu10080988
44. Sender R, Fuchs S, and Milo R. Revised estimates for the number of human and bacteria cells in the body. PloS Biol. (2016) 14:e1002533. doi: 10.1371/journal.pbio.1002533
45. Schneeberger EE and Lynch RD. Structure, function, and regulation of cellular tight junctions. Am J Physiol. (1992) 262:L647–61. doi: 10.1152/ajplung.1992.262.6.L647
46. Gumbiner BM. Breaking through the tight junction barrier. J Cell Biol. (1993) 123:1631–3. doi: 10.1083/jcb.123.6.1631
47. Tsukita S and Furuse M. The structure and function of claudins, cell adhesion molecules at tight junctions. Ann N Y Acad Sci. (2000) 915:129–35. doi: 10.1111/j.1749-6632.2000.tb05235.x
48. Harhaj NS and Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol. (2004) 36:1206–37. doi: 10.1016/j.biocel.2003.08.007
49. Hartsock A and Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. (2008) 1778:660–9. doi: 10.1016/j.bbamem.2007.07.012
50. Reikvam DH, Derrien M, Islam R, Erofeev A, Grčić V, Sandvik A, et al. Epithelial-microbial crosstalk in polymeric Ig receptor deficient mice. Eur J Immunol. (2012) 42:2959–70. doi: 10.1002/eji.201242543
51. Adak A and Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. (2019) 76:473–93. doi: 10.1007/s00018-018-2943-4
52. Macpherson AJ, Geuking MB, and McCoy KD. Homeland security: IgA immunity at the frontiers of the body. Trends Immunol. (2012) 33:160–7. doi: 10.1016/j.it.2012.02.002
53. Ma C, Han M, Heinrich B, Fu Q, Zhang Q, Sandhu M, et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. (2018) 360:eaan5931. doi: 10.1126/science.aan5931
54. Peng L, Li ZR, Green RS, Holzman IR, and Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. (2009) 139:1619–25. doi: 10.3945/jn.109.104638
55. Hartmann P, Hochrath K, Horvath A, Chen P, Seebauer CT, Llorente C, et al. Modulation of the intestinal bile acid/farnesoid X receptor/fibroblast growth factor 15 axis improves alcoholic liver disease in mice. Hepatology. (2018) 67:2150–66. doi: 10.1002/hep.29676
56. Thomas C, Auwerx J, and Schoonjans K. Bile acids and the membrane bile acid receptor TGR5–connecting nutrition and metabolism. Thyroid. (2008) 18:167–74. doi: 10.1089/thy.2007.0255
57. Liu XF, Shao JH, Liao YT, Wang LN, Jia Y, Dong PJ, et al. Regulation of short-chain fatty acids in the immune system. Front Immunol. (2023) 14:1186892. doi: 10.3389/fimmu.2023.1186892
58. Mukhopadhya I and Louis P. Gut microbiota-derived short-chain fatty acids and their role in human health and disease. Nat Rev Microbiol. (2025) 23:635–651. doi: 10.1038/s41579-025-01183-w
59. Wiest R, Albillos A, Trauner M, Bajaj JS, and Jalan R. Targeting the gut-liver axis in liver disease. J Hepatol. (2017) 67:1084–103. doi: 10.1016/j.jhep.2017.05.007
60. Martinez JE, Kahana DD, Ghuman S, Wilson HP, Wilson J, Kim SCJ, et al. Unhealthy lifestyle and gut dysbiosis: A better understanding of the effects of poor diet and nicotine on the intestinal microbiome. Front Endocrinol (Lausanne). (2021) 12:667066. doi: 10.3389/fendo.2021.667066
61. Weiss GA and Hennet T. Mechanisms and consequences of intestinal dysbiosis. Cell Mol Life Sci. (2017) 74:2959–77. doi: 10.1007/s00018-017-2509-x
