- Department of Dermatology, Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Hangzhou, China
Patients with Nagashima-type palmoplantar keratoderma (NPPK) experience progressive, painful hyperkeratosis and fissuring of palms and soles that limits daily activities Due to the incomplete understanding of its pathogenesis, there are currently no effective treatments for NPPK. We report a 26-year-old woman with lifelong, worsening palmoplantar keratoderma, nail dystrophy, and concomitant atopic dermatitis refractory to topical treatments. Next-generation sequencing revealed compound heterozygous mutations in SERPINB7 (c.796C>T, p.Arg266Ter) and filaggrin (FLG, c.3321delA, p.Gly1109GlufsTer13), while her asymptomatic parents and brother carried only single heterozygous variants, underscoring the digenic pathogenesis in our patient. After 42 weeks of dupilumab treatment, palmoplantar keratosis and nail changes had almost completely resolved, and the eruption resembled mild chronic eczema. Dupilumab therefore appears to be a safe and effective option for digenic NPPK complicated by atopic dermatitis and warrants further investigation in larger cohorts.
Introduction
Nagashima-type palmoplantar keratoderma (NPPK) is an autosomal recessive genetic disorder caused by homozygous or compound heterozygous mutations in the SERPINB7 (1, 2). It is clinically characterized by sharply demarcated erythema with mild-to-moderate hyperkeratosis on the palms and soles, often extending onto the dorsal aspects of the hands and feet. Patients may additionally develop concomitant fungal infections and eczematous lesions (3). Filaggrin (FLG), a key component of the cutaneous barrier, predisposes to atopic dermatitis when mutated, with FLG loss-of-function variants representing the strongest known genetic risk factor for the disease (4). Study identified a pathogenic SERPINB7 variant as a risk factor for atopic dermatitis (AD) development, indicating that AD and NPPK may share an underlying pathogenic mechanism (5).
Current management of NPPK is hindered by several unresolved challenges. The efficacy of topical medication such as salicylic acid, urea, and α-hydroxy acids, is modest and usually transient, failing to achieve sustained control. Systemic retinoids are limited by dose-dependent toxicities including mucocutaneous dryness, hyperlipidemia and teratogenicity, necessitating rigorous monitoring (6). Recent study has demonstrated that topical gentamicin can significantly ameliorate childhood-onset hyperkeratosis, erythema, maceration, and desquamation (7). The benefit is restricted to patients with nonsense mutations, and chronic topical gentamicin may foster bacterial resistance; therefore, its potential for inducing antimicrobial resistance warrants further investigation. No mechanism-based or gene-targeted therapy has yet reached the clinic, leaving an unmet therapeutic need for patients whose pain, fissuring, and recurrent infections severely erode quality of life.
Case presentation
A 26-year-old woman presented to our outpatient clinic with noticeable keratosis on the both palms, erythematous papules on the lateral hand edge, and occasional itching and tightness. She reported a history of erythema and keratosis on both palms, wrist flexures, thenar eminences, soles, and foot edges since childhood, which had gradually worsened over time. Previous topical treatments had alleviated keratosis and fissuring, but recent control of the rash was poor. Additionally, she noted gradual thickening, desquamation, and pruritus of the right fingernail over the past two years. On examination, well-demarcated keratotic erythema with marked hyperkeratosis and desquamation were observed in the aforementioned areas. Fungal examination of the fingernail was negative, but partial nail fissuring and dystrophy were present. The patient also had a history of atopic dermatitis for over a decade. Her IgE and eosinophil levels were within normal limits, and allergen testing revealed only a mild reaction to dust mites. Histopathological examination of the patient’s right palm during the treatment course revealed hyperkeratosis with parakeratosis, acanthosis, mild spongiosis, and perivascular mononuclear lymphocytic infiltration in the superficial dermis (Figure 1A). She was subsequently diagnosed with Nagashima-type Palmoplantar Keratoderma and atopic dermatitis.