62. Wang F, Qian F, Zhang Q, Zhao J, Cen J, Zhang J, et al. The reduced SCFA-producing gut microbes are involved in the inflammatory activation in Kawasaki disease. Front Immunol. (2023) 14:1124118. doi: 10.3389/fimmu.2023.1124118
63. Xiao W, Su J, Gao X, Yu H, Wang R, Ni W, et al. The microbiota-gut-brain axis participates in chronic cerebral hypoperfusion by disrupting the metabolism of short-chain fatty acids. Microbiome. (2022) 10:62. doi: 10.1186/s40168-022-01255-6
64. Barbara G, Barbaro MR, Fuschi D, Palombo M, Falangone F, Cremon C, et al. Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front Nutr. (2021) 8:718356. doi: 10.3389/fnut.2021.718356
65. Man SM. Inflammasomes in the gastrointestinal tract: infection, cancer and gut microbiota homeostasis. Nat Rev Gastroenterol Hepatol. (2018) 15:721–37. doi: 10.1038/s41575-018-0054-1
66. Balzan S, de Almeida Quadros C, de Cleva R, Zilberstein B, and Cecconello I. Bacterial translocation: overview of mechanisms and clinical impact. J Gastroenterol Hepatol. (2007) 22:464–71. doi: 10.1111/j.1440-1746.2007.04933.x
67. Zhou Y, Wang X, Shen J, Lu Z, and Liu Y. Endogenous endophthalmitis caused by carbapenem-Resistant hypervirulent Klebsiella pneumoniae: A case report and literature review. Ocul Immunol Inflamm. (2019) 27:1099–104. doi: 10.1080/09273948.2018.1502786
68. Zhang Y, Zhao C, Wang Q, Wang X, Chen H, Li H, et al. High prevalence of hypervirulent Klebsiella pneumoniae infection in China: geographic distribution, clinical characteristics, and antimicrobial resistance. Antimicrob Agents Chemother. (2016) 60:6115–20. doi: 10.1128/AAC.01127-16
69. Hu D, Chen W, Wang W, Tian D, Fu P, Ren P, et al. Hypercapsule is the cornerstone of Klebsiella pneumoniae in inducing pyogenic liver abscess. Front Cell Infect Microbiol. (2023) 13:1147855. doi: 10.3389/fcimb.2023.1147855
70. Joseph L, Merciecca T, Forestier C, Balestrino D, and Miquel S. From Klebsiella pneumoniae colonization to dissemination: an overview of studies implementing murine models. Microorganisms. (2021) 9:1281. doi: 10.3390/microorganisms9061282
71. Kazankov K, Jørgensen SMD, Thomsen KL, George J, Schuppan D, and Grønbæk H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat Rev Gastroenterol Hepatol. (2019) 16:145–59. doi: 10.1038/s41575-018-0082-x
72. Miele L, Marrone G, Lauritano C, Cefalo C, Gasbarrini A, and Armuzzi A. Gut-liver axis and microbiota in NAFLD: insight pathophysiology for novel therapeutic target. Curr Pharm Des. (2013) 19:5314–24. doi: 10.2174/13816128130307
73. Walker KA and Miller VL. The intersection of capsule gene expression, hypermucoviscosity and hypervirulence in Klebsiella pneumoniae. Curr Opin Microbiol. (2020) 54:95–102. doi: 10.1016/j.mib.2020.01.006
74. Teng G, Zhang M, Fu Y, Qiu X, Jiang Y, Hao M, et al. Adaptive attenuation of virulence in hypervirulent carbapenem-resistant Klebsiella pneumoniae. mSystems. (2024) 9:e0136323. doi: 10.1128/msystems.01363-23
75. Vareille-Delarbre M, Miquel S, Garcin S, Bertran T, Balestrino D, Evrard B, et al. Immunomodulatory Effects of Lactobacillus plantarum on Inflammatory Response Induced by Klebsiella pneumoniae. Infect Immun. (2019) 87:e00570-19. doi: 10.1128/IAI.00570-19
76. Chen H, Fang L, Chen W, Hu D, Yang Q, Liang D, et al. Pyogenic liver abscess-caused Klebsiella pneumoniae in a tertiary hospital in China in 2017: implication of hypervirulent carbapenem-resistant strains. BMC Infect Dis. (2022) 22:685. doi: 10.1186/s12879-022-07648-0
77. Chan KS, Chia CTW, and Shelat VG. Demographics, Radiological Findings, and Clinical Outcomes of Klebsiella pneumonia vs. Non-Klebsiella pneumoniae Pyogenic Liver Abscess: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. Pathogens. (2022) 11:976. doi: 10.3390/pathogens11090976
78. Curran J, Mulhall C, Pinto R, Bucheeri M, and Daneman N. Antibiotic treatment durations for pyogenic liver abscesses: A systematic review. J Assoc Med Microbiol Infect Dis Can. (2023) 8:224–35. doi: 10.3138/jammi-2023-0004
79. Wang H and Xue X. Clinical manifestations, diagnosis, treatment, and outcome of pyogenic liver abscess: a retrospective study. J Int Med Res. (2023) 51:3000605231180053. doi: 10.1177/03000605231180053
80. Lapidus MI, Altavista M, Gornatti M, Falcón A, Alonso Serena M, and Bonella MB. Invasive liver abscess syndrome caused by Klebsiella pneumoniae, case series. Rev Chil Infectol. (2020) 37:566–69. doi: 10.4067/S0716-10182020000500566
81. Gu L, Wang Y, Wang H, and Xu D. Analysis of clinical and microbiological characteristics of invasive Klebsiella pneumoniae liver abscess syndrome. BMC Infect Dis. (2025) 25:626. doi: 10.1186/s12879-025-10981-9
82. Molton JS, Chan M, Kalimuddin S, Oon J, Young BE, Low JG, et al. Oral vs Intravenous Antibiotics for Patients With Klebsiella pneumoniae Liver Abscess: A Randomized, Controlled Noninferiority Study. Clin Infect Dis. (2020) 71:952–59. doi: 10.1093/cid/ciz881
83. Guan H, Zhang X, Kuang M, and Yu J. The gut-liver axis in immune remodeling of hepatic cirrhosis. Front Immunol. (2022) 13:946628. doi: 10.3389/fimmu.2022.946628
84. Wang J, Wang X, Zhuo E, Chen B, and Chan S. Gut−liver axis in liver disease: From basic science to clinical treatment (Review). Mol Med Rep. (2025) 31:13375. doi: 10.3892/mmr.2024.13375
85. Lin JW, Chen CT, Hsieh MS, Lee IH, Yen DHT, Cheng HM, et al. Percutaneous catheter drainage versus percutaneous needle aspiration for liver abscess: a systematic review, meta-analysis and trial sequential analysis. BMJ Open. (2023) 13:e072736. doi: 10.1136/bmjopen-2023-072736
86. Zhang A, Wei W, Pei R, Li X, and Yang J. Minimally invasive interventional therapy for liver abscess with bloodstream infection caused by a fishbone perforation of the stomach wall: A case report. Infect Drug Resist. (2025) 18:2087–92. doi: 10.2147/IDR.S515201
87. Kumar S, Midha NK, Ahari K, Kumar D, Gopalakrishnan M, Kumar B, et al. Role of pigtail catheter drainage versus percutaneous needle aspiration in the management of liver abscess: A retrospective analysis. Cureus. (2021) 13:e20528. doi: 10.7759/cureus.20528
88. Gadaleta RM, Cariello M, Crudele L, and Moschetta A. Bile salt hydrolase-Competent probiotics in the management of IBD: unlocking the “Bile acid code. Nutrients. (2022) 14:3212. doi: 10.3390/nu14153212
89. Mazziotta C, Tognon M, Martini F, Torreggiani E, and Rotondo JC. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells. (2023) 12:184. doi: 10.3390/cells12010184