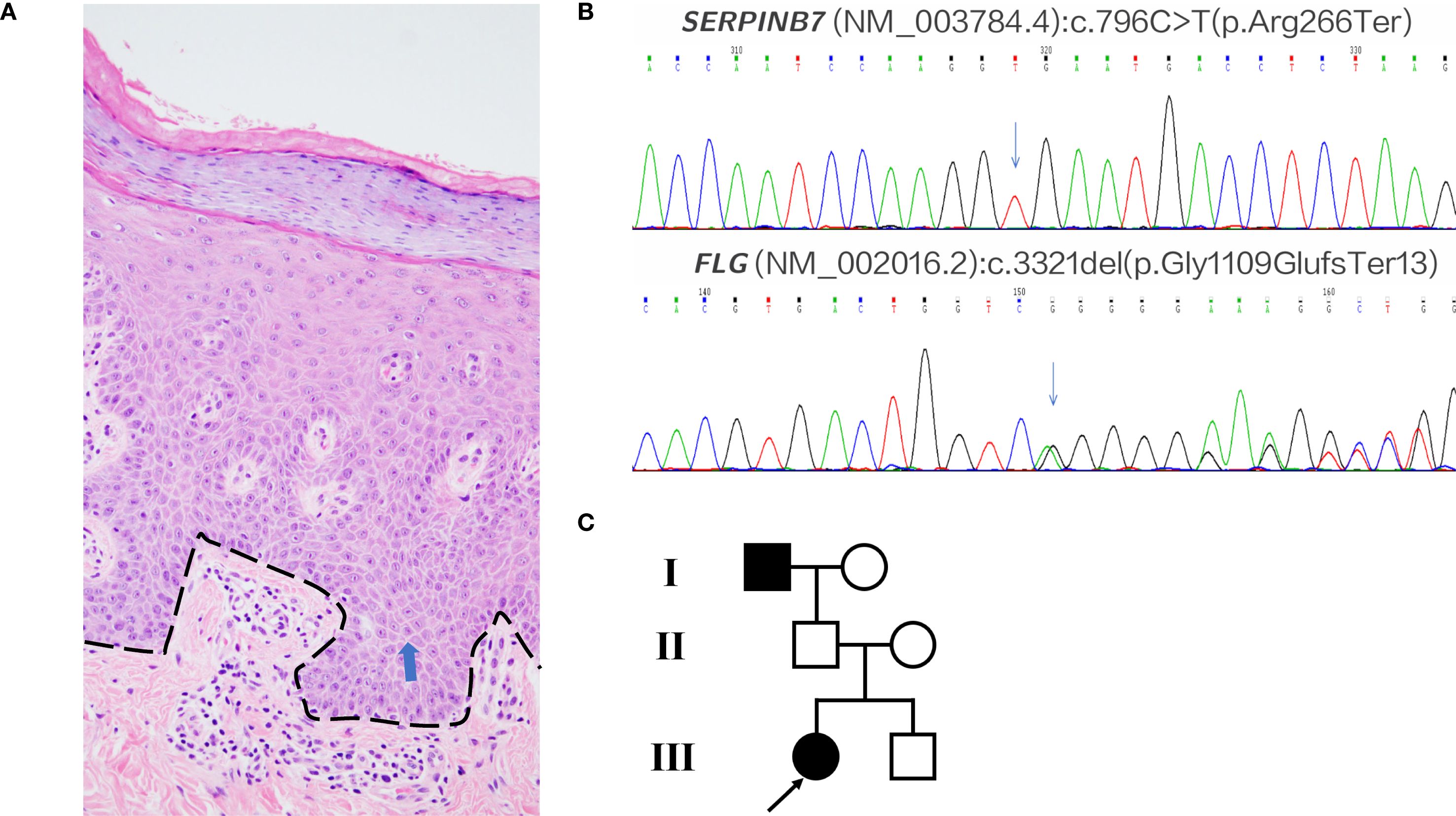
Figure 1. Histopathology and genetics. (A) Palm biopsy (H&E); dashed black line marks the dermal–epidermal junction; blue arrows indicate mild spongiosis. Family biopsies were declined. (B) Patient’s pathogenic variants: SERPINB7 c.796C>T and FLG c.3321delA. (C) Pedigree. Mother: FLG c.3321delA (het). Father: SERPINB7 c.796C>T (het). Brother: both variants (compound het).
Treatment
Given the rare co-occurrence of Nagashima-type palmoplantar keratoderma and severe atopic dermatitis in the patient, we performed Next-Generation Sequencing. The analysis revealed a SERPINB7 gene mutation (c.796C>T, p.Arg266Ter) and a Filaggrin (FLG) gene mutation (c.3321delA, p.Gly1109GlufsTer13) (Figure 1B). Additionally, we conducted medical history interviews and genetic testing on the patient’s immediate family members. The results revealed that the patient’s mother only had a heterozygous FLG mutation, the father only had a heterozygous SERPINB7 mutation while the younger brother had heterozygous mutations in both genes. None of these family members exhibited symptoms of palmoplantar keratoderma or atopic dermatitis. Notably, the patient reported a similar history of palmoplantar keratoderma in her deceased grandfather, though no genetic testing was performed (Figure 1C).
The patient had previously received upadacitinib 15 mg orally once daily but showed an inadequate response. Dupilumab therapy was initiated at a dose of 300 mg subcutaneously on April 10, 2024, followed by a maintenance dosing schedule of 300 mg every two weeks. The keratosis on palms and soles of the patient has shown significant improvement and fingernails essentially returned to normal state after dupilumab treatment (Figure 2).
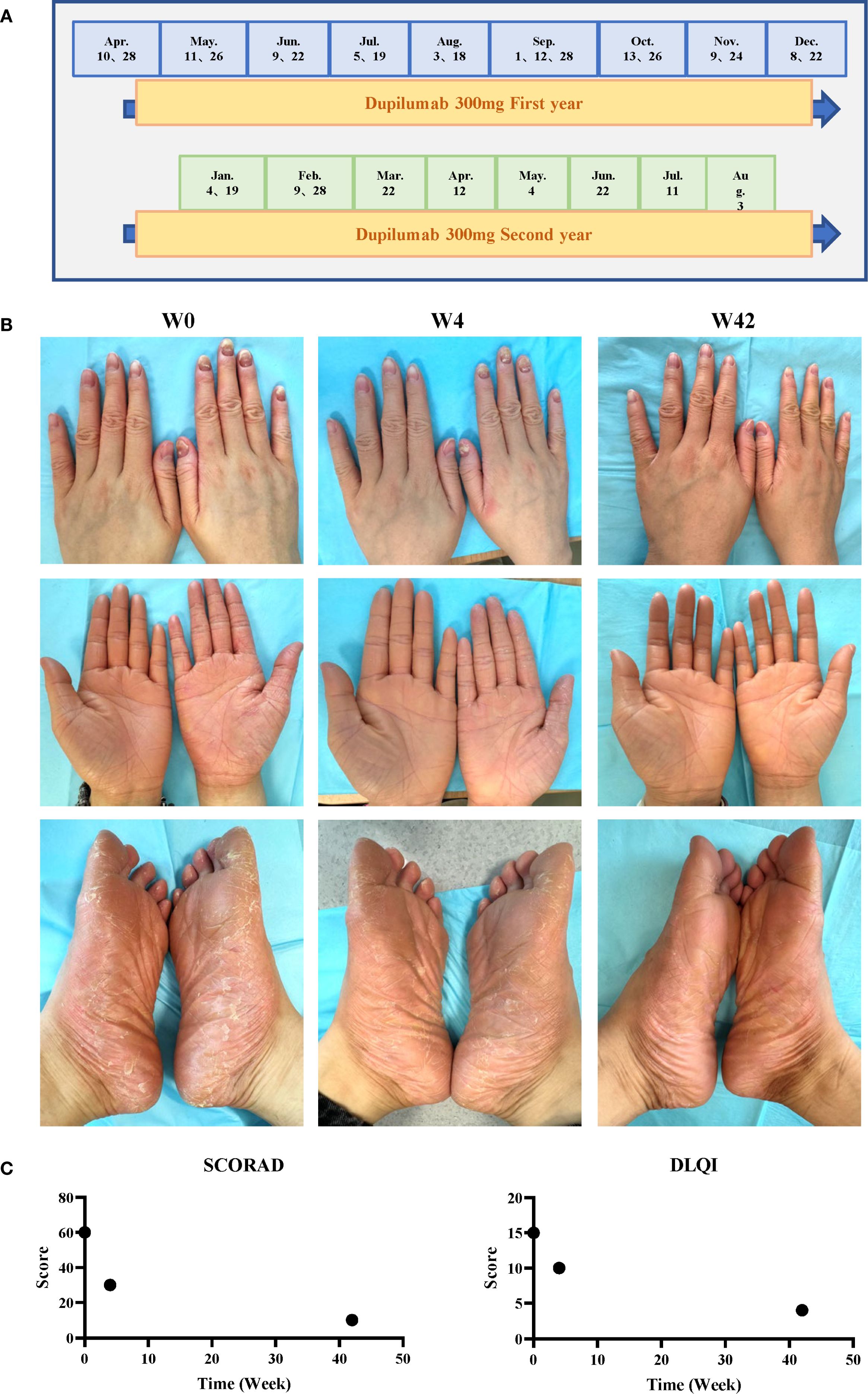
Figure 2. Treatment timeline and clinical outcomes. (A) Dupilumab dosing schedule. (B) Photographs obtained at baseline (week 0), after 4 doses (week 8), and after 22 doses (week 42). (C) Disease severity scores over time.