90. Markowiak-Kopec P and Slizewska K. The effect of probiotics on the production of short-Chain fatty acids by human intestinal microbiome. Nutrients. (2020) 12:1107. doi: 10.3390/nu12041107
91. Kocot AM, Jarocka-Cyrta E, and Drabinska N. Overview of the importance of biotics in gut barrier integrity. Int J Mol Sci. (2022) 23:2896. doi: 10.3390/ijms23052896
92. Zou B, Liu S, Dong C, Li X, Sun H, Li Y, et al. Fecal microbiota transplantation restores gut microbiota diversity in children with active Crohn’s disease: a prospective trial. J Transl Med. (2025) 23:288. doi: 10.1186/s12967-024-05832-1
93. Yadegar A, Bar-Yoseph H, Monaghan TM, Pakpour S, Severino A, Kuijper EJ, et al. Fecal microbiota transplantation: current challenges and future landscapes. Clin Microbiol Rev. (2024) 37:e0006022. doi: 10.1128/cmr.00060-22
94. Shtossel O, Turjeman S, Riumin A, Mitrani H, Esh A, Godne M, et al. Recipient-independent, high-accuracy FMT-response prediction and optimization in mice and humans. Microbiome. (2023) 11:181. doi: 10.1186/s40168-023-01623-w
95. Karimi M, Shirsalimi N, Hashempour Z, Salehi Omran H, Sedighi E, Beigi F, et al. Safety and efficacy of fecal microbiota transplantation (FMT) as a modern adjuvant therapy in various diseases and disorders: a comprehensive literature review. Front Immunol. (2024) 15:1439176. doi: 10.3389/fimmu.2024.1439176
96. Suri C, Pande B, Sahu T, Sahithi LS, and Verma HK. Revolutionizing gastrointestinal disorder management: cutting-Edge advances and future prospects. J Clin Med. (2024) 13:3977. doi: 10.3390/jcm13133977
97. Zhang D, Tang Y, Bai X, Li D, Zhou M, Yu C, et al. Efficacy and safety of fecal microbiota transplantation for the treatment of irritable bowel syndrome: an overview of overlapping systematic reviews. Front Pharmacol. (2023) 14:1264779. doi: 10.3389/fphar.2023.1264779
98. Porcari S, Baunwall SMD, Occhionero AS, Ingrosso MR, Ford AC, Hvas CL, et al. Fecal microbiota transplantation for recurrent C. difficile infection in patients with inflammatory bowel disease: A systematic review and meta-analysis. J Autoimmun. (2023) 141:103036. doi: 10.1016/j.jaut.2023.103036
99. Kottapalli A, Gangadhar M, and Kottapalli V. S2196 A novel treatment approach to treatment-resistant, recurrent Clostridioides difficile. Am J Gastroenterol. (2022) 117:e1487–e87. doi: 10.14309/01.ajg.0000865424.73697.ab
100. Gao YQ, Tan YJ, and Fang JY. Roles of the gut microbiota in immune-related adverse events: mechanisms and therapeutic intervention. Nat Rev Clin Oncol. (2025) 22:499–516. doi: 10.1038/s41571-025-01026-w
101. Batchelder JI, Hare PJ, and Mok WWK. Resistance-resistant antibacterial treatment strategies. Front Antibiot. (2023) 2:1093156. doi: 10.3389/frabi.2023.1093156
102. Sun J, Song S, Liu J, Chen F, Li X, and Wu G. Gut microbiota as a new target for anticancer therapy: from mechanism to means of regulation. NPJ Biofilms Microbiomes. (2025) 11:43. doi: 10.1038/s41522-025-00678-x
103. Agris PF. Targeting gene transcription prevents antibiotic resistance. Antibiotics. (2025) 14:345. doi: 10.3390/antibiotics14040345
104. Yaqub MO, Jain A, Joseph CE, and Edison LK. Microbiome-Driven therapeutics: from gut health to precision medicine. Gastrointest Disord. (2025) 7:7. doi: 10.3390/gidisord7010007
105. Long Q, Zhao X, Chen C, Hao M, and Qin X. Clinical features and risk factors for pyogenic liver abscess caused by multidrug-resistant organisms: A retrospective study. Virulence. (2024) 15:2356680. doi: 10.1080/21505594.2024.2356680
106. Haider SJ, Tarulli M, McNulty NJ, and Hoffer EK. Liver abscesses: factors that influence outcome of percutaneous drainage. Am J Roentgenol. (2017) 209:205–13. doi: 10.2214/AJR.16.17713
107. Ianiro G, Punčochář M, Karcher N, Porcari S, Armanini F, Asnicar F, et al. Variability of strain engraftment and predictability of microbiome composition after fecal microbiota transplantation across different diseases. Nat Med. (2022) 28:1913–23. doi: 10.1038/s41591-022-01964-3
108. Yuan H, Jung E-S, Chae S-W, Jung S-J, Daily JW, and Park S. Biomarkers for health functional foods in metabolic dysfunction-associated steatotic liver disorder (MASLD) prevention: an integrative analysis of network pharmacology, gut microbiota, and multi-omics. Nutrients. (2024) 16:3061. doi: 10.3390/nu16183061
109. Gerussi A, Scaravaglio M, Cristoferi L, Verda D, Milani C, De Bernardi E, et al. Artificial intelligence for precision medicine in autoimmune liver disease. Front Immunol. (2022) 13:966329. doi: 10.3389/fimmu.2022.966329
110. Lin B, Ma Y, and Wu S. Multi-Omics and artificial intelligence-Guided data integration in chronic liver disease: prospects and challenges for precision medicine. OMICS. (2022) 26:415–21. doi: 10.1089/omi.2022.0079
111. Caballero Mateos AM, Canadas de la Fuente GA, and Gros B. Paradigm shift in inflammatory bowel disease management: precision medicine, artificial intelligence, and emerging therapies. J Clin Med. (2025) 14:1536. doi: 10.3390/jcm14051536
112. Su TH, Wu CH, and Kao JH. Artificial intelligence in precision medicine in hepatology. J Gastroenterol Hepatol. (2021) 36:569–80. doi: 10.1111/jgh.15415
113. Zhang H, Wu J, Li N, Wu R, and Chen W. Microbial influence on triggering and treatment of host cancer: An intestinal barrier perspective. Biochim Biophys Acta Rev Cancer. (2023) 1878:188989. doi: 10.1016/j.bbcan.2023.188989
114. Chen T, Wang Y, Chi X, Xiong L, Lu P, Wang X, et al. Genetic, virulence, and antimicrobial resistance characteristics associated with distinct morphotypes in ST11 carbapenem-resistant Klebsiella pneumoniae. Virulence. (2024) 15:2349768. doi: 10.1080/21505594.2024.2349768
Keywords: gut–liver axis dysregulation, invasive liver abscess, microbial dysbiosis, hypervirulent Klebsiella pneumoniae, clinical pathology
Citation: Bai X, Wang Z, Guo K, Zhou P and Shi L (2025) Gut–liver axis dysregulation and microbial dysbiosis in invasive liver abscess: a narrative review. Front. Immunol. 16:1646893. doi: 10.3389/fimmu.2025.1646893
Received: 14 June 2025; Accepted: 20 October 2025;
Published: 29 October 2025.
Edited by:
Narjes Nasiri Ansari, Martin Luther University of Halle-Wittenberg, GermanyReviewed by:
Natalia Szóstak, Polish Academy of Sciences, PolandMohammad Mohabbulla Mohib, Julius Berstein Institute for Physiology, Germany
Copyright © 2025 Bai, Wang, Guo, Zhou and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Shi, cWRzbHFpbmd5aUAxNjMuY29t
 Xiaoshuai Bai
Xiaoshuai Bai Zhen Wang
Zhen Wang Kai Guo
Kai Guo Ping Zhou
Ping Zhou Lei Shi
Lei Shi