Discussion
NPPK is most prevalent among Asians, with estimated prevalences of 3.1 per 10,000 and 1.2 per 10,000 in the respective populations. Four recurrent SERPINB7 mutations—c.796C>T, c.522dupT, c.650_653delCTGT, and c.455G>T—account for 97.6% of all mutant alleles in Chinese patients, and the c.796C>T variant represents the founder mutation in Chinese and Japanese cohorts (3, 8). Outside Asia, NPPK is exceptionally rare; the first three Finnish cases were reported in 2020, carrying the homozygous founder mutation c.1136G>A (9). In 2023, four U.S. cases revealed novel variants c.806_818delinsT and c.828dup, presenting with painful, pruritic, malodorous fissures and hyperhidrosis; approximately 75% had comorbid atopic dermatitis (10).
The keratinization process and barrier function of the skin are interrelated. The abnormal hyperkeratosis in NPPK may potentially affect the integrity of the skin barrier, increasing the risk of atopic dermatitis. And FLG mutation c.3321delA has been reported in several patients with ichthyosis vulgaris and atopic dermatitis (11). From a genetic perspective, there may be some common regulatory genes or pathways between the two diseases that have not yet been clarified. To date, it’s the first reported case of combined SERPINB7 and FLG gene mutations.
Dupilumab works by targeting and binding to IL-4Rα and preventing IL-4 and IL-13 signaling. It has been approved for several allergic and inflammatory indications and is also used off-label for a variety of conditions such as bullous pemphigoid (12), Netherton syndrome (13) and Hailey-Hailey disease (14, 15). These off-label uses of dupilumab highlight its potential versatility in treating a range of conditions beyond its approved indications. Three considerations prompted its off-label use in our patient. Firstly, the patient had failed all conventional topical and systemic therapy and there is no targeted therapy approved for NPPK. Secondly, dupilumab has recently been reported to effectively improve bullous pemphigoid with hyperkeratosis and palmoplantar keratoderma (16), suggesting benefit in disorders of abnormal keratinisation driven by IL-4/IL-13 hyperactivity. Thirdly, the patient had atopic dermatitis that met standard prescribing criteria, making dupilumab a rational, dual-purpose therapeutic choice.
Over one year of follow-up, dupilumab markedly improved palmoplantar keratoderma in our patient with NPPK, without relapse or adverse events. These encouraging findings are tempered by the inherent limitations of a single-patient report: limited generalizability, absence of controls, and lack of validated biomarkers for objective quantification of response. Future multi-center, placebo-controlled trials with standardized clinical and molecular endpoints are essential to confirm efficacy, define optimal dosing, and elucidate the precise mechanisms through which IL-4/IL-13 inhibition benefits NPPK.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Sir Run Run Shaw Hospital ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CH: Writing – original draft, Writing – review & editing. HC: Investigation, Methodology, Project administration, Supervision, Writing – review & editing. XC: Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China (grant numbers: 82373491 and 82404156).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mizuno O, Nomura T, Suzuki S, Takeda M, Ohguchi Y, Fujita Y, et al. Highly prevalent SERPINB7 founder mutation causes pseudodominant inheritance pattern in Nagashima-type palmoplantar keratosis. Br J Dermatol. (2014) 171:847–53. doi: 10.1111/bjd.13076
2. Kubo A. Nagashima-type palmoplantar keratosis: a common Asian type caused by SERPINB7 protease inhibitor deficiency. J Invest Dermatol. (2014) 134:2076–9. doi: 10.1038/jid.2014.156
3. Liu J, Chen Z, Hu L, Song Z, Mo R, Shui-Lun L, et al. Investigation of Nagashima-type palmoplantar keratoderma in China: A cross-sectional study of 234 patients. J Dermatol. (2023) 50:375–82. doi: 10.1111/1346-8138.16621
4. Akiyama M. FLG mutations in ichthyosis vulgaris and atopic eczema: spectrum of mutations and population genetics. Br J Dermatol. (2010) 162:472–7. doi: 10.1111/j.1365-2133.2009.09582.x
5. Sliz E, Huilaja L, Pasanen A, Laisk T, Reimann E, Mägi R, et al. Uniting biobank resources reveals novel genetic pathways modulating susceptibility for atopic dermatitis. J Allergy Clin Immunol. (2022) 149:1105–1112.e9. doi: 10.1016/j.jaci.2021.07.043
6. Bodemer C, Steijlen P, Mazereeuw-Hautier J, and O’Toole EA. Treatment of hereditary palmoplantar keratoderma: a review by analysis of the literature. Br J Dermatol. (2021) 184:393–400. doi: 10.1111/bjd.19144
7. Wang S, Yang Z, Liu Y, Zhang H, Liu Z, Wang X, et al. Application of topical gentamicin ointment in the treatment of Nagashima-type palmoplantar keratosis in children with a nonsense mutation. Pediatr Investig. (2023) 7:163–7. doi: 10.1002/ped4.12389
8. Kubo A, Shiohama A, Sasaki T, Nakabayashi K, Kawasaki H, Atsugi T, et al. Mutations in SERPINB7, encoding a member of the serine protease inhibitor superfamily, cause Nagashima-type palmoplantar keratosis. Am J Hum Genet. (2013) 93:945–56. doi: 10.1016/j.ajhg.2013.09.015
9. Hannula-Jouppi K, Harjama L, Einarsdottir E, Elomaa O, Kettunen K, Saarela J, et al. Nagashima-type palmoplantar keratosis in Finland caused by a SERPINB7 founder mutation. J Am Acad Dermatol. (2020) 83:643–5. doi: 10.1016/j.jaad.2019.11.004
10. Braun M, Choate KA, and Mathes EF. Nagashima-type palmoplantar keratoderma: Case series and two novel variants. Pediatr Dermatol. (2023) 40:882–5. doi: 10.1111/pde.15265
11. Zhang J, Yao Y, Tan Y, Hu HY, Zeng LX, and Zhang GQ. Genetic analysis of seven patients with inherited ichthyosis and Nagashima−type palmoplantar keratoderma. Mol Med Rep. (2024) 30:111. doi: 10.3892/mmr.2024.13235
12. Abdat R, Waldman RA, de Bedout V, Czernik A, Mcleod M, King B, et al. Dupilumab as a novel therapy for bullous pemphigoid: A multicenter case series. J Am Acad Dermatol. (2020) 83:46–52. doi: 10.1016/j.jaad.2020.01.089
13. Steuer AB and Cohen DE. Treatment of netherton syndrome with dupilumab. JAMA Dermatol. (2020) 156:350–1. doi: 10.1001/jamadermatol.2019.4608
14. Alamon-Reig F, Serra-García L, Bosch-Amate X, Riquelme-Mc Loughlin C, and Mascaró JM Jr. Dupilumab in Hailey-Hailey disease: a case series. J Eur Acad Dermatol Venereol. (2022) 36:e776–9. doi: 10.1111/jdv.18350
15. Alzahrani N, Grossman-Kranseler J, and Swali R. Hailey-Hailey disease treated with dupilumab: a case series. Br J Dermatol. (2021) 185:680–2. doi: 10.1111/bjd.20475
Keywords: Chinese Nagashima-type palmoplantar keratoderma, atopic dermatitis, dupilumab, SERPINB7, FLG
Citation: Hua C, Cheng H and Chen X (2025) Dupilumab treatment for Chinese Nagashima-type palmoplantar keratoderma associated with atopic dermatitis: a case report. Front. Immunol. 16:1647441. doi: 10.3389/fimmu.2025.1647441
Received: 15 June 2025; Accepted: 09 September 2025;
Published: 25 September 2025.
Edited by:
Alpana Sharma, All India Institute of Medical Sciences, IndiaReviewed by:
Saeedeh Ghorbanalipoor, University of Veterinary Medicine Vienna, AustriaJean-Benoit Monfort, Hôpital Tenon AP-HP, France
Copyright © 2025 Hua, Cheng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Cheng, Y2hlbmdoYW8xQHpqdS5lZHUuY24=; Xianzhen Chen, MzMwOTAzMkB6anUuZWR1LmNu
 Chunting Hua
Chunting Hua Hao Cheng
Hao Cheng Xianzhen Chen*
Xianzhen Chen*